Abstract
Purpose:
To investigate the correlation between the medication regimen complexity index (MRCI) and adverse drug reactions (ADRs) among acquired immunodeficiency syndrome (AIDS) patients in the Chinese population.
Introduction:
The complexity of antiretroviral therapy (ART) regimens in individuals living with human immunodeficiency virus (HIV) presents significant challenges to medication management. To date, no studies have investigated the correlation between the MRCI and ADRs.
Methods:
This study retrospectively enrolled 1,010 patients. The MRCI was utilized to quantify the complexity of pharmacological treatment regimens. All suspected ADRs were assessed for causality using the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) system. Univariate and multivariate logistic regression analyses were conducted to identify risk factors associated with ADRs and MRCI.
Results:
Our findings demonstrate that the MRCI significantly increased from 4.09 ± 4.04 pre-admission to 6.09 ± 6.05 at discharge (P < 0.0001). Comorbidities (>6 diseases) (OR: 2.85, P < 0.001), tuberculosis medications (OR: 1.82, P < 0.001), and the number of medications administered during hospitalization (>19 drugs) (OR: 2.02, P < 0.001) were identified as independent factors influencing MRCI levels. The AUC for predicting adverse reactions using MRCI was 0.58 (P = 0.001), with an optimal cutoff value of 20.75. MRCI (>20.75) (OR: 1.42, P = 0.036) emerged as independent risk factors for adverse reactions in HIV/AIDS patients.
Conclusion:
In China, the MRCI of HIV/AIDS patients significantly increased after hospitalization. Further analysis indicates that patients with higher MRCI are more likely to experience ADRs.
Introduction
The advent of highly active antiretroviral therapy (HAART) has transformed HIV infection into a chronic manageable condition, enabling people living with Human Immunodeficiency Virus (HIV) to achieve near-normal life expectancy (Deeks et al., 2013; Poorolajal et al., 2016). HAART creates a potent attack by combining three or more drugs with different mechanisms, thereby maximally suppressing the HIV virus and preventing drug resistance (Lu et al., 2018). However, it inevitably entails the complexity of multiple - drug regimens. Particularly among elderly patients, who often present with age - related complications, this leads to complex polypharmacy regimens and an increased risk of drug interactions (Abdelbary et al., 2023; Back and Marzolini, 2020). The complexity of polypharmacy significantly elevates the incidence of adverse drug reactions (ADRs) (Yan et al., 2025). Existing research demonstrates that ADRs are prevalent among HIV-infected individuals undergoing antiretroviral therapy (ART) (Sherfa et al., 2021).
The complexity of medication regimens reflects the multidimensional nature of prescriptions, encompassing not only the quantity of drug classes but also various aspects of prescription practices. Currently, the Medication Regimen Complexity Index (MRCI) stands as the most widely utilized quantitative tool for assessing regimen complexity (George et al., 2004). This instrument has been translated and validated in multiple languages, including German (Stange et al., 2012), Spanish (Saez de la Fuente et al., 2016), Portuguese (Melchiors et al., 2007), Turkish (Okuyan et al., 2016), Korean (Lee et al., 2019), Japanese (Masumoto et al., 2021) and Chinese.
The MRCI influences patients’ risk of readmission, medication adherence, potential inappropriate medication use, quality of life, and other factors. (Abdelbary et al., 2023; González-Bueno et al., 2022; He et al., 2023; Sayın et al., 2022; Wilkening et al., 2020). Current research on MRCI values in patients with chronic conditions includes studies on chronic obstructive pulmonary disease (He et al., 2023), chronic heart failure (Wilkening et al., 2020) and acquired immunodeficiency syndrome (AIDS) (García et al., 2025). One study identified an MRCI threshold of 11.25 for HIV/AIDS patients meeting polypharmacy criteria (Morillo-Verdugo et al., 2019). Higher MRCI indices correlate with poorer quality of life in HIV-infected patients (Contreras-Macías et al., 2021). Furthermore, research demonstrates that elevated baseline MRCI are significantly associated with hospitalizations due to ADRs, with risk markedly increasing when MRCI exceed eight points (Pooja et al., 2024).
However, there is currently no published research investigating the association between the MRCI and the occurrence of ADRs in hospitalized HIV/AIDS patients, nor have studies examined factors influencing MRCI values in this population. This study employs MRCI to analyze the complexity of pharmacotherapy and its determinants in HIV/AIDS patients, while evaluating whether MRCI is correlated with ADRs during hospitalization among Chinese HIV/AIDS patients.
Methods
Study design and population
This study constitutes a retrospective investigation. The study cohort comprised 1,010 HIV/AIDS patients discharged from Changsha First Hospital between 1 January 2022 and 30 June 2022. All confirmed cases met the diagnostic criteria outlined in the Chinese Guidelines for HIV/AIDS Diagnosis and Treatment (Acquired Immunodeficiency Syndrome Professional Group et al., 2024). Generally, a diagnosis can be made when both the initial screening test for HIV antibodies and subsequent supplementary tests yield positive results. This study was approved by the institutional Ethics Committee of the First Hospital of Changsha (Ethics Approval Number: KX-2020048) and was conducted in accordance with the Declaration of Helsinki. All enrolled participants provided written informed consent.
All data have undergone encryption and anonymization processes to safeguard the privacy of participants. Upon completion of data analysis, all records were permanently deleted to ensure additional protection of subjects’ privacy.
Data collection and ADR assessment
The collected data encompassed the gender, age, medication allergy history, number of prescribed drugs, underlying diseases, complications, and the administration of antituberculosis medications during hospitalization among HIV/AIDS patients. All suspected ADRs in this study were retrieved from medical records. All suspected ADRs underwent causality assessment using the internationally recognized WHO-Uppsala Monitoring Centre (WHO-UMC) system (https://who-umc.org). Two clinical pharmacists and attending physicians jointly evaluated whether HIV patients had experienced ADRs based on established assessment criteria. Patients with causality assessments categorized as certain, probable, or possible were included in the study. When discrepancies arise among assessors regarding the identification or classification of adverse reactions, more authoritative experts will be consulted to make a new judgment.
MRCI calculation
Based on the relevant litterateur (García et al., 2025), we conducted MRCI scoring for eligible patients. The assessment comprises three primary components: Part A assigns higher weights to medications with inconvenient or more challenging administration forms (e.g., oral tablets score 1 point whereas metered-dose inhalers score 4 points). Part B focuses on medications requiring more frequent dosing or stricter administration intervals, where higher scores are allocated (e.g., twice-daily regimens score 2 points, while 12-h dosing intervals score 2.5 points). Part C further evaluates treatment regimens by assessing the presence of additional instructions, with examples including “split/crush tablets” (1 point) or “taper dose as directed” (2 points). The specific content can be found in Supplementary Table S1. MRCI values were calculated separately for the day prior to admission, the day of admission, and the day of discharge.
Data processing and statistical analysis
Statistical analysis was performed using SPSS 25.0 software. Quantitative data are presented as mean ± standard deviation (X ± s), while qualitative data are expressed as frequencies and percentages. SPSS 25.0 software analysis of the Receiver Operating Characteristic (ROC) curve. Between-group comparisons of quantitative data were conducted using t-tests, whereas chi-square tests were employed for qualitative data comparisons. Paired t-tests were utilized to analyze pre- and post-hospitalization MRCI changes. Factors with P-values <0.05 in between-group comparisons were incorporated into multivariate logistic regression analysis, with odds ratios (OR) and their 95% confidence intervals (CI) calculated. A P-value <0.05 was considered statistically significant.
Results
Participant characteristics and MRCI
This study enrolled 1,010 patients with a mean age of 48.0 ± 14.7 years, comprising 831 males (82.27%) and 179 females (17.73%). Among the patients included in this study, 61.19% of the AIDS patients had comorbidities. Additionally, newly diagnosed patients, patients admitted to the hospital due to ADRs, and those with poor treatment efficacy accounted for 34.75%, 3.56%, and 0.50% respectively. During hospitalization, the average number of medication types administered was 18.69 ± 12.45. Prior to hospitalization, the patient’s MRCI was (4.09 ± 4.04), which significantly increased to (6.09 ± 6.05) at discharge, demonstrating statistical significance (P < 0.0001) (Figure 1).
FIGURE 1
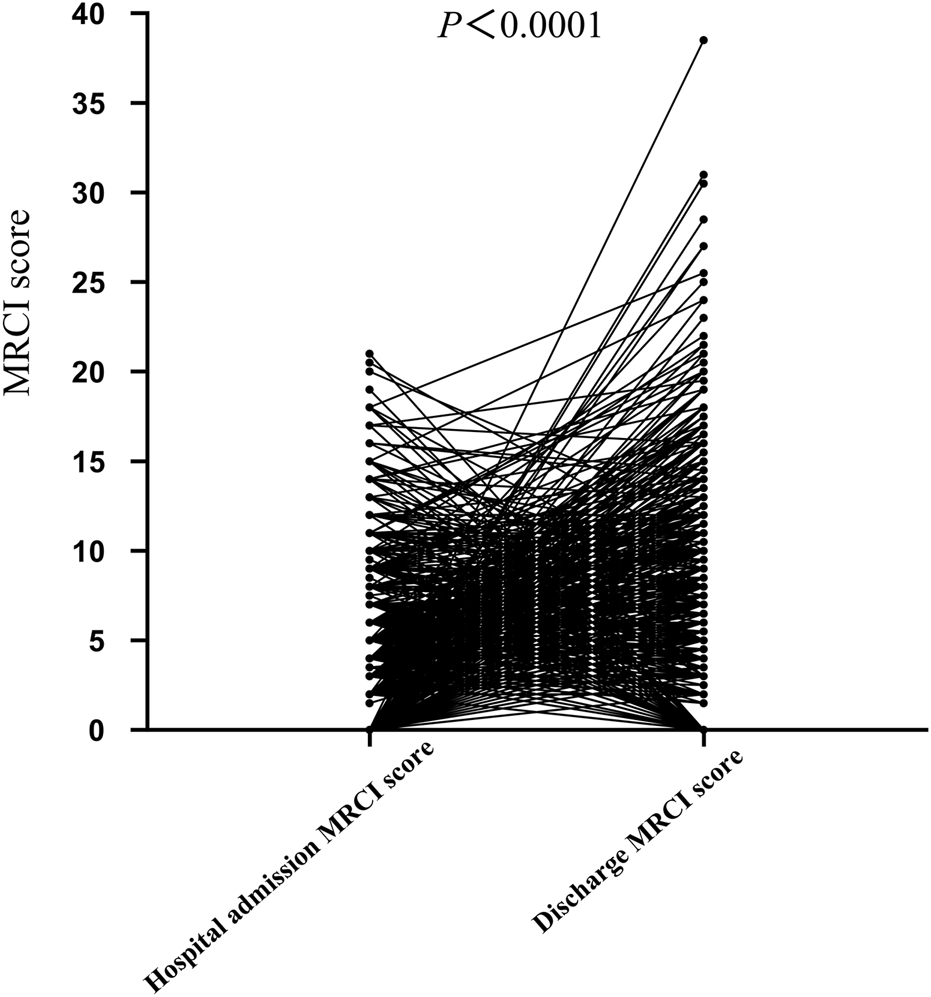
Comparison of MRCI values in HIV patients before admission and at discharge.
Based on the median MRCI at admission, patients were stratified into two cohorts: the low-MRCI group (<21) and the high-MRCI group (≥21). The results demonstrated that compared with the low-MRCI group, the high-MRCI group exhibited significantly higher incidences of complications (>6 diseases), tuberculosis drugs, number of drugs used in hospital (>19 drugs), and duration of HIV infection (>0.75 years). No statistically significant differences were observed between the two groups regarding age (≥65 years), gender, history of drug allergies, or underlying comorbidities (Table 1).
TABLE 1
| Characteristic | All patients (n = 1010) | Low-MRCI (n = 491) | High-MRCI (n = 519) | P |
|---|---|---|---|---|
| Age (≥65 years old) | 126 | 57 | 69 | 0.42 |
| Male | 831 | 405 | 426 | 0.87 |
| History of drug allergies | 18 | 7 | 11 | 0.41 |
| Underlying disease | 474 | 242 | 232 | 0.15 |
| Complications (>6 diseases) | 406 | 125 | 281 | <0.001 |
| Tuberculosis drugs | 316 | 111 | 205 | <0.001 |
| Number of drugs used in hospital (>19 drugs) | 496 | 171 | 325 | <0.001 |
| Duration of HIV infection (>0.75 years) | 498 | 265 | 233 | 0.004 |
Characteristics of patient between those with Low-MRCI and High-MRCI.
Data are n (%) or mean (+SD).
P value in bold italic shows that the variables are statistically significant.
Risk factors for MRCI in AIDS patients
We conducted further logistic regression analysis to investigate the risk factors influencing patients’ MRCI. Univariate analysis revealed that complications (>6 diseases), number of drugs used in hospital (>19 drugs), and duration of HIV infection (>0.75 years) demonstrated statistically significant associations with MRCI.
Multivariate logistic regression analysis indicated that complications (>6 diseases) (OR: 2.85, P < 0.001), tuberculosis drugs (OR: 1.82, P < 0.001), and the number of drugs used in the hospital (>19 drugs) (OR: 2.02, P < 0.001) were independent risk factors influencing the MRCI (Table 2).
TABLE 2
| Variable | Low-MRCI (n = 491) | High-MRCI (n = 519) | Univariate analysis | Multi-variate analysis | ||
|---|---|---|---|---|---|---|
| Or (95% CI) | P | Or (95% CI) | P | |||
| Age (≥65 years old) | 57 | 69 | | 0.42 | | |
| Male | 405 | 426 | | 0.87 | | |
| History of drug allergies | 7 | 11 | | 0.41 | | |
| Underlying disease | 242 | 232 | | 0.15 | | |
| Complications (>6 diseases) | 125 | 281 | 3.46 (2.65 4.51) | <0.001 | 2.85 (2.13 3.81) | <0.001 |
| Tuberculosis drugs | 111 | 205 | 2.24 (1.70 2.94) | <0.001 | 1.82 (1.35 2.42) | <0.001 |
| Number of drugs used in hospital (>19 drugs) | 171 | 325 | 3.14 (2.42 4.05) | <0.001 | 2.02 (1.52 2.68) | <0.001 |
| Duration of HIV infection (>0.75 years) | 265 | 233 | 1.44 (1.12 1.84) | 0.004 | | 0.069 |
Univariate and multivariate analysis of risk factor for MRCI values in the AIDS patients.
P value in bold italic shows that the variables are statistically significant.
Correlation of the MRCI with the ADRs among AIDS patients
This study evaluated the discriminatory efficacy of the MRCI in distinguishing patients at risk of adverse reactions through receiver operating characteristic (ROC) curve analysis. The analysis results indicated that the area under the curve (AUC) of the MRCI for predicting adverse reactions was 0.58 (95% confidence interval: 0.54–0.62; P = 0.001), and its optimal cut - off value was 20.75 (Figure 2).
FIGURE 2
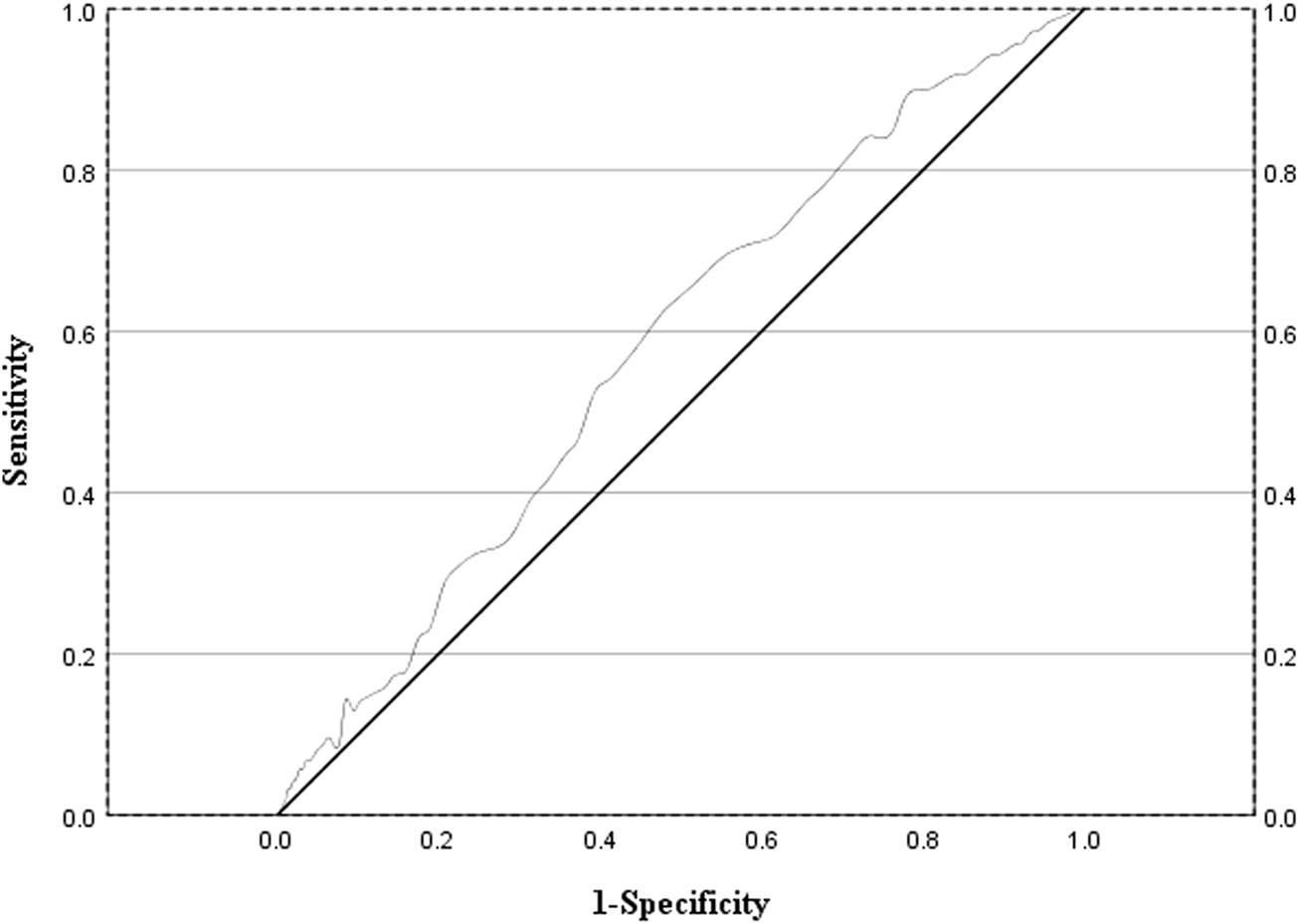
ROC curve evaluating the sensitivity and specificity of MRCI values in predicting the occurrence of adverse drug reactions in AIDS patients.
Among 1,010 patients, 210 experienced adverse reactions, with an incidence rate of adverse reactions at 20.79%. We classified the patients into two groups based on the occurrence of ADRs: the group with ADRs and the group without ADRs. Compared with the group without adverse reactions, the group with adverse reactions exhibited significant differences in terms of underlying diseases, complications (>6 diseases), and MRCI (>20.75) (Table 3).
TABLE 3
| Characteristic | All patients (n = 1010) | Patients with ADRs (n = 210) | Patients without ADRs (n = 800) | P |
|---|---|---|---|---|
| Age (≥65 years old) | 126 | 22 | 104 | 0.33 |
| Male | 831 | 173 | 658 | 0.96 |
| History of drug allergies | 18 | 1 | 17 | 0.11 |
| Underlying disease | 474 | 83 | 391 | 0.016 |
| Complications (>6 diseases) | 406 | 114 | 292 | <0.001 |
| Duration of HIV infection (>0.75 years) | 498 | 95 | 403 | 0.19 |
| MRCI (>20.75) | 519 | 131 | 388 | <0.001 |
Characteristics of patient between those with ADRs and without ADRs.
Data are n (%) or mean (+SD).
P value in bold italic shows that the variables are statistically significant.
To determine the correlation between MRCI (>20.75) and ADRs in AIDS patients, we conducted a regression analysis. Multivariate regression analysis indicated that underlying diseases (OR: 1.60, P = 0.004), complications (>6 diseases) (OR: 2.00, P < 0.001), and MRCI (>20.75) (OR: 1.42, P = 0.036) were independent risk factors for adverse reactions in AIDS patients (Table 4).
TABLE 4
| Variable | Patients with ADRs (n = 210) | Patients without ADRs (n = 800) | Univariate analysis | Multi-variate analysis | ||
|---|---|---|---|---|---|---|
| Or (95% CI) | P | Or (95% CI) | P | |||
| Age (≥65 years old) | 22 | 104 | | 0.33 | | |
| Male | 173 | 658 | | 0.97 | | |
| History of drug allergies | 1 | 17 | | 0.14 | | |
| Underlying disease | 83 | 391 | 1.46 (1.07 1.99) | 0.016 | 1.60 (1.16 2.20) | 0.004 |
| Complications (>6 diseases) | 114 | 292 | 2.07 (1.52 2.81) | <0.001 | 2.00 (1.45 2.78) | <0.001 |
| Duration of HIV infection (>0.75 years) | 95 | 403 | | 0.19 | | |
| MRCI (>20.75) | 131 | 388 | 1.76 (1.29 2.41) | <0.001 | 1.42 (1.02 1.98) | 0.036 |
Univariate and multivariate analysis of risk factor for ADRs in the AIDS patients.
P value in bold italic shows that the variables are statistically significant.
Discussion
People with AIDS typically require lifelong and complex ART regimens. Meanwhile, this population often suffers from multiple comorbidities, including cardiovascular diseases, metabolic disorders, mental disorders, and opportunistic infections. Such comorbid conditions have led to a significant increase in the use of non - antiretroviral medications (Abdelbary et al., 2023; Back and Marzolini, 2020). This study reveals that the MRCI, as a comprehensive assessment tool, is effective in quantifying medication complexity and predicting the risk of ADRs among AIDS patients. Compared with methods solely based on medication quantity statistics (such as the concept of polypharmacy), MRCI can more comprehensively and profoundly reflect the medication management challenges that AIDS patients encounter during actual drug treatment.
Research has indicated that the MRCI of AIDS patients range from 2 to 67.5 (Metz et al., 2014). A retrospective study found that the median MRCI of HIV - positive patients aged 18 and above who were receiving stable antiretroviral therapy was eight points (García et al., 2025). A multi - center cross - sectional study enrolled 74 HIV - infected individuals aged 65 years or older who were receiving antiretroviral therapy. The median complexity index of their treatment regimens was 13.0 (Gimeno-Gracia et al., 2020). Our research indicates that the average MRCI of AIDS patients during hospitalization is 20.61, which is consistent with the findings of Hirsch et al. (2014). The discrepancies in MRCI values across different studies may be attributed to the variations in the populations included. Our study solely encompasses inpatients, whereas other studies predominantly involve outpatients.
Further logistic regression analysis indicates that comorbidities, the use of anti - tuberculosis drugs, and the use of more than 19 types of in - hospital medications are independent predictors of high MRCI in AIDS patients. Among them, comorbidities or the requirement for multiple medications in anti - tuberculosis treatment is an important factor contributing to the complexity of the drug treatment regimen. In addition, the results of this study indicate that the MRCI of AIDS patients were 4.09 ± 4.04 before hospitalization and 6.09 ± 6.05 at the time of discharge (P < 0.0001). This is consistent with the findings of Poojar et al., which suggest that hospitalization increases the complexity of drug treatment regimens (Pooja et al., 2024).
This study also explored the relationship between MRCI and ADR. The area under the curve (AUC) of the MRCI for predicting adverse reactions is 0.58, and its optimal cut - off value is 20.75. Subsequently, we conducted a logistic regression analysis. The research results indicate that even after adjusting for potential confounding factors such as age, gender, and the number of comorbidities, MRCI >20.75 remains an independent predictor of the occurrence of ADRs. Clinicians can incorporate the calculation of patients’ MRCI values into routine drug evaluations to identify patients with an MRCI >20.75. For patients of this kind, clinicians can utilize professional drug interaction analysis tools or collaborate through multidisciplinary teams to identify potential risks of drug interactions (Conti et al., 2022; Varadarajan et al., 2025). This approach facilitates the early warning of adverse drug reactions in high - risk populations and enables targeted management based on risk stratification. Consequently, it can effectively reduce the incidence of adverse reactions and mitigate the associated risk of hospitalization.
Furthermore, in this study, most patients were HIV-infected individuals with comorbidities who likely presented with a high MRCI upon admission. For this population, clinicians should consider antiviral agents with a lower risk of drug interactions, such as integrase inhibitors, while avoiding combinations with overlapping toxicities (Tuan et al., 2024; Hidalgo-Tenorio and Martínez-Sanz, 2025; De Bellis et al., 2024). Concurrently, clinical pharmacists should perform medication reconciliation to comprehensively evaluate and optimize the therapeutic regimen, thereby contributing to the prevention of ADRs.
This study has certain limitations. Firstly, as a retrospective study, its inherent biases cannot be ignored. Secondly, the study cohort only includes patients from medical institutions in Hunan Province, resulting in limited geographical representativeness. Thirdly, there may be under - reporting of ADRs, and some cases may not be fully captured in medical records. Fourthly, this study did not conduct severity grading for the reported adverse events.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Hospital of Changsha. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JS: Writing – original draft. HQ: Writing – original draft, Data curation. JH: Data curation, Writing – original draft. YW: Data curation, Writing – original draft. YX: Writing – review and editing, Data curation. GH: Writing – review and editing, Data curation. SH: Writing – review and editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. The research is supported by Natural Science Foundation of Hunan Province (No. 2024JJ6080; No. 2025JJ80140) and the Research Program of Hunan Provincial Health Commission (D202303086966).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1721289/full#supplementary-material
References
1
Abdelbary A. Kaddoura R. Balushi S. A. Ahmed S. Galvez R. Ahmed A. et al (2023). Implications of the medication regimen complexity index score on hospital readmissions in elderly patients with heart failure: a retrospective cohort study. BMC Geriatr.23 (1), 377. 10.1186/s12877-023-04062-2
2
Acquired Immunodeficiency Syndrome Professional Group, Society of Infectious Diseases, Chinese Medical Association; Chinese Center for Disease Control and Prevention (2024). Chinese guidelines for diagnosis and treatment of human immunodeficiency virus infection/acquired immunodeficiency syndrome. Chin. J. Clin. Infect. Dis.17 (03), 161–190.
3
Back D. Marzolini C. (2020). The challenge of HIV treatment in an era of polypharmacy. J. Int. AIDS Soc.23 (2), e25449. 10.1002/jia2.25449
4
Conti V. Sellitto C. Torsiello M. Manzo V. De Bellis E. Stefanelli B. et al (2022). Identification of drug interaction adverse events in patients with COVID-19: a systematic review. JAMA Netw. Open5 (4), e227970. 10.1001/jamanetworkopen.2022.7970
5
Contreras-Macías E. Gutiérrez-Pizarraya A. RobustilloCortés M. A. Morillo-Verdugo R. (2021). High level of medication regimen complexity index correlate with worse quality of life in people living with HIV. Rev. Esp. Quimioter.34 (2), 93–99. 10.37201/req/097.2020
6
De Bellis E. Donnarumma D. Zarrella A. Mazzeo S. M. Pagano A. Manzo V. et al (2024). Drug-drug interactions between HIV antivirals and concomitant drugs in HIV patients: what we know and what we need to know. Pharmaceutics17 (1), 31. 10.3390/pharmaceutics17010031
7
Deeks S. G. Lewin S. R. Havlir D. V. (2013). The end of AIDS: HIV infection as a chronic disease. Lancet382 (9903), 1525–1533. 10.1016/S0140-6736(13)61809-7
8
García C. G. Contreras-Macías E. Naranjo-Pérez G. A. Morillo-Verdugo R. (2025). Comparison of perceived versus actual care complexity in HIV-Positive patients receiving antiretroviral treatment: the STRATPATIENT study. Rev. Esp. Quimioter.38 (4), 278–286. 10.37201/req/014.2025
9
George J. Phun Y. T. Bailey M. J. Kong D. C. Stewart K. (2004). Development and validation of the medication regimen complexity index. Ann. Pharmacother.38 (9), 1369–1376. 10.1345/aph.1D479
10
Gimeno-Gracia M. Sánchez-Rubio-Ferrández J. Robustillo-Cortés M. L. A. Morillo-Verdugo R. (2020). Prevalence of polypharmacy and pharmacotherapy complexity in elderly people living with HIV in Spain. POINT study. Farm Hosp.44 (4), 127–134. 10.7399/fh.11367
11
González-Bueno J. Sevilla-Sánchez D. Puigoriol-Juvanteny E. Molist-Brunet N. Codina-Jané C. Espaulella-Panicot J. (2022). Improving medication adherence and effective prescribing through a patient-centered prescription model in patients with multimorbidity. Eur. J. Clin. Pharmacol.78 (1), 127–137. 10.1007/s00228-021-03207-9
12
He R. Wang Y. Ren X. Huang K. Lei J. Niu H. et al (2023). Associations of medication regimen complexity with medication adherence and clinical outcomes in patients with chronic obstructive pulmonary disease: a prospective study. Ther. Adv. Respir. Dis.17, 17534666231206249. 10.1177/17534666231206249
13
Hidalgo-Tenorio C. Martínez-Sanz J. (2025). Simplification of antiretroviral therapy: comparative review of two-drug and three-drug regimens in HIV treatment. AIDS Rev.27 (1), 16–24. 10.24875/AIDSRev.M25000081
14
Hirsch J. D. Metz K. R. Hosokawa P. W. Libby A. M. (2014). Validation of a patient-level medication regimen complexity index as a possible tool to identify patients for medication therapy management intervention. Pharmacotherapy34 (8), 826–835. 10.1002/phar.1452
15
Lee S. Jang J. Yang S. Hahn J. Min K. L. Jung E. H. et al (2019). Development and validation of the Korean version of the medication regimen complexity index. PLoS One14 (5), e0216805. 10.1371/journal.pone.0216805
16
Lu D. Y. Wu H. Y. Yarla N. S. Xu B. Ding J. Lu T. R. (2018). HAART in HIV/AIDS treatments: future trends. Infect. Disord. Drug Targets18 (1), 15–22. 10.2174/1871526517666170505122800
17
Masumoto S. Sato M. Momo K. Matsushita A. Suzuki K. Shimamura H. et al (2021). Development of medication regimen complexity index: japanese version and application in elderly patients. Int. J. Clin. Pharm.43 (4), 858–863. 10.1007/s11096-020-01185-z
18
Melchiors A. C. Correr C. J. Fernández-Llimos F. (2007). Translation and validation into Portuguese language of the medication regimen complexity index. Arq. Bras. Cardiol.89 (4), 210–218. 10.1590/s0066-782x2007001600001
19
Metz K. R. Fish D. N. Hosokawa P. W. Hirsch J. D. Libby A. M. (2014). Patient-level medication regimen complexity in patients with HIV. Ann. Pharmacother.48 (9), 1129–1137. 10.1177/1060028014539642
20
Morillo-Verdugo R. Robustillo-Cortés M. A. Abdel-Kader Martín L. Álvarez de Sotomayor Paz M. Lozano de León Naranjo F. Almeida González C. V. (2019). Determination of a cutoff value for medication regimen complexity index to predict polypharmacy in HIV+ older patient. Rev. Esp. Quimioter.32 (5), 458–464.
21
Okuyan B. Babi B. Sancar M. Ay P. Yücel E. Yücel A. et al (2016). Validation of the Turkish version of medication regimen complexity index among elderly patients. J. Eval. Clin. Pract.22 (5), 732–736. 10.1111/jep.12526
22
Poojar B. Kamath A. Rao S. B. Ullal S. D. Ramapuram J. Yadiyal M. B. et al (2024). A prospective study of the medication regimen complexity index and hospitalization due to adverse drug reactions among people living with HIV. Med. Kaunas.60 (10), 1705. 10.3390/medicina60101705
23
Poorolajal J. Hooshmand E. Mahjub H. Esmailnasab N. Jenabi E. (2016). Survival rate of AIDS disease and mortality in HIV-Infected patients: a meta-analysis. Public Health139, 3–12. 10.1016/j.puhe.2016.05.004
24
Saez de la Fuente J. Such Diaz A. Cañamares-Orbis I. Ramila E. Izquierdo-Garcia E. Esteban C. et al (2016). Cross-cultural adaptation and validation of the medication regimen complexity index adapted to Spanish. Ann. Pharmacother.50 (11), 918–925. 10.1177/1060028016656385
25
Sayın Z. Sancar M. Özen Y. Okuyan B. (2022). Polypharmacy, potentially inappropriate prescribing and medication complexity in Turkish older patients in the community pharmacy setting. Acta Clin. Belg77 (2), 273–279. 10.1080/17843286.2020.1829251
26
Sherfa A. Haile D. Yihune M. Sako S. (2021). Incidence and predictors of adverse drug reaction (ADR) among adult HIV positive patients on anti-retroviral treatment in Arba Minch town public health facilities, southern Ethiopia: a retrospective cohort study, 2020. PLoS One16 (5), e0251763. 10.1371/journal.pone.0251763
27
Stange D. Kriston L. Langebrake C. Cameron L. K. Wollacott J. D. Baehr M. et al (2012). Development and psychometric evaluation of the German version of the medication regimen complexity index (MRCI-D). J. Eval. Clin. Pract.18 (3), 515–522. 10.1111/j.1365-2753.2011.01636.x
28
Tuan J. Igiraneza G. Ogbuagu O. (2024). Analysis of drug-drug interactions in patients with HIV and metabolic syndrome. Expert Opin. Drug Metab. Toxicol.20 (10), 953–965. 10.1080/17425255.2024.2401044
29
Varadarajan M. Blackburn S. Girometti N. Hicks A. Senkoro E. Candela C. et al (2025). Implementation of a multidisciplinary approach to care for people with HIV aged 80 years and over. Int. J. STD AIDS36 (1), 65–71. 10.1177/09564624241286558
30
Wilkening G. L. Brune S. Saenz P. F. Vega L. M. Kalich B. A. (2020). Correlation between medication regimen complexity and quality of life in patients with heart failure. Res. Soc. Adm. Pharm.16 (10), 1498–1501. 10.1016/j.sapharm.2020.01.003
31
Yan Z. Fan K. Q. Yu T. Su N. Zou Y. Xia L. (2025). Polypharmacy, drug-drug interactions and adverse drug reactions in older Chinese cancer patients: evidence from CHARLS. Front. Pharmacol.16, 1579023. 10.3389/fphar.2025.1579023
Summary
Keywords
adverse drug reactions, aids, HIV, Mrci, real-world
Citation
Sun J, Qi H, Huang J, Wang Y, Xiang Y, He G and Huang S (2026) Association of medication regimen complexity index with ADRs in HIV/AIDS patients: a retrospective cohort study. Front. Pharmacol. 16:1721289. doi: 10.3389/fphar.2025.1721289
Received
09 October 2025
Revised
17 December 2025
Accepted
23 December 2025
Published
12 January 2026
Volume
16 - 2025
Edited by
Sotero Serrate Mengue, Federal University of Rio Grande do Sul, Brazil
Reviewed by
Valeria Conti, University of Salerno, Italy
Emanuela De Bellis, University of Naples Federico II, Italy
Updates
Copyright
© 2026 Sun, Qi, Huang, Wang, Xiang, He and Huang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiqiong Huang, Inertia848223657@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.