Abstract
Objective:
To evaluate the relationship between early statin administration and hemorrhagic transformation (HT) in patients with acute ischemic stroke (AIS) patients following recanalization therapy.
Methods:
This retrospective study included AIS patients who underwent recanalization therapy (intravenous thrombolysis, endovascular treatment, or a combination of both) and categorized them into two groups based on whether statins were administered within 24 h of recanalization therapy. The primary outcome was the occurrence of HT during hospitalization. Secondary outcomes included in-hospital mortality, favorable clinical outcomes (mRS 0–2) at discharge, and neurological improvement 7 ± 2 days post-stroke (defined as a reduction of ≥4 points in NIHSS from baseline).
Results:
A total of 266 AIS patients were analyzed, with 164 (61.7%) receiving statins within 24 h (24 h-statins group). The 24 h-statins group demonstrated a significantly lower risk of HT compared to the non-24 h-statins group (4.9% vs. 21.6%, p < 0.001). In-hospital mortality was also lower in the 24 h-statins group, although not statistically significant (4.9% vs. 10.8%, p = 0.076). Favorable clinical outcomes were more frequent in the 24 h-statins group than in the non-24 h-statins group (60.5% vs. 36.7%, p < 0.001). Furthermore, a greater proportion of patients in the 24 h-statins group showed neurological improvement (51.8% vs. 35.1%, p = 0.019). Adjusted multivariate analysis revealed that early statin use was independently associated with a reduced risk of HT (OR 0.16, 95% CI 0.06–0.49, p < 0.001), as well as a positive association with favorable clinical outcomes (OR 3.63, 95% CI 1.42–9.28, p = 0.007) and neurological improvement (OR 5.23, 95% CI 1.96–13.91, p < 0.001). Subgroup analysis indicated that among patients with elevated low-density lipoprotein (LDL) levels, early statin therapy was linked to a lower risk of HT (P for interaction = 0.018).
Conclusion:
Early statin administration within 24 h of recanalization therapy, in AIS patients was associated with reduced risk of HT and improved neurological outcomes. For patients with elevated LDL levels, early statin therapy may further decrease the risk of HT.
1 Introduction
Recanalization therapy, including intravenous thrombolysis (IVT) and endovascular treatment (EVT), represents the most effective treatment for acute ischemic stroke (AIS) in the ultra-early stage. This approach rapidly restores cerebral blood flow and helps to minimize further neuronal injury (Powers et al., 2019; Herpich and Rincon, 2020). However, recanalization therapy carries an increased risk of hemorrhagic transformation (HT), a serious and often fatal complication following AIS (Maïer et al., 2020; Tian et al., 2022), posing significant threats to patient survival and health.
Statins, or 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are widely recognized for their beneficial role in preventing and managing cerebrovascular diseases (Amarenco et al., 2009; Åivo et al., 2023). Despite limited evidence, continuing or initiating statin therapy during the acute phase of ischemic stroke is considered reasonable (Powers et al., 2019). Most prior studies have suggested that statin use in AIS can improve patient prognosis (Hong and Lee, 2015; Åivo et al., 2023; Gao et al., 2024). However, controversy remains over whether statins increase the risk of HT in AIS patients, particularly among those undergoing recanalization therapy. While some studies indicated that combining statins with recanalization therapy does not elevate, and may even reduce the incidence of HT (Kang et al., 2015; Montaner et al., 2016; Tan et al., 2019). A meta-analysis reported that statins might raise the risk of HT (Hong and Lee, 2015). This increased risk may be linked to low lipid levels weakening endothelial integrity, potentially leading to vessel rupture (Lauer et al., 2015). These conflicting findings highlight the need for further research to evaluate the safety and efficacy of early statin use.
The objective of this study is to examine the relationship between early statin use and clinical outcomes, with a specific focus on HT in AIS patients following recanalization therapy.
2 Methods
This retrospective single-center cohort study was conducted with approval from the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (YE 2020-069).
2.1 Study participants
A total of 279 consecutive AIS patients admitted to Guangdong Provincial Hospital of Chinese Medicine between 1 January 2018 and 30 April 2020 were included. All patients were registered with the National Stroke Center (http://sinosc.chinasdc.cn). The inclusion criteria were: (1) diagnosis of AIS; (2) receiving recanalization therapy, including IVT, EVT, or a combination of both. The exclusion criteria included: (1) missing critical data, such as the timing of IVT or EVT; (2) final diagnosis not being AIS, for example, transient ischemic attack (TIA), internal carotid artery dissection, etc.,; (3) length of hospitalization duration of less than 1 day; (4) HT during the process of recanalization therapy.
2.2 Definition of statin use
The 24 h-statins group comprised patients who received statins within 24 h following recanalization therapy. The non-24 h-statins group included patients who began statin therapy more than 24 h after recanalization therapy or those who did not receive statin therapy during hospitalization.
2.3 Outcome
The primary outcome was HT, assessed based on clinical and CT criteria by experienced neurologists. According to the European Cooperative Acute Stroke Study (ECASS) criteria, hemorrhagic infarction (HI) 1 was defined as small petechiae along the infarct margins, while HI2 was described as confluent petechiae within the infarcted area without space-occupying effects. Parenchymal hemorrhage (PH) 1 was defined as blood clots covering ≤30% of the infarcted area with minimal space-occupying effects, whereas PH2 referred to blood clots affecting >30% of the infarcted area with significant space-occupying effects (Hacke et al., 1998). Secondary outcomes included in-hospital mortality, favorable clinical outcomes (modified Rankin Scale score 0–2 at discharge) (Haggag and Hodgson, 2022), and neurological improvement (defined as an National Institute of Health stroke scale (NIHSS) score improvement of ≥4 points from baseline to 7 ± 2 days after recanalization therapy) (Agarwal et al., 2020).
2.4 Data collection
Data were collected using a standardized Case Report Form, including variables such as age, gender, baseline NIHSS scores, blood pressure, medical history, prior use of antiplatelet or anticoagulants, vascular occlusion site, type of recanalization therapy, the time of recanalization therapy, in-hospital drug therapies, and blood test results.
2.5 Statistical analyses
All statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS) version 18.0 for Windows. Differences between the 24 h-statins group and the non-24 h-statins group were analyzed using the χ2 test, independent sample t-test, or Kruskal–Wallis test, as appropriate. Logistic regression models were utilized to examine the relationship between outcomes and statin use, calculating odds ratios (OR) with 95% confidence intervals (95% CI). In multivariate logistic regression model 1, adjustments were made for previously reported risk factors and all variables with p < 0.05 in univariate analyses. In model 2, adjustments included previously reported risk factors and variables with p < 0.1 in univariate analyses.
3 Results
3.1 Study population
Between 1 January 2018 and 30 April 2020, 279 consecutive AIS patients treated with IVT or EVT were screened. A total of 13 patients were excluded, including 8 with missing critical data, 1 with a length of hospitalization duration less than 1 day, 2 with a final diagnosis other than AIS and two who experienced HT during recanalization therapy. Ultimately, 266 patients were included in the analysis. Among these, 110 (41.4%) patients received IVT, 111 (41.7%) underwent EVT, and 45 (16.9%) received a combination of IVT and EVT. Of the 266 patients, 164 (61.7%) were in the 24 h-statins group, while 102 (38.3%) were in the non-24 h-statins group (Figure 1).
FIGURE 1
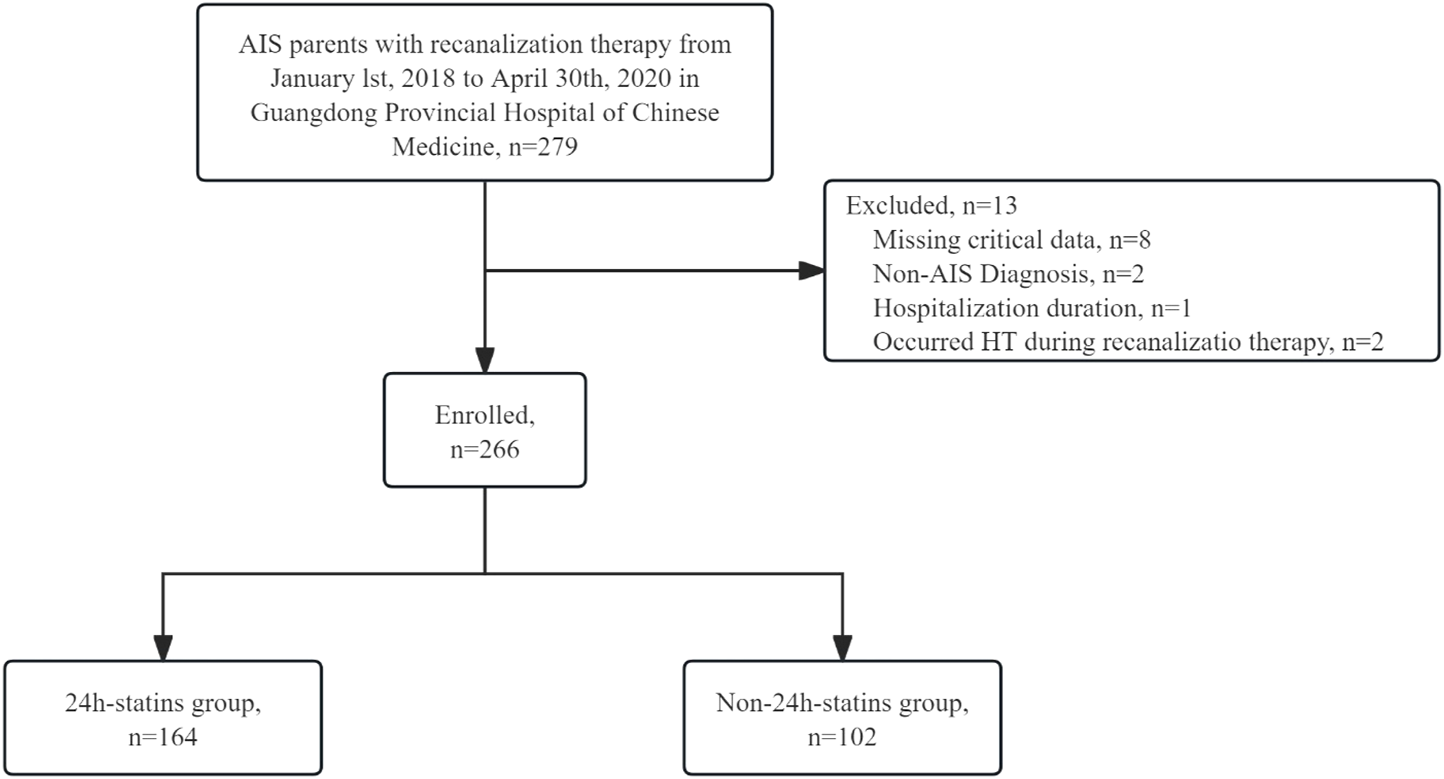
Study population AIS, acute ischemic stroke; HT = hemorrhagic transformation.
3.2 Baseline characteristics
The non-24 h-statins group included more male patients (62.7% vs. 57.3%, p = 0.381) and had a higher mean age (68.2 ± 12.8 years vs. 66.7 ± 11.4 years, p = 0.142) compared to the 24 h-statins group. A significantly higher proportion of patients in the non-24 h-statins group had a history of coronary heart disease (CHD) (21.6% vs. 12.2%, p = 0.041). Additionally, more patients in the non-24 h-statins group underwent combined recanalization therapy (24.5% vs. 12.2%, p = 0.009). Other patient characteristics, including medical history (ischemic stroke, diabetes, hypertension, atrial fibrillation, valvular heart disease), prior use of antiplatelet agents (9.8% vs. 6.1%, p = 0.265), prior use of anticoagulants (7.8% vs. 4.3%, p = 0.219), baseline SBP (152.6 ± 27.3 mmHg vs. 149.2 ± 23.9 mmHg, p = 0.413), baseline NIHSS scores (12.1 ± 7.2 vs. 10.8 ± 7.1, p = 0.104), vascular occlusion site, and antiplatelet or anticoagulant use within 24 h, showed no significant differences between the two groups. However, the non-24 h-statins group had significantly higher fibrinogen (FIB) levels (3.5 ± 1.0 g/L vs. 3.1 ± 1.1 g/L, p = 0.001) and longer activated partial thromboplastin time (APTT) (32.2 ± 6.2 s vs. 28.9 ± 6.4 s, p < 0.001) than the 24 h-statins group. No significant differences were observed in other baseline laboratory values (Table 1).
TABLE 1
| Characteristics | Total n = 266 | 24 h-statins group, n = 164 (61.7%) | Non-24 h-statins group n = 102 (38.3%) |
P value |
|---|---|---|---|---|
| Male, No. (%) | 158 (59.4) | 94 (57.3) | 64 (62.7) | 0.381 |
| Age, mean (SD) | 67.3 ± 12.0 | 66.7 ± 11.4 | 68.2 ± 12.8 | 0.142 |
| Medical history | ||||
| Ischemic stroke, No. (%) | 45 (16.9) | 28 (17.1) | 17 (16.7) | 0.931 |
| Diabetes, No. (%) | 59 (22.2) | 39 (23.8) | 20 (19.6) | 0.426 |
| Hypertension, No. (%) | 158 (59.4) | 95 (57.9) | 63 (61.8) | 0.535 |
| Coronary heart disease, No. (%) | 42 (15.8) | 20 (12.2) | 22 (21.6) | 0.041 |
| Atrial fibrillation, No. (%) | 58 (21.8) | 30 (18.3) | 28 (27.5) | 0.079 |
| Valvular heart disease, No. (%) | 37 (13.9) | 23 (14.0) | 14 (13.7) | 0.945 |
| Prior use of antiplatelet, No. (%) | 20 (7.5) | 10 (6.1) | 10 (9.8) | 0.265 |
| Prior use of anticoagulant, No. (%) | 15 (5.6) | 7 (4.3) | 8 (7.8) | 0.219 |
| SBP, mean (SD), mmHg | 150.5 ± 25.2 | 149.2 ± 23.9 | 152.6 ± 27.3 | 0.413 |
| DBP mean (SD), mmHg | 85.1 ± 14.5 | 85.2 ± 13.7 | 85.0 ± 15.8 | 0.747 |
| Stroke severity | ||||
| Baseline NIHSS score, mean (SD) | 11.3 ± 7.1 | 10.8 ± 7.1 | 12.1 ± 7.2 | 0.104 |
| Vascular occlusion site | ||||
| Anterior circulation, No. (%) | 163 (63.2) | 99 (61.5) | 64 (66.0) | 0.469 |
| Posterior circulation, No. (%) | 68 (26.4) | 42 (26.1) | 26 (26.8) | 0.899 |
| Type of Recanalization therapy | ||||
| IVT, No. (%) | 110 (41.4) | 72 (43.9) | 38 (37.3) | 0.284 |
| EVT, No. (%) | 111 (41.7) | 72 (43.9) | 39 (38.2) | 0.362 |
| IVT + EVT, No. (%) | 45 (16.9) | 20 (12.2) | 25 (24.5) | 0.009 |
| Process time, mean (SD) | ||||
| ONT, mean (SD), min | 149.20 ± 57.91 | 148.75 ± 52.40 | 149.84 ± 65.34 | 0.913 |
| OPT, mean (SD), min | 412.19 ± 245.96 | 440.23 ± 264.72 | 371.68 ± 211.52 | 0.077 |
| Antiplatelet use within 24h, No. (%) | 111 (41.7) | 74 (45.1) | 37 (36.3) | 0.155 |
| Anticoagulant use within 24h, No. (%) | 2 (0.8) | 1 (0.6) | 1 (1.0) | 1.000 |
| Blood test | ||||
| Hb, mean (SD), g/L | 136.3 ± 19.4 | 136.2 ± 19.4 | 136.4 ± 19.5 | 0.754 |
| PLT, mean (SD), /L | 230.8 ± 62.5 | 228.7 ± 57.9 | 234.5 ± 70.0 | 0.750 |
| INR, mean (SD) | 0.990 ± 0.109 | 0.987 ± 0.111 | 0.995 ± 0.106 | 0.367 |
| FIB, mean (SD), g/L | 3.3 ± 1.1 | 3.1 ± 1.1 | 3.5 ± 1.0 | 0.001 |
| APTT, mean (SD), s | 30.1 ± 6.5 | 28.9 ± 6.4 | 32.2 ± 6.2 | <0.001 |
| LDL, mean (SD), mmol/L | 2.9 ± 1.0 | 3.0 ± 1.0 | 2.9 ± 1.0 | 0.801 |
| Glu, mean (SD), mmol/L | 8.5 ± 3.7 | 8.5 ± 3.6 | 8.5 ± 3.9 | 0.676 |
| UA, mean (SD), μmol/L | 342.6 ± 110.1 | 342.2 ± 112.5 | 343.5 ± 106.3 | 0.742 |
| HCY, mean (SD), μmol/L | 11.7 ± 4.4 | 11.9 ± 4.6 | 11.6 ± 4.2 | 0.990 |
Clinical characteristics of the 24 h-statins group and the non-24 h-statins group.
SBP, systolic blood pressure; DBP, diastolic blood pressure; NIHSS, national institute of health stroke scale; IVT, intravenous thrombolysis; EVT, endovascular treatment; ONT, Onset-to-Needle Time, OPT, Onset-to-Procedure Time, Hb, hemoglobin, PLT, platelet; INR, international normalized ratio; FIB, fibrinogen; APTT, activated partial thromboplastin time; LDL, low density lipoprotein, Glu, glucose, UA, uric acid; HCY, homocysteine.
3.3 Comparison of outcomes
The overall occurrence rate of HT was 11.3% (n = 30), with a significantly lower rate observed in the 24 h-statins group (4.9%) compared to the non-24 h-statins group (21.6%, p < 0.001). Among the 19 patients (7.1%) who died before discharge, 8 (4.9%) were in the 24 h-statins group, while 11 (10.8%) were in the non-24 h-statins group (p = 0.076). A total of 134 patients (51.5%) achieved favorable clinical outcomes at discharge, with a higher proportion in the 24 h-statins group compared to the non-24 h-statins group (60.5% vs. 36.7%, p < 0.001). Among 98 patients (45.8%) who demonstrated neurological improvement 7 ± 2 days post-recanalization therapy, 71 (51.8%) were in the 24 h-statins group, compared to 27 (35.1%) in the non-24 h-statins group (p = 0.019) (Table 2).
TABLE 2
| Outcome, no. (%) | Total | 24 h-statins group (n = 164) | Non-24 h-statins group (n = 102) | P value | OR (95% CI) |
|---|---|---|---|---|---|
| Hemorrhagic transformation | 30 (11.3) | 8 (4.9) | 22 (21.6) | <0.001 | 0.2 (0.1–0.4) |
| HI | 10 (33.3) | 5 (62.5) | 5 (22.7) | 0.051 | 5.7 (1.0, 32.4) |
| HI1 | 2 (6.7) | 1 (12.5) | 1 (4.5) | 0.458 | 3.0 (0.2, 54.6) |
| HI2 | 8 (26.7) | 4 (50.0) | 4 (18.2) | 0.094 | 4.5 (0.8, 26.1) |
| PH | 20 (66.7) | 3 (37.5) | 17 (77.2) | 0.051 | 0.2 (0.0, 1.0) |
| PH1 | 5 (16.7) | 0 (0.0) | 5 (22.7) | 0.996 | 0.0 (0.0, Inf) |
| PH2 | 15 (50.0) | 3 (37.5) | 12 (54.5) | 0.413 | 0.5 (0.1, 2.6) |
| In-hospital mortality | 19 (7.1) | 8 (4.9) | 11 (10.8) | 0.076 | 0.4 (0.2–1.1) |
| Favorable clinical outcome | 134 (51.5) | 98 (60.5) | 36 (36.7) | <0.001 | 2.6 (1.6–4.4) |
| Neurologic improvement | 98 (45.8) | 71 (51.8) | 27 (35.1) | 0.019 | 2.0 (1.1–3.5) |
Outcomes of the 24 h-statins group and the non-24 h statins group.
CI, confidence interval; OR, odds ratio; HI, hemorrhagic infarction; PH, parenchymal hemorrhage.
3.4 Univariate analysis
Univariate analysis identified baseline NIHSS scores (OR 1.06, 95% CI 1.00–1.11) as being associated with an increased risk of HT, while IVT was associated with a lower risk of HT (OR 0.2, 95% CI 0.1–0.7). Baseline NIHSS scores (OR 1.1, 95% CI 1.0–1.2), EVT (OR 3.3, 95% CI 1.2–9.0), and female sex (male: OR 0.4, 95% CI 0.1–1.0) were associated with higher mortality. Regarding favorable clinical outcomes, baseline NIHSS scores (OR 0.8, 95% CI 0.8–0.9), hypertension (OR 0.6, 95% CI 0.3–0.9), CHD (OR 0.4, 95% CI 0.2–0.9), prior antiplatelet use (OR 0.3, 95% CI 0.1–1.0), anterior circulation stenosis (OR 0.4, 95% CI 0.2–0.6), posterior circulation stenosis (OR 0.5, 95% CI 0.3–0.9), EVT (OR 0.3, 95% CI 0.2–0.5), FIB levels (OR 0.7, 95% CI 0.5–0.9), and glucose levels (OR 0.9, 95% CI 0.8–1.0) were identified as risk factors. IVT (OR 3.9, 95% CI 2.3–6.6) emerged as a protective factor. For neurological improvement, higher age (OR 1.02, 95% CI 1.00–1.05), a medical history of atrial fibrillation (AF) (OR 2.0, 95% CI 1.1–3.8), higher baseline NIHSS scores (OR 1.15, 95% CI 1.09–1.20), and anterior circulation stenosis (OR 2.0, 95% CI 1.1–3.5) were associated with greater improvement. Patients who received IVT (OR 0.5, 95% CI 0.3–0.9) were associated with poorer improvement (Table 3).
TABLE 3
| Characteristics | Hemorrhagic transformation | In-hospital mortality | Favorable outcome | Neurologic improvement | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Demographic data | ||||||||
| Male | 0.6 (0.3–1.2) | 0.135 | 0.4 (0.1–1.0) | 0.044 | 1.3 (0.8–2.2) | 0.243 | 0.8 (0.5–1.4) | 0.477 |
| Age | 1.00 (0.97–1.03) | 0.868 | 1.03 (0.98–1.07) | 0.227 | 0.98 (0.96–1.00) | 0.055 | 1.02 (1.00–1.05) | 0.045 |
| Medical history | ||||||||
| Ischemic stroke | 1.3 (0.5–3.3) | 0.633 | 0.9 (0.3–3.3) | 0.892 | 0.7 (0.3–1.3) | 0.221 | 1.7 (0.8–3.5) | 0.144 |
| Diabetes | 1.6 (0.7–3.7) | 0.277 | 2.2 (0.8–5.8) | 0.118 | 0.7 (0.4–1.3) | 0.245 | 0.7 (0.4–1.3) | 0.263 |
| Hypertension | 1.4 (0.6–3.2) | 0.391 | 1.5 (0.6–4.1) | 0.409 | 0.6 (0.3–0.9) | 0.026 | 1.5 (0.9–2.6) | 0.148 |
| Coronary heart disease | 1.1 (0.4–3.0) | 0.889 | 1.0 (0.3–3.6) | 1.000 | 0.4 (0.2–0.9) | 0.017 | 1.4 (0.7–3.1) | 0.373 |
| Atrial fibrillation | 1.4 (0.6–3.2) | 0.495 | 1.7 (0.6–4.8) | 0.289 | 0.7 (0.4–1.3) | 0.245 | 2.0 (1.1–3.8) | 0.034 |
| Valvular heart disease | 1.7 (0.6–4.4) | 0.310 | 1.2 (0.3–4.2) | 0.806 | 0.7 (0.4–1.5) | 0.360 | 1.4 (0.7–2.9) | 0.363 |
| Prior use of antiplatelet | 0.9 (0.2–3.9) | 0.851 | 1.5 (0.3–7.0) | 0.608 | 0.3 (0.1–1.0) | 0.045 | 1.2 (0.4–3.5) | 0.744 |
| Prior use of anticoagulants | 3.1 (0.9–10.6) | 0.064 | 0.9 (0.1–7.4) | 0.941 | 1.1 (0.4–3.1) | 0.886 | 1.0 (0.3–3.1) | 0.979 |
| Blood pressure | ||||||||
| SBP | 1.00 (0.99–1.02) | 0.813 | 1.01 (0.99–1.03) | 0.273 | 0.99 (0.98–1.00) | 0.061 | 1.00 (0.99–1.01) | 0.688 |
| DBP | 1.00 (0.97–1.02) | 0.813 | 1.01 (0.98–1.04) | 0.434 | 0.99 (0.98–1.01) | 0.453 | 1.00 (0.99–1.02) | 0.686 |
| Stroke severity | ||||||||
| Baseline NIHSS score | 1.06 (1.00, 1.11) | 0.035 | 1.1 (1.0–1.2) | 0.003 | 0.8 (0.8–0.9) | <0.001 | 1.15 (1.09, 1.20) | <0.001 |
| Vascular occlusion site | ||||||||
| Anterior circulation | 1.9 (0.8–4.6) | 0.175 | 2.1 (0.7–6.7) | 0.192 | 0.4 (0.2–0.6) | <0.001 | 2.0 (1.1–3.5) | 0.022 |
| Posterior circulation | 0.6 (0.2–1.6) | 0.285 | 1.9 (0.7–5.0) | 0.217 | 0.5 (0.3–0.9) | 0.020 | 1.0 (0.5–1.8) | 0.889 |
| Type of recanalization therapy | ||||||||
| IVT | 0.2 (0.1–0.7) | 0.006 | 0.4 (0.1–1.1) | 0.073 | 3.9 (2.3–6.6) | <0.001 | 0.5 (0.3–0.9) | 0.014 |
| EVT | 2.0 (0.9–4.3) | 0.082 | 3.3 (1.2–9.0) | 0.020 | 0.3 (0.2–0.5) | <0.001 | 1.6 (0.9–2.7) | 0.105 |
| IVT + EVT | 2.0 (0.8–4.7) | 0.136 | 0.6 (0.1–2.5) | 0.447 | 0.7 (0.4–1.4) | 0.377 | 1.5 (0.7–3.1) | 0.272 |
| Process time | ||||||||
| ONT | 0.99 (0.97–1.00) | 0.245 | 1.00 (0.98–1.04) | 0.663 | 0.99 (0.97–1.00) | 0.157 | 1.01 (1.00–1.03) | 0.154 |
| OPT | 1.00 (0.98–1.01) | 0.670 | 1.00 (0.99–1.02) | 0.675 | 1.00 (0.99–1.01) | 0.673 | 1.00 (0.99–1.01) | 0.350 |
| Antiplatelet use within 24 h | 0.8 (0.4–1.7) | 0.551 | 1.0 (0.4–2.6) | 0.972 | 0.7 (0.4–1.1) | 0.121 | 1.0 (0.6–1.7) | 0.889 |
| Anticoagulant use within 24 h | 0.0 (0.0, Inf) | 0.990 | 0.0 (0.0, Inf) | 0.990 | 0.9 (0.1–15.2) | 0.965 | 1.2 (0.1–19.2) | 0.905 |
| Blood test | ||||||||
| Hb | 0.99 (0.97–1.01) | 0.235 | 0.99 (0.97–1.01) | 0.338 | 1.01 (0.99–1.02) | 0.397 | 0.99 (0.98–1.01) | 0.341 |
| PLT | 0.997 (0.991, 1.004) | 0.455 | 1.00 (0.99–1.01) | 0.874 | 0.998 (0.994, 1.002) | 0.277 | 0.995 (0.990, 1.001) | 0.081 |
| INR | 18.2 (0.8–430.5) | 0.072 | 6.5 (0.1–312.3) | 0.344 | 0.8 (0.1–7.8) | 0.834 | 7.6 (0.5–109.9) | 0.134 |
| FIB | 1.2 (0.8–1.7) | 0.309 | 1.0 (0.6–1.6) | 0.921 | 0.7 (0.5–0.9) | 0.003 | 0.9 (0.7–1.2) | 0.609 |
| APTT | 1.04 (0.99, 1.10) | 0.144 | 1.0 (0.9–1.1) | 0.991 | 1.01 (0.97, 1.05) | 0.632 | 1.03 (0.98, 1.07) | 0.219 |
| LDL | 0.7 (0.4–1.2) | 0.183 | 0.6 (0.3–1.2) | 0.128 | 1.3 (1.0–1.7) | 0.090 | 0.8 (0.6–1.1) | 0.249 |
| Glu | 1.0 (0.9–1.1) | 0.395 | 1.1 (1.0–1.2) | 0.063 | 0.9 (0.8–1.0) | 0.023 | 0.9 (0.8–1.0) | 0.004 |
| UA | 1.001 (0.996, 1.006) | 0.800 | 1.00 (0.99–1.01) | 0.832 | 0.999 (0.996–1.002) | 0.377 | 1.001 (0.998–1.004) | 0.605 |
| HCY | 1.0 (0.8–1.2) | 0.847 | 1.1 (1.0–1.3) | 0.130 | 0.94 (0.86, 1.03) | 0.217 | 1.0 (0.9–1.1) | 0.403 |
Univariate analysis of outcomes.
CI, confidence interval; OR, odds ratio; NA, not applicable; SBP, systolic blood pressure; DBP, diastolic blood pressure; NIHSS, national institute of health stroke scale; IVT, intravenous thrombolysis; EVT, endovascular treatment; ONT, Onset-to-Needle Time, OPT, Onset-to-Procedure Time, Hb, hemoglobin, PLT, platelet; INR, international normalized ratio; FIB, fibrinogen; APTT, activated partial thromboplastin time; LDL, low density lipoprotein, Glu, glucose, UA, uric acid; HCY, homocysteine.
3.5 Multivariate analysis
After adjusting for influencing factors, multivariate analysis revealed a negative association between the 24 h-statins group and the risk of HT (OR 0.16, 95% CI 0.06–0.49, p < 0.001). There was no significant association observed between statin use and in-hospital mortality (OR 0.43, 95% CI 0.13–1.43, p = 0.172). The 24 h-statins group demonstrated a positive correlation with favorable clinical outcomes (OR 3.63, 95% CI 1.42–9.28, p = 0.007) and neurological improvement (OR 5.23, 95% CI 1.96–13.91, p < 0.001) (Table 4).
TABLE 4
| Outcome | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| No. (%) | OR (95% CI) | P value | No. (%) | OR (95% CI) | P value | |
| Hemorrhagic transformation | 247 (92.9) | 0.16 (0.06, 0.49) | <0.001 | 247 (92.9) | 0.19 (0.08, 0.45) | <0.001 |
| In-hospital mortality | 247 (92.9) | 0.38 (0.12, 1.17) | 0.910 | 238 (89.5) | 0.43 (0.13, 1.43) | 0.172 |
| Favorable clinical outcome | 226 (85.0) | 2.88 (1.30, 6.38) | 0.009 | 183 (68.8) | 3.63 (1.42, 9.28) | 0.007 |
| Neurologic improvement | 194 (73.0) | 5.23 (1.96, 13.92) | 0.001 | 187 (70.3) | 5.23 (1.96, 13.91) | <0.001 |
Multivariate analysis of outcomes.
CI, confidence interval; OR, odds ratio.
Model 1: adjust for gender, age, SBP, prior use of antithrombotics, baseline NIHSS, coronary heart disease, IVT + EVT, FIB, level; APTT, level and other variables with P < 0.05 in the univariate analysis.
Model 2: adjust for gender, age, SBP, prior use of antithrombotics, baseline NIHSS, coronary heart disease, IVT + EVT, FIB, level; APTT, level and other variables with P < 0.1 in the univariate analysis.
3.6 Subgroup analysis
Further subgroup analysis was conducted on factors of concern related to hemorrhage, specifically HT outcomes. High low-density lipoprotein (LDL) was defined as an LDL level >3.37 mmol/L, based on laboratory reference values. No significant interaction was found between the 24 h-statins group and CHD, type of recanalization therapy, or antiplatelet use. However, subgroup analysis indicated that in patients with high LDL levels, early statin use was associated with a reduced risk of HT (P for interaction = 0.018) (Table 5).
TABLE 5
| Characteristics | 24 h-statins group | Non-24 h-statins group | Crude model | P For interaction | Model 1 | P For interaction | Model 2 | P For interaction | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||||||
| CHD, No. (%) | |||||||||||
| Yes | 20 (12.20) | 22 (21.57) | 0.2 (0.0, 1.4) | 0.107 | 0.817 | 0.0 (0.0, Inf) | 1.0000 | 0.217 | 0.0 (0.0, Inf) | 1.0000 | 0.223 |
| No | 144 (87.80) | 80 (78.43) | 0.2 (0.1, 0.4) | <0.001 | 0.2 (0.1, 0.6) | 0.0046 | 0.2 (0.0, 0.6) | 0.0036 | |||
| IVT, No. (%) | |||||||||||
| Yes | 72 (43.90) | 38 (37.25) | 0.1 (0.0, 0.3) | <0.001 | 0.515 | 0.1 (0.0, 0.5) | 0.0083 | 0.278 | 0.4 (0.1, 2.8) | 0.3498 | 0.193 |
| No | 92 (56.10) | 64 (62.75) | 0.2 (0.1, 0.4) | <0.001 | 0.1 (0.0, 0.5) | 0.0018 | 0.1 (0.0, 0.4) | 0.0010 | |||
| EVT, No. (%) | |||||||||||
| Yes | 72 (43.90) | 39 (38.24) | 0.4 (0.1, 1.2) | 0.097 | 0.340 | 1.0 (1.0, 1.0) | NA | 0.361 | 0.1 (0.0, 0.5) | 0.0045 | 0.357 |
| No | 92 (56.10) | 63 (61.76) | 0.3 (0.1, 0.9) | 0.038 | 0.2 (0.1, 1.0) | 0.0535 | 0.2 (0.0, 1.0) | 0.0492 | |||
| IVT + EVT, No. (%) | |||||||||||
| Yes | 20 (12.20) | 25 (24.51) | 0.4 (0.1, 2.0) | 0.281 | 0.470 | 0.2 (0.0, 1.4) | 0.1097 | 0.922 | 0.1 (0.0, 1.1) | 0.0603 | 0.717 |
| No | 144 (87.80) | 77 (75.49) | 0.2 (0.1, 0.4) | <0.001 | 0.2 (0.0, 0.6) | 0.0046 | 0.2 (0.0, 0.5) | 0.0041 | |||
| Antiplatelet use within 24h, No. (%) | |||||||||||
| Yes | 74 (45.12) | 37 (36.27) | 0.1 (0.0, 0.5) | 0.008 | 0.024 | 0.0 (0.0, 0.3) | 0.0027 | 0.065 | 0.0 (0.0, 0.3) | 0.0033 | 0.099 |
| No | 90 (54.88) | 65 (63.73) | 0.4 (0.1, 1.0) | 0.052 | 0.3 (0.1, 1.0) | 0.0528 | 0.3 (0.1, 0.9) | 0.0381 | |||
| LDL-high (>3.37 mmol/L), No. (%) | |||||||||||
| Yes | 42 (31.1) | 17 (21.8) | 0.0 (0.0, Inf) | 0.987 | 0.041 | 0.0 (0.0, Inf) | 1.0000 | 0.026 | 0.0 (0.0, Inf) | 1.0000 | 0.018 |
| No | 93 (68.89) | 61 (78.21) | 0.4 (0.2, 1.1) | 0.089 | 0.5 (0.1, 2.0) | 0.3284 | 0.5 (0.1, 2.1) | 0.3638 | |||
Subgroup analysis of hemorrhagic transformation.
CI, confidence interval; OR, odds ratio; NA, not applicable; IVT, intravenous thrombolysis; EVT, endovascular treatment; CHD, coronary heart disease.
Model 1: adjust for gender, age, SBP, prior use of antithrombotics, baseline NIHSS, coronary heart disease, IVT + EVT, FIB, level; APTT, level and other variables with P < 0.05 in the univariate analysis.
Model 2: adjust for gender, age, SBP, prior use of antithrombotics, baseline NIHSS, coronary heart disease, IVT + EVT, FIB, level; APTT, level and other variables with P < 0.1 in the univariate analysis.
4 Discussion
As we all know, statins are a cornerstone in the treatment of ischemic stroke and are widely used during the acute phase, recovery phase, and sequelae phase. The majority of the studies focuses on the efficacy of statins during the acute and recovery phases of ischemic stroke, while studies on the ultra-early phase are very limited. Currently, an increasing number of patients are receiving IVT, EVT, and bridging therapy. For these patients, it remains unclear whether early statin therapy can improve outcomes or increase the risk of intracranial hemorrhage. This uncertainty constitutes the primary objective and significance of the present study.
4.1 The association of statin use and the risk of HT
This study retrospectively analyzed 266 AIS patients treated with recanalization therapy and found that statin administration within 24 h after IVT or EVT was associated with a lower risk of HT, favorable clinical outcomes at discharge, and short-term neurological improvement, compared to patients in the non-24 h-statins group.
Since the rapid pro-fibrinolytic and anti-thrombotic mechanisms of statin treatment started may be protective during the acute phase (German and Liao, 2023). There remains a debate regarding whether statins treatment in the acute phase increases the risk of HT. Manuel Cappellari et al. (Cappellari et al., 2011) reported that AIS patients who received IVT and initiated statin use before hospitalization, continuing within 24 h of stroke onset, had a higher risk of symptomatic intracerebral hemorrhage. In contrast, a retrospective study (Gong et al., 2024) involving 510 cardioembolic stroke patients treated with EVT demonstrated that statin use within 7 days of the acute phase was associated with better clinical outcomes and a lower risk of symptomatic intracerebral hemorrhage compared to those who did not use statins. Similarly, the STARS trial (Montaner et al., 2016) confirmed that the combination of statins and IVT is safe and associated with a low incidence of HT. Different with the previous studies, our study not only encompasses patients with cardioembolic stroke but also investigated the effects of initiating statins within 24 h on HT and other clinical outcomes. Evidence from animal models also supports these results. In experiments involving early statin use during the acute phase of middle cerebral artery occlusion (MCAO) after IVT, statins did not increase and even reduced the risk of HT (Wu et al., 2019). Additionally, lovastatin use in intracerebral hemorrhage rat models did not exacerbate hemorrhage but instead inhibited further brain damage (Deng et al., 2023).
We hypothesize that statins reduce the risk of HT due to their pleiotropic effects. Statins exhibit anti-neuroinflammatory properties, improve endothelial dysfunction, and promote angiogenesis and tight vascular junctions (Reuter et al., 2015; Ma et al., 2021; Yang et al., 2021). These mechanisms may help reduce and repair damage to the blood-brain barrier caused by cerebral ischemia or reperfusion therapy. Further prospective cohort studies and interventional trials were needed to verify its efficacy.
4.2 The timepoint of statin use and prognosis
Current guidelines recommend initiating statin therapy for AIS patients during hospitalization (Powers et al., 2019). However, they do not provide specific recommendations regarding the optimal timing for statin initiation in AIS patients following recanalization therapy. Many studies have confirmed that initiating statin therapy within 72 h of recanalization may improve neurological outcomes. Jihoon Kang et al. (Kang et al., 2015) conducted a retrospective analysis of AIS patients undergoing recanalization therapy, including IVT and intra-arterial treatment. Their findings indicated that initiating statins on the first day after recanalization therapy was more effective in improving functional outcomes and reducing the risk of HT than starting statins on the second or third day. Similarly, a prospective study (Cappellari et al., 2013) of 2,072 AIS patients revealed that statin use within 72 h after IVT was associated with neurological improvement at 7 days, favorable functional outcomes at 3 months, and lower 90-day mortality. Statin use within 24 h demonstrated even greater efficacy. The ASCENT study (Havlíček et al., 2024) further reported that statin administration within 24 h after EVT was a significant predictor of favorable clinical outcomes at 3 months, with no evidence suggesting an association between statin use and symptomatic intracerebral hemorrhage. Our study further demonstrates that the use of statins within 24 h after revascularization therapy is safe and is associated with more favorable neurological outcomes. However, as these studies are primarily observational, further randomized controlled trials are required to verify it.
4.3 The interaction between LDL level and the risk of HT
Our subgroup analysis revealed that the effect of statins in reducing the risk of HT was more pronounced in patients with high LDL levels (>3.37 mmol/L). As is well known, following AIS, the damaged neurons release signals that activate the immune response, including the activation of glial cells and the release of various inflammatory mediators. These neuroinflammatory processes can further accelerate the disruption of the blood–brain barrier, exacerbate oxidative stress, and thereby cause secondary brain injury, such as the occurrence of HT (Rosell et al., 2008; Shi et al., 2019). This process is more intense in patients who have undergone reperfusion therapy (Khatri et al., 2012; Sun et al., 2018). Elevated plasma LDL levels can further stimulate the release of pro-inflammatory cytokines, accelerating the inflammatory response (Uno et al., 2005). Animal experimental studies have confirmed that dyslipidemia, characterized by high levels of LDL, can exacerbate brain injury in rats subjected to MCAO and reperfusion by inducing the formation of reactive oxygen species and promoting oxidative stress and inflammatory responses (Cao et al., 2015). Statins can mitigate brain injury induced by elevated LDL levels through their ability to reduce plasma LDL concentrations. In addition to their lipid-lowering effects, statins themselves also have anti-inflammatory and antioxidant properties (German and Liao, 2023). Therefore, in patients with high LDL levels, the use of statins can not only alleviate the enhanced inflammatory response caused by high LDL but also mitigate the adverse effects caused by cerebral ischemia/reperfusion. Hence, the effect of statins in reducing the risk of HT might be more pronounced in patients with high LDL levels (>3.37 mmol/L).
4.4 Limitations
There are limited studies exploring the relationship between early statin use and the prognosis of AIS patients undergoing EVT. Our findings suggest that statin use within 24 h of recanalization therapy is associated with a reduced risk of HT. However, several limitations must be acknowledged. First, as a single-center, retrospective study based on real-world data without long-term outcome data, such as 90-day mRS and mortality and the results should be interpreted with caution. Prospective studies are needed to further validate our findings. Secondly, this study defined statin use based solely on whether it was administered within 24 h, without considering factors such as the type of statin, dosage, or duration of use. These variables may significantly influence the effects of statins, and future research should aim to refine the definition of statin use by incorporating these critical aspects.
5 Conclusion
Our study demonstrated that in AIS patients undergoing recanalization therapy, statin use within 24 h was associated with a reduced risk of HT and improved neurological function. Subgroup analysis further indicated that in patients with elevated LDL levels, early statin use was linked to a lower risk of HT.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the ethic committee (YE2020‐069) of Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because our study was a retrospective cohort study.
Author contributions
BP: Data curation, Writing – original draft. JL: Data curation, Writing – original draft. XL: Data curation, Writing – original draft. HC: Data curation, Writing – original draft. LW: Methodology, Writing – review and editing. HX: Methodology, Writing – review and editing. YZ: Project administration, Supervision, Writing – review and editing. MZ: Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by “The National Science and Technology Major Project for the Prevention and Treatment of Cancer, Cardiovascular and Cerebrovascular, Respiratory and Metabolic Diseases (2024ZD0522100, 2024ZD0522102)”, “NATCM’s Project of High-level Construction of Key TCM Disciplines (zyyzdxk-2023154)”, “State Key Laboratory of Dampness Syndrome of Chinese Medicine (SZ2022KF22)”, “Guangzhou Science and Technology Project (2020KT1607).”
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Agarwal S. Scher E. Lord A. Frontera J. Ishida K. Torres J. et al (2020). Redefined measure of early neurological improvement shows treatment benefit of alteplase over placebo. Stroke51, 1226–1230. 10.1161/STROKEAHA.119.027476
2
Åivo J. Ruuskanen J. O. Tornio A. Rautava P. Kytö V. (2023). Lack of statin therapy and outcomes after ischemic stroke: a population-based study. Stroke54, 781–790. 10.1161/STROKEAHA.122.040536
3
Amarenco P. Benavente O. Goldstein L. B. Callahan A. Sillesen H. Hennerici M. G. et al (2009). Results of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial by stroke subtypes. Stroke40, 1405–1409. 10.1161/STROKEAHA.108.534107
4
Cao X.-L. Du J. Zhang Y. Yan J.-T. Hu X.-M. (2015). Hyperlipidemia exacerbates cerebral injury through oxidative stress, inflammation and neuronal apoptosis in MCAO/reperfusion rats. Exp. Brain Res.233, 2753–2765. 10.1007/s00221-015-4269-x
5
Cappellari M. Bovi P. Moretto G. Zini A. Nencini P. Sessa M. et al (2013). The THRombolysis and STatins (THRaST) study. Neurology80, 655–661. 10.1212/WNL.0b013e318281cc83
6
Cappellari M. Deluca C. Tinazzi M. Tomelleri G. Carletti M. Fiaschi A. et al (2011). Does statin in the acute phase of ischemic stroke improve outcome after intravenous thrombolysis? A retrospective study. J. Neurol. Sci.308, 128–134. 10.1016/j.jns.2011.05.026
7
Deng X. Yang J. Qing R. Yuan H. Yue P. Tian S. (2023). Suppressive role of lovastatin in intracerebral hemorrhage through repression of autophagy. Metab. Brain Dis.38, 361–372. 10.1007/s11011-022-01101-6
8
Gao Y. Jiang L. Pan Y. Chen W. Jing J. Wang C. et al (2024). Immediate- or delayed-intensive statin in acute cerebral ischemia: the INSPIRES randomized clinical trial. JAMA Neurol.81, 741–751. 10.1001/jamaneurol.2024.1433
9
German C. A. Liao J. K. (2023). Understanding the molecular mechanisms of statin pleiotropic effects. Arch. Toxicol.97, 1529–1545. 10.1007/s00204-023-03492-6
10
Gong C. Liu C. Wang Y. Chen L. Yuan J. Zhang J. et al (2024). Effect of statin treatment on clinical outcomes in cardioembolic stroke with endovascular thrombectomy. J. NeuroInterventional Surg.16, 947–954. 10.1136/jnis-2023-020619
11
Hacke W. Kaste M. Fieschi C. Von Kummer R. Davalos A. Meier D. et al (1998). Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet352, 1245–1251. 10.1016/S0140-6736(98)08020-9
12
Haggag H. Hodgson C. (2022). Clinimetrics: modified Rankin scale (mRS). J. Physiother.68, 281. 10.1016/j.jphys.2022.05.017
13
Havlíček R. Šaňák D. Černík D. Neradová J. Leško N. Gdovinová Z. et al (2024). Predictors of good clinical outcome after endovascular treatment for acute ischemic stroke due to tandem lesion in anterior circulation: results from the ASCENT study. Cardiovasc. Interv. Radiol.47, 218–224. 10.1007/s00270-023-03649-x
14
Herpich F. Rincon F. (2020). Management of acute ischemic stroke. Crit. Care Med.48, 1654–1663. 10.1097/CCM.0000000000004597
15
Hong K.-S. Lee J. S. (2015). Statins in acute ischemic stroke: a systematic review. J. Stroke17, 282–301. 10.5853/jos.2015.17.3.282
16
Kang J. Kim N. Park T. H. Bang O. Y. Lee J. S. Lee J. et al (2015). Early statin use in ischemic stroke patients treated with recanalization therapy: retrospective observational study. BMC Neurol.15, 122. 10.1186/s12883-015-0367-4
17
Khatri R. McKinney A. M. Swenson B. Janardhan V. (2012). Blood–brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology79, S52–S57. 10.1212/WNL.0b013e3182697e70
18
Lauer A. Greenberg S. M. Gurol M. E. (2015). Statins in intracerebral hemorrhage. Curr. Atheroscler. Rep.17, 46. 10.1007/s11883-015-0526-5
19
Ma G. Pan Z. Kong L. Du G. (2021). Neuroinflammation in hemorrhagic transformation after tissue plasminogen activator thrombolysis: potential mechanisms, targets, therapeutic drugs and biomarkers. Int. Immunopharmacol.90, 107216. 10.1016/j.intimp.2020.107216
20
Maïer B. Desilles J. P. Mazighi M. (2020). Intracranial hemorrhage after reperfusion therapies in acute ischemic stroke patients. Front. Neurol.11, 599908. 10.3389/fneur.2020.599908
21
Montaner J. Bustamante A. García-Matas S. Martínez-Zabaleta M. Jiménez C. De La Torre J. et al (2016). Combination of thrombolysis and statins in acute stroke is safe: results of the STARS randomized trial (stroke treatment with acute reperfusion and simvastatin). Stroke47, 2870–2873. 10.1161/STROKEAHA.116.014600
22
Powers W. J. Rabinstein A. A. Ackerson T. Adeoye O. M. Bambakidis N. C. Becker K. et al (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke50, e344–e418. 10.1161/STR.0000000000000211
23
Reuter B. Rodemer C. Grudzenski S. Meairs S. Bugert P. Hennerici M. G. et al (2015). Effect of simvastatin on MMPs and TIMPs in human brain endothelial cells and experimental stroke. Transl. Stroke Res.6, 156–159. 10.1007/s12975-014-0381-7
24
Rosell A. Cuadrado E. Ortega-Aznar A. Hernández-Guillamon M. Lo E. H. Montaner J. (2008). MMP-9–Positive neutrophil infiltration is associated to blood–brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke39, 1121–1126. 10.1161/STROKEAHA.107.500868
25
Shi K. Tian D.-C. Li Z.-G. Ducruet A. F. Lawton M. T. Shi F.-D. (2019). Global brain inflammation in stroke. Lancet Neurol.18, 1058–1066. 10.1016/S1474-4422(19)30078-X
26
Sun M.-S. Jin H. Sun X. Huang S. Zhang F.-L. Guo Z.-N. et al (2018). Free radical damage in ischemia‐reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid. Med. Cell. Longev.2018, 3804979. 10.1155/2018/3804979
27
Tan C. Liu X. Mo L. Wei X. Peng W. Wang H. et al (2019). Statin, cholesterol, and sICH after acute ischemic stroke: systematic review and meta-analysis. Neurol. Sci.40, 2267–2275. 10.1007/s10072-019-03995-0
28
Tian B. Tian X. Shi Z. Peng W. Zhang X. Yang P. et al (2022). Clinical and imaging indicators of hemorrhagic transformation in acute ischemic stroke after endovascular thrombectomy. Stroke53, 1674–1681. 10.1161/STROKEAHA.121.035425
29
Uno M. Kitazato K. T. Nishi K. Itabe H. Nagahiro S. (2005). Raised plasma oxidised LDL in acute cerebral infarction.
30
Wu Y. Lu D. Xu A. (2019). The effect of HMG-CoA reductase inhibitors on thrombolysis-induced haemorrhagic transformation. J. Clin. Neurosci.69, 1–6. 10.1016/j.jocn.2019.08.074
31
Yang Y. Yang L. Y. Salayandia V. M. Thompson J. F. Torbey M. Yang Y. (2021). Treatment with atorvastatin during vascular remodeling promotes pericyte-mediated blood-brain barrier maturation following ischemic stroke. Transl. Stroke Res.12, 905–922. 10.1007/s12975-020-00883-0
Summary
Keywords
acute ischemic stroke, statins, hemorrhagic transformation, intravenous thrombolysis, endovascular treatment
Citation
Pan B, Lan J, Li X, Chen H, Weng L, Xu H, Zhao Y and Zhao M (2025) Early statin use might reduce the hemorrhagic transformation among acute ischemic stroke patients with recanalization therapy: a retrospective cohort study. Front. Pharmacol. 16:1533905. doi: 10.3389/fphar.2025.1533905
Received
25 November 2024
Accepted
19 May 2025
Published
04 June 2025
Volume
16 - 2025
Edited by
Rui Liu, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Reviewed by
Shi-Jie Zhang, Guangzhou University of Chinese Medicine, China
Mingzhen Qin, Johns Hopkins University, United States
Updates
Copyright
© 2025 Pan, Lan, Li, Chen, Weng, Xu, Zhao and Zhao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Zhao, 376479808@qq.com; Yuanqi Zhao, zyq2022@gzucm.edu.cn
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.