- 1Department of Anaesthesia, Townsville University Hospital, Townsville, QLD, Australia
- 2Department of Anaesthesia, Geelong Hospital, Geelong, VIC, Australia
- 3Surgical Services, Townsville University Hospital, Townsville, QLD, Australia
- 4Department of Surgery, Townsville University Hospital, Townsville, QLD, Australia
- 5Sport and Exercise Science, James Cook University, Townsville, QLD, Australia
- 6Australian Institute of Tropical Health and Medicine, James Cook University, Townsville, QLD, Australia
There is substantial interest by clinicians to improve the health outcomes of older and frail patients following major surgery, with prehabilitation a potential and important component of future standard patient care. We studied the feasibility of a randomised controlled trial of pre-operative prehabilitation in frail patients scheduled for colorectal surgery in regional Australia. We conducted a single blind, parallel arm, randomised controlled trial in a regional referral centre where colorectal surgical patients aged over 50 were invited to participate and screened for frailty. Frail patients were randomised to undertake either a 4-week supervised exercise program with dietary advice, or usual care. The primary outcome was 6-min-walk-distance at baseline, pre-surgery (4 weeks later) and at follow-up (4–6 weeks post-operation). Secondary outcomes included physical activity level, health-related quality of life, and post-surgical complications. Feasibility outcomes were numbers of patients reaching each stage and barriers or reasons for withdrawal. Of 106 patients eligible for screening during the 2-year study period, only five were able to be randomised, of which one alone completed the entire study to follow-up. Fewer patients than expected met the frailty criteria (23.6%), and many (22.6%) were offered surgery in a shorter timeframe than the required 4 weeks. Physical and psychological aspects of frailty and logistical issues were key for patients declining study participation and/or not complying with the intervention and/or all outcome assessments. Feasibility for a large randomised controlled trial of prehabilitation for frail colorectal patients was poor (~5%) for our regional location. Addressing barriers, examination of a large, dense population base, and utilisation of a frailty-screening tool validated in surgical patients are necessary for future studies to identify the impact of prehabilitation for frail patients.
Introduction
Techniques in surgery and anaesthesia are continuously evolving, such that there are gradual improvements in outcomes, safety, and side-effect profiles over time. Examples of this are the development of minimally invasive procedures and fast-track programmes in colorectal cancer surgery, which have significantly reduced the surgical stress-response, the length of hospital stay and associated morbidity (1). Colorectal cancer is the third most commonly diagnosed cancer worldwide, and the fourth most common cause of cancer death (2). Age is a significant risk factor for colorectal cancer, with the majority of diagnoses in patients over the age of 60 (3). Nowadays, improvements in perioperative care have allowed major but potentially curative surgery to be offered to sections of the population who in previous generations may have been considered “too sick” or “too old”—in effect, too frail—to undergo large, invasive procedures under general anaesthesia (4).
Frailty is a clinically recognisable state of increased vulnerability to poor resolution of homeostasis after a stressor event such as surgery (5, 6). It results from aging-associated decline in reserve and function, as well as a variable burden of comorbidity across multiple physiologic systems, increasing the rate of adverse outcomes (5, 6). The prevalence of frailty in the developed world is increasing with the rate of frailty being 40–50% in patients diagnosed with colorectal cancer (7, 8). Frailty has been identified as an important risk factor for post-operative complications requiring intensive care support as evident in a study of 58,448 colectomies from the US National Surgical Quality Improvement Program database (9). In a systematic review, frail patients were generally at higher risk of complications perioperatively compared to their non-frail counterparts of the same age (10). It is now established that patients have reduced long-term survival after developing complications following major abdominal surgery, even if they survive to hospital discharge (11). Frailty is therefore an increasing clinical challenge perioperatively with ways to identify and optimise surgical recovery and beneficial outcomes in this susceptible group urgently required. Pre-operative exercise training, known as prehabilitation, is one possible method to gain these improvements.
Lower pre-operative exercise capacity and physical activity levels are reported to be independent predictors of mortality, discharge destination, and length of hospital stay for surgical patients in general (12). Frail patients are highly likely to experience lower exercise capacity and physical activity levels (6) and high rates of mortality and length of hospital stay following surgery (10). Therefore, a pre-operative focus on enhancing exercise capacity in frail patients may lead to beneficial outcomes, post-operatively. Exercise capacity can be modified through structured programs (13) with the frail elderly able to tolerate various exercise regimes (14). Further, studies of community and medical inpatients have demonstrated that multimodal interventions are feasible and beneficial in terms of reversing functional decline and improving quality of life (15, 16). Whilst studies have shown regular exercise to improve physical function with little harm during adjuvant chemotherapy for breast cancer patients (17), the evidence for prehabilitation to enhance post-operative function in other patients is less clear (18–21). A ~2.5-day shorter hospital stay was reported for frail colorectal patients following a novel trans-institutional, transdisciplinary model of care involving prehabilitation (20). A systematic review of prehabilitation studies with colorectal cancer patients aged over 60 years old reported that only 7% of the trials selected older patient groups, and frailty status was unspecified (22). The review concluded that prehabilitation was a possible strategy for enhancing physical performance pre-operatively in patients undergoing colorectal surgery, but there was no significant reduction in post-operative complications or length of hospital stay in a population aged over 60.
The type of exercise training protocols, the outcomes used and surgical case mix of the patients in prior prehabilitation studies have been diverse (18–23) with definitive benefits of prehabilitation still to be confirmed. Furthermore, the health economic benefits of pre-operative exercise training programs on patient care are unknown and encouraged (20), but if beneficial would provide an important healthcare incentive for prehabilitation to be part of standard patient care, especially for the frail elderly prior to major surgery.
This study aimed to assess the feasibility of a randomised controlled trial (RCT) of pre-operative prehabilitation in frail colorectal patients in regional Australia, including economic analysis. The feasibility study was required as this specific patient group had not previously been targeted for a RCT with an exercise intervention. Also, the planned location of regional Australia was unique, with a referral population of over 500,000 spread over an area of 80,000 km2. We hypothesised that compared to a control group of patients receiving usual care, frail patients undergoing elective colorectal surgery after a pre-operative tailored regimen of exercise with dietary advice would: demonstrate a greater functional walking capacity 28 days post-operatively (24); demonstrate an earlier recovery with improved post-operative 7-day physical activity levels; demonstrate improved health-related quality of life; and exhibit a reduced incidence of post-operative complications (25).
Materials and Methods
The feasibility study was carried out in a single centre—a university-affiliated, tertiary hospital in regional Queensland, Australia, between March 2016 and November 2017. Internationally recognised guidelines for feasibility studies were followed (26) to assess the possibility of conducting a larger pragmatic study looking at both the health and economic impacts of prehabilitation in frail patients. The feasibility study processes and patient pathway were planned as for the conduction of a larger study. This was a single blind, parallel arm, RCT in frail colorectal surgical patients with the trial prospectively registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12616000021471) in January 2016. Ethical approval was obtained from the Townsville Hospital and Health Service Human Research Ethics Committee (HREC/15/QTHS/176) with all patients providing written informed consent before their inclusion.
Patient inclusion criteria were as follows: patient undergoing colorectal surgery for cancer; frail or prefrail by Edmonton Frail Scale (EFS, >5 criteria) (27); able to attend exercise training in the regional city; and age ≥50. Exclusion criteria were: emergent or urgent surgery (<28 days wait); inability to speak English or documented learning impairment; and contraindications to prehabilitation based on medical comorbidities.
Patients attending colorectal outpatient clinics and booked for surgery were approached for screening and interviewed by a study nurse. Eligible consenting patients were then randomised (1:1) by computer-generated random numbers to receive either prehabilitation with dietary advice (intervention) or standard pre-operative care (control). Standard pre-operative care involved an ad-hoc, and as needed, program including continuation with medications and anxiety management if needed. Typically this care did not include assessment of frailty or dietary advice, unless signs of malnourishment were evident. Patients then underwent initial baseline assessments of exercise capacity via a 6-min walk test (6-MWT) and short physical performance battery (SPPB) (28), quality of life by EQ-5D (29), short-form 12 (30), and modified Barthel index (31), and 7-day physical activity levels using accelerometry (Sensewear Armband, BodyMedia Inc., Pittsburgh, PA). The assessments were repeated pre-operatively (~4 weeks after baseline) and at the follow-up appointment, 4–6 weeks after surgery. All assessors were blinded to group allocation.
The prehabilitation program consisted of three, 1-h sessions per week on non-consecutive days, for 4 weeks prior to surgery to increase muscular strength and cardiorespiratory/aerobic function (13). The sessions included a warm-up followed by a 30-min circuit consisting of strength and core/balance, and 20-min of aerobic exercise), and a cool down (Table 1). All sessions were fully tailored to the ability of the individual, updated as necessary, and supervised by qualified exercise physiologists. Dietary advice in accordance with Australian Dietary Guidelines (32) was provided to participants in the intervention group at the start of the 4-week program, and as needed, to ensure they could meet any increased metabolic needs.
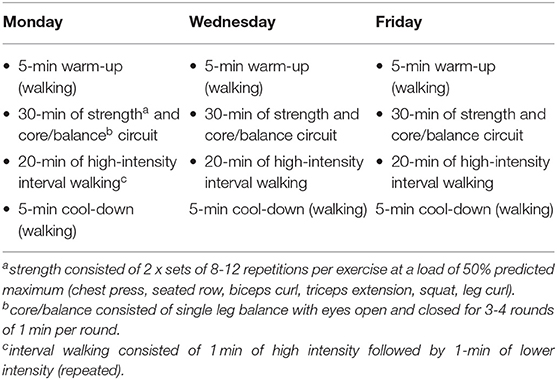
Table 1. Example of a typical week of the prescribed prehabilitation program for the intervention group.
The planned primary outcome for the RCT was the 6MWT distance at follow-up clinic with other data collected for secondary outcomes and economic analysis. Feasibility outcomes studied were as follows: number of patients able to be enrolled—i.e., meeting eligibility criteria for frailty screening, willing to be screened, meeting frailty criteria, and accepting randomisation; barriers to recruitment via open-ended questions; time required for screening and recruitment; proportion of patients enrolled and able to complete the study including all pre- and post-operative assessments, and undergoing surgery as scheduled; compliance with study protocols; reasons for patient early withdrawal; rate and type of post-operative complications, length of hospital stay, and readmission rate/reasons for frail patients; and economic analysis data collection: EQ-5D, SF-12, costs of inpatient care and complications, costs of intervention (i.e., salary, equipment, travel costs), and modified Barthel index as a measure of care dependence of patients in both groups.
Based upon historical records of annual major elective resection operations and the incidence of frailty at the tertiary hospital, and advice from colleagues at a large metropolitan hospital who care for similar patients, we expected to be able to recruit 50 patients (25 per group) over a 2-year period. Based upon this rate of recruitment, and with at least 75% of participants able to complete assessments required for the primary endpoint, a larger study involving other centres would be deemed feasible.
Results
Recruitment to this study was slow with initial barriers identified at the 4- and 8-month time points. These barriers were: shorter than expected operative times; lower incidence of frailty than expected; patients experiencing physical and psychological effects of frailty and disease; and logistical issue associated with recruitment process and follow-up. The predominant reasons for investigator-led exclusion were that 22.6% of screened patients had surgery scheduled within 4-weeks, not allowing time for the intervention, and 23.6% fell below the EFS cut-off for frailty (Supplementary Table 1). Patient reasons for not wanting to be involved in the study were assessed through semi-structured interviews and classified into four main categories:
• Physical effects of frailty and colorectal disease: Bowel symptoms such as diarrhoea and abdominal pain, and spontaneously occurring unrelated adverse events such as falls and hospital admissions.
• Psychological effects of frailty: Patients felt that they were a burden on family and friends, especially for transport. The exhaustion component of the frailty phenotype resulted in patients being less willing to participate in an exercise intervention, in fact several visibly recoiled at the mention of the word “exercise,” and many had been advised by family members and general practitioners that they needed to rest. Conversely, several patients commented that the telephone support and extra interactions with healthcare providers as a result being in the study were invaluable.
• Timing of recruitment: Patients were approached in a busy surgical clinic immediately after receiving the news that they had colorectal cancer and required major surgery. Many patients, understandably, were minimally able to process further information, and preferred not to consider involvement in research at that time. The time required for screening and baseline assessment, ~1 h, was also a deterrent to many.
• Logistics: The ability of the frail elderly to surmount logistical problems was limited, given their reduced independence with transport and potential memory problems.
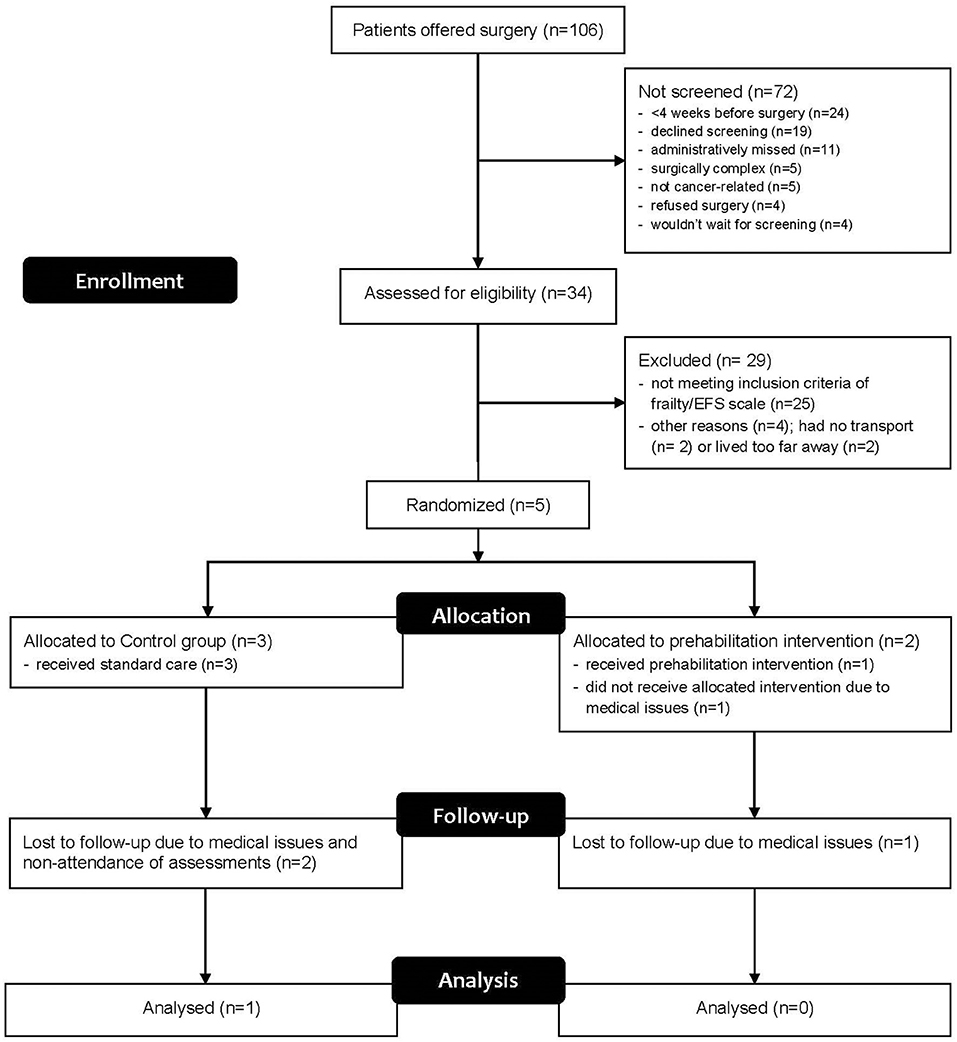
Subsequently, major amendments to the protocol were made and approved through local ethics and governance bodies, including: the inclusion of patients undergoing non-cancer colorectal surgery, such as for diverticular disease; reducing the EFS cut-off from >5 to 4, as many patients were not reaching eligibility criteria despite appearing frail to clinicians; and recruitment of exercise physiologists in surrounding towns (i.e., 100–900 km radius) to deliver the training and assessments closer to patients' residence, in order to surmount logistical issues related to travel. Despite these changes, substantial increases in patient recruitment were not achieved with similar issues raised as to those previously, and therefore the remainder of the feasibility outcomes could not be studied. The recruitment results for the feasibility study are shown in Figure 1. Only five patients out of a potential 106 were able to be randomised into the study.
Five patients were eventually recruited and one patient in the control group completed all assessments (Participant 4). All patients who undertook questionnaires and physical assessments completed these with no adverse reactions or problems. During the 6MWT, all patients walked without aids or stoppages and the distances recorded are shown in Table 2. Only one patient attended the prehabilitation sessions regularly, found them highly beneficial and was keen to continue with the program as a paying customer after surgery. The case narratives in Table 3 provide details on each participant's journey from recruitment to completion or withdrawal, giving a clear depiction of the types of issues encountered.

Table 2. Exercise capacity via 6-min walk test distance for participants at each assessment time point.
Discussion
The current study demonstrated poor feasibility of a RCT for pre-operative prehabilitation in frail colorectal patients within regional Australia. Over 2 years, only ~5% of eligible patients were willing to participate with significant barriers to participation identified. Consideration of barriers, population base, and frailty-screening tools for surgical patients are crucial to confirm the impact of prehabilitation for frail, elderly, colorectal patients.
There has been much interest in the past few years concerning frail patients and post-operative outcomes (33), with studies comparing frailty scores as outcome markers (8–10). Several studies examining prehabilitation in elderly colorectal patients have been conducted in metropolitan centres in Canada (19, 23, 34), the United Kingdom (35), Hungary (21), and a regional hospital in the Netherlands (12). These studies have demonstrated improved post-operative functional walking capacity (19, 21), and return to baseline exercise capacity after neoadjuvant chemoradiotherapy (35). Chia and colleagues (20) highlighted the potential of prehabilitation for frail colorectal patients with a ~2.5-day shorter hospital stay following a novel trans-institutional, transdisciplinary model of care. In contrast, no improvements were observed for duration of hospital stay or for post-operative mortality and morbidity following a trimodal (i.e., physical, emotional, and nutritional) prehabilitation program in non-frail colorectal patients (21). Whilst frailty may pose a significant confounding influence on patient outcomes, prehabilitation of frail patients may be associated with better outcomes from surgery (20, 36). Therefore, the current study aimed to expand upon these prior findings of frail colorectal patients (20) with a RCT within regional Australia however, significant barriers to recruitment were encountered. Notably, patients were scheduled for surgery within a short timeframe, due to both treatment urgency as well as efficiency drives to ensure a full operating schedule within the regional public health system (i.e., surgery brought forward to replace a cancelled surgery). The length of the prehabilitation program in the current study was in accordance with the average waiting period for colorectal surgery within the public health sector of regional Australia. Further, it aligned with other prehabilitation programs (33) and/or longer than other prehabilitation programs for abdominal surgery (37). The optimal length of prehabilitation has not been confirmed with recent reviews highlighting the large variability in prehabilitation programs for cancer and frail patients (33, 38). Therefore, it is generally accepted that surgery for treatment of cancer is offered with as minimal a delay as possible, in order to prevent progression of the disease. However, there is retrospective evidence that a delay of up to 12 weeks does not adversely affect outcomes for up to 5 years (39). Further, therapeutic delay (>35 days) led to similar overall or cancer-free survival in patients with primary colorectal cancer who underwent curative surgical treatments (40). Therefore, a dedicated commitment to prehabilitation (e.g., 2–12 weeks) as pre-surgical care may be fundamental to optimise patients' functional status, along with enhancement of many other risk factors such as nutritional status, psychological wellness, diabetes control, iron stores, and smoking status (21, 41, 42). Future studies comparing multimodal prehabilitation with a delay, vs. benefits of earlier surgery without prehabilitation, and identifying cohorts of patients who benefit from each strategy would be of great interest. While surgery timelines were a significant limitation, many patients in the current study were unwilling or unable to participate due to the physical and psychological effects of frailty and/or cancer (6, 43). Therefore, multidisciplinary approaches focusing on holistic care are necessary to support patients at this vulnerable time. The range of issues experienced by patients and encountered by clinicians in the current study pose significant challenges to research and future therapies to enhance surgical outcomes and long-term healthcare of these patients. Addressing these important barriers is vital for future robust RCT evaluations of prehabilitation in this vulnerable population (43).
Despite the aforementioned barriers, frail patients were able to complete exercise capacity assessments and undertake prehabilitation in accordance with recommended exercise guidelines (13). Recently, a small number of frail, older patients with colorectal cancer (n = 14) were able to complete an at-home, digital prehabilitation program of exercise and nutrition (20). Collectively, our and prior (20, 44) results highlighted that frailty alone is not a contraindication to prehabilitation but frail colorectal patients require additional support to engage with prehabilitation programs, as described above. Importantly, frailty needs to be identified initially by clinicians. A recent systematic review (33) highlighted that frailty should be assessed in surgical patients with an appropriate instrument. We elected to use the EFS as our tool to identify frailty as it has been the most validated in clinical and research use to date (27). However, the scale has been validated in medical patients rather than a surgical population and therefore we found that a significant proportion of our final cohort of patients (73.5%), although subjectively quite frail, did not score highly enough to be eligible for inclusion (Supplementary Table 1). Thus, tool selection is of paramount importance when classifying frailty, as one needs to identify a cohort of patients who are frail enough to have the potential for significant gains from increased exercise capacity, but not so frail that they are unable to be studied. In the current study, a large proportion of the screened patients were not frail enough by this tool to be included that may represent a bias. Those patients exhibiting greater frailty may be more likely to not consider enrolment in a prehabilitation program due to the physical and psychological effects of frailty and/or cancer (6, 43). Therefore, addressing the identified barriers at diagnosis may be crucial to engage patients with prehabilitation for benefits in this vulnerable population (43). For example, engaging family of the patient in all discussions, addressing expectations of patients in terms of effort needed and benefits of exercise to health, engaging medical/nursing staff to champion the program, and logistical issues such as transport and regular communication (43).
A range of tools to identify the clinical syndrome of frailty have been reported and validated over the last several years (27, 45, 46). They can be scored using objective measures, subjective questioning or mixed methods. Ideally they cover physical, psychological, and social domains as all three contribute to the frailty syndrome (6). Examples of mixed scores in widespread use are Fried's Phenotype of Frailty or the Frailty Index developed by Mitnitski (45), as well as the EFS (27). The Risk Analysis Index (47, 48) is a subjective frailty screening tool shown to have improved mortality outcomes when used as a trigger for senior pre-operative consultation in a large cohort of mixed surgical patients in a US Veteran Affairs hospital. The Risk Analysis Index could be a more appropriate choice of tool to identify frailty in a surgical cohort. Another approach to identify frailty could be based on objective markers of frailty such as muscular weakness and sarcopenia (46, 49, 50). Handgrip strength was reported to correlate negatively with overall frailty (50) while psoas muscle area was positively correlated with outcomes after major surgery (46, 49). Both of these musculoskeletal assessments are relatively simple and quick to assess however, only represent physical aspects of frailty. Therefore, the most appropriate frailty tool remains to be determined to assist clinicians with therapeutic options including prehabilitation.
As recently highlighted (33), there exists very little evidence for the use of prehabilitation for frail populations (i.e., five studies) with more RCT studies needed. Most studies to date have been conducted in metropolitan areas that likely can support the design of RCT studies. The proportion of colorectal cancer patients eligible and willing to travel to participate in our regional research study was as expected (~10% once eligibility modifications were in place) however, the absolute number was very small. Therefore, a larger, dense population base is warranted for future studies. Based upon our results, studies aiming to recruit 100 patients in a year should recruit from services that perform at least 1,000 colorectal surgeries annually. Also, as only one participant out of five managed to complete all the assessments to follow-up in our study, loss to follow-up rates may be high (up to 80%) and therefore may require an even larger population base. Future, large-scale studies of pre-operative prehabilitation in frail colorectal patients, possibly across multiple sites and/or metropolitan centres, are needed to confirm the health and economic benefits of prehabilitation for this patient group. A more pragmatic quality improvement initiative, such as prehabilitation embedded into routine care (20), may provide a valuable design for future studies to assist clinicians and patients with optimising healthcare delivery and outcomes. Further, the use of remote care (via technology) may support prehabilitation as routine care in the future. Recently, the use of a wearable (smartwatch/phone application) over 2-weeks was reported to increase physical activity levels and functional (aerobic) fitness of colorectal cancer patients (37). Incorporation of wearables and/or telehealth may support patients to undertake prehabilitation by overcoming issues identified in the current study (e.g., transport logistics, comfort, etc.). Future studies are encouraged to clarify the role of remote care to aid prehabilitation outcomes, especially in remote settings.
Finally, the current study highlighted the value of conducting a pilot study, especially for a vulnerable group of patients. Despite all good intentions to undertake a novel and important intervention within a regional setting, significant issues were encountered in this feasibility study that would limit a larger scale trial. Such learnings have provided clear direction and economic savings for the conduct of future trials in this topic by clinicians.
Conclusions
Improving surgical outcomes for high-risk, frail patients is a key health goal and prehabilitation interventions merit robust assessment in this group with the best patient-centred approach yet to be determined. Addressing barriers, examination of a large, dense population base and utilisation of a frailty-screening tool validated in surgical patients are necessary for future studies to clarify the impact of prehabilitation and those patients who can benefit most. Finally, a pilot or feasibility study can provide clinicians with valuable guidance when studying future “real-life” interventions for frail populations.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Townsville Hospital and Health Service Human Research Ethics Committee (HREC/15/QTHS/176). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
CF, SS, YH, and AL: conceptualisation. DN and AL: data collection. CF, SS, DN, YH, and AL: data analysis/interpretation and manuscript revisions. CF, SS, and AL: manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Townsville Hospital and Health Service Research Trust Fund (RG/2015/22) to SS, AL, CF, YH, and Australian and New Zealand College of Anaesthetists (ANZCA) Novice investigator grant (N17/001) to CF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge Kerrianne Watt and Kenny Lawson to their contribution to the original study design and plans for economic analysis; Crispin Mushaya for assistance with recruitment of patients; and David Story for his sound advice and encouragement throughout the process.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fresc.2021.650835/full#supplementary-material
References
1. Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, von Meyenfeldt MF, Ubbink DT, et al. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. (2006) 93:800–9. doi: 10.1002/bjs.5384
2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
3. Cancer Research UK. Bowel Cancer Statistics Cancer Research UK; 2015. (2018). Available online at: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer (accessed February 8, 2018).
4. Hamel MB, Henderson WG, Khuri SF, and Daley J. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc. (2005) 53:424–9. doi: 10.1111/j.1532-5415.2005.53159.x
5. Clegg A, Young J, Iliffe S, Rikkert MO, and Rockwood K. Frailty in elderly people. Lancet. (2013) 381:752–62. doi: 10.1016/S0140-6736(12)62167-9
6. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. (2011) 27:1–15. doi: 10.1016/j.cger.2010.08.009
7. Rockwood K, Song X, and Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. (2011) 183:E487–94. doi: 10.1503/cmaj.101271
8. Ommundsen N, Wyller TB, Nesbakken A, Jordhoy MS, Bakka A, Skovlund E, et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist. (2014) 19:1268–75. doi: 10.1634/theoncologist.2014-0237
9. Obeid NM, Azuh O, Reddy S, Webb S, Reickert C, Velanovich V, et al. Predictors of critical care-related complications in colectomy patients using the National Surgical Quality Improvement Program: exploring frailty and aggressive laparoscopic approaches. J Trauma Acute Care Surg. (2012) 72:878–83. doi: 10.1097/TA.0b013e31824d0f70
10. Lin HS, Watts JN, Peel NM, and Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. (2016) 16:157. doi: 10.1186/s12877-016-0329-8
11. Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. (2005) 242:326–43. doi: 10.1097/01.sla.0000179621.33268.83
12. Dronkers JJ, Chorus AM, van Meeteren NL, and Hopman-Rock M. The association of pre-operative physical fitness and physical activity with outcome after scheduled major abdominal surgery. Anaesthesia. (2013) 68:67–73. doi: 10.1111/anae.12066
13. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb
14. Chou CH, Hwang CL, and Wu YT. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: a meta-analysis. Arch Phys Med Rehabil. (2012) 93:237–44. doi: 10.1016/j.apmr.2011.08.042
15. Martinez-Velilla N, Casas-Herrero A, Zambom-Ferraresi F, Saez de Asteasu ML, Lucia A, Galbete A, et al. Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization: a randomized clinical trial. JAMA Intern Med. (2019) 179:28–36. doi: 10.1001/jamainternmed.2018.4869
16. Tarazona-Santabalbina FJ, Gomez-Cabrera MC, Perez-Ros P, Martinez-Arnau FM, Cabo H, Tsaparas K, et al. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc. (2016) 17:426–33. doi: 10.1016/j.jamda.2016.01.019
17. Markes M, Brockow T, and Resch KL. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. (2006) (4):CD005001. doi: 10.1002/14651858.CD005001.pub2
18. Lemanu DP, Singh PP, MacCormick AD, Arroll B, and Hill AG. Effect of preoperative exercise on cardiorespiratory function and recovery after surgery: a systematic review. World J Surg. (2013) 37:711–20. doi: 10.1007/s00268-012-1886-4
19. Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. (2014) 121:937–47. doi: 10.1097/ALN.0000000000000393
20. Chia CL, Mantoo SK, and Tan KY. 'Start to finish trans-institutional transdisciplinary care': a novel approach improves colorectal surgical results in frail elderly patients. Colorectal Dis. (2016) 18:O43–50. doi: 10.1111/codi.13166
21. Fulop A, Lakatos L, Susztak N, Szijarto A, and Banky B. The effect of trimodal prehabilitation on the physical and psychological health of patients undergoing colorectal surgery: a randomised clinical trial. Anaesthesia. (2020) 76:82–90. doi: 10.1111/anae.15215
22. Bruns ER, van den Heuvel B, Buskens CJ, van Duijvendijk P, Festen S, Wassenaar EB, et al. The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: a systematic review. Colorectal Dis. (2016) 18:O267–77. doi: 10.1111/codi.13429
23. Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim DJ, et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg. (2010) 97:1187–97. doi: 10.1002/bjs.7102
24. Moriello C, Mayo NE, Feldman L, and Carli F. Validating the six-minute walk test as a measure of recovery after elective colon resection surgery. Arch Phys Med Rehabil. (2008) 89:1083–9. doi: 10.1016/j.apmr.2007.11.031
25. Dindo D, Demartines N, and Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
26. Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. (2010) 10:1. doi: 10.1186/1471-2288-10-1
27. Petty DR, House A, Knapp P, Raynor T, and Zermansky A. Prevalence, duration and indications for prescribing of antidepressants in primary care. Age Ageing. (2006) 35:523–6. doi: 10.1093/ageing/afl023
28. Treacy D, and Hassett L. The short physical performance battery. J Physiother. (2018) 64:61. doi: 10.1016/j.jphys.2017.04.002
29. The EuroQol Group. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy. (1990) 16:199–208. doi: 10.1016/0168-8510(90)90421-9
30. Melville MR, Lari MA, Brown N, Young T, and Gray D. Quality of life assessment using the short form 12 questionnaire is as reliable and sensitive as the short form 36 in distinguishing symptom severity in myocardial infarction survivors. Heart. (2003) 89:1445–46. doi: 10.1136/heart.89.12.1445
31. Collin C, Wade DT, Davies S, and Horne V. The Barthel ADL index: a reliability study. Int Disabil Stud. (1988) 10:61–3. doi: 10.3109/09638288809164103
32. National Health and Medical Research Council. Australian Dietary Guidelines. Canberra, ACT: National Health and Medical Research Council (2013).
33. Baimas-George M, Watson M, Elhage S, Parala-Metz A, Vrochides D, and Davis BR. Prehabilitation in frail surgical patients: a systematic review. World J Surg. (2020) 44:3668–78. doi: 10.1007/s00268-020-05658-0
34. Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. (2013) 27:1072–82. doi: 10.1007/s00464-012-2560-5
35. West MA, Loughney L, Lythgoe D, Barben CP, Sripadam R, Kemp GJ, et al. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesth. (2015) 114:244–51. doi: 10.1093/bja/aeu318
36. Carli F, and Ferreira V. Prehabilitation: a new area of integration between geriatricians, anesthesiologists, and exercise therapists. Aging Clin Exp Res. (2018) 30:241–44. doi: 10.1007/s40520-017-0875-8
37. Waller E, Sutton P, Rahman S, Allen J, Saxton J, Aziz O, et al. Prehabilitation with wearables versus standard of care before major abdominal cancer surgery: a randomised controlled pilot study (trial registration: NCT04047524). Surg Endosc. (2021). doi: 10.1007/s00464-021-08365-6
38. Lee K, Zhou J, Norris MK, Chow C, and Dieli-Conwright CM. Prehabilitative exercise for the enhancement of physical, psychosocial, and biological outcomes among patients diagnosed with cancer. Curr Oncol Rep. (2020) 22:71. doi: 10.1007/s11912-020-00932-9
39. Curtis NJ, West MA, Salib E, Ockrim J, Allison AS, Dalton R, et al. Time from colorectal cancer diagnosis to laparoscopic curative surgery - is there a safe window for prehabilitation? Int J Colorectal Dis. (2018) 33:979–83. doi: 10.1007/s00384-018-3016-8
40. Strous MTA, Janssen-Heijnen MLG, and Vogelaar FJ. Impact of therapeutic delay in colorectal cancer on overall survival and cancer recurrence - is there a safe timeframe for prehabilitation? Eur J Surg Oncol. (2019) 45:2295–301. doi: 10.1016/j.ejso.2019.07.009
41. Arora RC, Brown CHt, Sanjanwala RM, and McKelvie R. “NEW” Prehabilitation: a 3-Way approach to improve postoperative durvival and health-related quality of life in cardiac surgery patients. Can J Cardiol. (2018) 34:839–49. doi: 10.1016/j.cjca.2018.03.020
42. Tew GA, Bedford R, Carr E, Durrand JW, Gray J, Hackett R, et al. Community-based prehabilitation before elective major surgery: the PREP-WELL quality improvement project. BMJ Open Qual. (2020) 9:e000898. doi: 10.1136/bmjoq-2019-000898
43. Freiberger E, Kemmler W, Siegrist M, and Sieber C. Frailty and exercise interventions : evidence and barriers for exercise programs. Z Gerontol Geriatr. (2016) 49:606–11. doi: 10.1007/s00391-016-1134-x
44. Bruns ERJ, Argillander TE, Schuijt HJ, van Duijvendijk P, van der Zaag ES, Wassenaar EB, et al. Fit4SurgeryTV at-home prehabilitation for frail older patients planned for colorectal cancer surgery: a pilot study. Am J Phys Med Rehabil. (2019) 98:399–406. doi: 10.1097/PHM.0000000000001108
45. Bouillon K, Kivimaki M, Hamer M, Sabia S, Fransson EI, Singh-Manoux A, et al. Measures of frailty in population-based studies: an overview. BMC Geriatr. (2013) 13:64. doi: 10.1186/1471-2318-13-64
46. Paknikar R, Friedman J, Cron D, Deeb GM, Chetcuti S, Grossman PM, et al. Psoas muscle size as a frailty measure for open and transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. (2016) 151:745–51. doi: 10.1016/j.jtcvs.2015.11.022
47. Hall DE, Arya S, Schmid KK, Blaser C, Carlson MA, Bailey TL, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. (2017) 152:175–82. doi: 10.1001/jamasurg.2016.4202
48. Hall DE, Arya S, Schmid KK, Carlson MA, Lavedan P, Bailey TL, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg. (2017) 152:233–40. doi: 10.1001/jamasurg.2016.4219
49. Englesbe MJ, Lee JS, He K, Fan L, Schaubel DE, Sheetz KH, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. (2012) 256:255–61. doi: 10.1097/SLA.0b013e31826028b1
Keywords: exercise, cancer, frailty, barriers, regional centre, quality of life
Citation: Furyk C, Senthuran S, Nye D, Ho YH and Leicht AS (2021) Prehabilitation for Frail Patients Undergoing Colorectal Surgery: Lessons Learnt From a Randomised Feasibility Study. Front. Rehabilit. Sci. 2:650835. doi: 10.3389/fresc.2021.650835
Received: 08 January 2021; Accepted: 09 April 2021;
Published: 10 May 2021.
Edited by:
Tanja Stamm, Medical University of Vienna, AustriaReviewed by:
Naoya Hasegawa, Hokkaido University, JapanRikke Helene Moe, Diakonhjemmet Hospital, Norway
Copyright © 2021 Furyk, Senthuran, Nye, Ho and Leicht. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anthony S. Leicht, YW50aG9ueS5sZWljaHRAamN1LmVkdS5hdQ==
†ORCID: Claire Furyk orcid.org/0000-0003-3945-7123
Siva Senthuran orcid.org/0000-0003-3767-4482
Yik H. Ho orcid.org/0000-0001-8870-6363
Anthony S. Leicht orcid.org/0000-0002-0537-5392
 Claire Furyk1,2†
Claire Furyk1,2† Anthony S. Leicht
Anthony S. Leicht