- 1Department of Clinical Sciences, Ajman University, Ajman, United Arab Emirates
- 2Center for Medical and Bio-Allied Health Sciences Research, Ajman University, Ajman, United Arab Emirates
The purpose of this study is to investigate the relationship between the ketogenic diet and periodontitis, as well as the nature of such relationship. Furthermore, emphasis was given to know whether ketogenic diet causes changes in oral health parameters and more specifically on periodontal health. Studies from 2010 to 2023 were reviewed and analyzed. Databases used to search included PubMed, Mednet, Scopus, Cochrane, and Embase. The literature reviewed was limited to randomized clinical trials, observational studies, and case-control studies. Of the eight studies included, three studies found that diets with similarities to the ketone-based diet could have a significant positive impact on periodontal health. One study pointed to the potential positive effect of a diet such as keto, but no definitive conclusion could be made. The current body of evidence concluded that there may be a relationship between keto and periodontitis, although the evidence is not consistent. It can be implied, however, that it is a positive relationship as ketogenic diet has been shown to have an anti-inflammatory effect, reducing inflammatory markers found in many diseases, including periodontitis.
Introduction
With the aging population increasing due to advances in healthcare and living conditions, chronic inflammatory diseases such as periodontitis have also been on the rise. The prevalence of moderate to severe periodontitis among adults aged 65 and over was 47.2% (1). This is significant because periodontitis is a leading cause of tooth loss, which can cause both functional and psychological issues, affecting quality of life. Over the recent years, people have been making more effort to retain their teeth due to aesthetic and social reasons. Contrary to popular belief, periodontitis does not only affect the older population. Although prevalence varies due to bias, it affects 20%–50% of the global population, including developing and developed countries (2). In light of this, there is a need for increased awareness and education about the advent of periodontitis and the risk factors that contribute to it.
Periodontitis is a chronic and debilitating oral disease characterized by the inflammation and eventual destruction of the periodontal tissue, including the gingiva, alveolar bone, and cementum, which support the teeth (2). The disease is mainly caused by accumulated dental plaque and subsequent mineralization into calculus (tartar), which harbors pathogenic microorganisms. Periodontitis begins as gingivitis or gum inflammation, then forms pockets that progress apically to the alveolar bone. Around 800 species of bacteria are involved, producing many virulent toxins and enzymes that incite a chronic inflammatory response in the host, destroying the periodontal tissue and, if left untreated, tooth loss (2).
The etiology of periodontitis is multi-factorial and encompasses a range of risk factors, such as genetics, hormonal fluctuations, stress, and, most importantly, diet (3). Some of these factors are modifiable, including smoking, oral hygiene, and, to some extent, diet. These factors are worth looking into, as understanding their correlation with periodontitis can lead us to a step closer to prevention. Although a good deal of research has been conducted over the past few years regarding the relationship between smoking and periodontitis, little attention has been given to diet as a risk factor.
Role of diet in the progression of periodontitis
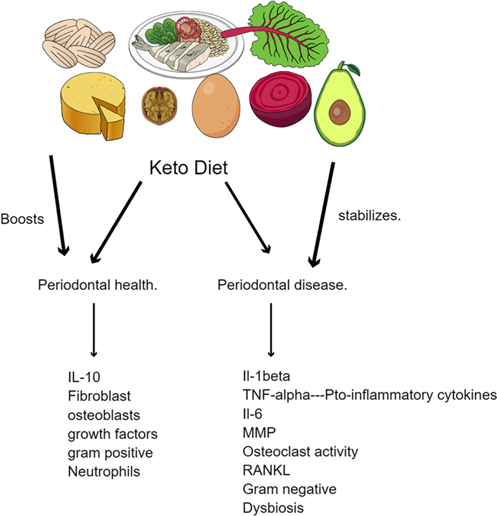
Interestingly, diet plays a crucial role in the advent of periodontal disease since food can act as a substrate for the oral microbiota and provide them with a favorable environment to grow and multiply (3). The oral microbiome is one of the most complex in the body, coming only second to the intestinal microbiota, and disruption of its harmony can promote periodontal disease (3). Our diet comprises micronutrients and macronutrients, which affect periodontal health (3, 4). Micronutrients, vitamins A, C, D, and E, and melatonin have antioxidant effects, slowing the inflammatory onset of periodontitis (4). As for macronutrients, these are required in large amounts and include minerals, carbohydrates, and fats. Studies have revealed a strong correlation between the incidence of periodontitis and dietary habits, particularly those high in refined carbohydrates and sugars. Oral microorganisms efficiently metabolize these dietary components into acid, lowering the oral cavity's pH. This leads to tooth enamel erosion and disruption of the oral microbiome, causing plaque formation and calculus accumulation. Processed foods, which have highly refined carbohydrates and are low in essential nutrients, have also been linked to the development of periodontitis (4, 5). These foods provide a substrate for oral microorganisms and contribute to nutrient deficiencies, hindering the ability of the host to produce a robust immune response to oral pathogens. It facilitates a bi-directional pathway whereby with the absence of essential nutrients, periodontal inflammation is seen with an increase in pro-inflammatory biomarkers such as IL-1β, IL-6 and Matrix metallo-proteinases and a biofilm which is dysbiotic. When essential nutrients are within acceptable limits it brings about the presence of predominantly synthetic cells contributing to periodontal health (Figure 1).
Conversely, a diet rich in omega-3, plant nitrates, and phytochemicals has been shown to protect against periodontitis (4). They are rich in vitamins and minerals, which support the host's immune system and provide a less favorable environment for oral pathogens (3, 4). This happens because dietary antioxidants can mitigate the harmful effects of inflammation through their ability to scavenge and neutralize free radicals, thereby reducing the risk of chronic inflammatory conditions such as periodontitis and maintaining cellular integrity. Free radicals cause oxidative damage to the gums and surrounding tissues. This effect can be reversed by consuming dietary antioxidants or reducing exposure to free-radical-generating sources such as tobacco and certain medications. These medications include chemotherapeutic, non-steroidal anti-inflammatory, anesthetic, and cardiovascular drugs (6). Phytochemical compounds found in plants have health-promoting properties, antioxidant, anti-inflammatory, and anticancer effects and have deterrent influence on pro-inflammatory activities (7). Vitamins A, C, and E have been linked to a reduced risk of periodontitis due to their antioxidant properties (4). In addition, a study following Danish students who incorporated whey protein, calcium, and vitamin D in their diet showed a reduced risk of severe periodontitis (7).
As for Carbohydrates, they are the body's primary source of energy and are crucial for proper function. Commonly used carbohydrate supplements include glucose, fructose, and maltodextrin. A diet that is high in sugar and processed carbohydrates can increase the risk of periodontal diseases. However, complex carbohydrates such as fruits and vegetables can help to maintain overall health and mitigate the risk of periodontal diseases (4). As public health awareness increases, people become more interested in leading a healthy lifestyle. With obesity reaching an all-time high, hypertension and type 2 diabetes are on the rise, which creates an urgency for both the management and the prevention of this epidemic. Various diets have emerged over the past few years, such as vegetarian, caloric restriction, and intermittent fasting. Diets such as vegetarian and Mediterranean diets emphasize the consumption of fruits, vegetables, and olive oil. As previously stated, such food groups have a lower risk of periodontitis (4).
However, today, it is considered one of the leading non-pharmacological therapies for the management of obesity (8, 9). A study on women following a very low-calorie ketogenic diet showed a significant reduction in weight, BMI, and waist circumference (10). The ketogenic diet consists of low carbohydrates (less than 10%), moderate protein, and high fat (8, 11). When the carbohydrates are reduced to less than 50 grams daily, the glycogen stores are depleted, and the body produces ketone bodies as an alternative energy source (8). Current evidence proves the ketogenic diet's efficacy in initiating and maintaining weight loss and its safety regarding the liver, thyroid, and kidney function in the long term (10).
Interestingly, the ketogenic diet has also positively affected cognitive function and cardiovascular risk factors (12). Additionally, the keto diet has been very popular with diabetic patients. Studies have linked keto with better glycemic control, probably due to the diet's reduced carbohydrate content (13). Diabetic patients who follow a ketogenic diet have been observed to experience weight loss, better-fasting glucose and insulin levels, lower cholesterol, and, in some cases, the ability to reduce or eliminate their diabetic medication (13). The ketogenic diet is still used as a therapeutic dietary approach to epilepsy today, as well as many other diseases, including polycystic ovary syndrome and even cancer.
On the other hand, the keto diet can have some potential harms and disadvantages. Potential harm is constipation due to a lack of fiber and fruits, vegetables, and whole grains (8). Diarrhea may also occur in some individuals due to the fat content present in the diet. Over time, a high protein and fat intake may also increase the risk of kidney stones (8). Other long-term issues associated with following the ketogenic diet include hepatic steatosis and hyperproteinemia. It is also worth mentioning that long-term adherence to the ketogenic diet is difficult, as it is pretty restrictive (8).
Relationship between keto and periodontitis
Despite the extensive research, little to no data exists regarding the keto diet's effect on clinical oral parameters. The current data needs to be more consistent. A study concerning patients following an anti-inflammatory, low-carbohydrate, vitamin-rich diet showed a clinically significant decrease in gum inflammation and reduced bleeding on probing (5). Another study, a randomized clinical trial in which subjects were put on a diet low in carbohydrates and rich in fibers and vitamins C and D, showed similar results (7). Oral parameters such as plaque index, bleeding on probing, and pocket depth were cut in half (7).
Only some studies have reported that following a keto diet might result in disparity among commonly seen clinical oral parameters such as salivation, healing, and keratinization (12, 14). Keto diet may contain saturated fat, which leads to an increase in LDL cholesterol in normal-weight healthy women (12). Much evidence suggests a connection between periodontal diseases and high cholesterol levels in the body (14). This relationship between periodontitis and lipid levels is likely a result of the systemic effects of inflammation. According to another study, elevated levels of LDL cholesterol in the serum were linked to clinical loss of attachment and gingival plaque (14).
It remains unclear whether diets such as keto affect oral parameters. Therefore, we aim to determine whether the keto diet is beneficial or detrimental to oral health. In addition, we would like to investigate the relationship between the keto diet and the progression of periodontitis. We would also like to determine the strength of the correlation, if it exists, between the keto diet as a modifiable risk factor and periodontitis.
Materials and methods
Studies from 2010 to 2023 were reviewed and analyzed. Databases used to search included PubMed, Mednet, Scopus, Cochrane, and Embase. The keywords used for the search included ((ketogenic diet) OR (keto diet)) AND (oral health). When typing keto diet OR Ketogenic diet AND periodontitis, the keywords showed only one result. We wanted to evaluate articles that mentioned the keto diet's significance in periodontitis conditions in their clinical trials. Hence, we used a broader terminology. The literature reviewed was limited to randomized clinical trials, observational studies, and case-control studies. Articles merely listing the advantages or disadvantages of the diet were excluded. Articles in English language only were selected. Narrative and systematic reviews were excluded. The review contained ten articles that were thoroughly read and analyzed (Figure 2).
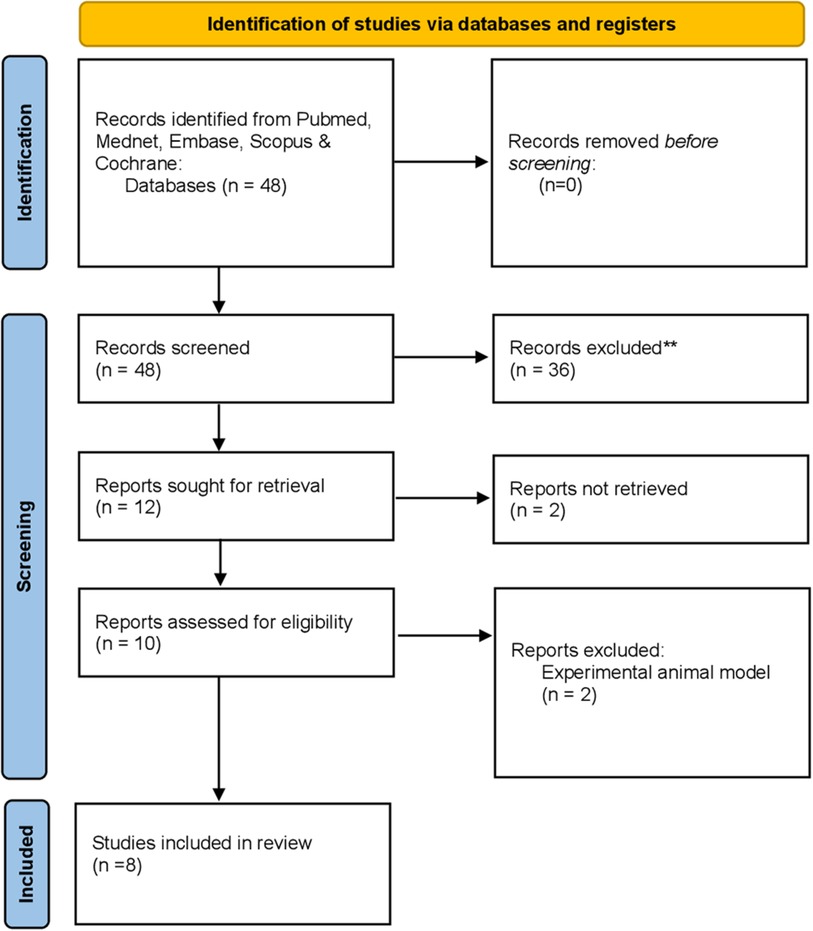
Figure 2. Flowchart highlighting the selection process of articles retrieved from digital databases.
Results
The present study investigates the relationship between the ketogenic diet and periodontitis. The relationship between diet and periodontal health has recently become a point of interest, with many interventional studies confirming this link. Of the eight studies included, only two directly investigated the potential relationship between keto and periodontitis (15–17). Another pilot trial found no changes in periodontal health in subjects after following a 6-week ketogenic diet (18). Three studies found that diets similar to the ketone-based diet could significantly positively impact periodontal health (16, 19, 20). These include two randomized clinical trials in which subjects following an oral-health-optimized diet containing reduced carbohydrates showed decreased potential periodontal bacterial species in the supragingival oral plaque and decreased periodontal inflammation (19, 20). One study pointed to the potential positive effect of a diet such as keto, but no definitive conclusion could be made (18). Another RCT is associated with using sugar substitutes such as erythritol, commonly used in the keto diet as sugar is restricted, with reduced plaque growth (21). One study showed that the ketogenic diet has an anti-inflammatory effect, which could be necessary as periodontitis is a chronic inflammatory disease (16, 20) (Table 1).
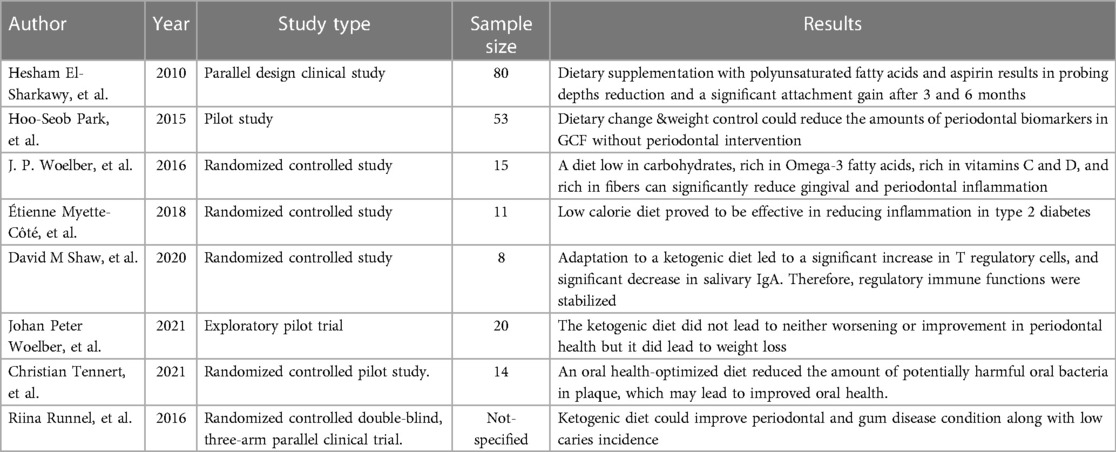
Table 1. List of clinical studies enumerating the role of ketogenic diet in periodontal disease progression.
Discussion
Like most chronic diseases, periodontitis can be influenced by many external factors. Research suggests that environmental factors, genetics, epigenetics, and lifestyle choices could contribute to the development of periodontitis by influencing the composition of the biofilm and the host's inflammatory immune response (22).
Periodontitis has been described as an inflammatory disease mainly resulting from the invasion of both the innate and the adaptive immune systems. The development of bacterial biofilm, sometimes called dental plaque, on the teeth's surface initiates the disease process. Pathogenic and commensal microorganisms coexist in the complex and dynamic community known as the bacterial biofilm. The expanding bacterial biofilm produces many virulence factors, including lipopolysaccharides, proteases, and toxins, and can start and maintain the inflammatory response in the periodontal tissues (22).
Neutrophils, macrophages, and T lymphocytes are just a few immune cells drawn to the infection site as part of the host response to the bacterial attack. The cytokines and chemokines these immune cells release increase the inflammatory response, activating osteoclasts and bone resorption (9). The periodontal fibroblast is one of the primary cells in the pathophysiology of periodontitis. These cells are the primary cell type in the periodontal ligament and are critical in maintaining periodontal tissue homeostasis. However, in periodontitis, pro-inflammatory cytokines, such as interleukin-1beta and tumor necrosis factor-alpha, can promote the destruction of the periodontal tissues (23).
In addition, osteoclasts play a significant role in periodontitis. The inflammatory cytokines released by immune cells and periodontal fibroblasts in periodontitis cause osteoclast activation. Osteoclast activation ultimately leads to the loss of alveolar bone, a defining feature of periodontitis (23). Furthermore, microbial colonization in periodontitis is a crucial part of the disease process in addition to the host response. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia are a few examples of pathogenic bacteria linked to periodontitis and are thought to contribute to the development and spread of the condition (24). These microorganisms create virulence factors that can undermine host defenses and aid in the survival of the pathogenic biofilm.
Therefore, microbial colonization and host response interact in a complicated way during the pathogenesis of periodontitis. Once bacterial biofilm builds up, immune cells become activated, pro-inflammatory cytokines are produced, and osteoclasts become active, which causes periodontal tissues to be destroyed. To create successful therapeutic approaches to treat periodontitis, it is essential to understand the pathophysiology of the disease and identify the major immune cell types and bacteria involved in the disease process (24).
Although the relationship between the ketogenic diet and periodontitis remains mainly unexplored, understanding the mechanisms of this diet and its impact on other chronic diseases could bring us closer to identifying a correlation. A ketogenic diet exerts various effects on health, including metabolism, brain function, and cancer prevention through ketosis. The pathogenesis of the ketogenic diet involves the production of ketone bodies, which are produced in the absence of carbohydrates. These ketone bodies are metabolized in the liver when the body is in a state of low carbohydrate availability or increased fatty acid breakdown. The body produces small amounts of ketone bodies that can yield 22 ATP each in health (25). This highly efficient process helps the body meet its energy demands when glucose availability is limited. Ketone body production is regulated mainly by insulin, which inhibits ketogenesis when glucose levels are high (26). For this reason, the ketogenic diet has been indicated for diabetic patients—according to a randomized clinical trial, a low-carbohydrate, high-fat diet led to better glycemic control and reduced inflammation (20).
One potential therapeutic effect of the ketogenic diet is a reduction in inflammation. Chronic inflammation is a significant contributor to periodontal disease, and studies have shown that the ketogenic diet does reduce inflammation in the body. This suggests that the diet may have a protective effect on the periodontium. The ketogenic diet has been observed to reduce the expression of various pro-inflammatory markers. These include cyclooxygenase 2, nuclear factor-k, and macrophage inflammatory protein 2. C-reactive protein has been linked to various diseases. Few studies suggest a ketogenic diet may reduce CRP levels, indicating a potential anti-inflammatory effect. A study where a hypocaloric carbohydrate diet was consumed for 12 weeks found that the C-reactive protein levels had significantly lowered (20, 26). A study demonstrated that reducing dietary carbohydrate intake improved pro-inflammatory markers such as TNF-α, IL-6, and IL-8 (27). Another cross-sectional study evaluated the effect of a low carbohydrate diet on two inflammatory markers, IL-1β and Galectin-3. This study further proved that LCD reduced the level of these two inflammatory markers (28).
On the other hand, the ketogenic diet may have adverse effects on periodontal health. It may lead to a decreased production of saliva. Saliva is crucial due to its cleansing effect and neutralizing acids in the mouth. Low salivary production can lead to dry mouth, erosive activity, gum disease, and other oral health problems (19). Additionally, it was found that a high-fat diet can induce periodontitis in mice by increasing the expression of LPS receptors, leading to an inflammatory response (29). It is important to note that this was just a high-fat diet, which shares similarities with the ketogenic diet but differs in various aspects.
Our review identified several studies showing the ketogenic diet's anti-inflammatory effects. This is consistent with a review article published in 2022 by Srivastava et al. This article found that the ketogenic diet has immune-modulatory and anti-inflammatory effects and is therefore beneficial in various chronic inflammatory diseases ranging from polycystic ovary syndrome to cardiovascular diseases and diabetes, and in our case, periodontitis (30). Similarly, our review contained studies that reported reductions in inflammatory markers in individuals following a ketogenic diet, which may contribute to improved periodontal health. Moreover, another review conducted by Pinto et al. (2018) found that the ketogenic diet has antioxidant and anti-inflammatory properties (6). While these studies suggest a possible anti-inflammatory effect of the keto diet on periodontitis, it is essential to note that the current evidence is minimal, and more research is required to fully evaluate the relationship between the ketogenic diet and periodontitis. In addition, many other factors, such as lifestyle and genetics, influence an individual's response to dietary interventions and conditions such as periodontitis. Therefore, while promising results, caution must be taken during interpretation.
Overall, we explored the potential impacts of the ketogenic diet on the periodontium. While several findings suggested a positive anti-inflammatory effect, as discussed above, some articles suggested a negative impact, and some suggested no relationship. Alternatively, Martinon et al. emphasize the importance of nutrient-dense diets containing calcium, polyphenols, and vitamins, which may not necessarily be present in a ketogenic diet (31). Furthermore, they discuss the potential benefits of dietary interventions such as probiotics, prebiotics, and plant-based diets in improving periodontal health (31, 32). Although both reviews emphasize different aspects of diet, both suggest that diet plays a role in the progression of periodontitis. Additionally, both studies recognize the importance of nutrition as a modifiable factor in the advent of periodontal disease.
There are very few interventional studies that directly investigate the nature of the relationship between the ketogenic diet and periodontitis. The current evidence is promising if such a relationship exists due to the ketogenic diet's anti-inflammatory and anti-oxidative effects. However, some studies have reported a potential negative impact of the ketogenic diet due to its high fat content. It cannot be denied that nutrition does play a vital role as a modifiable factor in periodontitis, but the results of specific dietary interventions such as keto remain unknown. More interventional studies with a larger population are needed to come up with a definitive conclusion.
Few limitations exist and must be considered while examining the studies' findings. With the majority of studies describing the effect of keto on other health conditions, there needs to be more scientific research regarding keto and periodontitis. It is also important to note that it is challenging to isolate dietary interventions, making it difficult to establish a correlation. Moreover, more interventional studies need to be conducted. Additionally, there may be individual variations in response to the ketogenic diet, impacting its efficacy in enhancing periodontal health.
Conclusion
Periodontitis has many factors that affect its progression, including lifestyle and nutrition. Growing evidence suggests that nutrition plays a crucial role in periodontitis. However, such a relationship is complex, and the evidence must be sufficient to use dietary interventions as the sole therapeutic approach to periodontitis. The current body of evidence concluded that there may be a relationship between keto and periodontitis, although the evidence is not consistent. It can be implied, however, that it is a positive relationship as the ketogenic diet has been shown to have an anti-inflammatory effect, reducing inflammatory markers found in many diseases, including periodontitis.
With the prevalence of periodontitis and the tremendous therapeutic potential of the ketogenic diet, this relationship cannot be ignored. More evidence is needed to confirm this correlation. Notably, more interventional studies with a larger population are needed to explore the ketogenic diet's effect on the periodontium properly.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
HT: Conceptualization, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. AS: Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. CR: Conceptualization, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. SV: Conceptualization, Formal Analysis, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. (2012) 91(10):914–20. doi: 10.1177/0022034512457373
2. Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci. (2017) 11(2):72–80. PMID: 28539867; PMCID: PMC5426403
3. Santonocito S, Giudice A, Polizzi A, Troiano G, Merlo EM, Sclafani R, et al. A cross-talk between diet and the oral microbiome: balance of nutrition on inflammation and immune system’s response during periodontitis. Nutrients. (2022) 14(12):2426. doi: 10.3390/nu14122426
4. Najeeb S, Zafar M, Khurshid Z, Zohaib S, Almas K. The role of nutrition in periodontal health: an update. Nutrients. (2016) 8(9):530. doi: 10.3390/nu8090530
5. Rajaram SS, Nisha S, Ali NM, Shashikumar P, Karmakar S, Pandey V. Influence of a low-carbohydrate and rich in omega-3 fatty acids, ascorbic acid, antioxidants, and fiber diet on clinical outcomes in patients with chronic gingivitis: a randomized controlled trial. J Int Soc Prev Community Dent. (2021) 11(1):58–67. doi: 10.4103/jispcd.JISPCD_365_20
6. Pinto A, Bonucci A, Maggi E, Corsi M, Businaro R. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer’s disease. Antioxidants (Basel). (2018) 7(5):63. doi: 10.3390/antiox7050063
7. Adegboye AR, Boucher BJ, Kongstad J, Fiehn NE, Christensen LB, Heitmann BL. Calcium, vitamin D, casein and whey protein intakes and periodontitis among danish adults. Public Health Nutr. (2016) 19(3):503–10. doi: 10.1017/S1368980015001202
8. Masood W, Annamaraju P, Uppaluri KR. Ketogenic Diet. Treasure Island (FL): StatPearls Publishing (2022).
9. Jia L, Han N, Du J, Guo L, Luo Z, Liu Y. Pathogenesis of important virulence factors of Porphyromonas gingivalis via toll-like receptors. Front Cell Infect Microbiol. (2019) 18(9):262. doi: 10.3389/fcimb.2019.00262
10. Tragni E, Vigna L, Ruscica M, Macchi C, Casula M, Santelia A, et al. Reduction of cardio-metabolic risk and body weight through a multiphasic very-low calorie ketogenic diet program in women with overweight/obesity: a study in a real-world setting. Nutrients. (2021) 13(6):1804. doi: 10.3390/nu13061804
11. Urbain P, Strom L, Morawski L, Wehrle A, Deibert P, Bertz H. Impact of a 6-week non-energy-restricted ketogenic diet on physical fitness, body composition and biochemical parameters in healthy adults. Nutr Metab (Lond). (2017) 14:17. doi: 10.1186/s12986-017-0175-5
12. Burén J, Ericsson M, Damasceno NRT, Sjödin A. A ketogenic low-carbohydrate high-fat diet increases LDL cholesterol in healthy, young, normal-weight women: a randomized controlled feeding trial. Nutrients. (2021) 13(3):814. doi: 10.3390/nu13030814
13. Alarim RA, Alasmre FA, Alotaibi HA, Alshehri MA, Hussain SA. Effects of the ketogenic diet on glycemic control in diabetic patients: meta-analysis of clinical trials. Cureus. (2020) 12(10):e10796. doi: 10.7759/cureus.10796
14. Meisel P, Kohlmann T, Wallaschofski H, Kroemer HK, Kocher T. Cholesterol, C-reactive protein, and periodontitis: HMG-CoA-reductase inhibitors (statins) as effect modifiers. Int Sch Res Notices Dent. (2011) 2011:125168. doi: 10.5402/2011/125168
15. El-Sharkawy H, Aboelsaad N, Eliwa M, Darweesh M, Alshahat M, Kantarci A, et al. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 fatty acids and low-dose aspirin. J Periodontol. (2010) 81(11):1635–43. doi: 10.1902/jop.2010.090628
16. Shaw DM, Merien F, Braakhuis A, Keaney L, Dulson DK. Adaptation to a ketogenic diet modulates adaptive and mucosal immune markers in trained male endurance athletes. Scand J Med Sci Sports. (2021) 31(1):140–52. doi: 10.1111/sms.13833
17. Woelber JP, Tennert C, Ernst SF, Vach K, Ratka-Krüger P, Bertz H, et al. Effects of a non-energy-restricted ketogenic diet on clinical oral parameters. An exploratory pilot trial. Nutrients. (2021) 13(12):4229. doi: 10.3390/nu13124229
18. Park HS, Nam HS, Seo HS, Hwang SJ. Change of periodontal inflammatory indicators through a 4-week weight control intervention including caloric restriction and exercise training in young Koreans: a pilot study. BMC Oral Health. (2015) 15(1):109. doi: 10.1186/s12903-015-0094-7
19. Woelber JP, Bremer K, Vach K, König D, Hellwig E, Ratka-Krüger P, et al. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—a randomized controlled pilot study. BMC Oral Health. (2016) 17(1):28. doi: 10.1186/s12903-016-0257-1
20. Myette-Côté É, Durrer C, Neudorf H, Bammert TD, Botezelli JD, Johnson JD, et al. The effect of a short-term low-carbohydrate, high-fat diet with or without postmeal walks on glycemic control and inflammation in type 2 diabetes: a randomized trial. Am J Physiol Regul Integr Comp Physiol. (2018) 315(6):R1210–9. doi: 10.1152/ajpregu.00240.2018
21. Runnel R, Mäkinen KK, Honkala S, Olak J, Mäkinen PL, Nõmmela R, et al. Effect of three-year consumption of erythritol, xylitol and sorbitol candies on various plaque and salivary caries-related variables. J Dent. (2013) 41(12):1236–44. doi: 10.1016/j.jdent.2013.09.007
22. Meyle J, Chapple I. Molecular aspects of the pathogenesis of periodontitis. Periodontol 2000. (2015) 69(1):7–17. doi: 10.1111/prd.12104
23. Ruth MR, Port AM, Shah M, Bourland AC, Istfan NW, Nelson KP, et al. Consuming a hypocaloric high fat low carbohydrate diet for 12 weeks lowers C-reactive protein, and raises serum adiponectin and high-density lipoprotein-cholesterol in obese subjects. Metab Clin Exp. (2013) 62(12):1779–87. doi: 10.1016/j.metabol.2013.07.006
24. Hienz SA, Paliwal S, Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res. (2015) 2015:615486. doi: 10.1155/2015/615486
25. Zhu H, Bi D, Zhang Y, Kong C, Du J, Wu X, et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct Target Ther. (2022) 7(1):11. doi: 10.1038/s41392-021-00831-w
26. Dhillon KK, Gupta S. Biochemistry, ketogenesis. Statpearls [internet]. Treasure Island (FL): StatPearls Publishing (2024). PMID: 29630231
27. Waldman HS, Heatherly AJ, Killen LG, Hollingsworth A, Koh Y, O'Neal EK. A 3-week, low-carbohydrate, high-fat diet improves multiple Serum inflammatory markers in endurance-trained males. J Strength Cond Res. (2022) 36(9):2502–8. doi: 10.1519/JSC.0000000000003761
28. Tavakoli A, Mirzababaei A, Sajadi F, Mirzaei K. Circulating inflammatory markers may mediate the relationship between low carbohydrate diet and circadian rhythm in overweight and obese women. BMC Womens Health. (2021) 21(1):87. doi: 10.1186/s12905-021-01240-5
29. Blasco-Baque V, Serino M, Vergnes JN, Riant E, Loubieres P, Arnal JF, et al. High-fat diet induces periodontitis in mice through lipopolysaccharides (LPS) receptor signaling: protective action of estrogens. PLoS One. (2012) 7(11):e48220. doi: 10.1371/journal.pone.0048220
30. Srivastava S, Pawar VA, Tyagi A, Sharma KP, Kumar V, Shukla SK. Immune modulatory effects of ketogenic diet in different disease conditions. Immuno. (2022) 3(1):1–15. doi: 10.3390/immuno3010001
31. Martinon P, Fraticelli L, Giboreau A, Dussart C, Bourgeois D, Carrouel F. Nutrition as a key modifiable factor for periodontitis and main chronic diseases. J Clin Med. (2021) 10(2):197. doi: 10.3390/jcm10020197
32. Tennert C, Reinmuth AC, Bremer K, Al-Ahmad A, Karygianni L, Hellwig E, et al. An oral health optimized diet reduces the load of potential cariogenic and periodontal bacterial species in the supragingival oral plaque: a randomized controlled pilot study. Microbiology Open. (2020) 9(8):e1056. doi: 10.1002/mbo3.1056
Keywords: ketogenic diet, ketone diet, oral health, periodontal health, periodontitis
Citation: Taher HA, Salah A, Rammal C and Varma SR (2024) Role of ketogenic diet and its effect on the periodontium. A scoping review. Front. Oral. Health 5:1364578. doi: 10.3389/froh.2024.1364578
Received: 2 January 2024; Accepted: 23 January 2024;
Published: 1 February 2024.
Edited by:
Santosh R. Patil, Saveetha Medical College & Hospital, IndiaReviewed by:
Manjusha Nambiar, Sri Rajiv Gandhi College of Dental Sciences and Hospital, IndiaBiju Thomas, AB Shetty Memorial Institute of Dental Sciences, India
© 2024 Taher, Salah, Rammal and Varma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sudhir Rama Varma cy52YXJtYUBham1hbi5hYy5hZQ==
 Hala Al Taher
Hala Al Taher Aya Salah1
Aya Salah1 Sudhir Rama Varma
Sudhir Rama Varma