- Nantong Mental Health Center (The Fourth People's Hospital of Nantong), Nangtong, Jiangsu, China
Background: Schizophrenia (SCZ) is a chronic and disabling psychiatric disorder. Modified Electroconvulsive Therapy (MECT), which involves electrical stimulation under general anaesthesia and muscle relaxation, is widely used to treat SCZ. Despite its rapid onset and robust therapeutic effect, the efficacy of MECT varies significantly between individuals. This study aimed to evaluate the clinical effectiveness of MECT in patients with SCZ and identify its influencing factors, with the goal of informing personalised treatment strategies.
Methods: This retrospective observational study included 237 inpatients with SCZ who received a full course of MECT at the Fourth People’s Hospital of Nantong between January 2023 and December 2024. Treatment response was evaluated using the Positive and Negative Syndrome Scale (PANSS) reduction rate. Patients were classified into effective (≥50% reduction) and ineffective (<50% reduction) groups. Demographic, clinical, and treatment-related variables were compared between groups, and multivariate logistic regression was used to identify predictors of treatment response.
Results: The overall effectiveness rate of MECT was 70.46%. Multivariate analysis identified age ≥50 years (OR = 0.111–0.078, P = 0.010–0.002) and illness duration ≥10 years (OR = 0.028–0.003, P < 0.05) as negative predictors of response. In contrast, first-episode SCZ (OR=6.537, P = 0.003), higher baseline positive symptom scores (OR = 1.325, P<0.001), and longer EEG seizure duration (OR = 1.183, P<0.001) were positive predictors. No significant associations were found for sex, education level, or stimulus parameters such as current or frequency.
Conclusion: MECT remains a clinically valuable intervention for SCZ, particularly in younger, first-episode patients with prominent positive symptoms. Treatment efficacy is influenced by age, illness duration, baseline symptom severity, and seizure quality. These findings support the need for personalised MECT protocols guided by clinical and electrophysiological characteristics.
1 Introduction
Schizophrenia (SCZ) is a chronic and disabling psychiatric disorder characterised by symptoms including positive symptoms (such as hallucinations, delusions, and disorganised thinking), negative symptoms (such as affective flattening and social withdrawal), and behavioural disturbances (1). Typically manifesting in adolescence or early adulthood, SCZ has become one of the global causes of disability (1, 2). Although antipsychotics have significantly improved clinical outcomes of patients (3), their adverse effects—particularly metabolic syndrome and extrapyramidal symptoms—remain a major clinical challenge (4). In this context, physical interventions have attention, and Modified Electroconvulsive Therapy (MECT) as a promising therapeutic approach owing to its unique mechanism of action (5, 6).
MECT involves the induction of brief, generalised cerebral seizures under anaesthesia, which may contribute to rebalancing neurotransmitter systems and modulating functional brain networks (7, 8). Existing evidence supports the efficacy of MECT in the treatment of SCZ episodes, agitation, and catatonia (8–11), and it is particularly beneficial for patients who are treatment-resistant or require urgent clinical intervention (12–15). Furthermore, several studies have demonstrated that MECT can significantly reduce the length of hospital stay in patients with schizophrenia, thereby alleviating the burden on families and healthcare systems (16–18).
Although previous studies have investigated individual predictors of MECT efficacy—for example patient demographics (age, illness duration), clinical measures (Positive and Negative Syndrome Scale scores) or single treatment parameters (stimulus intensity) (19, 20)—there is a clear lack of comprehensive, multivariable investigations that integrate these domains and examine their joint, potentially interacting effects. For instance, one influential study reported that bitemporal stimulus intensities above 1.5× the seizure threshold may accelerate improvement in positive symptoms, but it did not account for clinical or demographic covariates that could modify this relation (19).To address this evidence gap, the present retrospective study systematically evaluates how demographic characteristics, clinical phenotype and detailed MECT parameters jointly relate to symptomatic outcomes in patients with schizophrenia. Our objective is to generate robust, multivariable evidence to inform the optimisation and individualisation of MECT protocols in routine clinical practice.
2 Materials and methods
2.1 Participants
All consecutive adult in-patients diagnosed with schizophrenia at the Fourth People’s Hospital of Nantong between January 2023 and December 2024 were screened for eligibility (n = 451). Of these, 205 patients were excluded because they met one or more exclusion criteria; note that category counts are not mutually exclusive and therefore do not sum to the overall exclusion total. 9 patients were not evaluable in the primary analysis owing to dropout. The remaining 237 patients comprised the analysed sample (see Figure 1 for full details).
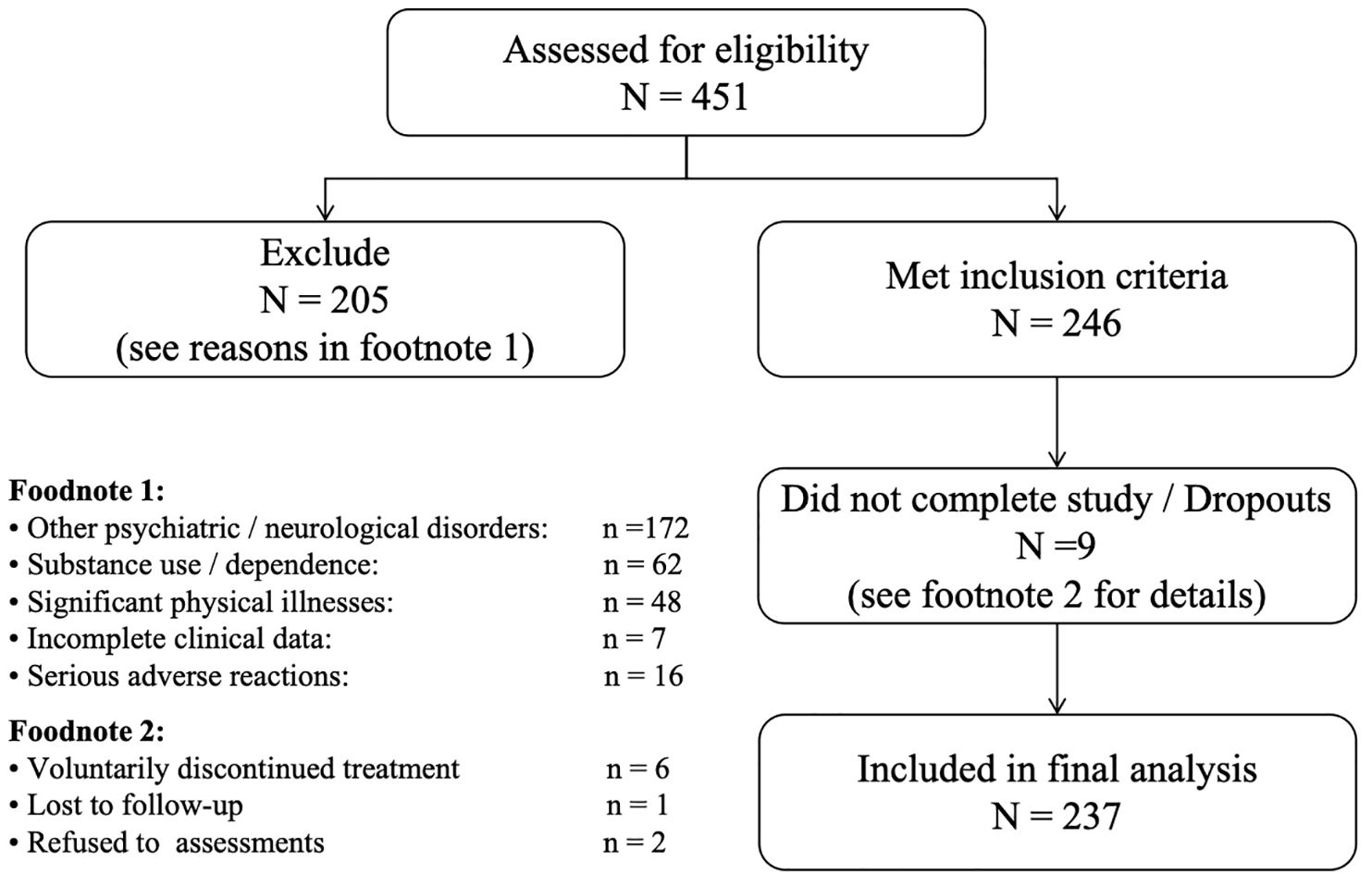
Figure 1. STROBE flow diagram of patient screening, exclusions, and inclusion in the final analysis.
1. Inclusion criteria: Patients who (a) were aged 18 - 65 years; (b) met the diagnostic criteria for SCZ according to either the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition or the International Classification of Diseases, Tenth Revision (21); (c) han no contraindications; (d) voluntarily accepted MECT and had not received MECT within the past six months; (e) completed ≥6 MECT sessions with complete pre- and post-treatment evaluation data; and (f) experienced no serious adverse events requiring treatment discontinuation occurred during the course.
2. Exclusion criteria: Patients with (a) other psychiatric disorders (e.g., bipolar disorder or major depressive episode) or neurological disorders (e.g. bipolar disorder, epilepsy); (b) substance use or drug dependence; (c) significant physical illnesses;(d) incomplete clinical data; or (e) serious adverse reactions to MECT.
3. Drop-out criteria: Patients who (a) voluntarily discontinued treatment; (b) lost to follow-up; or (c) who refused to complete post-treatment assessments.
2.2 Treatment procedure
All procedures were conducted in strict accordance with the Expert Consensus on Modified Electroconvulsive Therapy (2019 Edition) (7). Treatment was administered using a Thymatron IV device (Somatics, Lake Bluff, IL, USA). Pre-treatment protocols included fasting and water restriction, routine screening with Electrocardiograph, chest X-ray, and blood tests to exclude contraindications.
During treatment, vital signs were continuously monitored. Anaesthesia was induced using etomidate (0.16–0.2 mg/kg), muscle relaxation was achieved with succinylcholine (1.0 mg/kg), and atropine sulphate (0.01 mL/kg) was used to reduce airway secretions. Bilateral temporal electrodes were used to induce seizures (22). Initial stimulus dosing was calculated using an age-based formula, with subsequent adjustments based on seizure adequacy assessed by electroencephalographic (EEG) monitoring (18), as detailed in Supplementary 1 Seizure adequacy criteria and dose−titration algorithm.
MECT was administered three times during the first week (on alternate days) and twice weekly during the second week, resulting in a total of six sessions over two weeks. Each treatment was jointly supervised by an anaesthesiologist and a psychiatrist throughout the procedure (18).
2.3 Outcome assessment and grouping
The Positive and Negative Syndrome Scale (PANSS) was used to evaluate clinical efficacy. PANSS comprises three subscales: positive symptoms (7 items), negative symptoms (7 items), and general psychopathology (16 items), with total scores ranging from 30 to 210. Assessments were conducted at baseline (within 24 hours before the first MECT session) and post-treatment (within 24 hours after the final session).
Efficacy was determined based on the reduction rate of the PANSS total score, calculated as:
[(Baseline score − Post-treatment score)/Baseline score] × 100% (23).
We chose a ≥50% reduction in total PANSS score to define treatment response for three principal reasons. First, a 50% reduction is widely regarded in the MECT literature as representing a clinically meaningful improvement and is commonly used in studies that classify outcomes as “markedly effective” (≥75%), “effective” (50–74%), “improved” (25–49%) and “ineffective” (<25%) (24). This categorical scheme has precedent in prior MECT reports (11, 25, 26), facilitating direct comparability with the existing body of MECT evidence. Second, empirical work linking PANSS percentage change to the Clinical Global Impression (CGI) suggests that reductions in the order of ~40–50% correspond to CGI categories such as “much improved”, whereas smaller reductions of 20–30% typically reflect only minimal-to-moderate clinical change (27). Thus, a 50% threshold better captures treatment effects that are likely to be meaningful to clinicians and patients. Third, selecting ≥50% strikes a pragmatic balance between sensitivity and specificity in a treatment context where rapid and substantial symptomatic relief (rather than marginal change) is the clinical aim.
2.4 Observational variables
Demographic characteristics included age, sex, and education. Health-related variables comprised smoking, alcohol consumption, family history of mental illness, and body mass index (BMI). Smoking status was dichotomised based on history (smoker vs non-smoker). Alcohol use was assessed in accordance with WHO guidelines (Global Status Report on Alcohol and Health), categorised as non-drinker, moderate drinker, or harmful drinker. Harmful drinking was defined as a daily average alcohol intake of ≥61g for men and ≥41g for women.
Clinical characteristics included illness duration, first-episode status, and antipsychotic medication dose converted to olanzapine equivalents (28, 29) (see Supplementary Table S1). Baseline scores for PANSS positive, negative, general psychopathology, and total score were also collected.
MECT treatment parameters included EEG seizure duration (30), Average Seizure Energy Index (ASEI), Postictal Suppression Index (PSI), static and dynamic impedance, energy percentage, stimulus dose, current, frequency, and duration. The ASEI represents the averaged ictal EEG power, reflecting the overall “potency” of the seizure, and is calculated over the entire seizure duration. The PSI quantifies the proportion of abrupt termination of ictal activity versus a gradual, undifferentiated decline. Dynamic impedance is measured automatically by the Thymatron during stimulation, whereas static impedance whereas static impedance was measured after EEG electrode placement and prior to stimulation; both values are expressed in ohms (Ω).These definitions and calculations follow the Thymatron IV manual.
2.5 Assessment and quality control
All clinical evaluations were independently conducted by two psychiatrists with attending-level qualifications and formal training in PANSS assessment. Baseline assessments were conducted within 24 hours before MECT initiation, and post-treatment assessments were completed within 24 hours following the final session. Interviews using a standardised protocol.
Data were entered in real-time into an electronic database and double-checked by an independent data manager using a blinded approach. Ten percent of the sample was randomly selected for duplicate data entry verification before database locking.
2.6 Statistical analysis
Statistical analyses were performed STATA 16.0 (StataCorp LLC, USA). Continuous variables were inspected for normality using the Shapiro–Wilk test. Variables with approximately normal distributions are reported as mean ± standard deviation (SD) and compared using independent-samples t-tests. Variables with non-normal distributions are reported as median, interquartile range (IQR) and compared using the two-sided Mann–Whitney U test. Categorical variables are presented as counts (percentages) and compared by Pearson’s χ² test.
A multivariable logistic regression model was used to explore predictors of MECT efficacy. To address potential multicollinearity among predictors, Variance inflation factor (VIF) analysis was performed; variables with VIF > 5 were considered to exhibit multicollinearity. A two-tailed p-value of <0.05 was considered statistically significant.
Sensitivity analyses (1) a worst-case (intention-to-treat) sensitivity analysis in which patients who dropout (discontinued treatment or lacked post-treatment PANSS) were assumed to be non-responders; and (2) Using alternative responder thresholds ≥30%, Results from these sensitivity analyses are reported in the Supplementary Material. Robustness Analyses (1) Ordinal logistic model using the four pre-specified categories (≥75%, 50–74%, 25–49%, <25%); (2) Using PANSS total in place of subscales to test robustness; (3) Using beta regression to test robustness. All primary conclusions were robust to these alternative specifications.
2.7 Ethical statement
This study adhered to the principles of informed consent, autonomy, confidentiality, and beneficence. Written informed consent was obtained from all participants or their legal guardians, after clear explanation of the study’s objectives and procedures. Participation was voluntary and participants’ data were anonymised. The study protocol was reviewed and approved by the Ethics Committee of the Fourth People’s Hospital of Nantong City (Approval No. 2023-Ko37).
3 Results
Based on the percentage reduction in PANSS total scores, participants achieving a reduction rate of ≥50% (i.e. markedly effective or effective) were classified into the effective group (n = 167, 70.46%), while the remaining participants were assigned to the ineffective group (n = 70, 29.54%).
3.1 Comparison of baseline characteristics between groups
A comparison of demographic, health-related, and clinical characteristics between the effective and ineffective groups is presented in Table 1. The two groups were similar across most sociodemographic and clinical variables. The baseline differences of statistical significance were patient age distribution and first-episode status: a substantially greater proportion of non-responders were aged ≥50 years, whereas first-episode patients were more common among responders (both p < 0.05). All other baseline variables (sex, education, residence, BMI, family history, smoking and alcohol use, antipsychotic dose, and most illness-related categories) did not differ between groups (see Table 1).
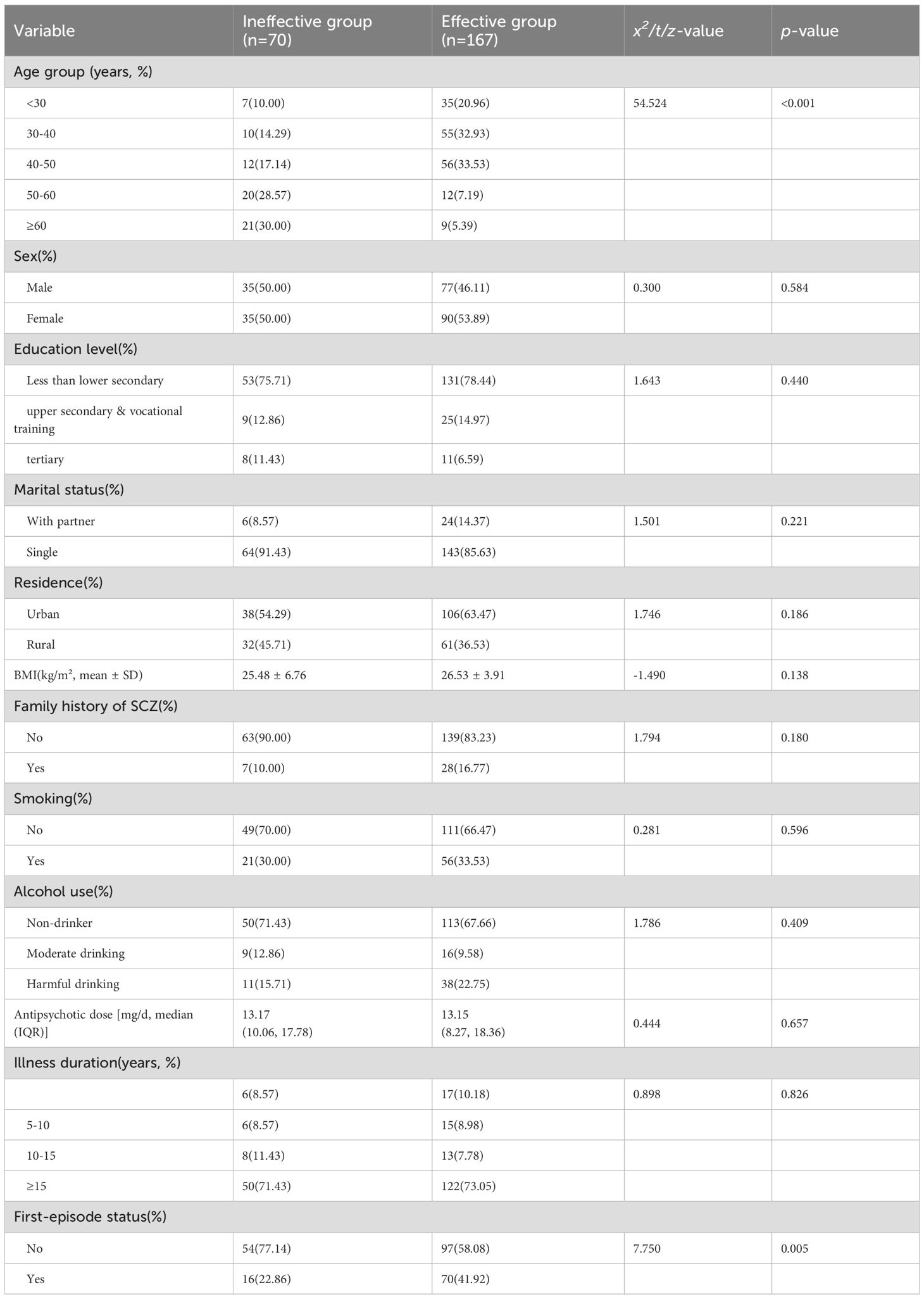
Table 1. Comparison of demographic and clinical characteristics between effective and ineffective groups.
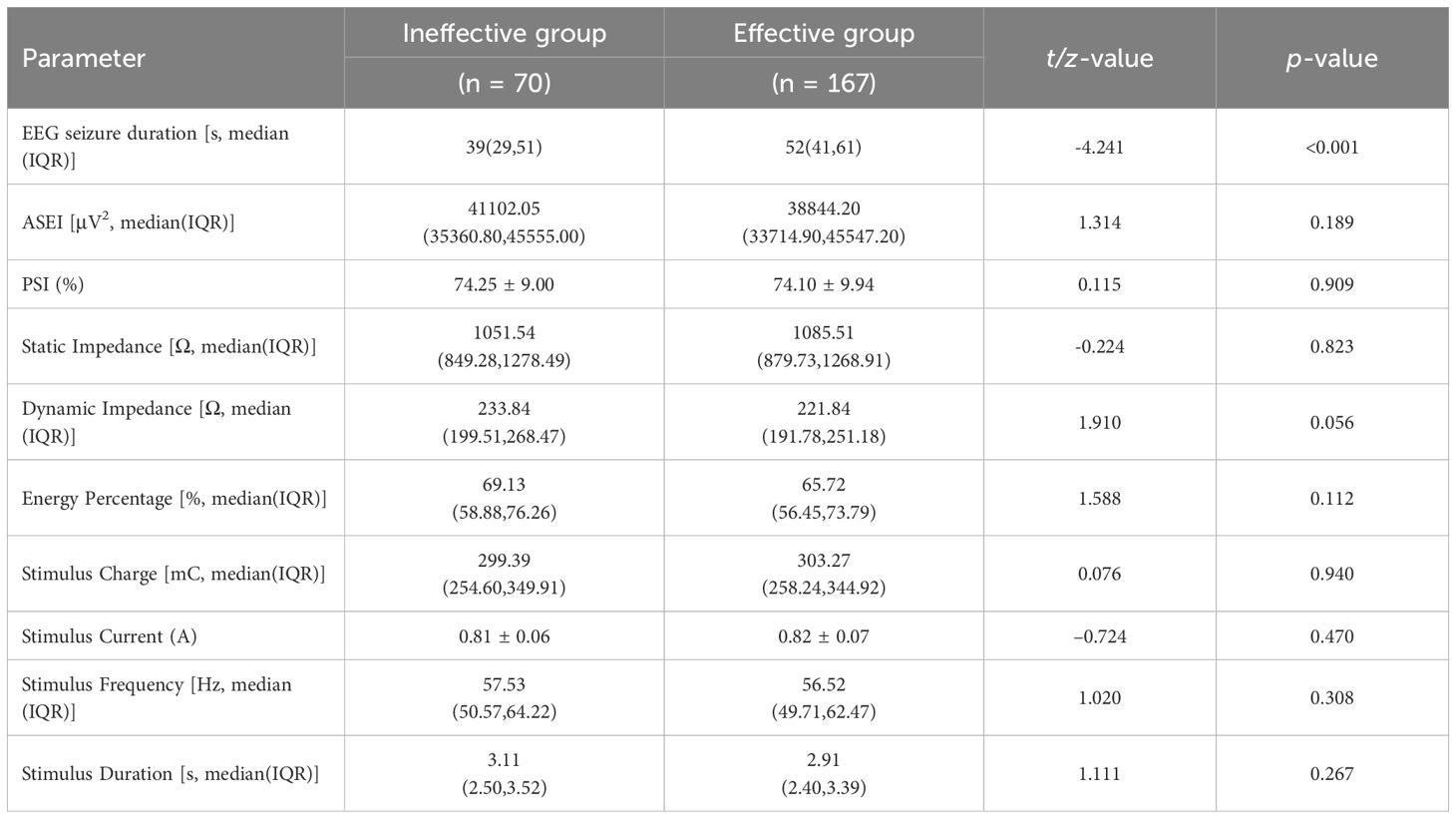
3.2 Comparison of MECT treatment parameters between groups.
The principal electrophysiological difference between groups was EEG seizure duration: responders had significantly longer than non-responders (p < 0.001). No other procedural or device parameters showed differences in comparisons (Table 2).
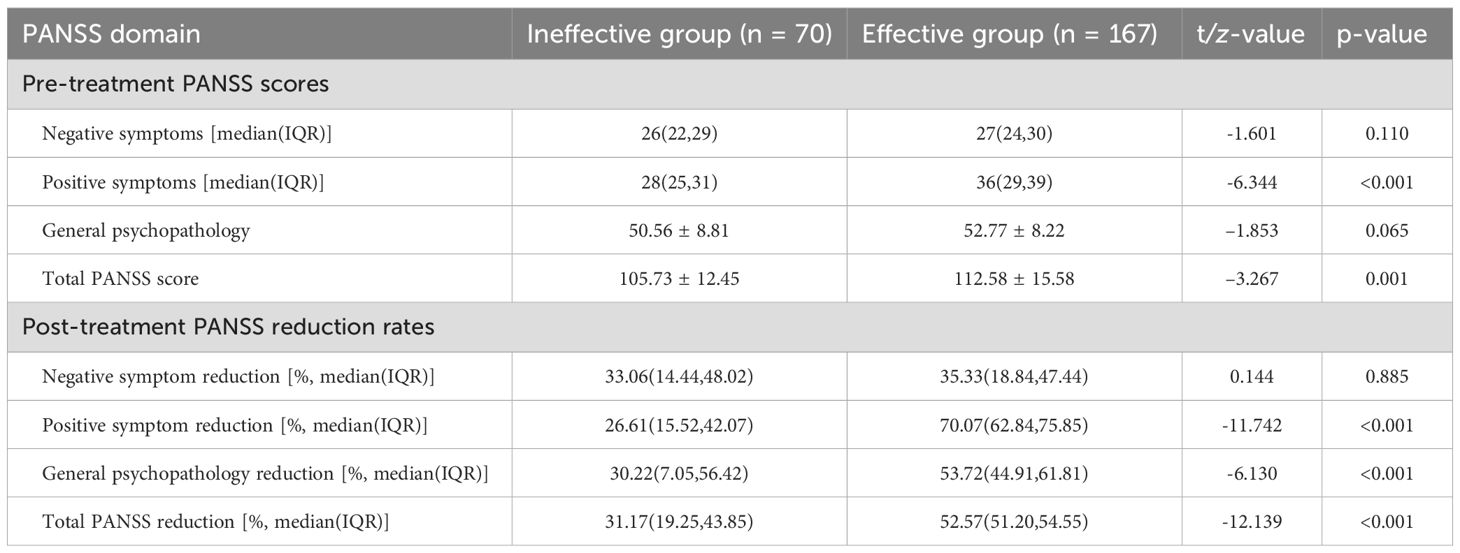
3.3 Comparison of PANSS reduction rates between groups before and after treatment.
At baseline, the effective group had higher PANSS positive and total scores. After the MECT course, responders exhibited substantially greater reductions in positive symptoms, general psychopathology and total PANSS score (all p < 0.001). There was no significant between-group difference in negative-symptom reduction (Table 3).
3.4 Multivariate logistic regression analysis
We fitted a multivariable logistic regression to identify independent predictors of achieving ≥50% PANSS reduction. After addressing severe multicollinearity among candidate predictors (see Supplementary Materials S3 Methods for VIF-based variable selection), the model retained clinical and electrophysiological covariates. Key independent predictors were: older age (≥50 years) and longer illness duration (≥10 years) - both associated with a lower likelihood of being a responder - and first-episode status, higher baseline positive-symptom severity, and longer EEG seizure duration, which were associated with increased odds of response (all P < 0.05), as shown in Table 4.
3.5 Sensitivity and robustness analysis
To test the robustness of these conclusions we (a) inspected VIFs and removed variables with extreme collinearity prior to reporting the final model (Supplementary Table S2), and (b) report sensitivity analyses (alternative response thresholds, continuous outcome modelling and worst-case imputation for dropouts) in the Supplementary Material S4. These supplementary analyses are included to address information-loss concerns inherent to dichotomisation and to verify that the identified direction of associations is robust to reasonable variations in modelling choices.
3.6 Adverse events
Among 237 patients (1,422 MECT sessions), no serious adverse events (AE) occurred that required permanent discontinuation. Overall, 68 patients (28.7%) experienced at least one non-serious AE. The commonest events were headache (n = 40; 16.9%), transient confusion/delirium (n = 18; 7.6%), and transient memory complaints (n = 12; 5.1%). The majority (82%) of events were mild and resolved within 72 hours with symptomatic treatment; 12% were moderate and required brief pharmacological therapy or extended observation. There were no statistically significant differences in overall AE incidence between the effective and ineffective groups (29.3% vs 27.1%, p = 0.55). Full AE details are provided in Supplementary Materials S5.
4 Discussion
This study confirmed a response rate of 70.46% for MECT in the treatment of SCZ, which aligns with previously reported ranging from 55.5% to 76.7% in both domestic and international literature (15, 23, 31). These findings further support the clinical value of MECT as an effective intervention for SCZ. Multivariate logistic regression identified age, duration of illness, first-episode status, severity of positive symptoms, and EEG seizure duration as predictors of treatment outcome, offering an evidence-based foundation for individualised treatment optimisation.
4.1 Demographic predictors of treatment response
Age ≥50 years emerged as a negative predictor of MECT efficacy (OR = 0.111–0.078), with younger patients demonstrating significantly greater symptomatic improvement—consistent with existing findings (32–35). These findings support a model of age-related responsiveness, suggesting that stimulation dosage should be adjusted by age, with older patients potentially requiring higher stimulus intensities to induce adequate therapeutic seizures. There is a clear need for age-adjusted dose–response algorithms to be established in future protocols.
Additionally, illness duration ≥10 years significantly reduced treatment efficacy (OR = 0.028–0.003), a result in line with previous studies (36). Chronicity in SCZ may decrease neuronal responsiveness to MECT. Notably, first-episode patients showed superior outcomes (OR = 6.537), likely due to lower seizure thresholds and higher neuroplasticity observed in early-onset patients (32). This supports the concept of a “critical treatment window,” highlighting the potential for early intervention to exert amplified effects and improve long-term outcomes (37).
Other demographic characteristics—including sex, education level, smoking and alcohol use, and family history of SCZ - were not independently associated with treatment efficacy (P > 0.05). While this finding is consistent with several prior reports (38–40), it is worth noting that one study found women to have lower seizure thresholds and more complete therapeutic seizures (41). These inconsistencies underscore the necessity of large-scale, multicentre randomised trials to determine sex-specific treatment responses and establish standardised evaluation frameworks.
4.2 Clinical characteristics and treatment response
MECT demonstrated substantial efficacy in reducing PANSS positive symptom scores, general psychopathology scores, and overall total scores. Although responders showed significantly greater post-treatment reductions in general psychopathology scores (Table 3), baseline general-psychopathology was not an independent predictor of achieving ≥50% PANSS reduction in our multivariable model. While higher baseline positive symptom scores were positively correlated with greater treatment response (OR = 1.325, p < 0.001), which was consistent with previous studies and may be involved in the recovery of cerebellar-cerebral connectivity following MECT (42).
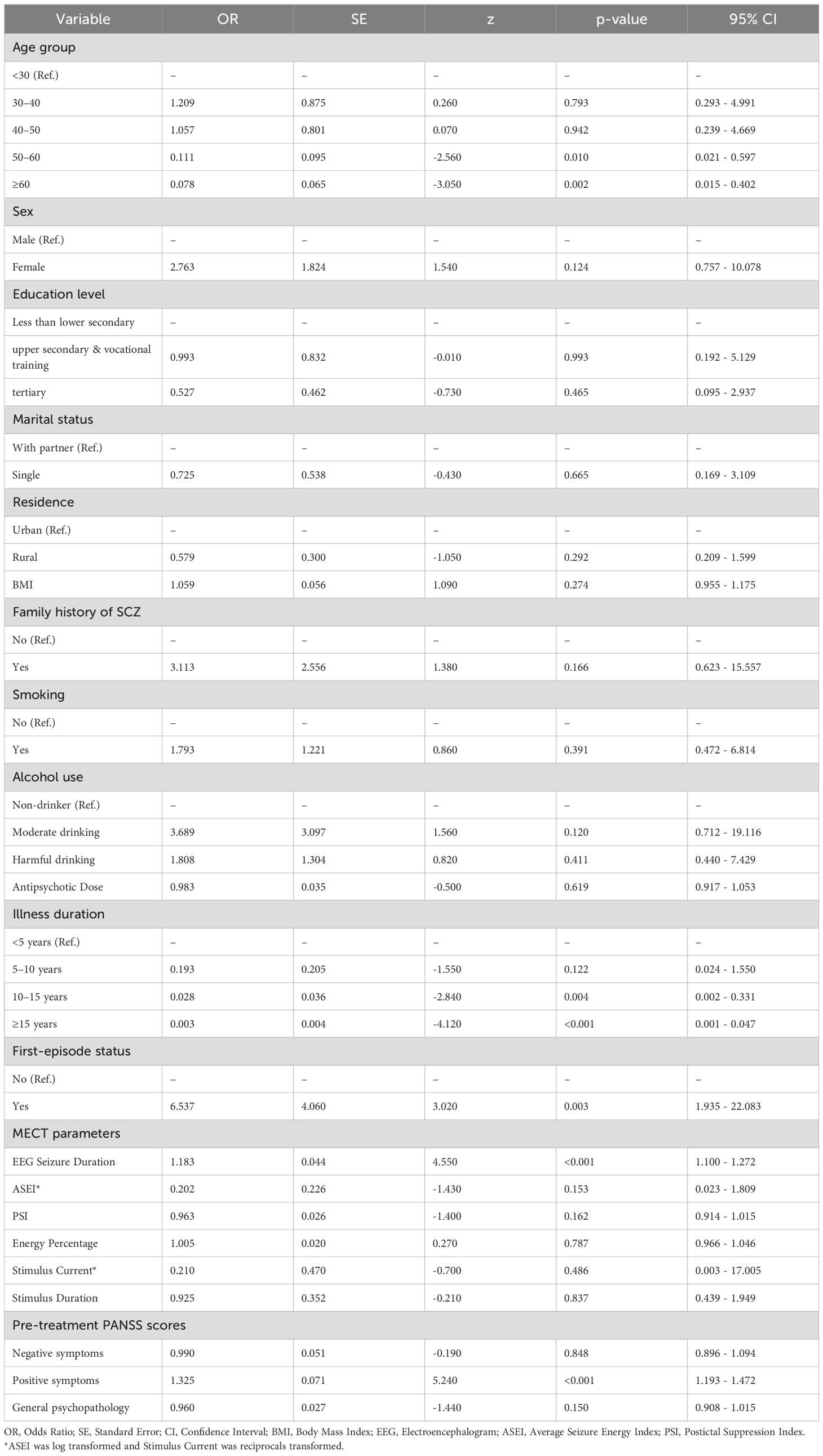
Table 4. Multivariate logistic regression analysis of factors associated with MECT treatment response.
4.3 Technical parameters and electrophysiological predictors
This study explored the association between MECT technical parameters and therapeutic efficacy in SCZ, identifying EEG seizure duration as a key electrophysiological predictor. The effective group exhibited a significantly longer EEG seizure duration compared to the ineffective group, with seizure duration strongly associated with treatment response (OR = 1.183, p < 0.001). These findings are consistent with prior research (43, 44) and suggest that monitoring EEG seizure duration provides useful information when titrating treatment.
No independent predictive value was found for the seizure index or suppression index, consistent with earlier studies (45, 46). This study employed a personalised parameter adjustment strategy, modifying stimulus charge, current, frequency, and duration to balance efficacy with tolerability. These clinician-led adjustments, although beneficial for individual outcomes, may obscure population-level dose–response relationships due to variability and limited sample size. This could explain why no significant associations were observed between stimulation parameters and clinical outcomes in our analysis. Future studies with larger cohorts are needed to elucidate the complex interplay between electrical dosing and neural plasticity.
4.4 Strengths and limitations
This study presents a comprehensive multidimensional evaluation, encompassing demographic, clinical, and electrophysiological factors, thereby enhancing the representativeness and practical relevance of the findings. The integration of diverse predictors strengthens the external validity and clinical applicability of the results. However, several limitations must be acknowledged:
First, the retrospective, observational design limits causal inference. Treatment assignment and parameter adjustments were determined by clinical teams rather than by protocolised allocation, so unmeasured confounding and indication bias may have influenced both treatment choices and outcomes despite multivariable adjustment. Second, the study was conducted at a single tertiary psychiatric hospital; local clinical practice, patient demographics and device-setting routines may differ from other centres, which restricts the generalisability of our findings. Third, we included only patients who completed the six-session MECT course with complete pre- and post-treatment PANSS data. This inclusion criterion risks survivorship (selection) bias: patients who discontinued early because of adverse events, clinical deterioration, practical reasons or early non-response were not represented in the primary analysis and may differ systematically from completers. Although we performed sensitivity analyses treating early dropouts as non-responders (reported in the Supplementary Material), prospective intention-to-treat data would more robustly estimate real-world effectiveness.Fourth, the study did not include formal cognitive assessments or longer-term follow-up. As a result, we cannot comment on the cognitive safety profile of the six-session regimen, nor on durability of response or relapse rates beyond the immediate post-treatment window. These outcomes are clinically important when weighing short-term symptom improvement against potential cognitive adverse effects and relapse prevention strategies. Fifth, although PANSS ratings were performed by two trained psychiatrists under a standardised protocol, we did not compute inter-rater reliability (e.g., ICC or kappa) for this dataset; future prospective work should include formal rater-training and reliability testing. Finally, several electrophysiological and device parameters (for example, seizure index, suppression index and impedance measures) are susceptible to measurement variability (influenced by electrode placement, muscle relaxation, amplifier settings and artefact) and were adjusted in real-time by clinicians; this pragmatic approach improves individual care but reduces the internal control required to precisely estimate dose–response relationships. Taken together, these limitations motivate prospective, multicentre, randomised or adaptive-design studies with standardised titration algorithms, comprehensive cognitive testing and longer follow-up to confirm and extend the present findings.
Future research should aim to address these limitations through multicentre, prospective, randomised controlled trials to validate the present findings. Additionally, exploring the associations between stimulation modalities (e.g., unilateral vs bilateral electrode placement, stimulation frequency variations) and both therapeutic outcomes and adverse effects will be essential. The integration of neuroimaging, electrophysiological indices, and biological markers may also enable the development of more personalised and precise MECT protocols.
5 Conclusion
MECT is an effective therapeutic strategy for patients with schizophrenia, particularly during the acute phase. Treatment efficacy is influenced by several key factors, including age, illness duration, first-episode status, baseline severity of positive symptoms, and EEG seizure duration. These variables should be considered when formulating individualised MECT treatment plans, with the aim of maximising clinical efficacy and informing optimised decision-making in psychiatric practice.
Data availability statement
The datasets presented in this article are not readily available because access requires approval from the Fourth People’s Hospital of Nantong City, Jiangsu Province, China. Requests to access the datasets should be directed to XW, eHVldGluZ193YW5nMjAyMUAxMjYuY29t.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Fourth People’s Hospital of Nantong City (approval no.: 2023-Ko37). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WZ: Formal Analysis, Writing – original draft, Data curation, Conceptualization. QJ: Supervision, Project administration, Conceptualization, Writing – review & editing. PZ: Writing – original draft, Data curation, Investigation, Conceptualization. CM: Software, Project administration, Writing – review & editing, Methodology. WX: Methodology, Validation, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by 2023 Annual General Research Project (Directive) -Nantong Municipal Health Commission (MS2023087).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1654151/full#supplementary-material
Abbreviations
SCZ, Schizophrenia; MECT, Modified Electroconvulsive Therapy; PANSS, Positive and Negative Syndrome Scale; CGI, Clinical Global Impression; EEG, Electroencephalogram; BMI, Body Mass Index; VIF, Variance Inflation Factor; ASEI, Average Seizure Energy Index; PSI, Postictal Suppression Index.
References
1. Lu L and Yu X. Guidelines for diagnosis and treatment of mental disorders (2020 edition) (2020). Available online at: https://www.nhc.gov.cn/wjw/c100175/202012/d21da62f7a654ae28650bc473f6d05e3/files/1644833637272_77437.pdf (Accessed June 10, 2025).
2. IHME. Institute for Health Metrics and Evaluation GBD Compare (2025). Available online at: https://vizhub.healthdata.org/gbd-compare/ (Accessed June 10, 2025).
3. Chinese Schizophrenia Coordination Group, Si T, and Li L. Expert consensus on long-acting injectable in the treatment of schizophrenia. Chin J Psychiatry. (2020) 53:99–110. doi: 10.3760/cma.j.cn113661-20190725-00246-1
4. Han H. Comparison of effects of aripiprazole and risperidone in treatment of schizophrenia. Med J Chin People’s Health. (2020) 32:83–4. doi: 10.3969/j.issn.1672-0369.2020.07.035
5. Wang H and Zhao Y. Clinical effects of MECT in treatment of negative symptoms of schizophrenia. J Guiyang Med Coll. (2016) 41:95–8.
6. Thomann PA, Wolf RC, Nolte HM, Hirjak D, Hofer S, Seidl U, et al. Neuromodulation in response to electroconvulsive therapy in schizophrenia and major depression. Brain Stimul. (2017) 10:637–44. doi: 10.1016/j.brs.2017.01.578
7. Chinese Association of Neurological Regulation Committee for Electroconvulsive Therapy and Nerve Stimulation, Chinese Association of Sleep Committee for Mental Psychology, and Chinese Association of Anesthesiology. Expert consensus on modified electroconvulsive therapy (2019). Trans Med J. (2019) 8:129–34. doi: 10.3969/j.issn.2095-3097.2019.03.001
8. Mukhtar F, Regenold W, and Lisanby SH. Recent advances in electroconvulsive therapy in clinical practice and research. Fac Rev. (2023) 12:13. doi: 10.12703/r/12-13
9. Lu J. Effect of psychological nursing interventions on effectiveness and quality of life in schizophrenia patients receiving modified electroconvulsive therapy. World J Clin cases. (2024) 12:2751–7. doi: 10.12998/wjcc.v12.i16.2751
10. Zhang H, Li H, Yu M, Yu M, Feng S, Tingting W, et al. Modified electroconvulsive therapy normalizes plasma GNA13 following schizophrenic relapse. J ECT. (2024) 40:286–92. doi: 10.1097/YCT.0000000000001050
11. Tao H, Zhou X, Liu Y, Wang Z, Liu Y, Su Z, et al. Clinical effect of modified electroconvulsive therapy on schizophrenia. Riv Psichiatr. (2023) 58:183–9. doi: 10.1708/4064.40481
12. Chiu Y-H, Hsu C-Y, Lu M-L, and Chen C-H. Augmentation strategies for clozapine-resistant patients with schizophrenia. Curr Pharm Des. (2020) 26:218–27. doi: 10.2174/1381612826666200110102254
13. Chen H-Y, Wang X-J, Guo P, Chen H-Y, Wei W-J, Chen Y, et al. Efficacy of modified electroconvulsive therapy in treatment-resistant schizophrenia. Alpha Psychiatry. (2024) 25:700–4. doi: 10.5152/alphapsychiatry.2024.231473
14. Arumugham SS, Praharaj SK, Shreekantiah U, Sreeraj VS, Roy C, Shenoy S, et al. Clinical efficacy and neurobiological correlates of electroconvulsive therapy in patients with clozapine-resistant/intolerant schizophrenia: study protocol of multi-site parallel arm double-blind randomized sham-controlled study. Wellcome Open Res. (2022) 7:212. doi: 10.12688/wellcomeopenres.18028.2
15. Watanabe M, Misawa F, and Takeuchi H. Real-world effectiveness of high-dose olanzapine and clozapine for treatment-resistant schizophrenia in Japan: A retrospective bidirectional mirror-image study. J Clin Psychopharmacol. (2024) 44:151–6. doi: 10.1097/JCP.0000000000001804
16. Tang C-d and Shen X-y. Cost-effectiveness analysis of MECT and risperidone on the treatment of schizophrenian. Strait Pharm. (2011) 23:181–3.
17. Ji P. Correlative factors and efficacy of modified electroconvulsive therapy in schizophrenia. Soochow: Soochow University (2018).
18. Grover S, Sahoo S, Rabha A, and Koirala R. ECT in schizophrenia: a review of the evidence. Acta Neuropsychiatr. (2019) 31:115–27. doi: 10.1017/neu.2018.32
19. Chen B, Tan XW, and Tor PC. Effects of dose on early treatment response to bifrontal electroconvulsive therapy in Schizophrenia: A retrospective study. Psychiatry Res. (2025) 350:116554. doi: 10.1016/j.psychres.2025.116554
20. Tan WJ, Choo KWX, Foo JHX, and Tor PC. Is there an optimal electrode placement for patients with schizophrenia undergoing electroconvulsive therapy? J ECT. (2025). doi: 10.1097/YCT.0000000000001108
21. WHO. The ICD-10 Classification of Mental and Behavioural Disorders. Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization (1992).
22. Kellner CH, Knapp R, Husain MM, Rasmussen K, Sampson S, Cullum M, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry. (2010) 196:226–34. doi: 10.1192/bjp.bp.109.066183
23. Ren J, Li Y, Wang T, Feng S, Xie H, Liang J, et al. Analysis of the efficacy of modified electroconvulsive therapy in schizophrenia patients across different genders. Chin J Nerv Ment Dis. (2025) 51:89–93. doi: 10.3969/j.issn.1002-0152.2025.02.004
24. Zhang F, Xu H, Liang L, Chen L, and Liao J. Therapeutic effect of ziprasidone combined with modified electroconvulsive therapy on patients with first episode schizophrenia and the impact on aggressive behavior and cognitive function. J Int Psychiatry. (2024) 51:1087–90.
25. Melzer-Ribeiro DL, Napolitano IC, Leite SA, Alencar de Souza JA, Vizzotto ADB, Di Sarno ES, et al. Randomized, double-blind, sham-controlled trial to evaluate the efficacy and tolerability of electroconvulsive therapy in patients with clozapine-resistant schizophrenia. Schizophr Res. (2024) 268:252–60. doi: 10.1016/j.schres.2023.11.009
26. Sinclair DJ, Zhao S, Qi F, Joeyi SK, and Clive EA. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. (2019) 3:CD011847. doi: 10.1002/14651858.CD011847.pub2
27. Leucht S, Kane J, Etschel E, Werner K, Johannes H, and Rolf RE. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacol. (2006) 31:2318–25. doi: 10.1038/sj.npp.1301147
28. WHO Collaborative Center for Drug Statistics Methodology. ATC/DDD Index 2025 (2024). Available online at: https://atcddd.fhi.no/atc_ddd_index/ (Accessed May 12, 2025).
29. Leucht S, Samara M, Heres S, and Davis JM. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull. (2016) 42 Suppl 1:S90–4. doi: 10.1093/schbul/sbv167
30. Exner J, Deuring G, Seifritz E, and Brühl AB. Dynamic impedance is correlated with static impedance and seizure quality parameters in bifrontal electroconvulsive therapy. Acta Neuropsychiatr. (2023) 35:177–85. doi: 10.1017/neu.2023.10
31. Wang J, Meng L, Xu Z, Ma Z, Zhang C, Zhong B, et al. Analysis of influencing factors of modified electroconvulsive therapy for schizophrenia. Chin J Nerv Ment Dis. (2021) 6:372–4. doi: 10.3969/j.issn.1002-0152.2021.06.009
32. Mahmood S, Tan X, Chen B, and Tor PC. The influence of age on ECT efficacy in depression, mania, psychotic depression and schizophrenia: A transdiagnostic analysis. J Psychiatr Res. (2024) 177:203–10. doi: 10.1016/j.jpsychires.2024.07.012
33. Yamasaki S, Aso T, Miyata J, Sugihara G, Hazama M, Nemoto K, et al. Early and late effects of electroconvulsive therapy associated with different temporal lobe structures. Transl Psychiatry. (2020) 10:344. doi: 10.1038/s41398-020-01025-8
34. Sgouros S, Goldin JH, Hockley AD, Wake MJ, and Natarajan K. Intracranial volume change in childhood. J Neurosurg. (1999) 91:610–6. doi: 10.3171/jns.1999.91.4.0610
35. Takamiya A, Plitman E, Chung JK, Chakravarty M, Graff-Guerrero A, Mimura M, et al. Acute and long-term effects of electroconvulsive therapy on human dentate gyrus. Neuropsychopharmacology. (2019) 44:1805–11. doi: 10.1038/s41386-019-0312-0
36. Altamura AC, Serati M, and Buoli M. Is duration of illness really influencing outcome in major psychoses? Nord J Psychiatry. (2015) 69:403–17. doi: 10.3109/08039488.2014.990919
37. Marshall M and Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. (2011) 2:CD004718. doi: 10.1002/14651858.CD004718.pub3
38. Parsanoglu Z, Balaban OD, Gica S, Atay OC, and Altin O. Comparison of the clinical and treatment characteristics of patients undergoing electroconvulsive therapy for catatonia indication in the context of gender. Clin EEG Neurosci. (2022) 53:175–83. doi: 10.1177/15500594211025889
39. Manohar H, Subramanian K, Menon V, and Kattimani S. Does gender influence electroconvulsive therapy sessions required across psychiatric diagnoses? A 5-year experience from a single center. J Neurosci Rural Pract. (2017) 8:427–30. doi: 10.4103/jnrp.jnrp_482_16
40. Chanpattana W and Sackeim HA. Electroconvulsive therapy in treatment-resistant schizophrenia: prediction of response and the nature of symptomatic improvement. J ECT. (2010) 26:289–98. doi: 10.1097/YCT.0b013e3181cb5e0f
41. Rasimas JJ, Stevens SR, and Rasmussen KG. Seizure length in electroconvulsive therapy as a function of age, sex, and treatment number. J ECT. (2007) 23:14–6. doi: 10.1097/01.yct.0000263254.21668.f0
42. Hu Q, Huang H, Jiang Y, Jiao X, Zhou J, Tang Y, et al. Temporoparietal connectivity within default mode network associates with clinical improvements in schizophrenia following modified electroconvulsive therapy. Front Psychiatry. (2021) 12:768279. doi: 10.3389/fpsyt.2021.768279
43. Ruangsetakit C and Ittasakul P. Response rate and factors associated with response in patients with schizophrenia undergoing bilateral electroconvulsive therapy. BJPsych Open. (2023) 9:e75. doi: 10.1192/bjo.2023.37
44. Li Y. Compare the clinical efficacy of modified electroconvulsive therapy with different duration in the treatment of refractory schizospermia. J Chin Res. (2018) 3:601–3. doi: 10.3969/j.issn.1671-7171.2018.03.065
45. Wu G, Ren C, and Mo L. Study of dexmedetomidine combined with propofol for electroconvulsive therapy without altering seizure duration. J Chongqing Med Univ. (2012) 37:162–4. doi: 10.3969/j.issn.0253-3626.2012.02.020
Keywords: schizophrenia, SCZ, MECT, electroconvulsive therapy, EEG seizure duration, PANSS, treatment predictors, personalised psychiatry
Citation: Wang Z, Qiu J, Zhang P, Chen M and Wang X (2025) Efficacy and influencing factors of modified electroconvulsive therapy for schizophrenia: a real-world retrospective observational study. Front. Psychiatry 16:1654151. doi: 10.3389/fpsyt.2025.1654151
Received: 26 June 2025; Accepted: 22 September 2025;
Published: 03 October 2025.
Edited by:
Joao Luciano De Quevedo, University of Texas Health Science Center at Houston, United StatesReviewed by:
Vijayalakshmi R., Saveetha College of Nursing, IndiaCiprian Ionut Bacila, Lucian Blaga University of Sibiu, Romania
Copyright © 2025 Wang, Qiu, Zhang, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiancheng Qiu, cWpjX3FxQDE2My5jb20=; Minmin Chen, ODE0NzgyNjYzQHFxLmNvbQ==; Xueting Wang, eHVldGluZ193YW5nMjAyMUAxMjYuY29t
†These authors have contributed equally to this work
 Zhiping Wang†
Zhiping Wang† Xueting Wang
Xueting Wang