Abstract
Background:
Cancer stem cells (CSC) are endowed with multipotency, self-renewal and unique tumorigenic potential. They have been identified as cells with high activity of aldehyde dehydrogenase (ALDH) and high expression of CD44 in head and neck squamous cell carcinoma (HNSCC). The objective of this work is to understand whether salivary gland adenoid cystic carcinoma contain CSCs and whether they exhibit a unique tumorigenic activity in this cancer.
Methods:
We used flow cytometry, salisphere and western blot assays with 3 human ACC cell lines (UM-HACC-2A, UM-HACC-14, UM-HACC-6) to characterize the impact of ALDH activity and CD44 expression. In vitro results were verified in vivo by orthotopic injection of cells retrieved from a patient-derived xenograft (PDX) model of ACC (UM-PDX-HACC-14) in the submandibular gland of SCID mice. Primary tumor and metastatic spread were evaluated by 2 pathologists blinded for experimental conditions.
Results:
The fraction of ALDHhighCD44high cells in UM-HACC-2A, UM-HACC-14 and UM-HACC-6 ranged from 3% to 8%. ALDHhighCD44high cells formed more salispheres and expressed higher levels of stem cell markers (e.g., Notch2, Bmi-1) compared to control ALDHlowCD44low cells. ALDHhighCD44high cells sorted from the ACC PDX tumors were more tumorigenic upon orthotopic transplantation into submandibular salivary glands and generated more lung metastases than control ACC cells. Strong ALDH1A1 staining was observed in the majority of salivary gland tumors and lung metastases generated by transplantation of ALDHhighCD44high cells.
Conclusions:
We conclude that salivary gland adenoid cystic carcinoma contain a small population of uniquely tumorigenic cells characterized by high ALDH activity and expression.
Introduction
There is a major need for a safe and effective therapy for patients with malignant salivary gland cancer. Adenoid cystic carcinoma (ACC) is a rare, slow-growing, cancer that is characterized by poor long-term outcome for patients (1, 2). ACC tumors are highly variable, consist of multiple cell types, arise from different primary sites, and tend to occur more frequently in women than men (1–5). In addition, patients with ACC tumors have diverse demographics (age, ethnicity, location of primary tumor), and have complex cases (grade, stage, +/−peri-neural, hematogenic invasion) making it difficult to identify effective treatments (1–7). The standard of care for ACC patients is surgery and radiation, with no effective and safe systemic therapies (5, 8–11). Identifying a “universal target” such as cancer stem cells, constitute an attractive strategy to overcome the intrinsic challenges associated with the slow, albeit relentless, pattern of progression exhibited by ACCs. Here, we investigated whether CSCs play a functional role in the pathobiology of ACC.
Cancer stem cells (CSC) are a rare and unique cell population found in many different solid tumors, including breast, glioblastoma, head and neck, prostate, lung, colon, pancreatic and liver cancer (12). CSC proliferate relatively slowly, are endowed with self-renewal, are multipotent, highly tumorigenic, express high levels of Bmi-1 and contribute to metastases, recurrence, and to chemoresistance (12, 13). Notably, Bmi-1 is a master regulator of self-renewal and stemness in both, physiological and malignant cells (13).
Cancer stem cells were first identified in solid tumors of breast cancer (14). Al-Hajj and colleagues identified CSCs as CD44 + CD24low in breast cancer (14). This tumorigenic cell population increased at very low numbers and generated phenotypically diverse tumors in vivo. ALDH1high cells also identified CSC in human breast cancer and head and neck tumors (15, 16). A few years later, Prince and colleagues identified CD44 + cells as CSCs in HNSCC tumors (17). CD44 is a transmembrane glycoprotein involved in cell survival, motility, and differentiation as a CSC marker in head and neck squamous cell carcinomas (18). The CD44 + cell population combined with ALDHhigh expression resulted in phenotypically diverse head and neck squamous cell carcinoma tumors upon serial dilution in vivo (19–22).
Adams and colleagues hypothesized that salivary gland CSCs might be responsible for treatment failure in these patients (23). Recent studies in head and neck squamous cell cancer and salivary gland mucoepidermoid carcinoma have shown ALDHhighCD44high cells exhibit characteristics consistent with CSCs and are resistant to platinum-based chemotherapy (24–26). Targeting HNSCC or mucoepidermoid carcinoma CSCs with IL-6R inhibitor (tocilizumab), small molecule inhibitors of MDM2-p53 interaction (MI-773, APG115), mTOR inhibitors (rapamycin, temsirolimus) reduced the CSC fraction, sensitized tumors to chemotherapy and inhibited tumor progression (26–30). Similarly, single agents such as small-molecule inhibitors of Bcl-2 or MDM2 inhibited ACC tumor growth in vivo (31, 32). Notably, a combination therapy using MI-773 and cisplatin significantly reduced CSC fraction, diminished tumor growth rates and prevented ACC recurrence for more than 300 days in mice (33). And finally, Sahara and colleagues reported significant reductions in CSC fraction and tumor recurrence using combination therapy of cisplatin and a small molecule inhibitor of a Bmi-1 (PTC-596, Unesbulin) in vivo (34).
Collectively, these studies suggest that targeting ACC CSCs might be beneficial for patients. A direct connection of ALDHhighCD44high expression to ACC CSC phenotype has been less clear. While some reports showed that human ACC ALDHhigh cells were more tumorigenic (35, 36), others reported no correlation between ALDH1 tumor cell expression and perineural invasion, patient survival in ACC or other clinical parameters (37). In addition, Notch and Nanog have also been implicated in salivary gland cancer stemness (38–40). Here, we used 3 unique human ACC cell lines in vitro, and a matching UM-PDX-HACC-14 model in vivo (41, 42) to test the hypothesis that ALDHhighCD44high cells function as uniquely tumorigenic CSCs in salivary gland adenoid cystic carcinoma.
Materials and methods
Cell culture, western analysis, STR profiling
Human salivary gland ACC cells (UM-HACC-2A, UM-HACC-14, UM-HACC-6) were cultured in Salivary Gland Medium (SGM) consisting of Dulbecco's Modified Eagle's Medium (DMEM) (Invitrogen, Wlatham, MA), supplemented with 1% L-glutamax (Invitrogen), 1% AAA antibiotic (Sigma-Aldrich), 1% Amphotericin B (Sigma-Aldrich), 10% Fetal Bovine Serum (FBS; R and D Systems), 20 ng/ml rhEGF (R&D Systems), 0.4 mg/ml human hydrocortisone (StemCell Technologies, Vancouver, Canada), 5 µg/ml human insulin (Sigma-Aldrich, St. Louis, MO) (25, 27, 34, 41, 42).
Proteins were extracted using NP-40 lysis buffer (24–34). Lysates were collected from cells grown in attached or ultra-low attachment (ULA) conditions (salispheres) or from ACC cells sorted for ALDH activity (Aldefluor; StemCell Technologies) and/or CD44-APC (BD Pharmingen) expression. Proteins were resolved on SDS-PAGE gels and membranes were probed using antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA), as follows: ALDH1/2 (SC-166362), ALDH1A1 (SC-374149), β-actin (SC-4778); and from Cell Signaling (Cell Signaling Technology, Danvers, MA), as follows:, STAT3 (30385), p-STAT3 (9138), Bmi-1 (6964), Nanog (4903), NOTCH1 (3608), NOTCH2 (4530). The identity and purity of ACC cells were routinely confirmed via STR profiling, (Genetica, Burlington, NC) (32, 41, 42).
Flow cytometry, orthotopic ACC model
For CSC analysis (2–4 × 105 UM-HACC-2A, UM-HACC-14, UM-HACC-6) or sorting (20–30 × 106) cells were preincubated with 10 µl of the ALDH inhibitor diethylaminobenzaldehyde (DEAB) for 10 min at 37°C, directly stained with Aldefluor (StemCell Technologies) for 30 min, CD44-APC (BD Pharmingen, Franklin Lanes, NJ, USA) for 10 min at 4°C, and DAPI (cell viability marker, Invitrogen) as previously described (25–31, 33, 34). ALDHlowCD44low, ALDHlowCD44high, ALDHhighCD44low and ALDHhighCD44high groups were analyzed or collected for experiments. For in vivo serial dilution experiments, UM-PDX-HACC-14 tumors were dissociated in collagenase-hylauronidase (StemCell Technologies), as previously described (25–30, 33, 34, 42). The UM-PDX-HACC-14 model was generated in our laboratory from a metastatic adenoid cystic carcinoma, as described (42). Positive staining for HLA isolated human cells and were incubated for 10″ at 4°C. The rationale for our first experiment was to inject serially diluted ALDHhighCD44high cells, as described (22, 25). Serial dilution experiments were performed by decreasing the number of cells by a factor of 10, starting from 1,500 ALDHhighCD44high cells. As controls, we injected 10-fold more ALDHlowCD44low cells than the highest number of ALDHhighCD44high cells (i.e., 15,000). Cells were injected directly into the mouse submandibular glands (n = 2/group) with a follow-up of 13 months (41, 42). In a second experiment designed to verify our initial in vivo results, we injected 40,000 or 4,000 ALDHhighCD44high or pooled cells (i.e., ALDHlowCD44low + ALDHlowCD44high + ALDHhighCD44low) and followed mice (n = 3/group) for up for 16 months. Autopsies were performed in all mice to examine for presence of metastases. All in vivo work was performed under an approved protocol according to the University of Michigan Animal Care and Use Committee (IACUC) guidelines. Animals were anesthetized during surgical procedures and received post-operatory analgesics to minimize discomfort or pain.
Immunohistochemistry
All mouse salivary and lung tissues were stained with Hematoxylin and Eosin (H and E) and evaluated by two experienced oral pathologists (FN, RC). ALDH1A1 (1:1,000, Santa Cruz, SC-374149) and CD44 expression (1:50, Cell Signaling, # 3570) staining of tumors was performed as previously described (42). Human tumors were confirmed by positive anti-human HLA class 1 ABC (1:25 EMR8-5; Abcam, Cambridge, UK) or Keratin-7 (1:50; Cell Signaling) staining and an absence of anti-mouse CD-45 (1:100 clone 30-F11; Biolegend, San Diego, CA, USA), a marker of mouse inflammatory cells (42).
Salisphere assays
Primary spheres were generated from unsorted or sorted UM-HACC cells (ALDHlowCD44low, ALDHlowCD44high, ALDHhighCD44low or ALDHhighCD44high) and grown in ultra-low attachment (ULA) conditions (25–30, 34). In vitro serial dilution studies used UM-HACC-2A (4,000, 400, 40 and 4) or UM-HACC-14 (10,000, 1,000, 100, 10) cells/well in 6-well ULA plates in triplicate and spheres were counted every 1–3 days (25–30, 34). To generate secondary spheres, sorted ALDHhighCD44high or ALDHlowCD44low (5.0 × 105–1 × 106) cells were cultured in T-75 ULA flasks for 4–7 days. Primary spheres were collected in a 40 µM cell strainer, washed in PBS, dissociated into single cells (FACSMAX, Genlantis, San Diego, CA) counted and re-plated into 6-well ULA plates for 7 days (25–30, 34). Salispheres were cultured in DMEM/F-12 (Invitrogen) supplemented with 20 ng/ml EGF (Sigma-Aldrich), 20 ng/ml basic fibroblast growth factor (bFGF; Millipore, Burlington, MA), 1% penicillin/streptomycin (Invitrogen), 1% glutamax (Invitrogen), 1% N-2 supplement (Invitrogen), 1 μM dexamethasone (Sigma-Aldrich), and 10 μg/ml insulin (Sigma-Aldrich) (25–30, 34).
Statistical analysis
All statistical analyses were performed using the Prism software (GraphPad; San Diego, CA). Comparisons between 2 experimental conditions were analyzed by paired t-tests, while one-way ANOVA followed by post-hoc tests was utilized for multiple group analyses. Statistical significance was determined at p < 0.05.
Results
Characterization of ALDH and CD44 in human salivary gland ACC cell lines
To begin to understand the pattern of expression of ALDH and CD44 in ACC, three human salivary gland ACC cell lines were evaluated using flow cytometry. The average percentage of CSC (ALDHhighCD44high) was approximately 5%, 7% and 3% in UM-HACC-2A, UM-HACC-14 and UM-HACC-6 cells respectively (Figure 1A). The gating strategy used to isolate CSC is shown in (Figure 1B). We observed that the proportion of ALDHlowCD44high was the highest (80%–90%), ALDHhighCD44low was minimal, and the percentage of ALDHlowCD44low ranged from 5% to 12% (Figure 1A). There were significantly higher levels of CSC (ALDHhighCD44high) in UM-HACC-2A and UM-HACC-14 cells when compared to UM-HACC-6 (p < 0.05). There were no significant differences in the non-CSC fraction (ALDHhighCD44low, ALDHlowCD44high, ALDHlowCD44low) for each UM-HACC line (p > 0.05). Each cell line was analyzed in triplicate experiments with reproducible results.
Figure 1

Baseline characterization of cancer stem cell fraction, sphere number and protein expression in UM-HACC cell lines. (A) Bar graph showing the cancer stem cell (CSC) and non-CSC percentages in flow cytometry sorted cells. (B) Representative flow cytometry gating schematics (C). Line and bar graphs depicting the average sphere number in unsorted UM-HACC cell lines. (D) Photomicrographs of spheres. (E) Western blots of UM-HACC cells grown in attached (AT) or ultra-low attachment (ULA) conditions. Statistical significance was defined at p < 0.05, as determined by one-way ANOVA followed by post-hoc analyses. NS, non-significant.
To determine if UM-HACC cell lines generate salispheres, 5,000 cells were cultured in ultra-low attachment plates. UM-HACC-14 cells formed the highest average number of salispheres when compared to UM-HACC-2A and UM-HACC-6 cells after 6 days (Figure 1C). Photomicrographs showed well-defined salispheres for all ACC cell lines (Figure 1D). Western blotting revealed an overall trend for enhanced stemness markers in ACC cells suspended in ultra-low attachment plates, as expected. We observed increased expression of Notch1, Notch2, and Nanog in salispheres compared to attached cells (Figure 1E). ALDH1A1 and ALDH1/2 were increased in UM-HACC-2A and UM-HACC-14 salispheres when compared to attached cells, while UM-HACC-6 salispheres had similar expression levels. Interestingly, we observed an additional, higher molecular weight band in UM-HACC-14 salispheres in the Western blot for ALDH1/2. Both constitutive p-STAT3 and total STAT3 expression were also upregulated in salispheres when compared to attached cells in the ACC cell lines evaluated here (Figure 1E). And finally, Bmi-1 levels were similar when attached cells were compared to suspended cells in our cell lines (Figure 1E).
ALDHhighCD44high ACC cells self-renew and show strong expression of stem cell markers
To determine if ALDHhighCD44high cells had enhanced in vitro stemness features, UM-HACC-2A, UM-HACC-14 and UM-HACC-6 cells were sorted for all four marker combinations (i.e., ALDHlowCD44low, ALDHlowCD44high, ALDHhighCD44low, ALDHhighCD44high) and then plated in ultra-low attachment conditions immediately after sorting (Figures 2A–C). The flow cytometry gating strategy for in vitro experiments is shown in (Supplementary Figure A1). We observed that ALDHhighCD44high cells formed the highest average salisphere number in UM-HACC-2A and UM-HACC-6 cells compared to all other groups (Figures 2A–C). UM-HACC-14 behaved slightly differently. In this case, ALDHhighCD44high and ALDHhighCD44low cells formed significantly more salispheres compared to ALDHlowCD44low cells but were not statistically different from each other (Figure 2B). Photomicrographs showed a trend for increased numbers of salispheres in ACC cells expressing high levels of ALDH activity (Figure 2D). We performed western analysis of several markers of stemness (i.e., Notch1, Notch2, ALDH1A1, ALDH1/2, Nanog, Bmi-1) in the 3 cell lines immediately after sorting for ALDH activity and CD44 expression. UM-HACC-2A and UM-HACC-14 sorted cells had similar protein expression profiles, i.e., Notch2, ALDH1/2, Bmi-1, p-STAT3, and STAT3 were increased in ALDHhighCD44high cells compared to ALDHlowCD44low cells (Figure 2E). Notch1 expression was reduced ALDHhighCD44high cells in UM-HACC-2A and UM-HACC-14, and upregulated in ALDHhighCD44high cells in the UM-HACC-6 cell line. Nanog expression was reduced in UM-HACC-2A ALDHhighCD44high cells and increased in UM-HACC-14 and UM-HACC-6 ALDHhighCD44high cells, when compared to ALDHlowCD44low cells. Interestingly, ALDHhighCD44high cells sorted from UM-HACC-6 cell line showed increased expression of all CSC markers evaluated here (i.e., Notch1, Notch2, ALDH1/2, Nanog, Bmi-1) when compared to ALDHlowCD44low cells (Figure 2E).
Figure 2
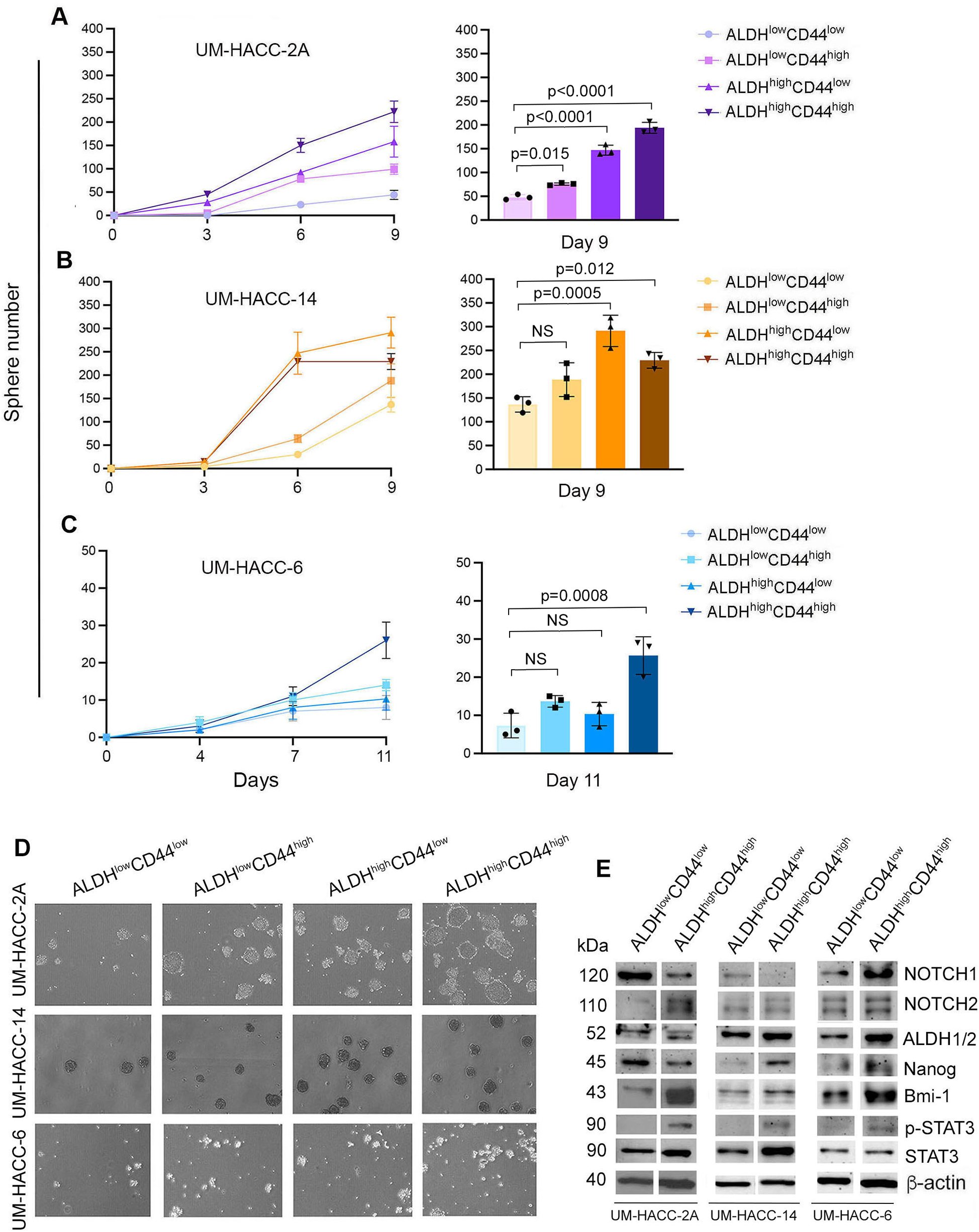
ALDHhighCD44high cells form more spheres and express higher levels of ALDH1-2, NOTCH2, p-STAT3 and Bmi-1 compared to non-CSC. (A–C) Line and bar graphs showing the number of spheres in UM-HACC-2A, UM-HACC-14 and UM-HACC-6 cell lines. Statistical significance was defined at p < 0.05, as determined by paired t-tests using ALDHlowCD44low cells as controls. NS, non-significant. (D) Photomicrographs of spheres generated from ALDHhighCD44high CSC and non-CSC (ALDHlowCD44low, ALDHlowCD44high, ALDHhighCD44low). (E) Western blot analysis in ALDHhighCD44high and ALDHlowCD44low cells.
To verify our initial results, we performed an independent serial dilution experiment in vitro with UM-HACC-2A and UM-HACC-14 cells sorted for ALDHhighCD44high and ALDHlowCD44low (Figures 3A,B). Here, we observed similar overall trends as those showed in the previous experiments, with the most noticeable differences observed at the higher numbers of cells plated. Interestingly, the salispheres generated by ALDHhighCD44high cells tended to be larger than those formed with ALDHlowCD44low cells (Figure 3C). To understand the impact of ALDH activity and CD44 expression on self-renewal of ACC cells, we sorted the 3 cell lines for ALDHhighCD44high or ALDHlowCD44low and expanded them as primary salispheres for 7 days. Then, we generated secondary spheres by dissociation of the primary salispheres, counting and re-plating the cells into new ultra-low attachment plates. The ALDHhighCD44high cells formed consistently more secondary salispheres than the ALDHlowCD44low cells in the ACC cell lines evaluated here (Figure 3E).
Figure 3
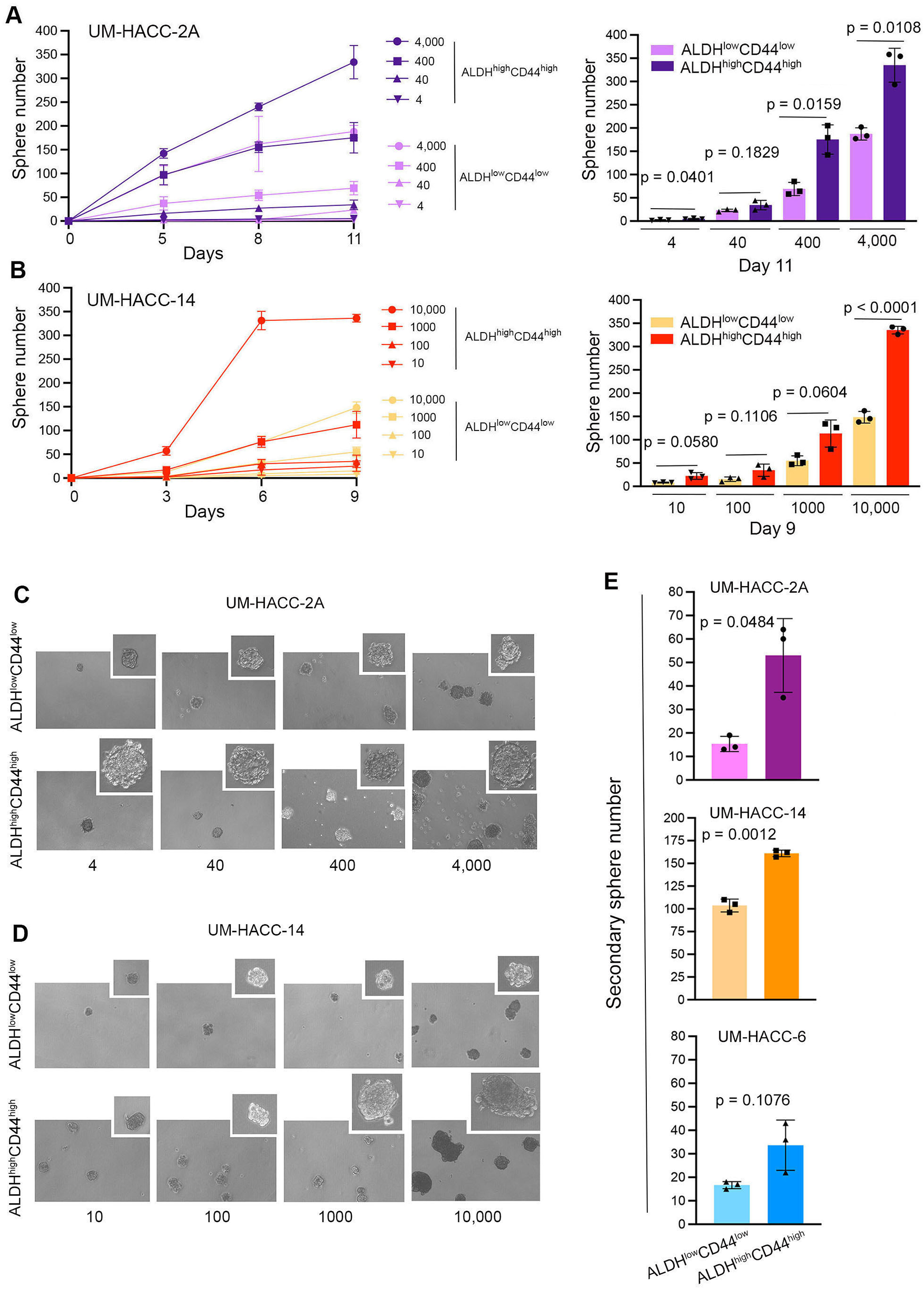
ALDHhighCD44high cells form more spheres and exhibit enhanced self-renewal capabilities compared to ALDHlowCD44low cells. (A,B) Line and bar graphs showing sphere number after sorting and serial dilution of UM-HACC-2A and UM-HACC-14 cells. p < 0.05. (C,D) Photomicrographs of spheres generated from ALDHhighCD44high and ALDHlowCD44low cells. (E) Bar graphs displaying the average number of secondary spheres generated with UM-HACC cell lines after 7 days.
ALDHhighCD44high ACC cells are highly tumorigenic and generate lung metastases
To understand whether our in vitro findings were consistent with the behavior of cells in vivo, we performed two independent serial dilution experiments involving the sorting of the ACC cells and transplantation into mice. Here, we grew ACC PDX tumors in mice (UM-PDX-HACC-14), retrieved them and dissociated the tumors to prepare single-cell suspensions, and then flow sorted these cells for ALDH activity and CD44 expression. The flow cytometry gating strategies used for the in vivo study is shown in Supplementary Figure 1B. These cells were injected into the submandibular salivary glands of mice immediately after sorting. Hematoxylin and Eosin staining of the mouse salivary glands showed human tumor cells (i.e., HLA-positive cells) upon injection of very low numbers (15–1,500) ALDHhighCD44high cells (Figures 4A–C). Strong expression of ALDH1A1 and minimal CD44 staining was present in salivary gland tumors generated with ALDHhighCD44high cells (Figures 4A–C). No staining of mouse CD45 confirmed that the cells growing tumors in the salivary glands were of human origin. As expected, there was minimal staining for Keratin-7, a marker used to detect differentiated tumor cells (42). These tumors grew very slowly and did not show palpability even after a 13-month follow-up, which is consistent with the slow growth rate observed in patients with ACC. Surprisingly, despite very small primary tumors, we observed that a significant number of mice exhibited lung metastases. We observed several lung metastases that stained positive with HLA and keratin 7 and showed no staining with mouse CD-45 (Figures 5A–C). Similar to the primary tumors in the salivary glands, strong ALDH1A1 staining and minimal CD44 expression was detected in the lung metastases (Figures 5A–C). Notably, some of these metastatic sites were localized in close proximity to nerves (Figures 5B,C).
Figure 4
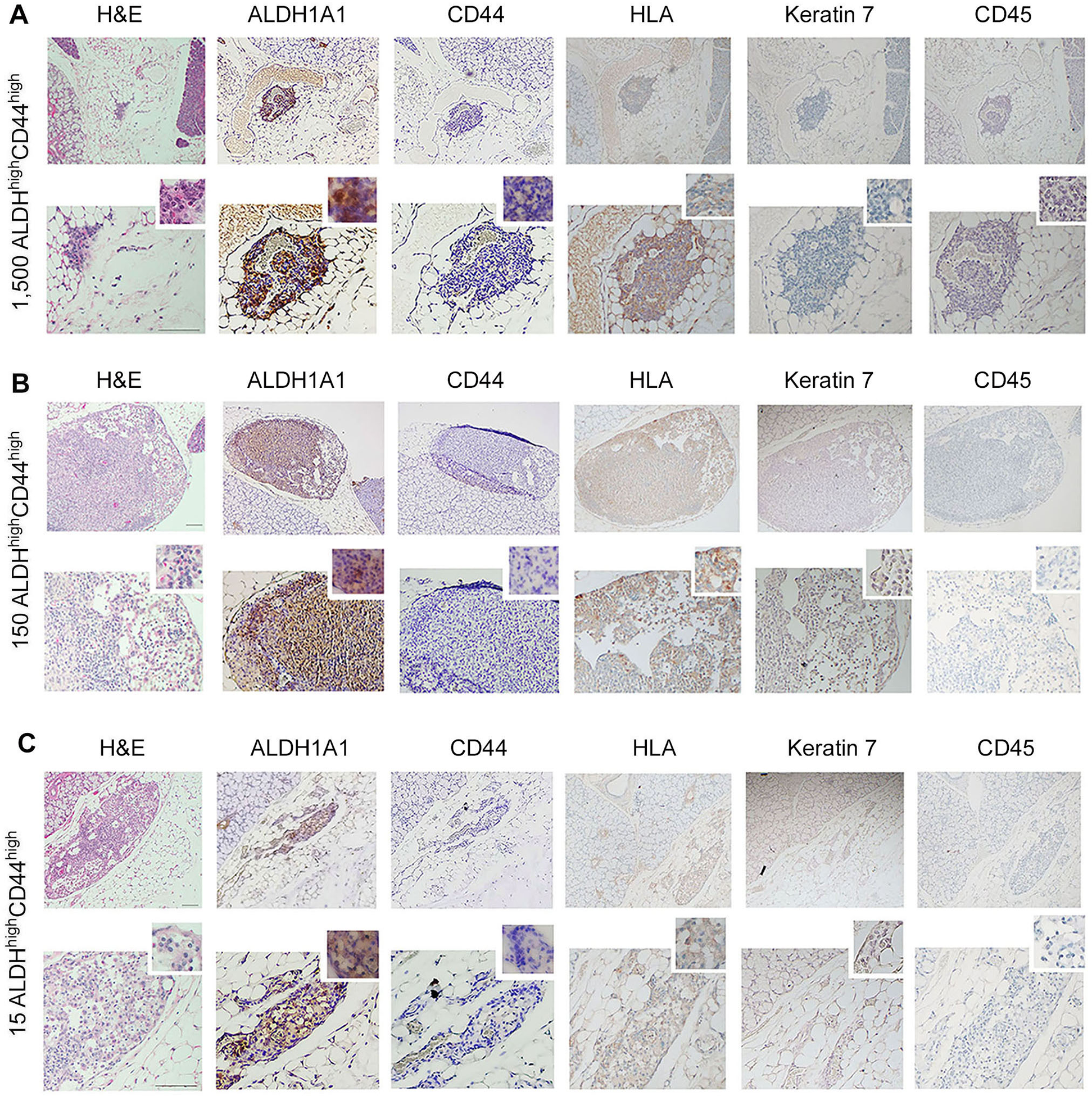
Tumorigenicity of UM-PDX-HACC-14 cancer stem cells in mouse salivary gland. (A-C) Photomicrographs of orthotopic tumors generated with 1,500, 150 and 15 UM-PDX-HACC-14 ALDHhighCD44high cells and stained with Hematoxylin/Eosin (H&E) or immunohistochemistry with anti-human ALDH1A1, CD44, HLA antibody, anti-Keratin 7 antibody and with anti-mouse CD45 antibody. All images are shown at 40× (top rows) and 100× magnification (bottom rows) with a 200× magnification shown in the smaller window. Scale bar represents 100 µm.
Figure 5
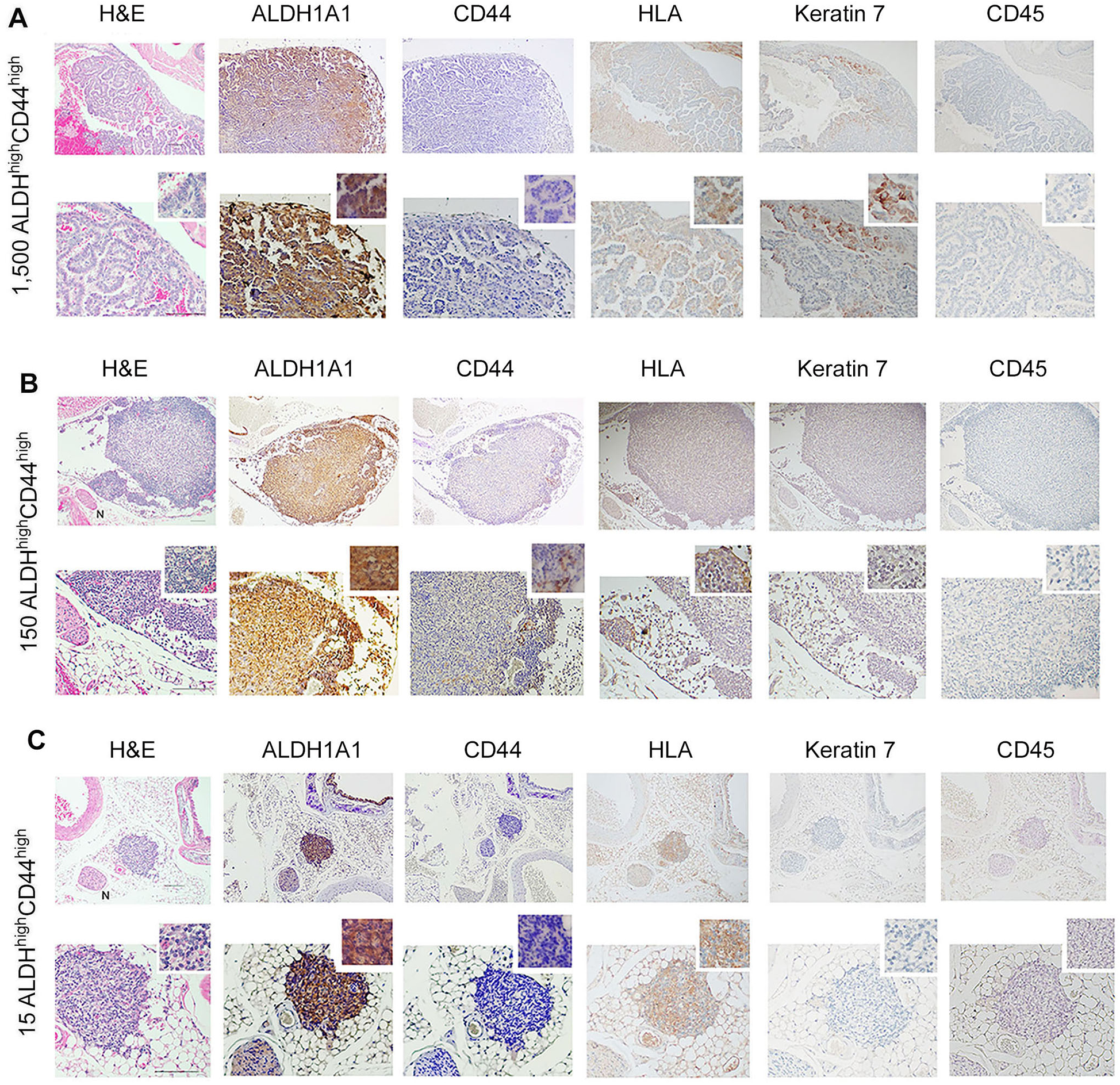
Metastatic capability of UM-PDX-HACC-14 cancer stem cells. (A-C) Photomicrographs of lung metastasis generated by orthotopic injection of 1,500, 150 and 15 UM-PDX-HACC-14 ALDHhighCD44high cells and stained with Hematoxylin/Eosin (H&E), or immunohistochemistry with anti-human ALDH1A1, CD44, HLA antibody, anti-Keratin 7 antibody and with anti-mouse CD45 antibody. N indicates a nerve in the H&E panel, 40× view (B,C). All images are shown at 40× (top rows) and 100× magnification (bottom rows) with a 200× magnification shown in the smaller window. Scale bar represents 100 µm.
To further explore the tumorigenic potential of ALDHhighCD44high ACC cells, we evaluated their tumorigenic potential against pooled cells (ALDHlowCD44low, ALDHlowC44high, ALDHhighCD44low) for 16 months in mice (Figures 6A,B). Hematoxylin and eosin staining revealed a salivary gland tumor with poorly differentiated, solid phenotype with low ALDH1A1 and strong CD44, HLA and keratin-7 expression (Figure 6A). In contrast, the injection of 15,000 ALDHlowCD44low cells resulted in a well-differentiated salivary gland tumor 13 months after injection (Figure 6B). Hematoxylin and eosin staining confirmed cribriform/tubular morphology, positive staining for HLA and keratin-7 and no CD45 staining (Figure 6B). We observed a palpable tumor 7 months after orthotopic injection of 4,000 ALDHhighCD44high cells (Figure 6C). When we combined both in vivo experiments together, a total of 12 mice injected with ALDHhighCD44high ACC cells resulted in 6 primary salivary gland tumors (50%) and 5 lung metastases (42%). In contrast, injection of 8 mice with control cells (i.e., ALDHlowCD44low or pooled cells) gave rise to 3 primary salivary gland tumors (37%) and 0 lung metastases (0%) (Figure 6D).
Figure 6
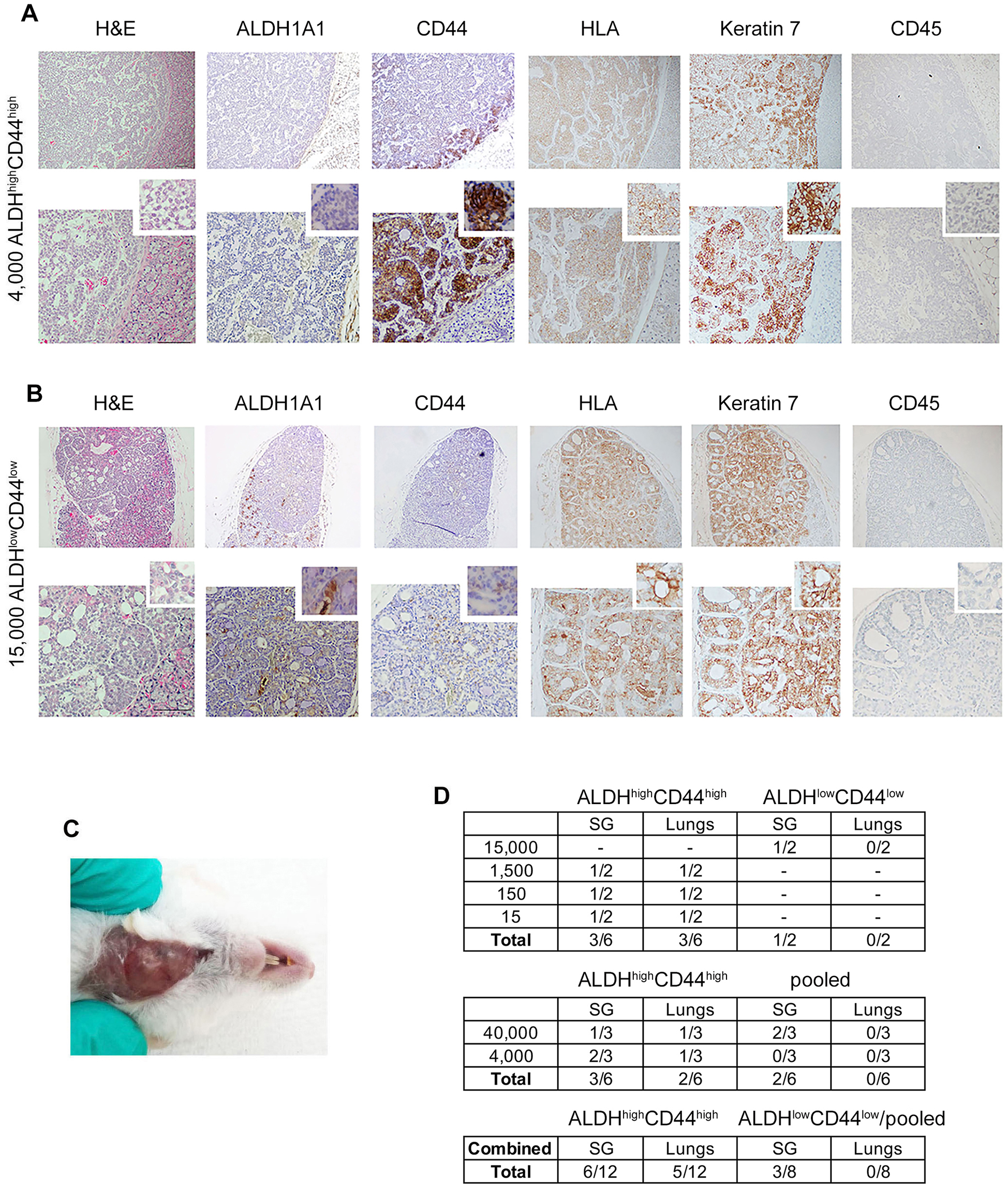
Different ACC tumor phenotypes generated with UM-PDX-HACC-14 cancer and non-cancer stem cells in mouse submandibular gland. (A) Photomicrographs of an orthotopic solid salivary gland tumor after injection of 4,000 ALDHhighCD44high (7 months). (B) Photomicrograph of an orthotopic cribriform/tubular salivary gland tumor after injection of 15,000 ALDHlowCD44low cells (13 months) stained with Hematoxylin/Eosin (H&E), or immunohistochemistry with anti-human ALDH1A1, CD44, HLA antibody, anti-Keratin 7 antibody and with anti-mouse CD45 antibody. All images are shown at 40× (top rows) and 100× magnification (bottom rows) with a 200× magnification shown in the smaller window. Scale bar represents 100 µm. (C) Photograph of the solid palpable salivary gland tumor of UM-PDX-HACC-14 ALDHhighCD44high cells in (A,D) table depicting the total number of mouse salivary gland and lung tumors after orthotopic injection of ALDHhighCD44high, ALDHlowCD44low or pooled cells (ALDHlowCD44low, ALDHlowCD44high, ALDHhighCD44low).
Discussion
The lack of better mechanistic understanding of the pathobiology of ACC has contributed to the poor long-term clinical outcome of patients with ACC. One of the key features of ACC is tumor cell heterogeneity, with tumors presenting in a cribriform or tubular pattern, while more undifferentiated tumors typically present a solid pattern. Whether or not ACC have uniquely tumorigenic cells and how these cells can be identified remains unclear. Here, we have shown that ALDHhighCD44high cells are more tumorigenic than controls. Further, we showed that orthotopic tumors generated by ALDHhighCD44high cells have a higher potential for metastatic spread to the lungs when compared with control cells. We report here that ALDHhighCD44high ACC cells generated more orthotopic tumors than ALDHlowCD44low cells, which is consistent with data published by Keysar and colleagues (36). We have also reported that ALDHhighCD44high ACC cells are more prone to metastatic spread to the lungs than ALDHlowCD44low cells.
Two recent studies have reported positive tumor cell staining for ALDH and CD44 in malignant salivary gland tumors (43, 44). Santos and colleagues reported variable ALDH expression in ACC tumors and consistent ALDH1 stromal cell expression correlated with reduced overall disease-free survival and advanced staging (44). We report here strong ALDH1A1 staining and lower CD44 staining in most tumors generated with ALDHhighCD44high cells. Similar trends were observed in metastatic lung tumors. Interestingly, even when we implanted a very low number of ALDHhighCD44high cells (15 cells) into the mouse submandibular gland, we observed metastatic spread to the lungs. While these correlational in vivo results suggest that ALDH1A1 (not CD44) is the primary “driver” of ACC tumorigenesis, additional mechanistic and in vivo studies should be done to verify this hypothesis. Considering that prevention and treatment of metastases require systemic therapies, these findings provide evidence in support of strategies for therapeutic targeting of ACC cancer stem cells (i.e., ALDHhighCD44highcells).
Studies have shown that several ALDH isoforms play a role in the pathobiology of cancer and in resistance to anti-cancer therapy (45). We observed here that the histological features of the tumors generated with orthotopic injection of ALDHhighCD44high cells matched the solid pattern exhibited by the patient that donated the tissues for the generation of the PDX model used here, UM-PDX-HACC-14 (42). The overall number of tumors generated with ALDHlowCD44low cells was smaller and their histological features were different than those observed in the tumors generated by the ALDHhighCD44high cells. The tumors generated with ALDHhighCD44high cells were of a solid, very aggressive phenotype, while the tumors generated with control cells presented with a less aggressive, cribriform/tubular histology. The connection of CSC to ACC histology sub-types was previously alluded to using immunohistochemistry for CD133 and CD44 in histological sections of 26 human ACC tumors (46). The authors showed that CD133 + and CD44 + cells lined cribriform pseudocysts and accumulated in tubular ACC. The results of this correlation study were confirmed by our results, which showed that CSCs defined by ALDH activity and CD44 expression generate solid, more aggressive ACC tumors while control cells generated a cribriform/tubular, more differentiated tumor. A limitation of our study is the small number ACC cell lines and animal models available for mechanistic studies. Nevertheless, we believe that the data presented here provide preliminary support for further exploration of the function of cancer stem cells in salivary gland adenoid cystic carcinoma.
The tumor progression observed in our experiments mimics the features of ACC tumors in patients (1–3, 6). While transplantation of HNSCC ALDHhighCD44high cells generates tumors within 4–6 months (22), we observed here that generation of orthotopic ACC tumors and lung metastases typically requires 13–16 months, which is approximately half of the life span of a mouse. This result is consistent with the slow, progressive disease and frequent lung metastases observed in patients with ACC. Notably, we unveiled here the generation of salivary gland ACC and lung metastases with very low ACC CSC cell numbers (as low as 15 cells). On the other hand, we have not observed bone or liver metastases in our studies. Another interesting feature is that we observed proximity between nerves and ACC cells in lung metastatic sites. This feature suggests that the orthotopic model reproduces, at least in part, the perineural invasion presented by the patient ACC that was used to generate our PDX model. Of note, perineural invasion is common feature of patients with ACC (1–3, 6, 42).
Progress in identifying and understanding the role of CSC in adenoid cystic carcinoma has been slow due to the lack of available authentic, non-transformed cell lines. Phuchareon and colleagues identified the cross contamination of 6 ACC cell lines widely used to study this disease (47). Here we report stemness of ACC using salisphere assays and analysis of cancer stem cell markers in vitro with our 3 UM-HACC cell lines and one matching PDX model (41, 42). The enhanced expression of Notch1, Notch2, and Nanog in salispheres and ALDHhighCD44high cells suggests the function of cancer stem cells in ACC. Of note, increased Notch1 and Notch2 in the 3 cell lines is particularly exciting, since they are therapeutic targets being studied in patients with ACC (48). We observed an extra band with slightly higher molecular weight in the western blot from UM-HACC-14 salispheres. The postulate that this extra band is likely due to enhanced expression of ALDH2 (56 kDa) in addition to ALDH1 (55 kDa). Of note, the antibody used here recognizes both, ALDH1 and ALDH2 isoforms. The extra band could also be due to post translational modification of ALDH1. For example, glycosylation events have been described for this molecule (45, 49).
When we sorted cancer stem cells (ALDHhighCD44high) from UM-HACC-6, we observed increased expression of CSC markers despite the fact this cell line has the lowest percentage of CSC and the slowest salisphere formation. This cell line is derived from an aggressive, recurrent ACC tumor with signs of perineural invasion and presence of lung metastases (32, 42). Orthotopic injection of UM-HACC-6 cells into mouse submandibular glands resulted in lung metastases within 6 months (42). We are currently performing additional studies to better understand the unique aspects of the stemness and tumorigenic potential of this ACC cell line.
The difficulty of diagnosing and treating ACC is related to the rarity of this tumor and the heterogeneity of tumor presentation. ACC tumor size, grade, stage, +/− metastases, (lymph or hematic spread), +/− perineural invasion, and fusion status makes predictions of rate of tumor progression and long-term prognosis very difficult. We have spent the last 15 years generating ACC cell lines. The UM-HACC-2A is c-MYB-NFIB positive cell line (32, 41, 42). The UM-HACC-14 cell line and UM-HACC-6 cell lines are fusion-negative (42). Despite differences in fusion status, tumor origin, location and grade, these three human ACC cell lines exhibit multipotent and self-renewing cancer stem cells with uniquely high tumorigenic potential. These findings suggest that cancer stem cells might be considered a common treatment target shared by diverse ACC tumor phenotypes. As such, the work presented here contributes to the knowledge of the pathobiology of this rare malignancy and suggest a new cellular target that can be explored to develop novel therapeutic strategies for patients with salivary gland adenoid cystic carcinoma.
Statements
Data availability statement
The datasets presented in this article are not readily available because there are no data sets associated with this work. Requests to access the datasets should be directed to not applicable.
Ethics statement
The studies involving humans were approved by human tumor specimen collection was performed under IRB-approved protocol (HUM00065996). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of our previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. The animal study was approved by University of Michigan IACUC (Institutional Animal Care and Use Committee-approved protocol (PRO00011044). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
KW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SS: Data curation, Methodology, Writing – review & editing. AH: Conceptualization, Data curation, Methodology, Writing – review & editing. FN: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. RC: Conceptualization, Formal analysis, Investigation, Methodology, Writing – review & editing. PP: Conceptualization, Formal analysis, Methodology, Writing – review & editing. JN: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by grants R01-DE021139 and R01-DE23220 from the NIH/NIDCR (J.E. Nör).
Acknowledgments
We thank the patients who generously provided the tumor samples used to generate the UM-HACC adenoid cystic carcinoma cell lines and PDX model. We also thank the surgeons, nurses and support staff who assisted with specimen collection and consent forms.
Conflict of interest
UM-HACC-2A cell line has been licensed by the University of Michigan and is available from Applied Biological Sciences, Inc.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2025.1570042/full#supplementary-material
Supplementary Figure 1(A) Representative flow plots and gating strategy for in vitro sorting of UM-HACC-2A for ALDH activity and CD44 expression. (B) Representative flow plots and gating strategy for sorting of UM-PDX-HACC-14 cells retrieved from mice for ALDH activity and CD44 expression.
References
1.
Bjørndal K Krogdahl A Therkildsen MH Charabi B Kristensen CA Andersen E et al Salivary adenoid cystic carcinoma in Denmark 1990–2005: outcome and independent prognostic factors including the benefit of radiotherapy. Results of the Danish head and neck cancer group (DAHANCA). Oral Oncol. (2015) 51(12):1138–42. 10.1016/j.oraloncology.2015.10.002
2.
Jaso J Malhotra R . Adenoid cystic carcinoma. Arch Pathol Lab Med. (2011) 135(4):511–5. 10.5858/2009-0527-RS.1
3.
Coca-Pelaz A Rodrigo JP Bradley PJ Vander Poorten V Triantafyllou A Hunt JL et al Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. (2015) 51(7):652–61. 10.1016/j.oraloncology.2015.04.005
4.
Seethala RR . An update on grading of salivary gland carcinomas. Head Neck Pathol. (2009) 3(1):69–77. 10.1007/s12105-009-0102-9
5.
Chen AM Granchi PJ Garcia J Bucci MK Fu KK Eisele DW . Local-regional recurrence after surgery without postoperative irradiation for carcinomas of the major salivary glands: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. (2007) 67(4):982–7. 10.1016/j.ijrobp.2006.10.043
6.
de Morais EF de Farias Morais HG de Almeida Freitas R Coletta RD . Prognostic significance of histopathological parameters for salivary gland adenoid cystic carcinoma. Dent J (Basel). (2023) 11(11): 262. 10.3390/dj11110262
7.
Friedrich RE Bleckmann V . Adenoid cystic carcinoma of salivary and lacrimal gland origin: localization, classification, clinical pathological correlation, treatment results and long-term follow-up control in 84 patients. Anticancer Res. (2003) 23(2A):931–40.
8.
Sahara S Herzog AE Nör JE . Systemic therapies for salivary gland adenoid cystic carcinoma. Am J Cancer Res. (2021) 11(9):4092–110.
9.
Vander Poorten V Bradley PJ Takes RP Rinaldo A Woolgar JA Ferlito A . Diagnosis and management of parotid carcinoma with a special focus on recent advances in molecular biology. Head Neck. (2012) 34(3):429–40. 10.1002/hed.21706
10.
Bradley PJ . Adenoid cystic carcinoma evaluation and management: progress with optimism!. Curr Opin Otolaryngl H and Neck Surge. (2017) 25(2):147–53. 10.1097/MOO.0000000000000347
11.
Surakanti SG Agulnik M . SG malignancies role for chemo and molecular targets. Sem in Oncol. (2008) 35(3):309–19. 10.1053/j.seminoncol.2008.03.009
12.
Hermann PC Bhaskar S Cioffi M Heeschen C . Cancer stem cells in solid tumors. Semin Cancer Biol. (2010) 20(2):77–84. 10.1016/j.semcancer.2010.03.004
13.
Herzog AE Somayaji R Nör JE . Bmi-1: a master regulator of head and neck cancer stemness. Front Oral Health. (2023) 16(4):1080255. 10.3389/froh.2023.1080255
14.
Al-Hajj M Wicha MS Benito-Hernandez A Morrison SJ Clarke MF . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. (2003) 100(7):3983–8. 10.1073/pnas.0530291100
15.
Russo JE Hilton J . Characterization of cytosolic aldehyde dehydrogenase from cyclophosphamide resistant L1210 cells. Cancer Res. (1988) 48(11):2963–8.
16.
Ginestier C Hur MH Charafe-Jauffret E Monville F Dutcher J Brown M et al ALDH1 Is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. (2007) 1(5):555–67. 10.1016/j.stem.2007.08.014
17.
Prince ME Sivanandan R Kaczorowski A Wolf GT Kaplan MJ Delerba P et al Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. (2007) 104(3):973–8. 10.1073/pnas.0610117104
18.
Cao L Hu X Zhang J Liang P Zhang Y . CD44(+) CD324(−) expression and prognosis in gastric cancer patients. J Surg Oncol. (2014) 110(6):727–33. 10.1002/jso.23690
19.
Clay MR Tabor M Owen JH Carey TE Bradford CR Wolf GT et al Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. (2010) 32(9):1195–201. 10.1002/hed.21315
20.
Davis SJ Divi V Owen JH Bradford CR Carey TE Papagerakis S et al Metastatic potential of cancer stem cells in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. (2010) 136(12):1260–6. 10.1001/archoto.2010.219
21.
Chinn SB Darr OA Owen JH Bellile E McHugh JB Spector ME et al Cancer stem cells: mediators of tumorigenesis and metastasis in head and neck squamous cell carcinoma. Head Neck. (2015) 37(3):317–26. 10.1002/hed.23600
22.
Krishnamurthy S Dong Z Vodopyanov D Imai A Helman JI Prince ME et al Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. (2010) 70(23):9969–78. 10.1158/0008-5472.CAN-10-1712
23.
Adams A Warner K Nör JE . Salivary gland cancer stem cells. Oral Oncol. (2013) 49(9):845–53. 10.1016/j.oraloncology.2013.05.013
24.
Nör C Zhang Z Warner KA Bernardi L Visioli F Helman JI et al Cisplatin induces bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia. (2014) 16(2):137–46. 10.1593/neo.131744
25.
Adams A Warner K Pearson AT Zhang Z Kim HS Basura G et al ALDH/CD44 identifies uniquely tumorigenic cancer stem cells in salivary gland mucoepidermoid carcinomas. Oncotarget. (2015) 6(29):26633–50. 10.18632/oncotarget.5782
26.
Herzog AE Warner KA Zhang Z Bellile E Bhagat MA Castilho RM et al The IL-6R and bmi-1 axis controls self-renewal and chemoresistance of head and neck cancer stem cells. Cell Death Dis. (2021) 12(11):988. 10.1038/s41419-021-04268-5
27.
Andrews A Warner K Rodriguez-Ramirez C Pearson AT Nör F Zhang Z et al Ablation of cancer stem cells by therapeutic inhibition of the MDM2-p53 interaction in mucoepidermoid carcinoma. Clin Cancer Res. (2019) 25(5):1588–600. 10.1158/1078-0432.CCR-17-2730
28.
Rodriguez-Ramirez C Zhang Z Warner KA Herzog AE Mantesso A Zhang Z et al P53 inhibits bmi-1-driven self-renewal and defines salivary gland cancer stemness. Clin Cancer Res. (2022) 28(21):4757–70. 10.1158/1078-0432.CCR-22-1357
29.
Andrade NP Warner KA Zhang Z Pearson AT Mantesso A Guimaraēs DM et al Survival of salivary gland cancer stem cells requires mTOR signaling. Cell Death Dis. (2021) 12(1):108. 10.1038/s41419-021-03391-7
30.
Nakano T Warner KA Oklejas AE Zhang Z Rodriguez-Ramirez C Shuman AG et al mTOR inhibition ablates cisplatin-resistant salivary gland cancer stem cells. J Dent Res. (2021) 100(4):377–86. 10.1177/0022034520965141
31.
Acasigua GA Warner KA Nör F Helman J Pearson AT Fossati AC et al BH3-mimetic small molecule inhibits the growth and recurrence of adenoid cystic carcinoma. Oral Oncol. (2015) 51(9):839–47. 10.1016/j.oraloncology.2015.06.004
32.
Warner KA Nör F Acasigua GA Martins MD Zhang Z McLean SA et al Targeting MDM2 for treatment of adenoid cystic carcinoma. Clin Cancer Res. (2016) 22(14):3550–9. 10.1158/1078-0432.CCR-15-1698
33.
Nör F Warner KA Zhang Z Acasigua GA Pearson AT Kerk SA et al Therapeutic inhibition of the MDM2-p53 interaction prevents recurrence of adenoid cystic carcinomas. Clin Cancer Res. (2017) 23(4):1036–48. 10.1158/1078-0432.CCR-16-1235
34.
Sahara S Warner KA Herzog AE Zhang Z Nör JE . Therapeutic inhibition of bmi-1 ablates chemoresistant cancer stem cells in adenoid cystic carcinoma. Oral Oncol. (2023) 142:106437. 10.1016/j.oraloncology.2023.106437
35.
Sun S Wang Z . ALDH high adenoid cystic carcinoma cells display cancer stem cell properties and are responsible for mediating metastasis. Biochem Biophys Res Commun. (2010) 396(4):843–348. 10.1016/j.bbrc.2010.04.170
36.
Keysar SB Eagles JR Miller B Jackson BC Chowdhury FN Reisinger J et al Salivary gland cancer patient-derived xenografts enable characterization of cancer stem cells and new gene events associated with tumor progression. Clin Cancer Res. (2018) 24(12):2935–43. 10.1158/1078-0432.CCR-17-3871
37.
Zhou JH Hanna EY Roberts D Weber RS Bell D . ALDH1 immunohistochemical expression and its significance in salivary adenoid cystic carcinoma. Head Neck. (2013) 35(4):575–8. 10.1002/hed.23003
38.
Drier Y Cotton MJ Williamson KE Gillespie SM Ryan RJ Kluk MJ et al An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat Genet. (2016) 48(3):265–72. 10.1038/ng.3502
39.
Wang Y Sun B Zhang C Xia R Sun J Gu T et al Genetic heterogeneity and therapeutic target detection through microdissection in solid-type adenoid cystic carcinoma. Pathology. (2022) 54(5):580–90. 10.1016/j.pathol.2021.12.292
40.
Destro Rodrigues MF Sedassari BT Esteves CM de Andrade NP Altemani A de Sousa SC et al Embryonic stem cells markers Oct4 and nanog correlate with perineural invasion in human salivary gland mucoepidermoid carcinoma. J Oral Pathol Med. (2017) 46(2):112–20. 10.1111/jop.12449
41.
Warner KA Oklejas AE Pearson AT Zhang Z Wu W Divi V et al UM-HACC-2A: MYB-NFIB fusion-positive human adenoid cystic carcinoma cell line. Oral Oncol. (2018) 87:21–8. 10.1016/j.oraloncology.2018.10.012
42.
Warner KA Herzog AE Sahara S Nör F Castilho RM Demirci H et al Establishment and characterization of cMYB-expressing human salivary adenoid cystic carcinoma cell lines (UM-HACC-14, UM-HACC-6) and matching patient-derived xenograft model (UM-PDX-HACC-14). Oral Surg Oral Med Oral Pathol Oral Radiol. (2024) 138(4):516–31. 10.1016/j.oooo.2024.06.005
43.
da Silva LP Lopes MLDS Sarmento ASC de Albuquerque Borges M de Moura SRS Sobral APV et al Increased expression of ALDH-1 is associated with clinical parameters of salivary glands neoplasms. Exp Mol Pathol. (2020) 117:104552. 10.1016/j.yexmp.2020.104552
44.
Santos AAD Mafra RP da Silva LP Pinto LP Freitas RA de Souza LB . Immunohistochemical comparative analysis of tumor stem cell biomarkers in pleomorphic adenoma, adenoid cystic carcinoma and mucoepidermoid carcinoma of salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol. (2023) 135:396–409. 10.1016/j.oooo.2022.09.038
45.
Zanoni M Bravaccini S Fabbri F Arienti C . Emerging roles of aldehyde dehydrogenase isoforms in anti-cancer therapy resistance. Front Med (Lausanne). (2022) 9:795762. 10.3389/fmed.2022.795762
46.
Fujita S Ikeda T . Cancer stem-like cells in adenoid cystic carcinoma of salivary glands: relationship with morphogenesis of histological variants. J Oral Pathol Med. (2012) 41(3):207–13. 10.1111/j.1600-0714.2011.01096.x
47.
Phuchareon J Ohta Y Woo JM Eisele DW Tetsu O . Genetic profiling reveals cross-contamination and misidentification of 6 adenoid cystic carcinoma cell lines: aCC2, ACC3, ACCM, ACCNS, ACCS and CAC2. PLoS One. (2009) 25(6):e6040. 10.1371/journal.pone.0006040
48.
Ferrarotto R Mishra V Herz E Yaacov A Solomon O Rauch R et al AL101, a gamma-secretase inhibitor, has potent antitumor activity against adenoid cystic carcinoma with activated NOTCH signaling. Cell Death Dis. (2022) 13(8):678. 10.1038/s41419-022-05133-9
49.
Song BJ Abdelmegeed MA Yoo SH Kim BJ Jo SA Jo I et al Post-translational modifications of mitochondrial aldehyde dehydrogenase and biomedical implications. J Proteomics. (2011) 18(12):2691–702. 10.1016/j.jprot.2011.05.013
Summary
Keywords
salivary gland cancer, cancer stemness, tumor-initiating cells, aldehyde dehydrogenase, tumor cell heterogeneity
Citation
Warner KA, Sahara S, Herzog AE, Nör F, Castilho RM, Polverini PJ and Nör JE (2025) Characterization of uniquely tumorigenic cancer stem cells in salivary gland adenoid cystic carcinoma. Front. Oral Health 6:1570042. doi: 10.3389/froh.2025.1570042
Received
02 February 2025
Accepted
15 April 2025
Published
30 April 2025
Volume
6 - 2025
Edited by
Shamshad Alam, University at Buffalo, United States
Reviewed by
Davide Lombardi, University of Brescia, Italy
Vishal Gupta, University at Buffalo, United States
Vasudha Mishra, The University of Chicago, United States
Kusmardi Kusmardi, University of Indonesia, Indonesia
Kousalya Lavudi, The Ohio State University, United States
Ieman Aljahdali, Taif University, Saudi Arabia
Updates
Copyright
© 2025 Warner, Sahara, Herzog, Nör, Castilho, Polverini and Nör.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Jacques E. Nör jenor@umich.edu
ORCID Kristy A. Warner orcid.org/0000-0001-6781-6109 Jacques E. Nör orcid.org/0000-0002-4056-0235
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.