- 1Department of Rehabilitation, Faculty of Applied Medical Sciences, Northern Border University, Arar, Saudi Arabia
- 2King Salman Center for Disability Research, Riyadh, Saudi Arabia
- 3Department of Physical Therapy and Health Rehabilitation, College of Applied Medical Sciences, Majmaah University, Al-Majmaah, Saudi Arabia
- 4Health and Basic Sciences Research Center, Majmaah University, Majmaah, Saudi Arabia
- 5Department of Rehabilitation, Ministry of National Guard Health Affairs, Taif, Saudi Arabia
- 6Department of Physical Therapy, College of Applied Medical Sciences, Qassim University, Buraydah, Saudi Arabia
- 7Department of Physical Therapy, Faculty of Allied Health Sciences, Kuwait University, Jabriya, Kuwait
- 8Department of Respiratory Therapy, College of Applied Medical Sciences, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- 9King Abdullah International Medical Research Center, Riyadh, Saudi Arabia
Background: Attention-deficit/hyperactivity disorder (ADHD) is a prevalent neurodevelopmental disorder affecting individuals across various age groups. Access to amphetamine (AMPH) stimulant is a critical component of evidence-based care for individuals with ADHD. In Saudi Arabia, despite clinical guidelines endorsing their use, the availability of AMPH-based stimulants remains limited.
Objective: This narrative review aims to explore the current regulatory and policy environment influencing AMPH medication availability for individuals diagnosed with ADHD in Saudi Arabia.
Methods: A narrative review methodology was adopted following the Scale for the Assessment of Narrative Review Articles guidelines. A literature search was conducted in PubMed, EBSCO, and PsycINFO using defined search terms related to ADHD, psychostimulants, and Saudi Arabia. Additional grey literature from key regulatory bodies, such as the Saudi Food and Drug Authority (SFDA), the Ministry of Health (MOH), and the Saudi ADHD Society, was also reviewed. Thirteen articles and reports met the eligibility criteria and were included for qualitative synthesis.
Results: Methylphenidate is the predominant stimulant prescribed for ADHD, while AMPH-based medications, such as lisdexamfetamine, are under prescribed due to regulatory restrictions, limited formulary inclusion, and supply inconsistencies. Policy reports from national institutions highlight persistent barriers to AMPH access, despite their inclusion in recent clinical practice guidelines. Prescription trends suggest significant treatment gaps for AMPH stimulants.
Conclusion: The available evidence suggests a likely shortfall in AMPH-based medications. in Saudi Arabia, despite global and local evidence supporting their efficacy. Our findings suggest the need for enhancing regulatory pathways regarding AMPH access and availability, which requires policy interventions targeting regulatory reform, formulary expansion, and improved awareness among AMPH prescribers.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent neurodevelopmental disorder that affects 5-8% of children and adolescents worldwide (1, 2) and 6.8% in adults (3), with substantial variation across regions and diagnostic criteria (4). Approximately, 2.6% of childhood ADHD cases persist into adulthood (3), contributing to the increased prevalence of ADHD among adults (3). In Saudi Arabia, the prevalence of ADHD among adolescents (12–18 years) and adults was estimated to be 11.29% (5), however, no accurate estimates of the national prevalence of ADHD among children (6–12 years) were researched, which warrants future studies. Typically, ADHD can be categorized based on the dominancy of its major symptoms, including inattention (predominantly inattentive) and hyperactivity-impulsivity (predominantly hyperactive/impulsive) or both (combined) (6). These ADHD symptoms were responsible for negative impacts on the individual’s personal life, contributing to academic underachievement (7, 8), impaired professional performance and unemployment (9), and/or suboptimal quality-of-life (10–12).
The first line of pharmacological treatment for children and adults with ADHD is psychostimulant medications which include methylphenidate-derived (MPH) and amphetamine-derived medications (AMPH) (13). Meta-analyses continue to show that AMPH-based stimulants, including mixed amphetamine salts and lisdexamfetamine demonstrate relatively large effect size in reducing ADHD symptoms, with standardized mean difference (SMD) ranging from-0.64 to -0.89 (14, 15). Generally, psychostimulant medications work on increasing dopamine and norepinephrine levels in the brain, thereby enhancing attention and impulse control (16). MPH increases norepinephrine and dopamine levels in the presynaptic axon by blocking the reuptake of these neurotransmitters. However, AMPH further increases secretion of these neurotransmitters through the inhibition of vesicular monoamine transporter 2 as well as through interruption of the electrochemical gradients necessary for vesicular transporter function (17). For individuals who are stimulants intolerant or have contraindications, non-stimulant alternatives such as atomoxetine, guanfacine, and clonidine are treatment options (18).
Despite the benefits of pharmacotherapy, concerns regarding their side effects, long-term efficacy, and potential misuse necessitate careful clinical assessment, and ongoing monitoring (19). Common side effects of psychostimulant medications include appetite suppression, sleep disturbances, and increased heart rate, meanwhile non-stimulant medications may cause fatigue, dizziness, or gastrointestinal discomfort (14). However, it has been shown that if left untreated, individuals with ADHD may face significant functional impairments across multiple domains of life, including academic (20), occupational (21), psychological (22, 23), and social aspects (24). Therefore, an evidence-based approach that includes individualized treatment planning, regular follow-ups, and consideration of non-pharmacological interventions is essential for optimizing outcomes in ADHD management. Such approach should include the provision of effective medications, like AMPH-derived medications, which would help in controlling ADHD symptoms.
Standard ADHD clinical practice guidelines (CPGs), such as the American Academy of Pediatrics (25) and the National Institute for Health and Clinical Excellence (NICE) (26), promote the use of AMPH-based stimulants for children aged 6 years and older. Limited access to AMPH-based stimulants for individuals with ADHD can lead to significant negative health outcomes and impose additional burdens on the healthcare system. Evidence found that using AMPH-based stimulants, in addition to effectively reducing core ADHD symptoms, have been associated with lower risks of suicidal behavior and psychiatric hospitalizations (27, 28).
According to the Centers for Disease Control and Prevention, restricted access to these medications can result in untreated or inadequately managed ADHD, leading to adverse outcomes including social and emotional impairments, increased risk of substance use, unintentional injuries, and suicide (29). From a healthcare system perspective, medication shortages and access barriers can force patients to seek alternative, often unregulated, sources for their medications, increasing the risk of exposure to counterfeit substances and associated health complications (29, 30). Furthermore, the lack of appropriate medication management may result in strain on healthcare resources due to the exacerbation of ADHD-related complications, thus elevating overall healthcare costs (29). Securing availability of AMPH-based stimulants in clinical practice is essential for optimizing health outcomes in individuals with ADHD and reducing the broader impact on the healthcare system.
Regulatory actions play a pivotal role in addressing medication shortages and prevent reliance on unregulated sources. Given the limited access of AMPH-based stimulants in Saudi Arabia, this study aims to synthesize existing knowledge on amphetamine availability for ADHD, explore the regulatory processes and healthcare system challenges that contribute to access barriers, and provide a policy recommendations on improving medication availability for this population.
Methods
Research question and study design
We reported this review in accordance with the Scale for the Assessment of Narrative Review Articles guidelines (SANRA) (31). SANRA is a validated tool designed to improve the quality, transparency, and reproducibility of narrative reviews (31). Key SANRA domains addressed in this review include justification of the article’s importance, appropriate referencing, transparent literature search, scientific reasoning, and balanced presentation of relevant literature (31). The aim of this review was to broadly understand the current literature on the availability of AMPH-derived stimulants for individuals with ADHD in Saudi Arabia. We selected to perform a narrative review after scoping searches revealed that the evidence base comprised a small number of observational studies and substantial grey literature (national guidelines, formulary documents, regulatory circulars). Given this heterogeneity, a formal systematic review would have yielded few studies and excluded critical policy sources. Narrative synthesis, guided by the SANRA quality framework (31), therefore offered the most suitable method for integrating multidisciplinary evidence and generating policy-relevant conclusions. In this narrative review, we summarized and reported the available published work as well as grey literature regarding challenges related to AMPH-based stimulants access and availability for physicians when treating individuals diagnosed with ADHD.
Search strategy
We comprehensively searched the current available literature for published work including PubMed, PsycINFO, and EBSCO from inception until March 1, 2025. Key terms (with their terminologies) included (ADHD OR “attention deficit hyperactivity disorder”) AND (psychostimulants OR amphetamine OR dextroamphetamine) AND (Saudi Arabia). Details on search terms were provided in Supplementary Table 1. Additionally, we searched other potentials sources for grey literature work such as regulatory agencies’ websites including the Saudi Ministry of Health (MOH; https://www.moh.gov.sa/Pages/Default.aspx(, Saudi Food and Drug Authority (SFDA; https://www.sfda.gov.sa/en), and the Saudi ADHD Society (https://adhd.org.sa/en/). The reference lists of the included studies were also carefully examined by two reviewers (the first author; MMA and the second author; NZA) manually to locate other potentially relevant studies.
Eligibility criteria
We included peer-reviewed articles that included participants diagnosed with ADHD. We also included national guidelines, formulary documents, regulatory circulars, and relevant grey literature that addressed AMPH-based treatment in ADHD and included data or commentary specific to Saudi Arabia. Articles without regional specificity, editorials, and non-medication-focused reports were excluded. Articles were included if they were written in English and Arabic.
Study selection and data extraction
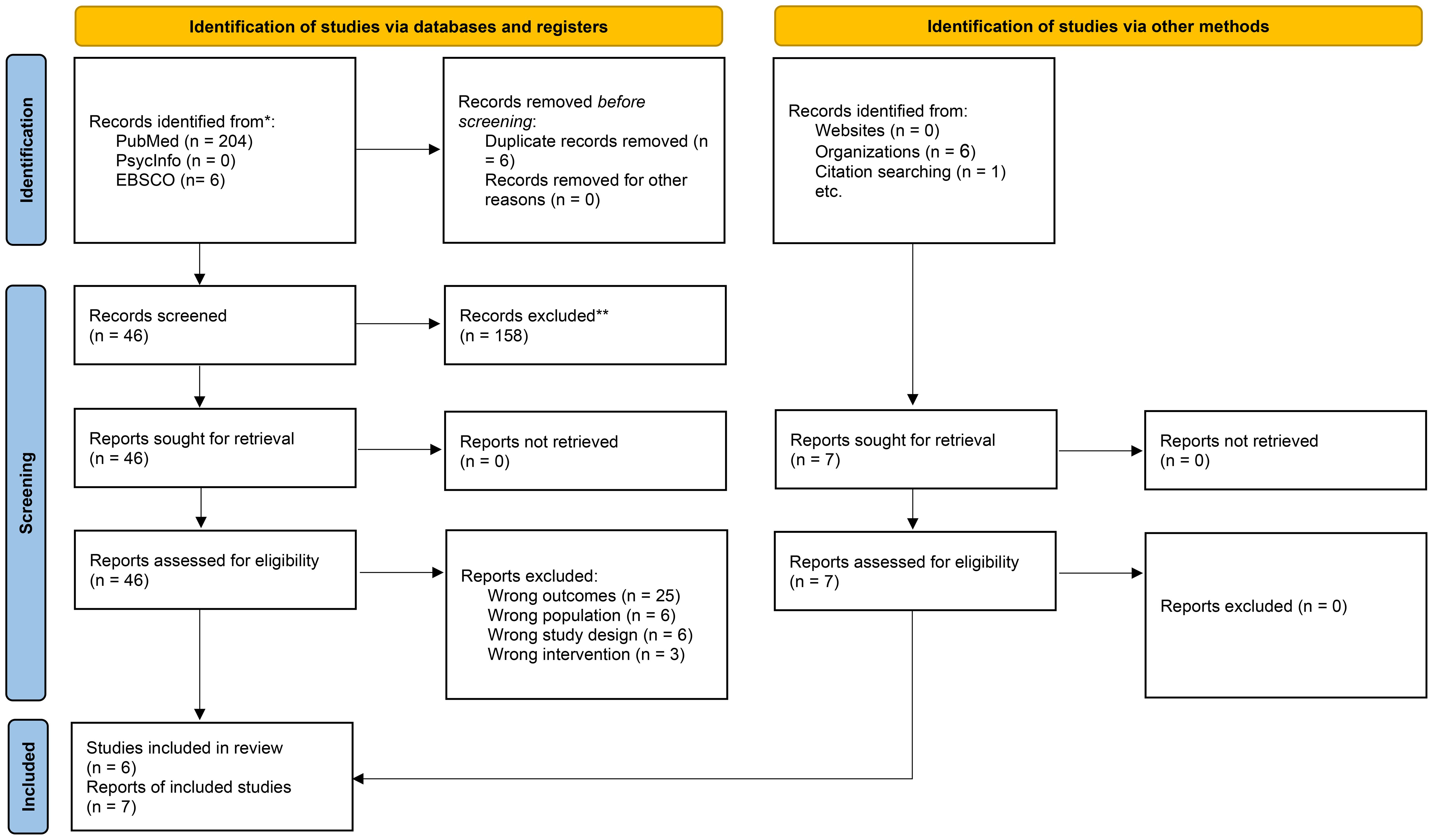
Two independent reviewers (the first author; MMA) and (the second author; NZA) screened all potential records retrieved through the search process. Initial screening was based on titles and abstracts. Full texts were retrieved for records that met the eligibility criteria or required further evaluation. Discrepancies during screening or full-text review were resolved through discussion or consultation with a third reviewer (the last author; MMA) when needed. The selection process is illustrated using a PRISMA-style flow diagram (see Figure 1). We extracted data using a standardized data extraction sheet. Data extracted included: study characteristics (author and year of publication), study design, as well as sample characteristics including age, sex, and percentage receiving treatment. Further, data extracted included treatment regimen and stimulant class (i.e., MPH or AMPH based stimulants). Finally, main findings related to AMPH availability in Saudi Arabia was reviewed for all included articles.
Data analysis and synthesis
In this narrative review, we conducted a qualitative synthesis from all included studies report AMPH-based stimulant availability for individuals with ADHD in Saudi Arabia. We conducted a qualitative narrative synthesis of the included sources, structured around three analytical domains:
1. Current prescribing and regulatory landscape for AMPH psychostimulants in Saudi Arabia.
2. Clinical practice context, including treatment guidelines and access pathways for AMPH treatment.
3. Systemic and policy-related barriers limiting availability and utilization of AMPH-based medications.
We reported our findings regarding: (i) study characteristics: authors (year), and study design; (ii) primary findings relevant to AMPH availability in Saudi Arabia; and (iii) clinical practice protocols and guidelines for individuals with ADHD in Saudi Arabia. Key findings were organized into thematic summaries with representative examples from included sources. No quantitative synthesis (meta-analysis) was performed due to the heterogeneity of study types and the nature of the data. Grey-literature sources were appraised based on their origin (e.g., government agency, academic society), internal consistency, relevance to the review question, and publication recency. Priority was given to documents issued by authorities that play key roles in ADHD diagnosis and management (e.g., Ministry of Health), or recognized scientific bodies. We recorded the issuing institution, year, and content scope for each document (Table 1).
Results
Search results
A total of 211 records were identified of which 204 records sourced from PubMed, six records from EBSCO, and one through citation search (manual search). Additionally, six records were identified from organizations’ work. After removing six duplicate records, a total of 205 sources remained for screening. During the title and abstract screening phase, 158 records were excluded due to irrelevance to our study’s aim, leaving 46 records for the full-text screening phase. Out of the 46 articles, 25 reports were excluded for reporting wrong outcomes, six reports included wrong populations, six reports used ineligible study design, and 3 reports investigations from wrong intervention, yielding 6 reports that were included in the analysis from the databases search. In addition, one report was included by the citation manual search and six reports were included from organizations’ work. The total number of articles/reports that were included in the final analysis is 13 (19, 32–43) (Figure 1).
Study characteristics
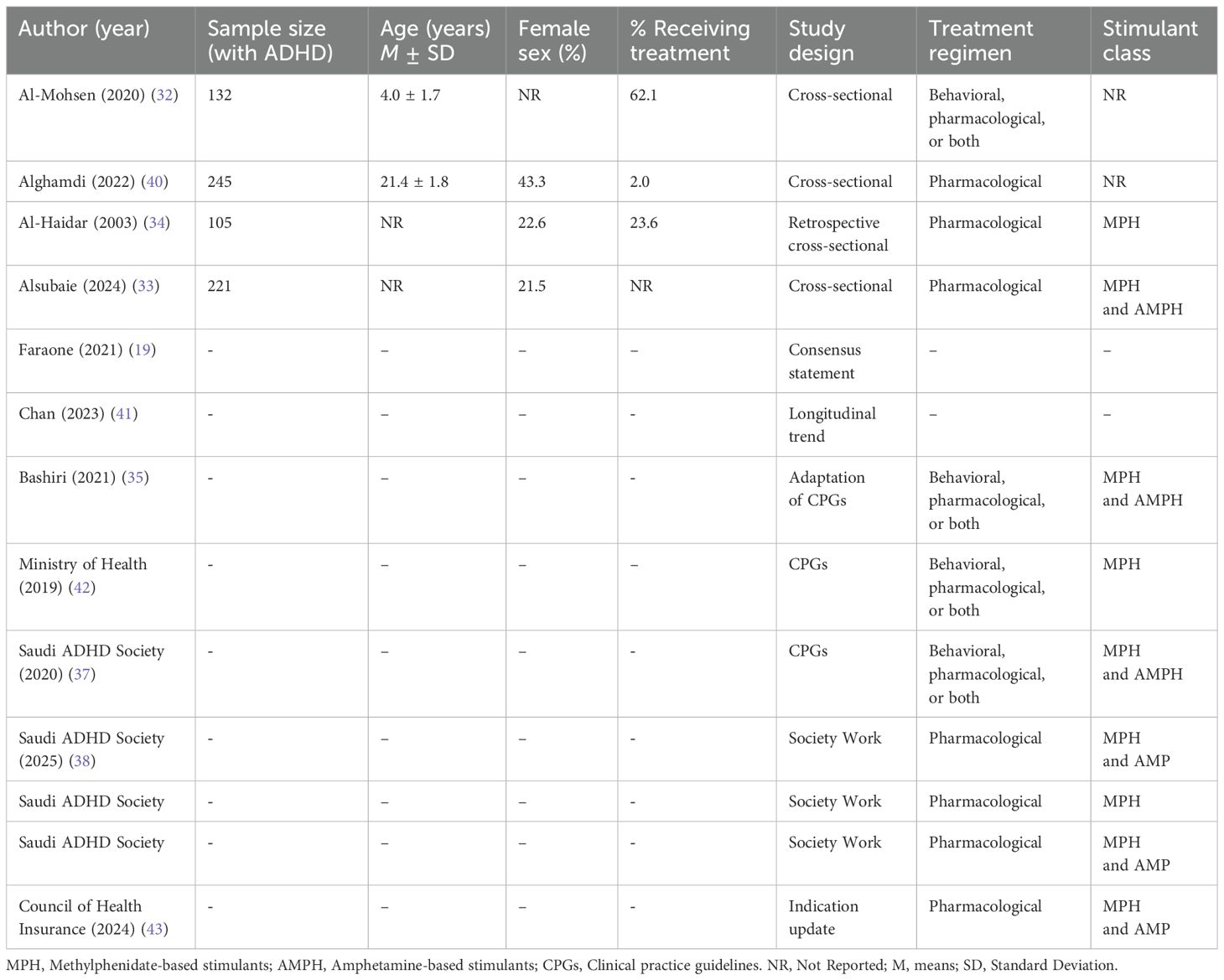
In Table 1, we summarize the main characteristics of the included study. Characteristics of the included studies/reports vary significantly in terms of sample size, study design, and/or treatment regimens. The studies that included participants’ data (n=4) had sample sizes varied between 105 and 245 individuals with ADHD, with all studies using a cross-sectional study design. The remaining studies consisted of consensus statements (19), longitudinal trend analyses (41), and guideline adaptations (35, 42, 43). The cross-sectional studies included children, adolescents, and adults with ADHD. Specifically, Al-Mohsen et al. (2020) (32) included young children (M age= 4.0 ± 1.7 years), whereas Alghamdi et al. (2022) (40) focused on older participants (M age= 21.4 ± 1.8 years). However, some studies, such as Al-Haidar (2003) et al. (34), included children through adolescents from early ages to 18 years, whereas Alsubaie et al. (2024) (33) included children and adults of ages less than 3 years to older than 17 years. The proportion of female participants in the included studies varied, although some studies did not report this information (sex distribution).
The percentage of participants receiving treatment also differed remarkably. Specifically, Al-Mohsen et al. (2020) (32) reported a relatively high treatment rate (62.1%), while Alghamdi et al. (2022) (40) found that only 2.0% of their sample was receiving pharmacological treatment. Al-Haidar et al. (2003) (34) indicated that 23.6% of patients were on medication, and Alsubaie et al. (2024) (33) did not provide details on medication received.
In terms of treatment regimens, many studies examined pharmacological treatments (33, 34, 36, 38–40, 43), while others investigated both pharmacological and behavioral treatments (32, 37, 42). The stimulant medications reported in the studies included MPH and AMPH. One study by Bashiri (2021) (35) and guidelines provided by the Saudi ADHD Society, acknowledged the use of both MPH and AMPH which were relatively recent. Other reports, such as the MOH guidelines, focused primarily on MPH without inclusion of AMPH stimulants.
Main findings related to AMPH availability
In Table 2, we summarize the main findings related to amphetamine availability for individuals with ADHD in Saudi Arabia. Al-Mohsen et al. (2020) (32) conducted a study on Saudi mothers’ perceptions of ADHD treatment. The study revealed that concerns regarding the side effects of stimulant medications were a major factor influencing treatment adherence. Furthermore, the study found that a limited number of mothers (<26%) were aware that MPH is a medication for ADHD. In addition, that study highlighted caregiver perceptions and stigma-related concerns were main barriers in managing ADHD among Saudi mothers. Alghamdi et al. (2022) (40) investigated ADHD prevalence among university students in Saudi Arabia. The findings indicated that only 2% of diagnosed students had ever received pharmacological treatment, suggesting significant under-prescription and limited access to AMPH stimulants.
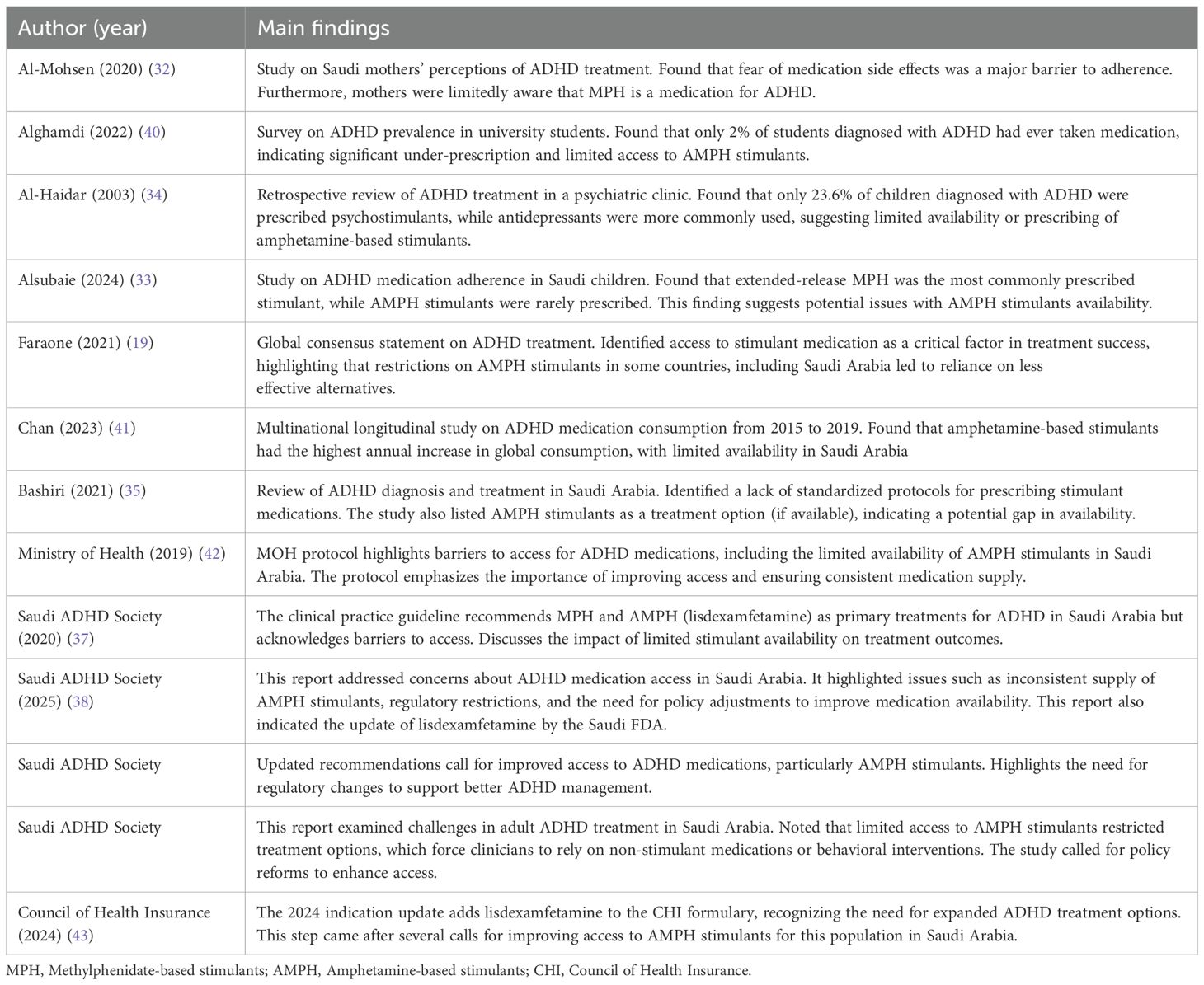
Table 2. Summary table of the main findings related to amphetamine availability for individuals with ADHD in Saudi Arabia.
Al-Haidar et al. (2003) (34) conducted a retrospective patient records review of ADHD treatment in a psychiatric clinic in Saudi Arabia. The study found that only 23.6% of children diagnosed with ADHD were prescribed psychostimulants, while antidepressants were more commonly used as an alternative. Alsubaie et al. (2024) (33) examined ADHD medication adherence of children in Saudi Arabia. The study found that extended-release MPH was the most commonly prescribed stimulant, while AMPH stimulants were rarely prescribed. Further, this study demonstrated that prescriber behavior, including concerns about medication side effects and parental hesitation may have an effect on prescribing AMPH stimulants.
Faraone et al. (2021) (19) identified access to stimulant medication as a critical factor for successful treatment outcomes. It highlighted that restrictions on AMPH stimulants in some countries, including Saudi Arabia, have led to increased reliance on non-stimulant alternatives, which may not be as effective. Bashiri et al. (2021) (35) reviewed ADHD diagnosis and treatment in Saudi Arabia and adapted the NICE guidelines related to ADHD diagnosis and management. This study identified a lack of standardized protocols for prescribing stimulant medications in Saudi Arabia. The study also listed AMPH stimulants as a treatment option (if available), indicating a potential gap in availability. Moreover, the study indicated other barriers to AMPH prescription, including clinical uncertainty and provider variability. Chan et al. (2023) (41) conducted a multinational longitudinal study on ADHD medication consumption from 2015 to 2019. The study found that AMPH -based stimulants had the highest annual increase in global consumption. However, their availability remained limited in Saudi Arabia, due to regulatory restrictions and supply inconsistencies.
The 2019 MOH Protocol for ADHD management (42) outlined several barriers to accessing ADHD medications in Saudi Arabia, including the limited availability of AMPH stimulants. The protocol emphasized the need for improved access and consistent medication supply to ensure effective ADHD management. The Saudi ADHD Society CPG (2020) (37) recommended MPH and AMPH stimulants (lisdexamfetamine) as primary treatments for ADHD in Saudi Arabia. However, the guidelines acknowledged existing barriers to accessing these medications and discussed the consequences of limited stimulant availability on treatment outcomes. The Council of Health Insurance (CHI) (2024) Indication Update (43) added lisdexamfetamine to the CHI formulary, recognizing the need for expanded ADHD treatment options. This step came after several calls for improving access to AMPH stimulants for this population in Saudi Arabia.
The Saudi ADHD Society has published three reports regarding AMPH limited availability (36, 38, 39). The first was the Saudi ADHD Society Updated Recommendations (44), which called for improved access to ADHD medications, particularly AMPH stimulants. This report highlighted the need for regulatory changes to support better ADHD management in Saudi Arabia. The second report of the Saudi ADHD Society (Adult Treatment) (36) examined challenges in adult ADHD treatment in Saudi Arabia. This report noted that limited access to AMPH stimulants restricted treatment options, which force clinicians to rely on non-stimulant medications or behavioral interventions. The report called for policy reforms to enhance access. The third report by the Saudi ADHD Society (Medication Availability FAQ) (38) addressed concerns about ADHD medication access in Saudi Arabia. It highlighted issues such as inconsistent supply of AMPH stimulants, regulatory restrictions, and the need for policy adjustments to improve medication availability. This report also indicated the update of lisdexamfetamine by the Saudi FDA.
Clinical practice protocols/guidelines
Both the MOH (42) and the Saudi ADHD Society (37) Clinical Practice Guidelines (CPGs) acknowledge that stimulant medications, specifically MPH and AMPH, are the primary pharmacological treatments for ADHD. The MOH Protocol notes that while MPH (e.g., Ritalin) is widely available and used as the first-line treatment, AMPH stimulants such as lisdexamfetamine and dexamfetamine are not as commonly prescribed. Additionally, the protocol highlights that the off-label use of second-generation antipsychotics (e.g., Risperidone, Aripiprazole) has increased, which may be partly due to barriers in accessing AMPH stimulants. Similarly, the Saudi ADHD Society CPG acknowledges that MPH is the most commonly used stimulant in Saudi Arabia, whereas AMPH stimulants such as lisdexamfetamine and dexamfetamine are less frequently prescribed. This CPG also states that AMPH stimulants should be considered for patients who do not respond adequately to MPH stimulants. Despite their efficacy, regulatory hurdles and concerns about potential misuse and diversion have made the use of AMPH stimulants more restrictive.
Both the MOH (42) and the Saudi ADHD Society CPGs (37) emphasize the necessity of AMPH stimulants for effective ADHD management. The MOH Protocol asserts that stimulant medications remain the most effective pharmacological treatment for ADHD, particularly for individuals who exhibit moderate to severe symptoms that interfere with daily functioning. The protocol further highlights that when MPH is ineffective or poorly tolerated, AMPH should be considered as an alternative. The Saudi ADHD Society CPG reinforces the need for expanded access to AMPH stimulants (37), citing evidence from international ADHD treatment guidelines, such as NICE and the American Academy of Pediatrics (AAP), that support their efficacy. They also stress that untreated or poorly managed ADHD can lead to poor academic performance, increased risk of comorbid psychiatric disorders, and higher rates of substance misuse and criminal behavior. Thus, the Saudi ADHD Society guideline (37) strongly recommends improving access to AMPH treatments to ensure optimal patient outcomes. Additionally, the Saudi ADHD Society CPG calls for regulatory changes to facilitate the approval and distribution of AMPH stimulants, ensuring that they are available as part of the national ADHD treatment framework. A few years after this call, the Saudi FDA has approved lisdexamfetamine for patients with ADHD in public and private healthcare settings.
Discussion
This narrative review highlights significant barriers to the availability of AMPH stimulants for individuals with ADHD in Saudi Arabia. The findings indicate that MPH is the most commonly prescribed stimulant, whereas AMPH, such as lisdexamfetamine and dexamfetamine, remain underutilized due to regulatory restrictions and supply limitations (32, 33). In addition, studies analyzing ADHD medication trends in Saudi Arabia have consistently reported low prescription rates of AMPH stimulants (41), with evidence suggesting that many patients lack access to optimal pharmacological treatment (34, 40). Furthermore, Saudi guideline for ADHD treatment, including the MOH Protocol and Saudi ADHD Society Protocol, indicated the critical role of stimulants in ADHD management but emphasized that the availability constraints continue to impact patient care (37, 42). International treatment guidelines, including NICE and AAP, recommend AMPH stimulants as effective treatments for ADHD (25, 26). Nonetheless, regulatory challenges in Saudi Arabia limit the availability of these medications, leading to increased reliance on less effective alternatives such as non-stimulant medications and off-label antipsychotic prescriptions (35). Recently, CHI’s 2024 indication update, which added lisdexamfetamine to the national formulary, represents a significant step forward in expanding treatment options (43). In fact, there is a dire need to expand the categories of AMPH stimulants, such as mixed amphetamine salts (Adderall), to ensure equitable access to evidence-based treatments for ADHD in Saudi Arabia.
The availability and regulation of AMPH stimulants for ADHD vary widely across countries, with notable differences between Saudi Arabia, the United States (US), the United Kingdom (UK), and other Gulf Cooperation Council countries. In the US, AMPH stimulants such as mixed amphetamine salts (Adderall) and lisdexamfetamine (Vyvanse) are widely prescribed as treatments for ADHD, following the AAP guidelines (25). In the UK, AMPH stimulants such as dexamfetamine and lisdexamfetamine are also included in NICE guidelines as recommended treatments for ADHD, particularly for patients who do not respond to MPH (26). However, both the US and the UK implemented stringent prescription and monitoring protocols to mitigate risks of misuse (25, 45). In Gulf countries such as the United Arab Emirates (UAE) and Kuwait, the availability of AMPH stimulants is also restricted (41). While the SFDA recently approved lisdexamfetamine for individuals with ADHD, challenges remain in ensuring widespread availability and physician prescribing confidence (38). Standard healthcare from evidence-based CPGs suggests that balancing regulatory control with improved access through specialist prescribing programs and prescription monitoring systems could improve ADHD management in Saudi Arabia.
The restricted availability of AMPH stimulants for ADHD in Saudi Arabia has significant impact on treatment outcomes. One of the primary concerns is the delay in initiation of effective pharmacological interventions. Research has shown that early intervention with psychostimulant medications, including AMPH, leads to enhanced academic performance, improved social functioning, and reduced risk of comorbid psychiatric disorders (19). However, due to the limited availability of these medications, many patients in Saudi Arabia may experience prolonged untreated ADHD symptoms, potential risks exist pertaining to academic underachievement, occupational difficulties, and social functioning.
The lack of AMPH has also resulted in increased reliance on off-label or non-stimulant medications. Patients who do not respond well to MPH often receive non-stimulant alternatives such as atomoxetine or even second-generation antipsychotics (40, 42). While non-stimulants can be effective in certain cases, they have a lower effect size compared to stimulant medications and may not provide sufficient symptom control for all individuals with ADHD (46). This situation forces clinicians to prescribe medications that may not align with the gold standard of ADHD treatment recommended by national and international CPGs.
Beyond regulatory limitations, multiple psychosocial and cultural factors contribute to reduced AMPH prescribing in Saudi Arabia. Researchers highlighted other barriers to prescribing AMPH, including physician reluctance, medicolegal risk, lack of stimulant prescribing experience, and concerns over perceived overdiagnosis (33) (35). Public stigma toward ADHD and psychostimulants was also described as a major barrier to family acceptance of medication (32). Such contextual barriers may explain the variability in prescribing rates, suggesting the need for both educational outreach programs.
Recent international studies have raised concerns that low stimulant consumption relative to diagnosed ADHD prevalence may signal under prescription or inappropriate treatment practices, particularly when low-psychostimulant consumption relative to non-stimulants is present. For example, a national-wide study from Slovenia (47) reported a decline of stimulant use in children and adolescents despite the increased ADHD diagnosis. This example highlights the need to analyze trends of ADHD treatment in Saudi Arabia, considering the ratio of stimulant to non-stimulant prescription.
Despite the growing body of literature on ADHD treatment, significant research gaps remain, particularly concerning the availability and efficacy of AMPH medications in Saudi Arabia. Addressing these gaps in future studies is crucial for optimizing ADHD management and ensuring equitable access to effective pharmacological treatments. One critical area of future research is conducting epidemiological studies to assess ADHD treatment gaps in Saudi Arabia. Although national data on ADHD prevalence exists for children, adolescents, and adults, there is limited information regarding the proportion of patients who receive optimal pharmacological treatment. Studies have shown that stimulant medications, particularly AMPH, are under-prescribed in Saudi Arabia compared to other countries (34, 40). Future research should explore the factors contributing to these disparities, such as regulatory restrictions, physician prescribing behaviors, and public perceptions of ADHD medications. Understanding these barriers through large-scale epidemiological surveys will provide valuable insights for policymakers to implement targeted interventions aimed at improving medication accessibility.
Investigating healthcare provider perspectives on psychostimulant prescriptions is essential for understanding prescribing patterns and potential hesitancies in using amphetamines for ADHD treatment. Previous research has indicated that concerns over misuse, addiction potential, and regulatory constraints influence prescribing behaviors among clinicians (35). Qualitative studies involving psychiatrists, pediatricians, and primary care physicians could help elucidate the factors that shape prescribing preferences and identify strategies to enhance physician confidence in managing ADHD with a broader range of stimulant options. Additionally, studies examining the impact of continuing medical education programs on psychostimulant prescription practices may provide insights into how physician training can improve evidence-based prescribing behaviors.
Study limitations
Several limitations should be acknowledged in this narrative review. First, potential biases may have been introduced during the identification, selection, and interpretation of the included literature. These biases could stem from the selective inclusion of studies, the inclusion of grey literature, or subjective judgment in evaluating the findings. As typical in narrative reviews, the interpretation of evidence and selection of themes may reflect author judgment rather than objective appraisal. Furthermore, the inclusion of grey literature and policy documents, although valuable, may have introduced variability in the quality and reliability of the information. Finally, the narrative approach allowed inclusion of policy documents and introduced subjectivity inherent in qualitative synthesis of evidence. Additionally, due to the nature of the included grey literature, reviewers were not blinded to document origin, which may introduce bias despite our use of independent screening and data verification. Therefore, the findings should be interpreted with caution.
Conclusion
This narrative review highlights the significant challenges surrounding the availability of AMPH stimulants for ADHD in Saudi Arabia. While MPH remains the most commonly prescribed stimulant, access to AMPH, including lisdexamfetamine and mixed amphetamine salts, remains limited in Saudi Arabia due to several factors, such as regulatory constraints and concerns about misuse. The limited access to these medications has critical implications for ADHD management, including delayed treatment and reliance on less effective treatment alternatives. Preliminary evidence supports the need for policy attention to AMPH availability. These policies should focus on reassessing psychostimulant medication efficacy and implementing robust prescription monitoring systems. Future research should focus on addressing treatment gaps through epidemiological studies and clinical trials to compare different psychostimulant classes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
MaMA: Investigation, Writing – review & editing, Conceptualization, Validation, Funding acquisition, Project administration, Writing – original draft, Methodology. NA: Writing – review & editing, Validation, Writing – original draft, Conceptualization. BA: Writing – original draft, Writing – review & editing. MKA: Writing – original draft, Writing – review & editing. SA: Writing – review & editing, Supervision, Writing – original draft. AA: Writing – review & editing, Writing – original draft. MoMA: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by King Salman Center for Disability Research (Award ID: KSRG-2024-244).
Acknowledgments
The authors extend their appreciation to the King Salman Center For Disability Research for funding this work through Research Group no KSRG-2024-224.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1624590/full#supplementary-material
Supplementary Table 1 | Search terms and database used in this narrative review.
Abbreviations
ADHD, Attention-Deficit/Hyperactivity Disorder; AMPH, Amphetamine-based stimulants; MPH, Methylphenidate-based stimulants; CPGs, Clinical Practice Guidelines; MOH, Ministry of Health (Saudi Arabia); SFDA, Saudi Food and Drug Authority; CHI, Council of Health Insurance; AAP, American Academy of Pediatrics; NICE, National Institute for Health and Care Excellence.
References
1. Thomas R, Sanders S, Doust J, Beller E, and Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. (2015) 135:e994–1001. doi: 10.1542/peds.2014-3482
2. Polanczyk GV, Willcutt EG, Salum GA, Kieling C, and Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol. (2014) 43:434–42. doi: 10.1093/ije/dyt261
3. Song P, Zha M, Yang Q, Zhang Y, Li X, and Rudan I. The prevalence of adult attention-deficit hyperactivity disorder: A global systematic review and meta-analysis. J Glob Health. (2021) 11:04009. doi: 10.7189/jogh.11.04009
4. Popit S, Serod K, Locatelli I, and Stuhec M. Prevalence of attention-deficit hyperactivity disorder (ADHD): systematic review and meta-analysis. Eur Psychiatry. (2024) 67:e68. doi: 10.1192/j.eurpsy.2024.1786
5. Altwaijri Y, Kazdin AE, Al-Subaie A, Al-Habeeb A, Hyder S, Bilal L, et al. Lifetime prevalence and treatment of mental disorders in Saudi youth and adolescents. Sci Rep. (2023) 13:6186. doi: 10.1038/s41598-023-33005-5
6. American Psychiatric Association p, American Psychiatric Association DSMTFa. Diagnostic and statistical manual of mental disorders: DSM-5™. 5th edition. Washington, DC: American Psychiatric Publishing, a division of American Psychiatric Association (2013).
7. Willoughby MT. Developmental course of ADHD symptomatology during the transition from childhood to adolescence: a review with recommendations. J Child Psychol Psychiatry. (2003) 44:88–106. doi: 10.1111/1469-7610.t01-1-00104
8. Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. (2012) 10:99. doi: 10.1186/1741-7015-10-99
9. Arnold LE, Hodgkins P, Kahle J, Madhoo M, and Kewley G. Long-term outcomes of ADHD: academic achievement and performance. J Atten Disord. (2020) 24:73–85. doi: 10.1177/1087054714566076
10. Levy S, Katusic SK, Colligan RC, Weaver AL, Killian JM, Voigt RG, et al. Childhood ADHD and risk for substance dependence in adulthood: a longitudinal, population-based study. PLoS One. (2014) 9:e105640. doi: 10.1371/journal.pone.0105640
11. Danckaerts M, Sonuga-Barke EJ, Banaschewski T, Buitelaar J, Döpfner M, Hollis C, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. (2010) 19:83–105. doi: 10.1007/s00787-009-0046-3
12. Biederman J, Newcorn J, and Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry. (1991) 148:564–77. doi: 10.1176/ajp.148.5.564
13. Faraone SV. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. (2018) 87:255–70. doi: 10.1016/j.neubiorev.2018.02.001
14. Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. (2018) 5:727–38. doi: 10.1016/S2215-0366(18)30269-4
15. Stuhec M, Lukić P, and Locatelli I. Efficacy, acceptability, and tolerability of lisdexamfetamine, mixed amphetamine salts, methylphenidate, and modafinil in the treatment of attention-deficit hyperactivity disorder in adults: A systematic review and meta-analysis. Ann Pharmacother. (2019) 53:121–33. doi: 10.1177/1060028018795703
16. Boland H, DiSalvo M, Fried R, Woodworth KY, Wilens T, Faraone SV, et al. A literature review and meta-analysis on the effects of ADHD medications on functional outcomes. J Psychiatr Res. (2020) 123:21–30. doi: 10.1016/j.jpsychires.2020.01.006
17. Childress AC. Stimulants. Child Adolesc Psychiatr Clin N Am. (2022) 31:373–92. doi: 10.1016/j.chc.2022.03.001
18. Pliszka SR. Non-stimulant treatment of attention-deficit/hyperactivity disorder. CNS Spectr. (2003) 8:253–8. doi: 10.1017/S1092852900018460
19. Faraone SV, Banaschewski T, Coghill D, Zheng Y, Biederman J, Bellgrove MA, et al. The World Federation of ADHD International Consensus Statement: 208 Evidence-based conclusions about the disorder. Neurosci Biobehav Rev. (2021) 128:789–818. doi: 10.1016/j.neubiorev.2021.01.022
20. Daley D and Birchwood J. ADHD and academic performance: why does ADHD impact on academic performance and what can be done to support ADHD children in the classroom? Child Care Health Dev. (2010) 36:455–64. doi: 10.1111/j.1365-2214.2009.01046.x
21. Biederman J, Petty CR, Evans M, Small J, and Faraone SV. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. (2010) 177:299–304. doi: 10.1016/j.psychres.2009.12.010
22. Shahnovsky O, Apter A, and Barzilay S. The association between hyperactivity and suicidal behavior and attempts among children referred from emergency departments. Eur J Invest Health Psychol Education. (2024) 14:2616–27. doi: 10.3390/ejihpe14100172
23. Caye A, Swanson J, Thapar A, Sibley M, Arseneault L, Hechtman L, et al. Life span studies of ADHD-conceptual challenges and predictors of persistence and outcome. Curr Psychiatry Rep. (2016) 18:111. doi: 10.1007/s11920-016-0750-x
24. Mikami AY. The importance of friendship for youth with attention-deficit/hyperactivity disorder. Clin Child Fam Psychol Rev. (2010) 13:181–98. doi: 10.1007/s10567-010-0067-y
25. Wolraich ML, Hagan JF Jr., Allan C, Chan E, Davison D, Earls M, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. (2019) 144:e20192528. doi: https://doi.org/10.1542/peds.2019-2528
26. NICE. National institute for health and care excellence: guidelines. In: Attention deficit hyperactivity disorder: diagnosis and management. National Institute for Health and Care Excellence (NICE) Copyright © NICE, London (2019). p. 2018.
27. Chang Z, Quinn PD, O’Reilly L, Sjölander A, Hur K, Gibbons R, et al. Medication for attention-deficit/hyperactivity disorder and risk for suicide attempts. Biol Psychiatry. (2020) 88:452–8. doi: 10.1016/j.biopsych.2019.12.003
28. Taipale H, Bergström J, Gèmes K, Tanskanen A, Ekselius L, Mittendorfer-Rutz E, et al. Attention-deficit/hyperactivity disorder medications and work disability and mental health outcomes. JAMA Network Open. (2024) 7:e242859–e. doi: 10.1001/jamanetworkopen.2024.2859
29. CDC. Disrupted Access to Prescription Stimulant Medications Could Increase Risk of Injury and Overdose. CDC (2024). Available online at: https://www.cdc.gov/han/2024/han00510.html (Accessed March 14, 2025).
30. WHO. Substandard and falsified medical products (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/substandard-and-falsified-medical-products (Accessed March 14, 2025).
31. Baethge C, Goldbeck-Wood S, and Mertens S. SANRA—a scale for the quality assessment of narrative review articles. Res Integrity Peer Review. (2019) 4:5. doi: 10.1186/s41073-019-0064-8
32. Al-Mohsin ZJ, Al-Saffar HA, Al-Shehri SZ, and Shafey MM. Saudi mothers’ perception of their children with attention-deficit hyperactivity disorder in Dammam, Al-Qatif, and Al-Khobar cities, Saudi Arabia. J Family Community Med. (2020) 27:46–52. doi: 10.4103/jfcm.JFCM_149_19
33. Alsubaie MA, Alshehri ZY, Alawadh IA, Abulreesh RY, Altaweel HM, and Alateeq DA. Treatment adherence and related factors among children with attention-deficit/hyperactivity disorder in Saudi Arabia. Patient Prefer Adherence. (2024) 18:337–48. doi: 10.2147/PPA.S443481
34. Al Haidar FA. Co-morbidity and treatment of attention deficit hyperactivity disorder in Saudi Arabia. East Mediterr Health J. (2003) 9:988–95. doi: 10.26719/2003.9.5-6.988
35. Bashiri FA, Albatti TH, Hamad MH, Al-Joudi HF, Daghash HF, Al-Salehi SM, et al. Adapting evidence-based clinical practice guidelines for people with attention deficit hyperactivity disorder in Saudi Arabia: process and outputs of a national initiative. Child Adolesc Psychiatry Ment Health. (2021) 15:6. doi: 10.1186/s13034-020-00351-5
36. Saudi ADHD Society. Adult Treatment. Available online at: https://cpg.adhd.org.sa/en/guideline/medication-tables/adult-treatment/:~:text=For%20adults%20with%20ADHD%2C%20drug,should%20be%20obtained%20and%20documented.
37. Saudi ADHD Society. Evidence-Based Clinical Practice Guideline for Management of Attention Deficit Hyperactivity Disorder ADHD in Saudi Arabia (2020). Available online at: https://adhd.org.sa/assets/resources/books/Saudi-ADHD-CPG-EN-1.7.0.pdf.
38. Saudi ADHD Society. Medication Availability FAQ (2025). Available online at: https://adhd.org.sa/en/adhd/treatment/medication-availability-faq/.
39. Saudi ADHD Society. The need for additional ADHD medications in Saudi Arabia: Saudi ADHD Society. Available online at: https://adhd.org.sa/en/adhd/treatment/medication-availability-faq/the-need-for-additional-adhd-medications-in-saudi-arabia/.
40. Alghamdi WA, Alzaben FN, Alhashemi HH, Shaaban SS, FaIraq KM, Alsuliamani AS, et al. Prevalence and correlates of attention deficit hyperactivity disorder among college students in Jeddah, Saudi Arabia. Saudi J Med Med Sci. (2022) 10:131–8. doi: 10.4103/sjmms.sjmms_654_21
41. Chan AYL, Ma TT, Lau WCY, Ip P, Coghill D, Gao L, et al. Attention-deficit/hyperactivity disorder medication consumption in 64 countries and regions from 2015 to 2019: a longitudinal study. EClinicalMedicine. (2023) 58:101780. doi: 10.1016/j.eclinm.2022.101780
42. MOH. MOH Protocols for the Management of Attention Deficit Hyperactive Disorder (ADHD) Across the Life Span (2019). Available online at: https://www.moh.gov.sa/en/Ministry/MediaCenter/Publications/Documents/MOH-Protocol-for-ADHD-Across-the-Life-Span.pdf.
43. Council of Health Insurance. Attention Deficit Hyperactivity Disorder CHI Formulary Indication Review (2024). Available online at: https://www.chi.gov.sa/Style%20Library/IDF_Branding/Indication/209%20-%20ADHD-Indication%20Update.pdf.
44. Saudi ADHD Society. The need for additional ADHD medications in Saudi Arabia. Available online at: https://adhd.org.sa/en/adhd/treatment/medication-availability-faq/the-need-for-additional-adhd-medications-in-saudi-arabia/.
45. Coghill D, Banaschewski T, Cortese S, Asherson P, Brandeis D, Buitelaar J, et al. The management of ADHD in children and adolescents: bringing evidence to the clinic: perspective from the European ADHD Guidelines Group (EAGG). Eur Child Adolesc Psychiatry. (2023) 32:1337–61. doi: 10.1007/s00787-021-01871-x
46. Faraone SV. Using meta-analysis to compare the efficacy of medications for attention-deficit/hyperactivity disorder in youths. P t. (2009) 34:678–94.
Keywords: attention deficit hyperactivity disorder, amphetamine, lisdexamfetamine, psychostimulants, access, ADHD
Citation: Alotaibi MM, Alrashdi NZ, Alzubaidi B, Almutairi MK, Alanazi SA, Almutairi AB and Alqahtani MM (2025) Challenges in amphetamine medication availability for individuals with ADHD: a narrative review of the current state of evidence. Front. Psychiatry 16:1624590. doi: 10.3389/fpsyt.2025.1624590
Received: 07 May 2025; Accepted: 07 July 2025;
Published: 23 July 2025.
Edited by:
Alessandra Maria Passarotti, University of Illinois Chicago, United StatesReviewed by:
Matej Stuhec, University of Maribor, SloveniaFrancesco Maria Boccaccio, University of Catania, Italy
Copyright © 2025 Alotaibi, Alrashdi, Alzubaidi, Almutairi, Alanazi, Almutairi and Alqahtani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mansour M. Alotaibi, bWFuc291ci5hbG90YWliaUBuYnUuZWR1LnNh
 Mansour M. Alotaibi
Mansour M. Alotaibi Naif Z. Alrashdi3,4
Naif Z. Alrashdi3,4 Bakriah Alzubaidi
Bakriah Alzubaidi Marzouq K. Almutairi
Marzouq K. Almutairi Sultan A. Alanazi
Sultan A. Alanazi