- 1Public Center of Experimental Technology, The School of Basic Medical Sciences, Southwest Medical University, Luzhou, China
- 2Department of Thoracic Surgery, Affiliated Hospital of Southwest Medical University, Luzhou, China
- 3State Key Laboratory of Quality Research in Chinese Medicine and Faculty of Chinese Medicine, Macau University of Science and Technology, Macau, China
- 4Rehabilitation Medicine Department, Affiliated Hospital Of Southwest Medical University, Luzhou, China
- 5Department of Traditional Chinese Medicine, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
Background: Regulatory T cells (Tregs), characterized by the transcription factor Foxp3, play a pivotal role in maintaining immune homeostasis, preventing autoimmunity, and contributing to tumor immune evasion. Traditional Chinese Medicine (TCM), with its long history of clinical application, exerts unique regulatory effects on immune responses. However, a comprehensive mechanistic synthesis of TCM-mediated Treg regulation remains lacking.
Methods: We reviewed studies from PubMed up to August 2025, focusing on molecular, cellular, and microbiota-related mechanisms by which TCM modulates Tregs. Identified evidence was synthesized into four major mechanisms and further integrated into three regulatory axes.
Results: TCM regulates Tregs through four mechanisms: (1) Foxp3 expression regulation mechanisms; (2) IL-2 receptor pathway mechanisms; (3) Regulation of other Treg surface molecules; and (4) Gut microbiota modulation mechanisms. These four mechanisms converge into three regulatory axes: the core execution axis (direct Foxp3 control), the upstream regulatory axis (cytokine and receptor crosstalk), and the cross-boundary integration axis (gut microbiota–immune interactions).
Conclusion: This review proposes an integrated framework that refines four regulatory mechanisms into three axes, highlighting the multi-layered and interconnected pathways through which TCM shapes Treg biology. This systems-level perspective provides a theoretical basis for developing TCM-derived strategies in immune-mediated diseases and cancer immunotherapy.
1 Introduction
Regulatory T cells (Tregs) are a specialized subset of CD4+ T cells characterized by the expression of the master transcription factor Foxp3 (Sakaguchi et al., 2008; Sakaguchi et al., 2010; Liston and Gray, 2014). These cells are defined by their potent immunosuppressive functions, which they mediate through the secretion of inhibitory cytokines direct cytolysis, inhibition of dendritic cell maturation, and metabolic disruption (Hatzioannou et al., 2021; Ou et al., 2023). Tregs play a crucial role in maintaining immune homeostasis, preventing autoimmune diseases, regulating inflammatory responses, and facilitating tumor immune evasion (Sakaguchi et al., 2020; Josefowicz et al., 2012). Their unique bidirectional function makes them potential therapeutic targets in various conditions, from autoimmune disorders to cancer (Mohr et al., 2019; Tanaka and Sakaguchi, 2017; Rezaei Kahmini et al., 2022; La, 2018; Li M. et al., 2020; Togashi et al., 2019; Esensten et al., 2018). Approaches aimed at either augmenting (Trotta et al., 2018; Zou et al., 2018) or reducing (Tanaka and Sakaguchi, 2017; Sugiyama et al., 2013) Treg numbers and modulating their suppressive activity represent novel therapeutic strategies already applied in clinical practice (Sakaguchi et al., 2020; Li C. et al., 2020; Liang et al., 2021).
As a natural product, Traditional Chinese Medicine (TCM) has demonstrated unique efficacy in the treatment of various diseases (Luo et al., 2019; Zhong et al., 2022; Chen et al., 2023). Numerous studies (Peng et al., 2022; Chen et al., 2019; Paik et al., 2019; Hoffman et al., 2020; Chen F. et al., 2021; Chen Y. et al., 2024) have demonstrated their ability to regulate various immune cells, including T cells (Ma et al., 2014), macrophages (Bamodu et al., 2019), dendritic cells (DCs) (Han et al., 2022) and natural killer (NK) cells (Lee et al., 2014). Like Tregs, these botanical drugs and their metabolites often display a dual regulatory capacity (Ma et al., 2013), either enhancing or suppressing immune responses depending on the specific disease context (Deng et al., 2022). However, while research has confirmed that TCM can modulate Tregs to alleviate autoimmune damage and enhance antitumor immune responses, a systematic and critical analysis of the specific pharmacological mechanisms by which their metabolites achieve this remains lacking.
Therefore, this review aims to provide a comprehensive and critically-assessed summary of the current evidence on how TCM botanical drugs and their metabolites modulate Tregs. In an era of precision medicine, understanding these specific regulatory mechanisms is crucial for developing more targeted and effective TCM-based immunotherapies. To achieve this, this review systematically integrates existing findings and proposes a hierarchical quad-mechanistic framework (master transcription factors, IL-2 receptor pathways, surface molecules, and gut microbiota) and three interconnected regulatory axes, aiming to provide a clear theoretical basis for future TCM-based immunotherapy research.
2 Search strategy and study selection
To ensure a comprehensive and non-biased collection of relevant literature, this review adhered to a systematic search and selection strategy.
2.1 Search strategy
The international literature search was performed in PubMed. The final search was conducted up to August 2025. We employed a combination of Medical Subject Headings (MeSH) terms and free-text keywords covering three conceptual domains: (1) Traditional Chinese Medicine (TCM) Metabolites: (“Traditional Chinese Medicine” OR “TCM” OR “Chinese herbal medicine” OR “botanical drug”OR “natural product”). (2) Target Immune Cells: (“Regulatory T cells”OR “Tregs” OR “Foxp3”). (3) Mechanism and Disease: (“Mechanism” OR “signaling pathway” OR “immunomodulation” OR “autoimmunity” OR “cancer” OR “inflammation” OR “gut microbiota”). The search was structured using Boolean operators (AND, OR) to ensure maximum sensitivity and relevance. A core search string in PubMed, for example, included: ((“TCM” OR “botanical drug” OR “natural product”) AND (“Treg” OR “regulatory T cells” OR “Foxp3”) AND (“signaling pathway” OR “mechanism”)).
2.2 Study selection process and data extraction
The final selection of included studies followed a rigorous three-step process. (1) Initial screening: two independent reviewers screened all titles and abstracts to remove duplicates and clearly irrelevant papers based on the exclusion criteria. (2) Full-text review: The same two reviewers independently assessed the full text of all potentially relevant articles. Discrepancies were resolved through discussion and consensus, or consultation with a third senior reviewer. (3) Final data collection (eligibility criteria): Only studies that explicitly elucidated the molecular signaling mechanisms underlying the regulation of Treg cells by TCM-derived metabolites were included for qualitative synthesis. A total of 82 studies met the inclusion criteria and were included in the qualitative analysis. For each included publication, the corresponding compound, experimental model type (animal, cellular, or human), dosing information, administration route, and mechanism of Treg regulation are summarized in Tables 1–3. The scientific rigor of each of the 82 included studies was critically appraised and assigned a rating (High/Moderate/Low Rigor). This assessment is presented in Supplementary Table S1. The appraisal criteria focused on: (1) Model Relevance (physiological context); (2) Controls and Completeness (clear dose-response and appropriate control groups); and (3) Mechanistic Depth (clear distinction between Treg phenotype and function reporting). This ensures that the foundation of our synthesis is transparently sourced and critically evaluated.
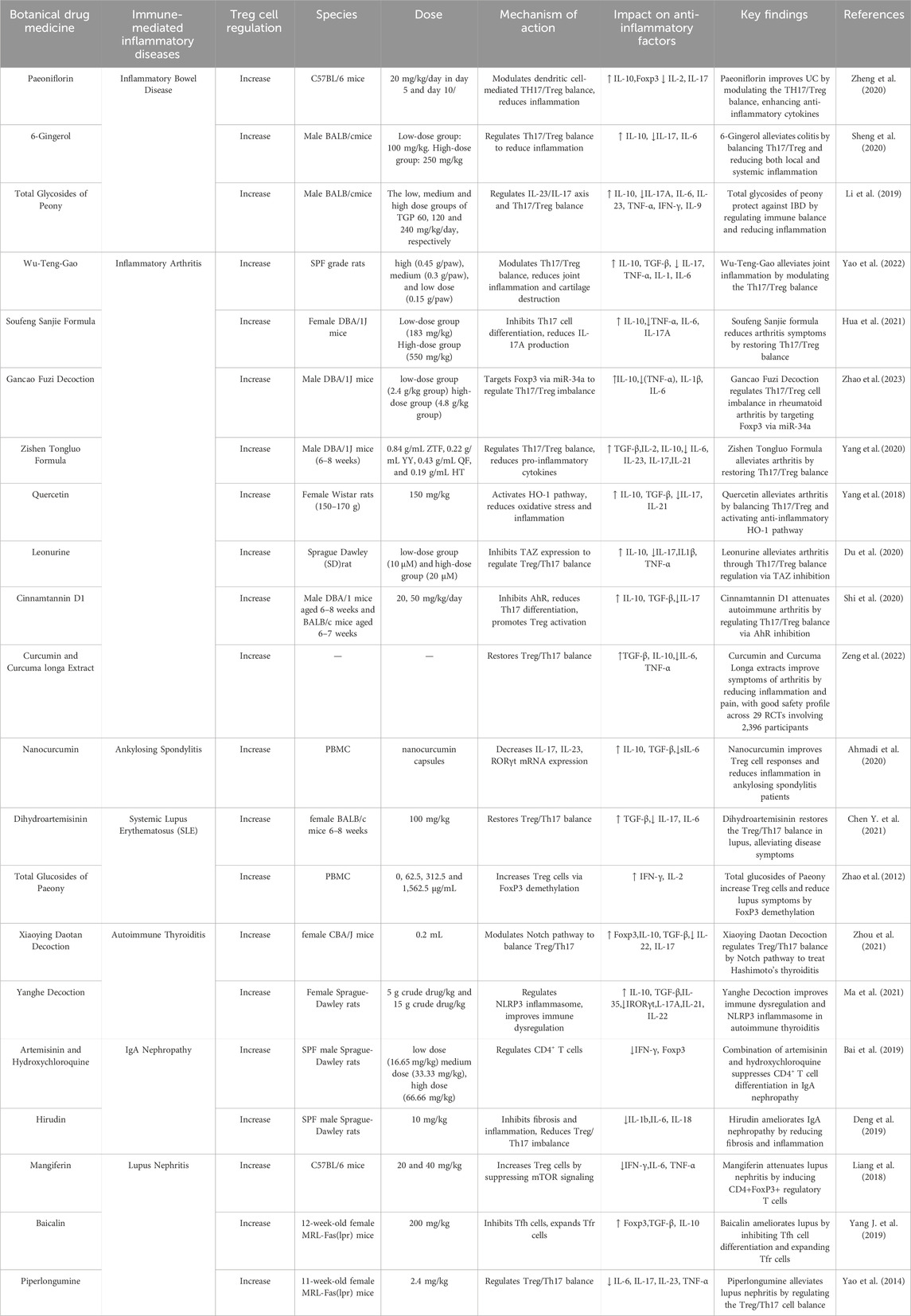
Table 1. TCMs exert therapeutic effects in immune-mediated autoinflammatory diseases by regulating Tregs.
3 TCM exerts therapeutic effects on various diseases by regulating Tregs
3.1 Immune-mediated autoinflammatory diseases
In immune-mediated inflammatory diseases, TCM can modulate Tregs and increase the secretion of anti-inflammatory cytokines (Sakaguchi et al., 2020; Esensten et al., 2018) to restore the Th17 (T helper 17 cell)/Treg balance, thereby suppressing excessive immune responses and alleviating inflammation (Xie et al., 2022; Xu Y. et al., 2020). Paeoniflorin, a monoterpene glycoside extracted from the roots of Paeonia lactiflora, can significantly promote the differentiation of CD4+CD25+Foxp3+ Tregs and suppress the production of Th17 cells, restoring the Th17/Treg balance for the treatment of inflammatory bowel disease (IBD). This leads to a marked reduction in histological scores in colitis models, revealing its potential anti-inflammatory mechanism (Zheng et al., 2020). Similarly, 6-gingerol, which is extracted from the rhizome of ginger, has been shown to reduce inflammation by modulating the Th17/Treg balance in a dextran sulfate sodium-induced colitis mouse model (Sheng et al., 2020). Additionally, total glycosides of peony (TGP) demonstrated significant efficacy in alleviating colitis induced by 2,4,6-trinitrobenzene-sulfonic acid, with higher doses providing effects comparable to sulfasalazine. These findings underscore the therapeutic potential of TGP in the management of IBD (Li et al., 2019).
In immune-mediated arthritis (IA), TCM also regulates immune responses by restoring the Th17/Treg balance, reducing joint inflammation, and preventing tissue damage (Li W. et al., 2023). In a collagen-induced arthritis mouse model, Wu Teng Gao can alleviate joint inflammation and bone destruction by increasing the number of Tregs and modulating the Th17/Treg balance (Yao et al., 2022). Other TCM compound formulations, such as Sou feng San Jie formula (Hua et al., 2021), Gan Cao Fu Zi decoction (Zhao et al., 2023), Zi Shen Tong Luo formula (Yang et al., 2020), as well as extracts like quercetin (Yang et al., 2018), leonurine (Du et al., 2020), and cinnamtannin D1 (Shi et al., 2020), have demonstrated anti-arthritis effects by rebalancing the Th17/Treg ratio. A meta-analysis evaluated the efficacy and safety of Curcumin and Curcuma longa extract in the treatment of arthritis, demonstrating significant improvements in arthritis symptoms and reductions in inflammation levels (Zeng et al., 2022). In patients with ankylosing spondylitis, curcumin significantly increases the number of Tregs, enhances the expression of transforming growth factor-β (TGF-β) and interleukin-10 (IL-10), and suppresses interleukin-6 (IL-6) levels (Ahmadi et al., 2020).
In the context of systemic lupus erythematosus (SLE), dihydroartemisinin (DHA), an extract isolated from the traditional Chinese botanical drug Artemisia annua L., has been shown to induce Treg differentiation, significantly increase the Treg ratio, and stimulate TGF-β secretion. When combined with prednisone, DHA exhibits a synergistic effect, enhancing therapeutic outcomes (Chen Y. et al., 2021). Additionally, TGP treatment significantly increased the proportion and number of Tregs among lupus CD4+ T cells, indicating that TGP may inhibit autoimmunity in SLE patients by promoting Treg differentiation (Zhao et al., 2012).
In autoimmune thyroiditis (AIT), TCM has demonstrated effective therapeutic effects on AIT (Zhou et al., 2024). Xiao Ying Dao tan decoction, which consists of herba Xia KuCao, Fritillaria (Tubeimu), and Bupleurum (Chai Hu), can effectively downregulate Notch protein expression in Hashimoto’s thyroiditis mouse models and thyroiditis cells while upregulating Treg cytokines and downregulating Th17 cytokines to treat Hashimoto’s thyroiditis (Zhou et al., 2021). Yang He decoction (composed of Radix Rehmanniae praeparata, Cortex Cinnamomi, Ephedra sinica stapf, Semen brassicae, Zingiber offcinale Rose, Radix Rhizoma glycyrrhizae, and Colla cornus cervi) alleviates experimental autoimmune thyroiditis in rats by restoring the Th17/Treg imbalance and improving the NLRP3 inflammasome (Ma et al., 2021).
In immunoglobulin A nephropathy (IgAN), the combination of artemisinin and hydroxychloroquine enhances Treg differentiation and reduces IgA immune complex and complement 3 deposition, which significantly improves renal dysfunction (Bai et al., 2019). In a bovine gamma-globulin-induced IgAN mouse model, hirudin reversed the BGG-induced reduction of CD4+CD25+Foxp3+ Tregs, thereby maintaining immune homeostasis to prevent IgAN (56). In lupus nephritis (LN), mangiferin increases the proportion of CD4+Foxp3+ Tregs and inhibits the mTOR/p70S6K pathway in FasL-deficient B6/gld mice, serving as a therapeutic agent for LN (57). Both baicalin (Yang J. et al., 2019) and piperlongumine (Yao et al., 2014) promote CD4+Foxp3+ Tregs accumulation, thereby alleviating LN.
For other immune-related diseases, such as primary Sjögren’s syndrome, systemic sclerosis, and psoriasis, TCM has demonstrated therapeutic effects by reducing Th17 cell levels and promoting Treg generation (Xu Y. et al., 2020; Jun et al., 2021).
In summary, TCM can alleviate autoimmune inflammation by promoting Treg differentiation, restoring the Th17/Treg balance, and enhancing Treg secretion of anti-inflammatory cytokines (Table 1). Moreover, these studies have expanded the application of TCM in treating diseases such as IBD, IA, and SLE. However, what role do TCMs play in treating tumors? How do they affect Tregs?
3.2 Tumors
In the TME, conventional T cells in the blood can be induced to differentiate into Tregs, leading to immunosuppression—one of the key mechanisms of tumor immune evasion (Sakaguchi et al., 2020; Toker and Ohashi, 2019; Campbell and Rudensky, 2020). Therefore, strategies aimed at reducing Treg differentiation or inhibiting Treg function offer promising therapeutic avenues for cancer treatment. TCM has also demonstrated significant antitumor effects in various cancers by modulating Treg activity (Tanaka and Sakaguchi, 2017; Zeng et al., 2020).
3.2.1 Lung cancer
Lung cancer is the most common cancer worldwide and threatens human life and health (Bray et al., 2024). In lung cancer treatment, TCM has demonstrated favorable therapeutic outcomes and synergistic effects, whether used in standard therapy, combination chemotherapy, targeted therapy, or immunotherapy. For example, the Fei Yan Ning Decoction has been found to enhance antitumor immune responses by reducing the proportion of CD4+CD25+ regulatory T cells and downregulating Foxp3 mRNA expression in mice bearing Lewis lung carcinoma (Guo et al., 2012). Fu Zheng Fan Gai Pill, when combined with the chemotherapeutic agent cyclophosphamide, significantly reduces the proportion of CD4+IL-17+ Th17 and CD4+CD25+Foxp3+ Tregs in the spleen and metastatic lesions of mice with Lewis lung cancer. Additionally, it inhibits Foxp3 and RORγt mRNA expression, thereby markedly reducing cancer growth and metastasis by suppressing the SOCS/JAK-STAT pathway and inflammatory cytokine responses (Liu et al., 2014). Both luteolin and apigenin significantly inhibit the proliferation of KRAS-mutant lung cancer cells and downregulate IFN-γ-induced PD-L1 expression, showing synergistic effects when combined with PD-1 inhibitors. In lung cancer mouse models treated with apigenin, the proportion of Tregs in the spleen and blood is reduced, further contributing to the antitumor activity of apigenin (Jiang et al., 2021). The aqueous extract of Taxus chinensis var. mairei, when combined with anti-PD-1 drugs in a mouse model of Lewis lung cancer, reduces the ratio of CD25+Foxp3+ Tregs and enhances synergistic effects by promoting antitumor immune responses (Dai et al., 2022). Berberine significantly inhibits the activation of myeloid-derived suppressor cells (MDSCs) and Tregs in the TME of a lung cancer mouse model, enhancing the immune activity of tumor-infiltrating T cells and shifting the immune microenvironment from immunosuppression to immune activation. Additionally, berberine reduces PD-L1 expression in cancer cells by inhibiting CSN5 deubiquitination, resulting in significant antitumor effects in Lewis tumor-bearing mice (Liu et al., 2020).
3.2.2 Breast cancer
Breast cancer is a leading cause of death among women worldwide, and its treatment has garnered significant attention from TCM researchers (Bray et al., 2024). Studies have indicated that artemisinin inhibits the growth of 4T1 breast cancer cells in vivo by enhancing T-cell activation and inhibiting the immunosuppressive activity of Tregs and MDSCs within the tumor (Cao et al., 2019). Oridonin suppresses Treg differentiation and attenuates their immunosuppressive function by reducing TGF-β receptor protein levels, thereby delaying the progression of triple-negative breast cancer. Moreover, oridonin exhibits synergistic effects with anti-PD-1 therapy, resulting in enhanced tumor regression when used in combination (Guo et al., 2020). Another formulation with synergistic anticancer effects is Aiduqing formula (ADQ), which is composed of oldenlandia diffusa, curcuma zedoaria, astragalus membranaceus and glycyrrhiza uralensis fisch. This formula works synergistically with paclitaxel to inhibit the development of breast cancer (Wang et al., 2018). Further research has shown that ADQ reshapes the immunosuppressive TME of breast cancer and inhibits breast cancer metastasis by reducing Treg differentiation and infiltration through suppression of the NF-κB/Foxp3 pathway. Additionally, ADQ has no significant hepatotoxicity, nephrotoxicity, or hematotoxicity, making it a promising treatment option for breast cancer (Li et al., 2021).
3.2.3 Colon cancer
As one of the top three malignant tumors in terms of global mortality, colon cancer has shown significant treatment responses to the regulatory effects of TCM(64). The botanical drug formula Yi-Yi-Fu-Zi-Bai-Jiang-San (Yi-Yi-ren, Fu-Zi, Bai-Jiang-Cao) reduces the expression of IL-6, CCXL13, and IL-10, thus mediating immune cell regulation. It also modulates the natural gut microbiota in mice and inhibits the proliferation of CD4+CD25+Foxp3+ Tregs that accumulate in the intestinal and mesenteric lymph nodes, indirectly suppressing the growth of colorectal cancer cells (Sui et al., 2020). Baicalein enhances the antitumor effects of immunotherapeutic agents by eliminating TNFR2-related Treg activity. The primary mechanism involves disrupting the TNF-TNFR2 interaction and inhibiting the phosphorylation of the downstream signaling component p38MAPK. In a CT26 colon cancer mouse model, baicalein treatment significantly improved the efficacy of CpG oligodeoxynucleotide tumor immunotherapy (Chen S. et al., 2022). Shenling Baizhu decoction also increases the abundance of the gut microbiota and modulates the tumor immune microenvironment by increasing the number of M1 macrophages while reducing the number of M2 macrophages and Tregs, thereby increasing the efficacy of tislelizumab and producing a synergistic antitumor effect (Deng et al., 2024).
3.2.4 Liver cancer
Liver cancer often has a hidden onset and is typically diagnosed at a late stage (Bray et al., 2024). Various TCMs have been shown to enhance antitumor immunity by m odulating Tregs and inhibiting the progression of liver cancer. Ganoderma lucidum polysaccharides can inhibit the expression of Notch1 and Foxp3 by upregulating the expression of miR-125b, thereby suppressing Treg function and inhibiting the growth of liver cancer cells (Li et al., 2015). Astragalus polysaccharide (APS), the primary active extract of Astragalus, can inhibit the growth and proliferation of CD4+CD25high Tregs and block their migration by suppressing SDF-1 or its receptor via the CXCR4/CXCL12 pathway. APS exerts antitumor effects by restoring the cytokine balance in the TME and reducing the immunosuppressive function of Tregs via Foxp3 mRNA inhibition (Li et al., 2012). Hydroxysafflor Yellow A (HSYA) demonstrated significant anti-hepatocarcinoma immunomodulatory activity. In the Hepa1-6 mouse model, HSYA could downregulate the FOXP3+ level, effectively suppressing the Treg cell ratio. Crucially, HSYA inhibited tumor growth while, in contrast to Cisplatin, did not cause body weight loss, highlighting its potential advantage in terms of lower toxicity (Wang Y. et al., 2024). Radix glycyrrhizae polysaccharide can inhibit the growth of hepatocellular carcinoma by downregulating Tregs in a liver cancer model mice and reducing the serum levels of IL-10 and TGF-β while upregulating the expression of the cytokines IL-2 and IL-12p70 (He et al., 2011). Similarly, Scutellaria barbata D. Don extract (SBE) suppressed liver cancer cell proliferation in vitro in a dose-dependent manner and inhibited tumor growth in H22 liver cancer-bearing mice. This effect is associated with SBE reducing Treg numbers and lowering the expression of IL-10, TGF-β, and IL-17A, while increasing IL-2 and IFN-γ levels in the serum (Kan et al., 2017). Additionally, Dahuang Zhechong Pill treatment in a mouse model of in situ liver cancer increased the number of Th1 cells in the peripheral blood and spleen, with increased IFN-γ secretion activating CD8 T cells and inhibiting Treg production, thereby suppressing hepatocellular carcinoma growth (Chen T. T. et al., 2022).
3.2.5 Other tumors
In addition to several high-incidence tumors, TCMs exert anticancer effects on various other tumors by regulating Tregs. In gastric cancer, modified Bu Zhong Yi Qi decoction reduced the proportions of CD8+PD-1+ T cells and PD-1+ Tregs in the peripheral blood of gastric cancer model mice through the PI3K/AKT pathway. It acts synergistically with 5-fluorouracil to inhibit gastric cancer progression and, as adjuvant therapy postchemotherapy, significantly prolongs the survival time of gastric cancer patients (Xu R. et al., 2020). In bladder cancer, lentinan is an isolated botanical drug metabolite that induces macrophage activation in a bladder cancer mouse model, promoting the proliferation of CD4+ and CD8+ T cells while upregulating the expression of IFN-γ and IL-2. Concurrently, it inhibited the proliferation of MDSCs and Tregs, as well as the expression of anti-inflammatory cytokines IL-10 and TGF-β. In combination with gemcitabine, lentinan activated immunity and had a synergistic effect on the suppression of bladder tumor growth in a mouse model (Sun et al., 2020). In cervical cancer, artesunate inhibits in situ tumor growth and exerts antitumor effects by suppressing PGE2 production in CaSki and HeLa cells and downregulating Foxp3 expression in T cells (Zhang et al., 2014). In melanoma, triptolide inhibits melanoma growth by suppressing the generation of regulatory T cells and the production of several cytokines, such as IL-10, TGF-β, and vascular endothelial growth factor (Liu B. et al., 2013). In head and neck squamous cell carcinoma, curcumin restored the cytotoxic function of effector T cells by modulating the expression of PD-1 and TIM-3 on CD4+or CD8+ T cells and CD4+CD25+Foxp3+ Tregs, promoting the expression of IFN-γ and granzyme B to exert its anticancer effects. Additionally, curcumin, in combination with PD-L1 antibodies, enhanced the cytotoxic activity of CD8+ T cells (Liu et al., 2021).
In summary, TCMs can suppress the differentiation of Tregs within the TME, attenuate their immunosuppressive effects, and enhance the body’s anticancer immune response. Moreover, TCM exhibits synergistic effects when combined with chemotherapy, targeted therapies, and immunotherapeutic agents across various types of tumors. (Table 2).
3.3 Other diseases
TCM exerts its therapeutic effects through the modulation of Tregs not only in autoimmune diseases and tumors, but also in other diseases. In metabolic diseases such as atherosclerosis, Bu Yang Huan Wu Decoction (BYHWD) promoted Treg differentiation, restored the immune balance among CD4+ T cells, regulated lipid metabolism, and inhibited inflammatory responses, thereby demonstrating the potential to increase plaque stability (Chen S. et al., 2021). Similarly, glycyrrhizin increased the expression of IL-10 and IL-2, and enhanced STAT5 phosphorylation in Tregs, which improved lipid metabolism abnormalities in Apoe−/− mice and inhibited vascular inflammation, potentially alleviating atherosclerotic lesions (Ding et al., 2018). In hepatitis, Astragalus polysaccharide (APS), extracted from the roots of the traditional botanical drug medicine Astragalus, significantly increased the production of antigen-specific antibodies, T-cell proliferation, and cytotoxic T lymphocyte activity when co-administered with recombinant hepatitis B surface antigen. Moreover, it reduced the expression of TGF-β and the proportion of CD4+CD25+Foxp3+ Tregs, thereby enhancing both humoral and cellular immune responses to hepatitis B surface antigen vaccination. This makes APS an effective adjuvant for hepatitis B subunit vaccines (Du et al., 2011). Water-extractable polysaccharides of Cistanche deserticola (WPCD) significantly upregulated IgG, IgG1, and IgG2a levels and enhanced the proliferation of T cells and B cells. WPCD also increased the production of IFN-γ and IL-4 in CD4+T cells, as well as the expression of IFN-γ in CD8+T cells, while elevating CD40 and CD80 expression in splenic DCs and decreasing Tregs frequencies. Additionally, the WPCD activated DCs through the TLR4 signaling pathway, thereby promoting both humoral and cellular immune responses and establishing it as a safe and effective vaccine adjuvant (Zhang et al., 2018). In stroke, Ginkgo biloba extract promoted the differentiation of CD4+T cells into Tregs by inhibiting HK2-mediated glycolysis. It increased the expression of the Treg transcription factor Foxp3 and the cytokine IL-10, while decreasing the expression of the Th17 transcription factor RORγt and the Th17-specific cytokine IL-17. This regulation of the Th17/Treg balance improved ischemia/reperfusion injury in mice (Hui et al., 2023). In asthma, Ephedrae herba polysaccharides regulate the imbalance of Th1/Th2 and Th17/Treg, jointly inhibiting inflammation, apoptosis, and reactive oxygen species production in ovalbumin-induced asthmatic rats (Zhang et al., 2022). In pneumonia, an botanical drug formula containing eight plants, compound 511, modulates the balance of Th1/Th2 and Th17/Treg through the PI3K/AKT/mTOR signaling pathway. It reduces Foxp3 and GATA3 mRNA levels and increases STAT3 and T-bet mRNA expression in the spleen, improving immune function and reducing lung inflammation caused by methicillin-resistant Staphylococcus aureus (Li Z. et al., 2023). In lung injury, Jiawei Maxing Shigan Tang (JMST) reduced the number of Tregs in lung tissue and alleviated the degree of pulmonary fibrosis. After intervention with JMST, the expression of Smad2/3, p-Smad2/3, Smad4, TGF-β1, vimentin, and α-SMA was significantly downregulated, whereas the expression of E-cadherin was upregulated. JMST alleviated radiation-induced lung injury by inhibiting epithelial-mesenchymal transition (EMT) through the TGF-β1/Smad pathway mediated by Tregs (Wang M. et al., 2024) (Table 3).
These findings suggest that various TCMs can modulate the quantity and function of Tregs through distinct regulatory mechanisms, providing therapeutic benefits across various diseases and effectively improving pathological conditions. Notably, the therapeutic effects of TCMs on the above diseases—whether autoimmune, neoplastic, or metabolic—are primarily achieved by modulating Treg cells. However, the specific ways in which TCMs regulate Treg cells (e.g., affecting their generation or modifying their function) remain to be systematically clarified. The following section will first focus on the two core aspects of TCM-mediated Treg regulation: the modulation of Treg generation and the regulation of Treg function, laying a foundation for further exploration of the underlying molecular mechanisms.
4 Pharmacological mechanisms of TCM metabolites in regulating Treg cells
It is evident from the literature summarized in the preceding sections that despite the chemical diversity of these metabolites, their regulatory effects primarily converge on two distinct aspects.
4.1 Phenotypic regulation (generation/number)
This involves controlling the differentiation, proliferation, and ultimately the number (frequency) of CD4+CD25+Foxp3+Treg cells, which is primarily driven by the regulation of the master transcription factor Foxp3. (1) Promotion (for immune-mediated inflammatory diseases): botanical drug(s) such as paeoniflorin (Zheng et al., 2020), curcumin (Zeng et al., 2022), mangiferin (Liang et al., 2018), Yun Nan Bai Yao (Ren et al., 2022). Licorice (Guo et al., 2015), ECa 233 (a standardized extract of C. asiatica) (Tawinwung et al., 2021), compound small peptide of Chinese medicine (Cui et al., 2023), promote Foxp3 expression and Treg proliferation. (2) Inhibition (for Tumor-Related Diseases): Conversely, metabolite(s) such as baicalin (Yang J. et al., 2019), berberine (Liu et al., 2020), and the Aiduqing formula (Li et al., 2021), Astragalus polysaccharides (Du et al., 2011), Cistanche deserticola (Zhang et al., 2018), compound 511 (Li Z. et al., 2023), and curcumin (Liao et al., 2018; Yang et al., 2008) inhibit Foxp3 expression and Treg proliferation.
4.2 Functional regulation (competence)
This refers to the adjustment of the Treg suppressive capacity, typically evidenced by the secretion of inhibitory cytokines. Firstly, the Treg suppressive function fundamentally relies on the stable expression of Foxp3 as discussed above (Sakaguchi et al., 2010; Hori et al., 2003). Secondly, it depends on inhibitory cytokines, especially IL-10 and TGF-β, which play a key role in suppressing inflammatory responses and promoting immune tolerance (Li C. et al., 2020; Akdis and Akdis, 2014; Josefowicz et al., 2012). Consequently, studies on TCM-mediated Treg functional regulation frequently examine the expression levels of Foxp3, IL-10, and TGF-β, which are often correlated. (1) Promotion of Cytokine Secretion: TCM metabolite(s) that promote IL-10 and TGF-β secretion include 6-Gingerol (Sheng et al., 2020), Wu Teng Gao (Yao et al., 2022), Soufeng Sanjie Formula (Hua et al. 2021), quercetin (Yang et al., 2018). (2) Inhibition of Cytokine Secretion: Conversely, metabolite like apigenin (Jiang et al., 2021), radix glycyrrhizae polysaccharide (He et al., 2011), and astragalus polysaccharides (Du et al., 2011), an cause Treg cells to reduce TGF-β and IL-10 secretion, thereby attenuating Treg suppressive function. (Table 4).
It is noteworthy that some TCM compounds, such as Curcumin (Zeng et al., 2022; Liao et al., 2018; Yang et al., 2008) exhibit the ability to both promote and inhibit Treg cell proliferation and function. This is not a contradictory result, but rather a demonstration of their bidirectional regulatory role manifesting in different doses and disease backgrounds.
In summary, these results suggest that TCM mainly regulate Tregs proliferation and function by modulating Foxp3 expression and the secretion of inhibitory cytokines.
4.3 Mechanisms of TCM in regulating Treg function
The specific mechanisms of action for different TCM metabolites are diverse. For instance, Gan Cao Fu Zi decoction (Zhao et al., 2023), total glucosides of paeony (Zhao et al., 2012), and oridonin (Guo et al., 2020), directly modulate Foxp3 expression to regulate Treg function, while others achieve this by targeting Treg surface receptors. CTD-1 promotes Treg differentiation and the secretion of inhibitory cytokines through AhR modulation (Shi et al., 2020), scutellarin (Chen S. et al., 2022) weakens Treg function by reducing TNFR2 receptor expression; while berberine (Liu et al., 2020), luteolin (Jiang et al., 2021), and Modified Bu Zhong Yi Qi Decoction (Xu R. et al., 2020) diminish Treg suppressive function by downregulating PD-1 receptor expression. Furthermore, Since IL-2 stimulation is crucial for both Treg differentiation and maintenance of Treg function, the regulation of IL-2R is more important than that of other Treg surface receptors (Ono, 2020). This regulation can also be achieved through the modulation of the gut microbiota. Consequently, based on these distinct mechanisms, this review classifies TCM -mediated Treg regulatory mechanisms into four major categories: (1) Foxp3 expression regulation mechanisms; (2) IL-2 receptor pathway mechanisms; (3) Regulation of other Treg surface molecules; and (4) gut microbiota modulation mechanisms.
4.3.1 Foxp3 expression regulation mechanisms
The marker of T-cell differentiation into Tregs is the expression of Foxp3, and the regulation of Foxp3 expression can be achieved through various mechanisms. Studies have shown that the regulatory element region of the Foxp3 locus contains a promoter and four conserved noncoding sequences (CNS0-3) (Kawakami et al., 2021; Bai et al., 2022). These cis-regulatory elements can be subject to DNA demethylation (Zong et al., 2021), histone modifications (Tao et al., 2007), and negative regulation by miRNAs (Yang L. P. et al., 2019), ensuring the stable transcription and expression of Foxp3. For instance, total glucosides of paeony promote the generation of Tregs by inducing Foxp3 expression through reducing the DNA methylation level of the Foxp3 promoter in lupus CD4 T cells (Zhao et al., 2012). Gancao Fuzi decoction may regulate the imbalance of Th17/Tregs in rheumatoid arthritis by promoting Foxp3 protein expression through the inhibition of miR-34a gene expression (Zhao et al., 2023).
However, how are these signals transmitted into the nucleus to achieve epigenetic regulation of the Foxp3 gene locus? Numerous studies suggest that TCMs transmit signals primarily through the TGF-β/Smad pathway and non-TGF-β/Smad pathways (Chen et al., 2003; Kanamori et al., 2016) (including the T-cell receptor (TCR) signaling pathway (Picca et al., 2006), and NOTCH1 pathway (Asano et al., 2008)), ultimately promoting Treg differentiation.
4.3.1.1 Classical pathway: TGF-β/Smad pathway
TGF-β plays an essential role in the transcription of Foxp3 and the generation of Tregs (Chen and Konkel, 2015). TGF-β binds to the TGF-β receptor (TGF-βR) on the surface of Tregs, activating Smad proteins. CNS1 serves as a platform for Smad2/3 molecules and induces Foxp3 expression in a TGF-β-dependent manner, thus promoting the generation of Tregs (Sanjabi et al., 2017; Wang et al., 2023a). This signaling pathway is the classical pathway for inducing Treg differentiation. Research has indicated that at low concentrations, TGF-β synergizes with IL-6 and IL-21 to promote IL-23R expression, fostering the differentiation of T cells into Th17 cells. Conversely, at high concentrations, TGF-β inhibits IL-23R expression, promoting the differentiation of Foxp3+ Tregs (Zhou et al., 2008). A study on mangiferin also demonstrated that adding MG, TGF-β1, or rapamycin to cell cultures significantly increased the percentage of CD4+Foxp3+ Tregs (Liang et al., 2018). Oridonin inhibits TGF-β1 signaling by promoting the degradation of the TGF-βRI and TGF-βRII proteins and reducing the phosphorylation of the Smad2 and Smad3 proteins. Through this pathway, it inhibits Foxp3 expression, further suppressing Treg polarization (Guo et al., 2020). Researchers have used different polar solvents to extract DaHuang ZheChong Pill, a formula composed of 12 TCMs, into four polar fractions: water-soluble metabolites (PW), ethyl acetate (PE), n-butanol (PB), and petroleum ether (PP). Both PW and PE significantly inhibited Treg differentiation. PE reduces TGF-β mRNA and protein levels, and inhibits the phosphorylation of Smad2 and Smad3, thus suppressing Treg differentiation. The water-soluble fraction (PW), on the other hand, primarily inhibits Treg differentiation by influencing hepatocellular carcinoma cell metabolism, improving TME acidity, and depleting glutamine (Wu et al., 2022). BYHWD also regulates Treg differentiation via this signaling pathway. In vivo experiments have demonstrated that BYHWD can upregulate the expression of TGF-β, Smad2, and Foxp3 in peripheral blood, spleen, and aorta. In vitro experiments confirmed that BYHWD can reverse the inhibition of the Foxp3/TGF-β/Smad2 pathway caused by blockers, thereby promoting Treg differentiation and improving atherosclerosis (Chen S. et al., 2021).
These findings suggest that TCM can modulate Treg generation by promoting or inhibiting Foxp3 expression through the TGF-β/Smad signaling pathway.
4.3.1.2 Non-classical pathway: T-cell receptor signaling and Notch1 signaling
TCR signaling is a key pathway within the non-TGF-β/Smad signaling network. During thymic development, Tregs initiate a series of intracellular signaling events through high-affinity interactions between the TCR and self-antigen-MHC complexes, inducing Foxp3 expression without the requirement for TGF-β costimulation (Picca et al., 2006; Kim and Leonard, 2007). The intracellular regulators activated by TCR signaling can be categorized into two main groups: (1) Transcription factors: nuclear factor kappa-B(NF-κB), hypoxia-inducible factor-1α(HIF-1α), myelocytomatosis oncogene (Myc), nuclear factors of activated T cells (NFAT). (2) Metabolic kinases: phosphoinositide 3-kinase (PI3K), protein kinase B/(Akt)、AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR). These regulators can directly regulate Foxp3 expression (Park and Pan, 2015).
NF-κB is one of the key transcription factors activated by TCR signaling, and upon activation, it induces the classical NF-κB signaling pathway (Fulford et al., 2015). The NF-κB transcription factor family consists of five members: c-Rel, p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), and RelB. The heterodimer of p65 (RelA) or c-Rel with p50 (NF-κB1) activates the classical NF-κB pathway, whereas the heterodimer formed by RelB and p52 (NF-κB2) activates the nonclassical pathway. c-Rel binds to the CNS3 enhancer region, promoting Foxp3 expression and thereby regulating Treg differentiation and function (Hövelmeyer et al., 2022; Oh et al., 2017; Long et al., 2009). The BuShen GuBiao Recipe can regulate Foxp3 expression via the NF-κB signaling pathway (Zhou et al., 2010), whereas the Ai Du Qing Formula can induce the differentiation of CD4+ T cells into Tregs by activating the NF-κB/Foxp3 pathway (Li et al., 2021). Myc and HIF-1α are transcription factors related to glucose metabolism that respond to TCR signaling (Chisolm and Weinmann, 2015). Ginkgo biloba extract promotes Foxp3 expression and Treg differentiation by inhibiting HIF-1α/HK2-mediated glycolysis (Hui et al., 2023). TCR signaling activates the PI3K/Akt/mTOR pathway, which is typically considered a negative regulator. When excessively activated, it suppresses Foxp3 expression, a topic that will be discussed in detail later.
Notch1 signaling represents another crucial non-TGF-β/Smad pathway involved in regulating Foxp3 transcription (Asano et al., 2008). Upon interaction between the Notch ligand and receptor, the Notch intracellular domain (NIC) is released into the cytoplasm. NIC then translocates into the nucleus, where it binds to the transcription factor RBP-J, forming an NIC-RBP-J complex that associates with the Foxp3 promoter to regulate its expression. This pathway exhibits a biphasic regulation: low-intensity Notch signaling activates the Foxp3 promoter via the NICD-RBP-J complex, whereas high-intensity signaling inhibits the promoter through HES (Ou-Yang et al., 2009; Vi et al., 2003). The TCM Xiaoying Daotan Tang and the levothyroxine sodium tablet (standard biomedical treatment) both effectively downregulate Notch1 protein expression. After treatment with Xiaoying Daotan Tang, the serum TGF-β levels in the mice increased significantly, whereas the Foxp3 and IL-10 levels did not. These finding indicate that Xiaoying Daotan Tang regulates the Treg/Th17 balance via the Notch1 signaling pathway and enhances TGF-β expression (Zhou et al., 2021). Ganoderma polysaccharide extract induces the expression of microRNA-125b, with Notch1 being a target of miR-125b. This interaction with the Notch1 receptor, via Notch1 signaling, inhibits Foxp3 expression, reduces regulatory T cell accumulation, and suppresses the growth of hepatocellular carcinoma cells (Li et al., 2015). Resveratrol ultrafine nanoemulsion (Res-mNE) induces Treg differentiation by inhibiting the Notch signaling pathway and activating Foxp3 expression, aiming to reverse the imbalance of Th17/Treg differentiation in immune thrombocytopenia (Cheng et al., 2023).
4.3.2 IL-2 receptor pathway mechanisms
The maintenance of Treg function requires IL-2 stimulation. Tregs possess a high density of IL-2 receptors (IL-2R), enabling them to competitively capture IL-2 and thereby suppress the activation of effector T cells. Additionally, mature Tregs need continuous IL-2 signaling to sustain their survival and suppressive function, which involves three major signaling axes via the IL-2 receptor: the STAT5, PI(3)K, and MAPK/ERK pathways (Sakaguchi et al., 2008; Fan et al., 2018; Ross and Cantrell, 2018). Each of these pathways regulates the development and maintenance of Treg function (Zorn et al., 2006; Osinalde et al., 2011).
4.3.2.1 JAK/STAT5 signaling
Upon signal stimulation, IL-2R activates Janus kinase (JAK), which subsequently activates signal transducer and activator of transcription 5 (STAT5). STAT5 directly binds to the promoter and enhancer elements of Foxp3, inducing its expression and promoting Treg differentiation (Jones et al., 2020; Passerini et al., 2008). Wuwei Xiaodu Drink regulates Foxp3 expression via the IL-2/STAT5 signaling pathway (Huang K. et al., 2022), Bufei Yishen formula induces Treg differentiation by increasing STAT5 phosphorylation levels, upregulating Foxp3 gene expression, and correcting the Th17/Treg imbalance, which in turn improves lung function and alleviates inflammation in chronic obstructive pulmonary disease rats (Peng et al., 2018).
4.3.2.2 PI3K/Akt/mTOR signaling
PI3K can be stimulated by various signals, activating the PI3K/Akt/mTOR pathway. When this pathway is activated via the IL-2 receptor, it induces aerobic glycolysis in Tregs, and elevated glycolysis is detrimental to the stability and suppressive function of the Treg lineage (Fan and Turka, 2018). Moreover, sustained TCR signaling through this pathway also inhibits Foxp3 expression, however, early blockade of TCR stimulation can restore Foxp3 expression (Sauer et al., 2008). Therefore, this pathway negatively regulates Foxp3 expression and Treg function (Luo and Li, 2013). Compound 511 reduces Foxp3 mRNA levels and Treg generation by regulating the PI3K/AKT/mTOR signaling pathway (Li Z. et al., 2023). Glycyrrhiza, along with its active metabolites Gly1 and isoliquiritigenin, promotes Treg generation by attenuating the TCR/Akt/mTOR axis (Guo et al., 2015). Mangiferin induces CD3+ T cell differentiation into CD4+Foxp3+ Tregs and promotes Treg proliferation by inhibiting mTOR and the downstream phosphorylation of P70S6K (Liang et al., 2018).
4.3.2.3 MAPK/ERK signaling
The mitogen-activated protein kinase (MAPK) pathway transmits signals from cell surface receptors to the DNA-binding protein chain in the nucleus (Ras-Raf-MEK-ERK pathway). It plays a regulatory role in processes such as cell proliferation and apoptosis (Gong et al., 2022; Bahar et al., 2023), and can also regulate Tregs. Dendrobium officinale national botanical drug drink reduces Foxp3 expression and modulates the balance between Th17 and Tregs via the SCFAs-GPR41/43-ERK1/2 pathway (Dong et al., 2024). Another study suggested that the p38 MAPK signaling pathway can activate TNF-mediated Treg proliferation, while SB203580, a p38 MAPK inhibitor, blocks LPS-induced Treg expansion and TNF expression in Tregs in vivo, thereby preventing TNF-mediated Treg proliferation (Chen and He, 2017). However, neither of these studies mentioned the involvement of IL-2R in the Treg process. Furthermore, this signaling pathway can be activated by TCRs, but the addition of exogenous IL-2 stimulation during TCR-CD3/CD28 stimulation significantly promotes Treg viability and expansion (Li et al., 2005). Moreover, in the TGF-β signaling pathway, inhibiting ERK activation enhances TGF-β-induced Foxp3 expression and Treg development (Liu H. et al., 2013). These findings suggest that the MAPK/ERK pathway is not a typical pathway regulated via the IL-2 receptor but rather acts as a bridge, integrating various regulatory pathways to modulate Tregs.
4.3.3 Regulation of other Treg surface molecules
Some key signaling axes mediated by cell surface molecules also modulate Treg differentiation and function, with the PD-1/PD-L1 regulatory axis being a prominent example (Gianchecchi and Fierabracci, 2018). For example, berberine specifically binds to CSN5 and inhibits its activity, destabilizing PD-L1 and preventing the activation of Tregs in the TME (69); modified Bu-Zhong-Yi-Qi Decoction inhibits tumor PD-L1 expression via the PI3K/AKT pathway, while also reducing PD-1 expression on Tregs, thereby weakening their immunosuppressive effects (Xu R. et al., 2020); similarly curcumin lowers PD-1 and TIM-3 expression on Tregs, reducing their suppressive function, although the study has not explored the precise regulatory mechanisms involved (Liu et al., 2021). Thus, the PD-1/PD-L1 axis, as a key regulatory axis for Treg suppressive function, can be modulated by various TCMs through different mechanisms. Many other similar cell surface molecules exist. For instance, scutellarin disrupts the TNF-TNFR2 interaction, reducing TNFR2 and Foxp3 expression, thereby lowering the proportion of tumor-infiltrating Tregs (Chen S. et al., 2022). Cinnamtannin D1 promotes Treg differentiation by inhibiting AhR expression and upregulating STAT5/Foxp3 (Shi et al., 2020).
While research on these cell surface molecule-mediated signaling pathways is not as systematic or extensive as that on the Foxp3 and IL-2R signaling pathways mentioned earlier, it still provides new research avenues for TCM researchers. A major emerging perspective is the regulation of the gut microbiota.
4.3.4 Gut microbiota modulation mechanisms
The gut microbiota plays a therapeutic role in various diseases, and TCM may also exert therapeutic effects by regulating the gut microbiome (Wei et al., 2024). Substantial research confirms that the regulatory effect of TCM botanical drugs and formulas on Treg cell differentiation is mediated by alterations to the gut microbiota. Multiple TCM botanical drug formulas have demonstrated this regulatory effect in animal models. For instance, Jiangu granule restores the abundance of the gut microbiota in rats, increase short-chain fatty acid content, reduce the permeability of the colonic epithelium to gut bacteria, increase the proportion of Tregs, and improve bone loss in ovariectomized rats (Sun et al., 2022). Similarly, Tongfu Lifei Decoction (Chen H. et al., 2024) and Modified Gegen Qinlian Decoction (Wang et al., 2021) were both observed to correct the Th17/Treg balance via gut microbiota modulation, alleviating sepsis-related intestinal mucosal injury and DSS-induced acute experimental colitis, respectively. Curcumin, a key metabolite from the botanical drug Curcuma longa L., likewise restored the Th17/Treg steady-state and upregulated the diversity and relative abundance of the gut microbiota in ulcerative colitis (UC) complicated by DM mice, effectively mitigating colitis in the Type 2 Diabetes Mellitus model (Xiao et al., 2022). Qingxie Fuzheng Granules (QFG) also rebalanced the Th17/Treg cell ratio through microbiota modulation in a cancer cachexia model (Jin et al., 2025). This collective evidence demonstrates that restoring the Th17/Treg balance remains a crucial checkpoint for various TCM botanical drugs to exert their therapeutic effects via the gut microbiota.
Focusing on this crucial checkpoint, researchers have also conducted in-depth mechanistic explorations. For example, studies on the anti-cachexia mechanism of QFG showed that it restored microbial balance by modulating dysbiosis (e.g., a decrease in Enterobacteriaceae and an increase in Lactobacillaceae). This action, coupled with the upregulation of tight junction proteins ZO-1, Occludin, and calprotectin, rebalanced the Th17/Treg cell ratio and inhibited IL-6/NF-κB signal transduction, thereby ameliorating cancer cachexia (Jin et al., 2025). This proposed mechanism effectively links the microbiota, intestinal barrier function, and inflammatory signaling pathways. Furthermore, Tuomin Dingchuan Decoction (Hong et al., 2025) was shown to promote Treg cell expansion via a Lactobacillus-dependent mechanism to alleviate asthma, underscoring the critical role of the Lactobacillus genus in Treg cell regulation. Shoutai pill (STP) (Xu et al., 2025) administration restores the gut microbial ecosystem and modulates the maternal Th17/Treg cell ratio through JAK2/STAT3 signaling, thus stabilizing immune tolerance in early pregnancy. The therapeutic efficacy is attributed to metabolites chlorogenic acid, isochlorogenic acid A, and desmoside VI. Licorice water extraction (Sh et al., 2022) significantly improved the species and quantity of probiotics in the gut microbiota of UC mice. LWE reversed the Th17/Treg cell differentiation imbalance, and its mechanism was linked to changes in the colonic expression of ROR-γt and Foxp3 proteins. Different therapeutic dosages of Chimonanthus nitens Oliv. Leaf Granules effectively improved the diversity and relative abundance of the gut microbiota in colitis mice. Specifically, Lachnospiraceae_NK4A136_group and Lachnospiraceae_UCG-006 were significantly enriched at the genus level, which correlated with Treg recruitment and the alleviation of oxidative stress damage (Huang J. Q. et al., 2022). The most direct mechanistic evidence comes from the research on fermented botanical drugs. Fermented Astragalus (FA), derived from the botanical drug Astragalus membranaceus (Fisch.) Bunge fermented with Lactobacillus plantarum, was analyzed to identify differential metabolites such as raffinose, progesterone, and uridine (11 in total). These metabolites were demonstrated to more effectively ameliorate DSS-induced colitis. Compared to unfermented Astragalus, FA-treated mice exhibited more pronounced gene expression of intestinal tight junction proteins (ZO-1, Occludin) and mucin-secreting proteins (MUC2). Concurrently, pro-inflammatory factors (TNF-α, IL-1β, IL-6, IL-17) were downregulated, while anti-inflammatory factors (IL-10, TGF-β) were upregulated. This indicates that these metabolites intervene in the inflammatory state by modulating the balance of Th1/Th2/Th17/Treg-related cytokines (Li et al., 2022). Collectively, these mechanistic studies suggest that the Lactobacillus genus may participate in the regulatory process of Treg cells. However, the exact, molecule-level mechanisms by which the gut microbiota, or specific microbial metabolites, regulate Tregs still warrant further, more systematic exploration.
5 Conclusion, limitation and prospect
5.1 Conclusion
This review highlights the distinctive immunoregulatory effect of TCM on Tregs, which can manifest as either immune-enhancing or immune-suppressive effects depending on the host’s pathological state. This characteristic reflects the fundamental TCM concept of “Fuzheng Quxie” (supporting the upright and dispelling the evil) and “Balancing Yin-Yang,” where the ultimate goal is to restore immune homeostasis rather than induce unidirectional stimulation or suppression. It should be emphasized that this “bidirectional regulation” represents a context-based hypothetical concept rather than a fully established molecular mechanism. Current evidence suggests that disease microenvironment, cytokine milieu, and pharmacological exposure may collectively determine the direction of Treg modulation, yet the key molecular determinants underlying this “directional switching” remain to be elucidated. Future mechanistic and systems-level studies are warranted to validate this hypothesis and identify the critical signaling pathways that mediate the context-dependent immunoregulatory effects of TCM.
Therefore, the “bidirectional regulation” should be regarded as an overarching characteristic of TCM’s holistic immunomodulation, rather than the property of a single compound. Several representative metabolites further exemplify this context-dependent characteristic. For instance, Curcumin and Glycyrrhizin (from Glycyrrhiza uralensis) display opposite effects on Tregs across different disease backgrounds: in autoimmune or inflammatory conditions, they can promote Treg differentiation and Foxp3 expression to restore immune tolerance; whereas in tumor models, they often suppress Treg accumulation in the tumor microenvironment, thereby activating anti-tumor immunity. This difference is not a contradiction but rather reflects the environment-dependent nature and flexibility of TCM’s immunomodulatory action. Understanding this bidirectionality is essential for the rational application of TCM based on specific disease characteristics, enabling the precise regulation of immune balance for optimal therapeutic outcomes (Figure 1).

Figure 1. Regulation of Treg Homeostasis by TCM Metabolites in Disease Contexts Traditional Chinese Medicines (TCMs) exhibit a regulatory effect on Treg cells through various pathways. By enhancing the expression of Foxp3, TCMs promote Treg cell differentiation and the secretion of inhibitory cytokines, thereby contributing to the treatment of immune-related autoimmune inflammation. Conversely, by suppressing Foxp3 expression, TCMs inhibit Treg cell differentiation and the release of inhibitory cytokines, thereby counteracting tumor immune evasion and restraining tumor growth. Created in BioRender.
The central contribution of this review is the systematic integration of the core mechanisms by which TCM botanical drugs and their metabolites modulate Treg cells, culminating in the proposal of a hierarchical four-dimensional mechanistic framework encompassing master transcription factors, IL-2 receptor pathways, other surface molecules, and the gut microbiota. This study strongly confirms that correcting the imbalance of the Th17/Treg ratio is the primary objective of various TCMs. This goal is achieved through a multi-layered intervention on Treg-targeted pathways, which can be summarized into three interconnected axes (Figure 2): (1) Core execution axis: Multi-Pathway Integration for Foxp3 Expression Regulation. This axis represents the most direct and primary molecular mechanism by which TCMs regulate Tregs. It involves the precise control of the master transcription factor Foxp3 through a multi-pathway approach, including both classical and non-classical signaling pathways. Whether TCMs induce Foxp3 expression through the typical TGF-β/Smad pathway (Chen and Konkel, 2015) or precisely modulate Foxp3 expression by intervening in the non-classic`al TCR (Picca et al., 2006), (Fulford et al., 2015) and Notch1 signaling pathways (Asano et al., 2008), this represents the most direct and primary action of TCMs in regulating Tregs. (2) Upstream regulatory axis: Crosstalk between surface molecules/inflammatory pathways and signaling pathways. This axis demonstrates how TCMs indirectly influence Foxp3 expression by modulating the cellular microenvironment and intercellular signals. These signaling pathways do not function in isolation but exhibit significant crosstalk. For example, the PD-1/PD-L1 axis, a surface molecule, participates in Treg cell proliferation by modulating the Notch pathway (Cai et al., 2019). Similarly, Notch1 signaling can enhance the effector function of TGF-β-mediated Tregs, as evidenced by the treatment of Hashimoto’s thyroiditis with Xiaoying Daotan Tang (Zhou et al., 2021). This crosstalk suggests that TCMs can indirectly affect Foxp3 expression and Treg function by regulating surface molecules like PD-1. Furthermore, the crosstalk between hyperactivated Notch3 and the classical NF-κB pathway upregulates Foxp3 expression, thereby enhancing the ability of Tregs to suppress protective anti-tumor immune responses within the tumor microenvironment (TME) (Ferrandino et al., 2018). Likewise, the inhibition of inflammatory pathways (NF-κB and JAK/STAT) creates and maintains a low-inflammatory microenvironment for Treg cells, acting as a synergistic effect. In addition, the IL-2R/STAT5 axis (Huang K. et al., 2022), as a core pathway for maintaining Treg function, is also an important target for TCMs (e.g., Bufei Yishen formula (Peng et al., 2018)). (3)Cross-boundary integration axis: Gut microbiota modulation of immune pathways. The regulatory effect of TCMs on Tregs is achieved by altering the structure and function of the gut microbiota (Wei et al., 2024), establishing a crucial cross-boundary mechanism within the quad-mechanistic framework. Existing research clearly reveals a complex chain of events by which TCMs modulate Tregs via the gut microbiota:1. Microbial effects and metabolic mediation: This mechanism involves TCM metabolites → promoting the abundance of specific probiotics (e.g., Lactobacillus (Hong et al., 2025; Li et al., 2022)) → increasing the concentration of microbial metabolites like short-chain fatty acids (SCFAs) (Dong et al., 2024) → acting on host receptors → regulating Foxp3 expression. Notably, SCFAs act on host cell surface GPRs receptors, serving as a key messenger connecting gut microbial metabolism with Treg differentiation. Microbial product-mediated TLR4/NF-κB Crosstalk: The regulation of the gut microbiota by TCMs essentially involves controlling the stimulation of host cells by microbial products. For instance, E. coli and its products can activate the TLR4/NF-κB signaling pathway, leading to a Th17/Treg imbalance. This suggests that TCMs, by improving the gut microbial structure, reduce the activation of the TLR4/NF-κB axis by microbial-associated molecular patterns (MAMPs), thereby synergistically maintaining Treg-mediated immune tolerance (Wang et al., 2023b). Synergistic Action on Intestinal Barrier: The ability of TCMs (e.g., QFG (155), FA (Li et al., 2022)) to repair intestinal tight junction proteins (upregulating ZO-1 and Occludin) is critical for reducing inflammatory stimulation. This maintenance of intestinal barrier function provides a stable physiological microenvironment for Treg cells, serving as an important synergistic foundation for sustaining their immunosuppressive activity.

Figure 2. Integrative Quad-Mechanistic Framework of TCM Treg Regulation via Core, Upstream, and Cross-Boundary Axes Mechanisms of TCM in regulating Treg cell. This figure illustrates the comprehensive hierarchical quad-mechanistic framework through which Traditional Chinese Medicine (TCM) botanical drugs and their metabolites modulate regulatory T (Treg) cell differentiation and function via three integrated and interactive axes: (1) Core Execution Axis (Direct molecular pathways controlling Foxp3): Classical pathway: TGF-β binds to its receptor (TGF-βR), activating Smad2/3 signaling, a primary inducer of Foxp3 expression. (e.g., Oridonin). Non-classical pathway: Notch1 signaling, where Notch intracellular domain (NIC) forms a complex with RBP-J. Low-intensity NIC-RBP-J promotes Foxp3, while high-intensity signaling inhibits it. (e.g., Mangiferin) (2) Upstream Regulatory Axis (Surface molecule crosstalk): Interactions involving PD-1and AhR affect Treg expansion. (e.g., Cinnamtannin D1, BuZhongYiQi Decoction). IL-2R signaling: IL-2 binding to IL-2R activates STAT5, crucial for Treg survival and suppressive function(e.g.,Wuwei Xiaodu Drink ), Inflammatory and metabolic pathway modulation: Signaling cascades PI3K/Akt/mTOR, and Ras/Raf/MEK/MAPK regulate Foxp3 by influencing glucose metabolism and inflammatory tone. TCM formulas (e.g., Bushen Gubiao Recipe, Ginkgo biloba extract) (3) Cross-Boundary Integration Axis (Gut microbiota): Microbial enrichment and metabolite production promote beneficial gut bacteria (e.g., Lactobacillaceae). Microbiota-immune crosstalk: Microbial products interact with host signaling (e.g., via NF-κB subunits p50 and p65/c-Rel) to regulate Foxp3, linking microbial metabolism to Treg-mediated immune tolerance: TCMs (e.g., JianGu granule, Curcumin). Created in BioRender.
5.2 Limitation
Despite this review systematically integrating the mechanisms of TCM regulating Tregs and proposing a hierarchical framework, we must acknowledge the following three inherent limitations of both current research and this review itself. (1) Weak causality chain in mechanistic validation: Most mechanistic findings in the existing literature remain at the level of correlation, lacking crucial causal validation. Specifically regarding this review’s core innovation—the Cross-boundary integration axis (Gut Microbiota-Treg axis)—studies generally lack functional intervention and verification using gold-standard methods (e.g., fecal microbiota transplantation), leaving the causal link between TCM-mediated microbial modulation and Treg regulation incomplete. (2) Metabolite complexity and insufficient quantitative analysis: Current mechanistic studies face the challenge of TCM’s high metabolite complexity, making it difficult to pinpoint which specific metabolites exert the dominant effects in vivo. Concurrently, research often relies on the analysis of single signaling pathways, lacking the use of multi-omics technologies (e.g., metabolomics, macro-genomics) for the systematic, quantitative resolution of the complex regulatory network. (3) Lack of pharmacokinetic data and clinical translation evidence: The majority of studies fail to fully explore the pharmacokinetics, ADME (absorption, distribution, metabolism, and excretion), and bioavailability of TCM metabolites or their metabolites at target tissues, hindering a complete understanding of the mechanism. More critically, as this review is predominantly based on cell and animal models, there is a scarcity of multicenter, high-quality Randomized Controlled Trials to fully validate the actual efficacy, safety, and dose dependency of TCM-mediated Treg regulation in human disease treatment.
5.3 Prospect
The application of TCM still faces significant challenges due to the chemical diversity and mechanistic complexity of its metabolites. Overcoming these hurdles requires advanced techniques to fully elucidate metabolite-specific effects and safety. To advance TCM-based immunotherapies, future research must prioritize: (1) Targeted discovery and mechanism elucidation of novel metabolites: Focusing on isolating novel, high-potency metabolites and precisely clarifying their specific mechanisms in regulating Tregs to establish a groundwork for drug development. (2) Validation of causal links and quantitative network analysis: Moving beyond correlation by applying gold-standard methods (e.g., fecal microbiota transplantation, sterile mouse models) to validate the causal link within the gut microbiota-Treg axis, alongside utilizing multi-omics technologies for the systematic, quantitative resolution of the complex Treg regulatory network. (3) Synergistic Studies with Immunotherapy and Clinical Translation: Exploring TCM’s synergy with existing immunotherapies (e.g., immune checkpoint inhibitors) to enhance efficacy and reduce side effects by balancing the Treg/Th17 ratio, thus positioning TCM as a safer key metabolite in comprehensive treatment regimens.
Author contributions
CW: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review and editing. M-JL: Methodology, Conceptualization, Project administration, Writing – original draft. YW: Investigation, Writing – review and editing. HL: Formal Analysis, Methodology, Writing – review and editing. Z-ZY: Methodology, Writing – review and editing. W-ZM: Conceptualization, Project administration, Supervision, Writing – review and editing. QY: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This work was supported by Sichuan Provincial Science and Technology Support Program (2025ZNSFSC0659); Luzhou Science and Technology Burean (2023SYF100, 2024SYF128), The Science and Technology Strategic Cooperation Programs of Luzhou Municipal People’s Government and Southwest Medical University (2023LZXNYDJ020; 2023LZXNYDJ029).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1527421/full#supplementary-material
References
Ahmadi, M., Hajialilo, M., Dolati, S., Eghbal-Fard, S., Heydarlou, H., Ghaebi, M., et al. (2020). The effects of nanocurcumin on Treg cell responses and treatment of ankylosing spondylitis patients: a randomized, double-blind, placebo-controlled clinical trial. J. Cell Biochem. 121 (1), 103–110. doi:10.1002/jcb.28901
Akdis, C. A., and Akdis, M. (2014). Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs. J. Clin. Invest 124 (11), 4678–4680. doi:10.1172/JCI78891
Asano, N., Watanabe, T., Kitani, A., Fuss, I. J., and Strober, W. (2008). Notch1 signaling and regulatory T cell function. J. Immunol. 180 (5), 2796–2804. doi:10.4049/jimmunol.180.5.2796
Bahar, M. E., Kim, H. J., and Kim, D. R. (2023). Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Sig Transduct. Target Ther. 8 (1), 455. doi:10.1038/s41392-023-01705-z
Bai, L., Li, H., Li, J., Song, J., Zhou, Y., Liu, B., et al. (2019). Immunosuppressive effect of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via regulating the differentiation of CD4+ T cell subsets in rats. Int. Immunopharmacol. 70, 313–323. doi:10.1016/j.intimp.2019.02.056
Bai, L., Hao, X., Keith, J., and Feng, Y. (2022). DNA methylation in regulatory T cell differentiation and function: challenges and opportunities. Biomolecules 12 (9), 1282. doi:10.3390/biom12091282
Bamodu, O. A., Kuo, K. T., Wang, C. H., Huang, W. C., Wu, A. T. H., Tsai, J. T., et al. (2019). Astragalus polysaccharides (PG2) enhances the M1 polarization of macrophages, functional maturation of dendritic cells, and T cell-mediated anticancer immune responses in patients with lung cancer. Nutrients 11 (10), 2264. doi:10.3390/nu11102264
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74 (3), 229–263. doi:10.3322/caac.21834
Cai, J., Wang, D., Zhang, G., and Guo, X. (2019). The role of PD-1/PD-L1 axis in treg development and function: implications for cancer immunotherapy. Onco Targets Ther. 12, 8437–8445. doi:10.2147/OTT.S221340
Campbell, C., and Rudensky, A. (2020). Roles of regulatory T cells in tissue pathophysiology and metabolism. Cell Metab. 31 (1), 18–25. doi:10.1016/j.cmet.2019.09.010
Cao, Y., Feng, Y. H., Gao, L. W., Li, X. Y., Jin, Q. X., Wang, Y. Y., et al. (2019). Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int. Immunopharmacol. 70, 110–116. doi:10.1016/j.intimp.2019.01.041
Chen, X., and He, T. (2017). The p38 MAPK inhibitor SB203580 abrogates TNF-mediated expansion of regulatory T cells. J. Immunol. 198 (1_Suppl. ment), 141.16. doi:10.4049/jimmunol.198.supp.141.16
Chen, W., and Konkel, J. E. (2015). Development of thymic Foxp3+ regulatory T cells: TGF-β matters. Eur. J. Immunol. 45 (4), 958–965. doi:10.1002/eji.201444999
Chen, W., Jin, W., Hardegen, N., Lei, K., Li, L., Marinos, N., et al. (2003). Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198 (12), 1875–1886. doi:10.1084/jem.20030152
Chen, L., Qi, Y, Qi, Z., Gao, K., Gong, R., Shao, Z., et al. (2019). A comparative study on the effects of different parts of Panax ginseng on the immune activity of cyclophosphamide-induced immunosuppressed mice. Molecules 24 (6), 1096. doi:10.3390/molecules24061096
Chen, F., Li, J., Wang, H., and Ba, Q. (2021). Anti-tumor effects of Chinese medicine compounds by regulating immune cells in microenvironment. Front. Oncol. 11, 746917. doi:10.3389/fonc.2021.746917
Chen, Y., Tao, T., Wang, W., Yang, B., and Cha, X. (2021). Dihydroartemisinin attenuated the symptoms of mice model of systemic lupus erythematosus by restoring the Treg/Th17 balance. Clin. Exp. Pharma Physio 48 (4), 626–633. doi:10.1111/1440-1681.13461
Chen, S., Wang, Y., Liang, C., Li, J., Li, Y., Wu, Q., et al. (2021). Buyang huanwu decoction ameliorates atherosclerosis by regulating TGF-β/Smad2 pathway to promote the differentiation of regulatory T cells. J. Ethnopharmacol. 269, 113724. doi:10.1016/j.jep.2020.113724
Chen, S., Li, R., Chen, Y., Chou, C. K., Zhang, Z., Yang, Y., et al. (2022). Scutellarin enhances anti-tumor immune responses by reducing TNFR2-expressing CD4+Foxp3+ regulatory T cells. Biomed. and Pharmacother. 151, 113187. doi:10.1016/j.biopha.2022.113187
Chen, T. T., Du, S. L., Wang, S. J., Wu, L., and Yin, L. (2022). Dahuang Zhechong pills inhibit liver cancer growth in a mouse model by reversing Treg/Th1 balance. Chin. J. Nat. Med. 20 (2), 102–110. doi:10.1016/S1875-5364(22)60160-2
Chen, J. F., Wu, S. W., Shi, Z. M., and Hu, B. (2023). Traditional Chinese medicine for colorectal cancer treatment: potential targets and mechanisms of action. Chin. Med. 18 (1), 14. doi:10.1186/s13020-023-00719-7
Chen, Y., Fan, W., Zhao, Y., Liu, M., Hu, L., and Zhang, W. (2024). Progress in the regulation of immune cells in the tumor microenvironment by bioactive compounds of traditional Chinese medicine. Molecules 29 (10), 2374. doi:10.3390/molecules29102374
Chen, H., Yu, Z., Qi, Z., Huang, X., and Gao, J. (2024). Tongfu Lifei decoction attenuated sepsis-related intestinal mucosal injury through regulating Th17/Treg balance and modulating gut microbiota. J. Interferon and Cytokine Res. 44 (5), 208–220. doi:10.1089/jir.2024.0001
Cheng, J., Wang, S., Lv, S. Q., Song, Y., and Guo, N. H. (2023). Resveratrol inhibits AhR/Notch axis and reverses Th17/Treg imbalance in purpura by activating Foxp3. Toxicol. Res. (Camb). 12 (3), 381–391. doi:10.1093/toxres/tfad021
Chisolm, D. A., and Weinmann, A. S. (2015). TCR-signaling events in cellular metabolism and specialization. Front. Immunol. 8. doi:10.3389/fimmu.2015.00292
Cui, Y., Zhang, L, Liu, Y., Liu, W., Shi, W., and Bao, Y. (2023). Compound small peptide of Chinese medicine alleviates cyclophosphamide induced immunosuppression in mice by Th17/Treg and jejunum intestinal flora. Front. Microbiol. 14, 1039287. doi:10.3389/fmicb.2023.1039287
Deng, B., Yang, B., Chen, J., Wang, S., Zhang, W., Guo, X., et al. (2022). Gallic acid induces T-helper-1-like Treg cells and strengthens immune checkpoint blockade efficacy. J. Immunother. Cancer 10 (10). e004037corr1. doi:10.1136/jitc-2021-004037
Dai, S., Liu, Y., Zhao, F., Wang, H., Shao, T., Xu, Z., et al. (2022). Aqueous extract of Taxus chinensis var. mairei targeting CD47 enhanced antitumor effects in non-small cell lung cancer. Biomed. and Pharmacother. 154, 113628. doi:10.1016/j.biopha.2022.113628
Deng, F., Zhang, J., Li, Y., Wang, W., Hong, D., Li, G., et al. (2019). Hirudin ameliorates immunoglobulin A nephropathy by inhibition of fibrosis and inflammatory response. Ren. Fail. 41 (1), 104–112. doi:10.1080/0886022X.2019.1583113
Deng, X., Zhang, C., Yang, Y., Wang, J., Ye, X., Gu, J., et al. (2024). Shenling Baizhu decoction (SLBZD) may play a synergistic role of tirelizumab in the treatment of colorectal cancer by influencing the imbalance of colon flora and tumor microenvironment. J. Cancer 15 (1), 30–40. doi:10.7150/jca.88854
Ding, J. W., Luo, C. Y., Wang, X. A., Zhou, T., Zheng, X. X., Zhang, Z. Q., et al. (2018). Glycyrrhizin, a high-mobility group box 1 inhibitor, improves lipid metabolism and suppresses vascular inflammation in apolipoprotein E knockout mice. J. Vasc. Res. 55 (6), 365–377. doi:10.1159/000495310
Dong, Y. J., Zhang, Y. P., Jiang, X. F., Xie, Z. Y., Li, B., Jiang, N. H., et al. (2024). Beneficial effects of Dendrobium officinale national herbal drink on metabolic immune crosstalk via regulate SCFAs-Th17/Treg. Phytomedicine 132, 155816. doi:10.1016/j.phymed.2024.155816
Du, X., Chen, X., Zhao, B., Lv, Y., Zhang, H., Liu, H., et al. (2011). Astragalus polysaccharides enhance the humoral and cellular immune responses of hepatitis B surface antigen vaccination through inhibiting the expression of transforming growth factor β and the frequency of regulatory T cells. FEMS Immunol. Med. Microbiol. 63 (2), 228–235. doi:10.1111/j.1574-695X.2011.00845.x
Du, Y. Y., Chen, Z. X., Liu, M. Y., Liu, Q. P., Lin, C. S., Chu, C. Q., et al. (2020). Leonurine regulates Treg/Th17 balance to attenuate rheumatoid arthritis through inhibition of TAZ expression. Front. Immunol. 11, 556526. doi:10.3389/fimmu.2020.556526
Esensten, J. H., Muller, Y. D., Bluestone, J. A., and Tang, Q. (2018). Regulatory T-cell therapy for autoimmune and autoinflammatory diseases: the next frontier. J. Allergy Clin. Immunol. 142 (6), 1710–1718. doi:10.1016/j.jaci.2018.10.015
Fan, M. Y., and Turka, L. A. (2018). Immunometabolism and PI(3)K signaling as a link between IL-2, Foxp3 expression, and suppressor function in regulatory T cells. Front. Immunol. 9, 69. doi:10.3389/fimmu.2018.00069
Fan, M. Y., Low, J. S., Tanimine, N., Finn, K. K., Priyadharshini, B., Germana, S. K., et al. (2018). Differential roles of IL-2 signaling in developing versus mature tregs. Cell Rep. 25 (5), 1204–1213.e4. doi:10.1016/j.celrep.2018.10.002
Ferrandino, F., Grazioli, P., Bellavia, D., Campese, A. F., Screpanti, I., and Felli, M. P. (2018). Notch and NF-κB: Coach and players of regulatory T-Cell response in cancer. Front. Immunol. 9, 2165. doi:10.3389/fimmu.2018.02165
Fulford, T. S., Ellis, D., and Gerondakis, S. (2015). Understanding the roles of the NF-κB pathway in regulatory T cell development, differentiation and function. Progress in molecular biology and translational science. Elsevier. 57–67. Available online at: https://linkinghub.elsevier.com/retrieve/pii/S1877117315001854
Gianchecchi, E., and Fierabracci, A. (2018). Inhibitory receptors and pathways of lymphocytes: the role of PD-1 in treg development and their involvement in autoimmunity onset and cancer progression. Front. Immunol. 9, 2374. doi:10.3389/fimmu.2018.02374
Gong, T., Si, K., Liu, H., and Zhang, X. (2022). Research advances in the role of MAPK cascade in regulation of cell growth, immunity, inflammation, and cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 47 (12), 1721–1728. doi:10.11817/j.issn.1672-7347.2022.220155
Guo, J., Wang, J., Zheng, Z., Wang, Q., and Dong, C. (2012). Effects of Chinese herbal medicine Feiyanning decoction on the ratio of CD4+CD25+ regulatory T cells and expression of transcription factor Foxp3 in mice bearing Lewis lung carcinoma. Zhong Xi Yi Jie He Xue Bao 10 (5), 584–590. doi:10.3736/jcim20120515
Guo, A., He, D., Xu, H. B., Geng, C. A., and Zhao, J. (2015). Promotion of regulatory T cell induction by immunomodulatory herbal medicine licorice and its two constituents. Sci. Rep. 15 (5), 14046. doi:10.1038/srep14046
Guo, J., Chen, T., Ma, Z., Qiao, C., Yuan, F., Liu, J., et al. (2020). Oridonin inhibits 4T1 tumor growth by suppressing Treg differentiation via TGF-β receptor. Int. Immunopharmacology 88, 106831. doi:10.1016/j.intimp.2020.106831
Han, S., Bi, S., Guo, T., Sun, D., Zou, Y., Wang, L., et al. (2022). Nano co-delivery of Plumbagin and Dihydrotanshinone I reverses immunosuppressive TME of liver cancer. J. Control Release 348, 250–263. doi:10.1016/j.jconrel.2022.05.057
Hatzioannou, A., Boumpas, A., Papadopoulou, M., Papafragkos, I., Varveri, A., Alissafi, T., et al. (2021). Regulatory T cells in autoimmunity and cancer: a duplicitous lifestyle. Front. Immunol. 12, 731947. doi:10.3389/fimmu.2021.731947
He, X., Li, X., Liu, B., Xu, L., Zhao, H., and Lu, A. (2011). Down-regulation of treg cells and Up-Regulation of Th1/Th2 Cytokine ratio were induced by polysaccharide from Radix Glycyrrhizae in H22 hepatocarcinoma bearing mice. Molecules 16 (10), 8343–8352. doi:10.3390/molecules16108343
Hoffman, R. D., Li, C. Y., He, K., Wu, X., He, B. C., He, T. C., et al. (2020). Chinese herbal medicine and its regulatory effects on tumor related T cells. Front. Pharmacol. 11, 492. doi:10.3389/fphar.2020.00492
Hong, Y., Yang, Z., Liu, Z., Li, N., Qin, J., Ge, D., et al. (2025). Tuo-min-ding-chuan decoction alleviates asthma via spatial regulation of gut microbiota and treg cell promotion. Pharm. (Basel). 18 (5), 646. doi:10.3390/ph18050646
Hori, S., Nomura, T., and Sakaguchi, S. (2003). Control of regulatory T cell development by the transcription factor Foxp3. Science 299 (5609), 1057–1061. doi:10.1126/science.1079490
Hövelmeyer, N., Schmidt-Supprian, M., and Ohnmacht, C. (2022). NF-κB in control of regulatory T cell development, identity, and function. J. Mol. Med. Berl. 100 (7), 985–995. doi:10.1007/s00109-022-02215-1
Hua, D., Yang, J., Meng, Q., Ling, Y., Wei, Q., Wang, Z., et al. (2021). Soufeng sanjie formula alleviates collagen-induced arthritis in mice by inhibiting Th17 cell differentiation. Chin. Med. 16 (1), 39. doi:10.1186/s13020-021-00448-9
Huang, K., Ren, H. Y., Lin, B. Y., Liu, Y. Y., and Guo, Q. F. (2022). Protective effects of Wuwei Xiaodu Drink against chronic osteomyelitis through Foxp3+CD25+CD4+ Treg cells via the IL-2/STAT5 signaling pathway. Chin. J. Nat. Med. 20 (3), 185–193. doi:10.1016/S1875-5364(22)60146-8
Huang, J. Q., Wei, S. Y., Cheng, N., Zhong, Y. B., Yu, F. H., Li, M. D., et al. (2022). Chimonanthus nitens oliv. Leaf granule ameliorates DSS-induced acute colitis through treg cell improvement, oxidative stress reduction, and gut microflora modulation. Front. Cell Infect. Microbiol. 12, 907813. doi:10.3389/fcimb.2022.907813
Hui, W., Huang, W., Zheng, Z., Li, Y., Li, P., and Yang, H. (2023). Ginkgo biloba extract promotes Treg differentiation to ameliorate ischemic stroke via inhibition of HIF -1α/HK2 pathway. Phytotherapy Res. 37 (12), 5821–5836. doi:10.1002/ptr.7988
Jiang, Z. B., Wang, W. J., Xu, C., Xie, Y. J., Wang, X. R., Zhang, Y. Z., et al. (2021). Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 1, 36–48. doi:10.1016/j.canlet.2021.05.019
Jin, Y., Lu, L., Hua, H., Chen, B., Fang, W., Lin, K., et al. (2025). Qingxie Fuzheng granules attenuate cancer cachexia by restoring gut microbiota homeostasis and suppressing IL-6/NF-κB signaling in colorectal adenocarcinoma. Hereditas 162 (1), 178. doi:10.1186/s41065-025-00541-1
Jones, D. M., Read, K. A., and Oestreich, K. J. (2020). Dynamic roles for IL-2-STAT5 signaling in effector and regulatory CD4+ T cell populations. J. Immunol. 205 (7), 1721–1730. doi:10.4049/jimmunol.2000612
Josefowicz, S. Z., Lu, L. F., and Rudensky, A. Y. (2012). Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564. doi:10.1146/annurev.immunol.25.022106.141623
jun, W. S., man, He Q., Zhang, Q., Fu, K., lan, Li R., Peng, W., et al. (2021). Traditional Chinese medicine is a useful and promising alternative strategy for treatment of Sjogren’s syndrome: a review. J. Integr. Med. 19 (3), 191–202. doi:10.1016/j.joim.2021.01.008
Kan, X., Zhang, W., You, R., Niu, Y., Guo, J., Xue, J., et al. (2017). Scutellaria barbata D. Don extract inhibits the tumor growth through down-regulating of Treg cells and manipulating Th1/Th17 immune response in hepatoma H22-bearing mice. BMC Complement. Altern. Med. 17 (1), 41. doi:10.1186/s12906-016-1551-9
Kanamori, M., Nakatsukasa, H., Okada, M., Lu, Q., and Yoshimura, A. (2016). Induced regulatory T cells: their development, stability, and applications. Trends Immunol. 37 (11), 803–811. doi:10.1016/j.it.2016.08.012
Kawakami, R., Kitagawa, Y., Chen, K. Y., Arai, M., Ohara, D., Nakamura, Y., et al. (2021). Distinct Foxp3 enhancer elements coordinate development, maintenance, and function of regulatory T cells. Immunity 54 (5), 947–961.e8. doi:10.1016/j.immuni.2021.04.005
Kim, H. P., and Leonard, W. J. (2007). CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J. Exp. Med. 204 (7), 1543–1551. doi:10.1084/jem.20070109
La, C. A. (2018). Tregs in SLE: an update. Curr. Rheumatol. Rep. 20 (2), 6. doi:10.1007/s11926-018-0714-8
Lee, J., Lee, S. J., and Lim, K. T. (2014). ZPDC glycoprotein (24 kDa) induces apoptosis and enhances activity of NK cells in N-nitrosodiethylamine-injected Balb/c. Cell Immunol. 289 (1–2), 1–6. doi:10.1016/j.cellimm.2014.03.002
Li, L., Godfrey, W. R., Porter, S. B., Ge, Y., June, C. H., Blazar, B. R., et al. (2005). CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood 106 (9), 3068–3073. doi:10.1182/blood-2005-04-1531
Li, Q., Bao, J., Li, X., Zhang, T., and Shen, X. (2012). Inhibiting effect of Astragalus polysaccharides on the functions of CD4+CD25 highTreg cells in the tumor microenvironment of human hepatocellular carcinoma. Chin. Med. J. Engl. 125 (5), 786–793. doi:10.3760/CMA.J.ISSN.0366-6999.2012.05.012
Li, A., Shuai, X., Jia, Z., Li, H., Liang, X., Su, D., et al. (2015). Ganoderma lucidum polysaccharide extract inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation and function by inducing microRNA-125b. J. Transl. Med. 13, 100. doi:10.1186/s12967-015-0465-5
Li, Q., Shan, Q., Sang, X., Zhu, R., Chen, X., and Cao, G. (2019). Total glycosides of peony protects against inflammatory bowel disease by regulating IL-23/IL-17 axis and Th17/Treg balance. Am. J. Chin. Med. 47 (1), 177–201. doi:10.1142/S0192415X19500095
Li, M., Zhou, X., Zhou, L., Yu, Z., Fu, L., and Yang, P. (2020). Meta-Analysis of changes in the number and proportion of regulatory T cells in patients with ankylosing spondylitis. Biomed. Res. Int. 2020, 8709804. doi:10.1155/2020/8709804
Li, C., Jiang, P., Wei, S., Xu, X., and Wang, J. (2020). Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 19 (1), 116. doi:10.1186/s12943-020-01234-1
Li, J., Wang, S., Wang, N., Zheng, Y., Yang, B., Wang, X., et al. (2021). Aiduqing formula inhibits breast cancer metastasis by suppressing TAM/CXCL1-induced Treg differentiation and infiltration. Cell Commun. Signal. CCS 19, 89. doi:10.1186/s12964-021-00775-2
Li, J., Ma, Y., Li, X., Wang, Y., Huo, Z., Lin, Y., et al. (2022). Fermented Astragalus and its metabolites regulate inflammatory status and gut microbiota to repair intestinal barrier damage in dextran sulfate sodium-induced ulcerative colitis. Front. Nutr. 9, 1035912. doi:10.3389/fnut.2022.1035912
Li W., W., Yu, L., Li, W., Ge, G., Ma, Y., Xiao, L., et al. (2023). Prevention and treatment of inflammatory arthritis with traditional Chinese medicine: underlying mechanisms based on cell and molecular targets. Ageing Res. Rev. 89, 101981. doi:10.1016/j.arr.2023.101981
Li, Z., Sun, Q., Liu, Q., Mu, X., Wang, H., Zhang, H., et al. (2023). Compound 511 ameliorates MRSA-induced lung injury by attenuating morphine-induced immunosuppression in mice via PI3K/AKT/mTOR pathway. Phytomedicine 108, 154475. doi:10.1016/j.phymed.2022.154475
Liang, C. L., Lu, W., Zhou, J. Y., Chen, Y., Zhang, Q., Liu, H., et al. (2018). Mangiferin Attenuates Murine Lupus nephritis by inducing CD4+Foxp3+ regulatory T cells via suppression of mTOR signaling. Cell Physiol. Biochem. 50 (4), 1560–1573. doi:10.1159/000494654
Liang, Z., Qian, C., Lan, T., Zeng, Q., Lu, W., and Jiang, W. (2021). Regulatory T cells: a new target of Chinese medicine in treatment of atherosclerosis. Chin. J. Integr. Med. 27 (11), 867–873. doi:10.1007/s11655-021-2877-9
Liao, F., Liu, L., Luo, E., and Hu, J. (2018). Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma. Archives Oral Biol. 92, 32–37. doi:10.1016/j.archoralbio.2018.04.015
Liston, A., and Gray, D. H. D. (2014). Homeostatic control of regulatory T cell diversity. Nat. Rev. Immunol. 14 (3), 154–165. doi:10.1038/nri3605
Liu, B., Zhang, H., Li, J., Lu, C., Chen, G., Zhang, G., et al. (2013). Triptolide downregulates Treg cells and the level of IL-10, TGF-β, and VEGF in melanoma-bearing mice. Planta Med. 79 (15), 1401–1407. doi:10.1055/s-0033-1350708
Liu, H., Yao, S., Dann, S. M., Qin, H., Elson, C. O., and Cong, Y. (2013). ERK differentially regulates T h17 and T regcell development and contributes to the pathogenesis of colitis. Eur. J. Immunol. 43 (7), 1716–1726. doi:10.1002/eji.201242889
Liu, S., Wang, X., and wang, Y. G. (2014). Action mechanism of Fuzheng Fangai Pill combined with cyclophosphamide on tumor metastasis and growth. Evi. Compl. Altern. Med. 2014. 494528. doi:10.1155/2014/494528
Liu, Y., Liu, X., Zhang, N., Yin, M., Dong, J., Zeng, Q., et al. (2020). Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm. Sin. B 10 (12), 2299–2312. doi:10.1016/j.apsb.2020.06.014
Liu, L., Lim, M. A., Jung, S. N., Oh, C., Won, H. R., Jin, Y. L., et al. (2021). The effect of Curcumin on multi-level immune checkpoint blockade and T cell dysfunction in head and neck cancer. Phytomedicine 92, 153758. doi:10.1016/j.phymed.2021.153758
Long, M., Park, S. G., Strickland, I., Hayden, M. S., and Ghosh, S. (2009). Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity 31 (6), 921–931. doi:10.1016/j.immuni.2009.09.022
Luo, C. T., and Li, M. O. (2013). Transcriptional control of regulatory T cell development and function. Trends Immunol. 34 (11), 531–539. doi:10.1016/j.it.2013.08.003
Luo, H., Vong, C. T., Chen, H., Gao, Y., Lyu, P., Qiu, L., et al. (2019). Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chin. Med. 14, 48. doi:10.1186/s13020-019-0270-9
Ma, H. D., Deng, Y. R., Tian, Z., and Lian, Z. X. (2013). Traditional Chinese medicine and immune regulation. Clin. Rev. Allerg. Immunol. 44 (3), 229–241. doi:10.1007/s12016-012-8332-0
Ma, P. F., Jiang, J., Gao, C., Cheng, P. P., Li, J. L., Huang, X., et al. (2014). Immunosuppressive effect of compound K on islet transplantation in an STZ-induced diabetic mouse model. Diabetes 63 (10), 3458–3469. doi:10.2337/db14-0012
Ma, B., Chen, D., Liu, Y., Zhao, Z., Wang, J., Zhou, G., et al. (2021). Yanghe decoction suppresses the Experimental autoimmune thyroiditis in rats by improving NLRP3 inflammasome and immune dysregulation. Front. Pharmacol. 12, 645354. doi:10.3389/fphar.2021.645354
Mohr, A., Atif, M., Balderas, R., Gorochov, G., and Miyara, M. (2019). The role of FOXP3+ regulatory T cells in human autoimmune and inflammatory diseases. Clin. Exp. Immunol. 197 (1), 24–35. doi:10.1111/cei.13288
Oh, H., Grinberg-Bleyer, Y., Liao, W., Maloney, D., Wang, P., Wu, Z., et al. (2017). An NF-κB transcription-factor-dependent lineage-specific transcriptional program promotes regulatory T cell identity and function. Immunity 47 (3), 450–465.e5. doi:10.1016/j.immuni.2017.08.010
Ono, M. (2020). Control of regulatory T-cell differentiation and function by T-cell receptor signalling and Foxp3 transcription factor complexes. Immunology 160 (1), 24–37. doi:10.1111/imm.13178
Osinalde, N., Moss, H., Arrizabalaga, O., Omaetxebarria, M. J., Blagoev, B., Zubiaga, A. M., et al. (2011). Interleukin-2 signaling pathway analysis by quantitative phosphoproteomics. J. Proteomics 75 (1), 177–191. doi:10.1016/j.jprot.2011.06.007
Ou, Q., Power, R., and Griffin, M. D. (2023). Revisiting regulatory T cells as modulators of innate immune response and inflammatory diseases. Front. Immunol. 14, 1287465. doi:10.3389/fimmu.2023.1287465
Ou-Yang, H. F., Zhang, H. W., Wu, C. G., Zhang, P., Zhang, J., Li, J. C., et al. (2009). Notch signaling regulates the FOXP3 promoter through RBP-J- and Hes1-dependent mechanisms. Mol. Cell Biochem. 320 (1–2), 109–114. doi:10.1007/s11010-008-9912-4
Paik, S., Choe, J. H., Choi, G. E., Kim, J. E., Kim, J. M., Song, G. Y., et al. (2019). Rg6, a rare ginsenoside, inhibits systemic inflammation through the induction of interleukin-10 and microRNA-146a. Sci. Rep. 9 (1), 4342. doi:10.1038/s41598-019-40690-8
Park, B. V., and Pan, F. (2015). Metabolic regulation of T cell differentiation and function. Mol. Immunol. 68 (2), 497–506. doi:10.1016/j.molimm.2015.07.027
Passerini, L., Allan, S. E., Battaglia, M., Di Nunzio, S., Alstad, A. N., Levings, M. K., et al. (2008). STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25− effector T cells. Int. Immunol. 20 (3), 421–431. doi:10.1093/intimm/dxn002
Peng, Z., Li, J., Tian, Y., Mao, J., Liu, X., Feng, S., et al. (2018). Restoring Th17/Treg balance via modulation of STAT3 and STAT5 activation contributes to the amelioration of chronic obstructive pulmonary disease by Bufei Yishen formula. J. Ethnopharmacology 217, 152–162. doi:10.1016/j.jep.2018.02.023
Peng, X., Tang, F., Yang, Y., Li, T., Hu, X., Li, S., et al. (2022). Bidirectional effects and mechanisms of traditional Chinese medicine. J. Ethnopharmacol. 298, 115578. doi:10.1016/j.jep.2022.115578
Picca, C. C., Larkin, J., Boesteanu, A., Lerman, M. A., Rankin, A. L., and Caton, A. J. (2006). Role of TCR specificity in CD4 + CD25 + regulatory Tcell selection. Immunol. Rev. 212 (1), 74–85. doi:10.1111/j.0105-2896.2006.00416.x
Ren, X., Zhang, M., Zhang, W., Xie, J., Luo, H., Zhang, H.-M., et al. (2022). Yunnan baiyao ameliorates rheumatoid arthritis in rats by shifting the Th17/Treg cell balance and preventing osteoclast differentiation. Evidence-based Complementary Altern. Med. eCAM 2022, 3764444. doi:10.1155/2022/3764444
Rezaei Kahmini, F., Shahgaldi, S., Azimi, M., and Mansourabadi, A. H. (2022). Emerging therapeutic potential of regulatory T (Treg) cells for rheumatoid arthritis: new insights and challenges. Int. Immunopharmacol. 108, 108858. doi:10.1016/j.intimp.2022.108858
Ross, S. H., and Cantrell, D. A. (2018). Signaling and function of Interleukin-2 in T lymphocytes. Annu. Rev. Immunol. 36, 411–433. doi:10.1146/annurev-immunol-042617-053352
Sakaguchi, S., Yamaguchi, T., Nomura, T., and Ono, M. (2008). Regulatory T cells and immune tolerance. Cell. 133 (5), 775–787. doi:10.1016/j.cell.2008.05.009
Sakaguchi, S., Miyara, M., Costantino, C. M., and Hafler, D. A. (2010). FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10 (7), 490–500. doi:10.1038/nri2785
Sakaguchi, S., Mikami, N., Wing, J., Tanaka, A., Ichiyama, K., and Ohkura, N. (2020). Regulatory T cells and human disease. Annu. Review immunology 38, 541–566. doi:10.1146/annurev-immunol-042718-041717
Sanjabi, S., Oh, S. A., and Li, M. O. (2017). Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb. Perspect. Biol. 9 (6), a022236. doi:10.1101/cshperspect.a022236
Sauer, S., Bruno, L., Hertweck, A., Finlay, D., Leleu, M., Spivakov, M., et al. (2008). T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. U. S. A. 105 (22), 7797–7802. doi:10.1073/pnas.0800928105
Shi, G., Kong, J., Wang, Y., Xuan, Z., and Xu, F. (2022). Glycyrrhiza uralensis Fisch. alleviates dextran sulfate sodium-induced colitis in mice through inhibiting of NF-κB signaling pathways and modulating intestinal microbiota. J. Ethnopharmacol. 298, 115640. doi:10.1016/j.jep.2022.115640
Sheng, Y., Wu, T., Dai, Y., Ji, K., Zhong, Y., and Xue, Y. (2020). The effect of 6-gingerol on inflammatory response and Th17/Treg balance in DSS-induced ulcerative colitis mice. Ann. Transl. Med. 8 (7), 442. doi:10.21037/atm.2020.03.141
Shi, C., Zhang, H., Wang, X., Jin, B., Jia, Q., Li, Y., et al. (2020). Cinnamtannin D1 attenuates autoimmune arthritis by regulating the balance of Th17 and treg cells through inhibition of aryl hydrocarbon receptor expression. Pharmacol. Res. 151, 104513. doi:10.1016/j.phrs.2019.104513
Sugiyama, D., Nishikawa, H., Maeda, Y., Nishioka, M., Tanemura, A., Katayama, I., et al. (2013). Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. U. S. A. 110 (44), 17945–17950. doi:10.1073/pnas.1316796110
Sui, H., Zhang, L., Gu, K., Chai, N., Ji, Q., Zhou, L., et al. (2020). YYFZBJS ameliorates colorectal cancer progression in ApcMin/+ mice by remodeling gut microbiota and inhibiting regulatory T-cell generation. Cell Commun. Signal 18 (1), 113. doi:10.1186/s12964-020-00596-9
Sun, M., Bu, R., Zhang, B., Cao, Y., Liu, C., and Zhao, W. (2020). Lentinan inhibits tumor progression by immunomodulation in a mouse model of bladder cancer. Integr. Cancer Ther. 19, 1534735420946823. doi:10.1177/1534735420946823
Sun, P., Zhang, C., Huang, Y., Yang, J., Zhou, F., Zeng, J., et al. (2022). Jiangu granule ameliorated OVX rats bone loss by modulating gut microbiota-SCFAs-Treg/Th17 axis. Biomed. and Pharmacother. 150, 112975. doi:10.1016/j.biopha.2022.112975
Tanaka, A., and Sakaguchi, S. (2017). Regulatory T cells in cancer immunotherapy. Cell Res. 27 (1), 109–118. doi:10.1038/cr.2016.151
Tao, R., De Zoeten, E. F., Özkaynak, E., Chen, C., Wang, L., Porrett, P. M., et al. (2007). Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 13 (11), 1299–1307. doi:10.1038/nm1652
Tawinwung, S., Junsaeng, D., Utthiya, S., and Khemawoot, P. (2021). Immunomodulatory effect of standardized C. asiatica extract on a promotion of regulatory T cells in rats. BMC Complement. Med. Ther. 21 (1), 220. doi:10.1186/s12906-021-03394-z
Togashi, Y., Shitara, K., and Nishikawa, H. (2019). Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat. Rev. Clin. Oncol. 16 (6), 356–371. doi:10.1038/s41571-019-0175-7
Toker, A., and Ohashi, P. S. (2019). Expression of costimulatory and inhibitory receptors in FoxP3+ regulatory T cells within the tumor microenvironment: implications for combination immunotherapy approaches. Adv. Cancer Res. 144, 193–261. doi:10.1016/bs.acr.2019.05.001
Trotta, E., Bessette, P. H., Silveria, S. L., Ely, L. K., Jude, K. M., Le, D. T., et al. (2018). A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat. Med. 24 (7), 1005–1014. doi:10.1038/s41591-018-0070-2
Vigouroux, S., Yvon, E., Wagner, H. J., Biagi, E., Dotti, G., Sili, U., et al. (2003). Induction of antigen-specific regulatory T cells following overexpression of a Notch ligand by human B lymphocytes. J. Virol. 77 (20), 10872–10880. doi:10.1128/jvi.77.20.10872-10880.2003
Wang, N., Yang, B., Zhang, X., Wang, S., Zheng, Y., Li, X., et al. (2018). Network Pharmacology-Based Validation of Caveolin-1 as a Key Mediator of Ai Du Qing Inhibition of Drug Resistance in Breast Cancer. Front. Pharmacol. 9, 1106. doi:10.3389/fphar.2018.01106
Wang, Y., Zhang, J., Xu, L., Ma, J., Lu, M., Ma, J., et al. (2021). Modified Gegen Qinlian Decoction Regulates Treg/Th17 Balance to Ameliorate DSS-Induced Acute Experimental Colitis in Mice by Altering the Gut Microbiota. Front. Pharmacol. 12, 756978. doi:10.3389/fphar.2021.756978
Wang, J., Zhao, X., and Wan, Y. Y. (2023). Intricacies of TGF-β signaling in Treg and Th17 cell biology. Cell Mol. Immunol. 20 (9), 1002–1022. doi:10.1038/s41423-023-01036-7
Wang, J., Yang, J., Xia, W., Zhang, M., Tang, H., Wang, K., et al. (2023b). Escherichia coli enhances Th17/Treg imbalance via TLR4/NF-κB signaling pathway in oral lichen planus. Int. Immunopharmacol. 119, 110175. doi:10.1016/j.intimp.2023.110175
Wang, Y., An, J., Zhou, J., Chang, L., Zhang, Q., and Peng, F. (2024). Hydroxysafflor yellow A: a natural pigment with potential anticancer therapeutic effect. Front. Pharmacol. 15, 1495393. doi:10.3389/fphar.2024.1495393
Wang, M., Feng, Y., Zhang, P., Shen, K., Su, J., Zhong, Y., et al. (2024). Jiawei Maxing Shigan Tang alleviates radiation-induced lung injury via TGF-β1/Smad signaling pathway mediated by regulatory T cells. J. Ethnopharmacol. 320, 117389. doi:10.1016/j.jep.2023.117389
Wei, X., Wang, F., Tan, P., Huang, H., Wang, Z., Xie, J., et al. (2024). The interactions between traditional Chinese medicine and gut microbiota in cancers: current status and future perspectives. Pharmacol. Res. 203, 107148. doi:10.1016/j.phrs.2024.107148
Wu, L., Yang, F. R., Xing, M. L., Lu, S. F., Chen, H. L., Yang, Q. W., et al. (2022). Multi-material basis and multi-mechanisms of the Dahuang Zhechong pill for regulating Treg/Th1 balance in hepatocellular carcinoma. Phytomedicine 100, 154055. doi:10.1016/j.phymed.2022.154055
Xiao, Q. P., Zhong, Y. B., Kang, Z. P., Huang, J. Q., Fang, W. Y., Wei, S. Y., et al. (2022). Curcumin regulates the homeostasis of Th17/Treg and improves the composition of gut microbiota in type 2 diabetic mice with colitis. Phytother. Res. 36 (4), 1708–1723. doi:10.1002/ptr.7404
Xie, F., Xiong, Q., Li, Y., Yao, C., Wu, R., Wang, Q., et al. (2022). Traditional Chinese medicine regulates Th17/Treg balance in treating inflammatory bowel disease. Evidence-based Complementary Altern. Med. eCAM 15. doi:10.1155/2022/6275136
Xu, Y.-Y., Wang, D., Liang, H., Liu, Z., Li, J., Wang, M., et al. (2020). The role of Th17/Treg axis in the traditional Chinese medicine intervention on immune-mediated inflammatory diseases: a systematic review. Am. Journal Chin. Medicine 48, 1–24. doi:10.1142/S0192415X20500275
Xu, R., Wu, J., Zhang, X., Zou, X., Li, C., Wang, H., et al. (2020). Modified Bu-zhong-yi-qi decoction synergies with 5 fluorouracile to inhibits gastric cancer progress via PD-1/PD- L1-dependent T cell immunization. Pharmacol. Res. 152, 104623. doi:10.1016/j.phrs.2019.104623
Xu, W., Li, B., Ge, Z., Li, L., He, X., Chen, X., et al. (2025). Shoutai pill exhibits anti-miscarriage efficacy through tripartite modulation of gut microbiota, systemic metabolism, and maternal-fetal immunity: a multi-omics analysis. Phytomedicine 145, 156991. doi:10.1016/j.phymed.2025.156991
Yang, Y., Paik, J. H., Cho, D., Cho, J. A., and Kim, C. W. (2008). Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. Int. Immunopharmacol. 8 (4), 542–547. doi:10.1016/j.intimp.2007.12.006
Yang, Y., Zhang, X., Xu, M., Wu, X., Zhao, F., and Zhao, C. (2018). Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect. Int. Immunopharmacol. 54, 153–162. doi:10.1016/j.intimp.2017.11.013
Yang, J., Yang, X., Yang, J., and Li, M. (2019). Baicalin ameliorates lupus autoimmunity by inhibiting differentiation of Tfh cells and inducing expansion of Tfr cells. Cell Death Dis. 10 (2), 140. doi:10.1038/s41419-019-1315-9
Yang, L. P., Lin, Q., and Mu, X. L. (2019). MicroRNA-155 and FOXP3 jointly inhibit the migration and invasion of colorectal cancer cells by regulating ZEB2 expression. Eur. Rev. Med. Pharmacol. Sci. 23 (14), 6131–6138. doi:10.26355/eurrev_201907_18426
Yang, P., Qian, F., Zhang, M., Xu, A. L., Wang, X., Jiang, B., et al. (2020). Zishen Tongluo formula ameliorates collagen-induced arthritis in mice by modulation of Th17/Treg balance. J. Ethnopharmacol. 250, 112428. doi:10.1016/j.jep.2019.112428
Yao, L., Chen, H., and Ma, Q. (2014). Piperlongumine alleviates lupus nephritis in MRL-Fas(lpr) mice by regulating the frequency of Th17 and regulatory T cells. Immunol. Lett. 161 (1), 76–80. doi:10.1016/j.imlet.2014.05.001
Yao, X., Wang, Q., Chen, C., Zeng, P., Hou, L., Zhou, J., et al. (2022). Wu-Teng-Gao external treatment improves Th17/Treg balance in rheumatoid arthritis. Evid. Based Complement. Altern. Med. 2022, 5105545. doi:10.1155/2022/5105545
Zeng, G., Jin, L., Ying, Q., Chen, H., Thembinkosi, M. C., Yang, C., et al. (2020). Regulatory T cells in cancer immunotherapy: basic research outcomes and clinical directions. Cancer Manag. Res. 12, 10411–10421. doi:10.2147/CMAR.S265828
Zeng, L., Yang, T., Yang, K., Yu, G., Li, J., Xiang, W., et al. (2022). Efficacy and safety of curcumin and Curcuma longa extract in the treatment of arthritis: a systematic review and meta-analysis of randomized controlled trial. Front. Immunol. 13, 891822. doi:10.3389/fimmu.2022.891822
Zhang, L., Liu, Z., Ye, J., Sha, M., Qian, H., Bu, X., et al. (2014). Artesunate exerts an anti-immunosuppressive effect on cervical cancer by inhibiting PGE 2 production and Foxp3 expression. Cell Biol. Int. 38 (5), 639–646. doi:10.1002/cbin.10244
Zhang, A., Yang, X., Li, Q., Yang, Y., Zhao, G., Wang, B., et al. (2018). Immunostimulatory activity of water-extractable polysaccharides from Cistanche deserticola as a plant adjuvant in vitro and in vivo. PLoS ONE 13. e0191356. doi:10.1371/journal.pone.0191356
Zhang, B., Zeng, M., Zhang, Q., Wang, R., Jia, J., Cao, B., et al. (2022). Ephedrae Herba polysaccharides inhibit the inflammation of ovalbumin induced asthma by regulating Th1/Th2 and Th17/Treg cell immune imbalance. Mol. Immunol. 152, 14–26. doi:10.1016/j.molimm.2022.09.009
Zhao, M., ping, L. G., ni, T. M., yan, L. S., Zhang, J., Cheng, W., et al. (2012). Total glucosides of paeony induces regulatory CD4+CD25+ T cells by increasing Foxp3 demethylation in lupus CD4+ T cells. Clin. Immunol. 143 (2), 180–187. doi:10.1016/j.clim.2012.02.002
Zhao, X., Yi, Y., Jiang, C., Huang, X., Wen, X., Liao, H., et al. (2023). Gancao Fuzi decoction regulates the Th17/Treg cell imbalance in rheumatoid arthritis by targeting Foxp3 via miR-34a. J. Ethnopharmacol. 301, 115837. doi:10.1016/j.jep.2022.115837
Zheng, K., Jia, J., Yan, S., Shen, H., Zhu, P., and Yu, J. (2020). Paeoniflorin ameliorates ulcerative colitis by modulating the dendritic cell-mediated TH17/Treg balance. Inflammopharmacol 28 (6), 1705–1716. doi:10.1007/s10787-020-00722-6
Zhong, H., Han, L., Lu, R. Y., and Wang, Y. (2022). Antifungal and immunomodulatory ingredients from traditional Chinese medicine. Antibiot. (Basel) 12 (1), 48. doi:10.3390/antibiotics12010048
Zhou, L., Lopes, J. E., Chong, M. M. W., Ivanov, I. I., Min, R., Victora, G. D., et al. (2008). TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453 (7192), 236–240. doi:10.1038/nature06878
Zhou, Y., Yu, J., Wu, J., Bai, L., li, H. L., Guang, Z., et al. (2010). Effects of Chinese herbal medicine Bushen Gubiao Recipe on toll-like receptor 4 and CD4(+)CD25(+)foxp3(+) regulatory T cells in mice with recurrent respiratory tract infections. Zhong Xi Yi Jie He Xue Bao 8 (11), 1053–1059. doi:10.3736/jcim20101109
Zhou, Y., Shen, H., Lan, W., Shi, Y., Yao, Q., and Wen, W. (2021). Mechanism of Xiaoying Daotan decoction in treating Hashimoto’s thyroiditis based on the Notch/Treg/Th17 pathway. Ann. Transl. Med. 9 (24), 1760. doi:10.21037/atm-21-6253
Zhou, L., Luo, J. L., Sun, A., Yang, H., Lin, Y., and Han, L. (2024). Clinical efficacy and molecular mechanism of Chinese medicine in the treatment of autoimmune thyroiditis. J. Ethnopharmacol. 323, 117689. doi:10.1016/j.jep.2023.117689
Zong, X., Hao, X., Xu, B., Crawford, J. C., Wright, S., Li, J., et al. (2021). Foxp3 enhancers synergize to maximize regulatory T cell suppressive capacity. J. Exp. Med. 218 (8), e20202415. doi:10.1084/jem.20202415
Zorn, E., Nelson, E. A., Mohseni, M., Porcheray, F., Kim, H., Litsa, D., et al. (2006). IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 108 (5), 1571–1579. doi:10.1182/blood-2006-02-004747
Keywords: traditional Chinese medicine, regulatory T cells, Foxp3, immunomodulation, mechanisms
Citation: Wang C, Liao M-J, Wu Y, Lin H, Ye Z-Z, Ma W-Z and Yuan Q (2025) Pharmacological mechanisms of traditional Chinese medicine metabolites in regulating Treg cells: an integrative pathway review. Front. Pharmacol. 16:1527421. doi: 10.3389/fphar.2025.1527421
Received: 13 November 2024; Accepted: 21 November 2025;
Published: 11 December 2025.
Edited by:
Javier Echeverria, University of Santiago, ChileReviewed by:
Fu Peng, Sichuan University, ChinaTara Fiyouzi, Complutense University of Madrid, Spain
Copyright © 2025 Wang, Liao, Wu, Lin, Ye, Ma and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Yuan, cWluZ3l1YW5Ac3dtdS5lZHUuY24=; Wen-Zhe Ma, d3ptYUBtdXN0LmVkdS5tbw==
 Chao Wang
Chao Wang Ming-Jie Liao1
Ming-Jie Liao1 Wen-Zhe Ma
Wen-Zhe Ma Qing Yuan
Qing Yuan

