- 1Department of Biomedical and Clinical Sciences, Pharmacovigilance & Clinical Research, International Centre for Pesticides and Health Risk Prevention, ASST Fatebenefratelli-Sacco, Università degli Studi di Milano, Milan, Italy
- 2Department of Biomedical and Clinical Sciences, Psychiatry Unit 2, ASST Fatebenefratelli-Sacco, Università degli Studi di Milano, Milan, Italy
- 3CRC “Aldo Ravelli” for Neurotechnology & Experimental Brain Therapeutics, Università degli Studi di Milano, Milan, Italy
- 4Scientific Institute, IRCCS E. Medea, Bosisio Parini, Italy
- 5Department of Psychiatry and Behavioral Sciences, Bipolar Disorders Clinic, Stanford Medical School, Stanford University, Stanford, CA, United States
- 6Centro per lo studio dei meccanismi molecolari alla base delle patologie neuro-psico-geriatriche, Università degli Studi di Milano, Milan, Italy
Introduction: Metformin has shown good efficacy in the management of antipsychotic-induced metabolic syndrome (MetS) in patients with schizophrenia or schizoaffective disorders. Its ability to induce antidepressant behavioural effects and improve cognitive functions has also been investigated: yet information has not been systematized. The aim of this study was therefore to investigate the effects of metformin on cognitive and other symptom dimension in schizophrenic patients treated with antipsychotics through a systematic review and meta-analysis.
Methods: We searched PubMed, ClinicalTrials.Gov, Embase, PsycINFO, and WHO ICTRP database up to February 2022, Randomised Controlled Trials (RCT) evaluating patients diagnosed with schizophrenia and related disorders, who were treated with metformin as add-on therapy to antipsychotics for the treatment of weight gain and in which changes in psychiatric symptoms and cognitive functions were evaluated.
Results: A total of 19 RCTs met the inclusion criteria. Meta-analysis was performed on 12 eligible studies. We found a positive trend after 24 weeks of treatment in schizophrenic patients with stable conditions [SMD (95%CI) = -0.40 (−0.82;0.01), OR (95%CI) = 0.5 (−2.4;3.4)]. Better performance was detected in the Brief Assessment of Cognition in Schizophrenia and Positive and Negative Syndrome Scale (PANSS) with low heterogeneity among studies. One study reported changes in BACS-verbal memory subdomain in favour of placebo [MD (95%CI) = -16.03 (-23.65;8.42)]. Gastrointestinal disorders, xerostomia, and extrapyramidal syndrome were the most reported adverse effects. Psychiatric adverse events were also described: in particular, symptoms attributable to a relapse of schizophrenia.
Conclusion: Some degree of efficacy was found for Metformin in improving cognitive and other symptom dimensions in patients with Schizophrenia. Given the clinical relevance of this potential pharmacological effect, longer specific studies using adequate psychometric scales are strongly recommended. Likewise, how metformin acts in this context needs to be evaluated in order to enhance its efficacy or find more efficacious drugs.
1. Introduction
Schizophrenia (SCZ) is a chronic disorder characterized by a combination of psychotic symptoms (i.e., hallucinations, delusions, and disorganization) and motivational and cognitive dysfunctions. It affects about 1% of the world’s population and it is considered a high-cost disease due to the lifelong clinical course and the need of healthcare resource utilization (1).
Patients with SCZ have a mortality rate 2.6 times higher than that in the general population, mostly due to the occurrence of cardiovascular diseases and metabolic syndrome (MetS). The latter disease frequently arises in patients with SCZ due to a dysregulated and unhealthy lifestyle (2, 3), but it is also related to the treatment with second-generation antipsychotics (SGAs) (4–6). Considering pharmacodynamic implications, occupancies of H1 histaminergic and M1/M3 cholinergic receptors represent risk factors for increased levels of total cholesterol, HDL, LDL, insulin, and triglycerides (7, 8). Therefore, metabolic adverse effects of SGAs contribute to long-term risk of mortality and to short-term risk of obesity and MetS (9, 10). Current therapeutic options for weight control consist in dietary support and regular exercise, which, however, may not be sufficient for antipsychotic induced MetS (11, 12).
Eighty% of schizophrenic patients are also affected by cognitive alterations (13). Unfortunately, available antipsychotic drugs are not only ineffective on cognitive impairment (14), but can also worsen it (15, 16). Moreover, pharmacological cognitive enhancers in SCZ have limited efficacy and tolerability issues (17–19). Cognitive remediation (CR), a behavioural training–based intervention (20, 21), is currently the best option to improve cognition (22, 23), but it was proved to be ineffective in patients with Mets (24, 25).
In order to prevent MetS in psychiatric patients, many drugs have been evaluated, especially among antidiabetic drugs, that showed efficacy, good tolerability and compliance (26). One of the most studied drugs for preventing and treating antipsychotic-induced weight gain and MetS is metformin, a biguanide drug used to treat DM2 because of its high efficacy in lowering plasma glucose levels. It also exerts additional metabolic effects such as weight loss, reduction of triglycerides and LDL levels while increasing HDL and sensitivity to insulin (27). There is evidence that metformin can control antipsychotic induced MetS in schizophrenic patients (28). Of importance, its ability to induce antidepressant behavioural effects and improve cognitive functions has also been investigated (29). According to recent preclinical and clinical findings, metformin can penetrate through the Blood–Brain Barrier (BBB) into the central nervous system (CNS) where it promotes neuroprotective, neurotrophic, neurogenetic and anti-inflammatory effects (30). Furthermore, metformin reduces the inflammatory markers p-IKB, IL-1, and VEGF in neuronal cells reducing the neuroinflammation, a driver for neurotoxicity and the development of neuropsychiatric diseases (31, 32). A recent review concludes that metformin may activate the AMP Protein Kinase (AMPK), an enzyme that regulates the metabolic process of lipids and carbohydrates, leading to potential cognitive properties (33). Consistent with this hypothesis, many studies conducted in murine models have demonstrated the potential positive effect of metformin on cognition in neurodegenerative disorders (34) as diverse as Alzheimer’s disease (35), and traumatic brain injuries (36). At the same time, animal studies have focused on the beneficial effects of metformin on the CNS even across different neuropsychiatric conditions, such as anxiety (37), depression (38), schizophrenia-like symptoms (39) and seizures (40).
In a population-based longitudinal cohort study of diabetic individuals, participants using metformin showed higher performances in neuropsychological tests involving cognitive functions, especially verbal learning, working memory and executive function; even after adjusting for behavioural lifestyle or clinical conditions, these results did not change (41). In diabetic patients metformin had a neuroprotective function for the prevention of dementia (42). An improvement in cognitive function through the use of metformin was observed also in Huntington’s disease (43) and in a small sample of patients diagnosed with Fragile X Syndrome (44). In a randomised controlled trial, treatment with metformin showed anti-depressive effects in depressive and diabetic patients (45). Other clinical trials and observational studies, however, did not confirm the efficacy of metformin on cognitive function or on prevention against any form of dementia (46); moreover, metformin monotherapy has also been found to have negative effects in diabetic patients increasing the risk of Parkinson disease (47).
Even though data from both preclinical and clinical studies on possible pro-cognitive effects of metformin provide contrasting outcomes no attempts have been done to date to systematize and weight the available knowledge. We have thus specifically investigated the effects of metformin on psychiatric and cognitive functions through a systematic review of literature and meta-analysis of clinical trials in a selected population composed by schizophrenic patients treated with antipsychotics.
2. Materials and methods
2.1. Literature search
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (48). We submitted our Protocol at the International Prospective Register of Ongoing Systematic Reviews (CRD42021250690). We searched up to February 2022 PubMed, ClinicalTrials.Gov, Embase, PsycINFO, WHO ICTRP database using a search string containing two sets of words referring to 1) schizophrenic patients and 2) metformin that were combined using the Boolean operator “AND.” There was no language, date, document type, or publication status limitations for inclusion of records. Additional articles were collected through the reference lists of reviews and eligible studies we found. We did not plan to contact authors for unpublished data. An example of a search string is fully described in the Supplementary Material S1.
We included only Randomised Controlled Trials (RCT) evaluating people diagnosed with SCZ and related disorders (such as schizoaffective disorder, schizophreniform disorder, and delusional disorder) who were treated with metformin as add-on therapy to antipsychotics. We did not use any criteria for age, nationality or sex of the participants, duration/stage of illness, treatment setting, current clinical state, or symptom clusters. We considered metformin compared to placebo or other types of pharmacological interventions for the treatment of weight gain. We considered behavioural interventions only when combined with a pharmacological intervention. Primary outcomes were the changes in psychiatric and cognitive scales: the psychometric properties of the measuring instrument should have been validated and the measuring instrument should have not been modified for that trial.
2.2. Data extraction and processing
All titles and abstracts were assessed independently by two authors (GM, RL) to identify potentially relevant articles. Studies fulfilling the eligibility criteria were included and their full texts were retrieved and reviewed in duplicate (GM, ER). Discrepancies during the check of the two-step independent screening were resolved through the discussion with a third author (VB). Data were extracted by two researchers (RL, VB) and disagreements were resolved by consensus and consultation with the expert group (MP, CC, GC).
For every study the following data were extracted: First author; Year; Study duration; Study type (blinding/design); Number of subjects; diagnosis; number of males; age; antipsychotic(s) used and dose; control/comparator/placebo group; concomitant drugs; additional behavioural interventions; all outcomes of interest; Adverse Drug Reactions. Endpoint data were mainly chosen, mean change data if the former was not available.
2.3. Risk of bias assessment
This study was designed as an Intention-To-Treat (ITT) analysis. The risk of bias of included studies was assessed by three authors (GM, RL, ER) by using the Cochrane risk-of-bias tool for randomised trials (RoB 2) (49). Disagreements were resolved by consensus among them and a further consultation with the expert group (CC and VB). We planned to conduct a sensitivity analysis excluding studies rated with a high risk of bias if the number of remaining studies exceeds three.
2.4. Meta-analysis
A meta-analysis was performed by using the generic inverse variance method with a random effect model combining psychiatric scales reported by each study, which were Brief Psychiatric Rating Scale (BPRS) (50), Positive and Negative Syndrome Scale (PANSS) (51), Clinical Global Impression Scale (CGI) (52), Global Assessment of Functioning (GAF) (53), Scale for the Assessment of Negative Symptoms (SANS) and Scale for the Assessment of Positive Symptoms (SAPS) (54), Brief Assessment of Cognition in SCZ (BACS) (55) and Patient Health Questionnaire-9 (PHQ9) (56). Among scales, when more than one tool was available, a priority order was defined considering their impact on cognitive assessment. The procedure is clearly described in Supplementary Material S2. Thus, the final priority order adopted was as follows: BACS composite T score > BACS verbal memory T score > PANSS> BPRS > GAF > CGI > SANS and SAPS > PHQ9.
Assumptions were made regarding missing SDs using data of similar studies in terms of population, number of patients, and the point estimate. Forest plots were created for the main outcomes. Sensitivity analyses were performed excluding these studies to check their influence in the results.
In order to help the reader in the interpretation of results we also provided Mean Differences (MDs) of subgroup analyses concerning single scales. In these analyses, we included all studies reporting results of the scale of interest, we did not follow the priority order reported above.
RevMan 5 was the chosen tool to perform the meta-analysis (57).
2.5. Meta-regression
It is known that the amount of adipose tissue of the patients impacts on the pharmacokinetic properties of antipsychotics and thus on their therapeutic effect (58–61).
We then explored the influence of the Body Mass Index (BMI) at baseline on the treatment effect (SMD) of our main meta-analysis to evaluate if a better response is more related to a better response to the psychiatric treatment than the efficacy of metformin in the regulation of psychiatric and cognitive symptoms. A random-effects meta-regression model with Knapp-Hartung method was performed. Data are provided by a regression bubble plot. The [meta] R package was used to perform meta-regression (62).
2.6. Post-hoc analysis
As specified in our protocol, we planned to check for eventual useful analyses that were not previously considered. We therefore decided to perform a sub-group analysis of studies reporting data at 12 and 24 weeks.
Since six studies reported an additional dietary and physical exercise control to patients, we also investigated the role of Lifestyle interventions in changes of scales with a sensitivity analysis.
2.7. Assessment of heterogeneity
We interpreted I2 estimate greater than or equal to 50% together with a statistically significant Chi2 statistic as evidence of substantial heterogeneity. We also visually inspected graphs to investigate the possibility of statistical heterogeneity and discussed it in the proper section.
2.8. Differences between protocol and review
We clearly state down below the deviations from the original protocol registered in PROSPERO:
• We included those studies in which less than 10% of patients were diagnosed with bipolar disorder.
• Our aim is to whether metformin as add-on therapy improved or not SCZ symptoms, with a particular interest in cognitive functions. Therefore, within psychiatric scales, when more than one tool was available in the study, we applied a priority order as described in the statistical analysis section. This was due to the presence of more than one outcome in several studies.
• We changed the statistical method of the meta-analysis due to the type of outcomes reported in the literature. We preferred the generic invariance methods instead of the Mantel–Haenszel because data were all reported in continuous variables (endpoint data or mean changes) and it wasn’t possible to convert them into dichotomous. However, we decided to provide Odds ratios (OR) by converting the results of total Standardized Mean Differences (SMDs) with the Hasselbach & Hedges’ method (63) in order to help the reader in the interpretation of our results:
• We provided sub-group analyses for each scale.
• Due to the paucity of studies retrieved, we could not perform neither funnel plots, nor analyses on the effects of high risk of bias and the role of diabetes.
3. Results
3.1. Literature search
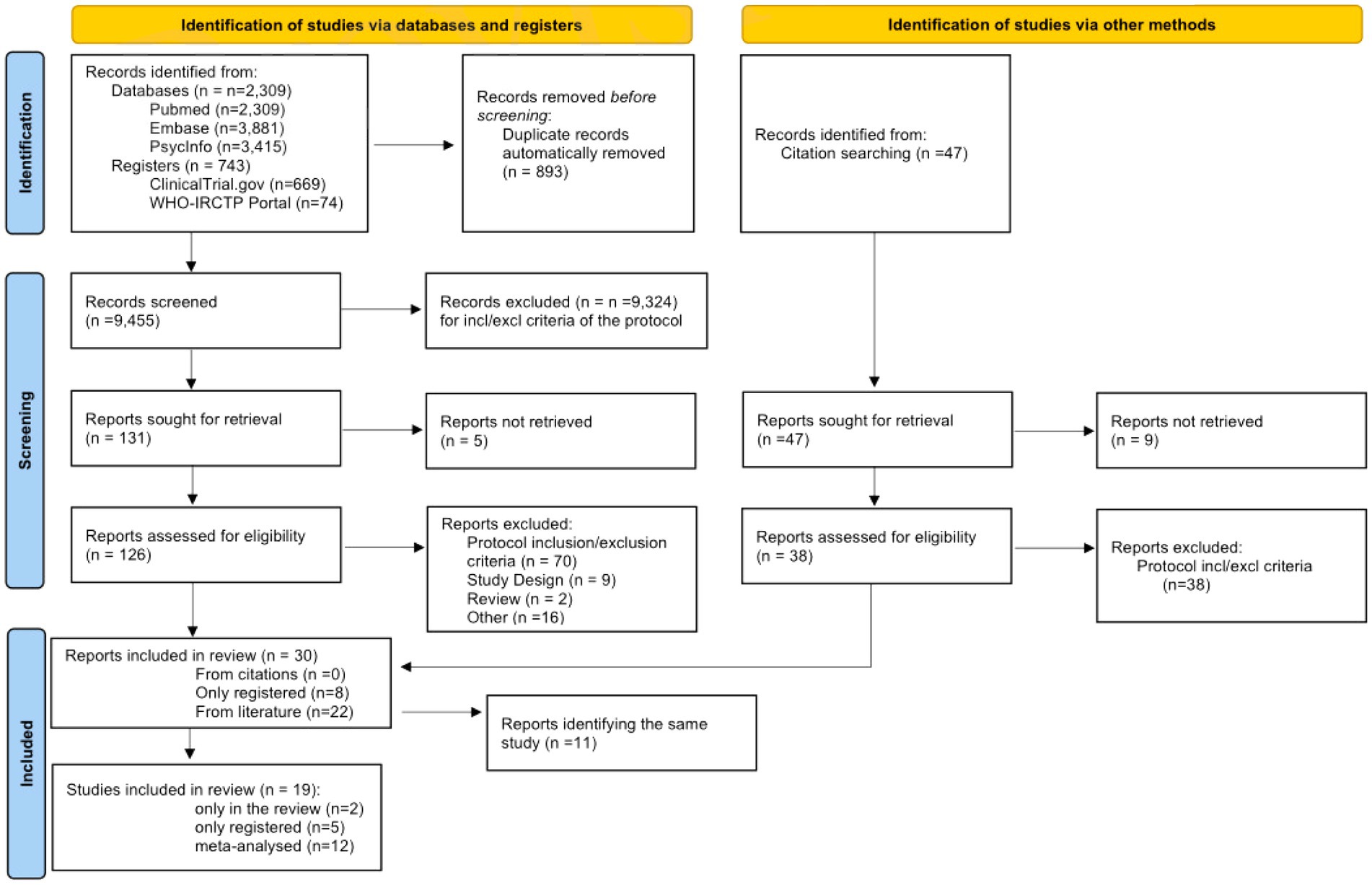
The study selection and screening process is presented in the PRISMA 2020 flowchart (Figure 1). The electronic search identified 9,605 records from literature databases and 743 trials in study registers. After duplicates removal, 9,455 records were screened. Thirty-eight records were retrieved by manual search in the reference lists of relevant reviews and included studies for full-text analysis. Nine-teen studies eventually met our eligibility criteria and were included in the review: 12 were eligible to perform meta-analysis, 2 reported only qualitative data, and 5 were only present in trial registers without any result. These latter trials will be described in a separate section.
3.2. Characteristics of the included studies (n = 14)
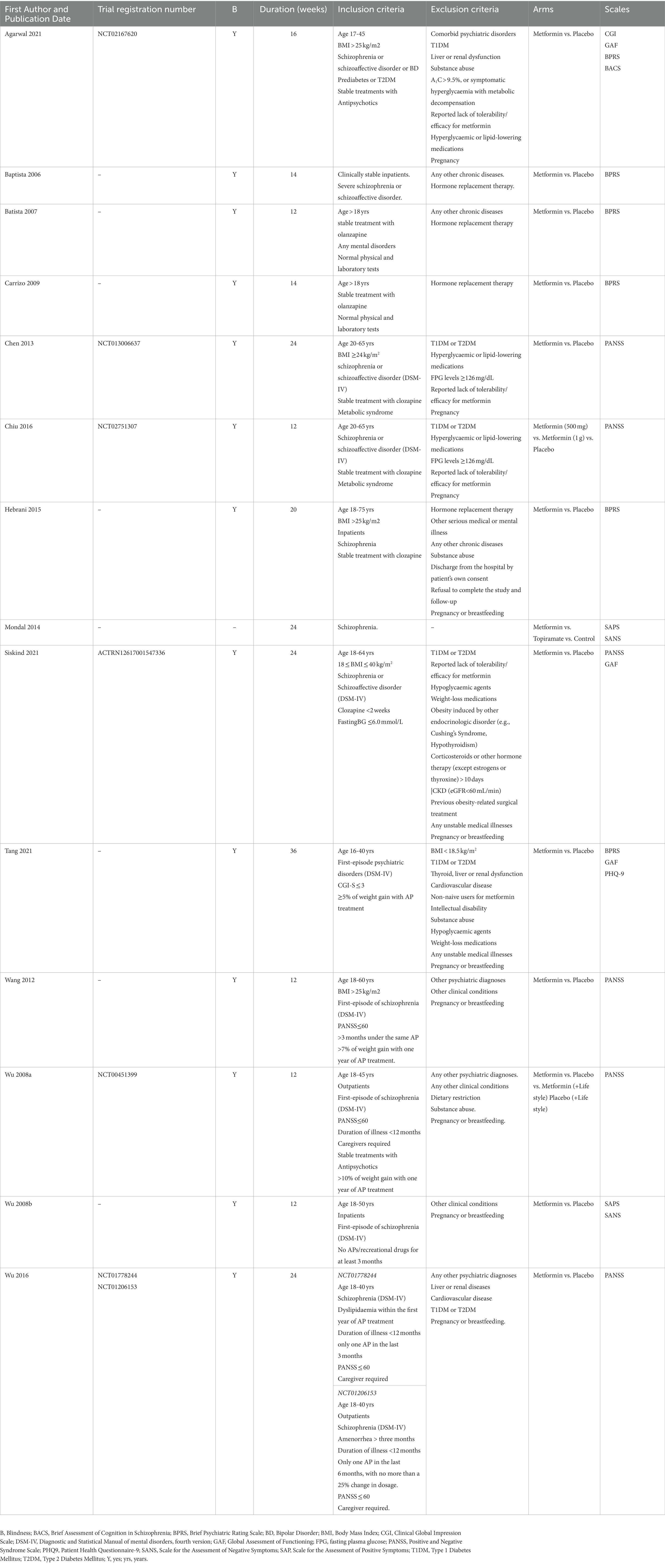
Full description of trials is available in Table 1 and Supplementary Material S1. All studies were designed as comparison between metformin and placebo, except two: Mondal et al. (64) that added one arm treated with topiramate, and Wu et al. (65) that explored the influence of lifestyle interventions. The duration of the included studies was between 12 weeks and 36 weeks. The prescribed dosage of metformin varied from 500 mg/die to 2000 mg/die. All studies included adult patients who were less than 80 years, diagnosed of SCZ (DSM-IV) and under stable treatment with antipsychotics. Four studies (66–69) included patients diagnosed with bipolar disorder and other psychiatric disorders (DSM-IV), however in a negligible percentage: as an example, Agarwal et al. 2 patients out of 30; Baptista et al. 4 patients out of 80. In 5 trials (66, 70–73) only overweight/obese patients were included, and 6 trials reported diabetes or prediabetes among the exclusion criteria (66, 69, 70, 72, 74, 75). All studies, except Mondal et al. (64), reported any other chronic disease (such as thyroid, liver or renal dysfunction, cardiovascular disease) and pregnancy among the exclusion criteria. The mean age at diagnosis was between 21 and 26 years. The disease duration varied from months (first episode) to more than two decades. Clozapine, olanzapine, aripiprazole, and risperidone were the most used antipsychotics. In 3 trials the concomitant use of mood stabilizers, benzodiazepines and antidepressants was permitted (65, 69, 75). Lifestyle intervention involving diet and physical exercise was provided in 6 trials (65, 66, 68, 72, 76, 77). All studies included validated psychometric scales for the clinical assessment; PANSS (65, 70, 72–74, 77) and BPRS (66–69, 71, 76) were the most used.
3.3. Scales
The following numerical results must be read as “metformin compared to placebo.”
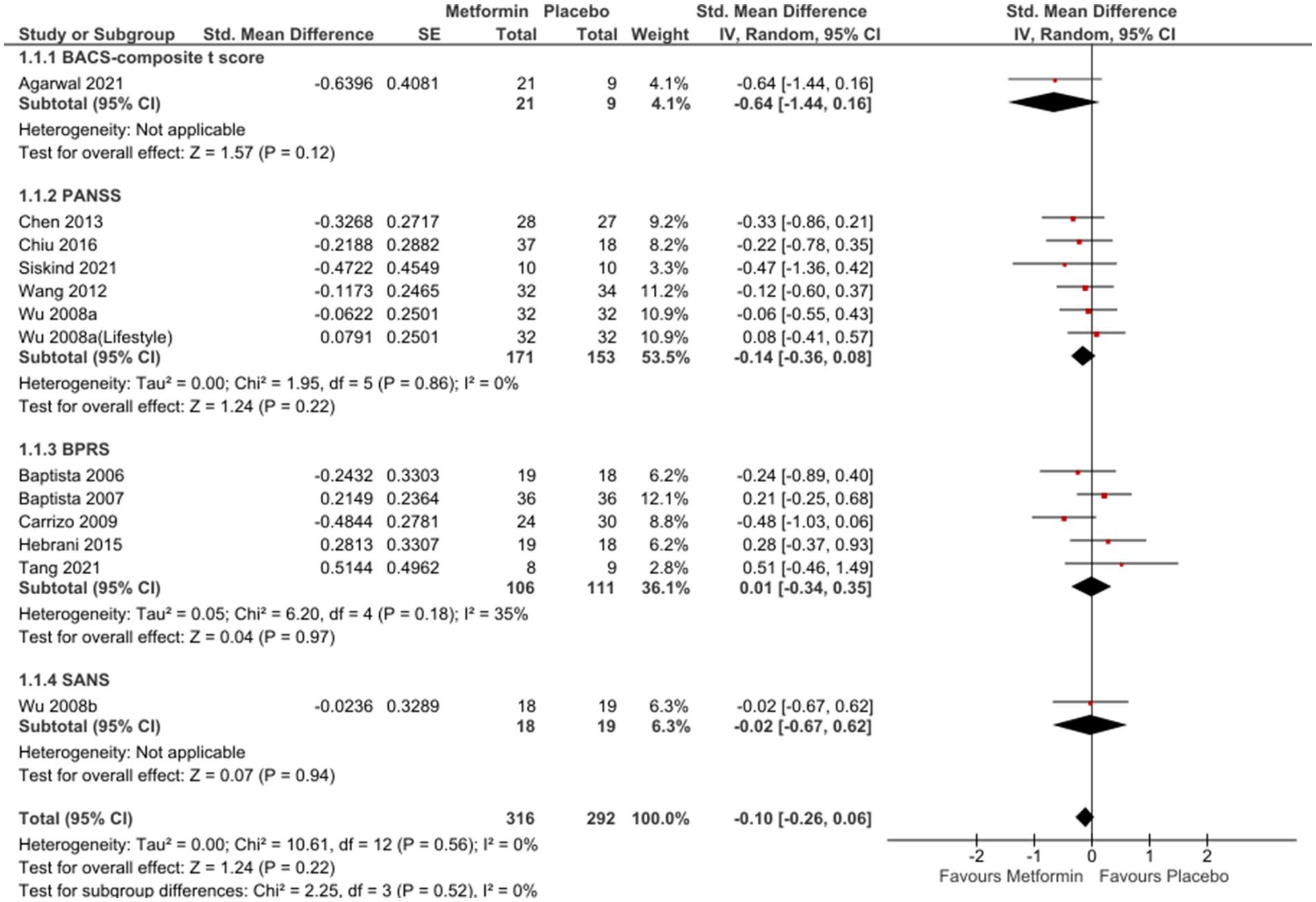
In the analysis of 12 studies (65–74, 76, 77), metformin resulted in a favorable position against placebo (Figure 2), even if not statistically significant [SMD (95% CI) = −0.10 (−0.26; 0.06), OR (95% CI) = 0.8 (−1.4; 3.1)]. No significant differences were seen when studies with missing SDs or those with lifestyle intervention were excluded [SMD (95% CI) = −0.09(−0.27; 0.09), OR (95% CI) = 0.9 (−1.5;3.16) and SMD (95% CI) = −0.02(−0.22; 0.19), OR (95%CI) =1.0 (−1.4; 3.3), respectively].
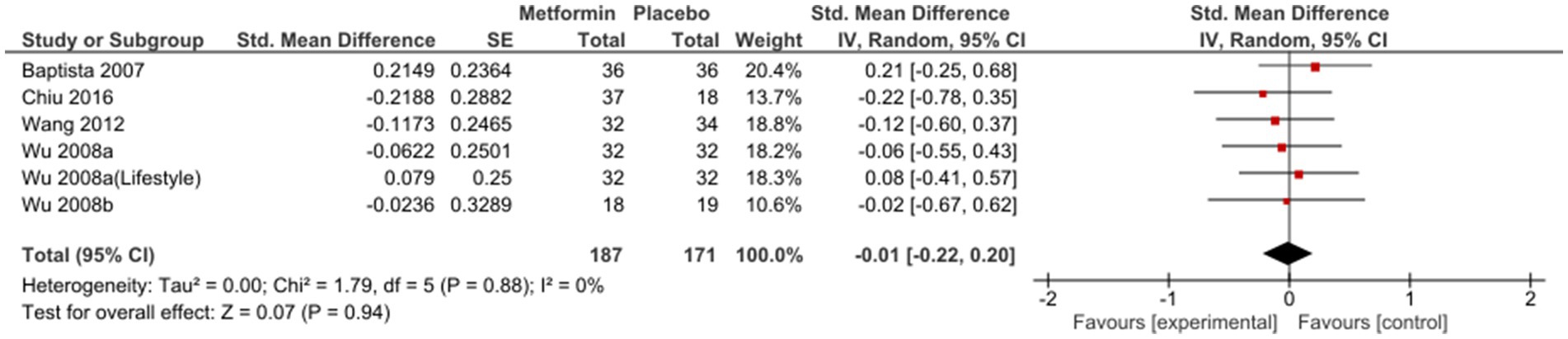
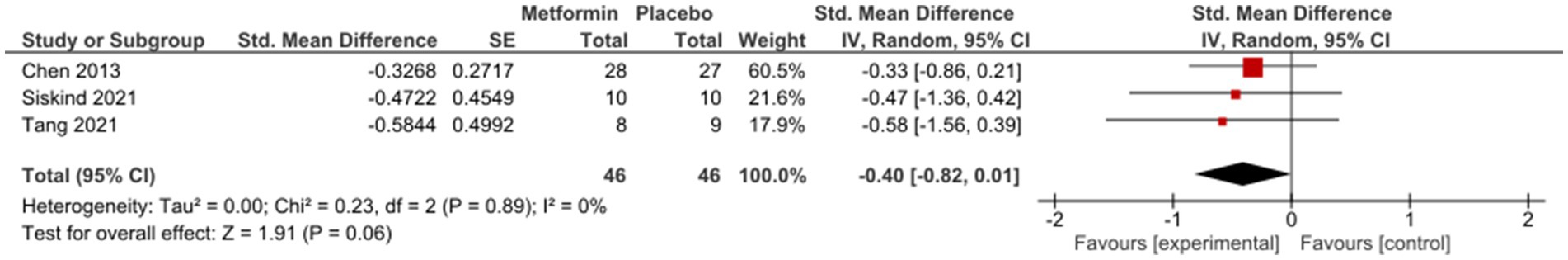
Sub-group analysis examining studies with same duration of follow-up found non-significant results at 12 weeks [SMD (95%CI) = −0.01(−0.22; 0.20), OR (95% CI) = 1.0 (−1.4; 3.4)] (Figure 3); however, a subsequent improvement at 24 weeks [SMD (95% CI) = −0.40 (−0.82; 0.01), OR (95% CI) = 0.5 (−2.4; 3.4)] (Figure 4). No significant differences were seen when studies with missing SDs or those with lifestyle intervention were excluded.
Forest plots of single-scale analyses of PANSS, BPRS and GAF are available in Supplementary Material (Figure S1-S3 respectively). Better performances were detected by BACS-composite t-score [MD (95%CI) = 1.26 (−0.42; 2.94)], result from one study (66) and PANSS [MD (95% CI) = −2.26 (−5.90; 1.39)], result from 5 studies (65, 70, 72–74), compared to BPRS [MD (95% CI) = −0.57 (−2.56; 1.41)], result from 6 studies (66–69, 71, 76). One study (66) reported changes in BACS-verbal memory in favour of placebo [MD (95% CI) = −16.03 (−23.65; 8.42)]; on the other hand, another study (77) described non-significant results related to SANS [MD (95% CI) = −0.05 (−1.38; 1.28)] and SAPS [MD (95%C I) = 0.09 (−0.67; 0.85)] and similar results were described by Mondal et al. (64) Three studies (66, 69, 72) reported a not significant improvement in GAF [MD (95% CI) = 0.35 (−2.51; 3.21)] and PHQ9 [MD (95% CI) = −2.50 (−1.70; 2.07)], only one study (69).
3.4. Metformin vs. topiramate
Only one study (64) compared metformin to another drug used to control the increase of weight in schizophraenic patients. The Authors did not report any quantitative result, they only state that no differences were found in SAPS and SANS scales among groups after 24 weeks.
3.5. The influence of BMI at baseline
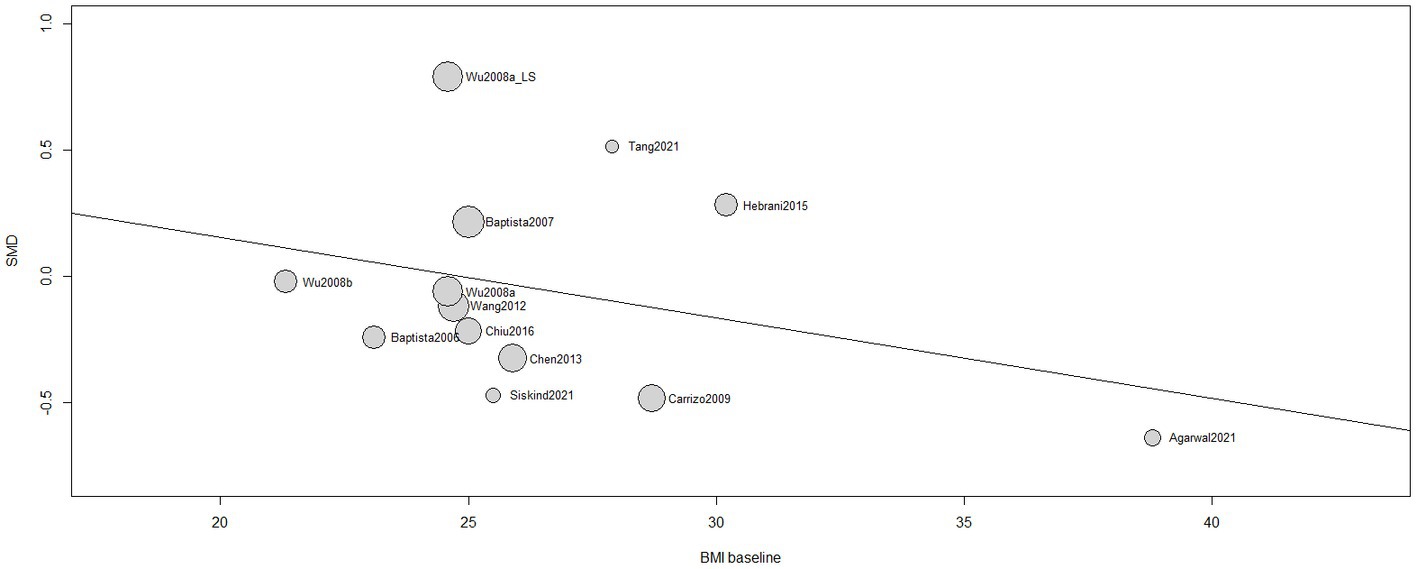
A non-significant influence of BMI at baseline in the treatment response [β (95%CI) = −0.0320 (−0.0982;0.0343), I2 = 45.05%, R2 = 4.60%, test of moderators: F = 1.1279; p = 0.3110] was found (Figure 5). Same results were reported by 5 single studies (66, 67, 75–77).
3.6. Adverse events
The general adverse events that were reported by authors were related to gastrointestinal discomfort, xerostomia, and extrapyramidal syndrome. Several psychiatric adverse events were also described, particularly some symptoms attributable to a relapse of SCZ (psychotic relapse/exacerbation, unstable/worsening of illness), others to mood alteration (depression, suicidality, irritated/bad mood), and finally some unspecific symptoms such as insomnia and agitation (Supplementary Table S2).
3.7. Studies in trial registers (n = 5)
Five trials were registered on clinicaltrial.gov (78). NCT01654640 was terminated because they were not able to recruit enough patients; in NCT02140788, the Principal Investigator left the Institution, and the trial was interrupted. NCT03271866, reported as “unknown status,” focuses on the effect of metformin on cognitive impairment. NCT03708549 is a phase 4 trial that is still recruiting; the aim of the study is to compare berberine and metformin and the evaluation of the PANSS is among the secondary outcomes. NCT04865835 is a phase I trial that has been completed and it is likely under review; however, the aim of this study is to evaluate in pharmacokinetic of a novel substance compared to metformin, which is not specifically our outcome of interest.
3.8. Risk of bias
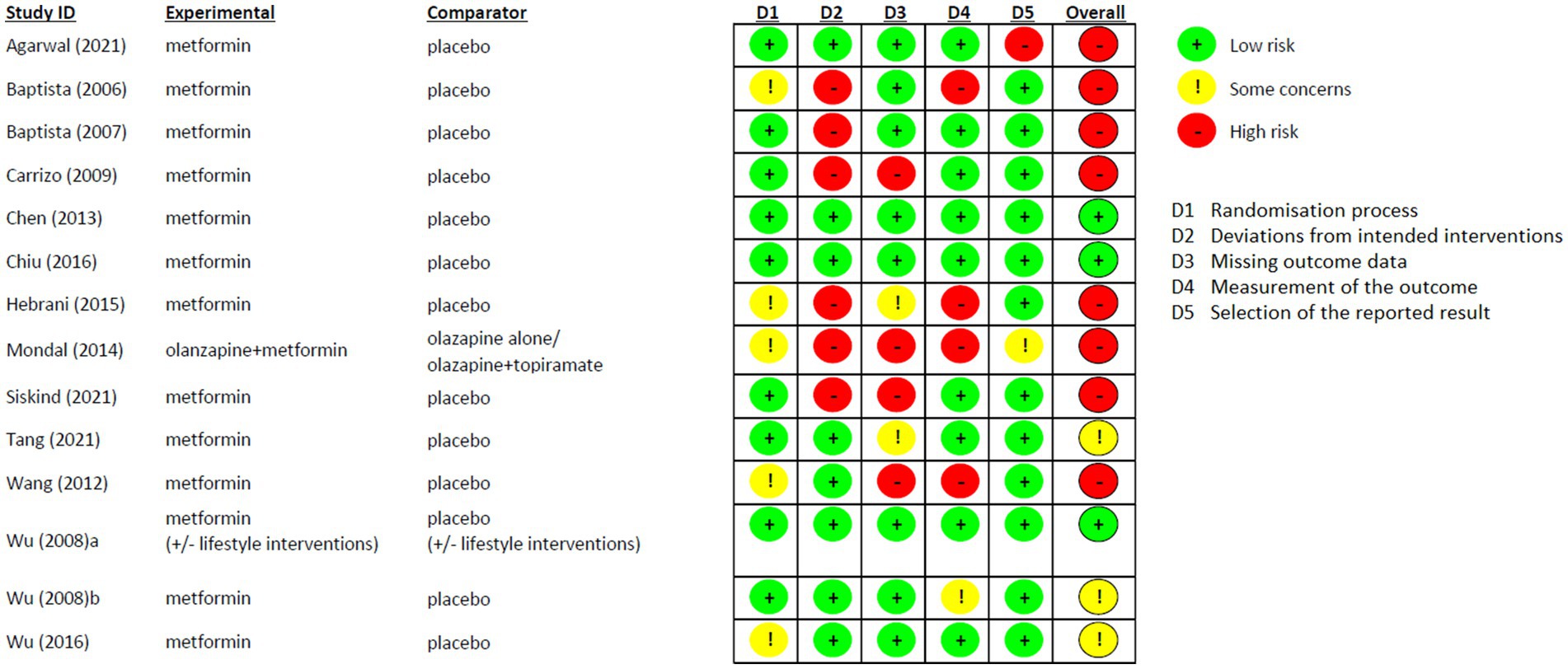
Risk of Bias of the included studies is shown in Figure 6. In general, most of the studies reported high risk of bias (8/14). However, the randomisation process was favorably assessed in all studies and the “Deviations from intended interventions” domain was the one that highly influenced the general results because of the Per Protocol analysis used in 6 studies (64, 67, 68, 71, 72, 76). Three studies were considered with low-risk of bias in all domains (65, 70, 74), and only one study was at real high-risk of bias, since none of the fields were assessed without any concern (64).
4. Discussion
Since its approval in 1958, metformin has become one of the most widely used therapy for DM2 and it still represents the first-line therapy. While improved mitochondrial metabolism and insulin signaling are generally suggested as mechanisms underlying beneficial pro-cognitive effects of antidiabetic drugs, other factors such as active adenosine 5′- monophosphate-activated protein kinase (AMPK) activation, modulation of microglial phenotype, mTOR inhibition, and increased autophagy in the brain might be involved (79). Because of these multiple mechanisms, many studies have already described potential effects of metformin in treating conditions other than diabetes (80–83); here we assessed for the first time through a systematic review the potential effects of metformin on cognitive functions and psychiatric symptoms in schizophrenic patients treated with antipsychotics.
A general positive trend was seen after 24 weeks of treatment [SMD (95%CI) = −0.40 (−0.82;0.01), OR (95% CI) = 0.5 (−2.4; 3.4)] in patients who were generally considered in stable conditions.
Unfortunately, the relatively short period of investigation of the included studies (only one study up to 36 weeks) could mask the neuroprotective effects of metformin since in previous RCTs they seemed to emerge after long-term use (6–8 years) (42). A better improvement was related to those scales allocated in the higher positions of our priority scale (BACS composite T score > BACS verbal memory T score > PANSS) with low heterogeneity among studies, then worsening while going further with the other psychiatric tools (BPRS > GAF > CGI > SANS and SAPS > PHQ9). Furthermore, among all cognitive domains assessed by the BACS (verbal memory, working memory, motor speed, attention, executive functions, and verbal fluency), only verbal memory was in favour of placebo [MD (95%CI) = −16.03 (−23.65; 8.42)] (66). This finding could indicate a greater influence of metformin on cognitive rather than psychiatric symptoms, but it is not possible to draw any conclusions since only one study (66) reported results for the BACS composite t-score [MD (95% CI) = 1.26 (−0.42; 2.94)]. This scale is specifically designed for the evaluation of cognitive functions, but the small sample size of this trial could be an important limit for the power of the performed analysis. It is interesting to note that we were able to retrieve another trial that was registered in 2017 (84): the aim of this study was to investigate the impact of metformin on cognitive impairment in schizophrenic patients with or without MetS. This 24-week trial should recruit 80 patients and compare metformin group versus controls on PANSS Scale, Calgary Depression Scale for SCZ (Chinese version) and MATRICS Consensus Cognitive Battery. Unfortunately, the last version of the protocol was submitted in 2020 and the recruiting status is unknown. We could not therefore include their findings in our analysis. However, some indirect clinical evidence on a potential enhancement of cognitive function may come from neurodegenerative disorders: metformin has shown potential therapeutic benefit against mild cognitive impairment and Alzheimer’s disease among diabetic patients (85), even if the use of metformin for prevention of dementia in older non-diabetic adults is not currently recommended (42).
No correlation was seen with the patients’ BMI at baseline, thus indicating no potential differences in the use of metformin in first-episode psychosis or under chronic treatment with SGAs. Literature findings report how the earlier the onset of SCZ and the longer its duration, the worse is the clinical response to antipsychotics (86). One of the hypotheses behind this evidence-based finding is that progressive brain tissue loss occurs in schizophrenic patients, and this neurobiological alteration would interfere with the effectiveness of metformin as much as antipsychotic therapy (87). Among the studies included in our analysis, only four used a first episode psychosis as an inclusion criterion (65, 69, 73, 77). Therefore, despite missing data, we can assume that most patients were enrolled after a duration of illness that could impact negatively on the efficacy of pharmacotherapy. Disease duration ranged from 6.8 months to 27.8 years and in seven studies it was not reported (64, 67–70, 72, 76). Further studies including disease onset and duration information or that include only first-episode patients are therefore recommended.
Regarding the antipsychotic drugs that were used in the included studies, all patients were mainly treated with SGAs, while only three studies (66, 69, 76) reported concurrent treatment with first-generation antipsychotics, confirming the known strong association between weight gain and SGAs (4). Among them, clozapine and olanzapine were responsible for the highest incidence of MetS, consistently with a recent network meta-analysis on glyco-metabolic adverse effects of antipsychotics (7).
As all the other drugs available on the market, metformin might cause adverse effects, although the most frequent ones are considered mild enough to recommend maintaining the use of metformin unless renal/hepatic function deterioration arises (88). Metformin doses that were used in all the included trials were in line with the latest recommendation (89) and no high-concerning adverse event was therefore reported. Gastrointestinal disorders were the most described events; this is not surprising as they are known to be very common at the start of the therapy and can be minimized by dose reduction, slower dose titration and after-meal administration (89). Physical symptoms, namely xerostomia, headache and extrapyramidal syndrome were also reported; this indicates that it is worth recommending caution and careful patients’ counseling before starting metformin, as adverse events may represent an additional risk factor for dropping out of the overall psychiatric treatment (90). Somehow unexpectedly, few psychiatric adverse events were reported, these were essentially from relapse of SCZ mood alteration, insomnia, and agitation. Based on the known mechanism of action of metformin a clear causal relationship between psychiatric symptoms and metformin appear improbable. Rather, it is likely that they arose due to the chronic course of the underlying psychiatric disease.
However, considering the observed adverse effects, it is important to assess the risk–benefit ratio of an add-on therapy with metformin. Metformin has proved its efficacy on cardiometabolic complications, which cause a three-times higher mortality risk in SCZ patients than that of the general population (91). When there is balance between the odds of therapeutic effects and the risk of adverse events, metformin administration in these patients seems beneficial, especially if metformin might exert improvements in pro-cognitive functions, which is of clinical relevance. However, such evidence is not yet solid enough and it is premature to propose a change in current clinical practice and in medical prescription at this stage. Further studies considering the benefit/risk ratio are warranted.
4.1. Strength and limitations
Chronic treatment with SGAs is essential in the control of psychotic symptoms and the prevention of relapses in SCZ. For this reason, it is widely adopted in clinical settings, even though it can increase the risk of MetS and negatively impact cognitive performance thus worsening the therapeutic compliance, already impaired by the pathology itself. Therefore, the identification of a treatment that can contrast dysmetabolism and cognitive impairment in psychiatric patients would have a high impact in psychiatric clinical practice. Not only has metformin previously shown to be effective in reducing MetS, but it is also considered a low-cost drug, with a well-known safety profile. Our primary aim was to verify the hypothesis, previously emerged from several preclinical and clinical studies, that metformin may exert pro-cognitive effects also in psychotic patients, with or without DM2. This meta-analysis, in addition to its clinical relevance, represents an original perspective in the current literature background.
The first obstacle in investigating our primary objective was that only one study (66) used a specific assessment instrument for cognitive function, the BACS. This is the most widely adopted and validated scale that assesses cognition’s domains most impaired and correlated with outcome of SCZ (55, 92). Unfortunately, it is still underused in clinical practice, while the clinical course and functioning of SCZ are usually assessed by several validated psychometric scales, the main ones being BPRS, PANSS, CGI, GAF. Most of these latter scales contain specific items concerning the patient’s cognitive asset. Thus, since partial scores of these items were not available in the analyzed studies, we applied the priority order described above, that is an original method in order not to neglect valuable information for our primary aim. However, further studies with appropriate scales are warranted.
Another limit of our analysis is the relatively short period of investigation (only one study up to 36 weeks) while neuroprotective effects of metformin observed in previous RCT seem to emerge after long-term use (8 and 6 years) (42). Only 3 RCTs were assessed with low risk of bias, and we could not perform any sensitive analysis excluding those with high risk. However, considering that our aim was defining changes in measurements, the most important domains for our results were the quality of the randomisation process (domain 1) and the measurement of the outcome (domain 4), which were both considered at low risk of bias in the 64% of the included studies.
5. Conclusion
Metformin has been previously shown to reduce weight gain and the risk of MetS in schizophrenic patients treated with SGA; our systematic review suggests that it may also improve psychiatric and cognitive symptoms in the same population. Given the clinical relevance of this potential pharmacological effect of metformin, longer specific studies exploring cognitive performance and using adequate psychometric scales are strongly recommended.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
VB, GC, RL, ER, GM, BB, MP, MN, SR, CC, BD’O, and EC contributed to the study conception and design. Material preparation and data collection were performed by VB, RL, GM, and ER. Data analyses were performed by VB, ER, and CC. The first draft of the manuscript was written by VB, RL, GC, and ER. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Università degli Studi di Milano (Piano di Sostegno alla Ricerca, LINEA 3 to CC) which are gratefully acknowledged. The funding public institutions had no role in any part of the work.
Acknowledgments
VB is enrolled in the PhD in Experimental and Clinical Pharmacological Sciences, Università degli Studi di Milano, which supports her fellowship. This work was supported by the Italian Ministry of health (Ministero della Salute- Ricerca Corrente) which are gratefully acknowledge. GC is supported by Fondazione Romeo ed Enrica Invernizzi (Milano, Italy).
Conflict of interest
BD’O has received lecture honoraria that are not related to the work submitted for publication, from Angelini, Janssen, Lundbeck, Bromatech, Otzuka, and Neuraxpharm.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1215807/full#supplementary-material
References
1. Charlson, FJ, Ferrari, AJ, Santomauro, DF, Diminic, S, Stockings, E, Scott, JG, et al. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr Bull. (2018) 44:1195–203. doi: 10.1093/SCHBUL/SBY058
2. Kahn, RS, Sommer, IE, Murray, RM, Meyer-Lindenberg, A, Weinberger, DR, Cannon, TD, et al. Schizophrenia. Nat Rev Dis Primers. (2015) 1:15067. doi: 10.1038/NRDP.2015.67
3. De Hert, M, Correll, CU, Bobes, J, Cetkovich-Bakmas, M, Cohen, DAN, Asai, I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. (2011) 10:52–77. doi: 10.1002/J.2051-5545.2011.TB00014.X
4. Leucht, S, Cipriani, A, Spineli, L, Mavridis, D, Örey, D, Richter, F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. (2013) 382:951–62. doi: 10.1016/S0140-6736(13)60733-3
5. DeJongh, BM. Clinical pearls for the monitoring and treatment of antipsychotic induced metabolic syndrome. Ment Health Clin. (2021) 11:311–9. doi: 10.9740/MHC.2021.11.311
6. Carli, M, Kolachalam, S, Longoni, B, Pintaudi, A, Baldini, M, Aringhieri, S, et al. Atypical antipsychotics and metabolic syndrome: from molecular mechanisms to clinical differences. Pharmaceuticals (Basel). (2021) 14:238. doi: 10.3390/PH14030238
7. Carnovale, C, Lucenteforte, E, Battini, V, Mazhar, F, Fornili, M, Invernizzi, E, et al. Association between the glyco-metabolic adverse effects of antipsychotic drugs and their chemical and pharmacological profile: a network meta-analysis and regression. Psychol Med. (2021) 52:3508–20. doi: 10.1017/S0033291721000180
8. Stahl, SM, Mignon, L, and Meyer, JM. Which comes first: atypical antipsychotic treatment or cardiometabolic risk? Acta Psychiatr Scand. (2009) 119:171–9. doi: 10.1111/J.1600-0447.2008.01334.X
9. Pillinger, T, McCutcheon, RA, Vano, L, Mizuno, Y, Arumuham, A, Hindley, G, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. (2020) 7:64–77. doi: 10.1016/S2215-0366(19)30416-X
10. Mauri, MC, Paletta, S, Di Pace, C, Reggiori, A, Cirnigliaro, G, Valli, I, et al. Clinical pharmacokinetics of atypical antipsychotics: an update. Clin Pharmacokinet. (2018) 57:1493–528. doi: 10.1007/S40262-018-0664-3
11. McCracken, E, and Monaghan, M, Dermatology SS-C in, 2018 undefined. Pathophysiology of the metabolic syndrome. Elsevier. Available at: https://www.sciencedirect.com/science/article/pii/S0738081X1730158X?casa_token=6K1vnLBRmg8AAAAA:x695EZyZQ81Fe6jRadR7rghlABiB7RLxjEdDtrlN2SYQfeC6Myk2GI5qs5szLKYxJaaICr22Iw (accessed April 11, 2023)
12. Cooper, SJ, Reynolds, GP, Barnes, TRE, England, E, Haddad, PM, Heald, A, et al. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol. (2016) 30:717–48. doi: 10.1177/0269881116645254
13. Psychiatry JM-J of C. (2007). The costs of schizophrenia. psychiatrist.com. Available at: https://www.psychiatrist.com/read-pdf/3879/ (Accessed April 11, 2023)
14. Harvey, PD, and Keefe, RSE. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry. (2001) 158:176–84. doi: 10.1176/APPI.AJP.158.2.176
15. Baldez, DP, Biazus, TB, Rabelo-da-Ponte, FD, Nogaro, GP, Martins, DS, Kunz, M, et al. The effect of antipsychotics on the cognitive performance of individuals with psychotic disorders: network meta-analyses of randomized controlled trials. Neurosci Biobehav Rev. (2021) 126:265–75. doi: 10.1016/J.NEUBIOREV.2021.03.028
16. Kaar, SJ, Natesan, S, McCutcheon, R, and Howes, OD. Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. (2020) 172:107704. doi: 10.1016/J.NEUROPHARM.2019.107704
17. Sinkeviciute, I, Begemann, M, Prikken, M, Oranje, B, Johnsen, E, Lei, WU, et al. Efficacy of different types of cognitive enhancers for patients with schizophrenia: a meta-analysis. NPJ Schizophr. (2018) 4:22. doi: 10.1038/S41537-018-0064-6
18. Harvey, PD, and Sand, M. Pharmacological augmentation of psychosocial and remediation training efforts in schizophrenia. FrontPsychiatry. (2017) 8:177. doi: 10.3389/FPSYT.2017.00177
19. Harvey, PD, Bowie, CR, McDonald, S, and Podhorna, J. Evaluation of the efficacy of BI 425809 pharmacotherapy in patients with schizophrenia receiving computerized cognitive training: methodology for a double-blind, randomized, parallel-group trial. Clin Drug Investig. (2020) 40:377–85. doi: 10.1007/S40261-020-00893-8
20. Lewandowski, KE. Cognitive remediation for the treatment of cognitive dysfunction in the early course of psychosis. Harv Rev Psychiatry. (2016) 24:164–72. doi: 10.1097/HRP.0000000000000108
21. Peyroux, E, and Franck, N. RC2S: a cognitive remediation program to improve social cognition in schizophrenia and related disorders. Front Hum Neurosci. (2014) 8:400. doi: 10.3389/FNHUM.2014.00400
22. Keepers, GA, Fochtmann, LJ, Anzia, JM, Benjamin, S, Lyness, JM, Mojtabai, R, et al. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. (2020) 177:868–72. doi: 10.1176/APPI.AJP.2020.177901
23. Takahashi, S, Keeser, D, Rauchmann, BS, Schneider-Axmann, T, Keller-Varady, K, Maurus, I, et al. Effect of aerobic exercise combined with cognitive remediation on cortical thickness and prediction of social adaptation in patients with schizophrenia. Schizophr Res. (2020) 216:397–407. doi: 10.1016/J.SCHRES.2019.11.004
24. Bosia, M, Buonocore, M, Bechi, M, Spangaro, M, Pigoni, A, Croci, M, et al. Cognitive remediation and functional improvement in schizophrenia: is it a matter of size? Eur Psychiatry. (2017) 40:26–32. doi: 10.1016/J.EURPSY.2016.06.007
25. Bosia, M, Buonocore, M, Bechi, M, Santarelli, L, Spangaro, M, Cocchi, F, et al. Improving cognition to increase treatment efficacy in schizophrenia: effects of metabolic syndrome on cognitive Remediation’s outcome. Front Psych. (2018) 9:647. doi: 10.3389/FPSYT.2018.00647
26. Cuomo, A, Bolognesi, S, Goracci, A, Ciuoli, C, Crescenzi, BB, Maina, G, et al. Feasibility, adherence and efficacy of Liraglutide treatment in a sample of individuals with mood disorders and obesity. Front Psychiatry. (2019) 9:784. doi: 10.3389/FPSYT.2018.00784
27. Siskind, DJ, Leung, J, Russell, AW, Wysoczanski, D, and Kisely, S. Metformin for clozapine associated obesity: a systematic review and Meta-analysis. PLoS One. (2016) 11:e0156208. doi: 10.1371/JOURNAL.PONE.0156208
28. de Silva, VA, Suraweera, C, Ratnatunga, SS, Dayabandara, M, Wanniarachchi, N, and Hanwella, R. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. (2016) 16:341. doi: 10.1186/S12888-016-1049-5
29. Battini, V, Van Manen, RP, Gringeri, M, Mosini, G, Guarnieri, G, Bombelli, A, et al. The potential antidepressant effect of antidiabetic agents: new insights from a pharmacovigilance study based on data from the reporting system databases FAERS and VigiBase. Front Pharmacol. (2023) 14:1128387. doi: 10.3389/FPHAR.2023.1128387
30. Cao, G, Gong, T, Du, Y, Wang, Y, Ge, T, and Liu, J. Mechanism of metformin regulation in central nervous system: progression and future perspectives. Biomed Pharmacother. (2022) 156:113686. doi: 10.1016/J.BIOPHA.2022.113686
31. De Oliveira, WH, De Santana Nunes, AK, De França, MER, Dos Santos, LA, Lós, DB, Rocha, SWS, et al. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. (2016) 1644:149–60. doi: 10.1016/J.BRAINRES.2016.05.013
32. Markowicz-Piasecka, M, Huttunen, KM, Mateusiak, L, Mikiciuk-Olasik, E, and Sikora, J. Is metformin a perfect drug? Updates in pharmacokinetics and pharmacodynamics. Curr Pharm Des. (2017) 23:2532–50. doi: 10.2174/1381612822666161201152941
33. Bridgeman, SC, Ellison, GC, Melton, PE, Newsholme, P, and Mamotte, CDS. Epigenetic effects of metformin: from molecular mechanisms to clinical implications. Diabetes Obes Metab. (2018) 20:1553–62. doi: 10.1111/DOM.13262
34. Du, MR, Gao, QY, Liu, CL, Bai, LY, Li, T, and Wei, FL. Exploring the pharmacological potential of metformin for neurodegenerative diseases. Front Aging Neurosci. (2022) 14:838173. doi: 10.3389/FNAGI.2022.838173
35. Campbell, JM, Stephenson, MD, de Courten, B, Chapman, I, Bellman, SM, and Aromataris, E. Metformin and Alzheimer’s disease, dementia and cognitive impairment: a systematic review protocol. JBI Database System Rev Implement Rep. (2017) 15:2055–9. doi: 10.11124/JBISRIR-2017-003380
36. Tao, L, Li, D, Liu, H, Jiang, F, Xu, Y, Cao, Y, et al. Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF-κB and MAPK signaling pathway. Brain Res Bull. (2018) 140:154–61. doi: 10.1016/J.BRAINRESBULL.2018.04.008
37. Sarkaki, A, Farbood, Y, Badavi, M, Khalaj, L, Khodagholi, F, and Ashabi, G. Metformin improves anxiety-like behaviors through AMPK-dependent regulation of autophagy following transient forebrain ischemia. Metab Brain Dis. (2015) 30:1139–50. doi: 10.1007/S11011-015-9677-X
38. Zhou, C, Kong, D, Xue, R, Chen, M, Li, G, Xu, Y, et al. Metformin enhances antidepressant/antipsychotic combination therapy of schizophrenia with comorbid depression in a murine model. Front Neurosci. (2020) 14:517. doi: 10.3389/FNINS.2020.00517
39. Wang, X, Luo, C, Mao, XY, Li, X, Yin, JY, Zhang, W, et al. Metformin reverses the schizophrenia-like behaviors induced by MK-801 in rats. Brain Res. (2019) 1719:30–9. doi: 10.1016/J.BRAINRES.2019.05.023
40. Mehrabi, S, Sanadgol, N, Barati, M, Shahbazi, A, Vahabzadeh, G, Barzroudi, M, et al. Evaluation of metformin effects in the chronic phase of spontaneous seizures in pilocarpine model of temporal lobe epilepsy. Metab Brain Dis. (2018) 33:107–14. doi: 10.1007/S11011-017-0132-Z
41. Herath, PM, Cherbuin, N, Eramudugolla, R, and Anstey, KJ. The effect of diabetes medication on cognitive function: evidence from the PATH through life study. Biomed Res Int. (2016) 2016:7208429. doi: 10.1155/2016/7208429
42. Campbell, JM, Stephenson, MD, De Courten, B, Chapman, I, Bellman, SM, and Aromataris, E. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and Meta-analysis. J Alzheimers Dis. (2018) 65:1225–36. doi: 10.3233/JAD-180263
43. Hervás, D, Fornés-Ferrer, V, Gómez-Escribano, AP, Sequedo, MD, Peiró, C, Millán, JM, et al. Metformin intake associates with better cognitive function in patients with Huntington’s disease. PLoS One. (2017) 12:e0179283. doi: 10.1371/JOURNAL.PONE.0179283
44. Dy, ABC, Tassone, F, Eldeeb, M, Salcedo-Arellano, MJ, Tartaglia, N, and Hagerman, R. Metformin as targeted treatment in fragile X syndrome. Clin Genet. (2018) 93:216–22. doi: 10.1111/CGE.13039
45. Guo, M, Mi, J, Jiang, QM, Xu, JM, Tang, YY, Tian, G, et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin Exp Pharmacol Physiol. (2014) 41:650–6. doi: 10.1111/1440-1681.12265
46. Tabatabaei Malazy, O, Bandarian, F, Qorbani, M, Mohseni, S, Mirsadeghi, S, Peimani, M, et al. The effect of metformin on cognitive function: a systematic review and meta-analysis. J Psychopharmacol. (2022) 36:666–79. doi: 10.1177/02698811211057304
47. Ping, F, Jiang, N, and Li, Y. Association between metformin and neurodegenerative diseases of observational studies: systematic review and meta-analysis. BMJ Open Diabetes Res Care. (2020) 8:e001370. doi: 10.1136/BMJDRC-2020-001370
48. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
49. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
50. Neuroscience NS-I in C. (2017) Precursors to the PANSS: The BPRS and its progenitors ncbi.nlm.nih.gov. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5788245/ (Accessed April 11, 2023).
51. Kay, S, Fiszbein, A, and Bulletin, LO-S. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia academic.oup.com. Available at: https://academic.oup.com/schizophreniabulletin/article-abstract/13/2/261/1919795 (accessed April 11, 2023)
52. Psychopharmacology a manual for, 1976 undefined. Clinical global impression. cir.nii.ac.jp. Available at: (https://cir.nii.ac.jp/crid/1572261550655271680)
53. Hall, RCW. Global assessment of functioningA modified scale. Psychosomatics. (1995) 36:267–75. doi: 10.1016/S0033-3182(95)71666-8
54. McAdams, LA, Harris, MJ, Heaton, SC, Bailey, A, Fell, R, and Jeste, DV. Validity of specific subscales of the positive and negative symptom scales in older schizophrenia outpatients. Schizophr Res. (1997) 27:219–26. doi: 10.1016/S0920-9964(97)00066-2
55. Keefe, RSE, Goldberg, TE, Harvey, PD, Gold, JM, Poe, MP, and Coughenour, L. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. (2004) 68:283–97. doi: 10.1016/j.schres.2003.09.011
56. Kroenke, K, Spitzer, RL, and Williams, JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/J.1525-1497.2001.016009606.X
58. Diaz, FJ, Josiassen, RC, and De Leon, J. The effect of body weight changes on Total plasma clozapine concentrations determined by applying a statistical model to the data from a double-blind trial. J Clin Psychopharmacol. (2018) 38:442–6. doi: 10.1097/JCP.0000000000000926
59. Greenberg, WM, and Citrome, L. Pharmacokinetics and pharmacodynamics of Lurasidone hydrochloride, a second-generation antipsychotic: a systematic review of the published literature. Clin Pharmacokinet. (2017) 56:493–503. doi: 10.1007/S40262-016-0465-5
60. Kuzin, M, Haen, E, Hiemke, C, Bochon, B, Bochon, K, Gründer, G, et al. Body mass index as a determinant of clozapine plasma concentrations: a pharmacokinetic-based hypothesis. J Psychopharmacol. (2021) 35:273–8. doi: 10.1177/0269881120985166
61. Paulzen, M, Haen, E, Stegmann, B, Hiemke, C, Gründer, G, Lammertz, SE, et al. Body mass index (BMI) but not body weight is associated with changes in the metabolism of risperidone a pharmacokinetics-based hypothesis. Psychoneuroendocrinology. (2016) 73:9–15. doi: 10.1016/J.PSYNEUEN.2016.07.009
62. Oh, H-S, and Kim, D. Meta: An R package for meta-analysis. cran.rstudio.org. (2007). Available at: https://cran.rstudio.org/doc/Rnews/Rnews_2007-3.pdf#page=40 (accessed April 11, 2023)
63. Da Costa, BR, Rutjes, AWS, Johnston, BC, Reichenbach, S, Nüesch, E, Tonia, T, et al. Methods to convert continuous outcomes into odds ratios of treatment response and numbers needed to treat: meta-epidemiological study. Int J Epidemiol. (2012) 41:1445–59. doi: 10.1093/IJE/DYS124
64. Mondal, H, Suhrita, P, and Guha, P. Role of metformin versus topiramate in preventing olanzapine associated weight gain and metabolic syndrome. Indian J Pharmacol. (2014):46, S21–S21.
65. Wu, RR, Zhao, JP, Jin, H, Shao, P, Fang, MS, Guo, XF, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. (2008) 299:185–93. doi: 10.1001/JAMA.2007.56-B
66. Agarwal, SM, Panda, R, Costa-Dookhan, KA, MacKenzie, NE, Treen, QC, Caravaggio, F, et al. Metformin for early comorbid glucose dysregulation and schizophrenia spectrum disorders: a pilot double-blind randomized clinical trial. Transl Psychiatry. (2021) 11:219. doi: 10.1038/S41398-021-01338-2
67. Baptista, T, Rangel, N, Fernández, V, Carrizo, E, El Fakih, Y, Uzcátegui, E, et al. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: a multicentric, double-blind, placebo-controlled trial. Schizophr Res. (2007) 93:99–108. doi: 10.1016/J.SCHRES.2007.03.029
68. Carrizo, E, Fernández, V, Connell, L, Sandia, I, Prieto, D, Mogollón, J, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. (2009) 113:19–26. doi: 10.1016/J.SCHRES.2009.05.007
69. Tang, C, Chua, YC, Abdin, E, Subramaniam, M, and Verma, S. Twenty-Four Week, Randomized, Double-Blind, Placebo-controlled trial of metformin for antipsychotic-induced weight gain in patients with first-episode psychosis: a pilot study. Int J Environ Res Public Health. (2021) 137, 19. doi: 10.3390/IJERPH19010137
70. Chen, CH, Huang, MC, Kao, CF, Lin, SK, Kuo, PH, Chiu, CC, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. (2013) 74:e424–30. doi: 10.4088/JCP.12M08186
71. Hebrani, P, and Manteghi, A FB-J of research in, 2015 undefined. Double-blind, randomized, clinical trial of metformin as add-on treatment with clozapine in treatment of schizophrenia disorder. researchgate.net. (2015) Available at: https://www.researchgate.net/profile/Fatemeh-Behdani/publication/279303570_Double-blind_randomized_clinical_trial_of_metformin_as_add-on_treatment_with_clozapine_in_treatment_of_schizophrenia_disorder/links/56502adc08ae4988a7a93cc3/Double-blind-randomized-clinical-trial-of-metformin-as-add-on-treatment-with-clozapine-in-treatment-of-schizophrenia-disorder.pdf (Accessed April 11, 2023)
72. Siskind, D, Russell, AW, Suetani, S, Flaws, D, Kisely, S, Moudgil, V, et al. CoMET: a randomised controlled trial of co-commencement of metformin versus placebo as an adjunctive treatment to attenuate weight gain in patients with schizophrenia newly commenced on clozapine. Ther Adv Psychopharmacol. (2021) 11:204512532110452. doi: 10.1177/20451253211045248
73. Wang, M, Hua, TJ, Zhu, G, Ming, LG, Fei, YH, and Zhen, WX. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. (2012) 138:54–7. doi: 10.1016/J.SCHRES.2012.02.021
74. Chiu, CC, Lu, ML, Huang, MC, Chen, PY, Lin, YK, Lin, SK, et al. Effects of low dose metformin on metabolic traits in clozapine-treated schizophrenia patients: an exploratory twelve-week randomized, double-blind, placebo-controlled study. PLoS One. (2016) 11:e0168347. doi: 10.1371/JOURNAL.PONE.0168347
75. Wu, RR, Zhang, FY, Gao, KM, Ou, JJ, Shao, P, Jin, H, et al. Metformin treatment of antipsychotic-induced dyslipidemia: an analysis of two randomized, placebo-controlled trials. Mol Psychiatry. (2016) 21:1537–44. doi: 10.1038/MP.2015.221
76. Baptista, T, Martínez, J, Lacruz, A, Rangel, N, Beaulieu, S, Serrano, A, et al. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Can J Psychiatr. (2006) 51:192–6. doi: 10.1177/070674370605100310
77. Wu, RR, Zhao, JP, Guo, XF, He, YQ, Fang, MS, Bin, GW, et al. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. Am J Psychiatry. (2008) 165:352–8. doi: 10.1176/APPI.AJP.2007.07010079
78. Home - ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ (accessed April 11, 2023)
79. Madhu, L, Kodali, M, and Shetty, A. Promise of metformin for preventing age-related cognitive dysfunction. Neural Regen Res. (2022) 17:503–7. doi: 10.4103/1673-5374.320971
80. Wu, L, Liu, Y, Huang, X, Lin, K, Liu, Y, Li, Z, et al. Oral contraceptives (OCs) in combination with metformin versus OCs alone on metabolism in nonobese polycystic ovary syndrome: a meta-analysis and systematic review of randomized controlled trials. Clin Endocrinol. (2023) 99:1, 3–16. doi: 10.1111/CEN.14895
81. Paridari, P, Jabermoradi, S, Gholamzadeh, R, Vazifekhah, S, Vazirizadeh-Mahabadi, M, Roshdi Dizaji, S, et al. Can metformin use reduce the risk of stroke in diabetic patients? A systematic review and meta-analysis. Diabetes Metab Syndr. (2023) 17:102721. doi: 10.1016/J.DSX.2023.102721
82. Zhang, Q, Zheng, J, Wang, W, Cornett, EM, Kaye, AD, Urits, I, et al. The anticancer effect of metformin combined with epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung Cancer patients with or without type 2 diabetes mellitus: a systematic review and Meta-analysis. Oncol Ther. (2022) 10:363–75. doi: 10.1007/S40487-022-00209-0
83. Taylor, J, Stubbs, B, Hewitt, C, Ajjan, RA, Alderson, SL, Gilbody, S, et al. The effectiveness of pharmacological and non-pharmacological interventions for improving Glycaemic control in adults with severe mental illness: a systematic review and Meta-analysis. PLoS One. (2017) 12:e0168549. doi: 10.1371/JOURNAL.PONE.0168549
84. Metformin treatment on cognitive impairment of schizophrenia co-morbid metabolic syndrome - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03271866 (accessed April 14, 2023)
85. Ng, TP, Feng, L, Yap, KB, Lee, TS, Tan, CH, and Winblad, B. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis. (2014) 41:61–8. doi: 10.3233/JAD-131901
86. Carbon, M, and Correll, CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. (2014) 16:505–24. doi: 10.31887/DCNS.2014.16.4/MCARBON
87. Nasrallah, HA, and Smeltzer, DJ. Contemporary diagnosis and management of the patient with schizophrenia. (2002). Available at: https://books.google.com/books/about/Contemporary_Diagnosis_and_Management_of.html?hl=it&id=PX5k5XtJ_XwC (accessed April 14, 2023)
88. Koslover, J, Bruce, D, Patel, S, and Webb, AJ. Metformin-‘BRAINS & AIMS’ pharmacological/prescribing principles of commonly prescribed (top 100) drugs: education and discussion. Br J Clin Pharmacol. (2023) 89:931–8. doi: 10.1111/BCP.15653
89. Fitzgerald, I, O’Connell, J, Keating, D, Hynes, C, McWilliams, S, and Crowley, EK. Metformin in the management of antipsychotic-induced weight gain in adults with psychosis: development of the first evidence-based guideline using GRADE methodology. Evid Based Ment Health. (2022) 25:15–22. doi: 10.1136/EBMENTAL-2021-300291
90. Taylor, D, Metformin for schizophrenia: an editorial comment to, Curtis, J, Newall, H, Shiers, D, and Samaras, K. Considering metformin in cardiometabolic protection in psychosis. Acta Psychiatr Scand. (2012) 126:233–4. doi: 10.1111/J.1600-0447.2012.01907.X
91. Foley, DL, and Morley, KI. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry. (2011) 68:609–16. doi: 10.1001/ARCHGENPSYCHIATRY.2011.2
Keywords: metformin, schizophrenia, meta-analysis, cognitive disorders, hypoglycemic drugs
Citation: Battini V, Cirnigliaro G, Leuzzi R, Rissotto E, Mosini G, Benatti B, Pozzi M, Nobile M, Radice S, Carnovale C, Dell’Osso B and Clementi E (2023) The potential effect of metformin on cognitive and other symptom dimensions in patients with schizophrenia and antipsychotic-induced weight gain: a systematic review, meta-analysis, and meta-regression. Front. Psychiatry. 14:1215807. doi: 10.3389/fpsyt.2023.1215807
Edited by:
Mirko Manchia, University of Cagliari, ItalyReviewed by:
Alessandro Cuomo, University of Siena, ItalyXiaoyan Ma, Tianjin Anding Hospital, China
Copyright © 2023 Battini, Cirnigliaro, Leuzzi, Rissotto, Mosini, Benatti, Pozzi, Nobile, Radice, Carnovale, Dell’Osso and Clementi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vera Battini, dmVyYS5iYXR0aW5pQHVuaW1pLml0
 Vera Battini
Vera Battini Giovanna Cirnigliaro2
Giovanna Cirnigliaro2 Rodolfo Leuzzi
Rodolfo Leuzzi Giulia Mosini
Giulia Mosini Marco Pozzi
Marco Pozzi Maria Nobile
Maria Nobile Sonia Radice
Sonia Radice Carla Carnovale
Carla Carnovale Bernardo Dell’Osso
Bernardo Dell’Osso Emilio Clementi
Emilio Clementi