- 1Food Safety Intervention Technologies Research Unit, U.S. Department of Agriculture, Wyndmoor, PA, United States
- 2Residue Chemistry and Predictive Microbiological Research Unit. U.S. Department of Agriculture, Agriculture Research Service, Eastern Regional Research Center, Wyndmoor, PA, United States
Cantaloupes, honeydew melons and watermelons inoculated with Salmonella cocktail at 4.5, 3.8, and 3.2 log10 CFU/cm2, respectively, were sanitized with 200 ppm chlorine before rinds removal, cutting, and juice preparation. Efficacy of 200 ppm chlorine in reducing transfer of Salmonella, aerobic mesophilic bacteria, yeast and mold, and Pseudomonas from the melon surfaces to freshly prepared fruit juice was investigated including the effect of waiting period before refrigeration of the juices. The melon juice filtrates were refrigerated immediately or stored at room temperature (~22°C) for 3 and 5 h before refrigeration. Average Salmonella bacteria recovered in fresh melon juice prepared from unwashed whole cantaloupes, watermelon and honeydew melons was 1.4, 0.5, and 0.4 log10 CFU/ml, respectively. Juices from unwashed inoculated melons had the highest bacterial populations and storage at an abusive temperature of 10°C led to proliferation. Holding these juices at room temperature for 5 h before refrigeration allowed Salmonella bacteria to increase by 0.5–0.8 log in cantaloupe juice and 0.3–0.5 log in watermelon and honeydew juices. No Salmonella bacteria was determined in fresh juices prepared from melons washed with chlorinated water. The results of this study showed that washing melons with 200 ppm chlorine before juice preparation and immediately refrigerating the juice will minimize the chances of Salmonella proliferation.
Introduction
Consumers are becoming health conscious and are demanding food products that are fresh and safe, and fresh cut fruits and juices are among the foods that are in high demand. Produce processors, and food manufacturers are responding to consumers' demand for safe juice or fruits which may be improperly processed and/or contaminated with human bacterial pathogens. Prepared fresh-cut melon in the supermarket is becoming very popular with the US consumer due to the benefits of a diet rich in fruits and vegetables (Ukuku et al., 2017). Foodborne illnesses due to consumption of fresh fruit and vegetable produce contaminated with bacteria is food safety concern in the United States. Several researchers have documented incidences of foodborne illnesses associated with consumption of fresh fruit and vegetable produce (Batz et al., 2004; CDC, 2011). The authors reported that consumption of bacteria contaminated fresh fruit and vegetable produce leads to 1.2 million illness per year, leading to 7,100 hospitalizations, and 134 human deaths. The authors also concluded that the cost associated with these illnesses was $1.4 billion per year (Batz et al., 2004; CDC, 2011). The reason for bacterial contamination of produce surfaces are mostly due to frequent contact with soil, insects, animals, or humans during growing or harvesting and in the processing plant (Castillo et al., 2004; Heaton and Jones, 2007; Ukuku et al., 2012). Therefore, surface microflora of fruits and vegetables is very important to fresh juice producers, processors and consumers when considering microbial food safety of fresh-cut fruits and or fruit juices. In a survey of imported 151 cantaloupe melons from Mexico, Costa Rica and Guatemala, FDA indicated that 5.3% of these melons was positives for Salmonella and 2% for Shigella (FDA, 2016) and several salmonellosis outbreaks has been associated with consumption of contaminated fresh-cut melon pieces (Ukuku, 2004) and fresh fruits and vegetable juices (Danyluk et al., 2012). Survival and growth of bacteria on fresh-cut melons was attributed to the nutrient composition of fresh-cut melons (FDA, 2015).
Methods of washing treatments for reducing bacterial populations on melon rind surfaces have been reported (Ukuku and Fett, 2004; Parnell et al., 2005) and the amount of chlorine in the wash water is usually up to 200 ppm as suggested in the Federal regulations (21 CFR-Part 173 and 21 CFR Part 178 permit). The chlorine wash treatments do not inactivate all bacterial populations on produce surfaces and can only achieve ~2–3 log reductions depending on type and method of application. Also, there is a concern of potential formation of harmful by-products by chlorine (CDC, 2016) prompting the need for alternative antimicrobial wash treatments. Prepared fresh-melon juice is becoming very popular with the U.S. due to the health benefits associated with drinking unpasteurized raw fresh juices. To date, most of the fresh-juices are prepared at home, bars, supermarkets and or by regional distributors.
Fresh juices prepared at home may be left at room temperature for several hours before consumption or refrigeration for later use. Bacterial populations on fruits surfaces that transferred to the juice during juice preparation can survive and grow when such juice is left standing at room temperature for hours before consumption. Previously, we reported transfer, survival and growth of Salmonella Poona from cantaloupe rind surfaces to fresh-cut pieces during fresh-cut preparation and later reported survival of this pathogen in fresh-cut pieces during storage at room or refrigeration temperatures (Ukuku, 2004). Similarly, there is limited information on the effect of soluble solids of fruit pulps or juices on the survival and growth of yeast and mold, aerobic mesophilic bacteria including human pathogenic bacteria. In this study, the impact of soluble solids of freshly prepared melon juice and the effect of time after preparation and before refrigeration and storage temperature at 5, 10, and 25°C on bacterial populations associated with each juice was investigated. The information on transfer and survival of spoilage and Salmonella bacteria in fresh cantaloupe, watermelon and honeydew juices and the effect of waiting period before refrigeration as presented in this study is aimed to provide the regulatory industry a base line data that should guide consumers, fresh juice industry and consumers in good manufacturing practices (GMP's) procedures for enhancing the microbial food safety of freshly prepared fruit juice.
Materials and Methods
Preparation of Bacterial Inoculum
Salmonella strains: Salmonella Newport 02-216, sprout related outbreak; Salmonella Poona 418, meat; Salmonella Hidalgo 02-517-2, cantaloupe; Salmonella Typhimurium 45, cantaloupe; Salmonella St. Paul FSIS 039, cantaloupe used in this study were maintained on Brain Heart Infusion Agar (BHIA, BBL/Difco, Sparks, MD) slants at 4°C. Each culture was grown on 5 ml Brain Heart Infusion Broth (BHIB, BBL/Difco) with two successive transfers by loop inocula to a new 5 ml BHIB. A final transfer of 0.2 ml from each strain was made into 20 ml BHIB with incubation at 36°C for 18 h under static conditions. The bacterial cells were centrifuged (10,000 × g, 10 min) at 4°C, and the cell pellets were harvested and washed in phosphate buffer saline (PBS) (BBL/Difco). The final bacterial concentration of each Salmonella strain was 2.5 × 109 CFU/ml, and were used to prepare a cocktail of 6.8 log CFU/ml Salmonella inoculum in 3 L of deionize distill water (ddH2O).
Inoculation of Melons With Salmonella Bacteria
Cantaloupes (Western shippers, 1,648–1,672 g), honeydew (Cucumis melo, 1,663–1,678 g), and watermelons (Citrullus lantus, 1,925–1,976 g) purchased from a local distributor were placed inside refrigerator at 4°C until used. Melons were removed from refrigerator and left at room temperature (22°C) for 18–20 h before inoculation with Salmonella bacteria prepared above. For inoculation treatment, two melons each were submerged in 3 L of 6.8 log CFU/ml bacterial inoculum stated above and agitated by stirring with a glove-covered hand for 5 min to ensure uniform inoculation (Ukuku et al., 2016). A total of ten melons each were inoculated with Salmonella bacteria. For washing treatment, a 200 ± 3 ppm chlorine was prepared from diluting Clorox [5.25% sodium hypochlorite (NaOCl), Clorox Company, Oakland CA] with sterile water. Total available chlorine in the solution was tested with a chlorine test kit (Hach Co., Ames, IA, a U.S. Environmental Protection Agency approved test kit). During washing treatments, two melons each were submerged in 3 L of ~200 ppm chlorine to minimize organic loading in the wash water and melons were washed inside a biosafety cabinet (Nuaire, Class II, Type A2, Plymouth, MN, USA) according to Ukuku and Fett (2004). All washed melons were air dried for 30 min inside the biosafety cabinet before rind removal and fresh-juice preparation.
Microbial Analysis of Whole Melon
Rind plugs from watermelon, honeydew and cantaloupe were randomly cut from the melon surfaces with sterilized stainless-steel cork-borer (22 mm in diameter). A total of rind plugs (70, with a total surface area of 266 cm2) per melon weighing ~25 g were homogenized in a waring commercial blendor (Dynamic Corp, New Hartford, CT, speed set at level 5, for 1 min) with 75 ml of 0.1% peptone water according to Ukuku and Fett (2004). Homogenized samples were diluted with 0.1% PW and a 0.1 ml aliquot was plated in duplicate on plate count agar (PCA, BBL/Difco Becton Dickinson Sparks, MD), with incubation at 30°C for 3 days for enumeration of aerobic mesophilic bacteria (Messer et al., 1984). Yeast and mold was enumerated according to Ukuku et al. (2012) on potato dextrose agar (PDA, BBL/Difco, Detroit, MI), amended with 10% tartaric acid to pH 3.5 (PDAA) and incubated at 25°C for 5 days. Similarly, Pseudomonas spp. were enumerated by plating 0.1 ml on Pseudomonas isolation agar (PIA, Difco/BBL) with incubation at 27°C for 3 days. Salmonella bacteria was enumerated on Xylose Lysine Sodium Tetradecylsufate (XLT4, BBL/Difco, Sparks, MD) agar incubated at 37°C for 24 h according to Ukuku et al. (2015). Colonies presumed to be Salmonella was confirmed by serological assays using latex agglutination (Oxoid, Ogdensburg, New York) and conventional biochemical methods of Andrews et al. (2018) in FDA Bacteriological Analytical Manual. The populations of Salmonella bacteria recovered on cantaloupes, honeydew and watermelons rind surfaces averaged 4.5, 3.8, and 3.2 log10 CFU/cm2, respectively.
Preparation of Melon Juices
Prior to fresh juice preparation, cutting boards and knives were sanitized in 200 ppm chlorine solution. Sanitized and un-sanitized whole cantaloupe, watermelon and honeydew melons were individually cut into four sections using sterile knives. The rinds were removed, and the interior flesh were further cut to generate average melon pieces of ~ 3 cm. The flesh of each melon's pieces was pooled (~500 g), and the pieces were put inside sterile filtered stomacher bag (Filtra, Fisher Scientific, Pittsburgh, PA, USA) containing 100 ml sterile water and pummeled for 2 min at medium speed with Stomacher model 400 (Dynatech Laboratories, Alexandria, VA). Filtrate per type of melon was poured into sterile 250 ml conical flasks (Fisher Scientific, Pittsburgh, PA, USA).
Microbiological Analysis of Melon Juice
Aliquots (0.1 ml) from the juices including the supernatants were plated on non-selective (PCA), and selective (PDAA, PIA, and XLT4) media depending on the microorganism of interest. Plating was done in duplicate and after appropriate incubations, CFU on each plate were counted and firmed as stated above.
Soluble Solids and PH
The pH of all melon juices and the soluble solids (°Brix) was measured using a Basic pH meter (Denver Instruments Inc., Arvada, CO, USA) and Abbe 3L refractometer (Spectronic Instruments, Rochester, NY, USA), respectively, as described by Sharma et al. (2005). Juice from each melon was split into two parts and one part was centrifuged at 1,000 g for 5 min at 5°C to sediment the soluble solids. The supernatants were collected and used as part of this study.
Effect of Waiting Period Before Refrigeration of Prepared Juices
All fresh juice was divided into different categories based on storage condition (5 or 10°C for 15 days). Some of the juices were placed inside the refrigerator immediately after preparation while some were left at room temperature (22°C) for 3 and 5 h before refrigeration and microbial determination. In another study, a serial dilution of Salmonella inoculum prepared above was added (50 μl) to cantaloupe, honeydew and water melon juices to achieve an average population of 2.1 log10 CFU/ml. All samples were taken, and 0.1 ml plated on XLT4 agar (Difco) with incubation as stated above to enumerate the colony forming unit (CFU).
Analysis of Data
Experiments were performed three times with duplicate analysis of samples during sampling time. Colony forming units was converted to log10 CFU/cm2 and log10 CFU/ml. All data generated were analyzed for analysis of variance (ANOVA) using Statistical Analysis System Program (SAS Institute, Cary, NC, USA), and each significant (p < 0.05) mean values for cells determined at specific waiting time before storage were calculated using Bonferroni LSD method (Miller, 1981).
Results and Discussion
Aerobic mesophilic bacteria, yeast and mold and Pseudomonas spp. on untreated whole cantaloupe, watermelon and honeydew rind surfaces and the populations transferred to fresh-cut pieces is shown in Table 1. Whole cantaloupe surfaces and cantaloupe fresh-cut pieces had the highest populations of aerobic mesophilic bacteria and the populations for each class of these organism was significantly (p < 0.05) higher in cantaloupe surfaces and fresh-cut pieces than whole watermelon and honeydew including their respective fresh-cut pieces (Table 1). The same trend for higher populations of yeast and mold and Pseudomonas spp. was observed on cantaloupes and fresh-cut pieces. Yeast and mold and Pseudomonas populations determined on watermelon and honeydew surfaces were not significantly (p > 0.05) different. Ukuku and Fett (2004) reported higher microbial populations on whole cantaloupe than honeydew melon rind surfaces and the results of this study agrees with our earlier report. The microbial populations determined on watermelons were significantly (p < 0.05) lower than cantaloupe and honeydew melons despite the lager surface area of the watermelon (Table 1).
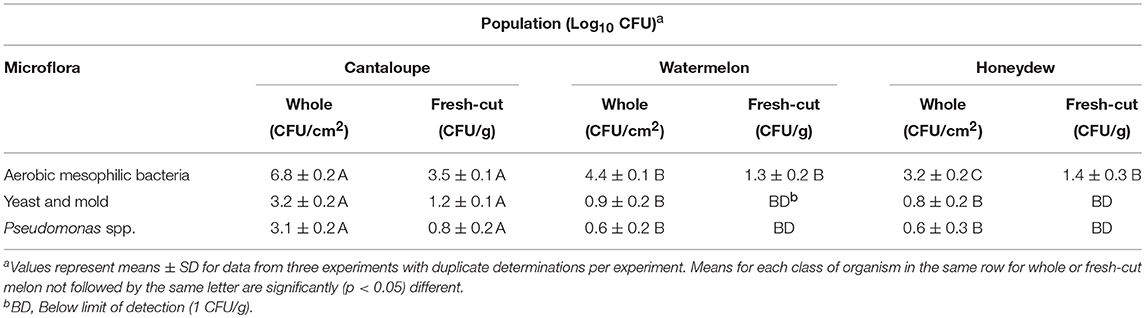
Table 1. Populations of aerobic mesophilic bacteria, yeast, and mold and pseudomonas spp. on unwashed cantaloupe, watermelon and honeydew rind surfaces and fresh-cut pieces.
Effect of 200 ppm chlorine wash on aerobic mesophilic population of each melons before fresh-cut preparation is shown in Table 2. The average surviving populations of aerobic mesophilic bacteria and yeast and mold and Pseudomonas were on cantaloupes, watermelons and honeydew melons after washing with 200 ppm chlorine was 4.4, 1.9, and 1.7 log10 CFU/cm2, respectively. Populations for aerobic mesophilic bacteria on watermelons treated with 200 ppm chlorine was 2.1 log10 CFU/cm2 while yeast and mold and Pseudomonas populations were below detection (< 1 CFU/cm2). The average surviving populations of aerobic mesophilic bacteria and yeast and mold and Pseudomonas on honeydew melons surfaces washed with 200 ppm chlorine averaged 1.9, 0.3, and 0.2 log10 CFU/cm2, respectively.
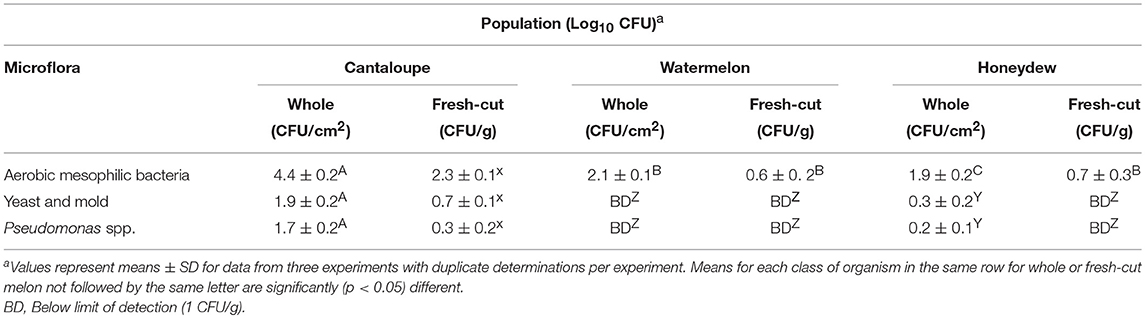
Table 2. Effect of 200 ppm chlorine wash on populations of aerobic mesophilic bacteria, yeast, and mold and pseudomonas spp. on cantaloupe, watermelon and honeydew rind surfaces and fresh-cut pieces.
Effect of Waiting Period on pH and Soluble Solids Before Refrigeration of Juice
The pH of cantaloupe juice and the soluble solids (°BRIX) determined immediately after preparation was higher than watermelon and honeydew melons (Table 3). Immediately after juice preparation, cantaloupe's pH and °BRIX values averaged 6.4 and 10.6, respectively, and was significantly different (p < 0.05) than watermelon and honeydew juices. There were no significant (p < 0.05) changes in pH and °BRIX values when the fresh juice was placed inside the refrigerator immediately after preparation and left refrigerated (5°C) for 5 h. However, holding melon juices at room temperature for 5 h before refrigeration led to significant (p < 0.05) changes in pH and °BRIX. The pH of cantaloupe and watermelon juices decreased slightly compared to day 0 while the °BRIX in all juices increased slightly. Similarly, the populations of aerobic mesophilic bacteria increased within this waiting period and was significantly (p < 0.05) higher than values in juices placed immediately inside refrigerator and those left at room temperature for 3 h before refrigeration. There were no significant (p > 0.05) changes in population of aerobic mesophilic bacteria in all juices that were placed inside the refrigerator immediately after preparation and determined after 3 h of refrigerated storage (Table 4).
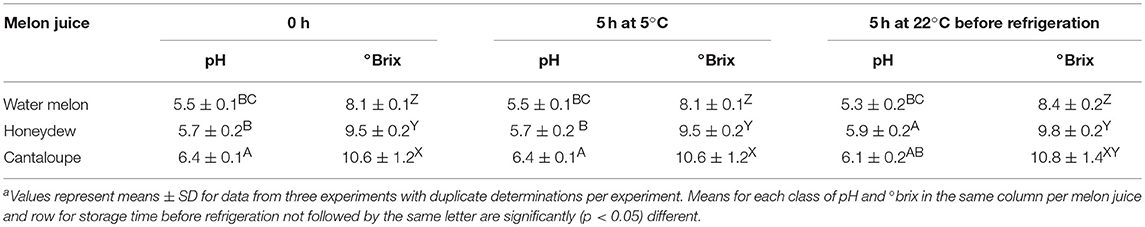
Table 3. Soluble solid (°brix) and pH of washed cantaloupe, watermelon, and honeydew melon juices immediately after preparation and storage at room temperature for 5 h before refrigerationa.
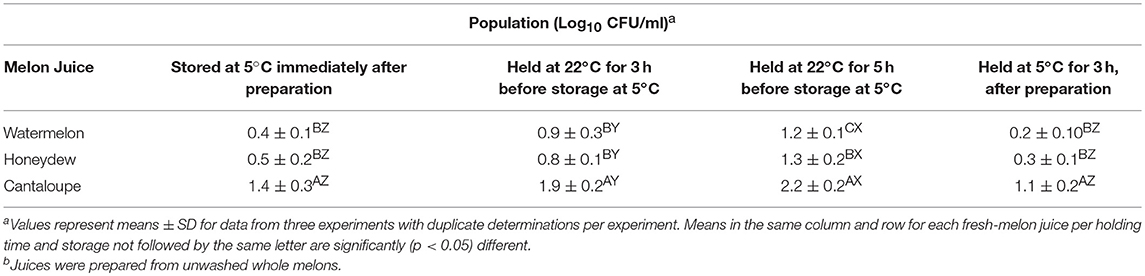
Table 4. Effect of waiting period on population of aerobic mesophilic bacteria of fresh- melon juice stored immediately at 5°C for 3 h or left at 22°C for 3 h before refrigerationb.
Effect of Waiting Period on Salmonella Bacteria in Juice
Populations of Salmonella in all fresh-melon juices recovered immediately after preparation and those determined in samples after waiting for 5 h at room temperature before refrigeration is shown in Table 5. All melon juice was prepared from control melons without 200 ppm chlorine wash treatments. Cantaloupe juice had the highest population of Salmonella bacteria compared to watermelon and honeydew juices. Salmonella bacteria in all juices left at room temperature for 3 and 5 h showed a slight increase and were significantly higher than juices stored immediately inside a refrigerator (5°C) or 3 h at room temperature after preparation. Holding cantaloupe juice at room temperature for 3 and 5 h before refrigeration led to 0.3 and 0.8 log increase of Salmonella bacteria (Table 4), respectively. A similar increase in the soluble solids of cantaloupe, honeydew, and watermelon juices was observed within the waiting period at room temperature before refrigeration (Table 3). It is appropriate to point out that after inoculation of all melons with Salmonella bacteria, cantaloupes fresh-cut pieces and juice had the highest population of Salmonella bacteria followed by honeydew and watermelon, respectively. In our earlier study, we reported transfer of Salmonella from melon rind surfaces to the interior flesh during fresh-cut preparation (Ukuku and Sapers, 2007).
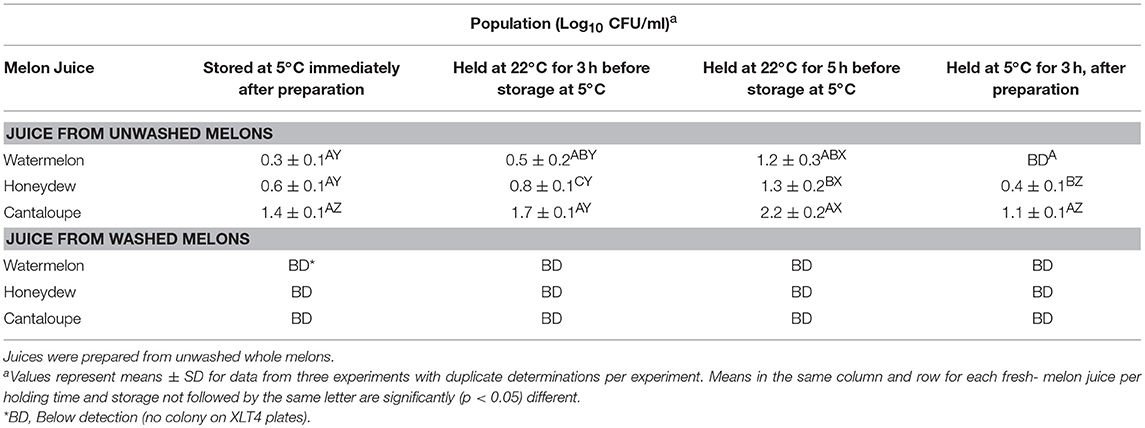
Table 5. Population of salmonella inoculated in fresh- melon juice immediately after preparation and storage at 5°C, and 22°C for 3 and 5 h before refrigeration.
Ukuku (2004) reported growth of Salmonella spp. in melon pieces stored at 23°C and Walsh et al. (2014) indicated that interior watermelon tissues support the growth of Salmonella bacteria during storage at 23°C. The authors did not report or make suggestions as to the influence of melons soluble solids may have had on the survival of Salmonella bacteria on the fresh-cut fruits. In our study, the 200 ppm chlorine wash reduced transfer of Salmonella populations on all melon rind surfaces to fresh-cut pieces. The population of Salmonella recovered from these fresh-cut pieces used in fresh juice preparation was <4 CFU/g for cantaloupes pieces and < 1 CFU/g for honeydew and watermelon pieces, respectively. When these fresh-cut pieces were used for preparing the juices, the pathogen was not detected on XLT4 plates (Table 5). It is possible that the residual Salmonella bacteria detected in fresh-cut pieces were diluted out during the juice preparation. The juice from these melons were similarly left at room temperature and or placed immediately inside refrigerator after preparation and still, no Salmonella bacteria was enumerated on XLT4 plates within the 5 h of waiting period at room temperature before refrigeration.
In another study, we deliberately contaminated the juices with Salmonella bacteria at 2.2 log CFU/ml. The 2.2 log CFU/ml Salmonella bacteria was chosen arbitrarily to allow us monitor the behavior of Salmonella bacteria in these juices during storage at 5°C (Figure 1). Aerobic mesophilic bacteria in cantaloupes juices was significantly (p < 0.05) different than populations in honeydew and watermelon juices. Populations for this native microflora grew in all juice during refrigerated storage at 5°C while the inoculated Salmonella bacteria survived with slightly higher numbers determined in in cantaloupe and honeydew juice at day 9. In juices stored at abusive temperature (10°C), growth populations for aerobic mesophilic bacteria in all juices were significantly (p < 0.05) higher than juices stored at 5°C for the same number of days (Figure 2). Salmonella bacteria in juices stored at abusive temperature (10°C) for 3 days slightly declined but increased in all juices after day 6. El-Safey (2013) reported variation in the survival of Salmonella spp. in fruit juices and concluded that survival was depended on pH, the type of strain, the type of juices and the incubation temperature. The author reported that Salmonella heidelberg survived up to 18 d in mango, guava, pineapple, and cocktail juices, 15 d in orange juice and 12 d in apple juice stored at 10°C. The author concluded that acid foods, especially if kept at refrigeration temperatures, support survival of Salmonella Heidelberg and may cause Salmonella heidelberg food poisoning and our current data agrees. The pH of cantaloupe, honeydew and watermelon juices in our study was 6.4, 5.7, and 5.5, respectively. The pH of cantaloupe and watermelon juice decreased by 0.2 point while honeydew melon juice increased by 0.2 point during 5 h waiting period before refrigeration. The juice storage time in El-Safey (2013) study and the type of juices studied was quite different from the juices in our study. However, El-Safey (2013) study and our current study reached a similar conclusion about survival of Salmonella in juices stored at 5 or 10°C and because of these small increases in Salmonella bacteria in fresh-melon juices, it is appropriate to follow the recommendation for immediate refrigeration of fresh-cut pieces and or juice after preparation (Ukuku and Sapers, 2007; FDA, 2008).
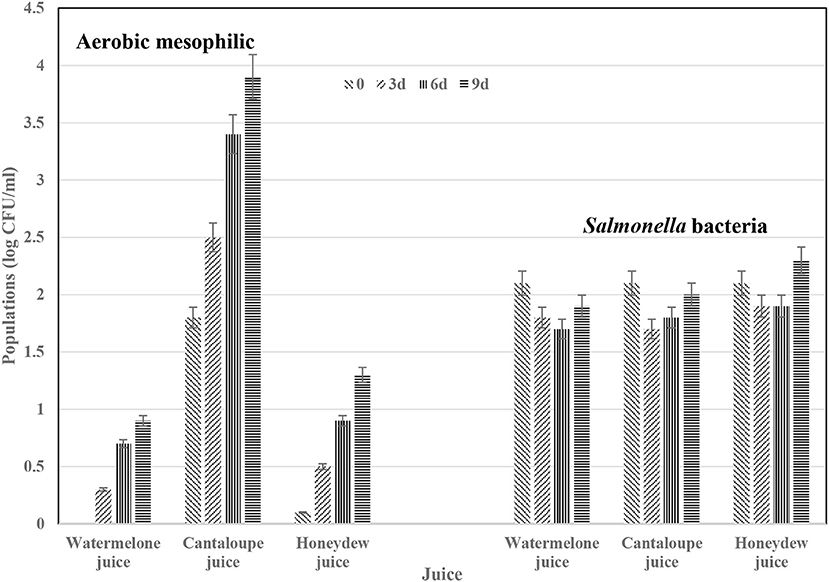
Figure 1. Survival of transferred aerobic mesophilic bacteria from melon rind surfaces during fresh-juice preparation with added Salmonella bacteria during storage at 5°C for 9 days. Values represent means ± SD for data from three experiments with duplicate determinations per experiment.
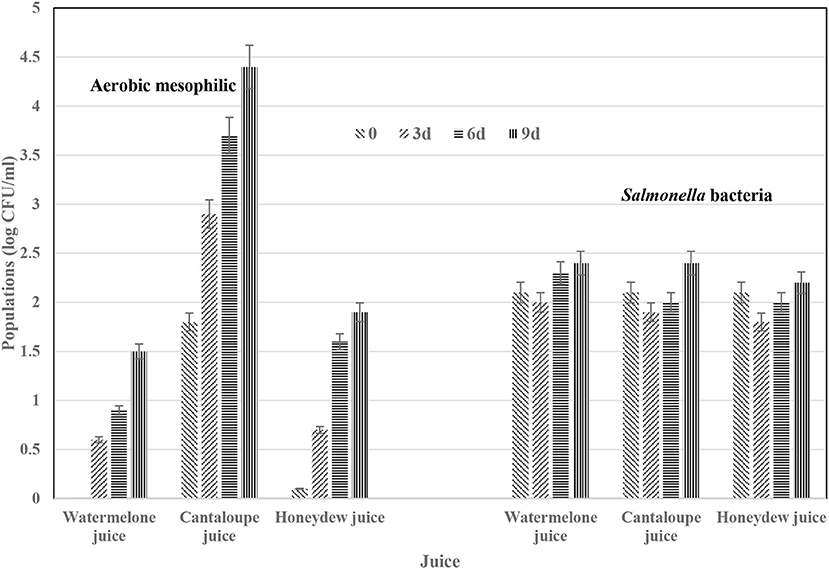
Figure 2. Survival of transferred aerobic mesophilic bacteria from melon rind surfaces during fresh-juice preparation with added Salmonella bacteria during storage at 10°C for 9 days. Values represent means ± SD for data from three experiments with duplicate determinations per experiment.
Salmonella bacteria in fruits accounted for 838 illnesses and 227 hospitalizations in United States from 2009 to 2015 (Dewey-Mattia et al., 2018). In a fruit and vegetable study on juice foodborne disease outbreak from 1922 to 2010, unpasteurized apple juice accounted for more outbreaks (Danyluk et al., 2010). The authors also, reported incidences of similar outbreaks associated with mixed fruit juice and watermelon juice, and the bacterial pathogens isolated from the apple juices were mostly E. coli O157:H7 bacteria while Salmonella spp. was reported in 18 cases of watermelon juice. Restaurants and sit-down dining accounted for 61 and 48% of foodborne outbreaks-associated illness in United States (Dewey-Mattia et al., 2018). The authors also, reported 636 and 561 outbreaks with 18,141 and 8,080 associated illnesses at catering/banquet facilities and private home, respectively while illnesses associated with fruits and vegetables accounted for 2,420 and 1,972. Consumers are becoming more health conscious thereby eating more foods described as “fresh” and tends to prepare such foods at home. Fruits and vegetables are mostly eaten raw or juiced and then drink therefore bacterial contamination and spoilage of melon juices was investigated. There is limited information on foodborne disease outbreaks related to cantaloupe and honeydew melon juices, respectively, and information presented in this study is intended to expound the knowledge base on what to do and or not do when preparing cantaloupe, honeydew and watermelon juices especially at home. It is true that Hazard Analysis and Critical Control Point (HACCP) regulation applies to domestic and imported juice products, still incidences of foodborne outbreaks related to fruits and vegetable juice occur probably due to inappropriate GMPs related to sanitation standard operating procedures (SSOPs).
Despite the washing treatment with ~200 ppm chlorine, the presence of aerobic mesophilic bacteria and Salmonella bacteria were detected on cantaloupe fresh-cut pieces than honeydew melon or watermelon. The inability of the washing treatment to inactivate all categories of microbes tested may be due to the rough surface of the cantaloupe rind compared to the waxy nature of honeydew and watermelon melon surfaces. Ukuku and Fett (2004) and Ukuku et al. (2017) reported that the extensive raised netting on the surface of cantaloupe melon provided more microbial attachments sites which helps to protect attached microbes from being washed off during chemical washing treatments concluding that that was the basis that led higher transfer of bacteria to fresh-cut during fresh-cut preparation. In a different study on fresh-cut cantaloupe pieces inoculated with Salmonella at 2 log CFU/g with storage at 23°C for 24 h, the bacterium increased to 5 log CFU/g of the fresh-cut (Parnell et al., 2005). Similarly, Jackson et al. (2013) reported a similar growth of Salmonella spp. on the edible portions of fruit. Spoilage of fresh-cut fruits are mostly associated with changes in color which can be attributed to oxidative browning and or microbial contamination (Barrett et al., 2010) and we are suggesting that similar oxidative and microbial contamination can be extended to explain the spoilage of fresh-juice. Barrett et al. (2010) suggested further evaluation of microflora of fresh-cut fruit pieces in other to set appropriate criteria for quality assessment. In our study, we investigated the efficacy of 200 ppm chlorine in reducing transfer of surface microflora of whole cantaloupe, honeydew melon and watermelon and the inoculated population of Salmonella bacteria on melons rind surfaces designated for fresh juice preparation. The 200 ppm chlorine used in this study was within the range reported in the literature (McGlynn, 2004, fapc-116, www.fapc.okstate.edu).
Melon rind surfaces are usually removed before fresh-cut and juice preparation and if proper washing treatments are not employed, microbial populations associated with the fruit surfaces or rinds (Fleming et al., 2005; Parnell et al., 2005; FDA, 2008; Ukuku et al., 2016) can end up in the juice. In our current study, we reported minimal transfer of residual surviving populations of aerobic mesophilic bacteria and inoculated Salmonella in the prepared melon juices and recommended immediate refrigeration after preparation to slow and or suppressed growth of bacteria. We recommended similar immediate refrigeration of apple cider juice amended with nisin-EDTA where we found that cold temperature inhibited growth of E. coli O157:H7, Salmonella and Listeria monocytogenes during refrigerated storage (Ukuku et al., 2009). In conclusion, immediate refrigeration of freshly prepared fresh-cut fruits and or juices prepared from cantaloupes, watermelon and honeydew fresh-cut pieces was able to inhibit growth of aerobic mesophilic bacteria, yeast and mold and Pseudomonas spp. including Salmonella bacteria. Washing all melons with 200 ppm chlorine before fresh-cut or juice preparation is highly recommended. Also, hot water (Ukuku et al., 2005; Solomon et al., 2006) and chlorine dioxide (Rodriguez et al., 2017) has been used to treat produces including whole melons before fresh-cut preparation. It is true that the 200 ppm chlorine did not inactivate all bacterial populations on melon surfaces, but the treatment was able to decrease transfer of bacterial populations from melon rind surfaces to the juice during melon juices preparation. Holding freshly prepared melon juices at 22°C for more than 3 h before refrigeration storage led to increase of the residual Salmonella population in the juice. More studies are needed to fully understand the relationship between the slight increase in soluble solids and the bacterial populations observed in juices held at room temperature for 3 to 5 h before refrigeration.
Author's Note
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Author Contributions
DU initiated the study based on prior work. MO and SM were involved in the experimental design and review of data. The study was performed in DU's Laboratory.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author appreciates the help of Dr. Michael Jordan and Ms. Sharon Abbott for supplying bacterial strains used in this study. We also thank Ms. Cara Heller, and Anita Parameswaran for excellent technical support.
References
Andrews, W. H., Wang, H., Jacobson, A., and Hammack, T. (2018). “Chapter 5: Salmonella” in FDA Bacteriological Analytical Manual, 8th Edn. Association of Official Analytical Chemists, ed M. D. Gaithersburg (Washington, DC: Food and Drug Administration), 5.01–5.19.
Barrett, D. M., Beaulieu, J. C., and Shewfelt, R. (2010). Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 50, 369–389. doi: 10.1080/10408391003626322
Batz, M. B., Hoffmann, S. A., Krupnick, A. J., Morris, J. G., Sherman, D. M., Taylor, M. R., et al. (2004). Identifying the Most Significant Microbiological Foodborne Hazards to Public Health: A New Risk Ranking Model. Food Safety Research Consortium Discussion Paper, No. 1. Available online at: http://www.rff.org/files/sharepoint/WorkImages/Download/FRSC-DP-01.pdf (Accessed May 19, 2015).
Castillo, A., Mercado, I., Lucia, L. M., Marti'Nez-Ruiz, Y., Ponce De León, J., Murano, E. A., et al. (2004). Salmonella contamination during production of cantaloupe: a binational study. J. Food Protec. 67, 713–720. doi: 10.4315/0362-028X-67.4.713
CDC (2011). Investigation update: Multistate Outbreak of Salmonella Panama Infections linked to Cantaloupe. National Center for Emerging and Zoonotic Infectious Diseases. Available online at: http://www.cdc.gov/salmonella/panama0311/062311/index.html (Accessed December 2, 2016).
CDC (2016). Disinfection by-Products. Available online at: https://www.cdc.gov/safewater/chlorination-byproducts.html (Accessed February 4, 2017).
Danyluk, M. D., Goodrich-Schneider, R. M., Schneider, K. R., Harris, L. J., and Worobo, R. W. (2012). Outbreaks of Foodborne Disease Associated with Fruit and Vegetable Juices, 1922–20101. FSHN12-04. Food Science and Human Nutrition Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. Available online at: http://edis.ifas.ufl.edu/ (Accessed September 26, 2018).
Danyluk, M. D., Interiano Villeda, L. O., Friedrich, L. M., Schneider, K. R., and Etxeberria, Ed. (2010). Natural-light labeling of tomatoes does not facilitate growth or penetration of Salmonella into the fruit. J. Food Protect. 73, 2276–2280. doi: 10.4315/0362-028X-73.12.2276
Dewey-Mattia, D., Manikonda, K., Hall, A. J., Wise, M. E., and Crowe, S. J. (2018). Surveillance for Foodborne Disease Outbreaks — United States, 2009–2015. MMWR Surveill. Summ. 67, 1–11. doi: 10.15585/mmwr.ss6710a1
El-Safey, E. M. (2013). Behavior of Salmonella heidelberg in fruit juices. Int. J. Nutr. Food Sci. 2, 38–44. doi: 10.11648/j.ijnfs.20130202.13
FDA (2008). Guidance for Industry: Guide to Minimize Microbial Food Safety Hazards of Fresh-cut Fruits and Vegetables. Available online at: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ProducePlantProducts/ucm064458.htm (Accessed on 5/12/14).
FDA (2015). Analysis and Evaluation of Preventive Control Measures for the Control and Reduction/Elimination of Microbial Hazards on Fresh and Fresh-Cut Produce: Chapter IV. Outbreaks Associated with Fresh and Fresh-Cut Produce. Incidence, Growth, and Survival of Pathogens in Fresh and Fresh-Cut Produce. Available online at: http://wayback.archive-it.org/7993/20170111183953/http:/www.fda.gov/Food/FoodScienceResearch/SafePracticesforFoodProcesses/ucm091265.htm (Accessed February 4, 2017).
FDA (2016). Import Alert #22-01. Available online at: http://www.accessdata.fda.gov/cms_ia/importalert_67.html (Accessed February 2, 2017).
Fleming, P., Pool, B., and Fruit, U. F. (2005). Commodity Specific Food Safety Guidelines for the Melon Supply Chain. Newark, DE: Produce Marketing Association; Washington, DC: United Fresh Fruit and Vegetable Association, 1–39.
Heaton, J. C., and Jones, K. (2007). Microbial contamination of fruit and vegetables and the behavior of enteropathogens in the phyllosphere: a review. J. Appl. Microbiol. 104, 613–626. doi: 10.1111/j.1365-2672.2007.03587.x
Jackson, B. R., Griffin, P. M., Cole, D., Walsh, K. A., and Chai, S. J. (2013). Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998–2008. Emerg Infect Dis. 19, 1239–1244. doi: 10.3201/eid1908
McGlynn, W. (2004). Guidelines for the Use of Chlorine Bleach as a Sanitizer in Food Processing Operations. Food Technology Fact Sheet. Available online at: http://www.ucfoodsafety.ucdavis.edu/files/26437.pdf
Messer, J. W., Peeler, J. T., and Gilchrist, J. E. (1984). “Aerobic Plate Count,” in Bacteriological Analytical Manual of the Division of Microbiology. Center for Food Safety and Applied Nutrition, ed U.S. FDA (Arlington, TX: AOAC), 4.01–4.10.
Miller, R. G. Jr. (1981). Simultaneous Statistical Inference, 2nd Edn. New York. NY: Springer-Verlag, 67–70.
Parnell, T. L., Harris, L. J., and Suslow, T. V. (2005). Reducing Salmonella on cantaloupes and honeydew melons using wash practices applicable to postharvest handling, foodservice, and consumer preparation. Int. J. Food Microbiol. 99, 59–70. doi: 10.1016/j.ijfoodmicro.2004.07.014
Rodriguez, A., Olanya, O. M., Annous, B. A., Cassidy, J. M., Orellana, L., and Niemira, B. A. (2017). Survival of Salmonella typhimurium on soybean sprouts after treatment with gaseous chlorine dioxide and biocontrol Pseudomonas bacteria. Food Sci. Biotechnol. 26, 513–520. doi: 10.1007/s10068-017-0071-9
Sharma, M., Adler, B. B., Harrison, M. D., and Beuchat, L. R. (2005). Thermal tolerance of acid-adapted and unadapted Salmonella, Escherichia coli O157:H7, and Listeria monocytogenes in cantaloupe juice and watermelon juice. Lett. Appl. Microbiol. 41, 448–453. doi: 10.1111/j.1472-765X.2005.01797.x
Solomon, E. B., Huang, L., Sites, J. E., and Annous, B. A. (2006). Thermal inactivation of Salmonella on cantaloupes using hot water. J. Food Sci. 71, M25–M30. doi: 10.1111/j.1365-2621.2006.tb08903.x
Ukuku, D. O. (2004). Effect of hydrogen peroxide treatment on microbial quality and appearance of whole and fresh-cut melons contaminated with Salmonella spp. melon pieces. Int. J. Food Microbiol. 95, 137–146. doi: 10.1016/j.ijfoodmicro.2004.01.021
Ukuku, D. O., Bari, M. L., Kawamoto, S., and Isshiki, K. (2005). Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int. J. Food Microbiol. 104, 225–233. doi: 10.1016/j.ijfoodmicro.2005.01.016
Ukuku, D. O., and Fett, W. F. (2004). Method of applying sanitizers and sample preparation affects recovery of native microflora and Salmonella on whole cantaloupe surfaces. J. Food Protec. 67, 999–1004. doi: 10.4315/0362-028X-67.5.999
Ukuku, D. O., Geveke, D. J., Chau, L., Bigley, A., and Niemira, B. A. (2017). Appearance and overall acceptability of fresh-cut cantaloupe pieces from whole melon treated with wet steam process. LWT Food Sci. Technol. 82, 235–242. doi: 10.1016/j.lwt.2017.04.033
Ukuku, D. O., Geveke, D. J., Chau, L., and Niemira, B. A. (2016). Microbial safety and overall quality of cantaloupe fresh-cut pieces prepared from whole fruit after wet steam treatment. I Int. J. Food Microbiol. 231, 86–92. doi: 10.1016/j.ijfoodmicro.2016.05.019
Ukuku, D. O., Huang, L., and Sommers, C. (2015). Effect of chemical sanitizers on survival and growth parameters of Escherichia coli O157:H7, Salmonella spp. and Listeria monocytogenes on cantaloupe fresh-cut pieces during storage. J. Food Prot. 78, 1288–1295. doi: 10.4315/0362-028X.JFP-14-233
Ukuku, D. O., Olanya, M., Geveke, D. J., and Sommers, C. H. (2012). Effect of native microflora, waiting period and storage temperature on Listeria monocytogenes serovars transferred from cantaloupe rind to fresh cut pieces during preparation. J. Food Prot. 75, 1912–1919. doi: 10.4315/0362-028X.JFP-12-191
Ukuku, D. O., and Sapers, G. M. (2007). Effect of time before storage and storage temperature on survival of Salmonella inoculated on fresh-cut melons. Food Microbiol. 24, 288–295. doi: 10.1016/j.fm.2006.04.007
Ukuku, D. O., Zhang, H. Q., and Lihan, H. (2009). Growth parameters of Escherichia coli, Salmonella enteritidis and Listeria monocytogenes, and aerobic mesophilic bacteria of apple cider amended with nisin-EDTA. Foodborne Pathog. Dis. 6, 487–494. doi: 10.1089/fpd.2008.0233
Keywords: storage, temperature, watermelon, honeydew, cantaloupe, juices, Salmonella
Citation: Ukuku DO, Mukhopadhyay S and Olanya M (2018) Reducing Transfer of Salmonella and Aerobic Mesophilic Bacteria on Melon Rinds Surfaces to Fresh Juice by Washing With Chlorine: Effect of Waiting Period Before Refrigeration of Prepared Juice. Front. Sustain. Food Syst. 2:78. doi: 10.3389/fsufs.2018.00078
Received: 08 August 2018; Accepted: 22 October 2018;
Published: 07 December 2018.
Edited by:
Karl Matthews, Rutgers University, The State University of New Jersey, United StatesReviewed by:
Keith Warriner, University of Guelph, CanadaCangliang Shen, West Virginia University, United States
Marilyn Erickson, University of Georgia, United States
Copyright © 2018 Ukuku, Mukhopadhyay and Olanya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dike O. Ukuku, ZGlrZS51a3VrdUBhcnMudXNkYS5nb3Y=
 Dike O. Ukuku
Dike O. Ukuku Sudarsan Mukhopadhyay2
Sudarsan Mukhopadhyay2