- 1School of Medicine and Surgery-University of Milano Bicocca, Milan, Italy
- 2Psychiatric Department, Azienda Socio Sanitaria Territoriale (ASST) di Monza, Monza, Italy
- 3Section of Psychiatry, Department of Neuroscience, Reproductive Sciences and Odontostomatology, University of Naples Federico II, Naples, Italy
- 4Department of Neuropsychiatry, Villa von Siebenthal Neuropsychiatric Hospital and Clinic, Genzano di Roma, Italy
- 5Mental Health Department, Azienda Sanitaria Locale Roma 1, Rome, Italy
- 6Santa Chiara University Hospital, University of Pisa, Pisa, Italy
- 7Department of Neuroscience, Imaging, and Clinical Sciences, University “G.d'Annunzio” Chieti-Pescara, Chieti, Italy
- 8Psychopharmacology, Drug Misuse and Novel Psychoactive Substances Research Unit, School of Life and Medical Sciences, University of Hertfordshire, Hatfield, United Kingdom
Patients with severe psychotic disorders such as schizophrenia, schizoaffective, and bipolar disorders frequently suffer from concomitant substance use disorders (SUDs)–Dual Disorder (DD) patients. In order to better understand current practices for management of patients with psychotic episodes and concomitant SUD in Italy, we carried out a survey of psychiatrists on current routine practice among prescribers. These aspects can help to identify at-risk patients, improve current prescribing practices, and favor early intervention. An ad hoc survey of 17 questions was administered to psychiatrists via electronic polling and on-line distribution; 448 completed questionnaires were collected. Comorbid substance abuse was most frequently diagnosed within the context of anxiety disorder (46%), followed by bipolar disorder (25%), and schizophrenia/schizoaffective disorder (12%). The vast majority of respondents felt that patient management was becoming more complex due to substance abuse. The areas reported to be most affected in patients with SUD were functioning, interpersonal relations, and impulsivity, while sensory perception disorders, ideation, agitation, and impulsivity were the most frequently reported symptoms. In the acute setting, haloperidol was used as the first-line agent of choice followed by aripiprazole and olanzapine. In the maintenance phase, aripiprazole was the dominantly used first-line agent, followed by olanzapine. Almost half of respondents used long-acting agents, while about one-third did not. Among those prescribing long-acting agents, efficacy, control of impulsivity, and control of specific symptoms were cited as motivators, while in the maintenance phase, better adherence, and tolerability were mainly cited. From the responses to the present survey, it is clear that the respondents are aware of the problem of SUD in psychotic patients. While treatment be optimized in terms of the choice and formulation of antipsychotics, greater emphasis should be placed on efficacy, tolerability, and the negative metabolic consequences of some antipsychotics. When considering the ideal antipsychotic, long-acting agents were considered to be superior in reducing relapse, even if current treatment guidelines often give preference to oral formulations.
Introduction
Patients affected by severe psychiatric disorders such as schizophrenia, schizoaffective, and bipolar disorders, with psychotic features, frequently suffer from concomitant substance use disorders (SUDs)–Dual Disorder (DD; nicotine excluded) patients. These are defined as conditions in which abuse of or dependence on substances such as alcohol, cocaine, opioids, phencyclidine, amphetamine, cannabis, or nicotine, negatively impacts on family and social life, work, and school. SUDs are also associated with impairment or distress (even if formal criteria of dependence are not necessarily met), and may causes financial problems (1).
WHO Mental Health surveys have suggested an association between psychotic experiences and SUDs, even if not all types of SUDs are associated with psychotic episodes (2). About 25–50% of psychotic patients are diagnosed with schizophrenia and a concomitant addiction disorder (3). Moreover, the prevalence of SUD is 25.1% in patients with schizophrenia and 20.1% in those with bipolar disorder, with young men affected by schizophrenia having the highest prevalence of non-alcohol drug-use disorder (4). Co-occurrent drug use is a frequent condition among patients presenting with a first episode of psychosis, with a prevalence ranging from 25 to 60% (5). In addition, substance abuse might trigger psychotic symptoms in certain individuals, since substances may be used to self-medicate psychotic symptoms (6, 7). Importantly, DD/psychosis is associated with more frequent psychotic relapses and emergency admissions, and with a tendency for chronicity (8); self-medication may also be related to the presence of psychotic symptoms (9). In this complex setting, adequate treatment is difficult to provide, which is further hampered by the known poor compliance of patients.
Psychotic patients with co-occurrent drug abuse may be more sensitive to the side effects of antipsychotics (i.e., extrapyramidal, cardiovascular, and metabolic) and therefore choosing agents proven with a low liability for such adverse events is warranted (10). Moreover, long acting antipsychotics with the above characteristics may be preferred in this clinical setting both reasons related to adherence and pharmacokinetic considerations (11).
There has been little effort to deliver a common clinical and procedural framework for DD patients and separate policies have focused on either severe mental health problems or addiction (12). At present, the aim of SUD treatment and prevention strategies has changed from a reduction in the use of substances to a greater prevention of the social and clinical consequences of substance use (12). While in some countries mental health services lead treatment programs, in others, such as Italy, addiction services normally care for people with SUD, including those with psychotic episodes. In Italy, indeed, the Drug Addiction Units [Servizi per le tossicodipendenze (SERT); and servizi per le dipendenze (SERD)] have generally been considered autonomous, and separate from Community Psychiatric Services, despite the frequent co-presence of a psychotic disorder and SUD. This has been increasingly recognized as ineffective, and the Departments of Mental Health (DSM) now allow access to users who were heretofore not treated at such centers. In addition, many traditional Therapeutic Communities are also recognizing the difficulty of working with patients with a psychotic disorder and SUD and have begun to adopt the services offered accordingly. In fact, DSM and SERD are increasingly called upon to adopt convergent guidelines and intervention procedures, for an integrated, multi-dimensional, and targeted interventions. This is especially relevant when managing chronic pathologies that require long-term treatment and the use of multiple resources.
In order to shed more light on the current practices for the management of patients with psychotic episodes and concomitant SUD in Italy, we have carried out a survey of psychiatrists on current routine practice among prescribers. Particular emphasis was put on clinical practice in patients suffering from schizophrenia/schizoaffective disorder and substance use (DD/psychosis). Beyond the Italian mental health and Addiction Service system, these aspects could be relevant to identify at-risk patients, improve current prescribing practices, and possibly implement early intervention programs.
Materials and Methods
General Description of Survey
This was an ad hoc survey consisting of 17 questions intended to investigate the models of care of psychotic patients with concomitant SUD in Italy regarding the prescribing practices of antipsychotic medications in psychotic patients with SUD. The survey was drafted by the authors of this article and made available for completion to psychiatrists throughout the country under the aegis of the Società Italiana Psichiatria delle Dipendenze1 The completed surveys were collected in two ways. First, through an electronic poll carried out at the national congress of Società Italiana di Psicopatologia (SOPSI) in 2017, where 98/350 participants responded to the questionnaire (28%). This percentage can be considered to be an adequate representation of participants based on literature data (12–15). In a second ad hoc, snowball sampling recruitment technique (in which participants were asked to recruit additional subjects from acquaintances), 5,155 psychiatrist subscribers to the Medikey newsletter (https://ssl.medikey.it/) were asked to fill in the questionnaire, of whom 350 (6.8%) did. Thus, a total of 448 completed questionnaires were collected. Participation in compilation of the questionnaires was entirely voluntary, and no incentive for participation was offered. A link was provided to an online participant information sheet, and upon consenting were participants redirected to the online questionnaire. There were no mandatory responses in the questionnaire and participants were free to withdraw at any stage in compilation, and participants wishing to withdraw could simply close the relevant page. All answers and questionnaires were completely anonymous, and other than the information detailed below no personal identifying information was collected.
The survey included a series of questions on the respondents' demographics (age, sex, specialty, position, type of setting, and geographic location), besides the 17 questions. Of these, questions 1–6 investigated the models of care of psychotic patients with concomitant SUD, questions 7–10 queried about the characteristics of patients seen in daily practice by psychiatrists, and questions 11–17 focused on the prescription practices and long-term formulations employed in the subgroup of patients with schizophrenia/schizoaffective disorder. The translated questionnaire is shown in Data Sheet 1 in Supplementary Material.
Results
Demographics of Respondents
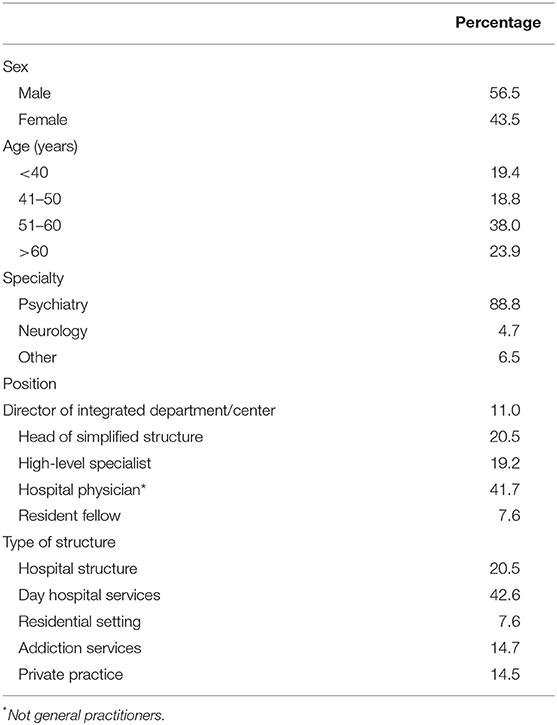
A total of 448 questionnaires were collected. As summarized in Table 1, 56.5% of the respondents were men and nearly 62% were older than 50 years; >40% responded that they were Head of Department or similar, while only 7.6% were resident fellows.
The type of settings of respondents was fairly well-balanced: indeed, over 40% worked in specialized centers, while the remainder was divided between local structures, private practice and addiction services, besides residential settings. The respondents were from all areas of Italy, although the highest percentages were from Lombardy (16.3%), Lazio (10.9%), Campania (9.4%), and Sicily (9.2%).
Models of Care of Psychiatric Patients With Concomitant SUD (Q1-Q6)
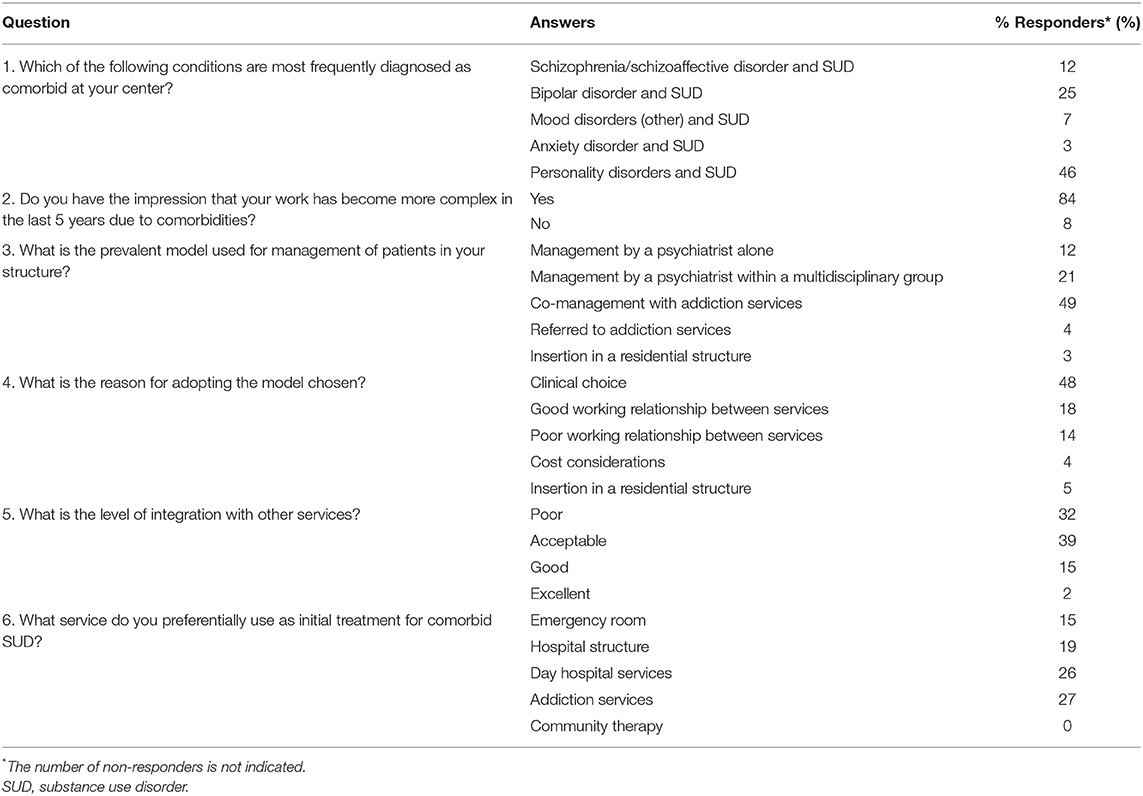
In the Italian practice, comorbid substance abuse was most frequently diagnosed within the context of anxiety disorder (46%), followed by bipolar disorder (25%), and schizophrenia/schizoaffective disorder (12%) (Q1, Table 2). Notably, 84.4% of respondents felt that patient management was becoming more complex due to substance abuse, while the reminder said “no” or “don't know” (Q2). With regard to the most prevalent type of structure for psychiatric patients with comorbid substance abuse in their center (Q3), 49% responded that co-management with addiction services was the optimal approach, followed by management by a psychiatrist within the context of a multidisciplinary group (21%). Only 12% felt that exclusive psychiatric management was the best approach. The clinical choice was overwhelmingly acknowledged as the major driver (48%) in the decision of the model of care, followed by the good working relationship between client services (18%); only 4% said that costs were a major motivator in their choice (Q4). The overall level of integration with other services (Q5) was mostly viewed as good/excellent (16.7%) or acceptable (39.3%). However, 31.9% deemed it as unacceptable. When asked in what context psychiatric patients with comorbid substance abuse are presented (Q6), a wide variety of responses was given: addiction services (27%), specialized center (26%), regional/local services (19%), and emergency services (15%).
Characteristics of Patients With Psychotic Episodes and SUD (Q7–Q10)
The areas reported to be the most affected in patients with comorbid substance abuse (Figure 1, Q7) were functioning, interpersonal relations, and impulsivity, while more heterogeneous responses were given for the remaining areas. Questions 8 and 9 were optional and are not presented herein as only a small number of responses were collected. With regard to the types of substances used in psychiatric patients, a wide variety of responses were seen (Figure 2, Q10). Cannabis and alcohol were among those most used, although stimulants were also used in more than 40% of patients. Fewer but significant proportions of patients were using opioids or other drugs, and even fewer new synthetic drugs or plant-derived hallucinogens. Of note, about 60% of patients had used more than one substance.
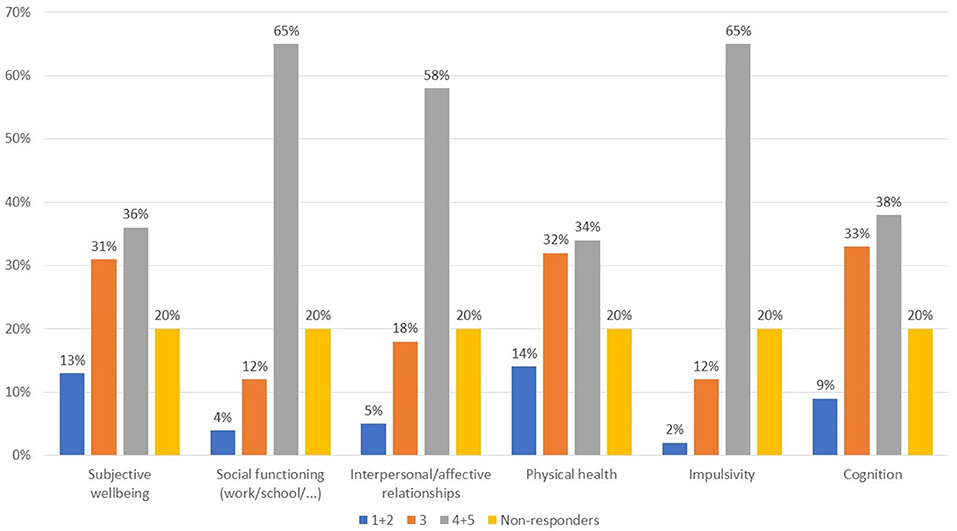
Figure 1. What areas are most compromised in psychotic patients with comorbid substance abuse? Value expressed on a scale of 1–5 where 1, not important; 2, not very important; 3, somewhat important; 4, important; 5, very important.
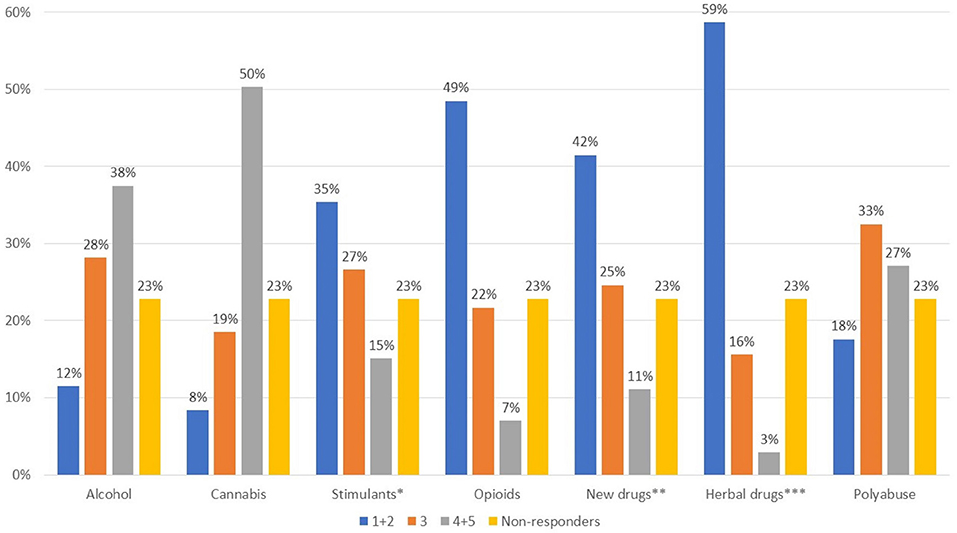
Figure 2. What type of substances are most frequently used by patients with psychotic episodes? Value expressed on a scale of 1–5 where 1, not used; 2, infrequent; 3, somewhat used; 4, often used; 5, very often used *cocaine, amphetamines; ** e.g., mephedrone, synthetic cannabinoids, Spice drugs, latest generation ecstasy derivatives, ketamine, and derivatives; ***hallucinogenic mushrooms, Salvia divinorum, Ayahuasca, Mitragyna speciosa-kratom.
Patients With Schizophrenia/Schizoaffective Disorder and SUD: Prescribing Practices and Long-Term Agents (Q11–Q17)
In patients with schizophrenia/schizoaffective disorder and comorbid substance abuse, the symptoms most frequently reported (Figure 3, Q11) were sensory perception disorders, ideation, agitation, and impulsivity. Of note, negative symptoms were predominant in a small proportion of subjects. In their daily practice, 51.9% of respondents used to treat differently this subgroup from other psychiatric patients, whereas 23.2% did not (25.0% did not respond) (Q12).
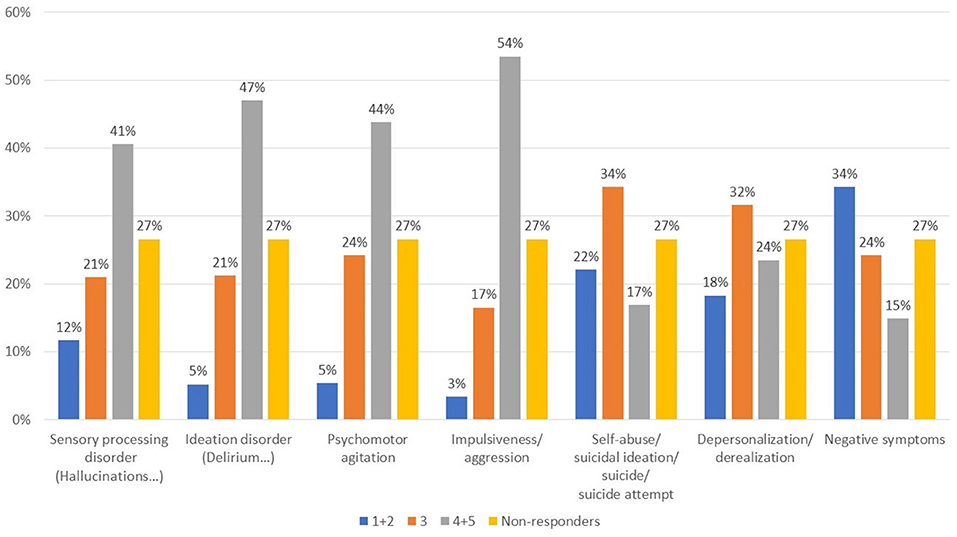
Figure 3. What symptoms are most represented in patients with schizophrenia/schizoaffective disorder and SUD? Value expressed on a scale of 1–5 where 1, rare; 2, infrequent; 3, somewhat frequent; 4, often; 5, very often.
In the acute setting (Figure 4A), haloperidol was widely seen as the first-line agent of choice followed by aripiprazole and olanzapine. Paliperidone was the most commonly chosen second-line agent. In the maintenance phase, aripiprazole was the prevalent choice as first-line agent, followed by olanzapine (Figure 4B). Quetiapine and risperidone were also frequently used.
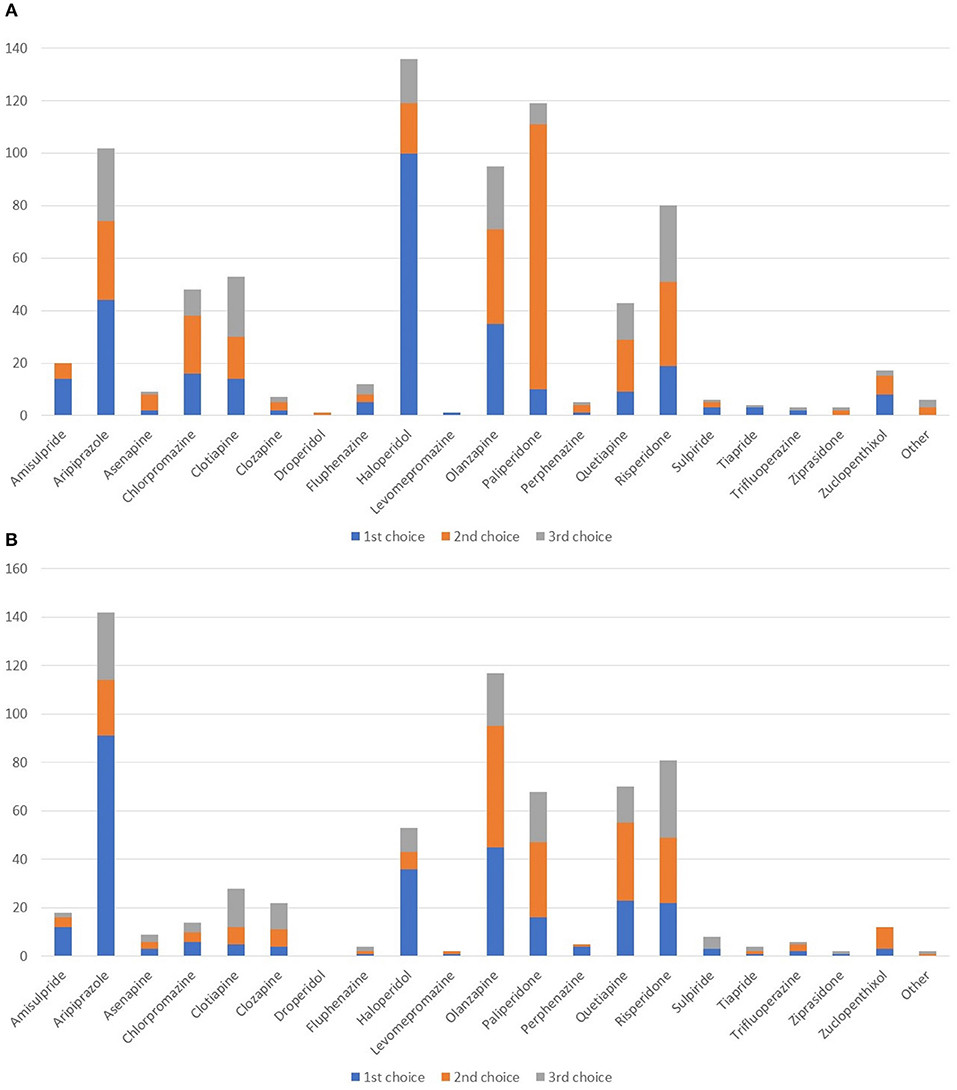
Figure 4. Which agents do you most often prescribe as first-, second-, or third-line in patients with schizophrenia/schizoaffective disorder and SUD in the acute (A) and in maintenance (B) setting?
The remaining questions queried about the use of long-acting agents 48.6% of respondents used long acting agents, while 35.0% did not (Q14). 74.9% prescribed prevalently an atypical agent, while 10.5, 8.2, and 6.4 stated that they usually preferred neither atypical or typical agents, a long-acting acting combined with an oral agent, and a typical agent, respectively. Figure 5 shows the agents rated as first, second, or third choice. Paliperidone was the long-acting agent most widely adopted in all three lines, followed by aripiprazole, haloperidol, and risperidone.
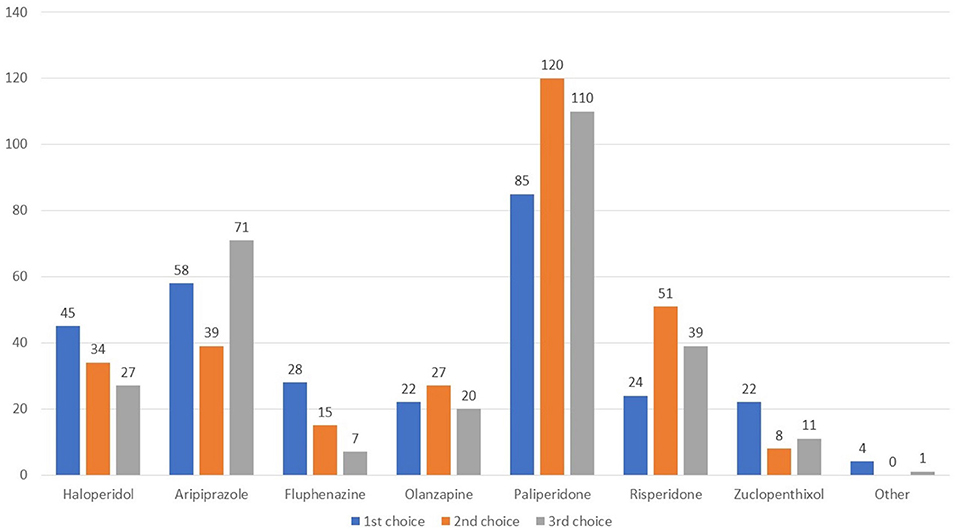
Figure 5. Which long-acting agent do you prefer to prescribe as first, second, and third line therapy?
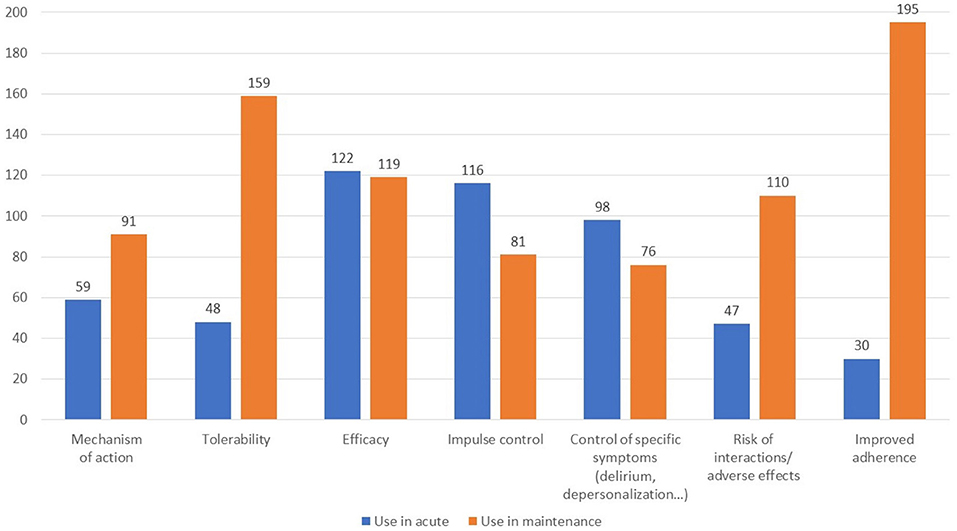
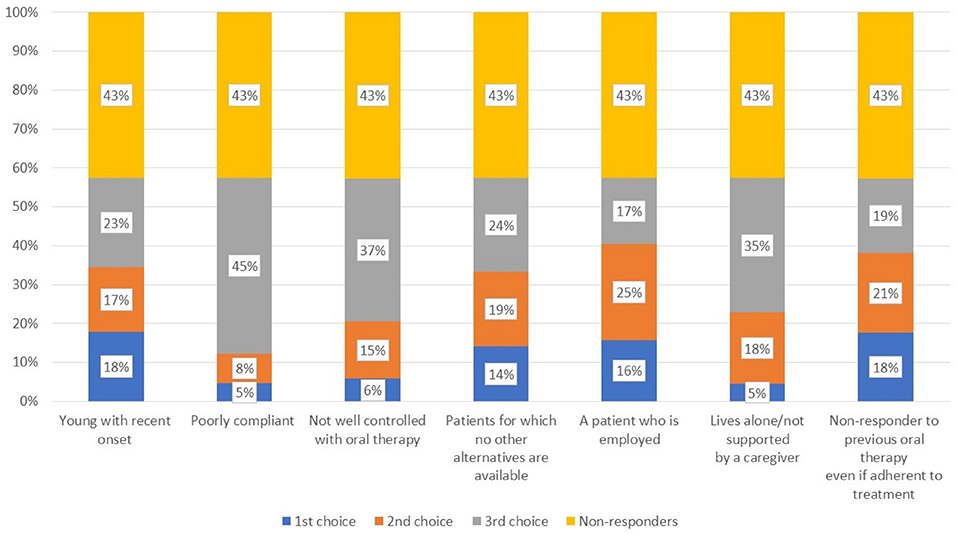
When asked about the reason for choosing a long-acting antipsychotic in the acute and maintenance phases (Q15), there was a wide range of responses for the former, most frequently citing efficacy, control of impulsivity, and control of specific symptoms (Figure 6). In the maintenance phase, better adherence and tolerability were the main drivers. Regarding the most suitable patient for a long-acting antipsychotic in those with and without comorbid substance use (Figure 7, Q16), a large proportion of participants did not respond, while many indicated the subjects with poor compliance and poorly controlled by oral therapies. In addition, respondents often cited those living alone or who were not well-supported by a caregiver as good candidates.
Finally, according to 43% of respondents the concomitant use of other psychoactive drugs in those being treated with long-acting antipsychotics is frequent, whereas another 43% responded no (Q17). Interestingly, there was little consensus on what types of drugs were more commonly used in concomitance (Figure 8).
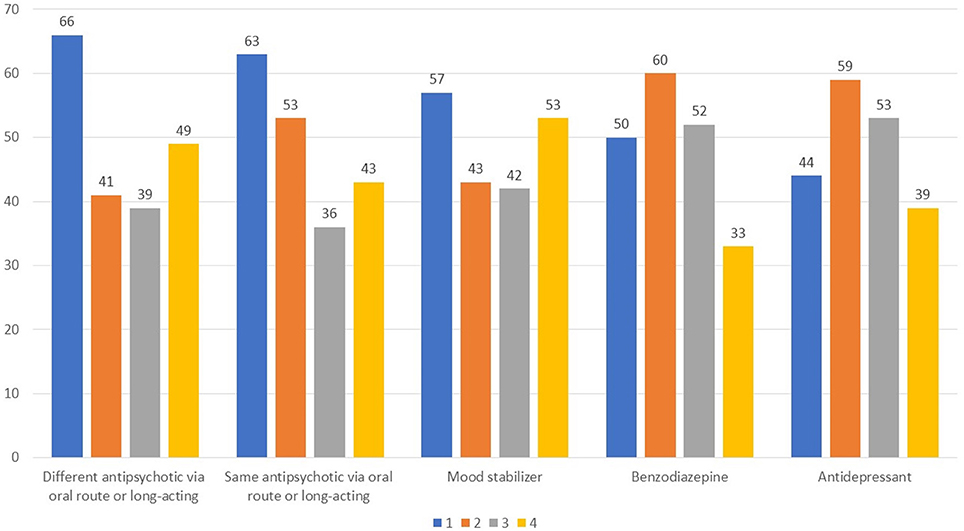
Figure 8. Which types of drugs are most widely used in combination with a long-acting antipsychotic? Value expressed on a scale of 1–4 to estimate the frequency, 1 for most frequent use and 4 for less frequent use.
Discussion
The respondents of the present survey were primarily psychiatrists working in a wide range of clinical settings significantly representing real-life practice. Addiction services do not commonly treat psychotic patients with SUD, representing only 14.7% of respondents. Indeed, from the present survey, the majority of the psychotic patients with SUD are treated at outpatient services and in hospital (63.1% combined) in line with what is believed to be the optimal approach, and much fewer in residential settings. In addition, general psychiatrists are likely to be those who formulate a diagnosis of SUD and psychosis, and prescribers may not always have adequate training on diagnosis of a DD.
It was our intention to gain more information about the current prescribing practices for patients with schizophrenia and SUD in Italy. The results of the present survey are consistent with previous studies indicating that SUD is common in patients with schizophrenia. Interestingly, the vast majority of respondents felt that management of comorbid SUD is becoming more complex. Thus, on one hand it is not surprising that many viewed the use of a multidisciplinary team as the optimal approach for such patients. It is also reassuring that costs did not appear to be a major motivator for this choice. As expected, interpersonal relationships and overall functioning were among the areas most seen to be affected by comorbid substance abuse, with patients abusing a variety of substances, most often alcohol and cannabis, but also other drugs. The majority of patients also had poly-substance abuse. The present survey can be seen as an extension of a previous one carried out over a decade ago on DDs in Italy (16).
It is noteworthy that negative symptoms were seen as predominant in only a minority of patients, with other symptoms being more prevalent, such as sensory perception disorders, ideation, agitation, and impulsivity. This could perhaps suggest that the antipsychotic treatment was not optimal in terms of the agent prescribed and/or dosing. However, such additional factors were not queried in the present survey. Nonetheless, in the acute phase haloperidol was often the agent of first choice, followed by the second-line agents aripiprazole and paliperidone. In the maintenance phase, aripiprazole appeared to be the first-line choice, followed by other second-line agents. This finding confirms that second-line agents are indeed widely prescribed in schizophrenic patients with comorbid substance abuse, and that those with lower metabolic impact are also favored. The prescribing preferences referred would also seem to indicate that the choice of agent is not specifically related to the presence of a single diagnosis of psychosis or DD/psychosis. Since reward deficiency syndrome is more easily established in patients with SUD, this suggests that the dopaminergic resources of those with SUD are already destabilized by substance use (16), with the development of anhedonia and/or hypophoria as probably the most relevant clinical symptoms (17). It is now commonly agreed that dopamine is a major neurotransmitter in terms of reward dependence, even if there is some controversy regarding its clinical modulation in treatment and prevention of prevent relapse for SUD. While many have advocated that medications blocking dopamine should be favored, it can also be argued that short-term blockade of D2 receptors is warranted, but that long-term treatment should activate and not completely inhibit D2 receptors in order to enhance the functional connectivity of brain reward circuits (16, 18–23).
Some psychoactive substances and medications can be related to “Reward Deficiency Syndrome,” which was originally described as an outcome of chronic alcohol and stimulant abuse, and a highly relevant topic of which clinicians should be aware within the context of SUD. In part, it links with the a-motivational syndrome (AS) (24), displayed as an expression of chronic cannabis intoxication, but which is closely related to post-withdrawal syndrome (PWS) described by Martin et al. as an enduring pathologic state in abstinent detoxified opiate addicts (25). From a withdrawal-related point of view, through each detoxification cycle the patient passes from the acute withdrawal state (counter-polar to intoxication—psychomotor retardation in case of cocaine acute withdrawal) to a later and enduring drug-free state featuring symptoms of anhedonia or hypophoria, looming as acquired discomfort related to the absence of drug-related stimulation. Hypophoria includes somatic, vegetative (sleep), mood, and anxiety symptoms such as susceptible or irritable (depressed) mood, amplified pain perception, inability to perform simple tasks, and make normal efforts, and inability to experience reward in any way other than substance use. This syndrome closely resembles the subthreshold symptoms of dysthymia and the residual symptoms of chronic bipolar disorder (26, 27). This is one of the possible ways of relapsing behavior.
With regards to the use of long-acting antipsychotics, roughly one-half responded that they use these agents in clinical practice, with clear preference for the use of an atypical agent in this formulation, in line with a recent study recently conducted in Italy (28). In this regard, a study comparing long-acting injectable formulations of aripiprazole and paliperidone in patients with comorbid psychosis and SUD found that both agents improved clinical status and QoL and reduced substance craving at 1 year (29). Among long-acting formulations, paliperidone was widely favored in the present survey. Respondents cited efficacy and better adherence as major motivators for prescription of a long-acting agent in the maintenance phase, along with good tolerability. Patients with poor compliance and those living alone with inadequate caregiver support were believed to be most suited for a long-acting antipsychotic. Lastly, there was little agreement as to which type of drugs were most commonly used in combination with a long-acting antipsychotic. This could be indicative that many different types of drugs are used in conjunction with long-acting agents, including oral antipsychotics, mood stabilizers, benzodiazepines, and antidepressants.
It is difficult to compare the present results with those in the literature as there is limited real-life data on prescribing habits in patients with schizophrenia and comorbid SUD. In a recent survey among French psychiatrists, most psychiatrists used second-generation antipsychotics, and preferentially an oral formulation, in the treatment of schizophrenia (30). Long-acting agents were prescribed in about one-third of schizophrenic patients, and the duration and type of practice did not influence the class or formulation of antipsychotics used. As perhaps can be inferred from the present survey, in that survey personal experience, government regulatory approval, and guidelines for the treatment of schizophrenia were the main factors that guided decision-making. However, that survey was not specific on patients with comorbid substance abuse. In a survey of practice in 7 Central and Eastern European Countries, oral atypical antipsychotics, mostly risperidone, olanzapine, clozapine, were among those most commonly prescribed for schizophrenia (31). As in the present survey, anxiolytics (70%), antidepressants (42%), and mood-stabilizers (27%) were commonly co-prescribed in the survey mentioned.
In previous survey of Italian psychiatrists treating schizophrenia, while efficacy and tolerability were among the most widely used factors used to evaluate treatment outcomes in patients with schizophrenia, overall quality of life and global functioning were also considered to be important. These findings were also mirrored in the present survey. Notwithstanding, some discrepancies in quality of care indicators have been revealed among Italian DSM, with a percentage of inappropriate interventions ranging from 5.9 to 66.8% for pharmacological interventions, with significant variability in monitoring of metabolic effects, psychosocial rehabilitation, family involvement, and work (32). These results underscore the greater need for monitoring of patients and better integration of mental health services, aspects which were also seen in the present survey as inadequate for a proportion of respondents.
In this regard, it is worthwhile to stress that while awareness is definitely increasing, psychiatrists should be vigilant to the adverse metabolic effects of some second-generation antipsychotics (33), especially the risk of reward deficiency syndrome in substance user psychotic patients treated with full blocking D2 antagonist. To improve the current situation, additional efforts are still needed in order to improve training programs as shown in a recent survey of 35 countries (34). In considering the ideal antipsychotic, long-acting agents are considered to be superior to their oral equivalents in reducing relapse, even if current schizophrenia treatment guidelines often give preference to oral formulations (35).
A relevant issue in long term therapy schizophrenia and drug addiction comorbidity is represented by treatment-resistant schizophrenia. Approximately 30% of schizophrenia patients do not respond or respond poorly to antipsychotics treatment (36), treatment resistant schizophrenia is defined as lack of response to at least two different antipsychotics at 600 mg chlorpromazine equivalent and for the duration of treatment of at least 6 weeks. These patients show reduction in milestone acquisition (37), more cognitive impairment (38), and functioning deficits as well as higher incidence of neurological soft signs (39) compared to patients who respond to antipsychotic treatment.
The relationship between treatment resistance and drug abuse is two-fold. Drug abuse or addiction, besides worsening adherence to treatment, can interfere with the efficacy of antipsychotics by worsening symptoms of the disease and/or interacting with the pharmacodynamics of antipsychotics (40). On the other hand, worsening of psychiatric symptoms may be relevant to further induce abuse and/or addiction. Long acting injectable antipsychotics could overcome some of these issues by improving adherence and therefore excluding false positive treatment-resistant patients, as well as possibly increasing the availability of the antipsychotics at the receptor level.
Overcoming these and other barriers in the future will undoubtedly help to optimize management of the difficult-to-treat group of patients with schizophrenia and comorbid substance abuse. From the responses to the present survey, it is clear that the respondents are aware of the problem of SUD in psychotic patients. Not only can treatment be optimized in terms of the choice and formulation of antipsychotics, but greater emphasis should be placed on treatment efficacy, tolerability, and especially the potentially negative metabolic consequences of antipsychotics, with the consequent need for better monitoring of patients. Despite the limitations of the present study, such as possible bias related to the survey techniques used and potential bias in the profile of responders, it seems clear that new treatment paradigms will be needed, and achieving these long-term goals will also require even greater awareness and training. The present survey nonetheless helps to better understand current routine practice among prescribers.
Author Contributions
All authors contributed to the design of the study, analyzed and interpreted the results, revised the manuscript in each section and approved the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Edra Spa provided technical support for the survey and medical writing and technical support for this article. The support by Edra spa was funded by Otsuka Pharmaceutical Italy and Lundbeck Italia.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2018.00575/full#supplementary-material
Data Sheet 1. Questionnaire administered.
Footnotes
References
1. Kerner B. Comorbid substance use disorders in schizophrenia: a latent class approach. Psychiatry Res. (2015) 225:395–401. doi: 10.1016/j.psychres.2014.12.006
2. Degenhardt L, Saha S, Lim CCW, Aguilar-Gaxiola S, Al-Hamzawi A, Alonso J, et al. The associations between psychotic experiences and substance use and substance use disorders: findings from the World Health Organization World Mental Health surveys. Addiction (2018) 113:924–34. doi: 10.1111/add.14145
3. Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. results from the epidemiologic catchment area (ECA) study. JAMA (1990) 264:2511–8. doi: 10.1001/jama.1990.03450190043026
4. Nesvag R, Knudsen GP, Bakken IJ, Hoye A, Ystrom E, Suren P, et al. Substance use disorders in schizophrenia, bipolar disorder, and depressive illness: a registry-based study. Soc Psychiatry Psychiatr Epidemiol. (2015) 50:1267–76. doi: 10.1007/s00127-015-1025-2
5. Delisi LE. The effect of cannabis on the brain: can it cause brain anomalies that lead to increased risk for schizophrenia? Curr Opin Psychiatry (2008) 21:140–50. doi: 10.1097/YCO.0b013e3282f51266
6. Archie S, Rush BR, Akhtar-Danesh N, Norman R, Malla A, Roy P, et al. Substance use and abuse in first-episode psychosis: prevalence before and after early intervention. Schizophr Bull. (2007) 33:1354–63. doi: 10.1093/schbul/sbm011
7. Rubino T, Parolaro D. Cannabis abuse in adolescence and the risk of psychosis: a brief review of the preclinical evidence. Prog Neuropsychopharmacol Biol Psychiatry (2014) 52:41–4. doi: 10.1016/j.pnpbp.2013.07.020
8. Gouzoulis-Mayfrank E, Konig S, Koebke S, Schnell T, Schmitz-Buhl M, Daumann J. Trans-sector integrated treatment in psychosis and addiction. Dtsch Arztebl Int. (2015) 112:683–91. doi: 10.3238/arztebl.2015.0683
9. Roncero C, Comin M, Daigre C, Grau-Lopez L, Martinez-Luna N, Eiroa-Orosa FJ, et al. Clinical differences between cocaine-induced psychotic disorder and psychotic symptoms in cocaine-dependent patients. Psychiatry Res. (2014) 216:398–403. doi: 10.1016/j.psychres.2014.01.026
10. Lambert M, Conus P, Eide P, Mass R, Karow A, Moritz S, et al. Impact of present and past antipsychotic side effects on attitude toward typical antipsychotic treatment and adherence. Eur Psychiatry (2004) 19:415–22. doi: 10.1016/j.eurpsy.2004.06.031
11. Papageorgiou G, Canas F, Zink M, Rossi A. Country differences in patient characteristics and treatment in schizophrenia: data from a physician-based survey in Europe. Eur Psychiatry (2011) 26:17–28. doi: 10.1016/S0924-9338(11)71710-2
12. Carra G, Bartoli F, Brambilla G, Crocamo C, Clerici M. Comorbid addiction and major mental illness in Europe: a narrative review. Subst Abus. (2015) 36:75–81. doi: 10.1080/08897077.2014.960551
14. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health (2008) 31:180–91. doi: 10.1002/nur.20247
15. Ventriglio A, Gentile A, Stella E, Bellomo A. Metabolic issues in patients affected by schizophrenia: clinical characteristics and medical management. Front Neurosci. (2015) 9:297. doi: 10.3389/fnins.2015.00297
16. Blum K, Febo M, Thanos PK, Baron D, Fratantonio J, Gold M. Clinically combating reward deficiency syndrome (RDS) with dopamine agonist therapy as a paradigm shift: dopamine for dinner? Mol Neurobiol. (2015) 52:1862–9. doi: 10.1007/s12035-015-9110-9
17. Martinotti G, Cloninger CR, Janiri L. Temperament and character inventory dimensions and anhedonia in detoxified substance-dependent subjects. Am J Drug Alcohol Abus. (2008) 34:177–83. doi: 10.1080/00952990701877078
18. Bacopoulos NC, Spokes EG, Bird ED, Roth RH. Antipsychotic drug action in schizophrenic patients: effect on cortical dopamine metabolism after long-term treatment. Science (1979) 205:1405–7. doi: 10.1126/science.38504
19. Acquas E, Carboni E, Di Chiara G. Profound depression of mesolimbic dopamine release after morphine withdrawal in dependent rats. Eur J Pharmacol. (1991) 193:133–4. doi: 10.1016/0014-2999(91)90214-B
20. Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and reward deficiency syndrome. Am J Med Genet B Neuropsychiatr Genet. (2005) 132B:29–37. doi: 10.1002/ajmg.b.30080
21. Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. (2007) 27:12700–6. doi: 10.1523/JNEUROSCI.3371-07.2007
22. Grob S, Pizzagalli DA, Dutra SJ, Stern J, Morgeli H, Milos G, et al. Dopamine-related deficit in reward learning after catecholamine depletion in unmedicated, remitted subjects with bulimia nervosa. Neuropsychopharmacology (2012) 37:1945–52. doi: 10.1038/npp.2012.41
23. Blum K, Thanos PK, Oscar-Berman M, Febo M, Baron D, Badgaiyan RD, et al. Dopamine in the brain: hypothesizing surfeit or deficit links to reward and addiction. J Reward Defic Syndr. (2015) 1:95–104. doi: 10.17756/jrds.2015-016
24. Rovai L, Maremmani AGI, Pacini M, Pani PP, Rugani F, Lamanna F. Negative dimensions in Psychiatry. Amotivation syndrome as a paradigme of negative symptoms in substance abuse. Riv Psichiatr. (2013) 48:1–9. doi: 10.1708/1228.13610
25. Martin WR, Jasinski DR. Physiological parameters of morphine dependence in man, early abstinence, protracted abstinence. J Psychiatr Res. (1969) 7:9–17.
26. Akiskal H, Judd LL, Gillin JC, Lemmi H. Subthreshold depressions: clinical and polysomnographic validation of dysthymic, residual and masked forms. J Affect Disord. (1997) 45:53–63. doi: 10.1016/S0165-0327(97)00059-1
27. Maremmani I, Pacini M. Understanding the pathogenesis of drug addiction in order to implement a correct pharmacological intervention. Heroin Addiction Rel Clinical Prob. (2003) 5:5–12.
28. Ostuzzi G, Mazzi MA, Terlizzi S, Bertolini F, Aguglia A, Bartoli F, et al. Factors associated with first- versus second-generation long-acting antipsychotics prescribed under ordinary clinical practice in Italy. PLoS ONE (2018) 13:e0201371. doi: 10.1371/journal.pone.0201371
29. Cuomo I, Kotzalidis GD, De Persis S, Piacentino D, Perrini F, Amici E, et al. Head-to-head comparison of 1-year aripiprazole long-acting injectable (LAI) versus paliperidone LAI in comorbid psychosis and substance use disorder: impact on clinical status, substance craving, and quality of life. Neuropsychiatr Dis Treat. (2018) 14:1645–56. doi: 10.2147/NDT.S171002
30. Samalin L, Garnier M, Auclair C, Llorca PM. Clinical decision-making in the treatment of schizophrenia: focus on long-acting injectable antipsychotics. Int J Mol Sci. (2016) 17:E1935. doi: 10.3390/ijms17111935
31. Szkultecka-Debek M, Miernik K, Stelmachowski J, Jakovljevic M, Jukic V, Aadamsoo K, et al. Treatment patterns of schizophrenia based on the data from seven Central and Eastern European Countries. Psychiatr Danub. (2016) 28:234–42.
32. Fantini G, Tibaldi G, Rucci P, Gibertoni D, Vezzoli M, Cifarelli L, et al. Quality of care indicators for schizophrenia: determinants of observed variations among Italian Departments of Mental Health. Results from the ETAS DSM study. Epidemiol Psychiatr Sci. (2017) 26:299–313. doi: 10.1017/S204579601600010X
33. Sugawara N, Yasui-Furukori N, Yamazaki M, Shimoda K, Mori T, Sugai T, et al. Attitudes toward metabolic adverse events among patients with schizophrenia in Japan. Neuropsychiatr Dis Treat (2016) 12:427–36. doi: 10.2147/NDT.S98711
34. Fiorillo A, Sampogna G, Del Vecchio V, Luciano M, Del Gaudio L, De Rosa C, et al. What is the current status of training and practice of early intervention in psychiatry? results from a survey in 35 countries. Early Interv Psychiatry (2015) 9:70–5. doi: 10.1111/eip.12085
35. Getzen H, Beasley M, D'mello DA. Barriers to utilizing long-acting injectable antipsychotic medications. Ann Clin Psychiatry (2014) 26:33–8.
36. Howes OD, Mccutcheon R, Agid O, De Bartolomeis A, Van Beveren NJ, Birnbaum ML, et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry (2017) 174:216–29. doi: 10.1176/appi.ajp.2016.16050503
37. Iasevoli F, Giordano S, Balletta R, Latte G, Formato MV, Prinzivalli E, et al. Treatment resistant schizophrenia is associated with the worst community functioning among severely-ill highly-disabling psychiatric conditions and is the most relevant predictor of poorer achievements in functional milestones. Prog Neuropsychopharmacol Biol Psychiatry (2016) 65:34–48. doi: 10.1016/j.pnpbp.2015.08.010
38. De Bartolomeis A, Balletta R, Giordano S, Buonaguro EF, Latte G, Iasevoli F. Differential cognitive performances between schizophrenic responders and non-responders to antipsychotics: correlation with course of the illness, psychopathology, attitude to the treatment and antipsychotics doses. Psychiatry Res. (2013) 210:387–95. doi: 10.1016/j.psychres.2013.06.042
39. De Bartolomeis A, Prinzivalli E, Callovini G, D'ambrosio L, Altavilla B, Avagliano C, et al. Treatment resistant schizophrenia and neurological soft signs may converge on the same pathology: Evidence from explanatory analysis on clinical, psychopathological, and cognitive variables. Prog Neuropsychopharmacol Biol Psychiatry (2018) 81:356–66. doi: 10.1016/j.pnpbp.2017.09.002
Keywords: schizophrenia, psychosis, psychotic disorder, substance use disorder, survey, management, dual disorder
Citation: Clerici M, de Bartolomeis A, De Filippis S, Ducci G, Maremmani I, Martinotti G and Schifano F (2018) Patterns of Management of Patients With Dual Disorder (Psychosis) in Italy: A Survey of Psychiatrists and Other Physicians Focusing on Clinical Practice. Front. Psychiatry 9:575. doi: 10.3389/fpsyt.2018.00575
Received: 31 July 2018; Accepted: 19 October 2018;
Published: 13 November 2018.
Edited by:
Carlos Roncero, Complejo Hospitalario de Salamanca, SpainReviewed by:
Nieves Martínez-Luna, Hospital Universitari Vall d'Hebron, SpainHussien Elkholy, Ain Shams University, Egypt
Copyright © 2018 Clerici, de Bartolomeis, De Filippis, Ducci, Maremmani, Martinotti and Schifano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni Martinotti, Z2lvdmFubmkubWFydGlub3R0aUBnbWFpbC5jb20=
 Massimo Clerici
Massimo Clerici Andrea de Bartolomeis
Andrea de Bartolomeis Sergio De Filippis
Sergio De Filippis Giuseppe Ducci5
Giuseppe Ducci5 Icro Maremmani
Icro Maremmani Giovanni Martinotti
Giovanni Martinotti Fabrizio Schifano
Fabrizio Schifano