- 1Department of General Psychiatry, The Affiliated Mental Health Center of Jiangnan University, Wuxi, Jiangsu, China
- 2School of Wuxi Medicine, Nanjing Medical University, Wuxi, Jiangsu, China
- 3Department of Medical Imaging, Shanghai Health and Medical Center, Wuxi, Jiangsu, China
Background: Schizophrenia patients with auditory verbal hallucinations exhibit brain structure abnormalities. However, the characterization of sulcal depth alterations and associated functional connectivity across the whole brain remains unclear.
Method: We recruited 38 schizophrenia patients with auditory verbal hallucinations and 31 schizophrenia patients without auditory verbal hallucinations. Magnetic resonance imaging data were collected on all participants, and clinical symptoms were assessed using standardized clinical scales. Structural abnormalities identified through sulcal depth analysis were localized to specific brain regions, which were subsequently selected as seed regions for functional connectivity analysis. Correlation analysis was employed to explore the associations between sulcal depth, functional connectivity, and the severity of clinical symptoms in individuals with schizophrenia with auditory verbal hallucinations.
Results: Schizophrenia patients with auditory verbal hallucinations exhibited significantly increased sulcal depth in left hemispheric regions including the lingual gyrus, cingulate gyrus, pericalcarine cortex, parahippocampal gyrus, superior frontal gyrus, cuneus, and precuneus, whereas decreased sulcal depth was observed in right hemispheric regions encompassing the superior parietal gyrus, superior frontal gyrus, lingual gyrus, lateral occipital cortex, fusiform gyrus, postcentral gyrus, middle frontal gyrus, precentral gyrus, inferior temporal gyrus, precuneus, and parahippocampal gyrus compared to schizophrenia patients without auditory verbal hallucinations. Seed-based functional connectivity analysis revealed widespread weakened connectivity in schizophrenia patients with auditory verbal hallucinations, particularly with the superior frontal gyrus, angular gyrus, putamen, and other regions. The increased sulcal depth cluster in schizophrenia patients with auditory verbal hallucinations was significantly correlated with negative syndromes and general psychopathology of Positive and Negative Syndrome Scale.
Conclusion: These findings highlight sulcal depth and associated functional connectivity abnormalities in schizophrenia patients with auditory verbal hallucinations, implicating early neurodevelopmental disturbances involving the default mode network and visual cortex. Sulcal depth may represent a promising biomarker for early diagnosis.
1 Introduction
Schizophrenia (SCZ) is a severe, chronic psychiatric disorder characterized by heterogeneous symptoms, including hallucinations, delusions, disorganized thinking, and affective flattening (1). Among these, auditory verbal hallucinations (AVH), defined as the perception of speech in the absence of external auditory stimuli, represent one of the most prevalent and clinically significant positive symptoms, affecting approximately 60–80% of individuals with SCZ (2, 3). These hallucinations frequently consist of negative or commanding content, which can cause substantial distress and functional impairment (4). AVH profoundly affects patients’ quality of life and is closely associated with increased risks of self-harm and suicide, as well as poorer long-term clinical outcomes (5). Despite the availability of various treatment approaches, including antipsychotic medications, brain stimulation techniques, and cognitive behavioral therapy, a considerable proportion of patients (around 30%) continue to experience persistent AVH that is resistant to conventional interventions (6). The persistence and severity of AVH in SCZ highlight a critical need to better understand their underlying neurobiological mechanisms.
Growing evidence suggested that AVH is linked to impairments in the brain’s auditory processing pathways (7, 8). Structural Magnetic Resonance Imaging (MRI) revealed consistent gray matter volume (GMV) reductions in key regions of SCZ patients with AVH, notably the left anterior insula and inferior frontal gyrus (9), with inverse correlations between AVH severity and GMV in the fusiform gyrus, inferior temporal gyrus (ITG), orbitofrontal gyrus, and superior frontal gyrus (SFG) (10). While GMV reflects broad neurodevelopmental changes, surface-based morphological analyses reveal more detailed cortical abnormalities, such as cortical thickness and gyrification index (11). Multiple neuroimaging studies identified reduced cortical thickness in SCZ patients with AVH, particularly in the temporal lobe and orbitofrontal cortex regions (12, 13). However, SCZ patients with AVH exhibit increased gyrification index specifically in the left superior parietal gyrus (SPG) and right anterior cingulate cortex (14). These diverse morphological observations highlight the need for more refined and in-depth investigations into cortical architecture, particularly through advanced surface-based metrics that can capture subtle structural variations beyond conventional measures.
Sulcal depth is a crucial morphological feature that reflects cortical complexity and folding patterns. Unlike other cortical measures, sulcal depth demonstrates remarkable neurodevelopmental stability, forming during fetal and infant stages before stabilizing in childhood and adolescence (15). Critically, this stability persists throughout the lifespan, a recent large-scale review confirming sulcal pits as fixed neuroanatomical markers (16). Notably, their integrity is maintained even in elderly populations, despite accelerated cortical atrophy typically emerging at the late age of 70 (17). Emerging evidence suggested that interindividual variations in sulcal depth correlate with cognitive function, intelligence, and neuropsychiatric disorders (18–20). Despite its potential significance, research on sulcal depth in SCZ patients with AVH remains limited, with only two studies examining sulcal pits to date. The first study (21) reported that the distribution of sulcal pits in the left superior temporal gyrus of SCZ patients with AVH was less than healthy controls, suggesting that the patients had an atypical morphological pattern. Another study (22) revealed that SCZ patients with AVH exhibited increased sulcal depth in the left inferior frontal cortex and prefrontal regions compared to SCZ patients who had never experienced AVH, which overlapped with Broca’s area and Brodmann area 47. Comparisons between SCZ patients with AVH and healthy controls revealed nearly identical results. These results indicated that early-emerging morphological abnormalities in language-related cortical areas may contribute to AVH vulnerability in SCZ.
Previous studies suggested that the structural characteristics of the cerebral cortex may influence brain function (23, 24). Abnormal cortical folding can alter the local neural circuit topology (25) and may disrupt the local microcircuit excitation and inhibition balance (26), leading to abnormal information output in specific brain regions, which can then spread through long-range connections to the whole-brain network, causing functional connectivity (FC) abnormalities. A recent study further supports this notion by showing that impaired corollary discharge and efference copy signals due to abnormal prefrontal-motor cortex connectivity lead to misinterpretation of self-generated speech as external voices, contributing to AVH (27). However, current studies focus on a single modality and selectively analyze brain regions, which fail to comprehensively capture the characteristics of sulcal depth across the whole brain. Importantly, the functional implications of abnormal sulcal depth remain largely unexplored. Addressing this critical knowledge gap is essential for advancing the development of more effective and targeted interventions to alleviate this debilitating symptom. Given that sulcal depth reflects cortical folding patterns and may index region-specific neurodevelopmental abnormalities, we hypothesized that structural deviations in sulcal depth would correspond to alterations in functional integration across neural circuits. To test this hypothesis, we conducted a comprehensive multimodal neuroimaging study combining whole-brain structural and functional analyses using seed-based FC analysis. First, we aimed to confirm whether SCZ patients with AVH demonstrate sulcal depth alterations consistent with previous findings. Subsequently, we examined whether corresponding FC abnormalities were present in these regions. Finally, we investigated relationships between these neuroimaging changes and clinical symptoms.
2 Materials and methods
2.1 Participants
All participants were recruited from the Mental Health Centre of Jiangnan University, China. The participants consisted of 38 SCZ patients with AVH (AVH patients) and 31 SCZ patients without AVH (NAVH patients). The study enrolled Han Chinese participants aged 18–65 years who were right-handed and had normal hearing. All patients were diagnosed with SCZ by senior psychiatrists in accordance with DSM-5 criteria (28). AVH patients were required to have a score ≥ 4 on the Positive and Negative Syndrome Scale (PANSS) P3 hallucination item, with clinical confirmation of AVH (29), while NAVH patients were required by a score = 1 on the PANSS P3 item hallucination and absence of all hallucination subtypes. Exclusion criteria included other psychiatric disorders (e.g., schizoaffective disorder, mood disorders, dementia, or substance dependence), severe or unstable somatic diseases (e.g., heart disease), narrow-angle glaucoma, a history of epilepsy or neuroleptic malignant syndrome, inability to adhere to prescribed medication, pregnancy or breastfeeding status, and contraindications for MRI.
This study was reviewed and approved by the Ethics Committee of the Affiliated Mental Health Center of Jiangnan University (Ethical approval number: WXMHCIRB2025LLky004). All participants signed written informed consent forms before the experiment.
2.2 Clinical assessment
A comprehensive psychiatric assessment was conducted for all participants by professional psychiatrists. We used PANSS to assess the positive syndromes (PANSS-P), negative syndromes (PANSS-N), and general psychopathology (PANSS-G) of SCZ patients and to identify whether they experience AVH. For AVH patients, we used the Hoffman Auditory Hallucination Rating Scale (HAHRS) to assess the severity of AVH (14). The scale can assess the frequency, reality, loudness, number of voices, length of words, attentional salience, and distress level of AVH. The Modified Overt Aggression Scale (30) and the Buss-Perry Aggression Questionnaire (31) were used to assess participants’ aggressive behavior. The Montreal Cognitive Assessment (32) was used to assess cognitive function. The Hamilton Rating Scale for Anxiety (33), the Hamilton Rating Scale for Depression (34) were used to assess emotional state. The Global Assessment Functioning Scale (35), the Social Disability Screening Schedule (36), and the Perceived Social Support Scale (PSSS) (37)were used to assess global function and social support. Medication doses of participants were calculated using the Olanzapine equivalent dose (38).
2.3 MRI data acquisition
Brain imaging was performed using a 3.0-Tesla MRI scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) at the Department of Medical Imaging, Shanghai Health and Medical Center, Wuxi, China. Before scanning, participants were briefed about the procedure to alleviate their anxiety and enhance cooperation. A custom-made sponge pad was used to securely fix the participants’ heads to minimize head motion artifacts in the images. Additionally, sponge earplugs were given to reduce the scanner noise. All participants completed the MRI in an awake state with eyes closed, and no overt signs of hallucinatory behavior (e.g., vocal responses or distressed movements) were observed during the scanning. The specific parameters for the three-dimensional T1-weighted images were as follows: echo time (TE) = 2.98 ms; repetition time (TR) = 2530 ms; flip angle (FA) = 7°; field of view (FOV) = 256×256 mm; slice thickness (ST) = 1.0 mm. The resting-state functional MRI acquisition parameters were as follows: TE = 30 ms, TR = 2000 ms, FA = 90°, FOV = 224×224 mm, ST = 3.5mm; slice number = 33 slices, with a total of 240 time points collected.
2.4 MRI data processing
T1-weighted images were processed with the CAT12 toolbox, which is based on SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). The key steps involved in this process are as follows. First, the DICOM files were converted into NIFITI images. Next, spatial normalization was used to align the images with the standard Montreal Neurological Institute template. Then, brain tissues were segmented into white matter, gray matter, and cerebrospinal fluid. The CAT12 toolbox offers a projection-based thickness technique to estimate the central surface of hemispheres in an integrated manner, which is a surface located within the gray matter, approximately midway between the gray-white matter boundary and the pial surface. Then, sulcal depth is calculated based on the Euclidean distance between the central surface and its convex hull (39). Finally, sulcal depth was smoothed using a Gaussian kernel with a full-width at half-maximum (FWHM) of 25 mm (40). To identify and name brain regions, we used the Desikan-Killiany atlas (41). This atlas provides detailed cortical parcellation based on anatomical features. Labels were assigned to cortical regions by matching the central surface with corresponding atlas regions.
Resting-state functional MRI images were processed with the CONN toolbox (https://web.conn-toolbox.org/). Brain regions exhibiting significant differences in sulcal depth were selected as seed regions for FC analysis. The processing steps of FC are briefly described as follows. The first step was to perform head motion correction. Then, spatial normalization was carried out to standardize the data and spatial smoothing using a Gaussian smoothing kernel with 8 mm FWHM (42). Next, the denoising was performed to remove noise from the data. Finally, frequencies below 0.008 Hz and above 0.09 Hz (43) were filtered out to eliminate irrelevant signals. All data underwent quality control, where the MaxMotion values exceeding the threshold of the third quartile plus three times the interquartile range or falling below the first quartile minus three times the interquartile range were excluded as extreme values (44).
2.5 Statistical analysis
Statistical analyses were performed using SPSS version 27.0 (IBM Corp., Armonk, NY, USA; http://www.spss.com). Demographic and clinical data were analyzed using independent two-sample t-tests for parametric continuous variables, Mann-Whitney U tests for nonparametric continuous variables, and chi-squared tests for categorical variables such as gender. The threshold for statistical significance was set at p < 0.05 (uncorrected).
Group differences in sulcal depth were evaluated by a general linear model (GLM), with age, gender, and years of education as covariates. The significance level was set at a cluster-level corrected p < 0.05 using False Discovery Rate (FDR) correction, following an initial vertex-wise threshold of p < 0.05. FC between the identified seed regions and all voxels across the whole brain was then evaluated. GLM was employed to compare FC between groups, with age, gender, and years of education included as covariates. The significance level was set at p < 0.05, corrected for multiple comparisons using cluster-level FDR correction, following an initial voxel-wise threshold of p < 0.05.
Correlation analyses were conducted to examine the relationships between the severity of clinical symptoms (measured by scores on HAHRS, PANSS-P3 hallucination, PANSS-P, PANSS-N, and PANSS-G subscales, PSSS) and both sulcal depth and FC among patients with AVH, controlling for age, gender, and years of education as covariates. For exploratory purposes, the significance threshold for these analyses was set at p < 0.05.
3 Results
3.1 Demographic and clinical characteristics
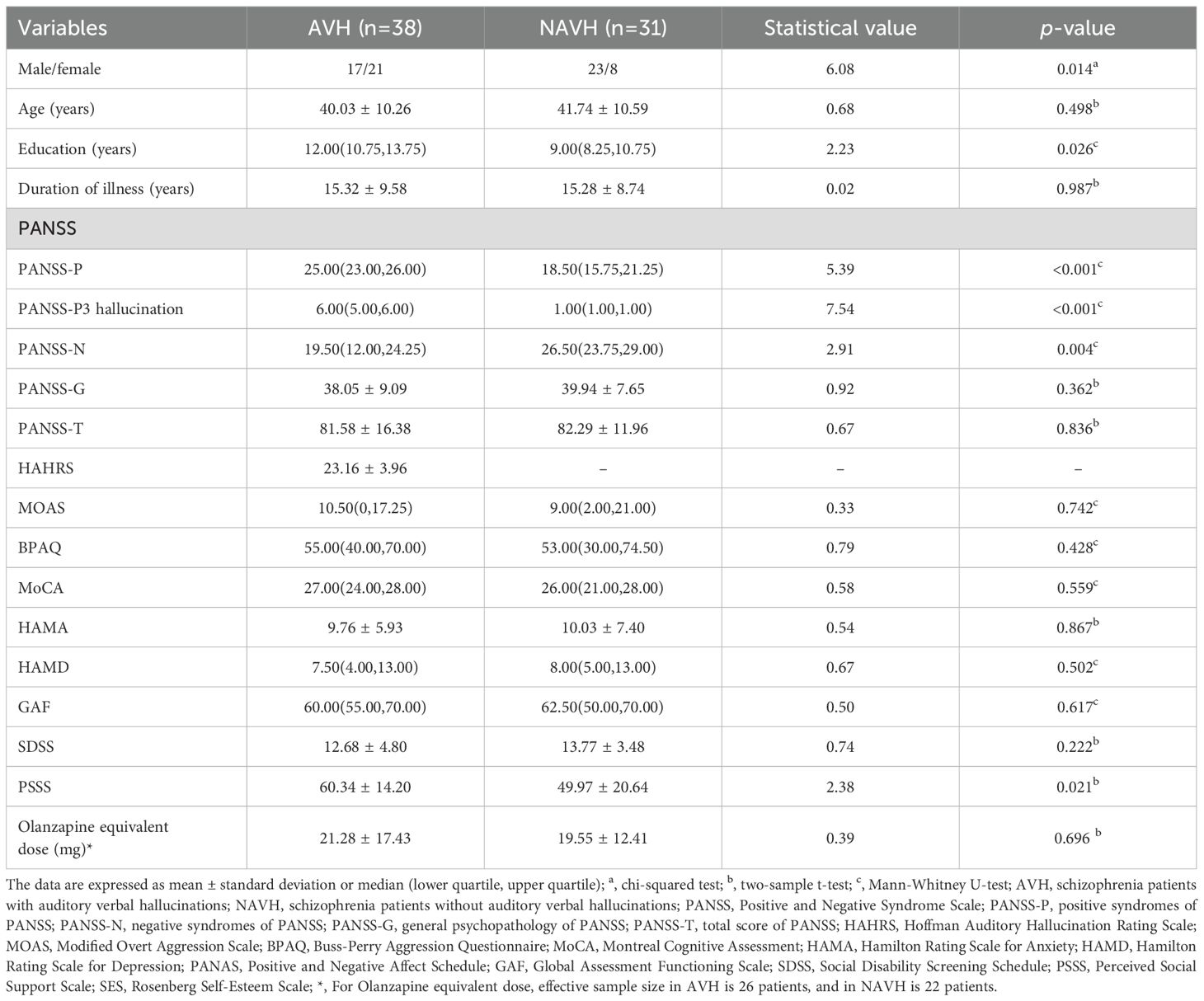
Demographic and clinical characteristics of the samples are summarized in Table 1. A total of 69 participants were enrolled, including 38 AVH patients and 31 NAVH patients. There was missing data for the Olanzapine equivalent dose of antipsychotic medications. Specifically, complete records were available for 26 AVH patients and 20 NAVH patients. The two groups exhibited significant differences in terms of gender and years of education. In AVH patients, there were more females than males. In NAVH patients, there were more males than females. Moreover, AVH patients had a higher level of education in our research. In terms of clinical assessments, significant differences were observed between the two groups in the PANSS-P, PANSS-P3 hallucination, PANSS-N, and PSSS. Compared to NAVH patients, AVH patients exhibited more severe positive symptoms and experienced more intense hallucinations. AVH patients also had fewer negative symptoms and reported higher levels of perceived social support. No statistically significant differences were found in other clinical measures between the groups.
3.2 Differences in sulcal depth between groups
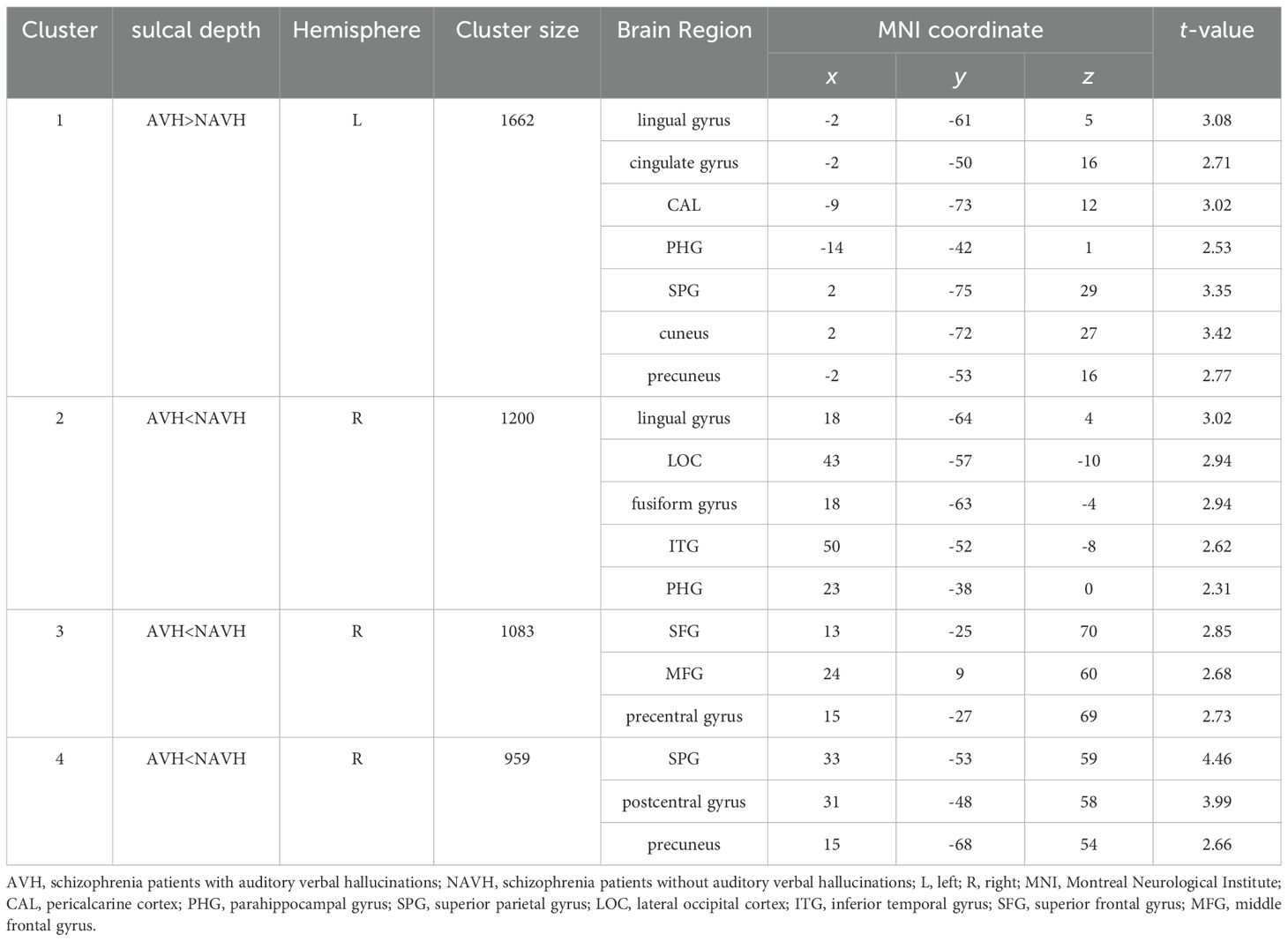
Compared with NAVH patients, AVH patients exhibited a cluster of increased sulcal depth in the left hemisphere, including the lingual gyrus, cingulate gyrus, pericalcarine cortex, parahippocampal gyrus (PHG), SPG, cuneus (CUN), and precuneus (PCUN) (Table 2 and Figure 1A). The mean values of the cluster between the two groups were significantly different (Figure 1C). Besides, AVH patients showed three clusters of decreased sulcal depth in the right hemisphere, particularly in the SPG, SFG, lingual gyrus, lateral occipital cortex, fusiform gyrus, postcentral gyrus, middle frontal gyrus (MFG), precentral gyrus (PreCG), ITG, PCUN, and PHG (Table 2 and Figure 1B). The mean values of the cluster between the two groups were significantly different (Figure 1D).
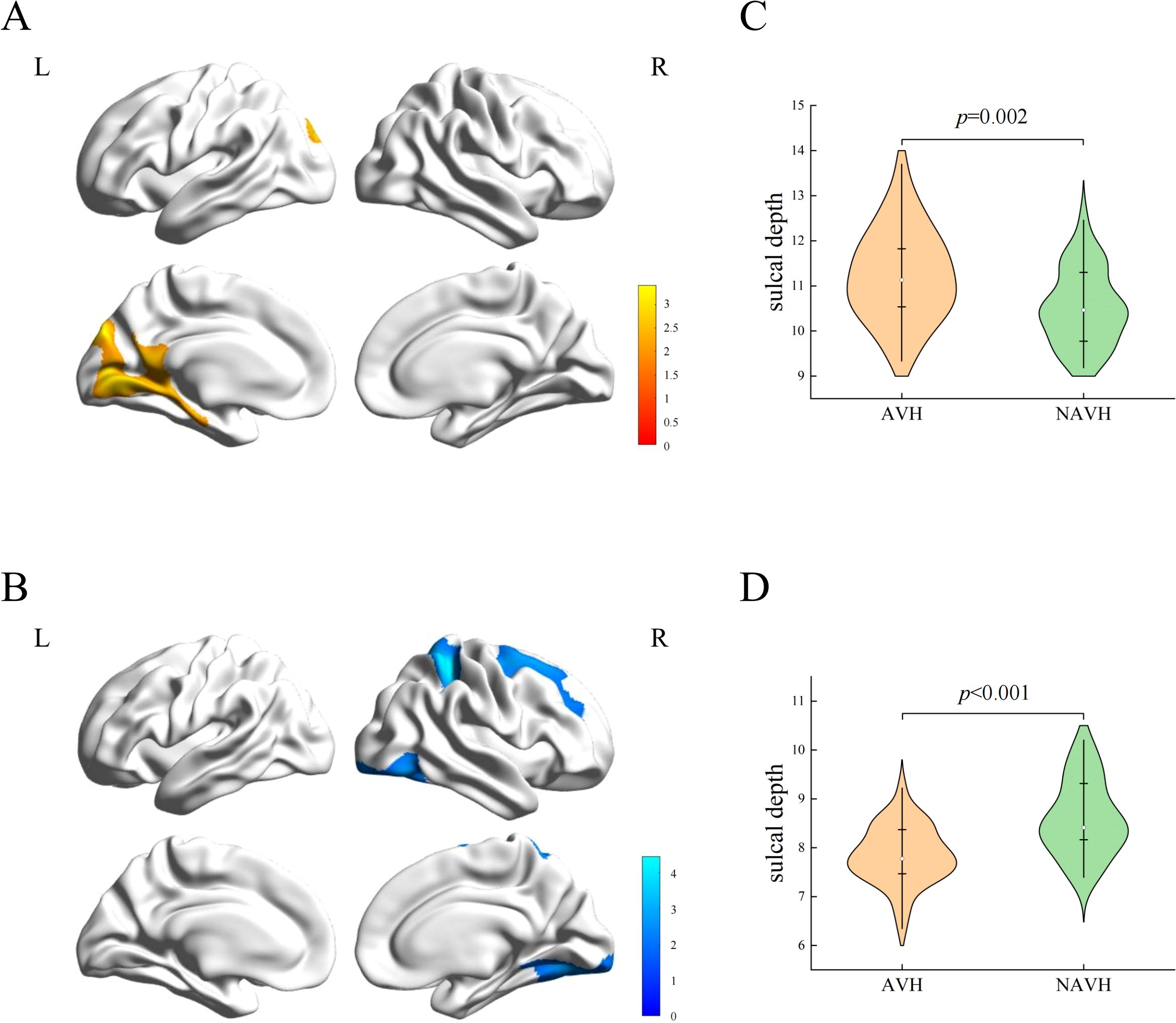
Figure 1. Sulcal depth differences between two groups. (A) The cluster of increased sulcal depth in AVH patients compared to NAVH patients. (B) The cluster of decreased sulcal depth in AVH patients compared to NAVH patients. (C) The difference in the mean values of the increased cluster between the two groups. (D) the difference in the mean values of the decreased clusters between the two groups.
3.3 FC abnormalities associated with differences in sulcal depth
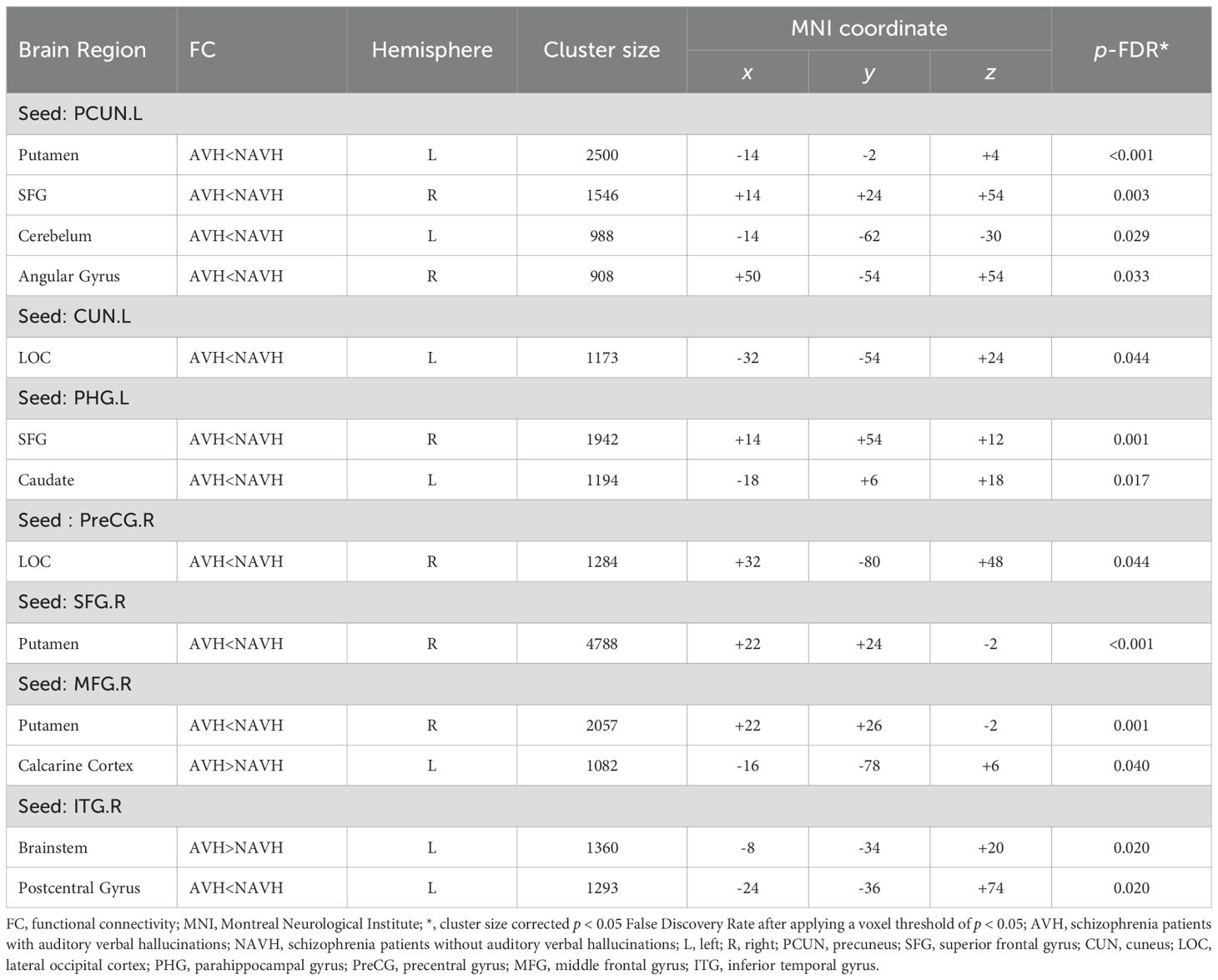
Based on sulcal depth results, brain regions with sulcal depth differences were defined as seed regions. We selected 18 seed regions (Figure 2, All seed regions), of which 7 seed regions exhibited FC abnormalities (Table 3; Figure 2), including left PHG, left CUN, left PCUN, right SFG, right MFG, right PreCG, and right ITG. FC analysis revealed widespread connectivity reductions in AVH patients compared to NAVH patients. Specifically, we observed reduced FC between left PHG and right SFG and left caudate, between left CUN and left lateral occipital cortex, between left PCUN and left putamen, right SFG, right angular gyrus, between right SFG and right putamen, between right MFG and right putamen, between right PreCG and right lateral occipital cortex, between right ITG and left postcentral gyrus. Conversely, increased FC was identified between right ITG and brainstem, as well as between the right MFG and left calcarine cortex.
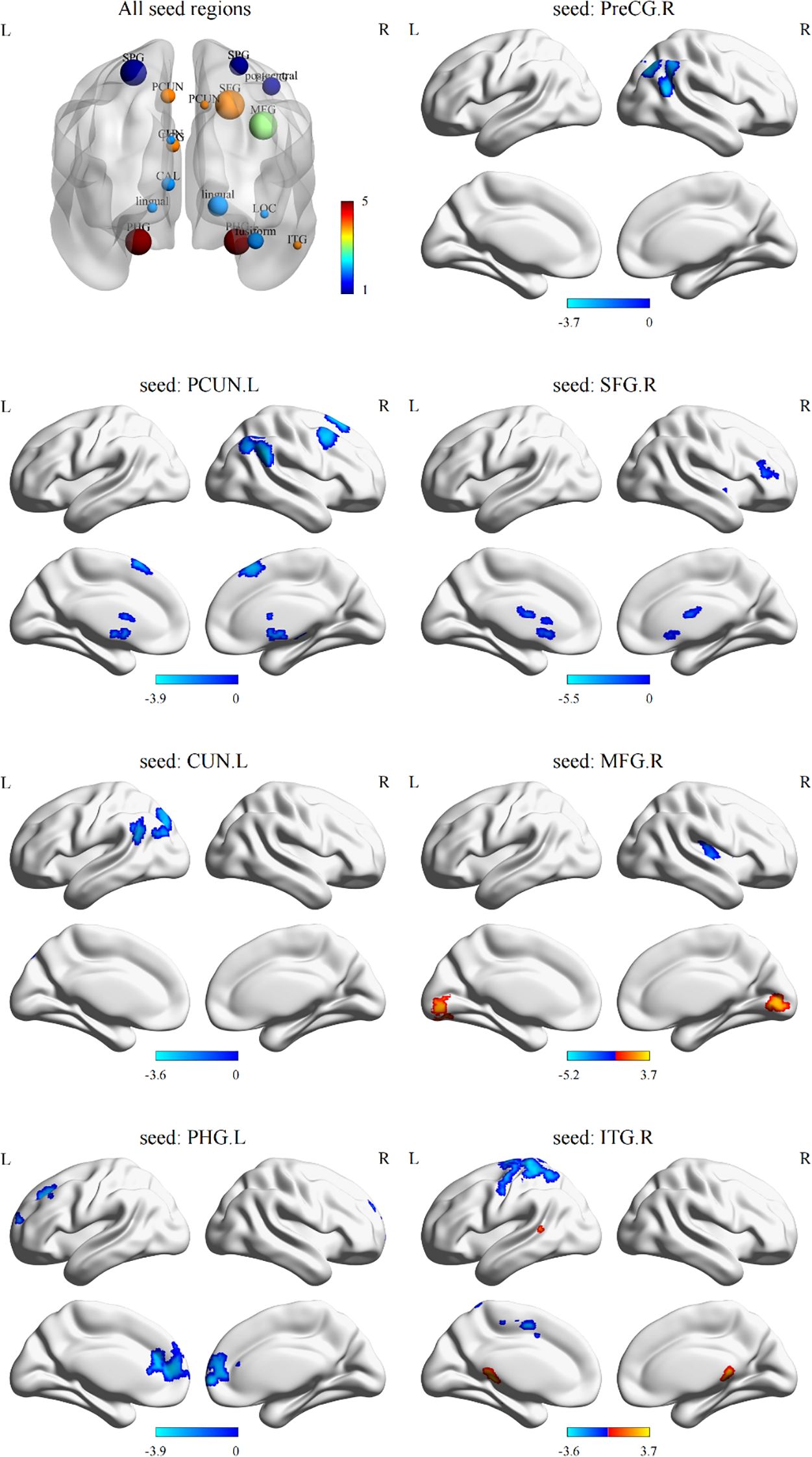
Figure 2. Differences in functional connectivity using sulcal depth differences as seed regions between two groups. All seed regions refer to the eighteen seed regions selected based on the brain regions with differences in sulcal depth. Among them, the following seven seed regions show abnormal functional connectivity. L, left hemisphere; R, right hemisphere; SPG, superior parietal gyrus; PCUN, precuneus; PreCG, precentral gyrus; SFG, superior frontal gyrus; MFG, middle frontal gyrus; CUN, cuneus; PCG, posterior cingulate gyrus; CAL, pericalcarine cortex; LOC, lateral occipital gyrus; PHG, parahippocampal gyrus; ITG, inferior temporal gyrus.
3.4 Correlation of sulcal depth and FC with psychiatric symptoms
The correlation analysis showed that the mean value of the increased sulcal depth cluster in AVH patients was positively correlated with the PANSS-N (r = 0.34, p = 0.045, uncorrected) (Figure 3A). This cluster also exhibited a significant positive correlation with the PANSS-G (r = 0.42, p = 0.013, uncorrected) (Figure 3B). The specific results regarding the correlation of sulcal depth and FC with psychiatric symptoms are presented in the Supplementary Materials (Supplementary Table S1). Regrettably, these results did not survive multiple comparison correction.
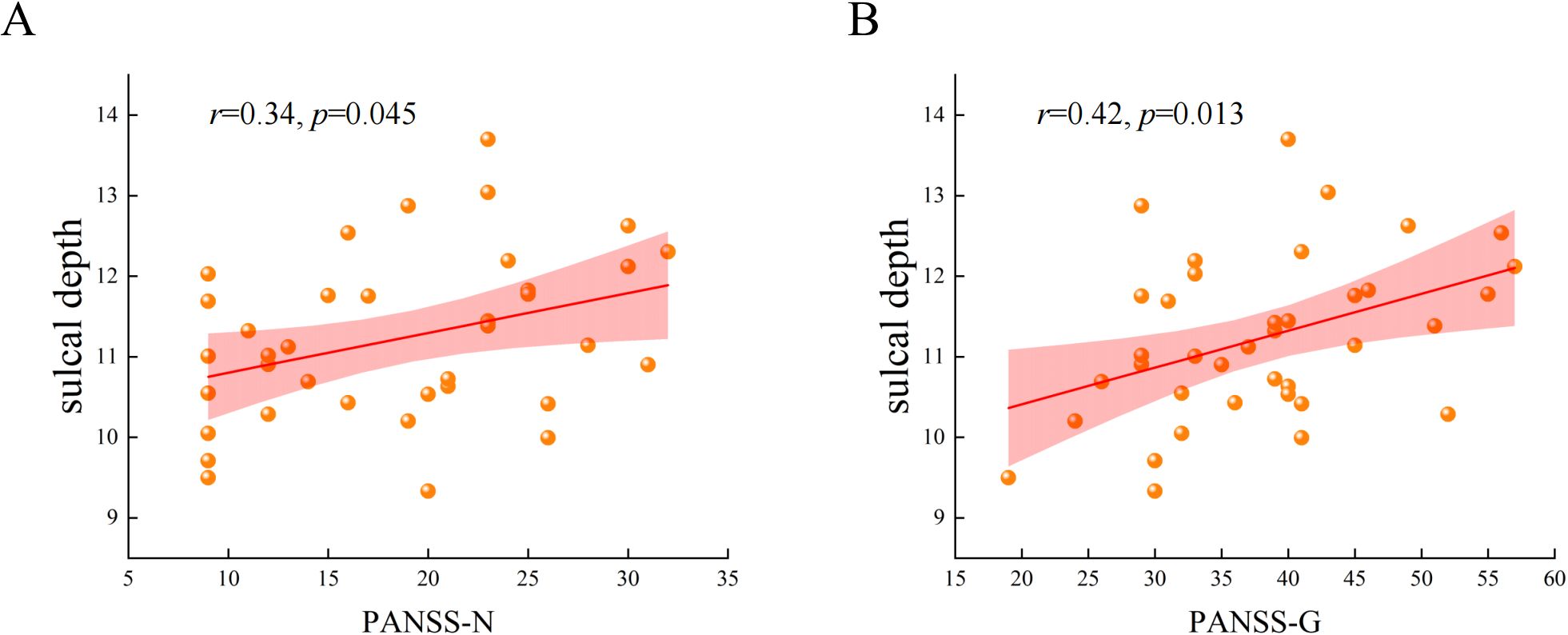
Figure 3. The correlation between the mean values of the increased cluster in sulcal depth with PANSS-N and PANSS-G in AVH patients. (A) The mean values of the increased cluster in sulcal depth were positively correlated with the scores of negative syndromes of Positive and Negative Syndrome Scale. (B) The mean values of the increased cluster in sulcal depth were positively correlated with the scores of general psychopathologies of Positive and Negative Syndrome Scale.
4 Discussion
This study analyzed the sulcal depth and associated FC in brain regions of AVH patients and NAVH patients and drew three conclusions. First, the cluster of increased sulcal depth in AVH patients was located in the left hemisphere, including the lingual gyrus, cingulate gyrus, pericalcarine cortex, PHG, SPG, CUN, and PCUN. Then, the clusters of decreased sulcal depth were observed in the right hemisphere, including SPG, SFG, lingual gyrus, lateral occipital cortex, fusiform gyrus, postcentral gyrus, MFG, PreCG, ITG, PCUN, and PHG. Secondly, our seed-based FC analysis revealed significantly decreased FC between the seed regions and several areas, such as the SFG, angular gyrus, putamen, and others. Third, in AVH patients, the brain regions with increased sulcal depth cluster were significantly correlated with PANSS-N and PANSS-G.
Our study identified abnormal sulcal depth in key regions of the default mode network (DMN), such as the PCUN, cingulate gyrus, and SPG, and found abnormal FC when using the PCUN as seed regions. DMN plays an important role in self-reference, social cognition, episodic and autobiographical memory, language and semantic memory, and mind wandering (45). Structurally, we observed abnormal sulcal depth alterations in key DMN regions, consistent with findings from Katharina et al. (46) and Shen et al. (14), who reported elevated local gyrification index in the PCUN, cingulate gyrus, and SPG. These structural changes may influence the pathogenesis of AVH in two critical ways. First, they impair spatial information processing and disrupt sensory integration. Second, they weaken self-referential processing, thereby exacerbating patients’ misattribution of hallucinatory sources (45, 47). Functionally, our study revealed three key connectivity abnormalities. First, the diminished FC from PCUN to putamen resonated with previous findings of causal disconnections in the striatal-DMN circuit (48), underscoring the crucial role of the striatal-DMN circuit in AVH neurobiology. Second, reduced FC between PCUN and angular gyrus reflects dysfunction in the lateral parietal system, which is a hub for internal speech monitoring and self-referential integration, suggesting impaired mechanisms for monitoring internally generated speech (49, 50). Third, the reduced FC between the PCUN and SFG in AVH patients may indicate that disruption in the crucial interplay and top-down modulation between the DMN and cognitive control network is disrupted. Xie et al. (51) found that low-frequency repetitive transcranial magnetic stimulation can improve the FC between these two regions in AVH patients and alleviate hallucination symptoms, further confirming their significant role in AVH.
Additionally, a notable reduction in FC was exhibited between the putamen and brain regions such as the SFG and MFG. Putamen is a key component of the striatum, the reduced FC may impair the striatum’s role in filtering and monitoring spontaneous activations. This phenomenon is consistent with the theory that cortico-striatal dysfunction disrupts gating mechanisms, allowing unmodulated internal signals to enter consciousness as hallucinations (52). Moreover, hallucinations are mediated by increased dopamine levels within the striatum (53), which is also an important target for antipsychotic medications. Chen et al. (54) proposed a significant correlation between the putamen-auditory cortex connectivity and the neuropathological mechanisms of AVH. Yang et al. also confirmed that the FC of prefrontal-striatal plays an important role in attention and executive dysfunction in SCZ patients (55). Our study further elucidates the significant role of the prefrontal-striatal neural circuits in the neuropathology of AVH.
We also found a significant decrease in sulcal depth in the PHG, which is anatomically connected to the cingulate gyrus, temporal lobe, and prefrontal cortex. PHG is a key component of the Papez circuit, which is a circuit believed to control memory, emotions, and motivations (56, 57). In addition, the PHG is associated with auditory working memory and related to the negative emotions associated with tinnitus (58, 59). A review (60) proposed that the PHG relays AVH memories to the auditory cortex to consolidate acoustic representations and maintain perceptual memory, which is mistakenly categorized as external stimuli. These findings support the theory the memory deficit model of source-monitoring problems (52). PHG is a key region within the medial temporal lobes, playing a crucial role in both relational encoding and memory retrieval. Damage or dysfunction in the PHG may lead to impaired binding and retrieval of memory features, thereby affecting an individual’s ability to accurately judge the source of memories (61). Moreover, reduced FC between PHG and SFG directly implicates dysfunctional prefrontal-limbic regulation. This may impair top-down modulation over memory integration processes, potentiating source monitoring failures through misattribution of internal representations.
In the study of cortical structure in AVH patients, we observed changes in sulcal depth in brain regions such as the lingual gyrus, pericalcarine cortex, lateral occipital cortex, fusiform gyrus, and CUN. These regions are primarily responsible for integrating and processing visual information. Previous studies reported that patients with AVH exhibit similarly aberrant FC to multiple brain regions when seeded in the auditory cortex. A widely accepted hypothesis suggested that disrupted FC between visual processing regions (occipital cortex) and auditory neural circuits may induce dysregulated phonological semantic integration mechanisms, potentially serving as a neurobiological substrate for the emergence of AVH (62). Other studies found reduced low-frequency fluctuation amplitude in the lingual gyrus and occipital cortex in SCZ patients (63), and hypoperfusion in the calcarine cortex in AVH patients (64). Lewis et al. (65) proposed that audiovisual synchrony enhances the connection between early visual and auditory areas to improve motion discrimination. Although AVH is classified as an auditory perceptual abnormality, the sensory systems of the brain do not operate independently. Changes in visual-related brain regions may disrupt the integration of perceptual information, thereby impairing normal auditory perception and leading to the occurrence of AVH.
The ITG is also a crucial part of the temporal lobe cortex and belongs to the ventral visual pathway. Although its primary functions are related to vision, it also collaborates with the auditory cortex during language processing, playing a significant role in multi-channel sensory integration, language, and semantic memory (66). Changes in its sulcal depth may affect the feature analysis and pattern recognition of auditory stimuli, leading to deviations in the brain’s processing of normal auditory information. This can cause the brain to misidentify internally generated abnormal signals as real sounds, triggering AVH. A meta-analysis found that SCZ patients generally have cortical thinning in the right temporal lobe (67). Xie et al. (64) reported increased cerebral blood flow in the left ITG and bilateral putamen in AVH patients. Additionally, our study discovered that in AVH patients, FC between the ITG and the brainstem is enhanced, while FC between the ITG and the postcentral gyrus is weakened. Previous studies reported that the increased FC between the ITG and the brainstem in AVH patients may be related to excessive bottom-up signal activation (68). The postcentral gyrus, a brain region associated with inner speech, is fundamental to its generation through its neural activity. Together with the PreCG, it is activated during AVH experiences and plays a significant role in processing and transmitting sensorimotor information (52). The reduced FC between the ITG and postcentral gyrus suggested impaired internal speech monitoring, hindering the verification of auditory perception authenticity and ultimately exacerbating AVH experiences (69, 70).
It is worth noting that our study found that the increased sulcal depth cluster in AVH patients (including the left cingulate gyrus, SPG, PCUN, PHG, etc.) was positively correlated with the PANSS-N and PANSS-G. This may suggest that in AVH patients, the cingulate gyrus is associated with negative symptoms such as apathy, the PHG is related to memory impairments and cognitive dysfunction, and the SPG may be linked to attention deficits and abnormalities in sensory information processing. This study unexpectedly found no significant correlation between the severity of AVH and the sulcal depth or FC. From a neurobiological mechanism perspective, FC changes might more likely reflect the transient neural state of AVH rather than the severity of chronic symptoms (71). Additionally, most AVH patients also present with other symptoms, such as delusions, a certain degree of confusion, and negative symptoms, this result may be related to the diversity of symptoms (72).
This study has several limitations that should be acknowledged. First, a significant limitation is the gender disparity between two groups, with a higher proportion of females in the AVH patients. This gender imbalance may reflect differences in disease characteristics or recruitment bias. Additionally, there was a slight mismatch in years of education between the two groups, which could potentially influence our findings. Although we included gender and years of education as covariates in our regression models, residual confounding may still exist. Future studies should aim to use more matched samples and conduct stratified analyses to explore whether gender or years of education independently influence AVH expression. Second, our study lacked healthy controls, limiting our ability to determine if the observed differences are specific to AVH or general to SCZ. However, our design aimed to explore the neural mechanisms underlying the AVH subtype within SCZ, shedding some light on potential biological correlations of AVH-related sulcal depth and FC through comparisons between the two SCZ groups. Future research should include diverse populations to better clarify specificity. Third, despite a series of analyses conducted, due to missing data on antipsychotic dosages, the potential confounding effects of antipsychotic medications on the study metrics have not been fully ruled out. In future studies, we plan to systematically record detailed antipsychotic dosage information or recruit more drug-naive patients with first-episode illnesses. Fourth, a key limitation is the inability to confirm whether participants were experiencing active AVH during scanning, which may have influenced the interpretation of FC alterations as either state or trait related. Despite these limitations, our study holds significant clinical and scientific value. This is the first research to utilize multimodal brain imaging techniques for demonstrating abnormal sulcal depth and associated FC abnormalities in SCZ patients with AVH.
5 Conclusions
In conclusion, this study is the first to reveal abnormalities in sulcal depth and associated FC abnormalities in SCZ patients with AVH, providing neuroimaging evidence for understanding the neural mechanisms underlying AVH. Our findings suggest that these abnormalities may originate early in neurodevelopment and involve key brain regions and networks, including the DMN, prefrontal-striatal pathway, visual cortex, and the limbic system. These systems may interact to disrupt self-monitoring and sensory processing, thereby contributing to the emergence of AVH. Sulcal depth may serve as a potential imaging biomarker for the early identification of individuals at risk for AVH. However, the utility of sulcal depth for guiding targeted neuromodulation, such as low-frequency repetitive transcranial magnetic stimulation, remains to be determined. While our whole-brain findings suggest broader network involvement beyond established targets, translating this into novel interventions requires future evidence demonstrating that sulcal morphology is modifiable and functionally relevant to outcomes. Our results thus provide a preliminary reference for exploring broader targets, meriting further investigation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of The Affiliated Wuxi Mental Health Center of Jiangnan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
ZG: Data curation, Writing – original draft. ZZ: Writing – review & editing. SW: Investigation, Writing – review & editing, Resources. LY: Visualization, Writing – review & editing. XG: Validation, Writing – review & editing. YX: Data curation, Writing – review & editing. YY: Investigation, Writing – review & editing. ZS: Writing – review & editing, Data curation. HH: Data curation, Writing – review & editing. LT: Writing – review & editing, Funding acquisition, Supervision, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Wuxi Taihu Talent Project (WXTTP2021), Medical Key Discipline Program of Wuxi Health Commission (FZXK2021012).
Acknowledgments
The authors express appreciation to all subjects included in our study for their participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XS declared a shared parent affiliation with the authors to the handling editor at the time of review.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1641190/full#supplementary-material
Supplementary Table 1 | Correlation of sulcal depth and FC with psychiatric symptoms in AVH patients.
References
1. Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, et al. Schizophrenia. Nat Rev Dis Primers. (2015) 1:15067. doi: 10.1038/nrdp.2015.67
2. Wei Y, Xue K, Yang M, Wang H, Chen J, Han S, et al. Aberrant cerebello-thalamo-cortical functional and effective connectivity in first-episode schizophrenia with auditory verbal hallucinations. Schizophr Bull. (2022) 48:1336–43. doi: 10.1093/schbul/sbab142
3. Ren H, Li Z, Li J, Zhou J, He Y, Li C, et al. Correlation between cortical thickness abnormalities of the olfactory sulcus and olfactory identification disorder and persistent auditory verbal hallucinations in Chinese patients with chronic schizophrenia. Schizophr Bull. (2024) 50:1232–42. doi: 10.1093/schbul/sbae040
4. Panikratova YR, Lebedeva IS, Akhutina TV, Tikhonov DV, Kaleda VG, and Vlasova RM. Executive control of language in schizophrenia patients with history of auditory verbal hallucinations: A neuropsychological and resting-state fmri study. Schizophr Res. (2023) 262:201–10. doi: 10.1016/j.schres.2023.10.026
5. Hua Q, Wang L, He K, Sun J, Xu W, Zhang L, et al. Repetitive transcranial magnetic stimulation for auditory verbal hallucinations in schizophrenia: A randomized clinical trial. JAMA network Open. (2024) 7:e2444215. doi: 10.1001/jamanetworkopen.2024.44215
6. Nathou C, Etard O, and Dollfus S. Auditory verbal hallucinations in schizophrenia: current perspectives in brain stimulation treatments. Neuropsychiatr Dis Treat. (2019) 15:2105–17. doi: 10.2147/ndt.S168801
7. Javitt DC and Sweet RA. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci. (2015) 16:535–50. doi: 10.1038/nrn4002
8. Zhuo C, Tian H, Fang T, Li R, Li Y, Kong L, et al. Neural mechanisms underlying visual and auditory processing impairments in schizophrenia: insight into the etiology and implications for tailoring preventive and therapeutic interventions. Am J Trans Res. (2020) 12:7657–69.
9. Romeo Z and Spironelli C. Hearing voices in the head: two meta-analyses on structural correlates of auditory hallucinations in schizophrenia. NeuroImage Clin. (2022) 36:103241. doi: 10.1016/j.nicl.2022.103241
10. Ren H, Li J, Zhou J, Chen X, Tang J, Li Z, et al. Grey matter volume reduction in the frontotemporal cortex associated with persistent verbal auditory hallucinations in Chinese patients with chronic schizophrenia: insights from a 3 t magnetic resonance imaging study. Schizophr Res. (2024) 269:123–9. doi: 10.1016/j.schres.2024.05.009
11. Matsuda Y and Ohi K. Cortical gyrification in schizophrenia: current perspectives. Neuropsychiatr Dis Treat. (2018) 14:1861–9. doi: 10.2147/ndt.S145273
12. Kose G, Jessen K, Ebdrup BH, and Nielsen MO. Associations between cortical thickness and auditory verbal hallucinations in patients with schizophrenia: A systematic review. Psychiatry Research-Neuroimaging. (2018) 282:31–9. doi: 10.1016/j.pscychresns.2018.10.005
13. Ren H, Wang Q, Li C, Li Z, Li J, Dai L, et al. Differences in cortical thickness in schizophrenia patients with and without auditory verbal hallucinations. Front Mol Neurosci. (2022) 15:845970. doi: 10.3389/fnmol.2022.845970
14. Shen X, Jiang F, Fang X, Yan W, Xie S, and Zhang R. Cognitive dysfunction and cortical structural abnormalities in first-episode drug-naïve schizophrenia patients with auditory verbal hallucination. Front Psychiatry. (2022) 13:998807. doi: 10.3389/fpsyt.2022.998807
15. Snyder WE, Vértes PE, Kyriakopoulou V, Wagstyl K, Williams LZJ, Moraczewski D, et al. A bimodal taxonomy of adult human brain sulcal morphology related to timing of fetal sulcation and trans-sulcal gene expression gradients. Neuron. (2024) 112:3396–411.e6. doi: 10.1016/j.neuron.2024.07.023
16. Hostalet N, Salgado-Pineda P, Martínez-Abadías N, and Fatjó-Vilas M. The sulcal pits as neurodevelopmental markers: A systematic review about their potential use in clinical practice. Prog Neuropsychopharmacol Biol Psychiatry. (2025) 137:111289. doi: 10.1016/j.pnpbp.2025.111289
17. Shen X, Liu T, Tao D, Fan Y, Zhang J, Li S, et al. Variation in longitudinal trajectories of cortical sulci in normal elderly. NeuroImage. (2018) 166:1–9. doi: 10.1016/j.neuroimage.2017.10.010
18. Shin SJ, Kim A, Han KM, Tae WS, and Ham BJ. Reduced sulcal depth in central sulcus of major depressive disorder. Exp Neurobiol. (2022) 31:353–60. doi: 10.5607/en22031
19. Lyu I, Kang H, Woodward ND, and Landman BA. Sulcal depth-based cortical shape analysis in normal healthy control and schizophrenia groups. Proc SPIE–the Int Soc Optical Eng. (2018), 10574. doi: 10.1117/12.2293275
20. Yao JK, Voorhies WI, Miller JA, Bunge SA, and Weiner KS. Sulcal depth in prefrontal cortex: A novel predictor of working memory performance. Cereb Cortex (New York NY: 1991). (2023) 33:1799–813. doi: 10.1093/cercor/bhac173
21. Lerosier B, Simon G, Takerkart S, Auzias G, and Dollfus S. Sulcal pits of the superior temporal sulcus in schizophrenia patients with auditory verbal hallucinations. AIMS Neurosci. (2024) 11:25–38. doi: 10.3934/Neuroscience.2024002
22. Salgado-Pineda P, Barbosa L, Hostalet N, García-León M, Fuentes-Claramonte P, Soler-Vidal J, et al. An ontogenetic role for broca’s and related speech areas in schizophrenic auditory hallucinations? Evidence from sulcal pits analysis. Psychiatry Res. (2025) 348:116502. doi: 10.1016/j.psychres.2025.116502
23. McKay DR, Kochunov P, Cykowski MD, Kent JW Jr., Laird AR, Lancaster JL, et al. Sulcal depth-position profile is a genetically mediated neuroscientific trait: description and characterization in the central sulcus. J neurosci: Off J Soc Neurosci. (2013) 33:15618–25. doi: 10.1523/jneurosci.1616-13.2013
24. Schmitt JE, Raznahan A, Liu S, and Neale MC. The heritability of cortical folding: evidence from the human connectome project. Cereb Cortex (New York NY: 1991). (2021) 31:702–15. doi: 10.1093/cercor/bhaa254
25. Allen P, Larøi F, McGuire PK, and Aleman A. The hallucinating brain: A review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. (2008) 32:175–91. doi: 10.1016/j.neubiorev.2007.07.012
26. Bridi MCD, Zong FJ, Min X, Luo N, Tran T, Qiu J, et al. Daily oscillation of the excitation-inhibition balance in visual cortical circuits. Neuron. (2020) 105:621–9.e4. doi: 10.1016/j.neuron.2019.11.011
27. Yang F, Zhu H, Cao X, Li H, Fang X, Yu L, et al. Impaired motor-to-sensory transformation mediates auditory hallucinations. PloS Biol. (2024) 22:e3002836. doi: 10.1371/journal.pbio.3002836
28. Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. Arlington: American Psychiatric Publishing (2013).
29. Kay SR, Fiszbein A, and Opler LA. The positive and negative syndrome scale (Panss) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
30. Yu T, Pei W, Xu C, Zhang X, and Deng C. Investigation of peripheral inflammatory biomarkers in association with violence in schizophrenia. BMC Psychiatry. (2024) 24:542. doi: 10.1186/s12888-024-05966-y
31. Buss AH and Perry M. The aggression questionnaire. J Pers Soc Psychol. (1992) 63:452–9. doi: 10.1037//0022-3514.63.3.452
32. Dahdouh O, Solh T, Lahoud C, Haddad C, and Hallit S. Association between cognition and color discrimination among Lebanese patients with schizophrenia. BMC Psychiatry. (2022) 22:606. doi: 10.1186/s12888-022-04245-y
33. Thompson E. Hamilton rating scale for anxiety (Ham-a). Occup Med (Oxford England). (2015) 65:601. doi: 10.1093/occmed/kqv054
34. Hamilton MA. Rating scale for depression. J neurol neurosurgery Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
35. Erzin G, Pries LK, Dimitrakopoulos S, Ralli I, Xenaki LA, Soldatos RF, et al. Association between exposome score for schizophrenia and functioning in first-episode psychosis: results from the athens first-episode psychosis research study. psychol Med. (2023) 53:2609–18. doi: 10.1017/s0033291721004542
36. Guaiana G, Abbatecola M, Aali G, Tarantino F, Ebuenyi ID, Lucarini V, et al. Cognitive behavioural therapy (Group) for schizophrenia. Cochrane Database systematic Rev. (2022) 7:Cd009608. doi: 10.1002/14651858.CD009608.pub2
37. Zimet GD, Powell SS, Farley GK, Werkman S, and Berkoff KA. Psychometric characteristics of the multidimensional scale of perceived social support. J Pers Assess. (1990) 55:610–7. doi: 10.1080/00223891.1990.9674095
38. Leucht S, Samara M, Heres S, and Davis JM. Dose equivalents for antipsychotic drugs: the ddd method. Schizophr Bull. (2016) 42 Suppl 1:S90–4. doi: 10.1093/schbul/sbv167
39. Dahnke R, Yotter RA, and Gaser C. Cortical thickness and central surface estimation. NeuroImage. (2013) 65:336–48. doi: 10.1016/j.neuroimage.2012.09.050
40. Li M, Yan J, Wen H, Lin J, Liang L, Li S, et al. Cortical thickness, gyrification and sulcal depth in trigeminal neuralgia. Sci Rep. (2021) 11:16322. doi: 10.1038/s41598-021-95811-z
41. Ghosh A, Kaur S, Shah R, Oomer F, Avasthi A, Ahuja CK, et al. Surface-based brain morphometry in schizophrenia vs. Cannabis-Induced Psychosis: A Controlled Comparison J Psychiatr Res. (2022) 155:286–94. doi: 10.1016/j.jpsychires.2022.09.034
42. Waner JL, Hausman HK, Kraft JN, Hardcastle C, Evangelista ND, O’Shea A, et al. Connecting memory and functional brain networks in older adults: A resting-state fmri study. GeroScience. (2023) 45:3079–93. doi: 10.1007/s11357-023-00967-3
43. Hallquist MN, Hwang K, and Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fmri preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. (2013) 82:208–25. doi: 10.1016/j.neuroimage.2013.05.116
44. Morfini F, Whitfield-Gabrieli S, and Nieto-Castañón A. Functional connectivity mri quality control procedures in conn. Front Neurosci. (2023) 17:1092125. doi: 10.3389/fnins.2023.1092125
45. Menon V. 20 years of the default mode network: A review and synthesis. Neuron. (2023) 111:2469–87. doi: 10.1016/j.neuron.2023.04.023
46. Kubera KM, Thomann PA, Hirjak D, Barth A, Sambataro F, Vasic N, et al. Cortical folding abnormalities in patients with schizophrenia who have persistent auditory verbal hallucinations. Eur neuropsychopharmacol: J Eur Coll Neuropsychopharmacol. (2018) 28:297–306. doi: 10.1016/j.euroneuro.2017.12.009
47. Binder JR, Desai RH, Graves WW, and Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex (New York NY: 1991). (2009) 19:2767–96. doi: 10.1093/cercor/bhp055
48. Huang H, Shu C, Chen J, Zou J, Chen C, Wu S, et al. Altered corticostriatal pathway in first-episode paranoid schizophrenia: resting-state functional and causal connectivity analyses. Psychiatry Res Neuroimaging. (2018) 272:38–45. doi: 10.1016/j.pscychresns.2017.08.003
49. Marino M, Spironelli C, Mantini D, Craven AR, Ersland L, Angrilli A, et al. Default mode network alterations underlie auditory verbal hallucinations in schizophrenia. J Psychiatr Res. (2022) 155:24–32. doi: 10.1016/j.jpsychires.2022.08.006
50. Humphreys GF, Lambon Ralph MA, and Simons JS. A unifying account of angular gyrus contributions to episodic and semantic cognition. Trends Neurosci. (2021) 44:452–63. doi: 10.1016/j.tins.2021.01.006
51. Xie Y, Guan M, He Y, Wang Z, Ma Z, Fang P, et al. The static and dynamic functional connectivity characteristics of the left temporoparietal junction region in schizophrenia patients with auditory verbal hallucinations during low-frequency rtms treatment. Front Psychiatry. (2023) 14:1071769. doi: 10.3389/fpsyt.2023.1071769
52l. Ćurčić-Blake B, Ford JM, Hubl D, Orlov ND, Sommer IE, Waters F, et al. Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Prog Neurobiol. (2017) 148:1–20. doi: 10.1016/j.pneurobio.2016.11.002
53. Schmack K, Bosc M, Ott T, Sturgill JF, and Kepecs A. Striatal dopamine mediates hallucination-like perception in mice. Sci (New York NY). (2021) 372:eabf4740. doi: 10.1126/science.abf4740
54. Chen C, Wang GH, Wu SH, Zou JL, Zhou Y, and Wang HL. Abnormal local activity and functional dysconnectivity in patients with schizophrenia having auditory verbal hallucinations. Curr Med Sci. (2020) 40:979–84. doi: 10.1007/s11596-020-2271-4
55. Yang KC, Yang BH, Liu MN, Liou YJ, and Chou YH. Cognitive impairment in schizophrenia is associated with prefrontal-striatal functional hypoconnectivity and striatal dopaminergic abnormalities. J Psychopharmacol (Oxford England). (2024) 38:515–25. doi: 10.1177/02698811241257877
56. Kamali A, Milosavljevic S, Gandhi A, Lano KR, Shobeiri P, Sherbaf FG, et al. The cortico-limbo-thalamo-cortical circuits: an update to the original papez circuit of the human limbic system. Brain topography. (2023) 36:371–89. doi: 10.1007/s10548-023-00955-y
57. Papez JW. A proposed mechanism of emotion. 1937. J neuropsychiatry Clin Neurosci. (1995) 7:103–12. doi: 10.1176/jnp.7.1.103
58. Kumar S, Gander PE, Berger JI, Billig AJ, Nourski KV, Oya H, et al. Oscillatory correlates of auditory working memory examined with human electrocorticography. Neuropsychologia. (2021) 150:107691. doi: 10.1016/j.neuropsychologia.2020.107691
59. Soni S, Muthukrishnan SP, Sood M, Kaur S, and Sharma R. Altered parahippocampal gyrus activation and its connectivity with resting-state network areas in schizophrenia: an eeg study. Schizophr Res. (2020) 222:411–22. doi: 10.1016/j.schres.2020.03.066
60. Berger JI, Billig AJ, Sedley W, Kumar S, Griffiths TD, and Gander PE. What is the role of the hippocampus and parahippocampal gyrus in the persistence of tinnitus? Hum Brain Mapp. (2024) 45:e26627. doi: 10.1002/hbm.26627
61. Mitchell KJ and Johnson MK. Source monitoring 15 years later: what have we learned from fmri about the neural mechanisms of source memory? psychol Bull. (2009) 135:638–77. doi: 10.1037/a0015849
62. Xue K, Chen J, Wei Y, Chen Y, Han S, Wang C, et al. Altered dynamic functional connectivity of auditory cortex and medial geniculate nucleus in first-episode, drug-naïve schizophrenia patients with and without auditory verbal hallucinations. Front Psychiatry. (2022) 13:963634. doi: 10.3389/fpsyt.2022.963634
63. Alonso-Solís A, Vives-Gilabert Y, Portella MJ, Rabella M, Grasa EM, Roldán A, et al. Altered amplitude of low frequency fluctuations in schizophrenia patients with persistent auditory verbal hallucinations. Schizophr Res. (2017) 189:97–103. doi: 10.1016/j.schres.2017.01.042
64. Xie Y, Guan M, Wang Z, Ma Z, Fang P, and Wang H. Cerebral blood flow changes in schizophrenia patients with auditory verbal hallucinations during low-frequency rtms treatment. Eur Arch Psychiatry Clin Neurosci. (2023) 273:1851–61. doi: 10.1007/s00406-023-01624-8
65. Lewis R and Noppeney U. Audiovisual synchrony improves motion discrimination via enhanced connectivity between early visual and auditory areas. J Neurosci. (2010) 30:12329–39. doi: 10.1523/jneurosci.5745-09.2010
66. Onitsuka T, Shenton ME, Salisbury DF, Dickey CC, Kasai K, Toner SK, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an mri study. Am J Psychiatry. (2004) 161:1603–11. doi: 10.1176/appi.ajp.161.9.1603
67. Zhao Y, Zhang Q, Shah C, Li Q, Sweeney JA, Li F, et al. Cortical thickness abnormalities at different stages of the illness course in schizophrenia: A systematic review and meta-analysis. JAMA Psychiatry. (2022) 79:560–70. doi: 10.1001/jamapsychiatry.2022.0799
68. Alderson-Day B, Diederen K, Fernyhough C, Ford JM, Horga G, Margulies DS, et al. Auditory hallucinations and the brain’s resting-state networks: findings and methodological observations. Schizophr Bull. (2016) 42:1110–23. doi: 10.1093/schbul/sbw078
69. Wang Z, Xue K, Kang Y, Liu Z, Cheng J, Zhang Y, et al. Altered intrinsic neural activity and its molecular analyses in first-episode schizophrenia with auditory verbal hallucinations. Front Neurosci. (2024) 18:1478963. doi: 10.3389/fnins.2024.1478963
70. Liang N, Liu S, Li X, Wen D, Li Q, Tong Y, et al. A decrease in hemodynamic response in the right postcentral cortex is associated with treatment-resistant auditory verbal hallucinations in schizophrenia: an nirs study. Front Neurosci. (2022) 16:865738. doi: 10.3389/fnins.2022.865738
71. Mo F, Zhao H, Li Y, Cai H, Song Y, Wang R, et al. Network localization of state and trait of auditory verbal hallucinations in schizophrenia. Schizophr Bull. (2024) 50:1326–36. doi: 10.1093/schbul/sbae020
Keywords: schizophrenia, auditory verbal hallucinations, sulcal depth, functional connectivity, default mode network, visual cortex
Citation: Guo Z, Zhou Z, Wang S, Yang L, Gao X, Xia Y, Yang Y, Shan Z, Huang H and Tian L (2025) Alterations in sulcal depth and associated functional connectivity in schizophrenia with auditory verbal hallucinations. Front. Psychiatry 16:1641190. doi: 10.3389/fpsyt.2025.1641190
Received: 04 June 2025; Accepted: 15 July 2025;
Published: 30 July 2025.
Edited by:
Massoud Stephane, Oregon Health and Science University, United StatesReviewed by:
Zhengcao Cao, Beijing Normal University, ChinaBaptiste Lerosier, Université de Caen Normandie, France
Xuran Shen, Nanjing Brain Hospital Affiliated to Nanjing Medical University, China
Copyright © 2025 Guo, Zhou, Wang, Yang, Gao, Xia, Yang, Shan, Huang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Tian, bGludGlhbkBqaWFuZ25hbi5lZHUuY24=
 Zhenru Guo
Zhenru Guo Zimo Zhou1
Zimo Zhou1 Lin Tian
Lin Tian