Abstract
Introduction:
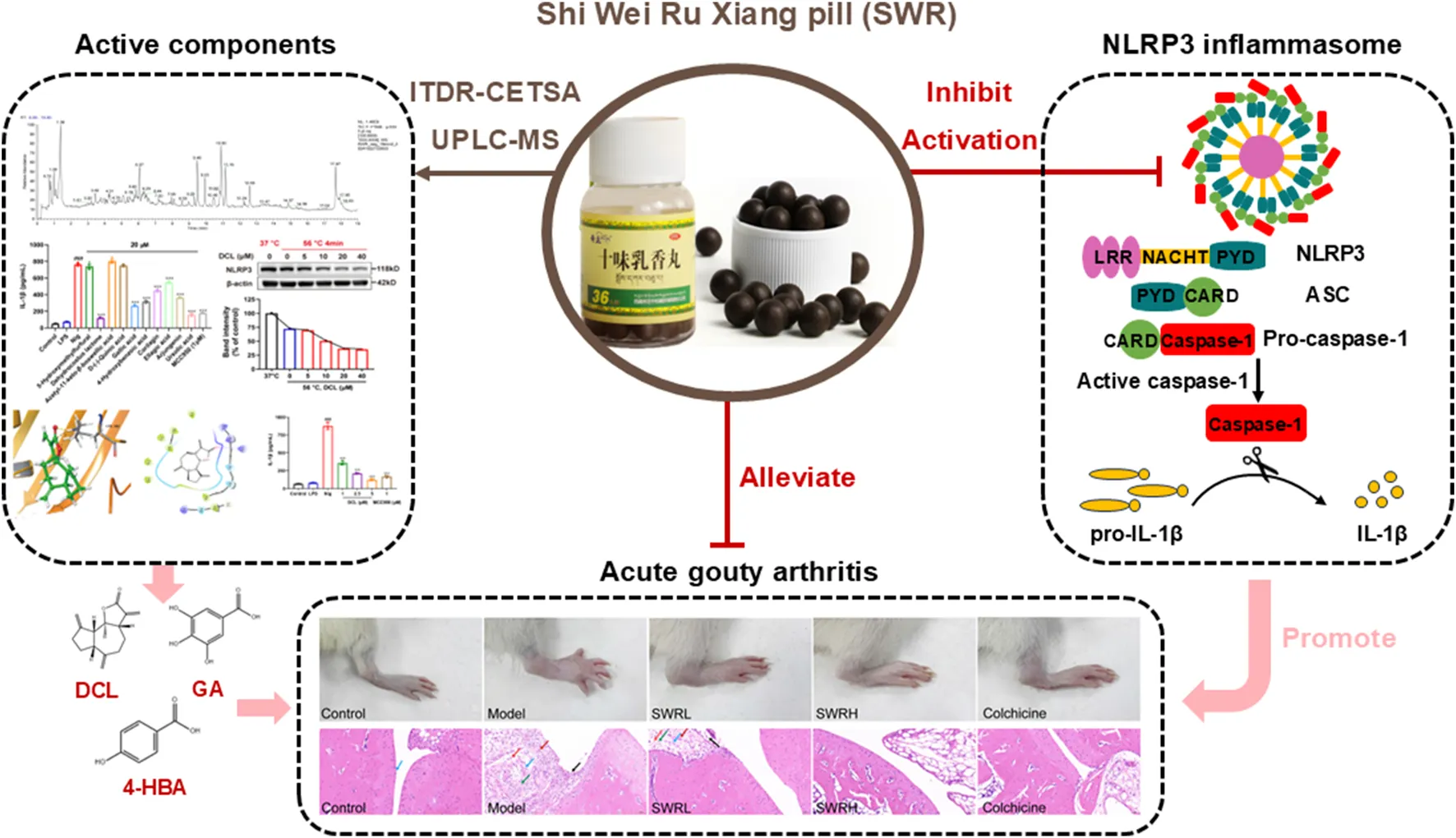
Shi Wei Ru Xiang pill (SWR) is commonly utilized in Tibetan medicine as a therapeutic intervention for “Huang-shui disease” and has been clinically validated as an effective treatment for acute gouty arthritis (AGA). Nevertheless, the underlying mechanisms of action and the active components of SWR in combating AGA remain unclear.
Methods:
In this study, the effects of SWR and its active components on AGA and NLRP3 inflammasome activation were investigated in monosodium urate (MSU)-induced AGA rats and lipopolysaccharide/nigericin-induced THP-1 cells. The chemical profile of SWR was characterized using UPLC-Q-Exactive Orbitrap MS.
Results:
The results indicated that SWR effectively suppressed pyroptosis, caspase-1 activity, and IL-1β production in THP-1 cells. Furthermore, SWR significantly suppressed NLRP3 inflammasome activation and attenuated ankle swelling in a rat AGA model. Specifically, SWR affected the priming and assembly phases to inhibit NLRP3 inflammasome activation. Surprisingly, SWR showed good liver and renal protective effects in AGA rats. A total of 58 compounds were identified in SWR by UPLC-MS analysis. Further pharmacological studies demonstrated that the phenolic compounds serve as active compounds responsible for the inhibition of inflammasome activation, including dehydrocostus lactone, gallic acid, 4-hydroxybenzoic acid.
Discussion:
This is the first study to comprehensively elucidate the therapeutic effects and underlying mechanisms of SWR against AGA by inhibiting NLRP3 inflammasome activation. This study strongly indicated that SWR could serve as a promising anti-inflammatory medicine with an acceptable safety profile for the treatment of AGA and other inflammatory disorders linked to NLRP3 inflammasome activation.
Highlights
• Integrated various approaches first revealed that the therapeutic effects and underlying mechanisms of SWR against AGA through inhibiting NLRP3 inflammasome activation.
• UPLC-MS analysis identified 58 compounds of SWR and revealed its active compounds.
• The binding of the active compounds of SWR to the NLRP3 protein was verified through molecular docking and isothermal dose-response cellular thermal shift assay (ITDR-CETSA).
1 Introduction
Acute gouty arthritis (AGA) is a prevalent inflammatory disease caused by the accumulation of monosodium urate (MSU) crystals in joints (Wang Y. et al., 2023). This disease is marked by significant swelling and intense discomfort in the lower extremity joints, and can eventually lead to deformity if left untreated (Keyβer, 2020). Recently, which has become a growing serious public health concern. In China, the prevalence of AGA in populations aged >40 years is approximately 46.3%, increasing annually (Filardo et al., 2016). It should be stressed that the therapeutic options for treating AGA are limited to colchicine, glucocorticoids, and nonsteroidal anti-inflammatory drugs (NSAIDs) (FitzGerald et al., 2020; Keller and Mandell, 2021). In addition, the clinical utilization of these medications is hindered by their respective hepatorenal toxicity, gastrointestinal adverse effects, and other complications (Ragab et al., 2017). Therefore, there is a pressing need to develop safer, more potent, and inexpensive therapeutic agents for patients with AGA.
AGA pathogenesis is characterized by an inflammatory response elicited by MSU crystal interactions with the local microenvironment, resulting in multiple pathological changes including cellular destruction, tissue injury, or functional impairment. It is commonly accepted that these changes are primarily associated with the overexpression of pro-inflammatory cytokines (Zhao et al., 2022) and the activation of nucleotide-oligomerization domain-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) inflammasome (Martinon et al., 2006). Among these inflammatory cytokines, interleukin-1β (IL-1β) serves as a central driver of joint inflammation associated with gout by facilitating vasodilatation and promoting the recruitment of monocytes and the infiltration of neutrophils to areas where MSU crystals are deposited (Dalbeth et al., 2021). Continuous IL-1β secretion further contributes to the production of enzymes responsible for matrix degradation, which ultimately destroys the cartilage and bone (Schlesinger and Thiele, 2010).
NLRP3 inflammasome is a multiprotein complex that consists of the innate immune sensor NLRP3, adaptor protein apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC), and the effector protein cysteinyl aspartate-specific proteinase-1 (caspase-1) (Lamkanfi and Dixit, 2014). As an important pattern recognition receptor in innate immunity, it is triggered by pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (Sharif et al., 2019; Swanson et al., 2019). The persistent and excessive NLRP3 inflammasome activation is well-established to be closely associated with the gout progression. Its activation facilitates the release of pro-inflammatory cytokine IL-1β, a key pro-inflammatory cytokine that mediates gout. Furthermore, its activation initiates pyroptosis, a form of programmed inflammatory cell death (Jiang et al., 2020; Liu et al., 2016). Therefore, inhibiting NLRP3 inflammasome activation could be of great significance for treating AGA. Recently, IL-1β inhibitors and biological drugs blocking NLRP3 activity have been proven to play an increasingly important role (Jena et al., 2021). Whereas, the high price, risk of severe infections, and potential to induce an exaggerated immune response in vivo have limited their clinical applications (Bettiol et al., 2019). Therefore, it is still attractive to explore novel therapeutic options for AGA by targeting NLRP3 inflammasome.
Owing to the unique geographic environment and regional lifestyle, the incidence rate of AGA in Tibet is significantly higher than that in other regions (Sun et al., 2018). Interestingly, for the treatment of AGA, Tibetan medicine has its characteristics and advantages. There are 22 types of commonly used patent medicines, the most frequently used of which are Twenty-Five Wei’ er Tea Pills, Gouty Decoction, and Shi Wei Ru Xiang Pill (SWR) (La et al., 2021). As a commonly used preparation for treating “Huang-shui disease” with a long history of clinical experience in Tibetan medicine, the prescription of SWR comes from the traditional Tibetan medical classics “Four Medical Tantras” (四部医典). Recent studies have demonstrated that SWR significantly inhibited collagen-induced arthritis in rats by suppressing MAPK and STAT3 signaling pathways (Xiong et al., 2023) and ameliorated hyperuricemia in mice by regulating the NF-κB and MAPK pathways (Li Q. et al., 2022). Although these previous studies have revealed the therapeutic effects and mechanisms of SWR on rheumatoid arthritis and hyperuricemia, the comprehensive therapeutic effects of SWR on AGA and underlying mechanisms remain largely unknown. In addition, the regulatory effects of SWR on NLRP3 inflammasome activation have not been reported. Notably, there are few reports concerning the active components of SWR in AGA treatment, which hinders its clinical application and further development. Therefore, it is necessary to reveal the therapeutic effect of SWR on AGA from a holistic perspective and explore the mechanism of action of SWR with a focus on the regulation of NLRP3 inflammasome activation.
In view of above perspective, in current study, a lipopolysaccharide (LPS)/Nigericin (Nig) induced inflammatory cell model in THP-1 cells and an MSU-induced AGA model in rats was adapted in the hope of further revealing its anti-inflammatory effect on AGA from a holistic perspective and mechanism of action with a focus on the regulation of NLRP3 inflammasome activation.
2 Materials and methods
2.1 The material and preparation of SWR samples
Shi Wei Ru Xiang Pill (SWR, Lot number 221045) was purchased from Tibet Qizheng Tibetan Medicine Co., Ltd. (Lhasa, Tibet, China) and deposited at the laboratory of the School of Chemical Engineering, Sichuan University (Sichuan, China). Its composition is presented in Table 1. According to preparation process included by the criteria of National Drug standards (WS3-BC-0211-95), the ten raw materials, except for Zhaxungao, are crushed into fine powder, sieved and mixed well, then mixed with Zhaxungao and an appropriate amount of water to form pills, the finished product is obtained after drying.
TABLE 1
| NO. | Chinese name | Latin name | Source species | Parts used | Weight (g) |
|---|---|---|---|---|---|
| 1 | Hezi | Chebulae Fructus | Terminalia chebular Retz. | Fruit | 150 |
| 2 | Yuganzi | Phyllanthi Fructus | Phyllanthus emblica L. | Fruit | 120 |
| 3 | Ruxiang | Olibanum | Boswellia carterii Birdw. | Resin | 100 |
| 4 | Kuanjinteng | Caulis Tinosporae Sinensis | Tinospora sinensis (Lour.) Merr. | Stem | 100 |
| 5 | Maohezi | Terminaliae Belliricae Fructus | Terminalia billirica (Gaertn.) Roxb. | Fruit | 100 |
| 6 | Muxiang | Aucklandiae Radix | Aucklandia lappa Decne. | Root | 85 |
| 7 | Juemingzi | Cassiae Semen | Cassia obtusifolia L. | Seed | 80 |
| 8 | Huangkuizi | Abelmoschus moschatus Medicus | Abelmoschus manihot (L.) Medic. | Seed | 80 |
| 9 | Baxiaga | Adhatoda vasica Nees | Veronica eriogyne H.Wink1. | Stem | 80 |
| 10 | Zhaxungao | Brag-zhun | Shilajit | Mineral | 50 |
The composition of Shi Wei Ru Xiang Pill (SWR).
For the preparation of SWR samples, the powders of SWR pills (5g) were soaked in 10 mL of dimethyl sulfoxide and extracted in an ultrasonic bath at room temperature (30 min × 3). The obtained extract was filtered using a 0.22 μm filter, which was stored at 4°C for further analysis.
2.2 Reagents and antibodies
Monosodium urate (MSU) was acquired from Aladdin Reagent Co., Ltd. (Shanghai, China). Phorbol 12-myristate 13-acetate (PMA) and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich Chemicals Co., Ltd. (St. Louis, USA). Adenosine triphosphate (ATP), Disuccinimidyl suberate (DSS), Propidium Iodide (PI) and NP-40 lysis buffer were purchased from Meilunbio (Dalian, China). Nigericin (Nig) was acquired from Invivogen (Toulouse, France). MCC950 was acquired from Med Chem Express (Trenton, USA). Hochest 33342 and Caspase 1 Activity Assay Kit were purchased from Beyotime Biotechnology (Shanghai, China). Colchicine and uric acid were purchased from Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Fetal bovine serum (FBS) was purchased from Gibco (10270-106, Brazil). CytoTox 96® Non-Radioactive Cytotoxicity Assay was acquired from Promega (Madison, USA). Human IL-1β ELISA kit, human TNF-α ELISA kit, rat IL-1β ELISA kit and rat TNF-α ELISA kit were supplied by 4A Biotech (Suzhou, China). Assay kits of UA, blood urea nitrogen (BUN), creatinine (Cr), alanine amino transferase (ALT), aspartate amino transferase (AST) were offered by Nanjing Jian Cheng Bioengineering Institute (Nanjing, China). Antibodies against GAPDH and β-actin were purchased from Abways (Shanghai, China). Anti-ASC antibody and anti-rat caspase-1 antibody was supplied by Santa Cruz Biotechnology (Dallas, USA). Anti-NLRP3 antibody was supplied by Abcam (Cambridge, UK). Anti-human caspase-1 antibody was purchased from Adipogen (San Diego, USA). Anti-IL-1β antibody was purchased from Affinity (Changzhou, China). Anti-GSDMD antibody was supplied by Huabio (Hangzhou, China). All other reagents and solvents were analytically pure and the ultra-pure water was used throughout the experiment.
Huamn THP-1 cells were acquired from Procell Life Science & Technology Co. Ltd. (Wuhan, China). The cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (Gibco, USA), 100 μg/mL streptomycin, and 100 units/mL penicillin in a humidified atmosphere of 95% air and 5% CO2 at 37 °C.
2.3 UPLC-Q-Orbitrap-MS analysis of SWR
UPLC-MS analysis was conducted using a Vanquish Liquid chromatography/Q Exactive Plus Orbitrap mass spectrometer. SWR (10 mg/mL) was prepared by dissolving it in MeOH, straining using a 0.22 μm filter, and analyzed using UPLC. The separation process utilized a HYPERSIL GOLD VANQUISH C18 column (2.1 mm × 100 mm, 1.9 μm) using a gradient elution comprising 0.1% aqueous formic acid (A) and acetonitrile (B) as follows: initially 2% B (0–3 min), increased to 30% B (3–5 min), raised to 70% B (5–14 min), elevated to 98% B (14–16 min), and decreased to 2% B (16–19 min). The column was maintained at 30°C with a 0.35 mL/min flow rate and injection volume of 2 µL. Mass spectrometry was performed using a Q Exactive Plus mass spectrometer fitted with an electrospray ionization source (Thermo Fisher Scientific, USA). Mass spectra were acquired across a range of 100.0–1,500.0 m/z, with chromatographic data collected in negative and positive ionization modes. Data processing and analysis were performed using the Xcalibur software.
2.4 Inflammasome stimulation
To induce NLRP3 inflammasome activation, THP-1 cells were cultured in 48-well plates and subsequently treated with 100 ng/mL PMA for 24 h to get differentiated cells. Following this incubation period, the cells were triggered with 1 μg/mL LPS for 3 h. Subsequently, the cells were incubated for an additional 1 h with 50 and 100 μg/mL SWR. The cells were then stimulated with 10 μM Nig for 40 min, 5 mM ATP for 1 h, 300 μg/mL aluminum salts (Alum) for 6 h, or 500 μg/mL MSU.
2.5 Cell viability assay
The cytotoxicity of SWR was determined using the MTT assay. The experimental procedures were conducted following the previously described method (Du et al., 2024).
2.6 Enzyme-linked immunosorbent assay (ELISA)
After stimulation, the cell culture supernatant was centrifuged to remove cellular debris. Rat blood samples were collected via the abdominal aorta and centrifuged for 15 min. The joint cavity of rats was thoroughly irrigated with sterile saline and the resulting lavage fluid was collected and centrifuged. The concentrations of IL-1β and TNF-α in the culture medium supernatants, rat serum, and joint cavities were quantified using commercial ELISA kits.
2.7 Cell pyroptosis assay
Cells were seeded at a density of 1 × 106/mL in 24-well plates. Differentiated THP-1 cells were primed for 3 h with LPS (1 μg/mL). Subsequently, cells were treated with SWR (50 and 100 μg/mL) for an additional hour, followed by stimulation with Nig (10 μM) for 40 min. The cell supernatant was discarded, and the cells were washed with PBS for three times. Propidium iodide (PI) (5 μg/mL) and Hoechst 33342 (5 μg/mL) were applied for 20 min and were then washed with PBS. Images were acquired using an ECLIPSE Ts2R/FL inverted microscope (Nikon, Japan).
2.8 Lactate dehydrogenase (LDH) assay
LPS-primed THP-1 cells were seeded at a density of 5 × 105/mL in 48-well plates. Cells were subsequently treated with Nig in the presence or absence of the SWR (50 and 100 μg/mL). Culture supernatants were collected and analyzed using an LDH cytotoxicity assay kit.
2.9 Measurement of soluble proteins p17 and p20
After inflammasome stimulation, the culture media were collected, 20% (v/v) trichloroacetic acid (TCA) was added, and the samples were incubated overnight at −20°C. The samples were centrifuged and the resulting pellets were washed three times with cold acetone. Finally, the pellets were resuspended in 1× loading buffer, and the concentrations of p17 and p20 were determined using Western blot analysis.
2.10 Western blot analysis
After inflammasome stimulation, the cells were lysed on ice with RIPA lysis for 30 min buffer and subsequently centrifuged. Synovial tissues from the rats were homogenized in RIPA lysis buffer. Total protein was recovered by centrifugation at 13,000 rpm for 15 min at 4 °C. The protein concentration was quantified using a BCA kit (Beyotime, China), and 5× loading buffer was added to the supernatant, followed by heating at 100°C for 10 min. Subsequent experiments were conducted using previously established methods (Du et al., 2024).
2.11 ASC oligomerization assay
The cells were lysed using NP-40 for 30 min. The resulting lysates were centrifuged and the pellets (NP-40 insoluble fraction) were resuspended in disuccinimidyl suberate (2 mM) and incubated for 30 min. Following incubation, the resuspended pellets were centrifuged and the cross-linked pellets were resuspended in 1× loading buffer for WB analysis.
2.12 Immunofluorescence
After inflammasome stimulation, cells were fixed with ice-cold methanol for 20 min. After blocking in 2.5% bovine serum albumin for 1 h at 25°C, the cells were incubated overnight with ASC antibodies at 4°C. After three washes with PBS, the cells were treated with secondary antibodies for 1 h at 25°C. Nuclei were stained for 10 min. Images were obtained using an ECLIPSE Ts2R/FL inverted microscope (Nikon, Japan).
2.13 Molecular docking
Molecular docking was conducted with the Glide module of Schrödinger (Schrödinger LLC, NY, USA) following a previously established method (Wang et al., 2025). The 3D structure of dehydrocostus lactone (DCL), 4-hydroxybenzoic acid (4-HBA), and gallic acid (GA) was drawn using ChemDraw software and subsequently optimized with the “LigPrep” module. The crystal structure of the human NLRP3 protein (PDB ID: 6NPY) was obtained from the RCSB Protein Data Bank (http://www.rcsb.org/pdb). The protein structure was processed using the Protein Preparation module, which included optimizing protein conformation, removing water molecules, minimizing energy, and adding any incomplete residues and hydrogen atoms. The pH value of the docking environment was set at 7.0 ± 0.5. While the binding site of ligands to NLRP3 was set as a grid box with dimensions 30 × 30 × 30 Å. All other parameters were set to their default values in Schrödinger.
2.14 Isothermal dose-response-cellular thermal shift assay (ITDR-CETSA)
To determine the binding between NLRP3 and active compounds, an ITDR-CETSA assay was performed (Tu et al., 2023). Briefly, LPS-primed THP-1 cells were lysed on ice for 30 min to extract proteins. The collected proteins (2.5 mg/mL) were incubated with DMSO or increasing concentrations of compounds for 1 h at room temperature. Later, the protein samples were heated at a single temperature point (56°C) for 4 min, after cooled at room temperature for 3 min, centrifuged at 15,000 rpm for 30 min to obtain soluble fractions, which were subsequently analyzed by Western blotting.
2.15 Animals
Thirty adult male Sprague-Dawley rats (250 g, 6–8 weeks old, specific pathogen-free grade) were procured from SPF Beijing Biotechnology Co., Ltd. (Animal Licence number SYXK 2023-0015, Beijing, China). The rats were contained in cages with a 12:12-h light-dark cycle, a temperature of 25°C ± 2°C, and a humidity of 55% ± 5%. Before the initiation of the experimental procedures, the rats were allowed a one-week acclimatization period, during which they had unrestricted access to diet and water. All protocols received approved and were adhered to the ethical guidelines of the Jiangxi Zhby Biotech Co., Ltd. Ethics Committee (No. LL-202405310001).
2.16 Establishment of MSU-induced acute gouty arthritis model
The rats were randomly assigned to five groups (n = 6): control, model (MSU), SWRL (400 mg/kg), SWRH (800 mg/kg), and colchicine (positive drug, 1 mg/kg). After 7 days acclimatization, the rats of the SWRL, SWRH, and colchicine groups were gavaged once daily for 6 days, whereas the rats of the remaining groups were gavaged an equivalent volume of 0.5% CMC-Na. One hour after drug administration on day 6, an AGA rat model was established by intra-articular injection of 0.2 mL of MSU suspension (25 mg/mL). Successful establishment of the model was confirmed by an observable contralateral protrusion in the joint cavity, along with redness and swelling of the joint. The hind paw volume of each group was measured at 0, 2, 4, 8, and 24 h following the MSU challenge using a toe volume-measuring instrument (KewBasis, China). According to Coderre’s method (Coderre and Wall, 1988), the gait of the rats was observed and scored as follows: grade 0, normal walking; 1 point, grade 1: slight lameness, the subject’s lower limbs were slightly bent, 2 points; grade 2: moderate lameness, the subject’s lower limbs just touched the ground, 3 points; grade 3: severe lameness, the subject’s lower limbs left the ground and walked on all three feet, 4 points. The joint inflammation index grading was as follows: grade 0 normal, 0 points; grade 1, mild swelling but bony signs with joint skin erythema, 2 points; grade 2, joint parts with limited swelling, bony signs disappeared, and joint erythema was obvious, 4 points; grade 3, joints outside the limbs with swelling, 6 points. Subsequently, all experimental groups were euthanized and tissues from the hind paws were homogenized. The supernatant obtained from the homogenate was collected for ELISA and Western blot analysis.
2.17 Histopathological study
Rat synovial tissue, liver, and right kidney were fixed with 4% paraformaldehyde for 48 h and then dehydrated with ethanol at a concentration gradient sequentially before being embedded in paraffin. These tissues were stained with hematoxylin and eosin (H&E) immediately after being sliced into 4 μm-thick sections. The pathological images of tissue sections were initially observed with an Olympus CKX53 inverted microscope (Tokyo, Japan) and then harvested by using the Pannoramic 250 digital slice scanner (3DHISTECH, Hungary) at a magnification of ×200.
2.18 Statistical analysis
All experimental analyses were conducted independently in triplicate. The in vitro data are presented as the mean ± standard deviation (SD), while the in vivo data are presented as the mean ± standard error of the mean (SEM) using GraphPad Prism 9.5 (San Diego, CA, USA). Significant differences among groups were assessed using a one-way analysis of variance (ANOVA). p-value <0.05 was considered statistically significant.
3 Results
3.1 Chemical profile of SWR by UPLC-MS analysis
The chemical profile of SWR was performed by UPLC-MS, and the chromatograms of positive and negative ion modes were shown in Figure 1. The Xcalibur software was utilized to process and analyze MS data. After testing, 58 identified compounds were matched and applied for qualitative analysis, whose detailed results were shown in Table 2. These compounds were mainly polyphenols and terpenoids, which was consistent with previous study (Li Q. et al., 2022).
FIGURE 1
TABLE 2
| Entry | Rt (min) | Quasi-molecular ion | m/z measured | m/z calculated | Error (ppm) | Molecular formula | Compound identification |
|---|---|---|---|---|---|---|---|
| 1 | 0.72 | [M-H]− | 180.0629 | 180.0634 | 2.69 | C6H12O6 | D-(+)-Galactose |
| 2 | 0.72 | [M+Na]+ | 193.0970 | 193.0950 | 10.28 | C7H15NO5 | (+)-valiolamine |
| 3 | 1.00 | [M-H]− | 192.0627 | 192.0634 | 3.56 | C7H12O6 | D-(-)-Quinic acid |
| 4 | 1.10 | [M-H]− | 129.0419 | 129.0426 | 5.32 | C5H7NO3 | 4-Oxoproline |
| 5 | 1.38 | [M-H]− | 170.0209 | 170.0215 | 3.65 | C7H6O5 | Gallic acid |
| 6 | 1.62 | [M+Na]+ | 150.0891 | 150.0892 | 0.71 | C6H14O4 | Triethylene glycol |
| 7 | 1.76 | [M+Na]+ | 126.0341 | 126.0317 | 19.11 | C6H6O3 | 5-Hydroxymethylfurfural |
| 8 | 2.54 | [M+H]+ | 191.0940 | 191.0946 | 3.26 | C11H13NO2 | 5-Methoxytryptophol |
| 9 | 3.20 | [M-H]− | 138.0311 | 138.0317 | 4.29 | C7H6O3 | 4-Hydroxybenzoic acid |
| 10 | 3.42 | [M-H]− | 179.0578 | 179.0582 | 2.44 | C9H9NO3 | Hippuric acid |
| 11 | 3.74 | [M+H]+ | 355.1774 | 355.1784 | 2.68 | C21H25NO4 | Tetrahydropalmatine |
| 12 | 3.76 | [M-H]− | 634.0818 | 634.0806 | 1.89 | C27H22O18 | Corilagin |
| 13 | 3.78 | [M+H]+ | 285.1356 | 285.1365 | 3.11 | C17H19NO3 | (R)-Coclaurine |
| 14 | 4.09 | [M+H]+ | 353.1254 | 353.1263 | 2.59 | C20H19NO5 | Protopine |
| 15 | 4.31 | [M-H]− | 302.0068 | 302.0063 | 1.78 | C14H6O8 | Ellagic acid |
| 16 | 4.37 | [M+H]+ | 217.2035 | 217.2042 | 3.10 | C12H27NO2 | (2S,3R)-2-aminododecane-1,3-diol |
| 17 | 4.51 | [M-H]− | 124.0518 | 124.0524 | 5.06 | C7H8O2 | 4-Methylcatechol |
| 18 | 4.76 | [M-H]− | 462.0805 | 462.0798 | 1.48 | C21H18O12 | Aureusidin 6-glucuronide |
| 19 | 4.98 | [M+H]+ | 211.0118 | 211.0126 | 3.57 | C9H9NOS2 | 2-(1,3-Benzothiazol-2-ylsulfanyl)ethanol |
| 20 | 5.33 | [M-H]− | 402.0954 | 402.0951 | 0.81 | C20H18O9 | Cerarvensin |
| 21 | 5.52 | [M+Na]+ | 227.1881 | 227.1885 | 1.86 | C13H25NO2 | Cyprodenate |
| 22 | 5.70 | [M+H]+ | 273.2658 | 273.2668 | 3.56 | C16H35NO2 | N-Lauryldiethanolamine |
| 23 | 5.75 | [M+H]+ | 317.2920 | 317.2930 | 3.11 | C18H39NO3 | 2-Amino-1,3,4-octadecanetriol |
| 24 | 5.76 | [M-H]− | 504.3458 | 504.3451 | 1.42 | C30H48O6 | Arjungenin |
| 25 | 6.07 | [M-H]− | 594.1377 | 594.1373 | 0.62 | C30H26O13 | Maritimetin 6- (6″-p-coumarylglucoside) |
| 26 | 6.44 | [M+Na]+ | 414.2035 | 414.2042 | 1.77 | C24H30O6 | Bis(4-ethylbenzylidene)sorbitol |
| 27 | 6.45 | [M-H]− | 284.0689 | 284.0685 | 1.51 | C16H12O5 | Glycitein |
| 28 | 6.47 | [M-H]− | 460.2103 | 460.2097 | 1.28 | C25H32O8 | Prednisolone hemisuccinate |
| 29 | 6.58 | [M-H]− | 158.1301 | 158.1307 | 3.66 | C9H18O2 | Nonanoic acid |
| 30 | 6.97 | [M+H]+ | 402.2371 | 402.2406 | 8.75 | C24H34O5 | Gamabufotalin |
| 31 | 7.07 | [M+H]+ | 230.1298 | 230.1307 | 3.82 | C15H18O2 | Dehydrocostus lactone |
| 32 | 7.20 | [M+H]+ | 282.1974 | 282.1984 | 3.42 | C20H26O | dehydroretinaldehyde |
| 33 | 7.52 | [M+Na]+ | 380.2559 | 380.2563 | 0.97 | C22H36O5 | Limaprost |
| 34 | 7.63 | [M-H]− | 298.2512 | 298.2508 | 1.37 | C18H34O3 | 2-Oxooctadecanoicacid |
| 35 | 7.95 | [M-H]− | 200.1772 | 200.1776 | 2.14 | C12H24O2 | Vulvic acid |
| 36 | 8.10 | [M-H]− | 354.2200 | 354.2195 | 1.43 | C23H30O3 | Etretinate |
| 37 | 8.31 | [M+Na]+ | 514.3499 | 514.3506 | 1.29 | C28H50O8 | Trihexyl 2-(butyryloxy)-1,2,3-propanetricarboxylate |
| 38 | 8.58 | [M-H]− | 470.3397 | 470.3396 | 0.20 | C30H46O4 | 3α-Hydroxyglycyrrhetinate |
| 39 | 8.97 | [M-H]− | 278.2250 | 278.2246 | 1.52 | C18H30O2 | α-Linolenic acid |
| 40 | 9.27 | [M+Na]+ | 396.2870 | 396.2876 | 1.44 | C23H40O5 | Pimilprost |
| 41 | 9.29 | [M-H]− | 228.2088 | 228.2089 | 0.56 | C14H28O2 | Myristic acid |
| 42 | 9.48 | [M-H]− | 340.2404 | 340.2402 | 0.51 | C23H32O2 | 2,2′-Methylenebis (4-methyl-6-tert-butylphenol) |
| 43 | 9.76 | [M+H]+ | 432.2841 | 432.2876 | 8.03 | C26H40O5 | Latanoprost |
| 44 | 9.93 | [M-H]− | 280.2403 | 280.2402 | 0.26 | C18H32O2 | Linoleic acid |
| 45 | 10.05 | [M+H]+ | 288.2444 | 288.2453 | 3.17 | C20H32O | Taxa-4(20) 11(12)-dien-5α-ol |
| 46 | 10.19 | [M+Na]+ | 582.3328 | 582.3345 | 2.95 | C38H46O5 | 1-[4-(3-{4-[(2S)-2,3-Dihydroxypropoxy]-3-methylphenyl}-3-pentanyl)-2-methylphenoxy]-3,3-dimethyl-4-(2-naphthyl)-2-butanone |
| 47 | 10.24 | [M-H]− | 456.3608 | 456.3603 | 1.00 | C30H48O3 | Ursolic acid |
| 48 | 10.45 | [M-H]− | 512.3500 | 512.3502 | 0.33 | C32H48O5 | Acetyl-11-keto-β-boswellic acid |
| 11.67 | [M+H]+ | 512.3491 | 2.09 | ||||
| 49 | 10.82 | [M-H]− | 454.3448 | 454.3447 | 0.24 | C30H46O3 | β-Elemonic acid |
| 12.05 | [M+H]+ | 454.3438 | 1.96 | ||||
| 50 | 10.90 | [M-H]− | 256.2403 | 256.2402 | 0.28 | C16H32O2 | Ethyl myristate |
| 51 | 11.16 | [M-H]− | 282.2558 | 282.2559 | 0.28 | C18H34O2 | Oleic acid |
| 52 | 11.57 | [M-H]− | 404.3138 | 404.3138 | 0.04 | C22H44O6 | Hexadecyl β-D-glucopyranoside |
| 53 | 12.24 | [M-H]− | 498.3713 | 498.3709 | 0.79 | C32H50O4 | Tsugaric acid A |
| 54 | 12.58 | [M-H]− | 284.2716 | 284.2715 | 0.25 | C18H36O2 | Octadecanoic acid |
| 55 | 12.59 | [M+H]+ | 283.2867 | 283.2875 | 2.86 | C18H37NO | Stearamide |
| 56 | 13.75 | [M+H]+ | 323.3179 | 323.3188 | 2.81 | C21H41NO | 1-(14-methylhexadecanoyl)pyrrolidine |
| 57 | 14.01 | [M+H]+ | 311.3179 | 311.3188 | 2.92 | C20H41NO | N,N-Dimethyloctadecanamide |
| 58 | 15.63 | [M-H]− | 340.3342 | 340.3341 | 0.21 | C22H44O2 | Docosanoic acid |
Identified compounds in SWR obtained by UPLC-MS.
3.2 SWR inhibited NLRP3 inflammasome activation
THP-1 cells were used to investigate the effects of SWR on NLRP3 inflammasome activation. The cell viability results revealed that SWR did not exhibit cytotoxicity at concentrations below 500 μg/mL (Figure 2A). While ELISA results indicated that IL-1β levels were elevated in the LPS plus Nig group relative to the control and LPS groups, confirming that the NLRP3 inflammasome activation model was successfully created. Next, we examined whether SWR (50 and 100 μg/mL) inhibited p20 cleavage and IL-1β secretion in THP-1 cells to verify the inhibitory effect of SWR on NLRP3 inflammasome activation. The results indicated that SWR dose-dependently suppressed IL-1β secretion without affecting TNF-α production (Figures 2B,C). In addition, SWR dose-dependently inhibited pro-caspase-1 (p45) form into p20, whereas intracellular pro-caspase-1 levels remained unchanged (p45) (Figures 2D–K). These findings meant that SWR inhibited NLRP3 inflammasome activation in THP-1 cells stimulated with LPS and Nig. Subsequently, we investigated whether SWR attenuated NLRP3 inflammasome activation triggered by other stimuli, including MSU, ATP, and Alum. As expected, SWR treatment effectively reduced the IL-1β maturation induced by MSU, ATP, and Alum in THP-1 cells (Figures 2L–O). Taken together, these observations suggest that SWR exhibits broad-spectrum inhibition of NLRP3 inflammasome activation.
FIGURE 2
3.3 SWR suppressed the cell pyroptosis induced by NLRP3 inflammasome activation
NLRP3 inflammasome activation initiates caspase-1 activation, which subsequently cleaves the N-terminus of Gasdermin D (GSDMD-NT) to form plasma membrane pores, which in turn induces cell pyrolysis and LDH release, leading to cell pyroptosis (Kelley et al., 2019). Following SWR treatment, THP-1 cell viability increased in macrophages stimulated with LPS and Nig (Figure 3A). Cell morphology observation indicated that 100 μg/mL SWR could effectively prevent cell swelling and rupture caused by LPS plus Nig treatment (Figure 3B). While the measurement of LDH release demonstrated that SWR inhibited LDH release (Figure 3C). Additionally, SWR significantly decreased GSDMD-NT expression (Figures 3D,E). THP-1 cells stained with PI displayed red fluorescence. As shown in Figures 3F,G, SWR showed concentration-dependent suppression of red fluorescence production in THP-1 cells, and the inhibition in the SWR (100 μg/mL) group was similar to that of MCC950. These phenomena implied that SWR could inhibit pyroptosis induced by NLRP3 inflammasome activation.
FIGURE 3
3.4 SWR inhibited NLRP3 inflammasome priming processes
In the process of NLRP3 inflammasome initiation, LPS stimulates the expression of NLRP3 and pro-IL-1β proteins through the TLR4/NF-κB pathway, which provides a sufficient protein environment for subsequent NLRP3 inflammasome assembly (Bauernfeind et al., 2009). Therefore, we investigated whether SWR influenced the LPS-induced priming stage of NLRP3 inflammasome activation. Given that TNF-α is a downstream product of the NF-κB signaling pathway and does not require NLRP3 inflammasome activation, the role of SWR in the priming process was examined by detecting the TNF-α, NLRP3, and pro-IL-1β levels. When THP-1 cells were treated with SWR before the LPS challenge, SWR significantly reduced the secretion of LPS-induced TNF-α (Figure 4B) and levels of pro-IL-1β and NLRP3 (Figures 4C–I). These findings meant that SWR suppressing NLRP3 inflammasome activation was related to the NF-κB signaling pathway, Most importantly, SWR inhibited the LPS-induced priming signal of NLRP3 inflammasome activation.
FIGURE 4
3.5 SWR inhibited NLRP3 inflammasome assembly
ASC oligomerization, which participates in NLRP3 inflammasome assembly, is an essential step in the cleavage of caspase-1. Toward unraveling how SWR suppressed NLRP3 activation. The impact of SWR on ASC oligomerization is evaluated. The results revealed that pretreatment with SWR prior to Nig stimulation significantly reduced ASC oligomerization (Figure 5A), indicating that SWR interfered with the upstream signaling pathway of ASC oligomerization. Further immunofluorescence analysis clarified a reduction in ASC speck formation in THP-1 cells stimulated with LPS plus Nig after pretreatment with SWR, suggesting that SWR also inhibited ASC formation (Figures 5B,C). These facts pointed out that SWR inhibits NLRP3 inflammasome activation by blocking ASC oligomerization.
FIGURE 5
3.6 SWR inhibited MSU-induced acute gouty arthritis in rats
Previous studies have revealed that the onset and progression of MSU-induced AGA are driven by the activation of the NLRP3 inflammasome (Szekanecz et al., 2019). In view of this, a rat model of AGA was established to evaluate the in vivo therapeutic efficacy of SWR. Rats were initially pretreated with SWR for 6 days, and the model was established by intra-articular injection of MSU 1 h after the final administration of SWR (Figure 6A). The doses of SWR (400 and 800 mg/kg) used in the experiments were triple and 1.5 times those clinically administered in adults. After MSU injection, the joints of the rats exhibited marked visible swelling (Figure 6B), representing that AGA was successfully induced. The joint volume in each group was measured using a toe volume-measuring instrument at 0, 2, 4, 8, and 24 h after MSU injection, and the degree of joint swelling was recorded. Compared to the model group, the joint swelling rate was significantly reduced in SWR-treated rats (Figures 6D,G). Encouragingly, the arthritis index was significantly increased in the model group of rats compared with that in the normal control group and significantly decreased after intervention with the positive drug colchicine and high-dose SWR (Figures 6C,F). In addition, the gait scores of rats in the model group were higher than those in the control group while the gait scores were significantly lower in the SWRH and colchicine groups than in the model group (Figures 6E,H). H&E staining images of the joint tissues showed that synovial cells within the knee joints exhibited varying degrees of necrosis, synovial tissue congestion, and inflammatory cell infiltration following MSU injection. Most importantly, inflammatory cell infiltration was markedly suppressed in the SWRH group (Figure 6I), demonstrating that SWR effectively alleviated AGA-related symptoms.
FIGURE 6
3.7 SWR inhibited NLRP3 inflammasome activation to prevent acute gouty arthritis in rats
Next, emphasis is given to the relationship of the therapeutic effect of SWR on AGA with the inhibition of inflammasome activation. As shown in Figures 7A,B, compared to the model group, the production of IL-1β and TNF-α in rat serum in the SWR and colchicine groups were significantly reduced, a gradient decrease in the SWRL and SWRH groups can be observed, in which the SWRH group exhibited therapeutic efficacy equivalent to that of the colchicine group. The expression of inflammasome-related markers in the synovial tissues of the model group increased significantly, indicating successful modeling (Figures 7C,G). Interestingly, oral administration of SWRL and SWRH significantly downregulated the protein expression of NLRP3, caspase-1, IL-1β, ASC, and GSDMD. Furthermore, SWRL and SWRH decreased IL-1β (Figure 7D) and TNF-α levels (Figure 7E) and caspase-1 activity (Figure 7F) in synovial tissues of rats with AGA. These variations were consistent with the findings of the cellular experiments, depicting that SWR could attenuate AGA by inhibiting the NLRP3 inflammasome activation and reducing the production of IL-1β and TNF-α.
FIGURE 7
3.8 SWR displayed good safety in rats with AGA
As displayed by H&E staining images of livers (Figure 8A), the hepatocyte membranes, and cords in the control group exhibited integrity and were organized systematically and devoid of significant pathological alterations, meanwhile the hepatic sinusoids maintained a structurally normal appearance. Noticeably, compared to control groups, slight lymphocytic infiltration and a small number of hepatocytes with mild edema were observed in the model group. The pathology of the liver in the SWR group was similar to that of the control group, no significant change can be observed in AST/ALT ratios between the SWR groups and the control group, which suggested the safe hepatic profile of SWR (Figures 8A,C,E,G).
FIGURE 8
H&E staining images showed renal pathological changes in rats with AGA, in which slight hydropic degeneration of renal tubular epithelial cells and a small amount of lymphocyte infiltration appeared. Administration of colchicine does not alleviate renal damage. Inspiringly, both SWRL and SWRH alleviated histopathological damage in the kidney compared to that of the colchicine group (Figure 8A). The good renal protective effect of SWR can be derived from a marked reduction in serum creatinine (Cr) and blood urea nitrogen (BUN) concentrations of the SWR-treated group (Figures 8D,F). Simultaneously, the elevated level of serum uric acid (UA) in AGA rats was significantly decreased by SWR treatment. These observations implied dual anti-inflammatory and UA-lowering effects of SWR.
3.9 Identification of active compounds of SWR against NLRP3 inflammasome activation
The above investigations has some interest findings, unfortunately, the active compounds of SWR against AGA remain unknown, particularly those related to the inhibition of NLRP3 inflammasome. The UPLC-MS analysis identified a total of 58 compounds, considering the commercial availability, ten compounds were selected and purchased for the subsequent bioactivity validation. Seven of them (Figure 9B), namely DCL, gallic acid (GA), 4-hydroxybenzoic acid (4-HBA), ursolic acid, arjungenin, corilagin, and ellagic acid, displayed good inhibitory activities on IL-1β production in LPS/Nig-induced THP-1 cells (Figure 9A). Further detailed evaluation of three potent compounds (DCL, GA, and 4-HBA) revealed their significant dose-dependent inhibition on IL-1β production, which disclosed they might possess good NLRP3 inflammasome inhibitory activity (Figure 9C). Molecular docking (Figure 9D) showed the strong interactions of three compounds with NLRP3 via hydrogen bonding. In brief, Lys322, ARG335, ASP272, GLN321, GLY271, and SER331 of NLRP3 protein are the key amino acids that interact with ligands. The carbonyl group of DCL, the free hydroxyl group of GA, and 4-HBA all form hydrogen bonds with the residue Lys322 of NLRP3. In addition, the presence of a salt bridge was observed in the binding mode of GA with NLRP3. These interaction modes indicated that the binding of three compounds to NLRP3 protein was quite stable. Subsequently, ITDR-CETSA analysis was adapted to determine whether the active compounds could directly bind to NLRP3 in the THP-1 cells. Transient heating at 56 °C than at 37 °C significantly caused NLRP3 protein denaturation, it is gratifying that incubation with GA and 4-HBA enhanced the resistance of NLRP3 to thermal denaturation. Additionally, incubation with DCL concentration-dependently reduced the thermal stability of NLRP3 (Figure 9E). These results indicate that GA, 4-HBA, and DCL can directly bind to NLRP3 in pathological environments, thereby inhibiting IL-1β production.
FIGURE 9
4 Discussion
AGA is considered a sterile autoimmune form of arthritis that involves rapid inflammation due to the MSU accumulating in the joints, marked by an abrupt onset of intense joint pain (Wang et al., 2024). Currently, treatment predominantly relies on pharmacological interventions including NSAIDs and colchicine. Notably, the unpleasant side effects cannot be ignored.
The NLRP3 inflammasome is a polyprotein composite primarily responsible for caspase-1 invasion, causing the release of proinflammatory cytokines and cell pyroptosis (Wang D. et al., 2020; Wang T. et al., 2023; Zou et al., 2023). Persistent and excessive activation of NLRP3 inflammasome can cause various inflammatory diseases such as Alzheimer’s disease and atherosclerosis (Daniels et al., 2016; Mangan et al., 2018). Recent studies have established an association between the NLRP3 inflammasome and the initiation and advancement of AGA (Lee et al., 2016; Szekanecz et al., 2019). Consequently, pharmacological agents targeting the inhibition of NLRP3 inflammasome have emerged as a potential therapeutic strategy for AGA (Zahid et al., 2019). Normally, the activation of NLRP3 inflammasome is divided into the priming phase and activation phase (Afonina et al., 2017). During the priming phase, PAMPs are detected by Toll-like receptors, resulting in the activation of the MAPK and NF-κB signaling pathways to upregulate NLRP3 and pro-IL-1β protein expression, of which LPS is a typical class of PAMPs (Zindel and Kubes, 2020). In the subsequent activation phase, signals, such as Nig and MSU, activate and assemble the NLRP3 inflammasome. Once NLRP3 inflammasomes are activated, pro-IL-1β is cleaved into the IL-1β form, expanding the inflammatory cascade in AGA (Jo et al., 2016).
Tibetan medicine displays positive clinical efficacy and low toxicity in preventing and treating AGA (Guo et al., 2022; Nima et al., 2024). SWR, a traditional Tibetan medicinal formulation, has been shown to be effective in the clinical management of AGA. It has recently been established that SWR could alleviate rheumatoid arthritis by restraining the MAPK and STAT3 signaling pathways, ameliorating HUA by regulating the MAPK and NF-κB signalling pathways (Li Q. et al., 2022; Xiong et al., 2023). It raised a question of whether SWR alleviated AGA via suppression of NLRP3 inflammasome activation. For these reasons, we embarked upon a systematic investigation of the anti-inflammatory effects of SWR on AGA rats and corresponding active compounds. Resulting observations unveiled that the suppression of NLRP3 inflammasome activation emerged as a critical mechanism contributing to the anti-AGA effects of SWR. SWR could both suppress NLRP3 inflammasome priming and activation processes and this inhibition is closely associated with its anti-inflammatory properties.
ASC is a crucial adaptor protein within the cytoplasm that connects pattern recognition receptors to pre-caspase-1. Upon triggering NLRP3 inflammasome sensors, ASC assembles into larger helical fibrils, forming ASC oligomers and specks that offer a molecular platform for autocatalytic cleavage and caspase-1 (Hoss et al., 2017). Thus, ASC oligomerization is essential for the activation of the NLRP3 inflammasome, and the regulation of ASC speckle formation is regarded as a promising approach for the treatment of inflammation. In this study, SWR suppressed ASC oligomerization, implying that it constrains NLRP3 inflammasome activation by obstructing inflammasome assembly. Unfortunately, the specific inhibition mechanism of ASC oligomerization remains unclear. More attention should be paid to this aspect.
It is widely known that AGA is an intense inflammation triggered by MSU sedimentation within the joints. MSU crystals are identified and phagocytosed by various intrinsic immune cells that enter the cell and activate intracellular signaling, including the formation of NLRP3 inflammasomes (Liu-Bryan, 2010). MSU crystals interact with intracellular NLRP3 inflammasomes and then secrete IL-1β into the extracellular compartment, resulting in neutrophil recruitment and the release of further inflammatory mediators, causing an amplified inflammatory cascade and accelerating the process of AGA (Torres et al., 2009). The above findings revealed that SWR effectively alleviated joint swelling and inflammatory pathological alterations in the synovial tissues of rats with AGA and the efficacy of the SWRL group was comparable to colchicine group. Meanwhile, SWR significantly suppressed caspase-1 activity and IL-1β production in the rat serum and paw tissue. Specifically, SWR decreased NLRP3, IL-1β, caspase-1, and ASC expression along with the cleavage of GSDMD-NT in vivo. These observations indicated that SWR alleviated MSU-induced AGA symptoms in rats through inhibiting NLRP3 inflammasome activation.
Colchicine, the first-line drug for AGA treatment, is highly effective but generated severe adverse effects (Terkeltaub, 2010). In clinical practice, the serum AST/ALT ratio reflects hepatic function (Chen et al., 2022), while renal function is assessed based on serum Cr and BUN (Patel et al., 2013; Zhong et al., 2022). No substantial liver damage and significant renal injury can be observed in vivo experiments. On the contrary, the colchicine group showed strong renal toxicity, whereas the SWRL and SWRH groups showed no hepatic injury and partial renal protection, as evidenced by the AST/ALT ratio, BUN and Cr levels, and histopathological results. Which confirms the favorable safety profile of SWR. HUA, characterized by an increased serum uric acid concentration, is considered a significant risk factor for AGA (Beyl et al., 2016; Mehmood et al., 2024). Approximately 5% of patients with HUA develop into AGA in a later period, experience significant pain, and have a diminished quality of life (Igel et al., 2017). So, in that sense, lowering serum UA levels may combat AGA. Notably, the significant serum UA-reducing effect of SWR was compatible with previous findings (Li Q. et al., 2022). It can be inferred that its alleviation of AGA was achieved by the dual anti-inflammatory and UA-lowering activities.
SWR is composed of ten materials, leading to its complex chemical profile. This complexity necessitates prompt identification of the active compounds responsible for its pharmacological effects. UPLC-MS analysis revealed 58 compounds, mainly polyphenols and terpenoids. Seven compounds (DCL, GA, 4-HBA, ursolic acid, arjungenin, corilagin, and ellagic acid) inhibited NLRP3 inflammasome activation. Among them, DCL was the most potent, corroborating previous findings (Chen et al., 2020). In addition, the inhibition of inflammasome activation of GA (Lin et al., 2020; Yu et al., 2023), 4-HBA, ursolic acid (Lei et al., 2023; Liu et al., 2024; Wang C. et al., 2020), corilagin (Li W. et al., 2022; Luo et al., 2022), and ellagic acid (Sun et al., 2021) has been reported previously. To our knowledge, this is the first report on the inhibitory activity of arjungenin (a common natural triterpenoid) against the NLRP3 inflammasome. Further molecular docking revealed that key hydrogen bonding interaction between NLRP3 and three active compounds (DCL, GA, and 4-HBA). Current findings and related reports demonstrate that phenolic are the main contributors in SWR to the amelioration of AGA by inhibiting the NLRP3 inflammasome activation.
It is worth noting that there are some limitations in this study. For example, due to the restriction of commercial availability, we only determined the bioactivities of 10 compounds that were available for purchase, and did not evaluate the activity of the remaining identified compounds. It will be necessary in the future to comprehensively evaluate the medicinal potential of SWR chemical components using virtual screening or artificial intelligence-driven drug screening methods. Some chemical components in SWR have been shown to suppress ROS production, including ursolic acid, 4-Hydroxybenzoic acid, corilagin and gallic acid, which might suggest that SWR can decrease ROS production (Kou et al., 2024; Lin et al., 2020; Liu et al., 2024; Luo et al., 2022). It is well-established that neutrophil extracellular traps (NETs) play a critical pathogenic role in gout by driving inflammation and tissue damage, which serves as an important biomarker and therapeutic target for gout and AGA (Liu et al., 2023; Remijsen et al., 2011; Schauer et al., 2014). Unfortunately, in this study, we mainly focused on its effect on NLRP3 inflammasome, with limited efforts on its impact on other pathological pathways of AGA. Future studies should switch to its regulation of ROS signaling and NETs, so as to provide a more comprehensive understanding of the anti-inflammatory mechanism of SWR.
In conclusion, current studies revealed that SWR significantly alleviated MSU-induced AGA symptoms in rats through dual UA-lowering and anti-inflammatory effects. The latter effect was primarily linked to its significant inhibition of NLRP3 inflammasome activation. The mechanism of action of SWR in treating AGA is detailed in Figure 10. SWR treatment effectively disrupted the inflammatory cascade initiated by the central inflammatory cytokine, IL-1β. Further investigation demonstrated that phenolics play key role in inhibition of inflammasome activation. This study offers a reasonable explanation for the effective and safe clinical use of SWR and provides valuable insights into its application.
FIGURE 10
Statements
Data availability statement
The raw Orbitrap data has been deposited to the MetaboLights repository, accession number MTBLS12617. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. The animal study was approved by Jiangxi Zhby Biotech Co., Ltd. Ethics Committee (No. LL-202405310001). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
NW: Data curation, Methodology, Visualization, Writing – original draft. PZ: Software, Visualization, Writing – review and editing. QY: Data curation, Methodology, Writing – review and editing. LL: Formal Analysis, Software, Writing – review and editing. HX: Formal Analysis, Validation, Writing – review and editing. CY: Resources, Writing – review and editing. YT: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review and editing. YL: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Tibet Autonomous Region Department of Science and Technology in China (Grant No. XZ202301ZY0034G).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1595578/full#supplementary-material
References
1
AfoninaI. S.ZhongZ.KarinM.BeyaertR. (2017). Limiting inflammation—the negative regulation of NF-κB and the NLRP3 inflammasome. Nat. Immunol.18, 861–869. 10.1038/ni.3772
2
BauernfeindF. G.HorvathG.StutzA.AlnemriE. S.MacDonaldK.SpeertD.et al (2009). Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol.183, 787–791. 10.4049/jimmunol.0901363
3
BettiolA.LopalcoG.EmmiG.CantariniL.UrbanM. L.VitaleA.et al (2019). Unveiling the efficacy, safety, and tolerability of anti-interleukin-1 treatment in monogenic and multifactorial autoinflammatory diseases. Int. J. Mol. Sci.20, 1898. 10.3390/ijms20081898
4
BeylR. N.HughesL.MorganS. (2016). Update on importance of diet in gout. Am. J. Med.129, 1153–1158. 10.1016/j.amjmed.2016.06.040
5
ChenY.LiR.WangZ.HouX.WangC.AiY.et al (2020). Dehydrocostus lactone inhibits NLRP3 inflammasome activation by blocking ASC oligomerization and prevents LPS-mediated inflammation in vivo. Cell Immunol.349, 104046. 10.1016/j.cellimm.2020.104046
6
ChenZ.MaY.CaiJ.SunM.ZengL.WuF.et al (2022). Serum biomarkers for liver fibrosis. Clin. Chim. Acta537, 16–25. 10.1016/j.cca.2022.09.022
7
CoderreT. J.WallP. D. (1988). Ankle joint urate arthritis in rats provides a useful tool for the evaluation of analgesic and anti-arthritic agents. Pharmacol. Biochem. Behav.29, 461–466. 10.1016/0091-3057(88)90004-4
8
DalbethN.GoslingA. L.GaffoA.AbhishekA. (2021). Gout. Lancet397, 1843–1855. 10.1016/S0140-6736(21)00569-9
9
DanielsM. J. D.Rivers-AutyJ.SchillingT.SpencerN. G.WatremezW.FasolinoV.et al (2016). Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat. Commun.7, 12504. 10.1038/ncomms12504
10
DuJ.WangN.YuD.HeP.GaoY.TuY.et al (2024). Data mining-guided alleviation of hyperuricemia by Paeonia veitchii Lynch through inhibition of xanthine oxidase and regulation of renal urate transporters. Phytomedicine124, 155305. 10.1016/j.phymed.2023.155305
11
FilardoG.KonE.LongoU. G.MadryH.MarchettiniP.MarmottiA.et al (2016). Non-surgical treatments for the management of early osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc.24, 1775–1785. 10.1007/s00167-016-4089-y
12
FitzGeraldJ. D.DalbethN.MikulsT.Brignardello-PetersenR.GuyattG.AbelesA. M.et al (2020). 2020 American college of rheumatology guideline for the management of gout. Arthritis Care Res. Hob.72, 744–760. 10.1002/acr.24180
13
GuoM.-X.ZhangM.-M.YangH.-Y.ZhangC.-L.ChengH.-Y.LiN.-Z.et al (2022). Lagotis brachystachya maxim attenuates chronic alcoholic liver injury combined with gouty arthritis in rats via its anti-inflammatory activity. Front. Pharmacol.13, 995777. 10.3389/fphar.2022.995777
14
HossF.Rodriguez-AlcazarJ. F.LatzE. (2017). Assembly and regulation of ASC specks. Cell. Mol. Life Sci.74, 1211–1229. 10.1007/s00018-016-2396-6
15
IgelT. F.KrasnokutskyS.PillingerM. H. (2017). Recent advances in understanding and managing gout. F1000Res6, 247. 10.12688/f1000research.9402.1
16
JenaM.TripathyA.MishraA.MaitiR. (2021). Effect of canakinumab on clinical and biochemical parameters in acute gouty arthritis: a meta-analysis. Inflammopharmacology29, 35–47. 10.1007/s10787-020-00753-z
17
JiangH.GongT.ZhouR. (2020). “Chapter Three - the strategies of targeting the NLRP3 inflammasome to treat inflammatory diseases,” in Adv. Immunol. Editors DongC.JiangZ. (Academic Press), 55–93.
18
JoE.-K.KimJ. K.ShinD.-M.SasakawaC. (2016). Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol.13, 148–159. 10.1038/cmi.2015.95
19
KellerS. F.MandellB. F. (2021). Management and cure of gouty arthritis. Med. Clin. North Am.105, 297–310. 10.1016/j.mcna.2020.09.013
20
KelleyN.JeltemaD.DuanY.HeY. (2019). The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci.20, 3328. 10.3390/ijms20133328
21
KeyβerG. (2020). Gout arthritis: pathogenesis, diagnostics and treatment. Dtsch. Med. Wochenschr.145, 991–1005. 10.1055/a-1036-8348
22
KouY.JingQ.YanX.ChenJ.ShenY.MaY.et al (2024). 4-Hydroxybenzoic acid restrains Nlrp3 inflammasome priming and activation via disrupting PU.1 DNA binding activity and direct antioxidation. Chem-Biol Interact.404, 111262. 10.1016/j.cbi.2024.111262
23
LaM.ZhenB.ZhuoG.LuorangY. (2021). Efficacy of Tibetan medicine in the treatment of gouty arthritis and analysis of treatment pattern. Asia-Pacific Tradit. Med.17, 11–14.
24
LamkanfiM.DixitV. M. (2014). Mechanisms and functions of inflammasomes. Cell157, 1013–1022. 10.1016/j.cell.2014.04.007
25
LeeH. E.YangG.KimN. D.JeongS.JungY.ChoiJ. Y.et al (2016). Targeting ASC in NLRP3 inflammasome by caffeic acid phenethyl ester: a novel strategy to treat acute gout. Sci. Rep.6, 38622. 10.1038/srep38622
26
LeiP.LiZ.HuaQ.SongP.GaoL.ZhouL.et al (2023). Ursolic acid alleviates neuroinflammation after intracerebral hemorrhage by mediating microglial pyroptosis via the NF-κB/NLRP3/GSDMD pathway. Int. J. Mol. Sci.24, 14771. 10.3390/ijms241914771
27
LiQ.LiuP.WuC.BaiL.ZhangZ.BaoZ.et al (2022a). Integrating network pharmacology and pharmacological validation to explore the effect of Shi Wei Ru Xiang powder on suppressing hyperuricemia. J. Ethnopharmacol.298, 115679. 10.1016/j.jep.2022.115679
28
LiW.YangK.LiB.WangY.LiuJ.ChenD.et al (2022b). Corilagin alleviates intestinal ischemia/reperfusion-induced intestinal and lung injury in mice via inhibiting NLRP3 inflammasome activation and pyroptosis. Front. Pharmacol.13, 1060104. 10.3389/fphar.2022.1060104
29
LinY.LuoT.WengA.HuangX.YaoY.FuZ.et al (2020). Gallic acid alleviates gouty arthritis by inhibiting NLRP3 inflammasome activation and pyroptosis through enhancing Nrf2 signaling. Front. Immunol.11, 580593. 10.3389/fimmu.2020.580593
30
LiuS.-j.GuoB.-d.GaoQ.-h.DengY.-j.YanB.ZengY.et al (2024). Ursolic acid alleviates chronic prostatitis via regulating NLRP3 inflammasome-mediated Caspase-1/GSDMD pyroptosis pathway. Phytother. Res.38, 82–97. 10.1002/ptr.8034
31
LiuW.PengJ.WuY.YeZ.ZongZ.WuR.et al (2023). Immune and inflammatory mechanisms and therapeutic targets of gout: an update. Int. Immunopharmacol.121, 110466. 10.1016/j.intimp.2023.110466
32
LiuX.ZhangZ.RuanJ.PanY.MagupalliV. G.WuH.et al (2016). Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature535, 153–158. 10.1038/nature18629
33
Liu-BryanR. (2010). Intracellular innate immunity in gouty arthritis: role of NALP3 inflammasome. Immunol. Cell Biol.88, 20–23. 10.1038/icb.2009.93
34
LuoT.ZhouX.QinM.LinY.LinJ.ChenG.et al (2022). Corilagin restrains NLRP3 inflammasome activation and pyroptosis through the ROS/TXNIP/NLRP3 pathway to prevent inflammation. Oxid. Med. Cell. Longev.2022, 1652244. 10.1155/2022/1652244
35
ManganM. S. J.OlhavaE. J.RoushW. R.SeidelH. M.GlickG. D.LatzE. (2018). Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov.17, 588–606. 10.1038/nrd.2018.97
36
MartinonF.PétrilliV.MayorA.TardivelA.TschoppJ. (2006). Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature440, 237–241. 10.1038/nature04516
37
MehmoodA.IftikharA.ChenX. (2024). Food-derived bioactive peptides with anti-hyperuricemic activity: a comprehensive review. Food Chem.451, 139444. 10.1016/j.foodchem.2024.139444
38
NimaC.WanmaL.JingX.DuojieC.GazangD.RenZ. (2024). Elucidating the mechanism of the Tibetan medicine sanguotang in treating gouty arthritis through network pharmacology and in vivo experiments. Am. J. Med. Sci.368, 68–79. 10.1016/j.amjms.2024.02.008
39
PatelS. S.MolnarM. Z.TayekJ. A.IxJ. H.NooriN.BennerD.et al (2013). Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J. Cachexia, Sarcopenia Muscle4, 19–29. 10.1007/s13539-012-0079-1
40
RagabG.ElshahalyM.BardinT. (2017). Gout: an old disease in new perspective – a review. J. Adv. Res.8, 495–511. 10.1016/j.jare.2017.04.008
41
RemijsenQ.BergheT. V.WirawanE.AsselberghB.ParthoensE.De RyckeR.et al (2011). Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res.21, 290–304. 10.1038/cr.2010.150
42
SchauerC.JankoC.MunozL. E.ZhaoY.KienhöferD.FreyB.et al (2014). Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med.20, 511–517. 10.1038/nm.3547
43
SchlesingerN.ThieleR. G. (2010). The pathogenesis of bone erosions in gouty arthritis. Ann. Rheum. Dis.69, 1907–1912. 10.1136/ard.2010.128454
44
SharifH.WangL.WangW. L.MagupalliV. G.AndreevaL.QiaoQ.et al (2019). Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature570, 338–343. 10.1038/s41586-019-1295-z
45
SunY.LuP.NiuH.ChenN.ChenX. (2018). Study on risk factors for indued hyperuricemia and gout in Tibet. Chin. Nurs. Res.32, 534–537.
46
SunZ.-R.LiuH.-R.HuD.FanM.-S.WangM.-Y.AnM.-F.et al (2021). Ellagic acid exerts beneficial effects on hyperuricemia by inhibiting xanthine oxidase and NLRP3 inflammasome activation. J. Agric. Food Chem.69, 12741–12752. 10.1021/acs.jafc.1c05239
47
SwansonK. V.DengM.TingJ. P. Y. (2019). The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol.19, 477–489. 10.1038/s41577-019-0165-0
48
SzekaneczZ.SzamosiS.KovácsG. E.KocsisE.BenkőS. (2019). The NLRP3 inflammasome - interleukin 1 pathway as a therapeutic target in gout. Arch. Biochem. Biophys.670, 82–93. 10.1016/j.abb.2019.01.031
49
TerkeltaubR. (2010). Update on gout: new therapeutic strategies and options. Nat. Rev. Rheumatol.6, 30–38. 10.1038/nrrheum.2009.236
50
TorresR.MacdonaldL.CrollS. D.ReinhardtJ.DoreA.StevensS.et al (2009). Hyperalgesia, synovitis and multiple biomarkers of inflammation are suppressed by interleukin 1 inhibition in a novel animal model of gouty arthritis. Ann. Rheum. Dis.68, 1602–1608. 10.1136/ard.2009.109355
51
TuY.TanL.TaoH.LiY.LiuH. (2023). CETSA and thermal proteome profiling strategies for target identification and drug discovery of natural products. Phytomedicine116, 154862. 10.1016/j.phymed.2023.154862
52
WangC.GaoY.ZhangZ.ChenC.ChiQ.XuK.et al (2020a). Ursolic acid protects chondrocytes, exhibits anti-inflammatory properties via regulation of the NF-κB/NLRP3 inflammasome pathway and ameliorates osteoarthritis. Biomed. Pharmacother.130, 110568. 10.1016/j.biopha.2020.110568
53
WangD.GaoQ.WangT.KanZ.LiX.HuL.et al (2020b). Green tea polyphenols and epigallocatechin-3-gallate protect against perfluorodecanoic acid induced liver damage and inflammation in mice by inhibiting NLRP3 inflammasome activation. Food Res. Int.127, 108628. 10.1016/j.foodres.2019.108628
54
WangN.LiuY.YangC.DuJ.YuD.HeP.et al (2025). Molecular insights into vasicine and butyrylcholinesterase interactions: a complimentary biophysical, multi-spectroscopic, and computational study. Int. J. Biol. Macromol.292, 139253. 10.1016/j.ijbiomac.2024.139253
55
WangS.LiuW.WeiB.WangA.WangY.WangW.et al (2024). Traditional herbal medicine: therapeutic potential in acute gouty arthritis. J. Ethnopharmacol.330, 118182. 10.1016/j.jep.2024.118182
56
WangT.XuH.DongR.WuS.GuoY.WangD. (2023a). Effectiveness of targeting the NLRP3 inflammasome by using natural polyphenols: a systematic review of implications on health effects. Food Res. Int.165, 112567. 10.1016/j.foodres.2023.112567
57
WangY.XuY.TanJ.YeJ.CuiW.HouJ.et al (2023b). Anti-inflammation is an important way that Qingre-Huazhuo-Jiangsuan recipe treats acute gouty arthritis. Front. Pharmacol.14. 10.3389/fphar.2023.1268641
58
XiongH.MengF.LuoM.ChenW.TianJ.ChenL.et al (2023). Anti-inflammatory and osteoprotective effects of Shi-Wei-Ru-Xiang pills on collagen-induced arthritis in rats via inhibiting MAPK and STAT3 pathways. J. Ethnopharmacol.300, 115693. 10.1016/j.jep.2022.115693
59
YuT.-Y.FengY.-M.KongW.-S.LiS.-N.SunX.-J.ZhouG.et al (2023). Gallic acid ameliorates dextran sulfate sodium-induced ulcerative colitis in mice via inhibiting NLRP3 inflammasome. Front. Pharmacol.14, 1095721. 10.3389/fphar.2023.1095721
60
ZahidA.LiB.KombeA. J. K.JinT.TaoJ. (2019). Pharmacological inhibitors of the NLRP3 inflammasome. Front. Immunol.10, 2538. 10.3389/fimmu.2019.02538
61
ZhaoJ.WeiK.JiangP.ChangC.XuL.XuL.et al (2022). Inflammatory response to regulated cell death in gout and its functional implications. Front. Immunol.13, 888306. 10.3389/fimmu.2022.888306
62
ZhongC.LongR.StewartG. S. (2022). The role of rumen epithelial urea transport proteins in urea nitrogen salvage: a review. Anim. Nutr.9, 304–313. 10.1016/j.aninu.2022.01.008
63
ZindelJ.KubesP. (2020). DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu. Rev. Pathology Mech. Dis.15, 493–518. 10.1146/annurev-pathmechdis-012419-032847
64
ZouJ.YangR.FengR.LiuJ.WanJ.-B. (2023). Ginsenoside Rk2, a dehydroprotopanaxadiol saponin, alleviates alcoholic liver disease via regulating NLRP3 and NLRP6 inflammasome signaling pathways in mice. J. Pharm. Analysis13, 999–1012. 10.1016/j.jpha.2023.05.005
Summary
Keywords
Shi Wei Ru Xiang pill, NLRP3 inflammasome, acute gouty arthritis, Tibetan medicine, monosodium urate
Citation
Wang N, Zhao P, Yin Q, Li L, Xu H, Yang C, Tu Y and Li Y (2025) Shi Wei Ru Xiang pill alleviates acute gouty arthritis through suppressing NLRP3 inflammasome activation. Front. Pharmacol. 16:1595578. doi: 10.3389/fphar.2025.1595578
Received
18 March 2025
Accepted
04 June 2025
Published
25 June 2025
Volume
16 - 2025
Edited by
Karl Tsim, Hong Kong University of Science and Technology, Hong Kong SAR, China
Reviewed by
Bingbing Sun, Dalian University of Technology, China
Xuxu Zhuang, University of Macau, China
Updates
Copyright
© 2025 Wang, Zhao, Yin, Li, Xu, Yang, Tu and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanbei Tu, yanbeitu@ujs.edu.cn; Yanfang Li, lyf471@vip.163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.