- 1Department of Pharmacy, Personalized Drug Research and Therapy Key Laboratory of Sichuan Province, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Pharmacy Department of Xinjiang Medical University Affiliated Traditional Chinese Medicine Hospital, Urumqi, Xinjiang, China
Background: Patients diagnosed with malignant tumors exhibit a markedly elevated risk of venous thromboembolism (VTE), which has a negative impact on their prognosis. Currently, there is no reliable predictive model specifically for thrombosis risk in lymphoma patients. This study aims to develop and validate a machine learning model leveraging real-world data, offering a dependable risk assessment tool for the early identification of VTE in lymphoma patients.
Methods: We retrospectively analyzed 605 hospitalized patients with lymphoma between January 2019 and June 2024. Candidate predictors included demographic characteristics, comorbidities and medical history, tumor-related factors, treatment-related factors, and laboratory parameters. The primary endpoint was the occurrence of VTE within 6 months after hospitalization for confirmed lymphoma. Model development incorporated three imputation methods, three sampling strategies, three feature selection approaches, and nine machine learning algorithms. Predictive performance was compared across all models.
Results: Combining different imputation, sampling, and feature selection strategies yielded 27 datasets, which were trained across nine algorithms to generate 243 models. The optimal model—Simp-SMOTE_rf_GBM, constructed using random forest imputation, SMOTE oversampling, and gradient boosting machine—achieved the highest predictive performance (AUC = 0.954). SHAP-based model interpretation identified nine key predictors ranked by importance: anticoagulant use, D-dimer, lactate dehydrogenase, central venous catheterization, carcinoembryonic antigen (CEA), Eastern Cooperative Oncology Group (ECOG) score, serum total protein (TP), total cholesterol (TC), and infectious disease.
Conclusion: This study established and validated a machine learning model for predicting VTE risk in lymphoma patients, with the optimal model demonstrating excellent discriminatory ability (AUC = 0.954). The model provides evidence to guide the timing and strategy of anticoagulation, supporting early VTE screening and risk stratification in clinical practice. Its implementation has important implications for improving patient outcomes and advancing public health.
Introduction
Cancer is one of the leading causes of global disease burden, accounting for approximately one-sixth of all deaths worldwide (Bray et al., 2021; 2024). Beyond its impact on health, cancer imposes a substantial economic burden and has become a major global public health concern (Chen et al., 2023). Within the spectrum of hematological malignancies, lymphoma exhibits the highest incidence globally (Ying X. H et al., 2024). Lymphoma comprises a heterogeneous group of malignancies arising from the lymphoid system and the potential to involve multiple anatomical sites, including lymph nodes, tonsils, spleen, and bone marrow (Bobillo et al., 2022). Recent epidemiological trends reveal a concerning 5% annual increase in lymphoma incidence worldwide (Meng, 2019). The 2020 Global Cancer Statistics Report documented approximately 630,000 incident lymphoma cases globally, with projections suggesting this burden will escalate to 910,000 cases by 2040 (Sung et al., 2021). In China, the incidence of lymphoma is also growing rapidly, ranking eighth among all cancer types (National Cancer Center, 2025).
VTE, comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication and a leading cause of mortality among hospitalized patients (Khan et al., 2013). Epidemiological data indicate that adult cancer patients face a 4 to 6.5-fold higher risk of VTE compared with noncancer populations (Kekre and Connors, 2019). Hematologic malignancies confer an even greater thrombotic risk than solid tumors (Blom et al., 2005), with lymphoma patients particularly predisposed to VTE, a risk that continues to rise annually (Wan, 2024). Reported incidence rates of VTE in lymphoma range from 5% to 17% (Sanfilippo et al., 2016). Notably, non-Hodgkin lymphoma (NHL) carries a higher thrombotic risk than Hodgkin lymphoma (HL). Mohren et al. reported a VTE incidence of 10.6% among patients with high-grade NHL, compared with 7.65% in HL and 5.8% in low-grade NHL (Mohren et al., 2005). A meta-analysis by Caruso et al. further confirmed this difference, with a thrombosis incidence rate of 6.5% in NHL patients and only 4.7% in HL patients (P < 0.001) (Caruso et al., 2010). Within NHL subtypes, difference persists: in a U.S. single-center retrospective study, the 1- and 5-year incidence of VTE in follicular lymphoma was 2.4% and 3.8%, respectively, markedly lower than 10.8% and 16.3% observed in patients with diffuse large B-cell lymphoma (DLBCL) (Dharmavaram et al., 2020). The occurrence of VTE not only leads to limb pain, impaired mobility, and reduced quality of life but also disrupts chemotherapy and is associated with inferior survival outcomes.
Although prophylactic anticoagulation can effectively prevent VTE and recurrence, it also increases the risk of bleeding. In contrast to solid malignancies, lymphoma exhibits greater bone marrow invasiveness, frequently resulting in thrombocytopenia and consequent bleeding diathesis, further exacerbating the economic and clinical burden (Shang et al., 2023). Consequently, achieving optimal risk-benefit equilibrium with prophylactic anticoagulation remains a pressing clinical dilemma and therapeutic challenge. International guidelines emphasize the need for accurate and efficient VTE risk assessment tools to identify high-risk patients and to inform tailored prevention and management strategies (Streiff et al., 2021).
Methods
Study population
We performed a retrospective cohort analysis of 605 lymphoma patients admitted to Sichuan Provincial People’s Hospital between January 2019 and June 2024. Inclusion criteria comprised: (1) Age ≥18 years; (2) Histopathologically confirmed lymphoma diagnosis according to the 2022 WHO Classification of Hematopoietic and Lymphoid Tumors (WHO-HAEM5) criteria. Exclusion criteria included: (1) Prior anticancer therapy at external institutions; (2) Secondary lymphoma or concurrent multiple primary malignancies; (3) VTE events diagnosed before lymphoma confirmation; (4) Incomplete hospitalization records; (5) Insufficient follow-up (<6 months) for VTE assessment. VTE occurrence within 6 months was ascertained through comprehensive review of electronic medical records (EMR), including inpatient documentation, outpatient visits, and confirmatory imaging studies (e.g., compression ultrasonography). The study protocol received approval from the Institutional Review Board of Sichuan Provincial People’s Hospital [Ethics Review (Research) No. 526 of 2024].
Data collection
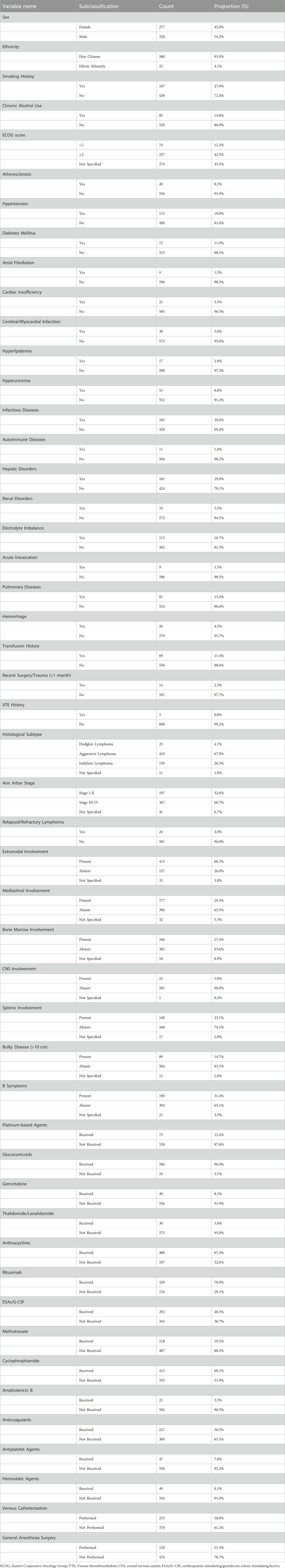
Potential VTE-associated predictors were identified through a systematic literature review and expert consultation. Clinical data were extracted retrospectively from the hospital’s EMR system. (1) Demographics: age, height, weight, Body mass index (BMI), sex, smoking status, chronic alcohol use (>5 years), and Eastern Cooperative Oncology Group (ECOG) score. (2) Comorbidities and medical history: hypertension, diabetes mellitus, active infections, hepatic disorders, electrolyte disturbances, pulmonary comorbidities, and prior transfusion history; (3) Tumor-related factors: tumor histological subtype, tumor stage, recurrence or refractory lymphoma, extranodal involvement, mediastinal involvement, bone marrow involvement, central nervous system involvement, splenic involvement, B symptoms, large mass (>10 cm); (4) Treatment-related factors: platinum-based drugs, anthracycline-based drugs, rituximab, erythropoietin/granulocyte colony-stimulating factor, etc.; (5) Laboratory parameters: D-dimer, Prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, white blood cell count (WBC), platelets, hemoglobin, neutrophils, monocytes, hypersensitive C-reactive protein (hs-CRP), erythrocyte sedimentation rate (ESR), etc. For patients with multiple admissions, only baseline data from the index hospitalization were analyzed. All patient identifiers were anonymized and replaced with unique study identification codes.
Data preprocessing
Because of missing data, class imbalance, and the high dimensionality of candidate predictors, data preprocessing included imputation, resampling, and feature selection to reduce the risk of overfitting and the “curse of dimensionality.” Comprehensive data preprocessing was through three key approaches: (1) Data imputation: K-nearest neighbors (KNN), random forest, and predictive mean matching; (2) Data sampling: random oversampling, SMOTE (Synthetic Minority Over-sampling Technique), and Borderline-SMOTE; (3) Feature selection: LASSO (Least Absolute Shrinkage and Selection Operator), ridge, and elastic net regression. Through a full factorial combination of these methods, we generated 27 distinct processed datasets for subsequent model development. Data cleaning methods and algorithm ID assignments are shown in Supplementary Table S1.
Model development and evaluation
Datasets were randomly split into training (80%) and test (20%) sets with stratified sampling to maintain outcome distribution. The training set facilitated model development using nine distinct machine learning (ML) algorithms, with the test set reserved for independent performance evaluation. The ML algorithms include logistic regression (LR), decision trees (DT), random forests (RF), support vector machines (SVM), naive Bayes (NB), KNN, gradient boosting machine (GBM), extreme gradient boosting (XGBoost), and adaptive boosting (AdaBoost). We employed the training set for model construction and utilized a 10-fold cross-validation approach coupled with a grid search strategy to optimize the parameters of the top-performing machine learning algorithm on the training set. Subsequently, feature selection was conducted using all available variables, followed by model rebuilding. Next, simplify the model by reducing the number of feature variables. The top nine ranked predictors were then used to rebuild simplified models with the five highest-performing algorithms, followed by hyperparameter optimization. This step assessed the trade-off between parsimony and predictive performance.
A comprehensive evaluation framework was employed to assess model performance across three critical dimensions: discrimination, calibration, and clinical utility. Discrimination was quantified using multiple metrics: accuracy, specificity, sensitivity (recall), positive predictive values (PPV), negative predictive values (NPV), F1-score, and area under the curve (AUC). The calculation formulas for evaluation metrics are provided in Supplementary Table S2. Clinical net benefit was rigorously evaluated through decision curve analysis (DCA) across clinically relevant probability thresholds. Model interpretability was achieved using Shapley Additive exPlanations (SHAP) analysis implemented in R (version 4.2.1). Feature importance was systematically ranked based on mean absolute SHAP values to identify the most influential predictors.
Statistical analysis
To ensure data integrity, dual independent data entry with cross-verification was performed by trained research assistants, followed by systematic quality control checks. Variables exceeding 80% missing data or exhibiting extreme outliers were excluded during preprocessing. Initial univariate analyses compared baseline characteristics between VTE and non-VTE cohorts to identify potential associations. Normally distributed continuous variables were expressed as mean ± standard deviation and compared using t-tests. Quantitative variables that did not follow a normal distribution were expressed as median and interquartile range and analyzed using the Kruskal–Wallis test. Categorical variables were presented as counts (percentages) and analyzed using Pearson’s χ2 or Fisher’s exact tests, as appropriate for expected cell frequencies. A two-sided α level of 0.05 defined statistical significance for all analyses. All data were analyzed using SPSS Statistics 26.0 software and R statistical software (version 4.0.3; https://www.r-project.org).
Result
Patient population characteristics
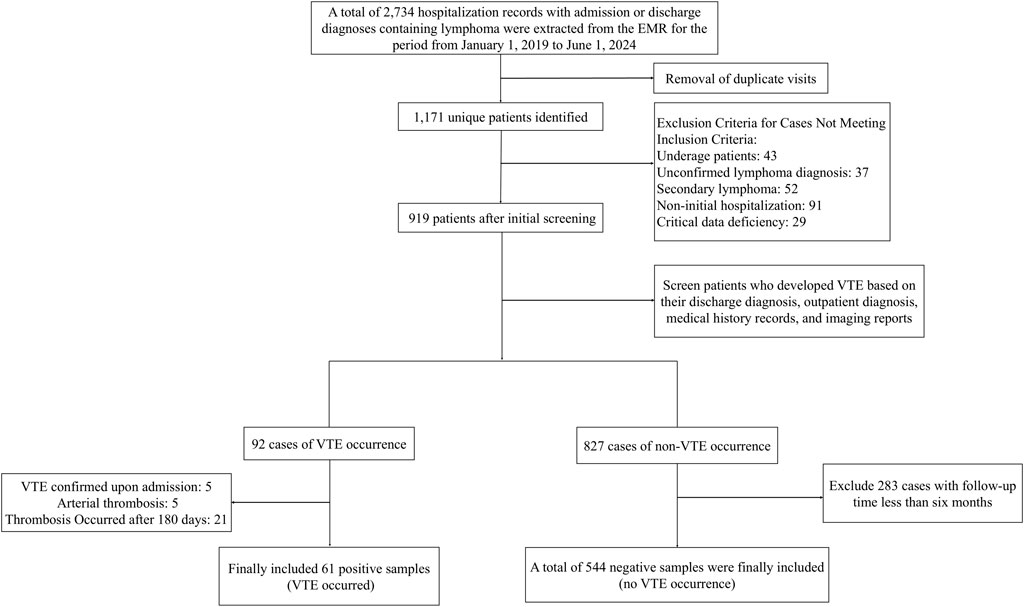
A total of 2,734 hospitalization records of patients with lymphoma were retrieved from the EMR system. After de-duplication, 1,171 valid records remained. The final analytic cohort comprised 605 eligible patients meeting all inclusion criteria. The cohort included 61 VTE cases (incidence 10.1%) and 544 matched controls. Figure 1 presents the patient selection process.
The final cohort comprised 605 patients (277 female [45.8%]; 328 male [54.2%]) with a mean age of 55.5 ± 14.5 years. Regarding histological classification: Aggressive NHL predominated (n = 410, 67.8%), followed by indolent NHL (n = 159, 26.3%) and HL (n = 25, 4.1%). Of patients with documented staging (n = 564), most presented with advanced disease (n = 367, 65.1%). Central venous access was utilized in 38.8% (n = 235) of cases. Complete baseline characteristics are summarized in Table 1.
Of the 61 patients with lymphoma complicated by VTE, 83.6% (n = 51) had aggressive histology. The VTE incidence peaked at 45.9% (28/61) by 30 days post-diagnosis, with subsequent rates of 27.9% at 90 days and 26.2% at 180 days. Advanced-stage disease (III-IV) conferred a higher VTE risk (70.5% vs. 18.0% in early-stage). VTE manifestations included: lower extremity DVT (49.2%, n = 30), upper extremity DVT (18.0%, n = 11), cervical venous thrombosis (9.8%, n = 6), PE (8.2%, n = 5), portal vein thrombosis (3.3%, n = 2), multisite thrombosis (11.5%, n = 7). Comprehensive VTE characteristics and comparative analyses are detailed in Supplementary Table S3 from the supplementary material.
Differences between patient groups
Univariate analysis demonstrated statistically significant differences (P < 0.05) between the two groups in baseline characteristics (age, ECOG score), comorbidities (diabetes, infectious diseases, electrolyte disturbances), tumor features [histological subtype, staging, central nervous system involvement (CNS)], treatment-related factors (transfusion history, EPO/G-CSF usage, central venous access, anticoagulant use), and laboratory parameters [D-dimer, fibrin degradation products (FDP), hs-CRP, LDH, red blood cell count, hematocrit, albumin, TP, serum calcium]. Detailed data are presented in Supplementary Table S4 from supplementary material.
Feature selection results
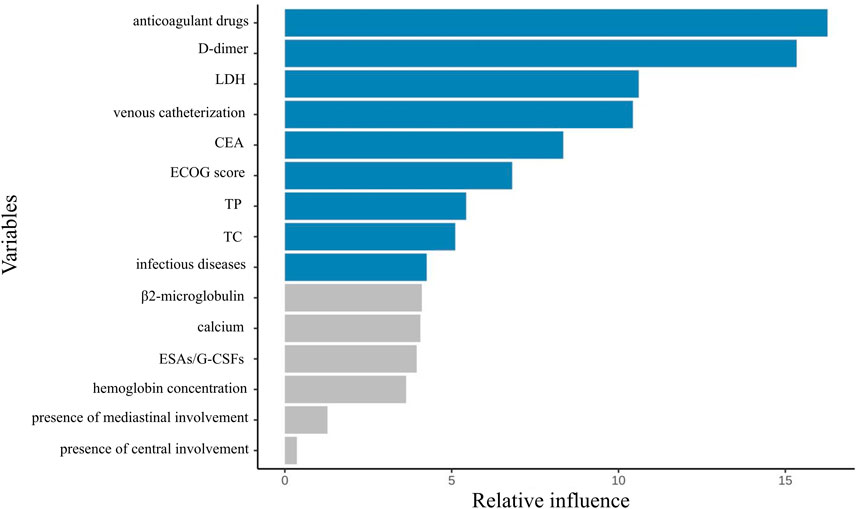
From an initial 63 candidate variables, feature selection was performed to reduce redundancy. Since the feature dimension was still redundant after screening, the study only presented the top 15 most important variables in the performance-optimal model constructed based on all variables, with the aim of improving analysis efficiency to meet actual analysis needs. Figure 2 illustrates the feature importance ranking. The weights of these features are ranked from highest to lowest as follows: anticoagulant drugs, D-dimer, lactate dehydrogenase levels, use of intravenous catheters, CEA, ECOG score, TP, TC, infectious diseases, β2-microglobulin, calcium, erythropoiesis-stimulating/granulocyte colony-stimulating factors (ESAs/G-CSFs), hemoglobin concentration, presence of mediastinal involvement, and presence of central involvement.
Model construction and evaluation results
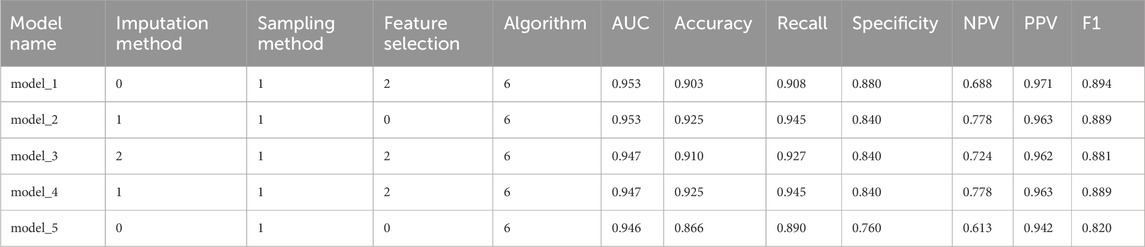
We ultimately constructed 243 models. In the training set, the five best models achieved AUCs of 0.987, 0.992, 0.993, 0.991, and 0.987, respectively (Figure 3). Performance was subsequently evaluated in the test set, with evaluation metrics for the top five models reported in Table 2 (See Supplementary Table S5 for the evaluation metrics of the other models) and their ROC curves shown in Figure 4. The optimal model combined k-nearest neighbors imputation, Synthetic Minority Over-sampling Technique (SMOTE), elastic-net–based feature selection, and a gradient boosting machine (GBM). In the test set, it achieved an AUC of 0.953 [95% confidence interval (CI): 0.932-0.974], accuracy of 0.903 (95% CI: 0.872-0.934), recall of 0.908 (95% CI: 0.864-0.952), and F1-score of 0.894 (95% CI: 0.853-0.935), significantly outperforming other approaches (P < 0.01). Feature-importance analysis identified the top nine predictors as venous catheterization, D-dimer, anticoagulant drugs, LDH, TP, β2-microglobulin, erythropoiesis or granulopoiesis-stimulating drugs, CEA, and ECOG score.
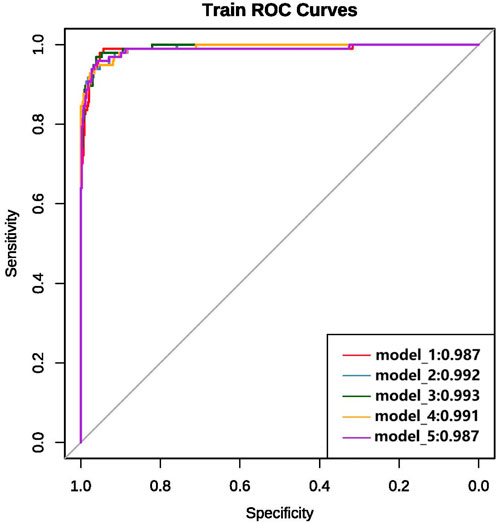
Figure 3. ROC curves and AUC values of the top five models on the training set after variable selection.
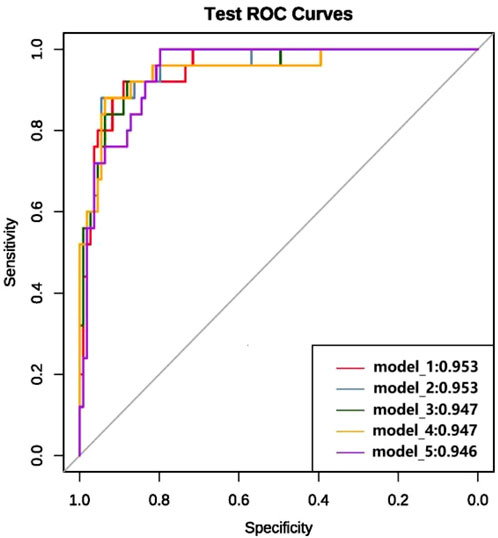
Figure 4. ROC curves and AUC values of the top five models in terms of modeling performance after full variable screening on the test set.
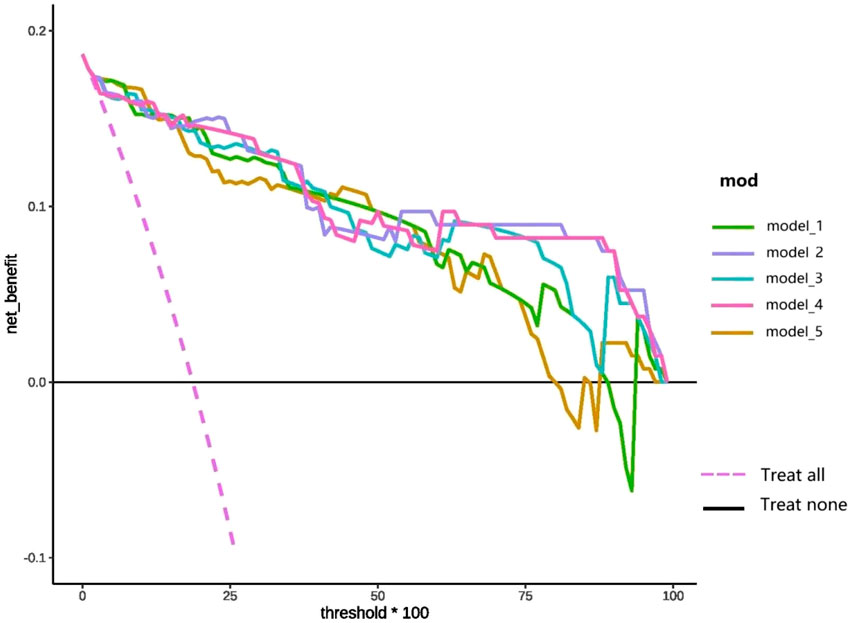
DCA of the top five prediction models following comprehensive feature selection is presented in Figure 5. As demonstrated in Figure 5, model_1 shows superior net benefit compared to the “treat-all” and “treat-none” reference lines across the 0%–85% probability threshold range. Similarly, when the threshold is within the 0%–75% probability range, model_5 has high clinical application value. Notably, models 2, 3, and four maintain clinical validity throughout the entire threshold spectrum (0%–100%), with models two and four consistently outperforming model three in terms of net benefit.
We employed SHAP analysis to quantify the relative contribution of each predictive feature in the model. Figure 6 presents the SHAP summary plot for the optimal model, displaying feature importance rankings derived from comprehensive feature selection. Global interpretation revealed the mean absolute SHAP values for each feature, ranked in descending order of contribution to model predictions. Venous catheterization emerged as the most influential predictor. Subsequent predictors included: D-dimer, anticoagulant drugs, LDH, TP, etc.
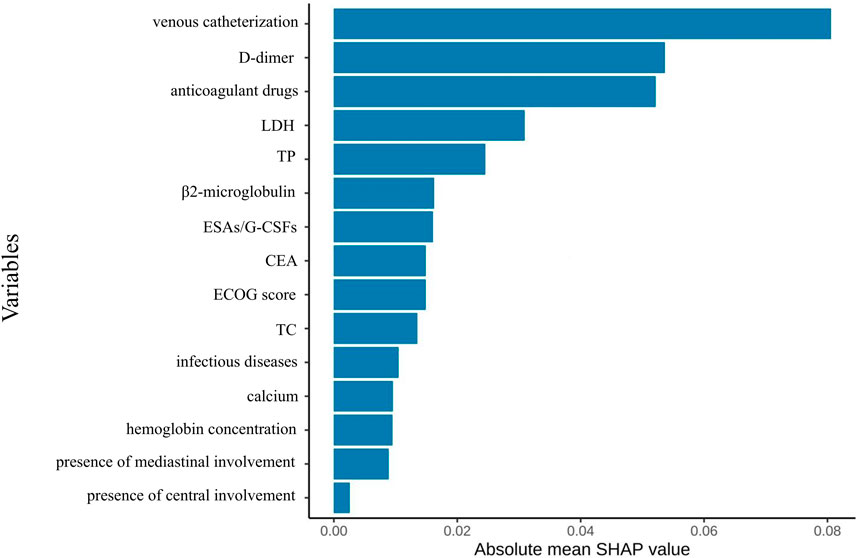
Figure 6. Feature importance ranking diagram of the optimal model constructed based on full variable feature selection.
Figure 7 presents the SHAP beeswarm plot of the optimal model derived from comprehensive feature selection, illustrating feature importance and effect directions across the entire test cohort. Venous catheterization had the strongest positive association with VTE risk, followed by D-dimer, anticoagulant use, LDH, and TP. Preventive anticoagulation, TP, and cholesterol were negatively associated with VTE, while mediastinal and CNS involvement showed weak predictive value.
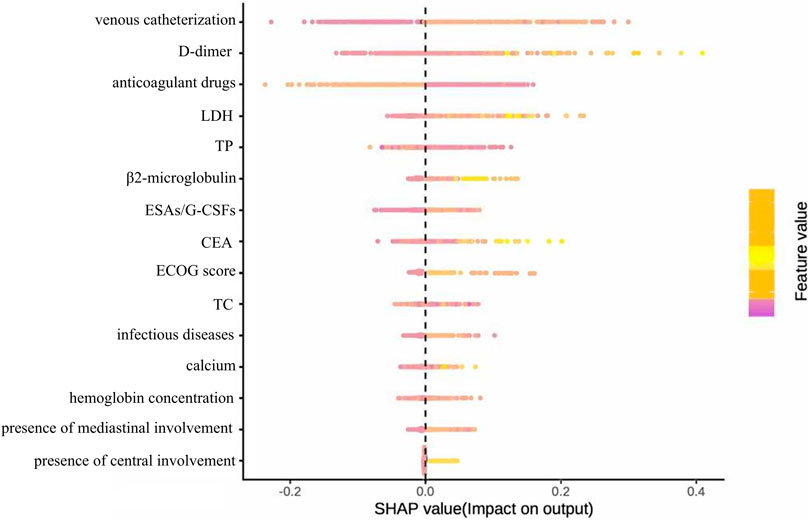
Figure 7. Summary diagram of the optimal model SHAP constructed based on full variable feature selection.
Simplified model results
To minimize overfitting and redundancy, simplified models were developed using only the top nine variables identified by the best-performing full model. Five simplified models were reconstructed using the same preprocessing methods and algorithms as their full counterparts. Performance metrics are presented in Table 3 and ROC curves in Figure 8. The simplified models bypassed feature selection by directly incorporating the predetermined top nine features from the comprehensive analysis. Models 1 and 2, differing only in feature selection approach but sharing k-NN imputation, SMOTE sampling, and GBM algorithm, yielded identical simplified versions (Simp-SMOTE_knn_GBM). Similarly, models four and 5 converged to the same simplified version. The Simp-SMOTE_rf_GBM model (random forest imputation + SMOTE + GBM) demonstrated superior performance across all metrics: AUC is 0.954 (95% CI: 0.932-0.976), Accuracy: 0.888, Sensitivity: 0.890, Specificity: 0.880, NPV:0.647, PPV: 0.970, and F1:0.885.
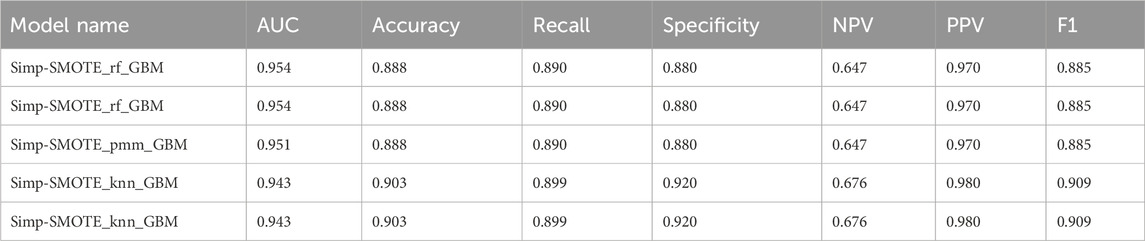
Table 3. Performance metrics results of the five simplified models reconstructed based on the first nine variables.
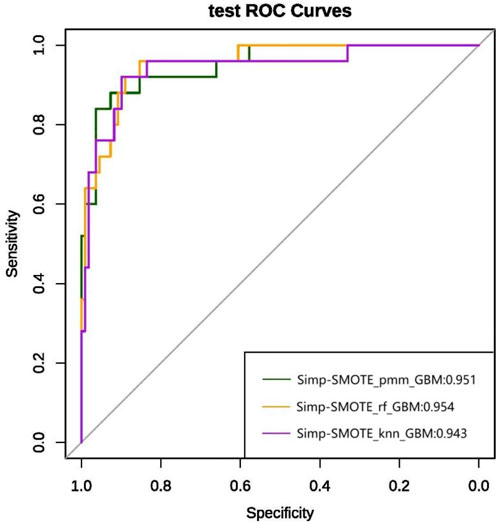
Figure 8. ROC curve and AUC values of the simplified model constructed based on the top nine variables in the test set.
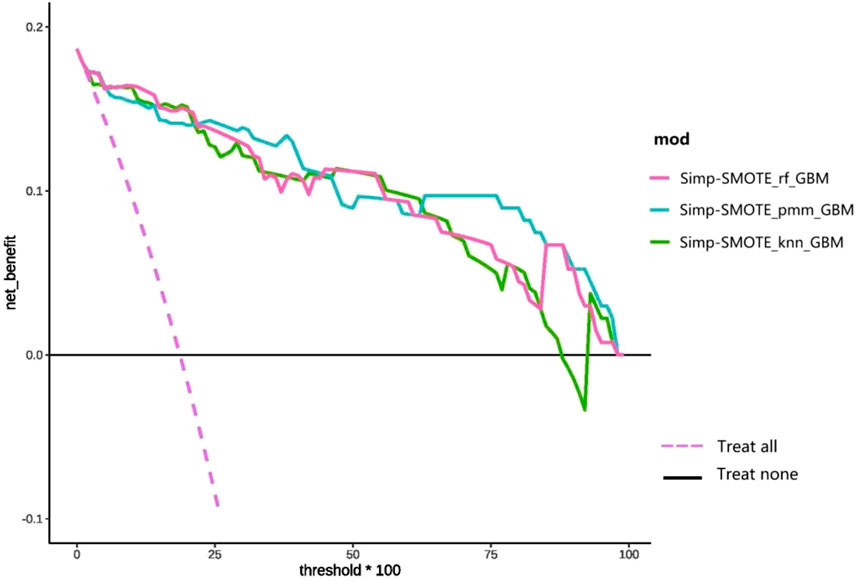
DCA (Figure 9) compares the clinical net benefit profiles of the simplified models across probability thresholds in the test set. The Simp-SMOTE_knn_GBM model demonstrated superior net benefit versus treat-all and treat-none strategies at probability thresholds of 15%–85%, suggesting optimal utility for intermediate-risk clinical decision-making. Both Simp-SMOTE_rf_GBM and Simp-SMOTE_pmm_GBM maintained clinical utility across the full threshold spectrum (0%–100%), with Simp-SMOTE_pmm_GBM showing consistently higher net benefit.
SHAP feature importance ranking for the simplified model (Figure 10) showed central venous catheterization as the strongest predictor, followed by anticoagulant use, D-dimer, LDH, TP, CEA, cholesterol, infectious disease, and ECOG performance status.
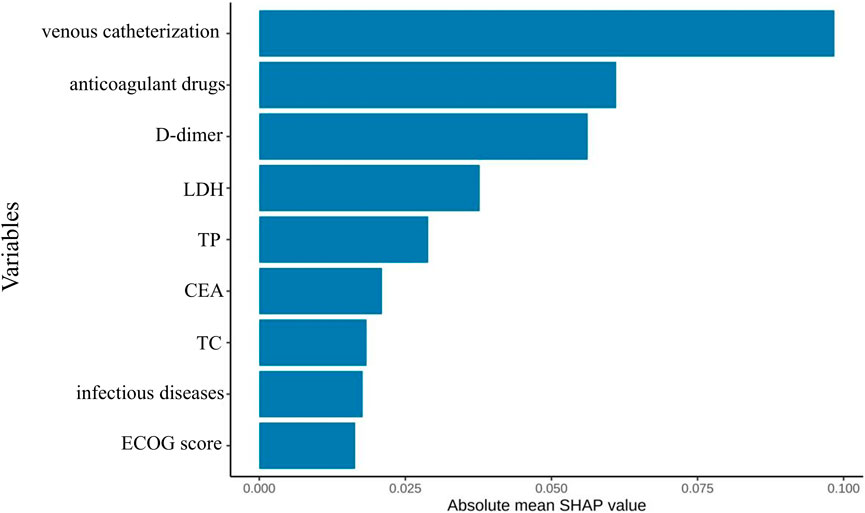
Figure 10. Optimal model feature importance ranking chart constructed based on the first nine feature variables.
Figure 11 presents the SHAP summary plot for the parsimonious VTE prediction model incorporating the top nine features, illustrating both feature importance and directionality of effects across the test set. The feature ranking of this simplified model differs from that of the optimal model constructed using full variable screening. Notably, active infection and TC emerged as new predictors in the simplified model, while relative importance shifted for D-dimer, therapeutic anticoagulation, and CEA. SHAP analysis revealed positive associations between VTE risk and: venous catheterization, D-dimer, LDH, CEA, and active infection. Conversely, therapeutic anticoagulation, TP, and TC demonstrated protective associations. The contribution of the ECOG score to the model output results is relatively insignificant.
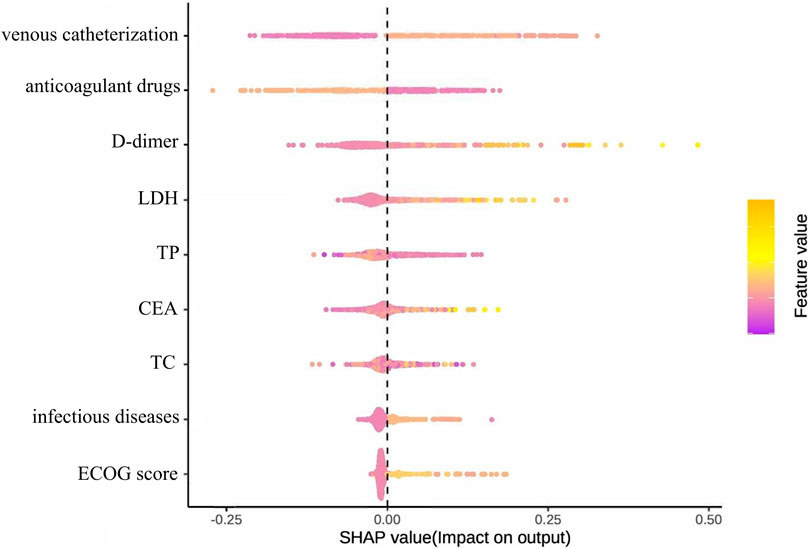
Figure 11. Summary diagram of the optimal model SHAP established based on the first nine feature variables.
Comparison of simplified and unsimplified model performance
We conducted a comprehensive performance comparison between the full-feature models and their simplified counterparts (using the top nine features) across five key metrics: AUC, sensitivity, specificity, accuracy, and F1-score, with detailed results shown in Figure 12. Figure 12 presents the comparative performance analysis across both training and test datasets, including 95% CI for all metrics. For the pre-simplification optimal model (a), the full-feature version demonstrated marginally superior performance compared to its simplified counterpart. Overall, the predictive performance of the simplified models differs only slightly from that of their corresponding original models, and some simplified models even outperform the original models in terms of performance metrics. These results suggest that feature reduction incurred minimal predictive penalty, while maintaining clinical utility through improved interpretability and computational efficiency.
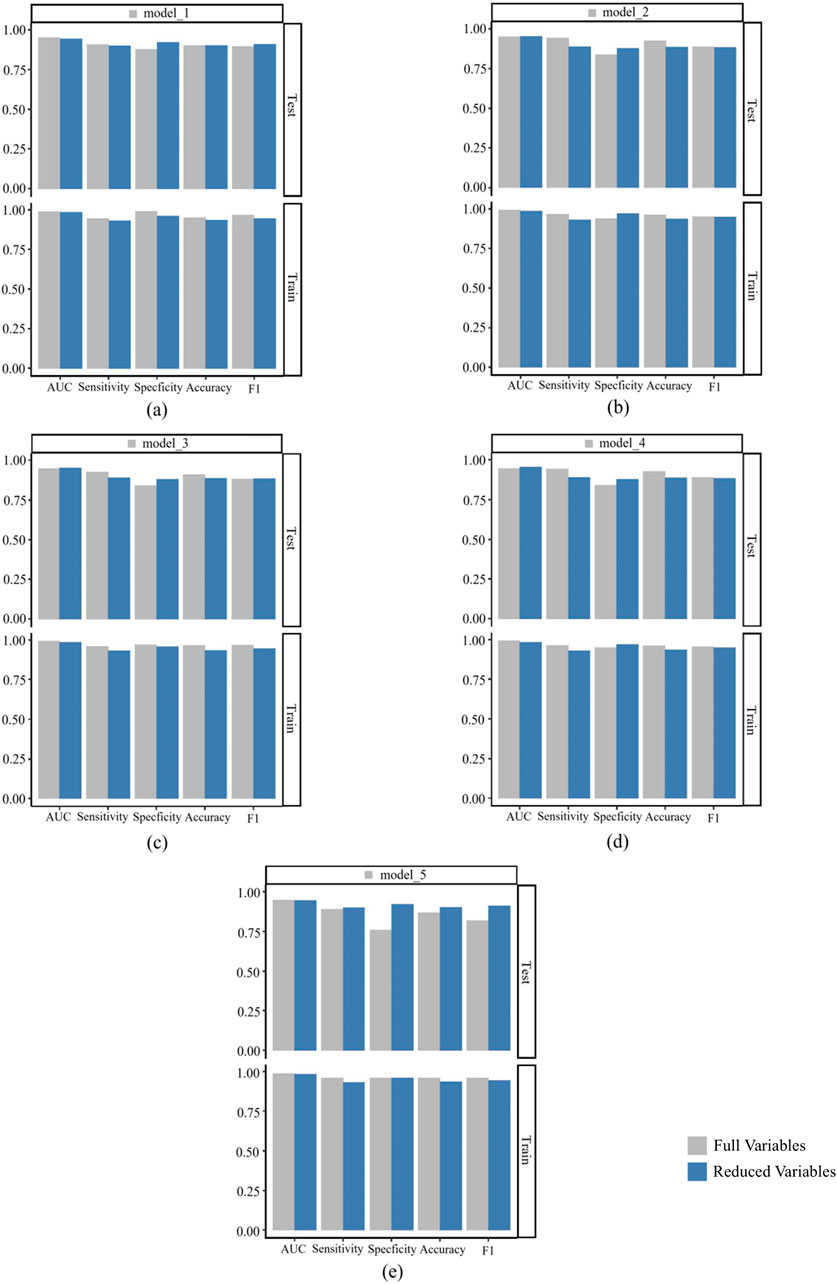
Figure 12. Comparison of performance indicators between the model built after full variable screening and the model built with the top nine variables. (a) is a comparison between model_1 and its corresponding simplified model; (b) is a comparison between model_2 and its corresponding simplified model; (c) is a comparison between model_3 and its corresponding simplified model; (d) is a comparison between model_4 and its corresponding simplified model; (e) is a comparison between model_5 and its corresponding simplified model.
Discussion
Lymphoma is the most common malignant tumor of the hematopoietic system, and factors such as the high tumor burden associated with the disease itself can increase the risk of VTE. In addition, common treatment methods such as surgery, chemotherapy, and immunotherapy may also increase the risk of VTE in patients. Current VTE prevention guidelines primarily target general chronic disease populations, leaving lymphoma-specific prevention strategies inadequately addressed—a critical gap in clinical practice. There are currently comprehensive risk assessment tools for VTE in patients with lymphoma. In recent years, researchers both domestically and internationally have developed numerous VTE risk assessment tools for cancer patients. Khorana et al. conducted a large-scale retrospective cohort study involving 66,106 cancer patients with neutropenia and developed a VTE risk scoring system suitable for outpatient chemotherapy cancer patients (Streiff et al., 2021). However, two critical limitations exist: (1) exclusion of hospitalized patients potentially underestimates true VTE risk (Louzada et al., 2012), and (2) Khorana scoring studies mostly involve solid tumour patients, with only a small proportion of lymphoma patients, raising questions about its applicability in the lymphoma population. Subsequent studies by Mohren et al. involved a VTE risk prediction analysis of 2,701 patients with malignant tumours undergoing chemotherapy. However, the lymphoma subgroup accounted for only 12.1% of the total sample (Caruso et al., 2010). The Ottawa score, designed to predict 6-month VTE recurrence post-anticoagulation in cancer patients, originally dichotomized patients into low- and high-risk categories. Subsequent refinements established three risk strata (low, moderate, high) (Louzada et al., 2012; den Exter et al., 2013). However, studies by Alatri et al. (2017) have shown that the improved Ottawa score has an AUC of 0.58 (95% CI: 0.56–0.61), indicating insufficient predictive performance. The accuracy and discriminative ability of the score in predicting VTE recurrence are generally poor, with low sensitivity, specificity, and positive predictive value. It is unable to accurately predict VTE recurrence in patients with cancer-related thrombosis, which could lead to biased clinical decision-making.
Due to natural differences in ethnicity and genetics, certain models are not applicable in Chinese cohorts. However, the universality of pan-cancer models has been called into question in the context of lymphoma. Validation studies demonstrate superior discrimination for lymphoma-specific models (e.g., TiC-LYMPHO: C-statistic 0.783, 95% CI: 0.752-0.814) versus pan-cancer tools in lymphoma populations (Bastos-Oreiro et al., 2021). Lymphoma-specific VTE risk factors (e.g., LDH, β2-microglobulin) differ substantially from solid tumors (Antic et al., 2016; Lim et al., 2016; Dharmavaram et al., 2020), explaining the poor performance of pan-cancer models. Key lymphoma biomarkers, including LDH and β2-microglobulin, demonstrate VTE associations (Yıldız et al., 2020), yet remain absent from general cancer models. Suboptimal risk stratification may cause both under-anticoagulation and over-treatment, adversely impacting clinical outcomes and healthcare costs. This study uses machine learning algorithms to analyse real-world medical data and incorporate multiple types of variables in order to develop a VTE risk prediction model for lymphoma patients. The model aims to provide clinicians with an intelligent decision-support tool that optimises strategies for preventing thrombosis and reduces the incidence of bleeding events.
Through systematic evaluation of 27 preprocessing pipelines and nine machine learning algorithms, we developed and validated 243 distinct prediction models. Although the optimal full-variable model (KNN interpolation + SMOTE + elastic network + gradient boosting machine) achieved an AUC of 0.953, there is a risk of feature redundancy. Therefore, based on feature importance, the top nine key variables were selected to rebuild a simplified model, ultimately obtaining the Simp-SMOTE_rf_GBM model (AUC = 0.954), which demonstrated superior predictive performance and practicality compared to the full-variable model. The simplified model showed robust external validity (calibration slope 0.98) while preserving accuracy (0.888) and sensitivity (0.890), making it particularly suitable for clinical implementation. Among the key features, venous catheterization, D-dimer, and LDH were positively correlated with VTE risk, while anticoagulant drugs and TP were negatively correlated. Feature importance hierarchy shifted in the simplified model, with TC and active infection replacing β2-microglobulin and hematopoietic growth factors. SHAP analysis further validated the contribution direction of these features. Key predictors such as venous catheterization, anticoagulant drugs, TC, and infectious diseases have special significance in clinical practice.
Venous catheterization is primarily used for patients requiring long-term intravenous infusion therapy, such as chemotherapy or intravenous nutrition. Currently, the most commonly used central venous catheter access routes in clinical practice include central venous catheters (CVCs) inserted via the internal jugular vein, subclavian vein, or femoral vein, and peripherally inserted central venous catheters (PICCs). Research data shows that venous catheterization may cause a 40%–80% decrease in venous blood flow rate. When combined with cancer-associated hypercoagulability, these hemodynamic changes synergistically increase thrombosis risk, potentially leading to life-threatening PE (Guan et al., 2018; Yang et al., 2020). Currently, multiple evidence-based medical studies have confirmed that venous catheterization is an independent risk factor for VTE in lymphoma patients (Park et al., 2012; Guan et al., 2018; Kirkizlar et al., 2020; Wan, 2024). Lymphoma patients receiving PICCs demonstrate a 5.25-fold increased VTE risk (95% CI 3.8-7.1) compared to non-catheterized patients (Zhang, 2025). Park et al. (2012) reported CVC-associated thrombosis risk elevation (OR 2.04, 95% CI 1.02-4.08, p = 0.042) in a prospective cohort of 452 lymphoma patients. A Chinese study, however, showed that the likelihood of VTE in lymphoma patients using CVC was 6.63 times higher than in those not using CVC (OR = 6.63, 95% CI: 2.24–19.57, p = 0.001) (Y et al., 2021).
According to clinical practice guidelines, the prophylactic use of anticoagulants can effectively reduce the risk of VTE and is an important protective factor (Streiff et al., 2021). Our findings corroborate guideline recommendations, showing significantly elevated VTE risk in patients without prophylaxis. Low molecular weight heparin (LMWH) drugs such as enoxaparin and nadroparin are currently the drugs of choice for prophylactic anticoagulant therapy. LMWHs exert their antithrombotic effect through selective inhibition of factor Xa and factor IIa, effectively interrupting the coagulation cascade. In recent years, direct oral anticoagulants (DOACs), such as rivaroxaban and apixaban, have become more widely used in clinical practice. These drugs offer advantages such as convenient administration and the elimination of the need for frequent monitoring of coagulation parameters. Studies have shown that they are as effective as LMWH at preventing blood clots in patients with malignant tumours (Agnelli et al., 2020). However, anticoagulation carries inherent bleeding risks, particularly in patients with thrombocytopenia, a history of gastrointestinal ulcers, or recent surgical procedures. Therefore, before initiating anticoagulant prophylaxis, it is essential to conduct a comprehensive assessment of the patient’s bleeding and thrombosis risks, and to develop a personalised treatment plan. Furthermore, the development of safer, easier-to-use anticoagulant drugs is necessary in order to provide lymphoma patients with an optimised thrombosis prevention regimen. Ying L et al. (2024) found that prophylactic anticoagulant use is a significant protective factor against PICC-related thrombosis in cancer patients. This factor served as the primary node in their decision tree model, underscoring its significant impact on thrombus formation. Furthermore, Boraks et al. (Boraks et al., 1998) demonstrated that prophylactic low-dose warfarin effectively reduces the incidence of catheter-related thrombosis. However, Heaton et al. (2002) found that a low-dose warfarin regimen (1 mg) did not significantly inhibit catheter-related thrombotic events in cancer patients. Therefore, the efficacy of prophylactic anticoagulation in reducing venous thrombosis incidence requires further validation and investigation in larger, prospective studies.
Lymphoma patients frequently exhibit compromised immune function and substantially elevated infection risk attributable to both the malignancy itself and treatment-related factors, including chemotherapy and immunosuppressive agents. Such infections further exacerbate VTE incidence, adversely impacting tumor prognosis. Multiple studies establish concomitant infections as significant risk factors for DVT in cancer patients (Chen et al., 2020; Wang et al., 2023). Patients with concomitant infections exhibit 2- to 3-fold higher VTE incidence compared to infection-free patients. This correlation is particularly pronounced within the initial 6 months post-diagnosis (Nakano et al., 2018). The prothrombotic effects of infection are multifactorial, encompassing oxidative stress, systemic inflammation, coagulation activation, and endothelial injury. Inflammatory responses—driven in part by neutrophil-derived cytokines such as interleukin-6 and tumor necrosis factor–α—induce tissue factor expression in circulating monocytes and promote release of tissue factor from monocytes and platelets, thereby activating the extrinsic coagulation pathway, fostering fibrin formation, and suppressing fibrinolysis to create a hypercoagulable state (Lim et al., 2016). In parallel, infection triggers innate immune pathways involving neutrophils and the formation of neutrophil extracellular traps (NETs), which help immobilize pathogens within the vasculature but also amplify thrombin generation and thrombosis (Longstaff et al., 2013). Together, these processes lead to endothelial injury, platelet activation and aggregation, increased procoagulant protein activity, and attenuation of anticoagulant mechanisms, culminating in thrombus formation (Beristain-Covarrubias et al., 2019). Overall, infection is both a potent precipitant of VTE in lymphoma and a key determinant of adverse prognosis.
CEA is a glycoprotein of the immunoglobulin superfamily that participates in cell adhesion, inflammatory signaling, and tumor progression (Kankanala et al., 2025). In a study of patients with lung cancer, multivariable analyses demonstrated a linear positive association between CEA concentration and pulmonary embolism, suggesting that elevated CEA may help identify individuals at increased risk of PE (Zhang et al., 2014). As a nonspecific tumor marker, CEA reflects tumor burden and growth kinetics and is widely used for diagnosis and prognostication. However, evidence linking CEA to VTE risk remains limited, and its clinical significance is not fully established. Large, prospective cohort studies are needed to clarify the mechanistic relationship between CEA and thrombogenesis.
Age (Chen et al., 2022),inherited predisposition (Sánchez Prieto et al., 2024),and reduced mobility (Saito et al., 2021) are established risk factors for VTE in patients with lymphoma. In one study, individuals with lymphoma or multiple myeloma who carried factor V Leiden and SERPINA10 variants had higher VTE incidence; other data indicate that the coexistence of cancer and factor V Leiden variants synergistically increases VTE risk (Gran et al., 2016). In our feature-selection pipeline, however, these variables were not retained in the final predictive model, likely due to sample characteristics, collinearity, or methodological constraints. Their exclusion does not diminish their clinical relevance in lymphoma, and their potential contributions warrant attention. To define the magnitude and independence of these associations, adequately powered, prospective, multicenter studies are needed.
This study has multifaceted clinical applicability. Firstly, the model helps to identify high-risk patients, supporting the personalisation of decisions regarding prophylactic anticoagulation. Secondly, it incorporates dynamic variables, such as changes in D-dimer levels, which enable ongoing risk assessment during treatment. Furthermore, the risk factors elucidated by the model could be used to refine VTE prevention strategies. For example, more aggressive prophylactic anticoagulation could be considered for patients requiring mandatory venous catheterisation. Finally, the simplified version of the model, which includes only nine readily available clinical variables, significantly enhances its feasibility for implementation in settings with limited resources.
Limitations
This study has limitations. Firstly, as this was a retrospective analysis, several clinically relevant variables, e.g., genetic risk scores, immunophenotypic scores (IPS), Throly scores and Khorana scores, were either unavailable or severely lacking because some assessments are not routinely performed in patients with lymphoma in the absence of clear clinical indications. Consequently, they were excluded from modelling and infrequently used medications were aggregated into composite categories. Secondly, VTE ascertainment from the electronic medical record may have missed events (e.g., non-specific symptoms, lack of screening or diagnoses made at outside institutions), which would lead to an underestimation of incidence. The small number of VTE cases also produced class imbalance, which may persist despite Synthetic Minority Over-sampling Technique (SMOTE) correction and could affect model performance. Thirdly, this was a single-centre study with a limited sample size and few VTE events. Future work should employ larger, prospective, multicentre cohorts to validate, refine and generalise the model.
Conclusion
This study developed a VTE risk prediction model specifically for lymphoma patients. With an AUC of 0.953, the optimized model exhibited outstanding discriminative capacity for lymphoma-associated VTE risk, providing an evidence-based framework to guide optimal timing of anticoagulation initiation and clinical strategy formulation. This model enables theoretically grounded and clinically actionable VTE risk stratification in lymphoma populations, advancing precision medicine approaches to ultimately enhance clinical outcomes.
Multicenter prospective validation warrants prioritization to establish model generalizability and robustness across heterogeneous healthcare environments. Concurrent integration of multi-omics data with emerging biomarkers—including circulating tumor DNA (ctDNA), microvesicles, and novel coagulation parameters (thrombin-antithrombin complex [TAT], plasmin-α2-plasmin inhibitor complex [PIC], thrombomodulin [TM], tissue plasminogen activator inhibitor complex [tPAI·C])—may substantially improve prognostic precision. Intervention trials should evaluate risk-stratified anticoagulation protocols via randomized controlled designs, validating their efficacy in optimizing hard clinical endpoints. Mechanistic studies must elucidate lymphoma-specific prothrombotic pathways, focusing on coagulation biomarkers (e.g., TAT, PIC) within tumor-associated thrombosis to inform targeted therapeutic development. These integrated approaches will accelerate the evolution of precision medicine in lymphoma-associated VTE management.
Data availability statement
The data for this study were obtained from Sichuan Provincial People’s Hospital and are not publicly available. However, they can be obtained from the corresponding author upon reasonable request.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Sichuan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because exemption from informed consent.
Author contributions
CH: Writing – original draft. YW: Writing – original draft. HZ: Writing – original draft. SL: Writing – original draft. FK: Writing – original draft. FC: Writing – original draft. LH: Writing – review and editing. Writing – original draft. QY: Writing – review and editing, Writing – original draft. GL: Writing – review and editing. XS: Writing – review and editing. YB: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Sichuan Provincial Natural Science Foundation (2022NSFSC0818), Chinese Pharmaceutical Association (CPA-Z05-ZC-2023-002), China International Medical Exchange Foundation (Z-2021-46-2101-2023), and Medical Research Project (2025083).
Acknowledgments
The authors would like to thank all researchers, research coordinators, and patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1691271/full#supplementary-material
References
Agnelli, G., Becattini, C., Meyer, G., Muñoz, A., Huisman, M. V., Connors, J. M., et al. (2020). Apixaban for the treatment of venous thromboembolism associated with cancer. N. Engl. J. Med. 382, 1599–1607. doi:10.1056/NEJMoa1915103
Alatri, A., Mazzolai, L., Font, C., Tafur, A., Valle, R., Marchena, P. J., et al. (2017). Low discriminating power of the modified Ottawa VTE risk score in a cohort of patients with cancer from the RIETE registry. Thromb. Haemost. 117, 1630–1636. doi:10.1160/TH17-02-0116
Antic, D., Milic, N., Nikolovski, S., Todorovic, M., Bila, J., Djurdjevic, P., et al. (2016). Development and validation of multivariable predictive model for thromboembolic events in lymphoma patients. Am. J. Hematol. 91, 1014–1019. doi:10.1002/ajh.24466
Bastos-Oreiro, M., Ortiz, J., Pradillo, V., Salas, E., Marínez-Laperche, C., Muñoz, A., et al. (2021). Incorporating genetic and clinical data into the prediction of thromboembolism risk in patients with lymphoma. Cancer Med. 10, 7585–7592. doi:10.1002/cam4.4280
Beristain-Covarrubias, N., Perez-Toledo, M., Thomas, M. R., Henderson, I. R., Watson, S. P., and Cunningham, A. F. (2019). Understanding infection-induced thrombosis: lessons learned from animal models. Front. Immunol. 10 10, 2569. doi:10.3389/fimmu.2019.02569
Blom, J. W., Doggen, C. J. M., Osanto, S., and Rosendaal, F. R. (2005). Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 293, 715–722. doi:10.1001/jama.293.6.715
Bobillo, S., Joffe, E., Lavery, J. A., Sermer, D., Ghione, P., Noy, A., et al. (2022). Clinical characteristics and outcomes of extranodal stage I diffuse large B-cell lymphoma in the rituximab era. Blood. 137 (1), 39–48. doi:10.1182/blood.2020005112
Boraks, P., Seale, J., Price, J., Bass, G., Ethell, M., Keeling, D., et al. (1998). Prevention of central venous catheter associated thrombosis using minidose warfarin in patients with haematological malignancies. Br. J. Haematol. 101, 483–486. doi:10.1046/j.1365-2141.1998.00732.x
Bray, F., Laversanne, M., Weiderpass, E., and Soerjomataram, I. (2021). The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 127, 3029–3030. doi:10.1002/cncr.33587
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263. doi:10.3322/caac.21834
Caruso, V., Di Castelnuovo, A., Meschengieser, S., Lazzari, M. A., de Gaetano, G., Storti, S., et al. (2010). Thrombotic complications in adult patients with lymphoma: a meta-analysis of 29 independent cohorts including 18 018 patients and 1149 events. Blood 115, 5322–5328. doi:10.1182/blood-2010-01-258624
Chen, P.-Y., Liu, Y.-H., Duan, C.-Y., Jiang, L., Wei, X.-B., Guo, W., et al. (2020). Impact of infection in patients with non-ST elevation acute coronary syndrome undergoing percutaneous coronary intervention: insight from a multicentre observational cohort from China. BMJ Open 10 10, e038551. doi:10.1136/bmjopen-2020-038551
Chen, Y., Lei, H., Wang, W., Zhu, J., Zeng, C., Lu, Z., et al. (2022). Characteristics and predictors of venous thromboembolism among lymphoma patients undergoing chemotherapy: a cohort study in China. Front. Pharmacol. 13, 901887. doi:10.3389/fphar.2022.901887
Chen, S., Cao, Z., Prettner, K., Kuhn, M., Yang, J., Jiao, L., et al. (2023). Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 9, 465–472. doi:10.1001/jamaoncol.2022.7826
den Exter, P. L., Kooiman, J., and Huisman, M. V. (2013). Validation of the Ottawa prognostic score for the prediction of recurrent venous thromboembolism in patients with cancer-associated thrombosis. J. Thromb. Haemost. 11, 998–1000. doi:10.1111/jth.12192
Dharmavaram, G., Cao, S., Sundaram, S., Ayyappan, S., Boughan, K., Gallogly, M., et al. (2020). Aggressive lymphoma subtype is a risk factor for venous thrombosis. Development of lymphoma - specific venous thrombosis prediction models. Am. J. Hematol. 95, 918–926. doi:10.1002/ajh.25837
Gran, O. V., Smith, E. N., Brækkan, S. K., Jensvoll, H., Solomon, T., Hindberg, K., et al. (2016). Joint effects of cancer and variants in the factor 5 gene on the risk of venous thromboembolism. Haematologica 101, 1046–1053. doi:10.3324/haematol.2016.147405
Guan, C. Y., Liao, H. T., Gao, W., and Wei, Y. P. (2018). Relationship between PICC catheter-related thrombosis and coagulation index changes in cancer patients. Chin. J. Pract. Nurs. 34, 848–852. doi:10.3760/cma.j.issn.1672-7088.2018.11.011
Heaton, D. C., Han, D. Y., and Inder, A. (2002). Minidose (1 mg) warfarin as prophylaxis for central vein catheter thrombosis. Intern Med. J. 32, 84–88. doi:10.1046/j.1445-5994.2002.00171.x
Kankanala, V. L., Zubair, M., and Mukkamalla, S. K. R. (2025). “Carcinoembryonic Antigen,” in. StatPearls. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK578172/.
Kekre, N., and Connors, J. M. (2019). Venous thromboembolism incidence in hematologic malignancies. Blood Rev. 33, 24–32. doi:10.1016/j.blre.2018.06.002
Khan, K., Hanna, G. G., Campbell, L., Scullin, P., Hussain, A., Eakin, R. L., et al. (2013). Re-challenge chemotherapy with gemcitabine plus carboplatin in patients with non-small cell lung cancer. Chin. J. Cancer 32, 539–545. doi:10.5732/cjc.013.10120
Kirkizlar, O., Alp Kirkizlar, T., Umit, E. G., Asker, I., Baysal, M., Bas, V., et al. (2020). The incidence of venous thromboembolism and impact on survival in hodgkin lymphoma. Clin. Lymphoma Myeloma Leuk. 20, 542–547. doi:10.1016/j.clml.2020.02.021
Lan, Y., Guan, J., and Zhu, J. (2021). Venous thromboembolic events in T-cell lymphoma patients: incidence, risk factors and clinical features. Leuk. Res. 103, 106537. doi:10.1016/j.leukres.2021.106537
Lim, S. H., Woo, S.-Y., Kim, S., Ko, Y. H., Kim, W. S., and Kim, S. J. (2016). Cross-sectional study of patients with diffuse large B-Cell lymphoma: assessing the effect of host status, tumor burden, and inflammatory activity on venous thromboembolism. Cancer Res. Treat. 48, 312–321. doi:10.4143/crt.2014.266
Longstaff, C., Varjú, I., Sótonyi, P., Szabó, L., Krumrey, M., Hoell, A., et al. (2013). Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J. Biol. Chem. 288, 6946–6956. doi:10.1074/jbc.M112.404301
Louzada, M. L., Carrier, M., Lazo-Langner, A., Dao, V., Kovacs, M. J., Ramsay, T. O., et al. (2012). Development of a clinical prediction rule for risk stratification of recurrent venous thromboembolism in patients with cancer-associated venous thromboembolism. Circulation 126, 448–454. doi:10.1161/CIRCULATIONAHA.111.051920
Meng, J. S. (2019). The epidemiological characteristics report of malignant lymphoma: analysis of 2,027 cases in a single institution. Master’s thesis. Wuhan: Huazhong University of Science and Technology. Available online at: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201901&filename=1018891461.nh.
Mohren, M., Markmann, I., Jentsch-Ullrich, K., Koenigsmann, M., Lutze, G., and Franke, A. (2005). Increased risk of thromboembolism in patients with malignant lymphoma: a single-centre analysis. Br. J. Cancer 92, 1349–1351. doi:10.1038/sj.bjc.6602504
Nakano, F., Matsubara, T., Ishigaki, T., Hatazaki, S., Mouri, G., Nakatsuka, Y., et al. (2018). Incidence and risk factor of deep venous thrombosis in patients undergoing craniotomy for brain tumors: a Japanese single-center, retrospective study. Thromb. Res. 165, 95–100. doi:10.1016/j.thromres.2018.03.016
National Cancer CenterLymphoma Expert Committee of National Cancer Quality Control Center (2025). Lymphoma expert committee of national cancer quality control center. Quality control index for standardized diagnosis and treatment of lymphoma in China (2022 edition). Chin. J. Oncol. 44, 628–633. doi:10.3760/cma.j.cn112152-20220511-00329
Park, L. C., Woo, S., Kim, S., Jeon, H., Ko, Y. H., Kim, S. J., et al. (2012). Incidence, risk factors and clinical features of venous thromboembolism in newly diagnosed lymphoma patients: results from a prospective cohort study with Asian population. Thromb. Res. 130, e6–e12. doi:10.1016/j.thromres.2012.03.019
Saito, M., Wages, N. A., and Schiff, D. (2021). Incidence, risk factors and management of venous thromboembolism in patients with primary CNS lymphoma. J. Neurooncol 154, 41–47. doi:10.1007/s11060-021-03791-x
Sánchez Prieto, I., Gutiérrez Jomarrón, I., Martínez Vázquez, C., Rodríguez Barquero, P., Gili Herreros, P., and García-Suárez, J. (2024). Comprehensive evaluation of genetic and acquired thrombophilia markers for an individualized prediction of clinical thrombosis in patients with lymphoma and multiple myeloma. J. Thromb. Thrombolysis 57, 984–995. doi:10.1007/s11239-024-02977-0
Sanfilippo, K. M., Wang, T. F., Gage, B. F., Luo, S., Riedell, P., and Carson, K. R. (2016). Incidence of venous thromboembolism in patients with non-Hodgkin lymphoma. Thromb. Res. 143, 86–90. doi:10.1016/j.thromres.2016.05.008
Shang, Q. W., Wang, A., Wang, W., and Zhang, Y. (2023). Research progress on risk factors and prediction models of venous thromboembolism in lymphoma. Int. J. Laboratory Med. 44, 2022–2028.
Streiff, M. B., Holmstrom, B., Angelini, D., Ashrani, A., Elshoury, A., Fanikos, J., et al. (2021). Cancer-associated venous thromboembolic disease, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 19, 1181–1201. doi:10.6004/jnccn.2021.0047
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Wan, Y. Z. (2024). Clinical features and risk factors in patients with lymphomacomplicated with venous thromboembolism. Master’s thesis. Jilin: Jilin University. doi:10.27162/d.cnki.gjlin.2023.003225
Wang, X. X., He, Y., Chu, J., Chen, C. M., and Wang, Y. F. (2023). Analysis of risk factors for central venous catheter-related thrombosis in malignant tumor patients and construction of a risk prediction model. Chin. J. New Clin. Med. 16, 1071–1076.
Yang, F. Y., Hua, R. Y., Wu, W. Y., Bi, D. F., Wu, Y., Wang, J. Y., et al. (2020). Establishment risk Predict. nomogram model Up. Extrem. venous thrombosis Assoc. Peripher. venous inserted central catheter cancer patients. Cancer Res. Clin. 32, 456–461. doi:10.3760/cma.j.cn115355-20200221-00065
Yıldız, A., Albayrak, M., Pala, Ç., Afacan Öztürk, H. B., Maral, S., Şahin, O., et al. (2020). The incidence and risk factors of thrombosis and the need for thromboprophylaxis in lymphoma and leukemia patients: a 9-year single-center experience. J. Oncol. Pharm. Pract. 26, 386–396. doi:10.1177/1078155219851540
Ying L, L., Han, Y., and Fu, X. W. (2024). Prediction risk of upper limb venous thrombosis after PICC catheterization in cancer patients using decision tree model. Hosp. Manag. Forum 41, 47–52.77
Ying X. H, X. H., Wu, J., Chu, H., and Han, S. Y. (2024). Analysis of disease perception status and influencing factors in patients with malignant lymphoma. Mod. Med. Health 40, 835–839.
Zhang, Y. H. (2025). Establishment and validation of a risk prediction model for venous thromboembolism in lymphoma patients. Master’s thesis. Nanchang: Nanchang University. doi:10.27232/d.cnki.gnchu.2024.004227
Keywords: lymphoma, venous thromboembolism, machine learning, predictive factors, predictive model
Citation: He C, Wang Y, Zhang H, Li S, Kang F, Cai F, Han L, Yin Q, Li G, Song X and Bian Y (2025) A study on a real-world data-based VTE risk prediction model for lymphoma patients. Front. Pharmacol. 16:1691271. doi: 10.3389/fphar.2025.1691271
Received: 23 August 2025; Accepted: 03 October 2025;
Published: 14 October 2025.
Edited by:
Zhiyao He, Sichuan University, ChinaReviewed by:
Tulika Seth, All India Institute of Medical Sciences, IndiaSwati Sharma, University of North Carolina at Chapel Hill, United States
Copyright © 2025 He, Wang, Zhang, Li, Kang, Cai, Han, Yin, Li, Song and Bian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Bian, Ymlhbnl1YW41NjdAMTI2LmNvbQ==; Xuewu Song, eHVlX3d1X3NvbmdAMTYzLmNvbQ==; Gang Li, bGlnYW5nNzQ5OEAxMjYuY29t
†These authors share first authorship
 Changli He
Changli He Yin Wang1†
Yin Wang1† Fengjiao Kang
Fengjiao Kang Lizhu Han
Lizhu Han Xuewu Song
Xuewu Song Yuan Bian
Yuan Bian