- 1Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- 2King Abdullah International Medical Research Center, Riyadh, Saudi Arabia
- 3Department of Biomedical Sciences, School of Health and Biomedical Sciences, RMIT University, Melbourne, VIC, Australia
- 4College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- 5Division of Neurology, Department of Medicine, King Abdulaziz Medical City, National Guard Health Affairs, Riyadh, Saudi Arabia
- 6Department of Pharmacology and Toxicology, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia
- 7College of Medicine, Aljouf University, Aljouf, Saudi Arabia
- 8College of Public Health and Health Informatics, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
Background: Dual antiplatelet therapy (DAPT) is commonly used for secondary stroke prevention, but the optimal timing and duration of treatment remain uncertain. This meta-analysis investigated the efficacy and safety of DAPT compared to any single antiplatelet therapy in stroke patients. We examined the effectiveness of DAPT versus monotherapy, stratified by stroke type, timing of intervention onset, and duration of DAPT.
Methods: We systematically searched electronic databases for randomized controlled trials (RCTs) comparing DAPT with any single antiplatelet therapy in stroke patients. Data from 30 RCTs involving 75,504 patients were pooled using a random-effects model. The key outcomes were recurrent ischemic stroke, major adverse cardiovascular events (MACE), and major bleeding. Additional studied outcomes included hemorrhagic stroke and mortality. Subgroup analysis examined the effectiveness of DAPT versus any single antiplatelet therapy, stratified by stroke type (ischemic stroke, lacunar stroke, and TIA or ischemic stroke), timing of intervention onset (within 12 h, 24 h, 48 h, 72 h, and 7–180 days), duration of DAPT (short-term: up to 30 days; long-term: beyond 30 days) and DAPT regimens (Aspirin and Clopidogrel, Aspirin and Cilostazol, Aspirin and Dipyridamole, and Clopidogrel and Cilostazol, etc.).
Results: DAPT significantly reduced recurrent ischemic stroke (RR 0.69, 95% CI 0.60–0.79) and MACE (RR 0.77, 95% CI 0.69–0.87), but did not significantly affect hemorrhagic stroke (RR 1.28, 95% CI 0.80–2.07), major bleeding (RR 1.10, 95% CI 0.91–1.33), or mortality (RR 1.01, 95% CI 0.88–1.15). Subgroup analyses showed that aspirin plus clopidogrel reduced recurrent stroke (RR 0.69, 95% CI 0.59–0.82) and MACE (RR 0.82, 95% CI 0.75–0.91). Early DAPT initiation (within 12–24 h) significantly reduced recurrent ischemic stroke (RR 0.73, 95% CI 0.57–0.92 and RR 0.66, 95% CI 0.52–0.84, respectively) and MACE (RR 0.78, 95% CI 0.62–0.98 and RR 0.81, 95% CI 0.72–0.93, respectively), but increased major bleeding (RR 2.32, 95% CI 1.10–4.86 and RR 1.34, 95% CI 1.20–1.49, respectively). Short-term DAPT (≤30 days) showed a greater reduction in recurrent ischemic events (RR 0.65, 95% CI 0.53–0.79) than long-term DAPT (>30 days; RR 0.72, 95% CI 0.60–0.86).
Conclusion: DAPT effectively reduces recurrent ischemic stroke and MACE, especially when initiated within 12–24 h using aspirin plus clopidogrel. Short-term DAPT (≤30 days) may be optimal for recurrent stroke prevention. Clinicians should carefully weigh benefits and risks when personalizing DAPT strategies.
Introduction
Individuals who have been diagnosed with a transient ischemic attack or small stroke are more likely to have repeated thrombotic episodes, especially in the 3 months that follow (Amarenco et al., 2016; Timp et al., 2019). The evidence from randomized controlled trials (RCTs) and subsequent meta-analyses have shown the efficacy and effectiveness of mono antiplatelet therapy such as aspirin or clopidogrel among patients with minor ischemic strokes or transient ischemic attacks (Collaboration, 2002; Xian et al., 2016). Antiplatelet therapy is a well-established pharmaceutical regime for reducing the probability of thrombotic events among patients diagnosed with ischemic stroke (Rothwell et al., 2007). The likely advantages of aspirin in alleviating the burden and reducing the severity of recurrent stroke are well documented in the scientific literature (Author anonymous, 1997; Rothwell et al., 2016). Pharmaceutical regimes, such as dipyridamole alone or in combination with aspirin, is not found to be superior in inhibiting the activity of platelets to mono therapy with aspirin (Rothwell et al., 2016). In addition, triple pharmaceutical regimen with antiplatelet drugs, such as combination of aspirin, clopidogrel, and dipyridamole increases the hazards of major bleeding among patients diagnosed with stroke (Bath et al., 2018; van Rein et al., 2019).
Since the antiplatelet drugs such as aspirin or clopidogrel do not antagonize the same steps in the platelet activation process, there is rationale to anticipate that combination of two antiplatelet agents may enhance antithrombotic efficacy (Furie et al., 2011). Hence, clinicians and experts in the field of cardiovascular diseases have shifted their focus in examining the role of dual antiplatelet therapy (DAPT) (e.g., aspirin and clopidogrel, aspirin and ticagrelor, aspirin and cilostazol, and aspirin and dipyridamole) in decreasing the prevalence and burden of recurrent ischemic strokes and associated comorbidities. Additionally, the emerging scientific evidence suggests the beneficial effect of dual antiplatelet therapy in reducing the incidence of recurrent ischemic stroke and other cardiovascular events among patients diagnosed with ischemic stroke. While the DAPT such as combination of aspirin and clopidogrel has been found successful among patients with acute coronary syndromes (Cuisset et al., 2017), therapeutic effect of the dual therapy among patients with ischemic stroke is not well studied. Therefore, it is important to determine whether these dual regimens can be considered appropriate alternatives to standard monotherapeutic antiplatelet regimens for the subsequent events of a stroke. Besides, while DAPT may provide additional protection against recurrence of stroke, the combination of two antiplatelet therapy may increase the risk of adverse events and complications. Thus, it would be helpful to assess the therapeutic efficacy and safety of DAPT by synthesizing the evidence from existing randomized controlled trials that have examined both efficacy and safety of combination of two antiplatelet therapies. Hence, we addressed this gap in knowledge by conducting a systematic review and meta-analysis of the randomized controlled trials examining the efficacy and safety of dual antiplatelet therapy among stroke patients. Additionally, in this comprehensive meta-analysis, we present a novel approach to evaluating the effectiveness of DAPT by incorporating an extensive subgroup analysis and including a broader range of studies. Our meta-analysis differentiates itself from previous research by stratifying data based on stroke type, timing of intervention onset, and duration of DAPT, areas previously unexplored in literature. This innovative methodology allows for a more nuanced understanding of DAPT’s efficacy across various clinical contexts. By integrating a wider array of studies and conducting detailed subgroup analyses, our meta-analysis offers valuable insights into optimizing DAPT regimens for preventing recurrent ischemic events, ultimately enhancing the precision and applicability of our findings to clinical practice. The findings of this review will help clinicians and policymakers to make informed evidence-based decisions for patients diagnosed with stroke.
Materials and methods
Data bases and search terms
We undertook the systematic review and meta-analysis using PRISMA (Preferred reporting items for Systematic reviews and meta-analysis) guidelines (Page et al., 2021). A comprehensive literature search was conducted to identify all relevant RCTs comparing DAPT with single antiplatelet therapy in stroke patients. The search encompassed three electronic databases: PubMed, Web of Science, and EMBASE, and spanned the period from January 2000 to December 2024 to include the most recent literature. The search strategy employed a combination of keywords and controlled vocabulary terms related to the population, intervention, comparison, and outcomes of interest (Supplementary Table S1). Specifically, terms related to stroke (including “stroke,” “ischemic stroke,” “cerebral infarction,” “cerebral ischemia,” and “transient ischemic attack”) were combined using Boolean operator “OR.” These population terms were then combined using “AND” with terms related to DAPT (“dual antiplatelet therapy,” “combined antiplatelet treatment,” “dual versus monoantiplatelet”). Similarly, terms related to single antiplatelet therapy (“single antiplatelet agent,” “antiplatelet agent”) were searched using “OR” and then combined with the population terms using “AND.” Individual searches for specific antiplatelet agents (aspirin, clopidogrel, ticagrelor, dipyridamole, and cilostazol) were also performed and combined with the population terms using “AND” to ensure comprehensive capture of relevant studies regardless of specific DAPT combinations. All searches were limited to studies involving adult populations (including young adult, middle aged, aged, and very elderly) and published between 2000 and December 2024. To identify relevant study designs, the term “randomized controlled trial” was included in the search strategy.
Eligibility criteria
We searched the databases using PICO framework, that helped us to apply the eligibility criteria using major concepts such as Population, Intervention, Comparison, and Outcome (Amir-Behghadami and Janati, 2020). PICO framework guided the development of our eligibility criteria, focusing on Population, Intervention, Comparison, and Outcome (Amir-Behghadami and Janati, 2020). We included randomized controlled trials (RCTs) involving patients with acute stroke, encompassing all subtypes (ischemic, hemorrhagic) and transient ischemic attacks (TIAs). The intervention of interest was dual antiplatelet therapy (DAPT), defined as the combination of two antiplatelet agents. This included, but was not limited to, the combination of aspirin with clopidogrel or ticagrelor. Other DAPT combinations, such as aspirin plus dipyridamole, were also eligible if evaluated in the included RCTs. The comparison group was single antiplatelet therapy. To avoid ambiguity, single antiplatelet therapy was defined as any antiplatelet medication administered as monotherapy (e.g., aspirin, clopidogrel, ticagrelor, cilostazol, dipyridamole). This definition clarifies that comparisons of DAPT to interventions other than single antiplatelet agents (such as thrombolysis or combination therapies involving non-antiplatelet drugs) were not eligible. The main outcomes were the occurrence of recurrent ischemic stroke during the follow-up period reported in each trial, major adverse cardiovascular events (MACE) (as specified by authors in their studies), and major bleeding (defined according to the criteria used in the original trials and details are provided in Supplementary Table S2). Other outcomes studied were all-cause mortality and hemorrhagic stroke. Only completed and peer-reviewed RCTs were eligible. Conference abstracts and other non-peer-reviewed materials were excluded to ensure the quality and reliability of the evidence, as abstracts often lack the detailed information necessary for rigorous assessment and data extraction. Studies were included only if they provided sufficient data to calculate pooled risk ratios (RRs) or hazard ratios (HRs), including the number of events and the total number of participants in each group. Studies were excluded if they: 1) evaluated interventions other than DAPT as defined above; 2) did not employ an RCT design; 3) were qualitative studies, reviews, editorials, or commentaries; 4) did not provide sufficient data for calculating effect measures; or 5) were published only as abstracts due to limited information.
Screening and data extraction
At the beginning, titles and abstracts of the articles were screened. This was followed by screening the full texts of the RCTs that were considered eligible based on the above-mentioned criteria. The data from eligible RCTs were extracted using the pre-defined data extraction form. We extracted data on study’s authors, year of publication, study setting or country where study was conducted, intervention, control, outcome, assessment tools to measure outcome, and key findings of the RCT. In addition, we also extracted raw data on the number of patients experiencing recurrent strokes in intervention and control arms along with the sample size of the randomized groups. This data helped us to calculate risk ration and 95% CIs in the meta-analysis.
Intervention and outcome under investigation
Primary intervention for this review and meta-analysis was dual antiplatelet therapy consisting of aspirin and clopidogrel or aspirin and ticagrelor. We studied the recurrence of ischemic stroke, MACE such as myocardial infarction and mortality due to MACE, and major bleeding that required blood transfusion or lead to compromised hemodynamic response or hypovolemic shock.
Statistical methods
Primary analysis
We performed the meta-analysis using random effect models proposed by Der Simonian and Laird, which allowed us to account for heterogeneity across studies. We pooled the results of individual RCTs and computed pooled summary measure-risk ratios (RRs) with 95% CIs. The weights to the individual RCTs were applied based on the sample size and number of events accrued in each group. We generated forest plots that helped visualize the overall pooled effect of DAPT on the primary and secondary outcomes defined in the methods. The RR in the forest plots represents the ratio of the risk of the outcome in the DAPT group compared to the risk in single antiplatelet therapy. An RR less than 1 indicates that DAPT is associated with a lower risk of the outcome. The 95% CI provides a range within which the true RR is likely to lie. Heterogeneity across RCTs was assessed using Q-test and I2 statistics. I2 value is reported in percentage and provides percentage of heterogeneity not explained by chance alone. Low, moderate, and high heterogeneity was suggested by 25%–50%, 50%–75%, >75%, respectively. In addition, we assessed publication bias using funnel plot and Egger’s regression test of asymmetry. Since the Egger’s test on its own is not powerful enough to detect publication bias, we visualized funnels plots and ran Egger’s regression test. We performed the analysis using R 4.2.2.
Subgroup analysis
In this study, subgroup analyses were conducted to evaluate the effectiveness of DAPT compared to monotherapy for most important outcomes including recurrent ischemic stroke, MACE, and major bleeding. These subgroup analyses were performed by stroke type (ischemic stroke, lacunar stroke, and TIA or ischemic stroke), timing of intervention onset (within 12 h, 24 h, 48 h, 72 h, and 7–180 days), duration of DAPT (short-term: up to 30 days; long-term: beyond 30 days), and DAPT regimens (Aspirin and Clopidogrel, Aspirin and Cilostazol, Aspirin and Dipyridamole, and Clopidogrel and Cilostazol, etc.). These analyses were performed to explore potential variations in treatment effects based on the underlying stroke etiology, the timing of therapy initiation, duration of treatment, and combination of DAPT regimen. For the subgroup analysis we estimated pooled RRs with 95% CIs. Heterogeneity was assessed using the I2 statistics, and subgroup differences were tested to determine if these factors influenced the treatment effect.
Study results
Search strategy
A systematic search of electronic databases was conducted to identify relevant studies for inclusion in this meta-analysis. The initial search yielded 3,568 records. After removing 1,013 duplicates, 2,555 unique studies remained. Titles and abstracts were screened, resulting in the exclusion of 2,386 irrelevant records. The remaining 169 potentially eligible studies were further assessed. Of these, 25 were review articles, 55 were observational studies, 11 were letters to the editor, 24 had irrelevant interventions, 14 had irrelevant outcomes, and 5 were study protocols. Following this screening process, 35 full-text articles were retrieved and assessed for eligibility. Five studies were excluded at this stage as they did not meet the pre-defined eligibility criteria. Finally, 30 studies met all eligibility criteria and were included in the systematic review and quantitative synthesis for the meta-analysis (Figure 1).
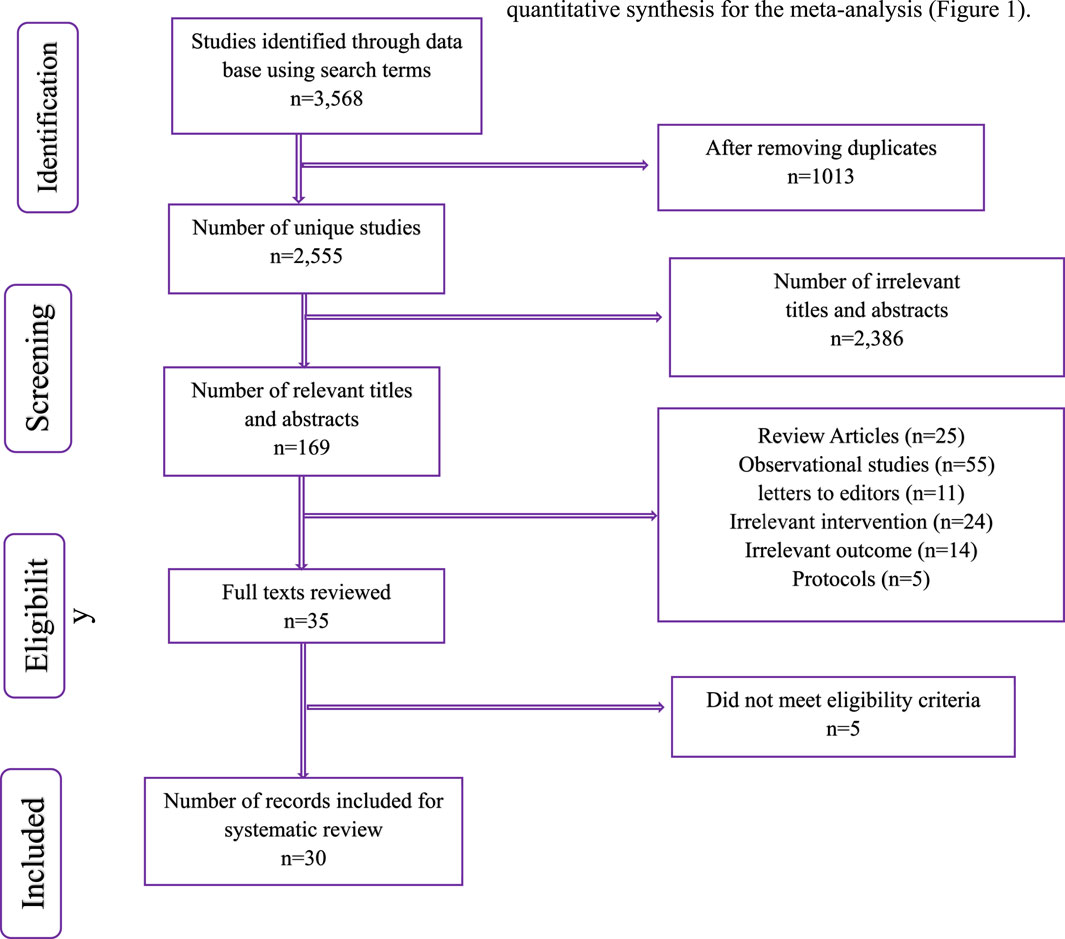
Figure 1. PRISMA Flow chart summarizing the identification and selection of records and final number of RCTs included in the quantitative synthesis for meta-analysis.
Characteristics of eligible studies
Table 1 summarizes the characteristics of various randomized controlled trials (RCTs) included in the systematic review and meta-analysis focusing on dual antiplatelet therapy among stroke patients (n = 30 RCTs; 75,504 patients). The sample size of RCTs ranged between 76 and 15, 604 and the total number of participants across all trials was considerable, amounting to 75,504, providing substantial statistical power for the meta-analysis. These RCTs were published between 2000 and 2024. Out of 30 RCTs, 11 RCTs were multi-country, 7 were conducted in China, 6 in Japan, 3 in Korea, and two in UK, and one was undertaken in North America, reflecting a global effort to address stroke prevention. The average age of participants varied between studies, generally falling in the 60 s (58–70 years), while the gender distribution showed a higher percentage of male participants in most studies. Blinding methods varied, with most studies employing double-blinded or open-label outcome researcher designs. Analysis types were primarily intention-to-treat (n = 19), with eight studies using per protocol analysis and three studies did not report any method of analysis. The rate of participants lost to follow-up also varied, with 11 studies reporting less than 1% while 9 studies reported higher percentages of at least 5% (Table 1).
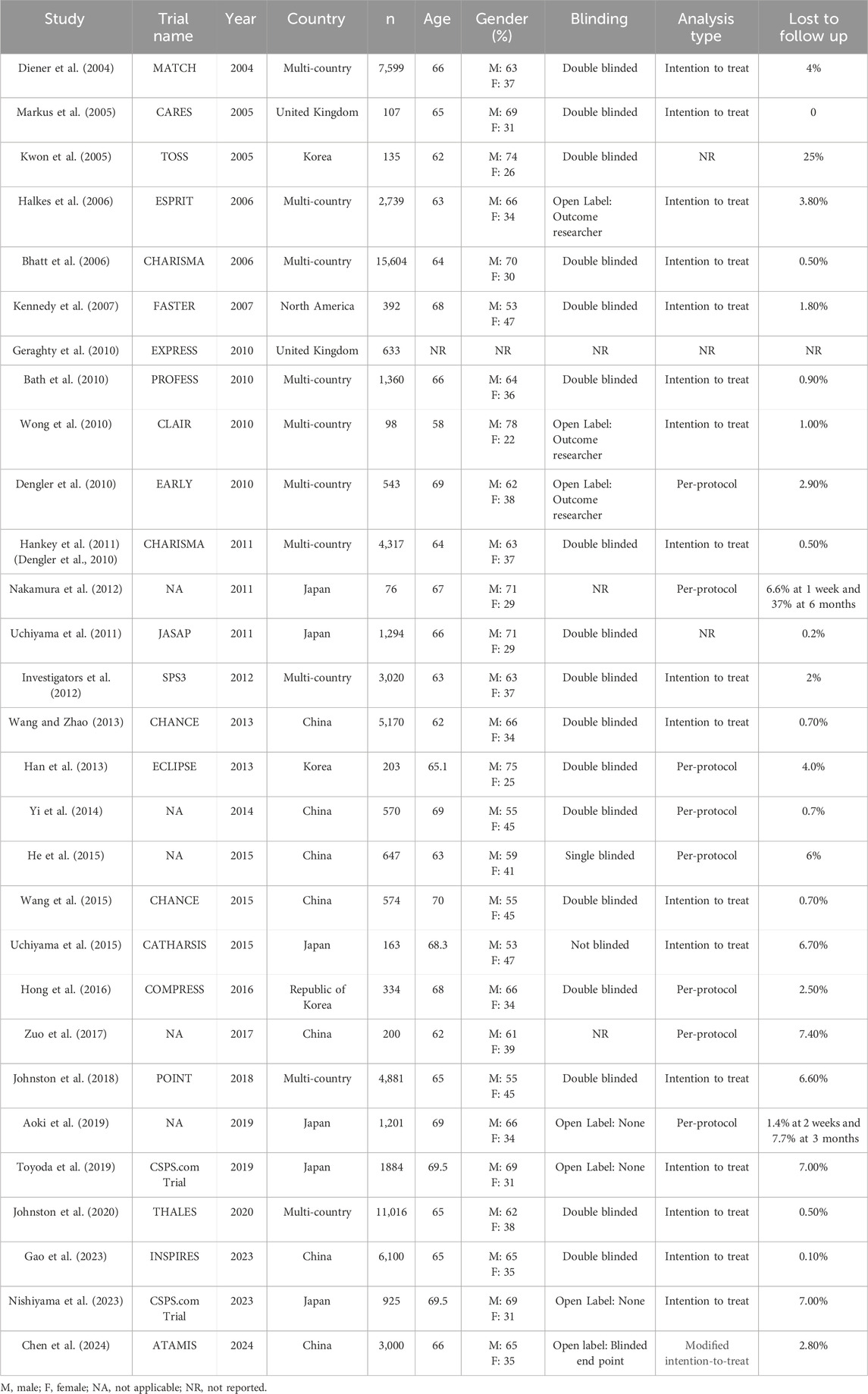
Table 1. Characteristics of the RCTs included in systematic review and meta-analysis of Dual antiplatelet therapy among stroke patients (n = 30).
Intervention and outcome details of included RCTs
Table 2 provides intervention and outcome details of the 30 included RCTs included in the review and meta-analysis focusing on dual antiplatelet therapy among stroke patients. All trials compared dual antiplatelet therapy (DAPT) to single antiplatelet therapy. The specific DAPT regimens varied, with the most common combination being aspirin plus clopidogrel (n = 18). Other DAPT regimens included aspirin plus dipyridamole (n = 4), aspirin plus cilostazol (n = 7), and aspirin and ticagrelor (n = 1). The comparator therapy in most trials was aspirin monotherapy (n = 24); however, some studies utilized clopidogrel or monotherapy (n = 4). Intervention duration also varied, with studies employing both short-term DAPT (ranging from 7 days to 30 days) and long-term DAPT (ranging from 1 month to 3.5 years). The timing of DAPT initiation post-stroke also showed variation, with some studies initiating treatment within 12–24 h (n = 11), while 5 RCTs initiated treatment within 48 h, and 8 RCTs started treatment within 72 h or beyond; however, 6 RCTs did not report the timing of onset. With respect to the type of stroke, 10 RCTs included participants with ischemic stroke, 3 RCTs had participants with lacunar stroke, and remaining included patients with ischemic stroke or transient ischemic attack (n = 17). The outcomes assessed across the trials encompassed a range of endpoints related to stroke recurrence, myocardial infarction, major cardiovascular events, death, and bleeding complications as shown in Table 2.
Overall pooled effect of DAPT on recurrent ischemic stroke
Figure 2 below summarizes the findings of a meta-analysis investigating the overall pooled effect of DAPT compared to any single antiplatelet therapy in reducing the risk of recurrent ischemic stroke. The meta-analysis pools data from 28 RCTs reported this primary outcome of recurrent ischemic stroke, encompassing a total of 37,283 participants each in intervention and control arm. For each RCT, the risk ratio (RR) and its associated 95% confidence interval (CI) are shown. The overall pooled RR for the meta-analysis is 0.69 (95% CI: 0.60–0.79). This indicates a statistically significant reduction in recurrent ischemic stroke associated with DAPT compared to monotherapy, as the CI does not cross 1. The heterogeneity among the studies is moderate, as indicated by an I2 value of 47%. This suggests some variability in the treatment effect across the trials, although it is not considered substantial.
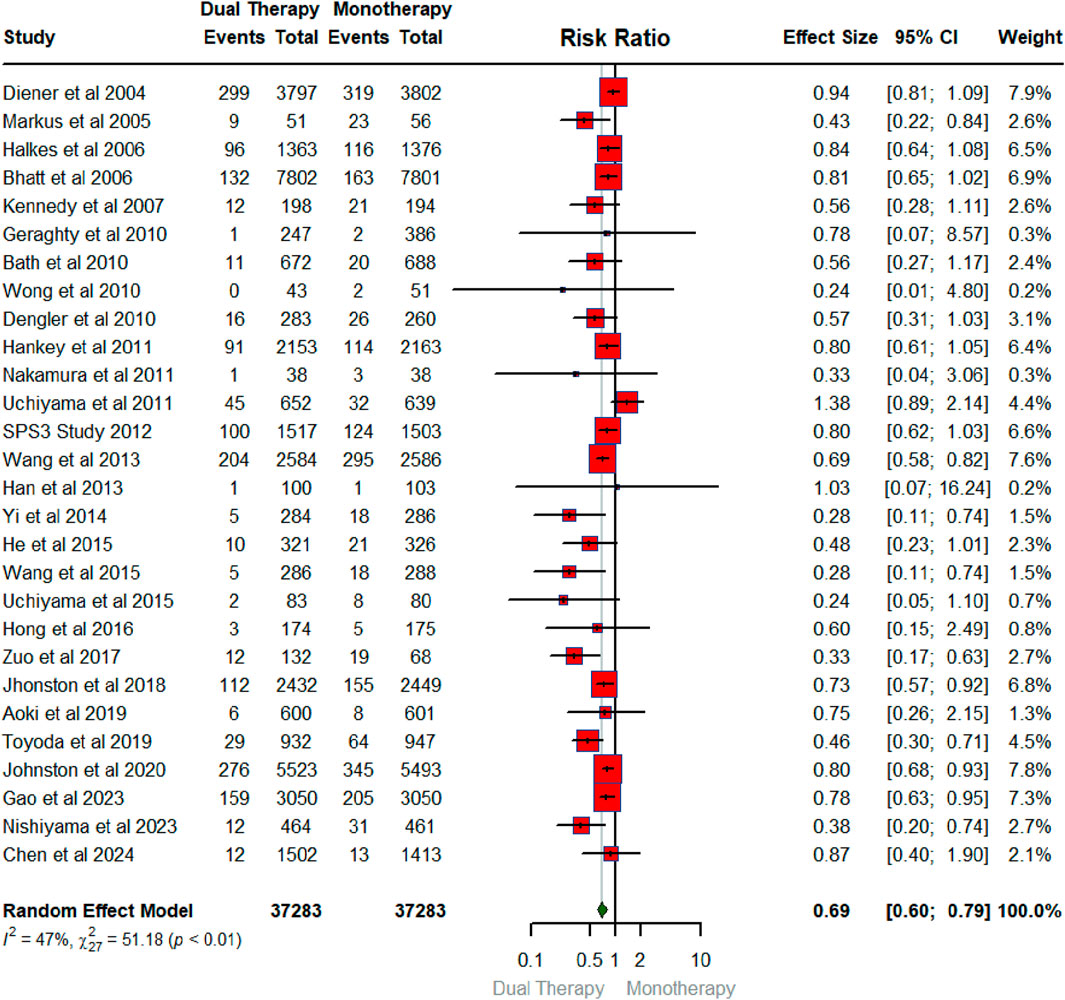
Figure 2. Forest plot illustrating the effect of dual antiplatelet vs. any single antiplatelet therapy in reducing the risk of recurrent ischemic stroke.
Subgroup analysis for recurrent ischemic stroke
Subgroup analysis for recurrent ischemic stroke by timing of DAPT initiation
The subgroup analysis by timing of DAPT initiation evaluated the effectiveness of DAPT compared to any single antiplatelet therapy in preventing recurrent ischemic strokes across different timings of initiating DAPT, reported by 23 studies (Supplemental Figure S1). Within 12 h, the pooled RR was 0.73 (95% CI: 0.57–0.92), demonstrating a statistically significant reduction in recurrent ischemic stroke risk favoring DAPT. For the 24-h subgroup, the pooled RR was 0.66 (95% CI: 0.52–0.84), indicating a significant reduction in risk with DAPT, though moderate heterogeneity was observed (I2 = 43%). While DAPT initiation within 48 h (RR 0.59, 95% CI 0.30–1.12), and 72 h (RR 0.76, 95% CI 0.57–1.03), and 7–180 days (RR 0.44, 95% CI 0.15–1.25), suggested potential reductions in recurrent ischemic stroke risk, the confidence intervals included 1, indicating uncertainty and statistically non-significant results (Supplementary Figure S1). Moderate heterogeneity was observed across subgroups (I2 = 47%), and the test for subgroup differences was significant (p < 0.01), indicating variability in effects across different time periods of commencing DAPT after stroke onset.
Subgroup analysis for recurrent ischemic stroke by DAPT duration
Supplementary Figure S2 reveals the findings of subgroup analysis (n = 28) where we compared the effectiveness of DAPT versus any single antiplatelet therapy in preventing recurrent ischemic stroke, stratified by the duration of intervention: short-term (up to 30 days) and long-term (DAPT beyond 30 days). For the short-term intervention subgroup, the pooled RR was 0.65 (95% CI: 0.53–0.79), demonstrating a 35% reduction in recurrent ischemic stroke risk with DAPT compared to monotherapy. This greater percentage reduction highlights the stronger protective effect of DAPT in the short-term setting. Heterogeneity in this subgroup was relatively low (I2 = 27%; p = 0.18), indicating consistent results across studies. For the long-term intervention subgroup, the pooled RR was 0.72 (95% CI: 0.60–0.86), showing a 28% reduction in recurrent ischemic stroke risk with DAPT (Supplementary Figure S2). While still significant, the reduction was less pronounced compared to the short-term subgroup. Substantial heterogeneity was observed in this subgroup (I2 = 53%; p < 0.01), suggesting variability in the effects across studies.
Subgroup analysis for recurrent ischemic stroke by stroke type
Supplementary Figure S3 illustrates the findings of subgroup analysis (n = 28) evaluating the effectiveness of DAPT compared to any single antiplatelet therapy in preventing recurrent ischemic events, stratified by stroke type: ischemic stroke, lacunar stroke, and TIA or ischemic stroke. For ischemic stroke, the pooled RR was 0.58 (95% CI: 0.38–0.88), indicating a 42% reduction in recurrent ischemic stroke risk with DAPT compared to monotherapy. However, substantial heterogeneity was observed (I2 = 59%, p < 0.01), suggesting variability in the effects across studies. For lacunar stroke, the pooled RR was 0.64 (95% CI: 0.22–1.84), showing a 36% reduction in recurrent stroke risk, though the confidence interval includes 1, indicating uncertainty. Heterogeneity was moderate (I2 = 53%, p = 0.12). For TIA or ischemic stroke, the pooled RR was 0.75 (95% CI: 0.66–0.84), demonstrating a 25% reduction in recurrent ischemic events with DAPT (Supplementary Figure S3). Heterogeneity in this subgroup was lower (I2 = 38%, p = 0.07), indicating more consistent results across studies. The test for subgroup differences was not significant (p = 0.37), suggesting that the type of stroke did not significantly influence the overall effect of DAPT compared to monotherapy.
Subgroup analysis for recurrent ischemic stroke by DAPT regimen combinations
This subgroup analysis (Supplementary Figure S4) of recurrent ischemic stroke risk reveals that the choice of DAPT regimen significantly influences patient outcomes. While aspirin plus cilostazol showed a trend towards reducing stroke risk, this was not statistically significant (RR 0.52, 95% CI 0.19–1.39). However, the combination of aspirin and clopidogrel demonstrated a significant reduction in recurrent stroke (RR 0.69, 95% CI 0.59–0.82), aligning with its widespread use in clinical practice. In contrast, aspirin plus dipyridamole did not have a significant effect on recurrent stroke (RR 0.82, 95% CI 0.43–1.58). Interestingly, aspirin plus ticagrelor indicated a potential benefit (RR 0.80, 95% CI 0.68–0.93), suggesting this combination may warrant further investigation. Similarly, clopidogrel plus cilostazol also showed a significant reduction in recurrent stroke risk (RR 0.44, 95% CI 0.15–1.25), although the results were statistically non-significant. Importantly, the analysis revealed significant heterogeneity across the different DAPT regimens (I2 = 47%, p < 0.01), and a test for subgroup differences was also significant (p < 0.01) as shown in Supplementary Figure S4.
Overall pooled effect of DAPT versus monotherapy on major adverse cardiovascular events (MACE)
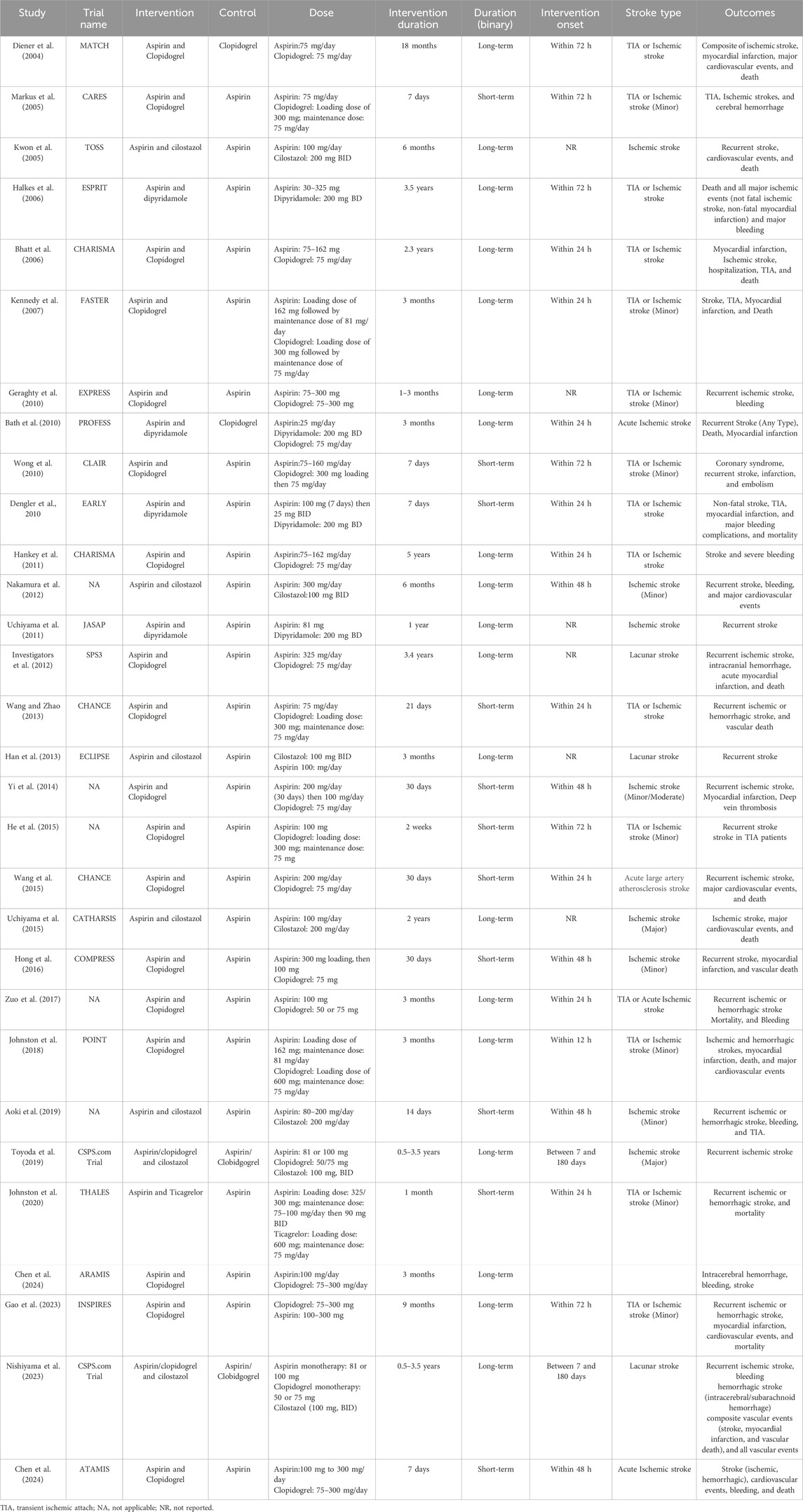
Figure 3 illustrates the pooled effect of DAPT versus any single antiplatelet therapy on MACE. The number of RCTs that contributed to MACE was 25 in total conducted between 2004 and 2024 with a sample size of 36,550 for intervention (DAPT therapy) and 36,468 for the control group (any single antiplatelet therapy). The findings showed that DAPT significantly reduced the risk of MACE by 23% (RR: 0.77; 95% CIs:0.69, 0.87). The heterogeneity statistics (I2 = 47%, X2: 44.88; P < 0.01) suggest small between-study variability.
Subgroup analysis for MACE
Subgroup analysis for MACE by timing of DAPT initiation
The subgroup analysis of MACE by timing of DAPT initiation (Supplementary Figure S5) reveals a compelling trend: earlier initiation appears to be associated with a greater reduction in MACE. Notably, initiating DAPT within 12 h shows a trend towards reducing MACE (RR 0.78, 95% CI 0.62–0.98), approaching statistical significance. Furthermore, when DAPT is initiated within 24 h, the reduction in MACE becomes statistically significant (RR 0.81, 95% CI 0.72–0.93). This highlights the potential importance of early DAPT intervention in mitigating adverse cardiovascular events. In contrast, initiating DAPT at later time points, such as within 48 h or 72 h, does not demonstrate a statistically significant effect on MACE. Similarly, initiating DAPT between 7 and 180 days did not show a statistically significant reduction (RR 0.49, 95% CI 0.16–1.49) in MACE (Supplementary Figure S5). Overall, there is moderate heterogeneity across the subgroups (I2 = 48%), and the test for subgroup differences is significant (p < 0.01), reinforcing the notion that the timing of DAPT initiation plays a crucial role in influencing MACE outcomes.
Subgroup analysis for MACE by DAPT duration
The subgroup analysis explored the impact of DAPT duration on MACE (Supplementary Figure S6). Interestingly, both long-term DAPT (n = 25,220) and short-term DAPT (n = 11,330) showed a statistically significant reduction in MACE, with a relative risk of 0.77 (95% CI 0.65–0.92) for long-term and 0.77 (95% CI 0.69–0.85) for short-term. While there was significant heterogeneity within the long-term studies (I2 = 63%, p < 0.01), the short-term studies showed no heterogeneity (I2 = 0%, p = 0.63). Overall, the analysis supports the benefit of DAPT in reducing MACE, regardless of the duration studied, with no significant difference detected between the long-term and short-term subgroups (p = 0.92) as shown in Supplementary Figure S6.
Subgroup analysis for MACE by stroke type
Supplementary Figure S7 presents a subgroup analysis of the pooled effect of DAPT versus monotherapy on MACE, stratified by stroke type: ischemic stroke, lacunar stroke, and TIA or ischemic stroke. For ischemic stroke, DAPT demonstrated a trend towards reducing MACE (RR 0.75, 95% CI 0.58–0.95), with moderate heterogeneity observed (I2 = 42%, p = 0.07). In the lacunar stroke subgroup, DAPT showed a statistically non-significant reduction in MACE (RR 0.62, 95% CI 0.01–69.06), with extremely high heterogeneity (I2 = 89%, p < 0.01). For TIA or ischemic stroke, DAPT significantly reduced MACE (RR 0.81, 95% CI 0.73–0.90), with low heterogeneity (I2 = 22%, p = 0.23) as illustrated in Supplementary Figure S7. The test for subgroup differences was not significant (p = 0.63), suggesting that the effect of DAPT on MACE did not significantly differ across the stroke subtypes analyzed.
Subgroup analysis for MACE by DAPT regimen combinations
Supplementary Figure S8 presents a subgroup analysis of the pooled effect of DAPT on MACE, stratified by DAPT regimen combinations. This subgroup analysis of DAPT regimens for MACE indicated that aspirin plus clopidogrel (RR 0.82, 95% CI 0.75–0.91) and aspirin plus ticagrelor (RR 0.81, 95% CI 0.70–0.95) significantly reduced MACE. Aspirin plus cilostazol (RR 0.66, 95% CI 0.30–1.46) and clopidogrel plus cilostazol (RR 0.49, 95% CI 0.16–1.49) showed trends towards reducing MACE, but these were not statistically significant. Aspirin plus dipyridamole did not show a significant effect (RR 0.87, 95% CI 0.60–1.25). There was significant heterogeneity across the different DAPT regimens (I2 = 47%, p < 0.01), and a significant test for subgroup differences (p < 0.01) as shown in Supplementary Figure S8, highlighting that the choice of DAPT regimen significantly impacts MACE outcomes.
Overall pooled effect of DAPT versus monotherapy on major bleeding
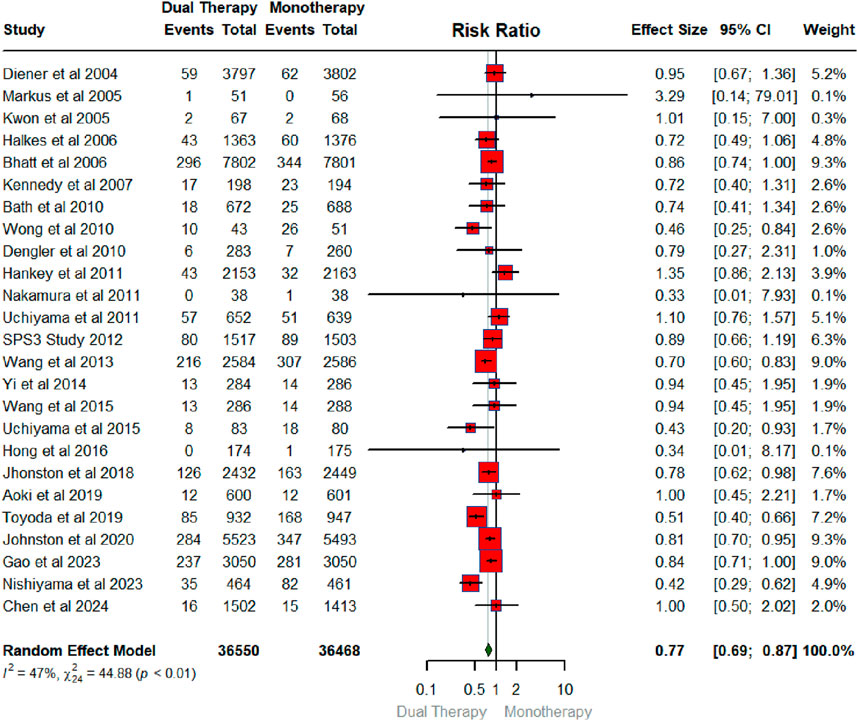
Figure 4 below exhibits the forest plot portraying the effect of DAPT on major bleeding. The number of RCTs that contributed to the major bleeding was 20 in total conducted between 2005 and 2024 with a sample size of 26,386 for intervention and 26,481 for the control group. The findings showed that DAPT did not have a significant effect on major bleeding (RR: 1.10; 95% CIs (0.91, 1.33). As shown in Figure 4, the heterogeneity statistics (I2 = 45%, X2:34.75; p-0.01) suggest low heterogeneity across the studies indicating a low between-study variability.
Subgroup analysis for major bleeding
Subgroup analysis for major bleeding by timing of DAPT initiation
Supplementary Figure S9 presents a subgroup analysis of the pooled effect of DAPT on major bleeding, stratified by the timing of DAPT initiation. Initiating DAPT within 12 (RR 2.32, 95% CI 1.10–4.86) and 24 h (RR 1.34, 95% CI 1.20–1.49) significantly increased the risk of major bleeding. However, initiating DAPT within 48 h (RR 0.96, 95% CI 0.52–1.78) and between 7 and 180 days (RR 0.78, 95% CI 0.12–5.21) showed a non-significant effect on major bleeding, with no significant heterogeneity (I2 = 0%). Initiating DAPT within 72 h showed a non-significant effect on major bleeding (RR 1.30, 95% CI 0.38–4.45), but with significant heterogeneity (I2 = 70%, p = 0.02). The test for subgroup differences was significant (p < 0.01), indicating that the timing of DAPT initiation significantly impacts the risk of major bleeding.
Subgroup analysis for major bleeding by DAPT duration
Supplementary Figure S10 presents a subgroup analysis of major bleeding events comparing DAPT to monotherapy, stratified by study duration (long-term vs short-term). In the long-term DAPT subgroup (n = 20,753 intervention, n = 20,942 control), there was a increase in major bleeding events with DAPT (RR 1.13, 95% CI 0.84–1.53), thought with statistically non-significant results and with substantial heterogeneity (I2 = 65%, p < 0.01). In the short-term DAPT subgroup (n = 5,633 intervention, n = 5,539 control), there was no statistically significant difference in major bleeding events between DAPT and monotherapy (RR 1.06, 95% CI 0.78–1.43), with no significant heterogeneity (I2 = 0%, p = 0.86) as shown in Supplementary Figure S10. The test for subgroup differences was not significant (p = 0.70), indicating no significant difference in major bleeding risk between long-term and short-term DAPT.
Subgroup analysis for major bleeding by stroke type
This subgroup analysis (Supplementary Figure S11) examined the influence of stroke type on major bleeding risk with DAPT compared to monotherapy. For ischemic stroke (RR 0.99, 95% CI 0.74–1.32) and lacunar stroke (RR 0.87, 95% CI 0.84–0.90), DAPT did not significantly affect major bleeding risk. However, in the TIA or ischemic stroke subgroup, DAPT appears to be associated with an increase in major bleeding (RR 1.34, 95% CI 0.92–1.94), though with statistically non-significant results (Supplementary Figure S11). This subgroup difference (p = 0.02) suggests that the risk of major bleeding with DAPT may vary depending on the specific type of stroke.
Subgroup analysis for major bleeding by DAPT regimen combinations
This subgroup analysis evaluated the impact of different DAPT regimens on major bleeding events (Supplementary Figure S12). Interestingly, none of the DAPT combinations showed a statistically significant effect on major bleeding. Specifically, aspirin plus cilostazol (RR 1.29, 95% CI 0.49–3.40), aspirin plus clopidogrel (RR 1.23, 95% CI 0.95–1.59), aspirin plus dipyridamole (RR 0.73, 95% CI 0.34–1.56), and clopidogrel plus cilostazol (RR 0.78, 95% CI 0.12–5.21) all had confidence intervals that crossed 1, indicating no significant difference in bleeding risk compared to monotherapy. However, there was significant heterogeneity observed in the aspirin plus clopidogrel subgroup (I2 = 54%, p = 0.01), suggesting variability in bleeding risk within this group. Furthermore, the test for subgroup differences was significant (p = 0.02), indicating that the risk of major bleeding may vary across different DAPT combinations.
Overall pooled effect of DAPT on hemorrhagic stroke
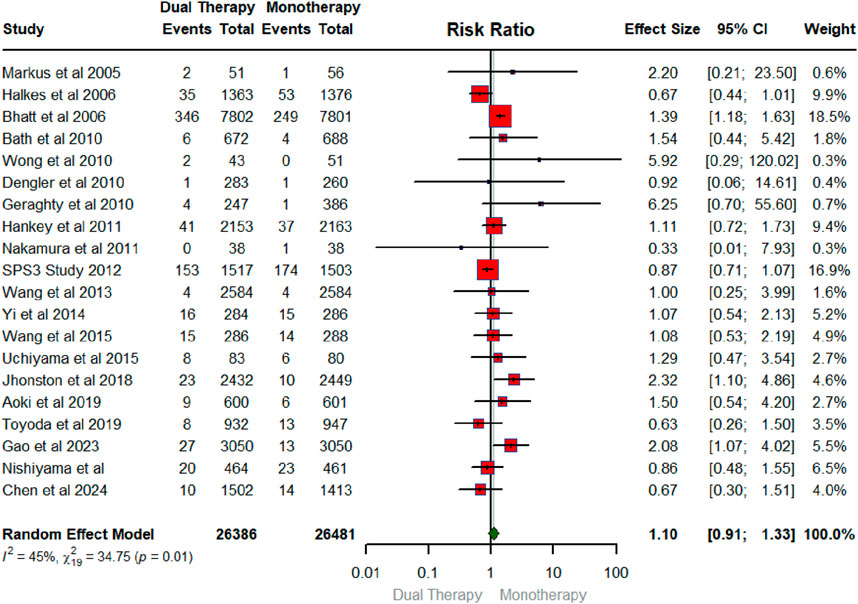
Figure 5 displays the forest plot illustrating the pooled effect of DAPT versus any single antiplatelet therapy on hemorrhagic stroke. The number of RCTs that contributed to the hemorrhagic stroke was 13 in total conducted between 2007 and 2024 with a sample size of 21,107 for intervention and 21,022 for the control group. Compared to monotherapy, DAPT did not have a significant effect on hemorrhagic stroke (RR: 1.28; 95% CIs (0.80, 2.07). The heterogeneity statistics (I2 = 48%, X2: 23.11; p:0.03), suggest that between-study variability was low for the RCTs examining hemorrhagic stroke.
Overall pooled effect of DAPT on mortality
Figure 6 below exhibits the forest plot illustrating the pooled effect of DAPT versus any single antiplatelet therapy on Death. The number of RCTs that measured mortality as an outcome was 18 in total conducted between 2004 and 2024 with a sample size of 32,417 for intervention and 32, 298 for the control group. The findings showed that DAPT had no significant effect on mortality (RR: 1.01; 95% CIs: 0.88, 1.15). The heterogeneity statistics (I2 = 27%, X2:23.30; p:0.14) indicate low heterogeneity across the studies with, suggesting very small between-study variability.
Findings of publication bias
The funnel plot (Figure 7) assesses the potential for publication bias in a meta-analysis of DAPT versus monotherapy for primary outcome. Visual inspection of the funnel plot, which plots the standard error of each study against its effect size, suggests asymmetry. This is supported by a statistically significant result from Egger’s linear regression test for funnel plot asymmetry (t = −3.41, df = 26, p = 0.0021). The test indicates a significant negative association between standard error and effect size, with a bias estimate of −1.1983 (standard error = 0.3512). This suggests that smaller studies (with larger standard errors) are more likely to report larger effect sizes favoring DAPT, a pattern indicative of potential publication bias where smaller, less precise studies with null or negative findings may be underreported or unpublished.
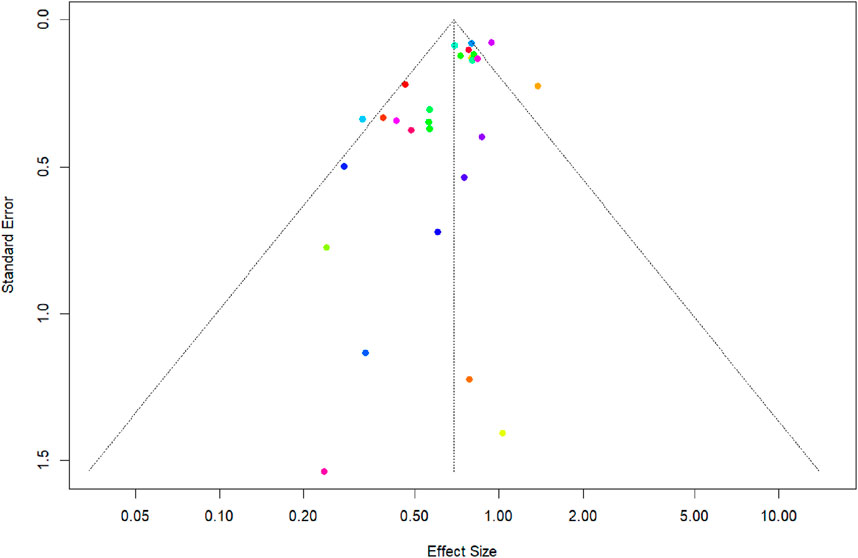
Figure 7. Funnel Plot and Egger’s regression test to assess the publication bias for recurrent ischemic stroke.
Discussion
This meta-analysis of 30 RCTs provides compelling evidence supporting the efficacy of DAPT compared to any single antiplatelet therapy in reducing the risk of recurrent ischemic stroke. The findings of the review and meta-analysis suggest that DAPT reduces the risk of recurrent ischemic stroke and cardiovascular outcomes. However, there is uncertainty about the role of dual antiplatelet therapy in reducing the risk of hemorrhagic stroke, major bleeding, and mortality. In fact, the direction of pooled risk ratio indicates that dual antiplatelet therapy may increase the risk of hemorrhagic stroke, however, the results are not statistically significant. Our analysis extends these findings by synthesizing a broader range of evidence and exploring the impact of key factors such as timing of intervention onset, stroke type, and treatment duration.
The mechanisms by which DAPT reduces the risk of recurrent ischemic stroke are multifactorial. DAPT, by combining two antiplatelet agents with different mechanisms of action, provides more comprehensive platelet inhibition compared to monotherapy (Brown et al., 2021). This synergistic effect reduces the likelihood of platelet activation and aggregation, key processes involved in thrombus formation and subsequent ischemic events (Chen et al., 2015). Specifically, aspirin inhibits cyclooxygenase (COX)-1, thereby reducing thromboxane A2 production, a potent vasoconstrictor and platelet activator (Simeone et al., 2022). Clopidogrel, ticagrelor, and other antiplatelet agents in the same class, inhibit the P2Y12 receptor on platelets, which is crucial for platelet aggregation and activation (Galli et al., 2024). The combination of these two mechanisms provides a more robust approach to preventing thrombus formation.
A key strength of our study lies in the detailed subgroup analyses, which offer valuable insights into the optimal utilization of DAPT, which offer valuable insights into the optimal utilization of DAPT. Our findings indicate that the benefit of DAPT is most pronounced, especially for outcomes such as recurrent ischemic stroke and MACE, when initiated early, specifically within 12 and 24 h of symptom onset. While a trend toward risk reduction was observed up to 72 h, the confidence intervals widened, suggesting greater uncertainty in the effect size. This observation underscores the importance of prompt DAPT administration following a stroke, aligning with the concept of “time is brain” (Gomez, 2018). The diminished effect observed with later initiation (7–180 days) warrants further investigation, though the wide confidence interval in this subgroup limits definitive conclusions. This finding may reflect the influence of other factors, such as stroke etiology or patient characteristics, on treatment response at later time points. Hence, the benefits of DAPT versus any single antiplatelet therapy with aspirin need to be interpreted cautiously after considering differences in the duration between randomization to DAPT and stroke onset.
However, our study finding suggested that Early DAPT (12–24 h) may increase bleeding risk, potentially due to disrupted hemostasis in the acute phase following stroke, especially when intracranial hemorrhage risk is elevated. In addition, the evidence suggests that patient characteristics like advanced age, low body weight, or comorbidities could further increase vulnerability (Khan et al., 2021). Careful patient selection and individualized risk assessment are crucial, particularly for those with higher baseline bleeding risks. Further research should identify which patients benefit most from early DAPT while minimizing bleeding.
Our analysis also explored the impact of DAPT duration, revealing that short-term DAPT (up to 30 days) appears to be associated with a greater reduction in recurrent ischemic events (compared to long-term DAPT (beyond 30 days). This observation raises the possibility that the maximal benefit of DAPT for secondary stroke prevention may be achieved with a shorter course of therapy. Upon critical analysis of the RCTs, it was found that the efficacy and safety of DAPT may not be found after the first 3 weeks of treatment onset. For example, findings from CHANCE and POINT trials suggest that DAPT versus monotherapy substantially reduces the risk of stroke for an initial 10 days to 2 weeks and the favorable effect of both DAPT and monotherapy appears to be the same after 10 days (Wang and Zhao, 2013; Johnston et al., 2018). This failure of DAPT to provide benefits after the first 3 weeks of treatment initiation is endorsed by a large SPS3 trial, a large multicenter trial with 3,020 patients (Investigators et al., 2012). More precisely, the findings of SPS3 trial documented that patient failed to benefit from dual antiplatelet therapy beyond the first 3 weeks of treatment onset. In fact, DAPT was found to be detrimental if the patients continued the DAPT beyond 3 weeks as it increased the risk of adverse events (Investigators et al., 2012). This finding contrasts somewhat with current clinical practice guidelines that often recommend long-term DAPT for secondary prevention (Chan et al., 2024), highlighting the need for further research to determine the optimal duration of DAPT and to explore the potential for tailoring treatment duration based on individual patient risk profiles. It is important to acknowledge that the definitions of short-term and long-term DAPT can vary across studies and clinical practice, and future research should aim to standardize these definitions to facilitate comparisons and improve clinical decision-making.
Lastly, our subgroup analyses revealed that the combination of aspirin and clopidogrel was particularly effective in reducing both recurrent stroke and MACE. This finding aligns with the widespread use of this DAPT regimen in clinical practice (Tan et al., 2015; Zhang et al., 2015; Paciaroni et al., 2019) and is likely influenced by the substantial number of studies investigating this combination, contributing to a larger sample size and increased statistical power. However, it is important to acknowledge that other DAPT combinations may also offer significant benefits but have not been as extensively studied. Therefore, we recommend conducting more RCTs to assess the efficacy and safety of alternative DAPT combinations, such as aspirin plus ticagrelor or clopidogrel plus cilostazol. This will provide a more comprehensive understanding of the comparative effectiveness of various DAPT regimens and allow for more personalized treatment strategies based on individual patient characteristics and risk profiles.
While the current review did not find a significant decrease in risk of hemorrhagic stroke and mortality with DAPT, the pooled risk ratio of greater than 1 suggests that DAPT may increase the risk of hemorrhagic stroke, though with statistically non-significant findings. As DAPT may decrease the risk of ischemic stroke and cardiovascular events, this could lead to a decreased mortality rate among these patients. Nonetheless, studies reporting on mortality as an outcome may have confounding factors such as patient’s age, gender, lifestyle, and socioeconomic status that could impact the true effects of DAPT on mortality. However, these findings are in agreement with existing evidence suggesting that long term DAPT versus monotherapy may not necessarily be favorable for reducing the risk of vascular events and mortality (Lee et al., 2013).
Strengths and limitations
Our meta-analysis boasts several significant strengths that enhance its credibility and value. First, we conducted an extensive subgroup analysis that stratified data based on stroke type (ischemic stroke, lacunar stroke, and TIA or ischemic stroke), timing of intervention onset (within 12 h, 24 h, 48 h, 72 h, and 7–180 days), and duration of DAPT (short-term: up to 30 days; long-term: beyond 30 days). This approach allowed for a more nuanced understanding of DAPT’s effectiveness across various clinical contexts, providing robust evidence for the optimization of treatment strategies.
Despite these strengths, several limitations of the current meta-analysis need to be considered. First, a meta-analysis is a technique that may be constrained by publication bias, methodological rigor, and the comprehensiveness of the search strategy. Second, limitations of the original trials may influence the pooled results of the meta-analysis. Third, the duration of the trials and follow-up times were not consistent across the RCTs included in this study, which may explain, in part, the heterogeneity across the studies. Forth, the findings may not be generalizable to patients with varying risk profiles, such as those with moderate to severe strokes, cardiometabolic strokes, and patients receiving additional anticoagulation or fibrinolytic drugs. Finally, a limitation of our meta-analysis is the inability to perform a detailed subgroup analysis based on the TOAST classification of stroke subtypes. The included RCTs did not consistently report detailed information on the specific TOAST categories (Large-artery atherosclerosis, Cardioembolism, Small-vessel occlusion, Stroke of other determined cause, and Stroke of undetermined cause). Consequently, we performed subgroup analyses using the available data, which primarily categorized strokes as ischemic, lacunar, or TIA. Future research should prioritize the reporting of stroke subtypes according to the TOAST criteria to facilitate more precise meta-analyses and a deeper understanding of the effects of DAPT on different stroke etiologies.
Despite these limitations, our study provides valuable insights into the optimal use of DAPT in secondary stroke prevention. Future research should focus on conducting larger RCTs to directly compare different DAPT regimens and durations, identifying biomarkers to predict response to DAPT, and investigating the role of DAPT in specific stroke subtypes. These findings have important implications for clinical practice and highlight the need for individualized treatment strategies based on stroke type, timing of intervention, and patient-specific factors.
Conclusion
Our findings affirm that DAPT reduces the risk of recurrent ischemic stroke and cardiovascular events. Nevertheless, there remains uncertainty regarding the role of DAPT in reducing the risk of hemorrhagic stroke, major bleeding episodes, and mortality. Given the high risk of recurrent stroke after a transient ischemic attack or ischemic stroke, a short course of DAPT may be beneficial. The timing of initiating DAPT is crucial, with the most favorable effects for recurrent ischemic stroke and MACE observed when treatment is started within 12–24 h post-stroke. The short-term use of DAPT (up to 30 days) was associated with a greater percentage reduction in recurrent ischemic stroke risk compared to long-term use, highlighting its stronger protective effect in the acute phase. This consistency in the short-term subgroup supports the efficacy of DAPT in preventing recurrent ischemic events during the critical early period following a stroke or TIA. We also found that aspirin plus clopidogrel is a good combination in reducing the risk of recurrent ischemic stroke and MACE. However, clinicians should carefully consider when and how to use DAPT, as it is not a one-size-fits-all solution. More research is needed to fine-tune the best ways to use DAPT for different types of strokes and patients.
Clinical implications and future directions
The findings underscore the potential of DAPT in reducing the risk of recurrent ischemic events, emphasizing the importance of early intervention. Given the high risk of recurrent stroke after a TIA or ischemic stroke, a short course of DAPT may be particularly beneficial. However, prolonged use of DAPT may increase the risk of hemorrhage or major bleeding compared to monotherapy, necessitating careful consideration of the risks and benefits based on the patient’s clinical profile. Individualized treatment strategies should be employed, taking into account stroke type, timing of intervention, and patient-specific factors to optimize outcomes. Future research should focus on conducting larger RCTs to directly compare different DAPT regimens and durations. Identifying biomarkers to predict response to DAPT and investigating the role of DAPT in specific stroke subtypes are also crucial areas for exploration. These efforts will provide a clearer understanding of the optimal use of DAPT in secondary stroke prevention and further refine clinical guidelines to enhance patient care.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
NMA: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review and editing. SF: Validation, Writing – review and editing. NDA: Data curation, Writing – review and editing, Software. HM: Software, Validation, Writing – review and editing, Data curation. FA: Data curation, Methodology, Writing – review and editing, Formal Analysis. JA: Formal Analysis, Writing – review and editing. AE-M: Formal Analysis, Methodology, Validation, Writing – review and editing
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank King Abdullah Medical Research Center for providing the publication fees for this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1516402/full#supplementary-material
References
Author anonyomus, (1997). CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet 349 (9066), 1641–1649.
Amarenco, P., Lavallée, P. C., Labreuche, J., Albers, G. W., Bornstein, N. M., Canhão, P., et al. (2016). One-year risk of stroke after transient ischemic attack or minor stroke. N. Engl. J. Med. 374 (16), 1533–1542. doi:10.1056/NEJMoa1412981
Amir-Behghadami, M., and Janati, A. (2020). Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 37, 387. doi:10.1136/emermed-2020-209567
Aoki, J., Iguchi, Y., Urabe, T., Yamagami, H., Todo, K., Fujimoto, S., et al. (2019). Acute aspirin plus cilostazol dual therapy for noncardioembolic stroke patients within 48 hours of symptom onset. J. Am. Heart Assoc. 8 (15), e012652. doi:10.1161/JAHA.119.012652
Bath, P. M., Cotton, D., Martin, R. H., Palesch, Y., Yusuf, S., Sacco, R., et al. (2010). Effect of combined aspirin and extended-release dipyridamole versus clopidogrel on functional outcome and recurrence in acute, mild ischemic stroke: PRoFESS subgroup analysis. Stroke 41 (4), 732–738. doi:10.1161/STROKEAHA.109.564906
Bath, P. M., Woodhouse, L. J., Appleton, J. P., Beridze, M., Christensen, H., Dineen, R. A., et al. (2018). Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet 391 (10123), 850–859. doi:10.1016/S0140-6736(17)32849-0
Bhatt, D. L., Fox, K. A. A., Hacke, W., Berger, P. B., Black, H. R., Boden, W. E., et al. (2006). Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N. Engl. J. Med. 354 (16), 1706–1717. doi:10.1056/NEJMoa060989
Brown, D. L., Levine, D. A., Albright, K., Kapral, M. K., Leung, L. Y., Reeves, M. J., et al. (2021). Benefits and risks of dual versus single antiplatelet therapy for secondary stroke prevention: a systematic review for the 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke 52 (7), e468–e479. doi:10.1161/STR.0000000000000377
Chan, B. P., Wong, L. Y. H., Tan, B. Y. Q., Yeo, L. L. L., and Venketasubramanian, N. (2024). Dual antiplatelet therapy for the acute management and long-term secondary prevention of ischemic stroke and transient ischemic attack, an updated review. J. Cardiovasc. Dev. Dis. 11 (2), 48. doi:10.3390/jcdd11020048
Chen, H.-S., Cui, Y., Wang, X. H., Ma, Y. T., Han, J., Duan, Y. J., et al. (2024). Clopidogrel plus aspirin vs aspirin alone in patients with acute mild to moderate stroke: the ATAMIS randomized clinical trial. JAMA neurol. 81 (5), 450–460. doi:10.1001/jamaneurol.2024.0146
Chen, Y., Xiao, Y., Lin, Z., Xiao, X., Bihl, J. C., Zhao, B., et al. (2015). The role of circulating platelets microparticles and platelet parameters in acute ischemic stroke patients. J. Stroke Cerebrovasc. Dis. 24 (10), 2313–2320. doi:10.1016/j.jstrokecerebrovasdis.2015.06.018
Collaboration, A. T. (2002). Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Bmj 324 (7329), 71–86. doi:10.1136/bmj.324.7329.71
Cuisset, T., Deharo, P., Quilici, J., Johnson, T. W., Deffarges, S., Bassez, C., et al. (2017). Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur. heart J. 38 (41), 3070–3078. doi:10.1093/eurheartj/ehx175
Dengler, R., Diener, H. C., Schwartz, A., Grond, M., Schumacher, H., Machnig, T., et al. (2010). Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 9 (2), 159–166. doi:10.1016/S1474-4422(09)70361-8
Diener, H.-C., Bogousslavsky, J., Brass, L. M., Cimminiello, C., Csiba, L., Kaste, M., et al. (2004). Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 364 (9431), 331–337. doi:10.1016/S0140-6736(04)16721-4
Furie, K. L.American Heart Association Stroke Council, Council on Cardiovascular Nursing (2011). Response to letter by naylor regarding article, guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 42 (1), 227–276. doi:10.1161/strokeaha.111.614933
Galli, M., Laudani, C., Occhipinti, G., Spagnolo, M., Gragnano, F., D'Amario, D., et al. (2024). P2Y12 inhibitor monotherapy after short DAPT in acute coronary syndrome: a systematic review and meta-analysis. Eur. Heart Journal-Cardiovascular Pharmacother. 10 (7), 588–598. doi:10.1093/ehjcvp/pvae057
Gao, Y., Chen, W., Pan, Y., Jing, J., Wang, C., Johnston, S. C., et al. (2023). Dual antiplatelet treatment up to 72 hours after ischemic stroke. N. Engl. J. Med. 389 (26), 2413–2424. doi:10.1056/NEJMoa2309137
Geraghty, O., Kennedy, J., Chandratheva, A., Marquardt, L., Buchan, A. M., and Rothwell, P. M. (2010). Preliminary evidence of a high risk of bleeding on aspirin plus clopidogrel in aspirin-naive patients in the acute phase after TIA or minor ischaemic stroke. Cerebrovasc. Dis. 29 (5), 460–467. doi:10.1159/000297961
Gomez, C. R. (2018). Time is brain: the stroke theory of relativity. J. Stroke Cerebrovasc. Dis. 27 (8), 2214–2227. doi:10.1016/j.jstrokecerebrovasdis.2018.04.001
Halkes, P. H., van Gijn, J., Kappelle, L. J., Koudstaal, P. J., and Algra, A. (2006). Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet 367 (9523), 1665–1673. doi:10.1016/S0140-6736(06)68734-5
Han, S. W., Lee, S. S., Kim, S. H., Lee, J. H., Kim, G. S., Kim, O. J., et al. (2013). Effect of cilostazol in acute lacunar infarction based on pulsatility index of transcranial Doppler (ECLIPse): a multicenter, randomized, double-blind, placebo-controlled trial. Eur. Neurol. 69 (1), 33–40. doi:10.1159/000338247
Hankey, G. J., Johnston, S. C., Easton, J. D., Hacke, W., Mas, J. L., et al. (2011). Trial Investigators, et al. (2011). Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. International Journal of Stroke. 6 (1), 3–9.
He, F., Xia, C., Zhang, J. H., Li, X. Q., Zhou, Z. H., Li, F. P., et al. (2015). Clopidogrel plus aspirin versus aspirin alone for preventing early neurological deterioration in patients with acute ischemic stroke. J. Clin. Neurosci. 22 (1), 83–86. doi:10.1016/j.jocn.2014.05.038
Hong, K. S., Lee, S. H., Kim, E. G., Cho, K. H., Chang, D. I., Rha, J. H., et al. (2016). Recurrent ischemic lesions after acute atherothrombotic stroke: clopidogrel plus aspirin versus aspirin alone. Stroke 47 (9), 2323–2330. doi:10.1161/STROKEAHA.115.012293
Investigators, s., Benavente, O. R., Hart, R. G., McClure, L. A., Szychowski, J. M., Coffey, C. S., et al. (2012). Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N. Engl. J. Med. 367 (9), 817–825. doi:10.1056/NEJMoa1204133
Johnston, S. C., Amarenco, P., Denison, H., Evans, S. R., Himmelmann, A., James, S., et al. (2020). Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N. Engl. J. Med. 383 (3), 207–217. doi:10.1056/NEJMoa1916870
Johnston, S. C., Easton, J. D., Farrant, M., Barsan, W., Conwit, R. A., Elm, J. J., et al. (2018). Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N. Engl. J. Med. 379 (3), 215–225. doi:10.1056/nejmoa1800410
Kennedy, J., Hill, M. D., Ryckborst, K. J., Eliasziw, M., Demchuk, A. M., Buchan, A. M., et al. (2007). Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 6 (11), 961–969. doi:10.1016/S1474-4422(07)70250-8
Khan, F., Tritschler, T., Kimpton, M., Wells, P. S., Kearon, C., Weitz, J. I., et al. (2021). Long-term risk for major bleeding during extended oral anticoagulant therapy for first unprovoked venous thromboembolism: a systematic review and meta-analysis. Ann. Intern. Med. 174 (10), 1420–1429. doi:10.7326/M21-1094
Kwon, S. U., Cho, Y. J., Koo, J. S., Bae, H. J., Lee, Y. S., Hong, K. S., et al. (2005). Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke 36 (4), 782–786. doi:10.1161/01.STR.0000157667.06542.b7
Lee, M., Saver, J. L., Hong, K. S., Rao, N. M., Wu, Y. L., and Ovbiagele, B. (2013). Risk–benefit profile of long-term dual-versus single-antiplatelet therapy among patients with ischemic stroke: a systematic review and meta-analysis. Ann. Intern. Med. 159 (7), 463–470. doi:10.7326/0003-4819-159-7-201310010-00006
Markus, H. S., Droste, D. W., Kaps, M., Larrue, V., Lees, K. R., Siebler, M., et al. (2005). Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation 111 (17), 2233–2240. doi:10.1161/01.CIR.0000163561.90680.1C
Nakamura, T., Tsuruta, S., and Uchiyama, S. (2012). Cilostazol combined with aspirin prevents early neurological deterioration in patients with acute ischemic stroke: a pilot study. J. Neurol. Sci. 313 (1-2), 22–26. doi:10.1016/j.jns.2011.09.038
Nishiyama, Y., Kimura, K., Otsuka, T., Toyoda, K., Uchiyama, S., Hoshino, H., et al. (2023). Dual antiplatelet therapy with cilostazol for secondary prevention in lacunar stroke: subanalysis of the CSPS. com trial. Stroke 54 (3), 697–705. doi:10.1161/STROKEAHA.122.039900
Paciaroni, M., Ince, B., Hu, B., Jeng, J. S., Kutluk, K., Liu, L., et al. (2019). Benefits and risks of clopidogrel vs. aspirin monotherapy after recent ischemic stroke: a systematic review and meta-analysis. Cardiovasc. Ther. 2019 (1), 1607181. doi:10.1155/2019/1607181
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 88, 105906. doi:10.1016/j.ijsu.2021.105906
Rothwell, P. M., Algra, A., Chen, Z., Diener, H. C., Norrving, B., and Mehta, Z. (2016). Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet 388 (10042), 365–375. doi:10.1016/S0140-6736(16)30468-8
Rothwell, P. M., Giles, M. F., Chandratheva, A., Marquardt, L., Geraghty, O., Redgrave, J. N. E., et al. (2007). Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 370 (9596), 1432–1442. doi:10.1016/S0140-6736(07)61448-2
Simeone, P., Liani, R., Tripaldi, R., Ciotti, S., Recchiuti, A., Abbonante, V., et al. (2022). Reduced platelet glycoprotein Ibα shedding accelerates thrombopoiesis and COX-1 recovery: implications for aspirin dosing regimen. Haematologica 108 (4), 1141–1157. doi:10.3324/haematol.2022.281006
Tan, S., Xiao, X., Ma, H., Zhang, Z., Chen, J., Ding, L., et al. (2015). Clopidogrel and aspirin versus aspirin alone for stroke prevention: a meta-analysis. PLoS One 10 (8), e0135372. doi:10.1371/journal.pone.0135372
Timp, J. F., Braekkan, S. K., Lijfering, W. M., van Hylckama Vlieg, A., Hansen, J. B., Rosendaal, F. R., et al. (2019). Prediction of recurrent venous thrombosis in all patients with a first venous thrombotic event: the Leiden Thrombosis Recurrence Risk Prediction model (L-TRRiP). PLoS Med. 16 (10), e1002883. doi:10.1371/journal.pmed.1002883
Toyoda, K., Uchiyama, S., Yamaguchi, T., Easton, J. D., Kimura, K., Hoshino, H., et al. (2019). Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial. Lancet Neurology 18 (6), 539–548. doi:10.1016/S1474-4422(19)30148-6
Uchiyama, S., Ikeda, Y., Urano, Y., Horie, Y., and Yamaguchi, T. (2011). The Japanese aggrenox (extended-release dipyridamole plus aspirin) stroke prevention versus aspirin programme (JASAP) study: a randomized, double-blind, controlled trial. Cerebrovasc. Dis. 31 (6), 601–613. doi:10.1159/000327035
Uchiyama, S., Sakai, N., Toi, S., Ezura, M., Okada, Y., Takagi, M., et al. (2015). Final results of cilostazol-aspirin therapy against recurrent stroke with intracranial artery stenosis (CATHARSIS). Cerebrovasc. Dis. extra 5 (1), 1–13. doi:10.1159/000369610
van Rein, N., Heide-Jørgensen, U., Lijfering, W. M., Dekkers, O. M., Sørensen, H. T., and Cannegieter, S. C. (2019). Major bleeding rates in atrial fibrillation patients on single, dual, or triple antithrombotic therapy: results from a Nationwide Danish Cohort Study. Circulation 139 (6), 775–786. doi:10.1161/CIRCULATIONAHA.118.036248
Wang, C., Yi, X., Zhang, B., Liao, D., Lin, J., and Chi, L. (2015). Clopidogrel plus aspirin prevents early neurologic deterioration and improves 6-month outcome in patients with acute large artery atherosclerosis stroke. Clin. Appl. Thrombosis/Hemostasis 21 (5), 453–461. doi:10.1177/1076029614551823
Wang, Y., and Zhao, X. (2013). Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N. Engl. J. Med. 369 (1), 1140–1149. doi:10.1016/j.jvs.2013.08.010
Wong, K. S., Chen, C., Fu, J., Chang, H. M., Suwanwela, N. C., Huang, Y. N., et al. (2010). Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 9 (5), 489–497. doi:10.1016/S1474-4422(10)70060-0
Xian, Y., Federspiel, J. J., Grau-Sepulveda, M., Hernandez, A. F., Schwamm, L. H., Bhatt, D. L., et al. (2016). Risks and benefits associated with prestroke antiplatelet therapy among patients with acute ischemic stroke treated with intravenous tissue plasminogen activator. JAMA neurol. 73 (1), 50–59. doi:10.1001/jamaneurol.2015.3106
Yi, X., Lin, J., Wang, C., Zhang, B., and Chi, W. (2014). A comparative study of dual versus monoantiplatelet therapy in patients with acute large-artery atherosclerosis stroke. J. Stroke Cerebrovasc. Dis. 23 (7), 1975–1981. doi:10.1016/j.jstrokecerebrovasdis.2014.01.022
Zhang, Q., Wang, C., Zheng, M., Li, Y., Li, J., Zhang, L., et al. (2015). Aspirin plus clopidogrel as secondary prevention after stroke or transient ischemic attack: a systematic review and meta-analysis. Cerebrovasc. Dis. 39 (1), 13–22. doi:10.1159/000369778
Zuo, F.-T., Liu, H., Wu, H. J., Su, N., Liu, J. Q., and Dong, A. Q. (2017). The effectiveness and safety of dual antiplatelet therapy in ischemic cerebrovascular disease with intracranial and extracranial arteriostenosis in Chinese patients: a randomized and controlled trail. Medicine 96 (1), e5497. doi:10.1097/MD.0000000000005497
Keywords: dual antiplatelet therapy, efficacy, safety, recurrent ischemic stroke, systematic review and meta-analysis
Citation: Alhawiti NM, Fouda S, Alotaibi ND, Madkhali HA, Alsharef FK, Alhawiti JM and El-Metwally A (2025) Tailoring dual antiplatelet therapy for stroke prevention: a meta-analysis of timing, duration, regimen, and stroke subtypes. Front. Pharmacol. 16:1516402. doi: 10.3389/fphar.2025.1516402
Received: 24 October 2024; Accepted: 03 April 2025;
Published: 24 April 2025.
Edited by:
Bum Joon Kim, University of Ulsan, Republic of KoreaReviewed by:
Ricardo Adrian Nugraha, Faculty of Medicine Universitas Airlangga, IndonesiaWookjin Yang, Asan Medical Center, Republic of Korea
Copyright © 2025 Alhawiti, Fouda, Alotaibi, Madkhali, Alsharef, Alhawiti and El-Metwally. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naif M. Alhawiti, aGF3aXRpbkBrc2F1LWhzLmVkdS5zYQ==, TmFpZi5hbG5hc3NhcnlAZ21haWwuY29t
 Naif M. Alhawiti
Naif M. Alhawiti Sherouk Fouda3
Sherouk Fouda3