- 1Department of Neurology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Institute for Brain Disorders, Beijing University of Chinese Medicine, Beijing, China
Background: Multiple sclerosis (MS) is an incurable, chronic, disabling disease that primarily affects young adults. Chinese herbal medicine (CHM) is increasingly recognized as a major form of complementary and alternative medicine; however, there is a limited number of systematic analyses regarding its therapeutic effects on MS.
Aim of the study: This study aimed to evaluate whether CHM, when used alongside conventional treatment, offers additional therapeutic benefits for patients with MS, focusing on clinical efficacy and safety.
Materials and methods: We searched eight databases from their inception until July 2025 to identify eligible randomized controlled trials (RCTs) involving CHM therapy for MS. Key outcomes assessed included the expanded disability status scale (EDSS), annual relapse rate (ARR), neurological signs scores, clinical symptoms scores, and modified fatigue impact scale (MFIS). The risk of bias was evaluated using the Cochrane Handbook for systematic reviews, while quantitative synthesis was performed with RevMan (version 5.4.1) and Stata (version 18.0) software. This review has been registered at the International Prospective Register of Systematic Reviews database (Registration No. CRD42024605890).
Results: This systematic review and meta-analysis included 28 RCTs involving a total of 1,971 participants. The combination of CHM and conventional therapy was superior to conventional therapy alone for individuals with MS. This was evidenced by a decrease in the EDSS (mean difference (MD) = −0.65; 95% confidence interval (CI): −0.96, −0.35; P < 0.0001; MD = −0.65, ARR (relative risk (RR) = 0.48; 95% CI: 0.31, 0.73; P = 0.0007), neurological signs scores (MD = −2.96; 95% CI: −4.52, −1.39; P = 0.0002), and MFIS (MD = −8.55; 95% CI: −10.20, −6.89; P < 0.00001). Additionally, there was an improvement in clinical effects (RR = 1.25; 95% CI: 1.19, 1.32; P < 0.00001). No serious adverse effect was reported.
Conclusion: Although the methodological quality of the included RCTs was relatively suboptimal, CHM therapy combined with conventional therapy manifests a promising effectiveness and safety for managing MS. Therefore, well-designed clinical studies are necessary to provide high-quality evidence.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024605890, identifer CRD42024605890.
1 Introduction
Multiple sclerosis (MS) is an autoimmune disease characterized by demyelinating lesions in the white matter of the central nervous system. It typically manifests between the ages of 18 and 40 (Jakimovski et al., 2024). MS is one of the most common causes of neurological disability among young adults, affecting more females than males (Tian et al., 2020). Although the exact cause of MS remains unclear, factors such as genetic susceptibility, environmental influences, infections, and vitamin D deficiency could increase susceptibility (Waubant et al., 2019). Clinical manifestations include paralysis, numbness, painful spasms, aphasia, visual impairment, ataxia, psychiatric symptoms, or intellectual disabilities (McGinley et al., 2021). According to the type of disease course, MS is divided into relapsing remitting MS, secondary progressive MS, primary progressive MS, and other types (Kolčava et al., 2020).
Despite ongoing advancements in treatment methods, MS remains an incurable, chronic, disabling disease. The primary objectives of treatment are to reduce the frequency of recurrence, reduce life burden, and improve quality of life. During acute exacerbations, hormone therapies are the first-line treatment. In the remission phase, disease-modifying therapies and symptomatic treatments are recommended. However, these treatments may present drawbacks, including a risk of opportunistic infections, secondary autoimmune reactions, and hormone-related side effects (Rae-Grant et al., 2018).
Patients suffering from MS tend to seek help from complementary and alternative medicine (CAM), which is used in 33%–80% of patients with MS in developed countries (Yadav et al., 2014; Liu et al., 2022). In Huangdi Neijing (2005), numerous classical symptom patterns consistent with those observed in MS are documented, such as “wei zheng” (flaccidity syndrome) and “shizhan hunmiao” (blurred vision due to deficiency). Treatment principles in traditional Chinese medicine (TCM) are based on syndrome differentiation, with common patterns including kidney yin deficiency, internal accumulation of turbid toxins, and phlegm-blood stasis. Herbal formulas are selected accordingly to tonify deficiency, eliminate turbid toxins, promote blood circulation, or clear heat and dampness. Modern pharmacological studies suggest that Chinese herbal medicine (CHM), a major form of CAM, may exert immunomodulatory and anti-inflammatory effects, providing a potentially valuable adjunct to conventional therapies in MS management. Baicalin is a bioactive flavonoid compound derived from the root of Scutellaria baicalensis Georgi. Zhang et al. (2015) found that intraperitoneal administration of baicalin (100 mg/kg) for 15 consecutive days significantly improved disease severity in Experimental Autoimmune Encephalomyelitis (EAE) mice induced by Myelin Oligodendrocyte Glycoprotein 35–55, an established animal model of MS. Similarly, matrine is a quinolizidine alkaloid component derived from the root of Sophora flavescens Aiton. Chu et al. (2021) administered matrine at 100 mg/kg intraperitoneally for 11 consecutive days in EAE mice, resulting in significantly reduced disease severity, decreased inflammatory infiltration, and attenuated demyelination. Two meta-analyses (Song et al., 2017; Liu et al., 2012) published 8 years ago indicated that CHM therapy positively affects patients with MS. Given the number of randomized controlled trials (RCTs) conducted in recent years, this study aimed to update the evidence and re-evaluate the impact of CHM therapy on MS.
2 Methods
2.1 Protocol registration
The systematic review and meta-analysis have been registered at the International Prospective Register of Systematic Reviews database (registration No. CRD42024605890). This study was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (Page et al., 2021) and PRISMA-CHM checklist (Zhang et al., 2020).
2.2 Eligibility criteria
1. Types of studies: RCTs were included regardless of language and publication status.
2. Types of participants: Patients diagnosed with MS were included without restrictions of gender, age, nationality, and geographical residence. There were also no limitations regarding disease course, duration, neurological disability, or comorbidities.
3. Types of interventions: The studies that used CHM as the intervention in the experimental group were included. According to The World Health Organization (2021), herbal medicines (HMs) encompass herbs, herbal materials, herbal preparations, and finished herbal products containing active ingredients derived from plants or other plant sources. CHM is a specific subset of HMs that is formulated and prescribed based on TCM, including syndrome differentiation and individualized or standardized herbal formulas. CHM interventions could be administered in various forms such as decoctions, granules, tablets, capsules, or other oral formulations. There were no restrictions on the frequency or dosage of CHM. The treatment course needed to last a minimum of 3 weeks.
4. Types of comparators: The studies that used conventional treatment (CT) with or without placebo as the intervention of the control group were included. If the experimental group received a combined treatment of CHM and CT, the CT should be administered equally across all trial groups.
5. Types of outcome measures: In this study, the Expanded Disability Status Scale (EDSS) was selected as the primary outcome. EDSS is the most widely used and internationally recognized clinical measure for evaluating neurological disability in patients with MS. Developed by Kurtzke (Kurtzke, 1983), the scale comprehensively evaluates eight functional systems, with a strong emphasis on ambulation. Its broad application in clinical trials and sensitivity to disease progression make it a robust indicator for treatment efficacy. Secondary outcomes of interest included annual relapse frequency and annual relapse rate (ARR), neurological signs scores, clinical symptoms scores, modified fatigue impact scale (MFIS), magnetic resonance imaging (new T2 lesions or gadolinium-enhanced lesions, change in volume of T2 lesions), scores of TCM syndrome, cognitive function, the MS functional composite (MSFC), pharmacodynamic biomarkers, clinical effect, and adverse effect.
2.3 Search strategy
Two independent reviewers (GXR and WYF) searched various electronic databases from inception to July 2025. The databases included PubMed, Cochrane Library, Web of Science, EMBASE, the Chinese National Knowledge Infrastructure, Wanfang Database, the Chinese Biomedical Database (SinoMed), and the Chinese Science and Technology Journals Database (VIP). No restrictions were imposed on the language of publication. The search terms used included MS and its synonyms, in combination with CHM or their proprietary names, utilizing MeSH and as free-text words. Chinese databases were also searched using the above search terms in Chinese. The detailed searching strategies are listed in the Supplementary Text (Supplementary Appendix 1).
2.4 Study selection and data extraction
According to prespecified selection criteria, two authors (GXR and WYF) independently reviewed the titles and abstracts of articles after deduplication. The articles that did not fulfil the inclusion criteria were removed. The remaining articles were screened with full text by the same two authors independently. Any disagreements in primary screening were to be discussed and resolved. A third review author (LJ) was consulted if required. For articles that met the inclusion criteria, two authors (GXR and JQY) extracted study characteristics and relevant outcome data. This included the first author’s name, publication year, sample size, age, gender, interventions and comparisons, and the course of treatment. The primary outcome was the EDSS, while secondary outcomes included annual relapse frequency (ARR), neurological signs scores, clinical symptoms scores, MFIS, MRI, scores of TCM syndrome, cognitive function, MSFC, and pharmacodynamic biomarkers. Adverse effects were also recorded. If patient data were reported in multiple articles, only the one with the most complete information was included.
2.5 Quality assessment
We conducted a methodological quality assessment of randomized trials using the Cochrane Handbook for Systematic Reviews of Interventions (Sterne et al., 2019). Two authors (GXR and WYF) independently assessed the risk of bias across five domains: (1) bias arising from the randomization process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in outcome measurement; (5) bias in the selection of reported results. Each domain was classified as having a “High risk,” “Low risk,” or “Some concerns”. According to the ROB 2.0 guidelines, a study was judged to have an overall low risk of bias (RoB) if it was rated as low risk across all domains for a given outcome. Studies with a high risk rating in any domain, or with “some concerns” in three or more domains, were considered to have a high overall RoB. Studies that had “some concerns” in at least one domain, but were not rated as high risk in any domain, were judged to have an overall RoB of “some concerns”. Any disagreements between the reviewers were resolved through discussion or arbitration by a third investigator (JQY).
2.6 Data synthesis
We analyzed the data using the RevMan (version 5.4.1) software. Relative risk (RR) was used as the effect size for binary variables, while the mean difference (MD) or standard mean difference (SMD) was employed for continuous variables. We used 95% confidence intervals (CIs) to represent the effect size interval for both variables. The Cochrane Q test and I2 values were used to assess the heterogeneity between trials. If P < 0.05 or I2 > 50%, the random effect model was selected to calculate the pooled effect size; otherwise, we used the fixed effects model. To visually present the results of the syntheses, we constructed forest plots. Subgroup analysis was conducted to identify possible causes of heterogeneity. A sensitivity analysis was performed by iteratively omitting one study at a time to determine the stability of the pooled effects. Finally, publication bias was quantitatively assessed using funnel plots and Egger’s tests, employing RevMan (version 5.4.1) and Stata (version 18.0) software.
2.7 Quality of evidence
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system was utilized by two independent reviewers to assess the quality of evidence for each outcome indicator. There are five evaluation aspects, including limitations, inconsistency, indirectness, imprecision, and other factors. The evidence was rated across four levels: Very low, low, medium, and high were rated for the above five domains.
3 Results
3.1 Study selection
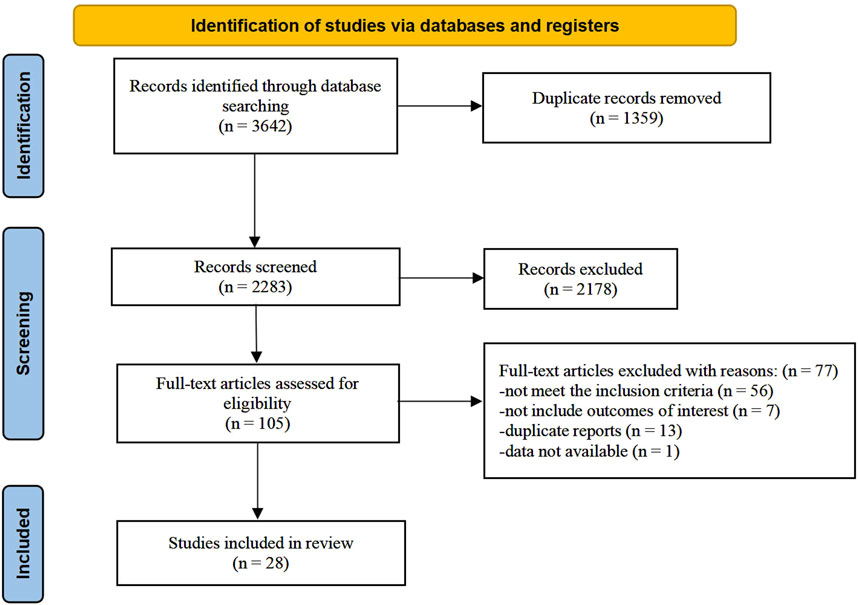
The research selection process is illustrated in Figure 1. According to our strategy, 3,642 records were retrieved. Among these, 1,359 records were removed due to duplication. After screening titles and abstracts, we excluded 2,178 papers for various reasons, including that they were not clinical trials, did not involve Chinese medicine interventions, or did not include participants with MS. We further evaluated 105 full-text articles for eligibility. The following factors led to the exclusion of 77 studies based on the selection criteria: 56 studies did not meet the inclusion criteria; seven studies did not address the relevant outcomes; 13 studies were duplicate reports, and the data of one study were unavailable (Bertoglio et al., 2016). Finally, 28 trials were included in the final analysis.
3.2 Study characteristics
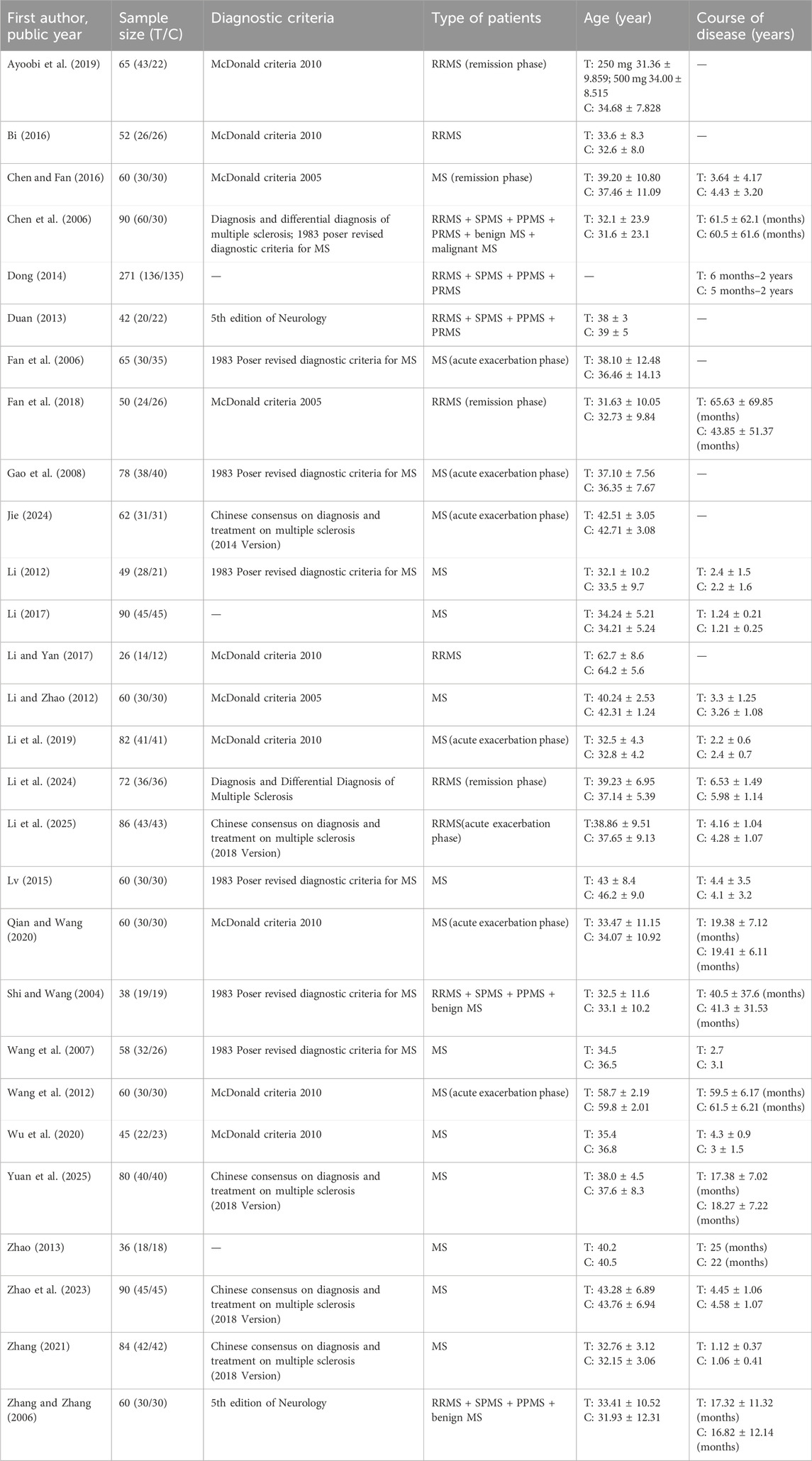
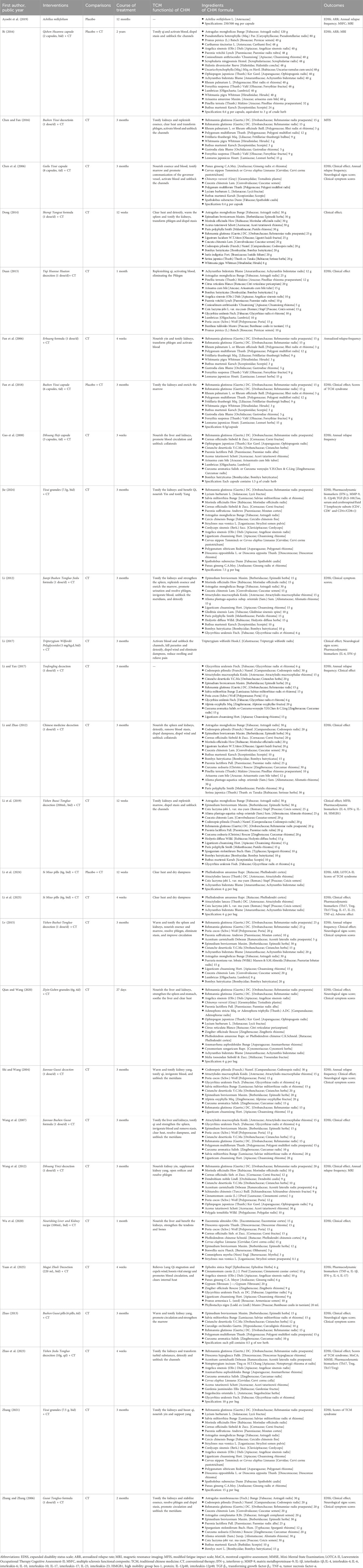
The characteristics of the 28 included RCTs are listed in Table 1. All 28 studies included were randomized clinical trials published between 2004 and 2025. A total of 27 studies were conducted in China, and one was conducted in Iran. Overall, 1971 patients were enrolled in this review, with a sample size ranging from 26 to 271. The intervention group comprised 1,013 patients, while the control group included 958 patients. Among the participants, 65.0% were female, and their ages ranged from 13 to 78 years. The medications used in the intervention group included herbal preparations and commercial Chinese polyherbal preparations. The composition of each herbal formula in each trial is listed in Table 2. The treatment duration varied from 3 weeks to 2 years, with most trials having a follow-up period of 3 months. In terms of outcome indicators, 23 RCTs reported EDSS, 3 RCTs provided ARR, 7 RCTs presented clinical symptom scores, and 5 RCTs reported neurological signs scores. Additionally, 18 RCTs evaluated clinical effects, 2 RCTs provided the MFIS, 3 RCTs presented MRI-related outcomes, 3 RCTs reported cognitive function outcomes, 4 RCTs presented scores of TCM syndrome, and 1 RCT reported MSFC. Furthermore, 6 RCTs presented pharmacodynamic biomarkers and 12 RCTs reported adverse effects.
3.3 Risk of bias assessment
The risk of bias assessment for the included RCTs is illustrated in Figure 2. In the domain of randomization process, only 7.1% of studies were judged as low risk, while 64.3% had some concerns, and 28.6% were assessed as high risk, mainly due to the use of inadequate sequence generation methods (such as hospital admission date, or the order of their visits) and insufficient reporting of allocation concealment. For deviations from intended interventions, 96.4% of studies were rated as low risk, indicating good adherence to the assigned interventions, with only 3.6% having some concerns. Regarding missing outcome data, 92.9% of studies were at low risk, 3.6% had some concerns, and 3.6% were judged as high risk due to incomplete outcome data. In the domain of measurement of the outcome, all studies (100%) were assessed as low risk. For selection of the reported result, 92.9% of studies were at low risk, while 7.1% were considered high risk because of potential selective reporting and lack of pre-specified analysis plans. Overall, only 7.1% of studies were rated as low risk of bias across all domains, while 57.1% had some concerns, and 35.7% were judged to be at high risk of bias.
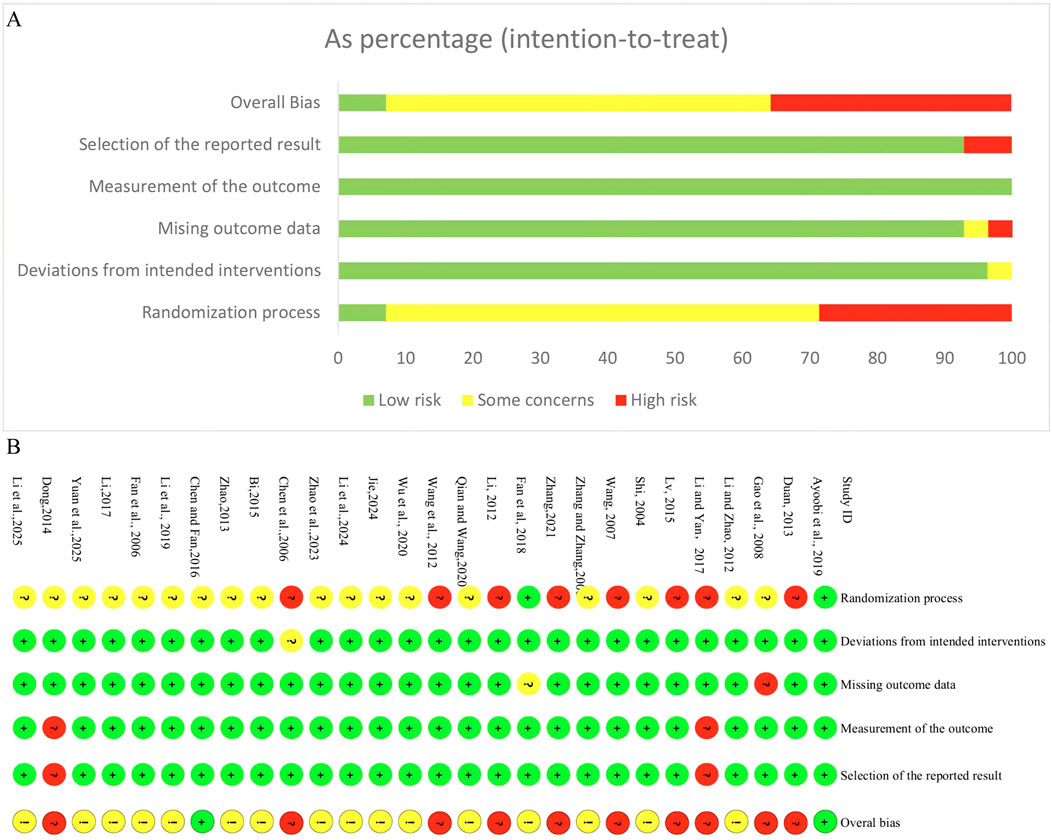
Figure 2. Risk of bias assessment for included RCTs. (A) Risk of bias graph for included RCTs. (B) Risk of bias summary for included RCTs.
3.4 Description of CHMs
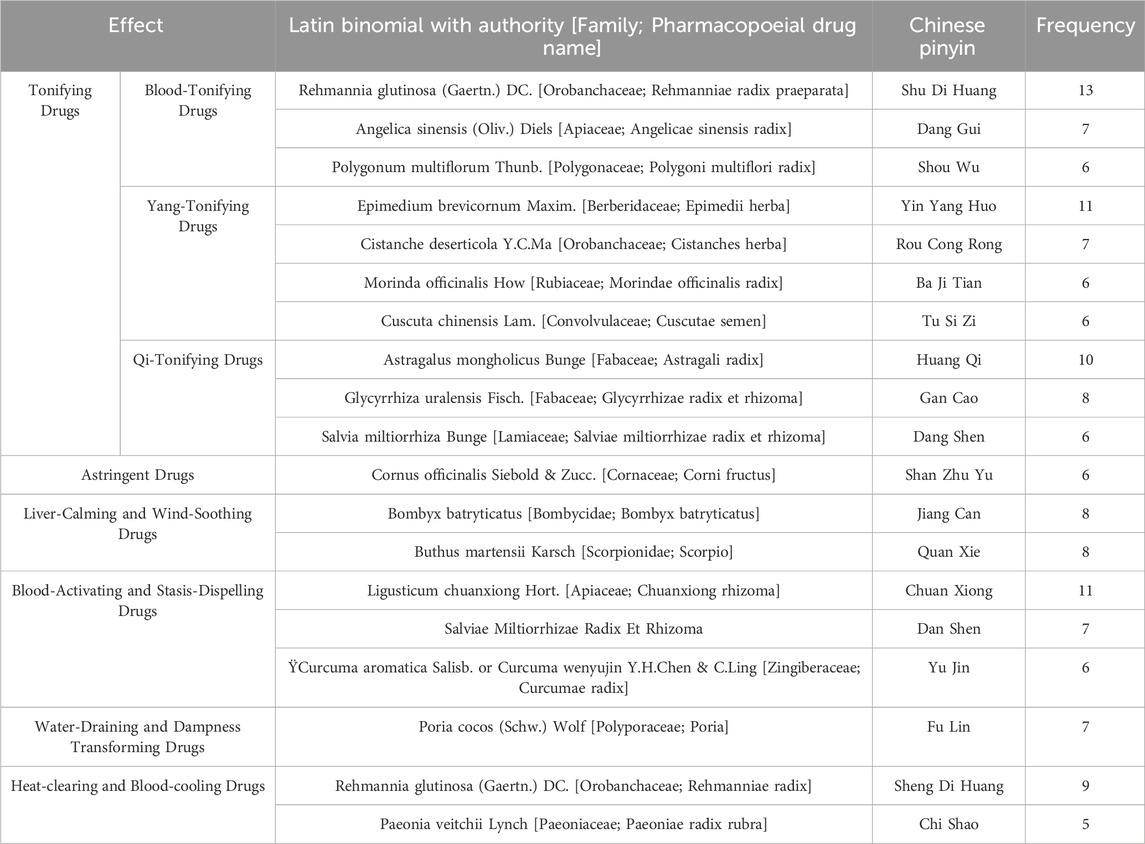
A total of 107 botanical drugs were included in the 28 studies. The top 19 most frequently used botanical drugs were as follows: Rehmannia glutinosa (Gaertn.) DC. (Orobanchaceae; Rehmanniae radix praeparata), Angelica sinensis (Oliv.) Diels (Apiaceae; Angelicae sinensis radix), Polygonum multiflorum Thunb. (Polygonaceae; Polygoni multiflori radix), Epimedium brevicornum Maxim. (Berberidaceae; Epimedii herba), Cistanche deserticola Y.C.Ma (Orobanchaceae; Cistanches herba), Morinda officinalis How (Rubiaceae; Morindae officinalis radix), Cuscuta chinensis Lam. (Convolvulaceae; Cuscutae semen), Astragalus mongholicus Bunge (Fabaceae; Astragali radix), Glycyrrhiza uralensis Fisch. (Fabaceae; Glycyrrhizae radix et rhizome), Salvia miltiorrhiza Bunge (Lamiaceae; Salviae miltiorrhizae radix et rhizome), Cornus officinalis Siebold & Zucc. (Cornaceae; Corni fructus), Bombyx batryticatus (Bombycidae; Bombyx batryticatus), Buthus martensii Karsch (Scorpionidae; Scorpio), Ligusticum chuanxiong Hort. (Apiaceae; Chuanxiong rhizome), Salviae Miltiorrhizae Radix Et Rhizoma, Curcuma aromatica Salisb. or Curcuma wenyujin Y.H.Chen & C. Ling (Zingiberaceae; Curcumae radix), Poria cocos (Schw.) Wolf (Polyporaceae; Poria), Rehmannia glutinosa (Gaertn.) DC. (Orobanchaceae; Rehmanniae radix), Paeonia veitchii Lynch (Paeoniaceae; Paeoniae radix rubra). These drugs were used at least five times (Table 3) and encompassed 66 single botanicals (Supplementary Appendix 2).
3.5 Outcome measures
3.5.1 EDSS
A total of 23 studies evaluated EDSS. Among these, 19 trials (Chen et al., 2006; Duan, 2013; Gao et al., 2008; Jie, 2024; Li and Yan, 2017; Li and Zhao, 2012; Li, 2012; Li et al., 2025; Lv, 2015; Qian and Wang, 2020; Shi and Wang, 2004; Wang et al., 2007; Wang et al., 2012; Wu et al., 2020; Yuan et al., 2025; Zhang and Zhang, 2006; Zhang, 2021; Zhao et al., 2023; Zhao, 2013) conducted conventional treatment add-on therapy. The qualitative summary replaced meta-analysis due to substantial heterogeneity (I2 = 99% in the pooling analysis of EDSS). Among the 19 trials, 18 reported that the combination of CHM with conventional therapy resulted in a significantly greater reduction in EDSS scores compared to conventional therapy alone. One trial (Li and Yan, 2017) also observed lower EDSS scores in the CHM group; however, there was no statistically significant difference between treatment and control groups.
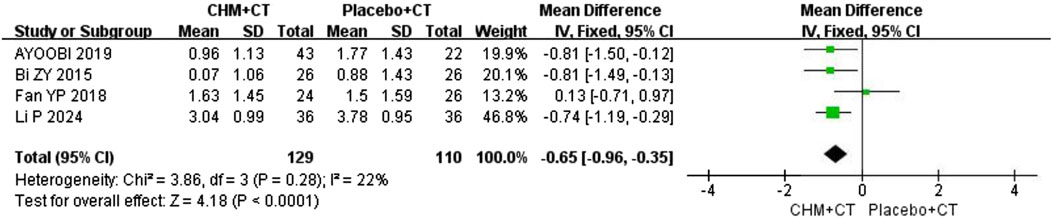
Additionally, four trials (Ayoobi et al., 2019; Bi, 2016; Fan et al., 2018; Li et al., 2024) compared CHM with a placebo. A fixed effect model was used due to the low heterogeneity (Cochrane Q test = 3.86, I2 = 22%, P = 0.28). The pooled results revealed that CHM therapy could decrease EDSS scores compared to placebo (MD = −0.65; 95% CI: −0.96, −0.35; P < 0.0001) (Figure 3).
3.5.2 Relapse
Eight trials reported on annual relapse frequency (Ayoobi et al., 2019; Chen et al., 2006; Fan et al., 2006; Gao et al., 2008; Li and Yan, 2017; Lv, 2015; Shi and Wang, 2004; Wang et al., 2012). Ayoobi et al. (2019) compared CHM with placebo (MD = −0.811; 95% CI: −1.26, −0.36; P < 0.0045), while other seven trials conducted conventional treatment add-on therapy. A random effect model was used due to the high heterogeneity (Cochrane Q test = 34.53, I2 = 83%, P < 0.00001). The pooled results indicated that adding CHM could decrease annual relapse frequency compared to CT alone (MD = −0.53; 95% CI: −0.76, −0.31; P < 0.00001) (Figure 4A). Moreover, sensitivity analysis indicated that after excluding the study by Wang et al. (2012), the heterogeneity significantly decreased (Cochrane Q test = 8.59, I2 = 42%, P = 0.13) (Figure 4B). Three trials reported on ARR (Ayoobi et al., 2019; Bi, 2016; Li et al., 2024). A fixed effect model was selected due to the low heterogeneity (Cochrane Q test = 0.74, I2 = 0%, P = 0.69). The pooled results indicated that CHM could significantly decrease ARR compared to placebo (RR = 0.48; 95% CI: 0.31, 0.73; P = 0.0007) (Figure 4C).
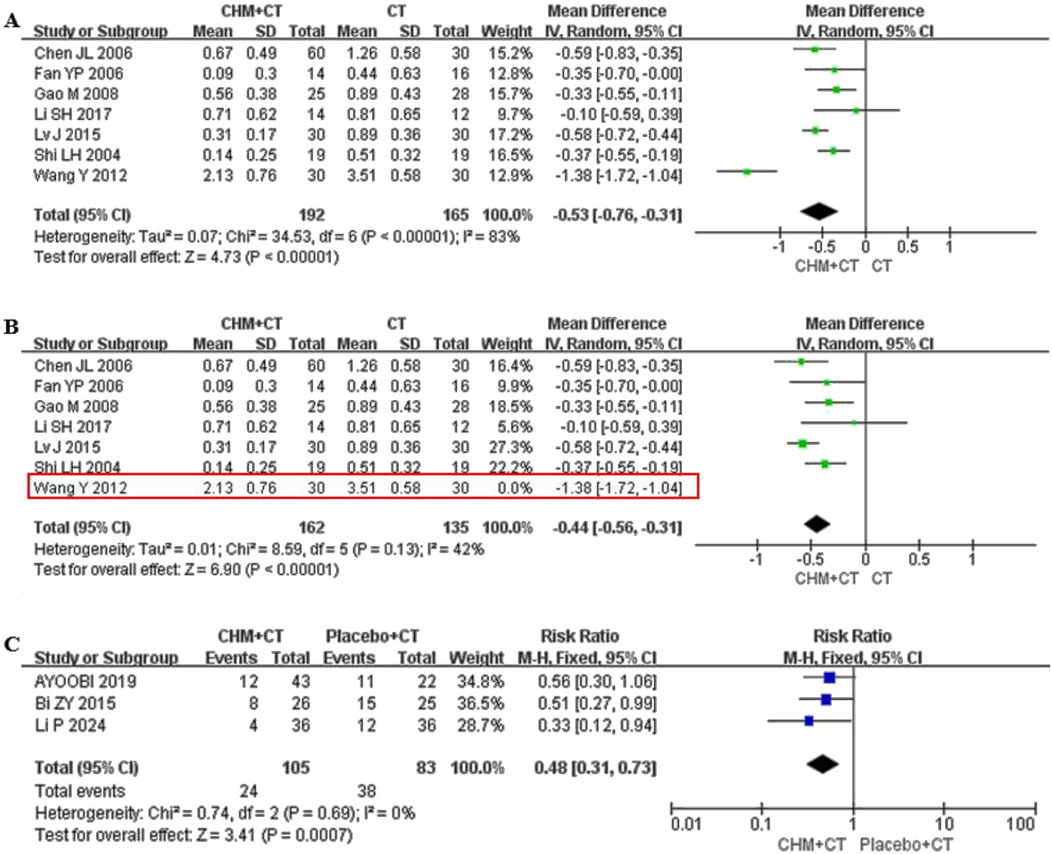
Figure 4. Effect of CHM on annual relapse frequency and ARR. (A) CHM + CT versus CT on annual relapse frequency. (B) Sensitivity analysis of the annual relapse frequency of CHM combined with CT and CT. (C) CHM + CT versus Placebo + CT on ARR.
3.5.3 Neurological signs score
The degree of neurological dysfunction was evaluated by the neurological signs score (range: 0–45 points, with higher scores indicating more severe neurological dysfunction). The grading is as follows: mild (0–15), moderate (16–30), and severe (31–45). Eight aspects were evaluated: Consciousness, gaze function, facial paralysis, speech, upper limb muscle strength, lower limb muscle strength, hand muscle strength, and walking ability. Five trials evaluated the neurological signs score (Lv, 2015; Qian and Wang, 2020; Shi and Wang, 2004; Zhao, 2013; Zhang and Zhang, 2006). A random effect model was conducted due to the high heterogeneity (Cochrane Q test = 23.98, I2 = 83%, P < 0.0001). The pooled results revealed that integrating CHM with CT therapy led to significant improvements in neurological function compared to CT alone (MD = −2.96; 95% CI: −4.52, −1.39; P = 0.0002) (Figure 5A).
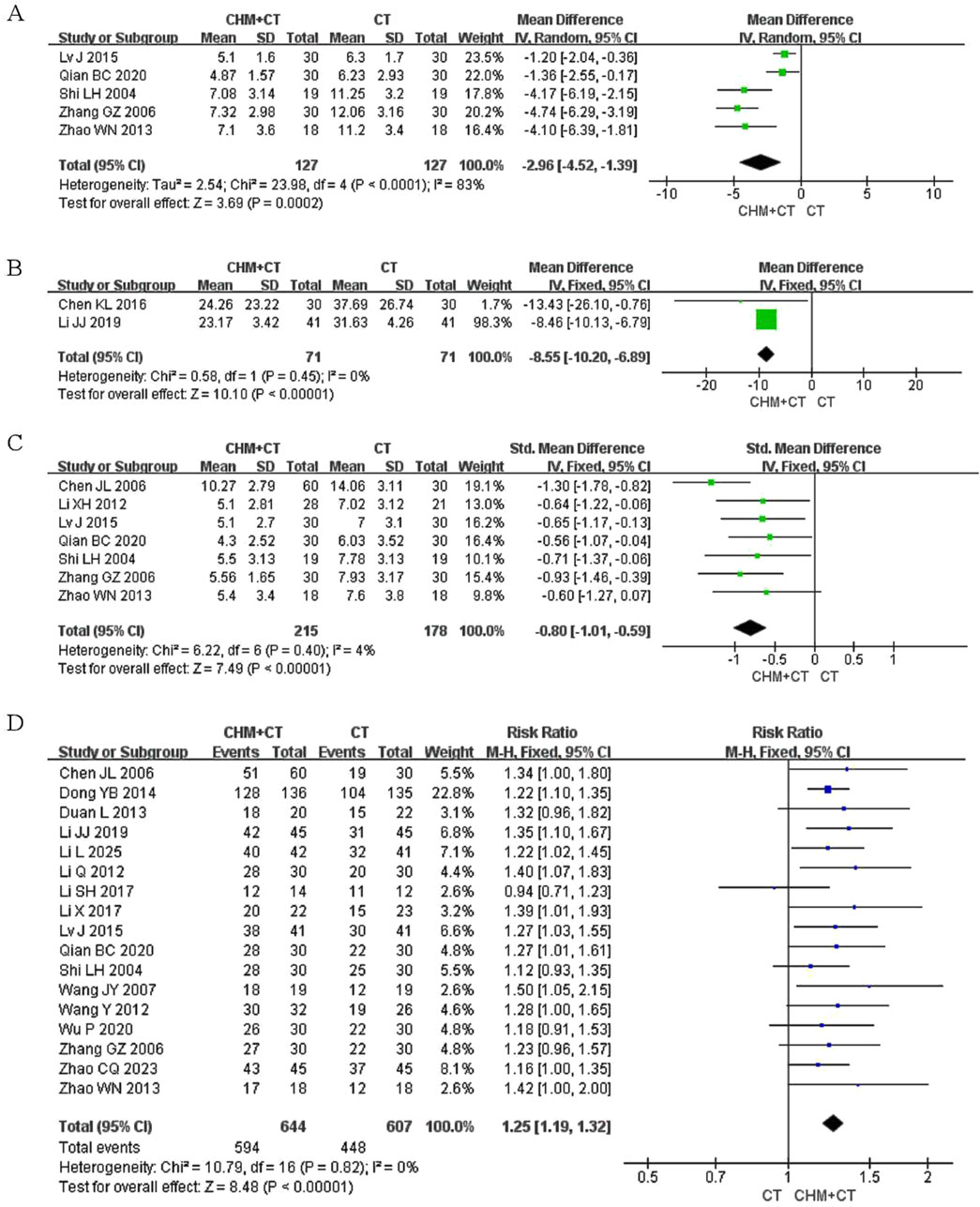
Figure 5. Effect of CHM on neurological signs score, MFIS, clinical symptom scores, and clinical effect. (A) CHM + CT versus CT on neurological signs score. (B) CHM + CT versus CT on MFIS. (C) CHM + CT versus CT on clinical symptom scores. (D) CHM + CT versus CT on clinical effect.
3.5.4 MFIS
Two trials reported on MFIS (Chen and Fan, 2016; Li et al., 2019). A fixed effect model was conducted due to the low heterogeneity (Cochrane Q test = 0.58, I2 = 0%, P = 0.45). The pooled results indicated that CHM could significantly relieve fatigue symptoms compared to CT alone (MD = −8.55; 95% CI: −10.20, −6.89; P < 0.00001) (Figure 5B).
3.5.5 Clinical symptom scores
Clinical symptoms were rated on a scale of 1–3 points based on their severity, classified as mild, moderate, and severe. Seven trials assessed clinical symptom scores (Chen et al., 2006; Li, 2012; Lv, 2015; Qian and Wang, 2020; Shi and Wang, 2004; Zhao, 2013; Zhang and Zhang, 2006). A fixed effect model was conducted due to the low heterogeneity (Cochrane Q test = 6.22, I2 = 4%, P = 0.40). The pooled results indicated that combining CHM with CT therapy could significantly improve clinical symptoms compared to CT alone (SMD = −0.80; 95% CI: −1.01, −0.59; P < 0.00001) (Figure 5C).
3.5.6 Magnetic resonance imaging
Three trials (Ayoobi et al., 2019; Bi, 2016; Wang et al., 2012) assessed MRI-related outcomes. Ayoobi et al. (2019) identified that the mean volume changes of lesions on T2-weighted scans significantly decreased in the CHM group compared to the placebo group at month 12 (p = 0.004). Bi ZY (Bi, 2016) reported that the number and volume of MRI lesions in the CHM group were significantly lower than those in the CT-only group at months 12 and 24. Wang et al. (2012) identified that 21 out of 30 patients in the CHM group exhibited a decrease in the number or scope of lesions on MRI, compared to 16 out of 30 patients in the control group.
3.5.7 Clinical effect
The clinical effect was assessed based on the improvement of symptoms (numbness or weakness in extremities, pain, difficulty walking or imbalance, dysarthria, dysphagia, and bladder dysfunction) and signs (spasticity, ataxia, sensory abnormalities, and cognitive impairment). This evaluation was reported in 17 trials following the intervention. Among these, 17 rials (Chen et al., 2006; Dong, 2014; Duan, 2013; Li, 2017; Li and Yan, 2017; Li and Zhao, 2012; Li et al., 2019; Li et al., 2025; Lv, 2015; Qian and Wang, 2020; Shi and Wang, 2004; Wang et al., 2007; Wang et al., 2012; Wu et al., 2020; Zhao, 2013; Zhao et al., 2023; Zhang and Zhang, 2006) conducted involved CT add-on therapy. A fixed effect model was carried out due to the low heterogeneity (Cochrane Q test = 10.79, I2 = 0%, P = 0.82). The pooled results indicated that integrated CHM with CT therapy could significantly improve clinical efficacy compared to CT alone (RR = 1.25; 95% CI: 1.19, 1.32; P < 0.00001) (Figure 5D). However, one trial (Fan et al., 2018) found non-significant improvement when compared with placebo.
3.5.8 Cognitive function
Three studies assessed cognitive impairment-related outcomes. Ayoobi et al. (2019) found that there was no statistically significant difference between CHM and placebo groups on the Mini-Mental State Examination (MMSE) task. Conversely, Zhao et al. (2023) identified that CHM and CT groups exhibited an increase in Montreal Cognitive Assessment (MoCA) and MMSE scores (P < 0.01). Furthermore, the MoCA and MMSE scores in the CHM group were higher than those in the CT alone group (P < 0.01). Li et al. (2024) found that the Loewenstein Occupational Therapy Cognitive Assessment-II scores of the CHM group were higher than those of the placebo group at month 12 (P = 0.003).
3.5.9 MSFC
Regarding the MSFC, only one study addressed this measure. Ayoobi et al. (2019) found that the MSFC z-score significantly increased in the group with CHM compared to placebo (p = 0.003) at month 12.
3.5.10 Scores of TCM syndrome
Four trials recorded the changes in symptoms (weakness, numbness, or stiffness in limbs, dizziness, pain, insomnia, fatigue, irritability, constipation, and shortness of breath) and signs (the appearance of tongue body and coating, the position, rate, and strength of pulse) based on TCM theory. Among these, two trials (Zhao et al., 2023; Zhang, 2021) conducted involved the CT add-on therapy. A fixed effect model was used due to the low heterogeneity (Cochrane Q test = 0.03, I2 = 0%, P = 0.85). The pooled results indicated that adding CHM could improve TCM syndrome compared to CT alone (SMD = −2.22; 95% CI: −2.61, −1.84; P < 0.00001) (Figure 6A). The other two trials (Fan et al., 2018; Li et al., 2024) compared CHM to placebo. Similarly, a fixed effect model was used due to the low heterogeneity (Cochrane Q test = 0.05, I2 = 0%, P = 0.82). The pooled results indicated that CHM could improve TCM syndrome compared to placebo (SMD = −0.63; 95% CI: −1.00, −0.27; P = 0.0007) (Figure 6B).
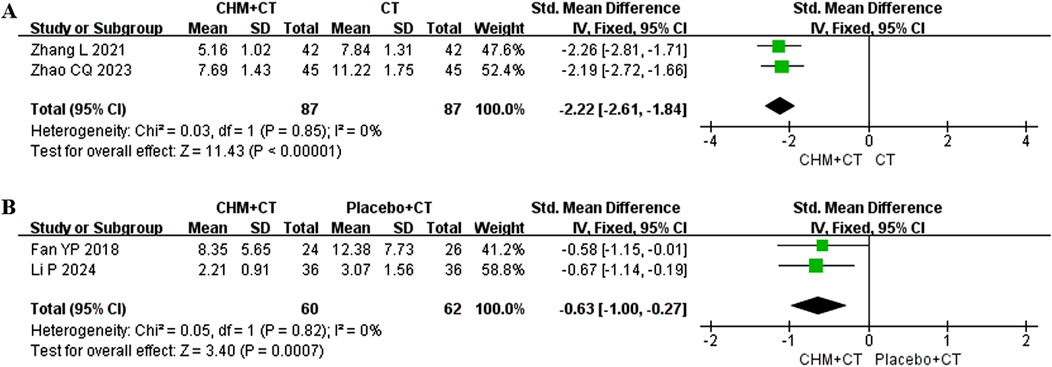
Figure 6. Effect of CHM on scores of TCM syndrome. (A) CHM + CT versus CT on scores of TCM syndrome. (B) CHM + CT versus placebo + CT on scores of TCM syndrome.
3.5.11 Pharmacodynamic biomarkers
Six trials (Jie, 2024; Li, 2017; Li et al., 2019; Li et al., 2025; Yuan et al., 2025; Zhao et al., 2023) reported significant differences in biofluid markers (P < 0.05) in the CHM group when compared with the CT group. These markers included inflammatory factors (interferon-γ↓, tumor necrosis factor-α↓, matrix metalloproteinase-9↓, interleukin-1β↓, interleukin-4↓, interleukin-6↓, interleukin-10↑, interleukin-17↓, interleukin-23↓ high mobility group box-1 protein↓, and interleukin-12p40 ↑), neuro-factors (transforming growth factor-β1↑, S-100 protein↓, and Tau protein↓), serum T lymphocyte subsets (Th17↓, Treg↑, Th17/Treg↓, CD8+ ↑, and CD4+/CD8+↓), and cerebrospinal fluid T lymphocyte subsets (CD4+↓, CD8+↑, and CD4+/CD8+↓).
3.5.12 Adverse effects
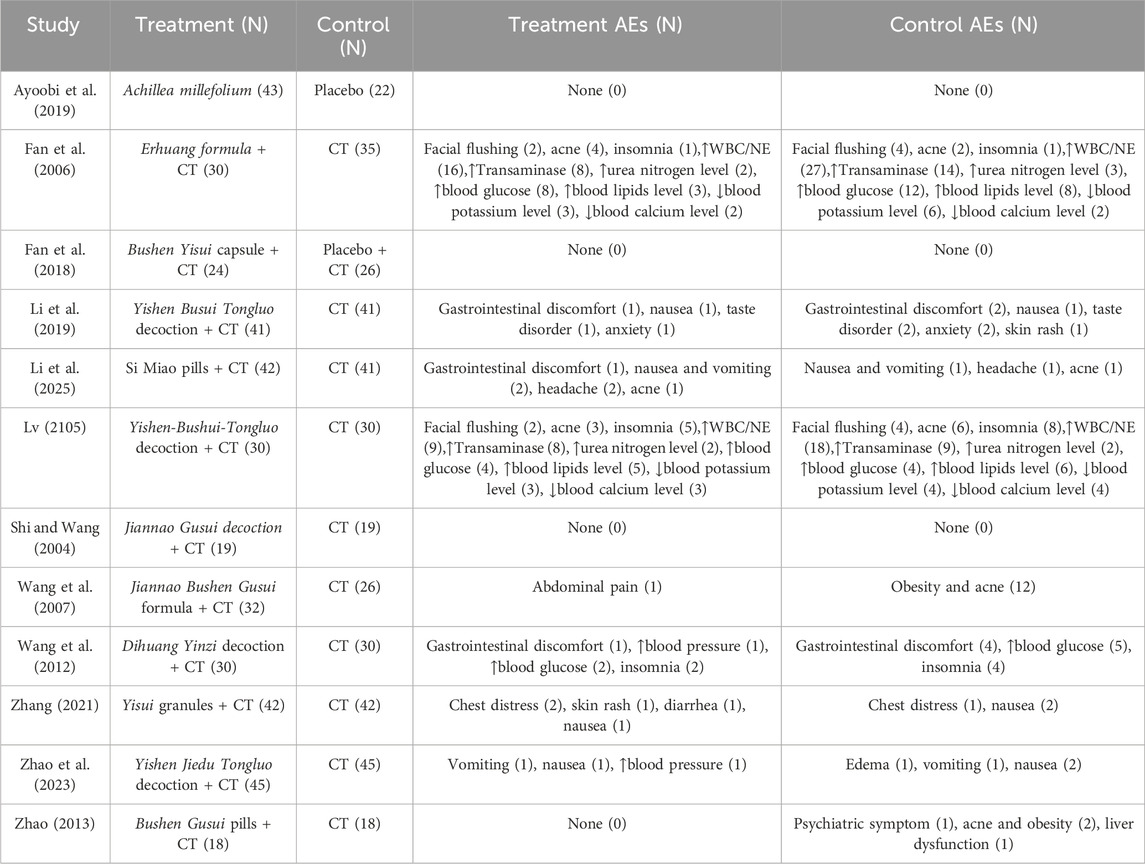
A total of 12 studies evaluated the safety of various treatments (Table 4). Among these, nine studies reported adverse events, while the remaining three reported none (Ayoobi et al., 2019; Fan et al., 2018; Shi and Wang, 2004). Six studies (Li et al., 2019; Li et al., 2025; Wang et al., 2007; Wang et al., 2012; Zhao, 2013; Zhao et al., 2023; Zhang, 2021) reported gastrointestinal reactions (abdominal discomfort, diarrhea, nausea and vomiting), anxiety, insomnia, edema, chest distress, taste disorders, headache, and rash. Two studies (Fan et al., 2006; Lv, 2015) reported hormone related adverse effect, including facial flushing, facial acne, obesity, abnormal laboratory tests (elevated transaminase, elevated urea nitrogen, elevated blood glucose, elevated blood lipids, decreased potassium, decreased blood calcium, elevated blood pressure, elevated white blood cells, and neutrophils). In these two studies, the incidence of elevated WBC or NE in the CHM group was significantly lower than that in the CT group (P < 0.05). There was no statistically significant difference in other hormone-related adverse effects between CHM and CT groups.
3.6 Publication bias
The funnel plot (Figures 7A–D) of annual relapse frequency, clinical symptom scores, neurological signs score and clinical effect, indicated a slight publication bias, while no statistically significant publication bias was detected through Egger’s test (P = 0.696, 0.232, 0.067, and 0.109, respectively) (Figures 8A–D).
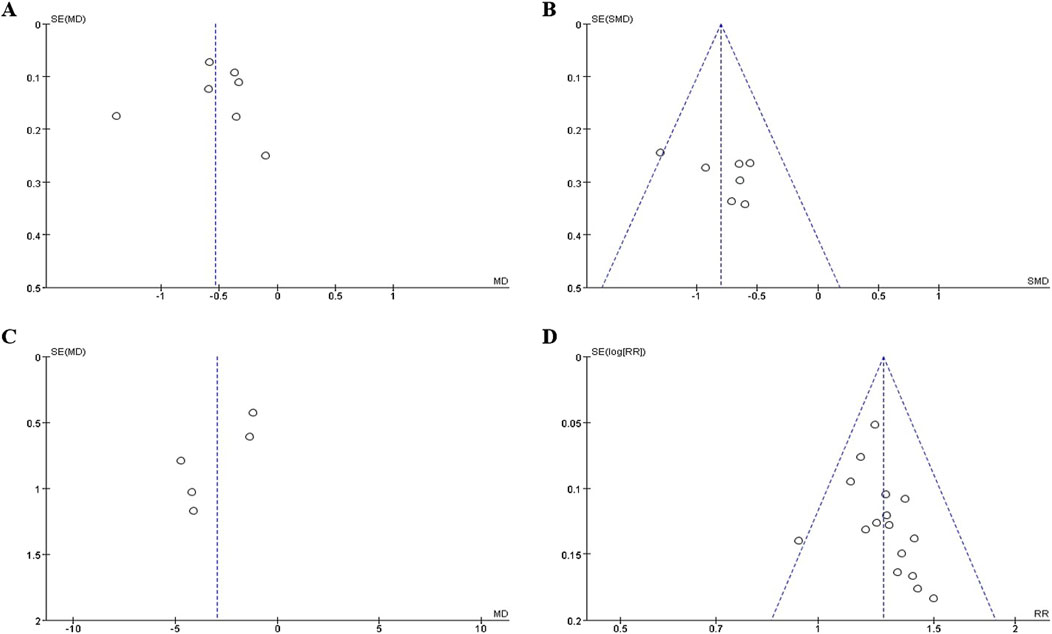
Figure 7. The funnel plot of outcomes: (A) annual relapse frequency; (B) clinical symptom scores; (C) neurological signs score; (D) clinical effect.
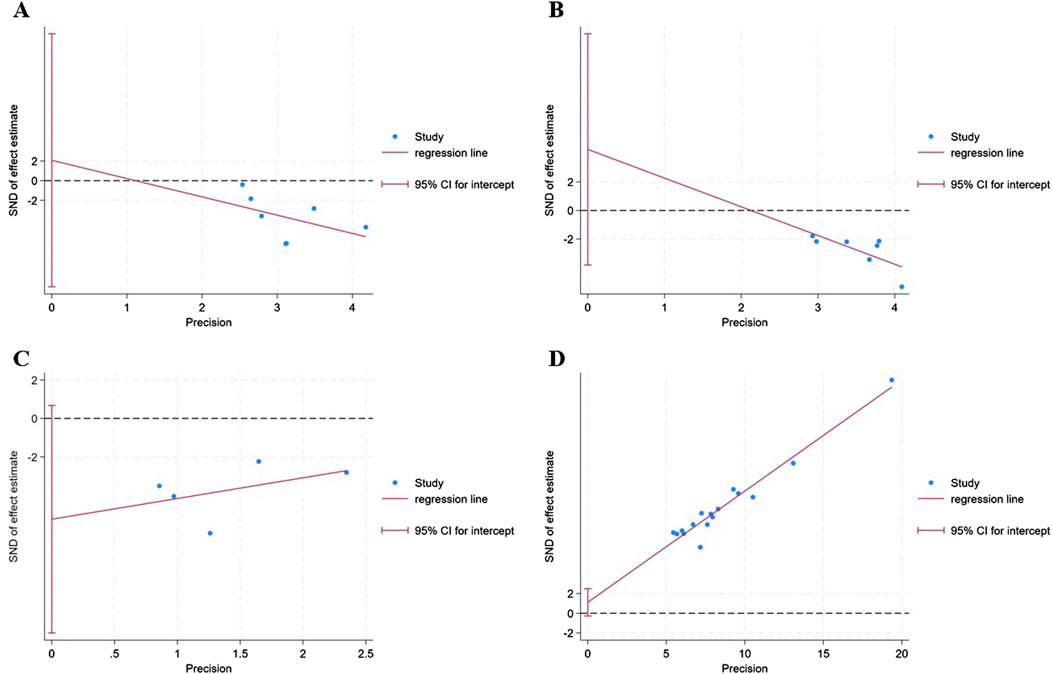
Figure 8. The Egger’s test of outcomes: (A) annual relapse frequency; (B) clinical symptom scores; (C) neurological signs score; (D) clinical effect.
3.7 Quality of evidence
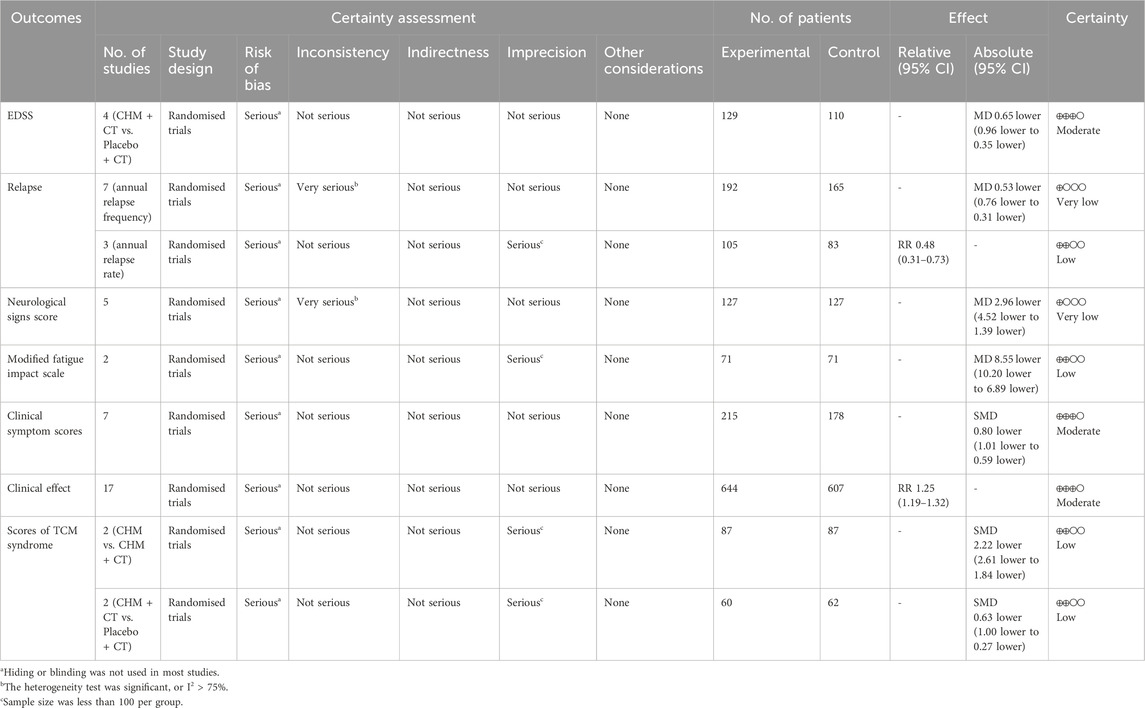
The GRADE system was used to assess the quality of evidence for all outcomes (Table 5).
4 Discussion
The systematic review summarized the efficacy and safety of CHM therapy for the treatment of MS. A total of 28 trials, involving 1,971 participants, were included in the analysis. Our findings suggested that CHM therapy may be effective in reducing the EDSS and relapse frequency. Additionally, it appeared to improve clinical symptom scores, TCM syndrome scores, neurological signs, cognitive function, and fatigue levels. Moreover, quite a few studies indicated that the clinical effect of adding CHM therapy was significantly favorable than that of the CT. However, the limited quality of the supporting data constrains the degree of confidence that can be attributed to the results. Additionally, only 12 out of 26 trials reported adverse events. Among these, one trial involving patients in the acute phase of RRMS reported no adverse events despite the use of corticosteroids, which may suggest underreporting rather than a true absence of adverse effects, particularly considering the known side effects of conventional therapies such as corticosteroids. Therefore, enhanced monitoring and standardized reporting of adverse events are necessary to ensure a reliable evaluation of the safety of these interventions.
Through a systematic review of the CHM therapies addressed in the RCTs, we found that the clinical treatment of MS in TCM mainly focuses on nourishing kidney yin and warming kidney yang. Additionally, it removes dampness, dispelling turbidity, and unblocking collaterals. This is consistent with the current mainstream understanding of the pathogenesis, which suggests that MS is caused by insufficient innate endowment and kidney qi deficiency. This condition leads to dysfunction of the organs, accumulation of dampness or dampness heat infiltration, the production of turbid toxins, damage to the Du meridian, brain, and spinal cord (Gao et al., 2022). Currently, experimental evidence has supported that CHM with the above effects can improve neurological deficits, reduce pathological damage such as central nervous system inflammatory cell infiltration and myelin sheath loss. Additionally, it can improve blood-brain barrier permeability in mice with experimental autoimmune encephalomyelitis, a traditional animal model of MS (Wu et al., 2012; Zhu et al., 2016). Moreover, several studies suggest that MS has a prodromal period (Makhani and Tremlett, 2021; Marrie et al., 2022). Managing risk factors during this prodromal phase, before a formal diagnosis of MS, and implementing secondary prevention strategies after a diagnosis of clinically isolated syndrome have gradually become research hotspots. Accordingly, the intervention time of CHM could potentially be initiated earlier, particularly during the stage when turbidity and toxicity are not abundant and healthy qi is not deficient. However, such attempts to treat MS using TCM must be supported by rigorous scientific evaluations in well-designed clinical studies.
Although the present study concluded that CHM demonstrates a good efficacy and safety profile in treating MS based on the results of available RCTs, the poor methodological quality and high clinical heterogeneity of the included trials require special attention. It is well-established that there are significant differences in pathological mechanisms, disease progression, and clinical manifestations among different clinical types of MS. When conducting clinical research, stratified analysis is usually necessary to evaluate the treatment efficacy more accurately, avoid bias and confounding, and increase the accuracy and reliability of the results. More precise and restrictive inclusion criteria for clinical trials should be established, as some RCTs included in this study did not adequately define the clinical characteristics necessary for participation, such as disease type, duration, and neurological function of patients with MS. Recruiting homogeneous subjects is necessary, particularly in clinical trials with a small sample size. Alternatively, the concept of treatment with syndrome differentiation guides the process of TCM diagnosis and treatment. The diagnostic criteria for syndrome differentiation play a crucial role in guiding medication choices. Consequently, a reliable TCM syndrome diagnosis and evaluation scale for MS is needed in the future to ensure consistency and reproducibility in research. Several included studies did not report the source or concentration of CHM preparations, contributing to increased heterogeneity of interventions. The uncertainty regarding herbal constituents and manufacturing processes may compromise the objective evaluation of CHM efficacy and reduce the credibility and reproducibility of the findings.
Regarding the selection of outcome measures, some included studies did not evaluate MRI and relapse rate. Currently, MS is mainly monitored and evaluated regularly through clinical, imaging, and biomarker dimensions to achieve no evidence of disease activity (NEDA) (Mayssam et al., 2020). This includes evaluating clinical recurrence (assessed by ARR), disability deterioration (assessed by EDSS), active lesions (evaluated through MRI), and biomarkers such as neurofilament light chain. We recommend incorporating NEDA as an efficacy indicator in future research on CHM. When evaluating the efficacy of CHM for MS, common measures include clinical symptom scores, TCM syndrome scores, and clinical effects. However, these assessments are subjective and influenced by evaluator experience and inconsistent standards, limiting comparability and reliability. Therefore, it is vital to establish standardized, objective criteria and improve evaluator training to enhance the accuracy and consistency of efficacy evaluations, thereby strengthening the foundation for clinical research and practice.
This systematic review has several limitations. First, the methodological quality of the included studies was assessed as low according to the Cochrane Handbook. The risk of bias assessment revealed that essential components of trial design—such as random sequence generation, allocation concealment, and blinding of participants and assessors—were either inadequately described or absent in a significant proportion of the studies. These deficiencies raise serious concerns about the internal validity of the reported outcomes. Particularly, the lack of blinding may introduce performance and detection biases, specifically in trials that rely heavily on subjective outcome measures. Second, the diagnostic criteria for some of the included studies were not specified, which may lead to bias. Third, the clinical types of MS population, the disability status of participants, the course of treatment, compositions of herbal formula, and dosage of CHM therapy varied among the included studies. This variability could further introduce bias. Moreover, incomplete reporting of relevant data further limited the feasibility of subgroup analyses and hindered comprehensive evidence synthesis. Fourth, the small sample size of included studies affected the pooled results. Lastly, although we did not restrict the language of the published literature, most included studies were from China, which limits the applicability of results to populations with different genetic and environmental risk factors for MS. Consequently, further rigorous RCTs that are well-designed, multi-center, and feature appropriate participant selection, long-term follow-up, and effective outcome measures are still required.
5 Conclusion
This systematic review and meta-analysis provide an updated and comprehensive evaluation of the clinical efficacy of CHM interventions for MS. The findings suggest that CHM therapy may play a role in alleviating the disability status and clinical symptoms, while also reducing relapse frequency with a low incidence of adverse effects. However, further well-designed RCTs with high-quality, appropriate participants and efficient outcome measures are warranted to strengthen clinical evidence of the efficacy and safety of CHM on MS management.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
XG: Formal Analysis, Writing – original draft, Software, Data curation, Writing – review and editing, Visualization. YW: Software, Formal Analysis, Writing – original draft, Data curation. QJ: Formal Analysis, Writing – original draft, Software, Data curation. YZ: Writing – original draft, Visualization, Software. MZ: Software, Writing – original draft, Visualization. JL: Methodology, Conceptualization, Investigation, Funding acquisition, Writing – review and editing. KS: Writing – review and editing, Funding acquisition, Conceptualization, Supervision. YG: Supervision, Funding acquisition, Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Capital’s Funds for Health Improvement and Research (Grant No.2024-1-4191), National High-level Chinese Medicine Hospital Clinical Study Specific Funding (Grant No.DZMG-ZLZX-25014), National Natural Science Foundation of China (Grant Nos. 82205034 and 82374365), Beijing-Tianjin-Hebei Basic Research Cooperation Funds (Grant No. J230035), The Basic scientific research operation cost project of Beijing University of Chinese Medicine (Grant No.2023-JYB-JBQN-017), and 2023 Science and Technology Innovation Project of Dongzhimen Hospital, Beijing University of Chinese Medicine (Grant No.DZMKJCX-2023-020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1635833/full#supplementary-material
Abbreviations
ARR, Annualized relapse rate; CAM, Complementary and alternative medicine; CHM, Chinese herbal medicine; CI, Confidence intervals; CNKI, The Chinese National Knowledge Infrastructure; CT, Conventional therapy; DMTs, Disease-modifying therapies; EDSS, Expanded disability status scale; GRADE, The Grading of Recommendations, Assessment, Development and Evaluation system; HMGB1, High mobility group box-1 protein; IFN-γ, Interferon-γ; IL-10, Interleukin-10; IL-12p40, Interleukin-12p40; IL-6, Interleukin-6; LOTCA-II, Loewenstein Occupational Therapy Cognitive Assessment-II; MD, Mean difference; MFIS, Modified Fatigue Impact Scale; MMP-9, Matrix metalloproteinase-9; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; MRI, Magnetic resonance imaging; MS, Multiple sclerosis; MSFC, Multiple sclerosis functional composite; NE, Neutrophil; NEDA, No evidence of disease activity; PPMS, Primary progressive MS; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses; RCT, Randomized controlled trials; RR, Relative risk; RRMS, Relapsing remitting multiple sclerosis; SinoMed, The Chinese Biomedical Database; SMD, Standard mean difference; SPMS, Secondary progressive multiple sclerosis; TCM, Traditional Chinese Medicine; TGF-β1, Transforming growth factor-β1; VIP, The Chinese Science and Technology Journals Database; WBC, White blood cell.
References
Ayoobi, F., Moghadam-Ahmadi, A., Amiri, H., Vakilian, A., Heidari, M., Farahmand, H., et al. (2019). Achillea millefolium is beneficial as an add-on therapy in patients with multiple sclerosis: a randomized placebo-controlled clinical trial. Phytomedicine 52, 89–97. doi:10.1016/j.phymed.2018.06.017
Bertoglio, J. C., Baumgartner, M., Palma, R., Ciampi, E., Cárcamo, C., Cáceres, D., et al. (2016). Andrographis paniculata decreases fatigue in patients with relapsing-remitting multiple sclerosis: a 12-month double-blind placebo-controlled pilot study. BMC Neurol. 16, 77. doi:10.1186/s12883-016-0595-2
Bi, Z. (2016). Clinical observation of methylprednisolone combined with Qi Shen Huan Wu capsule in the treatment of relapsing-remitting multiple sclerosis. Chin. Med. J. 387–388.
Chen, K., and Fan, Y. (2016). Effect of kidney-nourishing, phlegm-resolving and blood-activating method on quality of life of in patients with multiple sclerosis. Chin. Archives Traditional Chin. Med. 09, 2141–2144. doi:10.13193/j.issn.1673-7717.2016.09.026
Chen, J., Wang, D., and Li, Y. (2006). Clinical observation of Guilu Yisui capsules in the treatment of 60 cases of multiple sclerosis. New J. Traditional Chin. Med. 11, 31–32. doi:10.13457/j.cnki.jncm.2006.11.016
Chu, Y., Jing, Y., Zhao, X., Wang, M., Zhang, M., Ma, R., et al. (2021). Modulation of the HMGB1/TLR4/NF-κB signaling pathway in the CNS by matrine in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 352, 577480. doi:10.1016/j.jneuroim.2021.577480
Dong, Y. (2014). To observe the effect of methylprednisolone combined with Shenqi Yangsui formula in the treatment of multiple sclerosis. Cardiovasc. Dis. J. Integr. Traditional Chin. West. Med. 2 (17), 103–104. doi:10.16282/j.cnki.cn11-9336/r.2014.17.101
Duan, L. (2013). Clinical efficacy of Yiqi Huoxue Huatan decoction on multiple sclerosis. Hebei Med. J. 03, 460–461. doi:10.3969/j.issn.1002-7386.2013.03.086
Fan, Y., Wang, P., Zhang, X., Gong, H., Zhou, L., Liu, X., et al. (2006). Treatment of relapsing multiple sclerosis with Erhuang Formula. J. Beijing Univ. Traditional Chin. Med. 29 (04), 273–276.
Fan, Y., Chen, K., You, Y., Wang, S., Yang, T., and Wan, J. (2018). Clinical efficacy observation of Bushen Yisui capsule on relapsing remitting multiple sclerosis with syndrome of deficiency of kidney-liver yin. Chin. J. Traditional Chin. Med. 09, 4220–4223.
Gao, M., Lin, M., Zhang, K., Lin, Y., and Lv, N. (2008). Clinical observation of Dihuang compound (capsule) in the treatment of 38 cases of acute relapsing multiple sclerosis. Hunan J. Traditional Chin. Med. 06, 16–17. doi:10.16808/j.cnki.issn1003-7705.2008.06.008
Gao, Y., Xie, Y., Liu, J., and Rui, Y. (2022). Understanding the pathogenesis and staging treatment of multiple sclerosis. Beijing J. Traditional Chin. Med. 41 (7), 704–708. doi:10.16025/j.1674-1307.2022.07.001
Jakimovski, D., Bittner, S., Zivadinov, R., Morrow, S. A., Benedict, R. H. B., Zipp, F., et al. (2024). Multiple sclerosis. Lancet 403 (10422), 183–202. doi:10.1016/S0140-6736(23)01473-3
Jie, M. (2024). Observation on the effect of Yisui granules combined with methylprednisolone sodium succinate in the treatment of acute multiple sclerosis. Med. Innovation China 21 (23), 019–023. doi:10.3969/j.issn.1674-4985.2024.23.005
Kolčava, J., Kočica, J., Hulová, M., Dušek, L., Horáková, M., Keřkovský, M., et al. (2020). Conversion of clinically isolated syndrome to multiple sclerosis: a prospective study. Multiple Scler. Relat. Disord. 44, 102262. doi:10.1016/j.msard.2020.102262
Kurtzke, J. F. (1983). Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33 (11), 1444–1452. doi:10.1212/wnl.33.11.1444
Li, X. (2012). Clinical observation of Jianpi Bushen Tongluo Detox decoction in the treatment of 28 cases of multiple sclerosis. Guanming J. Chin. Med. 09, 1808–1809. doi:10.3969/j.issn.1003-8914.2012.09.050
Li, S. (2017). Clinical observation of Tripterygium Wilfordii polyglycosides in the treatment of multiple sclerosis. Res. Integr. Traditional Chin. West. Med. 9 (06), 288–289. doi:10.3969/j.issn.1674-4616.2017.06.003
Li, X., and Yan, B. (2017). Clinical experience of Toufengling in the treatment of multiple sclerosis. Chin. J. Geriatrics 19, 4912–4913. doi:10.3969/j.issn.1005-9202.2017.19.101
Li, Q., and Zhao, D. (2012). Integrated Chinese and Western medicine in the treatment of 30 cases of multiple sclerosis. Traditional Chin. Med. Res. 01, 22–23.
Li, J., Li, J., and Han, M. (2019). Effect of Yishen Busui Tongluo decoction with methylprednisolone on multiple sclerosis and its effect on the expression of inflammatory factors and HMGB1. Mod. J. Integr. Traditional Chin. West. Med. 28 (36), 4012–4016.
Li, P., Wang, J., and Li, Z. (2024). Exploring the clinical effects of Si Miao Pills in the treatment of multiple sclerosis (in remission). Liaoning J. Traditional Chin. Med. 1–9.
Li, L., Chen, X., and Nie, W. (2025). Clinical effect of Simiao Pill combined with conventional Western medicine in patients with multiple sclerosis. Int. Med. Health Bull. 31 (14), 2450–2455. doi:10.3760/cma.j.cn441417-20241119-14032
Liu, J., Gao, Y., Kan, B. H., and Zhou, L. (2012). Systematic review and meta-analysis of randomized controlled trials of Chinese herbal medicine in treatment of multiple sclerosis. J. Integr. Med. 10, 141–153. doi:10.3736/jcim20120204
Liu, J., Zhang, C., Xie, Y., Zhou, L., Guo, L., Li, B., et al. (2022). Demyelinating diseases of the central nervous system registry for patients with traditional Chinese medicine: rationale and design of a prospective, multicenter, observational study. Front. Pharmacol. 13, 981300. doi:10.3389/fphar.2022.981300
Lv, J. (2015). Clinical evaluation on Yishen-Bushui-Tongluo decoction in the treatment of multiple sclerosis. Int. J. Traditional Chin. Med. 37 (9), 788–791. doi:10.3760/cma.j.issn.1673-4246.2015.09.006
Makhani, N., and Tremlett, H. (2021). The multiple sclerosis prodrome. Nat. Rev. Neurol. 17 (8), 515–521. doi:10.1038/s41582-021-00519-3
Marrie, R. A., Allegretta, M., Barcellos, L. F., Bebo, B., Calabresi, P. A., Correale, J., et al. (2022). From the prodromal stage of multiple sclerosis to disease prevention. Nat. Rev. Neurol. 10, 1038. doi:10.1038/s41582-022-00686-x
Mayssam, E. N., Eid, C., Khoury, S. J., and Hannoun, S. (2020). No evidence of disease activity: is it an aspirational therapeutic goal in multiple sclerosis? Multiple Scler. Relat. Disord. 40, 101935. doi:10.1016/j.msard.2020.101935
McGinley, M. P., Goldschmidt, C. H., and Rae-Grant, A. D. (2021). Diagnosis and treatment of multiple sclerosis: a review. JAMA 325 (8), 765–779. doi:10.1001/jama.2020.26858
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev. Española Cardiol. 74 (9), 790–799. doi:10.1016/j.rec.2021.07.010
Qian, B., and Wang, B. (2020). Clinical efficacy of integrated traditional Chinese and Western medicine in the treatment of 30 cases of acute multiple sclerosis. Traditional Chin. Med. Res. 04, 16–18. doi:10.3969/j.issn.1001-6910.2020.04.07
Rae-Grant, A., Day, G. S., Marrie, R. A., Rabinstein, A., Cree, B. A., Gronseth, G. S., et al. (2018). Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology 90 (17), 777–788. doi:10.1212/WNL.0000000000005347
Shi, L., and Wang, Q. (2004). Preliminary study on the efficacy of integrated Chinese and Western medicine in preventing and treating relapses of multiple sclerosis. Guangxi J. Traditional Chin. Med. 02, 14–17.
Song, L., Zhou, Q., Wang, H., Liao, F., Hua, L., Zhang, H., et al. (2017). Chinese herbal medicine adjunct therapy in patients with acute relapse of multiple sclerosis: a systematic review and meta-analysis. Complementary Ther. Med. 31, 71–81. doi:10.1016/j.ctim.2017.02.004
Sterne, J., Savović, J., Page, M., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
The World Health Organization (2021). Traditional Chinese medicine could make “Health for one” true. Available online at: https://www.who.int/intellectualproperty/studies/Jia.pdf?ua=1.
Tian, D., Zhang, C., Yuan, M., Yang, X., Gu, H., Li, Z., et al. (2020). Incidence of multiple sclerosis in China: a nationwide hospital-based study. Lancet Regional Health West. Pac. 1, 100010. doi:10.1016/j.lanwpc.2020.100010
Wang, J., Zhang, C., and Zhang, Y. (2007). Clinical observation of integrated traditional Chinese and Western medicine in the treatment of 32 cases of multiple sclerosis. J. Mudanjiang Med. Coll. (02), 52–53. doi:10.13799/j.cnki.mdjyxyxb.2007.02.030
Wang, Y., Yang, X., and Chen, X. (2012). Clinical observation of the effect of Dihuang Yinzi combined with Western medicine in preventing relapses of multiple sclerosis. J. Sichuan Traditional Chin. Med. 09, 82–83.
Waubant, E., Lucas, R., Mowry, E., Graves, J., Olsson, T., Alfredsson, L., et al. (2019). Environmental and genetic risk factors for MS: an integrated review. Ann. Clin. Transl. Neurology 6 (9), 1905–1922. doi:10.1002/acn3.50862
Wu, Y., Gao, Y., Zhu, L., Lou, L., and Zhang, D. (2012). The expression of ZO-1 mRNA in the central nervous system and the effect of Yishendaluo decoction in experimental autoimmune encephalomyelitis. J. Emerg. Traditional Chin. Med. 21 (12), 1936–1938.
Wu, P., Xing, J., Chen, F., and Song, Q. (2020). Clinical observation on nourishing liver and kidney recipe in the treatment of multiple sclerosis. Chin. Med. Mod. Distance Educ. China 11, 84–86. doi:10.3969/j.issn.1672-2779.2020.11.034
Yadav, V., Bever, J. C., Bowen, J., Bowling, A., Weinstock-Guttman, B., Cameron, M., et al. (2014). Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development subcommittee of the American academy of neurology. Neurology 82, 1083–1092. doi:10.1212/WNL.0000000000000250
Yuan, Z., Ren, M., Yuan, W., Guo, D., Wu, M., Wang, X., et al. (2025). Clinical effect of Magui Zhuli decoction in the treatment of muliple sclerosis patients with spleen defciency and dampness-heat syndrome. Med. Front. 15 (08), 79–85. doi:10.20235/j.issn.2095-1752.2025.08.021
Zhang, L. (2021). Clinical evaluation of the effect of Yisui granules combined with methylprednisolone in the treatment of multiple sclerosis. Henan Med. Res. 08, 1488–1489. doi:10.3969/j.issn.1004-437X.2021.08.051
Zhang, G., and Zhang, J. (2006). Clinical research on the treatment of multiple sclerosis with Guishui Tongluo decoction. Chin. J. Emerg. Traditional Chin. Med. 06, 595–596.
Zhang, Y., Li, X., Ciric, B., Ma, C. G., Gran, B., Rostami, A., et al. (2015). Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis is mediated by SOCS3 regulatory pathway. Sci. Rep. 5 (1), 17407. doi:10.1038/srep17407
Zhang, X., Tan, R., Lam, W. C., Yao, L., Wang, X., Cheng, C. W., et al. (2020). PRISMA (preferred reporting items for systematic reviews and meta-analyses) extension for Chinese herbal medicines 2020 (PRISMA-CHM 2020). Am. J. Chin. Med. 48 (6), 1279–1313. doi:10.1142/S0192415X20500639
Zhao, W. (2013). Integrated Chinese and Western medicine treatment of 36 cases of multiple sclerosis. China J. Pharm. Econ. 01, 55–56.
Zhao, C., Zhao, L., Guo, X., Zhang, X., Shi, W., and Wen, J. (2023). The therapeutic effect of tonifying the kidney, detoxifying and dredging collaterals on multiple sclerosis and its influence on the levels of Th17/Treg cytokines. Chin. J. Microcirc. 04, 69–73. doi:10.3969/j.issn.1005-1740.2023.04.012
Keywords: multiple sclerosis, Chinese herbal medicine, complementary and alternative medicine, randomized controlled trials, meta-analysis
Citation: Guan X, Wu Y, Jia Q, Zheng Y, Zou M, Liu J, Sugimoto K and Gao Y (2025) Efficacy and safety of Chinese herbal medicine for multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 16:1635833. doi: 10.3389/fphar.2025.1635833
Received: 27 May 2025; Accepted: 15 August 2025;
Published: 22 September 2025.
Edited by:
Anthony Booker, University of Westminster, United KingdomReviewed by:
Hikmat Hadoush, Jordan University of Science and Technology, JordanHong Li, Southern Medical University, China
Copyright © 2025 Guan, Wu, Jia, Zheng, Zou, Liu, Sugimoto and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Liu, bGl1amlhODYwMzE0QDE2My5jb20=; Kazuo Sugimoto, cnVpeWlmZW5nQG1zbi5jb20=; Ying Gao, Z2FveWluZzk3M0AxMjYuY29t
 Xiaorui Guan
Xiaorui Guan Yufan Wu
Yufan Wu Qiuyang Jia1
Qiuyang Jia1 Jia Liu
Jia Liu Kazuo Sugimoto
Kazuo Sugimoto Ying Gao
Ying Gao