- 1Institute of Dentistry, School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, United Kingdom
- 2Center for Research in Oral Cancer, Faculty of Dental Sciences, University of Peradeniya, Kandy, Sri Lanka
- 3Oral and Maxillofacial Surgery Department, Horus University, New Damietta, Egypt
- 4NHS Education Scotland, NHS Grampian, Aberdeen, United Kingdom
- 5School of Dentistry, School of Health Sciences, College of Medicine and Health, University of Birmingham, Birmingham, United Kingdom
Objectives: Head and Neck Cancer (HNC) is a devastating disease with significant mortality and morbidity. Patients suffer from compromised quality of life, due to the impact of the disease and its treatment on oral health and related functions. The aim of this systematic review was to identify the effects of HNC on oral health related quality of life (OHQoL).
Methods: The protocol followed PRISMA-2020 guidelines. Literature search was conducted in electronic databases (PSYC-INFO, EMBASE, OVID-MEDLINE, SCOPUS, and WEB OF SCIENCE) at three time points, yielding 1198 records. Abstracts and full-texts were screened, and 101 eligible articles were identified. The risk of bias assessment was conducted using Joanna Briggs Institute critical appraisal tools. Narrative data synthesis was conducted under broad themes that influenced OHQoL; patient factors, diagnosis and treatment, and post treatment.
Results: Studies were published between 2001 and 2024, a growing interest in OHQoL research was noted over time. 70.3% of the studies used oral health impact profile (OHIP-14) for OHQoL assessment. Among patient factors, low socioeconomic status, being without a partner and underweight were associated with worse scores. OHQoL varied with anatomical location of HNC, treatment modalities and their side effects such as mucositis and xerostomia. Prosthetic rehabilitation positively influenced OHQoL post-treatment.
Conclusions: OHQoL assessment is critical in HNC patients from diagnosis, during treatment and beyond. It is influenced by factors related to sociodemographic, diagnosis, treatment, reconstruction and rehabilitation. The findings of this study can inform and guide clinicians to update supportive care and existing management of HNC and OPMD patients.
1 Introduction
Head and neck cancers (HNC) refer to malignancies arising in oral cavity (including mucosal lip), pharynx, larynx, and paranasal sinuses (1). The incidence of HNC is on the rise globally, with geographical variations due to differences in risk factors (2). Management strategies for HNC include surgery, radiotherapy, chemotherapy, immune therapy and combinations of different therapeutic approaches (3).
Quality of Life (QoL) is a concept that describes an individual's holistic well-being. It is influenced by biological factors, social determinants and interactions with the physical environment. Health related quality of life (HRQoL) assesses the impact of disease status or its treatment on physical, psychological and social well-being of individuals. Oral health related quality of life (OHQoL) is a subset of HRQoL, that focuses on oral health and how it affects well-being. Specifically, OHQoL subjectively evaluates patients’ oral health, and their reported comfort with function when eating, sleeping, and engaging in social interactions. It also evaluates patients’ self-esteem; and satisfaction with their oral health (4–6).
Although OHQoL is a subjective construct, researchers have developed questionnaire-based tools to objectively evaluate OHQoL. Two such commonly used tools are the Oral Health Impact Profile (OHIP) and the Oral Impacts on Daily Performances (OIDP) scales. Slade and Spencer developed the original OHIP questionnaire with 49 items and condensed it to a shorter 14-item version (7). The short form of OHIP is considered to be a useful instrument for use in clinical settings with good reliability, validity and precision (8). OHIP-14 covers seven main domains related to OHQoL, namely functional limitations, physical pain, psychological discomfort, physical disability, psychological disability, social disability, and handicap. Each domain is assessed via two questions and equally weighted. Due to the lack of questions related to esthetics in the OHIP tool, the Orofacial Esthetic Scale (OES) was developed (9). Other tools were also developed to help in the assessment of OHQoL including the Chewing Functional Questionnaire (CFQ) (10), and the Oral Mucositis-specific Quality of Life measure (OMQoL) (11). In 2004, Pace-Balzan and colleagues published a pilot study that used the Liverpool Oral Rehabilitation Questionnaire (LORQ) and subsequently developed the current version consisting of 40 items, LORQ version 3 (LORQv3), which captures patient's perception on oral problems and the success of their prosthetic rehabilitation (12). The European Organization for Research and Treatment of Cancer (EORTC) has developed several measurement tools to assess the quality of life in cancer patients. Specific modules to assess quality of life in HNC (EORTC QLQ-HN43/35) and quality of life related to oral health (EORTC QLQ—OH15) are available (13).
Patients with HNC are a group whose oral health and related well-being can be particularly affected due to the disease and its treatment. OHQoL has been researched at various stages of HNC, including at the time of diagnosing precursor lesions (oral potentially malignant disorders), cancer detection, treatment with various modalities, reconstruction and rehabilitation. The association of OHQoL with biological factors, treatment and post treatment related factors are not well documented in HNC patients. This could be due to a limited number of primary studies, heterogeneity on outcome measures and a lack of longitudinal data. In order to bridge this critical research gap, the current systematic review aimed to identify primary research that evaluated the OHQoL in HNC patients. The findings will help clinicians to comprehend various factors associated with OHQoL at different stages of HNC, which will enable appropriate modification of treatment strategies and management protocols to deliver optimum care and support for HNC patients.
2 Materials and methods
2.1 Protocol development
The study protocol was developed according to Preferred Reporting System for Systematic Reviews (PRISMA—2020) guidelines. The review protocol was not registered at the time of the study inception. The review question was defined according to the SPIDER format as follows: Sample of interest—Head and Neck Cancer, Phenomenon of Interest: Oral Health related Quality of Life, Design: any observational or interventional study designs, Evaluation: evaluation of OHQoL using validated tools, and Research type: primary research. The focused research question addressed in this review is “Which patient related factors, factors associated with diagnosis, treatment and post treatment affect OHQoL in patients with HNC?”.
2.2 Literature search
A comprehensive literature search was conducted in five electronic databases (PSYC INFO, EMBASE, OVID MEDLINE, SCOPUS, and WEB OF SCIENCE), to identify relevant literature at three time points. Controlled vocabulary search terms for head and neck cancer and oral health-related quality of life were used. Keywords included (“OHIP*”, OHIP, “Oral health impact profile”, “Oral health quality of life”, “OIDP*”, “Oral impact on daily performance” (Combined with OR) (group 1) AND (Cancer, Precancer*, Malig*, Premalig*, Neoplasms, Tumor*) (Combined with OR) (group 2), both groups were combined with AND Boolean. The detailed search strings used for each of the databases are presented in (Supplementary Table S1).
Literature search was conducted at three time points. First literature search was conducted to capture studies published from the inception of the databases up to January 2020. A second search was conducted in 2022 and captured articles from 2020 onwards. Search three was conducted in September 2024, to identify records published from 2023 up to the search date (September 2024). Three different researchers conducted the literature search. To ensure consistency, the original search was repeated at the time of the second and third searches, and comparisons were made to confirm reproducibility.
2.3 Screening and study selection
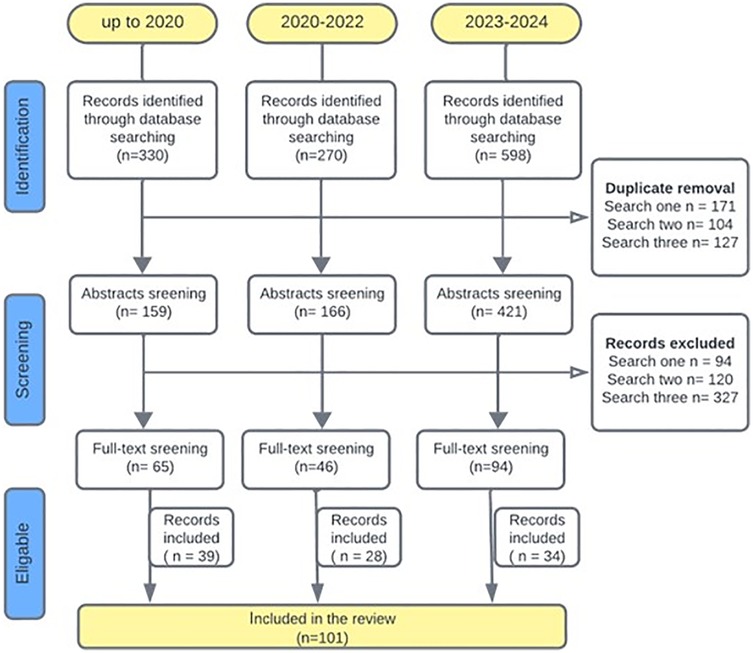
All records identified from the literature search were exported and managed through reference management software packages (Endnote and Rayyan.ai). Following duplicate removal, screening was performed at two stages: title and abstract screening and full text screening by independent reviewers using pre-defined selection criteria. The inclusion criteria were (1) studies including patients with HNC, (2) studies with human subjects aged 18 and above, and (3) studies that assessed OHQoL with a standardized tool. The exclusion criteria were (1) full text articles published in languages other than English, (2) studies including non-human participants, (3) studies that did not include HNC patients, (4) studies that did not report OHQoL and (5) case reports, case series, systematic reviews, meta-analysis, and conference proceedings. Details of literature searches and study selection are described in Figure 1, according to a modified PRISMA flow diagram.
2.4 Data extraction, quality assessment and data synthesis
The studies which met the inclusion criteria were retrieved and data extraction was performed using a pre-defined bespoke Microsoft Excel spreadsheet by independent reviewers. Any disagreements on screening and data extraction were resolved through discussion and random accuracy checks were performed by RAE and EG. Data extracted from included studies were: first author, manuscript title, year of publication, country where the study was conducted, study design, study groups, diagnosis, sample size, OHQoL assessment tool used, outcomes, any association between studied parameters, conclusions, recommendations, and limitations.
Risk of bias assessment was conducted for the included studies by independent reviewers using Joanna's Briggs Institute (JBI) quality appraisal tools. Since both observational and intervention study designs were included, JBI critical appraisal checklists for analytical cross sectional, case control, cohort and randomized controlled trial designs were used. Scores were presented as the statements marked as ‘Yes’ out of the total number of statements for each tool. Gradings for each study were decided following consensus agreement among the research team. For JBI-cross sectional tool, studies with 8–6 satisfied criteria were rated as good, 5–3 fair and <3 poor, JBI-cohort tool studies with 9–11 satisfied criteria were rated as good, 6–8 fair and <6 poor, for JBI-RCT tool studies satisfying 11–13 criteria were rated as good, 8–10 fair and <8 poor. For JBI-case control tool studies satisfying 10–8 were rated as good, 7–5 fair and less than 5 poor.
The included studies were of heterogenous nature; therefore, a meta-analysis and quantitative synthesis was not considered. A descriptive analysis and thematic data synthesis was conducted under three main headings: (1) patient related factors, (2) diagnosis and treatment, and (3) post treatment.
3 Results
3.1 Characteristics of the included studies
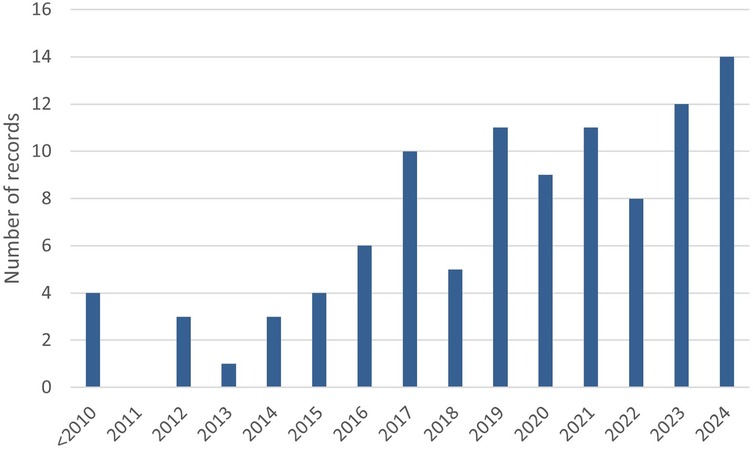
A total of n = 1,198 records were retrieved from literature searches conducted at three time points as described in Figure 1. Out of these, n = 402 duplicates were removed and a total of n = 205 full-text articles were screened from which n = 101 articles were included. The main reasons for excluding manuscripts following full text screening were: (1) OHQoL was not assessed with many study only assessing health related quality of life, (2) The studies were not relevant to the topic, for example not related to head and neck cancer, (3) Publications that did not provide data such as protocol studies, conference abstracts and reviews. The 101 selected studies were published between 2001 and 2024, and a year-wise breakdown of publications is presented in Figure 2. Data extracted from each included article are presented in (Supplementary Tables S2, S3).
A geographical distribution of the included studies is presented in Figure 3A. A breakdown of countries based on the WHO region classification (Figure 3B) showed that none of the studies were conducted in Africa, and a relatively small number of studies were conducted in South-East Asian region, where the incidence of head and neck cancer is high. Furthermore, based on the World Bank classification of countries, none of the studies were conducted in low-income countries, and the number of studies correlated with the increased income (Figure 2C).
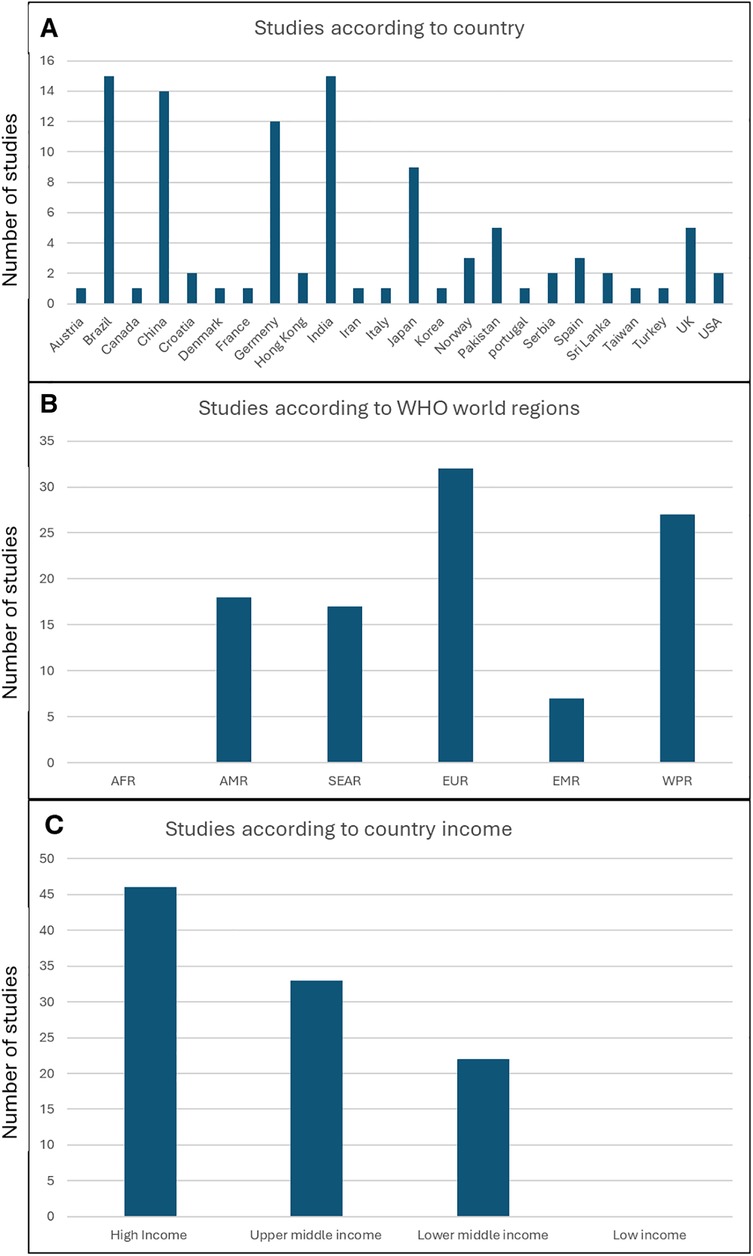
Figure 3. Geographical distribution of the studies (A) number of studies per country (B) number of studies per wHO world regions (C) number of studies according to country income classification. AFR, African region; AMR, region of the Americas; SEAR, South-East Asian region; EUR, European region; EMR, Easter Mediterranean region; WPR, Western Pacific region.
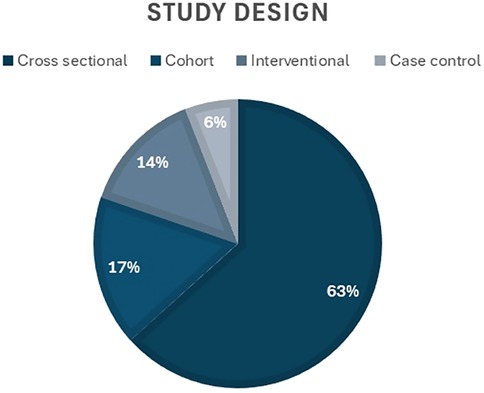
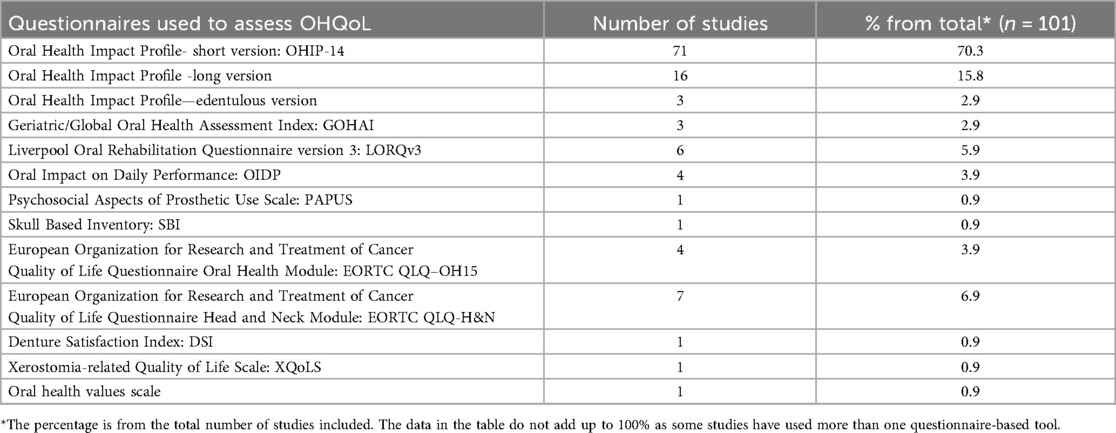
Regarding the study design, the majority of the studies (n = 64, 63.4%) were cross-sectional, while 17% (n = 17) were cohort, 14% (n = 14) used interventional designs, and 6% (n = 6) were case control (Figure 4). Thirteen questionnaire tools were used to assess OHQoL, with some studies reporting more than one tool. The frequency and percentage of studies using each tool is presented in Table 1.
More than two thirds of the studies used oral health impact profile short version (OHIP-14), for OHQoL assessment. This commonly used tool is simple and can be completed in less than 10 min and has been translated and validated in many languages including Tamil, Hindi, Sinhala, Spanish, Serbian, German, Japanese, Chinese, and Urdu demonstrating a good cross-cultural adaptability. The participants rate their problems on a five-point Likert scale coded as never (score 0), hardly ever (1), occasionally (2), fairly often (3) and very often (4). A higher score in OHIP tool indicates poor OHQoL.
The JBI critical appraisal checklists for randomised controlled trials (RCT), cohort, case control and analytical cross-sectional designs were used for quality appraisal. The majority of the studies were graded as good (n = 75, 74.3%) with low risk of bias, (n = 21, 20.8%) were rated as fair with moderate risk of bias, and (n = 5, 5.0%) were rated as poor with high risk of bias. Results of the quality assessment are presented in Supplementary Table S4.
The result summary of OHQoL measures reported in the studies are presented under the following main themes (1) patient related factors, (2) diagnosis and treatment, and (3) post treatment.
3.2 Patient-related factors (demographics and risk factors)
In this section, we will summarise the findings from the included studies that assessed OHQoL in relation to patient related factors (demographics) and HNC risk factors.
Regarding the association of OHQoL with sex, contradictory findings were reported. Worse OHQoL was reported in female patients compared to males in some studies (14, 15). On the other hand, a study reported that scores for physical disability, handicap were significantly higher in men than in women following glossectomy (16), and higher functional and physical disability were identified in males compared to females (16, 17). No significant difference was reported in OHQoL according to sex in another study (18).
In relation to age, OHQoL was reported to be worse in older patients compared to younger counterparts, while psychological issues were higher in younger patients (19). On the other hand, younger patients (age <55 years) had a better OHQoL after surgery as compared to older patients (16). Low body mass index (BMI) was a frequent finding in HNC patients (20, 21). Barrios and colleagues reported that malnutrition was a longer-term determinant of worse OHQoL (22). Patients with BMI less than 25 were more likely to have worse overall OHQoL (20).
HNC is a disease common in the low socioeconomic strata (23). Studies have reported associations between OHQoL and patient's social and economic factors (19, 21, 24). In the research by Binnal and colleagues, rural and poor socioeconomic background was associated with worse OHQoL, while urban residents had lower psychological discomfort when compared to rural patients (19). In addition to low socioeconomic status, being widowed and of a non-Caucasian ethnicity were associated with worse OHQoL (25). In support of the observation regarding marital status, married patients reported better OHQoL and mouth opening capacity compared to patients who were not married (21). Low education level, and lower income were identified as factors negatively affecting OHQoL (21, 24).
Risk factors for HNC include smoking, alcohol abuse, smokeless tobacco (SLT), chewing habits (especially betel) and HPV infections (26). Patients who used tobacco reported higher oral health-related social disability than those who never used tobacco (19). A positive association between duration of SLT usage and physical pain was reported, where the pain scores increased with increase duration of SLT use (19). Surprisingly, the same study reported that daily smokers and alcohol drinkers reported better OHQoL scores than occasional users.
3.3 Diagnosis and treatment
This section summarises the results related to patient diagnosis, including the stage of the disease and the impact of various treatment modalities on OHQoL.
Out of the included studies, only a small number (n = 8, 7.9%) reported data on OHQoL in oral potentially malignant disorders (OPMD) patients. In patients with oral lichen planus (OLP), correlation between OHQoL and disease severity was reported where the OHQoL declined with increasing severity (27–29). In patients with oral epithelial dysplasia, physical pain and psychological disability were the most compromised domains (30). Regarding different treatment modalities for OPMD, laser therapy resulted in significant improvement in OHQoL in leukoplakia patients (31, 32). Intralesional injections of dexamethasone and hyaluronidase produced similar results in oral submucous fibrosis patients (33).
The anatomical location of malignancy influenced OHQoL in HNC patients. Malignancies in the oropharynx and larynx were associated with worse OHQoL compared to lesions in the nasopharynx (24). Similarly, the lowest OHQoL was found in patients with oral cavity tumor site, compared to cancers located in the nasopharynx (34). Among malignancies arising in the oral cavity, tongue and the floor of the mouth reported a poorer OHQoL outcome than malignancies in the buccal mucosa (35). Maxillary defects resulted in better OHQoL outcomes compared to mandibular defects (36). Xiao and colleagues reported that malignancies involving the anterior skull base were more likely to have better OHQoL, than patients who had mid skull base involvement (37).
The primary treatment modality for HNC is surgical resection, followed by primary closure or reconstruction with flaps. Surgery can be combined with radiotherapy and/or chemotherapy. The choice of the treatment/s and their sequence is determined by the clinicians depending on several factors such as extent of tissue involvement of the tumor, co-morbidities and sometimes patient preference. Several studies reported that radiotherapy (RT) as the primary treatment or as an adjuvant to surgical therapy, resulted in a significant deterioration of the OHQoL compared to patients who did not undergo RT (16, 38–45). Patients OHQoL significantly fluctuated with time, during and after radiotherapy (46). Mucositis was a significant contributing factor for worse OHQoL during radiotherapy (39, 47, 48), together with xerostomia (49–51).
Radiation dose and technique impacted OHQoL as well. A 1,000 Gray (Gy) increase in RT dose was associated with a clinically evident change in difficulty of swallowing solid food, dry mouth, sticky saliva, and taste sensation problems (52). Another study reported that when the average dose received by maxillary anterior region is greater than 28.78 Gy, there was a tendency for deterioration in OHQoL (53). Compared to standard three beam RT technique, unilateral and bilateral neck RT with parotid-sparing techniques were successful in preserving salivary output, and lower radiation dose to contralateral parotid glands resulted in fewer xerostomia complaints one year post RT (49). In contrast, no significant variation of OHQoL was found according to the irradiation technique (3D-CRT vs. IMRT) in long-term survivors of HNC (34). Concurrent photobiomodualtion therapy was effective in preventing the negative impacts on OHQoL, mainly in the final stage of RT, with reduction of oral mucositis symptoms (54). In the study by de Oliveira and colleagues, researchers did not find a significant difference in OHQoL in relation to every day and alternate day photobiomodualtion therapy protocols (55). Topical application of pilocarpine spray stimulated whole salivary flow in HNC patients treated with RT (56). Jiang and colleagues introduced an integrated supportive programme for HNC patients undergoing RT, which included face to face education and telephone coaching which resulted in significant improvement in OHQoL and oral hygiene status, compared to standard care (57).
Oral health status of HNC patients influenced OHQoL, where poor oral hygiene resulted in worse OHQoL (21). Caries activity, periodontal disease and incidence of edentulism were high in individuals undergoing RT among HNC patients (58). Patients who suffered from trismus (41, 59), and xerostomia (43, 50) reported worse OHQoL compared to those who did not. Better OHQoL was reported in patients with more posterior functional tooth units (22); however, no association was found between the length of the dental arch and OHQoL post RT in HNC patients (60). Following intensity modulated RT, the compromised salivary flow was persistent with ocular dryness up to 6–24 months (45, 61).
Studies provide evidence regarding association between OHQoL and psychological status. In the study by Hassel and colleagues, compromised OHQoL predisposed to depression and anxiety (62). In a cohort of patients with oral epithelial dysplasia, nearly one fourth of patients were identified with concurrent psychological conditions such as anxiety, depression and emotional distress (30). In addition, the study by Li and colleagues identified that long term HNC survivors had reduced sleep quality. They reported that extensive neck dissection, poor mental health, and psychological disability are associated with poor sleep and suggested that maintaining good OHQoL could promote better sleep in HNC patients (63).
3.4 Post treatment
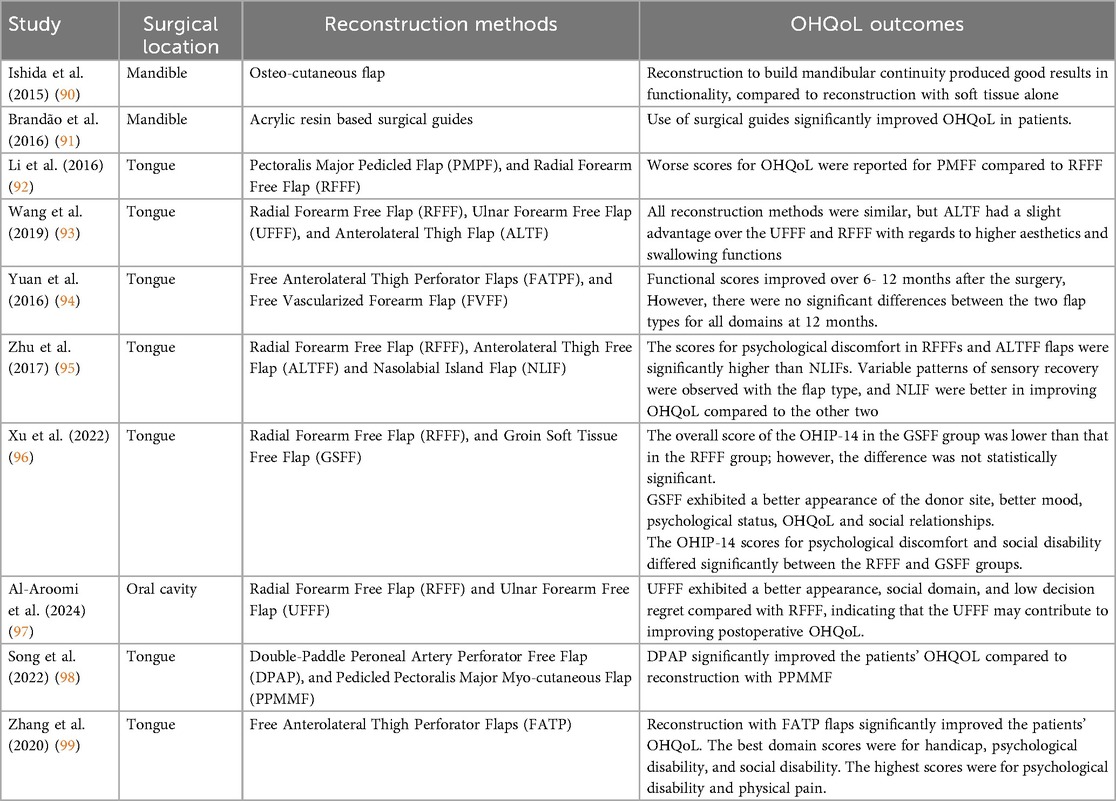
Many studies included in the systematic review assessed OHQoL in HNC patients post treatment. The results are summarised in this section. Reconstruction with flaps and rehabilitation with prosthesis are common clinical sequalae following primary surgical management in HNC patients. Findings from the studies comparing OHQoL outcomes in relation to different reconstruction methods are summarized in Table 2.
Several studies reported data on the effects of surgical techniques on OHQoL. A study compared variations of OHQoL according to the type of mandibulectomy, where AT group included defects of mandibular angle and mental tubercle, the Body group included defects involving mandibular body and the MR group included cases involved with marginal resection (64). The study disclosed that the Body group scored significantly higher than the AT group in the OHIP, and a significant difference was noted between the AT group and Body group in the physical and psychological disability domains. However, the body group showed higher stomatognathic performance than the AT group, despite a minor difference in the chewing performance between the MR and the Body groups (64). Not surprisingly, patients with concurrent gastrostomy, and tracheostomy reported worse OHQoL (24).
Several studies reported a significant improvement in all domains of OHQoL with prosthetic rehabilitation following surgery (8, 65–71); where the highest improvement was in psychological disability and handicap domains (65). Coward and colleagues found no statistically significant relations between the surface areas and mean depths of obturators with OHQoL (72). OHQoL improved following prosthetic rehabilitation for both maxillary and mandibular defects; however, the improvement of scores in different OHIP domains were variable between the two groups (67). Regarding the type of prosthesis, overdentures and fixed metal-acrylic resin prostheses were both acceptable treatments, while the implant-retained fixed metal-acrylic resin prostheses was more apt for managing the marginal mandibular defects (73). Implant-supported removable overdentures improved OHQoL outcomes in patients with reconstructed mandibles (74). A study by Stefano and colleagues (2019) provided evidence that palatal augmentation prosthesis is an effective therapeutic remedy to improve OHQoL in patients with absent or reduced lingual mobility following surgery (70). An obturator relined with soft silicone improved masticatory performance and OHQoL post-maxillectomy as opposed to acrylic resin prosthesis (75).
4 Discussion
Head and neck cancer is a debilitating disease, affecting vital functions in the body such as mastication, swallowing, phonation, aesthetics and appearance. Therefore, quality of life in affected individuals is a primary concern during disease management and survival. Health and oral health related quality of life is expected to deteriorate in HNC patients compared to disease free individuals; however, determinants of OHQoL in HNC are not well established. Hence, this systematic review aimed to identify factors that impact OHQoL in HNC patients.
Literature search was conducted at three different time points, which captured more than 1,000 records for initial screening. The year-wise distribution of studies indicated that there is a growing interest in OHQoL research. However, studies assessing OHQoL in oral potentially malignant disorders (OPMD) were less than 10%. This highlights a research gap that needs to be addressed. Evidence from OPMD studies indicates a variability of affected OHQoL domains with different clinical OPMD subtypes. Furthermore, OHQoL can be used as a useful adjunct measure to assess treatment response and can be incorporated as a standard clinical endpoint in care pathways for OPMD patients. We identified that OHQoL was used as a measure of the outcome of interventional studies comparing treatment methods for both HNC and OPMD. This is a positive trend, complying with the holistic approach of health as a construct that involves uplifting different domains pertaining to patient well-being, as opposed to absence of disease.
Results of the current review were organized under three main themes; namely OHQoL associated with patient related factors, diagnosis and treatment, and post treatment. Concerning the patient related factors, lower financial and social status were shown to be associated with worse OHQoL. HNC is more common among low socioeconomic groups, and financial strains due to cancer and lack of social support were identified as significant unmet needs in HNC survivors (76, 77). Spousal support was identified as a positive determinant for better OHQoL in HNC patients (21, 25), indicating that caring behavior and emotional support may help to maintain better OHQoL. A study assessing the psychological status of family caregivers of oral cancer patients reported that more than half of the caregivers experienced depression, anxiety, and perceived stress; iterating the need for comprehensive domestic and social support network for patients with HNC (78).
Interestingly, when we looked at the distribution of studies according to the WHO geographical regions and the World Bank classification of countries according to income, we identified a complete absence of studies in the African region and a smaller number of studies in the South-East Asian region, which has a high incidence of head and neck cancer, compared to the European and Western Pacific regions, where more studies on OHQoL in HNC have been conducted. Furthermore, our study showed that there were no studies conducted on this important topic in low-income countries, and that the number of studies correlated with income level in different countries. Given the association between low socioeconomic status and HNC, this clearly identifies a gap that needs to be addressed by conducting more research in the low income countries.
The current review did not identify conclusive evidence on the association of OHQoL with age and sex in HNC patients due to conflicting reports; however, significant evidence was reported for worse OHQoL associated with underweight or low BMI. With the compromised mastication, swallowing and salivary flow, maintaining adequate nutrition and optimum weight is a significant challenge for HNC patients. In agreement with the study reporting association between lower OHQoL and malnourishment in oral cancer patients (22), poor nutrition was a significant determinant of patient dissatisfaction, communication problems and poor decision making in HNC (79). In the study by Widaman and colleagues (2025), researchers reported that HNC patients undergoing professional nutritional assessment with dietician referrals demonstrated better satisfaction with care than those who were without nutritional assessment (79). These findings underscore the importance of incorporating nutritional assessment and dietary counselling in the management protocols for HNC patients.
When it comes to risk factors, we identified an unexpected finding, where daily users of tobacco and alcohol reported better OHQoL scores than occasional users (19). The reasons for these surprising results may be due to the euphoric effects, psychological coping mechanisms and socialization habits of tobacco and alcohol users and should be interpreted with caution.
OHQoL outcomes varied with the anatomical location of the malignancy in HNC. Mid skull base malignancies reported worse OHQoL measures compared to cancers involving anterior skull base (37); poor surgical access and higher number of vital structures in mid skull base may result in more surgical challenges and may compromise the success of the surgical procedure. Following reconstruction, maxillary defects resulted in better OHQoL outcomes compared to mandibular defects (36), this can be due improved blood supply in maxilla, better retention and enhanced acceptance of flaps. Defects of the maxilla, tongue and soft palate in addition to chemoradiotherapy were found to negatively affect the effectiveness of maxillofacial prosthetic treatment, while reconstructive surgery improved OHQoL (80).
Radiotherapy (RT) to the head and neck region resulted in significant deterioration of OHQoL. Radiation induces biological effects such as DNA damage, cell cycle arrest, cell death, and inflammation in irradiated areas and have a negative effect on tissues with rapid turnover. RT in the head and neck region may frequently present with complications such as oral and ocular mucosal irritation (mucositis), bone marrow suppression, fibrosis (in salivary glands leading to reduced salivary secretions), and necrosis (eg: osteoradionecrosis). Reduced salivary flow may cause taste disturbances, increase risk of caries and tooth loss, inflammation and irritation in oral mucosa can result in pain and burning sensation which may predispose to lack of satisfaction with food, these are integral components of OHQoL. Therefore, it is essential that supportive care is provided to patients to overcome these side effects. Artificial saliva (81), pilocarpine spray (56), local application of herbal products (82–84), close monitoring and individual coaching (57), may help to minimize radiation induced negative effects on OHQoL.
Both HNC and OPMD patients were reported with psychological symptoms such as low mood, anxiety and depression following diagnosis (30, 85). Poor OHQoL may be a predisposing factor for psychological distress (62). In addition, genetic links between psychological factors and quality of life in HNC patients have been identified (86). Psychological support and counselling is a critical need in HNC patients; the study by Nik and colleagues disclosed that psychosocial interventions enhanced post traumatic growth, and perceived spousal support reduced psychological complications such as depression, anxiety, and posttraumatic stress in HNC survivors (87).
In most of the studies included in the current systematic review, OHQoL assessment was conducted using the oral health impact profile short version (OHIP-14). Despite being a generic tool applicable for both healthy and disease populations, the ease of administration, coverage of seven domains, and cross-cultural adaptability has made it widely popular among researchers. A recent scoping review assessing different measures of OHQoL identified significant flows in the development and validation of tools used to measure OHQoL (88). Their study concludes that research reporting OHQoL in HNC may not comprehensively assess the full impact of the disease and its treatment on OHQoL, due to limitations and standardization issues in the assessment tools (88). Another scoping review therefore recommended using the Vanderbilt Head and Neck Symptom Survey version 2.0 (VHNSS 2.0) as it provides broader coverage of domains relevant to this patient group (89).
The findings of this systematic review should be interpreted with caution given the heterogeneity of the included studies, absence of uniform assessment measures and variation in the definition for OHQoL. Another limitation of the study, which resulted from the wide variation of the included studies, is the absence of quantitative data synthesis (e.g., meta-analysis) and temporal data analysis. This limits estimation of pooled effects and statistical interpretations, together with trends over time. Furthermore, a gray literature search was not conducted which could lead to a degree of bias.
5 Conclusions
OHQoL assessment is a critical need in HNC and OPMD patients and should be frequently monitored during the course of the disease, from the diagnosis stage, during the treatment and beyond. It is influenced by socioeconomic factors, body mass index, anatomical location of the malignancy, therapeutic modality, oral health status, reconstruction and rehabilitation methods. Compromised OHQoL can predispose to malnutrition, and psychological deterioration in patients. Nutritional assessment with dietary modifications, extended domestic and social support network, and psychological support are evidence-based recommendations to be incorporated into routine care pathways for HNC patients. Clinicians should be aware of the various factors related to HNC patients and how they influence OHQoL to devise tailored management protocols for individual patients. Increased awareness, together with the need for the modifications of determinants related to patient factors, diagnosis and treatment, and post treatment can help to improve the OHQoL of patients with HNC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
NP: Writing – original draft, Formal analysis, Data curation. SS: Writing – review & editing, Data curation, Writing – original draft, Formal analysis. KE: Data curation, Formal analysis, Writing – original draft. CC: Writing – review & editing, Data curation. EG: Methodology, Conceptualization, Writing – review & editing, Supervision. RA-E: Writing – original draft, Writing – review & editing, Conceptualization, Project administration, Formal analysis, Methodology, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
CC was supported by an INSPIRE Scheme funded summer project, Institute of Dentistry, University of Aberdeen.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2025.1691065/full#supplementary-material
References
1. Colevas AD, Cmelak AJ, Pfister DG, Spencer S, Adkins D, Birkeland AC, et al. NCCN Guidelines® insights: head and neck cancers, version 2.2025: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. (2025) 23:2–11. doi: 10.6004/jnccn.2025.0007
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Anderson G, Ebadi M, Vo K, Novak J, Govindarajan A, Amini A. An updated review on head and neck cancer treatment with radiation therapy. Cancers (Basel). (2021) 13:1–12. doi: 10.3390/cancers13194912
4. Baiju RM, Peter E, Varghese NO, Sivaram R. Oral health and quality of life: current concepts. J Clin Diagn Res JCDR. (2017) 11:ZE21–6. doi: 10.7860/JCDR/2017/25866.10110
5. Sischo L, Broder HL. Oral health-related quality of life: what, why, how, and future implications. J Dent Res. (2011) 90:1264–70. doi: 10.1177/0022034511399918
6. Oral Health in America - A Report of the Surgeon General. Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health. (2000).
7. Slade GD. Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol. (1997) 25:284–90. doi: 10.1111/j.1600-0528.1997.tb00941.x
8. Dholam KP, Dugad JA, Sadashiva KM. Impact of oral rehabilitation on patients with head and neck cancer: a study using the liverpool oral rehabilitation questionnaire and the oral health impact profile-14. J Prosthet Dent. (2017) 117:559–62. doi: 10.1016/j.prosdent.2016.06.019
9. Larsson P, John MT, Nilner K, Bondemark L, List T. Development of an orofacial esthetic scale in prosthodontic patients. Int J Prosthodont. (2010) 23:249–56.20552092
10. Fan Y, Shu X, Lo ECM, Leung KCM. Development and validation of a chewing function questionnaire for Chinese older adults. J Dent. (2021) 104:103520. doi: 10.1016/j.jdent.2020.103520
11. Al-Rudayni AHM, Gopinath D, Maharajan MK, Menon RK. Impact of oral mucositis on quality of life in patients undergoing oncological treatment: a systematic review. Transl Cancer Res. (2020) 9:3126–34. doi: 10.21037/tcr.2020.02.77
12. Pace-Balzan A, Cawood JI, Howell R, Lowe D, Rogers SN. The liverpool oral rehabilitation questionnaire: a pilot study. J Oral Rehabil. (2004) 31:609–17. doi: 10.1111/j.1365-2842.2004.01279.x
13. European Organisation for Research and Treatment of Cancer. Available online at: https://www.eortc.org/ (Accessed August 8, 2025).
14. de Melo NB, de Bernardino ÍM, de Melo DP, Gomes DQC, Bento PM. Head and neck cancer, quality of life, and determinant factors: a novel approach using decision tree analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. (2018) 126:486–93. doi: 10.1016/j.oooo.2018.07.055
15. Warhekar S, Pimpale G, Warhekar A, Ingole R, Ingole Y. Assessing the impact of precancerous lesions on oral health-related quality of life: a study at tertiary care hospital. J Pharm Bioallied Sci. (2024) 16:S165–7. doi: 10.4103/jpbs.jpbs_439_23
16. Zhu L, Zhang J, Chen W, Svensson P, Wang K. Sensory recovery and oral health-related quality of life following tongue reconstruction using non-innervated radial forearm free flaps. Oral Oncol. (2021) 121:105471. doi: 10.1016/j.oraloncology.2021.105471
17. Naidu G, Shukla S, Nagi R, Jain S, Makkad R. Evaluation of oral health related quality of life in subjects diagnosed with head and neck malignancies undergoing chemotherapy, radiotherapy, and surgery. J Indian Acad Oral Med Radiol. (2019) 31:228. doi: 10.4103/jiaomr.jiaomr_71_19
18. Rodrigues I, Botelho J, Machado V, Proença L, Mendes JJ, Zagalo C. Profiling oral health status, values, and related quality of life in patients with oral cancer: a pilot study. Front Oral Health. (2023) 4:1268657. doi: 10.3389/froh.2023.1268657
19. Binnal A, Rajesh G, Prakash Saxena P, Banerjee S, Denny C, Tadakamadla SK. Health-related quality of life among oral and oropharyngeal cancer patients: an exploratory study. Oral Dis. (2022) 28:585–99. doi: 10.1111/odi.13772
20. Huang B-S, Chung C-F, Chang Y-L, Lee L-Y, Peng H-L, Chen S-C. Body mass index and self-care behaviors related to oral health–related quality of life in patients with oral squamous cell carcinoma within three months posttreatment. Support Care Cancer. (2021) 29:2239–48. doi: 10.1007/s00520-020-05737-x
21. Qamar S, Rozi S, Sawani S, Awan M, Akhtar S, Siddiqui M, et al. Oral health related quality of life in head and neck cancer survivors within the first year following treatment: a cross-sectional study in Karachi, Pakistan. Sci Rep. (2024) 14:2560. doi: 10.1038/s41598-024-52813-x
22. Barrios R, Tsakos G, García-Medina B, Martínez-Lara I, Bravo M. Oral health-related quality of life and malnutrition in patients treated for oral cancer. Support Care Cancer. (2014) 22:2927–33. doi: 10.1007/s00520-014-2281-5
23. Conway DI, Petticrew M, Marlborough H, Berthiller J, Hashibe M, Macpherson LMD. Socioeconomic inequalities and oral cancer risk: a systematic review and meta-analysis of case-control studies. Int J Cancer. (2008) 122:2811–9. doi: 10.1002/ijc.23430
24. Čanković M, Tešić M, Jevtić M, Stevanović D, Jovanović M, Kostić D, et al. Predictors of health-related quality of life in serbian patients with head and neck cancer. Med Oral Patol Oral Cirugia Bucal. (2022) 27:e340–50. doi: 10.4317/medoral.25274
25. de Melo NB, de Sousa VM, Bernardino MM, de Melo DP, Gomes DQC, Bento PM. Oral health related quality of life and determinant factors in patients with head and neck cancer. Med Oral Patol Oral Cirugia Bucal. (2019) 24:e281–9. doi: 10.4317/medoral.22670
26. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primer. (2020) 6:92–92. doi: 10.1038/s41572-020-00224-3
27. Karbach J, Al-Nawas B, Moergel M, Daubländer M. Oral health-related quality of life of patients with oral lichen Planus, oral leukoplakia, or oral squamous cell carcinoma. J Oral Maxillofac Surg. (2014) 72:1517–22. doi: 10.1016/J.JOMS.2014.04.008
28. Stolte K, Danker K, Witt M, Ebhardt H, Dommisch H. Upregulation of psoriasin/S100A7 correlates with clinical severity in patients with oral lichen planus. Clin ORAL Investig. (2024) 28:318. doi: 10.1007/s00784-024-05717-z
29. Zucoloto ML, Shibakura MEW, Pavanin JV, Garcia FT, Da Silva Santos PS, Maciel AP, et al. Severity of oral lichen planus and oral lichenoid lesions is associated with anxiety. Clin Oral Investig. (2019) 23:4441–8. doi: 10.1007/s00784-019-02892-2
30. Alsoghier A, Riordain RN, Fedele S, Porter S. Psychosocial impacts of oral epithelial dysplasia. J Oral Pathol Med. (2021) 50:700–7. doi: 10.1111/jop.13173
31. Gabrić D. Evaluation of innovative digitally controlled er:yAG Laser in surgical treatment of oral leukoplakia – a preliminary study. Acta Clin Croat. (2019) 58:615–20. doi: 10.20471/acc.2019.58.04.07
32. Matulić N, Bago I, Sušić M, Gjorgievska E, Kotarac Knežević A, Gabrić D. Comparison of er:yAG and er,cr:ySGG Laser in the treatment of oral leukoplakia lesions refractory to the local retinoid therapy. Photobiomodulation Photomed Laser Surg. (2019) 37:362–8. doi: 10.1089/photob.2018.4560
33. Memon AB, Rahman AAU, Channar KA, Zafar MS, Kumar N. Evaluating the oral-health-related quality of life of oral submucous fibrosis patients before and after treatment using the OHIP-14 tool. Int J Environ Res Public Health. (2022) 19:1821. doi: 10.3390/ijerph19031821
34. Schweyen R, Kuhnt T, Wienke A, Eckert A, Hey J. The impact of oral rehabilitation on oral health-related quality of life in patients receiving radiotherapy for the treatment of head and neck cancer. Clin Oral Investig. (2017) 21:1123–30. doi: 10.1007/s00784-016-1874-4
35. Li W, Yang Y, Xu Z, Liu F, Cheng Y, Xu L, et al. Assessment of quality of life of patients with oral cavity cancer who have had defects reconstructed with free anterolateral thigh perforator flaps. Br J Oral Maxillofac Surg. (2013) 51:497–501. doi: 10.1016/j.bjoms.2012.09.005
36. Linsen S, Schmidt-Beer U, Fimmers R, Grüner M, Koeck B. Craniomandibular pain, bite force, and oral health-related quality of life in patients with jaw resection. J Pain Symptom Manage. (2009) 37:94–106. doi: 10.1016/j.jpainsymman.2006.12.019
37. Xiao Y, Liang Y, Yang L, Yang W, Liao G. Long-term quality of life in patients with maxillofacial malignancies who have undergone craniofacial resection: a cross-sectional survivorship study. J Oral Maxillofac Surg. (2019) 77:2573–83. doi: 10.1016/j.joms.2019.05.025
38. Aparna KS, Manjunath PP, Sowmya KR. Oral health Status and quality of life among head and neck cancer subjects receiving radiotherapy. J Int Dent Med Res. (2022) 15:263–7. ISSN 1309-100X
39. Caminha RDG, Caldas RJ, Bueno ICM, Scaraficci AC, Da Silva Santos PS. A time frame evaluation of the oral health-related quality of life among patients with head and neck cancer. Oral Oncol Rep. (2024) 11:100593. doi: 10.1016/j.oor.2024.100593
40. Fromm L, Gotfredsen K, Wessel I, Øzhayat EB. Oral health-related quality of life, oral aesthetics and oral function in head and neck cancer patients after oral rehabilitation. J Oral Rehabil. (2019) 46:738–46. doi: 10.1111/joor.12806
41. Gondivkar S, Gadbail A, Sarode S, Dasgupta S, Sharma B, Hedaoo A, et al. Prevalence of trismus and its impact on oral health-related quality of life in patients treated for oral squamous cell carcinoma. Asian Pac J Cancer Prev. (2021) 22:2437–44. doi: 10.31557/APJCP.2021.22.8.2437
42. Indrapriyadharshini K, Madankumar P, Karthikeyan G. Oral health-related quality of life in patients treated for oral malignancy at Kanchipuram district, India: a cross-sectional study. Indian J Cancer. (2017) 54:11. doi: 10.4103/ijc.IJC_116_17
43. Soldera EB, Ortigara GB, Bonzanini LIL, Schulz RE, Danesi CC, Antoniazzi RP, et al. Clinical and sociodemographic factors associated with oral health-related quality of life in survivors of head and neck cancer. Head Neck. (2020) 42:886–97. doi: 10.1002/hed.26063
44. Tesic M, Cankovic M, Jevtic M, Stevanovic D. Validation of the oral health impact profile - 14 in patients with head and neck cancer. Med Oral Patol Oral Cirugia Bucal. (2020) 25:e739–44. doi: 10.4317/medoral.23765
45. Westgaard KL, Hynne H, Amdal CD, Young A, Singh PB, Chen X, et al. Oral and ocular late effects in head and neck cancer patients treated with radiotherapy. Sci Rep. (2021) 11:4026. doi: 10.1038/s41598-021-83635-w
46. Kosgallana S, Jayasekara P, Abeysinghe P, Lalloo R. Oral health related quality of life of oral cancer patients treated with radiotherapy alone or with chemotherapy in a tertiary referral centre in Sri Lanka. BMC Oral Health. (2023) 23:162. doi: 10.1186/s12903-023-02854-x
47. Barkokebas A, Silva IHM, de Andrade SC, Carvalho AAT, Gueiros LAM, Paiva SM, et al. Impact of oral mucositis on oral-health-related quality of life of patients diagnosed with cancer. J Oral Pathol Med. (2015) 44:746–51. doi: 10.1111/jop.12282
48. Jung Y-S, Park E-Y, Sohn H-O. Oral health Status and oral health-related quality of life according to presence or absence of mucositis in head and neck cancer patients. J Cancer Prev. (2019) 24:43–7. doi: 10.15430/JCP.2019.24.1.43
49. Malouf JG, Aragon C, Henson BS, Eisbruch A, Ship JA. Influence of parotid-sparing radiotherapy on xerostomia in head and neck cancer patients. Cancer Detect Prev. (2003) 27:305–10. doi: 10.1016/S0361-090X(03)00095-3
50. Nascimento M, Farias A, Carvalho A, Albuquerque R, Ribeiro L, Leao J, et al. Impact of xerostomia on the quality of life of patients submitted to head and neck radiotherapy. Med Oral Patol Oral Cirugia Bucal. (2019) 24(6):e770–5. doi: 10.4317/medoral.23131
51. Winter A, Schulz SM, Schmitter M, Müller-Richter U, Kübler A, Kasper S, et al. Comprehensive geriatric assessment and quality of life aspects in patients with recurrent/metastatic head and neck squamous cell carcinoma (HNSCC). J Clin Med. (2023) 12:5738. doi: 10.3390/jcm12175738
52. Patton LL, Helgeson ES, Brennan MT, Treister NS, Sollecito TP, Schmidt BL, et al. Oral health-related quality of life after radiation therapy for head and neck cancer: the OraRad study. Support Care Cancer. (2023) 31:286. doi: 10.1007/s00520-023-07750-2
53. Yang J, Yang L, Han Q, Zhang Y, Tao Z, Zhou Y, et al. The dose limits of teeth protection for patients with nasopharyngeal carcinoma undergoing radiotherapy based on the early oral health-related quality of life. OPEN Med. (2023) 18(1):20230673. doi: 10.1515/med-2023-0673
54. Martins AFL, Morais MO, De Sousa-Neto SS, De Jesus APG, Nogueira TE, Valadares MC, et al. Photobiomodulation reduces the impact of radiotherapy on oral health-related quality of life due to mucositis-related symptoms in head and neck cancer patients. Lasers Med Sci. (2021) 36:903–12. doi: 10.1007/s10103-020-03167-z
55. Oliveira F, Borges M, Malta C, Moura J, Forte C, Barbosa J, et al. Comparison of a daily and alternate-day photobiomodulation protocol in the prevention of oral mucositis in patients undergoing radiochemotherapy for oral cancer: a triple-blind, controlled clinical trial. Med Oral Patol Oral Cirugia Bucal. (2024) 29(3):e430–40. doi: 10.4317/medoral.26436
56. Pereira RMDS, Bastos MDR, Ferreira MP, De Freitas O, De Macedo LD, De Oliveira HF, et al. Topical pilocarpine for xerostomia in patients with head and neck cancer treated with radiotherapy. Oral Dis. (2020) 26:1209–18. doi: 10.1111/odi.13343
57. Jiang N, Zhao Y, Mårtensson J, Stensson M. The effects of an integrated supportive programme on oral health in patients with head and neck cancer undergoing radiotherapy: a randomized controlled trial. Int J Dent Hyg. (2024) 22(4):878–86. doi: 10.1111/idh.12801
58. Santos PSS, Cremonesi AL, Quispe RA, Rubira CMF. The impact of oral health on quality of life in individuals with head and neck cancer after radiotherapy: the importance of dentistry in psychosocial issues. Acta Odontol Latinoam. (2017) 30(2):62–7. ISSN 1852-4834.29248940
59. Andreassen R, Hadler-Oslen E. Eating and speech problems in oral and pharyngeal cancer survivors - associations with treatment-related side-effects and time since diagnosis. Spec Care Dentist. (2022) 43(5):561–71. doi: 10.1111/scd.12791
60. Abed H, Reilly D, Burke M, Sharka R, Daly B. The association between dental arch length and oral health-related quality of life in head and neck cancer patients post-radiotherapy. Spec Care Dentist. (2023) 43:111–8. doi: 10.1111/scd.12755
61. Pow EHN, Kwong DLW, Sham JST, Lee VHF, Ng SCY. Can intensity-modulated radiotherapy preserve oral health-related quality of life of nasopharyngeal carcinoma patients? Int J Radiat Oncol. (2012) 83:e213–21. doi: 10.1016/j.ijrobp.2011.12.040
62. Hassel AJ, Danner D, Freier K, Hofele C, Becker-Bikowski K, Engel M. Oral health-related quality of life and depression/anxiety in long-term recurrence-free patients after treatment for advanced oral squamous cell cancer. J Cranio-Maxillofac Surg. (2012) 40:e99–e102. doi: 10.1016/j.jcms.2011.05.011
63. Li N, Otomaru T, Taniguchi H. Sleep quality in long-term survivors of head and neck cancer: preliminary findings. Support Care Cancer. (2017) 25:3741–8. doi: 10.1007/s00520-017-3804-7
64. Maeda M, Hirose M, Wada K, Kishimoto M, Akashi M, Kimoto A, et al. Elucidating the masticatory function and oral quality of life according to the range of mandibulectomy. J Oral Maxillofac Surg Med Pathol. (2018) 30:220–4. doi: 10.1016/j.ajoms.2018.01.004
65. Dholam K, Chouksey G, Dugad J. Impact of oral rehabilitation on patients with head and neck cancer: study of 100 patients with liverpool oral rehabilitation questionnaire and the oral health impact profile. Indian J Otolaryngol Head Neck Surg. (2020) 72:308–12. doi: 10.1007/s12070-020-01801-4
66. Ettl T. Impact of radiotherapy on implant-based prosthetic rehabilitation in patients with head and neck cancer: a prospective observational study on implant survival and quality of life-preliminary results. Facial Surg. (2016) 44(9):1453–62. doi: 10.1016/j.jcms.2016.07.016
67. Hagio M, Ishizaki K, Ryu M, Nomura T, Takano N, Sakurai K. Maxillofacial prosthetic treatment factors affecting oral health-related quality of life after surgery for patients with oral cancer. J Prosthet Dent. (2018) 119:663–70. doi: 10.1016/j.prosdent.2017.05.017
68. Kalaignan P, Shree Mohan J. Oral health related quality of life with mandibular resection prosthesis. Biomed Pharmacol J. (2018) 11:1423–8. doi: 10.13005/bpj/1506
69. Matapathi N, Shenoy V, Shenoy R, Miranda G, Upadhya M, Mehendale A, et al. Evaluation of the quality of life of patients with maxillofacial defects after prosthodontic rehabilitation: a cross-sectional study. J CANCER Res Ther. (2022) 18:S219–25. doi: 10.4103/jcrt.JCRT_889_20
70. Stefano DC, Francesca DA, Matteo A, Jamshir S, Michele F, Brauner E. Prosthetic rehabilitation with use of palatal augmentation prosthesis in patients affected by functional limitations of the tongue. J Int Dent Med Res. (2019) 12:607–11. ISSN 1309-100X
71. Yusa K, Yamanouchi H, Yoshida Y, Ishikawa S, Sakurai H, Iino M. Evaluation of quality of life and masticatory function in patients treated with mandibular reconstruction followed by occlusal rehabilitation with dental implants: a preliminary report. J Oral Maxillofac Surg Med Pathol. (2017) 29:499–503. doi: 10.1016/j.ajoms.2017.06.004
72. Coward TJ, Richards R, Fenlon MR, Scott BJJ. Effect of obturators on facial form following surgery for head and neck cancer and impact on the perception of appearance. J Dent. (2020) 92:103230. doi: 10.1016/j.jdent.2019.103230
73. Karayazgan-Saracoglu B, Atay A, Korkmaz C, Gunay Y. Quality of life assessment of implant-retained overdentures and fixed metal-acrylic resin prostheses in patients with marginal mandibulectomy. J Prosthet Dent. (2017) 118:551–60. doi: 10.1016/j.prosdent.2017.01.025
74. Kumar VV, Jacob PC, Ebenezer S, Kuriakose MA, Kekatpure V, Baliarsing AS, et al. Implant supported dental rehabilitation following segmental mandibular reconstruction- quality of life outcomes of a prospective randomized trial. J Cranio-Maxillofac Surg. (2016) 44:800–10. doi: 10.1016/j.jcms.2016.04.013
75. Yanamoto S, Soutome S, Murata M, Kawakita A, Yamaguchi E, Yoshida K, et al. Efficacy of silicone soft reliner on the obturator prosthesis after maxillectomy for oral malignant tumors: a single-arm prospective interventional study. Clin Exp Dent Res. (2020) 6:612–7. doi: 10.1002/cre2.326
76. Balogun Z, Gardiner L, Li J, Moroni E, Rosenzweig M, Nilsen M. Neighborhood deprivation and symptoms, psychological distress, and quality of life among head and neck cancer survivors. JAMA Otolaryngol-Head Neck Surg. (2024) 150:295–302. doi: 10.1001/jamaoto.2023.4672
77. O’Brien KM, Timmons A, Butow P, Gooberman-Hill R, O’Sullivan E, Balfe M, et al. Associations between neighbourhood support and financial burden with unmet needs of head and neck cancer survivors. Oral Oncol. (2017) 65:57–64. doi: 10.1016/J.ORALONCOLOGY.2016.12.019
78. Belapurkar P, Acharya S, Shukla S, Kumar S, Khurana K, Acharya N. Prevalence of anxiety, depression, and perceived stress among family caregivers of patients diagnosed with oral cancer in a tertiary care hospital in central India: a cross-sectional study. Cureus J Med Sci. (2023) 15(10):e47100. doi: 10.7759/cureus.47100
79. Widaman AM, Day AG, Kuhn MA, Dhaliwal R, Baracos V, Findlay M, et al. Poor nutrition status associated with low patient satisfaction six months into treatment for head and neck/esophageal cancer treatment: a prospective multicenter cohort study. Nutr Clin Pract. (2025) 40:405–19. doi: 10.1002/ncp.11211
80. Chiba T, Izumita K, Koyama S, Sato N, Tagaino R, Hatakeyama T, et al. Effects of maxillofacial prosthetic treatment on oral health-related quality of life and masticatory ability of patients with head and neck tumors. J ORAL Sci. (2024) 66:30–6. doi: 10.2334/josnusd.23-0162
81. Khamdi S, Matangkasombut O, Lam-Ubol A. Non-pharmacologic interventions for management of radiation-induced dry mouth: a systematic review. Oral Dis. (2024) 30:2876–93. doi: 10.1111/odi.14804
82. Chavan S, Bhuvad S, Kumbhlakar B, Auti J, Walunj T, Pathak S, et al. Antimicrobial and antioxidant potential of a standardized ayurvedic formulation explains its clinical efficacy as gargles in post-radiotherapy oral cancer patients. J Herb Med. (2021) 30:100510–100510. doi: 10.1016/j.hermed.2021.100510
83. Martins A, Pereira C, Morais M, de Sousa-Neto S, Valadares M, Freitas N, et al. Effects of a mucoadhesive phytomedicine (Curcuma longa L. And Bidens pilosa L.) on radiotherapy-induced oral mucositis and quality of life of patients undergoing head and neck cancer treatment: randomized clinical trial. Support Care Cancer. (2023) 31(9):517. doi: 10.1007/s00520-023-07971-5
84. Shah S, Rath H, Sharma G, Senapati SN, Mishra E. Effectiveness of curcumin mouthwash on radiation-induced oral mucositis among head and neck cancer patients: a triple-blind, pilot randomised controlled trial. Indian J Dent Res Off Publ Indian Soc Dent Res. (2020) 31:718–27. doi: 10.4103/ijdr.IJDR_822_18
85. Humphris GM, Ozakinci G. Psychological responses and support needs of patients following head and neck cancer. Int J Surg. (2006) 4:37–44. doi: 10.1016/j.ijsu.2005.12.004
86. Henry M, Chen L, Ducharme L, Devault-Tousignant C, Rosberger Z, Frenkiel S, et al. Genetic risk for depression and quality of life in patients with head and neck cancer. JAMA Otolaryngol-Head Neck Surg. (2024) 150:598–606. doi: 10.1001/jamaoto.2024.0376
87. Jaafar NR N, Hamid N A, Hamdan NA, Rajandram RK, Mahadevan R, Mohamad Yunus MR, et al. Posttraumatic growth, positive psychology, perceived spousal support, and psychological complications in head and neck cancer: evaluating their association in a longitudinal study. Front Psychol. (2022) 13:920691. doi: 10.3389/fpsyg.2022.920691
88. Patel J, Csikar J, Korfage A, Witjes M, Douglas G, Kanatas A. Do the existing quality of life tools appropriately measure oral health related quality of life in head and neck cancer? A scoping review. Br J Oral Maxillofac Surg. (2025) 63(6):415–22. doi: 10.1016/j.bjoms.2025.05.004
89. In ‘t Veld M, Jager DHJ, Chhangur CN, Ziesemer KA, Leusink FKJ, Schulten EAJM. Oral-Functioning questionnaires in patients with head and neck cancer: a scoping review. J Clin Med. (2023) 12:3964. doi: 10.3390/jcm12123964
90. Ishida S, Shibuya Y, Kobayashi M, Komori T. Assessing stomatognathic performance after mandibulectomy according to the method of mandibular reconstruction. Int J Oral Maxillofac Surg. (2015) 44:948–55. doi: 10.1016/j.ijom.2015.03.011
91. Brandão TB, Vechiato Filho AJ, Prado Ribeiro AC, Gebrim EMMS, Bodard A-G, Da Silva DP, et al. Evaluation of use of acrylic resin-based surgical guide in the function and quality of life provided by mandibular prostheses with microvascular free fibula flap: a four-year, randomized, controlled trial. J Prosthet Dent. (2016) 116:457–463.e2. doi: 10.1016/j.prosdent.2016.02.012
92. Li W, Zhang P, Li R, Liu Y, Kan Q. Radial free forearm flap versus pectoralis major pedicled flap for reconstruction in patients with tongue cancer: assessment of quality of lif. Med Oral Patol Oral Cirugia Bucal. (2016) 21(6):e737–42. doi: 10.4317/medoral.21274
93. Wang S, Yin S, Zhang Z, Su X, Xu Z. Quality of life after oral cancer resection and free flap reconstruction. J Oral Maxillofac Surg. (2019) 77:1724–32. doi: 10.1016/j.joms.2019.02.029
94. Yuan Y, Zhang P, He W, Li W. Comparison of oral function: free anterolateral thigh perforator flaps versus vascularized free forearm flap for reconstruction in patients undergoing glossectomy. J Oral Maxillofac Surg. (2016) 74:1500.e1–.e6. doi: 10.1016/j.joms.2016.03.039
95. Zhu L, Zhang J, Song X, Hou W, Wu S, Chen W, et al. Sensory recovery of non-innervated free flaps and nasolabial island flaps used for tongue reconstruction of oncological defects. J Oral Rehabil. (2017) 44:736–48. doi: 10.1111/joor.12510
96. Xu Q, Wang S-M, Liu Y-H, Yin S-C, Su X-Z, Xu Z-F. Comparison between the radial forearm and groin soft tissue free flaps for reconstruction in patients with oral cavity cancer: a quality of life analysis. Int J Oral Maxillofac Surg. (2022) 51:1289–95. doi: 10.1016/j.ijom.2022.04.011
97. Al-Aroomi M, Al-Worafi N, Ma Y, Alkebsi K, Mohamed A, Jiang C. Patient-reported outcomes after oral cancer reconstructions with radial and ulnar forearm-free flaps. Oral Dis. (2024) 30(8):4878–85. doi: 10.1111/odi.14968
98. Song P, Li J, Yang D, Hu K, Zhao T. Assessment of quality of life after soft tissue resection of head and neck carcinoma and reconstruction with double-paddle peroneal artery perforator free flap. Br J Oral Maxillofac Surg. (2023) 61:176–80. doi: 10.1016/j.bjoms.2022.10.008
Keywords: oral health related quality of life, oral health impact profile, head and neck cancer, oral cancer, oral potentially malignant disorders
Citation: Piyarathne N, Sinclair S, Elsayed KS, Cook C, Gupta E and Abu-Eid R (2025) Oral health–related quality of life in head and neck cancer: a systematic review. Front. Oral Health 6:1691065. doi: 10.3389/froh.2025.1691065
Received: 22 August 2025; Accepted: 28 October 2025;
Published: 19 November 2025.
Edited by:
Eliete Neves Da Silva Guerra, University of Brasilia, BrazilReviewed by:
Matthijs In 'T Veld, Amsterdam UMC, VUMC, NetherlandsLarissa Di Carvalho, University of Brasilia, Brazil
Copyright: © 2025 Piyarathne, Sinclair, Elsayed, Cook, Gupta and Abu-Eid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ekta Gupta, ZWt0YS5ndXB0YUBhYmRuLmFjLnVr; Rasha Abu-Eid, ci5hYnUtZWlkQGJoYW0uYWMudWs=
†These authors have contributed equally to this work
 Nadisha Piyarathne
Nadisha Piyarathne Serena Sinclair1,†
Serena Sinclair1,† Ekta Gupta
Ekta Gupta Rasha Abu-Eid
Rasha Abu-Eid