- 1Mental Health Institute of the Second Xiangya Hospital, Central South University, Changsha, China
- 2Chinese National Clinical Research Center on Mental Disorders, Chinese National Technology Institute on Mental Disorders, Hunan Key Laboratory of Psychiatry and Mental Health, Changsha, China
- 3Center for Medical Genetics, School of Life Sciences, Central South University, Changsha, China
- 4Department of Psychiatry, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 5Wuxi Mental Health Center, Nanjing Medical University, Wuxi, China
- 6Department of Psychiatry, Renmin Hospital of Wuhan University, Wuhan, China
Background: Dopaminergic and serotonergic systems play crucial roles in the pathophysiology of schizophrenia and modulate response to antipsychotic treatment. However, previous studies of dopaminergic and serotonergic genes expression are sparse, and their results have been inconsistent. In this longitudinal study, we aim to investigate the expressions of Catechol-O-methyltransferase (COMT), serotonin 2A receptor (5-HTR2A), and serotonin transporter gene (SLC6A4) mRNA in first-episode antipsychotic-naïve schizophrenia and to test if these mRNA expressions are associated with cognitive deficits and treatment outcomes or not.
Method: We measured COMT, 5-HTR2A, and SLC6A4 mRNA expressions in 45 drug-naive first-episode schizophrenia patients and 38 health controls at baseline, and repeated mRNA measurements in all patients at the 8-week follow up. Furthermore, we also assessed antipsychotic response and cognitive improvement after 8 weeks of risperidone monotherapy.
Results: Patients were divided into responders (N = 20) and non-responders groups (N = 25) according to the Remission criteria of the Schizophrenia Working Group. Both patient groups have significantly higher COMT mRNA expression and lower SLC6A4 mRNA expression when compared with healthy controls. Interestingly, responder patients have significantly higher levels of COMT and 5-HTR2A mRNA expressions than non-responder patients at baseline. However, antipsychotic treatment has no significant effect on the expressions of COMT, 5-HTR2A, and SLC6A4 mRNA over 8-week follow up.
Conclusion: Our findings suggest that dysregulated COMT and SLC6A4 mRNA expressions may implicate in the pathophysiology of schizophrenia, and that COMT and 5-HTR2A mRNA may be potential biomarkers to predict antipsychotic response.
Introduction
Schizophrenia is a complex and devastating psychiatric disorder affecting ~1% of the population. It is characterized by a set of psychotic symptoms such as hallucinations, delusions, cognitive impairments, and negative symptoms (1). Despite substantial efforts in recent decades, the pathophysiology of schizophrenia remains poorly delineated. So far, antipsychotic drugs are still the primary pharmacological treatment of schizophrenia. However, ~30% of the patients have not received satisfied symptomatic improvement after accepting antipsychotic medications with an adequate dosage and duration mainly due to the long-term morbidity and poor functional outcome in schizophrenia (2). Consequently, identifying reliable biomarkers for antipsychotic response has been considered as a promising strategy to improve the therapeutic effects to individual patients with the existing antipsychotic drugs.
Dysfunction in neurotransmitter systems is a well-established pathological feature of schizophrenia. Multiple lines of evidence indicated that dopaminergic and serotonergic systems and their interactions play critical roles in the mechanistic underpinnings as well as the antipsychotic response in schizophrenia (3–5). First, molecular imaging studies have reported the dysregulation of dopamine and neurotransmission in the striatum and prefrontal cortex (PFC) (3, 6–8), which is critical for cognition and emotional processing that has been implicated in the pathogenesis of schizophrenia. Second, cerebral dopamine and serotine receptor systems are the main targets of antipsychotic medications in treating schizophrenia. For example, the most effective atypical antipsychotics such as risperidone, olanzapine, and clozapine have the highest affinity to DAD2 and 5-HT2A receptors (9, 10). Moreover, the serotonin-dopamine interactions that modulate prefrontal brain activity have also received extensive focus as the development of treatment of schizophrenia (11, 12). Finally, drugs that drive dopamine/serotine release or increase dopamine/serotine transmission, such as amphetamine and lysergic acid diethylamide (LSD) will exacerbate psychosis in patients with schizophrenia and also can induce schizophrenic symptoms in healthy individuals at high doses (13, 14).
Candidate genetic association studies of dopaminergic and serotoninergic genes for schizophrenia and antipsychotic response have been widely conducted. Four candidate genes (DRD2, COMT, 5-HTR2A, and SLC6A4) from dopaminergic and serotoninergic pathways have received the most attention in the literature and have been supposed as the promising candidate genes for schizophrenia and treatment response (15–19). However, only DRD2 gene has been consistently replicated in studies with other strategies. For example, GWAS of schizophrenia found that variant of DRD2 is significantly associated with schizophrenia at the genome-wide significance level (20), and genetic drug-target networks analyses supported that DRD2 gene might play important and regulatory roles in the biological pathway of antipsychotic treatment (21–23). It is unsurprising for the limited successes in genetic association studies of schizophrenia owing to the heterogeneous nature of symptomatology and the small effect sizes of individual variants on phenotypic traits.
Recent evidence has shown that numerous GWAS significant risk variants locate in the non-coding regions and are enriched for expression quantitative trait locus (eQTLs) (20). In this regard, the application of gene expression analysis may provide an opportunity to investigate the pathogeneses of complicated clinical diseases such as schizophrenia. Many previous expression researches have reported that dysregulated expression of dopaminergic and serotoninergic genes in brain may implicated in the pathogenesis of schizophrenia (24–27). Furthermore, experimental evidences supported that antipsychotic treatment can regulate dopaminergic and serotoninergic genes expressions, implicating these genes might play roles in symptom improvement over antipsychotic treatment (28–30). Recently, many findings have indicated that peripheral gene expression may provide useful information on aberrant neurotransmission in neuropsychiatric diseases and monitor the drug response despite several methodological and theoretical limitations (31–33). Based on the above evidences, the present study measured COMT, 5-HTR2A, and SLC6A4 mRNA expressions in first-episode antipsychotic-naïve schizophrenia and followed up for their treatment response after 8 weeks' risperidone monotherapy. These three candidate genes were selected in our study because they have been supposed to be the most prominent in relation to disease pathophysiology and antipsychotic treatment but have been considerably inconsistent. The aims of this study are to explore (1) whether COMT, 5-HTR2A, and SLC6A4 mRNA expressions are altered in schizophrenia patients; (2) whether COMT, 5-HTR2A, and SLC6A4 mRNA expressions are associated with treatment outcomes, cognitive defects and psychopathological symptoms in schizophrenia; (3) whether antipsychotic treatment has effect on COMT, 5-HTR2A, and SLC6A4 mRNA expressions.
Materials and Methods
Subjects
Forty-five first-episode antipsychotic-naïve schizophrenia patients and thirty-eight age-matched controls were recruited in this study. Individuals were diagnosed with first-episode schizophrenia by experienced psychiatrists using the Structured Clinical Interview for DSM IV Disorders, and none of the patients was ever treated with antipsychotic medications or other psychotropics before. Psychotic symptoms severity was evaluated using the 30-item Positive and Negative Symptom Scale (PANSS) and only patients with PANSS score ≥65 were selected. Patients with any other DSM-IV diagnoses or clinically significant medical diseases were excluded. Healthy controls were screened using the SCID-NP (Non-Patient Edition) and were recruited if they are absent of any psychiatric disorder for the lifetime. Inclusion criteria for all participants were aged between 18 and 40 years, being right handed and having normal vision. The study design and procedures were in accordance with the Declaration of Helsinki, and approved by The Ethics Committee of the Second Xiangya Hospital of Central South University. All participants gave written informed consent after the procedures had been fully explained to participation in the study.
Clinical Assessment and Treatment Response
All patients were treated with risperidone monotherapy for 8 weeks. The dosage of risperidone was 2 mg per day initially and then was adjusted by the attending psychiatrist according to individual tolerance. For all patients, risperidone compliance was closely monitored by clinical interviews, and no mood stabilizers and antidepressants were given. Psychotic symptoms at baseline and after 8-week of treatment were evaluated using PANSS.
Treatment response at 8 weeks was evaluated with the Remission criteria of the Schizophrenia Working Group (RSWG) as the primary outcome (34). The criteria identifies an absolute threshold in the severity of symptoms and defines patients as responders if they simultaneously had a final score ≤ 3 for at least 6 months on eight core symptoms of the PANSS: P1, P2, P3, N1, N4, N6, G5, and G9. This study used the criteria without the duration requirement.
Cognitive Assessments
Cognitive assessments were conducted by two well-trained psychiatrists. The cognitive domains were selected for executive function, verbal fluency, attention, and processing speed, attention distribution, working memory, and motor speed. We assessed cognitive function using Wisconsin Card Sorting Test (WCST-C: categories and WCST-P: perseverative errors, 128 cards), Verbal Fluency Test (VFT), Trail Making Test (TMT-part A and TMT-part B), Digit Span Tests (DST-Forward and DST-Backward), and Stroop Tests (Stroop-W: words, Stroop-C: colors, and Stroop-I: interference). The details of cognitive assessments have been described elsewhere (35). All participants were assessed cognitive function at baseline and 36 patients repeated cognitive assessments after 8-week follow-up. Cognitive improvement was measured by using the difference of cognitive performance between follow-up and baseline.
Expression Analyses
Blood was collected from all participants in BD tubes before being processed. Total RNA sample was isolated by using the TRIZOL Reagent (Life Technologies). The RNA integrity was determined through electrophoresis on a 1.0 % agarose gel. The concentrations and purity of the RNA samples were determined using the Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Two micrograms of total RNA for each sample was used for complementary DNA (cDNA) synthesis using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA) in a total reaction volume of 20 μL, according to the manufacturer's protocol.
The mRNA expression of COMT, 5-HTR2A, and SLC6A4 were measured by quantitative polymerase chain reaction (qPCR). We designed different pairs of primers and probes to detect the targeting mRNA expression. The primers for COMT mRNA expression are: forward: 5′- TGGTACTGAAGGTGCCAGAC-3′; reverse: 5′-GTTCAGAGAGGTTAGCATGTCA-3′; probe: FAM-CCTGCTGACCTTCTGCGGCTCC-BHQ1. The primers for 5-HTR2A mRNA expression are: forward: 5′-AATAGCGACGGAGTGAATGA-3′; reverse: 5′-AGATAGGTGAAAACTTGCTCAG-3′; probe: FAM-TGCCTTCCACAGTTGCCACGG-BHQ1. The primers for SLC6A4 mRNA expression are: forward: 5′-GATGAGTTCCCACACGTCTG-3′; reverse: 5′-GTGACCAGGGATCCAAAGAAG-3′; probe: FAM-TGACCACGGCGAGCACGAACC-BHQ1. We selected the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as endogenous controls, because it is adequate expression in the blood and shows high stability between the different samples (36, 37). The GAPDH primers: forward: 5′-GTTCCAATATGATTCCACCCATG-3′; reverse: 5′-GGGATTTCCATTGATGACAAGC-3′; probe: FAM-CATGGCACCGTCAAGGCTGAGAAC-BHQ1. All qPCR were performed with TaqMan Universal PCR Master Mix on a Roche LightCycler® 480 machine (Roche Applied Science, Mannhein, Germany). Each sample was quantified in triplicate with an initial denaturing step of 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. The efficiencies of quantitative PCR amplifications for both target and reference genes were tested by a wide range of diluted cDNA dilution and ranges from 92 to 102%. Relative mRNA expression was calculated with comparative Ct method (ΔCt = Ct target–Ct reference; Supplementary file), and data were analyzed using the Light cycler® 480 software Version 1.5.
Data Analyses
All data were analyzed using the Statistical Package for Social Sciences (SPSS, version 20.0 for Windows). Kolmogorov-Smirnov tests were performed to detect the normal distribution of continuous variables, and data with skewed distribution were natural transformed prior to analysis. Data in schizophrenia patients (responders and non-responders) and controls were compared using chi-squared test for categorical variables, and independent Student's t-test or one-way ANOVA followed by the least significant difference (LSD) post-hoc analyses were applied for continuous variables. To examine the interaction between COMT, 5-HTR2A, and SLC6A4 mRNA expressions, partial correlation analyses between these three genes were tested in the patient and control groups separately controlling for age, gender and education. Furthermore, exploratory regression analyses were performed to examine the relationships of these three genes expressions with baseline clinical phenotypes (symptom severity and cognition) as well as cognitive improvement after treatment in patients. Correlations between baseline mRNA expression of COMT (or 5-HTR2A, SLC6A4) and clinical variables (baseline total PANSS score, baseline cognitive scores, or cognitive improvement) were computed by Stepwise multiple regression analyses, controlling for age, gender, education, and duration of illness. Bonferroni corrections were conducted to each test to adjust for multiple testing. Finally, we also performed repeated measures ANOVAs (RMANOVAs) to test longitudinal within-group changes (responders and non-responders), and group × time (baseline and follow-up) interaction for the levels of mRNA expression. All analyses were two-sided and p-value of 0.05 was considered statistically significant.
Results
Demographic Data
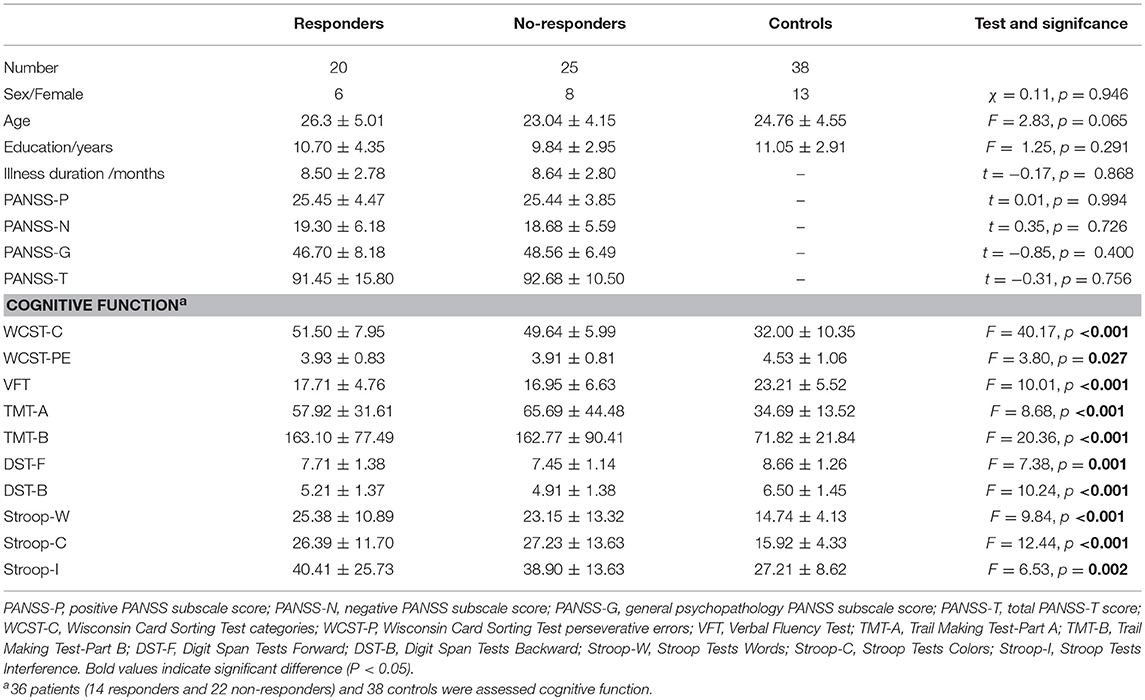
Demographic and clinical characteristics of all participants were shown in Table 1. According to RSWG criteria, 20 patients were classified as responders and 25 patients were classified as non-responders. There are no significant differences in age, sex, and education among three groups (Table 1). No significant differences of symptom severities at baseline between responders and no-responders in PANSS total symptoms (91.45 ± 15.80 vs. 92.68 ± 10.50, p = 0.756), PANSS positive symptoms (25.45 ± 4.47 vs. 25.44 ± 3.85, p = 0.994), PANSS negative symptoms (19.30 ± 6.18 vs. 18.58 ± 5.59, p = 0.726), and PANSS general symptoms (46.70 ± 8.18 vs. 48.56 ± 6.49, p = 0.400) were observed.
One-way ANOVAs for cognitive function found that there are significant differences among three groups in all items (Table 1). Among them, however, WCST-PE did not pass Bonferroni testing (p > 0.05). Furthermore, post-hoc analyses for cognitive function demonstrated no significant differences in WCST-C (d = 1.86, p = 0.539), WCST-PE (d = 0.02, p = 0.952), VFT (d = 0.76, p = 0.700), TMT-part A (d = −7.77, p = 0.442), TMT-part B (d = 0.33, p = 0.988), DST-Forward (d = 0.26, p = 0.545), DST-Backward (d = 0.31, p = 0.529), Stroop-W (d = 2.23, p = 0.476), Stroop-C (d = 0.84, p = 0.797) and Stroop-I (d = 1.51, p = 0.765) between responder and non-responder patients groups.
COMT, 5-HTR2A and SLC6A4 mRNA Expressions in Non-Responder and Responder Patients vs. Healthy Control Subjects
In the one-way ANOVAs, we found significant differences among responder, non-responder and control groups for the mRNA expressions of COMT (F = 61.05, p < 0.001), 5-HTR2A (F = 4.60, p = 0.013) and SLC6A4 (F = 13.73, p < 0.001). The post-hoc analyses showed that compared with controls, both responders and non-responders have significant higher COMT expression (d = 2.12, p < 0.001 and d = 1.60, p < 0.001) and lower SLC6A4 expression (d = −1.23, p = 0.001 and d = −1.66, P < 0.001). While a significant lower level of 5-HTR2A expression was observed in non-responders (d = −1.49, p = 0.01) when compared with controls, but not observed in responders (d = 0.40, p = 0.530). Interestingly, responders had significantly higher COMT (d = 0.52, p = 0.027) and 5-HTR2A (d = 1.89, p = 0.007) expressions when compared with non-responders. However, there were no significant difference in SLC6A4 expression (d = 0.43, p = 0.277) between responders and non-responders (Figure 1).
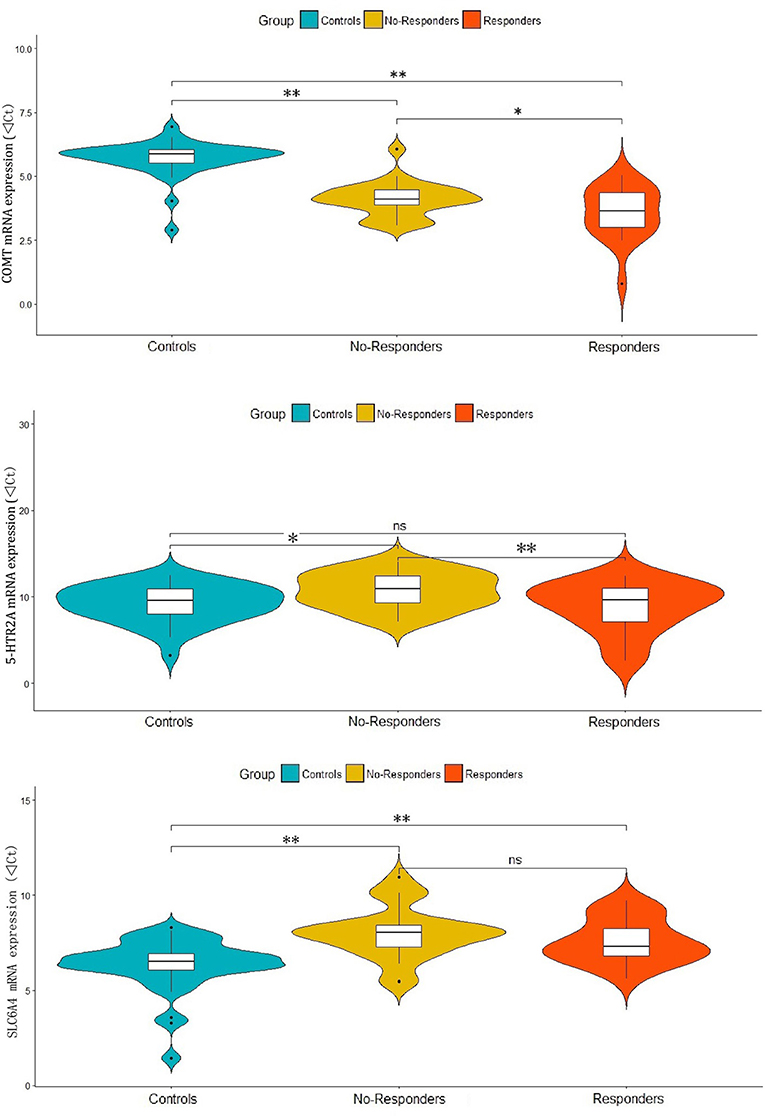
Figure 1. Differential expressions of COMT, 5-HTR2A and SLC6A4 in responder and non-responder of patients and healthy controls. **p-value < 0.01, *p-value < 0.05 and ns, no significance.
Interaction of COMT, 5-HTR2A, and SLC6A4 mRNA Expressions in Schizophrenia Patients and Controls
Partial correlation analyses showed significant positive correlations between 5-HTR2A and SLC6A4 mRNA expressions in both schizophrenia patients (r = 0.44, p = 0.004) and controls (r = 0.69, p < 0.001). These positive correlations remain significant after corrected with Bonferroni (p < 0.05). However, no significant correlation was observed among other genes (all p > 0.05).
Association of COMT, 5-HTR2A, and SLC6A4 mRNA Expressions With Baseline Symptom Severity, Cognitive Function, and Cognitive Improvement in Schizophrenia Patients
Stepwise multiple regression analyses found that no factor has a significant effect on the total PANSS score. In addition, analyses for cognitive function identified 5-HTR2A as the influencing factor for VFT (β = −0.47, p = 0.003), Stroop-W (β = 0.42, p = 0.012), Stroop-C (β = 0.36, p = 0.033) and Stroop-I (β = 0.45, p = 0.006). Similarly, age was identified as a influencing factor for DST-Backward (β = −0.39, p = 0.018) and TMT-part B (β = 0.47, p = 0.004). In addition, stepwise multiple regression analyses for cognitive improvement over 8-week treatment suggested SLC6A4 as a influencing factor for DST-Forward improvement (β = −0.45, p = 0.005), 5-HTR2A as a influencing factor for Stroop-I improvement (β = 0.40, p = 0.017), and education level as a influencing factor for Stroop-C improvement (β = −0.46, p = 0.005). However, all of these findings did not pass Bonferroni testing.
Antipsychotic Effect on COMT, 5-HTR2A, and SLC6A4 mRNA Expression in Schizophrenia Patients
We conducted RMANOVAs to evaluate the gene expression changes over 8 weeks in responders and non-responders. Analyses revealed that there were no significant group effect and time effect on COMT (p = 0.069 and p = 0.520, respectively), 5-HTR2A (p = 0.139 and P = 0.841, respectively) and SLC6A4 (p = 0.966 and p = 0.163, respectively) mRNA expressions with no group by time interaction (p = 0.471, p = 0.053 and p = 0.111, respectively).
Discussion
The present study has three major findings. (1) There are significantly higher COMT mRNA expression and lower SLC6A4 mRNA expression in schizophrenia patients, and a significant lower 5-HTR2A mRNA expression in non-responder patients when compared with healthy controls. (2) Responder patients have significantly higher levels of COMT and 5-HTR2A mRNA expressions than non-responder patients before antipsychotic treatment. (3) Antipsychotic treatment has no significant effect on the expressions of COMT, 5-HTR2A and SLC6A4 mRNA over 8-week follow up.
COMT is a catabolic enzyme involved in the degradation of cortical dopamine and plays a critical role in modulating the activity of prefrontal circuitry. Previous genetic association studies suggested that individuals with high-activity variants have a higher risk for schizophrenia (16). These findings are consistent with our result that schizophrenia patients have a significantly increased COMT mRNA expression compared with controls. However, prior studies have reported inconsistent results regarding COMT mRNA expression in schizophrenia. For example, although many studies reported no evidence of altered COMT mRNA expression in schizophrenia (38–40), some studies demonstrated a lower COMT mRNA expression in patients (24, 41). One possible explanation for these discrepancies is that several factors such as demographics, medications, disease state, tissues, and expression assays might confound these results across different studies.
More interestingly, we found that the aberrant COMT mRNA expression in responder patients is more serious than that of in no-responder patients, indicating that patients with higher level of COMT mRNA were more likely to have a better antipsychotic response. This finding is consistent with the tonic-phasic dopamine hypothesis regarding the antipsychotic response (42). According to this hypothesis, the high activity of COMT enzyme can increase phasic and decrease tonic dopamine transmission subcortically and reduce dopamine concentrations in PFC, then lead to an increased D2–mediated dopamine transmission (42). As a result, individuals with higher activity of COMT enzyme will have a better response to D2-blocking agents such as risperidone.
A further major finding of this study is that non-responder patients have a significantly lower 5-HTR2A mRNA expression when compared with responder patients. The serotonin 2A receptor is the most important excitatory receptor of serotonergic system and regulates serotonin transmission in the brain (3). In addition, the serotonin 2A receptor also interacts with dopamine systems and indirectly increases dopamine release in the PFC (11). As one of the main antipsychotic target, 5-HTR2A has been suggested as an important player in antipsychotic treatment. Numerous pharmacogenetic studies have reported that 5-HTR2A rs6314 T allele is associated with a poor treatment response to antipsychotic medicines (43–45). More recently, a study with converging evidence suggests rs6314 T allele significantly associated with decreased 5-HTR2A mRNA in postmortem prefrontal cortex and HeLa cells (46). Taken together, these evidences indirectly support our finding that decreased 5-HTR2A mRNA may predict a poor response to antipsychotic treatment.
The SLC6A4 gene encodes serotonin transporter that plays a critical role in serotonergic neurotransmission by the reuptake of serotonin from the synaptic cleft. Our study found decreased SLC6A4 mRNA expression in schizophrenia, which is consistent with a previous postmortem study(25), indicating that the reduced SLC6A4 mRNA may implicate in the pathophysiological process of schizophrenia. Interestingly, we found a significant positive correlation between 5-HTR2A and SLC6A4 mRNA expressions in all subjects. This is similar to a prior study which suggested that interaction between variants of 5-HTR2A and SLC6A4 polymorphisms might be involved in the etiology of schizophrenia (47). Further evidence showed that serotonin transporter binding has been reported to be heavily colocalized with 5-HT2A receptors in prefrontal regions (48), and the HTR2A gene appears to moderate serotonin transporter binding in key limbic regions (49). Taken together, these findings support that the serotonin transporter and 5-HT2A receptor are biologically and functionally linked although the precise mechanism in schizophrenia is still unclear.
Finally, our findings suggest that there is no significant effect of antipsychotic treatment on COMT, 5-HTR2A, and SLC6A4 mRNA expressions. Several previous studies found that there are prominent alterations in these genes expressions after antipsychotic treatment, but with exactly opposite effects (26, 41, 50). Furthermore, some studies reported these genes' expressions existing region-specific alterations along with antipsychotic treatment (51–53). Given that the relationships between antipsychotic drugs and dopaminergic/serotonergic genes expressions (COMT, 5-HTR2A, and SLC6A4) in schizophrenia remain inconclusive, it is necessary to elucidate the mechanisms of antipsychotic drugs implicated in dopaminergic/serotonergic genes expressions before any final conclusions to be made.
This study has some limitations. Firstly, our study investigated gene expression from a mixture of peripheral blood cells. Given that schizophrenia is one of brain disorders, there are evident limitations using peripheral blood cells to explore the disease pathophysiology although some studies have suggested blood shares significant gene expression similarities with brain tissues (54, 55). Secondly, there are potential eQTLs effects that may cofound our results; however we did not genotype the variants of targeted genes to preclude this confounding factor. Thirdly, the sample size in this study is relatively small, which might lead to insufficient statistical power to detect some true and slight differences. Finally, our study focused on three well-known candidate genes, it precludes the discovery of dopaminergic and serotonergic genes that may play important roles in disease pathophysiology and antipsychotic treatment. Therefore, our results should be cautiously interpreted with the consideration of these limitations.
In summary, dysregulated COMT and SLC6A4 mRNA expressions in schizophrenia provides further evidence supporting that these two genes may implicate in the pathophysiology of schizophrenia. The higher COMT and 5-HTR2A mRNA expressions in the responder patients indicate that these two genes mRNA may serve as predictors for antipsychotic treatment. However, the mechanisms of COMT and 5-HTR2A implicated in symptom improvement over antipsychotic treatment are still unknown, which deserve further investigation in the future.
Ethics Statement
This study was carried out with written informed consent from all subjec0ts. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by The Ethics Committee of the Second Xiangya Hospital of Central South University, China.
Author Contributions
JT, XC, MH, and XZ designed the study. MH, XZ, YH, HH, DW, JZ, HR, and LY collected the samples. ZL, YZ, XM acquired the data. ZL and YH analyzed the data and wrote the article. All authors have approved the submission.
Funding
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Hunan Provincial Innovation Foundation for Postgraduate (CX2016B064 to ZL), National Natural Science Foundation of China (81471361 to XC, 81371480 to JT), Wuhan Science and Technology Bureau grant (2017060201010169 to MH). China Scholarship Council Program (201706370045 to ZL, 201506370095 to MH), Natural Science Foundation of Hubei Province (132795 to XZ), Teachers funding project of Wuhan University (2042018kf0125 to XZ), the National Key R&D Program of China (2018YFC1314600 to XZ).The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2018.00577/full#supplementary-material
References
1. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet (2016) 388:86–97. doi: 10.1016/s0140-673601121-6
2. Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. (2005) 353:1209–23. doi: 10.1056/NEJMoa051688
3. Selvaraj S, Arnone D, Cappai A, Howes O. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci Biobehav Rev. (2014) 45:233–45. doi: 10.1016/j.neubiorev.2014.06.005
4. Renard J, Norris C, Rushlow W, Laviolette SR. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev. (2017) 75:157–65. doi: 10.1016/j.neubiorev.2017.02.006
5. Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. (2016) 17:524–32. doi: 10.1038/nrn.2016.57
6. Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatr. (2012) 698:776–86. doi: 10.1001/archgenpsychiatry.2012.169
7. Narendran R, Jedema HP, Lopresti BJ, Mason NS, Gurnsey K, Ruszkiewicz J, et al. Imaging dopamine transmission in the frontal cortex: a simultaneous microdialysis and [11C]FLB 457 PET study. Mol Psychiatr. (2014) 19:302–10. doi: 10.1038/mp.2013.9
8. Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatr. (2015) 72:316–24. doi: 10.1001/jamapsychiatry.2014.2414
9. Seeman P. Dopamine D2 receptors as treatment targets in schizophrenia. Clin Schizophr Relat Psychoses (2010) 4:56–73. doi: 10.3371/csrp.4.1.5
10. Meltzer HY. Serotonergic mechanisms as targets for existing and novel antipsychotics. Handb Exp Pharmacol. (2012) 212:87–124. doi: 10.1007/978-3-642-25761-2_4
11. Di Giovanni G, Esposito E, Di Matteo V. Role of serotonin in central dopamine dysfunction. CNS Neurosci Ther. (2010) 16:179–94. doi: 10.1111/j.1755-5949.2010.00135.x
12. de Bartolomeis A, Buonaguro EF, Iasevoli F. Serotonin-glutamate and serotonin-dopamine reciprocal interactions as putative molecular targets for novel antipsychotic treatments: from receptor heterodimers to postsynaptic scaffolding and effector proteins. Psychopharmacology (2013) 225:1–19. doi: 10.1007/s00213-012-2921-8
13. Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. (2008) 29:445–53. doi: 10.1016/j.tips.2008.06.006
14. Bramness JG, Gundersen OH, Guterstam J, Rognli EB, Konstenius M, Loberg EM, et al. Amphetamine-induced psychosis—A separate diagnostic entity or primary psychosis triggered in the vulnerable? BMC Psychiatr. (2012) 12:221. doi: 10.1186/1471-244x-12-221
15. Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. (2007) 113:296–320. doi: 10.1016/j.pharmthera.2006.08.004
16. Gonzalez-Castro TB, Hernandez-Diaz Y, Juarez-Rojop IE, Lopez-Narvaez ML, Tovilla-Zarate CA, Fresan A. The role of a catechol-o-methyltransferase (COMT) Val158Met genetic polymorphism in schizophrenia: a systematic review and updated meta-analysis on 32,816 subjects. Neuromol Med. (2016) 18:216–31. doi: 10.1007/s12017-016-8392-z
17. Gu L, Long J, Yan Y, Chen Q, Pan R, Xie X, et al. HTR2A-1438A/G polymorphism influences the risk of schizophrenia but not bipolar disorder or major depressive disorder: a meta-analysis. J Neurosci Res. (2013) 91:623–33. doi: 10.1002/jnr.23180
18. Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. (2008) 40:827–34. doi: 10.1038/ng.171
19. Yao J, Pan YQ, Ding M, Pang H, Wang BJ. Association between DRD2 (rs1799732 and rs1801028) and ANKK1 (rs1800497) polymorphisms and schizophrenia: a meta-analysis. Am J Med Genet B Neuropsychiatr Genet. (2015) 168B:1–13. doi: 10.1002/ajmg.b.32281
20. Ripke SNB, Corvin A, Walters JT, Farh KH, Holmans PA, Lee P, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature (2014) 511:421–7. doi: 10.1038/nature13595
21. Ruderfer DM, Charney AW, Readhead B, Kidd BA, Kahler AK, Kenny PJ, et al. Polygenic overlap between schizophrenia risk and antipsychotic response: a genomic medicine approach. Lancet Psychiatr. (2016) 3:350–7. doi: 10.1016/s2215-036600553-2
22. Gaspar H, Breen G. Drug enrichment and discovery from schizophrenia genome-wide association results: an analysis and visualisation approach. Sci Rep. (2017) 7:12460. doi: 10.1038/s41598-017-12325-3
23. Gao L, Feng S, Liu ZY, Wang JQ, Qi KK, Wang K. A computational network analysis based on targets of antipsychotic agents. Schizophr Res. (2018) 193:154–60. doi: 10.1016/j.schres.2017.07.041
24. Bray NJ, Buckland PR, Williams NM, Williams HJ, Norton N, Owen MJ, et al. A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am J Hum Genet. (2003) 73:152–61. doi: 10.1086/376578
25. Hernandez I, Sokolov BP. Abnormal expression of serotonin transporter mRNA in the frontal and temporal cortex of schizophrenics. Mol Psychiatr. (1997) 2:57–64. doi: 10.1038/sj.mp.4000215
26. Hernandez I, Sokolov BP. Abnormalities in 5-HT2A receptor mRNA expression in frontal cortex of chronic elderly schizophrenics with varying histories of neuroleptic treatment. J Neurosci Res. (2000) 59:218–25. doi: 10.1002/(sici)1097-4547(20000115)59:2<218::aid-jnr8>3.0.co;2-h
27. Mocci G, Jimenez-Sanchez L, Adell A, Cortes R, Artigas F. Expression of 5-HT2A receptors in prefrontal cortex pyramidal neurons projecting to nucleus accumbens. Potential relevance for atypical antipsychotic action. Neuropharmacology (2014) 79:49–58. doi: 10.1016/j.neuropharm.2013.10.021
28. Lipska BK, Lerman DN, Khaing ZZ, Weickert CS, Weinberger DR. Gene expression in dopamine and GABA systems in an animal model of schizophrenia: effects of antipsychotic drugs. Eur J Neurosci. (2003) 18:391–402. doi: 10.1046/j.1460-9568.2003.02738.x
29. Huang X-F, Han M, Huang X, Zavitsanou K, Deng C. Olanzapine differentially affects 5-HT2A and 2C receptor mRNA expression in the rat brain. Behav Brain Res. (2006) 171:355–62. doi: 10.1016/j.bbr.2006.03.040
30. Chen M-L, Chen C-H. Chronic antipsychotics treatment regulates MAOA, MAOB and COMT gene expression in rat frontal cortex. J Psychiatr Res. (2007) 41:57–62. doi: 10.1016/j.jpsychires.2005.03.005
31. Hayashi-Takagi A, Vawter MP, Iwamoto K. Peripheral biomarkers revisited: integrative profiling of peripheral samples for psychiatric research. Biol Psychiatr. (2014) 75:920–8. doi: 10.1016/j.biopsych.2013.09.035
32. Lai CY, Scarr E, Udawela M, Everall I, Chen WJ, Dean B. Biomarkers in schizophrenia: a focus on blood based diagnostics and theranostics. World J Psychiatr. (2016) 6:102–17. doi: 10.5498/wjp.v6.i1.102
33. Cattane N, Minelli A, Milanesi E, Maj C, Bignotti S, Bortolomasi M, et al. Altered gene expression in schizophrenia: findings from transcriptional signatures in fibroblasts and blood. PLoS ONE (2015) 10:e0116686. doi: 10.1371/journal.pone.0116686
34. Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatr. (2005) 162:441–9. doi: 10.1176/appi.ajp.162.3.441
35. Oral E, Canpolat S, Yildirim S, Gulec M, Aliyev E, Aydin N. Cognitive functions and serum levels of brain-derived neurotrophic factor in patients with major depressive disorder. Brain Res Bull. (2012) 88:454–9. doi: 10.1016/j.brainresbull.2012.03.005
36. Silver N, Best S, Jiang J, Thein SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol. (2006) 7:33. doi: 10.1186/1471-2199-7-33
37. Murthi P, Fitzpatrick E, Borg A, Donath S, Brennecke S, Kalionis B. GAPDH, 18S rRNA and YWHAZ are suitable endogenous reference genes for relative gene expression studies in placental tissues from human idiopathic fetal growth restriction. Placenta (2008) 29:798–801. doi: 10.1016/j.placenta.2008.06.007
38. Tunbridge E, Burnet PW, Sodhi MS, Harrison PJ. Catechol-o-methyltransferase (COMT) and proline dehydrogenase (PRODH) mRNAs in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and major depression. Synapse (2004) 51:112–8. doi: 10.1002/syn.10286
39. Dempster EL, Mill J, Craig IW, Collier DA. The quantification of COMT mRNA in post mortem cerebellum tissue: diagnosis, genotype, methylation and expression. BMC Med Genet. (2006) 7:10. doi: 10.1186/1471-2350-7-10
40. Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, et al. Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology (2003) 28:1521–30. doi: 10.1038/sj.npp.1300218
41. Noto C, Ota VK, Santoro ML, Gouvea ES, Silva PN, Spindola LM, et al. Depression, cytokine, and cytokine by treatment interactions modulate gene expression in antipsychotic naive first episode psychosis. Mol Neurobiol. (2016) 53:5701–9. doi: 10.1007/s12035-015-9489-3
42. Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology (2004) 29:1943–61. doi: 10.1038/sj.npp.1300542
43. Arranz MJ, Munro J, Owen MJ, Spurlock G, Sham PC, Zhao J, et al. Evidence for association between polymorphisms in the promoter and coding regions of the 5-HT2A receptor gene and response to clozapine. Mol Psychiatr. (1998) 3:61–6. doi: 10.1038/sj.mp.4000348
44. Olajossy-Hilkesberger L, Godlewska B, Schosser-Haupt A, Olajossy M, Wojcierowski J, Landowski J, et al. Polymorphisms of the 5-HT2A receptor gene and clinical response to olanzapine in paranoid schizophrenia. Neuropsychobiology (2011) 64:202–10. doi: 10.1159/000327602
45. Blasi G, Selvaggi P, Fazio L, Antonucci LA, Taurisano P, Masellis R, et al. Variation in dopamine D2 and serotonin 5-HT2A receptor genes is associated with working memory processing and response to treatment with antipsychotics. Neuropsychopharmacology (2015) 40:1600–8. doi: 10.1038/npp.2015.5
46. Blasi G, De Virgilio C, Papazacharias A, Taurisano P, Gelao B, Fazio L, et al. Converging evidence for the association of functional genetic variation in the serotonin receptor 2a gene with prefrontal function and olanzapine treatment. JAMA Psychiatr. (2013) 70:921–30. doi: 10.1001/jamapsychiatry.2013.1378
47. Sáiz PA, García-Portilla MP, Arango C, Morales B, Alvarez V, Coto E, et al. Association study of serotonin 2A receptor (5-HT2A) and serotonin transporter (5-HTT) gene polymorphisms with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatr. (2007) 31:741–5. doi: 10.1016/j.pnpbp.2007.01.012
48. Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature (2012) 489:391. doi: 10.1038/nature11405
49. David SP, Murthy NV, Rabiner EA, Munafó MR, Johnstone EC, Jacob R, et al. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J Neurosci (2005) 25:2586–90. doi: 10.1523/JNEUROSCI.3769-04.2005
50. Taraskina AE, Nasyrova RF, Zabotina AM, Sosin DN, Sosina capital Ka CAC, Ershov EE, et al. Potential diagnostic markers of olanzapine efficiency for acute psychosis: a focus on peripheral biogenic amines. BMC Psychiatr. (2017) 17:394. doi: 10.1186/s12888-017-1562-1
51. de Bartolomeis A, Marmo F, Buonaguro EF, Rossi R, Tomasetti C, Iasevoli F. Imaging brain gene expression profiles by antipsychotics: region-specific action of amisulpride on postsynaptic density transcripts compared to haloperidol. Eur Neuropsychopharmacol. (2013) 23:1516–29. doi: 10.1016/j.euroneuro.2012.11.014
52. Santoro ML, Ota VK, Stilhano RS, Silva PN, Santos CM, Diana MC, et al. Effect of antipsychotic drugs on gene expression in the prefrontal cortex and nucleus accumbens in the spontaneously hypertensive rat (SHR). Schizophr Res. (2014) 157:163–8. doi: 10.1016/j.schres.2014.05.015
53. von Wilmsdorff M, Manthey F, Bouvier ML, Staehlin O, Falkai P, Meisenzahl-Lechner E, et al. Effects of haloperidol and clozapine on synapse-related gene expression in specific brain regions of male rats. Eur Arch Psychiatry Clin Neurosci. (2018) 268:555–63. doi: 10.1007/s00406-018-0872-8
54. Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. Am J Med Genet Part B Neuropsychiatr Genet. (2006) 141:261–8. doi: 10.1002/ajmg.b.30272
Keywords: schizophrenia, COMT, 5-HTR2A, SLC6A4, antipsychotic response, cognitive deficits
Citation: Li Z, He Y, Han H, Zhou Y, Ma X, Wang D, Zhou J, Ren H, Yuan L, Tang J, Zong X, Hu M and Chen X (2018) COMT, 5-HTR2A, and SLC6A4 mRNA Expressions in First-Episode Antipsychotic-Naïve Schizophrenia and Association With Treatment Outcomes. Front. Psychiatry 9:577. doi: 10.3389/fpsyt.2018.00577
Received: 18 May 2018; Accepted: 22 October 2018;
Published: 13 November 2018.
Edited by:
Błazej Misiak, Wroclaw Medical University, PolandReviewed by:
Vanessa Kiyomi Ota, Federal University of São Paulo, BrazilXueqin Song, Zhengzhou University, China
Copyright © 2018 Li, He, Han, Zhou, Ma, Wang, Zhou, Ren, Yuan, Tang, Zong, Hu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinsong Tang, dGFuZ2ppbnNvbmdAY3N1LmVkdS5jbg==
Xiaofen Zong, em9uZ3hpYW9mZW5AZ21haWwuY29t
Maolin Hu, bWFvbGluaHVAaG90bWFpbC5jb20=
Xiaogang Chen, Y2hlbnhpYW9nYW5nQGNzdS5lZHUuY24=
 Zongchang Li1,2,3
Zongchang Li1,2,3 Hongying Han
Hongying Han Jinsong Tang
Jinsong Tang Xiaogang Chen
Xiaogang Chen