- 1Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Beijing University of Chinese Medicine, Beijing, China
Aim of the study: This systematic review and network meta-analysis aimed to evaluate the comparative effectiveness of traditional Chinese medicine injections (TCMIs) combined with angiotensin-converting enzyme inhibitors or angiotensin Ⅱ receptor blockers for diabetic nephropathy (DN).
Methods: Ten databases were searched. Primary endpoint indicators were urinary albumin excretion rate (UAER) and serum creatinine (Scr). Secondary endpoint indicators were blood urea nitrogen (BUN), urinary β2-microglobulin, total cholesterol, triglyceride, systolic blood pressure, and total effective rate. Cochrane risk of bias tool (version 2.0) was used to evaluate the quality of the studies. The GRADE method was used to assess the whole network. Finally, Stata 16.0 software was used to perform network meta-analysis.
Results: A total of 99 randomised controlled trials and ten TCMIs were included for analysis. Based on the surface under the cumulative ranking curve values, it was observed that the efficacy of the combination group was better than that of the control group. For the primary endpoints, the Shuxuetong and Shenkang injections were excellent in reducing UAER and Scr, respectively. The Danshen injection was the most effective for the total effective rate and BUN; the Shuxuetong, Yinxingdamo, Danshen-Chuanxiongqin, and Shuxuening injections were the most effective for total cholesterol, β2-microglobulin, triglyceride, and systolic blood pressure, respectively. In terms of dual indicators, for UAER and Scr, the Danshen injection may be the most effective treatment. In addition, no significant adverse reactions were reported in the relevant studies on the Huangqi and Gegensu injections, whereas the Yinxingdamo, Danshen-Chuanxiongqin, Shenkang, Shuxuetong, and Kudiezi injections demonstrated varying degrees of adverse reactions.
Conclusion: In this study, it is indicated that when combined with ACEI/ARB, the Shuxuetong, Shenkang, Danshen, Danshen-Chuanxiongqin, Yinxingdamo, and Shuxuening injections may confer advantages in improving DN indicators. However, due to limitations in the methodological quality of the included studies (especially deficiencies in randomisation and blinding) and the critical lack of reporting on key information regarding TCMI components, the reliability of these findings is compromised.
1 Introduction
Diabetic nephropathy (DN) is a disease in which persistent hyperglycaemia induces haemodynamic abnormalities, metabolic disturbances, and inflammatory responses, ultimately leading to renal dysfunction. Clinically, the disease manifests as persistent proteinuria with a progressive decrease in the glomerular filtration rate. DN is one of the most serious complications of diabetes and is the major cause of end-stage renal disease (Tuttle et al., 2014; Qi et al., 2020; Jung and Yoo, 2022). It is predicted that by the middle of the century, diabetes will affect approximately 700 million people worldwide and that approximately 40% of these will develop DN (Saeedi et al., 2019). DN affects more than 50% of patients entering the dialysis or kidney transplant programmes (DeFronzo et al., 2021). The incidence of the disease continues to increase, which not only exerts significant pressure on the global economy but also has a profound impact on the quality of life of patients. Therefore, early detection of the condition and optimisation of existing treatment regimens may delay the onset and progression of DN and reduce the number of deaths from the development of end-stage renal disease. Currently, treatment options for DN primarily focus on lifestyle modifications, glycaemic control, blood pressure management, and blockade of the renin–angiotensin system (Thomas et al., 2016).
Research has demonstrated that angiotensin-converting enzyme inhibitor (ACEI) or angiotensin Ⅱ receptor blocker (ARB) (collectively ACEI/ARB) possesses renoprotective properties and improves glomerular hyperfiltration to some extent (Wada and Makino, 2013; Deng et al., 2022). However, the use of renin–angiotensin system blockers may cause patients to experience adverse effects such as cough, laryngeal oedema, hyperkalaemia, and hypovolaemia (Bilen et al., 2023). The combination of ACEI and ARB may even worsen the development of DN (Fried et al., 2013). Therefore, although current therapies show some efficacy, their limitations have encouraged research workers to explore complementary treatment strategies. Traditional Chinese medicine (TCM) has provided a novel direction for research that may offer some degree of synergistic treatment. TCM injections (TCMIs) are sterile preparations extracted and purified from herbal medicines, enhancing the bioavailability and efficacy of herbal therapies (Xie et al., 2021; Long et al., 2023). Several meta-analyses have been conducted to show that a variety of injections are effective in lowering the urinary albumin excretion rate (UAER), blood urea nitrogen (BUN), or serum creatinine (Scr) in patients with DN, such as the Huangqi (HQ), Shenkang (SK), and Shuxuetong (ST) injections, among others (Wang R. H. et al., 2019; Wang and Xu, 2020; Zong et al., 2022). However, the comparison of the efficacy of different herbal injections for the treatment of DN is unclear. In this study, we used a network meta-analysis to synthesise evidence from relevant randomised controlled trials (RCTs) to investigate the optimal regimen of TCMIs by comparing the efficacy of different TCMIs in combination with ACEI/ARB in the treatment of DN.
2 Materials and methods
The network meta-analysis was registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) in September 2024 under the registration number INPLASY202490069 (refer to Supplementary Appendix Figure A1). In addition, in this study, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the extension statement for network meta-analyses (PRISMA-NMA) (Page et al., 2021). Details are provided in Supplementary Appendix Table A.1.
2.1 Standard evaluation of TCM
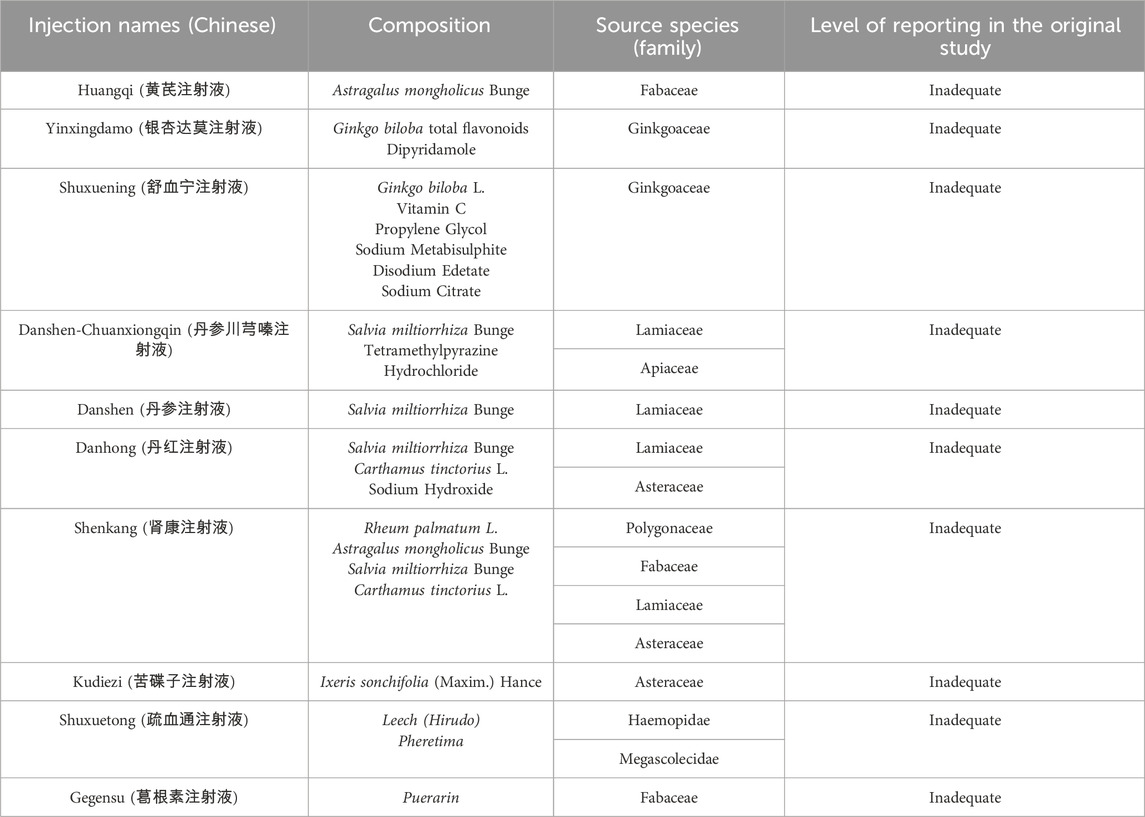
To enhance the accuracy, the TCMIs in this study were reported in accordance with the Consensus statement on the Phytochemical Characterisation of Medicinal Plant extracts (ConPhyMP). Concurrently, on 18 October 2024, we standardised the scientific names of the botanical drug components with reference to “A working list of all plants list” (http://www.theplantlist.org). In addition, plants name have been checked with “The World Flora Online” (WFO, http://www.worldfloraonline.org/). Summary tables describing the composition of the ingredients and how they were reported in the original study were prepared based on principles outlined in the four pillars of ethnopharmacology. The composition and standardised name for each injection are presented in Table 1. In addition, the TCMIs have been approved by the China Food and Drug Administration (CFDA) and have been widely used in clinical practice. Further information on the injections included in this study was obtained from the China Medical Information Platform (CMIP), details of which are given in Supplementary Appendix Tables A.2, A.3.
2.2 Search strategy
We conducted a comprehensive search across ten databases, including China National Knowledge Infrastructure (CNKI), the Chinese Scientific Journal database (VIP), Wanfang database, SinoMed, PubMed, Web of Science, Embase, Cochrane Library, Scopus, and Chinese Clinical Trial Registry (ChiCTR), from their inception to September 2024. Search terms included but were not limited to “diabetic nephropathy,” “diabetic kidney disease,” “glomerulosclerosis diabetic,” “injection,” “angiotensin-converting enzyme inhibitors,” “angiotensin receptor antagonists,” “ACEI,” “ARB,” “randomised controlled trial,” and “RCT”; further details are given in Supplementary Appendix Tables A.4–A.13.
2.3 Inclusion and exclusion criteria
2.3.1 Inclusion criteria
(i) Population: the study population met the diagnostic criteria of the Mogensen classification of DN (Mogensen, 1999), with no gender or age restrictions. (ii) Intervention and control: the control group underwent routine treatment plus ACEI/ARB, and the combination group was the control group plus TCMI. (iii) Outcomes: primary endpoints were UAER and Scr. Secondary endpoints were BUN, β2-microglobulin (β2-MG), total cholesterol (TC), triglyceride (TG), systolic blood pressure (SBP), and total effective rate (TER) (TER in this study was defined as an improvement in the patient’s symptoms and a decrease in UAER, BUN, or Scr). (iv) Study design: RCTs with or without blinding.
2.3.2 Exclusion criteria
The exclusion criteria included the following: non-randomised controlled trials; interventions involving other Chinese medical treatments or injections of other non-Chinese medicines; inability to extract valid primary data from the study; combination of other diseases affecting renal function in the included patients; duplication and inaccessibility of full text; and meta-analyses, reviews, case reports, animal experiments, conference papers, etc., and the number of included studies was less than two.
2.4 Study selection process and data extraction
All of the retrieved studies were imported into EndNote 20 (Clarivate Analytics, Philadelphia, PA, United States), followed by the removal of duplicate articles. The screening process comprised four steps: first, two reviewers independently screened titles and abstracts using inclusion and exclusion criteria. Second, the full text of the literature screened in the first step was downloaded and thoroughly reviewed to determine whether it met the inclusion criteria. Disagreements were resolved by discussion between the reviewers, and a third reviewer was consulted if necessary. Third, two reviewers performed data extraction for the included studies. The extracted data included the following: (i) basic information: first author and year of publication; (ii) patient aspects: sample size of each group, gender, mean age, duration of treatment, interventions (ACEI/ARB), and duration of disease; and (iii) outcome aspects: available outcome indicators included in each study. Fourth, in cases of missing data or incomplete reporting, we prioritized contacting the corresponding authors via email with standardized requests, allowing a 14-day response window and a maximum of two contact attempts. If no response was received, we used data imputation strategies according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al., 2024).
2.5 Risk of bias assessment
The quality of the included studies was individually assessed by two reviewers using the Cochrane risk of bias tool (version 2.0) (Sterne et al., 2019), including the randomisation process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of reported result. Risk of bias was categorised as “low risk,” “high risk,” and “some concerns.” In addition, the entire network was individually assessed by two reviewers using the GRADE approach (Puhan et al., 2014), which provided a framework for rating the certainty of each pairwise comparison evidence as “high,” “moderate,” “low,” or “very low.” Disagreements arising from the above assessments were resolved through discussion between the reviewers.
2.6 Statistical analysis
A random-effects model was used in this study, and we used Stata 16.0 software (Stata Corp, College Station, Texas, United States) to perform network meta-analyses. For dichotomous variables, based on the overall efficacy in patients with DN, the odds ratio (OR) with 95% confidence interval (95% CI) was used as the effect indicator. For continuous variables, such as TC, TG and SBP, the mean difference (MD) and 95% CI were calculated, but when data units were inconsistent, such as UAER, BUN, Scr, and β2-MG, the standardised mean difference (SMD) and 95% CI were calculated. We calculated the surface under the cumulative ranking curve (SUCRA) values to rank multiple interventions. SUCRA values of 100% and 0% were the outcomes of treatments with the best efficacy and the worst safety, respectively. We conducted a two-dimensional cluster analysis to further explore the rankings. We performed Egger’s regression test and created a funnel plot to evaluate potential publication bias in the intervention network. Finally, we tested the heterogeneity of effects across populations through subgroup analyses and used sensitivity analyses to verify the robustness of the results.
3 Results
3.1 Literature selection and study characteristics
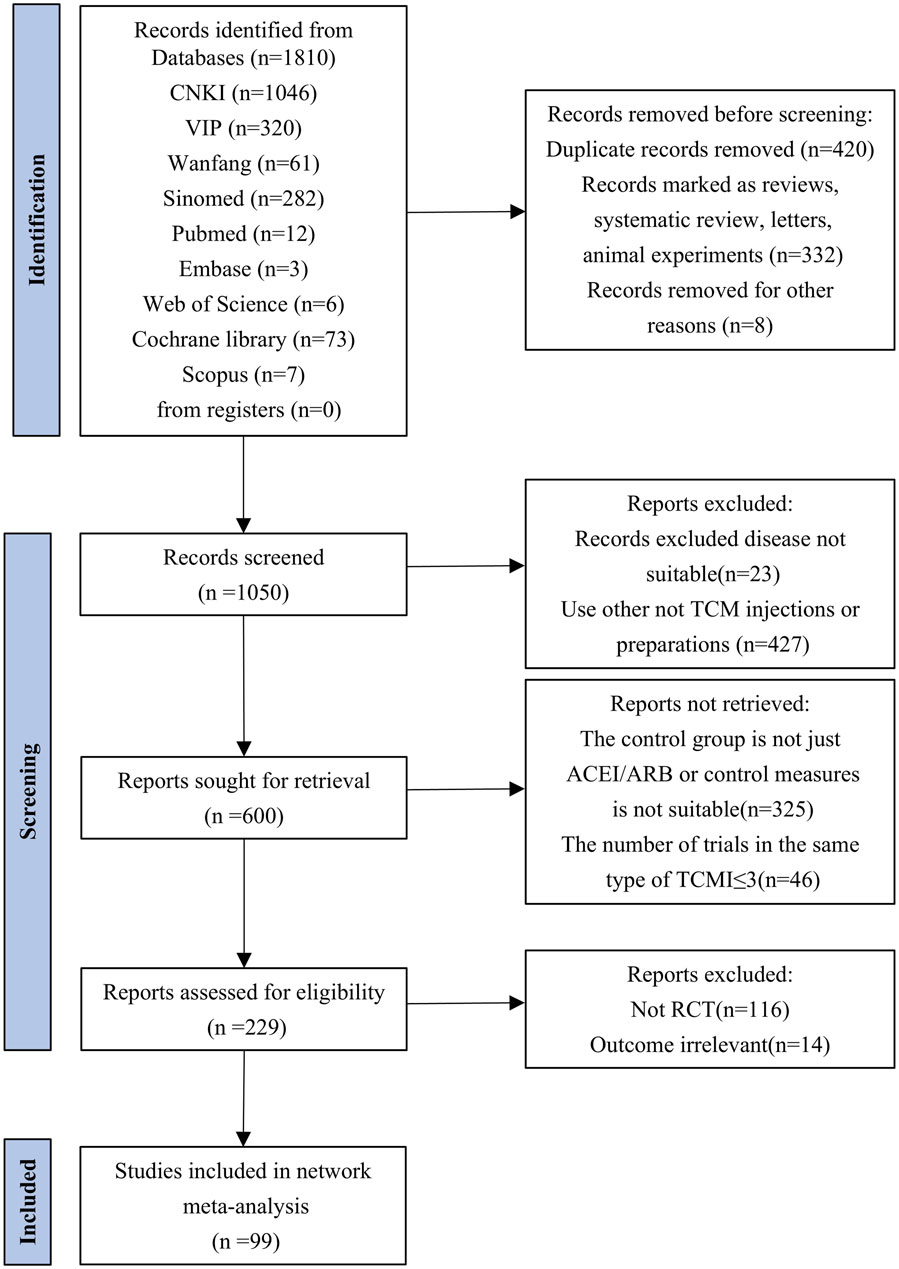
We retrieved 1,810 citations from the database. After excluding duplicates or ineligible records, 99 studies with 9,888 participants in 10 TCMIs met the eligibility criteria for inclusion in the network meta-analysis. The specific screening process is illustrated in Figure 1. Two updates were made during the screening process. All the included studies compared the effect of a single injection combined with ACEI/ARB versus ACEI/ARB alone. The specific interventions of the original study are given in Supplementary Appendix Table A.14. All the trials included in the analysis were RCTs, each with between 40 and 358 participants. In addition, all the trials were published between 2003 and 2024. The duration of treatment varied from 2 to 36 weeks. Baseline characteristics are given in Supplementary Appendix Table A.15. Among all the TCMIs, HQ was the treatment with the highest number of studies (33 RCTs), followed by the Yinxingdamo injection (YX) (17 RCTs), the SK injection (12 RCTs), the Danshen-Chuanxiongqin injection (DC) (nine RCTs), the Danhong injection (DH) (seven RCTs), the Danshen injection (DS) (five RCTs), the Shuxuening injection (SX) (four RCTs), the Kudiezi injection (KD) (four RCTs), the ST injection (four RCTs), and the Gegensu injection (GG) (four RCTs).
3.2 Quality assessment of evidence
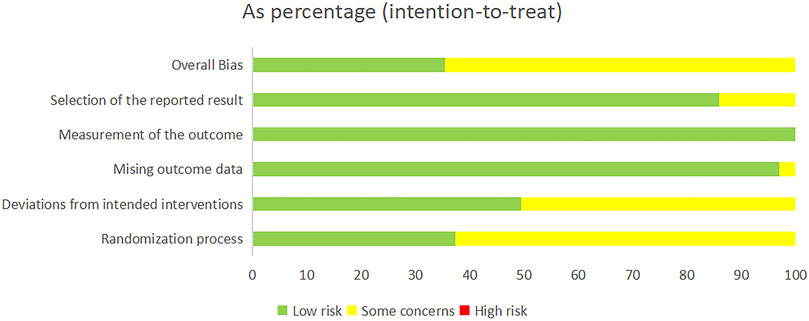
Among the 99 studies included, 37 studies provided detailed information on the method of randomisation. In contrast, the remaining studies only mentioned randomisation and did not provide specific information on the details of randomisation. Five studies (Xu, 2008; Rao et al., 2013; Zheng, 2013; Fan, 2017; Hao et al., 2022) reported programme concealment, allocation concealment, and measurement blinding. Three studies had incomplete data results, but this did not affect the completeness of the main outcome indicators (Wang, 2012; Zhao, 2014; Li and Zhang, 2015). In all the studies, the data were analysed using sound statistical methods. In addition, it was noted that the literature included was all from China, but none of the studies described a pre-established research plan or analysis protocol. As we all know, ChiCTR was established in 2005, and the requirements for selective reporting have become more standardised in the recent years. Consequently, the 14 older studies (Zhu et al., 2003; Huang et al., 2004; Jiang et al., 2005; Ma, 2006; Li, 2007; Yang et al., 2007; Cui, 2009; Li et al., 2009; Li, 2009; Liu et al., 2009; Tian et al., 2009; Xu, 2009; Yuan, 2009; Zhou et al., 2009) were identified as being at risk of selective reporting, whereas other trials exhibited a lower risk of such bias, as illustrated in Figure 2. The results of the risk of bias assessment for the included studies are given in Supplementary Appendix Table A.16. The presence of only indirect comparisons resulted in lower quality ratings for the vast majority of two-by-two comparisons. Details of the GRADE method of evidence assessment are given in Supplementary Appendix Tables A.17–A.24.
3.3 Network meta-analysis
3.3.1 UAER
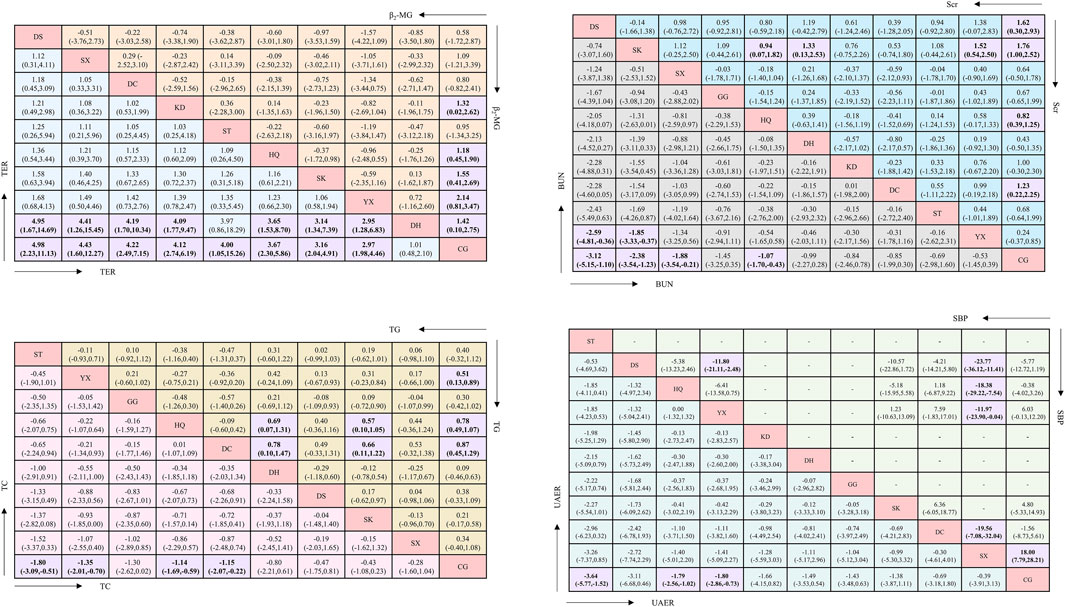
Regarding UAER, 49 studies with a total of 4,121 patients were included, evaluating eleven interventions with ten TCMIs, as illustrated in Figure 3A. The following combination groups did not demonstrate statistically significant difference in efficacy compared with the control group, including DS, KD, DH, GG, SK, DC, and SX. Three TCMIs were superior to ACEI/ARB alone in combination with ACEI/ARB: ST (SMD: −3.64, 95% CI: −5.77, −1.52) (p = 0.001), HQ (SMD: −1.79, 95% CI: −2.56, −1.02) (p < 0.001), and YX (SMD: −1.80, 95% CI: −2.86, −0.73) (p = 0.001), as illustrated in Figure 4. According to the SUCRA area, in combination with ACEI/ARB, ST (SUCRA = 90.2%) demonstrated the best therapeutic effect, followed by DS (SUCRA = 77.1%), HQ (SUCRA = 57.3%), YX (SUCRA = 57.2%), KD (SUCRA = 52.8%), DH (SUCRA = 49.1%), GG (SUCRA = 47.8%), SK (SUCRA = 46.8%), DC (SUCRA = 31.2%), SX (SUCRA = 29.0%), and ACEI/ARB (SUCRA = 11.5%), as illustrated in Figure 5A. Details are given in Supplementary Appendix Table A.25.
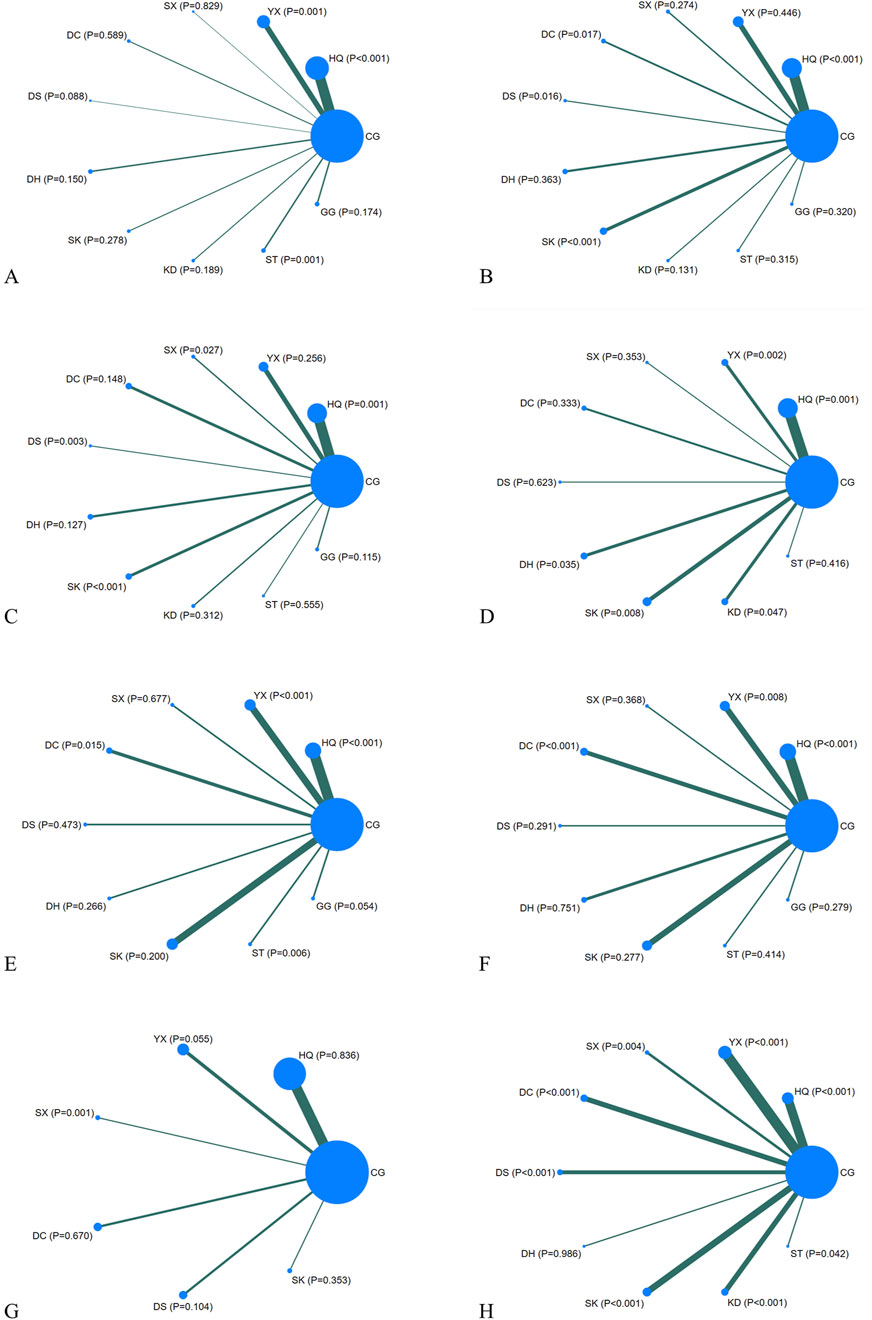
Figure 3. Network diagrams. CG, control group; ST, Shuxuetong injection; SK, Shenkang injection; YX, Yinxingdamo injection; DC, Danshen-Chuanxiongqin injection; DS, Danshen injection; HQ, Huangqi injection; GG, Gegensu injection; DH, Danhong injection; SX, Shuxuening injection; KD, Kudiezi injection. (A) Urinary albumin excretion rate; (B) serum creatinine; (C) blood urea nitrogen; (D) β2- microglobulin; (E) total cholesterol; (F) triglyceride; (G) systolic blood pressure; (H) total effective rate.
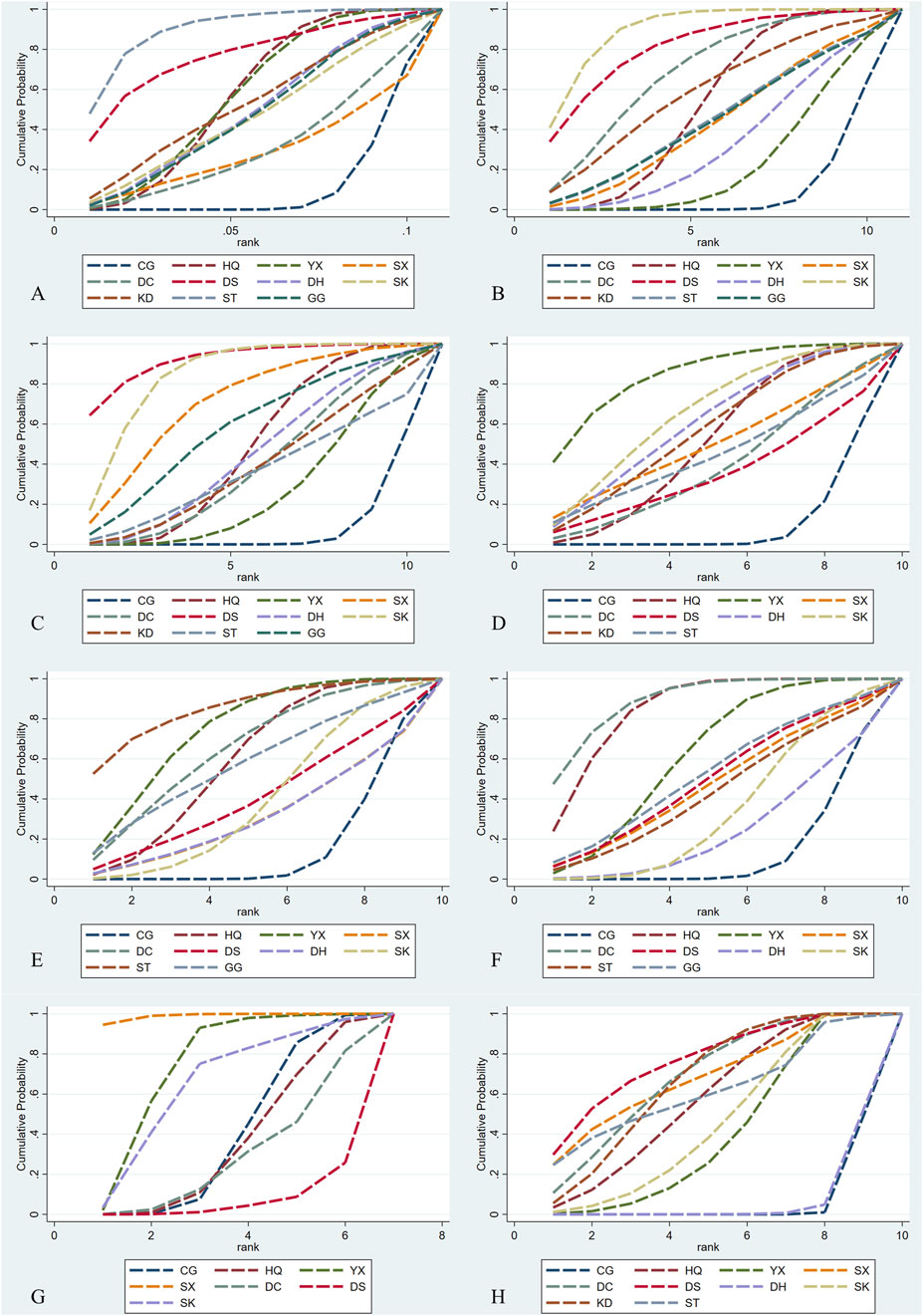
Figure 5. SCURA diagrams. CG, control group; ST, Shuxuetong injection; SK, Shenkang injection; YX, Yinxingdamo injection; DC, Danshen-Chuanxiongqin injection; DS, Danshen injection; HQ, Huangqi injection; GG, Gegensu injection; DH, Danhong injection; SX, Shuxuening injection; KD, Kudiezi injection. (A) Urinary albumin excretionrate; (B)serum creatinine; (C) blood urea nitrogen; (D)β2- microglobulin; (E) total cholesterol; (F) triglyceride; (G) systolic blood pressure; (H) total effective rate.
3.3.2 Scr
Regarding Scr, 78 studies with a total of 7,872 patients were included, evaluating eleven interventions in ten TCMIs, as illustrated in Figure 3B. The following combination groups demonstrated no statistically significant difference in efficacy compared to the control group: KD, ST, GG, SX, DH, and YX. Four TCMIs were superior to ACEI/ARB alone in combination with ACEI/ARB: SK (SMD: −1.76, 95% CI: −2.52, −1.00) (p < 0.001), DS (SMD: −1.62, 95% CI: −2.93, −0.30) (p = 0.016), DC (SMD: −1.23, 95% CI: −2.25, −0.22) (p = 0.017), and HQ (SMD: −0.82, 95% CI: −1.25, −0.39) (p < 0.001), as illustrated in Figure 4. According to the SUCRA area, in combination with ACEI/ARB, SK (SUCRA = 89.7%) demonstrated the best therapeutic effect, followed by DS (SUCRA = 81.2%), DC (SUCRA = 69.4%), KD (SUCRA = 58.3%), HQ (SUCRA = 52.7%), ST (SUCRA = 45.0%), GG (SUCRA = 44.7%), SX (SUCRA = 43.7%), DH (SUCRA = 32.9%), YX (SUCRA = 23.0%), and ACEI/ARB (SUCRA = 9.4%), as illustrated in Figure 5B. Details are given in Supplementary Appendix Table A.25.
3.3.3 BUN
Regarding BUN, 62 studies with a total of 6,115 patients were included, evaluating eleven interventions in ten TCMIs, as illustrated in Figure 3C. The following combination groups demonstrated no statistically significant difference in efficacy compared to the control group: GG, DH, KD, DC, ST, and YX. Four TCMIs were superior to ACEI/ARB alone in combination with ACEI/ARB: DS (MD: −3.12, 95% CI: −5.51, −1.10) (p = 0.003), SK (MD: −2.38, 95% CI: −3.54, −1.23) (p < 0.001), SX (MD: −1.88, 95% CI: −3.54, −0.21) (p = 0.027), and HQ (MD: −1.07, 95% CI: −1.70, −0.43) (p = 0.001), as illustrated in Figure 4. According to the SUCRA area, in combination with ACEI/ARB, DS (SUCRA = 92.3%) demonstrated the best therapeutic effect, followed by SK (SUCRA = 84.7%), SX (SUCRA = 71.2%), GG (SUCRA = 58.3%), HQ (SUCRA = 48.1%), DH (SUCRA = 45.0%), DC (SUCRA = 39.7%), KD (SUCRA = 39.0%), ST (SUCRA = 36.1%), YX (SUCRA = 27.8%), and ACEI/ARB (SUCRA = 7.8%), as illustrated in Figure 5C. Details are given in Supplementary Appendix Table A.25.
3.3.4 β2-MG
Regarding β2-MG, 28 studies with a total of 3,067 patients were included, evaluating ten interventions in nine TCMIs, as illustrated in Figure 3D. The following combination groups demonstrated no statistically significant difference in efficacy compared to the control group: SX, ST, DC, and DS. Five TCMIs were superior to ACEI/ARB alone in combination with ACEI/ARB: YX (SMD: −2.14, 95% CI: −3.47,-0.81) (p = 0.002), SK (SMD: −1.55, 95% CI: −2.69, −0.14) (p = 0.008), DH (SMD: −1.42, 95% CI: −2.75, −0.10) (p = 0.035), KD (SMD: −1.32, 95% CI: −2.62, −0.02) (p = 0.047), and HQ (SMD: −1.18, 95% CI: −1.90, −0.45) (p = 0.001), as illustrated in Figure 4. According to the SUCRA area, in combination with ACEI/ARB, YX (SUCRA = 84.4%) demonstrated the best therapeutic effect, followed by SK (SUCRA = 66.1%), DH (SUCRA = 61.1%), KD (SUCRA = 57.3%), HQ (SUCRA = 51.7%), SX (SUCRA = 49.9%), ST (SUCRA = 44.9%), DC (SUCRA = 39.2%), DS (SUCRA = 35.5%), and ACEI/ARB (SUCRA = 9.8%), as illustrated in Figure 5D. Details are given in Supplementary Appendix Table A.25.
3.3.5 TC
Regarding TC, 21 studies with a total of 2,270 patients were included, evaluating ten interventions in nine TCMIs, as illustrated in Figure 3E. The following combination groups demonstrated no statistically significant difference in efficacy compared to the control group: GG, DH, DS, SK, and SX. Four TCMIs were superior to ACEI/ARB alone in combination with ACEI/ARB: ST (MD: −1.80, 95% CI: −3.09, −0.51) (p = 0.006), YX (MD: −1.35, 95% CI: −2.01, −0.70) (p < 0.001), HQ (MD: −1.14, 95% CI: −1.69, −0.59) (p < 0.001), and DC (MD: −1.15, 95% CI: −2.07, −0.22) (p = 0.015), as illustrated in Figure 4. According to the SUCRA area, in combination with ACEI/ARB, ST (SUCRA = 85.3%) demonstrated the best therapeutic effect, followed by YX (SUCRA = 74.5%), DC (SUCRA = 65.2%), HQ (SUCRA = 59.4%), GG (SUCRA = 57.6%), DS (SUCRA = 40.7%), SK (SUCRA = 39.4%), DH (SUCRA = 31.6%), SX (SUCRA = 31.5%), and ACEI/ARB (SUCRA = 14.8%), as illustrated in Figure 5E. Details are given in Supplementary Appendix Table A.25.
3.3.6 TG
Regarding TG, 24 studies with a total of 2,388 patients were included, evaluating ten interventions in nine TCMIs, as illustrated in Figure 3F. The following combination groups demonstrated no statistically significant difference in efficacy compared to the control group: ST, DS, SX, GG, SK, and DH. Three TCMIs were superior to ACEI/ARB alone in combination with ACEI/ARB: DC (MD: −0.87, 95% CI: −1.29, −0.45) (p < 0.001), HQ (MD: −0.78, 95% CI: −1.07, −0.49) (p < 0.001), and YX (MD: −0.51, 95% CI: −0.89, −0.13) (p = 0.008), as illustrated in Figure 4. According to the SUCRA area, in combination with ACEI/ARB, DC (SUCRA = 89.1%) demonstrated the best therapeutic effect, followed by HQ (SUCRA = 84.7%), YX (SUCRA = 62.1%), GG (SUCRA = 52.3%), DS (SUCRA = 49.5%), SX (SUCRA = 47.1%), ST (SUCRA = 43.3%), SK (SUCRA = 34.3%), DH (SUCRA = 24.4%), and ACEI/ARB (SUCRA = 13.2%), as illustrated in Figure 5F. Details are given in Supplementary Appendix Table A.25.
3.3.7 SBP
Regarding SBP, 18 studies with a total of 1,746 patients were included, evaluating seven interventions in six TCMIs, as illustrated in Figure 3G. Three TCMIs in combination with ACEI/ARB were superior to ACEI/ARB alone: SX, YX, and SK, as illustrated in Figure 4. Unfortunately, only SX (MD: −18.00, 95% CI: −28.21, −7.79) (p = 0.001) demonstrated statistically significant difference. DS, DC, and HQ did not show a statistically significant therapeutic effect on lowering SBP compared with the control group. According to the SUCRA area, in combination with ACEI/ARB, SX (SUCRA = 98.9%) demonstrated the best therapeutic effect, followed by YX (SUCRA = 74.5%), SK (SUCRA = 65.4%), ACEI/ARB (SUCRA = 40.1%), HQ (SUCRA = 35.9%), DC (SUCRA = 28.6%), and DS (SUCRA = 6.5%), as illustrated in Figure 5G. Details are given in Supplementary Appendix Table A.25.
3.3.8 TER
Regarding TER, 35 studies with a total of 4,382 patients were included, evaluating ten interventions in nine TCMIs, as illustrated in Figure 3H. The comparison of the difference in TER between DH and ACEI/ARB alone was not statistically significant. The following combination groups demonstrated superior TER than the control group: DS (OR: 4.98, 95% CI: 2.23, 11.13) (p < 0.001), SX (OR: 4.43, 95% CI: 1.60, 12.27) (p = 0.004), DC (OR: 4.22, 95% CI: 2.49, 7.15) (p < 0.001), KD (OR: 4.12, 95% CI: 2.74, 6.19) (p < 0.001), ST (OR: 4.00, 95% CI: 1.05, 15.26) (p = 0.042), HQ (OR: 3.67, 95% CI: 2.30, 5.86) (p < 0.001), SK (OR: 3.16, 95% CI: 2.04, 4.91) (p < 0.001), and YX (OR: 2.97, 95% CI: 1.98, 4.46) (p < 0.001), as illustrated in Figure 4. This suggests a benefit in improving the main indicators and clinical symptoms of DN. According to the SUCRA area, in combination with ACEI/ARB, DS (SUCRA = 77%) had the best treatment outcome, followed by DC (SUCRA = 68.8%), SX (SUCRA = 68.6%), KD (SUCRA = 67.1%), ST (SUCRA = 61.9%), HQ (SUCRA = 57.7%), SK (SUCRA = 46.1%), YX (SUCRA = 40.6%), DH (SUCRA = 6.4%), and ACEI/ARB (SUCRA = 5.6%), as illustrated in Figure 5H. Details are given in Supplementary Appendix Table A.25.
3.4 Safety
A total of 30 studies involving adverse reactions (ARs) were reported. ARs included dizziness, cough, headache, rash, nausea, tinnitus, hypotension, tachycardia, itchiness, fatigue, shortness of breath, gastrointestinal discomfort, and elevated potassium in blood. ARs occurred in 59 of 1,803 patients in the combination group and 85 of 1,783 patients in the control group. The incidence of ARs in the combination group was 3.3%, which was lower than that observed in the control group (4.8%) (OR: 0.69, 95% CI: 0.50, 0.95) (p = 0.02). There were seven TCMIs in the study: HQ, YX, DC, DS, SK, KD, and ST. It is worth mentioning that no ARs were reported in the studies of HQ and GG. Fortunately, the symptoms observed were relatively mild and showed improvement with symptomatic treatment. Details of the reported safety issues are shown in Supplementary Appendix Table A.26.
3.5 Cluster analysis
According to KDIGO and ADA guidelines (de Boer et al., 2022), the diagnosis and staging of DN relies on the decreased glomerular filtration rate (based on Scr) and UAER. We employed cluster analysis to analyse interventions with multidimensional outcomes. In the combination group, for UAER and Scr, DS may represent the optimal treatment option, as illustrated in Figure 6.
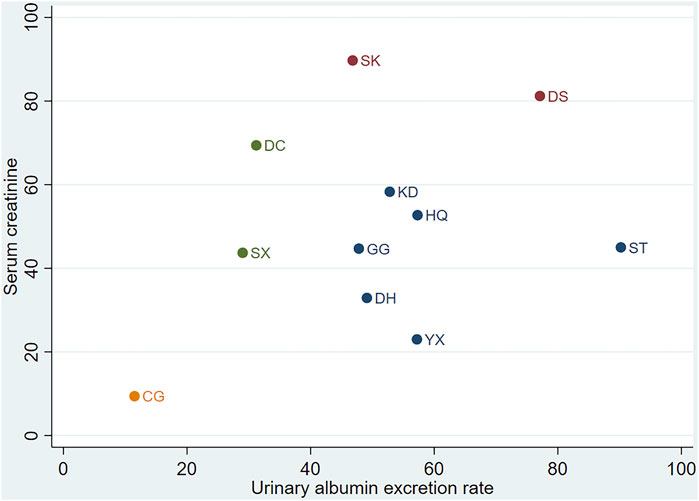
Figure 6. Cluster analysis. CG, control group; ST, Shuxuetong injection; SK, Shenkang injection; YX, Yinxingdamo injection; DC, Danshen-Chuanxiongqin injection; DS, Danshen injection; HQ, Huangqi injection; GG, Gegensu injection; DH, Danhong injection; SX, Shuxuening injection; KD, Kudiezi injection.
3.6 Publication bias
To assess potential publication bias, funnel plots were constructed for all outcome indicators, supplemented by Egger’s regression tests. The results showed that there was some publication bias in the results of UAER, TER, and SBP (p < 0.05) and no significant publication bias in Scr, BUN, β2-MG, TC, and TG (p > 0.05), as illustrated in Supplementary Appendix Figure A.2. The detailed results of Egger’s regression tests can be found in Supplementary Appendix Table A.27.
3.7 Tests of inconsistency and heterogeneity
This study did not form a closed loop, so inconsistency tests could not be performed. The results of the heterogeneity analysis showed that there was no significant heterogeneity in TER (I2 = 10.5%) and that there was significant heterogeneity in the other outcomes (I2 > 90%). More detailed information can be found in Supplementary Appendix Tables A.28–A.35. The source of heterogeneity was considered to be related to the stage of DN.
3.8 Subgroup analysis
We performed subgroup analyses using the specific stages of DN as an inclusion criterion. There were 48 trials of stage III, 34 trials of unknown stage, four trials of stage IV, and 13 trials of multiple stages. Unfortunately, a network meta-analysis of stage IV or multiple stages could not be performed for the primary endpoints because of the small number of included trials. The main results were as follows: in stage III, ST was the best treatment for UAER, which was consistent with the original network meta-analysis; for Scr, GG was the best treatment. In the unspecified stage, GG and DS were the best treatments for UAER and Scr, respectively. However, they were not fully consistent with the original results. Detailed information can be found in Supplementary Appendix Tables A.36–A.38.
3.9 Sensitivity analysis
It was taken into account that the concentration of the drug in the body may affect the results of the comparisons of efficacy. We excluded trials with an intervention period of less than 28 days and then performed a network meta-analysis, and the results of the primary endpoints were consistent with the original results. Specific information is provided in Supplementary Appendix Tables A.39, A.40.
4 Discussion
4.1 Main findings
TCMIs are sterile preparations derived from Chinese herbal materials through extraction and purification processes. They are formulated as solutions, emulsions for direct administration via an injection, or as powders or concentrated solutions that require reconstitution into solutions immediately before clinical use. As a new dosage form, TCMIs are considered a major innovation in the modernisation of Chinese medicine. It is increasingly being developed for a wide range of diseases (Geng et al., 2015; Liu et al., 2023; Ma et al., 2024). However, due to the complexity of TCMI’s composition, its quality control is still not fully resolved. Fortunately, the identification and exploration of relevant components has been ongoing. The CFDA has enhanced and standardized the protocols for the identification, testing, content determination, and pharmacological evaluation of TCMI components, thereby providing clearer evidence to support their safety profiles. Additional details can be found in Supplementary Appendix Tables A.2–A.4.
This network meta-analysis evaluated and compared the efficacy and safety of ten TCMIs in combination with ACEI/ARB in the treatment of DN. As is known, UAER is an important indicator of disease staging in DN. Scr, BUN, and β2-MG can reflect glomerular filtration function to some extent. Their monitoring is effective in predicting the progression of DN to a certain extent. ST and SK were excellent in reducing UAER and Scr, respectively. However, subgroup analysis was performed, and it was found that this result was not stable and could be affected by the stage of DN. The results of subgroup analysis need to be considered with caution. In the subgroup analyses, GG had a greater impact on the primary endpoints, probably because GG included only one trial in the primary endpoints. In addition, many studies did not report the stage of DN, which also affected the results of the subgroup analysis. DN is mostly undetected in stages Ⅰ and Ⅱ without obvious clinical manifestations, so the results of the original network meta-analysis may be more reliable than those of the subgroup analysis. DS demonstrated the highest TER in treating DN. However, this finding was based on only three RCTs of limited quality, so it should be treated with caution. Additionally, it exhibited the most significant effect in reducing BUN. YX was excellent in reducing β2-MG. For UAER and Scr, DS represented the most effective treatment option. The heterogeneity of several indicators in this study was high, and this study explored the source of heterogeneity by exploring the stage of DN. In fact, conventional treatments and the dosage of different TCMIs may also affect the results. However, the original study had limited coverage of conventional treatment and could not quantitatively compare its impact on outcomes. In addition, the issue of not being able to standardise the dosage of different TCMIs was not known to have an effect on the results. In addition, all of the original studies included in this meta-analysis were conducted in China, and many did not report the use of double-blind procedures or random allocation methods. Concerns have been raised regarding the reproducibility and quality of the original studies, which will affect the results of the meta-analysis. Therefore, more high-quality studies on TCMIs are needed in order to further validate these results.
Many diabetics with inadequate glycaemic control have abnormal lipid profiles. Studies have demonstrated that hypertriglyceridemia is closely related to atherosclerosis in diabetic patients (La Sala et al., 2019; Zhang et al., 2022). So, TC and TG may also reflect renal function to some extent. This study demonstrated that DC was the most effective in reducing TG, whereas ST exhibited superior efficacy in lowering TC. The evidence indicates that in diabetic and non-diabetic nephropathy, a more intensive blood pressure reduction below 140/90 mmHg may produce a favourable effect on renal function, and survival is multi-fold (Grassi et al., 2016). Therefore, blood pressure management is also important in slowing down the progression of DN. In this study, we demonstrated the significant efficacy of SX in lowering SBP in DN patients. However, this finding was based on only one RCT of limited quality, so it should be treated with caution. Furthermore, no significant ARs were observed in the relevant studies on HQ, whereas varying degrees of ARs were reported in others.
The aetiology of DN is complex, including inflammatory responses, dysregulation of glycolipid metabolism, oxidative stress, autophagy, endoplasmic reticulum stress, and immune responses. ST, SK, YX, and DS can optimally improve different renal function indices. Contemporary pharmacological research suggests that the diverse Chinese medicinal components present in these TCMIs may contribute to delaying the progression of DN through multiple pathways.
DS, a single herbal formulation, has shown superior efficacy in reducing BUN. It may preserve glomerular filtration integrity primarily through its anti-inflammatory targeting of the NF-κB/p38 MAPK pathway (Zhang et al., 2018). In contrast, SK, a multi-herbal compound, shows enhanced Scr-lowering effects through synergistic actions. Astragaloside IV inhibits Nox4 signalling-mediated oxidative stress and slows down podocyte apoptosis, whereas salvia components inhibit TGF-β/Smad3-driven fibrosis, collectively improving tubular secretory capacity and metabolic homoeostasis (Huang et al., 2023; Shen et al., 2023; Huang et al., 2024; Liu et al., 2024). This mechanistic divergence may underscore the glomerular-centric action of DS versus the multi-targeted tubulointerstitial remodelling of SK.
DC contains Salvia miltiorrhiza and Chuanxiongzine, which can effectively reduce TG. Danshen polysaccharide is the main active component in the aqueous extract of Salvia miltiorrhiza and can attenuate immune liver injury in mice by inhibiting the activation of the TLR4/MyD88 signalling pathway (Wang X. et al., 2019). Ligusticum chuanxiong alleviates liver injury by regulating nitric oxide biosynthesis and key pathways such as Toll-like receptor, NOD-like receptor, and TNF signalling pathways (Lu et al., 2023). The liver is the main site of cholesterol synthesis. Thus, both synergistically regulate cholesterol metabolism.
YX is an extract of Ginkgo biloba. Ginkgo biloba extracts attenuate β-glycerophosphate-induced vascular smooth muscle calcification by inhibiting the Wnt/β-catenin signalling pathway. It also inhibits extracellular matrix accumulation, epithelial–mesenchymal marker expression, and endoplasmic reticulum stress (Wang J. et al., 2019; Han et al., 2021). β2-MG passes easily through the glomerular filtration membrane, and almost all filtered β2-MG is reabsorbed and degraded by proximal tubular cells. It is therefore not difficult to understand why YX is more effective in reducing β2-MG. Moreover, Ginkgo biloba extract might exert its vascular protective effects by inducing vascular HO-1 expression (Chen et al., 2011). Therefore, the control of SBP is better in all the injections containing Ginkgo biloba extract, such as SX and YX.
ST is an extract of leeches and earthworms. It demonstrates dual efficacy in reducing UAER and TC. Hirudin protects the kidney and prevents proteinuria by inhibiting the p38 MAPK signalling pathway, reducing endoplasmic reticulum stress in podocytes and attenuating PAN damage to podocyte cytoskeletal proteins (Long et al., 2022). Aqueous extract of earthworms attenuates oxidative stress-induced renal injury by activating intrarenal Sirt1/Nrf2 cascade and ameliorating mitochondrial damage (Shu et al., 2024). In addition, the aqueous extract of earthworms reduces liver fibrosis in mice through the activation of the hepatic LKB1/Nrf2 signalling pathway, which suppresses hepatic stellate cell activation, thereby reducing triglyceride synthesis (Zhang T. et al., 2024).
4.2 Comparison with other studies
To the best of our knowledge, this study was the most comprehensive and up-to-date systematic review and network meta-analysis evaluating almost all the studies of TCMIs used in combination with ACEI/ARB for the treatment of DN. In the past, most of the studies of herbal injections combined with ACEI/ARB for the treatment of DN involved a single TCMI or several TCMIs (Zhu et al., 2022; Zhang Z. et al., 2024). A previous study conducted a network meta-analysis of TCM decoctions that had a large number of ingredients, and it was not possible to control for variable uniqueness. Therefore, there was some bias. The composition of TCMIs is more stable than that of TCM decoctions, which is of quantitative importance. Therefore, this study was well designed to investigate the optimal herbal injections for the treatment of DN in modern clinical applications.
4.3 Limitations of the study
First, “holistic diagnosis and treatment” was the essence of TCM. Unfortunately, this study included patients with DN who were given a single injection for treatment, which, to a certain extent, violated the essence in treating diseases in TCM and may have an impact on the results. Second, most of the included studies were of low quality and did not mention the specific method of randomisation allocation and blinding. Thus, not enough information was provided to accurately evaluate randomisation and allocation concealment by the research workers. In addition, some of the included trials did not mention the specific manufacturer of the drug. Third, DH, SX, KD, and GG were included in only four studies, and ST was included in only five studies. In addition, most of the studies did not have a clear safety evaluation. Fourth, previous RCTs have lacked head-to-head studies of herbal injections, adding to the uncertainty of the results. As no closed loops were formed in the analysis, inconsistency assessment was not feasible. Finally, all the literature included was from China.
5 Reflection on study design
During the design of the systematic review protocol, detailed definitions of inclusion and exclusion criteria (population, intervention, control, and outcome) were provided in this study, but a standardised PICOTS framework was not used to structure the research question. In future comparative effectiveness studies, clearly defining all elements of PICOTS in advance (particularly the environmental factors within the setting) will facilitate a more comprehensive understanding of the research context and potential sources of heterogeneity, and thereby enhance the rigour and transparency of the protocol design.
The reliability of our findings is severely limited by widespread methodological flaws in the included studies, particularly inadequate randomization, blinding, and critically insufficient reporting of TCMIs. This resulted in low or very low certainty of evidence (GRADE), rendering observed efficacy differences and treatment rankings highly uncertain and likely to change with future high-quality research. The fundamental limitation identified is inadequate TCMI reporting in original RCTs. Lack of chemical composition, source materials, manufacturing processes, and quality control details constitutes a critical weakness in the evidence base, which substantially compromises the reliability and translational potential. Therefore, a paramount priority for future high-quality RCTs must be the comprehensive and transparent reporting of all critical details pertaining to TCMIs used in research.
6 Conclusion
In this study, we indicate that when combined with ACEI/ARB, the Shuxuetong, Shenkang, Danshen, Danshen-Chuanxiongqin, Yinxingdamo, and Shuxuening injections may confer advantages in improving DN indicators. However, due to limitations in the methodological quality of the included studies (especially deficiencies in randomisation and blinding) and the critical lack of reporting on key information regarding TCMI components, the reliability of these findings is compromised.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further enquiries can be directed to the corresponding author.
Author contributions
AL: formal analysis, methodology, writing – original draft, and writing – review and editing. MW: data curation, software, investigation, writing – original draft, and writing – review and editing. CaW: data curation, software, and writing – review and editing. DY: data curation, software, and writing – review and editing. YT: investigation, software, and writing – review and editing. YJ: data curation, software, and writing – review and editing. CuW: data curation, software, and writing – review and editing. JG: data curation, software, and writing – review and editing. AS: software and writing – review and editing. XG: software and writing – review and editing. YG: funding acquisition, resources, supervision, and writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Administration of Traditional Chinese Medicine Young Qi Huang Scholars support project [National traditional Chinese medicine human education development (2020) No. 7]; the Leading Talent Training Program Project of Dongzhimen Hospital of Beijing University of Chinese Medicine (No. DZMG-LJRC0004); the Fundamental Research Funds for the Central Universities [grant number 2023-JYB-JBZD-010]; the China Postdoctoral Science Foundation [grant number 2024M750263]; and the Postdoctoral Fellowship Program of China Postdoctoral Science Foundation [grant number GZC20230324].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1543275/full#supplementary-material
Abbreviations
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blockers; ACEI/ARB, angiotensin-converting enzyme inhibitor or angiotensin II receptor blockers; ARs, adverse reactions; BUN, blood urea nitrogen; DN, diabetic nephropathy; DC, Danshen-Chuanxiongqin injection; DH, Danhong injection; GG, Gegensu injection; HQ, Huangqi injection; KD, Kudiezi injection; MD, mean difference; OR, odds ratio; TC, total cholesterol; TG, triglyceride; DS, Danshen injection; RCTs, randomised controlled trials; SBP, systolic blood pressure; Scr, serum creatinine; SK, Shenkang injection; ST, Shuxuetong injection; SX, Shuxuening injection; SUCRA, surface under the cumulative ranking curve; TCM, traditional Chinese medicine; TCMI, traditional Chinese medicine injection; TER, total effective rate; UAER, urinary albumin excretion rate; YX, Yinxingdamo injection; 95% CI, 95% confidence interval; β2-MG, β2-microglobulin.
References
Bilen, Y., Almoushref, A., Alkwatli, K., Osman, O., Mehdi, A., and Sawaf, H. (2023). Treatment and practical considerations of diabetic kidney disease. Front. Med. (Lausanne) 10, 1264497. doi:10.3389/fmed.2023.1264497
Chen, J. S., Huang, P. H., Wang, C. H., Lin, F. Y., Tsai, H. Y., Wu, T. C., et al. (2011). Nrf-2 mediated heme oxygenase-1 expression, an antioxidant-independent mechanism, contributes to anti-atherogenesis and vascular protective effects of Ginkgo biloba extract. Atherosclerosis 214 (2), 301–309. doi:10.1016/j.atherosclerosis.2010.11.010
Cui, B. S. (2009). Clinical observation of Astragalus injection combined with captopril in the treatment of early diabetic nephropathy. J. Clin. Exp. Med. 8 (11), 76–77.
de Boer, I. H., Khunti, K., Sadusky, T., Tuttle, K. R., Neumiller, J. J., Rhee, C. M., et al. (2022). Diabetes management in chronic kidney disease: a consensus report by the American diabetes association (ADA) and kidney disease: improving global outcomes (KDIGO). Diabetes Care 45 (12), 3075–3090. doi:10.2337/dci22-0027
DeFronzo, R. A., Reeves, W. B., and Awad, A. S. (2021). Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 17 (5), 319–334. doi:10.1038/s41581-021-00393-8
Deng, X., Li, D., Tang, Q., and Chen, Y. (2022). ACEI and ARB lower the incidence of end-stage renal disease among patients with diabetic nephropathy: a meta-analysis. Comput. Math. Methods Med. 2022, 6962654. doi:10.1155/2022/6962654
Fan, W. K. (2017). Observation on the effect of valsartan combined with danshen chuanxiongqin injection in the treatment of type 2 diabetes mellitus with microproteinuria. Chin. J. Mod. Drug. Appl. 11 (15), 107–108. doi:10.14164/j.cnki.cn11-5581/r.2017.15.060
Fried, L. F., Emanuele, N., Zhang, J. H., Brophy, M., Conner, T. A., Duckworth, W., et al. (2013). Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 369 (20), 1892–1903. doi:10.1056/NEJMoa1303154
Geng, T., Si, H., Kang, D., Li, Y., Huang, W., Ding, G., et al. (2015). Influences of Re Du Ning Injection, a traditional Chinese medicine injection, on the CYP450 activities in rats using a cocktail method. J. Ethnopharmacol. 174, 426–436. doi:10.1016/j.jep.2015.08.035
Grassi, G., Mancia, G., and Nilsson, P. M. (2016). Specific blood pressure targets for patients with diabetic nephropathy? Diabetes Care 39 (Suppl. 2), S228–S233. doi:10.2337/dcS15-3020
Han, J., Pang, X., Shi, X., Zhang, Y., Peng, Z., and Xing, Y. (2021). Ginkgo biloba extract EGB761 ameliorates the extracellular matrix accumulation and mesenchymal transformation of renal tubules in diabetic kidney disease by inhibiting endoplasmic reticulum stress. Biomed. Res. Int. 2021, 6657206. doi:10.1155/2021/6657206
Hao, J. L., Sun, X. J., and Tong, N. N. (2022). Effect of Shenkang injection on serum TGF-β1 and sICAM-1 levels in diabetic nephropathy patients. World. J. Integr. Tradit. West. Med. 17 (07), 1393–1396+1401. doi:10.13935/j.cnki.sjzx.220725
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2024). Cochrane handbook for systematic reviews of interventions version 6.5 (Cochrane). Available online at: https://www.training.cochrane.org/handbook.
Huang, W., Zhang, W., and Li, S. Y. (2004). Clinical observation on the combined treatment of diabetic nephropathy with Astragalus injection and Lotensin. Shandong J. Tradit. Chin. Med. (03), 161–162. doi:10.16295/j.cnki.0257-358x.2004.03.022
Huang, X., Gao, L., Deng, R., Peng, Y., Wu, S., Lu, J., et al. (2023). Huangqi-Danshen decoction reshapes renal glucose metabolism profiles that delays chronic kidney disease progression. Biomed. Pharmacother. 164, 114989. doi:10.1016/j.biopha.2023.114989
Huang, X., Peng, Y., Lu, L., Gao, L., Wu, S., Lu, J., et al. (2024). Huangqi-Danshen decoction against renal fibrosis in UUO mice via TGF-β1 induced downstream signaling pathway. Drug Des. Devel Ther. 18, 4119–4134. doi:10.2147/dddt.S457100
Jiang, L., Xu, Y., and Ouyang, F. (2005). Therapeutic efficacy of Astragalus injection and benazepril combination in the treatment of diabetic nephropathy. J. Emerg. Tradit. Chin. Med. 14 (2), 144–149. doi:10.3969/j.issn.1004-745X.2005.02.025
Jung, C. Y., and Yoo, T. H. (2022). Pathophysiologic mechanisms and potential biomarkers in diabetic kidney disease. Diabetes Metab. J. 46 (2), 181–197. doi:10.4093/dmj.2021.0329
La Sala, L., Prattichizzo, F., and Ceriello, A. (2019). The link between diabetes and atherosclerosis. Eur. J. Prev. Cardiol. 26 (2_Suppl.), 15–24. doi:10.1177/2047487319878373
Li, H. J. (2007). Clinical observation on the combination of Astragalus injection and captopril in the treatment of early diabetic nephropathy. J. Emerg. Tradit. Chin. Med. 16 (8).
Li, J. J., Zhou, L. J., and Shi, D. (2009). Clinical observation of Danhong injection combined with valsartan in the treatment of early DN. J. Pract. Diabetol. 5 (04), 31–32.
Li, S. B., and Zhang, T. Y. (2015). Astragalus injection in the treatment of diabetic nephropathy 30 cases of efficacy observation. Mod. Diagn. Treat. 26 (21), 4869–4870.
Li, S. H. (2009). Combination of ginkgolides injection and fosinopril for the treatment of diabetic nephropathy. Med. Forum 13 (02), 2–3.
Liu, H., An, N., Wang, L., Li, Y., Song, K., Sun, Y., et al. (2023). Protective effect of Xingnaojing injection on ferroptosis after cerebral ischemia injury in MCAO rats and SH-SY5Y cells. J. Ethnopharmacol. 301, 115836. doi:10.1016/j.jep.2022.115836
Liu, M., Di, Y. M., May, B., Zhang, A. L., Zhang, L., Chen, J., et al. (2024). Renal protective effects and mechanisms of Astragalus membranaceus for diabetic kidney disease in animal models: an updated systematic review and meta-analysis. Phytomedicine 129, 155646. doi:10.1016/j.phymed.2024.155646
Liu, W., Yang, Z. D., Wang, D. M., and Pang, S. H. (2009). Clinical observation on Danhong injection combined with enalapril in the treatment of type 2 diabetic nephropathy. China Pract. Med. 4 (11), 148–149.
Long, C., Feng, H., Liu, Z., Li, Z., Liu, J., Jiang, Y., et al. (2023). Efficacy of traditional Chinese medicine injection for diabetic kidney disease: a network meta analysis and systematic review. Front. Pharmacol. 14, 1028257. doi:10.3389/fphar.2023.1028257
Long, C., Lin, Q., Mo, J., Xiao, Y., and Xie, Y. (2022). Hirudin attenuates puromycin aminonucleoside-induced glomerular podocyte injury by inhibiting MAPK-mediated endoplasmic reticulum stress. Drug Dev. Res. 83 (4), 1047–1056. doi:10.1002/ddr.21932
Lu, L., Lu, T., Wu, Y., Wang, Y., Ke, X., and Yang, R. (2023). Research on the effectiveness and material basis of Ligusticum chuanxiong in alleviating acute liver injury. J. Ethnopharmacol. 314, 116643. doi:10.1016/j.jep.2023.116643
Ma, C. H. (2006). Clinical observation on the combined treatment of diabetic nephropathy with astragalus injection and enalapril. Chin. Pract. J. Rural. Doct. (02), 29–30.
Ma, Y., Bai, B., Liu, D., Shi, R., and Zhou, Q. (2024). Shenqi fuzheng injection reduces cisplatin-induced kidney injury via cGAS/STING signaling pathway in breast cancer mice model. Breast Cancer (Dove Med. Press) 16, 451–469. doi:10.2147/bctt.S475860
Mogensen, C. E. (1999). Microalbuminuria, blood pressure and diabetic renal disease: origin and development of ideas. Diabetologia 42 (3), 263–285. doi:10.1007/s001250051151
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (Clin. Res. Ed.) 372, n71. doi:10.1136/bmj.n71
Puhan, M. A., Schünemann, H. J., Murad, M. H., Li, T., Brignardello-Petersen, R., Singh, J. A., et al. (2014). A GRADE working group approach for rating the quality of treatment effect estimates from network meta-analysis. Bmj. (Clin. Res. Ed.) 349, g5630. doi:10.1136/bmj.g5630
Qi, S. S., Zheng, H. X., Jiang, H., Yuan, L. P., and Dong, L. C. (2020). Protective effects of chromium picolinate against diabetic-induced renal dysfunction and renal fibrosis in streptozotocin-induced diabetic rats. Biomolecules 10 (3), 398. doi:10.3390/biom10030398
Rao, G. F., Chen, E. F., Lin, H. Y., Wu, J. Y., and Song, D. G. (2013). Clinical observation of irbesartan combined with Kudiezi injection for early diabetic nephropathy. Chin. J. Clin. Pharmacol. 29 (10), 739–741. doi:10.13699/j.cnki.1001-6821.2013.10.009
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., et al. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes Federation diabetes atlas, 9(th) edition. Diabetes Res. Clin. Pract. 157, 107843. doi:10.1016/j.diabres.2019.107843
Shen, Q., Fang, J., Guo, H., Su, X., Zhu, B., Yao, X., et al. (2023). Astragaloside IV attenuates podocyte apoptosis through ameliorating mitochondrial dysfunction by up-regulated Nrf2-ARE/TFAM signaling in diabetic kidney disease. Free Radic. Biol. Med. 203, 45–57. doi:10.1016/j.freeradbiomed.2023.03.022
Shu, G., Wang, C., Song, A., Zheng, Z., Zheng, S., Song, Y., et al. (2024). Water extract of earthworms mitigates kidney injury triggered by oxidative stress via activating intrarenal Sirt1/Nrf2 cascade and ameliorating mitochondrial damage. J. Ethnopharmacol. 335, 118648. doi:10.1016/j.jep.2024.118648
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. (Clin. Res. ed.) 366, l4898. doi:10.1136/bmj.l4898
Thomas, M. C., Cooper, M. E., and Zimmet, P. (2016). Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 12 (2), 73–81. doi:10.1038/nrneph.2015.173
Tian, P. W., Wang, J. J., and Wang, X. Q. (2009). Astragalus combined with enalapril in the treatment of early diabetic nephropathy 40 cases of efficacy observation. J. Shanxi Coll. Tradit. Chin. Med. 10 (05), 39–40.
Tuttle, K. R., Bakris, G. L., Bilous, R. W., Chiang, J. L., de Boer, I. H., Goldstein-Fuchs, J., et al. (2014). Diabetic kidney disease: a report from an ADA consensus conference. Am. J. Kidney Dis. 64 (4), 510–533. doi:10.1053/j.ajkd.2014.08.001
Wada, J., and Makino, H. (2013). Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. (Lond) 124 (3), 139–152. doi:10.1042/cs20120198
Wang, F., and Xu, T. (2020). A Meta analysis on effectiveness and safety of shenkang injection in the treatment of diabetic nephropathy. Guangming. J. Chin. Med. 35 (17), 2639–2643.
Wang, G. C. (2012). Exploring the efficacy of valsartan combined with ginkgo dipyridamolum injection in patients with early diabetic nephropathy. China Pract. Med. 7 (25).
Wang, J., Qiu, X., Xu, T., Sheng, Z., and Yao, L. (2019a). Sclerostin/receptor related protein 4 and Ginkgo biloba extract alleviates β-Glycerophosphate-Induced vascular smooth muscle cell calcification by inhibiting Wnt/β-Catenin pathway. Blood Purif. 47 (Suppl. 1), 17–23. doi:10.1159/000496219
Wang, R. H., Xie, L. P., Su, Z. D., Xie, Y. X., Shi, W., Liu, S. H., et al. (2019b). Effect of combination of shuxuetong injection (SXT) and ACEI or ARB in treatment of diabetic nephropathy: a meta-analysis. Liaoning J. Tradit. Chin. Med. 46 (02), 225–230. doi:10.13192/j.issn.1000-1719.2019.02.001
Wang, X., Han, C., Qin, J., Wei, Y., Qian, X., Bao, Y., et al. (2019c). Pretreatment with Salvia miltiorrhiza polysaccharides protects from Lipopolysaccharides/d-Galactosamine-Induced liver injury in mice through inhibiting TLR4/MyD88 signaling pathway. J. Interferon Cytokine Res. 39 (8), 495–505. doi:10.1089/jir.2018.0137
Xie, F., Zhang, B., Dai, S., Jin, B., Zhang, T., and Dong, F. (2021). Efficacy and safety of Salvia miltiorrhiza (Salvia miltiorrhiza Bunge) and ligustrazine injection in the adjuvant treatment of early-stage diabetic kidney disease: a systematic review and meta-analysis. J. Ethnopharmacol. 281, 114346. doi:10.1016/j.jep.2021.114346
Xu, X. L. (2008). Clinical observation on effect of losartan associated with astragalus injection on early diabetic nephropathy. Chin. J. Pract. Intern. Med. 28 (S2), 42–44.
Xu, Z. X. (2009). Clinical observation on 28 cases of early diabetic nephropathy treated with valsartan combined with astragalus injection. Guangxi Med. J. 31 (6).
Yang, Q. H., Li, Y. S., and Zhang, S. Z. (2007). Efficacy observation on 35 cases of early diabetic nephropathy treated with Shu Xuening injection combined with Benadryl. J. New Chin. Med. (08), 92–93. doi:10.13457/j.cnki.jncm.2007.08.064
Yuan, D. Y. (2009). The efficacy of Puerarin injection combined with Benadryl in early diabetic nephropathy. Chin. J. Integr. Tradit. West. Nephrol. 10 (02), 167–168.
Zhang, H. F., Wang, Y. L., Gao, C., Gu, Y. T., Huang, J., Wang, J. H., et al. (2018). Salvianolic acid A attenuates kidney injury and inflammation by inhibiting NF-κB and p38 MAPK signaling pathways in 5/6 nephrectomized rats. Acta Pharmacol. Sin. 39 (12), 1855–1864. doi:10.1038/s41401-018-0026-6
Zhang, J., Xiao, Y., Hu, J., Liu, S., Zhou, Z., and Xie, L. (2022). Lipid metabolism in type 1 diabetes mellitus: pathogenetic and therapeutic implications. Front. Immunol. 13, 999108. doi:10.3389/fimmu.2022.999108
Zhang, T., Wang, C., Song, A., Lei, X., Li, G., Sun, H., et al. (2024a). Water extract of earthworms mitigates mouse liver fibrosis by potentiating hepatic LKB1/Nrf2 axis to inhibit HSC activation and hepatocyte death. J. Ethnopharmacol. 321, 117495. doi:10.1016/j.jep.2023.117495
Zhang, Z., Luo, L., Li, X., and Zhong, Y. (2024b). The effects of Salvia miltiorrhiza and ligustrazine injection combined with ACEI/ARB on diabetic kidney disease: a systematic review and meta-analysis. Med. (Baltimore) 103 (8), e35853. doi:10.1097/md.0000000000035853
Zhao, Z. M. (2014). Clinical analysis of irbesartan combined with danshen chuanxiongqin in the treatment of diabetic nephropathy. Diabetes New World 34 (22), 13–14. doi:10.16658/j.cnki.1672-4062.2014.22.091
Zheng, J. J. (2013). Effects of Kudiezi injection combined with Benazepril on urine protein and blood rheology in patients with type 2 diabetic nephropathy: a prospective clinical study. Pract. Clin. Med. 14 (05), 12–14+25.
Zhou, A. J., Zhou, G. Z., and Li, J. L. (2009). Effects of salvia injection and irbesartan on urine protein in patients with diabetic nephropathy. Clin. Educ. Gen. Pract. 7 (06), 595–596+600.
Zhu, Y. X., Liu, X., and Yang, S. R. (2003). Clinical observation of Puerarin injection and Enalaprilat in treatment of diabetic nephropathy. Chin. J. Integr. Tradit. West. Med. Intensive Crit. Care 10 (1), 52–53. doi:10.3321/j.issn:1008-9691.2003.01.018
Zhu, Z., Zhang, Q., Liu, L., Bao, P., Liu, S., Song, C., et al. (2022). Clinical efficacy and safety of astragalus injection combined with ACEI/ARB in the treatment of diabetic kidney disease: protocol for a systematic review and meta-analysis. Med. (Baltimore) 101 (49), e31490. doi:10.1097/md.0000000000031490
Keywords: traditional Chinese medicine injections, diabetic nephropathy, angiotensin-converting enzyme inhibitors, network meta-analysis, systematic review, angiotensin II receptor blockers
Citation: Li A, Wei M, Wu C, Yin D, Tang Y, Jiang Y, Wang C, Guo J, Sun A, Gu X and Gong Y (2025) Comparative effectiveness of traditional Chinese medicine injections combined with ACEI/ARB for diabetic nephropathy: A systematic review and network meta-analysis. Front. Pharmacol. 16:1543275. doi: 10.3389/fphar.2025.1543275
Received: 11 December 2024; Accepted: 12 June 2025;
Published: 18 July 2025.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Hewang Lee, George Washington University, United StatesYeshen Zhang, Central South University, China
Yunling Xu, Zhejiang Academy of Traditional Chinese Medicine, China
Yunhao Yi, Shandong University of Traditional Chinese Medicine, China
Copyright © 2025 Li, Wei, Wu, Yin, Tang, Jiang, Wang, Guo, Sun, Gu and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanbing Gong, Z3liXzEyMjZAMTYzLmNvbQ==
†These authors share first authorship
 Aijing Li
Aijing Li Maoying Wei
Maoying Wei Chan Wu
Chan Wu Dan Yin1
Dan Yin1 Yijia Jiang
Yijia Jiang Churan Wang
Churan Wang Jingyi Guo
Jingyi Guo Yanbing Gong
Yanbing Gong