- 1Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, Translational Addiction Medicine Branch, National Institute on Drug Abuse Intramural Research Program and National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research, National Institutes of Health, Baltimore, MD, United States
- 2Office of the Clinical Director, National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research, National Institutes of Health, Bethesda, MD, United States
- 3Department of Mental Health, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States
- 4Laboratory of Neurogenetics, National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research, National Institutes of Health, Rockville, MD, United States
- 5Medication Development Program, National Institute on Drug Abuse Intramural Research Program, National Institutes of Health, Baltimore, MD, United States
- 6Center for Alcohol and Addiction Studies, Department of Behavioral and Social Sciences, Brown University School of Public Health, Providence, RI, United States
- 7Division of Addiction Medicine, Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, MD, United States
- 8Department of Neuroscience, Georgetown University Medical Center, Washington, DC, United States
Comorbidity between alcohol use disorder (AUD) and other addictive and psychiatric disorders is highly prevalent and disabling; however, the underlying biological correlates are not fully understood. Leptin is a peptide hormone known for its role in energy homeostasis and food intake. Furthermore, leptin plays a key role in the activity of the hypothalamic-pituitary-adrenal (HPA) axis and of several neurotransmitter systems that regulate emotionality and behavior. However, human studies that have investigated circulating leptin levels in relation to AUD and affective disorders, such as anxiety and depression, are conflicting. Genetic-based analyses of the leptin gene (LEP) and leptin receptor gene (LEPR) have the potential of providing more insight into the potential role of the leptin system in AUD and comorbid psychopathology. The aim of the current study was to investigate whether genotypic variations at LEP and LEPR are associated with measures of alcohol use, nicotine use, anxiety, and depression, all of which represent common comorbidities with AUD. Haplotype association analyses were performed, using data from participants enrolled in screening and natural history protocols at the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Analyses were performed separately in European Americans and African Americans due to the variation in haplotype diversity for most genes between these groups. In the European American group, one LEP haplotype (EB2H4) was associated with lower odds of having a current AUD diagnosis, two LEPR haplotypes (EB7H3, EB8H3) were associated with lower cigarette pack years and two LEPR haplotypes (EB7H2, EB8H2) were associated with higher State-Trait Anxiety Inventory (STAI-T) scores. In the African American group, one LEP haplotype (AB2H8) was associated with higher cigarette pack years and one LEP haplotype (AB3H2) was associated with lower Fagerström Test for Nicotine Dependence (FTND) scores. Overall, this study found that variations in the leptin and leptin receptor genes are associated with measures of alcohol use, nicotine use, and anxiety. While this preliminary study adds support for a role of the leptin system in AUD and psychopathologies, additional studies are required to fully understand the underlying mechanisms and potential therapeutic implications of these findings.
Introduction
Alcohol use disorder (AUD) is highly prevalent in the U.S., affecting ~14.1 million adults (1). It is frequently co-morbid with other addictive and psychiatric disorders, and there is substantial evidence suggesting a bidirectional relationship between AUD and other psychopathologies (2). Intricately linked to social relationships, educational attainment, financial status, and general health outcomes, these disorders can lead to poor quality of life for both the affected individual and those close to them (3–5). Despite their high prevalence and comorbidity, the underlying biological correlates of these disorders are not fully understood.
The hormone leptin, a 167-amino acid protein encoded by the leptin gene (LEP), is primarily known for its role in regulating food intake and energy expenditure (6). Primarily secreted from white adipose tissue, leptin's effects are mediated through binding to leptin receptors expressed at the cell surface and found throughout the central (CNS) and peripheral nervous systems. In the CNS, highest levels of the leptin receptor gene (LEPR) expression are found in hypothalamic nuclei such as the paraventricular nucleus (PVN). This brain region plays a critical role in maintaining homeostasis. Furthermore, the PVN is an important driver of hypothalamic-pituitary-adrenal (HPA) responses (7–9). Abnormalities in HPA axis regulation have been implicated in the pathophysiology of anxiety, depression, and alcohol, nicotine and other substance use disorders (10–15). Furthermore, the HPA-axis-related hormones, cortisol and adrenocorticotropic hormone (ACTH), are inversely correlated with leptin levels (16). In addition to the HPA axis, leptin signals to brain regions involved in the regulation of stress, anxiety, emotion, and behavior, including the hippocampus, amygdala, substantia nigra, nucleus accumbens (NAc), and the ventral tegmental area (VTA) (17–19).
While the role of leptin in addiction remains to be fully confirmed, preclinical and clinical studies suggest that leptin may play a role in addictive behaviors and affective disorders (20, 21). Although there are some inconsistencies between studies, several have linked plasma leptin concentrations to alcohol consumption (22–24) and craving (25–30). Leptin levels have been found to be higher in individuals with alcohol dependence who are sweet preferring vs. those who are sweet averse (31). Additionally, plasma leptin levels have been found to be associated with craving of other substances such as nicotine (32, 33) and cocaine (34). Preclinical genetic studies further support a role of leptin in addictive behaviors; leptin-deficient ob/ob mice display reduced locomotor response to amphetamine, and this observation is normalized by leptin administration (19). Leptin signaling has also been shown to regulate cocaine expectancy, cocaine- conditioned reward, and cocaine seeking in rodents (35, 36). In addition to addictive behaviors, ob/ob mice exhibit increased anxiety, and treatment with leptin results in a reduction of anxiety-like behaviors (37). Wild-type mice also experienced anxiogenic-like effects after acute leptin administration (38). Interestingly, leptin administration has also been found to alleviate depressive symptoms in relevant animal models, such as tail suspension and forced swim tests (38). Collectively, these studies suggest a role for leptin in multiple psychopathologies.
While a growing body of preclinical research indicates a relationship between leptin signaling and neuropsychiatric disorders, studies in humans are limited and inconsistent, reporting higher, lower, or similar peripheral leptin levels in participants vs. controls (39–43). These inconsistencies could be due to a variety of factors, such as study length, time of day that leptin is measured, metabolic state of the participants, and/or the patient populations heterogeneity [see e.g., (31)]. An alternative approach to identify factors that may account for potential differences is to perform genetic analyses investigating the link between different components of the leptin system and behavioral outcomes. Therefore, we investigated potential associations between haplotype variation at LEP and LEPR, as well as behavioral phenotypes related to addiction, anxiety, and depression, with the aim of determining whether genetic variation in the leptin system might contribute to observable phenotypic differences in these measures.
Methods and Materials
Participants and Setting
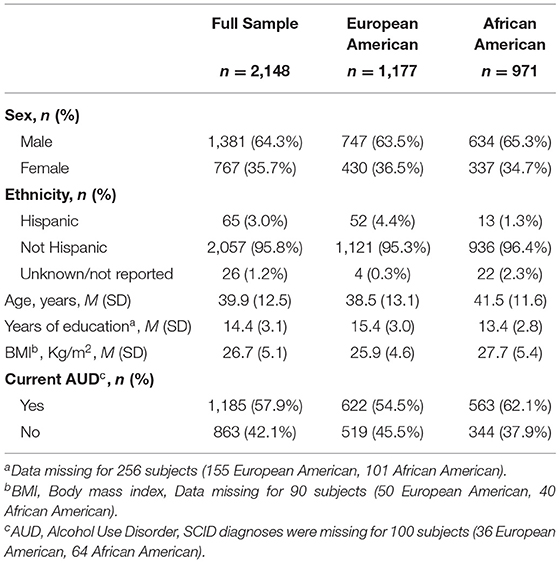
Participants included 2,148 individuals screened at the National Institutes of Health (NIH) Intramural Research Program, NIH Clinical Center (Bethesda, Maryland), for participation in research protocols at the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Study participants were screened from 2005 to 2018 under NIAAA screening and natural history protocols (98-AA-0009, 05-AA-0121, 14-AA-0181) approved by the appropriate NIH Institutional Review Board and registered at ClinicalTrials.gov (NCT00001673, NCT00106093, NCT02231840). All participants provided written informed consent for the use of their data, including behavioral and genome data. Demographic characteristics of the participants are summarized in Table 1.
Genotyping and Single Nucleotide Polymorphism Selection
Genotyping was performed at the NIAAA Laboratory of Neurogenetics on the Illumina OmniExpress BeadChip for the whole genome. The selected candidate gene regions, LEP and LEPR, were used for this study. Ancestry informative markers (AIMs) were extracted from the Illumina array and ancestral proportions were calculated for all participants (44). For quality control, participants with ≥ 3% of missing genome data were excluded from this study.
Single nucleotide polymorphisms (SNPs) covering regions from 30 Kb upstream to 30 Kb downstream of the target gene [LEP (7q32.1) and LEPR (1p31.3)] were selected. SNPs with a minor allele frequency of ≤ 0.05 and/or a call rate <90% were excluded from the analysis. Due to differences in haplotype block structure between people from European and African origin for most genes, participants were divided into European American and African American subgroups, based on self-reported race, and separate haplotype analyses were performed in each group. Three LEPR SNPs in the African American group violated Hardy-Weinberg Equilibrium but were not removed.
Haplotype Blocks and Association Analyses
Haploview (45) was used to analyze pair-wise linkage disequilibrium (LD) between all selected SNPs in the regions of LEP and LEPR. The pair-wise D′-values for the SNPs within each region were calculated using Haploview, and haplotype blocks were defined using the default D′/LOD method (45). The LD blocks are presented in Figures 1, 2. Haplotypes were generated separately for European American (n = 1,177) and African American (n = 971) groups to avoid type I error due to population stratification.
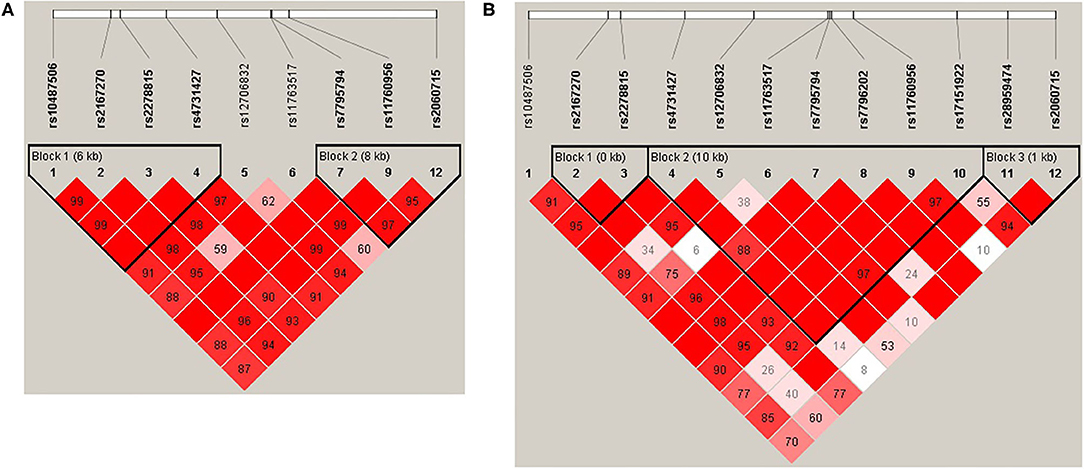
Figure 1. (A) LEP linkage disequilibrium (LD) plot for the European American group. (B) LEP LD plot for the African American group. Pairwise LD is represented as red for strong LD, blue for non-significant LD, and white for little or no LD.
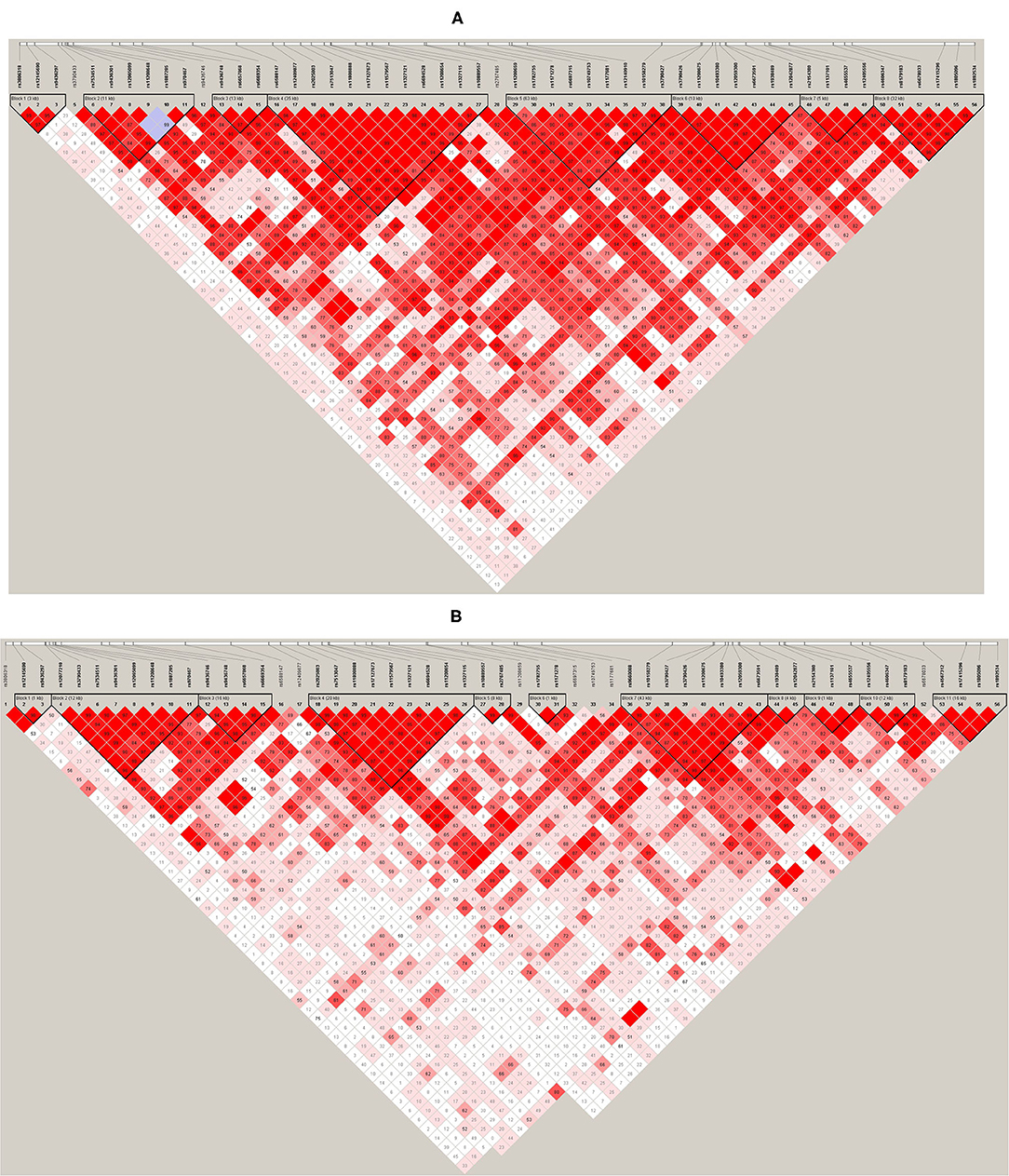
Figure 2. (A) LEPR linkage disequilibrium (LD) plot for the European American group. (B) LEPR LD plot for the African American group. Pairwise LD is represented as red for strong LD, blue for non-significant LD, and white for little or no LD.
The following assessments were selected for haplotype association analyses: (1) measures of alcohol use: current AUD from Diagnostic and Statistical Manual for Mental Disorders (DSM-IV diagnosis of either alcohol abuse or dependence and DSM-5 of AUD) (46, 47), the Alcohol Use Disorders Identification Test (AUDIT) (48), and number of heavy drinking days (defined as > 4 drinks per day for women and > 5 drink per day for men) and average drinks per drinking day as assessed by the 90-day alcohol Timeline Follow-Back (TLFB) (49); (2) measures of nicotine use: cigarette pack-years (number of cigarette packs smoked per day multiplied by the number of smoking years) and the Fagerström Test for Nicotine Dependence (FTND) (50); (3) a measure of anxiety: the trait scale from the State-Trait Anxiety Inventory (STAI-T) (51); and (4) a measure of depression: the Montgomery-Asberg Depression Rating Scale (MADRS) (52). Sample sizes for the haplotype association analyses vary between assessments due to missing data for each assessment.
Using the defined haplotype blocks, association analyses (linear or logistic regression, depending on the assessment) were then performed in PLINK v1.07 (53, 54) for each gene (LEP and LEPR) and each group (European American and African American). The p-values of each association test were corrected for multiple comparisons, using permutation tests (5,000 permutations). All models controlled for age, gender, years of education, and AIMs scores for Europe and Africa. Association analyses were performed for body mass index (BMI), and given no significant associations, BMI was not included as a covariate. A corrected p-value of < 0.05 was considered statistically significant.
Results
Haplotype Structure and Association Analyses
Haplotype structures differed between the European American and African American groups, as seen in Figures 1, 2. For LEPR, there were eight blocks in the European American group and eleven blocks in the African American group. For LEP, there were two blocks in the European American group and three blocks in the African American group.
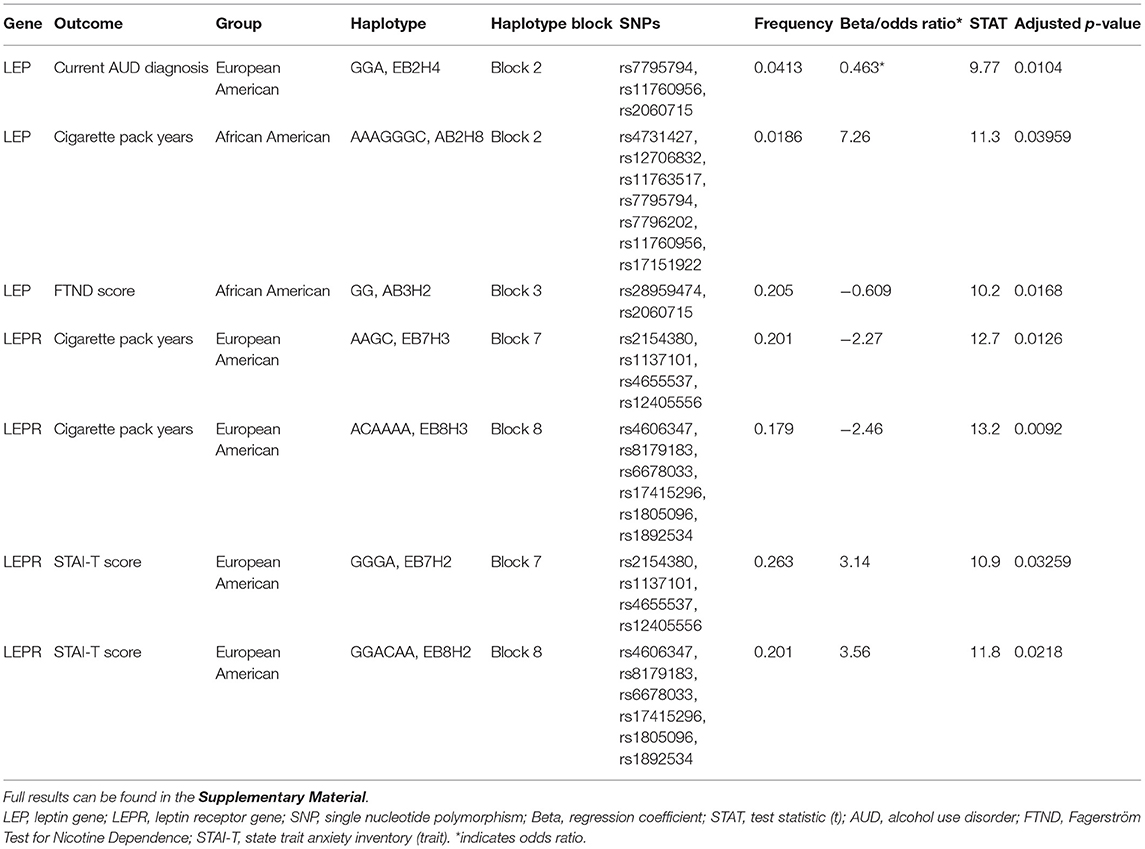
Significant results from the association analyses are displayed in Table 2 and detailed below. Full results can be found in the Supplementary Material. Briefly, a single LEP haplotype was associated with lower odds of having a current AUD diagnosis, two LEPR haplotypes were associated with lower cigarette pack years and two LEPR haplotypes were associated with higher STAI-T scores in the European American group. One LEP haplotype was associated with higher cigarette pack years and another LEP haplotype was associated with lower FTND scores in the African American group. No significant associations were found with AUDIT scores, number of heavy drinking days, average drinks per drinking day, or MADRS scores.
Leptin Gene
One LEP haplotype (EB2H4) was found to be significantly associated with lower odds of having a current AUD diagnosis in the European American group (n = 622 cases and 518 controls, p < 0.05). This haplotype was formed by rs7795794, rs11760956, and rs2060715.
One LEP haplotype (AB2H8) was significantly associated with higher cigarette pack years in the African American group (n = 848, p < 0.05). This haplotype was formed by rs4731427, rs12706832, rs11763517, rs7795794, rs7796202, rs11760956, and rs17151922. In addition, another LEP haplotype (AB3H2) was associated with lower FTND scores in the African American group (n = 404, p < 0.05). This haplotype was formed by rs28959474 and rs2060715.
Leptin Receptor Gene
Two LEPR haplotypes (EB7H3, EB8H3) were significantly associated with lower cigarette pack years in the European American group (n = 1034, p < 0.05, p < 0.01). Haplotype EB7H3 was formed by rs2154380, rs1137101, rs4655537, and rs12405556; haplotype EB8H3 was formed by rs4606347, rs8179183, rs6678033, rs17415296, rs1805096, and rs1892534.
Two LEPR haplotypes (EB7H2, EB8H2) were significantly associated with higher STAI-T scores in the European American group (n = 571, all p's < 0.05). Haplotype EB7H2 was formed by rs2154380, rs1137101 (Q223R), rs4655537, and rs12405556; and haplotype EB8H2 was formed by rs4606347, rs8179183 (K656N), rs6678033, rs17415296, rs1805096 (P1019P), and rs1892534.
Discussion
In this study, we examined whether genetic variations at LEP and LEPR are associated with AUD and measures related to commonly comorbid conditions, specifically cigarette smoking, anxiety, and depression. Our data showed that a single LEP haplotype was associated with risk for AUD in the European American group. Whilst no significant associations with AUD were found in the African American group, LEP haplotypes were significantly associated with measures of cigarette smoking. LEPR haplotypes in the European American group were also significantly associated with measures of cigarette smoking as well as anxiety.
We showed in the present study that AUD diagnosis was associated with genetic variation at the LEP gene in a European American sample. The mouse homolog of the LEP gene (Ob) was first mapped in 1995, and the structure of the human LEP gene was later described by Thompson et al., to have three exons separated by two introns (55). In the human gene, exon 1 is separated from exon 2 by a 10 kb intron (intron 1), in which the majority of SNPs in our haplotype blocks were located. We identified a haplotype in LEP (EB2H4) associated with lower odds of having a current diagnosis of AUD in the European American sample. The SNPs in this haplotype block were mostly located in intron 1 upstream from exon 2, with one variant (rs2060715) being located downstream from LEP in an intergenic region. Previous single SNP association studies have found the same SNP alleles to be associated with lower odds of breast cancer in women, bone mineral density in older men, and to be trending toward association with plasma leptin levels in post-menopausal women (56–58). Interestingly, a haplotype (AB3H2) in the African American sample contained the minor G allele at rs2060715 and was found to be associated with higher FTND score in our study, suggesting the presence of a common functional variant that may broadly contribute to addictive behaviors.
We also identified a LEP haplotype in the African American sample associated with nicotine use. In block 2, AB2H8 of the African American sample was associated with higher cigarette pack years. The SNPs in this haplotype were mainly intronic variants located in intron 1 (with the exception of one 3′ UTR variant - rs17151922). Notably, individuals with the CT genotype at rs11763517 were shown to have higher methylation levels at cg00666422, reduced performance in lung expiratory measures, and increased risk of asthma among 18-year-old subjects, particularly women (59). Other studies of these SNP alleles have identified phenotypic differences in systolic blood pressure and breast cancer in women, time to partial antidepressant treatment response, and above average olanzapine-induced weight gain in patients with schizophrenia and schizoaffective disorder (56, 58, 60). Lastly, another study reported reduced pain-induced dopamine release from the striatum and the NAc in the AA vs. GG genotype at rs12706832, which may imply decreased salience signaling to negative stimuli (61). Individuals with AA genotype at rs12706832 were additionally found to have lower LEP mRNA and protein expression, as well as lower basal and lipopolysaccharide-induced IL-6 levels, compared to individuals homozygous for the minor C allele. Our findings of increased cigarette pack years with this haplotype are interesting given that previous studies have found the individual SNPs in these haplotypes to be associated with lung function, dopamine signaling, and leptin secretion, which renders further investigation into the potential interaction between these identified phenotypes.
In addition to the leptin gene, we also identified haplotypes associated with cigarette use in the leptin receptor gene. The LEPR gene has an unusually complicated organization given that the proximal promoter produces two distinct transcripts through alternative splicing, the LEPR transcript and leptin receptor overlapping transcript (LEPROT, also known as endospanin-1) (62). LEPROT overlaps with the 5′ end of LEPR and appears to play a role in leptin receptor trafficking, cell surface expression, and leptin resistance. Additionally, the human LEPR gene itself can produce 4 isoforms through alternative splicing which vary in their C-terminal length and, thus, ability to activate downstream pathways (63). The haplotypes that we found to be associated with cigarette pack years were located in blocks 7 and 8, which contained SNPs spanning from intron 6 to the 3′ UTR end of LEPR (EB7H3 and EB8H3). EB7H3 and EB8H3 were both associated with lower cigarette pack years in the European American group. EB7H3 contains a known coding sequence variant, rs1137101 (A to G transition), that results in a glutamine to arginine substitution (Q223R) at the Corticotropin Releasing Hormone-1 (CRH1) domain of the leptin receptor. While molecular dynamic studies suggest that arginine substitution affects leptin receptor conformation (64), investigations into phenotypic differences resulting from this substitution have produced mixed findings. It is unlikely that our findings are the result of this amino acid substitution, given that another haplotype containing rs1137101 in block 7 (EB7H1) coding for the same amino acid was not associated with cigarette pack years. However, other studies of SNPs in EB7H3 have identified associations with C-reactive protein (CRP) levels and plasma leptin levels in women, soluble leptin receptor levels, E. histolytica infection in children, luminal A breast cancer, tolerance for exercise intensity, and exercise participation (56, 65–70). EB8H3 of LEPR also contained a missense variant, rs8179183 (G/C), which results in a lysine to asparagine (K656N) amino acid substitution and a negative to neutral charge change in the membrane-proximal extracellular region of the leptin receptor. Interestingly, in our analysis of cigarette pack years, haplotype EB8H3 was the only haplotype in block 8 containing the minor C allele resulting in the 656N protein, however the functional consequence of this protein, if any, remains unknown. Regarding the other SNPs in this haplotype, functional consequences identified to date include associations with plasma CRP and fibrinogen, osteoporosis, age of menarche, fasting glucose levels, early onset type II diabetes mellitus, and cancer (67, 71–77).
We identified two LEPR haplotypes in the European American sample that were associated with anxiety, as indicated by STAI-T score. These haplotypes were located in blocks 7 and 8 and contained SNPs spanning from intron 6 to the 3′ UTR region (EB7H2, EB8H2). Interestingly, we identified haplotypes in blocks 7 and 8 of LEPR in the European American sample that were also associated with lower cigarette pack years (EB7H3, EB8H3). Here, it is unlikely that the missense mutations coded for by haplotypes found in blocks 7 (EB7H2; 223R) and 8 (EB8H2; K656) underlie our observed associations with anxiety, as other haplotypes coding for the same amino acid change were not significantly associated with STAI-T score. Previous single SNP association studies have found the SNP alleles in these haplotypes to be associated with plasma leptin and soluble leptin receptor levels, plasma CRP, and other diseases, including breast cancer and diabetes mellitus, suggesting that further evaluation of the potential interaction between these phenotypes is needed. The role of leptin in anxiety remains to be fully understood. One study found no difference in leptin levels between children with anxiety disorders and controls (78). In adult participants, low cerebrospinal fluid (CSF) leptin in females who recently attempted suicide was associated with higher anxiety, while patients who had moderate to severe anxiety had a higher free leptin index, and leptin was positively associated with anxiety in overweight women (43, 79, 80). Our results add to this literature by suggesting that genetic variation at LEPR may play a role in anxiety disorders. Lastly, although previous literature has found some relationship between leptin and depression, we found no LEP or LEPR haplotype associations with MADRS scores.
We report here that genetic variation at LEP and LEPR was associated with AUD diagnosis, cigarette use, and anxiety. Numerous papers have identified a relationship between leptin and alcohol-related measures [for a recent review, see: (20)], wherein leptin appears to be negatively associated with alcohol craving in current drinkers, and an increase in leptin following alcohol abstinence appears to be positively associated with alcohol craving. Here, we identified only one LEP haplotype to be associated with a diagnosis of current AUD in the European American group but found no relationship with TLFB measures of alcohol drinking or AUDIT score. The majority of studies on the relationship between leptin and nicotine typically report the effect of nicotine use on leptin, given that nicotine is known to affect body weight, rather than the effect of leptin on nicotine craving and dependence. However, several studies document a direct relationship between leptin and nicotine craving during abstinence, and another study identified that the magnitude of increase in peripheral leptin, during early phases of withdrawal, is negatively associated with risk of smoking relapse (32, 81, 82). Our findings further suggest a role for the leptin system in nicotine use, as we identified two LEP haplotypes to be associated with nicotine dependence and cigarette pack years in the African American group.
Our identified haplotype associations with substance use and anxiety measures, along with findings from previous literature (20, 21, 32, 81–85) suggest that genetic variations within components of the leptin system (peptide/receptor) may be linked to these psychological and behavioral outcomes. These associations raise interesting questions about how anxiety may be linked to leptin and smoking, however, the causative effects of these associations cannot be delineated. Mechanistic studies are needed to understand the biological meaning and underpinnings of these findings. This notion should also be further explored, as the associations observed in this study were not consistent across measures, nor between groups (European American and African American). Additionally, although our sample size was large, replication of these findings in an independent sample and future studies with more comprehensive genetic approaches are imperative, given the limitations of candidate gene analyses, such as the possibility of false positive results. Finally, although this approach does allow for studying a more stable element of the leptin system, it remains unclear how these findings relate to functionality in leptin signaling activation via the peptide and/or the receptor, therefore causality cannot be implied.
In summary, our results suggest that LEP and LEPR are linked to some measures of substance use and anxiety. The mechanisms and casual factors underlying these behaviors include a complex combination of genetic and environmental factors; therefore, much additional work is needed to parse out the potential role of the leptin system in this regard.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The dataset is managed by and housed within the NIAAA Office of the Clinical Director. Requests to access these datasets should be directed to Melanie L. Schwandt, bWVsYW5pZXNAbWFpbC5uaWg=.gov.
Ethics Statement
The studies involving human participants were reviewed and approved by NIH Addictions Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
BB: data curation, formal analysis, methodology, and project administration. MS: data curation, formal analysis, investigation, methodology, project administration, resources, and supervision. MF and SD: formal analysis, investigation, and methodology. CH: data curation, formal analysis, investigation, and methodology. LL: conceptualization, investigation, methodology, project administration, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the intramural research programs of the National Institute on Alcohol Abuse and Alcoholism (BB, MS, MF, SD, CH, and LL) and the National Institute on Drug Abuse (BB, MF, SD, and LL). The funding organizations did not have any role in the study design, execution, or interpretation of the results.
Author Disclaimer
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the clinical, research, and technical staff involved in patient care and in data collection and in generating the clinical and human genetic data in the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism (NIAAA), in particular staff in the NIAAA Office of the Clinical Director, the NIAAA Clinical Core Laboratory and the NIAAA Laboratory of Neurogenetics. Furthermore, the authors would like to thank the clinical and other staff at the NIH Clinical Center.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.723059/full#supplementary-material
References
1. SAMHSA. National Survey on Drug Use and Health (NSDUH). Table 5.4A-Alcohol Use Disorder in Past Year among Persons Aged 12 or Older, by Age Group and Demographic Characteristics: Numbers in Thousands, 2018 and 2019. (2019). Available online at: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2018R2/NSDUHDetTabsSect5pe2018.htm#tab5-4a (accessed April 7, 2021).
2. Castillo-Carniglia A, Keyes KM, Hasin DS, Cerdá M. Psychiatric comorbidities in alcohol use disorder. Lancet Psychiatry. (2019) 6:1068–80. doi: 10.1016/S2215-0366(19)30222-6
3. Marazziti D, Dell'osso B, Catena Dell'Osso M, Consoli G, Del Debbio A, Mungai F, et al. Romantic attachment in patients with mood and anxiety disorders. CNS Spectrums. (2007) 12:751–6. doi: 10.1017/S1092852900015431
4. Esch P, Bocquet V, Pull C, Couffignal S, Lehnert T, Graas M, et al. The downward spiral of mental disorders and educational attainment: a systematic review on early school leaving. BMC Psychiatry. (2014) 14:237. doi: 10.1186/s12888-014-0237-4
5. Sareen J, Afifi TO, McMillan KA, Asmundson GJG. Relationship between household income and mental disorders: findings from a population-based longitudinal study. Arch Gen Psychiatry. (2011) 68:419–27. doi: 10.1001/archgenpsychiatry.2011.15
7. Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. (1998) 395:535–47. doi: 10.1002/(SICI)1096-9861(19980615)395:4<535::AID-CNE9>3.0.CO;2-2
8. Meister B. Control of food intake via leptin receptors in the hypothalamus. Vitam Hormones. (2000) 59:265–304. doi: 10.1016/S0083-6729(00)59010-4
9. Herman JP, Tasker JG. Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front Endocrinol (Lausanne). (2016) 7:137. doi: 10.3389/fendo.2016.00137
10. Buitelaar JK. The role of the HPA-axis in understanding psychopathology: cause, consequence, mediator, or moderator? Eur Child Adolesc Psychiatry. (2013) 22:387–9. doi: 10.1007/s00787-013-0441-7
11. Watson S, Mackin P. HPA axis function in mood disorders. Psychiatry. (2006) 5:166–70. doi: 10.1383/psyt.2006.5.5.166
12. Carlo F, Carolina Lo S, Lorenzo L, Francesco P, Lisa L, Lucia G, et al. The role of life events and HPA axis in anxiety disorders: a review. Curr Pharm Design. (2012) 18:5663–74. doi: 10.2174/138161212803530907
13. Stephens MAC, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. (2012) 34:468–83.
14. Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. (2017) 22:527–36. doi: 10.1038/mp.2016.120
15. Rohleder N, Kirschbaum C. The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers. Int J Psychophysiol. (2006) 59:236–43. doi: 10.1016/j.ijpsycho.2005.10.012
16. Licinio J, Mantzoros C, Negrão AB, Cizza G, Wong ML, Bongiorno PB, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. (1997) 3:575–9. doi: 10.1038/nm0597-575
17. Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. (2009) 297:E1247–59. doi: 10.1152/ajpendo.00274.2009
18. Valleau JC, Sullivan EL. The impact of leptin on perinatal development and psychopathology. J Chem Neuroanat. (2014) 61–62:221–32. doi: 10.1016/j.jchemneu.2014.05.001
19. Fulton S, Pissios P, Manchon Ramon P, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. (2006) 51:811–22. doi: 10.1016/j.neuron.2006.09.006
20. Bach P, Koopmann A, Kiefer F. The impact of appetite-regulating neuropeptide leptin on alcohol use, alcohol craving and addictive behavior: a systematic review of preclinical and clinical data. Alcohol Alcohol. (2021) 56:149–65. doi: 10.1093/alcalc/agaa044
21. Zarouna S, Wozniak G, Papachristou AI. Mood disorders: a potential link between ghrelin and leptin on human body? World J Exp Med. (2015) 5:103–9. doi: 10.5493/wjem.v5.i2.103
22. Obradovic T, Meadows GG. Chronic ethanol consumption increases plasma leptin levels and alters leptin receptors in the hypothalamus and the perigonadal fat of C57BL/6 mice. Alcohol Clin Exp Res. (2002) 26:255–62. doi: 10.1111/j.1530-0277.2002.tb02532.x
23. Otaka M, Konishi N, Odashima M, Jin M, Wada I, Matsuhashi T, et al. Effect of alcohol consumption on leptin level in serum, adipose tissue, and gastric mucosa. Digest Dis Sci. (2007) 52:3066–9. doi: 10.1007/s10620-006-9635-x
24. Pravdova E, Macho L, Fickova M. Alcohol intake modifies leptin, adiponectin and resistin serum levels and their mRNA expressions in adipose tissue of rats. Endocr Regul. (2009) 43:117–25.
25. Haass-Koffler CL, Aoun EG, Swift RM, de la Monte SM, Kenna GA, Leggio L. Leptin levels are reduced by intravenous ghrelin administration and correlated with cue-induced alcohol craving. Transl Psychiatry. (2015) 5:e646. doi: 10.1038/tp.2015.140
26. Bach P, Bumb JM, Schuster R, Vollstädt-Klein S, Reinhard I, Rietschel M, et al. Effects of leptin and ghrelin on neural cue-reactivity in alcohol addiction: two streams merge to one river? Psychoneuroendocrinology. (2019) 100:1–9. doi: 10.1016/j.psyneuen.2018.09.026
27. Kiefer F, Jahn H, Otte C, Demiralay C, Wolf K, Wiedemann K. Increasing leptin precedes craving and relapse during pharmacological abstinence maintenance treatment of alcoholism. J Psychiatr Res. (2005) 39:545–51. doi: 10.1016/j.jpsychires.2004.11.005
28. Kiefer F, Jahn H, Jaschinski M, Holzbach R, Wolf K, Naber D, et al. Leptin: a modulator of alcohol craving? Biol Psychiatry. (2001) 49:782–7. doi: 10.1016/S0006-3223(01)01081-2
29. Kraus T, Reulbach U, Bayerlein K, Mugele B, Hillemacher T, Sperling W, et al. Leptin is associated with craving in females with alcoholism. Addict Biol. (2004) 9:213–9. doi: 10.1111/j.1369-1600.2004.tb00535.x
30. Hillemacher T, Bleich S, Frieling H, Schanze A, Wilhelm J, Sperling W, et al. Evidence of an association of leptin serum levels and craving in alcohol dependence. Psychoneuroendocrinology. (2007) 32:87–90. doi: 10.1016/j.psyneuen.2006.09.013
31. Bouhlal S, Farokhnia M, Lee MR, Akhlaghi F, Leggio L. Identifying and characterizing subpopulations of heavy alcohol drinkers via a sucrose preference test: a sweet road to a better phenotypic characterization? Alcohol Alcohol. (2018) 53:560–9. doi: 10.1093/alcalc/agy048
32. von der Goltz C, Koopmann A, Dinter C, Richter A, Rockenbach C, Grosshans M, et al. Orexin and leptin are associated with nicotine craving: a link between smoking, appetite and reward. Psychoneuroendocrinology. (2010) 35:570–7. doi: 10.1016/j.psyneuen.2009.09.005
33. al'Absi M, Hooker S, Fujiwara K, Kiefer F, von der Goltz C, Cragin T, et al. Circulating leptin levels are associated with increased craving to smoke in abstinent smokers. Pharmacol Biochem Behav. (2011) 97:509–13. doi: 10.1016/j.pbb.2010.10.004
34. Martinotti G, Montemitro C, Baroni G, Andreoli S, Alimonti F, Di Nicola M, et al. Relationship between craving and plasma leptin concentrations in patients with cocaine addiction. Psychoneuroendocrinology. (2017) 85:35–41. doi: 10.1016/j.psyneuen.2017.08.004
35. Shen M, Jiang C, Liu P, Wang F, Ma L. Mesolimbic leptin signaling negatively regulates cocaine-conditioned reward. Translational Psychiatry. (2016) 6:e972. doi: 10.1038/tp.2016.223
36. You ZB, Wang B, Liu QR, Wu Y, Otvos L, Wise RA. Reciprocal inhibitory interactions between the reward-related effects of leptin and cocaine. Neuropsychopharmacology. (2016) 41:1024–33. doi: 10.1038/npp.2015.230
37. Asakawa A, Inui A, Inui T, Katsuura G, Fujino M, Kasuga M. Leptin treatment ameliorates anxiety in ob/ob obese mice. J Diab Compl. (2003) 17:105–7. doi: 10.1016/S1056-8727(02)00185-X
38. Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu X-Y. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology. (2010) 207:535–45. doi: 10.1007/s00213-009-1684-3
39. Deuschle M, Blum WF, Englaro P, Schweiger U, Weber B, Pflaum CD, et al. Plasma leptin in depressed patients and healthy controls. Horm Metab Res. (1996) 28:714–7. doi: 10.1055/s-2007-979885
40. Kraus T, Haack M, Schuld A, Hinze-Selch D, Pollmacher T. Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neuroendocrinology. (2001) 73:243–7. doi: 10.1159/000054641
41. Lawson EA, Miller KK, Blum JI, Meenaghan E, Misra M, Eddy KT, et al. Leptin levels are associated with decreased depressive symptoms in women across the weight spectrum, independent of body fat. Clin Endocrinol (Oxf). (2012) 76:520–5. doi: 10.1111/j.1365-2265.2011.04182.x
42. Antonijevic IA, Murck H, Frieboes RM, Horn R, Brabant G, Steiger A. Elevated nocturnal profiles of serum leptin in patients with depression. J Psychiatr Res. (1998) 32:403–10. doi: 10.1016/S0022-3956(98)00032-6
43. Cernea S, Both E, Hutanu A, Sular FL, Roiban AL. Correlations of serum leptin and leptin resistance with depression and anxiety in patients with type 2 diabetes. Psychiatry Clin Neurosci. (2019) 73:745–53. doi: 10.1111/pcn.12922
44. Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, et al. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. (2008) 43:505–15. doi: 10.1093/alcalc/agn032
45. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics (Oxford, England). (2005) 21:263–5. doi: 10.1093/bioinformatics/bth457
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association (2013).
47. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association (2000).
48. Babor TF, Kranzler HR, Lauerman RJ. Early detection of harmful alcohol consumption: comparison of clinical, laboratory, and self-report screening procedures. Addict Behav. (1989) 14:139–57. doi: 10.1016/0306-4603(89)90043-9
49. Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press (1992). p. 41–72.
50. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The fagerström test for nicotine dependence: a revision of the fagerström tolerance questionnaire. Br J Addict. (1991) 86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x
51. Spielberger CD. Manual for the State-Trait Inventory STAI (Form Y). Palo Alto, CA: Mind Garden (1983).
52. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. (1979) 134:382–9. doi: 10.1192/bjp.134.4.382
53. Purcell S. PLINK (v 1.07). Available online at: http://zzz.bwh.harvard.edu/plink/ (accessed May 27, 2021).
54. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. (2007) 81:559–75. doi: 10.1086/519795
55. Bruce Thompson D, Ravussin E, Bennett PH, Bogardus C. Structure and sequence variation at the human leptin receptor gene in lean and obese pima Indians. Human Mol Genet. (1997) 6:675–9. doi: 10.1093/hmg/6.5.675
56. Nyante SJ, Gammon MD, Kaufman JS, Bensen JT, Lin DY, Barnholtz-Sloan JS, et al. Common genetic variation in adiponectin, leptin, and leptin receptor and association with breast cancer subtypes. Breast Cancer Res Treat. (2011) 129:593–606. doi: 10.1007/s10549-011-1517-z
57. Zmuda JM, Yerges-Armstrong LM, Moffett SP, Klei L, Kammerer CM, Roeder K, et al. Genetic analysis of vertebral trabecular bone density and cross-sectional area in older men. Osteoporos Int. (2011) 22:1079–90. doi: 10.1007/s00198-010-1296-0
58. Ma D, Feitosa MF, Wilk JB, Laramie JM, Yu K, Leiendecker-Foster C, et al. Leptin is associated with blood pressure and hypertension in women from the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension. (2009) 53:473–9. doi: 10.1161/HYPERTENSIONAHA.108.118133
59. Mukherjee N, Lockett GA, Merid SK, Melén E, Pershagen G, Holloway JW, et al. DNA methylation and genetic polymorphisms of the Leptin gene interact to influence lung function outcomes and asthma at 18 years of age. Int J Mol Epidemiol Genet. (2016) 7:1–17.
60. Kloiber S, Ripke S, Kohli MA, Reppermund S, Salyakina D, Uher R, et al. Resistance to antidepressant treatment is associated with polymorphisms in the leptin gene, decreased leptin mRNA expression, and decreased leptin serum levels. Eur Neuropsychopharmacol. (2013) 23:653–62. doi: 10.1016/j.euroneuro.2012.08.010
61. Burghardt PR, Love TM, Stohler CS, Hodgkinson C, Shen PH, Enoch MA, et al. Leptin regulates dopamine responses to sustained stress in humans. J Neurosci. (2012) 32:15369–76. doi: 10.1523/JNEUROSCI.2521-12.2012
62. Bailleul B, Akerblom I, Strosberg AD. The leptin receptor promoter controls expression of a second distinct protein. Nucleic Acids Res. (1997) 25:2752–8. doi: 10.1093/nar/25.14.2752
63. Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. (1996) 93:8374–8. doi: 10.1073/pnas.93.16.8374
64. Daghestani M, Purohit R, Daghestani M, Daghistani M, Warsy A. Molecular dynamic (MD) studies on Gln233Arg (rs1137101) polymorphism of leptin receptor gene and associated variations in the anthropometric and metabolic profiles of Saudi women. PLoS ONE. (2019) 14:e0211381. doi: 10.1371/journal.pone.0211381
65. Ridker PM, Pare G, Parker A, Zee RY, Danik JS, Buring JE, et al. Loci related to metabolic-syndrome pathways including LEPR,HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women's Genome Health Study. Am J Hum Genet. (2008) 82:1185–92. doi: 10.1016/j.ajhg.2008.03.015
66. Duggal P, Guo X, Haque R, Peterson KM, Ricklefs S, Mondal D, et al. A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J Clin Invest. (2011) 121:1191–8. doi: 10.1172/JCI45294
67. Ortega-Azorín C, Coltell O, Asensio EM, Sorlí JV, González JI, Portolés O, et al. Candidate gene and genome-wide association studies for circulating leptin levels reveal population and sex-specific associations in high cardiovascular risk Mediterranean subjects. Nutrients. (2019) 11:2751. doi: 10.3390/nu11112751
68. Flack K, Pankey C, Ufholz K, Johnson L, Roemmich JN. Genetic variations in the dopamine reward system influence exercise reinforcement and tolerance for exercise intensity. Behav Brain Res. (2019) 375:112148. doi: 10.1016/j.bbr.2019.112148
69. Sun Q, Cornelis MC, Kraft P, Qi L, van Dam RM, Girman CJ, et al. Genome-wide association study identifies polymorphisms in LEPR as determinants of plasma soluble leptin receptor levels. Hum Mol Genet. (2010) 19:1846–55. doi: 10.1093/hmg/ddq056
70. De Moor MH, Liu YJ, Boomsma DI, Li J, Hamilton JJ, Hottenga JJ, et al. Genome-wide association study of exercise behavior in Dutch and American adults. Med Sci Sports Exerc. (2009) 41:1887–95. doi: 10.1249/MSS.0b013e3181a2f646
71. Mahajan A, Tabassum R, Chavali S, Dwivedi OP, Chauhan G, Ghosh S, et al. Common variants in CRP and LEPR influence high sensitivity C-reactive protein levels in North Indians. PLoS ONE. (2011) 6:e24645. doi: 10.1371/journal.pone.0024645
72. Liao WL, Chen CC, Chang CT, Wu JY, Chen CH, Huang YC, et al. Gene polymorphisms of adiponectin and leptin receptor are associated with early onset of type 2 diabetes mellitus in the Taiwanese population. Int J Obes (Lond). (2012) 36:790–6. doi: 10.1038/ijo.2011.174
73. Wu Y, McDade TW, Kuzawa CW, Borja J, Li Y, Adair LS, et al. Genome-wide association with C-reactive protein levels in CLHNS: evidence for the CRP and HNF1A loci and their interaction with exposure to a pathogenic environment. Inflammation. (2012) 35:574–83. doi: 10.1007/s10753-011-9348-y
74. Heikkilä K, Silander K, Salomaa V, Jousilahti P, Koskinen S, Pukkala E, et al. C-reactive protein-associated genetic variants and cancer risk: findings from FINRISK 1992, FINRISK 1997 and Health 2000 studies. Eur J Cancer. (2011) 47:404–12. doi: 10.1016/j.ejca.2010.07.032
75. Curocichin G, Wu Y, McDade TW, Kuzawa CW, Borja JB, Qin L, et al. Single-nucleotide polymorphisms at five loci are associated with C-reactive protein levels in a cohort of Filipino young adults. J Hum Genet. (2011) 56:823–7. doi: 10.1038/jhg.2011.106
76. Gajdos ZK, Butler JL, Henderson KD, He C, Supelak PJ, Egyud M, et al. Association studies of common variants in 10 hypogonadotropic hypogonadism genes with age at menarche. J Clin Endocrinol Metab. (2008) 93:4290–8. doi: 10.1210/jc.2008-0981
77. Lin CC, Li TC, Liu CS, Yang CW, Lin CH, Hsiao JH, et al. Associations of TNF-α and IL-6 polymorphisms with osteoporosis through joint effects and interactions with LEPR gene in Taiwan: Taichung Community Health Study for Elders (TCHS-E). Mol Biol Rep. (2016) 43:1179–91. doi: 10.1007/s11033-016-4037-4
78. Ozmen S, Seker A, Demirci E. Ghrelin and leptin levels in children with anxiety disorders. J Pediatr Endocrinol Metab. (2019) 32:1043–7. doi: 10.1515/jpem-2019-0229
79. Ambrus L, Westling S. Leptin, Anxiety symptoms, and hypothalamic-pituitary-adrenal axis activity among drug-free, female suicide attempters. Neuropsychobiology. (2019) 78:145–52. doi: 10.1159/000500737
80. Naufel MF, Boldarine VT, Oyama LM, do Nascimento CMO, Silva Dos Santos GM, Hachul H, et al. Age and leptinemia association with anxiety and depression symptoms in overweight middle-aged women. Menopause. (2019) 26:317–24. doi: 10.1097/GME.0000000000001210
81. Gomes Ada S, Toffolo MC, Keulen HV, Castro e Silva FM, Ferreira AP, Luquetti SC, et al. Influence of the leptin and cortisol levels on craving and smoking cessation. Psychiatry Res. (2015) 229:126–32. doi: 10.1016/j.psychres.2015.07.060
82. Lemieux A, Nakajima M, Hatsukami DK, Allen S, al'Absi M. Changes in circulating leptin levels during the initial stage of cessation are associated with smoking relapse. Psychopharmacology (Berl). (2015) 232:3355–61. doi: 10.1007/s00213-015-3989-8
83. Matarese G. Leptin and the immune system: how nutritional status influences the immune response. Eur Cytokine Netw. (2000) 11:7–14.
84. Pérez-Pérez A, Vilariño-García T, Fernández-Riejos P, Martín-González J, Segura-Egea JJ, Sánchez-Margalet V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. (2017) 35:71–84. doi: 10.1016/j.cytogfr.2017.03.001
Keywords: leptin, LEP, LEPR, alcohol, nicotine, anxiety
Citation: Browning BD, Schwandt ML, Farokhnia M, Deschaine SL, Hodgkinson CA and Leggio L (2021) Leptin Gene and Leptin Receptor Gene Polymorphisms in Alcohol Use Disorder: Findings Related to Psychopathology. Front. Psychiatry 12:723059. doi: 10.3389/fpsyt.2021.723059
Received: 09 June 2021; Accepted: 12 July 2021;
Published: 06 August 2021.
Edited by:
Estelle Barbier, Linköping University, SwedenCopyright © 2021 Browning, Schwandt, Farokhnia, Deschaine, Hodgkinson and Leggio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lorenzo Leggio, bG9yZW56by5sZWdnaW9AbmloLmdvdg==
 Brittney D. Browning
Brittney D. Browning Melanie L. Schwandt
Melanie L. Schwandt Mehdi Farokhnia
Mehdi Farokhnia Sara L. Deschaine1
Sara L. Deschaine1 Lorenzo Leggio
Lorenzo Leggio