- 1Standardization of Computational Anatomy Techniques for Cognitive and Behavioral Sciences (SoCAT) Lab, Department of Psychiatry, School of Medicine, Ege University, Izmir, Türkiye
- 2Department of Psychiatry, Izmir City Hospital, Izmir, Türkiye
- 3Department of Psychiatry, Ege University, Izmir, Türkiye
- 4Department of Child and Adolescent Psychiatry, University of Health Sciences, Erenkoy Mental and Neurological Diseases Research, Istanbul, Türkiye
- 5Faculty of Sport Sciences, Erciyes University, Kayseri, Türkiye
- 6Department of Radiology, Ege University, Izmir, Türkiye
- 7Department of Psychiatry and Behavioral Sciences, Mercer University, School of Medicine, Mocon, GA, United States
Introduction: Neuronal plasticity, or the ability to change and adapt in response to experiences, learning, or environment, is frequently disrupted in schizophrenia and contributes to disease-associated cognitive deficits and functional impairments.
Methods: In this study, we investigated the neuroplasticity alterations of schizophrenia patients in the cortico-striato-cerebellar circuits associated with implicit learning using a reward-enhanced Serial Reaction Time Task (SRTT) by resting-state functional MRI (rs-fMRI). Forty-two schizophrenia patients and 25 healthy controls underwent pre- and post-task rs-fMRI to evaluate changes in functional connectivity.
Results: Behavioral results indicated that all participants demonstrated shorter reaction times during sequential blocks, schizophrenia patients exhibited lower accuracy suggesting diminished implicit learning. Schizophrenia patients exhibited increased connectivity across cortico-striatocerebellar circuits, which became even more robust and widespread following task completion. Despite impaired performance, this post-task hyperconnectivity may reflect a compensatory mechanism attempting to recruit additional neural resources—albeit in a dysfunctional or inefficient manner. Data-driven analyses confirmed the post-task differences between groups, identifying task-induced connectivity changes in thalamo-cortico-cerebellar circuits as the strongest predictors of a group membership.
Discussion: These findings underscore the role of neuroplasticity impairments in schizophrenia-related cognitive deficits, highlighting potential neural markers for clinical differentiation and paving the way for targeted interventions.
1 Introduction
Schizophrenia is a mental disorder affecting approximately 1% of the global population, leading to significant functional impairments (1). Accumulated evidence suggests that cognitive symptoms are the mainstay factor for functional impairments in patients. Difficulties in acquiring, encoding, and retrieving new information—along with challenges in integrating previously learned material—are common cognitive impairments that significantly contribute to functional disability (2). It is thought that the cognitive symptoms stem from disrupted neuroplasticity, hindering the brain’s ability to process environmental stimuli essential for daily functioning (3). One major challenge in studying neuroplasticity in the in vivo brain is the limited availability of applicable tools. However, resting-state functional MRI (rs-fMRI), which captures changes in the functional coupling between brain regions following learning, has emerged as a promising method for investigating neuroplasticity-related alterations (4).
The Serial Reaction Time Task (SRTT) is commonly used to assess implicit learning—the unconscious acquisition of skills or knowledge—which is often impaired in individuals with schizophrenia. Reaction times (RTs) typically decrease as they become familiar with the predetermined sequence of cues but show a rebound when random stimuli are introduced. (5–7). The subject learns the predetermined sequence implicitly during the task, so, neuroplasticity is expected to occur in related brain regions. Neuroimaging studies revealed that SRTT primarily engages the motor cortex, premotor cortex (PMC), parietal cortex, basal ganglia, and medial temporal lobe (8–18). Indeed, theoretical models and experimental findings highlight the roles of parallel cortico-cerebellar and cortico-striatal circuits in procedural implicit learning (19–21). In the early stages of learning, the cerebellum facilitates motor adaptation and rapid skill acquisition, while the striatum plays a more prominent role in consolidating learned skills over time. Functional connectivity studies suggest a shift in connectivity during learning, decreased cerebellar and increased striatal connectivity to the motor cortex, reflecting the transition from adaptation to consolidation (19).
In the current study, we incorporated a reward-enhanced SRTT protocol between two rs-fMRI sessions to assess neuroplasticity deficits in the cortico-striato-cerebellar circuit and evaluate differences between schizophrenia patients and healthy controls. Based on the literature, we propose that the altered neuroplasticity in schizophrenia patients compared to healthy controls can be assessed by observing functional connectivity changes after SRTT. We included a reward component in SRTT to investigate its modulatory effect on learning and neuroplasticity, given that it has been shown to modulate both implicit and explicit learning (22) and enhance striatal-prefrontal connectivity in motor tasks (23). The use of a reward-enhanced SRTT may help to alleviate motivational deficits associated with negative symptoms and, in turn, enhance cortico-striatal activation during task performance. Thus, this study also highlights the potential role of motivation and reward mechanisms in mitigating these deficits.
2 Materials and methods
2.1 Participants
A sample of 44 participants with a DSM-5 diagnosis of schizophrenia (age; mean + SD: 35.23 ± 7.77) from the Department of Psychiatry, Ege University School of Medicine, and 26 right-handed healthy controls (age; mean + SD: 36.35 ± 8.04) with similar age, sex distribution, and education were enrolled in this study. The inclusion criteria were: 1) between 18 and 45 years, 2) diagnosis of schizophrenia for at least one year, and have no comorbid psychiatric diagnoses, including substance or alcohol use disorder, and no past present or past psychiatric diagnosis for the controls; 3) for patients, currently in remission according to Andreasen criteria (24) and clinically stable for the past three months (i.e. no changes in symptoms severity requiring interventions such as medication adjustments or hospitalization) 4) completing at least 8 years of basic primary school education 5) using the right hand. The exclusion criteria for both patient and control groups were: 1) unstable chronic or systemic medical diseases; 2) a history of loss of consciousness lasting longer than 3 minutes; 3) the presence of any lesion or space-occupying mass in the MRI; 4) any condition that prevents scanning in a magnetic field (such as having a pacemaker, prosthetics, pregnancy, or claustrophobia).
Participants were enrolled in the study between July 2020 and February 2023. The study was approved by the Ethics Committee of Ege University (approval number 19-12T/42, approval date December 11, 2019) and conducted following the principles of the Declaration of Helsinki from September 2020 to July 2024. After explaining the aim of the study, informed consent was obtained from the patients and their relatives.
2.2 Clinical assessments
Trained psychiatrists (ACH, FY) conducted interviews with all participants using the Structured Clinical Interview for DSM 5 (SCID) to confirm diagnoses. Patients were assessed with the following scales: the Positive and Negative Syndrome Scale (PANSS), the Calgary Depression Scale (CGS), the Extrapyramidal Symptom Rating Scale (ESRS), the Edinburgh Handedness Inventory (EHI) to confirm right-handedness, and the Montreal Cognitive Assessment (MoCA) within the week of MRI scans.
2.3 Experimental design and procedure
Two resting-state fMRI scans were conducted on participants—one before and one after the Serial Reaction Time Task (SRTT). (Figure 1).
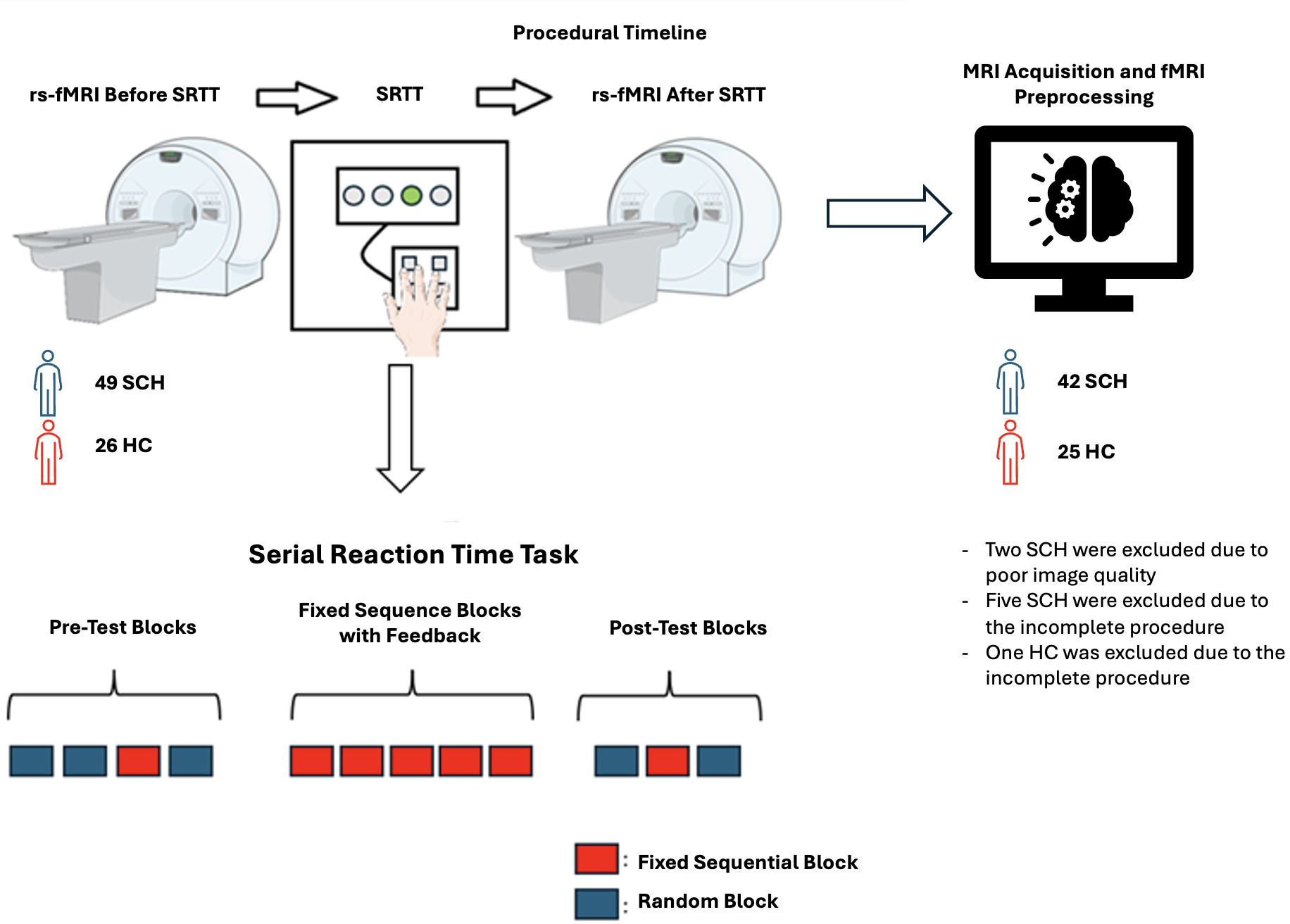
Figure 1. Experiment/Procedure Timeline (Figures contain modified Images from Servier Medical Art (https://smart.servier.com) licensed by a Creative Commons Attribution 3.0 Unported License.
2.3.1 Serial reaction time task
The Serial Reaction Time Task (SRTT) was developed in-house using the Borland Delphi software version 7. In this task, a visual cue appears at one of four horizontally arranged positions on a computer screen, labeled 1 through 4, corresponding to specific keys on the keyboard (‘g,’ ‘h,’ ‘j,’ ‘k’). At the onset of each trial, when the stimulus is presented (i.e., the gray box turns white), the participant must press the appropriate key using the designated fingers. A correct response within 800 ms causes the box to turn green, while a late or incorrect response triggers a red “error” message, which is displayed for 100 ms. The reaction time (RT), defined as the time taken to press the correct key, is recorded for each trial. Thus, reaction time, which is the primary performance measure, cannot exceed 800 ms.
The task consists of 12 blocks. Each block contains 96 trials and is separated by a 3000 ms rest period. Initially, the visual stimuli are presented randomly, followed by a fixed sequential order (e.g., 3-4-1-2-3-1-4-3-2-4-2-1) for implicit learning. Afterward, the sequence is randomized again, and the participant’s RT is recorded for each trial (4).
The first two blocks serve as “practice random blocks,” where stimuli are presented randomly, ensuring no stimulus appears more than twice consecutively. The third block introduces the “fixed sequential sequence” (e.g., 3-4-1-2-3-1-4-3-2-4-2-1), which is repeated eight times within the block. This block is referred to as the “pre-test fixed sequential block”. The fourth block returns to random stimuli. Blocks five through nine consist of the fixed sequential sequence, but with feedback provided during the 3000 ms rest periods between blocks. These blocks are referred to as the “fixed sequential blocks with feedback”. Feedback is contingent on the participant’s performance: if the error rate in a block is below 5%, the feedback message “you won money” is displayed; otherwise, “try harder!” is shown.
The tenth and twelfth blocks consist of random stimuli, while the eleventh block is another fixed sequential block without feedback. This block is referred to as the “post-test fixed sequential block”. Participants are informed prior to the task that they will earn a monetary reward; with the amount they can earn determined by their performance. After completing the task, participants were asked whether they could identify the presence of the sequence and, if they could, recall the sequence (25). Subsequently, all participants received a monetary reward of $10.
2.4 MRI acquisition and fMRI preprocessing
MRI Acquisition and fMRI preprocessing details are given in Supplementary Material with a standard minimal preprocessing driven by CONN’s default pipeline (26).
2.5 Image and statistical analysis
2.5.1 fMRI data analyses
Before and after the given task (SRTT), rs-fMRI was conducted to detect brain functional changes resulting from implicit learning in the schizophrenia group compared to the healthy control group. This analysis was performed using a 2x2 ANOVA generalized linear model (GLM) with the Functional Connectivity Toolbox (CONN Toolbox/(RRID: SCR_009550) release 22.a) based on MATLAB/Statistical Parametric Mapping (SPM/(RRID: SCR_007037) release 12.7771). Outliers were identified and removed through the CONN, yielding a final sample of 45 schizophrenia patients and 22 healthy controls.
Based on the previous studies about motor learning and executive tasks, we implemented ROI-to-ROI analysis of the posterior cingulate cortex (PCC), insular cortex (IC), thalamus, caudate nucleus, putamen, pallidum, nucleus accumbens, cerebellum’s subdivisions bilaterally and the left premotor area’s subdivisions (27) (Figure 2).
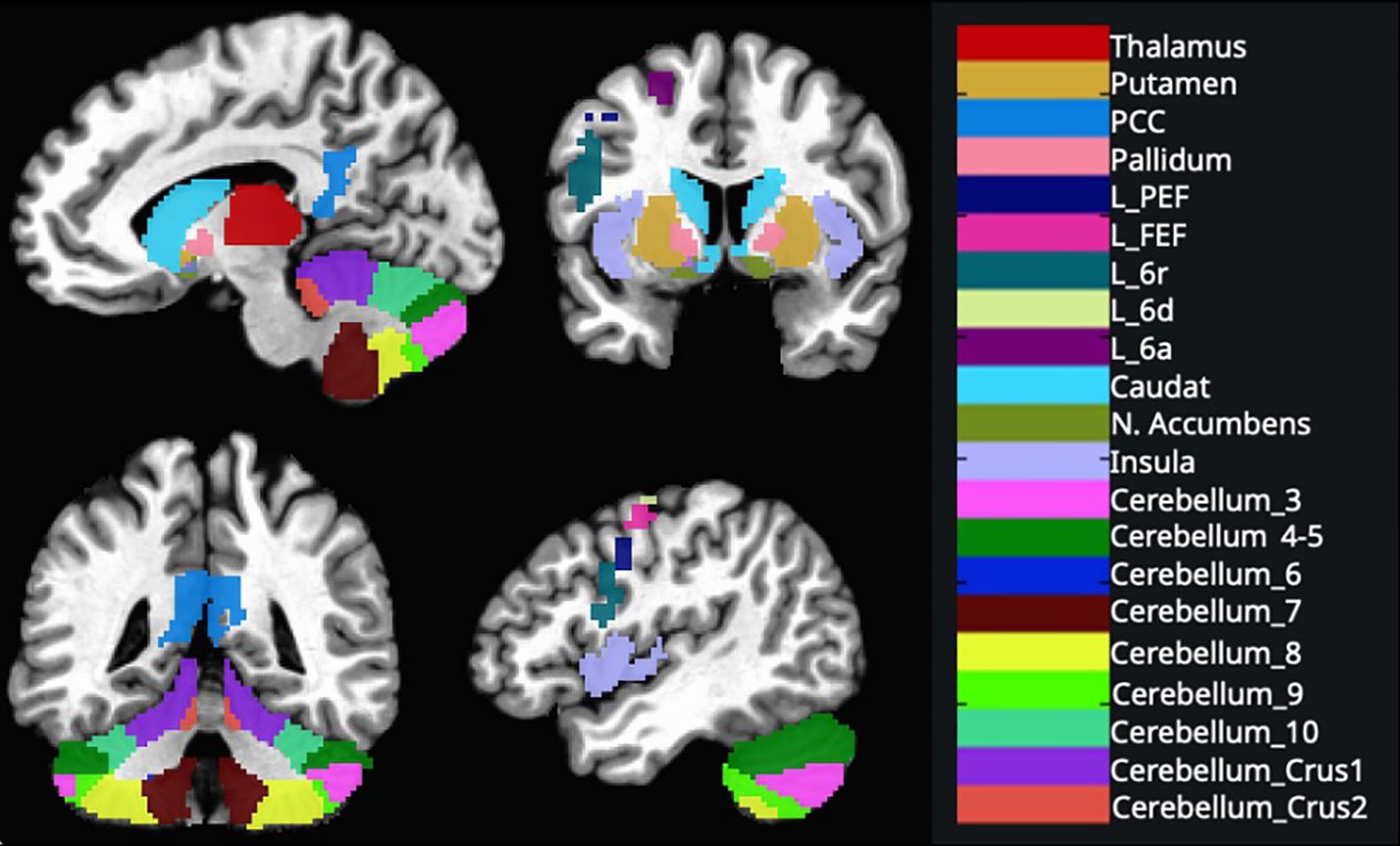
Figure 2. These regions were created using the AAL 3v1 atlas and overlaid on the MNI152 template brain and AFNI’s 3dcalc tool. (L, Left; FEF, frontal eye field; PCC, posterior cingulate cortex; PEF, posterior eye field) See Supplementary Material for ROI coordinates.
Functional and anatomical data were preprocessed using a modular preprocessing pipeline, which included realignment with correction of susceptibility distortion interactions, slice timing correction, outlier detection, direct segmentation and MNI-space normalization, and smoothing.
After preprocessing, data from 67 participants were used to generate seed-based connectivity maps (SBC) and estimate ROI-to-ROI connectivity matrices (RRC), characterizing functional connectivity patterns across 183 ROIs. In addition to the CONN toolbox default ROI parcellation, we prepared ROIs from glasser parcellation to investigate the premotor region more deeply.
The Glasser HCP atlas (MNI_Glasser_HCP_v1.0_LPI_2009c_resampled.nii.gz) in AFNI was used to distinguish the superior and inferior premotor subdivisions in the bilateral hemispheres. According to the Glasser atlas, the superior premotor subdivisions include 6d, 6a, and the frontal eye field (FEF), while the inferior premotor subdivisions include 6v, 6r, and the premotor eye field (PEF). These subdivisions were extracted for both the left and right hemispheres. Binary masks for each premotor region of interest (ROI) were created using AFNI’s 3dcalc tool.
Functional connectivity strength was represented by Fisher-transformed bivariate correlation coefficients computed between each pair of seed and target ROIs, modeling the association between their BOLD signal time series. For each condition (pre-task and post-task), connectivity was estimated using the default weighted correlation measures provided by the CONN toolbox. Group-level analyses were performed using a General Linear Model (GLM). Separate between-group comparisons (schizophrenia vs. control) were conducted for the pre-task and post-task resting-state conditions. For each ROI pair, connectivity values served as the dependent variable, with group as the independent variable. Inferences were assessed using cluster-level statistics across ROI-to-ROI connections based on parametric multivariate statistics (28), with a cluster-level false discovery rate (p-FDR < 0.05) threshold and connection-level p < 0.05 uncorrected threshold.
2.5.2 Behavioral data analyses
Behavioral data recorded during the SRTT were analyzed using a mixed-design variance analysis: 2 (group: control and schizophrenia) x 2 (sequence type: random and sequential) x 2 (test time: pre-test and post-test) to investigate whether there were differences in mean RT and the number of correct responses between groups in pre-test and post-test sequential and random blocks. Bonferroni correction was applied for Tukey’s post-hoc analyses. All results were considered significant at an alpha level of 0.05, and effect sizes were reported as partial eta squared.
To assess the changes in average RT and the number of correct responses during the five blocks of the fixed sequential sequence between the groups, each block was included in a 2 (group: control and schizophrenia) x 5 (block 5-6-7-8-9) ANOVA. Bonferroni correction was also applied for post-hoc analyses. Again, all results were considered significant at an alpha level of 0.05, and effect sizes were reported as partial eta squared. A correlation analysis was performed between the behavioral data and brain imaging results of the two groups.
We used the Shapiro-Wilk test was used to assess normality. Independent sample t-tests were conducted to compare the age differences, Pearson Chi-Square for gender differences, and Mann Whitney U for education year differences between patients and controls. We conducted a correlation analysis between age-task performance, clinical characteristics-functional connectivity, and behavioral data-functional connectivity with Pearson and Spearman correlation, respectively.
2.5.3 Classification method
Here, our primary aim was to investigate how neural alterations and behavioral outcomes after task completion contribute to the classification of subjects. Specifically, we analyzed “after > before” and “schizophrenia > control” contrasts using regions identified with p <.05, uncorrected, in CONN. We included both conditions separately without performing subtraction to evaluate whether post-task measurements were better predictors than pre-task measurements.
Subsequently, we applied a False Discovery Rate (FDR) correction and selected significant ROIs for further analysis. We included mean reaction time and accuracy rate as additional variables as part of a control analysis. Given the high multicollinearity among predictors, we employed Principal Component Analysis (PCA) with scaling to prepare the final predictor matrix.
For classification, we utilized the Support Vector Machine (SVM) approach. To classify the data, we implemented a Support Vector Machine (SVM) with a radial basis function (RBF) kernel using the caret package in R. Hyperparameter optimization was performed to tune the kernel width (σ) and the regularization parameter (C). A 10-fold cross-validation procedure repeated 10 times (i.e., 10x10 repeated cross-validation) was employed to ensure robust model evaluation and to mitigate overfitting. The tuning process involved a grid search over the σ∈ {0.01, 0.1, 0.5, 1} and C ∈ {0.01, 0.1, 1, 10, 100}.
Model performance during tuning was evaluated using the area under the receiver operating characteristic curve (AUC-ROC) as the metric. The optimal hyperparameters (sigma = 0.01 and C = 0.01) were selected based on this criterion. Subsequently, the dataset was randomly partitioned into training (80%) and testing (20%) sets using stratified sampling. The final SVM model was retrained on the training set using the optimal hyperparameters and evaluated on the testing set. After training and testing the model, we conducted a permutation analysis on the principal components to assess their relationship with clinical classification.
To evaluate the contribution of each principal component (PC) to the SVM classifier’s performance, we conducted a permutation-based feature importance analysis on the test set. This procedure quantifies how much the model’s predictive ability deteriorates when the values of a given PC are randomly permuted, thereby disrupting its relationship with the target variable.
Specifically, for each principal component, the values of the component in the test set were independently permuted while keeping all other components unchanged. Afterwards, predictions were generated using the trained SVM model on the permuted test data. The area under the receiver operating characteristic curve (AUC-ROC) was calculated based on those predictions. Lastly, the AUC drop was computed by subtracting the AUC of the permuted data from the original (unpermuted) AUC, representing the importance of that component. This process was repeated for each PC, and the resulting AUC drops were used to rank the components by their contribution to the model’s performance.
Finally, to enhance generalizability and reduce bias, we included all functional connectivity (FC) between regions—both pre-task and post-task—as predictors in the SVM model. This approach ensured that the classification was not limited to features that specifically differentiated the schizophrenia (SZ) and healthy control (HC) groups, thereby minimizing the risk of overfitting to group-specific patterns.
3 Results
3.1 Demographic and clinical characteristics
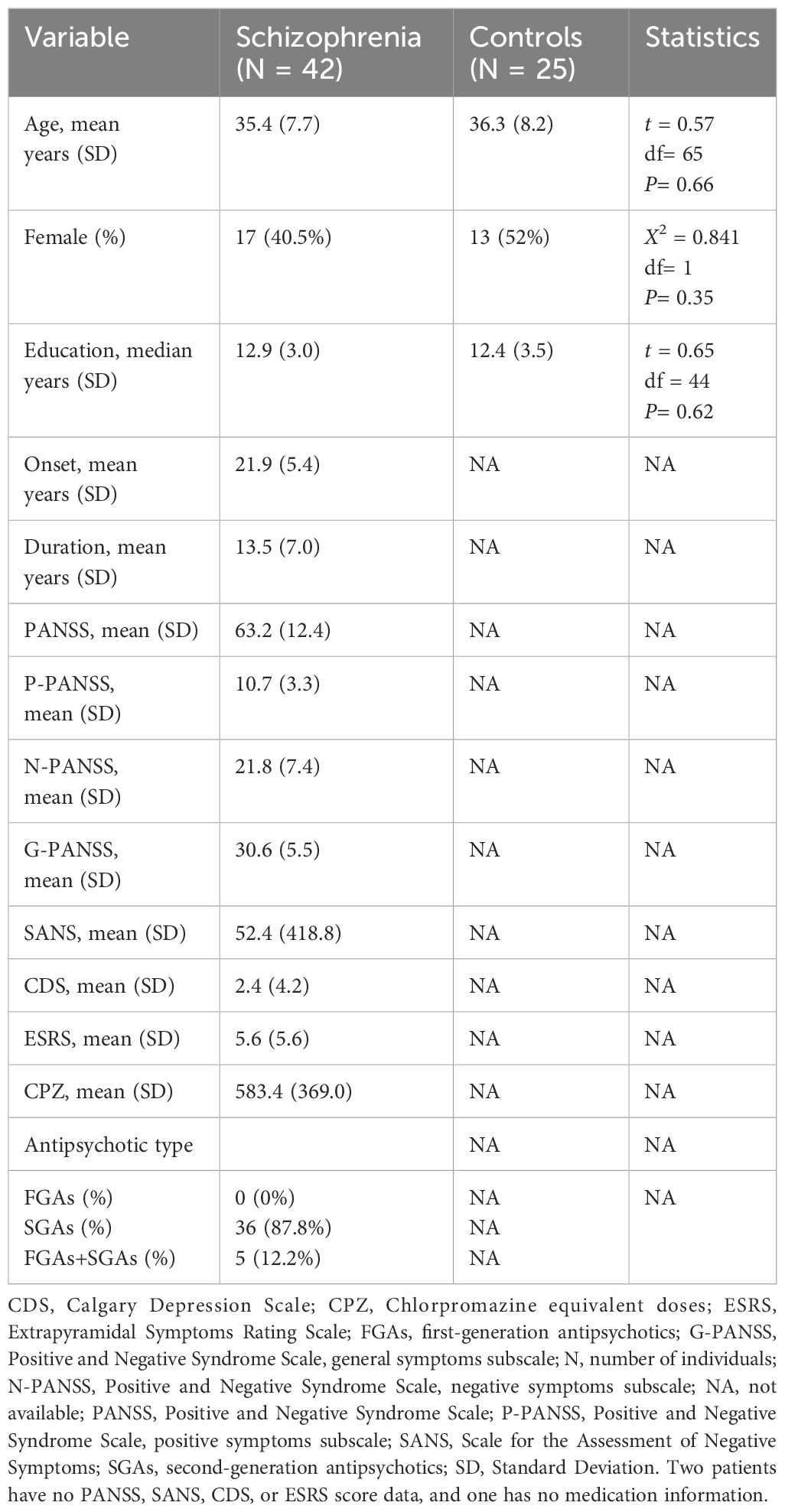
After excluding eight participants (two due to poor image quality and six for incomplete procedural data), the final sample consisted of 67 participants, with 42 patients and 25 controls. The two groups were similar in age, gender, and education (Table 1).
3.2 Behavioral data
3.2.1 SRTT performance in pre-test and post-test blocks: mean RT and correct responses
As expected, all the participants showed better performance for RT during the post-test compared to the pre-test (F(1,65) = 86.49, p < 0.001, η² = 0.57) (Figure 3a). There were no group effects among patients and controls, suggesting both groups showed similar performance (F(1,65) = 1.35, p = 0.40, η² = 0.02) (Figure 3b). Additionally, all participants showed better performance in the sequential blocks during both the pre-test and post-test (F (1,65) = 11.90, p = 0.003, η² = 0.15). Patients showed poorer performance than controls in the post-test for correct responses (F(1,65) = 7.72, MD = 10.91, SE = 3.92, p = 0.007) (Figure 3d), despite no significant difference between the groups in the pre-test (F(1,65) = 2.93, MD = 6.11, SE = 3.56, p = 0.09) (Figure 3c). This finding suggests that, compared to controls, patients demonstrated reduced effectiveness in sequence acquisition.
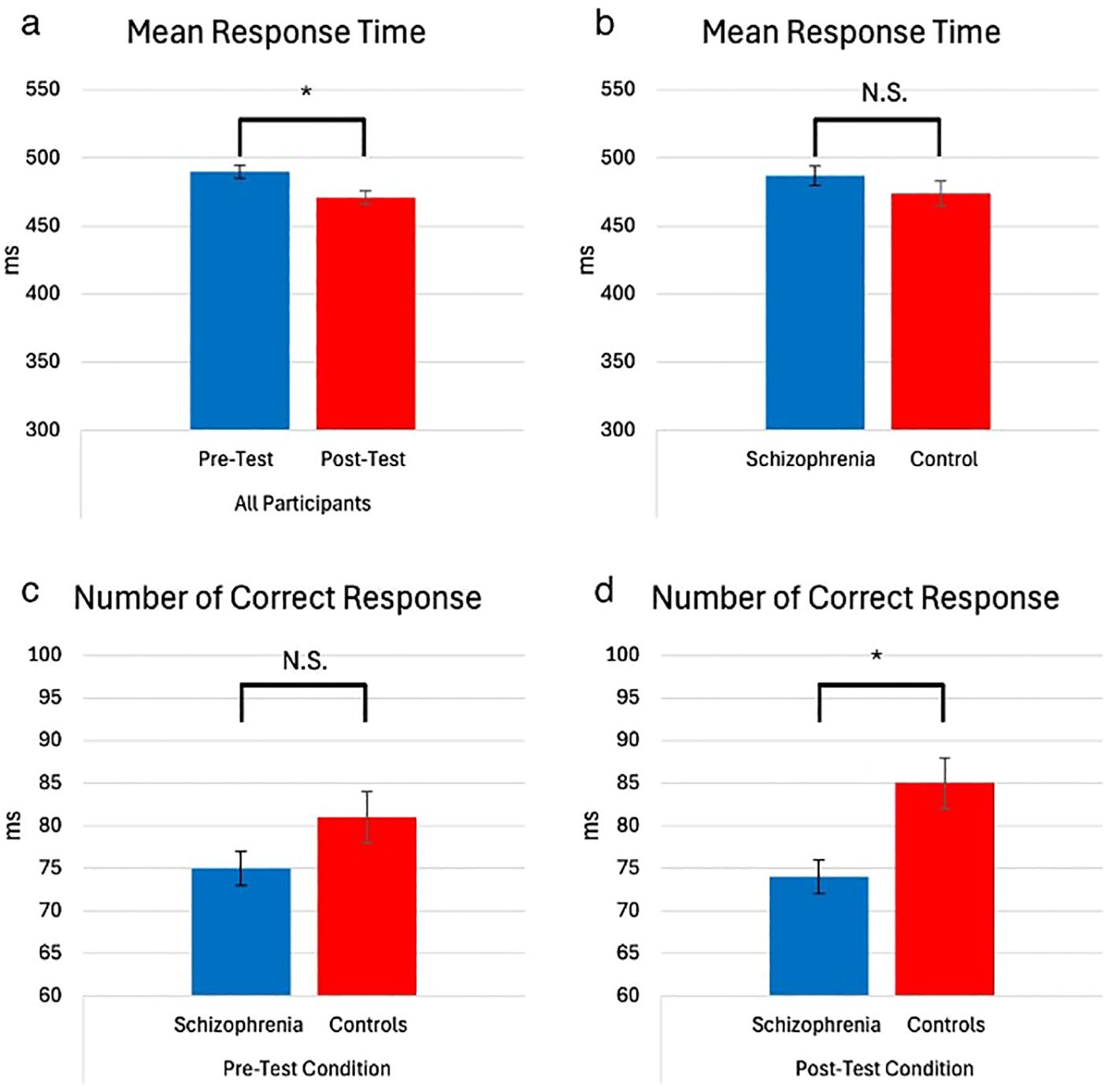
Figure 3. Mean RT (milliseconds) and number of correct responses of participants, test time, and group (with 95% Confidence Interval). (* = p<0.05) The main effect of test time on mean reaction time (RT). Participants showed significantly shorter RTs in the post-test (M = 471.21, SE = 5.88) compared to the pre-test (M = 490.25, SD = 5.73). No group-related interactions were observed, which mainly affected the number of correct responses. In the pre-test, no significant difference in performance between groups was observed (M_SCZ = 75.53, SE = 2.17 vs. M_HC = 81.64, SE = 2.82), but in the post-test, the control group outperformed the schizophrenia group (M_SCZ = 74.10, SE = 2.39 vs. M_HC = 85.02, SE = 3.10) (a) Mean Response Time – All Participants (Pre-Test vs. Post-Test). (b) Mean Response Time – Schizophrenia vs. Control. (c) Number of Correct Responses – Pre-Test Condition (Schizophrenia vs. Controls). (d) Number of Correct Responses – Post-Test Condition (Schizophrenia vs. Controls). NS, not significant.
3.2.2 SRTT performance in fixed sequential blocks with feedback: mean RT and correct responses
As the blocks progressed, both groups responded faster, and the mean reaction times gradually decreased (F (1,65) = 17.45, p < 0.001, η² = 0.21) (Figure 4a). There were no group effects on RT (main effect, F (1,65) = 2.44, p = 0.81; group effect, F (1,65) = 0.13, p = 0.95). Nevertheless, regardless of block progression, patients exhibited a lower number of correct responses compared to controls (Figure 4b).
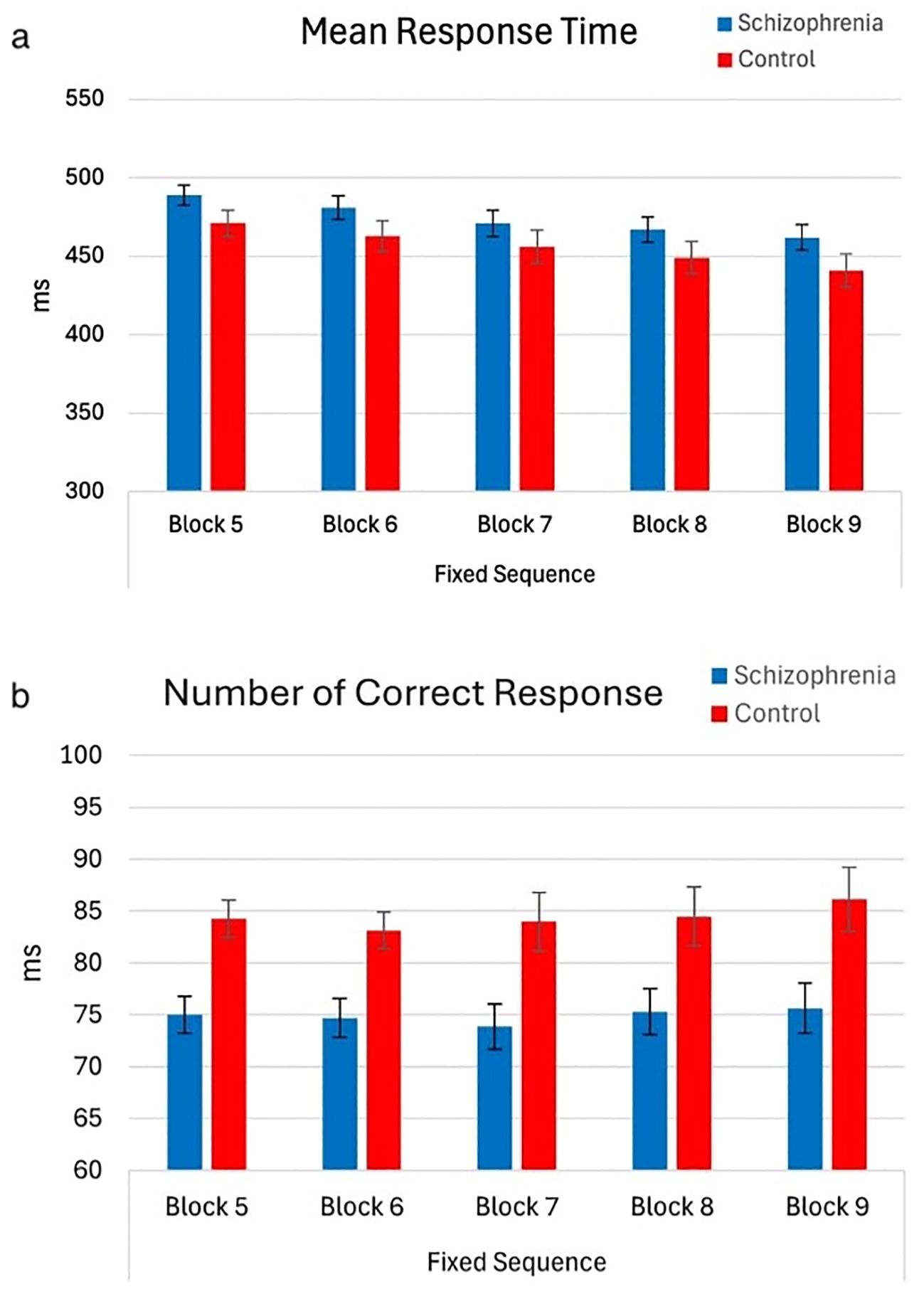
Figure 4. Mean RT and number of correct responses of participants by block order (with 95% Confidence Interval). The main effect of block order on mean reaction time (RT) during the fixed-test period. A significant decrease in mean RT was observed as blocks progressed, with Block 5 (M = 479.86, SE = 5.27) showing slower RTs compared to Block 6 (M = 471.79, SE = 6.17), Block 7 (M = 463.59, SE = 6.71), Block 8 (M = 457.97, SE = 6.51), and Block 9 (M = 451.34, SE = 6.63), indicating a gradual reduction in RTs across blocks. However, the main effect of the group on mean RT was not significant (F(1,65) = 2.44, p = 0.12), nor was the interaction between block order and group (F(1,65) = 0.13, p = 0.81). The main effect of the group on the number of correct responses during the fixed-test period. The number of correct responses in the schizophrenia group (Mean = 74.89, SE = 2.21) is lower than control (Mean = 84.38, SE = 2.86). However, the main effect of block order on the number of correct responses was not significant (F (1,65) = 1.52, p = 0.22), nor was the interaction between block order and group (F (1,65) = 0.38, p = 0.81) (a) Mean Response Time – Fixed Sequence (Blocks 5 to 9). (b) Number of Correct Responses – Fixed Sequence (Blocks 5 to 9).
3.3 Imaging results
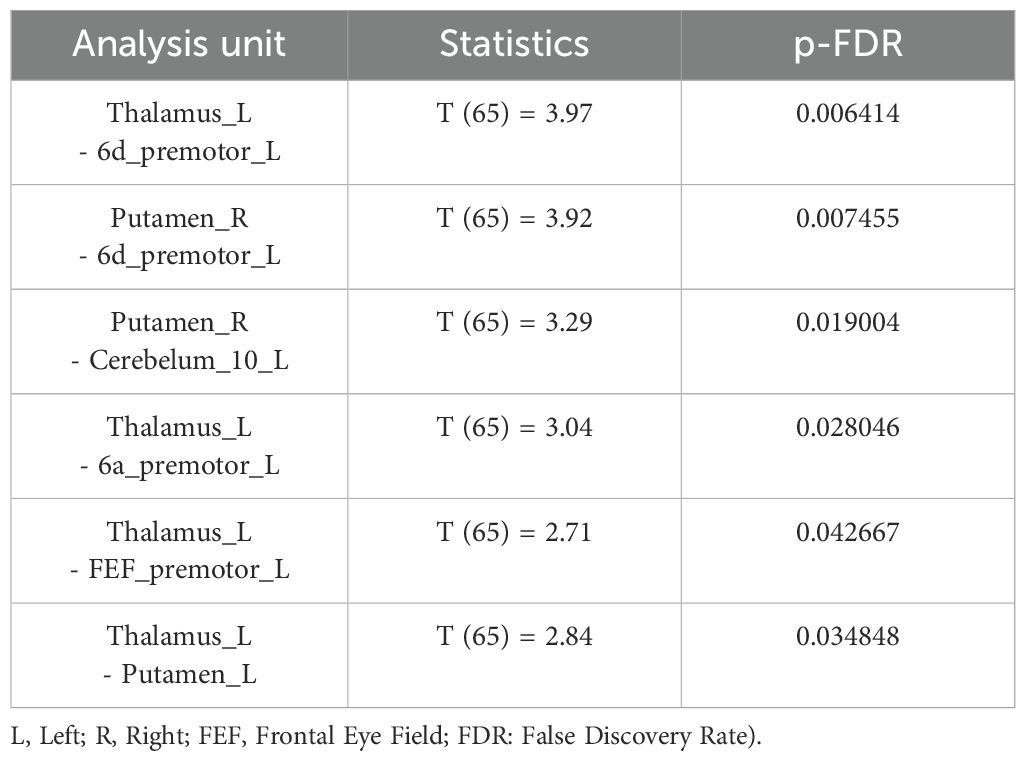
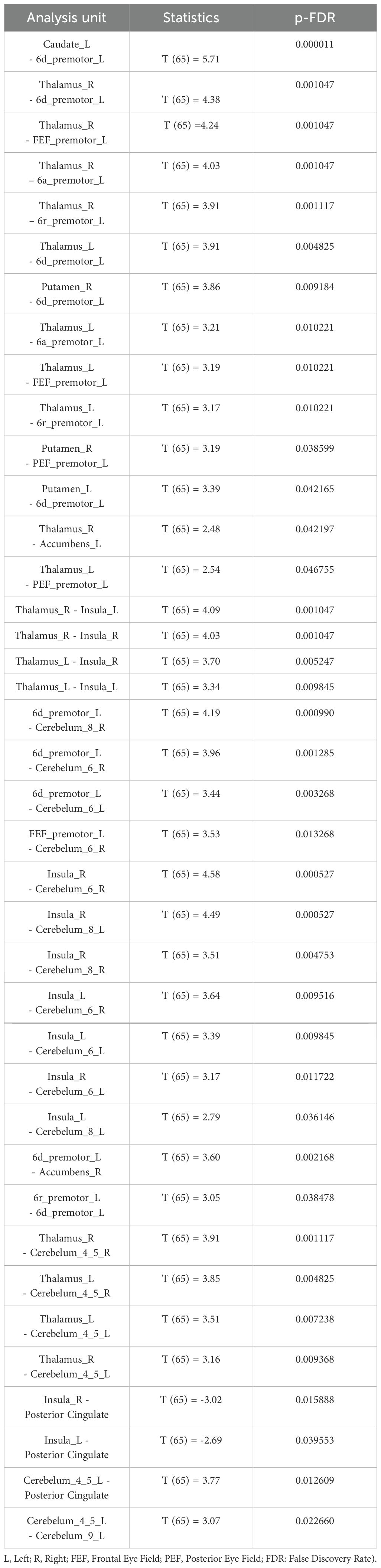
In the group comparison at the baseline, schizophrenia patients had higher connectivity between thalamus and putamen with left premotor regions (6a, 6d, FEF) (Table 2). Additionally, patients showed higher connectivity between thalamus with putamen and cerebellum (Table 2, Figure 5). After the task, patients showed increased connectivity between all subdivisions (6a, 6d, 6r, FEF, PEF) of the left premotor cortex (PMC) and the striatum (caudate and putamen), thalamus, cerebellum, and nucleus accumbens (Table 3, Figure 5). Notably, nucleus accumbens increased connectivity with thalamus. The cerebellum also exhibited increased intra-cerebellar functional connectivity and connectivity with the thalamus and PCC. Insula showed increased connectivity with the thalamus and cerebellum, while decreased connectivity between insula and PCC persisted.
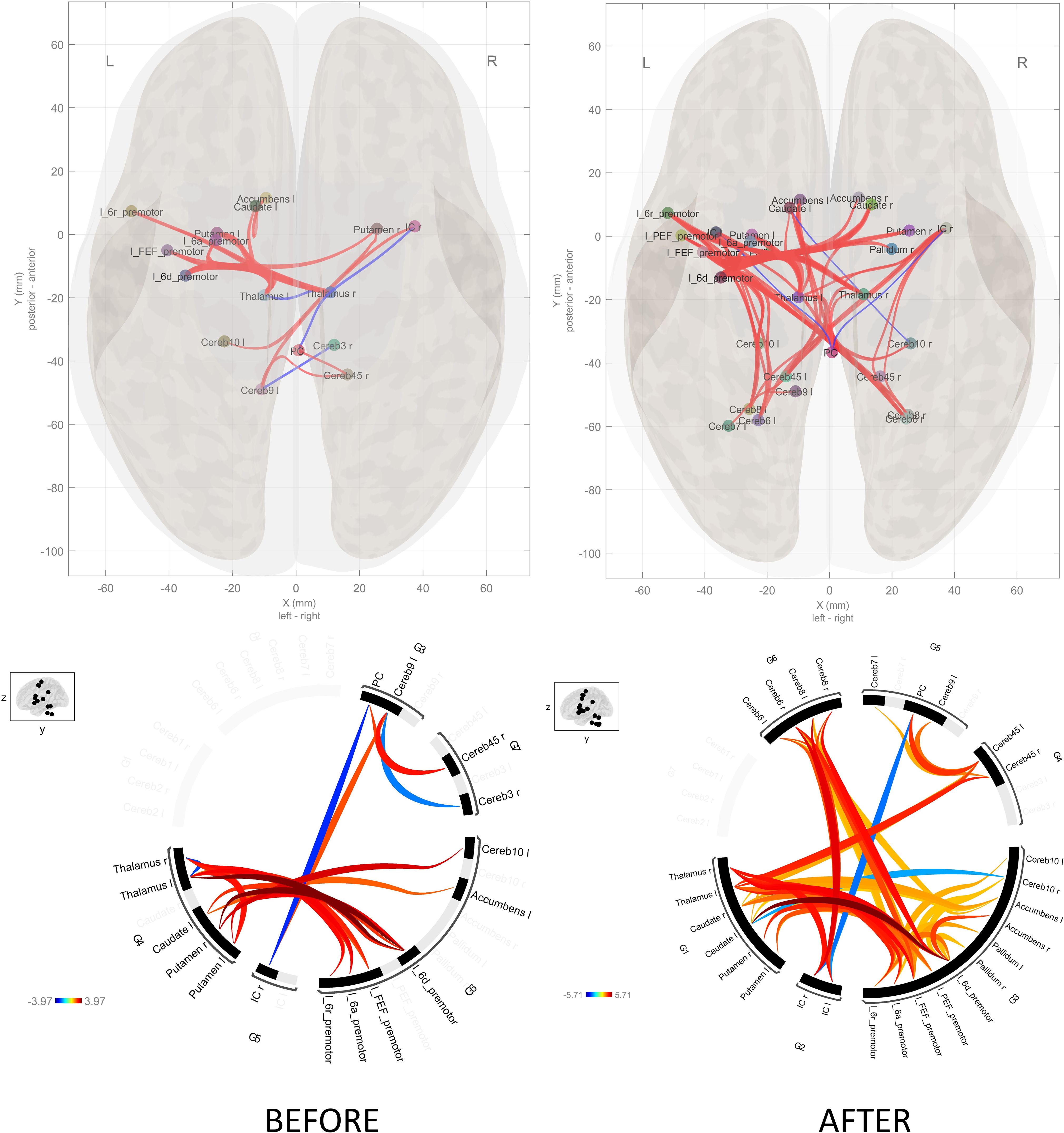
Figure 5. Schizophrenia versus controls connectome diagram before and after the SRTT. The connectome diagram demonstrates the summing of the functional connectivity of the healthy controls from the functional connectivity of the schizophrenia patients. There is an increase in functional connectivity after the SRTT.
We could not observe any difference between baseline and post-task within the groups.
3.4 Classification results
We conducted an analysis to evaluate how effectively task could distinguish between two clinical groups, using a classification method called Support Vector Machine (SVM). We built a model based on data from brain regions of interest (ROIs), including differences in activity before and after task, response times, and number of correct responses (see Method - Classification Method). To fine-tune the model, we optimized its settings to achieve the best performance, measured by an evaluation metric Area Under the Curve (AUC). The model performed well, achieving an overall AUC of 0.95 on train dataset, and a perfect score of 1 on the test dataset.
To evaluate the contribution of each principal component (PC) to the AUC, we permuted individual components to dissociate them from the group labels. The results showed a 0.3 drop in AUC on the test set when PC1 was excluded from the prediction, while no other principal components caused a significant drop. This indicates that PC1 influences group discrimination the most (See Figure 6A). The top five loading weights of PC1 were associated with the ‘after’ condition, including connectivities between Right Insular Cortex – Right Cerebellum 6 (weight (w) = 0.29), Right Thalamus – Right Insular Cortex (w= 0.26), Left Premotor 6d – Right Cerebellum 6 (w=0.26), Left Thalamus – Right Insular Cortex (w=0.25), and Right Thalamus – Left Insular Cortex (w=0.25), as shown by the thick lines in Figure 6B. These findings suggest that the thalamo-cortico-cerebellar circuit, also the premotor region, plays a significant role in differentiating schizophrenia from healthy controls. It is important to note that the behavioral data had a low weight on PC1, indicating that it is not a strong predictor for distinguishing between the clinical groups (Mean reaction time = 0.04, Number of correct responses = -0.05). A support vector machine (SVM) model classified patients and controls with high accuracy (AUC = 0.95–1), with task-induced connectivity changes in thalamo-cortico-cerebellar circuits emerging as the strongest predictors of a group membership. Although we conducted this study a priori, data-driven analyses confirmed the post-task differences between both groups. For a control analysis, when we included the all ROIs FC in pre- and post-task, AUC was 1 for the test group, highlighting that FC success for the group discrimination not only when group differences are considered as predictors.
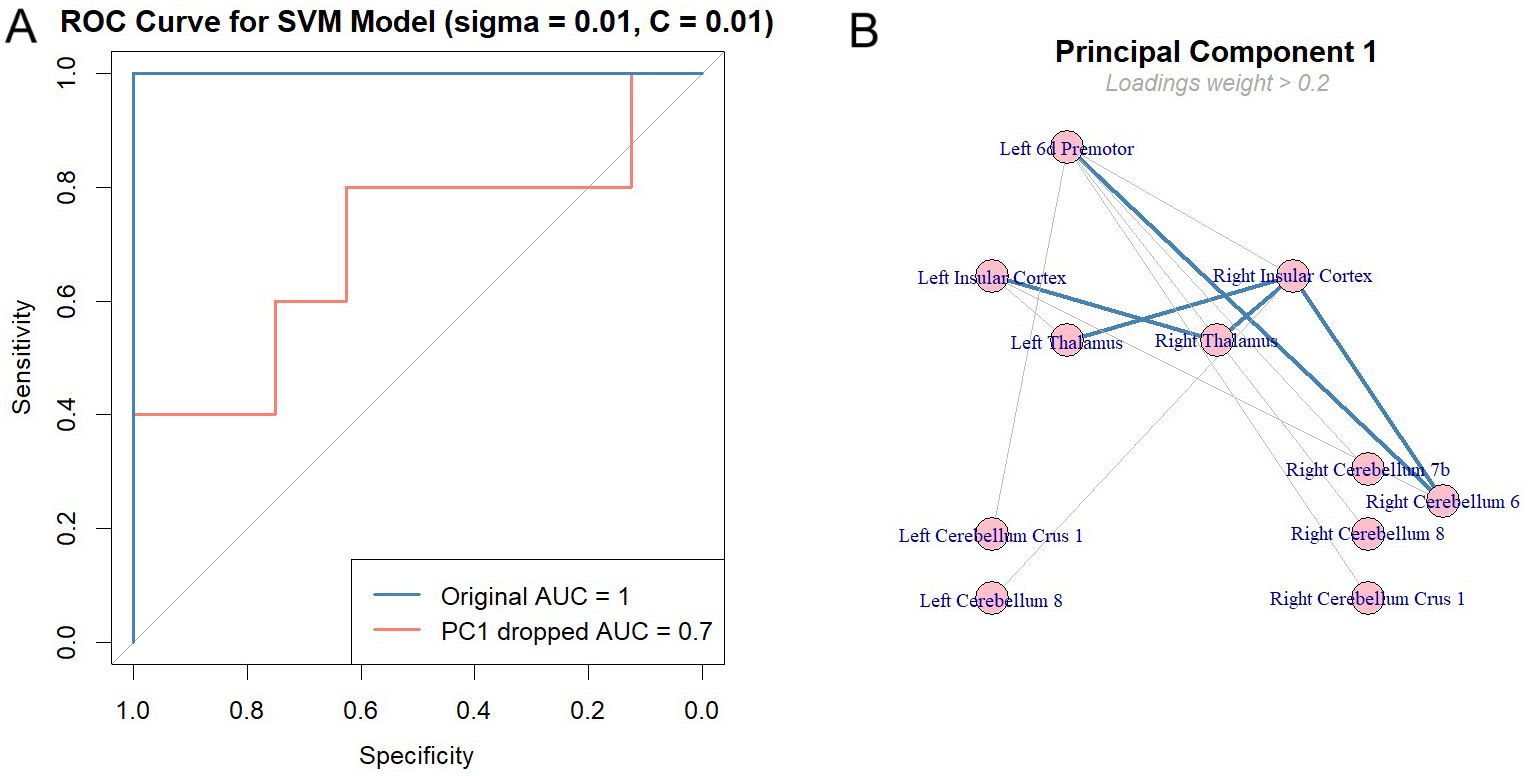
Figure 6. Classification of clinical groups by task effect. (A) The Support Vector Machine (SVM) model was trained on 80% of the data using a 10x10 nested cross-validation approach to ensure reliable and robust performance. Hyperparameters, specifically sigma and C, were optimized through a grid search, with both values fixed at 0.01 as they yielded the best performance. The model achieved an initial AUC of 1, predicting group classifications in the test set. To assess the contribution of each principal component (PC), we permuted them individually and observed the impact on AUC. Only the first principal component (PC1) caused a significant drop in AUC, decreasing it by 0.3. These results indicate that PC1 plays a substantial role in explaining the predictors’ variance and in maintaining the model’s ability to predict class labels accurately. (B) shows the components of PC1, with only loading weights greater than 0.2 included in the plot. Thicker lines showing the top 5 weights of the PC1 loadings. Results suggest that FC between thalamo-cortico-cerebellar loop elements with premotor cortex is responsible for the highly accurate predictions of the clinical groups.
4 Discussion
In this study, we examined the relationship between implicit learning of schizophrenia patients with neuroplasticity using a reward-enhanced SRTT via rs-fMRI. Our findings showed patients had a lower proportion of correct responses compared to controls, suggesting implicit learning deficits in schizophrenia. The observed baseline hyperconnectivity in cortico-striato-cerebellar regions became even more robust and widespread following task completion in patients. However, increased connectivity was not associated with the patients’ performance. It was further observed that hyperconnectivity after the task was a differentiating feature that could classify individuals into different groups.
A dissociation in behavioral performance was observed in schizophrenia patients. While their reduction in reaction times (RTs) across sequential compared to random blocks was comparable to that of healthy controls—suggesting partially preserved implicit learning—they demonstrated significantly lower accuracy in the sequential blocks. This discrepancy points to potential deficits in motor execution, attentional regulation, or the integration of learned sequences. Additionally, the inclusion of a reward component in the task, together with the group’s relatively low ESRS scores, may have influenced reaction time patterns positively in patients. However, the lack of sequence-specific improvement in the post-test condition, along with a lower proportion of correct responses, further supports the presence of subtle impairments in implicit learning among schizophrenia patients. Our findings are also consistent with the meta-analysis conducted by Siegert et al. (27). In reviewing the studies included in this meta-analysis, for example, Green et al. (29) reported that patients with chronic schizophrenia exhibited significantly lower levels of sequence learning compared to healthy controls. Similarly, Marvel et al. (30) found impaired implicit learning in a group of psychosis, although the inclusion of individuals with schizoaffective disorder was noted as a limitation. To eliminate the effects of medication, Exner et al. (31) investigated drug-naïve first-episode patients and reported that impairments in implicit learning may be related to the nature of the disorder. These findings were supported by Zedkova et al. (32), who also found impaired implicit learning in patients with chronic schizophrenia. Consistent with these findings, our study also showed that patients had lower accuracy compared to controls, suggesting implicit learning deficits.
Our finding of the increased baseline cortico-striato-cerebellar hyperconnectivity in schizophrenia patients has been reported previously from the onset of the disease and has been proposed as a potential soft neurological sign of the disorder (33–37). The observation of hyperconnectivity between the thalamus and cortical regions in the current study supports the idea that the excitatory-inhibitory imbalance between glutamatergic and GABAergic neurons leads to dysfunction in thalamo-cortical neurocircuits (38). Northoff et al. proposed that increased dopaminergic and decreased serotonergic signaling in psychotic disorders, including schizophrenia, may contribute to increased sensorimotor-thalamic functional connectivity (39).
In previous studies, healthy subjects have increased cortico-striato-cerebellar connectivity after SRTT and one study reported healthy controls have more cortico-striato-cerebellar connectivity compared to schizophrenia patients (40). However, we could not observed connectivity changes in the healthy controls. On the other hand, our schizophrenia patients had more widespread increased cortico-striato-cerebellar connectivity after SRTT. The reason for the discrepancy between our and the previous study might be due to the type antipsychotics that the patients were on. In the previous study, those who remained on typical antipsychotics continued to show a lack of procedural learning, while patients who switched to atypical antipsychotics demonstrated significant improvements in procedural learning, accompanied by increased activation in the superior-middle frontal gyrus, anterior cingulate, and striatum (41). In our study, the fact that all patients were using atypical antipsychotics may have contributed to the observed results, potentially due to the relatively weaker nigrostriatal D2 blockade associated with these medications.
Our results show that although cortico-striato-cerebellar hyperconnectivity present in schizophrenia patients, its lack of correlation with behavioral performance supports the dysfunctional brain networks hypothesis in schizophrenia (42). At the cellular level, the disturbances in the integration of electrical stimuli impair the consolidation of incoming information, resulting in excessive information overload in the brain. Consequently, even if connectivity within certain brain networks increases, functional impairments may still occur (43). Additionally, studies using psychedelic-induced psychosis models suggest that abnormal synaptogenesis leads to hyperconnectivity and increased neural entropy (44). However, this hyperconnectivity might not be functionally beneficial as showed in a previous study by Wu et al. (45), the finding that sensorimotor-thalamic hyperconnectivity was associated with poorer task performance further supports the dysfunctional brain networks hypothesis in schizophrenia patients.
In their study, Chang et al. (46) demonstrated the presence of microstructural abnormalities in the cerebellar peduncles of patients with schizophrenia. These abnormalities were specific to the middle and inferior cerebellar peduncles and were characterized by reduced fractional anisotropy and increased radial diffusivity. Notably, these changes were significantly associated with cognitive performance in healthy controls, but not in patients. This suggests that impaired cerebellar white matter integrity may underlie cognitive impairments in schizophrenia. Damage to the cerebellar pathways has been shown to impair efficient information processing, which, in turn, leads to increased functional connectivity within the circuit as a compensatory mechanism to maintain basic task performance.
Recent evidence from several neuropsychiatric disorders suggests that hyperconnectivity within large-scale brain networks may reflect a compensatory response to underlying structural or functional disturbances. For example, patients with mild traumatic brain injury showed increased functional connectivity between the inferior fronto-occipital fasciculus and primary sensorimotor along with cerebellar networks, which was associated with impaired information processing speed, suggesting a maladaptive compensatory mechanism (47). Similarly, patients with generalized tonic-clonic seizures showed increased variability in functional connectivity, particularly in the default mode, attention and cognitive control networks, reflecting a dynamic reorganization that may be related to both epileptic activity and cognitive dysfunction (48). Consistent with these findings, our study in schizophrenia revealed hyperconnectivity within the cortico-striato-cerebellar circuit, which we interpret as a compensatory adaptation to widespread disruptions in brain function. Taken together, these findings support the notion that increased network connectivity may serve as a general compensatory mechanism in neuropsychiatric disorders, albeit potentially associated with inefficient cognitive processing.
In this study, we employed a reward-related SRTT, which is expected to modulate neural activity in emotion-related regions. Our findings revealed reduced connectivity between the insula and the posterior cingulate cortex (PCC), a key region of the default mode network (DMN). This result aligns with Ebisch et al. (49), who reported similar disruptions in first-episode schizophrenia patients, suggesting that schizophrenia may impair the insula’s role in emotional processing (50).
Additionally, we observed increased insula-thalamus connectivity following the task, consistent with previous findings that connectivity in this pathway strengthens with feedback (51). This suggests that schizophrenia patients remain responsive to feedback mechanisms. Furthermore, we found enhanced connectivity between the insula, a core component of the salience network, and the cerebellum in schizophrenia. This may reflect a compensatory mechanism for executive dysfunction, potentially engaging the reward system.
While Chrobak et al. (52) reported no relationship between the sensorimotor network and SRTT performance, our findings suggest that using a reward-enhanced SRTT may have contributed to increased connectivity between the premotor cortex (PMC), a key sensorimotor region, and the nucleus accumbens. This indicates that despite neuroplasticity deficits, schizophrenia patients may still process rewards, albeit through alternative neural pathways.
Finally, contrary to the meta-analysis by Radua et al. (53), we found increased connectivity between the nucleus accumbens and both the thalamus and PMC. This discrepancy may stem from the fact that all participants in our study were treated with atypical antipsychotics, which are known to normalize striatal activity. Overall, our findings suggest that implicit learning deficits in schizophrenia are not solely attributable to motivational factors or negative symptoms, as often debated, but are likely rooted in fundamental neural dysfunctions, including impaired neuroplasticity.
Our findings demonstrate that SRTT-induced changes can predict clinical groups with high accuracy (AUC: 0.95–1). Key predictors included the PMC, bilateral insula, thalamus, and cerebellum (Figure 6). To assess the necessity of neuroimaging, behavioral metrics (reaction times, correct response rates) were included as predictors. However, their weak contributions to PC1, with significant weights (>0.2) only in the post-task fMRI condition, highlight the primacy of neural changes in classification. This suggests that fMRI-based functional connectivity provides deeper insights into neuroplasticity than behavioral measures alone, particularly in task-induced resting-state alterations. Further studies are needed to explore these interactions in greater detail and clarify their role in reward processing and its dysregulation in schizophrenia.
5 Limitations
This study has limitations, both in sample size and study design. One limitation is the inability to perform fMRI during the task. This was mainly due to the task’s total duration of approximately 75 minutes, making it challenging for patients to minimize their head movements. Relatively small sample size, the exclusion of left-handed participants, and the cross-sectional design decrease the generalizability of the findings. Additionally, the small sample size may contribute to an overestimation of the SVM results; however, the baseline AUC values appear sufficiently robust to support the findings. Similarly, external validation is needed further to support the effectiveness of SRTT in group distinction. Future research should use longitudinal designs and multimodal imaging to better understand neuroplasticity dynamics and implicit learning over time. Additionally, studying unmedicated patients or those at different illness stages may provide further insights.
6 Conclusion
This study provides evidence of altered neuroplasticity in schizophrenia, characterized by hyperconnectivity within cortico-striato-cerebellar circuits during implicit learning. While this adaptation may partly compensate for neuroplasticity deficits, it appears insufficient to fully support task-specific learning. These findings emphasize the importance of targeting neuroplasticity in the development of interventions, such as non-invasive brain stimulation techniques (TMS, tDCS) or pharmacological modulation of glutamate and dopamine systems, to help improve cognitive impairments in schizophrenia. These findings contribute to a growing understanding of the neural basis of implicit learning deficits in this population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Ege University (approval number 19-12T/42, approval date December 11, 2019). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AH: Conceptualization, Investigation, Resources, Writing – original draft. SS: Formal Analysis, Validation, Visualization, Writing – original draft. IS: Formal Analysis, Visualization, Writing – original draft. KK: Formal Analysis, Validation, Visualization, Writing – original draft. FY: Investigation, Writing – original draft. MEr: Investigation, Writing – original draft. YE: Data curation, Project administration, Writing – original draft. AY: Software, Writing – original draft. OK: Methodology, Supervision, Validation, Writing – original draft. MEk: Methodology, Writing – original draft. AG: Conceptualization, Data curation, Funding acquisition, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Bilimsel Araştırma Proje/No: TGA-2021-22567, which provided financial support for the submission and publication of this manuscript. The views expressed in this paper are those of the authors and do not necessarily reflect the official policy or position of Bilimsel Araştırma Proje.
Acknowledgments
The authors wish to acknowledge Duran Acikel for technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1600449/full#supplementary-material
References
1. McGrath J, Saha S, Chant D, and Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiologic Rev. (2008) 30:67–76. doi: 10.1093/epirev/mxn001
2. Carbon M and Correll CU. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS spectrums. (2014) 19 Suppl 1:38–53. doi: 10.1017/S1092852914000601
3. Lin C, Zhang X, and Jin H. The societal cost of schizophrenia: an updated systematic review of cost-of-illness studies. PharmacoEconomics. (2023) 41:139–53. doi: 10.1007/s40273-022-01217-8
4. Steel A, Silson EH, Stagg CJ, and Baker CI. Differential impact of reward and punishment on functional connectivity after skill learning. NeuroImage. (2019) 189:95–105. doi: 10.1016/j.neuroimage.2019.01.009
5. Nissen MJ and Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cogn Psychol. (1987) 19:1–32. doi: 10.1016/0010-0285(87)90002-8
6. Reiss JP, Campbell DW, Leslie WD, Paulus MP, Ryner LN, Polimeni JO, et al. Deficit in schizophrenia to recruit the striatum in implicit learning: a functional magnetic resonance imaging investigation. Schizophr Res. (2006) 87:127–37. doi: 10.1016/j.schres.2006.04.027
8. Albouy G, Fogel S, Pottiez H, Nguyen VA, Ray L, Lungu O, et al. Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PloS One. (2013) 8:e52805. doi: 10.1371/journal.pone.0052805
9. Albouy G, King BR, Maquet P, and Doyon J. Hippocampus and striatum: dynamics and interaction during acquisition and sleep-related motor sequence memory consolidation. Hippocampus. (2013) 23:985–1004. doi: 10.1002/hipo.22183
10. Breton J and Robertson EM. Dual enhancement mechanisms for overnight motor memory consolidation. Nat Hum Behav. (2017) 1:111. doi: 10.1038/s41562-017-0111
11. Hardwick RM, Rottschy C, Miall RC, and Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. NeuroImage. (2013) 67:283–97. doi: 10.1016/j.neuroimage.2012.11.020
12. Hardwick RM, Lesage E, Eickhoff CR, Clos M, Fox P, and Eickhoff SB. Multimodal connectivity of motor learning-related dorsal premotor cortex. NeuroImage. (2015) 123:114–28. doi: 10.1016/j.neuroimage.2015.08.024
13. Keele SW, Ivry R, Mayr U, Hazeltine E, and Heuer H. The cognitive and neural architecture of sequence representation. psychol Rev. (2003) 110:316–39. doi: 10.1037/0033-295x.110.2.316
14. King BR, Saucier P, Albouy G, Fogel SM, Rumpf JJ, Klann J, et al. Cerebral activation during initial motor learning forecasts subsequent sleep-facilitated memory consolidation in older adults. Cereb Cortex (New York N.Y.: 1991). (2017) 27:1588–601. doi: 10.1093/cercor/bhv347
15. Kornysheva K and Diedrichsen J. Human premotor areas parse sequences into their spatial and temporal features. eLife. (2014) 3:e03043. doi: 10.7554/eLife.03043
16. Schendan HE, Searl MM, Melrose RJ, and Stern CE. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. (2003) 37:1013–25. doi: 10.1016/s0896-6273(03)00123-5
17. Seger CA. The basal ganglia in human learning. Neuroscientist: A Rev J bringing neurobiology Neurol Psychiatry. (2006) 12:285–90. doi: 10.1177/1073858405285632
18. Wiestler T and Diedrichsen J. Skill learning strengthens cortical representations of motor sequences. eLife. (2013) 2:e00801. doi: 10.7554/eLife.00801
19. Doyon J, Penhune V, and Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. (2003) 41:252–62. doi: 10.1016/s0028-3932(02)00158-6
20. Hikosaka O, Nakamura K, Sakai K, and Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. (2002) 12:217–22. doi: 10.1016/s0959-4388(02)00307-0
21. Karni A, Meyer G, Jezzard P, Adams MM, Turner R, and Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. (1995) 377:155–8. doi: 10.1038/377155a0
22. Forano M and Franklin DW. Reward actively engages both implicit and explicit components in dual force field adaptation. J neurophysiology. (2024) 132:1–22. doi: 10.1152/jn.00307.2023
23. Calabro FJ, Montez DF, Larsen B, Laymon CM, Foran W, Hallquist MN, et al. Striatal dopamine supports reward expectation and learning: A simultaneous PET/fMRI study. NeuroImage. (2023) 267:119831. doi: 10.1016/j.neuroimage.2022.119831
24. Andreasen NC, Carpenter WT, Kane JM, Lasser RA, Marder SR, and Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. (2005) 162:441–9. doi: 10.1176/appi.ajp.162.3.441
25. Chrobak AA, Siuda-Krzywicka K, Siwek GP, Tereszko A, Janeczko W, Starowicz-Filip A, et al. Disrupted implicit motor sequence learning in schizophrenia and bipolar disorder revealed with ambidextrous Serial Reaction Time Task. Prog Neuropsychopharmacol Biol Psychiatry. (2017) 79:169–75. doi: 10.1016/j.pnpbp.2017.06.025
26. Nieto-Castanon A. “FMRI minimal preprocessing pipeline”. In: Handbook of functional connectivity Magnetic Resonance Imaging methods in CONN. Boston, USA: Hilbert Press (2020). p. 3–16. doi: 10.56441/hilbertpress.2207.6599
27. Siegert RJ, Weatherall M, and Bell EM. Is implicit sequence learning impaired in schizophrenia? A meta-analysis. Brain Cogn. (2008) 67:351–9. doi: 10.1016/j.bandc.2008.02.005
28. Jafri MJ, Pearlson GD, Stevens M, and Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. NeuroImage. (2008) 39:1666–81. doi: 10.1016/j.neuroimage.2007.11.001
29. Green MF, Kern RS, Williams O, McGurk S, and Kee K. Procedural learning in schizophrenia: evidence from serial reaction time. Cogn neuropsychiatry. (1997) 2:123–34. doi: 10.1080/135468097396360
30. Marvel CL, Schwartz BL, Howard DV, and Howard JH. Implicit learning of non-spatial sequences in schizophrenia. J Int Neuropsychol Society: JINS. (2005) 11:659–67. doi: 10.1017/S1355617705050861
31. Exner C, Weniger G, Schmidt-Samoa C, and Irle E. Reduced size of the pre-supplementary motor cortex and impaired motor sequence learning in first-episode schizophrenia. Schizophr Res. (2006) 84:386–96. doi: 10.1016/j.schres.2006.03.013
32. Zedkova L, Woodward ND, Harding I, Tibbo PG, and Purdon SE. Procedural learning in schizophrenia investigated with functional magnetic resonance imaging. Schizophr Res. (2006) 88:198–207. doi: 10.1016/j.schres.2006.06.039
33. Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. (2015) 72:882–91. doi: 10.1001/jamapsychiatry.2015.0566
34. Cai XL, Wang YM, Wang Y, Zhou HY, Huang J, Wang Y, et al. Neurological soft signs are associated with altered cerebellar-cerebral functional connectivity in schizophrenia. Schizophr Bull. (2021) 47:1452–62. doi: 10.1093/schbul/sbaa200
35. Cao H, Wei X, Hu N, Zhang W, Xiao Y, Zeng J, et al. Cerebello-thalamo-cortical hyperconnectivity classifies patients and predicts long-term treatment outcome in first-episode schizophrenia. Schizophr Bull. (2022) 48:505–13. doi: 10.1093/schbul/sbab112
36. Woodward ND, Karbasforoushan H, and Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. (2012) 169:1092–9. doi: 10.1176/appi.ajp.2012.12010056
37. Woodward ND and Heckers S. Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry. (2016) 79:1016–25. doi: 10.1016/j.biopsych.2015.06.026
38. Just N and Sonnay S. Investigating the role of glutamate and GABA in the modulation of transthalamic activity: A combined fMRI-fMRS study. Front Physiol. (2017) 8:30. doi: 10.3389/fphys.2017.00030
39. Martino M, Magioncalda P, Conio B, Capobianco L, Russo D, Adavastro G, et al. Abnormal functional relationship of sensorimotor network with neurotransmitter-related nuclei via subcortical-cortical loops in manic and depressive phases of bipolar disorder. Schizophr Bull. (2020) 46:163–74. doi: 10.1093/schbul/sbz035
40. Kumari V, Gray JA, Honey GD, Soni W, Bullmore ET, Williams SC, et al. Procedural learning in schizophrenia: a functional magnetic resonance imaging investigation. Schizophr Res. (2002) 57:97–107. doi: 10.1016/s0920-9964(01)00270-5
41. Kumari V, Ettinger U, Lee SE, Deuschl C, Anilkumar AP, Schmechtig A, et al. Common and distinct neural effects of risperidone and olanzapine during procedural learning in schizophrenia: a randomised longitudinal fMRI study. Psychopharmacology. (2015) 232:3135–47. doi: 10.1007/s00213-015-3959-1
42. Andreasen NC, Paradiso S, and O’Leary DS. Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. (1998) 24:203–18. doi: 10.1093/oxfordjournals.schbul.a033321
43. Forlim CG, Klock L, Gallinat J, and Kühn S. Altered resting-state functional connectivity in a thalamo-cortico-cerebellar network in patients with schizophrenia. Sci Rep. (2024) 14:26284. doi: 10.1038/s41598-024-78297-3
44. Atasoy S, Roseman L, Kaelen M, Kringelbach ML, Deco G, and Carhart-Harris RL. Connectome-harmonic decomposition of human brain activity reveals dynamical repertoire re-organization under LSD. Sci Rep. (2017) 7:17661. doi: 10.1038/s41598-017-17546-0
45. Wu G, Palaniyappan L, Zhang M, Yang J, Xi C, Liu Z, et al. Imbalance between prefronto-thalamic and sensorimotor-thalamic circuitries associated with working memory deficit in schizophrenia. Schizophr Bull. (2022) 48:251–61. doi: 10.1093/schbul/sbab086
46. Chang X, Jia X, Wang Y, and Dong D. Alterations of cerebellar white matter integrity and associations with cognitive impairments in schizophrenia. Front Psychiatry. (2022) 13:993866. doi: 10.3389/fpsyt.2022.993866
47. Jia X, Xie Y, Dong D, Pei H, Jiang S, Ma S, et al. Reconfiguration of dynamic large-scale brain network functional connectivity in generalized tonic-clonic seizures. Hum Brain Mapp. (2020) 41:67–79. doi: 10.1002/hbm.24787
48. Jia X, Chang X, Bai L, Wang Y, Dong D, Gan S, et al. A longitudinal study of white matter functional network in mild traumatic brain injury. J neurotrauma. (2021) 38:2686–97. doi: 10.1089/neu.2021.0017
49. Ebisch SJ, Mantini D, Northoff G, Salone A, De Berardis D, Ferri F, et al. Altered brain long-range functional interactions underlying the link between aberrant self-experience and self-other relationship in first-episode schizophrenia. Schizophr Bull. (2014) 40:1072–82. doi: 10.1093/schbul/sbt153
50. Pang L, Kennedy D, Wei Q, Lv L, Gao J, Li H, et al. Decreased functional connectivity of insular cortex in drug naïve first episode schizophrenia: in relation to symptom severity. PloS One. (2017) 12:e0167242. doi: 10.1371/journal.pone.0167242
51. Tsukamoto T, Kotani Y, Ohgami Y, Omura K, Inoue Y, and Aihara Y. Activation of insular cortex and subcortical regions related to feedback stimuli in a time estimation task: an fMRI study. Neurosci Lett. (2006) 399:39–44. doi: 10.1016/j.neulet.2006.01.061
52. Chrobak AA, Bielak S, Nowaczek D, Żyrkowska A, Styczeń K, Sobczak AM, et al. Is there a relationship between resting state connectivity within large-scale functional networks and implicit motor learning impairments in schizophrenia and bipolar disorder? Psychiatria polska. (2024), 1–12. doi: 10.12740/PP/OnlineFirst/192464
Keywords: schizophrenia, neuroplasticity, serial reaction time task, fMRI, implicit learning, neuroimaging, resting state, motor learning
Citation: Hinc AC, Selek S, Sungur I, Keskin K, Yazici F, Erata MC, Erdogan Y, Yilmaz A, Kitis O, Eker MC and Gonul AS (2025) The effect of implicit learning on functional connectivity in schizophrenia. Front. Psychiatry 16:1600449. doi: 10.3389/fpsyt.2025.1600449
Received: 26 March 2025; Accepted: 26 May 2025;
Published: 17 June 2025.
Edited by:
Guglielmo Lucchese, Universitätsmedizin Greifswald, GermanyReviewed by:
Chang Xuebin, Xi’an Jiaotong University, ChinaMario Tranfa, University of Naples Federico II, Italy
Copyright © 2025 Hinc, Selek, Sungur, Keskin, Yazici, Erata, Erdogan, Yilmaz, Kitis, Eker and Gonul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Saffet Gonul, YWxpLnNhZmZldC5nb251bEBlZ2UuZWR1LnRy
 Asli Ceren Hinc
Asli Ceren Hinc Simay Selek
Simay Selek Ibrahim Sungur
Ibrahim Sungur Kaan Keskin
Kaan Keskin Furkan Yazici
Furkan Yazici Mehmet Can Erata
Mehmet Can Erata Yigit Erdogan
Yigit Erdogan Alpaslan Yilmaz
Alpaslan Yilmaz Omer Kitis6
Omer Kitis6 Mehmet Cagdas Eker
Mehmet Cagdas Eker Ali Saffet Gonul
Ali Saffet Gonul