- First People’s Hospital of Linping District, Hangzhou, China
Background: Effective pain management following spinal surgery is crucial for preventing complications related to delayed mobilization. Magnesium sulfate (MgSO4) has shown promise as an analgesic agent, influencing neurotransmitter modulation and autonomic nervous system regulation. However, studies evaluating its effectiveness and safety in spinal surgery remain inconsistent, necessitating a comprehensive meta-analysis to assess its role.
Objective: This study aimed to perform a systematic meta-analysis to compare the safety and efficacy of magnesium sulfate against standard therapeutic options in spinal surgery.
Methods: The meta-analysis followed PRISMA guidelines. We performed data extraction and analysis using Review Manager version 5.4. The study population included patients undergoing spinal surgery, with the intervention group receiving intravenous magnesium sulfate at varying dosages or in combination with other agents. The comparison group received either a placebo or alternative treatments. Primary outcomes included pain intensity, opioid consumption, and safety parameters.
Results: Ten randomized controlled trials involving 641 patients were included. Magnesium sulfate administration significantly reduced pain scores at 24 h (MD −0.18, 95% CI: −0.34 to −0.02) and decreased opioid consumption (SMD −0.34, 95% CI: −1.07 to −0.35). Additionally, a significant reduction in muscle relaxant usage was observed (SMD −0.91, 95% CI: −0.66 to −0.10). When compared with dexmedetomidine, magnesium sulfate improved verbal response (MD 1.22, 95% CI: −0.16–2.61) and prolonged extubation time (MD 0.91, 95% CI: −0.98–2.80). No significant differences in hemodynamic parameters (heart rate and blood pressure) were observed between the groups.
Conclusion: Intravenous magnesium sulfate demonstrated significant benefits in reducing postoperative pain and opioid consumption, while also improving verbal response and orientation. These findings suggest that magnesium sulfate may serve as a valuable adjunct in the perioperative management of spinal surgery patients. Further research is required to confirm these results and establish optimal dosing protocols.
1 Introduction
Chronic low back pain represents a significant burden on healthcare systems worldwide (Shetty et al., 2022). The management of this condition requires a stepwise, progressive approach that prioritizes conservative treatments, including physiotherapy and lifestyle modifications. However, in severe and refractory cases, invasive interventions such as epidural steroid injections or surgical procedures may become necessary (Ketenci and Zure, 2021). While epidural steroid injections have demonstrated Level I evidence in treating radicular pain associated with disc herniation, concerns persist regarding their safety due to rare but potentially severe neurological complications (Helm et al., 2021).
Within this therapeutic landscape, various interventions for low back pain have been investigated. Ozone therapy, for instance, has shown promise in pain relief, though it remains controversial due to limited long-term evaluation and unclear mechanisms of action (de Andrade et al., 2019). Extensive research has been conducted on adjuvant medications in spine surgery, particularly focusing on ketamine and gabapentin use in adolescents undergoing spinal fusion for idiopathic scoliosis. These trials primarily evaluated the medications’ effectiveness in reducing postoperative pain and minimizing opioid consumption. Notably, these studies have demonstrated significant reductions in both opioid use and pain intensity during the initial 48 postoperative hours, accompanied by favorable safety profiles and low adverse event rates (Bas et al., 2023; Mariscal et al., 2022).
Postoperative pain management remains a critical concern for both clinicians and patients, as delayed mobilization can lead to serious adverse outcomes (Naftalovich et al., 2022). The impact of acute pain has been well-documented in numerous studies. Multiple therapeutic modalities, encompassing both pharmacological and non-pharmacological approaches, have been employed to address postoperative pain, which significantly influences patient prognosis and treatment outcomes (Mentes et al., 2008).
Magnesium, an inorganic ion, has diverse therapeutic applications, including the management of asthma exacerbations, hypokalemia, premature labor, myocardial protection following ischemia, postoperative acute pain control, and hemodynamic stabilization during intubation (Lysakowski et al., 2007). Among magnesium preparations, magnesium sulfate has gained particular attention, with its value being evaluated in numerous anesthesia-related investigations (Buvanendran et al., 2002; Ferasatkish et al., 2008). The anti-nociceptive properties of magnesium (Mg) are attributed to its antagonistic effect on N-Methyl-D-aspartate (NMDA) receptors. While preoperative magnesium administration has shown limited impact on postoperative pain, several clinical trials have demonstrated that magnesium infusion during general anesthesia reduces both anesthetic requirements and postoperative analgesic consumption (Ünlügenç et al., 2002). However, the effects of magnesium sulfate administration during regional anesthesia remain incompletely characterized.
Current evidence regarding magnesium sulfate use in spinal surgery is both conflicting and incomplete. While some studies have reported favorable outcomes, including improved hemostatic parameters, reduced intraoperative bleeding, and enhanced pain scores and patient satisfaction (Fathy et al., 2022; James et al., 1989), others have documented adverse effects such as prolonged emergence time and delayed recovery (Tsaousi et al., 2020). Despite these contradictory findings, no meta-analysis has specifically focused on magnesium sulfate use in spinal surgery. Previous literature reviews have noted “low statistical power” in existing studies, highlighting the need for a comprehensive analysis of available data to determine the effectiveness and safety profile of magnesium sulfate in spinal surgery procedures.
Therefore, this study aimed to conduct a comprehensive meta-analysis to evaluate the efficacy and clinical significance of magnesium sulfate administration in spinal surgery.
2 Materials and methods
2.1 Eligibility criteria
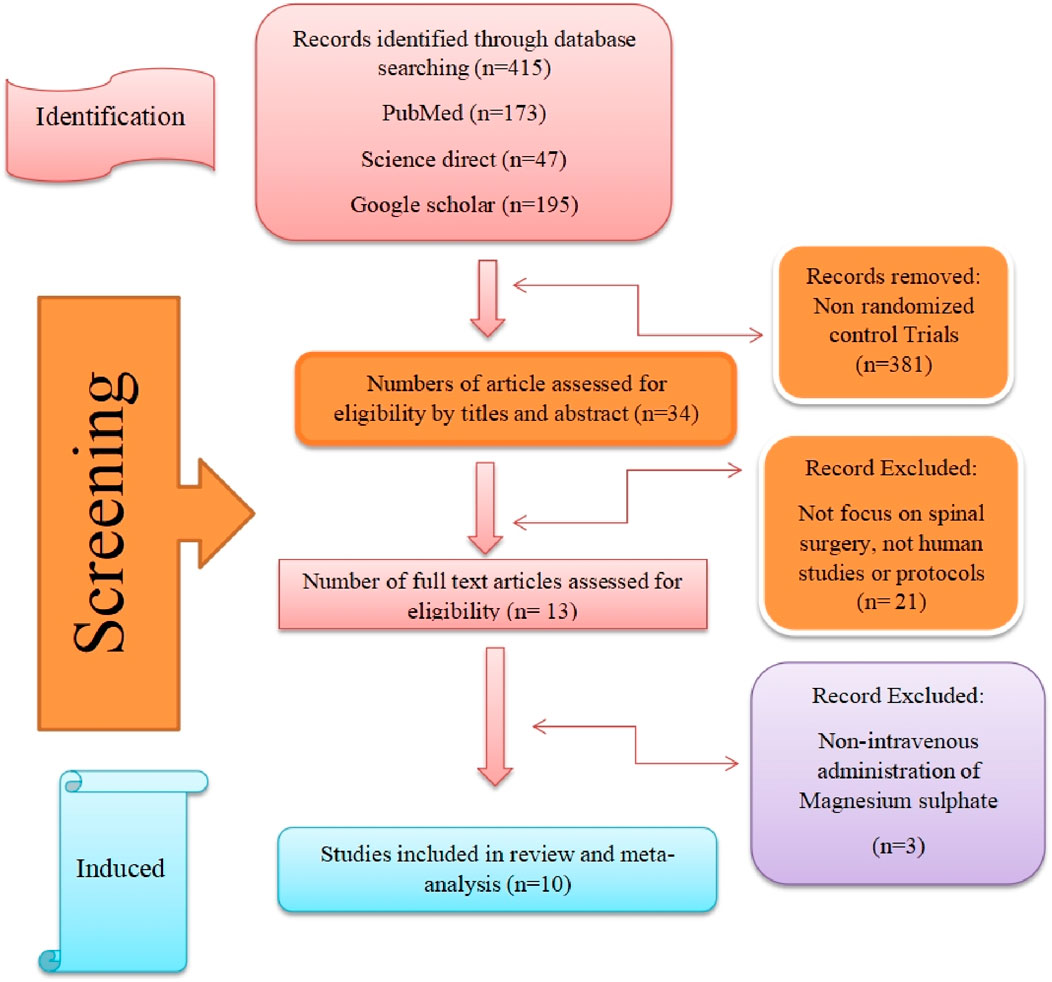
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The study selection process is illustrated in Figure 1 (Tang, 2009). Study inclusion criteria were developed using the PICOS framework. The study population comprised adult patients who underwent spinal surgery. The intervention group received intravenous magnesium sulfate (MgSO4) treatment in varying combinations or doses, while the comparison group received either placebo or alternative treatments. Primary outcomes included efficacy metrics (pain management, medication usage, hemodynamic parameters) and safety outcomes determined through complication analysis. Only randomized controlled trials (RCTs) were considered eligible for inclusion.
To minimize bias and prevent data duplication, studies with the following characteristics were excluded:
• Duplicate publications
• Non-randomized studies, due to their inherent limitations in establishing causal relationships and controlling for confounding variables
• Review articles, as they represent secondary analyses rather than primary data
• Studies with incomplete or missing data, to ensure result accuracy and consistency
• Studies that did not share variables with at least two other included studies, to enable meaningful data pooling and robust statistical analysis
2.2 Information sources and search strategy
A comprehensive literature search was conducted across multiple databases without language or publication date restrictions. The final search was performed in October 2023, encompassing PubMed, Google Scholar, ScienceDirect, and the Cochrane Library. The search strategy employed the Medical Subject Heading (MeSH) terms “Magnesium” and “spine” (detailed search strategy provided in Supplementary Material 1). Additionally, reference lists of included articles were manually screened to identify additional relevant studies.
Study selection was performed independently by two reviewers with Level V expertise (Tang, 2009; Chandler et al., 2019). In cases of disagreement, a third reviewer was consulted to reach consensus. The detailed study selection process is presented in the PRISMA flow diagram (Figure 1).
2.3 Data extraction and data items
Data extraction was conducted independently by two reviewers with Level V expertise, with discrepancies resolved through consultation with a third reviewer (Tang, 2009; Chandler et al., 2019). The following data were extracted from each included study:
• Study characteristics (author, location, study duration).
• Patient demographics (mean age, gender distribution).
• Clinical indicators (etiology, spinal drug dosage).
• Primary outcome measures:
o Pain assessment using Visual Analog Scale (VAS).
o Medication usage (opioids, muscle relaxants, remifentanil).
o Hemodynamic parameters (heart rate [HR], mean arterial pressure [MAP]).
o Recovery indicators (extubation time, verbal command response, orientation time).
2.4 Risk of bias assessment
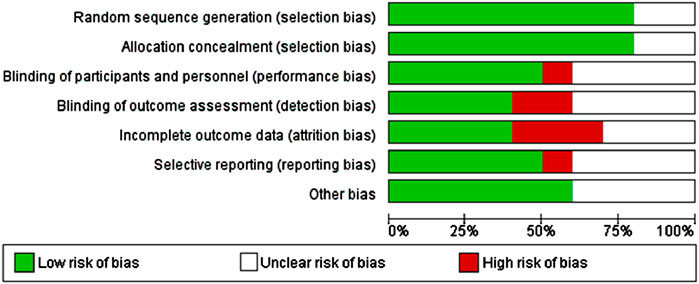
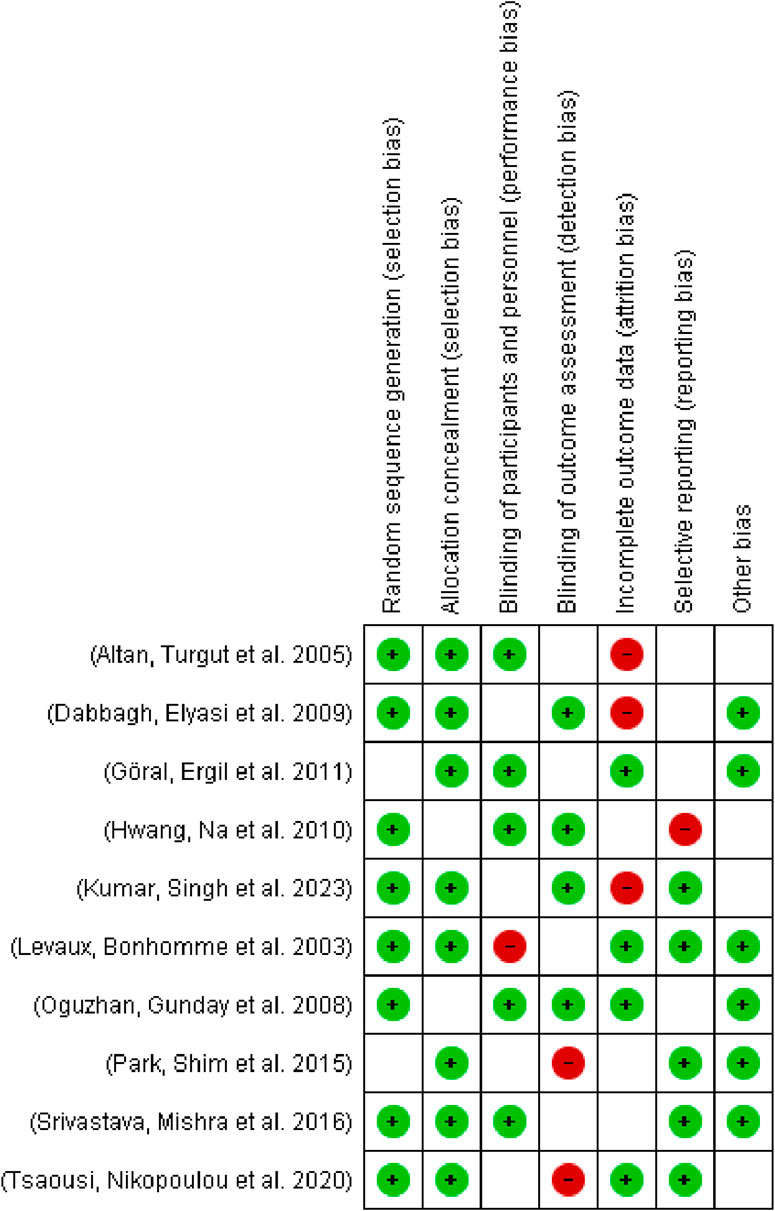
The risk of bias in included studies was evaluated using the Cochrane Collaboration’s risk of bias tool and analyzed using Review Manager software (Figure 2). Assessment domains included:
• Random sequence generation.
• Allocation concealment.
• Blinding of participants and personnel.
• Blinding of outcome assessments.
• Incomplete outcome data.
• Selective reporting.
2.5 Publication bias
Review Manager was used to assess publication bias through funnel plot analysis. Funnel plots provide a visual method for examining publication bias by plotting study precision (measured by sample size or standard error) against effect sizes. As shown in Figure 3, the asymmetrical distribution suggests the presence of publication bias, potentially due to the exclusion of smaller studies with non-significant results from the analysis. Additional subgroup analyses were performed to account for multiple time points of outcome measurement, enabling more comprehensive data interpretation.
2.6 Statistical analysis
Data analysis was performed using Review Manager 5.4 software. For continuous outcomes, mean differences were calculated, while odds ratios were computed for dichotomous outcomes. All results were presented with 95% confidence intervals. Heterogeneity was assessed using I2 and Chi2 tests, with I2 values > 25%, >50%, and >75% indicating low, moderate, and high heterogeneity, respectively. A fixed-effects model was employed in the absence of significant heterogeneity, while a random-effects model was used when heterogeneity was detected.
Data from figures were extracted using Web Plot Digitizer version 4.5. Missing data were handled according to Cochrane Handbook guidelines (Guyatt et al., 2013). Publication bias was assessed using Review Manager-generated funnel plots, with asymmetrical distribution indicating potential publication bias. Subgroup analyses were conducted to evaluate outcomes at different time points, enabling more detailed data analysis.
3 Results
3.1 Study selection
The systematic literature search initially identified 415 potentially relevant studies. After applying the clinical trial filter, 381 studies were excluded, leaving 34 studies for further evaluation. Following title and abstract screening, 21 studies were excluded based on predefined criteria: they were either study protocols, non-human studies, pharmacokinetic studies, or studies not focused on spinal surgery.
Of the remaining 13 studies that underwent full-text review, three were subsequently excluded due to:
• Non-shared outcome variables
• Duplicate publications
• Non-intravenous magnesium sulfate administration routes
• Significant heterogeneity in inclusion criteria
Manual screening of reference lists from included studies did not yield any additional eligible articles. Therefore, ten randomized controlled trials were ultimately included in the meta-analysis, as illustrated in the PRISMA flow diagram (Figure 1).
3.2 Study characteristics
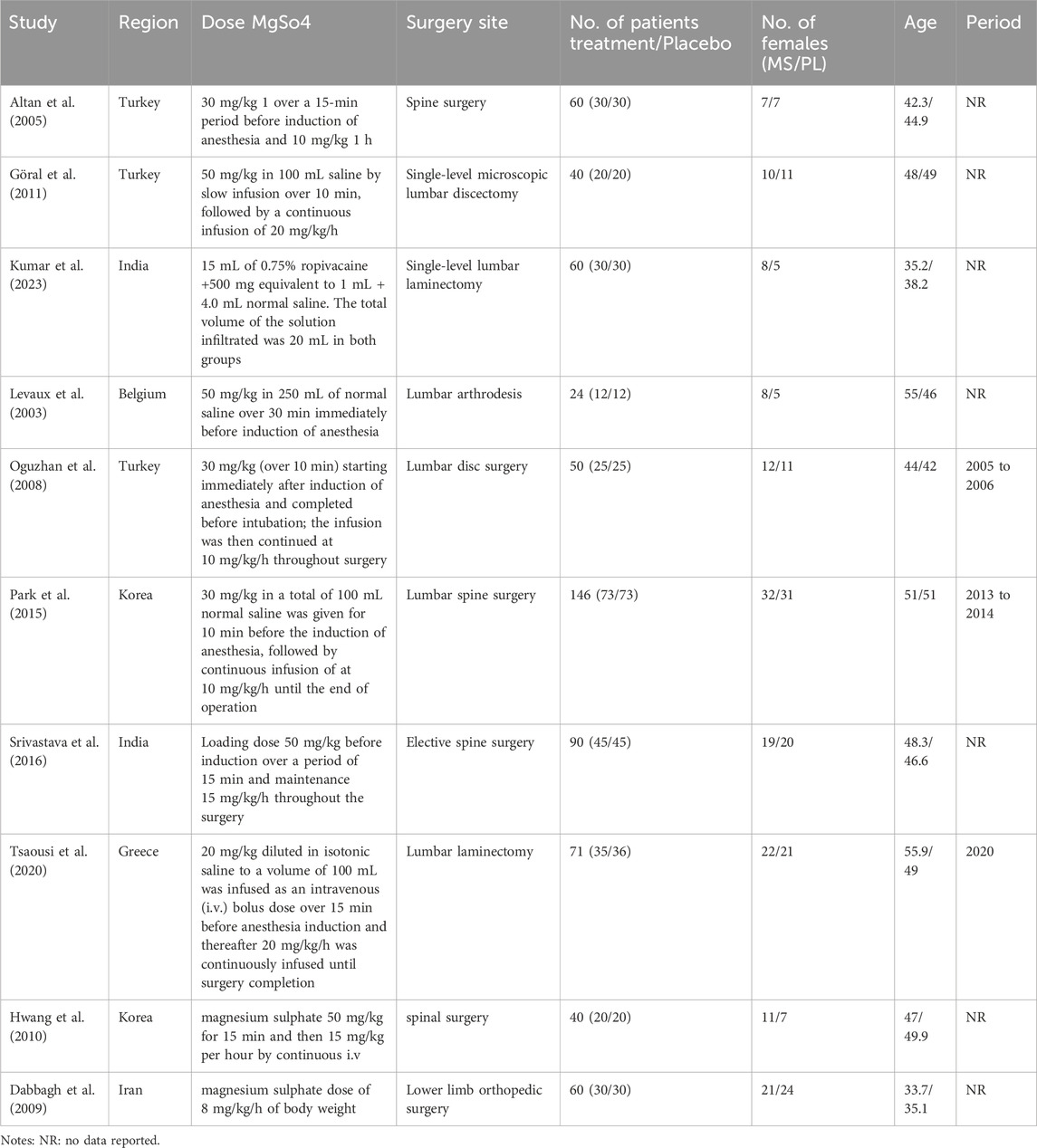
The primary characteristics of the included studies are summarized in Table 1. The meta-analysis comprised ten studies with a total of 641 participants: 295 in the magnesium sulfate (MS) group, 163 in the placebo group, 103 in the dexamethasone group, and 80 in the dexmedetomidine group. The mean age of participants ranged from 32.2 to 55.9 years, with 301 participants (46.9%) being female. Table 1 presents the detailed MS dosage regimens and surgical indications for each study. The risk of bias assessment for the included studies is presented in Figure 3.
A comparative analysis of different treatment approaches and anesthesia techniques in spinal surgery pain management is provided in Table 2. This analysis includes various interventions such as esketamine combined with pregabalin, dexmedetomidine, patient-controlled analgesia (PCA), and acupuncture, highlighting their respective outcomes and clinical significance in spinal surgery patients.
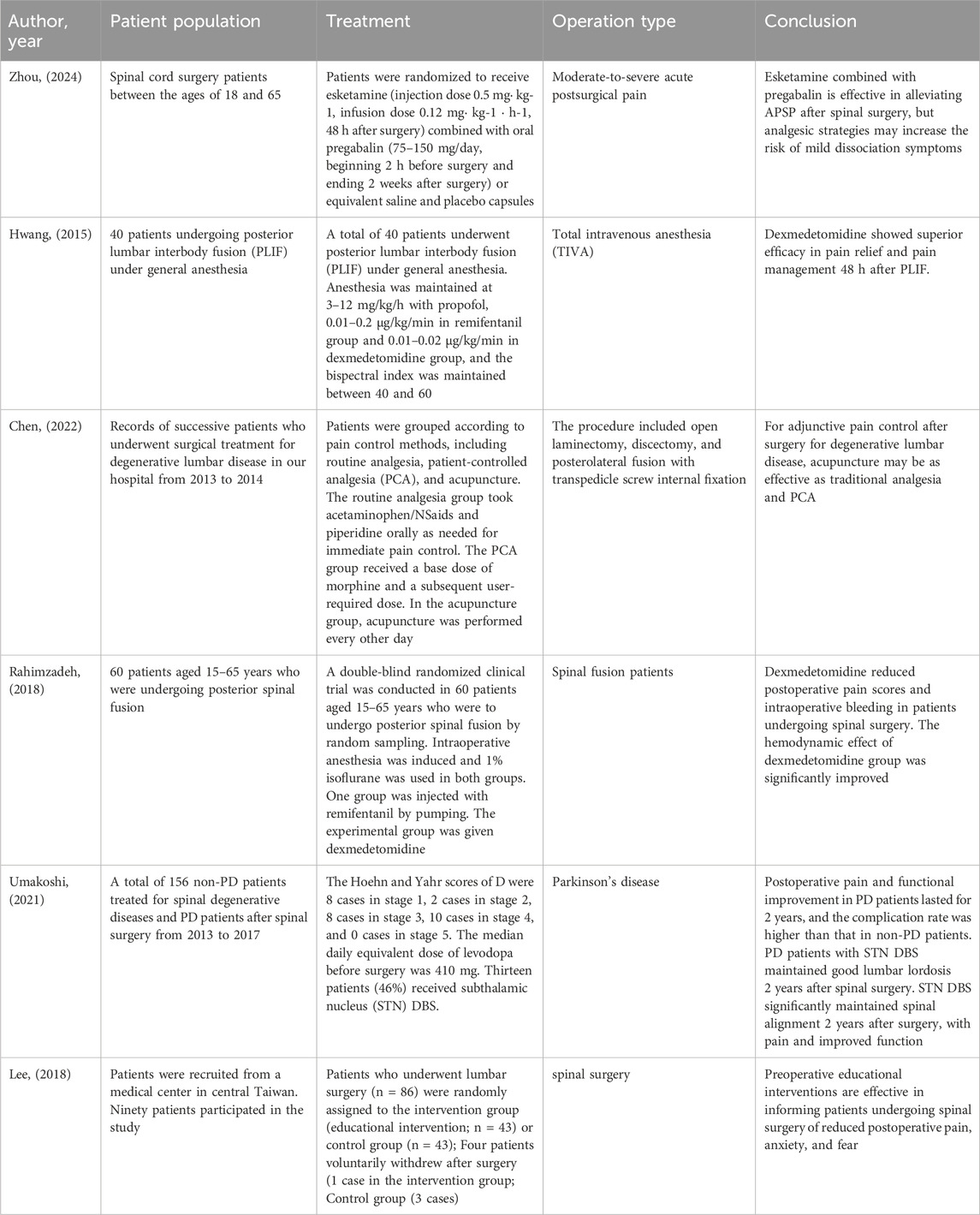
Table 2. A comparative of different treatments and anesthesia TechniquesSpinal in surgery pain management and outcomes.
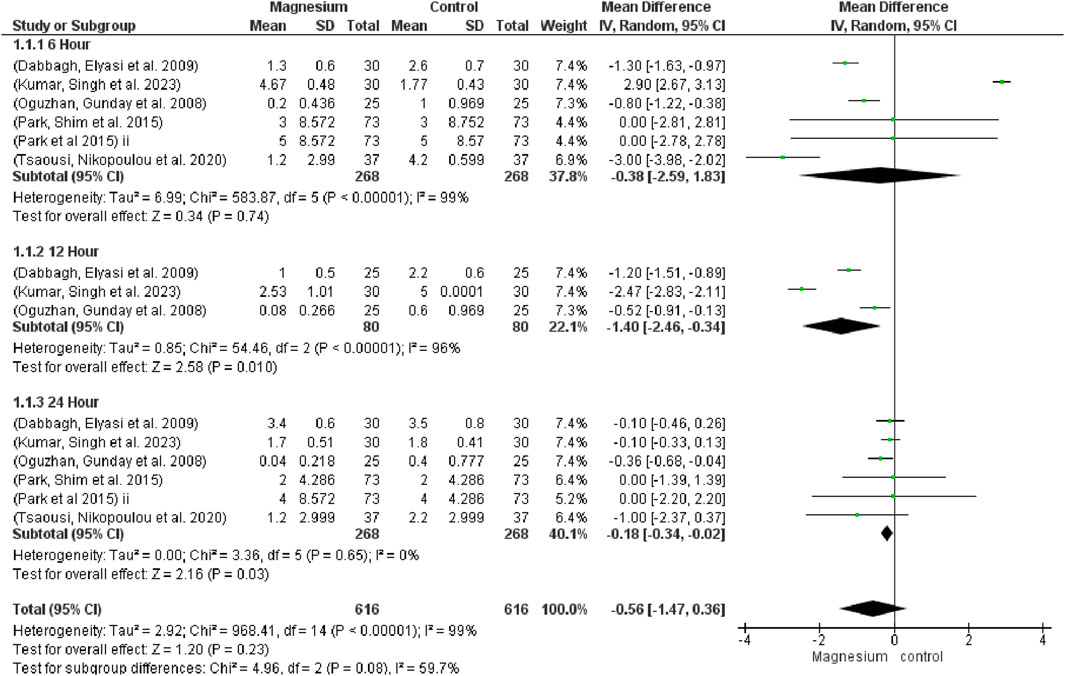
The global VAS (MD 0.56, 95% CI −1.47 to 0.36; participants = 1,232; studies = 15; I2 = 99%) showed no discernible differences (Figure 4). Comparably, at 6 h (MD −0.38, 95% CI −2.59 to 1.83; participants = 536; studies = 6; I2 = 99%) and 12 h (MD −1.40, 95% CI −2.64 to 0.34; participants = 160; studies = 3; I2 = 96%), no significant differences were discovered. After a full day, however, the magnesium group displayed noticeably less discomfort than the control group (MD−0.18, 95% CI−0.34, −0.02; participants, 536; studies, 6; I2 = 0%). When comparing MS with a placebo, MS showed a much higher global VAS reduction. There were no discernible changes between MS and dexmedetomidine. Likewise, no noteworthy distinctions were noted when contrasting MS with dexamethasone. The subgroup test is 1.20 and the overall effect test is 2.16.
3.2.1 Drug consumption
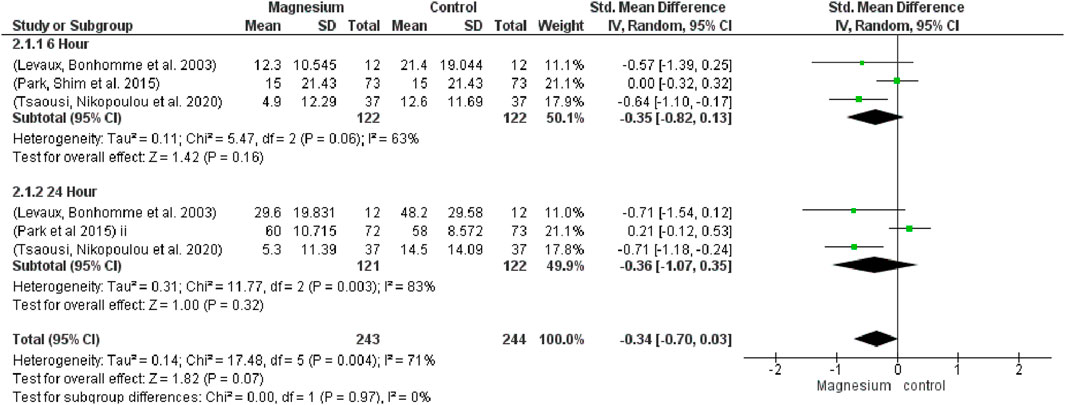
In relation to opioid consumption, no significant differences were observed at 6 h (SMD−0.35, 95%CI−0.82 to 0.13; participants = 244; studies = 3; I2 = 63%) or 24 h (SMD−0.36, 95% CI−1.07 to 0.35; participants = 243; studies = 3; I2 = 83%) (Figure 5). When magnesium was compared to a placebo, it was found to significantly reduce opioid consumption (SMD−0.66, 95%CI −0.95 to −0.38; participants = 196; studies = 6; I2 = 0%), both at 6 and 24 h (SMD−0.71, 95% CI −1.12 to −0.30; participants = 98; studies = 3; I2 = 0%). In terms of overall or at six or 24 h, there were no significant differences between MS and dexamethasone (SMD 0.10, 95% CI−0.13 to 0.33; participants = 292; studies = 6; I2 = 0%). Test results for the subgroup are 1.82 and the overall impact are 1.00.
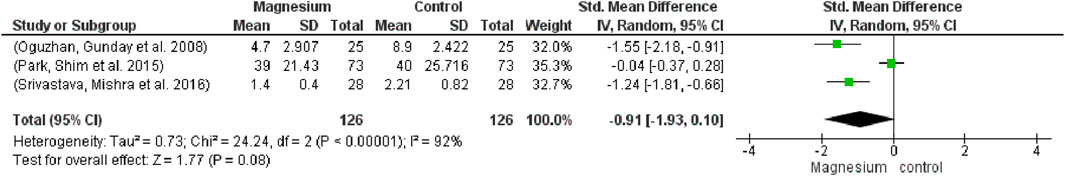
The MS group’s use of muscle relaxants was significantly lower among participants (252) and studies (3) (SMD −0.91, 95% CI−1.93 to −0.10; I2 = 92%) (Figure 6) MS had a significantly decreased consumption of muscle relaxants when compared to a placebo. The total impact test is 1.77 and the chi2 value is 0.73.
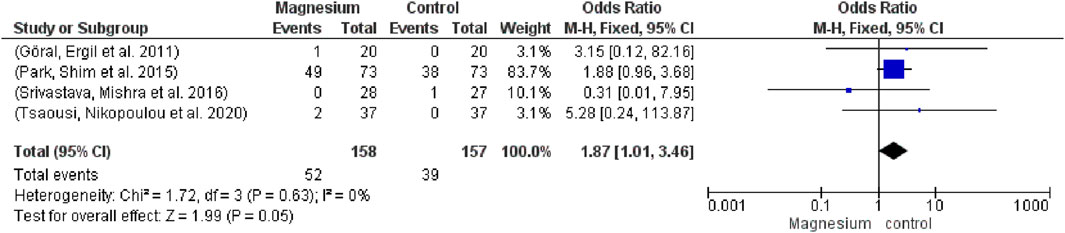
Vasoactive agent use did not differ significantly (OR 1.87, 95% CI 1.01 to 3.46; participants = 315; studies = 4; I2 = 0%) from (Figure 7). Not at all in contrast to a placebo. The chi2 value is 1.72 and the overall impact test is 1.99.
3.2.2 Hemodynamics
Regarding global heart rate (MD 2.37, 95% CI −0.66 to 5.10; participants = 797; studies = 17; I2 = 24%), there were no significant changes between the groups or in any of the follow-up time-based subgroups (Figure 8). The MS group displayed a considerably greater heart rate in comparison to the placebo. When MS was compared with clonidine, no discernible differences were found. Test results for subgroup difference are 0.40 and total effect are 0.55.
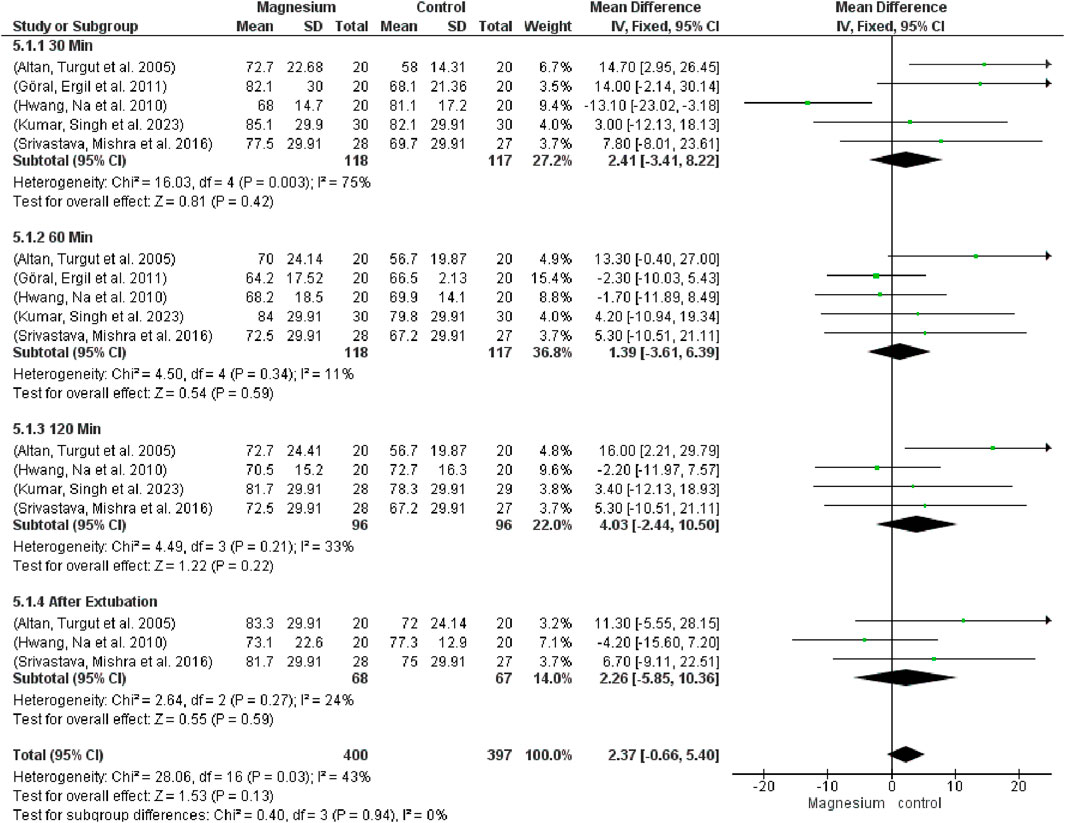
Figure 9 indicates that there were no significant variations in mean arterial pressure (MAP) across the groups (MD 1.81, 95% CI−2.55 to 6.17; 950 participants, 20 studies, and 57% I2). Notably, there were no variations when compared to the placebo group. MS displayed a considerably decreased MAP in contrast to clonidine. Regarding dexmedetomidine, there were no notable variations. The subgroup test is 0.55 while the overall effect test is 0.45.
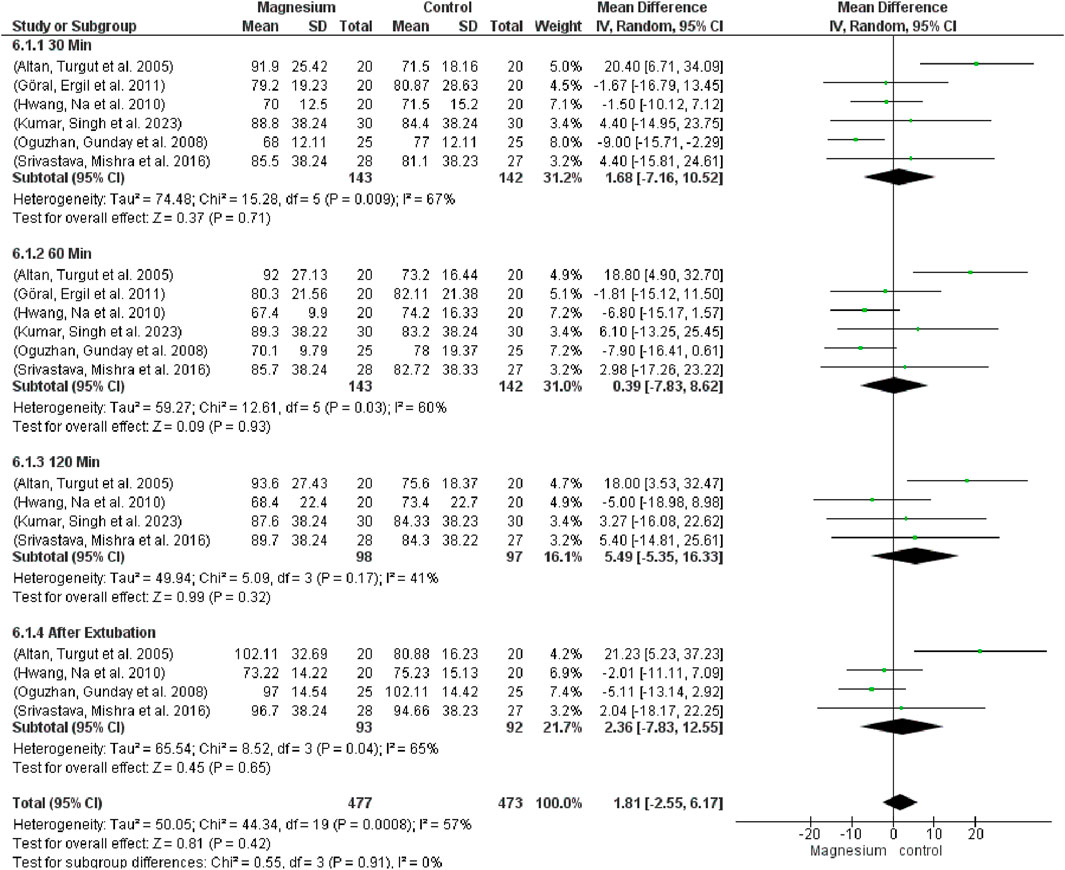
Figure 9. Forest plot express mean arterial pressure at 30 min, 60 min, 120 min, and after extubating.
3.2.3 Extubating time, response to verbal commands and orientation time
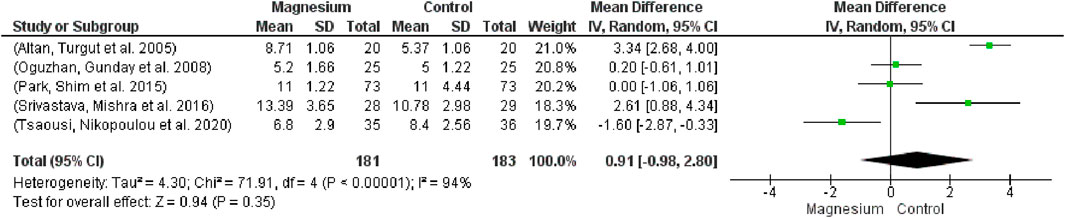
There was no statistically significant difference in extubating time between the groups (Figure 10; participants = 364, studies = 5, I2 = 94%; MD 0.91, 95% CI -0.98–2.80). With respect to the placebo group, there were no appreciable variations. The MS group had a considerably longer extubation time in comparison to dexmedetomidine. The overall effect test result is 0.94, and chi2 is 71.91.
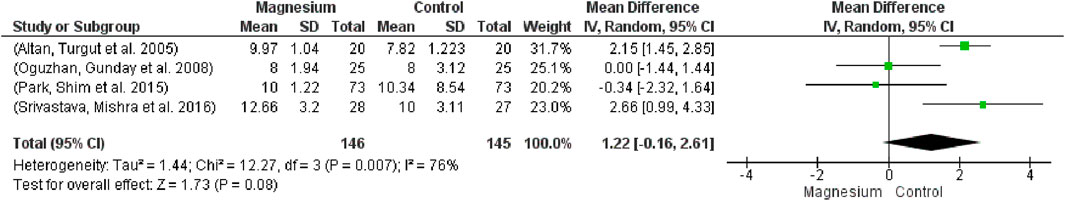
Figure 11 shows that the MS group’s responsiveness to verbal orders was considerably higher (MD 1.22, 95% CI -0.166 to 2.61; participants = 291; studies = 4; I2 = 76%). With respect to the placebo group, there were no appreciable variations. In contrast to the clonidine group, the MS group displayed a noticeably slower response time to spoken instructions. Chi2 is 12.27 and the test for the total effect is 1.73.
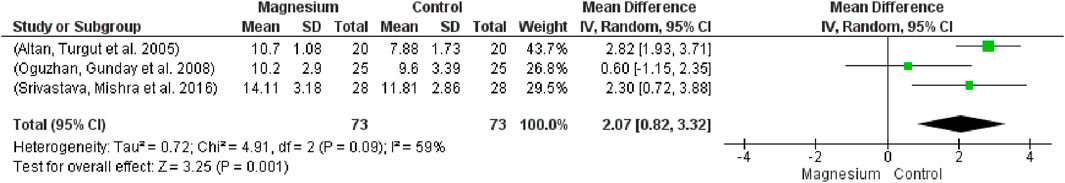
Additionally, the orientation time was substantially longer in the MS group compared to the placebo group (MD: 2.07, 95% CI 0.82 to 3.32; participants = 146; studies = 3; I2 = 59%) (Figure 12). The test effect was 3.25 overall, and the chi2 was 4.91.
3.2.4 Publication bias
Visual inspection of funnel plots revealed significant publication bias across most variables, as evidenced by notable asymmetry in the distribution of studies. Detailed analysis of these asymmetries and their implications is presented in the supplementary materials.
The therapeutic benefits of magnesium in postoperative pain management likely stem from its multifaceted neuroprotective and anti-inflammatory mechanisms (Hassan et al., 2020). Through the promotion of neuroplasticity, magnesium provides protection against neuronal deterioration and postoperative cognitive dysfunction (Hassan et al., 2020). Its antioxidant properties are demonstrated through the neutralization of reactive oxygen species (ROS), specifically via hydrogen peroxide (H2O2) elimination and hydrogen (H2) liberation, contributing significantly to its anti-inflammatory effects in treating intervertebral disc degeneration (IVDD) (Hassan et al., 2020; Zhang et al., 2023).
The neuroprotective profile of magnesium extends to its modulation of S100B protein levels, a recognized biomarker of oxidative stress and amyloid precursor (Zhang et al., 2023). Clinical investigations have revealed that magnesium’s efficacy in managing postoperative sore throat is comparable to corticosteroids, potentially contributing to enhanced patient satisfaction. This finding is particularly noteworthy given that patient-reported outcome measures (PROMs) were primarily limited to Visual Analog Scale (VAS) assessments (Park et al., 2015). The comparison with corticosteroids merits special attention, considering their adverse metabolic effects, particularly on glucose homeostasis. The therapeutic benefit appears to be mediated through magnesium’s anti-inflammatory properties, resulting in reduced postoperative nausea, vomiting, and subsequent throat discomfort (Lu et al., 2013; Mori et al., 2010).
A significant limitation across studies was the absence of minimum clinically important difference (MCID) assessments, highlighting a crucial area for future investigation. The marked reduction in opioid consumption observed with magnesium administration, compared to placebo, can be mechanistically explained by its non-competitive N-methyl-D-aspartate (NMDA) receptor antagonism. This mechanism results in decreased glutamate release and inhibition of excessive calcium influx into neurons, thereby providing neuroprotection and maintaining cellular integrity (Newcomer and Krystal, 2001). Similar opioid-sparing effects have been documented with other NMDA receptor antagonists, including gabapentin and ketamine (Newcomer and Krystal, 2001).
The neuroprotective properties of magnesium sulfate have broader clinical applications, as evidenced by its recommended use in reducing cerebral palsy incidence among preterm infants, particularly those at risk of delivery before 32 weeks gestation (Jameson and Bernstein, 2019). Through dual mechanisms of neuronal process stabilization and vasodilation, intracerebral magnesium sulfate reduces the incidence of cerebral vasospasm and delayed cerebral ischemia. Complementary intravenous hydrogen therapy may enhance these effects through additional antioxidant benefits, potentially improving clinical outcomes and reducing cerebral oxidative stress (Takeuchi et al., 2021).
3.2.5 Hemodynamic and cardiovascular effects
Our analysis revealed lower mean arterial pressure in the magnesium group compared to the corticosteroid group, attributable to magnesium’s vasodilatory properties. Magnesium exerts its hypotensive effect through multiple mechanisms: direct relaxation of blood vessels, calcium channel antagonism, and competition with sodium in vascular smooth muscle cells. Furthermore, magnesium enhances endothelial function and vascular wall integrity, promoting vasodilation and reducing inflammation (Houston, 2011). The observed increase in heart rate in our meta-analysis likely represents a compensatory response to this vasodilation, while the higher mean arterial pressure in the corticosteroid group can be attributed to adrenergic system activation.
The hypotensive properties of magnesium contribute to reduced perioperative bleeding (Rayssiguier et al., 2010). Additionally, magnesium modulates sympathetic nervous system activity through catecholamine blockade (Göral et al., 2011). While the magnesium group demonstrated prolonged activated partial thromboplastin time, other coagulation parameters remained comparable between groups (Wang et al., 2024).
3.2.6 Neuromuscular effects and safety considerations
Magnesium’s ability to reduce muscle relaxant requirements is attributed to its inhibition of acetylcholine receptor responses in muscle cells. This synergistic effect potentially allows for dose reduction of muscle relaxants during surgical procedures. However, careful dose titration of both vecuronium and magnesium sulfate is essential to prevent adverse effects and excessive muscle relaxation (Aal-Hamad et al., 2023).
3.2.7 Risk of hypermagnesemia
Careful monitoring of magnesium levels is crucial, as concentrations exceeding 4–5 mmol/L (9.7–12.2 mg/dL) are considered hazardous. Hypermagnesemia can manifest as weakness, nausea, dizziness, and confusion, with increased mortality risk in hospitalized patients. The prevalence ranges from 3.0% to 5.7%–9.3% in the general population, with higher rates in hospital settings. Patients with renal insufficiency require particularly vigilant monitoring and management to prevent serious complications (Srivastava et al., 2016).
3.2.8 Recovery period considerations
The prolonged recovery period observed in the magnesium group, characterized by delayed orientation time and verbal response, likely results from the combination of its hypotensive and muscle-relaxant effects. The vasodilatory properties may lead to reduced cerebral perfusion and oxygenation, contributing to extended recovery times and temporary cognitive effects. The muscle-relaxant properties may further impair patient responses during the recovery phase, affecting both verbal communication and orientation capabilities.
3.2.9 Comparative analysis with dexmedetomidine
Dexmedetomidine served as a key comparator in our meta-analysis, providing important insights into alternative therapeutic approaches. As an α2-adrenoceptor agonist, dexmedetomidine demonstrates significant efficacy in reducing surgical stress responses. Our analysis revealed that dexmedetomidine was more effective than magnesium in reducing both fentanyl and propofol consumption. Srivastav et al.'s findings demonstrated superior hemodynamic stability with dexmedetomidine compared to other interventions (Srivastava et al., 2016). This enhanced hemodynamic profile and greater efficacy in reducing opioid and propofol consumption can be attributed to dexmedetomidine’s high affinity and selectivity for α2-adrenergic receptors. However, these benefits are accompanied by an increased risk of bradycardia and hypotension compared to magnesium therapy (Srivastava et al., 2016).
3.2.10 Pain management and recovery characteristics
While Kumar’s study reported superior pain control with dexmedetomidine compared to magnesium plus ropivacaine combination, our meta-analysis found no significant differences in pain outcomes between the interventions. Notably, we observed increased muscle relaxant consumption in the dexmedetomidine group. In comparison with placebo, dexmedetomidine demonstrated advantages in orientation time and verbal response parameters. The prolongation of local anesthetic effects observed with dexmedetomidine can be attributed to its vasoconstrictive properties (Yoshitomi et al., 2008).
3.2.11 Safety profile and administration considerations
Recent meta-analyses have highlighted specific safety concerns with dexmedetomidine administration during spinal surgery. The risk of bradycardia and intraoperative hypotension is particularly pronounced when administered as a loading dose in combination with total intravenous anesthesia (Wang et al., 2024). These risks were notably elevated in patients receiving inhalation anesthesia. While the inhalation anesthesia subgroup demonstrated reduced blood loss, this effect was not observed consistently across all administration methods (Wang et al., 2024).
3.2.12 Multimodal applications and synergistic effects
Magnesium demonstrates significant potential for synergistic effects when combined with other analgesic modalities. The modest effects observed in comparisons between magnesium sulfate and corticosteroids may be attributed to limited sample sizes and short follow-up periods (typically 24 h). Evidence suggests enhanced patient outcomes when magnesium is combined with various analgesic interventions, including opioids, local anesthetics, and regional anesthesia techniques (Fairley et al., 2017). This multimodal approach may provide more comprehensive pain management strategies, though longer-term monitoring beyond the immediate postoperative phase is needed to fully evaluate these benefits.
3.2.13 Administration protocols and dosing strategies
Considerable variation exists in magnesium administration protocols (Tsaousi et al., 2020). Our meta-analysis evaluated various approaches, including:
• Loading doses: 30–50 mg/kg in saline, administered over 10–30 min pre-anesthesia.
• Maintenance infusions: 10–20 mg/kg/h throughout surgery.
• Alternative approaches: One study evaluated ropivacaine infiltration with 500 mg magnesium supplementation.
While visual analysis of forest plots did not reveal clear associations between administration protocols and outcomes, this observation requires cautious interpretation given the lack of formal statistical analysis.
3.2.14 Broader clinical applications
Magnesium’s therapeutic benefits extend beyond spinal surgery. Evidence demonstrates its efficacy in.
• Reducing postoperative atrial fibrillation in cardiac surgery (Fairley et al., 2017).
• Decreasing ventricular arrhythmias without additional adverse effects.
• Improving postoperative pain control in arthroscopic procedures when administered intra-articularly.
• Providing cartilage and chondrocyte protection, as supported by experimental studies (Zeng et al., 2016).
Meta-analyses have documented significant reductions in:
• 24-h morphine consumption.
• Time to first analgesic requirement.
• Postoperative shivering without increasing adverse effects such as bradycardia, nausea, or vomiting (Ng et al., 2020).
3.2.15 Surgical outcome analysis
Our study expanded upon previous meta-analyses by incorporating additional outcome measures, including remifentanil and muscle relaxant consumption. Key findings include.
• Peak pain reduction occurred at varying timepoints (6–12 h) across studies (Tsaousi et al., 2020; Kumar et al., 2023).
• Tsaousi et al. uniquely reported decreased extubation time with corresponding reductions in opioid and remifentanil use (Tsaousi et al., 2020).
• Variable outcomes in specific surgical subgroups:
o Microscopic surgery (Göral et al. (2011)): Limited comparable outcomes.
o Lumbar disc replacement: Significant improvements in pain control and reduced muscle relaxant requirements (Kumar et al., 2023).
o Broader surgical categories (“lumbar arthrodesis,” “spine surgery”): Limited comparative analysis due to heterogeneity (Morrison et al., 2013).
Unlike previous meta-analyses focusing on total anesthesia duration, our study specifically examined orientation and verbal response times, though findings aligned with established observations regarding prolonged anesthetic effects.
4 Limitations
There are several restrictions on this study. The restricted number of included studies hindered the ability to do sensitivity and consistent subgroup analysis, as well as to assess the impact of varying doses and surgical indications. To give more detailed advice, future research should describe findings according to the precise kind of surgery that was done. Additionally, rather than using formal procedures, publication bias was evaluated visually, and in several instances, there were just a few publications available for the control groups. Even though the established Cochrane guidelines were adhered to, challenges pertaining to missing data were also observed. Furthermore, several intriguing factors, including improved hemostatic parameters, decreased intraoperative bleeding, and patient satisfaction, were only reported in one trial and could not be compared via meta-analysis. Data on these characteristics should be used in future research. These restrictions should be considered when interpreting the findings, and they highlight the need for more study to address these restrictions in order to gain a more thorough understanding of the application of magnesium in the treatment of postoperative pain.
5 Conclusion
In conclusion, our meta-analysis showed that magnesium sulphate delivery following spinal surgery significantly reduced pain at 24 h and minimized the use of opioids and muscle relaxants when compared to placebo and other analgesics. Magnesium sulphate also extended orientation and reactions to spoken instructions. The groups’ heart rates and blood pressure did not differ significantly from one another. Without causing greater side effects, this multimodal strategy using magnesium sulphate seemed to reduce postoperative pain more well. These results imply that magnesium sulphate may improve recovery regimens optimised after spine surgery and solve current problems associated with opioid use. It is yet unknown how clinical improvements will translate to patient outcomes and what effect they will have on hospital stays, patient satisfaction, and care quality.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
ZJ: Writing – original draft, Conceptualization, Writing – review and editing, Formal Analysis, Supervision. JZ: Writing – original draft, Investigation, Conceptualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Thanks to all those who helped with the study but were not listed as co-authors due to insufficient contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1624119/full#supplementary-material
References
Aal-Hamad, A. H., Al-Alawi, A. M., Kashoub, M. S., and Falhammar, H. (2023). Hypermagnesemia in clinical practice. Medicina 59 (7), 1190. doi:10.3390/medicina59071190
Altan, T., Turgut, N., Yildiz, F., Balci, N., Ozyurek, Z., and Turhan, E. (2005). Effects of magnesium sulphate and clonidine on propofol consumption, haemodynamics and postoperative recovery. British Journal of Anaesthesia, 94 (4), 438–441. doi:10.1093/bja/aei070
Bas, J. L., Bas, P., Bonilla, F., Mariscal, G., Pérez, S., Bovea-Marco, M., et al. (2023). Efficacy of perioperative gabapentin use in patients with idiopathic scoliosis undergoing fusion surgery: a systematic review and meta-analysis. Eur. Spine J. 32 (7), 2521–2532. doi:10.1007/s00586-023-07764-8
Buvanendran, A., McCarthy, R. J., Kroin, J. S., Leong, W., Perry, P., and Tuman, K. J. (2002). Intrathecal magnesium prolongs fentanyl analgesia: a prospective, randomized, controlled trial. Anesth. and Analgesia 95 (3), 661–666. doi:10.1097/00000539-200209000-00031
Chandler, J., Cumpston, M., Li, T., Page, M. J., and Welch, V. (2019). Cochrane handbook for systematic reviews of interventions. Hoboken: Wiley.
Chen, L., Yu, L., Fan, Y., and Mao, J. (2022). A retrospective comparison of analgesic efficacy between acupuncture, patient-controlled analgesia, and conventional analgesia for postoperative pain management in spinal fusion surgery. Pain Research and Management, 2022, 8494206. doi:10.1155/2022/8494206
Dabbagh, A., Elyasi, H., Razavi, S. S., Fathi, M., and Rajaei, S. (2009). Intravenous magnesium sulfate for post-operative pain in patients undergoing lower limb orthopedic surgery. Acta Anaesthesiologica Scandinavica, 53 (8), 1088–1091. doi:10.1111/j.1399-6576.2009.02025.x
de Andrade, R. R., de Oliveira-Neto, O. B., Barbosa, L. T., Santos, I. O., de Sousa-Rodrigues, C. F., and Barbosa, F. T. (2019). Efetividade da ozonioterapia comparada a outras terapias para dor lombar: revisão sistemática com metanálise de ensaios clínicos randomizados. Rev. Bras. Anestesiol. 69, 493–501. doi:10.1016/j.bjan.2019.06.007
Fairley, J. L., Zhang, L., Glassford, N. J., and Bellomo, R. (2017). Magnesium status and magnesium therapy in cardiac surgery: a systematic review and meta-analysis focusing on arrhythmia prevention. J. Crit. care 42, 69–77. doi:10.1016/j.jcrc.2017.05.038
Fathy, W., Hussein, M., Ibrahim, R. E., Abdel-Aziz, M. M., Adel, S., Soliman, S. H., et al. (2022). Comparative effect of transforaminal injection of Magnesium sulphate versus Ozone on oxidative stress biomarkers in lumbar disc related radicular pain. BMC Anesthesiol. 22 (1), 254. doi:10.1186/s12871-022-01789-0
Ferasatkish, R., Dabbagh, A., Alavi, M., Mollasadeghi, G., Hydarpur, E., Moghadam, A., et al. (2008). Effect of magnesium sulfate on extubation time and acute pain in coronary artery bypass surgery. Acta Anaesthesiol. Scand. 52 (10), 1348–1352. doi:10.1111/j.1399-6576.2008.01783.x
Göral, N., Ergil, J., Alptekin, A., Özkan, D., Gürer, B., Dolgun, H., et al. (2011). Effect of magnesium sulphate on bleeding during lumbar discectomy. Anaesthesia 66 (12), 1140–1145. doi:10.1111/j.1365-2044.2011.06898.x
Guyatt, G. H., Thorlund, K., Oxman, A. D., Walter, S. D., Patrick, D., Furukawa, T. A., et al. (2013). GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles—continuous outcomes. J. Clin. Epidemiol. 66 (2), 173–183. doi:10.1016/j.jclinepi.2012.08.001
Hassan, W. F., Tawfik, M. H., Nabil, T. M., and Abd Elkareem, R. M. (2020). Could intraoperative magnesium sulphate protect against postoperative cognitive dysfunction? Minerva Anestesiol. 86 (8), 808–815. doi:10.23736/S0375-9393.20.14012-4
Helm, S., Harmon, P. C., Noe, C., Calodney, A. K., Abd-Elsayed, A., Knezevic, N. N., et al. (2021). Transforaminal epidural steroid injections: a systematic review and meta-analysis of efficacy and safety. Pain Physician 24 (S1), S209–S232.
Houston, M. (2011). The role of magnesium in hypertension and cardiovascular disease. J. Clin. Hypertens. 13 (11), 843–847. doi:10.1111/j.1751-7176.2011.00538.x
Hwang, J. Y., Na, H. S., Jeon, Y. T., Ro, Y. J., Kim, C. S., and Do, S. H. (2010). I.V. infusion of magnesium sulphate during spinal anaesthesia improves postoperative analgesia. British Journal of Anaesthesia, 104 (1), 89–93. doi:10.1093/bja/aep334
Hwang, S. H., Park, I. K., Kang, D. H., Park, H. J., and Lee, Y. J. (2015). Dexmedetomidine versus remifentanil in postoperative pain control after spinal surgery: a randomized controlled trial. Medicine, 94 (29), e1161. doi:10.1097/MD.0000000000001161
James, M. F., Beer, R. E., and Esser, J. D. (1989). Intravenous magnesium sulfate inhibits catecholamine release associated with tracheal intubation. Anesth. and Analgesia 68 (6), 772–776. doi:10.1213/00000539-198906000-00015
Jameson, R. A., and Bernstein, H. B. (2019). Magnesium sulfate and novel therapies to promote neuroprotection. Clin. perinatology 46 (2), 187–201. doi:10.1016/j.clp.2019.02.008
Ketenci, A., and Zure, M. (2021). Pharmacological and non-pharmacological treatment approaches to chronic lumbar back pain. Turkish J. Phys. Med. rehabilitation 67 (1), 1–10. doi:10.5606/tftrd.2021.8216
Kumar, M., Singh, R. B., Vikal, J. P., Yadav, J. B. S., and Singh, D. (2023). Comparison of ropivacaine plus dexmedetomidine and ropivacaine plus magnesium sulfate infiltration for postoperative analgesia in patients undergoing lumbar spine surgeries. Cureus 15 (3), e36295. doi:10.7759/cureus.36295
Lee, T. L., Chang, P. C., Huang, Y. T., Chen, Y. C., and Wang, J. J. (2018). Effects of preoperative pain education on postoperative pain control and anxiety in patients undergoing spinal surgery: A randomized controlled trial. Asian Spine Journal, 12 (1), 65–74. doi:10.4184/asj.2018.12.1.65
Levaux, C., Bonhomme, V., Dewandre, P. Y., Brichant, J. F., and Hans, P. (2003). Effect of intra-operative magnesium sulphate on pain relief and patient comfort after major lumbar orthopaedic surgery. Anaesthesia, 58 (2), 131–135. doi:10.1046/j.1365-2044.2003.02999.x
Lu, K.-z., Shen, H., Chen, Y., Li, M.-g., Tian, G.-p., and Chen, J. (2013). Ondansetron does not attenuate the analgesic efficacy of nefopam. Int. J. Med. Sci. 10 (12), 1790–1794. doi:10.7150/ijms.5386
Lysakowski, C., Dumont, L., Czarnetzki, C., and Tramèr, M. R. (2007). Magnesium as an adjuvant to postoperative analgesia: a systematic review of randomized trials. Anesth. and Analgesia 104 (6), 1532–1539. doi:10.1213/01.ane.0000261250.59984.cd
Mariscal, G., Morales, J., Pérez, S., Rubio-Belmar, P. A., Bovea-Marco, M., Bas, J. L., et al. (2022). Meta-analysis of the efficacy of ketamine in postoperative pain control in adolescent idiopathic scoliosis patients undergoing spinal fusion. Eur. Spine J. 31 (12), 3492–3499. doi:10.1007/s00586-022-07422-5
Mentes, O., Harlak, A., Yigit, T., Balkan, A., Balkan, M., Cosar, A., et al. (2008). Effect of intraoperative magnesium sulphate infusion on pain relief after laparoscopic cholecystectomy. Acta Anaesthesiol. Scand. 52 (10), 1353–1359. doi:10.1111/j.1399-6576.2008.01816.x
Mori, T., Koyama, N., Arendash, G. W., Horikoshi-Sakuraba, Y., Tan, J., and Town, T. (2010). Overexpression of human S100B exacerbates cerebral amyloidosis and gliosis in the Tg2576 mouse model of Alzheimer's disease. Glia 58 (3), 300–314. doi:10.1002/glia.20924
Morrison, A., Hunter, J., Halpern, S., and Banerjee, A. (2013). Effect of intrathecal magnesium in the presence or absence of local anaesthetic with and without lipophilic opioids: a systematic review and meta-analysis. Br. J. Anaesth. 110 (5), 702–712. doi:10.1093/bja/aet064
Naftalovich, R., Singal, A., and Iskander, A. J. (2022). Enhanced Recovery after Surgery (ERAS) protocols for spine surgery–review of literature. Anaesthesiol. Intensive Ther. 54 (1), 71–79. doi:10.5114/ait.2022.113961
Newcomer, J. W., and Krystal, J. H. (2001). NMDA receptor regulation of memory and behavior in humans. Hippocampus 11 (5), 529–542. doi:10.1002/hipo.1069
Ng, K. T., Yap, J. L., Izham, I. N., Teoh, W. Y., Kwok, P. E., and Koh, W. J. (2020). The effect of intravenous magnesium on postoperative morphine consumption in noncardiac surgery: a systematic review and meta-analysis with trial sequential analysis. Eur. J. Anaesthesiology| EJA 37 (3), 212–223. doi:10.1097/EJA.0000000000001164
Oguzhan, N., Gunday, I., and Turan, A. (2008). Effect of magnesium sulfate infusion on sevoflurane consumption, hemodynamics, and perioperative opioid consumption in lumbar disc surgery. Journal of Opioid Management, 4 (2), 105–110. doi:10.5055/jom.2008.0015
Park, J. H., Shim, J.-K., Song, J.-W., Jang, J., Kim, J. H., and Kwak, Y.-L. (2015). A randomized, double-blind, non-inferiority trial of magnesium sulphate versus dexamethasone for prevention of postoperative sore throat after lumbar spinal surgery in the prone position. Int. J. Med. Sci. 12 (10), 797–804. doi:10.7150/ijms.12831
Rahimzadeh, P., Faiz, S. H. R., Imani, F., Derakhshan, P., and Amniati, S. (2018). Comparative addition of dexmedetomidine and fentanyl to intrathecal bupivacaine in orthopedic procedure in lower limbs. BMC Anesthesiol. 18 (1), 62. doi:10.1186/s12871-018-0531-7
Rayssiguier, Y., Libako, P., Nowacki, W., and Rock, E. (2010). Magnesium deficiency and metabolic syndrome: stress and inflammation may reflect calcium activation. Magnesium Res. 23 (2), 73–80. doi:10.1684/mrh.2010.0208
Shetty, G. M., Jain, S., Thakur, H., and Khanna, K. (2022). Prevalence of low back pain in India: a systematic review and meta-analysis. Work 73 (2), 429–452. doi:10.3233/WOR-205300
Srivastava, V. K., Mishra, A., Agrawal, S., Kumar, S., Sharma, S., and Kumar, R. (2016). Comparative evaluation of dexmedetomidine and magnesium sulphate on propofol consumption, haemodynamics and postoperative recovery in spine surgery: a prospective, randomized, placebo controlled, double-blind study. Adv. Pharm. Bull. 6 (1), 75–81. doi:10.15171/apb.2016.012
Takeuchi, S., Kumagai, K., Toyooka, T., Otani, N., Wada, K., and Mori, K. (2021). Intravenous hydrogen therapy with intracisternal magnesium sulfate infusion in severe aneurysmal subarachnoid hemorrhage. Stroke 52 (1), 20–27. doi:10.1161/STROKEAHA.120.031260
Tang, J. B. (2009). Re: levels of experience of surgeons in clinical studies. J. Hand Surg. Eur. Volume 34 (1), 137–138. doi:10.1177/17531934097321
Tsaousi, G., Nikopoulou, A., Pezikoglou, I., Birba, V., and Grosomanidis, V. (2020). Implementation of magnesium sulphate as an adjunct to multimodal analgesic approach for perioperative pain control in lumbar laminectomy surgery: a randomized placebo-controlled clinical trial. Clin. Neurology Neurosurg. 197, 106091. doi:10.1016/j.clineuro.2020.106091
Umakoshi, N., Shimizu, T., Miyamoto, W., Kinoshita, M., Takao, M., Matsushita, T., et al. (2021). Spinal surgery in patients with Parkinson’s disease: Experiences with the challenges and outcomes. European Spine Journal, 30 (2), 452–461. doi:10.1007/s00586-020-06656-5
Ünlügenç, H., Gündüz, M., Özalevli, M., and Akman, H. (2002). A comparative study on the analgesic effect of tramadol, tramadol plus magnesium, and tramadol plus ketamine for postoperative pain management after major abdominal surgery. Acta Anaesthesiol. Scand. 46 (8), 1025–1030. doi:10.1034/j.1399-6576.2002.460817.x
Wang, M., Che, J.-X., Chen, L., Song, T.-T., and Qu, J.-T. (2024). Effect of dexmedetomidine on intraoperative hemodynamics and blood loss in patients undergoing spine surgery: a systematic review and meta-analysis. Chin. Med. Sci. J. 39 (1), 54–68. doi:10.24920/004294
Yoshitomi, T., Kohjitani, A., Maeda, S., Higuchi, H., Shimada, M., and Miyawaki, T. (2008). Dexmedetomidine enhances the local anesthetic action of lidocaine via an α-2A adrenoceptor. Anesth. and Analgesia 107 (1), 96–101. doi:10.1213/ane.0b013e318176be73
Zeng, C., Li, Y.-s., Wei, J., Xie, D.-x., Xie, X., Li, L.-j., et al. (2016). Analgesic effect and safety of single-dose intra-articular magnesium after arthroscopic surgery: a systematic review and meta-analysis. Sci. Rep. 6 (1), 38024. doi:10.1038/srep38024
Zhang, T., Wang, Y., Li, R., Xin, J., Zheng, Z., Zhang, X., et al. (2023). ROS-responsive magnesium-containing microspheres for antioxidative treatment of intervertebral disc degeneration. Acta Biomater. 158, 475–492. doi:10.1016/j.actbio.2023.01.020
Keywords: magnesium sulfate, spinal surgery, meta-analysis, postoperative pain, opioid consumption, systematic review, anesthesia
Citation: Jin Z and Zhao J (2025) Effectiveness and impact of intravenous magnesium sulfate in spinal surgery systematic review and meta-analysis. Front. Pharmacol. 16:1624119. doi: 10.3389/fphar.2025.1624119
Received: 07 May 2025; Accepted: 28 May 2025;
Published: 18 June 2025.
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
Sakarie Mustafe Hidig, Zhejiang University, ChinaAlshaimaa Abdel Fattah Kamel, Zagazig University, Egypt
Copyright © 2025 Jin and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoguo Jin, bGlucGluZzA4ODFAMTYzLmNvbQ==
 Zhaoguo Jin
Zhaoguo Jin Jianyong Zhao
Jianyong Zhao