- 1Department of Psychiatry, National Clinical Research Center for Mental Disorders, The Second Xiangya Hospital of Central South University, Changsha, China
- 2Hunan Key Laboratory of Psychiatry and Mental Health, Mental Health Institute of Central South University, China National Technology Institute on Mental Disorders, Hunan Technology Institute of Psychiatry, Changsha, Hunan, China
- 3Department of Radiology, Hunan Children’s Hospital, Changsha, China
Background: Dysregulation of immunity, such as levels of inflammatory factors, has been regarded as a sign of schizophrenia. Changes in cytokine levels are not only described in the early onset of disease, but also observed in ultra-high risk (UHR) individuals. This study aimed to investigate the potential of cytokines as biomarkers for psychotic disorders and in individuals at UHR of developing a psychotic disorder in the future.
Methods: The Luminex liquid chip technology was used to detect the concentrations of Interferon-gamma (INF-γ), Interleukin (IL)-2, Interleukin (IL)-4, Interleukin (IL)-6, Interleukin (IL)-17, Interleukin-1beta (IL-1β), and Tumor Necrosis Factor-beta (TNF-β) in the plasma of all subjects. Meanwhile, the plasma level of Tumor Necrosis Factor-Alpha (TNF-α) was measured with the enzyme-linked immunosorbent assay (ELISA) kits. Then, the levels of these cytokines were compared among patients with Drug-naïve first-episode schizophrenia (FES; n = 40), UHR population (UHR; n = 49), and healthy controls (HCs; n = 30). Baseline cytokine levels were compared among UHR individuals who later transitioned (UHR-T; n = 14), those who did not transition (UHR-NT; n = 35), and HCs (n = 30).
Results: Our analysis results showed that IL-1β levels were significantly higher in UHR group than HC group (p = 0.015). Meanwhile, TNF-α concentration was significantly increased in FES group compared with HC group (p = 0.027). IL-17 (p = 0.04) and TNF-β (p = 0.008) levels were significantly higher in UHR-T group compared with UHR-NT group.
Conclusion: In conclusion, our findings suggest that the immuno-inflammatory activation level is increased in the early stage of psychosis before psychotic conversion and the Drug-naïve FES. IL-1β and TNF-α are the representatives of the specific biomarkers for UHR and FES, respectively. IL-17 and TNF-β may be the potential selective predictive biomarkers for future transition in UHR individuals.
1 Introduction
Schizophrenia is a severe psychiatric disorder that severely impairs an individual’s social function, and it can induce disability and high expenses for patients for many years (1, 2). Schizophrenia is characterized by the intense clusters of symptoms, positive and negative symptoms, cognitive deficits, and both affective and aggressive clusters of symptoms (3). Early diagnosis and intervention of schizophrenia is the key to improving the clinical and functional outcomes of the disease (4). Over the last few decades, increasing attention has been paid to people presenting with potentially prodromal symptoms of psychosis (5, 6). It is reported in some research that, approximately 30% of the ultra-high risk (UHR) population will transition to psychosis within 2 years of follow-up (7–9). Therefore, identifying the biomarkers with convenient detection methods and clinical applications has become the focus and challenge of the current research.
The etiology of schizophrenia, though it remains unclear, is hypothesized to be related to the dysfunction of neurotransmitters and neurodevelopment (10, 11). The recent new theories of biological contributors to psychosis tend to concentrate on the role of immune dysfunction (12–14). Molecular genetic studies show that the histocompatibility complex (MHC) is closely associated with schizophrenia. The MHC gene, which is located on chromosome 6, is tightly linked to immunity (15). Sekar et al. demonstrated the impact of a particular mutation of the Complement 4 (C4) gene, which produced the complement protein C4, on synaptic pruning during the crucial stages of brain development, thus increasing the risk of schizophrenia (16). Evidence has identified aberrant immune function not only in the earliest stage of illness, but also before the onset of the disease (17). Some studies indicate that adjuvant treatment with immunomodulatory agents may be beneficial for improving clinical symptoms and social function (18–20). These results provide solid evidence that immunological dysfunction is a key player in the pathophysiology and etiology of psychiatric disorders.
Various cytokines have important functions in both adaptive and innate immune responses. Specifically, the key cytokines related to innate immunity include interleukin-1 beta (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-Alpha (TNF-α), interferon- gamma (IFN-γ), interleukin-2 (IL-2), interleukin-8 (IL-8), interleukin-12 (IL-12), and tumor necrosis factor-beta (TNF-β) from T helper-1 (Th-1) cells, whereas the major cytokines involved in adaptive immunity are interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-10 (IL-10) from T helper-2 (Th-2) cells (21, 22). Among them, IFN-γ, TNF-β, and IL-6 may be vital for the activation of Th-1 immunity and the subsequent inflammatory response. The main component of the Compensatory Immune-Regulatory Reflex System (CIRS), IL-4 (the cytokine produced by Th-2 cells), may have immune-regulatory effects (23). Abnormal cytokine levels have already been identified in first-episode psychosis (FEP) (24–26). Moreover, the interleukin-17 (IL-17) axis plays crucial roles in the pathogenesis of several mental disorders (27). As reported in a recent meta-analysis, the levels of IL-6, TNF-α, IFN-γ, IL-17, and transforming growth factor-beta (TGF-β) elevate in FEP compared with healthy controls (HCs) (28). In addition, several studies have demonstrated the systemic alterations of cytokine levels in UHR group, while the results remain controversial (29). A meta-analysis of studies on inflammatory biomarkers reports that IL-6 level increases in UHR group compared with control group, while interleukin-1β (IL-1β) level decreases in UHR group compared with control group (30). As not all UHR individuals will develop psychosis, it is imperative to identify inflammatory biomarkers that are significantly up-regulated in UHR individuals who later develop psychosis (UHR-T) (31). In a recent meta-analysis, the baseline levels of IL-1β, IL-7, IL-8, matrix metalloproteinase (MMP)-8, cortisol, albumin, and salivary cortisol are measured as predictors for UHR-T vs. UHR individuals who later did not transition (UHR-NT) (32). However, another research exhibits an unimportant trend for the higher blood IL-12, IL-1β, and IL-6 levels in converters vs. non-converters (33). The results are inconsistent due to cytokine alterations and are widely affected by potential confounders, including antipsychotics, smoking, and assay methodology.
Some relevant studies have been reported, but they are associated with some limitations, like the small number of cytokine types involved and the focus on a particular stage of schizophrenia. Moreover, many study subjects have received medications, while drug interference with inflammatory factors cannot be excluded. Based on the previously published studies, the present study tested the blood cytokine levels in UHR, Drug-naïve first-episode schizophrenia (FES), and HC groups. Thereafter, these cytokine concentrations were compared between UHR-T and UHR-NT subjects through a 2-year follow-up, aiming to identify cytokines as the potential biomarkers for high-risk psychosis and transition to psychotic disorder. It was hypothesized that pro-inflammatory analytes were up-regulated in early psychosis subjects than in controls and in UHR-T group than in UHR-NT group. Moreover, this study also attempted to determine whether specific changes of cytokine levels in UHR might predict the transition to psychosis.
2 Materials and methods
2.1 Participants
In this study, FES and UHR subjects were recruited from the outpatient clinics and inpatient units of the Second Xiangya Hospital of Central South University, whereas HC subjects were from the local community in the same region through advertisements. FES subjects developed acute psychosis. All participants were between 13 and 30 years old. Every participant received structured interviews conducted by experienced psychiatrists. This study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University and performed following the Declaration of Helsinki. All participants were aware of the risks and benefits of this study. Written informed consent was obtained from each subject.
The inclusion criteria for FES were the same as the diagnostic standard of schizophrenia from the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) (34). Using the Positive and Negative Symptom Scale (PANSS), the symptoms of FES patients were assessed (35). The Structured Interview was adopted for screening all UHR subjects for Prodromal Syndromes (SIPS). As a semi-structured interviewing method, SIPS is created by the PRIME Research Group of Yale University in the US to identify mental risk syndromes. Due to its high reliability and validity, it has been most extensively utilized both at home and abroad. Four elements make up its scale of prodromal symptoms (SOPS). To be specific, on a scale of 0 (no anomaly) to 6 (severe psychotic symptoms), the risk symptoms of psychosis and other symptoms from the last few months are classified as positive symptoms (P), negative symptoms (N), disintegrating symptoms (D), and general symptoms (G) (36). The UHR subjects satisfied the criteria for one of the three subsets, namely, Brief Intermittent Psychotic Syndrome (BIPS), Attenuated Positive Symptom Syndrome (APSS), and Genetic Risk and Deterioration Syndrome (GRDS). All HCs were confirmed to have no family history of psychiatric disorder in their first-degree relatives. The subject exclusion criteria included the history of being diagnosed with any psychiatric disorders and receiving corresponding treatments (including antipsychotics, antidepressants, and mood stabilizers), history of disturbance of consciousness for more than 5 min, major head trauma, history of serious organic diseases (like stroke and heart failure), history of immune system diseases (including bacterial or virus infection, systemic lupus erythematosus), and usage of anti-inflammatory drugs, immunosuppressive drugs or any other medications (oral or injectable) during the last 3 months.
UHR subjects were followed up at regular intervals to identify those who later transitioned to psychosis (UHR-T) and those who did not (UHR-NT). The transition to psychosis was defined by any DSM-V for a mental illness. Telephone assessments were performed monthly during the 2-year follow-up period, including the items of severity, frequency, duration of symptoms and mood, and sleep. The presence of a psychotic syndrome (POPS) questionnaire can exclude previous or current psychotic syndromes, and the diagnosis needs to be established in both (A) and (B). (A) Positive symptoms reach a certain level (score: level 6), such as abnormal thinking content (suspicion, delusion of victimization, delusion of exaggeration), perceptual abnormalities with a low degree of hallucination, and incoherent or incomprehensible speech; (B) at least one of the symptoms meeting criteria (A) occurs 4 days a week on average and lasts 1 h, with a duration of more than 1 month, or the symptoms are seriously confusing and dangerous. Transition to psychosis was comprehensively assessed every 3 months in the first year, every 6 months in the second year, and at the moment of transition.
Both assessments were conducted by two psychiatrists who passed conformance training with an internal conformance coefficient of 0.90 each.
2.2 Blood collection and analyses
All blood samples were collected at the baseline visit. Every participant was instructed to avoid caffeine and alcohol consumption, and physical exercise for at least 30 min before any blood sampling. After overnight fasting, blood was sampled from 8:00 a.m. to 10:00 a.m. The blood sample was later centrifuged at 3,000 g for 10 min, and the plasma was stored in a deep freezer (–80°C) before use. The Luminex assay was conducted to measure IL-1β, IL-2, IL-4, IL-6, IL-17, INF-γ, IL-10, IL-8, IL-12, and TNF-β levels at the same time following the manufacturer’s instructions. Both patient and reference serum samples were incorporated in each multi-bioassay plate. In addition, TNF-α level was measured by the high-sensitivity enzyme-linked immunosorbent assay (ELISA) kits in line with the manufacturer’s protocols. Standard curves were prepared by using 4- or 5-PL logistic regression, and data were investigated by the Luminex Flexmap 3D system. A total of 10 proteins were measured with the intra- and inter-assay coefficient of variance of < 10%. Meanwhile, three proteins (IL-10, IL-12, and IL-8) were excluded due to > 80% values below the limit of detection (LOD). For the specific assay, values below the LOD were replaced with the LOD. Six participants (including five FES and one UHR) were excluded because their samples failed quality control.
2.3 Statistical analysis
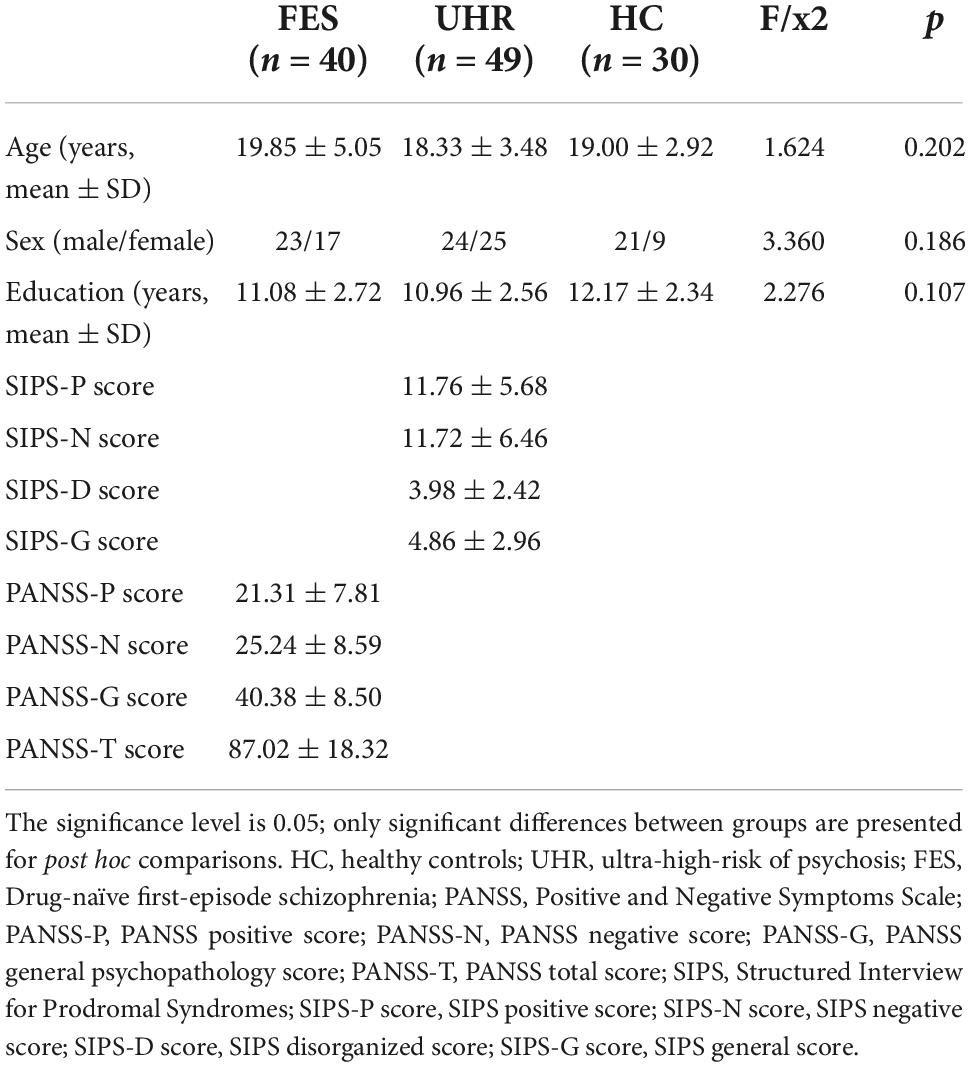
All statistical analyses were conducted with SPSS version 20, and an alpha level of 0.05 was adopted for all analyses. Group differences in age, sex, education, SIPS scores, and PANSS scores were assessed by independent-sample t-tests, chi-square test, and one-way analysis of variance (ANOVA). The mean concentration of each biomarker was log-transformed to decrease the data variability and make data more closely conform to the normal distribution. One-way ANOVA was conducted to assess group differences in biomarker concentrations, while post hoc comparisons were conducted by using the Bonferroni procedure. False Discovery Rate (FDR) correction was performed for each p-value to lower the false positive rate caused by multiple comparisons. The associations of baseline cytokines levels (IL-17, TNF-β), SIPS-N score, and outcomes of UHR subjects (UHR-T and UHR-NT) in 2 years were explored with binary logistic regression.
3 Results
3.1 Demographic and clinical characteristics
Initially, 142 participants were recruited in the project (including 45 FES subjects, 67 UHR subjects and 30 HCs). However, 23 participants were excluded because their samples failed the quality control criteria (n = 6, 5 from FES group and 1 from UHR group), dropped out in the year of follow-up (n = 4, all from UHR group), and insufficient follow-up time (n = 13, all from UHR group). Finally, 119 participants were included in the analysis, including 40 patients with Drug-naïve FES, 14 with UHR-T, 35 with UHR-NT, and 30 HCs. Table 1 displays the detailed characteristics of all participants. Finally, the clinical outcomes of 49 UHR subjects were obtained. There was no significant difference (p > 0.05) in age, gender, or education among the FES, UHR, and HC groups. The detailed demographic and clinical characteristics between UHR-T and UHR-NT groups were compared, as shown in Supplementary Table 1.
3.2 Cytokine levels among three groups (FES, UHR, HCs)
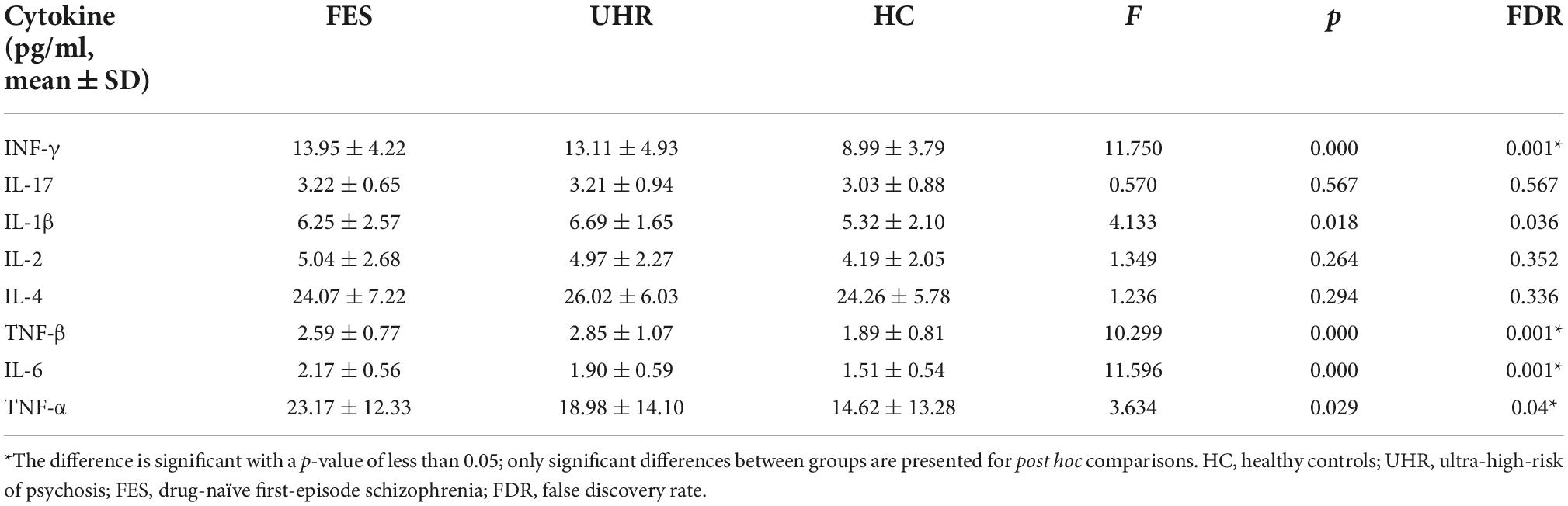
In our study, differences in the concentrations of several inflammatory analytes were statistically significant among the three groups (Figure 1 and Table 2), including IFN-γ, IL-1β, TNF-β, IL-6, and TNF-α. In Post hoc comparisons, the Bonferroni procedure revealed that FES patients and UHR subjects exhibited significantly higher mean concentrations of IFN-γ, TNF-β, and IL-6 than HC subjects. IL-1β concentration was significantly higher in UHR group than HC group, and TNF-α concentration was significantly increased in FES group compared with HC group.

Figure 1. Comparison of cytokines level among the three groups [FES, UHR, HCs, Cytokine concentration (pg/ml)]. Picture shows several significantly different inflammatory analytes between three groups, including IFN-γ, IL-1β, TNF-β, IL-6, and TNF-α, In Post hoc comparisons, the Bonferroni procedure revealed that FES patients and UHR subjects exhibited significantly higher mean concentrations of IFN-γ, TNF-β, and IL-6 than HC subjects. Besides, IL-1β concentration was significantly higher in UHR group than HC group, and TNF-α concentration remarkably increased in FES group relative to HC group. *The difference is significant with a p-value of less than 0.05. HC, healthy controls; UHR, ultra-high risk of psychosis; FES, Drug-naïve first-episode schizophrenia.
3.3 IL-17 and TNF-β levels were higher in the UHR-T group than in the UHR-NT groups
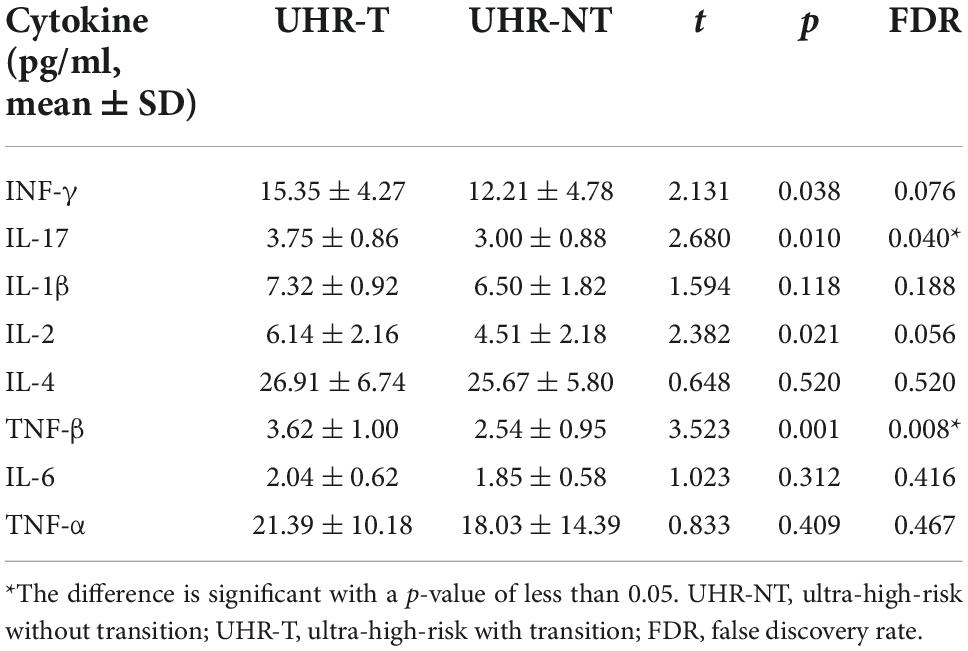
In our study, the concentrations of two inflammatory analytes, including IL-17 (p = 0.04) and IL-1β (p = 0.008), were of statistical differences among different groups after FDR correction (Figure 2 and Table 3). The concentrations of IL-17 and TNF-β were significantly up-regulated in UHR-T compared with UHR-NT groups.

Figure 2. Comparison of the levels of cytokines among the two groups [UHR-T and UHR-NT, Cytokine concentration (pg/ml)]. IL-17 levels were significantly higher in UHR-T group than UHR-NT group [p = 0.04, 3.750.86 vs. 3.000.88 pg/ml]. TNF-βlevels were significantly higher in UHR-T group than UHR-NT group [p = 0.008, 3.621.00 vs. 2.540.95]. *The difference is significant with a p-value of less than 0.05. **The difference is significant with a p-value of less than 0.01. UHR-NT, ultra-high-risk without transition; UHR-T, ultra-high-risk with transition.
3.4 The effects of N-scores, IL-17, and TNF-β levels on the conversion of psychosis
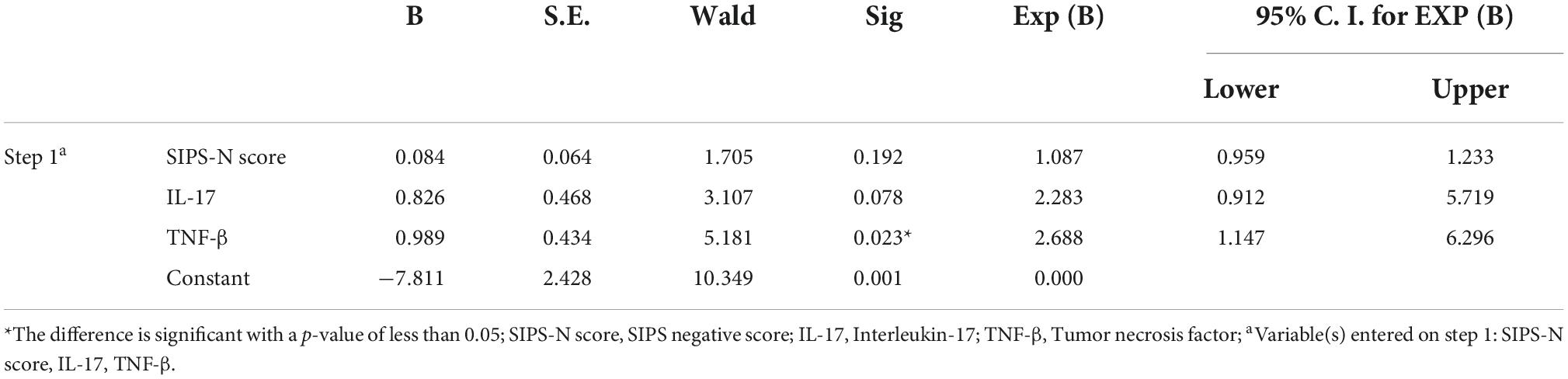
In this study, binary logistic regression was performed to assess the effects of N-scores, IL-17, and TNF-β levels on the conversion of psychosis in UHR subjects. Our results showed that the logistic model was statistically significant (χ2 = 16.517, p < 0.001). The model correctly classified 79.6% of the study subjects. The model sensitivity and specificity were 42.9 and 94.3%, whereas the positive predictive value (PPV) and negative predictive value (NPV) were 75 and 80.5%, respectively. Furthermore, the difference in TNF-β concentration was statistically significant among the three independent variables in the model. The risk of transition increased by 1.505 folds per unit increase in TNF-β (Table 4).
4 Discussion
This study investigated cytokines as the potential biomarkers for FES and UHR, and identified an inflammatory marker for distinguishing UHR-T from UHR-NT subjects during the 2-year follow-up period. A significant finding from our study indicated that the baseline levels of IL-17 and TNF-β were significantly different between individuals who converted and those who did not convert to psychosis. Although some previous studies have explored these biomarkers in patients with UHR or psychosis, our subjects did not take any drugs, making our findings more convincing. Previous studies indicate that drugs can alter the levels of inflammatory factors (37). Additionally, since IL-17 and TNF-β concentrations can be directly measured in most clinical laboratories, this study has important clinical implications since it may contribute to identifying UHR subjects who are at a greater risk of developing psychosis.
In our study, FES group showed significantly increased serum levels of TNF-a, TNF-β, IFN-γ, and IL-6 compared with HC group. UHR group presented higher IL-6, TNF-β, IFN-γ, and IL-1β levels than HC group, supporting the presumption of immune alterations in the early stage and even in the prodromal stage of the disease. Our results showed that, the increased levels of TNF-β and cytokines from the T helper type 1 immune response, particularly IFN-γ and IL-6, might indicate the aggravated pro-inflammatory process, which appeared to be related to the onset of a full-fledged psychotic episode that was apparent not only in our FEP group but also in UHR group. There was no significant difference in inflammatory factors between FES and UHR groups, while TNF-α concentration continuously increased in FES group. TNF-α is a major pro-inflammatory factor that can induce the production of a variety of cytokines (38). These results indicate that inflammatory factor concentrations have already increased in the UHR stage, and inflammation may be continuously aggravated as the disease progresses. Our findings support the microglial theory (39), which postulates that the activation of the central nervous system (CNS) microglia will produce pro-inflammatory cytokines including TNF-a and IFN-γ, resulting in aberrant neurogenesis and neuronal degeneration (40). Therefore, it is speculated that a large number of pro-inflammatory factors are released in UHR population, and a different type of immune response is activated. In this study, the Th1/Th2 balance is found to shift toward Th1 in psychotic patients. Inflammatory factors can induce the increased permeability of the blood-brain barrier (BBB) and activate the central immune system.
Moreover, our results also support the hypothesis of a TH1/TH2 imbalance; for instance, a 2015 systematic review shows the presence of Th2 drift in the serum of schizophrenic patients, while an overall immune imbalance in peripheral blood is dominated by Th1-type cytokines (41). In contrast, Th2-type cytokines have been reported to play a dominant role in schizophrenic patients (42–44). These results are inconsistent, but they have provided strong evidence for the presence of immune disorders in the early stage of schizophrenia. In this study, IL-4 concentrations in UHR and FES groups were not significantly changed compared with HC group, while their concentrations in FES and UHR groups showed a rising trend in relative to HC group. Combined with previous studies, UHR is activated by immune inflammation, leading to the increased production of acute phase proteins, such as MI macrophages (IL-1β, IL-6, TNF-β), which shifts toward Th1 in psychotic patients and then leads to activation of the compensatory immunomodulatory reflex system, resulting in the increased production of IL-4 and other anti-inflammatory cytokines. A new homeostasis set point can be formed between anti-inflammatory and pro-inflammatory cytokines, thereby influencing the development of the disease. Moreover, IL-1β level increased only in UHR group but not in FES group, while TNF-α level elevated only in FES group but not in UHR group. Therefore, it was hypothesized that the observed increases in IL-1β and TNF-α levels might represent the specific biomarkers for UHR and FES, respectively.
IL-17, which is produced by Th17 cells, plays a crucial role in various immune and inflammatory processes (45). Th17 cells entering the central center can directly act on endothelial cells by releasing inflammatory mediators like IL-17 and affect brain development together with microglial cells and other cytokines, finally causing changes in brain structure and function (46–48). Previous findings concerning IL-17 in schizophrenia have been inconsistent. In recent studies, individuals with anti-psychotic naïve psychosis show elevated IL-17 levels in the peripheral blood (49). Moreover, the reduced variability of IL-17 concentrations has been reported in subjects with psychosis (50, 51). Our results were in line with the recent meta-analysis, revealing no specific changes in IL-17 levels among FEP patients (52). We found that there was no significant difference in IL-17 levels between FES, UHR, and HC groups, but IL-17 levels were high in UHR-T group compared with the UHR-NT group. An explanation for this result is that IL-17 concentration might be influenced by other cytokines. Previous studies show that when IL-6 concentration reaches a certain level, it can co-form an internal environment with TGF-β to promote Th17 cell differentiation and IL-17 release (53). It is well known that RORt mRNA expression is sparked by TGF-a and IL-6. The precursor cells develop into Th17 subtype when RORt is over-expressed. The activation of STAT3, a crucial transcription factor that controls the effective up-regulation of RORt and Th17-associated genes like IL-17, is triggered by IL-6 and IL-21 (54, 55). In addition, IL-17 induces the production of IL-1β, IL-6, and TNF-α by interacting with its receptor, which can thus facilitate the expansion of the inflammatory process (56–59). Recent studies have reported that IL-17 is positively correlated with IL-6 concentration (60). Some studies also discover that IL-4 can down-regulate the expression of IL-17, possibly because that IL-4 induces the up-regulation of TGF-β and the down-regulation of IL-17 expression by activating regulatory T cells (61). The mechanism of action of IL-17 in the psychosis transition remains unknown. Emerging clinical and experimental evidence has suggested that maternal immune activation during pregnancy can lead to long-lasting changes in the developing brain, which last long after the initial inflammatory stressors are terminated; besides, these changes are associated with an increased risk of various psychiatric disorders (62). IL-17 can mediate various aspects of prenatal immune activation (63). At 24 h after poly I:C stimulation and 2 weeks after delivery, the cytokine indexes of mother mice and their progeny were detected, respectively. It was found that the proportion of CD4 + IL-17 cytokine was significantly higher than that in control group, indicating that immune-activated pregnant parents promoted Th17 cell differentiation in the progeny. Moreover, it increased the number of Th17 cells and IL-17 and caused an increased risk of schizophrenia in the offspring (64, 65).
Another important finding was that TNF-β levels were significantly different between individuals who converted and those who did not convert to psychosis. Furthermore, there was a statistically significant difference in plasma TNF-β levels among FES, UHR and HC groups. Previous studies have paid little attention to the potential role of elevated TNF-β in inducing the risk of a psychotic disorder. One study shows that there are no specific changes in TNF-β levels among subjects at high risk of psychosis (30) while Karanikas et al., who measured 12 circulating cytokines including TNF-β in males at the prodromal stage of psychosis, revealed that only IL-4 levels were significantly increased in UHR subjects than in HCs and schizophrenia patients (66). TNF-β is produced by lymphocytes, activated T cells and B cells, which has an important effect on cell proliferation, differentiation, lipid metabolism and neurotransmitter transmission. It has similar biological activity and effect on TNF-α (67). When bound to TNFR1, it induces the activation of NF-κB, p38α and MK2, promotes the transcription of target genes, the stability and translation of mRNA, and triggers the inflammatory response and cell death (68). Studies have found that the high level of TNF-β is a key acting factor in autoimmune diseases, and schizophrenia is closely related to autoimmune diseases. For example, a large sample study in Denmark based on 7,704 people found that the lifetime prevalence of autoimmune diseases in SCZ patients was 50%. Conversely, the relative risk of SCZ was increased by 45% if there was a history of autoimmune disease (69). The increase in TNF- levels in the UHR-T group might indicate a defective autoimmune mechanism in these people.
In addition, our results were consistent with previous studies that people with FES and a confirmed diagnosis of schizophrenia had elevated IL-6 levels in their peripheral blood (70–72) and cerebrospinal fluid (CSF) (73). According to a longitudinal follow-up study, subjects with high serum IL-6 levels at the age of 9 years had a twofold increased risk of psychosis at the age of 18 years old compared with HCs (74). Another previous research showed that compared with those who did not transition, at-risk mental state (ARMS) subjects who experienced psychosis had higher median IL-6 levels (75). However, there was no significant difference in IL-6 levels between the transition and non-transition groups in our study, probably due to the small sample size, and IL-6 was affected by several factors (76). Another explanation may be that IL-6 is not specific to schizophrenia; some studies have shown that IL-6 is altered in both bipolar disorder and depressed patients (77), and that IL-6 is correlated with negative and positive symptoms (78). The difference in IL-6 levels between the transition and non-transition groups in this study might be affected by mood or pathological symptoms. Our results might be explained by the fact that different types of immune response caused different levels of immune factors, while immune factors interacted with each other, and different factors were associated with different symptoms. For example, research has shown that excitation may be mediated by IL-17 and Uric acid (UA) interactions, whereas negative and cognitive effects may be associated with IL-6 and UA (79). To better understand the contributions of cytokines to positive feedback in schizophrenia, further research is needed.
5 Limitations
The pathogenesis of schizophrenia is complex, and the abnormal immune inflammatory system may be involved in the development of mental disorders including schizophrenia. Inflammatory cytokines can be transmitted in the immune inflammatory system, which can reflect the occurrence and development of inflammation. Nevertheless, due to the complexity of the immune inflammatory system and the multi-validity of cytokines, the existing clinical findings are inconsistent. In view of the limited number of studies, small cumulative sample size, and diversity of high-risk criteria, our findings should be interpreted with caution. Therefore, our results should be replicated in other samples before definitive conclusions are drawn. Moreover, in our study, the measurement methods of cytokines were inconsistent due to the deficiencies in the accuracy and precision of detection technology. In this regard, more accurate and sensitive technical methods and accurate statistical methods should be adopted for discussion. Finally, due to the multiplicity of the analyzed markers and various potential untested moderators such as the degree of certain psychopathological symptoms, coexisting physical health conditions, smoking habits, and dietary patterns in our study, it was not ruled out that the significant results on IL-17 and TNF-β might be affected by different variables. In the future, more investigations are needed to further clarify the clinical phenomena and the exact pathogenesis of cytokines in mental illness, aiming to explore more specific inflammatory biological markers for the precise diagnosis and treatment of individualized mental diseases.
6 Conclusion
This study reveals the immuno-inflammatory dysregulation in the early stage of psychosis before psychotic conversion and the first episode of schizophrenia. IL-17 and TNF-β levels significantly increase in UHR-T group compared with UHR-NT group, suggesting that IL-17 and TNF-β may be the potential selective predictive biomarkers for future transition in UHR individuals. In addition, IL-1β and TNF-α are identified as the representatives of the specific biomarkers for UHR and FES, respectively.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
LO, YH, and XC designed the study, analyzed, and discussed the experimental result. LY, LF, CL, ZZ, ZY, XM, and ZL collected the samples and clinical information. LO wrote the first draft of the manuscript. All authors contributed to the article and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (grant nos. 81871056 and 82101576), the National Key Research and Development Program of China (grant no. 2021YFE0191400), and the Fundamental Research Funds for the Central Universities of Central South University (grant no. 2021zzts0394).
Acknowledgments
We acknowledge all the participants for joining this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.1072380/full#supplementary-material
References
1. Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, et al. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr Bull. (2018). 44:1195–203. doi: 10.1093/schbul/sby058
2. Gbd 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/s0140-6736(18)32279-7
3. Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. (2022) 399:473–86. doi: 10.1016/s0140-6736(21)01730-x
4. Perkins DO, Gu H, Boteva K, Lieberman JA. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. (2005) 162:1785–804. doi: 10.1176/appi.ajp.162.10.1785
5. Schmidt SJ, Schultze-Lutter F, Schimmelmann BG, Maric NP, Salokangas RK, Riecher-Rössler A, et al. EPA guidance on the early intervention in clinical high risk states of psychoses. Eur Psychiatry. (2015) 30:388–404. doi: 10.1016/j.eurpsy.2015.01.013
6. Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. (2008) 65:28–37. doi: 10.1001/archgenpsychiatry.2007.3
7. Nelson B, Yuen HP, Wood SJ, Lin A, Spiliotacopoulos D, Bruxner A, et al. Long-term follow-up of a group at ultra high risk (”prodromal”) for psychosis: the PACE 400 study. Jama Psychiat. (2013) 70:793–802. doi: 10.1001/jamapsychiatry.2013.1270
8. Fusar-Poli P, Cappucciati M, Borgwardt S, Woods SW, Addington J, Nelson B, et al. Heterogeneity of psychosis risk within individuals at clinical high risk: a meta-analytical stratification. Jama Psychiat. (2016) 73:113–20. doi: 10.1001/jamapsychiatry.2015.2324
9. Zhang TH, Li HJ, Woodberry KA, Xu LH, Tang YY, Guo Q, et al. Two-year follow-up of a Chinese sample at clinical high risk for psychosis: timeline of symptoms, help-seeking and conversion. Epidemiol Psychiatr Sci. (2017) 26:287–98. doi: 10.1017/s2045796016000184
10. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. (2016) 388:86–97. doi: 10.1016/s0140-6736(15)01121-6
11. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. (2009) 35:549–62. doi: 10.1093/schbul/sbp006
12. Girgis RR, Kumar SS, Brown AS. The cytokine model of schizophrenia: emerging therapeutic strategies. Biol Psychiatry. (2014) 75:292–9. doi: 10.1016/j.biopsych.2013.12.002
13. Buckley PF. Neuroinflammation and schizophrenia. Curr Psychiatry Rep. (2019) 21:72. doi: 10.1007/s11920-019-1050-z
14. Jiang NM, Cowan M, Moonah SN, Petri WA Jr. The impact of systemic inflammation on neurodevelopment. Trends Mol Med. (2018) 24:794–804. doi: 10.1016/j.molmed.2018.06.008
15. Carmel M, Michaelovsky E, Weinberger R, Frisch A, Mekori-Domachevsky E, Gothelf D, et al. Differential methylation of imprinting genes and MHC locus in 22q11.2 deletion syndrome-related schizophrenia spectrum disorders. World J Biol Psychiatry. (2021) 22:46–57. doi: 10.1080/15622975.2020.1747113
16. Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. (2016) 530:177–83. doi: 10.1038/nature16549
17. Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry. (2016) 173:44–52. doi: 10.1176/appi.ajp.2015.14101358
18. Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med. (2019) 49:2307–19. doi: 10.1017/s0033291719001995
19. Cho M, Lee TY, Kwak YB, Yoon YB, Kim M, Kwon JS. Adjunctive use of anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Aust N Z J Psychiatry. (2019) 53:742–59. doi: 10.1177/0004867419835028
20. Sonnenschein SF, Grace A. Emerging therapeutic targets for schizophrenia: a framework for novel treatment strategies for psychosis. Expert Opin Ther Targets. (2021) 25:15–26. doi: 10.1080/14728222.2021.1849144
21. Lee N, Kim WU. Microbiota in T-cell homeostasis and inflammatory diseases. Exp Mol Med. (2017) 49:e340. doi: 10.1038/emm.2017.36
22. Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. (2015) 135:626–35. doi: 10.1016/j.jaci.2014.11.001
23. Maes M, Carvalho AF. The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol Neurobiol. (2018) 55:8885–903. doi: 10.1007/s12035-018-1016-x
24. Schiavone S, Trabace L. Inflammation, stress response, and redox dysregulation biomarkers: clinical outcomes and pharmacological implications for psychosis. Front Psychiatry. (2017) 8:203. doi: 10.3389/fpsyt.2017.00203
25. Pardo-de-Santayana G, Juncal-Ruiz M, Vázquez-Bourgon J, Riesco-Dávila L, Ortiz-Garcia de la Foz V, Pelayo-Terán JM, et al. Active psychosis and pro-inflammatory cytokines in first-episode of psychosis. J Psychiatr Res. (2021) 134:150–7. doi: 10.1016/j.jpsychires.2020.12.060
26. Kelsven S, de la Fuente-Sandoval C, Achim CL, Reyes-Madrigal F, Mirzakhanian H, Domingues I, et al. Immuno-inflammatory changes across phases of early psychosis: the impact of antipsychotic medication and stage of illness. Schizophr Res. (2020) 226:13–23. doi: 10.1016/j.schres.2020.01.003
27. Debnath M, Berk M. Functional implications of the IL-23/IL-17 immune axis in schizophrenia. Mol Neurobiol. (2017) 54:8170–8. doi: 10.1007/s12035-016-0309-1
28. Pillinger T, Osimo EF, Brugger S, Mondelli V, McCutcheon RA, Howes OD. A meta-analysis of immune parameters, variability, and assessment of modal distribution in psychosis and test of the immune subgroup hypothesis. Schizophr Bull. (2019) 45:1120–33. doi: 10.1093/schbul/sby160
29. Zhang T, Zeng J, Wei Y, Ye J, Tang X, Xu L, et al. Changes in inflammatory balance correlates with conversion to psychosis among individuals at clinical high-risk: a prospective cohort study. Psychiatry Res. (2022) 318:114938. doi: 10.1016/j.psychres.2022.114938
30. Park S, Miller BJ. Meta-analysis of cytokine and C-reactive protein levels in high-risk psychosis. Schizophr Res. (2020) 226:5–12. doi: 10.1016/j.schres.2019.03.012
31. Tomasik J, Rahmoune H, Guest PC, Bahn S. Neuroimmune biomarkers in schizophrenia. Schizophr Res. (2016) 176:3–13. doi: 10.1016/j.schres.2014.07.025
32. Khoury R, Nasrallah HA. Inflammatory biomarkers in individuals at clinical high risk for psychosis (CHR-P): state or trait? Schizophr Res. (2018) 199:31–8. doi: 10.1016/j.schres.2018.04.017
33. Lizano PL, Keshavan MS, Tandon N, Mathew IT, Mothi SS, Montrose DM, et al. Angiogenic and immune signatures in plasma of young relatives at familial high-risk for psychosis and first-episode patients: a preliminary study. Schizophr Res. (2016) 170:115–22. doi: 10.1016/j.schres.2015.12.001
34. Battle DE. Diagnostic and statistical manual of mental disorders (DSM). Codas. (2013) 25:191–2. doi: 10.1590/s2317-17822013000200017
35. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
36. Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. (2002) 159:863–5. doi: 10.1176/appi.ajp.159.5.863
37. Baumeister D, Ciufolini S, Mondelli V. Effects of psychotropic drugs on inflammation: consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology. (2016) 233:1575–89. doi: 10.1007/s00213-015-4044-5
38. Tseng WY, Huang YS, Lin HH, Luo SF, McCann F, McNamee K, et al. TNFR signalling and its clinical implications. Cytokine. (2018) 101:19–25. doi: 10.1016/j.cyto.2016.08.027
39. Mattei D, Notter T. Basic concept of microglia biology and neuroinflammation in relation to psychiatry. Curr Top Behav Neurosci. (2020) 44:9–34. doi: 10.1007/7854_2018_83
40. Ferro A, Auguste YSS, Cheadle L. Microglia, cytokines, and neural activity: unexpected interactions in brain development and function. Front Immunol. (2021) 12:703527. doi: 10.3389/fimmu.2021.703527
41. Guo J, Liu C, Wang Y, Feng B, Zhang X. Role of T helper lymphokines in the immune-inflammatory pathophysiology of schizophrenia: systematic review and meta-analysis. Nord J Psychiatry. (2015) 69:364–72. doi: 10.3109/08039488.2014.986761
42. Chiang SS, Riedel M, Schwarz M, Mueller N. Is T-helper type 2 shift schizophrenia-specific? Primary results from a comparison of related psychiatric disorders and healthy controls. Psychiatry Clin Neurosci. (2013) 67:228–36. doi: 10.1111/pcn.12040
43. Müller N, Riedel M, Ackenheil M, Schwarz MJ. The role of immune function in schizophrenia: an overview. Eur Arch Psychiatry Clin Neurosci. (1999) 249(Suppl 4):62–8. doi: 10.1007/pl00014187
44. Chaves C, Zuardi AW, Hallak JE. The role of inflammation in schizophrenia: an overview. Trends Psychiatry Psychother. (2015) 37:104–5. doi: 10.1590/2237-6089-2015-0007
45. Zhu S, Qian Y. IL-17/IL-17 receptor system in autoimmune disease: mechanisms and therapeutic potential. Clin Sci. (2012) 122:487–511. doi: 10.1042/cs20110496
46. Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. (2008) 453:1051–7. doi: 10.1038/nature07036
47. Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. (2007) 13:1173–5. doi: 10.1038/nm1651
48. Haqqani AS, Stanimirovic DB. Intercellular interactomics of human brain endothelial cells and th17 lymphocytes: a novel strategy for identifying therapeutic targets of CNS inflammation. Cardiovasc Psychiatry Neurol. (2011) 2011:175364. doi: 10.1155/2011/175364
49. Ding M, Song X, Zhao J, Gao J, Li X, Yang G, et al. Activation of Th17 cells in drug naïve, first episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. (2014) 51:78–82. doi: 10.1016/j.pnpbp.2014.01.001
50. Schwarz E, Guest PC, Rahmoune H, Harris LW, Wang L, Leweke FM, et al. Identification of a biological signature for schizophrenia in serum. Mol Psychiatry. (2012) 17:494–502. doi: 10.1038/mp.2011.42
51. Borovcanin M, Jovanovic I, Radosavljevic G, Djukic Dejanovic S, Bankovic D, Arsenijevic N, et al. Elevated serum level of type-2 cytokine and low IL-17 in first episode psychosis and schizophrenia in relapse. J Psychiatr Res. (2012) 46:1421–6. doi: 10.1016/j.jpsychires.2012.08.016
52. Fang X, Zhang Y, Fan W, Tang W, Zhang C. Interleukin-17 alteration in first-episode psychosis: a meta-analysis. Mol Neuropsychiatry. (2018) 3:135–40. doi: 10.1159/000481661
53. Midgley A, Barakat D, Braitch M, Nichols C, Nebozhyn M, Edwards LJ, et al. PAF-R on activated T cells: role in the IL-23/Th17 pathway and relevance to multiple sclerosis. Immunobiology. (2021) 226:152023. doi: 10.1016/j.imbio.2020.152023
54. McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. (2019) 50:892–906. doi: 10.1016/j.immuni.2019.03.021
55. Kumar R, Theiss AL, Venuprasad K. RORγt protein modifications and IL-17-mediated inflammation. Trends Immunol. (2021) 42:1037–50. doi: 10.1016/j.it.2021.09.005
56. Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL. IL-17 receptor-based signaling and implications for disease. Nat Immunol. (2019) 20:1594–602. doi: 10.1038/s41590-019-0514-y
57. Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. (2002) 71:1–8.
58. Ma X, Reynolds SL, Baker BJ, Li X, Benveniste EN, Qin H. IL-17 enhancement of the IL-6 signaling cascade in astrocytes. J Immunol. (2010) 184:4898–906. doi: 10.4049/jimmunol.1000142
59. Noguchi D, Wakita D, Tajima M, Ashino S, Iwakura Y, Zhang Y, et al. Blocking of IL-6 signaling pathway prevents CD4+ T cell-mediated colitis in a T(h)17-independent manner. Int Immunol. (2007) 19:1431–40. doi: 10.1093/intimm/dxm114
60. Borovcanin MM, Minic Janicijevic S, Jovanovic IP, Gajovic NM, Jurisevic MM, Arsenijevic NN. Type 17 immune response facilitates progression of inflammation and correlates with cognition in stable schizophrenia. Diagnostics. (2020) 10:926. doi: 10.3390/diagnostics10110926
61. Sarkar S, Tesmer LA, Hindnavis V, Endres JL, Fox DA. Interleukin-17 as a molecular target in immune-mediated arthritis: immunoregulatory properties of genetically modified murine dendritic cells that secrete interleukin-4. Arthritis Rheum. (2007) 56:89–100. doi: 10.1002/art.22311
62. Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. (2012) 72:1272–6. doi: 10.1002/dneu.22024
63. Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. (2016) 351:933–9. doi: 10.1126/science.aad0314
64. Mandal M, Marzouk AC, Donnelly R, Ponzio NM. Preferential development of Th17 cells in offspring of immunostimulated pregnant mice. J Reprod Immunol. (2010) 87:97–100. doi: 10.1016/j.jri.2010.06.156
65. Mandal M, Marzouk AC, Donnelly R, Ponzio NM. Maternal immune stimulation during pregnancy affects adaptive immunity in offspring to promote development of TH17 cells. Brain Behav Immun. (2011) 25:863–71. doi: 10.1016/j.bbi.2010.09.011
66. Karanikas E, Ntouros E, Oikonomou D, Floros G, Griveas I, Garyfallos G. Evidence for hypothalamus-pituitary-adrenal axis and immune alterations at prodrome of psychosis in males. Psychiatry Investig. (2017) 14:703–7. doi: 10.4306/pi.2017.14.5.703
67. Medler J, Wajant H. Tumor necrosis factor receptor-2 (TNFR2): an overview of an emerging drug target. Expert Opin Ther Targets. (2019) 23:295–307. doi: 10.1080/14728222.2019.1586886
68. Annibaldi A, Meier P. Checkpoints in TNF-induced cell death: implications in inflammation and cancer. Trends Mol Med. (2018) 24:49–65. doi: 10.1016/j.molmed.2017.11.002
69. Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. (2011) 168:1303–10. doi: 10.1176/appi.ajp.2011.11030516
70. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. (2011) 70:663–71. doi: 10.1016/j.biopsych.2011.04.013
71. Capuzzi E, Bartoli F, Crocamo C, Clerici M, Carrà G. Acute variations of cytokine levels after antipsychotic treatment in drug-naïve subjects with a first-episode psychosis: a meta-analysis. Neurosci Biobehav Rev. (2017) 77:122–8. doi: 10.1016/j.neubiorev.2017.03.003
72. Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. (2014) 155:101–8. doi: 10.1016/j.schres.2014.03.005
73. Gallego JA, Blanco EA, Husain-Krautter S, Madeline Fagen E, Moreno-Merino P, Del Ojo-Jiménez JA, et al. Cytokines in cerebrospinal fluid of patients with schizophrenia spectrum disorders: new data and an updated meta-analysis. Schizophr Res. (2018) 202:64–71. doi: 10.1016/j.schres.2018.07.019
74. Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. Jama Psychiat. (2014) 71:1121–8. doi: 10.1001/jamapsychiatry.2014.1332
75. Stojanovic A, Martorell L, Montalvo I, Ortega L, Monseny R, Vilella E, et al. Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology. (2014) 41:23–32. doi: 10.1016/j.psyneuen.2013.12.005
76. Koola MM. Cytokines in schizophrenia: hope or hype? Indian J Psychol Med. (2016) 38:97–100. doi: 10.4103/0253-7176.178766
77. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. (2016) 21:1696–709. doi: 10.1038/mp.2016.3
78. Goldsmith DR, Haroon E, Miller AH, Strauss GP, Buckley PF, Miller BJ. TNF-α and IL-6 are associated with the deficit syndrome and negative symptoms in patients with chronic schizophrenia. Schizophr Res. (2018) 199:281–4. doi: 10.1016/j.schres.2018.02.048
Keywords: ultra-high risk individual, schizophrenia, inflammation, cytokines, Drug-naïve first-episode
Citation: Ouyang L, Li D, Li Z, Ma X, Yuan L, Fan L, Yang Z, Zhang Z, Li C, He Y and Chen X (2022) IL-17 and TNF-β: Predictive biomarkers for transition to psychosis in ultra-high risk individuals. Front. Psychiatry 13:1072380. doi: 10.3389/fpsyt.2022.1072380
Received: 17 October 2022; Accepted: 29 November 2022;
Published: 16 December 2022.
Edited by:
Luca De Peri, Cantonal Sociopsychiatric Organization, SwitzerlandReviewed by:
Karina S. MacDowell, Universidad Complutense de Madrid, SpainMilica Milovan Borovcanin, University of Kragujevac, Serbia
Copyright © 2022 Ouyang, Li, Li, Ma, Yuan, Fan, Yang, Zhang, Li, He and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying He, eWluZ2hlQGNzdS5lZHUuY24=; Xiaogang Chen, Y2hlbnhpYW9nYW5nQGNzdS5lZHUuY24=
 Lijun Ouyang1,2
Lijun Ouyang1,2 David Li
David Li Lejia Fan
Lejia Fan Zhenmei Zhang
Zhenmei Zhang Xiaogang Chen
Xiaogang Chen