- 1Department of Orthopaedics, Nanjing Central Hospital, Nanjing, China
- 2Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China
Background: Traditional botanical drugs and medicinal plants, along with their metabolite extracts, have exhibited considerable potential in the management of knee osteoarthritis (KOA) due to their natural properties, favorable safety profiles, and minimal adverse effects.
Objective: This study aimed to evaluate the therapeutic efficacy of various botanical and medicinal plant extracts on KOA. Search Methods: We conducted a comprehensive literature search across PubMed, Embase, Cochrane Library, and Web of Science, focusing exclusively on randomized controlled trials (RCTs) that investigated the efficacy of botanical and medicinal plant extracts for KOA. Selection Criteria: Studies were included if they met the following criteria: (1) experimental groups receiving single botanical drugs or plant extracts for KOA; (2) control groups comprising patients receiving placebo or standard care; (3) clinical RCT designs; and (4) outcome measures including at least one of the following: Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Visual Analogue Scale (VAS), Short Form 36 Health Survey (SF-36), Knee injury and Osteoarthritis Outcome Score (KOOS), Lequesne’s Pain-Function Index (LPFI), Japanese Orthopaedic Association Score (JOA). Data Collection and Analysis: The methodological quality of the included studies was assessed using the Cochrane risk of bias tool, and data analysis was performed using appropriate statistical software.
Results: A total of 36 RCTs, encompassing 3,285 participants, were included in this review. Network meta-analysis revealed that compared to the placebo control group, Cucumis sativus (CS) extract [MD = 6.65, 95% CI = (3.83, 9.48)] significantly improved pain scores; Ashwagandha extract [MD = 4.16, 95% CI = (2.43, 5.90)] was more effective in reducing stiffness scores; and CS extract [MD = 4.28, 95% CI = (2.08, 6.49)] significantly improved function scores.
Conclusion: Based on Ranking Plot of the Network, we can state that CS extract is recommended as the most effective botanical and medicinal plant extract for KOA treatment. However, further studies are required to draw definitive conclusions. Given that there are only two studies with high homogeneity but small sample size for CS extract, the first result should be regarded as an exploratory signal and needs to be verified by a large sample multi-center RCT with independent teams.
Clinical Trial Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024617459, identifier CRD42024617459.
1 Introduction
Knee Osteoarthritis (KOA) is a degenerative disease primarily affecting the knee joint function of middle-aged and elderly populations (Giordano et al., 2020). Its pathological characteristics include joint space narrowing, subchondral bone sclerosis, and osteophyte formation, with clinical symptoms such as joint pain, restricted mobility, crepitus during movement, joint deformity, and muscle atrophy (Sharma, 2021). Remarkably, KOA has contributed more to the global burden of OA than any other site (Long et al., 2022). With the global trend of population aging, the prevalence of KOA continues to rise, becoming an increasingly serious public health issue worldwide (Menon and Mishra, 2018) and placing substantial pressure on healthcare systems and economies (Bedenbaugh et al., 2021; Jin et al., 2023). Data released by the World Health Organization in February 2021 reported that the number of osteoarthritis patients worldwide had reached approximately 343 million. In China, the overall prevalence of KOA is 8.1%, with rates of 5.2% in individuals aged 50 and above and rising to 11% in those aged 60 and older (Chen et al., 2022). By comparison, the overall prevalence rates of KOA in the United States and Europe are 4.3% and 5.3% (Chen et al., 2022), respectively, further highlighting the extensive impact of this condition.
Currently, the treatment of KOA primarily includes non-pharmacological interventions, pharmacological therapies, and surgical procedures (Charlesworth et al., 2019). Non-pharmacological interventions encompass patient education, moderate physical activity, mobility support, and physical therapy, aiming to reduce joint load, improve joint function, and enhance quality of life. Pharmacological therapies include oral administration of chondroprotective agents such as glucosamine and chondroitin sulfate, as well as analgesics like nonsteroidal anti-inflammatory drugs (NSAIDs) to alleviate pain and suppress inflammation (Kolasinski et al., 2020). Surgical interventions, such as joint replacement surgery, are typically recommended for patients with more severe conditions (Hannon et al., 2023). However, while conventional treatments can alleviate the symptoms of KOA to some extent, they cannot achieve a complete cure. Moreover, long-term use of certain medications may result in adverse effects, such as gastrointestinal discomfort and liver or kidney damage (da Costa et al., 2017). Therefore, the search for safer and more effective treatment options has become a major focus of current research efforts.
As there are currently no specific drugs for the treatment of KOA, traditional botanical drugs or medicinal plants and their extracted metabolites have shown significant potential due to their natural origins, high safety profile, and minimal side effects. The screening of botanical drugs and elucidation of their mechanisms of action are of great importance in the treatment of KOA (Wang et al., 2022). Many botanical and medicinal plant extracts, such as curcumin, ginger extract, and willow bark extract, have been found to possess anti-inflammatory, anti-swelling, and analgesic properties, effectively alleviating the symptoms of KOA. Numerous botanical drugs and their secondary metabolites, including extracts from Scutellaria baicalensis, turmeric, chicory root, frankincense, and ginger, have demonstrated potential therapeutic activity against KOA (Wang et al., 2022). The underlying mechanisms include reducing oxidative stress, inhibiting chondrocyte apoptosis, promoting autophagy, and modulating the NF-κB signaling pathway (Ziadlou et al., 2019). In recent years, significant progress has been made in the application of botanical drugs for the treatment of KOA (Walzer et al., 2015). Multiple clinical trials have indicated that botanical drugs or their active metabolites play an important role in alleviating KOA symptoms. Meta-analyses have shown that botanical medicines exhibit better efficacy in treating KOA compared to placebos and some conventional biomedicines (Lin et al., 2022). Additionally, combining traditional Chinese medicine with Biomedicine has proven more effective than using biomedicine alone (Liang et al., 2022). However, due to potential biases in the included studies, further research is needed to identify the active metabolites of botanical medicines and clarify their mechanisms of action.
Network meta-analysis (NMA) is an evidence-based technique used to compare the effectiveness of multiple interventions for a specific disease and to rank these treatments based on their efficacy (Rouse et al., 2017). In this study, we employed a network meta-analysis to compare different botanical and medicinal plant extracts, evaluate their effectiveness in treating patients with KOA, and provide patients and clinicians with a clearer understanding of the therapeutic potential of these extracts. Our objective is to assess the effects of these botanical and medicinal plant extracts on KOA patients and provide evidence-based recommendations for both patients and clinicians.
2 Materials and methods
2.1 Search strategies
Researchers retrieved data from four electronic databases (PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and Web of Science) spanning from their inception to October 2024. The search strategy was developed based on the PICOS framework: (P) Population: Knee Osteoarthritis; (I) Intervention: botanical and medicinal plant extracts; (C) Comparison: Placebo or conventional treatment measures; (O) Outcome: Functional scores of patients with knee osteoarthritis; (S) Study design: Randomized controlled trials (RCTs). The detailed search strategy is presented in Supplementary Table S5. The PICOTS framework is detailed in Supplementary Table S6.
2.2 Inclusion criteria
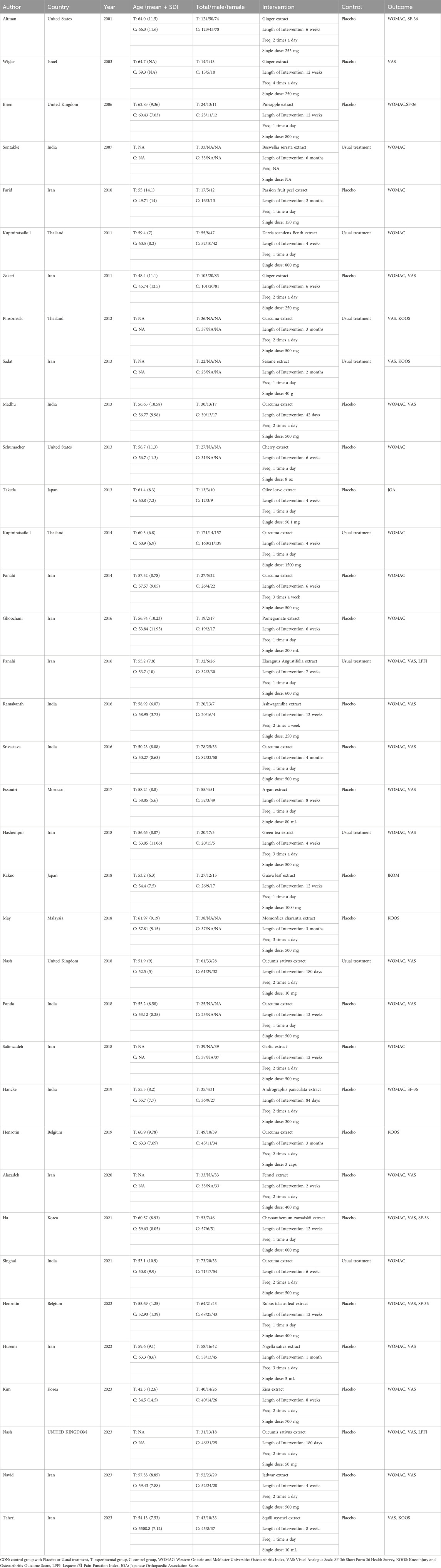
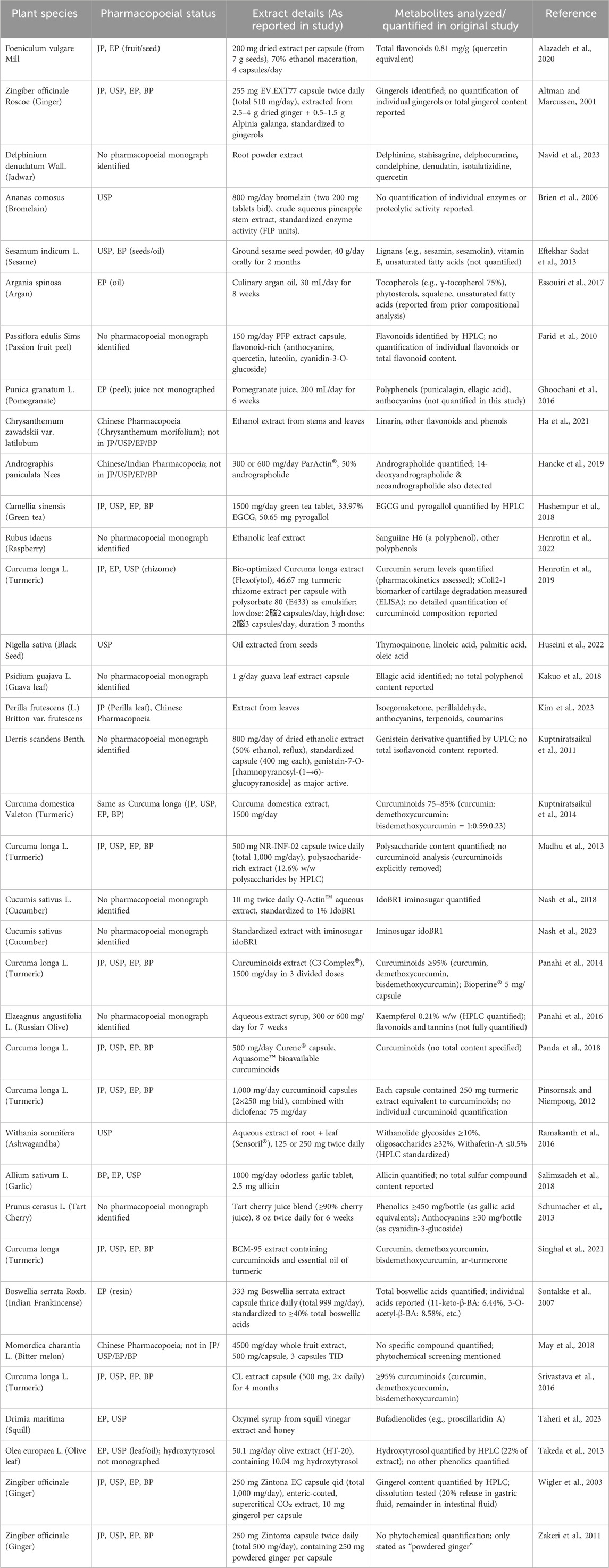
1. Experimental groups of various single botanical drugs or plant extracts for knee osteoarthritis included: Zingiber officinale Roscoe [Zingiberaceae; Zingiberis rhizoma] (Ginger), Ananas comosus (L.) Merr. [Bromeliaceae; Ananasis caulis] (Pineapple, bromelain), Boswellia serrata Roxb. ex Colebr. [Burseraceae; Olibanum indicum] (Indian frankincense, BS), Passiflora edulis Sims [Passifloraceae; Passiflorae edulis pericarpium] (Passion fruit peel, PFP), Derris scandens (Roxb.) Benth. [Fabaceae; Derridis scandentis caulis] (DSB), Curcuma longa L. [Zingiberaceae; Curcumae longae rhizoma] (Turmeric, Curcuma), Sesamum indicum L. [Pedaliaceae; Sesami semen] (Sesame), Prunus cerasus L. [Rosaceae; Pruni cerasi fructus] (Tart Cherry), Olea europaea L. [Oleaceae; Oleae folium] (Olive leaf, OL), Punica granatum L. [Lythraceae; Granati pericarpium] (Pomegranate), Elaeagnus angustifolia L. [Elaeagnaceae; Elaeagni angustifoliae fructus] (EA, Russian olive), Withania somnifera (L.) Dunal [Solanaceae; Withaniae radix] (Ashwagandha), Argania spinosa (L.) Skeels [Sapotaceae; Arganiae oleum] (Argan), Camellia sinensis (L.) Kuntze [Theaceae; Theae folium] (Green tea, GT), Psidium guajava L. [Myrtaceae; Psidii guajavae folium] (Guava leaf, GL), Momordica charantia L. [Cucurbitaceae; Momordicae fructus] (MC, bitter melon), Cucumis sativus L. [Cucurbitaceae; Cucumeris sativi fructus] (CS, cucumber), Allium sativum L. [Amaryllidaceae; Allii sativi bulbus] (Garlic), Andrographis paniculata (Burm.f.) Nees [Acanthaceae; Andrographidis herba] (AP), Foeniculum vulgare Mill. [Apiaceae; Foeniculi fructus] (Fennel), Chrysanthemum zawadskii var. latilobum [Asteraceae; Chrysanthemi flos] (CZ), Rubus idaeus L. [Rosaceae; Rubi idaei folium] (RIL, raspberry leaf), Nigella sativa L. [Ranunculaceae; Nigellae semen] (NS, black seed), Perilla frutescens (L.) Britton [Lamiaceae; Perillae folium] (Zisu), Delphinium denudatum Wall. ex Hook. f. and Thomson [Ranunculaceae; Delphinii radix] (Jadwar), and Drimia maritima (L.) Stearn [Asparagaceae; Scillae bulbus] (SO, Squill oxymel); All botanical drugs were selected and reported according to the guidelines of the “Consortium for Phytochemical Characterization of Medicinal Plants (ConPhyMP)”; (2) Control groups consisting of patients who received only placebo or usual treatment; (3) Study designs limited to clinical RCTs; and (4) Outcome measures that included at least one of the following: Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Visual Analogue Scale (VAS), Short Form 36 Health Survey (SF-36), Knee injury and Osteoarthritis Outcome Score (KOOS), Lequesne’s Pain-Function Index (LPFI), Japanese Orthopaedic Association Score (JOA).
2.3 Exclusion criteria
1. Studies with incomplete data or unreported data; (2) Non-RCTs (including quasi-RCTs, animal studies, protocols, conference abstracts, case reports or correspondence); (3) Study on mixed preparations of various botanical drugs or plant extracts; (4) Research on synthetic drugs with similar composition to botanical drugs or plant extracts.
2.4 Study selection
The EndNote reference management software was used to screen and exclude studies. Two researchers initially screened the titles of studies to identify duplicates, non-RCTs, review articles, conference papers, protocols, and correspondence. Subsequently, the abstracts of the studies were reviewed by the two researchers to determine eligible and ineligible studies. Finally, the full texts of the remaining studies were independently reviewed by the two researchers to further confirm the studies for inclusion. During this process, the two researchers independently conducted the screening and compared the remaining studies. If their decisions were consistent, the studies were included. In cases of discrepancies, a third researcher was consulted to resolve the issue through discussion.
2.5 Data extraction
This study utilized a standardized, pre-specified data extraction form with seven items to record the following information: (1) author, (2) publication year, (3) country, (4) study duration, (5) sample size, (6) mean age, and (7) details of the intervention.
2.6 Risk of bias in individual studies
Two researchers independently assessed the risk of bias (ROB) using the Cochrane Handbook version 5.1.0 to evaluate the ROB in RCTs. The assessment considered the following seven aspects: (1) generation of the random sequence; (2) allocation concealment; (3) blinding of participants; (4) blinding of personnel; (5) blinding of outcome assessors; (6) incomplete outcome data; and (7) selective reporting. Based on the number of metabolites with potentially high ROB, trials were categorized into three levels of ROB: high risk (five or more metabolites), moderate risk (three or four metabolites), and low risk (two or fewer metabolites) (Higgins et al., 2011).
2.7 Data analysis
In studies using botanical and medicinal plant extracts as interventions, all variables were continuous and were expressed as means with standard deviations (SD) (Li and Chen, 2021). Continuous variables were reported as either mean differences (MD, defined as the absolute difference between the means of the treatment and control groups, calculated using the same scale) or standardized mean differences (SMD, defined as the mean difference between groups divided by the standard deviation of outcomes among participants, used for pooling trial data from different scales) along with their 95% confidence intervals (CI) for analysis. The effect sizes are categorized as negligible (SMD <0.2), small (0.2–0.49), moderate (0.5–0.79), or significant (≥0.8). For interpretation, we apply the same evaluation criteria to the absolute values of SMD: negative SMD values (indicating better outcomes in the intervention group) are still assessed using the same evaluation standards after converting to absolute values. Given the potential heterogeneity across studies, a random-effects model was selected for analysis instead of a fixed-effects model (Jackson et al., 2011).
We used Stata software (version 15.1) in accordance with the PRISMA-NMA guidelines (Moher et al., 2015) to perform NMA aggregation and analysis within a Bayesian framework using Markov chain Monte Carlo (MCMC) simulation. To ensure the validity of the NMA, we carefully assessed transitivity and consistency, which are critical assumptions underlying indirect treatment comparisons. Transitivity was evaluated by examining the distribution of key effect modifiers (such as patient demographics, baseline severity of KOA, intervention doses, and study duration) across the included studies. We considered the assumption plausible if the populations, interventions, and outcomes were sufficiently similar to allow for meaningful indirect comparisons. Consistency was evaluated by comparing direct and indirect evidence using both local (node-splitting) and global inconsistency models. Statistical evidence for inconsistency was considered significant if p < 0.05. Deviations from transitivity or consistency assumptions were documented, and sensitivity analyses were performed to test the robustness of the results (Salanti et al., 2011).
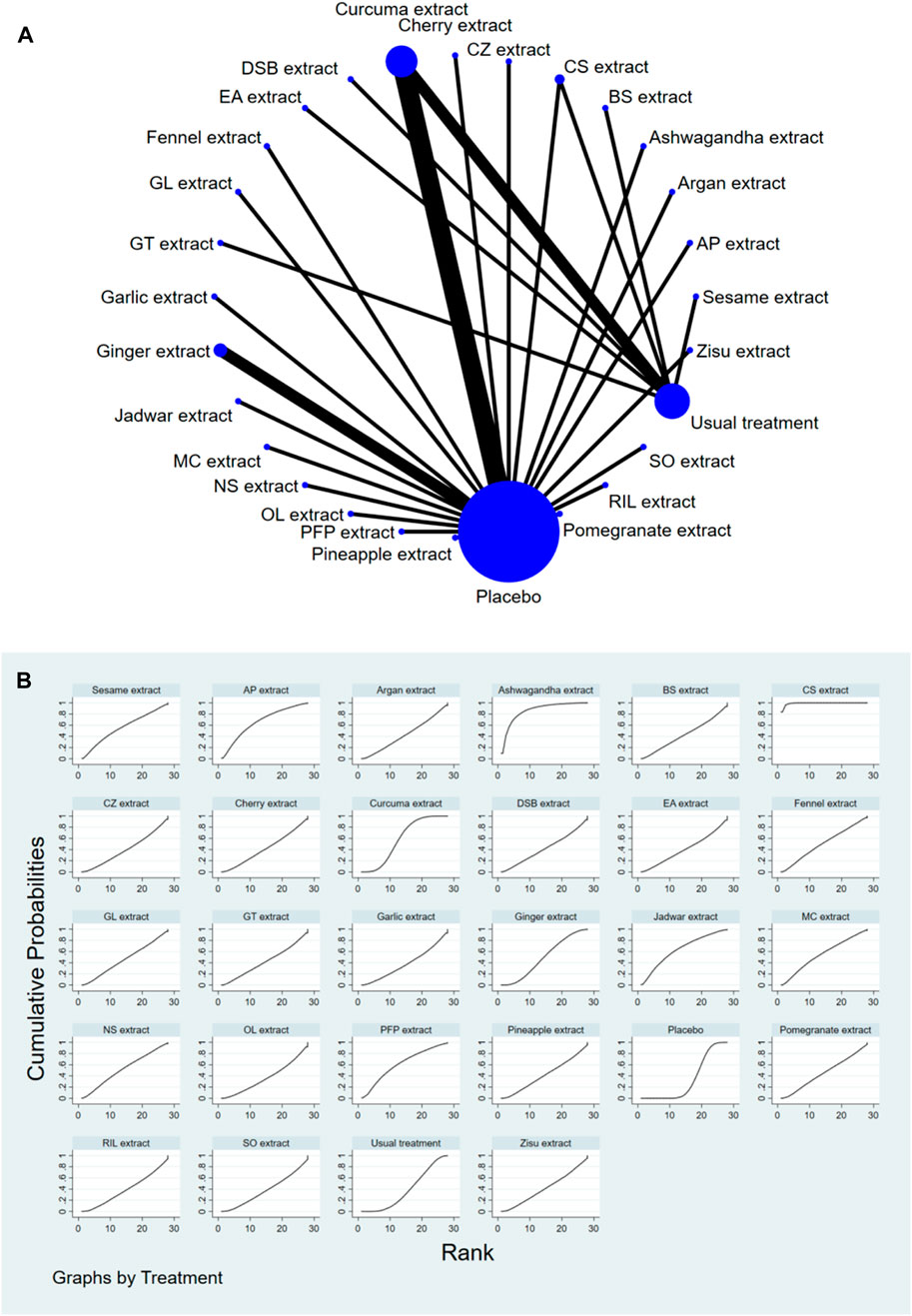
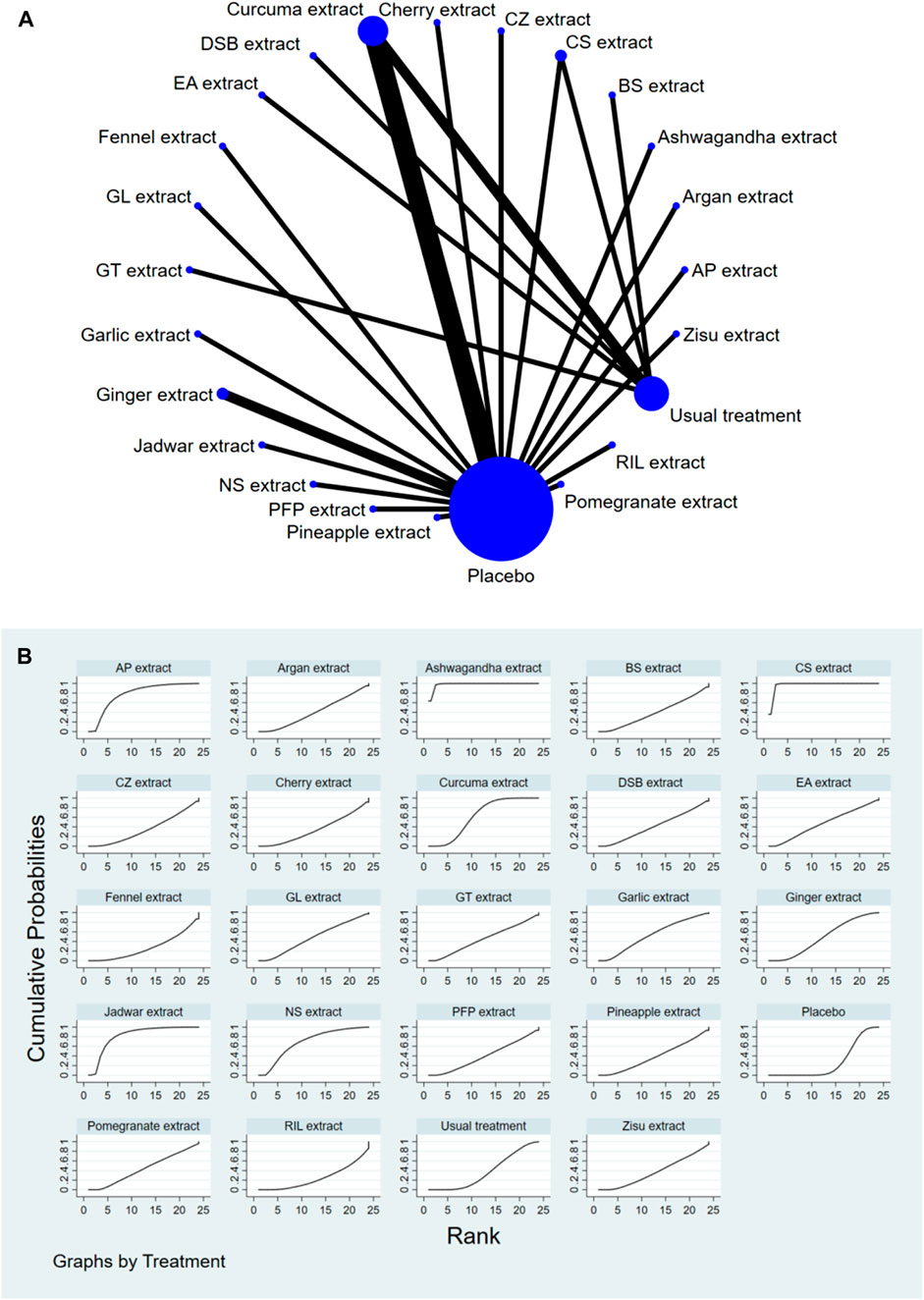
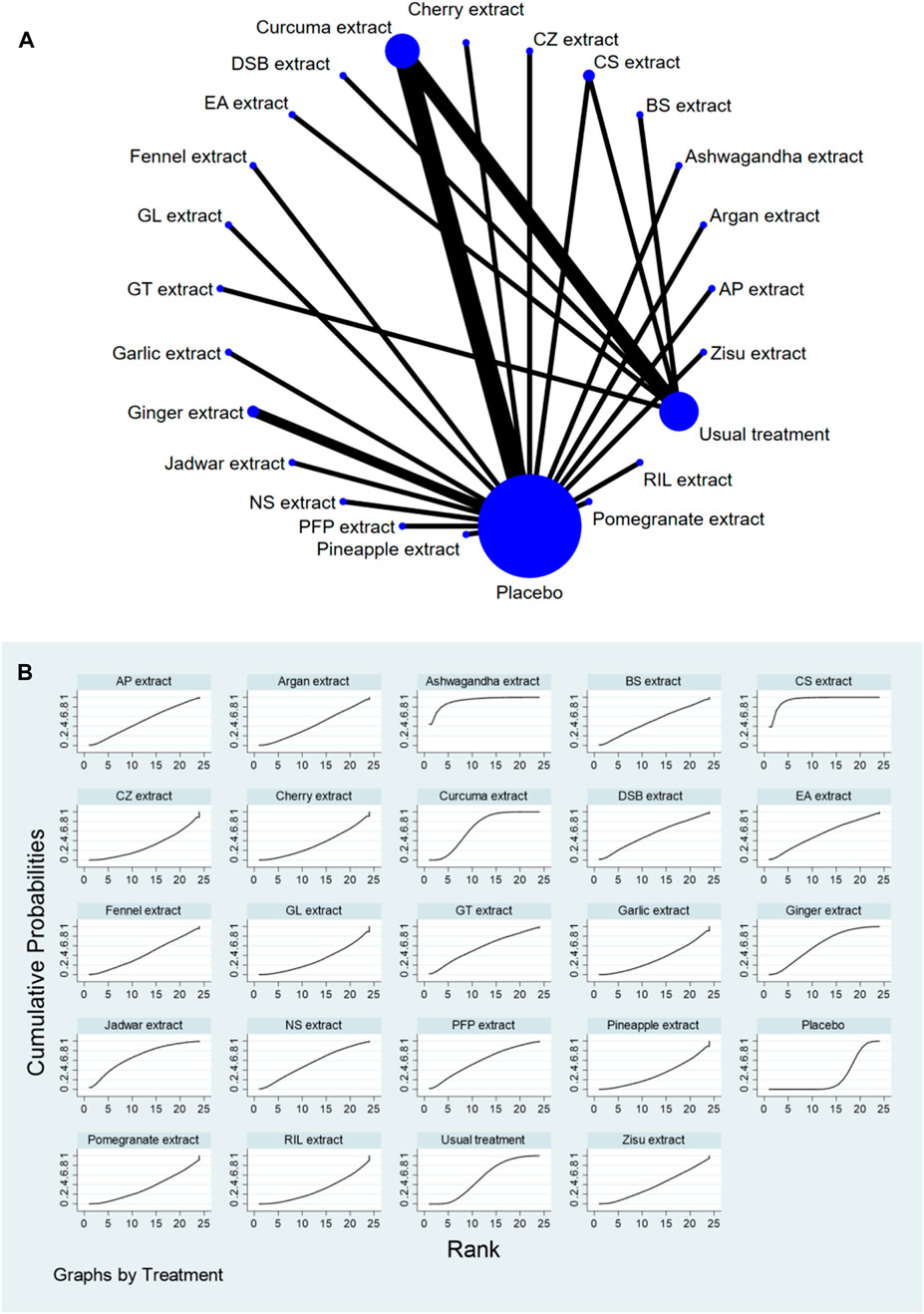
Stata software was used to generate and describe network diagrams for different exercise interventions. Each node in the generated network diagram represents a specific exercise intervention or control condition, and the lines connecting the nodes represent direct head-to-head comparisons between interventions. The size of each node and the thickness of the connecting lines were proportional to the number of studies included (Chaimani et al., 2013).
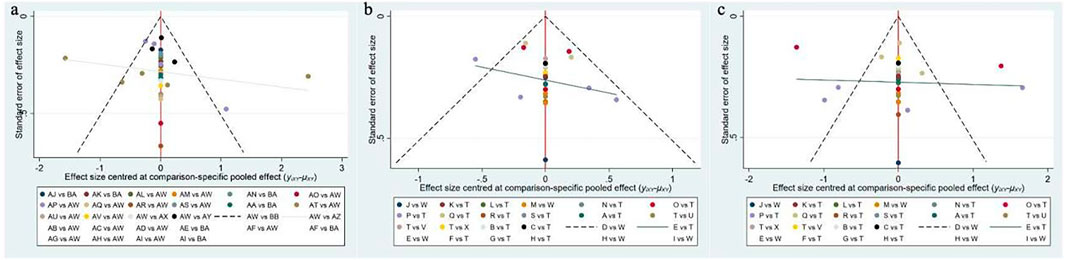
The intervention hierarchy was summarized and reported using P-scores. P-scores are considered frequency simulations under the surface of the cumulative ranking curve (SUCRA) values, measuring the certainty of one treatment being better than another across all competing treatments, on average. The P-score ranges from 0 to 1, where a score of 1 indicates the best treatment with no uncertainty, and a score of 0 indicates the worst treatment with no uncertainty. Although P-scores or SUCRA values can effectively be reinterpreted as percentages representing the effectiveness or acceptability of interventions, these scores should be interpreted cautiously unless there are clinically meaningful differences between interventions. To examine potential bias in small-scale studies, which could lead to publication bias in the NMA, a network funnel plot was generated and visually inspected for symmetry (Khera et al., 2016).
2.8 GRADE evidence assessment
We applied the GRading of Recommendations Assessment, Development and Evaluation (GRADE) framework to rate the certainty of evidence for the three primary outcomes: knee pain intensity, knee stiffness severity, and limitation of knee function. RCTs begin as high-certainty evidence. We then evaluated five potential downgrade domains: (1) risk of bias, (2) inconsistency, (3) indirectness, (4) imprecision, and (5) publication bias, lowering the grade one level for each serious limitation identified. All included studies were RCTs with no serious risk of bias, indirectness, or imprecision; however, serious inconsistency was present for every outcome. Consequently, the certainty of evidence was downgraded by one level for each outcome, resulting in a final rating of moderate certainty.
3 Results
3.1 Study identification and selection
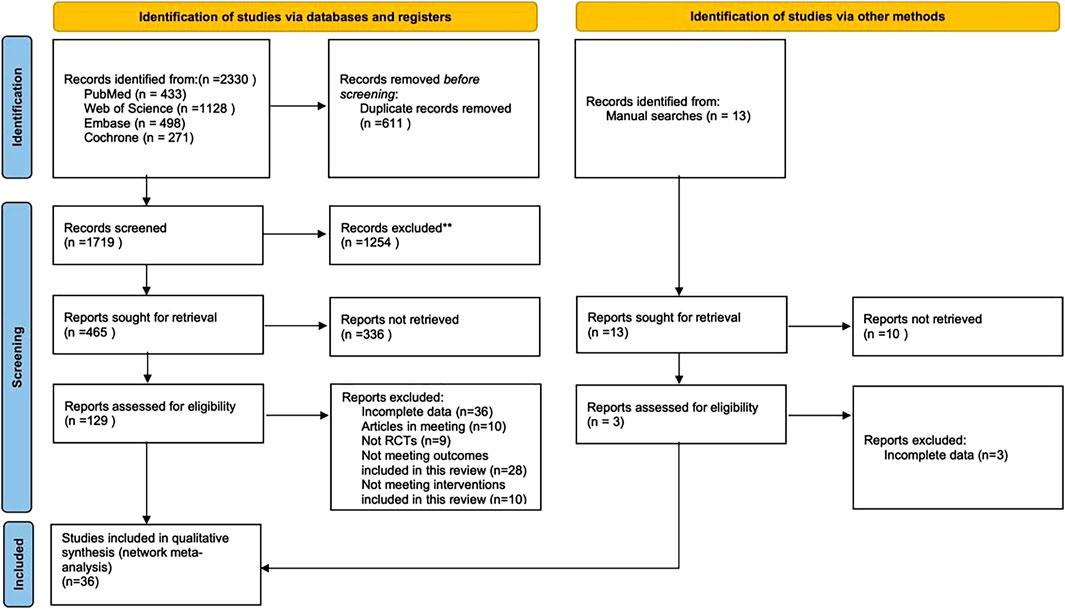
A total of 2,330 articles were retrieved from electronic databases, along with an additional 13 articles identified through manual searches. After removing duplicates, 1,732 articles remained for title and abstract screening, after which 1,603 were excluded. The remaining 129 articles underwent full-text review, resulting in the exclusion of another 93 articles for reasons including non-RCTs, incomplete data, conference papers, or failure to meet the inclusion criteria for interventions. Ultimately, 36 articles were included in this study (Figure 1).
3.2 Quality assessment of included studies
A total of 36 RCTs were included in this study. Overall, the quality of the included studies was moderate. Specific details will be presented in Table 1.
We used Grade to evaluate the evidence level of three indicators. Since there was no closed loop situation for all indicators, all inconsistent dimensions were downgraded. Specific details are presented. Specific details will be presented in Supplementary Table S1.
3.3 Characteristics of included studies
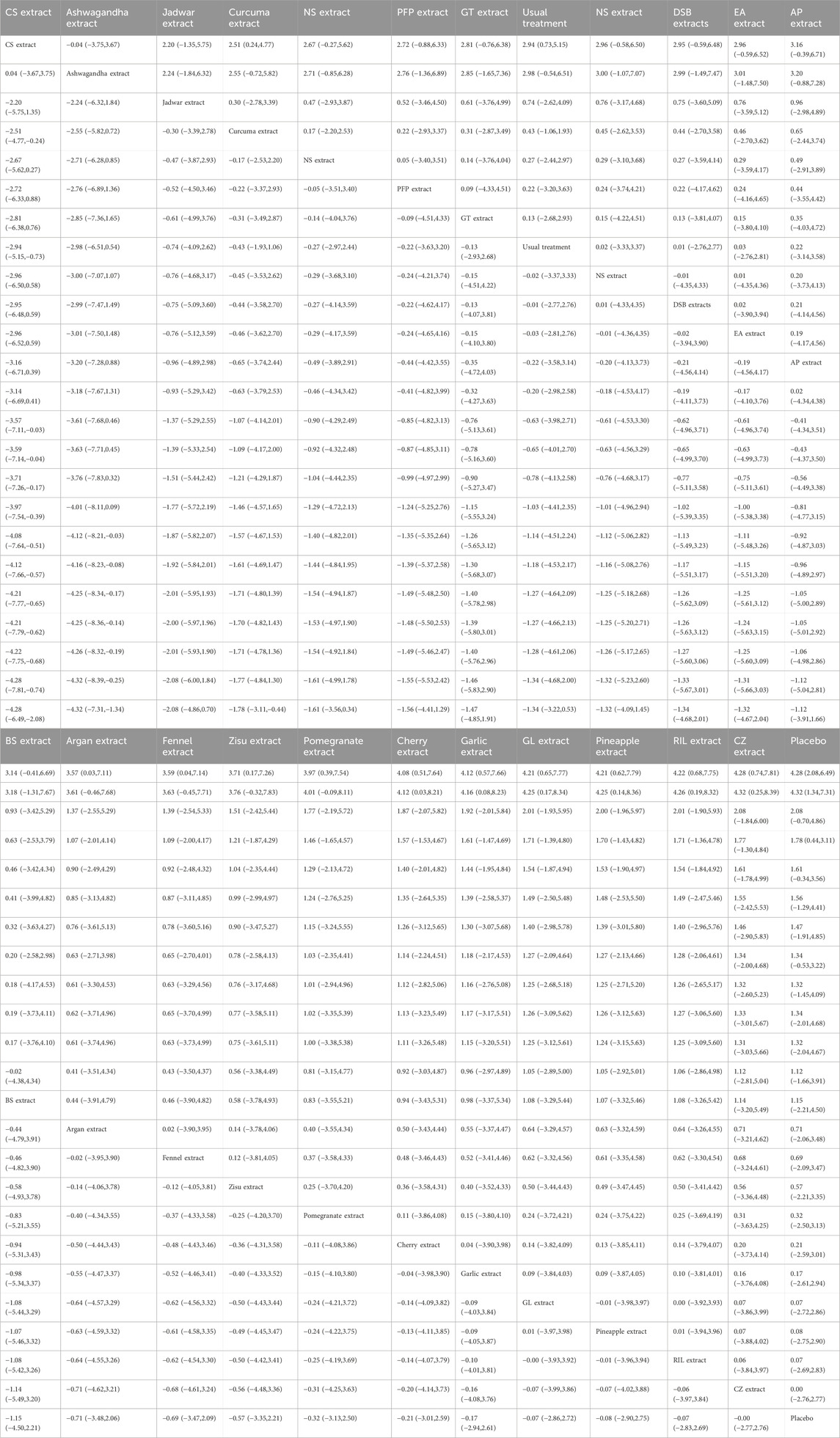
A total of 36 RCTs involving 3,285 patients diagnosed with KOA were included in this study. The interventions in the control group included Zingiber officinale Roscoe [Zingiberaceae; Zingiberis rhizoma] extract (3 studies) (Altman and Marcussen, 2001; Wigler et al., 2003; Zakeri et al., 2011), Ananas comosus (L.) Merr. [Bromeliaceae; Ananasis caulis] extract (1 study) (Brien et al., 2006), Boswellia serrata Roxb. ex Colebr. [Burseraceae; Olibanum indicum] extract (1 study) (Sontakke et al., 2007), Passiflora edulis Sims [Passifloraceae; Passiflorae edulis pericarpium] extract (1 study) (Farid et al., 2010), Derris scandens (Roxb.) Benth. [Fabaceae; Derridis scandentis caulis] extract (1 study) (Kuptniratsaikul et al., 2011), Curcuma longa L. [Zingiberaceae; Curcumae longae rhizoma] extract (8 studies) (Henrotin et al., 2019; Kuptniratsaikul et al., 2014; Madhu et al., 2013; Panahi et al., 2014; Panda et al., 2018; Pinsornsak and Niempoog, 2012; Singhal et al., 2021; Srivastava et al., 2016), Sesamum indicum L. [Pedaliaceae; Sesami semen] extract (1 study) (Eftekhar Sadat et al., 2013), Prunus cerasus L. [Rosaceae; Pruni cerasi fructus] extract (1 study) (Schumacher et al., 2013), Olea europaea L. [Oleaceae; Oleae folium] extract (1 study) (Takeda et al., 2013), Punica granatum L. [Lythraceae; Granati pericarpium] extract (1 study) (Ghoochani et al., 2016), Elaeagnus angustifolia L. [Elaeagnaceae; Elaeagni angustifoliae fructus] extract(1 study) (Panahi et al., 2016), Withania somnifera (L.) Dunal [Solanaceae; Withaniae radix] extract (1 study) (Ramakanth et al., 2016), Argania spinosa (L.) Skeels [Sapotaceae; Arganiae oleum] extract (1 study) (Essouiri et al., 2017), Camellia sinensis (L.) Kuntze [Theaceae; Theae folium] extract (1 study) (Hashempur et al., 2018), Psidium guajava L. [Myrtaceae; Psidii guajavae folium] extract (1 study) (Kakuo et al., 2018), Momordica charantia L. [Cucurbitaceae; Momordicae fructus] extract (1 study) (May et al., 2018), Cucumis sativus L. [Cucurbitaceae; Cucumeris sativi fructus] extract (2 studies) (Nash et al., 2018; Nash et al., 2023), Allium sativum L. [Amaryllidaceae; Allii sativi bulbus] extract (1 study) (Salimzadeh et al., 2018), Andrographis paniculata (Burm.f.) Nees [Acanthaceae; Andrographidis herba] extract (1 study) (Hancke et al., 2019), Foeniculum vulgare Mill. [Apiaceae; Foeniculi fructus] extract (1 study) (Alazadeh et al., 2020), Chrysanthemum zawadskii var. latilobum [Asteraceae; Chrysanthemi flos] extract (1 study) (Ha et al., 2021), Rubus idaeus L. [Rosaceae; Rubi idaei folium] extract (1 study) (Henrotin et al., 2022), Nigella sativa L. [Ranunculaceae; Nigellae semen] extract (1 study) (Huseini et al., 2022), erilla frutescens (L.) Britton [Lamiaceae; Perillae folium] extract (1 study) (Kim et al., 2023), Delphinium denudatum Wall. ex Hook. f. and Thomson [Ranunculaceae; Delphinii radix] extractt (1 study) (Navid et al., 2023), and Drimia maritima (L.) Stearn [Asparagaceae; Scillae bulbus] extract (1 study) (Taheri et al., 2023). Twenty-eight studies reported WOMAC as the outcome measure, 21 studies reported VAS, 5 studies reported KOOS, and 5 studies reported SF-36 as outcome measures. Of these, 28 studies were conducted in Asia, 2 in the Americas, 5 in Europe, and 1 in Africa. Since the age and gender distribution in the included studies are relatively consistent, transitivity is considered to be good. The characteristics of the included studies are summarized in Table 2. The pharmacopoeia and detailed characteristics of each plant extract are shown in Table 3.
3.4 Network meta-analysis
The complete NMA diagrams are presented in Figures 2A, 3A, 4A.
3.4.1 Knee joint pain score
The p-values for all indirect and direct comparisons among the studies were tested for consistency and inconsistency, with all p-values exceeding 0.05, indicating that the influence of inconsistency among the studies was acceptable. We conducted global inconsistency test and found that the p value was 0.63. The DIC of consistency model was 201.4, while that of inconsistency model was 208.9. ΔDIC was all >5, indicating that the consistency model was supported. Detailed information is provided in Supplementary Table S2.
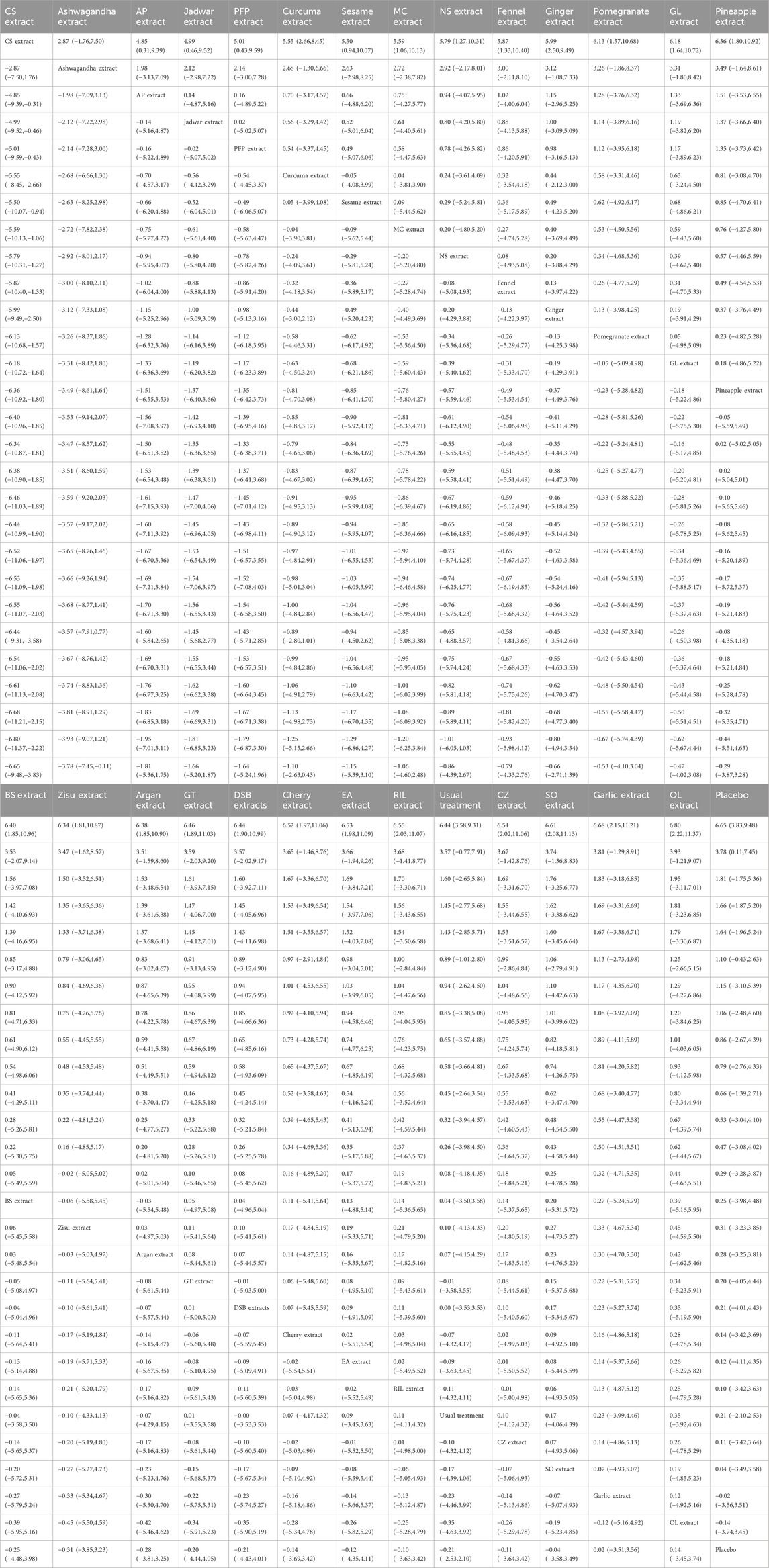
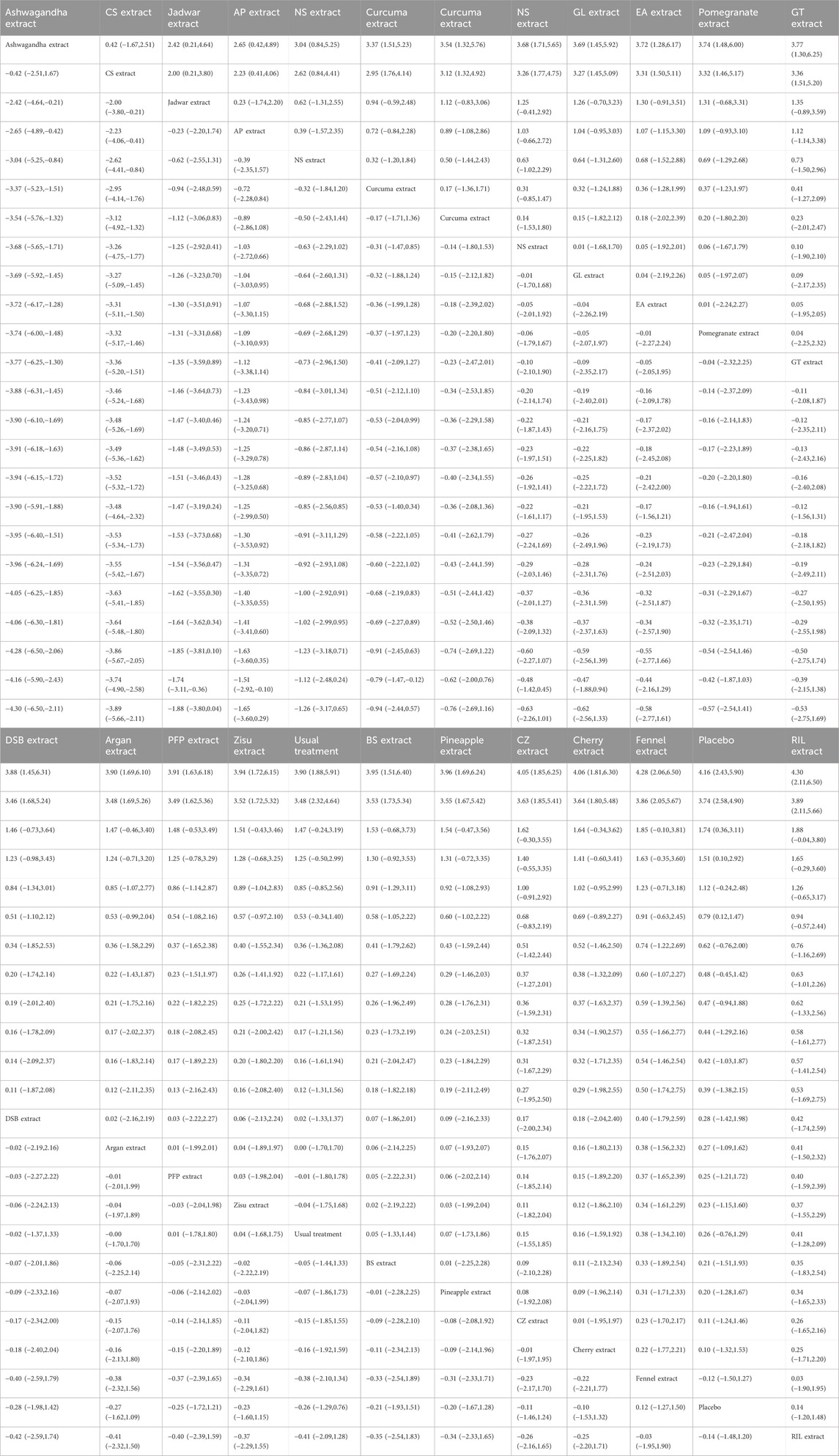
The results of the network meta-analysis showed that, compared to the control group using placebo, CS extract [SMD = 6.65, 95% CI = (3.83, 9.48)] and Ashwagandha extract [SMD = 3.78, 95% CI = (0.11, 7.45)] were more effective in reducing Pain scores. Compared to the control group receiving conventional treatment, CS extract [SMD = 6.44, 95% CI = (3.58, 9.31)] was also more effective in reducing Pain scores. In the probability ranking for reducing Pain scores among different botanical and medicinal plant extracts, CS extract ranked first in SUCRA (99.2%, see Figure 2B). The comparisons between the two different interventions are presented in Table 2.
3.4.2 Knee joint stiffness score
The p-values for all indirect and direct comparisons across the studies were tested for consistency and inconsistency, with all p-values exceeding 0.05, indicating that the consistency among the studies was acceptable. We conducted global inconsistency test and found that the p value was 0.57. The DIC of consistency model was 176.8, while that of inconsistency model was 184.3. ΔDIC was all >5, indicating that the consistency model was supported. Detailed information is provided in Supplementary Table S3.
The results of the network meta-analysis showed that, compared to the placebo control group, Ashwagandha extract [SMD = 4.16, 95% CI = (2.43, 5.90)], CS extract [SMD = 3.74, 95% CI = (2.58, 4.90)], Jadwar extract [SMD = 1.74, 95% CI = (0.36, 3.11)], AP extract [SMD = 1.51, 95% CI = (0.10, 2.92)], and Curcuma extract [SMD = 0.79, 95% CI = (0.12, 1.47)] were more effective in reducing Stiff scores. Compared to the control group receiving conventional treatment, Ashwagandha extract [SMD = 3.90, 95% CI = (1.88, 5.91)] and CS extract [SMD = 3.48, 95% CI = (2.32, 4.64)] were more effective in reducing Stiff scores. In the probability ranking for reducing Stiff scores among different botanical and medicinal plant extracts, Ashwagandha extract ranked first in SUCRA (98.3%, see Figure 3B). Comparisons between the two different interventions are presented in Table 4.
3.4.3 Knee joint function score
The p-values for all indirect and direct comparisons across the studies were tested for consistency and inconsistency, with all p-values exceeding 0.05, indicating that the consistency among the studies was acceptable. We conducted global inconsistency test and found that the p value was 0.43. The DIC of consistency model was 223.7, while that of inconsistency model was 230.1. ΔDIC was all >5, indicating that the consistency model was supported. Detailed information is provided in Supplementary Table S4.
The results of the network meta-analysis showed that, compared to the placebo control group, CS extract [SMD = 4.28, 95% CI = (2.08, 6.49)], Ashwagandha extract [SMD = 4.32, 95% CI = (1.34, 7.31)], and Curcuma extract [SMD = 1.78, 95% CI = (0.44, 3.11)] were more effective in reducing Function scores. Compared to the control group receiving conventional treatment, CS extract [SMD = 2.94, 95% CI = (0.73, 5.15)] was more effective in reducing Function scores. In the probability ranking for reducing Function scores among different botanical and medicinal plant extracts, CS extract ranked first in SUCRA (94.6%, see Figure 4B). Comparisons between the two different interventions are presented in Table 5.
3.5 Adverse events and safety outcomes
In terms of safety, the included botanical and plant-based preparations were generally well tolerated. Across trials, most adverse events were mild and gastrointestinal in nature. For example, ginger extract was associated with dyspepsia or heartburn in a small number of participants but without any treatment-related serious adverse events (Altman and Marcussen, 2001; Wigler et al., 2003). Bromelain mainly caused transient gastrointestinal upset without severe consequences (Brien et al., 2006). In a trial of curcumin co-administered with diclofenac, adverse events such as renal impairment and allergic swelling occurred only in the diclofenac group, whereas the combination group reported only a single case of hair loss (Pinsornsak and Niempoog, 2012). More recently, perilla extract was associated with only mild and unlikely drug-related adverse events and no laboratory abnormalities (Kim et al., 2023). Overall, no trial reported life-threatening or irreversible harms, and in several studies the safety profile compared favorably with conventional pharmacological comparators.
3.6 Publication bias test
We constructed independent funnel plots for all outcome measures to assess potential publication bias. Visual inspection of the funnel plots revealed no significant evidence of publication bias. Detailed information is presented in Figure 5.
4 Discussion
This study compared the efficacy of various botanical and medicinal plant extract interventions in treating patients with KOA. A total of 36 studies, comprising 26 different botanical and medicinal plant extracts and 3,285 patients diagnosed with KOA, were included. Our analysis indicated that CS extract was the most effective botanical and medicinal plant extract for reducing knee pain scores and functional impairment scores, while Ashwagandha extract demonstrated better efficacy in reducing knee stiffness scores. Overall, we consider CS extract to be the most suitable intervention for improving KOA symptoms. Given that there are only two studies with high homogeneity but small sample size for Cucumis sativus, the first result should be regarded as an exploratory signal and needs to be verified by a large sample multi-center RCT with independent teams.
From the results, it is evident that CS extract demonstrated the most significant therapeutic effect in alleviating pain scores among patients. Pain is the predominant symptom of KOA, typically characterized by mild to severe aching discomfort within the joint. In more severe instances, patients may experience tearing or pinprick sensations that persist even during rest and are often influenced by changes in weather conditions. The pathophysiology of KOA pain primarily arises from degenerative wear of joint cartilage, leading to an uneven articular surface that causes friction and impact during movement (Gelber, 2024). Furthermore, synovial inflammation due to cartilage damage releases inflammatory mediators that stimulate nerve endings, further contributing to joint pain (Tudorachi et al., 2021). CS extract contains a rare imino sugar amino acid, idoBR1 (Olajide et al., 2022), which has emerged as a novel anti-inflammatory molecule with excellent oral bioavailability and in vivo stability. Imino sugar amino acids are extremely rare in nature and exhibit no significant functional or structural similarity to current drugs for the treatment of osteoarthritis. Studies have shown that idoBR1 can effectively inhibit lipopolysaccharide (LPS)-induced production of the pro-inflammatory cytokine TNF-α in human serum and THP-1 cells, where elevated TNF-α levels are a key factor in degenerative changes (Nash et al., 2023). Additionally, idoBR1 was found to dose-dependently reduce LPS-induced TNF-α levels and significantly suppress multiple inflammatory markers, including interleukin-6 (IL-6), nitric oxide (NO), and transcription factor NF-κB, demonstrating its comprehensive anti-inflammatory potential(Nash et al., 2020; Olajide et al., 2022). The meta-analysis included two RCTs on CS extract, which showed that it exhibited the best therapeutic effects in improving pain. Additionally, its SUCRA ranking was second for alleviating knee stiffness. While Curcuma extract and BS extract have been promising in recent studies for KOA treatment (Liu et al., 2018; Yu et al., 2020), our study found CS extract to be more effective than both. In addition, other extracts are also considered to have certain clinical value, Ashwagandha extract can significantly reduce the release of pro-inflammatory cytokines such as IL-1β and TNF-α in synovial fluid monocytes by inhibiting the activation of the NF-κB signaling pathway, thereby alleviating the inflammatory response of knee joint synovium. It can also cooperate with chondroitin sulfate to upregulate the synthesis of glycosaminoglycans (GAGs) in articular cartilage and reduce the excessive production of nitric oxide (NO), thereby maintaining the homeostasis of cartilage matrix. The main active metabolite of Curcuma, curcumin, can block the NF-κB and MAPK cascades, inhibit the expression of cartilage-degrading enzymes such as MMP-3 and MMP-13, and thereby delay the destruction of articular cartilage. At the same time, curcumin can reduce oxidative stress damage in the joint cavity by enhancing the activity of superoxide dismutase (SOD) and reducing lipid peroxidation products, further improving the microenvironment of knee osteoarthritis.
Patients with KOA often experience joint stiffness, reduced fluidity of movement, and even locking sensations during activity, with stiffness being particularly prominent in the morning. These symptoms are often accompanied by joint swelling, pain, and restricted range of motion. The pathophysiology is complex, involving inflammation-induced synovial hyperplasia, congestion, and edema, along with structural changes such as capsular thickening and contracture, which significantly impair normal joint movement (Gelber, 2024). Inflammation may also lead to the accumulation of synovial fluid in the joint cavity, further exacerbating restrictions in joint mobility (Tudorachi et al., 2021). Prolonged inflammation can result in tension, spasms, and even atrophy of the surrounding muscles, further worsening joint stiffness and creating a vicious cycle. Ashwagandha (Indian ginseng) is a traditional Ayurvedic botanical drug. A previous study reported that Ashwagandha extract significantly reduced NO release in cartilage samples from osteoarthritis patients, demonstrating its anti-inflammatory effects (Sumantran et al., 2008). Clinically, it has been shown that after 4 and 8 weeks of Ashwagandha extract administration in KOA patients, patients showed statistically significant reductions in all efficacy variables in a dose-dependent manner (Ramakanth et al., 2016). However, studies on this botanical medicine remain limited, and its mechanisms and potential benefits in alleviating joint stiffness require further investigation.
Similarly, CS extract demonstrated the most effective treatment in reducing knee functional limitation scores. The degenerative wear and destruction of articular cartilage, reactive proliferation of subchondral bone forming osteophytes, and inflammatory reactions in the joint synovium result in abnormal joint structure, thereby affecting normal joint function (Gelber, 2024). The superior anti-inflammatory properties of CS extract exhibit better therapeutic outcomes in clinical practice compared to both placebo and chondroitin sulfate.
In conclusion, this study has a certain clinical significance. CS extract has a significant effect on relieving pain, stiffness and functional improvement of knee osteoarthritis, and it is a good natural extract for the treatment of KOA.
5 Strengths and limitations
Our study included 36 studies and 3,285 patients, representing a relatively large sample size. We summarized the efficacy of various botanical and medicinal plant extracts for treating KOA patients from databases, with each study comparing botanical and medicinal plant extracts to placebo or conventional treatment interventions, providing updated and more comprehensive evidence-based recommendations.
However, our study shares some common limitations with the studies on which it is based. Although we made every effort to control for heterogeneity when including the original studies, heterogeneity between studies was inevitable (e.g., differences in the proportion of studies conducted in various regions and gender disparities among participants). Although 36 studies were included in our analysis, the number of studies for each type of botanical and medicinal plant extract was relatively small, with most botanical and medicinal plant extracts supported by only 1–2 RCTs. In addition, certain studies had limited evaluation criteria, and their results should be interpreted with caution. This highlights the need for further research to expand the evidence base. Most of the clinical trials included in this systematic review were conducted by Asian research teams, and the subjects were mainly from Asian populations. Due to differences in genetic background, lifestyle, dietary habits, and physical constitution, the efficacy and safety of botanical interventions observed in Asian populations may not be directly extrapolated to other racial or regional populations. Therefore, the applicability of the existing evidence to non-Asian populations remains uncertain. Currently, nearly all plant extracts/formulations in the field are only compared with placebos or conventional treatments, resulting in a severe lack of head-to-head randomized controlled trials (RCTs). To reduce uncertainties from indirect comparisons, future studies should prioritize multi-arm designs or platform trials that directly compare 2-3 of the most promising botanical extracts. Standardizing dosage regimens, treatment durations, and outcome measures will enhance the robustness of network meta-analysis results.
6 Conclusion
Based on our findings, we recommend CS extract for patients seeking to alleviate knee pain and improve joint function, and Ashwagandha extract for those aiming to reduce knee stiffness. Overall, CS extract is the most recommended botanical and medicinal plant extract for osteoarthritis patients aiming to improve overall knee joint symptoms.
This study has been registered with PROSPERO, registration number: CRD42024617459.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
CY: Data curation, Project administration, Writing – original draft. XD: Formal Analysis, Supervision, Writing – original draft. YL: Writing – original draft, Visualization, Validation. FX: Resources, Software, Writing – original draft. HY: Writing – original draft, Methodology, Investigation. YZ: Funding acquisition, Writing – review and editing, Resources. TB: Conceptualization, Writing – review and editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Project supported by Nanjing medical science and technology development fund (Grant Nos. YKK24214).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1619589/full#supplementary-material
References
Alazadeh, M., Azadbakht, M., Niksolat, F., Asgarirad, H., Moosazadeh, M., Ahmadi, A., et al. (2020). Effect of sweet fennel seed extract capsule on knee pain in women with knee osteoarthritis. Complement. Ther. Clin. Pract. 40, 101219. doi:10.1016/j.ctcp.2020.101219
Altman, R. D., and Marcussen, K. C. (2001). Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 44 (11), 2531–2538. doi:10.1002/1529-0131(200111)44:11<2531::aid-art433>3.0.co;2-j
Bedenbaugh, A. V., Bonafede, M., Marchlewicz, E. H., Lee, V., and Tambiah, J. (2021). Real-world health care resource utilization and costs among US patients with knee osteoarthritis compared with controls. Clin. Outcomes Res. 13, 421–435. doi:10.2147/CEOR.S302289
Brien, S., Lewith, G., Walker, A. F., Middleton, R., Prescott, P., and Bundy, R. (2006). Bromelain as an adjunctive treatment for moderate-to-severe osteoarthritis of the knee: a randomized placebo-controlled pilot study. Qjm-an Int. J. Med. 99 (12), 841–850. doi:10.1093/qjmed/hcl118
Chaimani, A., Higgins, J. P., Mavridis, D., Spyridonos, P., and Salanti, G. (2013). Graphical tools for network meta-analysis in STATA. PLoS One 8 (10), e76654. doi:10.1371/journal.pone.0076654
Charlesworth, J., Fitzpatrick, J., Perera, N. K. P., and Orchard, J. (2019). Osteoarthritis-a systematic review of long-term safety implications for osteoarthritis of the knee. BMC Musculoskelet. Disord. 20 (1), 151. doi:10.1186/s12891-019-2525-0
Chen, N., Fong, D. Y. T., and Wong, J. Y. H. (2022). Secular trends in musculoskeletal rehabilitation needs in 191 countries and territories from 1990 to 2019. JAMA Netw. Open 5 (1), e2144198. doi:10.1001/jamanetworkopen.2021.44198
da Costa, B. R., Reichenbach, S., Keller, N., Nartey, L., Wandel, S., Juni, P., et al. (2017). Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 390 (10090), e21–e33. doi:10.1016/S0140-6736(17)31744-0
Eftekhar Sadat, B., Khadem Haghighian, M., Alipoor, B., Malek Mahdavi, A., Asghari Jafarabadi, M., and Moghaddam, A. (2013). Effects of sesame seed supplementation on clinical signs and symptoms in patients with knee osteoarthritis. Int. J. Rheum. Dis. 16 (5), 578–582. doi:10.1111/1756-185x.12133
Essouiri, J., Harzy, T., Benaicha, N., Errasfa, M., and Abourazzak, F. E. (2017). Effectiveness of argan oil consumption on knee osteoarthritis symptoms: a randomized controlled clinical trial. Curr. Rheumatol. Rev. 13 (3), 231–235. doi:10.2174/1573397113666170710123031
Farid, R., Rezaieyazdi, Z., Mirfeizi, Z., Hatef, M. R., Mirheidari, M., Mansouri, H., et al. (2010). Oral intake of purple passion fruit peel extract reduces pain and stiffness and improves physical function in adult patients with knee osteoarthritis. Nutr. Res. (New York, N.Y.) 30 (9), 601–606. doi:10.1016/j.nutres.2010.08.010
Gelber, A. C. (2024). Knee osteoarthritis. Ann. Intern Med. 177 (9), ITC129–ITC144. doi:10.7326/ANNALS-24-01249
Ghoochani, N., Karandish, M., Mowla, K., Haghighizadeh, M. H., and Jalalid, M. T. (2016). The effect of pomegranate juice on clinical signs, matrix metalloproteinases and antioxidant status in patients with knee osteoarthritis. J. Sci. Food Agric. 96 (13), 4377–4381. doi:10.1002/jsfa.7647
Giordano, R., Petersen, K. K., Andersen, H. H., Simonsen, O., and Arendt-Nielsen, L. (2020). Serum inflammatory markers in patients with knee osteoarthritis: a proteomic approach. Clin. J. Pain 36 (4), 229–237. doi:10.1097/AJP.0000000000000804
Ha, J. K., Kim, J. S., Kim, J. Y., Yun, J. B., Kim, Y. Y., and Chung, K. S. (2021). Efficacy of GCWB106 (Chrysanthemum zawadskii var. latilobum extract) in osteoarthritis of the knee: a 12-week randomized, double-blind, placebo-controlled study. Med. Baltim. 100 (26), e26542. doi:10.1097/md.0000000000026542
Hancke, J. L., Srivastav, S., Cáceres, D. D., and Burgos, R. A. (2019). A double-blind, randomized, placebo-controlled study to assess the efficacy of Andrographis paniculata standardized extract (ParActin®) on pain reduction in subjects with knee osteoarthritis. Phytother. Res. 33 (5), 1469–1479. doi:10.1002/ptr.6339
Hannon, C. P., Goodman, S. M., Austin, M. S., Yates, A., Guyatt, G., Aggarwal, V. K., et al. (2023). 2023 American college of rheumatology and American association of hip and knee surgeons clinical practice guideline for the optimal timing of elective hip or knee arthroplasty for patients with symptomatic moderate-to-severe osteoarthritis or Advanced symptomatic Osteonecrosis with secondary Arthritis for whom Nonoperative therapy is ineffective. Arthritis Rheumatol. 75 (11), 1877–1888. doi:10.1002/art.42630
Hashempur, M. H., Sadrneshin, S., Mosavat, S. H., and Ashraf, A. (2018). Green tea (Camellia sinensis) for patients with knee osteoarthritis: a randomized open-label active-controlled clinical trial. Clin. Nutr. 37 (1), 85–90. doi:10.1016/j.clnu.2016.12.004
Henrotin, Y., Malaise, M., Wittoek, R., de Vlam, K., Brasseur, J. P., Luyten, F. P., et al. (2019). Bio-optimized Curcuma longa extract is efficient on knee osteoarthritis pain: a double-blind multicenter randomized placebo controlled three-arm study. Arthritis Res. Ther. 21 (1), 179. doi:10.1186/s13075-019-1960-5
Henrotin, Y., Cozannet, R. L., Fança-Berthon, P., Truillet, R., Cohen-Solhal, M., DunnGalvin, G., et al. (2022). Rubus idaeus extract improves symptoms in knee osteoarthritis patients: results from a phase II double-blind randomized controlled trial. BMC Musculoskelet. Disord. 23 (1), 650. doi:10.1186/s12891-022-05612-2
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Huseini, H. F., Mohtashami, R., Sadeghzadeh, E., Shadmanfar, S., Hashem-Dabaghian, F., and Kianbakht, S. (2022). Efficacy and safety of oral Nigella sativa oil for symptomatic treatment of knee osteoarthritis: a double-blind, randomized, placebo-controlled clinical trial. Complement. Ther. Clin. Pract. 49, 101666. doi:10.1016/j.ctcp.2022.101666
Jackson, D., Riley, R., and White, I. R. (2011). Multivariate meta-analysis: potential and promise. Stat. Med. 30 (20), 2481–2498. doi:10.1002/sim.4172
Jin, X., Liang, W., Zhang, L., Cao, S., Yang, L., and Xie, F. (2023). Economic and humanistic burden of osteoarthritis: an updated systematic review of large sample studies. Pharmacoeconomics 41 (11), 1453–1467. doi:10.1007/s40273-023-01296-1
Kakuo, S., Fushimi, T., Kawasaki, K., Nakamura, J., and Ota, N. (2018). Effects of Psidium guajava Linn. leaf extract in Japanese subjects with knee pain: a randomized, double-blind, placebo-controlled, parallel pilot study. Aging Clin. Exp. Res. 30 (11), 1391–1398. doi:10.1007/s40520-018-0953-6
Khera, R., Murad, M. H., Chandar, A. K., Dulai, P. S., Wang, Z., Prokop, L. J., et al. (2016). Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA 315 (22), 2424–2434. doi:10.1001/jama.2016.7602
Kim, N., Kim, S.-Y., Kim, S.-W., Lee, J. M., Kim, S.-K., Park, M.-H., et al. (2023). Efficacy of Perilla frutescens (L.) Britton var. frutescens extract on mild knee joint pain: a randomized controlled trial. Front. Pharmacol. 14, 1114410. doi:10.3389/fphar.2023.1114410
Kolasinski, S. L., Neogi, T., Hochberg, M. C., Oatis, C., Guyatt, G., Block, J., et al. (2020). 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. Hob. 72 (2), 149–162. doi:10.1002/acr.24131
Kuptniratsaikul, V., Pinthong, T., Bunjob, M., Thanakhumtorn, S., Chinswangwatanakul, P., and Thamlikitkul, V. (2011). Efficacy and safety of Derris scandens Benth extracts in patients with knee osteoarthritis. J. Altern. Complement. Med. 17 (2), 147–153. doi:10.1089/acm.2010.0213
Kuptniratsaikul, V., Dajpratham, P., Taechaarpornkul, W., Buntragulpoontawee, M., Lukkanapichonchut, P., Chootip, C., et al. (2014). Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin. Interv. Aging 9, 451–458. doi:10.2147/cia.S58535
Li, D., and Chen, P. (2021). Effects of aquatic exercise and land-based exercise on cardiorespiratory fitness, motor function, balance, and functional independence in stroke patients-A meta-analysis of randomized controlled trials. Brain Sci. 11 (8), 1097. doi:10.3390/brainsci11081097
Liang, Y., Xu, Y., Zhu, Y., Ye, H., Wang, Q., and Xu, G. (2022). Efficacy and safety of Chinese herbal medicine for knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. Phytomedicine 100, 154029. doi:10.1016/j.phymed.2022.154029
Lin, Z., Zheng, J., Chen, M., Chen, J., and Lin, J. (2022). The efficacy and safety of Chinese herbal medicine in the treatment of knee osteoarthritis: an updated systematic review and meta-analysis of 56 randomized controlled trials. Oxid. Med. Cell Longev. 2022, 6887988. doi:10.1155/2022/6887988
Liu, X., Machado, G. C., Eyles, J. P., Ravi, V., and Hunter, D. J. (2018). Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis. Br. J. Sports Med. 52 (3), 167–175. doi:10.1136/bjsports-2016-097333
Long, H., Liu, Q., Yin, H., Wang, K., Diao, N., Zhang, Y., et al. (2022). Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the global burden of disease study 2019. Arthritis Rheumatol. 74 (7), 1172–1183. doi:10.1002/art.42089
Madhu, K., Chanda, K., and Saji, M. J. (2013). Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: a randomized placebo-controlled trial. Inflammopharmacology 21 (2), 129–136. doi:10.1007/s10787-012-0163-3
May, L. S., Sanip, Z., Shokri, A. A., Kadir, A. A., and Lazin, M. R. M. (2018). The effects of Momordica charantia (bitter melon) supplementation in patients with primary knee osteoarthritis: a single-blinded, randomized controlled trial. Complementary Ther. Clin. Pract. 32, 181–186. doi:10.1016/j.ctcp.2018.06.012
Menon, J., and Mishra, P. (2018). Health care resource use, health care expenditures and absenteeism costs associated with osteoarthritis in US healthcare system. Osteoarthr. Cartil. 26 (4), 480–484. doi:10.1016/j.joca.2017.12.007
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4 (1), 1. doi:10.1186/2046-4053-4-1
Nash, R. J., Azantsa, B. K., Sharp, H., and Shanmugham, V. (2018). Effectiveness of Cucumis sativus extract versus glucosamine-chondroitin in the management of moderate osteoarthritis: a randomized controlled trial. Clin. Interv. Aging 13, 2119–2126. doi:10.2147/cia.S173227
Nash, R. J., Bartholomew, B., Penkova, Y. B., Rotondo, D., Yamasaka, F., Stafford, G. P., et al. (2020). Iminosugar idoBR1 isolated from cucumber Cucumis sativus reduces inflammatory activity. ACS Omega 5 (26), 16263–16271. doi:10.1021/acsomega.0c02092
Nash, R. J., Mafongang, A., Singh, H., Singwe-Ngandeu, M., Penkova, Y. B., Kaur, T., et al. (2023). Standardised Ido-BR1 cucumber extract improved parameters linked to moderate osteoarthritis in a placebo-controlled study. Curr. Rheumatol. Rev. 19 (3), 345–351. doi:10.2174/1573397119666230206105703
Navid, R. B., Karimi, M., Ghojazadeh, M., Bagherzadeh-Karimi, A., Mohammadinasab, R., Dolati, S., et al. (2023). Effect of Delphinium denudatum Wall. (Jadwar) on knee osteoarthritis: a randomized double-blinded placebo-controlled clinical trial. Pharm. Sci. 29 (3), 338–345. doi:10.34172/PS.2022.26
Olajide, O. A., Iwuanyanwu, V. U., Banjo, O. W., Kato, A., Penkova, Y. B., Fleet, G. W. J., et al. (2022). Iminosugar amino acid idoBR1 reduces inflammatory responses in microglia. Molecules 27 (10), 3342. doi:10.3390/molecules27103342
Panahi, Y., Rahimnia, A.-R., Sharafi, M., Alishiri, G., Saburi, A., and Sahebkar, A. (2014). Curcuminoid treatment for knee osteoarthritis: a randomized double-blind placebo-controlled trial. Phytotherapy Res. 28 (11), 1625–1631. doi:10.1002/ptr.5174
Panahi, Y., Alishiri, G. H., Bayat, N., Hosseini, S. M., and Sahebkar, A. (2016). Efficacy of Elaeagnus Angustifolia extract in the treatment of knee osteoarthritis: a randomized controlled trial. Excli J. 15, 203–210. doi:10.17179/excli2015-639
Panda, S. K., Nirvanashetty, S., Parachu, V. A., Mohanty, N., and Swain, T. (2018). A randomized, double blind, placebo controlled, parallel-group study to evaluate the safety and efficacy of Curene® versus placebo in reducing symptoms of knee OA. Biomed Res. Int. 2018, 5291945. doi:10.1155/2018/5291945
Pinsornsak, P., and Niempoog, S. (2012). The efficacy of Curcuma Longa L. extract as an adjuvant therapy in primary knee osteoarthritis: a randomized control trial. J. Med. Assoc. Thail. = Chotmaihet thangphaet 95 (Suppl. 1), S51–S58.
Ramakanth, G. S. H., Uday Kumar, C., Kishan, P. V., and Usharani, P. (2016). A randomized, double blind placebo controlled study of efficacy and tolerability of Withaina somnifera extracts in knee joint pain. J. Ayurveda Integr. Med. 7 (3), 151–157. doi:10.1016/j.jaim.2016.05.003
Rouse, B., Chaimani, A., and Li, T. (2017). Network meta-analysis: an introduction for clinicians. Intern Emerg. Med. 12 (1), 103–111. doi:10.1007/s11739-016-1583-7
Salanti, G., Ades, A. E., and Ioannidis, J. P. (2011). Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J. Clin. Epidemiol. 64 (2), 163–171. doi:10.1016/j.jclinepi.2010.03.016
Salimzadeh, A., Alipoor, E., Dehghani, S., Yaseri, M., Hosseini, M., Feinle-Bisset, C., et al. (2018). The effect of 12-week garlic supplementation on symptom relief in overweight or obese women with knee osteoarthritis. Int. J. Clin. Pract. 72 (6), e13208. doi:10.1111/ijcp.13208
Schumacher, H. R., Pullman-Mooar, S., Gupta, S. R., Dinnella, J. E., Kim, R., and McHugh, M. P. (2013). Randomized double-blind crossover study of the efficacy of a tart cherry juice blend in treatment of osteoarthritis (OA) of the knee. Osteoarthr. Cartil. 21 (8), 1035–1041. doi:10.1016/j.joca.2013.05.009
Sharma, L. (2021). Osteoarthritis of the knee. N. Engl. J. Med. 384 (1), 51–59. doi:10.1056/NEJMcp1903768
Singhal, S., Hasan, N., Nirmal, K., Chawla, R., Chawla, S., Kalra, B. S., et al. (2021). Bioavailable turmeric extract for knee osteoarthritis: a randomized, non-inferiority trial versus paracetamol. Trials 22 (1), 105. doi:10.1186/s13063-021-05053-7
Sontakke, S., Thawani, V., Pimpalkhute, S., Kabra, P., Babhulkar, S., and Hingorani, L. (2007). Open, randomized, controlled clinical trial of Boswellia serrata extract as compared to valdecoxib in osteoarthritis of knee. Indian J. Pharmacol. 39 (1), 27–29. doi:10.4103/0253-7613.30759
Srivastava, S., Saksena, A. K., Khattri, S., Kumar, S., and Dagur, R. S. (2016). Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: a four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology 24 (6), 377–388. doi:10.1007/s10787-016-0289-9
Sumantran, V. N., Chandwaskar, R., Joshi, A. K., Boddul, S., Patwardhan, B., Chopra, A., et al. (2008). The relationship between chondroprotective and antiinflammatory effects of Withania somnifera root and glucosamine sulphate on human osteoarthritic cartilage in vitro. Phytother. Res. 22 (10), 1342–1348. doi:10.1002/ptr.2498
Taheri, M., Riahi, S. M., Meybodi, M. K. E., Ahmadian-Attari, M. M., Esmaeili, S., and Mokaberinejad, R. (2023). Effect of Squill oxymel on knee osteoarthritis: a triple-blind, randomized, controlled clinical trial. Traditional Integr. Med. 8 (2), 119–129. doi:10.18502/tim.v8i2.13077
Takeda, R., Koike, T., Taniguchi, I., and Tanaka, K. (2013). Double-blind placebo-controlled trial of hydroxytyrosol of Olea europaea on pain in gonarthrosis. Phytomedicine 20 (10), 861–864. doi:10.1016/j.phymed.2013.03.021
Tudorachi, N. B., Totu, E. E., Fifere, A., Ardeleanu, V., Mocanu, V., Mircea, C., et al. (2021). The implication of reactive oxygen species and antioxidants in knee osteoarthritis. Antioxidants (Basel) 10 (6), 985. doi:10.3390/antiox10060985
Walzer, S. M., Weinmann, D., and Toegel, S. (2015). Medical plant extracts for treating knee osteoarthritis: a snapshot of recent clinical trials and their biological background. Curr. Rheumatol. Rep. 17 (8), 54. doi:10.1007/s11926-015-0530-3
Wang, Z., Efferth, T., Hua, X., and Zhang, X. A. (2022). Medicinal plants and their secondary metabolites in alleviating knee osteoarthritis: a systematic review. Phytomedicine 105, 154347. doi:10.1016/j.phymed.2022.154347
Wigler, I., Grotto, I., Caspi, D., and Yaron, M. (2003). The effects of Zintona EC (a ginger extract) on symptomatic gonarthritis. Osteoarthr. Cartil. 11 (11), 783–789. doi:10.1016/s1063-4584(03)00169-9
Yu, G., Xiang, W., Zhang, T., Zeng, L., Yang, K., and Li, J. (2020). Effectiveness of Boswellia and Boswellia extract for osteoarthritis patients: a systematic review and meta-analysis. BMC Complement. Med. Ther. 20 (1), 225. doi:10.1186/s12906-020-02985-6
Zakeri, Z., Izadi, S., Bari, Z., Soltani, F., Narouie, B., and Ghasemi-Rad, M. (2011). Evaluating the effects of ginger extract on knee pain, stiffness and difficulty in patients with knee osteoarthritis. J. Med. Plants Res. 5 (15), 3375–3379. Available online at: http://www.academicjournals.org/JMPR.
Keywords: botanical extracts, knee osteoarthritis, system review, network, meta
Citation: Yan C, Du X, Liu Y, Xia F, Yu H, Zhu Y and Bao T (2025) Efficacy of botanical extracts for knee osteoarthritis: a network meta-analysis of randomized controlled trials. Front. Pharmacol. 16:1619589. doi: 10.3389/fphar.2025.1619589
Received: 28 April 2025; Accepted: 17 September 2025;
Published: 07 October 2025.
Edited by:
Mojtaba Vaismoradi, Nord University, NorwayReviewed by:
Eduardo Martins Sousa, Universidade CEUMA, BrazilHe Cai, Guizhou University of Traditional Chinese Medicine, China
Copyright © 2025 Yan, Du, Liu, Xia, Yu, Zhu and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianyi Bao, NTI2Njk0MjEwQHFxLmNvbQ==; Yongliang Zhu, NzUwMzI1NzNAcXEuY29t
†These authors have contributed equally to this work
 Chao Yan1†
Chao Yan1† Tianyi Bao
Tianyi Bao