Abstract
Chalcones isolated from natural sources are the primary metabolites of numerous biologically intriguing and pharmacologically essential drugs. Chalcones’ pharmacological properties are believed to result from a double bond conjugated to carbonyl functionality. This review aims to summarise the research findings, showing naturally occurring chalcones as a preferred scaffold in medicinal chemistry. Natural chalcones have an intense antimicrobial activity that targets many pathogens, including viruses, bacteria, fungi, and protozoa. Strong antibiotic qualities are exhibited by chalcones, including 4-hydroxyderricin, licochalcone A and C, isobavachalcone, and pinocembrin chalcone. Furthermore, chalcones are promising pharmacological agents for cancer treatment; they inhibit angiogenesis, decrease metastasis, and induce death in tumor cells via diverse mechanisms. Chalcones are also considered promising therapeutic agents for diabetes, neurodegenerative diseases, and cardiovascular diseases because of their anti-inflammatory and antioxidant characteristics and ability to modify enzyme functioning. This review emphasizes several aspects, such as the biosynthesis of chalcones, preparation of chalcone derivatives, isolation of chalcones, structural features of chalcones, structure-activity relationship study, the role of natural chalcones in managing various diseases and illustrates their action mechanism to control disease progression.
1 Introduction
Naturally occurring metabolites have historically been the principal origin of medications for treating human disease. Plants’ therapeutic properties have been recorded in Egyptian civilizations, Chinese medicine, Indian Ayurveda, and on Assyrian clay tablets dated around 2000 B.C. Natural metabolites originate from plants, marine life, and microorganisms and continue to be crucial in developing medications for treating most human diseases. Over half of the clinical medications permitted by the US Food and Drug Administration (FDA) have been developed from natural metabolites or their corresponding synthetic analogs (Newman and Cragg, 2016). For instance, around 200 natural antibiotics derived from microbial sources have been employed as medications (Wright, 2017). Chalcones are simple pharmacological scaffolds of several naturally occurring metabolites, and plants comprising chalcones have also been utilized in traditional medicine for decades (Zhuang et al., 2017a). Chalcones belong to the open chain flavonoid family, and chalcones are chemically 1,3-diaryl-2-propen-1-ones (Figure 1), in which a three-carbon α, β-unsaturated carbonyl scaffold connects the two aromatic rings (A and B). The primed numbers are assigned to the A ring, written to the left, and the unprimed numbers are assigned to the B-ring carbons (Figure 1). Bridge carbons are marked relative to the carbonyl function. Chalcones can exist as two isomers, trans (E) and cis (Z), however the trans (E) isomer has superior thermodynamic stability since there is no steric crowding amid the carbonyl group and ring B (Figure 1). The two aromatic rings of chalcones having a π-electron system experiences delocalization with the conjugated double bonds, which results in a negligible redox potential and a better possibility of enduring electron transfer. The pharmacological properties of chalcones are assumed to be due to the existence of a double bond in conjugation with carbonyl moiety, as steric hindrance or saturation of the double bond renders the activity significantly. The aromatic rings of naturally occurring chalcones are polyhydroxylated. β-Hydroxy chalcones (also known as dibenzoylmethanes) belong to a unique type of natural metabolites, and only a few β-hydroxy chalcones (such as pongamol, pongagallone a, pongagallone b, etc.) have been isolated from plants. They are typically found as diketo-ketoenolic tautomeric mixtures with E and Z configuration (Figure 2). The Z-isomer kinetically controlled product, which isomerizes to the thermodynamically more stable E-isomer. X-ray crystallographic study also supported this isomerization (Bukhari et al., 2013). The presence of an E isomer was also supported by mass spectral analysis, which showed mass ions, M-OMe or M-OH, depending on the substituent at the C-6′ site of the A-ring. The existence of a downfield H-bonded -OH proton near δ 15–17 and one olefinic proton near δ 7.0–8.5 in the 1H-NMR spectra is the characteristic of β-hydroxy chalcones having Z configuration (Nielsen and Houlihan, 2011a).
FIGURE 1
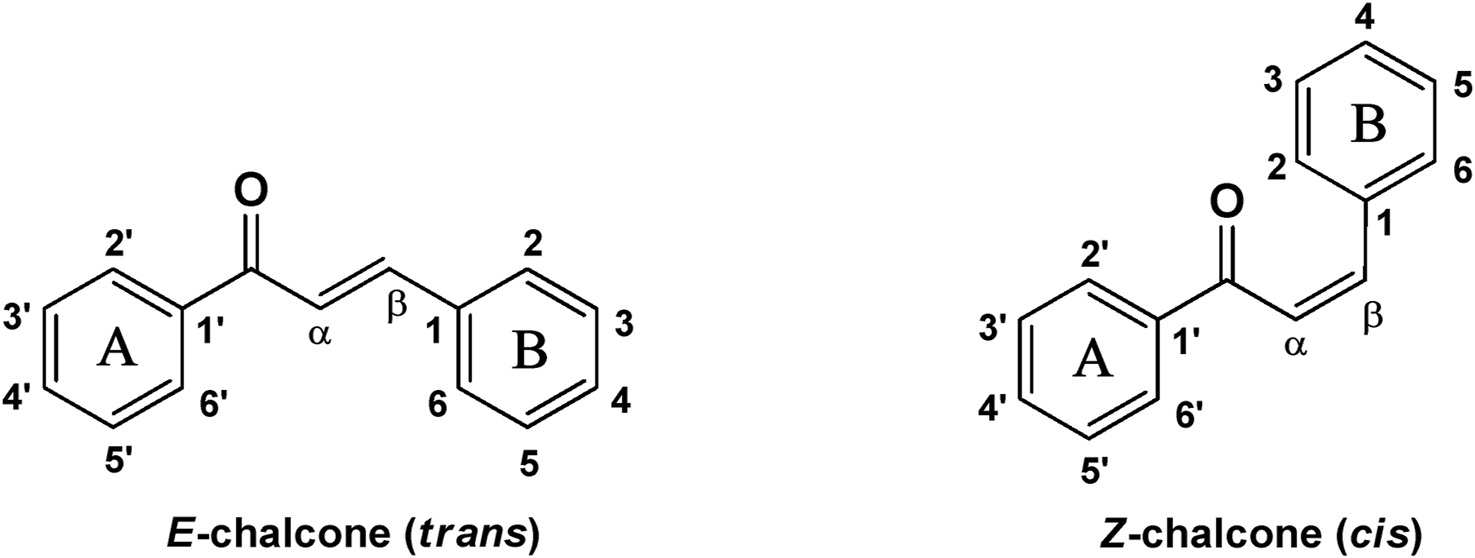
Chemical structure of chalcone (cis and trans).
FIGURE 2
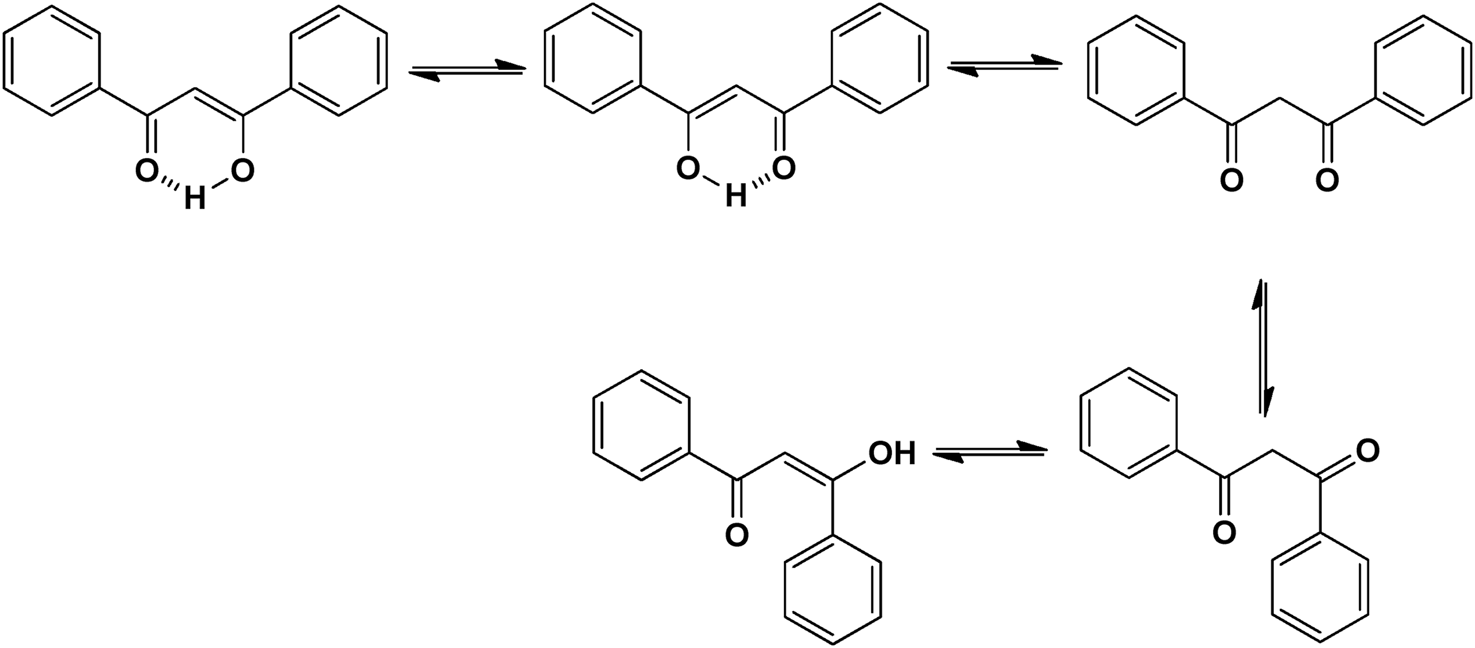
Tautomeric isomers and hydrogen bonded form of β-hydroxychalcone.
Scientists have been fascinated by chalcones, the building blocks of several pharmacologically intriguing metabolites extracted from natural sources, for decades. Researchers are still fascinated by the chemistry of chalcones in the 21st century, owing to their easy preparation and several replaceable hydrogens that generate an extensive range of derivatives and intriguing biological functions (Mazumder et al., 2024; Samota et al., 2024). Chalcones are present in various foods, including fruits, teas, vegetables, and several plants, which are synthetic precursors to the biosynthesis of isoflavonoids and flavonoids (Samota et al., 2024). The most significant number of naturally occurring chalcones has been extracted from species of the Asteraceae, Leguminosae, and Moraceae families. Chalcones family has been employed to treat numerous sicknesses for thousands of years, including diabetes, inflammation, and cancer, using botanical drugs and plants (Batovska and Todorova, 2010; Karthikeyan et al., 2014; Zhou, 2015). Recently, the chalcone derivatives have sparked a lot of consideration owing to their various pharmacological attributes, including anti-tumor, anti-inflammatory, and antimicrobial properties (Singh et al., 2014; Matos et al., 2015; Mahapatra et al., 2019; Ramadan et al., 2024). Metochalcone and sofalcone are chalcone-based drugs approved for clinical use (Figure 3) (Shigeru et al., 1991; Nowakowska, 2007a; Tanaka et al., 2009; Sahun et al., 2012a). The radical quenching characteristics of several chalcones’ phenolic moieties have sparked attention to employing the chalcone-rich plant extracts as medicines or preservatives of foods (Dhar DN, 1981). Butein, a chalcone derivative having four additional hydroxy groups at the 2′, 3, 4, and 4′position, has been usually utilized in Japan, Korea, and China for treating stomach cancer, pain, parasitic infections, thrombotic disease gastritis in addition to a food additive (Kang et al., 2004; Lee et al., 2004). Isoliquiritigenin, a liquorice chalcone, treats cardiovascular disorders as a phosphodiesterase III inhibitor (Wegener and Nawrath, 1997). Xanthine oxidase (SOGAWA et al., 1994), epoxide hydrolase (Morisseau et al., 1998), aldose reductase (IWATA et al., 1999), quinone reductase (Miranda et al., 2000a), and protein tyrosine kinase (Yang et al., 2001; Nerya et al., 2004a), are just a few of the essential enzymes found in biological systems that have been reported to be inhibited by derivatives of chalcone. In addition, several other pharmacological attributes of chalcones, including anti-inflammatory, antimicrobial, cytotoxic, and anti-cancer properties, find their medicinal applications for treating different diseases (Elias DW et al., 1999; Go et al., 2005). Aside from the various therapeutic characteristics of chalcone, it has a strong skin protection effect, which is an important component in enthanopharmacological research. In this regard, long-term UV (ultraviolet light) exposure on skin cells may result in chronic damage. In this context, an in-vitro investigation found that dihydrochalcones such as aspalathin and nothofagin extracted from Aspalathus linearis (Rooibos) could protect HaCaT and SK-MEL-1 skin cells. As a result, it was determined that these chalcones pre-treatment may be associated with greater cellular adaptability by decreasing lipid peroxidation and caspase 3 expression, potentially reducing UVB-mediated oxidative stress in human skin cells (Akinfenwa et al., 2021). Furthermore, an in-vivo model-based study found that hesperidin methyl chalcone (HMC) inhibits UVB-induced inflammation and oxidative stress. In this context, exposing hairless mice to a UVB irradiation level of 4.14 J/cm2 resulted in oxidative stress and skin inflammation. After treating the HMC, it was investigated that superoxide anion formation from UVB irradiation is reduced with a lower quantity of lipid hydroperoxides (Martinez et al., 2015). In addition to disease protection, isolated chalcones from plants may work as a skin protector. Numerous reviews have been published on synthetic and natural chalcones (Nasir Abbas Bukhari et al., 2012; Kamal et al., 2013; Sharma et al., 2013; Leon-Gonzalez et al., 2015; Mahapatra et al., 2015b; Das and Manna, 2016). This review will focus on recent breakthroughs in medicinal chemistry that have used naturally occurring chalcone as a privileged pharmacological scaffold and aims to initiate more pharmacological and medicinal research into the realm of chalcone chemistry.
FIGURE 3
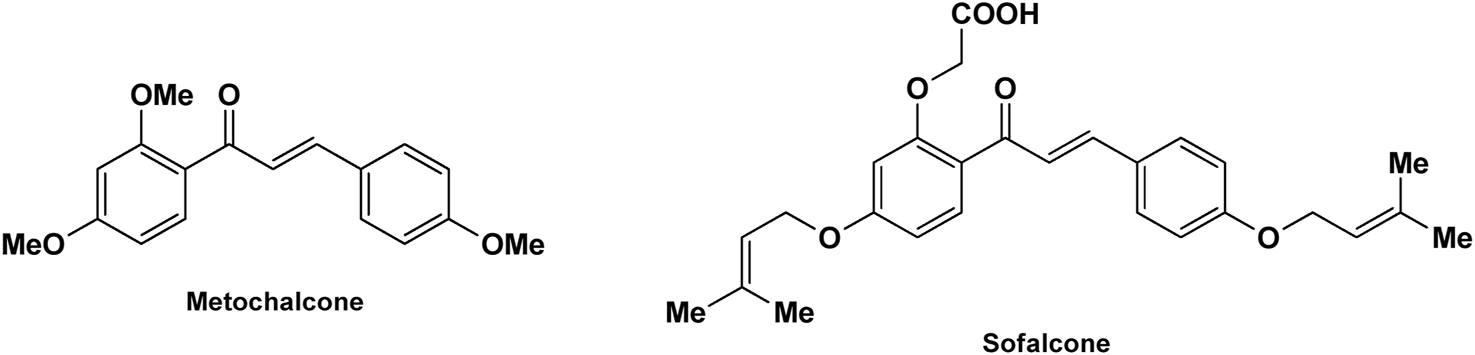
Metochalcone and sofalcone, two clinically permitted chalcone-based drugs.
The scope of the present review is wide-ranging and comprehends a multidisciplinary investigation of naturally occurring chalcones in the context of their clinical potential against various diseases. Focusing primarily on research articles published in the last 25 years, this review article attempts to showcase the most current developments using therapeutic potentials of naturally occurring chalcones in medicinal chemistry. The review presents the comprehensive features of naturally occurring chalcones, including their biosynthesis, synthetic approaches, antimicrobial, anti-cancer, antioxidant, anti-inflammatory, enzyme actions, antiobesity, cardioprotective activity, antidiabetic, and neuroprotective activity. In addition, this review also emphasizes the structural features of chalcones, structure-activity relationship (SAR: defines the relationship between the chemical structure and biological activity) studies, mechanism of actions, and marketed and clinically approved chalcones. The insights provided here aim to guide future research in exploring naturally occurring chalcones with enhanced pharmacological effectiveness against various diseases. Furthermore, this scientific literature review has been designed with essential studies on chalcone across 31-year (1993–2024) based on chemical structure, molecular mechanisms, and its application to various diseases as a therapeutic potential. A complete literature search was conducted using databases such as PubMed, Research Gate, ScienceDirect, and Springer Link to discuss the details and insights.
2 Biosynthesis of chalcone
Noel P Joseph et al. described the mechanism of the chalcone biosynthesis process by chalcone synthase in legume Medicago sativa plant (Jez and Noel, 2000). Chalcone synthase is a polyketide synthase type III enzyme found in all higher plants. This enzyme is also found in lower plants like liverwort Marchantia polymorpha. Structurally, it is a homodimer where a single monomer has 42–45 kDa molecular weight. Notably, some amino acid residues are also identified as situated in the active site of this enzyme, including Cys164, Phe215, His303, and Asn336 (Figure 4). In the biosynthetic mechanism, chalcone synthase transfers the coumaroyl scaffold from one 4-coumaroyl-coenzyme A (CoA) to its active site residue Cys164. Subsequently, the polyketide reaction occurs where an intermediate product forms as three malonyl-CoA thioesters. After this thioester-linked tetraketide formation, a cyclization reaction occurs generating a naringenin chalcone.
FIGURE 4
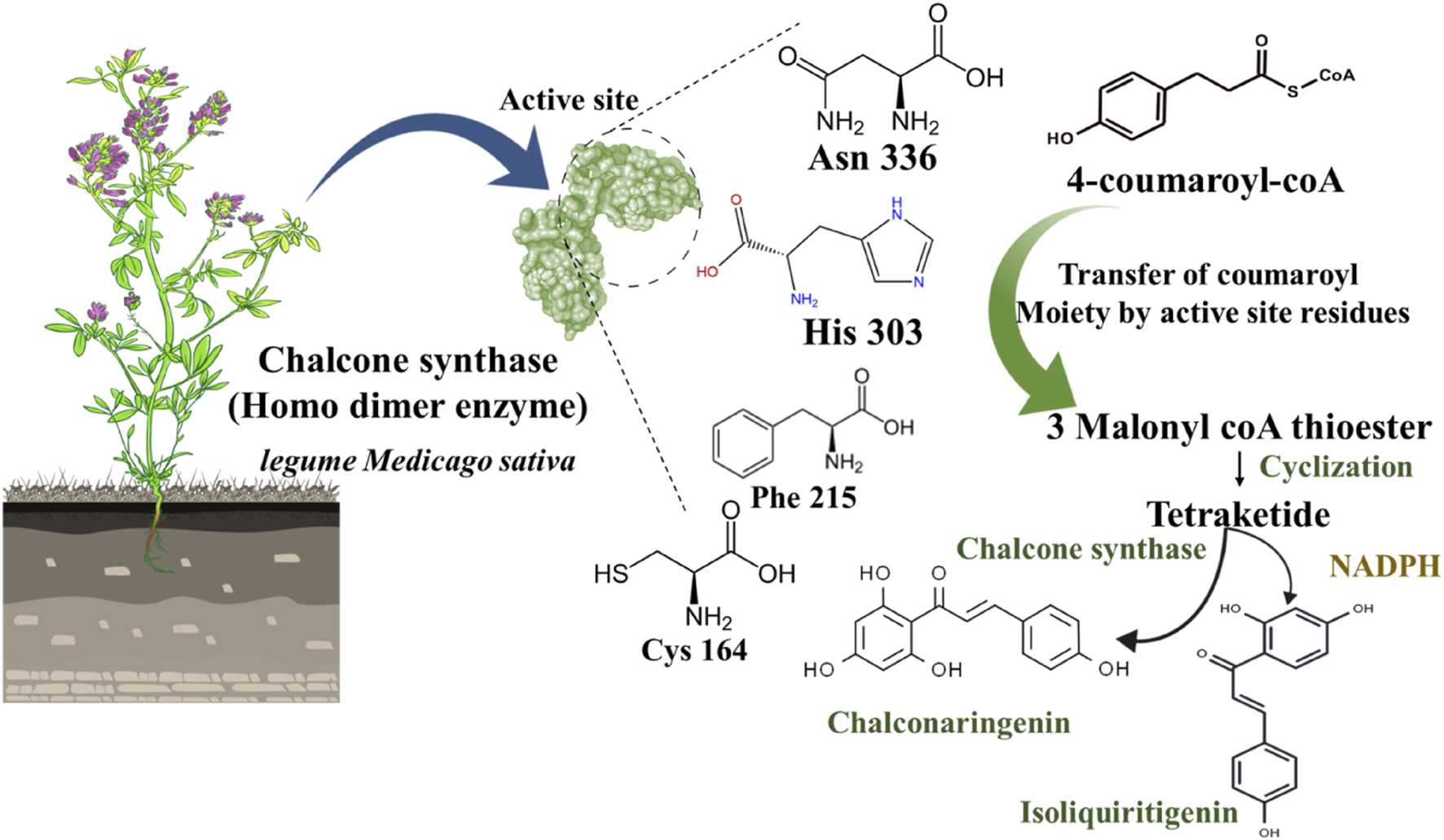
Schematic representation of chalcone biosynthesis in chalcone synthase and NADPH presence.
Further, this naringenin chalcone converts into the 6′-deoxy naringenin chalcone through chalcone reductase and chalcone synthase (Zhuang et al., 2017b). To delve into the depth of this biosynthetic pathway, a phenylpropanoid CoA (4-coumaroyl CoA) endures in a condensation reaction with three malonyl-CoA to form a tetraketide precursor which further goes into a cyclization reaction through a different pathway (Figure 4). Out of two distinct pathways, the primary path undergoes a cyclization process through chalcone synthase only to generate chalconaringenin. In the second pathway, the presence of nicotinamide adenine dinucleotide phosphate (NADPH: a co-enzyme that donates the hydrogens and electrons in anabolic metabolism) aids in the reduction reaction of tetraketide, and then it undergoes cyclization by chalcone synthase to form a 6′-deoxy chalcone (Figure 4) (Rammohan et al., 2020). Simultaneously, other molecules like phloroglucinols, benzophenones, and stilbenes are also synthesized as secondary metabolites. In this biosynthetic pathway, naringenin chalcone as a substrate produces flavonoids and isoflavonoids by chalcone synthase and chalcone isomerase (Zhuang et al., 2017b).
3 Various synthetic methods for the preparation of chalcones
Chalcones are considered a privileged scaffold and are typically employed in several pharmacological activities associated with drug discovery. As a result, researchers have kept looking for new advanced techniques and low-cost procedures for synthesizing chalcones and their derivatives. Chalcones are often synthesized by base or acid-catalyzed condensation processes. Conventional Claisen-Schmidt condensation is another method for the preparation of chalcone derivatives attributable to get higher yields than other procedures (Rammohan et al., 2020). The Suzuki coupling, Heck coupling, Wittig reaction, Friedel-Crafts acylation with cinnamonoyl chloride, Photo-Fries rearrangement of phenyl cinnamates, etc., are some well-known methods for the preparation of chalcone derivatives (Bukhari et al., 2013).
3.1 Claisen-schmidt reaction
The preparation of chalcone derivatives by the Claisen-Schmidt reaction comprises the condensation of derivatives of acetophenone and aldehyde in polar solvents in the presence of catalysts (acid or base). Usually, aq. NaOH or KOH or ethanolic NaOEt or potassium tert-butoxide is used to carry out the base-catalyzed Claisen-Schmidt reaction (Table 1) (Rammohan et al., 2020). The hydroxyl-substituted chalcone synthesis is commonly carried out using the base-catalyzed Claisen-Schmidt reaction, which usually provides good to outstanding yields. In the base-catalyzed Claisen-Schmidt reaction, the chalcone is formed from the aldol via the dehydration of enolate. In contrast, in an acid-catalyzed reaction, the chalcone is formed through an enol mechanism (Nielsen and Houlihan, 2011b).
TABLE 1
| Name of the reaction | Scheme | Reaction conditions | Solvent | Reference |
|---|---|---|---|---|
| Claisen-Schmidt Condensation |

|
Base catalysed (KOH, KOH, Ba(OH)2, Ca(OH)2, Sr(OH)2, CaO, NaH, LiHMDS, LiOH etc.) Acid catalysed (AlCl3, HCl, BF3-Et2O, SOCl2, p-TsOH etc.) |
Ethanol, methanol, THF Ethanol, methanol, dioxane, acetic acid, carbon disulphide |
Gaonkar and Vignesh (2017), Zhuang et al. (2017b) |
| Suzuki Coupling |

|
Catalyst: 3% PdCl2, base: Na2CO3 Catalyst: tetrakis(triphenylphosphine)palladium(0), base: CeCO3 |
Acetone/water = 3/1 Anhydrous toluene |
Bumagin and Korolev (1999), Haddach and McCarthy (1999), Eddarir et al. (2003) |
| Heck coupling |

|
Palladium catalyst Palladium catalyst, CO |
DMF, CH3CN Toluene |
Hird et al. (1993), Brennführer et al. (2009) |
| Wittig Reaction |

|
Benzene, THF | Ramirez and Dershowitz (1957) | |
| Sonogashira isomerization coupling |

|
PdCl2(PPh3)2, CuI | THF | Rammohan et al. (2020) |
| Julia-Kocienski Olefination |

|
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU), LiHMDS | DCM, THF, CHCl3, CH3CN | Kumar et al. (2010) |
| Friedel–Crafts Acylation with Cinnamoyl Chloride |

|
AlCl3 | Shotter et al. (1978) | |
| Photo-Fries Rearrangement of Phenyl Cinnamates |

|
h٧, Inert atmosphere (N2) | Benzene, CHCl3 | Obara et al. (1969), Ramakrishnan and Kagan (1970) |
Reaction scheme for synthesizing chalcone with different solvent systems.
3.2 Suzuki coupling
Two possible methods for synthesizing chalcone derivatives by Suzuki coupling are combining benzoyl chloride with phenylvinylboronic acid or cinnamoyl chloride with phenylboronic acid (Eddarir et al., 2003). The conditions of the Suzuki coupling reaction have an impact on the yield. For instance, coupling cinnamoyl chloride with phenylboronic acids under these conditions (acetone/water = 3/1; 3% PdCl2; Na2CO3) results in a moderate yield (23%–37%), whereas isolated yields of ∼50 and ∼90% are obtained under these conditions (anhydrous toluene; tetrakis (triphenylphosphine) palladium (0); CeCO3) (Table 1) (Haddach and McCarthy, 1999). Chalcones having electron-withdrawing or electron-donating moieties can also be synthesized via an extended Suzuki coupling procedure.
3.3 Heck coupling
Heck coupling provides an efficient way to synthesize chalcones by combining aryl vinyl ketones and aryl boronic acids over the formation of carbon-carbon bonds (Table 1) (Rammohan et al., 2020). Under catalytic conditions [Pd (OAc)2, Ph3P, K2CO3, DMF], aryl vinyl ketones are combined with ArI or aryl boronic acids to generate chalcones in good yields (Hird et al., 1993; Bumagin and Korolev, 1999). Chalcones have also been prepared by carbonylative Heck coupling using palladium catalysts and the carbonylative vinylation of aryl halides with styrene in carbon monoxide. While the metal-catalyzed Heck reaction is considered an extremely effective method for synthesizing chalcones, its use is restricted due to the scarcity of aryl vinyl ketones and the requirement for pressurized CO (Wu et al., 2010).
3.4 Wittig reaction
Chalcones can be synthesized via the Witting olefination reaction. The reaction between triphenylbenzoylmethylene phosphorane and benzaldehyde in tetrahydrofuran (THF) produced chalcones with 70% yield (Table 1) (RAMIREZ and DERSHOWITZ, 1957). Furthermore, a microwave-assisted synthesis of chalcones with a fast reaction time (5-6 min) and excellent yields was discovered. To obtain high yields, this creative endeavor enhances the reaction rates of the Wittig olefination reaction while decreasing the reaction time (Xu et al., 1995).
3.5 Sonogashira isomerization coupling
In the Sonogashira isomerization coupling reaction, the chalcone derivatives are prepared by treating ArX and aryl or alkenyl 1-propargyl alcohols in equimolar amounts catalyzed by PdCl2(PPh3)2 in THF (Table 1) (Rammohan et al., 2020).
3.6 Julia–Kocienski olefination
Julia–Kocienski olefination produces E-chalcones as the major product even at low temperatures. It involves directly coupling heteroaryl sulfonyl phenylethanone and aromatic aldehydes under basic conditions (Table 1) (Kumar et al., 2010).
3.7 Friedel-Crafts acylation with cinnamoyl chloride
By Friedel-Crafts acylation of an aromatic ether and cinnamoyl chloride, chalcone derivatives can be synthesized in the presence of a Lewis acid catalyst (AlCl3) (Table 1) (Shotter et al., 1978). Although this process was utilized to prepare highly substituted chalcones, it is a less popular procedure for the synthesis of chalcones.
3.8 Photo-fries rearrangement of phenyl cinnamates
Photo-Fries rearrangement was used to prepare 2-hydroxy substituted chalcones from phenyl-cinnamate under an inert atmosphere (N2) using benzene as a solvent (Table 1) (Obara et al., 1969). Alcohols and chloroform solvents can also perform the photo-fries rearrangement reaction of chalcones, increasing yields by up to 50% (Ramakrishnan and Kagan, 1970). This process is not commonly used because of its limitations, such as longer reaction time, poor yield, etc.
4 Role of naturally occurring chalcones in different pharmacological activities
Since natural metabolites have been revealed to have positive outcomes on an inclusive range of common and general diseases, such as cancer, cardiovascular disease, parasitic illnesses, type 2 diabetes mellitus, infectious diseases, and illnesses of the central nervous system, interest in and attraction toward naturally occurring metabolites have been steadily growing (Das et al., 2023c; Debnath et al., 2024; Sinha et al., 2024; Maity et al., 2025). These naturally occurring metabolites result from millions of centuries of evolution and natural selection, display efficacy and selectivity in interaction with biomolecular targets, and can efficiently avoid current antibiotic resistance. The chalcone-rich botanical drugs and plants were employed in traditional medicinal practice for eras. Naturally occurring chalcones were extracted for the first time in 1910 and attracted a lot of consideration because of their significant pharmacological attributes (Shimokoriyama M, 1962). Many chalcones also got formal medical approval for clinical trials against cancer, viral infections, and cardiovascular disorders (Salehi et al., 2021). In medicinal chemistry, chalcones are regarded as prime compounds for developing novel therapeutics (Zhuang et al., 2017b).
4.1 Antimicrobial activity of natural chalcones
Roughly 7.7 million of the approximately 13.7 million fatalities caused by infectious disease in 2019 were interrelated to 33 prevalent pathogens. Pseudomonas aeruginosa, Streptococcus pneumoniae, Klebsiella pneumoniae, Staphylococcus aureus, and Escherichia coli are responsible for 54.9% of these deaths, and the majority of deaths globally are caused by S. aureus infections (Adhikari et al., 2020; Ikuta KS et al., 2022; El-Helw et al., 2024). Furthermore, in medicinal chemistry, new advances in potential bioactive chalcone hybrids have been explored to play a vital role as antibacterial agents (Maurya and Agrawal, 2024). It has been found that chalcones are effective towards numerous gram-positive and negative bacteria, fungi, protozoa, and even viruses. Chalcone compounds like pinocembrin chalcone, 4′,6′-dihydroxy-3′,5′-dimethyl-2′-methoxychalcone, licochalcone A and C, isobavachalcone, 4-hydroxyderricin, xanthoangelol, xanthoangelol F, bavachalcone, broussochalcone B, panduratin A etc. are potent antibiotic in nature. They were found to exhibit their activity against numerous microbes, including staphylococcus, bacillus, mycobacterium, legionella, micrococcus, enterococcus and streptococcus, etc. (Bremner and Meyer, 1998; Sugamoto et al., 2011). Meyer and Bremner isolated, characterized and evaluated the antibacterial activity of pinocembrin chalcone and its isomer 5,7-dihydroxy flavanone. The pinocembrin chalcone was extracted from Helichrysum trilineatum and characterised by mass and NMR spectra (1H and 13C NMR). Antibacterial tests revealed that pinocembrin chalcone (1.0 μg) was effective in preventing S. aureus from growing but inactive against Candida species (Table 2) (Bremner and Meyer, 1998). The antibacterial property of pinocembrin chalcone might be aided by the presence of three phenolic -OH group and an α,β unsaturated ketone structure.
TABLE 2
| Name and structure of chalcone | Source | Smiles | Activity | Concentration and duration of treatment | Target organism | References |
|---|---|---|---|---|---|---|

Pinocembrin chalcone |
Helichrysum trilineatum DC (Family: Asteraceae) | OC1=CC(O)=CC(O)=C1C(/C=C/C2=CC=CC=C2)=O | Antibacterial. | 1.0 μg | Staphylococcus aureus | Bremner and Meyer (1998) |

Licochalcone A  Licochalcone C |
Glycyrrhiza inflata (Family: Fabaceae) | OC1=C(C(C)(C)C=C)C=C(/C=C/C(C2=CC=C(O)C=C2)=O)C(OC)=C1 OC1=CC=C(C(/C=C/C2=CC=C(O)C(C/C=C(C)\C)=C2OC)=O)C(OC)=C1 |
Antibacterial. Cause complete inhibition of the outgrowth of B. subtilis spores. |
2-3 μg/mL. | Bacillus subtilis | Tsukiyama et al. (2002) |
| Licochalcone A |
Glycyrrhiza uralensis
Fisch. ex DC. (Family: Fabaceae) |
OC1=C(C(C)(C)C=C)C=C(/C=C/C(C2=CC=C(O)C=C2)=O)C(OC)=C1 |
Antibacterial. Exerts antibacterial activity against human pathogenic Mycobacteria and Legionella species. |
1-4 mg/L. | Mycobacterium tuberculosis, Mycobacterium bovis; Legionella species (L. pneumophila, L. longbeacheae, L. bozemanii, L. wadsworthi, L. dumoffii, and L. feelei. | Friis-Møller et al. (2002) |

4′,6′-Dihydroxy-3′,5′-dimethyl-2′-methoxychalcone |
Dalea versicolor Zucc. (Family: Fabaceae) | OC1=C(C)C(O)=C(C(/C=C/C2=CC=CC=C2)=O)C(OC)=C1C | Antibacterial. | 30 μg/mL. |
Staphylococcus aureus, Bacillus cereus.
|
Belofsky et al. (2004) |

Isobavachalcone |
Angelica keiskei (Miq.) Koidz. (Family: Apiaceae) | OC1=CC=C(C(/C=C/C2=CC=C(O)C=C2)=O)C(O)=C1C/C=C(C)\C | Antibacterial | MIC 4 µg/mL. | Bacillus sublitis, Staphylococcus epidermidis, Micrococcus luteus. | Sugamoto et al. (2011) |

4-hydroxyderricin |
Angelica keiskei (Miq.) Koidz. (Family: Apiaceae) | OC1=C(C(/C=C/C2=CC=C(O)C=C2)=O)C=CC(OC)=C1C/C=C(C)/C | Antibacterial | MIC 2 µg/mL. | Bacillus sublitis, Staphylococcus epidermidis, Micrococcus luteus. | Sugamoto et al. (2011) |

Xanthoangelol |
Angelica keiskei (Miq.) Koidz. (Family: Apiaceae) | OC1=C(C/C=C(C)/CC/C=C(C)/C)C(O)=C(C(/C=C/C2=CC=C(O)C=C2)=O)C=C1 | Antibacterial | MIC 4 µg/mL. | Bacillus sublitis, Staphylococcus epidermidis, Micrococcus luteus. | Sugamoto et al. (2011) |

Xanthoangelol F |
Angelica keiskei (Miq.) Koidz. (Family: Apiaceae) | C/C(CC/C=C(C)/C)=C\CC1=C(OC)C=CC(C(/C=C/C2=CC=C(O)C=C2)=O)=C1O | Antibacterial | MIC 64 µg/mL. | Bacillus sublitis, Staphylococcus epidermidis, Micrococcus luteus. | Sugamoto et al. (2011) |

Bavachalcone |
Angelica keiskei (Miq.) Koidz. (Family: Apiaceae) | OC1=CC(OC)=C(C/C=C(C)/C)C=C1C(/C=C/C2=CC=C(O)C=C2)=O | MIC 4 µg/mL. | Bacillus sublitis, Staphylococcus epidermidis, Micrococcus luteus. | Sugamoto et al. (2011) | |

Broussochalcone B |
Angelica keiskei (Miq.) Koidz. (Family: Apiaceae) | OC1=C(C/C=C(C)/C)C=C(C(/C=C/C2=CC=C(O)C=C2)=O)C(O)=C1 | MIC 8-16 µg/mL. |
Bacillus sublitis, Staphylococcus epidermidis, Micrococcus luteus.
|
Sugamoto et al. (2011) | |
| Licochalcone A |
Glycyrrhiza uralensis Fisch. ex DC. (Family: Fabaceae) |
OC1=C(C(C)(C)C=C)C=C(/C=C/C(C2=CC=C(O)C=C2)=O)C(OC)=C1 |
Inhibits growth of protozoan. Reduced the rate of infection in macrophages -derived from human peripheral blood monocyte and U937 cells. |
5 μg/mL. |
Leishmania donovani, and promastigotes. |
Chen et al. (1993) |
| Licochalcone A |
Glycyrrhiza uralensis Fisch. ex DC. (Family: Fabaceae) |
OC1=C(C(C)(C)C=C)C=C(/C=C/C(C2=CC=C(O)C=C2)=O)C(OC)=C1 |
Antiprotozoal. Diminished the development of chloroquine-resistant (Dd2) and chloroquine-susceptible (3D7) Plasmodium falciparum strains. | 0.1-0.5 μg/mL (in vitro.) 10-15 mg/kg (in vivo.) |
Chloroquine-susceptible (3D7) and chloroquine-resistant (Dd2) Plasmodium strains
P. falciparum, and P. yoelii. |
Chen et al. (1994) |
| Licochalcone A |
Glycyrrhiza uralensis Fisch. ex DC. (Family: Fabaceae) |
OC1=C(C(C)(C)C=C)C=C(/C=C/C(C2=CC=C(O)C=C2)=O)C(OC)=C1 |
Antiprotozoal. Licochalcone A caused ultrastructural alteration in a dose-dependent manner without causing harm of macrophages. |
1 mg/mL. |
Leishmania sp. |
Zhai et al. (1995) |
| Licochalcone A |
Glycyrrhiza uralensis Fisch. ex DC. (Family: Fabaceae) |
OC1=C(C(C)(C)C=C)C=C(/C=C/C(C2=CC=C(O)C=C2)=O)C(OC)=C1 |
Antiprotozoal. It repressed the bc1 complex and complex II of Plasmodium falciparum mitochondria. | IC50 value for SQR activity inhibition is reported 1.30 µM. IC50 value for bc1 complex and DHOD inhibition is found between 0.077 to 0.10 µM. |
Plasmodium falciparum. | MI-ICHI et al. (2005) |

Xanthohumol |
Hop extract (Species not mentioned) (Family: Cannabaceae) | O=C(/C=C/C1=C(O)C=C(O)C=C1)C2=C(OC)C=C(O)C(C/C=C(C)\C)=C2O | Antiviral | BVDV (TI = 6.0), HSV-1 (TI = >1.9), HSV-2 (TI = >5.3) | Bovine viral diarrhoea virus, Herpes simplex virus-1, and Herpes simplex virus-2 | Buckwold et al. (2004) |
| Xanthohumol | Humulus lupulus L. (Family: Cannabaceae) | O=C(/C=C/C1=C(O)C=C(O)C=C1)C2=C(OC)C=C(O)C(C/C=C(C)\C)=C2O | Antiviral | EC50 = 20.74 µg/mL | Human immunodeficiency viruses-1 | Wang et al. (2004) |

2-Methoxy-3-methyl-4,6-dihydroxy-5-(3′-hydroxy)cinnamoylbenzaldehyde |
Desmos spp. [Desmos chinensis Lour. (Family: Annonaceae); Desmos grandifolius (Finet & Gagnep.) C.Y.Wu ex P.T.Li (Family: Annonaceae); Desmos dumosus (Roxb.) Saff. (Family: Annonaceae); and Desmos yunnanensis (Hu) P.T.Li (Family: Annonaceae)] |
O=C(/C=C(O)/C1=CC=C(O)C=C1)C2=C(O)C(C)=C(OC)C(C=O)=C2O | Antiviral | EC50 = 0.022 µg/mL | Human immunodeficiency viruses | Wu et al. (2003) |

Isoliquiritigenin |
Glycyrrhiza uralensis Fisch. ex DC. (Family: Fabaceae) | OC(C=C1O)=CC=C1C(/C=C/C2=CC=C(O)C=C2)=O | SAR based inhibition of neuraminidase activity. | IC50 = 9.0 µM | Influenza virus | Ryu et al. (2010b) |

Echinantin |
Glycyrrhiza inflata Batalin (Family: Fabaceae) |
O=C(/C=C/C1=C(OC)C=C(O)C=C1)C2=CC=C(O)C=C2 | Antiviral | IC50 = 2.49 ± 0.14 µg/mL | H1N1 influenza, H274Y mutant form of H1N1. | Dao et al. (2011) |

Xanthokeistal A |
Angelica keiskei (Miq.) Koidz. (Family: Apiaceae) | O=C(/C=C/C1=CC=C(O)C=C1)C2=C(O)C(C/C=C(C)/CCC(OC)OC)=C(O)C=C2 | Antiviral, causes inhibition of neuraminidase activity | IC50=12.3 μM | Influenza virus | Park et al. (2011) |
| Licochalcone A |
Glycyrrhiza inflata Batalin (Family: Fabaceae) Glycyrrhiza glabra L. (Family: Fabaceae) |
OC1=C(C(C)(C)C=C)C=C(/C=C/C(C2=CC=C(O)C=C2)=O)C(OC)=C1 |
Antibacterial | 25–250 µg/mL (G. inflata) 12.5–25µg/mL (G. glabra) | Streptococcus mutans, Lactobacillus buchneri, and Staphylococcus aureus. | van Dinteren et al. (2022) |

Kamalachalcone E  1-(5,7-dihydroxy-2,2,6-trimethyl-2H-1-benzo-pyran-8-yl)-3-phenyl-2-propen-1-one  Rottlerin  4′-hydroxyrottlerin. |
Mallotus philippinensis
(Lam.) Muell.Arg. (Family: Euphorbiaceae) |
OC(C=C1)=CC=C1/C=C/C(C2=C(OC(C)(C)C[C@@]34[H])C3=C(O[C@]5([H])[C@]4([H])C(C)(C)OC6=C5C(O)=C(CC7=C(O)C(C)=C(O)C(C(C)=O)=C7O)C(O)=C6C(/C=C/C8=CC=C(O)C=C8)=O)C(CC9=C(O)C(C(C)=O)=C(O)C(C)=C9O)=C2O)=O O=C(C1=C(O)C(C)=C(O)C2=C1OC(C)(C)C=C2)/C=C/C3=CC=CC=C3 O=C(C1=C(O)C(CC2=C(O)C(C)=C(O)C(C(C)=O)=C2O)=C(O)C3=C1OC(C)(C)C=C3)/C=C/C4=CC=CC=C4 O=C(C1=C(O)C(CC2=C(O)C(C)=C(O)C(C(C)=O)=C2O)=C(O)C3=C1OC(C)(C)C=C3)/C=C/C4=CC=C(O)C=C4 |
Antifungal | 8, 4 and 16 µg/mL | Cryptococcus neoformans, and Aspergillus fumigatus | Kulkarni et al. (2014) |

2ʹ,4ʹ-dihydroxy-3ʹ-methoxychalcone,  2ʹ,4ʹ-dihydroxychalcone |
Zuccagnia punctata Cav. (Family: Caesalpiniaceae) | OC(C=C1)=CC(O)=C1C(/C=C/C2=CC(OC)=CC=C2)=O OC(C=C1)=CC(O)=C1C(/C=C/C2=CC=CC=C2)=O |
Antifungal | 400 µg/mL | Candida Species (C. guilliermondii, C. tropicalis C. krusei, C. parasilopsis C. glabrata , C. albicans ) |
Gabriela et al. (2014) |
Plant source, doses, and antimicrobial roles of different natural chalcones.
Chalcone-induced suppression of O2 consumption in sensitive bacteria and prevention of NADH oxidation in bacterial membranes are the sources of chalcones’ antibacterial activity (Haraguchi et al., 1998b). The rhizome and root of the Glycyrrhiza species, liquorice, is a generally used botanical drug to cure a variety of ailments, such as gastrointestinal issues and arthritis (Pastorino et al., 2018). More than 600 bioactive metabolites were extracted from liquorice to date, including many retrochalcones such as licochalcone A, B, C, D, E, etc., (Yoon et al., 2007). The absence of a -OH moiety at the C-2′ and C-6′ sites make these retrochalcones differ from regular chalcones and makes them members of an uncommon phenolic family (Xiao et al., 2019). Retrochalcones are recognized for their photo reactivity due to α,β unsaturation. They can undergo photo-induced trans-to-cis isomerization via delocalization of electron, which is made possible by the conjugated carbonyl function. Licochalcone A effectively inhibits Tumor Necrosis Factor (TNF)-α, Interleukin (IL)-1β, and IL-6, three markers of inflammation. Licochalcone A, B, C, and D have demonstrated antiviral, antitrypanosomal, anti-cancer, anti-inflammatory, antidiabetic, and antibacterial properties (Rudrapal et al., 2021). In 1975, Saitoh discovered licochalcone A, a phenolic chalcone with two aromatic rings acting as the main structural unit from the root of Glycyrrhiza uralensis. Two chemically reactive double bonds are present in licochalcone A and its isomers: (1) the α,β unsaturation, which promotes trans-to-cis isomerization by absorbing long wavelength light; and (2) aliphatic side chain unsaturation, which can result in ring-closing with the -OH group at C-4 (Rozmer and Perjési, 2016; Ara et al., 2024). One of the main chalcones isolated from liquorice, licochalcone A, has been exposed to have numerous advantageous pharmacological activities, such as anti-inflammation, antioxidation, anti-cancer, antimicrobial properties etc., (Li M.-T. et al., 2022). Tsukiyama et al. reported that salt-, heat-, and protease-resistant licochalcone A exhibited antibacterial properties towards Gram-positive bacteria, particularly Bacillus species. The authors noted that in vitro, licochalcone A completely suppressed Bacillus subtilis’s vegetative cell development at concentrations of up to 3 μg/mL (Table 2). With minimum inhibitory concentrations (MICs) of 2 ∼ 3 μg/mL, licochalcone A exhibited efficacy towards all tested gram-positive bacteria, particularly against Bacillus species. However, at 50 μg/mL, it was ineffective against gram-negative bacteria (Tsukiyama et al., 2002). Moreover, licochalcone A, especially extracted from the Glycyrrhiza uralensis explored as an antimicrobial metabolite as it inhibits the growth of several species of Mycobacterium as well as Legionella with concentrations of 1–4 mg/L (Table 2) (Friis-Møller et al., 2002).
Belofsky et al. isolated and characterized 4′,6′-dihydroxy-3′,5′-dimethyl-2′-methoxychalcone along with six metabolites from the organic extracts of Dalea versicolor. Using NMR and HRMS methods, the extracted metabolite structures were identified. At very small doses (∼3.3 μg/mL), 4′,6′-dihydroxy-3′,5′-dimethyl-2′-methoxychalcone completely inhibited the growth of S. aureus when combined with a subinhibitory quantity of berberine (Table 2). Furthermore, 4′,6′-dihydroxy-3′,5′-dimethyl-2′-methoxychalcone was observed to enhance the effects of prescribed antibiotics berberine and some antibiotics (erythromycin and tetracycline); action mechanism of 4′,6′-dihydroxy-3′,5′-dimethyl-2′-methoxychalcone was consistent with blocking the NorA MDR efflux pump in S. aureus (Belofsky et al., 2004).
First isolated from Psoralea corylifolia in 1968, isobavachalcone is a prenylated chalcone (Bhalla et al., 1968). Sugamoto et al. synthesized and characterised prenyl or geranyl groups containing naturally occurring chalcones such as xanthoangelol F, bavachalcone, 4-hydroxyderricin, deoxyxanthoangelol H, xanthoangelol, xanthoangelol H, isobavachalcone, and broussochalcone B and assessed their antibacterial activities towards both gram-negative (Pseudomonas fluorescens, Proteus mirabilis, Escherichia coli) and gram-positive bacteria (Staphylococcus epidermidis, Bacillus subtilis, Micrococcus luteus). The chalcones were also prepared by the use of montmorillonite K10 as a catalyst in the [1,3]-sigmatropic rearrangement of 2′-prenyloxyacetophenone, 2′-prenyloxychalcones, or 2′-geranyloxychalcones. Although xanthoangelol, 4-Hydroxyderricin, bavachalcone, isobavachalcone, xanthoangelol F, and broussochalcone B were active against gram-positive bacteria, but displayed no activities towards gram-negative bacteria (Table 2). SAR study designated that prenyl group on the A-ring contributes to a rise in antibacterial action and 3′-geranylchalcone with 4′-hydroxy moiety containing xanthoangelol exhibited strong activity (Sugamoto et al., 2011). Isobavachalcone, kamalachalcone E, and geranyl-substituted chalcone derivatives etc. have been tested against various pathogenic fungal strains like Candida albicans, Cryptococcus neoformans, Trichophyton mentagrophytes, Cladosporium cladosporioides, Aspergillus fumigates, etc., (Bhakuni and Chaturvedi, 1984; ElSohly et al., 2001; Jayasinghe et al., 2004; Kulkarni et al., 2014). The ability of chalcone compounds to interact with intracellular thiols determines their antimicotic activity against Candida albicans. Many of the natural and synthetic chalcones inhibit the conversion of tubulin into microtubules, making them toxic for the growth and survival of fungus (Elias et al., 1999; Go et al., 2005).
Chalcone derivatives are found active against many protozoan species of genus Leishmania, Plasmodium responsible for leishmaniasis and malarial disease, respectively in humans (Sen and Chatterjee, 2011; Kumar et al., 2013). The antiprotozoal activity of licochalcone A is very well studied. It is reported to inhibit the development of Leishmania major and Leishmania donovani promastigotes and amastigotes germs and markedly reduces the contamination of cells (Table 2) (Chen et al., 1993). When it was administered in Plasmodium yoelii infected mice through intraperitoneal or oral route, the mice survived from the fatal Plasmodium yoelii infection (Table 2) (Chen et al., 1994). Licochalcone A reported to bring ultrastructural changes in leishmania cells, impairs respiratory function by inhibiting mitochondrial dehydrogenase, bc1 complex, complex II etc., (Zhai et al., 1995; MI-ICHI et al., 2005). First reported in 1993, Chen and his group provided proof of the antimalarial attributes of licochalcone A with strong activity towards human pathogenic protozoan Leishmania species, highlighting the potential of chalcones as an antimalarial drug. Furthermore, it was found that licochalcone A inhibited the growth of Plasmodium falciparum which is susceptible and resistant to chloroquine. In mice infected with Plasmodium yoelii YM, intraperitoneal injection of 15 mg/kg four times a day for 3 days resulted in a 93% clearance of parasites without any side effects. In the same experiment, oral lichochalcone A dosages of 450, 150, and 50 mg/kg/day were shown to almost completely eradicate the parasitemia, and by the end of the 21-day trial, there was no mortality (Table 2) (Chen et al., 1994).Lichochalcone A preferentially inhibits fumarate reductase (FRD: an enzyme that binds to membrane to catalyze the reduction of fumarate to succinate) in the respiratory system of the parasite, changing the ultrastructure as well as the mitochondrial function of the parasite (Zhai et al., 1995). Licochalcone A had an inhibitory impact on human pathogenic Legionella and Mycobacteria species. Legionella dumoffii, Legionella bozemanii, and other species were suppressed at concentrations of 1–4 mg/L, whereas Mycobacterium bovis, Mycobacterium tuberculosis, and BCG were repressed by less than 20 mg/L (Friis-Møller et al., 2002). The antimalarial effectiveness of licochalcone A was further demonstrated by Mi-Ichi et al. when they reported that the parasite Plasmodium yoelii was eliminated in mice by licochalcone A without causing any harmful side effects. The negligible IC50 results (0.10 µM) for licochalcone A suggested that the suppression of the Plasmodium bc1 complex (ubiquinol-cytochrome c reductase) may account for a significant portion of its antimalarial action (Table 2) (MI-ICHI et al., 2005).
Other chalcones reported for antiprotozoal activity include kanzonol C, isocordin, 5-prenylbutein, 5-deoxyabyssinin II, crotaorixin, medicagenin, xanthohumol, etc., (Christensen et al., 1994; Torres-Santos et al., 1999; Narender and Gupta, 2004; 2005; Yenesew et al., 2004; Frölich et al., 2005; Salem and Werbovetz, 2005; Borges-Argáez et al., 2007; Garcia et al., 2021). Some of these compounds impair with uptake of hypoxanthine, thymidine, interfere with the biosynthesis of polyamines, and haemin degradation leads to death of protozoan cell. Verzele et al. first characterized the structure of xanthohumol, but it was in the 1990s that the pharmacological benefits of xanthohumol were recognized (Verzele et al., 1957). The structure of xanthohumol comprised of a chain of flavonoids, one unsaturated double bond (α, β), a prenyl motif, and two aromatic rings substituted with -OH and -OCH3 moities organized in a trans position. Because of the existence of α,β-unsaturated ketone moiety, xanthohumol possesses pharmacological properties. Prenyl units and the -OCH3 group replace the aromatic ring in this molecule, making it more lipophilic and having a strong affinity for biological systems’ membranes (Oledzka, 2024). Xanthohumol and iso-xanthohumol exerts antiviral activity towards bovine viral diarrhea virus (BVDV), Hepatitis C virus (HCV), Rhinovirus, Herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) at micro-molar concentration (BUCKWOLD et al., 2004). The antiviral property of crude hop extracts and purified hop constituents was examined by Buckwold et al. None of the extracts were able to stop Human immunodeficiency viruses (HIV), Influenza (Flu)-A and B, Respiratory syncytial virus (RSV), or Yellow Fever Virus (YFV) from replicating. With an IC50 in the negligible µg/mL range, a xanthohumol contained hop extract showed mild to average antiviral efficacy towards BVDV (therapeutic index (TI) = 6.0), HSV-2 (TI = >5.3), Rhino (TI = 4.0), and HSV-1 (TI = >1.9). Xanthohumol was shown to be responsible for the antiviral action seen in the xanthohumol contained hop extract towards BVDV, HSV-1, and HSV-2 using ultra-pure preparations (>99% pure). Structure activity relationship study indicated that compared to the isomer iso-xanthohumol, xanthohumol was more effective antiviral agent towards several viruses. Furthermore, xanthohumol demonstrated antiviral efficacy towards CMV, indicating the possibility of a broader anti-herpesvirus antiviral effect (Table 2) (BUCKWOLD et al., 2004).
Xanthohumol (Figure 5) and other natural chalcones inhibit HIV-1 replication by modifying the action of viral reverse transcriptase (Figure 5) and inhibit HIV-1-induced cytopathic effects (Wu et al., 2003; WANG et al., 2004). Zheng and his group extracted xanthohumol from the hop Humulus lupulus and assessed its anti-HIV-1 efficacy. The authors attribute that, at non-cytotoxic concentrations, xanthohumol suppressed reverse transcriptase, viral p24 antigen synthesis, and cytopathic effects generated by HIV-1 in C8166 cells. The EC50 values for RT generation and the inhibition of HIV-1 p24 antigen synthesis were 0.50 μg/mL (1.22 µM) and 1.28 μg/mL (3.21 µM), respectively. Furthermore, with an EC50 of 20.74 μg/mL, xanthohumol suppressed HIV-1 replication in peripheral blood mononuclear cell (PBMC) (Table 2). Lee and his colleagues isolated sixteen flavonoids and their derivatives from Desmos spp. and in H9 lymphocyte cells for their ability to prevent HIV replication. It was found that β-Hydroxy chalcone 2-Methoxy-3-methyl-4,6-dihydroxy-5-(3′-hydroxy)cinnamoylbenzaldehyde showed a favorable therapeutic index (TI) and strong anti-HIV property (EC50 = 0.022 μg/mL) (Table 2) (Wu et al., 2003). The SAR study demonstrated that the chalcone skeleton’s C-2 methoxy group might be essential for its anti-HIV properties.
FIGURE 5
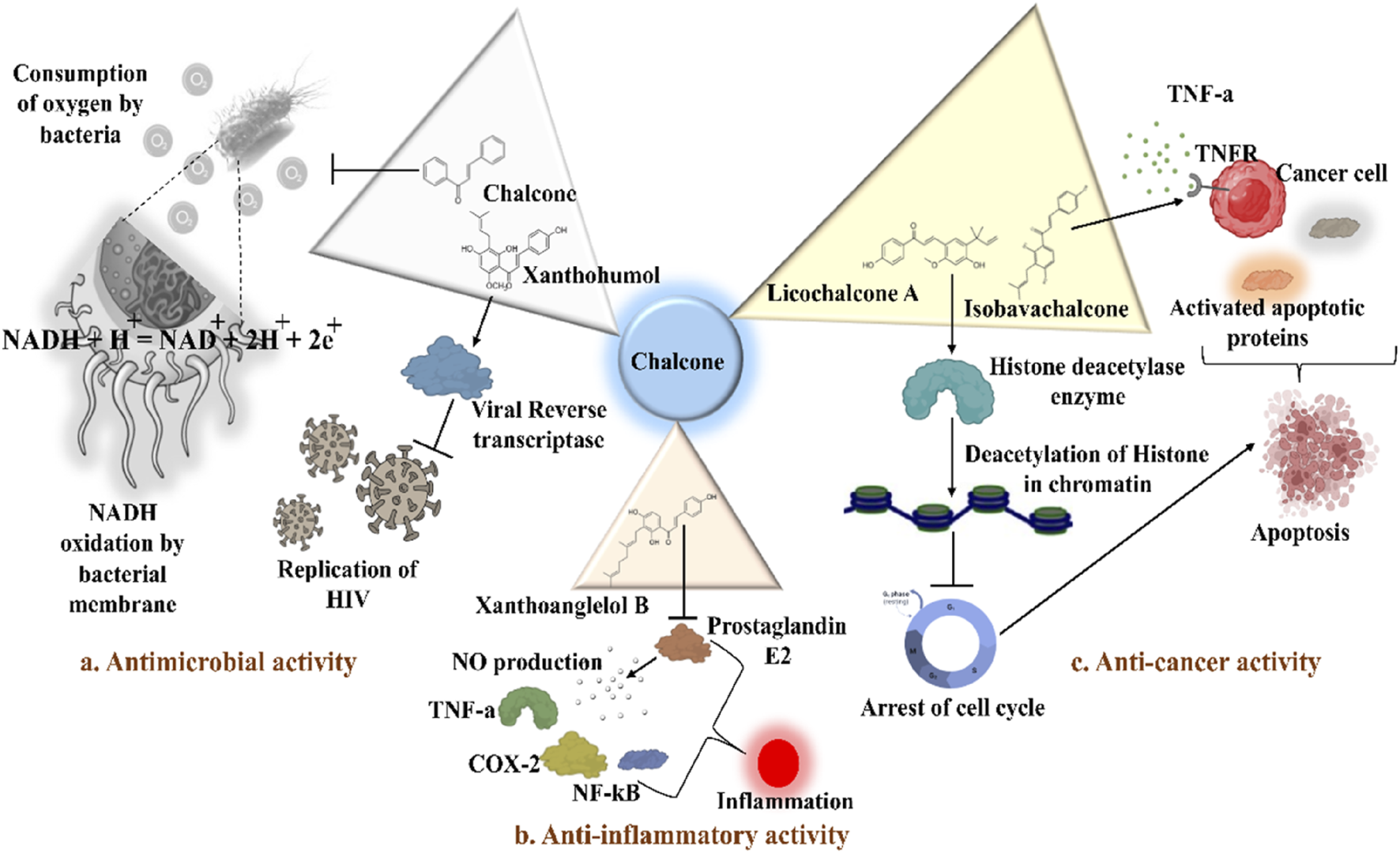
Distinct roles of naturally isolated chalcones as an antimicrobial, anti-inflammatory, and anti-cancer agent. Mechanism of different chalcones isolated from the natural sources. (a) Modulating the microbial membrane’s electron transport mechanism to inhibit the growth of microbes; (b) Regulating the enzymatic activity to control the inflammation; (c) Controlling the expression of several signaling proteins, and cell cycle phase transitions to eradicate the cancer cells.
Isoliquiritigenin, echinatin, and many other chalcones have been found to act against the influenza infection. They show strong inhibitory activity against neuraminidase activation and enhance the efficacy of antiviral drugs like oseltamivir (Ryu Y. B. et al., 2010; Dao et al., 2011; Park et al., 2011). Ryu et al. extracted eighteen polyphenols, including four chalcones from methanol extracts of Glycyrrhiza uralensis roots, and explored their neuraminidase repressive action. Experimental results suggested that isoliquiritigenin with an IC50 values of 9.0 µM among the chalcones had potent inhibitory activity. SAR studies demonstrated that the properties of chalcones are higher compared to their corresponding glycosides. Furthermore, methylation at the 2-OH reduced the inhibitory action, while increased -OH moieties at the 2 and 4′positions of chalcones augmented the repressive action (Table 2) (Ryu et al., 2010). Dao and associates isolated a novel licochalcone G and seven recognized chalcones from the acetone extract of Glycyrrhiza inflata and examined their anti-influenza activities. The chalcones’ structure was characterised by 1D and 2D NMR analysis, and it was validated by contrasting the spectroscopic and physicochemical analysis with those reported in the literature. With an IC50 = 2.49 ± 0.14 μg/mL, the most active chalcone echinantin, suppressed the neuraminidase (NA) produced from the new H1N1 influenza. Interestingly, echinantin maintained its potency in suppressing the H274Y mutant form’s activity, having an IC50 = 2.19 ± 0.06 μg/mL (Table 2) (Dao et al., 2011). Furthermore, the repressive activity of oseltamivir, a recognized competitive inhibitor in the presence of echinantin (at 1.35 μg/mL or 5 µM) was boosted remarkably on NAs of H9N2 (3.6-fold), H1N1 (7.0-fold), novel flu (WT) (3.7-fold), and tamiflu-resistant novel flu (H274Y) (52.6-fold) having IC50 from 4.94, 39.74, 21.09, and 5,132.85 ng/mL to 1.39, 5.69, 1.96, and 97.67 ng/mL, respectively. The authors assume that echinantin and oseltamivir may bind to distinct locations on the free and product-bound enzymes, each of which may function through a different inhibitory mechanism to cooperatively decrease NA activity.
Park et al. isolated a new chalcone xanthokeistal A having rare alkyl substitution with 6,6-dimethoxy-3-methylhex-2-enyl moiety along with five chalcones from Angelica keiskei and evaluated their potency against influenza virus neuraminidase inhibition (Table 2) (Park et al., 2011). With an IC50 of 12.3 µM, the most effective repressive effect was demonstrated by 2-hydroxy-3-methyl-3-butenyl alkyl (HMB) substituted chalcone xanthoangelol D. SAR studies indicated that for NA inhibition, the potency of substituted alkyl groups was as follows: HMB > 6-hydroxyl-3,7-dimethyl-octa-2,7-dienyl > dimethylallyl > geranyl.
Phenolic chalcones, for example, licochalcone A present in Glycyrrhiza spp. (particularly in the root region), have been investigated for their potential antimicrobial action towards Streptococcus mutans, Lactobacillus buchneri, and Staphylococcus aureus. Glycyrrhiza inflata, one of the two species of Glycyrrhiza, exhibits antimicrobial activity (MIC) at 25–250 μg/mL, while Glycyrrhiza glabra exhibits antimicrobial activity at 12.5–25 μg/mL (Table 2) (van Dinteren et al., 2022).
A study was conducted to isolate the new dimeric chalcone, kamalachalcone E, together with other compounds, including 1-(5,7-dihydroxy-2,2,6-trimethyl-2H-1-benzo-pyran-8-yl)-3-phenyl-2-propen-1-one, rottlerin, and 4′-hydroxyrottlerin, and studied the antifungal properties towards the Cryptococcus neoformans, and Aspergillus fumigatus. The structure of newly isolated kamalachalcone E was systematically characterised through 1D and 2D NMR studies, including HSQC, HMBC, COSY and ROESY experimentations. Interestingly, kamalachalcone E and 1-(5,7-dihydroxy-2,2,6-trimethyl-2H-1-benzo-pyran-8-yl)-3-phenyl-2-propen-1-one exhibited inhibitory property towards Aspergillus fumigatus, and Cryptococcus neoformans, and respectively with concentrations of 8, 4, and 16 μg/mL (Table 2). Interestingly, 4′-hydroxyrottlerin inhibited Thp-1 cell line proliferation by 54% at 100 μg/mL (Kulkarni et al., 2014). Similarly, another study was also conducted for antifungal study against different candida species for instance, C. krusei, C. albicans, C. glabrata, C. guilliermondii, C. parasilopsis. To delve into this study, Zuccagnia punctata Cav was taken and prepared it’s extract where 2ʹ,4ʹ-dihydroxy-3ʹ-methoxychalcone and 2ʹ,4ʹ-dihydroxychalcone have been isolated. These isolated chalcones were evaluated to prevent the growth of the above candida species. The MIC to eradicate 50% of the candida species population was 400 μg/mL (Table 2) (Gabriela et al., 2014).
Naturally occurring chalcones exhibit potent antibacterial, antifungal, antiviral, and antiprotozoal properties. Specific chalcones like pinocembrin chalcone, licochalcones, xanthohumol, and isoliquiritigenin have shown effectiveness against various bacteria, fungi, viruses, and protozoa. According to recent SAR studies, chalcone’s lipophilicity is influenced by the prenyl moiety on the A-ring and phenolic -OH groups, which is the reason for its antibacterial activity (Wang et al., 2023). Furthermore, the -OH group in the A ring’s 2-position and the prenyl group in the A ring’s 3-position boost the activity. In the 5′-position of the B ring, the propyl, prenyl, and hexyl groups are advantageous. Removing the prenyl moiety from the 5′-position of the B ring and methylating the -OH in the 4-position of the A ring both reduce activity. Prenyl moieties at the 3′and 2-positions of B rings and glycosyl in the A ring decrease the activities; if the prenyl group at the chalcones A-ring is further cyclized or oxygenated, the action will drop dramatically (Cui et al., 2015). They work by inhibiting microbial growth, disrupting cellular functions, and enhancing the efficacy of existing antimicrobial drugs. Their broad-spectrum activity highlights their potential as powerful natural antibiotics and therapeutic agents.
4.2 Tumor cell toxicity and chemopreventive attributes of naturally occurring chalcones
Cancer is the 2nd leading cause of mortality worldwide and is accountable for nearly one in every four premature deaths (22.8%) among those caused by noncommunicable diseases (NCDs: Such types of diseases that cannot be transmitted from one person to another) (Kocarnik et al., 2022). Globally, cancer claimed 9.7 million deaths in 2022, with an estimated 20 million new cases having been diagnosed (Siegel et al., 2022; Adhikari et al., 2025). Numerous factors might lead to cancer, and one of the most significant ones is chronic inflammation, which is related to the progression of cancer metastasis through dysregulating several cell signalling pathways (Nigam et al., 2023; Nath et al., 2025). Although platinum-based medication is one of the most advanced and widely used medications in clinical settings for treating a variety of human cancer types, it has significant adverse effects that limit its therapeutic usefulness (Nath et al., 2022; Adhikari et al., 2024a). As a result, drug resistance is becoming more and more widespread (Adhikari et al., 2019; Bhattacharjee et al., 2022; Das et al., 2023a; Nath et al., 2024). Plant-based drug development also gave rise to a stage for harmless anti-tumor medications by fully understanding the synergistic relationship between several anti-tumor botanical drugs or metabolites (Kaddah et al., 2021; Asma et al., 2022). The anti-cancer activities of more than 3,000 plant-based natural metabolites have been found. Among them, chalcone derivatives have demonstrated more cytotoxicity against various cancer cells than normal cells in both in vitro and in vivo studies, showing promising potential for anti-cancer therapeutics development (Ouyang et al., 2021). Furthermore, according to epidemiological research, eating a diet high in chalcones may lower your chance of developing malignancies in the breast, colon, lung, prostate, and pancreas (Prakash et al., 2013).
Tumor cytotoxicity and chemoprevention are among the enjoyable pharmacological activities of chalcones. Chemoprevention means preventing cancer from developing or delaying it with the use of various substances that impede cancer-initiating events (Benetou et al., 2015; Das et al., 2023b; Adhikari et al., 2024b; Bhattacharjee et al., 2024). It was reported that chalcones show inhibitory properties at micromolar concentrations by showing antimitotic activity; they arrest the cell cycle progression, inhibit transcription factors, induce mitochondrial uncoupling, and cause cellular apoptosis (Sharma et al., 2015). Chalcone treatment often leads to apoptosis of tumor cells via DNA disruption pathway characterized by nuclear condensation, DNA fragmentation, hypodiploid state, and upregulation of retinoblastoma (Rb) protein in tumor cells (Ramaiah et al., 2011). Several chalcones, such as Isobavachalcone (Figure 5), butein, licochalcone A, and xanthohumol, have been reported to enhance apoptosis in tumor cells by recruiting tumor necrosis factor-related apoptosis-inducing ligand (TRAIL: protein that may associate with certain different molecules in some cancer cells and responsible for inducing the apoptosis) (Figure 5) (Szliszka et al., 2009). Chalcones also inhibit histone deacetylase enzymes (HDACs: One type of evolutionarily conserved enzyme that aids in removing the acetyl groups from histones), blocking the deacetylation of histones in chromatin, causing changes in gene expression, resulting in cell cycle arrest, differentiation, and apoptosis of tumor cells (Kahyo et al., 2008; ORLIKOVA et al., 2012). Chalcones also hinder the initiation of nuclear factor kappa B (NF-κB: transcription factor that regulates the variety of cellular functions associated with promoter and enhancer regions of genes) as HDACs control the expression of the transcription factor NF-κB (ORLIKOVA et al., 2012).
Chalcones have been reported to hinder angiogenesis and cancer metastasis by controlling multiple signaling pathways. Natural chalcones originated from regulating the expression of many angiogenic factors, including epidermal growth factor receptor (EGFR: transmembrane protein of epidermal growth factor family), matrix metalloproteinases (MMPs: calcium-dependent zinc-containing endopeptidases that remodel the extracellular matrix proteins), vascular endothelial growth factor (VEGF), and also inhibit many numbers of signaling paths, for example, extracellular signal-regulated kinase (ERK)-1/2, NF-κB, and phosphoinositide-3-kinase–protein kinase B (P13-K/Akt: Cell signaling proteins that is responsible to enhance the growth of cancer cells) (MOJZIS et al., 2008). Isoliquiritigenin is an important chalcone derived from licorice root with promising anti-cancer action towards several malignant cells (Li et al., 2009; Bruyère et al., 2011; Wang et al., 2021). Isoliquiritigenin prevents migration and invasion in various tumor cells and demonstrates strong anti-cancer efficacy via several pathways, including apoptosis induction, the reduction of proliferation, and/or autophagy. Another study revealed that isoliquiritigenin, extracted from the Glycyrrhiza glabra showed the inhibitory activity of human acute promyelocytic leukemia cell line (HL-60) proliferation as well as decreased ROS production with induction of monocytic differentiation in leukemia cells. The reported effective concertation of this metabolite on HL-60 cells is 10 μg/mL (Table 3) (Li et al., 2009). In human U373glioblastoma cells, isoliquiritigenin exhibited cytostatic activity because it could overcome the cancer cells’ innate resistance to pro-apoptotic stimuli (Table 3) (Bruyère et al., 2011). Treatment with isoliquiritigenin cause apoptosis induction in cancer cells by preventing their proliferation and reducing inflammation. By reducing Psi(m) that causes apoptosis and inhibiting proliferation via the ERK/p38MAPK pathway, Zhang et al. reported that isoliquiritigenin (IC50 = 87.0 µM) repressed the C4-2, LNCaP prostate melanoma cells (Table 3) (Zhang et al., 2010).
TABLE 3
| Name and structure of chalcone | Source | Smiles | Activity | Drug concentration | Cell-line/Animal model of cancer | Mechanism of action | References |
|---|---|---|---|---|---|---|---|
| Isoliquiritigenin | Glycyrrhiza glabra L. (Family: Fabaceae) | OC(C=C1O) = CC = C1C(/C=C/C2 = CC = C(O)C=C2) = O | Inhibit the cell proliferation and decrease production of intracellular ROS. Also encourage the monocytic differentiation in HL-60 leukemia cells |
10 μg/mL | HL-60 (Cell line of Human acute promyelocytic leukemia) | Not known | Li et al. (2009) |
| Isoliquiritigenin | Synthesized, however the parent compound was from Calotropis procera (Aiton) W.T. Aiton (Family: Asclepiadaceae) | OC(C=C1O) = CC = C1C(/C=C/C2 = CC = C(O)C=C2) = O | Cytostatic effect of isoliquiritigenin delays the growth of U373glioblastoma | IC50 = 68 µM | U373 (Humanglioblastoma cell line) | Not known | Bruyère et al. (2011) |
| Isoliquiritigenin | Glycyrrhiza glabra L. (Family: Fabaceae) | OC(C=C1O) = CC = C1C(/C=C/C2 = CC = C(O)C=C2) = O | Prevent the proliferation of prostate tumor cells Effectively reduces ROS production |
10–100 μmol/L | C4-2 and LNCaP | Activation of pathways including adenosine monophosphate (AMP)-activated protein kinase (AMPK) and ERK cascades | Zhang et al. (2010) |
| Isoliquiritigenin | Glycyrrhiza uralensis Fisch. ex DC. (Family: Fabaceae) | OC(C=C1O) = CC = C1C(/C=C/C2 = CC = C(O)C=C2) = O | Downregulate proliferation of human umbilical vein endothelial induced by VEGF. | 5–20 µM | MDA-MB-231 and MCF-7 cells | It attenuated VEGF expression by inducing HIF-1a in breast cancer cells | Wang et al. (2013) |
| Xanthohumol | Humulus lupulus L. (Family: Cannabaceae) | O=C (/C=C/C1 = C(O)C=C(O)C=C1)C2 = C(OC)C=C(O)C(C/C=C(C)\C) = C2O | Anti-tumor activities with attenuation of colony formation, induced apoptosis, and reducing cell viability | 20 μM | A549, H520, and H358/old athymic nude mice | Dephosphorylation of forkhead box class O 3a (FOXO3a) and p53 upregulated modulator of apoptosis (PUMA) genes inhibits the Akt activity | Li et al. (2022b) |
| Xanthohumol | Humulus lupulus L. (Family: Cannabaceae) | O=C (/C=C/C1 = C(O)C=C(O)C=C1)C2 = C(OC)C=C(O)C(C/C=C(C)\C) = C2O | It induces apoptosis by increasing the DNA-damage response | 0.1–85 µM | SW620, SW480, and HT29 | Activation ATM signaling pathway | Scagliarini et al. (2020) |
| Licochalcone A | Glycyrrhiza inflata Batalin (Family: Fabaceae) | OC1 = C(C(C) (C)C=C)C=C (/C=C/C(C2 = CC = C(O)C=C2) = O)C(OC) = C1 | Antioxidant and anti-cancer activity | 58.79 ± 0.05 μg/mL (with PBS) 46.29 ± 0.05 μg/mL (without PBS) |
L-02 and HepG2 | Attenuation of p38/JNK/ERK signaling path and initiation of apoptotic cell death | Chen et al. (2017) |
| Licochalcone A | Glycyrrhiza uralensis Fisch. ex DC. (Family: Fabaceae) | OC1 = C(C(C) (C)C=C)C=C (/C=C/C(C2 = CC = C(O)C=C2) = O)C(OC) = C1 | Reduction of the proliferation of lung melanoma cells and induction of apoptosis | 20 µM | A549 and H460 | Suppress the expression of XIAP, Survivin, c-FLIPL, c-IAP1, c-IAP2, and RIP1 genes and attenuates the stability of Survivin, XIAP, RIP1 Suppression of ERK signaling proteins Downregulated activities of JNK pathway |
Luo et al. (2021) |
| Licochalcone A |
Glycyrrhiza uralensis
Fisch. ex DC. (Family: Fabaceae) |
OC1 = C(C(C) (C)C=C)C=C (/C=C/C(C2 = CC = C(O)C=C2) = O)C(OC) = C1 | Exerted HIF-1 repressive action in hypoxic tumor cells | IC50 = 10.6 and 13.7 μM | HCT116, H1299, and H322 | Reduction of hypoxia-induced HIF-1α accumulation It reduces the mitochondrial respiration-facilitated ATP production rate |
Park et al. (2021) |
| Licochalcone A | Glycyrrhiza glabra L (Family: Fabaceae) | OC1 = C(C(C) (C)C=C)C=C (/C=C/C(C2 = CC = C(O)C=C2) = O)C(OC) = C1 | Inhibition of PD-L1 expression | IC50 = 54 μM | A549, HeLa and Hep3B/BALB/c male nude mice | Inhibition of the interaction between p65 and Ras blocked the expression of PD-L1 Enhanced the activity of cytotoxic T cells to combat against the cancer cells |
Liu et al. (2021) |
| Licochalcone A | OC1 = C(C(C) (C)C=C)C=C (/C=C/C(C2 = CC = C(O)C=C2) = O)C(OC) = C1 | Anti-osteosarcoma activity | IC50 = 10.4 µM (MG63 cells) | MG63 cells | Induce cell cycle arrest at G2-M phase, and trigger the apoptosis | Rossi et al. (2022) | |
| Licochalcone A | OC1 = C(C(C) (C)C=C)C=C (/C=C/C(C2 = CC = C(O)C=C2) = O)C(OC) = C1 | Anti-osteosarcoma activity | IC50 between 5 and 20 µM (143B) | 143B cells MG63 cells |
Activate anti-cancer activity by inducing apoptosis and autophagy | Rossi et al. (2024) | |

Flavokawain B |
Alpinia pricei Hayata (Family: Zingiberaceae) | O=C(C1 = C(O)C=C(OC)C=C1OC)/C=C/C2 = CC = CC = C2 | Flavokawain B caused apoptosis induction in melanoma cells, accumulation of cells in G2/M stage and autophagy | Significant activity at 25 and 50 μM concentration | HCT116 | ROS production and GADD153 upregulation causes activation of mitochondrial apoptosis | Kuo et al. (2010) |

Cardamonin |
Artemisia absinthium L (Family: Asteraceae) | OC1 = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = CC(O) = C1 | Anti-cancer activity | A375 (IC50 = 2.43 μM) NHEM (IC50 = 12.87 μM) |
A375, NHEM, and NHDF cell lines | The dose-dependent enhanced caspase-3 activities and PARP cleavage Induce apoptosis in tumor cells |
Berning et al. (2019) |
| 2′,4'-dihydroxy-6′-methoxy-3′,5'-dimethylchalcone (DMC) | Cleistocalyx operculatus (Roxb.) Merr. & L.M.Perry (Family: Myrtaceae) | OC1 = C(C)C(O) = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = C1C | Cytotoxicity and anti-proliferation activity | IC50 = 14.2 ± 0.45 μM EC50 = 3.3 ± 0.14 μM |
K562 cell line | Suppress the Bcl-2 protein’s expression Not able to influence the Bax protein’s expression Lower ratio of Bcl-2/Bax and apoptosis induced |
Ye et al. (2005) |

2′,4'-dihydroxy-6′-methoxy-3′,5'-dimethylchalcone (DMC) |
Leaves of Syzygium samarangense (Blume) Merr. and L.M.Perry. (Family: Myrtaceae) | OC1 = C(C)C(O) = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = C1C | Induction of cell proliferation, cell-cycle distribution, and apoptosis | 40 μM | HCT116 and LOVO | Activated the cell cycle arrest at the G2/M phase | Ko et al. (2011) |
| 2′,4'-dihydroxy-6′-methoxy-3′,5'-dimethylchalcone (DMC) |
Cleistocalyx operculatus (Roxb.) Merr. and L.M.Perry (Family: Myrtaceae) |
OC1 = C(C)C(O) = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = C1C | Anti-cancer activity | IC50 = 10.5 ± 0.8 (PANC-1) and 12.2 ± 0.9 (MIA PACA2) µM | PANC-1 and MIA PACA2 | Induced caspase-3 activation leads to apoptosis of PANC-1 cells Triggered degradation of caspase-3 as well as proteolytic initiation of caspase-3 and -9 |
Tuan et al. (2019) |
| 2′,4'-dihydroxy-6′-methoxy-3′,5'-dimethylchalcone (DMC) | Syzygium nervosum (DC.) Kosterm (Family: Myrtaceae) | OC1 = C(C)C(O) = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = C1C | Anti-cancer activities towards cervical cancer cells | IC50 = 15.76 ± 1.49 (C-33A), 10.05 ± 0.22 (HeLa), and 18.31 ± 3.10 (SiHa) µM | C-33A, HeLa, and SiHa | DNA disruption and cell cycle arrest in the G0/G1 phase by DMC treatment | Utama et al. (2022) |
| 2′,4'-dihydroxy-6′-methoxy-3′,5'-dimethylchalcone (DMC) |
Cleistocalyx operculatus (Roxb.) Merr. and L.M.Perry (Family: Myrtaceae) |
OC1 = C(C)C(O) = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = C1C | Potent cytotoxic effect against multi-drug resistant BEL-7402/5- FU cells | IC50 = 47.24 ± 0.46 μM (BEL-7402) and 44.23 ± 3.50 μM (BEL-7402/5-FU) | BEL-7402 and BEL-7402/5-FU (multi-drug resistance cell line) | Induced apoptosis leads to the enhancement of ROS generation Cell cycle arrest in the G1 stage Enhanced p53 gene’s expression with suppression of NF-κB signaling cascades |
Ji et al. (2019) |
| 2′,4'-dihydroxy-6′-methoxy-3′,5'-dimethylchalcone (DMC) |
Cleistocalyx nervosum var. paniala (Roxb.) J.Parn. and Chantaranothai (Family: Myrtaceae) |
OC1 = C(C)C(O) = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = C1C | Anti-carcinogenic enzyme-inducing activity | IC50 = 15.76 µM (MRC5), and 15.10 ± 2.51 (SW620) µM | A549, HepG2, SW620, and MRC5/Male Wistar rats | Upregulation of detoxifying enzyme in rat livers | Vachiraarunwong et al. (2023) |
| 2′,4'-dihydroxy-6′-methoxy-3′,5'-dimethylchalcone (DMC) |
Cleistocalyx operculatus (Roxb.) Merr. and L.M.Perry (Family: Myrtaceae) |
OC1 = C(C)C(O) = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = C1C | Anti-cancer activities towards triple-negative breast cancer | IC50 = 34.95 ± 1.17 μM | MDA-MB-231, MCF 10A, and BT549 cells | Encouraged effective cell cycle arrest at G2/M stage Impair the microtubule polymerization through binding to β-tubulin protein Upregulation of pro-apoptotic proteins Bcl-2 related X protein (Bax) and caspase 3 |
Yu et al. (2024) |

2′-hydroxy-2,3,4′,6′-tetramethoxychalcone (HTMC) |
Caesalpinia pulcherrima (L.) Sw (Family: Fabaceae) |
O=C (/C=C/C1 = C(OC)C(OC) = CC = C1)C2 = C(OC)C=C(OC)C=C2O | Arrest cell cycle in G1 phase. Suppression of A549 cell growth in vitro condition along with A549 cells facilitated tumor in Balb/c mice Inhibit phosphorylation of cell cycle regulatory protein cdc2 and Rb and cause accretion of tumor suppresser genes p53 and p21 |
12.5 µM in-vitro 1 mg/kg body weight of mice |
A549 cell line Subcutaneously injected A549 cells mediated tumor in Balb/c mice |
Suppression of phosphorylation of cell cycle regulatory protein cdc2/CDK1 and Rb | Rao et al. (2010) |
| 2′,4′-dihydroxy-3′,5′-dimethyl-6′-methoxychalcone |
Syzygium samarangense (Blume) Merr. and L.M.Perry (Family: Myrtaceae) |
OC1 = C(C)C(O) = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = C1C | Displayed potent antioxidant and cytotoxic activity | IC50 = 10 µM | SW-480 | Not reported | Simirgiotis et al. (2008) |

Stercurensin |
Syzygium samarangense (Blume) Merr. and L.M.Perry (Family: Myrtaceae) |
OC1 = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = C(O)C=C1C | Displayed potent antioxidant and cytotoxic activity | IC50 = 35 µM | SW-480 | Not known | Simirgiotis et al. (2008) |
| Cardamonin |
Syzygium samarangense (Blume) Merr. and L.M.Perry (Family: Myrtaceae) |
OC1 = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = CC(O) = C1 | Displayed potent antioxidant and cytotoxic activity | IC50 = 35 µM | SW-480 | Not known | Simirgiotis et al. (2008) |

Hesperidin methyl chalcone |
Semi synthetic | OC1 = C(C(/C=C/C2 = CC(O) = C(OC)C=C2) = O)C(OC) = CC(O [C@@H]3O [C@H](CO [C@@H]4 [C@@H](O)[C@@H](O)[C@H](O)[C@@H](C)O4)[C@@H](O)[C@H](O)[C@H]3O) = C1 | Inhibit the cell viability of A549 melanoma cells | IC50 = 51.12 µM | Ehrlich ascites carcinoma murine model | Not Reported | (M.D. Rizvi et al., 2023) |

2′,3,4-trihydroxy-4′,6′-dimethoxychalcone (Chalcotatina) |
Chromolaena tacotana (Klatt) R.M.King and H.Rob. (Family: Asteraceae) | O=C (/C=C/C1 = CC = C(O)C(O) = C1)C2 = C(O)C=C(OC)C=C2OC | Anti-proliferative, autophagic, and apoptotic activity | IC50 = 42.8 µM | Breast cancer cells (MDA-MB-231) | Constantly interacted with anti-apoptotic proteins Bcl-2 | Mendez-Callejas et al. (2023) |

2′,4-dihydroxy-4′,6′-dimethoxy Chalcone |
Chromolaena tacotana (Klatt) R.M.King and H.Rob. (Family: Asteraceae) | O=C (/C=C/C1 = CC = C(O)C=C1)C2 = C(O)C=C(OC)C=C2OC | Anti-breast cancer activity | IC50 = 52.5 (MCF-7) µM and 66.4 (MDA-MB-231) µM | MCF-7 and MDA-MB-231 cells | Induction of cell cycle arrest in the G0/G1 stage Activation of autophagic protein microtubule-related protein 1A/1 B-light chain 3-II (LC3-II) |
Mendez-Callejas et al. (2024) |
Plant source, doses, and anti-cancer roles of different natural chalcones.
Isoliquiritigenin inhibits VEGF-induced proliferation of human umbilical vein endothelial cells (HUVECs) and also suppresses the sprouting of new blood vessels from VEGF-treated aortic rings in ex vivo studies. In addition, the administration of isoliquiritigenin in a dose-dependent manner in the mice with MDA-MB-231 xenograft tumor was able to diminish the growth of the tumor from day 16, with a 50%–65% reduction ratio than the vehicle groups. It is found to promote HIF-1α (Hypoxia-inducible factor-1α) to inhibit the expression of VEGF in breast cancer cells substantially and also interacted with VEGF receptor-2 (VEGFR-2) to block its kinase action (Table 3) (Wang et al., 2013).
Research has demonstrated the powerful antiaging, diabetic, inflammatory, antimicrobial, and cancer-preventing effects of xanthohumol, a prenylated chalcone in hop (Humulus lupulus L.). Growing data in recent years has indicated that xanthohumol has potent anti-cancer activities for several cancers, including glioblastoma, pancreatic cancer, hepatocellular carcinoma (HCC), thyroid cancer, cervical cancer, glioma, leukemia, breast cancer, cholangiocarcinoma (CCA), thyroid cancer, and ovarian cancer (Vesaghhamedani et al., 2022). Xanthohumol inhibits the development of cancer cells by inhibiting DNA synthesis, cell cycle arrest, and induction of apoptosis inhibition of aromatase activity (Miranda et al., 1999; Monteiro et al., 2007; Jiang et al., 2018). Li et al. and colleagues examined xanthohumol’s anti-cancer potential against human non-small cell lung cancer cells in both an in vitro and an in vivo model (Li et al., 2022). When xanthohumol was administered to A549, H520, and H358 cells in a dose-dependent method, the cells’ viability was considerably diminished. When exposed to the highest dose of 20 μM for 72 h, more than 85% of the cell viability was reduced, nearly preventing the cell growth. The number of colonies was significantly reduced after exposure to the highest dose of 20 μM for 72 h, with an inhibition rate on colony development of 95%. In the A549 (tumor volume of 221 mm3 compared to 632 mm3 in the control group) and H358 (tumor volume of 315 mm3 compared to 746 mm3 in the control group) xenograft models, xanthohumol at a dose of 10 mg/kg demonstrated excellent anti-tumor action, as the development of tumor was noticeably reduced (Scagliarini et al., 2020) (Table 3). In non-small cell lung cancer cells, xanthohumol triggered mitochondrial apoptosis by upregulating the expression of the p53-upregulated modulator of apoptosis. Anti-cancer attributes of xanthohumol towards colon cancer cells have also been evaluated (Scagliarini et al., 2020). In all three examined cell lines (SW480, SW620, and HT29), xanthohumol caused a potent and time-dependent reduction of cancer cell development starting at 5 µM. However, at concentrations greater than the previously established IC50, such as 30 μM, xanthohumol seemed toxic and inhibited many cells. Among the examined cell lines, xanthohumol was the most active towards SW620 cells (IC50 = 7 ± 1.38 μM after 72 h of treatment) (Table 3) (Scagliarini et al., 2020).
Interestingly, the DRI values of the anti-cancer drug 7-ethyl-10-hydroxycamptothecin indicated that synergistic interactions of xanthohumol with 7-ethyl-10-hydroxycamptothecin promoted the mortality of SW480 cells while potentially lowering the concentration of 7-ethyl-10-hydroxycamptothecin. The mechanistic study revealed that by triggering the ataxia telangiectasia mutated (ATM) pathway, xanthohumol exhibited its anti-cancer potential. Therefore, colorectal carcinoma (CRC) cells may become more sensitive to the anti-cancer drug 7-ethyl-10-hydroxycamptothecin, as a result of xanthohumol’s capacity to repair DNA disruption in melanoma cells.
Licochalcone A isolated from Glycyrrhiza glabra arrests cell cycle (Figure 5) in the G2/M stage, and causes apoptosis induction in several tumor cells (Deng et al., 2023). Treatment with licochalcone A inhibits phosphorylation of Rb, declines expression of transcription factor E2F, simultaneously reduces cyclin D1, and downregulates cyclin-dependent kinases (CDKs: cell cycle-regulating checkpoint proteins) 4 and 6, etc., (Fu et al., 2004). Chen and his group reported that HepG2 cells were repressed by licochalcone A in a dose-dependent way (Table 3) (Chen et al., 2017). This suppression was achieved by stopping the proliferation of cells and triggering apoptosis. In HepG2 cells, licochalcone A directly reduced MAPK signaling pathways, preventing proliferation and triggering apoptosis.
A study examined the anti-neoplastic activity of licochalcone A towards non-small cell lung carcinoma (NSCLC) cells (A549, H460, SPC-A1, H23, and H1299) (Table 3) (Luo et al., 2021). Using flow cytometry, it was confirmed that licochalcone A-induced apoptosis in A549 and H460 cells. In A549 and H460 cells, licochalcone A distinctly and time-dependently stimulated p38 and ERK. In addition, licochalcone A reduced the autophagy that was triggered by licochalcone A and inhibited jun N-terminal kinase (JNK: a cell signalling kinase protein that regulates the regulation of cellular senescence) activity. It also repressed the expression of cellular inhibitor of apoptosis protein 1 (c-IAP1), c-IAP2, X-linked inhibitor of apoptosis protein (XIAP), Survivin, cellular FLICE (FADD-like Il-1β-converting enzyme)-inhibitory protein (c-FLIPL), and receptor-interacting protein-1 (RIP1).
Mitochondrial malfunction is closely allied with the initiation of the mitochondrial apoptosis pathway. Park and colleagues reported that licochalcone A is the most prevailing bioactive metabolite in G. uralensis, which reduced the cancer cells’ growth and the activation of HIF-1α mediated by hypoxia (Park et al., 2021). Among the tested five major constituents of Glycyrrhiza uralensis, licochalcone A most effectively repressed HCT116 cell viability, having a GI50 value of 10.5 μM (Table 3). Moreover, licochalcone A demonstrated decreased viability of cells linked to tumor angiogenesis, such as smooth muscle cells (IC50 = 13.7 μM) and vascular endothelial cells (IC50 = 10.6 μM). Licochalcone A (2.5–25 μM) decreased ATP production and triggered mitochondrial disruption in H1299 and H322 lung melanoma cells by suppressing hypoxia-induced HIF-1α accretion and the expression of target genes glucose transporter 1 (GLUT1) and phosphoinositide-dependent kinase 1 (PDK1), leading to the instigation of the mitochondrial apoptosis and cancer cell apoptosis.
Liu and associates studied the anti-cancer activity both in vitro and in vivo of licochalcone A (Liu et al., 2021). In a tumor and T cell coculture model, licochalcone A inhibited the expression of programmed cell death ligand 1 (PD-L1), restoring T lymphocyte function. Flow cytometry result revealed that as the concentration of licochalcone A increased, the percentage of programmed cell death ligand 1 positive HCT116 cells decreased from 20.3% to 9.9%. Importantly, mice bearing HCT116 xenograft tumors were given licochalcone A, which suppressed tumor growth without causing cytotoxicity (Table 3). Additionally, it was also observed that licochalcone A inhibited the Ras/Raf/MEK and NF-κB signaling pathway, which is responsible for the proliferation of tumor cells.
Recently Rossi et al. investigated the anti-cancer efficacy of licochalcone A and several chalcone derivatives against multicellular tumor spheroids from MG63 and 143B osteosarcoma cell lines. In this study, it was also observed that licochalcone A able to arrest the cell cycle at G2-M phase in osteosarcoma cancer cells. Further, it induces the apoptosis to eradicate proliferation (Table 3) (Rossi et al., 2022). Remarkably, most of the chalcones had IC50 values between 5 and 20 µM against 143B osteosarcoma cell lines, indicating that they are all efficacious. Against the MG63 cells, licochalcone A at 10 µM inhibited the cell number to ∼40% compared to the control within 48 h. Furthermore, licochalcone A exhibited remarkable IC50 values of 10.4 µM against the MG63 cells (Table 3) (Rossi et al., 2024). Additionally, it was observed that after treating osteosarcoma cell lines with licochalcone A, it may function as an anti-proliferative agent by reducing cell invasion and activating apoptosis and autophagy.
Other potential chalcones with anti-cancer activity are isobavachalcone, xanthoangelol F, flavokawain B, cardamonin etc., (Akihisa et al., 2003; Kuo et al., 2010; Berning et al., 2019). Flavokawain B is a trans-chalcone substituted by -OH moiety at positions 2′and -OMe moieties at positions 4′and 6'. Flavokawain B, extracted from the Alpinia pricei Hayata displayed significant activity at concentrations of 25 and 50 μM to induce apoptosis and arrest the human colon cancer cell, HCT116, from G2 to M stage (Table 3). Moreover, this isolated metabolite is also capable of ROS generation with upregulation of the growth arrest and DNA damage-inducible gene 153 (GADD153). Therefore, Flavokawain B activates apoptosis based on mitochondria (Kuo et al., 2010). The chalcone cardamonin (2′,4′-dihydroxy-6′-methoxychalcone) was initially extracted from the flowers of Artemisia absinthium and it is frequently isolated from many plants in the Zingiberaceae family (Hatziieremia et al., 2006). Cardamonin can be isolated from plant sources using the micellar electrokinetic chromatography (MEKC) method (Liu et al., 2007). Kamiński and his group first reported the solid-state structure of cardamonin (Budziak et al., 2020). Two symmetry-independent molecules in the cardamonin crystal lattice are connected by hydrogen bonding and π···π stacking contacts, ensuing in two distinct conformations of the cardamonin molecules in the crystal structure. Furthermore, unlike in EtOH, where cardamonin mainly exists as monomers, cardamonin occurs in a dimeric state in water solutions. Cardamonin has been the topic of several analyses demonstrating its anti-cancer properties attributable to its capability to cause apoptosis in cancer cells. Cardamonin unveiled anti-cancer properties in different melanoma cell lines like lung cancer cells (A549, H460), ovarian cancer cells (SKOV3, A2780), gastric cancer cells (AGS, MGC-803, BGC-823), colon cancer cells (HCT-116, SW480, DLD1, LS174T), breast cancer cells (BT-549, SUM190, MCF7, COMA-1), prostate cancer cells (PC-3), colorectal cancer cells (HCT-15, HCT116, SW480, SW620), as well as nasopharyngeal cancer cells (CNE-1, CNE-2, HONE-1, SUNE-2), and leukemia (WEHI-3) etc., (Nawaz et al., 2020). Cardamonin suppresses the NF-κB pathway, which is known to generate reactive oxygen species (ROS). This affects cell development and triggers cell death in melanoma cells (Li et al., 2017). Additionally, it prevents the growth of cells by downregulating phosphorylated mammalian target of rapamycin (p-mTOR), protein kinase B (Akt/PKB), p70 Ribosomal Protein S6 Kinase (P70S6K), phosphatidylinositol 3-kinase (p-PI3K), and B cell lymphoma −2 (Bcl-2) (Shi et al., 2018). Berning and colleagues explored the anti-proliferative effect of cardamonin against A375 cancer cell lines and normal human epidermal melanocytes (NHEM) along with normal human dermal fibroblasts (NHDF) cell lines (Table 3) (Berning et al., 2019). Cardamonin had the most cytotoxic against A375 tumor cells (IC50 = 2.43 μM) and had less harmful effects against normal NHEM cell lines (IC50 = 12.87 μM). After 24 h of treatment, only around 5% of the tumor cells were viable, indicating that 20 µM of cardamonin had the maximum cytotoxic effect. The dose-dependent upsurge in caspase-3 actions and poly (ADP-ribose) polymerase (PARP) cleavage in the A375 cancer cells confirmed the induction of apoptosis, which was further established by the time-dependent rise in membrane blebbing following cardamonin treatment (Berning et al., 2019).
In recent day’s chemical engineering of chalcones manipulating the structure at aryl rings, addition of heteroaryl scaffolds, and conjugation with other molecules of pharmacological importance enhance the anti-cancer properties of chalcones (Karthikeyan et al., 2014). 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone (DMC), a chalcone present in leaves of Syzygium samarangense, seeds of Syzygium nervosum and buds of Cleistocalyx operculatus, exhibits potent anti-cancer properties against leukemia, liver, colorectal, pancreatic, and breast cancers (Ye et al., 2005). Similarly, Yang and his colleagues investigated the anti-cancer properties of 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone in LOVO and HCT116 human colorectal cancer cells (Table 3) (Ko et al., 2011). DMC repressed the cells growth dependent on concentration and time. Compared to the vehicle control, DMC suppressed cell growth in HCT116 and LOVO cells by 40% and 37%, respectively, after a 24-h treatment at a dosage of 40 μM. The authors ascribed that DMC can trigger autophagy and reduce the growth of HCT116 and LOVO cells by delaying the G2/M stage of the cell cycle. Tran and colleagues extracted 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethyl chalcone from the Cleistocalyx operculatus buds and characterized by mass and NMR (1H and 13C) spectroscopy and inspected anti-cancer properties on pancreatic cancer cell lines (Tuan et al., 2019). In vitro analysis indicated that in concentration-dependent ways, DMC noticeably repressed the proliferation of PANC-1 (IC50 = 10.5 ± 0.8 μM) and MIA PACA2 (IC50 = 12.2 ± 0.9 μM) cells (Table 3). The authors attribute that by activating caspase-3, DMC caused PANC-1 to undergo apoptosis. DMC increased the level of bak protein, caused the proteolytic initiation of caspase-3 and -9, degraded the substrate proteins caspase-3, and reduced the expression of bcl-2 in PANC-1 cells. DMC was also extracted from the seeds of S. nervosum and was characterized by 1H-NMR, 13C-NMR spectra, and 2D-NMR experiments, including COSY, HSQC, and HMBC (Utama et al., 2022). DMC showed promising anti-cancer activities against HeLa (IC50 = 10.05 ± 0.22), C-33A (IC50 = 15.76 ± 1.49), and SiHa cells (IC50 = 18.31 ± 3.10 µM), while cisplatin showed an IC50 = 9.93 ± 0.16 µM against HeLa cells (Table 3). HeLa cells treated with DMC exhibited DNA disruption, reduced cell division, and instigated apoptosis. DMC’s anti-cancer potential against a multidrug-resistant HCC cell line was reported by Lu and colleagues (Ji et al., 2019). In comparison to the generally utilized anti-cancer drug 5-fluorouracil (5-FU) (IC50 = 69.96 ± 10.69 μM against BEL-7402 and IC50 = 5,662.82 ± 245.77 μM against BEL-7402/5-FU), DMC exhibited concentration-dependently growth inhibition of BEL-7402 (IC50 = 47.24 ± 0.46 μM) and BEL7402/5-FU cells (IC50 = 44.23 ± 3.50 μM), suggesting that the BEL-7402/5-FU cells were more sensitive towards DMC (Table 3). BEL-7402/5-FU (5-FU resistant cancer cell line) cells had a resistance index of 80.95 against 5-FU, indicating that while both cell lines were susceptible to DMC, BEL-7402/5-FU cells have been unaffected to 5-FU. The authors attribute that the mechanism of DMC towards the multidrug-resistant BEL-7402/5-FU hepatocellular cancer cells was found to be via upregulating the ROS production within the cells and the mitochondria-dependent apoptotic pathway. Furthermore, it was shown that DMC inhibited the advancement of the cell cycle during the G1 stage through lowering the expression levels of associated proteins, such as phospho-glycogen synthase kinase 3 beta (p-GSK3β), cyclin D1, and CDK4. Vachiraarunwong and co-workers accessed the anti-cancer activities against colorectal carcinoma of CH2Cl2 extract of DMC extracted from the seeds of Cleistocalyx nervosum (Vachiraarunwong et al., 2023). In vitro studies revealed that DMC-suppressed colorectal carcinoma SW620 cells (IC50 = 15.10 ± 2.51 μM) were comparable to 5-FU (IC50 = 16.70 ± 6.74 μM), but less cytotoxic than 5-FU against noncancerous MRC-5 cells having IC50 of DMC (15.76 µM) was threefold more compared to 5-FU (5.78 µM) (Table 3). By promoting the metabolization of xenobiotics and inhibiting cell growth, DMC showed a chemopreventive result in the initial phases of colorectal carcinogenesis. Recently, it was reported that DMC exhibited a potent concentration-dependent cytotoxic effect in MDA-MB-231 cells (IC50 = 34.95 ± 1.17 μM) compared to MCF10A, MCF-7, BT549, and MDA-MB-468 cells (Table 3) (Yu et al., 2024). DMC worked as an anti-tumor agent by causing G2/M arrest in MDA-MB-231 cells. It also triggered G2/M stage arrest by blocking microtubule polymerization by binding with β-tubulin. Furthermore, it increased the production of ROS by suppressing catalase activity, which in turn controlled the PI3K signaling pathway and jointly caused cell cycle arrest.
In addition to MRC-5, SV-40 transfected Beas2B, and WI-38 cells normal cell lines, 2′-hydroxy-2,3,4′,6′-tetramethoxychalcone (HTMC) derived from Caesalpinia pulcherrima was tested for its anti-cancer properties against A549, H1299, and H1355 cancer cells (Rao et al., 2010). According to in vitro research, 2′-hydroxy-2,3,4′,6′-tetramethoxychalcone selectively killed cancer-derived cells instead of normal cell lines. The sensitivity of HTMC to A549 (IC50 = 47 μM) was higher than that of H1299 (IC50 = 48 μM) and H1355 (IC50 = 76 μM) cells (Table 3). In line with its in vitro efficacy, HTMC demonstrated powerful anti-cancer properties in the A549 tumor model in Balb/c mice, resulting in a noteworthy 33% decrease in tumor volume and no drop in body weight, health issues, or behavioral abnormalities. The mechanistic study indicated that cell cycle arrest in the G1 stage by inhibiting phosphorylation of cell cycle regulatory protein cdc2/CDK1 and Rb leads to a reduction of A549 cell growth (Rao et al., 2010). Syzygium samarangense has been recognized as a source of pharmacologically active chalcones. Various C-methylated chalcones such as stercurensin, cardamonin, and 2′,4′-dihydroxy-3′,5′-dimethyl-6′-methoxychalcone extracted from Syzygium samarangense displayed potent antioxidant and cytotoxic activity in human colon cancer cell SW-480 (IC50 = 10, 35, and 35 μM, respectively) (Table 3) (Simirgiotis et al., 2008). The methylated byproduct of hesperidin extracted from citrus foods is hesperidin methyl chalcone, which has an open ring with several -CH3 group substitutions. The semi-synthetic hesperidin methyl chalcone is derived from hesperidin (hesperidin-7-rhamnoglucoside), which undergoes methylation in alkaline condition to produce hesperidin methyl chalcone. Methylation produces a more water-soluble molecule and thus increases its absorption and bioavailability. It has been investigated for use as an analgesic and anti-inflammatory in treating numerous disorders (Guazelli et al., 2021). Rizvi and colleagues explored the anti-cancer activities of hesperidin methyl chalcone in vitro and in vivo. With an IC50 of 51.12 µM, it exhibited strong anti-cancer efficacy in lung cancer cell lines similar to that of the anti-cancer drug hesperetin (IC50 = 49.12 µM) (Table 3). In the in vivo studies, it was found that the survival of Ehrlich ascites carcinoma (EAC)-bearing mice was noticeably extended by 15 days of treatment with hesperidin methyl chalcone than EAC-bearing untreated mice. Furthermore, in EAC-bearing mice, hesperidin methyl chalcone effectively exhibited a strong anti-cancer activity, as seen by a prominent reduction in tumor volume and weight (M.D. Rizvi et al., 2023). Flavonoids having antioxidant and anti-cancer attributes are found in Chromolaena tacotana (Klatt). Gina Mendez-Callejas and co-workers recently isolated and characterized a novel chalcone 2′,3,4-trihydroxy-4′,6′-dimethoxychalcone (chalcotatina) from the C. tacotana plant. This novel chalcone exhibits anti-proliferative properties in the MDA-MB-231 triple-negative breast cancer cell line, having an IC50 of 42.8 µM (Table 3). Chalcotanina demonstrated a remarkable selectivity for the triple-negative breast cancer (TNBC) cell line, as evidenced by selectivity index values of 6.9, 9.1, and 5.3 in comparison to MCF-12F, MRC-5, and BHK-21 cells, respectively. Chalcotanina triggers apoptosis by activating caspases 3/7 and induces autophagy by altering the mTOR protein’s structural shape. Additionally, it alters the potential of the mitochondrial membrane and downregulates Bcl-2 anti-apoptotic members, which activates the intrinsic pathway (Mendez-Callejas et al., 2023). The same research group isolated and characterized 2′,4-dihydroxy-4′,6′-dimethoxy-chalcone (DDC) from the C. tacotana plant. The structure of the extracted chalcone was characterized by NMR (1H NMR and 13C NMR) spectra and high-resolution electrospray ionization mass spectrometry (HR-ESI-MS). Promising anti-cancer properties were exhibited by DDC against breast cancer cell lines MCF-7 (IC50 = 52.5 µM) and MDA-MB-231 (IC50 = 66.4 µM) compared non-tumor MCF-12F cells (IC50 = 232.8 µM) (Table 3). Selectivity index (SI) results for MCF-7, and MDA-MB-231 were 4.4 and 3.5, respectively than positive controls, resveratrol and paclitaxel. These findings imply that DDC may target breast cancer cells more successfully while having less of an effect on healthy cells. In breast cancer cells, DDC causes cell cycle arrest in the G0/G1 stage, alters the mitochondrial outer membrane potential (∆ψm), and initiates the mitochondrial route of death (Mendez-Callejas et al., 2024).
The paragraphs summarize the anti-cancer properties of various chalcones, focusing on their capability to prevent tumor development by suppressing the Ras/Raf/MEK and NF-κB signalling pathways. The SAR investigations indicated that structural modifications of both aryl rings, substitution of heteroaryl scaffolds for aryl rings, and hybridization by conjugation with other pharmacologically potential motifs significantly increase the anti-cancer properties of chalcones (Karthikeyan et al., 2014).
Furthermore, methoxy substitutions on both A and B rings and their substitution pattern significantly impact the anti-cancer activity of chalcones (Mahapatra et al., 2015b). Licochalcone A inhibits PD-L1 expression and restores T lymphocyte function, slowing tumor growth without toxicity. Chalcones like cardamonin and DMC also showed strong anti-cancer activity across numerous cancer cells, including lung, colorectal, and breast cancers. Notably, HTMC selectively killed cancer cells over normal cells, showing higher sensitivity in A549 lung cancer cells. HTMC also demonstrated significant tumor reduction in mice without adverse effects. Other chalcones like cardamonin, hesperidin methyl chalcone, and chalcotatina displayed significant anti-cancer activity in several melanoma cells. These findings suggest the potential of naturally occurring chalcones in cancer treatment.
4.3 Antioxidant and anti-inflammatory properties of natural chalcones
Chalcones are extensively recognized due to their anti-inflammatory and antioxidant activities. Natural chalcones are phenolic in nature and include one or more phenolic -OH in their structure. This generally allows them to scavenge free radicals naturally, which can be beneficial when dealing with oxidative stress. Numerous studies conducted in this field have confirmed the relation between inflammation, oxidative stress, and carcinogenesis. Persistent oxidative stress has been shown to cause chronic inflammation, which in turn may act as a mediator for numerous chronic illnesses, such as cancer. Numerous transcription aspects, such as NF-κB, HIF-1α, β-catenin/Wingless and Int-1 (Wnt), nuclear factor erythroid 2-related factor 2 (Nrf2), and many more, are induced by oxidative stress. This can result in the expression of hundreds of different gene products, including chemokines, growth factors, cell cycle regulatory biomolecules, inflammatory cytokines, and anti-inflammatory biomolecules (Reuter et al., 2010). Such misshapen gene expression sometimes leads to the alteration of a normal cell into neoplastic cells with altered proliferation and survival characteristics.
Chalcones have been found to counter many cancer-initiating processes and prevent carcinogenesis. The chemopreventive capacity of chalcone compounds is linked to their strong antioxidant and anti-inflammatory activities in biological systems (Orlikova et al., 2011). Owing to their potent antioxidant activities, chalcone compounds can scavenge free radicals and check oxidative stress; therefore, they can modulate many biological processes like diabetes, aging, inflammation, ischemic injury, cancer, and neurodegenerative illnesses. Free radicals and ROS (a strong reactive oxygen species like diatomic oxygen (O2), and hydrogen peroxide that is responsible for generating oxidative stress by damaging the DNA, lipids, and proteins) are related in various phases of carcinogenesis (Liou and Storz, 2010). ROS is well known for its cancer-promoting activity. Chalcones’ ability to reduce ROS generation is therefore crucial in preventing carcinogenesis. Various chalcones isolated from plants like licochalcone B and D, broussochalcone A, xanthokeismins A, B, and C, xanthoangelol B, etc. eported to have strong superoxide anion and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging action (Haraguchi et al., 1998a; Cheng et al., 2001). Aoki et al. extracted and characterized three new C-geranylated chalcones, designated as xanthokeismins, from the Angelica keiskei stems (Aoki et al., 2008). The structures of the new C-geranylated chalcones were well characterized by FT-IR, NMR (1H, 13C NMR, 1H-1H COSY, DEPT, and HMQC) and mass (MALDITOFMS) spectroscopic methods. All the extracted chalcones showed significant superoxide scavenging property (IC50 = 0.51–1.1 µM) superior to the positive control resveratrol (IC50 = 5.3 μM). Among the newly isolated C-geranylated chalcones, xanthokeismin A had the most significant superoxide-scavenging property (IC50 = 0.51 ± 0.023 μM) (Table 4). Furthermore, chalcone compounds have been demonstrated to block prostaglandin E2 (PGE2: involved in several biological functions, mostly in neurological inflammatory diseases) and nitric oxide (NO) synthesis, thereby abrogating inflammatory stimuli and mitigating the impact of inflammation (Figure 5) (Nowakowska, 2007b).
TABLE 4
| Name and structure of chalcone | Plant source | Smiles | Activity | Dose of drug/metabolite | Targeted model | Mechanism of action | References |
|---|---|---|---|---|---|---|---|

Xanthokeismins A  Xanthokeismins B  Xanthokeismins C |
Angelica keiskei (Miq.) Koidz. (Family: Apiaceae) | O=C (/C=C/C1 = CC = C(O)C=C1)C2 = C(O)C(C/C=C(C)/C/C=C/C(C) (O)C) = C(O)C=C2 O=C (/C=C/C1 = CC = C(O)C=C1)C2 = C(O)C ([C@H](O)[C@H](O3)C(C) (O)C/C=C/C(C) (O)C) = C3C = C2 O=C (/C=C/C1 = CC = C(O)C=C1)C2 = C(O)C ([C@H](O)[C@H](O3)C(C) (O)CCC(O)C(C) = C) = C3C = C2 |
Antioxidant | IC50 = 0.51 ± 0.023, 0.69 ± 0.017, and 1.1 ± 0.12 µM respectively for A, B and C | In vitro model | Scavenges superoxide radical | Aoki et al. (2008) |

Xanthoangelol B |
Angelica keiskei (Miq.) Koidz. (Family: Apiaceae) | O=C (/C=C/C1 = CC = C(O)C=C1)C2 = C(O)C(C/C=C(C)/CCC(O)C(C) = C) = C(O)C=C2 | Antioxidant | IC50 = 0.92 ± 0.16 µM | In vitro model | Superoxide radical scavenging | Aoki et al. (2008) |

3″,3″-dimethylpyrano [3′,4′]2,4,2′-trihydroxychalcone |
Artocarpus communis. J.R.Forst. and G.Forst. (Family: Moraceae) | O=C (/C=C/C1 = C(O)C=C(O)C=C1)C2 = C(O)C3 = C(OC(C) (C)C=C3)C=C2 | Anti-inflammatory. Inhibits NO generation from LPS initiated RAW264.7 cells | IC50 = 18.8 µM | RAW264.7 cells | Inhibition of iNOS. | Han et al. (2006) |

Isobacachalcone |
Artocarpus communis
J.R.Forst. and G.Forst. (Family: Moraceae) |
OC1 = C(C(/C=C/C2 = CC = C(O)C=C2) = O)C=CC(O) = C1C/C=C(C)/C | Anti-inflammatory | IC50 = 6.4 µM | RAW264.7 cells | Inhibition of iNOS. | Han et al. (2006) |

Morachalcone A |
Artocarpus communis. J.R.Forst. and G.Forst. (Family: Moraceae) | O=C (/C=C/C1 = CC = C(O)C=C1O)C2 = C(O)C(C/C=C(C)\C) = C(O)C=C2 | Anti-inflammatory | IC50 = 16.4 µM | RAW264.7 cells | Inhibition of iNOS. | Han et al. (2006) |

Gemichalcones B |
Artocarpus communis. J.R.Forst. and G.Forst. (Family: Moraceae) | O=C (/C=C/C1 = CC = C(O)C=C1)C2 = C(O)C(C/C=C(C)\COC(/C=C/C3 = CC = C(O)C=C3) = O) = C(O)C=C2 | Anti-inflammatory | IC50 = 9.3 µM | RAW264.7 cells | Inhibition of iNOS. | Han et al. (2006) |
| Xanthohumol | Humulus lupulus L (Family: Cannabaceae) | O=C (/C=C/C1 = C(O)C=C(O)C=C1)C2 = C(OC)C=C(O)C(C/C=C(C)\C) = C2O | Anti-inflammatory | 0.5–5 μg/mL | BV2 cells | Upregulation of downstream HO-1 factors Induction of Nrf2-ARE signaling pathway |
Lee et al. (2011) |
| Cardamonin | Alpinia conchigera Griff. (Family: Zingiberaceae) | OC1 = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = CC(O) = C1 | Anti-inflammatory Activity |
50 mg/kg 30 µM |
C57BL/6 Mice RAW264.7 cells |
Suppression of Nuclear Factor-B signal cascade Suppresses the expression of TNF-α, NO and inducible NO synthase and COX-2 |
Lee et al. (2006) |

2′,3′-dihydroxy-4′,6′-dimethoxychalcone (DDC) |
Perilla frutescens var. Crispa (Thunb.) H.Deane (Family: Lamiaceae) | OC1 = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = CC(OC) = C1O | Antioxidant activity | Less than 30 μM | PC12 cells | Activated Nrf2-antioxidant response element (ARE) | Izumi et al. (2012) |
| Hesperidin methyl chalcone | Semi synthetic | OC1 = C(C(/C=C/C2 = CC(O) = C(OC)C=C2) = O)C(OC) = CC(O [C@@H]3O [C@H](CO [C@@H]4 [C@@H](O)[C@@H](O)[C@H](O)[C@@H](C)O4)[C@@H](O)[C@H](O)[C@H]3O) = C1 | TNF α, IL-1β, IL-6, and IL-33 suppression in colon cells | Not reported | Male Swiss, C57BL/6 mice | Inhibits the colon’s NF-κB pathway | Guazelli et al. (2021) |
| Hesperidin methyl chalcone | Semi synthetic | OC1 = C(C(/C=C/C2 = CC(O) = C(OC)C=C2) = O)C(OC) = CC(O [C@@H]3O [C@H](CO [C@@H]4 [C@@H](O)[C@@H](O)[C@H](O)[C@@H](C)O4)[C@@H](O)[C@H](O)[C@H]3O) = C1 | Analgesic, anti-inflammatory, and antioxidant properties | 10, 30, or 100 mg/kg | Swiss mice | Attenuation of ROS production Impair macrophage NF-κB activation |
Rasquel-Oliveira et al. (2020) |
| Hesperidin methyl chalcone | Semi synthetic | OC1 = C(C(/C=C/C2 = CC(O) = C(OC)C=C2) = O)C(OC) = CC(O [C@@H]3O [C@H](CO [C@@H]4 [C@@H](O)[C@@H](O)[C@H](O)[C@@H](C)O4)[C@@H](O)[C@H](O)[C@H]3O) = C1 | Activate the Nrf2 signaling pathway Antioxidant activity |
0.03–3 mg/kg | Swiss mice | Dose-dependent alteration of diclofenac by reducing urea and creatinine levels Reduce the IL-6, IFN-γ, and IL-33 expression Upregulation of IL-10 with reduction of kidney swelling, and urine NGAL. |
Bussmann et al. (2022) |

Trans-Chalcone |
Piper methysticum G.Forst. (Family: Piperaceae); Didymocarpus corchorifolius Wall. ex A.DC. (Family: Gesneriaceae); and Aniba riparia (Nees) Mez (Family: Lauraceae)) | O=C (/C=C/C1 = CC = CC = C1)C2 = CC = CC = C2 | Activity of TC in inflammatory cytokines induced joint stiffness and flexion pain | 30, 60, and 120 mg/kg | Sprague−Dawley rats | Inhibition of IL-17, IL-1β, and IL-6 mRNA expression | Jabbar et al. (2024) |

Flavokawain A |
Piper methysticum G.Forst (Family: Piperaceae) |
O=C(C1 = C(O)C=C(OC)C=C1OC)/C=C/C2 = CC = C(OC)C=C2 | Antioxidant molecular mechanisms | 2–30 μM (in vitro) 30 mg/kg (in vivo) |
RAW264.7 cells and BALB/c female mice | Diminished the expression of TNF-α, IL-1β, and IL-6 Upregulation of IL-10 Impaired the LPS-triggered ROS production Inhibition of NF-κB (p65) pathway leads to attenuation of iNOS, COX-2, TNF-α, and IL-1β expression Triggered Nrf2 nuclear translocation leads to activating antioxidant proteins like HO-1, NQO-1, and γ-GCLC. |
Yang et al. (2020) |
 2-hydroxy-3,4,6-trimethoxychalcone 2-hydroxy-3,4,6-trimethoxychalcone |
Toussaintia orientalis Verdc. (Family: Annonaceae) | O=C(C1 = CC = CC = C1)/C=C/C2 = C(OC)C=C(OC)C(OC) = C2O | Anti-inflammatory activity | 30 μg/mL (Inhibition of COX-2) | Cell-free in-vitro assay and male Sprague-Dawley rats | Potent anti-inflammatory property by preventing the COX-2 expression | Nyandoro SS et al. (2012) |

Pongamol |
Pongamia pinnata (L.) Pierre (Family: Fabaceae) | O/C(C1 = CC = CC = C1) = C\C(C2 = CC = C(OC = C3)C3 = C2OC) = O | Anti-inflammatory property | IC50 = 72.2 μM (Inhibition of soy lipoxygenase-1) IC50 = 12.2 μg/mL (antioxidant activity) |
Wistar rats | Reduced the edema by74% in the ear and 55% in the paw Inhibition of lipoxygenase-1 |
Rekha et al. (2020) |
Plant source, doses, and antioxidative and anti-inflammatory roles of different naturally occurring chalcones.
Prenylated chalcones are substituted with at least one lipophilic side chain of variable lengths, and numerous studies have demonstrated that, compared to parent chalcones, the prenyl motif has many benefits. Prenylation often increases affinity to the target site’s cell membrane. Prenylation also boosts lipophilicity, which improves target protein interaction and affinity for biological membranes. The prenylated chalcones are more extensively found in tissues and accumulate longer than their parent chalcones (Sychrová et al., 2022). Han et al. were the first to describe the extraction and characterization of a new prenylated chalcone known as 3″,3″-dimethylpyrano [ 3′,4′]2,4,2′-trihydroxychalcone from the Artocarpus communis. Several other chalcones, such as isobacachalcone, morachalcone A, gemichalcones B, and C were also extracted and characterized from Artocarpus communis for the first time. The newly isolated 3″,3″-dimethylpyrano [ 3′,4′]2,4,2′-trihydroxychalcone was characterized by NMR (1H, 13C NMR, 1H–1H COSY, 1H-13C HSQC, and 1H-13C HMBC) and mass (FABMS and HRFABMS) spectroscopic method. The isolated chalcones reduced NO generation from LPS-activated RAW264.7 mouse macrophage cells by reducing inducible nitric oxide synthase (iNOS: an inflammatory molecule that aids in synthesizing the NO and leads to inflammation). 3′′,3′′-dimethylpyrano [3′,4′]2,4,2′-trihydroxychalcone, morachalcone A, and gemichalcones B, displayed promising selectivity indices (3.1, 3.5, and 3.8, respectively), which show their ability to inhibit iNOS without causing cytotoxicity (Table 4) (Han et al., 2006). Some of the potential chalcones that cause inhibition of NO production are broussochalcone A, xanthohumol, cardamonin, isoliquiritigenin, isobacachalcone, morachalcone A, gemichalcones B, (Miranda et al., 2000b; Zhao et al., 2003; Ban et al., 2004; Lee et al., 2006; Lee et al., 2011). Lee et al. described that in lipopolysaccharide (LPS)-triggered microglial BV2 cells, xanthohumol reduced the stimulation of NF-κB signaling and decreased the inflammatory factors NO, IL-1β, and TNF-α (Figure 5). Furthermore, in LPS-induced BV2 cells, xanthohumol augmented the nuclear translocation of NRF2 and stabilized its cytoplasmic level, activating the intracellular production of glutathione (GSH), heme oxygenase-1 (HO-1), and NAD(P)H quinone oxidoreductase 1 (NQO1) (Table 4). These findings suggest that xanthohumol protects against LPS-triggered brain injury (Lee et al., 2011). Ban and colleagues also reported that cardamonin mediates anti-inflammatory function via suppressing the nuclear translocation of NF-κB (Ban et al., 2004).
Through blocking NF-κB signaling, cardamonin extracted from Alpinia conchigera reduces the expression of TNF-α, inductive NO synthase, and COX-2. Cardamomin decreased the NF-κB reporter gene generated by LPS in a dose-dependent way, having an IC50 results of 1.2 µM. Moreover, cardamomin had IC50 of 1.0 and 1.5 µM, respectively, and reduced the production of TNF and NO-triggered by LPS in a dose-dependent way (Table 4). Additionally, pretreatment with cardamomin (50 mg/kg) significantly diminished the mortality caused by LPS in C57BL/6 mice. Although cardamomin (50 mg/kg) pretreatment decreased the mice’s serum level of TNF-α, it had no significant consequence on the LPS-induced death of C57BL/6 mice after treatment (Lee et al., 2006).
Isoliquiritigenin inhibits the expression of inflammation regulatory molecule intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on the cell surface (Tanaka et al., 2001). Another class of chalcone named mallotophilippens C, D, and E downregulate the expression of inflammatory molecules COX-2, IL-6, and IL-1beta (Daikonya et al., 2004). 2′,3′-dihydroxy-4′,6′-dimethoxychalcone (DDC) was extracted from green Perilla frutescens var. Crispa f. viridis and characterized by NMR (1H, 13C, DEPT, TOCSY, HMQC, and HMBC) and high-resolution MS analysis. The authors also prepared DDC by using the Friedel-Crafts method from 2′-hydroxy-4′,6′-dimethoxyacetophenone, and trans-cinnamoyl chloride, and proton NMR analysis revealed that the isolated and synthetic DDC had the same chemical shifts and peak pattern. Through the initiation of the Nrf2-antioxidant response element (Nrf2-ARE) path, DDC promotes an increase in the expression of antioxidant enzymes γ-glutamylcysteine synthetase, NQO1, and HO-1 (Table 4). The authors claim that by increasing the expression of many antioxidant proteins and inhibiting the generation of intracellular ROS, DDC enhanced cellular resistance to 6-OHDA-persuaded cytotoxic activity (Izumi et al., 2012). According to another study, Hesperidin methyl chalcone also demonstrates antioxidant properties, which help heal inflammatory colitis. Increases in ferric reducing antioxidant power (FRAP), 2.2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) scavenging, and GSH levels were indicative of an enhancement in total antioxidant capability following hesperidin methyl chalcone treatment. Hesperidin methyl chalcone suppresses the expression of multiple pro-inflammatory cytokines in the colon, such as TNF α, IL-1β, IL-6, and IL-33 (Table 4). The possibility that this substance inhibits the colon’s NF-κB pathway activity has also been explored (Guazelli et al., 2021).
In another study, an inflammation-induced mice model was administrated with varying dosages of hesperidin methyl chalcone (10, 30, or 100 mg/kg) to investigate the involvement of hesperidin methyl chalcone in zymosan-mediated inflammation (Table 4). The macrophage cell (RAW 264.7) was also employed to evaluate oxidative stress concurrently. Analysis of the antioxidative and anti-inflammatory tests revealed that the activation of macrophage NF-κB and the generation of ROS were reduced by hesperidin methyl chalcone treatment. Nevertheless, hesperidin methyl chalcone significantly decreased knee joint edema, mechanical hypersensitivity, and leukocyte recruitment while impairing pro-inflammatory cytokine expression (Rasquel-Oliveira et al., 2020).
Hesperidin methyl chalcone has also been exhibited to trigger the Nrf2 signaling pathway to protect against oxidative stress. Recently, analysis evaluated the function of hesperidin methyl chalcone in controlling the expression of cytokines to explore this situation further. Diclofenac (200 mg/kg) was provided orally to develop an inflammatory mouse model, which was subsequently treated with 0.3–3 mg/kg of hesperidin methyl chalcone. Subsequently, several biological parameters were examined, including kidney edema, histopathology, urine neutrophil gelatinase-associated lipocalin (NGAL), and Nrf2 mRNA expression. As a result, decreased urea levels and creatinine and lipid peroxidation, have been linked to the downregulation of IL-33, IL-6, and interferon-γ (IFN-γ) (Table 4). Not only may hesperidin methyl chalcone cause the Nrf2 pathways to be downregulated, but it also decreases the expression of downstream proteins, including HO-1, NQO1, and Kelch-like ECH-related protein 1 (Keap1) (Bussmann et al., 2022).
Another particular condition that can target the body’s immune system is autoimmune disease. Numerous immune cells and cytokines may contribute to developing these kinds of illnesses. Trans chalcone, a naturally occurring chalcone, was given orally to rats with joint tissue stiffness, an autoimmune disease caused by complete Freund’s adjuvant (CFA) to treat this disorder. Trans chalcone dosages were examined in increasing order of administration: 30 mg/kg, 60 mg/kg, and 120 mg/kg at the end. ELISA and RT-PCR were employed to measure the expression levels of IL-17, TNF-α, iNOS, and COX-2 to assess the outcome of trans chalcone efficacy (Table 4). Consequently, these pro-inflammatory cytokines decrease, demonstrating the efficaciousness of trans chalcone in reducing ameliorating joint stiffness (Jabbar et al., 2024).
Yang et al. demonstrated that flavokawain A, a central metabolite of chalcones (0.46%) isolated from kava extracts, enhanced the expression of antioxidant proteins in primary splenocytes (Yang et al., 2020). In vitro analysis demonstrated that nontoxic dosages of flavokawain A (2–30 μM) prominently repressed the release of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) while stimulating the production of interleukin-10, an anti-inflammatory cytokine (Table 4). In vitro results are supported by ex vivo results from primary splenocytes derived from oral flavokawain A-preadministered BALB/c mice, demonstrating that flavokawain A substantially repressed the secretion of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) in cells stimulated by LPS, Concanavalin A, or control. Significant reductions in the ratios of pro- and anti-inflammatory cytokines (IL-6/IL-10; TNF-α/IL-10) indicate the potent anti-inflammatory characteristics of flavokawain A. Moreover, in BALB/c mice given cholecystokinin (CCK) 8 to induce experimental pancreatitis, pre-treatment with flavokawain A led to decreased blood lipase levels. Another study demonstrated the anti-inflammatory role of isolated metabolites, including 2-hydroxy-3,4,6-trimethoxychalcone from the Toussaintia orientalis Verdc leaf extracts. Among these isolated metabolites, 2-hydroxy-3,4,6-trimethoxychalcone showed anti-inflammatory attributes in the inflammation-inducing enzyme COX-2 (Table 4) (Nyandoro SS et al., 2012). Pongamol is a β-hydroxybenzofuranchalcone with double bonds with -OH, -OMe, and ethylene moieties. It can be found in pure enol form in Tephrosia purpurea roots and Pongamia pinnata (karanjin) seeds. According to the solid-state structure of pongomal, the mean plane of the phenyl group is twisted by 27.9° from the mean plane of the β-hydroxy chalcone, which is twisted by 34.8° from the mean plane of the benzofuran moiety (Parmar et al., 1989). Recently, a study was conducted where pongamol, isolated from the Pongamia pinnata, interestingly showed anti-inflammatory activity and antioxidant activities, having an IC50 results of 12.2 μg/mL (Table 4). In this study Wistar rats were used to induce edema in the paw and ear by utilising Carrageenan and xylene, which get reduced after administrating the dihydropongamol with a concentration of 50 ppm (Rekha et al., 2020).
Several chalcones exhibit significant anti-inflammatory and antioxidant properties, including xanthohumol, isoliquiritigenin, and hesperidin methyl chalcone. The SAR analysis confirmed that the chalcones having –OH in the B-ring and –OCH3 in the para position of the A-ring were often potent antioxidant properties (Sivakumar et al., 2011). Furthermore, chalcones’ anti-inflammatory activity is enhanced by a -OH at p-position in ring-B. The anti-inflammatory action is further enhanced by substituting -OH and -OCH3 on both rings, suppressing NO generation (Ur Rashid et al., 2019). The existence of -OH and -OCH3 moieties in chalcones also represses the activity of adenosine receptors-A1, A2A and shows antioxidant and anti-inflammatory properties. They achieve this by inhibiting pro-inflammatory cytokines, suppressing enzymes like COX-2, and activating the Nrf2 pathway to boost antioxidant defenses. Studies in various models, including LPS-induced microglial cells and inflammation-induced mice, demonstrate these metabolites’ potential to diminish inflammation, oxidative stress, and related conditions such as autoimmune diseases and joint stiffness. Flavokawain A, another chalcone, also shows strong anti-inflammatory effects, enhancing antioxidant protein expression while reducing pro-inflammatory cytokines.
4.4 Chalcones as modifiers of enzyme actions in a biological system
Chalcones modify various enzyme actions and reshape the functions and metabolic activities within the biological body. Chalcone modifies the functions of almost all classes of enzymes, including oxidoreductases, cyclooxygenase and 5-lipoxygenase, aldose reductase, thioredoxin reductase, monoamine oxidase, proteases, esterase, etc., (Zhou, 2015). By modifying these enzyme activities, chalcone compounds can potentially change various metabolic events, biosynthesis of substances, disease development and progression, cancer, and many other pathological conditions. Chalcones have been found to modify mitochondrial enzymes, decrease cholesterol and melanin synthesis, modify estrogen biosynthesis, and many more. As the mechanism of modifying the enzyme actions, chalcone compound acts like competitive inhibitors and causes reversible inhibition of enzymes. Inhibiting some of the enzyme actions leads to the antidiabetic activity of the chalcone compounds, which we will discuss in the coming sections.
Many chalcones including isoliquiritigenin, 4-Hydroxychalcone, butein, 2,4,2′,4′-tetrahydroxy-3-(3-methyl-2-butenyl)-chalcone (TMBC), and 2′,4′,4-trihydroxychalcone etc., possess tyrosinase inhibitory activity (Nerya et al., 2003; 2004a; Zhang et al., 2009; Singh et al., 2020). Zhang and his group isolated TMBC, from the Morus nigra stem and characterized by NMR (1H and 13C NMR) and EIMS mass spectroscopic method (Zhang et al., 2009). It was found that TMBC reduced tyrosinase action and total melanin content of B16 cells without causing appreciable cytotoxicity. Furthermore, TMBC (IC50 = 0.95 ± 0.04 µM) was found to have around 25 times the potency of a recognised tyrosinase inhibitor kojic acid (IC50 = 24.88 ± 1.13 µM) (Table 5).
TABLE 5
| Name and structure of chalcone | Plant source | Smiles | Activity | Dose of tested drug | Target | Mechanism of action | References |
|---|---|---|---|---|---|---|---|

2,4,2′,4′-Tetrahydroxy-3-(3-methyl-2-butenyl)-chalcone (TMBC) |
Morus nigra L. (Family: Moraceae) | O=C (/C=C/C1 = C(O)C=C(O)C=C1)C2 = C(O)C(C/C=C(C)\C) = C(O)C=C2 | Inhibit both cellular tyrosinase action in tumor cells | IC50 = 0.95 ± 0.04 µM | B16 (melanoma cell) | Direct inhibition of tyrosinase activity | Zhang et al. (2009) |
Xanthohumol Xanthohumol B  Xanthohumol D |
Humulus lupulus L. (Family: Cannabaceae) | O = C (/C=C/C1 = C(O)C=C(O)C=C1)C2 = C(OC)C=C(O)C(C/C=C(C)\C) = C2O O=C (/C=C/C1 = CC = C(O)C=C1)C2 = C(O)C3 = C(OC(C) (C)C(O)C3)C=C2OC O=C (/C=C/C1 = CC = C(O)C=C1)C2 = C(O)C(CC(O)C(C) = C) = C(O)C=C2OC |
Induces the quinone reductase (QR) activity | Tested concentration of drug is 20 μM | Hepa 1c1c7 (mouse hepatoma cell line) | Not Known | Yu et al. (2014) |
| Isoliquiritigenin | Dipteryx odorata. (Aubl.) Forsyth f. (Family: Fabaceae) | OC(C=C1O) = CC = C1C(/C=C/C2 = CC = C(O)C=C2) = O | Induces quinine reductase activity | Tested concentration of drug is 2–30 μM | Hepa 1c1c7 cells | Not known | Cuendet et al. (2006) |
Isoliquiritigenin Butein  Homobutein |
Not reported | OC(C=C1O) = CC = C1C(/C=C/C2 = CC = C(O)C=C2) = O OC1 = CC = C(C(/C=C/C2 = CC = C(O)C(O) = C2) = O)C(O) = C1 OC1 = CC = C(C(/C=C/C2 = CC = C(O)C(OC) = C2) = O)C(O) = C1 |
Anti-inflammatory and anti-tumor activity | IC50 = 60–190 µM (with inhibition of HDAC) IC50 = 8–41 µM (TNFα-triggered NF-κB activation-inhibition) |
K562 cells | Inhibit the expression of Histone deacetylase enzymes Attenuated TNFα-triggered NF-κB activation |
ORLIKOVA et al. (2012) |
Morachalcone A 2,4,2′,4′-tetrahydroxychalcone |
Twigs of Morus alba L. (Family: Moraceae) | O=C (/C=C/C1 = CC = C(O)C=C1O)C2 = C(O)C(C/C=C(C)\C) = C(O)C=C2 O=C (/C=C/C1 = CC = C(O)C=C1O)C2 = C(O)C=C(O)C=C2 |
Tyrosinase Inhibition metabolites activity characterization | IC50 = 0.07 ± 0.02 µM (2,4,2′,4′-tetrahydroxychalcone) IC50 = 0.08 ± 0.02 µM (Morachalcone A) |
Not Reported | Inhibition of tyrosinase better than positive control kojic acid | Zhang et al. (2016) |

2′,4′,6′-trihydroxy-3′-prenylchalcone |
Helichrysum teretifolium (L.) Sweet (Family: Asteraceae) | O=C (/C=C/C1 = CC = CC = C1)C2 = C(O)C(C/C=C(C)/C) = C(O)C=C2O | Oxidative Stress as well as Skin Aging-associated enzymes inhibition | 4,529.01 ± 2.44; 4,170.66 ± 6.72 μM TE/g | Not Reported | Modulated biological activity than other isolated metabolites including isoxanthohumol from the same source | Popoola et al. (2015) |

2′4′-dihydroxy-6′-methoxy-chalcone |
Leaves and stems from Loranthus acutifolius Ruiz & Pav. (Family: Loranthaceae) | OC1 = CC(O) = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = C1 | Melanin production and tyrosinase inhibition activity | IC50 = 5.7 ± 0.02 μM (For tyrosinase inhibition) | B16-F10 cells | Melanin production and tyrosinase activity inhibition | Apaza Ticona et al. (2021) |
| Cardamonin | Not reported | OC1 = C(C(/C=C/C2 = CC = CC = C2) = O)C(OC) = CC(O) = C1 | Nrf2-mediated antioxidant enzymes biosynthesis in colon cancer cells | 50 µM | Caco-2 cells | Not elevated gene expression of TrxR1 and glutathione peroxidase 2 (GPx2) Attenuation of O-phosphoseryl-tRNA (Sec) kinase (PSTK) associated with the translation of selenoprotein mRNAs This metabolite interferes biosynthesis of Nrf2-regulated selenoenzymes |
De Spirt et al. (2016) |
Plant source, doses, and enzyme modifiers actions of different natural chalcones.
Tyrosinase is an important enzyme that works on substrates like l-tyrosine and l-DOPA to catecholates, benzoquinones etc., and act as a critical role in melanogenesis and skin pigmentation (Olivares and Solano, 2009). Expression of tyrosinase is also reported to increase during tumorigenesis, and it may accelerate tumor growth (Boyle et al., 2002). Development of effective tyrosinase inhibitors will therefore be useful in managing skin pigmentation and cosmetic industries and more. Chalcone compounds also cause inhibition of ornithine decarboxylase (Luyengi et al., 1994), aromatase, 17β-hydroxysteroid dehydrogenase (Le Bail et al., 2001) and 5α-reductase enzyme. These enzymes act in the biosynthetic pathway of sex steroids: aromatase in estrogen synthesis, 5α-reductase, and 17β-hydroxysteroid dehydrogenase in androgen synthesis. Chalcones isolated from Broussonetia papyrifera cause inhibition of aromatase enzyme, reducing estrogen biosynthesis in human placental microsomal preparation (Le Bail et al., 2001). Ornithine decarboxylase and aromatase activity increase during various types of cancer, mediate some molecular events in cancer pathogenesis, and thus can be a probable therapeutic target (Chumsri et al., 2011; Kim et al., 2017). Aromatase and ornithine decarboxylase inhibitors were very effective in treating cancer (Kim et al., 2017; Kharb et al., 2020). Chalcones also inhibit the enzyme cytochrome P450 1A (CYP1A), whose activity is essential for the metabolic initiation of polycyclic aromatic hydrocarbons and cause chemoprevention (Forejtníková et al., 2005). Essential functions in the redox control of multiple cellular signaling pathways comprised in proliferation and cell growth are played by thioredoxin reductase (TrxR: an essential protein that regulates the redox environment in cells) (Mahmood et al., 2013; Lu and Holmgren, 2014). Since many cancers overexpress the thioredoxin system, which confers drug resistance to cancer chemotherapy, there has been growing evidence in recent years that TrxR is a potential target for developing new anti-cancer drugs (Kim et al., 2005). Hatfield et al. provided proof of the physiological importance of TrxR for the progression of tumors, demonstrating that TrxR knockdown decreases the proliferation and DNA replication of melanoma cells and the development of tumors (Yoo et al., 2007). Xanthohumol, xanthohumol B and D, and xanthohmol analogs isolated from Humulus lupulus L. cause potent inhibition of the enzyme aromatase, quinone reductase, and thioredoxin reductase (Monteiro et al., 2007; Yu et al., 2014). An investigation into the potential effects of xanthohumol on the control of estrogen synthesis was conducted on the aromatase-expressing breast cancer cell Sk-Br-3 (Monteiro et al., 2007). According to the investigators, the aromatase activity in Sk-Br-3 was observed to be reduced by incubation with different dosages of xanthohumol (IC50 = 3.2 µM).
Further research on the relationship between the inhibition of cellular proliferation and aromatase inhibition showed that xanthohumol treatment on Sk-Br-3 cells for 72 h decreased protein synthesis dose-dependent (IC50 = 7.1 µM). Of all the substances tested, Sk-Br-3 cells treated with xanthohumol showed the greatest reduction in DNA synthesis (IC50 = 0.52 µM). The authors’ explanation of xanthohumol included reduction of the aromatase enzyme and a decrease in the rate of estrogen production (Monteiro et al., 2007).
Yu et al. isolated a novel prenylated chalcone xanthohumol, and the first new natural bichalcone humulusol having a prenyl substitution and methylene connector along with six known chalcones from Hmulus lupulus and evaluated their quinone reductase (QR) induction properties using hepa 1c1c7 cells (Table 5) (Yu et al., 2014). Newly isolated chalcones were characterized by HR-ESI-MS, and spectroscopic methods (1H NMR, 13C NMR, and HMBC). All the isolated chalcones demonstrated significant electrophilic ability, good solubility, and good QR induction activity. SAR studies on the isolated chalcones indicated that the QR induction activity is attributed to the trans double bond of the chalcones.
Another well-studied chalcone named isoliquiritigenin reported quinine reductase activity at micromolar concentration and was found helpful in cancer chemoprevention (Cuendet et al., 2006). It was found that isoliquiritigenin (2–30 μM), a monofunctional inducer with a maximum of 7 times induction at the highest tested concentration, could activate quinone reductase in Hepa 1c1c7 cells of the wild type, hence reducing the risk of cancer (Table 5). Furthermore, treatment with isoliquiritigenin (7.5–30 μM) did not exhibit any cytotoxicity and dramatically increased luciferase expression through interaction with ARE in a dose-dependent path.
A study explored the role of four major chalcones, including butein, isoliquiritigenin, glycoside marein, and homobutein out of 21 isolated natural chalcones for inhibiting the expression of HDACs, as HDACs regulate the NF-κB transcription factor for promoting the inflammation-mediated cancer progression. These four metabolites showed the IC50 = 60–190 µM as well as inhibit the TNFα-triggered NF-κB activation with IC50 value 8–41 µM. Interestingly, soliquiritigenin, butein, and homobutein showed an inhibitory role against the expression of total HDAC activities of classes I, II, and IV and TNFα-triggered NF-κB activity (Table 5) (ORLIKOVA et al., 2012). Zhang et al. also reported the inhibitory role of 2,4,2′,4′-tetrahydroxychalcone, and morachalcone A, isolated from the Morus alba L. towards tyrosinase. These metabolites disclosed a stronger repressive activity against tyrosinase than positive control kojic acid. 2,4,2′,4′-tetrahydroxychalcone showed IC50 = 0.07 ± 0.02 µM, and morachalcone A showed IC50 = 0.08 ± 0.02 µM (Table 5) (Zhang et al., 2016).
Furthermore, to study the reduction of oxidative stress and skin aging-associated enzymes, 2′,4′,6′-trihydroxy-3′-prenylchalcone extracted from the Helichrysum teretifolium methanolic extract. This metabolite showed modulated biological activity than other isolated metabolites, including isoxanthohumol from the same source. For instance, it exhibited some of the highest TEAC results (4,529.01 ± 2.44; 4,170.66 ± 6.72) μM TE/g (Table 5) (Popoola et al., 2015). Nevertheless, 2′4′-dihydroxy-6′-methoxy-chalcone isolated from the Loranthus acutifolius leaves and stems was also reported as a potential inhibitor in melanin production and tyrosinase activity. To delve into this activity, this metabolite was tested on B16-F10 cells, where it determined the IC50 value with 1.6 ± 0.03 μM. The prospect of tyrosinase inhibition by this same metabolite was also reported with IC50 = 5.7 ± 0.02 μM (Table 5) (Apaza Ticona et al., 2021). Interestingly, cardamonin a natural chalcone, was also reported as antioxidant activity by Nrf2 pathway-mediated enzymes (selenoenzymes) biosynthesis activation in 50 µM concentration in Caco-2 cell line (Table 5) (De Spirt et al., 2016).
Chalcones, such as isoliquiritigenin, TMBC, and xanthohumol, exhibit strong inhibitory effects on enzymes like tyrosinase, aromatase, and quinone reductase. These activities make them effective against skin pigmentation, cancer cell proliferation, and oxidative stress. The SAR studies about the tyrosinase inhibition potency indicate that the position of the -OH on A and B rings, having a substantial preference for a 4-substituted B ring, rather than a substituted A ring, is the most important factor in their efficacy; neither the number of -OH nor the incidence of a catechol moiety on ring B interrelated with enhancing tyrosinase reduction potency (Nerya et al., 2004b). TMBC, for example, is 25 folds more effective than kojic acid in preventing tyrosinase, a key enzyme in melanogenesis. Xanthohumol shows significant potential in reducing estrogen synthesis in breast cancer cells. Chalcones like butein and isoliquiritigenin also inhibit HDAC enzymes, further demonstrating their potential in cancer prevention and therapy.
4.5 Antiobesity and cardioprotective activity of naturally occurring chalcones
Cardiovascular diseases (CVDs) are among the foremost causes of human mortality globally, and CVDs caused 20.5 million deaths in 2021 alone, or over one-third of all fatalities worldwide, and are predicted to impact 23.3 million humans by year 2030 (Lindstrom M et al., 2022). Ischaemic heart disease caused by cholesterol deposition and the development of atherosclerotic plaque inside the coronary arteries has become one of the leading causes of death globally. Cardiovascular health is determined by numerous aspects, such as the cholesterol and triglyceride levels in the body, obesity, atherosclerosis of the blood vessels, and blood pressure, blood parameters, as well as the musculature of the heart, and all the factors regulating the myocardial activity. Variations in gene sequences may also be caused by chronic pathological diseases like cardiovascular disease. In this regard, a good number of studies found a link between the advancement of CVD and single-nucleotide polymorphisms (SNPs: variation in one building block of the genetic sequence) in inflammatory genes. Numerous SNPs linked to inflammatory molecules, for example, C-reactive protein (CRP), IL-10, IL-1, TNF-α, IL-6, and transforming growth factor-β (TGF-β), have been associated with the development of CVD (Deb et al., 2022). Chalcone compounds working on any of those mentioned above and also target the lipoprotein lipase (LPL), pancreatic lipase (PL), angiotensin-converting enzyme (ACE), cholesterol acyltransferase (ACAT), diacylglycerol acyltransferase (DGAT), cholesteryl ester transfer protein (CETP), thromboxane A2 (TXA2), calcium-potassium channel, and thromboxane B2 (TXB2), COX-1 can affect the cardiovascular health (Mahapatra and Bharti, 2016). Chalcones can be used as potential cardiovascular agents to treat and manage many cardiovascular disease conditions like the management of hypertension, prevention of atherosclerosis, impeding ischemia-induced myocardial infarction, and improving overall myocardial health (Mahapatra and Bharti, 2016; Khan et al., 2021; Maisto et al., 2023). Treatment with many chalcone compounds like 4-hydroxyderricin, xanthoangelol, and isoliquiritigenin reduced the ischemia-induced myocardial infarction and protected the antioxidant system (Ogawa et al., 2005; AN et al., 2006).
Chalcones also reduce blood pressure effectively and prevent atherosclerosis (Avila-Villarreal et al., 2013; Chen et al., 2020) by reducing the plasma cholesterol, low-density lipoprotein (LDL), and triglycerides levels (Ogawa et al., 2007; Birari et al., 2011). A 3-prenylated chalcone with a C5-isoprenoid unit at the 3-position is called 4-hydroxyderricin. In stroke-prone spontaneously hypertensive rats (SHRSP), Ogawa and colleagues extracted 4-hydroxyderricin from the Angelica keiskei stems and examined the dietetic results of 4-hydroxyderricin on lipid metabolism and blood pressure (Ogawa et al., 2005). The authors attributed that 4-hydroxyderricin (Figure 6) inhibited the rise in systolic blood pressure, lowered the levels of serum very-low-density lipoprotein (VLDL), and reduced hepatic triglyceride in SHRSP (Table 6). A study looking at the hepatic mRNA expression of lipid metabolism-related proteins suggested that the drop in serum VLDL levels could be caused by a significant decrease in microsomal triglyceride transfer protein, and the drop in hepatic triglyceride content could be triggered via significant decreases in fatty acid synthase and adipocyte determination in addition to differentiation factor 1.
FIGURE 6
Different naturally isolated chalcones in maintaining blood pressure and fat deposition. 4-hydroxyderricin, xanthoangelol, and isoliquiritigenin reduce the deposition of cholesterol and triglycerides in arteries to prevent high blood pressure. In contrast, licocholane A can also inhibit the differentiation of the adipocyte cells in the HFD-induced fatty mice model.
TABLE 6
| Name and structure of chalcone | Plant source | Smiles | Activity | Dose of drug | Target model | Mechanism of action | References |
|---|---|---|---|---|---|---|---|
| 4-hydroxyderricin | Angelica keiskei. (Miq.) Koidz. (Family: Apiaceae) | OC1 = C(C(/C=C/C2 = CC = C(O)C=C2) = O)C=CC(OC) = C1C/C=C(C)/C | Cardioprotective Reduction of serum VLDL and hepatic triglyceride |
Diet containing 0.07% 4-hydroxyderricin | Hypertensive rats | Reduced expression of mRNA is responsible for lipid metabolism such as microsomal triglyceride with downregulation of fatty acid synthase | Ogawa et al. (2005) |
| Xanthoangelol | Angelica keiskei. (Miq.) Koidz. (Family: Apiaceae) | OC1 = C(C/C=C(C)/CC/C=C(C)/C)C(O) = C(C(/C=C/C2 = CC = C(O)C=C2) = O)C=C1 | Cardioprotective Reduced serum LDL, triglyceride contents, and total cholesterol in liver tissue |
Diet containing 0.10% xanthoangelol |
Hypertensive rats | Increased hepatic PPARα mRNA expression as well as hepatic ACO and ACS mRNA. | Ogawa et al. (2005) |
| Isoliquiritigenin | Glycyrrhiza glabra. L. (Family: Fabaceae) | OC(C=C1O) = CC = C1C(/C=C/C2 = CC = C(O)C=C2) = O | Cardioprotective Attenuates severity of myocardial reperfusion |
20 mg/kg body weight | Rats with myocardial ischemia-reperfusion | Upregulation of metallothionein (MT) expression | AN et al. (2006) |
| Isoliquiritigenin | Glycyrrhiza glabra.L. (Family: Fabaceae) | OC(C=C1O) = CC = C1C(/C=C/C2 = CC = C(O)C=C2) = O | Antiobesity and lipid-lowering effects | IC50 for pancreatic lipase inhibition is 7.3 µM In vivo treatment dose is 30 mg/kg body weight/day |
Male SD rats fed | Inhibition of pancreatic lipase (PL) | Birari et al. (2011) |
| Licochalcone A | Glycyrrhiza sp. | OC1 = C(C(C) (C)C=C)C=C (/C=C/C(C2 = CC = C(O)C=C2) = O)C(OC) = C1 | Suppresses adipocyte differentiation | Treatment given are 5 and 10 mg/kg body weight | HFD-induced ICR mice | Downregulation of peroxisome proliferator-triggered receptor γ, fatty acid synthase, and other enzymes like stearoyl-CoA desaturase 1 to reduce obesity | Quan et al. (2012) |
| Licochalcone A | Glycyrrhiza uralensis.Fisch. ex DC. (Family: Fabaceae) | OC1 = C(C(C) (C)C=C)C=C (/C=C/C(C2 = CC = C(O)C=C2) = O)C(OC) = C1 | Decrease the level of plasma cholesterol | 10 mg/kg body weight | HFD-induced C57BL/6 mice | Instigates the expression of uncoupling protein 1 (UCP1) for ant-obesity in 3T3-L1 adipocyte cells | Lee et al. (2018) |
| Flavokawains A Flavokawains B  Flavokawains C |
Kaempferia angustifolia Roscoe (Family: Zingiberaceae) | O=C(C1 = C(O)C=C(OC)C=C1OC)/C=C/C2 = CC = C(OC)C=C2 O=C(C1 = C(O)C=C(OC)C=C1OC)/C=C/C2 = CC = CC = C2 O=C(C1 = C(O)C=C(OC)C=C1OC)/C=C/C2 = CC = C(O)C=C2 |
Cardioprotective | 10 μg/mL | 3T3-L1 murine model | Inhibited triglyceride accumulation | Hanif et al. (2022) |
| Licochalcone A | Glycyrrhiza uralensis Fisch. ex DC. (Family: Fabaceae) | OC1 = C(C(C) (C)C=C)C=C (/C=C/C(C2 = CC = C(O)C=C2) = O)C(OC) = C1 | Potential activity of ameliorating obesity | 1.5–12 μM (in HepG2 cells) | HepG2 hepatocytes Male C57BL/6 mice |
Triggered the sirt-1/AMPK path Downregulate the fatty acid chain synthesis Enhance lipolysis and β-oxidation in liver cells |
Liou et al. (2019) |
| Xanthohumol | Humulus lupulus L. (Family: Cannabaceae) | O=C (/C=C/C1 = C(O)C=C(O)C=C1)C2 = C(OC)C=C(O)C(C/C=C(C)\C) = C2O | Regulatory activity of lipid metabolism | 10 and 20 μM | HepG2 and Huh7 cells Zebrafish model |
Attenuated the expression of ANGPTL3 mRNA and protein | Gao et al. (2024) |
Plant source, doses, and cardioprotective roles of different natural chalcones.
The same authors also extracted xanthoangelol (Figure 6) from A. keiskei, and inspected the result of dietetic xanthoangelol on blood pressure and lipid metabolism in SHRSP (Ogawa et al., 2007). It was noted that the xanthoangelol increased the expression of LDL-receptor (LDL-R) mRNA in the liver, and it caused a decline in blood LDL. In addition to lowering liver weight in SHRSP, xanthoangelol (0.10%) also caused a decline in the hepatic cholesterol pool via increasing fecal cholesterol excretion (Table 6). Furthermore, xanthoangelol significantly enhanced the expression of peroxisome proliferator activated receptor-α (PPAR-α) in the liver in conjunction with increases in the expression of Acyl-coenzyme A oxidase (ACO) and Acyl-CoA synthetase (ACS) in the liver, suggesting an acceleration of fatty acid β-oxidation.
In an in vivo analysis of MI/R rats, An and his group observed that isoliquiritigenin (Figure 6) prominently decreased the myocardial infarct size and avoided arrhythmias caused by reperfusion (AN et al., 2006). Lactate dehydrogenase (LDH) and creatinine phosphokinase (CPK) activity in the isoliquiritigenin (20 mg/kg) group were 38.4% and 51.3% lower, respectively, than the vehicle group (Table 6). In isoliquiritigenin-treated groups, there was an increase in metallothionein (MT) synthesis along with enhanced JAK 2/STAT 3 phosphorylation, but not in COX-2 or iNOS production. AG490 can notably reduce isoliquiritigenin-induced cardioprotection and stop MT expression from rising and JAK 2/STAT 3 phosphorylation from happening.
Birari and colleagues extracted twelve flavonoids, including the chalcone isoliquiritigenin from the Glycyrrhiza glabra roots and examined the pancreatic lipase (PL) repressive action in vitro (Birari et al., 2011). Isoliquiritigenin among the evaluated metabolites exhibited a substantial inhibitory result towards PL, with an IC50 = 7.3 µM (Table 6). The antiobesity and lipid-lowering outcomes of chalcone isoliquiritigenin in male SD mouse fed a high-fat diet (HFD) were observed. These rats’ body weight enhanced by only 23.2 ± 3.6 g when supplemented with isoliquiritigenin, whereas the body weight of the HFD control group enhanced by 64.2 ± 0.5 g, suggesting the significance of the chalcone moiety as a cause for stopping obesity. Moreover, isoliquiritigenin decreased plasma total cholesterol to 84.6 ± 1.4 mg/dL and plasma total triglycerides to 128.8 ± 6.0 mg/dL.
The mechanistic study performed on antihypertensive action revealed reduction of ACE to blocking of calcium channel and β receptors by various chalcone compounds (Kumar et al., 2015; Goshain and Ahmed, 2019). Licochalcone A shows potent antiobesity property in HFD-fed ICR mice and suppresses adipocyte differentiation. Treatment with licochalcone A lowers gain in body weight and diminishes plasma levels of triglyceride, cholesterol, and nonesterified fatty acid. When compared to the HFD control mouse, the total fat volume of the mice given 5 and 10 mg/kg of licochalcone A reduced by 60% and 80%, respectively (Table 6) (Quan et al., 2012). Additionally, in a mouse model, the group provided 10 mg/kg licochalcone A had triglyceride, cholesterol, and no esterified fatty acid levels that were 14.0%, 48.2%, 58.9%, and 73.5% of body weight, respectively, lower than the control group. In 3T3-L1 adipocyte cells, a mechanistic study showed downregulation of sterol regulatory element-binding protein 1c, fatty acid synthase, glycerol-3-phosphate acyltransferase, stearoyl-CoA desaturase 1, and peroxisome proliferator-activated receptor γ. Additionally, it induced the expression of uncoupling protein 1 (UCP1) (Quan et al., 2012). Lee et al. also explored the antiobesity action of licochalcone A (Lee et al., 2018). Experimental results indicated that licochalcone A treatment causes browning of the subcutaneous adipocytes in the HFD-induced obesity mouse through promoting the expression of essential markers specific to brown adipocytes, which in turn drives the thermogenic gene program (Table 6). The authors attributed that licochalcone A may increase fat oxidation, lipolysis, and thermogenesis while decreasing lipogenesis.
Recently it was described that at 10 μg/mL concentration in a murine model of pre-adipocytes, a natural chalcone flavokawains A extracted from the rhizomes of the Indonesian terrestrial plant Kaempferia angustifolia exhibits antiobesity activity and reduced cytotoxic effect (EC50 = 39.2 µM) (Table 6) (Hanif et al., 2022). The authors also synthesized flavokawains A, flavokawains B, and flavokawains C to determine the activity of natural flavokawains A and investigated the results of electron-donating moieties of the B ring. Synthetic flavokawains A’s EC50 was 64.4 µM, comparable to natural flavokawains A’s value. It was also found that flavokawains C was more selective than the number flavokawains A, the authors attributed that the B ring’s reactivity has been decreased by a p-hydroxy (electron-donating) group and the antiobesity effect may be ascribed to the electron-donating moiety on the B ring.
A study revealed the potential antiobesity and protective role against nonalcoholic fatty liver diseases of licochalcone A, derived from Glycyrrhiza uralensis. This isolated metabolite was tested on HFD-mediated obesity-induced male C57BL/6 mice. Simultaneously, the oleic acid-induced fatty liver model was generated in the HepG2 cell line. After administration of licochalcone A (Figure 6), the weight of liver tissue decreased compared to high fat-induced fatty liver. Nevertheless, licochalcone A also reduced the expression of a transcription factor that helps in lipogenesis and synthesis of fatty acids.
Interestingly, it has also been observed that licochalcone A was able to activate the sirt-1/AMPK path to attenuate the fatty acid chain synthesis and to enhance the lipolysis and β-oxidation in hepatocytes (Table 6) (Liou et al., 2019). Another study was also conducted to evaluate the anti-atherosclerotic cardiovascular diseases where Hops (Humulus lupulus L.) was selected and isolated different metabolites, including xanthohumol. To delve into this context, HepG2 and Huh7 cells were used as in vitro models, and zebrafish were used as an in vivo model. As a result, xanthohumol reduced the expression of angiopoietin-like protein-3 (ANGPTL3) in both mRNA and protein level that function as inhibitor of lipoprotein lipase and thereby enhance the expression of lipoprotein lipase (Table 6) (Gao et al., 2024).
According to SAR research, prenylated chalcones have demonstrated superior pharmacokinetic and pharmacodynamic profiles compared to commercially available conventional medications. Prenylated chalcones including xanthohumol, xanthoangelol, isobavachalcone, 4-hydroxyderricin, and 2′,4′-dihydroxy-4-methoxy-3′-prenyldihydrochalcone had antiobesity potential and exhibited inhibition of AA (Arachidonic Acid), CETP, and DGAT. Calcium channel blockage requires the presence of a 1, 4-dihydropyridyl group at the 2- or 6-position of the A-ring, as well as a 4-OH substitution at B ring.3-, 4-, and 5-trimethoxy substitution is critical for inhibiting ACE, while the inhibition of PL requires the substitution of 3-OH or 4-OH at the B-ring and 2, 4-dihydroxy at the A-ring (Mahapatra and Bharti, 2016). Chalcones, such as 4-hydroxyderricin and xanthoangelol, exhibit significant cardiovascular benefits, including reducing blood pressure, cholesterol, and triglycerides and preventing atherosclerosis. Isoliquiritigenin shows potential in reducing myocardial infarct size and obesity. Licochalcone A, another chalcone, demonstrates antiobesity effects by promoting fat oxidation and thermogenesis while reducing lipogenesis. Chalcones like flavokawains and xanthohumol also show potential in protecting against nonalcoholic fatty liver disease and reducing atherosclerotic risk by regulating lipid metabolism pathways.
4.6 Antidiabetic roles of naturally occurring chalcones
Diabetes and related complications, such as diabetic neuropathy, retinopathy, and nephropathy, are main reasons of health suffering, morbidity, and mortality of diabetic patients. Globally, diabetes-related mortalities accounted for approximately 6.7 million deaths in 2021, and predicts that 537 million people globally were living with diabetes in 2021. If effective preventive measures are not taken, this figure is expected to rise to 643 million by 2030 (Federation ID, 2021). A variety of chalcone compounds exert antidiabetic activities (Rocha et al., 2020). Chalcones are among the molecules that have given promising results when examined for their antidiabetic actions (Mahapatra et al., 2015a). Antidiabetic property of chalcone derivatives is basically due to their inhibitory roles in glycolytic pathways and insulin-like activities (Rocha et al., 2020). Some of the important enzymes in this pathway are aldose reductase, α-amylase, α-glucosidase, etc. Apart from these enzymes the therapeutic target for the chalcones in control of diabetes also includes Sodium Glucose Cotransporter 2 (SGLT2), Glucose Transporter Type 4 (GLUT4), Peroxisome Proliferator-activated Receptor-gamma (PPAR-γ), Protein Tyrosine Phosphatase 1B (PTP1B), Dipeptidyl Peptidase 4 (DPP-4), and AMPK (Mahapatra et al., 2015a; Rocha et al., 2020).
Aldose reductase is one of the vital enzymes significantly related to the pathogenesis of diabetes mellitus. This enzyme catalyzes the reduction reaction of glucose and converts it into sorbitol, considered an aetiological factor for many diabetic complications. Therapeutic targeting of aldose reductase is therefore proposed as a noble strategy for treating diabetes mellitus (Suzen and Buyukbingol, 2003; Maccari and Ottanà, 2015). Aldose reductase inhibitors can be, therefore, the promising class of molecules for the treatment of diabetic patients. Similarly, α-glucosidase and α-amylase are the two other enzymes that make glucose available in the blood. Chalcones were found to inhibit not only the aldose reductase but also carbohydrate hydrolyzing enzymes, including α-glucosidase and α-amylase (Rocha et al., 2019) and also target many signaling pathways and proteins to mediate their antidiabetic effects (Adelusi et al., 2021).
In recent days, researchers are developing chalcone-based amines and hybrid chalcone molecules to increase the antidiabetic potential of existing chalcones (Mahapatra et al., 2017). From Sophora flavescens roots, Jung and associates isolated five prenylated flavanones and two prenylated chalcones, including β-hydroxy chalcone kuraridin, and investigated their repressive outcomes against the formation of advanced glycation endproducts (AGE), human recombinant aldose reductase (HRAR), and rat lens aldose reductase (RLAR) (Jung et al., 2010). All the isolated prenylated flavanones and chalcones act as strong inhibitor of RLAR and also inhibit AGE formation. When compared to the strong AR inhibitor epalrestat (IC50 = 0.28 µM), all tested prenylated metabolites, including kuraridin (IC50 = 0.27 µM) demonstrated considerable inhibitory effects (Table 7). SAR analysis on the isolated metabolites designated that the prenyl moiety at C-8 and the 3,4′-dihydroxyl moieties may, be responsible for the outcomes of diabetic complications, which include the suppression of RLAR, HRAR, and AGE production.
TABLE 7
| Name and structure of chalcone | Plant source | Smiles | Activity | Dose of drug | Target model | Actions | References |
|---|---|---|---|---|---|---|---|

Kuraridin |
Sophora flavescens Aiton (Family: Fabaceae) | O=C (/C(O) = C/C1 = CC = C(O)C=C1O)C2 = C(OC)C=C(O)C (CC(C(C) = C)C/C=C(C)/C) = C2O | Antidiabetic activity | IC50 = 0.27 µM | Lenses collected from Sprague–Dawley rat’s eyes | Suppression of RLAR, HRAR, and AGE production | Jung et al. (2010) |
| Isoliquiritigenin | Glycyrrhiza glabra.L. (Family: Fabaceae) | OC(C=C1O) = CC = C1C(/C=C/C2 = CC = C(O)C=C2) = O | Shows an antihyperglycemic effect | 200 mg/kg | Male swiss albino mice | Changes in intracellular enzyme activity like glucosidase | Gaur et al. (2014) |
| 4-hydroxyderricin (4-HD) | Angelica keiskei. (Miq.) Koidz. (Family: Apiaceae) | OC1 = C(C(/C=C/C2 = CC = C(O)C=C2) = O)C=CC(OC) = C1C/C=C(C)/C | Prevents progression of diabetes | Basal diets containing 0.15% of drug | KK-Ay/Ta mice (Genetically hyperglycemic) | Insulin-like action leads to increased uptake of glucose by cells | Enoki et al. (2007) |
| Xanthoangelol | Angelica keiskei. (Miq.) Koidz. (Family: Apiaceae) | OC1 = C(C/C=C(C)/CC/C=C(C)/C)C(O) = C(C(/C=C/C2 = CC = C(O)C=C2) = O)C=C1 | Prevents progression of diabetes | Basal diets containing 0.15% of drug | KK-Ay/Ta mice (Genetically hyperglycemic) | Mimic insulin actions | Enoki et al. (2007) |

2′,6′-dihydroxy-4′-methoxychalcone |
Piper Claussenianum. (Miq.) C.DC. (Family: Piperaceae) | OC1 = C(C(/C=C/C2 = CC = CC = C2) = O)C(O) = CC(OC) = C1 | Lowers the blood glucose levels | 2 mg/kg | Male Wistar rats (Streptozotocin induced diabetes) | Inhibition of enzymes like protein tyrosine phosphatase 1B, aldose reductase, and α-glucosidase Enhanced secretion of insulin |
Sudo et al. (2015) |
| Xanthohumol | Humulus lupulus L. (Family: Cannabaceae) | O=C (/C=C/C1 = C(O)C=C(O)C=C1)C2 = C(OC)C=C(O)C(C/C=C(C)\C) = C2O | Alleviates hyperglycemia | IC50 = 8.8 µM | Caco-2 cells | Inhibition of α-glucosidase enzyme Xanthohumol directly binds to the α-glucosidase to change the molecular structure for inhibiting the enzymes |
Liu et al. (2014b) |

Broussochalcone A |
Broussonetia papyrifera. (L.) L'Hér. ex Vent. (Family: Moraceae) | OC1 = CC(OC) = C(C/C=C(C)/C)C=C1C(/C=C/C2 = CC = C(O)C(O) = C2) = O | Antidiabetic | IC50 = 5.3 µM | In-vitro assay | Inhibition of α-glucosidase enzyme | Ryu et al. (2010a) |
| Isoliquiritigenin | Glycyrrhiza uralensis. Fisch. ex DC. (Family: Fabaceae) | OC(C=C1O) = CC = C1C(/C=C/C2 = CC = C(O)C=C2) = O | Antidiabetic activity by inhibiting hyperglycemia-induced inflammatory response | Up to 40 μM dose, no significant toxicity induced | H9c2 cells and male C57BL/6 mice | Attenuation of cardiac hypertrophy to protect the cardiac function Downregulation of mitogen-activated protein kinases and upregulation of Nrf2 signaling cascade |
Gu et al. (2020) |

New chalcone |
Cleistocalyx operculatus (Roxb.) Merr. and L.M.Perry (Family: Myrtaceae) | O=C (/C=C/C1 = CC = CC = C1)C2 = C(O)C(C=O) = C(O)C(C) = C2OC | Antidiabetic activity | IC50 value ranging from 0.9 ± 0.2 to 3.9 ± 0.7 μM | Inhibits the activity of protein tyrosine phosphatase 1B | Mai et al. (2024) |
Plant source, doses, and antidiabetic roles of different natural chalcones.
Isoliquiritigenin and its derivatives show very good antihyperglycemic properties against streptozotocin-nicotinamide-induced diabetic male Swiss albino mice (Table 7) (Gaur et al., 2014). SAR studies demonstrated that the occurrence of ether and ester moieties in isoliquiritigenin derivatives is vital for displaying the activity. Twenty types of chalcones are found in Angelica keiski, which is essential as a dietary supplement. Enoki and his group isolated two main chalcones 4-hydroxyderricin and xanthoangelol from Angelica keiskei ethanol extract (Enoki et al., 2007). Both the chalcones exhibited insulin-like actions by triggering PPAR-γ (Table 7). Furthermore, in genetically compromised KK-Ay mice, which acquire diabetes and exhibit hyperglycemia with aging due to resistance of insulin, 4-hydroxyderricin (0.15%) also reduced the progression of diabetes.
Another chalcone named 2′, 6′-dihydroxy-4′-methoxychalcone was described to prominently lesser the blood glucose levels in streptozotocin-induced diabetes rats model suppression of enzymes including protein tyrosine phosphatase 1B, α-glucosidase aldose reductase, and increased secretion of insulin (Sudo et al., 2015). After 12 days, the blood glucose levels of the rats administered 2′, 6′-dihydroxy-4′-methoxychalcone (2 mg/kg) dropped from 277.4 ± 7.7 mg/dL before treatment to 158.8 ± 9.2 mg/dL (Table 7).
Xanthohumol and papyriflavonol A alleviate hyperglycemia by inhibiting of α-glucosidase enzyme. The ability of xanthohumol (isolated from Humulus lupulus L.) to attach to α-glucosidase, decrease its hydrophobicity, and cause conformational changes in the enzyme structure to cause inhibition was studied by Liu and colleagues (Liu et al., 2014). The results demonstrated that xanthohumol inhibited α-glucosidase (IC50 = 8.8 μM) reversibly and noncompetitively (Table 7). Additionally, xanthohumol prevented glucose from being released from maltose on the apical side of the Caco-2 cell monolayer.
Ryu et al. isolated four chalcones and eight bioactive metabolites from the chloroform extract of Broussonetia papyrifera roots and examined their α-glucosidase inhibitory property (Ryu et al., 2010). 1H and 13C NMR, HMBC, HREIMS, and EIMS characterized the structure of the extracted metabolite. With an IC50 of 5.3 μM, the maximum effective chalcone-derived inhibitor was the prenylated chalcone broussochalcone A, which has a resorcinol moiety in the A ring and a catechol in the B ring (Table 7). From SAR studies it emerges that hydrophobic moieties around the aromatic core and a higher number of prenyl moieties enhance the effectiveness of the inhibitor. Furthermore, in kinetic studies, all the extracted chalcones displayed noncompetitive inhibition characteristics.
A natural chalcone, isoliquiritigenin, was evaluated for anti-inflammatory and antioxidant activity. To see these activities, streptozotocin-induced diabetic mice were used where isoliquiritigenin was administrated. Thus, high hyperglycemia was also induced using the H9c2 cells, which was generated from the embryonic rat heart. Consequently, isoliquiritigenin successfully inhibited apoptosis, fibrosis, and hypertrophy in H9c2 cells by lowering oxidative stress and the inflammatory response (Table 7). Additionally, it increased the Nrf2 signaling pathway and downregulated the MAPKs (Gu et al., 2020).
Recently, Mai and colleagues isolated four novel compounds (3 racemic chalcone-monoterpene hybrids and a novel chalcone) from the buds of Cleistocalyx operculatus and investigated the inhibitory effects on PTP1B. The isolated compounds’ structures were determined by analyzing NMR data and validated by computational techniques. The chalcone-myrcene compounds were found to be promising inhibitors by the in vitro PTP1B inhibitory experiment, and also isolated novel chalcone demonstrated good efficacy as well, with an IC50 of 3.9 ± 0.7 µM (Mai et al., 2024) (Table 7). PTP1B plays a significant role in insulin signal transduction, further regulating insulin receptor activity and its downstream signaling proteins well (Johnson et al., 2002).
Aldose reductase plays a vital role in diabetes, and inhibiting this enzyme, and others like α-amylase and α-glucosidase, is a promising strategy for treating diabetic complications. Chalcones have shown potential in inhibiting these enzymes and regulating various antidiabetic pathways. Chalcones’ structural modifications make them a prospective treatment candidate for diabetes control. The 2′-hydroxyl group is a crucial metabolite of natural chalcones, providing substantial activity through the formation of hydrogen bonds and maintaining the moiety’s structural stability. Chalcones with a 2′- and 4′-OH or with additional hydroxylation in ring B demonstrate strong antidiabetic properties (Rammohan et al., 2020). Furthermore, PTP1B inhibitory activity was significantly increased by adding two -OH groups to the A ring’s 2- and 4-positions. Moreover, the -OH group at 4 or 5 position of ring A and the -OCH3 group at 4 position of ring B in the chalcone were crucial for the activation of PPARγ(Mahapatra et al., 2015a). Chalcones like xanthohumol, 4-hydroxyderricin, and isoliquiritigenin exhibit strong inhibitory effects on diabetic markers and have shown positive results in reducing blood glucose levels, improving insulin sensitivity, and protecting against diabetes-related oxidative stress and inflammation.
4.7 Neuroprotective activities of naturally occurring chalcones
Neurodegenerative ailments like Alzheimer’s disease (Author Anonymous, 2024), and Parkinsons’s disease (PD) are very common among the aged person due to neuronal death. Global data as of 2020 indicated that 9.4 million people worldwide have Parkinson’s disease, with 930,000 of those cases occurring in the United States alone. Germany and Japan, probably in the hundreds of thousands. According to information from the WHO, approximately 329,000 fatalities worldwide occurred as a result of Parkinson’s disease till 2021 (https://ourworldindata.org/grapher/deaths-from-parkinsons-disease-ghe#sources-and-processing, n.d.). However, 6.9 million Americans are thought to be suffering from AD. By 2060, it is projected that this data might rise to 13.8 million. AD government data indicates that 119,399 deaths have been reported (2024 Alzheimer’s disease facts and figures, 2024). AD is a progressive neurologic syndrome that causes brain atrophy and leads to progressive deterioration of cognitive functions and memory loss. PD, in turn triggered due to loss of dopaminergic neurons in the substantia nigra. These settings are found to be contributed by neuroinflammation and oxidative stress in neuronal structures (Kim et al., 2012). Therefore, the agents that can check oxidative stress and neuroinflammation, in general, have neuroprotective activity and can give therapeutic benefits against neurodegenerative diseases. Many natural chalcones and their derivatives were reported to have neuroprotective action. The polar surface areas of chalcones are tiny, which helps them pass through the blood-brain barrier (BBB) and act on the central nervous system (CNS). This characteristic is mostly related to the two aromatic nuclei of the A and B rings’ hydrophobic nature (Mathew et al., 2015). The most often recommended course of treatment for AD is acetylcholinesterase inhibitors (AChEIs). It has been discovered that AChEIs increase attention span and slow the disease’s progression. Chalcones exhibit promising anti-neuroinflammatory action (suppression of iNOS or initiation of Nrf2 signaling) and are prospective enzyme inhibitors (MAO B, COMT, AChE), α-synuclein imaging probes, and antagonists of adenosine A1 and/or A2A receptors. Chalcones offer a powerful neuroprotective approach by regulating neurotrophic, inflammatory, and oxidative pathways.
In vitro and in vivo models of gliosis and neurodegeneration have been done by Kim et al. to assess licochalcone E’s capability to trigger the Nrf2/ARE path (Kim et al., 2012). Licochalcone E inhibited the inflammatory reactions that LPS caused in BV2 cells and shielded neuronal SH-SY5Y cells (Figure 6) from 6-OHDA cytotoxicity (Table 8). Licochalcone E stimulates the Nrf2-ARE pathway and upregulates NQO1 and HO-1 in downstream (Figure 6). Accompanying the in vivo 1-methyl-4-phenyl-1.2,3,6-tetrahydropyridine (MPTP) animal model, licochalcone E’s cytoprotective effect and upregulation of HO-1 and NQO1 were displayed. A specific HO-1 or NQO1 inhibitor, and siRNA-mediated Nrf2-silencing cells, were used to validate Nrf2’s role in licochalcone E’s cytoprotective and anti-inflammatory activities.
TABLE 8
| Name of chalcone | Plant source | Smiles | Activity | Dose of drug | Target | Mechanism of action | References |
|---|---|---|---|---|---|---|---|

Licochalcone E |
Glycyrrhiza inflata Batalin (Family: Fabaceae) | OC1 = CC = C(C(/C=C/C2 = C(OC)C=C(O)C ([C@@H](C)C(C) = C) = C2OC) = O)C=C1 | It shows neuroprotective effect against dopaminergic neurodegeneration | 10 mg/kg | Chemical induce neurodegenerative C57BL6 mice | Initiation of Nrf2-antioxidant response Up-regulations of the NQO1 and HO-1 |
Kim et al. (2012) |
| Butein | Rhus vernciflua Stokes (Family: Anacardiaceae) | OC1 = CC = C(C(/C=C/C2 = CC = C(O)C(O) = C2) = O)C(O) = C1 | Inhibit neuroinflammation and production of NO. Memory enhancing effects |
IC50 = 10.9 ± 2.3 µM | LPS induced BV2 cells (Mouse microglia cell line) Scopolamine induced memory-impaired Male ICR (Harlan Sprague–Dawley) mice |
Suppression of COX-2 as well as iNOS expression Activation of cAMP responsive neurotrophic factor (BDNF) pathway |
Cho et al. (2013) |

2.2′,5′-trihydroxychalcone (225THC) |
Botanical drugs extracted chemical marketed by Indofine Chemical Company Inc. | O=C (/C=C/C1 = CC = CC = C1O)C2 = C(O)C=CC(O) = C2 | Exerts anti-apoptotic and anti-inflammatory activity in primary rat neuronal cell culture Also inhibits LPS-mediated secretion of IL-6, and TNF-α |
50 or 500 μM | Primary Cultures of Microglia from Sprague Dawley rat pups | Not reported clearly | Jiwrajka et al. (2016) |
| Chalcones from Ashitaba (ChA) | Angelica keiskei (Miq.) Koidz. (Family: Apiaceae) | Neuroprotective effect | 300 or 600 mg/kg | C57BL/6 mice | Attenuated the extent of demyelination in TNFα (corpus callosum and brain levels) | Rowhanirad and Taherianfard (2023) | |
| Licochalcone A | Not reported | OC1 = C(C(C) (C)C=C)C=C (/C=C/C(C2 = CC = C(O)C=C2) = O)C(OC) = C1 | Antioxidant and neuroprotective | IC50 = 42.28 ± 0.06 μM (inhibiting the butyrylcholinesterase) IC50 23.41 ± 0.02 μM (inhibiting acetylcholinesterase) |
Not reported | It showed potent antioxidant properties, and AChE/BChE inhibition activity | Budziak-Wieczorek et al. (2023) |
Plant source, doses, and neuroprotective activity of different natural chalcones.
The neuroprotective actions of natural chalcones are because of their capability to prevent the enzyme monoamine oxidases (MAOs), and cholinesterases, reverse neuroinflammation, and prevent neuronal apoptosis. It is a fact that elevated monoamine oxidase activity is related with the accumulation of ROS, depression, neurodegeneration, and hampered cognitive abilities (Naoi et al., 2012). Encoded by the MOA-B gene, this enzyme is known for its vital role in the catabolism of biogenic amines, including 2-phenylethylamine, benzylamine, and dopamine, in the CNS. Similarly, cholinesterases, that catalyze the breakdown of the neurotransmitter acetylcholine are involved in many neuronal diseases. The use of cholinesterase inhibitors remains one of the important approaches for the treatment of AD and many others (Larner, 2010). Therefore, MAOs and cholinesterases are therapeutic targets for treating many neuronal or brain health problems. Identifying chalcone compounds inactivating monoamine oxidase and cholinesterase can be helpful in developing pharmaceuticals for various neuronal disorders, such as PD and AD, managing stress, neuroinflammation, etc., (Cesura, 2007; Chimenti et al., 2009). Numerous natural chalcones and their derivatives have been described to have inhibitory action on MAO-A and -B, AChE, and butyrylcholinesterase (BChE) (Tanaka et al., 1987; HARAGUCHI et al., 2004; Kang et al., 2012; Robinson et al., 2013; Liu et al., 2014; Zhang et al., 2019).
Chalcones and their derivatives also exert neuroprotective activity in in vitro neuronal cell culture and prevent inflammation and apoptosis by inhibiting iNOS and COX-2 expression (Figure 7) (Cho et al., 2013; Jiwrajka et al., 2016). Cho and colleagues isolated and characterized by spectroscopic methods chalcone butein along with flavonoid fisetin from ethyl acetate part of Rhus verniciflua bark and assessed their neuroprotective and anti-inflammatory activities (Cho et al., 2013). To delve into this study, it has been reported that a chalcone, 2,2′,5′-trihydroxychalcone isolated from the botanical drugs extraction showed anti-inflammatory and anti-apoptotic action in neural cells in 50 or 500 μM concentration (Table 8). It also inhibits the secretion of TNF-α as well as IL-6 pro-inflammatory cytokines that are triggered by LPS (Jiwrajka et al., 2016). The authors attributed that reducing the expression of this gene caused in a less neurotoxic microglial phenotype, which could prove advantageous for certain neurodegenerative illnesses where abnormal microglial inflammatory reactivity is connected. In in vivo system, the application of chalcones prevents toxic chemical-induced neurodegeneration, upregulates the secretion of neurotrophic factors as well and enhances memory (Kim et al., 2012; Cho et al., 2013; Liu et al., 2013).
FIGURE 7
(a) Licochalcone E triggers the upregulated expression of NQO1 and HO-1 by regulating the Nrf2-ARE path to prevent neural inflammation. (b) Chalcones are associated with treating neurodegenerative diseases like depression by modulating the role of different signaling proteins, enzymes, and molecules, including NF-κB, STAT3, MMP-9, COX-2, and iNOS.
Chalcone butein exhibited strong neuroprotective properties towards neuroinflammation and neuronal death caused by glutamate and LPS. The strong inhibitory properties of butein in vitro have been attributed to the cytotoxicity of chalcone, which has an α, β-unsaturated double bond, a 4-OH group, and no C ring. One typical example of a disease related to the central nervous system is multiple sclerosis. Currently, cuprizone-induced multiple sclerosis in C57BL6 mice, a multiple sclerosis model, can be reduced by chalcones isolated from the Ashitaba. It was observed that these chalcones can diminish the expression of TNF-α in serum and the brain. Importantly, it improved the behavioral responses significantly (Table 8) (Rowhanirad and Taherianfard, 2023).
The plant-derived chalcone cardamonin is present in some plant species, including Alpinia conchigera and Alpinia katsumadai. In addition to reducing oxidative stress and regulating inflammatory responses, this plant-derived chalcone can regulate brain diseases. It's fascinating to note that it can alter the expression of NF-κB and STAT3. Furthermore, cardamonin can change the expression of certain enzymes, including MMP-9 and COX-2, as well as proteins associated with apoptosis, including Bcl-2 and cyclin D1 (Table 8). Significantly, the therapeutic aspect of neurodegeneration has also been documented to benefit from its modulatory effects on miRNA (Barber et al., 2023).
A recent study explored the role of natural chalcones including cardamonin, isobavachalcone, xanthohumol, 2′-hydroxy-4,4′,6′-trimethoxychalcone, and licochalcone A as a neurodegenerative disease protector. For the first time, these isolated natural chalcones were characterized based on a spectroscopic study to analyze the structural features with variable numbers and positions of -OH moieties in rings. To delve into the antioxidant and anti-neurodegenerative properties, 1,1- diphenyl-2-picrylhydrazyl was used as a free-radical scavenging reagent. Thus, licochalcone A inhibited butyrylcholinesterase (IC50 = 42.28 ± 0.06 μM) and acetylcholinesterase (IC50 = 23.41 ± 0.02 μM), demonstrating possible antioxidant action with a neuroprotective role (Table 8) (Budziak-Wieczorek et al., 2023). The actions of naturally occurring chalcones in terms of their neuroprotective ability have been listed in Table 8.
Chalcones, natural metabolites with neuroprotective properties, can inhibit enzymes like MAOs and cholinesterases, which are crucial in managing AD and PD. Studies have shown that chalcones activate protective pathways, reduce neuroinflammation, and prevent neuronal apoptosis. According to the SAR studies AChE inhibitory activity was increased upon the existence of electron-donating groups, whereas activity was decreased upon the existence of electron-withdrawing groups (Aslan et al., 2019). Furthermore, the presence of several functional moieties originating from -O and -N improved the overall inhibitory characteristics of AChE. Moreover, by regulating the expression of the cell death signal factor, chalcones with a prenyl moiety also showed neuroprotective properties.
Specific chalcones, such as licochalcone E, cardamonin, and butein, have demonstrated neuroprotective and anti-inflammatory activity, highlighting their significance as therapeutic agents for neurodegenerative disorders.
5 Marketed and clinically approved chalcones
Several chalcones, including sofalcone, metochalcone, and hesperidin methylchalcone, have been approved for clinical uses and usage in clinical settings (Cheng et al., 2020). To prevent Helicobacter pylori from advancing the condition, sofalcone (Figure 8) has been approved as an anti-ulcer drug that enhances prostaglandin in the mucosal area (Higuchi et al., 2010). Conversely, hesperidin methylchalcone (Figure 8) was employed in a clinical trial (Weindorf and Schultz-Ehrenburg, 1987; Gomes et al., 2017), to treat chronic venous lymphatic varicosis, and metochalcone (Figure 8) was approved to treat cholera infections (Sahun et al., 2012b). These chalcones are used in various clinical applications (Jandial DD et al., 2014; Gomes et al., 2017). They can also be altered by the addition of functional groups such as phenyl, halogens, hydroxyl, aryls, and carboxyl (Gomes et al., 2017) to enhance their target specificity when combined with other molecules in potential therapeutic applications. Furthermore, by hybridizing chalcone with other anti-cancer drugs, which helps to overcome the drug resistance of melanoma cells, chalcones are turning into a very important molecule for developing novel anti-cancer therapeutics.
FIGURE 8
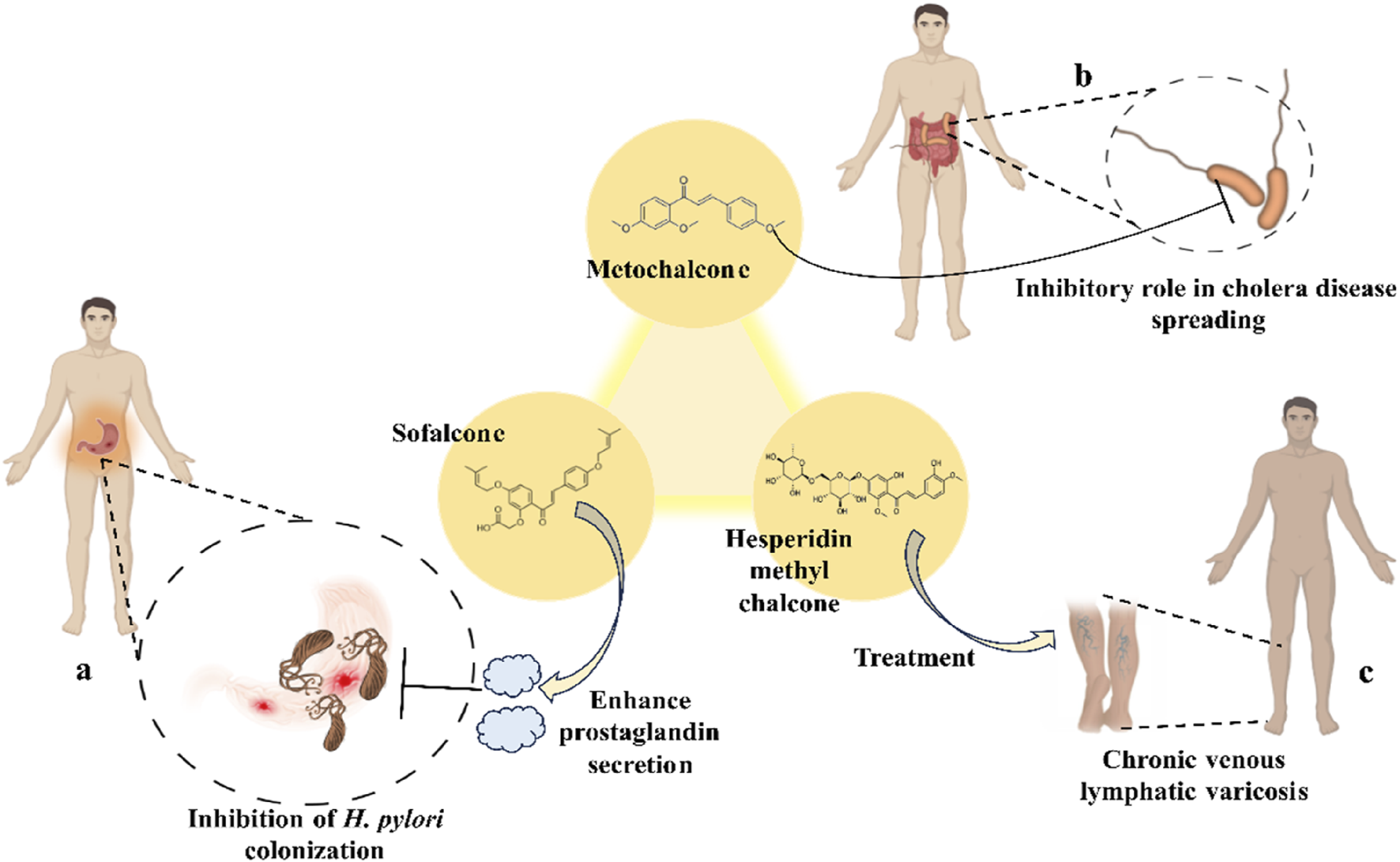
Role of marketed and clinically approved chalcones in different disease treatments. (a) Sofalcone can reduce the colonization of H. pylori by upregulating the expression of prostaglandin; (b) Metochalcone can also be a pharmacologically active reagent to treat cholera disease; (c) Hesperidin methyl chalcone has been applied clinically to treat chronic venous lymphatic varicosis.
6 Importance of chalcone as a traditional medicine over chemotherapy
Chalcone is an essential step in the flavonoid biosynthesis pathway, and plants containing chalcone have been utilized in traditional medicine since antiquity. The chemical structure of α,β-unsaturated ketones significantly influences pharmacological actions such as anti-cancer, antibacterial, immunosuppressive, and anti-inflammatory properties (Rudrapal et al., 2021). The privileged structure of a molecule is fundamental in medicinal chemistry, particularly in drug discovery. In this regard, chalcone is a typical simple scaffold as a natural form that can be utilized to synthesize a large number of derivatives. However, its conjugated structure with electron pulling and pushing functional groups on the benzene rings, can also be employed in imaging-based disease diagnosis (Zhuang et al., 2017c). It was also discovered that chalcone derivatives can be easily synthesized using various synthetic methods. These synthetic analogs have been demonstrated to have bioactivities to their natural counterparts, but with increased potency and lower toxicity (Jasim et al., 2021). On the other hand, synthetic compounds used for different medicinal purposes can have a variety of side effects, some of which are severe. As a result, chalcone-based conventional therapies may be a promising area of medicinal chemistry based on its unique structural feature, pharmacological activities, and less toxic characteristics.
7 Critical finding in chalcone-based drug designing
Chalcone is a highly effective plant-derived natural chemical due to its structural properties, which may be easily modified to produce a wide range of derivatives against various diseases such as bacterial infection, inflammatory diseases, cancer, neurological disorders, and so on. It was also shown that various plant-based chalcone derivatives can target cellular signaling pathways to modify metabolic activities, hence improving therapeutic efficacy. However, rigorous study has revealed that balancing the safety and efficacy of chalcone-based medicines is equally vital. In this connection, it was discovered that chalcone-based derivatives in plants can likewise produce the required effects without hurting the primary organs (Muller and Milton, 2012). However, this metabolite may also pose a hurdle because it has demonstrated distinct therapeutic windows in a number of in vitro and in vivo studies. Another crucial result that should be taken seriously is the contribution of multidrug resistance via chalcone derivatives in cancer cells when efflux pumps are overexpressed (Hba et al., 2023a). To investigate this context, it was first discovered that various possible chalcones have been employed as modulators of resistance to conventional medications by targeting P-glycoproteins (multidrug efflux transporters) (Bois et al., 1998; Parveen et al., 2014; Ngo et al., 2016), and multidrug resistance protein 1 (Nguyen et al., 2003; Lindamulage et al., 2017). However, these transporters contribute significantly to multidrug resistance in cancer cells by accumulating medicines (Xiao et al., 2021).
8 Limitations and problems raised in chalcone-based drug designing
In drug design, various constraints and issues that arise in the form of unwanted qualities such as local irritations, toxicity, short half-life, poor absorption, and, most critically, chemical instability and low water solubility. It has also been noted that some medications can be discovered in an inactive state that undergoes in vivo biotransformation through enzyme activity, facilitating drug accumulation at the site of action (Rautio et al., 2008; Jornada et al., 2015). In this regard, it was determined that some chalcone derivatives may work as good anti-inflammatory drugs by neutralizing CXCL12 (CXC motif chemokine ligand 12), preventing it from acting on CXCR4 and CXCR7 receptors. However, its decreased solubility in water, it cannot function adequately (Hachet-Haas et al., 2008). On the other hand, in drug synthesis, employing chalcone as a scaffold, green synthesis is becoming a significant feature in reducing drug toxicity. However, due to its low solubility in water, water cannot be used as a green solvent to synthesis chalcone derivatives, which is another limitation (Marotta et al., 2022).
9 Future perspective
Overall, chalcones’ complex role in treating various diseases provides an opportunity for more investigation into them during the drug discovery process. However, it has numerous obstacles, which have been addressed in the limitations and critical assessment of chalcone-based drug design. When it comes to their administration as possible medicinal agents, chalcones’ poor solubility and the extent of their dissolution in the gastrointestinal tract pose serious challenges (Hba et al., 2023b). These limitations can be changed with hydrophilic polymers, which improve the plant-derived compounds’ solubility, bioavailability, and pharmacokinetics in many therapeutic applications.
Additionally, to fully utilize chalcones’ medicinal potential and incorporate them into robust pharmaceutical formulations, their poor solubility, stability, and toxicity must be resolved. Nanoparticles (NPs) are a new delivery method for chalcones that researchers have recently begun using. The numerous advantages that NPs provide improve the solubility, effectiveness, and possible uses of chalcones in drug delivery, making them a fascinating and attractive field for pharmaceutical study. Future research should concentrate on enhancing chalcone solubility, figuring out the best therapeutic dosages, identifying resistance mechanisms, and investigating combination treatments that incorporate chalcones and chalcone-based nanoparticles. To realize the full therapeutic potential of chalcone-based pharmaceuticals and enhance human health outcomes, more investigation is needed into their mechanisms of action and optimization.
10 Conclusion
In conclusion, the various bioactive chalcones extracted from the different parts of plants display an extensive range of pharmacological properties, for example, anti-inflammatory, antiviral, anti-cancer, and antidiabetic properties. Researchers are becoming more interested in chalcones for developing pharmacological substances because of their superior bioavailability and high tolerance in the body. Numerous chalcones found in nature have demonstrated one or more pharmacological properties. Additionally, we provided an overview of the SAR studies and discussed the pharmacological potential of the various chalcones isolated from the plants. Chalcones are unique, versatile scaffolds that have undoubtedly demonstrated great promise in medicinal chemistry. Some chalcones have superior activity than conventional medications and may eventually be introduced to the market as novel drugs. These substances might act as lead compounds in the development of novel drugs. Licochalcones, for instance, has shown substantial potential in inhibiting the growth of protozoan species responsible for leishmaniasis and malaria and demonstrating anti-cancer and antimicrobial activities. Moreover, chalcones like isoliquiritigenin and xanthohumol have been identified as potent inhibitors of multiple signaling pathways related to cancer progression and inflammation, with notable effects on angiogenesis, apoptosis, and enzymatic activities related to tumor growth.
Plant-derived natural chalcone compounds exhibit significant potential for managing diabetes and its related complications and neurodegenerative diseases. Their antidiabetic effects are primarily attributed to their inhibitory roles in key enzymes and pathways related to glucose metabolism, such as aldose reductase, α-amylase, and α-glucosidase. Additionally, natural chalcones have shown promising neuroprotective activities by targeting enzymes like monoamine oxidases and cholinesterases, which are crucial for developing neurodegenerative disorders, for example, Alzheimer’s and Parkinson’s diseases.
This thorough analysis delves deeply into the medicinal chemistry of natural chalcones and their therapeutic potential. Chalcones have a variety of modes of action and show promise in pharmacological activities, as evidenced by the results of multiple investigations. This review underscores the therapeutic potential of naturally occurring chalcone compounds in developing novel therapeutics for treating various diseases, leveraging their capability to modulate key biological pathways. Although experimental studies have demonstrated numerous pharmacological activities of chalcones, additional comprehensive research investigations are necessary to address the toxicological and pharmacokinetic concerns, especially in preclinical and clinical studies.
Statements
Author contributions
SA: Conceptualization, Investigation, Project administration, Writing – original draft, Writing – review and editing. PN: Methodology, Writing – original draft. VD: Methodology, Writing – original draft. ND: Formal Analysis, Methodology, Writing – original draft. AB: Writing – review and editing. SP: Writing – review and editing. AD: Investigation, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- FDA
US Food and Drug Administration
- 1H-NMR
Proton Nuclear Magnetic Resonance
- SAR
Structure-activity Relationship
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate
- NaOH
Sodium hydroxide
- KOH
Potassium hydroxide
- NaOEt
Sodium Ethoxide
- PdCl2
Palladium(II) Chloride
- Na2CO3
Sodium Carbonate
- CeCO3
Cerium Cobalt
- Pd(OAc)2
Palladium(II) Acetate
- Ph3P
Triphenylphosphine
- K2CO3
Potassium Carbonate
- DMF
Dimethylformamide
- CO
Carbon Monoxide
- PdCl2(PPh3)2
bis(triphenylphosphine)palladium(II) dichloride
- THF
Tetrahydrofuran
- AlCl3
Aluminium Chloride
- N2
Nitrogen Gas
- Ba(OH)2
Barium Hydroxide
- Ca(OH)2
Calcium Hydroxide
- Sr(OH)2
Strontium Hydroxide
- CaO
Calcium Oxide
- NaH
Sodium hydride
- LiHMDS
Lithium Hexamethyldisilazide
- LiOH
Lithium Hydroxide
- HCl
Hydrogen Chloride
- BF3-Et2O
Boron Trifluoride Diethyl Etherate
- SOCl2
Thionyl chloride
- p-TsOH
para-Toluenesulfonic Acid
- CH3CN
Acetonitrile
- CuI
copper(I) iodide
- DBU
1,8-Diazabicyclo[5.4.0]undec-7-ene
- DCM
Dichloromethane
- CHCl3
Chloroform
- 13C NMR
Carbon-13 Nuclear Magnetic Resonance
- TNF-α
Tumor Necrosis Factor-α
- IL-1β
Interleukin-1 β
- IL-6
Interleukin-6
- MIC
Minimum Inhibitory Concentration
- HRMS
High-Resolution Mass Spectrometry
- NorA
A multidrug efflux pump expressed in methicillin-resistant Staphylococcus aureus (MRSA)
- MDR
Multidrug Resistance
- bc1 complex
The cytochrome bc1 complex
- Complex II
Complex II or succinate dehydrogenase
- P. yoelii YM
A clone of rodent malaria parasite Plasmodium yoelii
- FRD
Fumarate Reductase
- BCG
Bacillus Calmette-Guerin
- IC50
Half-maximal Inhibitory Concentration
- BVDV
Bovine Viral Diarrhea Virus
- HCV
Hepatitis C Virus
- HSV
Herpes Simplex Virus
- HIV
Human Immunodeficiency Viruses
- RSV
Rous Sarcoma Virus
- YFV
Yellow Fever Virus
- PBMC
Peripheral Blood Mononuclear Cell
- TI
Therapeutic Index
- NA
Neuraminidase
- H1N1
Type-1 hemagglutinin (H) protein and a type-1 neuraminidase (N) protein
- WT
Wild Type
- HMB
2-hydroxy-3-methyl-3-butenyl alkyl
- HSQC
Heteronuclear Single-Quantum Correlation Spectroscopy
- HMBC
Heteronuclear Multiple Bond Correlation
- COSY
Correlation Spectroscopy
- ROESY
Rotating Frame Overhauser Effect Spectroscopy
- NCDs
Noncommunicable Diseases
- DNA
Deoxyribonucleic acid
- Rb
Retinoblastoma
- TRAIL
Tumor Necrosis Factor-related Apoptosis-inducing Ligand
- HDACs
Histone Deacetylase Enzymes
- NF-κB
Nuclear Factor Kappa B
- EGFR
Epidermal Growth Factor Receptor
- MMPs
Matrix Metalloproteinases
- VEGF
Vascular Endothelial Growth Factor
- ERK
Extracellular Signal-regulated Kinase
- P13-K/Akt
Phosphoinositide-3-kinase–protein Kinase B
- MAPKs
Mitogen-activated Protein Kinases
- HUVECs
Human Umbilical Vein Endothelial Cells
- HIF-1α
Hypoxia-inducible Factor-1α
- TUNEL
Terminal Deoxynucleotidyl Transferase dUTP nick-end labeling
- HCC
Hepatocellular Carcinoma
- CCA
Cholangiocarcinoma
- DRI Values
Dietary Reference Intake Values
- ATM
Ataxia Telangiectasia Mutated
- CRC
Colorectal Carcinoma
- CDKs
Cyclin-dependent Kinases
- NSCLC
Nonsmall Cell Lung Carcinoma
- JNK
Jun N-terminal Kinase
- c-IAP
Cellular Inhibitor of Apoptosis Protein
- XIAP
X-linked Inhibitor of Apoptosis Protein
- c-FLIPL
Cellular FLICE (FADD-like Il-1β-converting enzyme)-inhibitory Protein
- RIP1
Receptor Interacting Protein-1
- Glut1
Glucose Transporter 1
- PDK1
Phosphoinositide-dependent Kinase 1
- Ras
Rat sarcoma
- Raf
Rapidly Accelerated Fibrosarcoma
- MEK or MAPKK
Mitogen-activated Protein Kinase Kinase
- PD-L1
Programmed Cell Death Ligand 1
- GADD153
Growth Arrest and DNA Damage-inducible Gene 153
- MEKC
Micellar Electrokinetic Chromatography
- ROS
reactive Oxygen Species
- p-mTOR
Phosphorylated Mammalian Target of Rapamycin
- Akt/PKB
Protein kinase B, a serine/threonine protein kinase that regulates many cellular functions
- P70S6K
p70 Ribosomal Protein S6 Kinase
- PI3K
Phosphatidylinositol 3-kinase
- BCL2
B-cell Lymphoma 2, a protein important for cell survival and apoptosis
- NHEM
Normal Human Epidermal Melanocytes
- NHDF
Normal Human Dermal Fibroblasts
- PARP
Poly (ADP-ribose) Polymerase
- DMC
2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone
- 5-FU
5-fluorouracil
- p-GSK3β
Phospho-glycogen Synthase Kinase 3 β
- CH2Cl2
Dichloromethane
- HTMC
2′-hydroxy-2,3,4′,6′-tetramethoxychalcone
- EAC
Ehrlich Ascites Carcinoma
- TNBC
Triple-negative Breast Cancer
- DDC
2′,4-dihydroxy-4′,6′-dimethoxy-chalcone
- FOXO3a
Forkhead Box Class O 3a
- PUMA
p53 Upregulated Modulator of Apoptosis
- Bax
Bcl-2 Associated X Protein
- LC3
Microtubule-associated Protein 1A/1 B-light Chain 3
- Wnt
Wingless and Int-1
- Nrf2
Nuclear Factor Erythroid 2-related Factor 2
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- FT-IR
Fourier Transform Infrared Spectroscopy
- DEPT
Distortionless Enhancement by Polarization Transfer
- HMQC
Heteronuclear Multiple-Quantum Coherence
- MALDI-TOF MS
Matrix-assisted Laser Desorption Ionization time-of-flight Mass Spectrometry
- PGE2
Prostaglandin E2
- NO
Nitric Oxide
- FABMS
Fast Atom Bombardment Mass Spectroscopy
- HRFABMS
High-resolution Fast Atom Bombardment Mass Spectroscopy
- iNOS
Inducible Nitric Oxide Synthase
- COX
Cyclooxygenase
- LPS
Lipopolysaccharide
- GSH
Glutathione
- HO-1
Heme Oxygenase-1
- NQO1
NAD(P)H Quinone Oxidoreductase 1
- ICAM-1
Intercellular Adhesion Molecule-1
- VCAM-1
Vascular Cell Adhesion Molecule-1
- MS
Mass Spectrometry
- Nrf2-ARE
Nrf2-antioxidant Response Element
- FRAP
Ferric Reducing Antioxidant Power
- ABTS
2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid
- NGAL
Neutrophil Gelatinase-associated Lipocalin
- IFN
Interferon
- Keap1
Kelch-like ECH-associated protein 1
- CFA
Complete Freund’s Adjuvant
- CCK
Cholecystokinin
- TMBC
2,4,2′,4′-Tetrahydroxy-3-(3-methyl-2-butenyl)-chalcone
- EIMS
Electron Ionization Mass Spectroscopy
- HREIMS
High Resolution Electron Ionization Mass Spectroscopy
- CYP1A
Cytochrome P450 1A
- TrxR
Thioredoxin Reductase
- QR
Quinone Reductase
- HR-ESI-MS
High-resolution Electrospray Ionization Mass Spectrometry
- GPx
Glutathione Peroxidase
- PSTK
Phosphoseryl-tRNA Kinase
- CVDs
Cardiovascular Diseases
- SNPs
Single Nucleotide Polymorphisms
- CRP
c - reactive protein
- TGF-β
Transforming Growth Factor- β
- LPL
Lipoprotein Lipase
- PL
Pancreatic Lipase
- ACE
Angiotensin-converting Enzyme
- ACAT
Acyl-coenzyme A cholesterol acyltransferase
- DGAT
Diacylglycerol Acyltransferase
- CETP
Cholesteryl Ester Transfer Protein
- TXA2
Thromboxane A2
- TXB2
Thromboxane B2
- LDL
Low-density Lipoprotein
- SHRSP
Stroke-prone Spontaneously Hypertensive Rats
- VLDL
Very-low-density Lipoprotein
- LDL-R
LDL-receptor
- PPAR-α
Peroxisome Proliferator Activated Receptor-α
- ACO
Acyl-coenzyme A Oxidase
- ACS
Acyl-CoA Synthetase
- LDH
Lactate Dehydrogenase
- CPK
Creatinine Phosphokinase
- JAK/STAT
Janus Kinase/Signal Transducer and Activator of Transcription
- MT
Metallothionein
- HFD
High-fat Diet
- UCP-1
Uncoupling Protein 1
- ANGPTL3
Angiopoietin-like Protein-3
- AA
Arachidonic Acid
- SGLT2
Sodium Glucose Cotransporter 2
- PPAR-γ
Peroxisome Proliferator-activated Receptor-gamma
- PTP1B
Protein Tyrosine Phosphatase 1B
- DPP-4
Dipeptidyl Peptidase 4
- AMPK
Adenosine Monophosphate (AMP)-activated Protein Kinase
- AGE
Advanced Glycation End-products
- HRAR
Human Recombinant Aldose Reductase
- RLAR
Rat Lens Aldose Reductase
- AD
Alzheimer’s Disease
- PD
Parkinsons’s Disease
- BBB
Blood-brain Barrier
- CNS
Central Nervous System
- AChE
Acetylcholinesterase
- AChEIs
Acetylcholinesterase Inhibitors
- MPTP
1-methyl-4-phenyl-1.2,3,6-tetrahydropyridine
- MAOs
Monoamine Oxidases
- BChE
Butyrylcholinesterase
References
1
Adelusi T. I. Du L. Chowdhury A. Xiaoke G. Lu Q. Yin X. (2021). Signaling pathways and proteins targeted by antidiabetic chalcones. Life Sci.284, 118982. 10.1016/j.lfs.2020.118982
2
Adhikari S. Bhattacharjee T. Butcher R. J. Porchia M. De Franco M. Marzano C. et al (2019). Synthesis and characterization of mixed-ligand Zn(II) and Cu(II) complexes including polyamines and dicyano-dithiolate(2-): in vitro cytotoxic activity of Cu(II) compounds. Inorganica Chim. Acta498, 119098. 10.1016/j.ica.2019.119098
3
Adhikari S. Bhattacharjee T. Gupta R. Daniliuc C.-G. Montazerozohori M. Naghiha R. et al (2020). Coordination framework of cadmium(II), harvested from dithiolate-imidazole binary ligand systems: crystal structure, Hirshfeld surface analysis, antibacterial, and DNA cleavage potential. Polyhedron192, 114838. 10.1016/j.poly.2020.114838
4
Adhikari S. Nath P. Das A. Datta A. Baildya N. Duttaroy A. K. et al (2024a). A review on metal complexes and its anti-cancer activities: recent updates from in vivo studies. Biomed. and Pharmacother.171, 116211. 10.1016/j.biopha.2024.116211
5
Adhikari S. Nath S. Kansız S. Balidya N. Paul A. K. Dege N. et al (2024b). Zinc(II) coordination compound with N′-(pyridin-2-ylmethylene)nicotinohydrazide: synthesis, crystal structure, computational and cytotoxicity studies. J. Inorg. Biochem.257, 112598. 10.1016/j.jinorgbio.2024.112598
6
Adhikari S. Nath S. Sen T. Raza R. Sahin O. Eftekhari-Sis B. et al (2025). A novel tetrazole–1,8-naphthyridine–amide hybrid: first structurally characterized tetrazolo[1,5-a]-derivative of naphthyridines with a luminescence activity, potency against COVID-19, and anticancer activity. J. Mol. Struct.1321, 139803. 10.1016/j.molstruc.2024.139803
7
Akihisa T. Tokuda H. Ukiya M. Iizuka M. Schneider S. Ogasawara K. et al (2003). Chalcones, coumarins, and flavanones from the exudate of Angelica keiskei and their chemopreventive effects. Cancer Lett.201, 133–137. 10.1016/S0304-3835(03)00466-X
8
Akinfenwa A. O. Abdul N. S. Marnewick J. L. Hussein A. A. (2021). Protective effects of linearthin and other chalcone derivatives from Aspalathus linearis (rooibos) against UVB induced oxidative stress and toxicity in human skin cells. Plants10, 1936. 10.3390/plants10091936
9
An W. Yang J. Ao Y. (2006). Metallothionein mediates cardioprotection of isoliquiritigenin against ischemia-reperfusion through JAK2/STAT3 activation. Acta Pharmacol. Sin.27, 1431–1437. 10.1111/j.1745-7254.2006.00419.x
10
Aoki N. Muko M. Ohta E. Ohta S. (2008). C -geranylated chalcones from the stems of angelica keiskei with superoxide-scavenging activity. J. Nat. Prod.71, 1308–1310. 10.1021/np800187f
11
Apaza Ticona L. Thiebaut Estrada C. Rumbero Sánchez Á. (2021). Inhibition of melanin production and tyrosinase activity by flavonoids isolated from Loranthus acutifolius. Nat. Prod. Res.35, 4690–4693. 10.1080/14786419.2019.1709185
12
Ara I. Turcio R. Islam T. Hossain Md. S. Hasan Md. K. (2024). Anti-aging related activities and health benefits of licochalcone A: a review. Clin. Complementary Med. Pharmacol.4, 100125. 10.1016/j.ccmp.2023.100125
13
Aslan H. E. Demir Y. Özaslan M. S. Türkan F. Beydemir Ş. Küfrevioğlu Ö. I. (2019). The behavior of some chalcones on acetylcholinesterase and carbonic anhydrase activity. Drug Chem. Toxicol.42, 634–640. 10.1080/01480545.2018.1463242
14
Asma S. T. Acaroz U. Imre K. Morar A. Shah S. R. A. Hussain S. Z. et al (2022). Natural products/bioactive compounds as a source of anticancer drugs. Cancers (Basel)14, 6203. 10.3390/cancers14246203
15
Author Anonymous (2024). Alzheimer’s disease facts and figures. Alzheimer’s and Dementia20, 3708–3821. 10.1002/alz.13809
16
Avila-Villarreal G. Hernández-Abreu O. Hidalgo-Figueroa S. Navarrete-Vázquez G. Escalante-Erosa F. Peña-Rodríguez L. M. et al (2013). Antihypertensive and vasorelaxant effects of dihydrospinochalcone-A isolated from Lonchocarpus xuul Lundell by NO production: computational and ex vivo approaches. Phytomedicine20, 1241–1246. 10.1016/j.phymed.2013.06.011
17
Ban H. S. Suzuki K. Lim S. S. Jung S. H. Lee S. Ji J. et al (2004). Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase and tumor necrosis factor-α by 2′-hydroxychalcone derivatives in RAW 264.7 cells. Biochem. Pharmacol.67, 1549–1557. 10.1016/j.bcp.2003.12.016
18
Barber K. Mendonca P. Soliman K. F. A. (2023). The neuroprotective effects and therapeutic potential of the chalcone cardamonin for Alzheimer’s disease. Brain Sci.13, 145. 10.3390/brainsci13010145
19
Batovska D. Todorova I. (2010). Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol.5, 1–29. 10.2174/157488410790410579
20
Belofsky G. Percivill D. Lewis K. Tegos G. P. Ekart J. (2004). Phenolic metabolites of daleaversicolor that enhance antibiotic activity against model pathogenic bacteria. J. Nat. Prod.67, 481–484. 10.1021/np030409c
21
Benetou V. Lagiou A. Lagiou P. (2015). Chemoprevention of cancer: current evidence and future prospects. F1000Res4, 916. 10.12688/f1000research.6684.1
22
Berning L. Scharf L. Aplak E. Stucki D. von Montfort C. Reichert A. S. et al (2019). In vitro selective cytotoxicity of the dietary chalcone cardamonin (CD) on melanoma compared to healthy cells is mediated by apoptosis. PLoS One14, e0222267. 10.1371/journal.pone.0222267
23
Bhakuni D. S. Chaturvedi R. (1984). Chemical constituents of Crotalaria madurensis. J. Nat. Prod.47, 585–591. 10.1021/np50034a003
24
Bhalla V. K. Nayak U. R. Dev S. (1968). Some new flavonoids from. Tetrahedron Lett.9, 2401–2406. 10.1016/S0040-4039(00)76141-7
25
Bhattacharjee T. Adhikari S. Sheikh A. H. Mahmoudi G. Mlowe S. Akerman M. P. et al (2022). Syntheses, crystal structures, theoretical studies, and anticancer properties of an unsymmetrical schiff base ligand N-2-(6-methylpyridyl)-2-hydroxy-1-naphthaldimine and its Ni(II) complex. J. Mol. Struct.1269, 133717. 10.1016/j.molstruc.2022.133717
26
Bhattacharjee T. Nath S. Baildya N. Das A. Pathak S. Molins E. et al (2024). Supramolecular assemblies of Zn(II) complex based on dithiolate-amine binary ligands: synthesis, crystal structure, Hirshfeld surface, DFT, molecular docking, and anticancer studies. Inorg. Chem. Commun.167, 112762. 10.1016/j.inoche.2024.112762
27
Birari R. B. Gupta S. Mohan C. G. Bhutani K. K. (2011). Antiobesity and lipid lowering effects of Glycyrrhiza chalcones: experimental and computational studies. Phytomedicine18, 795–801. 10.1016/j.phymed.2011.01.002
28
Bois F. Beney C. Boumendjel A. Mariotte A.-M. Conseil G. Di Pietro A. (1998). Halogenated chalcones with high-affinity binding to P-glycoprotein: potential modulators of multidrug resistance. J. Med. Chem.41, 4161–4164. 10.1021/jm9810194
29
Borges-Argáez R. Balnbury L. Flowers A. Giménez-Turba A. Ruiz G. Waterman P. G. et al (2007). Cytotoxic and antiprotozoal activity of flavonoids from Lonchocarpus spp. Phytomedicine14, 530–533. 10.1016/j.phymed.2006.11.027
30
Boyle J. L. Haupt H. M. Stern J. B. Multhaupt H. A. B. (2002). Tyrosinase expression in malignant melanoma, desmoplastic melanoma, and peripheral nerve tumors. Arch. Pathol. Lab. Med.126, 816–822. 10.1043/0003-9985(2002)126<0816:TEIMMD>2.0.CO;2
31
Bremner P. Meyer J. (1998). Pinocembrin chalcone: an antibacterial compound from Helichrysum trilineatum. Planta Med.64, 777. 10.1055/s-2006-957585
32
Brennführer A. Neumann H. Beller M. (2009). Palladium‐catalyzed carbonylation reactions of aryl halides and related compounds. Angew. Chem. Int. Ed.48, 4114–4133. 10.1002/anie.200900013
33
Bruyère C. Genovese S. Lallemand B. Ionescu-Motatu A. Curini M. Kiss R. et al (2011). Growth inhibitory activities of oxyprenylated and non-prenylated naturally occurring phenylpropanoids in cancer cell lines. Bioorg Med. Chem. Lett.21, 4174–4179. 10.1016/j.bmcl.2011.05.089
34
Buckwold V. Wilson R. Nalca A. Beer B. Voss T. Turpin J. et al (2004). Antiviral activity of hop constituents against a series of DNA and RNA viruses. Antivir. Res.61, 57–62. 10.1016/S0166-3542(03)00155-4
35
Budziak I. Arczewska M. Kamiński D. M. (2020). Structure and physical properties of cardamonin: a spectroscopic and computational approach. Molecules25, 4070. 10.3390/molecules25184070
36
Budziak-Wieczorek I. Kamiński D. Skrzypek A. Ciołek A. Skrzypek T. Janik-Zabrotowicz E. et al (2023). Naturally occurring chalcones with aggregation-induced emission enhancement characteristics. Molecules28, 3412. 10.3390/molecules28083412
37
Bukhari S. N. A. Jasamai M. Jantan I. Ahmad W. (2013). Review of methods and various catalysts used for chalcone synthesis. Mini Rev. Org. Chem.10, 73–83.
38
Bumagin N. A. Korolev D. N. (1999). Synthesis of unsymmetric ketones via ligandless Pd-catalyzed reaction of acyl chlorides with organoboranes. Tetrahedron Lett.40, 3057–3060. 10.1016/S0040-4039(99)00364-0
39
Bussmann A. J. C. Zaninelli T. H. Saraiva-Santos T. Fattori V. Guazelli C. F. S. Bertozzi M. M. et al (2022). The flavonoid hesperidin methyl chalcone targets cytokines and oxidative stress to reduce diclofenac-induced acute renal injury: contribution of the Nrf2 redox-sensitive pathway. Antioxidants11, 1261. 10.3390/antiox11071261
40
Cesura A. M. (2007). “Monoamine oxidase B,” in xPharm: the comprehensive pharmacology reference (Elsevier), 1–10. 10.1016/B978-008055232-3.60499-4
41
Chen M. Christensen S. B. Blom J. Lemmich E. Nadelmann L. Fich K. et al (1993). Licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species of Leishmania. Antimicrob. Agents Chemother.37, 2550–2556. 10.1128/AAC.37.12.2550
42
Chen M. Theander T. G. Christensen S. B. Hviid L. Zhai L. Kharazmi A. (1994). Licochalcone A, a new antimalarial agent, inhibits in vitro growth of the human malaria parasite Plasmodium falciparum and protects mice from P. yoelii infection. Antimicrob. Agents Chemother.38, 1470–1475. 10.1128/AAC.38.7.1470
43
Chen X. Liu Z. Meng R. Shi C. Guo N. (2017). Antioxidative and anticancer properties of Licochalcone A from licorice. J. Ethnopharmacol.198, 331–337. 10.1016/j.jep.2017.01.028
44
Chen Y.-F. Wu S.-N. Gao J.-M. Liao Z.-Y. Tseng Y.-T. Fülöp F. et al (2020). The antioxidant, anti-inflammatory, and neuroprotective properties of the synthetic chalcone derivative AN07. Molecules25, 2907. 10.3390/molecules25122907
45
Cheng P. Yang L. Huang X. Wang X. Gong M. (2020). Chalcone hybrids and their antimalarial activity. Arch. Pharm. Weinh.353, e1900350. 10.1002/ardp.201900350
46
Cheng Z.-J. Lin C.-N. Hwang T.-L. Teng C.-M. (2001). Broussochalcone A, a potent antioxidant and effective suppressor of inducible nitric oxide synthase in lipopolysaccharide-activated macrophages11Abbreviations: NF-κB, nuclear factor-κB; NO, nitric oxide; NOS, nitric oxide synthase; iNOS, inducible NOS; BCA, broussochalcone A; BHT, butylated hydroxytoluene; DPPH, diphenyl-2-picrylhydrazyl; L-NAME, NG-nitro-l-arginine methyl ester; LPS, lipopolysaccharide; MDA, malondialdyhyde; PDTC, pyrrolidine dithiocarbamate; TBARS. Biochem. Pharmacol.61, 939–946. 10.1016/S0006-2952(01)00543-3
47
Chimenti F. Fioravanti R. Bolasco A. Chimenti P. Secci D. Rossi F. et al (2009). Chalcones: a valid scaffold for monoamine oxidases inhibitors. J. Med. Chem.52, 2818–2824. 10.1021/jm801590u
48
Cho N. Lee K. Y. Huh J. Choi J. H. Yang H. Jeong E. J. et al (2013). Cognitive-enhancing effects of Rhus verniciflua bark extract and its active flavonoids with neuroprotective and anti-inflammatory activities. Food Chem. Toxicol.58, 355–361. 10.1016/j.fct.2013.05.007
49
Christensen S. Ming C. Andersen L. Hjørne U. Olsen C. Cornett C. et al (1994). An antileishmanial chalcone from Chinese licorice roots. Planta Med.60, 121–123. 10.1055/s-2006-959431
50
Chumsri S. Howes T. Bao T. Sabnis G. Brodie A. (2011). Aromatase, aromatase inhibitors, and breast cancer. J. Steroid Biochem. Mol. Biol.125, 13–22. 10.1016/j.jsbmb.2011.02.001
51
Cuendet M. Oteham C. P. Moon R. C. Pezzuto J. M. (2006). Quinone reductase induction as a biomarker for cancer chemoprevention. J. Nat. Prod.69, 460–463. 10.1021/np050362q
52
Cui Y. Taniguchi S. Kuroda T. Hatano T. (2015). Constituents of Psoralea corylifolia fruits and their effects on methicillin-resistant Staphylococcus aureus. Molecules20, 12500–12511. 10.3390/molecules200712500
53
Daikonya A. Katsuki S. Kitanaka S. (2004). Antiallergic agents from natural sources 9. Inhibition of nitric oxide production by novel chalcone derivatives from Mallotus philippinensis (euphorbiaceae). Chem. Pharm. Bull. (Tokyo)52, 1326–1329. 10.1248/cpb.52.1326
54
Dao T. T. Nguyen P. H. Lee H. S. Kim E. Park J. Lim S. I. et al (2011). Chalcones as novel influenza A (H1N1) neuraminidase inhibitors from Glycyrrhiza inflata. Bioorg Med. Chem. Lett.21, 294–298. 10.1016/j.bmcl.2010.11.016
55
Das A. Adhikari S. Deka D. Baildya N. Sahare P. Banerjee A. et al (2023a). An updated review on the role of nanoformulated phytochemicals in colorectal cancer. Med. (B Aires)59, 685. 10.3390/medicina59040685
56
Das A. Adhikari S. Deka D. Bisgin A. Paul S. Balidya N. et al (2023b). An updated review on recent advances in the usage of novel therapeutic peptides for breast cancer treatment. Int. J. Pept. Res. Ther.29, 32. 10.1007/s10989-023-10503-8
57
Das A. Deka D. Baildya N. Banerjee A. Bisgin A. Adhikari S. et al (2023c). BMAP-27 peptide reduces proliferation and increases apoptosis in primary and metastatic colon cancer cell lines. Int. J. Pept. Res. Ther.29, 100. 10.1007/s10989-023-10572-9
58
Das M. Manna K. (2016). Chalcone scaffold in anticancer armamentarium: a molecular insight. J. Toxicol.2016, 7651047. 10.1155/2016/7651047
59
Deb V. K. Rabbani A. Majid S. Abushouk A. Khan J. Al K. B. et al (2022). Role of single nucleotide polymorphisms of inflammatory molecules in susceptibility to cardiovascular diseases (CVDs). 1st Edition. Boca Raton, FL: CRC Press.
60
Debnath P. Deb V. K. Nath S. Guha A. Adhikari S. (2024). “Structure-activity relationship of plant-derived bioactive compounds in anti-cancer therapy,” in Plant derived bioactive compounds in human health and disease (Boca Raton: CRC Press), 66–94. 10.1201/9781003486237-5
61
Deng N. Qiao M. Li Y. Liang F. Li J. Liu Y. (2023). Anticancer effects of licochalcones: a review of the mechanisms. Front. Pharmacol.14, 1074506. 10.3389/fphar.2023.1074506
62
De Spirt S. Eckers A. Wehrend C. Micoogullari M. Sies H. Stahl W. et al (2016). Interplay between the chalcone cardamonin and selenium in the biosynthesis of Nrf2-regulated antioxidant enzymes in intestinal Caco-2 cells. Free Radic. Biol. Med.91, 164–171. 10.1016/j.freeradbiomed.2015.12.011
63
Dhar D. N. (1981). The chemistry of chalcones and related compounds. Wiley.
64
Eddarir S. Cotelle N. Bakkour Y. Rolando C. (2003). An efficient synthesis of chalcones based on the Suzuki reaction. Tetrahedron Lett.44, 5359–5363. 10.1016/S0040-4039(03)01140-7
65
El-Helw E. A. Alzahrani A. Y. Ramadan S. K. (2024). Synthesis and antimicrobial activity of thiophene-based heterocycles derived from thiophene-2-carbohydrazide. Future Med. Chem.16, 439–451. 10.4155/fmc-2023-0304
66
Elias D. W. Beazely M. A. Kandepu N. M. (1999). Bioactivities of chalcones. Curr. Med. Chem.6, 1125–1149. 10.2174/0929867306666220401182509
67
ElSohly H. N. Joshi A. S. Nimrod A. C. Walker L. A. Clark A. M. (2001). Antifungal chalcones from maclura tinctoria. Planta Med.67, 87–89. 10.1055/s-2001-10621
68
Enoki T. Ohnogi H. Nagamine K. Kudo Y. Sugiyama K. Tanabe M. et al (2007). Antidiabetic activities of chalcones isolated from a Japanese herb, angelica keiskei. J. Agric. Food Chem.55, 6013–6017. 10.1021/jf070720q
69
Federation I. D. (2021). IDF diabetes atlas. 10th Edition.
70
Forejtníková H. Lunerová K. Kubínová R. Jankovská D. Marek R. Kareš R. et al (2005). Chemoprotective and toxic potentials of synthetic and natural chalcones and dihydrochalcones in vitro. Toxicology208, 81–93. 10.1016/j.tox.2004.11.011
71
Friis-Møller A. Chen M. Fuursted K. Christensen S. B. Kharazmi A. (2002). In vitro antimycobacterial and antilegionella activity of licochalcone A from Chinese licorice roots. Planta Med.68, 416–419. 10.1055/s-2002-32087
72
Frölich S. Schubert C. Bienzle U. Jenett-Siems K. (2005). In vitro antiplasmodial activity of prenylated chalcone derivatives of hops (Humulus lupulus) and their interaction with haemin. J. Antimicrob. Chemother.55, 883–887. 10.1093/jac/dki099
73
Fu Y. Hsieh T. Guo J. Kunicki J. Lee M. Y. W. T. Darzynkiewicz Z. et al (2004). Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem. Biophys. Res. Commun.322, 263–270. 10.1016/j.bbrc.2004.07.094
74
Gabriela N. Rosa A. M. Catiana Z. I. Soledad C. Mabel O. R. Esteban S. J. et al (2014). The effect of Zuccagnia punctata, an Argentine medicinal plant, on vcirulence factors from Candida species. Nat. Prod. Commun.9, 933–936. 10.1177/1934578X1400900712
75
Gao W.-Y. Chen P.-Y. Hsu H.-J. Liou J.-W. Wu C.-L. Wu M.-J. et al (2024). Xanthohumol, a prenylated chalcone, regulates lipid metabolism by modulating the LXRα/RXR-ANGPTL3-LPL axis in hepatic cell lines and high-fat diet-fed zebrafish models. Biomed. and Pharmacother.174, 116598. 10.1016/j.biopha.2024.116598
76
Gaonkar S. L. Vignesh U. N. (2017). Synthesis and pharmacological properties of chalcones: a review. Res. Chem. Intermed.43, 6043–6077. 10.1007/s11164-017-2977-5
77
Garcia A. R. Oliveira D. M. P. Jesus J. B. Souza A. M. T. Sodero A. C. R. Vermelho A. B. et al (2021). Identification of chalcone derivatives as inhibitors of leishmania infantum arginase and promising antileishmanial agents. Front. Chem.8, 624678. 10.3389/fchem.2020.624678
78
Gaur R. Yadav K. S. Verma R. K. Yadav N. P. Bhakuni R. S. (2014). In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine21, 415–422. 10.1016/j.phymed.2013.10.015
79
Go M. Wu X. Liu X. (2005). Chalcones: an update on cytotoxic and chemoprotective properties. Curr. Med. Chem.12, 481–499. 10.2174/0929867053363153
80
Gomes M. Muratov E. Pereira M. Peixoto J. Rosseto L. Cravo P. et al (2017). Chalcone derivatives: promising starting points for drug design. Molecules22, 1210. 10.3390/molecules22081210
81
Goshain O. Ahmed B. (2019). Antihypertensive activity, toxicity and molecular docking study of newly synthesized xanthon derivatives (xanthonoxypropanolamine). PLoS One14, e0220920. 10.1371/journal.pone.0220920
82
Gu X. Shi Y. Chen X. Sun Z. Luo W. Hu X. et al (2020). Isoliquiritigenin attenuates diabetic cardiomyopathy via inhibition of hyperglycemia-induced inflammatory response and oxidative stress. Phytomedicine78, 153319. 10.1016/j.phymed.2020.153319
83
Guazelli C. F. S. Fattori V. Ferraz C. R. Borghi S. M. Casagrande R. Baracat M. M. et al (2021). Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem. Biol. Interact.333, 109315. 10.1016/j.cbi.2020.109315
84
Hachet-Haas M. Balabanian K. Rohmer F. Pons F. Franchet C. Lecat S. et al (2008). Small neutralizing molecules to inhibit actions of the chemokine CXCL12. J. Biol. Chem.283, 23189–23199. 10.1074/jbc.M803947200
85
Haddach M. McCarthy J. R. (1999). A new method for the synthesis of ketones: the palladium-catalyzed cross-coupling of acid chlorides with arylboronic acids. Tetrahedron Lett.40, 3109–3112. 10.1016/S0040-4039(99)00476-1
86
Han A.-R. Kang Y.-J. Windono T. Lee S. K. Seo E.-K. (2006). Prenylated flavonoids from the heartwood of Artocarpus communis with inhibitory activity on lipopolysaccharide-induced nitric oxide production. J. Nat. Prod.69, 719–721. 10.1021/np0600346
87
Hanif N. Iswantini D. Hioki Y. Murni A. Kita M. Tanaka J. (2022). Flavokawains, plant-derived chalcones, inhibit differentiation of murine pre-adipocytes. Chem. Lett.51, 54–57. 10.1246/cl.210615
88
Haraguchi H. Ishikawa H. Mizutani K. Tamura Y. Kinoshita T. (1998a). Antioxidative and superoxide scavenging activities of retrochalcones in Glycyrrhiza inflata. Bioorg Med. Chem.6, 339–347. 10.1016/S0968-0896(97)10034-7
89
Haraguchi H. Tanimoto K. Tamura Y. Mizutani K. Kinoshita T. (1998b). Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry48, 125–129. 10.1016/S0031-9422(97)01105-9
90
Haraguchi H. Tanaka Y. Kabbash A. Fujioka T. Ishizu T. Yagi A. (2004). Monoamine oxidase inhibitors from Gentiana lutea. Phytochemistry65, 2255–2260. 10.1016/j.phytochem.2004.06.025
91
Hatziieremia S. Gray A. I. Ferro V. A. Paul A. Plevin R. (2006). The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NFkappaB signalling pathways in monocytes/macrophages. Br. J. Pharmacol.149, 188–198. 10.1038/sj.bjp.0706856
92
Hba S. Ghaddar S. Wahnou H. Pinon A. El Kebbaj R. Pouget C. et al (2023a). Natural chalcones and derivatives in colon cancer: pre-clinical challenges and the promise of chalcone-based nanoparticles. Pharmaceutics15, 2718. 10.3390/pharmaceutics15122718
93
Hba S. Ghaddar S. Wahnou H. Pinon A. El Kebbaj R. Pouget C. et al (2023b). Natural chalcones and derivatives in colon cancer: pre-clinical challenges and the promise of chalcone-based nanoparticles. Pharmaceutics15, 2718. 10.3390/pharmaceutics15122718
94
Higuchi K. Watanabe T. Tanigawa T. Tominaga K. Fujiwara Y. Arakawa T. (2010). Sofalcone, a gastroprotective drug, promotes gastric ulcer healing following eradication therapy for Helicobacter pylori: a randomized controlled comparative trial with cimetidine, an H2 ‐receptor antagonist. J. Gastroenterol. Hepatol.25, S155–S160. 10.1111/j.1440-1746.2010.06232.x
95
Hird M. Toyne K. J. Gray G. W. (1993). Palladium-catalysed cross-coupling reactions in the synthesis of some high polarizability materials. Liq. Cryst.14, 741–761. 10.1080/02678299308027752
96
Ikuta K. S. Swetschinski L. R. Aguilar G. R. Sharara F. Mestrovic T. Gray A. P. et al (2022). Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet400, 2221–2248. 10.1016/S0140-6736(22)02185-7
97
Iwata S. Nagata N. Omae A. Yamaguchi S. Okada Y. Shibata S. et al (1999). Inhibitory effect of chalcone derivatives on recombinant human aldose reductase. Biol. Pharm. Bull.22, 323–325. 10.1248/bpb.22.323
98
Izumi Y. Matsumura A. Wakita S. Akagi K. Fukuda H. Kume T. et al (2012). Isolation, identification, and biological evaluation of Nrf2-ARE activator from the leaves of green perilla (Perilla frutescens var. crispa f. viridis). Free Radic. Biol. Med.53, 669–679. 10.1016/j.freeradbiomed.2012.06.021
99
Jabbar Z. Alamgeer A. Ullah A. Mahmoud M. H. Batiha G. E. et al (2024). Trans -chalcone (1–3-diphenyl-2-propen-1-one) as a therapeutic candidate in joint inflammation via reduction of TNF-α, IL-1β, IL-6, and IL-17 in rodents: an in vivo study by RT-PCR and ELISA analysis. ACS Omega9, 22123–22135. 10.1021/acsomega.4c00368
100
Jandial D. D. Blair C. A. Zhang S. Krill L. S. Zhang Y. B. Zi X. (2014). Molecular targeted approaches to cancer therapy and prevention using chalcones. Curr. Cancer Drug Targets14, 181–200. 10.2174/1568009614666140122160515
101
Jasim H. A. Nahar L. Jasim M. A. Moore S. A. Ritchie K. J. Sarker S. D. (2021). Chalcones: synthetic chemistry follows where nature leads. Biomolecules11, 1203. 10.3390/biom11081203
102
Jayasinghe L. Balasooriya B. A. I. S. Padmini W. C. Hara N. Fujimoto Y. (2004). Geranyl chalcone derivatives with antifungal and radical scavenging properties from the leaves of Artocarpus nobilis. Phytochemistry65, 1287–1290. 10.1016/j.phytochem.2004.03.033
103
Jez J. M. Noel J. P. (2000). Mechanism of chalcone synthase. pKa of the catalytic cysteine and the role of the conserved histidine in a plant polyketide synthase. J. Biol. Chem.275, 39640–39646. 10.1074/jbc.M008569200
104
Ji X. Wei X. Qian J. Mo X. Kai G. An F. et al (2019). 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone induced apoptosis and G1 cell cycle arrest through PI3K/AKT pathway in BEL-7402/5-FU cells. Food Chem. Toxicol.131, 110533. 10.1016/j.fct.2019.05.041
105
Jiang C.-H. Sun T.-L. Xiang D.-X. Wei S.-S. Li W.-Q. (2018). Anticancer activity and mechanism of xanthohumol: a prenylated flavonoid from hops (Humulus lupulus L.). Front. Pharmacol.9, 530. 10.3389/fphar.2018.00530
106
Jiwrajka M. Phillips A. Butler M. Rossi M. Pocock J. M. (2016). The plant‐derived chalcone 2,2′,5′‐trihydroxychalcone provides neuroprotection against toll‐like receptor 4 triggered inflammation in microglia. Oxid. Med. Cell Longev.2016, 6301712. 10.1155/2016/6301712
107
Johnson T. O. Ermolieff J. Jirousek M. R. (2002). Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat. Rev. Drug Discov.1, 696–709. 10.1038/nrd895
108
Jornada D. Dos Santos Fernandes G. Chiba D. De Melo T. Dos Santos J. Chung M. (2015). The prodrug approach: a successful tool for improving drug solubility. Molecules21, 42. 10.3390/molecules21010042
109
Jung H. A. Yoon N. Y. Kang S. S. Kim Y. S. Choi J. S. (2010). Inhibitory activities of prenylated flavonoids from Sophora flavescens against aldose reductase and generation of advanced glycation endproducts. J. Pharm. Pharmacol.60, 1227–1236. 10.1211/jpp.60.9.0016
110
Kaddah M. M. Fahmi A. A. Kamel M. M. Ramadan S. K. Rizk S. A. (2021). Synthesis, characterization, computational chemical studies and antiproliferative activity of some heterocyclic systems derived from 3-(3-(1,3-diphenyl-1H -pyrazol-4-yl)acryloyl)-2H -chromen-2-one. Synth. Commun.51, 1798–1813. 10.1080/00397911.2021.1904991
111
Kahyo T. Ichikawa S. Hatanaka T. Yamada M. K. Setou M. (2008). A novel chalcone polyphenol inhibits the deacetylase activity of SIRT1 and cell growth in HEK293T cells. J. Pharmacol. Sci.108, 364–371. 10.1254/jphs.08203FP
112
Kamal A. Kashi Reddy M. Viswanath A. (2013). The design and development of imidazothiazole–chalcone derivatives as potential anticancer drugs. Expert Opin. Drug Discov.8, 289–304. 10.1517/17460441.2013.758630
113
Kang D. G. Lee A. S. Mun Y. J. Woo W. H. Kim Y. C. Sohn E. J. et al (2004). Butein ameliorates renal concentrating ability in cisplatin-induced acute renal failure in rats. Biol. Pharm. Bull.27, 366–370. 10.1248/bpb.27.366
114
Kang J. Cho J. Curtis-Long M. Ryu H. Kim J. Kim H. et al (2012). Inhibitory evaluation of sulfonamide chalcones on β-secretase and acylcholinesterase. Molecules18, 140–153. 10.3390/molecules18010140
115
Karthikeyan C. Narayana Moorthy N. S. H. Ramasamy S. Vanam U. Manivannan E. Karunagaran D. et al (2014). Advances in chalcones with anticancer activities. Recent Pat. Anticancer Drug Discov.10, 97–115. 10.2174/1574892809666140819153902
116
Khan J. Deb P. K. Priya S. Medina K. D. Devi R. Walode S. G. et al (2021). Dietary flavonoids: cardioprotective potential with antioxidant effects and their pharmacokinetic, toxicological and therapeutic concerns. Molecules26, 4021. 10.3390/molecules26134021
117
Kharb R. Haider K. Neha K. Yar M. S. (2020). Aromatase inhibitors: role in postmenopausal breast cancer. Arch. Pharm. Weinh.353, e2000081. 10.1002/ardp.202000081
118
Kim H. I. Schultz C. R. Buras A. L. Friedman E. Fedorko A. Seamon L. et al (2017). Ornithine decarboxylase as a therapeutic target for endometrial cancer. PLoS One12, e0189044. 10.1371/journal.pone.0189044
119
Kim S. J. Miyoshi Y. Taguchi T. Tamaki Y. Nakamura H. Yodoi J. et al (2005). High thioredoxin expression is associated with resistance to docetaxel in primary breast cancer. Clin. Cancer Res.11, 8425–8430. 10.1158/1078-0432.CCR-05-0449
120
Kim S. S. Lim J. Bang Y. Gal J. Lee S.-U. Cho Y.-C. et al (2012). Licochalcone E activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: therapeutic relevance to neurodegenerative disease. J. Nutr. Biochem.23, 1314–1323. 10.1016/j.jnutbio.2011.07.012
121
Ko H. Kim Y. Amor E. C. Lee J. W. Kim H. Kim H. J. et al (2011). Induction of autophagy by dimethyl cardamonin is associated with proliferative arrest in human colorectal carcinoma HCT116 and LOVO cells. J. Cell Biochem.112, 2471–2479. 10.1002/jcb.23171
122
Kocarnik J. M. Compton K. Dean F. E. Fu W. Gaw B. L. Harvey J. D. et al (2022). Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol.8, 420–444. 10.1001/jamaoncol.2021.6987
123
Kulkarni R. R. Tupe S. G. Gample S. P. Chandgude M. G. Sarkar D. Deshpande M. V. et al (2014). Antifungal dimeric chalcone derivative kamalachalcone E from Mallotus philippinensis. Nat. Prod. Res.28, 245–250. 10.1080/14786419.2013.843178
124
Kumar A. Sharma S. Tripathi V. D. Srivastava S. (2010). Synthesis of chalcones and flavanones using Julia–Kocienski olefination. Tetrahedron66, 9445–9449. 10.1016/j.tet.2010.09.089
125
Kumar D. Kumar M. Kumar A. Singh S. (2013). Chalcone and curcumin derivatives: a way ahead for malarial treatment. Mini-Reviews Med. Chem.13, 2116–2133. 10.2174/13895575113136660101
126
Kumar H. Devaraji V. Joshi R. Jadhao M. Ahirkar P. Prasath R. et al (2015). Antihypertensive activity of a quinoline appended chalcone derivative and its site specific binding interaction with a relevant target carrier protein. RSC Adv.5, 65496–65513. 10.1039/C5RA08778C
127
Kuo Y.-F. Su Y.-Z. Tseng Y.-H. Wang S.-Y. Wang H.-M. Chueh P. J. (2010). Flavokawain B, a novel chalcone from Alpinia pricei Hayata with potent apoptotic activity: involvement of ROS and GADD153 upstream of mitochondria-dependent apoptosis in HCT116 cells. Free Radic. Biol. Med.49, 214–226. 10.1016/j.freeradbiomed.2010.04.005
128
Larner A. (2010). Cholinesterase inhibitors: beyond Alzheimer’s disease. Expert Rev. Neurother.10, 1699–1705. 10.1586/ern.10.105
129
Le Bail J.-C. Pouget C. Fagnere C. Basly J.-P. Chulia A.-J. Habrioux G. (2001). Chalcones are potent inhibitors of aromatase and 17beta-hydroxysteroid dehydrogenase activities. Life Sci.68, 751–761. 10.1016/S0024-3205(00)00974-7
130
Lee H. E. Yang G. Han S.-H. Lee J.-H. An T.-J. Jang J.-K. et al (2018). Anti-obesity potential of Glycyrrhiza uralensis and licochalcone A through induction of adipocyte browning. Biochem. Biophys. Res. Commun.503, 2117–2123. 10.1016/j.bbrc.2018.07.168
131
Lee I.-S. Lim J. Gal J. Kang J. C. Kim H. J. Kang B. Y. et al (2011). Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem. Int.58, 153–160. 10.1016/j.neuint.2010.11.008
132
Lee J.-C. Lee K.-Y. Kim J. Na C.-S. Jung N.-C. Chung G.-H. et al (2004). Extract from Rhus verniciflua Stokes is capable of inhibiting the growth of human lymphoma cells. Food Chem. Toxicol.42, 1383–1388. 10.1016/j.fct.2004.03.012
133
Lee J.-H. Jung H. S. Giang P. M. Jin X. Lee S. Son P. T. et al (2006). Blockade of nuclear factor-kappaB signaling pathway and anti-inflammatory activity of cardamomin, a chalcone analog from Alpinia conchigera. J. Pharmacol. Exp. Ther.316, 271–278. 10.1124/jpet.105.092486
134
Leon-Gonzalez A. Acero N. Muñoz-Mingarro D. Navarro I. Martín-Cordero C. (2015). Chalcones as promising lead compounds on cancer therapy. Curr. Med. Chem.22, 3407–3425. 10.2174/0929867322666150729114829
135
Li D. Wang Z. Chen H. Wang J. Zheng Q. Shang J. et al (2009). Isoliquiritigenin induces monocytic differentiation of HL-60 cells. Free Radic. Biol. Med.46, 731–736. 10.1016/j.freeradbiomed.2008.11.011
136
Li M.-T. Xie L. Jiang H.-M. Huang Q. Tong R.-S. Li X. et al (2022a). Role of licochalcone A in potential pharmacological therapy: a review. Front. Pharmacol.13, 878776. 10.3389/fphar.2022.878776
137
Li X. Jin L. Ma Y. Jiang Z. Tang H. Tong X. (2022b). Xanthohumol inhibits non-small cell lung cancer by activating PUMA-mediated apoptosis. Toxicology470, 153141. 10.1016/j.tox.2022.153141
138
Li Y. Qin Y. Yang C. Zhang H. Li Y. Wu B. et al (2017). Cardamonin induces ROS-mediated G2/M phase arrest and apoptosis through inhibition of NF-κB pathway in nasopharyngeal carcinoma. Cell Death Dis.8, e3024. 10.1038/cddis.2017.407
139
Lindamulage I. K. Vu H.-Y. Karthikeyan C. Knockleby J. Lee Y.-F. Trivedi P. et al (2017). Novel quinolone chalcones targeting colchicine-binding pocket kill multidrug-resistant cancer cells by inhibiting tubulin activity and MRP1 function. Sci. Rep.7, 10298. 10.1038/s41598-017-10972-0
140
Lindstrom M. DeCleene N. Dorsey H. Fuster V. Johnson C. O. LeGrand K. E. et al (2022). Global burden of cardiovascular diseases and risks collaboration, 1990-2021. J. Am. Coll. Cardiol.80, 2372–2425. 10.1016/j.jacc.2022.11.001
141
Liou C.-J. Lee Y.-K. Ting N.-C. Chen Y.-L. Shen S.-C. Wu S.-J. et al (2019). Protective effects of licochalcone A ameliorates obesity and non-alcoholic fatty liver disease via promotion of the sirt-1/AMPK pathway in mice fed a high-fat diet. Cells8, 447. 10.3390/cells8050447
142
Liou G.-Y. Storz P. (2010). Reactive oxygen species in cancer. Free Radic. Res.44, 479–496. 10.3109/10715761003667554
143
Liu H. Liu X. Fan H. Tang J. Gao X. Liu W.-K. (2014a). Design, synthesis and pharmacological evaluation of chalcone derivatives as acetylcholinesterase inhibitors. Bioorg Med. Chem.22, 6124–6133. 10.1016/j.bmc.2014.08.033
144
Liu L. Chen X. Hu Z. (2007). Separation and determination of alpinetin and cardamonin in Alpinia katsumadai Hayata by flow injection–micellar electrokinetic chromatography. Talanta71, 155–159. 10.1016/j.talanta.2006.03.032
145
Liu M. Yin H. Liu G. Dong J. Qian Z. Miao J. (2014b). Xanthohumol, a prenylated chalcone from beer hops, acts as an α-glucosidase inhibitor in vitro. J. Agric. Food Chem.62, 5548–5554. 10.1021/jf500426z
146
Liu X. Xing Y. Li M. Zhang Z. Wang J. Ri M. et al (2021). Licochalcone A inhibits proliferation and promotes apoptosis of colon cancer cell by targeting programmed cell death-ligand 1 via the NF-κB and Ras/Raf/MEK pathways. J. Ethnopharmacol.273, 113989. 10.1016/j.jep.2021.113989
147
Liu Y. Lian Z. Zhu H. Wang Y. Yu S. Chen T. et al (2013). A systematic, integrated study on the neuroprotective effects of hydroxysafflor Yellow A revealed by H1 NMR-based metabonomics and the NF-κB pathway. Evidence-Based Complementary Altern. Med.2013, 1–14. 10.1155/2013/147362
148
Lu J. Holmgren A. (2014). The thioredoxin antioxidant system. Free Radic. Biol. Med.66, 75–87. 10.1016/j.freeradbiomed.2013.07.036
149
Luo W. Sun R. Chen X. Li J. Jiang J. He Y. et al (2021). ERK activation-mediated autophagy induction resists licochalcone A-induced anticancer activities in lung cancer cells in vitro. Onco Targets Ther.13, 13437–13450. 10.2147/OTT.S278268
150
Luyengi L. Lee I.-S. Mar W. Fong H. H. S. Pezzuto J. M. Kinghorn A. D. (1994). Rotenoids and chalcones from Mundulea sericea that inhibit phorbol ester-induced ornithine decarboxylase activity. Phytochemistry36, 1523–1526. 10.1016/S0031-9422(00)89755-1
151
Maccari R. Ottanà R. (2015). Targeting aldose reductase for the treatment of diabetes complications and inflammatory diseases: new insights and future directions. J. Med. Chem.58, 2047–2067. 10.1021/jm500907a
152
Mahapatra D. K. Asati V. Bharti S. K. (2015a). Chalcones and their therapeutic targets for the management of diabetes: structural and pharmacological perspectives. Eur. J. Med. Chem.92, 839–865. 10.1016/j.ejmech.2015.01.051
153
Mahapatra D. K. Bharti S. K. (2016). Therapeutic potential of chalcones as cardiovascular agents. Life Sci.148, 154–172. 10.1016/j.lfs.2016.02.048
154
Mahapatra D. K. Bharti S. K. Asati V. (2015b). Anti-cancer chalcones: structural and molecular target perspectives. Eur. J. Med. Chem.98, 69–114. 10.1016/j.ejmech.2015.05.004
155
Mahapatra D. K. Bharti S. K. Asati V. Singh S. K. (2019). Perspectives of medicinally privileged chalcone based metal coordination compounds for biomedical applications. Eur. J. Med. Chem.174, 142–158. 10.1016/j.ejmech.2019.04.032
156
Mahapatra D. K. Chhajed S. S. Shivhare R. S. (2017). Development of Murrayanine-Chalcone hybrids: an effort to combine two privilege scaffolds for enhancing hypoglycemic activity. Int. J. Pharm. Chem. Analysis4, 30–34. 10.18231/2394-2797.2017.0008
157
Mahmood D. F. D. Abderrazak A. El Hadri K. Simmet T. Rouis M. (2013). The thioredoxin system as a therapeutic target in human health and disease. Antioxid. Redox Signal19, 1266–1303. 10.1089/ars.2012.4757
158
Mai V.-H. Ponce-Zea J. E. Doan T.-P. Vu Q. H. Ryu B. Lee C.-H. et al (2024). Chalcone-monoterpene derivatives from the buds of Cleistocalyx operculatus and their potential as protein tyrosine phosphatase 1B inhibitors. J. Nat. Prod.87, 1903–1913. 10.1021/acs.jnatprod.4c00249
159
Maisto M. Marzocchi A. Keivani N. Piccolo V. Summa V. Tenore G. C. (2023). Natural chalcones for the management of obesity disease. Int. J. Mol. Sci.24, 15929. 10.3390/ijms242115929
160
Maity S. Deb V. K. Mondal S. Chakraborty A. Pramanick K. Adhikari S. (2025). Leveraging supramolecular systems in biomedical breakthroughs. BioFactors51, e70005. 10.1002/biof.70005
161
Marotta L. Rossi S. Ibba R. Brogi S. Calderone V. Butini S. et al (2022). The green chemistry of chalcones: valuable sources of privileged core structures for drug discovery. Front. Chem.10, 988376. 10.3389/fchem.2022.988376
162
Martinez R. M. Pinho-Ribeiro F. A. Steffen V. S. Caviglione C. V. Vignoli J. A. Baracat M. M. et al (2015). Hesperidin methyl chalcone inhibits oxidative stress and inflammation in a mouse model of ultraviolet B irradiation-induced skin damage. J. Photochem Photobiol. B148, 145–153. 10.1016/j.jphotobiol.2015.03.030
163
Mathew B. Mathew G. E. Uçar G. Baysal I. Suresh J. Vilapurathu J. K. et al (2015). Development of fluorinated methoxylated chalcones as selective monoamine oxidase-B inhibitors: synthesis, biochemistry and molecular docking studies. Bioorg Chem.62, 22–29. 10.1016/j.bioorg.2015.07.001
164
Matos M. J. Vazquez-Rodriguez S. Uriarte E. Santana L. (2015). Potential pharmacological uses of chalcones: a patent review (from June 2011 – 2014). Expert Opin. Ther. Pat.25, 351–366. 10.1517/13543776.2014.995627
165
Maurya A. Agrawal A. (2024). Recent advancement in bioactive chalcone hybrids as potential antimicrobial agents in medicinal chemistry. Mini-Reviews Med. Chem.24, 176–195. 10.2174/1389557523666230727102606
166
Mazumder R. Ghosh A. Deb S. Ghosh R. (2024). Significance of chalcone scaffolds in medicinal chemistry. Top. Curr. Chem.382, 22. 10.1007/s41061-024-00468-7
167
Mendez-Callejas G. Piñeros-Avila M. Celis C. A. Torrenegra R. Espinosa-Benitez A. Pestana-Nobles R. et al (2024). Natural 2′,4-Dihydroxy-4′,6′-dimethoxy chalcone isolated from Chromolaena tacotana inhibits breast cancer cell growth through autophagy and mitochondrial apoptosis. Plants13, 570. 10.3390/plants13050570
168
Mendez-Callejas G. Piñeros-Avila M. Yosa-Reyes J. Pestana-Nobles R. Torrenegra R. Camargo-Ubate M. F. et al (2023). A novel tri-hydroxy-methylated chalcone isolated from Chromolaena tacotana with anti-cancer potential targeting pro-survival proteins. Int. J. Mol. Sci.24, 15185. 10.3390/ijms242015185
169
Mi‐Ichi F. Miyadera H. Kobayashi T. Takamiya S. Waki S. Iwata S. et al (2005). Parasite mitochondria as a target of chemotherapy: inhibitory effect of licochalcone A on the Plasmodium falciparum respiratory chain. Ann. N. Y. Acad. Sci.1056, 46–54. 10.1196/annals.1352.037
170
Miranda C. L. Aponso G. L. M. Stevens J. F. Deinzer M. L. Buhler D. R. (2000a). Prenylated chalcones and flavanones as inducers of quinone reductase in mouse Hepa 1c1c7 cells. Cancer Lett.149, 21–29. 10.1016/S0304-3835(99)00328-6
171
Miranda C. L. Stevens J. F. Helmrich A. Henderson M. C. Rodriguez R. J. Yang Y.-H. et al (1999). Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem. Toxicol.37, 271–285. 10.1016/S0278-6915(99)00019-8
172
Miranda C. L. Stevens J. F. Ivanov V. McCall M. Frei B. Deinzer M. L. et al (2000b). Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. J. Agric. Food Chem.48, 3876–3884. 10.1021/jf0002995
173
Mojzis J. Varinska L. Mojzisova G. Kostova I. Mirossay L. (2008). Antiangiogenic effects of flavonoids and chalcones. Pharmacol. Res.57, 259–265. 10.1016/j.phrs.2008.02.005
174
Monteiro R. Faria A. Azevedo I. Calhau C. (2007). Modulation of breast cancer cell survival by aromatase inhibiting hop (Humulus lupulus L.) flavonoids. J. Steroid Biochem. Mol. Biol.105, 124–130. 10.1016/j.jsbmb.2006.11.026
175
Morisseau C. Du G. Newman J. W. Hammock B. D. (1998). Mechanism of mammalian soluble epoxide hydrolase inhibition by chalcone oxide derivatives. Arch. Biochem. Biophys.356, 214–228. 10.1006/abbi.1998.0756
176
Muller P. Y. Milton M. N. (2012). The determination and interpretation of the therapeutic index in drug development. Nat. Rev. Drug Discov.11, 751–761. 10.1038/nrd3801
177
Naoi M. Maruyama W. Inaba-Hasegawa K. (2012). Type A and B monoamine oxidase in age-related neurodegenerative disorders: their distinct roles in neuronal death and survival. Curr. Top. Med. Chem.12, 2177–2188. 10.2174/156802612805219950
178
Narender T. Gupta S. (2004). A convenient and biogenetic type synthesis of few naturally occurring chromeno dihydrochalcones and their in vitro antileishmanial activity. Bioorg Med. Chem. Lett.14, 3913–3916. 10.1016/j.bmcl.2004.05.071
179
Narender T. Tanvir K. Srinivasa Rao M. Srivastava K. Puri S. K. (2005). Prenylated chalcones isolated from Crotalaria genus inhibits in vitro growth of the human malaria parasite Plasmodium falciparum. Bioorg Med. Chem. Lett.15, 2453–2455. 10.1016/j.bmcl.2005.03.081
180
Nasir Abbas Bukhari S. Jasamai M. Jantan I. (2012). Synthesis and biological evaluation of chalcone derivatives (mini review). Mini Rev. Med. Chem.12, 1394–1403. 10.2174/13895575112091394
181
Nath P. Datta A. Adhikari S. (2022). “Recent advances of metal-based anticancer agents and their in vivo potential against various types of malignancies,” in Handbook of animal models and its uses in cancer research (Singapore: Springer Nature Singapore), 1–28. 10.1007/978-981-19-1282-5_47-1
182
Nath P. Datta A. Sen T. Adhikari S. (2024). “Emergence of metal-based anticancer therapeutics: a promising perspective,” in Biomarkers in cancer detection and monitoring of therapeutics (Elsevier), 411–450. 10.1016/B978-0-323-95114-2.00012-1
183
Nath S. Sen T. Roy S. Baildya N. Borah P. Kaminsky W. et al (2025). Supramolecular Co (II) complex fabricated from adenine derivative: synthesis, crystal structure, hirshfeld surface, DFT optimization, anticancer, and molecular docking studies. Appl. Organomet. Chem.39. 10.1002/aoc.7846
184
Nawaz J. Rasul A. Shah M. A. Hussain G. Riaz A. Sarfraz I. et al (2020). Cardamonin: a new player to fight cancer via multiple cancer signaling pathways. Life Sci.250, 117591. 10.1016/j.lfs.2020.117591
185
Nerya O. Musa R. Khatib S. Tamir S. Vaya J. (2004a). Chalcones as potent tyrosinase inhibitors: the effect of hydroxyl positions and numbers. Phytochemistry65, 1389–1395. 10.1016/j.phytochem.2004.04.016
186
Nerya O. Musa R. Khatib S. Tamir S. Vaya J. (2004b). Chalcones as potent tyrosinase inhibitors: the effect of hydroxyl positions and numbers. Phytochemistry65, 1389–1395. 10.1016/j.phytochem.2004.04.016
187
Nerya O. Vaya J. Musa R. Izrael S. Ben-Arie R. Tamir S. (2003). Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J. Agric. Food Chem.51, 1201–1207. 10.1021/jf020935u
188
Newman D. J. Cragg G. M. (2016). Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod.79, 629–661. 10.1021/acs.jnatprod.5b01055
189
Ngo T.-D. Tran T.-D. Le M.-T. Thai K.-M. (2016). Computational predictive models for P-glycoprotein inhibition of in-house chalcone derivatives and drug-bank compounds. Mol. Divers20, 945–961. 10.1007/s11030-016-9688-5
190
Nguyen H. Zhang S. Morris M. E. (2003). Effect of flavonoids on MRP1-mediated transport in panc-1 cells. J. Pharm. Sci.92, 250–257. 10.1002/jps.10283
191
Nielsen A. T. Houlihan W. J. (2011a). “The aldol condensation,” in Organic reactions (Wiley), 1–438. 10.1002/0471264180.or016.01
192
Nielsen A. T. Houlihan W. J. (2011b). “The aldol condensation,” in Organic reactions (Wiley), 1–438. 10.1002/0471264180.or016.01
193
Nigam M. Mishra A. P. Deb V. K. Dimri D. B. Tiwari V. Bungau S. G. et al (2023). Evaluation of the association of chronic inflammation and cancer: insights and implications. Biomed. and Pharmacother.164, 115015. 10.1016/j.biopha.2023.115015
194
Nowakowska Z. (2007a). A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem.42, 125–137. 10.1016/j.ejmech.2006.09.019
195
Nowakowska Z. (2007b). A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem.42, 125–137. 10.1016/j.ejmech.2006.09.019
196
Nyandoro S. S. Nkunya M. H. H. Josepha C. C. Odalo J. O. Sattler I. (2012). New glucopyranosylglyceryl-N-octenyl adipate and bioactivity of retro and condensed chalcones from Toussaintia orientalis. Tanzan. J. Sci.38, 108–126.
197
Obara H. Takahashi H. Hirano H. (1969). The photo-fries rearrangement of hydroxyphenyl cinnamates. Bull. Chem. Soc. Jpn.42, 560–561. 10.1246/bcsj.42.560
198
Ogawa H. Ohno M. Baba K. (2005). Hypotensive and lipid regulatory actions of 4‐hydroxyderricin, a chalcone from Angelica keiskei, in stroke‐prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol.32, 19–23. 10.1111/j.1440-1681.2005.04147.x
199
Ogawa H. Okada Y. Kamisako T. Baba K. (2007). Beneficial effect of xanthoangelol, a chalcone compound from angelica keiskei, on lipid metabolism in stroke‐prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol.34, 238–243. 10.1111/j.1440-1681.2007.04578.x
200
Oledzka E. (2024). Xanthohumol—a miracle molecule with biological activities: a review of biodegradable polymeric carriers and naturally derived compounds for its delivery. Int. J. Mol. Sci.25, 3398. 10.3390/ijms25063398
201
Olivares C. Solano F. (2009). New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment. Cell Melanoma Res.22, 750–760. 10.1111/j.1755-148X.2009.00636.x
202
Orlikova B. Tasdemir D. Golais F. Dicato M. Diederich M. (2011). Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr.6, 125–147. 10.1007/s12263-011-0210-5
203
Orlikova B. Schnekenburger M. Zloh M. Golais F. Diederich M. Tasdemir D. (2012). Natural chalcones as dual inhibitors of HDACs and NF-κB. Oncol. Rep.28, 797–805. 10.3892/or.2012.1870
204
Ouyang Y. Li J. Chen X. Fu X. Sun S. Wu Q. (2021). Chalcone derivatives: role in anticancer therapy. Biomolecules11, 894. 10.3390/biom11060894
205
Park J.-Y. Jeong H. J. Kim Y. M. Park S.-J. Rho M.-C. Park K. H. et al (2011). Characteristic of alkylated chalcones from Angelica keiskei on influenza virus neuraminidase inhibition. Bioorg Med. Chem. Lett.21, 5602–5604. 10.1016/j.bmcl.2011.06.130
206
Park M. K. Ji J. Haam K. Han T.-H. Lim S. Kang M.-J. et al (2021). Licochalcone A inhibits hypoxia-inducible factor-1α accumulation by suppressing mitochondrial respiration in hypoxic cancer cells. Biomed. and Pharmacother.133, 111082. 10.1016/j.biopha.2020.111082
207
Parmar V. S. Rathore J. S. Jain R. Henderson D. A. Malone J. F. (1989). Occurrence of pongamol as the enol structure in Tephrosia purpurea. Phytochemistry28, 591–593. 10.1016/0031-9422(89)80057-3
208
Parveen Z. Brunhofer G. Jabeen I. Erker T. Chiba P. Ecker G. F. (2014). Synthesis, biological evaluation and 3D-QSAR studies of new chalcone derivatives as inhibitors of human P-glycoprotein. Bioorg Med. Chem.22, 2311–2319. 10.1016/j.bmc.2014.02.005
209
Pastorino G. Cornara L. Soares S. Rodrigues F. Oliveira M. B. P. P. (2018). Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phytotherapy Res.32, 2323–2339. 10.1002/ptr.6178
210
Popoola O. Marnewick J. Rautenbach F. Ameer F. Iwuoha E. Hussein A. (2015). Inhibition of oxidative stress and skin aging-related enzymes by prenylated chalcones and other flavonoids from Helichrysum teretifolium. Molecules20, 7143–7155. 10.3390/molecules20047143
211
Prakash O. Kumar A. Kumar P. Ajeet A. (2013). Anticancer potential of plants and natural products: a review. Am. J. Pharmacol. Sci.1, 104–115. 10.12691/ajps-1-6-1
212
Quan H.-Y. Baek N. I. Chung S. H. (2012). Licochalcone A prevents adipocyte differentiation and lipogenesis via suppression of peroxisome proliferator-activated receptor γ and sterol regulatory element-binding protein pathways. J. Agric. Food Chem.60, 5112–5120. 10.1021/jf2050763
213
Ramadan S. K. Rizk S. A. El-Helw E. A. E. (2024). Synthesis and biological applications of coumarinyl-chalcones. Curr. Org. Chem.28, 897–904. 10.2174/0113852728248318240418092208
214
Ramaiah M. J. Pushpavalli S. Krishna G. R. Sarma P. Mukhopadhyay D. Kamal A. et al (2011). Chalcone-imidazolone conjugates induce apoptosis through DNA damage pathway by affecting telomeres. Cancer Cell Int.11, 11. 10.1186/1475-2867-11-11
215
Ramakrishnan V. T. Kagan J. (1970). Photochemical synthesis of 2’-hydroxychalcones from phenyl cinnamates. J. Org. Chem.35, 2901–2904. 10.1021/jo00834a010
216
Ramirez F. Dershowitz S. (1957). Phosphinemethylenes.1 II. Triphenylphosphineacylmethylenes. J. Org. Chem.22, 41–45. 10.1021/jo01352a010
217
Rammohan A. Reddy J. S. Sravya G. Rao C. N. Zyryanov G. V. (2020). Chalcone synthesis, properties and medicinal applications: a review. Environ. Chem. Lett.18, 433–458. 10.1007/s10311-019-00959-w
218
Rao Y. K. Kao T.-Y. Ko J.-L. Tzeng Y.-M. (2010). Chalcone HTMC causes in vitro selective cytotoxicity, cell-cycle G1 phase arrest through p53-dependent pathway in human lung adenocarcinoma A549 cells, and in vivo tumor growth suppression. Bioorg Med. Chem. Lett.20, 6508–6512. 10.1016/j.bmcl.2010.09.056
219
Rasquel-Oliveira F. S. Manchope M. F. Staurengo-Ferrari L. Ferraz C. R. Saraiva-Santos T. Zaninelli T. H. et al (2020). Hesperidin methyl chalcone interacts with NFκB Ser276 and inhibits zymosan-induced joint pain and inflammation, and RAW 264.7 macrophage activation. Inflammopharmacology28, 979–992. 10.1007/s10787-020-00686-7
220
Rautio J. Kumpulainen H. Heimbach T. Oliyai R. Oh D. Järvinen T. et al (2008). Prodrugs: design and clinical applications. Nat. Rev. Drug Discov.7, 255–270. 10.1038/nrd2468
221
Rekha M. J. Bettadaiah B. K. Sindhu Kanya T. C. Govindaraju K. (2020). A feasible method for isolation of pongamol from karanja (Pongamia pinnata) seed and its anti-inflammatory activity. Ind. Crops Prod.154, 112720. 10.1016/j.indcrop.2020.112720
222
Reuter S. Gupta S. C. Chaturvedi M. M. Aggarwal B. B. (2010). Oxidative stress, inflammation, and cancer: how are they linked?Free Radic. Biol. Med.49, 1603–1616. 10.1016/j.freeradbiomed.2010.09.006
223
Rizvi M. D. Mudagal, M. P. Boregowda S. Hussain T. Al Hagbani T. Abdallah M. H. et al (2023). The flavonoid hesperidin methyl chalcone as a potential therapeutic agent for cancer therapy: molecular docking, in vitro cytotoxicity, and in vivo antitumor activity. Arabian J. Chem.16, 104769. 10.1016/j.arabjc.2023.104769
224
Robinson S. J. Petzer J. P. Petzer A. Bergh J. J. Lourens A. C. U. (2013). Selected furanochalcones as inhibitors of monoamine oxidase. Bioorg Med. Chem. Lett.23, 4985–4989. 10.1016/j.bmcl.2013.06.050
225
Rocha S. Ribeiro D. Fernandes E. Freitas M. (2020). A systematic review on anti-diabetic properties of chalcones. Curr. Med. Chem.27, 2257–2321. 10.2174/0929867325666181001112226
226
Rocha S. Sousa A. Ribeiro D. Correia C. M. Silva V. L. M. Santos C. M. M. et al (2019). A study towards drug discovery for the management of type 2 diabetes mellitus through inhibition of the carbohydrate-hydrolyzing enzymes α-amylase and α-glucosidase by chalcone derivatives. Food Funct.10, 5510–5520. 10.1039/C9FO01298B
227
Rossi M. Cappadone C. Picone G. Bisi A. Farruggia G. Belluti F. et al (2022). Natural-like chalcones with antitumor activity on human MG63 osteosarcoma cells. Molecules27, 3751. 10.3390/molecules27123751
228
Rossi M. Pellegrino C. Rydzyk M. M. Farruggia G. de Biase D. Cetrullo S. et al (2024). Chalcones induce apoptosis, autophagy and reduce spreading in osteosarcoma 3D models. Biomed. and Pharmacother.179, 117284. 10.1016/j.biopha.2024.117284
229
Rowhanirad S. Taherianfard M. (2023). The neuroprotective effects of Chalcones from Ashitaba on cuprizone‐induced demyelination via modulation of brain‐derived neurotrophic factor and tumor necrosis factor α. Brain Behav.13, e3144. 10.1002/brb3.3144
230
Rozmer Z. Perjési P. (2016). Naturally occurring chalcones and their biological activities. Phytochem. Rev.15, 87–120. 10.1007/s11101-014-9387-8
231
Rudrapal M. Khan J. Dukhyil A. A. B. Alarousy R. M. I. I. Attah E. I. Sharma T. et al (2021). Chalcone scaffolds, bioprecursors of flavonoids: chemistry, bioactivities, and pharmacokinetics. Molecules26, 7177. 10.3390/molecules26237177
232
Ryu H. W. Lee B. W. Curtis-Long M. J. Jung S. Ryu Y. B. Lee W. S. et al (2010a). Polyphenols from Broussonetia papyrifera displaying potent α-glucosidase inhibition. J. Agric. Food Chem.58, 202–208. 10.1021/jf903068k
233
Ryu Y. B. Kim J. H. Park S.-J. Chang J. S. Rho M.-C. Bae K.-H. et al (2010b). Inhibition of neuraminidase activity by polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioorg Med. Chem. Lett.20, 971–974. 10.1016/j.bmcl.2009.12.106
234
Sahu, N. K. Balbhadra S. Choudhary J. Kohli V. (2012a). Exploring pharmacological significance of chalcone scaffold: a review. Curr. Med. Chem.19, 209–225. 10.2174/092986712803414132
235
Sahu, N. K. Balbhadra S. Choudhary J. Kohli V. (2012b). Exploring pharmacological significance of chalcone scaffold: a review. Curr. Med. Chem.19, 209–225. 10.2174/092986712803414132
236
Salehi B. Quispe C. Chamkhi I. El Omari N. Balahbib A. Sharifi-Rad J. et al (2021). Pharmacological properties of chalcones: a review of preclinical including molecular mechanisms and clinical evidence. Front. Pharmacol.11, 592654. 10.3389/fphar.2020.592654
237
Salem M. M. Werbovetz K. A. (2005). Antiprotozoal compounds from psorothamnus polydenius. J. Nat. Prod.68, 108–111. 10.1021/np049682k
238
Samota M. K. Yadav D. K. Koli P. Kaur M. Kaur M. Rani H. et al (2024). Exploring natural chalcones: innovative extraction techniques, bioactivities, and health potential. Sustain. Food Technol.2, 1456–1468. 10.1039/D4FB00126E
239
Scagliarini A. Mathey A. Aires V. Delmas D. (2020). Xanthohumol, a prenylated flavonoid from hops, induces DNA damages in colorectal cancer cells and sensitizes SW480 cells to the SN38 chemotherapeutic agent. Cells9, 932. 10.3390/cells9040932
240
Sen R. Chatterjee M. (2011). Plant derived therapeutics for the treatment of Leishmaniasis. Phytomedicine18, 1056–1069. 10.1016/j.phymed.2011.03.004
241
Sharma R. Kumar R. Kodwani R. Kapoor S. Khare A. Bansal R. et al (2015). A review on mechanisms of anti tumor activity of chalcones. Anticancer Agents Med. Chem.16, 200–211. 10.2174/1871520615666150518093144
242
Sharma V. Kumar V. Kumar P. (2013). Heterocyclic chalcone analogues as potential anticancer agents. Anticancer Agents Med. Chem.13, 422–432. 10.2174/187152013804910424
243
Shi D. Zhao D. Niu P. Zhu Y. Zhou J. Chen H. (2018). Glycolysis inhibition via mTOR suppression is a key step in cardamonin-induced autophagy in SKOV3 cells. BMC Complement. Altern. Med.18, 317. 10.1186/s12906-018-2380-9
244
Shigeru M. Makoto M. Hironaka A. Susumu O. (1991). Inhibition of gastric H+,K+-ATPase by the anti-ulcer agent, sofalcone. Biochem. Pharmacol.42, 1447–1451. 10.1016/0006-2952(91)90458-H
245
Shimokoriyama M. (1962). “Flavanones, chalcones and aurones,” in The chemistry of flavonoid compounds (New York: The Macmillan Co).
246
Shotter R. G. Johnston K. M. Jones J. F. (1978). Reactions of unsaturated acid halides—IV. Tetrahedron34, 741–746. 10.1016/0040-4020(78)88113-7
247
Siegel R. L. Miller K. D. Fuchs H. E. Jemal A. (2022). Cancer statistics, 2022. CA Cancer J. Clin.72, 7–33. 10.3322/caac.21708
248
Simirgiotis M. J. Adachi S. To S. Yang H. Reynertson K. A. Basile M. J. et al (2008). Cytotoxic chalcones and antioxidants from the fruits of a Syzygium samarangense (Wax Jambu). Food Chem.107, 813–819. 10.1016/j.foodchem.2007.08.086
249
Singh L. R. Chen Y.-L. Xie Y.-Y. Xia W. Gong X.-W. Hider R. C. et al (2020). Functionality study of chalcone-hydroxypyridinone hybrids as tyrosinase inhibitors and influence on anti-tyrosinase activity. J. Enzyme Inhib. Med. Chem.35, 1562–1567. 10.1080/14756366.2020.1801669
250
Singh P. Anand A. Kumar V. (2014). Recent developments in biological activities of chalcones: a mini review. Eur. J. Med. Chem.85, 758–777. 10.1016/j.ejmech.2014.08.033
251
Sinha A. Deb V. K. Datta A. Yadav S. Phulkar A. Adhikari S. (2024). Evaluation of structural features of anabolic-androgenic steroids: entanglement for organ-specific toxicity. Steroids212, 109518. 10.1016/j.steroids.2024.109518
252
Sivakumar P. M. Prabhakar P. K. Doble M. (2011). Synthesis, antioxidant evaluation, and quantitative structure–activity relationship studies of chalcones. Med. Chem. Res.20, 482–492. 10.1007/s00044-010-9342-1
253
Sogawa S. Nihro Y. Ueda H. Miki T. Matsumoto H. Satoh T. (1994). Protective effects of hydroxychalcones on free radical-induced cell damage. Biol. Pharm. Bull.17, 251–256. 10.1248/bpb.17.251
254
Sudo G. Marques A. Pereira S. Paiva R. Cavalcante C. Sudo S. et al (2015). Hypoglycemic effect of the methanol flower extract of piper claussenianum and the major constituent 2′,6′-dihydroxy-4′-methoxychalcone in streptozotocin diabetic rats. Indian J. Pharm. Sci.77, 237–243. 10.4103/0250-474X.156624
255
Sugamoto K. Matsusita Y. Matsui K. Kurogi C. Matsui T. (2011). Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron67, 5346–5359. 10.1016/j.tet.2011.04.104
256
Suzen S. Buyukbingol E. (2003). Recent studies of aldose reductase enzyme inhibition for diabetic complications. Curr. Med. Chem.10, 1329–1352. 10.2174/0929867033457377
257
Sychrová A. Škovranová G. Čulenová M. Bittner Fialová S. (2022). Prenylated flavonoids in topical infections and wound healing. Molecules27, 4491. 10.3390/molecules27144491
258
Szliszka E. Czuba Z. P. Mazur B. Sedek L. Paradysz A. Krol W. (2009). Chalcones enhance TRAIL-induced apoptosis in prostate cancer cells. Int. J. Mol. Sci.11, 1–13. 10.3390/ijms11010001
259
Tanaka H. Nakamura S. Onda K. Tazaki T. Hirano T. (2009). Sofalcone, an anti-ulcer chalcone derivative, suppresses inflammatory crosstalk between macrophages and adipocytes and adipocyte differentiation: implication of heme-oxygenase-1 induction. Biochem. Biophys. Res. Commun.381, 566–571. 10.1016/j.bbrc.2009.02.086
260
Tanaka S. Kuwai Y. Tabata M. (1987). Isolation of monoamine oxidase inhibitors from Glycyrrhiza uralensis roots and the structure-activity relationship. Planta Med.53, 5–8. 10.1055/s-2006-962604
261
Tanaka S. Sakata Y. Morimoto K. Tambe Y. Watanabe Y. Honda G. et al (2001). Influence of natural and synthetic compounds on cell surface expression of cell adhesion molecules, ICAM-1 and VCAM-1. Planta Med.67, 108–113. 10.1055/s-2001-11514
262
Torres-Santos E. C. Moreira D. L. Kaplan M. A. C. Meirelles M. N. Rossi-Bergmann B. (1999). Selective effect of 2′,6′-dihydroxy-4′-methoxychalcone isolated from piper aduncum on leishmania amazonensis. Antimicrob. Agents Chemother.43, 1234–1241. 10.1128/AAC.43.5.1234
263
Tsukiyama R.-I. Katsura H. Tokuriki N. Kobayashi M. (2002). Antibacterial activity of licochalcone A against spore-forming bacteria. Antimicrob. Agents Chemother.46, 1226–1230. 10.1128/AAC.46.5.1226-1230.2002
264
Tuan H. Minh B. Tran P. Lee J. Oanh H. Ngo Q. et al (2019). The effects of 2′,4′-Dihydroxy-6′-methoxy-3′,5′- dimethylchalcone from Cleistocalyx operculatus buds on human pancreatic cancer cell lines. Molecules24, 2538. 10.3390/molecules24142538
265
Ur Rashid H. Xu Y. Ahmad N. Muhammad Y. Wang L. (2019). Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E2, inducible NO synthase and nuclear factor κb activities. Bioorg Chem.87, 335–365. 10.1016/j.bioorg.2019.03.033
266
Utama K. Khamto N. Meepowpan P. Aobchey P. Kantapan J. Sringarm K. et al (2022). Effects of 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone from Syzygium nervosum seeds on antiproliferative, DNA damage, cell cycle arrest, and apoptosis in human cervical cancer cell lines. Molecules27, 1154. 10.3390/molecules27041154
267
Vachiraarunwong A. Tuntiwechapikul W. Wongnoppavich A. Meepowpan P. Wongpoomchai R. (2023). 2,4’-dihydroxy-6’-methoxy-3’,5’-dimethylchalcone from Cleistocalyx nervosum var. paniala seeds attenuated the early stage of diethylnitrosamine and 1,2-dimethylhydrazine-induced colorectal carcinogenesis. Biomed. and Pharmacother.165, 115221. 10.1016/j.biopha.2023.115221
268
van Dinteren S. Meijerink J. Witkamp R. van Ieperen B. Vincken J.-P. Araya-Cloutier C. (2022). Valorisation of liquorice (Glycyrrhiza) roots: antimicrobial activity and cytotoxicity of prenylated (iso)flavonoids and chalcones from liquorice spent (G. glabra, G. inflata, and G. uralensis). Food Funct.13, 12105–12120. 10.1039/D2FO02197H
269
Verzele M. Stockx J. Fontijn F. Anteunis M. (1957). Xanthohumol, a new natural chalkone. Bull. Des. Sociétés Chim. Belg.66, 452–475. 10.1002/bscb.19570660137
270
Vesaghhamedani S. Ebrahimzadeh F. Najafi E. Shabgah O. G. Askari E. Shabgah A. G. et al (2022). Xanthohumol: an underestimated, while potent and promising chemotherapeutic agent in cancer treatment. Prog. Biophys. Mol. Biol.172, 3–14. 10.1016/j.pbiomolbio.2022.04.002
271
Wang K.-L. Yu Y.-C. Hsia S.-M. (2021). Perspectives on the role of isoliquiritigenin in cancer. Cancers (Basel)13, 115. 10.3390/cancers13010115
272
Wang S. Li C. Zhang L. Sun B. Cui Y. Sang F. (2023). Isolation and biological activity of natural chalcones based on antibacterial mechanism classification. Bioorg Med. Chem.93, 117454. 10.1016/j.bmc.2023.117454
273
Wang Z. Wang N. Han S. Wang D. Mo S. Yu L. et al (2013). Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway. PLoS One8, e68566. 10.1371/journal.pone.0068566
274
Wang Q. Ding Z. Liu J. Zheng Y. (2004). Xanthohumol, a novel anti-HIV-1 agent purified from Hops Humulus lupulus. Antivir. Res.64, 189–194. 10.1016/j.antiviral.2004.08.005
275
Wegener J. W. Nawrath H. (1997). Cardiac effects of isoliquiritigenin. Eur. J. Pharmacol.326, 37–44. 10.1016/S0014-2999(97)00134-9
276
Weindorf N. Schultz-Ehrenburg U. (1987). Controlled study of increasing venous tone in primary varicose veins by oral administration of Ruscus aculeatus and trimethylhespiridinchalcone. Z Hautkr62, 28–38.
277
Wright G. D. (2017). Opportunities for natural products in 21st century antibiotic discovery. Nat. Prod. Rep.34, 694–701. 10.1039/C7NP00019G
278
Wu J.-H. Wang X.-H. Yi Y.-H. Lee K.-H. (2003). Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorg Med. Chem. Lett.13, 1813–1815. 10.1016/S0960-894X(03)00197-5
279
Wu X.-F. Neumann H. Spannenberg A. Schulz T. Jiao H. Beller M. (2010). Development of a general palladium-catalyzed carbonylative Heck reaction of aryl halides. J. Am. Chem. Soc.132, 14596–14602. 10.1021/ja1059922
280
Xiao J. Gao M. Diao Q. Gao F. (2021). Chalcone derivatives and their activities against drug-resistant cancers: an overview. Curr. Top. Med. Chem.21, 348–362. 10.2174/1568026620666201022143236
281
Xiao Y. Han F. Lee I.-S. (2019). Microbial transformation of licochalcones. Molecules25, 60. 10.3390/molecules25010060
282
Xu C. Chen G. Huang X. (1995). Chalcones by the Wittig reaction of A stable ylide with aldehydes under microwave irradiation. Org. Prep. Proced. Int.27, 559–561. 10.1080/00304949509458500
283
Yang E. B. Guo Y. J. Zhang K. Chen Y. Z. Mack P. (2001). Inhibition of epidermal growth factor receptor tyrosine kinase by chalcone derivatives. Biochimica Biophysica Acta (BBA) - Protein Struct. Mol. Enzym.1550, 144–152. 10.1016/S0167-4838(01)00276-X
284
Yang H.-L. Yang T.-Y. Gowrisankar Y. V. Liao C.-H. Liao J.-W. Huang P.-J. et al (2020). Suppression of LPS-induced inflammation by chalcone flavokawain A through activation of nrf2/ARE-mediated antioxidant genes and inhibition of ROS/nfκ B signaling pathways in primary splenocytes. Oxid. Med. Cell Longev.2020, 3476212–3476214. 10.1155/2020/3476212
285
Ye C.-L. Qian F. Wei D.-Z. Lu Y.-H. Liu J.-W. (2005). Induction of apoptosis in K562 human leukemia cells by 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone. Leuk. Res.29, 887–892. 10.1016/j.leukres.2005.01.006
286
Yenesew A. Induli M. Derese S. Midiwo J. O. Heydenreich M. Peter M. G. et al (2004). Anti-plasmodial flavonoids from the stem bark of Erythrina abyssinica. Phytochemistry65, 3029–3032. 10.1016/j.phytochem.2004.08.050
287
Yoo M.-H. Xu X.-M. Carlson B. A. Patterson A. D. Gladyshev V. N. Hatfield D. L. (2007). Targeting thioredoxin reductase 1 reduction in cancer cells inhibits self-sufficient growth and DNA replication. PLoS One2, e1112. 10.1371/journal.pone.0001112
288
Yoon G. Kang B. Y. Cheon S. H. (2007). Topoisomerase i inhibition and cytotoxicity of licochalcones A and E fromGlycyrrhiza inflata. Arch. Pharm. Res.30, 313–316. 10.1007/BF02977611
289
Yu L. Zhang F. Hu Z. Ding H. Tang H. Ma Z. et al (2014). Novel prenylated bichalcone and chalcone from Humulus lupulus and their quinone reductase induction activities. Fitoterapia93, 115–120. 10.1016/j.fitote.2013.12.019
290
Yu T. Jiang Y. Guo M. Wei X. Xin X. An F. (2024). Edible‐plant derived 2′,4′‐dihydroxy‐6′‐methoxy‐3′,5′‐dimethylchalcone exert dual effects on invigorating triple‐negative breast cancer apoptosis. Food Bioeng.3, 222–235. 10.1002/fbe2.12092
291
Zhai L. Blom J. Chen M. Christensen S. B. Kharazmi A. (1995). The antileishmanial agent licochalcone A interferes with the function of parasite mitochondria. Antimicrob. Agents Chemother.39, 2742–2748. 10.1128/AAC.39.12.2742
292
Zhang L. Tao G. Chen J. Zheng Z.-P. (2016). Characterization of a new flavone and tyrosinase inhibition constituents from the twigs of Morus alba L. Molecules21, 1130. 10.3390/molecules21091130
293
Zhang X. Hu X. Hou A. Wang H. (2009). Inhibitory effect of 2,4,2’,4’-Tetrahydroxy-3-(3-methyl-2-butenyl)-chalcone on tyrosinase activity and melanin biosynthesis. Biol. Pharm. Bull.32, 86–90. 10.1248/bpb.32.86
294
Zhang X. Song Q. Cao Z. Li Y. Tian C. Yang Z. et al (2019). Design, synthesis and evaluation of chalcone Mannich base derivatives as multifunctional agents for the potential treatment of Alzheimer’s disease. Bioorg Chem.87, 395–408. 10.1016/j.bioorg.2019.03.043
295
Zhang X. Yeung E. D. Wang J. Panzhinskiy E. E. Tong C. Li W. et al (2010). Isoliquiritigenin, a natural anti‐oxidant, selectively inhibits the proliferation of prostate cancer cells. Clin. Exp. Pharmacol. Physiol.37, 841–847. 10.1111/j.1440-1681.2010.05395.x
296
Zhao F. Nozawa H. Daikonnya A. Kondo K. Kitanaka S. (2003). Inhibitors of nitric oxide production from hops (Humulus lupulus L.). Biol. Pharm. Bull.26, 61–65. 10.1248/bpb.26.61
297
Zhou B. Xing C. (2015). Diverse molecular targets for chalcones with varied bioactivities. Med. Chem. (Los Angeles)5, 388–404. 10.4172/2161-0444.1000291
298
Zhuang C. Zhang W. Sheng C. Zhang W. Xing C. Miao Z. (2017a). Chalcone: a privileged structure in medicinal chemistry. Chem. Rev.117, 7762–7810. 10.1021/acs.chemrev.7b00020
299
Zhuang C. Zhang W. Sheng C. Zhang W. Xing C. Miao Z. (2017b). Chalcone: a privileged structure in medicinal chemistry. Chem. Rev.117, 7762–7810. 10.1021/acs.chemrev.7b00020
300
Zhuang C. Zhang W. Sheng C. Zhang W. Xing C. Miao Z. (2017c). Chalcone: a privileged structure in medicinal chemistry. Chem. Rev.117, 7762–7810. 10.1021/acs.chemrev.7b00020
Summary
Keywords
chalcones, biosynthesis, pharmacological activity, structural features and antimicrobial activity, anti-inflammatory, anti-cancer
Citation
Adhikari S, Nath P, Deb VK, Das N, Banerjee A, Pathak S and Duttaroy AK (2025) Pharmacological potential of natural chalcones: a recent studies and future perspective. Front. Pharmacol. 16:1570385. doi: 10.3389/fphar.2025.1570385
Received
03 February 2025
Accepted
18 April 2025
Published
17 June 2025
Volume
16 - 2025
Edited by
Michał Tomczyk, Medical University of Bialystok, Poland
Reviewed by
Sayed K. Ramadan, Ain Sham University, Egypt
Hongliang Liu, Shandong University of Technology, China
Anand Maurya, Banaras Hindu University, India
Rishav Mazumder, Tripura University, India
Updates
Copyright
© 2025 Adhikari, Nath, Deb, Das, Banerjee, Pathak and Duttaroy.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suman Adhikari, sumanadhi@gmail.com; Surajit Pathak, drsurajitpathak@care.edu.in; Asim K. Duttaroy, a.k.duttaroy@medisin.uio.no
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.