Abstract
Background:
Postoperative depression (POD) represents a serious complication in surgical patients, exacerbating morbidity and mortality rates while imposing a substantial economic burden on healthcare systems. Despite its widespread clinical use, the role of esketamine, an NMDA receptor antagonist with rapid antidepressant effects, remains understudied in perioperative settings. Therefore, we conducted a systematic review and meta-analysis to assess the efficacy of esketamine on postoperative depression. To evaluate the effect of esketamine on the incidence and severity of postoperative depression in different types of surgery by randomized controlled trial, investigate whether esketamine can effectively reduce the postoperative depression score and the incidence of postoperative depression in the short and long term after use, to promote the application of perioperative analgesia-antidepressant combination.
Method:
Searched PubMed, the Cochrane Library, the Web of Science, and Medline to identify randomized controlled trials using the drug of esketamine and analyzed the data using Review Manager 5.3.
Results:
We included a total of 8 randomized controlled trials involving 1724 patients who met the criteria. The meta-analysis revealed that esketamine treatment, compared with control groups, significantly reduced POD. Improvements were observed at 1 week (RD -0.09, 95% CI [-0.13, -0.05], P < 0.0001, I²=84%), 2 weeks (RD -0.08, 95% CI [-0.13, -0.03], P < 0.00001, I²=97%), and long-term follow-up (RD -0.06, 95% CI [-0.10, -0.02], P=0.0002, I²=79%).
Conclusion:
Esketamine demonstrates efficacy in reducing POD incidence and severity, although its use is associated with an increased risk of adverse effects. Also, the method of drug injection, the duration of administration and the number of doses may have an effect on the results. Therefore, further exploration of appropriate dosing regimens and multi-modal strategies is necessary to mitigate adverse effects.
Systematic review registration:
https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024506329.
1 Introduction
Surgical interventions have become a cornerstone of modern medicine, anesthesia allowed them to perform more complex, invasive, and precise maneuvers than they had dared to attempt before (1). Perioperative treatment is a great challenge for anesthesiologists because it causes psychological stress reactions such as anxiety and depression and may increase the risk of postoperative complications (2).
Depression is a heterogeneous disorder that can manifest as low mood, loss of interest and pleasure in normal and pleasant activities, difficulty in thinking and decision-making, and disturbances in appetite and sleep (3, 4). Postoperative depression (POD) is defined as the onset or exacerbation of depressive symptoms within 30 days following surgery, due to its transient nature and direct association with surgical stress, anesthesia, and postoperative recovery, remains a serious problem for surgical patients. POD is associated with impaired immune function, elevated infection risks, and worsened oncologic outcomes (5). Major depressive disorder (MDD) is a common POD manifestation and can further aggravate its morbidity and mortality. MDD is one of the chronic mental disorders that induce disability, and projected to become the leading cause of global disability by 2030 (6), affecting 15-20% of the population and accounting for 50% of psychiatric suicides (7–12)”.
Despite the administration of diverse monoaminergic antidepressants, approximately one-third of MDD patients fail to achieve symptom relief. Patients with a major depressive episode who do not respond to an adequate duration of antidepressant treatment involving two medications are considered to have developed "treatment-resistant depression" (TRD) (13). Therefore, how to effectively prevent and lower the risk of postoperative depression is particularly important. Choosing appropriate anesthetic drugs during the perioperative period to prevent the occurrence of postoperative depression in patients has become an issue that anesthesiologists should consider.
Esketamine, the S-enantiomer of ketamine, exerts rapid antidepressant effects via N-methyl-D-aspartate (NMDA) receptor antagonism, enhancing prefrontal glutamate release and synaptic plasticity through BDNF-mTOR pathways (14–16). A study on the variability of patient responses to esketamine treatment using a machine learning model further suggest its potential for personalized psychiatry (17), while its dissociation and agitation are not very common among the side effects of esketamine, and there is no potential for addiction after its use. Therefore, these pieces of evidence indicate that esketamine for the treatment of depression is mild and acceptable (18).
Given the previously reported evidence regarding these complementary effects of esketamine as a postoperative antidepressant, intraoperative combination use of esketamine may be a promising approach to reduce the risk of postoperative major depression. However, there is a lack of meta-analyses on the efficacy of esketamine in the treatment of postoperative major depression. Therefore, the purpose of this study was to conduct a systematic review and meta-analysis of randomized controlled trials (RCTS) to investigate the efficacy of esketamine on postoperative depression, as well as the effects of different doses of esketamine on postoperative depression in patients and the risk of adverse effects.
2 Method and analysis
This meta-analysis was performed according to the recommendations in the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) statement and the guidelines described in the Cochrane Handbook (19).
2.1 Patient and public involvement
This work was based on published research data. Therefore, no patient or public involvement is required. Relevant results will be published in peer-reviewed journals.
2.2 Search strategy
We limited the search to peer-reviewed articles that were clinical studies with the use of a randomized controlled trial design and were published in English in the Cochrane Central Register of Controlled Trials, including four English electronic databases (PubMed, Cochrane Library, web of science, Medline). The search strategy was performed specifically for each database and included a combination of the medical subject headings and free text terms for (“esketamine” OR “s-ketamine” OR “L-ketamine” OR “ (–)-ketamine”) and (“depression” OR”depressive” OR “depressed “OR “postoperative depression”). The deadline for all retrieval was December 2023.
2.3 Inclusion/exclusion criteria
The following criteria were included:
(1) intervention: esketamine; (2) comparison: placebo, no intervention or other sedative hypnotics; (3) type of study: randomized controlled trial (RCT); (4) Comparison of the effects of postoperative depression with esketamine (experimental group) and the control group (no intervention or other sedative-hypnotic agents). (5) Postoperative depression (POD) diagnosed using validated scales (e.g., HAMD-17, EPDS) within 30 days postoperatively.
Exclusion criteria were as follows:
(1) Article did not conduct a randomized controlled trial; (2) Experimental design cannot extract relevant data on the results; (3) The article was themed in the form of a review or case report. (4) Specific types of surgeries or comorbidities may affect generalizability. (5) Patients with pre-existing psychiatric disorders.
2.4 Data extraction
We classified the extracted data using Microsoft Excel and then transcribed the data into the Review Manager (version 5.3) for statistical analysis. The data analyzed included name of the author, number and genders of the patients, types of surgery, types of anesthesia, time and doses of intervention for the esketamine group and the control group, postoperative depression scores, pain intensity scores, postoperative adverse effects, and time for follow-up. Two independent reviewers screened all the titles and abstracts to determine potential eligible articles. They independently applied the eligibility criteria to perform the final selection. When discrepancies occurred between both reviewers regarding the inclusion of the articles, they discussed and identified the reasons to either include or exclude the articles and then made the final decision. If they could not reach an agreement, the final decision was based on a third reviewer.
All rating scales on changes in depressive mood were extracted from the selected studies, including Edinburgh Postpartum Depression Scale (EPDS) scores, Montgomery-Absberg Depression Scale (MADRS), Beck Depression Scale (BDI), and Hamilton Depression Scale (HAMD-17) scores. Secondary outcomes included postoperative pain (visual analog scale) and adverse effects (e.g., headache, nausea). Three treatment time points (within 1 week, 2 weeks, and over 4 weeks) were selected to evaluate the effect of esketamine.
2.5 Strategy for data synthesis
The data were analyzed using the random-effects model to determine the pooled risk ratio (RR), mean difference (MD), and standardized mean difference (SMD). Additionally, the 95% confidence interval (CI) was reported for each outcome. A random-effects model was selected due to anticipated clinical heterogeneity (e.g., varied surgical types and dosing regimens).
We were unable to perform subgroup analyses of the effects of esketamine on depression because there were only one or two studies in each surgical procedure.
To further investigate the safety of perioperative use of esketamine for antidepressant effects, we conducted a subgroup analysis of adverse reactions following esketamine use. Statistical analysis was performed using the Review Manager (RevMan). Statistical significance was set with the probability value (p) of less than 0.05.
2.6 Risk of bias in individual studies
We assessed the risk of bias within individual trials using the Cochrane risk of bias tool for randomized controlled trials and independent assessment was conducted by two trained reviewers. Specifically, the risk of bias for each trial was classified as “low”, “high”, and “some concerns”. The risk of bias tool assesses indicators of selection bias, experimental bias, detection bias, and reporting bias. Any disagreement was resolved through discussion to reach a consensus (Figure 1).
Figure 1
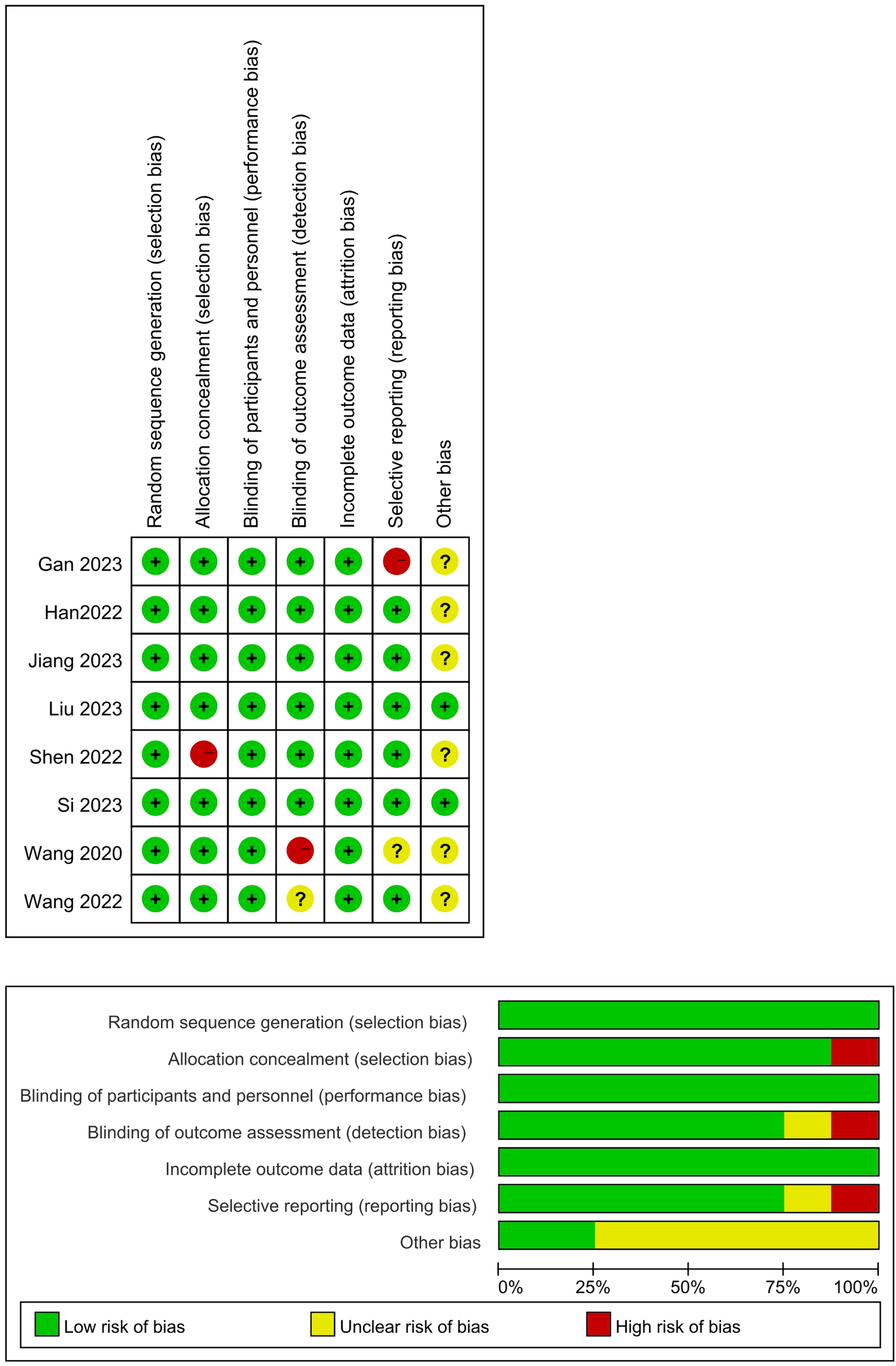
Quality assessment of the included studies. (+: Low risk of bias; –: High risk of bias; Yellow grid: Unclear risk of bias).
2.7 Assessment of heterogeneity
We assessed between-study heterogeneity using the I2 statistic, with 50% or higher values indicating significant heterogeneity.
3 Results
3.1 Study search and characteristics
A total of 3482 records were identified for preliminary screening. After screening the titles and abstracts, 8 eligible studies were included in this meta-analysis (Figure 2). Finally, the current meta-analysis included a total of 8 RCTs published between 2020 and 2023 with 1,724 participants (Table 1) (20–27).
Figure 2
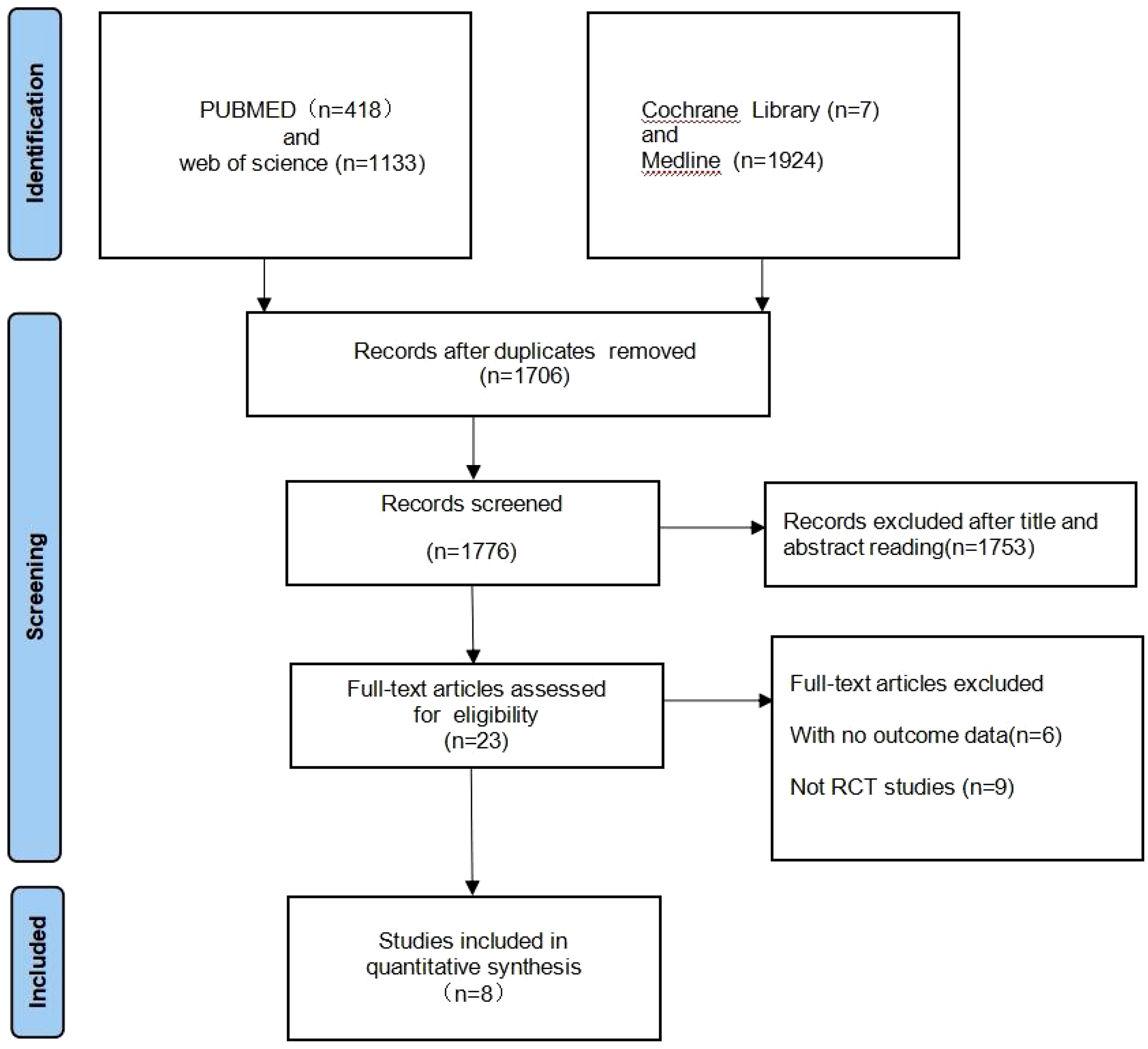
Flow chart for study selection. Total number of studies identified, screened, deemed eligible, and ultimately included is summarized.
Table 1
| First author | Year | Country | Study Design and sample size (S/C) | Age (S/C) | Sex | Surgery | Anesthesia | Ways of Esketamine injection | Time point of intervention | Intervention (S vs. C) | Depression Measurement | Pain Measurement | Follow up Period |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Han (20) |
2022 | china | RCT 275(122/153) | 31.64 ± 3.93 | F | cesarean section | Spinal anesthesia |
PCIA | after suturing the skin incision | S-ketamine 0.5mg/kg + sufentanil 2μg/kg + tropisetron 10mg vs. sufentanil 2μg/kg + tropisetron 10mg | EPDS | VAS | Post-op days 3,14 and 28 |
| 31.85 ± 4.16 | |||||||||||||
| Wang (21) |
2022 | china | RCT 156(39/40/38/39) | 27.9 ± 6.1 | F | cesarean section | Spinal anesthesia |
PCIA | at the time of surgical suture |
S-ket-low(0.1mg/kg)vs. S-ket-middle(0.2mg/kg)vs. S-ket-High(0.4mg/kg)vs. sufentanil 1.5μg/kg + totanisoltron 4mg |
PPD | / | Post-op week 1 and 6 |
| 28.3 ± 5.9 | |||||||||||||
| 28.8 ± 6.4 | |||||||||||||
| 29.1 ± 5.5 | |||||||||||||
| Gan (22) |
2023 | china | RCT 151(75/76) | 54 ± 11.7 | M+F | thoracoscopic lung cancer surgery |
General anesthesia |
Single dose + Continuous infusion+ PCIA |
Intraoperative-ly | S-KET 0.1mg/kg/h IV vs. normal saline | BDI-II | / | Post-op month 1,3 and hospital discharge |
| 53.8 ± 12.1 | |||||||||||||
| Yang (23) |
2023 | china | RCT 195(99/99/97) | 31.9 | F | cesarean section | Spinal anesthesia |
PCIA | after child birth |
S-ket-High(sufentanil 2.2μg/kg + esketamine 2.0 mg/kg), S-ket-low(sufentanil 2.2μg/kg+ esketamine 1.0 mg/kg) vs. sufentanil 2.2μg/kg | PDS | / | Post-op day7,42 |
| 31.7 | |||||||||||||
| 32.2 | |||||||||||||
| Jiang (24) |
2023 | china | RCT 105(35/35/35) | 32.77 ± 5.25 | F | patients with missed miscarriage |
General anesthesia |
Single dose | induction | Esketamine 0.3mg/kg + propofol 1.5–2mg/kg vs. Dezocine 0.08 mg/kg + propofol 1.5-2mg/kg vs. propofol 2.5–3mg/kg | EPDS | VSA | Post-op day7,42 |
| 32.29 ± 5.15 | |||||||||||||
| 30.71 ± 4.33 | |||||||||||||
| Shen (25) |
2022 | china | RCT 202(102/100) | 28.9 ± 3.9 | F | cesarean section | Spinal anesthesia |
Single dose | after child birth |
0.25 mg/kg esketamine vs. normal saline | EPDS | NRS | Post-op week 1,2 and4 |
| 29.6 ± 3.9 | |||||||||||||
| Liu (26) |
2023 | china | RCT 123(62/61) | 30.3 ± 4.1 | F | cesarean section | Spinal anesthesia |
Continuous infusion+ PCIA |
After clamping the neonatal umbilical cord |
100μg of sufentanil, 1.25 mg/kg of esketamine, and 8 mg of ondansetron vs. 100μg of sufentanil and 8 mg of ondansetron | EPDS | NRS | Post-op day 3,42 and months3,6 |
| 29.8 ± 4.2 | |||||||||||||
| Wang (27) | 2020 | china | RCT 417(104/104/104/105) | 48.53 ± 10.0 | F | Laparoscopic Total Hysterectomy |
General anesthesia |
Single dose | after 1h of analgesia |
0.5 mg/kg S-ketamine vs. 0.25 mg/kg S-ketamine vs. 0.5 mg/kg racemic ketamine vs. normal saline | HAMD-17 | VAS | Post-op days 1,2,3,5and7 |
| 48.11 ± 10.38 | |||||||||||||
| 47.07 ± 10.08 | |||||||||||||
| 46.27 ± 10.83 |
Characteristics of included studies.
3.2 Outcomes
All eight studies had a total of 1,724 participants, with 815 participants in the esketamine group and 909 participants in the placebo group. In the eight randomized controlled trials, esketamine was used for the experimental group. There were five studies on postpartum depression, three articles injected esketamine through PCIA, one article gave a single intravenous dose of esketamine, and one was injected with esketamine by continuous intravenous infusion and postoperative PCIA.
The remaining three studies were on thoracoscopic lung cancer, retained abortion, and laparoscopic total hysterectomy respectively. The experimental groups in all the three studies were injected with a single dose of intravenous esketamine. The ASA body status classification varied from I to III in eight randomized controlled trials, with ASA II being the most common. In eight randomized controlled studies, esketamine at 0.5mg/kg (20), or at low doses (0.1mg/kg), Medium dose (0.2mg/kg), High dose (0.4mg/kg) (21) or at a high dose (2.0 mg/kg), A low dose (1.0 mg/kg) combined with sufentanil at 2.2 μg/kg (23), 0.1mg/kg/h (22), 0.3mg/kg (24), 0.25 mg/kg (25), 0.25 mg/kg (27) with a single dose of esketamine and its control group.
The first part of our primary meta-analysis investigated the long-term differences in depression scores between esketamine and saline/deszocin/propofol for surgical patients within one week, two weeks, four weeks and beyond respectively. For the high, medium and low doses of esketamine, we included the data of the low dose group in the analysis and used the experimental data of the medium and high dose groups as a reference. From the 8 selected studies, a total of 815 patients received esketamine and 909 patients receiving saline, propofol, or deszocin were treated as control groups. The antidepressant effect was better in the esketamine group compared to the control group. Compared to placebo, POD 1week (RD -0.09, 95% CI [-0.13, -0.05], P <0.0001, I2 = 84%), POD2week (RD-0.08,95%CI [-0.13, -0.03], and P <0.00001, I2 = 97%) compared to placebo, Over the long term (RD-0.06, 95%CI [-0.10, -0.02], P=0.0002, I2 = 79%) had a positive effect (Figure 3).
Figure 3
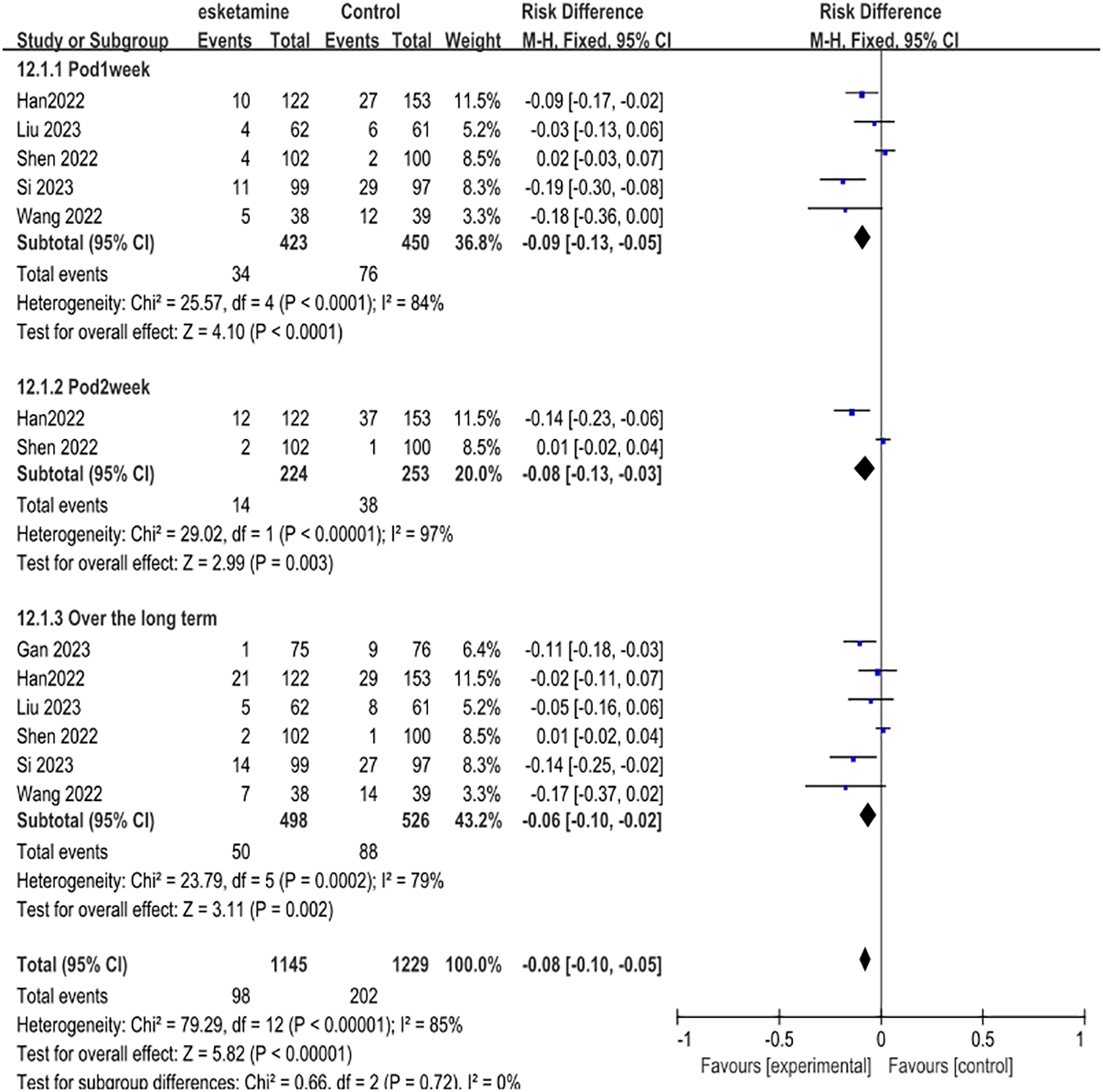
Meta-analysis of postoperative depression occurrence at one week, two weeks, and over the long term.
Only two studies had data for the EPDS score, with the duration within one week and one month respectively (Figure 4). 157 patients received esketamine and 188 patients receiving saline or propofol were included as controls. On the EPDS, 1week (SMD-0.61, 95%CI [-0.83, -0.39], P=0.52, I2 = 0), EPDS 1month (SMD-0.84,95%CI [-0.22,0.55], P <0.00001, I2 = 95%).
Figure 4
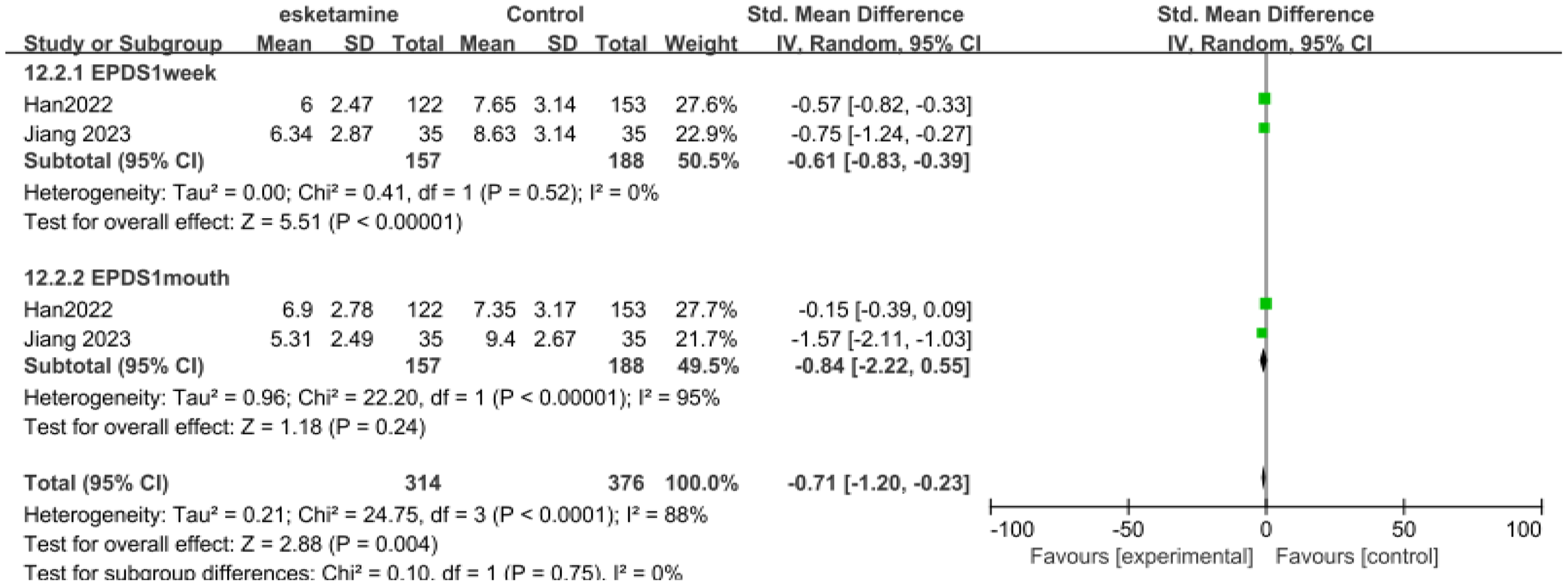
Meta-analysis of EPDS scores at one week and one month of follow-up.
3.3 Adverse effects
Next, we examined the adverse effects of esketamine, including headache and dizziness, nausea and vomiting, hallucinations, and pruritus. Compared with control group, the use of esketamine had a higher risk of side effects. Significantly increased the risk of headaches and dizziness (RR 2.11, 95% CI [1.47, 3.01], P = 0.007, I2 = 72%). However, Nausea and vomit (RR 0.95, 95% CI [0.74, 1.20], P = 0.46, I2 = 0%), Hallucinations (RR 1.77, 95% CI [0.91, 3.46], P =0.72, I2 = 0%), Pruritus (RR 0.43, 95% CI [0.06, 2.85], P =0.86, I2 = 0%) was not statistically significant (Figure 5).
Figure 5
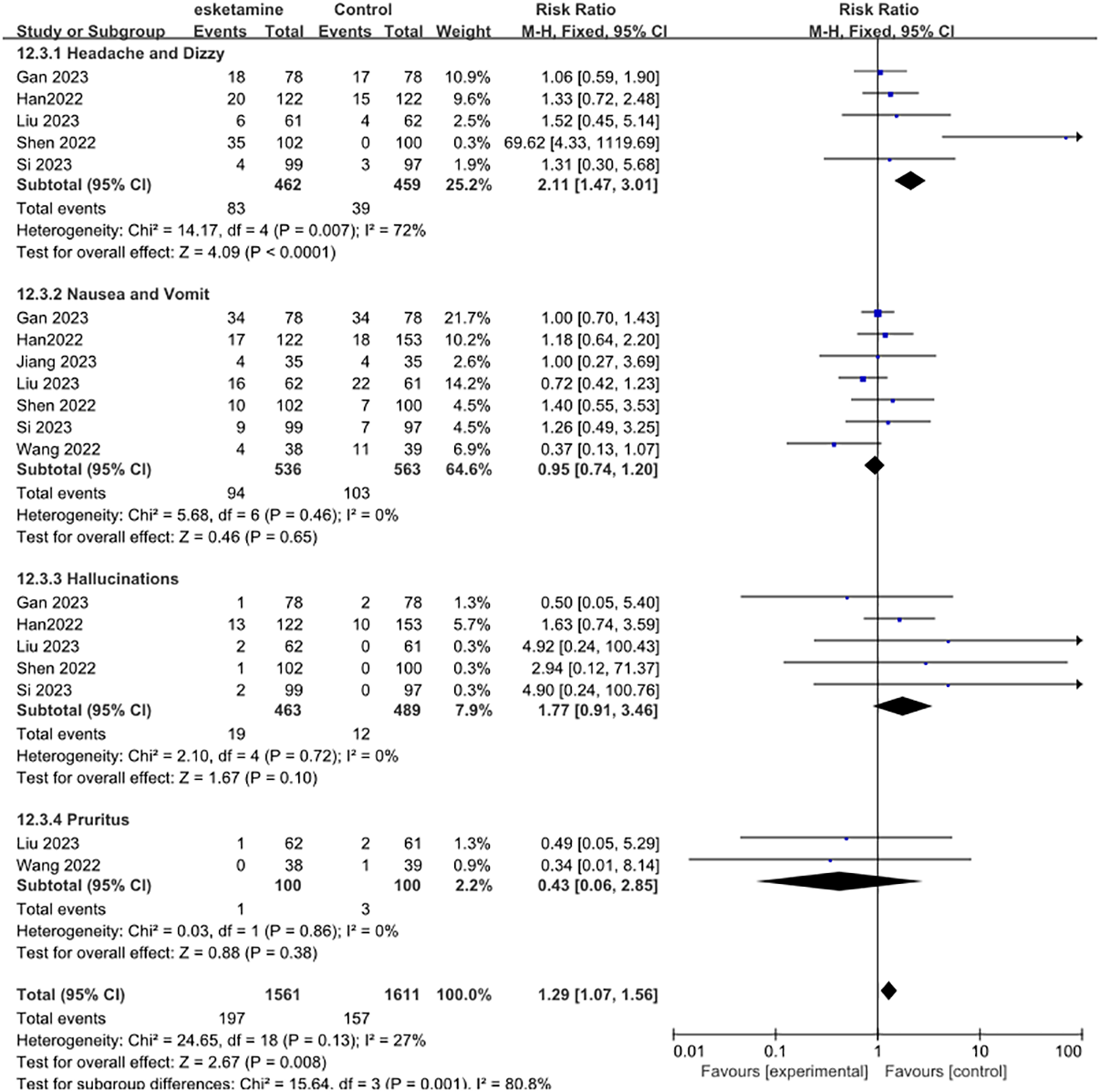
Meta-analysis of postoperative adverse effects. Including headache and dizzy, nausea and vomit, hallucinations, and pruritus.
4 Discussion
In this meta-analysis, we pooled current studies on the efficacy of esketamine on postoperative depression to look for evidence of its effectiveness and safety. Based on the included literature, we found that the use of esketamine could prevent or reduce the level of postoperative depression. In the short term (1 week) and more than 1 month, it can effectively reduce the postoperative depression score and the incidence of postoperative depression. Intraoperative intravenous administration of esketamine and post-operative addition of PCIA showed that esketamine significantly alleviated postpartum depression symptoms in cesarean section patients. Meanwhile, through studies (Han et al. (20), Wang et al. (21), Yang et al. (23), Liu et al. (26)), it was found that the antidepressant effect of Esketamine was more obvious in the short-term postoperative period.
Almost all the reviewed studies have demonstrated the antidepressant effect of esketamine. Meanwhile, we found that the higher the dose of esketamine, the stronger its antidepressant effect [Wang et al. (21), Yang et al. (23)], the dose-effect relationship that exists within a certain range. However, Compared with the control group, patients receiving esketamine treatment had a higher risk of adverse reactions such as headache, dizziness. The dose range of esketamine for patients included in the meta-analysis is 0.1 to 2 mg/kg, with the most commonly used dose being 0.2 mg/kg. It is generally believed that as the dosage increases, adverse reactions relatively increase. However, there was no significant difference between the high dose group (1mg/kg) and the low dose group (<0.4mg/kg) in this meta-analysis. It may be that differences in dosage and administration (PCIA, Single dose, Continuous infusion) lead to inconsistent results.
It is possible that the effects of intraoperative medication and surgical methods contribute to the lack of evidence, it may also be because intraoperative intravenous antiemetic drugs such as ondansetron reduced the occurrence of postoperative nausea and vomiting, which remains to be studied further. Therefore, the optimal dose of esketamine for the treatment of postoperative depression is not known. Further clinical studies are needed to determine the appropriate dose to treat postoperative depression to reduce the risk of adverse reactions.
Studies have shown that esketamine and its subtypes are effective interventions for reducing depression in depressed patients and have powerful and beneficial effects in the short term. Robustness to various symptoms and relatively sustained antidepressant effects are also their advantages (28, 29). But for different surgical methods, the efficacy of esketamine in preventing depression still needs to be observed through clinical trials. [Shen et al, (25)] found no significant difference in antidepressant treatment with esketamine during surgery, which may be due to a shorter observation period compared to patients in other studies, or due to the patients in this study were relatively healthy or had no symptoms of depression before surgery. In addition, differences in drug dosage and administration time may also lead to differences in results. At the same time, the different depression assessment scales used in the included studies increased heterogeneity. Second, differences in follow-up time may affect the antidepressant effects of esketamine. Therefore, it is possible to reduce the adverse effects of esketamine by adjusting the dosing schedule and combining with other general anesthesia drugs in a reasonable study design. At the same time, further clinical studies are needed to investigate the efficacy and duration of esketamine in the treatment of perioperative depression.
In previous clinical studies, the NMDA receptor antagonist ketamine has demonstrated antidepressant efficacy. And esketamine is a chiral cyclic hexone with strong analgesic effect, which is the dexter of ketamine, which has a separate anesthetic effect like ketamine. As a new generation of isomeric drugs, esketamine acts in a mechanism similar to racemic ketamine, but it binds more to NMDA receptor and μ receptor and is about twice higher than that of racemic ketamine. However, it has shorter recovery time than ketamine and less central nervous system side effects than ketamine (30). Sarasso P, et al. select two case studies, suggest that esketamine-induced disembodiment allows for a transient window of psychological plasticity and enhanced sensitivity, where the body recovers its permeability to affective affordances (31). In addition, the parasympathetic effect of esketamine does not inhibit the hemodynamics of patients, and it also has advantages in the prevention of postoperative delusion. Therefore, Esketamine has the following advantages: low incidence of side effects such as hallucinations and rapid postoperative recovery.
Now esketamine has received regulatory approval for use in the treatment of refractory depression in the United States and elsewhere. It was approved by The US Food and Drug Administration (FDA) in 2019 for treatment-resistant depression. Meanwhile, there is increasing evidence to evaluate the efficacy and safety of esketamine as a treatment for depression. The FDA trial did not show any sustained or significant reduction in cognitive processes, and some studies suggest that improvements in cognitive areas may be related to improvements in depressive symptoms. A recent Delphi Panel showed a widespread heterogeneity in the management of TRD patients, study revealed a high level of consensus and agreement was obtained about the identification of esketamine nasal spray as the best option to antidepressants, emphasizing its integration into routine care within outpatient settings (32). At the same time, brief increases in blood pressure (33, 34) and heart rate (35) should not be ignored during the use of esketamine. Headache and nausea are also common, but often the symptoms are mild, transient, and easily controlled (36). These need to be timely detected and actively handled by the anesthesiologist and the nursing team during the perioperative and postoperative care period.
This analysis has some limitations. The disadvantage of this meta-analysis is that the surgical methods included in the study are not comprehensive, resulting in a relatively small sample size. Only a few surgical methods cannot reflect the comprehensive efficacy of esketamine on the prevention and improvement of postoperative depression, so the accuracy of the conclusions still needs to be verified. Furthermore, underrepresentation of non-obstetric surgeries, the lack of diversity in surgical procedures represented in the included studies, lack of standardized preoperative depression screening and the variability in depressive symptom measurement tools used across studies, as this could impact generalizability. Meanwhile, in the included studies, different analyses were conducted on anesthesia methods, administration methods, and doses based on the research results. Secondly, the level of preoperative depression and postoperative follow-up treatment lack standardized procedures, therefore, the duration and effect of esketamine cannot be evaluated. Furthermore, the heterogeneity in the selection of postoperative depression assessment tools across these studies has introduced variability in directly comparing the efficacy of esketamine among different trials. Therefore, randomized controlled trials with a larger sample size and similar scales are needed to confirm whether perioperative use of esketamine can help reduce postoperative depression symptoms.
5 Conclusion
The current meta-analysis shows that the perioperative application of esketamine is effective in reducing postoperative depression scores and pain intensity, particularly in obstetric and oncologic surgeries. However, esketamine increased the risk of nausea and vomiting, headache, hallucinations, and dizziness compared with placebo. Therefore, assess the differences in the effects of drug administration at various time points before, during, and after surgery, studying the optimal dosage and administration time of esketamine for postoperative antidepressant effects, while considering how to reduce the occurrence of adverse reactions in patients is still an exploratory issue in future clinical practice. To conduct a multicenter, randomized controlled trial to explore the antidepressant effects of different doses of esketamine in the perioperative period, and to define the minimum effective dose and the maximum tolerated dose. Administer prophylactic medications concurrently during the perioperative period to mitigate the risk of adverse reactions.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
H-YL: Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Methodology, Resources, Software, Validation. W-JX: Data curation, Formal analysis, Investigation, Writing – review & editing. Y-MW: Investigation, Validation, Writing – review & editing. SX: Data curation, Formal analysis, Investigation, Writing – review & editing. H-LW: Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the project of the Hainan Province Clinical Medical Center, the project of the Health Commission of Hainan Province (22A200074 to H-LW), the funding for talent introduction by the Second Affiliated Hospital of Hainan Medical University (TP2022002 to H-LW), and the National Natural Science Foundation of China (82360015 to H-LW).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
McDowell JA . Two hundred years of surgery. New Engl J Med. (2012) 367:479. doi: 10.1056/NEJMc1206630
2
Guo J Qiu D Gu HW Wang XM Hashimoto K Zhang GF et al . Efficacy and safety of perioperative application of ketamine on postoperative depression: A meta-analysis of randomized controlled studies. Mol Psychiatry. (2023) 28:2266–76. doi: 10.1038/s41380-023-01945-z
3
Disner SG Beevers CG Haigh EA Beck AT . Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. (2011) 12:467–77. doi: 10.1038/nrn3027
4
Liu P Li P Li Q Yan H Shi X Liu C et al . Effect of pretreatment of S-ketamine on postoperative depression for breast cancer patients. J Invest Surg. (2021) 34:883–8. doi: 10.1080/08941939.2019.1710626
5
Ghoneim MM O’Hara MW . Depression and postoperative complications: an overview. BMC Surg. (2016) 16:5. doi: 10.1186/s12893-016-0120-y
6
Mathers CD Loncar D . Projections of global mortality and burden of disease from 2002 to 2030. PloS Med. (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
7
Cuijpers P Smit F . Excess mortality in depression: A meta-analysis of community studies. J Affect Disord. (2002) 72:227–36. doi: 10.1016/s0165-0327(01)00413-x
8
Kessler RC Berglund P Demler O Jin R Merikangas KR Walters EE . Lifetime prevalence and age-of-onset distributions of dsm-iv disorders in the national comorbidity survey replication. Arch Gen Psychiatry. (2005) 62:593–602. doi: 10.1001/archpsyc.62.6.593
9
Lopez AD Mathers CD Ezzati M Jamison DT Murray CJ . Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet (London England). (2006) 367:1747–57. doi: 10.1016/s0140-6736(06)68770-9
10
Amidfar M Réus GZ Quevedo J Kim YK . The role of memantine in the treatment of major depressive disorder: clinical efficacy and mechanisms of action. Eur J Pharmacol. (2018) 827:103–11. doi: 10.1016/j.ejphar.2018.03.023
11
Daly EJ Singh JB Fedgchin M Cooper K Lim P Shelton RC et al . Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry. (2018) 75:139–48. doi: 10.1001/jamapsychiatry.2017.3739
12
Kupferberg A Bicks L Hasler G . Social functioning in major depressive disorder. Neurosci Biobehav Rev. (2016) 69:313–32. doi: 10.1016/j.neubiorev.2016.07.002
13
Sforzini L Worrell C Kose M Anderson IM Aouizerate B Arolt V et al . A delphi-method-based consensus guideline for definition of treatment-resistant depression for clinical trials. Mol Psychiatry. (2022) 27:1286–99. doi: 10.1038/s41380-021-01381-x
14
Kaur U Pathak BK Singh A Chakrabarti SS . Esketamine: A glimmer of hope in treatment-resistant depression. Eur Arch Psychiatry Clin Neurosci. (2021) 271:417–29. doi: 10.1007/s00406-019-01084-z
15
Molero P Ramos-Quiroga JA Martin-Santos R Calvo-Sánchez E Gutiérrez-Rojas L Meana JJ . Antidepressant efficacy and tolerability of ketamine and esketamine: A critical review. CNS Drugs. (2018) 32:411–20. doi: 10.1007/s40263-018-0519-3
16
Johnston JN Zarate CA Jr. Kvarta MD . Esketamine in depression: putative biomarkers from clinical research. Eur Arch Psychiatry Clin Neurosci. (2024). doi: 10.1007/s00406-024-01865-1
17
Pettorruso M Guidotti R d’Andrea G De Risio L D’Andrea A Chiappini S et al . Predicting outcome with intranasal esketamine treatment: A machine-learning, three-month study in treatment-resistant depression (Esk-learning). Psychiatry Res. (2023) 327:115378. doi: 10.1016/j.psychres.2023.115378
18
Di Vincenzo M Martiadis V Della Rocca B Arsenio E D’Arpa A Volpicelli A et al . Facts and myths about use of esketamine for treatment-resistant depression: A narrative clinical review. Front Psychiatry. (2024) 15:1394787. doi: 10.3389/fpsyt.2024.1394787
19
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
20
Han Y Li P Miao M Tao Y Kang X Zhang J . S-ketamine as an adjuvant in patient-controlled intravenous analgesia for preventing postpartum depression: A randomized controlled trial. BMC Anesthesiol. (2022) 22:49. doi: 10.1186/s12871-022-01588-7
21
Wang W Xu H Ling B Chen Q Lv J Yu W . Effects of esketamine on analgesia and postpartum depression after cesarean section: A randomized, double-blinded controlled trial. Med (Baltimore). (2022) 101:e32010. doi: 10.1097/MD.0000000000032010
22
Gan SL Long YQ Wang QY Feng CD Lai CX Liu CT et al . Effect of esketamine on postoperative depressive symptoms in patients undergoing thoracoscopic lung cancer surgery: A randomized controlled trial. Front Psychiatry. (2023) 14:1128406. doi: 10.3389/fpsyt.2023.1128406
23
Yang SQ Zhou YY Yang ST Mao XY Chen L Bai ZH et al . Effects of different doses of esketamine intervention on postpartum depressive symptoms in cesarean section women: A randomized, double-blind, controlled clinical study. J Affect Disord. (2023) 339:333–41. doi: 10.1016/j.jad.2023.07.007
24
Jiang M Li Q Mao M Xu C Zhou R Wen Y et al . Evaluation of clinical effects of esketamine on depression in patients with missed miscarriage: A randomized, controlled, double-blind trial. J Affect Disord. (2023) 329:525–30. doi: 10.1016/j.jad.2023.02.127
25
Shen J Song C Lu X Wen Y Song S Yu J et al . The effect of low-dose esketamine on pain and post-partum depression after cesarean section: A prospective, randomized, double-blind clinical trial. Front Psychiatry. (2022) 13:1038379. doi: 10.3389/fpsyt.2022.1038379
26
Liu QR Zong QK Ding LL Dai HY Sun Y Dong YY et al . Effects of perioperative use of esketamine on postpartum depression risk in patients undergoing cesarean section: A randomized controlled trial. J Affect Disord. (2023) 339:815–22. doi: 10.1016/j.jad.2023.07.103
27
Wang J Wang Y Xu X Peng S Xu F Liu P . Use of various doses of S-ketamine in treatment of depression and pain in cervical carcinoma patients with mild/moderate depression after laparoscopic total hysterectomy. Med Sci Monit. (2020) 26:e922028. doi: 10.12659/MSM.922028
28
Ionescu DF Rosenbaum JF Alpert JE . Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin Neurosci. (2015) 17:111–26. doi: 10.31887/DCNS.2015.17.2/dionescu
29
Siegel AN Di Vincenzo JD Brietzke E Gill H Rodrigues NB Lui LMW et al . Antisuicidal and antidepressant effects of ketamine and esketamine in patients with baseline suicidality: A systematic review. J Psychiatr Res. (2021) 137:426–36. doi: 10.1016/j.jpsychires.2021.03.009
30
White PF . Ketamine and depression: an old drug in search of a clinical indication. J Clin Anesth. (2021) 75:110500. doi: 10.1016/j.jclinane.2021.110500
31
Sarasso P Billeci M Ronga I Raffone F Martiadis V Di Petta G . Disembodiment and affective resonances in esketamine treatment of depersonalized depression subtype: two case studies. Psychopathology. (2024) 57:480–91. doi: 10.1159/000539714
32
Maina G Adami M Ascione G Bondi E De Berardis D Delmonte D et al . Nationwide consensus on the clinical management of treatment-resistant depression in Italy: A delphi panel. Ann Gen Psychiatry. (2023) 22:48. doi: 10.1186/s12991-023-00478-7
33
Fedgchin M Trivedi M Daly EJ Melkote R Lane R Lim P et al . Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (Transform-1). Int J Neuropsychopharmacol. (2019) 22:616–30. doi: 10.1093/ijnp/pyz039
34
Popova V Daly EJ Trivedi M Cooper K Lane R Lim P et al . Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: A randomized double-blind active-controlled study. Am J Psychiatry. (2019) 176:428–38. doi: 10.1176/appi.ajp.2019.19020172
35
Wajs E Aluisio L Holder R Daly EJ Lane R Lim P et al . Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (Sustain-2). J Clin Psychiatry. (2020) 81(3). doi: 10.4088/JCP.19m12891
36
Nikayin S Murphy E Krystal JH Wilkinson ST . Long-term safety of ketamine and esketamine in treatment of depression. Expert Opin Drug Saf. (2022) 21:777–87. doi: 10.1080/14740338.2022.2066651
Summary
Keywords
esketamine, postoperative, depression, anesthesia, meta-analysis
Citation
Li H-Y, Xu W-J, Wang Y-M, Xie S and Wang H-L (2025) Efficacy of perioperative esketamine on postoperative depression: a systematic review and meta-analysis. Front. Psychiatry 16:1476449. doi: 10.3389/fpsyt.2025.1476449
Received
25 September 2024
Accepted
17 March 2025
Published
15 April 2025
Volume
16 - 2025
Edited by
Cicek Hocaoglu, Recep Tayyip Erdoğan University, Türkiye
Reviewed by
Vassilis Martiadis, Department of Mental Health, Italy
Lut Tamam, Çukurova University, Türkiye
Updates
Copyright
© 2025 Li, Xu, Wang, Xie and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan-Liang Wang, wanghuanliang@shhmu.net; Shuang Xie, xieshuang1985@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.