- 1Western Wheat Quality Laboratory, Agricultural Research Service, United States Department of Agriculture, Pullman, WA, United States
- 2Department of Plant Sciences, North Dakota State University, Fargo, ND, United States
Durum wheat is an important crop worldwide. In many areas, durum wheat appears to have competitive yield, and biotic and abiotic advantages over bread wheat. What limits durum production? In one respect, the comparatively more limited processing and food functionality. Two traits directly relate to these limitations: kernel texture (hardness) and gluten strength. We have addressed both using ph1b-mediated translocations from bread wheat. For kernel texture, ca. 28 Mbp of chromosome 5D short arm replaced about 20 Mbp of 5B short arm. Single Kernel Characterization System (SKCS) hardness was reduced from ca. 80 to 20 as the puroindolines were expressed and softened the endosperm. Break flour yields increased from 17 to >40%. Straight-grade flour had low starch damage (2%), and a mean particle size of 75 μm. Crosses with CIMMYT durum lines all produced soft kernel progeny and a high degree of genetic variance for milling and baking quality. Solvent Retention Capacities (SRC) and cookie diameters were similar to soft white hexaploid wheat, showing that soft durum can be considered a “tetraploid soft white spring wheat.” Regarding gluten strength, CIMMYT durums contributed a high genetic variance, with the “best” progeny exhibiting Na-dodecylsulfate (SDS) sedimentation volume, SRC Lactic Acid and Mixograph characteristics that were similar to medium-gluten-strength U.S. hard red winter. The best loaf volume among these progeny was 846 cm3 at ca. 12.8% flour protein. To further address the issue of gluten strength, Soft Svevo was crossed with durum lines possessing Dx2+Dy12 and Dx5+Dy10. Bread baking showed that Dx5+Dy10 was overly strong, whereas Dx2+Dy12 significantly improved bread loaf volume. The best progeny produced a loaf volume of 1,010 cm3 at 12.1% protein. As a comparison, the long-term in-house regression for loaf volume-flour protein for hard “bread” wheats is 926 cm3 at 12.1% protein. Obviously, from these results, excellent bread making potential has been achieved.
Introduction
High kernel hardness (texture) is a defining trait of durum wheat (Triticum turgidum subsp. durum) grain. Kernel texture dictates many aspects of durum milling and utilization, and in some ways, limits its culinary uses. Durum wheat also lacks the D genome, and thus it also lacks the Glu-D1 locus for the high molecular weight (HMW) glutenins Dx2+Dy12 and Dx5+Dy10. Consequently, the elasticity and extensibility of durum doughs are often viewed as inferior to bread wheat (Triticum aestivum) (Ammar et al., 2000). The research reviewed here shows how both kernel texture and dough rheology can be manipulated via ph1b-mediated homoeologous recombination and the transfer of genetic material from bread wheat to durum wheat.
Endosperm softness in wheat is controlled by the Puroinoline genes/proteins, Pina and Pinb, which reside at the Hardness (Ha) locus on the distal end of chromosome 5D short arm (5DS) (Morris, 2002; Bhave and Morris, 2008). When both genes are in a functional state, the endosperm is soft, but when either gene is absent or its sequence altered, harder endosperm is observed (Giroux and Morris, 1997, 1998; Morris and Beecher, 2012). When durum wheat formed, both genes from both diploid progenitors (A and S = B sub-genomes) were lost, and thus durum has the hardest kernels of all wheats. Because of the high kernel hardness of durum grain, roller milling does not aim to produce flour, but rather coarse semolina. Attempts to further reduce particle size of semolina result in unacceptably high starch damage and excessive dough water absorption.
Dough strength is a complex interplay between the HMW glutenin subunits, low molecular weight (LMW) glutenins, gliadins, and non-protein endosperm constituents. In bread wheat, the most prominent locus that contributes to dough elasticity and extensibility is Glu-D1, with two allelic variants, Dx2+Dy12 and Dx5+Dy10. In general, the Dx5+Dy10 allele is considered the “stronger” allele and is more desirable for bread quality.
Homoeologous Recombination
Homoeologous recombination in polyploid wheat can be achieved by eliminating the restrictive control of the Pairing homoeologous-1 (Ph1) locus, which restricts pairing to homologous chromosomes and prevents homoeologs from pairing. A line carrying an induced mutation in Ph1 (ph1b) was used in crossing a Langdon durum disomic substitution line carrying the pair of 5D chromosomes from Chinese Spring (Morris et al., 2011, 2015). Subsequently, several recombinant lines were isolated. Interestingly, all carry an identical 28 Mbp of 5DS which replaced 20 Mbp of 5BS (Boehm et al., 2017c; Ibba et al., in press). The specific cross-over occurred in a 39-bp region in the middle of a putative gene. The translocated 5DS fragment carries an entire and intact Ha locus with normal expression and endosperm softening.
Milling and Baking Performance
Soft kernel durum wheat was found to mill similar to soft white hexaploid wheats. Break flour yields increased from ~17% (normal durum) to >40% (Murray et al., 2016). Straight-grade flour had low starch damage (2%), and a mean particle size of 75 μm. Ash contents of flours from soft durums were lower than those obtained from hard durum. All crosses with a number of CIMMYT durum lines produced soft kernel progeny and a high degree of genetic variance for milling and baking quality (Boehm et al., 2017a,b). Family mean Single Kernel Characterization System (SKCS) hardness ranged from 5.8 to 23.0. Family mean break flour yields ranged from 38.2 to 42.8%. Ash and starch damage of the straight-grade flours were ~0.41 and ~1.5%, respectively. Solvent Retention Capacity (SRC) Water, Na-carbonate, and Sucrose were low and typical of soft white hexaploid wheats. Cookie diameters ranged from 9.16 to 9.48 cm, and were similar to soft white hexaploid wheat. Thus, soft durum can be considered a “tetraploid soft white spring wheat.” On a per unit weight of flour produced, soft durum required only from one-fifth to one-third the energy as hard durum (Heinze et al., 2016).
Durum wheat has variable but limited baking quality (Ammar et al., 2000). The very hard kernel texture affects milling, particle size, starch damage, and dough water absorption. Consequently, it is difficult to make direct comparisons between durum and soft durum. Murray et al. (2017) used near-isogenic lines of Svevo durum to show that the softer kernel was associated with about a 3% decrease in Na-dodecylsulfate (SDS) sedimentation volume, 17% lower SRC Water, 9% lower SRC Lactic acid, and about 10% lower SRC Sucrose. Dough water absorption on the 10-g Mixograph was 5% lower for soft durum flour, and about 10% lower on the 65-g Farinograph. Alveograph parameters were dramatically affected since analyses were performed at constant dough water absorption. Consequently, soft durum flours exhibited lower W and P, but similar L. Since the near-isogenic lines were at similar protein levels, differences were interpreted as being a direct result of “over hydrating” the soft durum doughs. Similarly, with AACCI 100-g “pup” bread loaf testing, optimum water absorption for durum flour was 66.5% and 58% for soft durum flour. Among the CIMMYT progeny, a wide range of SDS sedimentation volume and SRC Lactic Acid was observed. Similarly, bread loaf volumes varied significantly both within, but more so among families. Overall the loaf volume range for individual lines ranged from a very poor 629 cm3 to a moderate 864 cm3 at about 12% flour protein.
Introgression of Glu-D1
More recently, the Glu-D1 alleles Dx2+Dy12 and Dx5+Dy10 of (Lukaszewski, 2003) were introgressed into the soft kernel durum variety Soft Svevo (Figures 1, 2). Multiple full sibs possessing each glutenin allele were evaluated for milling and pan bread baking.
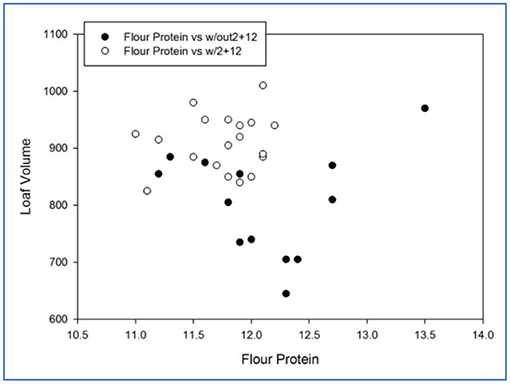
Figure 1. Bread loaf volume of soft durum lines with or without Dx2+Dy12 (flour protein in percent, loaf volume in cm3).
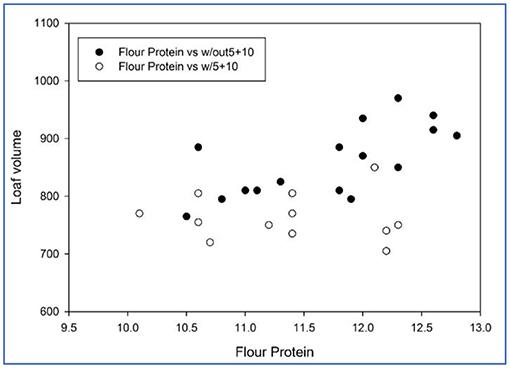
Figure 2. Bread loaf volume of soft durum lines with or without Dx5+Dy10 (flour protein in percent, loaf volume in cm3).
Those lines with allele Dx2+Dy12 exhibited superior loaf volumes (Figure 1). The best progeny line produced a loaf volume of 1,010 cm3 at 12.1% protein. As a comparison, the long-term in-house regression for loaf volume-flour protein for hard red “bread” wheats is 926 cm3 at 12.1% protein. Figure 2 shows the sibs with or without Dx5+Dy10. Across flour protein contents, those lines without the Glu-D1 translocation were superior. The lines with Dx5+Dy10 generally lacked extensibility, were termed “bucky” and could not reach full volume potential. This allele actually decreased bread quality.
And as described above, the best line derived from the CIMMYT crosses had a loaf volume of 864 cm3 at ~12% protein.
Obviously, from these results, excellent bread making potential can be achieved using Dx2+Dy12 in the Soft Svevo background. Sissons et al. (2019) however found no improvement in bread quality by adding Dx2+Dy12 or Dx5+Dy10 to hard Svevo.
Conclusions
The soft kernel trait in durum affects nearly every aspect of milling and baking quality. SKCS hardness, break flour yield and flour yield were similar to commercial soft white wheat cultivars. With the exception of dough water absorption, dough strength was essentially unchanged and reflected the inherent gluten properties of the durum background. That said, the introgression of Glu-D1 alleles dramatically changed dough strength and bread volume, with Dx2+Dy12 showing superiority over Dx5+Dy10. With the caveat of dough water absorption, soft kernel texture and bread quality are not in opposition to one another. The soft kernel trait itself appears to exert no negative affect on yield, agronomic performance or pest resistance (Kiszonas et al., 2019).
Author Contributions
CM wrote the manuscript. CM, AK, JM, JB, MI, and XC conceived the research. AK, JM, JB, MI, MZ, and XC performed the research and analyses, and contributed to the writing.
Funding
Funding was provided in part by USDA NIFA 2013-67013-21226 and 2019-67013-29164, and USDA ARS CRIS Proj. 2090 43440-007-00-D.
Conflict of Interest
CM is a co-inventor of soft durum wheat whose rights are assigned to the USDA.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Carlos Guzman, Karim Ammar, Claudia Carter, Teng Vang, Domenico Lafiandra, Marco Simeone, Leonard Joppa, Stacey Sykes, and the staff of the Western Wheat Quality Lab.
References
Ammar, K., Kronstad, W. E., and Morris, C. F. (2000). Breadmaking quality of selected durum wheat genotypes and its relationship with high molecular weight glutenin subunits, allelic variation and gluten protein polymeric composition. Cereal Chem. 77, 230–236. doi: 10.1094/CCHEM.2000.77.2.230
Bhave, M., and Morris, C. F. (2008). Molecular genetics of puroindolines and related genes: allelic diversity in wheat and other grasses. Plant Mol. Biol. 66, 205–219. doi: 10.1007/s11103-007-9263-7
Boehm, J. D. Jr., Ibba, M. I., Kiszonas, A. M., and Morris, C. F. (2017a). End-use quality of CIMMYT-derived soft-kernel durum wheat germplasm: I. Grain, milling and soft wheat quality. Crop. Sci. 57, 1475–1484. doi: 10.2135/cropsci2016.09.0774
Boehm, J. D. Jr., Ibba, M. I., Kiszonas, A. M., and Morris, C. F. (2017b). End-use quality of CIMMYT-derived soft-kernel durum wheat germplasm: II. Dough strength and pan bread quality. Crop Sci. 57, 1485–1494. doi: 10.2135/cropsci2016.09.0775
Boehm, J. D. Jr., Zhang, M., Cai, X., and Morris, C. F. (2017c). Molecular and cytogenetic characterization of the 5DS-5BS chromosome translocation conditioning soft kernel texture in durum wheat. Plant Genome 10, 1–11. doi: 10.3835/plantgenome2017.04.0031
Giroux, M. J., and Morris, C. F. (1997). A glycine to serine change in puroindoline b is associated with wheat grain hardness and low levels of starch-surface friabilin. Theor. Appl. Genet. 95, 857–864. doi: 10.1007/s001220050636
Giroux, M. J., and Morris, C. F. (1998). Wheat grain hardness results from highly conserved mutations in the friabilin components puroindoline a and b. Proc. Natl. Acad. Sci. U.S.A. 95, 6262–6266. doi: 10.1073/pnas.95.11.6262
Heinze, K., Kiszonas, A. M., Murray, J. C., Morris, C. F., and Lullien-Pellerin, V. (2016). Puroindoline genes introduced into durum wheat reduce milling energy and change milling behavior similar to soft common wheats. J. Cereal Sci. 71, 183–189. doi: 10.1016/j.jcs.2016.08.016
Ibba, M. I., Zhang, M., Cai, X., and Morris, C. F. (in press). Identification of a conserved ph1b-mediated 5DS-5BS crossing over site in soft-kernel durum wheat (Triticum turgidum subsp. durum) lines. Euphytica. doi: 10.1007/s10681-019-2518-y
Kiszonas, A. M., Higginbotham, R., Chen, X. M., Garland-Campbell, K., Bosque-Perez, N. A., Pumphrey, M., et al. (2019). Agronomic traits in durum wheat germplasm possessing puroindoline genes. Agron. J. 111, 1254–1265 doi: 10.2134/agronj2018.08.0534
Lukaszewski, A. (2003). Registration of six germplasms of durum wheat with introgressions of the Glu-D1 locus. Crop Sci. 43, 1138–1139. doi: 10.2135/cropsci2003.1138
Morris, C. F. (2002). Puroindolines: the molecular genetic basis of wheat grain hardness. Plant Mol. Biol. 48, 633–647. doi: 10.1023/A:1014837431178
Morris, C. F., and Beecher, B. S. (2012). The distal portion of the short arm of wheat (Triticum aestivum L.) chromosome 5D controls endosperm vitreosity and grain hardness. Theor. Appl. Genet. 125, 247–254. doi: 10.1007/s00122-012-1830-x
Morris, C. F., Casper, J., Kiszonas, A. M., Fuerst, E. P., Murray, J., Simeone, M. C., et al. (2015). Soft kernel durum wheat–a new bakery ingredient? Cereal Foods World 60, 76–83. doi: 10.1094/CFW-60-2-0076
Morris, C. F., Simeone, M. C., King, G. E., and Lafiandra, D. (2011). Transfer of soft kernel texture from Triticum aestivum to durum wheat, Triticum turgidum ssp. durum. Crop Sci. 51, 114–122. doi: 10.2135/cropsci2010.05.0306
Murray, J. C., Kiszonas, A. M., and Morris, C. F. (2017). Influence of soft kernel texture on the flour, water absorption, rheology, and baking quality of durum wheat. Cereal Chem. 94, 215–222. doi: 10.1094/CCHEM-06-16-0163-R
Murray, J. C., Kiszonas, A. M., Wilson, J. D., and Morris, C. F. (2016). Effect of soft kernel texture on the milling properties of soft durum wheat. Cereal Chem. 93, 513–517. doi: 10.1094/CCHEM-06-15-0136-R
Keywords: durum wheat, kernel texture, cookie quality, bread baking, gluten strength
Citation: Morris CF, Kiszonas AM, Murray J, Boehm J Jr, Ibba MI, Zhang M and Cai X (2019) Re-evolution of Durum Wheat by Introducing the Hardness and Glu-D1 Loci. Front. Sustain. Food Syst. 3:103. doi: 10.3389/fsufs.2019.00103
Received: 12 April 2019; Accepted: 23 October 2019;
Published: 15 November 2019.
Edited by:
Aldo Ceriotti, Italian National Research Council (CNR), ItalyReviewed by:
Somnath Mandal, Uttar Banga Krishi Viswavidyalaya, IndiaDidier Marion, INRA Centre Angers-Nantes Pays de la Loire, France
Copyright © 2019 Morris, Kiszonas, Murray, Boehm, Ibba, Zhang and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Craig F. Morris, Y3JhaWcubW9ycmlzQHVzZGEuZ292
 Craig F. Morris
Craig F. Morris Alecia M. Kiszonas
Alecia M. Kiszonas Jessica Murray
Jessica Murray Jeff Boehm Jr.
Jeff Boehm Jr. Maria Itria Ibba1
Maria Itria Ibba1 Mingyi Zhang
Mingyi Zhang