- 1Department of Oncology, Nicosia General Hospital, Nicosia, Cyprus
- 2Department of Psychiatry, Medical School, University of Cyprus, Nicosia, Cyprus
Introduction: This systematic review highlights that schizophrenia (SZ) manifests significant sex differences across neurobiological, clinical, treatment response, and adverse effect domains, underscoring the need for sex-specific considerations in both research and clinical practice.
Methods: A systematic search was conducted following PRISMA guidelines, primarily through Pubmed, PsycINFO, Web of Science, and the Cochrane Library. Eligible studies on sex differences in patients with schizophrenia were included. Main search keywords were schizophrenia, sex differences, sex, gender, neurobiology, symptomatology, epidemiology, treatment response, adverse effects.
Results: While lifetime prevalence is similar between men and women, the disorder’s trajectory diverges. Men typically experience illness onset approximately 3–5 years earlier than women, with more severe negative symptoms, worse social functioning, and higher rates of comorbid substance use disorders. By contrast, women often have later onset—including a secondary mid-life peak likely linked to declining estrogen—and tend to present with more affective symptoms. Neurobiologically, men with SZ exhibit more extensive structural brain abnormalities and cognitive impairment (especially in memory), whereas women benefit from a degree of neuroprotection possibly mediated by estrogen and distinct gene expression patterns. Hormonal influences appear pivotal, with estrogen’s neuroprotective effects potentially delaying onset and mitigating symptom severity in women, and low testosterone levels correlating with more pronounced negative symptoms in men. Treatment response also varies by sex: women with SZ generally respond to antipsychotics at lower doses with better clinical improvement and fewer relapses, whereas men often require higher doses due to faster drug metabolism and also typically face higher relapse risks. Notably, the treatment advantage of women diminishes after menopause. Adverse effect profiles differ as well: women with SZ are more prone to side effects such as antipsychotic-induced hyperprolactinemia, weight gain, and metabolic or cardiovascular complications, while men tend to experience more neurological side effects while also exhibiting lower treatment adherence.
Discussion: These multidimensional sex differences underscore that all aspects of schizophrenia—from pathophysiology and presentation to therapy and side effect management—must be viewed through a sex-specific lens. Tailoring interventions to the needs of men and women patients is essential for optimizing outcomes and advancing personalized care.
Introduction
Schizophrenia (SZ) is a severe psychiatric disorder with a complex etiology and heterogeneous clinical presentation. A consistent finding in the epidemiology of schizophrenia is that its incidence and course differ between men and women. Men have a higher incidence of schizophrenia (man-to-woman ratio ~1.4:1) and tend to develop the illness earlier in life (1). By contrast, women on average experience a later onset – often several years after the typical peak in men – and frequently display better premorbid social functioning (2, 3). Clinically, it can be argued that men more frequently present with prominent negative symptoms and poorer social adjustment, whereas women tend to exhibit more affective symptoms (e.g., depression) and acute psychotic features (4, 5). These differences may contribute to divergent outcomes: some studies report that women achieve higher initial remission rates and milder early-course illness severity (potentially due to estrogen’s neuroprotective effects) (6). Indeed, estrogen is hypothesized to delay illness onset and reduce symptom severity in women until menopause (7). Conversely, men often have a more chronic course with worse long-term functional outcomes, partly attributable to poorer premorbid development and greater substance use comorbidity (8, 9).
Growing recognition of these sex differences has spurred a robust body of literature exploring biological and psychosocial distinctions between men and women with schizophrenia. Evidence has emerged that sex-based factors can influence nearly every aspect of the disorder – from neurodevelopmental pathways and brain morphology to clinical symptomatology, treatment response, and side-effect profiles (9–11). For example, sex-specific genes and hormones may differentially impact brain structure and function, contributing to variations in disease risk and presentation (12). Likewise, gender-related social roles and behaviors (e.g., substance use patterns) can modify the clinical course (13). These differences have practical implications: women and men may respond differently to antipsychotic treatments and experience distinct adverse effect burdens (14, 15). Yet, historically, many treatment guidelines have not accounted for sex, and clinical trials often included predominantly men samples (16–18). There is a pressing need to synthesize current evidence on sex differences in schizophrenia to inform more personalized, gender-sensitive care (19–21).
Methods
This review was conducted following a systematic approach to identify and synthesize studies on sex differences in schizophrenia. A comprehensive literature search was performed in major databases including PubMed, PsycINFO, Web of Science, and the Cochrane Library. The search strategy combined keywords related to schizophrenia with terms denoting sex or gender differences and specific outcome domains. For example, we used queries such as “schizophrenia AND sex differences AND treatment response,” “schizophrenia AND gender AND symptomatology,” and “schizophrenia AND sex AND adverse effects” (7, 22). Reference lists of relevant articles and reviews were also hand-searched to ensure inclusion of all pertinent studies. The search covered articles published up to January 2025, with an emphasis on contemporary research (the last 10 years) while including seminal older studies for context. Information on the exact search procedure can be found in Figure 1.
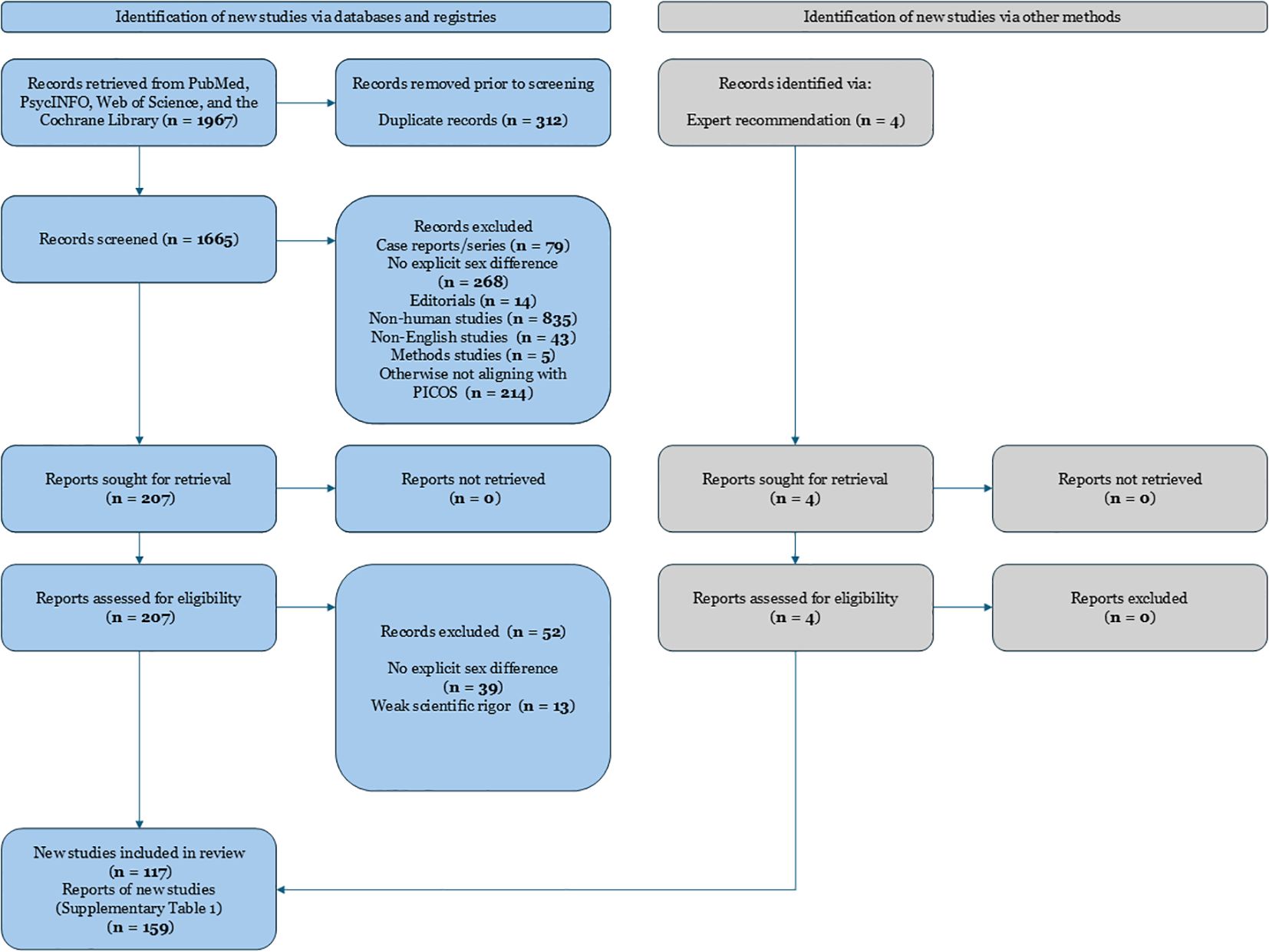
Figure 1. PRISMA 2020 flow diagram depicting the study selection process for a systematic review examining sex differences in schizophrenia. Records were identified from searches conducted in PubMed, PsycINFO, Web of Science, and the Cochrane Library. After removing duplicates and irrelevant records, full-text articles were assessed for eligibility. Main reasons for exclusion at the full-text stage included absence of explicit examination of sex differences, non-human studies, weak scientific rigor, non-English language, and studies not meeting PICOS criteria. Ultimately, 159 studies were included in the systematic review.
Inclusion criteria were defined according to the PICOS framework (Population, Intervention, Comparison, Outcome, Study design). We included peer-reviewed human studies (clinical trials, cohort studies, cross-sectional analyses, and meta-analyses) that explicitly examined differences between men and women with schizophrenia. Both chronic schizophrenia and first-episode psychosis populations were considered. Relevant outcomes included neurobiological measures (e.g., neuroimaging findings, genetic or hormonal factors), treatment outcomes (antipsychotic efficacy, response rates, relapse, and adherence), clinical symptomatology (positive, negative, and affective symptom profiles, age at onset, etc.), and adverse treatment effects (side-effect prevalence and severity). We excluded case reports, small case series, and studies conducted in animal models. Non-English articles, conference abstracts, and non-peer-reviewed sources were also excluded to ensure quality and reliability.
After removing duplicates, two reviewers (I.V. and V.K.) independently screened titles and abstracts for relevance. Studies that appeared to meet inclusion criteria were retrieved in full text and assessed for eligibility. Disagreements in study selection were resolved through discussion and consensus, consulting a third reviewer expert in the field if needed. Data extraction was performed using a standardized form, capturing key study characteristics and outcomes stratified by sex. We paid particular attention to extracting results on treatment response differences (e.g., differential efficacy or dosing requirements), symptom differences (such as variations in symptom severity or subtype by sex), and adverse effect profiles in men vs. women. The quality of included studies was appraised using criteria appropriate to each study’s design (for example, risk of bias tools for randomized trials and observational studies). We identified 159 relevant and recent studies that met inclusion criteria, encompassing a diverse range of methodologies and patient populations. Because this is a qualitative systematic review, no meta-analytic statistical pooling was performed; instead, findings are narratively synthesized. Our reporting follows PRISMA guidelines for systematic reviews, and a PRISMA flow diagram of study selection is provided (see Figure 1).
Results
The findings are organized below into four main subsections: Neurobiological Differences, Treatment Response, Symptomatology, and Adverse Effects, reflecting the domains of interest.
Neurobiological differences
Brain structure and neurodevelopment
Numerous studies have probed whether schizophrenia’s well-documented brain abnormalities manifest differently by sex. Overall, men and women with schizophrenia both exhibit structural brain changes relative to healthy controls, but there are subtle distinctions. For instance, evidence suggests that sex differences in brain morphology (such as volumetric differences in certain regions) and function seen in the general population tend to persist in patients with schizophrenia (6, 23). A recent large-scale neuroimaging meta-analysis found that man and woman schizophrenia patients showed divergent shape characteristics in deep brain structures that mirrored normal sexual dimorphism, implying that inherent neurodevelopmental sex differences are preserved to some degree in the illness (24). At the same time, some region-specific differences have been noted: the inferior parietal lobe volume reported that men with SZ lost the normal left-greater-than-right asymmetry seen in healthy men, whereas women did not exhibit this specific alteration (25). Women with SZ had decreased baseline activation of the dlPFC than men, a region that is involved in higher cognitive functions like working memory, flexibility, and planning (26). These findings provides further evidence that sex differences in brain morphology and function may contribute to the differing clinical expression of schizophrenia in men and women (27). It should be noted, however, that results across neuroimaging studies are not entirely consistent (25, 28). Differences that are observed often occur in regions that normally show sexual dimorphism (for example, limbic and cortical areas influenced by sex hormones), but normal sexual dimorphism in white matter is reverted in high risk individuals for schizophrenia emphasizing the neurodevelopmental aspect of this disorder (29).
Genetic and molecular factors
Emerging research indicates that genetic risk factors for schizophrenia might have sex-specific effects. A notable example concerns the complement component 4 (C4) gene, which is involved in synaptic pruning, which in turn is involved in the neurobiology of schizophrenia. Recent genetic analyses have suggested that certain C4 risk haplotypes for schizophrenia have a larger impact in men than in women, potentially contributing to the greater frequency of men in early-onset schizophrenia (30). Sex differences in immune function and complement protein levels (with adult women generally showing lower complement levels than men) might moderate the effect of these gene variants (31). Beyond C4, other gene-by-sex interactions have been explored. For instance, one study on the MTHFR gene (involved in folate metabolism) found that a functional polymorphism was associated with differences in symptom severity in men with SZ but not in women (12). Another investigation identified a sex-specific association involving the BDNF (brain-derived neurotrophic factor) gene: a particular BDNF variant predicted antipsychotic-induced weight gain in men but not in women, whereas low BDNF protein levels were linked to weight gain in women (32). These examples underscore that the genetic architecture of schizophrenia risk and treatment response may not be uniform across sexes. Epigenetic mechanisms and gene expression profiles also show sex-specific patterns in schizophrenia, although research in this area is still developing (33).
Hormonal differences
Perhaps the most evident biological disparities between men and women with schizophrenia involve gonadal hormones. The estrogen hypothesis of schizophrenia posits that estrogen confers a protective effect in women, delaying onset and attenuating symptoms until endogenous estrogen levels decline (34). Consistent with this, premenopausal women experience later onset and often less severe negative symptoms than age-matched men (35). Conversely, illness risk rises in women during low-estrogen phases (such as postpartum and postmenopause), aligning with the idea that estrogen withdrawal can precipitate or worsen psychosis (7, 36). In men, the role of testosterone and other androgens in schizophrenia is complex, with some evidence that low testosterone is linked to negative symptom severity, although findings are mixed (37). Recent clinical studies have directly measured sex hormone levels in first-episode patients to elucidate differences at illness onset. In one study of antipsychotic-naïve young patients from 2024, researchers found pronounced sex-specific hormonal disruptions at first episode (38). Young men with schizophrenia had significantly elevated estradiol levels compared to healthy men, while young women with SZ showed abnormal levels of multiple hormones relative to healthy women (38). These results indicate an overall gonadal hormone imbalance in early psychosis, with men uniquely exhibiting high estrogen and women exhibiting broader endocrine dysregulation. Hormonal fluctuations may also modulate symptoms during the course of the illness; for example, some women experience psychotic symptom exacerbation premenstrually when estrogen temporarily dips, and small trials of estrogen replacement or selective estrogen receptor modulators have shown a potential antipsychotic benefit in women (39).
Likewise, cognitive function profiles show subtle differences: some data indicate women with schizophrenia perform better on verbal memory tasks whereas men may have an advantage in visual-spatial processing, mirroring normal sex dimorphisms, though both sexes are impaired relative to controls (40). Notably, cognitive impairment in schizophrenia is a strong predictor of long-term functional outcome, making these sex-specific differences particularly relevant for designing targeted rehabilitation programs (41). Overall, while the core neuropathology of schizophrenia is shared by men and women, measurable biological differences in brain structure, genetics, and hormones likely underlie the distinct clinical profiles observed across sexes (42). Main findings in neurobiology are summarized in Figure 2.
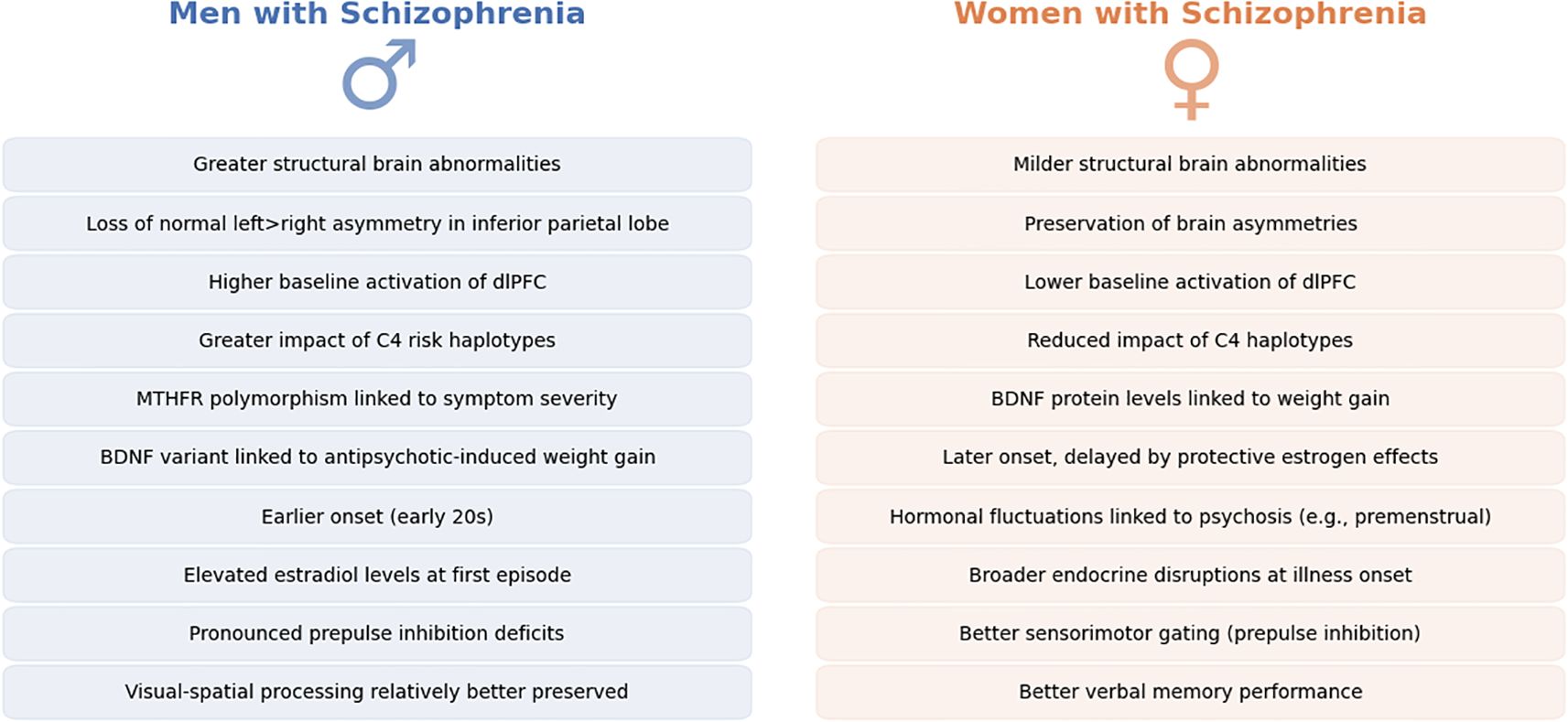
Figure 2. Neurobiological sex differences in Schizophrenia. This figure provides a side-by-side comparative overview of neurobiological differences observed between men (left, in blue) and women (right, in orange) diagnosed with schizophrenia. Each panel summarizes key findings from neuroimaging, genetic, hormonal, and cognitive studies reported in recent literature. Differences noted include variations in brain morphology, such as structural abnormalities and functional activation patterns, differential genetic influences, hormonal imbalances affecting disease onset and progression, and subtle distinctions in cognitive processing between sexes.
Treatment response
Antipsychotic efficacy and dosing
A growing literature indicates that men and women can respond differently to antipsychotic medications. Notably, pharmacokinetic differences between sexes often result in higher antipsychotic blood levels in women for a given dose. Women tend to have lower activity of certain drug-metabolizing liver enzymes (such as CYP1A2) and a higher body fat proportion, which can lead to slower clearance of antipsychotics (43). One clinical trial explicitly examining sex differences in antipsychotic treatment (the Best Strategy in Treatment-Resistant Schizophrenia [BeSt InTro] study) found that women achieved significantly higher plasma concentrations of various antipsychotics than men when treated at the same dose. In that study, dose-corrected serum levels of amisulpride were approximately 72% higher in women than in men, and levels of aripiprazole were about 56% higher in women, indicating markedly slower drug metabolism in women. Consequently, men often require higher doses to obtain therapeutic effects; for example, men with SZ needed moderately higher doses of aripiprazole to achieve comparable symptom control (44). By contrast, women face a risk of relative “overdosing” if dosages are not adjusted, given their greater drug exposure for the same dose (45).
Therapeutic efficacy
In terms of therapeutic efficacy, some antipsychotic agents appear to work better in one sex versus the other. For instance, amisulpride (a dopamine D2/D3 blocker) was found to be highly effective in men, producing a faster reduction in psychotic symptoms in men with SZ, whereas women derived less benefit from this drug (44, 46), 2007). Hoekstra et al. showed that men with SZ randomized to amisulpride had a significantly more rapid and robust symptom improvement than women (p = 0.003 for sex difference in speed of response). Moreover, amisulpride outperformed other medications (olanzapine and aripiprazole) in men but not in women (44). This suggests a potential sex-by-medication interaction where amisulpride is a preferential option for men with SZ. However, another study failed to uncover any sex differences in amisulpride treatment efficacy between men and women with SZ (47). Evidence indicates that women may respond more favorably to certain antipsychotics, such as paliperidone, particularly in first-episode populations. Women first-episode of psychosis (FEP) patients treated with paliperidone have been noted to achieve higher remission rates and symptom reduction than their men counterparts in some trials (16). Another study focused on improvement of symptomatology and found olanzapine to be superior to other antipsychotics in women after FEP (48) possibly due to differentiated metabolization rates (14). Similarly, clozapine, the prototypical treatment for treatment resistant refractory schizophrenia, has been reported in some cohorts to yield slightly higher response rates in women—possibly related to women’s higher clozapine blood levels for a given dose and clozapine’s interaction with estrogen—though findings on clozapine efficacy by sex are mixed (49, 50).
Symptom trajectory and outcomes
Beyond acute efficacy, long-term outcomes of treatment also might be influenced by sex. Women generally have better short-term outcomes early in schizophrenia—for example, a higher likelihood of achieving remission of positive symptoms in the first year (51). One large observational study noted that at one-year follow-up, a greater proportion of women were in remission and had improved functioning compared to men with equivalent treatment (52). This early advantage may reflect both biological factors (e.g., estrogen’s effects) and psychosocial factors (e.g., better premorbid skills aiding recovery). Adherence during the maintenance phase can also differ: some reports suggest men with SZ are slightly more likely to discontinue medication or relapse early, perhaps due to higher rates of substance abuse and poorer insight, whereas women, when not hampered by side effects, may adhere more consistently (53). Indeed, one analysis found that men were more prone to relapse after a medication dose reduction or discontinuation, whereas women maintained relapse-free stability longer when reducing antipsychotic dosage (54). This could be related to women’s generally lower dosing requirements; reducing the dose might affect men more adversely if they were just at the threshold of efficacy. On the other hand, among homeless patients with schizophrenia, one study found that homeless women had poorer treatment adherence than homeless men, possibly due to differing social support or victimization factors (55).
When examining long-term outcomes, some longitudinal studies have found that over decades of illness, outcome differences narrow or even reverse. For instance, a 10-year follow-up study reported that while women had better functional outcomes in early years, by the decade mark, men and women showed similar levels of clinical improvement and functional status on maintenance treatment. In the same study, there is also evidence that after mid-life (post-menopause), women’s illness severity can worsen relative to men’s, eroding earlier gains (56). A Finnish cohort study observed that women’s hospitalization rates and symptom severity increased after age 45, whereas men (who had already experienced a chronic course) changed little, resulting in mostly equivalent outcomes in later life (57). Such findings highlight that sex differences in schizophrenia are most pronounced in the early and mid-phases of illness, while late-life schizophrenia may converge across sexes. Nevertheless, some differences persist: for example, insight into illness and willingness to seek treatment have generally been found to be better in women with SZ, which can positively influence long-term adherence and outcomes (58). Women with SZ often engage more in psychosocial treatments and have stronger family support, factors linked to better prognosis. In contrast, men have higher rates of behaviors that complicate treatment (e.g., aggression, substance abuse, homelessness), which can lead to more frequent relapses or rehospitalizations (41).
Clinical management implications
The differential response patterns suggest that clinicians should consider sex when choosing and dosing antipsychotics. Women may achieve the same therapeutic benefit at lower doses and are at risk of overtreatment if dosages are not adjusted (due to higher blood levels), as previously mentioned (44). Conversely, men might require more aggressive dosing or preferential use of high-potency antipsychotics to reach symptom control (49, 59). Some experts have called for sex-specific treatment guidelines, noting that current prescribing practices often neglect these differences (53). For example, considering a trial of adjunctive estrogen or selective estrogen receptor modulators in women with persistent negative symptoms is an area of active investigation, given some positive trials showing estrogen’s augmentation effect (39). In men, ensuring adequate dosing and addressing comorbid substance use (which is more prevalent in men) are key for optimizing outcomes (6, 8, 60). Importantly, the goal is not to treat men and women wholly differently but to personalize treatment in recognition of sex-related tendencies. Both pharmacotherapy and psychosocial interventions (e.g., targeted compliance programs or gender-specific support groups) may benefit from tailoring to the unique needs and strengths of each sex. In summary, while antipsychotics remain the cornerstone of schizophrenia treatment for both men and women, accumulating evidence underscores that a “one-size-fits-all” approach may be suboptimal. Appreciating sex differences in pharmacokinetics and treatment response can guide more nuanced, effective management strategies for schizophrenia patients of each sex (61, 62). Treatment response sex-differences are presented in Figure 3.
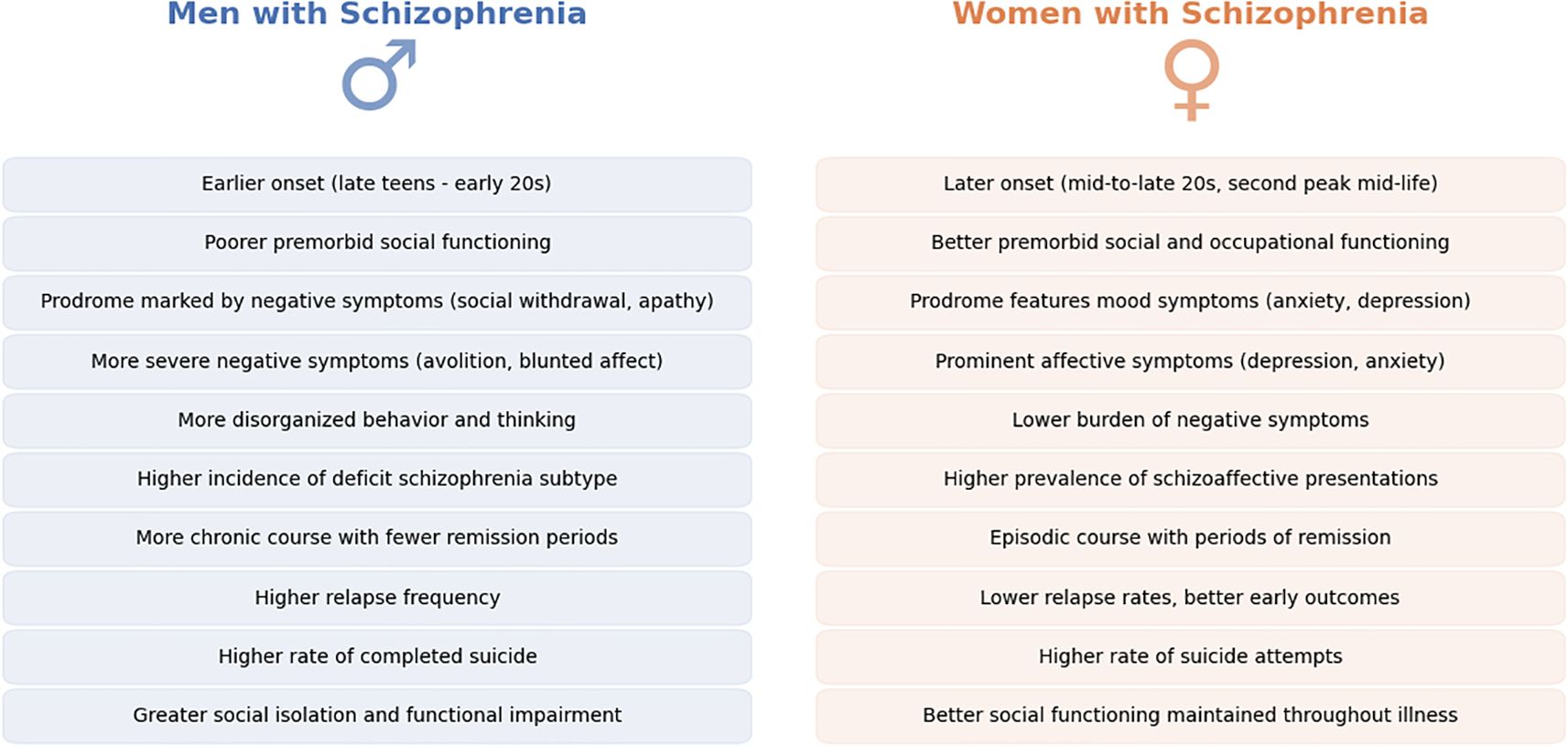
Figure 3. Sex differences in Schizophrenia symptomatology and illness course. This figure presents a comparative summary of key symptomatic and clinical course differences between men (left, in blue) and women (right, in orange) diagnosed with schizophrenia. Observations drawn from the literature highlight disparities in the age of illness onset, premorbid functioning, prodromal symptom profiles, predominant symptom types (e.g., affective versus negative symptoms), and patterns of illness progression and remission. The noted differences underscore variations in clinical expression and illness trajectory between sexes. Future clinical practices may benefit from systematically incorporating these symptom and illness-course sex differences, facilitating earlier and more personalized interventions tailored to each patient's specific needs.
Symptomatology
Age at onset
One of the most robust sex differences in schizophrenia is the age at illness onset. Men develop schizophrenia on average 3–5 years earlier than women (1, 63). Epidemiological studies consistently show a peak onset for men in late adolescence to early twenties, whereas the peak for women is in the mid-to-late twenties with a second smaller peak around mid-life (35). In a large cohort study the mean age of onset was approximately 23.7 years in men versus 27.6 years in women, a statistically significant gap (64). This delay in women has been attributed largely to the protective effect of estrogen during childbearing years (7). Interestingly, after women reach menopause (when estrogen levels drop), their incidence of schizophrenia can rise and sometimes even approach that of men, contributing to a later second peak of onset in women. Men, having no equivalent hormonal protection, experience their highest risk in early adulthood. The later onset in women often allows for better development of social and occupational skills before illness strikes, partially explaining their superior premorbid functioning (65).
Premorbid and prodromal features
The period before the onset of frank psychosis also shows sex-specific patterns. Men with SZ often exhibit more social withdrawal, academic difficulties, and schizoid or schizotypal traits in adolescence prior to diagnosis (64). They are more likely to have had behavioral problems or subtle developmental delays noted in childhood. Women, on the other hand, tend to have comparatively normal social development premorbidly; many have had stable relationships or employment prior to illness, reflecting better psychosocial adjustment. In clinical terms, men have a lower level of social development at illness onset, which subsequently impedes their further social maturation (66). This can lead to the often-observed outcome that schizophrenia’s social consequences (unemployment, isolation) are more severe in men. During the prodromal phase (the months or years of subtle symptoms before psychosis), some studies suggest men exhibit more negative symptoms (apathy, blunted affect) and unusual behavior, whereas women prodromally may experience more mood symptoms (e.g., anxiety, mild depression) or nonspecific physical complaints (67, 68). One interesting finding is that women in high-risk prodromal states are more likely to receive early interventions (such as antipsychotic treatment) than men (69). This could be because women’s prodromal symptoms bring them into contact with health services sooner, or clinicians might be more proactive in treating young women at risk. In any case, early intervention studies have noted that despite similar criteria, fewer men engage in preventive treatment during the prodrome, possibly contributing to a more abrupt and severe onset in men.
Clinical presentation and symptom profile
Once the illness is established, men and women can show distinct symptom profiles. Positive symptoms (hallucinations, delusions) are a prominent feature in both sexes, but some research suggests that women may have more affect-laden or auditory hallucinations, whereas men more often exhibit paranoid delusions (70, 71). In contrast, men with SZ exhibit more severe negative symptoms on average – such as flattened emotional expression, avolition, and social withdrawal and have different risk factors for developing this symptomatology than women (72–74). Men also more frequently meet criteria for the deficit subtype of schizophrenia (characterized by primary, enduring negative symptoms). For example, studies have found that deficit schizophrenia is disproportionately diagnosed in men, whereas women are more often diagnosed with non-deficit schizophrenia with more mood reactivity (75). Depressive symptoms and anxiety are reported more often by women with schizophrenia (3, 5). Women’s psychotic episodes commonly include prominent affective components (schizoaffective presentations are more frequent in women). Suicidality is another aspect with sex differences: while the overall suicide attempt rate in schizophrenia is high for both sexes, women may attempt suicide at slightly higher rates—often related to depressive symptoms—whereas men have a higher rate of completed suicide, potentially linked to greater social isolation and substance misuse (6, 63).
Some symptom differences emerge in specific contexts. For instance, the diagnostic subtype of schizophrenia has historically shown gender trends: earlier studies indicated that paranoid schizophrenia might be relatively more common in women (who often have later onset, more organized delusions), whereas disorganized or deficit presentations were more common in men (62) this has not been a universal finding; a study of first-episode patients in China found no sex difference in subtype at initial presentation, but in chronic samples, women were slightly more represented in paranoid subtype while men in negative/disorganized subtypes (41). Additionally, catatonic symptoms, though rare, have been reported more in men in some series. On the other hand, cognitive symptoms (neurocognitive deficits in memory, executive function) are a core feature for most patients and generally do not show large sex differences in magnitude – both men and women with schizophrenia suffer cognitive impairment relative to healthy controls. If anything, women’s slightly better premorbid verbal skills may translate to marginally better performance on verbal memory tests than men with schizophrenia, but when matched for education, cognitive profiles are largely comparable across sex (1, 64, 76).
Course of illness and recovery
Women not only present later but often have an episodic course with periods of remission, whereas men more often have a continuous course with residual symptoms. Clinical studies have observed that women are more likely to achieve remission of positive symptoms on treatment, especially in early and mid-life (51, 77). One large observational study noted that at one-year follow-up, a greater proportion of women were in remission and had improved functioning compared to men with equivalent treatment (52). This early advantage may reflect both biological factors (e.g., estrogen’s effects) and psychosocial factors (e.g., better premorbid skills aiding recovery). Adherence during the maintenance phase can also differ: some reports suggest that while men adhere better they are more likely to relapse early, perhaps due to higher rates of substance abuse and poorer insight, whereas women, possibly hampered by side effects, adhere less consistently (78, 79). On the same note, specific subpopulations continue this pattern – for example, among homeless patients with schizophrenia, one study found that homeless women had poorer treatment adherence than homeless men (55). Figure 4 highlights key differences in symptomatology between men and women.
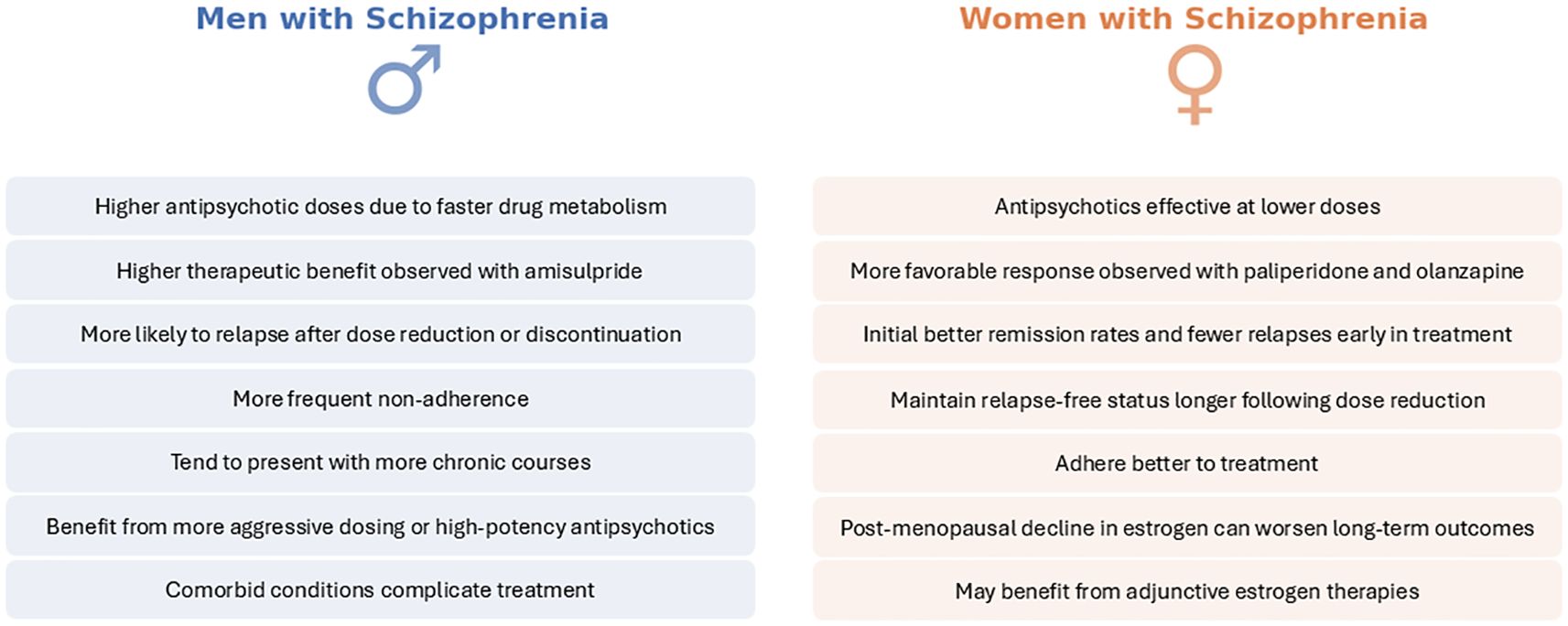
Figure 4. Sex differences in treatment response. This figure provides a concise side-by-side comparison of treatment response betwet men (left, blues) and women (right, orange). Key findings hghlight variations in antipsychotic efficiency and dosing, therapeutic efficiency and symptom trajectory and outcomes. Considering these sex-specific patterns during clinical assessment and therapeutic planning may enhance precision and effectiveness in managing schizophrenia across diverse patient populations.
Adverse effects
Metabolic side effects
One of the most concerning and well-studied long-term side effects of antipsychotic therapy is metabolic syndrome (weight gain, dyslipidemia, glucose intolerance). Studies have shown notable sex differences in metabolic outcomes. Women appear more significantly more vulnerable to antipsychotic-induced weight gain on certain medications (44, 80, 81). For example, in the previously mentioned randomized trial, women receiving amisulpride had significantly greater increases in body mass index (BMI) compared to men on the same treatment (44). Women’s BMI rose more and faster, especially with prolactin-elevating antipsychotics, suggesting a higher propensity for weight gain. Other research has found that atypical antipsychotics like clozapine and olanzapine confer a remarkably higher risk for metabolic dysfunction in women than in men. Women with SZ on these medications had higher odds of developing significant weight gain, hyperglycemia, and dyslipidemia relative to men with SZ on the same drugs (40). One proposed reason is that estrogen and other hormones in women might promote adipose deposition, or that women’s lower lean body mass results in greater effective drug concentration, amplifying metabolic side effects.
On the other hand, some data suggest that men are not immune to metabolic issues and may exceed women in certain parameters. For instance, a naturalistic study found that men with SZ were more likely to show elevations in cholesterol (especially LDL) and fasting glucose under long-term antipsychotic treatment, whereas women did not exhibit as steep an increase in those measures (82). Additionally, because men are more prone to abdominal (visceral) obesity when they gain weight, men with SZ might have a higher risk of cardiometabolic complications even if the degree of weight gain is similar. Indeed, one analysis reported that men with schizophrenia had a higher incidence of diabetes and dyslipidemia as well as more visceral fat tissue than women over a follow-up period, despite women gaining more weight in some cases (83). This highlights that the relationship between sex and metabolic side effects is complex: women gain more weight overall, but men might experience more adverse metabolic consequences per unit weight gained. It is worth noting that at least one study found no significant sex difference in the prevalence of metabolic syndrome among chronic patients – both sexes had high rates, likely due to universally poor lifestyle and antipsychotic effects (84). Thus, while trends exist with women often highlighted as higher risk for weight gain, clinicians should monitor metabolic health closely in all patients. Still, special vigilance is warranted for young women starting second-generation antipsychotics, as they can rapidly gain weight and develop metabolic syndrome unless preventive measures (diet, exercise, or preventative medication) are implemented (40).
Endocrine side effects (prolactin and sexual)
Prolactin elevation is a common antipsychotic side effect, especially with drugs like typical antipsychotics or risperidone and amisulpride that strongly block dopamine D2 receptors in the pituitary. Women are generally more susceptible to hyperprolactinemia on these medications (85). The high affinity for the dopamine receptor and subsequent agonism promotes prolactin production and the lack of 5-HTergic action does not inhibit it. Evidently, women more frequently experience hyperprolactinemia-related side effects such as menstrual irregularities (amenorrhea or oligomenorrhea), galactorrhea (milk discharge), and fertility issues (86). Many women with SZ on risperidone or amisulpride report loss of menstrual periods due to elevated prolactin. In contrast, men can also develop hyperprolactinemia, but the clinical sequelae differ – high prolactin in men may cause sexual dysfunction (e.g., decreased libido, erectile problems) and sometimes gynecomastia, though gynecomastia can occur in women as well (87, 88).
Despite such mixed findings, certain specific sexual side effects do show sex specificity. Erectile dysfunction and ejaculation difficulties are side effects unique to men, and antipsychotics—especially those raising prolactin or with alpha-adrenergic blocking properties—can cause impotence in a substantial fraction of men with SZ. Women do not experience erectile problems, but they may experience decreased sexual desire and arousal, as well as anorgasmia, which can be equivalent in impact (17, 19). Some evidence suggests that women are more likely to notice and be distressed by sexual side effects, potentially because men in this population may already have lower sexual activity due to negative symptoms or social factors. Also, hyperprolactinemia’s consequences like osteoporosis can differ: prolonged prolactin elevation leads to hypogonadism in both sexes, contributing to reduced bone density (89). Interestingly, one study found that men with SZ on prolactin-raising antipsychotics had higher rates of osteoporosis and low bone mineral density than women, which might reflect that clinicians are less likely to intervene for hypogonadism in men (90). This indicates that both sexes have unique vulnerabilities: women to reproductive side effects, and men to quietly developing bone loss or sexual dysfunction that they may not report.
Importantly, newer antipsychotics with partial dopamine agonism (such as aripiprazole) or those that spare prolactin (like quetiapine) can mitigate these endocrine side effects (91). Studies have shown that switching a patient (especially a woman with amenorrhea) from risperidone to a prolactin-sparing agent often restores normal menstrual function (86). Aripiprazole, in particular, can reduce prolactin levels in both men and women when added to or replacing a prolactin-elevating antipsychotic, with equivalent efficacy in normalizing prolactin across sexes (91). Thus, managing prolactin side effects may involve sex-specific considerations (e.g., ensuring premenopausal women maintain regular menses and bone health, and monitoring testosterone levels in men with long-term hyperprolactinemia).
Cardiovascular side effects
Antipsychotics can prolong the cardiac QT interval, raising the risk for a polymorphic ventricular tachycardia, torsade de pointes, an arrythmia which can be lethal if unmanaged. Female sex is a known risk factor for drug-induced QT prolongation in the general population (92, 93). This extends to the adverse effect profile for medications that alter heart repolarization such as antipsychotics. Women inherently have slightly longer baseline QT intervals than men, and estrogen may further lengthen cardiac repolarization (94). Research in schizophrenia patients indicates that severe QT prolongation (QTc > 500 ms) is observed more frequently in women on antipsychotics than in men (95). For example, a study in patients on long term antipsychotic therapy found that the prevalence of significant QTc prolongation was higher in women, despite similar drug regimens (96). Ziprasidone and clozapine have the highest rates of QT prolongation and cardiac toxicity (97) and ziprasidone was more frequently discontinued in women than in men with SZ (98), while women with SZ under clozapine treatment exhibit a more pronounced QT prolongation effect than men (99). This sex difference in cardiotoxicity risk is important for medication selection and ECG monitoring. Clinical guidelines usually recommend more cautious dose titration and periodic ECGs in women, especially if they are on multiple QT-prolonging drugs or have other risk factors (100). Men are not exempt from antipsychotic cardiac effects, but statistically, torsade de pointes has been reported more in women (101).
Neurological side effects
Antipsychotic-induced extrapyramidal symptoms (EPS), such as parkinsonism, tremor, and tardive dyskinesia (TD), also show some sex differences. Acute EPS (such as drug-induced parkinsonism) may be slightly more frequent in men, though evidence is not uniform (102, 103). Historically, TD was found to be more common in older women with schizophrenia, especially those who had long exposure to first-generation antipsychotics (104). Postmenopausal women were at highest risk for TD, possibly because estrogen has a dopamine-blocking effect; when estrogen levels drop, dopamine receptor supersensitivity (a cause of TD) might be unmasked (105). In the era of second-generation antipsychotics, TD incidence has declined, but some recent studies still find woman sex as a risk factor for tardive syndromes, particularly in those over 45 (57). On the other hand, acute EPS (such as drug-induced parkinsonism) may be slightly more frequent in men, though evidence is not uniform (103). One reason could be that men often receive higher doses and more high-potency antipsychotics (e.g., haloperidol) which cause EPS, whereas women more often receive atypical antipsychotics at moderate doses. Additionally, akathisia (restless agitation) has been reported more in men with SZ in some trials, potentially related to higher baseline motor activity or co-occurring substance use (102). These observations imply that long-term treatment plans must consider the differing risks: clinicians should monitor movement disorders closely in women and consider dose adjustments or switching medications if TD emerges, whereas men might benefit from adjunctive therapies aimed at mitigating acute EPS.
Other adverse effects
Some side effects are idiosyncratic but have noted sex biases. For example, it has been shown that women tend to exhibit less adverse effects from clozapine (49, 106). Blood dyscrasias like eosinophilia or agranulocytosis from clozapine have been reported more in one sex – men on clozapine had higher rates of neutropenia or other blood dyscrasias than women. The same large cohort study also reported that men had an elevated risk of cardiomyopathy and myocarditis (107). Liver enzyme elevations on certain antipsychotics might occur more in men (possibly due to higher alcohol use) (108). Sedation is a side effect that both men and women report, but some surveys suggest women may be more sensitive to its impact on daily functioning and quality of life (109). In contrast, men, who often are on higher doses, might experience more pronounced sedation when the dose is increased. Adherence-related effects (such as subjective feelings of dysphoria or cognitive dulling) could impact sexes differently: some evidence suggests that women are more likely to discontinue medication due to weight gain and menstrual side effects, whereas men are more likely to stop due to extrapyramidal side effects, sexual side effects or a subjective sense of emotional blunting (78, 88).
In summary, the adverse effect profiles of antipsychotic treatment in schizophrenia demonstrate clear sex differences. Women are more prone to weight gain, metabolic syndrome, hyperprolactinemia, sexual/reproductive disturbances, and QT prolongation whereas men are more prone to certain metabolic abnormalities (like dyslipidemia) and may experience more pronounced extrapyramidal symptoms. These differences necessitate sex-specific monitoring and management. For women, it is crucial to monitor weight, blood glucose/lipids, menstrual function, and bone density, and to consider adding a partial dopamine agonist or switching to prolactin-sparing and weight-neutral antipsychotics if problems arise (91, 110). For men, aggressive management of cardiovascular risk factors (given higher smoking rates and cholesterol findings) and vigilance for EPS and sexual side effects are key (111). With newer treatment options (e.g., partial agonists, statins, adjunct metformin for weight control), clinicians have tools to mitigate many side effects. The overarching principle is that being aware of a patient’s sex can guide anticipation of likely side-effect challenges—for instance, a young woman might need education about potential weight gain and contraceptive considerations, while a young man might require counselling on avoiding substances that exacerbate metabolic side effects. Tailoring side-effect management to sex not only improves quality of life but also enhances treatment adherence (112). Main findings are summarized in Figure 5.
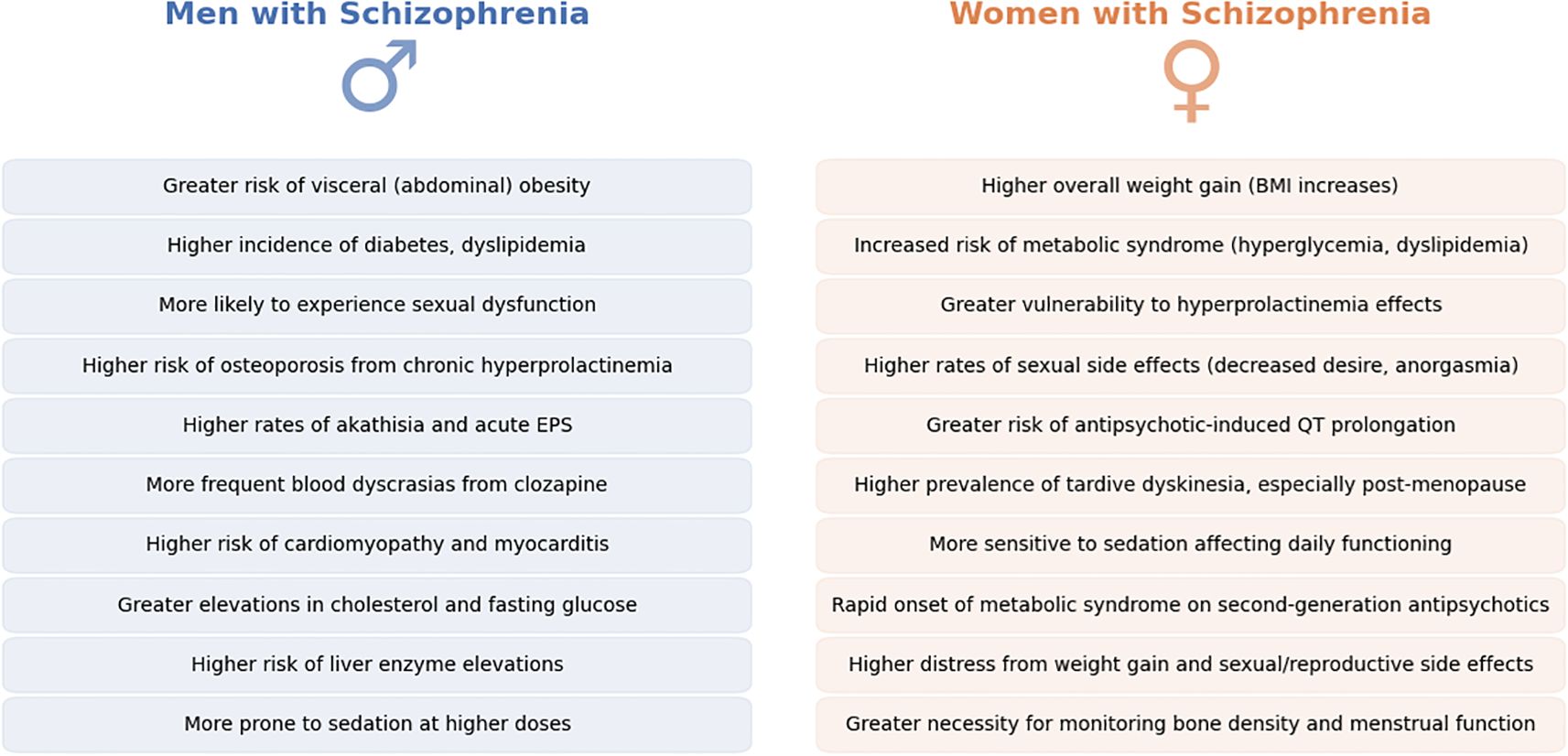
Figure 5. Sex differences in antipsychotic-induced adverse effects in Schizophrenia. This figure summarizes key sex-specific adverse effects associated with antipsychotic treatment in schizophrenia, contrasting men (left, blue) and women (right, orange). Differences span metabolic, endocrine, cardiovascular, and neurological domains, illustrating that men and women experience unique vulnerabilities when exposed to antipsychotic medications. Recognizing and anticipating these sex-related differences in clinical practice could facilitate more effective patient counseling, targeted monitoring, and proactive management of treatment-related complications, ultimately improving patient adherence and quality of life.
Discussion
This systematic review highlights significant sex differences in the neurobiology, clinical presentation, treatment response, and side-effect burden of schizophrenia. The compiled evidence confirms that sex is a crucial modulator of schizophrenia, influencing everything from age of onset to medication efficacy. Evaluating and adjusting for these differences has concrete implications for optimizing patient care.
One of the clearest patterns emerging from the literature is that women have advantages in some aspects of schizophrenia outcome (later onset, higher early remission, better social functioning), whereas men have advantages in fewer domains but may respond poorer to certain treatments (50, 68). For example, consistent evidence indicates that women’s later onset and preserved social skills often translate into a less disruptive initial course and a higher likelihood of early remission (51). This suggests that if interventions are provided promptly, women have a strong capacity for recovery. In contrast, men’s earlier onset and greater negative symptom burden contribute to a more chronic course and lower likelihood of full remission (4, 6, 63). However, on the treatment side, some evidence suggests that men may derive disproportionate benefit from particular antipsychotics such as amisulpride (44) and generally tolerate higher doses (owing to faster metabolism) (45). This information could be leveraged in clinical practice: for men with severe psychosis, choosing a high-efficacy antipsychotic and titrating to the upper therapeutic range might be justified, whereas for a woman patient, an agent with a favorable metabolic profile and a lower starting dose may be preferable.
The role of sex hormones, especially estrogen, emerges as a unifying theme influencing many differences. Estrogen’s protective effect in women provides a compelling explanation for later onset, fewer negative symptoms, and a different neurobiological trajectory (with less early neurodevelopmental disruption) (7, 105). Adding estrogen or selective estrogen modulators (such as raloxifene) to existing medication has shown promise in ameliorating symptoms in women, particularly for refractory negative symptoms (39, 113). This suggests that hormonal modulation could become a viable sex-specific treatment strategy. It also underscores the need for clinicians managing women with schizophrenia to be mindful of hormonal phases; for instance, the peripartum period and menopause require proactive planning, as relapse risk may be heightened when estrogen levels decline (35). Conversely, the findings regarding men prompt questions about whether androgens or other man-specific factors could be harnessed or managed; for example, testing for decreased testosterone and correcting it might improve motivation and general symptomatology in men with SZ. Emerging evidence suggests that testosterone may have therapeutic relevance in men with schizophrenia, particularly for mitigating negative symptoms (114). Several studies have documented low or dysregulated testosterone levels in male patients, often associated with greater severity of avolition, anhedonia, and cognitive deficits. Small clinical trials have explored testosterone augmentation or supplementation with androgen precursors like DHEA, reporting modest but promising improvements in negative symptoms and mood (37). The proposed mechanisms include modulation of dopaminergic and glutamatergic signaling, neuroprotection, and enhancement of neuroplasticity. While these interventions have generally been well tolerated in the short term, more robust, long-term studies are needed to establish efficacy, identify responsive subgroups, and ensure safety.
Another key discussion point is how sex differences intersect with current treatment guidelines and whether guidelines should be revised. Currently, standard schizophrenia treatment does not differentiate by sex—dosing recommendations and medication choices are largely uniform. However, it has been suggested that a one-size-fits-all approach may be suboptimal. For example, given women’s higher antipsychotic blood levels and greater susceptibility to side effects, guidelines could recommend initiating treatment at lower doses for women with slower titration, while men might require standard or even higher doses to achieve comparable efficacy. In practice, implementing sex-specific considerations could involve multidisciplinary collaboration, such as involving endocrinologists or OB/GYN’s for women with SZ and addiction specialists for men with SZ, to tailor treatment to the unique needs of each sex.
Gaps in current research became apparent during our review. Despite a growing number of studies, several areas remain underexplored or yield conflicting data. For instance, neuroimaging findings on sex differences are inconsistent—some studies show significant differences in brain connectivity and morphology, while others do not (23, 27, 115). Similarly, the influence of sex chromosomes and epigenetic regulation on schizophrenia risk is still poorly understood. Although there is considerable knowledge about young and middle-aged patients, research on aging women with schizophrenia is scarce; as more women with schizophrenia live into older age, understanding how post-menopausal hormonal changes affect cognitive and functional outcomes becomes increasingly important (15). Moreover, while some evidence suggests that men are more likely to relapse after a medication dose reduction, the factors driving this difference remain ambiguous (54, 116). Such discrepancies highlight that sex interacts with many other variables—such as age, genetics, and environmental factors—in complex ways.
It should also be noted that most studies in our review were conducted in high-income settings, and low- and middle-income countries were underrepresented. This geographic bias is an important limitation, as sociocultural and healthcare disparities in LMICs may shape sex differences in schizophrenia outcomes (117). Resource-limited healthcare systems often lack access to newer adjunctive treatments (e.g., estrogen or hormonal therapies), potentially limiting benefits that disproportionately aid female patients. Moreover, traditional gender roles and differing stigma in many LMIC communities can alter disease trajectories – women with schizophrenia might be more likely to be hidden or receive family care at home, whereas men unable to fulfill expected roles may experience greater social marginalization (118). Such factors could modulate observed sex-specific outcomes, underscoring that our findings may not fully generalize to LMIC settings. The is a great and urgent need for more inclusive research and caution in extrapolating results to regions where women and men with schizophrenia face distinct sociocultural challenges.
This review focused explicitly on sex differences between men and women with schizophrenia; however, a significant limitation is the exclusion of non-binary and gender-diverse populations. Most schizophrenia research, including the studies reviewed here, predominantly adheres to a binary understanding of sex and gender. Recent literature emphasizes that such a binary framework contributes to an evidence gap regarding the prevalence, symptomatology, treatment response, and side-effect profiles in transgender, non-binary, and gender-diverse individuals with schizophrenia. Indeed, existing epidemiological data suggest an elevated risk of schizophrenia-spectrum disorders among transgender individuals, but findings remain inconsistent due to methodological variations and inadequate differentiation of transgender from non-binary or other gender-diverse identities (119). Moreover, Nolan et al. (120) note that this exclusion limits the generalizability of research findings, potentially leaving clinical guidelines inadequately tailored to gender-diverse individuals, who might possess unique clinical characteristics and treatment needs (120).
Sex differences in schizophrenia are shaped not only by biological factors but also by sociocultural and evolutionary influences. Gender roles, such as caregiving responsibilities and societal expectations, affect symptom expression, treatment adherence, and clinical outcomes (121). Women, often more socially connected due to traditional caregiving roles, tend to seek help earlier, leading to better early outcomes but may face added stress from these roles, which can impact relapse risk. In contrast, men, who experience more externalizing behaviors and substance use due to societal pressures to maintain self-reliance, often face worse outcomes. Evolutionary pressures further contribute to these differences: women’s stress response, influenced by estrogen and oxytocin, provides protection against psychosis until menopause, explaining their later onset and less severe early course. Men, with no such hormonal safeguard, show earlier onset and more chronic symptoms, reflecting evolutionary trade-offs linked to stress resilience and reproductive priorities (122). These sociocultural and evolutionary perspectives highlight the need for gender-specific interventions to optimize outcomes in schizophrenia.
In terms of clinical implications, it is more than evident that a more personalized approach to treating schizophrenia in men and women will be more efficient (123). For women with SZ, clinicians might emphasize weight management from the outset, choose antipsychotics with lower prolactin elevation if fertility is a concern, and monitor for mood fluctuations that may necessitate adjunctive treatments. For men with SZ, screening for substance use and ensuring adequate dosing (perhaps with depot formulations) could be prioritized. Additionally, both sexes might benefit from psychosocial interventions tailored to their unique challenges—such as social skills training for men and trauma-informed care for women. Ultimately, these differences underscore the necessity for future clinical trials to stratify outcomes by sex and for clinical guidelines to incorporate sex-specific recommendations.
Conclusion
Men and women with schizophrenia differ significantly in epidemiology, clinical presentation, treatment response, and adverse effect profiles. Women tend to experience a later onset with better premorbid functioning and a more favorable early response to treatment, though they are at higher risk for metabolic and endocrine side effects. Men, by contrast, have an earlier onset, more pronounced negative symptoms, and often require higher doses for symptom control, but may be more vulnerable to substance use and certain neurological adverse effects. Recognizing these differences is crucial for tailoring treatment strategies and monitoring side effects in a sex-specific manner. Incorporating a sex-informed perspective into both clinical practice and future research will enhance personalized treatment approaches and ultimately improve outcomes for all individuals affected by schizophrenia.
Future directions
Further research is needed to elucidate the underlying mechanisms of sex differences in schizophrenia, including basic neuroscience, large-scale genomics, neuroimaging, and hormonal studies. Longitudinal investigations tracking patients from the prodromal phase through chronic stages will be essential to understand how early advantages or disadvantages evolve over time. Additionally, intervention trials should be designed to detect sex-specific effects, thereby informing updated, gender-sensitive clinical guidelines. Tailoring both pharmacological and psychosocial treatments to account for sex differences represents a promising step toward truly personalized care in schizophrenia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
IM: Writing – original draft, Conceptualization, Software, Validation, Funding acquisition, Investigation, Writing – review & editing, Resources, Formal Analysis, Methodology, Project administration, Data curation. VK: Software, Writing – review & editing, Investigation, Supervision, Funding acquisition, Writing – original draft, Conceptualization, Formal Analysis, Resources, Visualization, Data curation, Methodology, Project administration, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1594334/full#supplementary-material
References
1. Abel KM, Drake R, and Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. (2010) 22:417–28. doi: 10.3109/09540261.2010.515205
2. Arranz B, Safont G, Corripio I, Ramirez N, Duenas RM, Perez V, et al. Substance use in patients with first-episode psychosis: is gender relevant? J Dual Diagn. (2015) 11:153–60. doi: 10.1080/15504263.2015.1113761
3. Asif U, Saleem Z, Yousaf M, Saeed H, Hashmi FK, Islam M, et al. Genderwise clinical response of antipsychotics among schizophrenic patients: a prospective observational study from Lahore, Pakistan. Int J Psychiatry Clin Pract. (2018) 22:177–83. doi: 10.1080/13651501.2017.1395055
4. Groleger U and Novak-Grubic V. Gender, psychosis and psychotropic drugs: differences and similarities. Psychiatr Danub. (2010) 22:338–42. https://www.ncbi.nlm.nih.gov/pubmed/20562777.
5. Naguy A and Fatehi S. Schizophrenia in women-A different phenotype? Arch Womens Ment Health. (2016) 19:1153. doi: 10.1007/s00737-016-0662-0
6. Giordano GM, Bucci P, Mucci A, Pezzella P, and Galderisi S. Gender differences in clinical and psychosocial features among persons with schizophrenia: A mini review. Front Psychiatry. (2021) 12:789179. doi: 10.3389/fpsyt.2021.789179
7. Iqbal J, Huang GD, Xue YX, Yang M, and Jia XJ. Role of estrogen in sex differences in memory, emotion and neuropsychiatric disorders. Mol Biol Rep. (2024) 51:415. doi: 10.1007/s11033-024-09374-z
8. Boter H, Derks EM, Fleischhacker WW, Davidson M, Kahn RS, and Group ES. Generalizability of the results of efficacy trials in first-episode schizophrenia: comparisons between subgroups of participants of the European First Episode Schizophrenia Trial (EUFEST). J Clin Psychiatry. (2010) 71:58–65. doi: 10.4088/JCP.08m04506yel
9. Cannavo D, Minutolo G, Battaglia E, and Aguglia E. Insight and recovery in schizophrenic patients. Int J Psychiatry Clin Pract. (2016) 20:83–90. doi: 10.3109/13651501.2016.1141960
10. Camsari U, Viguera AC, Ralston L, Baldessarini RJ, and Cohen LS. Prevalence of atypical antipsychotic use in psychiatric outpatients: comparison of women of childbearing age with men. Arch Womens Ment Health. (2014) 17:583–6. doi: 10.1007/s00737-014-0465-0
11. Gogos A, Ney LJ, Seymour N, Van Rheenen TE, and Felmingham KL. Sex differences in schizophrenia, bipolar disorder, and post-traumatic stress disorder: Are gonadal hormones the link? Br J Pharmacol. (2019) 176:4119–35. doi: 10.1111/bph.14584
12. Gao J, Xiu MH, Liu DY, Wei CW, and Zhang X. Interactive effect of MTHFR C677T polymorphism and sex on symptoms and cognitive functions in Chinese patients with chronic schizophrenia. Aging (Albany NY). (2020) 12:10290–9. doi: 10.18632/aging.103248
13. Charlotte M, Schwartz E, Slade E, Medoff D, Li L, Dixon L, et al. Gender differences in mood stabilizer medications prescribed to Veterans with serious mental illness. J Affect Disord. (2015) 188:112–7. doi: 10.1016/j.jad.2015.08.065
14. Cheng F and Jones PB. Drug treatments for schizophrenia: pragmatism in trial design shows lack of progress in drug design. Epidemiol Psychiatr Sci. (2013) 22:223–33. doi: 10.1017/S204579601200073X
15. Zhang XY, Chen DC, Xiu MH, Yang FD, Haile CN, Kosten TA, et al. Gender differences in never-medicated first-episode schizophrenia and medicated chronic schizophrenia patients. J Clin Psychiatry. (2012) 73:1025–33. doi: 10.4088/JCP.11m07422
16. Chung YC, Cui Y, Kim MG, Kim YJ, Lee KH, and Chae SW. Early predictors of a clinical response at 8 weeks in patients with first-episode psychosis treated with paliperidone ER. J Psychopharmacol. (2016) 30:810–8. doi: 10.1177/0269881116654698
17. Ciocca G, Usall J, Dolz M, Limoncin E, Gravina GL, Carosa E, et al. Sexual dysfunctions in people with first-episode psychosis assessed according to a gender perspective. Riv Psichiatr. (2015) 50:239–44. doi: 10.1708/2040.22166
18. Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. (2020) 7:64–77. doi: 10.1016/S2215-0366(19)30416-X
19. Galbally M, Wynter K, Siskind D, Correll CU, Northwood K, and Every-Palmer S. Sex differences between female and male individuals in antipsychotic efficacy and adverse effects in the treatment of schizophrenia. CNS Drugs. (2024) 38:559–70. doi: 10.1007/s40263-024-01089-w
20. Gonzalez-Rodriguez A, Molina-Andreu O, Imaz Gurrutxaga ML, Catalan Campos R, and Arroyo MB. A descriptive retrospective study of the treatment and outpatient service use in a clinical group of delusional disorder patients. Rev Psiquiatr Salud Ment. (2014) 7:64–71. doi: 10.1016/j.rpsm.2013.01.004
21. Hong SI, Bennett D, and Rosenheck RA. Gender differences in outcomes of early intervention services for first episode psychosis. Early Interv Psychiatry. (2023) 17:715–23. doi: 10.1111/eip.13367
22. Salahuddin NH, Schutz A, Pitschel-Walz G, Mayer SF, Chaimani A, Siafis S, et al. Psychological and psychosocial interventions for treatment-resistant schizophrenia: a systematic review and network meta-analysis. Lancet Psychiatry. (2024) 11:545–53. doi: 10.1016/S2215-0366(24)00136-6
23. van Erp TGM, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol Psychiatry. (2018) 84:644–54. doi: 10.1016/j.biopsych.2018.04.023
24. Schijven D, Postema MC, Fukunaga M, Matsumoto J, Miura K, de Zwarte SMC, et al. Large-scale analysis of structural brain asymmetries in schizophrenia via the ENIGMA consortium. Proc Natl Acad Sci U S A. (2023) 120:e2213880120. doi: 10.1073/pnas.2213880120
25. Szeszko PR, Narr KL, Phillips OR, McCormack J, Sevy S, Gunduz-Bruce H, et al. Magnetic resonance imaging predictors of treatment response in first-episode schizophrenia. Schizophr Bull. (2012) 38:569–78. doi: 10.1093/schbul/sbq126
26. Kazinka R, Choi DS, Opitz A, and Lim KO. Individuals with psychosis receive less electric field strength during transcranial direct current stimulation compared to healthy controls. Schizophrenia. (2024) 10:111. doi: 10.1038/s41537-024-00529-2
27. Shenton ME, Whitford TJ, and Kubicki M. Structural neuroimaging in schizophrenia: from methods to insights to treatments. Dialogues Clin Neurosci. (2010) 12:317–32. doi: 10.31887/DCNS.2010.12.3/mshenton
28. Thompson PM, Jahanshad N, Ching CRK, Salminen LE, Thomopoulos SI, Bright J, et al. ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl Psychiatry. (2020) 10:100. doi: 10.1038/s41398-020-0705-1
29. Savadjiev P, Seidman LJ, Thermenos H, Keshavan M, Whitfield-Gabrieli S, Crow TJ, et al. Sexual dimorphic abnormalities in white matter geometry common to schizophrenia and non-psychotic high-risk subjects: Evidence for a neurodevelopmental risk marker? Hum Brain Mapp. (2016) 37:254–61. doi: 10.1002/hbm.23026
30. Chen CC, Howie J, Ebrahimi M, Teymouri K, Woo JJ, Tiwari AK, et al. Analysis of the complement component C4 gene with schizophrenia subphenotypes. Schizophr Res. (2024) 271:309–18. doi: 10.1016/j.schres.2024.07.039
31. Chen Y, Dai J, Tang L, Mikhailova T, Liang Q, Li M, et al. Neuroimmune transcriptome changes in patient brains of psychiatric and neurological disorders. Mol Psychiatry. (2023) 28:710–21. doi: 10.1038/s41380-022-01854-7
32. Zhang XY, Zhou DF, Wu GY, Cao LY, Tan YL, Haile CN, et al. BDNF levels and genotype are associated with antipsychotic-induced weight gain in patients with chronic schizophrenia. Neuropsychopharmacology. (2008) 33:2200–5. doi: 10.1038/sj.npp.1301619
33. Ori APS, Olde Loohuis LM, Guintivano J, Hannon E, Dempster E, St Clair D, et al. Meta-analysis of epigenetic aging in schizophrenia reveals multifaceted relationships with age, sex, illness duration, and polygenic risk. Clin Epigenet. (2024) 16:53. doi: 10.1186/s13148-024-01660-8
34. Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology. (2003) 28 Suppl 2:17–54. doi: 10.1016/s0306-4530(02)00125-7
35. Yasuda M, Kobayashi T, Kato S, and Kishi K. Clinical features of late-onset schizophrenia in Japan: comparison with early-onset cases. Psychogeriatrics. (2013) 13:244–9. doi: 10.1111/psyg.12032
36. Jesus Magueta S, Costa AL, Simões G, Gomes AI, Madaíl Grego C, and Garrido P. Hormones and psychosis: the role of estrogen in schizophrenia. Eur Psychiatry. (2024) 67:S175-6. doi: 10.1192/j.eurpsy.2024.386
37. Misiak B, Frydecka D, Loska O, Moustafa AA, Samochowiec J, Kasznia J, et al. Testosterone, DHEA and DHEA-S in patients with schizophrenia: A systematic review and meta-analysis. Psychoneuroendocrinology. (2018) 89:92–102. doi: 10.1016/j.psyneuen.2018.01.007
38. Hu Q, Wang J, Liang J, Xiu M, Zhang S, and Wu F. Gonadal hormone abnormalities in young patients with first-episode schizophrenia. Int J Neuropsychopharmacol. (2024) 27:pyae063. doi: 10.1093/ijnp/pyae063
39. Ghafari E, Fararouie M, Shirazi HG, Farhangfar A, Ghaderi F, and Mohammadi A. Combination of estrogen and antipsychotics in the treatment of women with chronic schizophrenia: a double-blind, randomized, placebo-controlled clinical trial. Clin Schizophr Relat Psychoses. (2013) 6:172–6. doi: 10.3371/CSRP.GHFA.01062013
40. Gardener EK, Carr AR, Macgregor A, and Felmingham KL. Sex differences and emotion regulation: an event-related potential study. PloS One. (2013) 8:e73475. doi: 10.1371/journal.pone.0073475
41. Xiang YT, Wang CY, Chiu HF, Weng YZ, Bo QJ, Chan SS, et al. Socio-demographic and clinical profiles of paranoid and nonparanoid schizophrenia: a prospective, multicenter study in China. Perspect Psychiatr Care. (2011) 47:126–30. doi: 10.1111/j.1744-6163.2010.00281.x
42. Ziemka-Nalecz M, Pawelec P, Ziabska K, and Zalewska T. Sex differences in brain disorders. Int J Mol Sci. (2023) 24:14571. doi: 10.3390/ijms241914571
43. Meibohm B, Beierle I, and Derendorf H. How important are gender differences in pharmacokinetics? Clin Pharmacokinet. (2002) 41:329–42. doi: 10.2165/00003088-200241050-00002
44. Hoekstra S, Bartz-Johannessen C, Sinkeviciute I, Reitan SK, Kroken RA, Loberg EM, et al. Sex differences in antipsychotic efficacy and side effects in schizophrenia spectrum disorder: results from the BeSt InTro study. NPJ Schizophr. (2021) 7:39. doi: 10.1038/s41537-021-00170-3
45. Seeman MV. The pharmacodynamics of antipsychotic drugs in women and men. Front Psychiatry. (2021) 12:650904. doi: 10.3389/fpsyt.2021.650904
46. Yang S, Wang H, Zheng GF, and Wang Y. Age, sex, and comedication effects on the steady-state plasma concentrations of amisulpride in chinese patients with schizophrenia. Ther Drug Monit. (2023) 45:676–82. doi: 10.1097/FTD.0000000000001089
47. Ji JW, Liu LY, Hao KR, Yu YL, Weng SZ, Wu JF, et al. Prediction of self-report cognitive function for the symptomatic remission in schizophrenia treated with amisulpride: a multicenter, 8-week case-control study. Psychiatr Q. (2021) 92:935–45. doi: 10.1007/s11126-020-09877-5
48. Ceskova E, Prikryl R, Libiger J, Svancara J, and Jarkovsky J. Gender differences in the treatment of first-episode schizophrenia: Results from the European First Episode Schizophrenia Trial. Schizophr Res. (2015) 169:303–7. doi: 10.1016/j.schres.2015.10.013
49. Alberich S, Fernandez-Sevillano J, Gonzalez-Ortega I, Usall J, Saenz M, Gonzalez-Fraile E, et al. A systematic review of sex-based differences in effectiveness and adverse effects of clozapine. Psychiatry Res. (2019) 280:112506. doi: 10.1016/j.psychres.2019.112506
50. Usall J, Suarez D, Haro JM, and Group SS. Gender differences in response to antipsychotic treatment in outpatients with schizophrenia. Psychiatry Res. (2007) 153:225–31. doi: 10.1016/j.psychres.2006.09.016
51. Dama M, Veru F, Schmitz N, Shah J, Iyer S, Joober R, et al. Sex differences in clinical and functional outcomes among patients treated in an early intervention service for psychotic disorders: an observational study. Can J Psychiatry. (2019) 64:708–17. doi: 10.1177/0706743719854069
52. Nejtek VA, Allison N, and Hilburn C. Race- and gender-related differences in clinical characteristics and quality of life among outpatients with psychotic disorders. J Psychiatr Pract. (2012) 18:329–37. doi: 10.1097/01.pra.0000419817.83375.c6
53. Olfson M, King M, and Schoenbaum M. Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry. (2015) 72:867–74. doi: 10.1001/jamapsychiatry.2015.0500
54. Xiang YT, Wang CY, Weng YZ, Bo QJ, Chiu HF, Zhao JP, et al. Sex differences in patients with schizophrenia: A prospective, multi-center study. Psychiatry Res. (2010) 177:294–8. doi: 10.1016/j.psychres.2010.03.014
55. Tinland A, Zemmour K, Auquier P, Boucekine M, Girard V, Loubiere S, et al. Homeless women with schizophrenia reported lower adherence to their medication than men: results from the French Housing First experience. Soc Psychiatry Psychiatr Epidemiol. (2017) 52:1113–22. doi: 10.1007/s00127-017-1411-z
56. Zhao J, Diao J, Li X, Yang Y, Yao Y, Shi S, et al. Gender differences in psychiatric symptoms and the social functioning of 610 patients with schizophrenia in urban China: A 10-year follow-up study. Neuropsychiatr Dis Treat. (2022) 18:1545–51. doi: 10.2147/NDT.S373923
57. Sommer IE, Brand BA, Gangadin S, Tanskanen A, Tiihonen J, and Taipale H. Women with schizophrenia-spectrum disorders after menopause: A vulnerable group for relapse. Schizophr Bull. (2023) 49:136–43. doi: 10.1093/schbul/sbac139
58. Ramsay G, Haime Z, Crellin NE, Stansfeld JL, Priebe S, Long M, et al. Recruitment to a trial of antipsychotic reduction: impact of an acceptability study. BMC Med Res Methodol. (2023) 23:78. doi: 10.1186/s12874-023-01881-0
59. Reeves S, Bertrand J, Obee SJ, Hunter S, Howard R, and Flanagan RJ. A population pharmacokinetic model to guide clozapine dose selection, based on age, sex, ethnicity, body weight and smoking status. Br J Clin Pharmacol. (2024) 90:135–45. doi: 10.1111/bcp.15691
60. Patel MX, Bowskill S, Couchman L, Lay V, Taylor D, Spencer EP, et al. Plasma olanzapine in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service 1999-2009. J Clin Psychopharmacol. (2011) 31:411–7. doi: 10.1097/JCP.0b013e318221b408
61. Ercis M, Sanchez-Ruiz JA, Webb LM, Solares-Bravo M, Betcher HK, Moore KM, et al. Sex differences in effectiveness and adverse effects of mood stabilizers and antipsychotics: A systematic review. J Affect Disord. (2024) 352:171–92. doi: 10.1016/j.jad.2024.02.038
62. Ochoa S, Usall J, Cobo J, Labad X, and Kulkarni J. Gender differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophr Res Treat. (2012) 2012:916198. doi: 10.1155/2012/916198
63. Ventura J, Subotnik KL, Han S, Hellemann GS, Green MF, and Nuechterlein KH. The relationship between sex and functional outcome in first-episode schizophrenia: the role of premorbid adjustment and insight. Psychol Med. (2023) 53:6878–87. doi: 10.1017/S0033291723000442
64. Norman RM, Malla AK, Manchanda R, and Townsend L. Premorbid adjustment in first episode schizophrenia and schizoaffective disorders: a comparison of social and academic domains. Acta Psychiatr Scand. (2005) 112:30–9. doi: 10.1111/j.1600-0447.2005.00555.x
65. Seeman MV. Current outcome in schizophrenia: women vs men. Acta Psychiatr Scand. (1986) 73:609–17. doi: 10.1111/j.1600-0447.1986.tb02732.x
66. Selvendra A, Toh WL, Neill E, Tan EJ, Rossell SL, Morgan VA, et al. Age of onset by sex in schizophrenia: Proximal and distal characteristics. J Psychiatr Res. (2022) 151:454–60. doi: 10.1016/j.jpsychires.2022.05.010
67. Simunovic Filipcic I, Ivezic E, Jaksic N, Mayer N, Grah M, Rojnic Kuzman M, et al. Gender differences in early onset of chronic physical multimorbidities in schizophrenia spectrum disorder: Do women suffer more? Early Interv Psychiatry. (2020) 14:418–27. doi: 10.1111/eip.12867
68. Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. (2022) 27:281–95. doi: 10.1038/s41380-021-01161-7
69. Zeng J, Raballo A, Gan R, Wu G, Wei Y, Xu L, et al. Antipsychotic exposure in clinical high risk of psychosis: empirical insights from a large cohort study. J Clin Psychiatry. (2022) 83. doi: 10.4088/JCP.21m14092
70. Deligiannidis KM, Rothschild AJ, Barton BA, Kroll-Desrosiers AR, Meyers BS, Flint AJ, et al. A gender analysis of the study of pharmacotherapy of psychotic depression (STOP-PD): gender and age as predictors of response and treatment-associated changes in body mass index and metabolic measures. J Clin Psychiatry. (2013) 74:1003–9. doi: 10.4088/JCP.13m08400
71. Segarra R, Ojeda N, Zabala A, Garcia J, Catalan A, Eguiluz JI, et al. Similarities in early course among men and women with a first episode of schizophrenia and schizophreniform disorder. Eur Arch Psychiatry Clin Neurosci. (2012) 262:95–105. doi: 10.1007/s00406-011-0218-2
72. Hajj A, Hallit S, Chamoun K, Sacre H, Obeid S, Haddad C, et al. Negative symptoms in schizophrenia: correlation with clinical and genetic factors. Pharmacogenomics. (2021) 22:389–99. doi: 10.2217/pgs-2020-0171
73. Mezquida G, Amoretti S, Bioque M, Garcia-Rizo C, Sanchez-Torres AM, Pina-Camacho L, et al. Identifying risk factors for predominant negative symptoms from early stages in schizophrenia: A longitudinal and sex-specific study in first-episode schizophrenia patients. Span J Psychiatry Ment Health. (2023) 16:159–68. doi: 10.1016/j.rpsm.2023.01.004
74. Wojciak P, Domowicz K, Andrzejewska M, and Rybakowski JK. Negative symptoms in schizophrenia, assessed by the brief negative symptom scale, self-evaluation of negative symptom scale, and social cognition: a gender effect. Int J Psychiatry Clin Pract. (2021) 25:252–7. doi: 10.1080/13651501.2020.1810278
75. Goldstein JM, Santangelo SL, Simpson JC, and Tsuang MT. The role of gender in identifying subtypes of schizophrenia: a latent class analytic approach. Schizophr Bull. (1990) 16:263–75. doi: 10.1093/schbul/16.2.263
76. Xiang YT, Weng YZ, Leung CM, Tang WK, Chan SS, Wang CY, et al. Gender differences in sociodemographic and clinical characteristic and the quality of life of Chinese schizophrenia patients. Aust N Z J Psychiatry. (2010) 44:450–5. doi: 10.3109/00048670903489858
77. Ružić MC, Gajšak LR, and Grošić V. Differences in psychotic disorders between men and women. Psychiatria Danubina. (2024) 36:S17.
78. Mosharraf N, Estevez TP, Cohen LJ, and Lantz M. Long-acting injectable antipsychotics in the geriatric population: A longitudinal study. Am J Geriatr Psychiatry. (2024) 32:1420–30. doi: 10.1016/j.jagp.2024.06.007
79. Raedler TJ, Schreiner A, Naber D, and Wiedemann K. Gender-specific effects in the treatment of acute schizophrenia with risperidone. Pharmacopsychiatry. (2006) 39:171–4. doi: 10.1055/s-2006-948327
80. Lee SY, Park MH, Patkar AA, and Pae CU. A retrospective comparison of BMI changes and the potential risk factors among schizophrenic inpatients treated with aripiprazole, olanzapine, quetiapine or risperidone. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:490–6. doi: 10.1016/j.pnpbp.2010.12.003
81. Verma S, Liew A, Subramaniam M, and Poon LY. Effect of treatment on weight gain and metabolic abnormalities in patients with first-episode psychosis. Aust N Z J Psychiatry. (2009) 43:812–7. doi: 10.1080/00048670903107609
82. Tylec A, Skalecki M, Ziemecki P, Brzozowska A, Dubas-Slemp H, and Kucharska K. Assessment of cardiovascular disease risk factors in patients treated for schizophrenia. Psychiatr Pol. (2019) 53:1305–19. doi: 10.12740/PP/OnlineFirst/95123
83. Konarzewska B, Stefanska E, Wendolowicz A, Cwalina U, Golonko A, Malus A, et al. Visceral obesity in normal-weight patients suffering from chronic schizophrenia. BMC Psychiatry. (2014) 14:35. doi: 10.1186/1471-244X-14-35
84. Schoretsanitis G, Drukker M, Van Os J, Schruers KRJ, and Bak M. No differences in olanzapine- and risperidone-related weight gain between women and men: a meta-analysis of short- and middle-term treatment. Acta Psychiatr Scand. (2018) 138:110–22. doi: 10.1111/acps.12879
85. Gopal S, Lane R, Nuamah I, Copenhaver M, Singh J, Hough D, et al. Evaluation of potentially prolactin-related adverse events and sexual maturation in adolescents with schizophrenia treated with paliperidone extended-release (ER) for 2 years: A post hoc analysis of an open-label multicenter study. CNS Drugs. (2017) 31:797–808. doi: 10.1007/s40263-017-0437-9
86. During SW, Nielsen MO, Bak N, Glenthoj BY, and Ebdrup BH. Sexual dysfunction and hyperprolactinemia in schizophrenia before and after six weeks of D(2/3) receptor blockade - An exploratory study. Psychiatry Res. (2019) 274:58–65. doi: 10.1016/j.psychres.2019.02.017
87. Schneider-Thoma J, Dong S, Efthimiou O, Siafis S, Hansen WP, Scheuring E, et al. Sexual dysfunction and other prolactin-related side effects of antipsychotic drugs in schizophrenia. F1000Research. (2025) 13:973. doi: 10.12688/f1000research.154742.2
88. Malik P, Kemmler G, Hummer M, Riecher-Roessler A, Kahn RS, Fleischhacker WW, et al. Sexual dysfunction in first-episode schizophrenia patients: results from European First Episode Schizophrenia Trial. J Clin Psychopharmacol. (2011) 31:274–80. doi: 10.1097/JCP.0b013e3182199bcc
89. Rey-Sanchez P, Lavado-Garcia JM, Canal-Macias ML, Gomez-Zubeldia MA, Roncero-Martin R, and Pedrera-Zamorano JD. Ultrasound bone mass in schizophrenic patients on antipsychotic therapy. Hum Psychopharmacol. (2009) 24:49–54. doi: 10.1002/hup.984
90. Hummer M, Malik P, Gasser RW, Hofer A, Kemmler G, Moncayo Naveda RC, et al. Osteoporosis in patients with schizophrenia. Am J Psychiatry. (2005) 162:162–7. doi: 10.1176/appi.ajp.162.1.162
91. Shim JC, Shin JG, Kelly DL, Jung DU, Seo YS, Liu KH, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry. (2007) 164:1404–10. doi: 10.1176/appi.ajp.2007.06071075
92. Liu Q, Yuan X, Sheng C, Cai W, Geng X, Liu H, et al. Effect of long-term use of antipsychotics on the ventricular repolarization index. BMC Psychiatry. (2024) 24:505. doi: 10.1186/s12888-024-05947-1
93. Ozeki Y, Fujii K, Kurimoto N, Yamada N, Okawa M, Aoki T, et al. QTc prolongation and antipsychotic medications in a sample of 1017 patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. (2010) 34:401–5. doi: 10.1016/j.pnpbp.2010.01.008
94. Hreiche R, Morissette P, and Turgeon J. Drug-induced long QT syndrome in women: review of current evidence and remaining gaps. Gend Med. (2008) 5:124–35. doi: 10.1016/j.genm.2008.05.005
95. Elliott A, Mork TJ, Hojlund M, Christensen T, Jeppesen R, Madsen N, et al. QTc interval in patients with schizophrenia receiving antipsychotic treatment as monotherapy or polypharmacy. CNS Spectr. (2018) 23:278–83. doi: 10.1017/S1092852917000402
96. Cao H, Zhou Y, Li T, Yao C, Yang W, Kong S, et al. The prevalence, risk factors and clinical correlates of QTc prolongation in chinese hospitalized patients with chronic schizophrenia. Front Psychiatry. (2021) 12:704045. doi: 10.3389/fpsyt.2021.704045
97. Friedrich ME, Winkler D, Konstantinidis A, Huf W, Engel R, Toto S, et al. Cardiovascular adverse reactions during antipsychotic treatment: results of AMSP, A drug surveillance program between 1993 and 2013. Int J Neuropsychopharmacol. (2020) 23:67–75. doi: 10.1093/ijnp/pyz046
98. Centorrino F, MacLean E, Salvatore P, Kidwell JE, Fogarty KV, Berry JM, et al. Ziprasidone: first year experience in a hospital setting. J Psychiatr Pract. (2004) 10:361–7. doi: 10.1097/00131746-200411000-00004
99. Yang FD, Wang XQ, Liu XP, Zhao KX, Fu WH, Hao XR, et al. Sex difference in QTc prolongation in chronic institutionalized patients with schizophrenia on long-term treatment with typical and atypical antipsychotics. Psychopharmacol (Berl). (2011) 216:9–16. doi: 10.1007/s00213-011-2188-5
100. Sala M, Vicentini A, Brambilla P, Montomoli C, Jogia JR, Caverzasi E, et al. QT interval prolongation related to psychoactive drug treatment: a comparison of monotherapy versus polytherapy. Ann Gen Psychiatry. (2005) 4:1. doi: 10.1186/1744-859X-4-1
101. Suzuki Y, Sugai T, Fukui N, Watanabe J, Ono S, Tsuneyama N, et al. Sex differences in the effect of four second-generation antipsychotics on QTc interval in patients with schizophrenia. Hum Psychopharmacol. (2013) 28:215–9. doi: 10.1002/hup.2309
102. Irving J, Colling C, Shetty H, Pritchard M, Stewart R, Fusar-Poli P, et al. Gender differences in clinical presentation and illicit substance use during first episode psychosis: a natural language processing, electronic case register study. BMJ Open. (2021) 11:e042949. doi: 10.1136/bmjopen-2020-042949
103. Tang CT, Chua EC, Chew QH, He YL, Si TM, Chiu HF, et al. Patterns of long acting injectable antipsychotic use and associated clinical factors in schizophrenia among 15 Asian countries and region. Asia Pac Psychiatry. (2020) 12:e12393. doi: 10.1111/appy.12393
104. Cassara CM, Xu J, Hall DB, Chen X, Young HN, and Caballero J. Use and discontinuation rates of long-acting injectable antipsychotics between race/ethnicity in older adults using medicaid databases. J Am Geriatrics Soc. (2025) 73:1454–61. doi: 10.1111/jgs.19386
105. Grigoriadis S and Seeman MV. The role of estrogen in schizophrenia: implications for schizophrenia practice guidelines for women. Can J Psychiatry. (2002) 47:437–42. doi: 10.1177/070674370204700504
106. Martini F, Spangaro M, Buonocore M, Bechi M, Cocchi F, Guglielmino C, et al. Clozapine tolerability in Treatment Resistant Schizophrenia: exploring the role of sex. Psychiatry Res. (2021) 297:113698. doi: 10.1016/j.psychres.2020.113698
107. Hollingworth SA, Winckel K, Saiepour N, Wheeler AJ, Myles N, and Siskind D. Clozapine-related neutropenia, myocarditis and cardiomyopathy adverse event reports in Australia 1993-2014. Psychopharmacol (Berl). (2018) 235:1915–21. doi: 10.1007/s00213-018-4881-0
108. Liao CC, Wu SY, and Chou WC. Liver enzyme elevations in patients with schizophrenia on long-term antipsychotic treatment: The impact of alcohol consumption. J Clin Psychopharmacol. (2015) 35:295–300. doi: 10.1097/JCP.0000000000000331
109. Seeman MV. Secondary effects of antipsychotics: women at greater risk than men. Schizophr Bull. (2009) 35:937–48. doi: 10.1093/schbul/sbn023
110. Edinoff AN, Silverblatt NS, Vervaeke HE, Horton CC, Girma E, Kaye AD, et al. Hyperprolactinemia, clinical considerations, and infertility in women on antipsychotic medications. Psychopharmacol Bull. (2021) 51:131–48.
111. Young SL, Taylor M, and Lawrie SM. First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. (2015) 29:353–62. doi: 10.1177/0269881114562090
112. Kim J, Ozzoude M, Nakajima S, Shah P, Caravaggio F, Iwata Y, et al. Insight and medication adherence in schizophrenia: An analysis of the CATIE trial. Neuropharmacology. (2020) 168:107634. doi: 10.1016/j.neuropharm.2019.05.011
113. Brand BA, de Boer JN, Marcelis MC, Grootens KP, Luykx JJ, and Sommer IE. The direct and long-term effects of raloxifene as adjunctive treatment for schizophrenia-spectrum disorders: A double-blind, randomized clinical trial. Schizophr Bull. (2023) 49:1579–90. doi: 10.1093/schbul/sbad058
114. Sisek-Sprem M, Krizaj A, Jukic V, Milosevic M, Petrovic Z, and Herceg M. Testosterone levels and clinical features of schizophrenia with emphasis on negative symptoms and aggression. Nord J Psychiatry. (2015) 69:102–9. doi: 10.3109/08039488.2014.947320
115. Salehi MA, Zafari R, Mohammadi S, Shahrabi Farahani M, Dolatshahi M, Harandi H, et al. Brain-based sex differences in schizophrenia: A systematic review of fMRI studies. Hum Brain Mapp. (2024) 45:e26664. doi: 10.1002/hbm.26664
116. Lipkovich I, Deberdt W, Csernansky JG, Buckley P, Peuskens J, Kollack-Walker S, et al. Predictors of risk for relapse in patients with schizophrenia or schizoaffective disorder during olanzapine drug therapy. J Psychiatr Res. (2007) 41:305–10. doi: 10.1016/j.jpsychires.2006.07.016
117. Ghebrehiwet S, Baul T, Restivo JL, Kelkile TS, Stevenson A, Gelaye B, et al. Gender-specific experiences of serious mental illness in rural Ethiopia: A qualitative study. Glob Public Health. (2020) 15:185–99. doi: 10.1080/17441692.2019.1680723
118. Mayston R, Kebede D, Fekadu A, Medhin G, Hanlon C, Alem A, et al. The effect of gender on the long-term course and outcome of schizophrenia in rural Ethiopia: a population-based cohort. Soc Psychiatry Psychiatr Epidemiol. (2020) 55:1581–91. doi: 10.1007/s00127-020-01865-1
119. Barr SM, Roberts D, and Thakkar KN. Psychosis in transgender and gender non-conforming individuals: A review of the literature and a call for more research. Psychiatry Res. (2021) 306:114272. doi: 10.1016/j.psychres.2021.114272
120. Nolan CJ, Roepke TA, and Perreault ML. Beyond the binary: gender inclusivity in schizophrenia research. Biol Psychiatry. (2023) 94:543–9. doi: 10.1016/j.biopsych.2023.03.018
121. Riecher-Rossler A, Butler S, and Kulkarni J. Sex and gender differences in schizophrenic psychoses-a critical review. Arch Womens Ment Health. (2018) 21:627–48. doi: 10.1007/s00737-018-0847-9
122. Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, and Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. (2000) 107:411–29. doi: 10.1037/0033-295x.107.3.411
Keywords: sex differences, schizophrenia, symptomatology, adverse effects, personalized medicine
Citation: Moniem I and Kafetzopoulos V (2025) Sex differences in schizophrenia: symptomatology, treatment efficacy and adverse effects. Front. Psychiatry 16:1594334. doi: 10.3389/fpsyt.2025.1594334
Received: 15 March 2025; Accepted: 23 April 2025;
Published: 16 June 2025.
Edited by:
Renato de Filippis, University Magna Graecia of Catanzaro, ItalyReviewed by:
Héctor Carceller, Universitat de València, SpainRen-Jun Feng, Hainan Medical University, China
Copyright © 2025 Moniem and Kafetzopoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vasilios Kafetzopoulos, a2FmZXR6b3BvdWxvcy52YXNpbGVpb3NAdWN5LmFjLmN5
 Ivi Moniem
Ivi Moniem Vasilios Kafetzopoulos
Vasilios Kafetzopoulos