- 1Institue of Physiological Chemistry, Faculty of Chemistry, University of Vienna, Vienna, Austria
- 2Vienna Doctoral School in Chemistry (DoSChem), University of Vienna, Vienna, Austria
- 3Symrise AG, Holzminden, Germany
- 4Leibniz Institute of Food Systems Biology, Technical University of Munich, Freising, Germany
- 5Institute of Clinical Nutrition, University of Hohenheim, Stuttgart, Germany
Introduction: In the context of epidermal inflammation, the inflammatory response not only involves the release of inflammatory cytokines like interleukin 8 (IL-8), but also modulation of tight junction protein expression levels. Previous studies showed that the tight junction protein claudin 1 (CLDN1) is upregulated during tumor necrosis factor α (TNFα)-induced inflammation by capsaicin in keratinocytes in a transient receptor potential channel vanilloid 1 (TRPV1)-dependent manner. However, the caveat with TRPV1 ligands is the undesired pain response elicited by the activation of neuronal TRPV1 channels. In this study, we hypothesized that also less or non-pungent homovanillic acid esters as structural analogs of capsaicin target CLDN1 upregulation during inflammation.
Methods: We aimed to identify beneficial structural characteristics by selecting homovanillic acid esters with different aliphatic tail structures and screening them for CLDN1 upregulation at early stages of TNFα-induced inflammation in basal keratinocytes.
Results: CLDN1 expression was upregulated independently of TRPV1 by compounds with a tail of 5 or 6 C-atoms, regardless of the presence of ramifications and double bonds with a maximum fold change of 2.05 ± 0.22 against control. The induction of CLDN1 expression was accompanied by increased expression of the differentiation marker involucrin (IVL).
Discussion: The results suggest that the homovanillic ester-induced CLDN1 upregulation is a result of increased differentiation of the basal keratinocytes towards the keratinocyte morphology present in the stratum granulosum (SG), where tight junctions are formed. In conclusion, homovanillic acid esters with a 5 or 6 C-atom long aliphatic chain induced CLDN1 expression, thereby stimulating keratinocyte differentiation, independent from TRPV1 activation.
1 Introduction
As the outermost layer of the skin, the epidermis serves as the primary point of contact for the body with bacterial infections, allergens, and other harmful external stimuli. When cells in the epidermis encounter these threats, the activation of receptors trigger signaling pathways that release a variety of inflammatory mediators rapidly (Chen et al., 2018; Banno et al., 2004). These mediators attract immune cells to the site of infection or injury, where they become activated to clear away dead cells and eliminate pathogens. TNFα is one of the key factors driving these inflammatory processes by orchestrating pathways that stimulate the release of inflammatory cytokines, regulate apoptosis, and induce tissue remodeling (Banno et al., 2004). Ultimately, the successful resolution of epidermal inflammation requires the restoration of keratinocyte barrier function (Chen et al., 2018). Claudin 1 (CLDN1) plays a critical role in skin barrier function by forming the intercellular strands of the tight junctions (TJ) that regulate permeability in the stratum granulosum (SG) as well as maintain stratum corneum (SC) integrity (Kirschner et al., 2013; Sugawara et al., 2013; Lynn et al., 2020). A differentiation-driven gene expression program in basal epidermal layer keratinocytes ensures CLDN1 presence in the SG, in conjunction with increasing expression of the differentiation marker involucrin (IVL) and halting the production of basal layer markers like keratin 14 (KRT14) (Watt, 1983; Fuchs, 1993). Tumor necrosis factor α (TNFα) favors this differentiation program via the nuclear factor kappa beta (NF-κB) pathway (Banno et al., 2004).
In a previous study, the effect of an increase in TNFα levels on the expression of CLDN1 was characterized using an inflammatory model of basal keratinocyte morphology. Due to their ability to differentiate in culture, the HaCaT keratinocyte cell line provided an adequate model for examining the impact of inflammation on differentiation-dependent cellular structures such as tight junctions (TJs) (Wilson, 2014). Specifically, incubation of HaCaT cells with 20 ng/mL TNFα for 48 h dose-dependently promoted CLDN1 expression, demonstrating that the inflammatory response impacts the production of TJ proteins already in lower epidermal layers to prevent a loss of barrier function (Cervantes Recalde et al., 2024). Additionally, TNFα-induced CLDN1 expression was enhanced by capsaicin and inhibited by capsazepine, indicating that TRPV1 activation also participates in the regulation of TJ proteins during inflammation (Cervantes Recalde et al., 2024). However, although capsaicin’s bioactivity is often attributed to its interaction with the TRPV1 channel, it is important to consider that capsaicin and structurally analog compounds of capsaicin may also trigger responses through TRPV1-independent mechanisms. Examples of this include the capsaicin-mediated inhibition of natural killer cell cytotoxicity, anti-tumor activity of capsaicin in oral cancer and counteraction of lipopolysaccharide (LPS)-induced hyperthermia in chicken (Gonzales et al., 2014; Nikami et al., 2008; Kim et al., 2014). This raises the question whether the promotion of CLDN1 during TNFα-induced inflammation can also be elicited by less or non-pungent structural analogs of capsaicin and if these effects are TRPV1 dependent.
From a structural perspective, the affinity for the TRPV1 channel can be fine-tuned by introducing modifications in three key pharmacophores that define structure-activity relationships in TRPV1 ligands: the aromatic “head” binding through hydrogen bonds the vanilloid pocket, a polar “neck”, and the aliphatic “tail” providing further biding affinity through Van der Waals interactions with surrounding residues (Caballero, 2022). Modifications to these regions will likely alter the molecule’s biological activity (Yang et al., 2015; Elokely et al., 2016). Capsaicin analogs with structural modifications have been demonstrated to have a reduced ability to activate the TRPV1 receptor in skin, eye and the oral cavity of mice (Meotti et al., 2014). For example, the direct replacement of the amide bond for an ester bond in the non-pungent analog capsiate changes the EC50 for TRPV1 activation from 0.099 µM (capsaicin) to 0.290 µM and evokes only a fraction of capsaicin’s pungency (Meotti et al., 2014; Kobata et al., 1998). This modification also diminished other TRPV1-induced effects such as intestinal fatty acid uptake or thermoregulation of gene expression in white adipose tissue (Lieder et al., 2019; Baskaran et al., 2018). The reduced affinity for the TRPV1 channel is not restricted to modifications in the neck pharmacophore of the ligand, as differences in the aliphatic tail also have consequences for the binding affinity (Chen et al., 2019).
Homovanillic acid esters have been characterized in the past as capsaicin or resiniferatoxin (RTX) analogues with reduced pungency to clarify the involvement of TRPV1 in pain and cell apoptosis (Macho et al., 2000; Liu et al., 1998; Appendino et al., 1996). These molecules have ester bonds instead of the amide bond characteristic in capsaicin and their tail pharmacophore can be synthetically modified with different moieties according to the needs of the researcher (Appendino et al., 1996). We, therefore, studied here the potential of less or non-pungent homovanillic acid esters as structural analogs of capsaicin to target CLDN1 upregulation in keratinocytes during inflammation. We aimed to achieve a better understanding of the structural characteristics that regulate CLDN1 expression in keratinocytes by evaluating the use of homovanillic acid esters with varying structural modifications in the tail pharmacophore characteristic of TRPV1 ligands (Figure 1). We hypothesized that homovanillic acid esters with defined structural features in their aliphatic tail induce CLDN1 expression in basal layer keratinocytes independent of TRPV1. Changes in inflammation and differentiation markers (i.e., chemokine (CXC motif) ligand 8 (CXCL8), IVL and KRT14) were documented along with CLDN1 expression in a basal layer keratinocyte inflammation model and structure-activity relationships (SAR) were drawn to identify CLDN1 promoting structures.
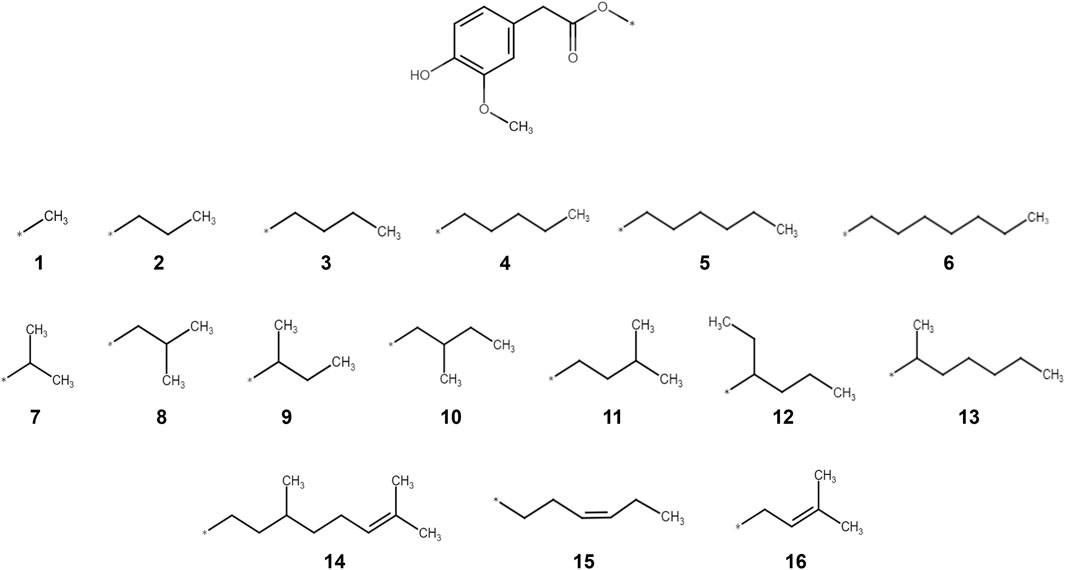
Figure 1. Molecular structures of the 16 homovanillic acid esters (1-16) used in the present study. The different homovanillic acid esters share the homovanillic ring “head” structure and an ester bond in the “neck” of the molecule. The different aliphatic tails are separately drawn, grouped according to chain length (1-6), ramifications (7-13) and double bonds (14-16).
2 Materials and methods
2.1 Chemicals
All chemicals used in this study were obtained at a ≥95% purity. TNFα (abcam) and capsazepine (Sigma Aldrich) were commercially purchased. Compounds 1 to 16 (Figure 1) were synthesized as described previously (Lieder et al., 2019). Treatment of the cell model with compounds 1-16 was done at a concentration of 10 µM using 0.1% dimethyl sulfoxide (DMSO) as solvent (Cervantes Recalde et al., 2024). Capsazepine was dissolved in 0.1% DMSO at a concentration of 1 µM and used in combination with the respective treatments with a resulting concentration of 0.2% DMSO. TNFα was used at a concentration of 20 ng/mL using double distilled water (ddH2O) as a solvent.
2.2 Cell culture
HaCaT keratinocyte cells (Cell Lines Service) were cultured under low calcium conditions (Wilson, 2014) using the keratinocyte growth medium 2 kit (PromoCell) that included basal medium supplemented with 5 μg/mL insulin, 0.33 μg/mL hydrocortisone, 0.004 mL/mL bovine pituitary extract, 10 µ/mL transferrin, 0.06 mM CaCl2, 0.125 ng/mL epidermal growth factor and 0.39 μg/mL epinephrine. Furthermore, a penicillin/streptomycin mix (Sigma Aldrich) was added at a concentration of 1% (v/v). Cells were cultured in a humidified and sterile incubator at 37°C at 5% CO2.
2.3 Treatment of cells with homovanillic acid esters and TNFα induction
The screening of the 16 homovanillic acid esters was performed on HaCaT keratinocytes seeded at a density of
2.4 Cell viability assay
The cell viability of the HaCaT keratinocytes was verified after use of compounds 1-16 at a concentration of 10 µM. This was done using an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Lieder et al., 2020). After a 24 h treatment with the different compounds (see 2.3) the medium was replaced with a 1 mg/mL MTT solution (Roth) in medium and incubated at 37°C and 5% CO2 for 15 min. The MTT solution was replaced with 100% DMSO for the dilution of the newly formed formazan crystals. The absorbance was measured simultaneously at 570 and 650 nm (reference wavelength) with a Spark® Multimode Microplate Reader (Tecan, Switzerland). Cell viability was calculated as percentage of the solvent control.
2.5 RNA isolation and RT-PCR
Sample RNA was isolated using the Monarch Total RNA Miniprep Kit (New England Biotechnologies) as stated in the manufacturer’s protocol. The quantification of the RNA concentration was carried out using a NanoQuant plate and a Spark® Multimode Microplate Reader at an absorbance of 260 nm combined with the assessment of the purity and integrity of the RNA using the 260/230 and 260/280 absorbance ratios. RNA reverse transcription was performed using 0.5 µg of the obtained RNA and processing it with the LunaScript RT SuperMix Kit (New England Biotechnologies) following the instructions provided by the manufacturer. The transcription took place in a thermal cycler C1000 Touch™ (BioRad) and the resulting cDNA was diluted 1:5 using RNase free water to be used as a template for real time qPCR (RT-qPCR) amplification. The cDNA templates were mixed with Luna Universal qPCR Master Mix (New England Biotechnologies) and the respective primer pairs (Sigma Aldrich) for CLDN1, CXCL8, IVL, KRT14, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and hypoxanthine phosphoribosyltransferase 1 (HPRT1) (Table 1). RT-qPCR measurement was done in a fluorescent quantitative detection system FQD-96A (Bioer) and the data analysis performed in LinRegPCR (version 2020.0) (Untergasser et al., 2021). The mRNA sample concentration (N0 values) was calculated using the CT values and the PCR efficiency followed by normalization against the geometric mean of the reference genes (GADPH and HPRT1).
Table 1. Primer pairs used for the characterization of the gene expression induced by TNFα treatment.
2.6 In vitro IL-8 ELISA assay
IL-8 cytokine release was assessed using a Human IL-8 ELISA Kit (abcam). HaCaT cells at a density of
2.7 Computational determination of physicochemical descriptors
Analysis on the structural characteristics of compounds 1 to 16 was done using the KNIME analytics platform 5 and the RDKit node was used to extract physicochemical descriptor information on the structures of compounds 1 to 16 (Berthold et al., 2008; Landrum et al., 2025). The descriptors were molecular weight in g/mol, SlogP, Labute’s approximate surface area (ASA), standard molecular refractivity (SMR), number of rotatable bonds, number of atoms and bond count. They were used for SAR analysis in combination with the experimentally obtained data of CLDN1 relative gene expression. Microsoft Excel was additionally used for tabulation and analysis of the data.
2.8 Statistical analysis
Statistical analysis was performed using Microsoft Excel and GraphPad Prism Version 10.1.1 software. Data is presented as mean +standard error of mean (SEM). Outliers were excluded after performing a ROUT test with a cut-off value (Q) of 5% (Motulsky and Brown, 2006). Gaussian distribution of the data was tested using a Shapiro-Wilk normality test. An F-test was used to check for equal variances between two groups and a Brown-Forsythe analysis of variance (ANOVA) test was applied when a larger group set was evaluated. Significant differences between two groups were evaluated using a Welch’s t-test or a Mann-Whitney t-test for non-parametric comparisons. Statistical analysis in comparisons between larger sets was achieved using a Brown-Forsythe and Welch ANOVA together with the Dunnett’s T3 multiple comparisons post hoc test. P-values of less than 0.05 were considered significant. Correlations were performed using a Spearman correlation between the average technical replicates of the 5 biological replicates for each treatment.
3 Results
3.1 The aliphatic tail in homovanillic acid esters can be modified to promote CLDN1 and CXCL8 gene expression
For the evaluation of structural characteristics important for the CLDN1 upregulating effect, a set of 16 compounds was selected based on the “head, neck and tail” model previously specified (Caballero, 2022). All compounds shared the same homovanillic ring “head” and an ester bond in the “neck” of the molecule, but the aliphatic tails of the different compounds varied according to chain length (1-6), ramification points (7-13) and double bonds (14-16) (Figure 1). Based on previous studies (Cervantes Recalde et al., 2024), the 16 compounds were applied for 24 h at a 10 µM concentration followed by a TNFα treatment for 6 h, respectively. Detrimental effects of the treatments on cell viability were excluded using MTT assays. None of the compounds showed adverse effects on the viability of HaCaT keratinocytes (Supplementary Figure S1) and baseline levels of CLDN1 and CXCL8 expression after TNFα induction were established (Supplementary Figure S2).
First, CXCL8 gene expression was measured and the related IL-8 cytokine release. Four compounds increased CXCL8 expression compared to cells only treated with TNFα for 6 h. Compounds 1, 3, 11 and 12 caused a relative fold increase of 1.67 ± 0.09, 1.59 ± 0.16, 1.83 ± 0.15 and 1.34 ± 0.06 as compared to the TNFα control set to one (Figure 2a). Compounds 7 and 14 were the only compounds which downregulated CXCL8 gene expression by a fold change of 0.79 ± 0.04 and 0.56 ± 0.07 against control, respectively (Figure 2a). When testing the IL-8 cytokine release, 7 and 14 reduced the release caused after TNFα treatment to 0.62 ± 0.05 and 0.73 ± 0.03-fold (Figure 2b). The expression of CLDN1 was significantly downregulated by 2 and 5 in the straight chain group by a fold change of 0.65 ± 0.03 and 0.59 ± 0.05 against control as well as 7 and 8 in the ramifications group by a fold change of 0.58 ± 0.16 and 0.85 ± 0.03, respectively (Figure 2a). However, compounds in all three groups, 1, 3, 4, 11, 12 and 15, upregulated the expression of CLDN1 in comparison to the TNFα control by a fold change of 1.28 ± 0.06, 1.88 ± 0.18, 2.00 ± 0.12, 1.90 ± 0.19, 1.46 ± 0.11 and 2.05 ± 0.22, respectively (Figures 2a). Therefore, structure differences in the tail of the tested homovanillic acid esters were shown to modulate CXCL8 and CLDN1 expression. The CXCL8 and CLDN1 gene expression correlated with a rs value of 0.61 (p < 0.0001) (Figure 2c). Consequently, differences in the aliphatic tail of the selected homovanillic acid esters resulted in similar changes in CLDN1 and CXCL8 gene expression during TNFα-induced inflammation when used as a 24 h pre-treatment.
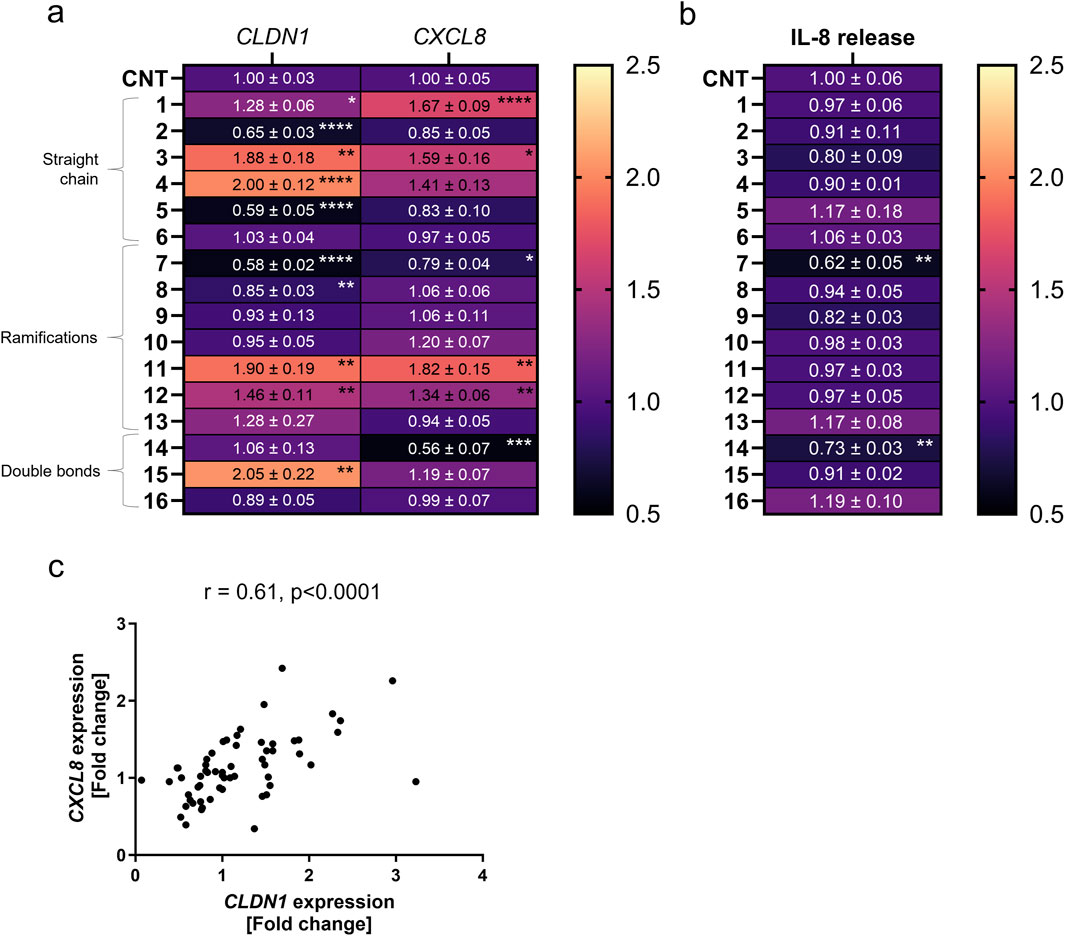
Figure 2. CXCL8 and CLDN1 gene expression is differentially influenced by pre-treatment with various homovanillic esters dependent on the structure of the side chain. HaCaT cells were pre-treated with 10 µM of compounds 1–16 before TNFα treatment for 6 h. (a) CXCL8 and CLDN1 gene expression in cells pre-treated with compounds 1-6 of increasing side chain length, compounds 7-13 with side chain ramifications and compounds 14-16 with side chains with double bonds. (b) IL-8 release in cells pre-treated with compounds 1-16. (c) Moderate correlation (r = 0.61) between the expression of both CXCL8 and CLDN1. CXCL8 and CLDN1 gene expression were measured using RT-qPCR and IL-8 release was measured using ELISA. Data presented as the fold change to the non-pretreated TNFα control (CNT) in a magma heatmap with an upper fold change limit of 2.5 (light orange) and a lower fold change limit of 0.5 (black). [Statistics: mean + SEM; technical replicates: 3, biological replicates: 4; Brown-Forsythe and Welch’s ANOVA with Dunnett’s T3 multiple comparisons post hoc test, *p < 0.05; **p < 0.01, ***p < 0.001, ****p < 0.0001; (c) Spearman correlation].
3.2 Structural characteristics in the homovanillic ester aliphatic tail can be modified to promote CLDN1 gene expression and reduce CXCL8 expression or IL-8 release
We then investigated how structural modifications of the homovanillic acid ester aliphatic tail—specifically chain length, branching, and double bonds—affect CXCL8 and CLDN1 gene expression (Figure 2a). The number of C-atoms in the aliphatic tail is provided in Table 2. The longest chains had 10 C-atoms in total, with the longest main chain comprising 8 C-atoms. Any tendencies in the structural features were evaluated considering all compounds and within individual groups classified by the chain length (1-6), ramification points (7-13) and double bonds (14-16).
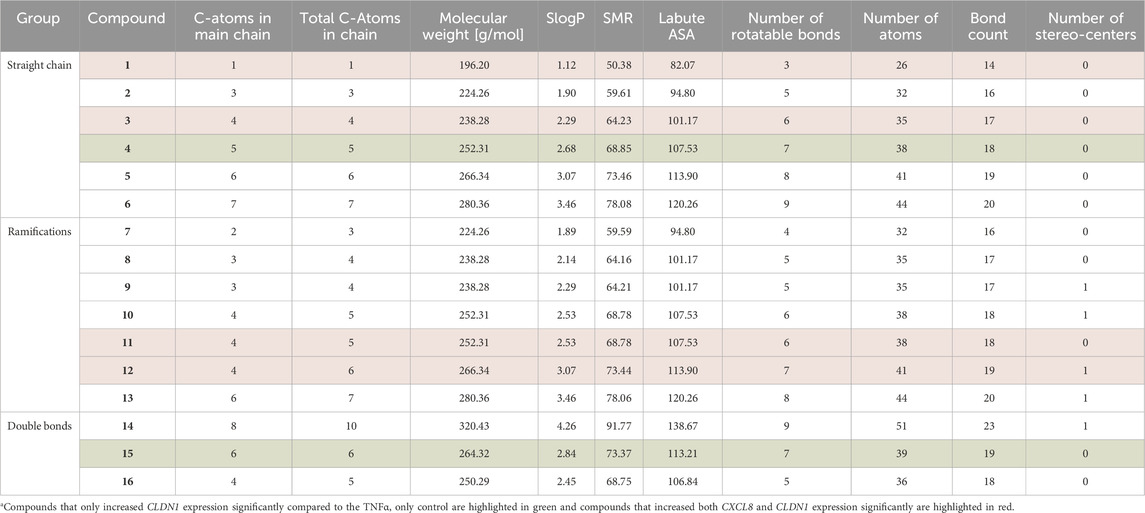
Table 2. Physicochemical descriptors of compounds 1-16 obtained through the RD kit in the KNIME analytics platform.
Treatment with compounds 3, 11 and 12 upregulated CXCL8 expression by a fold change of 1.59 ± 0.16, 1.82 ± 0.15 and 1.34 ± 0.06 compared to the TNFα control. These three compounds share the common feature of having 4 C-atoms in the main chain of the homovanillic acid ester tail. Compounds 10 and 4 also had the tendency to increase CXCL8 expression, albeit not significant, and shared in common with compound 11 the number of five total C-atoms in the tail structure. CXCL8 expression was upregulated by compounds with or without ramifications in the aliphatic chain but double bonds were not present in any of the compounds that increased CXCL8 expression. On the other hand, downregulation of CXCL8 was achieved when the cells were treated with compounds 7 (0.79 ± 0.04) and 14 (0.56 ± 0.07). These compounds did not share features other than the presence of ramifications in the chain. Both compounds also reduced IL-8 release by fold changes of 0.62 ± 0.05 and 0.73 ± 0.03 against control. Notably, only compounds that downregulated CXCL8 expression had a consequential effect on IL-8 release. The lowest value in the IL-8 release was obtained with the use of compound 14 which had the longest tail structure with 8 C-atoms in the main chain, 10 C-atoms in total. Therefore, CXCL8 expression was increased by compounds with predominantly 4–5 C-atoms in the aliphatic tail and downregulation of this gene did not show any distinctive pattern for structural features.
CLDN1 expression increased with the total number of C-atoms until the homovanillic acid ester tail had 5 C-atoms (straight chain group) or 6 C-atoms (ramifications or double bond groups) reaching the highest fold change versus TNFα control in compound 15 (2.05 ± 0.22) with 6 C-atoms and double bond in the aliphatic chain (Figure 2a). The lowest values were seen for aliphatic tails with 3 (C3) C-atoms. In the straight chain group, CLDN1 expression reached its highest values after treatment with 4 (C5) with a fold change of 2.00 ± 0.12 against the TNFα control. Within the ramification group, compound 11 (C5) induced the highest CLDN1 expression with a 1.90 ± 0.20 -fold increase in comparison to non-pre-treated cells followed by 12 (C6) and 13 (C7) with 1.46 ± 0.11 and 1.28 ± 0.27, respectively. Finally, the highest CLDN1 expression within the double bond group was induced by compound 15, with 6 C-atoms in the chain and one double bond, as it reached a 2.05 ± 0.22 -fold increase in comparison to the TNFα control. Thus, among the homovanillic esters tested in this study, the highest CLDN1 expression was induced with compounds with 5 or 6 C-atoms in the aliphatic tail.
Modulation of the CLDN1 gene expression did not follow a reproducible pattern when considering differences in tail structure for homovanillic acid esters from the ramifications (7-13) and the double bond (14-16) groups. Within the ramification group, the lowest values in CLDN1 expression are found after treatment with compounds 7 (0.58 ± 0.02) and 8 (0.85 ± 0.03), which have smaller side chains, and the largest after treatment with compounds 12 (1.46 ± 0.11) and 11 (1.90 ± 0.20), with 6 and 5 total C-atoms in the side chain. For compounds 8 and 10, the ramification was positioned in the second C-atom after the ester bond and both had similar fold change difference to the TNFα for CLDN1 expression with 0.85 ± 0.03 and 0.95 ± 0.05, respectively. For the isomers 10 and 11, the ramification changes from the second C-atom (10) after the ester bond to the third (11), but the fold change in CLDN1 expression is twice as high after treatment with 11. When considering the double bond group (14-16), the highest CLDN1 expression change was caused by 15 (2.05 ± 0.22) with 6 C-atoms in the chain and a double bond in third position after the ester bond. Compound 16 (0.89 ± 0.05), which has the same number of C-atoms as compound 11 (1.90 ± 0.20) but has a double bond in the second position after the ester bond, did not increase CLDN1 expression. Thus, ramifications or double bonds are no determinant for increased CLDN1 expression, although addition of ramifications or double bonds in the third position after the ester bond are characteristic of the homovanillic acid esters in the collection that favor upregulation of CLDN1 compared to the TNFα control.
Lastly, several physicochemical factors were calculated using the RDKit node on the KNIME analytics platform 5 (Table 2). Because the compound that downregulated CXCL8 expression the most was the largest compound, 14, it follows that the molecular weight, SlogP, SMR, Labout ASA, number of rotatable bonds, number of atoms, bond count and largest chain size would be more likely to favor downregulation of the gene. Compounds 6, 13 and 14 were the largest compounds within each category (unbranched, ramified and double bonded) but only compound 14 downregulated CXCL8 significantly. Nevertheless, no compound with a molecular weight higher than 280.36 g/mol upregulated CXCL8 expression. Among the factors that did not show any distinct patterns related to changes in CLDN1 expression were the molecular weight, the SlogP, the SMR, the Labout ASA, the number of atoms, the bond count and the number of stereocenters (Table 2). However, within the category of the number of rotatable bonds compounds with 6 and 7 rotatable bonds, except for 10, produced the highest fold change increase in CLDN1 expression compared to the control (Table 2). Thus, the number of rotatable bonds is an important characteristic of the homovanillic acid esters in this study that contributed to the upregulation of CLDN1 gene expression.
3.3 The TRPV1-inhibitor capsazepine did not affect the effects elicited by homovanillic acid esters
To test whether these achieved effects were TRPV1-dependent, compounds 4, 14 and 15 were applied as a 24 h pre-treatment in a concentration of 10 µM and in combination with 1 µM of the TRPV1 antagonist capsazepine (Cervantes Recalde et al., 2024). Compounds 4 and 15 increased CLDN1 gene expression the most among the 16 evaluated compounds, whereas compound 14 attenuated IL-8 expression and release. Co-treatment with the TRPV1 antagonist capsazepine did not downregulate the CLDN1 expression caused by any of the compounds, but 14 and 15 showed an additive effect by a 1.08 ± 0.02 and 1.30 ± 0.07-fold increase compared to cells that were treated with the compounds solely (Figure 3a–c). IL-8 release was not altered by the addition of capsazepine to the pre-treatment (Figure 3d–f). As a result, the regulation of CLDN1 gene expression by 4, 14 and 15 is not dependent on the TRPV1 channel nor is the decrease in IL-8 release by 14. Homovanillic acid esters, therefore, regulate CLDN1 expression through an inflammatory pathway, possibly connected but not reliant on TRPV1 activation.
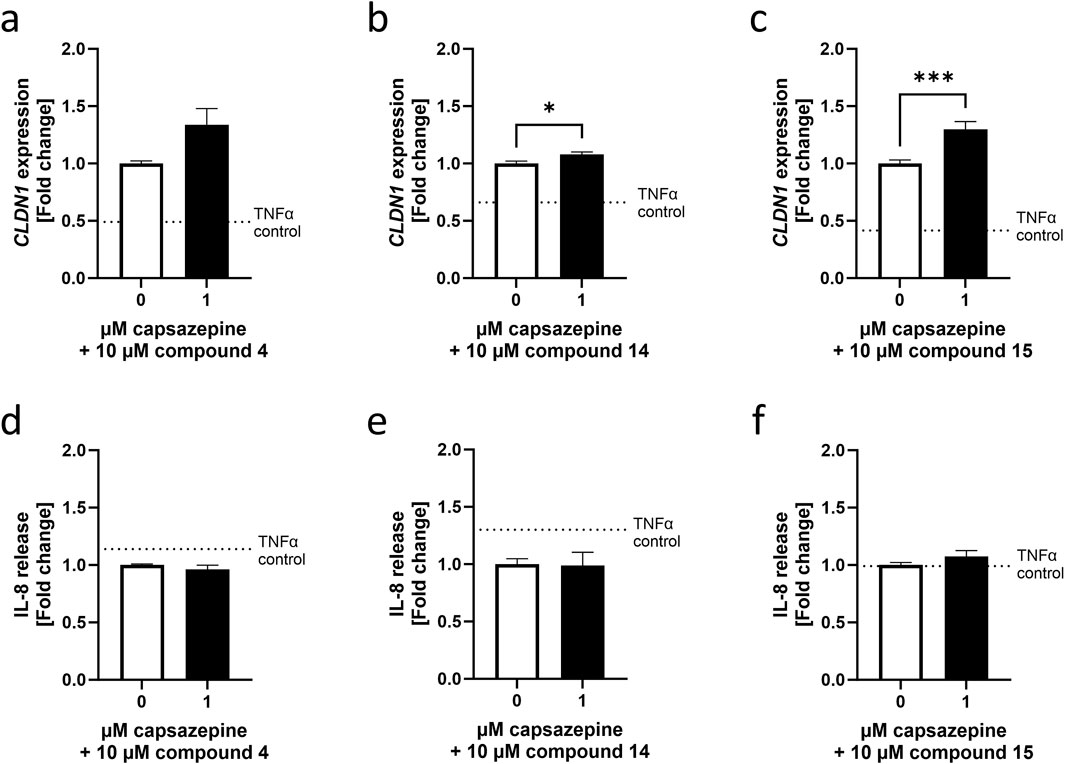
Figure 3. Homovanillic ester effect on CLDN1 expression is independent from TRPV1 channel. A 10 µM pre-treatment of HaCaT cells with compound 4, compound 14 or compound 15 in combination with 1 µM TRPV1 antagonist capsazepine was performed in HaCaT cells before induction of inflammation with 20 ng/mL TNFα. Combination of capsazepine with compound 4 resulted in no significant changes in (a) CLDN1 gene expression or (d) IL-8 release compared to cells pre-treated without capsazepine. Combination of capsazepine with compound 14 resulted in (b) significantly increased CLDN1 expression but (e) no alteration of IL-8 release in comparison to the samples that did not receive capsazepine in the pre-treatment. Finally, CLDN1 was significantly increased in samples pre-treated with (c) a combination of compound 15 and capsazepine compared to samples only treated with compound 15 but there was no significant change in IL-8 release (f). White bars represent the values obtained by pre-treatment with the compounds alone and black bars represent values obtained from cells pre-treated with the respective compound in combination with capsazepine. CLDN1 was measured using RT-qPCR and IL-8 release was measured using ELISA. Data presented as fold change of the control pre-treatment without capsazepine. (Statistics: mean + SEM; technical replicates: 3, biological replicates: 5; (a) Mann–Whitney t-test (b) unpaired t-test or (c–e) Welch’s t-test, *p < 0.05, ***p < 0.001).
3.4 Regulation of CLDN1 expression correlates with the expression of differentiation marker IVL but not with the basal keratinocyte marker KRT14
The NF-κB pathway is activated by TNFα during inflammation and is known to regulate keratinocyte differentiation (Banno et al., 2005). Because TJs are a characteristic of differentiated keratinocytes populating the SG (McGrath et al., 2010), we investigated whether the increase in CLDN1 expression may be sign of the keratinocytes shifting towards a differentiated state. Two well-documented markers of differentiation used in HaCaT cells are IVL and KRT14 (Watt, 1983; Fuchs, 1993; Ghahary et al., 2001). Whilst IVL expression increases in keratinocytes that have left the basal epidermal layer, KRT14 is limited to basal layer keratinocytes (Watt, 1983; Fuchs, 1993). These markers were selected to assess the tendency towards differentiation of HaCaT keratinocytes after establishing the effect of a 6 h TNFα treatment (20 ng/mL) on their gene expression (Supplementary Figure S3). This was followed by the evaluation of a 24 h pre-treatment with four of the compounds that upregulated CLDN1 expression (i.e. 3, 4, 11 and 15) and one compound that downregulated CLDN1 expression (i.e. 7), respectively, and the results were calculated as fold change to the TNFα control (Figure 4a).
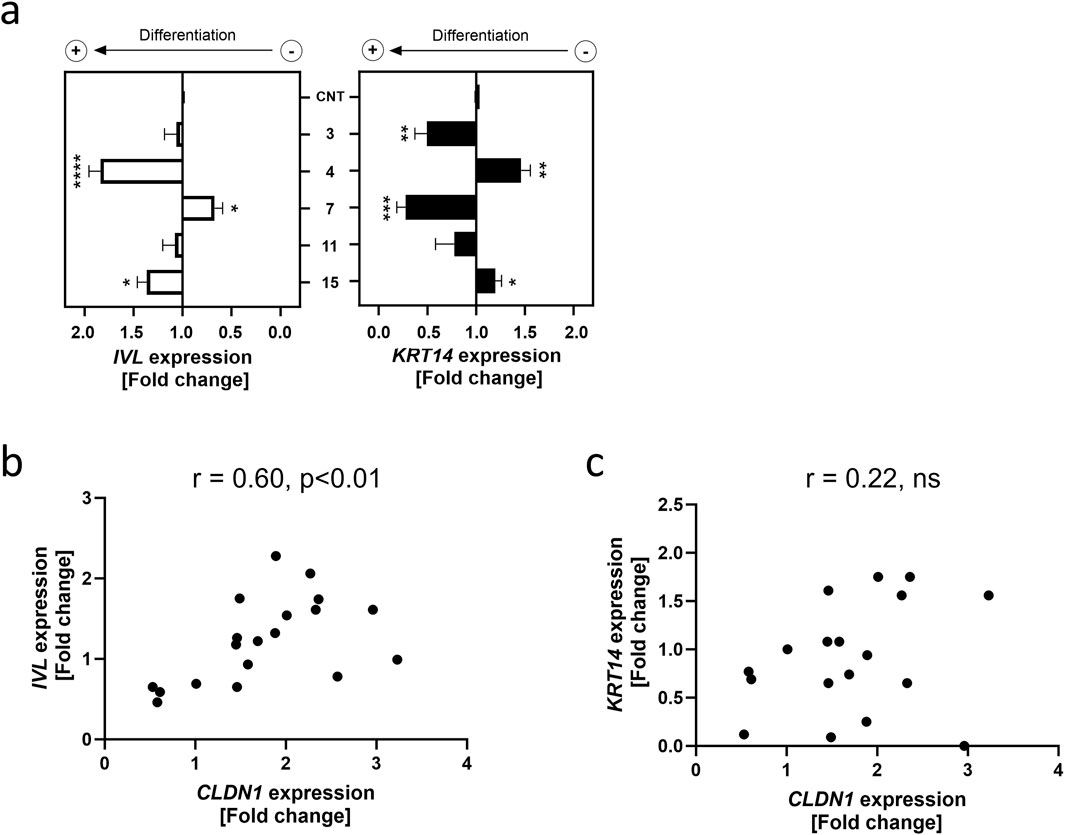
Figure 4. CLDN1 expression correlates to differentiation marker gene expression. (a) IVL and KRT14 gene expression after a 24 h treatment with compounds 3, 4, 7, 11 and 15 at a common concentration of 10 µM or without pre-treatment (CNT) followed by a 6 h treatment with 20 ng/mL TNFα. Data presented as the fold change to the non-pretreated TNFα control (CNT). (b) Correlation plot of IVL expression against CLDN1 expression (c) Correlation plot of KRT14 expression against CLDN1 expression. IVL and KRT14 gene expressions were measured using RT-qPCR. [Statistics: mean + SEM; technical replicates: 3, biological replicates: 4; (a) Brown-Forsythe and Welch’s ANOVA with Dunnett’s T3 multiple comparisons post hoc test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; (b,c) Pearson correlation].
The SG differentiation marker IVL was upregulated 1.84 ± 0.12-fold by a pre-treatment with 4 and 1.37 ± 0.10-fold by a pre-treatment with 15 compared to the TNFα control. Compound 7 downregulated IVL expression to 0.67 ± 0.08-fold of the TNFα control. Contrastingly, the basal layer marker KRT14 was significantly upregulated by a 1.46 ± 0.10-fold change to the TNFα control in cells pre-treated with 4 and downregulated in cells treated with 3 and 7 to a fold change of 0.50 ± 0.12 and 0.28 ± 0.09 of the TNFα control, respectively. There was a significant correlation between CLDN1 and IVL gene expression with a rs value of 0.60 (p < 0.01), however, no association between CLDN1 expression and KRT14 was found (Figures 4b,c). These results show that aliphatic tail modifications of homovanillic acid esters had an effect on CLDN1 expression which is associated with changes in the differentiation marker IVL, but not KRT14.
4 Discussion
The TJ protein CLDN1 is essential for the formation of the TJ in the SG and for their role in epidermal permeability and therefore constitutes an important target for skin barrier recovery (Sugawara et al., 2013). During inflammation, CLDN1 is upregulated presumably as a consequence of the promotion of differentiation elicited by the TNFα-mediated activation of the NF-κB pathway (Cervantes Recalde et al., 2024; Banno et al., 2005; Bhat et al., 2016) and can be further potentiated by activation of the TRPV1 channel (Cervantes Recalde et al., 2024). Although promising, capsaicin and other known TRPV1 ligands may trigger an undesired pain response through their action on neuronal TRPV1 channels present in the skin. The identification of structural components that support CLDN1 production without eliciting negative side effects can pave the way for the development of safe, non-irritating active compounds for the promotion of the skin barrier function. This study focused on the potential of homovanillic acid esters, non-pungent capsaicin analogues, to alter the expression of CLDN1 in keratinocytes stimulated with TNFα. A total of 16 compounds were selected according to the “head, neck and tail” structural moieties characteristic of TRPV1 ligands (Caballero, 2022; Yang et al., 2015), and analyzed based on the modifications that potentially contribute to higher levels of CLDN1 expression in HaCaT keratinocytes of basal epidermal layer morphology. Compounds 1-16 all shared an homovanillic acid ring as “head” and an ester bond as “neck” but the “tail” structures vary according to length, ramifications and double bonds. We hypothesized that structural features in the tail of the compounds are related to higher levels of CLDN1 expression in undifferentiated HaCaT keratinocytes when used as a 24 h treatment preceding 6 h TNFα-mediated inflammation and this increase is independent of TRPV1.
In a previous study, we demonstrated that a 24 h pre-treatment with 10 µM capsaicin before TNFα-induced inflammation reduced the release of the inflammatory cytokine IL-8 after 6 h by approximately 40% and this was accompanied by a marked increase in CLDN1 gene expression (Cervantes Recalde et al., 2024). To test whether these effects could be also achieved using less pungent structurally related compounds, we investigated the impact on CLDN1 and CXCL8 expression as well as IL-8 release of a 24 h pre-treatment with the homovanillic acid esters 1-16 with different aliphatic tail structures varying in lengths, ramifications and double bonds. Regulation of CLDN1 was evaluated 6 h after induction of inflammation by TNFα. Baseline levels of TNFα had indeed strong increases in the expression of CLDN1 and CXCL8 already at 6 h after induction (Supplementary Figure S2). The shortest tail structure had 1 C-atom (1) whilst the largest structure had 8 C-atoms in the carbon chain (14). Out of the 16 homovanillic acid esters, 10 modulated CLDN1 expression. Compounds 2, 5, 7 and 8 reduced CLDN1 expression, whereas compounds 1, 3, 4, 11, 12, and 15 upregulated its expression. CXCL8 expression was upregulated by compounds 1, 3, 11, 12 and downregulated by compounds 7 and 14. Compounds 7 and 14 also reduced IL-8 release. When SAR analysis was conducted to identify structural components in the hydrophobic tail of the homovanillic acid esters, it was discovered that the most conducive structural characteristic towards higher levels of CLDN1 expression was a tail size of 5 or 6 C-atoms with 6 or 7 rotatable bonds. Modifications in the tail that reduced the size or amount of rotatable bonds to these numbers potentiated CLDN1 expression. Similar effects were found with the addition of ramifications or double bonds in the third position after the ester bond. CXCL8 was predominantly upregulated by compounds with 4 C-atoms in the main aliphatic chain or 5 total C-atoms in the tail structure. The largest compound, 14, was also the compound that downregulated CXCL8 and IL-8 release the most out of all compounds. Therefore, homovanillic acid ester structures targeting CLDN1 upregulation might benefit from a design with a shorter aliphatic tail, whereas structures targeting CXCL8 downregulation would require larger tail moieties to avoid enhancing the expression of this gene during TNFα induced inflammation.
However, short aliphatic tails in TRPV1 ligands result in a reduced affinity to the channel (Gavva et al., 2004), raising the possibility that the TRPV1 channel is not involved in eliciting the observed effects. To evaluate this, the participation of the channel was tested by using the TRPV1 antagonist capsazepine in combination with compounds 4 and 15, which upregulated CLDN1 expression, and compound 14 which reduced IL-8 release after induction of inflammation with TNFα. The participation of TRPV1 in these effects could not be verified as the addition of capsazepine to the compound pre-treatment did not counteract the effect elicited by the compound alone but potentiated the increase in CLDN1 expression even further. The additive effect on CLDN1 expression seen in cells due to capsazepine suggests that the TRPV1 antagonist may contribute to the regulation of CLDN1 through a synergistic mechanism extending beyond direct TRPV1 channel interaction. Further experiments using a TRPV1 knock out or a gene silencing model can deliver more accurate information on the participation of the channel in these effects.
By further considering the gene expression tendencies shown by the selected compounds it was discovered that CXCL8 and CLDN1 expression showed a moderate correlation. This correlation could be explained by their downstream position to TNFα in the NF-κB and mitogen-activated protein kinase (MAPK) pathways (Figure 5) (Chen et al., 2018; Sabio and Davis, 2014; Webster and Vucic, 2020). TNFα activates the NF-kB pathway in keratinocytes, mediating the release of pro-inflammatory cytokines such as IL-8 (Chen et al., 2018; Banno et al., 2005). The MAPK pathway is also activated leading to pro-inflammatory cytokine expression and cell differentiation accompanied by TJ protein expression (Qi and Elion, 2005; Bongki and Breton, 2016). The alterations in CLDN1 expression induced by homovanillic acid esters during inflammation, therefore, could be attributed to enhanced differentiation of keratinocytes due to the necessary production of TJ proteins like CLDN1 in the burgeoning SG. To investigate this, we selected markers for differentiation (IVL) and proliferation (KRT14) and assessed whether the homovanillic acid esters promoted differentiation or whether they reinforced the proliferative state. IVL is a precursor protein of the cornified envelope (or SC) and is found predominantly in the outer layer of skin biopsies (Watt, 1983; Murphy et al., 1984). KRT14 is a widely used marker to identify cells in basal layers of the skin that are not undergoing differentiation but are rather more likely to remain in the basal layer and undergo proliferation (Fuchs, 1993). The baseline values of a TNFα treatment for 6 h correspond to an increased IVL expression compared to the untreated control as well as a downregulation of KRT14 expression vs. untreated control consistent with the previously documented effect of TNFα in keratinocytes where differentiation is promoted and proliferation inhibited (Banno et al., 2005) (Supplementary Figure S3). After pre-treatment with selected homovanillic acid compounds, IVL was downregulated by 7 as well as upregulated by 4 and 15, corresponding to the tendency of these compounds to alter CLDN1 expression in comparison to the TNFα treatment as control. On the other hand, KRT14 expression was upregulated by compound 4 and downregulated by compounds 3 and 7. The expression of IVL was correlated to the expression pattern of CLDN1, but KRT14 did not show any correlation. This suggests that the regulation of CLDN1 expression by compounds 7, 4 and 15 acts on components of the NF-κB and MAPK pathways related to keratinocyte differentiation (Banno et al., 2005; Bongki and Breton, 2016; Li et al., 2021; Shin et al., 2013).
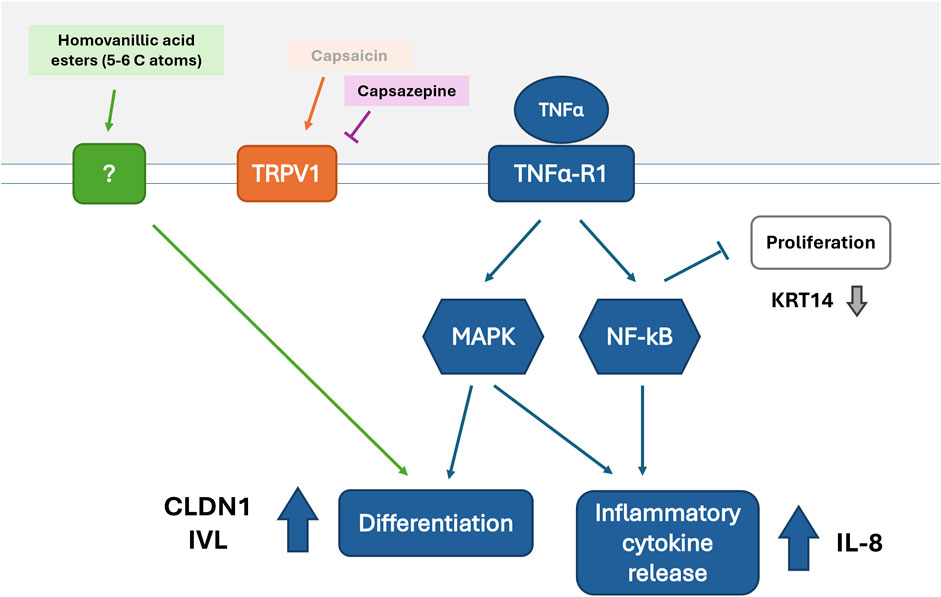
Figure 5. Potential mechanism of action for homovanillic acid esters leading to increased CLDN1 expression during TNFα induced inflammation.
Considering all this, we propose a possible mechanism of action (Figure 5) where upregulation of CLDN1 and CXCL8 is seen during inflammation induced by TNFα through the activation of the NF-κB and MAPK pathways and the consequential increase in inflammatory cytokine release (e.g., IL-8) and differentiation (Sabio and Davis, 2014; Webster and Vucic, 2020). The pathways leading to differentiation are strengthened by the inhibition of keratinocyte proliferation through NF-κB’s action (Takao et al., 2003). The increase in differentiation promotes TJ protein expression (CLDN1) as well as the expression of other proteins required to form the cornified envelope or SC (e.g., IVL). On the other hand, downregulation of proliferative activity leads to decrease or stagnation of the basal production of keratins (e.g., keratin 14) by keratinocytes. Treatment with homovanillic acid esters with aliphatic tails of 5–6 C-atoms intervenes components regulating differentiation downstream of TNFα by an alternative mechanism that does not require TRPV1 activation resulting in increased expression of CLDN1. Future studies should explore this hypothesized mechanism by investigating individual components of the NF-kB and MAPK pathways.
The evaluation of the structural features of the homovanillic acid esters in this study led to non-pungent capsaicin analog structures that promoted CLDN1 expression and provided information on characteristic features that lead to elevated gene expression levels of this TJ protein. Our findings on CLDN1 promotion by these compounds show that comparable effect sizes to those of capsaicin can be achieved (Cervantes Recalde et al., 2024), but direct comparisons of capsaicin and homovanillic acid esters would be needed to confirm the effectivity of the compounds in relation to well-known TRPV1 ligands. This, in combination with a broader selection of structural modifications in the homovanillic acid ester molecule as well as metabolites stemming from esterase activity would enable a more holistic picture on which molecular characteristics lead to targeted modulation of CLDN1. This study focused exclusively on the effects of the selected homovanillic acid esters on keratinocytes after a 6-h pre-treatment with TNFα, leaving the effects of the individual compounds on untreated keratinocytes unexplored. However, the increases of CXCL8 and CLDN1 expression due to the TNFα treatment were overwhelmingly superior to that of capsaicin (Supplementary Figure S2). This suggests that the effect size of homovanillic acids on healthy keratinocytes might be overpowered by TNFα, but this needs to be confirmed through experimentation.
Furthermore, the study design aimed to study compounds with modifications that purposedly reduce the affinity for the TRPV1 channel in order to determine whether non-pungent compounds affected CLDN1 gene expression during inflammation. This marker was used for its robustness and prevalence in both inflammation and TJ function (Furuse et al., 2002; Bergmann et al., 2020; Arnold et al., 2024). Nevertheless, we recognize the need for further research on a wider variety of TJ proteins, in particular ZO-1 and claudin-4, to further substantiate these findings and provide a more comprehensive understanding of the effect of homovanillic acid ester pre-treatment on TJs. This is also required regarding the use of IVL and KRT14 as markers for keratinocyte differentiation versus proliferation, which could be complemented by the inclusion of other differentiation markers like transglutaminase-1, loricrin or filaggrin. It is also important to mention that a 6 h timeframe is likely not long enough to verify keratinocyte differentiation with morphological changes and protein abundance measurements. In our previous work, CLDN1 protein abundance changes were only verifiable 48 h after induction of TNFα, and morphological changes regarding differentiation were limited (Cervantes Recalde et al., 2024; Saha et al., 2022). Longer incubation times would be required in future to explore the mechanisms that lead to the effects of homovanillic acid ester treatment on keratinocyte differentiation during inflammation. Notwithstanding this, we do believe a closer look towards the effects of phenolic compounds on keratinocyte differentiation needs to be taken, because of the promising data presented in this and other studies (Petersen et al., 2011; Wang et al., 2014).
This study provides novel evidence for homovanillic acid esters with characteristic structural motifs, e.g., 5 or 6 C-atoms in the aliphatic ester tail, to have the potential to support skin barrier recovery and TJ development. The suggested intervention of homovanillic acid esters in differentiation-related pathways invites an extended investigation and further characterization in vitro and ex vivo experiments could lead the discovery of important molecules for effective skin repair treatments that can bypass capsaicin’s adverse effects.
In conclusion, the structure-activity relationship between homovanillic acids and CLDN1 expression was determined in HaCaT keratinocytes of basal layer morphology at early stages of TNFα-induced inflammation. CLDN1 expression was modulated by several of the tested homovanillic acid esters without involvement of the TRPV1 channel. The structural characteristics of compounds that upregulated CLDN1 expression were 5 or 6 C-atoms in the aliphatic tail of the ester with ramifications or double bonds in the third C-atom after the ester bond. Because of the short length of the aliphatic tail, it is possible that the affinity for the TRPV1 channel is reduced in comparison with known TRPV1 ligands. Nevertheless, an alternative NF-κB pathway regulation by the compounds targeting differentiation of HaCaT keratinocytes as evidenced by the increase in IVL expression is conceivable. This gene is not expressed in basal keratinocytes but was induced in correlation to the expression of CLDN1 advocating for differentiation towards SG morphology where TJs are formed. This presents a first hint on the potential of homovanillic acid esters to aid in epidermal barrier recovery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
MC: Formal Analysis, Data curation, Methodology, Writing – original draft, Visualization, Investigation, Conceptualization. EB: Writing – review and editing, Investigation. JH: Project administration, Writing – review and editing, Data curation, Conceptualization. DS: Resources, Conceptualization, Writing – review and editing. VS: Conceptualization, Writing – review and editing, Funding acquisition, Supervision, Resources. BL: Supervision, Conceptualization, Project administration, Writing – review and editing, Funding acquisition, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by Symrise AG. The Symrise AG had no role in the data collection, statistical analyses, interpretation, and the decision to publish the results. Publishing fees were supported by the Funding Programme Open Access Publishing of the University of Hohenheim.
Acknowledgments
We are grateful to Symrise AG, Germany, for the financial support and providing the homovanillic acid esters used in the present study.
Conflict of interest
Authors JH and DS were employed by Symrise AG.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1629941/full#supplementary-material
References
Appendino, G., Cravotto, G., Palmisano, G., Annunziata, R., and Szallasi, A. (1996). Synthesis and evaluation of phorboid 20-homovanillates: discovery of a class of ligands binding to the vanilloid (capsaicin) receptor with different degrees of cooperativity. J. Med. Chem. 39 (16), 3123–3131. doi:10.1021/jm960063l
Arnold, K. A., Moran, M. C., Shi, H., van Vlijmen-Willems, I. M. J. J., Rodijk-Olthuis, D., Smits, J. P. H., et al. (2024). CLDN1 knock out keratinocytes as a model to investigate multiple skin disorders. Exp. Dermatol. 33 (5), e15084. doi:10.1111/exd.15084
Banno, T., Gazel, A., and Blumenberg, M. (2004). Effects of tumor necrosis Factor-α (TNFα) in epidermal keratinocytes revealed using global transcriptional profiling. J. Biol. Chem. 279 (31), 32633–32642. doi:10.1074/jbc.M400642200
Banno, T., Gazel, A., and Blumenberg, M. (2005). Pathway-specific profiling identifies the NF-κB-dependent tumor necrosis factor α-regulated genes in epidermal keratinocytes. J. Biol. Chem. 280 (19), 18973–18980. doi:10.1074/jbc.M411758200
Baskaran, P., Covington, K., Bennis, J., Mohandass, A., Lehmann, T., and Thyagarajan, B. (2018). Binding efficacy and thermogenic efficiency of pungent and nonpungent analogs of capsaicin. Molecules 23 (12), 3198. doi:10.3390/molecules23123198
Bergmann, S., Von Buenau, B., Vidal-Y-Sy, S., Haftek, M., Wladykowski, E., Houdek, P., et al. (2020). Claudin-1 decrease impacts epidermal barrier function in atopic dermatitis lesions dose-dependently. Sci. Rep. 10 (1), 2024. doi:10.1038/s41598-020-58718-9
Berthold, M. R., Cebron, N., Dill, F., Gabriel, T. R., Kötter, T., Meinl, T., et al. (2008). KNIME: the konstanz information miner. Berlin, Heidelberg: Springer, 319–326.
Bhat, A. A., Ahmad, R., Uppada, S. B., Singh, A. B., and Dhawan, P. (2016). Claudin-1 promotes TNF-α-induced epithelial-mesenchymal transition and migration in colorectal adenocarcinoma cells. Exp. Cell Res. 349 (1), 119–127. doi:10.1016/j.yexcr.2016.10.005
Bongki, K. S. B., and Breton, S. (2016). The MAPK/ERK-Signaling pathway regulates the expression and distribution of tight junction proteins in the mouse proximal epididymis. Biol. Reproduction 94 (1), 22. doi:10.1095/biolreprod.115.134965
Caballero, J. (2022). A new era for the design of TRPV1 antagonists and agonists with the use of structural information and molecular docking of capsaicin-like compounds. J. Enzyme Inhibition Med. Chem. 37 (1), 2169–2178. doi:10.1080/14756366.2022.2110089
Cervantes Recalde, M. F., Schmidt, J., Girardi, C., Massironi, M., Rechl, M. L., Hans, J., et al. (2024). Capsaicin attenuates the effect of inflammatory cytokines in a HaCaT cell model for basal keratinocytes. Front. Pharmacol. 15, 1474898. doi:10.3389/fphar.2024.1474898
Chen, K., Feng, L., Feng, S., Yan, Y., Ge, Z., Li, Z., et al. (2019). Multiple quantitative structure–pungency correlations of capsaicinoids. Food Chem. 283, 611–620. doi:10.1016/j.foodchem.2019.01.078
Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., et al. (2018). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9 (6), 7204–7218. doi:10.18632/oncotarget.23208
Elokely, K., Velisetty, P., Delemotte, L., Palovcak, E., Klein, M. L., Rohacs, T., et al. (2016). Understanding TRPV1 activation by ligands: insights from the binding modes of capsaicin and resiniferatoxin. Proc. Natl. Acad. Sci. U. S. A. 113 (2), E137–E145. doi:10.1073/pnas.1517288113
Fuchs, E. (1993). Epidermal differentiation and keratin gene expression. J. Cell Sci. 1993 (17), 197–208. doi:10.1242/jcs.1993.supplement_17.28
Furuse, M., Hata, M., Furuse, K., Yoshida, Y., Haratake, A., Sugitani, Y., et al. (2002). Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell Biol. 156 (6), 1099–1111. doi:10.1083/jcb.200110122
Gao, W., Wu, D., Wang, Y., Wang, Z., Zou, C., Dai, Y., et al. (2018). Development of a novel and economical agar-based non-adherent three-dimensional culture method for enrichment of cancer stem-like cells. Stem Cell Res. Ther. 9 (1), 243. doi:10.1186/s13287-018-0987-x
Gavva, N. R., Klionsky, L., Qu, Y., Shi, L., Tamir, R., Edenson, S., et al. (2004). Molecular determinants of vanilloid sensitivity in TRPV1. J. Biol. Chem. 279 (19), 20283–20295. doi:10.1074/jbc.M312577200
Ghahary, A., Marcoux, Y., Karimi-Busheri, F., and Tredget, E. E. (2001). Keratinocyte differentiation inversely regulates the expression of involucrin and transforming growth factor beta1. J. Cell. Biochem. 83 (2), 239–248. doi:10.1002/jcb.1223
Gonzales, C. B., Kirma, N. B., De La Chapa, J. J., Chen, R., Henry, M. A., Luo, S., et al. (2014). Vanilloids induce oral cancer apoptosis independent of TRPV1. Oral Oncol. 50 (5), 437–447. doi:10.1016/j.oraloncology.2013.12.023
Kim, H. S., Kwon, H.-J., Kim, G. E., Cho, M.-H., Yoon, S.-Y., Davies, A. J., et al. (2014). Attenuation of natural killer cell functions by capsaicin through a direct and TRPV1-independent mechanism. Carcinogenesis 35 (7), 1652–1660. doi:10.1093/carcin/bgu091
Kirschner, N., Rosenthal, R., Furuse, M., Moll, I., Fromm, M., and Brandner, J. M. (2013). Contribution of tight junction proteins to ion, macromolecule, and water barrier in keratinocytes. J. Investigative Dermatology 133 (5), 1161–1169. doi:10.1038/jid.2012.507
Kobata, K., Todo, T., Yazawa, S., Iwai, K., and Watanabe, T. (1998). Novel capsaicinoid-like substances, capsiate and dihydrocapsiate, from the fruits of a nonpungent cultivar, CH-19 sweet, of pepper (Capsicum annuum L.). J. Agric. Food Chem. 46 (5), 1695–1697. doi:10.1021/jf980135c
Landrum, G., Tosco, P., Kelley, B., Rodriguez, R., Cosgrove, D., Vianello, R., et al. (2025). RDKit: open-source cheminformatics. Available online at: https://www.rdkit.org.
Li, J., Wang, H., Zhang, L., An, N., Ni, W., Gao, Q., et al. (2021). Capsaicin affects macrophage anti-inflammatory activity via the MAPK and NF-κB signaling pathways. Int. J. Vitam. Nutr. Res. 93 (4), 289–297. doi:10.1024/0300-9831/a000721
Lieder, B., Hans, J., Hentschel, F., Geissler, K., and Ley, J. (2019). Biological evaluation of natural and synthesized homovanillic acid esters as inhibitors of intestinal fatty acid uptake in differentiated Caco-2 cells. Molecules 24 (19), 3599. doi:10.3390/molecules24193599
Lieder, B., Hoi, J., Burian, N., Hans, J., Holik, A.-K., Marquez, L. R. B., et al. (2020). Structure-dependent effects of cinnamaldehyde derivatives on TRPA1-Induced serotonin release in human intestinal cell models. J. Agric. Food Chem. 68 (13), 3924–3932. doi:10.1021/acs.jafc.9b08163
Liu, L., Szallasi, A., and Simon, S. A. (1998). A non-pungent resiniferatoxin analogue, phorbol 12-phenylacetate 13 acetate 20-homovanillate, reveals vanilloid receptor subtypes on rat trigeminal ganglion neurons. Neuroscience 84 (2), 569–581. doi:10.1016/s0306-4522(97)00523-x
Lynn, K. S., Peterson, R. J., and Koval, M. (2020). Ruffles and spikes: control of tight junction morphology and permeability by claudins. Biochimica Biophysica Acta Biomembr. 1862 (9), 183339. doi:10.1016/j.bbamem.2020.183339
Macho, A., Lucena, C., Calzado, M. A., Blanco, M., Donnay, I., Appendino, G., et al. (2000). Phorboid 20-homovanillates induce apoptosis through a VR1-independent mechanism. Chem. Biol. 7 (7), 483–492. doi:10.1016/s1074-5521(00)00132-0
McGrath, J. A., and Uitto, J. (2010). “Anatomy and organization of human skin,” in Rook’s textbook of dermatology. Editors S. B. T. Burns, N. Cox, and C. Griffiths (Oxford, United Kingdom: Blackwell Publishing Ltd.).
Meotti, F. C., Lemos de Andrade, E., and Calixto, J. (2014). TRP modulation by natural compounds. Handb. Exp. Pharmacol. 223, 1177–1238. doi:10.1007/978-3-319-05161-1_19
Motulsky, H. J., and Brown, R. E. (2006). Detecting outliers when fitting data with nonlinear regression – a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinforma. 7 (1), 123. doi:10.1186/1471-2105-7-123
Murphy, G. F., Flynn, T. C., Rice, R. H., and Pinkus, G. S. (1984). Involucrin expression in normal and neoplastic human skin: a marker for keratinocyte differentiation. J. Investigative Dermatology 82 (5), 453–457. doi:10.1111/1523-1747.ep12260945
Nikami, H., Mahmoud, M. E., Shimizu, Y., Shiina, T., Hirayama, H., Iwami, M., et al. (2008). Capsaicin pretreatment attenuates LPS-induced hypothermia through TRPV1-independent mechanisms in chicken. Life Sci. 82 (23-24), 1191–1195. doi:10.1016/j.lfs.2008.04.003
Petersen, R. K., Christensen, K. B., Assimopoulou, A. N., Fretté, X., Papageorgiou, V. P., Kristiansen, K., et al. (2011). Pharmacophore-driven identification of PPARγ agonists from natural sources. J. Computer-Aided Mol. Des. 25 (2), 107–116. doi:10.1007/s10822-010-9398-5
Qi, M., and Elion, E. A. (2005). MAP kinase pathways. J. Cell Sci. 118 (16), 3569–3572. doi:10.1242/jcs.02470
Sabio, G., and Davis, R. J. (2014). TNF and MAP kinase signalling pathways. Seminars Immunol. 26 (3), 237–245. doi:10.1016/j.smim.2014.02.009
Saha, K., Sarkar, D., Khan, U., Karmakar, B. C., Paul, S., Mukhopadhyay, A. K., et al. (2022). Capsaicin inhibits inflammation and gastric damage during H pylori infection by targeting NF-kB–miRNA axis. Pathogens 11 (6), 641. doi:10.3390/pathogens11060641
Shin, Y.-H., Namkoong, E., Choi, S., Bae, J.-S., Jin, M., Hwang, S.-M., et al. (2013). Capsaicin regulates the NF-κB pathway in salivary gland inflammation. J. Dent. Res. 92 (6), 547–552. doi:10.1177/0022034513487376
Soares, D. J., Walker, J., Pignitter, M., Walker, J. M., Imboeck, J. M., Ehrnhoefer-Ressler, M. M., et al. (2014). Pitanga (Eugenia uniflora L.) fruit juice and two major constituents thereof exhibit anti-inflammatory properties in human gingival and oral gum epithelial cells. Food Funct. 5 (11), 2981–2988. doi:10.1039/c4fo00509k
Sugawara, T., Iwamoto, N., Akashi, M., Kojima, T., Hisatsune, J., Sugai, M., et al. (2013). Tight junction dysfunction in the stratum granulosum leads to aberrant stratum corneum barrier function in claudin-1-deficient mice. J. Dermatological Sci. 70 (1), 12–18. doi:10.1016/j.jdermsci.2013.01.002
Takao, J. Y. T., Das, A., Shikano, S., Bonkobara, M., Ariizumi, K., Cruz, P. D., et al. (2003). Expression of NF-kappaB in epidermis and the relationship between NF-kappaB activation and inhibition of keratinocyte growth. Br. J. Dermatology 148 (4), 680–688. doi:10.1046/j.1365-2133.2003.05285.x
Untergasser, A., Ruijter, J. M., Benes, V., and Van Den Hoff, M. J. B. (2021). Web-based LinRegPCR: application for the visualization and analysis of (RT)-qPCR amplification and melting data. BMC Bioinforma. 22 (1), 398. doi:10.1186/s12859-021-04306-1
Walker, J., Ley, J. P., Schwerzler, J., Lieder, B., Beltran, L., Ziemba, P. M., et al. (2017). Nonivamide, a capsaicin analogue, exhibits anti-inflammatory properties in peripheral blood mononuclear cells and U-937 macrophages. Mol. Nutr. Food Res. 61 (2). doi:10.1002/mnfr.201600474
Wang, L. W. B., Pferschy-Wenzig, E. M., Blunder, M., Liu, X., Malainer, C., Blazevic, T., et al. (2014). Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochem. Pharmacol. 92 (1), 73–89. doi:10.1016/j.bcp.2014.07.018
Watt, M. F. (1983). Involucrin and other markers of keratinocyte terminal differentiation. J. Investigative Dermatology 81 (1), 100s–103s. doi:10.1111/1523-1747.ep12540786
Webster, J. D., and Vucic, D. (2020). The balance of TNF mediated pathways regulates inflammatory cell death signaling in healthy and diseased tissues. Front. Cell Dev. Biol. 8, 365. doi:10.3389/fcell.2020.00365
Wilson, V. G. (2014). Growth and differentiation of HaCaT keratinocytes. Methods Mol. Biol. 1195, 33–41. doi:10.1007/7651_2013_42
Yang, F., Xiao, X., Cheng, W., Yang, W., Yu, P., Song, Z., et al. (2015). Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat. Chem. Biol. 11 (7), 518–524. doi:10.1038/nchembio.1835
Keywords: skin, inflammation, TRPV1, claudin 1, homovanillic acid ester
Citation: Cervantes Recalde MF, Bogensperger EZ, Hans J, Stuhlmann D, Somoza V and Lieder B (2025) Role of homovanillic acid esters in the regulation of skin inflammatory pathways and their effect on tight junction protein expression. Front. Pharmacol. 16:1629941. doi: 10.3389/fphar.2025.1629941
Received: 16 May 2025; Accepted: 04 July 2025;
Published: 21 July 2025.
Edited by:
Galina Sud’ina, Lomonosov Moscow State University, RussiaReviewed by:
Kai Wei Tang, Chia Nan University of Pharmacy and Science, TaiwanXinyi Shao, First Affiliated Hospital of Chongqing Medical University, China
Daniel Tortolani, University of Teramo, Italy
Copyright © 2025 Cervantes Recalde, Bogensperger, Hans, Stuhlmann, Somoza and Lieder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara Lieder, YmFyYmFyYS5saWVkZXJAdW5pLWhvaGVuaGVpbS5kZQ==
 Maria Fernanda Cervantes Recalde
Maria Fernanda Cervantes Recalde Elena Zoe Bogensperger1
Elena Zoe Bogensperger1 Joachim Hans
Joachim Hans Barbara Lieder
Barbara Lieder