- 1Unit of Psychiatry, Department of Medicine (DAME), University of Udine, Udine, Italy
- 2Department of Chemical Sciences and Materials Technologies, Institute of Biomolecular Chemistry, National Research Council (CNR), Pozzuoli, Italy
- 3Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom
Introduction: The endocannabinoid (eCB) system disruption has been suggested to underpin the development of psychosis, fueling the search for novel, better-tolerated antipsychotic agents that target the eCB system. Among these, palmitoylethanolamide (PEA), an N-acylethanolamine (AE) with neuroprotective, anti-inflammatory, and analgesic properties, has drawn attention for its antipsychotic potential.
Methods: This Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020-compliant systematic review aimed at reappraising all clinical and preclinical studies investigating the biobehavioral role of PEA in psychosis.
Results: Overall, 13 studies were eligible for data extraction (11 human, 2 animal). Observational studies investigating PEA tone in psychosis patients converged on the evidence for increased PEA plasma (6 human) and central nervous system (CNS; 1 human) levels, as a potential early compensatory response to illness and its severity, that seems to be lost in the longer-term (CNS; 1 human), opening to the possibility of exogenously supplementing it to sustain control of the disorder. Consistently, PEA oral supplementation reduced negative psychotic and manic symptoms among psychosis patients, with no serious adverse events (3 human). No PEA changes emerged in either preclinical psychosis model (2 animal) studied.
Discussion: Evidence supports PEA signaling as a potential psychosis biomarker, also indicating a therapeutic role of its supplementation in the disorder.
Systematic review registration: https://doi.org/10.17605/OSF.IO/AFMTK.
1. Introduction
Psychotic disorders—non-affective (e.g., schizophrenia (SCZ), schizophreniform disorder) and affective psychoses (e.g., bipolar disorder (BPAD), major depressive disorder with psychotic symptoms)—are a heterogeneous group of disabling mental health disturbances (1), sharing common phenomenological, neurobiological, and genetic characteristics (2–5). These conditions generally emerge between late adolescence and early adulthood (6), with a lifetime prevalence exceeding 3% (7–9), and severely affect the patients' and their families' quality of life. The dopaminergic and glutamatergic hypotheses still play a pivotal role in the attempt to describe the neurobiological mechanisms underlying psychosis (10–13), with potential for an integrated model explaining both positive (e.g., delusions, hallucinations) and negative (e.g., restricted emotional expression, avolition) psychotic symptoms (12).
To date, antipsychotic (AP) medications represent the cornerstone treatment for these conditions, although not always devoid of suboptimal clinical response and unpleasant side-effects (14, 15). Therefore, the exploration of other perturbed systems potentially underpinning psychotic disorders has aimed at identifying novel therapeutic targets. The endocannabinoid (eCB) system has been recognized as a mediator of the dopaminergic and glutamatergic systems via the cannabinoid receptor 1 (CB1) in the central nervous system (CNS), and found to be altered in the early phases of the disorder (16–19). Consistently, accumulating evidence has highlighted the therapeutic potential of the eCB system modulation. Particularly, cannabidiol (CBD) has shown promising results for both psychosis and clinical high-risk (CHR) for psychosis state (20–23). Further, reduced diversity of gut microbiota and gut-brain axis metabolic alterations associated have been indicated as having a putative role in the patho-etiological cascade toward psychosis (24). To this end, reduced microbiota diversity has been observed to contribute to common SCZ negative symptoms such as anhedonia and amotivation via eCB-like compound palmitoylethanolamide (PEA) fecal levels, warranting the possibility to target the gut microbiota-eCB axis (25). Finally, growing evidence emphasizes the importance of inflammation and oxidative stress in the stages preceding psychosis onset and throughout illness progression (26, 27).
PEA is an N-acylethanolamine (AE), produced “on demand” by different cell types as a response to actual or potential damage (28, 29). Importantly, PEA has been proven to down-regulate central and peripheral activity of mast cells and non-neuronal cells (e.g., astrocytes, microglia) (30–32) and to exert protective functions against glutamate neuro-toxicity, accounting for its naturally-occurring anti-inflammatory, analgesic, and anticonvulsant properties (33). It directly activates the Peroxisome Proliferator Activated Receptor-α (PPAR-α) and the GPR55, allosterically modulates the Transient Receptor Potential Vanilloid 1 (TRPV1), and indirectly interacts with CB1 and cannabinoid receptor 2 (CB2) (32, 34, 35). Due to the shared pharmacodynamic properties, PEA is considered as the endogenous equivalent of CBD (36, 37). A growing body of literature has confirmed the role of PEA in most neurobiological mechanisms underpinning several neuropsychiatric conditions both in clinical and preclinical settings (38–40).
1.1. Objectives
The effect of PEA over neuroinflammation and glutamate signaling may represent a promising biobehavioral mechanism underlying its clinical utility in psychosis. This systematic review aimed to collect and discuss all available clinical and preclinical data generated by studies investigating the role of PEA in non-affective and affective psychoses. We reviewed all interventional and observational studies, employing either retrospective or prospective methodological approaches with any PEA neuro-biological correlates investigated in psychosis.
2. Materials and methods
2.1. Inclusion and exclusion criteria
All clinical and preclinical evidence about the topic was gathered and systematically reviewed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (41). Inclusion criteria were defined as follows: 1. analytic, observational, and interventional studies; 2. studies assessing (i) acute or long-term effects of palmitoylethanolamide (PEA) administration over psychosis-related biological underpinnings (e.g., neuroimmune disruption, hypothalamus-pituitary-adrenal axis dysregulation); and behavioral features (e.g., negative psychotic symptoms, manic symptoms); or (ii) PEA and PEA signaling-related molecular marker (e.g., other endocannabinoids/acylethanolamines, PEA-related enzymes) modulation in peripheral tissues (e.g., plasma, serum), or in the central nervous system (e.g. cerebrospinal fluid, brain tissue) in psychosis and related conditions. Exclusion criteria were defined as outlined: 1. studies in which (i) PEA was not the intervention or outcome of interest (e.g., studies evaluating only exogenous cannabinoid administration or assessing endogenous cannabinoid levels); and (ii) PEA bio-behavioral correlates were not investigated with reference to psychosis; and (iii) PEA bio-behavioral correlates were not directly reported on; 2. reviews; 3. systematic reviews; 4. meta-analyses.
2.2. Search strategy and data extraction
A literature search was performed using electronic databases (PubMed, Scopus, and Web of Science) for any published original study written in English, on 16 January 2023. In order to be as much inclusive as possible, a combination of broad-meaning terms describing and/or concerning PEA (“palmitoylethanolamide,” “palmitylethanolamide,” “N-2-hydroxyethyl-hexadecanamide,” “N-2-hydroxyethyl-palmitate,” “N-palmitoylethanolamine,” “PEA,” and “palmitoyl-ethanolamine”) and psychosis (“schizophreni*,” ‘psychosis,” “psychoses,” “psychotic,” “bipolar,” “mania,” “manic”) was adopted. Reference lists of all selected studies were scrutinized to identify any adjunctive eligible evidence. Data screening and extraction were conducted according to a two-step selection process (conventional double-screening), performed by two researchers (RB and MC) independently from each other. In the instances of conflicting opinions regarding papers' inclusion, a consensus was sought through discussion with a third senior reviewer (MB).
2.3. Risk of bias assessment
In light of the methodological heterogeneity of collected evidence, quality of studies assessment was conducted in accordance to an adapted and suitably flexible set of criteria suggested by the Agency for Healthcare Research and Quality (AHRQ) guidance (42), in line with previous research in the field (38–40). Risk of systematic bias across human studies was ruled out by screening all papers for potential confounding factors (e.g., gender, age, smoking status, level of education). Furthermore, factors possibly accounting for similarities and differences between all studies were assessed, extracting information about study characteristics, including study design (e.g., observational, interventional), defined study population (for human studies: e.g., schizophrenia (SCZ) patients, clinical high-risk (CHR) subjects; for animal studies: e.g., mouse or rat model), age or developmental stage, gender, adequate psychosis model (for animal studies only: e.g., maternal deprivation, methylazoxymethanol acetate (MAM) prenatal exposure), PEA measure (e.g., PEA dosage and administration route, PEA assessment in tissues), adequate PEA evaluation (e.g., time of exposure, single or multiple assessments), defined control group, comparability of subjects (for human studies only), exclusion criteria/adjusting factors (for human studies only), statistical analyses, and declaration of fundings/sponsorship. The full study protocol is available at https://doi.org/10.17605/OSF.IO/AFMTK.
3. Results
3.1. Study selection
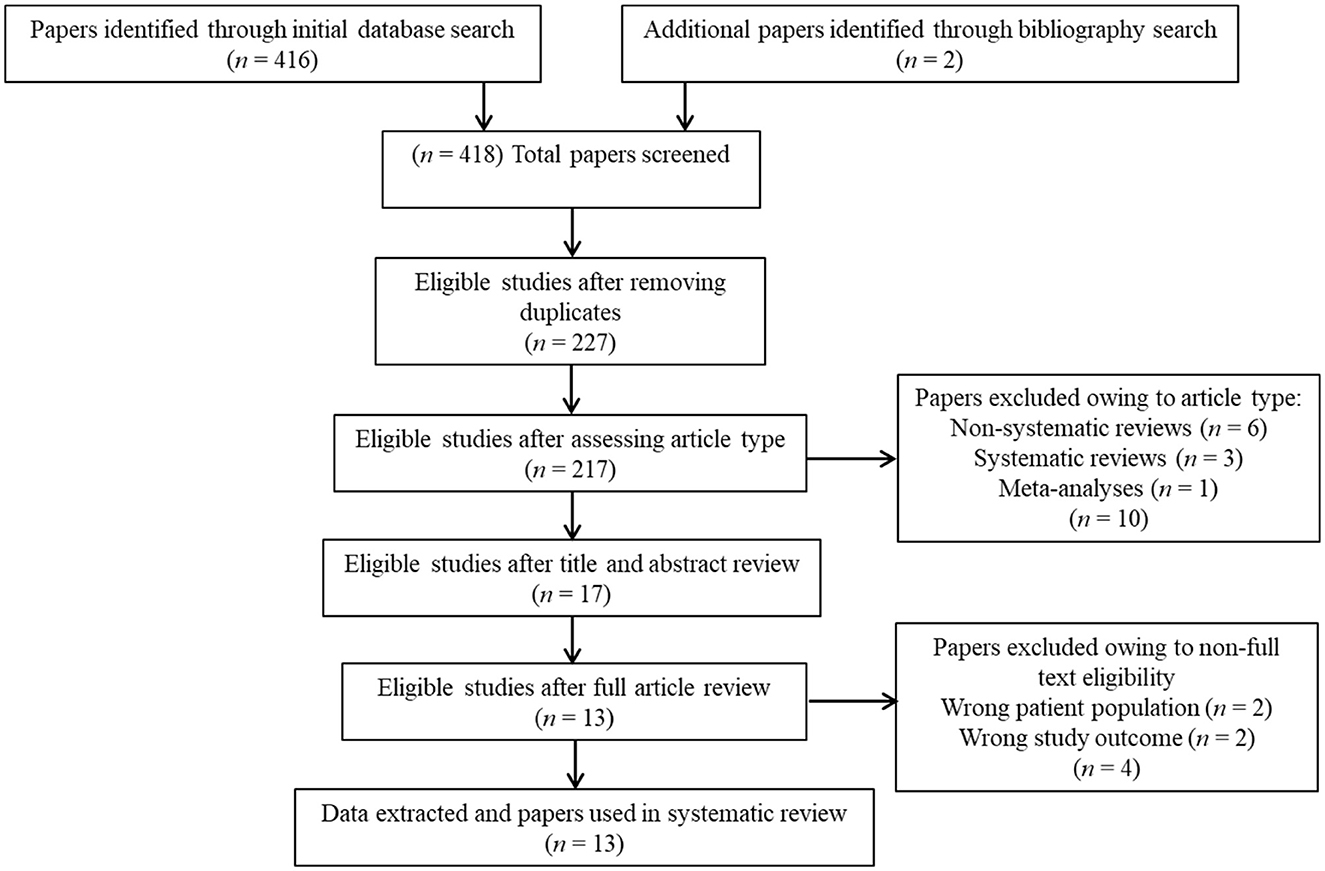
Overall, 418 studies were retrieved through the initial data search. After removing duplicates as well as excluding articles owing to article type (e.g., non-systematic reviews, systematic reviews, meta-analyses), titles, abstracts, or full texts of all records were examined against the inclusion and exclusion criteria following a three-step screening process (Figure 1). A final list of thirteen studies was used for systematic analysis in this review, including 11 studies conducted only in human populations and two studies performed in animal models, exploring various aspects of palmitoylethanolamide (PEA) signaling pathway (Table 1). These include (i) in vivo PEA add-on treatment exposure in humans with different types of psychoses (e.g., non-affective psychosis, affective psychosis) or psychotic symptoms (e.g., hallucinations) (3 studies; Table 1); (ii) PEA quantitative blood assessment in humans with psychosis clinical high-risk (CHR) state (1 study; Table 1); (iii) PEA quantitative blood assessment in humans with different types of psychoses (e.g., non-affective psychosis, affective psychosis) at different stages of illness (5 studies; Table 1); (iv) PEA quantitative central nervous system (CNS; e.g., brain tissue, cerebrospinal fluid) assessment in humans with schizophrenia (SCZ; 2 studies; Table 1); (v) PEA quantitative brain tissue assessment in animal models of SCZ (2 studies; Table 1). Additional data on methodological quality of studies conducted in humans and animals are reported in Tables 2, 3. A brief synthesis of the main results is presented below and summarized in Table 1.
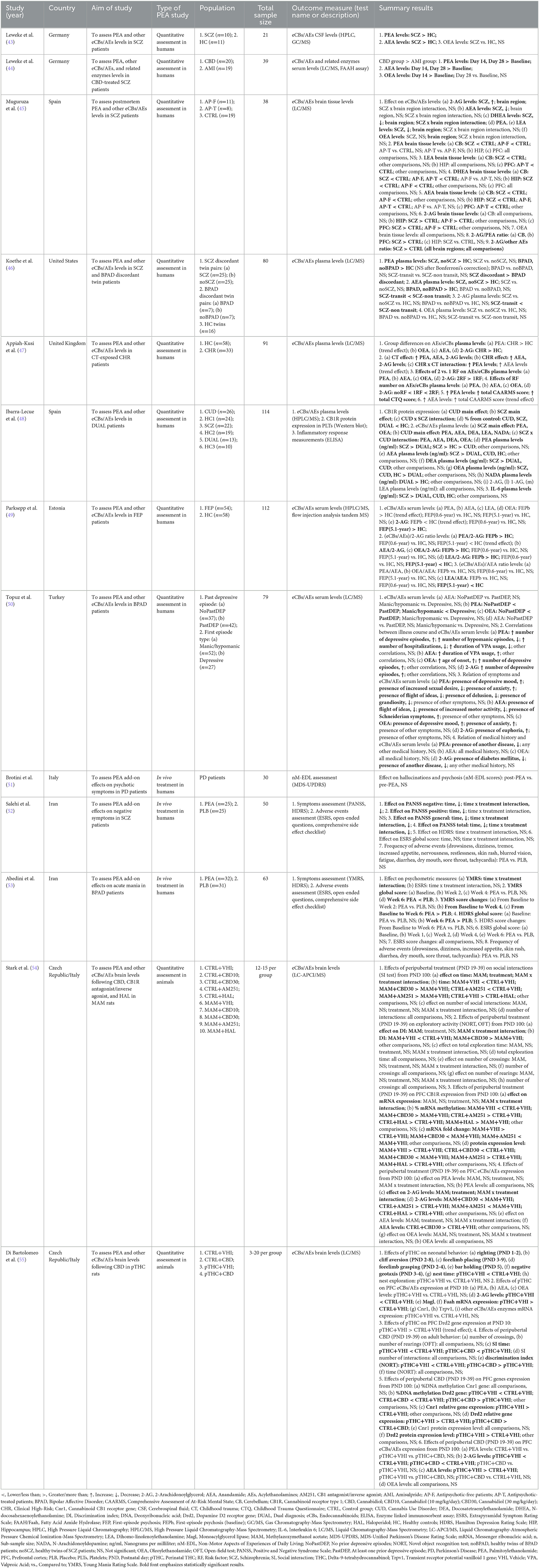
Table 1. Summary of clinical and preclinical studies investigating palmitoylethanolamide and its correlations to psychotic disorders.
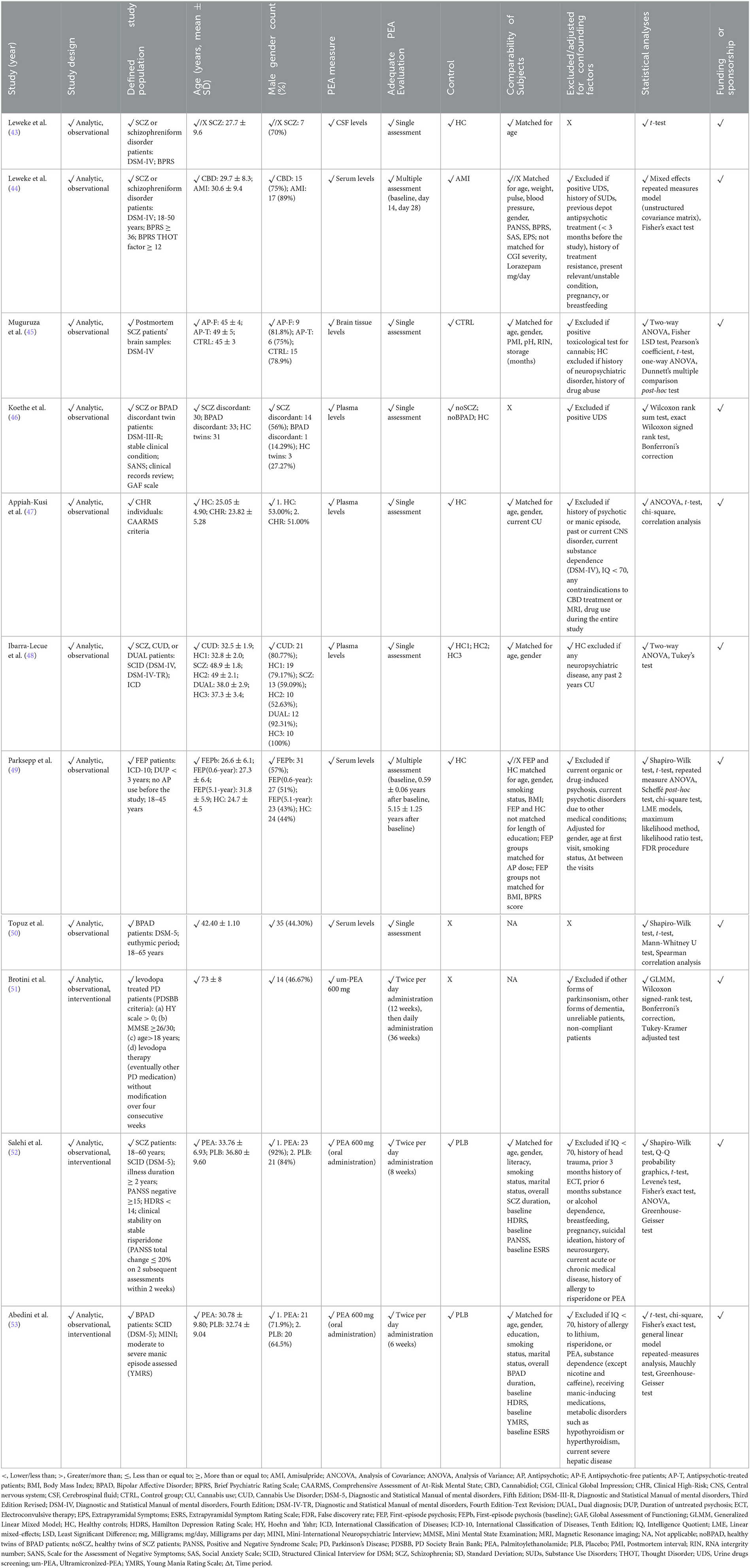
Table 2. Methodological quality of clinical studies investigating palmitoylethanolamide and its correlations to psychotic disorders.

Table 3. Methodological quality of preclinical studies investigating palmitoylethanolamide and its correlations to psychotic disorders.
3.2. In vivo palmitoylethanolamide (PEA) add-on treatment exposure in humans with different types of psychoses or psychotic symptoms
Three human studies have addressed this area (Table 1) using similar but not overlapping methodologies in terms of study population [chronic schizophrenia (SCZ) patients (52), bipolar disorder (BPAD) patients with manic symptoms (53), Parkinson's disease (PD) patients (51)], PEA formulation [oral native PEA (52, 53), oral Ultramicronized (um)-PEA (51)], PEA dosage [600 milligrams (mg) daily (51), 600 mg twice/daily (51–53)], and PEA period of exposure [6 weeks (53), 8 weeks (52), 12 months (51)]. Apart from a single study lacking a controlled condition (51), all studies adopted a randomized, double-blind, placebo-controlled design (52, 53). Overall, results indicated a beneficial effect of PEA adjunctive therapy on residual negative and general psychopathological symptoms, but not positive symptoms, of risperidone-treated SCZ patients (52), as well as on manic symptoms of lithium- plus risperidone-treated BPAD patients (53). Coherent data emerged regarding depressive symptomatology, which did not appear to be improved in both SCZ and BPAD patients treated with PEA add-on as compared to placebo (52, 53). A single study addressing the effect of PEA over non-motor symptoms among levodopa-treated PD patients, showed no reduction in the number of subjects presenting with hallucinations and psychosis (51). Noteworthy, PEA was well-tolerated, in the absence of extrapyramidal symptoms or any other relevant side effect across the three studies, and for the entire duration of the compound administration.
3.3. Palmitoylethanolamide quantitative blood assessment in humans with psychosis clinical high-risk state
This systematic reappraisal identified a single human study specifically assessing peripheral blood PEA levels in individuals suffering from CHR state, as compared to healthy controls (HC) (47) (Table 1). PEA levels tended to be elevated in CHR patients, even though just approaching statistical significance. Intriguingly, PEA levels appeared to be significantly higher in those who were both CHR and had been exposed to childhood trauma (CT), compared to individuals having none of the above-mentioned risk factors or one risk factor alone. Furthermore, a significant positive correlation between PEA levels and the severity of CHR state and CT was observed (47).
3.4. Palmitoylethanolamide quantitative blood assessment in humans with different types of psychoses at different stages of illness
Most of the studies retrieved investigated peripheral blood PEA levels in patients with non-affective [e.g., schizophrenia (SCZ) (44, 46, 48, 49) and schizophreniform psychosis (44)] or affective [e.g., bipolar disorder (BPAD) (46, 50)] psychoses at different stages of illness (Table 1). Two studies converged on the evidence of higher PEA levels in SCZ patients than in healthy controls (HC) (46, 48), one of which further suggesting significantly higher PEA plasma concentration in SCZ patients compared to patients meeting criteria for cannabis use disorder (CUD) or dual diagnosis of CUD and SCZ (48). Interestingly, compared to HC, unaffected twin siblings of SCZ patients also showed increased PEA levels, that did not differ from those of patients (46). Remarkably, antipsychotic (AP)-naïve first-episode psychosis (FEP) patients compared to HC showed a tendency to elevated PEA plasma levels and a significantly higher PEA/2-arachidonoylglycerol (2-AG) ratio, with both that subsided an average of 0.6 and 5.1 years after the initiation of AP treatment (49). The modulating effect of AP treatment over acylethanolamines (AEs) levels was also investigated through a double-blind, randomized, parallel-group, controlled clinical trial, showing elevated serum PEA concentration in cannabidiol (CBD)-treated SCZ patients, compared with those treated with the antipsychotic amisulpride (44). Studies measuring PEA levels among BPAD patients showed a less pronounced increase in affected and unaffected siblings of illness-discordant twin couples, when compared to HC (46). Further, a higher PEA plasma concentration was found in BPAD patients having first episode as depression than in those who had their first episode as mania (50) as well as in those who had at least one depressive episode than in patients who had no prior depressive episodes (50). Finally, PEA levels were increased according to the number of depressive episodes and the presence of depressive mood and anxiety, while inversely correlating with the number of hypomanic episodes, the number of hospitalizations, the duration of valproate (VPA) treatment, sexual desire, presence of flight of ideas, delusion, and grandiosity (50).
3.5. Palmitoylethanolamide quantitative central nervous system assessment in humans with schizophrenia
Two studies analyzed PEA levels in the CNS of patients with psychosis (43, 45) (Table 1). In particular, PEA levels were reported to be elevated in the cerebrospinal fluid (CSF) of SCZ and schizophreniform psychosis patients compared with healthy controls (HC) (43). Conversely, a study on postmortem brain samples from subjects diagnosed with SCZ compared to controls indicated lower PEA levels in the cerebellum of antipsychotic (AP)-free patients only (45). No significant differences in PEA brain quantification were detected among all study groups in the other brain areas investigated (45).
3.6. Palmitoylethanolamide quantitative brain tissue assessment in animal models of schizophrenia
In total, two studies evaluated PEA levels in the prefrontal cortex (PFC) (54, 55), hippocampus (HIP) (54), and nucleus accumbens (NAc) (54) of rats exposed to either prenatal methylazoxymethanol acetate (MAM) (54) or perinatal delta-9-tetrahydrocannabinol (THC) (55), which present with many SCZ-relevant biobehavioral deficits at neonatal age (55) and adulthood (54, 55) (Table 1). While modulating endocannabinoids [eCBs; e.g., anandamide (AEA) and 2-acylglycerol (2-AG) (54, 55)] and other acylethanolamines [AEs; e.g., oleoylethanolamide (OEA) (54)], both MAM and THC exposure did not significantly affect PEA levels in all investigated brain areas, as neither did the peripubertal exposure to cannabidiol (CBD) (54, 55), Cannabinoid receptor type 1 (CB1)-antagonist/agonist AM251 (54), and first-generation antipsychotic haloperidol (HAL) (54), compared to control conditions.
4. Discussion
This is the first systematic review of all evidence exploring the biobehavioral correlates of palmitoylethanolamide (PEA) in psychosis across human and animal studies. Unlike previous research in this field (38–40), the greatest majority of records included consisted of human studies. Existing reviews focusing on the role of the major phytocannabinoid cannabidiol (CBD) as a potential treatment for schizophrenia (SCZ) patients (20, 56, 57) gathered still preliminary evidence supporting its antipsychotic (AP) efficacy, while highlighting its advantageous side effect profile and good tolerability. Targeting similar pathways, PEA may be considered as a viable alternative to CBD with implications for many therapeutic areas, due to its established safety and the development of formulations maximizing its bioavailability (33, 37).
Overall, the present review demonstrated that PEA may be involved in different psychotic phenotypes. Also, it found initial evidence that PEA levels may reflect the severity of the disorder as well as the stage of illness. Evidence was obtained from both interventional studies addressing the AP potential of PEA supplementation, and observational studies of PEA tone in peripheral blood and in the central nervous system (CNS) in the context of clinical high-risk (CHR) for psychosis and psychotic disorders at different stages of illness.
Some important findings from this systematic review deserve to be highlighted. First, despite its promise, evidence regarding the therapeutic potential of PEA supplementation for psychosis is still limited (51–53) and provides findings about selective efficacy on specific symptoms. In particular, PEA add-on to conventional psychotropic medications did not significantly reduce positive psychotic symptoms (51–53), while ameliorating negative psychotic (52) and acute manic (53) symptoms. Importantly, while not being the focus of this review, results presented here are inconclusive about a potential role of PEA in ameliorating depressive symptoms among psychosis patients (53). Also, in only one study participants were clearly asked to avoid forms of cognitive behavioral therapies during the trial period (52), thus requiring future interventional studies to clearly rule out a potential masking effect of add-on psychotherapy over symptoms.
Second, a line of research investigating endocannabinoids (eCBs) tone in blood among subjects with genetic vulnerability to psychosis (i.e., unaffected twins of SCZ patients) (46), CHR individuals (47), untreated first-episode psychosis (FEP) patients (49), and longer-course SCZ patients (46, 48), converged on the evidence of increased PEA plasma levels as compared to healthy subjects. Also, among CHR patients, the more severe the clinical picture (i.e., greater symptom severity) and the risk profile (i.e., more severe childhood trauma), the higher were the PEA levels (47). Based on PEA-related neurobiological mechanisms, the finding of augmented PEA release across different populations may reflect an endogenous attempt to restore homodynamic balance under disease conditions (28, 29). However, follow-up studies revealed that PEA plasma levels are no longer heightened in AP-treated SCZ patients after 0.6 years of treatment, and further decreased at 5.1 years from baseline (49), potentially suggesting that such biological self-regulation of PEA levels is lost as the diseases progresses. Interestingly, a 2- to 4-week CBD treatment among SCZ patients was associated with higher PEA levels (44) when compared with a 2- to 4-week AP treatment (44), possibly indicating a CBD-specific property in sustaining PEA tone.
Further, differently from SCZ spectrum disorders, PEA plasma level increase was less pronounced in bipolar disorder (BPAD) patients and unaffected twins of BPAD patients, when compared to healthy subjects (46), perhaps accounting for existing phenotypical discrepancies within the affective psychosis group. In fact, PEA plasma levels were greater among BPAD patients having first episode as depression and increased consistently as a function of the lifetime number of depressive episodes (50). Instead, the occurrence of positive psychotic symptoms, manic symptoms, and hypomanic episodes, was correlated with reduced PEA tone (50). Based on evidence that BPAD patients with manic onset have an older age at diagnosis and a longer duration of untreated illness than those with depressive onset (58), and that polarity of episodes over time often reflects polarity at onset (59), such different findings among BPAD patients may suggest a changing pattern in PEA levels over time. In other words, PEA plasma levels would be higher in the first phases of the disorder, while declining because of disease progression, in line with what observed for non-affective psychosis, and thus strengthening the rationale for its supplementation.
Third, CNS PEA levels were found to be augmented in the cerebrospinal fluid (CSF) of young adults with SCZ and schizophreniform disorder (43), whereas reduced in postmortem cerebellar samples of AP-free middle-aged SCZ patients (45), as compared to healthy controls. Similarly, brain PEA levels did not differ between AP-treated middle-aged SCZ patients and healthy controls (45). Again, it can be assumed that PEA levels are elevated in the early stages of psychosis, potentially reflecting an innate compensatory mechanism, before dropping concomitantly to disease progression. With reference to AP treatment, while on one hand it did not appear to increase PEA levels, on the other it is not clear if it prevented a more pronounced longer-term decrease (45).
Only two studies assessed PEA levels in the prefrontal cortex (PFC) of preclinical models of psychosis. They did not show any significant differences as compared to the control condition (54, 55). Further animal studies will need to yield a more robust understanding of the neurobiological mechanisms involving PEA in psychosis.
The findings of this review should be seen considering some strengths and limitations. Despite supporting PEA tone alterations in psychosis and effectiveness of PEA supplementation as a therapeutic strategy, research in this field needs to be expanded, especially to fully comprehend the potential of PEA supplementation as add-on therapy for psychosis across all its symptoms dimensions. Indeed, evidence points toward a beneficial effect of PEA over negative psychotic symptoms and acute manic symptoms, reasonably due to the protective role of the compound against altered neuroinflammation and related mechanisms (28, 29), while PEA effect over positive psychotic symptoms remains to be clarified. Further, future follow-up studies will have to investigate whether PEA effect is sustained in the longer-term. Besides, to date, evidence of PEA effect as monotherapy in the clinical stages preceding full-blown psychosis onset to prevent the risk of progression is totally lacking and is worth of exploration. Finally, PEA levels appear to be altered in psychosis. However, further clarification is needed as to whether PEA tone is altered to a different extent depending on the stage of illness and whether this can be considered a biomarker of psychosis. In line with this, whether any AP treatment may be beneficial through PEA tone modulation requires additional investigation. Biobehavioral comparisons between CBD and PEA are also worth of consideration, especially whether CBD antipsychotic effects are mediated by PEA signaling and similar effects can be obtained directly supplementing PEA. The latter would be inevitably more advantageous, due to its safer profile and shorter biological distance from the therapeutic target.
5. Conclusions
This systematic review provided a first overview of all observational and experimental studies of PEA and its biobehavioral correlates in psychosis. Although in its infancy and still limited, research in this field is primarily carried out on humans and provides evidence for both alterations in PEA signaling, implications for psychosis-related behavioral features, and benefits from PEA supplementation. In particular, PEA may be useful to improve negative psychotic symptoms and manic symptoms. Noteworthy, no serious adverse events were reported across all human studies investigating its administration, further supporting PEA potential effectiveness and elevated safety as a therapeutic intervention in psychosis.
Author contributions
Conceptualization, methodology, validation, resources, writing—review and editing, and visualization: RB, FP, AC, SB, MB, and MC. Software, data curation, writing—original draft preparation, supervision, and project administration: RB and MC. Investigation: RB, MB, and MC. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
MC has been a consultant/advisor to GW Pharma Limited, F. Hoffmann-La Roche Limited, and GW Pharma Italy SRL, outside of this work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kraepelin E. Die Erscheinungsformen des Irreseins: (The manifestations of insanity). Hist Psychiatry. (1992) 3:509–29. doi: 10.1177/0957154X9200301208
2. Potash JB, Bienvenu OJ. Neuropsychiatric disorders: Shared genetics of bipolar disorder and schizophrenia. Nat Rev Neurol. (2009) 5:299–300. doi: 10.1038/nrneurol.2009.71
3. Craddock N, O'Donovan MC, Owen MJ. Psychosis genetics: modeling the relationship between schizophrenia, bipolar disorder, and mixed (or “schizoaffective”) psychoses. Schizophr Bull. (2009) 35:482–90. doi: 10.1093/schbul/sbp020
4. Craddock N, Owen MJ. The Kraepelinian dichotomy - going, going but still not gone. Br J Psychiatry. (2010) 196:92–5. doi: 10.1192/bjp.bp.109.073429
5. Ivleva EI, Morris DW, Moates AF, Suppes T, Thaker GK, Tamminga CA. Genetics and intermediate phenotypes of the schizophrenia–bipolar disorder boundary. Neurosci Biobehav Rev. (2010) 34:897–921. doi: 10.1016/j.neubiorev.2009.11.022
6. Fusar-Poli L, Aguglia A, Albert U. Editorial: Early identification of affective and non-affective psychoses: from psychopathology to biomarkers. Front Psychiatry. (2023) 14:1144943. doi: 10.3389/fpsyt.2023.1144943
7. Perala J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsa E, Pirkola S, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. (2007) 64:19–28. doi: 10.1001/archpsyc.64.1.19
8. Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. (2013) 382:1575–86. doi: 10.1016/S0140-6736(13)61611-6
9. Vargas TG, Mittal VA. The critical roles of early development, stress, and environment in the course of psychosis. Annu Rev Dev Psychol. (2022) 4:423–45. doi: 10.1146/annurev-devpsych-121020-032354
10. Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. (2012) 69:776–86. doi: 10.1001/archgenpsychiatry.2012.169
11. Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. (2014) 383:1677–87. doi: 10.1016/S0140-6736(13)62036-X
12. Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol. (2015) 29:97–115. doi: 10.1177/0269881114563634
13. Howes OD, McCutcheon R, Owen MJ, Murray RM. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. (2017) 81:9–20. doi: 10.1016/j.biopsych.2016.07.014
14. Andrews G, Sanderson K, Corry J, Issakidis C, Lapsley H. Cost-effectiveness of current and optimal treatment for schizophrenia. Br J Psychiatry. (2003) 183:427–35. doi: 10.1192/bjp.183.5.427
15. Obradovic M, Mrhar A, Kos M. Cost-effectiveness of antipsychotics for outpatients with chronic schizophrenia. Int J Clin Pract. (2007) 61:1979–88. doi: 10.1111/j.1742-1241.2007.01431.x
16. Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, et al. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. (2004) 29:2108–14. doi: 10.1038/sj.npp.1300558
17. Koethe D, Hoyer C, Leweke FM. The endocannabinoid system as a target for modelling psychosis. Psychopharmacology. (2009) 206:551–61. doi: 10.1007/s00213-009-1591-7
18. Appiah-Kusi E, Leyden E, Parmar S, Mondelli V, McGuire P, Bhattacharyya S. Abnormalities in neuroendocrine stress response in psychosis: the role of endocannabinoids. Psychol Med. (2016) 46:27–45. doi: 10.1017/S0033291715001786
19. Colizzi M, McGuire P, Pertwee RG, Bhattacharyya S. Effect of cannabis on glutamate signalling in the brain: a systematic review of human and animal evidence. Neurosci Biobehav Rev. (2016) 64:359–81. doi: 10.1016/j.neubiorev.2016.03.010
20. Schubart CD, Sommer IE, Fusar-Poli P, de Witte L, Kahn RS, Boks MP. Cannabidiol as a potential treatment for psychosis. Eur Neuropsychopharmacol. (2014) 24:51–64. doi: 10.1016/j.euroneuro.2013.11.002
21. McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. (2018) 175:225–31. doi: 10.1176/appi.ajp.2017.17030325
22. Bhattacharyya S, Wilson R, Appiah-Kusi E, O'Neill A, Brammer M, Perez J, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. (2018) 75:1107–17. doi: 10.1001/jamapsychiatry.2018.2309
23. Appiah-Kusi E, Petros N, Wilson R, Colizzi M, Bossong MG, Valmaggia L, et al. Effects of short-term cannabidiol treatment on response to social stress in subjects at clinical high risk of developing psychosis. Psychopharmacology (Berl). (2020) 237:1121–30. doi: 10.1007/s00213-019-05442-6
24. Kelly JR, Minuto C, Cryan JF, Clarke G, Dinan TG. The role of the gut microbiome in the development of schizophrenia. Schizophr Res. (2021) 234:4–23. doi: 10.1016/j.schres.2020.02.010
25. Minichino A, Jackson MA, Francesconi M, Steves CJ, Menni C, Burnet PWJ, et al. Endocannabinoid system mediates the association between gut-microbial diversity and anhedonia/amotivation in a general population cohort. Mol Psychiatry. (2021) 26:6269–76. doi: 10.1038/s41380-021-01147-5
26. Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. (2015) 2:258–70. doi: 10.1016/S2215-0366(14)00122-9
27. Fraguas D, Diaz-Caneja CM, Ayora M, Hernandez-Alvarez F, Rodriguez-Quiroga A, Recio S, et al. Oxidative stress and inflammation in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull. (2019) 45:742–51. doi: 10.1093/schbul/sby125
28. Petrosino S, Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol. (2017) 174:1349–65. doi: 10.1111/bph.13580
29. Petrosino S, Schiano Moriello A. Palmitoylethanolamide: a nutritional approach to keep neuroinflammation within physiological boundaries: a systematic review. Int J Mol Sci. (2020) 21:526. doi: 10.3390/ijms21249526
30. Skaper SD, Facci L. Mast cell-glia axis in neuroinflammation and therapeutic potential of the anandamide congener palmitoylethanolamide. Philos Trans R Soc Lond B Biol Sci. (2012) 367:3312–25. doi: 10.1098/rstb.2011.0391
31. Skaper SD, Facci L, Giusti P. Glia and mast cells as targets for palmitoylethanolamide, an anti-inflammatory and neuroprotective lipid mediator. Mol Neurobiol. (2013) 48:340–52. doi: 10.1007/s12035-013-8487-6
32. Petrosino S, Schiano Moriello A, Verde R, Allara M, Imperatore R, Ligresti A, et al. Palmitoylethanolamide counteracts substance P-induced mast cell activation in vitro by stimulating diacylglycerol lipase activity. J Neuroinflammation. (2019) 16:274. doi: 10.1186/s12974-019-1671-5
33. Clayton P, Hill M, Bogoda N, Subah S, Venkatesh R. Palmitoylethanolamide: A Natural Compound for Health Management. Int J Mol Sci. (2021) 22:305. doi: 10.3390/ijms22105305
34. De Petrocellis L, Davis JB, Di Marzo V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett. (2001) 506:253–6. doi: 10.1016/S0014-5793(01)02934-9
35. Petrosino S, Schiano Moriello A, Cerrato S, Fusco M, Puigdemont A, De Petrocellis L, et al. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br J Pharmacol. (2016) 173:1154–62. doi: 10.1111/bph.13084
36. Couch DG, Cook H, Ortori C, Barrett D, Lund JN, O'Sullivan SE. Palmitoylethanolamide and cannabidiol prevent inflammation-induced hyperpermeability of the human gut in vitro and in vivo: a randomized, placebo-controlled, double-blind controlled trial. Inflamm Bowel Dis. (2019) 25:1006–18. doi: 10.1093/ibd/izz017
37. Clayton P, Subah S, Venkatesh R, Hill M, Bogoda N. Palmitoylethanolamide: a potential alternative to cannabidiol. J Diet Suppl. (2021) 5:1–26. doi: 10.1080/19390211.2021.2005733
38. Colizzi M, Bortoletto R, Costa R, Zoccante L. Palmitoylethanolamide and its biobehavioral correlates in autism spectrum disorder: a systematic review of human and animal evidence. Nutrients. (2021) 13:1346. doi: 10.3390/nu13041346
39. Bortoletto R, Balestrieri M, Bhattacharyya S, Colizzi M. Is it time to test the antiseizure potential of palmitoylethanolamide in human studies? A systematic review of preclinical evidence. Brain Sci. (2022) 12:101. doi: 10.3390/brainsci12010101
40. Colizzi M, Bortoletto R, Colli C, Bonomo E, Pagliaro D, Maso E, et al. Therapeutic effect of palmitoylethanolamide in cognitive decline: a systematic review and preliminary meta-analysis of preclinical and clinical evidence. Front Psychiatry. (2022) 13:8122. doi: 10.3389/fpsyt.2022.1038122
41. Parums DV. Editorial: review articles, systematic reviews, meta-analysis, and the updated preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines. Med Sci Monit. (2021) 27:e934475. doi: 10.12659/MSM.934475
42. West S, King V, Carey TS, Lohr KN, McKoy N, Sutton SF, et al. Systems to rate the strength of scientific evidence. Evid Rep Technol Assess. (2002) 47:1–11.
43. Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport. (1999) 10:1665–9. doi: 10.1097/00001756-199906030-00008
44. Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. (2012) 2:e94. doi: 10.1038/tp.2012.15
45. Muguruza C, Lehtonen M, Aaltonen N, Morentin B, Meana JJ, Callado LF. Quantification of endocannabinoids in postmortem brain of schizophrenic subjects. Schizophr Res. (2013) 148:145–50. doi: 10.1016/j.schres.2013.06.013
46. Koethe D, Pahlisch F, Hellmich M, Rohleder C, Mueller JK, Meyer-Lindenberg A, et al. Familial abnormalities of endocannabinoid signaling in schizophrenia. World J Biol Psychiatry. (2019) 20:117–25. doi: 10.1080/15622975.2018.1449966
47. Appiah-Kusi E, Wilson R, Colizzi M, Foglia E, Klamerus E, Caldwell A, et al. Childhood trauma and being at-risk for psychosis are associated with higher peripheral endocannabinoids. Psychol Med. (2020) 50:1862–71. doi: 10.1017/S0033291719001946
48. Ibarra-Lecue I, Unzueta-Larrinaga P, Barrena-Barbadillo R, Villate A, Horrillo I, Mendivil B, et al. Cannabis use selectively modulates circulating biomarkers in the blood of schizophrenia patients. Addict Biol. (2022) 27:3233. doi: 10.1111/adb.13233
49. Parksepp M, Haring L, Kilk K, Koch K, Uppin K, Kangro R, et al. The expanded endocannabinoid system contributes to metabolic and body mass shifts in first-episode schizophrenia: a 5-year follow-up study. Biomedicines. (2022) 10:243. doi: 10.3390/biomedicines10020243
50. Topuz RD, Gorgulu Y, Uluturk MK. Could serum endocannabinoid and N-acylethanolamine levels be important in bipolar disorder? World J Biol Psychiatry. (2022) 24:314–20. doi: 10.1080/15622975.2022.2111713
51. Brotini S, Schievano C, Guidi L. Ultra-micronized Palmitoylethanolamide: an efficacious adjuvant therapy for Parkinson's disease. CNS Neurol Disord Drug Targets. (2017) 16:705–13. doi: 10.2174/1871527316666170321124949
52. Salehi A, Namaei P, TaghaviZanjani F, Bagheri S, Moradi K, Khodaei Ardakani MR, et al. Adjuvant palmitoylethanolamide therapy with risperidone improves negative symptoms in patients with schizophrenia: a randomized, double-blinded, placebo-controlled trial. Psychiatry Res. (2022) 316:114737. doi: 10.1016/j.psychres.2022.114737
53. Abedini T, Hosseyni R, Ghannadi F, Moghaddam HS, Ardakani MK, Talaei A, et al. Efficacy and safety of palmitoylethanolamide as an adjunctive treatment for acute mania: a randomized, double-blind, placebo-controlled trial. Psychiatry Clin Neurosci. (2022) 76:505–11. doi: 10.1111/pcn.13441
54. Stark T, Ruda-Kucerova J, Iannotti FA, D'Addario C, Di Marco R, Pekarik V, et al. Peripubertal cannabidiol treatment rescues behavioral and neurochemical abnormalities in the MAM model of schizophrenia. Neuropharmacology. (2019) 146:212–21. doi: 10.1016/j.neuropharm.2018.11.035
55. Di Bartolomeo M, Stark T, Maurel OM, Iannotti FA, Kuchar M, Ruda-Kucerova J, et al. Crosstalk between the transcriptional regulation of dopamine D2 and cannabinoid CB1 receptors in schizophrenia: analyses in patients and in perinatal Δ9-tetrahydrocannabinol-exposed rats. Pharmacol Res. (2021) 164:105357. doi: 10.1016/j.phrs.2020.105357
56. Bonaccorso S, Ricciardi A, Zangani C, Chiappini S, Schifano F. Cannabidiol (CBD) use in psychiatric disorders: a systematic review. Neurotoxicology. (2019) 74:282–98. doi: 10.1016/j.neuro.2019.08.002
57. Ghabrash MF, Coronado-Montoya S, Aoun J, Gagne AA, Mansour F, Ouellet-Plamondon C, et al. Cannabidiol for the treatment of psychosis among patients with schizophrenia and other primary psychotic disorders: a systematic review with a risk of bias assessment. Psychiatry Res. (2020) 286:112890. doi: 10.1016/j.psychres.2020.112890
58. Wang Z, Cao Y, Zhu Y, Li K, Jiang X, Zhuo C, et al. Differences in demographic and clinical characteristics of patients with depressive vs. manic first episode of bipolar disorder. Front Psychiatry. (2021) 12:616415. doi: 10.3389/fpsyt.2021.616415
59. Perugi G, Micheli C, Akiskal HS, Madaro D, Socci C, Quilici C, et al. Polarity of the first episode, clinical characteristics, and course of manic depressive illness: a systematic retrospective investigation of 320 bipolar I patients. Compr Psychiatry. (2000) 41:13–8. doi: 10.1016/S0010-440X(00)90125-1
Keywords: Schizophrenia, bipolar disorder, major depressive disorder, antipsychotics, cannabidiol, nutraceuticals
Citation: Bortoletto R, Piscitelli F, Candolo A, Bhattacharyya S, Balestrieri M and Colizzi M (2023) Questioning the role of palmitoylethanolamide in psychosis: a systematic review of clinical and preclinical evidence. Front. Psychiatry 14:1231710. doi: 10.3389/fpsyt.2023.1231710
Received: 30 May 2023; Accepted: 30 June 2023;
Published: 18 July 2023.
Edited by:
Teresa Sanchez-Gutierrez, International University of La Rioja, SpainReviewed by:
Vincenzo Micale, University of Catania, ItalyMassimo Tusconi, University of Cagliari, Italy
Copyright © 2023 Bortoletto, Piscitelli, Candolo, Bhattacharyya, Balestrieri and Colizzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Riccardo Bortoletto, Ym9ydG9sZXR0by5yaWNjYXJkb0BzcGVzLnVuaXVkLml0; Marco Colizzi, bWFyY28uY29saXp6aUB1bml1ZC5pdA==
 Riccardo Bortoletto
Riccardo Bortoletto Fabiana Piscitelli
Fabiana Piscitelli Anna Candolo1
Anna Candolo1 Sagnik Bhattacharyya
Sagnik Bhattacharyya Matteo Balestrieri
Matteo Balestrieri Marco Colizzi
Marco Colizzi