- 1Medical Sciences Division, Northern Ontario School of Medicine University, Sudbury, ON, Canada
- 2School of Natural Sciences, Laurentian University, Sudbury, ON, Canada
- 3Health Sciences North Research Institute, Sudbury, ON, Canada
- 4Department of Medicine, McMaster University, Hamilton, ON, Canada
Background: Oral candidiasis is a common fungal infection that disproportionately affects older adults, immunosuppressed individuals, and patients undergoing cancer treatment. Despite its prevalence, diagnosis and treatment remain challenging due to the diverse symptom presentation and potential for antifungal resistance.
Objective: This study aimed to systematically evaluate which clinical signs and symptoms are most predictive of oral Candida infections, with a specific focus on identifying features associated with antifungal treatment failure. A secondary objective was to assess whether underlying medical conditions, including frailty and comorbidities, influence infection susceptibility or resolution following therapy.
Methods: A cohort of 57 patients aged 65 years and older (mean age 74) was enrolled through oncology and hospitalist clinics in Northern Ontario. The majority (65%) were actively receiving cancer treatment. Participants underwent clinical assessment for oral candidiasis signs and symptoms, and fungal swabs were taken at baseline and two-week follow-up. Fungal species identification and treatment outcomes were recorded.
Results: The majority of infections involved Candida albicans and responded to standard antifungal treatment. In contrast, infections involving Nakaseomyces glabratus and Pichia kudriavzevii tended to persist, consistent with known antifungal resistance. Symptomatically, pseudomembranous candidiasis—characterized by white plaques, coated tongue, and taste disturbance—was more likely to resolve, while erythematous features such as angular cheilitis and oral redness were associated with persistent infection. Although 45% of patients were classified as moderately to severely frail, frailty status was not significantly associated with infection persistence or resistance.
Conclusion: These findings underscore the clinical variability of oral candidiasis and highlight the need for rapid molecular diagnostic tools at the point of care to distinguish infection types and guide appropriate therapy, particularly in older and medically complex populations.
1 Introduction
Oral candidiasis is an infection caused by the pathogenic overgrowth of fungal Candida species. Candidiasis results in local oral pain and discomfort, enhanced oral dryness, loss of taste, and aversion to food, and may lead to secondary complications such as dehydration, malnutrition, and decreased quality of life (1, 2). Furthermore, fungal infections in the oral cavity pose a risk of further consequences such as invasion into the bloodstream or to internal organ systems including the gut and digestive system, sepsis and higher mortality (3–7).
Common Candida species can typically live on the host's mucosal tissues in a commensal population with no pathogenicity or symptoms (8). In fact, approximately half of adults, depending on the community, have species like Candida albicans existing in their oral cavity without adverse consequences to their human health (8, 9). Good oral hygiene practices and dental care are an effective way to prevent fungal overgrowth and to maintain microbial homeostasis and overall good health (6, 10, 11). This includes proper dental brushing techniques, and proper care of dentures like leaving dentures out at night (12).
Numerous factors predispose an individual to developing fungal overgrowth. Infant and older adult populations, and palliative patients are highly susceptible to oral Candida infections, in addition to those with xerostomia (dry mouth) and disorders associated with immunosuppression and the endocrine system like HIV and cancer (2, 12–14). Factors such as polypharmacy, excessive alcohol use, lack of proper oral care, smoking, poor denture hygiene, poor nutrition and long-term steroid use or recent antibiotic and antifungal treatment can further increase susceptibility (9, 12, 15).The overall impact of different factors and how they can cause the development of infection when present in combination is still unclear.
Typical oral candidiasis symptoms in addition to dry mouth are taste disturbance and burning mouth, while angular cheilitis, removeable plaques, and oral redness are further signs. Cases can vary based on location (acute/local or systemic) and type (pseudomembranous or erythematous (9). Pseudomembranous infections which some clinicians refer to as oral thrush for acute cases, present as white lesions that resemble plaques. They can also occur chronically and become hyperplastic where the clinician cannot effectively wipe away the lesions (5). Alternatively, erythematous infections present with oral redness and burning mouth due to inflammation (16). Angular cheilitis presents with these signs at the edges of mouth with possible additional cracking (16). Accurate diagnosis requires an experienced clinician who can assess the patient's medical history for risk factors that can contribute to the development of Candida infection in addition to considering the range of signs and symptoms (9). Still, microbiological techniques which take time and are costly may be ultimately required to definitively identify if there is Candida overgrowth or an alternate condition present. This can make choosing an appropriate treatment difficult at the initial point of care as no one factor guarantees a Candida infection.
The main objective of this study was to determine what oral candidiasis signs and symptoms could serve as indicators for Candida infections especially those that are resistant to antifungal treatment. Additionally, the impact of medical conditions and frailty (physical and age-related changes that can increase the risk of illness and declining health) that may contribute to oral candidiasis and the ability to treat it successfully was assessed.
2 Materials and methods
2.1 Patients and setting
Eligible participants were identified through the North Eastern Cancer Center (NECC) Dental Clinic and Symptom Management Clinic and the Health Sciences North (HSN) Hospitalist program, all in Sudbury, Ontario, Canada. Enrollment occurred from June 2019 to September 2021. The main exclusion criterion was that individuals must not have received an antifungal treatment within two weeks prior to recruitment. The participant inclusion criteria was: age of 65 years or older currently attending the NECC Dental Oncology Clinic, Symptoms Management Clinic or HSN Hospitalist program; consent for the retrieval of two mouth swabs at the initial visit and follow-up; must be a candidate for fluconazole treatment with at least one oral candidiasis symptom present at the time of recruitment; able to attend a follow-up visit two weeks after the initial visit; and have the cognitive capacity to complete questionnaires.
Participants were surveyed to collect demographic data for their age, sex, smoking status, clinical frailty scale (CFS) (17) scores and presence of other disease comorbidities (such as high blood pressure, cardiovascular disease, diabetes, cancer etc.). Frailty scores were measured in three categories: Fit/low (CFS of 1–4), moderate (CFS of 5 or 6), severe (CFS of 7–9). Nutrition intake was assessed between normal and compromised. Oral hygiene was assessed as good, fair and poor. Smoking status was recorded as former, current or non-smoker. For dentition, patients were categorized either dentate (having natural teeth), partially dentate or edentate (no natural teeth).
2.2 Symptoms and fungal surveillance
The following symptoms were recorded: dry mouth, taste disturbance and burning mouth. Signs of oral candidiasis that were recorded were: angular cheilitis, coated tongue, oral redness and removable plaques (oral or oropharyngeal). An oral fluconazole treatment was prescribed as the first line of treatment for most cases displaying at least one sign or symptom. Nystatin (polyene) or Nizoral (ketoconazole) were prescribed where a topical treatment appeared more suitable depending on the location and severity of physical symptoms.
There was a follow-up after two weeks of using the prescribed antifungal treatment to assess if the infection was resolved. In cases where infections did not resolve, alternate antifungals micafungin-S (echinocandin), miconazole or clotrimazole were prescribed at the follow-up. Swab samples retrieved from the initial visit (and follow up if it persisted) were processed by the Health Sciences North (HSN) microbiology lab. Swabs were collected intraorally from the bilateral buccal mucosa and dorsum of the tongue. For fungal culturing, the samples were streaked onto Sabouraud Dextrose Agar (SDA) plates and incubated at 35°C for 48 h. Single clonal colonies were then selected for species identification using the VITEK® MS system (bioMérieux, France), a matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) platform. Each colony was transferred to a target slide, overlaid with a matrix solution (α-cyano-4-hydroxycinnamic acid), and allowed to dry. The samples were analyzed using the VITEK MS instrument, and species identification was determined by comparing protein mass spectral profiles against the IVD database provided by the manufacturer. Identifications with a confidence value of ≥95% were considered valid. Additionally, Glycerol stocks were created for each sample from the SDA plate colonies and stored at −80°C for future use.
2.3 Statistical analysis
Continuous data was summarized as the mean and standard deviation, and categorical as the count and frequency. Combinations of patient indications were presented as an upset plot, generated using the R package “ComplexHeatmap”. Associations between patient indications (dependant variable) and Candida species identified at baseline were estimated by logistic regression, with exception of the total indication count, which was estimated by ordered logistic regression. For the association between patient characteristics or indications and the persistence of Candida infection at follow-up (dependant variable), univariate estimates were calculated by logistic regression. All analyses were performed in R v4.5.0.
3 Results
3.1 Patients enrolled
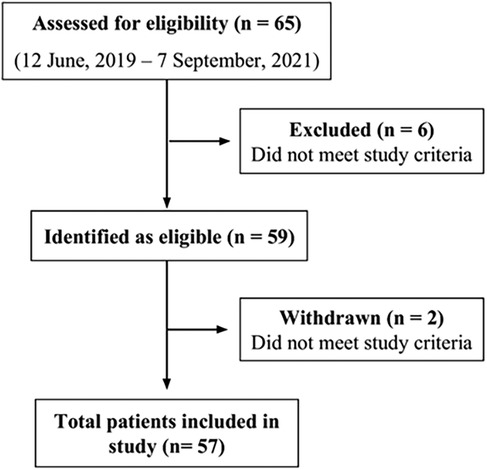
Of 65 patients approached for enrollment, 57 met the inclusion criterion and were enrolled in the study (Figure 1). Patients' ages ranged from 65–94 years. 33 individuals were aged between 65 and 74 years, 22 were aged between 75 and 84 years and 2 were 85 years or older. The mean age of patients participating in the study was 74 years old, with about half of patients of either sex (47% for female and 53% male) (Table 1). The majority of participants were undergoing cancer treatment (65%). Of these 37 participants, lung cancer was most common (n = 10) followed by tongue (n = 4) and myeloma, tonsil and prostate (n = 3 for each). Two participants each had mouth, breast or kidney cancer. There was one participant each with pelvis, bladder, skin, cheek, stomach, rectum, ear, and esophagus cancer. The cancer stage for the majority of cases was unknown (n = 22), followed by stage IV (n = 11), stage II (n = 3) and stage III (n = 1). (Supplementary Table S1) About half of participants had a frailty score of fit/low (53%), while 26% and 19% had a frailty score of mild/moderate or severe, respectively (with 1.8% missing data). For dentition status, 36% were completely dentate, 19% had partial dentition, and 24% were edentate (with 22.8% missing data). More than half (60%) had compromised nutrition intake, while for oral hygiene, most participants had fair status (61.4%) and the rest either had poor (19.3%) or good (19.3%). Only 12% of participants were current smokers, and 47% had been former smokers. Patients had individually different complex medical histories and various cardiac, vascular, pulmonary, renal, neuromuscular, liver, gastrointestinal, rheumatologic, and mental health conditions were cited among the patient cohort (Supplementary Table S2). The most common comorbid conditions were high blood pressure (n = 31), diabetes (n = 17), cardiovascular disease (n = 13) and gastroesophageal reflux disease (GERD) (n = 13).
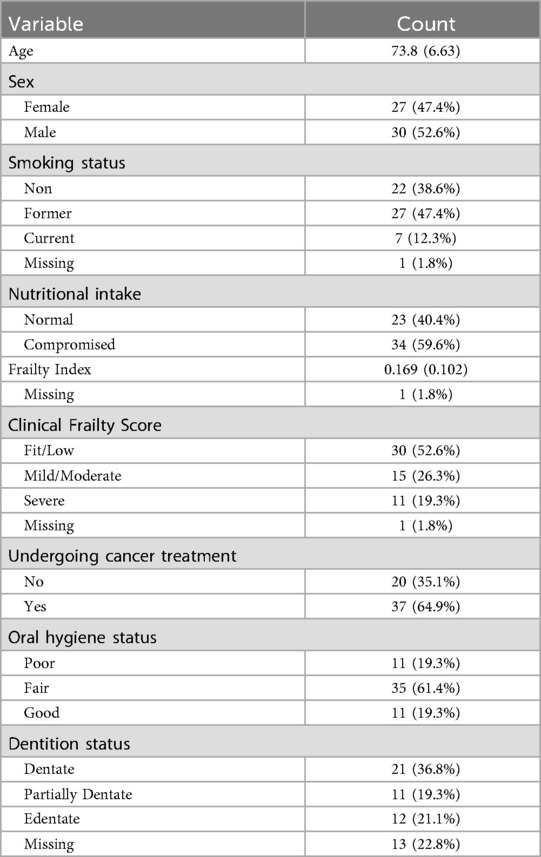
Table 1. Summary of patient characteristics at baseline (n = 57). Continuous data summarized as the mean (standard deviation), and categorical data as the count (frequency).
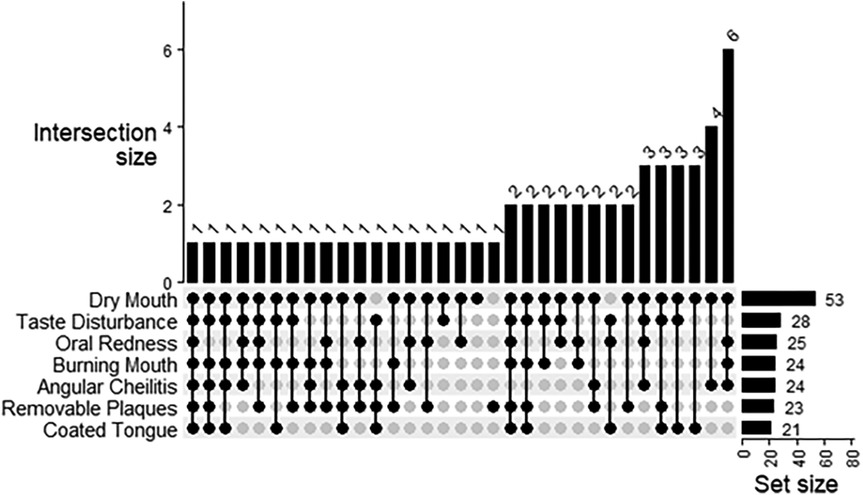
3.2 Baseline fungal screening and symptomology
Almost all the participants presented with the dry mouth symptom (93.0%) (Figure 2). About half of participants experienced taste disturbance (50.9%), while about 40% of patients had burning mouth (42.1%), angular cheilitis (42.1%), oral redness (43.9%) or removable plaques (40.4%). A coated tongue was present least frequently (36.8%). Only one participant presented with all six symptoms of oral candidiasis (Figure 2). The most frequent combination of symptoms was dry mouth, angular cheilitis, redness and burning (n = 6). Using logistic regression analysis, the odds of experiencing redness [OR (95% CI) = 5.7 (1.57, 27.6)] or removeable plaques [8.3 (2.0, 57.1)] was significantly higher with the presence of any Candida species infection (Figure 3). The total number of indications present was also significantly associated with the presence of any Candida species, where the odds of an increased indication count is 3.9-times (95% CI = 1.32, 11.8) higher. There was a similar result if Candida albicans was specifically present at baseline [oral redness: OR (95% CI) = 4.5 (1.44, 16.4); removable plaques: 5.3 (1.61, 21.5); and total indication count: 5.9 (1.98, 17.4)] in addition to significantly increased odds of having burning mouth [4.0 (1.28, 14.6)] (Figure 3). No significant associations were observed between patient indications and species other than Candida albicans.
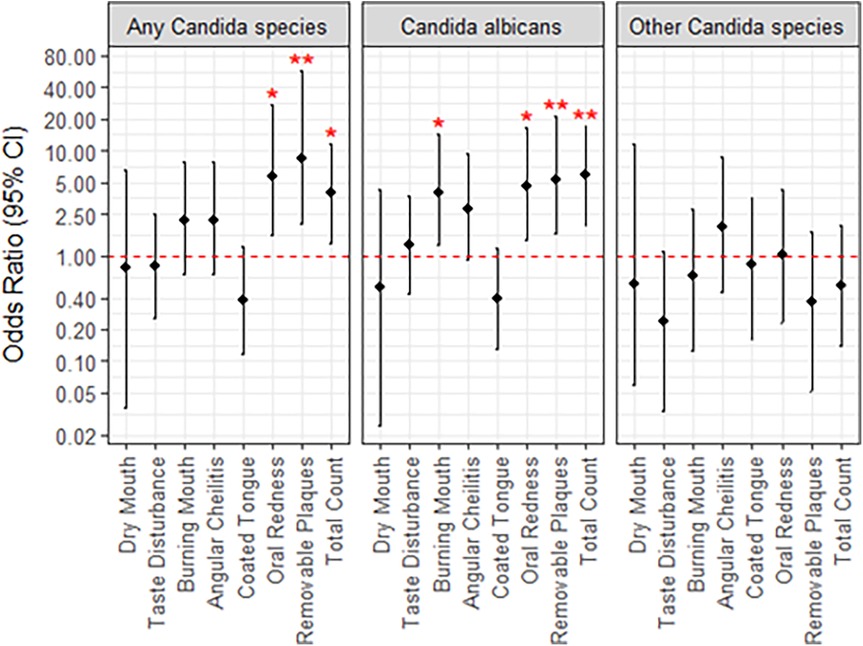
Figure 3. Univariate associations between Candida species detected at baseline and patient indications. Lack of significance is indicated by overlap with the red, dotted line, and asterisks; **, p < 0.01; *, p < 0.05.
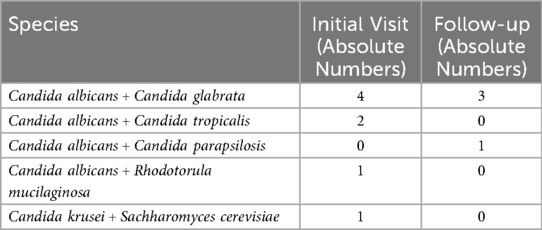
Most infections (57.6%) were treated with the first-line antifungal agent fluconazole. Nystatin or Nizoral was prescribed in 32.3% of cases as alternatives, typically based on infection severity and anatomical location within the oral cavity. In total, approximately 65 clinical isolates were retrieved from the initial visit (n = 48) and follow-ups (n = 15). From the baseline swab samples, 17 (29.8%) were identified as normal flora since the concentration of Candida did not surpass the pathological threshold (Table 2). The majority of abnormal flora samples contained Candida albicans (61.0%). The second most common species was Nakaseomyces glabratus (n = 6,) and there were a few other relevant species: Pichia kudriavzevii (n = 1), Candida tropicalis (n = 2), and Candida lusitanae (n = 1) (Table 2). Eight infections at the initial visit and four infections at the follow-up contained more than one fungal species (Table 3).
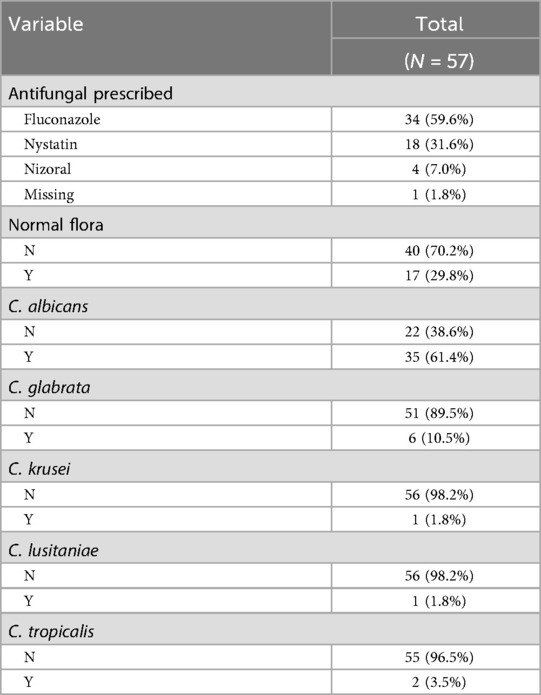
Table 2. Summary of antifungal treatment and detected species at baseline. Candida species present in oral candidiasis samples.
3.3 Follow-up outcomes and regression analysis
After two weeks, many infections were successfully treated and no remaining Candida was detected, thus there was no follow-up sample collected. Additionally, 12 patients were deceased before the follow-up or otherwise did not attend. After the follow-up was completed, 15 patients (26.3%) had a persistent Candida infection (5 C. albicans, 2 N. glabratus, 3 C. albicans + N. glabratus, 2 C. parapsilosis, 1 C. albicans + C. parapsilosis, 1 P. kudriavzevii and 1 C. tropicalis) while it was resolved in 18 patients (31.6%). If the patient presented with oral redness [OR (95% CI) = 4.68 (1.09, 22.9)] or angular cheilitis [6.50 (1.47, 34.3)]) at the initial visit, there were significantly increased odds that the infection would be unresolved at the two-week follow up (Figure 4). Alternately, patients presenting with coated tongue were less likely to have their Candida infection resolved by the time of the follow-up [0.08 (0.01, 0.51)](Figure 4). In terms of the Candida species present for unresolved infections at follow up, there was no significant association with C. albicans, N. glabratus, or other Candida species. Patients with a confirmed N. glabratus infection at baseline were significantly more likely to be infected at follow-up [OR = 9.4 (1.27, 196.2)], although confidence intervals were particularly wide. Of the other patient factors considered, such as age, smoking status and antifungal prescribed, only sex was found to be significantly associated to resolution of infection at follow-up (Supplementary Figure S1). Specifically, males were found to be less likely than females [0.2 (0.04, 0.86)]. Univariate estimates for the indications oral redness, angular cheilitis and coated tongue were slightly reduced after adjusting for male sex, although all retained significance at p < 0.20.
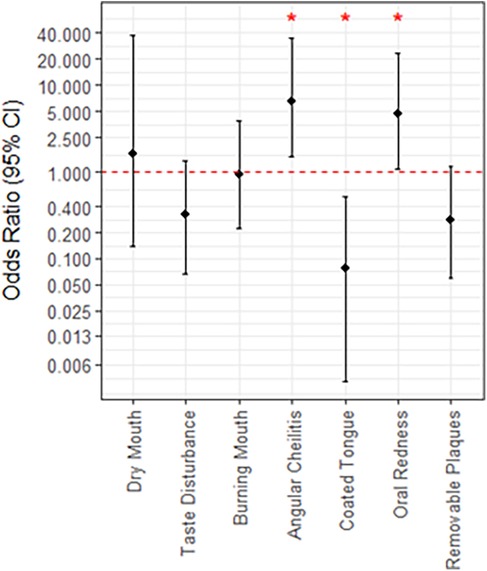
Figure 4. Associations between patient indications at baseline and the persistence of Candida infection at follow up. Lack of significance is indicated by overlap with the red, dotted line, and asterisks; *, p < 0.05.
4 Discussion
Overall, this study aimed to identify which signs and symptoms of oral candidiasis in addition to what risk factors can aid in correctly diagnosing a Candida infection and can predict whether the prescribed treatment will be successful. We found that a variety of risk factors may be present, and no single factor was significantly associated with the presence of a Candida infection. Alternately, the presence of oral redness or removeable plaques did increase the likelihood that there was Candida overgrowth and this was further increased with a higher total count of signs and symptoms present. There was a similar result for Candida albicans specifically, except burning mouth also increased the likelihood. At the follow-up after a two-week treatment, those who initially presented with oral redness or angular cheilitis had significantly increased odds that infection would not be resolved. In comparison, the presence of a coated tongue suggested increased odds that the infection would be cleared up.
4.1 Signs and symptoms of oral candidiasis
The results indicate that Candida infections do not all present uniformly as demonstrated by the varying combinations of oral candidiasis signs and symptoms. Removeable plaques are one of the most salient indicators in diagnosing this infection, however our results indicate the importance of considering all possible features. Diagnosis of pathogenic Candida strains could be missed since almost all patients had dry mouth and plaques cannot form without adequate saliva. In fact, the most frequent combinations of symptoms (dry mouth, angular cheilitis, oral redness and burning mouth) did not include plaques. These appear to be just as relevant for accurate diagnosis as indicated by the strong statistical association with the presence of plaques, oral redness and the total count of signs and symptoms with increased odds of a Candida infection.
Dry mouth was the most ubiquitous of the symptoms that were assessed as it was experienced by 90% of our patient population. The lack of variable variance likely resulted in no statistical significance when considering chances of having a Candida infection. Nonetheless, this symptom calls for closer examination as there was no case of oral candidiasis without this feature. A study by Alt-Epping (et al., 2012), had a similar finding, where almost 90% of their patient cohort also had dry mouth. Their cohort had a higher frequency of taste disturbance as well (∼80%) in comparison to the approximate 50% of our study (18). The symptom of xerostomia can have a negative consequence where those lacking adequate saliva cannot benefit from the natural and protective antifungal proteins like histatins, defensins and lysozymes secreted in saliva (10). Targeting treatment to restore salivary gland functioning could aid in prevention of future infection (18).
4.2 Factors associated with increased risk of oral candidiasis
Oral Candida infections can occur with equal frequency in both males and females as our patient survey data demonstrates (9). In terms of other health factors like frailty scores and oral hygiene, there were no statistically significant predictors of a persistent Candida infection. This highlights that many older adult patients susceptible to pathogenic Candida infections will have a complex medical history and the range of health factors needs to be considered on a case-by-case basis.
Frailty is a complex, age-related syndrome of enhanced vulnerability to a myriad of health outcomes and has been described as the “most problematic expression of aging” (19). Elevated frailty has been linked to microbial dysbiosis, oral disease and infection, emphasizing its important role in driving disease risk beyond what can be attributable to age (20, 21). This is a bidirectional relationship, as poor oral health such as loss of natural teeth also exacerbates frailty, leading to worse health outcomes including earlier mortality (22). Frailty was not significantly associated with infection persistence or resistance in the 45% of patients in our study classified as moderately to severely frail. The limited size of the patient cohort may attribute to the lack of significance that was determined.
Diabetes is an additional condition that is associated with a higher prevalence of oral candidiasis and oral symptoms like dry mouth (23). Higher concentrations of glucose in saliva and blood can enable the pathogenic switch of Candida spp. that can turn into an invasive infection (24). One study found in diabetes patients that Candida species have higher enzymatic activity (hemolytic and phospholipases) which can break down membranes and invade epithelial tissues (24). In our cohort, 18 of the 57 patients had diabetes, which could increase their susceptibility to chronic and invasive infection.
4.3 Follow-up outcomes
The sign of white plaques trended towards being cleared up by the follow-up exhibiting that pseudomembranous infections can be easier to adequately treat. Coated tongue and taste disturbance symptoms also trended similarly which correlates well with the overall presentation of this infection type. In this case, the topical antifungals are quite effective. The odds of having no Candida infection at the follow-up was higher with a coated tongue present possibly because this symptom can occur due to other reasons like poor oral hygiene or xerostomia (25–27).
Alternatively, erythematous candidiasis with symptoms like angular cheilitis can be more difficult to treat and can occur chronically (28). The significant odds of persistent Candida infection for this symptom and redness are understandably correlated as one of the key features of angular cheilitis is the redness and cracking at the edges of the mouth. In these cases, the clinician may not accurately diagnose the presence of oral candidiasis and topical antifungals are not sufficient to fully resolve the infection. Prescribing a systemic antifungal would be a better choice for C. albicans strains. If the infection contains intrinsically resistant species like N. glabratus or P. kudriavzevii, then an alternate prescription is required such as an echinocandin. Nakaseomyces glabratus and Pichia kudriavzevii have been reclassified taxonomically, but they have still been included in this analysis alongside other Candida species for consistency with historical literature. Both species are clinically relevant as fungal pathogens that cause oral candidiasis.
There was no significant correlation between the type of Candida species and whether the infection was resolved at the two-week follow-up. This corroborates that most C. albicans are susceptible to antifungals and do not have innate resistance to the azole or polyene drug class (2). For N. glabratus, there were 5 out of 6 total N. glabratus infections still present at the follow-up, likely due to its intrinsic azole resistance. Notably, Candida parapsilosis presented in three follow up infections and none were identified at baseline. This suggests a mycobiome shift during antifungal treatment that enabled this species to colonize the infection over C. albicans. Recent studies indicate the increasing emergence of fluconazole-resistant C. parapsilosis strains when previously this species was typically susceptible to first-line treatment (29, 30).
One of the cleared infections with initial C. albicans and N. glabratus strains instead presented with another fluconazole-resistant species P. kudriavzevii at the follow up. Additionally, some patients can have numerous pathological Candida species comprising the infection, which further emphasizes the usefulness of a sensitive and quick point-of-care species identification to prescribe antifungal treatment that can treat the entire infection.
4.4 Conclusions and future directions
This study's featured strengths include real world setting, longitudinal design and comprehensive characterization of patients and clinical Candida samples. The data contributes to filling in the gaps of knowledge specifically for Northern Ontario cohorts. Limitations include the sample size and wide range of medical history which made it more difficult to ascertain health factors as indicators for oral candidiasis Adequate sample numbers in future studies could enable segregation of participants based on cancer types or other immunocompromising conditions. The study's applications are also limited by geographic location, as it is well known that the prevalence of different Candida species can vary based on this factor. For both Canada and the United States, Candida albicans is usually cited as the most common pathogenic species followed by Nakaseomyces glabratus (31–33). Furthermore, the limited number of persistent cases at follow-up reduced the statistical power of the analysis. Again, higher sample numbers could better indicate if there are clinical signs and symptoms that significantly correlate with less common species that display more antifungal resistance. This is of particular importance considering the rising incidence of uncommon Candida spp. and its impact on higher mortality (34). Also, numerous patients exhibiting signs and symptoms of infection had only normal flora identified. Indeed, numerous disorders like Sjorgen disease can present with similar features that are not directly a result of Candida overgrowth (35). Future studies should consider a more holistic approach for studying the oral microbiome which may reveal what other organisms are involved in maintaining normal floral homeostasis.
In summary, our study emphasizes the variation in presentation of oral candidiasis and the need for molecular diagnostic tools that can aid the physician in accurate diagnosis. Physicians need to consider a host of factors when treating patients showing signs or symptoms to prescribe the appropriate antifungal. Furthermore, there are Candida species with innate resistance to first line agents as well as strains with acquired resistant mutations that are difficult to pre-emptively identify without microbiological culturing and testing (2). Current methods for identifying oral candidiasis require a skilled lab technician to culture strains from a swab and then using the VITEK® assay which identifies fungal species based on a mass-spectrometry protein profile (2).
When patients presented with Candida albicans as the primary infection, the standard fluconazole treatment typically worked sufficiently. Erythematous infections with symptoms like angular cheilitis and oral redness trended towards persisting, especially when treated with topical agents like nystatin or Nizoral. A systemic treatment of fluconazole may have been more effective. However, intrinsically resistant species like Nakaseomyces glabratus (formerly known as Candida glabrata) were not effectively treated with fluconazole. In fact, 83% of Nakaseomyces glabratus infections (5/6) persisted at the follow-up and required an alternate antifungal treatment to be prescribed. The clinical indicators of oral candidiasis cannot distinguish between these various species and molecular species characterization would be required to identify antifungal-resistant species.
The development of a point-of-care species identification could enable clinicians to more accurately assess Candida infections and prescribe the appropriate treatment. Other species that are resistant to fluconazole include Pichia kudrivzevii (formerly known as Candida krusei) and the recently emerged multi-drug resistant Candida auris. More than 95% of P. kudriavzevii isolates and about 90% of C. auris strains are typically resistant to fluconazole (36, 37). Though Candida auris has not yet been identified in the oral cavity of a human patient, it was retrieved from the oral cavity of a dog in Kansas (38). Being able to accurately identify this species along with the more expected Candida species would be helpful for monitoring the potential spread and evolving modes of transmission (39).
There are few in-depth analyses that consider the variety of oral candidiasis signs and symptoms, patient health survey data, and microbiological testing of samples. Future studies will include susceptibility testing, and molecular gene expression data for the samples collected with a focus on resistant strains. This combination of analyses will help to elucidate the pathophysiology of oral candidiasis infections in susceptible populations like immunocompromised and older adults.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Health Sciences North Research Ethics Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KC: Writing – original draft, Formal analysis, Methodology, Data curation, Conceptualization, Visualization, Investigation, Writing – review & editing. CV: Conceptualization, Methodology, Investigation, Writing – review & editing, Data curation, Funding acquisition, Writing – original draft, Resources, Visualization, Formal analysis. SS: Conceptualization, Visualization, Investigation, Writing – review & editing, Funding acquisition, Project administration, Writing – original draft, Data curation, Methodology. DB-K: Methodology, Data curation, Conceptualization, Funding acquisition, Writing – original draft, Investigation, Writing – review & editing. MK: Methodology, Writing – review & editing, Writing – original draft, Data curation, Visualization, Formal analysis. KV: Writing – original draft, Conceptualization, Investigation, Funding acquisition, Formal analysis, Writing – review & editing. VA: Writing – review & editing, Funding acquisition, Supervision, Writing – original draft, Resources, Project administration. RS: Funding acquisition, Conceptualization, Methodology, Writing – review & editing, Writing – original draft. ST: Formal analysis, Validation, Project administration, Resources, Supervision, Methodology, Writing – review & editing, Investigation, Conceptualization, Writing – original draft, Funding acquisition. DS: Conceptualization, Supervision, Funding acquisition, Writing – review & editing, Writing – original draft, Methodology, Resources, Data curation, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the following agencies and grants: 1. the Northern Ontario Academic Medicine Association (NOAMA) AFP Innovation Fund (A-22-14), 2. the Northern Ontario School of Medicine University (NOSM U) Faculty Association Research Development Award, 3. the Northern Ontario Heritage Fund Corporation (NOHFC) Internship Fund (7400852), 4. the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN-2022-03159) and 5. the Northern Cancer Foundation.
Acknowledgments
C.P.V is supported as the HSN Foundation Research Chair in Healthy Aging. We thank Logan Bach for screening and recruiting patients, obtaining samples, filling out the questionnaires with the participants and collecting chart data. We also thank Jacob Bennett, Emelie Cote, Massimo Marrone and Michael Reich for their contributions in assisting with data collection and medical chart extraction, and ethics administration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2025.1661524/full#supplementary-material
References
1. Bensadoun RJ, Patton LL, Lalla RV, Epstein JB. Oropharyngeal candidiasis in head and neck cancer patients treated with radiation: update 2011. Support Care Cancer. (2011) 19:737–44. doi: 10.1007/s00520-011-1154-4
2. Czajka KM, Venkataraman K, Brabant-Kirwan D, Santi SA, Verschoor C, Appanna VD, et al. Molecular mechanisms associated with antifungal resistance in pathogenic Candida species. Cells. (2023) 12:2655. doi: 10.3390/cells12222655
3. Lichtenstern C, Herold C, Mieth M, Brenner T, Decker S, Busch CJ, et al. Relevance of Candida and other mycoses for morbidity and mortality in severe sepsis and septic shock due to peritonitis. Mycoses. (2015) 58(7):399–407. doi: 10.1111/myc.12331
4. Yamaguchi M, Katsuno T, Iwagaitsu S, Nobata H, Kinashi H, Banno S, et al. Oral candidiasis is a significant predictor of subsequent severe infections during immunosuppressive therapy in anti-neutrophil cytoplasmic antibody-associated vasculitis. BMC Infect Dis. (2019) 19(1):664. doi: 10.1186/s12879-019-4300-0
6. Patel M. Oral cavity and Candida albicans: colonisation to the development of infection. Pathogens. (2022) 11:335. doi: 10.3390/pathogens11030335
7. Ferreira DT, da Silva PV, de Oliveira Junior HCC, Rocha KAP, da Silva DR, de Souza Pitangui N, et al. Can there be a relationship between oral candidiasis and candidemia in ICU patients? Curr Fungal Infect Rep. (2023) 17:195–201. doi: 10.1007/s12281-023-00470-4
8. Samaranayake L. Commensal oral Candida in Asian cohorts. Int J Oral Sci. (2009) 1:2–5. doi: 10.4248/ijos.08006
9. Taylor MT, Brizuela M, Raja A. Oral candidiasis. In: Raja A, Brizuela M, editors. StatPearls. Treasure Island, FL: StatPearls Publishing (2023). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK545282/
10. Vila T, Rizk AM, Sultan AS, Jabra-Rizk MA. The power of saliva: antimicrobial and beyond. PLoS Pathog. (2019) 15(11):e1008058. doi: 10.1371/journal.ppat.1008058
11. Vila T, Sultan AS, Montelongo-Jauregui D, Jabra-Rizk MA. Oral candidiasis: a disease of opportunity. J Fungi. (2020. ) 6:15. doi: 10.3390/jof6010015
12. Garcia-Cuesta C, Sarrion-Pérez MG, Bagán JV. Current treatment of oral candidiasis: a literature review. J Clin Exp Dent. (2014) 6(5):e576. doi: 10.4317/jced.51798
13. Taverne-Ghadwal L, Kuhns M, Buhl T, Schulze MH, Mbaitolum WJ, Kersch L, et al. Epidemiology and prevalence of oral candidiasis in HIV patients from Chad in the post-HAART era. Front Microbiol. (2022) 13:844069. doi: 10.3389/fmicb.2022.844069
14. Noël de Tilly A, Tharmalingam S. Review of treatments for oropharyngeal fungal infections in HIV/AIDS patients. Microbiol Res (Pavia). (2022) 13(2):219–34. doi: 10.3390/microbiolres13020019
15. Hellstein JW, Marek CL. Candidiasis: red and white manifestations in the oral cavity. Head Neck Pathol. (2019) 13:25–32. doi: 10.1007/s12105-019-01004-6
16. Patil S, Rao RS, Majumdar B, Anil S. Clinical appearance of oral Candida infection and therapeutic strategies. Front Microbiol. (2015) 6:1391. doi: 10.3389/fmicb.2015.01391
17. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ Can Med Assoc J. (2005) 173(5):489–95. doi: 10.1503/cmaj.050051
18. Alt-Epping B, Nejad RK, Jung K, Groß U, Nauck F. Symptoms of the oral cavity and their association with local microbiological and clinical findings-a prospective survey in palliative care. Support Care Cancer. (2012) 20:531–7. doi: 10.1007/s00520-011-1114-z
19. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. (2013) 381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9
20. Oh J, Robison J, Kuchel GA. Frailty-associated dysbiosis of human microbiotas in older adults in nursing homes. Nat Aging. (2022) 2:876. doi: 10.1038/s43587-022-00289-7
21. Zakaria MN, Furuta M, Takeshita T, Shibata Y, Sundari R, Eshima N, et al. Oral mycobiome in community-dwelling elderly and its relation to oral and general health conditions. Oral Dis. (2017) 23(7):973–82. doi: 10.1111/odi.12682
22. Yang M, Liu Y, Watanabe Miura K, Matsumoto M, Jiao D, Zhu Z, et al. Frailty risk patterns and mortality prediction in community-dwelling older adults: a 3-year longitudinal study. J Am Med Dir Assoc. (2025) 26(1):105359. doi: 10.1016/j.jamda.2024.105359
23. Mohammed L, Jha G, Malasevskaia I, Goud HK, Hassan A. The Interplay Between Sugar and Yeast Infections: Do Diabetics Have a Greater Predisposition to Develop Oral and Vulvovaginal Candidiasis? Cureus. (2021).
24. Nouraei H, Jahromi MG, Jahromi LR, Zomorodian K, Pakshir K. Potential pathogenicity of Candida species isolated from oral cavity of patients with diabetes mellitus. Biomed Res Int. (2021) 2021(1):9982744. doi: 10.1155/2021/9982744
25. Martins M de L da C, Barros Cd. (No) oral health in palliative care patients: predisposing factors and treatment. J Palliat Care. (2023) 40:113–9. doi: 10.1177/08258597231212305
26. Van Tornout M, Dadamio J, Coucke W, Quirynen M. Tongue coating: related factors. J Clin Periodontol. (2013) 40(2):180–5. doi: 10.1111/jcpe.12031
27. Buranarom N, Komin O, Matangkasombut O. Hyposalivation, oral health, and Candida colonization in independent dentate elders. PLoS One. (2020) 15(11 November):e0242832. doi: 10.1371/journal.pone.0242832
28. Williams D, Lewis M. Pathogenesis and treatment of oral candidosis. J Oral Microbiol. (2011) 3:5771. doi: 10.3402/jom.v3i0.5771
29. Escribano P, Guinea J. Fluconazole-resistant Candida parapsilosis: a new emerging threat in the fungi arena. Front Fungal Biol. (2022) 3:1010782. doi: 10.3389/ffunb.2022.1010782
30. Daneshnia F, de Almeida Júnior JN, Ilkit M, Lombardi L, Perry AM, Gao M, et al. Worldwide emergence of fluconazole-resistant Candida parapsilosis: current framework and future research roadmap. Lancet Microbe. (2023) 4:e470–80. doi: 10.1016/S2666-5247(23)00067-8
31. McTaggart LR, Cabrera A, Cronin K, Kus JV. Antifungal susceptibility of clinical yeast isolates from a large Canadian reference laboratory and application of whole-genome sequence analysis to elucidate mechanisms of acquired resistance. Antimicrob Agents Chemother. (2020) 64(9):10–1128. doi: 10.1128/AAC.00402-20
32. Fuller J, Dingle TC, Bull A, Shokoples S, Laverdière M, Baxter MR, et al. Species distribution and antifungal susceptibility of invasive Candida isolates from Canadian hospitals: results of the CANWARD 2011–16 study. J Antimicrob Chemother. (2019) 74:iv48–54. doi: 10.1093/jac/dkz287
34. Pinho S, Miranda IM, Costa-de-Oliveira S. Global epidemiology of invasive infections by uncommon Candida species: a systematic review. J Fungi. (2024) 10:558. doi: 10.3390/jof10080558
35. Ergun S, Çekici A, Topcuoglu N, Migliari DA, Külekçi G, Tanyeri H, et al. Oral status and Candida colonization in patients with Sjögren’s syndrome. Med Oral Patol Oral Cir Bucal. (2010) 15(2):310–5. doi: 10.4317/medoral.15.e310
36. Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front Microbiol. (2017) 7:2173. doi: 10.3389/fmicb.2016.02173
38. White TC, Esquivel BD, Rouse Salcido EM, Schweiker AM, dos Santos AR, Gade L, et al. Candida auris detected in the oral cavity of a dog in Kansas. mBio. (2024) 15(2):e0308023. doi: 10.1128/mbio.03080-23
Keywords: Nakaseomyces glabratus, Pichia kudriavzevii, Candida albicans, antifungal resistance, oral candidiasis, older adults, pseudomembranous candidiasis, erythematous candidiasis
Citation: Czajka KM, Verschoor CP, Santi SA, Brabant-Kirwan D, Kusnierczyk MH, Venkataraman K, Appanna VD, Singh R, Tharmalingam S and Saunders DP (2025) Signs and symptoms of oral candidiasis associated with health factors and resistant Candida infections in a Northern Ontario patient cohort. Front. Oral Health 6:1661524. doi: 10.3389/froh.2025.1661524
Received: 7 July 2025; Accepted: 25 August 2025;
Published: 22 September 2025.
Edited by:
Subhabrata Maiti, Saveetha Dental College and Hospitals, IndiaCopyright: © 2025 Czajka, Verschoor, Santi, Brabant-Kirwan, Kusnierczyk, Venkataraman, Appanna, Singh, Tharmalingam and Saunders. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deborah P. Saunders, RFNhdW5kZXJzQGhzbnN1ZGJ1cnkuY2E=
 Karolina M. Czajka
Karolina M. Czajka Chris P. Verschoor
Chris P. Verschoor Stacey A. Santi3
Stacey A. Santi3 Vasu D. Appanna
Vasu D. Appanna Sujeenthar Tharmalingam
Sujeenthar Tharmalingam Deborah P. Saunders
Deborah P. Saunders