- 1Addiction and Mental Health Services, Metro South Health Services, Brisbane, QLD, Australia
- 2School of Medicine, University of Queensland, Brisbane, QLD, Australia
- 3School of Exercise and Nutrition Sciences, Queensland University of Technology, Brisbane, QLD, Australia
- 4Queensland Institute of Medical Research, Mental Health and Complex Disorders, Brisbane, QLD, Australia
- 5School of Psychiatry, Faculty of Medicine, UNSW, Sydney, NSW, Australia
Purpose: People with severe mental illness (SMI) experience poor physical health and premature mortality, contributed significantly by modifiable lifestyle risk factors such as poor nutrition, low cardiorespiratory fitness, and physical inactivity. Lifestyle interventions can reduce cardiometabolic risk and confer a range of other positive mental and physical health benefits. We assessed the feasibility, acceptability, safety, and preliminary effectiveness of a lifestyle (combined dietary and exercise) intervention lead by senior exercise and dietetics students in a residential mental health rehabilitation setting.
Design: Single arm, prospective study evaluating outcomes pre and post a 10-week dietary and exercise intervention.
Method: People with SMI from three residential rehabilitation units participated in a mixed aerobic and resistance training exercise intervention three times per week that was combined with a dietary intervention (six individual and group sessions). Primary outcome considerations were feasibility (recruitment, retention, and participation rates), acceptability, and adverse events. Secondary outcomes were preliminary effectiveness; (functional exercise capacity, volume of exercise, and metabolic markers), psychiatric symptoms, quality of life, and attitudes to exercise.
Results: Forty-two participants were recruited (92% primary diagnosis of schizophrenia). Intervention feasibility was supported by high levels of recruitment (68%), retention (77%), and participation (70% exercise, 65% diet sessions); and the absence of serious adverse events. Significant improvements in functional exercise capacity, volume of exercise, general psychiatric symptoms, and negative psychotic symptoms occurred. Anthropometric and metabolic blood markers did not change. While the intervention was acceptable to participants, motivation for and perceived value of exercise reduced over 10 weeks.
Conclusions: A brief pragmatic student-led lifestyle intervention integrated into usual mental health care was feasible, acceptable, safe, and scalable across two additional mental health residential rehabilitation sites, and resulted in physical and mental health improvements. Increased frequency of dietary sessions and length of dietary intervention may improve metabolic outcomes in the future. People with SMI living in residential rehabilitation units should have access to lifestyle programs to address modifiable lifestyle risk factors. While this brief intervention was feasible and acceptable, this study highlights some of the challenges associated with maintaining motivation for healthy lifestyles for people with SMI. Longer term investigation of real-world lifestyle interventions is warranted, together with additional interventions that may support people with SMI to sustain motivation to address lifestyle factors.
Clinical Trial Registration: The trial was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR), Unique Identifier: ACTRN 12618000478213, http://www.anzctr.org.au Universal trial number (UTN)—U1111-1211-4009.
Introduction
People with severe mental illness (SMI) have poor physical health and experience premature mortality of approximately 15–20 years compared to the general population (1); up to 85% of this prematurely mortality has been attributed to physical illnesses such as cardiometabolic disease (CMD), and cancer (2). Modifiable risk factors such as physical inactivity, poor nutrition, and low cardiorespiratory fitness play a significant contribution to this increased risk (3, 4). While the poor physical health of people with SMI has gained increasing international recognition (5), there is evidence that physical health disparities persist and the mortality gap for people with SMI may actually be widening (1). Addressing modifiable risk factors and improving the physical health of people with SMI should be a primary concern in a mental health setting (5).
Several meta-analyses of lifestyle (diet and exercise) interventions for people with SMI have demonstrated improvements in metabolic risk factors, such as waist circumference, body mass index (BMI), fasting blood glucose, triglycerides, and blood pressure (6–8). A recent meta-review found lifestyle interventions were comparable to pharmacotherapy for cardiometabolic risk reduction (9). However, the majority of lifestyle interventions have been conducted in outpatient community settings, or with early psychosis populations (10).
One setting in which few lifestyle interventions have been conducted are residential rehabilitation and supported housing units for people with SMI. People accessing these services typically experience significant burden from negative and cognitive symptoms, and have lower functioning than people living in community settings (11, 12). In Australia, Community Care Units (CCU’s) are a type of residential rehabilitation which provide independent housing in the community, with access to 24 h on-site multidisciplinary mental health staff, but do not provide food or meals.
Previous lifestyle studies conducted within supported housing, residential rehabilitation, or long stay inpatient units have broadly employed two different methods; either via evaluating the effect of the addition of combined structured nutrition and exercise interventions provided directly to residents with SMI (13, 14), or by more broadly focusing on attempts to utilize existing resources to improve the environment and culture of facilities, via policy change and by upskilling mental health staff to promote healthy behaviors for residents of the facilities (15–17). Both types of studies have found various improvements in components of the metabolic syndrome [weight, BMI, waist circumference, blood pressure, high density lipoprotein (HDL) cholesterol], (13–17) and objective physical activity (15). In an Australian context, to date, no combined diet and exercise interventions have been formally evaluated within a residential rehabilitation setting.
Our team conducted a small (n = 10) exercise physiology student-led pilot supervised exercise intervention (three sessions per week of supervised exercise) in 2016 in a single residential rehabilitation unit, Coorparoo CCU (18). We found the intervention to be feasible and improved functional exercise capacity and total exercise volume/week. However, no changes in metabolic risk factors were observed. We postulated that this may be due to the poor diet quality of the participants (19), and the addition of a dietary intervention may be beneficial.
In this follow-up study, we expanded on the pilot study by including (i) the dietary intervention (six individual and group sessions), and (ii) two additional CCU sites. Research from large scale clinical trials are often significantly resourced compared to usual clinical settings, with interventions typically led by external research teams, which limits generalizability to usual care settings (20, 21). There are increasing calls for the research agenda of lifestyle interventions to include studies that report outcomes from pragmatic and real-world settings (22, 23). Hence, in the current study, we implemented and evaluated the feasibility of a 10-week, exercise physiology and dietetics student-led, pragmatic lifestyle intervention integrated within the usual mental health care setting of three CCU’s.
Based on the results of our pilot exercise study, we hypothesize that the combined dietary and exercise lifestyle intervention will be feasible and acceptable increase fitness (measured via functional exercise capacity) and the amount of exercise of participants. Further, we hypothesize the addition of a dietary component to the intervention will decrease metabolic risk factors of participants with SMI located within CCU’s.
Materials and Methods
Study Design
This study is a single arm, prospective trial. The intervention consisted of a 10-week lifestyle intervention conducted on-site at three different CCU’s. This study was approved by the Metro South Human Research Ethics Committee in March 2016 (HREC 16/QPAH/042).
Participants
Participants were residents of three CCU’s within the Metro South Addiction and Health Services in Brisbane, Australia. The residents were initially assessed by the CCU consultant psychiatrist, using the Diagnostic Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and admitted to a CCU with a diagnosis of SMI (schizophrenia, schizoaffective disorder, bipolar disorder, or chronic major depression) for psychosocial rehabilitation. Admission criteria for CCU’s stipulate that residents with a history of co-morbid substance abuse are able to access residential services provided they do not use substances on site or access rehabilitation services while intoxicated and are engaged in an active process of seeking help to manage their substance abuse disorder from CCU staff or other agencies. The maximum number of residents living in a CCU at any one time is 20, with an expected length of stay of approximately 12 months.
Participants were recruited to the study via verbal invitation from research staff located at each CCU and referral by staff members. Recruitment occurred over a 3-week period prior to the intervention. Inclusion criteria for the study were: (i) a current resident of the CCU (ii) able to provide informed consent, and (iii) aged between 18–65 years. Residents were excluded if they were: (i) currently engaging in exercise of ≥150 min/week of low-to-moderate intensity or ≥75 min/week of vigorous intensity exercise, (ii) considered high risk for aggression or suicide by the treating psychiatrist, (iii) assessed to have an acutely unstable mental state by the treating psychiatrist, (iv) pregnant, or (v) classified as high risk on the Adult Pre-Exercise Screening System (APSS), remove a space after (APSS) (24) and not granted medical clearance for exercise by a general practitioner (GP).
Residents with a history of substance abuse were not excluded from access to the lifestyle intervention provided they were able to agree to CCU admission criteria regarding substance abuse.
Participants were provided with a participant information sheet and written informed consent was obtained.
Exercise Intervention
Three, 1 h group exercise sessions per week were offered, (45-min session with a 5-min warm up and a 10-min cool down) involving circuit training with a combination of aerobic and strength exercises in the shared courtyard of each CCU for 10 consecutive weeks.
Individuals who missed a session or preferred to exercise alone were offered individual sessions. Further details of the exercise intervention are published elsewhere (25).
Drawing on earlier research, the intervention included several evidence-based motivational strategies to increase participants’ adherence to the program (26). These strategies included validation and encouragement of participants, goal setting, individualization of the program and assistance to identify and problem solve barriers for exercise engagement.
Additionally, exercise circuit stations were designed such that they could be modified (regressed/progressed) to suit individual participant capacity and preference which is an important factor in the design of exercise interventions for people with SMI (26, 27).
The sessions were led by two fourth (final) year exercise physiology (EP) students at each CCU undertaking a 12-week practicum placement, under the supervision of university lecturers and a part time accredited exercise physiologist (AEP).
Dietary Intervention
Final year masters of dietetics (MDiet) students attended CCU’s fortnightly on rotation from their university, and collaborated with mental health staff to offer the dietary intervention on-site at each of the CCU’s setting. The dietary intervention ran concurrently with the 10-week exercise intervention, with an individual dietary assessment and a healthy cooking/eating group occurring on the same day, once per fortnight, on six occasions. While food was provided for the purpose of the cooking group, residents of CCU’s are supported to shop and cook independently, and no food is provided on site for other meals.
There were three components of the diet intervention:
1. Group education on basic healthy nutrition on the following structured topics: portion size, recommended daily intake, daily energy requirements, benefits of fiber and how to increase daily consumption, label reading, healthy snack and breakfast preparation, incorporation of more vegetables, food storage, and weight loss principles.
2. Cooking skills demonstration where participants practiced basic cooking skills under the supervision of the MDiet students followed by a shared eating experience. Participants were provided with a copy of the recipe broken down into simple steps for future use.
3. On the same day as the cooking group. MDiet students offered individualized dietary assessment and counseling session based on participants’ goals.
Any participants discharged from the CCU prior to the study completion were invited to continue attending both the dietary and exercise sessions at the CCU’s.
The student placements were an opportunity for mutual collaboration and capacity building for both EP students, Mdiet students, and mental health multidisciplinary staff regarding physical activity, nutrition, and mental health concepts (28).
Outcomes
Primary Outcomes: Feasibility, Safety, and Acceptability
Feasibility was determined through procedural statistics (recruitment, retention, participation rates). Previous studies were used to determine thresholds for indicative feasibility a priori.
1. Retention: Study feasibility was determined if >70% of the sample complete the study. Previous feasible lifestyle interventions had retention rates of 73%–78% over the first 6 months (29–31).
2. Recruitment: We considered the intervention feasible if the majority of CCU residents (>60%) were recruited. Recruitment is based on the percentage of participants who consent to the intervention as a proportion of residents residing in a CCU during the recruitment phase 3 weeks prior to study onset.
3. Participation rates: The intervention was considered feasible if participants completed ≥65% of the exercise and diet interventions. Fitness improvements have been reported in rehabilitation studies in which participants completed ≥66% of 3*/week classes (32) and weight loss in participants who participated in ≥ 66% of group and individual diet interventions over the first 6 months (30).
Safety was reported as the number of adverse events (new injuries, exacerbation of pre-existing conditions), experienced during the intervention as a proportion of the total number of participants recruited into the trial.
Acceptability of the exercise (questionnaire adapted from previous exercise research in this population), (33) and dietary components of the program were assessed via questionnaires on a 5-point Likert Scale (strongly disagree, disagree, neutral, agree, strongly disagree). Responses were collapsed into three categories: disagree/strongly disagree, neutral, and agree/strongly agree.
Secondary Outcomes (Preliminary Effectiveness)
1. Functional exercise capacity; a submaximal proxy measure of fitness (34), was measured via the distance walked during the 6 Minute Walk Test (6MWT). The 6MWT was performed according to the ATS guidelines (35)
2. Total psychiatric symptoms was measured via the Brief Psychiatric Rating Scale (BPRS), (36), a semi-structured interview.
3. Negative symptoms was measured via the Scale for the Assessment of Negative Symptoms (SANS), (37).
4. Physical activity levels and sedentary behavior was measured using the Simple Physical Activity Questionnaire (SIMPAQ). The SIMPAQ is a researcher-administered self-report questionnaire assessing time (average minutes and hours) spent in bed, sitting or lying down, napping during the day, walking, structured exercise, and incidental activities completed in the previous week (38).
5. Fasting blood lipids: (total cholesterol, HDL, low-density lipoprotein, and triglycerides) and blood glucose. Venous blood was collected from a trained phlebotomist from a community collection center and analyzed using standard clinical laboratory procedures
6. Anthropometrics: BMI, waist circumference, height (meters), and body weight (kg) were measured on an electronic scale and wall-mounted stadiometer, respectively, for the calculation of BMI, (weight/height2). Waist circumference was measured using a retractable anthropometry tape (39)
7. Quality of life: was assessed using the AQol-8D, a reliable and valid instrument, which is particularly suitable when psychosocial elements of health are of importance. It involves questions assessing eight dimensions of mental and physical health; three of the dimensions (independent living, pain, senses) make up a physical super-dimension; the other five (mental health, happiness, coping, relationships, and self-worth) a mental super-dimension. Each dimension has high reliability (Cronbach’s α=0.61–0.96), and a demonstrated ability to distinguish general population scores from mental health patients. Dimension scores are placed scale of 0–1, where 1 is optimal health (40).
8. Participants’ motivations toward exercise was measured by the Behavioral Exercise Regulations Questionnaire (BREQ-2), (41). The BREQ-2 comprises 19 items to assess six domains of motivation; amotivation external, introjected, identified, integrated and intrinsic behavioral regulations (42). Responses to items are scored on a 5-point scale ranging from 0 = “not true for me” to 4 = “very true for me, with an average score created for each of the six domains, with higher scores indicating higher ratings in each of these domains.
Statistical Analysis
Results were analyzed using Statistical Package for the Social Sciences (SPSS, version 26, IBM). Normality of the resulting model residuals were assessed using the Shapiro-Wilk test and via inspection of histogram and quintile-quintile plots. Continuous data was analyzed pre- and post-intervention by paired t-test, or where data were not normally distributed, a Wilcoxon signed rank test. Categorical data was analyzed by chi-squared test. All tests were two-tailed with an alpha of 0.05 applied as the criterion for statistical significance. The Bonferroni correction was conducted to consider significance after multiple analyses performed on the same data. This was achieved by dividing the per analysis rate (αPC = 0.05) by the number of statistical analyses performed for all outcome measures. Given 25 statistical tests were performed, individual outcomes remained significant after Bonferroni corrections if P ≤ 002, which was indicated in the respective tables with a superscript a. Effect sizes for parametric data were calculated using the Cohen’s d statistic (cut points; low = < 0.4, medium = 0.4–0.6, large = > 0.6) and nonparametric data (z/√n), (cut points; small 0.1, medium 0.3, large 0.5).
Results
Baseline Demographics
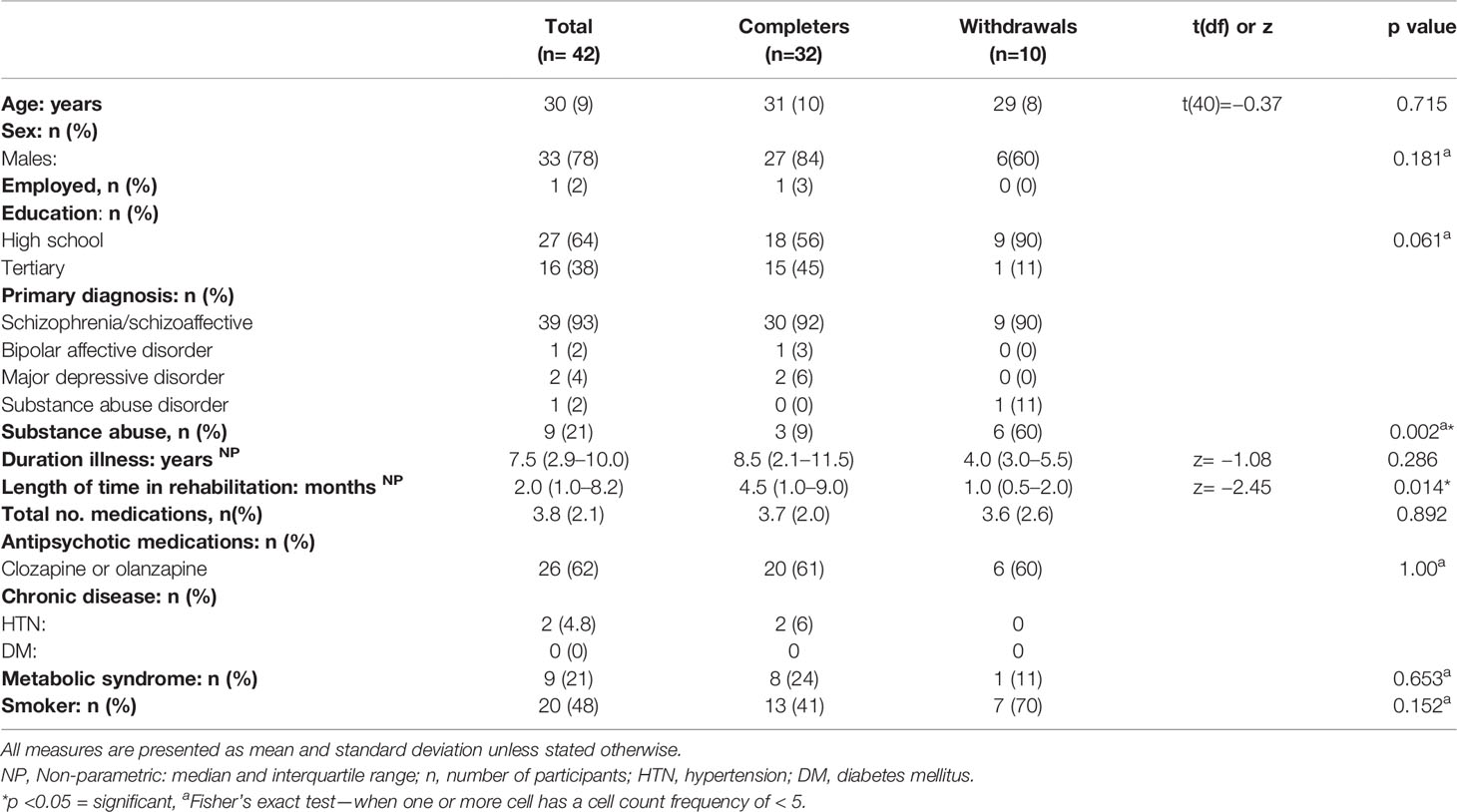
The primary SMI diagnosis of participants in this study was schizophrenia (93%), with a median of with a median of 7.5 years of illness [inter-quartile range (IQR) = 2.9–10.0 years]. Of the 42 participants recruited to this study, 26 (62%) were taking clozapine or olanzapine, atypical antipsychotic medications with the highest propensity for weight gain, with an average of 3.6 (SD = 2.1) medications per participant.
There were no significant differences between participants who completed or withdrew from the intervention in terms of age, total number of medications, duration of illness, sex, employment, education, diagnosis, smoking status, chronic disease, or antipsychotic medications (Table 1). However, participants with a diagnosis of substance abuse were more likely to be in the withdrawal group (p=0.002). Further, there was a significant difference between those who completed follow up assessments and those who did not in total time in rehabilitation, (completers; median 4.5 months, IQR 1.0–9.0), participants who withdrew, median = 1.0 month, IQR 0.5–2.0; p=0.014).
Feasibility
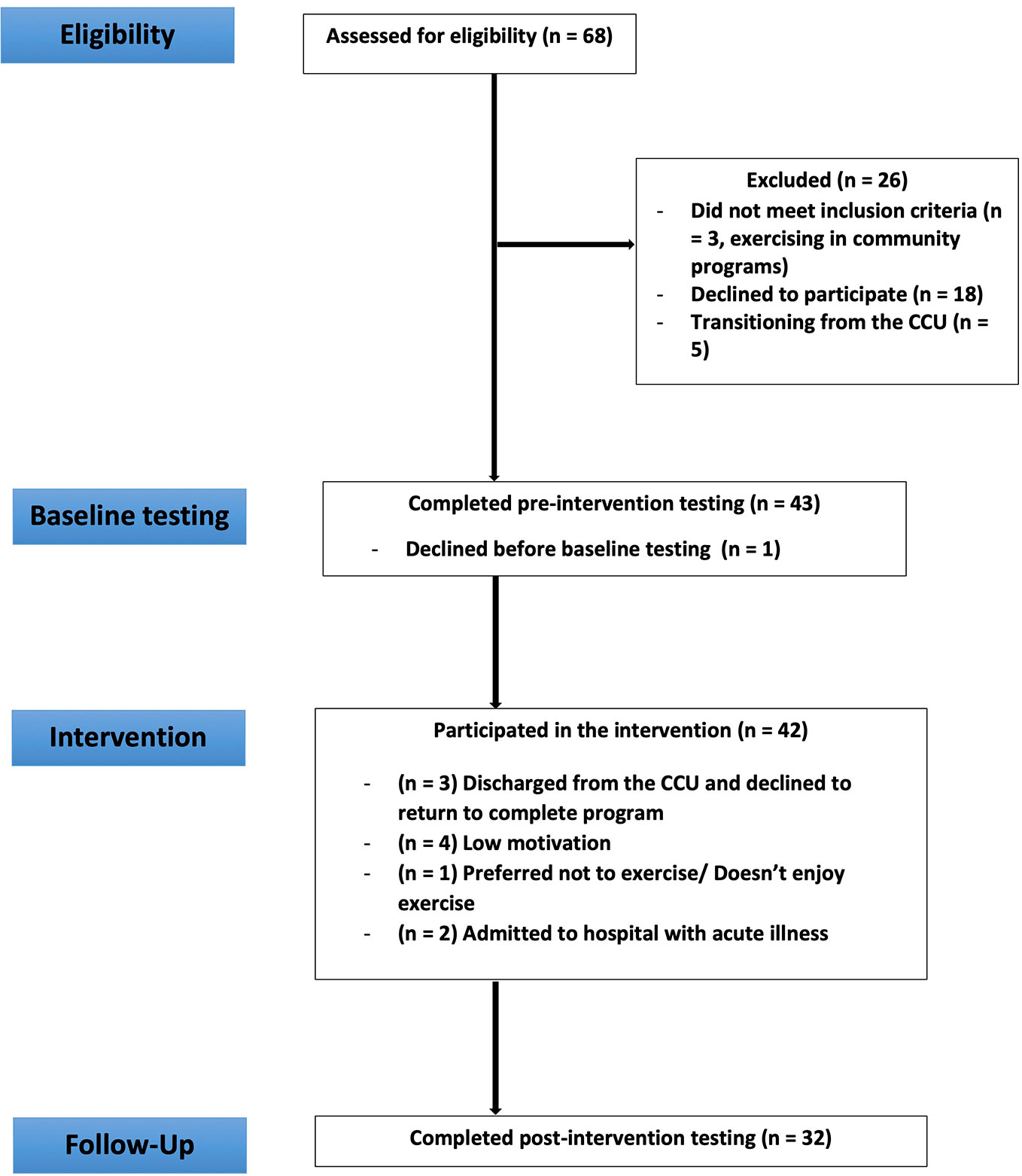
Of the 68 eligible residents, 42 participants were recruited and completed baseline measures (recruitment rate = 61.8%). Of the 42 recruited participants, 32 completed post-intervention measures and 10 withdrew (retention rate of 77%). Reasons for withdrawal are provided in Figure 1.
Participation rate for the 30 available exercise sessions was 70% (of those completing the intervention), (median = 21 sessions, IQR 13.5–30.0). Attendance to the six fortnightly individual and group dietary session was a median of 4 (IQR 3–4).
Acceptability
Exercise acceptability questionnaires were completed by 50% (n=21) of the sample, (65% of completers), and 62% (n=26) completed the diet acceptability questionnaires, (81% of completers) See Supplementary Tables 1–4.
Of participants who completed the acceptability questionnaires, the majority expressed satisfaction (86%), enjoyment (90%), desire to continue exercising after the study (71%), and enjoyed the group setting (86%). More participants enjoyed resistance components such as stretch bands (71%), hand weights (76%), and the medicine ball (67%) as compared to cardio elements such as treadmill (47%) or seated bicycle (38%) and more than half preferred more sports to be included. Over half of participants reported they would be confident to complete this exercise on their own and when they were having a bad day (57%).
Of the participants who completed the diet acceptability questionnaire, the majority (88%) would recommend the program to others and noticed improvements in themselves (81%) following the healthy eating program. Eighty-five percent of the participants reported improvement in nutritional knowledge, and 92% of them were willing to continue to use their new skills post-intervention. The dietary intervention was sufficiently challenging for most participants (70%).
Safety
There were no new injuries or adverse events that resulted in intervention withdrawal. Of the 32 completers, three (9.4%) experienced an exacerbation of pre-existing musculoskeletal issues that required individual modifications to the prescribed exercises without the need for medical intervention. Two participants had an increase in knee pain and one experienced exacerbation of back pain. Modifications were made to avoid aggravating exercises.
Preliminary Effectiveness
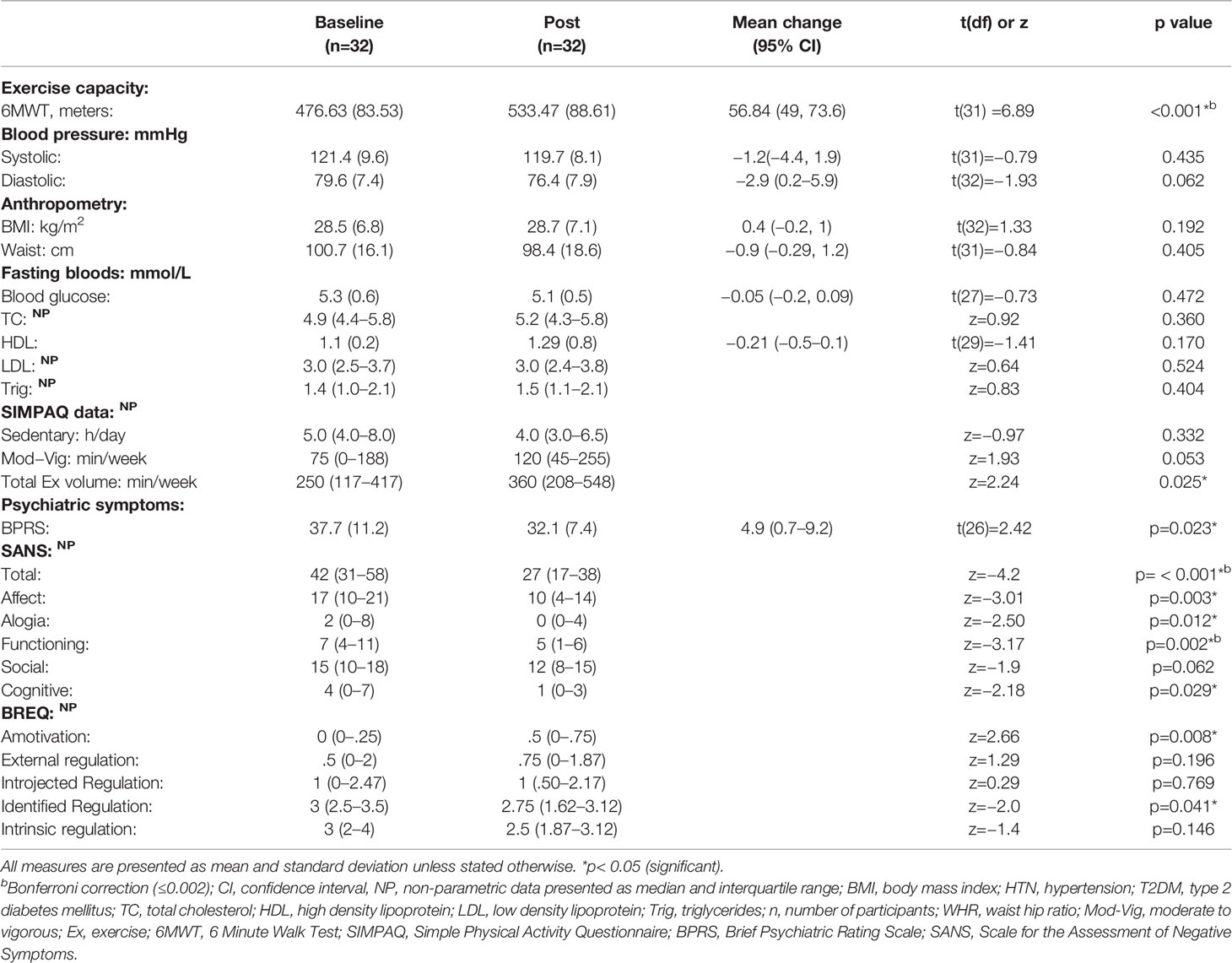
There was a significant increase in functional exercise capacity as measured by 6MWT, mean difference = 56.8 m (95% CI 40–73.66) p< 0.001, large effect size (Cohen’s d = 0.7). Total minutes of exercise increased by 110 minutes per week, post intervention, z=2.24 (p=0.025), medium effect size (r= 0.39) See Table 2.
All measures of psychiatric symptoms reduced following the intervention. Total symptoms reduced by 4.9 points (95% CI 0.7–9.2; p = 0.023), medium effect size (Cohen’s d = 0.6). With respect to negative symptoms, each category of the SANS was reduced with a significant total mean reduction of 15.4 points (95% CI 10.5–20.2; p< 0.001) and large effect size (Cohen’s d = 0.9).
There was a change in the physical SuperDimension of the AQOL-8D, (combined physical health domain: reduced pain, increase in senses; hearing/vision/communication, increase in functioning in independent living skills, mean difference −0.04 (SD 0.12), p = 0.038, small effect (Cohen’s d =0.33), however no change in the psychological SuperDimension or any other individual domain was observed, see Supplementary Table 5.
With respect to attitudes to exercise, there was an increase in amotivation (lack of intention to begin exercise) following the intervention (“I can’t see why I would bother exercising, I don’t see the point”), z=2.66, p=0.008, medium effect (r= 0.47), and a reduction in identified regulation (“I value the benefits of exercise, it’s important to exercised regularly”), z= −2.0, p=0.041, medium effect (r= 0.35). There were no other significant differences for any other domain of the BREQ-2.
There were no significant differences from pre- to post-intervention in any of the other outcome measures (sedentary behavior, anthropometric measures, or markers of metabolic health).
Discussion
Implementation of a brief student-led lifestyle (combined dietary and exercise) intervention integrated into usual mental health care within three residential rehabilitation settings was feasible, safe, and acceptable with no significant adverse events resulting in drop out. We found increases in functional exercise capacity, total exercise volume, and a decrease in negative and total psychiatric symptoms, confirming and extending our 2018 pilot exercise findings across two additional rehabilitation settings. However, addition of the dietary intervention did not improve markers of metabolic health or anthropometric measures.
The recruitment rate of the combined diet and exercise lifestyle intervention (68.0%) was similar to previously reported lifestyle interventions conducted within both outpatient (30, 43) and residential rehabilitation settings (13, 14), (63–70%), indicating the large majority of people accessing rehabilitation facilities may be interested in participating in lifestyle interventions. This is encouraging given that rehabilitation facilities typically have a holistic focus and are often resourced with shared cooking facilities making these settings opportune for implementation of lifestyle interventions (43).
Our retention rates (77%) were high and comparable with other lifestyle intervention studies in SMI populations (73–84%) (29, 30, 43, 44). Features of our program that may have promoted participant retention included support by mental health staff to participate, provision of the intervention on site, and integration into usual mental health care (26).
Participation rate for those completing the exercise intervention was 70%, and was broadly comparable to the 72% reported in a meta-analysis of exercise interventions for people with SMI (45). Of the 32 completers, one third (9) of participants attended 100% of the sessions and were observed to make friendships with other participants during partnering and teamwork involved with circuit sessions, which likely facilitated autonomous motivation to exercise (46). As was highlighted in previous pilot work, (25) an advantage of the student placement design was that EP students had the flexibility to offer both group and individual make-up sessions which increased participation rates. Broadening the exercise program to include less structured exercise formats and greater variety (45), i.e. more sports and use of green spaces (47), in keeping with participants’ preferences, may increase participation further.
For those who completed exercise questionnaires, there was overall acceptance and enjoyment of the exercise program. Confidence to exercise alone and when having a bad day was particularly encouraging as exercise could be included as a coping strategy for symptom exacerbations in the setting of treatment resistance, commonly encountered by residents in residential facilities (12). We found resistance training elements were popular; future exercise interventions could focus on expanding the provision of resistance training, particularly given emerging evidence for this type of training for depressive symptoms, quality of life, and symptoms of psychosis (48–50).
While the brief intervention was acceptable to participants, the most common reason for withdrawal was a lack of motivation to exercise. Additionally, for those completing the intervention we found an increase in amotivation and decrease in the value of exercise on the BREQ-2 questionnaire over ten weeks. Together, these findings confirm the challenges people with SMI can face regarding motivation to adapt and sustain a healthy lifestyle (27), which raises two points. Firstly, while participation was high over ten weeks, amotivation was already increasing which may impact longer term engagement. The vast majority of lifestyle intervention evidence has been reported from studies under 6 months (10), hence longer pragmatic interventions need to be evaluated, as these findings may have a significant impact on lasting health benefits for people with SMI. Secondly, these findings highlight the importance of addressing autonomous motivation when delivering lifestyle interventions (51). While we employed motivational strategies, students leading the lifestyle intervention were not fully qualified; and interventions delivered by qualified practitioners may improve adherence and motivation (52). In future, studies could also investigate the use of objective physical activity measurement devices (i.e. pedometers or accelerometers) and mobile phone apps to target autonomous motivation to address lifestyle behaviors (53–55). Additionally, in future, utilizing principles of co-design, by consulting service users regarding optimal development and implementation of exercise interventions, may increase autonomous motivation to engage (23, 56, 57).
There was broad level of acceptance of the dietetic program by those completing acceptability questionnaires: the majority felt that they had improved their eating habits and would continue to use healthy cooking skills independently, however this was not assessed objectively.
While no adverse events resulted in participant withdrawal, several participants experienced exacerbations of pre-existing injuries that required program modifications. This highlights the importance of exercise professionals prescribing and delivering exercise programs for people with SMI, who are known to experience higher levels of pain and physical comorbidity than the general population (26, 58, 59). Nonetheless, all participants with pre-existing injuries were able to complete a modified program, in keeping with several recent studies suggesting that exercise is safe in people with SMI (22, 51).
Consistent with our hypothesis, and pilot study findings, we found a significant increase in functional exercise capacity (26) of 57 m—these findings are in keeping with the literature regarding exercise interventions and people with SMI (26, 60). Increases of greater than 50 m (achieved by over half of those completing our program) have been associated with clinically significant improvements in walking capacity in people with cardiovascular disease (29, 61) and at improving self-perceived fitness levels in other disease populations (62). Our results were higher than several outpatient lifestyle interventions (increases of 7–34 m), (29, 43, 44), however the average BMI of participants in these studies (BMI 34–37) was higher than ours (BMI 28.5), where obesity is known to cause impairments in functional exercise capacity (63). Our functional exercise capacity increases may also have been higher than other lifestyle studies in outpatients and residential rehabilitation studies due to the greater frequency of sessions we offered (three supervised sessions per week versus one), (13, 29, 44). Engagement in three or more sessions per week has been associated with greater fitness improvements for people with SMI (60), which may have relevance when planning exercise interventions in a residential rehabilitation setting.
There were no significant anthropometric or metabolic changes following our 10-week study. Previous lifestyle interventions in both residential and outpatient rehabilitation settings have demonstrated various reductions in anthropometric (weight, BMI, waist) and metabolic syndrome components in people with SMI (13, 14, 30, 64). There may be several reasons for this finding.
Firstly, people accessing 24 h residential services can have substantial motivational and cognitive deficits (11, 65) and may require more intensive support to integrate changes into their diet. The number of diet intervention sessions we offered (six same-day cooking groups and individual sessions) may have been insufficient to facilitate behavior change. Previous research in both a supported housing and outpatient rehabilitation setting demonstrated, improvements in metabolic syndrome parameters (HDL, systolic BP, body weight) occurred after substantially more diet interventions than our study employed (35 group sessions and 21 combined individual and group sessions respectively), (13, 30).
Secondly, whereas weight loss tends to peak early after lifestyle interventions in the general population, weight loss may occur more gradually for people with SMI (14, 30, 66), hence our 10-week intervention may have been too short to detect significant change.
Thirdly, CCU’s provide support to residents to cook and shop independently but do not provide food on site. Successful weight loss has been reported in rehabilitation settings where food was provided (14, 30) and hence caloric intake and nutrition quality could be modified more easily than in a CCU where participants had to shop for and cook their own food. Healthy meal delivery to replace meals for some CCU residents who experience ongoing barriers to cooking healthy meals regularly despite attempted engagement in rehabilitation to increase independent cooking skills (ie prominent negative and cognitive symptoms), may be an option to improve metabolic outcomes for some residents in future, which may be preferable to “take-away” and discretionary food, with the caveat that this option may increase dependency on external services and need to be weighed carefully against the priority of rehabilitation services to increase independent functioning.
Finally, our sample included participants with chronic illness (mean 8.5 years) in which more than half (62%) were taking the most metabolically unfavorable medications (clozapine and olanzapine), known to substantially increase body weight via hunger and metabolic disturbances (67, 68). Greater anthropometric reductions have been found in samples where olanzapine and clozapine prescriptions were much lower (30) and when dietary interventions have been conducted at antipsychotic initiation, highlighting the importance of early intervention (69).
Of note, our participants increased their fitness while maintaining their weight during the 10-week intervention. This may be a more realistic cardiometabolic disease risk reduction goal for people living with longer term exposure to metabolically unfavorable antipsychotics, and particularly in this population with high levels of treatment resistance (11), where switching to antipsychotics with a more favorable cardio-metabolic profile may not be possible due risk of mental state deterioration.
There was a significant reduction in negative symptoms of schizophrenia and general psychiatric symptoms following the lifestyle intervention, consistent with the literature regarding exercise interventions and people with SMI (45, 70). While we observed improvements in social functioning during exercise participation in a subset of our participants, this was a small, uncontrolled study and improvements in symptoms could have occurred due to other aspects of the rehabilitation treatments available at the CCU. We found the physical health component of quality of life improved, confirming the physical health focus of the intervention, however none of the psychological quality of life domains improved. This may relate to the length of the intervention (14, 71), that the lifestyle intervention was not specifically designed to improve psychological constructs (72), or that weight loss did not occur in our participants (a pathway to improved self-efficacy and quality of life) (73).
This brief student-led diet and exercise intervention was found to be feasible, acceptable, and safe for senior students to lead while on practicum placement and could be replicated across residential rehabilitation facilities with minimal resources. Results of this brief study led to the development of an ongoing relationship with local universities providing senior MDiet and EP students on practicum placement at each of the sites throughout the year, resulting in the provision of student-led diet and exercise service to people living in CCU’s. It is beyond the scope of this study to report cost-effectiveness data, however future studies should address this as it is a key factor in determining successful implementation (23). While senior MDiet, EP students and mental health staff were afforded mutual learning opportunities in this novel design, qualified exercise physiology and dieticians could achieve better adherence and physical and mental health outcomes (52, 69).
This was a small, unblinded, uncontrolled pilot study. Psychiatric symptom scales were most prone to observer bias, and these results should be interpreted with caution and future real-world studies in a residential setting should include a control group. Preliminary effectiveness revealed improvements in a number of mental and physical health outcomes, however when multiple analyses were corrected for, only exercise capacity and negative symptoms remained significant; this was a small feasibility study and larger studies are required to confirm our results.
Additionally, while motivational strategies were employed by students, we did not utilize a specific behavior change theory to inform intervention design, limiting discussion regarding implications for clinical practice in light of the reduction in motivation we found and also limiting potential replication of the design in real world settings. Future lifestyle intervention studies in residential settings should utilize and articulate a clear behavior change model (74). While commonly utilized, physical activity self-report measures are prone to recall bias (75), particularly in this population of people with SMI utilizing residential services who can experience significant cognitive impairment. Future studies using device-based measures, (ie accelerometry), could detect differences in sedentary behavior and physical activity more objectively, and particularly as recent research has reported accelerometry may be the most preferable measure of PA by people with SMI (76). Participants reported improvements in their diet following the intervention, however we did not measure energy consumption, diet quality or macro- and micro-nutrient intake, and this should be an important focus in future dietary interventions. Finally, the lower completion rate of the acceptability questionnaires may not have been representative of the entire sample recruited into the study.
Conclusion
Implementation of a brief student-led lifestyle intervention within mental health residential rehabilitation facilities for people with SMI is feasible, safe, scalable, and may improve functional exercise capacity, exercise participation, and negative symptoms. This pragmatic study may be relevant to other CCU’s and other residential rehabilitation units. People living in CCU’s may require more intensive dietetic support to positively impact metabolic risk. Longer duration of lifestyle interventions and investigation of additional interventions to support sustaining healthy lifestyles are warranted.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Metro South Human Research Ethics Committeein March 2016: (HREC 16/QPAH/042). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
NK wrote the protocol in conjunction with DS. CD, NK, SP, CC, and SS collected data. NK, CD, and HF entered data. NK, HF, and DS performed statistical analyses in conjunction with TS. CD supervised the exercise intervention. NK and HF wrote the first draft of the manuscript. All authors NK, HF, CD, TS, JC, SS, DS, FD, CC, and SR revised the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge the hard work and commitment of senior exercise physiology students of the Queensland University of Technology and Masters of Dietetics students from University of Queensland on practicum placement for leading the lifestyle intervention in conjunction with clinical mental health rehabilitation staff from Metro South Addiction and Mental Health services.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00319/full#supplementary-material
References
1. Oakley P, Kisely S, Baxter A, Harris M, Desoe J, Dziouba A, et al. Increased mortality among people with schizophrenia and other non-affective psychotic disorders in the community: A systematic review and meta-analysis. J Psychiatr Res (2018) 102:245–53. doi: 10.1016/j.jpsychires.2018.04.019
2. Suetani S, Rosenbaum S, Scott JG, Curtis J, Ward PB. Bridging the gap: What have we done and what more can we do to reduce the burden of avoidable death in people with psychotic illness? Epidemiol Psychiatr Sci (2016) 25(3):205–10. doi: 10.1017/S2045796015001043
3. Ringen PA, Faerden A, Antonsen B, Falk RS, Mamen A, Rognli EB, et al. Cardiometabolic risk factors, physical activity and psychiatric status in patients in long-term psychiatric inpatient departments. Nord J Psychiatry (2018) 72(4):296–302. doi: 10.1080/08039488.2018.1449012
4. Vancampfort D, Knapen J, Probst M, van Winkel R, Deckx S, Maurissen K, et al. Considering a frame of reference for physical activity research related to the cardiometabolic risk profile in schizophrenia. Psychiatry Res (2010) 177(3):271–9. doi: 10.1016/j.psychres.2010.03.011
5. Firth J, Siddiqi N, Koyanagi A, Siskind D, Rosenbaum S, Galletly C, et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry (2019) 6(8):675–712. doi: 10.1016/S2215-0366(19)30387-6
6. Bruins J, Jörg F, Bruggeman R, Slooff CJ, Corpeleijn E, Pijnenborg M. The effects of lifestyle interventions on (long-term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: A meta-analysis. PloS One (2014) 9(12):1932–6203. doi: 10.1371/journal.pone.0112276
7. Caemmerer J, Correll CU, Maayan L. Acute and maintenance effects of non-pharmacologic interventions for antipsychotic associated weight gain and metabolic abnormalities: A meta-analytic comparison of randomized controlled trials. Schizophr Res (2012) 140(1-3):159–68. doi: 10.1016/j.schres.2012.03.017
8. Singh VK, Karmani S, Malo PK, Virupaksha HG, Muralidhar D, Venkatasubramanian G, et al. Impact of lifestyle modification on some components of metabolic syndrome in persons with severe mental disorders: A meta-analysis. Schizophr Res (2018) 202:17–25. doi: 10.1016/j.schres.2018.06.066
9. Vancampfort D, Firth J, Correll CU, Solmi M, Siskind D, De Hert M, et al. The impact of pharmacological and non-pharmacological interventions to improve physical health outcomes in people with schizophrenia: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry (2019) 18(1):53–66. doi: 10.1002/wps.20614
10. Speyer H, Jakobsen AS, Westergaard C, Nørgaard HCB, Pisinger C, Krogh J, et al. Lifestyle Interventions for Weight Management in People with Serious Mental Illness: A Systematic Review with Meta-Analysis, Trial Sequential Analysis, and Meta-Regression Analysis Exploring the Mediators and Moderators of Treatment Effects. Psychother Psychosomatics (2019), 88(6):350–62. doi: 10.1159/000502293
11. Meehan T, Stedman T, Parker S, Curtis B, Jones D. Comparing clinical and demographic characteristics of people with mental illness in hospital- and community-based residential rehabilitation units in Queensland. Aust Health Rev (2017) 41(2):139–43. doi: 10.1071/AH15207
12. Parker S, Hopkins G, Siskind D, Harris M, McKeon G, Dark F, et al. A systematic review of service models and evidence relating to the clinically operated community-based residential mental health rehabilitation for adults with severe and persisting mental illness in Australia. BMC Psychiatry (2019) 19(1):55. doi: 10.1186/s12888-019-2019-5
13. Forsberg KA, Björkman T, Sandman PO, Sandlund M. Physical health-a cluster randomized controlled lifestyle intervention among persons with a psychiatric disability and their staff. Nordic J Psychiatry (2008) 62(6):486–95. doi: 10.1080/08039480801985179
14. Melamed Y, Stein-Reisner O, Gelkopf M, Levi G, Sivan T, Ilievici G, et al. Multi-Modal Weight Control Intervention for People with Persistent Mental Disorders. Psychiatr Rehabil J (2008) 31(3):194–200. doi: 10.2975/31.3.2008.194.200
15. Deenik J, Tenback DE, Tak ECPM, Rutters F, Hendriksen IJM, van Harten PN. Changes in physical and psychiatric health after a multidisciplinary lifestyle enhancing treatment for inpatients with severe mental illness: The MULTI study I. Schizophr Res (2018) 204:360–7. doi: 10.26226/morressier.5d1a038457558b317a140e33
16. Hjorth P, Davidsen AS, Kilian R, Pilgaard Eriksen S, Jensen SO, Sørensen HØ, et al. Improving the physical health of long-term psychiatric inpatients. Aust New Z J Psychiatry (2014) 48(9):861–70. doi: 10.1177/0004867414533011
17. Looijmans A, Stiekema APM, Bruggeman R, van Der Meer L, Stolk RP, Schoevers RA, et al. Changing the obesogenic environment to improve cardiometabolic health in residential patients with a severe mental illness: cluster randomised controlled trial. Br J Psychiatry: J Ment Sci (2017) 211(5):296–303. doi: 10.1192/bjp.bp.117.199315
18. Korman NH, Shah S, Suetani S, Kendall K, Rosenbaum S, Dark F, et al. Evaluating the Feasibility of a Pilot Exercise Intervention Implemented Within a Residential Rehabilitation Unit for People With Severe Mental Illness: GO HEART: (Group Occupational Health Exercise and Rehabilitation Treatment). Front Psychiatry (2018) 9:343. doi: 10.3389/fpsyt.2018.00343
19. Teasdale SB, Ward P, Samaras K, Firth J, Stubbs B, Tripodi E, et al. Dietary intake of people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry (2019) 214(5):251–9. doi: 10.1192/bjp.2019.20
20. Gaughran F, Stahl D, Ismail K, Greenwood K, Atakan Z, Gardner-Sood P, et al. Randomised control trial of the effectiveness of an integrated psychosocial health promotion intervention aimed at improving health and reducing substance use in established psychosis (IMPaCT).(Report). BMC Psychiatry (2017) 17(1). doi: 10.1186/s12888-017-1571-0
21. Crawford MJ, Barnicot K, Patterson S, Gold C. Negative results in phase III trials of complex interventions: Cause for concern or just good science? Br J Psychiatry (2016) 209(1):6–8. doi: 10.1192/bjp.bp.115.179747
22. Czosnek L, Lederman O, Cormie P, Zopf E, Stubbs B, Rosenbaum S. Health benefits, safety and cost of physical activity interventions for mental health conditions: A meta-review to inform translation efforts. Ment Health Phys Activity (2019) 16:140–51. doi: 10.1016/j.mhpa.2018.11.001
23. Deenik J, Czosnek L, Teasdale SB, Stubbs B, Firth J, Schuch FB, et al. From impact factors to real impact: translating evidence on lifestyle interventions into routine mental health care. Trans Behav Med (2019). doi: 10.1093/tbm/ibz067
24. (ESSA) ESSA. Australia EaSS. Adult Pre Screening Tool 2019 [updated 24.5.19. Available from: https://www.essa.org.au/Public/ABOUT_ESSA/Adult_Pre-Screening_Tool.aspx.
25. Korman NH, Shah S, Suetani S, Kendall K, Rosenbaum S, Dark F, et al. Evaluating the Feasibility of a Pilot Exercise Intervention Implemented Within a Residential Rehabilitation Unit for People With Severe Mental Illness: GO HEART: (Group Occupational Health Exercise and Rehabilitation Treatment). Front Psychiatry (2018) 9. doi: 10.3389/fpsyt.2018.00343
26. Vancampfort D, Rosenbaum S, Probst M, Soundy A, Mitchell AJ, De Hert M, et al. Promotion of cardiorespiratory fitness in schizophrenia: a clinical overview and meta-analysis. Acta Psychiatrica Scand (2015) 132(2):131–43. doi: 10.1111/acps.12407
27. Firth J, Rosenbaum S, Stubbs B, Gorczynski P, Yung AR, Vancampfort D. Motivating factors and barriers towards exercise in severe mental illness: a systematic review and meta-analysis. (2016) Psychol Med 46: (14):2869–81. doi: 10.1017/S0033291716001732
28. Das P, Naylor C, Majeed A. Bringing together physical and mental health within primary care: a new frontier for integrated care. J R Soc Med (2016) 109(10):364–6. doi: 10.1177/0141076816665270
29. Bartels SJ, Pratt SI, Aschbrenner KA, Barre LK, Jue K, Wolfe RS, et al. Clinically Significant Improved Fitness and Weight Loss Among Overweight Persons With Serious Mental Illness. Psychiatr Serv (2013) 64(8):729–36. doi: 10.1176/appi.ps.003622012
30. Daumit GL, Dickerson FB, Wang N-Y, Dalcin A, Jerome GJ, Anderson CAM, et al. A Behavioral Weight-Loss Intervention in Persons with Serious Mental Illness. New Engl J Med (2013) 368(17):1594–602. doi: 10.1056/NEJMoa1214530
31. Green CA, Yarborough BJH, Leo MC, Yarborough MT, Stumbo SP, Janoff SL, et al. The STRIDE Weight Loss and Lifestyle Intervention for Individuals Taking Antipsychotic Medications: A Randomized Trial. Am J Psychiatry (2015) 172(1):71–81. doi: 10.1176/appi.ajp.2014.14020173
32. Jerome GJ, Young DR, Dalcin AT, Wang N-Y, Gennusa J, Goldsholl S, et al. Cardiorespiratory benefits of group exercise among adults with serious mental illness. Psychiatry Res (2017) 256:85–7. doi: 10.1016/j.psychres.2017.06.019
33. Chapman JJ, Coombes JS, Brown WJ, Khan A, Chamoli S, Pachana NA, et al. The feasibility and acceptability of high-intensity interval training for adults with mental illness: A pilot study. Ment Health Phys Activity (2017) 13:40–8. doi: 10.1016/j.mhpa.2017.09.007
34. Vancampfort D, Probst M, Scheewe T, Knapen J, De Herdt A, De Hert M. The functional exercise capacity is correlated with global functioning in patients with schizophrenia. Acta Psychiatrica Scand (2012) 125(5):382–7. doi: 10.1111/j.1600-0447.2011.01825.x
35. Crapo RO, Casaburi R, Coates AL, Enright PL, MacIntyre NR, McKay RT, et al. ATS statement: Guidelines for the six-minute walk test. Am J Respiratory Crit Care Med (2002) 166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102
36. Crippa JAS, Sanches RF, Hallak JEC, Loureiro SR, Zuardi AW. A structured interview guide increases Brief Psychiatric Rating Scale reliability in raters with low clinical experience. Acta Psychiatrica Scand (2001) 103(6):465–70. doi: 10.1034/j.1600-0447.2001.00185.x
37. Lyne J, Renwick L, Grant T, Kinsella A, McCarthy P, Malone K, et al. Scale for the Assessment of Negative Symptoms structure in first episode psychosis. Psychiatry Res (2013) 210(3):1191–7. doi: 10.1016/j.psychres.2013.09.008
38. Rosenbaum S, Ward PB. The Simple Physical Activity Questionnaire. Lancet Psychiatry (2016) 3(1):e1–e. doi: 10.1016/S2215-0366(15)00496-4
39. Coombes J. (2013). ESSA’s student manual for health, exercise and sport assessment. Skinner T, Exercise, Sports Science A, editors. Elsevier Australia.
40. Richardson J, Iezzi A, Khan MA, Maxwell A. Validity and reliability of the assessment of quality of life (AQoL)-8D multi-attribute utility instrument. Patient: Patient-Centered Outcomes Res (2014) 7(1):85. doi: 10.1007/s40271-013-0036-x
41. Markland D, Tobin V. A modification to the behavioural regulation in exercise questionnaire to include an assessment of amotivation. J Sport Exercise Psychol (2004) 26(2):191–6. doi: 10.1123/jsep.26.2.191
42. Wilson PM, Rodgers WM, Loitz CC, Scime G. “It’s Who I Am … Really!’The Importance of Integrated Regulation in Exercise Contexts. J Appl Biobehav Res (2006) 11(2):79–104. doi: 10.1111/j.1751-9861.2006.tb00021.x
43. Daumit GL, Dalcin AT, Jerome GJ, Young DR, Charleston J, Crum RM, et al. A behavioral weight- loss intervention for persons with serious mental illness in psychiatric rehabilitation centers. Int J Obesity (2010) 35(8):1114. doi: 10.1038/ijo.2010.224
44. Bartels SJ. Can Behavioral Health Organizations Change Health Behaviors? The STRIDE Study and Lifestyle Interventions for Obesity in Serious Mental Illness. Am J Psychiatry (2015) 172(1):9–11. doi: 10.1176/appi.ajp.2014.14101246
45. Firth J, Cotter J, Elliott R, French P, Yung AR. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med (2015) 45(7):1343–61. doi: 10.1017/S0033291714003110
46. Vancampfort D, De Hert M, Vansteenkiste M, De Herdt A, Scheewe TW, Soundy A, et al. The importance of self-determined motivation towards physical activity in patients with schizophrenia. Psychiatry Res (2013) 210(3):812–8. doi: 10.1016/j.psychres.2013.10.004
47. Barton J, Pretty J. What is the best dose of nature and green exercise for improving mental health? A multi-study analysis. Environ Sci Technol (2010) 44(10):3947–55. doi: 10.1021/es903183r
48. Gordon B, McDowell C, Hallgren M, Meyer J, Lyons M, Herring M. Association of Efficacy of Resistance Exercise Training With Depressive SymptomsMeta-analysis and Meta-regression Analysis of Randomized Clinical Trials. JAMA Psychiatry (2018) 75(6):566. doi: 10.1001/jamapsychiatry.2018.0572
49. Andrade E Silva B, Cassilhas RC, Attux C, Cordeiro Q, Gadelha AL, Telles BA, et al. A 20-week program of resistance or concurrent exercise improves symptoms of schizophrenia: results of a blind, randomized controlled trial. Rev Bras Psiquiatria (2015) 37(4):271–9. doi: 10.1590/1516-4446-2014-1595
50. Strassnig M, Signorile J, Gonzalez C, Harvey PD. Physical performance and disability in schizophrenia. Schizophr Res: Cogn (2014) 1(2):112–21. doi: 10.1016/j.scog.2014.06.002
51. Stubbs B, Vancampfort D, Hallgren M, Firth J, Veronese N, Solmi M, et al. EPA guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur Psychiatry (2018) 54:124–44. doi: 10.1016/j.eurpsy.2018.07.004
52. Vancampfort D, Rosenbaum S, Schuch F, Ward P, Probst M, Stubbs B. Prevalence and predictors of treatment dropout from physical activity interventions in schizophrenia: a meta-analysis. Gen Hosp Psychiatry (2016) 39:15–23. doi: 10.1016/j.genhosppsych.2015.11.008
53. Naslund JA, Aschbrenner KA. Digital technology for health promotion: opportunities to address excess mortality in persons living with severe mental disorders. Evid Based Ment Health (2019) 22(1):17. doi: 10.1136/ebmental-2018-300034
54. Naslund JA, Aschbrenner KA, Scherer EA, McHugo GJ, Marsch LA, Bartels SJ. Wearable devices and mobile technologies for supporting behavioral weight loss among people with serious mental illness. Psychiatry Res (2016) 244:139–44. doi: 10.1016/j.psychres.2016.06.056
55. Chapman JJ, Suetani S, Siskind D, Kisely S, Breakspear M, Byrne JH, et al. Protocol for a randomised controlled trial of interventions to promote adoption and maintenance of physical activity in adults with mental illness. BMJ Open (2018) 8(9):e023460. doi: 10.1136/bmjopen-2018-023460
56. Wheeler A, Roennfeldt H, Slattery M, Krinks R, Stewart V. Codesigned recommendations for increasing engagement in structured physical activity for people with serious mental health problems in Australia. Health Soc Care Community (2018) 26(6):860–70. doi: 10.1111/hsc.12597
57. Matthews E, Cowman M, Denieffe S. Using experience-based co-design for the development of physical activity provision in rehabilitation and recovery mental health care. J Psychiatr Ment Health Nursing (2017) 24(7):545–52. doi: 10.1111/jpm.12401
58. Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry (2011) 10(1):52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x
59. Stubbs B, Mitchell AJ, De Hert M, Correll CU, Soundy A, Stroobants M, et al. The prevalence and moderators of clinical pain in people with schizophrenia: A systematic review and large scale meta-analysis. Schizophr Res (2014) 160(1-3):1–8. doi: 10.1016/j.schres.2014.10.017
60. Vancampfort D, Rosenbaum S, Schuch F, Ward P, Richards J, Mugisha J, et al. Cardiorespiratory Fitness in Severe Mental Illness: A Systematic Review and Meta-analysis. Sports Med (2017) 47(2):343–52. doi: 10.1007/s40279-016-0574-1
61. Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE. The six-minute walk test: a useful metric for the cardiopulmonary patient. Melbourne, Australia: Blackwell Publishing Asia (2009) p. 495–501.
62. Cannistra LMC, Brian MD, Myers J. Interpreting Small Differences in Functional Status: The Six Minute Walk Test in Chronic Lung Disease Patients. J Cardiopulmonary Rehab (1998) 18(2):153. doi: 10.1097/00008483-199803000-00009
63. Vancampfort D, Probst M, Sweers K, Maurissen K, Knapen J, De Hert M. Relationships between obesity, functional exercise capacity, physical activity participation and physical self-perception in people with schizophrenia. Acta Psychiatrica Scand (2011) 123(6):423–30. doi: 10.1111/j.1600-0447.2010.01666.x
64. Jörg F, Looijmans A, Stiekema A, Van Der Meer L, Schoevers R, Corpeleijn E. Changing the obesogenic environment to improve cardiometabolic health in residential patients with a severe mental Illness: ELIPS, a randomized controlled trial. Eur Psychiatry (2017) 41(sS):S267–S8. doi: 10.1016/j.eurpsy.2017.02.088
65. Stephen P, Dan S, Daniel FH, Frances D, Gemma M, Nicole K, et al. A Comprehensive Cohort Description and Statistical Grouping of Community-Based Residential Rehabilitation Service Users in Australia. Front Psychiatry (2019) 10:798. doi: 10.3389/fpsyt.2019.00798
66. Tsai AG, Wadden TA. Systematic review: An evaluation of major commercial weight loss programs in the United States.(Author Abstract). Ann Internal Med (2005) 142(1):56. doi: 10.7326/0003-4819-142-1-200501040-00012
67. Daumit GL, Dalcin AT, Jerome GJ, Young DR, Charleston J, Crum RM, et al. A behavioral weight-loss intervention for persons with serious mental illness in psychiatric rehabilitation centers. Int J Obes (Lond) (2011) 35(8):1114–23. doi: 10.1038/ijo.2010.224
68. Meehan T, Jones D, Stedman T. Metabolic risk in patients participating in residential rehabilitation programs: how are we doing? Australas Psychiatry (2019) 27(2):179–82. doi: 10.1177/1039856218815762
69. Teasdale SB, Ward PB, Rosenbaum S, Samaras K, Stubbs B. Solving a weighty problem: systematic review and meta-analysis of nutrition interventions in severe mental illness. Br J Psychiatry: J Ment Sci (2017) 210(2):110–8. doi: 10.1192/bjp.bp.115.177139
70. Biddle GFS. Exercise as an adjunct treatment for schizophrenia: A review of the literature. J Ment Health (1999) 8(5):441–57. doi: 10.1080/09638239917157
71. Battaglia G, Alesi M, Inguglia M, Roccella M, Caramazza G, Bellafiore M, et al. Soccer practice as an add-on treatment in the management of individuals with a diagnosis of schizophrenia.(Original Research)(Report). Neuropsychiatr Dis Treat (2013) 9:595. doi: 10.2147/NDT.S44066
72. Stiekema APM, Looijmans A, van Der Meer L, Bruggeman R, Schoevers RA, Corpeleijn E, et al. Effects of a lifestyle intervention on psychosocial well-being of severe mentally ill residential patients: ELIPS, a cluster randomized controlled pragmatic trial. Schizophr Res (2018) 199:407–13. doi: 10.1016/j.schres.2018.02.053
73. Vazin R, McGinty EE, Dickerson F, Dalcin A, Goldsholl S, Oefinger Enriquez M, et al. Perceptions of Strategies for Successful Weight Loss in Persons With Serious Mental Illness Participating in a Behavioral Weight Loss Intervention: A Qualitative Study. Psychiatr Rehabil J (2016) 39(2):137–46. doi: 10.1037/prj0000182
74. Romain AJ, Caudroit J, Hokayem M, Bernard P. Is There Something Beyond Stages of Change in the Transtheoretical Model? The State of Art for Physical Activity. Can J Behav Sci Can Des Sci Du Comportement (2018) 50(1):42–53. doi: 10.1037/cbs0000093
75. Firth J, Stubbs B, Vancampfort D, Schuch FB, Rosenbaum S, Ward PB, et al. The Validity and Value of Self-reported Physical Activity and Accelerometry in People With Schizophrenia: A Population-Scale Study of the UK Biobank. Schizophr Bull (2018) 44(6):1293–300. doi: 10.1093/schbul/sbx149
76. Suetani S, Chapman J, Korman N, Chapman C, Dodd C, Dark F, et al. A comparison study of three physical activity measurement tools examining acceptability in people with psychosis. Australas Psychiatry: Bull R Aust New Z Coll Psychiatrists (2019), 1039856219881957:1–5. doi: 10.1177/1039856219881957
Keywords: severe mental illness, schizophrenia, exercise, diet, rehabilitation, lifestyle intervention, physical activity, student placement
Citation: Korman N, Fox H, Skinner T, Dodd C, Suetani S, Chapman J, Parker S, Dark F, Collins C, Rosenbaum S and Siskind D (2020) Feasibility and Acceptability of a Student-Led Lifestyle (Diet and Exercise) Intervention Within a Residential Rehabilitation Setting for People With Severe Mental Illness, GO HEART (Group Occupation, Health, Exercise And Rehabilitation Treatment). Front. Psychiatry 11:319. doi: 10.3389/fpsyt.2020.00319
Received: 29 January 2020; Accepted: 31 March 2020;
Published: 28 April 2020.
Edited by:
Helen Killaspy, University College London, United KingdomReviewed by:
Ellie Mary Forrey, Monash University, AustraliaLisette Van Der Meer, University of Groningen, Netherlands
Copyright © 2020 Korman, Fox, Skinner, Dodd, Suetani, Chapman, Parker, Dark, Collins, Rosenbaum and Siskind. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicole Korman, bi5rb3JtYW5AdXEuZWR1LmF1
 Nicole Korman
Nicole Korman Harley Fox
Harley Fox Tina Skinner
Tina Skinner Cassandra Dodd1
Cassandra Dodd1 Stephen Parker
Stephen Parker Frances Dark
Frances Dark Simon Rosenbaum
Simon Rosenbaum Dan Siskind
Dan Siskind