Abstract
Shenfu injection (SFI), composed of ginseng and aconite, is a Chinese patent developed from the classic traditional prescription Shenfu Decoction created more than 700 years ago. SFI has been widely used in China for over 30 years for treating cardiovascular diseases. The main components in it include ginsenosides and aconitum alkaloids. In recent years, the role of SFI in the treatment of cardiovascular diseases has attracted much attention. The pharmacological effects and therapeutic applications of SFI in cardiovascular diseases are summarized here, highlighting pharmacological features and potential mechanisms developments, confirming that SFI can play a role in multiple ways and is a promising drug for treating cardiovascular diseases.
1 Introduction
Cardiovascular diseases (CVDs) remain the predominant cause of mortality and morbidity worldwide over the past 20 years, including atherosclerosis, coronary heart disease, arrhythmia, hypertension, cardiomyopathy, stroke and heart failure (Parikh et al., 2018; Benjamin et al., 2019; Feng et al., 2019; Makhmudova et al., 2021). According to the World Health Organization (WHO) Report 2021, noncommunicable diseases (NCDs) kill more than 40 million people every year, and CVDs are the world’s leading cause of death, accounting for almost one in three of all reported deaths globally. Data from the World Heart Report 2023 shows that 20.5 million people died from CVDs in 2021 (Mariappan et al., 2023). CVDs are caused by a variety of pathological factors, such as atherosclerosis, hypertension, hyperlipidemia, diabetes mellitus and so on, associated with energy metabolism disorder, mitochondrial structure abnormality, oxidative stress injury, cardiomyocyte apoptosis, inflammatory reaction, but the specific pathogenesis has not yet been fully elucidated (Parikh et al., 2018; Benjamin et al., 2019; Feng et al., 2019; Makhmudova et al., 2021). Based on the complex pathophysiologcial mechanisms, there are numerous drugs recommended for the treatment of CVDs, including angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists, β-receptor antagonists, vasodilators, diuretics, α-receptor antagonists, positive inotropes, lipid-lowering drugs, antiarrhythmics, calcium channel blockers, etc. However, their potential serious adverse effects caused by these drug, such as hyperkalemia, cardiac depression, and electrolyte disturbance, cannot be ignored (Alhawassi et al., 2018; Núñez-Acevedo et al., 2018; Pall et al., 2021). Therefore, folk medicine is widely used to treat CVDs, among which traditional Chinese medicine (TCM) is well known in the world. Along with the long history of development for TCM, some classic recipes for the treatment of CVDs have been used in the clinic since then. Zhigancao Decoction, originated from Treatise on Febrile Diseases in the Eastern Han Dynasty (25–280 AD), is composed of Glycyrrhiza uralensis Fisch [Leguminosae; Glycyrrhizae Radix et Rhizoma], Zingiber officinale Rosc [Zingiberaceae; Zingiberis Rhizoma Recens], Cinnamomum cassia Presl [Lauraceae; Cinnamomi Ramulus], Panax ginseng C.A.Mey [Araliaceae; Ginseng Radix et Rhizoma Rubra], Rehmannia glutinosa Libosch [Scrpophulariaceae; Rehmanniae Radix], Equus asinus L [Equidae; Asini Corii Colla], Ophiopogon japonicus (L.f) Ker-Gawl [Liliaceae; Ophiopogonis Radix], Cannabis sativa L [Moraceae; Cannabis Fructus], Ziziphus jujuba Mill [Rhamnaceae; Jujubae Fructus], and used to treat arrhythmia and heart failure (Xiong, 2019; Zhang N. et al., 2021; Wu et al., 2021; Yang Y. et al., 2022). Xuefu Zhuyu Decoction, recorded in the classic Yi Lin Gai Cuo in the Qing dynasty (1830 AD), composed of eleven commonly used herbs, including Prunus persica (L.) Batsch [Rosaceae; Persicae Semen], Carthamus tinctorius L [Compositae; Carthami Flos], Angelica sinensis (Oliv.) Diels [Umbelliferae; Angelicae Sinensis Radix], Rehmannia glutinosa Libosch [Scrpophulariaceae; Rehmanniae Radix], Achyranthes bidentata Bl [Amaranthaceae; Achyranthis Bidentatae Radix], Ligusticum chuanxiong Hort [Umbelliferae; Chuanxiong Rhizoma], Platycodon grandiflorum (Jacq.) A. DC [Campanulaceae; Platycodonis Radix], Paeonia lactiflora Pall [Ranunculaceae; Paeoniae Radix Rubra], Citrus aurantium L [Rutaceae; Aurantii Fructus], Glycyrrhiza uralensis Fisch [Leguminosae; Glycyrrhizae Radix et Rhizoma], Bupleurum chinense DC [Umbelliferae; Bupleuri Radix], is used to treat hyperlipidemia and coronary heart disease (Wang and Qiu, 2019; Zhang S. et al., 2021; Yang et al., 2023). Zhenwu Decoction, was firstly recorded in Treatise on Febrile Diseases. It inculdes five herbs: Poria cocos (Schw.) Wolf [Polyporaceae; Poria], Paeonia lactiflora Pall [Ranunculaceae; Paeoniae Radix Alba], Zingiber officinale Rosc [Zingiberaceae; Zingiberis Rhizoma Recens], Aconitum carmichaelii Debx [Ranunculaceae; Aconiti Lateralis Radix Praeparata], Atractylodes macrocephala Koidz [Compositae; Atractylodis Macrocephalae Rhizoma], which is applied to treat chronic heart failure (Tang et al., 2018; Han et al., 2022). It is believed in TCM that CVDs is related to the imbalance of Qi, Xue, Yin and Yang in the human body. When Qi and Yang is insufficient, CVDs are prone to occur.
SFI (Figure 1) is widely used in China to treat numerous ailments, including shock (Zhang X. et al., 2020; Wang et al., 2022; Zhang and Li, 2023), pulmonary fibrosis (Liu et al., 2021), sepsis (Luo et al., 2021; Li X. et al., 2022; Xu et al., 2022), pneumonia (Niu et al., 2021; Shi et al., 2022), cancer (Gao and Zhang, 2023; Wen et al., 2023), cercerebral infarction (Zhou et al., 2020), CVDs, and has shown promising results. With development of pharmacological research, SFI has been identified as an effective drug for the treatment of CVDs.This paper reviews the latest reports in the past 20 years (2003–2022) from PubMed, Web of Science, and National Knowledge Infrastructure (CNKI) using the keywords “Shenfu injection” and “cardiovascular diseases”. The pharmacological action and therapeutic application of SFI in treating CVDs were discussed, and its pharmacological characteristics and potential mechanism was emphasized.
FIGURE 1
Shenfu injection.
2 SFI -basic characteristics and history of use
SFI is a commonly used traditional Chinese medicine injection that has been used in clinical for over 30 years (Liu et al., 2021). It originated from the traditional Chinese classical formula “Shenfu Decoction", which was first recorded in Yan’s Prescriptions for Rescuing Lives in the Song Dynasty (1253 AD). SFI is composed of Panax ginseng C.A.Mey [Araliaceae; Ginseng radix et rhizoma rubra] (RG) (Figure 2) and Aconitum carmichaelii Debx [Ranunculaceae; Aconiti lateralis radix praeparata] (RA) (Figure 3), which has the function of restoring Yang and invigorating Qi (Pei et al., 2021; Zhou et al., 2022). The existing studies reported that RG can be used to treat coronary heart disease and atherosclerosis by reducing blood lipid levels and improving inflammation (Hernández-García et al., 2019; Lu et al., 2019; Im, 2020). Additionally, it can inhibit arrhythmia by affecting the ion channels, such as activating potassium channel while blocking calcium channel and sodium current (Liu Z. et al., 2019; Gou et al., 2020). Furthermore, by ameliorating mitochondrial function and reducing oxidative damage in cardiomyocytes, it can prevent ventricular remodeling and heart failure. Moreover, it has the potential to improve the function of vascular endothelial cells, thereby lowering blood pressure (Yang F. et al., 2022; Liu et al., 2022). Meanwhile, RA has cardiotonic effects by accelerating β-adrenergic receptor synthesis (Tong et al., 2021), has anti-inflammatory effects through the Toll-like receptor4/Nuclear factor κB (TLR4/NF-κB) pathway (Yan et al., 2020), and has anti-arrhythmic effects (Wang et al., 2023). SFI, composed of RA and RG, is a common drug for the treatment of CVDs.
FIGURE 2

Ginseng Radix et Rhizoma Rubra.
FIGURE 3

Aconiti lateralis radix praeparata.
Modern chemical studies have shown that SFI mainly contains ginsenosides, aconite alkaloids, organic acids, nucleosides, amino acids and other components (Song et al., 2015). Ginsenosides and aconite alkaloids are the main active components of SFI. The content of ginsenosides is 676–742 μg/mL, and the content of aconite alkaloids is 3–7 μg/mL (Yang et al., 2014; Ge et al., 2015; Song et al., 2015). It is known that aconite has certain toxicity, and the use of RG and RA in combination can achieve the effect of potentiation and detoxification. Ginsenosides can promote the metabolism of the toxic component aconitine, prolong the elimination half-life of active ingredients such as hypaconitine, benzoylmesaconine and songorine, and significantly increase the in vivo exposure of active ingredients. At the same time, some studies have found that ginseng can inhibit the ion disorders, toxicity in calcineurin-nuclear factor of activated T cells (CaN-NFAT3) pathway and inhibition of the cytochrome P450 2J3 (CYP2J3) expression caused by aconitine, and enhance the antioxidant effect of myocardial cells (Liu et al., 2020; Yang et al., 2021; Chen Z. Y. et al., 2022; Bao et al., 2023). Therefore, the compatibility of aconite and ginseng has the effect of ‘reducing toxicity and increasing efficiency’.
3 Bioavailability and metabolism of SFI
Pharmacokinetic data of rodents show that aconitum alkaloids can be rapidly eliminated after intravenous injection of SFI. Protopanaxatriol (PPT) ginsenosides such as ginsenoside Re (Figure 4), Rg1 (Figure 5) and Rg2 (Figure 6) can be rapidly excreted into bile when ginsenosides was given to rats (Cai et al., 2022). The elimination rate of protopanaxadiol ginsenosides such as ginsenoside Rb1 (Figure 7), Rd (Figure 8) and Rh2 (Figure 9) is slower than that of PPT ginsenosides (Li et al., 2015; Zhang et al., 2016; Shen et al., 2021). The pharmacokinetic properties of ginsenosides (ginsenoside Rg1, ginsenoside Rb1, ginsenoside Rc (Figure 10)) and aconitine alkaloids (benzoylmesaconine (Figure 11), aconitine (Figure 12)) in SFI showed a linear relationship in the dose range of 2–8 mL/kg (Zhang et al., 2016; Li S. et al., 2022).
FIGURE 4
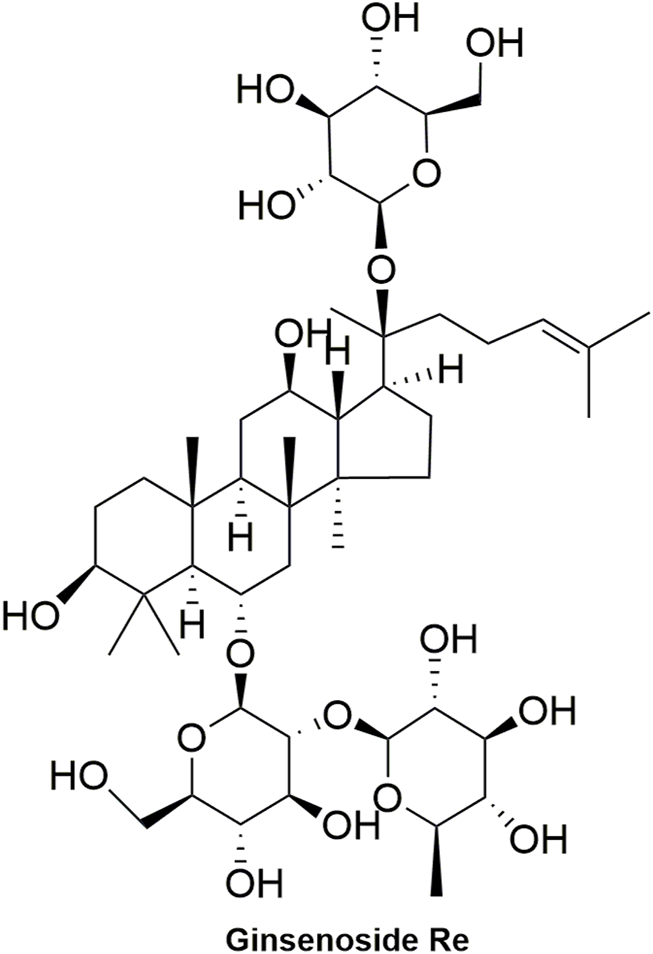
Structural formula of ginsenoside Re.
FIGURE 5
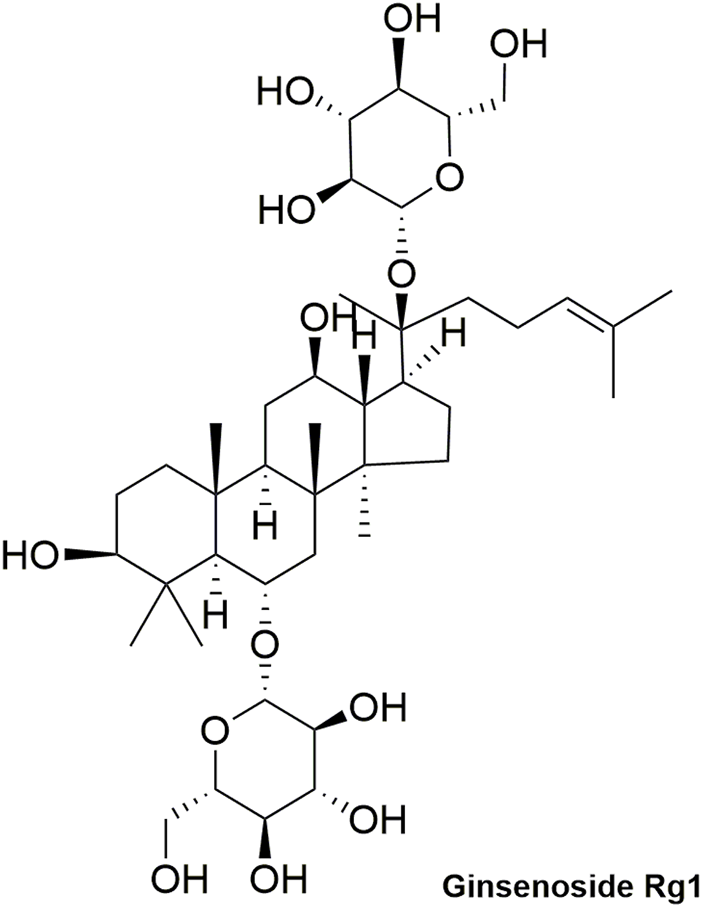
Structural formula of ginsenoside Rg1.
FIGURE 6
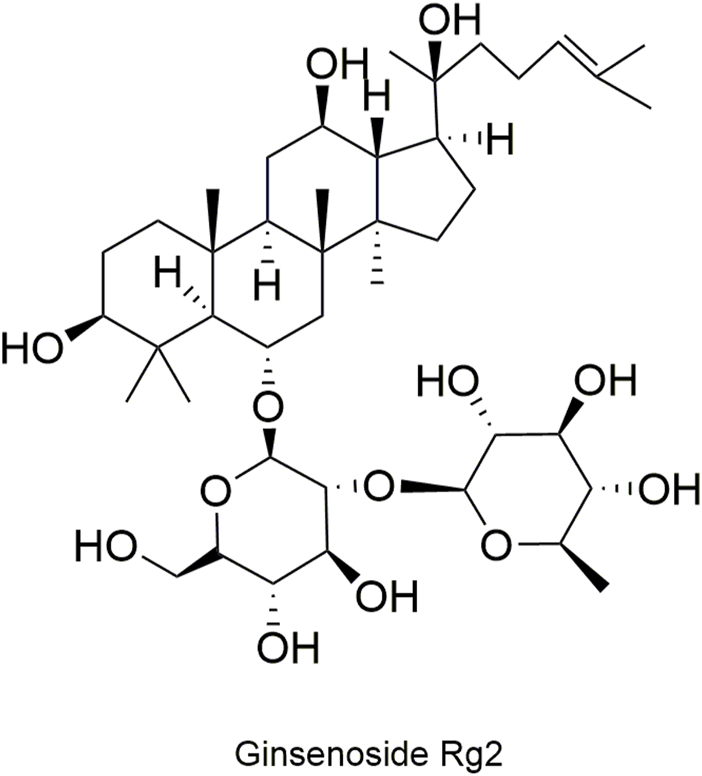
Structural formula of ginsenoside Rg2.
FIGURE 7
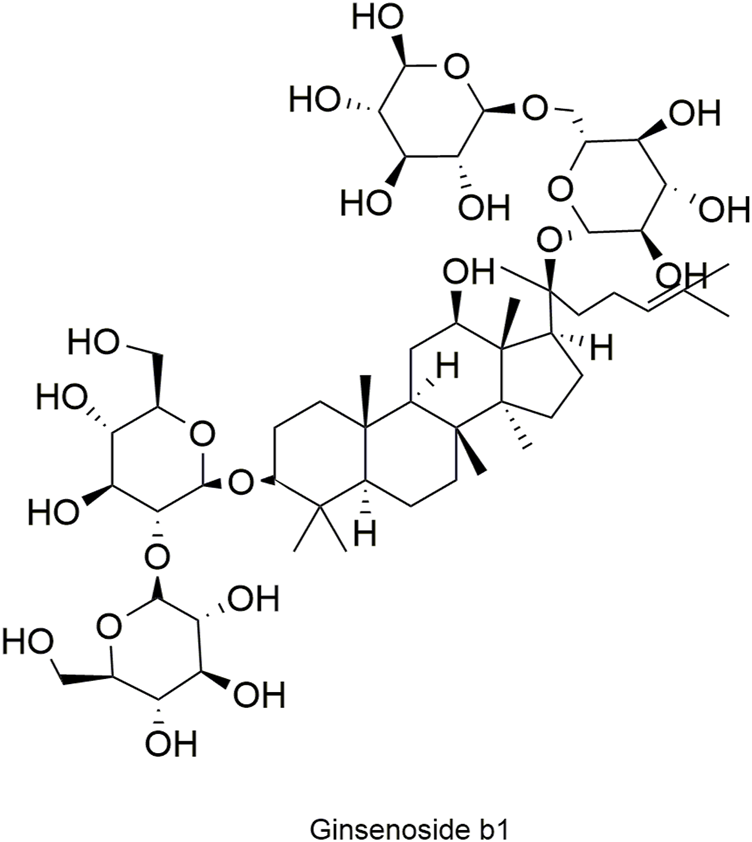
Structural formula of ginsenoside Rb1.
FIGURE 8
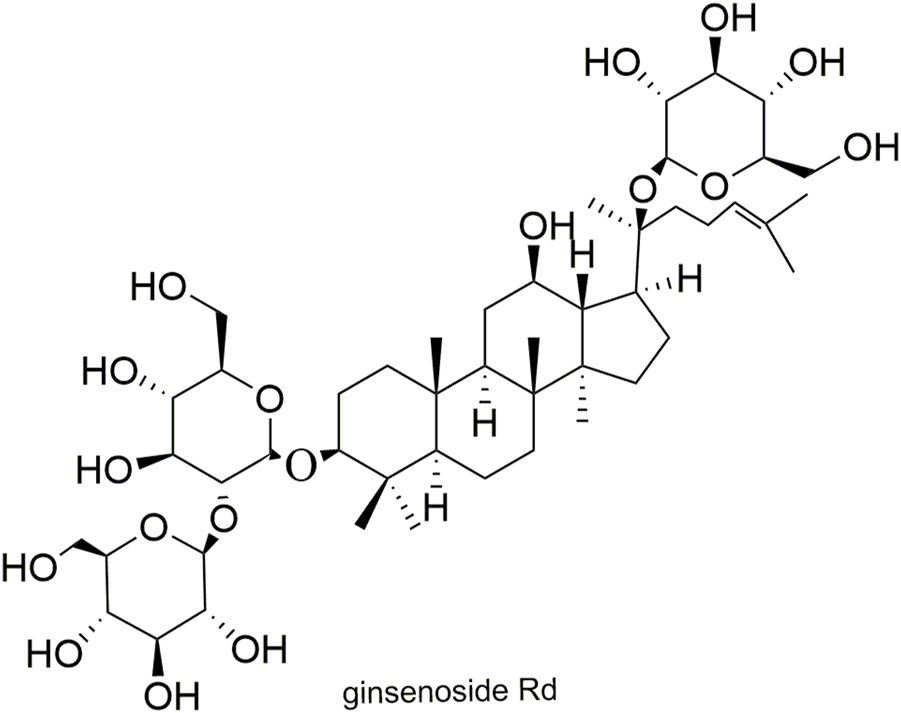
Structural formula of ginsenoside Rd.
FIGURE 9
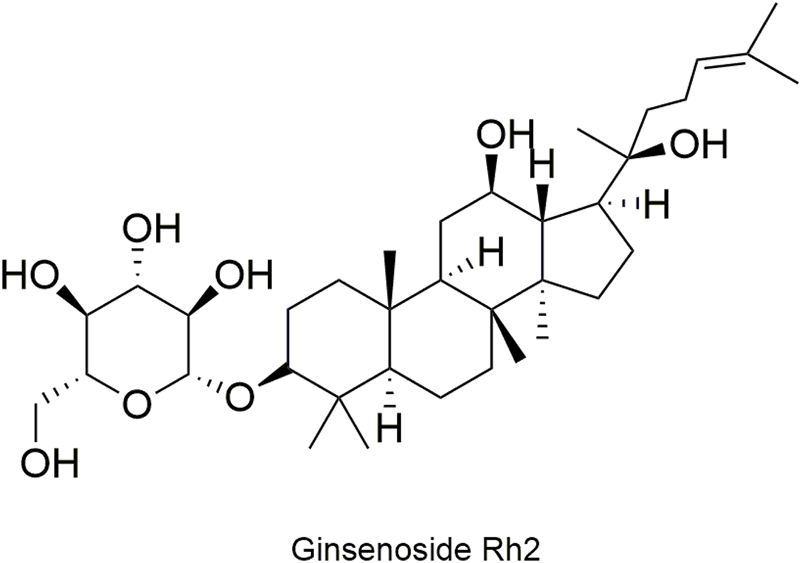
Structural formula of ginsenoside Rh2.
FIGURE 10
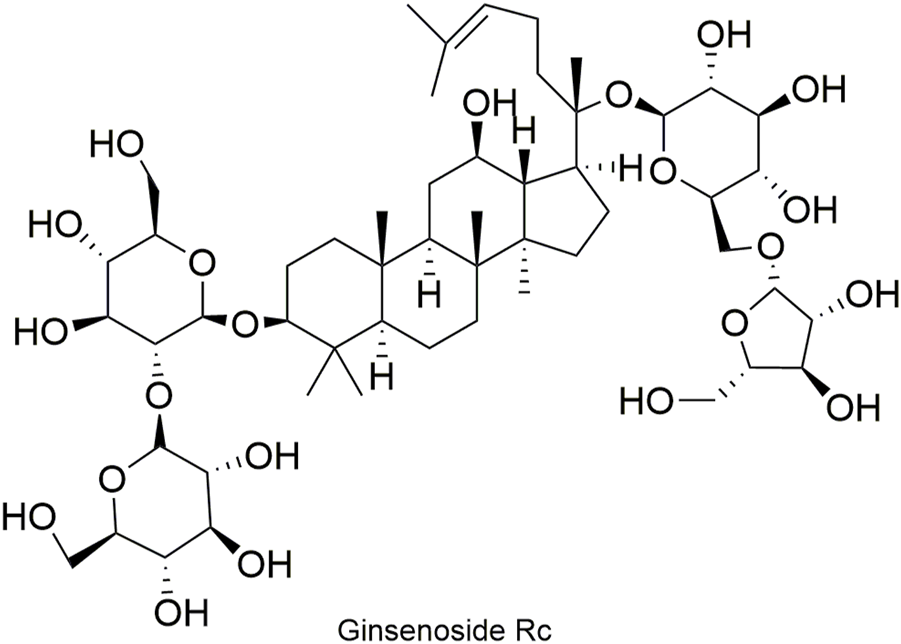
Structural formula of ginsenoside Rc.
FIGURE 11
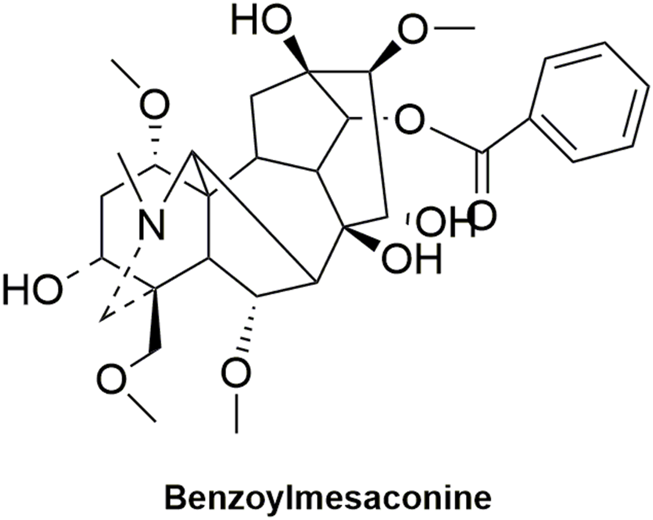
Structural formula of benzoylmesaconine.
FIGURE 12
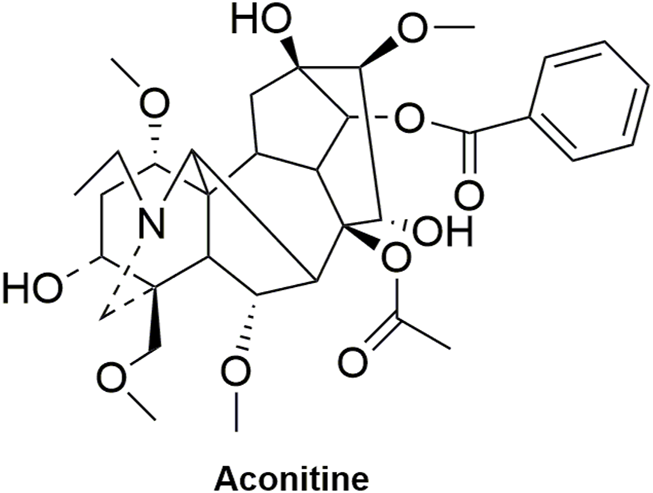
Structural formula of aconitine.
Modern studies have shown that SFI is almost safe at conventional therapeutic doses, and the incidence of adverse reactions is relatively low (0.076%), such as rash, itching, nausea, vomiting, dizziness, abdominal pain, and palpitation (Wang Z. F. et al., 2017).
4 Pharmacological activities of SFI on CVDs
Many studies have confirmed that SFI has therapeutic effects on a variety of CVDs, such as myocardial hypertrophy, heart failure, ischemia-reperfusion injury, cardiac arrest, and arrhythmia. Its mechanism of action is mainly related to reducing inflammation through NF-κB signaling pathway, oxidative stress by reducing free radical damage, dilating blood vessels by increasing nitric oxide (NO) content, decreasing fibrosis through TGF-𝛽/Smads signaling pathway and reducing apoptosis by increasing the expression of apoptosis proteins (Figure 13).
FIGURE 13
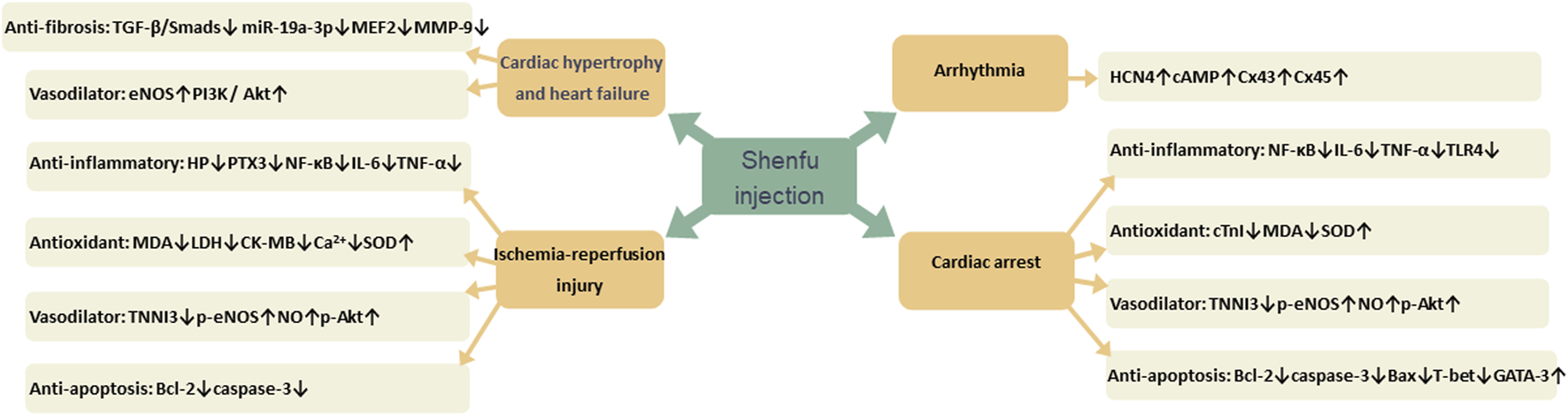
The pharmacological effect of Shenfu injection (SFI) on cardiovascular diseases.
4.1 Cardiac hypertrophy and heart failure
Cardiac hypertrophy is mainly manifested as thickening of ventricular walls and an increase in cardiomyocyte size, closely related to cardiac fibrosis and heart failure (Feng et al., 2019). As time progresses and in settings of sustained stress, cardiac hypertrophy and fibrosis will eventually lead to heart failure (Gallo et al., 2019; Zhao D. et al., 2021; Methatham et al., 2021). Inhibiting cardiac hypertrophy and fibrosis is an effective way to treat heart failure.
TGF-𝛽/Smads plays a key role in the pathogenesis of myocardial fibrosis. The previous research suggested that TGF-𝛽1 bind to receptor, recruited and phosphorylated type I receptor, induced phosphorylation of Smad2 and Smad3. The phosphorylated Smad2 and Smad3 formed a trimer complex with Smad4. Then the complex transferred into the nucleus and regulated the transcription of target genes, regulating the synthesis of collagen fibers and the activation of fibroblasts. Smad7 can competitively bind to the type I receptor of TGF-𝛽1 and inhibit the signal transduction of TGF-𝛽1/Smads pathway (Stewart et al., 2018; Wang L. et al., 2021). Ni et al. (2017) found that SFI can effectively improve cardiac function in the rate model of congestive heart failure (CHF) and attenuate ventricular remodeling and myocardial fibrosis by regulating TGF-𝛽/Smads signaling pathway, upregulating Smad7 and downregulating TGF-𝛽1, Smad2 and Smad3 gene expression.
Inflammatory response is trigged in the myocardial infarction area which can result in cardiac remodeling and heart failure, accumulating high levels of monocytes and neutrophils (Halade and Lee, 2022). Inflammatory cells can also stimulate repair pathways, with increasing the content of extracellular matrix in the myocardium, including matrix metalloproteinase-9 (MMP-9) and collagen (Valiente-Alandi et al., 2018). SFI has anti-inflammatory effect, which can reduce the content of inflammatory factors such as tumor necrosis factor-α (TNF-α), interleukin-1𝛽 (IL-1𝛽) and interleukin-6 (IL-6) in serum of rats, and also reduce the expression of fibronectin, collagen I, collagen III and MMP-9 protein (Ni et al., 2017; Guo et al., 2022).
Endothelial function is essential for maintaining normal vasomotor function, and disruption of this function can result in vasomotor dysfunction (Sabe et al., 2022). When heart failure occurs, endothelial function is impaired, with vasoconstrictor substances increasing, and vasodilator substances decreasing (Monteiro et al., 2019). NO is the most famous vasodilator, while endothelin-1 (ET-1) is the most widely recognized vasoconstrictor (Miyauchi and Sakai, 2019). The production of NO mainly depends on the activity and quantity of endothelial nitric oxide synthase (eNOS). When the expression of eNOS mRNA increases, the number of eNOS synthesis increases. When eNOS binds with calmodulin (CaM), the activity of eNOS enchance, adversely, when binds with caveolin-1 (Cav-1), decrease. The phosphorylation of eNOS depends on the phosphatidylinositol 3-kinase/protein kinase B (PI3K-Akt) signaling pathway, which produces phosphorylated tyrosine residues, thereby provides an anchor site for the recruitment of PI3K to the membrane (Garcia and Sessa, 2019; Suvorava et al., 2022). Zhu et al. (2020) found that SFI can increase the expression of eNOS mRNA and CaM and promote NO synthesis, decrease the expression of Cav-1 and ET-1 content, promote eNOS phosphorylation via the PI3K/Akt signaling pathway.
MicroRNAs (miRNAs), encoded by myosin heavy chain (MHC) genes, are important regulatory factors of CVDs (Omidkhoda et al., 2019). MiR19a-3p plays an important role in cardiac hypertrophy. Myocytes specific enhancer factor 2A (MEF2A) is a target gene of miR-19a-3p and is highly expressed in cardiac hypertrophy while miR-19a-3p has low expression (Mao et al., 2018). SFI can upregulate the expression of miR-19a-3p, and downregulate the expression of MEF2A and 𝛽-myosin heavy chain (𝛽-MHC), so attenuate cardiac hypertrophy (Mao et al., 2018).
4.2 Ischemia-reperfusion injury
Myocardial ischemia-reperfusion injury is a significant factor that has a negative impact on the prognosis of myocardial infarction patients, causing myocardial stunning, no-reflow phenomena, reperfusion arrhythmia, and even permanent cardiomyocyte death. Therefore, it is critical to understand the mechanism of myocardial ischemia reperfusion and develop efficient treatments (Mokhtari-Zaer et al., 2018; Deng, 2021).
Apoptosis is involved in the pathogenesis of various CVDs and plays an important role in myocardial ischemia-reperfusion injury. Bcl-2 family proteins are important regulators of the process, prevent apoptosis by acting upstream of apoptosis proteins, such as caspase-3 and caspase-9 (King et al., 2023; Sahoo et al., 2023). Previous research found that SFI can upregulate the anti-apoptosis protein Bcl-2 and inhibit the consecutive activation of caspase-3 and caspase-9, both of which are intimately associated to apoptosis (Cao et al., 2005; Wang et al., 2009; Guo et al., 2016).
Oxidative stress is a risk factor for CVDs, and abnormally increased reactive oxygen species (ROS) is the main cause of oxidative stress. ROS combined with proteins and lipids damage cardiomyocytes (Peng et al., 2022). The superoxide dismutase (SOD) is the major antioxidant enzyme that degrade superoxide (Eleutherio et al., 2021). The glutathione system is widely recognized as one of the most potent endogenous antioxidant systems within cardiovascular system. Glutathione, one of the endogenous antioxidant molecules, can directly scavenge ROS caused by myocardial ischemia (Panday et al., 2020; Tan et al., 2023). Taurine is a common endogenous sulfur-containing amino acid with antioxidant activity and can inhibit the abnormal increase of ROS (Li et al., 2020). SFI dramatically decreased glutathione and taurine, increased SOD activity, and inhibited the rise in malondialdehyde (MDA), which is closely related to oxidative stress (Zheng et al., 2004; Cao et al., 2005; Wu et al., 2019).
Numerous studies have revealed that NO is a vasodilator with the ability to operate on cardiomyocytes and vascular endothelium via a variety of signaling pathways (Boycott et al., 2020; Cyr et al., 2020). The action of eNOS is primarily responsible for NO generation. NO generated by eNOS phosphorylation induces soluble guanylate cyclase (sGC) to create cyclic guanosine monophosphate (cGMP), a second messenger with cardiovascular protective properties (Mount et al., 2007; Zhang Q. et al., 2020; Lee et al., 2021). SFI activated eNOS phosphorylation via Akt, thereby promoting the production of NO (Wu et al., 2011; Wang et al., 2018).
4.3 Cardiac arrest
Cardiac arrest (CA), one of the leading causes of death, has a significant impact on the public health, particularly due to its persistent increase worldwide (Vazquez and Sudhir, 2023). Post-cardiac arrest syndrome (PCAS) is a group of diseases characterized by systemic ischemia/reperfusion injury, hypoxic brain injury and myocardial dysfunction after cardiac arrest (Jou et al., 2020). It is associated with cardiovascular ischemia/reperfusion injury and cardiovascular toxicity, including factors such as excessive activation of inflammatory cytokines and catecholamines (Lazzarin et al., 2022). Matrix metalloproteinases, tumor necrosis factor, and interleukins each have a special prognostic function in PCAS. High inflammatory cytokine levels have been linked to poor neurologic and/or death outcomes (Jou et al., 2020).
NF-κB signaling pathway is one of the important pathways regulating inflammation and plays a key regulatory role in the occurrence and development of various CVDs (Cheng et al., 2023). Study have reported that in cardiac arrest swine, SFIremarkedly decreased levels of many inflammatory cytokines, such as TNF-α, IL-6, mRNA and protein levels of myocardial TLR4 and NF-κB (Gu et al., 2021). TLR4, as a ‘portal’ protein, regulates the initiation of the inflammatory chain reaction of the body‘s immunity and mediates the inflammatory response (Fitzgerald and Kagan, 2020).
Na+-K+-ATPase enzyme and Ca2+-ATPase enzyme, ubiquitous enzymes in the heart, play a crucial role in process of CVDs (Fedosova et al., 2021). Na+-K+-ATPase transports two Na+ ions extrude out of the cell in exchange for one K+ ions, thereby maintaining the concentration gradients across the cell membrane (Fedosova et al., 2021; Obradovic et al., 2023). Ca2+-ATPase, a crucial role for cellular Ca2+ homeostasis, maintains normal intracellular calcium concentration and prevents calcium overload (Nguyen et al., 2023; Ye et al., 2023). It is reported that SFI increased Na+-K-ATPase and Ca2+-ATPase activity (Ji et al., 2011).
4.4 Arrhythmia
The normal electrical activity of the heart is initiated by special pacemaker cells located in the sinoatrial node (Liang et al., 2021). Dysfunction or loss of pacemaker cells can cause arrhythmia (Liu and Yuan, 2021b). The transplantation of stem cells is regarded as a kind of feasible treatment for arrhythmia (Sattayaprasert et al., 2020). It has been reported that bone marrow mesenchymal stem cells (BMSCs) with specific phenotypes can be transformed into pacemaker-like cells after special treatment (Chauveau et al., 2014). Moreover, HMSCs possess the capability to regulate arrhythmia substrates by altering their secretory groups in diseases (Sattayaprasert et al., 2020).
In vitro, SFI can activate inward pacemaker current of BMSCs in a concentration-dependent manner, increase HCN4 expression and cAMP content in BMSCs, induce BMSCs proliferation, promote their differentiation into pacemaker-like cells (Zhao X. et al., 2021). The HCN4 gene serves as the molecular basis for the pacemaker current, contributing significantly to inward current during depolarization and playing a crucial role in the generation and autonomous regulation of heart rate (Bucchi et al., 2012; D'Souza et al., 2021; Hoekstra et al., 2021). Bone marrow mesenchymal stem cells treated with SFI retained the function of sinoatrial node in rabbits with sinoatrial node syndrome, improved the expression of HCN4 gene and gap junction proteins (Cx43 and Cx45), and significantly upregulated the expression of cAMP in sinoatrial node (Chen Q. et al., 2022).
In addition, SFI has certain pharmacological effects on nervous system, respiratory system and digestive system. For example, SFI has a protective effect on lipopolysaccharide-induced septic shock in rabbits (Liu X. et al., 2019). It can reduce bile duct injury in rats with acute obstructive cholangitis (Tan et al., 2019) and increase the level of acetylcholine in acute liver injury in septic young rats (Wu et al., 2022). It also has a protective effect on lung and intestinal epithelial injury in mice with acute gastrointestinal injury (Zheng et al., 2022).
5 Clinical trial of SFI in CVDs
There are many clinical trials related to SFI, and nine clinical trials have been conducted to study its role in the treatment of CVDs. These clinical trials have demonstrated that SFI can improve cardiac function and corresponding indicators in patients with CVDs, including heart failure, myocardial infarction, cardiac arrest after resuscitation, coronary syndrome, coronary heart disease and other diseases (Table 1).
TABLE 1
| Disease | Number of patients | Dose of SFI (mL) | Duration | Route of administration | Outcome measures | References |
|---|---|---|---|---|---|---|
| acute heart failure | 80 patients | 50 | 7 days | i.v | cardiac function, clinical symptoms and quality of life | Wang et al. (2019b) |
| 55 patients | 100 | 24 h | i.v | CI, cardiac output and stroke volume index | Li et al. (2022a) | |
| Chronic heart failure | 80 patients | 50 | 7 days | i.v | LVEF, LVED, BNP,Fas, TNF-α, IL-6, mortality, readmission rate | Liu et al. (2015) |
| 171 patients | 60 | 2 weeks | i.v | the all-cause mortality,6MWT | Wang et al. (2017a) | |
| 55 patients | 50 | 7 days | i.v | Cardiacfunction, LVEF, NT-proBNP, TNF-α, IL-6 | Gao et al. (2021) | |
| 91 patients | 40 | 7 days | i.v | Cardiac function, LVEF, NT-proBNP, BNP | Guo et al. (2021) | |
| coronary syndrome | 74 patients | 40 | 4–6 h | i.v | the level of NGAL in urine | Guo et al. (2017) |
| myocardial infarction | 20 patients | 80 | 5 days | i.v | the area of myocardial infarction | Wang et al. (2021b) |
| cardiac arrest | 492 patients | 100 | 28 days | i.v | 28-day and 90-day survival rates, the mechanical ventilation time and hospitalization time | Zhang et al. (2017) |
Clinical trial of SFI in cardiovascular disease.
5.1 The effect of SFI in patients with acute heart failure
Infusion of SFI in 80 patients with acute heart failure can improve cardiac function, clinical symptoms and quality of life (Wang et al., 2019b). Fifty patients with acute decompensated heart failure were treated with combination therapy. Compared with simple infusion of levosimendan, the improvement of hemodynamic parameters including CI, cardiac output and stroke volume index was more significant, especially in patients with acute decompensated heart failure with hypotension (Li M. et al., 2022).
5.2 The effect of SFI in patients with chronic heart failure
SFI was used to treat 80 patients with acute exacerbation of chronic heart failure, which could improve the symptoms, quality of life, exercise tolerance, improve left ventricular ejective fraction (LVEF), reduce left ventricular end diastolic diameter (LVED), plasma brain natriuretic peptide (BNP) and cytokine Fas, TNF-α, IL-6 levels, reduce mortality and readmission rate (Liu et al., 2015). SFI was administered to 171 patients suffering from chronic heart failure on the basis of Western medicine. Compared with Western medicine alone, it could reduce the all-cause mortality by 30.99%, increase the 6-min walking distance (6MWT) and improve the quality of life (Wang X. et al., 2017). Patients with coronary heart disease complicated with chronic heart failure were treated with SFI and furosemide injection for 7 days. The effect was better than that of furosemide injection alone in improving cardiac function, LVEF, N-terminal B-type natriuretic peptide (NT-proBNP), TNF-α, IL-6 (Gao et al., 2021). For 7 days, SFI and sodium nitroprusside were administered intravenously to 91 patients who had coronary heart disease and chronic heart failure. The effect was better than that of sodium nitroprusside injection alone in improving cardiac function, LVEF, NT-proBNP and BNP (Guo et al., 2021).
5.3 The effect of SFI in patients with other CVDs
Infusion of SFI 1h before coronary angiography in 74 patients with coronary syndrome undergoing percutaneous coronary intervention (PCI) significantly reduced the level of neutrophil gelatinase-associated lipocalin (NGAL) in urine and effectively prevent contrast-induced acute kidney injury (Guo et al., 2017). SFI was used to treat patients with ST-segment elevation myocardial infarction before PCI and maintained for 5 days after PCI. Compared with patients treated with placebo, SFI reduced the area of myocardial infarction (Wang X. et al., 2021). A total of 492 cardiac arrest patients received bi-daily intravenous SFI infusions over a span of 28 days. The 28-day and 90-day survival rates were improved, the mechanical ventilation time and hospitalization time were shortened, and the recovery of spontaneous circulation after cardiac arrest was effectively improved (Zhang et al., 2017).
6 Concluding remarks and future perspectives
The results of numerous research studies in the past have demonstrated that SFI exerts varying degrees of therapeutic effects on various types of CVDs, such as heart failure, myocardial hypertrophy, myocardial ischemia, cardiac arrest, arrhythmia, and so forth. SFI plays a therapeutic role through multiple different targets, such as TGF-Smads, PI3K-Akt, eNOS-Akt pathway, and so on. SFI has been used in China for more than 30 years. It is a commonly used drug for clinical treatment of CVDs. No serious adverse reactions have been found so far.
In general, SFI is a promising drug for the treatment of CVDs. However, SFI has the characteristics of multi-component, multi-target and multi-pathway, which increases the difficulty of research. There is still a lack of in-depth study on the mechanism of SFI. In addition, large-scale, high-quality, multi-center clinical trials are needed to determine the comparison of SFI with traditional CVDs treatment regimens.
Statements
Author contributions
F-FX: Writing–original draft. X-FX: Writing–original draft. H-YH: Writing–original draft. R-ST: Writing–review and editing. CP: Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Sichuan Province Natural Science Foundation Program (Grant NO. 24NSFSC0627); “Xinglin Scholars” Nursery Talent Special Program (grant number MPRC2023032).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Alhawassi T. M. Krass I. Pont L. G. (2018). Antihypertensive-related adverse drug reactions among older hospitalized adults. Int. J. Clin. Pharm.40, 428–435. 10.1007/s11096-017-0583-7
2
Bao Y. Zhang R. Jiang X. Liu F. He Y. Hu H. et al (2023). Detoxification mechanisms of ginseng to aconite: a review. J. Ethnopharmacol.304, 116009. 10.1016/j.jep.2022.116009
3
Benjamin E. J. Muntner P. Alonso A. Bittencourt M. S. Callaway C. W. Carson A. P. et al (2019). Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation139, e56–e528. 10.1161/cir.0000000000000659
4
Boycott H. E. Nguyen M. N. Vrellaku B. Gehmlich K. Robinson P. (2020). Nitric oxide and mechano-electrical transduction in cardiomyocytes. Front. Physiol.11, 606740. 10.3389/fphys.2020.606740
5
Bucchi A. Barbuti A. Difrancesco D. Baruscotti M. (2012). Funny current and cardiac rhythm: insights from HCN knockout and transgenic mouse models. Front. Physiol.3, 240. 10.3389/fphys.2012.00240
6
Cai J. Huang K. Han S. Chen R. Li Z. Chen Y. et al (2022). A comprehensive system review of pharmacological effects and relative mechanisms of Ginsenoside re: recent advances and future perspectives. Phytomedicine102, 154119. 10.1016/j.phymed.2022.154119
7
Cao J. Zheng C. D. Zhang G. X. Zhang Y. J. Min S. (2005). Protective effect of Shenfu injection on myocardial mitochondria injured by ischemia-reperfusion in rabbits. Chin. Med. J. Engl.118, 505–507.
8
Chauveau S. Brink P. R. Cohen I. S. (2014). Stem cell-based biological pacemakers from proof of principle to therapy: a review. Cytotherapy16, 873–880. 10.1016/j.jcyt.2014.02.014
9
Chen Q. Kang L. Li Y. Lin Z. Chu Q. Cai Y. et al (2022a). Effect of shenfu injection on differentiation of bone marrow mesenchymal stem cells into pacemaker-like cells and improvement of pacing function of sinoatrial node. Oxid. Med. Cell. Longev.2022, 4299892. 10.1155/2022/4299892
10
Chen Z. Y. Wei X. Y. Qiu Z. D. Huang Y. Tan T. Feng Y. L. et al (2022b). Compatibility of fuzi and ginseng significantly increase the exposure of aconitines. Front. Pharmacol.13, 883898. 10.3389/fphar.2022.883898
11
Cheng W. Cui C. Liu G. Ye C. Shao F. Bagchi A. K. et al (2023). NF-κB, A potential therapeutic target in cardiovascular diseases. Cardiovasc. Drugs Ther.37, 571–584. 10.1007/s10557-022-07362-8
12
Cyr A. R. Huckaby L. V. Shiva S. S. Zuckerbraun B. S. (2020). Nitric oxide and endothelial dysfunction. Crit. Care. Clin.36, 307–321. 10.1016/j.ccc.2019.12.009
13
Deng J. (2021). Advanced research on the regulated necrosis mechanism in myocardial ischemia-reperfusion injury. Int. J. Cardiol.334, 97–101. 10.1016/j.ijcard.2021.04.042
14
D'Souza A. Wang Y. Anderson C. Bucchi A. Baruscotti M. Olieslagers S. et al (2021). A circadian clock in the sinus node mediates day-night rhythms in Hcn4 and heart rate. Heart rhythm.18, 801–810. 10.1016/j.hrthm.2020.11.026
15
Eleutherio E. C. A. Silva Magalhães R. S. de Araújo Brasil A. Monteiro Neto J. R. de Holanda Paranhos L. (2021). SOD1, more than just an antioxidant. Arch. Biochem. Biophys.697, 108701. 10.1016/j.abb.2020.108701
16
Fedosova N. U. Habeck M. Nissen P. (2021). Structure and function of Na,K-ATPase-The sodium-potassium pump. Compr. Physiol.12, 2659–2679. 10.1002/cphy.c200018
17
Feng X. Sureda A. Jafari S. Memariani Z. Tewari D. Annunziata G. et al (2019). Berberine in cardiovascular and metabolic diseases: from mechanisms to therapeutics. Theranostics9, 1923–1951. 10.7150/thno.30787
18
Fitzgerald K. A. Kagan J. C. (2020). Toll-like receptors and the control of immunity. Cell180, 1044–1066. 10.1016/j.cell.2020.02.041
19
Gallo S. Vitacolonna A. Bonzano A. Comoglio P. Crepaldi T. (2019). ERK: a key player in the pathophysiology of cardiac hypertrophy. Int. J. Mol. Sci.20, 2164. 10.3390/ijms20092164
20
Gao W. Zhang K. (2023). Network meta-analysis of 8 types of traditional Chinese medicine injection combined with chemotherapy in colorectal cancer treatment. J. Cancer Res. Clin. Oncol.149, 9823–9838. 10.1007/s00432-023-04892-y
21
Gao Y. Gao Y. Zhu R. Tan X. (2021). Shenfu injection combined with furosemide in the treatment of chronic heart failure in patients with coronary heart disease: a protocol of randomized controlled trial. Med. Baltim.100, e24113. 10.1097/md.0000000000024113
22
Garcia V. Sessa W. C. (2019). Endothelial NOS: perspective and recent developments. Br. J. Pharmacol.176, 189–196. 10.1111/bph.14522
23
Ge A. H. Li J. Donnapee S. Bai Y. Liu J. He J. et al (2015). Simultaneous determination of 2 aconitum alkaloids and 12 ginsenosides in Shenfu injection by ultraperformance liquid chromatography coupled with a photodiode array detector with few markers to determine multicomponents. J. Food Drug Anal.23, 267–278. 10.1016/j.jfda.2014.10.013
24
Gou D. Pei X. Wang J. Wang Y. Hu C. Song C. et al (2020). Antiarrhythmic effects of ginsenoside Rg2 on calcium chloride-induced arrhythmias without oral toxicity. J. Ginseng Res.44, 717–724. 10.1016/j.jgr.2019.06.005
25
Gu W. Hou X. M. Li C. S. (2021). Effects of shenfu injection on inflammatory response during post-resuscitation myocardial dysfunction after cardiac arrest in swine. Chin. J. Integr. Med.27, 417–423. 10.1007/s11655-021-2855-2
26
Guo B. Yang T. Nan J. Huang Q. Wang C. Xu W. (2021). Efficacy and safety of Shenfu injection combined with sodium nitroprusside in the treatment of chronic heart failure in patients with coronary heart disease: a protocol of randomized controlled trial. Med. Baltim.100, e24414. 10.1097/md.0000000000024414
27
Guo F. Wang X. Guo Y. Wan W. Cui Y. Wang J. et al (2022). Shenfu administration improves cardiac fibrosis in rats with myocardial ischemia-reperfusion through adenosine A(2a) receptor activation. Hum. Exp. Toxicol.41, 9603271221077684. 10.1177/09603271221077684
28
Guo Z. Niu D. Yu Y. Zhen D. Li W. (2017). Effects of hydration combined with Shenfu injection on contrast-induced acute kidney injury in acute coronary syndrome patients undergoing percutaneous coronary intervention. Biomed. Rep.7, 477–481. 10.3892/br.2017.986
29
Guo Z. J. Wu C. J. Li C. S. (2016). Shen-Fu Injection alleviates post-resuscitation myocardial dysfunction by up-regulating expression of sarcoplasmic reticulum Ca(2+)-ATPase. Chin. J. Integr. Med.22, 503–509. 10.1007/s11655-015-2156-8
30
Halade G. V. Lee D. H. (2022). Inflammation and resolution signaling in cardiac repair and heart failure. EBioMedicine79, 103992. 10.1016/j.ebiom.2022.103992
31
Han Y. Huang L. Zhong G. Chang X. Zhu Q. Xu M. et al (2022). Evaluation of the safety and efficacy of Zhenwu decoction as adjuvant therapy for the treatment of heart failure with reduced ejection fraction: a protocol for systematic review and meta-analysis. Med. Baltim.101, e28672. 10.1097/md.0000000000028672
32
Hernández-García D. Granado-Serrano A. B. Martín-Gari M. Naudí A. Serrano J. C. (2019). Efficacy of Panax ginseng supplementation on blood lipid profile. A meta-analysis and systematic review of clinical randomized trials. J. Ethnopharmacol.243, 112090. 10.1016/j.jep.2019.112090
33
Hoekstra M. van Ginneken A. C. G. Wilders R. Verkerk A. O. (2021). HCN4 current during human sinoatrial node-like action potentials. Prog. Biophys. Mol. Biol.166, 105–118. 10.1016/j.pbiomolbio.2021.05.006
34
Im D. S. (2020). Pro-resolving effect of ginsenosides as an anti-inflammatory mechanism of Panax ginseng. Biomolecules10, 444. 10.3390/biom10030444
35
Ji X. F. Yang L. Zhang M. Y. Li C. S. Wang S. Cong L. H. (2011). Shen-fu injection attenuates postresuscitation myocardial dysfunction in a porcine model of cardiac arrest. Shock35, 530–536. 10.1097/SHK.0b013e31820e2058
36
Jou C. Shah R. Figueroa A. Patel J. K. (2020). The role of inflammatory cytokines in cardiac arrest. J. Intensive Care Med.35, 219–224. 10.1177/0885066618817518
37
King L. E. Hohorst L. García-Sáez A. J. (2023). Expanding roles of BCL-2 proteins in apoptosis execution and beyond. J. Cell Sci.136, jcs260790. 10.1242/jcs.260790
38
Lazzarin T. Tonon C. R. Martins D. Fávero E. L. Jr. Baumgratz T. D. Pereira F. W. L. et al (2022). Post-cardiac arrest: mechanisms, management, and future perspectives. J. Clin. Med.12, 259. 10.3390/jcm12010259
39
Lee G. H. Kim C. Y. Zheng C. Jin S. W. Kim J. Y. Lee S. Y. et al (2021). Rutaecarpine increases nitric oxide synthesis via eNOS phosphorylation by TRPV1-dependent CaMKII and CaMKKβ/AMPK signaling pathway in human endothelial cells. Int. J. Mol. Sci.22, 9407. 10.3390/ijms22179407
40
Li M. Zhang Y. Wan Q. Li Y. Qu T. Yuan F. (2022a). Use of levosimendan combined with Shenfu injection to treat acute heart failure patients with hypotension: a prospective randomized controlled single-blind study. BMC Cardiovasc Disord.22, 130. 10.1186/s12872-022-02572-2
41
Li S. Yu L. Shi Q. Liu Y. Zhang Y. Wang S. et al (2022b). An insight into current advances on pharmacology, pharmacokinetics, toxicity and detoxification of aconitine. Biomed. Pharmacother.151, 113115. 10.1016/j.biopha.2022.113115
42
Li W. Yang J. Lyu Q. Wu G. Lin S. Yang Q. et al (2020). Taurine attenuates isoproterenol-induced H9c2 cardiomyocytes hypertrophy by improving antioxidative ability and inhibiting calpain-1-mediated apoptosis. Mol. Cell. Biochem.469, 119–132. 10.1007/s11010-020-03733-7
43
Li X. Huang F. Zhu L. Luo T. Zhang Y. Gu H. et al (2022c). Effects of combination therapy with Shenfu Injection in critically ill patients with septic shock receiving mechanical ventilation: a multicentric, real-world study. Front. Pharmacol.13, 1041326. 10.3389/fphar.2022.1041326
44
Li Z. Zhang R. Wang X. Hu X. Chen Y. Liu Q. (2015). Simultaneous determination of seven ginsenosides in rat plasma by high-performance liquid chromatography coupled to time-of-flight mass spectrometry: application to pharmacokinetics of Shenfu injection. Biomed. Chromatogr.29, 167–175. 10.1002/bmc.3272
45
Liang D. Xue Z. Xue J. Xie D. Xiong K. Zhou H. et al (2021). Sinoatrial node pacemaker cells share dominant biological properties with glutamatergic neurons. Protein Cell12, 545–556. 10.1007/s13238-020-00820-9
46
Liu C. Hou Y. Wang X. Zhao Z. Liu Z. Zhai J. et al (2015). Clinical assessment of Shenfu injection loading in the treatment of patients with exacerbation of chronic heart failure due to coronary heart disease: study protocol for a randomized controlled trial. Trials16, 222. 10.1186/s13063-015-0729-7
47
Liu L. Hu J. Mao Q. Liu C. He H. Hui X. et al (2022). Functional compounds of ginseng and ginseng-containing medicine for treating cardiovascular diseases. Front. Pharmacol.13, 1034870. 10.3389/fphar.2022.1034870
48
Liu M. Li Y. Tang Y. Zheng L. Peng C. (2020). Synergistic effect of Aconiti Lateralis Radix Praeparata water-soluble alkaloids and Ginseng Radix et Rhizoma total ginsenosides compatibility on acute heart failure rats. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci.1137, 121935. 10.1016/j.jchromb.2019.121935
49
Liu P. Yang S. Wang Z. Dai H. Wang C. (2021). Feasibility and mechanism analysis of shenfu injection in the treatment of idiopathic pulmonary fibrosis. Front. Pharmacol.12, 670146. 10.3389/fphar.2021.670146
50
Liu X. Liu R. Dai Z. Wu H. Lin M. Tian F. et al (2019a). Effect of Shenfu injection on lipopolysaccharide (LPS)-induced septic shock in rabbits. J. Ethnopharmacol.234, 36–43. 10.1016/j.jep.2019.01.008
51
Liu Y. Yuan X. (2021). Logic analysis of arrhythmia triggered by pacemaker special functions - an educational presentation. Braz. J. Cardiovasc. Surg.36, 412–415. 10.21470/1678-9741-2020-0630
52
Liu Z. Song L. Zhang P. Cao Z. Hao J. Tian Y. et al (2019b). Ginsenoside Rb1 exerts antiarrhythmic effects by inhibiting I(Na) and I(CaL) in rabbit ventricular myocytes. Sci. Rep.9, 20425. 10.1038/s41598-019-57010-9
53
Lu S. Luo Y. Zhou P. Yang K. Sun G. Sun X. (2019). Ginsenoside compound K protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury via inhibition of nuclear factor-κB, p38, and JNK MAPK pathways. J. Ginseng Res.43, 95–104. 10.1016/j.jgr.2017.09.004
54
Luo S. Gou L. Liu S. Cao X. (2021). Efficacy and safety of Shenfu injection in the treatment of sepsis: a protocol for systematic review and meta-analysis. Med. Baltim.100, e27196. 10.1097/md.0000000000027196
55
Makhmudova U. Schulze P. C. Lütjohann D. Weingärtner O. (2021). Phytosterols and cardiovascular disease. Curr. Atheroscler. Rep.23, 68. 10.1007/s11883-021-00964-x
56
Mao Z. J. Zhang Q. L. Shang J. Gao T. Yuan W. J. Qin L. P. (2018). Shenfu Injection attenuates rat myocardial hypertrophy by up-regulating miR-19a-3p expression. Sci. Rep.8, 4660. 10.1038/s41598-018-23137-4
57
Mariappan V. Srinivasan R. Pratheesh R. Jujjuvarapu M. R. Pillai A. B. (2023). Predictive biomarkers for the early detection and management of heart failure. Heart fail. Rev.10.1007/s10741-023-10347-w
58
Methatham T. Tomida S. Kimura N. Imai Y. Aizawa K. (2021). Inhibition of the canonical Wnt signaling pathway by a β-catenin/CBP inhibitor prevents heart failure by ameliorating cardiac hypertrophy and fibrosis. Sci. Rep.11, 14886. 10.1038/s41598-021-94169-6
59
Miyauchi T. Sakai S. (2019). Endothelin and the heart in health and diseases. Peptides111, 77–88. 10.1016/j.peptides.2018.10.002
60
Mokhtari-Zaer A. Marefati N. Atkin S. L. Butler A. E. Sahebkar A. (2018). The protective role of curcumin in myocardial ischemia-reperfusion injury. J. Cell Physiol.234, 214–222. 10.1002/jcp.26848
61
Monteiro J. P. Bennett M. Rodor J. Caudrillier A. Ulitsky I. Baker A. H. (2019). Endothelial function and dysfunction in the cardiovascular system: the long non-coding road. Cardiovasc. Res.115, 1692–1704. 10.1093/cvr/cvz154
62
Mount P. F. Kemp B. E. Power D. A. (2007). Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J. Mol. Cell Cardiol.42, 271–279. 10.1016/j.yjmcc.2006.05.023
63
Nguyen H. T. Noriega Polo C. Wiederkehr A. Wollheim C. B. Park K. S. (2023). CDN1163, an activator of sarco/endoplasmic reticulum Ca(2+) ATPase, up-regulates mitochondrial functions and protects against lipotoxicity in pancreatic β-cells. Br. J. Pharmacol.180, 2762–2776. 10.1111/bph.16160
64
Ni J. Shi Y. Li L. Chen J. Li L. Li M. et al (2017). Cardioprotection against heart failure by shenfu injection via TGF-β/smads signaling pathway. Evid. Based Complement. Altern. Med.2017, 7083016. 10.1155/2017/7083016
65
Niu L. Xiao L. Zhang X. Liu X. Liu X. Huang X. et al (2021). Comparative efficacy of Chinese herbal injections for treating severe pneumonia: a systematic review and bayesian network meta-analysis of randomized controlled trials. Front. Pharmacol.12, 743486. 10.3389/fphar.2021.743486
66
Núñez-Acevedo B. Domínguez-Ortega J. Rodríguez-Jiménez B. Kindelan-Recarte C. Pérez-Fernández M. A. (2018). Severe and rare adverse reaction to hydrochlorothiazide. Rev. Alerg. Mex.65, 442–445. 10.29262/ram.v65i4.363
67
Obradovic M. Sudar-Milovanovic E. Gluvic Z. Banjac K. Rizzo M. Isenovic E. R. (2023). The Na(+)/K(+)-ATPase: a potential therapeutic target in cardiometabolic diseases. Front. Endocrinol. (Lausanne).14, 1150171. 10.3389/fendo.2023.1150171
68
Omidkhoda N. Wallace Hayes A. Reiter R. J. Karimi G. (2019). The role of MicroRNAs on endoplasmic reticulum stress in myocardial ischemia and cardiac hypertrophy. Pharmacol. Res.150, 104516. 10.1016/j.phrs.2019.104516
69
Pall A. H. Rasmussen E. R. Wadelius M. (2021). Pharmacogenetics of angiotensin-converting enzyme inhibitor-induced angioedema. Pharmacogenomics22, 319–321. 10.2217/pgs-2021-0036
70
Panday S. Talreja R. Kavdia M. (2020). The role of glutathione and glutathione peroxidase in regulating cellular level of reactive oxygen and nitrogen species. Microvasc. Res.131, 104010. 10.1016/j.mvr.2020.104010
71
Parikh M. Netticadan T. Pierce G. N. (2018). Flaxseed: its bioactive components and their cardiovascular benefits. Am. J. Physiol. Heart Circ. Physiol.314, H146–h159. 10.1152/ajpheart.00400.2017
72
Pei H. Ma Y. Wang L. Wang L. Xu L. Wang R. (2021). Effects of Shenfu injection on inflammatory factors and immune function in children with Mycoplasma pneumoniae: a protocol for a double-blind, randomized controlled trial. Med. Baltim.100, e27585. 10.1097/md.0000000000027585
73
Peng M. L. Fu Y. Wu C. W. Zhang Y. Ren H. Zhou S. S. (2022). Signaling pathways related to oxidative stress in diabetic cardiomyopathy. Front. Endocrinol. (Lausanne).13, 907757. 10.3389/fendo.2022.907757
74
Sabe S. A. Feng J. Sellke F. W. Abid M. R. (2022). Mechanisms and clinical implications of endothelium-dependent vasomotor dysfunction in coronary microvasculature. Am. J. Physiol. Heart Circ. Physiol.322, H819–h841. 10.1152/ajpheart.00603.2021
75
Sahoo G. Samal D. Khandayataray P. Murthy M. K. (2023). A review on caspases: key regulators of biological activities and apoptosis. Mol. Neurobiol.60, 5805–5837. 10.1007/s12035-023-03433-5
76
Sattayaprasert P. Vasireddi S. K. Bektik E. Jeon O. Hajjiri M. Mackall J. A. et al (2020). Human cardiac mesenchymal stem cells remodel in disease and can regulate arrhythmia substrates. Circ. Arrhythm. Electrophysiol.13, e008740. 10.1161/circep.120.008740
77
Shen B. Q. Qu C. Mi L. Wang H. Y. Yang H. (2021). Simultaneous quantification of twenty-eight components of Shenfu Injection in rat plasma by UHPLC-QQQ MS and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal.203, 114211. 10.1016/j.jpba.2021.114211
78
Shi S. Wang F. Chen B. Pan J. Luo D. Pei C. et al (2022). Efficacy and safety of shenfu injection for severe pneumonia in the elderly: a systematic review and meta-analysis based on western and eastern medicine. Front. Pharmacol.13, 779942. 10.3389/fphar.2022.779942
79
Song Y. Zhang N. Shi S. Li J. Zhang Q. Zhao Y. et al (2015). Large-scale qualitative and quantitative characterization of components in Shenfu injection by integrating hydrophilic interaction chromatography, reversed phase liquid chromatography, and tandem mass spectrometry. J. Chromatogr. A1407, 106–118. 10.1016/j.chroma.2015.06.041
80
Stewart A. G. Thomas B. Koff J. (2018). TGF-β: master regulator of inflammation and fibrosis. Respirology23, 1096–1097. 10.1111/resp.13415
81
Suvorava T. Metry S. Pick S. Kojda G. (2022). Alterations in endothelial nitric oxide synthase activity and their relevance to blood pressure. Biochem. Pharmacol.205, 115256. 10.1016/j.bcp.2022.115256
82
Tan H. Y. Li P. Z. Gong J. P. Yang K. (2019). Shenfu injection attenuates bile duct injury in rats with acute obstructive cholangitis. Surg. Infect. (Larchmt).20, 424–430. 10.1089/sur.2018.304
83
Tan M. Yin Y. Ma X. Zhang J. Pan W. Tan M. et al (2023). Glutathione system enhancement for cardiac protection: pharmacological options against oxidative stress and ferroptosis. Cell Death Dis.14, 131. 10.1038/s41419-023-05645-y
84
Tang Q. Wang Y. Li K. (2018). Zhenwu decoction for chronic heart failure: protocol for a systematic review and meta-analysis. Med. Baltim.97, e11559. 10.1097/md.0000000000011559
85
Tong H. Zhu J. Gong F. Zhong L. Xu T. (2021). Relationship between cardiotonic activity of Fuzi (Radix Aconiti Lateralis Preparata) and its fingerprint determined by liquid chromatography-mass spectrometry. J. Tradit. Chin. Med.41, 140–149. 10.19852/j.cnki.jtcm.2021.01.016
86
Valiente-Alandi I. Potter S. J. Salvador A. M. Schafer A. E. Schips T. Carrillo-Salinas F. et al (2018). Inhibiting fibronectin attenuates fibrosis and improves cardiac function in a model of heart failure. Circulation138, 1236–1252. 10.1161/circulationaha.118.034609
87
Vazquez A. R. Sudhir A. (2023). Cardiac arrest as a public health issue. Emerg. Med. Clin. North Am.41, 405–411. 10.1016/j.emc.2023.05.003
88
Wang L. Wang H. L. Liu T. T. Lan H. Y. (2021a). TGF-beta as a master regulator of diabetic nephropathy. Int. J. Mol. Sci.22, 7881. 10.3390/ijms22157881
89
Wang M. Hu W. J. Zhou X. Yu K. Wang Y. Yang B. Y. et al (2023). Ethnopharmacological use, pharmacology, toxicology, phytochemistry, and progress in Chinese crude drug processing of the lateral root of Aconitum carmichaelii Debeaux. (Fuzi): a review. J. Ethnopharmacol.301, 115838. 10.1016/j.jep.2022.115838
90
Wang S. Liu G. Chen L. Xu X. Jia T. Zhu C. et al (2022). EFFECTS OF SHENFU INJECTION ON SUBLINGUAL MICROCIRCULATION IN SEPTIC SHOCK PATIENTS: A RANDOMIZED CONTROLLED TRIAL. Shock58, 196–203. 10.1097/shk.0000000000001975
91
Wang S. Qiu X. J. (2019). The efficacy of Xue Fu Zhu Yu prescription for hyperlipidemia: a meta-analysis of randomized controlled trials. Complement. Ther. Med.43, 218–226. 10.1016/j.ctim.2019.02.008
92
Wang X. Hou Y. Mao J. Zhang Y. Li Z. Zhao Y. et al (2017a). Western medication plus Traditional Chinese Medicine preparations in patients with chronic heart failure: a prospective, single-blind, randomized, controlled, and multicenter clinical trial. J. Tradit. Chin. Med.37, 756–766. 10.1016/s0254-6272(18)30038-4
93
Wang X. Miao H. Yan Y. Guo R. Gong W. He Y. et al (2021b). Effect of shenfu injection on reperfusion injury in patients undergoing primary percutaneous coronary intervention for st segment elevation myocardial infarction: a pilot randomized clinical trial. Front. Cardiovasc. Med.8, 736526. 10.3389/fcvm.2021.736526
94
Wang X. Zhao Z. Mao J. Du T. Chen Y. Xu H. et al (2019b). Randomized, double-blinded, multicenter, placebo-controlled trial of shenfu injection for treatment of patients with chronic heart failure during the acute phase of symptom aggravation (Yang and Qi deficiency syndrome). Evid. Based Complement. Altern. Med.2019, 9297163. 10.1155/2019/9297163
95
Wang Y. L. Wang C. Y. Zhang B. J. Zhang Z. Z. (2009). Shenfu injection suppresses apoptosis by regulation of Bcl-2 and caspase-3 during hypoxia/reoxygenation in neonatal rat cardiomyocytes in vitro. Mol. Biol. Rep.36, 365–370. 10.1007/s11033-007-9188-x
96
Wang Y. Y. Li Y. Y. Li L. Yang D. L. Zhou K. Li Y. H. (2018). Protective effects of shenfu injection against myocardial ischemia-reperfusion injury via activation of eNOS in rats. Biol. Pharm. Bull.41, 1406–1413. 10.1248/bpb.b18-00212
97
Wang Z. F. Yu J. Y. Xie Y. M. (2017b). Clinical safety imtensive hospital monitoring on Shenfu injection with 30 106 cases. Zhongguo Zhong Yao Za Zhi42, 2871–2876. 10.19540/j.cnki.cjcmm.20170705.008
98
Wen L. Xie L. Gong F. Zhang S. Xi T. (2023). Efficacy and safety of Chinese medicine injections in combination with docetaxel and cisplatin for non-small cell lung cancer: a network meta-analysis. Front. Pharmacol.14, 1277284. 10.3389/fphar.2023.1277284
99
Wu H. Dai Z. Liu X. Lin M. Gao Z. Tian F. et al (2019). Pharmacodynamic evaluation of shenfu injection in rats with ischemic heart failure and its effect on small molecules using matrix-assisted laser desorption/ionization-mass spectrometry imaging. Front. Pharmacol.10, 1424. 10.3389/fphar.2019.01424
100
Wu J. Yu W. Zhu J. Liu Y. Shen Y. Sun M. et al (2022). Effect of Shenfu injection on the level of acetylcholine in acute liver injury of young rats with sepsis. Minerva Gastroenterol. (Torino)68, 367–369. 10.23736/s2724-5985.21.03064-3
101
Wu Q. Zhang Q. Li Y. Yu L. Zhang Y. Ao M. (2021). A systematic review and meta-analysis of high-frequency prescription of zhigancao decoction combined with conventional western medicine in the treatment of chronic heart failure. Evid. Based Complement. Altern. Med.2021, 7140044. 10.1155/2021/7140044
102
Wu Y. Xia Z. Y. Meng Q. T. Zhu J. Lei S. Xu J. et al (2011). Shen-Fu injection preconditioning inhibits myocardial ischemia-reperfusion injury in diabetic rats: activation of eNOS via the PI3K/Akt pathway. J. Biomed. Biotechnol.2011, 384627. 10.1155/2011/384627
103
Xiong X. J. (2019). Exploration on connotation of Zhigancao Decoction formula syndrome from the perspective of modern pathophysiology and severe cases of critical care and its clinical efficacy on cardioversion,maintenance of sinus rhythm,hemostasis,increasing platelets count,and tonifying deficiency. Zhongguo Zhong Yao Za Zhi44, 3842–3860. 10.19540/j.cnki.cjcmm.20190416.501
104
Xu C. Xia Y. Jia Z. Wang S. Zhao T. Wu L. (2022). The curative effect of Shenfu-injection in the treatment of burn sepsis and its effect on the patient's immune function, HMGB, and vWF. Am. J. Transl. Res.14, 2428–2435.
105
Yan P. Mao W. Jin L. Fang M. Liu X. Lang J. et al (2020). Crude radix Aconiti lateralis preparata (fuzi) with Glycyrrhiza reduces inflammation and ventricular remodeling in mice through the TLR4/NF-κB pathway. Mediat. Inflamm.2020, 5270508. 10.1155/2020/5270508
106
Yang F. Yang M. Y. Le J. Q. Luo B. Y. Yin M. D. Chao L. et al (2022a). Protective effects and therapeutics of ginsenosides for improving endothelial dysfunction: from therapeutic potentials, pharmaceutical developments to clinical trials. Am. J. Chin. Med.50, 749–772. 10.1142/s0192415x22500318
107
Yang H. Liu L. Gao W. Liu K. Qi L. W. Li P. (2014). Direct and comprehensive analysis of ginsenosides and diterpene alkaloids in Shenfu injection by combinatory liquid chromatography-mass spectrometric techniques. J. Pharm. Biomed. Anal.92, 13–21. 10.1016/j.jpba.2013.12.041
108
Yang L. Huang G. Y. Wang Y. G. Han B. Q. Zheng B. Zhu J. M. et al (2021). Efficacy of renshen (radix ginseng) plus fuzi (radix Aconiti lateralis preparata) on myocardial infarction by enhancing autophagy in rat. J. Tradit. Chin. Med.41, 909–918. 10.19852/j.cnki.jtcm.2021.06.009
109
Yang Y. Ge F. L. Huang Q. Zeng R. Zhang X. Y. Liu P. et al (2022b). Randomized controlled trials of zhigancao decoction combined with metoprolol in the treatment of arrhythmia: a systematic review and meta-analysis. Front. Cardiovasc. Med.9, 795903. 10.3389/fcvm.2022.795903
110
Yang Y. Su C. Zhang X. Z. Li J. Huang S. C. Kuang H. F. et al (2023). Mechanisms of Xuefu Zhuyu Decoction in the treatment of coronary heart disease based on integrated metabolomics and network pharmacology approach. J. Chromatogr. B Anal. Technol. Biomed. Life Sci.1223, 123712. 10.1016/j.jchromb.2023.123712
111
Ye B. Zhou H. Chen Y. Luo W. Lin W. Zhao Y. et al (2023). USP25 ameliorates pathological cardiac hypertrophy by stabilizing SERCA2a in cardiomyocytes. Circ. Res.132, 465–480. 10.1161/circresaha.122.321849
112
Zhang M. Q. Li C. S. (2023). Therapeutic effects of shenfu injection in shock. Chin. J. Integr. Med.29, 1142–1146. 10.1007/s11655-023-3631-2
113
Zhang N. Zhao Y. Liu Y. Tang N. Zheng W. Mao M. et al (2021a). A double-blinded, placebo-controlled randomized trial evaluating the efficacy and safety of Zhigancao Tang granules for treating HFpEF: study protocol for a randomized controlled trial. Trials22, 293. 10.1186/s13063-021-05232-6
114
Zhang Q. Li C. Shao F. Zhao L. Wang M. Fang Y. (2017). Efficacy and safety of combination therapy of shenfu injection and postresuscitation bundle in patients with return of spontaneous circulation after in-hospital cardiac arrest: a randomized, assessor-blinded, controlled trial. Crit. Care. Med.45, 1587–1595. 10.1097/ccm.0000000000002570
115
Zhang Q. Lyu W. Yu M. Niu Y. (2020a). Sulfur dioxide induces vascular relaxation through PI3K/Akt/eNOS and NO/cGMP signaling pathways in rats. Hum. Exp. Toxicol.39, 1108–1117. 10.1177/0960327120911428
116
Zhang S. Chen Z. L. Tang Y. P. Duan J. L. Yao K. W. (2021b). Efficacy and safety of xue-fu-zhu-yu decoction for patients with coronary heart disease: a systematic review and meta-analysis. Evid. Based Complement. Altern. Med.2021, 9931826. 10.1155/2021/9931826
117
Zhang X. Guo T. Zhang K. Guo W. An X. Gao P. (2020b). Effect of shenfu injection on microcirculation in shock patients: a protocol for systematic review and meta-analysis. Med. Baltim.99, e22872. 10.1097/md.0000000000022872
118
Zhang Y. Tian D. Huang Y. Li L. Mao J. Tian J. et al (2016). Pharmacokinetic evaluation of Shenfu Injection in beagle dogs after intravenous drip administration. Acta. Pharm. Sin. B6, 584–592. 10.1016/j.apsb.2016.05.006
119
Zhao D. Zhong G. Li J. Pan J. Zhao Y. Song H. et al (2021a). Targeting E3 ubiquitin ligase WWP1 prevents cardiac hypertrophy through destabilizing DVL2 via inhibition of K27-linked ubiquitination. Circulation144, 694–711. 10.1161/circulationaha.121.054827
120
Zhao X. Chu Q. Wu W. Wu H. Wang S. Qing L. et al (2021b). Shenfu injection: a famous Chinese prescription that promotes HCN4 activity in bone marrow mesenchymal stem cells. Evid. Based Complement. Altern. Med.2021, 9912844. 10.1155/2021/9912844
121
Zheng S. Y. Sun J. Zhao X. Xu J. G. (2004). Protective effect of shen-fu on myocardial ischemia-reperfusion injury in rats. Am. J. Chin. Med.32, 209–220. 10.1142/s0192415x04001874
122
Zheng Y. Zheng M. Shao J. Jiang C. Shen J. Tao R. et al (2022). Upregulation of claudin-4 by Chinese traditional medicine Shenfu attenuates lung tissue damage by acute lung injury aggravated by acute gastrointestinal injury. Pharm. Biol.60, 1981–1993. 10.1080/13880209.2022.2128824
123
Zhou D. Xie L. Wang Y. Wu S. Liu F. Zhang S. et al (2020). Clinical efficacy of tonic traditional Chinese medicine injection on acute cerebral infarction: a bayesian network meta-analysis. Evid. Based Complement. Altern. Med.2020, 8318792. 10.1155/2020/8318792
124
Zhou W. Chen Z. Fang Z. Xu D. (2022). Network analysis for elucidating the mechanisms of Shenfu injection in preventing and treating COVID-19 combined with heart failure. Comput. Biol. Med.148, 105845. 10.1016/j.compbiomed.2022.105845
125
Zhu J. Song W. Xu S. Ma Y. Wei B. Wang H. et al (2020). Shenfu injection promotes vasodilation by enhancing eNOS activity through the PI3K/Akt signaling pathway in vitro. Front. Pharmacol.11, 121. 10.3389/fphar.2020.00121
Summary
Keywords
Shenfu injection, cardiovascular disease, heart failure, pharmacology, TCM (traditional Chinese medicine), clinical trial
Citation
Xu F-F, Xie X-F, Hu H-Y, Tong R-S and Peng C (2024) Shenfu injection: a review of pharmacological effects on cardiovascular diseases. Front. Pharmacol. 15:1279584. doi: 10.3389/fphar.2024.1279584
Received
18 August 2023
Accepted
31 January 2024
Published
14 February 2024
Volume
15 - 2024
Edited by
Yusof Kamisah, Faculty of Medicine Universiti Kebangaan Malaysia, Malaysia
Reviewed by
Songqi Tang, Hainan Medical University, China
Ahmad Khusairi Azemi, Universiti Malaysia Terengganu, Malaysia
Updates
Copyright
© 2024 Xu, Xie, Hu, Tong and Peng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong-Sheng Tong, tongrs@126.com; Cheng Peng, pengchengchengdu@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.