- 1Department of Orthopaedics, Tianjin Hospital, Tianjin, China
- 2Department of Pharmacy, Suqian First Hospital, Suqian, China
- 3Tianjin Institute of Orthopedics in Traditional Chinese and Western Medicine, Tianjin, China
Background: The objective of this study was to conduct a systematic review and network meta-analysis (NMA) to compare the efficacy of various anti-osteoporosis drugs in preventing femoral periprosthetic bone loss following total hip arthroplasty (THA).
Methodology: Randomized controlled trials (RCTs) assessing the clinical efficacy of various anti-osteoporosis drugs and control treatments in preventing periprosthetic bone loss following THA were identified. Outcomes evaluated included bone mineral density (BMD) at 6 months, 12 months, 24 months, and 5–10 years. The network meta-analysis was conducted using Stata 13.0 and R-3.5.1 software with the “gemtc” package.
Results: A total of 33 RCTs with 1,169 patients were included. At 6 months, alendronate, alendronate + alfacalcidol, denosumab, ibandronate, raloxifene, teriparatide + alendronate, and zoledronic acid were beneficial in increasing BMD, with denosumab ranking highest (based on surface under the cumulative ranking curve (SUCRA) values). At 12 months, alendronate, alendronate + alfacalcidol, denosumab, ibandronate, risedronate, and zoledronic acid showed benefits, with alendronate + alfacalcidol ranking highest (SUCRA = 0.97). For 24-month BMD, teriparatide + alendronate ranked highest (SUCRA = 0.82). Analysis of BMD at 5–10 years, involving four studies on alendronate, pamidronate, and placebo, indicated that alendronate achieved the highest SUCRA value (0.87).
Conclusion: Both denosumab and bisphosphonates are effective in preventing femoral periprosthetic bone loss following THA. Denosumab was the most efficient agent for increasing BMD at 6 months post-THA, while alendronate combined with alfacalcidol or feriparatide was most efficient at 12 months and 24 months. More high-quality direct comparisons and long-term follow-up studies are needed to determine the optimal drug and dosage for THA patients.
Introduction
Total hip arthroplasty (THA) is a widely performed surgical procedure designed to relieve pain and improve quality of life for individuals with conditions like osteoarthritis and osteonecrosis of the femoral head (ONFH) (Gao et al., 2017; Anderl et al., 2022; Knutsen et al., 2017). In the United States alone, more than 230,000 primary THA surgeries are performed annually, with the global figure exceeding 500,000. This number is expected to rise by approximately 10% each year (Su et al., 2021; Sariali et al., 2020). Despite its success, the long-term durability of THA is often compromised by progressive bone loss around the implant. Studies report that bone mineral density (BMD) in the proximal femur can decline by up to 40% within the first year after surgery compared to immediately post-operation (Yan et al., 2017; Gerhardt et al., 2019). This bone loss is believed to contribute to aseptic loosening and late-stage implant-related fractures, posing significant challenges to implant longevity (Zhang et al., 2018; Tapaninen et al., 2012). Consequently, various strategies, such as the utilization of bisphosphonates, denosumab, and anabolic osteoporosis drugs, have been employed to preserve bone mass and enhance the stability of the implant, ultimately leading to improved outcomes in THA (Tran et al., 2016).
Bisphosphonates, which are antiresorptive agents, can bind to hydroxyapatite and inhibit farnesyl-pyrophosphate synthase, an enzyme involved in the mevalonate pathway. These agents are frequently utilized in the management of osteoporosis and various metabolic bone disorders (Wang et al., 2018). Recent research has indicated that bisphosphonates possess the potential to decrease the likelihood of fractures and prolong the lifespan of implants (Shi et al., 2018a). Numerous studies, including randomized controlled trials (RCTs), have been conducted to explore the effects of bisphosphonates on the preservation of periprosthetic bone mineral density following THA (Shi et al., 2018b; Lin et al., 2012; Bhandari et al., 2005).
Multiple meta-analyses have been conducted to compare the efficacy of bisphosphonates versus control in mitigating bone loss subsequent to THA (Shi et al., 2018b; Zhao et al., 2015; Di Martino et al., 2024; Hatano et al., 2025). However, the indiscriminate amalgamation of outcomes with differing levels of evidence may undermine the obtained effect size. Additionally, the aggregation of results from diverse follow-up periods without proper categorization diminishes the reliability of the findings. Apart from qualitative analyses, the literature only provides two meta-analyses that compare bisphosphonates for bone loss following THA and total knee arthroplasty (Shi et al., 2018b; Bhandari et al., 2005). Regrettably, the aforementioned studies failed to distinguish between THA and total knee arthroplasty, resulting in ambiguity during the interpretation process.
Denosumab, a fully humanized monoclonal antibody, acts as an osteoprotegerin (OPG) mimicker by targeting the receptor activator of nuclear factor kappa B ligand (RANKL), which influences the activity, survival, and recruitment of osteoclasts (Deeks, 2018). In a network meta-analysis encompassing ten drugs, denosumab exhibited the most effective efficacy in preventing and tolerating BMD loss in the hip and femur (Migliorini et al., 2021).
Anabolic osteoporosis medications, such as teriparatide, are typically prescribed exclusively for patients who have confirmed and severe osteoporosis (Dong et al., 2024; Roy and Mazumdar, 2023). Teriparatide, a synthetic form of human parathyroid hormone (PTH) (1–34), is the sole authorized anabolic therapy for osteoporosis in multiple countries (Mineta et al., 2025). Research has demonstrated its efficacy in reducing the likelihood of both vertebral and nonvertebral fractures in individuals with osteoporosis induced by glucocorticoids (Liu et al., 2020).
However, a significant proportion of these articles have been subjected to placebo comparisons, while direct comparisons between different pharmacological interventions, including the utilization of innovative drugs, have been lacking. Moreover, the scarcity of evidence and the absence of direct statistical analysis pose challenges for physicians in determining the most effective treatment.
A network meta-analysis (NMA) is a methodological approach that facilitates the simultaneous comparison of multiple treatments within a single meta-analysis (Shim et al., 2019). In line with this approach, the present systematic review and NMA aim to compare different anti-osteoporosis drugs in terms of femoral periprosthetic bone loss following THA. Through the implementation of this network meta-analysis, it becomes possible to directly compare and forecast the most efficacious anti-osteoporosis drugs following THA.
Methods
This systematic review was written according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) checklist and A Measurement Tool to Assess Systematic Reviews 2 (AMSTAR2) (Shea et al., 2007). This systematic review protocol has been registered on PROSPERO (www.crd.york.ac.uk/prospero/) with the number CRD42023453329 (Page et al., 2021).
Search strategy
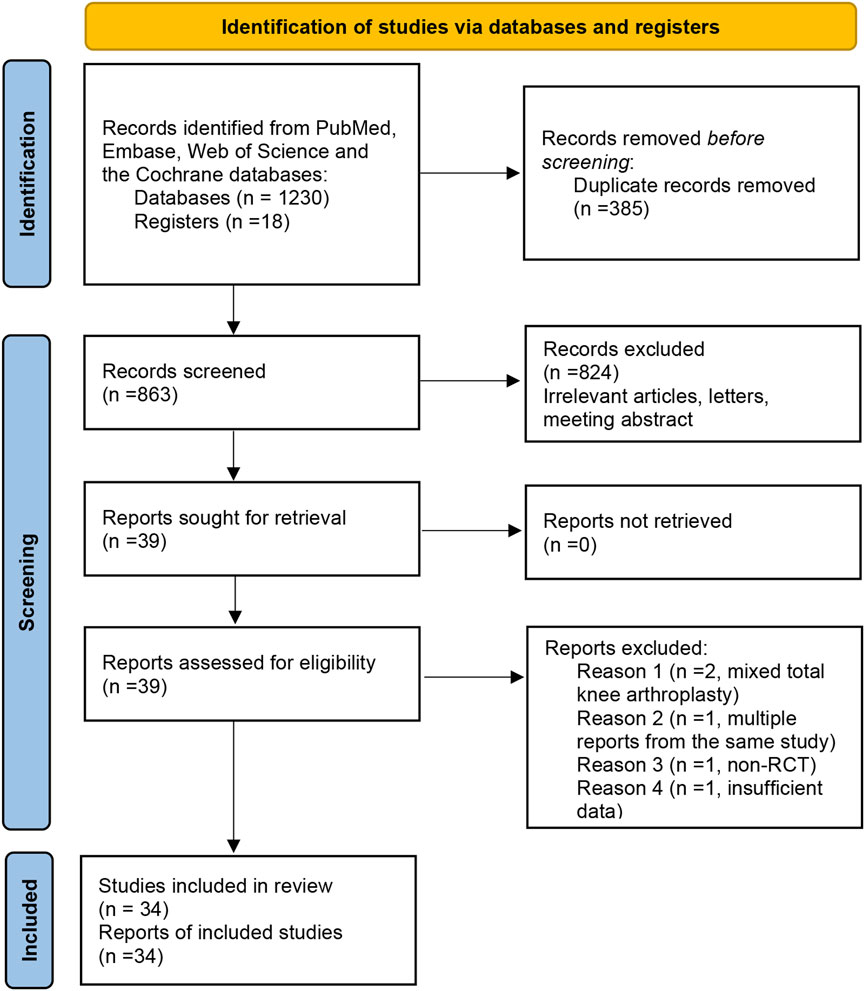
The first two authors (Zhi-hu Zhao and Tao Ling) independently searched the PubMed, Embase, Web of Science, and the Cochrane databases from the date of their inception to November 2024. Reference lists of previously published systematic reviews were also reviewed for potentially relevant studies. The terms used for screening THA were “THA or THR OR total hip arthroplasty OR total hip replacement OR ‘Arthroplasty, Replacement, Hip’ [Mesh]”, based on a previous systematic review (Shi et al., 2018b). The terms used for screening bisphosphonates were “alendronate OR pamidronate OR etidronate OR zoledronate OR clodronate OR bisphosphonate OR statin”. The term “alfacalcidol OR risedronate OR teriparatide OR denosumab OR simvastatin” was added in the searching process to identify other relevant studies. To enhance the scope of the search for pertinent studies, the terms “BMD,” “bone density,” “osteoporosis,” and “periprosthetic fracture” were employed in querying relevant databases. Keywords and medical subject headings (MeSH) terms were used to identify relevant literature. All retrieved citations were imported into EndNote X7 software (EndNote Clarivate Analytics, USA), a reference management software, for deduplication and organization. The subsequent screening process was conducted using this software to manage the literature and generate the final reference list. A flow diagram of the literature selection process can be seen in Figure 1.
Inclusion and exclusion criteria
The inclusion criteria: (1) all studies included in this NMA were RCTs, irrespective of the language or publication status; (2) Participants were adult patients (typically over 20 years of age) who underwent primary total hip arthroplasty (THA); (3) Studies using any of the following interventions were included: alendronate, etidronate, zoledronic acid, alfacalcidol, alendronate + alfacalcidol, risedronate teriparatide, denosumab pamidronate, clodronate, simvastatin ibandronate and placebo; (4) The BMD around the stem was assessed at least 6 months after THA.
The exclusion criteria: (1) non-randomized controlled study; (2) patients in the study were revision THA or had a metabolic bone disorder other than osteoporosis; (3) studies with insufficient data. The full texts of all included articles were obtained via institutional library access or the authors’ user profiles on the ResearchGate platform.
Study selection
To complete the study selection, two authors (Songqing Ye and Wei Luo) utilized EndNote (Thomson Reuters, New York, NY, United States) to organize all studies. First, the “remove duplicates” command was used, and duplicates were removed. We then reviewed abstracts of the remaining records to remove studies based on the inclusion and exclusion criteria. Finally, the full texts of potentially eligible studies were retrieved and reviewed by two independent reviewers (Jian-xiong Ma and Wei Luo), and any discrepancies during the selection process were resolved by Xinlong Ma.
Data extraction
The first two authors (Zhi-hu Zhao and Tao Ling) independently reviewed all titles and abstracts. In case of inadequate information, the full text was retrieved for further screening. The same authors independently extracted data from eligible studies, including the name of the first author, treatment, control, sample size of treatment and control, mean age of the patients, surgery, intervention duration, follow-up, results, and level of evidence. Primary outcomes were periprosthetic BMD at 6 months, 12 months, 24 months, and 5–10 years. Dual-energy X-ray absorptiometry (DXA) was used to measure periprosthetic BMD in the entire hip. To reduce bias from varying baseline data time points, BMD (g/cm2) was used instead of percentage change in BMD.
Quality assessment
The first two reviewers (Zhi-hu Zhao and Tao Ling) independently used the Cochrane’s risk of bias tool to evaluate the methodological quality of the selected RCTs. A low, unclear, or high risk of bias value was assigned to the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. At the end of quality assessment, a consensus on the final evaluation was reached; any disagreements were resolved by the judgment of another author. Kappa values were used to measure the degree of agreement between the two reviewers and were rated as follows: fair, 0.40 to 0.59; good, 0.60 to 0.74; and excellent, 0.75 or more (Higgins et al., 2011).
Assessing the transitivity assumption in network meta-analysis
A fundamental concept in network meta-analysis is the notion that all patients within a network should, in principle, be equally eligible to receive any of the available treatments. This principle is often referred to as “jointly randomizable” (Ahn and Kang, 2021). Essentially, it implies that all patients within our network should, theoretically, have the option to receive any of the anti-osteoporotic agents described in the analysis. To assess the validity of the transitivity assumption, which underpins the network meta-analysis, we evaluated whether the participants in the identified studies were jointly randomizable. We also determined whether effect modifiers were distributed consistently across different treatment options in the network (Efthimiou et al., 2016). We tested the distribution of commonly espoused effect modifiers (i.e., publication year, age, and sample size) to ensure that they were balanced and that our estimates were not confounded.
Statistically analysis
Time points of interest included 6 months, 12 months, 24 months, and 5–10 years postoperatively. In the absence of standard deviation (SD), the median value of SDs from other studies in the same comparison was borrowed. For data described as median and range, mean and SD were estimated according to a previous manner (Hozo et al., 2005). Weighted mean difference (WMD) and 95% credible intervals (CrI) were used as pooled effect size measures. Indirect network meta-analysis (NMA) was conducted on the “gemtc” and “rjags” packages in R (version 3.5.1, https://www.r-project.org/) (van Valkenhoef et al., 2012). A Bayesian random-effects analysis, which was based on the Markov chain Monte Carlo (MCMC) simulation from the posterior distribution, was adopted to calculate the estimates of relative effects and all model parameters. The probability of the best treatment was then ranked based on NMA results using the surface under the cumulative ranking curve (SUCRA). The larger the SUCRA, the higher the treatment in the hierarchy for an outcome. To evaluate the heterogeneity, the mtc.anohe command of the R package of “gemtc” was utilized by reporting the heterogeneity variance parameter I2. I2 > 50% was regarded as significant heterogeneity, and the random-effects model was utilized; otherwise, the fixed-effect model was utilized. To measure the consistency of results between direct and indirect meta-analyses, the node-splitting method or a comparison of effect sizes obtained from direct and indirect results was applied when applicable (Dias et al., 2010).
We performed a random-effects network meta-regression within a Bayesian hierarchical framework using the gemtc package in R. The gemtc package automatically determines uninformative prior distributions for all parameters in our model, which are commonly applied in NMA. We ran the Markov chain Monte Carlo simulation with four chains for each model, using 100,000 iterations, a burn-in of 5000 iterations, and extraction of every 10th value. We assessed convergence with the Gelman–Rubin–Brooks plot and potential scale reduction factor (a threshold of <1.05 indicates adequate convergence). We specified our assumptions (common or exchangeable covariate-comparison interaction) for each network meta-regression model once the data were available so as to make best use of the available data. We assumed coherent relative treatment effects estimated at the covariate value 0 and coherent regression coefficients for the treatment effect by covariate interaction. The network plot and funnel plots were generated using STATA 13.0 software (StataCorp LLC, College Station, TX, USA).
Results
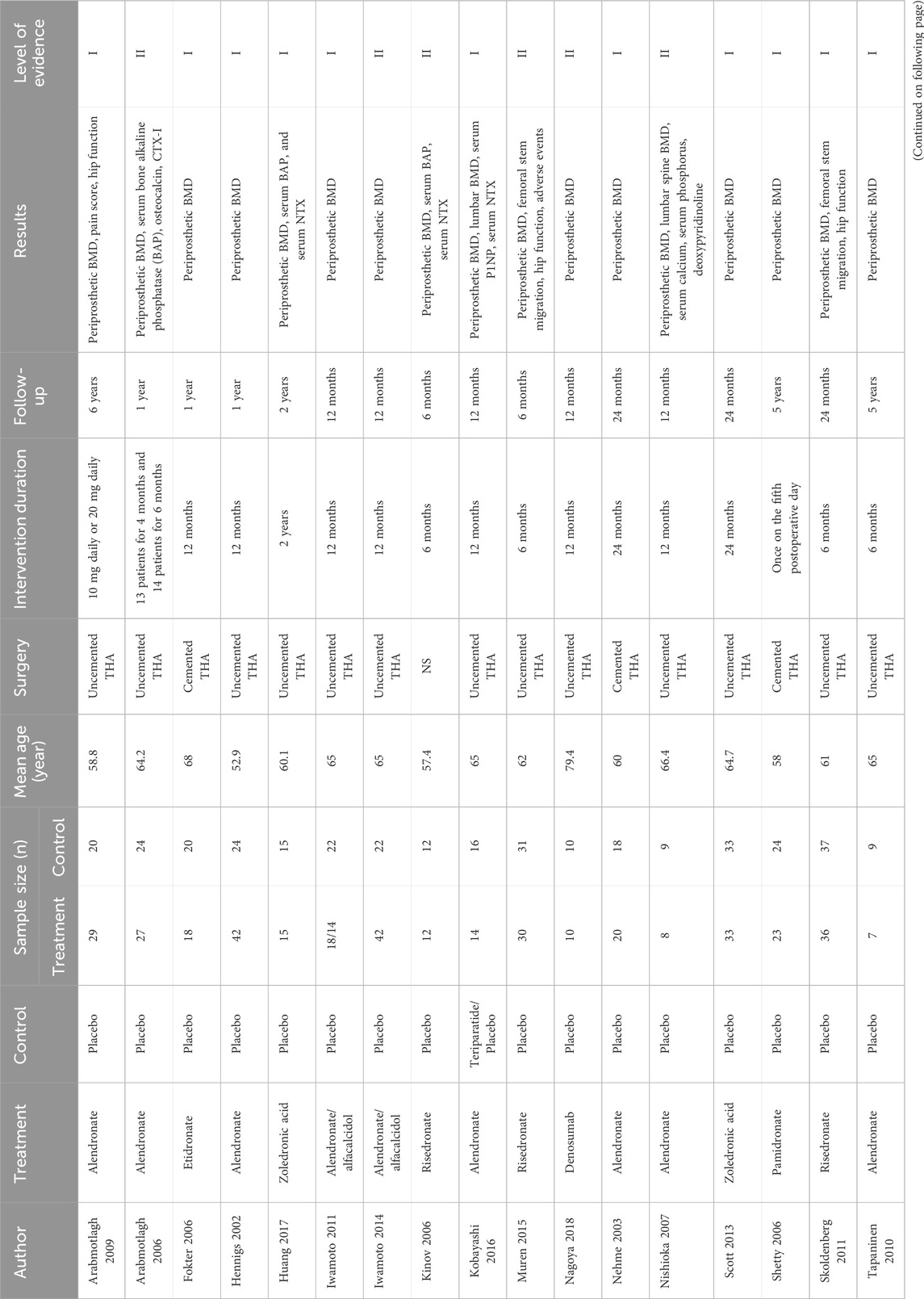
A total of 1,248 titles were obtained by primary search, including 1230 electronic records and 18 additional records from other sources. A total of 855 records were identified after duplicates were removed. Finally, 33 studies with 1,169 patients were included (Zhang et al., 2018; Arabmotlagh et al., 2009; Arabmotlagh et al., 2006; Arnala, 2012; Aro et al., 2018; Fokter et al., 2005; Gong et al., 2020; Hennigs et al., 2002; Huang et al., 2017; Iwamoto et al., 2011; Iwamoto et al., 2014; Kinov et al., 2006; Kobayashi et al., 2016; Morita et al., 2020; Muren et al., 2015; Nagoya et al., 2018; Nehme et al., 2003; Nishioka et al., 2007; Nyström et al., 2020; Scott et al., 2013; Shetty et al., 2006; Skoldenberg et al., 2011; Tapaninen et al., 2010; Trevisan et al., 2010; Venesmaa et al., 2001; Wilkinson et al., 2005; Yamaguchi et al., 2004; Yamaguchi et al., 2003; Yamasaki et al., 2007; Yang et al., 2019; Yukizawa et al., 2017; Zhou et al., 2019; Yamaguchi et al., 2005). Nine RCTs compared alendronate to placebo for BMD loss after THA. One RCT compared etidronate to placebo for BMD loss after THA. Three studies compared zoledronic acid to a placebo for BMD loss after THA. Five studies compared risedronate to placebo for BMD loss after THA. One study compared simvastatin to a placebo for BMD loss after THA. One study compared alendronate to teriparatide for BMD loss after THA. One study compared denosumab to placebo for BMD loss after THA. One study compared pamidronate to a placebo for BMD loss after THA. One study compared ibandronate and clodronate to a placebo for BMD loss after THA. Follow-up duration ranged from 6 months to 10 years (Table 1).
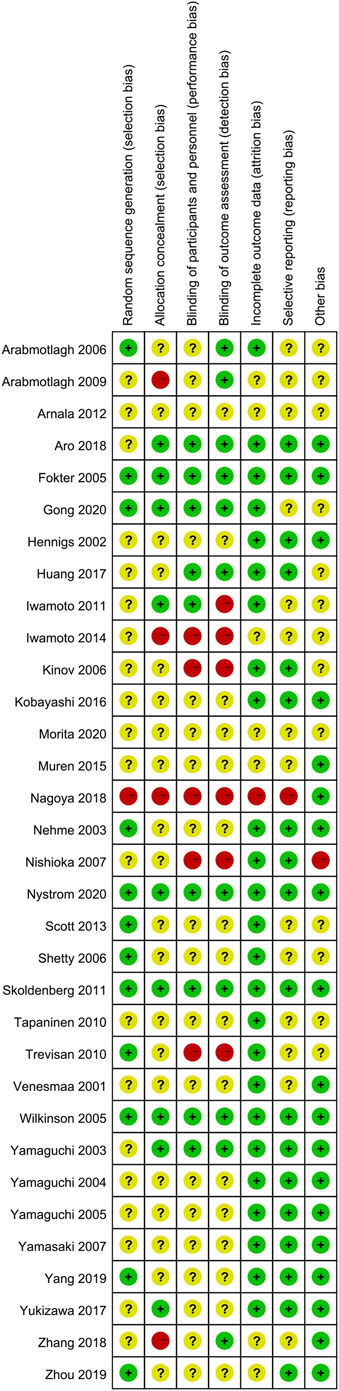
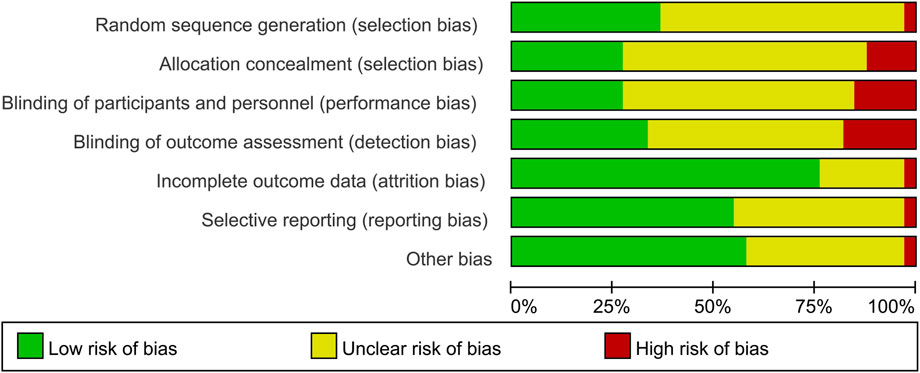
Only 12 studies reported random sequence generation, which was classified as low risk of bias. Among the 34 studies included, one was evaluated as “high risk” in terms of selection bias related to random sequence generation. The remaining studies were categorized as having an unclear risk of bias. In terms of selection bias associated with allocation concealment, 26.5% of the studies were classified as low risk, 11.8% as high risk, and 60.5% as unclear risk of bias. Blinding of participants and personnel emerged as the most commonly identified factor associated with potential bias, with 10 studies deemed to have a low risk of bias due to participant blinding (29.4%). Additionally, a total of 12 studies were identified as having a low risk of bias for outcome assessment blinding (35.3%). A total of seven studies were classified as unclear risk of bias for incomplete outcome data. A total of eight studies were classified as low risk of bias for selective reporting, and 20 studies were classified as low risk of bias for other bias (Figures 2, 3). The overall kappa value regarding the evaluation of risk of bias of included RCTs was 0.827, indicating an excellent degree of agreement between the two reviewers.
Assessment of transitivity
After grouping the studies by treatment comparison and inspecting the distribution of possible effect modifiers, there were no significant differences between the demographic characteristics for all treatments (Supplementary Figure S1). Therefore, they were judged to be sufficiently similar to be jointly synthesized in a network meta-analysis.
Network structure diagrams
In this article, 15 different therapeutic drugs, namely, alendronate, alendronate + alfacalcidol, alfacalcidol, calcitonin, clodronate, denosumab, etidronate, ibandronate, pamidronate, raloxifene, risedronate, simvastatin, teriparatide, teriparatide + alendronate, zoledronic acid, and placebo, were finally enrolled. Four clinical outcomes comprising BMD at 6 months, 12 months, 24 months, and 5–10 years were ultimately evaluated. As displayed in Figure 4, the network structure diagrams detail the direct comparisons between different drugs in the four clinical outcomes, respectively. In addition, the numbers show the number of direct comparisons. Line thicknesses are proportional to the number of direct comparisons. Circle diameters are proportional to the number of patients treated who were included in this network meta-analysis.
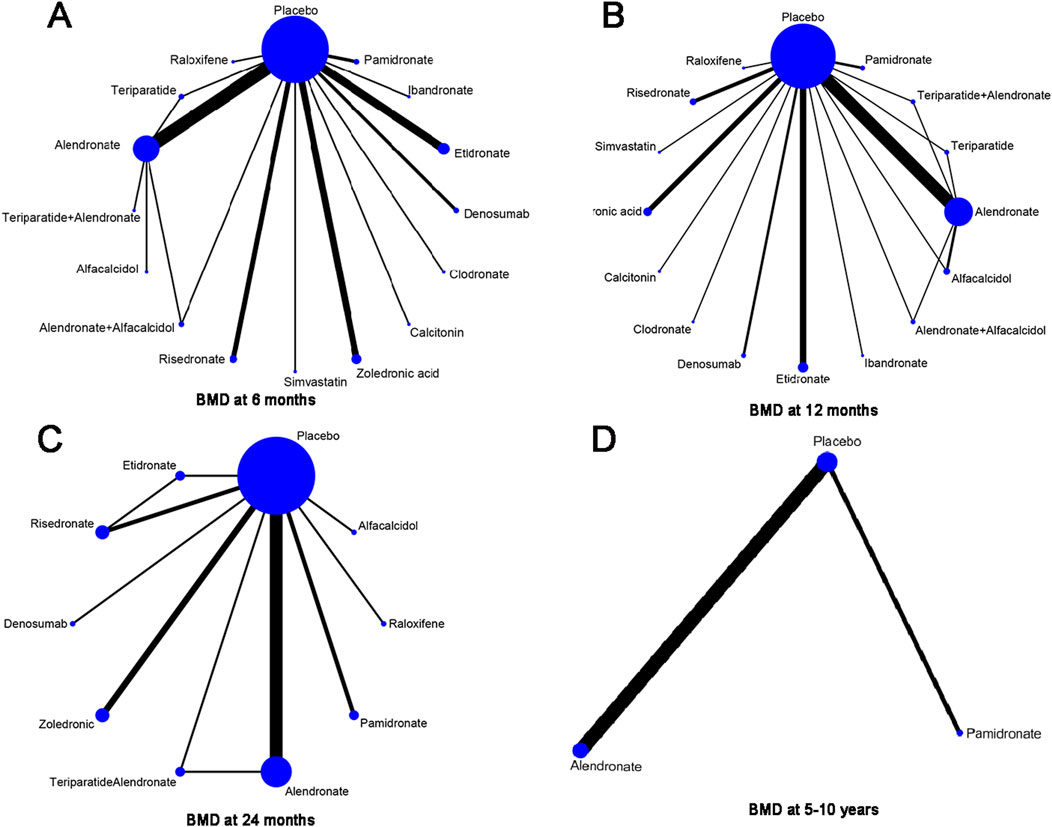
Figure 4. Network structure diagrams. (A) BMD at 6 months; (B) BMD at 12 months; (C) BMD at 24 months; and (D) BMD at 5–10 years. The numbers showed the number of direct comparisons. Line thicknesses are proportional to the number of direct comparisons. Circle diameters are proportional to the treatment numbers.
BMD at 6 months
A total of 31 RCTs, including 15 drugs (alendronate, alendronate + alfacalcidol, alfacalcidol, calcitonin, clodronate, denosumab, etidronate, ibandronate, pamidronate, raloxifene, risedronate, simvastatin, teriparatide, teriparatide + alendronate, zoledronic acid, and placebo) contributed to the clinical outcome of the BMD at 6 months. The network meta-analysis demonstrated that, compared with the control group, alendronate (WMD = 0.24, 95% CrI: 0.17, 0.32), alendronate + alfacalcidol (WMD = 0.24, 95% CrI: 0.04, 0.44), denosumab (WMD = 0.44, 95% CrI: 0.31, 0.57), ibandronate (WMD = 0.38, 95% CrI: 0.20, 0.56), raloxifene (WMD = −0.2, 95% CrI: 0.00, 0.40), teriparatide + alendronate (WMD = 0.21, 95% CrI: 0.02, 0.40), and zoledronic acid (WMD = 0.12, 95% CrI: 0.01, 0.23) exhibited a beneficial role in increasing the BMD at 6 months (Table 2). Figure 5A summarizes the heterogeneity between different comparisons of drugs. There was high heterogeneity between alendronate and placebo (I2 = 80.5%), pamidronate and placebo (I2 = 93.6%), denosumab and placebo (I2 = 83.9%), risedronate and placebo (I2 = 87.2%), and zoledronic acid and placebo (I2 = 69.0%). Thus, we applied the random-effect model for the cumulative values. As shown in Figures 5B,C, the SUCRA values of the five interventions demonstrated that denosumab ranked the highest SUCRA values of the BMD at 6 months (0.97).
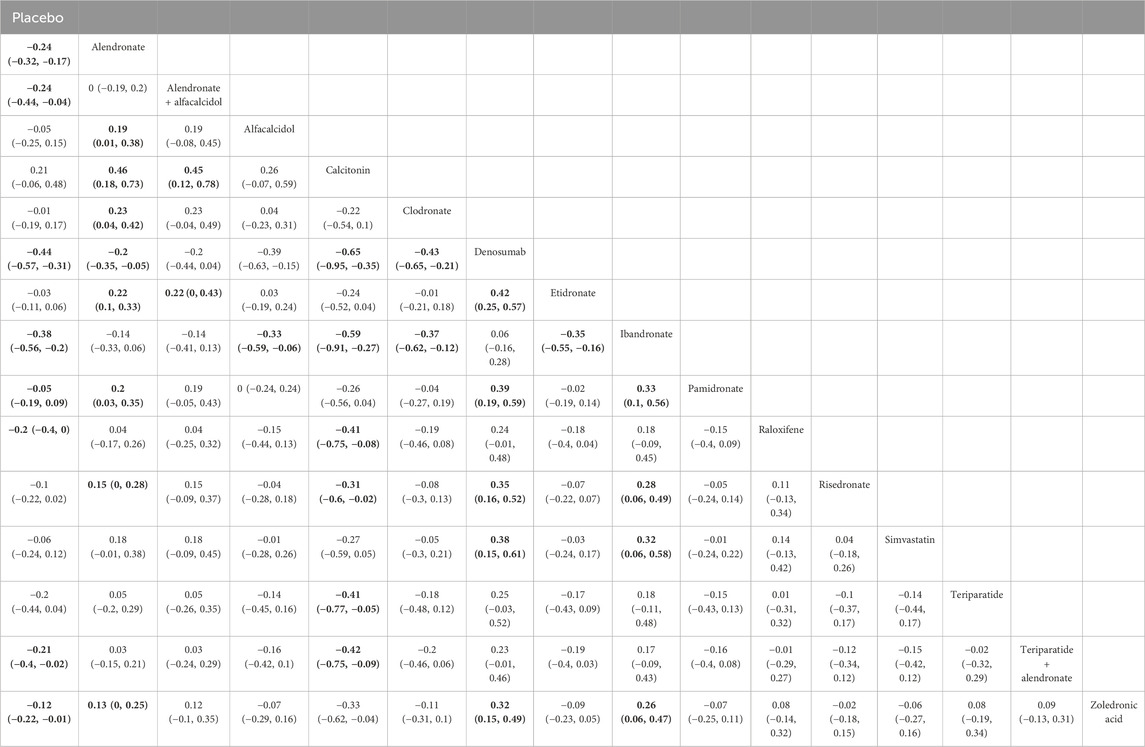
Table 2. Efficacy of different comparisons of drugs by WMDs and corresponding 95% CrIs for BMD at 6 months. Bold fonts indicate p-value <0.05.
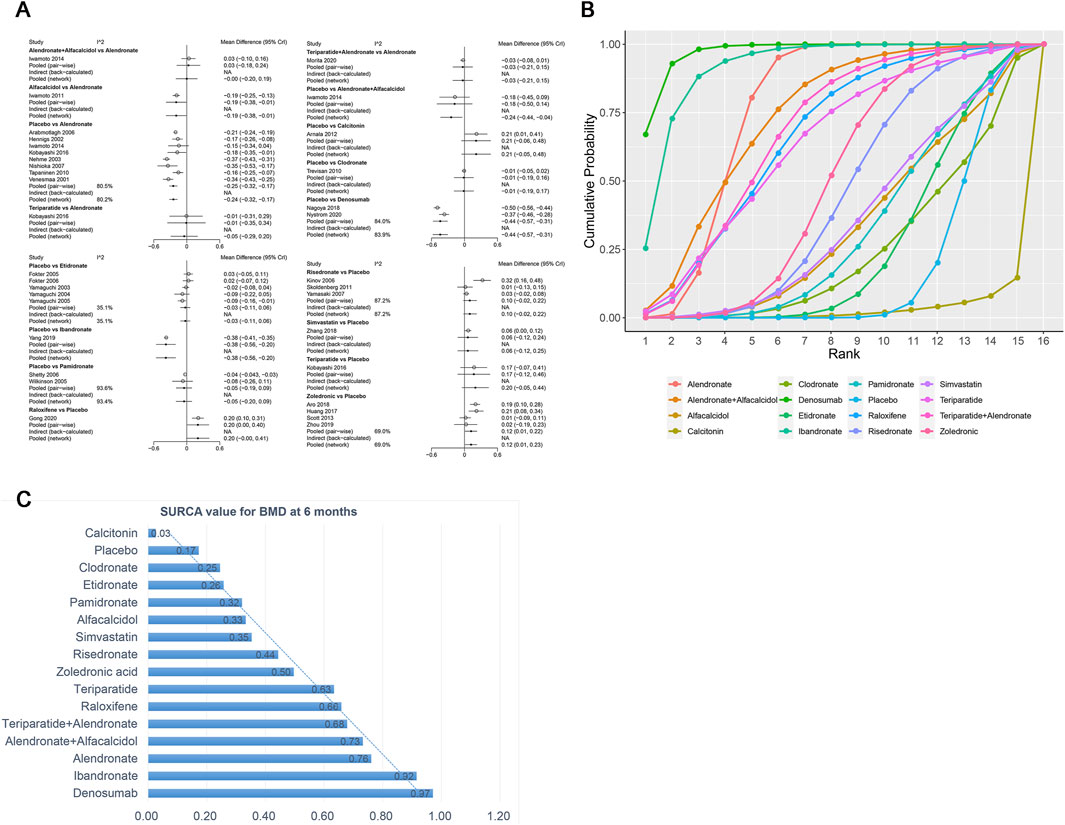
Figure 5. (A) Heterogeneity between different comparisons of drugs for BMD at 6 months. (B) Surface under the cumulative ranking curve (SUCRA) probabilities of different drugs for BMD at 6 months. (C) SUCRA values of the different drugs for BMD at 6 months.
BMD at 12 months
The network meta-analysis demonstrated that, compared with the control group, alendronate (WMD = 0.17, 95% CrI: 0.07, 0.27), alendronate + alfacalcidol (WMD = 0.6, 95% CrI: 0.29, 0.89), denosumab (WMD = 0.36, 95% CrI: 0.13, 0.58), ibandronate (WMD = 0.38, 95% CrI: 0.07, 0.69), risedronate (WMD = −0.30, 95% CrI: −0.49, -0.11), and zoledronic acid (WMD = −0.18, 95% CrI: −0.35, -0.01) exhibited a beneficial role in increasing the BMD at 12 months (Table 3). Figure 6A summarizes the heterogeneity between different comparisons of drugs. There was high heterogeneity between alendronate and placebo (I2 = 91.0%), etidronate and placebo (I2 = 69.7%), zoledronic acid and placebo (I2 = 70.6%), pamidronate and placebo (I2 = 97.4%), and risedronate and placebo (I2 = 94.7%). Thus, we applied the random effect model for the cumulative values.
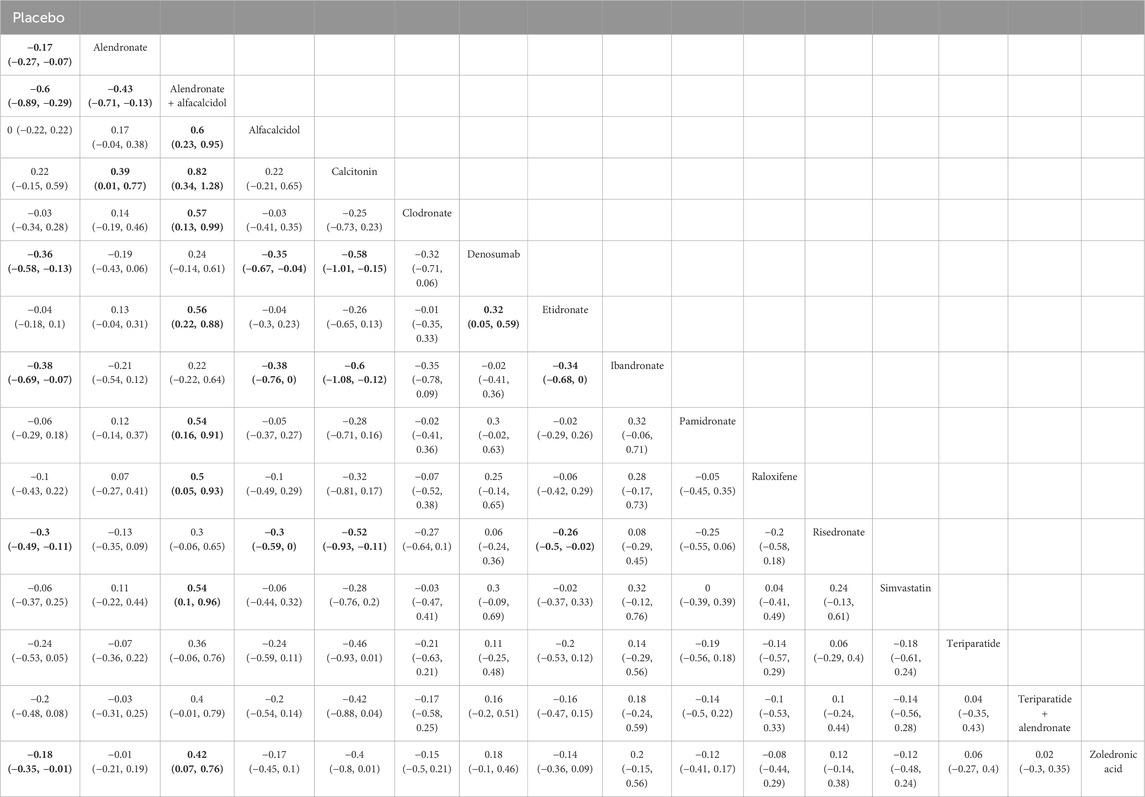
Table 3. Efficacy of different comparisons of drugs by WMDs and corresponding 95% CrIs for BMD at 12 months; Bold fonts indicate p-value <0.05.
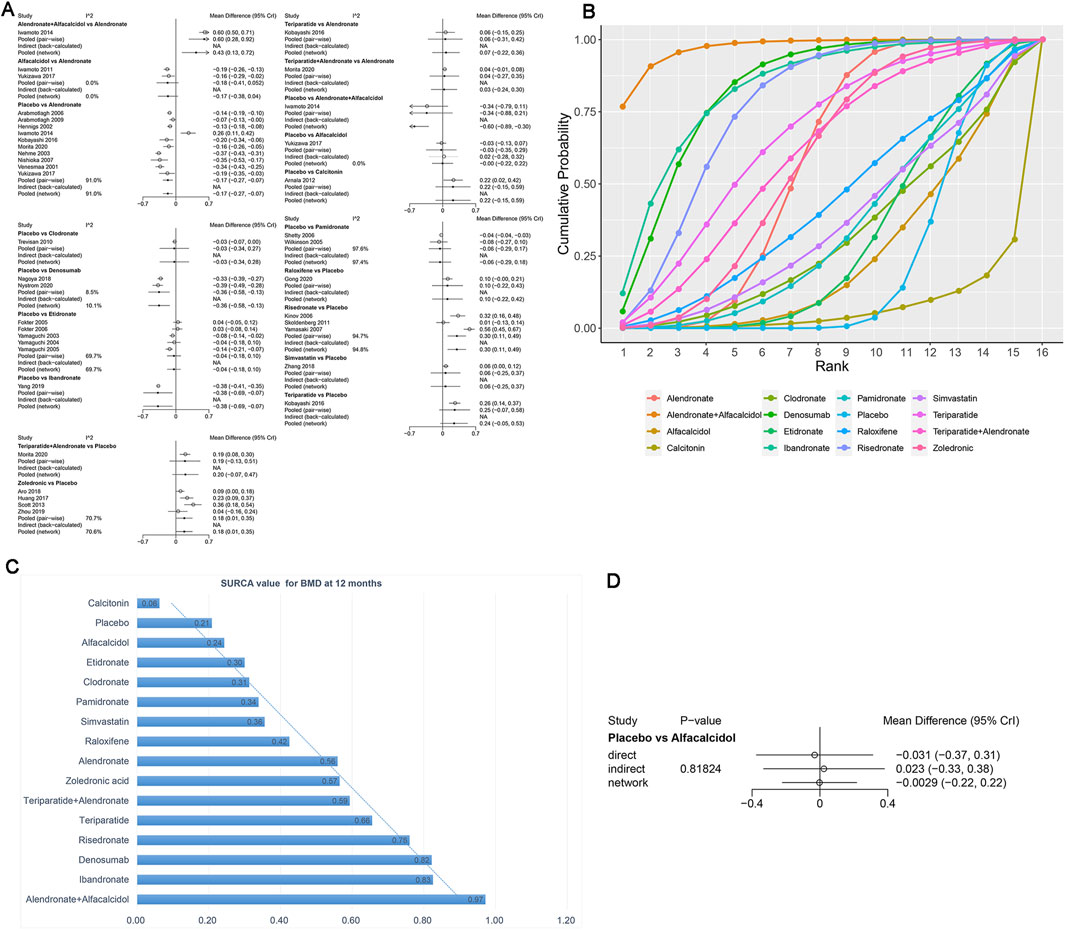
Figure 6. (A) Heterogeneity between different comparisons of drugs for BMD at 12 months. (B) Surface under the cumulative ranking curve (SUCRA) probabilities of different drugs for BMD at 12 months. (C) SUCRA values of the different drugs for BMD at 12 months. (D) Node-splitting method in comparisons between direct and indirect evidence for BMD at 12 months.
As shown in Figures 6B,C, the SUCRA values of the five interventions demonstrated that alendronate + alfacalcidol was most effective for BMD at 12 months (SUCRA = 0.97), followed by ibandronate (SUCRA = 0.83) and denosumab (SUCRA = 0.82).
We used the node-splitting method and its Bayesian p-value to report the inconsistency of our results. The confidence intervals from direct and indirect evidence are, in general, consistent, with minor differences (Figure 6D, p = 0.82).
BMD at 24 months
Sixteen studies that examined nine drugs (alendronate, alfacalcidol, denosumab, etidronate, pamidronate, raloxifene, risedronate, teriparatide + alendronate, zoledronic acid, and placebo) were included in the analysis regarding BMD at 24 months. According to our results, patients using teriparatide + alendronate had the highest BMD at 24 months compared to placebo (teriparatide + alendronate: WMD = 0.17, 95% CrI 0.08, 0.26; denosumab: WMD = 0.17, 95% CrI 0.04, 0.30; alendronate: WMD = 0.12, 95% CrI 0.07, 0.18; raloxifene: WMD = 0.16, 95% CrI 0.04, 0.29, zoledronic acid: WMD = 0.14, 95% CrI 0.07, 0.21, Table 4). There was no heterogeneity between the included treatments. Thus, we applied the fixed effect model for the cumulative values (Figure 7A).
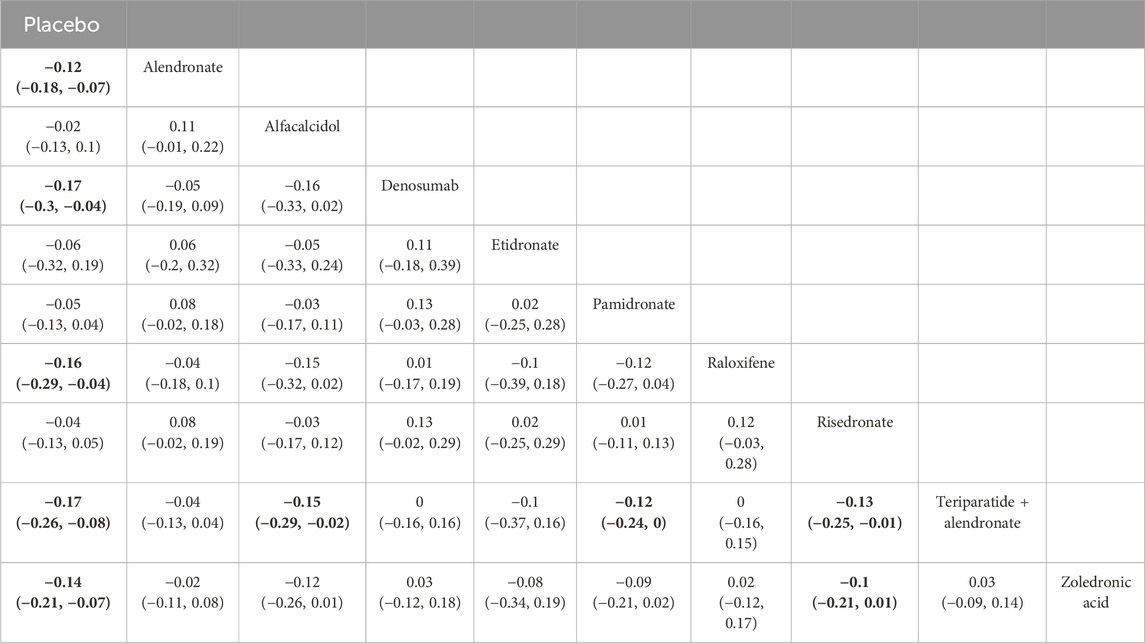
Table 4. Efficacy of different comparisons of drugs by WMDs and corresponding 95% CrIs for BMD at 24 months. Bold fonts indicate p-value <0.05.
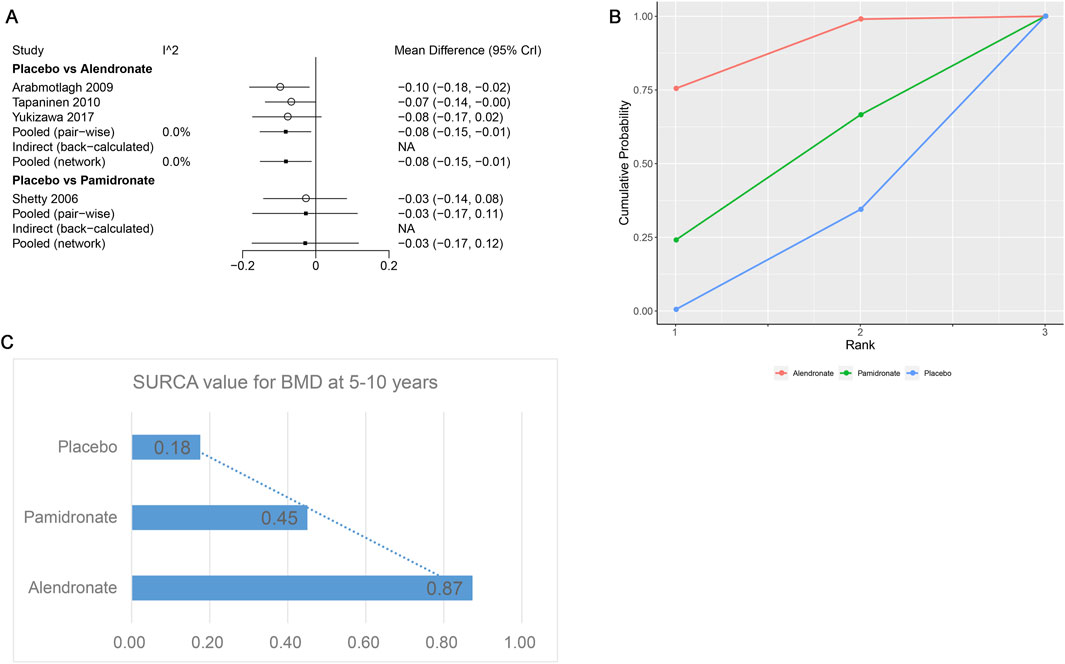
Figure 7. (A) Heterogeneity between different comparisons of drugs for BMD at 24 months. (B) Surface under the cumulative ranking curve (SUCRA) probabilities of different drugs for BMD at 24 months. (C) SUCRA values of the different drugs for BMD at 24 months.
As shown in Figures 7B,C, the SUCRA values of the ten interventions demonstrated that teriparatide + alendronate was most effective for BMD at 24 months (SUCRA = 0.82), followed by raloxifene (SUCRA = 0.77) and denosumab (SUCRA = 0.79).
BMD at 5–10 years
Four studies examining two drugs (alendronate, pamidronate, and placebo) were involved in the analysis regarding BMD at 5–10 years. According to our results, patients using alendronate had the highest BMD at 5–10 years compared to other drugs (alendronate: WMD = 0.08, 95% CrI 0.01, 0.15; pamidronate: WMD = 0.03, 95% CrI −0.11, 0.17, Table 5). There was no heterogeneity between the included treatments (Figure 8A). Thus, we applied the fixed-effect model for the cumulative values. As shown in Figures 8B,C, the SUCRA values of the two interventions demonstrated that alendronate achieved the highest SUCRA values of the BMD at 5–10 years (0.87).
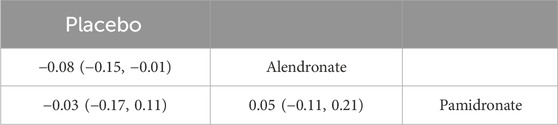
Table 5. Efficacy of different comparisons of drugs by WMDs and corresponding 95% CrIs for BMD at 5–10 years. Bold fonts indicate p-value <0.05.
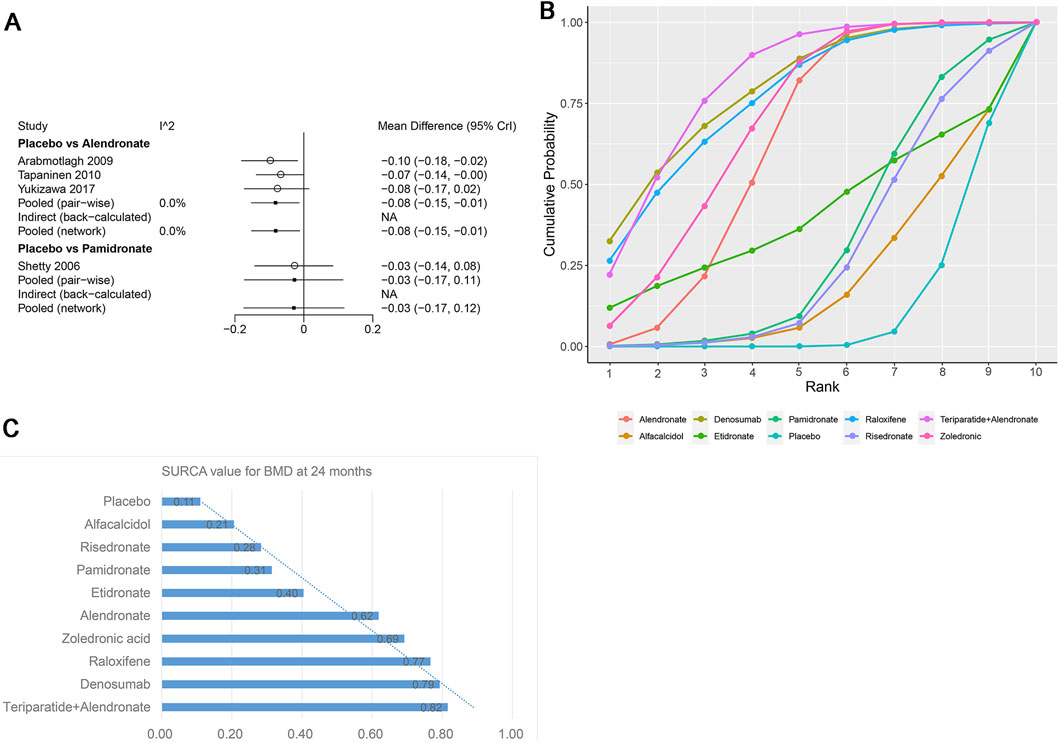
Figure 8. (A) Heterogeneity between different comparisons of drugs for BMD at 5–10 years. (B) Surface under the cumulative ranking curve (SUCRA) probabilities of different drugs for BMD at 5–10 years. (C) SUCRA values of the different drugs for BMD at 5–10 years.
Publication bias and meta-regression
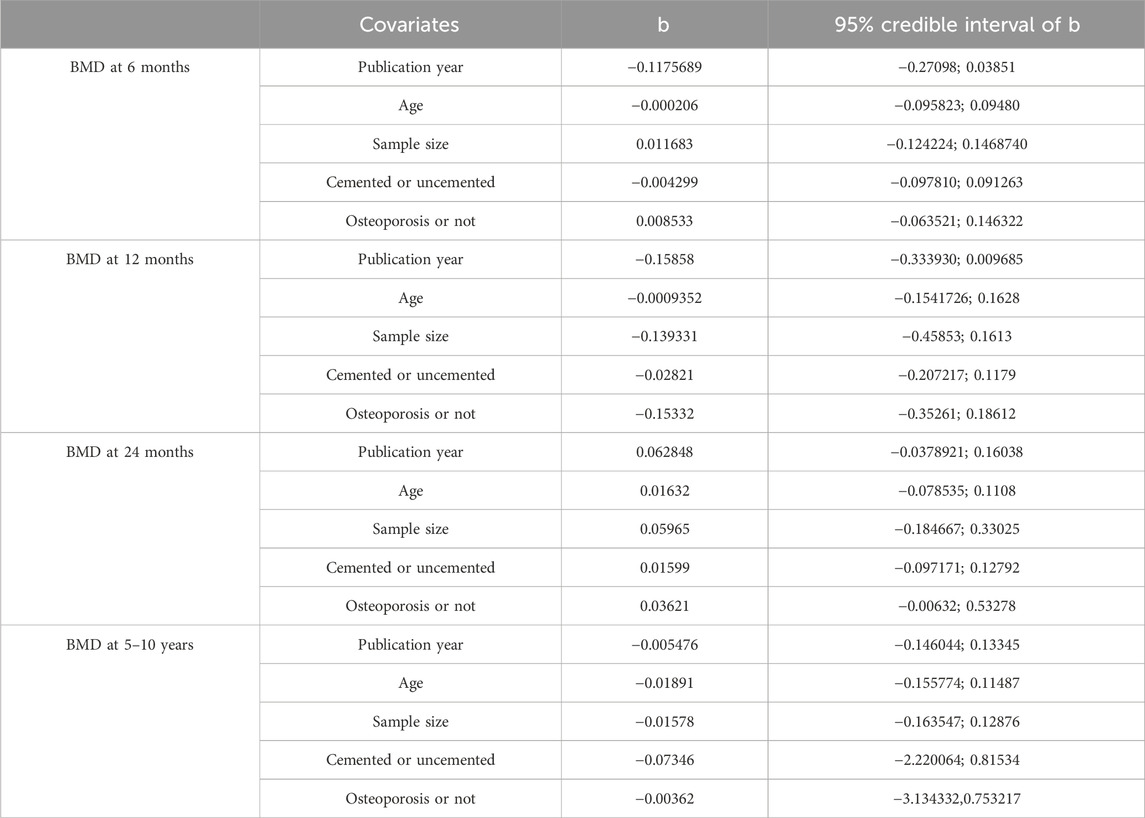
Figure 9 exhibited that there was no publication bias in the included studies. We conducted meta-regression analyses on BMD at 6 months, 12 months, 24 months, and 5–10 years according to the publication year, age, sample size, prosthesis type (cemented or uncemented), and with or without osteoporosis. We noted that the conclusions on the outcomes did not change substantially after accounting for potential effect modifiers (Table 6).
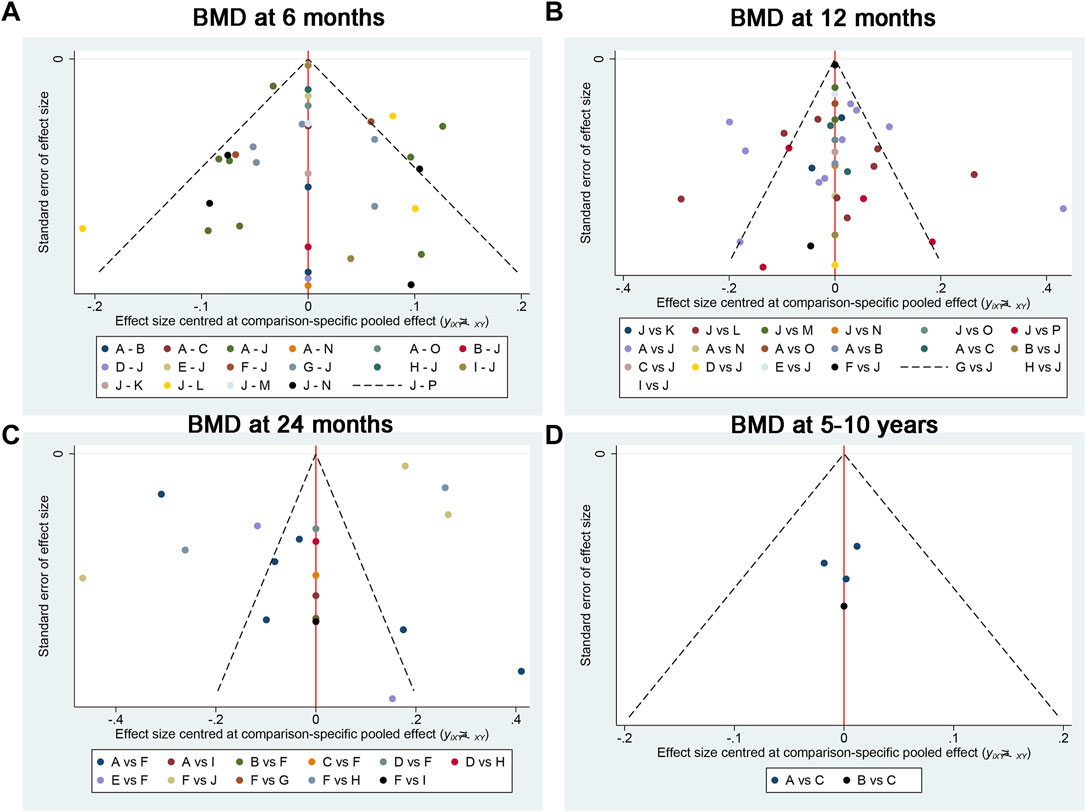
Figure 9. Publication bias between the included studies for (A) BMD at 6 months, (B) BMD at 12 months, (C) BMD at 24 months, and (D) BMD at 5–10 years. A, alendronate; B, alendronate + alfacalcidol; C, alfacalcidol; D, calcitonin; E, clodronate; F, denosumab; G, etidronate; H, ibandronate; I, pamidronate; J, raloxifene; K, risedronate; L, simvastatin; M, teriparatide; N, teriparatide + alendronate; O, zoledronic acid; H, placebo.
Discussion
Main findings
This is the first systematic review with network meta-analysis (NMA) comparing the effects of different anti-osteoporosis drugs on femoral periprosthetic bone loss following THA. By synthesizing data with the highest level of evidence, we found that both denosumab and bisphosphonates can effectively prevent bone loss after THA. Denosumab was identified as the most effective agent for increasing BMD at 6 months post-THA. Alendronate, when combined with alfacalcidol or teriparatide, was found to be the most effective for increasing BMD at 12 months and 24 months after THA.
Compared with previous meta-analyses
The present study is the first systematic review and network meta-analysis that compared anti-osteoporotic drugs on the preservation of periprosthetic BMD followed-up for long-term efficiency after THA. Our findings both align with and extend the conclusions of earlier traditional meta-analyses. Previous meta-analyses, such as those by Shi et al. (2018b) and Zhao et al. (2015), consistently demonstrated that bisphosphonates are effective in reducing periprosthetic bone loss after THA compared to placebo. Our results confirm the efficacy of bisphosphonates, particularly alendronate and zoledronic acid, across various time points.
However, a key advancement of our network meta-analysis is the ability to rank multiple treatments simultaneously, including newer agents like denosumab and combination therapies. While prior meta-analyses primarily focused on bisphosphonate-versus-placebo comparisons, our NMA reveals that denosumab may offer superior early BMD preservation compared to individual bisphosphonates. This finding does not necessarily contradict previous studies but rather refines and expands upon them by incorporating a broader spectrum of interventions. For instance, the superior ranking of combination therapies (alendronate + alfacalcidol at 12 months and teriparatide + alendronate at 24 months) highlights potential synergistic effects that were not extensively evaluated in earlier pairwise meta-analyses due to the scarcity of head-to-head trials.
Our analysis regarding certain drugs like clodronate, etidronate, pamidronate, and risedronate, which showed no significant difference from placebo in some comparisons, underscores the heterogeneity in efficacy among different bisphosphonates, a nuance that could not be fully elucidated in traditional meta-analyses that often grouped them together. Therefore, rather than contradicting previous findings, this NMA provides a more granular and hierarchical perspective on the comparative effectiveness of anti-osteoporosis medications for this specific clinical indication, leveraging both direct and indirect evidence to inform treatment choices more precisely.
Our primary finding is that denosumab most significantly preserves periprosthetic BMD among anti-osteoporotic drugs (SUCRA values at 6 months, 12 months, and more than 2 years postoperatively were 97.1%, 82.2%, and 79.2%, respectively). Several large clinical trials have shown the advantages of denosumab compared with bisphosphonates in postmenopausal women (Suzuki et al., 2018a; Suzuki et al., 2018b; Nakamura et al., 2017). Treatment with denosumab illustrated that the early migration of the tibial component had reduced in total knee arthroplasty, as determined using radiostereometric analysis (Ledin et al., 2017). Nystrom et al. (Ledin et al., 2017) reported that denosumab potently prevents early periprosthetic bone loss after uncemented THA by measuring periprosthetic BMD by DXA. They analyzed the periprosthetic standardized uptake value (SUV) by [18F] sodium fluoride (18F NaF) positron emission tomography/CT (F-PET). Our study found that denosumab showed a significant increase in the preservation efficiency compared with bisphosphonates, such as alendronate, zoledronate, pamidronate, etidronate, and risedronate. The pronounced efficacy of denosumab in preserving periprosthetic bone mineral density (BMD) at 6 months post-THA can be attributed to its distinct mechanism of action.
Denosumab is a fully human monoclonal antibody that specifically targets the receptor activator of nuclear factor kappa B ligand (RANKL) (Aapro et al., 2025). By binding to RANKL, denosumab prevents its interaction with the RANK receptor on osteoclast precursors and mature osteoclasts, thereby inhibiting osteoclast formation, function, and survival (Singh et al., 2025). This rapid and direct blockade of the RANK/RANKL pathway results in a swift reduction in bone resorption. Unlike bisphosphonates, which must be incorporated into the bone matrix and internalized by osteoclasts to induce apoptosis, denosumab acts systemically and does not require bone binding, allowing for a more immediate antiresorptive effect. This rapid onset of action likely explains its superior performance in the early postoperative period, a critical phase characterized by accelerated periprosthetic bone turnover due to surgical trauma and adaptive bone remodeling.
Alendronate had the third-highest ranking at 12 months and 24 months after THA. Several studies reported that alendronate reduced periprosthetic bone loss after THA (Nishioka et al., 2007; Yukizawa et al., 2017; Venesmaa et al., 2001). Zoledronate was more effective than placebo at all follow-up time points. The loss of periprosthetic BMD after THA could be effectively reversed using zoledronate (Zhou et al., 2019). Clodronate, etidronate, pamidronate, and risedronate showed no significant difference compared with placebo in our network analysis. Iwamoto et al. confirmed that alfacalcidol did not show any effects in any regions after hybrid-type THA (Iwamoto et al., 2011).
Teriparatide is another promising potent drug in terms of preventing periprosthetic bone loss after THA (SUCRA values at 6 months and 12 months postoperatively were 63.3% and 65.5%, respectively). Kobayashi et al. (2016) reported that the application of teriparatide alone showed good protection results after THA for osteoporotic patients. Switching administration of teriparatide before alendronate had a significant effect on BMD of the lumbar spine and zones 1 and 7 at 2 years postoperatively, and the study found that the combination was more effective than alendronate alone (Morita et al., 2020). However, in our network analysis, there was no significant difference between teriparatide and alendronate. More high-quality trials comparing two drugs are needed for a solid result.
Patients taking raloxifene reported higher improvement in periprosthetic BMD for postmenopausal women compared with placebo at 24 months postoperatively (Gong et al., 2020). SUCRA values at 6 months, 12 months, and more than 2 years postoperatively were 83.1%, 58.5% and 83.9%, respectively. Therefore, raloxifene is possibly well suited to prevent periprosthetic bone loss after THA in postmenopausal women.
Calcitonin was even worse than a placebo, and the reduction was significantly different at 12 months postoperatively. Arnala (2012) reported that Nasal salmon calcitonin 200 IU on a daily basis did not promote any additional calcium substitution to prevent bone loss after hip replacement. It seems that calcitonin does not promote any BMD added value to prevent bone loss after THA.
While this network meta-analysis demonstrates the efficacy of several pharmacologic interventions in preserving periprosthetic BMD, the ultimate clinical goal is to prevent adverse events such as aseptic loosening and periprosthetic fractures. Our review of the available data on these hard endpoints highlights a critical gap in the current literature. The included RCTs were primarily designed and powered to detect differences in BMD and lacked the long-term follow-up necessary to assess rare clinical events. Consequently, while denosumab and bisphosphonates show significant benefits for BMD preservation, the current evidence is insufficient to confirm that these benefits directly translate to a reduced risk of loosening or fracture. The pathway from reduced bone loss to improved implant survival is protracted and multifactorial. Future research prioritizing long-term follow-up and the analysis of large-scale registry data is essential to validate the effect of anti-osteoporosis treatments on these critical patient-centered outcomes.
There are several limitations related to the inferences provided in the present network meta-analysis. First, the baseline characteristics of the included trials are various, including gender, ages of patients, primary diseases, type of prosthesis, and dose of medicine, which would lead to bias. Second, the analysis of long-term efficacy (5–10 years) is based on a very sparse network, including only two active drugs (alendronate and pamidronate) from four studies. The ranking of alendronate as the best intervention for this time point should therefore not be overinterpreted as it primarily reflects the scarcity of long-term comparative data rather than established clinical superiority. Third, most of the included studies involve active agents versus a placebo. The lack of head-to-head trials with large samples increases the risk of bias.
Strengths
Despite these limitations, our study has several notable strengths. It is the first NMA to provide a comprehensive ranking of a wide array of anti-osteoporosis medications for this specific clinical application. We employed robust statistical methods to synthesize both direct and indirect evidence, thus strengthening the comparative conclusions. The analyses included multiple follow-up time points, offering valuable insights into the temporal efficacy of different treatments, which is crucial for informing clinical decision-making regarding treatment initiation and duration.
Conclusion
This network meta-analysis provides comparative effectiveness estimates for various anti-osteoporosis treatments aimed at preserving femoral periprosthetic BMD following THA. Our results suggest that denosumab is the most effective agent for increasing BMD at 6 months postoperatively. For the 12- and 24-month periods, combination therapies, particularly alendronate with alfacalcidol or teriparatide, appear to yield superior BMD preservation compared to monotherapies. However, it is important to note that these findings regarding combination regimens are based on a limited number of studies and thus should be interpreted with caution until further high-quality direct-comparison trials are available. Based on the current evidence, a potential clinical strategy could involve initiating denosumab within the first 6 months after THA to capitalize on its early efficacy, followed by transition to a combination regimen such as alendronate with alfacalcidol or teriparatide for mid- to long-term management, especially in patients at high risk of periprosthetic bone loss. Nonetheless, the optimal timing, sequence, and patient selection for such therapeutic approaches remain to be established. Future research should prioritize head-to-head RCTs comparing active treatments, standardize outcome reporting, and include longer follow-up durations to better inform clinical decision-making and treatment guidelines.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Z-HZ: Investigation, Methodology, Supervision, Writing – original draft, Writing – review and editing. TL: Data curation, Investigation, Methodology, Writing – original draft. SY: Writing – original draft, Writing – review and editing, Supervision, Conceptualization, Investigation, Software. WL: Investigation, Methodology, Writing – original draft. J-XM: Software, Supervision, Writing – original draft. XM: Data curation, Formal Analysis, Investigation, Methodology, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study was sponsored by grants from the National Natural Science Foundation of China (82302701), Tianjin Health Research Project (TJWJ2022QN051 and TJWJ2022MS025), Tianjin Health Science and Technology Project (ZC20096 and ZC20191), Tianjin Municipal Science and Technology Bureau Project (22JCQNJC01060, 22ZYJDSY00110), Tianjin Youth Medical Cutting-edge (TJSQNYXXR-D2-131 and TJSQNYXXR-D2-136), and the Technology Fund of Tianjin Hospital (TJYY2117).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1566890/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | Demographic characteristics for all treatments. (A) Number of randomized for BMD at 6 months; (B) Publication year for BMD at 6 months; (C) Mean age for BMD at 6 months; (D) Number of randomized for BMD at 12 months; (E) Publication year for BMD at 12 months; (F) Mean age for BMD at 12 months; (G) Number of randomized for BMD at 24 months; (H) Publication year for BMD at 24 months; (I) Mean age for BMD at 24 months; (J) Number of randomized for BMD at 5–10 years; (K) Publication year for BMD at 5–10 years; (L) Mean age for BMD at 5–10 years.
References
Aapro, M., Hadji, P., Santini, D., Schmidmaier, R., and Eastell, R. (2025). Biosimilars in osteoporosis treatment: focus on denosumab. Expert Opin. Biol. Ther. 25, 887–898. doi:10.1080/14712598.2025.2540471
Ahn, E., and Kang, H. (2021). Concepts and emerging issues of network meta-analysis. Korean J. Anesthesiol. 74, 371–382. doi:10.4097/kja.21358
Anderl, C., Steinmair, M., and Hochreiter, J. (2022). Bone preservation in total hip arthroplasty. J. Arthroplasty 37, 1118–1123. doi:10.1016/j.arth.2022.01.077
Arabmotlagh, M., Rittmeister, M., and Hennigs, T. (2006). Alendronate prevents femoral periprosthetic bone loss following total hip arthroplasty: prospective randomized double-blind study. J. Orthop. Res. 24, 1336–1341. doi:10.1002/jor.20162
Arabmotlagh, M., Pilz, M., Warzecha, J., and Rauschmann, M. (2009). Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty. J. Orthop. Res. 27, 183–188. doi:10.1002/jor.20748
Arnala, I. O. (2012). Salmon calcitonin (miacalcic ns 200 IU) in prevention of bone loss after hip replacement. Scand. J. Surg. 101, 249–254. doi:10.1177/145749691210100405
Aro, E., Moritz, N., Mattila, K., and Aro, H. T. (2018). A long-lasting bisphosphonate partially protects periprosthetic bone, but does not enhance initial stability of uncemented femoral stems: a randomized placebo-controlled trial of women undergoing total hip arthroplasty. J. Biomech. 75, 35–45. doi:10.1016/j.jbiomech.2018.04.041
Bhandari, M., Bajammal, S., Guyatt, G. H., Griffith, L., Busse, J. W., Schünemann, H., et al. (2005). Effect of bisphosphonates on periprosthetic bone mineral density after total joint arthroplasty. A meta-analysis. J. Bone Jt. Surg. Am. 87, 293–301. doi:10.2106/jbjs.d.01772
Deeks, E. D. (2018). Denosumab: a review in postmenopausal osteoporosis. Drugs Aging 35, 163–173. doi:10.1007/s40266-018-0525-7
Di Martino, A., Valtetsiotis, K., Rossomando, V., Brunello, M., Bordini, B., D'Agostino, C., et al. (2024). Efficacy of bisphosphonates in total hip arthroplasty patients: systematic review and meta-analysis. Biomedicines 12, 1778. doi:10.3390/biomedicines12081778
Dias, S., Welton, N. J., Caldwell, D. M., and Ades, A. E. (2010). Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 29, 932–944. doi:10.1002/sim.3767
Dong, L., Jiang, L., Xu, Z., and Zhang, X. (2024). Denosumab, teriparatide and bisphosphonates for glucocorticoid-induced osteoporosis: a Bayesian network meta-analysis. Front. Pharmacol. 15, 1336075. doi:10.3389/fphar.2024.1336075
Efthimiou, O., Debray, T. P., van Valkenhoef, G., Trelle, S., Panayidou, K., Moons, K. G. M., et al. (2016). GetReal in network meta-analysis: a review of the methodology. Res. Synth. Methods 7, 236–263. doi:10.1002/jrsm.1195
Fokter, S. K., Komadina, R., Repse-Fokter, A., Yerby, S. A., Kocijancic, A., and Marc, J. (2005). Etidronate does not suppress periprosthetic bone loss following cemented hip arthroplasty. Int. Orthop. 29, 362–367. doi:10.1007/s00264-005-0018-2
Gao, J., Gao, C., Li, H., Wang, G. S., Xu, C., and Ran, J. (2017). Effect of zoledronic acid on reducing femoral bone mineral density loss following total hip arthroplasty: a meta-analysis from randomized controlled trails. Int. J. Surg. 47, 116–126. doi:10.1016/j.ijsu.2017.08.559
Gerhardt, D. M., Smolders, J. M., Roovers, E. A., Rijnders, T. A., and van Susante, J. L. (2019). Changes in periacetabular bone mineral density five years after resurfacing hip arthroplasty versus conventional total hip arthroplasty. Hip Int. 29, 153–160. doi:10.1177/1120700018808023
Gong, L., Zhang, Y. Y., Yang, N., Qian, H. J., and Tan, M. S. (2020). Raloxifene prevents early periprosthetic bone loss for postmenopausal women after uncemented total hip arthroplasty: a randomized placebo-controlled clinical trial. Orthop. Surg. 12, 1074–1083. doi:10.1111/os.12696
Hatano, M., Koizumi, Y., Yamamoto, N., Miyoshi, K., Kawabata, K., Tanaka, T., et al. (2025). Anti-osteoporotic drug efficacy for periprosthetic bone loss after total hip arthroplasty: a systematic review and network meta-analysis. J. Orthop. Sci. 30, 126–135. doi:10.1016/j.jos.2024.01.011
Hennigs, T., Arabmotlagh, M., Schwarz, A., and Zichner, L. (2002). Dose-dependent prevention of early periprosthetic bone loss by alendronate. Z Orthop. Ihre Grenzgeb 140, 42–47. doi:10.1055/s-2002-22090
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 343, d5928. doi:10.1136/bmj.d5928
Hozo, S. P., Djulbegovic, B., and Hozo, I. (2005). Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 5, 13. doi:10.1186/1471-2288-5-13
Huang, T. W., Wang, C. J., Shih, H. N., Chang, Y., Huang, K. C., Peng, K. T., et al. (2017). Bone turnover and periprosthetic bone loss after cementless total hip arthroplasty can be restored by zoledronic acid: a prospective, randomized, open-label, controlled trial. BMC Musculoskelet. Disord. 18, 209. doi:10.1186/s12891-017-1577-2
Iwamoto, N., Inaba, Y., Kobayashi, N., Ishida, T., Yukizawa, Y., and Saito, T. (2011). A comparison of the effects of alendronate and alfacalcidol on bone mineral density around the femoral implant and in the lumbar spine after total hip arthroplasty. J. Bone Jt. Surg. Am. 93, 1203–1209. doi:10.2106/jbjs.i.01714
Iwamoto, N., Inaba, Y., Kobayashi, N., Yukizawa, Y., Ike, H., Ishida, T., et al. (2014). The effectiveness of mono or combined osteoporosis drug therapy against bone mineral density loss around femoral implants after total hip arthroplasty. J. Bone Min. Metab. 32, 539–544. doi:10.1007/s00774-013-0526-x
Kinov, P., Tivchev, P., Doukova, P., and Leithner, A. (2006). Effect of risedronate on bone metabolism after total hip arthroplasty: a prospective randomised study. Acta Orthop. Belg 72, 44–50.
Knutsen, A. R., Lau, N., Longjohn, D. B., Ebramzadeh, E., and Sangiorgio, S. N. (2017). Periprosthetic femoral bone loss in total hip arthroplasty: systematic analysis of the effect of stem design. Hip Int. 27, 26–34. doi:10.5301/hipint.5000413
Kobayashi, N., Inaba, Y., Uchiyama, M., Ike, H., Kubota, S., and Saito, T. (2016). Teriparatide Versus alendronate for the preservation of bone mineral density after total hip arthroplasty - a randomized controlled trial. J. Arthroplasty 31, 333–338. doi:10.1016/j.arth.2015.07.017
Ledin, H., Good, L., and Aspenberg, P. (2017). Denosumab reduces early migration in total knee replacement. Acta Orthop. 88, 255–258. doi:10.1080/17453674.2017.1300746
Lin, T., Yan, S. G., Cai, X. Z., and Ying, Z. M. (2012). Bisphosphonates for periprosthetic bone loss after joint arthroplasty: a meta-analysis of 14 randomized controlled trials. Osteoporos. Int. 23, 1823–1834. doi:10.1007/s00198-011-1797-5
Liu, Z., Zhang, M., Shen, Z., Ke, J., Zhang, D., and Yin, F. (2020). Efficacy and safety of 18 anti-osteoporotic drugs in the treatment of patients with osteoporosis caused by glucocorticoid: a network meta-analysis of randomized controlled trials. PLoS One 15, e0243851. doi:10.1371/journal.pone.0243851
Migliorini, F., Maffulli, N., Colarossi, G., Eschweiler, J., Tingart, M., and Betsch, M. (2021). Effect of drugs on bone mineral density in postmenopausal osteoporosis: a Bayesian network meta-analysis. J. Orthop. Surg. Res. 16, 533. doi:10.1186/s13018-021-02678-x
Mineta, K., Nishisho, T., Okada, M., Kamada, M., and Sairyo, K. (2025). Real-world safety and effectiveness of romosozumab following daily or weekly administration of teriparatide in primary and secondary osteoporosis. Bone 193, 117392. doi:10.1016/j.bone.2025.117392
Morita, A., Kobayashi, N., Choe, H., Ike, H., Tezuka, T., Higashihira, S., et al. (2020). Effect of switching administration of alendronate after teriparatide for the prevention of BMD loss around the implant after total hip arthroplasty, 2-year follow-up: a randomized controlled trial. J. Orthop. Surg. Res. 15, 17. doi:10.1186/s13018-020-1547-5
Muren, O., Akbarian, E., Salemyr, M., Bodén, H., Eisler, T., Stark, A., et al. (2015). No effect of risedronate on femoral periprosthetic bone loss following total hip arthroplasty. A 4-year follow-up of 61 patients in a double-blind, randomized placebo-controlled trial. Acta Orthop. 86, 569–574. doi:10.3109/17453674.2015.1041846
Nagoya, S., Tateda, K., Okazaki, S., Kosukegawa, I., Shimizu, J., and Yamashita, T. (2018). Restoration of proximal periprosthetic bone loss by denosumab in cementless total hip arthroplasty. Eur. J. Orthop. Surg. Traumatol. 28, 1601–1607. doi:10.1007/s00590-018-2223-x
Nakamura, Y., Suzuki, T., Kamimura, M., Murakami, K., Ikegami, S., Uchiyama, S., et al. (2017). Vitamin D and calcium are required at the time of denosumab administration during osteoporosis treatment. Bone Res. 5, 17021. doi:10.1038/boneres.2017.21
Nehme, A., Maalouf, G., Tricoire, J. L., Giordano, G., Chiron, P., and Puget, J. (2003). Effect of alendronate on periprosthetic bone loss after cemented primary total hip arthroplasty: a prospective randomized study. Rev. Chir. Orthop. Reparatrice Appar. Mot. 89, 593–598.
Nishioka, T., Yagi, S., Mitsuhashi, T., Miyamoto, M., Tamura, T., Kobayashi, T., et al. (2007). Alendronate inhibits periprosthetic bone loss around uncemented femoral components. J. Bone Min. Metab. 25, 179–183. doi:10.1007/s00774-006-0743-7
Nyström, A., Kiritopoulos, D., Ullmark, G., Sörensen, J., Petrén-Mallmin, M., Milbrink, J., et al. (2020). Denosumab prevents early periprosthetic bone loss after uncemented total hip arthroplasty: results from a randomized placebo-controlled clinical trial. J. Bone Min. Res. 35, 239–247. doi:10.1002/jbmr.3883
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Roy, A. N., and Mazumdar, I. (2023). Effects of teriparatide treatment on bone mineral density in patients with osteoporosis: a short-term dose-response study. Cureus 15, e45662. doi:10.7759/cureus.45662
Sariali, E., Gaujac, N., Grimal, Q., and Klouche, S. (2020). Pre-operative bone mineral density is a predictive factor for excellent early patient-reported outcome measures in cementless total hip arthroplasty using a proximally fixed anatomic stem. A prospective study at two year minimum follow-up. Int. Orthop. 44, 2253–2259. doi:10.1007/s00264-020-04683-x
Scott, D. F., Woltz, J. N., and Smith, R. R. (2013). Effect of zoledronic acid on reducing femoral bone mineral density loss following total hip arthroplasty: preliminary results of a prospective randomized trial. J. Arthroplasty 28, 671–675. doi:10.1016/j.arth.2012.08.007
Shea, B. J., Grimshaw, J. M., Wells, G. A., Boers, M., Andersson, N., Hamel, C., et al. (2007). Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 7, 10. doi:10.1186/1471-2288-7-10
Shetty, N., Hamer, A. J., Stockley, I., Eastell, R., and Willkinson, J. M. (2006). Clinical and radiological outcome of total hip replacement five years after pamidronate therapy. A trial extension. J. Bone Jt. Surg. Br. 88, 1309–1315. doi:10.1302/0301-620x.88b10.17308
Shi, M., Chen, L., Wu, H., Wang, Y., Wang, W., Zhang, Y., et al. (2018a). Effect of bisphosphonates on periprosthetic bone loss after total knee arthroplasty: a meta-analysis of randomized controlled trials. BMC Musculoskelet. Disord. 19, 177. doi:10.1186/s12891-018-2101-z
Shi, M., Chen, L., Xin, Z., Wang, Y., Wang, W., and Yan, S. (2018b). Bisphosphonates for the preservation of periprosthetic bone mineral density after total joint arthroplasty: a meta-analysis of 25 randomized controlled trials. Osteoporos. Int. 29, 1525–1537. doi:10.1007/s00198-018-4488-7
Shim, S. R., Kim, S. J., Lee, J., and Rücker, G. (2019). Network meta-analysis: application and practice using R software. Epidemiol. Health 41, e2019013. doi:10.4178/epih.e2019013
Singh, M., Sabharwal, S., Jamwal, N., Gupta, M., and Garg, K. (2025). Comparison of efficacy and safety of romosozumab versus denosumab for treatment of postmenopausal osteoporosis: a meta-analysis. J. Midlife Health 16, 235–246. doi:10.4103/jmh.jmh_108_25
Skoldenberg, O. G., Salemyr, M. O., Boden, H. S., Ahl, T. E., and Adolphson, P. Y. (2011). The effect of weekly risedronate on periprosthetic bone resorption following total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. J. Bone Jt. Surg. Am. 93, 1857–1864. doi:10.2106/jbjs.j.01646
Su, R., Feng, W., Liu, X., Song, Y., Xu, Z., and Liu, J. G. (2021). Early rehabilitation and periprosthetic bone environment after primary total hip arthroplasty: a randomized controlled trial. Orthop. Surg. 13, 1521–1531. doi:10.1111/os.12984
Suzuki, T., Nakamura, Y., Tanaka, M., Kamimura, M., Ikegami, S., Uchiyama, S., et al. (2018a). Comparison of the effects of denosumab with either active vitamin D or native vitamin D on bone mineral density and bone turnover markers in postmenopausal osteoporosis. Mod. Rheumatol. 28, 376–379. doi:10.1080/14397595.2017.1308454
Suzuki, T., Nakamura, Y., and Kato, H. (2018b). Vitamin D and calcium addition during denosumab therapy over a period of four years significantly improves lumbar bone mineral density in Japanese osteoporosis patients. Nutrients 10, 272. doi:10.3390/nu10030272
Tapaninen, T. S., Venesmaa, P. K., Jurvelin, J. S., Miettinen, H. J. A., and Kröger, H. P. J. (2010). Alendronate reduces periprosthetic bone loss after uncemented primary total hip arthroplasty - a 5-year follow-up of 16 patients. Scand. J. Surg. 99, 32–37. doi:10.1177/145749691009900108
Tapaninen, T., Kröger, H., Jurvelin, J., and Venesmaa, P. (2012). Femoral neck bone mineral density after resurfacing hip arthroplasty. Scand. J. Surg. 101, 211–215. doi:10.1177/145749691210100312
Tran, P., Zhang, B. X., Lade, J. A., Pianta, R. M., Unni, R. P., and Haw, C. S. (2016). Periprosthetic bone remodeling after novel short-stem neck-sparing total hip arthroplasty. J. Arthroplasty 31, 2530–2535. doi:10.1016/j.arth.2016.04.023
Trevisan, C., Ortolani, S., Romano, P., Isaia, G., Agnese, L., Dallari, D., et al. (2010). Decreased periprosthetic bone loss in patients treated with clodronate: a 1-year randomized controlled study. Calcif. Tissue Int. 86, 436–446. doi:10.1007/s00223-010-9356-1
van Valkenhoef, G., Lu, G., de Brock, B., Hillege, H., Ades, A. E., and Welton, N. J. (2012). Automating network meta-analysis. Res. Synth. Methods 3, 285–299. doi:10.1002/jrsm.1054
Venesmaa, P. K., Kroger, H. P., Miettinen, H. J., Jurvelin, J. S., Suomalainen, O. T., and Alhav, E. M. (2001). Alendronate reduces periprosthetic bone loss after uncemented primary total hip arthroplasty: a prospective randomized study. J. Bone Min. Res. 16, 2126–2131. doi:10.1359/jbmr.2001.16.11.2126
Wang, M., Wang, L., and Ye, R. (2018). Risedronate reduces postoperative bone resorption after cementless total hip arthroplasty: a systematic review and meta-analysis. Int. J. Surg. 52, 189–200. doi:10.1016/j.ijsu.2018.02.007
Wilkinson, J. M., Eagleton, A. C., Stockley, I., Peel, N. F. A., Hamer, A. J., and Eastell, R. (2005). Effect of pamidronate on bone turnover and implant migration after total hip arthroplasty: a randomized trial. J. Orthop. Res. 23, 1–8. doi:10.1016/j.orthres.2004.06.004
Yamaguchi, K., Masuhara, K., Yamasaki, S., Nakai, T., and Fuji, T. (2003). Cyclic therapy with etidronate has a therapeutic effect against local osteoporosis after cementless total hip arthroplasty. Bone 33, 144–149. doi:10.1016/s8756-3282(03)00085-1
Yamaguchi, K., Masuhara, K., Yamasaki, S., Fuji, T., and Seino, Y. (2004). Effects of discontinuation as well as intervention of cyclic therapy with etidronate on bone remodeling after cementless total hip arthroplasty. Bone 35, 217–223. doi:10.1016/j.bone.2004.03.017
Yamaguchi, K., Masuhara, K., Yamasaki, S., and Fuji, T. (2005). Efficacy of different dosing schedules of etidronate for stress shielding after cementless total hip arthroplasty. J. Orthop. Sci. 10, 32–36. doi:10.1007/s00776-004-0854-8
Yamasaki, S., Masuhara, K., Yamaguchi, K., Nakai, T., Fuji, T., and Seino, Y. (2007). Risedronate reduces postoperative bone resorption after cementless total hip arthroplasty. Osteoporos. Int. 18, 1009–1015. doi:10.1007/s00198-007-0339-7
Yan, S. G., Li, D., Yin, S., Hua, X., Tang, J., and Schmidutz, F. (2017). Periprosthetic bone remodeling of short cementless femoral stems in primary total hip arthroplasty: a systematic review and meta-analysis of randomized-controlled trials. Med. Baltim. 96, e8806. doi:10.1097/md.0000000000008806
Yang, D. F., Zhang, Y., and Ruan, W. (2019). Preventive effect of ibandronate on bone loss around prosthesis stem after hip replacement. J. Trauma. Surg. 21, 351–354. doi:10.1016/s1473-3099(19)30301-9
Yukizawa, Y., Inaba, Y., Kobayashi, N., Choe, H., Kubota, S., and Saito, T. (2017). Efficacy of alendronate for the prevention of bone loss in calcar region following total hip arthroplasty. J. Arthroplasty 32, 2176–2180. doi:10.1016/j.arth.2017.02.036
Zhang, X., Sun, Y., Xie, H., Liu, J., Zhao, Y., and Xu, Z. (2018). The effect of simvastatin on periprosthetic bone mineral density in the hypercholesterolaemic patients after total hip arthroplasty. Int. Orthop. 42, 59–64. doi:10.1007/s00264-017-3551-x
Zhao, X., Hu, D., Qin, J., Mohanan, R., and Chen, L. (2015). Effect of bisphosphonates in preventing femoral periprosthetic bone resorption after primary cementless total hip arthroplasty: a meta-analysis. J. Orthop. Surg. Res. 10, 65. doi:10.1186/s13018-015-0206-8
Zhou, W., Liu, Y., Guo, X., Yang, H., Xu, Y., and Geng, D. (2019). Effects of zoledronic acid on bone mineral density around prostheses and bone metabolism markers after primary total hip arthroplasty in females with postmenopausal osteoporosis. Osteoporos. Int. 30, 1581–1589. doi:10.1007/s00198-019-05005-7
Keywords: total hip arthroplasty, meta-analysis, bone mineral density, network, anti-osteoporosis
Citation: Zhao Z-H, Ling T, Ye S, Luo W, Ma J-X and Ma X (2025) Effectiveness of treatments to prevent femoral periprosthetic bone loss following total hip arthroplasty: a network meta-analysis. Front. Pharmacol. 16:1566890. doi: 10.3389/fphar.2025.1566890
Received: 26 January 2025; Accepted: 09 October 2025;
Published: 27 November 2025.
Edited by:
Alessandro Ruggiero, University of Salerno, ItalyReviewed by:
Bo Shuai, Huazhong University of Science and Technology, ChinaJuliana Ebling Brondani, Federal University of Minas Gerais, Brazil
Copyright © 2025 Zhao, Ling, Ye, Luo, Ma and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinlong Ma, eTI0NjAxM0B0anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Zhi-Hu Zhao
Zhi-Hu Zhao Tao Ling
Tao Ling Songqing Ye
Songqing Ye Wei Luo
Wei Luo Jian-Xiong Ma
Jian-Xiong Ma Xinlong Ma
Xinlong Ma