- 1Health, Medical and Neuropsychology Unit, Faculty of Social and Behavioral Sciences, Leiden University, Leiden, Netherlands
- 2Leiden Institute for Brain and Cognition, Leiden University, Leiden, Netherlands
- 3Department of Psychiatry, Leiden University Medical Center, Leiden, Netherlands
- 4Medical Delta, Leiden University, Technical University Delft, Rotterdam University, Rotterdam, Netherlands
Background: Nocebo and placebo effects, i.e., adverse or beneficial treatment effects, respectively, putatively due to expectancies can modulate pain and itch. These effects can generalize within the pain or itch modality. Predicting the induction and generalization of these effects can be helpful in clinical practice. This study aims to investigate whether psychological characteristics related to the fear-avoidance model predict the induction and generalization of nocebo and placebo effects on pain and itch in young healthy participants.
Methods: Data from two previous experiments were analyzed. In Experiment 1, we induced nocebo and placebo effects on heat pain and tested generalization to pressure pain and to cowhage-evoked itch (n = 33 in a nocebo group, n = 32 in a placebo group). In Experiment 2, we induced nocebo effects on cowhage-evoked itch and tested generalization to mechanical itch and to mechanical touch (n = 44). Potential predictors were anxiety- and stress symptoms, attention to pain/itch, and pain/itch catastrophizing. Multiple regression analyses were performed.
Results: For nocebo effects, none of the individual psychological characteristics significantly predicted induction of nocebo effects nor their generalization. For placebo effects, only less stress symptoms, lower attention to pain, and higher pain catastrophizing weakly predicted a stronger generalization of placebo effects from heat pain to pressure pain.
Conclusion: The tested psychological characteristics may not play an important role in the induction and generalization of nocebo and placebo effects in healthy individuals. However, firm conclusions cannot be drawn with the current sample. Future studies should validate findings in larger and more diverse samples.
Introduction
Placebo effects and nocebo effects, the beneficial and adverse treatment outcomes that cannot be ascribed to active treatments ingredients, respectively, can decrease and increase symptoms like pain and itch (1–3) by expectancy mechanisms. Expectancies can be effectively shaped by verbal suggestion (via providing explicit information) and classical conditioning (via repeatedly pairing a neutral stimulus with an unconditioned stimulus that naturally evokes a specific response) (2, 3). Recently, placebo and nocebo effects were found to generalize within the pain and itch modalities (4–6). This phenomenon is called response generalization, where similar placebo/nocebo effects can be found on perception of a novel stimulus that is different from the original stimulus for which placebo/nocebo effects were evoked (7). For instance, patients who experienced negative treatment outcomes may be prone to experience also similar negative treatment outcomes for similar symptoms, presumably mediated by expectancies. The susceptibility to placebo and nocebo effects as well as their generalization varies across individuals (8), making it difficult to harness them in clinical settings. It can be valuable to identify those individuals who are more sensitive to induction and generalization of placebo and nocebo effects.
Although mixed, evidence has shown that psychological characteristics related to the fear-avoidance model such as affective factors (including anxiety- and stress symptoms) and cognitive factors (including attention and catastrophizing) may be associated with placebo and nocebo effects on pain (1, 9–14), So far, most of what we know about the findings of predictors comes from the study of these effects on pain (11, 13–15). Only few studies explored the role of predictors in induction of placebo and nocebo effects on itch (12). Given the history of inconsistent findings on the predictors for placebo/nocebo effects and the paucity of studies on predicting these effects on itch, it is important to extend the current understanding of the relations between cognitive-affective factors and placebo/nocebo effects.
Cognitive-affective factors beyond expectancies may also influence generalization of placebo/nocebo effects from one symptom to similar symptoms. This is indirectly supported by research into fear generalization because of closely overlapping experimental procedures used when examining classical conditioning and generalization of (pain-related) fear and of placebo and nocebo effects (16, 17). Specifically, pain-related fear may arise as a by-product of the procedure of pain-related conditioning in placebo/nocebo effects, and one recent experimental study showed that pain-related fear can contribute to nocebo hyperalgesia (18). Therefore, it is reasonable to assume that the factors that influence fear generalization such as affect (e.g., anxiety- and stress symptoms) (19, 20) and cognitions (e.g., attention) (21), may also be associated with generalization of placebo/nocebo effects. However, no studies have explored predictors for generalization of placebo and nocebo effects on somatosensory sensations yet. Understanding whether and how psychological characteristics are involved in the induction and generalization of placebo/nocebo effects could be clinically relevant to foster the efficacy of positive treatment outcomes and minimize the severity of negative treatment outcomes within or across symptoms.
Our aims were to explore whether psychological characteristics can predict the induction and generalization of placebo and nocebo effects on somatosensory sensations in young healthy participants. Specifically, we explore if anxiety- and stress symptoms, as well as attention, and catastrophizing can predict (1) induction and generalization of nocebo effects (primary objective), (2) induction and generalization of placebo effects (secondary objective), (3) expected nocebo and placebo effects as well as generalization (exploratory objective). Given indirect support from the fear-avoidance model (22, 23), we would expect that these cognitive-affective factors may positively predict nocebo effects (and generalization) and negatively predict placebo effects (and generalization). To this end, in two different experiments [from which the findings on nocebo and placebo effects have been published in separate articles (4, 24)] we first measured individual psychological characteristics with self-report questionnaires. In the first experiment, we consecutively induced nocebo and placebo effects on heat pain and tested generalization of nocebo and placebo effects to pressure pain and to cowhage-evoked itch (4). In the second experiment, we induced nocebo effects on cowhage-evoked itch and tested generalization of nocebo effects to mechanical itch and to mechanical touch (24).
Materials and methods
A brief summary of the two experiments (i.e., the information of participants and the experimental designs) can be found below. The procedures have been extensively described in our previous publications (4, 24), and are briefly repeated in Supplementary Appendix Method.
Participants
The sample size calculations were conducted for the main (placebo/nocebo) outcomes of two experiments (4, 24). Specifically, each group (placebo or nocebo) in experiment 1 would require 34 participants (4), and experiment 2 would require 44 participants (24). Post-hoc power analyses suggest that these sample sizes are sufficient to detect large effect sizes (f2 > 0.35) for multiple regression analyses with 4 predictors (α = 0.05, power = 0.8). However, sample sizes of >25 should be sufficient to conduct multiple regressions (25). All participants (English-speaking) were between 18 and 35 years old. All participants were recruited via an online recruitment system (Sona Systems, Tallinn, Estonia) and through flyers posted in and around the university. Exclusion criteria were: current physical or mental illness, suffering from chronic itch (≥6 weeks), currently using medication or psychoactive drugs, being pregnant or lactating. Additionally, experiment 1 also excluded participants who were suffering from chronic pain (≥6 months), and experiment 2 excluded participants when they experienced spontaneous itch ≥3 on a 0 (not itch at all)-10 (worst itch imaginable) numerical rating scale (NRS) at the start of the testing session or cowhage insensitivity. Both experiments were approved by the Psychology Research Ethics Committee of Leiden University (CEP19-1205/571 and CEP18-1218/491). The experiments were conducted at Leiden University, the Netherlands. All participants provided their written informed consent. A data-blind preregistration for the current study was published at AsPredicted (#71238.1 None of the currently reported analyses had been conducted prior to pre-registration).
Study designs
Both experiments used a within-subject design. Noteworthy, participants received neither verbal suggestions nor conditioning regarding the stimuli used for investigating generalization. All stimuli were applied in a pseudorandom order.
Experiment 1
The experiment had two independent groups (i.e., nocebo group and placebo group). During the experiment, we first induced nocebo and placebo effects on heat pain, and then tested generalization to pressure pain and to cowhage-evoked itch. All participants underwent a design consisting of 3 parts (see Figure 1). Part 1 comprised an induction phase and a test phase, where participants either received a negative expectation induction (nocebo group) or a positive expectation induction (placebo group) by verbal suggestion and conditioning (see Supplementary Appendix Method) regarding heat pain stimuli and tested on heat pain stimuli (see Supplementary Appendix Method). Part 2 comprised a short version of the conditioning in part 1 (Reinstatement in Figure 1) and a test phase to test generalization to pressure pain stimuli (see Supplementary Appendix Method). Part 3 comprised the same short version of the conditioning in part 1 (Reinstatement in Figure 1) and a test phase to test generalization to cowhage-evoked itch (see Supplementary Appendix Method).
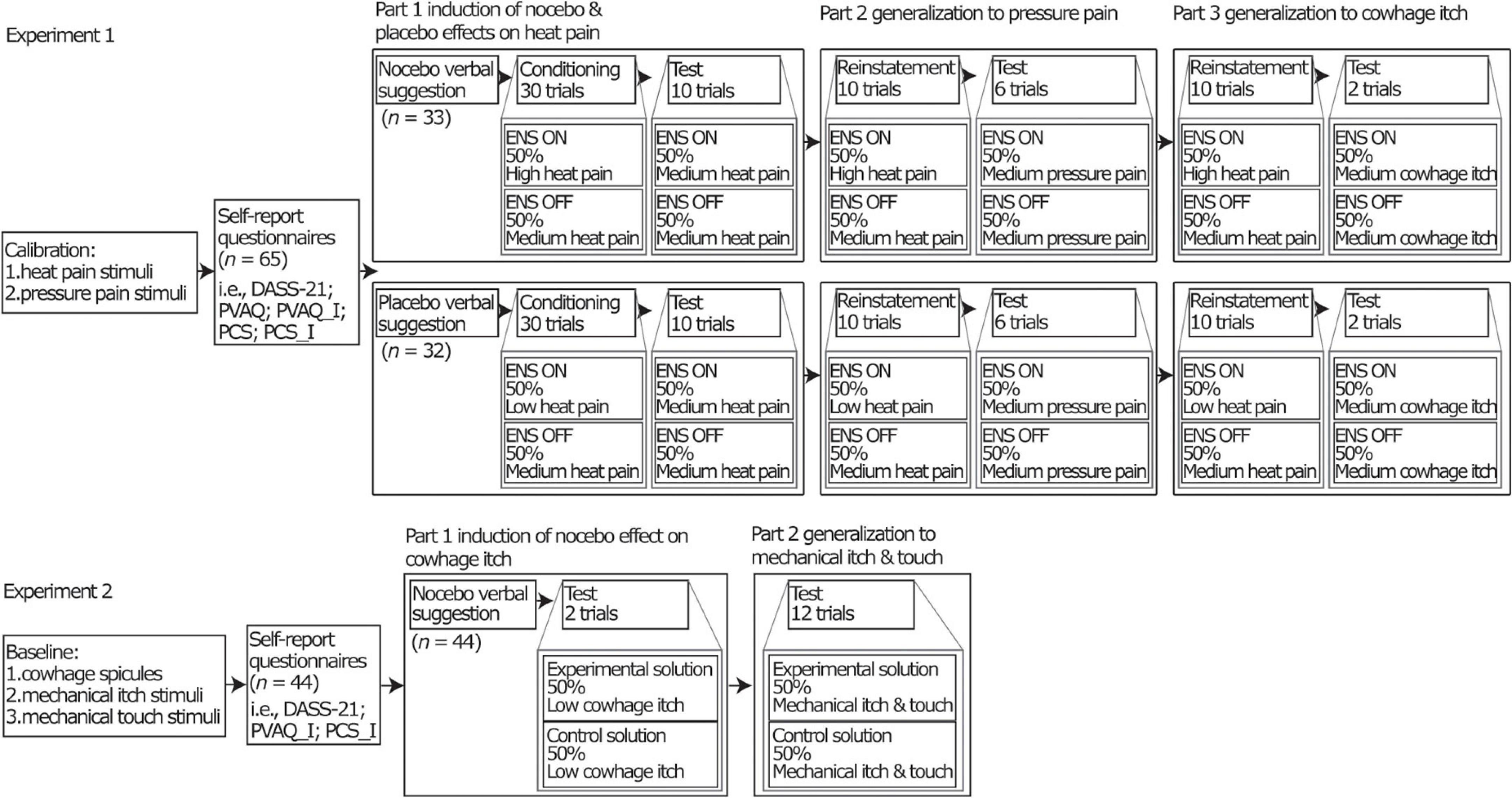
Figure 1. Overview of the full design of the two separate experiments. For experiment 1, “ENS” was functioned as a placebo treatment. “ON” and “OFF” indicated the sham (de)activation of the ENS device. “ON” represents an experimental trial and “OFF” represents a control trial. The ENS device was a transcutaneous electrical nerve stimulation (TENS) device (model EM80, Beurer, Germany). Participants rated their pain intensities on a 0 (no pain at all)-10 (worst pain imaginable) numerical rating scale (NRS). Low (NRS 0.5-2), moderate (NRS 3-4.5), and high (NRS 5.5-7) heat pain intensities were individually calibrated. Moderate pressure pain intensity (NRS 3-4.5) was individually determined. For experiment 2, “experimental solution” was served as a nocebo treatment. “Experiment solution” represents an experiment trial and “control solution” represents a control trial. Throughout both experiments, participants received all stimuli in half of experimental trials and in half of control trials in all phases. DASS-21, The 21-item version of the Depression Anxiety Stress Scale; PVAQ, The Pain Vigilance and Awareness Questionnaire; PCS, The Pain Catastrophizing Scale; PVAQ-I, itch-adjusted version of the PVAQ; PCS-I, itch-adjusted version of the PCS. For more details of the design for two experiments see (4, 24).
Experiment 2
We first induced nocebo effects on cowhage-evoked itch and then tested generalization to mechanical itch and to mechanical touch. The design included 2 parts. Part 1 comprised an induction and a test phase, where participants received a negative expectation induction by verbal suggestion (see Supplementary Appendix Method) on cowhage-evoked itch and tested on cowhage-evoked itch. Part 2 comprised a test phase to test generalization to mechanical itch and mechanical touch (see Supplementary Appendix Method).
Assessment of predictors
Psychological characteristics, specifically anxiety-, stress-, depressive symptoms, attention to pain/itch, pain/itch catastrophizing were measured with the questionnaires described below. In experiment 1, all mentioned questionnaires were administered. In experiment 2, all questionnaires except those pertaining specifically to pain were administered. All questionnaires were administered in English and completed using Qualtrics (Qualtrics, Provo, United States) on a desktop computer in the lab before administering somatosensory stimuli in both experiments.
Anxiety-, stress-, and depressive symptoms
The 21-item version of the Depression Anxiety Stress Scale (DASS-21) was used to measure the frequency and severity of experiencing negative emotions over the previous week. The scale consists of subscales of anxiety (e.g., “I was aware of dryness of my mouth”), depression (e.g., “I felt that life was meaningless”), and stress (e.g., “I found it hard to wind down”). Each item was rated on a Likert scale from 0 (did not apply to me at all) to 3 (applied to me very much, or most of the time). Seven items per scale were summed and doubled to be equivalent to the full DASS version. The scores of each subscale theoretically range from 0 to 42, with higher scores indicating greater state anxiety, stress, and depression, respectively (26, 27). Cronbach’s alpha of the subscales in both experiments ranged from 0.69 to 0.78, except from the subscale depression in experiment 1 in the placebo group (Cronbach’s alpha = 0.52).
Attention to pain
The Pain Vigilance and Awareness Questionnaire (PVAQ) was used to measure the frequency of self-reported attentional habits with a focus on pain and changes in pain. This scale consists of 16 items, e.g., “I am very sensitive to pain.” Each item was rated on a Likert scale from 0 (never) to 5 (always). All items were summed, with a theoretical range from 0 to 80, with higher scores indicating a higher focus on pain sensations (28). Cronbach’s alpha of the PVAQ was 0.84 in experiment 1 and 0.85 in experiment 2.
Attention to itch
The PVAQ was adjusted to pertain itch (PVAQ-I) by only replacing the word “pain” with “itch” for all items, e.g., “I am very sensitive to itch” (29). Cronbach’s alpha of the PVAQ-I was 0.83 in experiment 1 and 0.86 in experiment 2.
Pain catastrophizing
The Pain Catastrophizing Scale (PCS) was used to measure catastrophizing about pain experienced in daily life. This scale consists of 13 items, e.g., “I become afraid that the pain will get worse.” Each item was rated on a Likert scale from 0 (not at all) to 4 (all the time). All items were summed, with a theoretical range from 0 to 52, with higher scores indicating more pain catastrophizing (30). Cronbach’s alpha of the PCS was 0.85 in experiment 1 and 0.93 in experiment 2.
Itch catastrophizing
The PCS was adjusted to pertain itch (PCS-I) by only replacing the word “pain” with “itch” for all items, e.g., “I become afraid that the itch will get worse” (29, 31). Cronbach’s alpha of the PCS-I was 0.84 in experiment 1 and 0.92 in experiment 2.
Statistical analysis
All analyses were performed using R (Version 3.6.3, Vienna, Austria) for Windows. Nocebo and placebo effects were defined as the difference in scores between experimental and control trials during the test phases in both experiments (4, 24). Furthermore, we defined generalization responders as participants who reported higher sensation scores in experimental trials in the testing generalization phases in the nocebo group or lower scores in the placebo group when compared to control trials. Due to a low-reliability of the DASS-21’s subscale depression, this subscale was removed as predictor from all analyses. Assumption checks included normality, linearity, homoscedasticity, and multicollinearity. All assumptions were met in this study. Influential values were checked by Cook’s distance (>0.5 considered as influential values, see Supplementary Appendix Figures). In case of influential values, the main outcomes would be conducted with and without influential values. Given the small sample size, regression analyses were conducted with bootstrapping (2,000 samples with reporting 95% confidence intervals (CIs)]. The statistically significant level was set at p < 0.05.
To check whether psychological characteristics were related to the induction and generalization of nocebo and placebo effects and to check the intercorrelations between predictors for each model, Pearson correlation coefficients (normal distribution) were calculated.
To examine the primary objective of exploring predictors for the induction and generalization of nocebo effects, multiple regression analyses were performed in which the psychological characteristics (i.e., anxiety-, stress symptoms, attention to pain/itch, pain/itch catastrophizing) were entered into the model simultaneously (i.e., forced entry) as predictors. Dependent outcomes were nocebo effects on heat pain, nocebo effects on cowhage-evoked itch, generalization of nocebo effects to pressure pain, to cowhage-evoked itch, and to mechanical itch and touch. Note that, in experiment 2, we observed that mechanical stimuli induced impure sensations at baseline (i.e., the mechanical touch filaments evoked itch and the mechanical itch filaments did not evoke itch at baseline). Therefore, we selected those filaments that evoked either touch or itch at baseline for each individual (“individualized mechanical touch/itch filaments”) to assess the nocebo effects evoked in the test phase and included these outcomes as dependent variables in present analyses (24). Further, note that psychological characteristics related to pain were not used to predict dependent outcomes related to itch, and vice versa for itch. An overview of the specific predictors and dependent outcomes is reported in Supplementary Appendix Table 1.
To examine the secondary objective of exploring predictors for placebo effects, the same method and predictors as described in the primary objective were used, except that the dependent outcomes were placebo effects on heat pain as well as generalization of placebo effects to pressure pain and cowhage-evoked itch (see Supplementary Appendix Table 1).
To examine the exploratory objectives of exploring predictors for expected itch and pain (referred to expected nocebo and placebo effects in the remainder), the same method and predictors as described in the primary objective were used, except that the dependent outcomes were the expected itch and pain intensities.
Results
Sample characteristics
In experiment 1, 33 participants were included in the nocebo group and 32 participants in the placebo group. In experiment 2, 44 participants were included. Due to the sensitivity check in which those participants were excluded who did not perceive the baseline stimuli as intended, e.g., mechanical itch stimuli not evoking itch (24), 29 participants were included in the analyses of the models related to mechanical touch, and 39 participants in the analyses of the models related to mechanical itch. Participants’ demographics and spontaneous fatigue/pain/itch levels are reported in Supplementary Appendix Table 2.
Induced and generalized nocebo and placebo effects
Induction and generalization of nocebo and placebo effects were previously reported (4, 24). A summary of descriptive results of all stimuli scores by group and trial type are reported in Supplementary Appendix Tables 3, 4. In short, in experiment 1, both nocebo and placebo effects were significantly induced on heat pain as hypothesized. As also hypothesized, nocebo and placebo effects significantly generalized from heat pain to pressure pain, but contrary to our hypothesis they did not generalize to cowhage-evoked itch. In experiment 2, nocebo effects were significantly induced on cowhage-evoked itch as hypothesized. As also hypothesized, nocebo effects from cowhage-evoked itch significantly generalized to mechanical itch, but contrary to our hypothesis nocebo effects did not generalize to mechanical touch. In both experiments, at least 60% of participants were classified as generalization responders for each generalization effect, despite a lack of generalization effects across modalities at the group level. Frequencies of participants showing generalization per effect are reported in Supplementary Appendix Table 5.
Predictors and intercorrelations
Tables 1, 2 display an overview of mean, standard deviations, observed range, and intercorrelations of dependent outcomes and the relevant predictors in both experiments. Regarding nocebo effects, the correlation coefficients showed that none of the predictors was significantly associated with induction and generalization of nocebo effects. Regarding placebo effects, only stress symptoms were significantly associated with generalization of placebo effects to pressure pain (r = −0.39, p = 0.03).
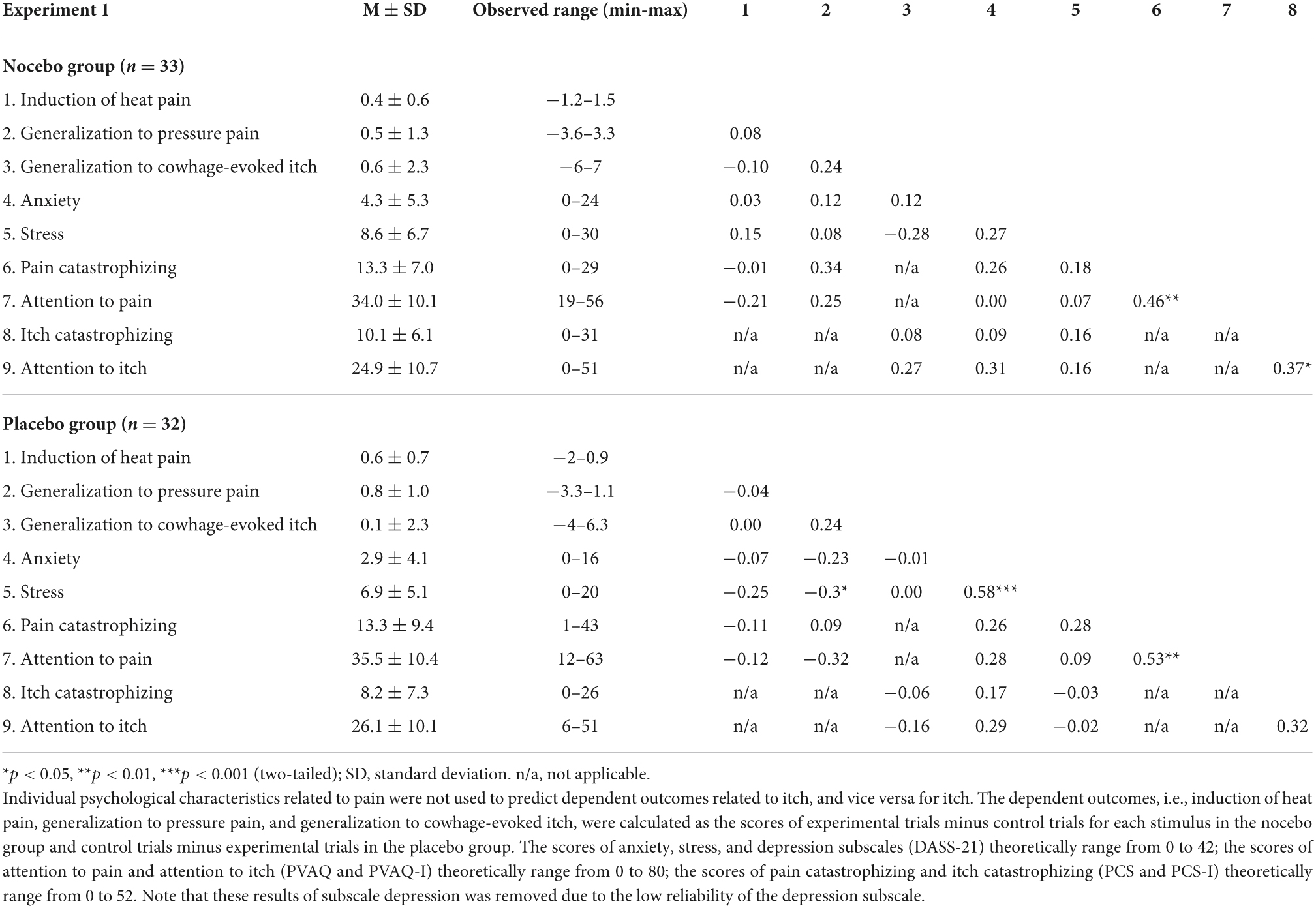
Table 1. Mean ± SD and intercorrelations of predictors and dependent outcomes in the nocebo and the placebo group in experiment 1.
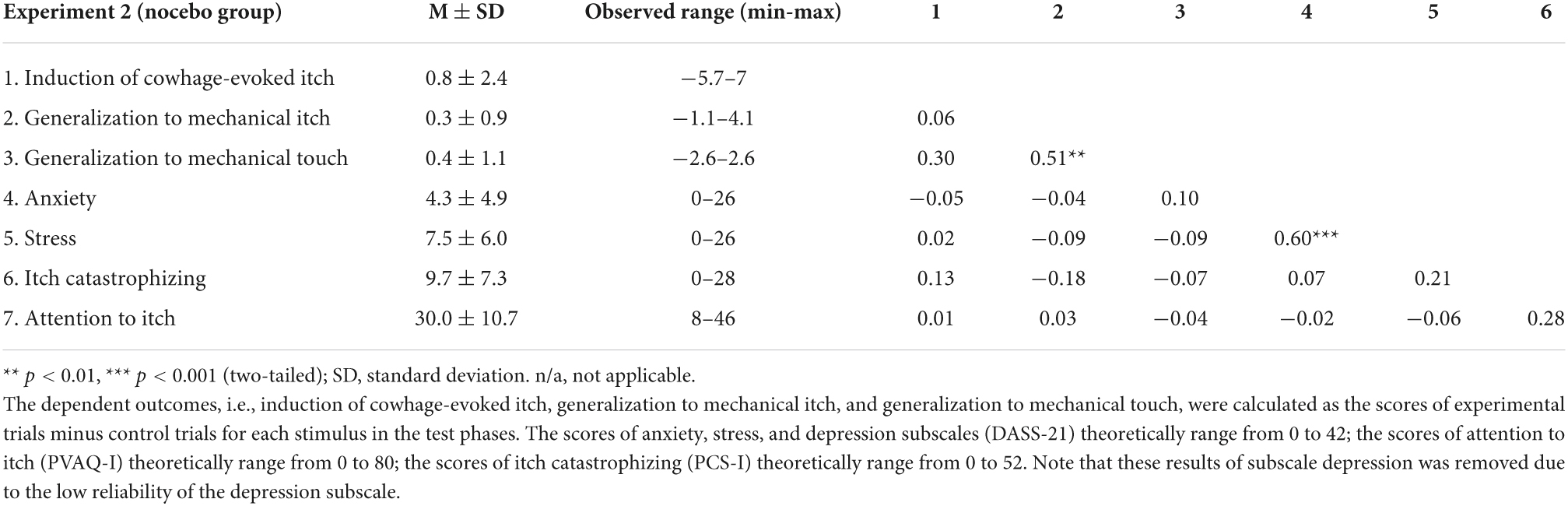
Table 2. Mean (M) ± SD and intercorrelations of predictors and dependent outcomes in experiment 2 (n = 44).
Regression analyses
Table 3 displays the results of regression analyses regarding induction and generalization of nocebo and placebo effects. The results of regression analyses regarding expected nocebo and placebo effects are listed in Supplementary Appendix Table 6.
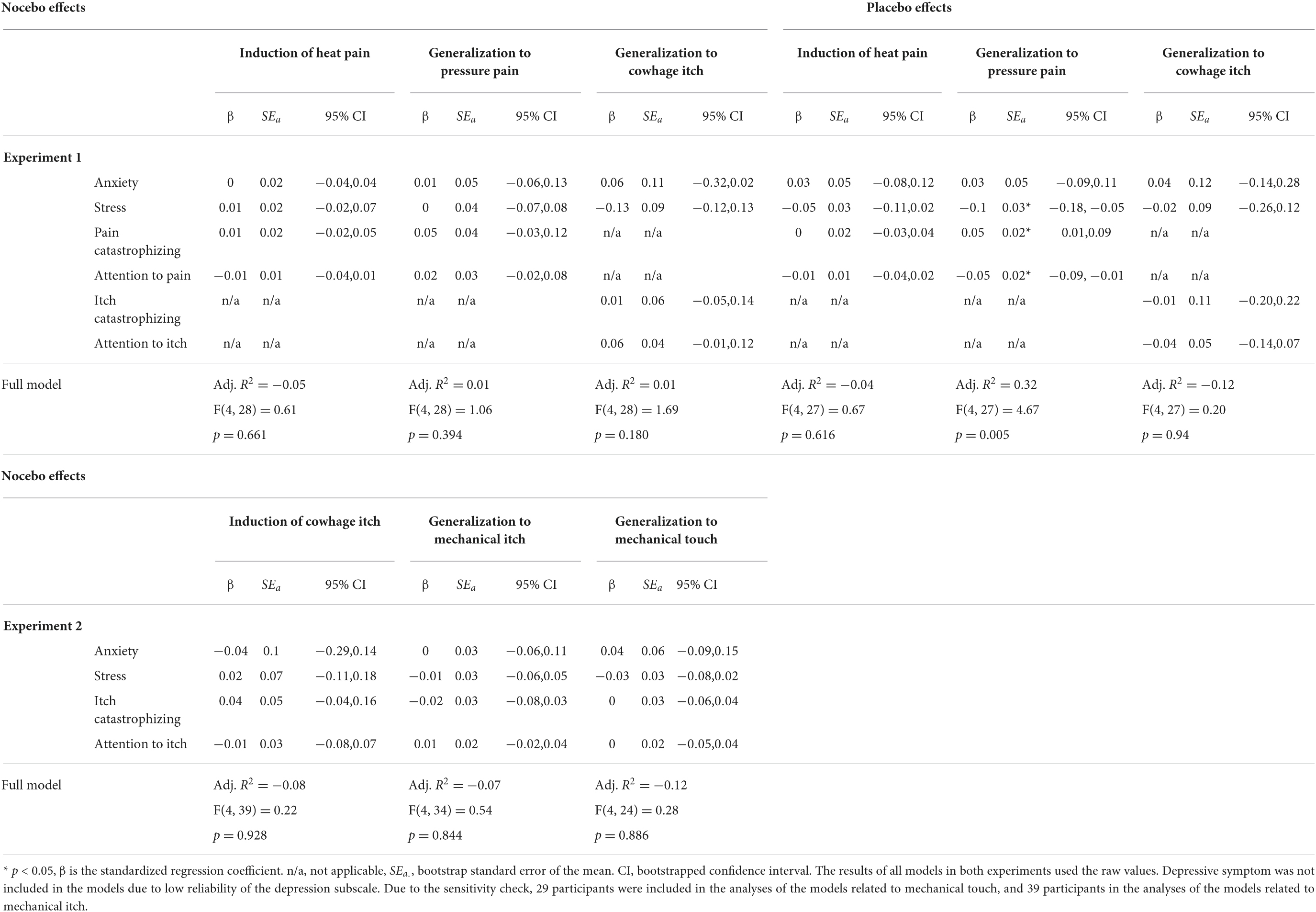
Table 3. An overview of multiple regression analyses via forced entry to predict induction of nocebo and placebo effects on heat pain and their generalization to pressure pain and to cowhage-evoked itch in experiment 1 (n = 33 in the nocebo group, n = 32 in the placebo group), and to predict induction of nocebo effects on cowhage-evoked itch and their generalization to mechanical itch and to mechanical touch in experiment 2 (n = 44).
Regarding the primary objective concerning nocebo effects, in line with the results from the correlations, multiple regression analyses indicated that the studied psychological characteristics predicted neither induction of nocebo effects on heat pain and cowhage-evoked itch, nor generalization of nocebo effects within modalities (i.e., from heat pain to pressure pain and from cowhage-evoked itch to mechanical itch) or across modalities (i.e., from heat pain to cowhage-evoked itch and from cowhage-evoked itch to mechanical touch) (Table 3). Influential values were observed in the model of generalization of nocebo effects to cowhage-evoked itch, but removal of the influential values did not lead to different results.
Regarding the secondary objective concerning placebo effects, multiple regression analyses showed that lower stress symptoms (β = −0.1, 95% CI [−0.18, −0.05]), less attention to pain (β = −0.05, 95% CI [−0.09, −0.01]), and higher pain catastrophizing (β = 0.05, 95% CI [0.01, 0.09]), predicted stronger generalization of placebo effects to pressure pain (full model: F(4,27) = 4.67, p = 0.005, Adj. R2 = 0.32) (Table 3).
Regarding the exploratory objective concerning expected nocebo and placebo effects, multiple regression analyses showed that lower itch catastrophizing (β = −0.15, 95% CI [−0.28, −0.04]) and higher attention to itch (β = 0.07, 95% CI [0.01, 0.14]) predicted higher expectancies of nocebo effects on cowhage-evoked itch (generalization) (full model: F(4,28) = 3.27, p = 0.025, Adj. R2 = 0.22) (Supplementary Appendix Table 6). Similar analyses showed that less attention to itch (β = −0.06, 95% CI [−0.12, −0.02]) alone predicted higher expectancies of nocebo effects on mechanical sensations (generalization) (full model: F(4,39) = 1.72, p = 0.166, Adj. R2 = 0.06) (Supplementary Appendix Table 6).
Discussion
The current study aimed to explore predictors for induction and generalization of nocebo and placebo effects within and across pain and itch modalities. Our results showed that anxiety-, stress symptoms, pain/itch catastrophizing, and attention to pain/itch did not significantly predict, with relatively small confidence intervals, induction of nocebo and placebo effects. Regarding generalization, only lower stress symptoms, lower attention to pain, and higher pain catastrophizing weakly predicted a stronger generalization of placebo effects from heat pain to pressure pain. These findings and their implications should be interpreted with caution, considering the sample was limited in size and consisted of young healthy individuals.
Regarding nocebo effects, the findings that the psychological characteristics did not predict nocebo effect induction are in line with several previous studies indicating the lack of significant associations between psychological characteristics and nocebo effects (1, 9, 14, 32). Moreover, no significant predictors were found for generalization of nocebo effects within and across the pain and itch modalities. This may be partly caused by our target sample of young healthy individuals who have, unsurprisingly, low levels of negative affect and cognitions. It should be noted that nocebo effects were not found to generalize across modalities. Therefore, replication is necessary before drawing a conclusion. The exploratory analyses of the prediction of participants’ expectancies showed that lower itch catastrophizing and higher attention to itch predicted higher expectancies of nocebo effects on cowhage-evoked itch (generalization). As the overall pooled associations were small and the (directions of) predictors were not consistently found for generalization across the two experiments, these findings should be interpreted with caution. From a hypothesis-generating perspective, the current study paves the way to further explore potential predictors of generalization of nocebo effects.
Regarding placebo effects, the findings that the psychological characteristics did not predict placebo effect induction contrasts with some previous research with comparable sample sizes e.g., (11, 33). However, two recent studies with large cohorts yielded mixed results, with one study (N = 397) reporting negative associations between negative affect (including anxiety-, and stress symptoms) and placebo effects (10) and one reporting (N = 624) null associations (14). Further research herein may examine possible interactions between multiple predictors and explore other potential predictors (e.g., fear). Regarding generalization of placebo effects, there are some indications for psychological characteristics that may explain small parts of the variance. Specifically, stronger generalization of placebo effects within the pain modality may be predicted by lower stress symptoms, less attention to pain, and higher pain catastrophizing. One potential explanation could be that people with lower stress symptoms and less attention to symptoms, may tend to focus on positive information and avoid harmful information (34, 35). However, the result also showed that higher pain catastrophizing may be relevant to a stronger generalization of placebo effects, which contrasts with theory (36) and previous research (37). As these predictors only explained a small part of the variance, no firm conclusions can be drawn from these findings. Further research is warranted to validate these findings. Moreover, the exploratory results did not suggest that psychological characteristics predict expectancies of the induction and generalization of placebo effects. One possible explanation is that the psychological characteristics measured in this study may be less relevant in the facilitation/inhibition of positive expectancies (38, 39). More research is warranted, as a better understanding of individual responses could foster the efficacy of positive treatment outcomes.
Limitations and suggestions for future studies
First, given the limited sample size and the inclusion of only young healthy participants, variances in the characteristics could have been restricted, and false negative findings might have occurred. Besides, representativeness of the demographics and psychological characteristics could limit the generalizability of the current findings to the general population or patient populations. Further studies should include more variance in characteristics such as age and health status (40, 41). Besides, although our sample size met a minimum requirement (N = 25) for multiple regressions with multiple predictors (25) and our study was of exploratory, hypothesis-generating nature, only large effects may be detected with this small sample and thus results should be interpreted with caution. Future research with larger cohorts is required, for instance in the forms of meta-analyses on individual data. Second, considering the low reliability of the depression subscale (depressive symptoms were removed from all analyses), further studies may use other questionnaires such as Positive and Negative Affect Schedule e.g., (42). Also, the generally low levels of cognitive-affective factors could not provide a comprehensive insight into their predictive value. It may be helpful to include participants at different baseline levels of cognitive-affective factors. Next to self-report measurements, experimental research directly manipulating factors such as anxiety- and stress symptoms and assessing effects on induction and generalization of nocebo and placebo effects seems to be currently lacking. Third, the lack of generalization of nocebo effects across modalities at the group level may have affected the current results. However, psychological characteristics may still help to distinguish individuals who tend to generalize and those who do not at the individual level, although this study did not provide a clear pattern. Finally, it is common that prediction research, including ours, only included few potential predictors at once. However, it appears suboptimal to account for only few factors to predict nocebo and placebo effects as well as their generalization, especially in clinical settings. Future studies are recommended to not only examine multiple psychological characteristics at once, but also to combine these characteristics with other factors such as personality traits, e.g., (15, 43) genetic variants, e.g., (44, 45) doctor-patient relationships, e.g., (46, 47) treatment history, e.g., (48) and various contextual variables e.g., (49), to get a comprehensive multifaceted structure of predicting nocebo and placebo effects.
Suggestions for future research
Some suggestions need to be discussed. On top, assessing changes in dynamic individual characteristics, such as state anxiety and state fear, before versus after the nocebo and placebo manipulations could provide more insight into the underlying dynamics of nocebo and placebo effects as well as their generalization. Second, as different mechanisms are supposed to underlie nocebo and placebo effects (3), it is recommended to assess different predictors for nocebo and placebo effects, e.g., anxiety for nocebo effects and optimism for placebo effects (32, 33). Another recommendation to advance the field is to systematically test theoretical models such as the fear-avoidance model e.g., (22) and a predictive coding framework regarding symptom perception e.g., (50). Finally, including patient samples would be an important next step. For instance, patients with chronic itch due to atopic dermatitis appear to be more sensitive to nocebo-like effects on itch than healthy individuals (51, 52). Assessing the predictors in patients’ treatment outcomes as well as subsequent treatment outcomes would contribute to identifying patients who are sensitive to nocebo and placebo effects. This could eventually provide individualized interventions to increase treatment effectiveness.
Conclusion
This study suggests that the psychological characteristics may not (or only weakly) predict the induction and generalization of nocebo and placebo effects in young healthy individuals. Given the current restrictions to the sample, however, it cannot be ruled out that these characteristics do play a significant role in placebo and nocebo effects on pain and itch and their generalization. The current study can be a starting point for further exploring the relevance of these predictors for generalization of nocebo and placebo effects. Exploring the predictors for nocebo and placebo effects as well as their generalization would contribute to helping treatment outcomes in the clinic and establishing individualized treatments schemes, thereby helping increase the success of treatments.
Data availability statement
Data not included in the article/Supplementary material will be made available through Dataverse (https://dataverse.nl/dataverse/leidenuniversity). Further inquiries also can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Psychology Research Ethics Committee of Leiden University. The participants provided their written informed consent to participate in this study.
Author contributions
LW analyzed the data and drafted the manuscript. All authors have critically revised, edited, and approved the final manuscript.
Funding
This work was supported by the China Scholarship Council (CSC; grant number: 201706990035) granted to LW and by a VICI grant from the Netherlands Organization for Scientific Research (NWO; grant number: 45316004) granted to AE.
Acknowledgments
We thank all the student assistants who were involved in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.838578/full#supplementary-material
Footnotes
References
1. Wolters F, Peerdeman KJ, Evers AWM. Placebo and nocebo effects across symptoms: from pain to Fatigue, Dyspnea, Nausea, and Itch. Front Psychiatry. (2019) 10:470. doi: 10.3389/fpsyt.2019.00470
2. Blythe JS, Peerdeman KJ, Veldhuijzen DS, van Laarhoven AIM, Evers AWM. Placebo and nocebo effects on itch: a review of experimental methods. Itch. (2019) 4:e27–27. doi: 10.1097/itx.0000000000000027
3. Colagiuri B, Schenk LA, Kessler MD, Dorsey SG, Colloca L. The placebo effect: from concepts to genes. Neuroscience. (2015) 307:171–90. doi: 10.1016/j.neuroscience.2015.08.017
4. Weng L, Peerdeman KJ, Della Porta D, Van Laarhoven AIM, Evers AWM. Can placebo and nocebo effects generalize within pain modalities and across somatosensory sensations? Pain. (2022) 163:548–59. doi: 10.1097/j.pain.0000000000002390
5. Bartels DJP, Van Laarhoven AIM, Stroo M, Hijne K, Peerdeman KJ, Donders ART, et al. Minimizing nocebo effects by conditioning with verbal suggestion: a randomized clinical trial in healthy humans. PLoS One. (2017) 12:e0182959. doi: 10.1371/journal.pone.0182959
6. Carlino E, Guerra G, Piedimonte A. Placebo effects: from pain to motor performance. Neurosci Lett. (2016) 632:224–30. doi: 10.1016/j.neulet.2016.08.046
7. Shepard RN. Stimulus and response generalization: deduction of the generalization gradient from a trace model. Psychol Rev. (1958) 65:242–56. doi: 10.1037/h0043083
8. Kaptchuk TJ, Kelley JM, Deykin A, Wayne PM, Lasagna LC, Epstein IO, et al. Do “placebo responders” exist? Contemp Clin Trials. (2008) 29:587–95. doi: 10.1016/j.cct.2008.02.002
9. Geers AL, Faasse K, Guevarra DA, Clemens KS, Helfer SG, Colagiuri B. Affect and emotions in placebo and nocebo effects: what do we know so far? Soc Personal Psychol Compass. (2021) 15:1–19. doi: 10.1111/spc3.12575
10. Wang Y, Chan E, Dorsey SG, Campbell C, Colloca L. placebo responders versus non-responders: a cross-sectional cohort study for psychological determinants. Pain. (2021) 163:1078–90. doi: 10.1097/j.pain.0000000000002478
11. Flaten MA, Aslaksen PM, Lyby PS, Bjørkedal E. The relation of emotions to placebo responses. Philos Trans R Soc B Biol Sci. (2011) 366:1818–27. doi: 10.1098/rstb.2010.0407
12. Bartels DJP, Van Laarhoven AIM, Van De Kerkhof PCM, Evers AWM. Placebo and nocebo effects on itch: effects, mechanisms, and predictors. Eur J Pain. (2016) 20:8–13. doi: 10.1002/ejp.750
13. Horing B, Weimer K, Muth ER, Enck P. Prediction of placebo responses: a systematic review of the literature. Front Psychol. (2014) 5:1079. doi: 10.3389/fpsyg.2014.01079
14. Feldhaus MH, Horing B, Sprenger C, Büchel C. Association of nocebo hyperalgesia and basic somatosensory characteristics in a large cohort. Sci Rep. (2021) 11:1–12. doi: 10.1038/s41598-020-80386-y
15. Peciña M, Azhar H, Love TM, Lu T, Fredrickson BL, Stohler CS, et al. Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology. (2013) 38:639–46. doi: 10.1038/npp.2012.227
16. Janssens T, Meulders A, Cuyvers B, Colloca L, Vlaeyen JWS. Placebo and nocebo effects and operant pain-related avoidance learning. Pain Rep. (2019) 4:1–9. doi: 10.1097/PR9.0000000000000748
17. Meulders A, Jans A, Vlaeyen JWS. Differences in pain-related fear acquisition and generalization: an experimental study comparing fibromyalgia patients and healthy controls. Pain. (2015) 2:108–22. doi: 10.1016/j.pain.0000000000000016
18. Thomaidou MA, Veldhuijzen DS, Meulders A, Evers AWM. An experimental investigation into the mediating role of pain-related fear in boosting nocebo hyperalgesia. Pain. (2021) 162:287–99. doi: 10.1097/j.pain.0000000000002017
19. Kausche FM, Zerbes G, Kampermann L, Müller JC, Wiedemann K, Büchel C, et al. Acute stress leaves fear generalization in healthy individuals intact. Cogn Affect Behav Neurosci. (2021) 21:372–89. doi: 10.3758/s13415-021-00874-0
20. Sep MSC, Steenmeijer A, Kennis M. The relation between anxious personality traits and fear generalization in healthy subjects: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2019) 107:320–8. doi: 10.1016/j.neubiorev.2019.09.029
21. Asok A, Kandel ER, Rayman JB. The neurobiology of fear generalization. Front Behav Neurosci. (2019) 12:329. doi: 10.3389/fnbeh.2018.00329
23. Vlaeyen JWS, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. (2012) 153:1144–7. doi: 10.1016/j.pain.2011.12.009
24. Weng L, Laarhoven AIM, Peerdeman KJ, Evers AWM. Induction and generalization of nocebo effects on itch. Exp Dermatol. (2022) 31:878–89. doi: 10.1111/exd.14522
25. Jenkins DG, Quintana-Ascencio PF. A solution to minimum sample size for regressions. PLoS One. (2020) 15:e0229345. doi: 10.1371/journal.pone.0229345
26. Norton PJ. Depression anxiety and stress scales (DASS-21): psychometric analysis across four racial groups. Anxiety Stress Coping. (2007) 20:253–65. doi: 10.1080/10615800701309279
27. Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the depression anxiety stress scales (DASS) with the beck depresion and anxiety inventories. Behav Res Ther. (1995) 33:335–43. doi: 10.1016/0005-7967(94)00075-U
28. Roelofs J, Peters ML, McCracken L, Vlaeyen JWS. The pain vigilance and awareness questionnaire (PVAQ): further psychometric evaluation in fibromyalgia and other chronic pain syndromes. Pain. (2003) 101:299–306. doi: 10.1016/S0304-3959(02)00338-X
29. van Laarhoven AIMM, van Damme S, Lavrijsen APMM, van Ryckeghem DM, Crombez G, Evers AWMM. Attentional processing of itch. Psychol Res. (2018) 82:876–88. doi: 10.1007/s00426-017-0878-2
30. Sullivan MMJLM, Bishop SRS, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. (1995) 7:524–32. doi: 10.1037//1040-3590.7.4.524
31. Andersen HH, van Laarhoven AIM, Elberling J, Arendt-Nielsen L. Modulation of itch by conditioning itch and pain stimulation in healthy humans. J Pain. (2017) 18:1437–50. doi: 10.1016/j.jpain.2017.07.002
32. Kern A, Kramm C, Witt CM, Barth J. The influence of personality traits on the placebo/nocebo response: a systematic review. J Psychosom Res. (2020) 128:109866. doi: 10.1016/j.jpsychores.2019.109866
33. Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol. (2007) 20:435–9. doi: 10.1097/ACO.0b013e3282b972fb
34. Andreotti C, Garrard P, Venkatraman SL, Compas BE. Stress-related changes in attentional bias to social threat in young adults: Psychobiological associations with the early family environment. Cognit Ther Res. (2015) 39:332–42. doi: 10.1007/s10608-014-9659-z
35. Crombez G, Van Ryckeghem DML, Eccleston C, Van Damme S. Attentional bias to pain-related information: a meta-analysis. Pain. (2013) 154:497–510. doi: 10.1016/j.pain.2012.11.013
36. Darnall BD, Colloca L. Optimizing Placebo and Minimizing Nocebo to Reduce Pain, Catastrophizing, and Opioid Use: A Review of the Science and an Evidence-Informed Clinical Toolkit. 1st ed. (Vol. 139). Amsterdam: Elsevier Inc (2018). doi: 10.1016/bs.irn.2018.07.022
37. Sullivan MJL, Lynch ME, Clark AJ, Mankovsky T, Sawynok J. Catastrophizing and treatment outcome: differential impact on response to placebo and active treatment outcome. Contemp Hypn. (2008) 25:129–40. doi: 10.1002/ch.365
38. Schut C, Rädel A, Frey L, Gieler U, Kupfer J. Role of personality and expectations for itch and scratching induced by audiovisual itch stimuli. Eur J Pain. (2016) 20:14–8. doi: 10.1002/ejp.751
39. Vase L, Skyt I, Petersen GL, Price DD. Placebo and nocebo effects in chronic pain patients: how expectations and emotional feelings contribute to the experience of pain. Zeitschrift Psychol J Psychol. (2014) 222:135–9. doi: 10.1027/2151-2604/a000181
40. Wrobel N, Fadai T, Sprenger C, Hebebrand J, Wiech K, Bingel U. Are children the better placebo analgesia responders? An experimental approach. J Pain. (2015) 16:1005–11. doi: 10.1016/j.jpain.2015.06.013
41. Enck P, Klosterhalfen S. Does sex/gender play a role in placebo and nocebo effects? Conflicting evidence from clinical trials and experimental studies. Front Neurosci. (2019) 13:160. doi: 10.3389/fnins.2019.00160
42. Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. (2004) 43:245–65. doi: 10.1348/0144665031752934
43. Corsi N, Colloca L. Placebo and nocebo effects: the advantage of measuring expectations and psychological factors. Front Psychol. (2017) 8:308. doi: 10.3389/fpsyg.2017.00308
44. Yu R, Gollub RL, Vangel M, Kaptchuk T, Smoller JW, Kong J. Placebo analgesia and reward processing: integrating genetics, personality, and intrinsic brain activity. Hum Brain Mapp. (2014) 35:4583–93. doi: 10.1002/hbm.22496
45. Frisaldi E, Shaibani A, Benedetti F. Understanding the mechanisms of placebo and nocebo effects. Swiss Med Wkly. (2020) 150:1–10. doi: 10.4414/smw.2020.20340
46. Evers AWM. Using the placebo effect: how expectations and learned immune function can optimize dermatological treatments. Exp Dermatol. (2017) 26:18–21. doi: 10.1111/exd.13158
47. Czerniak E, Biegon A, Ziv A, Karnieli-Miller O, Weiser M, Alon U, et al. Manipulating the placebo response in experimental pain by altering doctor’s performance style. Front Psychol. (2016) 7:874. doi: 10.3389/fpsyg.2016.00874
48. Müller M, Kamping S, Benrath J, Skowronek H, Schmitz J, Klinger R, et al. Treatment history and placebo responses to experimental and clinical pain in chronic pain patients. Eur J Pain. (2016) 20:1530–41. doi: 10.1002/ejp.877
49. Palese A, Rossettini G, Colloca L, Testa M. The impact of contextual factors on nursing outcomes and the role of placebo/nocebo effects: a discussion paper. Pain Rep. (2019) 4:1–9. doi: 10.1097/PR9.0000000000000716
50. Van den Bergh O, Witthöft M, Petersen S, Brown RJ. Symptoms and the body: taking the inferential leap. Neurosci Biobehav Rev. (2017) 74:185–203. doi: 10.1016/j.neubiorev.2017.01.015
51. Napadow V, Li A, Loggia MLL, Kim J, Mawla I, Desbordes G, et al. The imagined itch: brain circuitry supporting nocebo-induced itch in atopic dermatitis patients. Allergy Eur J Allergy Clin Immunol. (2015) 70:1485–92. doi: 10.1111/all.12727
Keywords: predictors, nocebo effects, placebo effects, pain, itch, pruritus, generalization
Citation: Weng L, van Laarhoven AIM, Peerdeman KJ and Evers AWM (2022) Do individual psychological characteristics predict induction and generalization of nocebo and placebo effects on pain and itch? Front. Psychiatry 13:838578. doi: 10.3389/fpsyt.2022.838578
Received: 18 December 2021; Accepted: 05 July 2022;
Published: 04 August 2022.
Edited by:
Chamindi Seneviratne, University of Maryland Baltimore, United StatesReviewed by:
Stefan Schmidt, University of Freiburg Medical Center, GermanyJesper Elberling, University of Copenhagen, Denmark
Copyright © 2022 Weng, van Laarhoven, Peerdeman and Evers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingling Weng, bC53ZW5nQGZzdy5sZWlkZW51bml2Lm5s
 Lingling Weng
Lingling Weng Antoinette I. M. van Laarhoven
Antoinette I. M. van Laarhoven Kaya J. Peerdeman
Kaya J. Peerdeman Andrea W. M. Evers
Andrea W. M. Evers