- Department of Urology, The First Hospital of Jilin University, Changchun, China
Purpose: On 12 April 2019, erdafitinib gained the first FDA approval as the second-line treatment for adult patients with locally advanced or metastatic urothelial cancer following progression during or after at least one previous line of platinum-based chemotherapy. However, the long-term safety profile of erdafitinib in a large patient population remains unexplored. The current study aimed to assess the adverse events (AEs) associated with erdafitinib through data mining of the US Food and Drug Administration Adverse Event Reporting System (FAERS).
Method: The reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network (BCPNN), and the multi-item gamma Poisson shrinker (MGPS) algorithms based on disproportionality were employed to quantify the signals of erdafitinib-associated AEs.
Results: A total of 6,322,279 reports of AEs were retrieved from the FAERS database spanning 2019 to 2022, out of which, 700 reports of erdafitinib as the “primary suspected” were identified. These erdafitinib-induced AEs were observed across 24 targeted system organ classes (SOCs). After conforming to the four algorithms at the same time, a total of 441 signals of erdafitinib-induced AEs were detected across 23 SOCs. Notably, signals associated with metabolism and nutrition disorders, eye disorders, and skin and subcutaneous tissue disorders were among the most prevalent. The median onset time for AEs was found to be 54 days [interquartile range (IQR) 17–112 days], with a majority of AEs occurring within the initial 6 months after initiating erdafitinib (37.23% within the first month, 15.53% within the second month, and 16.79% within the third month).
Conclusion: The findings of this study align with existing clinical observations, offering a comprehensive long-term post-marketing safety evaluation of erdafitinib. The results provide valuable evidence to enhance the understanding of erdafitinib’s safety profile, aiding further research and guiding clinical practice.
Introduction
In 2023, bladder cancer (BC) stands as the seventh most prevalent malignant neoplasm in the United States, projecting an estimated 82,290 new cases and 16,710 fatalities (Siegel et al., 2023). Among the histological types, urothelial carcinoma (UC) represents the prevailing subtype, accounting for approximately 90% or more of BC cases (Bin Riaz et al., 2021). Unfortunately, about 30% of diagnoses manifest as muscle-invasive BC at the initial stage, predominantly presenting as locally advanced or metastatic disease, entailing a relative 5-year overall survival rate of merely 15% (Nadal and Bellmunt, 2019; Morales-Barrera et al., 2020).
Historically, cisplatin-based regimens have served as the first-line chemotherapy option for metastatic UC (mUC), revealing an overall response rate (ORR) of 50% and a median progression-free survival of 7 months (Roberts et al., 2006). However, almost half of the patients cannot undergo cisplatin chemotherapy due to factors such as renal impairment, inadequate treatment response, etc (Marandino et al., 2019). In recent years, the emergence of immune checkpoint inhibitors (ICIs) and fibroblast growth factor receptor inhibitors (FGFRs) has introduced novel therapeutic avenues for advanced UC management (Patel et al., 2020). FDA approvals have positioned ICIs as second-line systemic treatment for mUC. Although there is a durable response in some immunotherapy, many people will not benefit from immunotherapy (McConkey et al., 2016). Notably, gene expression profiling has facilitated the identification of specific UC subtypes (Robertson et al., 2017). For instance, the luminal I subtype exhibits reduced PD-L1 expression, rendering it less responsive to ICIs, whereas it manifests a higher FGFR3 mutation rate, including FGFR3 mutations and FGFR2/3 fusions (McConkey et al., 2015). Approximately 20% of patients with advanced UC exhibit alterations in FGFR, with FGFR2/3 gene fusion and mutation being the most prevalent, particularly in luminal I type, thus prompting downstream signaling pathway changes and instigating carcinogenesis (Haugsten et al., 2010; Knowles and Hurst, 2015). Consequently, FGFRs offer a promising therapeutic target for advanced UC treatment.
Erdafitinib, a potent and orally available FGFR one to four tyrosine kinase inhibitor (Garje et al., 2020), obtained its inaugural global approval by the FDA on 12 April 2019. It was indicated for treating adult patients with locally advanced or mUC harboring FGFR3 or FGFR2 genetic alterations, which progressed during or after at least one prior platinum-based chemotherapy, including cases within 12 months post-neoadjuvant or platinum-based adjuvant chemotherapy (Markham, 2019). The efficacy and safety of erdafitinib in this patient cohort were assessed in a multicenter, open-label, phase 2 study (BLC2001-NCT02365597) (Loriot et al., 2019). While generally well-tolerated as with other biological agents, erdafitinib exhibited some adverse reactions associated with its mechanism of action, dosage, or other factors. The most common AEs observed in the BLC2001 study included hyperphosphatemia (78%), stomatitis (35%), diarrhea (54%), nausea (22%), dry mouth (46%), dysgeusia (41%), fatigue (33%), nail changes (19%), elevated alanine aminotransferase (19%), and anemia (22%) (Peng et al., 2022).
It is essential to consider that prior investigations on erdafitinib were primarily based on clinical trials conducted under different specific conditions, and the AEs observed in such trials may not fully reflect all the AEs encountered in clinical practice (Fang et al., 2023). In addition, the limited sample size and follow-up time of these clinical trials may not adequately capture the full spectrum of observable AEs. More importantly, the onset time of adverse drug reactions (ADRs) associated with erdafitinib remains unknown. Therefore, exploring potential ADR signals linked to erdafitinib through data mining algorithms in large post-marketing samples is of paramount importance. The Federal Adverse Event Reporting System (FAERS) is a publicly accessible repository for spontaneous reports of post-marketing AEs submitted to the US FDA (Grace et al., 2020). As the largest pharmacovigilance database globally, FAERS serves as a highly effective tool for monitoring drug-related AEs. Therefore, the purpose of this study is to evaluate the AEs associated with erdafitinib via FAERS data mining, thereby providing valuable insights for future clinical safety monitoring and risk assessment endeavors.
Materials and methods
Study design and data source
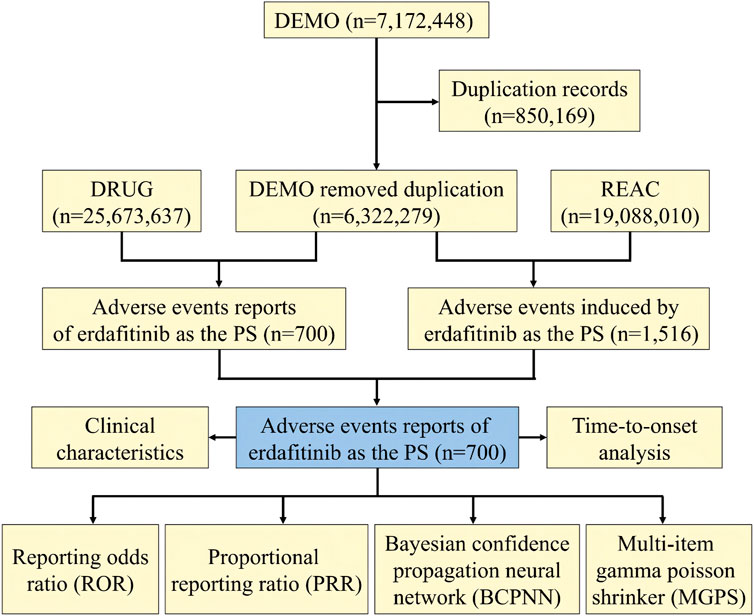
We conducted a retrospective pharmacovigilance study based on the FAERS database, with data covering the period from 2019 to 2022. The FAERS data files contain seven types of datasets: patient demographic and administrative information (DEMO), drug information (DRUG), coded for the adverse events (REAC), patient outcomes (OUTC), report sources (RPSR), therapy start dates and end dates for reported drugs (THER), and indications for drug administration (INDI), and deleted cases (Yang et al., 2022). To facilitate statistical analysis, all data from the FDA were imported into MySQL software (v8.0; Oracle, Sweden), followed by a deduplication process (Shu et al., 2022b). Given that the FDA data receives submissions from various sources, potential duplicate reports needed reprocessing. In compliance with FDA recommendations, we selected the most recent FDA_DT when the CASEID was the same and opted for the higher PRIMARYID. By adhering to FDA guidelines and identifying instances where FDA_DT and CASEID were identical, we successfully eliminated duplicate reports originating from different individuals and institutions (Shu et al., 2022a). Consequently, the number of reports was streamlined, resulting in a total of 6,322,279 unique reports. A comprehensive flowchart outlining the meticulous processes of data extraction, processing, and analysis has been meticulously depicted in Figure 1.
Adverse events and drug identification
The AEs reports available in the FAERS database have been meticulously coded according to the Medical Dictionary for Regulatory Activities (MedDRA) classification system. MedDRA’s structural hierarchy is organized into five hierarchical structures, namely, system organ class (SOC), high-level group term (HLGT), high-level term (HLT), preferred term (PT), and lowest-level term (LLT) (Mascolo et al., 2021). In the context of the current study focusing on associated-erdafitinib AEs retrieved from the FAERS database, both SOC and PT were standardized and classified to serve as the primary subjects for data analysis and research exploration. We identified cases in the DRUG files using generic names (erdafitinib in the drug name) and trade names (Balversa in the drug list) and selected role_cod as the PS to improve accuracy.
Data mining
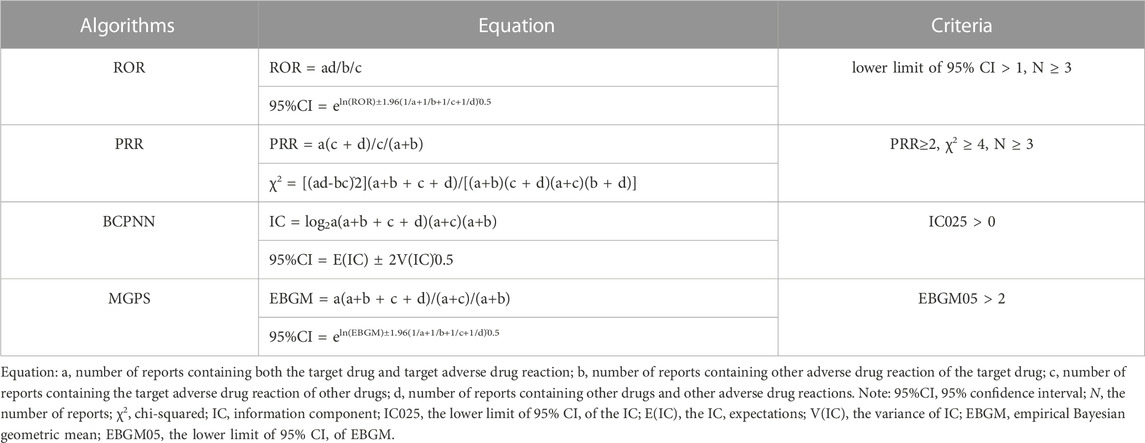
In the pursuit of pharmacovigilance studies, disproportionality analysis has gained wide acceptance. This approach compares the proportion of specific ADRs related to single or multiple drugs with the proportion of ADRs attributed to the same drug reported in the entire database (Hu et al., 2020). In our study, we used a disproportionality analysis to identify potential signals between erdafitinib and all AEs. The reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network (BCPNN), and the muti-item gamma Poisson shrinker (MGPS) algorithms are four major specific indices that were used to evaluate the signals of erdafitinib-related AEs (Guo et al., 2022). Among them, the ROR was utilized as one of the crucial indicators to evaluate the correlation between erdafitinib and AEs. ROR is a statistical measure utilized in pharmacovigilance to evaluate the association between a specific adverse event and a particular drug, in comparison to all other drugs in a database. In statistics, a higher ROR typically indicates a stronger association between the drug and the AE. The detailed computational equations and criteria for these four algorithms are shown in Table 1. Generally speaking, when the incidence of a specific AE related to erdafitinib significantly exceeds the background frequency within the database and reaches a certain threshold or standard, the higher the value of these four parameters, the stronger the signal (Yang et al., 2022).
Furthermore, the onset time of erdafitinib-related AEs was calculated. Time to onset was defined as the interval between START_DATE (the start of erdafitinib use) and EVENT_DATE (the appearance of AEs). Measures were taken to exclude false and inaccurate AE occurrence information. MYSQL 8.0, Navicat Premium 15, Microsoft EXCEL 2019, and GraphPad Prism 8 were used for statistical processing and data analysis.
Results
Descriptive analysis
During the period spanning from 2019 to 2022, this study garnered a total of 6,322,279 reports from the FAERS database, diligently excluding duplicate data and procuring 700 AEs reports for erdafitinib as the PS (Figure 1). The clinical characteristics of events associated with erdafitinib were described in Table 2. Among these AEs, the proportion of males (51.57%) marginally surpassed that of females (33.71%). From the age distribution, the majority of patients experiencing AEs were aged over 65. A total of 232 (33.14%) death cases and 125 (17.86%) hospitalization cases were reported. Other serious consequences included life-threatening and disability. These AEs were primarily reported from five countries, with the United States (78.71%) accounting for the majority, followed by France (6.43%), Israel (2.29%), Spain (1.71%), and Brazil (1.57%). In addition, consumers (45.86%) and physicians (33.71%) were the main contributors in terms of reported occupations. In terms of reporting year, the highest number of AEs surfaced in 2020 (33.43%), followed by 2022 (31.43%), 2021 (26.43), and 2019 (8.71%).
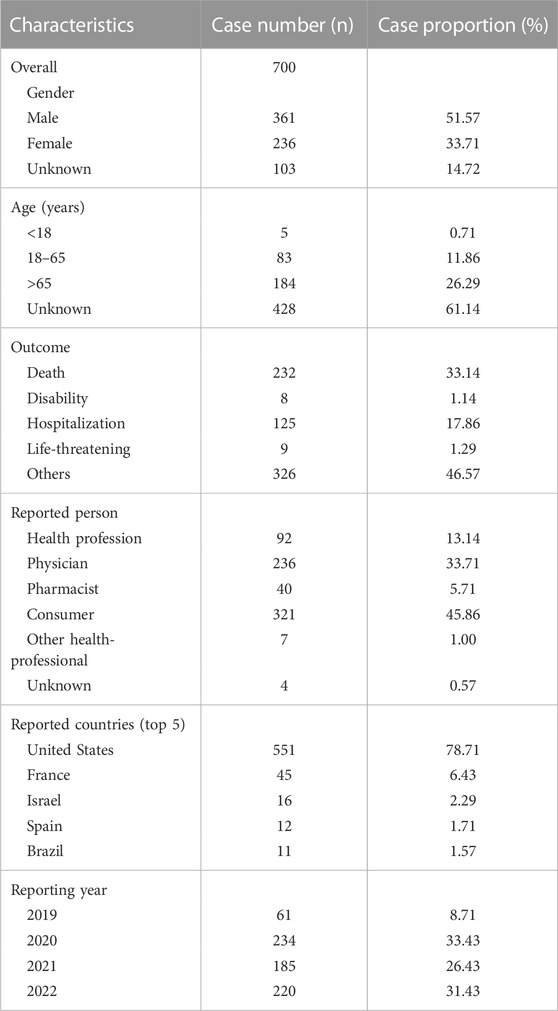
TABLE 2. Basic characteristics of patients with erdafitinib-associated adverse events from the FAERS database.
Disproportionality analysis signal strengths of AEs related-erdafitinib at the SOC level were described in Table 3. Statistically, AEs associated with erdafitinib were mainly distributed in 24 distinct system organs. The significant SOCs that satisfied at least one of the four calculation criteria encompassed Skin and subcutaneous tissue disorders, Eye disorders, Metabolism and infestations, General disorders and administration site condition, Gastrointestinal disorders, Surgical and medical procedures, and Hepatobiliary disorders.
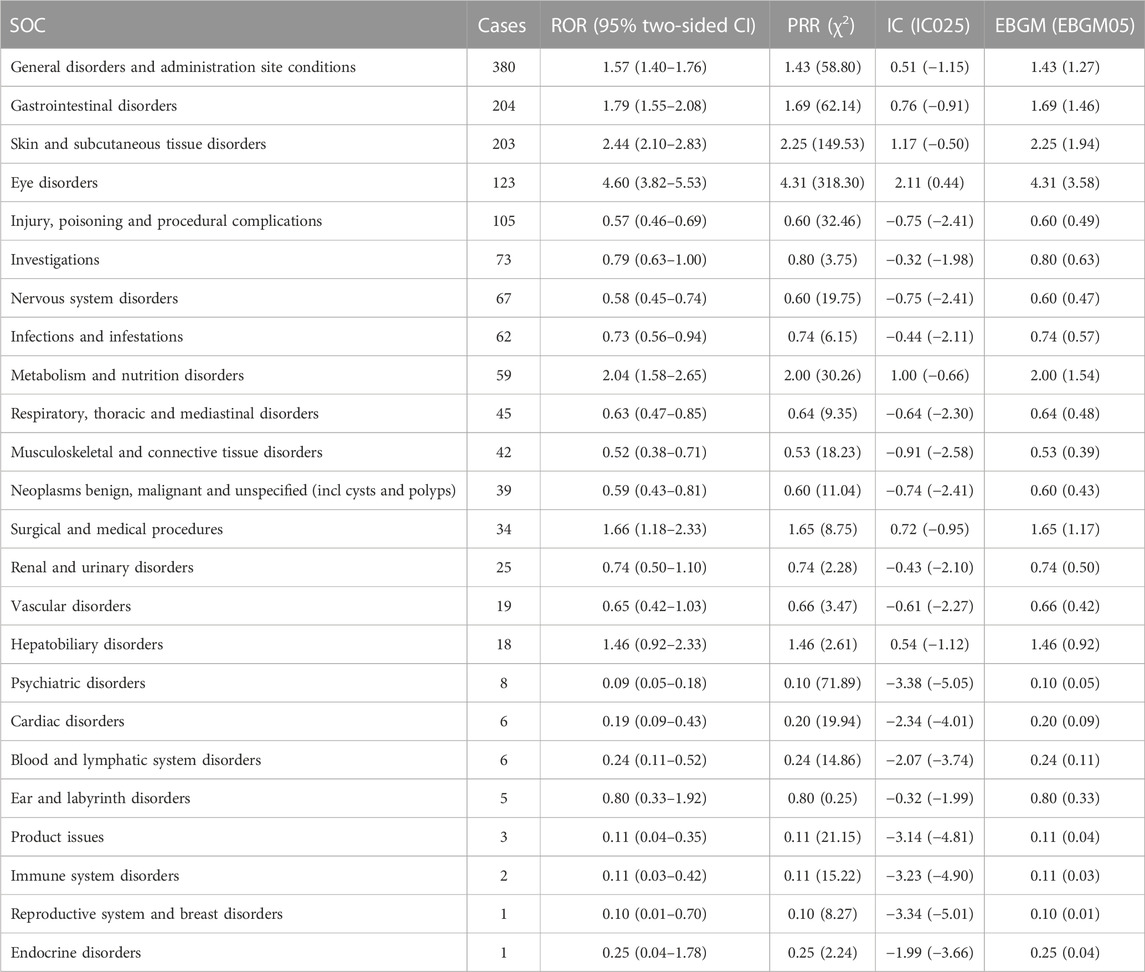
TABLE 3. Signal strength of AEs of erdafitinib at the system organ class (SOC) level in FAERS database.
Upon simultaneous application of the four algorithms, a total of 441 signals corresponding to erdafitinib-induced AEs were detected, spanning 23 SOCs (Supplementary Table S1). The number of reporting PTs exceeding 10 was described in Table 4. Our study observed PTs encompassing hyperphosphatemia, stomatitis, diarrhea, nausea, dry mouth, fatigue, nail changes (disorder or discoloration), dry eye, onycholysis, decreased appetite, and taste disorder, which aligned with the documented AEs listed in the erdafitinib label. It is worth noting that since FAERS includes PTs that are related to medical and health, we also collected some signals that were not related to drugs, which mainly included injury, poisoning and procedural complications, and surgical and medical procedures.
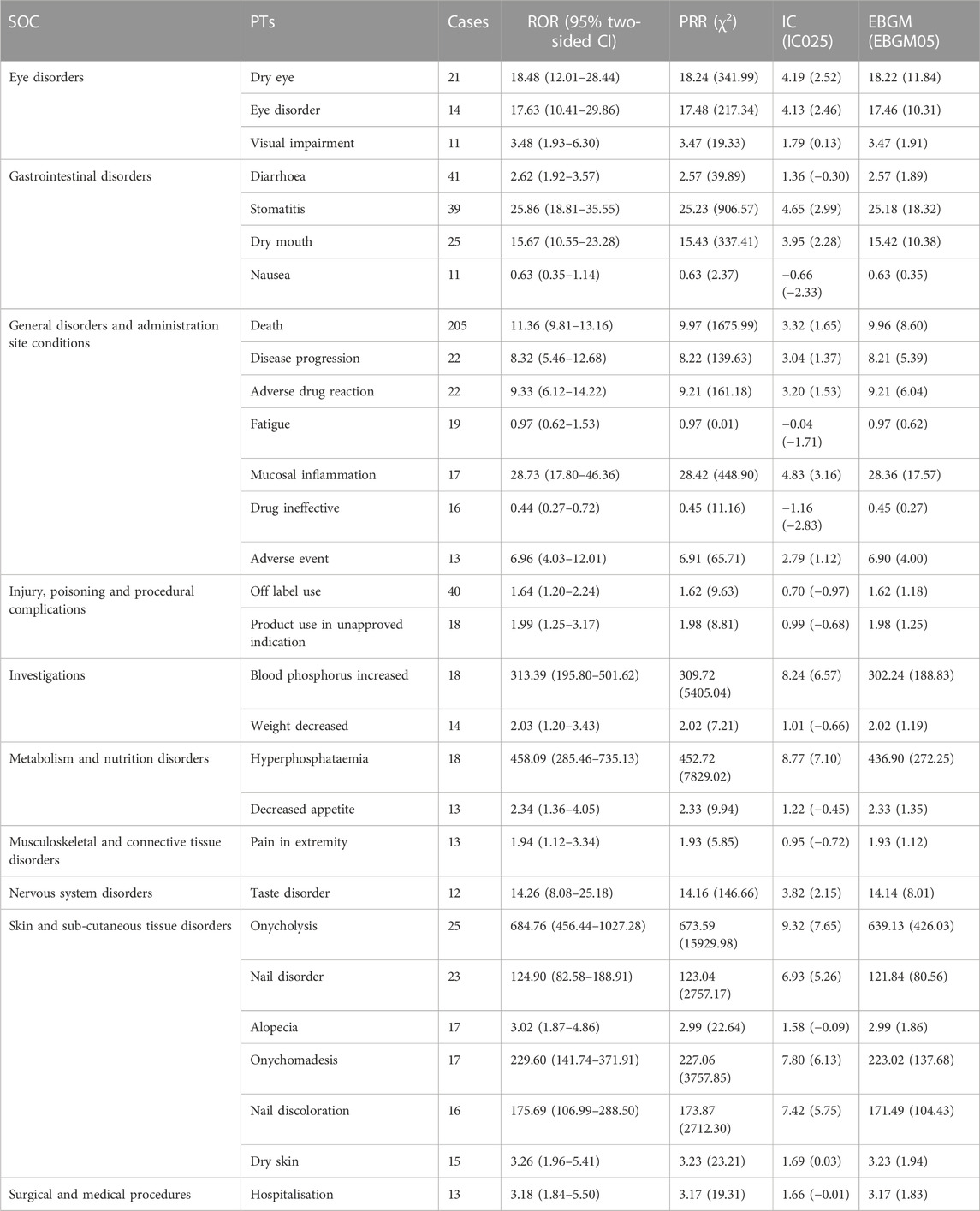
TABLE 4. Signal strength of reports of erdafitinib at the preferred term (PT) level in the FAERS database. The number of reporting PTs >10 were showed.
Onset time of events
To discern the onset time of erdafitinib-associated AEs, data were meticulously collected from the database. Subsequent to the removal of duplicate and erroneous reports, a total of 137 AEs provided information on the onset time. The median onset time was calculated at 54 days [interquartile range (IQR) 17–112 days]. As illustrated in Figure 2, a substantial portion of the AEs manifested within the first (n = 51, 37.23%), second (n = 21, 15.53%), and third months (n = 23, 16.79%) after the initiation of erdafitinib treatment. It merits attention that after 1 year of erdafitinib treatment, 2.92% (n = 4) of AEs were still reported in our study.
Discussion
Previous research on erdafitinib has primarily centered on clinical trials, mechanisms, and literature analysis, with limited emphasis on real-world investigations. To the best of our knowledge, this study is the first pharmacovigilance endeavor utilizing the FAERS database to explore potential associations between erdafitinib and AEs to evaluate the drug’s post-marketing safety. Our study results unveiled that, among the total reports examined, 361 instances (comprising 51.57% of the cohort) pertained to males, while 236 cases (constituting 33.71% of the cohort) pertained to females, both of whom exhibited erdafitinib-induced AEs. Notably, the occurrence of these AEs was more prevalent among males, potentially attributed to the elevated incidence of bladder cancer in the male demographic. This heightened prevalence led to a greater utilization of erdafitinib within the male population, consequently resulting in a larger male patient cohort within the purview of our investigation. Additionally, a higher proportion of AEs was observed among individuals aged over 65, warranting vigilant monitoring of AEs in elderly male patients. The early identification of AEs holds critical importance, as these events may pose life-threatening risks or contribute to disease progression.
According to disproportionation analysis, at the SOC level, the most common and meaningful signals were eye diseases, metabolic and nutritional diseases, and skin and subcutaneous tissue disorders. Hyperphosphatemia, skin and nail changes, and eye disorders were identified as typical ADRs of EFGR inhibitors. In phase I and phase II clinical trial, the above AEs were also confirmed to have occurred, which was consistent with our results (Marandino et al., 2019). Eye diseases were recurrently observed in the drug label of erdafitinib, with dry eye, eye diseases, and visual impairment comprising the most frequently reported AEs within the SOC of ocular diseases. In I and phase II studies, dry eye, and blurred vision were observed in almost more than half of the patients (Loriot et al., 2019). Our findings affirm the prevalence of dry eye as the most common ocular abnormality, and the signal intensities measured as ROR 18.48 (12.01–28.44), PRR 18.24 (341.99), IC 4.19 (2.52), and EBGM 18.22 (11.84), respectively. Besides, approximately 25% of patients exhibited central serous retinopathy or retinal pigment epithelium detachment (D'Angelo et al., 2020). Studies have found that ocular toxicity, including central serous retinopathy, is the class effect of known mitogen-activated protein kinase pathway inhibitors (Loriot et al., 2019), potentially related to erdafitinib’s inhibition of FGFR-related downstream pathways. Normally, central serous retinopathy is temporary, and visual impairment will improve gradually following appropriate dose reduction or discontinuation. Therefore, as a precautionary measure, baseline ophthalmic examination should be repeated monthly during the initial 4 months of erdafitinib treatment, followed by evaluations every 3 months (Sayegh et al., 2022).
In addition, metabolism and nutritional diseases, particularly hyperphosphatemia and decreased appetite, emerged as other important and non-negligible AEs signals related to erdafitinib. Hyperphosphatemia is a recognized type of toxicity attributed to FGFR inhibitors and has been reported in about 77% of patients (Montazeri and Bellmunt, 2020). In our study, despite the relatively few reports on hyperphosphatemia (18), it exhibited a significant signal intensity, with ROR 458.09 (285.46–735.13), PRR 452.72 (7829.02), IC 8.77 (7.10), and EBGM 436.90 (272.25), respectively. Studies have found that FGFR regulates the excretion of phosphate in serum in renal tubules, and inhibition of the FGFR pathway in the renal tubules can lead to hyperphosphatemia (D’Alessandro et al., 2015). Subsequently, a correlation study between erdafitinib plasma concentration and serum phosphate levels demonstrated a significant association (Bahleda et al., 2019). In a further phase I study (NCT01962532) involving Japanese patients with advanced or refractory solid tumors, a single oral dose of 2, 4 and 6 mg erdafitinib was associated with increased plasma phosphate concentration, but no clear dose-response relationship was observed (Markham, 2019). Thus, it is recommended that patients taking erdafitinib should adhere to a dietary phosphate intake of 600–800 mg per day, diligently monitor serum phosphate levels on time, and discontinue treatment if necessary.
Another noteworthy observation pertains to the long-term use of erdafitinib, which carries the risk of dermal AEs, with onycholysis, nail discoloration, alopecia, and dry skin being the most prevalent within the skin and subcutaneous tissue diseases. In a multicenter phase I trial of erdafitinib (JNJ-42756493), 43% of patients experienced skin changes, with dry skin being the most common (29%), and 33% of patients reported nail changes, with onycholysis (11%) and nail dystrophy (9%) being the most frequent occurrences (Bahleda et al., 2019). In this study, onycholysis exhibited the highest number of reported cases in the category of skin and subcutaneous tissue diseases and showed a strong correlation with signal intensity, indicated by ROR 684.76 (456.44–1027.28), PRR 673.59 (15929.98), IC 9.32 (7.65), EBGM 639.13 (426.03), respectively. These findings align with the outcomes reported in the aforementioned clinical trials. However, the underlying pathophysiological mechanisms responsible for these dermal-related AEs have yet to be conclusively determined (Lacouture et al., 2021). Several possible pathological mechanisms have been proposed, such as erdafitinib-induced inhibition of the FGFR pathway in keratinocytes, which may lead to disorders in hair follicle homeostasis and epidermal proliferation and/or differentiation, alongside downregulation of tight junction gene expression, as demonstrated in FGFR-deficient mice (Yang et al., 2010). Therefore, the occurrence of skin and subcutaneous tissue disorders constitutes a significant event necessitating due attention.
Moreover, gastrointestinal diseases also manifested common AEs on the label of erdafitinib, with dry mouth and stomatitis, respectively (Bahleda et al., 2019), among them, dry mouth, typically observed at grade 1 or 2, which was common in patients treated with FGFR inhibitors, occurred in approximately 31%–46% of patients with UC (Lacouture et al., 2021). As evidenced by our study, the dry mouth was detected as obvious signal strength being ROR 15.76 (10.55–23.28), PRR 15.43 (337.41), IC 3.95 (2.28), and EBGM 15.42 (10.38), respectively. Research has found that FGF and FGFRs play a central role in the morphogenesis of salivary gland branches where the destruction of these factors or their receptors probably has been shown to have an impact on salivary gland function, causing the performance of dry mouth (Prochazkova et al., 2018). In addition, in our study, death-related AEs showed obvious signals, which were rare in erdafitinib-related AEs and may be related to the patient’s disease progression rather than the drug itself.
Despite the existence of clinical trials and normative reports, patients receiving erdafitinib treatment also showed hematological abnormalities, especially decreased hemoglobin (35%), decreased platelet count (19%), decreased white blood cells (19%), and neutrophils (10%) (Roubal et al., 2020). However, these AEs did not appear as significant signals in our data analysis. Similarly, several common AEs listed on the drug label, such as hand and foot syndrome, abnormal liver function (including elevated ALT and AST), diarrhea, or constipation, were not detected as signals in our study. These phenomena can be explained by the fact that AEs of all drugs in the FAERS database are quite common. The signal scores can be attenuated due to a high volume of reports for AEs related to various drugs (Fang et al., 2023). Disproportionation analysis requires a higher (or lower) frequency of AE reporting for a particular drug. The absence of signals does not imply the absence of correlation AEs but rather indicates that these AEs do not exhibit a disproportionate relationship (Markham, 2019; Fang et al., 2023).
This research indicated that the median onset time of erdafitinib-induced AEs following initial treatment was 54 days [(IQR) 17–112 days], with a majority of AEs occurring within the first (n = 51, 37.23%), second (n = 21, 15.53%), third months (n = 23, 16.79%). These findings were consistent with the previous report in an experimental environment where 27% of patients developed symptoms of central serous retinopathy at 53 days (15% of patients taking 8 mg per day, 12% of patients taking 9 mg per day) (Peng et al., 2022). However, it should be noted that our study included only 137 AE reports with recorded onset times, potentially limiting the accurate reflection of actual onset times and warranting further verification. Therefore, it is essential for clinicians to be vigilant about the onset time, proactively identify and prevent AEs, and promptly implement effective measures.
At present, there is a scarcity of studies focusing on the safety of erdafitinib in real-world large samples. In this regard, our research is particularly noteworthy as it represents the first large-scale investigation into post-marketing AEs of erdafitinib based on the FAERS database. By conducting data mining on the FAERS database, our study systematically explored and analyzed the common signals of adverse reaction to erdafitinib, such as hyperphosphatemia, dry mouth, and eye diseases, as well as other meaningful AEs reports and their respective onset times. This research contributes valuable insights into the clinical safety profile of erdafitinib for future reference.
However, there are still some limitations in our research. First of all, due to the FAERS database relies on a spontaneous reporting system, gathering information from different countries and occupations, which may lead to issues such as underreporting, and incomplete or inaccurate reports, thus potentially affecting the robustness of our research findings. Therefore, some degree of deviation in the result was expected. Secondly, several unmeasured confounding factors, such as potential drug-drug interactions and patient comorbidities that could impact AEs, were not accounted for in our data analysis. Thirdly, it is crucial to emphasize that disproportionality analysis, although valuable in assessing signal intensity, does not provide quantitative risk or prove causal relationships between AEs and targeted drugs. Nevertheless, the FAERS database remains an important tool for post-marketing safety surveillance of drugs.
Conclusion
This study utilized the comprehensive FAERS database, spanning from 2019 to 2022, to acquire a total of 6,322,279 reports after removing duplicates, and successfully identified 700 AE reports associated with erdafitinib as the PS. Through the application of disproportionality analysis, erdafitinib-related AE signals were rigorously explored and quantified, encompassing the onset time and safety signal spectrum of AEs. Notably, common AEs identified at the SOC level included hyperphosphatemia, eye diseases, and dry mouth. It is worth mentioning that the AEs reported in this study demonstrated a considerable concordance with the previously reported clinical trial outcomes and the erdafitinib label provided by the manufacturer. This extensive post-marketing safety surveillance significantly contributes to a more profound comprehension of erdafitinib’s safety profile, thus offering valuable evidence to inform future research and clinical practice in the field.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
TY: Writing–original draft. FL: Investigation, Writing–review and editing. YH: Funding acquisition, Writing–review and editing. HG: Writing–review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. The study was financially supported by the National Natural Science Foundation of China (Grant Nos. 52173281 and 52203166), the Science and Technology Development Program of Jilin Province (Grant No. YDZJ202201ZYTS610), and Talent Reserve Program (TRP), The First Hospital of Jilin University (No. JDYYCB-2023004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1266890/full#supplementary-material
References
Bahleda, R., Italiano, A., Hierro, C., Mita, A., Cervantes, A., Chan, N., et al. (2019). Multicenter phase I study of erdafitinib (JNJ-42756493), oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced or refractory solid tumors. Clin. Cancer Res. 25 (16), 4888–4897. doi:10.1158/1078-0432.CCR-18-3334
Bin Riaz, I., Khan, A. M., Catto, J. W., and Hussain, S. A. (2021). Bladder cancer: shedding light on the most promising investigational drugs in clinical trials. Expert Opin. Investig. Drugs 30 (8), 837–855. doi:10.1080/13543784.2021.1948999
D’alessandro, C., Piccoli, G. B., and Cupisti, A. (2015). The “phosphorus pyramid”: a visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 16, 9. doi:10.1186/1471-2369-16-9
D'angelo, A., Bagby, S., Galli, I. C., Bortoletti, C., and Roviello, G. (2020). Overview of the clinical use of erdafitinib as a treatment option for the metastatic urothelial carcinoma: where do we stand. Expert Rev. Clin. Pharmacol. 13 (10), 1139–1146. doi:10.1080/17512433.2020.1823830
Fang, Z., Xu, Z., Zhu, W., Yu, M., and Ji, C. (2023). A real-world disproportionality analysis of apalutamide: data mining of the FDA adverse event reporting system. Front. Pharmacol. 14, 1101861. doi:10.3389/fphar.2023.1101861
Garje, R., An, J., Obeidat, M., Kumar, K., Yasin, H. A., and Zakharia, Y. (2020). Fibroblast growth factor receptor (FGFR) inhibitors in urothelial cancer. Oncologist 25 (11), e1711–e1719. doi:10.1634/theoncologist.2020-0334
Grace, E., Goldblum, O., Renda, L., Agada, N., See, K., Leonardi, C., et al. (2020). Injection site reactions in the federal adverse event reporting system (FAERS) post-marketing database vary among biologics approved to treat moderate-to-severe psoriasis. Dermatol Ther. (Heidelb) 10 (1), 99–106. doi:10.1007/s13555-019-00341-2
Guo, M., Shu, Y., Chen, G., Li, J., and Li, F. (2022). A real-world pharmacovigilance study of FDA adverse event reporting system (FAERS) events for niraparib. Sci. Rep. 12 (1), 20601. doi:10.1038/s41598-022-23726-4
Haugsten, E. M., Wiedlocha, A., Olsnes, S., and Wesche, J. (2010). Roles of fibroblast growth factor receptors in carcinogenesis. Mol. Cancer Res. 8 (11), 1439–1452. doi:10.1158/1541-7786.MCR-10-0168
Hu, Y., Gong, J., Zhang, L., Li, X., Li, X., Zhao, B., et al. (2020). Colitis following the use of immune checkpoint inhibitors: a real-world analysis of spontaneous reports submitted to the FDA adverse event reporting system. Int. Immunopharmacol. 84, 106601. doi:10.1016/j.intimp.2020.106601
Knowles, M. A., and Hurst, C. D. (2015). Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 15 (1), 25–41. doi:10.1038/nrc3817
Lacouture, M. E., Sibaud, V., Anadkat, M. J., Kaffenberger, B., Leventhal, J., Guindon, K., et al. (2021). Dermatologic adverse events associated with selective fibroblast growth factor receptor inhibitors: overview, prevention, and management guidelines. Oncologist 26 (2), e316–e326. doi:10.1002/onco.13552
Loriot, Y., Necchi, A., Park, S. H., Garcia-Donas, J., Huddart, R., Burgess, E., et al. (2019). Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 381 (4), 338–348. doi:10.1056/NEJMoa1817323
Marandino, L., Raggi, D., Giannatempo, P., Fare, E., and Necchi, A. (2019). Erdafitinib for the treatment of urothelial cancer. Expert Rev. Anticancer Ther. 19 (10), 835–846. doi:10.1080/14737140.2019.1671190
Markham, A. (2019). Erdafitinib: first global approval. Drugs 79 (9), 1017–1021. doi:10.1007/s40265-019-01142-9
Mascolo, A., Scavone, C., Ferrajolo, C., Rafaniello, C., Danesi, R., Del Re, M., et al. (2021). Immune checkpoint inhibitors and cardiotoxicity: an analysis of spontaneous reports in eudravigilance. Drug Saf. 44 (9), 957–971. doi:10.1007/s40264-021-01086-8
Mcconkey, D. J., Choi, W., Ochoa, A., Siefker-Radtke, A., Czerniak, B., and Dinney, C. P. (2015). Therapeutic opportunities in the intrinsic subtypes of muscle-invasive bladder cancer. Hematol. Oncol. Clin. North Am. 29 (2), 377–394. doi:10.1016/j.hoc.2014.11.003
Mcconkey, D. J., Choi, W., Shen, Y., Lee, I. L., Porten, S., Matin, S. F., et al. (2016). A prognostic gene expression signature in the molecular classification of chemotherapy-naive urothelial cancer is predictive of clinical outcomes from neoadjuvant chemotherapy: a phase 2 trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with bevacizumab in urothelial cancer. Eur. Urol. 69 (5), 855–862. doi:10.1016/j.eururo.2015.08.034
Montazeri, K., and Bellmunt, J. (2020). Erdafitinib for the treatment of metastatic bladder cancer. Expert Rev. Clin. Pharmacol. 13 (1), 1–6. doi:10.1080/17512433.2020.1702025
Morales-Barrera, R., Suarez, C., Gonzalez, M., Valverde, C., Serra, E., Mateo, J., et al. (2020). The future of bladder cancer therapy: optimizing the inhibition of the fibroblast growth factor receptor. Cancer Treat. Rev. 86, 102000. doi:10.1016/j.ctrv.2020.102000
Nadal, R., and Bellmunt, J. (2019). Management of metastatic bladder cancer. Cancer Treat. Rev. 76, 10–21. doi:10.1016/j.ctrv.2019.04.002
Patel, V. G., Oh, W. K., and Galsky, M. D. (2020). Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J. Clin. 70 (5), 404–423. doi:10.3322/caac.21631
Peng, J., Sridhar, S., Siefker-Radtke, A. O., Selvarajah, S., and Jiang, D. M. (2022). Targeting the FGFR pathway in urothelial carcinoma: the future is now. Curr. Treat. Options Oncol. 23 (9), 1269–1287. doi:10.1007/s11864-022-01009-4
Prochazkova, M., Prochazka, J., Marangoni, P., and Klein, O. D. (2018). Bones, glands, ears and more: the multiple roles of FGF10 in craniofacial development. Front. Genet. 9, 542. doi:10.3389/fgene.2018.00542
Roberts, J. T., Von Der Maase, H., Sengelov, L., Conte, P. F., Dogliotti, L., Oliver, T., et al. (2006). Long-term survival results of a randomized trial comparing gemcitabine/cisplatin and methotrexate/vinblastine/doxorubicin/cisplatin in patients with locally advanced and metastatic bladder cancer. Ann. Oncol. 17 (5), v118–v122. doi:10.1093/annonc/mdj965
Robertson, A. G., Kim, J., Al-Ahmadie, H., Bellmunt, J., Guo, G., Cherniack, A. D., et al. (2017). Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 171 (3), 540–556.e25. doi:10.1016/j.cell.2017.09.007
Roubal, K., Myint, Z. W., and Kolesar, J. M. (2020). Erdafitinib: a novel therapy for FGFR-mutated urothelial cancer. Am. J. Health Syst. Pharm. 77 (5), 346–351. doi:10.1093/ajhp/zxz329
Sayegh, N., Tripathi, N., Agarwal, N., and Swami, U. (2022). Clinical evidence and selecting patients for treatment with erdafitinib in advanced urothelial carcinoma. Onco Targets Ther. 15, 1047–1055. doi:10.2147/OTT.S318332
Shu, Y., He, X., Liu, Y., Wu, P., and Zhang, Q. (2022a). A real-world disproportionality analysis of olaparib: data mining of the public version of FDA adverse event reporting system. Clin. Epidemiol. 14, 789–802. doi:10.2147/CLEP.S365513
Shu, Y., He, X., Wu, P., Liu, Y., Ding, Y., and Zhang, Q. (2022b). Gastrointestinal adverse events associated with semaglutide: a pharmacovigilance study based on FDA adverse event reporting system. Front. Public Health 10, 996179. doi:10.3389/fpubh.2022.996179
Siegel, R. L., Miller, K. D., Wagle, N. S., and Jemal, A. (2023). Cancer statistics, 2023. CA Cancer J. Clin. 73 (1), 17–48. doi:10.3322/caac.21763
Yang, J., Meyer, M., Muller, A. K., Bohm, F., Grose, R., Dauwalder, T., et al. (2010). Fibroblast growth factor receptors 1 and 2 in keratinocytes control the epidermal barrier and cutaneous homeostasis. J. Cell Biol. 188 (6), 935–952. doi:10.1083/jcb.200910126
Keywords: erdafitinib, FAERS, adverse event, data mining, pharmacovigilance
Citation: Yuan T, Li F, Hou Y and Guo H (2023) Adverse events in patients with advanced urothelial carcinoma treated with erdafitinib: a retrospective pharmacovigilance study. Front. Pharmacol. 14:1266890. doi: 10.3389/fphar.2023.1266890
Received: 25 July 2023; Accepted: 10 November 2023;
Published: 21 November 2023.
Edited by:
Alfredo Vannacci, University of Florence, ItalyReviewed by:
Scot Niglio, National Cancer Institute (NIH), United StatesEli Ehrenpreis, Advocate Lutheran General Hospital, United States
Copyright © 2023 Yuan, Li, Hou and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuchuan Hou, aG91eWNAamx1LmVkdS5jbg==; Hui Guo, Z3VvaHVpMzE1QGpsdS5lZHUuY24=
 Tengfei Yuan
Tengfei Yuan Faping Li
Faping Li Yuchuan Hou
Yuchuan Hou Hui Guo*
Hui Guo*