Abstract
Bisphenol A (BPA) and tributyltin (TBT) are two endocrine disrupting compounds (EDC) that have opposite effects on estrogen signaling. BPA is an estrogen agonist that binds to all estrogen receptor types. TBT is an aromatase inhibitor that binds to the enzyme aromatase, preventing the synthesis of estrogen from testosterone. Both estrogen receptors and aromatase are localized to the retina and estrogen signaling is required for proper eye and retinal neurogenesis. Abnormal eye growth and retinal changes are reported immediately after developmental exposure to either EDC consistent with the role of estrogen in proper neurogenesis. In this review, we examine the impact of BPA and TBT exposure on the development and function of the visual system. We focus primarily on zebrafish but include data from other species to show trends across vertebrates. We discuss a case study designed to determine if a transient developmental exposure to BPA or TBT has persistent effects that are evident in adults and if these latent outcomes reflect the opposite impact of these compounds on estrogen signaling. Surprisingly, although some opposing outcomes were observed, most differences in adult retinal function were similar between the two compounds, with varying effects noted by concentration and exposure age. Overall, we conclude that developing zebrafish retina is sensitive to EDCs that target estrogenic pathways. However, these findings cannot be explained by estrogenic modulation alone, suggesting additional mechanisms beyond their current established roles.
1 Introduction
Endocrine disrupting compounds (EDCs) are natural or synthetic compounds (Niehs, 2025) structurally similar to natural hormones (Ahn and Jeung, 2023). EDCs can influence the synthesis, action, and/or downstream signaling pathways of hormones (Bertram et al., 2022; Ahn and Jeung, 2023) by either blocking enzyme activity, antagonizing receptors, or mimicking endogenous ligands (Wingfield and Mukai, 2009; De Coster and van Larebeke, 2012). Exposure is a significant public health concern as EDCs are linked to certain cancers and cardiovascular problems, as well as metabolic, reproductive, and neurological disorders (Kumar et al., 2020; Bertram et al., 2022; Ahn and Jeung, 2023). Early life/developmental stages are the most sensitive to EDC exposure, with both immediate (Kahn et al., 2020; Puri et al., 2023) and latent/later life effects reported (Kumar et al., 2020; Duh-Leong et al., 2023). Research has shown that neurodevelopment is particularly vulnerable to EDC exposure (Damiano et al., 2025).
EDCs are prevalent and persistent contaminants in aquatic environments (Le Page et al., 2011; Soffker and Tyler, 2012) with landfills, wastewater effluent, and agricultural/road/roof runoff serving as primary sources (Kumar et al., 2020; Bertram et al., 2022). Human exposure occurs significantly via ingestion and inhalation; fetal exposure also occurs as many EDCs can cross the placenta (Bertram et al., 2022; Ahn and Jeung, 2023). Non-human animal exposure occurs through ingestion, inhalation, and/or uptake across the gills (Bertram et al., 2022). Importantly, low doses of EDCs are reported to have significant effects on both humans and animal models (Puri et al., 2023), suggesting non-linear and non-monotonic effects of these compounds (Kumar et al., 2020; Duh-Leong et al., 2023).
Many of the identified EDCs target estrogen signaling pathways, resulting in significant effects on both development and adult reproductive physiology (Mericskay et al., 2005; Dickerson et al., 2011; Jasarevic et al., 2011; Meeker, 2012; Damiano et al., 2025). Some EDCs specifically interfere with estrogen synthesis; while others bind estrogen receptors, resulting in aberrant downstream signaling and gene transcription. Outside the reproductive axis, proper estrogen signaling is important for neurogenesis (Wesselman and Wingert, 2024) and EDCs affecting estrogen pathways have both overt and subtle effects on the visual system (Dong et al., 2006; Hamad et al., 2007; Wang et al., 2012).
In this review, we focus on the impact of EDC exposure on development and function of the visual system. We discuss two well-known compounds–bisphenol A and tributyltin–which have opposite effects on estrogen signaling. Bisphenol A (BPA) is a weak estrogen agonist (Chapin et al., 2008) that increases estrogen-dependent signaling. Tributyltin (TBT), on the other hand, prevents the synthesis of estrogen (estradiol or E2) from testosterone (Matthiessen and Gibbs, 1998; McAllister and Kime, 2003; McGinnis and Crivello, 2011). Given their contrasting effects, we examine if developmental exposure to environmentally relevant levels of BPA and TBT cause opposing functional differences in the visual system. We have structured our review to provide background on both BPA and TBT, discuss estrogen signaling within retina, summarize reported effects of EDC exposure on retinal development and function, and then review our recent findings as a case study. Though our work focuses on zebrafish (Danio rerio), we also include data from other species to show trends across vertebrates.
2 Bisphenol A (BPA)
BPA is a synthetic compound widely used in the manufacturing of industrial/household plastics, epoxy resin, and the lining of food and drink containers (Geens et al., 2012). As an environmental pollutant, BPA leaches into aquatic environments from industrial and material waste, leading to long term consequences to the local environment’s food web. On top of the environmental impact, human exposure to BPA is common (Rubin, 2011). There are several recent, comprehensive reviews of BPA’s negative effects on human health (Lin et al., 2023; Hyun and Ka, 2024; Triswindyaningrum et al., 2025).
Human exposure to BPA can occur via inhalation, ingestion, or dermal contact (Lin et al., 2023; Hyun and Ka, 2024), though the primary pathway is through ingestion, as BPA leaches from food containers, canned goods, and thermal papers into food products (Geens et al., 2012; Hyun and Ka, 2024). Human exposure is significant, with over 90% of people worldwide showing measurable levels of BPA in their tissues (Lin et al., 2023; Triswindyaningrum et al., 2025) and recent estimates suggest the world average daily human intake of BPA is 2.53 µg/p/day (Wang et al., 2020). BPA is readily absorbed in the gastrointestinal tract due to its lipophilicity and undergoes metabolism in the liver, forming BPA-glucuronide and BPA-sulfate (Almeida et al., 2018). Although these conjugates are traditionally considered inactive, deconjugation can occur via tissue-specific enzymes or gut microbiota, regenerating biologically active BPA (Dekant and Volkel, 2008). BPA and its metabolites can cross the placenta (Almeida et al., 2018), with detectable levels in the fetal circulation, amniotic fluid, breast milk, and neonatal urine, highlighting particular risk during early development (Vandenberg et al., 2007). Humans exposed to BPA before birth show increased anxiety and depression as children (Hyun and Ka, 2024) and in utero exposure, particularly during the third trimester, is associated with an increased risk of preterm births (Lin et al., 2023).
A central concern regarding BPA toxicity is the substantial evidence that low concentrations can induce significant biological effects (Vandenberg et al., 2012) and the life stages that are most sensitive are embryonic and early postnatal periods (Rubin, 2011). Low-dose BPA exposure has been linked to altered neurobehavior, metabolic disturbances, reproductive tract abnormalities, and long-term physiological deficits across numerous vertebrate models (Rubin, 2011; Pironti et al., 2021; Hyun and Ka, 2024) and in humans (Mustieles et al., 2015; Lin et al., 2023; Hyun and Ka, 2024). These outcomes identify a non-monotonic dose response relationship for BPA (Vandenberg et al., 2012; Lagarde et al., 2015). Importantly, environmentally relevant BPA exposures often fall within the low-dose range where non-monotonic responses are most prominent, increasing ecological and human-health relevance.
Although BPA is not as persistent as legacy contaminants like polychlorinated bisphenols (PCB), another EDC, continuous release from wastewater, plastic degradation, and landfill leachate has resulted in widespread and persistent environmental contamination (Almeida et al., 2018). BPA concentrations in streams range from 0.14 to 12 μg/L. BPA levels measured in human urine samples range widely (0.4–149 μg/L) with a geometric mean of 2.6 μg/L (Vandenberg et al., 2010). BPA accumulates in aquatic systems, where it affects both micro- and macro-organisms by altering growth, reproduction, and endocrine function (Pironti et al., 2021). In fish and amphibians, for example, chronic BPA exposure has been associated with disrupted gonadal development, abnormal steroidogenesis, impaired reproductive behavior, and long-term physiological deficits (Corrales et al., 2015). These findings suggest that BPA poses significant risk not only to individual organisms but also to broader ecosystem structure and function.
3 Tributyltin (TBT)
TBT has industrial uses in antifouling paints, lumber and textile production, and as a stabilizer in PVC production. Its use on marine structures and ship hulls allows it to readily leach into the water, where it quickly settles and adheres to marine sediment, creating a bioavailable sink (Hoch, 2001). This sequestration prevents degradation and increases the risk of chemical resuspension, especially around major ports where there is increased ship traffic. In 2008, the International Convention of the Control of Harmful Antifouling Systems on Ships (AFS Convention–International Maritime Organization) banned the use of TBT on registered ships worldwide. Despite global restrictions, regulations are not strictly enforced, and TBT based paints are still being produced and are available for purchase (Uc-Peraza et al., 2022). Measurable TBT levels have been found along the coasts of several different countries. Major ports are especially susceptible, but alarmingly, some environmentally protected areas are just as contaminated (Castro et al., 2021). Researchers found that 77% of samples collected from marine protected areas along the Latin American coast had TBT levels ranging from 0.002–0.05 μg/g (Castro et al., 2021). TBT levels collected from the surface waters of India and China, reached 0.342 and 0.977 μg/L respectively (Gui-bin et al., 2001; Garg et al., 2011). In Brazil, sediment samples collected from Vitoria range from 0.006–0.0211 µg Sn/g dry weight (Abreu et al., 2021) and in Ceará, samples collected after dredging activities reached 0.0526 µg Sn/g dry weight (Moreira et al., 2021).
Out of all organotin compounds, TBT is regarded as the most toxic due to its slow degradation, environmental persistence, bioaccumulation potential, and ability to cross the blood brain barrier (Rouleau et al., 2003; Al-shatri et al., 2015). Human exposure to TBT occurs through dietary intake, direct exposure to treated products, and dust inhalation (Sousa et al., 2014). Cohort studies examining human placentas obtained after birth have detected measurable levels of TBT in Scandinavian countries (Rantakokko et al., 2013; Rantakokko et al., 2014). A Danish study specifically discovered that the human placental concentration of TBT was inversely associated with thyroid hormones (Li et al., 2018), which are critical for fetal development. Exposure to TBT during gestation in humans has been linked to reduced fetal weight and placental abnormalities (Adeeko et al., 2003; Liu et al., 2021). And more recently, the impact of TBT on mammalian cells was reviewed by (Correia et al., 2025).
4 Estrogen receptor types and signaling mechanism
Estrogen is present in three forms in vertebrates: E1 (estrone), E2 (estradiol; 17β-estradiol), and E3 (estriol) (Bondesson et al., 2015; Nazari and Suja, 2016). E2 is the major biological form in reproductively active females (Nazari and Suja, 2016). All estrogens are synthesized from cholesterol, with the final step in E2 synthesis being the aromatization of testosterone by the enzyme aromatase (Simpson et al., 2002; Nuzzi and Caselgrandi, 2022). Circulating E2 binds to one of several intracellular estrogen receptors (ER), which are ligand-activated transcription factors. Activated (ligand-bound) ERs then dimerize and bind to estrogen response elements (ERE) on DNA, affecting gene transcription and/or rapidly altering membrane potentials (Belcher and Zsarnovszky, 2001; Cascio et al., 2015). In mammals and birds, there is a single aromatase gene (cyp19a1) (Bondesson et al., 2015; Cascio et al., 2015); whereas, in zebrafish there are two aromatase genes, cyp19a1a (aromatase A, gonadal aromatase) and cyp19a1b (aromatase B, brain aromatase) (Chiang et al., 2001; Wesselman and Wingert, 2024). Estrogen binds to two ER isoforms in mammals: ERα and ERβ (Rubin, 2011; Rochester, 2013). In zebrafish, there are three ER isoforms, one for ERα (esr1) and two for ERβ (ERβ1 or esr2b and ERβ2 or esr2a) (Menuet et al., 2002; Menuet et al., 2004; Bondesson et al., 2015) and zebrafish ERs have high sequence homology with mammalian ER (Lassiter et al., 2002; Wesselman and Wingert, 2024). E2 can also bind to a membrane-bound G-protein coupled estrogen receptor (GPER), triggering different intracellular cascades (Belcher and Zsarnovszky, 2001) involving MAPK/ERK and P13K/AKT signaling pathways (Lim et al., 2002; Gorelick et al., 2008; Bouskine et al., 2009; Cohen et al., 2022). All vertebrates have a single copy/isoform of GPER (Bondesson et al., 2015).
Most of the circulating E2 is synthesized in the ovaries (Nazari and Suja, 2016). However, local synthesis can also occur. The aromatase enzyme is present in brains of mammals, birds, fish, and amphibians (Pelligrini et al., 2005; Bondesson et al., 2015). In mammals and birds aromatase is localized to nerve cells; in fish, particularly zebrafish, aromatase is localized to radial glial cells in the brain (Menuet et al., 2005). Aromatase-positive radial glial cells are progenitor cells (Chapouton et al., 2010) that proliferate throughout life in the fish and developmental aromatase expression is estrogen-dependent (Kishida et al., 2001; Menuet et al., 2005; Sawyer et al., 2006; Vosges et al., 2010; Chung et al., 2011; Brion et al., 2012).
4.1 BPA and TBT have opposite effects on estrogenic mechanisms
BPA binds to both ERα and ERβ (Rubin, 2011; Rochester, 2013), mimicking endogenous estrogen, though BPA is reported to have greater affinity for ERβ (Ben-Jonathan and Steinmetz, 1998; Vandenberg et al., 2009). Overall, for both ERs, the affinity for BPA is weak compared to E2, though BPA binding can induce transcription of estrogen responsive genes (Kishida et al., 2001; Richter et al., 2007; Chung et al., 2011; Kinch et al., 2015; Cano-Nicolau et al., 2016; Qiu et al., 2016). Beyond this classic genomic pathway, BPA can also bind GPER (Mustieles et al., 2015), initiating rapid non-genomic responses (Lim et al., 2002; Gorelick et al., 2008; Bouskine et al., 2009). The ability of BPA to interact with estrogen receptors is suggested to be causal for some adverse effects in humans (Almeida et al., 2018).
The aromatase enzyme binds testosterone and catalyzes the formation of E2 (Simpson et al., 2002; Nuzzi and Caselgrandi, 2022). Aromatase is also able to synthesize E1 from androstenedione, with the resulting E1 being converted to E2 (Luu-The, 2013). Thus, EDCs that bind aromatase disrupt E2 synthesis. TBT is one of these compounds. As an aromatase inhibitor, TBT reversibly binds to the active site on aromatase, preventing substrate (testosterone) binding (Cheng and Yang, 2023). Consequently, E2 levels are reduced and testosterone levels are increased after TBT exposure (McGinnis and Crivello, 2011) which causes masculinization in gastropods (Horiguchi, 2006) and zebrafish (McAllister and Kime, 2003; Santos et al., 2006; McGinnis and Crivello, 2011). TBT concentrations as low as 0.003–0.005 μg/L have deleterious consequences for marine and freshwater organisms (Al-shatri et al., 2015). Filter and sediment feeding organisms are especially at risk and TBT has been detected in both target and non-target organisms (Hoch, 2001).
4.2 Estrogen in retina
The general structure and function of the vertebrate retina is highly conserved (Baden et al., 2020). Rod and cone photoreceptors have their cell bodies in the distal outer nuclear layer (ONL) and synapse onto second-order bipolar and horizontal cells in the outer plexiform layer (OPL). Bipolar cell somata are in the inner nuclear layer (INL) and these cells are presynaptic to amacrine and ganglion cells in the inner plexiform layer (IPL). Ganglion cell axons leave the eye, forming the optic nerve. Signaling from photoreceptors to bipolar cells to ganglion cells is direct and glutamatergic; horizontal and amacrine cells, with cell bodies in the INL, are local circuit neurons that provide inhibitory feedback to the photoreceptor-bipolar-ganglion cell pathway in the OPL and IPL, respectively. Primate retinas, including humans, have three cone types (L, Long wavelength sensitive or “red” cones | M, Mid-wavelength sensitive “green” cones | S, Short wavelength sensitive or “blue” cones) (Mustafi et al., 2009), non-primate mammals have two cone types (M and S) (Lindenau et al., 2019), and zebrafish have four cone types (L, M, S, and a UV sensitive cone) (Robinson et al., 1993). Multiple bipolar, horizontal, amacrine, and ganglion cell types are reported in all species, contributing to complex and diverse levels of processing within the retina (Baden et al., 2020).
While circulating E2 can reach the retina through the blood (Chaychi et al., 2015), it is also locally synthesized (Nuzzi et al., 2018). In rats, aromatase is found in distal retinal neurons (photoreceptors, horizontal and bipolar cells) within the ONL, OPL, and INL (Cascio et al., 2015; Valero-Ochando et al., 2024). Goldfish retina contains aromatase-positive horizontal, bipolar, and amacrine cells, as well as aromatase-containing ganglion cell axons (Gelinas and Callard, 1993; Callard et al., 1995). Aromatase transcripts have been identified in zebrafish (Sawyer et al., 2006), along with esr1 and esr2b (Cohen et al., 2025). Inhibition of aromatase changes the thickness of the IPL, INL, and OPL in both zebrafish and rats (Salyer et al., 2001; Hamad et al., 2007).
E2 exposure affects photoreceptor gene expression (Hao et al., 2013; Cohen et al., 2022), suggesting ER are present in that retinal layer. ERα, ERβ, and GPER are present in mouse retina, with GPER (Jiang et al., 2019; Li and Li, 2020a; Li and Li, 2020b; Pinon-Teal and Ogilvie, 2024) and ERβ expressed on ganglion cells (Rodriguez-Ramirez et al., 2025). In rat and human retinas, ERα is expressed in the OPL and in amacrine and ganglion cells in inner retina and ERβ is expressed in the IPL, with colocalization of these ER types observed on some amacrine and ganglion cell types (Cascio et al., 2015). GPER has been identified in the retinas of zebrafish (Liu et al., 2009; Jayasinghe and Volz, 2012; Shi et al., 2013) and goldfish (Mangiamele et al., 2017).
While estrogen is present in both males and females, the higher circulating E2 levels in females are suggested to underlie sex-differences in retinal function. ERG recordings from female Sprague-Dawley rats (age 2–6.5 months) are larger in amplitude than age-matched males (Chaychi et al., 2015). Similarly, multifocal ERGs recorded from men and women <50 years of age identified shorter implicit times in women participants (Ozawa et al., 2014). Increased E2 levels in female Tungara frogs during the reproductive season is associated with an increase in retinal sensitivity (Leslie et al., 2021). Estrogen is also neuroprotective in retina (Bondesson et al., 2015; Cascio et al., 2015). E2 helps regulate the blood-retinal-barrier and protect photoreceptors from glutamate-induced damage (Nuzzi and Caselgrandi, 2022). In a mouse retinopathy of maturity model, activation of GPER on ganglion cells and astrocytes decreased endoplasmic reticulum stress response to hypoxia (Li and Li, 2020a) and decreased apoptosis (Li and Li, 2020b). Activation of GPER on mouse ganglion cells was also protective against NMDA-mediated neurotoxicity (Jiang et al., 2019); while activation of ERβ was protective after optic nerve crush (Rodriguez-Ramirez et al., 2025). In vitro, cultured mouse Muller glial cells were protected from oxidative stress by E2 treatment (Tawarayama et al., 2024). Given this latter role of E2, it is not surprising that age-related changes in estrogen levels are associated with neurodegenerative retinal diseases in humans (Gupta et al., 2005; Nuzzi et al., 2018) and rodents (Rowe et al., 2025) or that estrogen modulation as a breast cancer treatment has been associated with a variety of retinal complications (Eisner et al., 2008; Eisner and Luoh, 2011; Moschos et al., 2012).
5 EDC exposure and retinal development
Retinal development has been extensively studied in zebrafish because their rapid external development and transparent eggs allow a real-time observation of events which are difficult to do in mammals (Kimmel et al., 1995; Phillips and Westerfield, 2014). The initial work documenting photoreceptor development (Branchek and Bremiller, 1984), followed by electron microscopic analysis (Schmitt and Dowling, 1994; 1999), identified that zebrafish retinal development begins at 24 hpf (hours post fertilization). At 32 hpf ganglion cell axons project to the brain (Stuermer, 1988) and by 72 hpf, the larvae have hatched and all retinal layers are present (Schmitt and Dowling, 1999). One day later (96 hpf), optokinetic responses, a retina-based response where the eyes track a moving stimulus, can be recorded. By 7 dpf, zebrafish have exhausted their yolk sac, begin feeding, and visually guided optomotor responses can be recorded (Chiang et al., 2001; Neuhauss, 2003; Muto et al., 2005).
Development of estrogen signaling occurs in parallel with zebrafish retinal development. Zygotic synthesis of ER mRNA begins ∼24–48 hpf (Bardet et al., 2002; Lassiter et al., 2002; Tingaud-Sequeira et al., 2004; Mouriec et al., 2009a). Cyp19a1b is detected at 32 hpf in larvae with expression upregulated by E2 treatment at ∼25 hpf (Balshaw, 2023). All zebrafish ER types are expressed and functional in brain at 24–26 hpf (Balshaw, 2023). GPER mRNA can be identified in the eye ∼24–26 hpf (Jayasinghe and Volz, 2012; Shi et al., 2013). At 5 dpf, aromatase protein can be detected in ganglion cells and in the INL using immunocytochemistry (Ulhaq and Kishida, 2026).
There is a similar overlap of estrogen and eye development in mammals. In mice, ER expression begins at E (embryonic day) 9.5 when ERα is identified in the heart. ERβ is identified in the brain beginning at E10.5 and remains the only ER expressed in that tissue until E16.5 when ERα expression was identified (Lemmen et al., 1999). The presumptive neural retina is distinguished in the optic cup at ∼E9.5. From E11 to E18 neurogenesis of retinal ganglion cells, horizontal cells, amacrine cells, and cone photoreceptors occurs and the first ganglion cell axons leave the eye ∼ E11.5. A second wave of retinal neurogenesis occurs from postnatal day (P) 0 – P7 when rods, bipolar cells, and Muller glia are formed (Zhang et al., 2011; Heavner and Pevny, 2012). In humans, the presumptive neural retina forms around 4–5 weeks of gestation and retinal differentiation begins ∼47 days with rods and cones evident at weeks 10–15 (Graw, 2010). At 9 weeks, the placenta is the major source of fetal estrogen; fetal ERα and ERβ transcripts are identified at 13 weeks (Bondesson et al., 2015).
Given the overlap in timing, it is not surprising that developmental disruption of estrogen signaling either by blocking aromatase activity or antagonizing estrogen receptors has adverse effects on retinal/eye development. Exposure to aromatase inhibitors from 24 to 120 hpf thins ONL, IPL, and GCL in zebrafish (Hamad et al., 2007). Morpholino knockdown of aromatase B (cyp19a1b) decreased overall eye and optic nerve development, reduced thickness of the INL and the IPL, increased apoptosis in the eye, and caused deficits in visual based behaviors. These effects were mediated by ERβ (Ulhaq and Kishida, 2026). Aberrant activation of GPER causes concentration-dependent effects on survival and morphology during zebrafish embryogenesis (Jayasinghe and Volz, 2012). Larval exposure to 4-OH-A, an aromatase inhibitor, reduced the thickness of retinal layers (Hamad et al., 2007) and caused visual deficits in adults (Gould et al., 2017). In another teleost, medaka, adult exposure to EE2, a potent estrogenic EDC, caused generational effects that delayed eye pigmentation in larvae and changed expression of the genes associated with synaptic structure, synaptic transmission, and eye structure and development (Qin et al., 2023).
Polychlorinated biphenyls (PCBs) are another well-known EDC (Ulbrich and Stahlmann, 2004) that are estrogenic (Ma and Sassoon, 2006), able to bind ER (Damiano et al., 2025), and effect eye development. Examination of retina morphology and ultrastructure in zebrafish larvae exposed to PCB1254 from 0 to 96 hpf revealed delayed retinal layer development and smaller photoreceptor outer segments at 72 hpf and irregularly arranged photoreceptors and larger photoreceptor and ganglion cell layers at 96 hpf (Wang et al., 2011). Zebrafish larvae exposed to PCB1254 until 7 dpf displayed concentration-dependent decreases in optomotor responses and in the expression of photoreceptor-specific genes (Zhang et al., 2015). Adult female offspring of pregnant Long-Evans rats fed either PCB77 or PCB47 showed decreased scotopic ERG b-wave amplitudes at high illuminance levels (for PCB77) and increased b-wave latency at low illuminance levels (for PCB47) (Kremer et al., 1999). Immunocytochemical analysis of brains of adult offspring of pregnant Sprague Dawley rats fed a mix of 14 different PCB congeners during pregnancy and lactation revealed region-specific reductions in endothelial cell size, increases in GAD67 immunoreactivity, and decreased lipofuscin autofluorescence (Rustom and Reynolds, 2025).
5.1 Specific effects of BPA, TBT, and related compounds on retinal development
Developmental exposure to both BPA and TBT cause immediate effects on neurogenesis, with many of the studies performed in zebrafish. Early BPA exposure reduces zebrafish eye diameter (Crowley-Perry et al., 2021; Volz et al., 2024) and affects retinal layer thickness (Volz et al., 2024). Zebrafish larvae exposed to BPA showed changes to red (L) and UV cones within the retinal mosaic (Qiu et al., 2025). Chronic 8 days BPA exposure thinned the IPL and impaired responses to red and green (M cone) light (Volz et al., 2024). Similarly, adult male zebrafish exposed to BPA for 7 weeks showed altered color preference (Li et al., 2017). BPA increased ERα and ERβ expression, but decreased locomotor behavior in larvae (dos Santos et al., 2022). BPA analogs also alter visual function. Chronic BPS exposure until 6 dpf reduced thickness of the ganglion cell layer (Gu et al., 2019) and BPS exposure for 120 days disorganized the ONL, thinned the IPL and GCL, and altered adult spectral sensitivities (Liu et al., 2018). BPS exposure from 2 to 5 dpf disrupts spacing and alters signaling of red and UV cones (Qiu et al., 2023); differences in optic nerve structure were noted in a separate study that chronically exposed larvae until 6 dpf (Gu et al., 2019). Exposure to TBBPA until 5 dpf decreased both eye size and optokinetic responses in zebrafish (Baumann et al., 2016).
TBT exposure during early life stages causes abnormal and delayed eye development (Hano et al., 2007), thinning of the cornea (Wang and Huang, 1999), and retina-specific defects (Fent and Meier, 1992; Wang and Huang, 1999; Wester et al., 2004; Dong et al., 2006).Chronic 5 days TBT exposure (0–120 hpf) increased apoptosis in zebrafish retina, with the greatest difference observed at 60 hpf when there was a 2× increase in macrophages (Dong et al., 2006). In tropical guppies, a 7 days exposure to TBT decreased retinal pigment epithelium, disorganized the photoreceptor layer, and caused vacuoles in the IPL (de Paulo et al., 2020). In minnows, embryonic-larval exposure to TBT similarly reduced the amount of pigment and disrupted retinal layering (Fent and Meier, 1992) and a 7 days exposure decreased retinomotor responses in tiger perch (Wang and Huang, 1999). TBT exposure increased oxidative stress in the eyes of medaka juveniles (Shi et al., 2021) and disrupted the blood-brain-barrier in rats (Mitra et al., 2013; Mitra et al., 2015). Eyes of neonatal ICR mice exposed to trimethyltin (TMT), a related organotin compound, until P14 had thinner retinal layers, increased apoptosis, increased overall glutamate levels, and decreased micro-ERG b-wave amplitude, consistent with neurotoxicity (Kim et al., 2023). TMT exposure during zebrafish development caused concentration-dependent effects that reduced vision-based behaviors, thinned retinal layers, formed pyknotic nuclei, and distorted the boundaries of the IPL and OPL (Kim et al., 2019). A 14-day exposure to Triphenyltin (TPT) altered expression of genes involved in polarity during retinal development in zebrafish (Li and Li, 2020a).
6 Case study: Does transient early life BPA/TBT exposure cause persistent effects in zebrafish
6.1 Premise and experimental design
Most published reports of BPA and TBT induced effects typically occurred immediately after exposure, and exposure was often chronic, lasting multiple days. In contrast, our approach assessed whether a transient, 24 h developmental exposure to either compound was sufficient to cause long-term changes in adult retinal function and anatomy. We also sought to determine if these compounds have opposite sequelae in retina, given their contrasting effects on estrogen signaling.
Compound exposure occurred when larvae were either 3 dpf or 7 dpf because these ages represent key time points in both visual system and estrogen pathway development, as noted above. Exposure lasted for 24 h. The concentrations used for each compound are environmentally relevant and in agreement with previous studies (Fent and Meier, 1992; Kolpin et al., 2002; Schmidt et al., 2004; el Hassani et al., 2005; Schmidt et al., 2005; Dong et al., 2006; Oliver et al., 2011; Saili et al., 2012; Wang et al., 2013; Borges et al., 2014; Hayashi et al., 2015; Kinch et al., 2015; Weber et al., 2015; Cano-Nicolau et al., 2016; Santangeli et al., 2016). Our BPA concentrations were 0.228 μg/L (0.001 µM) and 22.8 μg/L (0.1 µM), with DMSO (0.0003%) as the vehicle control (Cohen et al., 2025); TBT concentrations were 0.04 μg/L (0.12 nM) and 0.4 μg/L (1.2 nM), with 0.1% ethanol as the vehicle control (Jensen et al., 2025). After the 24 h exposure, treated larvae were returned to system water and allowed to grow undisturbed until adulthood (≥3–4 months of age). In adults, we examined retinal anatomy (using H&E staining of retinal sections) and function [using electroretinograms (ERGs)] to identify if there were persistent outcomes resulting from transient developmental exposure.
6.2 Early life exposure to either BPA or TBT altered adult retinal function
To determine if developmental exposure to BPA or TBT altered retinal function, we used ERGs. The ERG response components include an initial negative a-wave (photoreceptor response) at light ON, followed by a positive b-wave (ON-bipolar cell response), and a positive d-wave (OFF-bipolar cell response) at the end of the light pulse (Perlman, 2007; Nelson and Singla, 2009). We decided to focus on ERGs as differences in b- and d-waves (reflecting ON and OFF bipolar cell responses) have been associated with retinal diseases in animal models (Constable et al., 2023; Aviles et al., 2025; Medina Arellano et al., 2025) and in the clinic (Cornish et al., 2021; Chiang et al., 2022; Constable et al., 2023).
ERGs were recorded from adult zebrafish that had been developmentally exposed to either BPA or TBT when they were 3 dpf or 7 dpf. ERGs were recorded as described in (Nelson and Singla, 2009; Cohen et al., 2025; Jensen et al., 2025). Briefly, eye cups, with the cornea and lens removed, were placed onto a piece of 0.45 µm black filter paper in the recording chamber and perfused (0.3 mL/min inflow; 4 mL/min outflow) with MEM solution that had been equilibrated with 95%O2/5%CO2. A tungsten recording electrode was placed into the eye cup to record responses to 300 ms flashes of white light presented individually at 7 different brightness levels (ND 3.0 – ND 6.0 in 0.5 ND increments) on an infrared background (RG780 filter). Each flash was presented 4 times for each brightness level, and the entire protocol was repeated 10 times for a total of 280 retinal responses per eye. Recordings were amplified using a DAM80 amplifier (WPI) and Digidata 1440A (Axon Instruments). Data was collected using pCLAMP software (Axon) and analyzed in Origin. For each recording, b-wave amplitudes were measured from the a-wave trough to the peak of the b-wave (50–200 ms after stimulus onset); d-wave amplitude was measured as the peak occurring within 25–300 ms after stimulus offset. Technical replicates were excluded from analysis if b-waves were negative or the response occurred outside the time intervals. Amplitudes and implicit times (= time to peak amplitude) were analyzed using SPSS or R software, and graphs were made in Excel. One-way ANOVAs were used to assess differences in b- and d-wave responses for each age and compound. Results were considered significant if the p-value was ≤0.05.
For both compounds, exposure to the lower concentration (0.228 μg/L BPA; 0.04 μg/L TBT) at 3 dpf had greater effects than the higher concentrations (Figure 1), revealing non-monotonic effects. Exposure to 0.04 μg/L TBT at 3 dpf increased b-wave response amplitude (Figure 1A, p < 0.001) and b-wave peak time (Figure 1B, p = 0.019) compared to vehicle controls and 0.4 μg/L TBT exposed tissue. However, TBT exposure at 3 dpf did not alter OFF-bipolar d-wave responses (Figures 1C,D; N’s for the different groups: vehicle = 6 | 0.04 μg/L TBT = 6 | 0.4 μg/L TBT = 6). Exposure to 0.228 μg/L BPA at this age increased ON-bipolar cell b-wave (Figure 1E; p < 0.001) and OFF-bipolar cell d-wave (Figure 1G; p = 0.003) response amplitudes; d-wave response time was also faster than vehicle controls (Figure 1H; p = 0.005; vehicle = 10 | 0.228 μg/L BPA = 6 | 22.8 μg/L BPA = 18). Exposure to 22.8 μg/L BPA at 3 dpf decreased b-wave amplitude (p < 0.001).
FIGURE 1
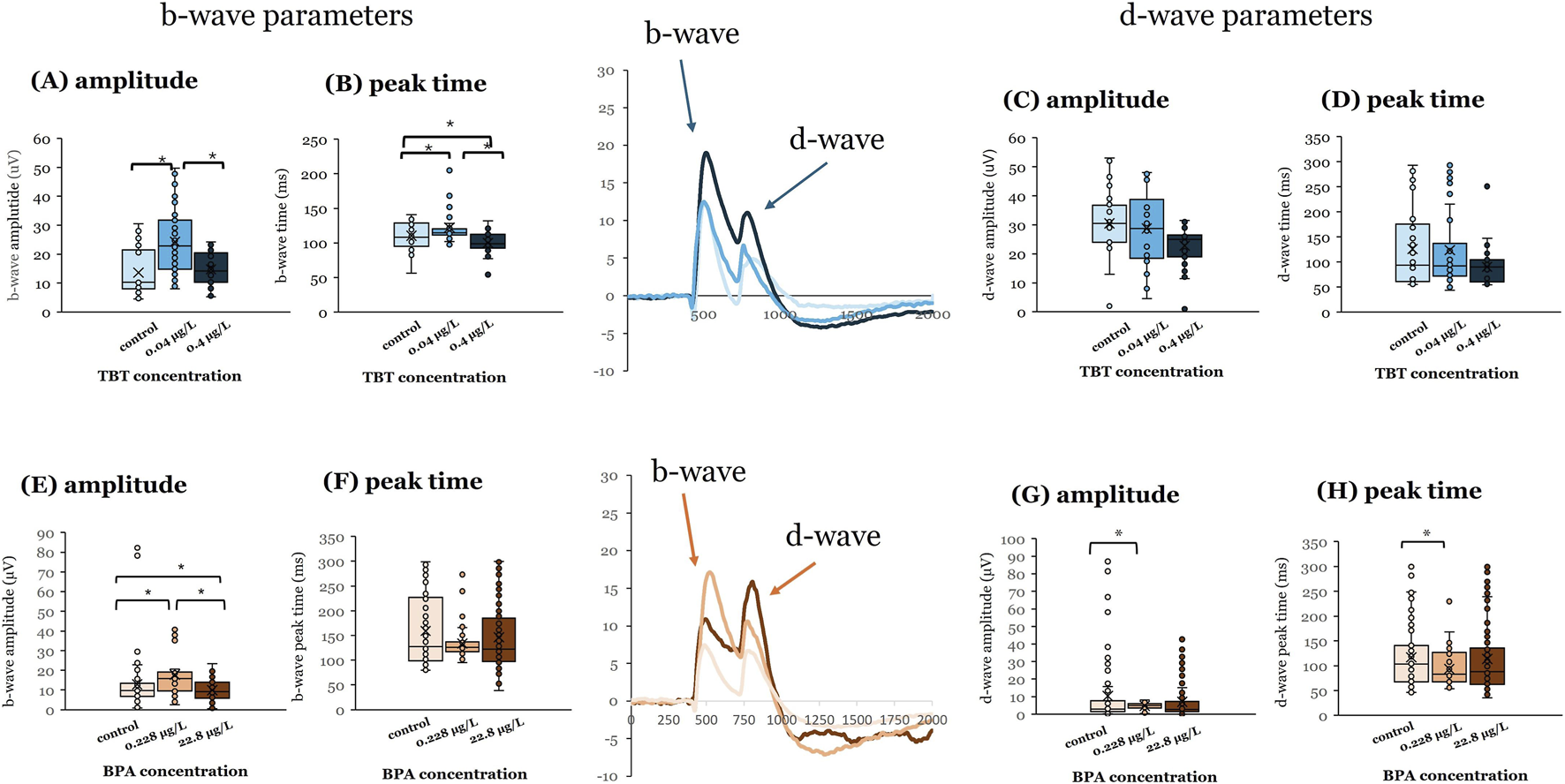
BPA or TBT exposure at 3 dpf enhances ON-bipolar responses. Electroretinograms (ERGs) were recorded from adult eyecups to assess retinal function. Zebrafish larvae were exposed to bisphenol A (BPA) at either 0.228 μg/L or 22.8 μg/L, (control = 0.0003% DMSO) or tributyltin (TBT) at 0.04 μg/L or 0.4 μg/L (control = 0.1% ethanol) for 24 h when they were 3 days postfertilization (dpf). Larvae were then placed into system water and allowed to grow to adulthood (≥3–4 months pf) when ERGs were recorded. ERGs were elicited in response to a white light stimulus at 7 different brightness levels. Responses shown are from the brightest stimulus level (ND3.0) as that is the largest response. Representative mean ERGs at the brightest stimulus level are shown in the center of the figure. ERG b-wave amplitude and peak time and d-wave amplitude and peak time were quantified. (A) b-wave amplitude, (B) b-wave peak time, (C) d-wave amplitude, and (D) d-wave peak time measured in adults developmentally exposed to TBT (blue bars). (E) b-wave amplitude, (F) b-wave peak time, (G) d-wave amplitude, and (H) d-wave peak time measured in adults developmentally exposed to BPA (orange bars). Significant differences (asterisks) were determined for each parameter using a one-way ANOVA evaluated at α = 0.05. (Parts of this figure are taken from Cohen et al. (2025) and Jensen et al. (2025).
Exposure to 0.04 μg/L TBT at 7 dpf also affected adult ERG responses (Figure 2). This TBT concentration increased b-wave (Figure 2A; p ≤ 0.001) and d-wave response amplitudes (Figure 2C; p ≤ 0.001) and delayed d-wave peak time (Figure 2D; p ≤ 0.001; vehicle = 4 | 0.04 μg/L TBT = 7 | 0.4 μg/L TBT = 5). For all three variables, responses measured after 0.04 μg/L TBT exposure were significantly different from both vehicle controls and the 0.4 μg/L treatment group. In contrast to TBT, it was the higher BPA concentration (22.8 μg/L) that significantly altered ERG responses in adult retinas when exposure occurred at 7 dpf. ERG b-wave amplitude was increased (Figure 2E; p < 0.001) compared to the 0.228 μg/L treatment group, whereas d-wave response amplitude was decreased (Figure 2E; p < 0.001) compared to both 0.228 μg/L BPA and vehicle controls. Exposure to 22.8 μg/L BPA at 7 dpf also quickened (reduced) the response time of the d-wave peak compared to the 0.228 μg/L BPA treatment group (Figure 2H; p = 0.02; vehicle = 6 | 0.228 μg/L BPA = 13 | 22.8 μg/L BPA = 5). Time to b-wave peak was not affected by either BPA (Figure 2F) or TBT exposure at 7 dpf (Figure 2B).
FIGURE 2
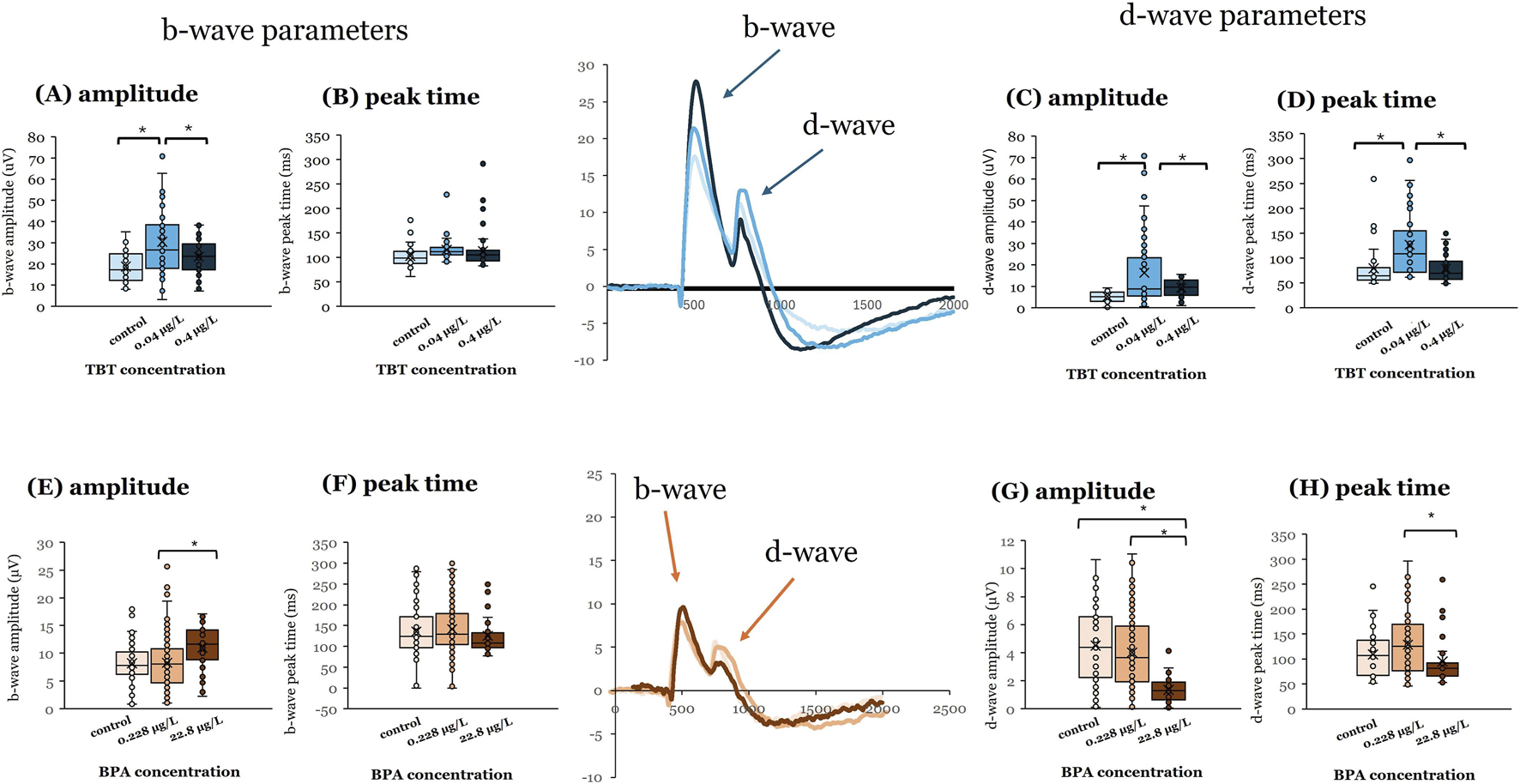
BPA or TBT exposure at 7 dpf affects OFF-bipolar cell responses. Electroretinograms (ERGs) were recorded from adult eyecups to assess retinal function. Zebrafish larvae were exposed to bisphenol A (BPA) at either 0.228 μg/L or 22.8 μg/L, (control = 0.0003% DMSO) or tributyltin (TBT) at 0.04 μg/L or 0.4 μg/L (control = 0.1% ethanol) for 24 h when they were 7 days postfertilization (dpf). Larvae were then placed into system water and allowed to grow to adulthood (≥3–4 months pf) when ERGs were recorded. ERGs were elicited in response to a white light stimulus at 7 different brightness levels. Responses shown are from the brightest stimulus level (ND3.0) as that is the largest response. Representative mean ERGs at the brightest stimulus level are shown in the center of the figure. ERG b-wave amplitude and peak time and d-wave amplitude and peak time were quantified. (A) b-wave amplitude, (B) b-wave peak time, (C) d-wave amplitude, and (D) d-wave peak time measured in adults developmentally exposed to TBT (blue bars). (E) b-wave amplitude, (F) b-wave peak time, (G) d-wave amplitude, and (H) d-wave peak time measured in adults developmentally exposed to BPA (orange bars). Significant differences (asterisks) were determined for each parameter using a one-way ANOVA evaluated at α = 0.05. (Parts of this figure are taken from Cohen et al. (2025) and Jensen et al. (2025).
6.3 Developmental exposure to TBT or BPA caused age-dependent differences in retinal anatomy
To determine if the observed physiological differences reflected compound-induced changes in retinal anatomy, we measured the thicknesses of the different layers in H&E stained adult retinal sections (Figure 3). The only retinal layer found to be sensitive to compound exposure was the IPL, where axon terminals of ON- and OFF-bipolar cells are located. The anatomical change in retinal layer thickness for the IPL was different for the two compounds. BPA exposure at 3 dpf, but not 7 dpf, significantly decreased IPL thickness in adult retinas (Figure 3A; p = 0.042). In contrast, TBT exposure at 7 dpf significantly increased IPL thickness (Figure 3B; p = 0.002).
FIGURE 3
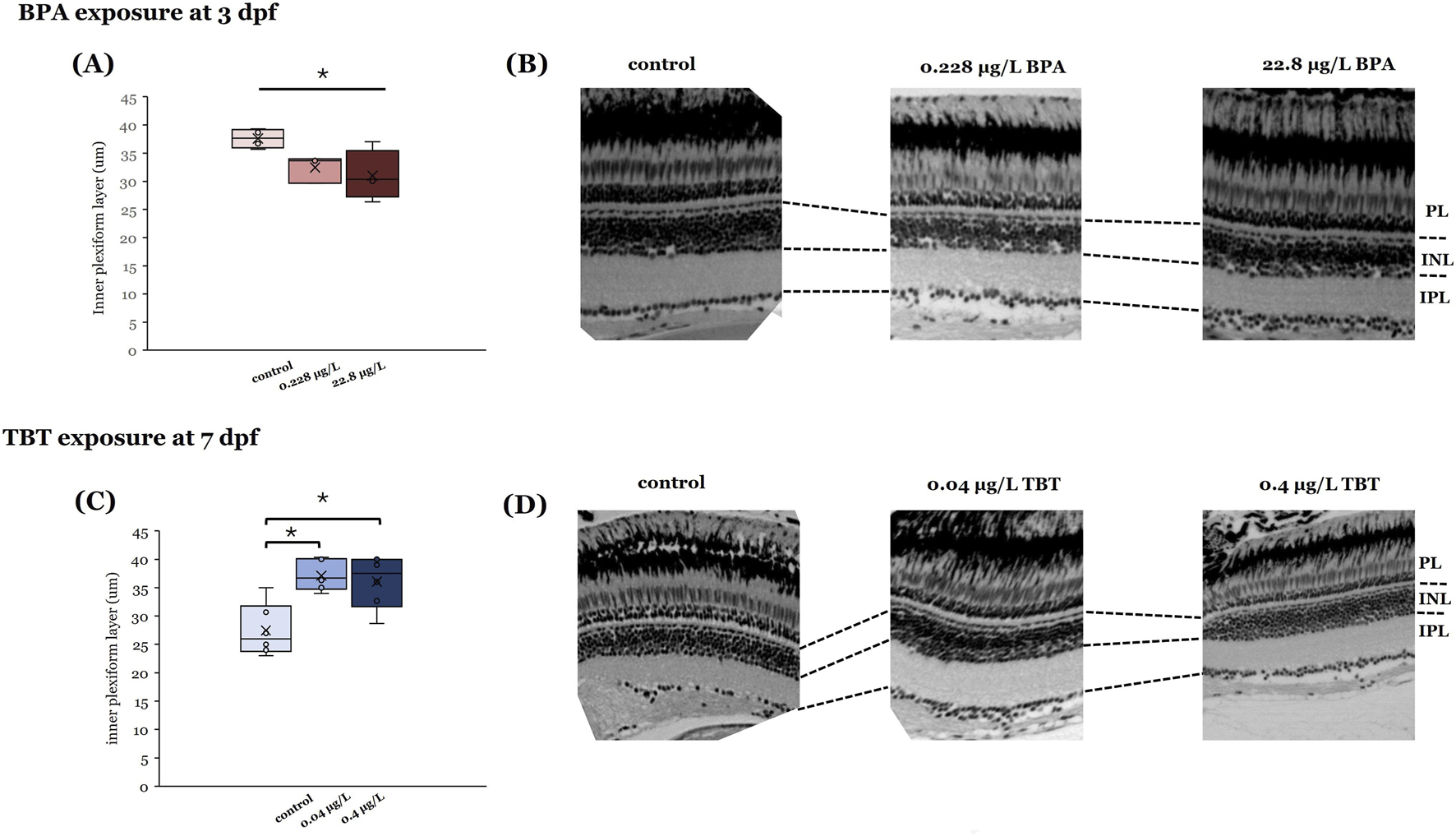
Effects of BPA and TBT on retinal anatomy are age dependent. Retinal sections taken from adults developmentally exposed to either BPA (A,B) or TBT (C,D) to determine if there are changes in retinal structure. Measurements of individual retinal layers (at the right) identified significant differences in the inner plexiform layer (IPL) only. Adults exposed to BPA when they were 3 days postfertilization (dpf) (A,B) showed a significant reduction in IPL thickness. In contrast, adults exposed to TBT when they were 7 dpf (C,D) showed a significant increase in IPL thickness. Measurements were made from 3 different retinas per treatment group per age. Significant differences (asterisks) were determined for each parameter using a one-way ANOVA evaluated at α = 0.05. (Parts of this figure are taken from Cohen et al. (2025) and Jensen et al. (2025).
6.4 Developmental EDC exposure altered protein and mRNA expression of estrogen signaling components
We recently reported (Cohen et al., 2025) changes in estrogen signaling components in adult retinas following exposure to BPA at either 3 dpf or 7 dpf. Retinal homogenates collected from adults exposed to 0.228 μg/L BPA at 3 dpf showed a significant increase in mRNA expression of aromatase (cyp19a1b), and a significant decrease in protein levels of ERβ, p-ERK and p-JNK (Cohen et al., 2025) indicating effects on genomic/nuclear signaling and the activation of either membrane bound ER or GPER (Prossnitz and Barton, 2014). In contrast, retinal homogenates from adult fish exposed when they were 7 dpf did not show differences in aromatase, esr1, or esr2a expression though ERβ protein levels were increased. Exposure to 0.228 μg/L BPA at 7 dpf decreased p-ERK levels, while exposure to 22.8 μg/L BPA increased p-JNK levels. The two BPA concentrations also differentially effected p-AKT levels, with increased p-AKT protein observed in retinas treated with 22.8 μg/L BPA but decreased protein levels observed in the 0.288 μg/L treatment group (Cohen et al., 2025). BPA-induced changes in AKT levels are important to note, as this pathway is associated with GPER activation and neuroprotection in mouse retina (Jiang et al., 2019).
We have not completed a similar molecular analysis of retinal homogenates from adults developmentally exposed to TBT, and no similar analysis is reported in the literature. However, we can anticipate TBT-induced effects on estrogen signaling components, as a transcriptomic analysis of whole zebrafish embryos exposed from 2 to 5 dpf revealed TBT exposure altered genes involved in development and immune and inflammatory responses (Martinez et al., 2020). Further, given that TBT is an aromatase inhibitor (Matthiessen and Gibbs, 1998; McAllister and Kime, 2003; McGinnis and Crivello, 2011) and that aromatase and ER are present in zebrafish retina (Menuet et al., 2002; Sawyer et al., 2006; Cohen et al., 2025), it is likely that estrogen levels/signaling will be reduced by early life TBT exposure, as reported in juvenile Atlantic salmon (Lyssimachou et al., 2006).
7 ERGs reveal persistent effects of BPA and TBT that were concentration and age dependent
Our data suggests similarities in functional outcomes to early life EDC exposure in zebrafish retina with respect to age of exposure and effective concentration for both BPA and TBT. ERG recordings from adult retinas following developmental exposure to BPA or TBT, identified age-dependent effects (Figure 4). In general, the ON-bipolar cell b-wave was sensitive to both compounds, regardless of exposure age. The OFF-bipolar d-wave, in contrast, appeared more sensitive to compound exposure at the later exposure age. Exposure to either 0.228 μg/L BPA or 0.04 μg/L TBT at 3 dpf enhanced adult b-wave amplitudes. This TBT concentration also delayed b-wave peak time but had no effect on d-wave responses. BPA, on the other hand, increased and delayed OFF-bipolar d-wave responses. These effects of TBT and BPA exposure at 3 dpf are evident in the mean traces shown in Figure 1. Thus, only ON-bipolar cells appear sensitive to TBT exposure at 3 dpf; while both ON and OFF-bipolar cells are sensitive to BPA. Exposure to either compound at 7 dpf affected both the b-wave and d-wave components of the adult ERG, with all OFF-bipolar response components showing significant differences. Adult retinas exposed to 0.04 μg/L (the lower concentration) TBT showed increased and delayed d-wave responses, as did ERG b-waves. In contrast, significant differences in adults previously exposed to BPA were only observed in the high (22.8 μg/L) treatment group which decreased d-wave amplitude compared to vehicle controls but increased b-wave amplitude. These age-dependent effects were also evident in the anatomical data. BPA exposure at 3 dpf also reduced the thickness of the retinal IPL; while TBT exposure at 7 dpf increased IPL thickness.
FIGURE 4
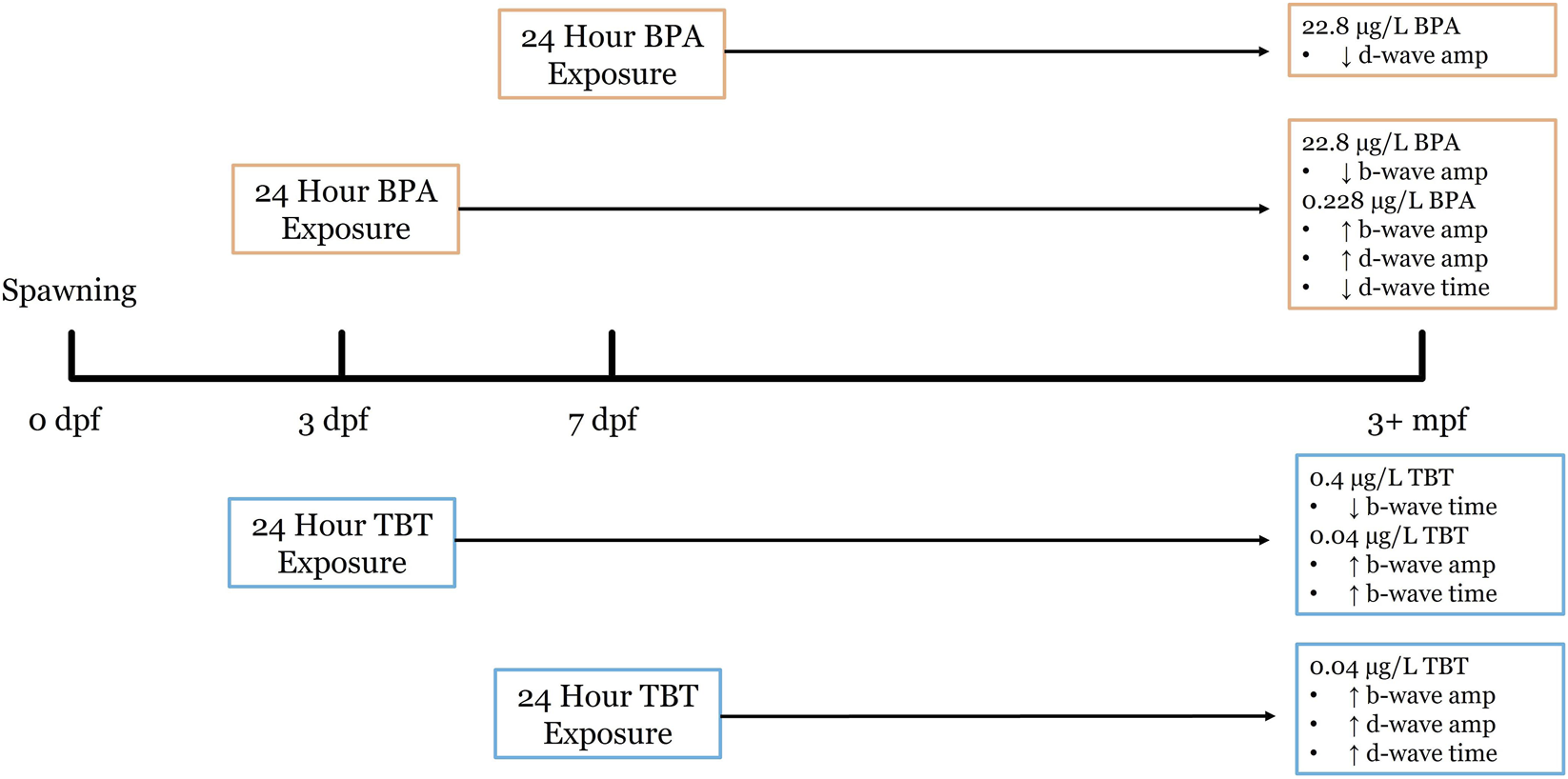
Developmental exposure timeline for BPA and TBT and resulting long-term retinal functional outcomes in zebrafish. This timeline illustrates the experimental design used to assess the long-term effects of early life exposure of BPA and TBT at our two exposure timepoints. Embryos were maintained in an incubator from 0 days post fertilization (dpf) to 10 dpf, before being transferred in the aquatic care facility, where they remained until 3 months post fertilization (mpf). Both BPA and TBT had 24 h exposures beginning at either the 3 dpf or the 7 dpf. Electroretinography (ERG) was performed at 3+ mpf to quantify long-term change in bipolar cells. High concentrations of BPA (0.1 μM) at 7 dpf result in an increase in b-wave amplitude and a decrease in d-wave time. While low concentration (0.228 ug/L) at 3 dpf BPA exposure increase b-wave amplitude and d-wave amplitude, with a decrease in d-wave time. High concentration of TBT (0.4 μg/L) at 7 dpf showed an increase in b-wave amplitude, d-wave amplitude, and d-wave time. As opposed to high concentration at 3 dpf which showed a decrease in b-wave amplitude.
We also identified concentration-dependent effects. Developmental exposure to 0.04 μg/L TBT appears more deleterious than 0.4 μg/L as all ERG components were enhanced by the lower concentration, regardless of exposure age. Similarly, exposure to the lower 0.228 μg/L BPA concentration at 3 dpf consistently enhanced all ERG responses. These results describe nonmonotonic responses, as exposure to the lower dose caused more effects (Canesi and Fabbri, 2015). We did observe changes in ERG responses in adults exposed to the 22.8 μg/L BPA treatment, however, with this dose enhancing b-wave amplitudes but reducing d-wave responses when exposure occurred at the later age. Nonmonotonic effects of EDC’s have been reported (Vandenberg, 2014) including in retina (Kang et al., 2024). The suggested mechanisms underlying these responses include different affinities for/differential activation of receptors (Villar-Pazos et al., 2017; Vandenberg, 2022) and differences in pathway activation between central EDC effects, which would trigger regulatory negative feedback, and peripheral EDC effects, which would be stimulatory (Shi et al., 2025). G-protein coupled receptors can be desensitized, where continued exposure to an EDC inactivates the receptor, causing larger concentrations to be less effective (Vandenberg, 2022). In contrast, nuclear receptors can be downregulated as EDC concentration increases, decreasing the effects of higher doses (Vandenberg, 2022). These mechanisms all describe the impact of the EDC binding to a receptor, which is the case for BPA and ER. In fact, nonmonotonic responses of BPA are well reported, occurring in ∼20% of reports (Vandenberg, 2014). Given that BPA is known to have differential affinities for ERα/β (Ben-Jonathan and Steinmetz, 1998; Vandenberg et al., 2009) and that BPA can bind GPER, we suggest that differential binding to these receptors and/or receptor desensitization may underlie the BPA-induced nonmonotonic effects observed here. Determining the mechanisms of TBT’s nonmonotonic effects is more difficult, as TBT does not bind ER. Here, the observed responses may reflect competition between TBT and the natural substrate for the enzyme active site. Further, the ability of both BPA and TBT to bind thyroid receptors, and influence other cellular mechanism, must also be considered, as nonmonotonic responses can also occur when a single outcome (such as retinal function) is influenced by more than one pathway (Vandenberg, 2022).
The increase in b-wave amplitude in response to the lower concentrations of both BPA and TBT reveals similar, not opposite effects on retina function (Figure 5). While unexpected, we identified three studies with similar results. Antagonistic effects were not observed in neonatal (PN 1–16) female rats after co-exposure to a BPA/TBT mixture (Yang et al., 2019). Chronic (2–5 dpf) exposure to BPA and TBT in zebrafish revealed only concentration dependent effects, with higher doses of TBT needed to produce the same effects as low BPA (Martinez et al., 2019). Finally, a metabolomic analysis of zebrafish larvae exposed to either compound from 48 to 120 hpf revealed some overlap with regard to which metabolites/metabolic pathways were altered (Ortiz-Villanueva et al., 2018). These reports, together with our data, suggest that BPA and TBT may be differentially targeting the same and/or different pathways. These pathways, which include estrogen disruption, are described below.
FIGURE 5
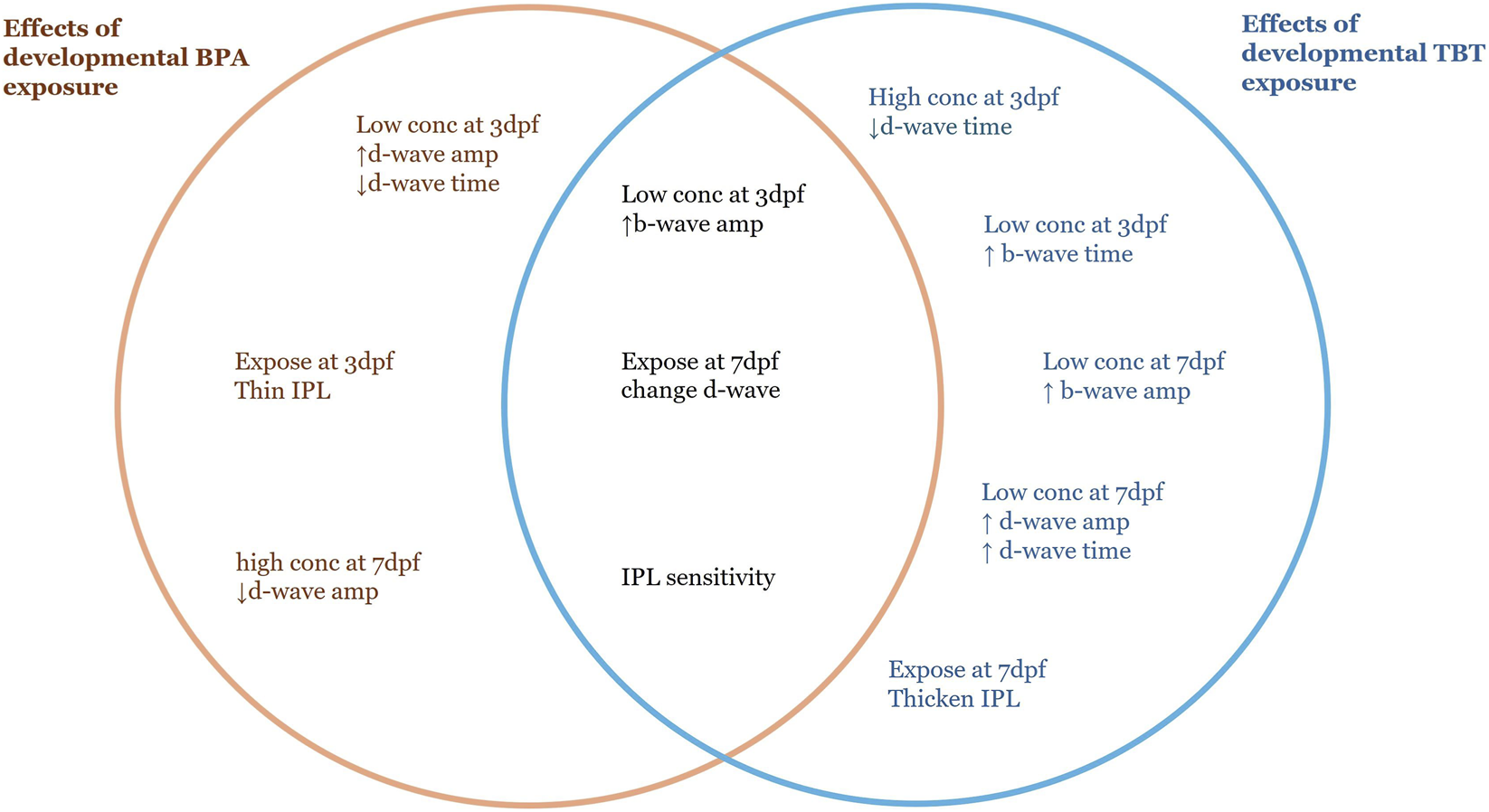
Summary diagram comparing the effects of developmental BPA and TBT exposure. Items on the left (orange text) were observed only in adult retinas developmentally exposed to BPA. Items on the right (blue text) were observed only in adult retinas developmentally exposed to TBT. Items in the center (black text) are common to both. Differences were in comparison to the relative vehicle control. For BPA: low conc = 0.228 μg/L; high conc = 22.8 μg/L; control = 0.0003% DMSO. For TBT: low conc = 0.04 μg/L; high conc = 0.4 μg/L; control = 0.1% ethanol. ↑, increase in amplitude or time; ↓, decrease in amplitude or time; Dpf, days postfertilization; IPL, inner plexiform layer.
8 Possible mechanisms of long-term functional effects resulting from acute developmental EDC exposure
8.1 Developmental exposure to BPA or TBT alters estrogen signaling pathways in retina causing latent physiological changes
Exposure to BPA and TBT, if only transiently, likely had a direct effect on retinal estrogen signaling. All estrogen receptor types are present in vertebrate retinas (Cascio et al., 2015) and aromatase is found in rat INL and OPL (Valero-Ochando et al., 2024) where bipolar cells and their dendrites are located. Importantly, estrogen signaling components are present in zebrafish retina at our exposure ages, including nuclear ER (Bardet et al., 2002; Lassiter et al., 2002; Tingaud-Sequeira et al., 2004), aromatase (Mouriec et al., 2009b; Cohen et al., 2025) and GPER (Shi et al., 2013). Developmental BPA exposure reduces eye size (Crowley-Perry et al., 2021; Volz et al., 2024) and affects brain development in zebrafish (Nakamura et al., 2006; Vandenberg et al., 2009; Zhou et al., 2009; Kim et al., 2011; Tse et al., 2013). Similarly, developmental TBT exposure delays eye development (Hano et al., 2007) and has retina-specific effects (Fent and Meier, 1992; Wang and Huang, 1999; Wester et al., 2004; Dong et al., 2006).
Our molecular analysis identified aromatase (cyp19a1b) and ER (esr1, esr2a) mRNA and/or protein (ERβ) in adult zebrafish retinal homogenates and found that developmental BPA exposure altered expression of some of these estrogen responsive genes (Cohen et al., 2025). We also observed differences in MAPK and AKT pathway activation (phosphorylation) suggesting BPA was also binding to GPER. In mammalian retina activation of GPER and/or ERβ is neuroprotective to retinal ganglion cells (Jiang et al., 2019; Rodriguez-Ramirez et al., 2025). BPA exposure (0.228 μg/L) at 3 dpf decreased ERβ, but increased p-ERK and p-JNK levels; while exposure to the same concentration at 7 dpf increased ERβ protein and decreased p-ERK, suggesting age-dependent differences ER/GPER activation by 0.228 μg/L BPA. BPA concentration-dependent effects were also observed for AKT pathway activation, with an increase in p-AKT (associated with pathway activation) found in retinas treated with 22.8 μg/L at 7 dpf and decreased levels in the 7 dpf 0.228 μg/L group (Cohen et al., 2025). GPER activation associated with PI3K/AKT pathway activation is neuroprotective (Jiang et al., 2019), suggesting a possible effect of BPA exposure. Together, this data suggests that a brief early life BPA exposure can cause latent effects on estrogen pathways in retina, with BPA concentration determining which specific ER/GPER is/are activated.
With regard to TBT, the presence of aromatase in zebrafish (Cohen et al., 2025) and mammalian (Cascio et al., 2015) retinas suggests a direct effect on estrogen synthesis. In his review of estrogenic signaling in mammalian retina, Cascio et al. (2015) indicates the retinal INL is the layer where most local E2 is synthesized. Goldfish retina contains aromatase-positive bipolar, horizontal, and amacrine cells in the INL (Gelinas and Callard, 1993; Callard et al., 1995), suggesting E2 synthesis also occurs in the INL in fish retina. Importantly, the INL is where bipolar cells are located. The changes in ERG b- and d-waves, which reflect bipolar cell responses, in reponse to TBT exposure suggest the INL may be targeted by compound exposure. Inhibition of aromatase changed the thickness of the IPL and INL in zebrafish and rats (Salyer et al., 2001; Orger and Baier, 2005) in support of this hypothesis. The IPL is where ERβ is located in mammals (Cascio et al., 2015) and our anatomical data indicates that the IPL is sensitive to TBT (and BPA) exposure, suggesting that TBT may selectively target the inner retina.
8.2 Long-term retina-specific effects of developmental BPA/TBT exposure are estrogen independent
While BPA and TBT are classified as EDCs, both compounds have a variety of effects. TBT exposure induces oxidative stress (Zhang et al., 2017) and increases levels of glucose and creatine kinase in the blood (Li and Li, 2021). TBT exposure increased permeability of the blood brain barrier, lipid peroxidation, glial activation and autophagy in rats (Mitra et al., 2013; Mitra et al., 2015). TBT exposure also causes apoptosis in isolated mammalian cells (Correia et al., 2025) and disrupts thyroid signaling in mice (Sharan et al., 2014), rats (Rodrigues-Pereira et al., 2022) and zebrafish (Li and Li, 2021). BPA can binding to thyroid receptors (Almeida et al., 2018; dos Santos et al., 2022) and to thyroid hormone transport proteins such as transthyretin, displacing thyroxine (T4) (Lim et al., 2002; Fujimoto et al., 2004; Brent, 2023). BPA working through thyroid hormone receptor β (thrb), in particular, is required for proper development of cone photoreceptors in zebrafish (Suzuki et al., 2013; Qiu et al., 2025) and mice (Roberts et al., 2006).
Other reports indicate BPA and TBT exposure alter levels of the neurotransmitters glutamate, dopamine, and GABA (Aoshima et al., 2001; Tsunoda et al., 2006; Choi et al., 2007; Almeida et al., 2018; Liu et al., 2020; Tu et al., 2020; Hyun and Ka, 2024). BPA and TBT impact these neurotransmitter systems by either blocking compound synthesis or binding to receptors. In Xenopus oocytes (Aoshima et al., 2001) and dissociated rat CA3 neurons (Choi et al., 2007), BPA had concentration dependent effects on GABAA receptor mediated responses. In rodent brain, BPA decreased TH-immunoreactivity (Ishido et al., 2007). GABA and dopamine synthesis decreased, and glutamate synthesis increased, in zebrafish larvae exposed to BPA from 5 to 8 dpf (Kim et al., 2020). Similarly, adult male zebrafish exposed to TBT had decreased expression of genes involved in dopamine, GABA and serotonin synthesis (Tu et al., 2020). Developmental TBT exposure blocked ligand (glutamate) binding to NMDA receptors in rodent brain membrane preparations (Konno et al., 2005).
As in the rest of the brain, retinal signaling depends on the release of neurotransmitters. Photoreceptor synapses onto bipolar and horizontal cells are glutamatergic, with glutamate release decreasing in response to light stimulation–the basis for ON and OFF bipolar cell responses (i.e., ERG b- and d-waves, respectively). Zebrafish retinal bipolar cells also express GABA and dopamine receptors (Connaughton, 2011). In zebrafish retina, GABA is localized to horizontal cells in outer retina and amacrine cells in inner retina (Connaughton et al., 1999; Yazulla and Studholme, 2001; Marc and Cameron, 2002). Dopamine is present in retinal amacrine and interplexiform cells (Connaughton et al., 1999; Yazulla and Studholme, 2001; Marc and Cameron, 2002). Amacrine and interplexiform processes are found in the IPL, the retinal layer found to be sensitive to BPA and TBT exposure. Thus, BPA/TBT exposure could be impacting bipolar cell responses by changing modulatory (GABA, dopamine) inputs or altering photoreceptor glutamate release.
Dopamine is synthesized in a 3-step sequence from the amino acid phenylalanine, with tyrosine hydroxylase (TH) the rate limiting enzyme in the pathway (Daubner et al., 2011). GABA is synthesized from glutamate via the enzyme glutamic acid decarboxylase (GAD) (Zhang et al., 2024). We observed changes in the protein levels of both enzymes in adult retinal homogenates that were exposed to 0.228 μg/L BPA exposure at 3 dpf. TH protein levels were increased, while GAD levels were decreased (Cohen et al., 2025). These enzymes are the rate limiting enzymes in the synthesis of dopamine and GABA, respectively, suggesting a direct BPA-induced effect on neurotransmitter synthesis in exposed retinas. Exposure to the higher BPA concentration (22.8 μg/L) at 3 dpf increased GAD levels in the same homogenates, identifying concentration dependent effects.
In amphibian retina, blocking GABAA receptors increased ERG b- and d-wave amplitudes (Hirasawa et al., 2021). We observed a thinner IPL and a decrease in GAD protein levels in adult retinal homogenates developmentally exposed to 0.228 μg/L BPA at 3 dpf (Cohen et al., 2025), suggesting a BPA-induced decrease in GABA synthesis, which may contribute to the enhanced b- and d-wave responses that were observed. Adult retinas treated with the higher concentration of BPA (22.8 μg/L) at 3 dpf displayed reduced b-wave amplitude but increased GAD protein levels, consistent with an increase in GABAergic inhibition. Both concentrations of BPA increased TH protein levels in retinal homogenates exposed at 3 dpf (Cohen et al., 2025), suggesting a BPA-induced increase in dopamine levels. Elevated dopamine levels reduce gap junctional coupling of (McMahon, 1994) and block GABA release from retinal horizontal cells (Yazulla and Kleinschmidt, 1982; Calaza et al., 2006), contributing to the observed ERG responses. TBT exposure similarly influences both dopamine and GABA. We observed an increase in IPL thickness due to TBT exposure at 7 dpf. This could suggest an increase in GABA and/or dopaminergic inner neurons and their synaptic connections or an increase in bipolar cell synaptic contacts. TBT is reported to increase dopamine levels in zebrafish (Liu et al., 2020) but decrease GABA levels (Tu et al., 2020). The increase in ERG response amplitudes we observed in retinas exposed to the 0.04 μg/L TBT treatment suggest a decrease in inhibition, consistent with these reports.
Alternatively, BPA/TBT could be targeting photoreceptor responses, causing a downstream effect on the postsynaptic bipolar cells which would alter b- and d-wave responses. Examining ERG a-wave response amplitudes and timing suggest this possibility (Cohen et al., 2025; Jensen et al., 2025). For example, exposure to 0.228 μg/L BPA at 3 dpf increased both b-wave and d-wave amplitudes. Photoreceptor a-wave amplitude was also increased in this BPA treatment group, suggesting a downstream effect. Similarly, exposure to 22.8 μg/L BPA at 7 dpf quickened both a-wave and d-wave implicit times. Exposure to the higher TBT concentration (0.4 μg/L) at 3 dpf similarly quickened b-wave and a-wave peak times. The lower TBT concentration (0.04 μg/L) at 7 dpf increased and delayed adult a-wave and d-wave responses. These similarities suggest that, for some of the outcomes measured, the change in bipolar cell responses may be indirect and downstream of a direct impact on photoreceptors. BPA exposure in zebrafish larvae altered red (L) and UV cone morphology and disrupted the retinal cone mosaic (Qiu et al., 2025), suggesting a direct effect of BPA on photoreceptors. Photoreceptor sensitivity to TBT is highly likely, given the localization of aromatase to the ONL in some vertebrate retinas (Valero-Ochando et al., 2024) and the identification of aromatase (cyp19a1b) in zebrafish retina (Cohen et al., 2025).ERG.
Taken together, the changes in ERG b- and d-wave responses in adult retinas from fish developmentally exposed to BPA or TBT could be due to EDC-induced changes in neurotransmitter release/levels within the retina.
8.3 BPA and TBT-induced changes in retinal function are due to a direct effect on retinal bipolar cells
Finally, both BPA and TBT are known to directly alter neuronal activity, suggesting that the ERG differences observed may reflect a direct effect of these EDCs on retinal bipolar cells. TBT application can increase calcium levels in isolated DRG neurons (Fross et al., 2021) and reduce Na+/K+ pump activity in brain (Zhang et al., 2008; Li et al., 2015). BPA inhibited L-, N-, and T-type Ca+2 channels in isolated GH3 cells and reduced L-type Ca+2 channel amplitude in cardiac myocytes (Deutschmann et al., 2013) and rat aortic smooth muscle (Feiteiro et al., 2018). These actions of BPA are due to the compound binding to and stabilizing calcium channels in the resting state (Deutschmann et al., 2013). BPA application increases the amplitude of BK potassium channels in AD 293 cells by binding to both intracellular and extracellular sites on the channel (Rottgen et al., 2014), and inhibits TTX-sensitive and TTX-insensitive Na+ channels in DRG neurons via PKA and PKC dependent pathways (Wang et al., 2011). Zebrafish bipolar cells express depolarization elicited calcium (T-type or L-type) and potassium (sustained IK or transient IA) channels (Connaughton and Maguire, 1998). Patch clamp analysis of adult zebrafish bipolar cells revealed BPA-induced effects (Cohen et al., 2025). Developmental BPA exposure reduced L-type Ca+2 current amplitude regardless of exposure age; whereas outward rectifying K+ currents (IK) were reduced when developmental BPA exposure occurred at 3 dpf but increased when exposure occurred at 7 dpf. These differences in voltage gated currents measured in tissue collected from adults at the later age were mostly observed in response to 22.8 μg/L BPA exposure, suggesting concentration-dependent effects, as reported in other preparations (Wang et al., 2011; Deutschmann et al., 2013; Feiteiro et al., 2018). In isolated pancreatic β-cells, concentration dependent effects of BPA on R-type Ca+2 channels were reported, with exposure to a low (1 nM) dose of BPA decreasing current amplitude (Villar-Pazos et al., 2017). In this preparation, BPA did not directly interact with the Ca+2 channel, but induced effects through differential activation of ERβ and ERα (Villar-Pazos et al., 2017).
Changes in voltage gated currents across a population of retinal cells would affect ERG responses. Increases in b-wave amplitude, for example, could reflect increased internal calcium levels, as reported to occur after TBT exposure (Fross et al., 2021). A reduced current through voltage-gated calcium channels coupled with a reduced outward rectifying K+ current would lower overall bipolar cell responses. Alternatively, a reduced calcium current and enhanced outward K+ current would reduce and delay neuronal responses. These latter options were observed in BPA treated adult retinas.
9 Translational implications and future directions
Endocrine disrupting compounds are a public health concern worldwide because of persistent exposure, diverse point sources, and the presence of significant low dose effects (Niehs, 2025). Two well-known EDCs, BPA and TBT, are of primary concern and were the focus of this review. BPA is ubiquitous as it is still continually used for industrial and household products. The effect of BPA on animals is well-reported and recent reports show effects of early life BPA exposure in humans (Lin et al., 2023; Hyun and Ka, 2024; Triswindyaningrum et al., 2025). In contrast, while TBT use has been banned for almost 20 years, the slow degradation of this chemical coupled with recent increases in environmental concentrations and measurable levels in humans (Correia et al., 2025) keep TBT a chemical of concern.
Our goal was to review estrogen signaling in retina and identify how BPA and TBT exposure alter these pathways. These compounds have opposite effects on estrogen signaling pathways. Interestingly, it does not appear that exposure to these compounds cause opposite outcomes. Developmental exposure to either compound showed similar effects on adult retinal ERG responses, with enhancement of ON-bipolar cell b-wave responses observed for the lower concentrations used. While we did observe a few contrasting outcomes, they depended on age of exposure (i.e., IPL thickness) and/or concentration (i.e., d-wave responses). It is important to note that while environmentally relevant levels of both compounds were used, these levels were several orders of magnitude different from each other. Nonetheless, both compounds displayed developmental effects. While bath application of the compounds likely resulted in systemic differences, our focus on the retina identifies specific effects on neuronal signaling in this tissue. Current experiments are continuing to elucidate the long-term effects of these compounds by examining changes in gene expression, individual neuronal responses, and larval differences.
Overall, our findings suggest that the retina is extremely sensitive to EDCs, and that adverse outcomes due to BPA and/or TBT exposure should include analysis of long-term developmental effects focused on the visual system.
Statements
Author contributions
JJ: Conceptualization, Formal Analysis, Methodology, Writing – original draft, Writing – review and editing. LO: Conceptualization, Writing – original draft, Writing – review and editing. RH: Formal Analysis, Methodology, Writing – review and editing. PO: Formal Analysis, Methodology, Writing – review and editing. VC: Conceptualization, Data curation, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. The work was supported by NEI R15EY029866-01.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abreu F. Batista R. Castro I. Fillmann G. (2021). Legacy and emerging antifouling biocide residues in a tropical estuarine system (Espirito Santo state, SE, Brazil). Mar. Poll. Bull.166, 112255. 10.1016/j.marpolbul.2021.112255
2
Adeeko A. Li D. Forsyth D. Casey V. Cooke G. Barthelemy J. et al (2003). Effects of in utero tributyltin chloride exposure in the rat on pregnancy outcome. Toxicol. Sci.74, 407–415. 10.1093/toxsci/kfg131
3
Ahn C. Jeung E.-B. (2023). Endocrine-disrupting chemicals and disease endpoints. Int. J. Mol. Sci.24, 5342. 10.3390/ijms24065342
4
Al-Shatri M. Abdulmumin A. Basheer C. Al-Arfaj A. Al-Tawabini B. (2015). Assessment of tributyltin and triphenyltin compounds and their main degradation products in Saudi Coastal waters. Arab. J. Sci. Eng.40, 2959–2967. 10.1007/s13369-015-1673-2
5
Almeida S. Raposo A. Almeida-Gonzalez M. Carrascosa C. (2018). Bisphenol A: food exposure and impact on human health. Comp. Rev. Food Sci. Food Saf.17, 1503–1517. 10.1111/1541-4337.12388
6
Aoshima H. Hossain S. Imamura H. Shingai R. (2001). Effects of bisphenol A and its derivatives on the response of GABAA erceptors expressed in Xenopus oocytes. Biosci. Biotechnol. Biochem.65, 2070–2077. 10.1271/bbb.65.2070
7
Aviles E. Wang S. Patel S. Cordero S. Shi S. Lin L. et al (2025). ERG responses to high-frequency flickers require FAT3 signaling in mouse retinal bipolar cells. J. Gen. Physiol.157, e202413642. 10.1085/jgp.202413642
8
Baden T. Euler T. Berens P. (2020). Understanding the retinal basis of vision across species. Nat. Rev. Neurosci.21, 5–20. 10.1038/s41583-019-0242-1
9
Balshaw A. G. (2023). The role of estrogen receptors in anxiety disorders: an investigation in zebrafish (Doctoral dissertation, Stellenbosch: Stellenbosch University).
10
Bardet P.-L. Horard B. Robinson-Rechavi M. Laudet V. Vanacker J.-M. (2002). Characterization of oestrogen receptors in zebrafish (Danio rerio). J. Mol. Endocrinol.28, 153–163. 10.1677/jme.0.0280153
11
Baumann L. Ros A. Rehberger K. Neuhauss S. Segner H. (2016). Thyroid disruption in zebrafish (Danio rerio) larvae: different molecular response patterns lead to impaired eye development and visual functions. Aquat. Toxicol.172, 44–55. 10.1016/j.aquatox.2015.12.015
12
Belcher S. Zsarnovszky A. (2001). Estrogenic actions in the brain: estrogen, phytoestrogens, and rapid intracellular signaling mechanisms. J. Pharmacol. Exp. Ther.299, 408–414. 10.1016/S0022-3565(24)29245-4
13
Ben-Jonathan N. Steinmetz R. (1998). Xenoestrogens: the emerging story of bisphenol A. TEM9, 124–128. 10.1016/s1043-2760(98)00029-0
14
Bertram M. G. Gore A. C. Tyler C. R. Brodin T. (2022). Endocrine‐disrupting chemicals. Current Biology32 (13), R727–R730. 10.1016/j.cub.2022.05.063
15
Bondesson M. Hao R. Liu C.-Y. Williams C. Gustafsson J.-A. (2015). Estrogen receptor signaling during vertebrate development. Biochimica Biophysica Acta1849, 142–151. 10.1016/j.bbagrm.2014.06.005
16
Borges A. Oliver A.L.-S. Gallego-Gallegos M. Munoz-Olivas R. Vale M. Camara C. (2014). Transformation of tributyltin in zebrafish eleutheroembryos (Danio rerio). Biol. Trace Elem. Res162, 317–323. 10.1007/s12011-014-0144-z
17
Bouskine A. Nebout M. Brucker-Davis F. Benahmed M. Fenichel P. (2009). Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ. Health Perspect.117, 1053–1058. 10.1289/ehp.0800367
18
Branchek T. Bremiller R. (1984). The development of photoreceptors in the zebrafish, Brachydanio rerio. I. Structure. J. Comp. Neurol.224, 107–115. 10.1002/cne.902240109
19
Brent G. (2023). A historical reflection on scientific aadvances in understanding thyroid hormone action. Thyroid33, 1140–1149. 10.1089/thy.2022.0636
20
Brion F. Le Page Y. Piccini B. Cardoso O. Tong S.-K. Chung B.-C. et al (2012). Screening estrogenic activities of chemicals or mixtures in vivo using transgenic (cyp19a1b-GFP) zebrafish embryos. PLoS One7, e36069. 10.1371/journal.pone.0036069
21
Calaza K. Gardino P. De Mello F. (2006). Transporter mediated GABA release in the retina: role of excitatory amino acids and dopamine. Neurochem. Int.49, 769–777. 10.1016/j.neuint.2006.07.003
22
Callard G. Kruger A. Betka M. (1995). The goldfish as a model for studying neuroestrogen synthesis, localization, and action in the brain and visual system. Environ. Health Perspect.7, 51–57. 10.1289/ehp.95103s751
23
Canesi L. Fabbri E. (2015). Environmental effects of BPA: focus on aquatic species. Dose Response2015, 1–14. 10.1177/1559325815598304
24
Cano-Nicolau J. Vaillant C. Pellegrini E. Charlier T. Kah O. Coumailleau P. (2016). Estrogenic effects of several BPA analogs in the developing zebrafish brain. Front. Neurosci.10, 112. 10.3389/fnins.2016.00112
25
Cascio C. Deidda I. Russo D. Guarneri P. (2015). The estrogenic retina: the potential contribution to healthy aging and age-related neurodegenerative diseases in retina. Steroids103, 31–41. 10.1016/j.steroids.2015.08.002
26
Castro I. Machado F. De Sousa G. Paz-Villarraga C. Fillmann G. (2021). How protected are marine protected areas: a case study of tributyltin in Latin America. J. Environ. Manag.278, 111543. 10.1016/j.jenvman.2020.111543
27
Chapin R. Adams J. Boekelheide K. Gray J. Hayward S. Lees P. et al (2008). NTP-CERHR expret panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res. B Dev. Reprod. Toxicol.83, 157–395. 10.1002/bdrb.20147
28
Chapouton P. Skupien P. Hesl B. Coolen M. Moore J. Madelaine R. et al (2010). Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J. Neurosci.30, 7961–7974. 10.1523/JNEUROSCI.6170-09.2010
29
Chaychi S. Polosa A. Lachapelle P. (2015). Differences in retinal structure and function between aging male and female Sprague-Dawley rats are strongly influenced by estrus cycle. PLoS One10, e0136056. 10.1371/journal.pone.0136056
30
Cheng S. Yang J. (2023). A theoretical study of organotin binding in aromatase. Int. J. Mol. Sci.24, 8954. 10.3390/ijms24108954
31
Chiang E.-L. Yan Y.-L. Tong S.-K. Hsiao P.-H. Guigen Y. Postlethwait J. et al (2001). Characterization of duplicated zebrafish cyp19 genes. J. Exp. Zool.290, 709–714. 10.1002/jez.1121
32
Chiang T.-K. White K. Kurup S. Yu M. (2022). Use of visual electrophysiology to monitor retinal and optic nerve toxicity. Biomolecules12, 1390. 10.3390/biom12101390
33
Choi I.-S. Cho J.-H. Park E.-J. Park J.-W. Kim S.-H. Lee M.-G. et al (2007). Multiple effects of bisphenol A, and endocrine disruptor, on GABAA receptors in acutely dissociated rat CA3 pyramidal neurons. Neurosci. Res.59, 8–17. 10.1016/j.neures.2007.05.003
34
Chung E. Genco M. Megrelis L. Ruderman J. (2011). Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proc. Natl. Acad. Sci.108, 17732–17737. 10.1073/pnas.1115187108
35
Cohen A. Popowitz J. Delbridge-Perry M. Rowe C. Connaughton V. (2022). The role of estrogen and thyroid hormones in zebrafish visual system function. Front. Pharmacol.13, 837687. 10.3389/fphar.2022.837687
36
Cohen A. Sherffius A. Jensen J. Harris R. Burch E. Foster G. et al (2025). Loss of adult visual resonses by developmental BPA exposure is correlated with altered estrogenic signaling. Commun. Biol.8, 847. 10.1038/s42003-025-08245-y
37
Connaughton V. (2011). Bipolar cells in the zebrafish retina. Vis. Neurosci.28, 77–93. 10.1017/S0952523810000295
38
Connaughton V. Maguire G. (1998). Differential expression of voltage-gated K+ and Ca+2 currents in bipolar cells in the zebrafish retinal slice. Eur. J. Neurosci.10, 1350–1362. 10.1046/j.1460-9568.1998.00152.x
39
Connaughton V. Behar T. Liu W.-L. Massey S. (1999). Immunocytochemical localization of excitatory and inhibitory neurotransmitters in the zebrafish retina. Vis. Neurosci.16, 483–490. 10.1017/s0952523899163090
40
Constable P. Lim J. Thompson D. (2023). Retinal electrophysiology in central nervous system disorders. A review of human and mouse studies. Front. Neurosci.17, 1215097. 10.3389/fnins.2023.1215097
41
Cornish E. Vaze A. Jamieson R. Grigg J. (2021). The electroretinogram in the genomics era: outer retinal disorders. Eye35, 2406–2418. 10.1038/s41433-021-01659-y
42
Corrales J. Kristofco L. Steele W. Yates B. Breed C. Williams E. et al (2015). Global assessment of bisphenol A in the environment. Dose Response13, 1559325815598308. 10.1177/1559325815598308
43
Correia L. De Moraes T. Dos Santos Pereira A. De Aguiar G. De Barros Viana M. Robiero D. et al (2025). Tributyltin induces apoptosis in mammalian cells in vivo: a scoping review. Rev. Environ. Health40, 197–203. 10.1515/reveh-2023-0152
44
Crowley-Perry M. Barberio A. Zeino J. Winston E. Connaughton V. (2021). Zebrafish optomotor response and morphology are altered by transient, developmental exposure to bisphenol-A. J. Dev. Biol.9, 14. 10.3390/jdb9020014
45
Damiano A. Caioni G. D'addario C. Merola C. Francioso A. Amorena M. (2025). The invisible influence: can endocrine disruptors reshape behaviors across generations?Stresses5, 46. 10.3390/stresses5030046
46
Daubner S. Le T. Wang S. (2011). Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys.508, 1–12. 10.1016/j.abb.2010.12.017
47
De Coster S. Van Larebeke N. (2012). Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J. Env. Pub Health2012, 1–52. 10.1155/2012/713696
48
De Paulo D. Mariz Jr, C. De Melo Alves M. Alves R. Batista R. Fillmann G. et al (2020). Histological and behavioral toxicity of tributyltin in the tropical guppy Poecilia vivipara. Environ. Toxicol. Chem.39, 1953–1963. 10.1002/etc.4808
49
Dekant W. Volkel W. (2008). Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmentale exposures. Toxicol. Appl. Pharmacol.228, 114–134. 10.1016/j.taap.2007.12.008
50
Deutschmann A. Hans M. Meyer R. Haberlein H. Swandulla D. (2013). Bisphenol A inhibits voltage-activated Ca+2 channels in vitro: mechanisms and structural requirements. Mol. Pharmacol.83, 501–511. 10.1124/mol.112.081372
51
Dickerson S. Cunningham S. Patisaul H. Woller M. Gore A. (2011). Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology152, 581–594. 10.1210/en.2010-1103
52
Dong W. Muramoto W. Nagai Y. Takehana K. Stegeman J. Teraoka H. et al (2006). Retinal neuronal cells is a toxicological target of tributyltin in developing zebrafish. J. Vet. Med. Sci.68, 573–579. 10.1292/jvms.68.573
53
Dos Santos B. Ivantsova E. Guzman A. Martyniuk C. (2022). Critical review of the toxicity mechanisms of bisphenol F in zebrafish (Danio rerio): knowledge gaps and future directions. Chemosphere297, 134132. 10.1016/j.chemosphere.2022.134132
54
Duh-Leong C. Maffini M. Kassotis C. Vandenberg L. Trasande L. (2023). The regulation of endocrine-disrupting chemicals to minimize their impact on health. Nat. Rev. Endocrinol.19, 600–614. 10.1038/s41574-023-00872-x
55
Eisner A. Luoh S.-W. (2011). Breast cancer medications and vision: effects of treatments for early-stage disease. Curr. Eye Res.36, 867–885. 10.3109/02713683.2011.594202
56
Eisner A. Falardeau J. Toomey M. Vetto J. (2008). Retinal hemorrhages in anastrozole users. Optom. Vis. Sci.85, E301–E308. 10.1097/OPX.0b013e31816bea3b
57
el Hassani L. H. Frenich A. Vidal J. Muros M. Benjiba M. (2005). Study of the accumulation of tributyltin and triphenyltin compounds and their main metabolites in the sea bass, Dicentrachus labrax, under laboratory conditions. Sci. Total Environ.348, 191–198. 10.1016/j.scitotenv.2004.12.061
58
Feiteiro J. Mariana M. Gloria S. Cairrao E. (2018). Inhibition of L-type calcium channels by bisphenol A in rat aorta smooth muscle. J. Toxicol. Sci.43, 579–586. 10.2131/jts.43.579
59
Fent K. Meier W. (1992). Tributyltin-induced effects on early life stages of minnows Phoxinus phoxinus. Arch. Environ. Contam. Toxicol.22, 428–438. 10.1007/BF00212563
60
Fross S. Mansel C. Mccormick M. Vohra B. (2021). Tributyltin alters calcium levels, mitochondrial dynamics, and activates calpains within dorsal root ganglion neurons. Toxicol. Sci.180, 342–355. 10.1093/toxsci/kfaa193
61
Fujimoto N. Jinno N. Kitamura S. (2004). Activation of estrogen response element dependent transcription by thyroid hrmone with increase in estrogen receptor levels in a rat pituitary cell line, GH3. J. Endocrinol.181, 77–83. 10.1677/joe.0.1810077
62
Garg A. Meena R. Jadhav S. Bhosle N. (2011). Distribution of butyltins in the waters and sediments along the coast of India. Mar. Poll. Bull.62, 423–431. 10.1016/j.marpolbul.2010.12.003
63
Geens T. Aerts D. Berthot C. Bourguignon J. Goeyens L. Lecomte P. et al (2012). A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol.20, 3725–3740. 10.1016/j.fct.2012.07.059
64
Gelinas D. Callard G. (1993). Immunocytochemical and biochemical evidence for aromatase in neurons of the retina, optic tectum and retinotectal pathways in goldfish. J. Neuroendocrinol.5, 635–641. 10.1111/j.1365-2826.1993.tb00533.x
65
Gorelick D. Watson W. Halpern M. (2008). Androgen receptor gene expression in the developing and adult zebrafish brain. Dev. Dyn.237, 2987–2995. 10.1002/dvdy.21700
66
Gould C. Wiegand J. Connaughton V. (2017). Acute developmetnal exposure to 4-hydroxyandrostenedione has a long term effect on visually-guided behaviors. Neurotoxicol Teratol.64, 45–49. 10.1016/j.ntt.2017.10.003
67
Graw J. (2010). Eye development. Current topics in developmental biology, 90, 343–386. https://doi.org/10.1016/S0070-2153(10)90010-0
68
Gu J. Zhang J. Chen Y. Want H. Guo M. Wang L. et al (2019). Neurobehavioral effects of bisphenol S exposure in early life stages of zebrafish larvae (Danio rerio). Chemosphere217, 629–635. 10.1016/j.chemosphere.2018.10.218
69
Gui-Bin J. Qun-Fang Z. Di-Jing W. (2001). Occurrence of butyltin compounds in the waters of the selected lakes, rivers and costal environments from China. Environ. Poll.115, 81–87. 10.1016/S0269-7491(01)00088-4
70
Gupta P. Johar K. Nagpal K. Vasavada A. (2005). Sex hormone receptors in the human eye. Surv. Ophthalmol50, 274–284. 10.1016/j.survophthal.2005.02.005
71
Hamad A. Kluk M. Fox J. Park M. Turner J. (2007). The effects of aromatase inhibitors and selective estrogen receptor modulators on eye development in the zebrafish (Danio rerio). Curr. Eye Res.32, 819–827. 10.1080/02713680701573712
72
Hano T. Oshima Y. Kim S. Satone H. Oba Y. Kitano T. et al (2007). Tributyltin causes abnormal development in embryos of medaka, Oryzias latipes. Chemosphere69, 927–933. 10.1016/j.chemosphere.2007.05.093
73
Hao R. Bondesson M. Singh A. Riu A. Mccollum C. Knudsen T. et al (2013). Identification of estrogen target genes during zebrafish embryonic development through transcriptomic analysis. PLoS One8, e79020. 10.1371/journal.pone.0079020
74
Hayashi L. Sheth M. Young A. Kruger M. Wayman G. Coffin A. (2015). The effect of the aquatic contaminants bisphenol-A and PCB-95 on the zebrafish lateral line. Neurotoxicol46, 125–136. 10.1016/j.neuro.2014.12.010
75
Heavner W. Pevny L. (2012). Eye development and retinogenesis. Cold Spring Harb. Perspect. Biol.4, a008391. 10.1101/cshperspect.a008391
76
Hirasawa J. Miwa N. Watanabe S.-I. (2021). GABAergic and glycinergic systems regulate ON-OFF electroretinogram by cooperatively modulating cone pathways in the amphibian retina. Eur. J. Neurosci.53, 1428–1440. 10.1111/ejn.15054
77
Hoch M. (2001). Organotin compounds in the environment - an overview. Appl. Geochem16, 719–743. 10.1016/s0883-2927(00)00067-6
78
Horiguchi T. (2006). Masculinization of female gastropod mollusks induced by organotin compounds, focusing on mechanisms of action of tributyltin and triphenyltin for development of imposex. Environ. Sci.13, 77–87.
79
Hyun S.-A. Ka M. (2024). Bisphenol A (BPA) and neurological disorders: an overview. Int. J. Biochem. Cell Biol.173, 106614. 10.1016/j.biocel.2024.106614
80
Ishido M. Yonemoto J. Morita M. (2007). Mesencephalic neurodegeneration in the orally administered bisphenol A-caused hyperactive rats. Toxicol. Lett.173, 66–72. 10.1016/j.toxlet.2007.06.014
81
Jasarevic E. Sieli P. Twellman E. Welsh T. Jr Schachtman T. Roberts R. et al (2011). Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc. Natl. Acad. Sci.108, 11715–11720.
82
Jayasinghe B. Volz D. (2012). Abberant ligand-induced activation of G-protein couopled estrogen receptor 1 (GPER) results in developmental malformations during vertebrate embryogenesis. Toxicol. Sci.125, 262–273. 10.1093/toxsci/kfr269
83
Jensen J. Owrang P. Sherffius A. Selby C. Fleming N. Ouellette L. et al (2025). Early life tributyltin exposure has long term physiological effects on the zebrafish (Danio rerio) visual system. Comp. Biochem. Physiol. C301, 110416. 10.1016/j.cbpc.2025.110416
84
Jiang M. Xueyun M. Zhao Q. Li Y. Xing Y. Deng Q. et al (2019). The neuroprotective effecs of novel estrogen receptor GPER1 in mouse retinal ganglion cell degeneration. Exp. Eye Res.189, 107826. 10.1016/j.exer.2019.107826
85
Kahn L. Philippat C. Nakayama S. Slama R. Trasande L. (2020). Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol.8, 703–718. 10.1016/S2213-8587(20)30129-7
86
Kang H. Park S. Kim D. Choi Y.-H. (2024). Exposure to per- and polyfluoroalkyl substances and age-related macular degeneration in the U.S. middle-aged and older adults. Chemosphere364, 143167. 10.1016/j.chemosphere.2024.143167
87
Kim M. Park H. Gong E. Choi S. Kim H. Lee J. (2011). Exposure to bisphenol A appears to impair hippocampal neurogenesis and spatial learning and memory. Food Chem. Toxicol.49, 3383–3389. 10.1016/j.fct.2011.09.017
88
Kim J. Kim C.-Y. Oh H. Ryu B. Kim U. Lee J. et al (2019). Trimethyltin chloride induces reactive oxygen species-mediated apoptosis in retinal cells during zebrafish eye development. Sci. Total Environ.653, 36–44. 10.1016/j.scitotenv.2018.10.317
89
Kim S. Hwang K.-S. Yang J. Chae J. Kim G. Kan H. et al (2020). Neurochemical and behavioral analysis by acute exposure to bisphenol A in zebrafish larvae model. Chemosphere239, 124751. 10.1016/j.chemosphere.2019.124751
90
Kim J.-S. Ryu B. Bang J. Kim C.-Y. Park J.-H. (2023). Postnatal exposure to trimethyltin chloride induces retinal developmental neurotoxicity in mice via glutamate and its transporter related changes. Reprod. Toxicol.119, 108395. 10.1016/j.reprotox.2023.108395
91
Kimmel C. Ballard W. Kimmel S. Ullmann B. Schilling T. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn.203, 253–310. 10.1002/aja.1002030302
92
Kinch C. Ibhazehiebo K. Jeong J.-H. Habibi H. Kurrasch D. (2015). Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc. Natl. Acad. Sci.112, 1475–1480. 10.1073/pnas.1417731112
93
Kishida M. Mclellan M. Miranda J. Callard G. (2001). Estrogen and xenoestrogens upregulate the brain aromatase isoform (P450aromB) and perturb markers of early development in zebrafish (Danio rerio). Comp. Biochem. Physiol. B129, 261–268. 10.1016/s1096-4959(01)00319-0
94
Kolpin D. W. Furlong E. T. Meyer M. T. Thurman E. M. Zaugg S. D. Barber L. B. Buxton H. T. (2002). Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999– 2000: A national reconnaissance. Environmental science & technology. 36(6), 1202–1211. https://doi.org/10.1021/es011055j
95
Konno N. Tsunoda M. Sugita-Konishi Y. (2005). Effect of tributyltin compound on N-methyl-D-aspartate (NMDA) receptors in brain of preweanling mice. Environ. Health Prev. Med.10, 335–337. 10.1007/BF02898194
96
Kremer H. Lilienthal H. Hany J. Roth-Harer A. Winneke G. (1999). Sex-dependent effects of maternal PCB exposure on the electroretinogram in adult rats. Neurotoxicol Teratol.21, 13–19. 10.1016/s0892-0362(98)00030-0
97
Kumar M. Sarma D. Subham S. Kumawat M. Verma V. Prakash A. et al (2020). Environmental endocrine-disrupting chemical exposure: role in non-communicable diseases. Front. Public Health8, 553850. 10.3389/fpubh.2020.553850
98
Lagarde F. Beausoleil C. Belcher S. Belzunces L. Emond C. Guerget M. et al (2015). Non-monotonic dose-response relationships and endocrine disruptors: a qualitaqtive method of assessment. Environ. Health14, 13. 10.1186/1476-069X-14-13
99
Lassiter C. Kelley B. Linney E. (2002). Genomic structure and embryonic expression of estrogen receptor beta a (ERBa) in zebrafish (Danio rerio). Gene299, 141–151. 10.1016/s0378-1119(02)01050-8
100
Le Page Y. Vosges M. Servili A. Brion F. Kah O. (2011). Neuroendocrine effects of endocrine disruptors in teleost fish. J. Toxicol. Env. Health B14, 370–386. 10.1080/10937404.2011.578558
101
Lemmen J. Broekhof J. Kuiper G. Gustafsson J.-A. Van Der Saag P. Van Der Burg B. (1999). Expression of estrogen receptor alpha and beta during mouse embryogenesis. Mech. Dev.81, 163–167. 10.1016/s0925-4773(98)00223-8
102
Leslie C. Walkowski W. Rosencrans R. Gordon W. Bazan N. Ryan M. et al (2021). Estrogenic modulation of retinal sensitivity in reproductive female Tungara frogs. Integr. Comp. Biol.61, 231–239. 10.1093/icb/icab032
103
Li P. Li Z.-H. (2020a). Tributyltin induces the tissue-specific stresses in zebrafish, a study in various tissues of muscle, gill and intestine. Bull. Environ. Contamin Toxicol.105, 847–852. 10.1007/s00128-020-03048-9
104
Li P. Li Z.-H. (2020b). Toxicity evaluation of triphenyltin in zebrafish larvae by embryonic malformation, retinal development and GH/IGF axis. Fish. Physiol. Biochem.46, 2101–2107. 10.1007/s10695-020-00861-1
105
Li Z.-H. Li P. (2021). Effects of the trybutyltin on the blood parameters, immune responses and thyroid hormone system in zebrafish. Environ. Pollut.268, 115707. 10.1016/j.envpol.2020.115707
106
Li Z.-H. Li P. Shi Z.-C. (2015). Chronic exposure to tributyltin induces brain functional damage in juvenile common carp (Cyprinus carpio). PLoS One10, e0123091. 10.1371/journal.pone.0123091
107
Li X. Sumn M.-Z. Li X. Zhang S.-H. Dai L.-T. Liu X.-Y. et al (2017). Impact of low-dose chronic exposure to bisphenol A (BPA) on adult male zebrafish adaptation to the environmental complexity: disturbing the color preference patterns and reliving the anxiety behavior. Chemosphere186, 295–304.
108
Li Z.-M. Hernandez-Moreno D. Main K. Skakkebaek N. Kiviranta H. Toppari J. et al (2018). Association of in utero persistent organic pollutant exposure with placental thyroid hormones. Endocrinol159, 3473–3481. 10.1210/en.2018-00542
109
Liu Z.-H. Li Y.-W. Hu W. Chen Q.-L. Shen Y.-J. (2020). Mechanisms involved in tributyltin-enhanced aggressive behaviors and fear responses in male zebrafish. Aquat. Toxicol.220, 105408. 10.1016/j.aquatox.2020.105408
110
Lim W. Nguyen N. Yang H. Scanlan T. Furlow J. (2002). A thyroid hormone antagonist that inhibits thyroid hormone action in vivo. J. Biol. Chem.277, 35664–35670. 10.1074/jbc.M205608200
111
Lin M.-H. Lee C.-Y. Chuang Y.-S. Shih C.-L. (2023). Exposure to bisphenol A associated with multiple health-related outcomes in humans: an umbrella review of systematic reviews with meta-analysis. Environ. Res.237, 116900. 10.1016/j.envres.2023.116900
112
Lindenau W. Kuhrt H. Ulbricht E. Kormer K. Bringmann A. Reichenbach A. (2019). Cone-to-Muller cell ratio in the mammalian retina: a survey of seven mammals with different lifestyle. Exp. Eye Res.181, 38–48. 10.1016/j.exer.2019.01.012
113
Liu X. Zhu P. Sham K. Yuen J. Xie C. Zhang Y. et al (2009). Identification of a membrane estrogen receptor in zebrafish with homology to mammalian GPER and its high expression in early germ cells of the testis. Biol Reprod80, 1253–1261. 10.1095/biolreprod.108.070250
114
Liu W.-L. Zhang X. Wei P. Tian H. Wang W. Ru S. (2018). Long-term exposure to bisphenol S damages the visual system and reduces the tracking capability of male zebrafish (Danio rerio). J. Appl. Toxicol.38, 248–258. 10.1002/jat.3519
115
Liu H. Jiang W. Ye Y. Yang B. Shen X. Lu S. et al (2021). Maternal exposure to tributyltin during early gestation increases adverse pregnancy outcomes by impairing placental development. Environ. Toxicol.36, 1303–1315. 10.1002/tox.23127
116
Luu-The V. (2013). Assessment of steroidogenesis and steroidogenic enzyme functions. J. Steriod Biochem. Molec Biol.117, 176–182. 10.1016/j.jsbmb.2013.05.017
117
Lyssimachou A. Jenssen B. Arukwe A. (2006). Brain cytochrome P450 aromatase gene isoforms and activity levels in Atlantic Salmon after waterborne exposure to nominal environmental concentrations of the pharmaceutical ethynylestradiol and antifoulant tributylin. Toxicol. Sci.91, 82–92. 10.1093/toxsci/kfj136
118
Ma R. Sassoon D. (2006). PCBs exert an estrogenic effect through repression of the Wnt7a signaling pathway in the female reproductive tract. Environ. Health Perspect.114, 898–904. 10.1289/ehp.8748
119
Mangiamele L. Gomez J. Curtis N. Thompson R. (2017). GPER/GPR30, a membrane estrogen receptor, is expressed in the brain and retina of a social fish (Carassius auratus) and colocalizes with isotocin. J. Comp. Neurol.525, 252–270. 10.1002/cne.24056
120
Marc R. Cameron D. (2002). A molecular phenotype atlas of the zebrafish retina. J. Neurocytol.30, 593–654. 10.1023/a:1016516818393
121
Martinez R. Herrero-Nogareda L. Van Antro M. Campos M. Casado M. Barata C. et al (2019). Morphometric signatures of exposure to endocrine disrupting chemicals in zebrafish eleutheroembryos. Aquat. Toxicol.214, 105232. 10.1016/j.aquatox.2019.105232
122
Martinez R. Codina A. Barata C. Tauler R. Pina B. Navarro-Martin L. (2020). Transcriptomic effects of tributyltin (TBT) in zebrafish eleutheroembryos. A functional benchmark dose analysis. J. Hazard Mat.398, 122881. 10.1016/j.jhazmat.2020.122881
123
Matthiessen P. Gibbs P. (1998). Critical appraisal of the evidence for tributyltin-mediated endocrine disruption in mollusks. Environ. Toxicol. Chem.17, 37–43. 10.1897/1551-5028(1998)017<0037:caotef>2.3.co;2
124
Mcallister B. Kime D. (2003). Early life exposure to environmental levels of the aromatase inhibitor tributyltin causes masculinisation and irreversible sperm damage in zebrafish (Danio rerio). Aquat. Toxicol.65, 309–316. 10.1016/s0166-445x(03)00154-1
125
Mcginnis C. Crivello J. (2011). Elucidating the mechanisms of action of tributyltin (TBT) in zebrafish. Aquat. Toxicol.103, 25–31. 10.1016/j.aquatox.2011.01.005
126
Mcmahon D. (1994). Modulation of electrical synaptic transmission in zebrafish retinal horizontal cells. J. Neurosci.14, 1722–1734. 10.1523/JNEUROSCI.14-03-01722
127
Medina Arellano A. Albert-Garay J. Huerta N. Hernandez K. Ruiz-Cruz M. Ochoa-De La Paz L. (2025). From neurotransmission to retinal pathophysiology: unraveling the role of GABA receptors in retinal disease progression. J. Neurochem.169, e70198. 10.1111/jnc.70198
128
Meeker J. (2012). Exposure to environmental endocrine disruptors and child development. Arch. Pediatr. Adolesc. Med.166, 952–958.
129
Menuet A. Pellegrini E. Anglade I. Blaise O. Laudet V. Kah O. et al (2002). Molecular characterization of three estrogen receptor forms in zebrafish: binding characteristics, transactivation properties, and tissue distributions. Biol Reprod66, 1881–1892. 10.1095/biolreprod66.6.1881
130
Menuet A. Le Page Y. Torres O. Kern L. Kah O. Pakdel F. (2004). Analysis of the estrogen regulation of the zebrafish estrogen receptor (ER) reveals distinct effects of ERa, ERb1, and ERb2. J. Mol. Endocrinol.32, 975–986. 10.1677/jme.0.0320975
131
Menuet A. Pelligrini E. Brion F. Gueguen M.-M. Anglade I. Pakdel F. et al (2005). Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J. Comp. Neurol.485, 304–320. 10.1002/cne.20497
132
Mericskay M. Carta L. Sassoon D. (2005). Diethylstilbesterol exposure in utero: a paradigm for mechanisms leading toa dult disease. Birth Defects Res. A73, 133–135. 10.1002/bdra.20121
133
Mitra S. Gera R. Siddiqui W. Khandelwal S. (2013). Tributyltin induces oxidative damage, inflammation and apoptosis via disturbance of blood-brain barrier and metal homeostasis in cerebral cortex of rat brain: an in vivo and in vitro study. Toxicol310, 39–52. 10.1016/j.tox.2013.05.011
134
Mitra S. Siddiqui W. Khandelwal S. (2015). C-phycocyanin protects against acute tributyltin chloride neurotoxicity by modulatin glial cell activity along with its anti-oxidant and anti-inflammatory property: a comparative efficacy evaluation with N-acetyl cysteine in adult rat brain. Chemico-Biol Interact.238, 138–150. 10.1016/j.cbi.2015.06.016
135
Moreira L. Castro I. Fillmann G. Peres T. Cavalcante Belmino I. Sasaki S. et al (2021). Dredging impacts on the toxicity and development of sediment quality values in a semi-arid region (Ceara state, NE Brazil). Environ. Res.193, 110525.
136
Moschos M. Chatziralli I. Zagouri F. Zografos G. (2012). Macular oedema due to letrozole: a first case report. Clin. Exp. Optom.95, 646–650. 10.1111/j.1444-0938.2012.00771.x
137
Mouriec K. Gueguen M.-M. Manuel C. Percevault F. Thieulant M.-L. Pakdel F. et al (2009a). Androgens upregulate cyp19a1b (Aromatase B) gene expression in the brain of zebrafish (Danio rerio) through estrogen receptors. Biol Reprod80, 889–896. 10.1095/biolreprod.108.073643
138
Mouriec K. Lareyre J. Tong S.-K. Le Page Y. Vaillant C. Pelligrini E. et al (2009b). Early regulation of brain aromatase (cyp19a1b) by estrogen receptors during zebrafish development. Dev. Dyn.238, 2641–2651. 10.1002/dvdy.22069
139
Mustafi D. Engel A. Palczewski K. (2009). Structure of cone photoreceptos. Progr Ret Eye Res.28, 289–302. 10.1016/j.preteyeres.2009.05.003
140
Mustieles V. Perez-Lobato R. Olea N. Fernandez M. (2015). Bisphenol A: human exposure and neurobehavior. Neurotoxicol49, 174–187. 10.1016/j.neuro.2015.06.002
141
Muto A. Orger M. Wehman A. Smear M. Kay J. Page-Mccaw P. et al (2005). Forward genetic analysis of visual behavior in zebrafish. PLoS Genet.1, e66. 10.1371/journal.pgen.0010066
142
Nakamura K. Itoh K. Yaoi T. Fujiwara Y. Sugimoto T. Fushiki S. (2006). Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of bisphenol A. J. Neurosci. Res.84, 1197–1205. 10.1002/jnr.21020
143
Nazari E. Suja F. (2016). Effects of 17B-estradiol (E2) on aqueous organisms and its treatment problem: a review. Rev. Environ. Health31, 465–491. 10.1515/reveh-2016-0040
144
Nelson R. Singla N. (2009). A spectral model for signal elements isolated from zebrafish photopic electroretinogram. Vis. Neurosci.26, 349–363. 10.1017/S0952523809990113
145
Neuhauss S. (2003). Behavioral genetic approaches to visual system development and function in zebrafish. J. Neurobiol.54, 146–160. 10.1002/neu.10165
146
Niehs (2025). Endocrine disruptors. Natl. Inst. Environ. Health Sci. Available online at: https://www.niehs.nih.gov/health/topics/agents/endocrine (Accessed May 1, 2025).
147
Nuzzi R. Caselgrandi P. (2022). Sex hormones and their effects in ocular disorders and pathophysiology: current aspects and our experience. Int. J. Mol. Sci.23, 3269. 10.3390/ijms23063269
148
Nuzzi R. Scalabrin S. Becco A. Panzica G. (2018). Gonadal hormones and retinal disorders: a review. Front. Endocrinol.9, 66. 10.3389/fendo.2018.00066
149
Oliver A.L.-S. Sanz-Landaluze J. Munoz-Olivas R. Guinea J. Camara C. (2011). Zebrafish larvae as a model for the evaluation of inorganic arsenic and tributyltin bioconcentration. Water Res.45, 6515–6524. 10.1016/j.watres.2011.09.052
150
Orger M. Baier H. (2005). Channeling of red and green cone inputs to the zebrafish optomotor response. Vis. Neurosci.22, 275–281. 10.1017/S0952523805223039
151
Ortiz-Villanueva E. Jaumot J. Martinez R. Navarro-Martin L. Pina B. Tauler R. (2018). Assessment of endocrine disruptors effects on zebrafish (Danio rerio) embryos by untargeted LC-HRMS metabolomic analysis. Sci. Total Environ.635, 156–166. 10.1016/j.scitotenv.2018.03.369
152
Ozawa G. Bearse M. J. Harrison W. Bronson-Castain K. Schneck M. Barez S. et al (2014). Differences in neuroretinal function between adult males and females. Optom. Vis. Sci.91, 602–607. 10.1097/OPX.0000000000000255
153
Pelligrini E. Menuet A. Lethimonier C. Adrio F. Gueguen M.-M. Tascon C. et al (2005). Relationships between aromatase and estrogen receptors in the brain of teleost fish. Gen. Comp. Endocrinol.142, 60–66. 10.1016/j.ygcen.2004.12.003
154
Perlman I. (2007). The electroretinogram: ERG. Webvision: the organization of the retina and visual system [Internet]. Webvision The Organization Retina Visual System.
155
Phillips J. Westerfield M. (2014). Zebrafish models in translational research: tipping the scales toward advancements in human health. Dis. Models Mech.7, 739–743. 10.1242/dmm.015545
156
Pinon-Teal W. Ogilvie J. (2024). G protein-coupled estrogen receptor expression in postnatal developing mouse retina. Front. Ophthal4, 1331298. 10.3389/fopht.2024.1331298
157
Pironti C. Ricciardi M. Motta O. Miele Y. Pronto A. Montano L. (2021). Microplastics in the environment: intake through the food web, human exposure and toxicological effects. Toxics9, 224. 10.3390/toxics9090224
158
Prossnitz E. Barton M. (2014). Estrogen biology: new insights into GPER function and clinical opportunities. Mol. Cell Endocrinol.389, 71–83. 10.1016/j.mce.2014.02.002
159
Puri M. Gandhi K. Kumar M. (2023). A global overview of endocrine disrupting chemicals in the environment: occurrence, effects, and treatment methods. Int. J. Environ. Sci. Technol.20, 12875–12902. 10.1007/s13762-022-04636-4
160
Qin X. Lin H. Cao Y. Wu R. Lai K. Kong R. (2023). Embryo developmental toxicity in marine medaka (Oryzias melastigma) due to parental and embryonic 17alpha-ethinylestradiol exposure. Sci. Total Environ.861, 160594. 10.1016/j.scitotenv.2022.160594
161
Qiu W. Zhao Y. Yang M. Farajzadeh M. Pan C. Wayne N. (2016). Actions of bisphenol A and bisphenol S on the reproductive neuroendocrine system during early development in zebrafish. Endocrinology157, 636–647. 10.1210/en.2015-1785
162
Qiu L. Wei S. Yang Y. Zhang R. Ru S. Zhang X. (2023). Mechanism of bisphenol S exposure on color sensitivity of zebrafish larvae. Environ. Poll316, 120670. 10.1016/j.envpol.2022.120670
163
Qiu L. Yu P. Li Q. Wen C. Wang H. Zhao D. et al (2025). Comparative the effect of bisphenol A and bisphenol S on the development and spectral sensitivity of cone photoreceptors in zebrafish larvae (Danio rerio). Sci. Total Environ.290, 117737. 10.1016/j.ecoenv.2025.117737
164
Rantakokko P. Main K. Wohlfart-Veje C. Kiviranta H. Airaksinen R. Vartiainen T. et al (2013). Association of placenta organotin concentrations with congenital cryptorchidism and reproductive hormone levels in 280 newborn boys from Denmark and Finland. Hum. Reprod.28, 1647–1660. 10.1093/humrep/det040
165
Rantakokko P. Main K. Wohlfart-Veje C. Kiviranta H. Airaksinen R. Vartiainen T. et al (2014). Association of placental organotin concentrations with growth and ponderal index in 110 newborn boys from Finland during the first 18 months of life: a cohort study. Environ. Health13, 45. 10.1186/1476-069X-13-45
166
Richter C. Taylor J. Ruhlen R. Welshons W. Vom Saal F. (2007). Estradiol and bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ. Health Perspect.115, 902–908. 10.1289/ehp.9804
167
Roberts M. M S. D F. G D. E. T R. (2006). Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc. Natl. Acad. Sci.103, 6218–6223.
168
Robinson J. Schmitt E. Harosi F. Reece R. Dowling J. (1993). Zebrafish ultraviolet visual pigment: absorption spectrum, sequence, and localization. Proc. Natl. Acad. Sci.90, 6009–6012. 10.1073/pnas.90.13.6009
169
Rochester J. (2013). Bisphenol A and human health: a review of the literature. Reprod. Toxicol.42, 132–155. 10.1016/j.reprotox.2013.08.008
170
Rodrigues-Pereira P. Andrade M. Santos-Silva A. Teixeira M. Soares P. Graceli J. et al (2022). Subacute and low-dose tributyltin exposure disturbs the mammalian hypothalamus-pituitary-thyroid axis in a sex-dependent manner. Comp. Biochem. Physiol. C254, 109279. 10.1016/j.cbpc.2022.109279
171
Rodriguez-Ramirez K. Galindo-Romero C. Lucas-Ruiz F. Vidal-Sanz M. Agudo-Barriuso M. (2025). Neuroprotection of retinal ganglion cells by agonism of the beta but not the alpha oestrogen receptor in the axotomized retina of male and female mice. Acta Ophthalmol.103, 571–579. 10.1111/aos.17454
172
Rottgen T. Fancher I. Asano S. Widlanski T. Dick G. (2014). Bisphenol A activates BK channels through effects on alpha and beta 1 subunits. Channels8, 249–257. 10.4161/chan.27709
173
Rouleau C. Xiong Z.-H. Pacepavicius G. Huang G.-L. (2003). Uptake of waterborne tributyltin in the brain of fish: axonal transport as the proposed mechanism. Environ. Sci. Technol.37, 3298–3302. 10.1021/es020984n
174
Rowe A. Velasquez M. Aumeier J. Reyes S. Yee T. Nettesheim E. et al (2025). Female sex hormones exacerbate retinal neurodegeneration. Sci. Adv.11, eadr6211. 10.1126/sciadv.adr6211
175
Rubin B. (2011). Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J. Steriod Biochem. Molec Biol.127, 27–34. 10.1016/j.jsbmb.2011.05.002
176
Rustom N. Reynolds J. (2025). Develomental polychlorinated biphenyl mixture exposure promotes selective neural alterations: an immunohistochemical study in adult rat offspring. J. Dev. Orig. Health Dis.16, 1–22. 10.1017/S2040174425100329
177
Saili K. Corvi M. Weber D. Patel A. Das S. Przybyla J. et al (2012). Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicol291, 83–92. 10.1016/j.tox.2011.11.001
178
Salyer D. Lund T. Fleming D. Lephart E. Horvath T. (2001). Sexual dimorphism and aromatase in the rat retina. Dev. Brain Res.126, 131–136. 10.1016/s0165-3806(00)00147-4
179
Santangeli S. Maradonna F. Gioacchini G. Cobellis G. Piccinetti C. Valle L. et al (2016). BPA-induced deregulation of epigenetic patterns: effects on female zebrafish reproduction. Sci. Rep.6, 21982. 10.1038/srep21982
180
Santos M. Micael J. Carvalho A. Morabito R. Booy P. Massanisso P. et al (2006). Estrogens counteract the masculinizing effect of tributyltin in zebrafish. Comp. Biochem. Physiol. C142, 151–155. 10.1016/j.cbpc.2005.11.014
181
Sawyer S. Gerstner K. Callard G. (2006). Real-time PCR analysis of cytochrome P450 aromatase expression in zebrafish: gene specific tissue distribution, sex differences, developmental programming, and estrogen regulation. Gen. Comp. Endocrinol.147, 108–117. 10.1016/j.ygcen.2005.12.010
182
Schmidt K. Steinberg C. Pflugmacher S. Staaks G. (2004). Xenobiotic substances such as PCB mixtures (Aroclor 1254) and TBT can influence swimming behavior and biotransformation activity (GST) of carp (Cyprinis carpio). Environ. Toxicol.19, 460–470. 10.1002/tox.20051
183
Schmidt K. Staaks G. Pflugmacher S. Steinberg C. (2005). Impact of PCB mixture (Aroclor 1254) and TBT and a mixture of both on swimming behavior and body growth ane enzymatic biotransformation activities (GST) of young carp (Cyprinis carpio). Aquat. Toxicol.71, 49–59. 10.1016/j.aquatox.2004.10.012
184
Schmitt E. Dowling J. (1994). Early eye morphogenesis in the zebrafish, Brachydanio rerio. J. Comp. Neurol.344, 532–542. 10.1002/cne.903440404
185
Schmitt E. Dowling J. (1999). Early retinal development in the zebrafish, Danio rerio: Ligth and electron microscopic analysis. J. Comp. Neurol.404, 515–536. 10.1002/(SICI)1096-9861(19990222)404:4<515::AID-CNE8>3.0.CO;2-A
186
Sharan S. Nikhil K. Roy P. (2014). Disruption of thyroid hormone functions by low dose exposure of tributyltin: an in vitro and in vivo approach. Gen. Comp. Endocrinol.206, 155–165. 10.1016/j.ygcen.2014.07.027
187
Shi Y. Liu X. Zhu P. Li J. Sham K. Cheng S. et al (2013). G-protein-coupled estrogen receptor1 is involved in brain development during zebrafish (Danio rerio) embryogenesis. Biochem. Biophys. Res. Comm.435, 21–27. 10.1016/j.bbrc.2013.03.130
188
Shi Y. Chen C. Li M. Liu L. Dong K. Chen K. et al (2021). Oral exposure to tributyltin induced behavioral abnormalities and oxidative stress in the eyes and brains of juvenile Japanese medaka (Oryzias latipes). Antioxidants10, 1647. 10.3390/antiox10111647
189
Shi Z. Xiao S. Zhang Q. (2025). Interference with systematic negative feedback as a potential mechanism for nonmonotonic dose-responses of endocrine-disrupting chemicals. Toxicol. Sci.206, 354–372. 10.1093/toxsci/kfaf060
190
Simpson E. Clyne C. Rubin G. Boon W. Robertson K. Britt K. et al (2002). Aromatase - a brief overview. Ann. Rev. Physiol.64, 93–127. 10.1146/annurev.physiol.64.081601.142703
191
Soffker M. Tyler C. (2012). Endocrine disrupting chemicals and sexual behaviors in fish - a critical review on effects and possible consequences. Crit. Rev. Toxicol.42, 653–668. 10.3109/10408444.2012.692114
192
Sousa A. Pastorinho M. Takahashi S. Tanabe S. (2014). History on organotin compounds, from snails to humans. Environ. Chem. Lett.12, 117–137. 10.1007/s10311-013-0449-8
193
Stuermer C. (1988). Retinotopic organizaiton of the developing retinotectal projection in the zebrafish embryo. J. Neurosci.8, 4513–4530. 10.1523/JNEUROSCI.08-12-04513
194
Suzuki S. Bleckert A. Williams P. Takechi M. Kawamura S. Wong R. (2013). Cone photoreceptor types in zebrafish are generated by symmetric terminal divisions of dedicated precursors. Proc. Natl. Acad. Sci.110, 15109–15114. 10.1073/pnas.1303551110
195
Tawarayama H. Uchida K. Hasegawa H. Yoshida M. Inoue-Yanagimachi M. Sato W. et al (2024). Estrogen, via ESR2 receptor, prevents oxidative stress-induced Muller cell death and stimulates FGF2 production independently of NRF2, attenuating retinal degeneration. Exp. Eye Res.248, 110103. 10.1016/j.exer.2024.110103
196
Tingaud-Sequeira A. Andre M. Forgue J. Barthe C. Babin P. (2004). Expression patterns of three estrogen receptor genes during zebrafish (Danio rerio) development: evidence for high expression in neuromasts. Gene Expr. Patterns4, 561–568. 10.1016/j.modgep.2004.02.002
197
Triswindyaningrum O. Sulistiyani S. Darundiati Y. (2025). Systematic review: impact of bisphenol-A (BPA) exposure on human health. J. Environ. Health17, 85–98. 10.20473/jkl.v17i1.2025.85-98
198
Tse W. Yeung B. Wan H. Wong C. (2013). Early embryogenesis in zebrafish is affected by bisphenol A exposure. Biol. Open2, 466–471. 10.1242/bio.20134283
199
Tsunoda M. Aizawa Y. Konno N. Kimura K. Sugita-Konishi Y. (2006). Subacute administration of tributyltin chloride modulates neurotransmitters and their metabolites in discrete brain regions of maternal mice and their F1 offspring. Toxicol. Ind. Health22, 15–25. 10.1191/0748233706th240oa
200
Tu X. Li Y.-W. Chen Q.-L. Shen Y.-J. Liu Z.-H. (2020). Tributyltin enhanced anxiety of adult male zebrafish through elevating cortisol level and disruption of serotonin, dopamine and gamma-aminobutyric acid neurotransmitter pathways. Ecotoxicol. Environ. Saf.203, 111014. 10.1016/j.ecoenv.2020.111014
201
Uc-Peraza R. Castro I. Fillmann G. (2022). An absurd scenario in 2021: banned TBT-based antifouling products still available on the market. Sci. Total Environ.805, 150377. 10.1016/j.scitotenv.2021.150377
202
Ulbrich B. Stahlmann R. (2004). Developmental toxicity of polychlorinated biphenyls (PCBs): a systematic review of experimental data. Arch. Toxicol.78, 252–268. 10.1007/s00204-003-0519-y
203
Ulhaq Z. Kishida M. (2026). Estrogen produced in the retina by brain aromatase plays a significant role in zebrafish eye development. BBA - Gene Regul. Mech.1869, 195133. 10.1016/j.bbagrm.2025.195133
204
Valero-Ochando J. Canto A. Lopez-Pedrajas R. Almansa I. Miranda M. (2024). Role of gonadal steroid hormones in the eye: therapeutic implications. Biomolecules14, 1262. 10.3390/biom14101262
205
Vandenberg L. N. (2014). Non-monotonic dose reponses in studies of endocrine disrupting chemicals: bisphenol A as a case study. Dose Response12, 259–276. 10.2203/dose-response.13-020.Vandenberg
206
Vandenberg L. N. (2022). Low dose effects and nonmonotonic dose responses for endocrine disruptors, In Endocrine disruption and human health, 141–163. Academic Press.
207
Vandenberg L. N. Hauser R. Marcus M. Olea N. Welshons W. (2007). Human exposure to bisphenol A (BPA). Reprod. Toxicol.24, 139–177. 10.1016/j.reprotox.2007.07.010
208
Vandenberg L. Maffini M. Sonnenschein C. Rubin B. Soto A. (2009). Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr. Rev.30, 75–95. 10.1210/er.2008-0021
209
Vandenberg L. Chahout B. Heindel J. Padmanabhan V. Paumgartten F. Schoenfelder G. (2010). Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect.118, 1055–1070. 10.1289/ehp.0901716
210
Vandenberg L. Colborn T. Hayes T. Heindel J. Jacobs J. Lee D.-H. et al (2012). Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev.33, 378–455. 10.1210/er.2011-1050
211
Villar-Pazos S. Martinez-Pinna J. Castellano-Munoz M. Alonso-Magdalena P. Marroqui L. Quesada I. et al (2017). Molecular mechanisms involved in the non-monotonic effect of bisphenol-a on Ca+2 entry in mouse pancreatic B-cells. Sci. Repts7, 11770. 10.1038/s41598-017-11995-3
212
Volz S. Poulsen R. Hansen M. Holbech J. (2024). Bisphenol A alters retinal morphology, visually guided behavior, and thyroid hormone levels in zebrafish larvae. Chemosphere348, 140776. 10.1016/j.chemosphere.2023.140776
213
Vosges M. Le Page Y. Chung B.-C. Combarnous Y. Porcher J.-M. Kah O. et al (2010). 17α−ethinylestradiol disrupts the ontogeny of the forebrain GnRH system and the expression of brain aromatase during early development of zebrafish. Aquat. Toxicol.99, 479–491. 10.1016/j.aquatox.2010.06.009
214
Wang W.-Y. Huang B.-Q. (1999). TBT (tributyltin) toxicity to the visual and olfactory functions of tigerperch (Terapon jarbua Forsskal). Zool. Stud.38, 189–195.
215
Wang Q. Cao J. Zhu Q. Luan C. Chen X. Yi X. et al (2011). Inhibition of voltage-gated sodium channels by bisphenol A in mouse dorsal root ganglion neurons. Brain Res.1378, 1–8. 10.1016/j.brainres.2011.01.022
216
Wang Y.-P. Hong Q. Qin D. Kou C.-Z. Zhang C.-M. Guo M. et al (2012). Effects of embryonic exposure to polychlorinated biphenyls on zebrafish (Danio rerio) retinal development. J. Appl. Toxicol.32, 186–193. 10.1002/jat.1650
217
Wang X. Dong Q. Chen Y. Jiang H. Xiao Q. Wang Y. et al (2013). Bisphenol A affects axonal growth, musculature and motor behavior in developing zebrafish. Aquat. Toxicol.142-143, 104–113. 10.1016/j.aquatox.2013.07.011
218
Wang H. Liu Z.-H. Zhang J. Huang R.-P. Yin H. Dang Z. (2020). Human exposure to bisphenol A and its analogues: understandings from human urinary excretion data and wastewater-based epidemiology. Environ. Sci. Pollut. Res.27, 3247–3256. 10.1007/s11356-019-07111-9
219
Weber D. Hoffmann R. Hoke E. Tanguay R. (2015). Bisphenol A exposure during early development induces sex-specific changes in adult zebrafish social interactions. J. Toxicol. Env. Health A78, 50–66. 10.1080/15287394.2015.958419
220
Wesselman H. Wingert R. (2024). Estrogen signaling in development: recent insights from the zebrafish. Int. J. Dev. Biol.68, 1–7. 10.1387/ijdb.230116rw
221
Wester P. Van Der Ven L. Vos J. (2004). Comparative toxicological pathology in mammals and fish: some examples with endocrine disruptors. Toxicol205, 27–32. 10.1016/j.tox.2004.06.063
222
Wingfield J. Mukai M. (2009). Endocrine disruption in the context of life cycles: perception and transduction of environmental cues. Gen. Comp. Endocrinol.163, 92–96. 10.1016/j.ygcen.2009.04.030
223
Yang Z. Shi J. Guo Z. Chen M. Wang C. He C. et al (2019). A pilot study on polycystic ovarian syndrome caused by neonatal exposure to tributyltin and bisphenol A in rats. Chemosphere231, 151–160. 10.1016/j.chemosphere.2019.05.129
224
Yazulla S. Kleinschmidt J. (1982). Dopamine blocks carrier-mediated release of GABA from retinal horizontal cells. Brain Res.233, 211–215. 10.1016/0006-8993(82)90944-1
225
Yazulla S. Studholme K. (2001). Neurochemical anatomy of the zebrafish retina as determined by immunocytochemistry. J. Neurocytol.30, 551–592. 10.1023/a:1016512617484
226
Zhang J. Zuo Z. Chen R. Chen Y. Wang C. (2008). Tributyltin exposure causes brain damage in Sebastiscus marmoratus. Chemosphere73, 337–343. 10.1016/j.chemosphere.2008.05.072
227
Zhang X. Serb J. Greenlee M. (2011). Mouse retinal development: a dark horse model for systems biology research. Bioinform Biol. Insights5, 99–113. 10.4137/BBI.S6930
228
Zhang X. Hong Q. Yang L. Zhang M. Guo X. Chi X. et al (2015). PCB1254 exposure contributes to the abnormalities of optomotor responses and influence of photoreceptor cell development in zebrafish larvae. Ecotoxicol. Environ. Saf.118, 133–138. 10.1016/j.ecoenv.2015.04.026
229
Zhang J. Zhang C. Sun P. Huang M. Fan M. Liu M. (2017). RNA-sequencing and pathway analysis reveal alteration of hepatic steroid biosynthesis and retinol metabolism by tributyltin exposure in male rare minnow (Gobiocypris rarus). Aquat. Toxicol.188, 109–118. 10.1016/j.aquatox.2017.03.015
230
Zhang Q. Zhu L. Li H. Chen Q.-L. Li N. Li J. et al (2024). Insights and progress on the biosynthesis, metabolism, and physiological functions of gamma-aminobutyric acid (GABA): a review. PeerJ12, e18712. 10.7717/peerj.18712
231
Zhou R. Zhang Z. Zhu Y. Chen L. Sokabe M. Chen L. (2009). Deficits in development of synaptic plasticity in rat dorsal striatum following prenatal and neonatal exposure to low-dose bisphenol A. Neuroscience159, 161–171. 10.1016/j.neuroscience.2008.12.028
Summary
Keywords
bisphenol A, Danio rerio , ERG, estrogen, tributyltin
Citation
Jensen JS, Ouellette L, Harris R, Owrang P and Connaughton VP (2026) Disruption of estrogen signaling by developmental exposure to BPA and TBT causes long term functional deficits in zebrafish retina. Front. Pharmacol. 17:1750796. doi: 10.3389/fphar.2026.1750796
Received
20 November 2025
Revised
18 January 2026
Accepted
20 January 2026
Published
17 February 2026
Volume
17 - 2026
Edited by
Alejandra M. Pacchioni, Universidad Nacional de Rosario, Argentina
Reviewed by
Mingzhe Yuan, Ningbo University, China
Vessy Yancheva, University of Plovdiv Paisii Hilendarski, Bulgaria
Updates
Copyright
© 2026 Jensen, Ouellette, Harris, Owrang and Connaughton.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: V. P. Connaughton, vconn@american.edu
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.