- 1Department of Psychological Medicine, Institute of Psychiatry, Psychology & Neuroscience, King's College London, London, United Kingdom
- 2The National Institute for Health Research Health Protection Research Unit in Emergency Preparedness and Response, King's College London, London, United Kingdom
Background: Nocebo effects contribute to a large proportion of the non-specific side-effects attributed to medications and are mainly generated through negative expectations. Previous reviews show that interventions designed to change participants' expectations have a small effect on pain experience. They are also effective in reducing side-effects caused by exposure to sham medications. To date, there has been no review of the influence of such interventions on symptoms attributed to real medicinal treatments.
Objective: To review studies using a randomized controlled design testing the effect of brief psychological interventions compared to usual practice on the side-effect experience to medicinal treatments in healthy volunteers and patients.
Methods: We searched Web of Science, Scopus, Medline, PsycINFO, PsycARTICLES, and Cochrane CENTRAL using search terms for randomized controlled trials along with “nocebo,” “placebo effect,” “medication,” “side-effects,” and associated terms. Studies were eligible if they studied a human population, used an active medicine, delivered a brief psychological intervention intended to influence side-effect reporting compared to usual care or no intervention, and used a randomized controlled design. Because of the heterogeneity of the literature we used a narrative synthesis and assessed evidence quality using the GRADE approach.
Results: Our database search and supplementary search of the reference sections of included studies retrieved 50,140 citations. After screening, full text review and manual reference searches, 27 studies were included. The quality of the studies and evidence was judged to be low. The strongest and most consistent effect came from omitting side-effect information, although surprisingly de-emphasizing side-effects did not affect side-effect reporting. Other techniques, including priming, distraction, and altering the perception of branding, produced mixed results.
Conclusion: Brief psychological interventions can influence side-effect reporting to active medications. Research is currently investigating new ways to de-emphasize side-effects whilst still upholding informed consent, but larger confirmatory trials with suitable control groups are needed. The literature in this area would be improved by more detailed reporting of studies.
Introduction
Nocebo effects, sometimes dubbed the placebo effect's “evil twin,” are the experience of noxious symptoms in response to an inert exposure (1). Nocebo effects can also refer to negative clinical outcomes which are not attributable to the actual pharmacological or physiotherapeutic action of an intervention (2). It is estimated that between 38 and 100% of side-effects reported to drugs taken for a large range of medical conditions are related to the treatment context, rather than the active ingredients of the medication itself (3).
These nocebo-related side-effects are important, as they can affect a patient's well-being (4) and influence their decision as to whether to adhere to their treatment regimen (5, 6). For example adverse media coverage surrounding the safety of statins and their reported side-effects has resulted in around 2,00,000 patients who are no longer taking their statins as directed leading to a predicted increase of 2,000 cardiovascular events in the next decade (7). This is despite the fact that most of these side-effects are probably nocebo-related (8). Perhaps unsurprisingly, side-effects can also result in substantial additional health care costs in terms of additional primary care and hospital visits and also the cost of wasted medication due to non-adherence (9).
Of the multiple factors that may contribute to the development of nocebo effects, expectations of symptoms appear to be the main contributor. These can be generated through verbal and written suggestions about what symptoms to expect, be implied by the apparent dose of a drug, and be learnt through classical conditioning and social observation (10). Studies have used these psychological mechanisms as a means to alter peoples' experience of experimentally induced pain (11), as well as pain following acute medical procedures, such as injections (12) and surgery (13). These effects have been studied in multiple reviews, showing that brief psychological interventions designed to change expectation of pain following treatment have a small but reliable effect on relieving patients' pain compared to usual care (14–16).
However, to our knowledge, there has been no review of whether such interventions can alter patient experience ofside-effects to medicinal treatments. Although evidence demonstrates that such interventions can be effective in altering side-effects reported following exposure to inert substances (10), it is also important to assess if these effects can be transferred to clinical practice. We therefore set out to review studies using a randomized controlled design testing the effect of brief psychological interventions compared to usual practice on the side-effect experience to medicinal treatments in healthy volunteers and patients. To answer the question: can brief psychological interventions influence the side-effect experience to medications?
Methods
Our reporting of this systematic review adheres to the standards for the Preferred Reporting Items for Systematic reviews and Meta-Analyses (17). The protocol for this review was prospectively registered on PROSPERO (CRD42018091903).
Identification of Studies
We searched the following electronic databases with a predefined search strategy: Web of Science, Scopus, OvidSp (Medline, PsycINFO, and PsycARTICLES) and Cochrane CENTRAL. We included Web of Science and Scopus for their coverage of the sciences and social sciences. OvidSp was chosen for its coverage of journals chiefly in the area of health sciences, and also for its inclusion of the databases PsycINFO and PsycARTICLES. Cochrane CENTRAL was included due to its coverage of randomized controlled trials and because it includes records which are derived from other sources to the ones already chosen.
In preliminary work we tested a variety of search strategies in an effort to balance specificity and sensitivity. Our final search strategy used the recommended search terms to identify randomized controlled trials (18) along with the terms and associated words for “nocebo,” “placebo effect,” “medication,” and “side-effects.” We used separate search strategies for each of the databases as these needed to be modified due to differences in MeSH terms, boolean operators and wildcards. A copy the search strategy we used for Medline can be seen in the Supplementary Material.
Review Process
The search was initially carried out on 22nd March 2018 and updated on 22nd June 2018 following the identification of a relevant study published between this time. The initial electronic searches were combined using EndNote and duplicates were identified and deleted. The titles and abstracts of citations were then screened for potential relevance. If relevance was not clear from the abstract, the study was taken forward to the full text review. All full text versions of papers that were potentially relevant were then screened in relation to the inclusion criteria. Papers that met the inclusion criteria had their reference sections manually searched for other studies that could be included.
Selection Criteria
Studies were eligible for inclusion in this review if they met the criteria below.
Population
Human population (healthy volunteers, patients and children were allowed).
Exposure
Active medicinal treatment (i.e., contains a pharmacological agent), associated with side-effects.
Intervention
A brief, psychological intervention delivered in one session and that could be feasibly introduced within a single doctor-patient consultation or treatment appointment. By psychological we mean an intervention that targets certain psychological processes, such as cognitive expectations, attention or learning. Interventions requiring biological or chemical stimuli were excluded because these are not purely psychological. As we wanted to identify interventions that could be easily incorporated into clinical practice, in-depth psychological interventions, such as cognitive behavior therapy, mindfulness, relaxation training or guided imagery, or that consisted of intensive educational packages were excluded as these typically are not delivered in one session and often take place over the course of a treatment.
Comparator
Usual care. We excluded studies with control conditions involving a different type of intervention.
Outcome
We included studies with an outcome of side-effects measured via self-report or inferred through objective measures. We followed the NICE (19) definition of a side-effect as “An effect of a drug (or treatment or intervention) that is additional to the main intended effect. It could be good, bad or neutral, depending on the circumstances.” For some studies, e.g., those concerning infant experience to vaccinations, side-effects were measured within minutes of the procedure. We excluded these on the bases that the “side-effects” were presumably related to the insertion of the needle rather than the effects of the vaccine itself.
Study Design
Used an experimental design in which participants were randomized or quasi randomized to receive the intervention or the control condition.
Other Criteria
Published in the English language.
Data Extraction
We extracted data from the final set of studies using a data extraction table developed for this systematic review. Data extracted included the study design and methodology, main demographics of participants, description of intervention and control conditions, side-effect measures, statistical approach and results. We also extracted details about the mode of the intervention, its content and duration.
Quality Assessment
We assessed the quality of all eligible studies using the Cochrane Collaboration's Risk of Bias tool for randomized controlled trials (20).
Data Synthesis and Analysis
Due to the heterogeneity in the interventions that we included and the way that side-effects were measured, scored and analyzed, we used a narrative synthesis to analyse the results. There is no general consensus on the best way to carry out a narrative synthesis for systematic reviews (21). As such we decided to use a weight of evidence approach by identifying the quality of evidence for each type of intervention reviewed. To do this we used the GRADE approach (22) which is a transparent framework used to grade the quality of evidence included in systematic reviews and the strength of recommendations.
Results
Search Results
The database search retrieved 50,133 citations and searching the reference lists of included studies retrieved another 7, giving a total of 50,140. After removing duplicates 40,346 citations remained. After screening titles and abstracts, we reviewed the full text of 63 articles relating to 66 studies. Of these, 39 studies were excluded for not meeting the inclusion criteria, resulting in a total of 26 articles reporting on 27 studies. One article (23) reported results on two separate studies and is referred to in the text and tables as Study 1 or Study 2 where necessary. The number of studies at each stage of the search strategy and the reasons for exclusion are shown in Figure 1.
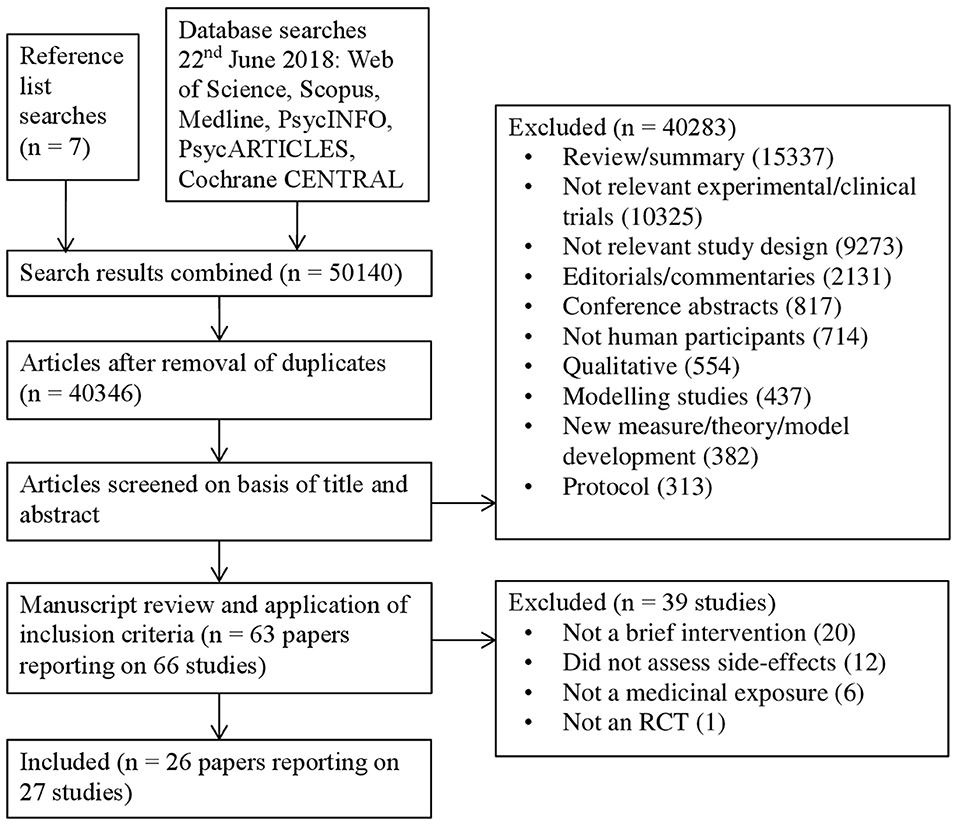
Figure 1. Flow diagram of the selection process of studies including the number of events and reasons for exclusion.
Study Characteristics
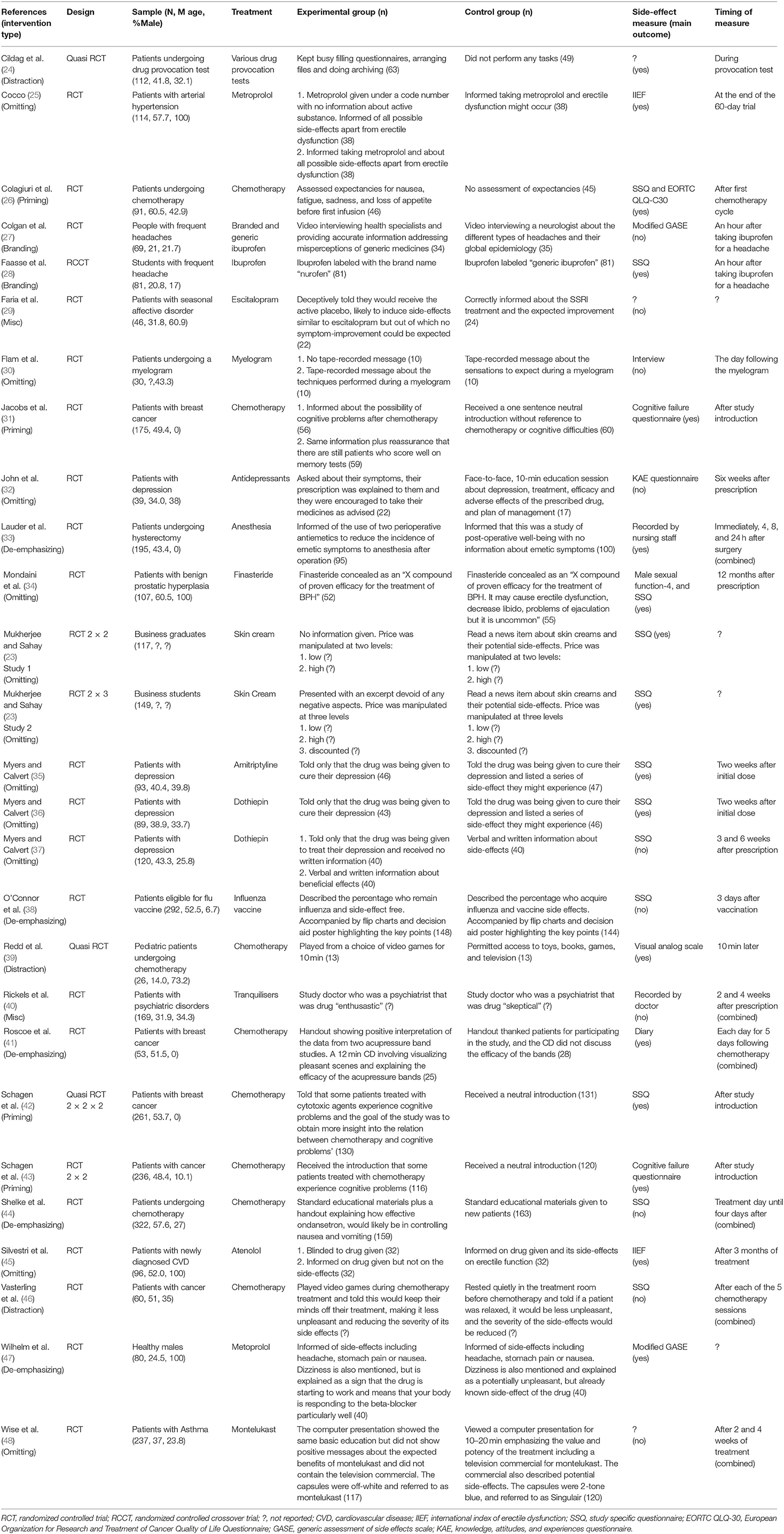
See Table 1 for a full summary of the characteristics of the included studies. The 27 studies included in the review reported on a total of 3,459 participants. There was a range of patient groups and treatments under investigation. The most common of these were patients with cancer receiving chemotherapy (26, 31, 39, 41–44, 46), and patients with depression prescribed anti-depressants (29, 32, 35–37). All studies used a between participants RCT design apart from Cildag et al. (24), Myers and Calvert (36), Redd et al. (39), and Schagen et al. (42) which used a quasi-randomized approach, and Faasse et al. (28) who used a within-subjects RCT design. Some studies used a factorial design in their RCT involving different experimental conditions or baseline variables entered as independent factors (23, 31, 39, 41–43). In these cases, we have reported the main effects of the relevant intervention under investigation.
There were a variety of interventions used by included studies. We looked for common themes and content of the various interventions and were able to group the studies into five different types, these were: priming, distraction, branding, omitting side-effects, and de-emphasizing side-effects, plus a miscellaneous group.
The majority of studies used an un-validated questionnaire specifically designed for their study to measure side-effects, and measures were generally completed within days/weeks following treatment initiation.
Quality Assessment
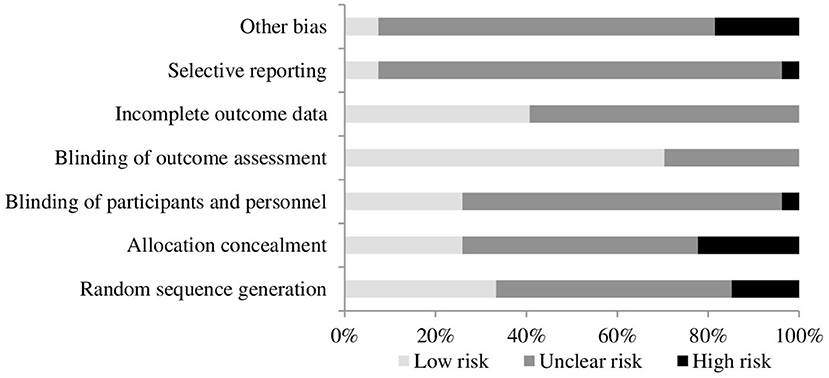
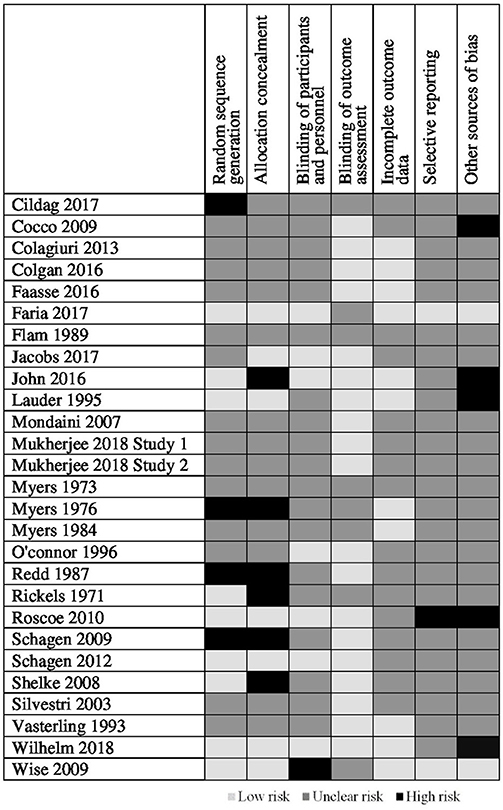
The quality of included studies was poor (see Figures 2, 3). The main problem was a lack of clear reporting within the papers. Over half of the studies neglected to mention how they carried out randomization, and four were at high risk for using a quasi-randomized approach. Because of the unclear reporting of random sequence generation, the risk for allocation concealment bias followed a similar pattern, and six studies were at high risk because their randomization approach allowed research staff to foresee subsequent allocations. For blinding of participants and personnel, studies often failed to state whether the experimenters were blind to the manipulation that accompanied the active treatment, leaving the risk of bias unclear. Only seven studies used adequate blinding procedures, with one not using blinding at all. Nineteen studies used side-effect measures which were completed by participants, as such blinding of the outcome assessment was judged unlikely to influence these results. For the remaining eight studies it was unclear if participants filled in the measures themselves or if they were administered by a blind/non-blind member of the study team. For 16 studies, drop outs were not addressed, or if they were, the paper typically failed to explain how this affected the results, leaving the risk of bias unclear; the remaining 11 studies provided adequate information and reasoning behind drop outs. Only two studies had lodged a protocol in a publicly accessible registry before the start of recruitment, leaving us unable to assess the risk for selective reporting for the remaining studies, apart from one in which there was a change in the prespecified primary analysis suggesting there was a high risk of bias.
Quality of the Evidence
The quality of evidence regarding priming, distraction, omitting side-effect information and de-emphasizing side effects, and doctor characteristic intervention(s) was very low. This is because most of the information came from studies at low or unclear risk of bias, in which plausible bias could alter the results. There was also some evidence of inconsistency and imprecision in the results due to opposite findings, wide confidence intervals and some small studies which may not have been adequately powered. Due to the broad nature of this systematic review, there is no evidence of indirectness, as all included studies helped to answer the question. It is plausible however, there may have been some publication bias due to the preponderance of smaller studies.
The quality of evidence regarding the branding intervention studies was low. This was graded similarly due to the reasons discussed for the above interventions, however the inconsistency in the results could perhaps be explained by differences in the interventions, and we judged that the small studies were probably due to this literature representing an early evidence base, rather than publication bias.
Finally, the quality of evidence for the deception intervention was moderate. There was some imprecision evident and the sample size was small, however the study was judged to have a low overall risk of bias, and there was no evidence of indirectness. As only one study was included, inconsistency and publication bias could not be determined.
Effect of Interventions on Side-Effect Reporting
Priming
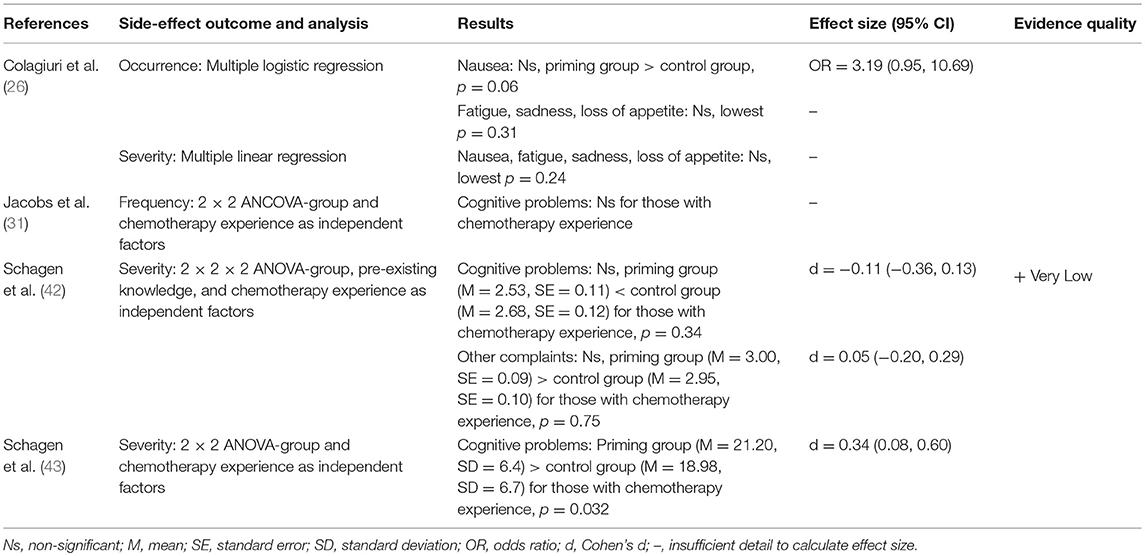
Four studies looked at the effect of priming on side-effect reporting following chemotherapy with mixed results (see Table 2). Colagiuri et al. (26) found a slight trend for priming patients by assessing their expectancies for side-effects vs. no assessment on subsequent nausea. Jacobs et al. (31) and Schagen et al. (42) found no indication of an effect of priming patients by mentioning that chemotherapy is associated with cognitive problems on retrospectively reported cognitive side-effects. However, Schagen et al. (43) in a similar study did find a small effect of priming leading to increased reporting of previous cognitive side-effects to chemotherapy compared to those in a control group who received no such information.
Distraction
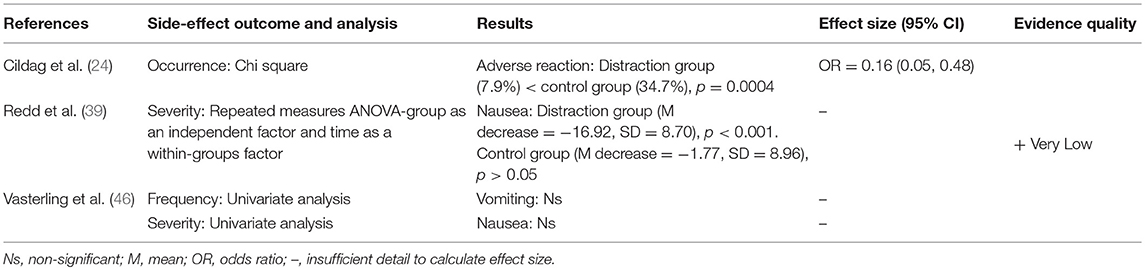
Three studies looked at the effect of distraction on side-effect reporting following chemotherapy and drug provocation tests, showing some evidence that distraction can reduce side-effect reporting (see Table 3). Cildag et al. (24) found that keeping patients busy with filling/archiving files significantly reduced the occurrence of adverse reactions compared to a control group, but only by a small amount. Redd et al. (39) found that distracting pediatric cancer patients with video games significantly reduced chemotherapy nausea from baseline, compared to those in the control group. However, for adult cancer patients, Vasterling et al. (46) found that video games were not effective in reducing chemotherapy nausea or vomiting compared to a control group.
Branding
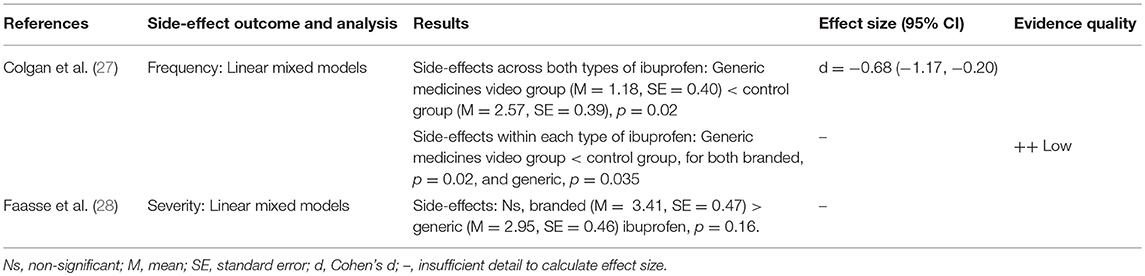
Two studies looked at the effect of branding on side-effect reporting to ibuprofen showing some evidence that branding can affect side-effect reporting (see Table 4). Colgan et al. (27) found that a video designed to correct participants' beliefs about generic medicines significantly reduced side effects for both branded and generic ibuprofen compared to those in a control group, showing a large effect. However, Faasse et al. (28) found that simply changing the labeling of ibuprofen from branded to generic did not significantly affect side-effect reporting.
Omitting Side-Effect Information
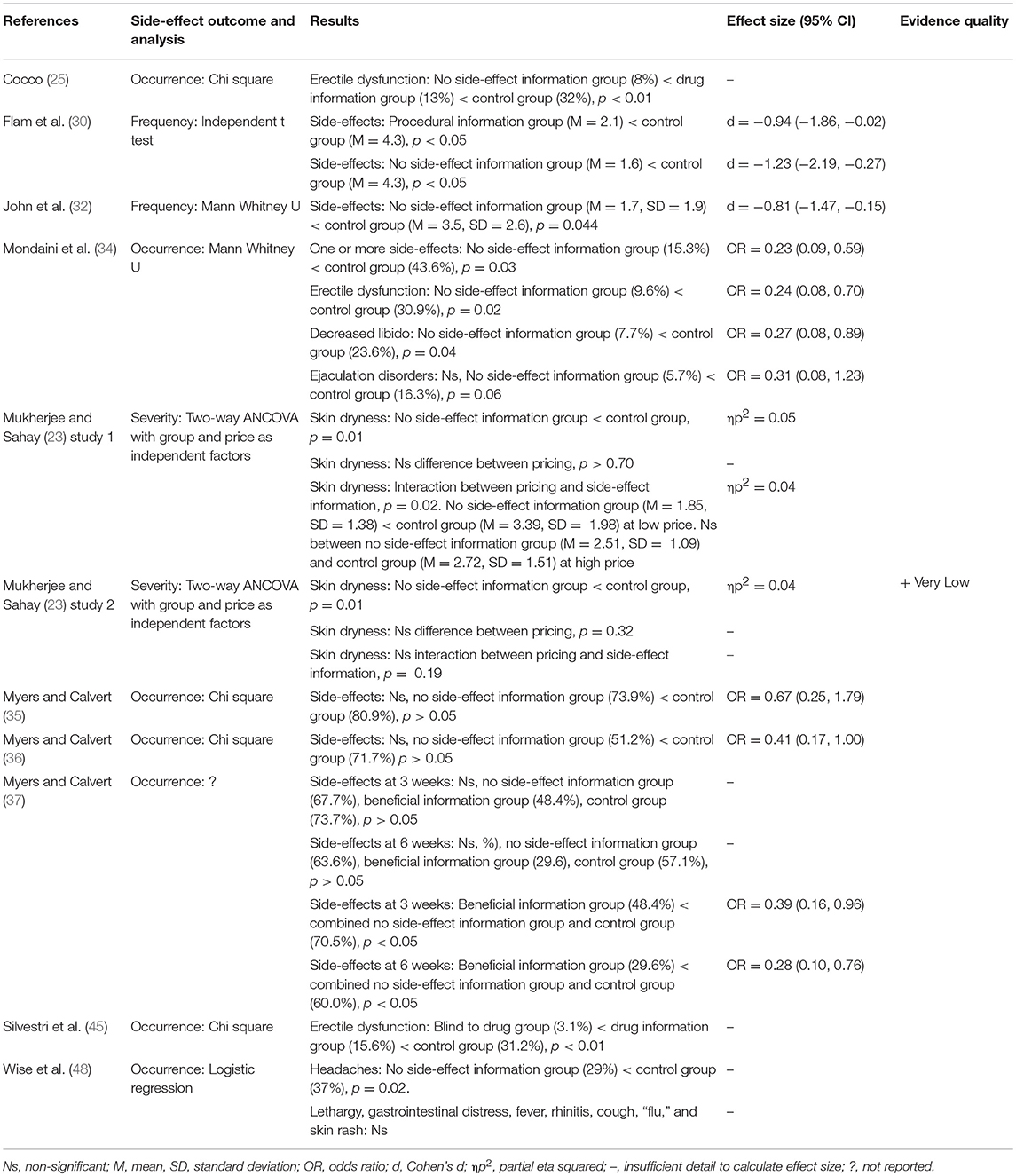
Eleven studies looked at the effect of omitting side-effect information on side-effect reporting to a range of different treatments, showing that omitting side-effects significantly decreases side-effect reporting (see Table 5). Eight studies found that not informing patients about potential side-effects significantly decreased side-effect reporting to metropolol (25), a myelogram (30), antidepressants (32), finasteride (34), skin cream (23) (study 1 and 2), atenolol (45), and montekulast (48) compared to a control group which received side-effect information, each showing large effect sizes. Similarly Myers and Calvert (36) found a trend for a decrease in side-effect reporting when patients were not informed about the side-effects to the antidepressant dothiepin compared to a control group, and Myers and Calvert (37) found that side-effects significantly decreased to dothiepin when comparing the group that only received beneficial information to groups that received no information and side-effect information. Only one study, Myers and Calvert (35), found no effect of side-effect information on subsequent side-effect reporting.
De-emphasizing Side-Effects
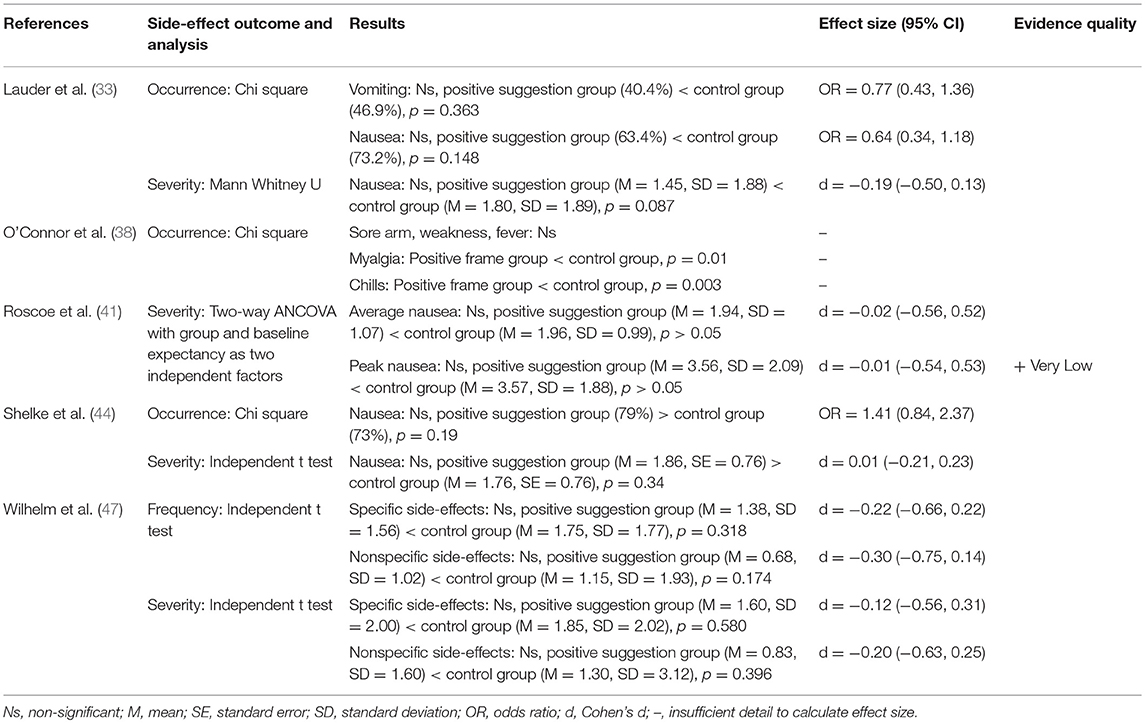
Five studies looked at the effect of de-emphasisng side-effects on side-effect reporting to range of different treatments, showing evidence that this seems to have no effect (see Table 6). Three studies found that informing patients of side-effects but in a way that does not make them seem as bad had no effect on side-effect reporting to anesthesia (33), or chemotherapy (41, 44), however this was compared to a control group that did not receive any information about side-effects. O'Connor et al. (38) found that positively framing side-effects to emphasize those that remain side-effect free and comparing to a control group that received standard information about side-effects significantly reduced side-effect reporting to the flu vaccine. Wilhelm et al. (47) found that positively framing side-effects by explaining they are a sign that the drug is working did not significantly reduce side-effects to metoprolol compared to those who received standard information.
Other Interventions
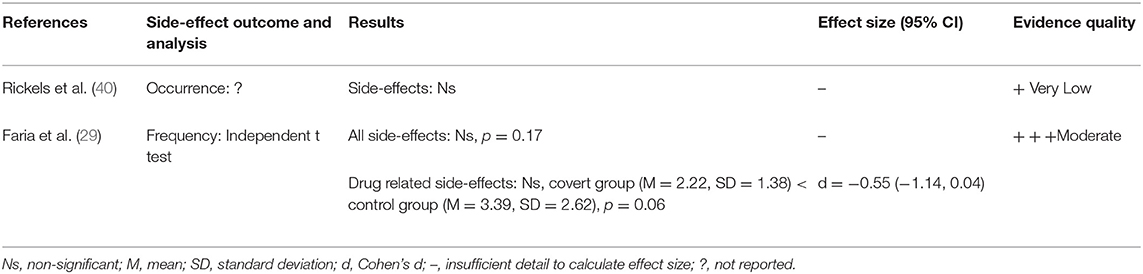
Two other studies investigated interventions which do not fall into the above categories (see Table 7). Faria et al. (29) deceptively told seasonal affective disorder patients that they would receive an active placebo which would produce similar side-effects to escitalopram when in fact they received the active drug itself and found this showed a trend in decreasing reported side-effects compared to a control group who were correctly informed. Rickels et al. (40) found no effect of the prescribing psychiatrist being a drug “enthusiast” or drug “skeptical” on reported side-effects to tranquilisers among psychiatric patients.
Discussion
Summary of Main Findings
Although previous literature has looked at altering side-effects generated in response to inert exposures, it is important to test if these interventions also work in the clinical setting and affect side-effects to real medications which may be initiated or exacerbated through a nocebo effect. This can then provide the basis for introducing into clinical practice strategies to reduce these side-effects. Unfortunately, the quality of the studies identified in this review were generally low quality mainly due to the lack of clear reporting, inadequate randomization and allocation procedures, and unpowered effects. Our overriding recommendation, therefore, is that additional, better quality work is needed in this field.
This point notwithstanding, from the results of the included studies, the strongest and most consistent effect in altering side-effects experienced following medical treatments was omitting information about side-effects. Other techniques, such as priming, distraction and altering the perceptions of branding produced mixed results. More tentatively, studies which investigated over the counter medications, common prescription medications, and vaccines seemed to be more susceptible to these interventions than those which studied chemotherapy.
The finding that omitting side-effect information produced the most consistent and strongest effect supports the evidence from the literature on inert exposures (10) which recommends that in order to reduce side-effects induced by nocebo effects we should avoid giving suggestions of side-effects associated with medications to patients. It also echoes what is found in experimental nocebo studies which find that altering information about side-effects alters side-effect experience to infrasound (49), and electrical pain stimuli (50). In addition, this supports previous work showing that interventions designed to change patients' expectations of pain by altering verbal suggestions about the pain to expect after a treatment or procedure can relieve (placebo) or increase (nocebo) patients baseline pain depending on the suggestion (15, 16), highlighting the role that expectations play in both placebo and nocebo effects. Perhaps unsurprisingly no study looked at the effect of omitting information about side-effects to chemotherapy, and therefore we cannot say if the results extend to chemotherapy too. However, as chemotherapy is already well-known for its side-effects, it may be that omitting side-effect information would do little to alter subsequent side-effect reporting in this group.
Not mentioning side-effects to patients in order to reduce these effects is ethically problematic and may not meet the requirements of informed consent, something which has been widely discussed in the literature (51, 52). An alternative approach is to explain the potential side-effects to patients in a way that de-emphasizes them and reduces their apparent likelihood or severity (53). At first look, the results of studies which have used this approach do not appear promising. Most studies have showed no effect of de-emphasizing side-effects on subsequent side-effect experience. However, this might be an artifact relating to the design of these studies, in which the groups that received the de-emphasized side-effect information were compared to a control groups that received no side-effect information at all. Explaining side-effects to patients, albeit in a positive light, is still likely to increase the perceived likelihood of side-effects compared to not describing side-effects. It would be interesting for future studies to test the effects of de-emphasizing side-effects of medication compared to a suitable control group which receives standard side-effect information. In other studies which used an appropriate control group, positive framing of side-effects was shown to be beneficial, a finding that has also been reproduced in healthy adults taking an inert tablet (54). There is also scope for further investigations about framing the side-effects of medication as a sign that the drug is working. This was investigated in a pilot study that, although not powered to find an effect, nonetheless showed a decrease in side-effect measures among participants who believed the medicine to be harmful (47). This idea of de-emphasizing side-effects has shown some promise in the placebo literature on pain, in which positive messages which focus more on the beneficial outcome of treatments rather than the potential side-effects may be more effective in relieving patients pain compared to usual care messages (14).
Priming patients by informing them about the side-effects to chemotherapy and then asking them to recall side-effects, or by asking about their expectations of chemotherapy side-effects overall showed little impact on side-effect reporting. This may be due to the treatment under investigation. Chemotherapy is a high-profile treatment, and as such it is likely patients are already aware of the side-effects that accompany it, limiting the effect that priming could have. In experimental studies, priming patients using pain-related fear has been shown to increase sensitivity to heat stimuli (55). It may be that priming patients about side-effects to lower profile drugs find more promising effects.
Distraction techniques have been shown to be effective in the field of pain research for example experimental and needle-related pain (56, 57), but in terms of medication side-effects, the evidence base is not as large, limiting conclusions. From the results, it seems that distraction tasks should be relevant to the patients to have the greatest chance of being effective. For example, while video games are suitable for reducing side-effects to chemotherapy in pediatric patients they are less effective in adults (39, 46).
The effect of branding on side-effects shows some effect, something also reflected in the inert literature (58). However given the early evidence base, future studies are needed to test the effects of branding on prescribed drugs, and interventions to alter patients' perceptions of prescribed generic drugs.
Quality of Original Research
It is possible that some of our conclusions may be due to differences in quality between those studies that found an effect and those that did not. We did not observe any clear trend for lower quality studies to report more or fewer significant results than higher quality studies. However, overall the quality of the studies included in this review was limited due to poor reporting of key issues in experimental research, such as randomization, allocation concealment, blinding, and not registering a study protocol prior to initiating recruitment. In addition, the quality of evidence from these studies was low, partially due to these risk of bias issues, but also the fact that the samples sizes of studies were relatively small, adding to evidence of imprecision and indirectness due to the wide confidence intervals, and sometimes contradictory findings.
Quality of This Review
Search strategies for systematic reviews based on nocebo effects are difficult to balance in terms of their specificity and sensitivity (10). In this instance we deliberately opted for a broad search strategy in order to identify as many relevant studies as possible. Due to time constraints, screening, data extraction and quality assessment were done by primarily one author. However, there were regular weekly meetings with both authors to discuss screening, data extraction, quality assessment and writing up of the results, allowing us to resolve any issues as they arose.
Other limitations of the review reflect the way we grouped the results. We aggregated studies based on the type of intervention under investigation. These groupings contained different side-effect outcomes, treatments and participants. It is possible that interactions exist between these variables and the interventions under investigation. Unfortunately, due to the small number of studies investigating each intervention, we did not have enough data to explore this in any depth. However, it does appear that chemotherapy might not be as susceptible to brief psychological interventions compared to prescription and over-the-counter drugs.
Implications and Future Directions
Not mentioning potential side-effects to patients has the most consistent effect in reducing side-effects to medical treatments, especially for over-the-counter and prescription drugs. Whether this meets ethical or regulatory requirements is debatable, however (53, 59). De-emphasizing side-effects through positive framing has potential and could be introduced within doctor-patient consultations and in accompanying patient information leaflets for patients to read at home. Further testing of this method especially in terms of reframing side-effects as signs that the drug is working is needed in an adequately powered trial. In addition, it is important for future studies testing ways of de-emphasizing side-effects to adequately compare them to a control group that receives the standard side-effect information.
Besides framing, it is also important for doctors to consider patients beliefs about generic medicines if prescribing generic drugs or switching patients from a previously branded medication to a generic. Colgan et al. (27) suggest that a simple explanation of how the pharmacological ingredients in generic drugs do not actually differ with branded drugs would be useful. So far, the effects of branding have been studied in over the counter and inert tablets: research with prescribed medication is now needed. In addition, distraction could be beneficial for use if age appropriate tasks are used.
Finally, only one study investigated the effect of doctor characteristics on side-effects. This represents a surprising gap in the literature. Doctor characteristics, such as empathy have been shown to be important in benefitting patients for a range of clinical conditions, especially pain (60). We believe this is an important avenue for future research to investigate in terms of benefitting patients by reducing medication side-effects.
Conclusion
This review was restricted by the quality and heterogeneity of the included studies, limiting the conclusions that can be drawn. It does however, provide an indication of which brief psychological interventions are effective in reducing side-effects to active medical treatment. The clearest effect was from omitting information about side-effects to participants before exposure. Although withholding side-effect information would be one way to reduce this, it is ethically and legally problematic. Current work is looking at how we can effectively de-emphasize side-effects while still giving patients the information needed for informed consent, and this shows promise. Potential strategies include positively framing the risk of side-effects, focusing more on the benefits of the drug, and framing side-effects in terms of signs that the drug is working. However further research is needed in larger trials with suitable control groups. There is also a gap for future research to consider doctor characteristics, such as empathy, as a means of reducing patients' experience of side-effects. Finally, better reporting of studies is essential in future, allowing for more concrete determinations of study quality.
Author Contributions
RKW and GJR developed the initial research question for the systematic review and the search strategy. RKW carried out the search, screening, data extraction and quality assessment with regular input from GJR. RKW wrote the first draft which was subsequently revised by GJR.
Funding
RKW and GJR are affiliated to the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Emergency Preparedness and Response at King's College London in partnership with Public Health England (PHE), in collaboration with the University of East Anglia and Newcastle University. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2018.00775/full#supplementary-material
References
2. Kaptchuk TJ. Powerful placebo: the dark side of the randomised controlled trial. Lancet (1998) 351:1722–5. doi: 10.1016/s0140-6736(97)10111-8
3. Mahr A, Golmard C, Pham E, Iordache L, Deville L, Faure P. Types, frequencies, and burden of nonspecific adverse events of drugs: analysis of randomized placebo-controlled clinical trials. Pharmacoepidemiol. Drug Saf. (2017) 26:731–41. doi: 10.1002/pds.4169
4. Ammassari A, Murri R, Pezzotti P, Trotta MP, Ravasio L, De Longis P, et al. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J Acquir Immune Defic Syndr. (2001) 28:445–9. doi: 10.1097/00042560-200112150-00006
5. Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients' adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS ONE (2013) 8:e80633. doi: 10.1371/journal.pone.0080633
6. Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. (2013) 4:91. doi: 10.3389/fphar.2013.00091
7. Horton R. Offline: lessons from the controversy over statins. Lancet (2016) 388:1040. doi: 10.1016/S0140-6736(16)31583-5
8. Pedro-Botet J, Rubies-Prat J. Statin-associated muscle symptoms: beware of the nocebo effect. Lancet (2017) 389:2445–6. doi: 10.1016/s0140-6736(17)31163-7
9. National Institute For Health and Clinical Excellence. (2009). Medicines Adherence: Involving Patients in Decisions About Prescribed Medicines and Supporting Adherence. Available online at: http://www.nice.org.uk/guidance/cg76/resources/guidance-medicines-adherence-pdf
10. Webster RK, Weinman J, Rubin GJ. A systematic review of factors that contribute to nocebo effects. Health Psychol. (2016) 35:1334–55. doi: 10.1037/hea0000416
11. Klinger R, Soost S, Flor H, Worm M. Classical conditioning and expectancy in placebo hypoalgesia: a randomized controlled study in patients with atopic dermatitis and persons with healthy skin. Pain (2007) 128:31–9. doi: 10.1016/j.pain.2006.08.025
12. Varelmann D, Pancaro C, Cappiello EC, Camann WR. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. (2010) 110:868–70. doi: 10.1213/ANE.0b013e3181cc5727
13. Benedetti F, Maggi G, Lopiano L, Lanotte M, Rainero I, Vighetti S, et al. Open versus hidden medical treatments: the patient's knowledge about a therapy affects the therapy outcome. Prev. Treat. (2003) 6:1a. doi: 10.1037/1522-3736.6.1.61a
14. Howick J, Lewith G, Mebius A, Fanshawe TR, Bishop F, van Osch M, et al. Positive messages may reduce patient pain: a meta-analysis. Eur J Integr Med. (2017) 11:31–8. doi: 10.1016/j.eujim.2017.03.005
15. Mistiaen P, van Osch M, van Vliet L, Howick J, Bishop FL, Di Blasi Z, et al. The effect of patient-practitioner communication on pain: a systematic review. Eur J Pain (2016) 20:675–88. doi: 10.1002/ejp.797
16. Peerdeman KJ, van Laarhoven AIM, Keij SM, Vase L, Rovers MM, Peters ML, et al. Relieving patients' pain with expectation interventions: a meta-analysis. Pain (2016) 157:1179–91. doi: 10.1097/j.pain.0000000000000540
17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:97. doi: 10.1371/journal.pmed.1000097
18. Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int J Epidemiol. (2002) 31:150–3. doi: 10.1093/ije/31.1.150
19. NICE. (2018). Glossary. Retrieved from: https://www.nice.org.uk/Glossaryletter=S
20. Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
21. Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Britten N, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: Final Report (2006). Retrieved from Swindon.
22. Grade Working Group. Grading quality of evidence and strength of recommendations. BMJ (2004) 328:1490. doi: 10.1136/bmj.328.7454.1490
23. Mukherjee S, Sahay A. Nocebo effects from negative product information: when information hurts, paying money could heal. J. Cons. Market. (2018) 35:32–9. doi: 10.1108/JCM-11-2015-1609
24. Cildag S, Senturk T, Sargin G. The effects of distraction on symptoms during drug provocation test. Clujul Medical (2017) 90:18–21. doi: 10.15386/cjmed-688
25. Cocco G. Erectile dysfunction after therapy with metoprolol: the Hawthorne effect. Cardiology (2009) 112:174–7. doi: 10.1159/000147951
26. Colagiuri B, Dhillon H, Butow PN, Jansen J, Cox K, Jacquet J. Does assessing patients' expectancies about chemotherapy side effects influence their occurrence? J Pain Symptom Manage. (2013) 46:275–81. doi: 10.1016/j.jpainsymman.2012.07.013
27. Colgan SL, Faasse K, Pereira JA, Grey A, Petrie KJ. Changing perceptions and efficacy of generic medicines: an intervention study. Health Psychol. (2016) 35:1246–53. doi: 10.1037/hea0000402
28. Faasse K, Martin LR, Grey A, Gamble G, Petrie KJ. Impact of brand or generic labeling on medication effectiveness and side effects. Health Psychol. (2016) 35:187–90. doi: 10.1037/hea0000282
29. Faria V, Gingnell M, Hoppe JM, Hjorth O, Alaie I, Frick A, et al. Do you believe it? Verbal suggestions influence the clinical and neural effects of escitalopram in social anxiety disorder: a randomized trial. EBio Med. (2017) 24:179–88. doi: 10.1016/j.ebiom.2017.09.031
30. Flam B, Spice-Cherry P, Amsel R. Effects of preparatory information of a myelogram on patients' expectations and anxiety levels. Patient Educ Couns. (1989) 14:115–26. doi: 10.1016/0738-3991(89)90047-5
31. Jacobs W, Das E, Schagen SB. Increased cognitive problem reporting after information about chemotherapy-induced cognitive decline: the moderating role of stigma consciousness. Psychol Health (2017) 32:78–93. doi: 10.1080/08870446.2016.1244535
32. John AP, Singh NM, Nagarajaiah Andrade C. Impact of an educational module in antidepressant-naive patients prescribed antidepressants for depression: pilot, proof-of-concept, randomized controlled trial. Indian J. Psychiatry (2016) 58:425–31. doi: 10.4103/0019-5545.196710
33. Lauder GR, McQuillan PJ, Pickering RM. Psychological adjunct to perioperative antiemesis. Br J Anaesth. (1995) 74:266–70. doi: 10.1093/bja/74.3.266
34. Mondaini N, Gontero P, Giubilei G, Lombardi G, Cai T, Gavazzi A, et al. Finasteride 5 mg and sexual side effects: How many of these are related to a nocebo phenomenon? J Sex Med. (2007) 4:1708–12. doi: 10.1111/j.1743-6109.2007.00563.x
35. Myers ED, Calvert EJ. The effect of forewarning on the occurrence of side-effects and discontinuance of medication in patients on amitriptyline. Br J Psychiatry (1973) 122:461–4. doi: 10.1192/bjp.122.4.461
36. Myers ED, Calvert EJ. The effect of forewarning on the occurrence of side-effects and discontinuance of medication in patients on dothiepin. J Int Med Res. (1976) 4:237–40. doi: 10.1177/030006057600400405
37. Myers ED, Calvert EJ. Information, compliance and side-effects: a study of patients on antidepressant medication. Br J Clin Pharmacol. (1984) 17:21–5. doi: 10.1111/j.1365-2125.1984.tb04993.x
38. O'Connor AM, Pennie RA, Dales RE. Framing effects on expectations, decisions, and side effects experienced: the case of influenza immunization. J Clin Epidemiol. (1996) 49:1271–6. doi: 10.1016/S0895-4356(96)00177-1
39. Redd WH, Jacobsen PB, Die-Trill M, Dermatis H, McEvoy M, Holland JC. Cognitive/attentional distraction in the control of conditioned nausea in pediatric cancer patients receiving chemotherapy. J Consult Clin Psychol. (1987) 55:391–5. doi: 10.1037/0022-006X.55.3.391
40. Rickels K, Lipman RS, Park LC, Covi L, Uhlenhuth EH, Mock JE. Drug, doctor warmth, and clinic setting in the symptomatic response to minor tranquilizers. Psychopharmacologia (1971) 20:128–52. doi: 10.1007/BF00404367
41. Roscoe JA, O'Neill M, Jean-Pierre P, Heckler CE, Kaptchuk TJ, Bushunow P, et al. An exploratory study on the effects of an expectancy manipulation on chemotherapy-related nausea. J Pain Symptom Manage. (2010) 40:379–90. doi: 10.1016/j.jpainsymman.2009.12.024
42. Schagen SB, Das E, van Dam FSAM. The influence of priming and pre-existing knowledge of chemotherapy-associated cognitive complaints on the reporting of such complaints in breast cancer patients. Psychooncology (2009) 18:674–8. doi: 10.1002/pon.1454
43. Schagen SB, Das E, Vermeulen I. Information about chemotherapy-associated cognitive problems contributes to cognitive problems in cancer patients. Psychooncology (2012) 21:1132–5. doi: 10.1002/pon.2011
44. Shelke AR, Roscoe JA, Morrow GR, Colman LK, Banerjee TK, Kirshner JJ. Effect of a nausea expectancy manipulation on chemotherapy-induced nausea: a university of Rochester cancer center community clinical oncology program study. J Pain Symptom Manage. (2008) 35:381–7. doi: 10.1016/j.jpainsymman.2007.05.008
45. Silvestri A, Galetta P, Cerquetani E, Marazzi G, Patrizi R, Fini M, et al. Report of erectile dysfunction after therapy with beta-blockers is related to patient knowledge of side effects and is reversed by placebo. Eur Heart J. (2003) 24:1928–32. doi: 10.1016/j.ehj.2003.08.016
46. Vasterling J, Jenkins RA, Tope DM, Burish TG. Cognitive distraction and relaxation training for the control of side-effects due to cancer-chemotherapy. J Behav Med. (1993) 16:65–80. doi: 10.1007/bf00844755
47. Wilhelm M, Rief W, Doering BK. Decreasing the burden of side effects through positive message framing: an experimental proof-of-concept study. Int J Behav Med. (2018) 25:381–9. doi: 10.1007/s12529-018-9726-z
48. Wise RA, Bartlett SJ, Brown ED, Castro M, Cohen R, Holbrook JT, et al. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J Allergy Clin Immunol. (2009) 124:436–44, 444e431–8. doi: 10.1016/j.jaci.2009.05.041
49. Crichton F, Dodd G, Schmid G, Gamble G, Cundy T, Petrie KJ. The power of positive and negative expectations to influence reported symptoms and mood during exposure to wind farm sound. Health Psychol. (2014) 33:1588–92. doi: 10.1037/hea0000037
50. Laarhoven AI, Vogelaar ML, Wilder-Smith OH, Riel PL, Kerkhof PC, Kraaimaat FW, et al. Induction of nocebo and placebo effects on itch and pain by verbal suggestions. Pain (2011) 152:1486–94. doi: 10.1016/j.pain.2011.01.043
51. Colloca L. Tell me the truth and I will not be harmed: informed consents and nocebo effects. Am J Bioeth. (2017) 17:46–8. doi: 10.1080/15265161.2017.1314057
52. Wells RE, Kaptchuk TJ. To tell the truth, the whole truth, may do patients harm: the problem of the nocebo effect for informed consent. Am J Bioeth. (2012) 12:22–9. doi: 10.1080/15265161.2011.652798
53. Webster RK, Weinman J, Rubin GJ. Explaining all without causing unnecessary harm: is there scope for positively framing medical risk information? Patient Educ Couns. (2018). doi: 10.1016/j.pec.2018.09.014. [Epub ahead of print].
54. Webster RK, Weinman J, Rubin GJ. Positively framed risk information in patient information leaflets reduces side effect reporting: a double-blind randomized controlled trial. Ann Behav Med. (2018) 52:kax064. doi: 10.1093/abm/kax064
55. Kirwilliam SS, Derbyshire SWG. Increased bias to report heat or pain following emotional priming of pain-related fear. Pain (2008) 137:60–5. doi: 10.1016/j.pain.2007.08.012
56. DeMore M, Cohen LL. Distraction for pediatric immunization pain: a critical review. J Clin Psychol Med Settings (2005) 12:281–91. doi: 10.1007/s10880-005-7813-1
57. Malloy KM, Milling LS. The effectiveness of virtual reality distraction for pain reduction: a systematic review. Clin Psychol Rev. (2010) 30:1011–8. doi: 10.1016/j.cpr.2010.07.001
58. Faasse K, Cundy T, Gamble G, Petrie KJ. The effect of an apparent change to a branded or generic medication on drug effectiveness and side effects. Psychosom Med. (2013) 75:90–6. doi: 10.1097/PSY.0b013e3182738826
59. Dyer C. Doctors should not cherry pick what information to give patients, court rules. Br Med J. (2015) 350. doi: 10.1136/bmj.h1414
Keywords: review, side-effects, medicine, nocebo effect, interventions, side-effect information
Citation: Webster RK and Rubin GJ (2019) Influencing Side-Effects to Medicinal Treatments: A Systematic Review of Brief Psychological Interventions. Front. Psychiatry 9:775. doi: 10.3389/fpsyt.2018.00775
Received: 24 October 2018; Accepted: 24 December 2018;
Published: 05 February 2019.
Edited by:
Luana Colloca, University of Maryland, Baltimore, United StatesReviewed by:
Meike C. Shedden-Mora, University Medical Center Hamburg-Eppendorf, GermanyKarin Meissner, Ludwig Maximilian University of Munich, Germany
Copyright © 2019 Webster and Rubin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rebecca K. Webster, UmViZWNjYS53ZWJzdGVyQGtjbC5hYy51aw==
 Rebecca K. Webster
Rebecca K. Webster G. James Rubin
G. James Rubin