- 1School of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
Introduction: To evaluate the efficacy and safety of Rhodiola crenulata extract (RCE) for the treatment of patients with acute high altitude disease (AHAD).
Methods: This study systematically retrieved randomized controlled trials (RCTs) published prior to September 2024 from eight distinct databases. It included AHAD patients, with the control group receiving either conventional western medicine (WM) or placebo, and the experimental group receiving RCE alone or in conjunction with WM. The primary outcomes were arterial oxygen saturation (SaO2) and arterial partial pressure of oxygen (PaO2). The secondary outcomes were total clinical efficacy, systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR). Adverse events incidence was analyzed to assess safety. The meta-analysis was performed with Review Manager 5.4, and the evidence’s certainty was assessed using the GRADE approach.
Results: This study included 19 eligible RCTs with 1,690 participants. In improving SaO2, PaO2 and total clinical efficacy, no significant differences were found between RCE and WM, but RCE was more effective than placebo. RCE showed no significant effect in reducing SBP, DBP and HR. Regarding safety, the experimental group demonstrated superior performance compared to the control group.
Conclusion: RCE may enhance blood oxygen levels and mitigate clinical symptoms in the treatment of AHAD with favorable safety. Nonetheless, it is imperative to undertake further rigorous RCTs to validate these findings.
Systematic Review Registration:: https://www.crd.york.ac.uk/PROSPERO/myprospero, identifier CRD42024593081.
1 Introduction
High altitude disease (HAD) is an idiopathic disease that occurs in high-altitude regions, with hypoxia being the primary cause (Bärtsch and Gibbs, 2007; Wu, 2014; Zhaxi et al., 2024). Acute high-altitude disease (AHAD) can occur with initial or rapid exposure to high altitudes, and in serious cases, it may result in pulmonary and cerebral edema, which could be life-threatening (Pena et al., 2022). AHAD is a syndrome primarily marked by headache, along with symptoms like nausea, fatigue, dyspnea, insomnia, and dizziness (Wu et al., 2018). In 2000, over 100 million people traveled to high-altitude areas, a trend that continues to grow, especially in regions like the Qinghai-Tibet Plateau (Faulhaber et al., 2014; Ma et al., 2022; Zhaxi et al., 2024). AHAD typically appears within 6 h of ascending above 2,500 m, peaking within 12–96 h. It affects over 25% of those reaching 3,500 m and more than 50% at elevations above 6,000 m, indicating a significant impact on a significant portion of the population (Wu et al., 2018; Guo et al., 2024).
Currently, the conventional treatment drugs for AHAD include acetazolamide, dexamethasone, aminophylline, etc (Imray et al., 2010; Hung et al., 2019). They exert effects that enhance anti-hypoxia capacity, increase blood flow, and improve acid-base balance (Ren and Wang, 2011). Although these medications act rapidly, they have significant side effects and are primarily used for emergency situations in AHAD (Ren and Wang, 2011). Given the limitations of current pharmacological approaches, attention has turned toward traditional herbal remedies with historical usage in high-altitude regions, such as Rhodiola crenulata.
Rhodiola crenulata (Hook.f. and Thomson) H.Ohba (World Flora Online, 1976), a traditional Chinese medicinal plant, thrives at 3,000-4,000 m and is used in Tibet to fight fatigue and adapt to high altitudes (Ren, 2022). At present, extracts derived from R. crenulata have been incorporated into a variety of Chinese patent medicine, and R. crenulata extract (RCE) has become a leading remedy in China for preventing and treating altitude sickness (Chen, 2013; Feng et al., 2013; Tang et al., 2015; Li et al., 2023). Research showed that RCE contained various bioactive compounds, including salidroside and gallic acid. It offers immunomodulatory, antioxidant, anti-inflammatory, and neuroprotective benefits, protecting the heart, brain, blood vessels, and other organs from damage (Hsu et al., 2017; Ren, 2022; Si et al., 2022; Wang, 2023; Hou et al., 2024). Thus, RCE may provide clinical benefits to patients in the treatment of symptoms of altitude sickness such as headache, nausea, anorexia, gastrointestinal discomfort, insomnia, fatigue, and hair loss (Zhang and Shu, 2011; Liu et al., 2022).
Currently, numerous clinical studies on RCE for AHAD have been published. Nevertheless, only a single systematic review of the quality of its research findings and methodologies exists, and it was published at an early stage (Cao et al., 2015). This suggested that the translation of evidence concerning the efficacy of RCE in treating AHAD has not been adequately addressed (Liu and Gong, 2024; Zhang et al., 2024). Consequently, this study conducted a systematic review and meta-analysis to assess the efficacy and safety of RCE in the treatment of AHAD, with the objective of providing robust and practical evidence for clinical application.
2 Materials and methods
2.1 Study registration and reporting guideline
The protocol for this systematic review, registered with PROSPERO (CRD42024593081) on 1 October 2024, adheres to the PRISMA 2020 guidelines (Page et al., 2021).
2.2 Ethical statement
Since this study is a literature review, it did not require ethical approval.
2.3 Inclusion criteria
2.3.1 Type of study
The study included randomized controlled trials (RCTs) that were published in either English or Chinese.
2.3.2 Participants
The diagnosis of followed the diagnostic criteria outlined in the 2018 Lake Louise Acute Mountain Sickness Score (Roach et al., 2018) and Guidelines for the Diagnosis, Prevention and Treatment of High Altitude Disease (2014) (Wu, 2014). No restrictions exist regarding age, gender, nationality, birth location, or ethnic origin. The nomenclature, classification and relevant diagnostic criteria are listed in Supplementary Material S1.
2.3.3 Intervention and comparison
The control group was given either conventional western medicine (WM) or placebo. Western medicine encompasses conventional drugs, excluding traditional Chinese medicine (TCM), such as acetazolamide, dexamethasone, and aminophylline, used for AHAD. The intervention group was administered RCE (all dosage forms, such as capsules and aqueous extracts, were included) or a combination of WM and RCE, without consideration for the dosage, duration, or frequency of RCE administration. Comprehensive information regarding the RCE is available in Supplementary Material S2.
2.3.4 Outcomes
1) Primary outcomes: arterial oxygen saturation (SaO2) and arterial partial pressure of oxygen (PaO2).
2) Secondary outcomes: total clinical efficacy, systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR).
The criteria for evaluating total clinical efficacy are as follows: ①Significant Effective: the patient’s symptoms and signs completely recovered; ②Effective: the patient’s symptoms and signs were basically recovered; ③Invalid: the patient’s symptoms and signs did not return to normal, and his condition worsened. Total clinical efficacy = number of effective cases/total number of cases × 100%.
3) Safety outcome: The incidence of adverse events.
4) Other outcomes: To comprehensively assess the efficacy and safety of RCE in treating AHAD, all reported outcomes from the RCTs were included in the analysis.
2.4 Exclusion criteria
1) Incomplete or inaccurate data, including missing baseline data, mean, SD, etc.
2) Interventions included other TCM components or therapies other than RCE.
3) Single-arm studies.
4) For duplicate publications, only one was included.
2.5 Information source and search strategy
Eight databases, including CNKI, VIP, WF, SinoMed, PubMed, Embase, Cochrane Library, and Web of Science, were comprehensively searched for RCTs from their inception until September 2024. Search conducted between September 1 and 10, 2024. The reference lists from the included trials were manually reviewed to find any additional relevant studies. The search strategy is detailed using PubMed as an example. Strategies for other databases can be found in Supplementary Material S3.
#1″Altitude Sickness” [MeSH Terms] OR “Altitude Diseases” [Title/Abstract] OR “Sickness, Altitude” [Title/Abstract] OR “Diseases, Altitude” [Title/Abstract] OR “Altitude Hypoxia” [Title/Abstract] OR “Altitude Hypoxias” [Title/Abstract] OR “Hypoxia, Altitude” [Title/Abstract] OR “Mountain Sickness” [Title/Abstract] OR “Sickness, Mountain” [Title/Abstract].
#2″R. crenulata” [MeSH Terms] OR “Rhodiola rosea” [Title/Abstract] OR “Roseroot” [Title/Abstract] OR “Roseroots” [Title/Abstract] OR “Hongjingtian” [Title/Abstract] OR “Hong jing tian” [Title/Abstract].
#3″randomized controlled trial” [Publication type] OR “randomized clinical trial” [Publication type] OR “randomized trial” [Publication type] OR “clinical trial” [Publication type] OR “randomized controlled trial” [Title/Abstract] OR “randomized clinical trial” [Title/Abstract] OR “randomized trial” [Title/Abstract] OR “clinical trial” [Title/Abstract].
#4 #1 AND #2 AND #3.
2.6 Study screening and data extraction
The study’s screening protocol involved: 1) Reviewing titles and abstracts to choose studies that met the inclusion criteria; 2) Examining the full text if additional information was required. Design the standard data extraction table, including: title, primary author, publication year, source, sample size, age, gender, disease diagnosis, diagnostic criteria, disease duration, course of treatment, follow-up and outcomes. If complete data were unavailable, the authors would be emailed; lack of response would result in exclusion from the study. The processes of study screening, data extraction, and risk of bias assessment were independently conducted by two researchers. Discrepancies were resolved through discussion or, if necessary, by consulting a third researcher.
2.7 Risk of bias assessment
The risk of bias of RCTs was assessed by using the Cochrane Collaboration Risk of Bias tool (https://methods.cochrane.org/bias/risk-bias-tool) for randomized controlled trials 2.0 (RoB 2.0) (Sterne et al., 2019). Bias was assessed in each of the five domains using specific signaling questions:
1) randomization process (selection bias);
2) deviations from the intended interventions (performance bias);
3) missing outcome data (attrition bias);
4) measurement of the outcome (detection bias);
5) selection of the reported outcome (reporting bias).
Bias risk in each domain was described as “low risk,” “some concerns,” or “high risk.” The overall risk of bias for each study was determined by assessing the risk of bias in each domain.
2.8 Statistical analysis
The meta-analysis was conducted using Review Manager software (Cochrane Collaboration, version 5.4) along with Stata 15.0. For dichotomous variables, the relative risk (RR) was used as the effect measure for analysis. For continuous variables, meta-analysis used the mean and standard deviation (SD) of pre- and post-treatment differences (Mean ± SD), employing the mean difference (MD) or standardized mean difference (SMD) as effect statistics. When the outcome was evaluated utilizing consistent measurement methods and units, MD was employed; otherwise, SMD was chosen. Statistical analysis results were expressed using 95% confidence intervals (CI). Statistical heterogeneity was evaluated using the P value and the I2 statistic. A threshold of I2 ≤ 50% and P > 0.05 indicated low heterogeneity among the included studies, thereby warranting the use of a fixed-effects model for the synthesis of results. Otherwise, it was considered that the included studies had high heterogeneity, and subgroup or sensitivity analyses would be employed to investigate possible origins of this heterogeneity. If the source of heterogeneity cannot be determined, the outcomes will be combined using a random-effects model. In this study, the I2 statistic for all primary and secondary outcomes exceeded 50%. Subsequent sensitivity analyses, which accounted for variables such as age, dosage form, intervention dosage, and studies identified as having a high risk of bias, did not result in a significant reduction in heterogeneity. Consequently, a random-effects model was utilized for the meta-analysis.
For analyses incorporating data from over 10 studies, funnel plots and Egger’s test were employed to evaluate the presence of publication bias. A P-value of less than 0.05 from Egger’s test was interpreted as evidence suggesting the existence of publication bias. Due to the fact that none of the analyzed outcomes encompassed more than ten studies, an assessment of publication bias was not performed. This study aims to investigate the efficacy of three distinct interventions: RCE vs. placebo, RCE vs. WM and RCE + WM vs. WM. In addition, subgroup analyses will be performed to assess the efficacy of varying treatment durations (≤7 days and >7 days) utilizing the same intervention.
2.9 Certainty of evidence
The certainty of the evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, which takes into account five factors: risk of bias, imprecision, inconsistency, indirectness, and publication bias. The principal findings were delineated in the Summary of Findings table, which was generated using the GRADE Pro GDT software (http://gradepro.org). Evidence certainty is ranked as high, moderate, low, or very low based on the available evidence (Balshem et al., 2011).
3 Results
3.1 Study screening
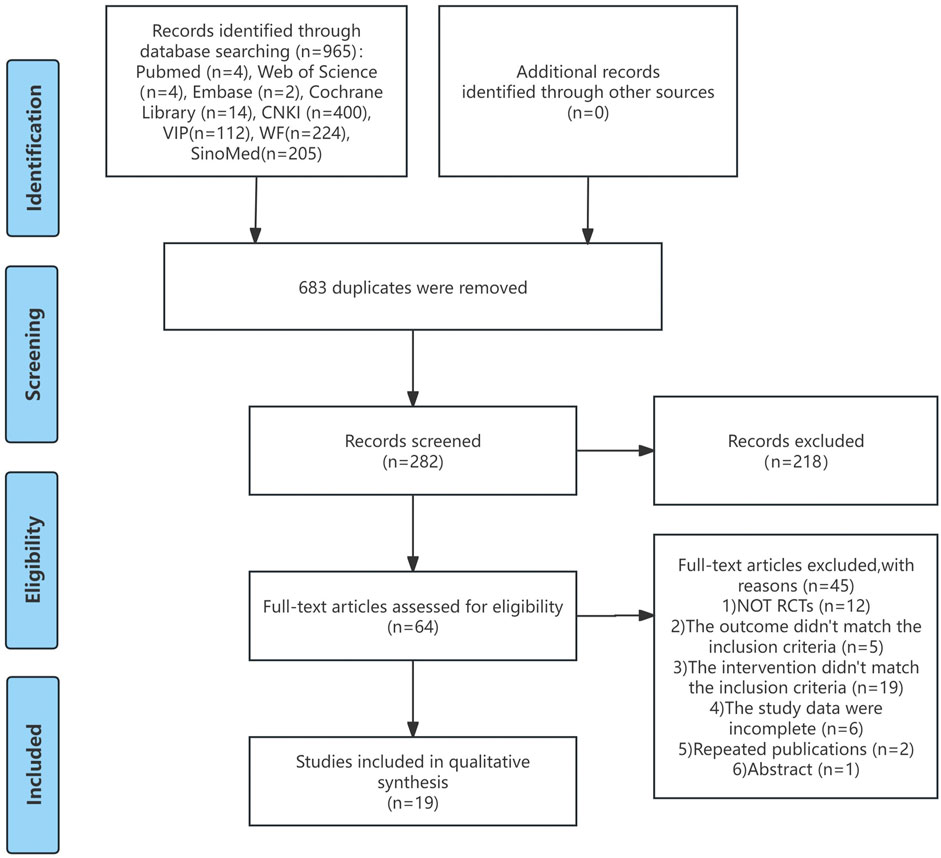
A comprehensive search across eight databases yielded 965 articles. Following the removal of 683 duplicate entries, title and abstract screening resulted in the exclusion of 218 studies. A full-text assessment led to the exclusion of 45 articles that failed to meet the inclusion criteria, including non-RCTs, misaligned interventions and outcomes, incomplete data, duplicates, and reviews. Ultimately, 19 RCTs, all published in Chinese, were included in the analysis. Figure 1 depicts the study screening process.
3.2 Study characteristics
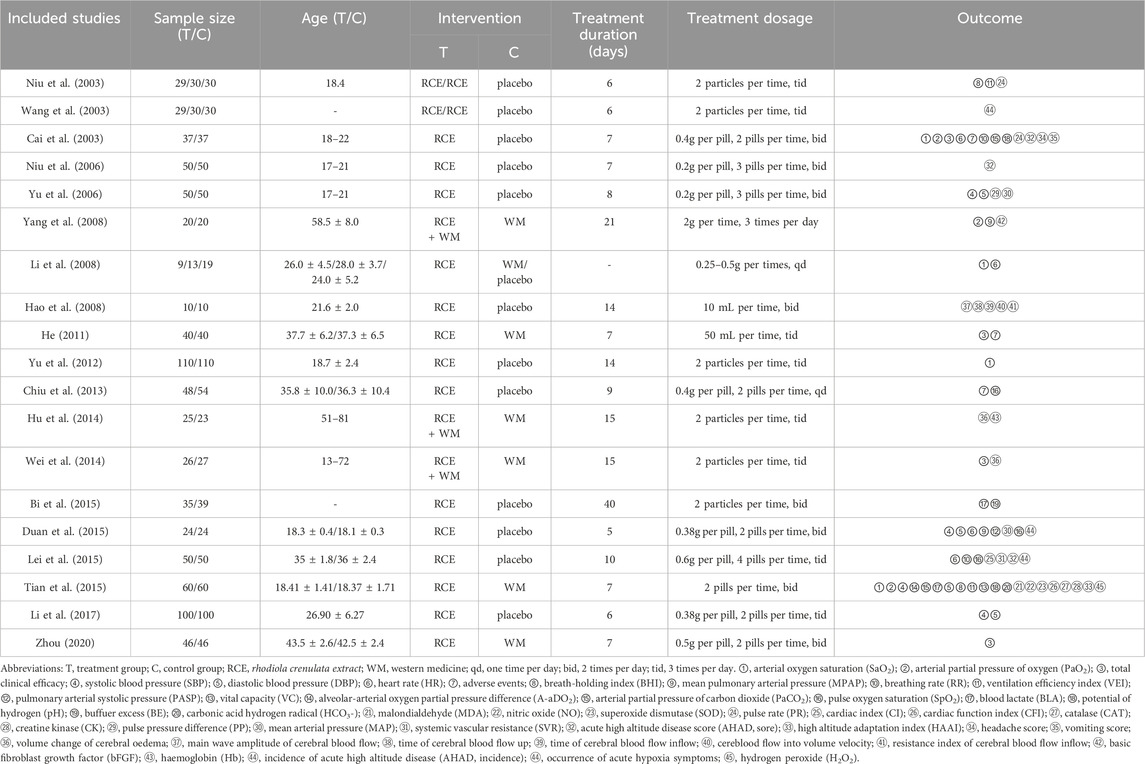
Table 1 presents a comprehensive summary of the characteristics of the studies included in this analysis. All 19 RCTs published between 2003 and 2020. All studies are from China, which limits global representativeness. These RCTs collectively involved a total of 1,690 participants, with 871 individuals assigned to the intervention group and 819 to the control group. Four RCTs with 314 participants compared RCE with WM, thirteen RCTs with 1,244 participants compared RCE with placebo (one study compared RCE with both WM and placebo), and three RCTs with 141 participants compared RCE plus WM with WM. WM included acetoamide, aminophylline, edaravone, trimetazidine, among other conventional pharmaceuticals. Placebo included medical starch capsules and purified water. The duration of the treatment regimen varied from 5 days to 3 months. No significant disparities were observed in the baseline characteristics across all the studies.
3.3 Risk of bias assessment
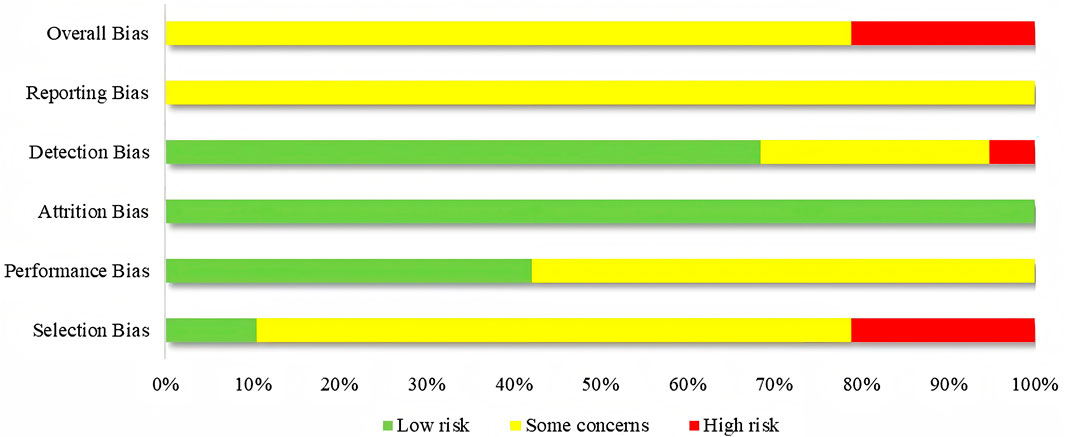
The 19 studies were assessed based on the five domains of ROB 2.0. Seventeen studies exhibited issues with the randomization process, with 13 studies only mentioning randomization without detailing the method of implementation. Furthermore, none of the 17 studies looked into how allocation concealment was implemented. Eleven studies faced issues with deviations from the intended interventions, potentially resulting in a divergence from the intended intervention due to the lack of blinding. In terms of missing outcome data, none of the 19 studies reported any dropout cases, and all outcome data were complete. Issues with measurement of the outcome were present in six studies, with one study being at high risk of bias due to vague criteria for measuring outcomes. All 19 studies faced issues with selection of the reported results, as none of the studies had established a predetermined protocol. Among the overall risk of bias, 15 studies were rated as some concerns, and four studies were rated as high risk. The findings suggested that the principal limitations affecting the quality of the study were attributable to biases in selection and reporting. Furthermore, almost all studies were published in Chinese, potentially introducing language bias and limiting the generalizability of the findings. Figure 2 presents the assessment of bias risk for the included studies.
3.4 Meta-analysis results
3.4.1 Primary outcomes
3.4.1.1 SaO2
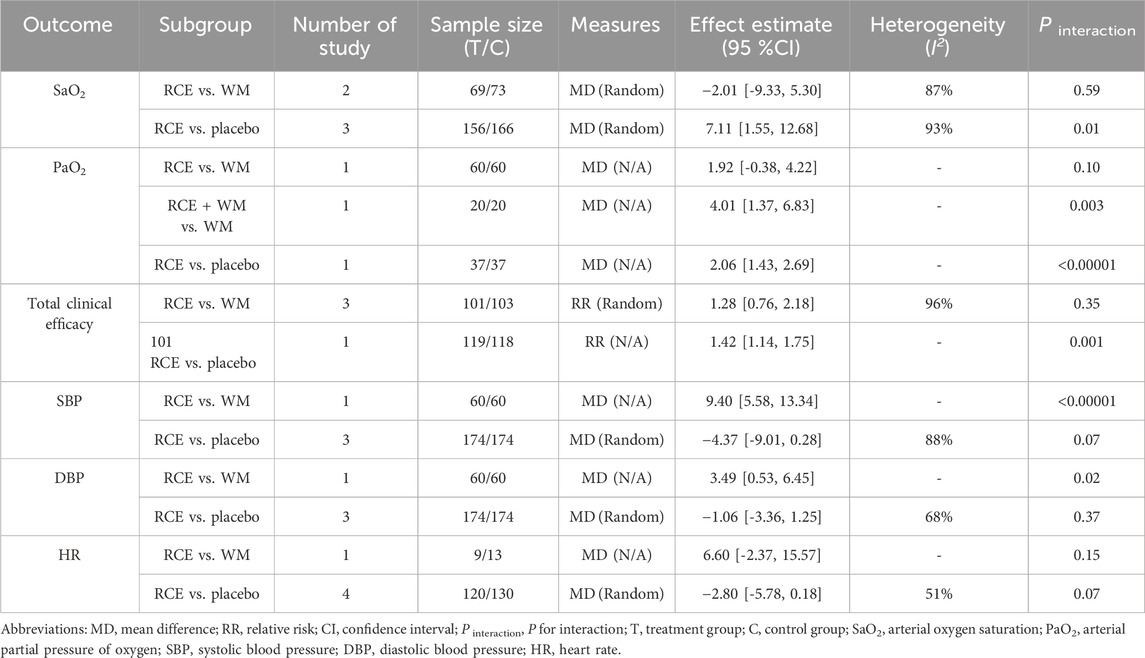
SaO2 was reported in 4 RCTs including 464 participants (Cai et al., 2003; Li et al., 2008; Yu et al., 2012; Tian et al., 2015), with three being two-arm trials and one a three-arm trial (Figure 3). Two RCTs reported RCE vs. WM (including 142 participants). The heterogeneity test indicated significant heterogeneity among the studies (P = 0.006, I2 = 87%). The sensitivity analysis showed that removing any single study did not decrease the significant heterogeneity of the combined results. Therefore, a random-effects model was employed to pool the results. The results of the meta-analysis revealed that there was no statistically significant difference between the two groups [MD = −2.01, 95%CI (−9.33, 5.30), p = 0.59], implying that RCE showed efficacy comparable to WM in improving SaO2 levels. Three RCTs reported RCE vs. placebo (including 322 participants). The heterogeneity test indicated significant heterogeneity among the studies (P < 0.00001, I2 = 93%). The sensitivity analysis showed that removing any single study did not decrease the significant heterogeneity of the combined results. Therefore, a random-effects model was employed to pool the results. The results of the meta-analysis revealed that RCE demonstrated significant efficacy in improving SaO2 [MD = 7.11, 95%CI (1.55, 12.68), p = 0.01]. Despite the statistical improvement in SaO2 with RCE compared to placebo, the high heterogeneity and risk of bias among the studies prevent a robust conclusion.
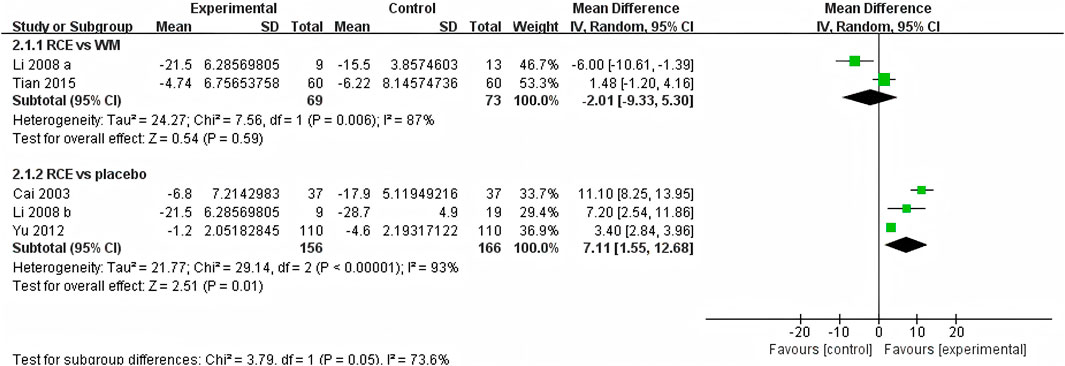
Figure 3. Forest plot of the effect of Rhodiola crenulata extract (RCE) vs. western medicine (WM) and RCE vs. placebo on arterial oxygen saturation (SaO2).
3.4.1.2 PaO2
PaO2 was reported in 3 RCTs including 234 participants (Cai et al., 2003; Yang et al., 2008; Tian et al., 2015) (Figure 4). One RCT reported RCE vs. WM (including 120 participants), the meta-analysis indicated that there was no significant difference between the two groups [MD = 1.92, 95%CI (−0.38, 4.22), p = 0.10], implying that RCE showed efficacy comparable to WM in improving PaO2 levels. One RCT reported RCE + WM vs. WM (including 40 participants), the meta-analysis indicated that RCE demonstrated significant efficacy in improving PaO2 levels [MD = 4.10, 95%CI (1.37. 6.83), p = 0.003]. One RCT reported RCE vs. placebo (including 74 participants), the meta-analysis indicated that RCE demonstrated significant efficacy in improving PaO2 levels [MD = 2.06, 95%CI (1.43, 2.69), p < 0.00001]. Despite the statistical improvement in PaO2 with RCE combined with WM compared to WM, as well as between RCE compared to placebo, the high heterogeneity and risk of bias among the studies prevent a robust conclusion.
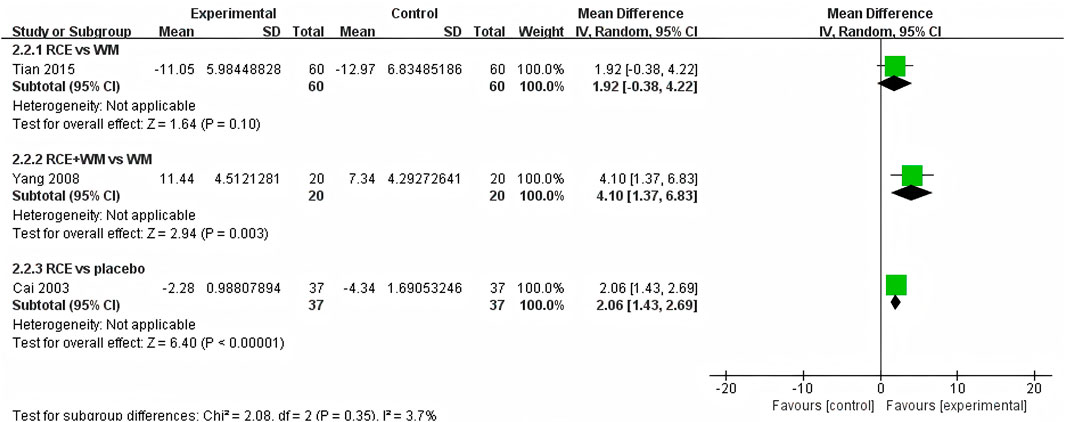
Figure 4. Forest plot of the effect of Rhodiola crenulata extract (RCE) vs. western medicine (WM), RCE combined with WM vs. WM and RCE vs. placebo on arterial partial pressure of oxygen (PaO2).
3.4.2 Secondary outcomes
3.4.2.1 Total clinical efficacy
The total clinical efficacy was reported in 4 RCTs including 278 participants (Cai et al., 2003; He, 2011; Wei et al., 2014; Zhou, 2020) (Figure 5). Three RCTs reported RCE vs. WM (including 204 participants). The heterogeneity test indicated significant heterogeneity among the studies (P < 0.00001, I2 = 96%). The sensitivity analysis showed that removing any single study did not decrease the significant heterogeneity of the combined results. Therefore, a random-effects model was employed to pool the results. The results of the meta-analysis revealed that there was no statistically significant difference between the two groups [RR = 1.28, 95%CI (0.76, 2.18), p = 0.35], suggesting that RCE showed efficacy comparable to WM on total clinical efficacy. One RCT reported RCE vs. placebo (including 74 participants), the meta-analysis revealed a significant improving effect of RCE on total clinical efficacy [RR = 1.42, 95%CI (1.14, 1.75), p = 0.001]. Despite the statistical improvement on total clinical efficacy compared to placebo, the small sample size of the study and risk of bias among the studies prevent a robust conclusion.
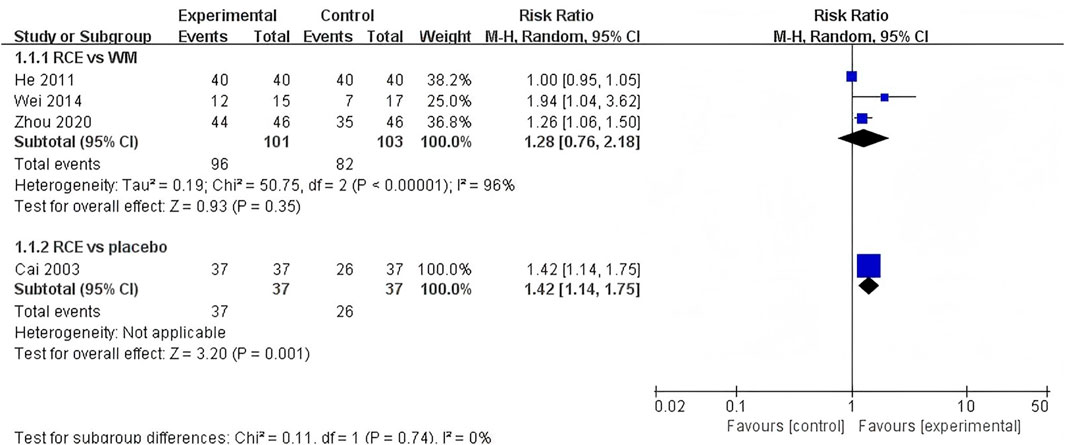
Figure 5. Forest plot of the effect of Rhodiola crenulata extract (RCE) vs. western medicine (WM) and RCE vs. placebo on total clinical efficacy.
3.4.2.2 SBP
SBP was reported in 4 RCTs including 468 participants (Yu et al., 2006; Duan et al., 2015; Tian et al., 2015; Li et al., 2017) (Figure 6). One RCT reported RCE vs. WM (including 120 participants), the meta-analysis indicated that WM exhibits superior efficacy compared to RCE in reducing SBP [MD = 9.46, 95%CI (5.58, 13.34), p < 0.00001]. Three RCTs reported RCE vs. placebo (including 348 participants). The heterogeneity test indicated significant heterogeneity among the studies (P = 0.0002, I2 = 88%). The sensitivity analysis showed that removing any single study did not decrease the significant heterogeneity of the combined results. Therefore, a random-effects model was employed to pool the results. The results of the meta-analysis revealed that there was no statistically significant difference between the two groups [MD = −4.37, 95%CI (−9.01, 0.28), p = 0.07]. Nevertheless, it is important to acknowledge that the substantial heterogeneity and potential for bias present in the studies may hinder the formulation of robust conclusions.
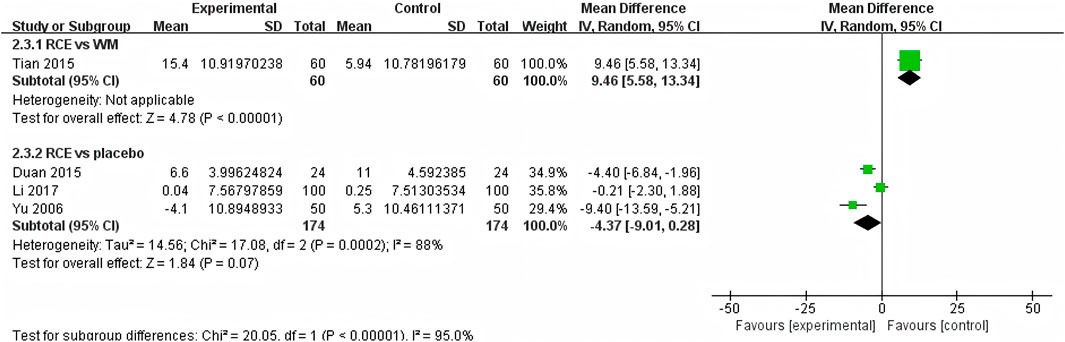
Figure 6. Forest plot of the effect of Rhodiola crenulata extract (RCE) vs. western medicine (WM) and RCE vs. placebo on systolic blood pressure (SBP).
3.4.2.3 DBP
DBP was reported in 4 RCTs including 468 participants (Yu et al., 2006; Duan et al., 2015; Tian et al., 2015; Li et al., 2017) (Figure 7). One RCT reported RCE vs. WM (including 120 participants), the meta-analysis indicated that WM exhibits superior efficacy compared to RCE in reducing DBP [MD = 3.49, 95%CI (0.53, 6.45), p = 0.02]. Three RCTs reported RCE vs. placebo (including 348 participants). The heterogeneity test indicated significant heterogeneity among the studies (P = 0.05, I2 = 68%). The sensitivity analysis showed that removing any single study did not decrease the significant heterogeneity of the combined results. Therefore, a random-effects model was employed to pool the results. The results of the meta-analysis revealed that there was no statistically significant difference between the two groups [MD = −1.06, 95%CI (−3.36, 1.25, p = 0.37]. Nevertheless, it is important to acknowledge that the substantial heterogeneity and potential for bias present in the studies may hinder the formulation of robust conclusions.
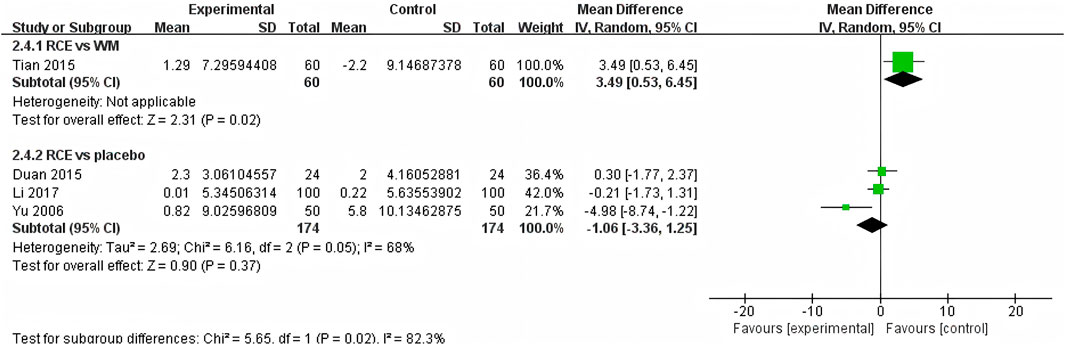
Figure 7. Forest plot of the effect of Rhodiola crenulata extract (RCE) vs. western medicine (WM) and RCE vs. placebo on diastolic blood pressure (DBP).
3.4.2.4 HR
HR was reported in 4 RCTs including 272 participants (Cai et al., 2003; Li et al., 2008; Duan et al., 2015; Lei et al., 2015), with three being two-arm trials and one a three-arm trial (Figure 8). One RCT reported RCE vs. WM (including 22 participants), the meta-analysis indicated that there was no significant difference between the two groups [MD = 6.60, 95%CI (−2.37, 15.57), p = 0.15]. Three RCTs reported RCE vs. placebo (including 250 participants). The heterogeneity test indicated significant heterogeneity among the studies (P = 0.11, I2 = 51%). The sensitivity analysis showed that removing any single study did not decrease the significant heterogeneity of the combined results. Therefore, a random-effects model was employed to pool the results. The results of the meta-analysis indicated that there was no statistically significant difference observed between the two groups [MD = −2.80, 95%CI (−5.78, 0.18, p = 0.07]. Nevertheless, it is important to acknowledge that the substantial heterogeneity and potential for bias present in the studies may hinder the formulation of robust conclusions.
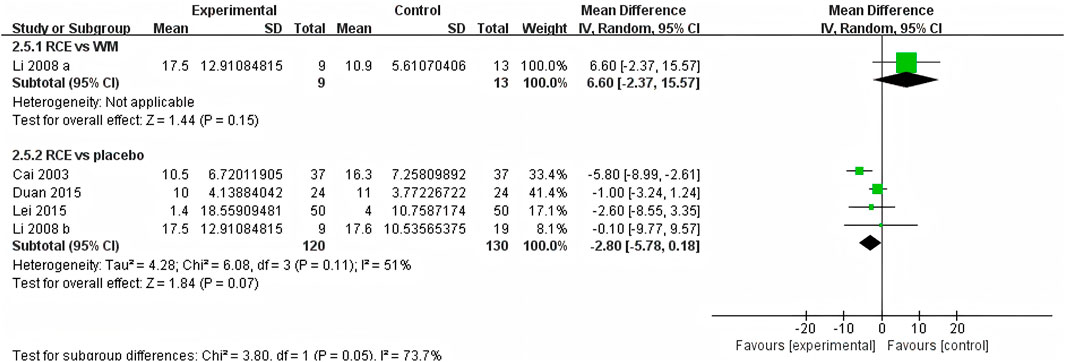
Figure 8. Forest plot of the effect of Rhodiola crenulata extract (RCE) vs. western medicine (WM) and RCE vs. placebo on heart rate (HR).
A summary table of primary and secondary outcomes are shown in Table 2.
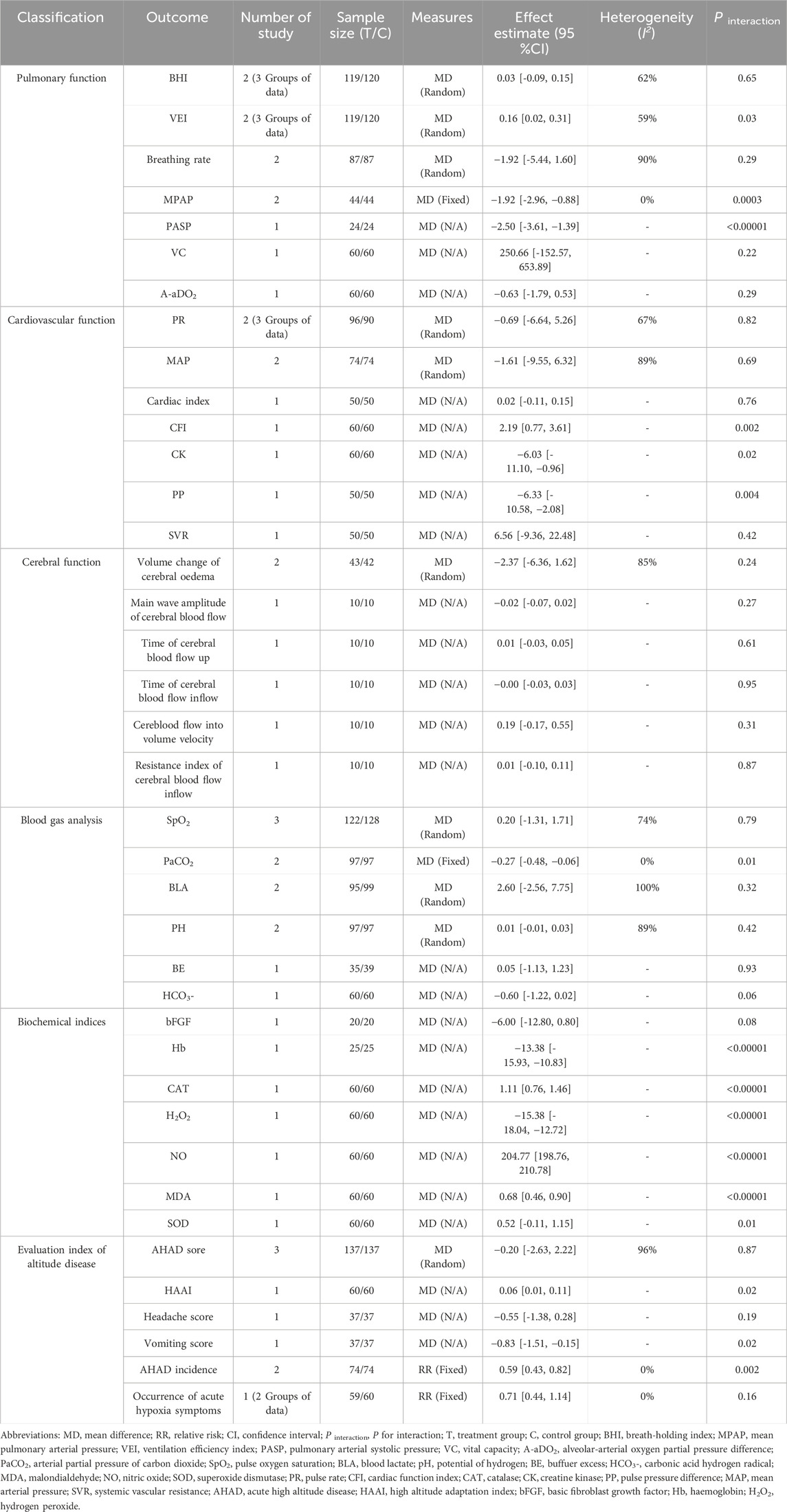
3.4.3 Other outcomes
The meta-analysis results indicated that RCE demonstrated efficacy across these outcomes, including pulmonary function (increasing VEI, reducing MPAP and PASP), cardiovascular function (reducing PP, decreasing CK and increasing CFI), blood gas analysis (decreasing PaCO2), biochemical indices (increasing NO, decreasing H2O2 and Hb) and evaluation index of altitude disease (increasing HAAI, decreasing vomiting score and the incidence of AHAD). However, the efficacy of reducing CAT and MDA levels was limited. The remaining results did not demonstrate a statistically significant difference. The results are shown in Table 3.
3.5 Adverse events
Three studies (Cai et al., 2003; He, 2011; Chiu et al., 2013) reported adverse events occurring during the treatment process. In the treatment group, six out of 125 participants (4.8%) experienced adverse events, with the primary adverse events being dizziness and drowsiness. In the control group, 14 out of 131 participants (10.7%) experienced adverse events, with the primary adverse events identified being headache, xerostomia, and gastrointestinal reactions. All of which were mild and self-limiting in nature, with no reports of severe cases. The findings demonstrated that the incidence of adverse events in the experimental group was significantly lower compared to the control group.
3.6 Sensitivity analyses
The sensitivity analyses indicated that the results of the meta-analysis remained consistent regardless of the exclusion of any individual study, thereby suggesting the robustness of the findings. Detailed data pertaining to the sensitivity analyses are presented in Supplementary Materia S5.
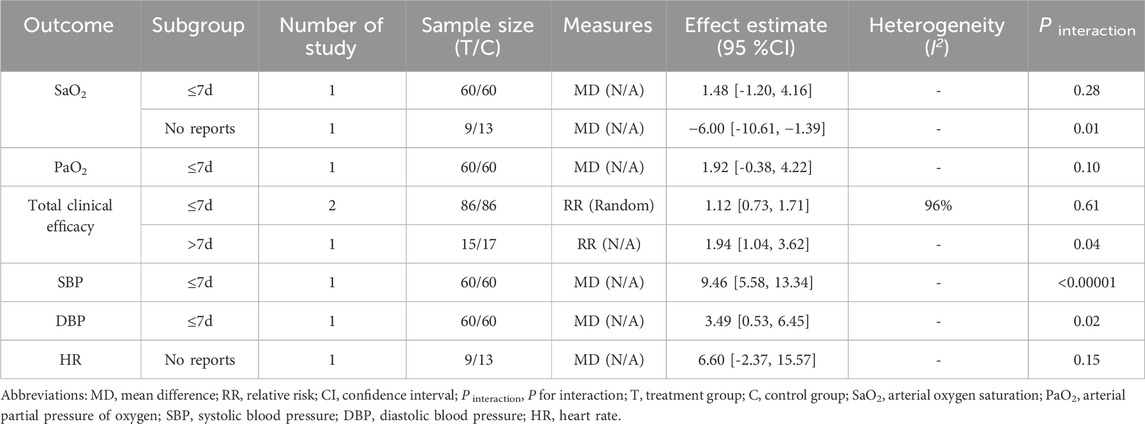
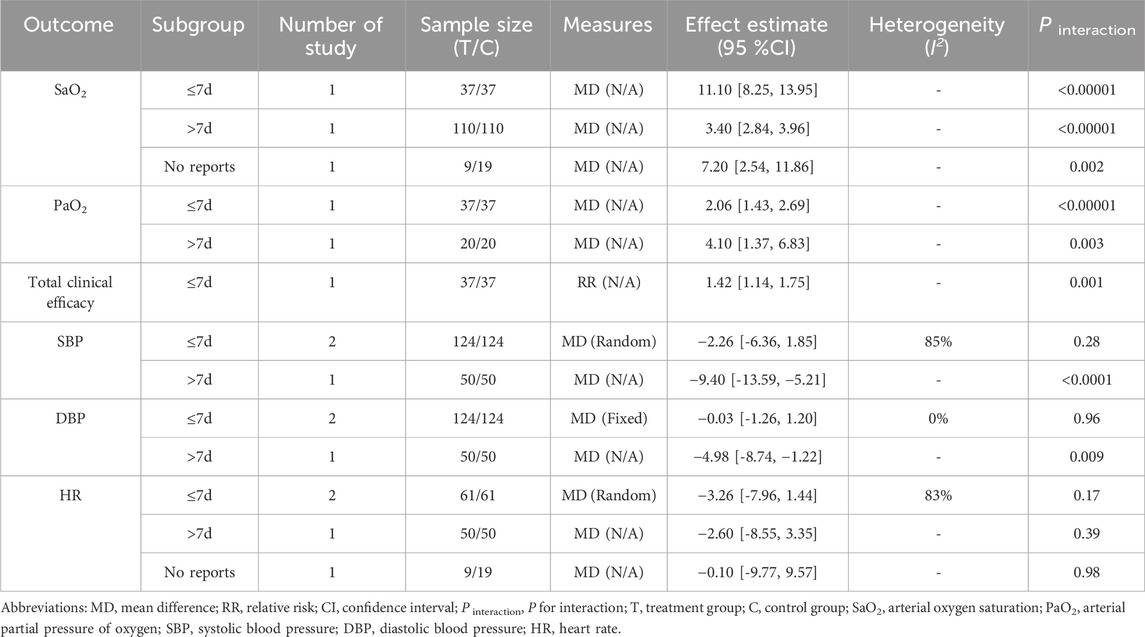
3.7 Subgroup analysis
Subgroup analyses for both primary and secondary outcomes were carried out depending on the treatment durations (≤7 days, >7 days, and no reports) within the same intervention comparisons (RCE vs. WM, RCE vs. placebo) (Table 4; Table 5). In the RCE vs. WM group, the meta-analysis showed no significant difference in total clinical efficacy between the groups for treatment durations of ≤7 days, but RCE is significantly more effective for durations >7 days; the other outcomes showed no statistical significance due to the lack of comparison of treatment durations. In the RCE vs. placebo group, the meta-analysis demonstrated that RCE showed significant efficacy in improving SaO2 and PaO2, regardless of whether the treatment lasted ≤7 days or >7 days; for reducing SBP and DBP, no statistically significant differences were observed between groups for treatment durations of ≤7 days, but RCE showed significant efficacy for durations >7 days; and regarding HR reduction, there were no statistically significant differences between the two groups for treatment durations of ≤7 days and >7 days; the total clinical efficacy showed no statistical significance due to the lack of comparison of treatment durations.
3.8 Publication bias
Due to the limited sample size of the included studies, an assessment of publication bias was not performed.
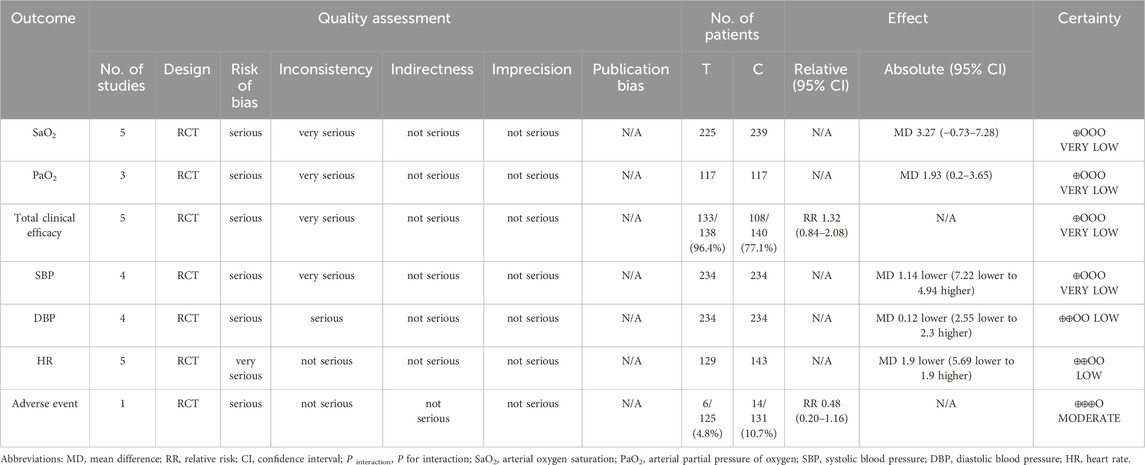
3.9 Certainty of evidence
The certainty of evidence regarding the outcomes was evaluated with the GRADE methodology, as detailed in Table 6. The results demonstrated that the evidences for adverse events were considered to be of moderate certainty. In contrast, the evidences for DBP and HR were assessed as having low certainty, while the evidences for SaO2, PaO2, SBP and total clinical efficacy were deemed to have very low certainty. The downgrade was primarily due to uncertainty associated with bias and inconsistency.
4 Discussion
4.1 Efficacy of RCE for AHAD
In the context of AHAD, hypoxia represents the predominant pathological mechanism and the most frequently observed clinical symptom, while SaO2 and PaO2 are the main indicators reflecting the oxygen content in the human body (Wu, 2014). The results indicated that RCE was significantly more effective than the placebo and may be as effective as WM in improving SaO2 and PaO2 levels. This suggested that RCE may improve blood oxygen levels in patients with AHAD. The primary mechanisms are linked to salidroside, the principal constituent of RCE, which decreases oxygen consumption, scavenges free radicals, mitigates lipid peroxidation reactions, and prevents hemorheological changes induced by hypoxic conditions (Chen et al., 2017; Ma et al., 2019; Hou et al., 2024). It is essential to recognize that the mechanism underlying AHAD cannot be entirely equated with hypoxia. SaO2 and PaO2 do not fully capture the therapeutic efficacy related to AHAD (Burtscher et al., 2004; Loeppky et al., 2008). Consequently, when citing this article, it is imperative to consider analyses of additional indicators.
AHAD often presents with symptoms like headache, nausea, loss of appetite, digestive issues, insomnia, fatigue, and hair loss due to hypoxia, cold, and radiation (Zhang and Shu, 2011). The meta-analysis demonstrated that RCE exhibited significant efficacy in improving the total clinical efficacy, and it might effectively reduce and shorten high altitude reactions. This efficacy may be closely associated with RCE’s pharmacological properties, including immune regulation, antioxidative activity, anti-inflammatory effects, anti-apoptotic mechanisms, and neuroprotective functions (Yang et al., 2015; Si et al., 2022). However, In terms of reducing SBP and DBP, RCE’s efficacy was inferior to that of WM, and it did not demonstrate significant efficacy relative to placebo. RCE also showed no significant effect in reducing HR. The results indicated that RCE may not have an advantage in acutely reducing elevated SBP, DBP, and HR. This lack of efficacy may be due to its slow action, which fails to counteract the quick damage caused by hypoxia. Its complex makeup, poor specificity, and low concentration of active ingredients also likely reduce its efficacy (Yu et al., 2006; Li et al., 2008). The observed lack of efficacy might also be attributed to confounding variables, including methodological limitations, the formulation type, the route of administration, and the dosage. We also recommend strengthening the comparison with WM, highlighting any potential synergies in combined use.
High-altitude conditions cause various pathophysiological changes in cardiac, pulmonary, and cerebral tissues due to hypoxia acclimatization (Liu et al., 2016). This environment also disrupts oxidative stress balance, resulting in altered blood gas and biochemical parameters (Yang et al., 2011). The results of the meta-analysis demonstrated that RCE was effective in enhancing pulmonary function, cardiovascular function, blood gas analysis, biochemical indices and evaluation index of altitude disease. Previous studies have also shown that RCE may enhance blood oxygen and hemodynamics to boost heart function, lower pulmonary artery pressure and ease vascular tension, thereby preventing and treating AHAD (Yang et al., 2015; Liu et al., 2016). However, its efficacy in improving indicators of brain function did not reach statistical significance.
4.2 Safety of RCE for AHAD
According to the results, the occurrence of adverse events was infrequent and of mild severity during the treatment of AHAD with RCE. These results suggested that incorporating RCE into the treatment regimen does not appear to elevate the incidence of additional safety events, thereby implying that RCE may have a favorable safety profile. Nevertheless, given the limited number of studies reporting adverse events that were included in the analysis, this conclusion should be interpreted with caution. Simultaneously, the inconsistencies in standardized reporting of adverse reactions in RCTs may contribute to underreporting. We suggested that future research adhere to international guidelines, such as CONSORT-Harms, to enhance the rigor of safety evaluations. Previous research indicated that RCE may be a contributing factor to adverse events, including rashes, headaches, dizziness, palpitations, nausea, vomiting, anaphylactoid reactions, dyspnea, among others (Wang et al., 2019). Adverse events associated with RCE affect multiple physiological systems, with systemic damage representing the highest incidence, predominantly occurring in elderly individuals (Li et al., 2015). The incidence of these adverse events may be attributed to drug interactions and delayed metabolism in the elderly population. Consequently, it is imperative to exercise caution when administering drug combinations, and particular vigilance should be applied when treating patients with hepatic or renal insufficiency (Li et al., 2015). The adverse reactions associated with RCE continue to be a subject of debate, necessitating further research to elucidate this issue. Additionally, reinforce that use in elderly individuals or those with comorbidities requires special monitoring, especially given altered hepatic and renal metabolism.
4.3 Risk of bias
Despite our efforts to mitigate bias throughout the research process, certain factors proved to be unavoidable. Most studies exhibited bias in the randomization process, primarily due to the randomization methodology and the concealment of allocation. Over half of the studies exhibited bias in deviations from the intended interventions, attributable to the absence of blinding. More than 30% of the studies exhibited bias in measurement of the outcome, primarily due to improper measurement methods. All studies demonstrated selection bias in the reported results, primarily due to the absence of a predetermined plan. Furthermore, the lack of intention-to-treat analysis in these studies may lead to an overestimation of the efficacy of RCE. Consequently, the results of this study warrant careful interpretation. This highlighted the critical importance of employing instruments such as the Cochrane Risk of Bias 2.0 (RoB 2.0) in forthcoming research assessments. Moreover, we recommend the prospective registration of studies in databases such as ClinicalTrials.gov or ChiCTR to reduce selection and reporting bias.
4.4 Certainty of evidence
The GRADE system was used to assess the certainty of evidence for both primary and secondary outcomes, as well as adverse events. The low certainty of most results made us cautious about the results, primarily due to bias risk and inconsistency. Firstly, the study found that among the overall risks of bias for all outcomes, more than two-thirds were rated as issues of concern. Consequently, the certainty of all outcomes was downgraded by one level. Secondly, in terms of inconsistency in evidence, the heterogeneity test of four outcomes showed that I2 exceeding 75%, and the evidence was downgraded by two levels. And due to the heterogeneity test of two outcomes showing I2 > 50% and <75%, the evidence was downgraded by one level. Due to downgrading, the certainty of the results in this study is affected, and therefore the results should be interpreted with caution. Conducting high-quality RCTs is crucial to enhance the reliability of evidence related to RCE in AHAD. We recommend that future studies increase sample size and standardize clinical outcomes, as heterogeneity may have contributed to the low confidence in the evidence.
4.5 Heterogeneity between the included studies
Addressing clinical heterogeneity, this review implemented strict eligibility criteria regarding participants, interventions, comparisons, outcomes, and study designs. Furthermore, the study performed a sensitivity analysis and a subgroup analysis stratified by the treatment duration. However, the clinical heterogeneity observed in some of the results remained inadequately explained, possibly due to the following two factors. Firstly, there were demographic differences among study participants, but age, gender, and comorbidity details were hard to differentiate. Secondly, the intervention measures employed for the control groups in this study comprised WM or placebo. Although the analyses were conducted separately for each, variations in the implementation of WM and placebo were observed across different studies. Future studies must implement stricter control over interventions in the control group, such as clearly defining western medicine treatments or placebo protocols. We also recommend multicenter studies to minimize population heterogeneity, including factors like gender, age, ethnicity, and local altitude.
4.6 Clinical implications
The results of this study indicated that RCE may substantially improve hypoxia resulting from high-altitude environments, enhance organ function and optimize physiological and biochemical parameters, and demonstrated favorable safety. Consequently, RCE exhibited potential as a clinical therapeutic agent for the prevention of altitude sickness and the mitigation of symptoms associated with AHAD. It also illustrated the potential applicability of Rhodiola crenulata extract (RCE) as an adjunct to standard treatment in areas with limited access to western medicine. Moreover, the subgroup analysis revealed that RCE’s therapeutic efficacy on AHAD was notably improved when the treatment duration surpassed 7 days, suggesting that a longer treatment course might result in better efficacy of RCE in the treatment of AHAD. This suggested that the treatment duration (exceeding 7 days) may be a key variable for efficacy, which may have implications for prophylactic use logistics before travel to high altitudes.
4.7 Strengths and limitations
This study constituted a pioneering systematic review and meta-analysis examining the efficacy of RCE in the treatment of AHAD. We meticulously implemented stringent inclusion and exclusion criteria to mitigate the confounding effects of other traditional Chinese medicine interventions. Our analysis incorporated data from 1,690 participants across 19 randomized controlled trials, yielding a substantial sample size that provides compelling clinical evidence regarding the effectiveness of RCE in the management of AHAD. Additionally, the influence of treatment duration on the efficacy of RCE in the management of AHAD was explored through a subgroup analysis.
Nevertheless, this review was subject to several limitations. First, the low quality of the included studies undermined the credibility of the research findings. Second, all 19 RCTs included in this study were conducted exclusively in China, with no representation from other countries. Third, this analysis was influenced to several confounding variables, such as the inconsistent dosages, administration frequency and treatment durations of RCE reported in the original studies, the omission of participants’ prior experience with herbal medicine, and the lack of documentation regarding the altitudes at the journey’s commencement and destination. These factors may compromise the accuracy of the analysis. Fourth, notwithstanding the implementation of sensitivity and subgroup analyses, the meta-analysis results for both primary and secondary outcomes exhibited significant heterogeneity, the origins of which were difficult to determine. Fifth, the absence of follow-up time reported in the studies hinders the assessment of RCE’s long-term prognosis in patients.
4.8 Future perspectives
We recommend broader geographic representation in future studies (beyond China). To enhance the quality of literature, it is recommended that future studies will employ more robust research designs, reinforce quality control throughout the research implementation process, and undertake multi-center, large-sample, double-blind RCTs, and include international research. In the design of clinical trials, it is advisable to incorporate functional and quality-of-life outcomes alongside laboratory indicators. Additionally, metrics for evaluating long-term efficacy, such as recurrence rates and mortality, should be included.
Furthermore, the pharmacological mechanism through which RCE exerts its therapeutic effects in the treatment of AHAD requires further investigation. It is recommended that future studies explore the specific molecular pathways of RCE in both animal and human models, with a particular focus on its antioxidant, anti-apoptotic, and vascular mechanisms. Employing preclinical AMS models in conjunction with neuroinflammatory or mitochondrial biomarkers could significantly enhance the understanding of RCE’s mechanisms. Additionally, there is a need to develop standardized RCE formulations with rigorous quality control of active compounds to ensure consistency and efficacy in research and therapeutic applications.
5 Conclusion
This systematic review and meta-analysis suggesed that R. crenulata extract may offer therapeutic potential for Acute high altitude disease, particularly by improving blood oxygenation and alleviating clinical symptoms, with a favorable safety profile. Notably, prolonged use appears to enhance its efficacy. However, the overall certainty of the evidence remained low due to methodological limitations in the included studies. Therefore, there is an urgent need for robust, well-designed, multicenter randomized clinical trials to validate these findings and clarify the long-term safety and effectiveness of RCE in managing AHAD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
ZG: Methodology, Resources, Software, Visualization, Writing – original draft, Writing – review and editing. YL: Methodology, Resources, Software, Visualization, Writing – original draft, Writing – review and editing. WL: Data curation, Investigation, Writing – review and editing. WS: Data curation, Investigation, Writing – review and editing. XZ: Formal Analysis, Validation, Writing – review and editing. HL: Formal Analysis, Methodology, Writing – review and editing. HZ: Funding acquisition, Project administration, Resources, Validation, Writing – review and editing. TZ: Conceptualization, Project administration, Supervision, Validation, Writing – review and editing. WP: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Tianjin Education Commission Research Program Project (grant number 2023KJ145) and the Natural Science Foundation of Tianjin Municipality (grant number 23ZYJDSS00020).
Acknowledgments
We express our gratitude for the financial support provided for this study and acknowledge the contributions of all authors in its successful completion.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1595953/full#supplementary-material
Abbreviations
AHAD, acute high-altitude disease; PaO2, arterial partial pressure of oxygen; CAT, catalase; PASP, pulmonary arterial systolic pressure; CFI, cardiac function index; PP, pulse pressure difference; CI, confidence interval; PRISMA, 2020, Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020; CK, creatine kinase; RCE, R. crenulata extract; CNKI, China National Knowledge Infrastructure Database; RCT, randomized controlled trial; DBP, diastolic blood pressure; ROB 2.0) the revised Cochrane risk of bias tool for randomized trials 2.0; GRADE, Grading of Recommendations Assessment, Development and Evaluation; RR, relative risk; HAAI, high altitude adaptation index; SaO2, oxygen saturation; AHAD, high altitude diseases; SBP, systolic blood pressure; Hb, haemoglobin; SinoMed, Chinese Biomedical Literature Database; H2O2, hydrogen peroxide; TCM, Traditional Chinese Medicine; HR, heart rate; VEI, ventilation efficiency index; MD, mean difference; VIP, VIP Database for Chinese Technical Periodicals; MDA, malondialdehyde; WF, Wanfang Database; MPAP, mean pulmonary arterial pressure; WM, western medicine; NO, nitric oxide; PaCO2, arterial partial pressure of carbon dioxide.
References
Balshem, H., Helfanda, M., J.Schunemann, H., D.Oxman, A., Kunz, R., Brozek, J., et al. (2011). GRADE guidelines: 3. Rating the quality of evidence. Chin. J. Evidence-Based Med., 451–455. doi:10.3969/j.issn.1672-2531.2011.04.017
Bärtsch, P., and Gibbs, S. J. (2007). Effect of altitude on the heart and the lungs. Circulation 116, 2191–2202. doi:10.1161/CIRCULATIONAHA.106.650796
Bi, X., Liu, Q., Jin, feng, and Huang, L. (2015). Experimental study on the anti-fatigue role of Jujube extract cyclic adenosine monophosphate under plateau stress. Food Nutr. China 21, 81–84.
Burtscher, M., Flatz, M., and Faulhaber, M. (2004). Prediction of susceptibility to acute mountain sickness by SaO2 values during short-term exposure to hypoxia. High Alt. Med. and Biol. 5, 335–340. doi:10.1089/ham.2004.5.335
Cai, J., Li, Q., and Hong, Y. (2003). The influence of Rhodiola on the healthy human body on the plateau. Chin. Mag. Clin. Med. Prof., 11917–11919.
Cao, F., Jiang, X., Xiong, H., and Xiong, W. (2015). A systematic analysis of Rhodiola rosea in the prevention of acute high altitude disease. Chin. J. Lung Dis. (Electronic Edition), 425–430. doi:10.3877/cma.j.issn.1674-6902.2015.04.004
Chen, Q., Zhu, J., Li, Z., Li, M., and Hu, H. (2017). Effect of rhoside on the content of MMP-9 and TIMP-1 in hypoxic-ischemic brain tissue of neonatal rats. Asia-Pacific Tradit. Med. 13, 6–8. doi:10.11954/ytctyy.201713003
Chen, Z. (2013). Progress in the clinical application of Rhodiola rosea. Strait Pharm. J. 25, 106–107.
Chiu, T.-F., Chen, L. L.-C., Su, D.-H., Lo, H.-Y., Chen, C.-H., Wang, S.-H., et al. (2013). Rhodiola crenulata extract for prevention of acute mountain sickness: a randomized, double-blind, placebo-controlled, crossover trial. BMC Complementary Altern. Med. 13, 298. doi:10.1186/1472-6882-13-298
Duan, W., Sun, S., Hui, Z., Gao, Z., Hu, J., Jin, F., et al. (2015). Effect of baicalin capsules on acute mountain sickness in healthy young males at acute high altitude exposure. Med. J. Chin. People’s Armed Police Force 26, 75–78. doi:10.14010/j.cnki.wjyx.2015.01.024
Faulhaber, M., Wille, M., Gatterer, H., Heinrich, D., and Burtscher, M. (2014). Resting arterial oxygen saturation and breathing frequency as predictors for acute mountain sickness development: a prospective cohort study. Springer Sci. Bus. Media LLC 18, 669–674. doi:10.1007/s11325-013-0932-2
Feng, B., Liu, Z., Xing, Y., Gao, A., Zhu, H., and Wang, J. (2013). Advance in studies on effect of traditional Chinese (Tibetan) medicines in prevention and treatment of acute altitude sickness. China J. Chin. Materia Medica 38, 1876–1880. doi:10.4268/cjcmm20131207
Guo, Y., Liu, X., Zhang, Q., Shi, Z., Zhang, M., and Chen, J. (2024). Can acute high-altitude sickness be predicted in advance. Rev. Environ. Health 39, 27–36. doi:10.1515/reveh-2022-0117
Hao, L., Zhang, G., Liu, F., Mu, J., Chen, L., Xing, X., et al. (2008). Effect of three anti-hypoxia preparations on human cerebral blood flow at altitude. J. Prev. Med. Chin. People’s Liberation Army, 30–32. doi:10.3969/j.issn.1001-5248.2008.01.008
He, Y. (2011). Analysis of the clinical efficacy of Rhodiola rosea for acute altitude sickness. China Health Ind. 65. doi:10.16659/j.cnki.1672-5654.2011.13.017
Hou, Y., Fan, F., Xie, N., Zhang, Y., Wang, X., and Meng, X. (2024). Rhodiola crenulata alleviates hypobaric hypoxia-induced brain injury by maintaining BBB integrity and balancing energy metabolism dysfunction. Phytomedicine 128, 155529. doi:10.1016/j.phymed.2024.155529
Hsu, S.-W., Chang, T.-C., Wu, Y.-K., Lin, K.-T., Shi, L.-S., and Lee, S.-Y. (2017). Rhodiola crenulata extract counteracts the effect of hypobaric hypoxia in rat heart via redirection of the nitric oxide and arginase 1 pathway. BMC Complementary Altern. Med. 17, 29. doi:10.1186/s12906-016-1524-z
Hu, Q., Wei, L., Dong, H., Li, C., Ye, D., Li, Z., et al. (2014). Efficacy analysis of Edaravone and Rhodiola capsule in the treatment of high hypertensive cerebral hemorrhage. Chin. J. Clin. Ration. Drug Use 7, 32–33. doi:10.15887/j.cnki.13-1389/r.2014.04.134
Hung, H. P., Lin, C. F., Tsai, C. H., Chao, H. S., Chou, C. W., and Chang, S. C. (2019). The usefulness of prophylactic use of acetazolamide in subjects with acute mountain sickness. J. Chin. Med. Assoc. 82, 126–132. doi:10.1097/JCMA.0000000000000014
Imray, C., Wright, A., Subudhi, A., and Roach, R. (2010). Acute mountain sickness: pathophysiology, prevention, and treatment. Prog. Cardiovasc Dis. 52, 467–484. doi:10.1016/j.pcad.2010.02.003
Lei, Y., Zhang, L., Hu, Y., Li, S., and Qian, R. (2015). Protective effects of prophylactic caplendus rhodiola capsules on acute mountain sickness. Chin. J. Clin. Electron. Ed. 9, 2296–2299. doi:10.3877/cma.j.issn.1674-0785.2015.12.007
Li, L., Lin, L., Wen, B., Zhao, P., Liu, D., Pang, G., et al. (2023). Promising natural medicines for the treatment of high-altitude illness. High Alt. Med. and Biol. 24, 175–185. doi:10.1089/ham.2022.0139
Li, L., Wang, L., Zhu, X., Guan, Y., Fan, R., and Jin, F. (2015). Analysis of 719 reports of adverse drug reactions/events induced by Sofren injection. Chin. J. Pharmacovigil. 12, 679–682. doi:10.19803/j.1672-8629.2015.11.010
Li, S., Jin, G., and Li, W. (2008). Prevention of AMS and enhancement of exercise ability by Rhodiola. J. Prev. Med. Chin. People’s Liberation Army, 246–249. doi:10.3969/j.issn.1001-5248.2008.04.004
Li, W., Zhang, Y., and Liu, J. (2017). Effect of Rhodiola extract capsules on the blood pressure of youths after acute exposure to high altitude. China and Foreign Med. Treat. 36, 146–148. doi:10.16662/j.cnki.1674-0742.2017.10.146
Liu, C., Huang, S., Du, R., Li, T., and Jiang, M. (2022). Visual analysis of research progress in Rhodiola based on CiteSpace. Cent. South Pharm. 20, 1192–1197. doi:10.7539/j.issn.1672-2981.2022.05.040
Liu, P., Cai, H., Liu, Z., Zhang, L., Zeng, Z., and Ge, C. (2016). Effect of Rhodiola on serum SOD,MDA,OX-LDL and MMP-2 levels in patients with acute cerebral infarctionin plateau areas. Chin. J. General Pract. 14, 176–178. doi:10.16766/j.cnki.issn.1674-4152.2016.02.003
Liu, X., and Gong, T. (2024). Artificial intelligence and evidence-basedresearch will promote the development oftraditional medicine. Acupuncture and Herbal Medicine 4, 134–135.
Loeppky, J. A., Icenogle, M. V., Charlton, G. A., Conn, C. A., Maes, D., Riboni, K., et al. (2008). Hypoxemia and acute mountain sickness: which comes first. High Alt. Med. and Biol. 9, 271–279. doi:10.1089/ham.2008.1035
Ma, D., Wang, L., Jin, Y., Gu, L., Yin, G., Wang, J., et al. (2022). Chemical characteristics of Rhodiola crenulata and its mechanism in acute mountain sickness using UHPLC-Q-TOF-MS/MS combined with network pharmacology analysis. J. Ethnopharmacol. 294, 115345. doi:10.1016/j.jep.2022.115345
Ma, S., Liu, Y., Tian, X., Li, X., Chai, Y., Liu, C., et al. (2019). Comparative study on the effects of Rhodiola broken decoction and traditional decoction on myocardial fine protective effect of Hypoxia-H9c2. Asia-Pacific Tradit. Med. 15, 18–23. doi:10.13863/j.issn1001-4454.2019.02.013
Niu, W., Wang, Y., Cao, Z., Yu, S., and Zhang, L. (2006). Experimental effects of Shulikang capsule on prevention of acute high atitude reaction. J. High Alt. Med., 2–4. doi:10.3969/j.issn.1007-3809.2006.03.002
Niu, W., Wang, Y., Wang, H., Chen, N., and Zhang, J. (2003). The influence of the Tibetan medicine on the ventilation efficiency index of people who quickly enter the plateau. J. High Alt. Med., 2–5.
Page, J. M., McKenzie, E. J., Bossuyt, M. P., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pena, E., El, S. A., Siques, P., and Brito, J. (2022). Oxidative stress and diseases associated with high-altitude exposure. Antioxidants (Basel) 11, 267. doi:10.3390/antiox11020267
Ren, W. (2022). Study of the mechanism by which Rhodiola rosea extract improves the tolerance of high-altitude hypoxia in mice. Northwest Univ. Natl. doi:10.27408/d.cnki.gxmzc.2022.000343
Ren, W., and Wang, J. (2011). Pharmacological prophylaxis of acute mountain sickness. Prog. Mod. Biomed. 11, 1187–1190+1200. doi:10.13241/j.cnki.pmb.2011.06.034
Roach, C. R., Hackett, H. P., Oelz, O., Bärtsch, P., Luks, A. M., MacInnis, M. J., et al. (2018). The 2018 lake louise acute mountain sickness score. High. Alt. Med. Biol. 19, 4–6. doi:10.1089/ham.2017.0164
Si, Y., Liu, D., Yan, X., and Pan, F. (2022). Mechanism of Rhodiola rosea on preventing and treating acute mountain sickness based on network pharmacology and molecular docking. Mod. Traditional Chin. Med. Materia Medica-World Sci. Technol. 25, 144–155. doi:10.11842/wst.20211207001
Sterne, J. A. C., Savović, J., Page, J. M., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Tang, R., Qu, Y., Xu, H., Yan, L., Yan, T., Jiang, Y., et al. (2015). Preventive effect and mechanism of salidroside on acute mountain sickness. Chin. J. Clin. Pharmacol. Ther., 822–827.
Tian, J., Guo, M., Zhou, X., Wang, Y., Liu, L., Liu, C., et al. (2015). Comparative study of cardiopulmonary protective effects of Trimetazidine and Rodiola in acute highland recruits. J. Logist. Univ. PAP Medical Sci. 24, 529–532+542. doi:10.16548/j.2095-3720.2015.07.005
Wang, lingdi, Xie, yangming, Wang, lianxin, and Wang, huan (2019). China journal of traditional Chinese medicine. China J. Traditional Chin. Med. 34, 95–99.
Wang, Y., Niu, W., Zhang, J., Wang, H., and Chen, N. (2003). Effect of new Tibetan medicine on hypoxia of recruits exposed rapidly to high altitude. J. High Alt. Med., 14–16. doi:10.3969/j.issn.1007-3809.2003.02.005
Wang, Z. (2023). Effect of Salidroside on prevention and treatment of high altitude pulmonary. Gansu Univ. Traditional Chin. Med. doi:10.27026/d.cnki.ggszc.2023.000004
Wei, L., Dong, H., Ye, D., Li, Z., Second, O., Jia, F., et al. (2014). Efficacy analysis of edaravone and rhodiola treatment in patients after plateau severe craniocerebral trauma, 17, 87–88. doi:10.3969/j.issn.1673-5110.2014.03.048
World Flora Online (1976). Rhodiola crenulata. Available online at: https://www.worldfloraonline.org/(Accessed September 10, 2024).
Wu, T. (2014). Guidelines for the diagnosis, prevention and treatment of high altitude disease. Lanzhou: Lanzhou University Press. doi:10.CNKI/:SUN:QHYZ.0.2017-01-043
Wu, Y., Zhang, C., Chen, Y., and Luo, Y.-J. (2018). Association between acute mountain sickness (AMS) and age: a meta-analysis. Mil. Med. Res. 5, 14. doi:10.1186/s40779-018-0161-x
Yang, P., Peng, J., Chen, D., and Zhu, K. (2015). Effect of Rhodiola rosea injection on VEGF in rats undergoing myocardial ischemic preconditioning. Mod. J. Integr. Traditional Chin. West. Med. 24, 710–712. doi:10.3969/j.issn.1008-8849.2015.07.010
Yang, S., Feng, E., Yan, Z., He, W., Tian, Z., Yin, H., et al. (2011). Effect of oxidative stress in development of acute high altitude response during the process of strong physical work at high altitude. Chin. J. Appl. Physiology 27, 457–460. doi:10.13459/j.cnki.cjap.2011.04.027
Yang, S., Shen, J., Guo, Z., Feng, E., Wang, S., Dong, H., et al. (2008). The relationship between change of serum basic fibroblast growth factor level and pulmonary arterial pressure and its intervention in patients with chronic cor pulmonale at high altitude areas. Chin. J. Clin. Med., 47–49.
Yu, J., Mi, Y., Wei, J., Lin, F., Liu, C., Feng, B., et al. (2012). Effect of thrombolytic capsule on blood oxygen saturation of recruits in Tibet. Clin. J. Med. Officers 40, 591–593. doi:10.3969/j.issn.1671-3826.2012.03.028
Yu, S., Niu, W., Wang, Y., Zhang, L., and Cao, Z. (2006). Effects of Shulikang capsule on blood pressure of young men exposed rapidly to high altitude. Med. J. Natl. Defending Forces Southwest China, 382–384. doi:10.3969/j.issn.1004-0188.2006.04.012
Zhang, Y., and Shu, Y. (2011). Pharmacological effects and clinical application of TCM in preventing altitude sickness against altitude sickness. J. High Alt. Med. 21, 57–62. doi:10.3969/j.issn.1007-3809.2011.01.026
Zhang, Z., Li, R., Chen, Y., Yang, H., Fitzgerald, M., Wang, Q., et al. (2024). Integration of traditional, complementary, andalternative medicine with modern biomedicine: the scientization, evidence, and challenges forintegration of traditional Chinese medicine. Acupuncture and Herbal Medicine 4, 68–78.
Zhaxi, Q., Rob, G., Ci, bai, and Huang, J. (2024). Research status of drug prevention and treatment of altitude sickness. Chin. J. Clin. Pharmacol. 40, 1689–1692. doi:10.13699/j.cnki.1001-6821.2024.11.032
Keywords: Rhodiola rosea, roseroot, altitude sickness, acute mountain sickness, AMS
Citation: Gao Z, Liu Y, Liao W, Song W, Zhang X, Lin H, Zhang H, Zhang T and Pang W (2025) Efficacy and safety of Rhodiola crenulata extract in the treatment of acute high altitude disease, based on studies involving populations in China: A systematic review and meta-analysis. Front. Pharmacol. 16:1595953. doi: 10.3389/fphar.2025.1595953
Received: 18 March 2025; Accepted: 21 May 2025;
Published: 13 June 2025.
Edited by:
Anthony Booker, University of Westminster, United KingdomReviewed by:
Dale R. Wagner, Utah State University, United StatesDiego Elias Pereira, Federal University of Paraíba, Brazil
Kuo-Cheng Wu, University of Taipei, Taiwan
Copyright © 2025 Gao, Liu, Liao, Song, Zhang, Lin, Zhang, Zhang and Pang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Zhang, emhhbmd0YW90Y21AMTYzLmNvbQ==; Wentai Pang, cHd0dGNtQHRqdXRjbS5lZHUuY24=
†These authors share first authorship
 Zixuan Gao
Zixuan Gao Yaoyuan Liu
Yaoyuan Liu Weiwen Liao1
Weiwen Liao1 Han Zhang
Han Zhang