Abstract
Introduction:
Phytoestrogens, with estrogenic activity, are commonly found across the Fabaceae family. Here we develop methods that use phylogeny and ethnomedicinal information in order to identify candidate species for novel phytoestrogens.
Method:
We selected Fabaceae species traditionally used as aphrodisiacs or with applications to control fertility (aphrodisiac-fertility species), to create a cross-cultural dataset of ethnomedicinal use. Using a phylogeny of the Fabaceae, “hot nodes” methods were used to identify lineages with a higher number of species with aphrodisiac-fertility uses. The known distribution of estrogenic flavonoids was used to determine whether the phytoestrogen-containing species was associated with aphrodisiac-fertility “hot nodes”. Additionally, we examined the overlap of aphrodisiac-fertility uses with neurological applications, hypothesising that such species may have bioactive compounds with estrogenic properties. Lastly, the “aphrodisiac-fertility hot node” lineages without previously known estrogenic flavonoids were identified.
Results:
We showed species in aphrodisiac-fertility hot nodes were more likely to contain estrogenic flavonoids (21% of species), a major group of phytoestrogens, compared to Fabaceae in the phylogeny (11% of species). Additionally, when aphrodisiac fertility species are limited to those with neurological applications, 62% of the species within hot nodes contain estrogenic flavonoids.
Discussion:
We recognised 43 high-priority hot nodes, these lineages might represent promising targets for future studies on phytoestrogens. The results demonstrated the combining phylogenetic and ethnomedicinal data to guide the discovery of estrogenic flavonoids with therapeutic potential.
Introduction
Natural products continue to provide promising leads for drug discovery (Newman and Cragg, 2020). However, whether natural product research is practicable for drug discovery (Amirkia and Heinrich, 2015), and whether traditional uses in ethnomedicine can guide the discovery of new chemical compounds remains controversial (Gertsch, 2012; Gurib-Fakim, 2006; Saslis-Lagoudakis et al., 2011; Sucher, 2013; Skirycz et al., 2016). Devising strategies to target species for evaluation is an area of research interest (Fabricant and Farnsworth, 2001; Holzmeyer et al., 2020). Ethnobotanically-guided screening is one such approach (Fabricant and Farnsworth, 2001). Strategies incorporating phylogenies alongside ethnobotanical use data have been adopted (Pellicer et al., 2018; Saslis-Lagoudakis et al., 2012; Souza et al., 2018). Here we apply phylogenetic methods to ethnobotanical use data, to explore whether they can more effectively target bioactive plant products.
Plant lineages that contain significantly more species with ethnomedicinal use were first referred to as hot nodes for bioprospecting by Saslis-Lagoudakis et al. (2011). Since then, hot nodes have been identified for different groups of medicinal plants from other parts of the world, and at varying taxonomic levels. At the generic level, hot nodes for potential anti-inflammatory compounds have been described for genus Euphorbia L. (Ernst et al., 2016), for species of interest to treat malaria in genus Artemisia L. (Pellicer et al., 2018), and for putative antioxidant and antidiabetic bioactivity for genus Allium L. (Teotia et al., 2024). At a higher taxonomic level, hot nodes in the orchid subtribe Coelogyninae that may show antimicrobial properties were identified based on ethnomedicinal uses (Wati et al., 2021). Geographically-focused studies have examined cross-cultural patterns between Nepal, South Africa and New Zealand (Saslis-Lagoudakis et al., 2012), whilst others have focused on the Brazilian Fabaceae (Souza et al., 2018), the Chinese Lamiaceae (Zaman et al., 2022) of whole medicinal floras (South Africa, Yessoufou et al., 2015; Atienza-Barthelemy et al., 2024), or pharmacopoeias (China, Zaman et al., 2021; India; Yao et al., 2023). Global studies include a study of angiosperms to identify hot nodes for psychoactive activity (Halse-Gramkow et al., 2016), for antimalarial properties (Milliken et al., 2021) and cancer (Thompson and Hawkins, 2024). Some of these studies have sought to validate the hot node method; for example, confidence in the hot node method is increased where hot nodes include a higher proportion of plant drugs in clinical trials (Ernst et al., 2016; Pellicer et al., 2018; Souza and Hawkins, 2017) or where there is cross-cultural convergence (Saslis-Lagoudakis et al., 2012). At least one study has used a literature search to show that hot node species have relevant biological activity (Teotia et al., 2024). Pellicer et al. (2018) screened for artemisinin in fifteen species, finding four of seven species from hot nodes and five of eight from outside hot nodes contained artemisinin. Their interpretation was that in this case–where a molecule of interest is common throughout the genus - the hot node approach is not effective. Given the increasing application of the hot node method, further tests of its validity are crucial.
Phytoestrogens (PE) are plant-derived compounds that have similar functions to estrogen. By binding to the estrogen receptor, estrogen (estradiol, E2) or PEs can activate estrogen-responsive genes, which in turn encode proteins that maintain bone, reproductive health, cognition, and cardiovascular function (O’Donnell et al., 2007; West et al., 2009). Consuming one common dietary source of PEs, soybean, can offer a range of health benefits, one of which is alleviating the symptoms of menopause (Branca and Lorenzetti, 2005). These symptoms include hot flashes, night sweats, vaginal dryness, mood changes, difficulty sleeping, anxiety and decreased libido (Booyens et al., 2022). Several medicinal plant drugs containing PEs are also used to reduce hot flashes and night sweats (Hajirahimkhan et al., 2013), vaginal dryness (Rosa Lima et al., 2014), and cardiovascular disease (Rossouw et al., 2007). The varying interactions of PEs with estrogen receptors suggest that different PEs may have specific functions or roles in various tissues (Ceccarelli et al., 2022; Kiyama, 2022). Because PEs can have both therapeutic and cancer risks (Maggiolini et al., 2002; Umehara et al., 2008; Ye and Shaw, 2019), characterising the diversity of PEs to identify therapeutically optimal molecules is desirable. However, the studies of PEs for postmenopausal symptoms comprise a small number of plants. PEs appear to be distributed throughout the Fabaceae, though most plant sources remain uncharacterised, suggesting there are molecules yet unknown (Dixon, 2004; Rutz et al., 2022). Strategies to identify likely sources of novel PEs are therefore needed.
Here, we propose a strategy for identifying potential sources of therapeutically optimal, novel PEs for estrogen-related symptoms. A lack or excess of phytoestrogens, particularly from soybean-based foods, has been shown to suppress sexual behaviour development in both male and female rodents during puberty, suggesting that optimal concentrations of PEs can modulate estrogen-driven behaviours (Khan et al., 2008; Kudwa et al., 2007; Sandhu et al., 2020). Additionally, chemically isolated PEs such as genistein and daidzein have been shown to produce an anxiolytic-like effect in mice, indicating their potential role in reducing anxiety-related behaviours (Rodríguez-Landa et al., 2009; Zeng et al., 2010). The effects of PEs on socio-sexual behaviour may be mediated through a set of hypothalamic or hypothalamic-linked areas in the brain called the social behaviour network (SBN; Newman, 1999), and applications of plant drugs for neurological symptoms might affect the same regions (O’Donnell et al., 2007; West et al., 2009). Treatments for menopausal symptoms are very rarely described in ethnobotanical literature, but plants with hormone-modulating properties or those with estrogenic activity may be used as aphrodisiacs or to enhance fertility. Since these applications are directly relevant to sexual behaviour and are often well-documented in traditional medicine, we propose that the exploration of aphrodisiac fertility (AF) as a therapeutic category in ethnomedicine could highlight high-activity PEs that may act predominantly in the CNS. Additionally, neurological applications that regulate CNS activity (Dong and Nao, 2023) may intersect with these therapeutic uses, focusing on plants that have specific effects on the CNS. Species with AF use that also have neurological applications could therefore be of particular interest, as candidates for neuro-selective estrogens.
The Fabaceae is a large, widely distributed family comprising approximately 18,000 species, several of which are economically important for food and medicine (Lewis, 2005). Fabaceae plants are rich in alkaloids, flavonoids, saponins, tannins, glycosides, and other phytochemicals that contribute to their medicinal properties (Wink, 2013). Several studies show that the family Fabaceae is over-represented in medicinal floras (Moerman, 1991; Moutouama and Gaoue, 2024; Saslis-Lagoudakis et al., 2011). The species diversity, widespread distribution, and numerous reported uses (Souza and Hawkins, 2017)availability of phylogenetic information (Azani et al., 2017), and multiple reports of estrogenic compounds within this family (Kiyama, 2017; Kiyama, 2022) have motivated us to focus on this family.
The main objective of this study is to test whether phylogenetic methods may be useful to prioritise species for screening for PEs that could be therapeutically useful. A secondary objective is to identify and highlight candidate species that have not been the focus of research relevant to the identification of therapeutic PEs. Here we identify species traditionally used for AF purposes and for closely related applications to enhance fertility, and that also have neurological applications. We test the hypothesis that hot nodes identified using ethnomedicinal data include more species known to have PEs than a random sample. The distribution of PEs was according to a database of natural products, the LOTUS database (Rutz et al., 2022). We suggest that the phylogenetic analyses can highlight ethnomedicinally important lineages that are putative sources of novel PEs.
Methods
Data collection
Species-level data for flowering plants used as medicine were gathered from recent and comprehensive systematic reviews for Brazil (Souza et al., 2018), China (Zaman et al., 2021), the Greco-Roman Mediterranean (Leonti et al., 2024), the sub-Saharan region of Africa (Ajao et al., 2019) and Thailand (Phumthum et al., 2018).
We compiled a list of aphrodisiac-fertility (AF) species in Fabaceae from these sources by using the search terms “aphrodisiac,” “sexual intercourse,” “libido,” “fertility,” and “sterilisation.” AF plants are those that stimulate sexual desire. Aphrodisiac use refers to sexual desire within the psychological category. However, aphrodisiacs have also been used in other categories, such as fertility, erectile dysfunction, menstrual disorders, and pregnancy, which fall under the genital system and pregnancy categories. For a more extensive search, we included fertility properties in the search terms because sexual desire and fertility are related to each other (Berger et al., 2016) and estrogen and PEs affected both sexual desire and fertility (Najaf Najafi and Ghazanfarpour, 2018; Scavello et al., 2019).
Whether the species with AF use had other therapeutic uses was recorded from the original sources and by Google Scholar and PubMed searches. Other uses were classified into ten therapeutic applications (general, blood, digestive, eye, circulatory, muscular, neurological, psychological, respiratory, skin, nutritional, and urinary) according to the ICPC-3 International Classification of Primary Care (van Boven and Ten Napel, 2021).
The list of known PEs (Supplementary Material S1), particularly flavonoids, was obtained by referencing a review on estrogenic flavonoids (Kiyama, 2022). These compounds were then cross-referenced with the LOTUS initiative database, a database which includes 750,000 referenced structure-organism pairs (Rutz et al., 2022). We used “stringdist_left_join” function from the “fuzzyjoin” package with a maximum difference of two characters between words in R (Robinson et al., 2020) to extract Angiosperm species containing estrogenic flavonoids (Supplementary Material S2).
Phylogenetic analysis
We utilised a large time-calibrated phylogeny of the rosids comprising nearly 20,000 species (Sun et al., 2020), and pruned it to retain only the species in Fabaceae from our data using the “keep.tip” function from the “ape” package in R (Paradis et al., 2004). The final phylogeny included 5,626 (31%) of approximately 18,000 Fabaceae species and 651 (85%) of the 765 Fabaceae genera. We used this phylogeny, the list of AF species and the list of species with estrogenic flavonoids in our analyses.
The D statistic was calculated as an estimate of the phylogenetic signal of the AF species using the “phylo.d” function from the “caper” package in R (Fritz and Purvis, 2010).
We predicted the hot nodes for AF use at the species level using the “hot.nodes” function developed by (Molina-Venegas et al., 2020). Hot nodes were considered only if they contained fewer than 100 species, following (Halse-Gramkow et al., 2016). Hot nodes were recognised according to the number of species within the lineage, rather than across the entire genus where genera were split between nodes. To determine whether screening known AF species or species that belong to AF hot nodes is an efficient bioprospecting strategy, we calculated the percentage of known estrogenic flavonoids by the species that belonged to these groups. These percentages were compared to the overall percentages of species in the family known to contain estrogenic flavonoids, as well as to the percentage of species within the phylogeny that possess estrogenic flavonoids. We refer to these percentages as “search efficiency” (Souza et al., 2018).
We supposed that those AF hot nodes that contained no known estrogenic flavonoids might be the sources of novel estrogenic flavonoids, and made species lists for these nodes.
The predicted lineages, hot nodes and the phylogenetic distributions of species containing estrogenic flavonoids were visualised using the Interactive Tree of Life v5 (Letunic and Bork, 2021).
Results
AF species and species with known estrogenic flavonoids
According to the five sources, 183 species belonging to 64 genera were the source of AF medicines. Eight were from Brazil, 122 were from China, seven were from the Graeco-Roman Mediterranean, 28 were sub-Saharan, and 19 were from Thailand (Supplementary Material S3). We were able to identify 638 species that were recorded to produce estrogenic flavonoids, showing approximately 11% of the 5,626 species of Fabaceae phylogeny are known to produce estrogenic flavonoids (Supplementary Material S3). Fifty-five (30%) of the species used as AFs were known to have estrogenic flavonoids, and these represented 35 genera; we consider screening AF species to have 30% efficiency (Figure 1).
FIGURE 1
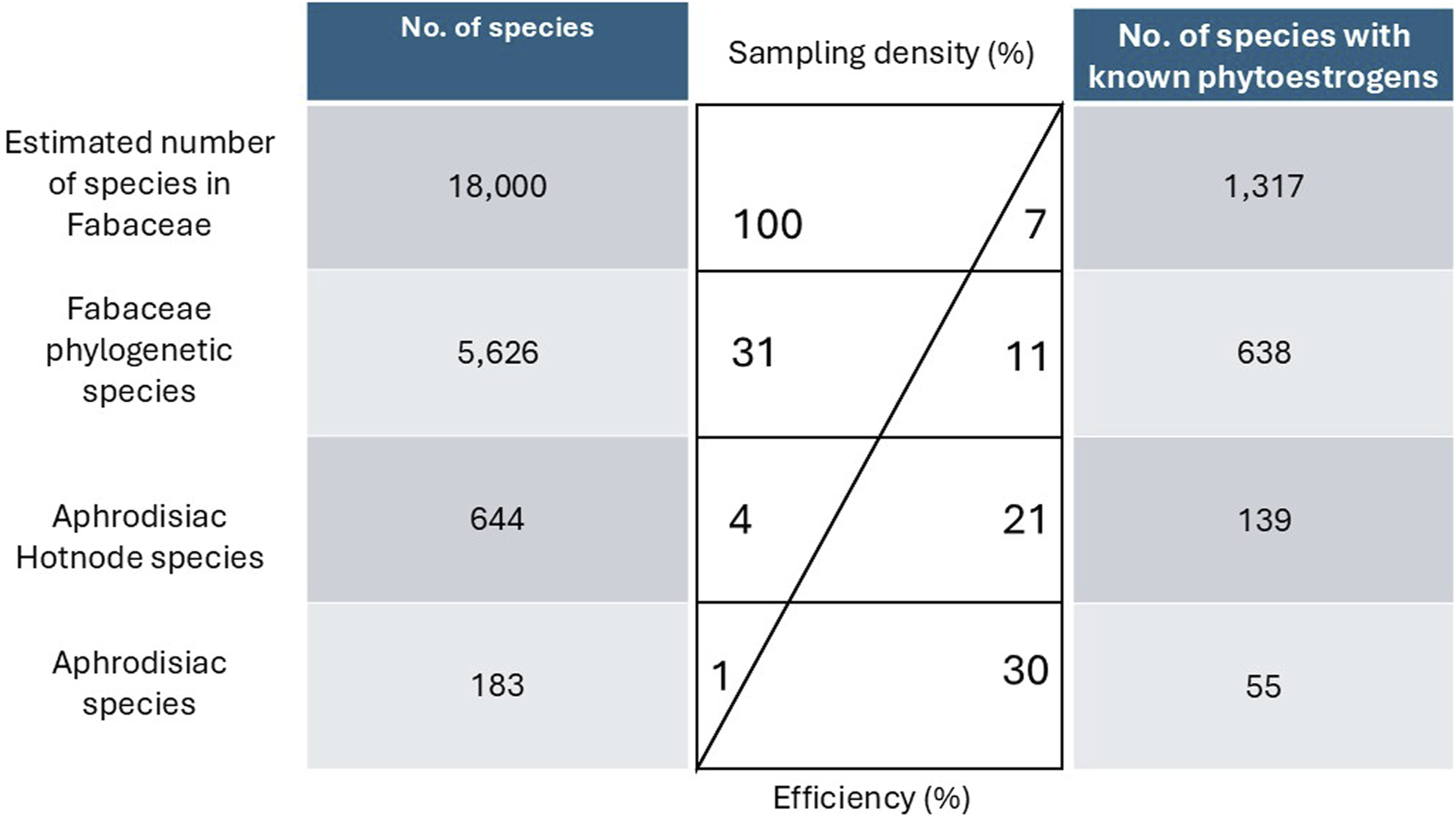
The comparison of aphrodisiac-fertility species (AF) and species that contained estrogenic flavonoid. The first column shows the number of species screened, the central table indicates the sample density and the efficiency of the species with known estrogenic flavonoids by the number of species screened, and the last column shows the number of species with known estrogenic flavonoids.
Predicting lineages with elevated bioprospecting potential
Of the 183 AF species, 106 (57%) were included in the phylogeny. The estimated D statistic for these species was 0.70, indicating a weak to moderate phylogenetic signal for the AF trait. The “hot.nodes” function identified 319 AF hot nodes. AF hot nodes are nested, so our analysis identified 43 highest-level AF hot nodes (Figure 2). These 43 hot nodes comprise 644 species in 142 genera, of which 139 species were known to contain estrogenic flavonoids (21% efficiency; Figure 1). The average number of species in the higher-level AF hot nodes was 29.86 ± 52.27. Of the 43 AF hot nodes, there were 12 that did not include any species known to have estrogenic flavonoids according to the LOTUS initiative database; the average number of hot node species known to have estrogenic flavonoids was 11.49 species, with a standard deviation of 31.77.
FIGURE 2
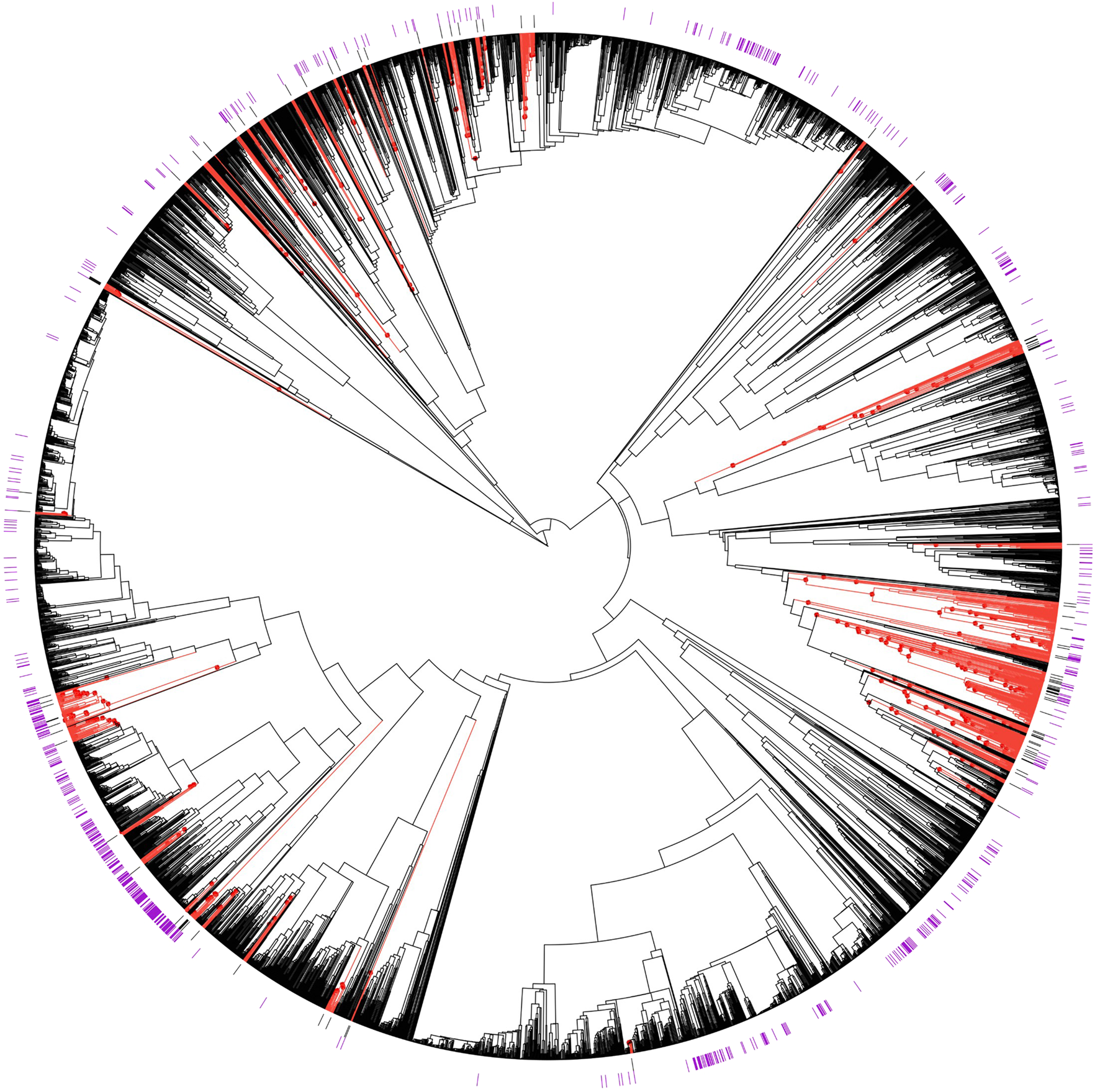
Phylogenetic distributions of species with aphrodisiac-fertility applications and species containing estrogenic flavonoids relative to hot nodes for aphrodisiac-fertility use. Species with traditional aphrodisiacs (black bars) and species containing estrogenic flavonoids (purple bars) are indicated on the phylogeny of Fabaceae plants. Hot node lineages for “aphrodisiac-fertility” identified by the “hot.nodes” function developed by (Molina-Venegas et al., 2020) are shown as red dots and branches.
Of the 43 highest-level hot nodes, 31 correspond to species known to contain estrogenic flavonoids (Table 1, Supplementary Material S4). There were 12 that did not include any species known to contain estrogenic flavonoids, Table 2 shows these AF hot nodes. The number of species in them ranges from two to 25, with two of the smallest AF hot nodes only including two species, and one node has three species. The first hot node was a sub-family of Dialioideae Legume Phylogeny Working Group, and the third cluster contained the genus Delonix Raf. The sixth and seventh clusters were in the genera Vachellia Wight and Arn., and the eighth cluster included Senegalia Raf. and relatives. The ninth cluster was in the genus Poiretia Sm. The 10th and 11th clusters were in the genus Indigofera L., while the last cluster was in the genus Sesbania Adans.
TABLE 1
| Species | Estrogenic-flavonoids | Bioactivities |
|---|---|---|
| Glycyrrhiza glabra L. | Glabrene, Licochalcone a, Galangin, Formononetin, Pinocembrin, Glabridin, Hispaglabridin A, Glabrol, Rutin, Hispaglabridin B, Isobavachromene, Isoliquiritigenin, Naringenin, Genistein, Astragalin, Prunetin, Liquiritigenin, Glyasperin C, Isobavachalcone, Medicarpin, Glycycoumarin, Wighteone, Afrormosin, and Phaseolin. | Antioxidant, Anti-inflammatory, Antitussive and Expectorant, Antiulcerative, Antimicrobial, Antiviral, Hepatoprotective, Anticarcinogenic, Antimutagenic, Neuroprotective, Sedative, and Antidepressive (Pastorino et al., 2018) |
| Phaseolus vulgaris L. | Rutin, Coumestrol, Kaempferol, Quercetin, Astragalin, Vestitone, Pelargonidin, Cianidanol, Kievitone, Luteolin, Genistein, Phaseolin, Daidzein, L-Epicatechin, Taxifolin, Apigenin, Naringenin, Myricetin, Delphinidin 3-glucoside, Hesperetin, and Cyanidin, Pelargonidin 3-glucoside. | Immunogenicity, Anticarcinogenic, Antiviral, Antimicrobial, Cardioprotective, Antidiabetic, and Anti-obesity (He et al., 2018; Rodríguez et al., 2022; Peddio et al., 2022) |
| Glycine max (L.) Merr. | Kaempferol, Daidzein, Formononetin, Genistein, Coumestrol, Naringenin, Glyceollin, Glycitein, Quercetin, Isoliquiritigenin, Cianidanol, Rutin, Afrormosin, Fisetin, Glyceollidin II, Isoformononetin, Astragalin, and Glyceollin II, Vitexin. | Estrogenic, Anti-estrogenic, Anti-thrombotic, Postmenopausal Relief, Antimutagenic, Antihypertensive, Antioxidant, Neuroprotective, Immunoregulatory, Anticancer, Anti-obesity, and Anti-arteriosclerosis (Rizzo and Baroni, 2018; Cao et al., 2019; Kim et al., 2021) |
| Pueraria montana (Lour.) Merr. | Coumestrol, Formononetin, Tectoridin, Daidzein, Glycitein, Tectorigenin, Kakkalide, Genistein, Isoliquiritigenin, Isoformononetin, Sissotrin, Irisolidone, Apigenin, Quercetin, Baicalein, and Medicarpin. | Anti-alcoholism, Antioxidant, Hepatoprotective, Antidiabetic, Neuroprotective, Cardioprotective, Nephroprotective, Anti-inflammatory, Mutagenic, Anticancer, Antibacterial, and Anti-osteoporosis (Wang et al., 2020) |
| Cicer arietinum L. | Calycosin, Pratensein, Sissotrin, Genistein, Formononetin, Quercetin, Vestitone, Daidzein, Garbanzol, Astragalin, Isorhamnetin, Medicarpin, Cianidanol, Naringenin, Isoliquiritigenin, and Kaempferol. | Antioxidant, Antitumor, Antiproliferative, Anti-inflammatory, and Antibacterial (Wang et al., 2021) |
| Cullen corylifolium (L.) Medik. | Corylifol A, Neobavaisoflavone, Psoralidin, Bavachin, Daidzein, Bavachalcone, Coumestrol, Corylin, Bavachinin, Isobavachalcone, Isobavachromene, Genistein, Isobavachin, and Astragalin. | Anti-osteoporosis, Antitumor, Antiviral, Antibacterial, Anti-inflammatory, Anticancer, Anti-vitiligo, and Antidepressant-like (Qi et al., 2023; Ren et al., 2020; Sharifi-Rad et al., 2020) |
| Butea monosperma (Lam.) Kuntze | Genistein, Formononetin, Prunetin, Butin, Cajanin, Butein, Daidzein, Medicarpin, Isoliquiritigenin, Liquiritigenin, Afrormosin, Isoformononetin, and Kaempferide. | Antibacterial, Antiviral, Anticancer, Anti-inflammatory, Antioxidant, and Neuropathic Pain Relief (Forouzanfar and Hosseinzadeh, 2018; Hiremath et al., 2024) |
| Vicia faba L. | Neohesperidin dihydrochalcone, Kaempferol, Cyanidin, Astragalin, Formononetin, Myricetin, Luteolin, Cianidanol, Butein, Apigenin, Chrysoeriol, and Quercetin. | Antioxidant, Antidiabetic, Cholesterol-lowering, Anti-inflammatory, Anticancer, Antihypertensive, and Antimicrobia (Martineau-Côté et al., 2022) |
| Andira inermis (W.Wright) Kunth ex DC. | Engeletin, Formononetin, Taxifolin, Afrormosin, Pratensein, Calycosin, Astilbin, Prunetin, Genistein, and Daidzein. | Hypoglycemic, Antioxidant, Hematological, and Antiplasmodial (Egua et al., 2020; Gayus Aminu et al., 2024) |
| Spatholobus suberectus Dunn | Liquiritigenin, Formononetin, Genistein, Daidzein, Calycosin, Afrormosin, Taxifolin, Butin, and Butein | Neuroprotective, Antioxidant, Antitumor, Antiviral, Antidiabetic, and Anti-inflammatory (Huang et al., 2023; Zhang et al., 2022) |
| Trigonella foenum-graecum L. | Luteolin, Kaempferol, Rutin, Daidzein, Formononetin, Astragalin, Calycosin, Quercetin, and Irilone. | Antimicrobial, Anticancer, Antioxidant, Neuroprotective, Hormonal, and Anti-obesity (Visuvanathan et al., 2022) |
| Flemingia macrophylla (Willd.) Kuntze ex Merr | Lupinalbin A, Genistein, Kushenol E, Auriculasin, Prunetin, 6,8-Diprenylorobol, Flemiphilippinin C, Flemiphilippinin A, and Flemichin D. | Antioxidant, Antityrosinase, and Antidiabetic (Gahlot et al., 2013; Wang et al., 2012; Fatema et al., 2024) |
| Vigna radiata (L.) R.Wilczek. | Kaempferol, Quercetin, Formononetin, Astragalin, Kievitone, Daidzein, Rutin, and Genistein. | Hypoglycemic, Hypolipidemic, Hepatoprotective, Antihypertensive, Anticancer, Immunomodulatory, and Anti-melanogenesis (Hou et al., 2019) |
The well-characterized species from aphrodisiac-fertility hot nodes corresponded with estrogenic flavonoids in the LOTUS Initiative database (Rutz et al., 2022) and their bioactivities. The species were selected based on the highest number of estrogenic flavonoids identified in the LOTUS Initiative database, excluding species within the same genus.
TABLE 2
| High-level hot nodes | Number of nested hot nodes | Species |
|---|---|---|
| 1. Dialioideae nodes | 4 | Apuleia leiocarpa (Vogel) J.F.Macbr., Dialium guineense Willd., Dicorynia guianensis Amshoff, Distemonanthus benthamianus Baill., Koompassia excelsa (Becc.) Taub., Labichea punctata Benth., Martiodendron parviflorum (Amshoff) Köppen, Storckiella australiensis J.H.Ross and B.Hyland, Petalostylis labicheoides R.Br., and Zenia insignis Chun |
| 2. Clitoria node | 1 | Chamaecrista acosmifolia (Mart. Ex Benth.) H.S.Irwin and Barneby, and Clitoria guianensis (Aubl.) Benth. |
| 3. Delonix nodes | 3 | Colvillea racemosa Bojer, Delonix boiviniana (Baill.) Capuron, D. brachycarpa (R.Vig.) Capuron, D. edulis (H.Perrier) Babineau and Bruneau., D. elata (L.) Gamble, D. floribunda (Baill.) Capuron, D. pumila Du Puy, Phillipson and R.Rabev., D. regia (Bojer ex Hook.) Raf.a, and D. velutina Capuron |
| 4. Entada node | 1 | Entada elephantina (Burch.) S.A.O’Donnell and G.P.Lewis, and E. abyssinica Steud. Ex A.Rich. |
| 5. Alantsilodendron node | 1 | Alantsilodendron pilosum Villiers, Dichrostachys spicata (F.Muell.) Domin, and Vachellia nilotica (L.) P.J.H.Hurter and Mabb. |
| 6. Vachellia borleae nodes | 5 | Vachellia borleae (Burtt Davy) Kyal. and Boatwr., V. dyeri (P.P.Sw. Ex Coates Palgr.) Kyal. and Boatwr., V. flava (Forssk.) Kyal. and Boatwr., V. karroo (Hayne) Banfi and Galassoa, V. kirkii (Oliv.) Kyal. and Boatwr., V. leucophloea (Roxb.) Maslin, Seigler and Ebinger, and V. robbertsei (P.P.Sw. Ex Coates Palgr.) Kyal. and Boatwr. |
| 7. Vachellia caven nodes | 3 | Neltuma laevigata (Humb. and Bonpl. Ex Willd.) Britton and Rose, Vachellia caven (Molina) Seigler and Ebinger, V. bravoensis (Isely) Seigler and Ebinger, V. etbaica (Schweinf.) Kyal. and Boatwr., V. farnesiana (L.) Wight and Arn., and V. schaffneri (S.Watson) Seigler and Ebinger. |
| 8. Senegalia nodes | 7 | Acacia pulchella R.Br., A. scleroxyla Tussac, Senegalia burkei (Benth.) Kyal. and Boatwr., S. caffra (Thunb.) P.J.H.Hurter and Mabb., S. dudgeonii (Craib) Kyal. and Boatwr., S. erubescens (Welw. Ex Oliv.) Kyal. and Boatwr., S. ferruginea (DC.) Pedley, S. fleckii (Schinz) Boatwr., S. galpinii (Burtt Davy) Seigler and Ebinger, S. goetzei (Harms) Kyal. and Boatwr., S. hereroensis (Engl.) Kyal. and Boatwr., S. laeta (R.Br. Ex Benth.) Seigler and Ebinger, S. macrostachya (Rchb. Ex DC.) Kyal. and Boatwr., S. mellifera (Vahl) Seigler and Ebinger, S. modesta (Wall.) P.J.H.Hurter, S. nigrescens (Oliv.) P.J.H.Hurter, S. polyacantha (Willd.) Seigler and Ebinger, S. robynsiana (Merxm. and A.Schreib.) Kyal. and Boatwr., S. senegal (L.) Britton, S. welwitschii (Oliv.) Kyal. and Boatwr., Parasenegalia muricata (L.) Seigler and Ebinger, P. vogeliana (Steud.) Seigler and Ebinger, Prosopis cineraria (L.) Druce, and Vachellia sieberiana (DC.) Kyal. and Boatwr. |
| 9. Poiretia nodes | 1 | Poiretia angustifolia Vogel, P. latifolia Vogel, P. punctata (Willd.) Desv., and P. tetraphylla (Poir.) Burkart |
| 10. Indigofera amblyantha nodes | 6 | Indigofera amblyantha Craib, I. cassioides Rottler ex DC., I. cylindracea Graham ex Baker, I. decora Lindl., I. dosua Buch.-Ham. ex D.Don, I. grandiflora B.H.Choi and S.K.Cho, I. hebepetala Benth. Ex Baker, I. heterantha Wall. Ex Brandis, I. himalayensis Ali, I. kirilowii Palib, I. koreana Ohwi, I. lacei Craib, I. nigrescens Kurz ex King and Prain, I. pendula Franch., I. thibaudiana DC. and I. venulosa Champ. Ex Benth. |
| 11. Indigofera bemarahaensis nodes | 5 | Indigofera bemarahaensis Du Puy and Labat, I. exellii Torre, I. glandulosa J.C.Wendl., I. leucoclada Baker, I. squalida Prain, I. prostrata Willd., and I. psoraloides (L.) L. |
| 12. Sesbania nodes | 4 | Sesbania campylocarpa (Domin) N.T.Burb., S. bispinosa (Jacq.) W.Wight, S. brachycarpa F.Muell., S. formosa (F.Muell.) N.T.Burb., S. grandiflora (L.) Poir., S. microphylla Harm., and S. transvaalensis J.B.Gillett |
The clusters of hot nodes that include no species recorded as having estrogenic flavonoids in the LOTUS initiative database (Rutz et al., 2022). High-level hot nodes were named by tribe or by most represented genus. If a hot node is repeated in another named node, the descendant node is named alphabetically by the genus appearing first.
Neurological uses.
Neurological applications of AF plants
There were 18 of the 165 AF species (10.9%) that also had neurological applications. Of these 18 species, 13 were found in the AF hot nodes, and of those 13, there were eight species (62%) that have been shown to contain estrogenic flavonoids. The eight plants were Peltophorum africanum Sond, Senna siamea (Lam.) H.S.Irwin and Barneby, Senna petersiana (Bolle) Lock, Mundulea sericea (Willd.) A. Chev., Abrus precatorius L., Glycyrrhiza glabra L., Vicia sativa L., and Mimosa pudica L. In comparison, only 22% of the 165 AF species without neurological applications found estrogenic flavonoids.
The frequency of other therapeutic applications of the AF medicinal plants is shown in Figure 3. 40 AF plants were used to treat “general” disorders, so this was the most common category of use for AF. The second most common category was “digestive” disorders; the “neurological” categories were the next most frequently cited, with 19 AF species reported as used for disorders in each of these categories.
FIGURE 3
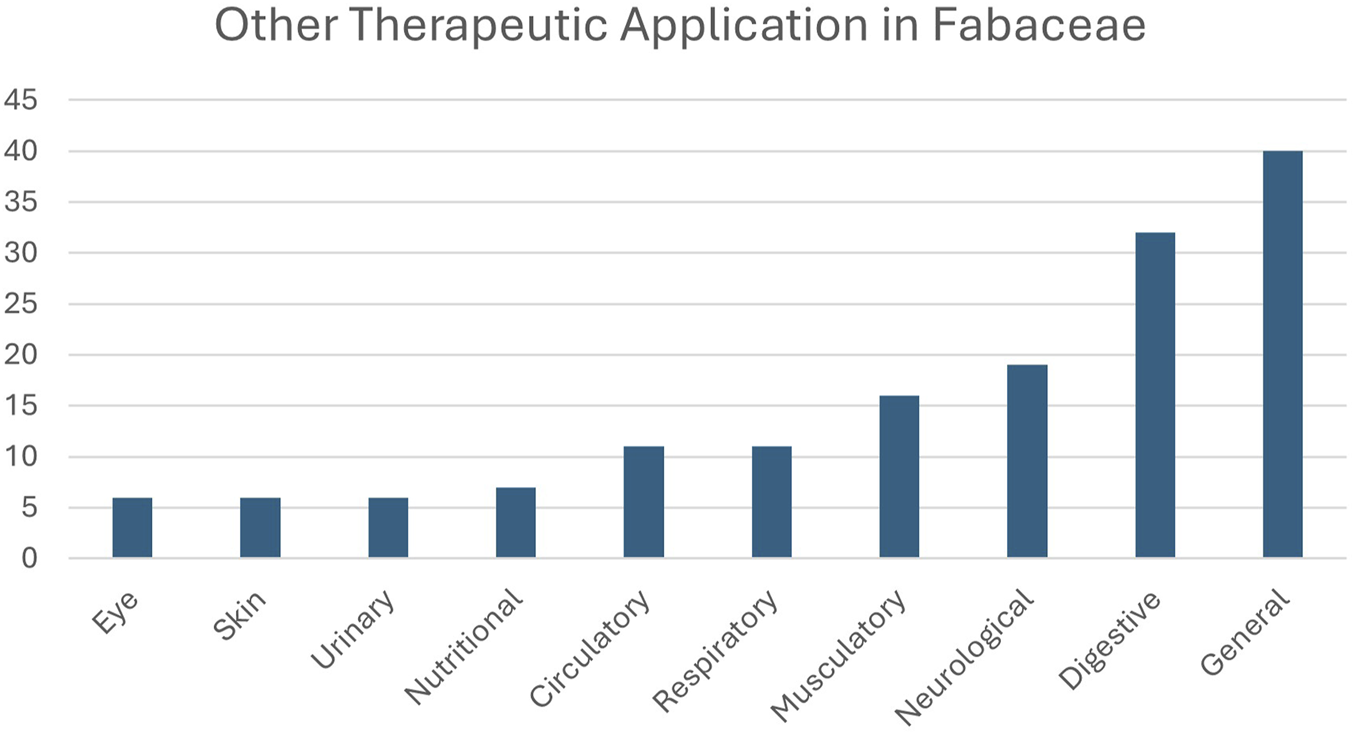
Therapeutic applications of aphrodisiac-fertility plants of Fabaceae. The ten therapeutic categories follow the ICPC-3 International Classification of Primary Care (van Boven and Ten Napel, 2021).
Discussion
The efficiency of phylogenetic prediction
Species with aphrodisiac and fertility uses appear good candidates for the discovery of novel estrogenic flavonoids. A major challenge in drug discovery from plants is the need to select strategically which species to screen, given the impracticality of evaluating all species (Verpoorte, 2000). Plant-derived drugs have been discovered through random or systematic screening of local floras (Perdue and Hartwell, 1969; Spjut, 1985; Barclay and Perdue Jr, 1976) and therapeutic applications in ethnomedicine have also been used in screening programmes to discover new drug leads for many decades. Optimisation of the screening process could include focusing on ethnomedicinal species or plant lineages to which they most frequently belong. This study focused on plants with aphrodisiac and fertility applications in the context of phylogeny to predict lineages with estrogenic flavonoids. Even before we incorporated a phylogenetic framework and hot node analysis, we found that 30% of AF species produce estrogenic flavonoids, compared to 11% of species in phylogenetic tree, demonstrating an increased frequency of estrogenic flavonoid-containing species amongst species with AF applications.
Our study reported a D statistic of 0.70, which is a weak to moderate phylogenetic signal for the AF trait (Fritz and Purvis, 2010). However, our community phylogenetic statistics highlighted a pattern of “clusters,” since both MPD and MNTP are positive, encouraging us to explore the distribution of estrogenic flavonoids relative to hot nodes for the AF trait. The D statistic is rarely reported in studies of specific therapeutic applications, but one example also includes weak to moderate values: in the genus Euphorbia L. D statistic values were all weak apart from one moderate value (Ernst et al., 2016). Our finding, that 21% of species in AF hot nodes have estrogenic flavonoids compared to 11% overall, appears to validate the hot node method, even though the D statistic was suggestive of only moderate predictive power. The search efficiency of 21% for screening hot node species is lower than the search efficiency of 30% for the direct screening of AF species. However, hot node species represent more than three times as many candidates for screening without a correspondingly large decrease in known estrogenic flavonoids. We propose that, at least in the case of the Fabaceae, screening hot node species as well as ethnomedicinal species is strategic. Accessing species in sufficient quantity for screening is not trivial, so being able to have more than three times as many candidates without a correspondingly large decrease in the identification of species with PEs would be valuable.
In this study, we show that considering the overlap between a pair of therapeutic applications can enhance the effectiveness of phylogenetic search strategies. The second application we explore here is the application for neurological therapeutic needs. Our data show that when AF applications overlap with neurological uses, these plants are more likely to include estrogenic flavonoids, suggesting a potential dual role in both reproductive and neurological health. We found that 62% of the AF species that are found in hot nodes and that have neurological applications are known to contain estrogenic flavonoids. This is markedly higher than the 22% with phytoestrogens found among the 165 AF species alone. Considering the other therapeutic applications of the AF species, we show that use for neurological disorders is the second most common specific application, after digestive applications. This appears to be an elevated frequency, for example, in comparison to a ranking of ninth in a study of all therapeutic applications of Brazilian Fabaceae (Souza and Hawkins, 2017), further supporting the view that neurological and AF applications highlight plants with similar bioactivity. Here we highlight the two AF hot nodes which meet the criteria of hot-node inclusion and neurological use, but which have not been tested for estrogenic flavonoids: Delonix nodes and Vachillia borleae nodes. A literature survey revealed two species, one from each hot node, Delonix regia (Bojer ex Hook.) Raf. and Vachellia karroo (Hayne) Banfi and Galasso, which did contain estrogenic flavonoids. Delonix regia (Bojer ex Hook.) Raf. contains quercetin and its derivatives (Modi et al., 2016) and V. karroo (Hayne) Banfi and Galasso has epicatechin (Maroyi, 2017); both are estrogenic flavonoids (Kiyama, 2022). Neither species was included in our list of estrogenic flavonoid species because the natural product database was incomplete (Rutz et al., 2022). Our study shows that the database has been sufficient to validate the use of the AF category in hot node analysis. Going forward, it is likely that analyses of this kind for other flowering plant families would use the LOTUS initiative database, as it is a current and freely available resource.
In our study, we use knowledge of whether plants have estrogenic flavonoids to show that ethnomedicinal uses have predictive power. The presence of estrogenic-flavonoid compounds (Kiyama, 2022) was determined using the LOTUS initiative database (Rutz et al., 2022). Other studies which have sought to validate the hot node method have made comparisons to plant drugs in clinical trials (Pellicer et al., 2018; Ernst et al., 2016; Souza and Hawkins, 2017). Given the increasing application of the hot node method, validation is crucial, so a critical consideration of assumptions related to validation is important. The increase in “search efficiency” we find, from 11% to 30%, is interpreted here as the power of traditional medicine in phytochemical prediction. However, it could also represent a screening bias. There are several biases that one should be aware of, and that might suggest caution in the interpretation of studies such as ours.
Species distributions follow a hollow curve, with some species being highly abundant whilst most are relatively rare (McGill et al., 2007). Widespread species are more likely to be recognised for their properties and included in local medicinal floras, because of both their accessibility and visibility in ethnobotanical field studies (Stepp and Moerman, 2001). For instance, 26% of North American weedy species were reported to have ethnomedicinal use, compared to only 8% of native flora (Moerman, 2005). Furthermore, the species that are used in ethnomedicine may be more likely to be screened because of their perceived value and because they are easily accessible species, contributing to a sampling bias favouring common, weedy species. The size of the genus may also influence research screening effort. Spjut (1985) observed that most genera were represented by only one or two species in screening efforts, whereas the majority of screened species came from large genera distributed across both tropical and temperate phytogeographic regions. While widespread species are better studied and more frequently reported, rare species often remain undocumented, and indigenous perceptions—critical to ethnomedicine—cannot be fully quantified (Phillips and Gentry, 1993). It might be argued that an elevated percentage of species known to have PEs in a hot node, relative to the percentage in the family as in tree, could be because a large proportion of the species in the family are not studied because they are rare. The elevated proportion in the hot nodes would therefore be because there are more ethnobotanically-used species which are screened more often, both because of their medicinal uses and because they are widespread. More sophisticated models are needed to consider the distribution of plants. However, whilst we recognise this caveat, we do consider the raised search efficiency to validate our method. Three factors in addition to the elevated frequency of species with known PEs are relevant here. Firstly, there is an elevated frequency of a secondary therapeutic application, neurological application, and we had predicted these two uses would be attributed to the same underlying phytochemistry. Secondly, our hot node data are drawn from a cross-cultural sample. This is important because it allows us to discover lineages independently, where cultural beliefs about virility might result in biases in studies of a single culture. Such culture-specific beliefs might be expected for aphrodisiac application, for example, it is well known that bitter tonics are attributed aphrodisiac properties specifically in West Africa and the Caribbean (van Andel et al., 2012). Thirdly, we did find that there were PEs in species we predicted to have them, even when these were not recorded by the LOTUS initiative database. Ultimately, the strongest test of the method may be to assay the plants. Pellicer et al. (2018) screened for artemisinin in fifteen species but did not find that species from hot nodes were significantly more likely to have this bioactive molecule. Whether this is an issue specific to congeneric species, where the biosynthetic pathways needed to produce a bioactive are shared, will be determined by further tests of this kind.
We show that predictive methods of the kind we carry out here merit further investigation. However, the search for therapeutically relevant small molecules has ethical dimensions. The data we analyse here are publicly available data describing ethnomedicinal plant use. Much of these data are available as the result of ethnobotanical research, perhaps motivated by a perceived need to preserve ethnomedicinal knowledge that was experiencing rapid erosion (McManis and Ong, 2018; Schultes, 2007). The ethical dimensions of placing data in the digital commons are now under scrutiny (Mulatinho Simoes and Birchfield, 2024). Where research in this area is carried out by national programmes, in China and India, for example, the twin aims of validating and preserving traditional medicine systems can be met, whilst any commercial benefits remain in-country. In our study, the data that we use comes from multiple cultures, and species are highlighted that may not have documented, relevant traditional use. Pellicer et al. (2018) recognise this as an ethical “grey area” yet not addressed and we further highlight this issue here.
Whether the ethical dimensions of the kind of analysis we present here become the specific focus of rethinking protections for knowledge holders may depend on whether these methods enter the commercial sphere. At present, to the best of our knowledge, work of the kind we present here remains in the academic literature. However, the hot node approach has the advantage of highlighting a broader range of species within the same lineage as known ethnobotanical species. While easily accessible AF plants have been well-characterized in local and regional studies (Ajao et al., 2019; Ganie et al., 2019). Other AF plants remain understudied, perhaps due to the limited availability of plant material. These local and regional studies of local plants could include screening of species that are not used medicinally but that are highlighted by phylogenetic studies. In this way, hot node studies use global data to highlight locally available species to incorporate into local and regional research. Hot nodes provide a better way of identifying species that are a random selection, thus reducing unproductive screening in national programmes.
Even where a wider number of species might be targetted, practical limitations such as the season-dependent chemical composition of plant material, which restrict the time window for recollection, remain. Although many plant-derived natural products have already been isolated and characterized, the amounts available were usually insufficient for extensive testing across a wide range of biological activities (Atanasov et al., 2015). In addition to the accessibility of plant material, the quality was also important. The available plant material often varied in quality and composition, which could hinder the accurate assessment of its therapeutic claims. Chemical composition was influenced not only by species identity and harvest time but also by factors such as soil composition, altitude, climate, processing, and storage conditions (Atanasov et al., 2015). Furthermore, during extraction and isolation processes, compounds could transform and degrade, further complicating the evaluation of their potential therapeutic benefits (Kingston, 2011). These challenges in devising and implementing a screening programme highlight how important it may be to widen the pool of targeted species.
Ethnobotany and phytochemistry of priority AF hot nodes
This study devised, tested and demonstrated the utility of a method to use aphrodisiac-fertility AF hot nodes to discover PEs. To demonstrate that the method works, we have necessarily focused on a plant family that has been well-studied, so the distribution of PEs is well-known. Nevertheless, we identified AF hot nodes that did not include any species known to contain estrogenic flavonoids according to the LOTUS initiative database. These priority AF hot nodes were investigated in more detail, and several were shown to include estrogenic flavonoids.
The Dialioideae nodes include Apuleia leiocarpa (Vogel) J.F.Macbr., the bark of this species was used in Peru as a drug to help expel the placenta during childbirth (Odonne et al., 2013), and the root bark of Distemonanthus benthamianus Baill. was used for pain relief (Ajibesin et al., 2008). Phytochemical studies of A. leiocarpa (Vogel) J.F.Macbr. have revealed the presence of flavones (Braz Filho and Gottlieb, 1971), although their estrogenic activities have not yet been investigated.
The Delonix nodes include trees native to Madagascar and East Africa. The most well-known species of the twelve species in the genus, D. regia (Bojer ex Hook.) Raf., has been used in traditional medicine globally and extensively studied for its phytochemical properties (Modi et al., 2016). Ethnobotanical reports show that its flowers have been used to treat gynaecological disorders (Vidyasagar and Prashantkumar, 2007). Studies have identified flavonoids such as leucocyanidin, cyanidin, and quercetin and their derivatives in the plant (Adjé et al., 2010). Another species, Delonix elata (L.) Gamble, has been researched for its mosquito-repellent properties (Govindarajan et al., 2015). While D. regia (Bojer ex Hook.) Raf. and D. elata (L.) Gamble have been extensively studied, other species within the genus have received less attention.
The genera Acacia,Senegalia, and Vachellia are found in the Vachellia borleae nodes, Vachellia caven nodes, and Senegalia nodes, and were previously grouped as a single genus that was segregated due to its non-monophyly (Kyalangalilwa et al., 2013). These genera are found in Australia, Africa, and other tropical regions (Maslin et al., 2003), and have been widely used in traditional medicine across these areas. Phytochemical investigations have identified flavonoids such as apigenin, catechin, epicatechin, kaempferol, naringenin, quercetin, and myricetin derivatives in species from both Africa and Australia (Subhan et al., 2018). One species, Vachellia nilotica (L.) P.J.H.Hurter and Mabb., has been particularly well studied and used to treat a range of conditions, including its use as an aphrodisiac. Research on V. nilotica (L.) P.J.H.Hurter and Mabb. indicates that it possesses anti-inflammatory, antioxidant, antidiarrheal, antihypertensive, and antispasmodic properties, in addition to antibacterial, anthelmintic, anticancer, and acetylcholinesterase (AChE) inhibitory activities (Rather et al., 2015).
The Poiretia nodes consist of twelve endemic species to tropical regions of the Americas. Ethnobotanical reports highlight the use of Poiretia species for treating musculoskeletal ailments (Geck et al., 2016). However, some species, such as Poiretia bahiana C. Mueller, contain sabinene, a toxic monoterpene (Araújo et al., 2009). Moreover, P. bahiana C. Mueller also contains isoflavonoids with antifungal properties (Araújo et al., 2021). Another species, Poiretia latifolia Vogal, contains monoterpenes such as limonene, trans-dihydrocarvone, and carvone, which also exhibit antifungal activities (Nunes Alves Paim et al., 2018).
The Indigofera nodes are found in the genus Indigofera, one of the largest genera within the Fabaceae family (Schrire, 2013) and widely used for medicinal purposes (Gerometta et al., 2020). Several species have been employed as aphrodisiacs, including Indigofera aspalathoides Vahl ex DC. in India (Prabhu et al., 2014), Indigofera cordifolia B. Heyne ex Roth in Cameroon, Kenya, and Tanzania (Ajao et al., 2019), and Indigofera flavicans Baker in Botswana (Ajao et al., 2019). Additionally, I. cordifolia B. Heyne ex Roth has been used as an abortifacient in India (Jain et al., 2004) and Indigofera sanguinea N.E.Br. in Swaziland (Amusan et al., 2002). Phytochemical studies of various species in the genus have identified numerous flavonoids and isoflavonoids, including apigenin, kaempferol, luteolin, quercetin, genistein, coumestrol, formononetin, and their derivatives (Gerometta et al., 2020).
Finally, the species from Sesbania nodes are found in tropical and subtropical regions worldwide (Govaerts, 2024). This genus has been used in traditional medicine for treating malaria (Budiarti et al., 2020), dermatology (Mutheeswaran et al., 2011), and headaches (Chellappandian et al., 2012). Phytochemical studies have shown that Sesbania species contain isoflavonoids (Hasan et al., 2012), though much of their potential pharmacological applications remain unexplored.
Despite the documented medicinal uses of many AF species in hot nodes, significant gaps remain in the phytochemical and pharmacological study, particularly regarding their potential estrogenic activities. The Dialioideae Legume Phylogeny Working Group subfamily, for instance, has shown promising preliminary results in identifying flavones in A. leiocarpa (Vogel) J.F.Macbr., yet its estrogenic potential remains untested. Similarly, while D. regia (Bojer ex Hook.) Raf. has been extensively studied, other species within the genus Delonix Raf. have not received the same attention. This lack of comprehensive research creates a valuable opportunity for further exploration, especially given the known pharmacological relevance of flavonoids. Investigating underexplored genera like Poiretia Sm., Indigofera L., and Sesbania Adams could yield novel estrogenic flavonoids and other bioactive compounds with potential therapeutic applications. As well as suggesting these lineages of the Fabaceae should be studied, we suggest that our methods could also be applied to other families which as less well known, and that this might increase the chances of identifying novel PEs.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
KT: Conceptualization, Data curation, Formal Analysis, Methodology, Visualization, Writing – original draft, Writing – review and editing. JT: Methodology, Writing – review and editing. NV: Conceptualization, Methodology, Supervision, Writing – review and editing. JH: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We are thankful to Assistant Professor Methee Phumthum, Mahidol University, for providing Thai ethnobotanical data and advising on this literature.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1583365/full#supplementary-material
SUPPLEMENTARY DATA SHEET S1The list of known PEs.
SUPPLEMENTARY DATA SHEET S2Angiosperm species containing estrogenic flavonoids.
SUPPLEMENTARY DATA SHEET S3The list of species of Fabaceae phylogeny containing estrogenic flavonoids.
SUPPLEMENTARY DATA SHEET S4The list of species in hot nodes containing estrogenic flavonoids.
References
1
AdjéF.LozanoY. F.LozanoP.AdimaA.ChematF.GaydouE. M. (2010). Optimization of anthocyanin, flavonol and phenolic acid extractions from Delonix regia tree flowers using ultrasound-assisted water extraction. Industrial Crops Prod.32, 439–444. 10.1016/j.indcrop.2010.06.011
2
AjaoA. A.SibiyaN. P.MoteeteeA. N. (2019). Sexual prowess from nature: a systematic review of medicinal plants used as aphrodisiacs and sexual dysfunction in sub-Saharan Africa. South Afr. J. Bot.122, 342–359. 10.1016/j.sajb.2018.08.011
3
AjibesinK. K.EkpoB. A.BalaD. N.EssienE. E.AdesanyaS. A. (2008). Ethnobotanical survey of Akwa Ibom state of Nigeria. J. Ethnopharmacol.115, 387–408. 10.1016/j.jep.2007.10.021
4
AmirkiaV.HeinrichM. (2015). Natural products and drug discovery: a survey of stakeholders in industry and academia. Front. Pharmacol.6, 237. 10.3389/fphar.2015.00237
5
AmusanO. O. G.DlaminiP. S.MsonthiJ. D.MakhubuL. P. (2002). Some herbal remedies from Manzini region of Swaziland. J. Ethnopharmacol.79, 109–112. 10.1016/S0378-8741(01)00381-6
6
AraújoF. M.PassosM. D. G. V. M.LimaE. D. O.RoqueN. F.GuedesM. L. S.Souza-NetaL. C. D.et al (2009). Composition and antimicrobial activity of essential oils from Poiretia bahiana C. Müller (Papilionoideae-Leguminosae). J. Braz. Chem. Soc.20, 10. 10.1590/S0103-50532009001000006
7
AraújoF. M.RibeiroP. R.GuedesM. L. S.YoungM. C. M.MartinsD. (2021). A new isoflavone glucoside and other compounds from Poiretia bahiana C. Mueller: chemophenetics, fragmentation pattern and biogenetic implications. Fitoterapia153, 104977. 10.1016/j.fitote.2021.104977
8
AtanasovA. G.WaltenbergerB.Pferschy-WenzigE.-M.LinderT.WawroschC.UhrinP.et al (2015). Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv.33, 1582–1614. 10.1016/j.biotechadv.2015.08.001
9
Atienza-BarthelemyD.MacíaM. J.Molina-VenegasR. (2024). Hot node limitations and impact of taxonomic resolution on phylogenetic divergence patterns: a case study on Ecuadorian ethnomedicinal flora. Plants, People, Planet, 7(3), 644–653. 10.1002/ppp3.10594
10
AzaniN.BabineauM.BaileyC. D.BanksH.BarbosaA. R.PintoB.et al (2017). A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny: the Legume Phylogeny Working Group (LPWG). TAXON66, 44–77. 10.12705/661.3
11
BarclayA. S.PerdueJ. R. R. E. (1976). Distribution of anticancer activity in higher plants. Cancer Treat. Rep.60, 1081–1113.
12
BergerM. H.MessoreM.PastuszakA. W.RamasamyR. (2016). Association between infertility and sexual dysfunction in men and women. Sex. Med. Rev.4, 353–365. 10.1016/j.sxmr.2016.05.002
13
BooyensR. M.EngelbrechtA.-M.StraussL.PretoriusE. (2022). To clot, or not to clot: the dilemma of hormone treatment options for menopause. Thrombosis Res.218, 99–111. 10.1016/j.thromres.2022.08.016
14
BrancaF.LorenzettiS. (2005). Health effects of phytoestrogens. Diet Diversification and health promotion: European Academy of nutritional sciences (EANS) conference, Vienna, May 2004, S.Karger AG.
15
Braz FilhoR.GottliebO. R. (1971). The flavones of Apuleia leiocarpa. Phytochemistry10, 2433–2450. 10.1016/S0031-9422(00)89891-X
16
BudiartiM.MaruzyA.MujahidR.SariA. N.JokopriyambodoW.WidayatT.et al (2020). The use of antimalarial plants as traditional treatment in Papua Island, Indonesia. Heliyon6, e05562. 10.1016/j.heliyon.2020.e05562
17
CaoZ.-H.Green-JohnsonJ. M.BuckleyN. D.LinQ.-Y. (2019). Bioactivity of soy-based fermented foods: a review. Biotechnol. Adv.37, 223–238. 10.1016/j.biotechadv.2018.12.001
18
CeccarelliI.BiolettiL.PepariniS.SolomitaE.RicciC.CasiniI.et al (2022). Estrogens and phytoestrogens in body functions. Neurosci. Biobehav. Rev.132, 648–663. 10.1016/j.neubiorev.2021.12.007
19
ChellappandianM.MutheeswaranS.PandikumarP.DuraipandiyanV.IgnacimuthuS. (2012). Quantitative ethnobotany of traditional siddha medical practitioners from Radhapuram taluk of Tirunelveli district, Tamil Nadu, India. J. Ethnopharmacol.143, 540–547. 10.1016/j.jep.2012.07.014
20
DixonR. A. (2004). Phytoestrogens. Annu. Rev. Plant Biol.55, 225–261. 10.1146/annurev.arplant.55.031903.141729
21
DongX.NaoJ. (2023). Relationship between the therapeutic potential of various plant-derived bioactive compounds and their related microRNAs in neurological disorders. Phytomedicine108, 154501. 10.1016/j.phymed.2022.154501
22
EguaM. O.NwinyiF. C.OkwocheO. J.MondayO. M.GaniyatA. M.OkwudiliO. S.et al (2020). Evaluation of Andira inermis stem bark extract for hypoglycaemic and antioxidant effects. Clin. Phytosci.6, 76. 10.1186/s40816-020-00225-5
23
ErnstM.Saslis-LagoudakisC. H.GraceO. M.NilssonN.SimonsenH. T.HornJ. W.et al (2016). Evolutionary prediction of medicinal properties in the genus Euphorbia L. Sci. Rep.6, 30531. 10.1038/srep30531
24
FabricantD. S.FarnsworthN. R. (2001). The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect.109, 69–75. 10.1289/ehp.01109s169
25
FatemaK.SharminA. A.SharnaJ. F.HaqueM. A.RahmanM. M.SarkerS.et al (2024). Antioxidant and antidiabetic effects of Flemingia macrophylla leaf extract and fractions: in vitro, molecular docking, dynamic simulation, pharmacokinetics, and biological activity studies. BioResources19, 4960–4983. 10.15376/biores.19.3.4960-4983
26
ForouzanfarF.HosseinzadehH. (2018). Medicinal herbs in the treatment of neuropathic pain: a review. Iran. J. Basic Med. Sci.21, 347–358. 10.22038/ijbms.2018.24026.6021
27
FritzS. A.PurvisA. (2010). Selectivity in mammalian extinction risk and threat types: a new measure of phylogenetic signal strength in binary traits. Conserv. Biol.24, 1042–1051. 10.1111/j.1523-1739.2010.01455.x
28
GahlotK.LalV.JhaS. (2013). Total Phenolic content, flavonoid content and in vitro antioxidant activities of Flemingia species (Flemingia chappar, Flemingia macrophylla and Flemingia strobilifera). Res. J. Pharm. Technol.6, 516–523.
29
GanieA. H.TaliB. A.ShapooG. A.NawchooI. A.KhurooA. A. (2019). Ethno-survey of traditional use of plants as aphrodisiacs in Kashmir Himalaya, India. J. Herb. Med.17-18, 100256. 10.1016/j.hermed.2019.100256
30
Gayus AminuP.ObidahW.Idris AliyuK. (2024). Effects of aqueous extract of Andira inermis stem bark on some reproductive parameters of male Wistar rats. Int. J. Sci. Res. Technol.6. 10.70382/tijsrat.v06i9.006
31
GeckM. S.Reyes GarcíaA. J.CasuL.LeontiM. (2016). Acculturation and ethnomedicine: a regional comparison of medicinal plant knowledge among the Zoque of southern Mexico. J. Ethnopharmacol.187, 146–159. 10.1016/j.jep.2016.04.036
32
GeromettaE.GrondinI.SmadjaJ.FrederichM.Gauvin-BialeckiA. (2020). A review of traditional uses, phytochemistry and pharmacology of the genus Indigofera. J. Ethnopharmacol.253, 112608. 10.1016/j.jep.2020.112608
33
GertschJ. (2012). Cross-cultural comparisons of medicinal floras—what are the implications for bioprospecting?J. Ethnopharmacol.139, 685–690. 10.1016/j.jep.2011.09.017
34
GovaertsR. (2024). The world checklist of vascular plants WCVP. Kew: Royal Botanic Gardens.
35
GovindarajanM.RajeswaryM.SivakumarR. (2015). Repellent properties of Delonix elata (L.) Gamble (family: Fabaceae) against malaria vector Anopheles stephensi (liston) (Diptera: Culicidae). J. Saudi Soc. Agric. Sci.14, 128–133. 10.1016/j.jssas.2013.08.005
36
Gurib-FakimA. (2006). Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol. Aspects Med.27, 1–93. 10.1016/j.mam.2005.07.008
37
HajirahimkhanA.DietzB. M.BoltonJ. L. (2013). Botanical modulation of menopausal symptoms: mechanisms of action?Planta Medica79, 538–553. 10.1055/s-0032-1328187
38
Halse-GramkowM.ErnstM.RønstedN.DunnR. R.Saslis-LagoudakisC. H. (2016). Using evolutionary tools to search for novel psychoactive plants. Plant Genet. Resour.14, 246–255. 10.1017/S1479262116000344
39
HasanN.OsmanH.MohamadS.ChongW. K.AwangK.ZahariluddinA. S. M. (2012). The chemical components of Sesbania grandiflora root and their antituberculosis activity. Pharmaceuticals5, 882–889. 10.3390/ph5080882
40
HeS.SimpsonB. K.SunH.NgadiM. O.MaY.HuangT. (2018). Phaseolus vulgaris lectins: a systematic review of characteristics and health implications. Crit. Rev. Food Sci. Nutr.58, 70–83. 10.1080/10408398.2015.1096234
41
HiremathK. Y.VeeranagoudarD. K.BojjaK. S. (2024). Butea monosperma as a collective phytomedicine and environmentally sustainable, conservative, and beneficial plant. Archives Razi Inst.79, 465–474. 10.32592/ari.2024.79.3.465
42
HolzmeyerL.HartigA.-K.FrankeK.BrandtW.Muellner-RiehlA. N.WessjohannL. A.et al (2020). Evaluation of plant sources for antiinfective lead compound discovery by correlating phylogenetic, spatial, and bioactivity data. Proc. Natl. Acad. Sci. U. S. A.117, 12444–12451. 10.1073/pnas.1915277117
43
HouD.YousafL.XueY.HuJ.WuJ.HuX.et al (2019). Mung bean (Vigna radiata L.): bioactive polyphenols, polysaccharides, peptides, and health benefits. Nutrients11, 1238. 10.3390/nu11061238
44
HuangX.FeiQ.YuS.LiuS.ZhangL.ChenX.et al (2023). A comprehensive review: botany, phytochemistry, traditional uses, pharmacology, and toxicology of Spatholobus suberectus vine stems. J. Ethnopharmacol.312, 116500. 10.1016/j.jep.2023.116500
45
JainA.KatewaS. S.ChaudharyB. L.GalavP. (2004). Folk herbal medicines used in birth control and sexual diseases by tribals of southern Rajasthan, India. J. Ethnopharmacol.90, 171–177. 10.1016/j.jep.2003.09.041
46
KhanA.BellefontaineN.DecatanzaroD. (2008). Onset of sexual maturation in female mice as measured in behavior and fertility: interactions of exposure to males, phytoestrogen content of diet, and ano-genital distance. Physiology Behav.93, 588–594. 10.1016/j.physbeh.2007.10.019
47
KimI.-S.YangW.-S.KimC.-H. (2021). Beneficial effects of soybean-derived bioactive peptides. Int. J. Mol. Sci.22, 8570. 10.3390/ijms22168570
48
KingstonD. G. I. (2011). Modern natural products drug discovery and its relevance to biodiversity conservation. J. Nat. Prod.74, 496–511. 10.1021/np100550t
49
KiyamaR. (2017). Estrogenic terpenes and terpenoids: pathways, functions and applications. Eur. J. Pharmacol.815, 405–415. 10.1016/j.ejphar.2017.09.049
50
KiyamaR. (2022). Estrogenic flavonoids and their molecular mechanisms of action. J. Nutr. Biochem.114, 109250. 10.1016/j.jnutbio.2022.109250
51
KudwaA. E.BoonW. C.SimpsonE. R.HandaR. J.RissmanE. F. (2007). Dietary phytoestrogens dampen female sexual behavior in mice with a disrupted aromatase enzyme gene. Behav. Neurosci.121, 356–361. 10.1037/0735-7044.121.2.356
52
KyalangalilwaB.BoatwrightJ. S.DaruB. H.MaurinO.Van Der BankM. (2013). Phylogenetic position and revised classification of Acacia s.l. (Fabaceae: mimosoideae) in Africa, including new combinations in Vachellia and Senegalia. Botanical J. Linn. Soc.172, 500–523. 10.1111/boj.12047
53
LeontiM.BakerJ.StaubP.CasuL.HawkinsJ. (2024). Taste shaped the use of botanical drugs. Elife12. 10.7554/eLife.90070
54
LetunicI.BorkP. (2021). Interactive Tree of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res.49, W293–W296. 10.1093/nar/gkab301
55
LewisG. P. (2005). Legumes of the world. Richmond, UK: Royal Botanic Gardens, Kew.
56
MaggioliniM.StattiG.VivacquaA.GabrieleS.RagoV.LoizzoM.et al (2002). Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. J. Steroid Biochem. Mol. Biol.82, 315–322. 10.1016/S0960-0760(02)00230-3
57
MaroyiA. (2017). Acacia Karroo Hayne: ethnomedicinal uses, phytochemistry and pharmacology of an important medicinal plant in southern Africa. Asian Pac. J. Trop. Med.10, 351–360. 10.1016/j.apjtm.2017.03.021
58
Martineau-CôtéD.AchouriA.KarbouneS.L’HocineL. (2022). Faba bean: an untapped source of quality plant proteins and bioactives. Nutrients14, 1541. 10.3390/nu14081541
59
MaslinB. R.MillerJ. T.SeiglerD. S. (2003). Overview of the generic status of Acacia (leguminosae: mimosoideae). Aust. Syst. Bot.16, 1–18. 10.1071/sb02008
60
McgillB. J.EtienneR. S.GrayJ. S.AlonsoD.AndersonM. J.BenechaH. K.et al (2007). Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett.10, 995–1015. 10.1111/j.1461-0248.2007.01094.x
61
McmanisC. R.OngB. (2018). Routledge handbook of biodiversity and the law. London and New York: Routledge.
62
MillikenW.WalkerB. E.HowesM.-J. R.ForestF.Nic LughadhaE. (2021). Plants used traditionally as antimalarials in Latin America: mining the tree of life for potential new medicines. J. Ethnopharmacol.279, 114221. 10.1016/j.jep.2021.114221
63
ModiA.MishraV.BhattA.JainA.MansooriM. H.GurnanyE.et al (2016). Delonix regia: historic perspectives and modern phytochemical and pharmacological researches. Chin. J. Nat. Med.14, 31–39. 10.3724/SP.J.1009.2016.00031
64
MoermanD. E. (1991). The medicinal flora of native North America: an analysis. J. Ethnopharmacol.31, 1–42. 10.1016/0378-8741(91)90141-Y
65
MoermanD. (2005). “Dilemmas and solutions,” in North American plant knowledge. Handbook on medicinal plants (Binghamton, NY: The Haworth Press), 125–138.
66
Molina-VenegasR.FischerM.MollelN. P.HempA. (2020). Connecting plant evolutionary history and human well-being at Mt. Kilimanjaro, Tanzania. Botanical J. Linn. Soc.194, 397–409. 10.1093/botlinnean/boaa049
67
MoutouamaJ. K.GaoueO. G. (2024). A phylogenetic evaluation of non-random medicinal plants selection around an African biosphere reserve. People Nat.6, 260–268. 10.1002/pan3.10559
68
Mulatinho SimoesR.BirchfieldV. L. (2024). Biodiversity and the digital transformation. Environ. Ethics46, 47–69. 10.5840/enviroethics202422270
69
MutheeswaranS.PandikumarP.ChellappandianM.IgnacimuthuS. (2011). Documentation and quantitative analysis of the local knowledge on medicinal plants among traditional Siddha healers in Virudhunagar district of Tamil Nadu, India. J. Ethnopharmacol.137, 523–533. 10.1016/j.jep.2011.06.003
70
Najaf NajafiM.GhazanfarpourM. (2018). Effect of phytoestrogens on sexual function in menopausal women: a systematic review and meta-analysis. Climacteric21, 437–445. 10.1080/13697137.2018.1472566
71
NewmanD. J.CraggG. M. (2020). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod.83, 770–803. 10.1021/acs.jnatprod.9b01285
72
NewmanS. W. (1999). The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci.877, 242–257. 10.1111/j.1749-6632.1999.tb09271.x
73
Nunes Alves PaimL. F.Flores Dalla LanaD.GiarettaM.Jacobi DanielliL.Meneghello FuentefriaA.Anders ApelM.et al (2018). Poiretia latifolia essential oil as a promising antifungal and anti-inflammatory agent: chemical composition, biological screening, and development of a nanoemulsion formulation. Industrial Crops Prod.126, 280–286. 10.1016/j.indcrop.2018.10.016
74
OdonneG.ValadeauC.Alban-CastilloJ.StienD.SauvainM.BourdyG. (2013). Medical ethnobotany of the chayahuita of the paranapura basin (Peruvian amazon). J. Ethnopharmacol.146, 127–153. 10.1016/j.jep.2012.12.014
75
O’DonnellE.HarveyP. J.GoodmanJ. M.De SouzaM. J. (2007). Long-term estrogen deficiency lowers regional blood flow, resting systolic blood pressure, and heart rate in exercising premenopausal women. Am. J. Physiol. Endocrinol. Metab.292, E1401–E1409. 10.1152/ajpendo.00547.2006
76
ParadisE.ClaudeJ.StrimmerK. (2004). APE: analyses of phylogenetics and evolution in R language. Bioinformatics20, 289–290. 10.1093/bioinformatics/btg412
77
PastorinoG.CornaraL.SoaresS.RodriguesF.OliveiraM. (2018). Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phytother. Res.32, 2323–2339. 10.1002/ptr.6178
78
PeddioS.PadigliaA.CanneaF. B.CrnjarR.ZamW.Sharifi-RadJ.et al (2022). Common bean (Phaseolus vulgaris L.) α-amylase inhibitors as safe nutraceutical strategy against diabetes and obesity: an update review. Phytother. Res.36, 2803–2823. 10.1002/ptr.7480
79
PellicerJ.Saslis-LagoudakisC. H.CarrióE.ErnstM.GarnatjeT.GraceO. M.et al (2018). A phylogenetic road map to antimalarial Artemisia species. J. Ethnopharmacol.225, 1–9. 10.1016/j.jep.2018.06.030
80
PerdueR. J.HartwellJ. (1969). The search for plant sources of anticancer drugs. Morris Arbor. Bull.20, 35–53.
81
PhillipsO.GentryA. H. (1993). The useful plants of Tambopata, Peru: II. Additional hypothesis testing in quantitative ethnobotany. Econ. Bot.47, 33–43. 10.1007/BF02862204
82
PhumthumM.SrithiK.IntaA.JunsongduangA.TangjitmanK.PongamornkulW.et al (2018). Ethnomedicinal plant diversity in Thailand. J. Ethnopharmacol.214, 90–98. 10.1016/j.jep.2017.12.003
83
PrabhuS.VijayakumarS.YabeshJ. E. M.RavichandranK.SakthivelB. (2014). Documentation and quantitative analysis of the local knowledge on medicinal plants in Kalrayan hills of Villupuram district, Tamil Nadu, India. J. Ethnopharmacol.157, 7–20. 10.1016/j.jep.2014.09.014
84
QiS.-Z.LiB.-B.LiuM.-S.ZhangZ.MiaoS.GongK.-K. (2023). Chemical constituents from the seeds of Cullen corylifolium and their inhibitory activity on diacylglycerol acyltransferase. Nat. Prod. Res.37, 1601–1607. 10.1080/14786419.2022.2103126
85
RatherL. J.Shahid UlI.MohammadF. (2015). Acacia nilotica (L.): a review of its traditional uses, phytochemistry, and pharmacology. Sustain. Chem. Pharm.2, 12–30. 10.1016/j.scp.2015.08.002
86
RenY.SongX.TanL.GuoC.WangM.LiuH.et al (2020). A review of the pharmacological properties of psoralen. Front. Pharmacol.11, 571535. 10.3389/fphar.2020.571535
87
RizzoG.BaroniL. (2018). Soy, soy foods and their role in vegetarian diets. Nutrients10, 43. 10.3390/nu10010043
88
RobinsonD.BryanJ.EliasJ. (2020). fuzzyjoin: join tables together on inexact matching (Version 0.1.6) [R package]. CRAN. Available online at: https://CRAN.R-project.org/package=fuzzyjoin (accessed January 20, 2025).
89
RodríguezL.MendezD.MontecinoH.CarrascoB.ArevaloB.PalomoI.et al (2022). Role of Phaseolus vulgaris L. In the prevention of cardiovascular diseases-cardioprotective potential of bioactive compounds. Plants (Basel)11, 186. 10.3390/plants11020186
90
Rodríguez-LandaJ. F.Hernández-FigueroaJ. D.Hernández-CalderónB. D. C.SaavedraM. (2009). Anxiolytic-like effect of phytoestrogen genistein in rats with long-term absence of ovarian hormones in the black and white model. Prog. Neuro-Psychopharmacology Biol. Psychiatry33, 367–372. 10.1016/j.pnpbp.2008.12.024
91
Rosa LimaS. M. R.BernardoB. F. A.YamadaS. S.ReisB. F.Da SilvaG. M. D.Longo GalvãoM. A. (2014). Effects of Glycine max (L.) Merr. soy isoflavone vaginal gel on epithelium morphology and estrogen receptor expression in postmenopausal women: a 12-week, randomized, double-blind, placebo-controlled trial. Maturitas78, 205–211. 10.1016/j.maturitas.2014.04.007
92
RossouwJ. E.PrenticeR. L.MansonJ. E.WuL.BaradD.BarnabeiV. M.et al (2007). Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA297, 1465–1477. 10.1001/jama.297.13.1465
93
RutzA.SorokinaM.GalgonekJ.MietchenD.WillighagenE.GaudryA.et al (2022). The LOTUS initiative for open knowledge management in natural products research. Elife11, e70780. 10.7554/eLife.70780
94
SandhuK. V.DemirayY. E.YanagawaY.StorkO. (2020). Dietary phytoestrogens modulate aggression and activity in social behavior circuits of male mice. Hormones Behav.119, 104637. 10.1016/j.yhbeh.2019.104637
95
Saslis-LagoudakisC. H.SavolainenV.WilliamsonE. M.ForestF.WagstaffS. J.BaralS. R.et al (2012). Phylogenies reveal predictive power of traditional medicine in bioprospecting. Proc. Natl. Acad. Sci. U. S. A.109, 15835–15840. 10.1073/pnas.1202242109
96
Saslis-LagoudakisC. H.WilliamsonE. M.SavolainenV.HawkinsJ. A. (2011). Cross-cultural comparison of three medicinal floras and implications for bioprospecting strategies. J. Ethnopharmacol.135, 476–487. 10.1016/j.jep.2011.03.044
97
ScavelloI.MaseroliE.Di StasiV.VignozziL. (2019). Sexual health in menopause. Med. Kaunas.55, 559. 10.3390/medicina55090559
98
SchrireB. (2013). A review of tribe indigofereae (Leguminosae–Papilionoideae) in southern Africa (including South Africa, Lesotho, Swaziland and Namibia; excluding Botswana). South Afr. J. Bot.89, 281–283. 10.1016/j.sajb.2013.06.014
99
SchultesR. E. (2007). Amazonian ethnobotany and the search for new drugs. in Ciba foundation symposium 185 ‐ ethnobotany and the search for new drugs. New York: John Wiley & Sons. 106–115. 10.1002/9780470514634.ch8
100
Sharifi-RadJ.KamilogluS.YeskaliyevaB.BeyatliA.AlfredM. A.SalehiB.et al (2020). Pharmacological activities of psoralidin: a comprehensive review of the molecular mechanisms of action. Front. Pharmacol.11, 571459. 10.3389/fphar.2020.571459
101
SkiryczA.KierszniowskaS.MéretM.WillmitzerL.TzotzosG. (2016). Medicinal bioprospecting of the Amazon rainforest: a modern Eldorado. Trends Biotechnol.34, 781–790. 10.1016/j.tibtech.2016.03.006
102
SouzaE. N. F.HawkinsJ. A. (2017). Comparison of herbarium label data and published medicinal use: herbaria as an underutilized source of ethnobotanical information. Econ. Bot.71, 1–12. 10.1007/s12231-017-9367-1
103
SouzaE. N. F.WilliamsonE. M.HawkinsJ. A. (2018). Which plants used in ethnomedicine are characterized? Phylogenetic patterns in traditional use related to research effort. Front. Plant Sci.9, 834. 10.3389/fpls.2018.00834
104
SpjutR. W. (1985). Limitations of a random screen: search for new anticancer drugs in higher plants. Econ. Bot.39, 266–288. 10.1007/BF02858796
105
SteppJ. R.MoermanD. E. (2001). The importance of weeds in ethnopharmacology. J. Ethnopharmacol.75, 19–23. 10.1016/S0378-8741(00)00385-8
106
SubhanN.BurrowsG. E.KerrP. G.ObiedH. K. (2018). “Chapter 9 - phytochemistry, ethnomedicine, and pharmacology of Acacia,” in Studies in natural products chemistry. Editor AttaU. R. R. (Amsterdam: Elsevier).
107
SucherN. J. (2013). The application of Chinese medicine to novel drug discovery. Expert Opin. Drug Discov.8, 21–34. 10.1517/17460441.2013.739602
108
SunM.FolkR. A.GitzendannerM. A.SoltisP. S.ChenZ.SoltisD. E.et al (2020). Recent accelerated diversification in Rosids occurred outside the tropics. Nat. Commun.11, 3333. 10.1038/s41467-020-17116-5
109
TeotiaD.AgrawalA.GoyalH.JainP.SinghV.VermaY.et al (2024). Pharmacophylogeny of genus Allium L. J. King Saud Univ. - Sci.36, 103330. 10.1016/j.jksus.2024.103330
110
ThompsonJ. B.HawkinsJ. A. (2024). Phylogeny and bioprospecting: the diversity of medicinal plants used in cancer management. Plants, People, Planet7, 147–158. 10.1002/ppp3.10566
111
UmeharaK.NemotoK.KimijimaK.MatsushitaA.TeradaE.MonthakantiratO.et al (2008). Estrogenic constituents of the heartwood of Dalbergia parviflora. Phytochemistry69, 546–552. 10.1016/j.phytochem.2007.07.011
112
Van AndelT.MitchellS.VolpatoG.VandebroekI.SwierJ.RuysschaertS.et al (2012). In search of the perfect aphrodisiac: parallel use of bitter tonics in West Africa and the Caribbean. J. Ethnopharmacol.143, 840–850. 10.1016/j.jep.2012.08.008
113
Van BovenK.Ten NapelH. (2021). ICPC-3 international classification of primary Care: user manual and classification. Boca Raton, FL: CRC Press.
114
VerpoorteR. (2000). Pharmacognosy in the new millennium: leadfinding and biotechnology. J. Pharm. Pharmacol.52, 253–262. 10.1211/0022357001773931
115
VidyasagarG. M.PrashantkumarP. (2007). Traditional herbal remedies for gynecological disorders in women of Bidar district, Karnataka, India. Fitoterapia78, 48–51. 10.1016/j.fitote.2006.06.017
116
VisuvanathanT.ThanL. T. L.StanslasJ.ChewS. Y.VellasamyS. (2022). Revisiting trigonella Foenum-graecum L.: pharmacology and therapeutic potentialities. Plants (Basel)11, 1450. 10.3390/plants11111450
117
WangB.JuangL.YangJ.ChenL.TaiH.HuangM. (2012). Antioxidant and antityrosinase activity of Flemingia macrophylla and Glycine tomentella roots. Evidence‐Based Complementary Altern. Med.2012, 431081. 10.1155/2012/431081
118
WangJ.LiY.LiA.LiuR. H.GaoX.LiD.et al (2021). Nutritional constituent and health benefits of chickpea (Cicer arietinum L.): a review. Food Res. Int.150, 110790. 10.1016/j.foodres.2021.110790
119
WangS.ZhangS.WangS.GaoP.DaiL. (2020). A comprehensive review on Pueraria: insights on its chemistry and medicinal value. Biomed. Pharmacother.131, 110734. 10.1016/j.biopha.2020.110734
120
WatiR. K.De GraafE. F.BogarínD.HeijungsR.Van VugtR.SmetsE. F.et al (2021). Antimicrobial activity of necklace orchids is phylogenetically clustered and can be predicted with a biological response method. Front. Pharmacol.11, 586345. 10.3389/fphar.2020.586345
121
WestS. L.ScheidJ. L.De SouzaM. J. (2009). The effect of exercise and estrogen on osteoprotegerin in premenopausal women. Bone44, 137–144. 10.1016/j.bone.2008.09.008
122
WinkM. (2013). Evolution of secondary metabolites in legumes (Fabaceae). South Afr. J. Bot.89, 164–175. 10.1016/j.sajb.2013.06.006
123
YaoR.HeinrichM.ZhangB.WeiX.QiY.GaoW. (2023). Single botanical drugs in the Ayurvedic Pharmacopoeia of India—a quantitative ethnobotanical analysis. Front. Pharmacol.14, 1136446. 10.3389/fphar.2023.1136446
124
YeH.ShawI. C. (2019). Food flavonoid ligand structure/estrogen receptor-α affinity relationships – toxicity or food functionality?Food Chem. Toxicol.129, 328–336. 10.1016/j.fct.2019.04.008
125
YessoufouK.DaruB. H.MuasyaA. M. (2015). Phylogenetic exploration of commonly used medicinal plants in South Africa. Mol. Ecol. Resour.15, 405–413. 10.1111/1755-0998.12310
126
ZamanW.YeJ.AhmadM.SaqibS.ShinwariZ. K.ChenZ. (2022). Phylogenetic exploration of traditional Chinese medicinal plants: a case study on Lamiaceae (Angiosperms). Pak. J. Bot.54 (19). 10.30848/PJB2022-3
127
ZamanW.YeJ.SaqibS.LiuY.ShanZ.HaoD.et al (2021). Predicting potential medicinal plants with phylogenetic topology: inspiration from the research of traditional Chinese medicine. J. Ethnopharmacol.281, 114515. 10.1016/j.jep.2021.114515
128
ZengS.TaiF.ZhaiP.YuanA.JiaR.ZhangX. (2010). Effect of daidzein on anxiety, social behavior and spatial learning in male Balb/cJ mice. Pharmacol. Biochem. Behav.96, 16–23. 10.1016/j.pbb.2010.03.015
129
ZhangF.GanesanK.LiuQ.ChenJ. (2022). A review of the pharmacological potential of spatholobus suberectus dunn on cancer. Cells11, 2885. 10.3390/cells11182885
Summary
Keywords
phytoestrogen, fabaceae, ethnobotany, phylogeny, flavonoids, bioprospecting, hot nodes
Citation
Thaweepanyaporn K, Thompson JB, Vasudevan N and Hawkins JA (2025) Phylogeny, ethnomedicinal use and the distribution of phytoestrogens in the Fabaceae. Front. Pharmacol. 16:1583365. doi: 10.3389/fphar.2025.1583365
Received
25 February 2025
Accepted
16 May 2025
Published
26 May 2025
Volume
16 - 2025
Edited by
Da-Cheng Hao, Dalian Jiaotong University, China
Reviewed by
Richard Spjut, World Botanical Associates, Inc., United States
Sumera Nazneen, Osmania University, India
Sujatha Govindaraj, Bharathidasan University, India
Updates
Copyright
© 2025 Thaweepanyaporn, Thompson, Vasudevan and Hawkins.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kongkidakorn Thaweepanyaporn, k.thaweepanyaporn@pgr.reading.ac.uk
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.