- 1Department of Sleep Disorders, Hefei Fourth People’s Hospital, Hefei, China
- 2Anhui Mental Health Center, Hefei, China
- 3Department of Radiology, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Background: Depression has been linked to vitamin D deficiency. However, little attention was paid to the neural substrate underlying this association.
Methods: Fifty patients with major depressive disorder (MDD) were enrolled in this study. High-resolution structural magnetic resonance imaging was performed to calculate total intracranial volume (TIV). Peripheral venous blood samples were collected to measure serum vitamin D concentration. Hamilton Rating Scale for Depression (HAMD) was used to assess severity of depression symptoms. The relationship among TIV, serum vitamin D concentration, and HAMD score was examined using correlation, linear regression, and mediation analyses.
Results: In patients with MDD, HAMD score was negatively correlated with TIV and serum vitamin D concentration, and TIV was positively correlated with serum vitamin D concentration. Linear regression analyses showed that TIV and serum vitamin D concentration were significant predictors of HAMD score. Importantly, mediation analysis revealed that TIV significantly mediated the relationship between serum vitamin D concentration and HAMD score.
Conclusion: Our findings suggest that TIV may serve as a potential neural biomarker for monitoring responses to adjuvant therapy of vitamin D in patients with MDD.
Introduction
Major depressive disorder (MDD) is a severe mental disorder, which diminishes the functioning and quality of life (1) and leads to a heavy social and economic burden (2). A recent systematic review and network meta-analysis has found that almost all antidepressants are more efficacious than placebo in adults with MDD and thus antidepressants are used more frequently than other interventions (3). Another network meta-analysis has found that although fluoxetine might reduce depressive symptoms in children and adolescents with MDD, most antidepressants do not seem to be suitable as routine treatment options for MDD in young people because of poor efficacy and tolerability (4). The strength and limitation of antidepressants’ action are suggestive of other modifiable risk factors that have not been fully considered but can be targeted to prevent and treat depression. For example, there is evidence that some nutritional components (5), such as vitamin D, might play an important role in mental health and neuropsychiatry disorders including MDD (6–9).
Vitamin D consists of two forms: ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). Vitamin D2 is present in plants and some types of fish, and vitamin D3 is produced when the skin is exposed to ultraviolet B (UVB) rays from sunlight (10). It is estimated that 12 min of midday exposure to the sun without sunscreen is equivalent to 3,000 IU of vitamin D a day intake of the diet (11). 25-Hydroxyvitamin D [25(OH)D] is the main circulating metabolite of vitamin D and the serum 25(OH)D level is the most reliable marker of vitamin D status (12). The discovery of vitamin D metabolites in cerebrospinal fluid in healthy adults suggests that vitamin D may play a role in brain development (13). Furthermore, the identification of vitamin D receptor (VDR) in the human central nervous system (CNS) indicates that vitamin D may have a functional role in the nervous system (14, 15). VDR is expressed in the prefrontal cortex, cingulate cortex, basal forebrain, amygdala, caudate/putamen, thalamus, substantia nigra, lateral geniculate nuclei, hypothalamus, cerebellum, and hippocampus (16, 17). VDR expression in the prefrontal cortex and limbic system illustrates the role of vitamin D in maintaining affect, emotional, and cognitive functions (14, 18, 19). Over the past decade, there is increasing interest in the interaction between vitamin D deficiency and the development or clinical manifestations of psychiatric disorders, especially depression. Several review and meta-analysis literatures have suggested that depression is linked to vitamin D deficiency (20, 21) and that vitamin D supplements are beneficial for the treatment of depression (22, 23). However, the neural substrate underlying the association between vitamin D and depression remains indiscernible.
Prior neuroimaging studies have demonstrated that serum vitamin D level alterations are associated with brain volume changes (24–27) and patients with MDD have widespread cortical and subcortical volume abnormalities (28–34). Given these findings, one may speculate that there is an association among serum vitamin D concentration, severity of depressive symptoms, and total intracranial volume (TIV, a global and unbiased volumetric parameter) in patients with MDD. In this study, we aimed to clarify this issue and hypothesized that TIV would mediate the relationship between serum vitamin D concentration and severity of depressive symptoms.
Materials and Methods
Participants
We consecutively recruited patients from the outpatient and inpatient departments of Hefei Fourth People’s Hospital. A total of 50 right-handed patients with MDD were comprised in this study. Two well-trained clinical psychiatrists confirmed the diagnoses of depression in accordance with the International Classification of Diseases (ICD-10) criteria. Exclusion criteria for all participants included 1) the presence of other psychiatric disorders such as bipolar disorder, schizophrenia, anxiety disorders, substance-induced mood disorder, substance abuse, or dependence; 2) a history of significant physical or neurological diseases; 3) a history of head injury with loss of consciousness; and 4) contraindications for MRI (such as pregnancy). The severity of depression and anxiety symptoms was captured by using the 24-item Hamilton Rating Scale for Depression (HAMD) (35) and the 14-item Hamilton Rating Scale for Anxiety (HAMA) (36). All patients were receiving their regular antidepressant medications (see Table S1 in the Supplementary Materials). However, combination therapies were frequently used and there was a considerable variability in the administered drugs and the current medication duration, which prevents us from testing differences between patients with distinct medication regimens. This more natural setting allows us to perform a more conservative analysis to identify aberrations in patients with MDD and thus should better reflect the overall population of patients with MDD. This study was approved by the ethics committee of The First Affiliated Hospital of Anhui Medical University. All participants provided written informed consent after they had been given a complete description of the study.
Image Acquisition
MRI scans were acquired using a 3.0-T MR system (Discovery MR750w, General Electric, Milwaukee, WI, USA) with a 24-channel head coil. Earplugs were used to reduce scanner noise, and tight but comfortable foam padding was used to minimize head motion. During the scans, all participants were instructed to keep their eyes closed, relax but not fall asleep, think of nothing in particular, and move as little as possible. High-resolution 3D T1-weighted structural images were acquired by employing a brain volume (BRAVO) sequence with the following parameters: repetition time (TR) = 8.5 ms; echo time (TE) = 3.2 ms; inversion time (TI) = 450 ms; flip angle (FA) = 12°; field of view (FOV) = 256 mm × 256 mm; matrix size = 256 × 256; slice thickness = 1 mm, no gap; 188 sagittal slices; and acquisition time = 296 s.
Image Processing
Voxel-based morphometry (VBM) analysis was performed using the CAT12 toolbox (http://www.neuro.uni-jena.de/cat) implemented in the Statistical Parametric Mapping software (SPM12, http://www.fil.ion.ucl.ac.uk/spm). First, all the structural T1-weighted images were corrected for bias-field inhomogeneities. Second, these images were segmented into gray matter, white matter, and cerebrospinal fluid using the “new-segment” approach. Finally, TIV was calculated as the sum of gray matter, white matter, and cerebrospinal fluid volumes.
Serum Vitamin D Concentration Measurement
After an overnight fasting period, peripheral venous blood samples (2 ml) were collected from all patients in the morning of MRI scanning. Samples were sent to the Department of Clinical Laboratory, Hefei Fourth People’s Hospital immediately for centrifugation and serum was separated. Vitamin D [25(OH)D] was measured in serum using a chemiluminescence immunoassay (CLIA) technique in a fully automated Maglumi 1000 analyzer (SNIBE Co., Ltd., China). Internal quality control provided by the manufacturer was used to assure quality. Vitamin D level was stratified as follows: 30–100 ng/ml (75–250 nmol/L) as sufficiency, 20–29 ng/ml (50–72.5 nmol/L) as insufficiency, <20 ng/ml (50 nmol/L) as deficiency (37).
Statistical Analysis
The statistical analyses were performed by using the SPSS 23.0 software package (SPSS, Chicago, IL). Pearson’s correlation analyses were performed to examine the associations among TIV, serum concentration of vitamin D, and HAMD score in the patients. Linear regression analyses were used to assess the predictive values of serum vitamin D concentration and TIV for HAMD score. In addition, to test whether the association between variables was mediated by other variables, mediation analysis was performed using the PROCESS macro (http://www.processmacro.org/) developed by Hayes (38, 39), a versatile modeling tool freely available for SPSS. The PROCESS uses an ordinary least squares path analytic framework to estimate direct and indirect mediation effects. In the mediation analysis model, all paths were reported as unstandardized ordinary least squares regression coefficients, namely, total effect of X on Y (c) = indirect effect of X on Y through M (a × b) + direct effect of X on Y (c′). The significance analysis was based on 5,000 bootstrap realizations, and the significance of indirect effects was assessed by bootstrap 95% confidence interval (CI). In the PROCESS analysis, a significant indirect effect is indicated when the bootstrap 95% CI does not include zero. In this study, only variables that showed a significant correlation with others in the correlation analyses were considered independent, dependent, or mediating variables in the mediation analysis. Finally, we divided the patients into two subgroups (patients with non-severe depressive symptoms: HAMD score < 24; patients with severe depressive symptoms: HAMD score ≥ 24) (40) and tested differences in serum vitamin D concentration and TIV between them. For these analyses, P < 0.05 was considered to indicate statistical significance.
Results
Participant Characteristics
The characteristics of participants are shown in Table 1. The mean age was 42.10 years (SD: 10.52 years) and 62% of the participants were females. The mean educational level was 9.30 years (SD: 3.80 years); HAMD, 29.42 (SD: 11.59); HAMA, 19.06 (SD: 7.41); illness duration, 66.33 months (SD: 74.66 months); onset age, 36.68 years (SD: 10.80 years); episode number, 2.5 (SD: 1.62); and TIV, 1,458.00 cm3 (SD: 157.90 cm3). Serum concentration of vitamin D was available for 45 of 50 patients with a mean value of 44.37 nmol/L (SD: 11.47 nmol/L). Moreover, among the 45 patients, 15 (33.33%) were classified as insufficiency and 30 were classified (66.67%) as deficiency.
Correlation and Linear Regression Analyses
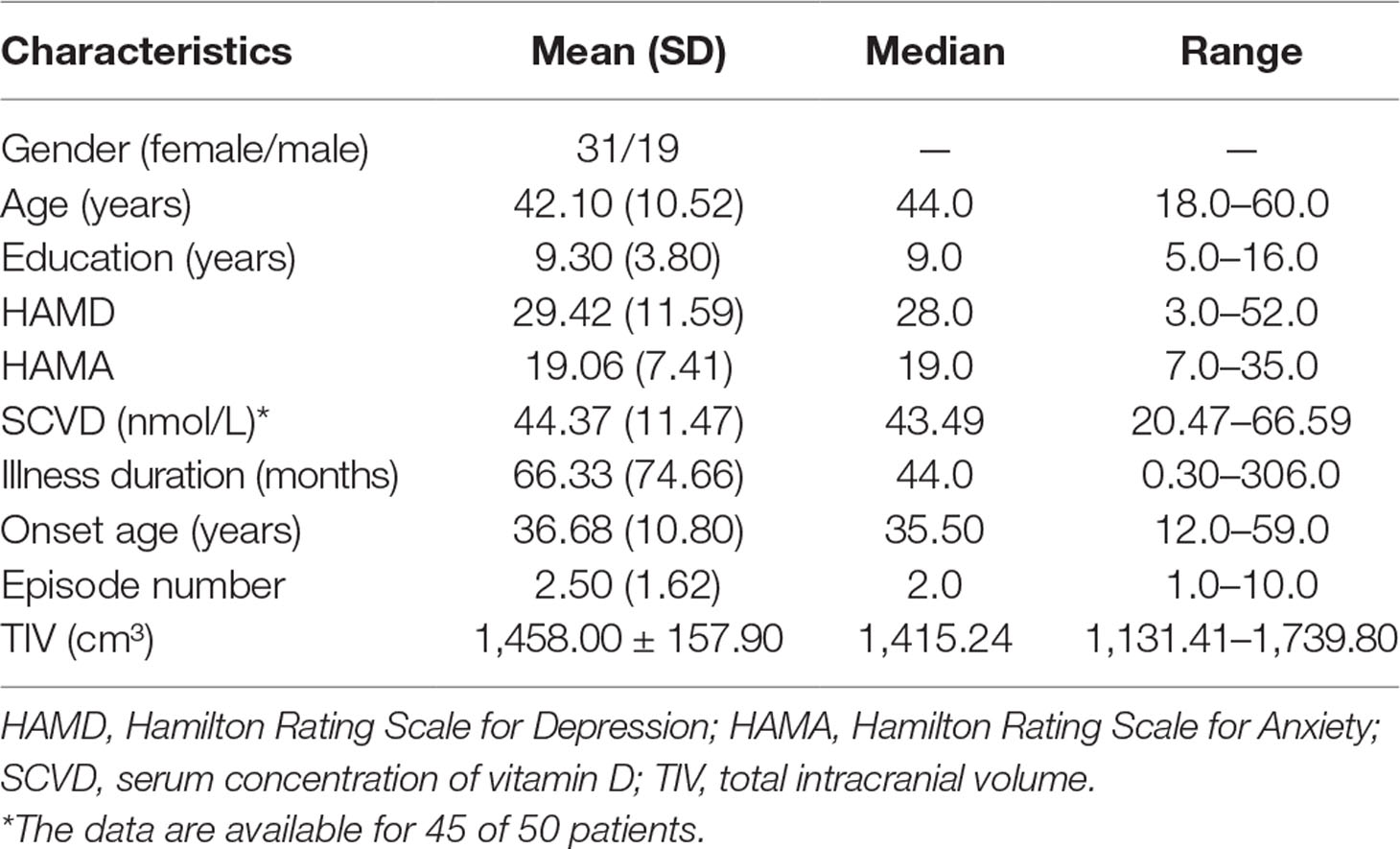
There were significant negative correlations between HAMD score and TIV (r = −0.526, P < 0.001; Figure 1A) and between HAMD score and serum vitamin D concentration (r = −0.334, P = 0.025; Figure 1B) in the patients with MDD. In addition, there was a positive correlation between TIV and serum vitamin D concentration (r = 0.445, P = 0.002; Figure 1C) in the patient group. Linear regression analyses showed that TIV (β = −0.039, t = −4.282, P < 0.001, R2 = 0.276) and serum concentration of vitamin D (β = −0.328, t = −2.322, P = 0.025, R2 = 0.111) were significant predictors of HAMD score, respectively. When TIV and serum concentration of vitamin D were included in a linear regression model (R2 = 0.238), TIV remained predictive of HAMD score (β = −0.029, t = −2.640, P = 0.012), but serum concentration of vitamin D was not a significant predictor (β = −0.154, t = −1.043, P = 0.303).
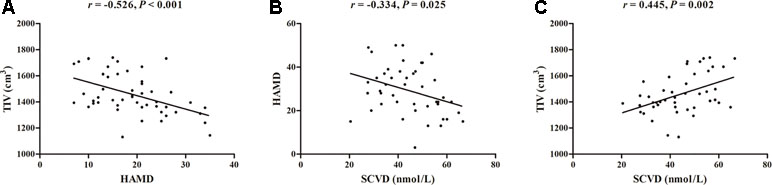
Figure 1 Correlations among TIV, HAMD, and SCVD in patients with major depressive disorder. (A) Scatter plot of the association between TIV and HAMD. (B) Scatter plot of the association between SCVD and HAMD. (C) Scatter plot of the association between SCVD and TIV. Abbreviations: TIV, total intracranial volume; HAMD, Hamilton Rating Scale for Depression; SCVD, serum concentration of vitamin D.
Mediation Analysis
The mediation analysis model and the investigated variables are described in Figure 2. There was a significant negative indirect effect of the serum vitamin D concentration on HAMD score through TIV in patients with MDD, i.e., the total effect of serum vitamin D concentration on HAMD score was significant (path c = −0.328, SE = 0.141, P = 0.025), and was fully mediated by the TIV (indirect effect = −0.1741, SE = 0.0931, 95% CI: −0.3984, −0.0326; path c′ = −0.154, SE = 0.148, P = 0.303).
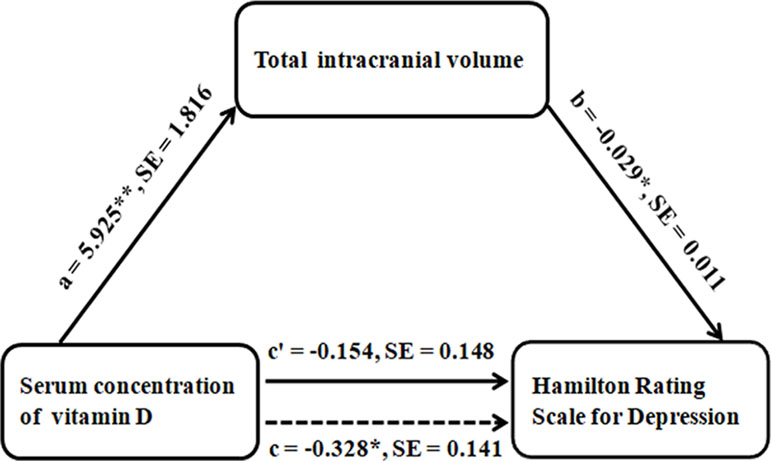
Figure 2 Graphical representation of the mediation analysis between SCVD and HAMD, with TIV as the mediator: estimates of the mediated, direct, and total effects. All paths are reported as unstandardized ordinary least squares regression coefficients. *P < 0.05, **P < 0.005. Abbreviations: SCVD, serum concentration of vitamin D; HAMD, Hamilton Rating Scale for Depression; TIV, total intracranial volume; SE, standard error.
Comparison Between Patients With Severe and Non-Severe Depressive Symptoms
Patients with severe depressive symptoms (N = 32, 1,407.01 ± 139.59 cm³) exhibited reduced TIV (two-sample t test, t = −3.347, P = 0.002) compared with patients with non-severe depressive symptoms (N = 18, 1,548.68 ± 150.79 cm³). A trend toward decreased serum concentration of vitamin D (two-sample t test, t = −1.928, P = 0.060) was found in patients with severe depressive symptoms (N = 30, 42.11 ± 9.97 nmol/L) compared with non-severe depressive symptoms (N = 15, 48.90 ± 13.23 nmol/L).
Discussion
Our main findings are twofold: 1) in patients with MDD, more severe depressive symptoms were related to smaller TIV and lower serum vitamin D concentration, and smaller TIV was related to lower serum vitamin D concentration; 2) TIV was a significant mediator of the association between serum vitamin D concentration and severity of depressive symptoms in the patients.
There are several lines of evidence in support of a plausible association between lower serum vitamin D concentration and the development and clinical manifestations of depression. For example, observational studies have reported that serum concentration of vitamin D was lower among patients with depression when compared to healthy controls (41, 42). Vitamin D has been proven to have neuroprotective effects, and higher serum vitamin D concentrations are related to a reduced risk of depression (19, 43–46). Combined, these previous reports are compatible with our findings that the MDD patients had vitamin D insufficiency or deficiency and lower serum vitamin D concentration was associated with greater severity of depressive symptoms.
A meta-analysis has demonstrated that vitamin D supplementation (≥800 I.U. daily) is somewhat favorable in the management of depression and the effect size is even comparable to that of antidepressant medication (23). There is current evidence that adjunctive use of vitamin D with antidepressants can reduce depressive symptoms more effectively (47). Song et al. have found that vitamin D supplements can predict lower depressive symptoms and reduce cardiac events for MDD patients (48). Wong et al. have revealed that vitamin D supplementation is effective in treating depression and maintaining optimum vitamin D levels is a highly feasible approach to prevent depression (49). However, a meta-analysis did not support the evidence for the efficacy of vitamin D in improving depression among adults (50). Therefore, the eventual impact of a vitamin D supplementation on depressed patients remains uncertain and needs to be investigated further.
The relationship between brain and vitamin D has attracted intense interest from neuroimaging researchers. For instance, previous studies have revealed that vitamin D depletion is associated with smaller brain volume and larger lateral cerebral ventricles (24). In older adults, vitamin D deficiency has be found to be linked to lower hippocampal volume (25) and increased white matter abnormality volume (26). In healthy young women, lower serum vitamin D level has been shown to be associated with greater TIV as well as total cortical gray and cerebral white matter volumes (27). A previous review has highlighted the effects of vitamin D on brain development and adult brain function (51). Therefore, the current finding of a positive correlation between TIV and serum vitamin D concentration in MDD patients not only supports consistent findings in healthy population but also adds to the current knowledge of vitamin D–brain association in a clinical population.
Brain structural studies have revealed widely distributed gray matter volume reductions in patients with MDD, and the affected brain regions mainly comprise orbitofrontal cortex, prefrontal cortex, insula, supplementary motor area, middle temporal gyrus, hippocampus, parahippocampal gyrus and fusiform gyrus, thalamus, cingulate cortex, amygdala, and striatum (28–34, 52, 53). In this study, we found that patients with severe depressive symptoms exhibited reduced TIV compared with those with non-severe depressive symptoms. The inverse correlation between TIV and depression severity implies that lower TIV may render the patients into a risk of experiencing more severe depressive symptoms.
Notably, further mediation analysis indicates that the relationship between serum vitamin D concentration and severity of depressive symptoms can be fully mediated by TIV, which is the most pivotal finding with clinical relevance. On one hand, it provides preliminary evidence that the effects of vitamin D on depression appear to have a neuroanatomical basis, though the relationship between them is complex. On the other hand, TIV can serve as a sensitive and objective imaging biomarker capable of capturing depressive symptom changes along with serum vitamin D level alteration, and thus might be used in a clinical setting to monitor MDD patients’ responses to adjuvant therapy of vitamin D.
Our results need to be interpreted in light of several limitations. First, the fairly modest sample size did not allow us to perform a full analysis of the potential effects of medication and illness duration. Future studies in a larger sample of medication-naive, first-episode patients with MDD are warrant to test the stability of our results. Second, this study is a cross-sectional design, which makes it difficult to draw a conclusion about the causal relationship. Longitudinal studies are required to establish the direction of causality. Finally, this study lacks normal randomized controlled data, which precludes us from exploring the relationship between serum vitamin D concentration and TIV in healthy subjects.
In conclusion, we demonstrated a negative effect of serum vitamin D concentration on severity of depressive symptoms in patients with MDD, which was mediated by TIV. This finding may help to establish TIV as a potential neural biomarker for monitoring responses to adjuvant therapy of vitamin D in patients with MDD.
Data Availability Statement
The datasets for this manuscript are not publicly available because this is to protect the patient’s privacy. Requests to access the datasets should be directed to
Ethics Statement
This study was approved by the ethics committee of The First Affiliated Hospital of Anhui Medical University. All participants provided written informed consent after they had been given a complete description of the study.
Author Contributions
YYu, JZ, D-mZ, and WZ conceptualized and designed the study. D-mZ and WZ were responsible for conducting the analyses, preparing the first draft of the manuscript, and preparing the manuscript for submission. YYu and JZ were responsible for obtaining funding for the study, supervising the analyses, and editing drafts of the manuscript. WZ, BZ, YZ, CZ, YW, and YYa were responsible for data collection and initial data preprocessing. All authors contributed to and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (grant numbers: 81801679, 81571308, and 8177817) and the Hefei Municipal Health Planning Commission Applied Medicine Research Project (grant numbers: hwk2016yb010 and hwk2018yb001).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2019.00322/full#supplementary-material
References
1. Sherchand O, Sapkota N, Chaudhari RK, Khan SA, Baranwal JK, Pokhrel T, et al. Association between vitamin D deficiency and depression in Nepalese population. Psychiatry Res (2018) 267:266–71. doi: 10.1016/j.psychres.2018.06.018
2. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (2016) 388:1545–602. doi: 10.1016/S0140-6736(16)31678-6
3. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet (2018) 391:1357–66. doi: 10.1016/S0140-6736(17)32802-7
4. Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet (2016) 388:881–90. doi: 10.1016/S0140-6736(16)30385-3
5. Libuda L, Antel J, Hebebrand J, Focker M. Nutrition and mental diseases: focus depressive disorders. Nervenarzt (2017) 88:87–101. doi: 10.1007/s00115-016-0262-2
6. Ganji V, Milone C, Cody MM, McCarty F, Wang YT. Serum vitamin D concentrations are related to depression in young adult US population: the third national health and nutrition examination survey. Int Arch Med (2010) 3:29. doi: 10.1186/1755-7682-3-29
7. Williams JA, Sink KM, Tooze JA, Atkinson HH, Cauley JA, Yaffe K, et al. Low 25-hydroxyvitamin D concentrations predict incident depression in well-functioning older adults: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci (2015) 70:757–63. doi: 10.1093/gerona/glu184
8. Focker M, Antel J, Ring S, Hahn D, Kanal O, Ozturk D, et al. Vitamin D and mental health in children and adolescents. Eur Child Adolesc Psychiatry (2017) 26:1043–66. doi: 10.1007/s00787-017-0949-3
9. Kocovska E, Gaughran F, Krivoy A, Meier UC. Vitamin-D deficiency as a potential environmental risk factor in multiple sclerosis, schizophrenia, and autism. Front Psychiatry (2017) 8:47. doi: 10.3389/fpsyt.2017.00047
10. Kulie T, Groff A, Redmer J, Hounshell J, Schrager S. Vitamin D: an evidence-based review. J Am Board Fam Med (2009) 22:698–706. doi: 10.3122/jabfm.2009.06.090037
11. Holick MF. Vitamin D: a millenium perspective. J Cell Biochem (2003) 88:296–307. doi: 10.1002/jcb.10338
13. Balabanova S, Richter HP, Antoniadis G, Homoki J, Kremmer N, Hanle J, et al. 25-Hydroxyvitamin D, 24,25-dihydroxyvitamin D and 1,25-dihydroxyvitamin D in human cerebrospinal fluid. Klin Wochenschr (1984) 62:1086–90. doi: 10.1007/BF01711378
14. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat (2005) 29:21–30. doi: 10.1016/j.jchemneu.2004.08.006
15. Sutherland MK, Somerville MJ, Yoong LK, Bergeron C, Haussler MR, McLachlan DR. Reduction of vitamin D hormone receptor mRNA levels in Alzheimer as compared to Huntington hippocampus: correlation with calbindin-28k mRNA levels. Brain Res Mol Brain Res (1992) 13:239–50. doi: 10.1016/0169-328X(92)90032-7
16. Harris HW JP, Holmes V, Weisler RH, Patkar AA. Vitamin D deficiency and psychiatric illness: supplementation might help patients with depression, seasonal mood disturbances. Curr Psychiatry (2013) 12:18.
17. Nimitphong H, Holick MF, Vitamin D. neurocognitive functioning and immunocompetence. Curr Opin Clin Nutr Metab Care (2011) 14:7–14. doi: 10.1097/MCO.0b013e3283414c38
18. Di Somma C, Scarano E, Barrea L, Zhukouskaya VV, Savastano S, Mele C, et al. Vitamin D and neurological diseases: an endocrine view. Int J Mol Sci (2017) 18:2482. doi: 10.3390/ijms18112482
19. Schlogl M, Holick MF. Vitamin D and neurocognitive function. Clin Interv Aging (2014) 9:559–68. doi: 10.2147/CIA.S51785
20. Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry (2013) 202:100–7. doi: 10.1192/bjp.bp.111.106666
21. Sotodehasl N MF, Tamadon MR. Vitamin D deficiency and depression: a short review article. Middle East J Rehabil Health Stud (2015) 2(3):e26961. doi: 10.17795/mejrh-26961
22. Shaffer JA, Edmondson D, Wasson LT, Falzon L, Homma K, Ezeokoli N, et al. Vitamin D supplementation for depressive symptoms: a systematic review and meta-analysis of randomized controlled trials. Psychosom Med (2014) 76:190–6. doi: 10.1097/PSY.0000000000000044
23. Spedding S. Vitamin D and depression: a systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients (2014) 6:1501–18. doi: 10.3390/nu6041501
24. Annweiler C, Annweiler T, Montero-Odasso M, Bartha R, Beauchet O. Vitamin D and brain volumetric changes: systematic review and meta-analysis. Maturitas (2014) 78:30–9. doi: 10.1016/j.maturitas.2014.02.013
25. Karakis I, Pase MP, Beiser A, Booth SL, Jacques PF, Rogers G, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: the Framingham Heart Study. J Alzheimers Dis (2016) 51:451–61. doi: 10.3233/JAD-150991
26. Annweiler C, Bartha R, Karras SN, Gautier J, Roche F, Beauchet O. Vitamin D and white matter abnormalities in older adults: a quantitative volumetric analysis of brain MRI. Exp Gerontol (2015) 63:41–7. doi: 10.1016/j.exger.2015.01.049
27. Plozer E, Altbacker A, Darnai G, Perlaki G, Orsi G, Nagy SA, et al. Intracranial volume inversely correlates with serum 25(OH)D level in healthy young women. Nutr Neurosci (2015) 18:37–40. doi: 10.1179/1476830514Y.0000000109
28. Schmaal L, Veltman DJ, van Erp TGM, Sämann PG, Frodl T, Jahanshad N, et al., Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry (2015) 21:806–12. doi: 10.1038/mp.2015.69
29. Zaremba D, Enneking V, Meinert S, Forster K, Burger C, Dohm K, et al. Effects of cumulative illness severity on hippocampal gray matter volume in major depression: a voxel-based morphometry study. Psychol Med (2018) 48:2391–98. doi: 10.1017/S0033291718000016
30. Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry (2008) 13:993–1000. doi: 10.1038/mp.2008.57
31. Arnone D, McIntosh AM, Ebmeier KP, Munafo MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol (2012) 22:1–16. doi: 10.1016/j.euroneuro.2011.05.003
32. Nugent AC, Davis RM, Zarate CA Jr., Drevets WC. Reduced thalamic volumes in major depressive disorder. Psychiatry Res (2013) 213:179–85. doi: 10.1016/j.pscychresns.2013.05.004
33. Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry (2013) 21:184–95. doi: 10.1016/j.jagp.2012.10.019
34. Arnone D, Job D, Selvaraj S, Abe O, Amico F, Cheng Y, et al. Computational meta-analysis of statistical parametric maps in major depression. Hum Brain Mapp (2016) 37:1393–404. doi: 10.1002/hbm.23108
35. Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry (1988) 45:742–47. doi: 10.1001/archpsyc.1988.01800320058007
36. Thompson E. Hamilton Rating Scale for Anxiety (HAM-A). Occup Med (Lond) (2015) 65:601. doi: 10.1093/occmed/kqv054
37. Ringe JD, Kipshoven C. Vitamin D-insufficiency: an estimate of the situation in Germany. Dermatoendocrinol (2012) 4:72–80. doi: 10.4161/derm.19829
38. Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: Guilford Press (2013).
39. Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr (2009) 76:408–20. doi: 10.1080/03637750903310360
40. Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord (2013) 150:384–8. doi: 10.1016/j.jad.2013.04.028
41. Hoogendijk WJ, Lips P, Dik MG, Deeg DJ, Beekman AT, Penninx BW. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry (2008) 65:508–12. doi: 10.1001/archpsyc.65.5.508
42. Jozefowicz O, Rabe-Jablonska J, Wozniacka A, Strzelecki D. Analysis of vitamin D status in major depression. J Psychiatr Pract (2014) 20:329–37. doi: 10.1097/01.pra.0000454777.21810.15
43. Kalueff AV, Eremin KO, Tuohimaa P. Mechanisms of neuroprotective action of vitamin D(3). Biochemistry (Mosc) (2004) 69:738–41. doi: 10.1023/B:BIRY.0000040196.65686.2f
44. Kesby JP, Eyles DW, Burne TH, McGrath JJ. The effects of vitamin D on brain development and adult brain function. Mol Cell Endocrinol (2011) 347:121–7. doi: 10.1016/j.mce.2011.05.014
45. Ju SY, Lee YJ, Jeong SN. Serum 25-hydroxyvitamin D levels and the risk of depression: a systematic review and meta-analysis. J Nutr Health Aging (2013) 17:447–55. doi: 10.1007/s12603-012-0418-0
46. Jaaskelainen T, Knekt P, Suvisaari J, Mannisto S, Partonen T, Saaksjarvi K, et al. Higher serum 25-hydroxyvitamin D concentrations are related to a reduced risk of depression. Br J Nutr (2015) 113:1418–26. doi: 10.1017/S0007114515000689
47. Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry (2016) 173:575–87. doi: 10.1176/appi.ajp.2016.15091228
48. Song EK, Wu JR, Moser DK, Kang SM, Lennie TA. Vitamin D supplements reduce depressive symptoms and cardiac events in heart failure patients with moderate to severe depressive symptoms. Eur J Cardiovasc Nurs (2018) 17:207–16. doi: 10.1177/1474515117727741
49. Wong SK, Chin KY, Ima-Nirwana S. Vitamin D and depression: the evidence from an indirect clue to treatment strategy. Curr Drug Targets (2018) 19:888–97. doi: 10.2174/1389450118666170913161030
50. Gowda U, Mutowo MP, Smith BJ, Wluka AE, Renzaho AM. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition (2015) 31:421–9. doi: 10.1016/j.nut.2014.06.017
51. Eyles DW, Burne TH, McGrath JJ, Vitamin D. Effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol (2013) 34:47–64. doi: 10.1016/j.yfrne.2012.07.001
52. Zhang H, Li L, Wu M, Chen Z, Hu X, Chen Y, et al. Brain gray matter alterations in first episodes of depression: a meta-analysis of whole-brain studies. Neurosci Biobehav Rev (2016) 60:43–50. doi: 10.1016/j.neubiorev.2015.10.011
Keywords: major depressive disorder, magnetic resonance imaging, vitamin D, total intracranial volume, mediator
Citation: Zhu D, Zhao W, Zhang B, Zhang Y, Yang Y, Zhang C, Wang Y, Zhu J and Yu Y (2019) The Relationship Between Serum Concentration of Vitamin D, Total Intracranial Volume, and Severity of Depressive Symptoms in Patients With Major Depressive Disorder. Front. Psychiatry 10:322. doi: 10.3389/fpsyt.2019.00322
Received: 27 February 2019; Accepted: 26 April 2019;
Published: 09 May 2019.
Edited by:
Michele Fornaro, New York State Psychiatric Institute (NYSPI), United StatesReviewed by:
Stefano Novello, University of Naples Federico II, ItalyAnnalisa Anastasia, Villa Camaldoli Foundation, Italy
Copyright © 2019 Zhu, Zhao, Zhang, Zhang, Yang, Zhang, Wang, Zhu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiajia Zhu emh1amlhamlhZ3JhZHVhdGVAMTYzLmNvbQ==
Yongqiang WXVjanIueXV5b25ncWlhbmdAdmlwLjE2My5jb20=
†These authors have contributed equally to this work.
 Dao-min Zhu1,2†
Dao-min Zhu1,2† Yongqiang Yu
Yongqiang Yu