Abstract
Multiple sclerosis (MS) is an immune-mediated demyelinating disease of the central nervous system. Studies have shown that MS disrupts several social cognitive abilities [including empathy and theory of mind (ToM)]. Overall ToM deficits in MS are well documented, but how the specific ToM subcomponents and empathic capacity are affected remains unclear. For this meta-analysis, we searched PubMed, Web of Science, and Embase from inception to July 2020. Effect sizes were calculated using Hedges g with a random-effects model. Thirty-three studies were included. Relative to healthy controls (HCs), patients with MS were moderately impaired in overall empathy (g = −0.67), overall ToM (g = −74), cognitive ToM (g = −0.72), and the overlapping domains of cognitive empathy/affective ToM (g = −0.79); no group differences were identified for affective empathy (g = −0.19). Compared with HCs, patients with relapsing-remitting MS (RRMS) and progressive MS were impaired in overall empathy, overall ToM, cognitive ToM, and cognitive empathy/affective ToM, without significant RRMS–progressive MS differences in impairment degree. We conducted the first meta-analytic review investigating the empathy and ToM functioning patterns in patients with MS and examined the overlapping and distinct subcomponents of these constructs. The findings suggest differential impairment of the core aspects of social cognitive processing in patients with MS, which may importantly inform the development of structured social cognitive MS interventions.
Introduction
Multiple sclerosis (MS) is an immune-mediated demyelinating disease of the central nervous system (1), which is characterized by multifocal destruction of the myelin sheath and axonal loss (2, 3). Patients usually develop sensorimotor, visual, and emotional symptoms as well as cognitive impairment, leading to functional disability and reduced quality of life (QoL) (4). The precise etiology of MS remains unclear, and the prognosis is variable and unpredictable.
Cognitive impairment has been recognized as a common symptom in MS, with an estimated lifetime occurrence of 40–65% (5–7). The cognitive domains generally affected include executive functioning, information processing speed, attention, and memory (8, 9), and social cognition (10–14). Social cognition, a basic means for the individual to perceive, encode, store, retrieve, and regulate information regarding other people and the self (15), has a remarkable impact on interpersonal communication and QoL (16–19). Social cognition is a multidimensional construct, mainly involving four dimensions: empathy, theory of mind (ToM), social perception and social knowledge, and attribution bias (15, 17).
One core aspect of social cognition, i.e., empathy, refers to the ability to understand and identify the mental states of others, as well as the ability to share the feelings of others (20). It is a complex construct with multiple components, usually including affective and cognitive domains. Emotional empathy is described as “I feel your feelings” and can be regarded as primitive empathy, while cognitive empathy refers to “I understand your feelings” and can be regarded as advanced empathy (21–23). This is significant in clinical practice, as any deficit in cognitive or affective empathy can lead to atypical emotional reactions, but the clinical treatment implications differ (20, 24). Recently, several studies have assessed empathy deficits in patients with MS with inconsistent findings. For example, Realmuto et al. (25) and van der Hiele et al. (26) found no differences between patients with MS and healthy controls (HCs) in terms of empathy, whereas Kraemer et al. (27) found moderate impairment in empathy in patients with MS compared to HCs. These inconsistent findings may be related to low statistical power, as many of these studies enrolled small sample sizes. To answer important clinical questions, a quantitative meta-analysis is needed to test the magnitude and significance of empathy in MS to increase the statistical power and refine the conclusions derived from the inconsistent findings of the previous studies.
ToM, another core domain of social cognition, refers to the ability to attribute mental states (beliefs, intentions, and desires) to others and to use the attributions to understand and predict behavior (28, 29). Like empathy, ToM can also be divided into affective and cognitive components (30). Affective ToM refers to the capacity to understand others' emotional states, and cognitive ToM is the ability to infer other's thoughts, intentions, and beliefs (31). To our knowledge, two recent meta-analyses examined ToM differences between patients with MS and HCs. Cotter et al. and Bora et al. calculated the overall ToM score based on numerous different ToM tasks (a combination of affective ToM and cognitive ToM tasks) and found that patients with MS have ToM deficits (32, 33). However, it remains unclear whether these defects were attributable to only one or both subcomponents, as no specific subgroup analysis was conducted for affective and cognitive ToM.
Notably, although there are differences between cognitive empathy and affective ToM in definition (34), these two constructs are difficult to distinguish at a purely behavioral level of assessment because they both involve attribution of another's emotional states (35). Additionally, overlap between cognitive empathy and affective ToM has often been noted (24, 36, 37). Therefore, in this study, we considered cognitive empathy and affective ToM to be interchangeable.
To this end, the present study aimed to provide the first meta-analytic integration of broader empathy and ToM in MS with the affective and cognitive subcomponents of both these abilities distinguished. Moreover, specific subgroup analyses for the overlapping components (cognitive empathy and affective ToM) and separate components (cognitive ToM and affective empathy) were also conducted. Besides, considering that MS is a heterogeneous disease, with subtypes and diverse trajectories, in influx between relapse, remission, stability, and progression (38, 39), we performed subgroup analyses (including of relapsing-remitting MS [RRMS] and progressive MS (including progressive primary MS and secondary progressive MS).
In addition, studies have reported that certain clinical behavioral symptoms may have a significant relationship with social cognition (40–44). Clinically, depression and anxiety are common behavioral symptoms in patients with MS (45–50), and it has been reported that social cognitive deficits are significantly related to the severity of depressive symptoms or anxiety symptoms in some diseases (40–42, 44). So far, the exact relationship between social cognitive performance and the severity of depression or anxiety in patients with MS remains unclear. Therefore, we evaluated the effect of potential variables [such as sex (ratio of female patients in the MS group), mean age, education level, disease duration, Expanded Disability Status Scale (EDSS) score, quality assessment score, severity of depression, and severity of anxiety] on social cognition. With this meta-analysis, we hope to promote a more comprehensive and nuanced understanding of how these two core domains of social cognition are affected in MS.
Methods
Study Registration
This study was performed per the Preferred Reporting Items of Systematic Review and Meta-Analysis (PRISMA) guidelines (51). This protocol was prospectively registered at the International Platform of Registered Systematic Review and Meta-analysis Protocols (ID: INPLASY202070029) and has been released in the journal of Medicine (52).
Data Sources and Study Selection
A systematic literature search was conducted across the PubMed, Web of Science, and Embase databases from inception to July 2020. The following search terms were used: “multiple sclerosis” or “MS” or “clinically isolated syndrome” combined with: “social cognition” or “theory of mind” or “ToM” or “mentalizing” or “mentalizing” or “Reading the Mind in the Eyes Test” or “Faux pas task” or “False Belief” or “the Awareness of Social Inference Test” or “Virtual Assessment of Mentalising Ability” or “the Movie for the Assessment of Social Cognition” or “picture sequencing task” or “Cartoon Test” or “Hinting Test” or “Strange Stories Test” or “facial expression*” or “prosody” or “pragmatic impairment” or “non-literal language” or “sarcas*” or “lie*” or “joke*” or “empath*” or “perspective taking” or “Peer-Report Social Functioning Scale.” Furthermore, other resources, such as the reference lists of all included studies, were searched manually.
Inclusion Criteria
Studies were included if they met four criteria. First, the study should have compared patients with MS to a matched HC group. Second, the study should have assessed empathy performance or ToM performance using standard measures. Third, the study should have provided sufficient data to calculate the effect sizes of empathy or ToM. Fourth, the study should have been published in a peer-reviewed journal in English.
Exclusion Criteria
Studies were excluded for three reasons. First, if the participant overlapped with a participant in another study with a larger sample size. Second, if they lacked an HC group. Third, if they included <10 participants to ensure the reliability of the outcome (29).
Screening and Data Extraction
Article retrieval, screening, data extraction, and quality evaluation were independently completed by two investigators. The relevant data extracted included: (a) Title information, such as first author, publication year, and title; (b) Sample characteristics from the MS and HC groups, such as sample size, sex (female and male), mean age, education level, disease duration, EDSS scores, severity of depression, and severity of anxiety; (c) For both empathy and ToM, tasks were divided into affective and cognitive subcomponents, and the classification was based on the nature of the task and the information provided by the author of the original article; (d) The data used for calculating the effect sizes of empathy or ToM. Any disagreements were first discussed between these two investigators, and further disagreements were arbitrated by a third investigator.
Study Quality Assessment
To assess study quality, a nine-star protocol was used based on the Newcastle-Ottawa Scale for case-control studies. Studies with ≥7 stars were considered high-quality (53).
Statistical Analysis
Meta-analyses were conducted using the Stata 15.0 software package (54). The effect size (Hedges g) and 95% confidence interval (CI) were calculated to estimate differences in ToM and empathy between the MS and HC groups (55). The magnitude of Hedges g could be interpreted using Cohen d effect size conventions, and effect sizes were deemed small, moderate, or large when their values were equal to or larger than 0.2, 0.5, or 0.8, respectively (56).
When studies did not provide a total mean score on a particular measure but reported subscores (i.e., individual ToM tasks presented separately), pooled effect sizes were aggregated by computing the mean effect size (and standard error) (57). Similarly, when studies reported the effect size per subgroup [i.e., by clinical subtypes (relapsing MS and progressive MS)], data were pooled into an overall effect size (57). Meta-analyses were completed using a random-effects model, as it better accommodates heterogeneous effect distributions.
The degree of heterogeneity within effect size estimates was tested with the I2 statistic, and the degree of heterogeneity was deemed low, moderate, or large when I2 was equal to or larger than 0, 50, or 75%, respectively (58).
To assess the risk of publication bias, Egger's test was used. For this analysis, significance indicates that bias may be present [p < 0.05; (59)]. Additionally, the trim-and-fill analyses were applied, providing effect sizes adjusted for publication bias (60).
Meta-regression analyses were conducted to investigate whether demographic and clinical variables (including age, sex, education level, disease duration, and EDSS score, quality assessment score, severity of depression, and severity of anxiety) explained the variance in any of the effects identified. As a measure of severity of depression or anxiety, according to accepted cut-off scores of depression or anxiety rating scales used, studies were classified as no symptoms = 0, mild symptoms = 1, moderate symptoms = 2, severe symptoms = 3 (41, 61–63). For each of these analyses, a minimum of 10 data points was required for each relevant predictor variable and the social cognitive ability under assessment (64).
Results
Study Characteristics
The flow chart of the study selection process is shown in Figure 1. In total, 34,365 potentially eligible articles were retrieved. After the removal of duplicates, 29,601 articles remained, which were then subjected to title and abstract screening. Of these, 43 initially met the inclusion criteria. Three of these studies did not include an HC group (65–67); another three were excluded for lack of sufficient data to calculate the effect sizes and standard errors of empathy or ToM (18, 68, 69). Four studies were excluded, as their samples overlapped with those of other studies (14, 70–72). Eventually, 33 studies with 1,568 patients with MS (mean age = 40.71 years, SD = 9.63 years, 70.4% female) and 1,283 HCs (mean age = 39.18 years, SD = 9.91 years, 65.3% female) were included in the meta-analysis [Table 1; (4, 11–13, 25–27, 46, 48, 50, 63, 73–94)].
Figure 1

PRISMA flowchart displaying study screening and selection process.
Table 1
| Study | HCs groups | MS groups | Task | Type | ||||
|---|---|---|---|---|---|---|---|---|
| Sample (female) | Age (years, SD) | Sample (female) | Age (years, SD) | Duration (years, SD) | EDSS (SD) | |||
| Banati et al. (63) | 35 (18) | 33.40 (7.80) | 40 (29) | 36.20 (9.40) | NA | NA | Faux pas task | CogToM |
| Adult Faces task | CogEmp/AffTom | |||||||
| RMET | CogEmp/AffTom | |||||||
| Baron-Cohen's EQ | Mixed Emp | |||||||
| Batista et al. (73) | 60 (40) | 36.10 (9.40) | 60 (40) | 37.20 (7.50) | 10.6 (6.6) | 2 (0.75) | RMET | CogEmp/AffTom |
| ToM videos test | CogToM | |||||||
| Bisecco et al. (74) | 25 (18) | 37.83 (11.95) | 41 (27) | 34.18 (10.27) | 8.8 (8.2) | 2.375 (1.625) | RMET | CogEmp/AffTom |
| ToM picture sequencing task | CogToM | |||||||
| Chanial et al. (75) | 21 (16) | 33.90 (7.00) | 21 (15) | 38.80 (5.50) | 10 (7) | 4.2 (2) | Faux pas task | CogToM |
| Charvet et al. (76) | 32 (23) | 15.69 (2.94) | 28 (19) | 16.29 (3.12) | 2.82 (2.51) | 1.5 (1) | RMET | CogEmp/AffTom |
| Faux pas task | CogToM | |||||||
| FB Task | CogToM | |||||||
| Empathy Systemizing Quotient | Mixed Emp | |||||||
| Czekóová et al. (77) | 43 (25) | 34.70 (11.00) | 43 (31) | 35.80 (8.00) | 7.5 (4.4) | 2.5 (1.5) | RMET | CogEmp/AffTom |
| Dulau et al. (78) | 60 (35) | 43.20 (9.30) | 60 (43) | 46.50 (10.60) | 14.4 (9.4) | 3.5 (1.5) | Faux pas task | CogToM |
| RMET | CogEmp/AffTom | |||||||
| Attribution of intentions | CogToM | |||||||
| García et al. (79) | 106 | NA | 35 | NA | NA | NA | RMET | CogEmp/AffTom |
| Genova et al. (80) | 15 (5) | 38.90 (13.10) | 15 (11) | 49.50 (8.00) | 17.98 (10.3) | NA | TASIT-SIE-Feel | CogEmp/AffTom |
| TASIT-SIE-Do | CogToM | |||||||
| TASIT-SIE-Say | CogToM | |||||||
| TASIT-SIE-Think | CogToM | |||||||
| Genova and McDonald (13) | 15 (11) | 45.60 (11.70) | 17 (9) | 51.90 (9.20) | 13.8 (9.5) | NA | TASIT-SIM | Mixed ToM |
| TASIT-SIE | Mixed ToM | |||||||
| Gleichgerrcht et al. (81) | 38 (33) | 39.30 (8.10) | 38 (33) | 42.30 (11.30) | 1.6 (8.7) | 1.66 (1.6) | IRI Empathic Concern | AffEmp |
| IRI Personal distress | AffEmp | |||||||
| IRI Perspective Taking | CogEmp/AffTom | |||||||
| IRI Fantasy | CogEmp/AffTom | |||||||
| Goitia et al. (82) | 42 (29) | 37.10 (10.70) | 36 (30) | 39.20 (10.20) | 9.3 (7.3) | NA | Faux pas task | CogToM |
| Golde et al. (83) | 30 (19) | 39.57 (8.36) | 30 (18) | 40.20 (9.87) | 8.23 (5.04) | 1.875 (1) | MASC | Mixed ToM |
| MET-Cognitive empathy | CogEmp/AffTom | |||||||
| MET-Emotional empathy | AffEmp | |||||||
| Henry et al. (84) | 30 (19) | 44.30 (9.55) | 27 (18) | 47.00 (11.01) | 7 (6.08) | 1.9 (1.98) | RMET | CogEmp/AffTom |
| Henry et al. (4) | 30 (21) | 38.60 (13.90) | 64 (50) | 42.40 (9.80) | 9.1 (5.37) | 2.3 (1.7) | First-order FB task | CogToM |
| Second-order FB task | CogToM | |||||||
| Faux pas task | CogToM | |||||||
| Henry et al. (48) | 33 (24) | 43.70 (10.50) | 62 (36) | 46.80 (10.90) | 11.4 (9.4) | 3.8 (1.8) | First-order FB task | CogToM |
| Second-order FB task | CogToM | |||||||
| Faux pas task | CogToM | |||||||
| Ignatova et al. (50), EDSS < 3.5 | 36 (24) | 42.40 (12.30) | 18 (13) | 41.90 (11.60) | 7.06 (4.3) | 1.86 (0.8) | RMET | CogEmp/AffTom |
| Faux pas task | CogToM CogToM | |||||||
| ToM cartoons | CogToM | |||||||
| Self-Compassion Scale | Mixed Emp | |||||||
| Ignatova et al. (50), EDSS ≥ 3.5 | 36 (24) | 42.40 (12.30) | 18 (11) | 43.70 (8.50) | 11.17 (7.45) | 4.56 (0.95) | RMET | CogEmp/AffTom |
| Faux pas task | CogToM CogToM | |||||||
| ToM cartoons | CogToM | |||||||
| Self-Compassion Scale | Mixed Emp | |||||||
| Isernia et al. (85) | 26 (19) | 51.35 (12.42) | 42 (24) | 52.38 (10.31) | 21.24 (10.94) | 5.25 (1.75) | RMET | CogEmp/AffTom |
| Faux pas task-Intention | CogToM | |||||||
| Faux pas task-Emotion | CogEmp/AffTom | |||||||
| Strange Stories-Double bluff | CogToM | |||||||
| Strange Stories-White lie | CogToM | |||||||
| Strange Stories-Misunderstanding | CogToM | |||||||
| Strange Stories-Emotions | CogEmp/AffTom | |||||||
| MASC-Thoughts | CogToM | |||||||
| MASC-Intention | CogToM | |||||||
| MASC-Affective | CogEmp/AffTom | |||||||
| Kraemer et al. (27) | 25 (11) | 33.44 | 25 (15) | 30.92 | 1.24 (0.25) | 0.94 (0.63) | MASC | Mixed ToM |
| Baron-Cohen's EQ | Mixed Emp | |||||||
| Labbe et al. (86) | 45 (22) | 37.58 (12.00) | 47 (26) | 36.28 (10.21) | 4.28 (3.65) | 1.75 (1.25) | Faux pas task | CogToM |
| Lancaster et al. (87) | 15 | 45.60 (11.67) | 15 | 48.93 (8.60) | 14.43 (9.09) | NA | VAMA cognitive | CogToM |
| VAMA affective | CogEmp/AffTom | |||||||
| Mike et al. (88) | 24 (13) | 36.81 (7.27) | 49 (31) | 39.82 (9.31) | 9.49 (6.19) | 2.43 (1.71) | Faux pas task | CogToM |
| Adult Faces task | CogEmp/AffTom | |||||||
| RMET | CogEmp/AffTom | |||||||
| Neuhaus et al. (11) | 34 (22) | 43.90 (12.50) | 35 (22) | 43.80 (12.13) | 12.9 (9.6) | 3.125 (1.63) | Faux pas task | CogToM |
| ToM cartoons | CogToM | |||||||
| ToM-Inference test | CogToM | |||||||
| RMET | CogEmp/AffTom | |||||||
| Ouellet et al. (46), MS– | 20 (10) | 48.50 (8.20) | 26 (15) | 45.20 (7.3) | 10.2 (8.1) | 3.8 (2.7) | Faux pas task | CogToM |
| Strange Stories-Mental task | CogToM | |||||||
| ToM-Conversations and Insinuations | Mixed ToM | |||||||
| Ouellet et al. (46), MS+ | 20 (10) | 48.50 (8.20) | 15 (12) | 43.6 (8.3) | 6.2 (4.6) | 2.8 (2.2) | Faux pas task | CogToM |
| Strange Stories | CogToM | |||||||
| ToM-Conversations and Insinuations | Mixed ToM | |||||||
| Parada-Fernández et al. (89) | 40 (20) | 50.78 (10.08) | 45 (29) | 49.44 (9.44) | NA | NA | RMET | CogEmp/AffTom |
| Patil et al. (90) | 38 (31) | 39.30 (8.10) | 38 (33) | 42.3 (11.3) | 10.6 (8.7) | 1.66 (1.6) | IRI Empathic Concern | AffEmp |
| IRI Personal distress | AffEmp | |||||||
| IRI Perspective Taking | CogEmp/AffTom | |||||||
| IRI Fantasy | CogEmp/AffTom | |||||||
| Pitteri et al. (12) | 38 (28) | 37.10 (8.90) | 31 (24) | 36.3 (7.6) | 7 (4.5) | 1 (0.875) | RMET | CogEmp/AffTom |
| Baron-Cohen's EQ | Mixed Emp | |||||||
| Pöttgen et al. (91) | 45 (31) | 42.50 (10.45) | 45 (31) | 42.42 (10.66) | 8.46 (6.18) | 3.47 (1.63) | MASC-Thoughts | CogToM |
| MASC-Intention | CogToM | |||||||
| MASC-Affective | CogEmp/AffTom | |||||||
| Raimo et al. (92) | 40 (31) | 40.20 (11.40) | 40 (29) | 40.58 (11.51) | 8.23 (7.48) | 2.44 (1.48) | Advanced Test of ToM | CogToM |
| ToM picture sequencing task | CogToM | |||||||
| RMET | CogEmp/AffTom | |||||||
| Emotion attribution task | CogEmp/AffTom | |||||||
| Realmuto et al. (25) | 45 (32) | 33.04 (7.73) | 45 (31) | 34.22 (7.35) | 9.72 (6.22) | 2.06 (1.46) | RMET | CogEmp/AffTom |
| SET-identifying intentions | CogToM | |||||||
| SET-emotional states | CogEmp/AffTom | |||||||
| Roca et al. (93) | 16 | 40.88 (9.95) | NA | 40.67 (9.53) | 5.05 (3.75) | 0.58 (0.99) | Faux pas task-Intention | CogToM |
| Faux pas task-Emotion | CogEmp/AffTom | |||||||
| van et al. (26) | 128 (94) | NA | 278 (216) | NA | Baron-Cohen's EQ | Mixed Emp | ||
| Vanotti et al. (94) | 53 | 36.40 (10.90) | 121 | 42.3 (4.1) | 3.53 (0.34) | 2.1 (1.5) | RMET | CogEmp/AffTom |
Individual study characteristics.
SD, standard deviation; NA, not available; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; HCs, healthy controls; ToM, theory of mind; EMP, empathy; CogToM, Cognitive ToM; CogEmp/AffTom, Cognitive empathy/Affective ToM; AffEmp, affective empathy; FB, False Belief; RMET, Reading the Mind in the Eyes Test; IRI, Interpersonal Reactivity Index; TASIT, the Awareness of Social Inference Test; SIM, Social Inference-Minimal Test; SIE, Social Inference-Enriched; EQ, Empathy Quotient; MASC, the Movie for the Assessment of Social Cognition; MET, Multifaceted Empathy Test; VAMA, Virtual Assessment of Mentalizing Ability; SET, Story-based Empathy task.
Study Quality Assessment
The results of the study quality assessment are shown in Table 2. The mean score was 7.11 (SD = 0.83), and 29 of the 35 case-control studies were awarded ≥7 stars and considered of high quality.
Table 2
| Study | S1 | S2 | S3 | S4 | C | E1 | E2 | E3 | Sum |
|---|---|---|---|---|---|---|---|---|---|
| Banati et al. (63) | ⋆ | — | — | ⋆ | ⋆ — | ⋆ | ⋆ | ⋆ | 6 |
| Batista et al. (73) | ⋆ | — | ⋆ | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Bisecco et al. (74) | ⋆ | ⋆ | — | ⋆ | ⋆ — | ⋆ | ⋆ | ⋆ | 7 |
| Chanial et al. (75) | ⋆ | ⋆ | — | ⋆ | — — | ⋆ | ⋆ | ⋆ | 6 |
| Charvet et al. (76) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ — | ⋆ | ⋆ | ⋆ | 8 |
| Czekóová et al. (77) | ⋆ | ⋆ | — | ⋆ | ⋆ — | ⋆ | ⋆ | ⋆ | 7 |
| Dulau et al. (78) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| García et al. (79) | ⋆ | — | — | ⋆ | — — | ⋆ | ⋆ | ⋆ | 5 |
| Genova et al. (80) | ⋆ | — | — | ⋆ | — ⋆ | ⋆ | ⋆ | ⋆ | 6 |
| Genova and McDonald (13) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Gleichgerrcht et al. (81) | ⋆ | ⋆ | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Goitia et al. (82) | ⋆ | — | ⋆ | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Golde et al. (83) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Henry et al. (84) | ⋆ | — | ⋆ | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Henry et al. (4) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Henry et al. (48) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Ignatova et al. (50), EDSS < 3.5 | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Ignatova et al. (50), EDSS ≥ 3.5 | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Isernia et al. (85) | ⋆ | ⋆ | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Kraemer et al. (27) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Labbe et al. (86) | ⋆ | — | — | ⋆ | ⋆ — | ⋆ | ⋆ | ⋆ | 6 |
| Lancaster et al. (87) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Mike et al. (88) | ⋆ | — | — | ⋆ | ⋆ — | ⋆ | ⋆ | ⋆ | 6 |
| Neuhaus et al. (11) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Ouellet et al. (46), MS– | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Ouellet et al. (46), MS+ | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Parada-Fernández et al. (89) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Patil et al. (90) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Pitteri et al. (12) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Pottgen et al. (91) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Raimo et al. (92) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 9 |
| Realmuto et al. (25) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Roca et al. (93) | ⋆ | — | ⋆ | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| van et al. (26) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 9 |
| Vanotti et al. (94) | ⋆ | — | — | ⋆ | ⋆ ⋆ | ⋆ | ⋆ | ⋆ | 7 |
Quality evaluation of included studies.
We herein selected “age” as the most important adjusting factor and selected “education level” as other controlled factor. S1, Is the case definition adequate?; S2, Representativeness of the cases; S3, Selection of Controls; S4, Definition of Controls; C, Comparability of cases and controls on the basis of the design or analysis; E1, Ascertainment of exposure; E2, Same method of ascertainment for cases and controls; E3, Non-Response rate. The ⋆ means one point. The — means no score.
Empathy and ToM in Patients With MS vs. HCs
Table 3 reports the key results from this meta-analysis. Compared to HCs, patients with MS were impaired in overall empathy, with this deficit being moderate in magnitude (g = −0.67, 95% CI [−0.84, −0.50], K = 27; see Figure 2). Patients with MS were also moderately impaired in their overall ToM ability (g = −0.74, 95% CI [−0.88, −0.61], K = 34; see Figure 3). Examining the overlapping and distinct subcomponents of these constructs revealed that MS was associated with moderate deficits in cognitive ToM (g = −0.72, 95% CI [−0.92, −0.51], K = 22; see Figure 4) and cognitive empathy/affective ToM (g = −0.79, 95% CI [−0.96, −0.62], K = 25; see Figure 5). However, no group differences were evident for affective empathy (g = −0.19, 95% CI [−0.63, 0.26], K = 3; see Figure 6). There was no heterogeneity across studies for affective empathy (I2 = 0) and moderate heterogeneity across studies for overall ToM (I2 = 70.8%), overall empathy (I2 = 74.1%), and affective ToM/cognitive empathy (I2 = 68.9%), but there was significant heterogeneity in studies for cognitive ToM (I2 = 82.3%). Egger's test was not significant for overall empathy, overall ToM, cognitive ToM, or cognitive empathy/affective ToM. Egger's test was only significant for affective empathy (p = 0.005). However, a trim-and-fill analysis did not result in imputation of any studies, and the effect size remained similar (g = −0.23, 95% CI [–0.63, 0.18]).
Table 3
| Subcomponent | K | N in MS groups | N in HCs groups | g | 95% CI | Test for Heterogeneity | Assess risk of publication bias | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | I2 statistic,% | Egger's test P-Value | Trim and fill imputed g | |||||
| overall empathy | 27 | 1,275 | 1,110 | −0.67 | −0.84 | −0.50 | 74.1 | 0.099 | No change |
| overall ToM | 34 | 1,295 | 1,208 | −0.74 | −0.88 | −0.61 | 70.8 | 0.303 | No change |
| CogToM | 22 | 805 | 710 | −0.72 | −0.92 | −0.51 | 82.3 | 0.062 | No change |
| CogEmp/AffTom | 25 | 972 | 957 | −0.79 | −0.96 | −0.62 | 68.9 | 0.244 | No change |
| AffEmp | 3 | 106 | 106 | −0.19 | −0.63 | 0.26 | 0 | 0.005 | Similar |
Mean effects for ToM and empathy subcomponents comparing participants with multiple sclerosis against healthy controls and tests for publication bias.
MS, multiple sclerosis; HCs, healthy controls; ToM, theory of mind; CI, confidence interval; CogToM, cognitive ToM; CogEmp/AffToM, cognitive empathy/affective ToM; AffEmp, affective empathy; g, Hedges g; K, the number of studies; N, the number. Trim and fill: look for missing studies to left of mean, using random effects model. Imputed mean is random effects.
Figure 2
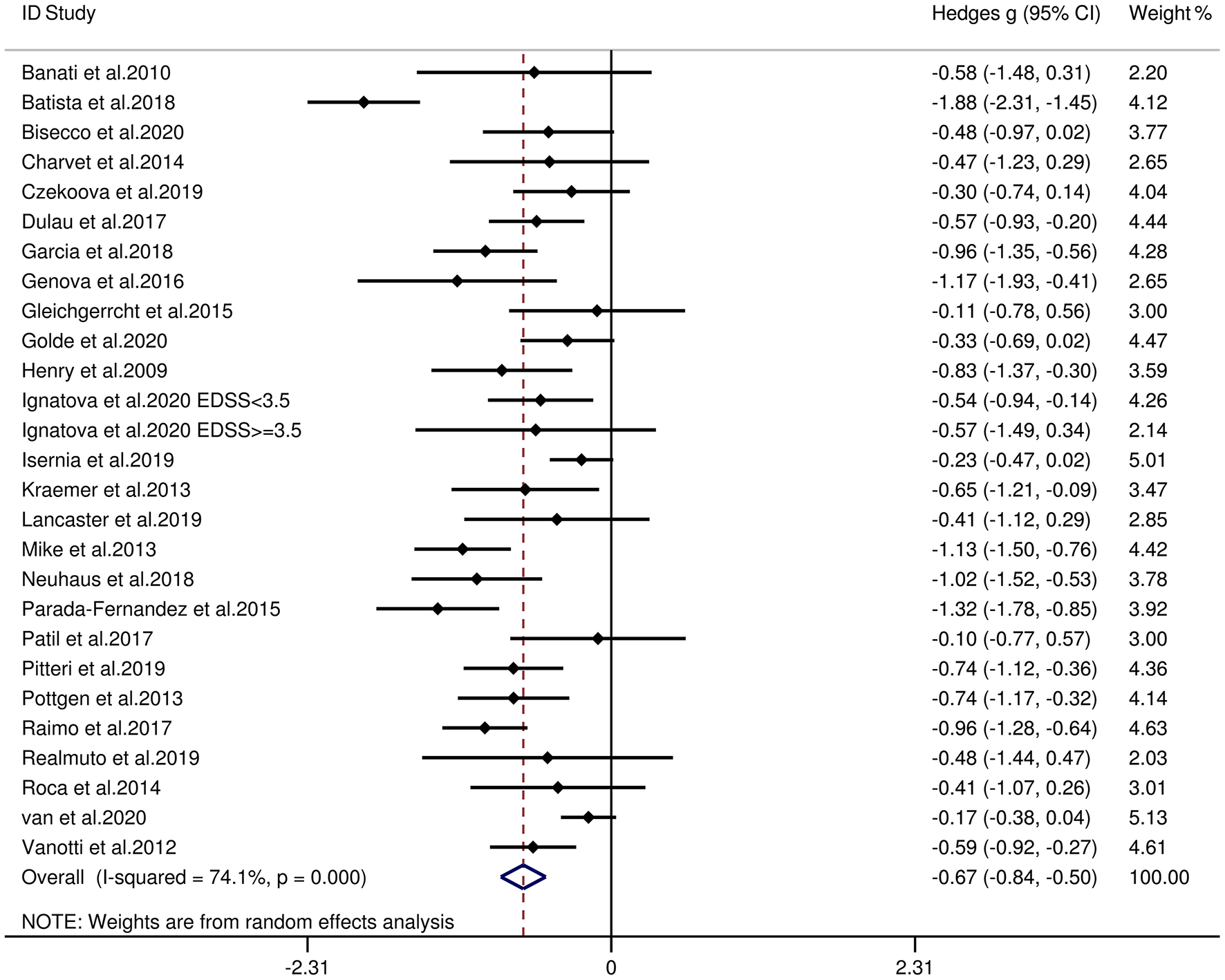
Forest plots showing effect size estimates (Hedges g) for overall empathy differences between MS and healthy controls. CI, confidence interval; MS, multiple sclerosis; ToM, theory of mind.
Figure 3
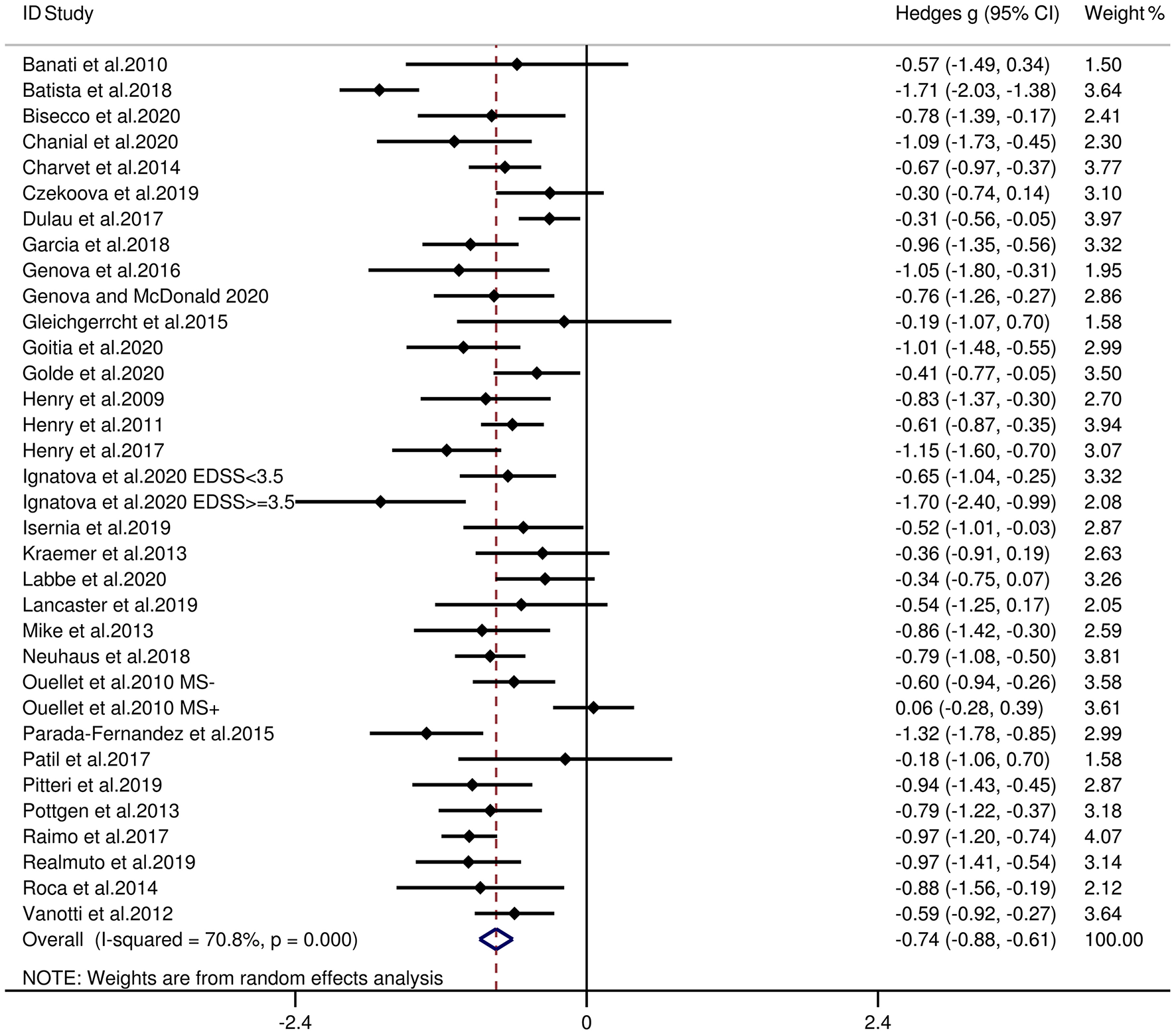
Forest plots showing effect size estimates (Hedges g) for overall ToM differences between MS and healthy controls. CI, confidence interval; MS, multiple sclerosis; ToM, theory of mind.
Figure 4

Forest plots showing effect size estimates (Hedges g) for cognitive ToM differences between MS and healthy controls. CI, confidence interval; MS, multiple sclerosis; ToM, theory of mind.
Figure 5

Forest plots showing effect size estimates (Hedges g) for cognitive empathy/affective ToM differences between MS and healthy controls. CI, confidence interval; MS, multiple sclerosis; ToM, theory of mind.
Figure 6

Forest plots showing effect size estimates (Hedges g) for affective empathy differences between MS and healthy controls. CI, confidence interval; MS, multiple sclerosis; ToM, theory of mind.
Empathy and ToM in Patients With RRMS vs. HCs
Table 4 reports the key results from this meta-analysis. Relative to HCs, patients with RRMS exhibited low impairment in overall empathy (g = −0.43, 95% CI [−0.57, −0.29], K = 17), and moderate impairment in overall ToM ability (g = −0.67, 95% CI [−0.82, −0.52], K = 20). Examining the overlapping and distinct subcomponents of these constructs revealed that RRMS was associated with significant and large-sized deficits in cognitive ToM (g = −0.83, 95% CI [−1.17, −0.50], K = 11) and cognitive empathy/affective ToM (g = −0.59, 95% CI [−0.77, −0.41], K = 15). However, no group differences were evident for affective empathy (g = −0.19, 95% CI [−0.63, 0.26], K = 3). There was no heterogeneity across studies for affective empathy (I2 = 0), low heterogeneity across studies for overall empathy (I2 = 39.1%), and moderate heterogeneity across studies for overall ToM (I2 = 53.3%) and affective ToM/cognitive empathy (I2 = 52.2%), but there was significant variation among studies for cognitive ToM (I2 = 85.4%). Egger's test was not significant for overall ToM, overall empathy, cognitive ToM, or affective ToM/cognitive empathy. Egger's test was only significant for affective empathy. However, a trim-and-fill analysis did not result in imputation of any studies, and the effect size remained similar.
Table 4
| Subcomponent | K | N in RRMS groups | N in HCs groups | g | 95% CI | Test for Heterogeneity | Assess risk of publication bias | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | I2 statistic,% | Egger's test P-Value | Trim and fill imputed g | |||||
| Overall empathy | 17 | 858 | 772 | −0.43 | −0.57 | −0.25 | 39.1 | 0.094 | No change |
| Overall ToM | 20 | 758 | 794 | −0.67 | −0.82 | −0.52 | 53.3 | 0.282 | No change |
| CogToM | 11 | 357 | 466 | −0.83 | −1.17 | −0.50 | 85.4 | 0.053 | No change |
| CogEmp/AffTom | 15 | 555 | 619 | −0.59 | −0.77 | −0.41 | 52.2 | 0.172 | No change |
| AffEmp | 3 | 106 | 106 | −0.19 | −0.63 | 0.26 | 0 | 0.005 | Similar |
Mean effects for ToM and empathy subcomponents comparing participants with relapsing-remitting multiple sclerosis against healthy controls and tests for publication bias.
RRMS, relapsing-remitting multiple sclerosis; HCs, healthy controls; ToM, theory of mind; CI, confidence interval; CogToM, cognitive ToM; CogEmp/AffToM, cognitive empathy/affective ToM; AffEmp, affective empathy; g, Hedges g; K, the number of studies; N, the number. Trim and fill: look for missing studies to left of mean, using random effects model. Imputed mean is random effects.
Empathy and ToM in Patients With Progressive MS vs. HCs
Table 5 reports the key results from this meta-analysis. Relative to HCs, patients with progressive MS exhibited a moderate-sized deficit in overall empathy (g = −0.50, 95% CI [−0.73, −0.27], K = 4), overall ToM ability (g = −0.75, 95% CI [−1.08, −0.41], K = 7, cognitive ToM (g = −0.72, 95% CI [−1.15, −0.29], K = 6), and affective ToM/cognitive empathy (g = −0.50, 95% CI [−0.73, −0.27], K = 4). No analysis for affective empathy was conducted, as no such studies were included in the meta-analysis. There was no heterogeneity across studies for overall empathy (I2 = 0) and affective ToM/cognitive empathy (I2 = 0) and moderate heterogeneity across studies for overall ToM (I2 = 65%), but there was significant variation among studies for cognitive ToM (I2 = 75.5%). Egger's test was not significant for overall ToM, overall empathy, cognitive ToM, or affective ToM/cognitive empathy.
Table 5
| Subcomponent | K | N in progressive MS groups | N in HCs groups | g | 95% CI | Test for Heterogeneity | Assess risk of publication bias | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | I2 statistic,% | Egger's test P-Value | Trim and fill imputed g | |||||
| overall empathy | 7 | 61 | 101 | −0.50 | −0.73 | −0.27 | 0 | 0.366 | No change |
| overall ToM | 4 | 124 | 149 | −0.75 | −1.08 | −0.41 | 64.9 | 0.091 | No change |
| CogToM | 6 | 107 | 134 | −0.72 | −1.15 | −0.29 | 75.5 | 0.196 | No change |
| CogEmp/AffTom | 4 | 61 | 101 | −0.50 | −0.73 | −0.27 | 0 | 0.366 | No change |
Mean effects for ToM and empathy subcomponents comparing participants with progressive multiple sclerosis against healthy controls and tests for publication bias.
Progressive MS, progressive multiple sclerosis; HCs, healthy controls; ToM, theory of mind; CI, confidence interval; CogToM, cognitive ToM; CogEmp/AffToM, cognitive empathy/affective ToM; AffEmp, affective empathy; g, Hedges g; K, the number of studies; N, the number. Trim and fill: look for missing studies to left of mean, using random effects model. Imputed mean is random effects.
Empathy and ToM in Patients With RRMS vs. Patients With Progressive MS
Table 6 reports the key results from this meta-analysis. Relative to patients with progressive MS, patients with RRMS showed no difference in overall empathy (g = 0.21, 95% CI [−0.23, 0.65], K = 2), overall ToM (g = 0.11, 95% CI [−0.09, 0.31], K = 3), cognitive ToM (g = 0.05, 95% CI [−0.14, 0.24], K = 3), and affective ToM/cognitive empathy (g = 0.21, 95% CI [−0.23, 0.65], K = 2). No analysis for affective empathy was conducted, as no such studies were included in this meta-analysis. There was low heterogeneity across studies for overall ToM (I2 = 23.7%) and cognitive ToM (I2 = 15.4%) and moderate heterogeneity across studies for overall empathy (I2 = 63.2%) and cognitive empathy/affective ToM (I2 = 63.2%). Egger's test was not significant for overall ToM and cognitive ToM.
Table 6
| Subcomponent | K | N in RRMS groups | N in progressive MS groups | g | 95% CI | Test for Heterogeneity | Assess risk of publication bias | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | I2 statistic,% | Egger's test P-Value | Trim and fill imputed g | |||||
| Overall empathy | 2 | 56 | 46 | 0.21 | −0.23 | 0.65 | 63.2 | ||
| Overall ToM | 3 | 87 | 77 | 0.11 | −0.09 | 0.31 | 23.7 | 0.303 | No change |
| CogToM | 3 | 87 | 77 | 0.05 | −0.14 | 0.24 | 15.4 | 0.062 | No change |
| CogEmp/AffTom | 2 | 56 | 46 | 0.21 | −0.23 | 0.65 | 63.2 | ||
Mean effects for ToM and empathy subcomponents comparing participants with relapsing-remitting multiple sclerosis against progressive multiple sclerosis and tests for publication bias.
RRMS, relapsing-remitting multiple sclerosis, progressive MS progressive multiple sclerosis; ToM, theory of mind; CI, confidence interval; CogToM, cognitive ToM; CogEmp/AffToM, cognitive empathy/affective ToM; AffEmp, affective empathy; g, Hedges g; K, the number of studies; N, the number. Trim and fill: look for missing studies to left of mean, using random effects model. Imputed mean is random effects.
Meta-Regression Analyses
Meta-regression analyses showed that the included variables did not account for significant variance across studies. The variables (age, sex, education level, disease duration, EDSS score, quality assessment score, severity of depression, and severity of anxiety) did not account for significant variance in overall empathy(p = 0.871, 0.218, 0.582, 0.996, 0.712, 0.318, 0.671, and 0.871, respectively), overall ToM (p = 0.825, 0.341, 0.832, 0.245, 0.527, 0.535, 0.068, and 0.224, respectively), cognitive ToM (p = 0.961, 0.418, 0.89, 0.997, 0.831, 0.098, 0.423, NA, respectively), or cognitive empathy/affective ToM (p = 0.548, 0.516, 0.61, 0.634, 0.549, 0.589, 0.48, and 0.872, respectively). No meta-regression analyses were conducted for the severity of anxiety in cognitive ToM, as fewer than 10 studies contributed to the data for this subcomponent.
Discussion
To our knowledge, this was the first meta-analysis to investigate the patterns of empathy and ToM functioning in patients with MS. The meta-analysis included 33 studies, with combined samples of 1,568 individuals with MS and 1,283 HCs. Relative to the HC group, the MS group showed moderate impairments in both overall empathy (g = −0.67) and overall ToM (g = −0.74). Among the overlapping and distinct subcomponents of these constructs, MS was associated with moderate impairment in cognitive ToM (g = −0.72) and cognitive empathy/affective ToM (g = −0.79), but no significant difference was found in affective empathy. Subgroup analyses showed that compared with the HCs, patients with RRMS and progressive MS were both impaired in overall empathy, overall ToM, cognitive ToM, and cognitive empathy/affective ToM, and there was no statistical difference between RRMS and progressive MS in the degree of impairment. Meta-regression analysis indicated that the examined variables (age, sex, education level, disease duration, EDSS score, quality assessment score, severity of depression, and severity of anxiety) did not affect the magnitude of the effect sizes observed.
For overall empathy, a moderate effect size was found (g = −0.67). When focusing on the subcomponents of empathy, patients with MS were found to have moderate impairment in cognitive empathy; however, there was no difference in affective empathy. The quantitative findings support the conclusions of previous qualitative studies, indicating that cognitive and affective empathy are separate and have different requirements for effortful processing (81, 83, 90). Specifically, cognitive empathy/affective ToM, requiring attention and time, is a slow and laborious process, while affective empathy, operating with minimal conscious awareness, is an automatic and spontaneous response (95). Therefore, these two empathic components may present different challenges for patients with MS. As affective empathy has low cognitive requirements, it might be expected that this ability remains relatively preserved in MS. This disconnection between the cognitive and affective subcomponents of empathy has been confirmed in several other neurological diseases. For example, in patients with Alzheimer's disease, there was a moderate-sized deficit in cognitive empathy/affective ToM, but no impairment in affective empathy (96). Patients with Parkinson's disease, compared to HCs, PD had significant impairment in cognitive empathy/affective ToM, but no group differences were identified in affective empathy (97). However, the findings should be interpreted with caution in this meta-analysis due to the limited number of included studies contributing to the effect size of affective empathy (K = 3).
The results pertaining to overall ToM impairment supported the findings of Bora et al. (32) and Cotter et al. (33), showing that overall ToM is moderately impaired in MS. When considering the sub-components of ToM, some previous studies have suggested that the domains of cognitive and affective ToM are dissociated, and the function of cognitive empathy/affective ToM in MS is conserved, but cognitive ToM is impaired (74, 85, 88, 93). However, findings from the current quantitative meta-analysis do not support this suggestion, which showed that patients of MS had moderate impairment in both cognitive and affective ToM and their degrees of defect were close (g = −0.72 and g = −0.79, respectively). This impairment may be related to white matter (WM) damage in MS. On the macro-structure, ToM impairment is associated with T1 and T2 lesions (65, 71, 88); on the microstructure, ToM impairment is shown to be related to the disconnection with the social brain network caused by diffuse normal-appearing white matter damage in MS, especially in tracts of limbic pathways (uncinate fasciculus, fornix) and callosal interhemispheric fibers (corpus callosum, tapetum) (72), which play a key role in social and communication skills or emotional processing (98–100). In addition, gray matter (GM) pathology is considered to have an important role in ToM impairment. GM atrophy was found in the cingulate, orbitofrontal, cerebellar cortex, and insula decreased was found (65, 66, 72), which are involved in cognitive and affective ToM network (101). Several studies based on magnetic resonance imaging (MRI) have found that amygdala atrophy is the main predictor of ToM impairment in MS (66, 72). Besides, one resting-state functional MRI study found that there was an association between ToM impairment and functional connectivity changes in the default mode network, executive network, and limbic network in MS (74).
In the subgroup meta-analyses, the results showed that compared with HCs, patients with RRMS and progressive MS were impaired in overall empathy, overall ToM, cognitive ToM, and cognitive empathy/affective ToM, and there was no statistical difference between RRMS and progressive MS in overall empathy, overall ToM, cognitive ToM, and cognitive empathy/affective ToM. This result is inconsistent with the previous quantitative results of Bora et al., which indicated that social cognition tended to be more impaired in progressive MS in comparison to RRMS. However, it should be noted that the aforementioned Bora et al. study calculated a social cognition score based on numerous very different ToM tasks and facial emotion recognition tasks (another core domain of social cognition). Besides, due to the limited number of included studies contributing to the comparison between RRMS and progressive MS (K = 3 in this study, K = 5 in the study by Bora et al.), we should cautiously interpret the results.
Our meta-analysis findings may contribute to the development of cognitive rehabilitation for MS. Several studies have shown that cognitive rehabilitation intervention may have a positive impact on MS symptoms (102–104). In particular, depression symptoms, anxiety, fatigue, pain, physical vitality, and sleep quality improved significantly after most of the cognitive rehabilitation intervention (104–107). Besides, studies have shown that cognitive rehabilitation can improve the cognitive function in patients with MS, mainly focusing on general cognitive functions such as memory, executive function, attention, and processing speed (102, 104, 108–112). However, there are few studies about how the cognitive interventions affect social cognitive in MS. Our meta-analytic findings can broaden the theoretical understanding of MS, which may help improve or formulate cognitive intervention strategies.
Limitations
The current meta-analysis has some limitations. First, although 33 studies were included in this meta-analysis, only three contributed to the mean effect size for affective empathy between patients with MS and HCs. In addition, only three studies provided data comparing RRMS and progressing MS; hence, more research in this area is needed in the future. Second, we only included cross-sectional studies, while more longitudinal studies are needed to investigate the dynamic changes in empathy and ToM function in patients of MS. Third, although we investigated some demographic and clinical variables (including age, sex, education level, disease duration, EDSS scores, severity of depression, and severity of anxiety) that may affect empathy and ToM function, other factors [such as prior substance abuse or some other behavioral symptoms (including apathy, inflexible, obsessive, sometimes with flattened affect, suspiciousness, etc.)] were not examined due to the limited data available in the original studies (67, 113, 114). Further studies are required to comprehensively elucidate the potential effects of these factors on empathy- and ToM-associated features in MS. Fifth, there was heterogeneity between the individual tasks for the assessment of ToM or empathy, and further development of standardized batteries for ToM/empathy assessment in MS is needed. For example, the Measurement and Treatment Research to Improve Cognition in Schizophrenia Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) (115), which makes it possible to standardize the evaluation of cognitive outcomes in schizophrenia, may also be adapted for MS.
Conclusions
The results of this meta-analysis suggest that patients with MS exhibited moderate impairment in broad constructs of ToM and empathy and the ToM subcomponents (cognitive ToM and affective ToM/cognitive empathy), but no significant impairment in affective empathy. These quantitative results suggest a differential impairment of the core aspects of social cognitive (including empathy and ToM) processing in patients with MS, which may greatly inform the development of structured social cognitive interventions in MS.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
ZY and GW: study design and critical revision of the manuscript. XL, XZ, QL, PZ, JZ, and PP: analysis and interpretation of data. XL and XZ: drafting of the manuscript. All authors: approval of the final version for submission.
Funding
This work was supported by Jiangsu Commission of Health (LGY20180390).
Acknowledgments
We thank all the authors of the studies included. This protocol was prospectively registered at the International Platform of Registered Systematic Review and Meta-analysis Protocols (ID: INPLASY202070029) and has been released in the journal of Medicine (52).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
RolfLMurisAHTheunissenRHuppertsRDamoiseauxJSmoldersJ. Vitamin D(3) supplementation and the IL-2/IL-2R pathway in multiple sclerosis: attenuation of progressive disturbances?J Neuroimmunol. (2018) 314:50–7. 10.1016/j.jneuroim.2017.11.007
2.
BuchananRJMindenSLChakravortyBJHatcherWTyryTVollmerT. A pilot study of young adults with multiple sclerosis: demographic, disease, treatment, and psychosocial characteristics. Disabil Health J. (2010) 3:262–70. 10.1016/j.dhjo.2009.09.003
3.
MotlRWSandroffBMKwakkelGDalgasUFeinsteinAHeesenCet al. Exercise in patients with multiple sclerosis. Lancet Neurol. (2017) 16:848–56. 10.1016/s1474-4422(17)30281-8
4.
HenryATourbahAChaunuMPRumbachLMontreuilMBakchineS. Social cognition impairments in relapsing-remitting multiple sclerosis. J Int Neuropsychol Soc. (2011) 17:1122–31. 10.1017/s1355617711001147
5.
BenedictRHCookfairDGavettRGuntherMMunschauerFGargNet al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc. (2006) 12:549–58. 10.1017/s1355617706060723
6.
SanfilipoMPBenedictRHWeinstock-GuttmanBBakshiR. Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology. (2006) 66:685–92. 10.1212/01.wnl.0000201238.93586.d9
7.
ChalahMAAyacheSS. Deficits in social cognition: an unveiled signature of multiple sclerosis. J Int Neuropsychol Soc. (2017) 23:266–86. 10.1017/s1355617716001156
8.
JongenPJTer HorstATBrandsAM. Cognitive impairment in multiple sclerosis. Minerva Med. (2012) 103:73–96.
9.
MillerEMorelARedlickaJMillerISalukJ. Pharmacological and non-pharmacological therapies of cognitive impairment in multiple sclerosis. Curr Neuropharmacol. (2018) 16:475–83. 10.2174/1570159x15666171109132650
10.
PhillipsLHHenryJDScottCSummersFWhyteMCookM. Specific impairments of emotion perception in multiple sclerosis. Neuropsychology. (2011) 25:131–6. 10.1037/a0020752
11.
NeuhausMBaguttiSYaldizliÖZwahlenDSchaubSFreyBet al. Characterization of social cognition impairment in multiple sclerosis. Eur J Neurol. (2018) 25:90–6. 10.1111/ene.13457
12.
PitteriMGenovaHLengenfelderJDeLucaJZiccardiSRossiVet al. Social cognition deficits and the role of amygdala in relapsing remitting multiple sclerosis patients without cognitive impairment. Mult Scler Relat Disord. (2019) 29:118–23. 10.1016/j.msard.2019.01.030
13.
GenovaHMMcDonaldS. Social cognition in individuals with progressive multiple sclerosis: a pilot study using TASIT-S. J Int Neuropsychol Soc. (2020) 26:539–44. 10.1017/s1355617719001371
14.
IserniaSCabinioMPirastruAMendozziLDi DioCMarchettiAet al. Theory of mind network in multiple sclerosis: a double disconnection mechanism. Soc Neurosci. (2020) 15:544–57. 10.1080/17470919.2020.1766562
15.
GreenMFHoranWPLeeJ. Social cognition in schizophrenia. Nat Rev Neurosci. (2015) 16:620–31. 10.1038/nrn4005
16.
RaoSMLeoGJEllingtonLNauertzTBernardinLUnverzagtF. Cognitive dysfunction in multiple sclerosis. II Impact on employment and social functioning. Neurology. (1991) 41:692–6. 10.1212/wnl.41.5.692
17.
GreenMFPennDLBentallRCarpenterWTGaebelWGurRCet al. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. (2008) 34:1211–20. 10.1093/schbul/sbm145
18.
KrauseMWendtJDresselABerneiserJKesslerCHammAOet al. Prefrontal function associated with impaired emotion recognition in patients with multiple sclerosis. Behav Brain Res. (2009) 205:280–5. 10.1016/j.bbr.2009.08.009
19.
BoraEWalterfangMVelakoulisD. Theory of mind in Parkinson's disease: a meta-analysis. Behav Brain Res. (2015) 292:515–20. 10.1016/j.bbr.2015.07.012
20.
HenryJDvon HippelWMolenberghsPLeeTSachdevPS. Clinical assessment of social cognitive function in neurological disorders. Nat Rev Neurol. (2016) 12:28–39. 10.1038/nrneurol.2015.229
21.
WondraJDEllsworthPC. An appraisal theory of empathy and other vicarious emotional experiences. Psychol Rev. (2015) 122:411–28. 10.1037/a0039252
22.
BartochowskiZGatlaSKhouryRAl-DahhakRGrossbergGT. Empathy changes in neurocognitive disorders: a review. Ann Clin Psychiatry. (2018) 30:220–32.
23.
ChenJ. Empathy for distress in humans and rodents. Neurosci Bull. (2018) 34:216–36. 10.1007/s12264-017-0135-0
24.
DvashJShamay-TsoorySG. Theory of mind and empathy as multidimensional constructs. Top Langu Disord. (2014) 34:282–95. 10.1097/TLD.0000000000000040
25.
RealmutoSDodichAMeliRCanessaNRagonesePSalemiGet al. Moral Cognition and multiple sclerosis: a neuropsychological study. Archiv Clin Neuropsychol. (2019) 34:319–26. 10.1093/arclin/acy047
26.
van der HieleKvan EgmondEEAJongenPJvan der KlinkJJLBeenakkerEACvan EijkJJJet al. Empathy in multiple sclerosis–correlates with cognitive, psychological and occupational functioning. Mult Scler Relat Disord. (2020) 41:102036. 10.1016/j.msard.2020.102036
27.
KraemerMHeroldMUekermannJKisBWiltfangJDaumIet al. Theory of mind and empathy in patients at an early stage of relapsing remitting multiple sclerosis. Clin Neurol Neurosurg. (2013) 115:1016–22. 10.1016/j.clineuro.2012.10.027
28.
McDonaldSFlanaganS. Social perception deficits after traumatic brain injury: interaction between emotion recognition, mentalizing ability, and social communication. Neuropsychology. (2004) 18:572–9. 10.1037/0894-4105.18.3.572
29.
LeppanenJSedgewickFTreasureJTchanturiaK. Differences in the theory of mind profiles of patients with anorexia nervosa and individuals on the autism spectrum: a meta-analytic review. Neurosci Biobehav Rev. (2018) 90:146–63. 10.1016/j.neubiorev.2018.04.009
30.
YiZZhaoPZhangHShiYShiHZhongJet al. Theory of mind in Alzheimer's disease and amnestic mild cognitive impairment: a meta-analysis. Neurol Sci. (2020) 41:1027–39. 10.1007/s10072-019-04215-5
31.
HeitzCNobletVPhillippsCCretinBVogtNPhilippiNet al. Cognitive and affective theory of mind in dementia with Lewy bodies and Alzheimer's disease. Alzheimers Res Ther. (2016) 8:10. 10.1186/s13195-016-0179-9
32.
BoraEÖzakbaşSVelakoulisDWalterfangM. Social cognition in multiple sclerosis: a meta-analysis. Neuropsychol Rev. (2016) 26:160–72. 10.1007/s11065-016-9320-6
33.
CotterJFirthJEnzingerCKontopantelisEYungARElliottRet al. Social cognition in multiple sclerosis: a systematic review and meta-analysis. Neurology. (2016) 87:1727–36. 10.1212/wnl.0000000000003236
34.
SingerT. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci Biobehav Rev. (2006) 30:855–63. 10.1016/j.neubiorev.2006.06.011
35.
BensalahLCailliesSAnduzeM. Links among cognitive empathy, theory of mind, and affective perspective taking by young children. J Genet Psychol. (2016) 177:17–31. 10.1080/00221325.2015.1106438
36.
Shamay-TsoorySGAharon-PeretzJPerryD. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. (2009) 132(Pt 3):617–27. 10.1093/brain/awn279
37.
PreckelKKanskePSingerT. On the interaction of social affect and cognition: empathy, compassion and theory of mind. Curr Opin Behav Sci. (2018) 19:1–6. 10.1016/j.cobeha.2017.07.010
38.
JacquesFLublinF. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. (2015) 84:963. 10.1212/01.wnl.0000462309.76486.c5
39.
MartinSJMcglassonSHuntDOverellJ. Cerebrospinal fluid neurofilament light chain in multiple sclerosis and its subtypes: a meta-analysis of case–control studies. J Neurol Neurosurg Psychiatry. (2019) 90:1059–67. 10.1136/jnnp-2018-319190
40.
AchimAMOuelletRLavoieMAVallièresCJacksonPLRoyMA. Impact of social anxiety on social cognition and functioning in patients with recent-onset schizophrenia spectrum disorders. Schizophr Res. (2013) 145:75–81. 10.1016/j.schres.2013.01.012
41.
BoraEBerkM. Theory of mind in major depressive disorder: a meta-analysis. J Affect Disord. (2016) 191:49–55. 10.1016/j.jad.2015.11.023
42.
BoraEKoseS. Meta-analysis of theory of mind in anorexia nervosa and bulimia nervosa: a specific Impairment of cognitive perspective taking in anorexia nervosa?Int J Eat Disord. (2016) 49:739–40. 10.1002/eat.22572
43.
GkikaSWittkowskiAWellsA. Social cognition and metacognition in social anxiety: a systematic review. Clin Psychol Psychother. (2018) 25:10–30. 10.1002/cpp.2127
44.
AlviTKourosCDLeeJFulfordDTabakBA. Social anxiety is negatively associated with theory of mind and empathic accuracy. J Abnorm Psychol. (2020) 129:108–13. 10.1037/abn0000493
45.
LimaFSSimioniSBruggimannLRuffieuxCDudlerJFelleyCet al. Perceived behavioral changes in early multiple sclerosis. Behav Neurol. (2007) 18:81–90. 10.1155/2007/674075
46.
OuelletJScherzerPBRouleauIMétrasPBertrand-GauvinCDjerroudNet al. Assessment of social cognition in patients with multiple sclerosis. J Int Neuropsychol Soc. (2010) 16:287–96. 10.1017/s1355617709991329
47.
MavrogiorgouPBethgeMLuksnatSNalatoFJuckelGBrüneM. Social cognition and metacognition in obsessive–compulsive disorder: an explorative pilot study. Eur Archiv Psychiatry Clin Neurosci. (2016) 266:209–16. 10.1007/s00406-016-0669-6
48.
HenryATourbahAChaunuMPBakchineSMontreuilM. Social cognition abilities in patients with different multiple sclerosis subtypes. J Int Neuropsychol Soc. (2017) 23:653–64. 10.1017/S1355617717000510
49.
MisirEBoraEAkdedeBB. Relationship between social-cognitive and social-perceptual aspects of theory of mind and neurocognitive deficits, insight level and schizotypal traits in obsessive-compulsive disorder. Compreh Psychiatry. (2018) 83:1–6. 10.1016/j.comppsych.2018.02.008
50.
IgnatovaVGSurchevJKStoyanovaTGVassilevPMHaralanovLHTodorovaLP. Social cognition impairments in patients with multiple sclerosis: comparison with grade of disability. Neurol India. (2020) 68:94–8. 10.4103/0028-3886.279700
51.
MoherDLiberatiATetzlaffJAltmanDG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097
52.
LinXZhangXLiuQZhaoPZhongJPanPet al. Empathy and theory of mind in multiple sclerosis: a protocol for systematic review and meta-analysis. Medicine. (2020) 99:e21773. 10.1097/MD.0000000000021773
53.
StangA. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z
54.
MasiAQuintanaDSGlozierNLloydARHickieIBGuastellaAJ. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry. (2015) 20:440–6. 10.1038/mp.2014.59
55.
HedgesLV. Distribution theory for glass's estimator of effect size and related estimators. J Educ Stats. (1981) 6:107–28.
56.
CohenJ. Statistical Power Analysis for the Behavioural Sciences. New York, NY: Academic Press (1988).
57.
VelikonjaTFettAKVelthorstE. Patterns of nonsocial and social cognitive functioning in adults with autism spectrum disorder: a systematic review and meta-analysis. JAMA Psychiatry. (2019) 76:135–51. 10.1001/jamapsychiatry.2018.3645
58.
HigginsJPThompsonSG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. 10.1002/sim.1186
59.
EggerMDavey SmithGSchneiderMMinderC. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. 10.1136/bmj.315.7109.629
60.
DuvalSTweedieR. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. 10.1111/j.0006-341x.2000.00455.x
61.
SpielbergerCD. STAI Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press (1970).
62.
BeckATSteerRA. Beck Depression Inventory: Manual. Vol. 8. San Antonio TX: Harcourt Brace Jovanovich (1996). p. 77–100.
63.
BanatiMSandorJMikeAIllesEBorsLFeldmannAet al. Social cognition and Theory of Mind in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. (2010) 17:426–33. 10.1111/j.1468-1331.2009.02836.x
64.
HigginsJGreenS. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Cochrane book series (2009).
65.
ChalahMAKauvPLefaucheurJPHodelJCréangeAAyacheSS. Theory of mind in multiple sclerosis: a neuropsychological and MRI study. Neurosci Lett. (2017) 658:108–13. 10.1016/j.neulet.2017.08.055
66.
CiampiEUribe-San-MartinRVásquezMRuiz-TagleALabbeTCruzJPet al. Relationship between social cognition and traditional cognitive impairment in progressive multiple sclerosis and possible implicated neuroanatomical regions. Multiple Scler Relat Disord. (2018) 20:122–8. 10.1016/j.msard.2018.01.013
67.
GenovaHMLancasterKLengenfelderJBoberCPDeLucaJChiaravallotiND. Relationship between social cognition and fatigue, depressive symptoms, and anxiety in multiple sclerosis. J Neuropsychol. (2020) 14:213–25. 10.1111/jnp.12185
68.
KrämerMBraunerSHeroldMDiehlRRDziobekIBerlitP. theory of mind, perception of affective prosody and empathy in secondary progressive multiple sclerosis. Aktuelle Neurol. (2014) 41:208–16. 10.1055/s-0034-1370029
69.
FabriTLDattaRO'MahonyJBar-OrAYehEAArnoldDL. Memory and identification of emotional expression in pediatric-onset multiple sclerosis. Multiple Scler J. (2018) 24:340–1. 10.1177/1352458518798590
70.
HenryABakchineSMaaroufAChaunuMPRumbachLMagninEet al. Facial emotion recognition and faux pas interpretation in multiple sclerosis. Brain Impairment. (2015) 16:158–72. 10.1017/BrImp.2015.33
71.
BatistaSAlvesCD'AlmeidaOCAfonsoAFélix-MoraisRPereiraJet al. Disconnection as a mechanism for social cognition impairment in multiple sclerosis. Neurology. (2017) 89:38–45. 10.1212/WNL.0000000000004060
72.
BatistaSd'AlmeidaOCAfonsoAFreitasSMacárioCSousaLet al. Impairment of social cognition in multiple sclerosis: amygdala atrophy is the main predictor. Multiple Scler. (2017) 23:1358–66. 10.1177/1352458516680750
73.
BatistaSFreitasSAfonsoAMacárioCSousaLCunhaLet al. Theory of mind and executive functions are dissociated in multiple sclerosis. Arch Clin Neuropsychol. (2018) 33:541–51. 10.1093/arclin/acx101
74.
BiseccoAAltieriMSantangeloGDi NardoFDocimoRCaiazzoGet al. Resting-state functional correlates of social cognition in multiple sclerosis: an explorative study. Front Behav Neurosci. (2020) 13:276. 10.3389/fnbeh.2019.00276
75.
ChanialCBasaglia-PappasSJacquelineSBoulangeAGourdonCDonyaSet al. Assessment of implicit language and theory of mind in multiple sclerosis. Ann Phys Rehabil Med. (2020) 63:111–5. 10.1016/j.rehab.2019.08.005
76.
CharvetLEClearyREVazquezKBelmanALKruppLB. Social cognition in pediatric-onset multiple sclerosis (MS). Multiple Scler. (2014) 20:1478–84. 10.1177/1352458514526942
77.
CzekóováKShawDJSaxunováKDufekMMarečekRVaníčekJet al. Impaired self-other distinction and subcortical gray-matter alterations characterize socio-cognitive disturbances in multiple sclerosis. Front Neurol. (2019) 10:525. 10.3389/fneur.2019.00525
78.
DulauCDeloireMDiazHSaubusseACharre-MorinJProuteauAet al. Social cognition according to cognitive impairment in different clinical phenotypes of multiple sclerosis. J Neurol. (2017) 264:740–8. 10.1007/s00415-017-8417-z
79.
GarcíaMRuedaDSRosenbaumKCassaráFPSinayVTorralvaTet al. The reduced version of the reading the mind in the eyes test. It's utility in evaluating complex emotion recognition in relapsing remitting multiple sclerosis. Neurology. (2018) 90.
80.
GenovaHMCagnaCJChiaravallotiNDDeLucaJLengenfelderJ. Dynamic assessment of social cognition in individuals with multiple sclerosis: a pilot study. J Int Neuropsychol Soc. (2016) 22:83–8. 10.1017/S1355617715001137
81.
GleichgerrchtETomashitisBSinayV. The relationship between alexithymia, empathy and moral judgment in patients with multiple sclerosis. Eur J Neurol. (2015) 22:1295–303. 10.1111/ene.12745
82.
GoitiaBBrunoDAbrevayaSSedeñoLIbáñezAManesFet al. The relationship between executive functions and fluid intelligence in multiple sclerosis. PLoS ONE. (2020) 15:e0231868. 10.1371/journal.pone.0231868
83.
GoldeSHeineJPöttgenJMantwillMLauSWingenfeldKet al. Distinct Functional connectivity signatures of impaired social cognition in multiple sclerosis. Front Neurol. (2020) 11:507. 10.3389/fneur.2020.00507
84.
HenryJDPhillipsLHBeattyWWMcDonaldSLongleyWAJoscelyneAet al. Evidence for deficits in facial affect recognition and theory of mind in multiple sclerosis. J Int Neuropsychol Soc. (2009) 15:277–85. 10.1017/S1355617709090195
85.
IserniaSBaglioFd'ArmaAGroppoEMarchettiAMassaroD. Social mind and long-lasting disease: focus on affective and cognitive theory of mind in multiple sclerosis. Front Psychol. (2019) 10:218. 10.3389/fpsyg.2019.00218
86.
LabbeTPZuritaMMontalbaCCiampiELCruzJPVasquezMet al. Social cognition in multiple sclerosis is associated to changes in brain connectivity: a resting-state fMRI study. Multiple Scler Relat Disord. (2020) 45:102333. 10.1016/j.msard.2020.102333
87.
LancasterKStoneEMGenovaHM. Cognitive but not affective theory of mind deficits in progressive MS. J Int Neuropsychol Soc. (2019) 25:896–900. 10.1017/s1355617719000584
88.
MikeAStrammerEAradiMOrsiGPerlakiGHajnalAet al. Disconnection mechanism and regional cortical atrophy contribute to impaired processing of facial expressions and theory of mind in multiple sclerosis: a structural MRI study. PLoS ONE. (2013) 8:e8242210.1371/journal.pone.0082422
89.
Parada-FernándezPOliva-MacíasMAmayraILópez-PazJFLázaroEMartínezÓet al. Accuracy and reaction time in recognition of facial emotions in people with multiple sclerosis. Rev Neurol. (2015) 61:433–40. 10.33588/rn.6110.2015225
90.
PatilIYoungLSinayVGleichgerrchtE. Elevated moral condemnation of third-party violations in multiple sclerosis patients. Soc Neurosci. (2017) 12:308–29. 10.1080/17470919.2016.1175380
91.
PöttgenJDziobekIRehSHeesenCGoldSM. Impaired social cognition in multiple sclerosis. J Neurol Neurosurg Psychiatry. (2013) 84:523–8. 10.1136/jnnp-2012-304157
92.
RaimoSTrojanoLPappacenaSAlaiaRSpitaleriDGrossiDet al. Neuropsychological correlates of theory of mind deficits in patients with multiple sclerosis. Neuropsychology. (2017) 31:811–21. 10.1037/neu0000372
93.
RocaMManesFGleichgerrchtEIbáñezAGonzález De ToledoMEMarencoVet al. Cognitive but not affective theory of mind deficits in mild relapsing-remitting multiple sclerosis. Cognit Behav Neurol. (2014) 27:25–30. 10.1097/WNN.0000000000000017
94.
VanottiSRojasGAllegriRCaceresF. How behavioral and quality of life changes affect social cognition in multiple sclerosis patients. Neurology. (2012) 78:P02.042. 10.1212/WNL.78.1
95.
YuCLChouTL. A dual route model of empathy: a neurobiological prospective. Front Psychol. (2018) 9:2212. 10.3389/fpsyg.2018.02212
96.
DemichelisOPCoundourisSPGraingerSAHenryJD. Empathy and theory of mind in Alzheimer's disease: a meta-analysis. J Int Neuropsychol Soc. (2020) 26:963–77. 10.1017/s1355617720000478
97.
CoundourisSPAdamsAGHenryJD. Empathy and theory of mind in Parkinson's disease: a meta-analysis. Neurosci Biobehav Rev. (2020) 109:92–102. 10.1016/j.neubiorev.2019.12.030
98.
PaulLKBrownWSAdolphsRTyszkaJMRichardsLJMukherjeePet al. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. (2007) 8:287–99. 10.1038/nrn2107
99.
Von Der HeideRJSkipperLMKlobusickyEOlsonIR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. (2013) 136(Pt 6):1692–1707. 10.1093/brain/awt094
100.
DowneyLEMahoneyCJBuckleyAHGoldenHLHenleySMSchmitzNet al. White matter tract signatures of impaired social cognition in frontotemporal lobar degeneration. Neuroimage Clin. (2015) 8:640–51. 10.1016/j.nicl.2015.06.005
101.
Abu-AkelAShamay-TsooryS. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia. (2011) 49:2971–84. 10.1016/j.neuropsychologia.2011.07.012
102.
O'BrienARChiaravallotiNGoveroverYDelucaJ. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: a review of the literature. Arch Phys Med Rehabil. (2008) 89:761–9. 10.1016/j.apmr.2007.10.019
103.
PagniniFBosmaCMPhillipsDLangerE. Symptom changes in multiple sclerosis following psychological interventions: a systematic review. BMC Neurol. (2014) 14:222. 10.1186/s12883-014-0222-z
104.
SeselALSharpeLNaismithSL. Efficacy of psychosocial interventions for people with multiple sclerosis: a meta-analysis of specific treatment effects. Psychother Psychosom. (2018) 87:105–11. 10.1159/000486806
105.
MitoloMVenneriAWilkinsonIDSharrackB. Cognitive rehabilitation in multiple sclerosis: a systematic review. J Neurol Sci. (2015) 354:1–9. 10.1016/j.jns.2015.05.004
106.
van den AkkerLEBeckermanHColletteEHTwiskJWBleijenbergGDekkerJet al. Cognitive behavioral therapy positively affects fatigue in patients with multiple sclerosis: results of a randomized controlled trial. Mult Scler. (2017) 23:1542–53. 10.1177/1352458517709361
107.
ChalahMAAyacheSS. Cognitive behavioral therapies and multiple sclerosis fatigue: a review of literature. J Clin Neurosci. (2018) 52:1–4. 10.1016/j.jocn.2018.03.024
108.
FinkFRischkauEButtMKleinJElingPHildebrandtH. Efficacy of an executive function intervention programme in MS: a placebo-controlled and pseudo-randomized trial. Mult Scler. (2010) 16:1148–51. 10.1177/1352458510375440
109.
MattioliFStampatoriCZanottiDParrinelloGCapraR. Efficacy and specificity of intensive cognitive rehabilitation of attention and executive functions in multiple sclerosis. J Neurol Sci. (2010) 288:101–5. 10.1016/j.jns.2009.09.024
110.
MattioliFStampatoriCScarpazzaCParrinelloGCapraR. Persistence of the effects of attention and executive functions intensive rehabilitation in relapsing remitting multiple sclerosis. Mult Scler Relat Disord. (2012) 1:168–73. 10.1016/j.msard.2012.06.004
111.
HanssenKTBeiskeAGLandrøNIHofossDHessenE. Cognitive rehabilitation in multiple sclerosis: a randomized controlled trial. Acta Neurol Scand. (2016) 133:30–40. 10.1111/ane.12420
112.
LincolnNBBradshawLEConstantinescuCSDayFDrummondAEFitzsimmonsDet al. Cognitive rehabilitation for attention and memory in people with multiple sclerosis: a randomized controlled trial (CRAMMS). Clin Rehabil. (2020) 34:229–41. 10.1177/0269215519890378
113.
KratzALBraleyTJFoxen-CraftEScottEMurphyJF IIIMurphySL. How do pain, fatigue, depressive, and cognitive symptoms relate to well-being and social and physical functioning in the daily lives of individuals with multiple sclerosis?Arch Phys Med Rehabil. (2017) 98:2160–6. 10.1016/j.apmr.2017.07.004
114.
HenryATourbahACamusGDeschampsRMailhanLCastexCet al. Anxiety and depression in patients with multiple sclerosis: the mediating effects of perceived social support. Mult Scler Relat Disord. (2019) 27:46–51. 10.1016/j.msard.2018.09.039
115.
NuechterleinKHGreenMFKernRSBaadeLEBarchDMCohenJDet al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. (2008) 165:203–13. 10.1176/appi.ajp.2007.07010042
Summary
Keywords
multiple sclerosis, empathy, theory of mind, meta-analysis, cognitive, affective
Citation
Lin X, Zhang X, Liu Q, Zhao P, Zhong J, Pan P, Wang G and Yi Z (2021) Empathy and Theory of Mind in Multiple Sclerosis: A Meta-Analysis. Front. Psychiatry 12:628110. doi: 10.3389/fpsyt.2021.628110
Received
13 November 2020
Accepted
18 March 2021
Published
09 April 2021
Volume
12 - 2021
Edited by
Philipp Kanske, Technische Universität Dresden, Germany
Reviewed by
Katja Koelkebeck, LVR Hospital Essen, Germany; Michele Poletti, Local Health Authority of Reggio Emilia, Italy
Updates
Copyright
© 2021 Lin, Zhang, Liu, Zhao, Zhong, Pan, Wang and Yi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: ZhongQuan Yi yizhongquan@163.com
This article was submitted to Social Cognition, a section of the journal Frontiers in Psychiatry
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.