- Department of Food Science, Center for Food Safety, University of Arkansas, Fayetteville, AR, United States
The landscape of commercial poultry production is changing due to increasing trends in consumer preference for organic sources of poultry products. This is in part due to perceptions regarding food safety and environmental issues, along with concerns for livestock animal welfare. Consequently, alternative poultry production systems such as small-scale farming and mobile poultry processing units (MPPUs) have achieved a certain level of popularity. However, these alternative production systems, like conventional poultry processing systems, face food safety concerns, due to potential of Campylobacter and Salmonella prevalence. Unlike stationary processing systems, MPPUs may have limited access to sanitation products as they often attempt to comply with organic processing regulations. They may also have limited access to a consistent, high quality water supply which may pose additional food safety and microbial contamination concerns. Due to these food safety concerns and potential limitations on traditional sanitizers, botanicals, organic acids, dry acids, bacteriocins, and phages may offer alternative potential solutions to ensure poultry product safety. The objective of this review is to discuss food safety concerns within alternative poultry processing systems, particularly MPPUs, and describe potential sanitizer strategies.
Introduction
Consumer preferences are leading to changes throughout the poultry industry (Northcutt and Jones, 2004; Fanatico et al., 2005; Meneses et al., 2017). From 2016 to 2017 the amount of organic poultry production has increased by 76%, holding a 2016 market share of $750 million (Philips, 2017). This is compared to an increase of the total organic agricultural farming market share which rose only 23% (Philips, 2017). This is partially influenced by consumers believing the higher cost of pasture flock chicken at market implies a product higher in quality (Hanning et al., 2010; Van Loo et al., 2012). Also, chickens typically considered free range and raised outdoors in pasture flocks are perceived by consumers as being in a more “natural” environment (Fanatico, 1998; Jacob et al., 2008; Hilimire, 2012). These flocks can be processed for retail using systems such as mobile poultry processing units (MPPUs) described in research studies (Trimble et al., 2013; O'Bryan et al., 2017). Use of MPPUs for small scale pasture flocks is potentially advantageous, due to the limited scale of the operational production of these small farms and potential geographical distance from large-scale processing facilities (O'Bryan et al., 2014).
To ensure consumer acceptance of these emerging alternative production systems, public health, and environmental impacts must be evaluated (Hanning et al., 2010; Van Loo et al., 2013; Park et al., 2016). Studies have noted pathogenic Campylobacter and Salmonella within pasture and free-range flocks at higher or similar rates than traditional poultry farming (Avrain et al., 2003; Colles et al., 2008; Hanning et al., 2010). However, like other organically focused processing facilities, the intervention strategies for sanitation in the MPPUs are more limited compared to traditional poultry processing due to the more restrictive requirements for sanitizers and their practical usage within MPPUs (O'Bryan et al., 2014; National Organic Program, 2018). There are other factors to consider as well. For instance, water usage, and therefore water-based sanitization, can be particularly limited in MPPUs as the inherent quality of the water, including source, pressure, and composition varies from location to location (United States Department of Agriculture Food Safety and Inspection Service., 2010). While conventional production systems may use water extensively with an estimated 21 to 30 L per bird, and processors often integrate water use within their Hazard Analysis and Critical Control Points (HACCP) plan, small-scale and mobile processing facilities are not necessarily afforded this possibility (Northcutt and Jones, 2004; O'Bryan et al., 2014; Sanitation Performance Standards Compliance Guide, 2016; Luján-Rhenals et al., 2017). This water limitation can be a challenge for ensuring contaminant-free products. Therefore, the objectives of this review are to explore food safety concerns within alternative poultry production processing systems, which can be affected by the source of the water being utilized and discuss sources of antimicrobials and sanitizers that may offer potential applications to overcome food safety issues associated with these systems.
Mobile Poultry Processing Systems
Due to growing interest in sustainable small-scale poultry farming there is a need for mobile processing systems due to the limitations of centralized processing facilities (Ollinger et al., 2005; Van Loo et al., 2013; O'Bryan et al., 2014). Zezima (2010) reported a 23% decrease in the number of slaughterhouses from 1992 to 2008 despite the growth of small-scale livestock farmers in the U.S. To fill this gap in the poultry processing chain, MPPUs have been used to logistically address geographical limitations of stationary centralized processing facilities, which can be several hundred miles away from some small-scale farmers (Ollinger et al., 2005; O'Bryan et al., 2014). Mobile poultry processing units can remedy this need and are described in detail in O'Bryan et al. (2014). Briefly, MPPUs are trailers that can be anywhere from 5 to 11 meters long and generally include kill cones, scalders, pickers, evisceration tables, and chill tanks. There is typically a trough for catching blood during the bleed step and after scalding and picking the birds are eviscerated, rinsed with processing water, and placed in a chill tank or ice bucket (New Entry Sustainable Farming Project, 2012; O'Bryan et al., 2014).
There is a wide variation in MPPU designs that are well-detailed in the New Entry Sustainable Farming Project (2012). One of the differences includes whether the processing unit is open (outdoor) or enclosed. While there are practical advantages to both, from an environmental standpoint, there are concerns regarding used contaminated processing water overflowing or splashing onto the ground around open-air systems (New Entry Sustainable Farming Project, 2012). Further variations include the decision to capture all solid waste onboard for disposal offsite, setting up stations on the ground behind the MPPUs and the equipment on board (New Entry Sustainable Farming Project, 2012). For instance, scalders can range from a pot of hot water to motor based rotary scalders (New Entry Sustainable Farming Project, 2012). For evisceration, a shackle system may be employed for large throughput MPPUs, while others may opt for using evisceration tables (New Entry Sustainable Farming Project, 2012). Each design choice could drastically impact food safety requirements. For example, while an evisceration table may be more pratical, there is a greater need to sanitize the surface of the table to prevent contamination compared to if the caracasses were hanging (Fanatico., 2003a).
Moreover, water usage varies greatly between locations of these MPPUs. Often water is procured by running a food-grade lead-free hose directly to each spot where water is needed from either municipal, well, or ground water sources (New Entry Sustainable Farming Project, 2012). This means that pressure can vary depending on the location of the unit and within the unit itself. Mobile poultry processing units may possess a booster pump to counteract weak on-site water, but this is not a requirement for processing (New Entry Sustainable Farming Project, 2012). Depending on state regulations, MPPUs may not be required to have backflow devices which prevent used water from flowing back into the potable water supply (O'Bryan et al., 2014). Furthermore, it is possible some water is lost through dissipation into the ground during application around the unit, which is especially likely for setups that place stations adjacent to the processing unit (New Entry Sustainable Farming Project, 2012). When considering these factors, and others such as manual washing of carcasses and utilizing an ice bucket instead of a chiller tank with mechanical stirrers, along with the uniqueness of each MPPU, the assessment of water management in each system should be independently evaluated compared to more consistent high-volume water applications associated with conventional poultry processing systems.
Water Sources and Food Safety Concerns in Mobile Poultry Processing Systems
An overlying issue for local poultry production and processing operations is the variation in available water sources and the potential differences in water quality which could influence food safety. Information on water sources for MPPUs is limited but, like large-scale processing facilities, water use during processing is essential (Micciche et al., 2018a). Unlike large-scale stationary facilities with access to consistent water treatment facilities, the water source(s) for pasture flock production and processing is much more likely to be variable simply due to their mobile nature and the geographical differences associated with fresh water quality (Gwin, 2008). Over 15% of the population of the United States is not served by public water systems and instead use private wells, which are exempt from the Safe Water Drinking Act (Center for Disease Control, 2013). Many of these wells are dug instead of drilled, which can lead to bacterial contamination especially if the well is shallow (< 10 meters deep) (Sawyer et al., 2003; Centers for Disease Control Prevention, 2008). The Centers for Disease Control (CDC) recommended that wells are tested for microbial populations such as coliforms, along with nitrates (Sawyer et al., 2003; Centers for Disease Control Prevention, 2008). While Campylobacter and Salmonella can inhabit the poultry GIT and are typically associated with food-borne outbreaks, outbreaks of contaminated drinking water leading to gastroenteritis have also been noted (O'Reilly et al., 2007; Park et al., 2011). Salmonella particularly has the potential to survive and express virulence genes in laboratory water microcosms originating from poultry water as well as fresh water from rivers and streams (Nutt et al., 2002, 2003).
Ground water sources supply approximately 50% of the U.S. population of the U.S. but they also can suffer from contamination by agricultural runoff or industrial waste (Waller, 1994). This effect can be particularly exacerbated if the water source comes from or is in contact with an open body of water such as a river or a lake (Waller, 1994; Centers for Disease Control Prevention, 2008). Gannon et al. (2004) was able to isolate Salmonella and E. coli O157: H7 from 5% and 17% of river samples taken across two years from the Little Bow River (Alberta, Canada). During the summer months, the prevalence increased to 20 and 30.2% respectively, and it was concluded these bacterial populations might pose a threat to humans if this water is used in irrigation or processing of raw vegetables (Gannon et al., 2004). Certainly, such a risk would also exist if this type of water source was used for pasture flock poultry processing. Out of 72 sampling sites on the Little River (South Georgia, U.S.), 79% were found positive for Salmonella ranging in population levels anywhere from 2.5 Most Probable Number (MPN)/L to 36.3 MPN/L (Haley et al., 2009). An additional study of the region isolated 32 distinct serovars from water sources in the region across 344 isolates. These represented 15 of the top 20 isolates linked to human cases (Baird-Parker, 1990; Maurer et al., 2015). In the U.S., 32 incidents of waterborne outbreaks occurred in drinking water from 2011 to 2012 and numerous other studies have reported Salmonella and non-Salmonella pathogen contamination on vegetables due to contaminated irrigation water (Proctor et al., 2001; Greene et al., 2008; Emch and Waite-Cusic, 2016).
Other sources of fresh water, such as springs, may require continuous disinfection to prevent microbial buildup. However, this may not be the case if the mobile processing facility is located near a city, where municipal water that is often chlorinated up to 4 ppm could be used for poultry processing (United States Environmental Protection Agency, 2013). These concentrations have been shown to be greatly effective against typical planktonic water contaminants (Dunlop et al., 2002). As such, the quality and water pressure may vary significantly and the type of water (well, ground, or municipal water) could greatly impact the chemical and microbial composition of these waters. Furthermore, groundwater and well water may have no additional sanitizers added, unless the farm has added a personal sanitation system, and the waters are unlikely monitored for pathogens or spoilage organisms (Gannon et al., 2004). Because these waters are utilized on farm they may directly impact the product safety during processing through MPPUs. Food safety concerns are increased for MPPU not only due to potential for pathogen contamination from processing water but also during the processing of organic and pasture flocks.
Food Safety Concerns With Free-Range and Pasture Flock Processing
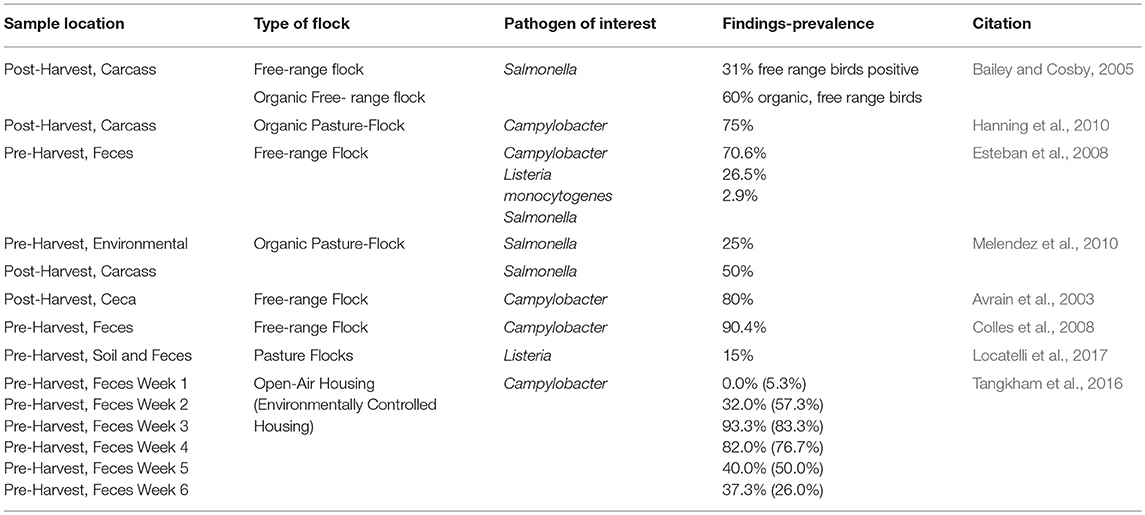
Mobile poultry processing units service small-scale poultry farms, which includes organic farming and pasture flocks (Berlin et al., 2009; O'Bryan et al., 2014). This helps meet a gap in the industry, but higher pathogen counts within free-range or pasture flocks have been reported in some studies (Table 1) (Bailey and Cosby, 2005; Trimble et al., 2013). As stated in Kijlstra et al. (2009) consumer preferences indicates an improvement in animal welfare is necessary. However, free-range systems may create or reintroduce risk and discussion regarding food safety issues within these systems is also important (Kijlstra et al., 2009). Data regarding the prevalence of pathogens within pasture or free-range flocks is limited (Kijlstra et al., 2009; Locatelli et al., 2017). Pathogen prevalence is a concern however, as their presence may lead to contamination in processing facilities and on the finished product. Part of this concern is related to the somewhat vague nature of poultry husbandry management strategies for pasture flock operations. The only requirement for farming to qualtify as free-range is that the chickens have access to the outdoors (Bailey and Cosby, 2005; Van Loo et al., 2012). For pasture farming, birds are provided routine access to pasture land which they may be able to graze for at least one third of their life (Glatz et al., 2005). The reason pasture flock and free-range birds could have higher concentrations of foodborne pathogens is due in part to their potential contact with wild birds, rodents, and insects and other vectors which may carry these pathogens (Berg, 2001; Hanning et al., 2010). In 2014, the USDA reported the prevalence rate of Salmonella amongst conventional poultry processing to be 3.7% (United States Department of Agriculture., 2016). However, significantly higher rates of Salmonella prevalence within pasture flocks have been observed in some studies (Table 1; Bailey and Cosby, 2005; Esteban et al., 2008; Melendez et al., 2010). Lower rates of Salmonella in free range flocks but no other foodborne pathogens were reported in one pre-harvest study conducted in Spain compared to rates in non-organic conventionally reared poultry (Esteban et al., 2008). However, high concentrations of Campylobacter have also been observed but were considered similar enough to the prevalence in conventionally reared organic chicken (Cui et al., 2005; Hanning et al., 2010). In an open-aired housing system Campylobacter prevalence was found to be higher at the end of a 6 week study compared to an environmentally regulated control (Tangkham et al., 2016). Additionally, pasture flock bird carcasses processed by MPPUs have been shown in some research studies to exhibit greater prevalence of Campylobacter but not Salmonella when compared to conventional slaughter facilities (Trimble et al., 2013). To confront these potential food safety issues additional sanitizers should be employed to ensure product safety (Carrasco et al., 2012). Carrasco et al. (2012) reviewed cross-contamination events within conventional poultry processing. They assert that if Salmonella, and presumably other pathogens, are present within the gastrointestinal tract (GIT) of the bird then an increase of prevalence on the carcass may be observed. This can lead to direct cross-contamination within the chiller tank as many carcasses may come into direct contact with each other or indirect contact through contaminated water in the tank (Carrasco et al., 2012). Proper sanitation in the wash solutions and of the equipment, equipment design and control of ingredients can help mitigate this risk (Carrasco et al., 2012). However, pathogens and chemical contaminants may still be present after processing and proper disposal of the wastewater must also be considered. Large-scale poultry processing plants often have their own wastewater treatment facilities, but small-scale and mobile poultry processing units may have to make do with limited proper disposal sites (Fanatico., 2003a).
Disposal of Wastewater in Mobile Poultry Processing Systems
In addition to the food safety issues associated with water sources used for processing there are potential food safety and environmental concerns related to water being discharged post processing in the form of wastewater. Despite state regulations, it has been noted that wastewaters from MPPUs may be applied to gardens as fertilizer or discharged onto private property according to federal regulations (Fanatico, 2003b; Hoppe, 2010; O'Bryan et al., 2014). Such practices raise concerns due to the increased biochemical oxygen demands (BOD) of liquid poultry wastewater, caused by high blood and fat content, and can be of considerable concern from a food and water safety standpoint (Kiepper et al., 2008; Turan, 2009). With chicken blood containing a BOD of over 90,000 mg/L, and consisting of 8% of the live broiler weight, poultry processing water is often contaminated with this pollutant (Kiepper et al., 2008). While this waste water may be high in nutrient value, with a very high BOD, there will be limited oxygen available (Kim et al., 2003). This can lead to anaerobic conditions which will prevent the breakdown of ammonium produced by nitrification to nitrates (Turan, 2009). While plants can use ammonia as a nutrient source, high concentrations can be phytotoxic and decrease the value of the fertilizer (Turan, 2009). Municipal treated wastewater, which is frequently deposited into bodies of fresh water or used in agriculture in arid regions, have a BOD of < 20 mg/L (National Research Council, 2003). Therefore, before application to gardens it is important to minimize the BOD and pathogen contamination of MPPU waste water. It does not appear that there are any formal regulations or requirements detailing how MPPUs should reduce BOD or microbial contamination prior to application to gardens or private land. Furthermore, there are significant environmental concerns with some of these dumping areas (Pellow, 2004). They may be near bodies of water used for recreational purposes or sources of drinking water. Even if the water is not disposed within close proximity of a pond, leeching into the soil and groundwater sources raises concerns. For instance, to prevent source contamination through water backflow, a gap is left between the fill container and the hose (Gwin, 2008). However, it is difficult to provide general recommendations to the practice of dumping wastewater on the ground as the variety of soils will determine the leaching capability of contaminated water (Ding et al., 2010). Wastewater may also be discharged into a municipal sewage system, but this may be costly as rates are usually much greater for treating water with high organic matter (Fanatico., 2003a). In an effort to prevent dumping of wastewater potentially contaminated with human-borne pathogens, as well as mitigate food safety issues and deliver a safe product, chemical disinfection methods may need to be employed.
Conventional Sanitation Approaches for Microbial Decontamination in MPPUs
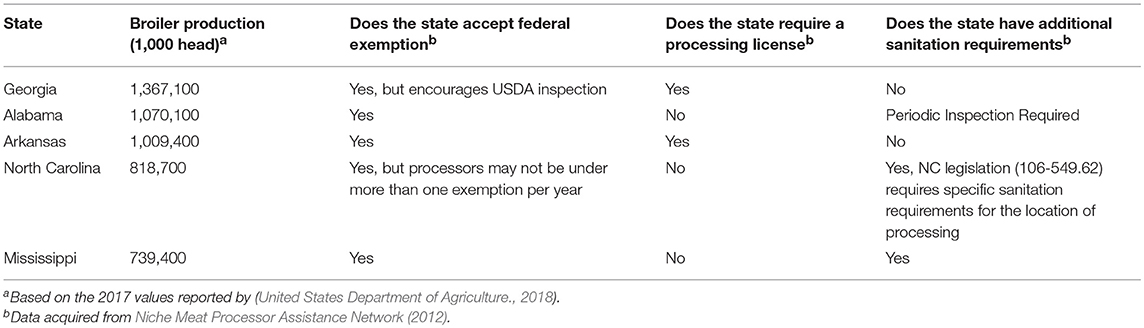
State laws considering sanitation requirements for MPPUs are detailed in and verified through the Niche Meat Processor Assistance Network (2012). The state regulations regarding MPPUs for the five states that produce the most commercial broiler chickens (by individual bird numbers) are detailed in Table 2. Regulations may vary significantly from state to state. Some states, such as Arkansas, strictly follow the federal sanitation requirements (9 CFR 416) but in Iowa, custom-exempt facilities, which an MPPU may fall under, have individual sanitation standard operating procedures (SSOP) (Iowa Meat and Poultry Inspection legislation, Chapter 76) (Niche Meat Processor Assistance Network, 2012). In Maine, for facilities that process < 1,000 birds annually, hot water is considered an appropriate sanitation method and ice may not be re-used, which differs from the USDA regulations that allow ice reuse (9 CFR 416.2 g).
Chemical Sanitation
To prevent microbial contamination during poultry processing from any source, chlorine-based sanitizers have been traditionally used due to cost-effectiveness (Northcutt and Jones, 2004). Microbial contamination, particularly foodborne pathogens Salmonella and Campylobacter, can be especially concerning as there have comprised 23,662 and 2,395 of the confirmed cases of foodborne illness from 2009 to 2015 respectively (Dewey-Mattia et al., 2018). Salmonella and Campylobacter, primarily acquired from poultry, results in an estimated $3.6 and $1.9 billion annual cost to the U.S. (United States Department of Agriculture., 2017). Because chlorine and peracetic acid (PAA), can be utilized in organic processing, these sanitizers have the potential for use in MPPUs to eliminate these pathogens (7 CFR 205.605) (Northcutt and Jones, 2004; United States Department of Agriculture Food Safety and Inspection Service., 2010; O'Bryan et al., 2014). However, chlorine is considered a class 3 health hazard that could cause serious temporary or moderate residual injury on brief exposure (United States Department of Agriculture., 2015). As a consequence, chlorine gas, a common by-product of sodium hypochlorite and acid, should never exceed 1 ppm according to Occupational Safety and Health Administrations (OSHA) Permissible Exposure Limit (PEL) (Occupational Safety Health Administration., 2017) to prevent lung damage and chemical burns (Center for Disease Control, 2013). However chlorine concentrations are often recommended at 50 to 100 ppm to eliminate Salmonella contamination on processing surfaces (Casani et al., 2005; Micciche et al., 2018a). In areas with high organic matter, such as a chiller tank, chloroform as a disinfection byproduct can be formed which poses additional worker health concerns (Tsai et al., 1992; Casani et al., 2005). Furthermore, many stainless steel alloys are susceptible to corrosion in the presence of high concentrations of chlorine (>5 mg/L), and those alloys that are resistant are more costly (Avery et al., 1998). Due to the risk of chemical spills during transport, tight spaces that exist on closed MPPUs and the potentially non-corrosion resistant piping, chlorine may not be best suited for sanitization of these processing units.
The other traditional chemical sanitizer utilized in conventional poultry processing that is also used in organic poultry production is PAA (Bauermeister et al., 2008; Micciche et al., 2018a; National Organic Program, 2018). The antimicrobial PAA is a synthetic substance approved for organic livestock production and has been approved by the Food and Drug Administration for use up to 2,000 ppm (21 CFR 173.370) (Kim et al., 2017; Micciche et al., 2018a; National Organic Program, 2018). Studies have demonstrated that its acidifying properties and membrane oxidation capabilities are effective in inhibiting Salmonella and Campylobacter contamination of poultry products (Kitis, 2004; Oyarzabal, 2005; Bauermeister et al., 2008; Mani-Lopez et al., 2012). In the chiller tank 200 ppm PAA, along with hydrogen peroxide (H2O2), did not impact sensory characteristics of poultry products (Bauermeister et al., 2008). Despite its ability to reduce pathogen concentrations without negatively impacting product quality, PAA is caustic to exposed skin and respiratory systems of personnel and can be corrosive to equipment (Casani et al., 2005; Peracetic acid-MSDS, 2013; Micciche et al., 2018a). Peracetic acid also degrades to water, oxygen, and acetic acid (Kitis, 2004; Warburton, 2014).
Physical Treatment Methods
Physical treatment methods such as filtration and ultraviolet light (UV) have also been suggested for food processing wastewater treatment. Filtration systems are often favored because they can remove oils, organic compounds, and macrosolutes (Meneses et al., 2017). Filtration, namely ultrafiltration, can be utilized to remove pathogens from processing waters, however a buildup of microorganisms has been shown to significantly reduce the flow rate (Lo et al., 1996; James et al., 2000; Bohdziewicz and Sroka, 2005). Saravia et al. (2005) concluded that only large-scale processing operators would be able to use filtration in a cost-efficient manner. With an estimated initial cost of $65,000 and variable energy and filter costs, this system is not currently practical in an MPPU, with processing units ranging in startup costs of $35,000 to $90,000 (Saravia et al., 2005; New Entry Sustainable Farming Project, 2012).
Ultraviolet light (UV) has also been utilized to disinfect food surfaces and liquid beverages (Koutchma, 2008). On pork skin Salmonella and Escherichia coli were significantly reduced by UV irradiation (Wong et al., 1998). Listeria monocytogenes and Salmonella were also reduced on poultry carcasses (Wallner-Pendleton et al., 1994; Kim et al., 2002). However, in the presence of UV, meat discoloration is observed due to chemical alterations of myoglobin to metmyoglobin (Hood, 1980; Bertelsen and Boegh-Soerensen, 1986; Djenane et al., 2001). After brief exposure, meat was discolored in the presence of UV light at 254 nm (Hood, 1980). Furthermore, as determined in the beverage industry, the presence of organic solutes, such as those observed in processing waters, drastically reduce the transmission and therefore performance of UV as a sanitizer and needs to be coupled with an additional chemical sanitizer to be effective (Koutchma, 2008; Selma et al., 2008).
While conventional sanitation approaches represent different levels of effectiveness, practical issues such as cost and quality impact on pasture flock poultry meat products must be considered as well. Given the variability in water source quality and the requirements needed to achieve sufficient food safety mitigation, other options for microbial decontamination should be examined. Ideally an optimal sanitizer would be cost-efficient, safe to handle, signficantly reduce pathogen contamination of product and processing water without damaging the product, and have the ability to generate water of sufficient quality that could potentially be reused in processing. To acomplish these ideals several sanitizers may need to be employed in the form of a multiple hurdle approach (Mendoza et al., 2004; Ricke et al., 2005). In the following sections potential alternatives to conventional methodologies for decontamination of pasture flock poultry processing water and meat product safety are discussed.
Alternative Acidifiers Used in Poultry Production
Organic acids include short chain fatty acids (SCFAs) that contain two to five carbon chains such as propionic acid, butyric acid, and lactic acid (Cherrington et al., 1991; Ricke, 2003). In addition to SCFAs, organic acids also entail medium chain fatty acids (MCFAs) which are 6 to 12 carbons in length, and long chain fatty acids (LCFAs ≥ 13C) (Beermann et al., 2003). Short chain fatty acids are produced in the gastrointestinal tract (GIT) of chickens and humans and have been extensively studied as potential poultry GIT modifiers for pre-harvest intervention as animal feed additives (Cherrington et al., 1991; Ricke, 2003; Van Immerseel et al., 2006; Dittoe et al., 2018a). These fatty acids have also been utilized as sanitizers, although less information is available from the literature. They are on the list of allowed substances for organic livestock production, presumably due to the possibility of their being generated through microbial fermentation (21 CFR 205.605) (National Organic Program, 2018). Organic acids, in general, are all GRAS certified except mandelic acid (Tamblyn and Conner, 1997; Center for Food Safety Applied Nutrition, 2018).
Most of the research conducted with organic acid applications has involved conventionally processed poultry. When Salmonella was attached to the skin, concentrations up to and exceeding 4% of SCFAs were required to generate 2 log reductions (Tamblyn and Conner, 1997). However, acetic acid concentrations of 0.6% utilized in an air injection system reduced Salmonella incidence to 8% on broiler carcasses compared to 96% in the control (Dickens and Whittemore, 1994). In scalder water the D52 value of Salmonella Typhimurium decreased from 29.05 min to 3.56 and 1.30 min when 0.1 and 0.2% of acetic acid was added respectively (Okrend et al., 1986). In Izat et al., (1989), 1% acetic acid did not reduce the incidence of Salmonella on carcasses vs. the control. Compared to controls, lactic acid significantly reduced the presence of experimentally inoculated Salmonella on chicken carcasses in the chiller to below the level of detection on 12/12 samples (0.5% lactic acid) and 11/12 samples (1.0% lactic acid) (Izat et al., 1989). While this indicates lactic acid has the potential to be used in poultry processing, employment at these concentrations led to discoloration in the abdominal region of the carcass (Woolthuis and Smulders, 1985; Izat et al., 1989). Lactic acid treatment does have merit in non-poultry processing. For example, on beef carcasses, 2% lactic and acetic acid also reduced E. coli and Salmonella significantly compared to the control (Hardin et al., 1995). Lactic acid also demonstrated a significantly more pronounced antimicrobial activity against E. coli compared to acetic acid across multiple different cuts of the beef carcass (Hardin et al., 1995).
Other organic acids such as medium chain fatty acids (MCFAs) and long chain fatty acids (LCFAs) also possess antimicrobial properties (Kabara et al., 1972; Greenway and Dyke, 1979). Linoleic acid was shown to not only be inhibitory to Staphylococcus aureus, but its inhibitory effects were more pronounced on penicillin resistance strains (Greenway and Dyke, 1979). This implies the plasmid conferring resistance to penicillin alters the cellular membrane, allowing for linolenic acid to be more effective in cellular membrane disruption (Greenway and Dyke, 1979). This likely occurred in the uptake of the acid into the membrane of cells causing changes in permeability (Greenway and Dyke, 1979). Lauric and myristic acids inhibit Clostridium perfringens at concentrations of 0.1 to 0.2 mg/ml (Skrivanová et al., 2006). However, the lipopolysaccharide layer of Gram-negative bacteria has been shown to elicit resistance against MCFAs and LCFAs by preventing the fatty acids from crossing the cell membrane (Sheu and Freese, 1973; Cherrington et al., 1991; Dittoe et al., 2018a). Citric acid, another organic acid, has been shown to inhibit C. perfringens at a concentration of 4 mg/ml and has antimicrobial properties against Gram-negative bacteria (Skrivanová et al., 2006; Over et al., 2009; DoleŽalová et al., 2010). In broth culture, 5-log reductions of both E. coli and S. Typhimurium were observed after 24 h incubation with 0.75% of citric acid (Over et al., 2009). After 24 h, using a chicken meat model system, 3% citric acid was effective in reducing E. coli and S. Typhimurium when vacuum infused into the meat (Over et al., 2009). DoleŽalová et al. (2010) also tested 10% concentrations of citric acid on chilled chicken products and observed an extension of shelf life and reduction in microbial populations, but noted this concentration generates unacceptable sensory characteristics. These results demonstrate that there is potential to utilize organic acids as sanitizers in the poultry processing operations, but further research is needed to ensure product quality and efficacy across a spectrum of pathogens.
Inorganic acids also offer potential as acidifiers. For example, sodium bisulfate has been evaluated for use in poultry processing. The dry solid acid is advantageous for transport and is GRAS certified with use in a wide range of food and beverages (21 CFR 582.1095) (Calvo et al., 2010; Kassem et al., 2012; United States Department of Agriculture., 2015; Jones-Hamilton., 2018). Sodium bisulfate is also considered natural according to the International Association of Natural Product Producers (IANPP) and the FDA (Kim et al., 2018). Through degradation into sulfate, hydrogen, and sodium, SBS acidifies water without adding potentially toxic compounds to the product or producing off-flavors (Sun et al., 2008). It has also been considered a safer choice by the Environmental Protection Agency (EPA) for use as an antimicrobial and processing aid (United States Environmental Protection Agency, 2018). The sanitizing effects of SBS have been demonstrated against Salmonella and Listeria. On apples, 60 ppm PAA was supplemented with 1% SBS to reduce Listeria innocua counts over 5 logs for up to seven days of storage (Kim et al., 2018). This same effect was observed up to 14 days when 3% SBS was utilized (Kim et al., 2018). Using a spray of 10% SBS, Yabin et al. (1997) reduced S. Typhimurium on chicken carcasses by 2.4-log CFU. A 2-log CFU reduction of artificially inoculated Salmonella Enteritidis on poultry drumsticks was observed using 1% SBS (Dittoe et al., 2018b) and Micciche et al. (2018b) reported complete reductions of 8 log CFU/100 mL of inoculated Salmonella Typhimurium in poultry processing reuse water microcosms.
Amplon™ is another inorganic acidifier that has potential use in poultry processing (Zoetis, 2018). Consisting of inorganic buffering salts and H2SO4, Amplon™is USDA approved as an antimicrobial and processing aid (FSIS 7120.1) and is GRAS (Kim et al., 2017; Center for Food Safety Applied Nutrition, 2018; Zoetis, 2018). Scott et al. (2015) inoculated chicken wings with Salmonella and found that a 20s dip with Amplon™adjusted to a pH of 1.1 reduced Salmonella concentrations by 1.6 log CFU/mL after a 24 h storage time. This reduction was not statistically different from a 20s dip with 700 ppm PAA and was shown to outperform cetylpyridinium chloride as an antimicrobial against Salmonella on the carcass (Scott et al., 2015). Using Amplon™or PAA in a pilot poultry processing plant yielded detectable reductions of Campylobacter both in the post-chiller and as a spray (Kim et al., 2017). However, PAA was able to reduce aerobic plate counts in this study while no reductions were found with Amplon™ (Kim et al., 2017). Based on microbiome analysis, the Amplon™spray but not the post-chiller dip was found to significantly reduce levels of Proteobacteria but not Firmicutes (Kim et al., 2017). While further research must be performed, inorganic acids, along with other acidifiers, either alone or combined seem promising as alternative sanitizers that may be utilized in mobile poultry processing units. However, research needs to be conducted specifically on their application in pasture flock poultry processing operations.
Bacteriocins
Bacteriocins are produced by microorganisms to inhibit similar bacterial strains and often function by using specific cell-surface receptors (Bruno and Montville, 1993; Sirsat et al., 2009). While one bacteriocin (Nisin) is currently considered GRAS, and the others are generally considered natural due to their historical use since ancient times, they are not currently approved for use in organic livestock production (Cleveland et al., 2001; Joerger, 2003; National Organic Program, 2018; United States Food Drug Administration, 2018). Bacteriocins generated by Gram-positive organisms are typically lower in molecular weight than those generated by Gram-negative organisms (Sirsat et al., 2009; Yang et al., 2014). Bacteriocins are heterogeneous and can consist of short peptide chains (< 50 amino acids) to peptides possessing molecular weights up to 90,000 Daltons (Joerger, 2003).
Colicins, produced by E. coli, were the first extensively studied group of bacteriocins and were documented in 1925 by Gratia (Gratia, 1925; Cascales et al., 2007; Sirsat et al., 2009). Their mode of inhibition functions by preventing cell wall synthesis or ribonuclease activity (Cleveland et al., 2001; Cascales et al., 2007). Colicin, and bacteriocins in general, have a very narrow range of effectiveness (Cascales et al., 2007). For instance, S. Paratyphi B strains were found to be sensitive to colicin B by Fredericq (1953), unlike S. Typhimurium which was resistant (Fredericq, 1953; Atkinson, 1970; Graham and Stocker, 1977). However, further studies have found that it is not colicin B that S. Paratyphi B is sensitive to but rather colicin M which is produced in tandem with colicin B (Graham and Stocker, 1977). This narrow range can be advantageous as it can preserve microbial populations that are not harmful or pathogenic and may in fact be potentially beneficial such as starter cultures in fermented meat products, but still target specific pathogens. If poultry meats derived from pasture flock birds are to be further processed into some type of fermented final retail product, such as in the form of sausages, this selectivity could be advantageous for ensuring food safety from pathogens such as Listeria monocytogenes which can occur in poultry including pasture flock raised birds (Milillo et al., 2012; Ricke et al., 2013; Rothrock Jr et al., 2017).
Nisin is one bacteriocin that exhibits a broader range (Campos et al., 2011). It, like many other bacteriocins, is produced by lactic acid bacteria (LAB) (Joerger, 2003; Campos et al., 2006). Nisin is generally regarded as safe (GRAS) and it is not on the prohibited list of non-synthetic substances in organic livestock production (7 CFR 205.604). However, it is not included on the list of allowed substances for use in processing of foods labeled as “organic” because it is considered “synthetic” as it can be genetically engineered and produced using methodologies that do not comply with organic standards (United States Department of Agriculture., 1995; National Organic Program, 2018). Nisin is produced by the Gram-positive bacteria Lactobacillus lactis and has been proven to be effective against the Gram-positive pathogen Listeria monocytogenes on cheese, sausage, and fish (Davies et al., 1997; Nykänen et al., 2000; Geornaras et al., 2006; Abdollahzadeh et al., 2014). Nisin disrupts the cytoplasmic membrane and destroys the membrane electrostatic potential causing cell death (Ruhr and Sahl, 1985). Geornaras et al. (2006), applied 5000 international units of nisin/mL to entire smoked sausages by immersion for 2 min before vacuum packing and storing at 10°C for 48 days. Reductions of L. monocytogenes were observed at levels of 2 to 3 log CFU/cm2, which were originally inoculated with 3 to 4 log CFU/cm2. In turkey processing, 2.5 mg/L of nisin reduced Listeria populations by 1 log after addition to scalder water (Mahadeo and Tatini, 1994). A 3-log reduction of Salmonella was observed on the 5.12 cm2 pieces of chicken skin after 72 h when treated with 100 μg/mL nisin in combination with 5.0 mM EDTA and 0.5% Tween 80 (Natrajan and Sheldon, 2000). However, these supplemental chemicals appeared to be necessary to achieve significant log reductions of Gram-negative pathogens with nisin (Stevens et al., 1991; Sirsat et al., 2009). A 3.2 to 6.9 log reduction of Salmonella was observed when treated with 50 mg/mL of nisin and 20 mg/ml of EDTA. However, < 1 log reductions were observed when treated with the same concentration of nisin without supplementation of EDTA, which suggests a potential synergistic application (Stevens et al., 1991). The antimicrobial activity of nisin appears to be neutralized when in the presence of 25 mM magnesium or 100 mM sodium or potassium (Elliason and Tatini, 1999). Therefore, the supplementation of the chelating agent, EDTA, may be necessary to allow for antimicrobial activity (Elliason and Tatini, 1999).
Other bacteriocins have been identified for Salmonella and Campylobacter inhibition. Lactobacillus plantarum KLDS1.0391 produces Plantaricin MG which has been shown to damage the cytoplasmic membrane of Salmonella by utilizing the proton motive force of the microorganism's ion gradient (Gong et al., 2010a). This bacteriocin also produced a zone of inhibition of 16 mm in agar well diffusion test using S. Typhimurium and a 96.8% reduction using a concentration of 0.5 mg bacteriocin/mL (Gong et al., 2010b). Bacteriocins generated from Bacillus circulans and Paenibacillus polymyxa reduced Campylobacter populations below 2 log CFU/g of chicken cecal counts compared to 6 log CFU populations/g of cecal contents in the control birds (Cole et al., 2006). Furthermore, the bacteriocin produced by Paenibacillus polymyxa was shown to reduce several different strains of Campylobacter in chicken feces below 2 log CFU/g compared to 6 to 8 log in the control flock (Stern et al., 2005). Neither of these has currently been approved as GRAS (FDA, 2018).
However, there are also several disadvantages and limitations with bacteriocins. Nisin is currently the only GRAS certified bacteriocin, and as with most bacteriocins, the spectrum of activity is narrow (Riley and Wertz, 2002). Furthermore, there is a high cost associated with commercial production as these peptides must be acquired from cultured bacteria and purified without damaging the structure (Bradshaw, 2003). This is coupled with the low yield that traditional purification methods provide and loss of activity due to chemical and physical changes that can occur at various processing steps (Jung et al., 1992; Schillinger et al., 1996; Carolissen-Mackay et al., 1997; Davidson et al., 2005; Fahim et al., 2016). Currently the cost likely renders them unsuitable for cost-effective use within MPPUs. Additionally, bacteriocin activity declines over time due to degradation of the compound in food systems as interactions with lipids, proteins, and proteolytic enzymes can rapidly destroy the molecule, requiring constant application of the sanitizer (Bradshaw, 2003; Mahapatra et al., 2005). These disadvantages could greatly impact their effectiveness in water applications during processing for small-scale production systems such as MPPUs when organic loads increase over time. Some of these limitations may be overcome with further development and refinement of bacteriocin properties.
One promising avenue is the use of Nano formulations to encapsulate the bacteriocins (Fahim et al., 2016). While Nano formulations are synthetic, their use in encapsulation of natural product may be approved for application in pasture flock production. However, this must be verified. By integrating approved nanotechnology properties with bacteriocins, protection from degradation and effective delivery can be achieved (Farokhzad and Langer, 2009). For instance, the antimicrobial activity of BLS P40, produced by Bacillus licheniformis, was maintained for 30 days compared to 20 days for the non-encapsulated version (Teixeira et al., 2008). Nisin loaded onto chitosan-based nanoparticles also had four times lower the minimum inhibitory concentration than that of free nisin (Zohri et al., 2010). However, as Fahim et al. (2016) documented, this technique needs to be optimized as several studies found that the non-encapsulated bacteriocin was comparable or even more effective than the encapsulated version.
Botanicals
Another option for sources of sanitizers for water application during alternative poultry processing are the various compounds originating from a wide range of plants. Plant-derived products, or botanicals, have been utilized in food for centuries for flavor enhancement and extension of shelf life (Billing and Sherman, 1998; Smid and Gorris, 1999; Cutter, 2000; Draughon, 2004; Ricke et al., 2005). In 2012, over 1,600 botanicals were marketed as dietary supplements for human consumption and many are organic and generally regarded as safe by the FDA (Food Processing Staff., 2012; Diaz-Sanchez et al., 2015; Center for Food Safety Applied Nutrition, 2018; National Organic Program, 2018). The four major classifications of botanicals are: (1) herbs, such as flowering plants, (2) botanicals, which are parts of a plant such as the bark and roots, (3) essential oils, which are volatile plant compounds, and (4) oleoresins, such as balsam (Windisch and Kroismayr, 2006; Bajpai et al., 2012). Essential oils, including eugenol, thymol, and cinnamaldehyde, are some of the more frequently examined series of compounds for screening and characterization of their antimicrobial properties. Essential oils are slightly soluble in water, usually have a perceived pleasant odor or taste, and can be extracted using distillation and maceration techniques (Kelkar et al., 2006; Shannon et al., 2011; Calo et al., 2015).
These compounds have been explored for both preharvest and postharvest applications in poultry. In their comprehensive review, Diaz-Sanchez et al. (2015) discussed the application of botanicals to poultry feed and subsequent antimicrobial activities upon administration to birds and thus will not be discussed in the current review. Less information is available regarding plant-based derivative use in poultry processing. Chouliara et al. (2007) reported a 1 to 5 log CFU/g reductions of aerobic microbial populations using oregano oil and modified atmosphere packaging (MAP) for the extension of shelf-life of chicken breast. This study demonstrated that the introduction of oregano oil drastically reduced microbial populations. For instance, after nine days MAP retained 6.1 log CFU/g of aerobic microorganisms, but when 0.1 and 1% oregano oil were added, these populations were reduced to 5.77 and 2.75, respectively. The shelf life storage was also extended by approximately 3 days when the 0.1% oregano oil was combined with any of the packaging conditions. Plate assays also demonstrated that 1 and 2% concentrations of both oregano and rosemary oil were effective in inhibiting Staphylococcus aureus and Salmonella Typhimurium, and rosemary was also effective against Listeria monocytogenes and Escherichia coli O157:H7 (Morsy et al., 2014). (Friedman et al., 2002) also found over 27 essential oils and 12 other botanical compounds exhibiting some level of efficacy against Campylobacter jejuni, S. Typhimurium, L. monocytogenes and E. coli.
Non-poultry water sanitizer applications have also been documented that may offer potential approaches for alternative poultry processing water-based amendments. For example, in the produce industry, carrots were found to have similar microbial populations when treated with chlorine, oregano, or a combination of oregano and thyme (Gutierrez et al., 2009). Significant alterations of sensory characteristics using these compounds were also not observed (Gutierrez et al., 2009). Additionally, a 2-log reduction of E. coli was detected on lettuce and baby carrots when a 0.1% thymine oil wash was applied (Singh et al., 2002). Further reductions were also observed when additional washes containing ozone or chlorine dioxide were applied (Singh et al., 2002). Essential oils such as oregano oil can be emulsified and nanoemulsions have been shown even at low concentrations (0.05%) to decrease L. monocytogenes, S. Typhimurium, and E. coli O157:H7 on lettuce by 3.44, 2.31, and 3.05 log CFU/g, respectively (Bhargava et al., 2015). These effects improved to 3.57, 3.26, and 3.35 log CFU/g, respectively, when the concentration doubled (Bhargava et al., 2015).
While the use of botanicals for poultry production is still in its initial phases, the potential to use organic and natural products to reduce pathogenic microorganisms in poultry processing is certainly of noted interest (Ernst, 2015). However, one significant drawback is the potential for off-odors or flavors that can be generated when using sufficient quantities of these botanicals (Calo et al., 2015; Ernst, 2015). For instance, despite its antimicrobial properties, 1% oregano oil used in packaging renders the product inedible (Chouliara et al., 2007). However, this disadvantage may be a benefit for the alternative poultry processing industry under certain circumstances where unique branding strategies are being pursued for gaining marketing competiveness. For instance, thyme oil was found to improve the sensory characteristics of organically aqua-cultured seabass (Kostaki et al., 2009). With many botanicals sold on the organic and natural market, they have the potential to be marketed as natural antimicrobials (Calo et al., 2015; Diaz-Sanchez et al., 2015; Ernst, 2015). Alterations of flavor would not be attractive for large-scale conventional poultry processing where meat product sensory consistency and uniformity is considered critical. However, provided the flavor is palatable, small-scale facilities that process only a few hundred to a few thousands of birds a year have the opportunity to market their product as being perceived unique by promoting the botanical generated flavor as a readily identifiable local product. In short, the ability to generate unique flavors and other sensory properties offer economic attractiveness as brand identification for the development of niche markets but would depend on consumer receptiveness to such products.
Bacteriophage Administration
The use of bacteriophages for treatment both in the medical field and in reducing foodborne pathogens has been controversial (Loc-Carrillo and Abedon, 2011). Phages or bacteriophages are viruses that have a protein coat and enclose a nucleic acid (Shors, 2001). They can exist as virulent or lytic phages, which replicate within the bacterial host and lyse the cell, or temperate phages which insert themselves, as a prophage, into the host genome (Shors, 2001). When the host cell is stressed these prophages can become active, initiate replication, and ultimately lead to cell death (Shors, 2001). The therapeutic potential for phages is believed to be initially considered in 1917 by d'Herelle when he observed lytic phages attacking Bacillus spp. (Summers, 1999; Greer, 2005). This began the idea of phage therapy, which involves the addition of bacteriophages that specifically target species of bacteria.
Phages have been found to be ubiquitous in commercial poultry products, and therefore they represent a potential indigenous intervention that can be used in the alternative poultry industry without introducing a foreign entity into the product, which in turn may alleviate consumer concerns (Atterbury et al., 2003a; Greer, 2005). There is a precedent for this perception based on studies in live poultry production. Phage therapy has been used as an alternative to antibiotics in broiler chickens to reduce E. coli, Salmonella, and Campylobacter as well as improve body weight (Higgins et al., 2002; Goode et al., 2003; Huff et al., 2005). These studies supported the consensus opinion that phage treatment represented a promising alternative to reducing pathogens in broilers as well as improve overall bird growth. A further attraction would be their administration to live pasture flock birds prior to entering processing at an MPPU to reduce pathogen loads before evisceration.
Certainly, there is potential for more direct bacteriophage application against carcass foodborne pathogen contamination in the processing of pasture flock birds as well. However, much of the research thus far has been conducted with conventionally produced poultry products. Several studies have investigated the use of phages to eliminate bacteria on the chicken carcass post-harvest (Greer, 2005). After 24 h storage at 4°C, < 1 log CFU reductions were observed in Campylobacter counts on artificially inoculated chicken skin that was inoculated with Bacteriophage ϕ2 107 PFU/mL (Atterbury et al., 2003b). (Goode et al., 2003), reported similar results. By using C. jejuni typing phage 12673 at 109 to 1010 CFU/mL, they detected a 2 log CFU reduction of Campylobacter when 104 CFU were inoculated onto broiler carcass skin (Goode et al., 2003). No S. Enteritidis was recovered by Goode et al. (2003) when 1 log CFU/cm2 of S. Enteritidis were inoculated onto the broiler skin, and either P22 or 29C phage was used. Furthermore, using chicken breast as a medium, Spricigo et al. (2013) observed 2.2 and 0.9 log CFU/g reductions of S. Typhimurium and S. Enteritidis using phage therapy. The Salmonella inoculated chicken breasts were immersed in a solution for 5 min containing 109 PFU/mL of three bacteriophages (UAB_Phi 20, UAB_Phi78, and UAB_Phi87) with a multiplicity of infection, or ratio of phage to bacteria, of 1,000 (Spricigo et al., 2013). Similar studies will be needed to elucidate if and where phage therapy would best be implemented in alternative poultry processing operations.
There may also be a precedent for further development for alternative poultry retail products as phages have been shown to be effective in other non-poultry retail foods. In 2006, the Food and Drug Administration approved ListShield™, a bacteriophage cocktail for the reduction of Listeria on meat and poultry products during processing (Sulakvelidze, 2011; Ricke et al., 2012). Spricigo et al. (2013), observed reductions of S. Typhimurium and S. Enteritidis on pig skin, < 4 and 2 log CFU/cm2, lettuce, 3.9 and 2.2 log CFU/g, and eggs, 0.9 log CFU/cm2 (Pao et al., 2004). In the agricultural field phages applied to mustard and broccoli seeds suppressed Salmonella growth by 1.37 and 1.50 log CFU. In the dairy industry, the use of phages significantly decreased the numbers of S. Enteritidis (Modi et al., 2001). When inoculated with 104 CFU/mL, which is unlikely to be encountered under the typical conditions of commercial cheese production, the control group retained 103 CFU/g after 99 days of storage compared to the 108 PFU/mL SJ2 phage inoculated cheese which retained only 50 CFU/g (Modi et al., 2001; Greer, 2005). Furthermore, Abuladze et al., 2008, E. coli O157:H7 reported that contaminated hard surfaces (glass coverslips and gypsum boards) that were treated with different levels (1010, 109, and 108 PFU/ml) of phage for 5 min resulted in reductions of 85 to 100%. This was performed by inoculating 1 × 107 CFU/mL to the hard surface and, after drying, 100 ul of the cocktail of ECML-4, ECML-117, and ECML-134 was applied (Abuladze et al., 2008). This effect may apply to stainless-steel surfaces of poultry processing equipment. Abuladze et al. (2008) also tested tomato, broccoli, ground beef, and spinach samples using the cocktail above and E. coli O157:H7 and reported reductions ranging from 94 to 100%.
Indeed, there are several advantages to utilizing bacteriophages, especially in alternative poultry processing water where there is limited availability of antimicrobials. Due to their low toxicity, specific host range, and the lack of toxic by-products, large quantities of phages could be added to processing waters with less risk to workers than traditional sanitizers (Kutter et al., 2010; Loc-Carrillo and Abedon, 2011). For these reasons phages also have comparatively lower environmental impacts compared to chemical sanitizers and antibiotics (Ding and He, 2010). Due to their size, they can also be used in combination with filtration systems allowing them to pass through and continue their administration in a reuse water system. This concept is further promoted by the idea of only having to apply the phage treatment once, as the phages can replicate provided their target host-pathogen remains present (Capparelli et al., 2010). Water, especially a chiller tank is an ideal vehicle for pathogen-phage interaction, as it allows the phage to have maximum contact between it and the target pathogen due to the diffusion of the phage throughout the water (Sutherland et al., 2004). However, perhaps one of the most potentially beneficial properties of phages is their ability to clear biofilms (Sutherland et al., 2004). While the diversity of biofilms and phages is extensive, it is known that some phages possess polysaccharases or polysaccharide lyases which can degrade capsules and exo-polysaccharide matrices (Hughes et al., 1998; Kimura and Itoh, 2003).
Furthermore, many biofilms have water filled channels which allow phages easy access to bacterial cells (Wood et al., 2000). Doolittle et al. (1996) demonstrated that T4 phage infected E. coli host cells would readily attach to an E. coli-based biofilm and the phage would spread throughout the biofilm independent of the exo-mucosal layer. This effect can be beneficial for the food industry as traditional sanitizers are less efficacious against biofilms than planktonic bacteria (Scher et al., 2005; Deborde and Von Gunten, 2008).
While further research is needed to elucidate the value of phage therapy in alternative poultry processing waters, there is potential for their use as alternative sanitizers. However, there are some drawbacks. For instance, isolation of phages can be difficult to achieve and depending on the approach, costly (Clokie and Kropinski, 2009). This cost can occur when characterizing the phage as morphology, protein profiles, mechanism of infection, and genome-characterization beyond full genome sequencing is often necessary (Krylov, 2001; Clokie and Kropinski, 2009). Furthermore, due to their narrow host range, a cocktail of multiple phages in high concentrations would be needed to effectively eliminate the pathogens of interest from the food product (Goodridge, 2010; Kutateladze and Adamia, 2010; Ricke et al., 2012).
Moreover, of the literature currently available, most papers dealing with phages in food borne settings have only investigated artificial inocula of bacteria and usually at bacterial population levels (3 to 5 log CFU) that are unlikely to occur in the industry (Greer, 1988, 2005). Also, despite the highly selective nature of phages and the inability to directly impact eukaryotic cells, consumers could equate bacteriophages to human pathogen associated viruses (Shors, 2001; Sklar and Joerger, 2001; Greer, 2005). However as Górski et al. (2018) points out, phage therapy has been recently gaining more attention in public media outlets, which potentially, in time, will reduce any public resistance to its use in food or indeed medical settings. These factors mean that further investigation and awareness of phage therapy is needed before its full potential in the food industry can be implemented.
Conclusions
Mobile Poultry Processing Units should meet food quality standards and ensure a product that is safe for human consumption. However, these units face other challenges that are not as prominent in conventional poultry systems. Water use in units MPPUs varies in quality and may introduce microorganisms, and even pathogens, to the processing system as many of the water sources are not regulated and may not be routinely monitored for microbial contamination. Furthermore, there is some evidence that organic and pasture flock poultry can have higher counts of foodborne pathogens, such as Salmonella and Campylobacter. In conventional systems chlorine, UV, filtration, and or peracetic acid may be utilized either as primary means of sanitation or generation of processing reuse water. However, the physical treatment systems involving UV or filtration are not easily scalable and thus cost-inefficient for small scale MPPUs. Conventional chemical sanitizers are not always user friendly when applied by inexperienced personnel and could produce by-products that can be a concern for worker safety. A potential solution is to consider alternative more user-friendly sanitizers that could be added to their local sources of water during processing that would be acceptable for the classification of their retail product as a naturally produced and processed meat source.
There are several potential candidates, and all have advantages and drawbacks. Certain chemicals may be applicable, but regulatory constraints and practical application must be considered. Bacteriocins and bacteriophages are biological in origin but may not always be acceptable from a regulatory standpoint. In some cases, they may be too species or even strain-specific for broad spectrum use to reduce general bacterial loads. Organic acids offer a much more broad-spectrum efficacy but may be caustic for routine use in certain applications. Botanicals appear to be promising, but considerably more chemical characterization and mechanism research will need to be done before these can be commercially marketed for wide-scale application. Also, the sensory impact will need to be considered before implementation of specific botanical compounds.
Due to the limitations inherent with any antimicrobial along with the challenges unique to pasture flock processing designing overall sanitation strategies that offer some flexibility to meet individual geographical location requirements may be necessary in the form of a multiple hurdle application. For example, it is anticipated that realistic multiple hurdle applications involving combinations of different antimicrobials will need to be explored to optimize efficacy by taking advantage of different mechanistic properties of different antimicrobials. It may be that a broad-spectrum acidifier in larger quantities could be employed in the water source for reducing general bacterial loads prior to use in the MPPU followed by applying lesser quantities of more microbial target specific sanitizers in the various MPPU processing stages. This, in turn, would hopefully generate alternative processing aids that will allow MPPUs to meet quality standards and ensure consumer safety but be both economical and practical. A risk assessment detailing how effective these interventions would be at each processing step within small processing units, would generate vital information to ensure consumer safety.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Author AM is supported by a University Distinguished Doctoral Fellowship and from the Department of Food Science at the University of Arkansas.
References
Abdollahzadeh, E., Rezaei, M., and Hosseini, H. (2014). Antibacterial activity of plant essential oils and extracts: the role of thyme essential oil, nisin, and their combination to control Listeria monocytogenes inoculated in minced fish meat. Food Control 35, 177–183. doi: 10.1016/j.foodcont.2013.07.004
Abuladze, T., Li, M., Menetrez, M. Y., Dean, T., Senecal, A., and Sulakvelidze, A. (2008). Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157: H7. Appl. Environ. Microbiol. 74, 6230–6238. doi: 10.1128/.01465-08
Atkinson, N. (1970). Colicin-like antibiotics and bacteriophages of Salmonellas. Immunol. Cell Biol. 48:199. doi: 10.1038/icb.1970.19
Atterbury, R. J., Connerton, P. L., Dodd, C. E., Rees, C. E., and Connerton, I. F. (2003a). Isolation and characterization of Campylobacter bacteriophages from retail poultry. Appl. Environ. Microbiol. 69, 4511–4518. doi: 10.1128/AEM.69.8.4511-4518.2003
Atterbury, R. J., Connerton, P. L., Dodd, C. E., Rees, C. E., and Connerton, I. F. (2003b). Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 69, 6302–6306. doi: 10.1128/AEM.69.10.6302-6306.2003
Avery, R., Lamb, S., and Kobrin, G. (1998). Effect of chlorine on common materials in fresh water. Mater. Perform. 37, 52–56.
Avrain, L., Humbert, F., L'Hospitalier, R., Sanders, P., Vernozy-Rozand, C., and Kempf, I. (2003). Antimicrobial resistance in Campylobacter from broilers: association with production type and antimicrobial use. Vet. Microbiol. 96, 267–276. doi: 10.1016/j.vetmic.2003.07.001
Bailey, J. S., and Cosby, D. E. (2005). Salmonella prevalence in free-range and certified organic chickens. J. Food Prot. 68, 2451–2453. doi: 10.4315/0362-028X-68.11.2451
Baird-Parker, A. C. (1990). Foodborne salmonellosis. Lancet 336, 1231–1235. doi: 10.1016/0140-6736(90)92844-8
Bajpai, V. K., Baek, K. H., and Kang, S. C. (2012). Control of Salmonella in foods by using essential oils: a review. Food Res. Int. 45, 722–734. doi: 10.1016/j.foodres.2011.04.052
Bauermeister, L. J., Bowers, J. W., Townsend, J. C., and McKee, S. R. (2008). The microbial and quality properties of poultry carcasses treated with peracetic acid as an antimicrobial treatment. Poult. Sci. 87, 2390–2398. doi: 10.3382/ps.2008-00087
Beermann, C., Jelinek, J., Reinecker, T., Hauenschild, A., Boehm, G., and Klör, H. U. (2003). Short term effects of dietary medium-chain fatty acids and n-3 long-chain polyunsaturated fatty acids on the fat metabolism of healthy volunteers. Lipids Health Dis. 2:10. doi: 10.1186/1476-511X-2-10
Berg, C. (2001). Health and welfare in organic poultry production. Acta Vet. Scand. 43:S37. doi: 10.1186/1751-0147-43-S1-S37
Berlin, L., Lockeretz, W., and Bell, R. (2009). Purchasing foods produced on organic, small and local farms: A mixed method analysis of New England consumers. Renew. Agricult. Food Syst. 24, 267–275. doi: 10.1017/S1742170509990111
Bertelsen, G., and Boegh-Soerensen, L. (1986). “The effect of lighting on color of beef,”in Proceedings of the I.I.R-Meeting (Bristol).
Bhargava, K., Conti, D. S., da Rocha, S. R., and Zhang, Y. (2015). Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 47, 69–73. doi: 10.1016/j.fm.2014.11.007
Billing, J., and Sherman, P. W. (1998). Antimicrobial functions of spices: why some like it hot. Q. Rev. Biol. 73, 3–49. doi: 10.1086/420058
Bohdziewicz, J., and Sroka, E. (2005). Treatment of wastewater from the meat industry applying integrated membrane systems. Process Biochem. 40, 1339–1346 doi: 10.1016/j.procbio.2004.06.023
Bradshaw, J. (2003). Cationic antimicrobial peptides. BioDrugs 17, 233–240. doi: 10.2165/00063030-200317040-00002
Bruno, M. E., and Montville, T. J. (1993). Common mechanistic action of bacteriocins from lactic acid bacteria. Appl. Environ. Microbiol. 59, 3003–3010.
Calo, J. R., Crandall, P. G., O'Bryan, C. A., and Ricke, S. C. (2015). Essential oils as antimicrobials in food systems–A review. Food Control 54, 111–119. doi: 10.1016/j.foodcont.2014.12.040
Calvo, M. S., Gerry, A. C., McGarvey, J. A., Armitage, T. L., and Mitloehner, F. M. (2010). Acidification of calf bedding reduces fly development and bacterial abundance. J. Dairy Sci. 93, 1059–1064. doi: 10.3168/jds.2009-2797
Campos, C. A., Castro, M. P., Gliemmo, M. F., and Schelegueda, L. I. (2011). Use of Natural Antimicrobials for the Control of Listeria monocytogenes in Foods. Science Against Microbial Pathogens: Communicating Current Research and Technological Advances. (Badajoz: Formatex).
Campos, C. A., Rodríguez, Ó., Calo-Mata, P., Prado, M., and Barros-Velázquez, J. (2006). Preliminary characterization of bacteriocins from Lactococcus lactis, Enterococcus faecium and Enterococcus mundtii strains isolated from turbot (Psetta maxima). Food Res. Int. 39, 356–364. doi: 10.1016/j.foodres.2005.08.008
Capparelli, R., Nocerino, N., Lannaccone, M., Ercolini, D., Parlato, M., Chiara, M., and Iannelli, D. (2010). Bacteriophage therapy of Salmonella enterica: a fresh appraisal of bacteriophage therapy. J. Infect. Dis. 201, 52–61. doi: 10.1086/648478
Carolissen-Mackay, V., Arendse, G., and Hastings, J. W. (1997). Purification of bacteriocins of lactic acid bacteria: problems and pointers. Int. J. Food Microbiol. 34, 1–16. doi: 10.1016/S0168-1605(96)01167-1
Carrasco, E., Morales-Rueda, A., and García-Gimeno, R. M. (2012). Cross-contamination and recontamination by Salmonella in foods: a review. Food Res. Intern. 45, 545–556. doi: 10.1016/j.foodres.2011.11.004
Casani, S., Rouhany, M., and Knøchel, S. (2005). A discussion paper on challenges and limitations to water reuse and hygiene in the food industry. Water Res. 39, 1134–1146. doi: 10.1016/j.watres.2004.12.015
Cascales, E., Buchanan, S. K., Duché, D., Kleanthous, C., Lloubes, R., Postle, K. Cavard, D., et al. (2007). Colicin biology. Microbiol. Mol. Biol. Rev. 71, 158–229. doi: 10.1128/MMBR.00036-06
Center for Disease Control (2013). Facts About Chlorine. Available online at: https://emergency.cdc.gov/agent/chlorine/basics/facts.asp (Accessed on October 26, 2018).
Center for Food Safety Applied Nutrition (2018). GRAS Substances (SCOGS) Database. Available online at: https://www.fda.gov/food/ingredientspackaginglabeling/gras/scogs/default.htm (Accessed on September 13, 2018).
Centers for Disease Control and Prevention (2008). “Chapter 8 Rural water supplies and Water-quality issues,” in Healthy Housing Reference Manual (Atlanta, GA: US Department of Health and Human Services), 131–142.
Cherrington, C. A., Hinton, M., Mead, G. C., and Chopra, I. (1991). “Organic acids: chemistry, antibacterial activity and practical applications,” in Advances in Microbial Physiology, Vol. 32, ed A. H. Rose (London, UK: Academic Press), 87–108.
Chouliara, E., Karatapanis, A., Savvaidis, I. N., and Kontominas, M. G. (2007). Combined effect of oregano essential oil and modified atmosphere packaging on shelf-life extension of fresh chicken breast meat, stored at 4 C. Food Microbiol. 24, 607–617. doi: 10.1016/j.fm.2006.12.005
Cleveland, J., Montville, T. J., Nes, I. F., and Chikindas, M. L. (2001). Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 71, 1–20. doi: 10.1016/S0168-1605(01)00560-8
Clokie, M. J., and Kropinski, A. M. (2009). Bacteriophages. Methods and Protocols. Isolation, Characterization and Interactions. New York, NY: Humana Press.
Cole, K., Farnell, M. B., Donoghue, A. M., Stern, N. J., Svetoch, E. A., Eruslanov, B. N., et al. (2006). Bacteriocins reduce Campylobacter colonization and alter gut morphology in turkey poults. Poult. Sci. 85, 1570–1575. doi: 10.1093/ps/85.9.1570
Colles, F. M., Jones, T. A., McCarthy, N. D., Sheppard, S. K., Cody, A. J., Dingle, K. E., et al. (2008). Campylobacter infection of broiler chickens in a free-range environment. Environ. Microbiol. 10, 2042–2050. doi: 10.1111/j.1462-2920.2008.01623.x
Cui, S., Ge, B., Zheng, J., and Meng, J. (2005). Prevalence and antimicrobial resistance of Campylobacter spp. and Salmonella serovars in organic chickens from Maryland retail stores. Appl. Environ. Microbiol. 71, 4108–4111. doi: 10.1128/AEM.71.7.4108-4111.2005
Cutter, C. N. (2000). Antimicrobial effect of herb extracts against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella Typhimurium associated with beef. J. Food Prot. 63, 601–607. doi: 10.4315/0362-028X-63.5.601
Davidson, P. M., Sofos, J. N., and Branen, A. L. (eds.) (2005). Antimicrobials in Food. Boca Raton, FL: CRC Press.
Davies, E. A., Bevis, H. E., and Delves-Broughton, J. (1997). The use of the bacteriocin, nisin, as a preservative in ricotta-type cheeses to control the food-borne pathogen Listeria monocytogenes. Lett. Appl. Microbiol. 24, 343–346. doi: 10.1046/j.1472-765X.1997.00145.x
Deborde, M., and Von Gunten, U. (2008). Reactions of chlorine with inorganic and organic compounds during water treatment—kinetics and mechanisms: a critical review. Water Res. 42, 13–51. doi: 10.1016/j.watres.2007.07.025
Dewey-Mattia, D., Manikonda, K., Hall, A. J., Wise, M. E., and Crowe, S. J. (2018). Surveillance for foodborne disease outbreaks—United States, 2009–2015. MMWR Surv. Summar. 67:1. doi: 10.15585/mmwr.ss6710a1
Diaz-Sanchez, S., D'Souza, D., Biswas, D., and Hanning, I. (2015). Botanical alternatives to antibiotics for use in organic poultry production. Poult. Sci. 94, 1419–1430. doi: 10.3382/ps/pev014
Dickens, J. A., and Whittemore, A. D. (1994). The effect of acetic acid and air injection on appearance, moisture pick-up, microbiological quality, and Salmonella incidence on processed poultry carcasses. Poult. Sci. 73, 582–586. doi: 10.3382/ps.0730582
Ding, C., and He, J. (2010). Effect of antibiotics in the environment on microbial populations. Appl. Microbiol. Biotechnol. 87, 925–941. doi: 10.1007/s00253-010-2649-5
Ding, Y., Liu, Y. X., Wu, W. X., Shi, D. Z., Yang, M., and Zhong, Z. K. (2010). Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water Air Soil Pollut. 213, 47–55. doi: 10.1007/s11270-010-0366-4
Dittoe, D. K., Atchley, J. A., Feye, K. M., Knueven, C. J., and Ricke, S. C. (2018b). Effect of sodium bisulfate salt on mitigating the presence of an antibiotic resistant strain of Salmonella Enteritidis on chicken drug sticks (Abs. #163). Poultry Science Association Annual Meeting, San Antonio, TX, July 22nd – 26th.
Dittoe, D. K., Ricke, S. C., and Kiess, A. S. (2018a). Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Frontiers in Veterinary Science 5, 216. doi: 10.3389/fvets.2018.00216
Djenane, D., Sánchez-Escalante, A., Beltrán, J. A., and Roncalés, P. (2001). Extension of the retail display life of fresh beef packaged in modified atmosphere by varying lighting conditions. J. Food Sci. 66, 181–186. doi: 10.1111/j.1365-2621.2001.tb15603.x
DoleŽalová, M., Molatov,á, Z., Bunka, F., Brezina, P., and Marounek, M. (2010). Effect of organic acids on growth of chilled chicken skin microflora. J. Food Saf. 30, 353–365. doi: 10.1111/j.1745-4565.2009.00212.x
Doolittle, M. M., Cooney, J. J., and Caldwell, D. E. (1996). Tracing the interaction of bacteriophage with bacterial biofilms using fluorescent and chromogenic probes. J. Ind. Microbiol. 16, 331–341. doi: 10.1007/BF01570111
Dunlop, P. S. M., Byrne, J. A., Manga, N., and Eggins, B. R. (2002). The photocatalytic removal of bacterial pollutants from drinking water. J. Photochem. Photobiol. A Chem. 148, 355–363. doi: 10.1016/S1010-6030(02)00063-1
Elliason, D. J., and Tatini, S. R. (1999). Enhanced inactivation of Salmonella typhimurium and verotoxigenic Escherichia coli by nisin at 6 5°C. Food Microbiol. 16, 257–267. doi: 10.1006/fmic.1998.0226
Emch, A. W., and Waite-Cusic, J. G. (2016). Conventional curing practices reduce generic Escherichia coli and Salmonella spp. on dry bulb onions produced with contaminated irrigation water. Food Microbiol. 53, 41–47. doi: 10.1016/j.fm.2015.08.004
Ernst, M. (2015). Managing Risk: Costs, Regulations and Food Safety for On-farm Poultry Processing in Tennessee. University of Tennessee Institute of Agriculture.
Esteban, J. I., Oporto, B., Aduriz, G., Juste, R. A., and Hurtado, A. (2008). A survey of food-borne pathogens in free-range poultry farms. Int. J. Food Microbiol. 123, 177–182. doi: 10.1016/j.ijfoodmicro.2007.12.012
Fahim, H. A., Khairalla, A. S., and El-Gendy, A. O. (2016). Nanotechnology: a valuable strategy to improve bacteriocin formulations. Front. Microbiol. 7:1385. doi: 10.3389/fmicb.2016.01385
Fanatico, A. (1998). Sustainable Chicken Production: Livestock Production Guide. Fayetteville, AR: Appropriate Technology Transfer for Rural Areas (ATTRA)
Fanatico, A. C. (2003b). Small Scale Poultry Processing. Available online at: https://attra.ncat.org/attra-pub/summaries/summary. php?pub = 235 (Accessed 25 May 2018).
Fanatico, A. C., Cavitt, L. C., Pillai, P. B., Emmert, J. L., and Owens, C. M. (2005). Evaluation of slower-growing broiler genotypes grown with and without outdoor access: meat quality. Poult. Sci. 84, 1785–1790. doi: 10.1093/ps/84.11.1785
Fanatico. (2003a). Small-Scale Poultry Processing Processing. Available online at: https://sd.appstate.edu/sites/sd.appstate.edu/files/poultryprocess.pdf (Accessed September 30, 2018)
Farokhzad, O. C., and Langer, R. (2009). Impact of nanotechnology on drug delivery. ACS Nano 3, 16–20. doi: 10.1021/nn900002m
Food Processing Staff. (2012). How the Food Industry Defines Botanicals. Available online at: https://www.foodprocessing.com/articles/2012/defining-botanicals/ (Accessed September 21, 2018).
Fredericq, P. (1953). Recherches sur les caractères et la distribution des souches productrices de diverses colicines dans les selles normales et pathologiques. Bull. Acad. R. Med. Belg. 18, 126–139.
Friedman, M., Henika, P. R., and Mandrell, R. E. (2002). Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 65, 1545–1560. doi: 10.4315/0362-028X-65.10.1545
Gannon, V. P., Graham, T. A., Read, S., Ziebell, K., Muckle, A., Mori, J., et al. (2004). Bacterial pathogens in rural water supplies in southern Alberta, Canada. J. Toxicol. Environ. Health Part A 67, 1643–1653. doi: 10.1080/15287390490492421
Geornaras, I., Skandamis, P. N., Belk, K. E., Scanga, J. A., Kendall, P. A., Smith, G. C., and Sofos, J. N. (2006). Post-processing application of chemical solutions for control of Listeria monocytogenes, cultured under different conditions, on commercial smoked sausage formulated with and without potassium lactate–sodium diacetate. Food Microbiol. 23, 762–771. doi: 10.1016/j.fm.2006.01.008
Glatz, P. C., Ru, Y. J., Miao, Z. H., Wyatt, S. K., and Rodda, B. J. (2005). Integrating poultry into a crop and pasture farming system. Int. J. Poult. Sci. 4, 187–191. doi: 10.3923/ijps.2005.187.191
Gong, H. S., Meng, X. C., and Wang, H. (2010a). Mode of action of plantaricin MG, a bacteriocin active against Salmonella Typhimurium. J. Basic Microbiol. 50, S37–S45. doi: 10.1002/jobm.201000130
Gong, H. S., Meng, X. C., and Wang, H. (2010b). Plantaricin MG active against Gram-negative bacteria produced by Lactobacillius plantarum KLDS1. 0391 isolated from “Jiaoke”, a traditional fermented cream from China. Food Control 21, 89–96. doi: 10.1016/j.foodcont.2009.04.005
Goode, D., Allen, V. M., and Barrow, P. A. (2003). Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 69, 5032–5036. doi: 10.1128/AEM.69.8.5032-5036.2003
Goodridge, L. D. (2010). Designing phage therapeutics. Curr. Pharmaceut. Biotechnol. 11, 15–27. doi: 10.2174/138920110790725348
Górski, A., Miedzybrodzki, R., Łobocka, M., Głowacka-Rutkowska, A., Bednarek, A., Borysowski, J., et al. (2018). Phage therapy: what have we learned?. Viruses 10, 288–316. doi: 10.3390/v10060288
Graham, A. C., and Stocker, B. A. D. (1977). Genetics of sensitivity of Salmonella species to colicin M and bacteriophages T5, T1, and ES18. J. Bacteriol. 130, 1214–1223.
Gratia, A. (1925). Sur un remarquable exemple d'antagonisme entre deux souches de coilbacille. CR Seances Soc. Biol. Fil. 93, 1040–1041.
Greene, S. K., Daly, E. R., Talbot, E. A., Demma, L. J., Holzbauer, S., Patel, N. J., et al. (2008). Recurrent multistate outbreak of Salmonella Newport associated with tomatoes from contaminated fields, 2005. Epidemiol. Infect. 136, 157–165. doi: 10.1017/S095026880700859X
Greenway, D. L., and Dyke, K. G. (1979). Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. Microbiology 115, 233–245.
Greer, G. G. (1988). Effect of phage concentration, bacterial density, and temperature on phage control of beef spoilage. J. Food Sci. 53, 1226–1227. doi: 10.1111/j.1365-2621.1988.tb13570.x
Greer, G. G. (2005). Bacteriophage control of foodborne bacteria. J. Food Prot. 68, 1102–1111. doi: 10.4315/0362-028X-68.5.1102
Gutierrez, J., Bourke, P., Lonchamp, J., and Barry-Ryan, C. (2009). Impact of plant essential oils on microbiological, organoleptic and quality markers of minimally processed vegetables. Innov. Food Sci. Emerg. Technol. 10, 195–202. doi: 10.1016/j.ifset.2008.10.005
Gwin, L. (2008). Mobile Slaughter and Processing. Available online at: http//www.nichemeatprocessing.org/mobile-unit-overview (Accessed September 30, 2018).
Haley, B. J., Cole, D. J., and Lipp, E. K. (2009). Distribution, diversity, and seasonality of waterborne Salmonellae in a rural watershed. Appl. Environ. Microbiol. 75, 1248–1255. doi: 10.1128/AEM.01648-08
Hanning, I., Biswas, D., Herrera, P., Roesler, M., and Ricke, S. C. (2010). Prevalence and characterization of Campylobacter jejuni isolated from pasture flock poultry. J. Food Sci. 75, M496–M502. doi: 10.1111/j.1750-3841.2010.01747.x
Hardin, M. D., Acuff, G. R., Lucia, L. M., Oman, J. S., and Savell, J. W. (1995). Comparison of methods for decontamination from beef carcass surfaces. J. Food Prot. 58, 368–374. doi: 10.4315/0362-028X-58.4.368
Higgins, J. P., Higgins, S. E., Guenther, K. L., Huff, W. E., and Hargis, B. M. (2002). Evaluation of bacteriophage treatment as a method to reduce culturable Salmonella in poultry carcass rinse water. Poult. Sci. 81(Suppl. 1):130.
Hilimire, K. (2012). The grass is greener: Farmers' experiences with pastured poultry. Renew. Agricult. Food Syst. 27, 173–179. doi: 10.1017/S1742170511000287
Hood, D. E. (1980). Factors affecting the rate of metmyoglobin accumulation in prepackaged beef. Meat Sci. 4, 247–265. doi: 10.1016/0309-1740(80)90026-1
Hoppe, R. A. (2010). Structure and Finances of US Farms: Family Farm Report (2010 ed.). Diane Publishing Darby, PA. Available online at: https://www.nytimes.com/2010/03/28/us/28slaughter.html (Accessed May 2018).
Huff, W. E., Huff, G. R., Rath, N. C., Balog, J. M., and Donoghue, A. M. (2005). Alternatives to antibiotics: utilization of bacteriophage to treat colibacillosis and prevent foodborne pathogens. Poult. Sci. 84, 655–659. doi: 10.1093/ps/84.4.655
Hughes, K. A., Sutherland, I. W., and Jones, M. V. (1998). Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology 144, 3039–3047. doi: 10.1099/00221287-144-11-3039
Izat, A. L., Colberg, M., Adams, M. H., Reiber, M. A., and Waldroup, P. W. (1989). Production and processing studies to reduce the incidence of salmonellae on commercial broilers. J. Food Prot. 52, 670–673. doi: 10.4315/0362-028X-52.9.670
Jacob, M. E., Fox, J. T., Reinstein, S. L., and Nagaraja, T. G. (2008). Antimicrobial susceptibility of foodborne pathogens in organic or natural production systems: an overview. Foodborne Pathog. Dis. 5, 721–730. doi: 10.1089/fpd.2008.0095
James, C., Goksoy, E. O., Corry, J. E. L., and James, S. J. (2000). Surface pasteurization of poultry meat using steam at atmospheric pressure. J. Food Eng. 45, 111–117. doi: 10.,1016/S0260-8774(00)00048-0
Joerger, R. D. (2003). Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 82, 640–647. doi: 10.1093/ps/82.4.640
Jones-Hamilton. (2018). Specialty Products of Jones Hamilton. Available online at: http://www.jones-hamilton.com/spd/specialty-products-of-jones-hamilton.html (Accessed on May 26, 2018).
Jung, D. S., Bodyfelt, F. W., and Daeschel, M. A. (1992). Influence of fat and emulsifiers on the efficacy of nisin in inhibiting Listeria monocytogenes in fluid milk. J. Dairy Sci. 75, 387–393. doi: 10.3168/jds.S0022-0302(92)77773-X
Kabara, J. J., Swieczkowski, D. M., Conley, A. J., and Truant, J. P. (1972). Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 2, 23–28. doi: 10.1128/AAC.2.1.23
Kassem, I. I., Sanad, Y., Stonerock, R., and Rajashekara, G. (2012). An evaluation of the effect of sodium bisulfate as a feed additive on Salmonella enterica serotype Enteritidis in experimentally infected broilers. Poult. Sci. 91, 1032–1037. doi: 10.3382/ps.2011-01935
Kelkar, V. M., Geils, B. W., Becker, D. R., Overby, S. T., and Neary, D. G. (2006). How to recover more value from small pine trees: essential oils and resins. Biomass Bioenergy 30:316e320. doi: 10.1016/j.biombioe.2005.07.009
Kiepper, B. H., Merka, W. C., and Fletcher, D. L. (2008). Proximate composition of poultry processing wastewater particulate matter from broiler slaughter plants. Poult. Sci. 87, 1633–1636. doi: 10.3382/ps.2007-00331
Kijlstra, A., Meerburg, B. G., and Bos, A. P. (2009). Food safety in free-range and organic livestock systems: Risk management and responsibility. J. Food Prot. 72, 2629–2637. doi: 10.4315/0362-028X-72.12.2629
Kim, B. H., Chang, I. S., Gil, G. C., Park, H. S., and Kim, H. J. (2003). Novel BOD (biological oxygen demand) sensor using mediator-less microbial fuel cell. Biotechnol. Lett. 25, 541–545. doi: 10.1023/A:1022891231369
Kim, S. A., Park, S. H., Knueven, C., Basel, R., and Ricke, S. C. (2018). A decontamination approach using a combination of bisulfate of soda and peracetic acid against Listeria innocua inoculated on whole apples. Food Control 84, 106–110. doi: 10.1016/j.foodcont.2017.07.036
Kim, S. A., Park, S. H., Lee, S. I., Owens, C. M., and Ricke, S. C. (2017). Assessment of chicken carcass microbiome responses during processing in the presence of commercial antimicrobials using a next generation sequencing approach. Sci. Rep. 7:43354. doi: 10.1038/srep43354
Kim, T., Silva, J. L., and Chen, T. C. (2002). Effects of UV irradiation on selected pathogens in peptone water and on stainless steel and chicken meat. J. Food Prot. 65, 1142–1145. doi: 10.4315/0362-028X-65.7.1142
Kimura, K., and Itoh, Y. (2003). Characterization of poly-γ-glutamate hydrolase encoded by a bacteriophage genome: possible role in phage infection of Bacillus subtilis encapsulated with poly-γ-glutamate. Appl. Environ. Microbiol. 69, 2491–2497. doi: 10.1128/AEM.69.5.2491-2497.2003
Kitis, M. (2004). Disinfection of wastewater with peracetic acid: a review. Environ. Int. 30, 47–55. doi: 10.1016/S0160-4120(03)00147-8
Kostaki, M., Giatrakou, V., Savvaidis, I. N., and Kontominas, M. G. (2009). Combined effect of MAP and thyme essential oil on the microbiological, chemical and sensory attributes of organically aquacultured sea bass (Dicentrarchus labrax) fillets. Food Microbiol. 26, 475–482. doi: 10.1016/j.fm.2009.02.008
Koutchma, T. (2008). UV light for processing foods. Ozone Sci Eng. 30, 93–98. doi: 10.1080/01919510701816346
Krylov, V. N. (2001). Phagotherapy in terms of bacteriophage genetics: hopes, perspectives, safety, limitations. Genetika 37, 869–887. doi: 10.1023/A:1016716606135
Kutateladze, M., and Adamia, R. (2010). Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol. 28, 591–595. doi: 10.1016/j.tibtech.2010.08.001
Kutter, E., De Vos, D., Gvasalia, G., Alavidze, Z., Gogokhia, L., Kuhl, S., and Abedon, S. T. (2010). Phage therapy in clinical practice: treatment of human infections. Curr. Pharm. Biotechnol. 11, 69–86. doi: 10.2174/138920110790725401
Lo, Y. M., Yang, S. T., and Min, D. B. (1996). Kinetic and feasibility studies of ultrafiltration of viscous xanthan gum fermentation broth. J. Membr. Sci. 117, 237–249. doi: 10.1016/0376-7388(96)00067-1
Locatelli, A., and Lewis, M. A. (2017). The distribution of Listeria in pasture-raised broiler farm soils is potentially related to university of Vermont medium enrichment bias toward Listeria innocua over Listeria monocytogenes. Front. Vet. Sci. 4:227. doi: 10.3389/fvets.2017.00227
Loc-Carrillo, C., and Abedon, S. T. (2011). Pros and cons of phage therapy. Bacteriophage 1, 111–114 doi: 10.4161/bact.1.2.14590
Luján-Rhenals, D., Morawicki, R., Van Loo, E., and Ricke, S. C. (2017). “Energy and water use in poultry processing,” in Achieving Sustainable Production of Poultry Meat, Vol 1, Safety, Quality and Sustainability, ed S. C. Ricke (Cambridge: Burleigh Dodds Science Publishing Limited).
Mahadeo, M., and Tatini, S. R. (1994). The potential use of nisin to control Listeria monocytogenes in poultry. Lett. Appl. Microbiol. 18, 323–326. doi: 10.1111/j.1472-765X.1994.tb00879.x
Mahapatra, A. K., Muthukumarappan, K., and Julson, J. L. (2005). Applications of ozone, bacteriocins and irradiation in food processing: a review. Crit. Rev. Food Sci. Nutr. 45, 447–461 doi: 10.1080/10408390591034454
Mani-Lopez, E., García, H. S., and López-Malo, A. (2012). Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 45, 713–721. doi: 10.1016/j.foodres.2011.04.043
Maurer, J. J., Martin, G., Hernandez, S., Cheng, Y., Gerner-Smidt, P., Hise, K. B., et al. (2015). Diversity and persistence of Salmonella enterica strains in rural landscapes in the Southeastern United States. PLoS ONE 10:e0128937. doi: 10.1371/journal.pone.0128937
Melendez, S. N., Hanning, I., Han, J., Nayak, R., Clement, A. R., Wooming, A., et al. (2010). Salmonella enterica isolates from pasture-raised poultry exhibit antimicrobial resistance and class I integrons. J. Appl. Microbiol. 109, 1957–1966. doi: 10.1111/j.1365-2672.2010.04825.x
Mendoza, J. L., Bard, D. E., Mumford, M. D., and Ang, S. C. (2004). Criterion-related validity in multiple-hurdle designs: estimation and bias. Organization. Res. Methods 7, 418–441. doi: 10.1177/1094428104268752
Meneses, Y. E., Stratton, J., and Flores, R. A. (2017). Water reconditioning and reuse in the food processing industry: current situation and challenges. Trends in Food Sci. Technol. 61, 72–79. doi: 10.1016/j.tifs.2016.12.008
Micciche, A. C., Feye, K. M., Rubinelli, P. M., Wages, J. A., Knueven, C., and Ricke, S. C. (2018a). The implementation and food safety issues associated with poultry processing reuse water for conventional poultry production systems in the United States. Front. Sustain. Food Syst. 2:70. doi: 10.3389/fsufs.2018.00070
Micciche, A. C., Feye, K. M., Wages, J. A., and Knueven, C. J. Ricke, S. C. (2018b). Impact of acid treatments on the microbial populations of commercial poultry processing re-use water microcosms (Abs. 86), in Poultry Science Association Annual Meeting, San Antonio, TX, July 22nd – 26th.
Milillo, S. R., Stout, J. C., Hanning, I. B., Clement, A., Fortes, E. D., Den Bakker, H. C., et al. (2012). Listeria monocytogenes and hemolytic Listeria innocua in poultry. Poult. Sci. 91, 2158–2163. doi: 10.3382/ps.2012-02292
Modi, R., Hirvi, Y., Hill, A., and Griffiths, M. W. (2001). Effect of phage on survival of Salmonella enteritidis during manufacture and storage of cheddar cheese made from raw and pasteurized milk. J. Food Prot. 64, 927–933. doi: 10.4315/0362-028X-64.7.927
Morsy, M. K., Khalaf, H. H., Sharoba, A. M., El-Tanahi, H. H., and Cutter, C. N. (2014). Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. J. Food Sci. 79, M675–M684. doi: 10.1111/1750-3841.12400
National Research Council (2003). “Chapter: 3 municipal wastewater and sludge treatment,” in Use of Reclaimed Water and Sludge in Food Crop Production (Washington, DC: The National Academies Press), 44–55.
Natrajan, N., and Sheldon, B. W. (2000). Efficacy of nisin-coated polymer films to inactivate Salmonella typhimurium on fresh broiler skin. J. Food Prot. 63, 1189–1196. doi: 10.4315/0362-028X-63.9.1189
New Entry Sustainable Farming Project (2012). Building an On-farm Poultry Processing Facility. Available online at: http://www.sare.org/Learning-Center/Project-Products/Northeast-SARE-Project-Products/Buildingan-On-Farm-Poultry-Processing-Facility (Accessed 18 February 2014).
Niche Meat Processor Assistance Network (2012). State Poultry Processing Regulations. Available online at: www.nichemeatprocessing.org (Accessed 18 December 2014).
Northcutt, J. K., and Jones, D. R. (2004). A survey of water use and common industry practices in commercial broiler processing facilities. J. Appl. Poult. Res. 13, 48–54. doi: 10.1093/japr/13.1.48
Nutt, J. D., Medvedev, K. L., Woodward, C. L., Pillai, S. D., and Ricke, S. C. (2002). Assessment of laboratory media controls for determining Salmonella virulence potential of poultry water sources using a hilA:lacZY fusion strain. J. Rapid Methods Automat. Microbiol. 10, 173–184. doi: 10.1111/j.1745-4581.2002.tb00024.x
Nutt, J. D., Pillai, S. D., Woodward, C. L., Sternes, K. L., Zabala-Dıaz, I. B., Kwon, Y. M., et al. (2003). Use of a Salmonella typhimurium hilA fusion strain to assess effects of environmental fresh water sources on virulence gene expression. Water Res. 37, 3319–3326. doi: 10.1016/S0043-1354(03)00244-6
Nykänen, A., Weckman, K., and Lapveteläinen, A. (2000). Synergistic inhibition of Listeria monocytogenes on cold-smoked rainbow trout by nisin and sodium lactate. Int. J. Food Microbiol. 61, 63–72. doi: 10.1016/S0168-1605(00)00368-8
O'Bryan, C. A., Crandall, P., Jaroni, D., Ricke, S. C., and Gibson, K. E. (2017). Assessment of nitrogen and phosphorus loads present in environments impacted by alternative poultry processing operations utilized in pasture-raised poultry production. Renew. Agricult. Food Syst. 32, 33–42. doi: 10.1017/S1742170515000514
O'Bryan, C. A., Crandall, P. G., Davis, M. L., Kostadini, G., Gibson, K. E., Alali, W. Q., et al. (2014). Mobile poultry processing units: a safe and cost-effective poultry processing option for the small-scale farmer in the United States. World's Poult. Sci. J. 70, 787–802. doi: 10.1017/S0043933914000853
Occupational Safety Health Administration. (2017). United States Department of Labor. Available online at: https://www.osha.gov/dts/chemicalsampling/data/CH_226500.html (Accessed on May 12, 2018).
Okrend, A. J., Johnston, R. W., and Moran, A. B. (1986). Effect of acetic acid on the death rates at 52 C of Salmonella newport, Salmonella typhimurium and Campylobacter jejuni in poultry scald water. J. Food Protect. 49, 500–503. doi: 10.4315/0362-028X-49.7.500
Ollinger, M., MacDonald, J. M., and Madison, M. (2005). Technological change and economies of scale in US poultry processing. Am. J. Agricult. Econom. 87, 116–129. doi: 10.1111/j.0002-9092.2005.00706.x
O'Reilly, C. E., Bowen, A. B., Perez, N. E., Sarisky, J. P., Shepherd, C. A., Miller, M. D., et al. (2007). A waterborne outbreak of gastroenteritis with multiple etiologies among resort island visitors and residents: Ohio, 2004. Clin. Infect. Dis. 44, 506–512. doi: 10.1086/511043
Over, K. F., Hettiarachchy, N., Johnson, M. G., and Davis, B. (2009). Effect of organic acids and plant extracts on Escherichia coli O157: H7, Listeria monocytogenes, and Salmonella Typhimurium in broth culture model and chicken meat systems. J. Food Sci. 74, M515–M521. doi: 10.1111/j.1750-3841.2009.01375.x
Oyarzabal, O. A. (2005). Reduction of Campylobacter spp. by commercial antimicrobials applied during the processing of broiler chickens: a review from the United States perspective. J. Food Protect. 68, 1752–1760. doi: 10.4315/0362-028X-68.8.1752
Pao, S., Rolph, S. P., Westbrook, E. W., and Shen, H. (2004). Use of bacteriophages to control Salmonella in experimentally contaminated sprout seeds. J. Food Sci. 69, M127–M130. doi: 10.1111/j.1365-2621.2004.tb10720.x
Park, S. H., Hanning, I., Jarquin, R., Moore, P., Donoghue, D. J., Donoghue, A. M., and Ricke, S. C. (2011). Multiplex PCR assay for the detection and quantification of Campylobacter spp., Escherichia coli O157: H7, and Salmonella serotypes in water samples. FEMS Microbiol. Lett. 316, 7–15. doi: 10.1111/j.1574-6968.2010.02188.x
Park, S. H., Lee, S. I., and Ricke, S. C. (2016). Microbial populations in naked neck chicken ceca raised on pasture flock fed with commercial yeast cell wall prebiotics via an Illumina MiSeq platform. PLoS ONE 11:e0151944. doi: 10.1371/journal.pone.0151944
Pellow, D. N. (2004). The politics of illegal dumping: an environmental justice framework. Qualit. Soc. 27, 511–525. doi: 10.1023/B:QUAS.0000049245.55208.4b
Peracetic acid-MSDS (2013). No. SLP5503; Sciencelab.com, Inc. 14025 Smith Rd. Houston, Texas 77396 May 12,. Available online at: https://www.sciencelab.com/msds.php?msdsId=9926439 (accessed 5/15/18).
Philips, R. (2017). 2016 Sales of U.S. Certified Organic Agricultural Production Up 23 Percent from Previous Year California continues to lead in certified farms, acres, sales. Available online at: https://www.nass.usda.gov/Newsroom/archive/2017/09_20_2017.php (Accessed on August 26, 2018).
Proctor, M. E., Hamacher, M., Tortorello, M. L., Archer, J. R., and Davis, J. P. (2001). Multistate outbreak of Salmonella serovar Muenchen infections associated with alfalfa sprouts grown from seeds pretreated with calcium hypochlorite. J. Clin. Microbiol. 39, 3461–3465. doi: 10.1128/JCM.39.10.3461-3465.2001
Ricke, S., Koo, O., and Keeton, J. (2013). “Fermented meat, poultry, and fish products,” in Food Microbiology, eds M. Doyle and R. Buchanan (Washington, DC: ASM Press), 857–880.
Ricke, S. C. (2003). Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poultry Sci. 82, 632–639. doi: 10.1093/ps/82.4.632
Ricke, S. C., Hererra, P., and Biswas, D. (2012). “Chapter 23: Bacteriophages for potential food safety applications in organic meat production,” in Organic Meat Production and Processing, eds S. C. Ricke, E. J. Van Loo, M. G. Johnson, and C. A. O'Bryan (Hoboken, NJ: John Wiley & Sons), 407–424.
Ricke, S. C., Kundinger, M. M., Miller, D. R., and Keeton, J. T. (2005). Alternatives to antibiotics: chemical and physical antimicrobial interventions and foodborne pathogen response. Poult. Sci. 84, 667–675. doi: 10.1093/ps/84.4.667
Riley, M. A., and Wertz, J. E. (2002). Bacteriocins: evolution, ecology, and application. Annu. Rev. Microbiol. 56, 117–137. doi: 10.1146/annurev.micro.56.012302.161024
Rothrock Jr, M. J., Davis, M. L., Locatelli, A., Bodie, A., McIntosh, T. G., Donaldson, J. R., and Ricke, S. C. (2017). Listeria occurrence in poultry flocks: detection and potential implications. Front. Vet. Sci. 4:125. doi: 10.3389/fvets.2017.00125
Ruhr, E., and Sahl, H. G. (1985). Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob. Agents Chemother. 27, 841–845. doi: 10.1128/AAC.27.5.841
Sanitation Performance Standards Compliance Guide (2016). Available online at: https://www.fsis.usda.gov/wps/portal/fsis/topics/regulatory-compliance/compliance-guides-index/sanitation-performance-standards/sanitation-compliance-guide
Saravia, H., Houston, J. E., Toledo, R., and Nelson, H. M. (2005). “Economic feasibility of recycling chiller water in poultry processing plants by ultrafiltration,” in Proceedings of the 2005 Georgia Water Resources Conference, held April 25-27, 2005, at the University of Georgia. ed J. Kathryn Hatcher, (Athens: Institute Ecology, The University of Georgia).
Sawyer, C., McCarty, P., and Parkin, G. (2003). “Chapter: 8 water treatment,” in Chemistry for Environmental Engineering and Science, 8th Edn. (New York, NY: McGraw-Hill), 209–239.
Scher, K., Römling, U., and Yaron, S. (2005). Effect of heat, acidification, and chlorination on Salmonella enterica serovar Typhimurium cells in a biofilm formed at the air-liquid interface. Appl. Environ. Microbiol. 71, 1163–1168. doi: 10.1128/AEM.71.3.1163-1168.2005
Schillinger, U., Geisen, R., and Holzapfel, W. H. (1996). Potential of antagonistic microorganisms and bacteriocins for the biological preservation of foods. Trends Food Sci. Technol. 7, 158–164. doi: 10.1016/0924-2244(96)81256-8
Scott, B. R., Yang, X., Geornaras, I., Delmore, R. J., Woerner, D. R., Reagan, J. O., et al. (2015). Antimicrobial efficacy of a sulfuric acid and sodium sulfate blend, peroxyacetic acid, and cetylpyridinium chloride against Salmonella on inoculated chicken wings. J. Food Prot. 78, 1967–1972. doi: 10.4315/0362-028X.JFP-15-170
Selma, M. V., Allende, A., López-Gálvez, F., Conesa, M. A., and Gil, M. I. (2008). Disinfection potential of ozone, ultraviolet-C and their combination in wash water for the fresh-cut vegetable industry. Food Microbiol. 25, 809–814. doi: 10.1016/j.fm.2008.04.005
Shannon, E. M., Milillo, S. R., Johnson, M. G., and Ricke, S. C. (2011). Efficacy of cold pressed terpeneless valencia oil and its primary components on inhibition of Listeria species by direct contact and exposure to vapors. J. Food Sci. 76, M500–M503. doi: 10.1111/j.1750-3841.2011.02337.x
Sheu, C. W., and Freese, E. (1973). Lipopolysaccharide layer protection of Gram-negative bacteria against inhibition by long-chain fatty acids. J. Bacteriol. 115, 869–875.
Shors, T. (2001). “The best for last: bacteriophages,” in Understanding Viruses ed M. Johnson (Burlington, MA: Jones and Bartlett Publishers), 642–657.
Singh, N., Singh, R. K., Bhunia, A. K., and Stroshine, R. L. (2002). Efficacy of chlorine dioxide, ozone, and thyme essential oil or a sequential washing in killing Escherichia coli O157: H7 on lettuce and baby carrots. LWT Food Sci. Technol. 35, 720–729. doi: 10.1006/fstl.2002.0933
Sirsat, S. A., Muthaiyan, A., and Ricke, S. C. (2009). Antimicrobials for foodborne pathogen reduction in organic and natural poultry production. J. Appl. Poult. Res. 18, 379–388. doi: 10.3382/japr.2008-00140
Sklar, I. B., and Joerger, R. D. (2001). Attempts to utilize bacteriophage to combat Salmonella enterica serovar Entertidis infection in chickens. J. Food Saf. 21, 15–29. doi: 10.1111/j.1745-4565.2001.tb00305.x
Skrivanová, E., Marounek, M., Benda, V., and Brezina, P. (2006). Susceptibility of Escherichia coli, Salmonella sp. and Clostridium perfringens to organic acids and monolaurin. Vet. Med. 51, 81–88. doi: 10.17221/5524-VETMED
Smid, E. J., and Gorris, L. G. M. (1999). “Natural antimicrobials for food preservation,” in Handbook of Food Preservation, ed M. S. Rahman (New York, NY: Marcel Dekker), 285–308.
Spricigo, D. A., Bardina, C., Cortés, P., and Llagostera, M. (2013). Use of a bacteriophage cocktail to control Salmonella in food and the food industry. Int. J. Food Microbiol. 165, 169–174. doi: 10.1016/j.ijfoodmicro.2013.05.009
Stern, N. J., Svetoch, E. A., Eruslanov, B. V., Kovalev, Y. N., Volodina, L. I., Perelygin, V. V., et al. (2005). Paenibacillus polymyxa purified bacteriocin to control Campylobacter jejuni in chickens. J. Food Prot. 68, 1450–1453. doi: 10.4315/0362-028X-68.7.1450
Stevens, K. A., Sheldon, B. W., Klapes, N. A., and Klaenhammer, T. R. (1991). Nisin treatment for inactivation of Salmonella species and other Gram-negative bacteria. Appl. Environ. Microbiol. 57, 3613–3615.
Sulakvelidze, A. (2011). Safety by nature: potential bacteriophage applications-bacteriophages offer opportunities for safely managing bacterial infections. Microbe 6:122. doi: 10.1128/microbe.6.122.1
Summers, W. C. (1999). Felix d'Herelle and the Origins of Molecular Biology. New Haven, CT: Yale University Press.
Sun, H., Pan, Y., Zhao, Y., Jackson, W. A., Nuckles, L. M., Malkina, I. L. Mitloehner, F. M., et al. (2008). Effects of sodium bisulfate on alcohol, amine, and ammonia emissions from dairy slurry. J. Environ. Qual. 37, 608–614. doi: 10.2134/jeq2006.0446
Sutherland, I. W., Hughes, K. A., Skillman, L. C., and Tait, K. (2004). The interaction of phage and biofilms. FEMS Microbiol. Lett. 232, 1–6. doi: 10.1016/S0378-1097(04)00041-2
Tamblyn, K. C., and Conner, D. E. (1997). Bactericidal activity of organic acids against Salmonella typhimurium attached to broiler chicken skin. J. Food Prot. 60, 629–633. doi: 10.4315/0362-028X-60.6.629
Tangkham, W., Janes, M., and Lemieux, F. (2016). Prevalence and distribution of Campylobacter jejuni in small-scale broiler operations. J. Food Prot. 79, 75–81. doi: 10.4315/0362-028X.JFP-15-331
Teixeira, M. L., dos Santos, J., Silveira, N. P., and Brandelli, A. (2008). Phospholipid nanovesicles containing a bacteriocin-like substance for control of Listeria monocytogenes. Innov. Food Sci. and Emerg. Technol. 9, 49–53. doi: 10.1016/j.ifset.2007.05.001
Trimble, L. M., Alali, W. Q., Gibson, K. E., Ricke, S. C., Crandall, P., Jaroni, D., and Berrang, M. (2013). Salmonella and Campylobacter prevalence and concentration on pasture-raised broilers processed on-farm, in a mobile processing nit, and at small USDA-inspected facilities. Food Control 34, 177–182. doi: 10.1016/j.foodcont.2013.04.024
Tsai, L. S., Schade, J. E., and Molyneux, B. T. (1992). Chlorination of poultry chiller water: chlorine demand and disinfection efficiency. Poult. Sci. 71, 188–196. doi: 10.3382/ps.0710188
Turan, N. G. (2009). Nitrogen availability in composted poultry litter using natural amendments. Waste Manage. Res. 27, 19–24. doi: 10.1177/0734242X07087993
United States Department of Agriculture Food Safety Inspection Service. (2010). Mobile Slaughter Compliance Guide. Available online at: http://www.fsis.usda.gov/PDF/Compliance_Guide_Mobile_Slaughter.pdf (Accessed May 2018).
United States Department of Agriculture. (1995). Petitioned Substances: National Organic Standards Board-Nisin. Available online at: https://www.ams.usda.gov/rules-regulations/organic/national-list/n (Accessed September 29, 2018).
United States Department of Agriculture. (2015). Sodium Bisulfate - Agricultural Marketing Service. Available online at: https://www.ams.usda.gov/sites/default/files/media/Sodium%20Bi%20report%202015.pdf (Accessed May 26, 2018).
United States Department of Agriculture. (2016). Serotypes Profile of Salmonella Isolates from Meat and Poultry Products January 1998 through December 2014. Available online at: https://www.fsis.usda.gov/wps/portal/fsis/topics/data-collection-and-reports/microbiology/annual-serotyping-reports (Accessed October 31, 2018)
United States Department of Agriculture. (2017). Cost Estimates of Foodborne Illnesses. Available online at: https://www.ers.usda.gov/data-products/cost-estimates-of-foodborne-illnesses/. (Accessed September 22, 2018).
United States Department of Agriculture. (2018). Poultry - Production and Value Summary 2017. (Accessed October 31, 2018) Available online at: http://usda.mannlib.cornell.edu/usda/current/PoulProdVa/PoulProdVa-04-27-2018.pdf
United States Environmental Protection Agency (2013). Basic Information About Disinfectants in Drinking Water: Chloramine, Chlorine and Chlorine dioxide. Available online at: https://www.epa.gov/dwstandardsregulations (Accessed September 25, 2018).
United States Environmental Protection Agency (2018), July 30. Safer Chemical Ingredients List. Available online at: https://www.epa.gov/saferchoice/safer-ingredients (Accessed September 25, 2018).
United States Food Drug Administration (2018). GRAS Substances (SCOGS) Database. Available online at: https://www.fda.gov/food/ingredientspackaginglabeling/gras/scogs/default.htm (Accessed August 31, 2018).
Van Immerseel, F., Russell, J. B., Flythe, M. D., Gantois, I., Timbermont, L., Pasmans, F., et al. (2006). The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol. 35, 182–188. doi: 10.1080/03079450600711045
Van Loo, E. J., Alali, W., and Ricke, S. C. (2012). Food safety and organic meats. Annu. Rev. Food Sci. Technol. 3, 203–225. doi: 10.1146/annurev-food-022811-101158
Van Loo, E. J., Alali, W. Q., Welander, S., O'Bryan, C. A., Crandall, P. G., and Ricke, S. C. (2013). Consumers' interest in locally raised, small-scale poultry in Georgia. Agricult. Food Anal. Bacteriol. 3, 94–102.
Waller, R. (1994). Ground Water and the Rural Homeowner-United States Geological Survey. Available online at: https://pubs.usgs.gov/gip/gw_ruralhomeowner/ (Accessed October 31, 2018).
Wallner-Pendleton, E. A., Sumner, S. S., Froning, G. W., and Stetson, L. E. (1994). The use of ultraviolet radiation to reduce Salmonella and psychrotrophic bacterial contamination on poultry carcasses. Poult. Sci. 73, 1327–1333. doi: 10.3382/ps.0731327
Warburton, R. (2014). Peracetic Acid in the Fresh Food Industry. Food Safety Magazine. Available online at: www.foodsafetymagazine.com/signature-series/peraceticacid-in-the-fresh-food-industry/
Windisch, W., and Kroismayr, A. (2006). “The effects of phytobiotics on performance and gut function in monogastrics,” in World Nutrition Forum: The Future of Animal Nutrition (Vienna), 85–90.
Wong, E., Linton, R. H., and Gerrard, D. E. (1998). Reduction of Escherichia coli and Salmonella senftenbergon pork skin and pork muscle using ultraviolet light. Food Microbiol. 15, 415–423. doi: 10.1006/fmic.1998.0185
Wood, S. R., Kirkham, J., Marsh, P. D., Shore, R. C., Nattress, B., and Robinson, C. (2000). Architecture of intact natural human plaque biofilms studied by confocal laser scanning microscopy. J. Dent. Res. 79, 21–27. doi: 10.1177/00220345000790010201
Woolthuis, C. H., and Smulders, F. J. (1985). Microbial decontamination of calf carcasses by lactic acid sprays. J. Food Prot. 48, 832–837. doi: 10.4315/0362-028X-48.10.832
Yabin, L.i., Slavik, M. F., Walker, J. T., and Xiong, H. (1997). Pre-chill spray of chicken carcasses to reduce Salmonella typhimurium. J. Food Sci. 62, 605–607. doi: 10.1111/j.1365-2621.1997.tb04441.x
Yang, S. C., Lin, C. H., Sung, C. T., and Fang, J. Y. (2014). Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front. Microbiol. 5:241. doi: 10.3389/fmicb.2014.00241
Zoetis (2018). Amplon. Available online at: https://www.zoetisus.com/products/food-safety/amplon.aspx (Accessed September 28, 2018).
Zohri, M., Alavidjeh, M. S., Haririan, I., Ardestani, M. S., Ebrahimi, S. E. S., Sani, H. T., et al. (2010). A comparative study between the antibacterial effect of nisin and nisin-loaded chitosan/alginate nanoparticles on the growth of Staphylococcus aureus in raw and pasteurized milk samples. Probiotics Antimicrob. Proteins 2, 258–266. doi: 10.1007/s12602-010-9047-2
Keywords: reuse water, mobile poultry-processing unit, organic, poultry processing, bacteriocins, botanicals, organic acids, bacteriophage
Citation: Micciche AC, Rubinelli PM and Ricke SC (2018) Source of Water and Potential Sanitizers and Biological Antimicrobials for Alternative Poultry Processing Food Safety Applications. Front. Sustain. Food Syst. 2:82. doi: 10.3389/fsufs.2018.00082
Received: 05 October 2018; Accepted: 20 November 2018;
Published: 06 December 2018.
Edited by:
Joshua B. Gurtler, Agricultural Research Service, United States Department of Agriculture, United StatesReviewed by:
Keith Warriner, University of Guelph, CanadaTam L. Mai, IEH laboratories and Consultant Group, United States
Copyright © 2018 Micciche, Rubinelli and Ricke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven C. Ricke, c3JpY2tlQHVhcmsuZWR1
 Andrew C. Micciche
Andrew C. Micciche Peter M. Rubinelli
Peter M. Rubinelli Steven C. Ricke
Steven C. Ricke