- 1Center for Evidence-Based Medicine and Clinical Research, Hubei Provincial Clinical Research Center of Central Nervous System Repair and Functional Reconstruction, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, China
- 2Department of Neurosurgery, Hubei Provincial Clinical Research Center of Central Nervous System Repair and Functional Reconstruction, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, China
Objective: To evaluated the effectiveness and safety of single anti-seizure medication (ASM) when used as adjunctive therapy for drug-resistant focal epilepsy.
Methods: We conducted a comprehensive search of PubMed, EMbase, and the Cochrane Library from their inception until 12 February, 2025, to identify randomized controlled trials (RCTs) meeting our criteria. The trials were analyzed for their use of ASMs in treating drug-resistant focal epilepsy. Inclusion criteria comprised: 1) Participants aged 12 years or older with drug-resistant focal epilepsy; 2) Incorporation of an additional single ASM as an adjunct to the existing antiepileptic treatment regimen; 3) Comparison with placebo or continuation of the original antiepileptic regimen without a new ASM; 4) Primary outcome as a 50% response rate, with safety as a secondary outcome, encompassing dizziness, somnolence, headache, ataxia, diplopia, fatigue, and nausea; and 5) Study design limited to RCTs. The surface under the cumulative ranking curve (SUCRA) was employed to rank the effectiveness and safety of the ASMs.
Results: A total of 53 RCTs involving 17 ASMs as adjunctive therapy and placebo were analyzed. Compared to placebo, the following ASMs demonstrated statistically significant effectiveness in achieving a 50% response rate: brivaracetam (RR = 2.07, 95% CI: 1.53–2.81), cenobamate (RR = 2.12, 95% CI: 1.56–2.88), eslicarbazepine acetate (RR = 1.95, 95% CI: 1.41–2.70), gabapentin (RR = 2.30, 95% CI: 1.76–3.02), lacosamide (RR = 2.22, 95% CI: 1.47–3.35), lamotrigine (RR = 1.55, 95% CI: 1.00–2.40), levetiracetam (RR = 2.43, 95% CI: 1.88–3.15), oxcarbazepine (RR = 3.03, 95% CI: 2.08–4.40), perampanel (RR = 1.72, 95% CI: 1.21–2.44), pregabalin (RR = 2.06, 95% CI: 1.70–2.50), rufinamide (RR = 2.28, 95% CI: 1.20–4.31), tiagabine (RR = 4.07, 95% CI: 2.03–8.18), topiramate (RR = 3.10, 95% CI: 2.44–3.95), vigabatrin (RR = 2.34, 95% CI: 1.58–3.46), and zonisamide (RR = 2.40, 95% CI: 1.76–3.27). Based on SUCRA rankings, tiagabine (92.7%) exhibited the most favorable therapeutic outcome, followed by topiramate (87.3%), oxcarbazepine (83%), and levetiracetam (62.8%). The ASMs with the least favorable therapeutic effects were placebo (1.1%), lamotrigine (17.8%), and perampanel (24.7%).
Conclusion: The network meta-analysis revealed topiramate, tiagabine, oxcarbazepine, and levetiracetam as the four most effective adjuvant ASM treatments for drug-resistant focal epilepsy. However, it is noteworthy that topiramate and oxcarbazepine were associated with a higher incidence of somnolence. Additionally, comprehensive safety data for tiagabine and levetiracetam are lacking, necessitating further research. Larger studies are required to solidify these findings and better understand the safety profiles of all involved ASMs.
Introduction
Epilepsy was stands as one of the most prevalent brain disorders worldwide, impacting over 70 million individuals across all age groups, from infants and young children to the elderly, to varying degrees. The most frequent form of epilepsy in humans was focal epilepsy, which comprises more than half of all cases and poses the greatest therapeutic challenge when treated with anti-epileptic medications (Gooley et al., 2022; Engel, 2004). Focal seizures typically originated in a confined area of the cerebral cortex and subsequently propagate to adjacent regions, encompassing both the surrounding cortical tissue and subcutaneous structures (Jenssen et al., 2011). The most typical pathological conditions associated with focal epilepsy include traumatic brain injuries, tumors, and vascular malformations (Bernasconi and Bernasconi, 2022). Meanwhile, drug-resistant epilepsy referred to cases where seizures persist despite adjustments to anti-seizure medication (ASM) therapy, rendering seizure freedom highly improbable with further pharmacological interventions.
Over the past few decades, remarkable progress had been achieved in the treatment of epilepsy, with approximately 30 ASMs now clinically available. These ASMs had played a pivotal role in decreasing the frequency and severity of seizures, ultimately enhancing the quality of life for epilepsy patients (Löscher and Klein, 2021). A study revealed that topiramate, levetiracetam, pregabalin, and oxcarbazepine offered advantages over other ASMs in terms of adverse reactions and treatment risks. Conversely, rufinamide demonstrated suboptimal treatment effectiveness and a high risk of severe, urgent headaches (Zhao et al., 2017). Another meta-analysis (Hu et al., 2018) found that brivaracetam, levetiracetam, oxcarbazepine, vigabatrin, and topiramate exhibited reliable effectiveness, with levetiracetam being the most well-tolerated. Additionally, the study suggested that levetiracetam, vigabatrin, and gabapentin offered the best balance of short-term effectiveness and tolerability, while oxcarbazepine was effective but poorly tolerated (Bodalia et al., 2013). Despite consistent findings highlighted levetiracetam’s effectiveness, the efficacy of other ASMs as adjunctive therapy remained controversial due to factors such as limited sample sizes, unclear outcome definitions, and variations in patient populations. To provided clinicians with more authoritative and efficient guidelines, an updated and comprehensive network meta-analysis was conducted to evaluate the effectiveness and safety of adding a new single ASM to an existing anti-epileptic regimen for drug-resistant focal epilepsy among the various available options.
Methods
This study was conducted in accordance with the extended Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines specifically tailored for network meta-analyses of healthcare interventions (Hutton et al., 2015).
Search strategy
As of 12 February, 2025, we involved a network meta-analysis by searching to identify related RCTs in the PubMed, EMbase and Cochrane Library. The MeSH and keywords used in the search were “drug-resistant,” “medication-resistant,” “intractable,” “refractory,” “uncontrolled,” “drug refractory,” “pharmacoresistant,” “complex,” “partial,” “partial-onset,” “focal,” “epilepsy,” “seizure,” and “randomized controlled trial.” The literature search strategies were showed in Supplementary Method S1.
Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) Population: Participants with drug-resistant focal epilepsy (age ≥12 years). 2) Intervention: Incorporating an additional single ASM as an adjunct to the existing antiepileptic treatment regimen. 3) Comparison: Placebo or no new ASM as adjunctive therapy to an existing anti-epileptic regimen. 4) Outcomes: All studies included at least one effectiveness or safety outcomes. Effectiveness outcome was defined as 50% response rate, and was used as the primary outcome. Safety outcomes were used as the secondary outcomes, including dizziness, somnolence, headache, ataxia, diplopia, fatigue and nausea. 5) Study designs: Randomised controlled trials (RCTs).
The exclusion criteria included duplicate studies, no specific descriptions of ASMs as adjunctive therapy, studies with missing data, conference proceedings, and publications that are solely accessible in the abstract form.
Data collection and processing
Five authors (Nian-Jia Deng, Xin-Yi Li, Zhi-Xin Zhang, Chen-Yang Xian-Yu, Yu-Ting Tao), in consensus, independently filtrate the literature and strictly extracted data in accordance with the predetermined inclusion criteria. Any potential conflicts or differences of opinion among the authors were resolved through a process of deliberation and consultation involving a fourth author (Yu-Tong Ma). The fundamental information of each study was extracted, including the year, sex ratio of participants, mean age, median duration of epilepsy (years), main inclusion criteria, comparison measures, and sample size.
Quality assessment
Two reviewers independently assessed the risk of bias of the included studies (RoB-2) (Sterne et al., 2019). The RoB-2 evaluated studies in five domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in outcome measurements, and bias in the selection of the reported results. There were “yes,” “probably yes,” “probably no,” “no,” and “no information” to answer the signal questions in the above domains. Notably, the consequences for bias risk were the same for “yes” and “probably yes” replies as they were for “no” and “probably no”. Additionally, the “probably” versions would typically imply that a judgment had been made. Following the completion of the signaling questions, a risk-of-bias assessment was made, and each domain was given one of three levels: low risk of bias, some concerns or high risk of bias.
Statistical analysis
All dichotomous outcomes were employed for relative risk (RR) with 95% confidence intervals (CI), with a significant level of P < 0.05. I2 was used to detect the magnitude of heterogeneity. Additionally, the I2 statistic was used, where I2 values of ≥40% were indicative of significant heterogeneity (Higgins and James, 2011), the random effects model was employed. Otherwise, the fixed effects model was used. Network meta-analyses offer trustworthy proof for both direct and indirect comparisons of many interventions (Lu and Ades, 2004). The “loop inconsistency” method was employed for test of consistency equations when the treatment effects around a loop (Song et al., 2011). By definition, the surface under the cumulative ranking curve (SUCRA) values reflect the effectiveness and safety of ASMs as adjunctive therapy; thus, a rank plot with larger SUCRA scores implies more effective or safe ASMs as adjunctive therapy (Rücker and Schwarzer, 2015). Furthermore, a network funnel plot was used to detect any potential publication bias. All statistical analyses were conducted using STATA 15.0 and R 4.2.2, and it obtained a copyright license.
Results
Search results
In total, 5,303 relevant studies were retrieved, of which 1,759 were removed as duplicates. For participants who met the diagnostic standard for drug-resistant focal epilepsy, quantitative data was obtained for the network meta-analysis by scrutinizing the relevant literature titles, abstracts and full-text evaluations. Finally, a total of 53 studies comprising 13,700 participants with 17 ASMs as adjunctive therapy and placebo were involved in this study (Figure 1).
Basic characteristics and quality assessment
Table 1 showed the primary attributes characteristics of the included studies, incorporating the quantity of study (n = 53), study year, sex ratio of participants, mean age, median duration of epilepsy (years), main inclusion criteria, comparison measures, and sample size. Active ASMs as adjunctive therapy, including brivaracetam, cenobamate, eslicarbazepine acetate, gabapentin, lacosamide, lamotrigine, levetiracetam, natalizumab, oxcarbazepine, perampanel, pregabalin, remacemid, rufinamide, tiagabine, topiramate, vigabatrin and zonisamide were incorporated in the network meta-analysis. An assessment of the risk of bias from randomized trials was conducted utilizing the latest RoB-2 assessment tool (Supplementary Table S1).
Effective outcome
50% Response rate
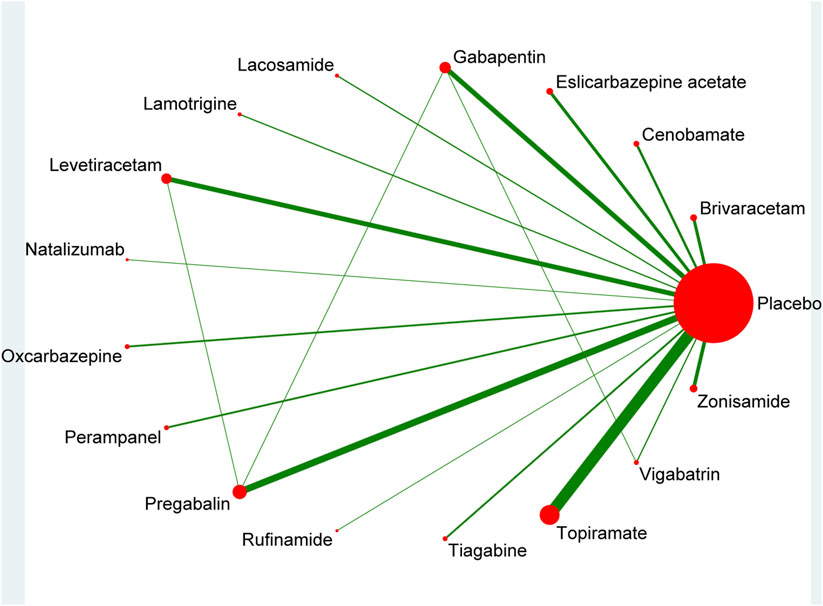
The pool of 46 RCTs (Gabapentin in Partial Epilepsy, 1990; Anhut et al., 1994; The US Gabapentin Study Group No. 5, 1993; Schmidt et al., 1993; French et al., 1996; Privitera et al., 1996; Faught et al., 1996; Tassinari et al., 1996; Sharief et al., 1996; Faught, 1997; Ben-Menachem, 1997; Uthman et al., 1998; Bruni et al., 2000; Cereghino et al., 2000; Yen et al., 2000; Shorvon et al., 2000; Lindberger et al., 2000; Barcs et al., 2000; French et al., 2003; Arroyo et al., 2004; Sackellares et al., 2004; Brodie, 2004; Brodie et al., 2005; Beydoun et al., 2005; Tsai et al., 2006; Yamauchi et al., 2006; Naritoku et al., 2007; Zhou et al., 2008; Wu et al., 2009; Xiao et al., 2009; Lee et al., 2009; Gil-Nagel et al., 2009; Ben-Menachem et al., 2010; French et al., 2010; Biton et al., 2011; Zaccara et al., 2014; French et al., 2014; Hogan et al., 2014; Klein et al., 2015; French et al., 2016; Hong et al., 2016; Nishida et al., 2018; Krauss et al., 2020; Chung et al., 2020; French et al., 2021; Baulac et al., 2010), including 12,120 study participants, contributed to the analysis of the 50% response rate. Figure 2 illustrated a network plot of 50% response rate assessment of 16 eligible ASMs as adjunctive therapy and placebo for the treatment of drug-resistant focal epilepsy.
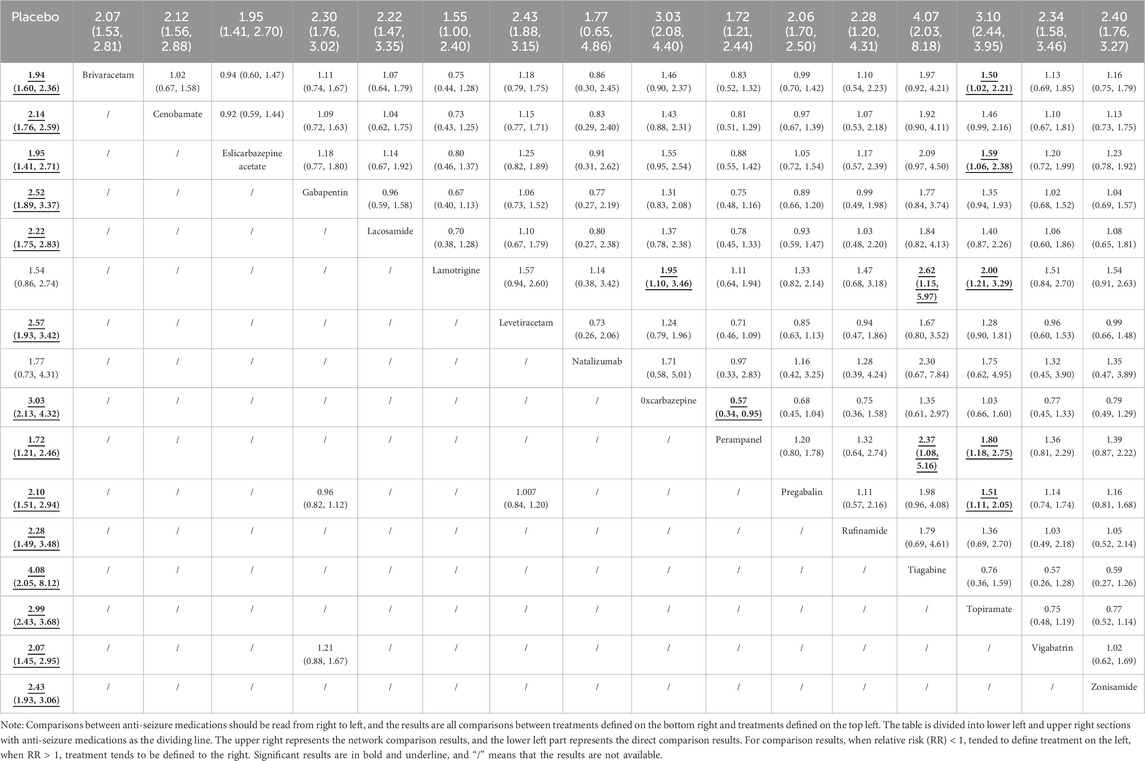
As shown in Table 2, the consequence of direct comparisons showed that the following ASMs as adjunctive therapy, including brivaracetam, cenobamate, eslicarbazepine acetate, gabapentin, lacosamide, levetiracetam, oxcarbazepine, perampanel, pregabalin, rufinamide, tiagabine, topiramate, vigabatrin and zonisamide, demonstrated statistically significant in 50% response rate than that of placebo. Nevertheless, the other results were no statistically significant differences.
Compared with placebo in the network meta-analysis, ASMs as adjunctive therapy, including brivaracetam, cenobamate, eslicarbazepine acetate, gabapentin, lacosamide, lamotrigine, levetiracetam, oxcarbazepine, perampanel, pregabalin, rufinamide, tiagabine, topiramate, vigabatrin, and zonisamide, demonstrated statistically significant in 50% response rate, as detailed in Table 2. The results of other ASMs as adjunctive therapy were shown in Table 2.
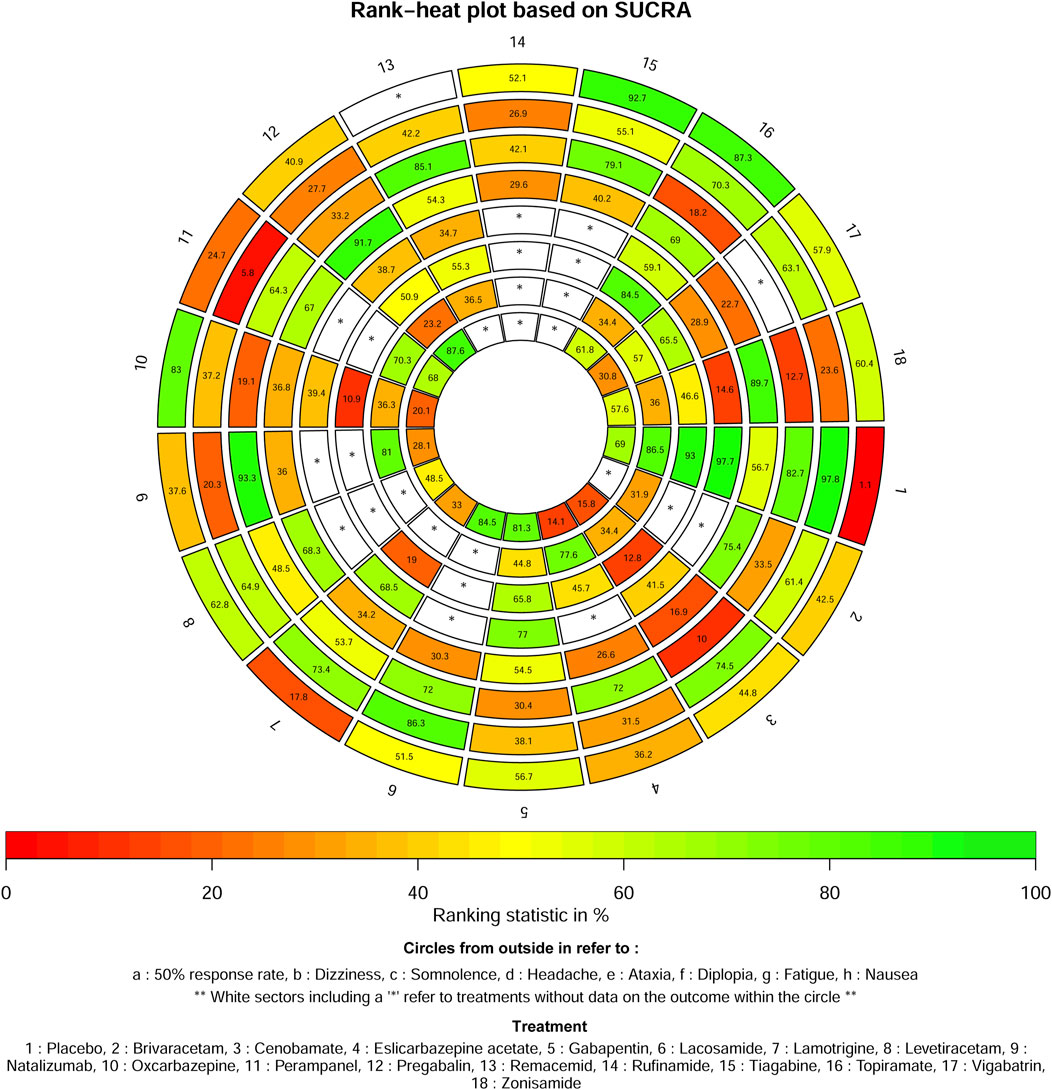
The ASMs as adjunctive therapy were assessed and graded based on the SUCRA, with tiagabine (92.7%) demonstrating the most optimal therapeutic outcome, subsequent to topiramate (87.3%), oxcarbazepine (83%) and levetiracetam (62.8%). The three ASMs as adjunctive therapy with the worst therapeutic effects were placebo (1.1%), lamotrigine (17.8%) and perampanel (24.7%) in Figure 3.
Safety outcomes
Dizziness
A total of 45 studies (Gabapentin in Partial Epilepsy, 1990; Anhut et al., 1994; The US Gabapentin Study Group No. 5, 1993; Schmidt et al., 1993; Privitera et al., 1996; Faught et al., 1996; Tassinari et al., 1996; Uthman et al., 1998; Bruni et al., 2000; Cereghino et al., 2000; Yen et al., 2000; Shorvon et al., 2000; Barcs et al., 2000; French et al., 2003; Arroyo et al., 2004; Brodie, 2004; Brodie et al., 2005; Beydoun et al., 2005; Tsai et al., 2006; Yamauchi et al., 2006; Naritoku et al., 2007; Wu et al., 2009; Xiao et al., 2009; Lee et al., 2009; Gil-Nagel et al., 2009; Ben-Menachem et al., 2010; French et al., 2010; Biton et al., 2011; Zaccara et al., 2014; French et al., 2014; Klein et al., 2015; French et al., 2016; Hong et al., 2016; Nishida et al., 2018; Krauss et al., 2020; Chung et al., 2020; French et al., 2021; Baulac et al., 2010; Matsuo et al., 1993; Ben-Menachem et al., 1996; Kälviäinen et al., 1998; Chadwick et al., 2000; Guberman et al., 2002; Peltola et al., 2009; Inoue et al., 2021) comprising 12,608 participants contributed to the analysis of the safety outcome of dizziness. Supplementary Figure S1 illustrated a network plot of the safety outcomes dizziness assessment of 17 eligible ASMs as adjunctive therapy and placebo for the treatment of drug-resistant focal epilepsy.
As shown in Supplementary Table S2, the consequence of direct comparisons showed that, compared with placebo, the following ASMs as adjunctive therapy demonstrated statistically significant in dizziness: cenobamate, eslicarbazepine acetate, gabapentin, levetiracetam, oxcarbazepine, perampanel, pregabalin, remacemid, rufinamide, tiagabine, topiramate and zonisamide. Nevertheless, the other results were no statistically significant differences.
The findings of the network meta-analysis indicated that, compared with placebo, ASMs as adjunctive therapy, including brivaracetam, cenobamate, eslicarbazepine acetate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, perampanel, pregabalin, remacemid, rufinamide, tiagabine, topiramate and zonisamide, demonstrated statistically significant in dizziness (Supplementary Table S2). The results of other ASMs as adjunctive therapy were shown in Supplementary Table S2.
According to the SUCRA, all ASMs as adjunctive therapy assessed for the safety outcome of dizziness were rated, with placebo (97.8%) exhibiting the best therapeutic benefit, subsequently followed by lacosamide (86.3%), cenobamate (74.5%) and lamotrigine (73.4%). The three ASMs as adjunctive therapy with the worst therapeutic effects were perampanel (5.8%), natalizumab (20.3%) and zonisamide (23.6%) (Figure 3).
Somnolence
A total of 42 studies (Gabapentin in Partial Epilepsy, 1990; Anhut et al., 1994; The US Gabapentin Study Group No. 5, 1993; Schmidt et al., 1993; Privitera et al., 1996; Faught et al., 1996; Tassinari et al., 1996; Sharief et al., 1996; Cereghino et al., 2000; Shorvon et al., 2000; Barcs et al., 2000; French et al., 2003; Arroyo et al., 2004; Brodie, 2004; Brodie et al., 2005; Beydoun et al., 2005; Tsai et al., 2006; Yamauchi et al., 2006; Naritoku et al., 2007; Wu et al., 2009; Xiao et al., 2009; Lee et al., 2009; Gil-Nagel et al., 2009; Ben-Menachem et al., 2010; French et al., 2010; Biton et al., 2011; Zaccara et al., 2014; French et al., 2014; Klein et al., 2015; French et al., 2016; Hong et al., 2016; Nishida et al., 2018; Krauss et al., 2020; Chung et al., 2020; French et al., 2021; Baulac et al., 2010; Matsuo et al., 1993; Kälviäinen et al., 1998; Chadwick et al., 2000; Guberman et al., 2002; Peltola et al., 2009; Inoue et al., 2021) encompassing 12,163 participants contributed to the analysis of the safety outcome of somnolence. Supplementary Figure S2 illustrated a network plot of safety outcomes somnolence assessment of 16 eligible ASMs as adjunctive therapy and placebo for the treatment of drug-resistant focal epilepsy.
In the results of direct comparisons, compared with placebo, ASMs as adjunctive therapy including cenobamate, gabapentin, levetiracetam, oxcarbazepine, pregabalin, topiramate and zonisamide demonstrated statistically significant in somnolence (Supplementary Table S3). Nevertheless, the other results were no statistically significant differences.
The findings of the network meta-analysis indicated that, compared with placebo, ASMs as adjunctive therapy, including brivaracetam, cenobamate, gabapentin, levetiracetam, oxcarbazepine, pregabalin, topiramate and zonisamide, demonstrated statistically significant in somnolence (Supplementary Table S3). The results of other ASMs as adjunctive therapy were shown in Supplementary Table S3.
The ASMs as adjunctive therapy were ranked based on the SUCRA and the results indicate that natalizumab (93.3%) exhibited the most favourable therapeutic effect, subsequent to remacemide (85.1%), placebo (82.7%) and tiagabine (79.1%). The three ASMs as adjunctive therapy with the worst therapeutic effects were cenobamate (10%), zonisamide (12.7%) and topiramate (18.2%) in Figure 3.
Headache
A total of 38 studies (Anhut et al., 1994; The US Gabapentin Study Group No. 5, 1993; Privitera et al., 1996; Faught et al., 1996; Tassinari et al., 1996; Sharief et al., 1996; Bruni et al., 2000; Cereghino et al., 2000; Yen et al., 2000; Shorvon et al., 2000; Barcs et al., 2000; French et al., 2003; Arroyo et al., 2004; Brodie et al., 2005; Tsai et al., 2006; Yamauchi et al., 2006; Naritoku et al., 2007; Wu et al., 2009; Lee et al., 2009; Gil-Nagel et al., 2009; Ben-Menachem et al., 2010; French et al., 2010; Biton et al., 2011; Zaccara et al., 2014; Klein et al., 2015; French et al., 2016; Hong et al., 2016; Nishida et al., 2018; Krauss et al., 2020; Chung et al., 2020; French et al., 2021; Baulac et al., 2010; Matsuo et al., 1993; Ben-Menachem et al., 1996; Kälviäinen et al., 1998; Chadwick et al., 2000; Peltola et al., 2009; Inoue et al., 2021) encompassing 11,011 participants contributed to the analysis of the headache safety outcome. Supplementary Figure S3 illustrated a network plot of the safety outcomes headache assessment of 17 eligible ASMs as adjunctive therapy and placebo for the treatment of drug-resistant focal epilepsy.
In the results of direct comparisons, compared with placebo, ASMs as adjunctive therapy including pregabalin, demonstrated statistically significant in headache (Supplementary Table S4). Nevertheless, the other results were no statistically significant differences.
The findings of the network meta-analysis indicated that, compared with placebo, ASMs as adjunctive therapy, including pregabalin, demonstrated statistically significant in headache (Supplementary Table S4). The results of other ASMs as adjunctive therapy were shown in Supplementary Table S4.
The ASMs as adjunctive therapy were ranked based on the SUCRA, with pregabalin (91.7%) showing the best therapeutic effect, subsequent to zonisamide (89.7%), brivaracetam (75.4%) and topiramate (69%). The three ASMs as adjunctive therapy exhibiting the most unfavorable therapeutic effects were cenobamate (16.9%), vigabatrin (22.7%) and eslicarbazepine acetate (26.6%) in Figure 3.
Ataxia
12 studies (Anhut et al., 1994; The US Gabapentin Study Group No. 5, 1993; Faught et al., 1996; Bruni et al., 2000; Barcs et al., 2000; French et al., 2003; Brodie, 2004; Beydoun et al., 2005; Krauss et al., 2020; Baulac et al., 2010; Matsuo et al., 1993; Chadwick et al., 2000) encompassing 3,596 study participants contributed to the analysis of the safety outcome of ataxia. Supplementary Figure S4 illustrated a network plot of safety outcomes ataxia assessment of 9 eligible ASMs as adjunctive therapy and placebo for the treatment of drug-resistant focal epilepsy.
In the results of direct comparisons, compared with placebo, ASMs as adjunctive therapy including cenobamate, gabapentin, oxcarbazepine, pregabalin, topiramate, zonisamide, demonstrated statistically significant in ataxia (Supplementary Table S5). Nevertheless, the other results were no statistically significant differences.
The findings of the network meta-analysis indicated that, compared with placebo, ASMs as adjunctive therapy, including cenobamate, gabapentin, lamotrigine, oxcarbazepine, pregabalin, topiramate, zonisamide, demonstrated statistically significant in ataxia (Supplementary Table S5). The results of other ASMs as adjunctive therapy were shown in Supplementary Table S5.
The ASMs as adjunctive therapy were ranked based on the SUCRA, with the placebo (97.7%) demonstrating optimal therapeutic effectiveness, subsequent to gabapentin (77%) and lamotrigine (68.5%). The three ASMs as adjunctive therapy with the worst therapeutic effects were zonisamide (14.6%), vigabatrin (28.9%) and remacemide (34.7%) in Figure 3.
Diplopia
The safety outcome study of diplopia included 16 studies (Anhut et al., 1994; The US Gabapentin Study Group No. 5, 1993; Privitera et al., 1996; Faught et al., 1996; Bruni et al., 2000; Barcs et al., 2000; Arroyo et al., 2004; Beydoun et al., 2005; Yamauchi et al., 2006; Gil-Nagel et al., 2009; Ben-Menachem et al., 2010; Krauss et al., 2020; Baulac et al., 2010; Matsuo et al., 1993; Kälviäinen et al., 1998; Chadwick et al., 2000) with 4,487 participants. Supplementary Figure S5 illustrated a network plot of the safety outcomes diplopia assessment of 10 eligible ASMs as adjunctive therapy and placebo for the treatment of drug-resistant focal epilepsy.
In the results of direct comparisons, compared with placebo, ASMs as adjunctive therapy including oxcarbazepine cenobamate, eslicarbazepine acetate, gabapentin, lamotrigine, oxcarbazepine, pregabalin and topiramate, demonstrated statistically significant in diplopia (Supplementary Table S6). Nevertheless, the other results were no statistically significant differences.
The findings of the network meta-analysis indicated that, compared with placebo, ASMs as adjunctive therapy, including cenobamate, eslicarbazepine acetate, gabapentin, lamotrigine, oxcarbazepine, pregabalin and topiramate, demonstrated statistically significant in diplopia (Supplementary Table S6). The results of other ASMs as adjunctive therapy were shown in Supplementary Table S6.
The ASMs as adjunctive therapy were ranked based on the SUCRA, with the placebo (93%) demonstrating optimal therapeutic effectiveness, subsequent to topiramate (84.5%) and gabapentin (65.8%). The three ASMs as adjunctive therapy with the worst therapeutic effects were oxcarbazepine (10.9%), cenobamate (12.8%) and lamotrigine (19%) in Figure 3.
Fatigue
A total of 22 studies (Anhut et al., 1994; The US Gabapentin Study Group No. 5, 1993; Schmidt et al., 1993; Privitera et al., 1996; Faught et al., 1996; Tassinari et al., 1996; Sharief et al., 1996; Bruni et al., 2000; Barcs et al., 2000; Brodie, 2004; Lee et al., 2009; Ben-Menachem et al., 2010; French et al., 2010; French et al., 2014; Klein et al., 2015; Nishida et al., 2018; Krauss et al., 2020; Chung et al., 2020; French et al., 2021; Ben-Menachem et al., 1996; Chadwick et al., 2000; Guberman et al., 2002) comprising 5,800 participants contributed to the analysis of the safety outcome of fatigue. Supplementary Figure S6 illustrated a network plot of the safety outcomes fatigue assessment of 12 eligible ASMs as adjunctive therapy and placebo for the treatment of drug-resistant focal epilepsy.
In the results of direct comparisons, compared with placebo, ASMs as adjunctive therapy including brivaracetam, cenobamate, gabapentin, oxcarbazepine, topiramate and zonisamide, demonstrated statistically significant in fatigue (Supplementary Table S7). Nevertheless, the other results were no statistically significant differences.
The findings of the network meta-analysis indicated that, compared with placebo, ASMs as adjunctive therapy, including brivaracetam, cenobamate, gabapentin, oxcarbazepine, topiramate, and zonisamide, demonstrated statistically significant in fatigue (Supplementary Table S7). The results of other ASMs as adjunctive therapy were shown in Supplementary Table S7.
The ASMs as adjunctive therapy were ranked based on the SUCRA, with the placebo (86.5%) demonstrating optimal therapeutic effectiveness, subsequent to natalizumab (81%) and eslicarbazepine acetate (77.6%). The three ASMs as adjunctive therapy with the worst therapeutic effects were pregabalin (23.2%), brivaracetam (31.9%) and cenobamate (34.4%), in Figure 3.
Nausea
A total of 21 studies (Anhut et al., 1994; Schmidt et al., 1993; Tassinari et al., 1996; Yen et al., 2000; Shorvon et al., 2000; Barcs et al., 2000; Brodie et al., 2005; Yamauchi et al., 2006; Naritoku et al., 2007; Gil-Nagel et al., 2009; Ben-Menachem et al., 2010; Zaccara et al., 2014; French et al., 2014; Nishida et al., 2018; Krauss et al., 2020; Chung et al., 2020; French et al., 2021; Matsuo et al., 1993; Kälviäinen et al., 1998; Peltola et al., 2009; Inoue et al., 2021) encompassing 6,235 participants contributed to the safety outcome of nausea. Supplementary Figure S7 illustrated a network plot of the safety outcomes nausea assessment of 13 eligible ASMs as adjunctive therapy and placebo for the treatment of drug-resistant focal epilepsy.
In the results of direct comparisons, compared with placebo, ASMs as adjunctive therapy, including lamotrigine and oxcarbazepine demonstrated statistically significant in nausea (Supplementary Table S8). Nevertheless, the other results were no statistically significant differences.
The findings of the network meta-analysis indicated that, compared with placebo, ASMs as adjunctive therapy, cenobamate, eslicarbazepine acetate, lamotrigine and oxcarbazepine demonstrated statistically significant in nausea (Supplementary Table S8). In addition, except for a limited number of combination comparisons between active ASMs as adjunctive therapy and placebo, no statistically significant differences were found for the remaining comparisons between active ASMs as adjunctive therapy and placebo in Supplementary Table S8. The results of other ASMs as adjunctive therapy were shown in Supplementary Table S8.
The ASMs as adjunctive therapy were ranked based on the SUCRA, with pregabalin (87.6%) demonstrating optimal therapeutic effectiveness, subsequent to lacosamide (84.5%) and gabapentin (81.3%). The three ASMs as adjunctive therapy exhibiting the worst therapeutic outcomes were eslicarbazepine acetate (14.1%), cenobamate (15.8%) and oxcarbazepine (20.1%) in Figure 3.
Test of inconsistency
Since closed loops were not formed for the outcomes of ataxia, fatigue, and diplopia, it was not possible to assess the inconsistency of these loops. Additionally, closed-loop structures were identified for the outcomes of a 50% response rate and adverse events (including dizziness, somnolence, headache, and nausea), and rigorous loop-consistency evaluation revealed no detectable inconsistencies within these loops.
Publication bias
No publication bias were revealed in the network funnel plot of all outcomes (Supplementary Figures S8–S15).
Discussion
While ASMs as adjunctive therapy remained the primary approach for managing epilepsy, some drugs inevitably caused varying degrees of harm to patients. Therefore, physicians must meticulously select specific drugs for treating epilepsy (Iyer and Marson, 2014). The study conducted an evidence-based assessment of comparative effectiveness and safety of ASMs as adjunctive therapy in drug-resistant focal epilepsy. The pertinent findings were as follows: tiagabine, topiramate, zonisamide, levetiracetam, rufinamide, and oxcarbazepine were more effective in controlling seizure frequency (as assessed by seizure-free analysis), whereas lacosamide was less effective than all other ASMs when used as adjunctive therapy.
Tiagabine was mechanistically one of the most precise ASMs in clinical use, but its use was limited to adjunctive therapy for partial seizures with or without secondary generalization in adolescents and adults (Mengel, 1994). Studies had demonstrated that adding tiagabine can reduce the frequency of seizures in individuals with drug-resistant focal seizures (Bresnahan et al., 2019). Another study found that, in the study population, short-term treatment with tiagabine at low doses had no cognitive or electroencephalogram adverse effects compared to placebo. Furthermore, tiagabine therapy did not result in worsening of cognitive function when used at high doses during long-term follow-up (Kälviäinen et al., 1996). Similarly, this study confirmed the substantial superiority of tiagabine in terms of therapeutic effectiveness.
Notably, in this study, topiramate achieved a high ranking for this outcome in 50% response rate (SUCRA: 87.3%), suggesting it may be a favorable first-choice option for this particular outcome. Furthermore, despite the risk of adverse events, such as dizziness, headache, ataxia, and diplopia, topiramate, demonstrated the highest safety profile and the lowest incidence of these events. One study found that when used in the management of drug-resistant focal epilepsy, topiramate could reduce the intensity and frequency of seizures while promoting overall stability, making it an effective, safe, and well-tolerated option for controlling disease progression (Viteva and Zahariev, 2020).
In the current study, levetiracetam exhibited an effective of 50% response rate and a relatively low risk profile (Marson et al., 2021). One study indicated that patients treated with levetiracetam were more prone to experiencing nausea (Zhao et al., 2017). Although levetiracetam lacked approval from the Food and Drug Administration (FDA) as a standalone treatment, it had been frequently used as a first-line ASM in the United States for both focal and generalized tonic-clonic seizures, and as an initial monotherapy in Europe (Abou-Khalil, 2019). Levetiracetam had minimal drug interactions and can be considered as the drug of choice for elderly individuals and fertile women (Sen et al., 2024). However, the findings also revealed that severe psychiatric symptoms, such as anger, violence, and even suicidal thoughts, may occur with levetiracetam administration. In most cases, these mental symptoms can be alleviated or disappear after reducing the dose or discontinuing the drug, but some patients may experience severe mental conditions that negatively impact their quality of life (Tao et al., 2024).
Gabapentin had proven effective as an adjunctive treatment for individuals with drug-resistant focal epilepsy and was generally well-tolerated. However, its used during pregnancy may pose risks to fetal neurodevelopment and congenital malformations (Honybun et al., 2024; Christensen et al., 2024). Some studies (Nakajima-Ohyama et al., 2024) had suggested that gabapentin can improve delirium and serve as a safe alternative therapy, but dose adjustments may be necessary to prevent sleepiness. It is important to note that gabapentin was associated with a higher incidence of dizziness, fatigue, and somnolence compared to placebo (Panebianco et al., 2021), and clinicians and patients should be vigilant of these symptoms during its use.
Pregabalin had demonstrated significant effectiveness in reducing the frequency of seizures in adults with drug-resistant focal epilepsy, but it also carried adverse reactions such as ataxia, dizziness, nausea, and weight gain (Panebianco et al., 2022). When combined with zonisamide, pregabalin had achieved impressive and sustained seizure control in patients with drug-resistant focal epilepsy, with minimal complications and fully reversible effects (Taghdiri et al., 2015).
Oxcarbazepine was an oral medication utilized for the treatment of focal-onset epilepsy, serving both as a monotherapy and an adjunctive therapy (Beydoun et al., 2020). Notably, other studies had indicated that oxcarbazepine exhibited superior overall effectiveness and was associated with fewer adverse events, such as vomiting, compared to other treatments (Zhang et al., 2022). However, it was crucial to acknowledge that our study included relatively small sample sizes for each drug, which may have introduced potential biases in the results. Consequently, further research was required to comprehensively evaluate the effectiveness and safety of oxcarbazepine.
Zonisamide, due to its adverse effects, was unlikely to emerge as the first-line treatment for focal epilepsy (Reimers and Ljung, 2019). Among other treatment options, brivaracetam, considered the second generation of levetiracetam, was a new ASM (Verrotti et al., 2021) that demonstrated high tolerability and effectiveness, particularly for adults with drug-resistant focal epilepsy (Bresnahan et al., 2022). Nevertheless, contrary to preclinical studies suggesting brivaracetam’s potential as an ideal treatment for focal epilepsy (Russo et al., 2017), this study found that the ASM was less effective in practical applications.
Monotherapy was widely accepted as the conventional primary treatment approach for epilepsy. However, when the initial administration of ASMs as adjunctive therapy proved ineffective, the option of employing combination therapy was contemplated. In cases where monotherapy was not controlled, the combination of lamotrigine and levetiracetam was considered. This combination regimen had the highest rate of seizure freedom both before and during pregnancy. Although the effectiveness of either ASM as adjunctive therapy alone may have been similar to that of sodium valproate in the treatment of generalized epilepsy, combination therapy with multiple agents was believed to have better effectiveness (Cohen et al., 2024). For patients who failed to respond to dual therapy, the prognosis could be improved through the reasonable selection of triple therapy, with about 15% of patients with refractory focal epilepsy achieving seizure-free status under triple therapy (Cai et al., 2024).
As indicated in clinical guidelines (Kanner et al., 2018), the following medications were effective in reducing the frequency of treatment-resistant adult focal epilepsy (Level A): immediate-release pregabalin, perampanel, and vigabatrin (though vigabatrin was not considered a first-line treatment). Medications that could reduce the frequency of treatment-resistant adult focal epilepsy (Level B) included lacosamide, eslicarbazepine, extended-release topiramate, and levetiracetam (used as add-on therapy for treatment-resistant childhood focal epilepsy). Perampanel and vigabatrin were found to be effective as add-on treatments for intractable focal epilepsy in adults, whereas oxcarbazepine required a high dose and its efficacy was dose-dependent. The drugs recommended in this study differed from those in the guidelines for several reasons. Firstly, the overall population studied varied, including differences in age and the severity of epilepsy. Secondly, the underlying anti-epileptic medication regimen was unclear. Thirdly, there may have been variations in the amount of adjuvant therapy used across different studies. Fourthly, the quality of research evidence varied across studies. Finally, the small sample size may have affected the accuracy of the results. By expanding the discussion of clinical implications, this study provides a broader and more specific analysis of controversial drugs from previous meta-analyses, making our findings more actionable and relevant to clinicians and patients. This will help ensure that our study has a meaningful impact on the management of drug-refractory focal epilepsy and ultimately improves patient outcomes.
This study had several limitations. Firstly, it lacked sufficient data and subgroup analyses regarding the ethnicity and comorbidities of the participants, which could have substantially impacted the overall conclusion. Secondly, the route of administration may have influenced the potential for side effects associated with each medication, dose, and treatment duration, potentially leading to significant differences among the studies included. Thirdly, we did not evaluate the etiology of drug resistance in drug-resistant focal epilepsy. Fourthly, patient heterogeneity, such as age and comorbidities, was not discussed, which could affect the generalizability of the findings. Fifthly, because some confounding factors were not mentioned in the original studies, subgroup analyses could not be performed. Finally, due to the lack of other safety data, some adverse event outcomes were excluded from the study for comparison, resulting in incomplete conclusions regarding safety.
Conclusion
This network meta-analysis provided an overview of the 50% response rate and tolerability of the ASMs used in drug-resistant focal seizures, aiming to offer more authoritative and effective guidance for clinical medication guidelines. The analysis demonstrated that topiramate, tiagabine, oxcarbazepine, and levetiracetam were the four most effective adjuvant treatments for ASMs. However, it was important to note that topiramate and oxcarbazepine were associated with a higher risk of somnolence. Furthermore, there was a lack of comprehensive safety data for tiagabine and levetiracetam, necessitating further research in this area. Larger sample studies were still needed to strengthen the support for these findings and to gain a better understanding of the safety profiles of all the ASMs involved.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
N-JD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review and editing. X-YL: Data curation, Formal Analysis, Methodology, Project administration, Software, Writing – review and editing. ZX-Z: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Writing – original draft. C-YX-Y: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. Y-TT: Data curation, Investigation, Methodology, Resources, Software, Writing – original draft. Y-TM: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. H-JL: Data curation, Formal Analysis, Investigation, Project administration, Software, Writing – original draft. T-YG: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Writing – original draft. XL: Data curation, Formal Analysis, Investigation, Software, Validation, Writing – original draft. JL: Conceptualization, Investigation, Methodology, Project administration, Resources, Visualization, Writing – review and editing. CZ: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review and editing. S-LH: Conceptualization, Data curation, Methodology, Project administration, Resources, Software, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Leading scientific research projects of Shiyan City in 2021 (No. 21Y16) and the College students Innovation and entrepreneurship training program project from Hubei University of Medicine in 2022 (No. X202213249005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1500475/full#supplementary-material
References
Abou-Khalil, B. W. (2019). Update on antiepileptic drugs 2019. Contin. Minneap. Minn 25 (2), 508–536. doi:10.1212/CON.0000000000000715
Anhut, H., Ashman, P., Feuerstein, T. J., Sauermann, W., Saunders, M., and Schmidt, B. (1994). Gabapentin (Neurontin) as add-on therapy in patients with partial seizures: a double-blind, placebo-controlled study. The International Gabapentin Study Group. Epilepsia 35 (4), 795–801. doi:10.1111/j.1528-1157.1994.tb02513.x
Arroyo, S., Anhut, H., Kugler, A. R., Lee, C. M., Knapp, L. E., Garofalo, E. A., et al. (2004). Pregabalin add-on treatment: a randomized, double-blind, placebo-controlled, dose-response study in adults with partial seizures. Epilepsia 45 (1), 20–27. doi:10.1111/j.0013-9580.2004.31203.x
Barcs, G., Walker, E. B., Elger, C. E., Scaramelli, A., Stefan, H., Sturm, Y., et al. (2000). Oxcarbazepine placebo-controlled, dose-ranging trial in refractory partial epilepsy. Epilepsia 41 (12), 1597–1607. doi:10.1111/j.1499-1654.2000.001597.x
Baulac, M., Leon, T., O'Brien, T. J., Whalen, E., and Barrett, J. (2010). A comparison of pregabalin, lamotrigine, and placebo as adjunctive therapy in patients with refractory partial-onset seizures. Epilepsy Res. 91 (1), 10–19. doi:10.1016/j.eplepsyres.2010.05.008
Ben-Menachem, E. (1997). Clinical efficacy of topiramate as add-on therapy in refractory partial epilepsy: the European experience. Epilepsia 38 (Suppl. 1), S28–S30. doi:10.1111/j.1528-1157.1997.tb04514.x
Ben-Menachem, E., Gabbai, A. A., Hufnagel, A., Maia, J., Almeida, L., and Soares-da-Silva, P. (2010). Eslicarbazepine acetate as adjunctive therapy in adult patients with partial epilepsy. Epilepsy Res. 89 (2-3), 278–285. doi:10.1016/j.eplepsyres.2010.01.014
Ben-Menachem, E., Henriksen, O., Dam, M., Mikkelsen, M., Schmidt, D., Reid, S., et al. (1996). Double-blind, placebo-controlled trial of topiramate as add-on therapy in patients with refractory partial seizures. Epilepsia 37 (6), 539–543. doi:10.1111/j.1528-1157.1996.tb00606.x
Bernasconi, A., and Bernasconi, N. (2022). The role of MRI in the treatment of drug-resistant focal epilepsy. Eur. Neurol. 85 (5), 333–341. doi:10.1159/000525262
Beydoun, A., DuPont, S., Zhou, D., Matta, M., Nagire, V., and Lagae, L. (2020). Current role of carbamazepine and oxcarbazepine in the management of epilepsy. Seizure 83, 251–263. doi:10.1016/j.seizure.2020.10.018
Beydoun, A., Uthman, B. M., Kugler, A. R., Greiner, M. J., Knapp, L. E., Garofalo, E. A., et al. (2005). Safety and efficacy of two pregabalin regimens for add-on treatment of partial epilepsy. Neurology 64 (3), 475–480. doi:10.1212/01.WNL.0000150932.48688.BE
Biton, V., Krauss, G., Vasquez-Santana, B., Bibbiani, F., Mann, A., Perdomo, C., et al. (2011). A randomized, double-blind, placebo-controlled, parallel-group study of rufinamide as adjunctive therapy for refractory partial-onset seizures. Epilepsia 52 (2), 234–242. doi:10.1111/j.1528-1167.2010.02729.x
Bodalia, P. N., Grosso, A. M., Sofat, R., Macallister, R. J., Smeeth, L., Dhillon, S., et al. (2013). Comparative efficacy and tolerability of anti-epileptic drugs for refractory focal epilepsy: systematic review and network meta-analysis reveals the need for long term comparator trials. Br. J. Clin. Pharmacol. 76 (5), 649–667. doi:10.1111/bcp.12083
Bresnahan, R., Martin-McGill, K. J., Hutton, J. L., and Marson, A. G. (2019). Tiagabine add-on therapy for drug-resistant focal epilepsy. Cochrane database Syst. Rev. 10 (10), Cd001908. doi:10.1002/14651858.CD001908.pub4
Bresnahan, R., Panebianco, M., and Marson, A. G. (2022). Brivaracetam add-on therapy for drug-resistant epilepsy. Cochrane database Syst. Rev. 3 (3), Cd011501. doi:10.1002/14651858.CD011501.pub3
Brodie, M. J. (2004). Zonisamide clinical trials: European experience. Seizure 13 (Suppl. 1), S66–S72. doi:10.1016/j.seizure.2004.04.010
Brodie, M. J., Duncan, R., Vespignani, H., Solyom, A., Bitenskyy, V., and Lucas, C. (2005). Dose-dependent safety and efficacy of zonisamide: a randomized, double-blind, placebo-controlled study in patients with refractory partial seizures. Epilepsia 46 (1), 31–41. doi:10.1111/j.0013-9580.2005.14704.x
Bruni, J., Guberman, A., Vachon, L., and Desforges, C. (2000). Vigabatrin as add-on therapy for adult complex partial seizures: a double-blind, placebo-controlled multicentre study. The Canadian Vigabatrin Study Group. The Canadian Vigabatrin Study Group. Seizure 9 (3), 224–232. doi:10.1053/seiz.2000.0381
Cai, A. J., Gao, K., Zhang, F., and Jiang, Y. W. (2024). Recent advances and current status of gene therapy for epilepsy. World J. Pediatr. 20 (11), 1115–1137. doi:10.1007/s12519-024-00843-w
Cereghino, J. J., Biton, V., Abou-Khalil, B., Dreifuss, F., Gauer, L. J., and Leppik, I. (2000). Levetiracetam for partial seizures: results of a double-blind, randomized clinical trial. Neurology 55 (2), 236–242. doi:10.1212/wnl.55.2.236
Chadwick, D., Smith, D., Crawford, P., and Harrison, B. (2000). Remacemide hydrochloride: a placebo-controlled, one month, double-blind assessment of its safety, tolerability and pharmacokinetics as adjunctive therapy in patients with epilepsy. Seizure 9 (8), 544–550. doi:10.1053/seiz.2000.0448
Christensen, J., Zoega, H., Leinonen, M. K., Gilhus, N. E., Gissler, M., Igland, J., et al. (2024). Prenatal exposure to antiseizure medications and fetal growth: a population-based cohort study from the Nordic countries. Lancet Reg. Health Eur. 38, 100849. doi:10.1016/j.lanepe.2024.100849
Chung, S. S., French, J. A., Kowalski, J., Krauss, G. L., Lee, S. K., Maciejowski, M., et al. (2020). Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology 94 (22), e2311–e2322. doi:10.1212/WNL.0000000000009530
Cohen, J. M., Alvestad, S., Suarez, E. A., Schaffer, A., Selmer, R. M., Havard, A., et al. (2024). Comparative risk of major congenital malformations with antiseizure medication combinations vs valproate monotherapy in pregnancy. Neurology 102 (2), e207996. doi:10.1212/WNL.0000000000207996
Engel, J. (2004). Models of focal epilepsy. Suppl. Clin. Neurophysiology 57, 392–399. doi:10.1016/s1567-424x(09)70376-9
Faught, E. (1997). Efficacy of topiramate as adjunctive therapy in refractory partial seizures: United States trial experience. Epilepsia 38 (Suppl. 1), S24–S27. doi:10.1111/j.1528-1157.1997.tb04513.x
Faught, E., Wilder, B. J., Ramsay, R. E., Reife, R. A., Kramer, L. D., Pledger, G. W., et al. (1996). Topiramate placebo-controlled dose-ranging trial in refractory partial epilepsy using 200-400-and 600-mg daily dosages. Topiramate YD Study Group. Neurology 46 (6), 1684–1690. doi:10.1212/wnl.46.6.1684
French, J., Brandt, C., Friedman, D., Biton, V., Knapp, L., Pitman, V., et al. (2014). Adjunctive use of controlled-release pregabalin in adults with treatment-resistant partial seizures: a double-blind, randomized, placebo-controlled trial. Epilepsia 55 (8), 1220–1228. doi:10.1111/epi.12690
French, J., Glue, P., Friedman, D., Almas, M., Yardi, N., Knapp, L., et al. (2016). Adjunctive pregabalin vs gabapentin for focal seizures: interpretation of comparative outcomes. Neurology 87 (12), 1242–1249. doi:10.1212/WNL.0000000000003118
French, J. A., Cole, A. J., Faught, E., Theodore, W. H., Vezzani, A., Liow, K., et al. (2021). Safety and efficacy of natalizumab as adjunctive therapy for people with drug-resistant epilepsy: a phase 2 study. Neurology 97 (18), e1757–e1767. doi:10.1212/WNL.0000000000012766
French, J. A., Costantini, C., Brodsky, A., and von Rosenstiel, P.N01193 Study Group (2010). Adjunctive brivaracetam for refractory partial-onset seizures: a randomized, controlled trial. Neurology 75 (6), 519–525. doi:10.1212/WNL.0b013e3181ec7f7f
French, J. A., Kugler, A. R., Robbins, J. L., Knapp, L. E., and Garofalo, E. A. (2003). Dose-response trial of pregabalin adjunctive therapy in patients with partial seizures. Neurology 60 (10), 1631–1637. doi:10.1212/01.wnl.0000068024.20285.65
French, J. A., Mosier, M., Walker, S., Sommerville, K., and Sussman, N. (1996). A double-blind, placebo-controlled study of vigabatrin three g/day in patients with uncontrolled complex partial seizures. Vigabatrin Protocol 024 Investigative Cohort. Neurology 46 (1), 54–61. doi:10.1212/wnl.46.1.54
Gabapentin in Partial Epilepsy (1990). UK gabapentin study group. Lancet (London, England) 335 (8698), 1114–1117. doi:10.1016/0140-6736(90)91123-R
Gil-Nagel, A., Lopes-Lima, J., Almeida, L., Maia, J., and Soares-da-Silva, P.BIA-2093-303 Investigators Study Group (2009). Efficacy and safety of 800 and 1200 mg eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures. Acta neurol. Scand. 120 (5), 281–287. doi:10.1111/j.1600-0404.2009.01218.x
Gooley, S., Crompton, D. E., and Berkovic, S. F. (2022). ILAE Genetic Literacy Series: familial focal epilepsy syndromes. Epileptic Disord. Int. Epilepsy J. Videotape. 24 (2), 221–228. doi:10.1684/epd.2021.1393
Guberman, A., Neto, W., and Gassmann-Mayer, C.EPAJ-119 Study Group (2002). Low-dose topiramate in adults with treatment-resistant partial-onset seizures. Acta neurol. Scand. 106 (4), 183–189. doi:10.1034/j.1600-0404.2002.02071.x
Higgins, J. P., and James, T. (2011). Cochrane handbook for systematic reviews of interventions 5 (1). Available online at: https://handbook-5-1.cochrane.org/ (accessed March, 2011).
Hogan, R. E., Blatt, I., Lawson, B., Nagaraddi, V., Fakhoury, T. A., Anders, B., et al. (2014). Efficacy of once-daily extended-release topiramate (USL255): a subgroup analysis based on the level of treatment resistance. Epilepsy and Behav. 41, 136–139. doi:10.1016/j.yebeh.2014.09.061
Hong, Z., Inoue, Y., Liao, W., Meng, H., Wang, X., Wang, W., et al. (2016). Efficacy and safety of adjunctive lacosamide for the treatment of partial-onset seizures in Chinese and Japanese adults: a randomized, double-blind, placebo-controlled study. Epilepsy Res. 127, 267–275. doi:10.1016/j.eplepsyres.2016.08.032
Honybun, E., Cockle, E., Malpas, C. B., O’Brien, T. J., Vajda, F. J., Perucca, P., et al. (2024). Neurodevelopmental and functional outcomes following in utero exposure to antiseizure medication: a systematic review. Neurology 102 (8), e209175. doi:10.1212/WNL.0000000000209175
Hu, Q., Zhang, F., Teng, W., Hao, F., Zhang, J., Yin, M., et al. (2018). Efficacy and safety of antiepileptic drugs for refractory partial-onset epilepsy: a network meta-analysis. J. Neurology 265 (1), 1–11. doi:10.1007/s00415-017-8621-x
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162 (11), 777–784. doi:10.7326/M14-2385
Inoue, Y., Liao, W., Wang, X., Du, X., Tennigkeit, F., Sasamoto, H., et al. (2021). Safety and efficacy of adjunctive lacosamide in Chinese and Japanese adults with epilepsy and focal seizures: a long-term, open-label extension of a randomized, controlled trial. Epilepsy Res. 176, 106705. doi:10.1016/j.eplepsyres.2021.106705
Iyer, A., and Marson, A. (2014). Pharmacotherapy of focal epilepsy. Expert Opin. Pharmacother. 15 (11), 1543–1551. doi:10.1517/14656566.2014.922544
Jenssen, S., Roberts, C. M., Gracely, E. J., Dlugos, D. J., and Sperling, M. R. (2011). Focal seizure propagation in the intracranial EEG. Epilepsy Res. 93 (1), 25–32. doi:10.1016/j.eplepsyres.2010.10.008
Kälviäinen, R., Aikiä, M., Mervaala, E., Saukkonen, A. M., Pitkänen, A., and Riekkinen, P. J. (1996). Long-term cognitive and EEG effects of tiagabine in drug-resistant partial epilepsy. Epilepsy Res. 25 (3), 291–297. doi:10.1016/s0920-1211(96)00084-8
Kälviäinen, R., Brodie, M. J., Duncan, J., Chadwick, D., Edwards, D., and Lyby, K. (1998). A double-blind, placebo-controlled trial of tiagabine given three-times daily as add-on therapy for refractory partial seizures. Epilepsy Res. 30 (1), 31–40. doi:10.1016/s0920-1211(97)00082-x
Kanner, A. M., Ashman, E., Gloss, D., Harden, C., Bourgeois, B., Bautista, J. F., et al. (2018). Practice guideline update summary: efficacy and tolerability of the new antiepileptic drugs II: treatment-resistant epilepsy: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology and the American epilepsy society. Neurology 91 (2), 82–90. doi:10.1212/WNL.0000000000005756
Klein, P., Schiemann, J., Sperling, M. R., Whitesides, J., Liang, W., Stalvey, T., et al. (2015). A randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia 56 (12), 1890–1898. doi:10.1111/epi.13212
Krauss, G. L., Klein, P., Brandt, C., Lee, S. K., Milanov, I., Milovanovic, M., et al. (2020). Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neuro. 19 (1), 38–48. doi:10.1016/S1474-4422(19)30399-0
Lee, B. I., Yi, S., Hong, S. B., Kim, M. K., Lee, S. A., Lee, S. K., et al. (2009). Pregabalin add-on therapy using a flexible, optimized dose schedule in refractory partial epilepsies: a double-blind, randomized, placebo-controlled, multicenter trial. Epilepsia 50 (3), 464–474. doi:10.1111/j.1528-1167.2008.01954.x
Lindberger, M., Alenius, M., Frisén, L., Johannessen, S. I., Larsson, S., Malmgren, K., et al. (2000). Gabapentin versus vigabatrin as first add-on for patients with partial seizures that failed to respond to monotherapy: a randomized, double-blind, dose titration study. GREAT Study Investigators Group. Gabapentin in refractory epilepsy add-on treatment. Epilepsia 41 (10), 1289–1295. doi:10.1111/j.1528-1157.2000.tb04607.x
Löscher, W., and Klein, P. (2021). The pharmacology and clinical efficacy of antiseizure medications: from bromide salts to cenobamate and beyond. CNS drugs 35 (9), 935–963. doi:10.1007/s40263-021-00827-8
Lu, G., and Ades, A. E. (2004). Combination of direct and indirect evidence in mixed treatment comparisons. Statistics Med. 23 (20), 3105–3124. doi:10.1002/sim.1875
Marson, A., Burnside, G., Appleton, R., Smith, D., Leach, J. P., Sills, G., et al. (2021). The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet (London, England) 397 (10282), 1375–1386. doi:10.1016/S0140-6736(21)00246-4
Matsuo, F., Bergen, D., Faught, E., Messenheimer, J. A., Dren, A. T., Rudd, G. D., et al. (1993). Placebo-controlled study of the efficacy and safety of lamotrigine in patients with partial seizures. U.S. Lamotrigine Protocol 0.5 Clinical Trial Group. Neurol. 43 (11), 2284–2291. doi:10.1212/wnl.43.11.2284
Mengel, H. (1994). Tiagabine. Epilepsia 35 (Suppl. 5), S81–S84. doi:10.1111/j.1528-1157.1994.tb05976.x
Nakajima-Ohyama, K. C., Shizusawa, Y., Uchiyama, S., Kishi, Y., and Tanimukai, H. (2024). Usefulness of gabapentin as an alternative/adjunct therapy for delirium: a retrospective observational study. J. Nippon. Med. Sch. 91 (2), 233–240. doi:10.1272/jnms.JNMS.2024_91-214
Naritoku, D. K., Warnock, C. R., Messenheimer, J. A., Borgohain, R., Evers, S., Guekht, A. B., et al. (2007). Lamotrigine extended-release as adjunctive therapy for partial seizures. Neurology 69 (16), 1610–1618. doi:10.1212/01.wnl.0000277698.33743.8b
Nishida, T., Lee, S. K., Inoue, Y., Saeki, K., Ishikawa, K., and Kaneko, S. (2018). Adjunctive perampanel in partial-onset seizures: Asia-Pacific, randomized phase III study. Acta Neurol. Scand. 137 (4), 392–399. doi:10.1111/ane.12883
Panebianco, M., Al-Bachari, S., Hutton, J. L., and Marson, A. G. (2021). Gabapentin add-on treatment for drug-resistant focal epilepsy. Cochrane Database Syst. Rev. 1 (1), Cd001415. doi:10.1002/14651858.CD001415.pub4
Panebianco, M., Bresnahan, R., and Marson, A. G. (2022). Pregabalin add-on for drug-resistant focal epilepsy. Cochrane database Syst. Rev. 3 (3), Cd005612. doi:10.1002/14651858.CD005612.pub5
Peltola, J., Coetzee, C., Jiménez, F., Litovchenko, T., Ramaratnam, S., Zaslavaskiy, L., et al. (2009). Once-daily extended-release levetiracetam as adjunctive treatment of partial-onset seizures in patients with epilepsy: a double-blind, randomized, placebo-controlled trial. Epilepsia 50 (3), 406–414. doi:10.1111/j.1528-1167.2008.01817.x
Privitera, M., Fincham, R., Penry, J., Reife, R., Kramer, L., Pledger, G., et al. (1996). Topiramate placebo-controlled dose-ranging trial in refractory partial epilepsy using 600-800-and 1,000-mg daily dosages. Topiramate YE Study Group. Neurology 46 (6), 1678–1683. doi:10.1212/wnl.46.6.1678
Reimers, A., and Ljung, H. (2019). An evaluation of zonisamide, including its long-term efficacy, for the treatment of focal epilepsy. Expert Opin. Pharmacother. 20 (8), 909–915. doi:10.1080/14656566.2019.1595584
Rücker, G., and Schwarzer, G. (2015). Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 15, 58. doi:10.1186/s12874-015-0060-8
Russo, E., Citraro, R., and Mula, M. (2017). The preclinical discovery and development of brivaracetam for the treatment of focal epilepsy. Expert Opin. Drug Discov. 12 (11), 1169–1178. doi:10.1080/17460441.2017.1366985
Sackellares, J. C., Ramsay, R. E., Wilder, B. J., Browne, T. R., and Shellenberger, M. K. (2004). Randomized, controlled clinical trial of zonisamide as adjunctive treatment for refractory partial seizures. Epilepsia 45 (6), 610–617. doi:10.1111/j.0013-9580.2004.11403.x
Schmidt, D., Jacob, R., Loiseau, P., Deisenhammer, E., Klinger, D., Despland, A., et al. (1993). Zonisamide for add-on treatment of refractory partial epilepsy: a European double-blind trial. Epilepsy Res. 15 (1), 67–73. doi:10.1016/0920-1211(93)90011-u
Sen, A., Chowdhary, N., Hallab, A., Romoli, M., Cross, J. H., and Cappello, B. (2024). Equitable access to levetiracetam for people with epilepsy. Lancet Neuro. 23 (11), 1076–1077. doi:10.1016/S1474-4422(24)00376-4
Sharief, M., Viteri, C., Ben-Menachem, E., Weber, M., Reife, R., Pledger, G., et al. (1996). Double-blind, placebo-controlled study of topiramate in patients with refractory partial epilepsy. Epilepsy Res. 25 (3), 217–224. doi:10.1016/s0920-1211(96)00029-0
Shorvon, S. D., Löwenthal, A., Janz, D., Bielen, E., and Loiseau, P. (2000). Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. European Levetiracetam Study Group. Epilepsia 41 (9), 1179–1186. doi:10.1111/j.1528-1157.2000.tb00323.x
Song, F., Xiong, T., Parekh-Bhurke, S., Loke, Y. K., Sutton, A. J., Eastwood, A. J., et al. (2011). Inconsistency between direct and indirect comparisons of competing interventions: meta-epidemiological study. BMJ Clin. Res. Ed 343, d4909. doi:10.1136/bmj.d4909
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ Clin. Res. Ed 366, l4898. doi:10.1136/bmj.l4898
Taghdiri, M. M., Bakhshandeh Bali, M. K., Karimzadeh, P., Ashrafi, M. R., Tonekaboni, S. H., and Ghofrani, M. (2015). Comparative efficacy of zonisamide and pregabalin as an adjunctive therapy in children with refractory epilepsy. Iran. J. Child Neurology 9 (1), 49–55.
Tao, K., Chen, H., Chen, Y., Gu, Y., and Wang, X. (2024). Levetiracetam induces severe psychiatric symptoms in people with epilepsy. Seizure 116, 147–150. doi:10.1016/j.seizure.2022.12.002
Tassinari, C. A., Michelucci, R., Chauvel, P., Chodkiewicz, J., Shorvon, S., Henriksen, O., et al. (1996). Double-blind, placebo-controlled trial of topiramate (600 mg daily) for the treatment of refractory partial epilepsy. Epilepsia 37 (8), 763–768. doi:10.1111/j.1528-1157.1996.tb00649.x
The US Gabapentin Study Group No. 5 (1993). Gabapentin as add-on therapy in refractory partial epilepsy: a double-blind, placebo-controlled, parallel-group study. Neurology 43 (11), 2292–2298. doi:10.1212/wnl.43.11.2292
Tsai, J. J., Yen, D. J., Hsih, M. S., Chen, S. S., Hiersemenzel, R., Edrich, P., et al. (2006). Efficacy and safety of levetiracetam (up to 2000 mg/day) in Taiwanese patients with refractory partial seizures: a multicenter, randomized, double-blind, placebo-controlled study. Epilepsia 47 (1), 72–81. doi:10.1111/j.1528-1167.2006.00372.x
Uthman, B. M., Rowan, A. J., Ahmann, P. A., Leppik, I. E., Schachter, S. C., Sommerville, K. W., et al. (1998). Tiagabine for complex partial seizures: a randomized, add-on, dose-response trial. Archives Neurology 55 (1), 56–62. doi:10.1001/archneur.55.1.56
Verrotti, A., Grasso, E. A., Cacciatore, M., Matricardi, S., and Striano, P. (2021). Potential role of brivaracetam in pediatric epilepsy. Acta neurol. Scand. 143 (1), 19–26. doi:10.1111/ane.13347
Viteva, E., and Zahariev, Z. (2020). Topiramate effectiveness as add-on therapy in Bulgarian patients with drug-resistant epilepsy. Folia Medica. 62 (4), 712–722. doi:10.3897/folmed.62.e50175
Wu, X. Y., Hong, Z., Wu, X., Wu, L. W., Wang, X. F., Zhou, D., et al. (2009). Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in Chinese patients with refractory partial-onset seizures. Epilepsia 50 (3), 398–405. doi:10.1111/j.1528-1167.2008.01729.x
Xiao, Z., Li, J. M., Wang, X. F., Xiao, F., Xi, Z. Q., Lv, Y., et al. (2009). Efficacy and safety of levetiracetam (3,000 mg/Day) as an adjunctive therapy in Chinese patients with refractory partial seizures. Eur. Neurol. 61 (4), 233–239. doi:10.1159/000197109
Yamauchi, T., Kaneko, S., Yagi, K., and Sase, S. (2006). Treatment of partial seizures with gabapentin: double-blind, placebo-controlled, parallel-group study. Psychiatry Clin. Neurosci. 60 (4), 507–515. doi:10.1111/j.1440-1819.2006.01553.x
Yen, D. J., Yu, H. Y., Guo, Y. C., Chen, C., Yiu, C. H., and Su, M. S. (2000). A double-blind, placebo-controlled study of topiramate in adult patients with refractory partial epilepsy. Epilepsia 41 (9), 1162–1166. doi:10.1111/j.1528-1157.2000.tb00321.x
Zaccara, G., Almas, M., Pitman, V., Knapp, L., and Posner, H. (2014). Efficacy and safety of pregabalin versus levetiracetam as adjunctive therapy in patients with partial seizures: a randomized, double-blind, noninferiority trial. Epilepsia 55 (7), 1048–1057. doi:10.1111/epi.12679
Zhang, Y. J., Lu, X. M., Li, P. W., Guo, C. A., and Wan, D. J. (2022). Oxcarbazepine versus carbamazepine for the treatment of post-stroke epilepsy: a systematic review and meta-analysis. Turk. Neurosurg. 32 (2), 176–184. doi:10.5137/1019-5149.JTN.34664-21.3
Zhao, T., Feng, X., Liu, J., Gao, J., and Zhou, C. (2017). Evaluate the efficacy and safety of anti-epileptic medications for partial seizures of epilepsy: a network meta-analysis. J. Cell. Biochem. 118 (9), 2850–2864. doi:10.1002/jcb.25936
Keywords: drug-resistant focal seizures, anti-seizure medication, topiramate, levetiracetam, gabapentin, pregabalin
Citation: Deng N-J, Li X-Y, Zhang Z-X, Xian-Yu C-Y, Tao Y-T, Ma Y-T, Li H-J, Gao T-Y, Liu X, Luo J, Zhang C and Hu S-L (2025) Effectiveness and safety of single anti-seizure medication as adjunctive therapy for drug-resistant focal epilepsy based on network meta-analysis. Front. Pharmacol. 16:1500475. doi: 10.3389/fphar.2025.1500475
Received: 23 September 2024; Accepted: 14 April 2025;
Published: 25 April 2025.
Edited by:
Hua-Jun Feng, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Angelo Russo, IRCCS Institute of Neurological Sciences of Bologna (ISNB), ItalyVishal Sondhi, Armed Forces Medical College, India
Copyright © 2025 Deng, Li, Zhang, Xian-Yu, Tao, Ma, Li, Gao, Liu, Luo, Zhang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Luo, dGFpaGVob3NwaXRhbEB5ZWFoLm5ldA==; Chao Zhang, emhhbmdjaGFvMDgwM0AxMjYuY29t; Sheng-Li Hu, aHVzaGVuZ2xpd3VAMTYzLmNvbQ==
 Nian-Jia Deng1
Nian-Jia Deng1 Teng-Yu Gao
Teng-Yu Gao Xin Liu
Xin Liu Chao Zhang
Chao Zhang