- 1Periodontology Department, Faculty of Dentistry, Istanbul University, Istanbul, Türkiye
- 2Department of Oral Biology, College of Dentistry, Dow International Dental College, Karachi, Pakistan
- 3Dar Al Uloom University, Riyadh, Saudi Arabia
- 4Prosthodontis & Orthodontist & GP (PGG) Dental Clinic, Istanbul, Türkiye
- 5Wits Oral Health Centre/School of Oral Health Sciences, Faculty of Health Sciences, Gauteng Department of Health, University of the Witwatersrand, Johannesburg, South Africa
- 6Oral and Maxillofacial Surgery, College of Dentistry, Gulf Medical University, UAE Gulf Medical University, Ajman, United Arab Emirates
- 7Department of Orthodontics, Pediatric and Community Dentistry, College of Dental Medicine, University of Sharjah, Sharjah, United Arab Emirates
- 8Marigold Dental and Orthodontic Clinic, Abu Dhabi, United Arab Emirates
- 9Biomaterials Department, Faculty of Dentistry, Cairo University, Cairo, Egypt
- 10Faculty of Dentistry, Oral and Maxillofacial Surgery Department, King Abdulaziz University, Jeddah, Saudi Arabia
- 11Kenya Dental Association, Nairobi, Kenya
- 12Department of Child Dental Health, Faculty of Dentistry, Bayero University, Kano/Aminu Kano Teaching Hospital, Kano, Nigeria
- 13Haleon, Weybridge, United Kingdom
- 14Medical Affairs, Haleon, Dubai, United Arab Emirates
Dentine hypersensitivity (DH) is a common yet often overlooked oral condition that causes pain and discomfort, negatively impacting quality of life. The prevalence of DH in the Middle East and Africa (MEA) region is relatively higher than in European countries, highlighting the need for interventions to reduce the disease burden associated with DH. A systematic approach and a thorough understanding of the condition are required for proper diagnosis and management. However, the lack of specific treatment guidelines in the MEA region poses a challenge for clinicians in identifying and managing DH. To address this, an advisory board panel of 12 dental experts from 8 MEA countries developed these consensus recommendations for DH diagnosis and management. This paper presents an overview of the clinical presentation, diagnostic challenges, and management strategies specific to the MEA region. It provides evidence-based recommendations and a simplified algorithm to guide clinicians in diagnosing and managing DH effectively. The panel underscored the importance of early diagnosis, preventive education, behavioral modification, and personalized treatment interventions, including self-care home-based therapies, for optimal DH management. Additionally, the panel emphasized the need for heightened public awareness and the integration of DH education into dental professional curricula.
1 Introduction
Dentine hypersensitivity (DH) is one of the most common discomforting oral conditions that causes pain (1). It affects quality of life (QoL) of patients and if not addressed, it can become a long term problem, with symptoms potentially worsening over time and negatively impacting daily activities such as speaking, eating, drinking, and toothbrushing (1–4).
Studies indicate that DH is relatively common in the Middle East and Africa (MEA) region (1, 5–7). For instance, a large survey of dental patients across several Arab countries found that roughly one-third of individuals had experienced DH, with prevalence as high as ∼13.9% in Saudi Arabia and ∼15% in Oman (7). These rates exceed many published figures from European or Asian populations (1, 8–11). In fact, global DH prevalence estimates are highly variable, a recent meta-analysis reported values ranging from about 1.3% to 92.1% (mean ≈33%), reflecting major differences in study methods, populations, and diagnostic criteria (1). Notably, most prevalence studies have been conducted outside the MEA region (8–11), and few rigorous surveys exist locally (6, 7). Since DH symptoms often go unreported by patients and may not be specifically assessed by clinicians, many cases likely go undocumented. Thus, even the relatively high prevalence reported for the MEA probably underestimate the true burden of DH in these countries (12). In other words, the real prevalence of DH in MEA is likely higher than current estimates imply, highlighting the need for targeted interventions and further epidemiological research. Diagnosing DH requires a systematic clinical approach to rule out other sources of dental pain (such as caries or pulpitis) (13). However, the MEA region generally lacks standardized, region-specific guidelines or training for DH, making its identification and management more challenging (14). In routine practice, this has led DH being frequently under-recognized (12, 14). Improving clinician awareness and establishing clear diagnostic protocols are therefore critical steps to uncovering the true extent of DH in MEA region (14).
This paper aims to provide evidence-based recommendations for the diagnosis and clinical management of DH in the MEA region. These recommendations are intended to help oral healthcare professionals (dentists and dental hygienists) and policymakers, offering a foundation for informed, evidence-based decisions. While they are not intended to establish a mandatory standard of care, these recommendations provide valuable insights to enhance patient care and support oral health practitioners.
2 Panel composition
On June 29th, 2024, an advisory board meeting was convened in Dubai through funding and support provided by Haleon. The panel comprised 12 distinguished dental professionals from 8 countries across the MEA region (Supplementary Table 1). Panelists represented a wide range of dental specialties, including periodontics and implantology, restorative dentistry, orthodontics, pediatric and community dentistry, and oral and maxillofacial surgery. Members were selected based on their clinical expertise, academic contributions, and representation across various dental specialties, ensuring a multidisciplinary approach throughout the discussion.
The meeting aimed to address the challenges in diagnosing and managing DH in the MEA region. Panelists engaged in structured deliberations focused on the clinical presentation of DH, diagnostic barriers, and available management strategies relevant to the regional context. Their discussions were guided by a thorough evaluation of existing international guidelines, a comprehensive literature review, and their collective clinical experiences.
The outcome of this collaboration is a set of practical, evidence-informed recommendations tailored to the MEA region. These include simplified diagnostic and management algorithms to support clinicians in delivering personalized care for DH patients. The recommendations presented in this review were developed through a structured process involving expert discussions and a comprehensive review of the literature. To ensure objectivity and scientific rigor, the draft recommendations were independently reviewed by all participating experts, including those unaffiliated with the sponsor. Each expert provided feedback, and the draft was revised accordingly to reflect a balanced and evidence-based consensus. To ensure transparency and inclusivity, all the dissenting opinions and minority viewpoints were documented and were considered in the final consensus.
3 Literature search
An electronic search was conducted on PubMed, Google Scholar, and ProQuest without time restrictions, using combinations of the following basic terms and Medical Subject Headings (MeSH) keywords: “Dentine hypersensitivity”, “Dentine sensitivity”, “Tooth sensitivity”, “tooth hypersensitivity”, “root sensitivity”, teeth, tooth, dental, enamel, “dentin*”. The search was restricted to clinical studies, reviews, meta-analyses, or any existing guidelines published in English. The inclusion criteria encompassed studies involving adult patients diagnosed with DH, focusing on prevalence, etiological factors, clinical features, underlying mechanism, and diagnostic barriers. We also considered studies exploring the screening, diagnosis, treatment strategies and awareness efforts among dental professionals and the public. The exclusion criteria eliminated studies involving dental pain from other causes unrelated to DH. The search and selection process of articles is presented as Supplementary Figure 1. The potential articles were reviewed to gather information and to inform the panel on the latest relevant findings on DH and served to underpin the expert opinions. Expert opinions or recommendations were graded from A to C based on the level of associated evidence, or as a good practice point (GPP), based on the grading method adapted from National Collaborating Centre for Mental Health (Supplementary Table 2) (15).
4 Discussion
4.1 Dentine hypersensitivity remains an underdiagnosed condition with a prevalence that varies across different MEA regions
4.1.1 Prevalence
Identifying the precise proportion of the population suffering from DH posed a challenge during the literature search. Zeola et al. conducted a meta-analysis that estimated a global prevalence ranging widely from 1.3% to 92.1% (1). This broad range was attributed to variations in study characteristics, participant age, study size, and recruitment method (1). Most of the included studies focused on Europe (40%) and Asia (38%), followed by America (13%), Africa (6%), and Oceania (3%) (1). On average, DH affects about 33% of adults globally, highlighting its significance as a widespread clinical issue (1).
Studies across the MEA region are scarce, with DH prevalence ranging from 16.3% in Nigeria to 35.4% in Saudi Arabia and 49.8% in Oman (Figure 1) (7, 16–20). Variations in socio-economic status, lifestyle factors, dietary habits, oral hygiene practices, and access to healthcare services likely explain these differences. Limited awareness of proper oral health practices and inadequate healthcare infrastructure in these countries may also contribute to the observed disparities (21).
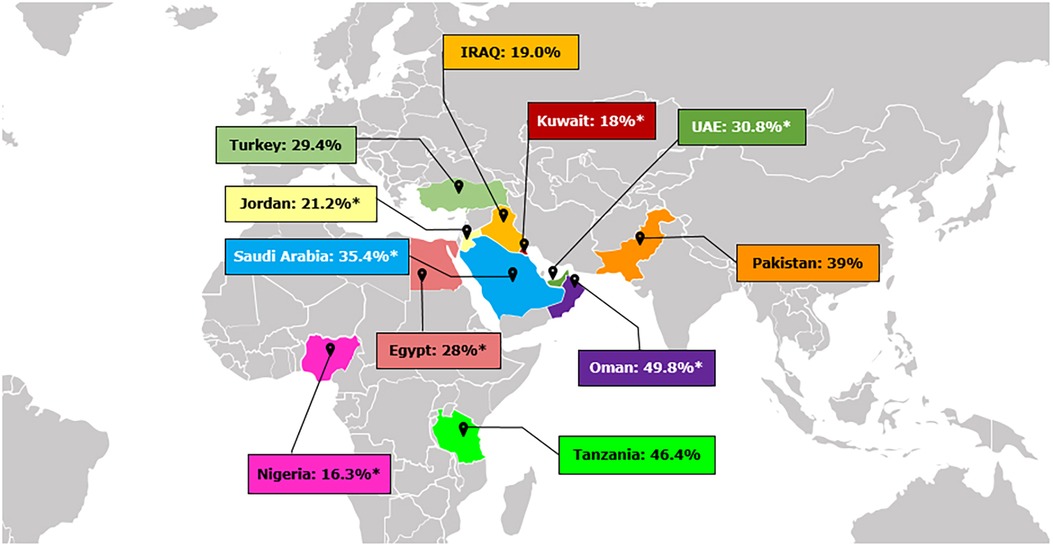
Figure 1. Prevalence of dentine hypersensitivity in different countries across Middle East and Africa (7, 16–20). *Indicates prevalence of DH measured by Schiff Scale and reflects percent people with Schiff 2 and 3.
Notably, DH shows a higher prevalence in women (5, 22) and tends to peak between 30 and 40 years of age (5). Older adults often experience reduced sensitivity, possibly due to changes in biomedical factors e.g., the development of secondary dentine and tubular sclerosis (5).
4.1.2 Etiological or predisposing factors
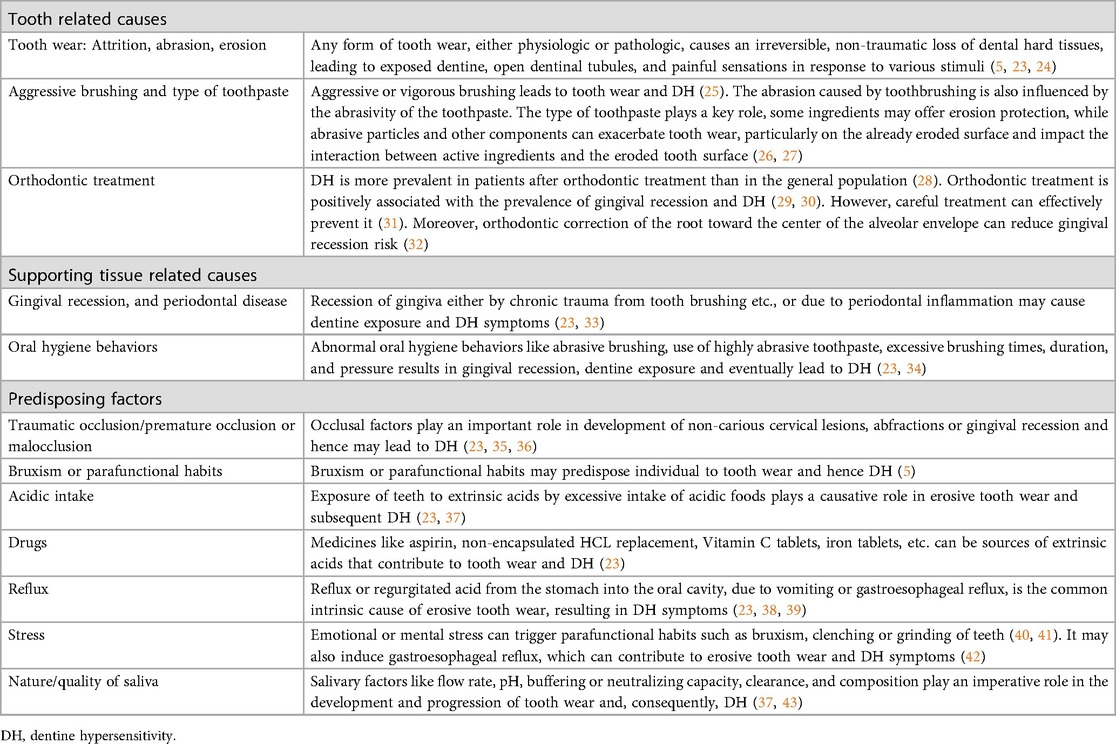
DH primarily develops from dentine exposure due to the loss of enamel or cementum, particularly in cases of gingival recession. This exposure opens the dentinal tubules to both the oral cavity and the pulp, leading to sensitivity (2, 5). The panel suggested the importance of classifying the etiology of DH for accurate diagnosis and effective treatment. They classified these etiologies into two main categories: tooth-related and soft tissue-related causes (Table 1) (2, 5, 23). Tooth related causes include tooth wear, orthodontic treatment, aggressive tooth brushing (2, 5, 23). Soft tissue-related causes encompass gingival recession, and periodontal disease (2, 5, 23). Several factors can predispose or provoke DH including traumatic or premature occlusion, malocclusion, bruxism, acidic intake, medications, gastroesophageal reflux, stress, and saliva quality (2, 5, 23).
4.1.3 Clinical features and definition
DH is a chronic or long-lasting condition, subjective in nature, characterized by acute episodes of brief and intense pain in teeth that occurs from exposed dentine (3, 44). This pain is triggered by different external and non-noxious or non-harmful stimuli (44). It generally occurs in otherwise healthy teeth wherein the pain cannot be attributed to any other dental condition and may negatively affect QoL (44).
In 2002, the Canadian Advisory Board on DH updated the initial definition of DH by Holland GR, 1997 as “short, sharp pain arising from exposed dentine in response to stimuli typically thermal, evaporative, tactile, osmotic or chemical and which cannot be ascribed to any other form of dental defect or disease” (2).
After thorough consideration of the literature and an in-depth discussion, the panel recommended adding a few elements to the Canadian Advisory Board definition. The final agreed-upon definition was “a short, sharp, acute pain, subjective in nature arising from exposed dentine in otherwise healthy teeth in response to non-noxious stimuli, typically thermal, evaporative, tactile, osmotic or chemical and which cannot be ascribed to any other form of dental defect or disease.” This definition not only challenges clinicians to consider other potential causes of pain associated with tooth sensitivity, but also emphasizes the importance of differential diagnosis as well as, acknowledges individual differences in pain response to DH.
4.1.4 Mechanisms underlying DH
The panel agreed that the most widely accepted mechanism of DH is the hydrodynamic theory proposed by Brännström (2, 5, 23). According to this theory, fluid flow within the dentinal tubules near the exposed surface increases or changes direction due to thermal, tactile, or osmotic stimuli. This alteration stimulates the baroreceptors or A-delta fibers around the odontoblasts, resulting in neural discharge. For DH to occur, the dentinal tubules must be open at both the surface and within the pulp (2, 5, 23). Some argued that this theory does not explain sensitivity in endodontically treated teeth. In such cases, the odontoblast receptor (OR) theory may apply. This theory suggests that exposed odontoblastic processes on the dentine surface are stimulated by various stimuli, leading to the release of neurotransmitters toward the nerve endings (23, 45).
4.1.5 Barriers of DH diagnosis
One of the main barriers in DH identified by the panel was the lack of communication between patients and their dental practitioners (46). DH often is not perceived as a serious condition. Some individuals consider DH to be an inevitable aspect of aging leading them to develop coping behaviors rather than self-reporting their symptoms (47, 48). Others may endure the sensitivity but choose not to discuss it, either due to anxiety or because they prioritize other dental concerns that they may perceive as more serious (46). Moreover, many patients remain unaware that DH is both treatable and preventable (49), while many professionals also exhibit gaps in their knowledge of its prevention, diagnosis, and management (50).
The panel suggested that clinicians initiate conversations about the impact of DH on QoL to help patients better relate to the condition. Cost and time are additional barriers (46, 51). In busy dental practices, clinicians have limited time during consultations (46). In some countries, where the majority of the population relies on the public dental facilities, patients often present with severe dental conditions alongside DH. However, DH is not typically addressed in treatment plans, as clinicians are focused on more invasive dental procedures (46, 52).
The panel emphasized the need to involve other healthcare professionals such as dental hygienists in the consultation and diagnosis of DH as a mean to reduce the cost barrier. Additionally, the panel highlighted the lack of formal training on DH diagnosis and consequently the inadequate clinical skills of the clinicians, as additional factors that contribute to underdiagnosis of DH (14). Studies in the MEA region are needed to specifically identify the barriers to DH diagnosis and suggest more effective methods of patient-clinical communication to address the reported high prevalence of DH in the region (14).
4.2 Early, periodic, and comprehensive screening
4.2.1 Screening
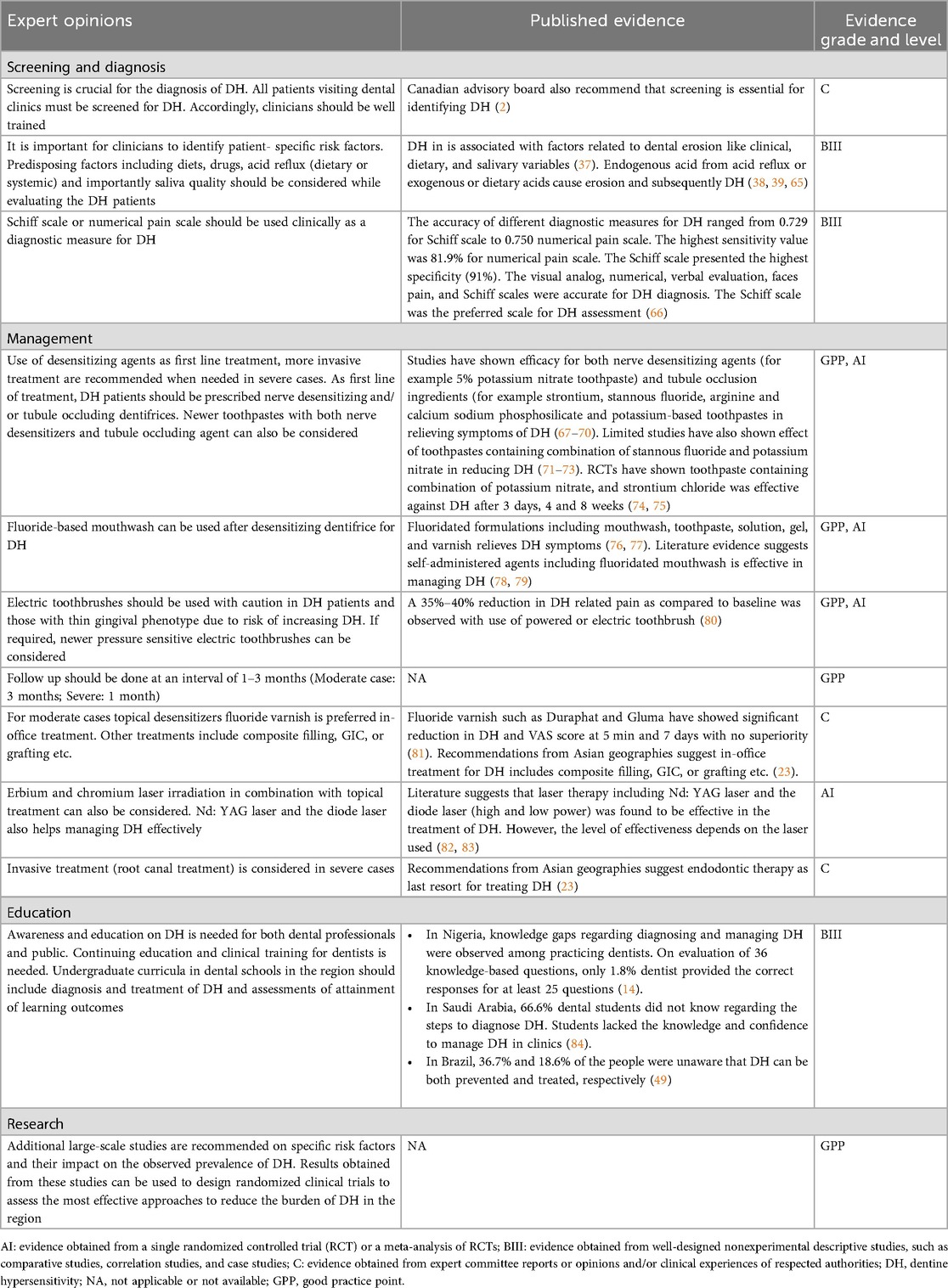
Many patients with DH may not self-report their condition, leading to overlooked early detection and prevention. The panel advised that screening for DH should be performed for all patients visiting dental clinics (2, 23). Considering the limited time during consultations, the panel suggested asking one screening question, such as “do you have sensitive teeth?” Clinicians can then proceed based on the patient's response. The panel also recommended pre-consultation aid, such as questionnaires that the patient can respond to while waiting to see his/her dentist, or before they arrive for their appointment (53). These responses can be reviewed by the clinician to maximize the use of appointment time. This approach could be economically beneficial in countries where the majority of patients visit public oral health facilities. Additionally, the panel also emphasized the importance of patient education as a mean to prevent the occurrence of DH or reduce its burden on affected patients. Equally important is ensuring that dental professionals are adequately trained to recognize and diagnose DH. Several studies have shown that knowledge gaps among clinicians, can limit accurate diagnosis and management of DH. Investigations outside the MEA region (50, 54, 55) and within MEA countries (14, 56) highlight that both undergraduate training and continuing education are essential to improve diagnostic consistency. Thus, alongside patient education, strengthening professional training is critical to ensure early and reliable detection of DH.
4.2.2 History taking
Comprehensive clinical history taking is crucial in patients complaining of DH-related pain. The panel suggested that clinicians should inquire about the type and duration of pain, its triggering factors, relieving factors, and any prior treatments the patient may have received (2, 23). Moreover, a comprehensive medical history taking is necessary to accurately identify DH underlying risk factors. For example, inhalation therapies for asthma can reduce salivary buffering capacity and flow, thereby leading to increased erosive wear and consequently DH (57). Clinicians should also consider the patient's dietary habits (e.g., frequent intake of acidic foods and beverages such as carbonated drinks, citrus fruits, and vinegar-containing products), lifestyle factors (e.g., stress levels that may contribute to bruxism or clenching, smoking, alcohol consumption), and oral health behaviors (e.g., aggressive or improper toothbrushing techniques, frequency and duration of brushing, use of abrasive whitening toothpastes, or overuse of dental floss or interdental aids). Each of these factors can predispose to enamel/dentine wear or gingival recession and thereby contribute to DH (2, 23).
DH episodes are often short-lived, and patients may develop coping behaviors, such as, avoiding cold foods or eating and drinking from the opposite side of the mouth (58). The unpleasant symptoms of DH impacts QoL, making QoL assessment essential in clinical practice, as it can influence the treatment plan (4, 59). The Dentine Hypersensitivity Experience Questionnaire (DHEQ) is a widely accepted and validated tool that evaluates the subjective impact of DH in five areas of life: functional restrictions (e.g., slower eating), coping behaviors (e.g., warming food and drinks), emotional impact (e.g., annoyance), social impact (e.g., difficulty conversing), and personal identity impacts (e.g., feeling old) (60). While the original version of DHEQ has 48 items and can be time consuming limiting its clinical utility, a shorter 15-item version (DHEQ-15) was developed for use in general dental practices (60). However, the panel raised concern about its applicability in routine consultations due to time constraints. Consequently, there is a recognized need to further simplify DHEQ to enhance its utility in everyday clinical practice.
4.2.3 Clinical examination and exclusion of other conditions
To properly diagnose DH, it is essential to exclude other conditions that cause similar pain, e.g., cracked tooth, dental caries, root resorption, defective or fractured restorations, post-operative sensitivity (from restorative, periodontal and bleaching procedures), dental trauma, occlusal trauma, cervical plaque, gingivitis, periodontal disease, marginal leakage, pulpitis, enamel or dentine hypoplasia, hypomineralization and other pathologic conditions like cysts (2, 13, 23, 52). In addition to visual and tactile examination, various diagnostic methods can be employed to exclude other conditions, including percussion, palpation, vitality test, radiographic examination, and transillumination (2, 13, 23, 52).
4.2.4 Diagnostic measures
Owing to its subjective nature, quantifying DH, and its impact on patients in clinical setting can be challenging. Therefore, clinicians must rely on the patient's self-report of pain. Stimulus-based diagnostic tests are valuable for diagnosing DH (2, 13, 23, 52). The panel recommended starting with a simple mechanical or tactile stimulus test. This involves using a sharp explorer or conventional or pressure-sensitive dental probe to gently move over the exposed dentine in a mesio-distal direction while observing the patients' response (2, 13, 23, 52).
As a next step, the panel recommended performing a cold air blast test using three-way syringe, which is commonly used by clinicians and in clinical studies (1, 14). The panel agreed that Schiff scoring, which is response-based assessment, requires minimum time to implement clinically. The panel also recommended using a numerical rating scale, where patients can self-assess DH pain from 0 to 10, in which 0 would indicate no pain and 10 indicates severe pain. The Schiff scale has been reported to have high specificity (91%), while the numerical scale has high sensitivity (81.9%) (61). The numerical rating can be particularly useful for assessing treatment progress and follow-up; a lower rating during follow-up visits would indicate an improvement in DH symptoms.
The panel also suggested exploring the aid of clinical photographs, intraoral scanners and AI based intraoral scanning software in future for DH diagnosis (62). These technologies may serve as efficient pre-screening tools that can also save time (62).
To assess and record DH, the panel suggested documenting the severity, location (localized or generalized) and whether it is primary (no previous treatment) or secondary (prior treatment). The findings should be recorded clearly so that other clinicians can easily understand and localize the problem. Imperial charting systems, such as periodontal charting for mobility and furcation involvement, could be adapted for recording DH (63). Currently, there is no objective classification system for DH severity analogous to those available for gingival recession, thus clinicians must rely on their judgment to classify or quantify DH. Manual or electronic health record systems, like Axium or Doc 32, which offer customization, can be utilized (64). These systems should be adaptable to the clinical setting and include an editable section for clinicians to write recommendations or treatment notes. Proper documentation of DH findings allows for comparison between initial and subsequent visits, facilitating the monitoring of treatment progress.
4.3 Optimizing DH management with a simplified algorithm is essential
Treatment for DH should follow a step-by-step approach, beginning with simple strategies such as patient education and behavior modification, followed by self-care home-based treatments, and progressing to more complex in-office treatment as needed (13, 23). The complexity as well as the invasiveness of treatment strategies should be chosen based on the severity or extent of DH. The Canadian Advisory Board on DH has previously recommended reversible procedures to be employed before nonreversible ones depending on the severity and extent of each condition (2). A simplified algorithm has been developed to provide evidence-based practical guidance for clinicians (Figure 2). Clinicians should consider the following approaches for the prevention and management of DH (Table 2).
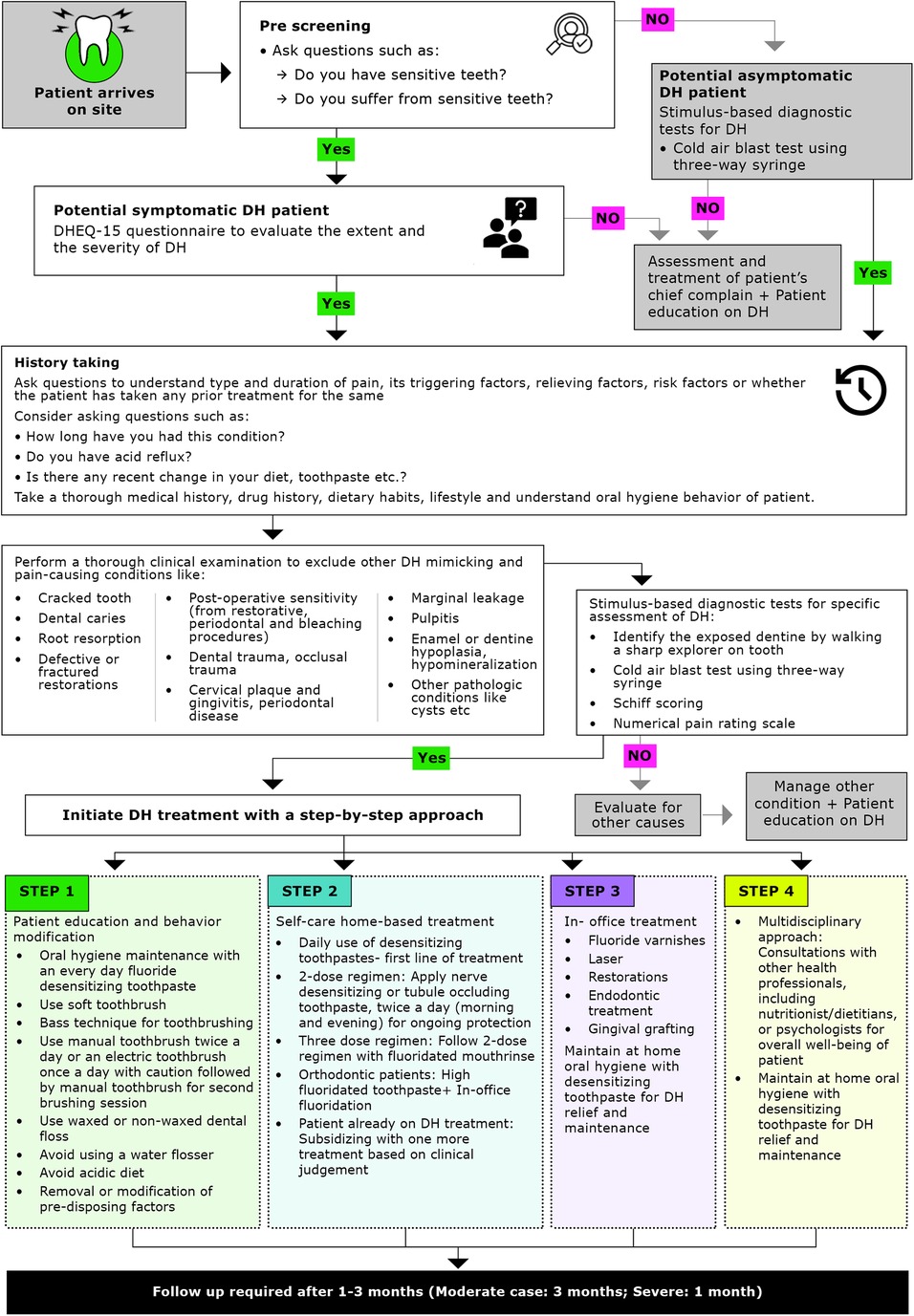
Figure 2. Simplified algorithm for management of dentine hypersensitivity. DH, dentine hypersensitivity; DHEQ, dentine hypersensitivity experience questionnaire.
4.3.1 Patient education and behavior modification
A dedicated counseling session for patient education and behavior modification is essential for the prevention and management of DH. The panel recommended educating patients on behavioral modifications, focusing on the following areas:
4.3.1.1 Oral hygiene maintenance and toothbrushing
The panel emphasized the importance of maintaining good oral hygiene and suggested training patients on proper brushing techniques, including how to position the brush, apply pressure, and effectively clean their teeth. The Bass technique was agreed as the correct method for brushing, as horizontal brushing may lead to increased gingival recession, cervical abrasions and exacerbate DH (85). It is advised that patients use a soft-bristled toothbrush and avoid highly abrasive teeth whitening toothpaste, as these products often contain harsh, coarse granules that can increase DH (2, 23). Regular dental check-ups and monitoring of plaque indices are also essential for effective management (2, 23).
4.3.1.2 Electric toothbrush
The panel emphasized that when recommending an electric toothbrush, it is important to advise the patient to use it gently and passively, without applying additional pressure as is done with manual toothbrushing (86, 87). The brush tip is designed to do the work, and any additional pressure can lead to gingival recession. An electric toothbrush should be used cautiously in people with DH or a thin gingival phenotype, as additional pressure may aggravate DH or cause gingival recession. The use of a pressure-sensitive toothbrush can help control the amount of pressure applied (88). The panel prefers that patients with DH use an electric toothbrush once daily to help minimize brushing pressure. For the second daily brushing, patients may use a manual toothbrush, or alternatively, use a manual toothbrush for both brushing sessions.
4.3.1.3 Interdental aid
The panel advised against the use of water flossers in people with DH, as the water spray can act as a thermal stimulus, triggering DH pain. Instead, they recommended the use of waxed or non-waxed floss (23).
4.3.1.4 Dietary habits
It is essential to educate patients on avoiding acidic foods and beverages such as fizzy drinks, acidic beverages, lemon juice with vinegar etc. (2, 23). Clinicians should advise patients to limit frequent intake of highly acidic, low-mineral foods and drinks that increase erosive wear and may exacerbate DH, for example, carbonated soft drinks (including diet sodas), energy/sports drinks, fruit juices and citrus fruits, sour candies/lozenges, vinegar-based foods, and frequent sipping of acidic beverages. In contrast, calcium/phosphate-rich foods (milk, cheese, plain yogurt) are less erosive and can aid remineralization (23, 74–89).
When complete avoidance is impractical, recommend harm-reduction measures: drink acidic beverages with meals, use a straw, avoid swishing, rinse with water or a fluoride mouthwash after exposure, chew sugar-free gum to stimulate saliva, consume a calcium-rich snack afterward, and delay toothbrushing for 30–60 min after acidic intake. These strategies help balance nutritional benefits (e.g., fruit consumption) with enamel protection (2, 23).
4.3.1.5 Removal of predisposing factors
Selective occlusal correction to resolve premature contacts should be considered with caution in cases of trauma from occlusion or premature contacts. For individuals with bruxism or other parafunctional habits, the use of an occlusal splint night guard is recommended. Any other predisposing factor identified during history taking and clinical evaluation should be addressed, removed, or modified as necessary (2, 23).
4.3.2 Self-care home-based treatment
Desensitizing agents are the most widely used among all available self-care home-based regimes (Figure 3). The daily use of desensitizing toothpastes is a non-invasive, inexpensive, and effective first-line treatment for DH. Desensitizers with tubule-occluding agents, such as strontium acetate, stannous fluoride, and more recently calcium sodium phosphosilicate, reduce fluid flow across the dentinal tubules by blocking them while nerve desensitizers containing potassium salts suppress nerve stimulation associated with intratubular fluid movements (90). Toothpastes containing stannous chloride can also reduce DH and improve oral health-related QoL (91, 92). However, no clinical studies have assessed the desensitizing effect of this formulation (91, 93, 94). Desensitizing toothpaste can be applied with a toothbrush or directly on sensitive areas using a fingertip before brushing (95). Patients should be advised to continue using desensitizing toothpaste even after symptoms have subsidized. Discontinuing may result in the recurrence of DH, possibly due to the gradual reopening of dentine tubules after stopping the use of desensitizing toothpaste (96). For patients undergoing orthodontic treatment, high-fluoridated toothpaste, along with in-office fluoridation should be considered.
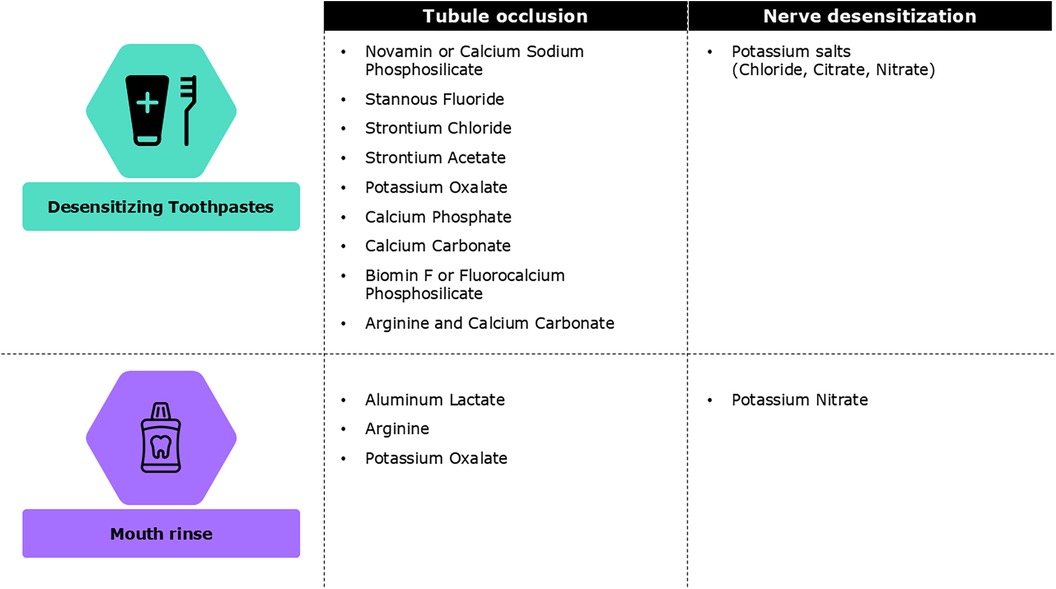
Figure 3. Different types of self-care home based treatments for dentine hypersensitivity (23).
If the patient is already undergoing treatment for DH, clinical judgement should guide whether to continue the current treatment or adjust it. Adding a fluoride mouthwash or considering an in-office treatment could be beneficial (23).
4.3.3 In- office treatment
In cases where there is no improvement in DH symptoms despite self-care home-based treatments, or if the symptoms are worsening, in-office treatments may be necessary. These can include the professional application of fluoride varnishes, topical desensitizing agents that contain glutaraldehyde and hydroxyethyl methacrylate (HEMA), laser therapy, and restorative interventions when indicated. Highly-fluoridated toothpastes are typically prescription home products and should not be listed as in-office materials. Fluoride varnishes and glutaraldehyde/HEMA products have been widely reported as effective in clinical studies (97–81). Clinicians should note that some in-office desensitizing agents may affect the bond strength of subsequently placed resin composites; when adhesive restorations are planned, follow manufacturer recommendations, consider delaying bonding procedures, or perform appropriate surface management to minimise bonding interference (98). In cases of extreme sensitivity, local anaesthesia may be used to apply desensitizing materials. For severe cases, endodontic treatment or extraction may be considered. If gingival recession is present, any existing root caries should be treated first. In cases with aesthetic concerns, gingival grafting may be considered (2, 13, 23, 52, 81).
4.3.4 Multidisciplinary approach
To promote the overall wellbeing of the patient, consultations with other health professionals, including nutritionist/dietitians, or psychologists (to address stress, which is one of the causative factors of DH) must be considered.
4.3.5 Follow-up
Regular follow-up for DH patients is essential for achieving better patient outcomes. Follow-up must be scheduled every 2–3 months. However, in severe cases, monthly follow-ups may be necessary. It is not mandatory for a patient to visit the clinic in person for every follow-up; this can also be managed through telephonic interviews conducted by dental hygienists or assistants.
4.4 Raising DH awareness among dentists and the public
4.4.1 Education for dental professionals
The panel highlighted concerns regarding the education, awareness, and clinical application of DH among dental students (Table 2). They emphasized that DH should be integrated into the curricula of public health, endodontic, and periodontal specialties, adopting an interdisciplinary approach. It was suggested that DH be considered an essential component of dental pedagogy.
Tutorial-based education on DH alone is insufficient, its importance and clinical application should be conveyed through problem-based learning. As clinical education typically begins later in the curriculum, DH should be introduced to students in their later years. Implementing a competency-based approach to DH education is recommended. Both dental hygienists and clinicians should be trained in managing DH. Scientific organizations could play a crucial role by discussing DH at national and international conferences or symposiums to raise awareness and advance knowledge.
4.4.2 Education for public
The panel discussed the importance of public education and awareness in the prevention and management of DH (Table 2). Increased awareness can lead to greater seriousness about the condition, which in turn raises the demand for DH management. It is crucial to advertise on social media with clear messages that DH treatment is effective, rather than merely discounting the symptoms. The panel also suggested that dental clinics should display anatomical charts to raise awareness about healthy gums and teeth, as well as DH in relation to gingival recession. Educational materials should be made available on dental clinics' websites or through health associations to further public awareness.
4.5 Limitations
The expert panel included representatives from eight MEA countries, the literature cited in this review is predominantly derived from studies conducted outside the MEA region. The limited availability of region-specific data across many MEA contexts presents a broader challenge. While prevalence figure from countries such as Nigeria, Saudi Arabia, and Oman are included, some MEA nations remain underrepresented. We acknowledge that the consensus-based recommendations may not fully capture the diversity and unique characteristics of all regional populations. This limitation underscores the need for more locally driven research to strengthen the evidence base and ensure future guidelines are more representative of the entire MEA region.
5 Conclusion
DH is an increasingly prevalent clinical condition that affects QoL. The expert panel's recommendations provide a structured, evidence-based framework for diagnosing and managing DH in the MEA region. By offering a simplified diagnostic and treatment algorithm, the guidelines address the unique factors contributing to DH, such as dietary habits, oral behaviors.
The expert panel underscores the importance of identifying underlying predisposing or etiologic factors to ensure accurate diagnosis and effective management. Given the subjective nature of DH and the tendency for symptoms to be underreported, routine screening all dental patients is recommended. A comprehensive clinical history and thorough clinical examination are vital to differentiate DH from other conditions with similar presentations. Stimulus-based diagnostic tests, along with clear documentation of key findings (e.g., location and severity), support accurate diagnosis and facilitate appropriate follow-up.
Management of DH should follow a stepwise approach, beginning with the elimination of modifiable risk factors and the promotion of optimal oral hygiene practices. First-line treatment should include home-based self-care strategies such as twice-daily use of desensitizing toothpaste (tubule occluding or nerve desensitizers) and/or fluoridated mouthwash. For more persistent or severe cases, escalation to in-office interventions may be necessary. A multidisciplinary approach, engaging dental specialists, nutritionists, and other healthcare professionals can support holistic patient care, addressing both oral and systemic contributors to DH.
Overall, the panel emphasizes the importance of early diagnosis, preventive education, and tailored personalized interventions to ensure effective DH management. Integration of DH awareness into both public health initiatives and professional dental education is crucial to reducing DH prevalence and improving QoL for affected individuals across the MEA region.
Author contributions
AC: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. AS: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. AA: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. AG: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. EP: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. HM: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. MA: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. NB: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. NH: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. SO: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. TT: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. TA: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. CP: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. AH: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. JT: Conceptualization, Data curation, Investigation, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The advisory board meeting comprising 12 experts from 8 countries in the MEA region was convened in Dubai through funding and support provided by Haleon. The open access fee was funded by Haleon.
Acknowledgments
We gratefully acknowledge the support of “Center for advanced professional practices-CAPP” to organize the advisory board meeting and Dr. Shaista Siddiqui for moderating the meeting. Professional writing and editorial support were provided by Dr. Swati Gupta, Dr. Abhijeet Dhiman, Dr. Richa Kumari and Nitu Bansal from Knowledge Centre, under the direction of the authors, following an expert panel meeting supported by Dr. Swati Gupta, Dr. Ankita Srivastava, Dr. Kritika Sharma and Nitu Bansal from Knowledge Centre, WNS Global Services and funded by Haleon.
Conflict of Interest
Authors JT, AH and CP are employees of Haleon.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2025.1663984/full#supplementary-material
References
1. Zeola LF, Soares PV, Cunha-Cruz J. Prevalence of dentin hypersensitivity: systematic review and meta-analysis. J Dent. (2019) 81:1–6. doi: 10.1016/j.jdent.2018.12.015
2. Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. (2003) 69(4):221–6.12662460
3. Idon PI, Sotunde OA, Ogundare TO. Beyond the relief of pain: dentin hypersensitivity and oral health-related quality of life. Front Dent. (2019) 16(5):325–34. doi: 10.18502/fid.v16i5.2272
4. Bekes K, Hirsch C. What is known about the influence of dentine hypersensitivity on oral health-related quality of life? Clin Oral Investig. (2013) 17(Suppl 1):S45–51. doi: 10.1007/s00784-012-0888-9
5. Splieth CH, Tachou A. Epidemiology of dentin hypersensitivity. Clin Oral Investig. (2013) 17 (Suppl 1):S3–8. doi: 10.1007/s00784-012-0889-8
6. Katirci G, Celik EU. The prevalence and predictive factors of dentine hypersensitivity among adults in Turkey. BMC Oral Health. (2023) 23(1):474. doi: 10.1186/s12903-023-03137-1
7. Awad MA, Kassas DE, Harthi LA, Abraham S, Al-Khalifa K, Khalaf M, et al. Dentine hypersensitivity and dentine exposure in Arab patient populations. J Oral Rehabil. (2020) 47(4):473–9. doi: 10.1111/joor.12927
8. Wang Y, Que K, Lin L, Hu D, Li X. The prevalence of dentine hypersensitivity in the general population in China. J Oral Rehabil. (2012) 39(11):812–20. doi: 10.1111/j.1365-2842.2012.02334.x
9. Cunha-Cruz J, Wataha JC, Heaton LJ, Rothen M, Sobieraj M, Scott J, et al. The prevalence of dentin hypersensitivity in general dental practices in the northwest United States. J Am Dent Assoc. (2013) 144(3):288–96. doi: 10.14219/jada.archive.2013.0116
10. West NX, Sanz M, Lussi A, Bartlett D, Bouchard P, Bourgeois D. Prevalence of dentine hypersensitivity and study of associated factors: a European population-based cross-sectional study. J Dent. (2013) 41(10):841–51. doi: 10.1016/j.jdent.2013.07.017
11. Tengrungsun T, Jamornnium Y, Tengrungsun S. Prevalence of dentine hypersensitivity among Thai dental patients at the faculty of dentistry, Mahidol university. Southeast Asian J Trop Med Public Health. (2012) 43(4):1059–64.23077831
12. Izhar F, Nazir MA, Majeed A, Almas K. A study of dentists about their knowledge and practice of dentine hypersensitivity. Eur J Dent. (2019) 13(4):540–6. doi: 10.1055/s-0039-1697110
13. Liu X-X, Tenenbaum HC, Wilder RS, Quock R, Hewlett ER, Ren Y-F. Pathogenesis, diagnosis and management of dentin hypersensitivity: an evidence-based overview for dental practitioners. BMC Oral Health. (2020) 20(1):220. doi: 10.1186/s12903-020-01199-z
14. Oderinu OH, Sede MA, Oginni AO, Adegbulugbe IC, Uti OG, Olusile AO, et al. Knowledge, diagnosis and management of dentine hypersensitivity: a national survey of dentists in Nigeria. Int Dent J. (2017) 67(5):287–93. doi: 10.1111/idj.12302
15. National Collaborating Centre for Mental Health (UK). Self-Harm: The Short-Term Physical and Psychological Management and Secondary Prevention of Self-Harm in Primary and Secondary Care. Leicester, UK: British Psychological Society (UK), National Institute for Health and Care Excellence: Guidelines (2004).
16. Demirci M, Karabay F, Berkman M, Özcan İ, Tuncer S, Tekçe N, et al. The prevalence, clinical features, and related factors of dentin hypersensitivity in the Turkish population. Clin Oral Investig. (2022) 26(3):2719–32. doi: 10.1007/s00784-021-04245-4
17. Savage KO, Oderinu OH, Oginni AO, Uti OG, Adegbulugbe IC, Dosumu OO. Dentine hypersensitivity and associated factors: a Nigerian cross-sectional study. Pan Afr Med J. (2019) 33:272. doi: 10.11604/pamj.2019.33.272.18056
18. Carneiro LC, Minja AA. Prevalence of dentine hypersensitivity among adult dental patients in Dar-es-salaam, Tanzania. Dental Res Oral Health. (2020) 3(2):74–82. doi: 10.26502/droh.0022
19. Neelofar N, Mahirah I, Khan JMI, Pashmina N, Ambereen H, Aqsa K, et al. Rate and factors associated with dentine hypersensitivity among Pakistani patients: a cross sectional study: incidence of dentine hypersensitivity among Pakistani patients. Pak BioMed J. (2022) 5:219–23. doi: 10.54393/pbmj.v5i6.586
20. Kadhim MA. Prevalence and associated factors of dentin hypersensitivity in IRAQI students. Azerb Med J. (2023) 63(05):1.
21. Morgano SM, Doumit M, Shammari KFA, Al-Suwayed A, Al-Suwaidi A, Debaybo D, et al. Burden of oral disease in the Middle East: opportunities for dental public health. Int Dent J. (2010) 60(3S1):197–9. doi: 10.1016/S0020-6539(20)34125-3
22. Colak H, Aylikci BU, Hamidi MM, Uzgur R. Prevalence of dentine hypersensitivity among university students in Turkey. Niger J Clin Pract. (2012) 15(4):415–9. doi: 10.4103/1119-3077.104514
23. Grover V, Kumar A, Jain A, Chatterjee A, Grover HS, Pandit N, et al. ISP good clinical practice recommendations for the management of dentin hypersensitivity. J Indian Soc Periodontol. (2022) 26(4):307–33. doi: 10.4103/jisp.jisp_233_22
24. Nadine S, Amaechi BT, David B, Rabelo BMA, Saads CT, Carolina G, et al. Terminology of erosive tooth wear: consensus report of a workshop organized by the ORCA and the cariology research group of the IADR. Caries Res. (2020) 54(1):2–6. doi: 10.1159/000503308
25. Addy M. Tooth brushing, tooth wear and dentine hypersensitivity–are they associated? Int Dent J. (2005) 55(4 Suppl 1):261–7. doi: 10.1111/j.1875-595X.2005.tb00063.x
26. Wiegand A, Schlueter N. The role of oral hygiene: does toothbrushing cause harm? Monogr Oral Sci. (2025) 33:32–7. doi: 10.1159/000543551
27. Wiegand A, Schlueter N. The role of oral hygiene: does toothbrushing harm? Monogr Oral Sci. (2014) 25:215–9. doi: 10.1159/000360379
28. Dalmolin AC, Finkler BC, Almeida CV, Bechtold LB, Silva KR, Centenaro GG, et al. Prevalence of dentin hypersensitivity after orthodontic treatment: a cross-sectional study. Am J Orthod Dentofacial Orthop. (2023) 164(3):431–40. doi: 10.1016/j.ajodo.2023.02.018
29. Slutzkey S, Levin L. Gingival recession in young adults: occurrence, severity, and relationship to past orthodontic treatment and oral piercing. Am J Orthod Dentofacial Orthop. (2008) 134(5):652–6. doi: 10.1016/j.ajodo.2007.02.054
30. Lin YH, Gillam DG. The prevalence of root sensitivity following periodontal therapy: a systematic review. Int J Dent. (2012) 2012:407023. doi: 10.1155/2012/407023
31. Jati AS, Furquim LZ, Consolaro A. Gingival recession: its causes and types, and the importance of orthodontic treatment. Dental Press J Orthod. (2016) 21(3):18–29. doi: 10.1590/2177-6709.21.3.018-029.oin
32. Godtfredsen LM, Mette R, Birte M. The role of orthodontics in the repair of gingival recessions. Am J Orthod Dentofacial Orthop. (2020) 157(1):29–34. doi: 10.1016/j.ajodo.2019.01.023
33. Fukumoto Y, Horibe M, Inagaki Y, Oishi K, Tamaki N, Ito H-O, et al. Association of gingival recession and other factors with the presence of dentin hypersensitivity. Odontology. (2014) 102(1):42–9. doi: 10.1007/s10266-012-0099-5
34. Kumar S, Gopalkrishna P, Syed AK, Sathiyabalan A. The impact of toothbrushing on oral health, gingival recession, and tooth wear-a narrative review. Healthcare (Basel). (2025) 13(10). doi: 10.3390/healthcare13101138
35. Duangporn D, Arthur M, Hong PP, Man LEC, Chun-Hung C. Occlusal stress is involved in the formation of non-carious cervical lesions. A systematic review of abfraction. Am J Dent. (2017) 30(4):212–20.29178704
36. Prasad DK, Shetty NS, Solomon EGR. The influence of occlusal trauma on gingival recession and gingival clefts. J Indian Prosthodont Soc. (2013) 13(1):7–12. doi: 10.1007/s13191-012-0158-1
37. Shitsuka C, Mendes FM, Corrêa MSNP, Leite MF. Exploring some aspects associated with dentine hypersensitivity in children. ScientificWorldJournal. (2015) 2015:764905. doi: 10.1155/2015/764905
38. Chakraborty A, Anjankar AP. Association of gastroesophageal reflux disease with dental erosion. Cureus. (2022) 14(10):e30381. doi: 10.7759/cureus.30381
39. Wilder-Smith CH, Materna A, Martig L, Lussi A. Longitudinal study of gastroesophageal reflux and erosive tooth wear. BMC Gastroenterol. (2017) 17(1):113. doi: 10.1186/s12876-017-0670-1
40. Rabia M, Afifa E, Naila U, Simra K, Mahgul A, Sabaiyna S. Association between stress and parafunctional habits among undergraduate healthcare students of Pakistan. J Bahria Univ Med Dental Coll. (2023) 13(02):115–20. doi: 10.51985/JBUMDC2022117
41. Almutairi AF, Albesher N, Aljohani M, Alsinanni M, Turkistani O, Salam M. Association of oral parafunctional habits with anxiety and the big-five personality traits in the Saudi adult population. Saudi Dent J. (2021) 33(2):90–8. doi: 10.1016/j.sdentj.2020.01.003
42. Wickramasinghe N, Thuraisingham A, Jayalath A, Wickramasinghe D, Samarasekara N, Yazaki E, et al. The association between symptoms of gastroesophageal reflux disease and perceived stress: a countrywide study of Sri Lanka. PLoS One. (2023) 18(11):e0294135. doi: 10.1371/journal.pone.0294135
43. Buzalaf MAR, Hannas AR, Kato MT. Saliva and dental erosion. J Appl Oral Sci. (2012) 20(5):493–502. doi: 10.1590/S1678-77572012000500001
44. Dimitrios D, Olga G, Charis B. Dentin hypersensitivity: etiology, diagnosis and contemporary therapeutic approaches—a review in literature. Appl Sci (Basel). (2023) 13(21):11632. doi: 10.3390/app132111632
45. Corrêa DG, Ballassini AH, Trindade C-NJ, Pedroso TC. Dentin hypersensitivity: expression of neuron/odontoblast receptors and release of neuropeptide in dental pulp. Braz J Oral Sci. (2025) 24:e258423. doi: 10.20396/bjos.v24i00.8678423
46. Asimakopoulou K, West N, Davies M, Gupta A, Parkinson C, Scambler S. Why don’t dental teams routinely discuss dentine hypersensitivity during consultations? A qualitative study informed by the theoretical domains framework. J Clin Periodontol. (2024) 51(2):118–26. doi: 10.1111/jcpe.13885
47. Fatima AB, Nabeela AUK, Talha N. Dentine hypersensitivity and its associated factors: a cross-sectional study conducted on patients visiting dental hospital of Karachi, Pakistan. JPDA. (2019) 148:253–6.
48. Saadaldina SA, Eldwakhly E, Alnazzawi AA, Alharbi RA, Alghamdi BK, Hammad OAA, et al. Awareness and practice of oral health measures in Medina, Saudi Arabia: an observational study. Int J Environ Res Public Health. (2020) 17(23). doi: 10.3390/ijerph17239112
49. Mosquim V, Carneiro GU, Foratori-Junior GA, Honório HM, Gillam DG, Wang L. Knowledge and attitudes on preventing and treating dentin hypersensitivity and its predicting factors: a cross-sectional study with Brazilian citizens. Eur J Dent. (2023) 17(3):855–62. doi: 10.1055/s-0042-1757905
50. Mosquim V, Zabeu GS, Jacomine JC, Santin DC, Honório HM, Wang L. Brazilian undergraduates’ and dentists’ knowledge on preventing, diagnosing and managing dentin hypersensitivity: a cross-sectional questionnaire study. Int J Dent Hyg. (2025) 23(1):3–13. doi: 10.1111/idh.12833
51. Hill KB, Chadwick B, Freeman R, O’Sullivan I, Murray JJ. Adult dental health survey 2009: relationships between dental attendance patterns, oral health behaviour and the current barriers to dental care. Br Dent J. (2013) 214(1):25–32. doi: 10.1038/sj.bdj.2012.1176
52. Gillam DG. Current diagnosis of dentin hypersensitivity in the dental office: an overview. Clin Oral Investig. (2013) 17(Suppl 1):S21–9. doi: 10.1007/s00784-012-0911-1
53. Gulnur Z, Karina T, Temirlan K, Perizat K. Pre-consultation history taking systems and their impact on modern practices: advantages and limitations. J Clin Med Kazakhstan. (2023) 20(6):26–35. doi: 10.23950/jcmk/13947
54. Amarasena N, Spencer J, Ou Y, Brennan D. Dentine hypersensitivity—Australian dentists’ perspective. Aust Dent J. (2010) 55(2):181–7. doi: 10.1111/j.1834-7819.2010.01223.x
55. Exarchou C, Betsani I, Sakellari D, Chatzopoulou D, Gillam D. A survey of dentists in the management of dentine hypersensitivity: a questionnaire-based study. Eur J Dent. (2019) 13(3):383–90. doi: 10.1055/s-0039-1694306
56. Benoist FL, Ndiaye FG, Faye B, Bane K, Ngom PI, Ndong PMK. Knowledge of and management attitude regarding dentin hypersensitivity among dentists from a west African country. J Contemp Dent Pract. (2014) 15(1):86–91. doi: 10.5005/jp-journals-10024-1493
57. Farag ZHA, Awooda EM. Dental erosion and dentin hypersensitivity among adult asthmatics and non-asthmatics hospital-based: a preliminary study. Open Dent J. (2016) 10:587–93. doi: 10.2174/1874210601610010587
58. Zeb A, Amirzadah S, Ahmad F, Afridi F, Nahmeed U, Munir A. Frequency of dentine hypersensitivity and their associated risk factors amongst the patients visiting the dental outpatient department. Pak J Med Health Sci. (2022) 16(10):578. doi: 10.53350/pjmhs221610578
59. Gillam D. The impact of dentine hypersensitivity on the quality of life: an overview. Clin Oral Sci Dent. (2021) 4.
60. Arheiam A, ELTantawi M, ELkadiki N, Elhashani A, Baker SR. Cross-cultural adaptation of the Arabic version of the DHEQ-15. Int J Dent Hyg. (2022) 20(3):527–33. doi: 10.1111/idh.12579
61. Gernhardt CR. How valid and applicable are current diagnostic criteria and assessment methods for dentin hypersensitivity? An overview. Clin Oral Investig. (2013) 17(Suppl 1):S31–40. doi: 10.1007/s00784-012-0891-1
62. O’Toole S, Marro F, Loomans BAC, Mehta SB. Monitoring of erosive tooth wear: what to use and when to use it. Br Dent J. (2023) 234(6):463–7. doi: 10.1038/s41415-023-5623-1
63. Preshaw PM. Detection and diagnosis of periodontal conditions amenable to prevention. BMC Oral Health. (2015) 15(Suppl 1):S5. doi: 10.1186/1472-6831-15-S1-S5
64. Dolce MC, Parker JL, Jason S, Ramos CR, DaSilva JD. The adaptation and implementation of a medical–dental electronic health record in an academic dental center. ACI Open. (2019) 3(01):e37–43. doi: 10.1055/s-0039-1688935
65. Davari A, Ataei E, Assarzadeh H. Dentin hypersensitivity: etiology, diagnosis and treatment; a literature review. J Dent (Shiraz). (2013) 14(3):136–45.24724135
66. Rocha MOC, Cruz AACF, Santos DO, Douglas-DE-Oliveira DW, Flecha OD, Gonçalves PF. Sensitivity and specificity of assessment scales of dentin hypersensitivity—an accuracy study. Braz Oral Res. (2020) 34:e043. doi: 10.1590/1807-3107bor-2020.vol34.0043
67. Schiff T, Bonta Y, Proskin HM, DeVizio W, Petrone M, Volpe AR. Desensitizing efficacy of a new dentifrice containing 5.0% potassium nitrate and 0.454% stannous fluoride. Am J Dent. (2000) 13(3):111–5.11763944
68. Kobler A, Kub O, Schaller H-G, Gernhardt CR. Clinical effectiveness of a strontium chloride- containing desensitizing agent over 6 months: a randomized, double-blind, placebo-controlled study. Quintessence Int. (2008) 39(4):321–5.19081901
69. Maillard M, Bandiaky ON, Maunoury S, Alliot C, Alliot-Licht B, Serisier S, et al. The effectiveness of calcium phosphates in the treatment of dentinal hypersensitivity: a systematic review. Bioengineering. (2023) 10(4). doi: 10.3390/bioengineering10040447
70. Haleema S, Gillam DG. Review of selected over-the-counter toothpastes in the management of dentine hypersensitivity. J Dent Maxillofac Res. (2024) 7(1):1–4. Available online at: https://researchopenworld.com/wp-content/uploads/2024/05/JDMR-7-713-1.pdf
71. Pollard AJ, Khan I, Davies M, Claydon N, West NX. Comparative efficacy of self-administered dentifrices for the management of dentine hypersensitivity—a systematic review and network meta-analysis. J Dent. (2023) 130:104433. doi: 10.1016/j.jdent.2023.104433
72. Sowinski J, Ayad F, Petrone M, DeVizio W, Volpe A, Ellwood R, et al. Comparative investigations of the desensitising efficacy of a new dentifrice. J Clin Periodontol. (2001) 28(11):1032–6. doi: 10.1111/j.1600-051X.2001.281107.x
73. NCT03943095. Study to Characterize the efficacy of a dual active dentifrice for the relief of dentin hypersensitivity. Randomized Controlled Examiner-Blind Phase II Exploratory Clinical Study to Characterize the Efficacy Profile of an Experimental Dual Active Combination Dentifrice for the Relief of Dentin Hypersensitivity, in Subjects With Clinically Diagnosed Dentin Hypersensitivity. Available online at: https://clinicaltrials.gov/study/NCT03943095 (Accessed April 4, 2025).
74. Li J, Han Q, Zhang L, Zhang J, Zhong Y. Efficacy of a toothpaste containing paeonol, potassium nitrate, and strontium chloride on dentine hypersensitivity: a double-blind randomized controlled trial in Chinese adults. Heliyon. (2023) 9(4):e14634. doi: 10.1016/j.heliyon.2023.e14634
75. Liu H, Hu D. Efficacy of a commercial dentifrice containing 2% strontium chloride and 5% potassium nitrate for dentin hypersensitivity: a 3-day clinical study in adults in China. Clin Ther. (2012) 34(3):614–22. doi: 10.1016/j.clinthera.2012.01.027
76. Petersson LG. The role of fluoride in the preventive management of dentin hypersensitivity and root caries. Clin Oral Investig. (2013) 17(Suppl 1):S63–71. doi: 10.1007/s00784-012-0916-9
77. Yates RJ, Newcombe RG, Addy M. Dentine hypersensitivity: a randomised, double-blind placebo-controlled study of the efficacy of a fluoride-sensitive teeth mouthrinse. J Clin Periodontol. (2004) 31(10):885–9. doi: 10.1111/j.1600-051X.2004.00581.x
78. Molina A, García-Gargallo M, Montero E, Tobías A, Sanz M, Martín C. Clinical efficacy of desensitizing mouthwashes for the control of dentin hypersensitivity and root sensitivity: a systematic review and meta-analysis. Int J Dent Hyg. (2017) 15(2):84–94. doi: 10.1111/idh.12250
79. West NX, Seong J, Davies M. Management of dentine hypersensitivity: efficacy of professionally and self-administered agents. J Clin Periodontol. (2015) 42(Suppl 16):S256–302. doi: 10.1111/jcpe.12336
80. Hefti AF, Stone C. Power toothbrushes, gender, and dentin hypersensitivity. Clin Oral Investig. (2000) 4(2):91–7. doi: 10.1007/s007840050122
81. Sivaramakrishnan G, Sridharan K. Fluoride varnish versus glutaraldehyde for hypersensitive teeth: a randomized controlled trial, meta-analysis and trial sequential analysis. Clin Oral Investig. (2019) 23(1):209–20. doi: 10.1007/s00784-018-2428-8
82. Tabatabaei MH, Chiniforush N, Hashemi G, Valizadeh S. Efficacy comparison of Nd:YAG laser, diode laser and dentine bonding agent in dentine hypersensitivity reduction: a clinical trial. Laser Ther. (2018) 27(4):265–70. doi: 10.5978/islsm.27_18-OR-24
83. Cattoni F, Ferrante L, Mandile S, Tetè G, Polizzi EM, Gastaldi G. Comparison of lasers and desensitizing agents in dentinal hypersensitivity therapy. Dent J (Basel). (2023) 11(3). doi: 10.3390/dj11030063
84. MAjeeD AD, Alshwaimi EM, Nazir MA, Almas KH. Dental students’ perception of dentine hypersensitivity and awareness about its management. J Clin Diagnostic Res. (2019) 13(8):15–9. doi: 10.7860/JCDR/2019/39928.13080
85. Hye-Jeong B, Hee LC. Proper tooth-brushing technique according to patient’s age and oral status. Korean Acad Prev Dent. (2020) 16(4):149–53. doi: 10.15236/ijcpd.2020.16.4.149
86. Addy M, Hunter ML. Can tooth brushing damage your health? Effects on oral and dental tissues. Int Dent J. (2003) 53(Suppl 3):177–86. doi: 10.1111/j.1875-595X.2003.tb00768.x
87. Agrawal S. Toothbrushes and tooth brushing methods: a periodontal review. J Clin Stud Med Case Rep. (2022) 9(1):1–12.
88. Ng C, Tsoi JKH, Lo ECM, Matinlinna AJP. Safety and design aspects of powered toothbrush-a narrative review. Dent J (Basel). (2020) 8(1). doi: 10.3390/dj8010015
89. Yousefi B, Mehran M, Sadabadi Y, Banakar M, Haghgoo R. Effect of cheese and casein phosphopeptide-amorphous calcium phosphate on erosive lesions of primary teeth enamel following exposure to amoxicillin and ibuprofen syrups: an in vitro study. Dent Res J (Isfahan). (2024) 21:25. doi: 10.4103/drj.drj_247_23
90. Jang J-H, Oh S, Kim H-J, Kim D-S. A randomized clinical trial for comparing the efficacy of desensitizing toothpastes on the relief of dentin hypersensitivity. Sci Rep. (2023) 13(1):5271. doi: 10.1038/s41598-023-31616-6
91. Mosquim V, Santin DC, Jacomine JC, Zabeu GS, Gillam DG, Hill RG, et al. Metals, fluoride and bioactive glass on dentin hypersensitivity and quality of life: a 6-month double-blind randomized clinical trial. J Dent. (2025) 160:105931. doi: 10.1016/j.jdent.2025.105931
92. Cruz LPDD, Hill RG, Chen X, Gillam DG. Dentine tubule occlusion by novel bioactive glass-based toothpastes. Int J Dent. (2018) 2018:5701638. doi: 10.1155/2018/5701638
93. Ji-Hyun B, Young-Kyun K, Seung-Kwon M. Desensitizing toothpaste versus placebo for dentin hypersensitivity: a systematic review and meta-analysis. J Clin Periodontol. (2015) 42(2):131–41. doi: 10.1111/jcpe.12347
94. Castro MC, Joseph RJ, Targino FR, Jens SH. Formulations of desensitizing toothpastes for dentin hypersensitivity: a scoping review. J Appl Oral Sci. (2022) 30:e20210410. doi: 10.1590/1678-7757-2021-0410
95. Schiff T, Delgado E, Zhang YP, DeVizio W, Cummins D, Mateo LR. The clinical effect of a single direct topical application of a dentifrice containing 8.0% arginine, calcium carbonate, and 1450ppm fluoride on dentin hypersensitivity: the use of a cotton swab applicator versus the use of a fingertip. J Clin Dent. (2009) 20(4):131–6.19831166
96. Satyapal T, Mali R, Mali A, Patil V. Comparative evaluation of a dentifrice containing calcium sodium phosphosilicate to a dentifrice containing potassium nitrate for dentinal hypersensitivity: a clinical study. J Indian Soc Periodontol. (2014) 18(5):581–5. doi: 10.4103/0972-124X.142447
97. Osmari D, Fraga S, Ferreira ACDO, Eduardo CDP, Marquezan M, Silveira BLD. In-office treatments for dentin hypersensitivity: a randomized split-mouth clinical trial. Oral Health Prev Dent. (2018) 16(2):125–30. doi: 10.3290/j.ohpd.a40299
Keywords: dentine hypersensitivity, dentine hypersensitivity experience questionnaire (DHEQ), Middle East and Africa (MEA) region, tooth sensitivity, diagnostic methods, clinical management
Citation: Cekici A, Shaikh A, Alsayed A, Gokbuget AY, Patel E, Marei H, Awad M, Banday N, Habib NA, Osailan SM, Theuri T, Adeyemi TE, Parkinson CR, Hamdy A and Thomas J (2025) Evidence-based recommendations for diagnosing and managing dentine hypersensitivity in clinical practice: insights from the Middle East and Africa. Front. Oral Health 6:1663984. doi: 10.3389/froh.2025.1663984
Received: 11 July 2025; Accepted: 20 October 2025;
Published: 7 November 2025.
Edited by:
Reisha Rafeek, The University of the West Indies St. Augustine, Trinidad and TobagoReviewed by:
Stanley Chibuzor Onwubu, Durban University of Technology, South AfricaVictor Mosquim, Universidade de São Paulo, Brazil
Copyright: © 2025 Cekici, Shaikh, Alsayed, Gokbuget, Patel, Marei, Awad, Banday, Habib, Osailan, Theuri, Adeyemi, Parkinson, Hamdy and Thomas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juhi Thomas, anVoaS54LnRob21hc0BoYWxlb24uY29t
 Ali Cekici
Ali Cekici Amynah Shaikh2
Amynah Shaikh2 Hesham Marei
Hesham Marei Manal Awad
Manal Awad Charlie R. Parkinson
Charlie R. Parkinson Ahmed Hamdy
Ahmed Hamdy Juhi Thomas
Juhi Thomas