Abstract
Introduction:
Coronavirus disease 2019 (COVID-19) is an epidemic respiratory disease caused due to the infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In China, the National Health Commission of China announced that patients with COVID-19 who were treated with traditional Chinese medicines (TCMs) combined with antiviral drugs effectively alleviated their symptoms and recovered. Among these TCMs, Xuebijing (XBJ) injection plays an important role in the treatment of patients with COVID-19. However, this was a puzzle that what will be the clinical efficacy and safety of XBJ injection for COVID-19 treatment, and what are the potential mechanisms behind XBJ injection?
Methods:
To search for articles on “Xuebijing injection in the treatment of COVID-19” in PubMed, use the following query: (Xuebijing injection OR Xuebijing) AND (COVID-19 OR SARS-CoV-2 OR severe pneumonia). We added filters for “Clinical Trial,” “Randomized Controlled Trial,” or “Review” to focus on specific study types, and limit the search to recent years (2010–2025) and English-language articles for more targeted results.
Results:
XBJ injection in combination with regular therapy has been shown to improve overall efficacy, reduce 28-day mortality, improve lung CT recovery and reduce pro-inflammatory markers in patients with COVID-19. The high affinity for angiotensin converting enzyme 2, inhibition of neutrophil extracellular trap release and prevention of cell death and inflammation may be the main molecular mechanisms of XBJ injection in the treatment of COVID-19.
Conclusion:
This review synthesizes the current evidence on the clinical efficacy and safety of XBJ injection in the treatment of COVID-19. Our analysis indicates that XBJ injection, when used in combination with standard therapy, significantly improves overall efficacy, reduces 28-day mortality, enhances lung CT recovery, and decreases pro-inflammatory markers such as C-reactive protein (CRP) and interleukin-6 (IL-6). These findings suggest that Xuebijing injection is a promising adjunctive treatment for COVID-19, particularly in severe cases, although it must be confirmed through rigorous pharmacological and clinical studies.
1 Introduction
Coronavirus disease 2019 (COVID-19) is an epidemic respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (Yüce et al., 2021; Lino et al., 2025). COVID-19 is highly contagious, characterized by atypical clinical symptoms and easily missed diagnosis, which seriously affects the socio-economic development and health of the population (Hadj, 2022; de Melo et al., 2025a). As of October 2021, the number of confirmed COVID-19 infections has exceeded 242 million globally, with nearly five million deaths reported across multiple countries, according to the World Health Organization (WHO) and other international health agencies (Sharma et al., 2021). Although COVID-19 vaccination can partially reduce the incidence and severity of the disease, it cannot prevent infection (Fernandes et al., 2022). In the early stage of COVID-19, there are mainly fever, fatigue, dry cough and other manifestations, and such as upper respiratory tract symptoms (nasal congestion, runny nose, etc.) is less common; in addition, some patients will also have varying degrees of hypoxia. Many patients will develop respiratory distress 1 week after the onset of the disease and may even progress to septic shock, acute respiratory distress syndrome (ARDS), or even death (Szafran et al., 2023). This pandemic has highlighted the significant role of infectious agents, including viruses and bacteria, in the progression and severity of various diseases. Historically, we have faced numerous disease outbreaks, such as the West Nile Virus, sexually transmitted infections, measles, malaria, and others, each posing unique challenges to public health (Cilloniz et al., 2016; Sharma et al., 2022; Singh et al., 2022; Heidecke et al., 2025). Therefore, it is necessary to provide active treatment and achieve early detection, early isolation, and early treatment (Radcliffe et al., 2023).
SARS-CoV-2 is a coronavirus of the genus β, with an envelope, and the particles are presented as elliptical or round, in addition, most of them are polymorphic, with a general diameter of 60–140 nm; and in terms of genetic characterization, it is different from SARSr-CoV (Chen et al., 2023; Shahi et al., 2023). SARS-CoV-2 has been reported to be >85% homologous to the bat SARS-like coronavirus (bat-SL-CoVZC45) (Cilibrasi and Vitányi, 2022; de Melo et al., 2025b). Some studies suggest that SARS-CoV-2 is sensitive to heat and ultraviolet light and can be killed by lipid solvents such as ethanol, 75% ethanol, and peroxyacetic acid (Mathew et al., 2023). Drug repurposing has emerged as a vital strategy in addressing emerging and challenging diseases, including COVID-19. By identifying new therapeutic uses for existing drugs, this approach can accelerate the development of treatments and reduce the time and cost associated with drug discovery (Singh et al., 2022). Additionally, computational approaches have been employed to predict the role of pathogens in various diseases, such as the targeting of Mycoplasma hominis proteins in prostate cancer etiology (Heidecke et al., 2025; Khan et al., 2017; Khan et al., 2016a) and the implications of Helicobacter pylori proteins in gallbladder cancer (Khan et al., 2016b). Antiviral drugs are mostly used to treat COVID-19, such as ritonavir and α-interferon nebulization, but the therapeutic effect is not exact. In China, the National Health Commission of China announced that patients with COVID-19 who were treated with traditional Chinese medicine (TCM) combined with antiviral drugs had their symptoms effectively alleviated and recovered (Liu, 2020; Zhang et al., 2025). Among these TCMs, Xuebijing (XBJ) injection plays a critical role in the treatment of patients with COVID-19, which is recommended by the National Health Commission of China to treat severe and critical cases of COVID-19 (China NHCotPsRo, 2022).
XBJ injection is a Chinese botanical medicine developed by Prof. Jinda Wang based on the principle of “Three Evidences and Three Methods” and the theory of “simultaneous treatment of bacteria and virus” and Xuefu Zhuyu decoction with the effects of dispersing toxins, resolving blood stasis, and activating collaterals (Zhang et al., 2023; Liao et al., 2025). XBJ injection is composed of five traditional Chinese botanical drugs, including Angelica sinensis (Oliv.) Diels (Angelicae sinensis Radix, Danggui), Ligusticum chuanxiong Hort. (Chuanxiong rhizoma, Chuanxiong), Paeonia lactiflora Pall. (Paeoniae radix Rubra, Chishao), Carthamus tinctorius L. (Carthami Flos, Honghua), and Salvia miltiorrhiza Bge. (Salviae miltiorrhizae Radix Et Rhizoma, Danshen) (Li et al., 2021; Cui et al., 2025). These herbs are known for their anti-inflammatory, antioxidant, and antiviral properties. On 12 April 2020, the National Drug Administration (NMPA) of China approved the use of XBJ for “COVID-19 pneumonia with severe, critically ill systemic inflammatory response syndrome or/and multi-organ failure”. However, what is the clinical efficacy and safety of XBJ injection for COVID-19 treatment and what are the potential effects behind XBJ injection? This review summarizes the basic and clinical research progress of XBJ injection for COVID-19 treatment to date, and expects to provide clinical evidence for the effects and safety of clinical use of XBJ injection for COVID-19 patients.
2 Potential related metabolites of XBJ injection
To search for articles on “Xuebijing injection in the treatment of COVID-19,” we conducted a comprehensive literature search using PubMed. The query used was: (Xuebijing injection OR Xuebijing) AND (COVID-19 OR SARS-CoV-2 OR severe pneumonia). We added filters for “Clinical Trial,” “Randomized Controlled Trial,” or “Review” to focus on specific study types and limited the search to recent years (2010–2025) and English-language articles for more targeted results. While we acknowledge that other databases such as Embase, Web of Science, Scopus, Cochrane Library, and CNKI may also contain relevant studies, we chose to focus on PubMed due to its extensive coverage of high-quality biomedical literature, ease of access, and the efficiency of its search tools. This approach allowed us to efficiently identify and analyze a substantial number of relevant studies for our review. In the pharmacological studies reviewed, the dose range tested varied from 10 μM to 100 µM for in vitro studies and 10 mg/kg to 100 mg/kg for in vivo studies. The minimal active concentration was reported to be 10 µM in several in vitro studies. The models used included HEK-293T cells, Vero-E6 cells, and various animal models such as mice and rats. Both positive and negative controls were used in these studies, with durations ranging from 24 h to 14 days. The type of extract used was specified in each study, and basic pharmacological data were provided to assess the claims.
Artificial intelligence (AI) and bioinformatics play a pivotal role in managing newly arising diseases and epidemics. These technologies enable the rapid identification of potential drug targets and the prediction of pathogen behavior, facilitating the development of effective treatment strategies (Zhou et al., 2020). More than 100 metabolites have been discovered in XBJ injection as it is a chemically complex botanical injection (Chen et al., 2025). Among them, danshensu, hydroxysafflor yellow A, paeoniflorin, ferulic acid, and senkyunolide I have been characterized as the major related metabolites of XBJ injection (Li et al., 2019; Li et al., 2018; Chen et al., 2010; Zuo et al., 2019; Chen et al., 2012). Advanced analytical techniques such as ultra-high performance liquid chromatography-high resolution hybrid quadrupole-orbitrap mass spectrometry (UHPLC-Q-Orbitrap MS) and ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS) have further characterized additional metabolites, such as gallic acid, 5-hydroxymethylfurfural, matrine, tanshinone IIA, protocatechuic aldehyde, caffeic acid, galuteolin, apigenin, benzoylpaeoniflorin, kaempferol, and ethyl ferulate (Sun et al., 2017; Zuo et al., 2017a; Huang et al., 2013; Ouyang and He, 2018; Zuo et al., 2017b; Ji et al., 2010; Huang et al., 2011). These metabolites have demonstrated potential pharmacological activities in vitro or in animal models, including inhibition of inflammation, prevention of cell death, and enhancement of immune function, though their clinical relevance remains to be established.
Through pharmacokinetic investigation, 10 phthalides and 17 danshen catechols, including nine major metabolites, have been discovered in sepsis treatments (Zhang et al., 2018; Li et al., 2016). Meanwhile, 18 monoterpene glycosides have been discovered by LC/TOF-MS in XBJ antiseptic injection in humans and rats (Cheng et al., 2016). In addition, one of the most pharmaceutically relevant metabolites in XBJ injection for the treatment of traumatic brain injury, hydroxysafflor yellow A, has been shown to cross the blood-brain barrier, suggesting the potential use of XBJ injection in brain diseases (Sheng et al., 2020). A summary of the potential related metabolites of XBJ injection is shown in Table 1. In addition, Li et al. recently reported an aggregation-induced emission sensor combined with UHPLC-Q-TOF/MS that can rapidly identify anticoagulants from XBJ injection (Li et al., 2023). The total inhibition rate of the six mixed standards was approximately 60% of the inhibition rate of XBJ injection, providing a novel, inexpensive, and simple method for monitoring thrombin activity and screening agents from XBJ injection. The following is a list of important active metabolites and their underlying mechanisms (Table 2). While analytical methods such as UPLC-Q-TOF-MS and RP-HPLC are essential for identifying and quantifying metabolites, they do not provide direct evidence of therapeutic activity. Therefore, we have focused on studies that provide in vitro, in vivo, and clinical evidence for the activity of these metabolites.
TABLE 1
| Disease | Methods | Metabolites | Potential related metabolites | References |
|---|---|---|---|---|
| Sepsis | Literature-mining and in vitro experiments | 12 | Hydroxysafflor yellow A, paeoniflorin, oxypaeoniflorin, albiflorin, senkyunolides I and G, tanshinol, salvianolic acid B, protocatechuic acid, ferulic acid, senkyunolide I-7-O-β-glucuronide, and 3-O-methyltanshinol | Li et al. (2019) |
| N/A | UHPLC-Q exactive hybrid quadrupole-orbitrap high-resolution MS | 162 (including 38 major metabolites) | Gallic acid, 5-hydroxymethylfurfural, matrine, salvianic acid A sodium, tanshinol, protocatechuic acid, protocatechuic aldehyde, tetramethylpyrazine, catechin, chlorogenic acid, caffeic acid, oxypaeoniflorin, hydroxysafflor yellow A, albiflorin, paeoniflorin, ferulic acid, rutin, hyperin, quercetin, luteolin-O-glc, senkyunolide I/H, rosmarinic acid, crocin I, salvianolic acid B, luteolin, salvianolic acid A, naringenin, paeonol, apigenin, benzoylpaeoniflorin, kaempferol, ethyl ferulate, ligustilide, butylidenephthalide, tanshinone I, cryptotanshinone, levistolide A, and tanshinoneαA | Sun et al. (2017) |
| N/A | HPLC-DAD | 5 | Danshensu, hydroxysafflor yellow A, paeoniflorin, ferulic acid, and senkyunolide I | Li et al. (2018) |
| N/A | UHPLC-Q-Orbitrap MS | 30 | Gallic acid, 5-hydroxymethylfurfural, sodium danshensu, tanshinol, protocatechuic acid, hydroxysafflor yellow A, oxypaeoniflorin, chlorogenic acid, catechinic acid, hyperoside, rutin, albiflorin, protocatechuic aldehyde, caffeic acid, galuteolin, salvianolic acid B, rosmarinic acid, ferulic acid, salvianolic acid a, senkyunolide I, quercetin, luteolin, benzoylpaeoniflorin, apigenin, naringenin, ethyl ferulate, paeonol, butylidenephthalide, cryptotanshinone, and tanshinone IIA | Zuo et al. (2017a) |
| N/A | UPLC-Q-TOF-MS | 13 | Uridine, gallic acid, guanosine, danshensu, protocatechualdehyde, oxypaeoniflorin, hydroxysafflor yellow A, paeoniflorin, ferulic acid, safflor yellow A, senkyunolide I, senkyunolide H, and salvianolic acid B | Huang et al. (2013) |
| N/A | RP-HPLC | 5 | hydroxysafflor yellow A, paeoniflorin, ferulic acid, benzoic acid, and danshensu | Chen et al. (2010) |
| Sepsis | Pharmacokinetic investigation | 10 Phthalides | Senkyunolide I, senkyunolide H, senkyunolide G, senkyunolide N, 3-hydroxy-3-n-butylphthalide, Z-6,7-epoxyligustilide, 6,7-dihydroxyligustilide, senkyunolide A, senkyunolide J, and 4-hydroxy-3-nbutylphthalide | Zhang et al. (2018) |
| Sepsis | Pharmacokinetic investigation | 17 Danshen catechols (including 9 major metabolites) | Tanshinol, salvianolic acid B, protocatechuic acid, isosalvianolic acid C, rosmarinic acid, protocatechuic aldehyde, salvianolic acid D, salvianolic acid C, and lithospermic acid | Li et al. (2016) |
| Sepsis | LC/TOF-MS | 18 monoterpene glycosides | Mudanpioside F, 1-O-β-D-glucopyranosyl-paeonisuffrone, desbenzoylpaeoniflorin, albiflorin, paeoniflorin, oxypaeoniflorin, oxypaeoniflorin isomer, ortho-oxypaeoniflorin, mudanpioside E, 6′-O-galloyl-desbenzoylpaeoniflorin, benzoylpaeoniflorin, benzoyloxypaeoniflorin, mudanpioside C, mudanpioside J, galloylpaeoniflorin, isomer of galloylpaeoniflorin or galloylalbiflorin, and galloyloxypaeoniflorin | Cheng et al. (2016) |
| N/A | UHPLC-Q-Orbitrap HRMS | 4 | Hydroxysafflor yellow A, oxypaeoniflorin, ferulic acid, and benzoylpaeoniflorin | Zuo et al. (2019) |
| N/A | HPLC-MS/MS | 9 | Ferulic acid, benzoylpaeoniflorin, danshensu, chlorogenic acid, rosmarinic acid, hydroxy-methyl safflower yellow A, paeoniflorin, albiflorin, and oxypaeoniflorin | Ouyang and He (2018) |
| N/A | UHPLC-Q-Orbitrap HRMS | 12 | Gallic acid, hydroxysafflor yellow A, oxypaeoniflorin, chlorogenic acid, rutin, luteoloside, albiflorin, hyperoside, rosmarinic acid, ferulic acid, salvianolic acid A, and benzoylpaeonif-lorin | Zuo et al. (2017b) |
| N/A | HPLC-MS/MS | 4 | Danshensu, hydroxysafflor yellow A, paeoniflorin, and ferulic acid | Chen et al. (2012) |
| Traumatic brain injury | LC-MS/MS | 1 | Hydroxysafflor yellow A | Sheng et al. (2020) |
| N/A | UPLC-Q-TOF-MS | 11 | Paeoniflorin, senkyunolide I, safflor yellow A, danshensu, uridine, ferulic acid, salvianolic acid B, uridine, senkyunolide H, gallic acid, and protocatechuic aldehyde | Ji et al. (2010) |
| N/A | HPLC-ESI-MS | 21 | Uridine, gallic acid, guanosine, danshensu, protocatechuic aldehyde, hydroxysafflor yellow A, oxypaeoniflorin, 6-hydroxykaemferol 3,6,7-tri-O-β-D-glucopyranoside, 6-hydroxykaemferol 3,6-Di-O-β-D-glucopyranosyl-7-O-β-D-glucuronopyranoside, caffeic acid, albiflorin, 4′,5,6,7-Tetrahydroxyflavanone 6,7-Di-O-β-D-glucopyranoside, paeoniflorin, ferulic acid, galloylpaeoniflorin, anhydrosafflor yellow B, safflor yellow A, senkyunolide I, senkyunolide H, salvianolic acid B, and benzoylpaeoniflorin | Huang et al. (2011) |
| N/A | UPLC-QTOF-MS | 5 | ML 334, Deoxynyboquinone, Tanshinone IIA, Luteolin, Baicalein | Lin et al. (2025) |
Related metabolites of XBJ injection.
UPLC-Q-TOF-MS, ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry; UHPLC-Q-Orbitrap HRMS, ultra-high performance liquid chromatography-Q, executive hybrid quadrupole-orbitrap high-resolution accurate mass spectrometry; UHPLC-Q-Orbitrap MS, ultra-high performance liquid chromatography-Q, executive hybrid quadrupole-orbitrap mass spectrometry; HPLC-ESI-MS, high-performance liquid chromatography electrospray ionization-tandem mass spectrometry.
TABLE 2
| Metabolite names | Chemical structures | Molecular mechanisms |
|---|---|---|
| Danshensu |
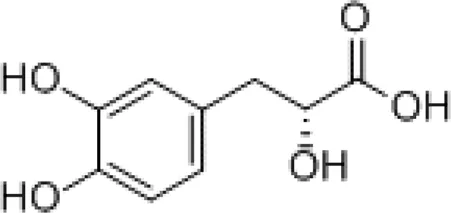
|
Preventing SARS-CoV-2 from entering certain cells |
| Hydroxysafflor yellow A |
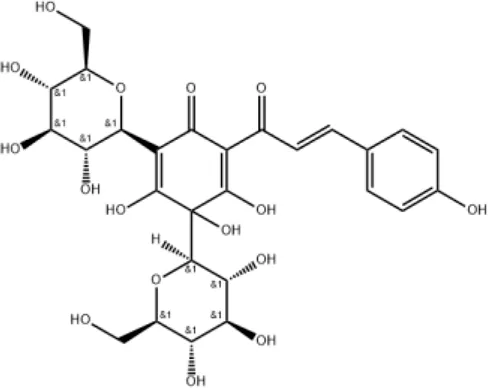
|
Inhibiting TLR4-dependent pathways and activating antioxidant mechanisms |
| Paeoniflorin |
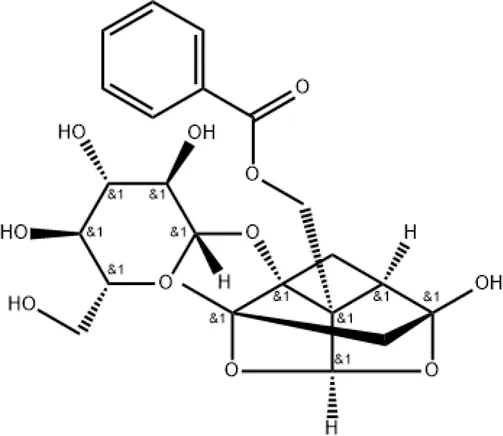
|
Regulating MAPK/NF-κB, PI3K/AKT/mTOR, and JAK2/STAT3 pathways |
| Ferulic acid |
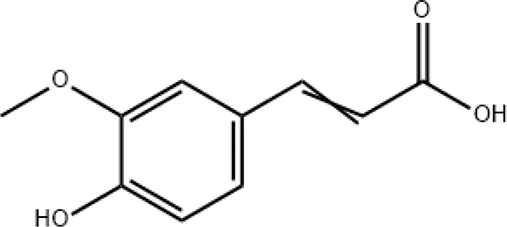
|
Targeting autophagy and protecting against intracerebral hemorrhages |
| Senkyunolide I |
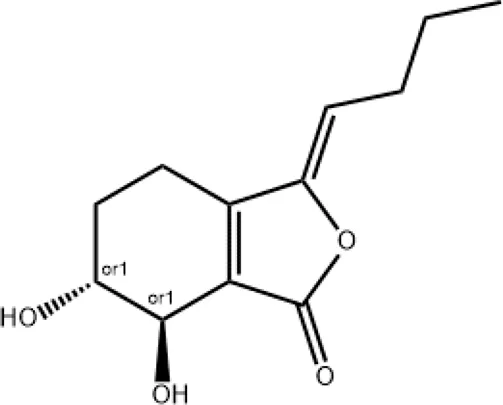
|
Preventing the formation of neutrophil extracellular traps |
| Baicalein |
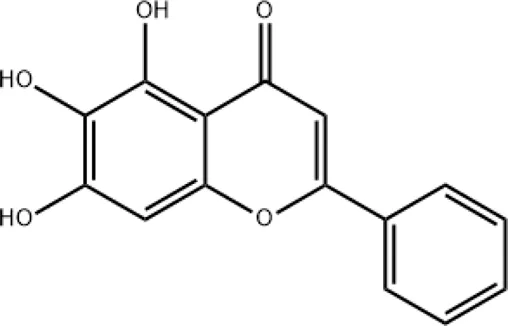
|
Targeting the KEAP1 protein, thereby activating the NRF2/KEAP1 signaling pathway |
A list of important active metabolites and their underlying mechanisms.
2.1 Danshensu
Danshensu is a pure molecule derived from the root of S. miltiorrhiza and is also one of the main active metabolites in XBJ injection (Zhang et al., 2019). In vitro studies have shown that danshensu inhibits the entry of SARS-CoV-2 into ACE2-overexpressed cells (Bourgonje et al., 2020). Both oral and intravenous pretreatment with danshensu dose-dependently ameliorated the pathological changes in mice infected with pseudotyped SARS-CoV-2 (Wang et al., 2022). Meanwhile, a study by Wang et al. showed that danshensu is a covalent inhibitor of 3-chymotrypsin-like protease against SARS-CoV-2 (Wang et al., 2022).
2.2 Hydroxysafflor yellow A
Hydroxysafflor yellow A can be extracted from the flower of C. tinctorius L and is also one of the main active metabolites in XBJ injection (He et al., 2021). Hydroxysafflor yellow A could reduce pathological changes, pulmonary edema, pulmonary vascular permeability, levels of inflammatory mediators, and myeloperoxidase (MPO) activity in mice with acute lung injury (ALI) induced by lipopolysaccharide (LPS) or bleomycin (Wu et al., 2012; Sun et al., 2010). The mechanisms involve inhibition of TLR4-dependent MAPK and NF-κB pathways (Liu et al., 2014). In addition, hydroxysafflor yellow A (15 mg/kg) can inhibit lung injury in an oleic acid-induced ALI rat model by activating antioxidant enzymes and inactivating the inflammatory response via the cAMP/PKA pathway (Wang et al., 2013).
2.3 Paeoniflorin
Paeoniflorin can be extracted from Paeoniae Radix Rubra (Chishao in Chinese) and is also one of the main active metabolites in XBJ injection. It has various pharmacological effects and has been shown to protect mice against LPS-induced ALI (Zhou et al., 2011). The anti-inflammatory mechanism of paeoniflorin is associated with the regulation of MAPK/NF-κB, PI3K/AKT/mTOR, and JAK2/STAT3 pathways (Zhang and Wei, 2020). However, the mechanisms of paeoniflorin in the treatment of COVID-19 remain to be elucidated.
2.4 Ferulic acid
Ferulic acid (4-hydroxy-3-methoxycinnamic acid) is a phenolic bioactive metabolite belonging to a group of hydroxycinnamates and is also one of the main active metabolites in XBJ injection. It has high antioxidant activity, antibacterial, anti-inflammatory, neuro- and photoprotective, antidiabetic, anticancer, and skin-whitening effects (Stompor-Gorący and Machaczka, 2021). Salman et al. investigated the effects of ferulic acid on anti-SARS-CoV-2 (Salman et al., 2020). A preclinical finding showed that ferulic acid attenuated COVID-19 by targeting autophagy (Pang et al., 2022). In addition, ferulic acid has been reported to have neuroprotective potentials against COVID-19 intracerebral hemorrhage (Dong et al., 2023).
2.5 Senkyunolide I
Senkyunolide I can be extracted from Ligusticum Chuanxiong hort and is also one of the main active metabolites in XBJ injection (Li et al., 2012). Senkyunolide I has the effects of anti-migraine, anti-inflammation, anti-oxidation and sedation (Hu et al., 2016; Hu et al., 2015; Wang et al., 2011). In a study be Zha et al., Senkyunolide I protected against ALI via inhibiting formation of neutrophil extracellular traps in a murine model of cecal ligation and puncture (Zha et al., 2021). However, the mechanisms of paeoniflorin in the treatment of COVID-19 remain to be elucidated.
2.6 Baicalein
Baicalein can be extracted from Honghua/Chishao. According to Lin et al. (Lin et al., 2025), baicalein plays an important role in treating SARS-CoV-2 infection. Baicalein targets the KEAP1 protein, thereby activating the NRF2/KEAP1 signaling pathway. This pathway increases the expression of antioxidant enzymes, such as heme oxygenase-1. This enhances the cells’ antioxidant capacity and reduces oxidative stress damage.
However, several metabolites identified in XBJ injection, such as ferulic acid, are known to be pan-assay interfering substances (PAINS) in in vitro assays (Sifontes-Rodríguez et al., 2025). PAINS are compounds that exhibit activity across a wide range of assays, often due to nonspecific interactions rather than specific biological activity. This can lead to false-positive results in high-throughput screening and other in vitro studies, potentially inflating the perceived effects of certain metabolites. The presence of PAINS in XBJ injection raises important questions about the validity of conclusions drawn from in vitro studies. While these compounds may show promising results in laboratory settings, their true therapeutic potential must be validated through in vivo and clinical studies to avoid false positives. For instance, ferulic acid has been shown to exhibit antioxidant and anti-inflammatory properties in in vitro assays. However, its effectiveness in a biological context may be limited by its nonspecific interactions, which could interfere with accurate assessment of its therapeutic potential. To critically assess the impact of PAINS on the interpretation of the effects of XBJ injection, the therapeutic potential of metabolites identified in vitro should be confirmed through in vivo animal models and clinical trials. This step is crucial to differentiate between true biological activity and nonspecific interactions caused by PAINS. Moreover, researchers should be cautious about selectively reporting positive results from in vitro studies without considering the potential for PAINS interference. A comprehensive evaluation of both positive and negative findings is necessary to provide a balanced view of the metabolite’s effects. To mitigate the impact of PAINS, the use of orthogonal assays that are less susceptible to nonspecific interactions is recommended. This approach can help confirm the specificity of the observed effects and provide more reliable data. When reviewing the literature on XBJ injection, it is important to critically evaluate studies that rely solely on in vitro findings. The presence of PAINS should be considered as a potential confounding factor, and conclusions should be drawn with caution until in vivo and clinical data are available.
In summary, although in vitro studies have identified several metabolites in XBJ injection with potential therapeutic effects, the presence of PAINS necessitates a cautious approach. Further confirmation through in vivo and clinical investigations is essential to validate the true effects of these metabolites and to ensure that the conclusions drawn are not influenced by nonspecific interactions.
3 Research on material basis
XBJ injection is composed of five traditional Chinese medicinal materials. The main components of these raw materials include danshensu, hydroxysafflor yellow A, paeoniflorin, ferulic acid, and senkyunolide I, etc. These components have higher content in the medicinal materials and possess clear pharmacological activities. During the preparation process of XBJ injection, the transfer and degradation of the main components are analyzed. Through high-performance liquid chromatography (HPLC) and mass spectrometry (MS), some components would undergo a certain degree of degradation during the extraction and purification processes. For example, the water-soluble components in Salvia miltiorrhiza may partially transform into other compounds during the high-temperature extraction process (Liu et al., 2025). By optimizing the extraction process, the degradation of components is minimized and a component traceability chain is established to ensure that the changes of components from raw materials to finished products can be effectively tracked. A component traceability chain from “raw materials-intermediates-finished products” is established. By analyzing the components at each stage, the patterns of component changes during the preparation process are clarified. The establishment of this traceability chain helps us to better control the product quality and ensure the stability and consistency of the components in XBJ injection.
4 Potential targets of XBJ injection
Network analysis and molecular docking analysis approaches were used to investigate the active metabolites, potential molecular targets, and predictive mechanisms of XBJ injection (Lin et al., 2025; Tianyu and Liying, 2021; Xing et al., 2020; Niu et al., 2021; Zheng et al., 2020; Tao and Jiming, 2022). These studies identified the potential related metabolites using online databases, including Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, http://tcmspw.com/tcmsp.php), Encyclopedia of Traditional Chinese Medicine (ETCM, http://www.nrc.ac.cn:9090/ETCM/index.php/Home/Index/index.html), PubChem (https://pubchem.ncbi.nlm.nih.gov/), etc., and found that luteolin and quercetin might be the main metabolites in XBJ injection, as well as cryptotanshinone, ferulic acid, rutin, and tanshinone IIA, although they are still pending experimental validation. The potential targets of XBJ injection could be tumor necrosis factor (TNF), mitogen-activated protein kinase 1 (MAPK1), Caspase-3 (CASP3), epidermal growth factor receptor (EGFR), interleukin-1β (IL1B), c-Jun (JUN), mitogen-activated protein kinase 8 (MAPK8), myeloperoxidase (MPO), prostaglandin-Endoperoxide Synthase 2 (PTGS2), nuclear factor kappa-b subunit RelA (RELA), and tumor protein p53 (TP53). Zhao et al. suggested that the potential molecular mechanisms by which XBJ injection inhibits COVID-19 are by acting on AKT serine/threonine kinase 1 (AKT1) (Tianyu and Liying, 2021). Xing et al. found that TNF, MAPK1, and interleukin-6 (IL-6) may be the key to the treatment of COVID-19 (Xing et al., 2020). Niu et al. suggested that XBJ could inhibit COVID-19 by down-regulating IL-6 (Niu et al., 2021). Zheng et al. showed that glyceraldehyde-3-phosphate dehydrogenase (GAPDH), albumin (ALB), TNF, EGFR, and MAPK1 might be involved in the regulation of COVID-19 by XBJ injection (Zheng et al., 2020). For the treatment of COVID-19-induced acute respiratory distress syndrome (ARDS), 56 targets of XBJ injection have been identified, among which AKT1, TNF, CASP3, and signal transducer and activator of transcription 3 (STAT3) are the main targets (Tao and Jiming, 2022). The following gene ontology (GO) biological process enrichment analysis and Kyoto Gene and Genome Encyclopedia (KEGG) enrichment analysis clarified some important pathways. Interestingly, the TNF signaling pathway seemed to play a critical role in XBJ injection treatment for COVID-19, which deserves further investigation. The results of network analysis and molecular docking analysis of XBJ injection in the above five articles are shown in Table 3 and Figure 1.
TABLE 3
| Classification* | Predicted metabolites | Predicted target molecules** | GO biological process enrichment analysis*** | KEGG enrichment analysis*** |
|---|---|---|---|---|
| Existing in 5 articles | Luteolin Quercetin Tanshinone IIA |
TNF | - | - |
| Existing in 4 articles | Cryptotanshinone Ferulic acid Rutin |
MAPK1 | response to lipopolysaccharide | TNF signaling pathway |
| Existing in 3 articles | 5-Hydroxymethyl furfural Albiflorin Apigenin Benzoylpaeoniflorin Caffeic acid Chlorogenic acid Danshensu Ethyl ferulate Gallic acid Hydroxysafflor yellow A Naringenin Paeoniflorin Paeonol Protocatechuic acid Protocatechuic aldehyde Rosmarinic acid Salvianolic acid A Salvianolic acid B Senkyunolide I |
CASP3 EGFR IL1B JUN MAPK8 MPO PTGS2 RELA TP53 |
regulation of inflammatory response regulation of protein serine/threonine kinase activity response to molecule of bacterial origin |
Hepatitis C |
| Existing in 2 articles | Butylidenephthalide Catechinic acid Galuteolin Hyperoside Kaempferol Matrine Oxypaeoniflorin Tanshinol, Baicalein |
AKT1 AR BCL2L1 CA2 CASP8 CAT CCL2 CTNNB1 EGF ESR1 FGF2 GSK3B IFNG IL2 IL6 MAPK14 MAPK3 MMP1 PPARG SELE STAT3 KEAP1 |
cell chemotaxis cellular response to biotic stimulus cytokine activity regulation of leukocyte migration regulation of neuron death response to oxidative stress response to reactive oxygen species |
Apoptosis C-type lectin receptor signaling pathway HIF-1 signaling pathway IL-17 signaling pathway |
Network analysis and molecular docking analysis between XBJ injection and COVID-19.
*The five articles include references (Tianyu and Liying, 2021; Xing et al., 2020; Niu et al., 2021; Zheng et al., 2020; Tao and Jiming, 2022). **The molecules in bold represent the focused targets in the five articles. ***Refence (Niu et al., 2021) is excluded due to the unavailable data. GO, gene ontology; KEGG, kyoto gene and genome encyclopedia.
FIGURE 1
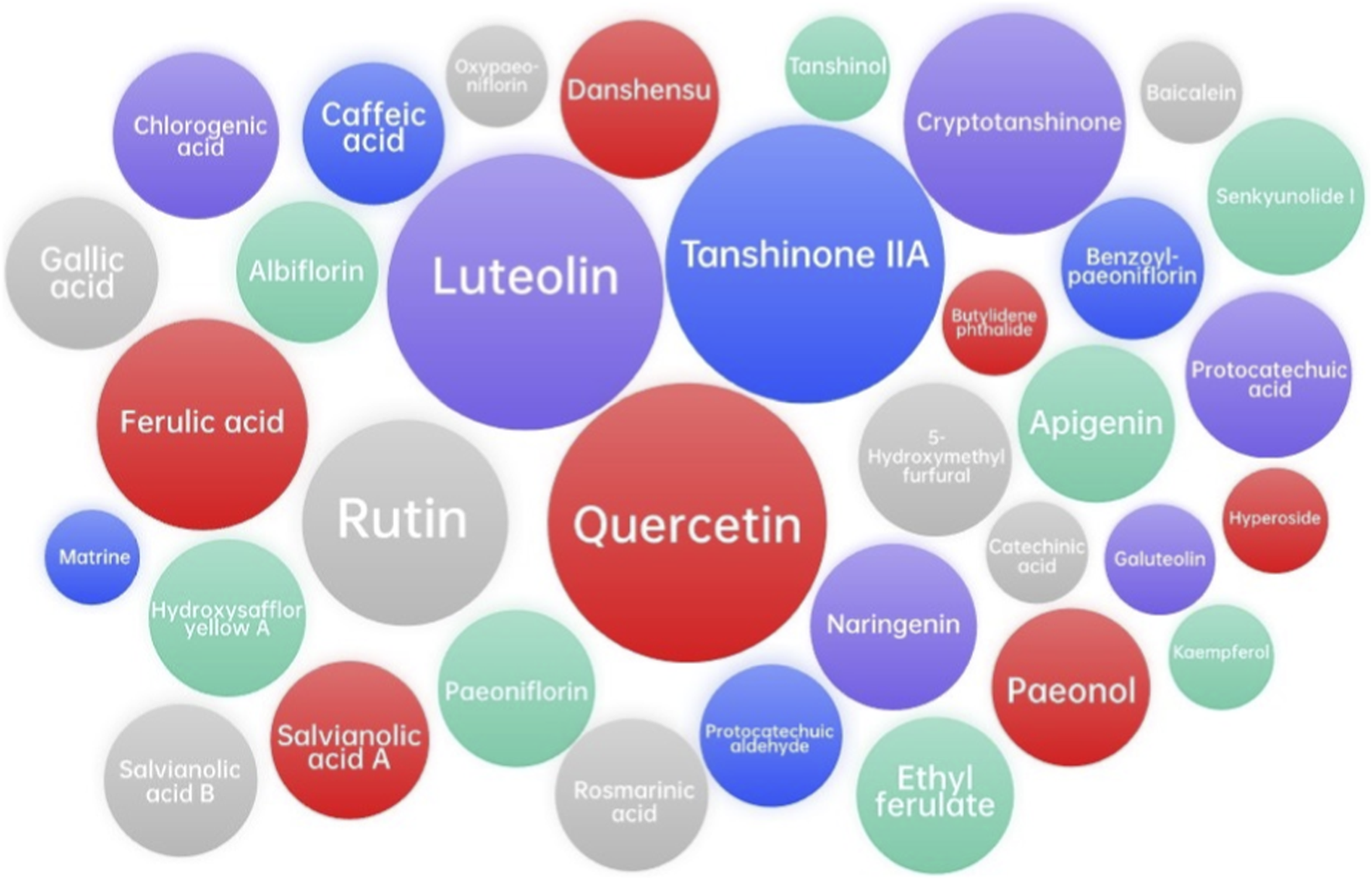
Major related metabolites from network analysis and molecular docking analysis between XBJ injection and COVID-19. However, further confirmation through in vivo and clinical investigations is essential to validate the true effects of these metabolites and to ensure that the conclusions drawn are not influenced by nonspecific interactions.
The network analysis reveals that XBJ injection might targets multiple key molecular pathways. By modulating the activity of proteins such as TNF, MAPK1, CASP3, EGFR, IL1B, MPO, and RELA, XBJ injection can potentially reduce inflammation, prevent excessive immune activation, and promote tissue repair. These network-based predictions offer hypothetical insights into possible modes of action, which require further experimental and pharmacological validation.
4.1 Finding of basic studies
Pharmacological studies have shown that XBJ injection can activate the phagocytosis function of the reticuloendothelial system and also strengthen the humoral immune function; thus, it can treat bacteria and viruses, activate blood circulation, relieve blood congestion, and clear fever and toxins (Cheng and Yu, 2021). In addition, XBJ injection can inhibit the elevation of serum tumor necrosis factor alpha (TNF-α) levels caused by endotoxin and promote the decrease of C-reactive protein (CRP) levels, which is a sensitive indicator commonly used to evaluate the degree of inflammatory response and shows a significant positive correlation with leukocyte levels (Song et al., 2020). When the individual is attacked by SARS-CoV-2, CRP levels tend to rise, leading to severe inflammation and exacerbation of COVID-19.
The spike protein (S protein) is responsible for coronavirus entry into host cells. Because angiotensin converting enzyme 2 (ACE2) can bind to the receptor binding domain (RBD) of the S protein, it was identified as the critical functional receptor for SARS-CoV-2, making it the important intervention target for COVID-19. The metabolites of anhydrosafflor yellow B, salvianolic acid B, and rutin in XBJ injection showed high affinity for ACE2 in the treatment of COVID-19, suggesting that it may be a molecular mechanism of XBJ injection for COVID-19 treatment (Zhang and Qi, 2023). The release of neutrophil extracellular traps (NETs) is a major cause of organ failure and mortality in sepsis, as well as in lung injury during COVID-19. In septic mouse models, XBJ injection reduced neutrophil recruitment and chemokines, including CSF-3, CSF-2, CXCL-3, and CXCL-2, in the lungs. It could also inhibit NET formation by reducing the expressions of citrullinated histone H3 (CitH3), MPO, and neutrophil elastase (NE) and normalizing sepsis-induced overexpression of targeting gasdermin D (GSDMD) (Shang et al., 2022). In addition, XBJ injection could significantly protect cells from SARS-CoV-2-induced cell death and inhibit the average size and number of plaques in vitro (Ma et al., 2020). It inhibited the release of inflammatory mediators, including TNF-α, IL-6, MIP-1β, RANTES, and IP-10, induced by SARS-COV-2 in Huh-7 cells. Taken together, the high affinity for ACE2, inhibition of NET release, and prevention of cell death and inflammation may be the main molecular mechanisms of XBJ injection in the treatment of COVID-19 (Figure 2).
FIGURE 2
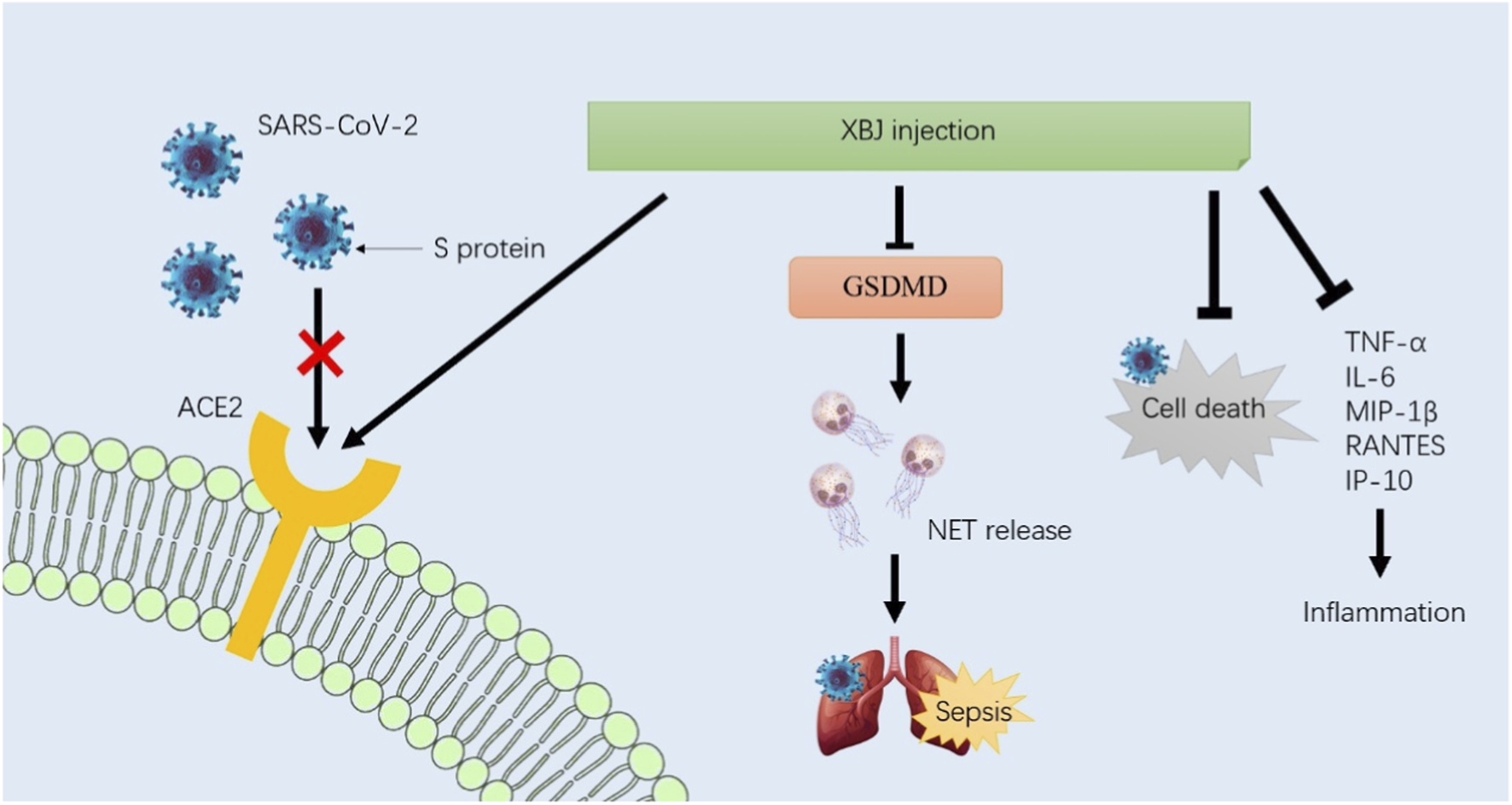
Based on the basic studies, the high affinity for ACE2, inhibition of NET release, and prevention of cell death and inflammation may be the main molecular mechanisms of XBJ injection in the treatment of COVID-19. Future research should further elucidate these mechanisms and identify patient subgroups that are most likely to benefit from XBJ injection.
In summary, XBJ injection exerts its therapeutic effects through a multi-target mechanism that includes inhibition of viral entry, reduction of inflammation, protection against cell death, and promotion of tissue repair. These mechanisms collectively contribute to the management and treatment of COVID-19, particularly in severe cases, although it must be confirmed through rigorous pharmacological and clinical studies. Future research should further elucidate these mechanisms and identify patient subgroups that are most likely to benefit from XBJ injection.
5 Findings of clinical studies
Several randomized controlled trials (RCTs) have been conducted to evaluate the therapeutic effects of XBJ injection in the treatment of COVID-19. In patients with confirmed and suspected COVID-19, administration of XBJ injection for 5 days to 2 weeks was shown to increase overall therapeutic efficacy (Chen et al., 2020; Li et al., 2022). However, there was no significance in the incidence of complications with or without XBJ injection. Both studies suggested that XBJ injection could reduce CRP levels as well as erythrocyte sedimentation rate (ESR), TNF-α, and IL-6 levels. In severe COVID-19 patients, 7–14 days of XBJ injection could attenuate major clinical symptoms and reduce the length of ICU stay (Luo et al., 2021; Wen et al., 2020). However, it did not significantly reduce 28-day mortality or time to negative nucleic acid test. In addition to the decrease in CRP and ESR levels, these severe patients showed an increase in lymphocyte levels after XBJ injection.
Two cohort studies showed a higher 28-day discharge rate (66.7% vs. 22.2%), 28-day survival rate (91.7% vs. 81.9%), and overall efficacy (68.2% vs. 50.0%) after XBJ injection (Liu et al., 2021; Zhang et al., 2020). However, the potential mechanisms of XBJ injection have not been well discussed. A retrospective study showed that 16 days of XBJ injection shortened the time of SARS-CoV-2 RNA clearance and improved CT imaging results without causing adverse events related to liver and kidney function (Wang et al., 2021). Patients also showed reductions in CRP and serum ferritin levels. A case-control study showed that XBJ injection significantly lowered body temperature, while it was not significant in making nucleic acid tests negative (Guo et al., 2020). An observational study by Ma et al. further investigated the potential mechanisms of XBJ injection in COVID-19 treatment in 11 patients (Ma et al., 2020). They found that XBJ injection could protect cells from virus-induced cell death and reduce pro-inflammatory cytokine levels, although it was not significant for white blood cell (WBC), neutrophil count, CRP, and procalcitonin (PCT) levels. Research included 455 participants with COVID-19 showed that neutrophil to lymphocyte platelet ratio (NLPR) is the most reliable inflammatory marker for predicting prognosis among individuals with COVID-19, and can accurately identify individuals who may benefit from XBJ injection (Liao et al., 2025). A summary of the clinical trials is provided in Table 4.
TABLE 4
| Research type | Patients | Intervention/Control | Intervention | Control | XBJ dosage | Outcomes | Potential mechanisms | Ref. |
|---|---|---|---|---|---|---|---|---|
| RCT | severe COVID-19 patients | 29/28 | + XBJ | Regular treatments | 50 mL, q12h,14 days | 1. Attenuating main clinical symptoms 2. Decrease in length of ICU hospitalization stay 3. Not significantly reducing 28-day mortality |
1. Decrease in IL-6, IL-8, and TNF-α levels 2. Increase in lymphocyte levels 3. Decrease in CRP levels |
Luo et al. (2021) |
| Retrospective study | COVID-19 patients | 23/32 | + XBJ and other traditional Chinese medicine | Regular treatments | 100 mL, bid, 16 days | 1. Shorter time of SARS-CoV-2 RNA clearance (12 days vs. 15.5 days) 2. Improvements in CT imaging results 3. Not significant in adverse events of liver and renal functions |
decrease in CRP and serum ferritin levels | Wang et al. (2021) |
| Case-control study | COVID-19 patients | 16/16 | + XBJ | Regular treatments | 100 mL, bid, ≥7 days | 1. Improvements in body temperature 2. Improvements in CT imaging results 3. Not significant in nucleic acid test turning to negative |
1. Decrease in IL-6 levels 2. Decrease in TNF-α and IL-10 |
Guo et al. (2020) |
| Observation study | severe COVID-19 patients | 11 | Regular treatments + XBJ | - | 100 mL, q12h, 7 days | 1. Improvements in PSI grade and PSI score 2. Improvements in lung injury |
1. Protecting cells from virus-induced cell death 2. Improvements in the oxygenation index, PaCO2, and lymphocyte count 3. Decrease in TNF-α, IP-10, MIP-1β, and RANTES levels 4. Not significant in WBC, neutrophil count, CRP, and PCT |
Ma et al. (2020) |
| RCT | severe COVID-19 patients | 20/20 | + XBJ | Regular treatments | 50 mL, bid, 7 days | 1. Decrease in APACHE II score 2. Improvements in conditions of patients 3. Not significant in nucleic acid test turning to negative |
1. Increase in white blood cell and lymphocyte counts 2. Decrease in CRP and ESR levels |
Wen et al. (2020) |
| Cohort study | severe COVID-19 patients | 72/72 | + XBJ | Regular treatments | 100 mL, bid, ≥1 day | 1. Improvements in PSI risk score 2. Higher 28-day discharge rate (66.7% vs. 22.2%) 3. Higher 28-day survival rate (91.7% vs. 81.9%) |
not mentioned | Liu et al. (2021) |
| RCT | COVID-19 patients | 15/15 | + XBJ | Regular treatments | 100 mL, bid, 2 weeks | 1. Higher overall efficiency (73.33% vs. 53.33%) 2. Not significant in occurrence of complications |
decrease in CRP levels | Chen et al. (2020) |
| Cohort study | common COVID-19 patients | 22/22 | + XBJ | Regular treatments | 50 mL, bid, 7 days | 1. Improvements in CT imaging results 2. Higher overall efficiency (68.2% vs. 50.0%) 3. Not significant in occurrence of complications |
not mentioned | Zhang et al. (2020) |
| RCT | patients suspected of COVID-19 | 24/24 | + XBJ | Regular treatments | 50 mL, bid, 5 days | 1. Higher overall efficiency (95. 8% vs. 83. 3%) | 1. Decrease in CRP levels 2. Decrease in ESR, TNF-α, and IL-6 levels |
Li et al. (2022) |
| retrospective study | common COVID-19 patients | 455 | + XBJ | Regular treatments | - | 1. Providing XBJ injection to patients with NLPR >3.29 was associated with a lower risk of 60-day all-cause mortality | reduce in NLPR | Liao et al. (2025) |
Clinical trials in XBJ injection for COVID-19 treatment.
Regular treatments include nutritional support, oxygen therapy, nebulization, antibiotics, non-invasive and invasive ventilation if necessary, resolving phlegm and cough, and maintaining electrolyte balance. NLPR, neutrophil to lymphocyte platelet ratio.
Based on these clinical trials, a meta-analysis was performed to evaluate the effects and safety of XBJ injection in COVID-19 patients. Sun et al. analyzed seven studies (204 patients in the XBJ group and 183 patients in the control group) and found that XBJ injection could reduce 28-day mortality, CRP, ESR, and IL-6 levels, and increase peripheral blood leukocyte and lymphocyte counts (Sun et al., 2023). Meanwhile, the side effects of XBJ injection were not obvious. However, there was no evidence that XBJ injection could improve the nucleic acid conversion rate and CT results of COVID-19 patients. Luo et al. analyzed nine studies (230 patients in the XBJ group and 227 patients in the control group) and found that XBJ injection had a higher overall effect rate and a lower 28-day mortality rate, and it could increase the lung CT recovery rate and decrease the CRP rate (Luo et al., 2022). However, there was no significant difference in nucleic acid negative conversion rate or WBC level. The ESR of patients with severe COVID-19 and other types was significantly decreased by XBJ injection. Similar to Sun’s study, the side effects of XBJ injection were not obvious compared to the control group. Taken together, these studies indicated that XBJ injection has advantages in improving therapeutic effects, reducing 28-day mortality, improving lung recovery, and reducing pro-inflammatory factors in patients with COVID-19.
6 Comparative analysis of XBJ injection in COVID-19 and other related conditions
XBJ has garnered significant attention for its potential therapeutic effects in treating severe infections, including COVID-19 and other related conditions, such as severe community-acquired pneumonia (SCAP) and sepsis.
XBJ’s therapeutic effects in SCAP are primarily attributed to its ability to downregulate key inflammatory pathways. For instance, XBJ has been shown to inhibit the expression of TLR4 and NF-κB, which are critical in mediating the inflammatory response (Song et al., 2022). Additionally, XBJ can modulate the balance of Th17 and T regulatory cells, thereby reducing the severity of the inflammatory cascade. These mechanisms collectively contribute to the improvement of clinical outcomes in SCAP patients.
A large multicenter randomized controlled trial showed that XBJ injection significantly improved the pneumonia severity index (PSI) risk rating and reduced the 28-day mortality rate in patients with SCAP (Song et al., 2019). Specifically, the 28-day mortality rate was reduced from 24.63% to 15.87% in patients treated with XBJ, representing an absolute reduction of 8.76%. Furthermore, XBJ administration led to a shorter duration of mechanical ventilation and ICU stay, with median values of 11.0 days and 12 days, respectively, compared to 16.5 days and 16 days in the placebo group. These findings highlight the clinical benefits of XBJ injection in managing SCAP.
In sepsis, XBJ injection exerts its therapeutic effects through multiple pathways. It protects endothelial cells, improves microcirculation, and alleviates coagulopathy, which are critical in preventing the progression of organ dysfunction (Song et al., 2022). XBJ also downregulates the expression of HMGB1 and RAGE, key mediators of sepsis-induced organ injury. Moreover, it promotes the polarization of macrophages towards the M2 phenotype, which is associated with tissue repair and anti-inflammatory effects. These mechanisms collectively contribute to the mitigation of the systemic inflammatory response syndrome (SIRS) and improve survival rates in sepsis patients.
The EXIT-SEP trial, a large-scale randomized controlled trial, showed that adjunctive therapy with XBJ injection significantly reduced 28-day mortality in sepsis patients (Lou et al., 2025). Post-hoc analysis of this trial further revealed that XBJ was particularly effective in patients with respiratory dysfunction, acidosis, and shock. These findings suggest that XBJ injection can be a valuable adjunctive therapy in specific sepsis subgroups, potentially improving treatment efficiency and reducing healthcare costs.
Therefore, XBJ injection has shown promising therapeutic effects in both SCAP and sepsis through its anti-inflammatory and immune-modulating properties. Clinical trials have showed significant improvements in key outcomes, such as mortality rates, duration of mechanical ventilation, and ICU stay. Future research should focus on further elucidating the specific mechanisms of action and identifying patient subgroups that are most likely to benefit from XBJ injection. This approach could pave the way for more personalized and effective treatment strategies in managing severe infections.
7 Discussion
Discrepancies in the pharmacological effects of XBJ injections for treating SARS-CoV-2 infections, as observed in clinical studies, are due to various factors. Some studies show significant improvements in certain indicators, while others do not (Ji et al., 2025). These factors include differences in study design. For example, RCTs versus cohort or retrospective studies may introduce selection bias and confounding factors. Sample size also plays a crucial role; smaller studies may lack the statistical power to detect significant effects, whereas larger studies are more likely to reveal such improvements. Additionally, patient population heterogeneity, including variations in disease severity, comorbidities, and prior treatments, can influence the observed outcomes. The duration and dosage of the XBJ injection used in the studies may also contribute to inconsistent results because different regimens could affect the therapeutic response.
Furthermore, the clinical significance of the findings should be carefully interpreted. While some studies report improvements in indicators such as inflammatory markers (e.g., C-reactive protein and interleukin-6) and clinical symptoms, the impact on long-term patient outcomes, such as mortality and quality of life, is unclear (Lin et al., 2025). Research limitations include publication bias, whereby studies with positive results are more likely to be published, as well as reliance on non-blinded assessments, which could introduce observer bias. The generalizability of the findings may also be limited by the specific patient populations and settings in which the studies were conducted. Future research should address these limitations by conducting large-scale, multicenter, rigorously designed trials to provide definitive evidence on the effects and safety of using the XBJ injection to treat SARS-CoV-2 patients.
A comprehensive evaluation of any therapeutic intervention, including the XBJ injection, requires an understanding of its associated benefits and risks (Li et al., 2025). Regarding the treatment of SARS-CoV-2 infection, existing research has primarily examined the XBJ injection’s effects and mechanisms. However, potential adverse reactions and drug interactions have not been extensively explored (Hu et al., 2025). This knowledge gap significantly impacts clinicians’ and researchers’ ability to make informed decisions about using XBJ injections.
Adverse reactions to the injection may include allergic responses, gastrointestinal disturbances, and other systemic effects (Bin et al., 2025). However, such occurrences have not been widely reported in the existing literature. Clinicians and researchers must also consider potential interactions between XBJ and antiviral medications or other treatments commonly used to manage the disease. These interactions could affect the pharmacokinetics or pharmacodynamics of XBJ or concomitant medications, leading to suboptimal therapeutic outcomes or an increased risk of adverse effects.
In the published clinical trials, no serious adverse reaction events were observed. A few patients reported mild allergic reactions and gastrointestinal discomfort, but these reactions were transient and did not significantly affect the treatment. In combination with the research on the material basis, we analyzed the components that may pose safety risks. For example, ferulic acid, as a phenolic compound, may have certain cytotoxicity at high doses (Sun et al., 2025). However, the content of ferulic acid in XBJ injection is far below the dose that may cause toxicity. In addition, we also conducted safety assessments on other components and believe that the safety of XBJ injection is relatively high at the current dosage. Although no serious adverse reactions have been found in the current clinical studies, there are still shortcomings in safety research. For example, there is a lack of long-term safety monitoring data and insufficient research on the control of impurities. We suggest that long-term safety monitoring studies should be carried out in the future, focusing on the potential risks of long-term use of XBJ injection. At the same time, the control of impurities should be strengthened to ensure the quality and safety of the product.
Despite the promising findings from various studies, several limitations and challenges remain. Many of the in vitro studies lack validation through in vivo and clinical trials. Additionally, the dose range tested, minimal active concentration, and the use of appropriate controls are often not clearly reported, which limits the reliability of the findings. It should be emphasized that network analysis is a hypothesis-generating tool rather than definitive evidence of pharmacological mechanism. All predicted targets and pathways require experimental validation before any mechanistic claims can be made.
To address these concerns, future studies should incorporate detailed monitoring and reporting of adverse events associated with XBJ injections. This would involve conducting prospective trials with robust safety endpoints and pharmacovigilance metabolites. Investigating the potential for drug interactions through in vitro studies and clinical pharmacokinetic evaluations would also provide valuable insights into the safe and effective use of XBJ injections in combination with other treatments.
While PubMed is a comprehensive and widely-used database for biomedical research, our reliance on a single database may introduce selection bias. PubMed’s focus on English-language articles and its specific indexing criteria could potentially exclude relevant studies published in other languages or indexed in other databases such as Embase, Web of Science, Scopus, Cochrane Library, or CNKI. Future research should consider a broader range of databases to ensure a more comprehensive evaluation of the clinical efficacy and safety of XBJ injection in the treatment of COVID-19.
8 Conclusion and perspectives
XBJ injection, derived from traditional Chinese medicine, has emerged as a promising therapeutic option for COVID-19 treatment. This study provides a comprehensive synthesis of the key findings related to the material basis, mechanisms of action, clinical efficacy, and safety profile of XBJ injection.
The related metabolites of XBJ injection have been extensively characterized through various analytical techniques, including HPLC and MS. Major metabolites identified include danshensu, hydroxysafflor yellow A, paeoniflorin, ferulic acid, and senkyunolide I, among others. These metabolites exhibit a range of pharmacological activities, such as anti-inflammatory, antioxidant, and antiviral properties, which contribute to the therapeutic effects of XBJ injection in treating COVID-19.
Network analysis and molecular docking were employed to predict potential targets and explore preliminary associations between XBJ injection and COVID-19-related pathways. Key molecular targets identified include TNF, MAPK1, CASP3, EGFR, IL1B, JUN, MAPK8, MPO, PTGS2, RELA, TP53, and AKT1. These targets might be involved in pathways of COVID-19 pathogenesis, such as inflammation, immune response, and cell survival. XBJ injection might modulate these pathways by inhibiting pro-inflammatory cytokines, preventing excessive immune activation, and promoting tissue repair. While network analysis suggests possible interactions with inflammatory pathways, these remain speculative and must be confirmed through rigorous pharmacological and clinical studies.
Clinical trials have showed the therapeutic efficacy of XBJ injection in COVID-19 patients. Studies show that XBJ injection, when used in combination with standard therapy, significantly improves overall efficacy, reduces 28-day mortality, and enhances lung CT recovery. Meta-analyses of multiple studies further support these findings, indicating that XBJ injection can reduce inflammatory markers such as CRP and IL-6 levels, while improving clinical outcomes.
The safety profile of XBJ injection is also noteworthy. Clinical trials and observational studies have consistently reported a lack of significant adverse effects associated with XBJ injection. No severe adverse events related to liver or kidney function were observed, suggesting that XBJ injection is well-tolerated in COVID-19 patients. This favorable safety profile, combined with its therapeutic benefits, makes XBJ injection a potentially valuable addition to the treatment regimen for COVID-19.
While the current evidence supports the therapeutic potential of XBJ injection in COVID-19 treatment, further research is needed to fully elucidate its mechanisms of action and optimize clinical application. Large-scale, multicenter, randomized controlled trials are warranted to provide more definitive evidence on the effects and safety of XBJ injection. Additionally, studies should focus on identifying patient populations most likely to benefit from XBJ injection and exploring potential drug interactions.
In summary, XBJ injection might have significant potential in improving clinical outcomes and reducing mortality in COVID-19 patients. Its multi-target mechanism of action, predicted by network analysis and clinical studies, highlights its value in managing the complex pathophysiology of COVID-19. Future research should continue to explore the therapeutic potential of XBJ injection and its role in the broader context of COVID-19 treatment strategies.
Statements
Author contributions
ZZ: Formal Analysis, Writing – original draft, Methodology, Investigation. XL: Investigation, Writing – review and editing, Methodology. JZ: Formal Analysis, Methodology, Writing – review and editing. YL: Conceptualization, Writing – review and editing, Supervision.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Bin Y. Peng R. Lee Y. Lee Z. Liu Y. (2025). Efficacy of xuebijing injection on pulmonary ventilation improvement in acute pancreatitis: a systematic review and meta-analysis. Front. Pharmacol.16, 1549419. 10.3389/fphar.2025.1549419
2
Bourgonje A. R. Abdulle A. E. Timens W. Hillebrands J. L. Navis G. J. Gordijn S. J. et al (2020). Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol.251 (3), 228–248. 10.1002/path.5471
3
Chen Y. Li Y. Chen X. Wang L. Sun C. Yan W. et al (2010). Development and validation of a HPLC method for the determination of five bioactive compounds in the “Xuebijing” injection. Anal. Lett.43 (15), 2456–2464. 10.1080/00032711003698739
4
Chen X. Wang X. Luo J. (2012). In vivo pharmacokinetic study of Xuebijing injection in rats. Yao Wu Fen Xi Za Zhi32 (5), 744–748. 10.16155/j.0254-1793.2012.05.004
5
Chen L. Liu H. Xiao G. (2020). Efficacy of Xuebijing injection in the treatment of COVID-19 and its effect on CRP. Zhong Guo Chu Fang. Yao18 (10), 110–111. 10.3969/j.issn.1671-945X.2020.10.057
6
Chen R. Kezhekkekara S. G. Kunasekaran M. P. MacIntyre C. R. (2023). Universal masking during COVID-19 outbreaks in aged care settings: a systematic review and meta-analysis. Ageing Res. Rev.93, 102138. 10.1016/j.arr.2023.102138
7
Chen Z. Zheng R. Jiang H. Zhang X. Peng M. Jiang T. et al (2025). Therapeutic efficacy of Xuebijing injection in treating severe acute pancreatitis and its mechanisms of action: a comprehensive survey. Phytomedicine140, 156629. 10.1016/j.phymed.2025.156629
8
Cheng C. Yu X. (2021). Research progress in Chinese herbal medicines for treatment of sepsis: pharmacological action, phytochemistry, and pharmacokinetics. Int. J. Mol. Sci.22 (20), 11078. 10.3390/ijms222011078
9
Cheng C. Lin J. Z. Li L. Yang J. L. Jia W. W. Huang Y. H. et al (2016). Pharmacokinetics and disposition of monoterpene glycosides derived from Paeonia lactiflora roots (Chishao) after intravenous dosing of antiseptic XueBiJing injection in human subjects and rats. Acta Pharmacol. Sin.37 (4), 530–544. 10.1038/aps.2015.103
10
China NHCotPsRo (2022). Guidelines for the diagnosis and treatment of COVID-19 (9th Edition). Available online at: http://wwwnhcgovcn/yzygj/s7653p/202203/b74ade1ba4494583805a3d2e40093d88shtml.
11
Cilibrasi R. L. Vitányi P. M. B. (2022). Fast phylogeny of SARS-CoV-2 by compression. Entropy (Basel)24 (4), 439. 10.3390/e24040439
12
Cilloniz C. Martin-Loeches I. Garcia-Vidal C. San J. A. Torres A. (2016). Microbial etiology of pneumonia: epidemiology, diagnosis and resistance patterns. Int. J. Mol. Sci.17 (12), 2120. 10.3390/ijms17122120
13
Cui J. Deng Y. Li X. Gao L. Li J. Li Z. et al (2025). Herbal-based Xuebijing injection ameliorated vascular endothelial dysfunction via inhibiting ACLY/MYB/RIG-I axis in sepsis-associated lung injury. Phytomedicine140, 156573. 10.1016/j.phymed.2025.156573
14
de Melo B. P. da Silva J. A. M. Rodrigues M. A. Palmeira J. D. F. Amato A. A. Argañaraz G. A. et al (2025a). SARS-CoV-2 spike protein and long COVID-Part 2: understanding the impact of spike protein and cellular receptor interactions on the pathophysiology of long COVID syndrome. Viruses17 (5), 619. 10.3390/v17050619
15
de Melo B. P. da Silva J. A. M. Rodrigues M. A. Palmeira J. D. F. Saldanha-Araujo F. Argañaraz G. A. et al (2025b). SARS-CoV-2 spike protein and long COVID-Part 1: impact of spike protein in pathophysiological mechanisms of long COVID syndrome. Viruses17 (5), 617. 10.3390/v17050617
16
Dong Q. Tan Y. Tang G. Wu Z. Li A. Qin X. et al (2023). Neuroprotective potentials of ferulic acid against intracerebral hemorrhage COVID-19 through using network pharmacology approach and molecular docking analysis. Curr. Res. Toxicol.5, 100123. 10.1016/j.crtox.2023.100123
17
Fernandes Q. Inchakalody V. P. Merhi M. Mestiri S. Taib N. Moustafa Abo El-Ella D. et al (2022). Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med.54 (1), 524–540. 10.1080/07853890.2022.2031274
18
Guo H. Zheng J. Huang G. Xiang Y. Lang C. Li B. et al (2020). Xuebijing injection in the treatment of COVID-19: a retrospective case-control study. Ann. Palliat. Med.9 (5), 3235–3248. 10.21037/apm-20-1478
19
Hadj H. I. (2022). Covid-19 vaccines and variants of concern: a review. Rev. Med. Virol.32 (4), e2313. 10.1002/rmv.2313
20
He Y. Q. Zhou C. C. Yu L. Y. Wang L. Deng J. L. Tao Y. L. et al (2021). Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res.163, 105224. 10.1016/j.phrs.2020.105224
21
Heidecke J. Wallin J. Fransson P. Singh P. Sjödin H. Stiles P. C. et al (2025). Uncovering temperature sensitivity of West Nile virus transmission: novel computational approaches to mosquito-pathogen trait responses. PLoS Comput. Biol.21 (3), e1012866. 10.1371/journal.pcbi.1012866
22
Hu Y. Duan M. Liang S. Wang Y. Feng Y. (2015). Senkyunolide I protects rat brain against focal cerebral ischemia-reperfusion injury by up-regulating p-Erk1/2, Nrf2/HO-1 and inhibiting caspase 3. Brain Res.1605, 39–48. 10.1016/j.brainres.2015.02.015
23
Hu Y. Y. Wang Y. Liang S. Yu X. L. Zhang L. Feng L. Y. et al (2016). Senkyunolide I attenuates oxygen-glucose deprivation/reoxygenation-induced inflammation in microglial cells. Brain Res.1649 (Pt A), 123–131. 10.1016/j.brainres.2016.08.012
24
Hu Y. Xu Y. Gao J. Ling B. Pan S. Liu S. et al (2025). Integrated metabolomics and network pharmacology reveal the mechanisms of Xuebijing in counteracting sepsis-induced myocardial dysfunction. J. Ethnopharmacol.347, 119729. 10.1016/j.jep.2025.119729
25
Huang H. Ji L. Song S. Wang J. Wei N. Jiang M. et al (2011). Identification of the major constituents in Xuebijing injection by HPLC-ESI-MS. Phytochem. Anal.22 (4), 330–338. 10.1002/pca.1284
26
Huang H. Wang J. Fu J. Z. Wang L. Q. Zhao H. Z. Song S. Y. et al (2013). Simultaneous determination of thirteen main components and identification of eight major metabolites in Xuebijing Injection by UPLC/Q-TOF. J. Anal. Chem.68 (4), 348–356. 10.1134/S1061934813040023
27
Ji L. Huang H. Jiang M. Bai G. Luo G. (2010). Simultaneous HPLC determination of 11 essential compounds in Xuebijing injection. Zhongguo Zhong Yao Za Zhi35 (18), 2395–2398.
28
Ji Y. Song H. Li L. (2025). Traditional Chinese medicine for sepsis: advancing from evidence to innovative drug discovery. Crit. Care29 (1), 193. 10.1186/s13054-025-05441-4
29
Khan S. Zakariah M. Palaniappan S. (2016a). Computational prediction of Mycoplasma hominis proteins targeting in nucleus of host cell and their implication in prostate cancer etiology. Tumour Biol.37 (8), 10805–10813. 10.1007/s13277-016-4970-9
30
Khan S. Imran A. Khan A. A. Abul K. M. Alshamsan A. (2016b). Systems biology approaches for the prediction of possible role of Chlamydia pneumoniae proteins in the etiology of lung cancer. PLoS One11 (2), e0148530. 10.1371/journal.pone.0148530
31
Khan S. Zakariah M. Rolfo C. Robrecht L. Palaniappan S. (2017). Prediction of mycoplasma hominis proteins targeting in mitochondria and cytoplasm of host cells and their implication in prostate cancer etiology. Oncotarget8 (19), 30830–30843. 10.18632/oncotarget.8306
32
Li W. Tang Y. Chen Y. Duan J. A. (2012). Advances in the chemical analysis and biological activities of chuanxiong. Molecules17 (9), 10614–10651. 10.3390/molecules170910614
33
Li X. Cheng C. Wang F. Huang Y. Jia W. Olaleye O. E. et al (2016). Pharmacokinetics of catechols in human subjects intravenously receiving XueBiJing injection, an emerging antiseptic herbal medicine. Drug Metab. Pharmacokinet.31 (1), 95–98. 10.1016/j.dmpk.2015.10.005
34
Li D. Cao X. Pu W. (2018). Determination of the active ingredients in Xuebijing injection by HPLC method. Zhong Guo Xian Dai Zhong Yao20 (9), 1157–1160. 10.13313/j.issn.1673-4890.20180316003
35
Li J. Olaleye O. E. Yu X. Jia W. Yang J. Lu C. et al (2019). High degree of pharmacokinetic compatibility exists between the five-herb medicine XueBiJing and antibiotics comedicated in sepsis care. Acta Pharm. Sin. B9 (5), 1035–1049. 10.1016/j.apsb.2019.06.003
36
Li C. Wang P. Li M. Zheng R. Chen S. Liu S. et al (2021). The current evidence for the treatment of sepsis with Xuebijing injection: bioactive constituents, findings of clinical studies and potential mechanisms. J. Ethnopharmacol.265, 113301. 10.1016/j.jep.2020.113301
37
Li J. Pang Y. Yang B. (2022). Clinical study on the treatment of 24 suspected cases of COVID-19 with Xuebijing injection. Hunan Zhong Yi Za Zhi38 (1), 11–13. 10.16808/j.cnki.issn1003-7705.2022.01.003
38
Li Z. Wang B. Sun K. Yin G. Wang P. Yu X. A. et al (2023). An aggregation-induced emission sensor combined with UHPLC-Q-TOF/MS for fast identification of anticoagulant active ingredients from traditional Chinese medicine. Anal. Chim. Acta1279, 341799. 10.1016/j.aca.2023.341799
39
Li J. Shen M. Yin Z. (2025). Exploring the mechanism of action of xuebijing injection in treating acute respiratory distress syndrome based on network pharmacology and animal experiments. J. Inflamm. Res.18, 6037–6047. 10.2147/jir.S507468
40
Liao M. Zhang L. T. Bai L. J. Wang R. Y. Liu Y. Han J. et al (2025). Xuebijing injection reduces COVID-19 patients' mortality as influenced by the neutrophil to lymphocyte platelet ratio. J. Integr. Med.23, 282–288. 10.1016/j.joim.2025.04.002
41
Lin T. S. Cai X. X. Wang Y. B. Xu J. T. Xiao J. H. Huang H. Y. et al (2025). Identifying baicalein as a key bioactive compound in XueBiJing targeting KEAP1: implications for antioxidant effects. Antioxidants (Basel)14 (3), 248. 10.3390/antiox14030248
42
Lino M. M. Mather S. Trani M. Chen Y. Caubel P. De Bernardi B. (2025). Challenges and innovations in pharmacovigilance and signal management during the COVID-19 pandemic: an industry perspective. Vaccines (Basel)13 (5), 481. 10.3390/vaccines13050481
43
Liu L. (2020). Traditional Chinese medicine contributes to the treatment of COVID-19 patients. Chin. Herb. Med., 12(2): 95–96. 10.1016/j.chmed.2020.04.003
44
Liu Y. L. Liu Y. J. Liu Y. Li X. S. Liu S. H. Pan Y. G. et al (2014). Hydroxysafflor yellow A ameliorates lipopolysaccharide-induced acute lung injury in mice via modulating toll-like receptor 4 signaling pathways. Int. Immunopharmacol.23 (2), 649–657. 10.1016/j.intimp.2014.10.018
45
Liu X. Song Y. Guan W. Qiu H. Du B. Li Y. et al (2021). A multicenter prospective cohort study of Xuebijing injection in the treatment of severe coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue33 (7), 774–778. 10.3760/cma.j.cn121430-20210514-00714
46
Liu L. W. Du X. R. Chu R. He X. Y. Chen Y. W. You H. Q. et al (2025). DNA methylation controls the expression of tanshinone synthesis genes and the tanshinone accumulation in Salvia miltiorrhiza and Salvia bowleyana. Plant J.123 (6), e70494. 10.1111/tpj.70494
47
Lou X. Chen H. Shi N. Yu R. Li S. Yang Y. et al (2025). Treatment effects of Xuebijing injection in patients with sepsis by clinical phenotype: a post hoc analysis of the EXIT-SEP trial. EClinicalMedicine86, 103341. 10.1016/j.eclinm.2025.103341
48
Luo Z. Chen W. Xiang M. Wang H. Xiao W. Xu C. et al (2021). The preventive effect of Xuebijing injection against cytokine storm for severe patients with COVID-19: a prospective randomized controlled trial. Eur. J. Integr. Med.42, 101305. 10.1016/j.eujim.2021.101305
49
Luo T. Yang X. Yang L. Qin X. Cui J. (2022). Meta-Analysis of efficacy and safety of xuebijing in the treatment of coronavirus disease 2019. Zhong Yao Yao Li Yu Lin. Chuang38 (5), 136–141. 10.13412/j.cnki.zyyl.2022.05.011
50
Ma Q. Qiu M. Zhou H. Chen J. Yang X. Deng Z. et al (2020). The study on the treatment of Xuebijing injection (XBJ) in adults with severe or critical Corona Virus Disease 2019 and the inhibitory effect of XBJ against SARS-CoV-2. Pharmacol. Res.160, 105073. 10.1016/j.phrs.2020.105073
51
Mathew D. S. Pandya T. Pandya H. Vaghela Y. Subbian S. (2023). An overview of SARS-CoV-2 etiopathogenesis and recent developments in COVID-19 vaccines. Biomolecules13 (11), 1565. 10.3390/biom13111565
52
Niu W. H. Wu F. Cao W. Y. Wu Z. G. Chao Y. C. Liang C. (2021). Network pharmacology for the identification of phytochemicals in traditional Chinese medicine for COVID-19 that may regulate interleukin-6. Biosci. Rep.41 (1). 10.1042/bsr20202583
53
Ouyang H. Z. He J. (2018). Simultaneous determination of nine constituents of Xuebijing Injection in rat plasma and their pharmacokinetics by LC-MS/MS. Zhongguo Zhong Yao Za Zhi43 (17), 3553–3561. 10.19540/j.cnki.cjcmm.20180611.010
54
Pang G. Yi T. Luo H. Jiang L. (2022). Preclinical findings: the pharmacological targets and molecular mechanisms of ferulic acid treatment for COVID-19 and osteosarcoma via targeting autophagy. Front. Endocrinol. (Lausanne)13, 971687. 10.3389/fendo.2022.971687
55
Radcliffe C. Malinis M. Azar M. M. (2023). Antiviral treatment of Coronavirus Disease-2019 Pneumonia. Clin. Chest Med.44 (2), 279–297. 10.1016/j.ccm.2022.11.008
56
Salman S. Shah F. H. Idrees J. Idrees F. Velagala S. Ali J. et al (2020). Virtual screening of immunomodulatory medicinal compounds as promising anti-SARS-CoV-2 inhibitors. Future Virol.15 (5), 267–275. 10.2217/fvl-2020-0079
57
Shahi F. Rasti M. Moradi M. (2023). Overview of the different methods for RNA preparation in COVID-19 diagnosis process during the pandemic. Anal. Biochem.686, 115410. 10.1016/j.ab.2023.115410
58
Shang T. Zhang Z. S. Wang X. T. Chang J. Zhou M. E. Lyu M. et al (2022). Xuebijing injection inhibited neutrophil extracellular traps to reverse lung injury in sepsis mice via reducing Gasdermin D. Front. Pharmacol.13, 1054176. 10.3389/fphar.2022.1054176
59
Sharma A. Ahmad F. I. Lal S. K. (2021). COVID-19: a review on the novel coronavirus disease evolution, transmission, detection, control and prevention. Viruses13 (2), 202. 10.3390/v13020202
60
Sharma H. Ilyas A. Chowdhury A. Poddar N. K. Chaudhary A. A. Shilbayeh S. A. R. et al (2022). Does COVID-19 lockdowns have impacted on global dengue burden? A special focus to India. BMC Public Health22 (1), 1402. 10.1186/s12889-022-13720-w
61
Sheng C. Peng W. Xia Z. Wang Y. (2020). Plasma and cerebrospinal fluid pharmacokinetics of hydroxysafflor yellow A in patients with traumatic brain injury after intravenous administration of Xuebijing using LC-MS/MS method. Xenobiotica50 (5), 545–551. 10.1080/00498254.2019.1668983
62
Sifontes-Rodríguez S. Meneses-Gómez S. Escalona-Montaño A. R. Sánchez-Almaraz D. A. Pérez-Olvera O. Cañón Rosas A. R. et al (2025). PubChem BioAssays 1063: a poorly exploited source of new antileishmanial compounds. J. Parasitol. Res.2025, 6338486. 10.1155/japr/6338486
63
Singh M. Jayant K. Singh D. Bhutani S. Poddar N. K. Chaudhary A. A. et al (2022). Withania somnifera (L.) Dunal (Ashwagandha) for the possible therapeutics and clinical management of SARS-CoV-2 infection: Plant-based drug discovery and targeted therapy. Front. Cell Infect. Microbiol.12, 933824. 10.3389/fcimb.2022.933824
64
Song Y. Yao C. Yao Y. Han H. Zhao X. Yu K. et al (2019). XueBiJing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit. Care Med.47 (9), e735–e743. 10.1097/ccm.0000000000003842
65
Song Y. Zhang M. Yin L. Wang K. Zhou Y. Zhou M. et al (2020). COVID-19 treatment: close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int. J. Antimicrob. Agents56 (2), 106080. 10.1016/j.ijantimicag.2020.106080
66
Song Y. Wang X. Chen C. Wei T. Lang K. Yang D. et al (2022). Weaker response to XueBiJing treatment in severe community-acquired pneumonia patients with higher body mass index or hyperglycemia: a post hoc analysis of a randomized controlled trial. Front. Pharmacol.13, 755536. 10.3389/fphar.2022.755536
67
Stompor-Gorący M. Machaczka M. (2021). Recent advances in biological activity, new formulations and prodrugs of ferulic acid. Int. J. Mol. Sci.22 (23), 12889. 10.3390/ijms222312889
68
Sun C. Y. Pei C. Q. Zang B. X. Wang L. Jin M. (2010). The ability of hydroxysafflor yellow a to attenuate lipopolysaccharide-induced pulmonary inflammatory injury in mice. Phytother. Res.24 (12), 1788–1795. 10.1002/ptr.3166
69
Sun Z. Zuo L. Sun T. Tang J. Ding D. Zhou L. et al (2017). Chemical profiling and quantification of XueBiJing injection, a systematic quality control strategy using UHPLC-Q Exactive hybrid quadrupole-orbitrap high-resolution mass spectrometry. Sci. Rep.7 (1), 16921. 10.1038/s41598-017-17170-y
70
Sun W. Zhao Y. Liao L. Zhao Z. Chen S. Yan X. et al (2023). Effectiveness and safety of Xuebijing injection for patients with coronavirus disease 2019: a systematic review and metaanalysis. J. Tradit. Chin. Med.43 (4), 631–639. 10.19852/j.cnki.jtcm.20230517.002
71
Sun M. H. Chen K. J. Tsao Y. T. Sun C. C. Lai J. Y. Lin C. J. et al (2025). Surface moieties drive the superior protection of curcumin-derived carbon quantum dots against retinal ischemia-reperfusion injury. J. Mater Chem. B13 (13), 4225–4237. 10.1039/d4tb02364a
72
Szafran A. Dahms K. Ansems K. Skoetz N. Monsef I. Breuer T. et al (2023). Early versus late tracheostomy in critically ill COVID-19 patients. Cochrane Database Syst. Rev.11 (11), Cd015532. 10.1002/14651858.Cd015532
73
Tao P. Jiming L. (2022). Molecular mechanisms revealed by network pharmacology of xuebijing on the treatment of acute respiratory distress syndrome caused by novel coronavirus infection. Eur. Rev. Med. Pharmacol. Sci.26 (8), 2651–2661. 10.26355/eurrev_202204_28594
74
Tianyu Z. Liying G. (2021). Identifying the molecular targets and mechanisms of xuebijing injection for the treatment of COVID-19 via network parmacology and molecular docking. Bioengineered12 (1), 2274–2287. 10.1080/21655979.2021.1933301
75
Wang Y. H. Liang S. Xu D. S. Lin X. He C. Y. Feng Y. et al (2011). Effect and mechanism of senkyunolide I as an anti-migraine compound from Ligusticum chuanxiong. J. Pharm. Pharmacol.63 (2), 261–266. 10.1111/j.2042-7158.2010.01191.x
76
Wang C. Huang Q. Wang C. Zhu X. Duan Y. Yuan S. et al (2013). Hydroxysafflor yellow A suppresses oleic acid-induced acute lung injury via protein kinase A. Toxicol. Appl. Pharmacol.272 (3), 895–904. 10.1016/j.taap.2013.07.021
77
Wang Y. Lu C. Li H. Qi W. Ruan L. Bian Y. et al (2021). Efficacy and safety assessment of severe COVID-19 patients with Chinese medicine: a retrospective case series study at early stage of the COVID-19 epidemic in Wuhan, China. J. Ethnopharmacol.277, 113888. 10.1016/j.jep.2021.113888
78
Wang W. Li S. S. Xu X. F. Yang C. Niu X. G. Yin S. X. et al (2022). Danshensu alleviates pseudo-typed SARS-CoV-2 induced mouse acute lung inflammation. Acta Pharmacol. Sin.43 (4), 771–780. 10.1038/s41401-021-00714-4
79
Wen L. Zhou Z. Jiang D. Huang K. (2020). Effect of Xuebijing injection on inflammatory markers and disease outcome of coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue32 (4), 426–429. 10.3760/cma.j.cn121430-20200406-00386
80
Wu Y. Wang L. Jin M. Zang B. X. (2012). Hydroxysafflor yellow A alleviates early inflammatory response of bleomycin-induced mice lung injury. Biol. Pharm. Bull.35 (4), 515–522. 10.1248/bpb.35.515
81
Xing Y. Hua Y. R. Shang J. Ge W. H. Liao J. (2020). Traditional Chinese medicine network pharmacology study on exploring the mechanism of Xuebijing Injection in the treatment of coronavirus disease 2019. Chin. J. Nat. Med.18 (12), 941–951. 10.1016/s1875-5364(20)60038-3
82
Yüce M. Filiztekin E. Özkaya K. G. (2021). COVID-19 diagnosis -A review of current methods. Biosens. Bioelectron.172, 112752. 10.1016/j.bios.2020.112752
83
Zha Y. F. Xie J. Ding P. Zhu C. L. Li P. Zhao Z. Z. et al (2021). Senkyunolide I protect against lung injury via inhibiting formation of neutrophil extracellular trap in a murine model of cecal ligation and puncture. Int. Immunopharmacol.99, 107922. 10.1016/j.intimp.2021.107922
84
Zhang B. Qi F. (2023). Herbal medicines exhibit a high affinity for ACE2 in treating COVID-19. Biosci. Trends17 (1), 14–20. 10.5582/bst.2022.01534
85
Zhang L. Wei W. (2020). Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther.207, 107452. 10.1016/j.pharmthera.2019.107452
86
Zhang N. Cheng C. Olaleye O. E. Sun Y. Li L. Huang Y. et al (2018). Pharmacokinetics-Based identification of potential therapeutic phthalides from XueBiJing, a Chinese herbal injection used in sepsis management. Drug Metab. Dispos.46 (6), 823–834. 10.1124/dmd.117.079673
87
Zhang J. Zhang Q. Liu G. Zhang N. (2019). Therapeutic potentials and mechanisms of the Chinese traditional medicine Danshensu. Eur. J. Pharmacol.864, 172710. 10.1016/j.ejphar.2019.172710
88
Zhang C. Li Z. Zhang S. (2020). Clinical efficacy observation of Xuebijing in the treatment of COVID-19. Zhong Guo Yi Yao Xue Za Zhi40 (9), 964–967. 10.13286/j.1001-5213.2020.09.03
89
Zhang M. Zheng R. Liu W. J. Hou J. L. Yang Y. L. Shang H. C. (2023). Xuebijing injection, a Chinese patent medicine, against severe pneumonia: current research progress and future perspectives. J. Integr. Med.21 (5), 413–422. 10.1016/j.joim.2023.08.004
90
Zhang H. Ge C. Fisher D. Hien N. T. T. Musabaev E. Pronyuk K. et al (2025). Antiviral treatment for viral pneumonia: current drugs and natural compounds. Virol. J.22 (1), 62. 10.1186/s12985-025-02666-1
91
Zheng W. J. Yan Q. Ni Y. S. Zhan S. F. Yang L. L. Zhuang H. F. et al (2020). Examining the effector mechanisms of Xuebijing injection on COVID-19 based on network pharmacology. BioData Min.13, 17. 10.1186/s13040-020-00227-6
92
Zhou H. Bian D. Jiao X. Wei Z. Zhang H. Xia Y. et al (2011). Paeoniflorin protects against lipopolysaccharide-induced acute lung injury in mice by alleviating inflammatory cell infiltration and microvascular permeability. Inflamm. Res.60 (10), 981–990. 10.1007/s00011-011-0359-9
93
Zhou Y. Wang F. Tang J. Nussinov R. Cheng F. (2020). Artificial intelligence in COVID-19 drug repurposing. Lancet Digit. Health2 (12), e667–e676. 10.1016/s2589-7500(20)30192-8
94
Zuo L. Sun Z. Hu Y. Sun Y. Xue W. Zhou L. et al (2017a). Rapid determination of 30 bioactive constituents in XueBiJing injection using ultra high performance liquid chromatography-high resolution hybrid quadrupole-orbitrap mass spectrometry coupled with principal component analysis. J. Pharm. Biomed. Anal.137, 220–228. 10.1016/j.jpba.2017.01.024
95
Zuo L. Zhong Q. Wang Z. Sun Z. Zhou L. Li Z. et al (2017b). Simultaneous determination and pharmacokinetic study of twelve bioactive compounds in rat plasma after intravenous administration of Xuebijing injection by UHPLC-Q-Orbitrap HRMS. J. Pharm. Biomed. Anal.146, 347–353. 10.1016/j.jpba.2017.09.010
96
Zuo L. Sun Z. Wang Z. Ding D. Xu T. Liu L. et al (2019). Tissue distribution profiles of multiple major bioactive components in rats after intravenous administration of Xuebijing injection by UHPLC-Q-Orbitrap HRMS. Biomed. Chromatogr.33 (2), e4400. 10.1002/bmc.4400
Summary
Keywords
angiotensin converting enzyme 2, COVID-19, severe pneumonia, traditional Chinese medicine, Xuebijing injection
Citation
Zhang Z, Li X, Zhou J and Li Y (2026) Xuebijing injection in the treatment of COVID-19: An update on clinical studies, potentially active metabolites and mechanisms. Front. Pharmacol. 16:1667022. doi: 10.3389/fphar.2025.1667022
Received
18 July 2025
Revised
28 November 2025
Accepted
28 November 2025
Published
14 January 2026
Volume
16 - 2025
Edited by
Javier Echeverria, University of Santiago, Chile
Reviewed by
Shahanavaj Khan, Indian Institute of Health and Technology, India
Yaolei Li, National Institutes for Food and Drug Control, China
Mansoor Khaledi, Shahrekord University of Medical Sciences, Iran
Updates
Copyright
© 2026 Zhang, Li, Zhou and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yupeng Li, liyupeng200@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.