- 1Department of Urology, Second Hospital of Shanxi Medical University, Taiyuan, China
- 2Department of Cardiology, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, China
- 3Department of Gastroenterology, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, China
- 4Department of General Surgery, Second Hospital of Shanxi Medical University, Taiyuan, China
Introduction: Epirubicin is widely used in many malignancies with good efficacy and tolerability. However, investigations about adverse events (AEs) using real-world information are still insufficient.
Methods: We extracted Epirubicin-related reports submitted between the first quarter of 2014 and first quarter of 2023 from FAERS database. Four algorithms were utilized to evaluate whether there was a significant correlation between Epirubicin and AEs.
Results: After de-duplicating, a total of 3919 cases were extracted. Among the 3919 cases, we identified 1472 AEs, 253 of which were found to be adverse drug reactions (ADRs) associated with Epirubicin. We analysed the occurrence of Epirubicin-induced ADRs and found several unexpected significant ADRs, such as hepatic artery stenosis, hepatic artery occlusion, intestinal atresia and so on. Interestingly, we found gait apraxia, a neurological condition, was also significantly associated with Epirubicin. To our knowledge, there haven't studies that have reported an association between gait disorders and the usage of epirubicin.
Discussion: Our study identified new unexpected significant ADRs related to Epirubicin, providing new perspectives to the clinical use of Epirubicin.
Introduction
Epirubicin, the 4′-epimer of doxorubicin, was introduced into clinical use more than 40 years ago and soon established a vital place in cancer chemotherapy (Mouridsen et al., 1990; Pavone-MacalusoTripi et al., 1993). Epirubicin and doxorubicin have different properties due to the different spatial orientation of the hydroxyl group at 4'. Compared to doxorubicin, epirubicin has been shown to cause less myelosuppression, fewer cardiac toxicities, fewer non-hematologic toxicities, and less nausea and vomiting (Henderson et al., 2003; Burnell et al., 2010). The anti-tumor mechanisms of epirubicin appear to intercalate with DNA, inhibit the activity of topoisomerase II, generate free radicals and oxygen, and thus disturb the synthesis of DNA, RNA, and proteins, resulting in its anti-tumor function (Khasraw et al., 2012).
Since its approval by the U.S. Food and Drug Administration (FDA), epirubicin has been widely used to treat many malignancies, including bladder cancer, breast cancer, liver cancer, and non-small cell lung cancer (NSCLC) (Plosker and Faulds, 1993; de Azambuja et al., 2009). Bladder cancer accounts for approximately 90%–95% of urothelial cancers and 4% of malignant cancers in adults (Richters et al., 2020). Since 1985, many studies have shown that epirubicin has good therapeutic effects and high efficacy in preventing bladder cancer recurrence (Tripi et al., 1986; Oosterlinck et al., 1993). Previous clinical trials have shown that, in patients with bladder cancer, the main adverse events of epirubicin are hematuria, cystitis, urinary tract infection, and systemic side effects, which include fever, tiredness, nausea, abdominal pain, and headache—listed on the instruction label (Onrust et al., 1999; Hendricksen et al., 2008). Hepatocellular carcinoma (HCC) accounts for approximately 90% of liver cancer (Forner et al., 2018). Transarterial chemoembolization using doxorubicin (TACE-DOX) is recommended for the therapy of hepatocellular carcinoma (Laface et al., 2021). According to Ellis et al. (1995), epirubicin is well tolerated by patients with hepatocellular carcinoma and has a good curative effect. A study published in 2015 suggested that, compared to patients with hepatocellular carcinoma treated with miriplatin, patients treated with miriplatin-plus-epirubicin were more likely to develop adverse events such as neutropenia and anorexia (Tawada et al., 2015). These adverse events (AEs) have been marked on the instruction label; however, it is worth noting that the majority of safety-related information comes from premarket randomized controlled trials (RCTs) and a few case reports on AEs; these lack extensive real-world data.
A thorough understanding of their possible side effects is essential for the safe use of drugs. Given the limitations of clinical trials and case reports, public databases containing extensive information on drug use, such as the FDA Adverse Event Reporting System (FAERS), have emerged. FAERS contains large-scale information submitted by healthcare professionals, consumers, and manufacturers; therefore, it can help identify AEs that have not been marked on the instruction label (Sakaeda et al., 2013). In this study, the AEs of epirubicin were identified and analyzed using four rigorous algorithms based on the FAERS database. AEs not specified on the medicine labels were observed when the safety of epirubicin was reviewed and analyzed. The findings of this study offer suggestions for the therapeutic application of epirubicin.
Methods
Data source and preprocessing
The FAERS database, containing various reports of spontaneous adverse events and side effects submitted by consumers, healthcare professionals, and manufacturers, has worldwide coverage and large-scale information, making it suitable for the early detection of safety signals and timely characterization of the safety profile (Ji et al., 2019).
This study aimed to evaluate the safety of epirubicin. AE data related to epirubicin submitted between the first quarter of 2014 and the first quarter of 2023 from the FAERS database were extracted and subsequently preprocessed using SAS and Navicat for MySQL software. After cleaning and standardization, it was mapped to MedDRA to identify adverse drug reactions (ADRs) (Ma et al., 2005). Significant ADRs were calculated and mapped to preferred terms (PTs) and system organ class (SOC), which represent the different levels of MedDRA (Brown, 2004). The specific information extracted for this study included age, sex, and country. Serious clinical outcomes were classified as disability, death, hospitalization, and life-threatening events.
Statistical analysis
Multiple algorithms, including ROR, PRR, MHRA, and BCPNN, were used to assess whether epirubicin was significantly associated with AEs. In the formula, the value a represents the total number of AEs for epirubicin, b represents the total number of cases for this specific AE for all other drugs, c represents the total number of other AEs for epirubicin, and d represents the total number of other AEs for all other drugs (Almenoff et al., 2006). The specific formulae are as follows.
ROR
If the lower limit of 95% CI > 1 and a≥3, the ROR should be considered a signal.
PRR
If a≥3 and the lower limit of 95% CI > 1, the PRR should be considered a signal.
BCPNN
If IC-2SD ≤ 0, it should be identified as no signal (−); if 0< IC-2SD ≤ 1.5, it should be identified as a weak signal (+); if 1.5< IC-2SD ≤ 3, it should be identified as a medium signal (++); if IC-2SD > 3, it should be identified as a strong signal (+++). IC-2SD > 0 is identified as the cutoff value for a positive signal.
EBGM
If EBGM05 > 2, it should be considered a signal.
Results
Descriptive analysis
A total of 13,703,053 reports submitted between the first quarters of 2014 and 2023 were extracted from the FAERS database. After deduplicating, 3,919 cases were used for subsequent analyses. Among these, we identified 1,472 AEs, of which 253 were found to be ADRs associated with epirubicin. The clinical characteristics of the patients are summarized in Table 1.
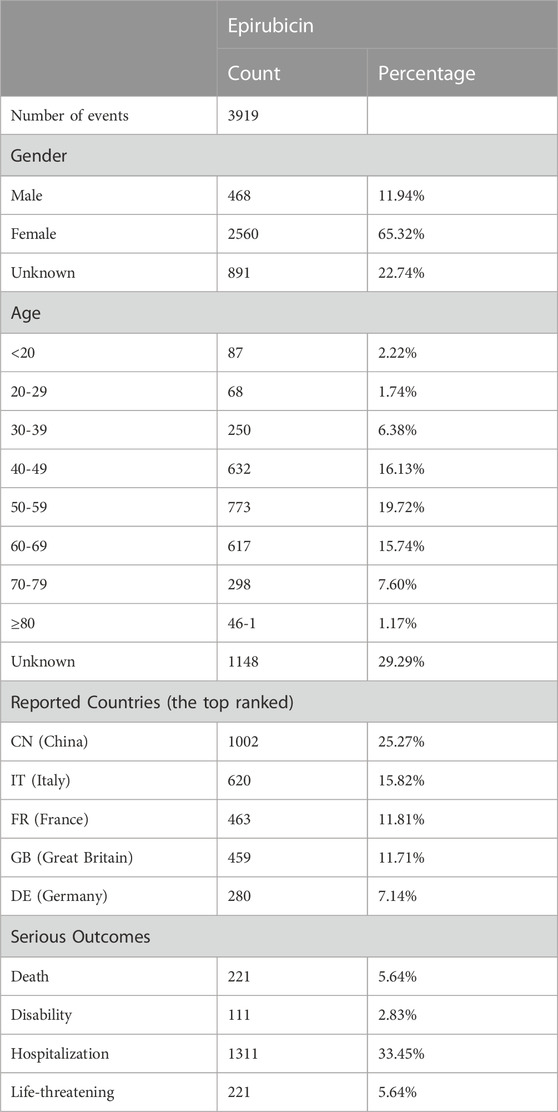
TABLE 1. The characters of case reports associated with Epirubicin as primary suspected drug from 2014 Q1 to 2023 Q1.
Of the 3,919 cases, 2,560 were females (65.32%) and 468 were males (11.94%); 891 were unspecified. The analysis showed that individuals aged 50–59 were more likely to experience AEs, accounting for 773 (19.72%) cases. The five countries with the highest frequency of epirubicin use were China (1,002, 25.27%), Italy (620, 15.82%), France (463, 11.81%), Great Britain (459, 11.71%), and Germany (280, 7.14%). Our results showed that the top five severe outcomes were hospitalization (1,311, 33.45%), life-threatening events (221, 5.64%), death (221, 5.64%), and disability (111, 2.83%).
Signal detections at the system organ class level
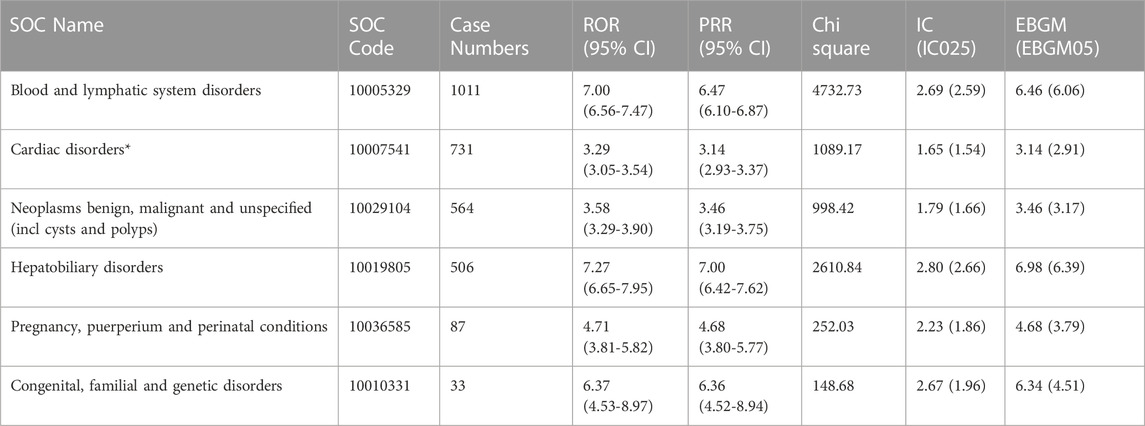
As shown in Table 2, the top three ADRs ranked by case numbers at the SOC level were blood and lymphatic system disorders [case numbers: 1011, ROR (95% CI) = 7.00 (6.56–7.47), PRR (95% CI) = 6.47 (6.10–6.87), IC (IC025) = 2.69 (2.59), and EBGM (EBGM05) = 6.46 (6.06)], cardiac disorders [case numbers: 731, ROR (95% CI) = 3.29 (3.05–3.54), PRR (95% CI) = 3.14 (2.93–3.37), IC (IC025) = 1.65 (1.54), and EBGM (EBGM05) = 3.14 (2.91)], and neoplasms benign, malignant, and unspecified (including cysts and polyps) [case numbers: 564, ROR (95% CI) = 3.58 (3.29–3.90), PRR (95% CI) = 3.46 (3.19–3.75), IC (IC025) = 1.79 (1.66), and EBGM (EBGM05) = 3.46 (3.17)]. The epirubicin label includes a list of these items. However, the medication label for epirubicin does not include all SOC keywords, such as hepatobiliary illnesses, pregnancy, puerperium, perinatal ailments, and congenital, familial, and genetic disorders.
Signal detections ranked by the EBGM at the prefer term level
In our study, four algorithms were used to analyze drug reactions and evaluate their compliance with various screening criteria. The signals identified by the four algorithms are listed in Table 3 and Supplementary Table S1. At the PT level, 253 ADRs were associated with 25 SOCs. As shown in Table 3, the top five strongest signal ADRs, ranked by the EBGM algorithm at the PT level—the most stringent algorithm—were hepatic artery stenosis [case numbers: 8, EBGM: 468.01 (219.99)], endocardial fibrosis [case numbers: 3, EBGM: 426.22 (125.53)], gait apraxia [case numbers: 3, EBGM: 426.22 (125.53)], cardiac perfusion defect [case numbers: 3, EBGM: 331.50 (99.81)], and hepatic artery occlusion [Case numbers: 3, EBGM: 308.64 (93.40)].
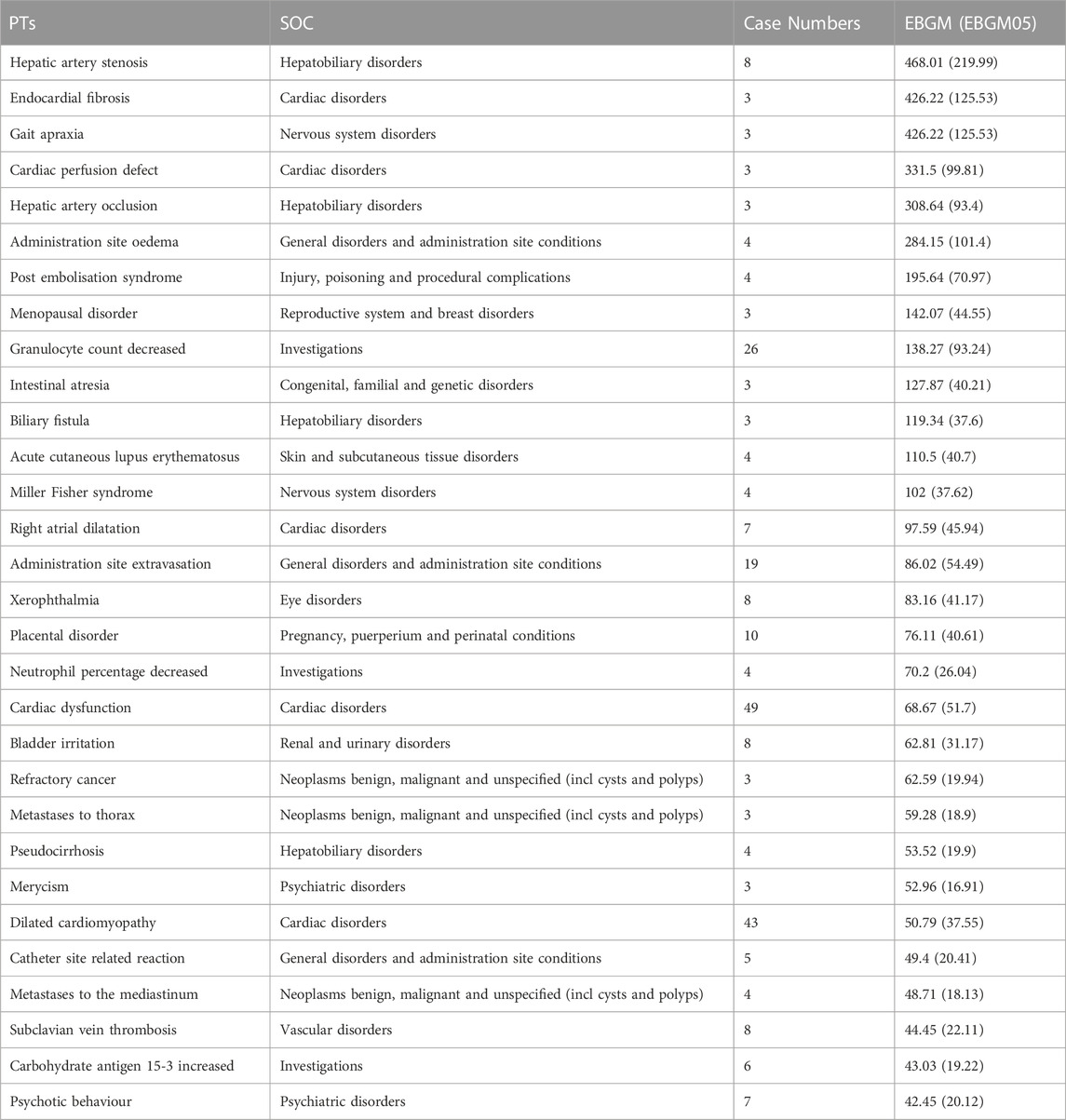
TABLE 3. The top 30 signal strength of AEs of Epirubicin ranked by EBGM at the PTs level in the FAERS database.
Hepatic artery stenosis and occlusion are associated with hepatobiliary disorders, whereas gait apraxia is associated with nervous system disorders. To our knowledge, neither hepatobiliary disorders nor nervous system disorders are included in the epirubicin label; therefore, they could be identified as potential new ADRs of epirubicin. Moreover, several newly detected ADRs have been found to be associated with other SOCs such as intestinal atresia, Miller Fisher syndrome, and merycism. Please refer to Supplementary Table S1 for the EBGM rankings of other PTs. Additionally, 390 ADRs were identified by ROR and PRR, and 348 ADRs were identified by ROR, PRR, and BCPNN (Supplementary Tables S1–S5).
Other drugs and gait apraxia
The occurrence of gait apraxia, a neurological disorder, suggests that epirubicin may damage the nervous system; this attracted our attention. To further explain the conclusions of our study, we reviewed the FAERS database to identify other drugs that may cause gait apraxia. Medical records submitted between the first quarter of 2014 and the first quarter of 2023 of patients who developed adverse events of gait apraxia were collected. The medications used in these patients were also collected. We found that 17 drugs, including epirubicin, gabapentin, and imatinib, were reported to be associated with gait apraxia (Supplementary Table S6). Carbidopa\levodopa contains two ingredients, so it was excluded. We further conducted ADR analyses of the 16 drug types and found that gabapentin could cause gait apraxia [case numbers: 3, ROR: 35.32 (10.54), PRR: 35.22 (10.54), BCPNN: 4.50 (3.41), and EBGM: 162.03 (38.72)].
Discussion
Despite being approved by the FDA, serious but less-common safety problems can emerge once the drug is post-marketed (Garashi et al., 2022; Kong et al., 2023). This phenomenon may be partly caused by: i) a larger patient population, making the detection of rare AEs more feasible; ii) a wider range of patients with underlying diseases and co-medications, making it easier to detect interactions between drugs and diseases, as well as drugs and drugs; iii) drug abuse; and iv) drug misuse (Schick et al., 2017). Given these problems, the public FAERS database could help investigation into less common adverse drug reactions and provide guidance for clinical drug use. To our knowledge, this is the first study to investigate epirubicin-related AEs using real-world data from the FAERS database.
Our study analyzed the signal strength of AEs ranked by case number, the three most frequent of which were blood and lymphatic system disorders, cardiac disorders, and neoplasms benign, malignant, and unspecified (including cysts and polyps) consistent with the drug instructions (Schneeweiss et al., 2018). In addition, the number of AEs associated with hepatobiliary disorders and the signal intensity were both high, indicating that these disorders were closely related to epirubicin.
Among the top five results generated from all four algorithms and ranked by the value of the EBGM, which is a stringent algorithm, we found that hepatic artery stenosis and hepatic artery occlusion were associated with hepatobiliary disorders, and gait apraxia was associated with nervous system disorders. Neither type of disorder was listed on the epirubicin label.
Hepatic artery stenosis and occlusion may be partly due to transarterial chemotherapy (Matsui et al., 2017a; Matsui et al., 2017b). Hepatocellular carcinoma (HCC), one of the most common cancers worldwide, has left many people annually facing the threat of death (Okuda et al., 1985; Bosch et al., 2004). Hepatic artery damage (HAD) associated with transcatheter arterial chemoembolization (TACE) (Idée and Guiu, 2013) may affect clinical treatment outcomes. A previous study comprehensively analyzed the occurrence of HAD in 33 patients treated with TACE alone using a mixture of epirubicin, iodized oil, and gelatin sponge (Maeda et al., 2008). The results of this study indicated that accumulated epirubicin dose was significantly associated with HAD (p = 0.001). Our study also suggests that hepatic artery stenosis and occlusion are potential side effects of epirubicin. Therefore, during the course of clinical use, more attention should be paid to liver artery damage caused by epirubicin.
Other drugs associated with gait apraxia were also investigated. Gabapentin, an anticonvulsant, has been widely reported to be associated with gait apraxia (Meng et al., 2014; Wiffen et al., 2017). Based on the four algorithms used in our study, we also identified that gabapentin can cause gait apraxia, which is consistent with previous studies, indicating the high robustness and reliability of the four algorithms. We also found that the patient IDs reported for other drugs were inconsistent with those reported for epirubicin, indicating that gait apraxia caused by epirubicin was not caused by other drug ingredients.
Gait apraxia has been widely used to describe gait disturbances accompanied by normal-pressure hydrocephalus, cerebral small vessel disease, and other frontal disorders (Dale et al., 2018). Gait apraxia, a nervous system disease, may indicate that epirubicin has damaged the nervous system. Gait apraxia is also referred to as a gait disturbance and is associated with many signs, including locomotor abnormalities, disequilibrium, loss of gait ignition, and inappropriate postural responses (Della Sala et al., 2002). It is widely accepted that injury to the frontal lobe and cerebellum and damage to the connections between the frontal lobe, basal ganglia, cerebellum, and brainstem may be the cause of gait apraxia (Thompson, 2012). Since its approval for cancer treatment, epirubicin-induced cerebral lesions have attracted increasing attention. Although the blood–brain barrier restricts many therapeutic molecules from entering the brain, chemotherapy, including epirubicin therapy, can damage the microscopic structure of the cerebral tissue through direct neurotoxicity (Meyers, 2008). The following reports support this hypothesis. Chen et al. (2023) found that, compared with breast cancer survivors who did not receive chemotherapy (docetaxel and epirubicin), survivors who received chemotherapy had reduced white matter integrity in the middle frontal gyrus (MFG). Deprez et al. (2012) found that, compared with controls, patients with breast cancer who were exposed to chemotherapy (epirubicin, fluorouracil, and cyclophosphamide) had significant decreases in fractional anisotropy (FA) in the frontal WM tracts after treatment, suggesting poor connectivity of the brain microstructure. Considering the important role of the frontal lobe and the connections between it, the basal ganglia, and the cerebellum, it is reasonable to conclude that epirubicin may cause gait apraxia.
To date, we have not found studies that have shown that gait apraxia is associated with the use of epirubicin, which seems to indicate that there is only a correlation, not a causation. However, we still pay more attention to the occurrence of gait apraxia associated with the clinical use of epirubicin.
It is worth emphasizing that we found many ADRs that have not been listed on the drug label, such as intestinal atresia and Miller Fisher syndrome, which should be paid closer attention in future clinical drug use.
This study has some limitations that should be considered. First, although ROR and PRR have high stability and specificity, BCPNN has higher sensitivity, stability, and specificity than ROR and PRR (Zhang et al., 2023). To make our results more robust, we used four algorithms to filter the AEs associated with the use of epirubicin: PRR, ROR, BCPNN, and EBGM. AEs were considered to have occurred only when all four algorithms met simultaneously, thereby guaranteeing the accuracy of their detection. Second, owing to the small number of patients treated with epirubicin, we could not determine the incidence of adverse reactions. Third, because the sources of reports in the FAERS are diverse, there are differences in the quality of data submitted by reporters, leading to omissions and errors. Fourth, we could not completely avoid the occurrence of false-positive signals, although four algorithms were utilized. Moreover, each patient’s additional prescription drugs may not be fully disclosed in their medical records. It is therefore difficult to determine whether an adverse reaction was caused by the target drug, a companion drug, or a combination of the two. Therefore, more effort should be made to address these limitations and investigate the safety of epirubicin.
Conclusion
The findings of this study provide several insights into the clinical applications of epirubicin. Several unexpected AEs such as hepatic artery stenosis, gait apraxia, and intestinal atresia are associated with the use of epirubicin. Clinicians should be careful when prescribing epirubicin to patients to prevent the emergence of these AEs. Further clinical studies are required to validate these findings. To better protect patients, the safety of epirubicin must be continuously explored in the real world.
Data availability statement
The data analyzed in this study were obtained from the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS: https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers). The following licenses/restrictions apply. Users of these files need to be familiar with creation of relational databases using applications such as ORACLE\xAE, Microsoft Office Access, MySQL\xAE, and IBM DB2, or the use of ASCII files with SAS\xAE analytic tools. A simple search of FAERS data cannot be performed with these files by those unfamiliar with the creation of relational databases. However, a summary FAERS report for a product can be obtained by sending a Freedom of Information Act (FOIA) request to the FDA. You can also request individual case reports by submitting a FOIA request listing the case report number(s). Requests to access these datasets should be directed to the FDA: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
Author contributions
WW, XG, and YFZ conceived the project and conducted the analyses. WW, SW, LPS completed the manuscript. PBH collected the data and proofread the results of the data processing. JQW, QG, and PBH reviewed and revised this article. All authors contributed to the article and approved the submitted version.
Funding
This study was privately funded by JW and QG.
Acknowledgments
The authors are grateful for the help of the Bioinfor-medical Centre.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1249845/full#supplementary-material
References
Almenoff, J. S., LaCroix, K. K., Yuen, N. A., Fram, D., and DuMouchel, W. (2006). Comparative performance of two quantitative safety signalling methods: Implications for use in a pharmacovigilance department. Drug Saf. 29 (10), 875–887. doi:10.2165/00002018-200629100-00005
Bosch, F. X., Ribes, J., Díaz, M., and Cléries, R. (2004). Primary liver cancer: Worldwide incidence and trends. Gastroenterology 127 (51), S5–S16. doi:10.1053/j.gastro.2004.09.011
Brown, E. G. (2004). Using MedDRA: Implications for risk management. Drug Saf. 27 (8), 591–602. doi:10.2165/00002018-200427080-00010
Burnell, M., Levine, M. N., Chapman, J. A. W., Bramwell, V., Gelmon, K., Walley, B., et al. (2010). Cyclophosphamide, epirubicin, and Fluorouracil versus dose-dense epirubicin and cyclophosphamide followed by Paclitaxel versus Doxorubicin and cyclophosphamide followed by Paclitaxel in node-positive or high-risk node-negative breast cancer. J. Clin. Oncol. Official J. Am. Soc. Clin. Oncol. 28 (1), 77–82. doi:10.1200/JCO.2009.22.1077
Chen, V. C. H., Chuang, W., Chen, C. W., Tsai, Y. H., McIntyre, R. S., and Weng, J. C. (2023). Detecting microstructural alterations of cerebral white matter associated with breast cancer and chemotherapy revealed by generalized q-sampling MRI. Front. Psychiatry 14, 1161246. doi:10.3389/fpsyt.2023.1161246
Dale, M. L., Curtze, C., and Nutt, J. G. (2018). Apraxia of gait- or apraxia of postural transitions? Park. Relat. Disord. 50, 19–22. doi:10.1016/j.parkreldis.2018.02.024
de Azambuja, E., Paesmans, M., Beauduin, M., Vindevoghel, A., Cornez, N., Finet, C., et al. (2009). Long-term benefit of high-dose epirubicin in adjuvant chemotherapy for node-positive breast cancer: 15-year efficacy results of the Belgian multicentre study. J. Clin. Oncol. Official J. Am. Soc. Clin. Oncol. 27 (5), 720–725. doi:10.1200/JCO.2008.17.2155
Della Sala, S., Francescani, A., and Spinnler, H. (2002). Gait apraxia after bilateral supplementary motor area lesion. J. Neurology, Neurosurg. Psychiatry 72 (1), 77–85. doi:10.1136/jnnp.72.1.77
Deprez, S., Amant, F., Smeets, A., Peeters, R., Leemans, A., Van Hecke, W., et al. (2012). Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J. Clin. Oncol. Official J. Am. Soc. Clin. Oncol. 30 (3), 274–281. doi:10.1200/JCO.2011.36.8571
Ellis, P. A., Norman, A., Hill, A., O'Brien, M. E., Nicolson, M., Hickish, T., et al. (1995). Epirubicin, cisplatin and infusional 5-fluorouracil (5-FU) (ECF) in hepatobiliary tumours. Eur. J. Cancer (Oxford, Engl. 1990) 31A (10), 1594–1598. doi:10.1016/0959-8049(95)00323-b
Forner, A., Reig, M., and Bruix, J. (2018). Hepatocellular carcinoma. Lancet (London, Engl. 391 (10127), 1301–1314. doi:10.1016/S0140-6736(18)30010-2
Garashi, H. Y., Steinke, D. T., and Schafheutle, E. I. (2022). A systematic review of pharmacovigilance systems in developing countries using the WHO pharmacovigilance indicators. Ther. Innovation Regul. Sci. 56 (5), 717–743. doi:10.1007/s43441-022-00415-y
Henderson, I. C., Berry, D. A., Demetri, G. D., Cirrincione, C. T., Goldstein, L. J., Martino, S., et al. (2003). Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J. Clin. Oncol. Official J. Am. Soc. Clin. Oncol. 21 (6), 976–983. doi:10.1200/JCO.2003.02.063
Hendricksen, K., Witjes, W. P. J., Idema, J. G., Kums, J. J. M., van Vierssen Trip, O. B., de Bruin, M. J. F. M., et al. (2008). Comparison of three schedules of intravesical epirubicin in patients with non-muscle-invasive bladder cancer. Eur. Urol. 53 (5), 984–991. doi:10.1016/j.eururo.2007.12.033
Idée, J. M., and Guiu, B. (2013). Use of lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: A review. Crit. Rev. Oncology/hematology 88 (3), 530–549. doi:10.1016/j.critrevonc.2013.07.003
Ji, H. H., Tang, X. W., Dong, Z., Song, L., and Jia, Y. T. (2019). Adverse event profiles of anti-CTLA-4 and anti-PD-1 monoclonal antibodies alone or in combination: Analysis of spontaneous reports submitted to FAERS. Clin. Drug Investig. 39 (3), 319–330. doi:10.1007/s40261-018-0735-0
Khasraw, M., Bell, R., and Dang, C. (2012). Epirubicin: Is it like doxorubicin in breast cancer? A clinical review. Breastedinbg. Scotl. 21 (2), 142–149. doi:10.1016/j.breast.2011.12.012
Kong, L., Rong, L., Xie, M., and Wang, M. (2023). Temporal offset association between the number of irinotecan-related adverse reactions and pharmacogenomic studies: A cross-correlation analysis. Saudi Pharm. J. SPJ Official Publ. Saudi Pharm. Soc. 31 (1), 180–183. doi:10.1016/j.jsps.2022.11.016
Laface, C., Laforgia, M., Molinari, P., Ugenti, I., Gadaleta, C. D., Porta, C., et al. (2021). Hepatic arterial infusion of chemotherapy for advanced hepatobiliary cancers: State of the art. Cancers 13 (12), 3091. doi:10.3390/cancers13123091
Ma, W., Wei, M., Moore, R., Ganesan, V., and Nelson, S. (2005). RxNorm: Prescription for electronic drug information exchange. IEEE Comput. Soc. 7, 17–23. doi:10.1109/mitp.2005.122
Maeda, N., Osuga, K., Mikami, K., Higashihara, H., Onishi, H., Nakaya, Y., et al. (2008). Angiographic evaluation of hepatic arterial damage after transarterial chemoembolization for hepatocellular carcinoma. Radiat. Med. 26 (4), 206–212. doi:10.1007/s11604-007-0216-5
Matsui, Y., Figi, A., Horikawa, M., Jahangiri Noudeh, Y., Tomozawa, Y., Hashimoto, K., et al. (2017a). Arteriopathy after transarterial chemo-lipiodolization for hepatocellular carcinoma. Diagnostic Interventional Imaging 98 (12), 827–835. doi:10.1016/j.diii.2017.10.010
Matsui, Y., Figi, A., Horikawa, M., Jahangiri Noudeh, Y., Tomozawa, Y., Hashimoto, K., et al. (2017b). Arteriopathy after transarterial chemo-lipiodolization for hepatocellular carcinoma. diagn. Interv. Imaging 98 (12), 827–835. doi:10.1016/j.diii.2017.10.010
Meng, F. Y., Zhang, L. C., Liu, Y., Pan, L. H., Zhu, M., Li, C. L., et al. (2014). Efficacy and safety of gabapentin for treatment of postherpetic neuralgia: A meta-analysis of randomized controlled trials. Minerva Anestesiol. 80 (5), 556–567.
Meyers, C. A. (2008). How chemotherapy damages the central nervous system. J. Biol. 7 (4), 11. doi:10.1186/jbiol73
Mouridsen, H. T., Alfthan, C., Bastholt, L., Bergh, J., Dalmark, M., Eksborg, S., et al. (1990). Current status of epirubicin (Farmorubicin) in the treatment of solid tumours. Acta Oncol. Stockh. Swed. 29 (3), 257–285. doi:10.3109/02841869009089998
Okuda, K., Ohtsuki, T., Obata, H., Tomimatsu, M., Okazaki, N., Hasegawa, H., et al. (1985). Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 56 (4), 918–928. doi:10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e
Onrust, S. V., Wiseman, L. R., and Goa, K. L. (1999). Epirubicin: A review of its intravesical use in superficial bladder cancer. Drugs & Aging 15 (4), 307–333. doi:10.2165/00002512-199915040-00006
Oosterlinck, W., Kurth, K. H., Schröder, F., Bultinck, J., Hammond, B., and Sylvester, R. (1993). A prospective European Organization for Research and Treatment of Cancer Genitourinary Group randomized trial comparing transurethral resection followed by a single intravesical instillation of epirubicin or water in single stage Ta, T1 papillary carcinoma of the bladder. J. Urology 149 (4), 749–752. doi:10.1016/s0022-5347(17)36198-0
Pavone-Macaluso, S., Tripi, M., Ingargiola, G. B., Corselli, G., Pavone, C., and Serretta, V. (1993). Current views on intravesical treatment and chemoprophylaxis of superficial bladder cancer. The present role of epirubicin and doxorubicin. J. Chemother. (Florence, Italy) 5 (3), 207–211. doi:10.1080/1120009x.1993.11739234
Plosker, G. L., and Faulds, D. (1993). Epirubicin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cancer chemotherapy. Drugs 45 (5), 788–856. doi:10.2165/00003495-199345050-00011
Richters, A., Aben, K. K. H., and Kiemeney, L. A. L. M. (2020). The global burden of urinary bladder cancer: An update. World J. Urology 38 (8), 1895–1904. doi:10.1007/s00345-019-02984-4
Sakaeda, T., Tamon, A., Kadoyama, K., and Okuno, Y. (2013). Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10 (7), 796–803. doi:10.7150/ijms.6048
Schick, A., Miller, K. L., Lanthier, M., Dal Pan, G., and Nardinelli, C. (2017). Evaluation of pre-marketing factors to predict post-marketing boxed warnings and safety withdrawals. Drug Saf. 40 (6), 497–503. doi:10.1007/s40264-017-0526-1
Schneeweiss, A., Chia, S., Hickish, T., Harvey, V., Eniu, A., Waldron-Lynch, M., et al. (2018). Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur. J. Cancer 89, 27–35. doi:10.1016/j.ejca.2017.10.021
Tawada, A., Chiba, T., Ooka, Y., Kanogawa, N., Saito, T., Motoyama, T., et al. (2015). Transarterial chemoembolization with miriplatin plus epirubicin in patients with hepatocellular carcinoma. Anticancer Res. 35 (1), 549–554.
Thompson, P. D. (2012). Frontal lobe ataxia. Handb. Clin. Neurology 103, 619–622. doi:10.1016/B978-0-444-51892-7.00044-9
Tripi, M., Falletta, C., Corselli, G., Pavone, C., and Pavone-Macaluso, M. (1986). 4'-epidoxorubicin for intravesical instillation in urotheliomas of the bladder. Minerva Urologica E Nefrologica = Italian J. Urology Nephrol. 38 (4), 379–383.
Wiffen, P. J., Derry, S., Bell, R. F., Rice, A. S., Tölle, T. R., Phillips, T., et al. (2017). Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 6 (6), CD007938. doi:10.1002/14651858.CD007938.pub4
Keywords: epirubicin, FDA Adverse Event Reporting System database, safety, reporting odds ratio (ROR), proportional reporting ratio (PRR), bayesian confidence propagation neural network (BCPNN), empirical Bayes geometric mean (EBGM)
Citation: Wang W, Guan X, Wang S, Shi L, Zhu Y, Hua P, Guo Q and Wang J (2023) Epirubicin and gait apraxia: a real-world data analysis of the FDA Adverse Event Reporting System database. Front. Pharmacol. 14:1249845. doi: 10.3389/fphar.2023.1249845
Received: 30 June 2023; Accepted: 24 August 2023;
Published: 14 September 2023.
Edited by:
Tayyaba Iftikhar, Shenzhen University, ChinaReviewed by:
Thomas Hsueh, Taipei City Hospital, TaiwanGuldem Mercanoglu, University of Health Sciences, Türkiye
Copyright © 2023 Wang, Guan, Wang, Shi, Zhu, Hua, Guo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingqi Wang, ZHJ3YW5nanFAMTI2LmNvbQ==; Qiang Guo, bGxncWlhbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Wei Wang
Wei Wang Xin Guan
Xin Guan Shuang Wang
Shuang Wang Lipeng Shi4
Lipeng Shi4 Yanfei Zhu
Yanfei Zhu Qiang Guo
Qiang Guo Jingqi Wang
Jingqi Wang