- 1Department of Psychiatry, Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China
- 2Department of Psychology and Cognitive Science, East China Normal University, Shanghai, China
- 3Department of Geriatric Psychiatry, Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China
Objective: Cognitive deficits present transdiagnostic characteristic and partly explain the poor functional outcomes of patients with mental disorders. Understanding the relationships between neurocognition, social cognition, and global function may help identify new cognitive intervention targets. We aimed to model the complex interrelationships among these variables with Gaussian Graphical Modeling in a transdiagnostic sample.
Methods: A total of 482 individuals were included in this study, comprising 281 patients with first-episode schizophrenia, 128 patients with bipolar disorder, and 73 patients with major depressive disorder. The Wechsler Adult Intelligence Scale, the MATRICS Consensus Cognitive Battery, and the Global Assessment of Functioning Scale were evaluated. The interaction and centrality indexes of cognitive and global function were analyzed by network analysis.
Results: In the transdiagnostic network, speed of processing (SOP) and verbal learning (Vrbl) exhibited higher centrality indexes. The cognitive nodes closely associated with global function included working memory (WM), and attention/vigilance (AV). When subjects were modeled separately by gender, no significant differences were found between males and females.
Conclusion: The close connections between WM, AV, and global function as well as the high centrality indexes of SOP and Vrbl suggest that these domains share aspects of pathophysiology in schizophrenia and mood disorder. However, the data-driven approach limited our interpretation of the results. Theory-driven model should be further validated to elucidate causal pathways and find more promising approaches to recovery.
Introduction
Previous studies have consistently shown that individuals with mental disorders often experience cognitive deficits to varying degrees. These deficits are not specific to any one disorder but rather exhibit transdiagnostic characteristic. This perspective has been further corroborated by subsequent studies (1–3). Notably, a large meta-analysis conducted in 2021 analyzed executive function, working memory (WM), and speed of processing (SOP) across 12 mental disorders. The study not only proposed but also validated the C-factor hypothesis, which underscores the transdiagnostic nature of cognitive impairment (4).
The clinical remission rates of patients with schizophrenia and depression after 1 year were 39.2% and 42.3%, while the function recovery were only 17% and 38.1% (5). An array of variables such as untreated duration, simultaneous remission of positive and negative symptoms, and most cognitive variables have been pointed as important dimensions impacting the functional recovery of patients with first-episode psychosis (6). It has been suggested that cognitive function may be a better predictor of global function than the severity of affective symptoms in bipolar disorder (7). Researchers (8) divided the predictors of remission into non-modifiable factors (such as sex, age) and modifiable factors (such as education, disease symptoms, work status), they found that female sex and older age were predictors of positive clinical outcomes in depression, schizophrenia spectrum, and substance use. There were many researches on gender differences in cognitive function of mental disorders. But the results so far were inconsistent, most supported that women perform better than men on certain cognitive domains (9, 10). The cognitive functions’ transdiagnostic nature and their impact on global function make it becoming one of the important targets in clinical intervention. Therefore, understanding the important distribution characteristics of cognitive domains and their relationships with global function are of great significance for future precise intervention. Previous researches had predominantly focused on exploring the characteristics of cognitive impairment (11) and comparing the extent of cognitive deficits across different mental disorders (12). However, these studies have generally failed to provide a comprehensive understanding of the relationships among the impaired cognitive domains, especially from a perspective of transdiagnostic.
The concept of transdiagnostic was firstly appeared in a report on cognitive behavioral therapy for patients with eating disorders (13), the authors suggested that anorexia nervosa, bulimia nervosa and atypical eating disorder share similar psychopathology, clinical features, disease maintenance factors, and finally proposed a new transdiagnostic cognitive behavioral therapy. Subsequently, transdiagnostic researches expanded to depression, anxiety, substance use, and schizophrenia spectrum disorders (14, 15).The transdiagnostic framework has also evolved beyond its initial focus on early psychotherapy interventions (16–18) to cross-sectional factors of disease maintenance or recurrence (19–21), clinical features (22, 23), and cognitive function (4, 24).
Network analysis can reveal the relationships among various variables more intuitively and is suitable for transdiagnostic studies because of some similar viewpoints. The network theory of psychopathology believes that mental disorders are formed by the interaction of symptoms. The mutual feedback between symptoms will play an important role in the persistence of mental disorders (25). A number of studies have used network analysis to assess the relationships between the symptoms of mental disorders in order to understand which symptoms are more important in the disease (26), which symptoms act as a bridge between different clusters (27). S. Galderisi et al. (28) studied the relationship between personal resources, environmental factors, psychopathology and real-life functions in schizophrenia with network analysis. They found that functional ability and daily living skills were at the core of the network and highly correlated, while positive symptoms in psychopathology were at the periphery of the network. Another study (29) added cognitive function dimension to the network analysis, and found that both neurocognition (NC) and social cognition (SC) were important contributors to functional outcomes, WM was particularly related to functional outcomes. Current research samples about network analysis are relatively single, few studies have conducted network analysis from a cross-diagnostic perspective. S. Fritze et al. (30) modeled the relationships between sensorimotor, cognition and global function in a transdiagnostic sample of 212 patients with mental disorders, they found sensorimotor symptoms may represent a transdiagnostic therapeutic target. However, the unbalanced diagnostic groups limited the inference of the results. Our study aimed to examine the relationships between a broader range of cognitive domains and global functioning within a larger transdiagnostic sample as well as investigate the impact of gender on these networks. Our goals can be divided into the following three points: 1. Establish the network of cognitive and global function. 2. Identify key cognitive subtypes influencing global functioning. 3. Explore the gender differences in cognitive and global function networks.
Methods
Study participants
A total of 482 subjects, including 281 first-episode schizophrenia, 128 bipolar disorder patient (depressive phase), and 73 major depressive disorder patients were recruited in this study. All the patients were from the outpatient and inpatient departments of Nanjing Brain Hospital, and the healthy controls were recruited from the surrounding community. Inclusion criteria for the patients were as following: Fulfilling DSM-5 criteria for bipolar disorder (depressive phase), major depressive disorder or schizophrenia. The Chinese Han population, aged 16–45, right-handed. Education years ≥ 8 years. No previous exposures to any psychotropic drugs including benzodiazepines or other physical therapy. Exclusion criteria: History of neurological disease or other major physical disease. History of alcohol or substance abuse. Intelligence quotient (IQ) < 70. The Inclusion criteria for the healthy controls were identical to those of the patients’ group, with the exception being the absence of a psychotic disorder. This study was approved by the Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University (Ethics number: 2021-KY075-01). All subjects signed informed consent, and subjects under the age of 18 were signed by their guardians.
Clinical assessments
All assessments were completed by physicians trained in standardized neuropsychological tests on the same day as enrollment. The MATRICS Consensus Cognitive Battery (MCCB) and the Wechsler Adult Intelligence Scale (WAIS) were used to assess cognitive function and IQ. Additionally, the global assessment of functioning (GAF) was used to evaluate global function of participants. MCCB was originally recommended for assessing cognitive function in schizophrenia (31). However, recent studies have demonstrated that the MCCB also exhibits relatively high internal consistency and reliability among patients with bipolar disorder and depression (32, 33). The MCCB includes seven cognitive domains, namely, SOP, WM, attention/vigilance (AV), Vrbl, visuospatial memory (Vis), reasoning and problem solving (RPS), and SC. Higher scores in these domains indicate better cognitive functioning. For the purposes of this study, the T-scores of each cognitive subdomain were calculated and used as the final inclusion metric. The GAF is a widely used clinical scale for assessing overall functioning (34). It measures symptoms severity and functional outcomes on a scale ranging from 0 to 100, with higher scores indicating less severity of symptoms and better function outcome.
Statistical analysis
Firstly, a descriptive analysis of demographic data was conducted with SPSS27.0. Nest, R (version 4.3.3) and R-Studio were used to perform network analysis. The qgraph package was used to estimate and visualize network. In the network, each visual item is called a node, and the lines between nodes are called edges which are also the variables we want to evaluate. The blue edge indicates a positive correlation, and the red indicates negative. The thicker of the edges, the stronger of the correlation. To avoid false-positive relationships, we used the extended Bayesian information criterion (EBIC) lasso to regularize the network (35–37), which can assign zero values to edges with lower edge weights, and create a more compact network graph. We calculated the centrality indexes of strength and expected influence. Strength centrality is the sum of absolute values of all edge weights connected to it, which can reflect the importance of a node in the whole network. The expected influence is the average value of the influence of a node on all other nodes, which reflects the degree of activation and inhibition of the node on the whole network. Then the accuracy of edge-weights and stability of centrality indexes were estimated by bootstrapping the 95% confidence intervals (CI) and case dropping bootstrapping. A narrower CI indicates a more accurate estimation of the edge weight. The correlation stability coefficient (CS-C) was used to quantify the stability of centrality indexes. We used recommended CS-C cut-off values (optimal CS-C>0.5, minimum CS-C>0.25) (29). To verify whether the model is driven by diagnostics, we repeated the network analysis in schizophrenia and mood disorders (including major depressive disorder and bipolar disorder). Finally, the Network Comparison Test package was used to analyze the differences between the networks of males and females from four aspects (the network structure invariance test, the global strength invariance test, the edge invariance test, and the centrality invariance test). p<0.05 was considered statistically significant.
Results
Clinical and demographic characteristics
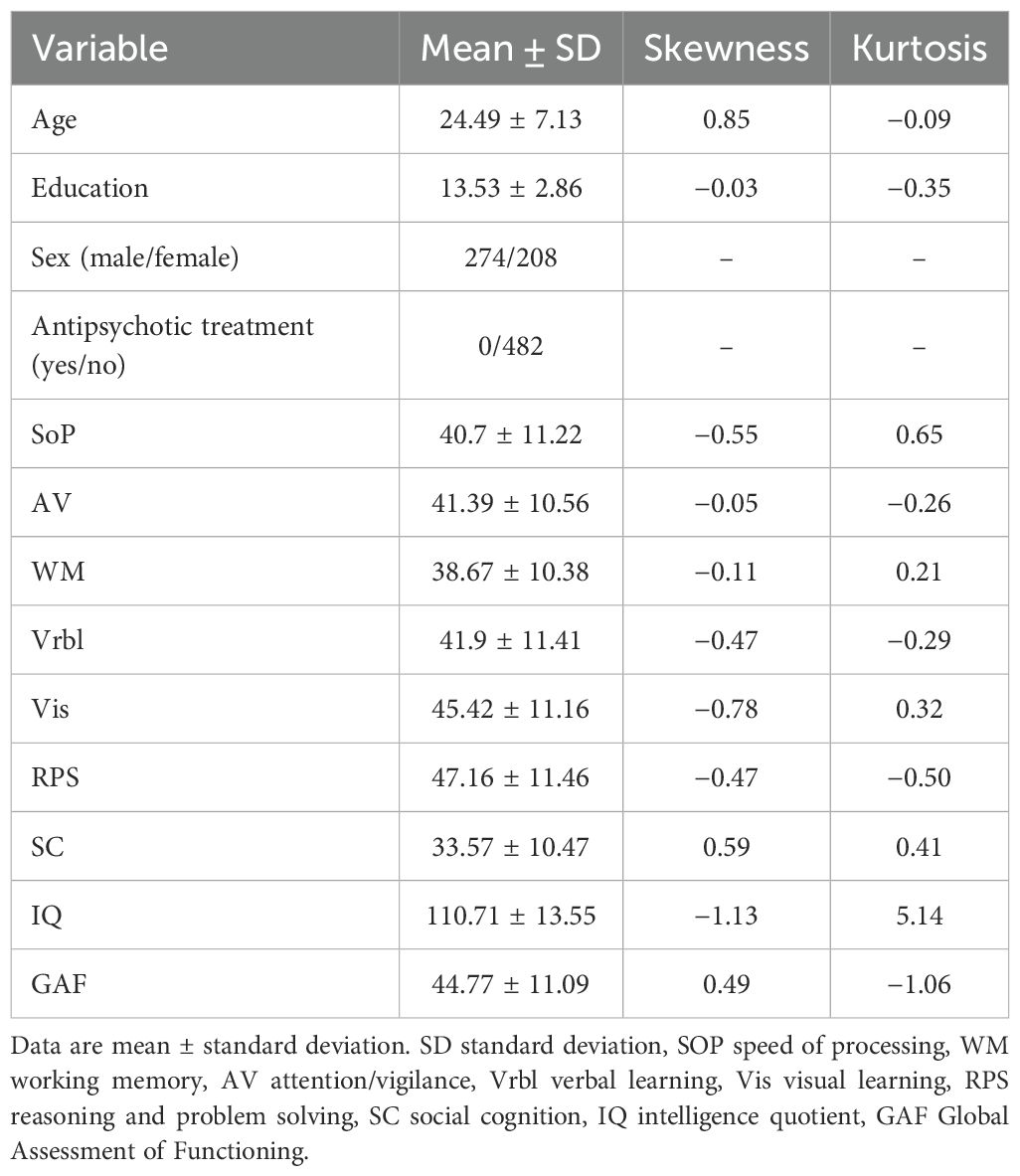
Data of 482 patients were included in the analyses. The mean age and education of the entire sample were 24.49 ± 7.14 years and 13.58 ± 2.86 years. Approximately 56.8% of the sample were male. Table 1 shows the detailed demographic and clinical characteristics as well as the skewness and kurtosis of each variable. No data-transformation procedure was performed because of the acceptable range of skewness (cutoff is 2.0) and kurtosis (cutoff is 7.0) (38). The group differences between all the variables can be seen in Supplementary Table S1.
Network analysis
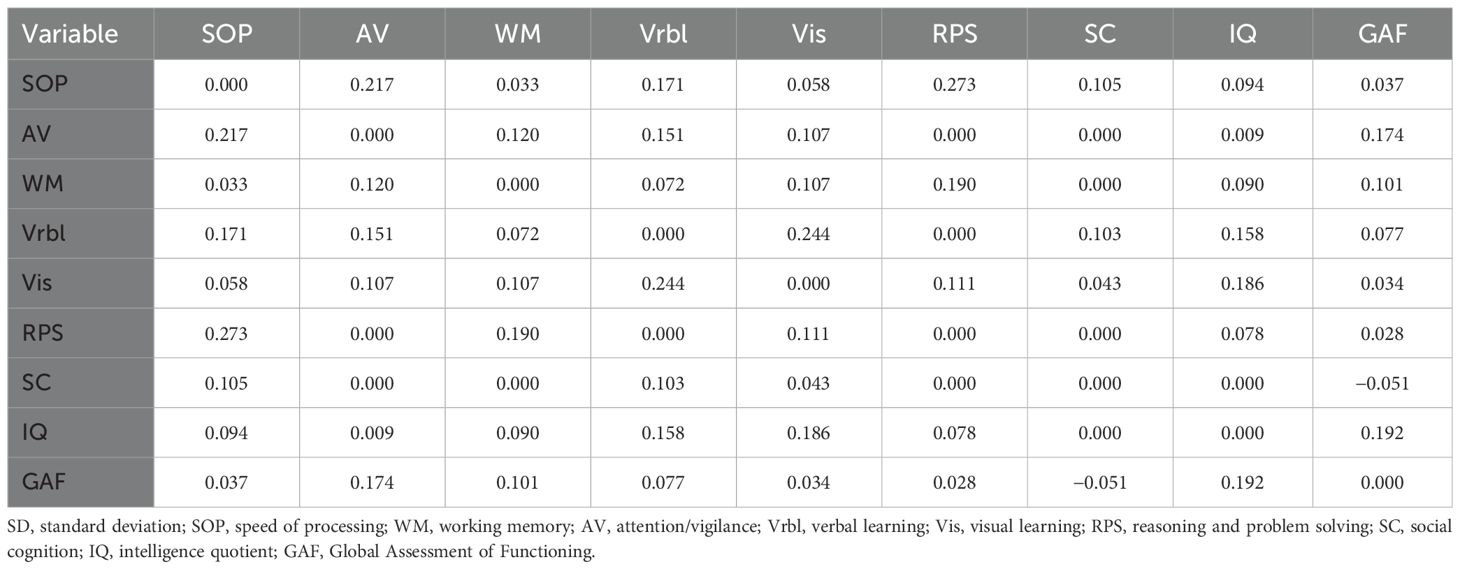
As shown in Figure 1, the network consisted of IQ, the seven cognitive domains of MCCB, and the GAF. The GAF was positively correlated with IQ, AV, and WM, specific edge weights can be seen in Table 2.
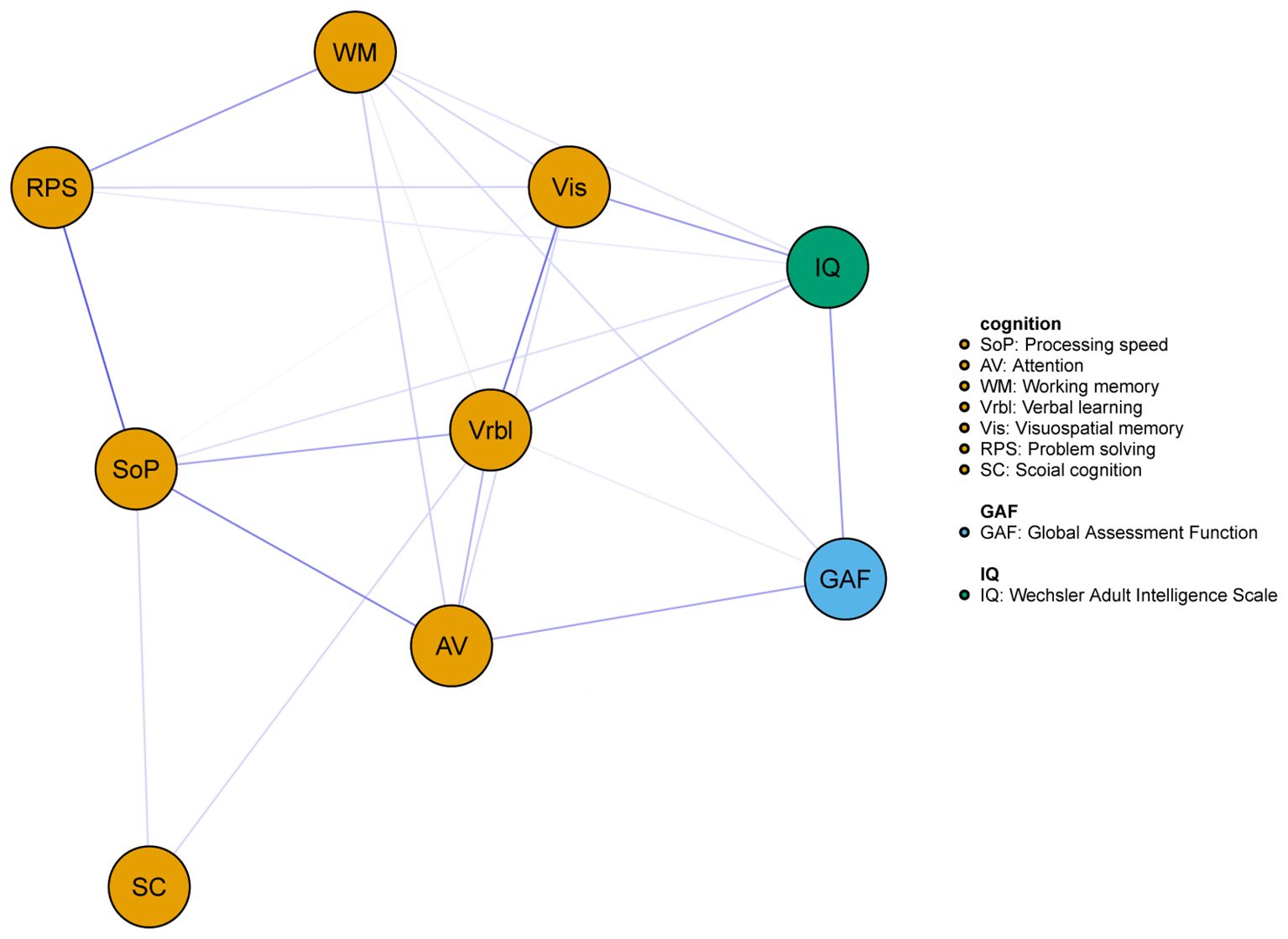
Figure 1. Network of the cognitive and global function for all subjects. Blue edges represent positive associations whereas red edges represent negative associations, and thickness of an edge represents the strength of association between two nodes.
The centrality index of each node in the network for all subjects were shown in Figure 2. In this network model, the top three nodes with the strongest strength centrality were Vrbl, SOP, and RPS, while SOP, Vrbl and Vis occupied the top three positions of expected influence. SC had the lowest centrality indexes both in strength and expected influence. The estimation of the accuracy and stability of the network showed that the 95% CI value of the edge weight was relatively narrow. The CS-C of centrality indexes were as following, strength: 0.595, expected influence: 0.672. These results indicated that the overall network stability was acceptable. For details, see Supplementary Figure S1. When repeating the network analysis in patients with schizophrenia (n=281) and mood disorders (including major depressive disorder and bipolar disorder, n=201), only the network of schizophrenia (Supplementary Figure S7) was stable. The SOP and Vrbl had the highest expected influence and strength centrality index respectively (Supplementary Figure S10). The network results of mood disorder were not shown here due to instability.
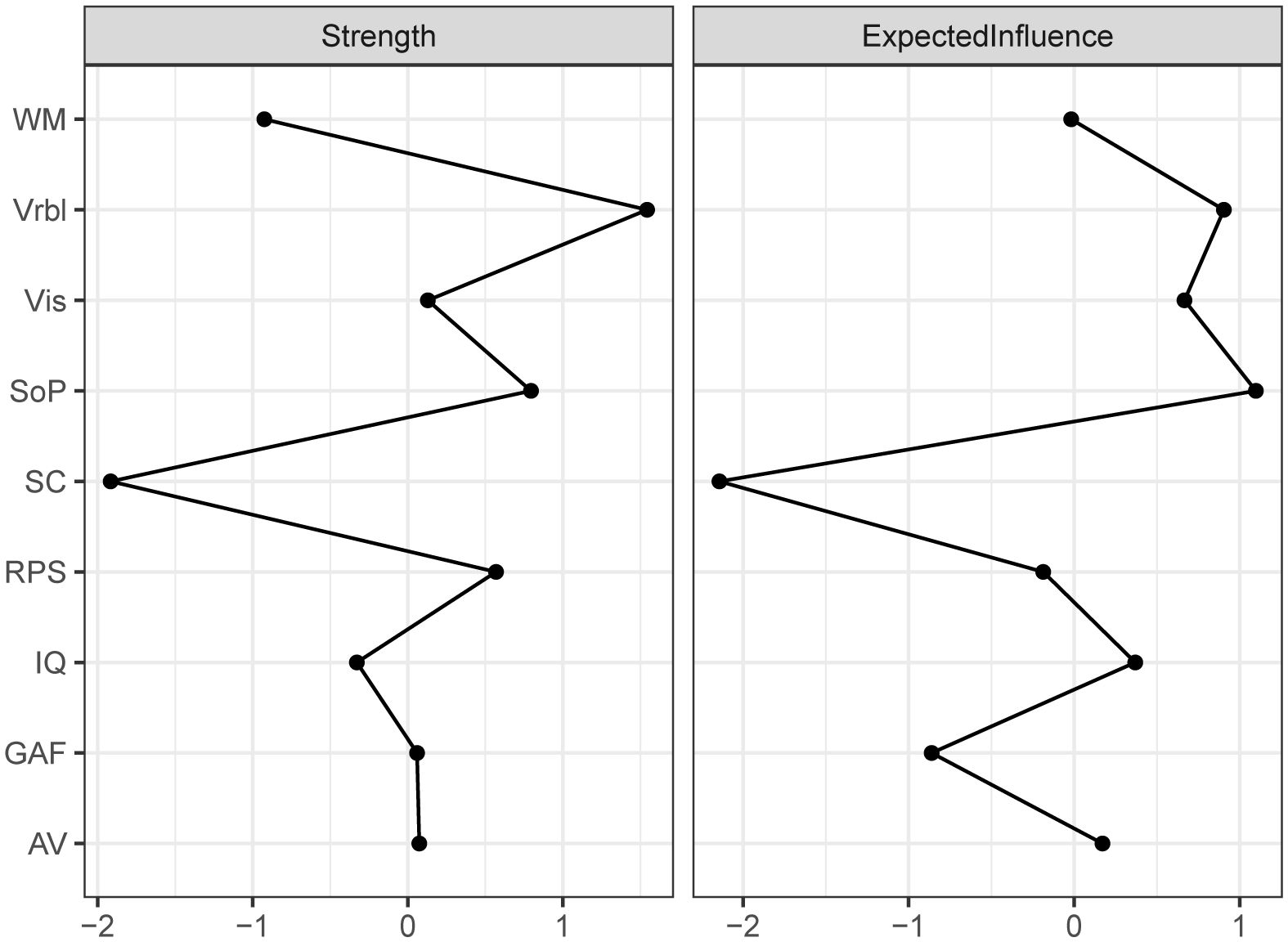
Figure 2. Centrality of the network for all subjects. Depicting the Strength and Expected influence of each node. WM working memory, Vrbl verbal learning, Vis visual learning, SOP speed of processing, SC social cognition, RPS reasoning and problem solving, IQ intelligence quotient, GAF Global Assessment of Functioning, AV attention/vigilance.
The networks for different genders were constructed respectively, then the accuracy and stability of the two networks were estimated. The CS-C of centrality indexes in the males were as following, strength: 0.518, expected influence: 0.595. While females were 0.438 and 0.438. Both the strength and expected influence indexes were within the acceptable range, details can be found in Supplementary Figure S3 and Supplementary Figure S5. As we can see in Figure 3, the global function in the male network was positively correlated with IQ (edge weight: 0.223), AV (0.162), and Vrbl (0.142). For the females, the nodes with stronger correlation are IQ (0.186), AV (0.145), and WM (0.132). When comparing the network structures of men and women, no significant differences were found. The centrality index of each node in the male and female networks can be found in Figure 4.
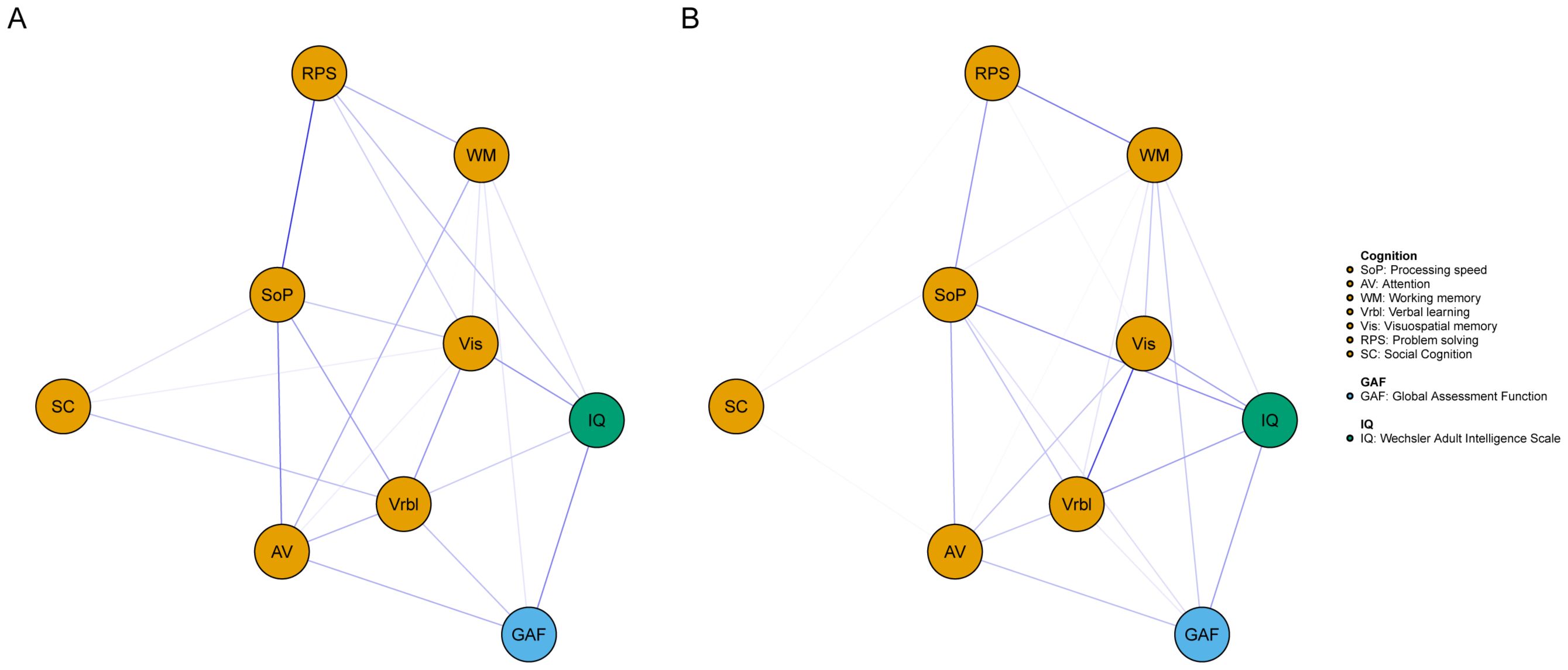
Figure 3. Network of cognitive and global function for different genders. A= male, B= female. Blue edges represent positive associations whereas red edges represent negative associations, and thickness of an edge represents the strength of association between two nodes.
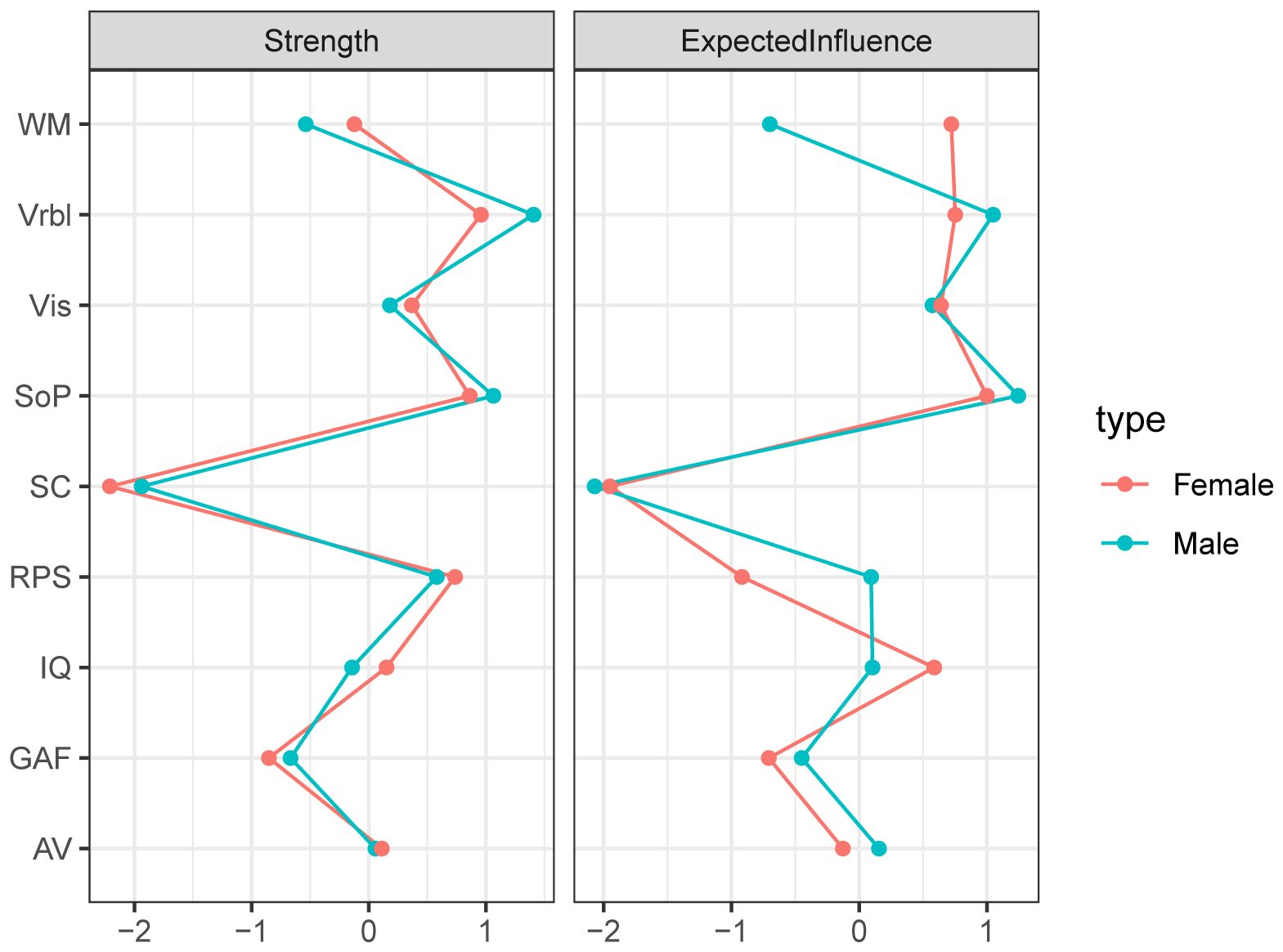
Figure 4. Centrality of the network for different genders. Depicting the Strength and Expected influence of each node, with red nodes representing female and green nodes representing male.
Discussion
In this study, we used network analysis to investigate the interrelationships between various cognitive domains and global function as well as explore significant differences in transdiagnostic networks between males and females. The results showed that SOP and Vrbl had relatively higher centrality indexes both in strength and expected influence across all networks, while the centrality indexes of SC were the lowest.
SOP involves a series of mental processes such as the acquisition, encoding and storage of input information. Consistent with several previous studies (11, 39), we also utilized the Trail Making Test, Symbol Coding, and Category Fluency from the MCCB as measures of information processing speed. While SOP is considered a limited cognitive resource for learning and memory (40), some researchers have demonstrated that it can account for the individual differences in Vrbl of older adults to some extent (41). A study examining the rates of cognitive impairment across different psychiatric disorders found that the cognitive tests with higher impairment in clinical groups mainly involved SOP, Vrbl, WM, and verbal fluency (42).The deficits of SOP and Vrbl usually have higher effect sizes in people with mental disorders (11, 43),and are related to functional outcomes (44, 45). M. Lindgren et al. (46) conducted a follow-up study and found that cognitive function, especially the SOP and SC at baseline can predict outcomes one year later in patients with first-episode psychosis, the authors suggested that both SOP and SC should be considered primary cognitive targets for clinical intervention. Our results further support the potential value of SOP and Vrbl as transdiagnostic targets for cognitive intervention in terms of the significance of the network structure.
Cognitive deficits are common among individuals with mental disorders (47, 48), the relationship between NC, SC and global function has always been a clinical concern. In our results, the node of SC was at a relatively marginal position in the network. The remaining six nodes of NC and IQ were more closely connected and exhibited stronger correlations with global function. SC appeared to influence global function indirectly through certain NC variables, such as SOP and Vrbl. These findings align with the structural view of previous studies, which suggest that NC and SC are related but largely independent constructs (49, 50). The processing of social cognition is typically predicated on the foundation of neurocognitive abilities. Only by focusing on individuals’ language, expressions, actions, and other details, can one integrate numerous pieces of information and feed them back to the brain to make corresponding judgments and inferences, which is a manifestation of SC. However, some researchers have argued that SC may be a stronger predictor of functional outcomes in schizophrenia spectrum disorders than NC, which acts as a mediating factor between functional outcomes and NC (51). Recently, network analysis has been employed to investigate the relationships between NC, SC, clinical symptoms, and functional outcomes in a sample of 408 patients with schizophrenia spectrum disorders. The results indicated that both SC and NC contribute to functional outcomes, with social skills and functional ability potentially serving as bridges connecting NC, SC, and functional outcomes (29). In our results, the correlations between SC and global function were so low in all networks that they were less evident in the network diagram. These are inconsistent with those of previous studies. As we all know, the SC includes emotional processing, theory of mind, social perception, social knowledge and attribution bias (52). The SC assessment in the MCCB primarily focuses on emotional processing. Functional outcomes, on the other hand, have been divided into four categories based on different measurements: community function, social behavior in the environment, social problem-solving, and social skills (50, 51). The authors have even called for considering the type of functional outcome when planning intervention measures in research. In our study, we used relatively single tools to measure SC and global function due to data-driven approach, so there are fewer nodes representing these two clusters. This may partly explain the inconsistencies with previous studies. Besides, the heterogeneity of assessment tools and different research methods may also contribute the inconsistency.
The study of gender differences may be a window into the pathogenesis of some mental disorders, and could help develop specific treatment strategies for these disorders. Consistent with prior research (53), we did not find gender differences in the network of cognitive and global function in terms of global node strength and edge weights. The network theory of psychopathology (25) pointed that a strongly connected network tends to transition to a disordered state when disturbed by an external field, while a weakly connected network has more flexibility and can quickly recover to a stable state when disturbed. Based on this theory, we speculate that the same external factors may have little different effect on the cognitive and global functional networks for different genders. However, as mentioned in network theory, external factors not only include changes or stress in some social environment, but also those from the body, such as inflammation and changes in specific brain areas. This underscores the need to incorporate a broader range of research variables into future network analysis studies to identify more therapeutic targets.
The primary strength of our study lies in the integration of the transdiagnostic concept with network analysis methodology. Besides, the influence of medications on cognition was minimized, as none of the participants had received antipsychotic drugs or other sleep-promoting medications before enrollment.
Through these approaches, our study displayed the relatively important cognitive nodes of patients with mental disorders, revealed the relationships between NC, SC and global function, and compared the differences of networks with different genders. It may provide us some ideas for understanding the cognitive network structures of different genders and selecting transdiagnostic cognitive intervention targets. Of course, there are still some limitations in this study. First of all, the design of cross-sectional research will hinder our judgment on the causal relationships and the direction of influence between cognitive nodes. Second, although we used well-validated tasks to measure cognitive, the data-driven approach limited the number of nodes for SC and global function, and failed to take into account other factors affecting global function, such as psychopathology, family environment, and general clinical information. Therefore, our results should be interpreted with caution. The theory-driven model should be further validated in the future. In addition, further intervention studies combining longitudinal design, imaging or electroencephalogram research tools and network analysis are needed to elucidate causal pathways and find more promising approaches to recovery.
Conclusion
The close connections between WM, AV, and global function as well as the high centrality indexes of SOP and Vrbl suggest that these domains share aspects of pathophysiology in schizophrenia and mood disorder. However, the data-driven approach limited our interpretation of the results. Theory-driven model should be further validated to elucidate causal pathways and find more promising approaches to recovery.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YZ: Formal Analysis, Writing – original draft, Data curation, Conceptualization. RZ: Data curation, Conceptualization, Writing – review & editing. LN: Writing – review & editing, Conceptualization, Data curation. ZX: Software, Writing – original draft, Formal Analysis. SL: Funding acquisition, Writing – review & editing, Data curation. SX: Funding acquisition, Supervision, Writing – review & editing. XZ: Supervision, Conceptualization, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82371510), Social Development Foundation of Jiangsu Province, China (No. BE2023668), Nanjing Major Science and Technology Project (Life and Health, No 202305035), Medical Science and Technology Development Foundation, Nanjing Municipality Health Bureau (YKK23134 and ZKX21033).
Acknowledgments
We would like to express our deep gratitude to all the researchers and subjects who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1643369/full#supplementary-material
References
1. Bingham KS, Dawson DR, Mulsant BH, Banerjee S, and Flint AJ. Relationships among history of psychosis, cognition and functioning in later-life remitted major depression. Am J geriatric Psychiatry. (2021) 29:144–55. doi: 10.1016/j.jagp.2020.06.014
2. Benzina N, Mallet L, Burguière E, N’Diaye K, and Pelissolo A. Cognitive dysfunction in obsessive-compulsive disorder. Curr Psychiatry Rep. (2016) 18:80. doi: 10.1007/s11920-016-0720-3
3. Ramey T and Regier PS. Cognitive impairment in substance use disorders. CNS spectrums. (2019) 24:102–13. doi: 10.1017/s1092852918001426
4. Abramovitch A, Short T, and Schweiger A. The C Factor: Cognitive dysfunction as a transdiagnostic dimension in psychopathology. Clin Psychol review. (2021) 86:102007. doi: 10.1016/j.cpr.2021.102007
5. Spellmann I, Schennach R, Seemüller F, Meyer S, Musil R, Jäger M, et al. Validity of remission and recovery criteria for schizophrenia and major depression: comparison of the results of two one-year follow-up naturalistic studies. Eur Arch Psychiatry Clin Neurosci. (2017) 267:303–13. doi: 10.1007/s00406-016-0741-2
6. Santesteban-Echarri O, Paino M, Rice S, González-Blanch C, McGorry P, Gleeson J, et al. Predictors of functional recovery in first-episode psychosis: A systematic review and meta-analysis of longitudinal studies. Clin Psychol review. (2017) 58:59–75. doi: 10.1016/j.cpr.2017.09.007
7. Baune BT and Malhi GS. A review on the impact of cognitive dysfunction on social, occupational, and general functional outcomes in bipolar disorder. Bipolar Disord. (2015) 17 Suppl 2:41–55. doi: 10.1111/bdi.12341
8. Solmi M, Cortese S, Vita G, De Prisco M, Radua J, Dragioti E, et al. An umbrella review of candidate predictors of response, remission, recovery, and relapse across mental disorders. Mol Psychiatry. (2023) 28:3671–87. doi: 10.1038/s41380-023-02298-3
9. Menghini-Müller S, Studerus E, Ittig S, Valmaggia LR, Kempton MJ, van der Gaag M, et al. Sex differences in cognitive functioning of patients at-risk for psychosis and healthy controls: Results from the European Gene-Environment Interactions study. Eur psychiatry: J Assoc Eur Psychiatrists. (2020) 63:e25. doi: 10.1192/j.eurpsy.2019.10
10. Huang D, Lai S, Zhong S, Zhang Y, He J, Yan S, et al. Sex-differential cognitive performance on MCCB of youth with BD-II depression. BMC Psychiatry. (2024) 24:345. doi: 10.1186/s12888-024-05701-7
11. Zhang H, Wang Y, Hu Y, Zhu Y, Zhang T, Wang J, et al. Meta-analysis of cognitive function in Chinese first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) profile of impairment. Gen Psychiatry. (2019) 32:e100043. doi: 10.1136/gpsych-2018-100043
12. Li W, Zhou FC, Zhang L, Ng CH, Ungvari GS, Li J, et al. Comparison of cognitive dysfunction between schizophrenia and bipolar disorder patients: A meta-analysis of comparative studies. J Affect Disord. (2020) 274:652–61. doi: 10.1016/j.jad.2020.04.051
13. Fairburn CG, Cooper Z, and Shafran R. Cognitive behaviour therapy for eating disorders: a “transdiagnostic” theory and treatment. Behav Res Ther. (2003) 41:509–28. doi: 10.1016/s0005-7967(02)00088-8
14. Sloan E, Hall K, Moulding R, Bryce S, Mildred H, and Staiger PK. Emotion regulation as a transdiagnostic treatment construct across anxiety, depression, substance, eating and borderline personality disorders: A systematic review. Clin Psychol review. (2017) 57:141–63. doi: 10.1016/j.cpr.2017.09.002
15. Weintraub MJ, Ichinose MC, Zinberg J, Done M, Morgan-Fleming GM, Wilkerson CA, et al. App-enhanced transdiagnostic CBT for adolescents with mood or psychotic spectrum disorders. J Affect Disord. (2022) 311:319–26. doi: 10.1016/j.jad.2022.05.094
16. McIntosh VVW, Jordan J, Carter JD, Frampton CMA, McKenzie JM, Latner JD, et al. Psychotherapy for transdiagnostic binge eating: A randomized controlled trial of cognitive-behavioural therapy, appetite-focused cognitive-behavioural therapy, and schema therapy. Psychiatry Res. (2016) 240:412–20. doi: 10.1016/j.psychres.2016.04.080
17. Boswell JF, Farchione TJ, Sauer-Zavala S, Murray HW, Fortune MR, and Barlow DH. Anxiety sensitivity and interoceptive exposure: a transdiagnostic construct and change strategy. Behav Ther. (2013) 44:417–31. doi: 10.1016/j.beth.2013.03.006
18. Espejo EP, Gorlick A, and Castriotta N. Changes in threat-related cognitions and experiential avoidance in group-based transdiagnostic CBT for anxiety disorders. J Anxiety Disord. (2017) 46:65–71. doi: 10.1016/j.janxdis.2016.06.006
19. Reininghaus U, Daemen M, Postma MR, Schick A, Hoes-van der Meulen I, Volbragt N, et al. Transdiagnostic ecological momentary intervention for improving self-esteem in youth exposed to childhood adversity: the SELFIE randomized clinical trial. JAMA Psychiatry. (2024) 81:227–39. doi: 10.1001/jamapsychiatry.2023.4590
20. Puttevils L, Vanderhasselt MA, and Vervaet M. Investigating transdiagnostic factors in eating disorders: Does self-esteem moderate the relationship between perfectionism and eating disorder symptoms? Eur eating Disord review: J Eating Disord Assoc. (2019) 27:381–90. doi: 10.1002/erv.2666
21. McFarlane T, Olmsted MP, and Trottier K. Timing and prediction of relapse in a transdiagnostic eating disorder sample. Int J eating Disord. (2008) 41:587–93. doi: 10.1002/eat.20550
22. MacNamara A, Klumpp H, Kennedy AE, Langenecker SA, and Phan KL. Transdiagnostic neural correlates of affective face processing in anxiety and depression. Depression anxiety. (2017) 34:621–31. doi: 10.1002/da.22631
23. Samtani S, McEvoy PM, Mahoney AEJ, Werner-Seidler A, Li SSY, McGill BC, et al. Examining a transdiagnostic measure of repetitive thinking in depressed, formerly depressed and never-depressed individuals. J Affect Disord. (2018) 229:515–22. doi: 10.1016/j.jad.2017.12.081
24. Sabharwal A, Szekely A, Kotov R, Mukherjee P, Leung HC, Barch DM, et al. Transdiagnostic neural markers of emotion-cognition interaction in psychotic disorders. J Abnormal Psychol. (2016) 125:907–22. doi: 10.1037/abn0000196
25. Borsboom D. A network theory of mental disorders. World Psychiatry. (2017) 16:5–13. doi: 10.1002/wps.20375
26. Galimberti C, Bosi MF, Caricasole V, Zanello R, Dell’Osso B, and Viganò CA. Using network analysis to explore cognitive domains in patients with unipolar versus bipolar depression: a prospective naturalistic study. CNS spectrums. (2020) 25:380–91. doi: 10.1017/s1092852919000968
27. Yuan D, Wu J, Li S, Zhang R, Zhou X, and Zhang Y. Network analysis of cold cognition and depression in middle-aged and elder population: the moderation of grandparenting. Front Public Health. (2023) 11:1204977. doi: 10.3389/fpubh.2023.1204977
28. Galderisi S, Rucci P, Kirkpatrick B, Mucci A, Gibertoni D, Rocca P, et al. Interplay among psychopathologic variables, personal resources, context-related factors, and real-life functioning in individuals with schizophrenia: A network analysis. JAMA Psychiatry. (2018) 75:396–404. doi: 10.1001/jamapsychiatry.2017.4607
29. Hajdúk M, Penn DL, Harvey PD, and Pinkham AE. Social cognition, neurocognition, symptomatology, functional competences and outcomes in people with schizophrenia - A network analysis perspective. J Psychiatr Res. (2021) 144:8–13. doi: 10.1016/j.jpsychires.2021.09.041
30. Fritze S, Brandt GA, Volkmer S, Daub J, Krayem M, Kukovic J, et al. Deciphering the interplay between psychopathological symptoms, sensorimotor, cognitive and global functioning: a transdiagnostic network analysis. Eur Arch Psychiatry Clin Neurosci. (2024) 274:1625–37. doi: 10.1007/s00406-024-01782-3
31. Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. (2004) 56:301–7. doi: 10.1016/j.biopsych.2004.06.023
32. Liang S, Yu W, Ma X, Luo S, Zhang J, Sun X, et al. Psychometric properties of the MATRICS Consensus Cognitive Battery (MCCB) in Chinese patients with major depressive disorder. J Affect Disord. (2020) 265:132–38. doi: 10.1016/j.jad.2020.01.052
33. Burdick KE, Goldberg TE, Cornblatt BA, Keefe RS, Gopin CB, Derosse P, et al. The MATRICS consensus cognitive battery in patients with bipolar I disorder. Neuropsychopharmacology. (2011) 36:1587–92. doi: 10.1038/npp.2011.36
34. Niv N, Cohen AN, Sullivan G, and Young AS. The MIRECC version of the Global Assessment of Functioning scale: reliability and validity. Psychiatr Serv (Washington D.C.). (2007) 58:529–35. doi: 10.1176/ps.2007.58.4.529
35. Epskamp S, Borsboom D, and Fried EI. Estimating psychological networks and their accuracy: A tutorial paper. Behav Res Methods. (2018) 50:195–212. doi: 10.3758/s13428-017-0862-1
36. Epskamp S and Fried EI. A tutorial on regularized partial correlation networks. psychol Methods. (2018) 23:617–34. doi: 10.1037/met0000167
37. Zhang M, Zhang D, and Wells MT. Variable selection for large p small n regression models with incomplete data: mapping QTL with epistases. BMC Bioinf. (2008) 9:251. doi: 10.1186/1471-2105-9-251
38. Curran PJ, West SG, and Finch JF. The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. J psychol Methods. (1996) 1:16–29. doi: 10.1037/1082-989X.1.1.16
39. Zhang T, Wei Y, Tang X, Cui H, Hu Y, Xu L, et al. Cognitive impairments in drug-naive patients with first-episode negative symptom-dominant psychosis. JAMA network Open. (2024) 7:e2415110. doi: 10.1001/jamanetworkopen.2024.15110
40. Salthouse TA. The processing-speed theory of adult age differences in cognition. psychol Rev. (1996) 103:403–28. doi: 10.1037/0033-295x.103.3.403
41. Rast P. Verbal knowledge, working memory, and processing speed as predictors of verbal learning in older adults. Dev Psychol. (2011) 47:1490–8. doi: 10.1037/a0023422
42. Stainton A, Chisholm K, Griffiths SL, Kambeitz-Ilankovic L, Wenzel J, Bonivento C, et al. Prevalence of cognitive impairments and strengths in the early course of psychosis and depression. Psychol Med. (2023) 53:5945–57. doi: 10.1017/s0033291723001770
43. Liang S, Xing X, Wang M, Wei D, Tian T, Liu J, et al. The MATRICS consensus cognitive battery: psychometric properties of the chinese version in young patients with major depression disorder. Front Psychiatry. (2021) 12:745486. doi: 10.3389/fpsyt.2021.745486
44. Braund TA, Tillman G, Palmer DM, and Harris AWF. Verbal memory predicts treatment outcome in syndromal anxious depression: An iSPOT-D report. J Affect Disord. (2020) 260:245–53. doi: 10.1016/j.jad.2019.09.028
45. Lystad JU, Falkum E, Haaland V, Bull H, Evensen S, Bell MD, et al. Neurocognition and occupational functioning in schizophrenia spectrum disorders: The MATRICS Consensus Cognitive Battery (MCCB) and workplace assessments. Schizophr Res. (2016) 170:143–9. doi: 10.1016/j.schres.2015.12.002
46. Lindgren M, Holm M, Kieseppä T, and Suvisaari J. Neurocognition and social cognition predicting 1-year outcomes in first-episode psychosis. Front Psychiatry. (2020) 11:603933. doi: 10.3389/fpsyt.2020.603933
47. Kahn RS and Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. (2013) 70:1107–12. doi: 10.1001/jamapsychiatry.2013.155
48. Szmulewicz AG, Lomastro MJ, Valerio MP, Igoa A, and Martino DJ. Social cognition in first episode bipolar disorder patients. Psychiatry Res. (2019) 272:551–54. doi: 10.1016/j.psychres.2019.01.002
49. Mehta UM, Thirthalli J, Subbakrishna DK, Gangadhar BN, Eack SM, and Keshavan MS. Social and neuro-cognition as distinct cognitive factors in schizophrenia: a systematic review. Schizophr Res. (2013) 148:3–11. doi: 10.1016/j.schres.2013.05.009
50. Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, and Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. (2011) 35:573–88. doi: 10.1016/j.neubiorev.2010.07.001
51. Halverson TF, Orleans-Pobee M, Merritt C, Sheeran P, Fett AK, and Penn DL. Pathways to functional outcomes in schizophrenia spectrum disorders: Meta-analysis of social cognitive and neurocognitive predictors. Neurosci Biobehav Rev. (2019) 105:212–19. doi: 10.1016/j.neubiorev.2019.07.020
52. Green MF, Olivier B, Crawley JN, Penn DL, and Silverstein S. Social cognition in schizophrenia: recommendations from the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophr bulletin. (2005) 31:882–7. doi: 10.1093/schbul/sbi049
Keywords: neurocognition, social cognition, global function, network analysis, intervention target
Citation: Zhu Y, Zhang R, Ni L, Xie Z, Lu S, Xie S and Zhang X (2025) The relationship between cognitive and global function in patients with schizophrenia and mood disorders: a transdiagnostic network analysis. Front. Psychiatry 16:1643369. doi: 10.3389/fpsyt.2025.1643369
Received: 08 June 2025; Accepted: 15 July 2025;
Published: 30 July 2025.
Edited by:
Tianhong Zhang, Shanghai Jiao Tong University, ChinaCopyright © 2025 Zhu, Zhang, Ni, Xie, Lu, Xie and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiping Xie, eGllc2hpcGluZ0Buam11LmVkdS5jbg==; Xiangrong Zhang, ZHJ4cnpAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Yuanyuan Zhu1†
Yuanyuan Zhu1† Rongrong Zhang
Rongrong Zhang Shiping Xie
Shiping Xie Xiangrong Zhang
Xiangrong Zhang