- 1Department of Brain and Cognitive Sciences, College of Natural Science, Seoul National University, Seoul, South Korea
- 2Department of Psychiatry, College of Medicine, Seoul National University, Seoul, South Korea
- 3Department of Neuropsychiatry, Seoul National University Hospital, Seoul, South Korea
- 4Institute of Human Behavioral Medicine, SNU-MRC, Seoul, South Korea
Objective: Numerous reports on neurocognitive functioning deficits in individuals at clinical high risk (CHR) and first-episode psychosis (FEP) patients suggest particular deficits in executive functioning (EF). However, to date, most of the studies have administered a single or a few EF tests to participants, and few investigations have examined the different components of EF to identify specific subdomains of relative strength and weakness.
Method: Forty CHR subjects, 85 FEP patients, and 85 healthy controls (HCs) were assessed with a neuropsychological battery to elucidate the profiles of EF in the subdomains of shift, attention, fluency, and planning.
Results: In the subdomains of shift, attention, and fluency, CHR individuals and FEP patients showed deficits compared to HC. The post hoc analysis revealed that CHR individuals had comparable attention shifting and phonemic fluency compared to FEP. CHR showed intermediate deficits between FEP and HCs in spatial working memory and semantic fluency, and the largest effect size was observed in semantic fluency both for CHR and FEP.
Conclusion: Overall, the findings of this study, in addition to providing detailed profiles of EF in prodromal and early psychosis patients, highlight the informative value of the specific subdomains of semantic fluency and spatial working memory.
Introduction
Impaired cognition across a range of cognitive domains is the hallmark of schizophrenia. Cognitive deficits in attention, learning, memory, and executive functioning (EF) have moderate to large effect sizes of impairments (1). Further, the deficits, especially in domains of attention, processing speed, working memory, verbal declarative memory, and EF are identified not only in chronic schizophrenia patients (2) but also prior to the onset of the disorder (3–5). In fact, distinctive patterns of cognitive deficits at different stages of the disorder exist, and they form before the onset of clinical symptoms, as early as the first episode of psychosis (FEP) (6, 7) or even before the prodromal state (8), the latter of which is also referred to as clinical high risk (CHR) for psychosis (9, 10). The FEP patients show cognitive deficits across on almost all cognitive domains, which are comparable deficits to the fully established disorder, and CHR individuals show intermittent degree of deficits.
Recent efforts have aimed at elucidating neurocognitive deficits prior to psychosis onset in CHR individuals who exhibit clinical features, such as symptoms and behaviors, that place them at increased risk for developing psychosis. To date, studies report small to medium impairments of approximately 0.3–0.6 standard deviations (SDs) below healthy controls (HCs) across various cognitive domains in CHR individuals (11–13) and larger sizes of impairment in FEP patients of 1.0–1.5 SDs below HCs (14–17). However, despite acknowledging the importance of understanding detailed cognitive patterns of CHR individuals and FEP patients, few studies have investigated both groups concurrently. Further, the few articles that exist report huge variations in reported effect sizes, which hinder us from clearly understanding the whole picture. This is most noticeable especially in the domain of EF. Significant factors contributing to this phenomenon are inconsistencies in definition of EF and its subdomains.
Disorders of EF are the most commonly observed cognitive deficits in schizophrenia (18). These disorders may be objectified by neuropsychological tests to examine different aspects of EF. This is because EF refers to complex mental processes that orchestrate purposeful and goal-directed activity that intrinsically underlies almost all of our neuropsychological functioning—verbal fluency, working memory, attention, and planning, to name a few. Further, disturbances in EF lead to impairment in second-order cognitive processes such as memory, language, or emotion, which may eventually produce psychosis symptoms such as hallucinations, negative symptoms, and dysexecutive behaviors (18). A number of reviews and meta-analyses have been conducted, but despite much effort, the current understanding of EF deficits in schizophrenia is limited. This is due to the inconsistencies of EF constructs between studies and the small number of studies evaluating EF across accepted domains of interest. In general, studies investigating EF of psychosis patients have employed a limited range of tasks and yet refer to EF as whole. Further, currently available tests can only capture one subdomain of EF, requiring administration of various tests to obtain a comprehensive view on EF. Hence, the currently available results are speculated to be more related to the variability in difficulty levels of the tests or to the dysfunction degree in the different subfunctions being measured in the investigation group. Thus, there exists criticism of this approach of investigation (19) in favor of moving toward building an overall framework of the EF profile in psychosis.
The consequences of the inconsistencies are best exemplified as discrepancies of EF effect sizes in several review studies. In both groups of FEP and CHR, a range of neurocognitive functions, such as verbal fluency and memory are consistently reported to have large effect sizes. In studies where these functions are grouped under EF, the effect sizes are reported as high (13), whereas in cases where these are classified as separate or are not measured, the effect sizes of EF are reported as low (12). Hence, so far, investigations have led to only crude conclusions that EF of CHR individuals is deficient. Thus, it is necessary to conduct a thorough examination of EF of CHR individuals, FEP patients and HCs, to reveal specific strengths and weaknesses on a subdomain basis that lie in their EF, compared to HCs, and to elucidate the common aspects and distinctive features of EF profiles in CHR individuals and FEP patients.
Therefore, in this study, we examined the EF profile, including all the subdomains of function, of both CHR and FEP individuals who were at different psychosis illness stages and compared their abilities to those of an HC group. We hypothesized that CHR individuals would show poorer performance compared to HCs but better performance compared to FEP patients. The subdomain of 1) shifting was measured by the Trail Making Test (TMT) and Wisconsin Card Sorting Test (WCST), 2) attention was measured by the Stop Signaling Test (SST), 3) fluency was measured by the Controlled Oral Word Association (COWA) test, and 4) planning was measured by the Rey–Osterrieth Complex Figure Test (RCFT) and Spatial Working Memory (SWM) Test.
Methods
Participants
Forty subjects at CHR, 85 FEP patients, and 85 HCs who participated in the prospective and longitudinal high-risk cohort study conducted at the Seoul Youth Clinic were involved in this research (20). All participants made initial contact with the Seoul Youth Clinic by telephone, by website (http://www.youthclinic.org), or by a referral from a local clinic.
Rigorous clinical interviews were administered to all FEP and CHR individuals by experienced psychiatrists using the Structured Clinical Interview for Diagnostic and statistical manual of mental disorders (DSM-IV) Axis I (SCID-I) disorders to identify past and current psychiatric illnesses. For FEP, inclusion criteria were having schizophreniform disorder, schizophrenia, or schizoaffective disorder in accordance with the DSM-IV criteria with a duration of symptoms of less than 2 years. At the time of assessment, 77.6% (n = 66) were receiving atypical antipsychotic medication, with a mean olanzapine-equivalent dose of 10.1 mg/day (SD = 11.3 mg/day), and 19 were not receiving any antipsychotic medication.
The CHR individuals were administered the validated Korean version of the Structured Interview of Prodromal Symptoms (SIPS). To be included, they had to fulfill at least one of the three established criteria for prodromal psychosis state: attenuated positive symptoms, brief intermittent psychotic symptoms (BIPS) below the threshold required for a DSM-IV Axis I psychotic disorder diagnosis, or a 30% decline in global functioning over the past year as well as a diagnosis of schizotypal personality disorder or a first-degree relative with psychosis.
HCs were recruited through an Internet advertisement. Exclusion criteria for HCs included past or current SCID-I Non-Patient Edition (SCID-NP) axis I diagnoses and any first- to third-degree biological relative with a psychotic disorder. The common exclusion criteria for all participants are as follows: substance use disorder, neurological disease, significant head injury accompanying loss of consciousness, evidence of significant medical illnesses that could manifest as psychiatric symptoms, and intellectual disability (IQ < 70). Informed consent was obtained from all subjects, in writing, and the study was conducted in accordance with the Declaration of Helsinki. The study was also approved by the Institutional Review Board of the Seoul National University Hospital.
Clinical and Neurocognitive Function Assessments
The Positive and Negative Syndrome Scale (PANSS) and the Global Assessment of Functioning (GAF) were administered to both CHR and FEP groups. To estimate each subject’s IQ, the Korean version of the Wechsler Adult Intelligence Scale (K-WAIS) was administered.
To assess EF and its subdomains, the following neuropsychological tasks were administered: 1) To assess participants’ attention shifting, the TMT (21) Part A and Part B were administered, and scores from Part A were subtracted from Part B, which enabled acquisition of the TMT B-A score. 2) Participants’ cognitive flexibility was measured by administering the WCST (22), in which the number of perseverative responses was calculated. 3) Response inhibition was evaluated with the SST (23), for which we calculated the stop signal reaction time (SST SSRT). 4) A verbal fluency test, the COWA (24), was administered. It measured the spontaneous oral generation of words within a fixed time span based on phonemic (phonological fluency, COWA Word) or semantic criteria (semantic fluency, COWA Category). 5) To evaluate participants’ visual memory, the RCFT (25), in which we calculated immediate and delayed scores and organization strategy scores, was given. 6) Participants’ working memory was assessed by administering the SWM (26), in which the error scores were calculated (see Table 1).
Statistical Analysis
Data analysis was conducted using Statistical package for the social sciences (SPSS) V.24.0 (SPSS Inc., 2016; PC version). Neuropsychological variables were assessed for normality (skewing and kurtosis). The test scores were standardized to the performance of the control group (z scored), and error scores were sign-changed to provide a standard metric for comparison across tests. A series of univariate analysis of covariance (ANCOVA) tests was conducted to examine differences in EF subdomain performances, with group (CHR, FEP, and HCs) as a between-participant factor and test performance scores as dependent variables. Furthermore, the demographic variables of sex and olanzapine-equivalent doses, which were significantly different between groups, were included as covariates. To detail group differences, post hoc Bonferroni-corrected pairwise comparisons were used. Multiple testing was controlled by the stepdown Bonferroni–Holm procedure (starting alpha level 0.05/7 = 0.007). Descriptive statistics for these variables are shown in Table 2. Effect sizes are reported as Cohen’s d.
Results
Demographic and Clinical Characteristics
There were no significant differences in age, years of education, or parental socioeconomic status in the participating individuals. Both the CHR and FEP groups had a significantly lower current IQ than the control sample, and FEP had a lower current IQ than CHR (F2,207 = 22.59, p < .001). Significant differences were also found in the demographic variable of sex: there were more females in the FEP than CHR and HC groups (F2,207 = 9.68, p < .01) (Table 2).
Neuropsychological Functioning Tests
Each of the ANCOVAs demonstrated a significant main effect of group (Attention: F2,202 = 9.092, p < .001; Shifting: F4,404 = 10.5, p < .001; Fluency: F4,404 = 10.169, p < .001; Planning: F4,404 = 11.526, p < .001) except for RCFT (Table 3).
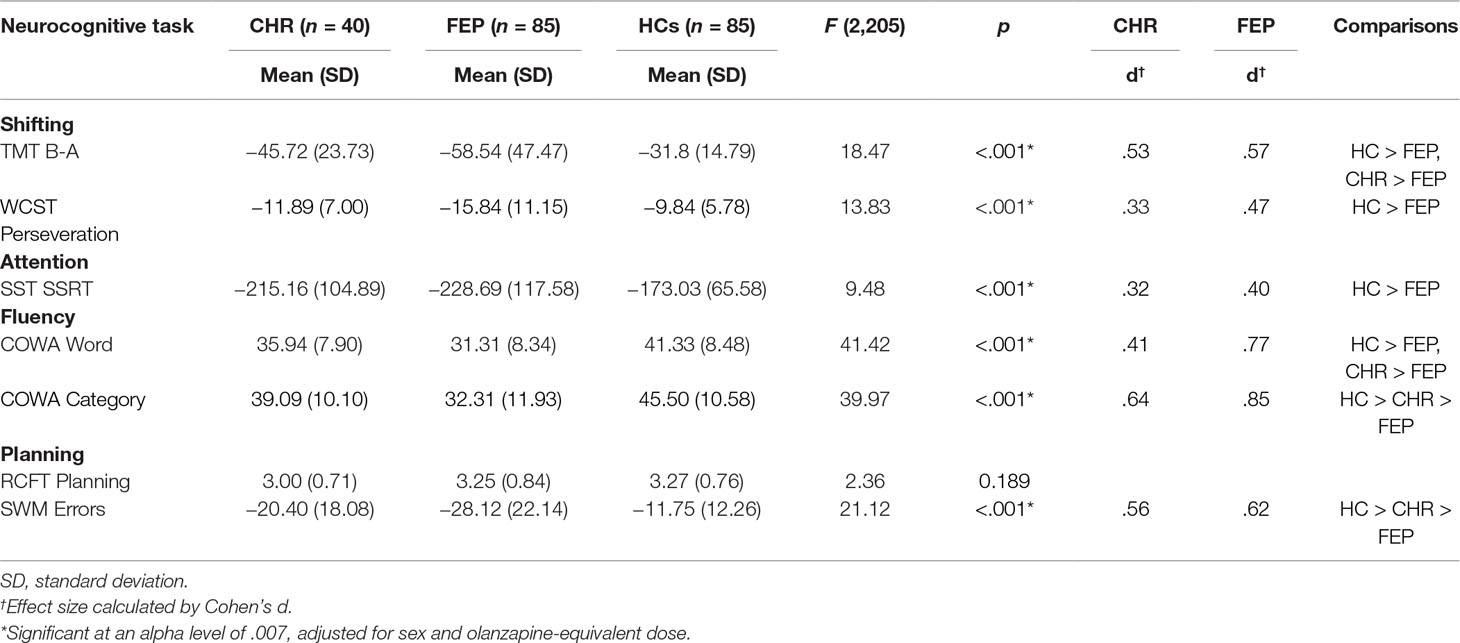
Table 3 Means of raw scores or scaled scores of CHR, FEP, and HCs for each of the executive function domains.
Follow-up analyses on the subdomain of shifting showed higher abilities of HCs compared to FEP in attention shifting (p < .001) and cognitive flexibility (p < .001) measured by subtracting the TMT-A score from the TMT-B scores and by the WCST perseveration score, respectively. CHR individuals showed better ability to shift their attention compared to FEP (p = .01). Impaired performance of inhibiting responses, compared to HCs, was observed in FEP (p < .001). Furthermore, post hoc analyses on fluency showed FEP had the most impaired semantic word fluency, with CHR having an intermediate level between the FEP and HCs. However, for phonemic fluency, both HCs and CHR were better than FEP (p < .001; p = .037, respectively). Lastly, for the planning subdomain, CHR showed intermediate spatial working memory ability, with HCs showing the best and FEP showing the worst (Figure 1). For Z-score means of each EF domain for CHR individuals and FEP patients, please refer to Supplementary Table 1.
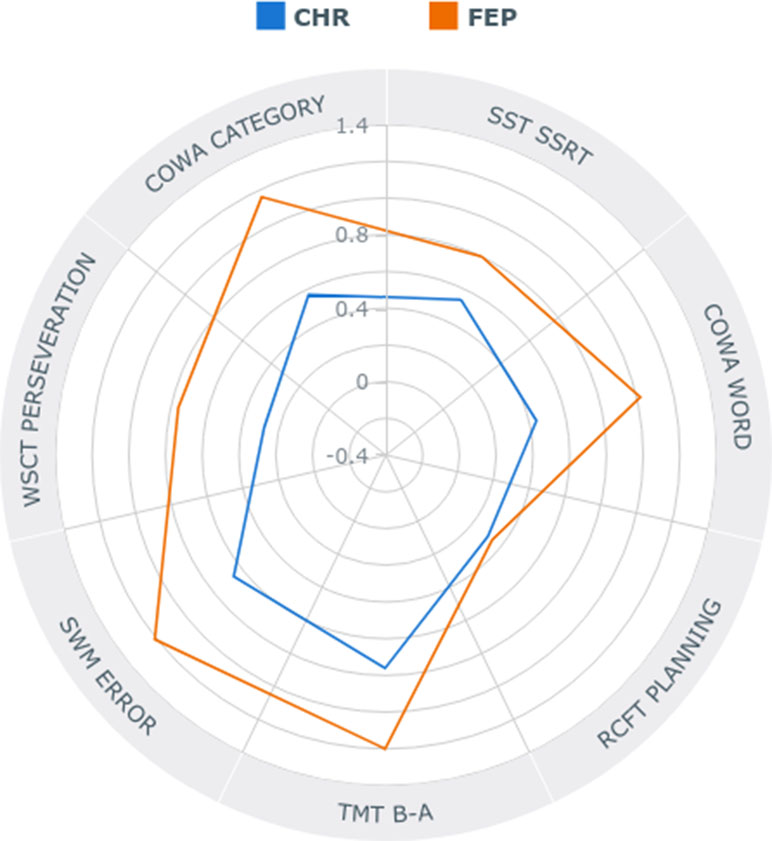
Figure 1 Radar plot showing specific profiles of executive functioning in individuals at clinical high risk (CHR) for psychosis and first-episode psychosis (FEP) in absolute z-scores.
Effect Size Analysis
Table 3 lists effect sizes as Cohen’s d corresponding to those group differences that remained significant after adjusting for multiple testing. The FEP and CHR groups demonstrated performance deficits with respective effect sizes from 0.4 and 0.32 (inhibitory control of attention) to 0.62 and 0.56 (spatial working memory) and to 0.85 and 0.64 (semantic fluency) compared with HCs (Table 3).
Addition of IQ as a Covariate
Supplementary Table 2 describes the results when IQ was added as a covariate in our analysis, as well as sex and olanzapine-equivalent dose.
Discussion
The present study examined the subdomains of EF in CHR and FEP to elucidate the patterns of EF profiles. The findings, in line with other studies, suggest broad impairment of EF performance in early psychotic individuals and individuals at risk for psychosis. However, despite showing similar patterns of deficits at a subdomain level, when each functioning within each subdomain was examined, different patterns were revealed between prodromal and early psychosis, with variable effect sizes. Gradual impairments, from prodromal to early psychosis, were observed in spatial working memory and semantic fluency. The latter functioning also had the largest effect sizes in both groups, suggesting it as a highly sensitive functioning for detection of both CHR individuals and FEP patients. Attention shifting and phonemic fluency were impaired in FEP patients but comparable in CHR individuals. Further, the integrities of cognitive flexibility and response inhibition were not detected for CHR individuals. Overall, the findings of this study highlight the informative values and the detailed nature of EF in early psychosis and individuals at risk for psychosis.
Abundant literature reveals substantial impairments in a range of cognitive functions in FEP patients (8, 19, 27, 28). In line with the literature, we also found broad impairments of EF in FEP patients in areas of attention shifting, cognitive flexibility, inhibition of attention, spatial working memory, and fluencies in phonemics and semantics (8, 29). Unlike the current literature, we did not find a significant deficit in the planning ability of FEP patients (30, 31). We cautiously speculate that this was due to the sensitivity of the test utilized in this study. Whereas the past studies (30, 31) have administered the Tower of London or modified versions of it to measure planning deficits of FEP patients, we utilized the planning score derived from RCFT. Although RCFT is a valid measure of one’s visuospatial abilities, organizing skills, and planning abilities, most studies in the field of psychosis research have utilized its measures of copy and recall (32–34), rather than planning. Although the planning measure is indeed a reliable and sensitive measure in the research field of obsessive-compulsive disorders (35), the results of our current study lead us to cautiously speculate that the planning measure of RCFT may not be sensitive enough to detect planning deficits in FEP patients or may capture different aspects of planning than the Tower of London test.
The CHR individuals showed fluctuations in their EF profiles, and there were three distinctive patterns: 1) comparable abilities to HCs in attention shifting and phonemic fluency; 2) significant deficits compared to HCs but still outperforming FEP patients in semantic fluency and spatial working memory; and 3) no statistically evident detection of significant deficits or preservation in cognitive flexibility or inhibition of attention. This pattern of fluctuations, as well as elucidating the strengths and weaknesses of EF function in CHR individuals, is evidence that there exist areas where currently employed tests fail to detect the subtle deficits.
We found significantly comparable ability of phonemic fluency in CHR individuals. Indeed, this is one of the most consistent findings in cognition studies of CHR individuals (36–38). Further, we found significantly disturbed semantic fluency in the same group. This phenomenon of comparable phonemic fluency but disturbed semantic fluency is one of the consistent findings in cognition studies of CHR individuals (36–38). This is thought to be due to the different underlying mechanisms involved in retrieving the stored information. While semantic fluency highly depends on activation flow through the semantic network, phonemic fluency depends on search and retrieval from the lexicon using phonemic or orthographic cues. Furthermore, on a brain-circuit level, unlike phonological processing, which requires activation of a number of fontal and temporal sites, semantic processing requires middle and superior temporal sites (39), and semantic processing involves a high degree of interhemispheric connectivity (40). Evidence suggests frontal bilaterality in verbal fluency in CHR individuals (41), and their semantic fluency deficit may be the result of the failure of lateralization (38), upon which semantic fluency partly depends. Further investigation is required to elucidate the similarities and differences in the neural mechanisms underlying semantic and phonemic fluency in psychosis patients.
Currently, semantic fluency deficit is considered as a candidate trait marker in schizophrenia. A meta-analysis by Szoke et al. (42) that investigated longitudinal studies of cognitive performance of schizophrenia patients showed that, unlike phonological fluency, semantic fluency remained stable, suggesting it is a persistent cognitive deficit that may be considered a potential trait marker in schizophrenia. Our results showing altered semantic fluency in CHR subjects and FEP patients further support this notion, together with the literature findings reporting deficits in first-degree relatives and the stability of the aforementioned results in schizophrenia patients (43, 44). Further, in a longitudinal study design for 2 years, we have previously reported persistent semantic fluency deficits in CHR subjects (45). To add more, in a recent meta-analysis by Fusar-Poli et al. (12), a range of neuropsychological functions was investigated in CHR individuals, and verbal fluency functioning, as well as working memory, was reported as one of the factors associated with the transition to psychosis, suggesting that it may be useful for early intervention.
We found significant deficits of spatial working memory in CHR individuals and FEP patients compared to HCs, with CHR individuals having significantly better performance than FEP patients. Indeed, the deficit in visuospatial working memory is fundamental to schizophrenia (46). Mounting evidence exists for spatial working memory deficits in CHR individuals (12), FEP patients (47, 48), people at genetic risk for schizophrenia (49), and individuals with schizotypal personality disorder (50), suggesting its function as a cognitive marker of an increased vulnerability to disease. In a recent meta-analysis by Fusar-Poli et al. (12), CHR individuals had impaired working memory compared to controls, with an effect size of 0.36, and the CHR individuals who subsequently transitioned to psychosis had poorer working memory than the individuals who did not. However, there also exists evidence otherwise, and authors have failed to see spatial working memory as a possible indicator of psychosis onset (51). Functional neuroimaging studies also report altered regional brain activation during working memory performance in CHR individuals compared to controls, but similar to the current study’s pattern, it was to a lesser degree than in FEP patients (52–54).
Our findings should be interpreted in the context of several limitations. First, the planning factor was based upon errors in planning ability. In future studies, it would be beneficial to examine specific aspects of planning to tease apart the components of planning (e.g., formation versus execution of a plan). Furthermore, additional measures of planning exist (e.g., Tower of London) that may reveal planning abilities in other contexts. Similarly, additional measures, such as sustained attention, divided attention, and selective attention, could be employed for the attention factor to capture specific attentional deficits in psychosis patients. Second, this study, being a cross-sectional study, cannot account for the potential variability that may be present within individuals and does not suggest direct evidence that EF functions decline with the onset of psychosis or that the psychosis onset affects EF functions. Longitudinal studies suggest no cognitive decline from the psychosis prodrome to the FEP (8, 55, 56) and no associations between EF and psychotic (57). Further, we have not collected the data on how many patients declined participation. Thus, there may be potential recruitment bias. Lastly, different cognitive types of CHR or FEP may be present. Studies have reported the existence of different cognitive subtypes in CHR and FEP (2, 58–61). For example, we have reported baseline differences in neurocognitive functioning between remitting and nonremitting CHR individuals in a longitudinal study. The CHR nonconverters who later remitted did not show any significant baseline cognitive deficits compared to HCs (62).
The current study, as well as suggesting EF requires a thorough examination in individuals at different stages of psychosis illness, also highlights the significance of examining different components of EF to identify specific subdomains of relative strength and weakness, rather than administering a single or a few tests and summarizing that EF is globally intact or deficient. Further, this approach can be taken to a global level, when applied across multiple psychiatric disorders, and be utilized to provide evidence of disorder-specific profiles, which duly supports the current movement toward a shared-assessment approach. The clinicians, then, may be able to select tests that are most sensitive to disorder-specific patterns. The present findings of individuals at different psychosis illness stages indicate that semantic fluency and spatial working memory can be utilized to distinguish individuals at different stages of psychosis. Overall, the findings of this study highlight the informative value and the detailed nature of EF in individuals with early psychosis and individuals at risk for psychosis, especially in the subdomains of semantic fluency and spatial working memory.
Ethics Statement
This study was carried out in accordance with the recommendations of the Seoul National University Hospital Institutional Review Board with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Seoul National University Hospital Institutional Review Board.
Author Contributions
WH contributed to performing the literature search, data analysis, interpretation of the results and to drafting the manuscript. TL and JK contributed to the study design, analysis plans and to editing the manuscript. JL contributed to collecting the clinical information of the participants. JK contributed to the data collection and data analysis. WS contributed to the data collection, analysis plan and to editing the manuscript. MK contributed to the editing the manuscript.
Funding
The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (grant no. 2017M3C7A1029610).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2019.00356/full#supplementary-material
References
1. Conn PJ, Tamminga C, Schoepp DD, Lindsley C. Schizophrenia: moving beyond monoamine antagonists. Mol Interv (2008) 8(2):99–107. doi: 10.1124/mi.8.2.7
2. Raffard S, Bayard S. Understanding the executive functioning heterogeneity in schizophrenia. Brain Cogn (2012) 79(1):60–9. doi: 10.1016/j.bandc.2012.01.008
3. Kuperberg G, Heckers S. Schizophrenia and cognitive function. Curr Opin Neurobiol (2000) 10(2):205–10. doi: 10.1016/S0959-4388(00)00068-4
4. Breton F, Plante A, Legauffre C, Morel N, Ades J, Gorwood P, et al. The executive control of attention differentiates patients with schizophrenia, their first-degree relatives and healthy controls. Neuropsychologia (2011) 49(2):203–8. doi: 10.1016/j.neuropsychologia.2010.11.019
5. Freedman D, Brown AS. The developmental course of executive functioning in schizophrenia. Int J Dev Neurosci (2011) 29(3):237–43. doi: 10.1016/j.ijdevneu.2010.11.003
6. Flashman LA. Disorders of awareness in neuropsychiatric syndromes: an update. Curr Psychiatry Rep (2002) 4(5):346–53. doi: 10.1007/s11920-002-0082-x
7. Addington J, Brooks BL, Addington D. Cognitive functioning in first episode psychosis: initial presentation. Schizophr Res (2003) 62(1–2):59–64. doi: 10.1016/S0920-9964(02)00340-7
8. Bora E, Murray RM. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr Bull (2014) 40(4):744–55. doi: 10.1093/schbul/sbt085
9. Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rossler A, Schultze-Lutter F, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry (2013) 70(1):107–20. doi: 10.1001/jamapsychiatry.2013.269
10. Schultze-Lutter F, Michel C, Schmidt SJ, Schimmelmann BG, Maric NP, Salokangas RK, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry (2015) 30(3):405–16. doi: 10.1016/j.eurpsy.2015.01.010
11. Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des (2012) 18(4):399–415. doi: 10.2174/138161212799316019
12. Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry (2012) 69(6):562–71. doi: 10.1001/archgenpsychiatry.2011.1592
13. Woodberry KA, Seidman LJ, Giuliano AJ, Verdi MB, Cook WL, McFarlane WR. Neuropsychological profiles in individuals at clinical high risk for psychosis: relationship to psychosis and intelligence. Schizophr Res (2010) 123(2–3):188–98. doi: 10.1016/j.schres.2010.06.021
14. Corigliano V, De Carolis A, Trovini G, Dehning J, Di Pietro S, Curto M, et al. Neurocognition in schizophrenia: from prodrome to multi-episode illness. Psychiatry Res (2014) 220(1–2):129–34. doi: 10.1016/j.psychres.2014.07.067
15. Jahshan C, Heaton RK, Golshan S, Cadenhead KS. Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology (2010) 24(1):109–20. doi: 10.1037/a0016791
16. Woodberry KA, McFarlane WR, Giuliano AJ, Verdi MB, Cook WL, Faraone SV, et al. Change in neuropsychological functioning over one year in youth at clinical high risk for psychosis. Schizophr Res (2013) 146(1–3):87–94. doi: 10.1016/j.schres.2013.01.017
17. Zhang T, Li H, Stone WS, Woodberry KA, Seidman LJ, Tang Y, et al. Neuropsychological Impairment in prodromal, first-episode, and chronic psychosis: assessing RBANS Performance. PLoS One (2015) 10(5):e0125784. doi: 10.1371/journal.pone.0125784
18. Orellana G, Slachevsky A. Executive functioning in schizophrenia. Front Psychiatry (2013) 4:35. doi: 10.3389/fpsyt.2013.00035
19. Bozikas VP, Andreou C. Longitudinal studies of cognition in first episode psychosis: a systematic review of the literature. Aust N Z J Psychiatry (2011) 45(2):93–108. doi: 10.3109/00048674.2010.541418
20. Kwon JS, Byun MS, Lee TY, An SK. Early intervention in psychosis: insights from Korea. Asian J Psychiatr (2012) 5(1):98–105. doi: 10.1016/j.ajp.2012.02.007
21. Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Tucson: Neuropsychology Press (1985).
22. Heaton R, Chelune G, Talley J, Kay G, Curtiss GJI. Wisconsin Card Sorting Test Manual. Odessa, FL: Psychological Assessment Resources (1981).
23. Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci (2003) 6(2):115–6. doi: 10.1038/nn1003
24. Strauss E, Sherman EM, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. New York: Oxford University Press (2006).
25. Osterrieth PA. Le test de copie d’une figure complexe; contribution à l’étude de la perception et de la mémoire. Arch Psychol (1944) 30(1944):206–356.
26. Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia (1990) 28(10):1021–34. doi: 10.1016/0028-3932(90)90137-D
27. Fitzgerald D, Lucas S, Redoblado MA, Winter V, Brennan J, Anderson J, et al. Cognitive functioning in young people with first episode psychosis: relationship to diagnosis and clinical characteristics. Aust N Z J Psychiatry (2004) 38(7):501–10. doi: 10.1080/j.1440-1614.2004.01403.x
28. Aas M, Dazzan P, Mondelli V, Melle I, Murray RM, Pariante CM. A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation. Front Psychiatry (2014) 4:182. doi: 10.3389/fpsyt.2013.00182
29. Hutton SB, Puri BK, Duncan LJ, Robbins TW, Barnes TR, Joyce EM. Executive function in first-episode schizophrenia. Psychol Med (1998) 28(2):463–73. doi: 10.1017/S0033291797006041
30. Ayesa-Arriola R, Rodriguez-Sanchez JM, Gomez-Ruiz E, Roiz-Santianez R, Reeves LL, Crespo-Facorro B. No sex differences in neuropsychological performance in first episode psychosis patients. Prog Neuropsychopharmacol Biol Psychiatry (2014) 48:149–54. doi: 10.1016/j.pnpbp.2013.09.009
31. Huddy VC, Hodgson TL, Kapasi M, Mutsatsa SH, Harrison I, Barnes TRE, et al. Gaze strategies during planning in first-episode psychosis. J Abnorm Psychol (2007) 116(3):589–98. doi: 10.1037/0021-843X.116.3.589
32. Becker HE, Nieman DH, Wiltink S, Dingemans PM, de Fliert JR, Velthorst E, et al. Neurocognitive functioning before and after the first psychotic episode: does psychosis result in cognitive deterioration? Psychol Med (2010) 40(10):1599–606. doi: 10.1017/S0033291710000048
33. Gold S, Arndt S, Nopoulos P, O’Leary DS, Andreasen NC. Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. Am J Psychiatry (1999) 156(9):1342–8.
34. Rodriguez-Sanchez JM, Perez-Iglesias R, Gonzalez-Blanch C, Pelayo-Teran JM, Mata I, Martinez O, et al. 1-year follow-up study of cognitive function in first-episode non-affective psychosis. Schizophr Res (2008) 104(1–3):165–74. doi: 10.1016/j.schres.2008.05.020
35. Shin MS, Park SJ, Kim MS, Lee YH, Ha TH, Kwon JS. Deficits of organizational strategy and visual memory in obsessive-compulsive disorder. Neuropsychology (2004) 18(4):665–72. doi: 10.1037/0894-4105.18.4.665
36. Gourovitch ML, Goldberg TE, Weinberger DR. Verbal fluency deficits in patients with schizophrenia: semantic fluency is differentially impaired as compared with phonologic fluency. Neuropsychology (1996) 10(4):573–7. doi: 10.1037//0894-4105.10.4.573
37. Magaud E, Kebir O, Gut A, Willard D, Chauchot F, Olie JP, et al. Altered semantic but not phonological verbal fluency in young help-seeking individuals with ultra high risk of psychosis. Schizophr Res (2010) 123(1):53–8. doi: 10.1016/j.schres.2010.05.005
38. Phillips TJ, James ACD, Crow TJ, Collinson SL. Semantic fluency is impaired but phonemic and design fluency are preserved in early-onset schizophrenia. Schizophr Res (2004) 70(2–3):215–22. doi: 10.1016/j.schres.2003.10.003
39. Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, et al. Cerebral organization of component processes in reading. Brain (1996) 119 (Pt 4):1221–38. doi: 10.1093/brain/119.4.1221
40. Faust M, Chiarello C. Sentence context and lexical ambiguity resolution by the two hemispheres. Neuropsychologia (1998) 36(9):827–35. doi: 10.1016/S0028-3932(98)00042-6
41. Kircher TT, Brammer M, Tous Andreu N, Williams SC, McGuire PK. Engagement of right temporal cortex during processing of linguistic context. Neuropsychologia (2001) 39(8):798–809. doi: 10.1016/S0028-3932(01)00014-8
42. Szoke A, Meary A, Trandafir A, Bellivier F, Roy I, Schurhoff F, et al. Executive deficits in psychotic and bipolar disorders - implications for our understanding of schizoaffective disorder. Eur Psychiatry (2008) 23(1):20–5.
43. Aukes MF, Alizadeh BZ, Sitskoorn MM, Selten JP, Sinke RJ, Kemner C, et al. Finding suitable phenotypes for genetic studies of schizophrenia: heritability and segregation analysis. Biol Psychiatry (2008) 64(2):128–36. doi: 10.1016/j.biopsych.2007.12.013
44. Snitz BE, Macdonald AW 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull (2006) 32(1):179–94. doi: 10.1093/schbul/sbi048
45. Shin YS, Kim SY, Lee TY, Hur JW, Shin NY, Kim SN, et al. Longitudinal change in neurocognition and its relation to symptomatic and functional changes over 2 years in individuals at clinical high-risk for psychosis. Schizophr Res (2016) 174(1–3):50–7. doi: 10.1016/j.schres.2016.03.024
46. Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry (2003) 160(12):2209–15. doi: 10.1176/appi.ajp.160.12.2209
47. Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med (2009) 39(6):889–905. doi: 10.1017/S0033291708004558
48. Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol (2005) 114(4):599–611. doi: 10.1037/0021-843X.114.4.599
49. Saperstein AM, Fuller RL, Avila MT, Adami H, McMahon RP, Thaker GK, et al. Spatial working memory as a cognitive endophenotype of schizophrenia: assessing risk for pathophysiological dysfunction. Schizophr Bull (2006) 32(3):498–506. doi: 10.1093/schbul/sbj072
50. Farmer CM, O’Donnell BF, Niznikiewicz MA, Voglmaier MM, McCarley RW, Shenton ME. Visual perception and working memory in schizotypal personality disorder. Am J Psychiatry (2000) 157(5):781–8. doi: 10.1176/appi.ajp.157.5.781
51. Goghari VM, Brett C, Tabraham P, Johns L, Valmaggia L, Broome M, et al. Spatial working memory ability in individuals at ultra high risk for psychosis. J Psychiatr Res (2014) 50:100–5. doi: 10.1016/j.jpsychires.2013.12.010
52. Allen P, Chaddock CA, Howes OD, Egerton A, Seal ML, Fusar-Poli P, et al. Abnormal relationship between medial temporal lobe and subcortical dopamine function in people with an ultra high risk for psychosis. Schizophr Bull (2012) 38(5):1040–9. doi: 10.1093/schbul/sbr017
53. Broome MR, Fusar-Poli P, Matthiasson P, Woolley JB, Valmaggia L, Johns LC, et al. Neural correlates of visuospatial working memory in the ‘at-risk mental state’. Psychol Med (2010) 40(12):1987–99. doi: 10.1017/S0033291710000280
54. Broome MR, Matthiasson P, Fusar-Poli P, Woolley JB, Johns LC, Tabraham P, et al. Neural correlates of executive function and working memory in the ‘at-risk mental state’. Br J Psychiatry (2009) 194(1):25–33. doi: 10.1192/bjp.bp.107.046789
55. Addington J, Saeedi H, Addington D. The course of cognitive functioning in first episode psychosis: changes over time and impact on outcome. Schizophr Res (2005) 78(1):35–43. doi: 10.1016/j.schres.2005.05.008
56. Carrion RE, Walder DJ, Auther AM, McLaughlin D, Zyla HO, Adelsheim S, et al. From the psychosis prodrome to the first-episode of psychosis: no evidence of a cognitive decline. J Psychiatr Res (2018) 96:231–8. doi: 10.1016/j.jpsychires.2017.10.014
57. Barder HE, Sundet K, Rund BR, Evensen J, Haahr U, Velden Hegelstad W, et al. Ten year neurocognitive trajectories in first-episode psychosis. Front Hum Neurosci (2013) 7:643. doi: 10.3389/fnhum.2013.00643
58. Lewandowski KE, Sperry SH, Cohen BM, Ongur D. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychol Med (2014) 44(15):3239–48. doi: 10.1017/S0033291714000774
59. Wells R, Swaminathan V, Sundram S, Weinberg D, Bruggemann J, Jacomb I, et al. The impact of premorbid and current intellect in schizophrenia: cognitive, symptom, and functional outcomes. NPJ Schizophr (2015) 1:15043. doi: 10.1038/npjschz.2015.43
60. Carrion RE, McLaughlin D, Auther AM, Olsen R, Correll CU, Cornblatt BA. The impact of psychosis on the course of cognition: a prospective, nested case-control study in individuals at clinical high-risk for psychosis. Psychol Med (2015) 45(15):3341–54. doi: 10.1017/S0033291715001233
61. Barbato M, Colijn MA, Keefe RS, Perkins DO, Woods SW, Hawkins KA, et al. The course of cognitive functioning over six months in individuals at clinical high risk for psychosis. Psychiatry Res (2013) 206(2–3):195–9. doi: 10.1016/j.psychres.2012.10.013
Keywords: executive function, psychosis, clinical high risk, neurocognition, semantic fluency, spatial working memory, first-episode psychosis
Citation: Hwang WJ, Lee TY, Shin W-G, Kim M, Kim J, Lee J and Kwon JS (2019) Global and Specific Profiles of Executive Functioning in Prodromal and Early Psychosis. Front. Psychiatry 10:356. doi: 10.3389/fpsyt.2019.00356
Received: 20 December 2018; Accepted: 07 May 2019;
Published: 21 May 2019.
Edited by:
Kelly Anne Allott, University of Melbourne, AustraliaReviewed by:
Gaelle Eve Doucet, Icahn School of Medicine at Mount Sinai, United StatesWenche Ten Velden Hegelstad, Stavanger University Hospital, Norway
Copyright © 2019 Hwang, Lee, Shin, Kim, Kim, Lee and Kwon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Soo Kwon, a3dvbmpzQHNudS5hYy5rcg==
 Wu Jeong Hwang
Wu Jeong Hwang Tae Young Lee
Tae Young Lee Won-Gyo Shin
Won-Gyo Shin Minah Kim
Minah Kim Jihyang Kim
Jihyang Kim Junhee Lee
Junhee Lee Jun Soo Kwon
Jun Soo Kwon