- 1Department of Nursing, Nantong Health College of Jiangsu Province, Nantong, Jiangsu, China
- 2Department of Laboratory Medicine, Haimen Hospital Affiliated to Xinglin College of Nantong University, Nantong, Jiangsu, China
- 3Department of General Surgery, The Sixth People’s Hospital of Nantong, Nantong, Jiangsu, China
Background: To evaluate the effects of different types of nucleos(t)ide analogs on the survival rate of patients with hepatitis B virus-associated hepatocellular carcinoma (HBV-HCC) after radical resection through a network meta-analysis.
Methods: PubMed, Embase, the Cochrane Library, and CNKI databases were searched up to 6 March 2024. The NOS was used to assess the risk of bias in cohort studies, while the ROB tool in Review Manager was employed for randomized controlled trials. Data on overall survival (OS) and recurrence-free survival (RFS) were extracted from the literature to pool hazard ratios (HRs) and corresponding 95% CrIs. Meta-analysis was performed via R.
Results: 24 studies involving 9,787 HBV-HCC patients were included. Compared with the control group, antiviral therapies using telbivudine (HR [95% CrI] = 0.23 [0.12,0.44]), tenofovir disoproxil fumarate (HR [95% CrI] = 0.40 [0.30,0.52]), lamivudine (HR [95% CrI] = 0.50 [0.34, 0.75]), adefovir (HR [95% CrI] = 0.55 [0.38,0.79]), and entecavir (HR [95% CrI] = 0.55 [0.43,0.71]) significantly improved OS. Among these, telbivudine (98.22%) and tenofovir disoproxil fumarate (76.12%) demonstrated superior effects in improving OS. Compared with the control group, antiviral therapies using telbivudine (HR [95% CrI] = 0.45 [0.28,0.70]), tenofovir disoproxil fumarate (HR [95% CrI] = 0.52 [0.44,0.62]), entecavir (HR [95% CrI] = 0.65 [0.55,0.77]),adefovir (HR [95% CrI] = 0.79 [0.65,0.94]),and lamivudine (HR [95% CrI] = 0.82 [0.71, 0.94]) significantly improved RFS. Telbivudine (SUCRA, 93.22%) and tenofovir disoproxil fumarate (SUCRA, 85.37%) exhibited superior effects in improving RFS.
Conclusion: When compared to other nucleos(t)ide analogs, telbivudine and tenofovir disoproxil fumarate exhibited the most notable effects.
Systematic Review: Identifier CRD42024612794.
1 Introduction
In Asia, hepatocellular carcinoma (HCC) is most frequently caused by hepatitis B virus (HBV) infection. Treatments such as radical resection, transarterial chemoembolization (TACE), and radiofrequency ablation (RFA) can effectively improve the prognosis of patients with hepatitis B virus-associated hepatocellular carcinoma (HBV-HCC). Nevertheless, despite treatment, the 5-year survival rate remains at only 50%, with a recurrence rate surpassing 70% (Shen et al., 2018). Research has demonstrated that postoperative antiviral therapy using nucleos(t)ide analogs such as lamivudine (LAM), entecavir (ENT), adefovir (ADV), telbivudine (Ldt), and tenofovir disoproxil fumarate (TDF) can suppress the replication of hepatitis B virus deoxyribonucleic acid (HBV-DNA), reduce the HBV-DNA level in the body, improve overall survival (OS) and recurrence-free survival (RFS) in HBV-HCC patients (Choi et al., 2021; He et al., 2019; Huang et al., 2013; Kao et al., 2023; Li et al., 2023; Linye et al., 2023; Qi et al., 2021; Qi et al., 2020; Ren et al., 2018; Rui et al., 2017; Shen et al., 2022; Wang et al., 2022; Xiao et al., 2021; Xu et al., 2019; Zhong et al., 2016; Chen, 2015; Cheng et al., 2011; Ding et al., 2014; Fang et al., 2012; Lin et al., 2016; Zhang, 2015; Huang et al., 2015; Ke et al., 2013; Zhang et al., 2014; Chen et al., 2017), and thereby improve patient prognosis. However, no consensus has been reached on which specific antiviral drug offers the optimal effect for improving the prognosis. Some studies comparing tenofovir and ENT have suggested that TDF is superior to ENT in improving the survival rate and reducing the recurrence rate of HBV-HCC patients (Hu et al., 2022). However, research has reported no significant differences in OS or recurrence rate between the two drugs (Li et al., 2023). Similarly, studies comparing the efficacy of ENT and LAM have reported conflicting results. Research has found similar effects of ENT and LAM on OS in HBV-HCC patients (Shin et al., 2012), while ENT has been reported in other research to provide superior effects for improving OS compared to LAM (Kim et al., 2016). Therefore, we utilized a systematic review and network meta-analysis to integrate existing evidence, aiming to identify the optimal antiviral treatment for affected patients.
2 Methods
2.1 Data and methods
The present study was carried out as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Liberati et al., 2009), and was pre-registered with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42024612794).
2.2 Literature retrieval
Literature searches were conducted across PubMed, Embase, the Cochrane Library, and CNKI databases, with the search cut-off date on 6 December 2024. The search strategy was based on MeSH and free-text terms, with no language restrictions. The primary search terms included but were not limited to: Hepatitis B virus, hepatocellular carcinoma, overall survival, recurrence-free survival, disease-free survival, progression-free survival, and their corresponding free-text terms.
2.3 Literature screening
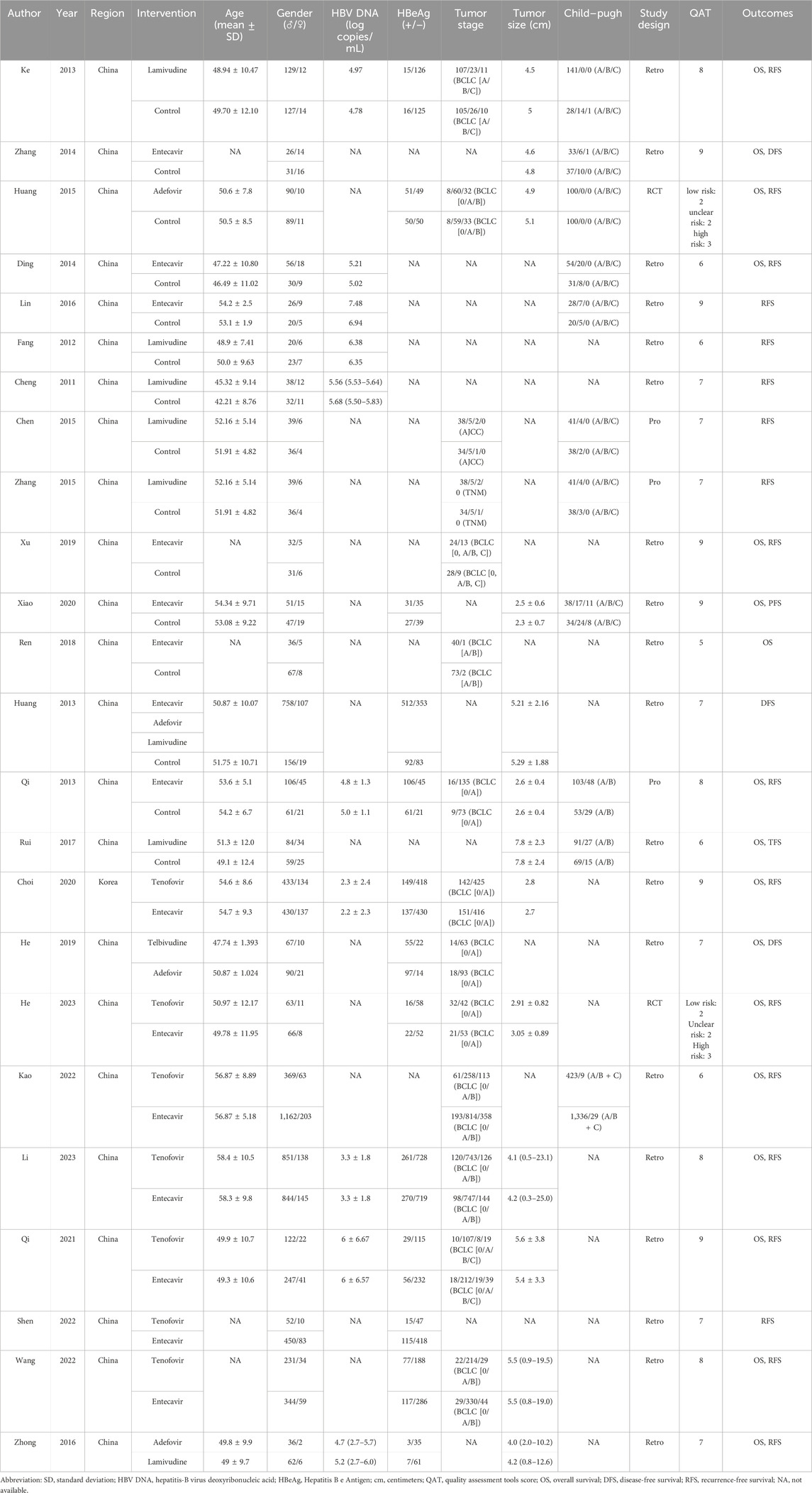
The articles were first independently evaluated by two researchers, and a third researcher was responsible for consolidating the findings. Studies were included if they met the following criteria (Shen et al., 2018): they were randomized controlled trials (RCTs) or cohort studies (Choi et al., 2021); they involved patients with HBV-HCC who received radical resection as part of their treatment (He et al., 2019); they provided relatively complete follow-up data on OS or RFS (Huang et al., 2013); they used postoperative nucleos(t)ide analog antiviral therapy as the control. Exclusion criteria were as follows (Shen et al., 2018): Patients infected with other types of hepatitis viruses other than hepatitis B virus (Choi et al., 2021); Patients received preoperative antiviral therapy (He et al., 2019); Different patients were treated with different types of antiviral drugs after surgery and were not grouped according to the type; (Huang et al., 2013); Studies that failed to specify the type of antiviral drugs to be used (Kao et al., 2023); The patients received liver transplantation and interferon antiviral therapy (Li et al., 2023); The patients did not receive radical resection. Ultimately, 24 eligible articles were included.
2.4 Risk of bias assessment
The selected studies were independently assessed for risk of bias by two researchers, and the results were consolidated by a third researcher. All RCTs were evaluated using the Risk of Bias (ROB) tool in Review Manager, while cohort studies were assessed using the Newcastle-Ottawa Scale (NOS) (Lo et al., 2014). The ROB tool included the following evaluation criteria (Shen et al., 2018): method of random sequence generation (Choi et al., 2021); allocation concealment (He et al., 2019); blinding of participants (Huang et al., 2013); blinding of outcome assessors (Kao et al., 2023); completeness of outcome data (Li et al., 2023); selective outcome reporting (Linye et al., 2023); other potential sources of bias. The NOS included the following evaluation criteria (Shen et al., 2018): representativeness of the exposed group (Choi et al., 2021); representativeness of the non-exposed group (He et al., 2019); ascertainment of exposure (Huang et al., 2013); demonstration that the outcome of interest was not present at the start of the study (Kao et al., 2023); comparability of exposed and non-exposed groups in study design and statistical analysis (Li et al., 2023); assessment of outcome (Linye et al., 2023); adequacy of follow-up duration (Qi et al., 2021); completeness of follow-up in both exposed and non-exposed groups. The results of the risk of bias assessment are presented in Table 1.
2.5 Data extraction
Using a pre-designed data collection sheet, data extraction was completed independently by two researchers. Any discrepancies in the extracted data were resolved by consulting a third researcher to ensure consistency. The main extracted data included (Shen et al., 2018): General study information: first author, publication year, and study region (Choi et al., 2021); Basic participant information: sample size, gender composition, age, HBV DNA level, hepatitis B e antigen (HBeAg) status, Child-Pugh score (a clinical grading system used to assess liver function based on parameters such as bilirubin, albumin, prothrombin time, ascites, and hepatic encephalopathy), and antiviral therapies (He et al., 2019); Tumor characteristics: tumor size and stage (Huang et al., 2013); Outcome measures: OS, RFS, or progression-free survival (PFS).
2.6 Statistical analysis
In this study, R language was used as the primary data analysis tool, and the “gemtc” package was applied to perform Bayesian network meta-analysis to systematically synthesize and compare the effectiveness of multiple treatment regimens. Model convergence quality was first assessed by calculating the potential scale reduction factor (PSRF). A PSRF value close to 1 indicated that the simulation process had achieved good convergence, ensuring the stability and reliability of the statistical inference results. Then, the ranking of treatment regimens included in the studies was visualized using two methods: first, by calculating and reporting surface under the cumulative ranking curve (SUCRA) values to quantify the overall ranking of each antiviral drug; and second, by utilizing relative effect forest plots and league tables to compare the relative effectiveness of different types of antiviral drugs. Additionally, DFS, RFS, and TFS were combined for statistical analysis. Next, if a closed-loop structure was identified in the network diagram, the “mtc.nodesplit” function was used to perform inconsistency testing. This test was to examine significant differences between indirect and direct evidence to evaluate whether the consistency assumption of the network meta-analysis was valid. At last, the “mtc.anohe” function was applied to perform heterogeneity testing, assessing whether the variability among the included studies exceeded the range of random variation. Potential sources of heterogeneity were further explored to interpret the results more comprehensively.
3 Results
3.1 Basic information of included studies
A total of 5,355 articles were retrieved from the above-mentioned databases. After removing 2,461 duplicates using Endnote 20, 2,894 articles remained. In the first round of screening, 2,857 articles that did not meet the study criteria were excluded after reviewing titles and abstracts, leaving 37 articles. In the second round of screening, after full-text reviews, we excluded 8 articles in which patients had received preoperative antiviral therapy and 5 articles where the types of antiviral drugs were not specified. Ultimately, 24 articles (Choi et al., 2021; He et al., 2019; Huang et al., 2013; Kao et al., 2023; Li et al., 2023; Linye et al., 2023; Qi et al., 2021; Qi et al., 2020; Ren et al., 2018; Rui et al., 2017; Shen et al., 2022; Wang et al., 2022; Xiao et al., 2021; Xu et al., 2019; Zhong et al., 2016; Chen, 2015; Cheng et al., 2011; Ding et al., 2014; Fang et al., 2012; Lin et al., 2016; Zhang, 2015; Huang et al., 2015; Ke et al., 2013; Zhang et al., 2014) meeting the criteria were included in the study. The 24 included studies were published between 2011 and 2023, involving a total of 9,787 patients. The literature retrieval and screening process are illustrated in Figure 1. The included patients were predominantly from China, except for one study conducted in South Korea. The average age of the patients was approximately 50 years, and most were classified as Child-Pugh grade A. The majority of the studies were retrospective. Detailed baseline characteristics are presented in Table 1.
3.2 Risk of bias assessment
Of the 22 cohort studies included, 6 studies achieved a score of 9 on the NOS, 4 studies scored 8, 7 studies received a score of 7, 4 studies scored 6, and 1 study obtained a score of 5. Some studies had issues, including incomplete control of confounding factors, lack of descriptions regarding loss to follow-up, or short follow-up durations. The two included RCTs were rated as having a low risk of bias in 2 domains, unclear risk in 2 domains, and high risk in 3 domains. Both RCTs were open-label studies, with a high risk of bias in the domains of blinding and allocation concealment.
3.3 Effect of antiviral therapy on OS in HBV-HCC patients
For OS, a total of 17 studies, including 7,890 patients and five intervention measures, were included in the analysis (Figure 2A). Heterogeneity analysis and inconsistency testing using the node-splitting method demonstrated that the network meta-analysis satisfied the assumptions of homogeneity and consistency. Compared with the control group, antiviral therapies using Ldt (HR [95% CrI] = 0.23 [0.12, 0.44]), TDF (HR [95% CrI] = 0.40 [0.30, 0.52]), LAM (HR [95% CrI] = 0.50 [0.34, 0.75]), ADV (HR [95% CrI] = 0.55 [0.38, 0.79]), and ENT (HR [95% CrI] = 0.55 [0.43, 0.71]) significantly improved OS (Figure 2B; Table 2). The SUCRA-based probability rankings were consistent with the trends observed in the forest plot and league table. Ldt showed the best effect in improving OS (SUCRA = 98.22%).
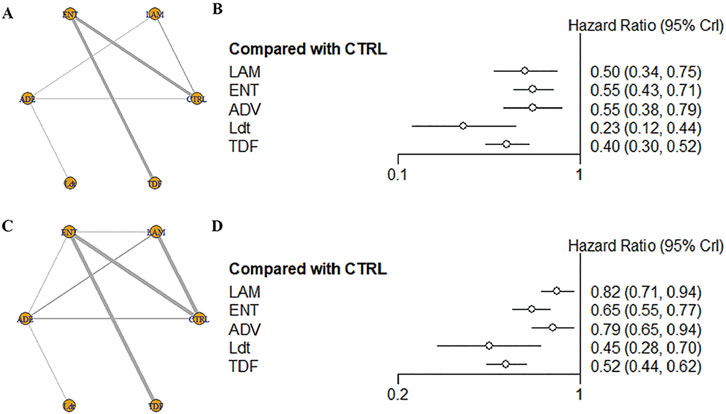
Figure 2. (A) Network structure diagram of different antiviral therapies; (B) Forest plot of different antiviral therapies; (C) Network structure diagram of different antiviral therapies; (D) Forest plot of different antiviral therapie.
3.4 Effect of antiviral therapy on RFS in HBV-HCC patients
For RFS, a total of 23 studies, including 9,671 patients and five intervention measures, were included in the analysis (Figure 2C). Heterogeneity analysis and inconsistency testing using the node-splitting method demonstrated that the network meta-analysis satisfied the assumptions of homogeneity and consistency. Given that most studies reported RFS, we treated DFS and TFS as RFS for analysis. Compared with the control group, antiviral therapies using Ldt (HR [95% CrI] = 0.45 [0.28, 0.70]), TDF (HR [95% CrI] = 0.52 [0.44, 0.62]), ENT (HR [95% CrI] = 0.65 [0.55, 0.77]), ADV (HR [95% CrI] = 0.79 [0.65, 0.94]), and LAM (HR [95% CrI] = 0.82 [0.71, 0.94]) significantly improved RFS (Figure 2D and Supplementary Table S1). The SUCRA-based probability rankings were consistent with the trends observed in the forest plot and league table. Ldt showed the best effect in improving RFS (SUCRA = 93.22%).
4 Discussion
The results of this study indicated that nucleos(t)ide analogs, such as LAM, ENT, ADV, Ldt, and TDF, significantly improved OS and RFS in HBV-HCC patients after radical resection, compared to those who did not receive nucleos(t)ide analog antiviral therapy. A key mechanism of HBV-induced carcinogenesis is the extensive replication of HBV in the body following infection. This process leads to the integration of HBV DNA into the host hepatocyte genome, triggering the activation of proto-oncogenes and impairing the function of tumor suppressor genes, thereby driving the development and progression of liver cancer (Jiang et al., 2021). In HBV-HCC patients, after taking nucleos(t)ide analog antiviral drugs, these analogs are converted into triphosphate compounds through catalysis by cellular kinases. Due to the absence of a 3′-hydroxyl group in their ribose molecules, the triphosphate compounds, once integrated into viral DNA, cause the termination of DNA chain elongation. This process inhibits HBV-DNA replication and effectively reduces the HBV viral load (Levrero and Zucman-Rossi, 2016). Studies have found that high HBV viral load in the serum can promote the growth and metastasis of HCC (Su et al., 2013; Huang et al., 2011). Radical resection alters the immune status of HBV-HCC patients, making them prone to HBV reactivation and high HBV viral load replication postoperatively (Li et al., 2020; Wang et al., 2023). Nucleos(t)ide analogs, which can inhibit HBV-DNA replication, effectively reduce the HBV-DNA level, thereby improving OS and RFS in these patients. Additionally, treatment with nucleos(t)ide analogs has been shown to reduce the levels of regulatory T cells (Tregs) in the peripheral blood of patients with chronic hepatitis B (He and Zhao, 2019; Xu, 2021). Research has indicated that CD4+CD25high regulatory T cells suppress Th cell responses and HBV-specific cytotoxic T lymphocyte-mediated immune responses, which are considered key contributors to HBV immune tolerance (Cheng et al., 2023; Yu, 2018). Nucleos(t)ide analogs can decrease the levels of peripheral Tregs, thereby reducing immune tolerance, modulating the patient’s immune status (He and Zhao, 2019), and facilitating viral clearance in HBV-HCC patients. This mechanism contributes to the improvement of OS and RFS in these patients.
Furthermore, our study findings suggested that different types of nucleos(t)ide analogs exhibited varying effects on improving OS and RFS in HBV-HCC patients. LAM, ENT, and ADV were less effective than Ldt in improving OS, while ENT was less effective than TDF. In terms of improving RFS, LAM was less effective than ENT, and both LAM and ADV were less effective than Ldt. Moreover, LAM, ENT, and ADV were all less effective than TDF. These differences may be attributed to variations in the specific mechanisms by which different nucleos(t)ide analogs inhibit HBV-DNA replication. LAM is a cytosine analog that inhibits DNA replication by competitively binding to the active site of HBV reverse transcriptase, thereby blocking its activity. ADV is an adenosine monophosphate analog that competes with natural nucleoside triphosphates (NTPs) to inhibit HBV DNA polymerase. ENT is a guanosine analog that competes with dGTP for HBV DNA polymerase. Ldt is a thymidine deoxynucleotide analog that competes with HBV’s natural substrate, thymidine 5′-adenosine. TDF is a diphosphate of fovir, which competes for incorporation into the viral DNA chain. Due to the absence of a 3′-OH group, tenofovir blocks DNA chain elongation and inhibits viral replication (Hruba et al., 2023; Zhang et al., 2019). Since HBV is a retrovirus, its reverse transcriptase lacks proofreading capability and cannot correct mismatched nucleotides (Yasutake et al., 2020). This characteristic leads to the presence of viral strains with diverse genetic sequences in HBV-HCC patients. During long-term postoperative use of nucleos(t)ide analogs, drug-resistant strains with greater survival ability and replication potential are gradually selected. Consequently, drug resistance frequently develops, leading to reduced sensitivity of the virus to antiviral therapy (Song et al., 2012; Liu et al., 2021). Clinical trial evidence has indicated that the rates of drug resistance vary depending on the type of nucleos(t)ide analog used in treatment. During LAM treatment, the drug resistance rate was approximately 20% after 1 year and increased to as high as 70% after 5 years. Furthermore, about 50% of patients who develop LAM resistance would also develop resistance to ENT within 5 years of treatment (Amini-Bavil-Olyaee et al., 2010). For ADV, the resistance rate was around 2% after 2 years and approximately 29% after 5 years (Roediger et al., 2022). During Ldt, the resistance rate was 11% after 2 years (Tacke and Kroy, 2016). For ENT, the resistance rate was 1.2% after 5 years of treatment (Takayama et al., 2021), while TDF showed a similarly low resistance rate during treatment (Li et al., 2021). This may be related to the poorer effectiveness of LAM in improving OS and RFS, compared to the better outcomes observed with Ldt, TDF, and ENT. The differences in resistance rates among various nucleos(t)ide analogs may be attributed to their specific resistance mechanisms and mutation sites. For LAM, resistance is primarily associated with mutations at rtM204I/V in the POL/RT region. ADV resistance is linked to mutations at rtN236T in the D domain. ENT resistance develops after the emergence of the rtM204V + rtL180M mutations. Ldt resistance is associated with mutations at rtL80I and rtL80V, while TDF resistance is related to mutations at rtP177G and rtF249A (Li et al., 2021).
This study has certain limitations. First, most of the included studies are cohort studies, with relatively few RCTs addressing the topic. Second, some of the included studies had small sample sizes. The study did not ascertain the effects of other non-nucleoside antiviral drugs, such as interferons, or combination therapy regimens due to the limited number of studies. All participants were from East Asian populations, limiting the generalizability of the conclusions. Caution is needed when applying these findings to populations in other regions. Future research should consider including multinational data to enhance global applicability. Third, many of the included RCTs were open-label designed, with no mention of allocation concealment or blinding methods, raising the possibility of implementation and measurement biases, which could affect the reliability of the results. Fourth, the outcome measures did not account for the incidence of adverse events, as only a few studies provided relevant data. Fifth, owing to the small number of included studies, publication bias was not examined. Sixth, excluding preoperative antiviral therapy may affect the external validity of the findings, as preoperative treatment is clinically significant in preventing viral reactivation and improving prognosis. Future research could consider evaluating the impact of preoperative treatment.
5 Conclusion
Different types of nucleos(t)ide analogs can improve both OS and RFS in HBV-HCC patients after radical resection. Although the findings of this study suggest that Ldt and TDF exhibit the most notable effects on OS and RFS in HBV-HCC patients, it may cause drug resistance. As the Ldt therapy is used continuously, drug resistance may occur, which may limit its long-term effectiveness. Due to the limitations of this study, the results of this study should be interpreted with caution. Clinicians should be cautious when applying these findings to long-term treatment plans. Further research is needed to explore strategies to prevent and manage drug resistance in these patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
HQ: Supervision, Writing – original draft, Writing – review and editing. YZ: Formal Analysis, Investigation, Writing – review and editing. FX: Methodology, Writing – review and editing. SX: Conceptualization, Funding acquisition, Resources, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was suppoeted by Scientific research project of Nantong Health Committee [grant number QA2020040] and Nantong Health and Wellness Committee Surface Program [grant number MB2020049].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1647888/full#supplementary-material
References
Amini-Bavil-Olyaee, S., Vucur, M., Luedde, T., Trautwein, C., and Tacke, F. (2010). Differential impact of immune escape mutations G145R and P120T on the replication of lamivudine-resistant hepatitis B virus e antigen-positive and -negative strains. J. Virol. 84 (2), 1026–1033. doi:10.1128/jvi.01796-09
Chen, Y. (2015). Role of antiviral treatment in hepatitis related liver cancer recurrence. Chin. J. Biochem. Pharm. 35 (01), 97–99.
Chen, X. X., Cheng, J. W., Huang, A., Zhang, X., Wang, J., Fan, J., et al. (2017). The effect of antiviral therapy on patients with hepatitis B virus-related hepatocellular carcinoma after curative resection: a systematic review and meta-analysis. Onco Targets Ther. 10, 5363–5375. doi:10.2147/ott.S150281
Cheng, F., Lyu, L., Sun, B. C., Li, G. Q., Li, X. C., Zhang, F., et al. (2011). A study of antiviral therapy in prevention of tumor recurrence after radical liver resection of hepatocellular carcinoma with high load of hepatitis B virus DNA. J. Nanjing Medicial Univ. 31 (06), 882–4+8.
Cheng, B. Q., Zhang, M., Wu, D. D., Huang, C., Huang, H., Chen, S. M., et al. (2023). Analysis of chronic hepatitis B disease progression with low ALT level by HBcrAg expression level combined with CD4+CD25+regulatory T cells. Fujian Med. J. 45 (05), 20–22. doi:10.3969/j.issn.1002-2600.2023.05.008
Choi, J., Jo, C., and Lim, Y. S. (2021). Tenofovir Versus entecavir on recurrence of hepatitis B virus–related hepatocellular carcinoma after surgical resection. Hepatology 73 (2), 661–673. doi:10.1002/hep.31289
Ding, C., Pan, F., Hu, H. Z., Xiong, R. H., Li, S., Jiang, Y., et al. (2014). Efficacy of antiviral therapy in hepatocellular carcinoma patients with high HBV DNA levels after radical resection. J. Clin. Hepatology 30 (07), 656–659. doi:10.3969/j.issn.1001-5256.2014.07.021
Fang, L., Zhou, Y., Wang, Y., and Li, X. Y. (2012). Influence of antiviral therapy on the clinical postoperative prognosis of primary liver cancer with positive HBV DNA. Clin. Med. & Eng. 19 (07), 1079–1081. doi:10.3969/j.issn.1674-4659.2012.07.1079
He, J. N., and Zhao, S. S. (2019). Effect of the nucleoside analogues in the treatment of chronic hepatitis B and its correlation with regulatory T cells of peripheral blood. J. Bengbu Med. Coll. 44 (03), 335–337. doi:10.13898/j.cnki.issn.1000-2200.2019.03.015
He, L., Xia, Z., Shen, J., Zhang, X., Peng, W., Li, C., et al. (2019). The different effects of adefovir dipivoxil and telbivudine on the prognosis of hepatitis b virus-related hepatocellular carcinoma patients after curative resection. Med. Baltim. 98 (6), e14386. doi:10.1097/MD.0000000000014386
Hruba, L., Das, V., Hajduch, M., and Dzubak, P. (2023). Nucleoside-based anticancer drugs: mechanism of action and drug resistance. Biochem. Pharmacol. 215, 115741. doi:10.1016/j.bcp.2023.115741
Hu, Z., Zeng, H., Hou, J., Wang, J., Xu, L., Zhang, Y., et al. (2022). Tenofovir vs. Entecavir on outcomes of Hepatitis B virus-related hepatocellular carcinoma after radiofrequency ablation. Viruses 14 (4), 656. doi:10.3390/v14040656
Huang, Y., Tong, S., Tai, A. W., Hussain, M., and Lok, A. S. (2011). Hepatitis B virus core promoter mutations contribute to hepatocarcinogenesis by deregulating SKP2 and its target, p21. Gastroenterology 141 (4), 1412–1421. doi:10.1053/j.gastro.2011.06.048
Huang, G., Yang, Y., Shen, F., Pan, Z. Y., Fu, S. Y., Lau, W. Y., et al. (2013). Early viral suppression predicts good postoperative survivals in patients with hepatocellular carcinoma with a high baseline HBV-DNA load. Ann. Surg. Oncol. 20 (5), 1482–1490. doi:10.1245/s10434-012-2803-7
Huang, G., Lau, W. Y., Wang, Z. G., Pan, Z. Y., Yuan, S. X., Shen, F., et al. (2015). Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann. Surg. 261 (1), 56–66. doi:10.1097/sla.0000000000000858
Jiang, Y., Han, Q., Zhao, H., and Zhang, J. (2021). The mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatocell. Carcinoma 8 (8), 435–450. doi:10.2147/jhc.S307962
Kao, W. Y., Tan, E. C. H., Lee, H. L., Huang, Y. H., Huo, T. I., Chang, C. C., et al. (2023). Entecavir versus tenofovir on prognosis of hepatitis B virus-related hepatocellular carcinoma after curative hepatectomy. Alimentary Pharmacol. Ther. 57 (11), 1299–1312. doi:10.1111/apt.17438
Ke, Y., Ma, L., You, X. M., Huang, S. X., Liang, Y. R., Xiang, B. D., et al. (2013). Antiviral therapy for hepatitis B virus-related hepatocellular carcinoma after radical hepatectomy. Cancer Biol. Med. 10 (3), 158–164. doi:10.7497/j.issn.2095-3941.2013.03.006
Kim, J. H., Sinn, D. H., Kim, K., Kim, H., Gwak, G. Y., Paik, Y. H., et al. (2016). Lamivudine versus Entecavir for newly diagnosed hepatitis B virus-related hepatocellular carcinoma. Gut Liver 10 (6), 939–947. doi:10.5009/gnl15527
Levrero, M., and Zucman-Rossi, J. (2016). Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 64 (1 Suppl. l), S84-S101–S101. doi:10.1016/j.jhep.2016.02.021
Li, C., Li, Z. C., Ma, L., Li, L. Q., Zhong, J. H., Xiang, B. D., et al. (2020). Perioperative antiviral therapy improves the prognosis of HBV DNA-negative patients with HBV-related hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 14 (8), 749–756. doi:10.1080/17474124.2020.1784727
Li, Y. H., Ge, F. L., Chen, R. J., Bai, Z. F., Li, Y., Zhao, J., et al. (2021). Research progress on nucleos(t)ide analogues resistance mechanism and mutation sites. Chin. J. Pharmacovigil. 18 (08), 795–799. doi:10.19803/j.1672-8629.2021.08.22
Li, P., Wang, Y., Yu, J., Yu, J., Tao, Q., Zhang, J., et al. (2023). Tenofovir vs Entecavir among patients with HBV-Related HCC after resection. JAMA Netw. Open 6 (10), e2340353. doi:10.1001/jamanetworkopen.2023.40353
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 6 (7), e1000100. doi:10.1371/journal.pmed.1000100
Lin, Q., Li, M. J., Li, H. Q., and Wei, G. (2016). Clinical study of entecavir on treating hepatitis B related hepatic carcinoma after radical operation. Chin. J. New Clin. Med. 9 (02), 139–142. doi:10.3969/j.issn.1674-3806.2016.02.14
Linye, H., Zijing, X., Xiaoyun, Z., Zhihui, L., Tianfu, W., and Chuan, L. (2023). Tenofovir versus entecavir on the prognosis of hepatitis B-related hepatocellular carcinoma after surgical resection: a randomised controlled trial. Int. J. Surg. 109 (10), 3032–3041. doi:10.1097/js9.0000000000000554
Liu, Y., Chen, R., Liu, W., Si, L., Li, L., Li, X., et al. (2021). Investigation of multidrug-resistance mutations of hepatitis B virus (HBV) in a large cohort of chronic HBV-infected patients with treatment of nucleoside/nucleotide analogs. Antivir. Res. 189, 105058. doi:10.1016/j.antiviral.2021.105058
Lo, C. K., Mertz, D., and Loeb, M. (2014). Newcastle-ottawa scale: comparing reviewers' to authors' assessments. BMC Med. Res. Methodol. 14, 45. doi:10.1186/1471-2288-14-45
Qi, W., Zhang, Q., Xu, Y., Wang, X., Yu, F., Zhang, Y., et al. (2020). Peg-interferon and nucleos(t)ide analogue combination at inception of antiviral therapy improves both anti-HBV efficacy and long-term survival among HBV DNA-positive hepatocellular carcinoma patients after hepatectomy/ablation. J. Viral Hepat. 27 (4), 387–396. doi:10.1111/jvh.13236
Qi, W., Shen, J., Dai, J., Wu, Y., Zhang, Y., Leng, S., et al. (2021). Comparison of nucleoside and nucleotide analogs in the recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection: a multicenter study. Cancer Med. 10 (23), 8421–8431. doi:10.1002/cam4.4348
Ren, Y., Liu, J. Y., Zhang, Z. M., Ouyang, G. X., Li, J. H., and Xiang, B. D. (2018). Influence of antiviral therapy on postoperative liver function and prognosis of HBV DNA-negative patients with HBV related hepatocellular carcinoma. Chin. J. Cancer Prev. Treat. 25 (5), 349–352.
Roediger, R., Smyth, E. K., and Dieterich, D. (2022). Adefovir for lamivudine-resistant hepatitis B. Antivir. Ther. 27 (2), 13596535211067605. doi:10.1177/13596535211067605
Rui, S., Yan, J., Zhang, H., Wang, Z., and Zhou, W. (2017). Intermediate-stage hepatocellular carcinoma patients with a high HBV-DNA load may benefit from postoperative anti-hepatitis B virus therapy. Med. Baltim. 96 (30), e7608. doi:10.1097/md.0000000000007608
Shen, J., Liu, J., Li, C., Wen, T., Yan, L., and Yang, J. (2018). The prognostic significance of serum HBeAg on the recurrence and long-term survival after hepatectomy for hepatocellular carcinoma: a propensity score matching analysis. J. Viral Hepat. 25 (9), 1057–1065. doi:10.1111/jvh.12911
Shen, J., Qi, W., Dai, J., Leng, S., Jiang, K., Zhang, Y., et al. (2022). Tenofovir vs. entecavir on recurrence of hepatitis B virus-related hepatocellular carcinoma beyond Milan criteria after hepatectomy. Chin. Med. J. Engl. 135 (3), 301–308. doi:10.1097/CM9.0000000000001864
Shin, H. S., Kim, S. U., Park, J. Y., Kim, D. Y., Han, K. H., Chon, C. Y., et al. (2012). Antiviral efficacy of lamivudine versus entecavir in patients with hepatitis B virus-related advanced hepatocellular carcinoma. J. Gastroenterol. Hepatol. 27 (9), 1528–1534. doi:10.1111/j.1440-1746.2012.07145.x
Song, Z. L., Cui, Y. J., Zheng, W. P., Teng, D. H., and Zheng, H. (2012). Diagnostic and therapeutic progress of multi-drug resistance with anti-HBV nucleos(t)ide analogues. World J. Gastroenterol. 18 (48), 7149–7157. doi:10.3748/wjg.v18.i48.7149
Su, C. W., Chiou, Y. W., Tsai, Y. H., Teng, R. D., Chau, G. Y., Lei, H. J., et al. (2013). The influence of hepatitis B viral load and Pre-S deletion mutations on post-operative recurrence of hepatocellular carcinoma and the tertiary preventive effects by anti-viral therapy. PLoS One 8 (6), e66457. doi:10.1371/journal.pone.0066457
Tacke, F., and Kroy, D. C. (2016). Treatment for hepatitis B in patients with drug resistance. Ann. Transl. Med. 4 (18), 334. doi:10.21037/atm.2016.09.19
Takayama, H., Komura, T., Kagaya, T., Sugimoto, S., Orita, N., Asahina, Y., et al. (2021). Clinical features and resistance to Entecavir monotherapy of patients with hepatitis B. Can. J. Gastroenterol. Hepatol. 2021, 3259833. doi:10.1155/2021/3259833
Wang, X. H., Hu, Z. L., Fu, Y. Z., Hou, J. Y., Li, W. X., Zhang, Y. J., et al. (2022). Tenofovir vs. entecavir on prognosis of hepatitis B virus-related hepatocellular carcinoma after curative resection. J. Gastroenterol. 57 (3), 185–198. doi:10.1007/s00535-022-01855-x
Wang, S., Zhang, L. T., Gao, X. Q., and Zhou, D. (2023). Effect of preoperative antiviral therapy on viral reactivation and liver function in patients with HBV-DNA negative hepatocellular carcinoma. Fertil. Health 29 (06), 37–39. doi:10.3969/j.issn.1006-9488.2023.06.013
Xiao, C., Huang, L., Xiao, N., and Lun, J. (2021). Effect of radical resection combined with antiviral therapy in patients with hepatitis B virus-associated hepatocellular carcinoma and prognostic analysis. J. BUON 25 (6), 2576–2583.
Xu, F. (2021). Analyzing the effects of interferon and nucleotide analogs on peripheral blood regulatory T cell levels in patients with chronic hepatitis B. China Pract. Med. 16 (24), 157–159. doi:10.14163/j.cnki.11-5547/r.2021.24.055
Xu, M., Zhou, Z., Xu, R., Zhang, H., Lin, N., and Zhong, Y. (2019). Antiviral therapy predicts the outcomes following resection of hepatocellular carcinoma in patients negative for HBV DNA: a propensity score matching analysis. World J. Surg. Oncol. 17 (1), 45. doi:10.1186/s12957-019-1577-9
Yasutake, Y., Hattori, S. I., Tamura, N., Matsuda, K., Kohgo, S., Maeda, K., et al. (2020). Structural features in common of HBV and HIV-1 resistance against chirally-distinct nucleoside analogues entecavir and lamivudine. Sci. Rep. 10 (1), 3021. doi:10.1038/s41598-020-59775-w
Yu, X. (2018). Comparative analysis of the distribution characteristics of CD8∼+CD28∼- and CD4∼+CD25∼(high) regulatory T cells in peripheral blood of hepatitis B patients and their clinical significance. Suzhou: Soochow University.
Zhang, X. Y. (2015). The role of antiviral treatment in HBV related liver cancer recurrence. J. Trop. Med. 15 (07), 946–9+1008.
Zhang, Z. Y., Zhou, Z. Q., and Zhou, G. W. (2014). Higher efficacy of antiviral therapy after major hepatectomy in patients with hepatitis B virus-related hepatocellular carcinoma of less than 3 cm. Eur. J. Gastroenterol. Hepatol. 26 (10), 1116–1124. doi:10.1097/meg.0000000000000153
Zhang, J. F., An, H. L., Su, R., and Lyu, W. F. (2019). Progress in research on hepatitis B virus resistance. J. Mol. Diagnosis Ther. 11 (05), 434–440. doi:10.3969/j.issn.1674-6929.2019.05.020
Zhong, J. H., Ke, Y., Zhu, S. L., Wang, L., Luo, C. P., Gong, W. F., et al. (2016). Adefovir dipivoxil is less expensive than lamivudine and associated with similar prognosis in patients with hepatitis B virus-related hepatocellular carcinoma after radical resection. Onco Targets Ther. 9, 6897–6907. doi:10.2147/ott.S120062
Keywords: hepatitis B virus, hepatocellular carcinoma, antiviral therapy, nucleos(t)ideanalogs, recurrence-free survival
Citation: Qiu H, Zhang Y, Xu F and Xue S (2025) Different nucleos(t)ide analogs in resected hepatitis B virus-associated hepatocellular carcinoma: a systematic review. Front. Pharmacol. 16:1647888. doi: 10.3389/fphar.2025.1647888
Received: 30 June 2025; Accepted: 30 October 2025;
Published: 14 November 2025.
Edited by:
Piyameth Dilokthornsakul, Chiang Mai University, ThailandReviewed by:
H. Syed Iqbal, YR Gaitonde Centre for AIDS Research and Education, IndiaYutang Li, Fudan University, China
Copyright © 2025 Qiu, Zhang, Xu and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songhui Xue, bWlhb21pYW8yMDE0MDMxOUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Hongquan Qiu
Hongquan Qiu Yu Zhang
Yu Zhang Fengxia Xu3
Fengxia Xu3